Note: Descriptions are shown in the official language in which they were submitted.
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
1
LIQUID CARRIER FOR ORAL DELIVERY OF A PHARMACOLOGICALLY ACTIVE AGENT
FIELD OF THE INVENTION
The present invention relates to a liquid carrier for oral delivery of a
pharmacologically active agent
dissolved or suspended therein. The invention also relates to a method of
manufacture of the carrier
and a pharmaceutical composition comprising the carrier and a
pharmacologically active agent
dissolved or suspended therein. Furthermore, the invention relates to uses of
the pharmaceutical
composition.
BACKGROUND OF THE INVENTION
Pharmaceutically active agents can be administered perorally dissolved or
suspended in a lipid carrier.
This kind of administration is advantageous for active agents that are desired
not to be released in
quantity prior to their passage through the duodenum. It is also useful for
active agents, which are
poorly soluble or practically insoluble in aqueous liquids but are at least
somewhat soluble in lipid
carriers.
A problem with lipids is their early and variable (from person to person)
degradation during passage
through the upper gastrointestinal tract, and the concomitant early and
unpredictable release of the
active agent. This unpredictability may have contributed to the reluctance to
use lipid carriers in the
pharmaceutical field.
The degradation of known lipid carriers is due to the sensitivity of their
ester linkages to gastric and
jejunal lipases. Since there is substantial variation from person to person in
regard of the excretion of
gastrointestinal enzymes, lipid carriers may be degraded at substantially
differing rates by different
persons, and their contents thus released in an unpredictable manner.
Moreover, structurally differing triglycerides are degraded in the
gastrointestinal tract at substantially
different rates (Knutsson L et al., Gastrointestinal metabolism of a vegetable-
oil emulsion in healthy
subjects. Am J Clin Nutr doi: 10.3945). This suggests that the chemical
composition of triglycerides in
a lipid carrier should be strictly controlled to improve the gastrointestinal
degradation rate of the
carrier.
OBJECTS OF THE INVENTION
One object of the present invention is to provide a liquid carrier of the
aforementioned kind, which
has improved stability against degradation in the gastrointestinal tract, in
particular against
degradation in the upper gastrointestinal tract, and a method for its
production.
Another object of the present invention is to provide a corresponding
pharmaceutical composition
and a method for its production.
A further object of the invention is to provide uses of the vehicle and of the
composition.
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
2
Additional objects of the invention will become evident from the study of the
following summary of
the invention, the description of preferred embodiments thereof, and the
appended claims.
SUMMARY OF THE INVENTION
According the present invention is disclosed a fluid carrier comprising a
first liquid and a second
liquid, the first liquid consisting of an open-chain silicone oil of the
formula [(CH3)351-0]-[(CH2)251-O]n-
[Si(CH3)3] and the second liquid consisting of or comprising a polar lipid
material. The polar lipid
material of the second liquid and the non-volatile silicone oil of the first
liquid are substantially
immiscible. In this application, "substantially immiscible" designates a
degree of miscibility of less
than 1 % by weight, that is, each of the liquids is incapable of dissolving
more than 1 % by weight of
the other liquid. The fluid carrier of the invention is useful in the
preparation of pharmaceutical
compositions for peroral administration, such as the compositions described
below.
When submitting them to a dispersing treatment, such as by a stirring at a
high shear rate, the two
immiscible liquids of the fluid carrier form unstable dispersions. In this
application is understood by
"unstable dispersion" a dispersion comprising two immiscible liquids, which
separates into its
components within a week or a month when stored at room temperature (20 C).
The non-volatile silicone oil of the first liquid is a dimethicone.
Dimethicones are widely used in
pharmaceutical and personal care applications. They are mixtures of fully
methylated linear siloxane
polymers, i.e., polydimethylsiloxanes, and are available in different nominal
viscosities, ranging from
about 1 cSt (centistoke) to about 100,000 cSt. In the art dimethicones with
high viscosities are known
to be used in topical formulations as emollient and antifoaming agent. They
are also known to be
used therapeutically in oral formulations for the treatment of flatulence.
Dimethicones with a
nominal viscosity of 50 cSt or lower are intended for external use only. The
silicone oil of the
invention has a viscosity of 50 cSt or more, preferably of 100 cSt or more.
Dimethicones are physiologically and chemically inert materials, which are not
metabolized by the
body upon oral ingestion. They leave the body unaltered with the faeces.
Dimethicones are generally
regarded to be essentially non-toxic and non-irritant. They protect the active
substance through the
upper gastrointestinal tract, whereas the polar lipid material promotes the
dissolution of the
formulation in the gut as well as the uptake and thereby the oral
bioavailability.
The polar lipid material of the second liquid can be described as lipids
capable of interaction with
water (as defined in D. Small, The Physical Chemistry of Lipids. Plenum Press
1986, section 4.3), for
example formed of membrane lipid(s), that is, lipid constituents of biological
membranes. Membrane
lipids contain a polar, hydrophilic head group and one or more lipophilic
hydrocarbon chains. This
combination makes the membrane lipid molecules amphipathic and enables them to
associate both
with water and oil. Such membrane lipids can be classified according to their
chemical structure,
which is a function of how the polar head group is linked to the lipophilic
chains. Sphingolipids (linked
by sphingosine) and glycerolipids (linked by glycerol) are the two main
groups. Depending on the
characteristics of the polar head group sphingolipids and glycerolipids can be
further classified as
phospholipids comprising a phosphate ester head group and glycolipids
comprising a carbohydrate
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
3
head group. Depending on the specific nature of the carbohydrate group,
membrane lipids are
sometimes called, for instance, galactolipids, which are glycerolipids with
galactose in the polar head
group. Examples of common membrane lipids are phosphatidylcholine (PC),
phosphatidylethanolamine (PE), and digalactosyldiacylglycerol (DGDG). Membrane
lipids of interest
can be extracted from, for example, egg yolk (egg lecithin), milk and dairy
products, soybeans (soy
lecithin), other oil crops, oat kernels, and other cereal and grains. These
extracts can be further
treated to obtain, for instance, PC from soy beans and galactolipids from
oats. Preferred polar lipids
are galactolipids, in particular galactolipids from oat kernels, or
phospholipids from soybeans (soy
lecithin or soy-PC). Examples of synthetic phospholipids comprise
dioleoylphosphatidylcholine and
dioleoylphosphatidylethanolamine. Other lipids capable of interaction with
water are
monoglycerides, for example monooleylglycerol.
The first liquid is preferably comprised by the vehicle in an amount of from
50 % by weight to 90 % by
weight. The second liquid is preferably comprised by the vehicle in an amount
of from 10 % by weight
to 50 % by weight. It is preferred by the vehicle to not comprise more than 10
% of components other
than the first and second lipids, more preferred not more than 5 % by weight
or 2 % by weight or
even less than 1 % by weight.
The fluid carrier of the invention is characterised to comprise two immiscible
liquids, which form
dispersions that separate into their components in a short time (days to weeks
when stored at room
temperature).
The fluid carrier of the invention provides for superior incorporation of dry
powders, resulting in good
stability of the suspensions formed.
According to an important aspect of the invention the fluid carrier, when
mixed with a finely
dispersed solid, e.g. a fine powder, insoluble in the liquids, forms a stable
creamy or ointment¨like
suspension.
When stored at room temperature, the stable creamy or ointment-like stable
suspension of the
invention is stable for a month or several months and even for a year or two
years or more, that is,
does not separate into its components.
Depending on the chemical and physical nature of the solid and its particle
size, a minimum amount
of the solid is required to form the stable suspension of the invention. For a
given substance of given
physical and chemical nature as well of particle size, this minimum amount can
be easily determined
by experimentation, which is within the reach of a person skilled in the art.
With some substances,
such as hydrocortisone, stable suspensions are obtained by incorporating as
little as 3 % by weight of
the substance into the fluid carrier. A preferred average particle size (with
50% or more of the
particles being below average) is one of less than 550 m or 250 p.m, in
particular of less than 1001.1m
or 20 p.m, most preferred of less than 5 p.m or 2 pm. The stabilizing effect
of the particulate solid of
the invention can also be obtained by a mixture of particulate substances,
such as a particulate
pharmacologically or cosmetically active agent, for instance hydrocortisone,
and a particulate
pharmaceutically or cosmetically acceptable excipient, such as
microcrystalline cellulose.
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
4
The fluid carrier of the invention comprising a storage-stabilizing amount of
a particulate solid
incorporated to it is termed first composition of the invention. The
incorporated particulate solid can
be a pharmacologically active agent or a mixture of pharmacologically active
agent and
pharmaceutically acceptable excipient. The first composition of the invention
is of a creamy or pasty
or ointment-like nature. It can be administered orally as such or in a
capsule, for instance a hard or
soft gelatin capsule.
A pharmaceutically or cosmetically acceptable excipient for use in the
invention is preferably a
traditional pharmaceutical tablet excipient essentially insoluble in the first
and second liquids, that is,
of a solubility (w/w) of less than 1.0, 0.5 or 0.1 %, preferably of less than
0.01 %, selected from filler,
binder, glidant, anti-adherent, lubricant, disintegrant, anti-oxidant, and
their mixtures. Colorants and
flavourings may be used as supplementary excipients in addition to the
aforementioned traditional
excipients. The excipient can comprise one or more of silicon dioxide,
titanium dioxide, aluminium
oxide, calcium sulphate, calcium carbonate, dibasic calcium phosphate
dihydrate, microcrystalline
cellulose, powdered cellulose, cyclodextrin, bentonite, kaolin, lactose,
magnesium aluminium silicate,
magnesium carbonate, magnesium oxide, magnesium trisilicate, and talc.
According to a further preferred aspect of the invention is disclosed a
mouldable second composition
of the invention obtained by incorporating an amount of particulate
pharmacologically active agent
or a combination of pharmacologically active agent and pharmaceutically
acceptable excipient into
the composition in excess of an amount required for obtaining the stable
creamy or ointment-like
suspension of the invention. The second composition of the invention is
mouldable at room
temperature like a dough or potter's clay. The mouldable composition can be
extruded from a nozzle,
and the extrudate segmented. The segments of the size of a medical tablet for
peroral administration
can be rounded off mechanically in suitable pharmaceutical equipment after
adding an anti-adherent
like finely dispersed calcium carbonate, silica or talc. The so obtained
tablet cores can be covered with
a desired single layer or multi-layered coat, for instance a sugar coat or an
enteric coat. The tablets
formed are storage-stable, that is, can be stored in a closed container at
room temperature for a year
or two years or more without suffering a loss of pharmacologically active
agent exceeding 5 % or 10 %
by weight. Alternatively, the aforementioned segments or coarse particles of
uniform weight of the
second mouldable composition can be formed into tablets of uniform shape by
pressing them into
moulds of desired shape, removing excess composition, and expelling the so
formed tablets from the
moulds. The mouldable second composition of the invention comprises at least
75 % by weight, more
preferred at least 85 % by weight, and most preferred at least 90 % by weight
of particulate
pharmacologically active agent or a combination of particulate
pharmacologically active agent and
particulate pharmaceutical excipient.
In the following the invention will be explained in more detail by reference
to a drawing and a
number of examples.
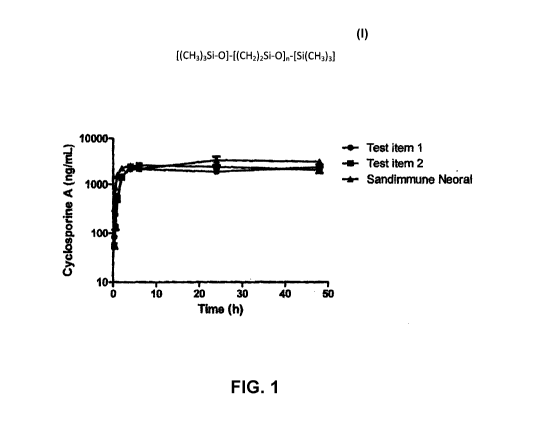
DESCRIPTION OF THE FIGURE
The Figure illustrates the gastrointestinal absorption of cyclosporine A
comprised by a composition of
the invention in comparison with two prior art compositions.
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
DESCRIPTION OF PREFERRED EMBODIMENTS
Materials. Dimethicones of different viscosities were obtained from Dow
Corning (DC 200 Fluids).
Akoline MCM and Capmul MCM EP (medium-chain monoglycerides) were obtained from
AAK,
5 Sweden and Abitec Corp., USA, respectively. Tween 80, monoolein (technical
grade), cholesterol and
hydrocortisone were obtained from Sigma-Aldrich. Potato starch was obtained
from KMC (Pharma
M20). Dextrose was obtained from Risenta, Sweden. Phosal 50 PG, a standardised
mixture of at least
50 % by weight of phosphatidylcholine, propylene glycol, sunflower mono- &
diglycerides, and
ascorbyl palmitate, was obtained from Phospholipid GmbH, Germany. Lipoid S 35
FS and
Phospholipon 50 were obtained from Lipoid AG, Switzerland.
Table 1. Vehicle composition components
Short name Supplier, Trade name Chemical name, CAS No. Lot No.
DC 200A Dow Corning , DC 200 Dimethicone, 9006-65-9 5627357
Fluid 1 000 cSt
1DC 200B Dow Corning , DC 200 Dimethicone, 9006-65-9
1 Fluid 12 500 cSt
1DC 200C Dow Corning , DC 200 Dimethicone, 9006-65-9
Fluid 100 000 cSt
Rape seed oil Eldorado Food grade rape seed oil purchased
in a grocery store
DOPC Lipoid DOPC Dioleoylphosphatidylcholine, 566073-
1/32
10015-85-7
DOPE Lipoid DOPE Dioleoylphosphatidylethanolamine, 656006-
0012
2462-63-7
MOG Fluka (Sigma-Aldrich),
Monooleoylglycerol, 25496-72-4 1384627
Monoolein
MCM Aarhus Karlshamn, Akoline Medium chain monoglycerides
8192270
MCM
100 Lipoid 5100 Soy bean lecithin, 8002-43-5 790551-
7/910
S35 Lipoid S 35 FS
Phospholipon Lipoid Phospholipon 50
Phosal 50 PG Phospholipid GmbH At least 50 % phosphatidylcholine,
propylene glycol, sunflower mono- &
diglycerides, ascorbyl palmitate
ween 80 Sigma-Aldrich Polysorbate 80, 9005-65-6
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
6
Table 2. Model substances for incorporation into the vehicle of the invention
'Model substance CAS No Supplier 'Lot No
Wavelength, nm*
1
Cholesterol 57-88-5 Sigma-Aldrich 057K0683
Hydrocortisone 50-23-7 Sigma-Aldrich 010M1568
Potato starch KMC (Pharma Sigma
M20) BCBC0008V
Dextrose 14431-43-7 Risenta
Cyanocobalamin (Vitamin B12) 68-19-9 Sigma-Aldrich 030M1567 550
Methylene blue 61-73-4 Sigma-Aldrich BCBD4688V
599
Vanillin 121-33-5 Sigma-Aldrich 580107-209 310
Tyrosine 60-18-4 Sigma-Aldrich 1437266V 278
* Wavelength used in the dissolution experiments described below.
EXAMPLE 1. Vehicles
A number of vehicles of the invention are listed in Table 3. Vehicle 6 does
not comprise dimethicone
and is not a vehicle of the invention.
Table 3. Vehicles
No. Composition Appearance after mixing Appearance on
standing
(days to weeks)
1 75 % DC 200 Fluid 12,500 cSt, Opaque homogeneous paste, Two liquid
layers
25 % Akoline MCM ointment-like consistency
2 75 % DC 200 Fluid 100,000 Opaque homogeneous
paste, Two liquid layers
cSt, 25 % Akoline MCM ointment-like consistency
3 75 % DC 200 Fluid 12,500 cSt, Opaque homogeneous paste, Two liquid
layers
25 % Monoolein ointment-like consistency
4 75 % DC 200 Fluid 12,500 cSt, Opaque homogeneous paste, Two liquid
layers
25 % Tween 80 ointment-like consistency
5 75 % DC 200 Fluid 12,500 cSt, Opaque homogeneous paste, Two liquid
layers
25 % Tween 80 ointment-like consistency
6 75 % Phosal 50 PG, 25 % Opaque homogeneous paste, Two liquid
layers
Akoline MCM ointment-like consistency
7 75 % DC 200 Fluid 1000 cSt, Opaque homogeneous paste, Two liquid
layers
25 % Akoline MCM liquid consistency
8 75 % DC 200 Fluid, 500 cSt, 25 Opaque homogeneous paste, Two liquid
layers
% Akoline MCM liquid consistency
Vehicle No. 7 was filled in a hard-gelatin capsule (Licaps, size 1; Capsugel)
and stored at room
temperature for more than 3 months without any noticeable detrimental effect
on the capsule.
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
7
EXAMPLE 2. Dispersions of solid powderous agents in vehicles of Table 3
The powderous agents used are practically insoluble in the vehicles. Mixing of
the relatively unstable
liquid (pasty) compositions of Table 3 with solid powderous agents resulted in
storage-stable creamy
suspensions or mouldable masses (Table 4).
Table 4. Storage-stable suspensions (compositions of the invention)
No. Vehicle (Table 1) Incorporated Amount of incorporated agent Appearance
agent
1 1 Dextrose 21 % Smooth paste
2 1 Dextrose 38 % Smooth paste
3 2 Dextrose 25 % Smooth viscous paste
4 1 Potato starch 25 % Smooth paste
5 1 Cholesterol 25 % Smooth paste
6 1 Hydrocortisone 3 % Smooth paste
7 1 Cellulose powder 93 % Mouldable mass
Hydrocortisone 1 %
8 5 Cellulose powder 90 % Mouldable mass
Tyrosine 5%
EXAMPLE 3. Compositions of the invention comprising astaxanthin
In Table 5 several formulations with AstaREAL, a powder rich in the natural
antioxidant astaxanthin,
are presented. Compositions No. 1 and 2 show that the solubility of AstaREAL
in water and ethanol is
poor. Compositions No. 3 and 4, containing two phospholipid materials both
resulted in slurries,
which sedimented on standing. On the other hand, compositions No. 5 to 10 were
all stable for
several months and may be administered orally to a mammal, either by mixing
with food and/or a
foodstuff, or by means of capsules or syringes.
Table 5. AstaREAL compositions (batch size 5-10 g if not otherwise stated)
No. Batch AstaREAL Vehicle Treatment Appearance,
Appearance,
(% by w.) at start 4 months at 20
C
1 ACA100511-1 24.2 Water Mixing with Dark red slurry
Sediment, clear
spatula supernatant
2 ACA100511-2 24.7 Et0H abs. Mixing with Dark red
slurry Sediment, dark
spatula red
supernatant
3 ACA100514-1 18.2 75 % Lipoid S 35 FS, Dissolution in Dark red
viscous Dark red slurry
%Akoline MCM Et0H abs.; rotary slurry with sediment
evaporation
4 ACA100514-2 19.7 75 % Akoline MCM, Dissolution in Dark red
viscous Dark red slurry
25 % Phospholipon ETOH abs.; rotaryslurry with sediment
50 evaporation
5 ACA100526-2 21.2 75 % DC 200 Fluid Mixing with Dark red
slurry, Unchanged
100,000 cSt, 25 % spatula smooth ointment-
Akoline MCM like consistency
CA 02833594 2013-10-18
WO 2012/144943
PCT/SE2012/000054
8
6 ACA100527-1 17.7 50 % DC 200 Fluid Mixing with Dark
red slurry, Unchanged
100,000 cSt, 50 % spatula ointment-like
Akoline MCM consistency, signs
of separ.
7 ACA100527-2 17.6 75 % DC 200 Fluid Mixing with Dark
red slurry, Unchanged
12,500 cSt, 25 % spatula smooth ointment-
Akoline MCM like consistency
8 ACA100527-3 21.2 75 % DC 200 Fluid Mixing with Dark
red slurry, Unchanged
12,500 cSt, 25 % spatula firm ointment-like
Phosal 50 PG consistency
9 ACA100611 24.9 75 % Mineral oil, 25 Mixing with Dark
red slurry, Unchanged
% Akoline MCM spatula smooth
consistency
ACA100922 19.5 65 % DC 200 Fluid Mixing with Dark red
slurry, Unchanged
(500 g) 12,500 cSt, 5 % DC spatula and smooth ointment-
200 Fluid 1 000 cSt, spoon like consistency
5 % DC 200 Fluid
100,000 cSt, 25 %
Akoline MCM
EXAMPLE 4. Dissolution testing of model substances in vehicles of the
invention
5 The dissolution behavior of compositions prepared according to the
invention was studied according
to the following procedure.
Carriers of the invention were prepared by mixing silicone oil and lipid, and
by mixing silicone oil, lipid
and ethanol. A weighed amount of the model substance was added to the mixture.
If necessary, the
10 model substance was ground in a mortar prior to addition in order to
obtain sufficiently small
particles. If ethanol had been added when preparing the carrier it was
evaporated in a rotary
evaporator. The composition of the invention was obtained in form of a paste-
like to semi-fluid
suspension.
A 250 ml beaker was filled with 200 ml of deionised water and placed on a
magnetic stirrer with
temperature control. The temperature in the dissolution medium was set to 37 C
and the stirring rate
to 114 rpm. The fluid in the beaker was continuously sampled by means of a
capillary tube and a
peristaltic pump (Gilson Minipulse 3) and passed through a UV detector
(Shimadzu SPD-10A), and
returned to the beaker. When a stable baseline had been obtained, 200 mg of
the formulation was
added to the beaker. The absorbance was continuously recorded. The half life
(tip) of model
substance release from the formulation was calculated as the time required for
reaching half the
expected absorbance of the total amount of added substance.
As can be seen from the large differences in half live observed in the
dissolution experiments
presented in Tables 6-8, the ratio of lipid to silicone oil can substantially
affect dissolution (release)
behavior. Furthermore, there is a great variation in dissolution behavior of
different model substances
incorporated in one and the same carrier. The experiments also demonstrate
that the nature of the
oily component (silicone or triglyceride oil) substantially effects
dissolution (Table 6).
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
9
Table 6. Release of tyrosine from silicone oil/polar lipid/tyrosine
compositions of the invention
Composition X DC 2008 Lipid 1 % Lipid 1 Lipid 2 % Lipid 2 %
Tyrosine tip (min)
KL-Old-1 67.6 % MCM -23.8% 8.6% 13
KL-Old-3 69.0 % MCM 12.0% MOG -10.3% 8.7% 79
KL-01d-4 65.5 %1 'MCM 14.2% S100 11.0% 9.3% 459
KL-01n-2 67.7 %2 MCM 12.1% DOPE 11.2% 9.0% 15633
KL-Old-2 69.0 % -MOG 21.5% 9.5% 711
KL-Old-5 MOG 12.7/0 DOPC 11.9/0
0 0 8.5%
66.9 %3 1210
'Ethanol (0.16 g/g silicone oil) was used as mixing aid. 2 Ethanol (1.32 g/g
silicone oil) was used as
mixing aid. 3 Ethanol (0.36 g/g silicone oil) was used as mixing aid.
Table 7. Release of vanillin from silicone oil/polar lipid/vanillin
compositions of the invention
Composition % DC 2008 Lipid 1 % Lipid 1 Lipid 2 % Lipid 2 96 Vanillin
tip(min)
.KL-01b-9 71.8%' MCM 13.7% S100 13.3% 1.2% -93
KL-01n-3 73.5 %2 'MCM -12.6% DOPE 12.6% 1.2% 42
KL-01)-1 74.6 % MOG 24.4% 1.0% 73
1Ethanol (0.21 g/g silicone oil) was used as mixing aid. 2 Ethanol (1.21 g/g
silicone oil) was used as
mixing aid.
Table 8. Release of methylene blue from silicone oil/polar lipid/methylene
blue compositions of the
invention
Composition % DC 2008 Lipid 1 96 Lipid 1 Lipid 2 % Lipid 2 % Methylene blue
tip(min)
KL-Ole-1 74.4% -MCM 24.1% 1.5% 2
KL-01p-2 88.2%1 'MCM 5.1% S100 5.5% 1.1% 104
KL-01p-1 48.8% MCM -37.1% S100 12.6% 1.5% 6
=KL-Ole-2 74.0% -MOG 24.6% 1.4%
99
KL-Ole-3 75.0% MCM 12.2% MOG 12.0% 0.9% 3
KL-01e-5 73.7%2 MOG 13.4% DOPC 11.4% 1.6% 1579
1 Ethanol (0.16 g/g silicone oil) was used as mixing aid.2 Ethanol (0.49 g/g
silicone oil) was used as
mixing aid.
CA 02833594 2013-10-18
WO 2012/144943 PCT/SE2012/000054
Table 9. Release of Vitamin 12 (cyanocobalamin) from silicone oil/polar lipid
/cobalamin
compositions of the invention
'Composition Silicone oil % S. oil Lipid 1 % Lipid 1 Lipid 2 % Lipid 2 % Vit.
B12 tiA (min)
KL-01g-1 DC 200B 73.9% MCM 25.6% 0.5% 2
KL-01g-2 DC 200B 75.2% MOG 24.2% 0.6% 270
'KL-01m-1 'DC 200B 74.2%1 MOG 12.6% S100 12.1% 1.0% 34
KL-01m-3 Rape seed oil 74.2%1 MOG 12.6% S100 12.2% 1.0%
1359
1 Ethanol (0.20 g/g silicone oil) was used as mixing aid.
5 EXAMPLE 5. Gastrointestinal absorption of cyclosporine A
Gastro-intestinal absorption of cyclosporine comprised by a composition of the
invention was
compared with the absorption from two prior art compositions.
10 Commercial prior art composition A. Sandimmune Neoral, oral solution
(Novartis, lot HS5107, expiry
date November 2013). The known composition is a clear, low viscous solution of
100 mg cyclosporine
A per ml, the excipients consisting of a-tocopherol, water-free ethanol,
propylene glycol, corn oil, and
macroglycerol hydroxystearate. Prior to use the solution was diluted to 1:1 by
weight with 10 % (w/w)
of aqueous ethanol. Accordingly, the cyclosporine concentration was 50 mg/g.
The ethanol content
was at least 50 mg/g.
Cyclosporine stock solution. A stock solution of cyclosporine A was prepared
by mixing 2.00 g of
cyclosporine A USP/EP (Abbot Laboratories) with 2.0 g of 99.9 % (w/w) ethanol
and ultrasonicating
the mixture at 40 C for a few minutes until a clear oil had been formed. The
solution contained about
500 mg/g of cyclosporine A.
Prior art composition B. Sesame oil (45 g) and melted Capmul MCM EP (15 g)
were mixed. The clear
oily formed (9.00 g) was mixed with 1.00 g cyclosporine stock solution to
provide composition B in
form of a clear oil.
Composition of the invention C. The pharmaceutical carrier was prepared by
mixing in a 100 ml glass
beaker 45 g of silicone oil, DC 200 Fluid 500 cSt, and 15 g of lipid, Capmul
MCM EP. Prior to mixing
Capmul MCM EP was melted in a microwave oven. Blending 1.00 g of cyclosporine
A stock solution
and 9.00 g of the mixture rendered a milky emulsion.
Animal tests. The concentration of cyclosporine A was determined (LC-MS/MS,
Method PHARM 1326)
in whole rats after administrating a single oral dose of 100 mg/kg by gavage.
Twelve male Sprague
Dawley rats of about 200 g weight were divided into three groups of four
animals for testing one
formulation by group. Blood was sampled at 15 and 30 min, and at 1, 2, 4, 6,
24 and 48 hrs after
administration. A control sample was taken prior to administration. Mean
cyclosporine A
CA 02833594 2013-10-18
WO 2012/144943
PCT/SE2012/000054
11
concentrations for each group are illustrated in the Figure. Cyclosporine A
was absorbed after oral
administration from all three formulations.