Note: Descriptions are shown in the official language in which they were submitted.
CA 02835434 2013-11-07
WO 2012/158983
PCT/US2012/038462
-1-
IMPROVED PLATELET STORAGE USING A SIALIDASE INHIBITOR
RELATED APPLICATIONS
This application is a continuation-in-part of U.S. Application No. 13/474,473,
filed May 17,
2012; a continuation-in-part of U.S. Application No. 13/474,627, filed May 17,
2012; and a
continuation-in-part of U.S. Application No. 13/474,679, filed May 17, 2012,
and claims the benefit of
U.S. Provisional Application No. 61/613,876, filed March 21, 2012; U.S.
Provisional Application No.
61/613,837, filed March 21, 2012; U.S. Provisional Application No. 61/503,984,
filed July 01, 2011;
and U.S. Provisional Application No. 61/487,077, filed May 17, 2011.
The entire teachings of the above applications are incorporated herein by
reference.
GOVERNMENT SUPPORT
The invention was supported, in whole or in part, by a grant No. 3R01HL089224-
0351 from
National Heart, Lung, and Blood Institute. The Government has certain rights
in the invention.
BACKGROUND OF THE INVENTION
Collected platelets intended for transfusion are highly perishable. Platelets
are non-nucleated
bone marrow-derived blood cells that protect injured mammals from blood loss
by adhering to sites of
vascular injury and by promoting the formation of plasma fibrin clots. Humans
depleted of circulating
platelets by bone marrow failure suffer from life threatening spontaneous
bleeding, and less severe
deficiencies of platelets contribute to bleeding complications following
trauma or surgery.
As the count of circulating platelets falls (e.g., ¨70,000 per L), patients
become increasingly
susceptible to cutaneous bleeding. Patients with platelet counts of less than
20,000 per iut are highly
susceptible to spontaneous hemorrhage from mucosal surfaces, especially when
the thrombocytopenia
is caused by a bone marrow disorder or failure. The platelet deficiencies
associated with bone marrow
disorders such as aplastic anemia, acute and chronic leukemia, metastatic
cancer, and deficiencies
resulting from cancer treatment such as ionizing radiation or chemotherapy all
contribute to a major
CA 02835434 2013-11-07
WO 2012/158983 -2-
PCT/US2012/038462
public health problem. Patients that suffer from thrombocytopenia associated
with major surgery,
injury, and sepsis also require significant numbers of platelet transfusions.
A major advance in medical care half a century ago was the development of
platelet
transfusions to correct such platelet deficiencies, resulting in about 2.6
million platelet transfusions in
the United States per year at current transfusion rates. However, platelets
collected for transfusion are
highly perishable because, upon storage at or below room temperature, they
quickly lose in vivo
hemostatic activity. Hemostatic activity broadly refers to the ability of a
population of platelets to
mediate bleeding cessation.
Platelets, unlike all other transplantable tissues, do not tolerate
refrigeration and disappear
rapidly from the circulation of recipients if subjected to even very short
periods of chilling.
Importantly, the cooling effect that shortens platelet survival is thought to
be irreversible and cooled
platelets become unsuitable for transfusion. One of the first visible effects
of platelet impairment is an
irreversible conversion from a discoid morphology towards a spherical shape,
and the appearance of
spiny projections on the surface of platelets due to calcium dependent
gelsolin activation and
phosphoinositide-mediated actin polymerization. When platelets are exposed to
temperatures lower
than 20 C, they rapidly undergo such modifications in shape.
The need to keep platelets at room temperature prior to transfusion has
imposed a unique set of
costly and complex logistical requirements for platelet storage. Because
platelets are metabolically
active at room temperature, they require constant agitation in gas permeable
containers to allow for the
exchange of gases to prevent the toxic consequences of metabolic acidosis.
Room temperature storage
conditions result in macromolecular degradation and reduced hemostatic
functions of platelets, a set of
defects known as "the storage lesion." In addition, storage at room
temperature encourages the growth
of bacteria thereby creating a higher risk of bacterial infection, which
effectively limits the duration of
such storage to about 5 days. In this regard, bacterial contamination of
platelets is by far the most
frequent infectious complication of blood component use. At current rates,
from one in 1,000 to one in
2,000 units of platelets are contaminated sufficiently with bacteria to pose a
significant risk to the
recipient.
Thus, there remains a pressing need to develop agents, solutions and methods
to improve or
prolong in vivo hemostatic activity of human platelets upon storage at or
below room temperature.
There is a further and more significant need to do so and inhibit bacterial
proliferation.
SUMMARY OF THE INVENTION
CA 02835434 2013-11-07
WO 2012/158983 -3-
PCT/US2012/038462
The present invention relates to a platelet additive solution (PAS) that
includes an amount of
one or more sialidase inhibitors and optionally one or more glycan-modifying
agents; and one or more
of PAS components that includes a salt (e.g., sodium source, a chloride
source, a potassium source, a
magnesium source, a calcium source, and a combination thereof), a citrate
source (e.g., monosodium
citrate, disodium citrate, trisodium citrate, citric acid, and a combination
thereof), and/or a carbon
source (e.g., acetate, glucose, sucrose and any combination thereof). The PAS
of the present invention
is maintained at a pH ranging between about 6.4 and about 7.6. In one
embodiment, the PAS of the
present invention further includes a phosphate source (e.g., sodium
monophosphate, diphosphate,
triphosphate, and a combination thereof). An acetate source can include, for
example, sodium acetate,
potassium acetate, magnesium acetate, or a combination thereof In an aspect,
the sodium source can
be sodium chloride, sodium citrate, sodium acetate, sodium phosphate or a
combination thereof
Similarly, the chloride source can be sodium chloride, magnesium chloride,
potassium chloride, or a
combination thereof. The potassium source, in an example, can be potassium
chloride, potassium
citrate, potassium acetate, potassium phosphate, potassium sulfate or a
combination thereof. Examples
of sources of magnesium include magnesium chloride, magnesium citrate,
magnesium sulfate and a
combination thereof In an embodiment, the calcium source encompasses calcium
chloride, calcium
acetate, calcium citrate or a combination thereof.
In a particular embodiment, the PAS of the present invention includes an
amount of one or
more sialidase inhibitors and optionally one or more glycan-modifying agents;
a sodium source in the
amount ranging between about 100 mM and about 300 mM; a chloride source in the
amount ranging
between about 40 mM and about 110 mM; a citrate source in the amount ranging
between about 2 mM
and about 20 mM; an acetate source in the amount ranging between about 10 mM
and about 50 mM; a
phosphate source in the amount ranging between about 5 mM and about 50 mM; a
potassium source in
the amount ranging between about 0.5 mM and about 10 mM; a magnesium source in
the amount
ranging between about 0.5 mM and about 2.5 mM; and a calcium source in the
amount ranging
between about 0.5 mM and about 2.5 mM; wherein the PAS is maintained at a pH
ranging between
about 6.4 and about 7.6 (e.g., about 7.1 to about 7.4, or about 7.2).
In yet another embodiment, the present invention pertains to platelet
compositions having
isolated platelets; the PAS of the present invention; and plasma, wherein the
platelet composition is
maintained at a pH ranging between about 6.4 and about 7.6. In an aspect, the
plasma is present in an
amount ranging between about 1% and about 50% by volume (e.g., between 20% and
40% plasma, or
CA 02835434 2013-11-07
WO 2012/158983 -4-
PCT/US2012/038462
about 30% plasma). In yet another embodiment, the platelet additive solution
is present in an amount
ranging between about 50% and about 99% by volume.
The present invention further relates to a bag or container suitable for
platelet storage having
the PAS of the present invention. The bag or container can further include
isolated platelets and/or be
maintained at a pH ranging between about 6.4 and about 7.6.
The present invention relates to a method of storing platelets, wherein
isolated platelets are
obtained from one or more donors. The method includes the steps of contacting
the isolated platelets
with the PAS, described herein. The sialidase inhibitor can be e.g., fetuin,
2,3-dehydro-2-deoxy-N-
acetylneuraminic acid (DANA) or a pharmaceutically acceptable salt thereof;
Oseltamivir (ethyl
(3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)-cyclohex-1-ene-1-
carboxylate); Zanamivir
((2R,3R,4S)-4-guanidino-3-(prop-1-en-2-ylamino)-2-((1R,2R)-1,2,3-
trihydroxypropyl)-3,4-dihydro-
2H-pyran-6-carboxylic acid); Laninamivir ((4S,5R,6R)-5-acetamido-4-
carbamimidamido-6-[(1R,2R)-
3-hydroxy-2-methoxypropy1]-5,6-dihydro-4H-pyran-2-carboxylic acid); and
Peramivir ((lS,2S,3S,4R)-
3- [(1S)-1-acetamido-2-ethyl-butyl] -4-(diaminomethylideneamino)-2-hydroxy-
cyclopentane-1-
carboxylic acid) or a pharmaceutically acceptable salt thereof In an
embodiment, the sialidase
inhibitor is the sodium salt of 2,3-dehydro-2-deoxy-N-acetylneuraminic acid.
The method allows
isolated platelets to be stored for a period of about 1 to about 21 days. The
isolated platelets are stored
a temperature of between about 1 C and about 24 C. The method, in an
embodiment, includes the
steps of cooling the platelet composition to a temperature below room
temperature; storing the platelet
composition for a period of time; and then rewarming the platelet composition
back to room
temperature. In an aspect, the population of platelets is treated with the
sialidase inhibitor within a time
period, wherein the time period is in a range between about 1 minute to about
8 hours.
The present invention relates to methods for preparing platelets for storage,
wherein isolated
platelets are obtained from one or more donors. The methods include contacting
the isolated platelets
with one or more sialidase inhibitors, as described herein, and optionally one
or more glycan-modifying
agents. The glycan-modifying agent can be, for example, CMP-sialic acid, a CMP-
sialic acid precursor,
UDP-galactose or a combination thereof. In an aspect, an enzyme that converts
the CMP-sialic acid precursor to
CMP-sialic acid can also be added to the platelets.
The present invention also relates to methods of preparing platelets for
storage. The method includes the
steps of isolating platelets from one or more individuals; and contacting the
isolated platelets with a sialidase
inhibitor in an amount sufficient to reduce hydrolysis of sialic acid residues
from platelet surface glycans. In an
aspect, a reduction in platelet receptors (e.g., GPIba loss, GPV loss or both)
loss occurs.
CA 02835434 2013-11-07
WO 2012/158983 -5-
PCT/US2012/038462
Encompassed by the present invention are methods for increasing the in vivo
circulation time of isolated
platelets. These methods include the following steps: obtaining isolated
platelets from one or more individuals;
treating the platelets with an amount of one or more sialidase inhibitors, and
optionally one or more glycan-
modifying agents to thereby obtain treated platelets; and transfusing the
treated platelets into an individual in
need thereof to thereby obtain transfused platelets. The circulation time of
the transfused platelets is longer, as
compared to platelets not subjected to a sialidase inhibitor. The methods
include, in one aspect, storing the
platelet composition at room temperature for a period of time. In another
aspect, the methods include cooling
the platelet composition to a temperature below room temperature; storing the
platelet composition for a period
of time; and then rewarming the platelet composition back to room temperature.
Yet another embodiment of the present invention includes a platelet
preparation having a sialidase
inhibitor and a population of platelets; wherein the stable platelet
preparation is prepared by the
following method: obtaining a population of platelets from a donor; and
treating the platelets with an
effective amount of a sialidase inhibitor. Such a platelet preparation is
suitable for administration to a
human after storage without significant loss of hemostatic function or without
a significant increase in
platelet clearance in the human as compared to untreated platelets. Similarly,
a platelet preparation of
the present invention also includes platelets isolated from a donor; and an
amount of one or more
sialidase inhibitors and optionally one or more glycan-modifying agents.
The present invention further relates to kit and systems. The kits of the
present invention include a sterile
container capable of receiving and containing a population of platelets,
wherein the container is substantially
closed to the environment, and a sterile quantity of a sialidase inhibitor, as
described herein, and optionally
suitable packaging materials, and instructions for use. In an example, the
container is suitable for the cold
storage of platelets. The kit can further include an amount of at least one
glycan-modifying agent such as CMP-
sialic acid, a CMP-sialic acid precursor, UDP galactose or a combination
thereof. Similarly, the present
invention includes a system that has a sterile bag or container suitable for
blood storage; and an amount of one
or more sialidase inhibitors, as described herein, and optionally one or more
glycan-modifying agents.
The present invention relates to methods for reducing sialidase activity and
inhibiting
proliferation of one or more bacteria in a platelet product preparation from
one or more donors. The
methods include the steps of contacting the platelet product preparation with
an amount of a sialidase
inhibitor, as described herein, to thereby obtain a sialidase treated platelet
product preparation; wherein
the sialidase activity is reduced and the proliferation of one or more
bacteria is inhibited, as compared
to a platelet product preparation not subjected to a sialidase inhibitor. The
type of bacteria inhibited
include those commonly found in platelet product preparations. Examples of
such bacteria include:
Aspergillus, Bacillus sp, Bactero ides eggerthii, Candida albicans,
Citrobacter sp, Clostridium
CA 02835434 2013-11-07
WO 2012/158983 -6-
PCT/US2012/038462
perfringens, Corynebacterium sp, Diphtheroid, Enterobacter aero genes,
Enterobacter amnigenus,
Enterobacter cloacae, Enterococcus avium, Enterococcus faecalis, Escherichia
coli, Fusobacterium
spp., Granulicatella adiacens, Heliobacter pylori, Klebsiella sp, (K.
pneumonia, K. oxytoca),
Lactobacillus sp, Listeria sp, Micrococcus sp, Peptostreptococcus, Proteus
vulgaris, Pseudomonas sp,
Pseudomys oralis, Propionibacterium sp, Salmonella sp, Serratia sp, Serratia
marcescens
Staplhylococcus sp (Coagulase-negative Staphylococcus, Staphylococcus
epidermidis, Staphylococcus
aureus), Streptococcus sp, (S. gallolyticus, S. bovis, S. pyo genes, S.
viridans), and Yersinia
enterocolitica. The methods further include the steps of assessing the
sialidase inhibitor-treated platelet
product preparation for bacterial proliferation, and comparing the assessment
to a control. One or more
glycan-modifying agents can be added to the platelets. Such glycan modifying
agents include, for
example, CMP-sialic acid, a CMP-sialic acid precursor, UDP galactose or a
combination thereof In an
aspect, an enzyme that converts the CMP-sialic acid precursor to CMP-sialic
acid can also be added to
the platelets.
The present invention encompasses methods for inhibiting bacterial
proliferation in platelets
during storage, wherein isolated platelets are obtained from one or more
donors. The methods involve
contacting the isolated platelets with an amount of one or more sialidase
inhibitors, as described herein,
and optionally one or more glycan-modifying agents; and assessing bacterial
proliferation in the
isolated platelets at one or more time points; wherein bacterial proliferation
in the isolated platelets is
inhibited. In an aspect, the platelet preparation is contacted with the
sialidase inhibitor in an amount
sufficient to reduce hydrolysis of sialic acid residues from platelet surface
glycans. The isolated
platelets, in an embodiment, can be stored for a period of about 1 to about 21
days. The isolated
platelets can be stored, for example, at a temperature of between about 1 C
and about 24 C. In one
embodiment, the method involves storing the platelet composition at room
temperature for a period of
time. In another embodiment, the method involves cooling the platelet
composition to a temperature
below room temperature; storing the platelet composition for a period of time;
and then rewarming the
platelet composition back to room temperature prior to transfusion to an
individual.
Yet another embodiment of the present invention relates to methods of
preparing platelets for
storage during which sialidase activity is reduced and bacterial proliferation
is inhibited, wherein
isolated platelets are obtained from one or more donors. The method includes
the steps of contacting
the isolated platelets with one or more sialidase inhibitors, and optionally
one or more glycan-
modifying agents; and assessing bacterial proliferation in the isolated
platelets, wherein bacterial
proliferation in the isolated platelets is inhibited, as compared to a
control. The present invention
CA 02835434 2013-11-07
WO 2012/158983 -7-
PCT/US2012/038462
further include methods of increasing the storage time of a population of
platelets by obtaining a
population of platelets from one or more individuals; and treating the
platelets with an effective amount
of a sialidase inhibitor to thereby obtain treated platelets. In an aspect,
the population of platelets is
treated with the sialidase inhibitor within a time frame, wherein the time
frame is in a range between
about 1 minute to about 8 hours. These steps can also be used for methods of
increasing the storage
time of a population of platelets by reducing sialidase activity and
inhibiting bacterial proliferation.
Methods of transfusing isolated platelets are also encompassed by the present
invention. The
methods include the steps of obtaining isolated platelets from one or more
individuals; treating the
platelets with an amount of one or more sialidase inhibitors, and optionally
one or more glycan-
modifying agents to thereby obtain treated platelets exhibiting inhibited
bacterial proliferation; and
transfusing the treated platelets into an individual in need thereof; wherein
the bacterial proliferation is
inhibited, as compared to platelets not subjected to the sialidase inhibitor.
The present invention pertains to methods of maintaining hemostatic activity
of platelets transfused
into a recipient after being stored. This method is performed by contacting
isolated platelets with an
amount of a sialidase inhibitor to thereby obtain treated platelets; storing
the treated platelets for a
period of between about 1 and 14 days; transfusing the stored, treated
platelets to the recipient in need
thereof to thereby obtain transfused platelets; wherein the transfused
platelets can activate and form a
clot, as compared to that of platelets not subjected to a sialidase inhibitor.
Platelet preparations are also encompassed by the present invention. Such
platelet preparations
include a sialidase inhibitor and a population of platelets; wherein the
stable platelet preparation is
prepared by the methods described herein. Additionally, platelet preparations
of the present invention
include platelets isolated from a donor; and an amount of one or more
sialidase inhibitors and
optionally one or more glycan-modifying agents; wherein the platelet
preparation exhibits inhibited
bacterial proliferation, as compared to a platelet preparation not treated
with a sialidase inhibitor.
BRIEF DESCRIPTION OF THE DRAWINGS
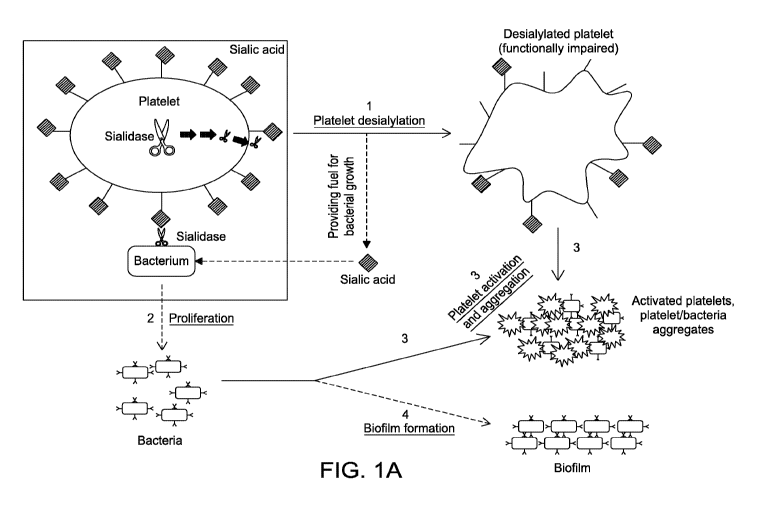
Figs. 1A-C are schematics depicting a sialylated platelet containing
intracellular sialidase and
sialidase containing bacteria. (A) Both bacterial and platelet derived
sialidases remove sialic acid from
platelet surfaces, leading to the formation of platelet with impaired function
(1). The released sialic
acids support the proliferation of contaminating bacteria (short-dashed line
and 2), which leads to
platelet activation (3), formation of platelet-bacteria aggregates (3) and
biofilm formation (long-dashed
CA 02835434 2013-11-07
WO 2012/158983 -8-
PCT/US2012/038462
line and 4). (B) Desialylated platelets are recognized and removed from the
circulation by phagocytes
upon transfusion. (C) Addition of the sialidase inhibitor DANA (sodium salt of
2,3-dehydro-2-deoxy-
N-acetylneuraminic acid) inhibits the sialidase activities, derived from
platelets and bacteria,
prevents the platelets from desialylation so that platelets are not recognized
by phagocytic cells after
transfusion.
Fig. 2 is a bar graph showing that human platelets lose sialic acid during
storage at 4 C.
Platelet concentrates (A: Donor A and B: Donor B) were stored at 4 C for 5
days in the absence of
exogenous nucleotide sugar (a), in the presence of CMP-sialic acid (CMP-SA)
and UDP-galactose
(UDP-Gal) (b) or UDP-Gal alone (c). Sialic acid content of platelets at day 0
was set to 100%.
Fig. 3 is line graphs showing that the human platelet sialidase surface
activity increases
following cold storage. (A) depicts the analysis of fresh platelets, with or
without permeabilization.
(B) depicts the analysis of fresh intact platelets (Donors A and B) at pH 5
and 6. (C) depicts the
corresponding analysis of intact platelets (Donors A and B) after storage at 4
C for 5 days.
Fig. 4 shows immunofluoresence micrographs of fixed, non-permeabilized,
resting room
temperature (RT) (left panels) and refrigerated (right panels) human platelets
demonstrating the
presence of sialidase Neu3, but not Neul on their surfaces. Refrigeration
(48h) of platelets increases
sialidase (Neul) surface fluorescence, i.e exposure. Anti-Neul antibody was
used in the upper panels.
Anti-Neu3 antibody was used in the lower panels.
Fig. 5 shows that mouse platelet sialidase surface activity increases
following 48 h cold
storage and rewarming. Platelet-derived sialidase activity was measured in
fluorescence (Absorption
Intensity (Al)) over 0-2.5 h at room temperature. Platelet storage at cold
temperatures (4 C, darker
circles) was compared with fresh platelets (RT, lighter circles). As a
control, sialidase activity
(Clostridium perfringens (Component H)) was measured over the same time period
(inset).
Fig. 6 shows that fetuin competes for sialidase surface activity during
platelet storage and thus
inhibits the hydrolysis of sialic acid from platelet glycans. The left pair of
bars represents the 0-
galactose exposure on fresh platelets (0) in the absence (Control) or presence
of fetuin (Fetuin). The
right pair of bars represents the I3-galactose in the absence (Control) or
presence of fetuin following
platelet refrigeration for 48 h. Sialic acid loss, i.e., I3-galactose
exposure, is measured by RCA I
binding.
CA 02835434 2013-11-07
WO 2012/158983 -9-
PCT/US2012/038462
Fig. 7 shows that the sialidase inhibitor DANA increases mouse platelet life
span in vivo. The
bottom line represents the control platelet life span (Control). The top line
represents the platelet life
span upon addition of DANA (DANA).
Fig. 8 (A) represents the schematic structure of the primary GPIba structure
and 0- and N-
linked glycans. (B) represents the biosynthetic modifications of terminal
Ga1131,4G1cNAc
(lactosaminoglycan/LacNAc) and the Core-1 0-glycan.
Fig. 9 shows that human platelets contain the sialidases Neul and Neu3 by
Western blot
analysis of total platelet lysates.
Fig. 10 shows that human platelets release Neul into plasma upon long-term
refrigeration as
analyzed by Western blot. Platelets and their corresponding plasma were
analyzed at day 0 and
following platelet refrigeration for 1, 2 and 5 days.
Fig. 11 (A) depicts the characterization of platelet glycosyltransferases
(GTs). Human total
platelet lysates were subjected to SDS-PAGE and were immunoblotted with
monoclonal antibodies:
anti-GalNAc transferases (Ga1NAc-T1, -T2, -T3), 134Gal-Transferasel (134Gal-
T1), and
sialyltransferase ST3Ga1-1 (B) Platelets secrete GTs. Resting platelets were
maintained at 37 C or
activated via the thrombin receptor PAR-1 with 25 ILIM TRAP, for 5 min.
Maximal release was
observed after 1 min. The Enzymatic Activity in counts per minute (CPM) was
measured in the
pelleted platelet fraction (P), or in their corresponding bathing media (M).
The media was clarified at
100,000 xg for 90 min to eliminate microparticles prior to activity
measurements.
Fig. 12 depicts that endogenous platelet sialyltransferases incorporate sialic
acid into platelet
surface receptors. (A) Active human platelets surface sialyltransferase
incorporated FITC-conjugated
CMP-SA (FITC-SA) into resting (dotted line) or TRAP-activated platelets. FITC
alone (Control) was
added to resting (dotted line) or TRAP activated platelets (solid line). (B)
shows immunoblots of
lysates from resting (Rest) or TRAP-activated platelets (TRAP), treated with
FITC (C) or FITC-CMP-
sialic acid (S) or left untreated (-) with antibodies to FITC, GPIba, aIIb,
and vWf. The blots shown are
representative of two experiments. Actin is shown as a loading control.
Fig. 13 shows that platelets lose GPIba and GPV receptors during storage at
room temperature
(A) or under refrigeration (B). Expression of mouse vWf receptor complex
components (GPIba,
GPIbI3, GPIX, GPV), GPVI and a11b133 was measured by flow cytometry before and
after platelet
CA 02835434 2013-11-07
WO 2012/158983 -10-
PCT/US2012/038462
storage in the cold at the indicated time points. Results are expressed as
means SD, n = 5.
Glycoprotein expression on freshly isolated platelets was set as 100%.
Fig. 14 shows that inhibition of metalloprotease-mediated GPIba shedding alone
does not
improve mouse platelet recovery and survival. (A) GPIba and (B) GPV surface
expression were
assessed by flow cytometry. Wild-type mouse platelet rich plasma was stored
for 0, 24 and 48 h at 4 C
in the presence of DMSO (Control) or 100 ILIM of the metalloproteinase
inhibitor GM6001 (n = 6).
Surface expression of (C) GPIba and (D) GPV was determined by flow cytometry
on freshly isolated
or 24 and 48 h refrigerated platelet rich plasma from TACE+1+ and TACEAZ11/AM
mice. Results are the
mean + s.e.m. n = 5. (C, Inset) Immunoblot for GPIba in lysates from TACE'/
and TACEAzil/Am
platelets stored for 3, 24 and 48 h in the cold. (E) Fluorescently-labeled (5-
chloromethyl fluorescein
diacetate, CMFDA) fresh PRP (RT) or platelets from stored platelet rich plasma
in the absence (48 h)
or presence of 100 ILIM GM6001(48h + GM6001), were infused into wild-type mice
(108 platelets/10
gm of body weight). Blood was drawn at the indicated time points, and
platelets were immediately
analyzed by flow cytometry. Results are mean percentage CMFDA-labeled
platelets s.e.m. The
percentage of CMFDA positive fresh platelets at time 5 min post-transfusion
was set as 100%. n = 5.
*P < .05. Cold-stored platelets are compared. (F) Fluorescently-labeled
(CMFDA) fresh platelets
(TACE'/' RT and TACE-/- RT) or platelets from stored platelet rich plasma
(TACE'/' 48h and TACE-/-
48h) were infused intravenously into wild type mice (108 platelets/10 gm of
body weight). Blood was
drawn at the indicated time points, and platelets were immediately analyzed by
flow cytometry.
Results are mean percentage CMFDA-labeled platelets s.e.m. The percentage of
CMFDA positive
fresh TACE+1+ platelets at 5 min post-transfusion was set as 100 A. n = 5.
Fig. 15 shows that sialidase-treated TACEAm/Azn platelets are rapidly cleared
from the
circulation. (A) Flow cytometric analysis of f3-galactose exposure on
glycoproteins, as detected with
ECL FITC-labeled lectin. Lectin binding to TACE (white bars) or TACEAzil/Am
(black bars) platelets
treated or not with a2-3,6,8,9-sialidase (Neu). The ratio of mean fluorescence
intensity binding to
untreated TACE platelets is shown. Histograms report the mean s.e.m. for
three separate
experiments. *P < 0.05, **P < 0.01, ***P < 0.001. (B) GPIba, GPV, and (411,133
surface expression
was assessed by flow cytometry. TACE'/' (not shown) and TACEAzil/Am platelets
were treated with
sialidase (5 mU/mL) (black bars) or not (white bars). Results are expressed
relative to the amount of
GPIba on TACEAz"zn platelets (mean % relative to control s.e.m.). n = 3. (C)
Fresh, room
CA 02835434 2013-11-07
WO 2012/158983 -11-
PCT/US2012/038462
temperature and fluorescently-labeled (CMFDA) TACE ' ' and TACEAm/Azn
platelets were treated with
a2-3,6,8,9- Sialidase (5 mU/mL) (filled symbols) or left untreated (open
symbols) were infused
intravenously into TACE ' mice (108 platelets/10 g of body weight). Blood was
drawn at the indicated
time points, and the platelets were immediately analyzed by flow cytometry.
Results are expressed as
the mean percentage CMFDA-labeled platelets s.e.m. The percentage of CMFDA
positive untreated
TACE ' platelets at 5 min post-transfusion was set as 100%. Each point
represents 4 mice. n.s. not
significant, ***P < .0001. Sialidase treated TACE ' and TACEAziliAm were
compared.
Fig. 16 shows that neuraminidase treatment of platelets increases I3-galactose
exposure (loss of
sialic acid) as measured by ECL fluorescence lectin binding. Flow cytometric
analysis of I3-galactose
or 13-G1cNAc exposure on platelet glycoproteins, as detected with ECL I (open
bars) or s-WGA (closed
bars) FITC-labeled lectins. Lectin binding to fresh mouse platelets in the
presence and absence of a2-
3,6,8,9- Sialidase from A. ureafaciens (Neu) at the indicated concentrations,
n = 5.
Fig. 17 shows the dose dependent loss of platelet GPIba and GPV receptors with
increasing
neuraminidase concentrations. GPIba and GPV surface expression on freshly
isolated mouse platelets
was assessed by flow cytometry. Surface receptor expression in the presence
and absence of a2-
3,6,8,9- sialidase (Neu) at the indicated concentrations. The mean
fluorescence of receptor expression
at time 0 was set as 100%. n = 4.
Fig. 18 shows that DANA inhibits the exposure of I3-galactose by neuraminidase
treatment.
Flow cytometric analysis of I3-galactose or 13-G1cNAc exposure on mouse
platelet glycoproteins, as
detected above in the presence (Neu) and absence (Control) of 5 mU a2-3,6,8,9-
sialidase (Neu) and the
competitive sialidase inhibitor DANA (Neu + DANA). n = 4.
Fig. 19 shows that DANA inhibits the loss of platelet GPIba, GPV, GPIX and
allb133receptors
induced by neuraminidase treatment. Surface receptor expression (GPIba, GPV,
GPIX and allb133) was
measured by flow cytometry on mouse platelets in the presence (grey bars) and
absence (open bars) of
5 mU a2-3,6,8,9-sialidase. Receptor expression on platelets treated with
sialidase and DANA is also
shown (black bars). The mean fluorescence of receptor expression on untreated
platelets was set as
100%. n = 4.
Fig. 20 depicts a non-reduced immunoblot blot of total platelet lysates
(INPUT), supernatants
(SUPERNATANT) and the corresponding platelets pellet (PELLET) showing that
DANA inhibits the
CA 02835434 2013-11-07
WO 2012/158983 -12-
PCT/US2012/038462
loss of platelet GPIba induced by neuraminidase (NA) treatment. Control
represents untreated
samples.
Fig. 21 is a graph showing that addition of DANA completely rescues the in
vivo recovery and
survival of mouse platelets treated with neuraminidase. Control depicts the
survival of non-treated
fresh room temperature platelets.
Fig. 22 is a graph showing that platelet GPIba and GPV receptor loss during
storage at room
temperature is inhibited by the addition of DANA.
Fig. 23 is a bar graph depicting the effect of neuraminidase treatment on f3-
galactose exposure
in the presence of 100 ILLM metalloproteinase (MP) inhibitor GM6001. 13-
Galactose exposure was
measured by fluorescently-labeled RCA-1 lectin binding.
Fig. 24 is a bar graph depicting the effect of neuraminidase treatment on
platelet GPIba and
GPV receptor surface expression in the presence of 100 ILLM metalloproteinase
(MP) inhibitor GM6001.
The receptor expression on MP inhibitor-neuraminidase was set to 100%.
Fig. 25 is a bar graph depicting the effects of recombinant TACE (ADAM17)
(TACE) and
recombinant TACE and DANA (TACE + DANA) on platelet GPIba and GPV receptor
surface
expression. The fact that inhibition of sialic acid loss prevents receptor
cleavage by the
metalloproteinase TACE shows that sialic acid has to be hydrolyzed from
glycoproteins before the
proteolysis of GPIba and GPV. The receptors GPIX and aIIN33 were not affected
by treatment with
recombinant TACE (not shown).
Fig. 26 is a bar graph depicting the quantification of free sialic acid (FSA)
in fresh platelet
samples and stored samples at 4 C and RT for the indicated time points. FSA
concentrations are also
shown on the top of each bar graph. Note that FSA detected in RT-stored
platelet samples was much
higher when compared to samples stored at 4 C for equivalent time periods.
Fig. 27 are photographs showing the time required to detect bacteria in
platelet samples (Time
of color detection = TOCD) stored at 4 C or at RT in the presence or absence
of a sialidase inhibitor,
DANA. The bacterial concentration in the test sample is inversely proportional
to the onset time of
color development, i.e., shorter time of color detection = higher
concentration of bacteria; longer time
color detection = lower concentration of bacteria. Selected pictures for the
analysis of Day 9 samples
CA 02835434 2013-11-07
WO 2012/158983 -13-
PCT/US2012/038462
are shown (A-C). Bacteria were detected using an assay technology as described
in Example 6. D is a
bar graph showing the quantification of the bacterial analysis in platelet
samples stored at 4 C or RT in
the presence or absence of sialidase inhibitor DANA. TOCD (min) was plotted
against the platelet
samples. Note that the time required for in RT stored samples with DANA is
equivalent to 4 C stored
samples, indicating that DANA inhibits bacterial growth as effectively as 4 C-
storage.
Fig. 28 is a line graph depicting the survival of mouse platelets stored for
48 h by refrigeration
in the absence (48h) or presence of 1 mM DANA (48h + DANA) in the storage
solution. The survival
of fresh, isolated platelets (RT) is shown for comparison, n = 7 for each
survival graph.
Fig. 29 is a flow cytometry analysis of fresh platelet (Fresh platelets) size
and density (A) and
the combined effect of DANA, sialylactose and glucose on stabilizing RT-stored
mouse platelet
integrity, as judged by their size (FSC) and density (S SC). Analysis of mouse
platelets stored for 48 h
at RT in the absence (- preservatives) (B) and presence (+ preservatives) (C)
of sialylactose, glucose
and DANA. The corresponding platelet numbers are shown below the dot plots.
The concentration of
the preservatives is also shown.
Fig. 30 is a flow cytometry dot plot analysis of mouse platelets stored at RT
for 48 h in the
absence (0 mM DANA) or presence of DANA at the indicated concentrations. Note
that 0.1 mM
DANA efficiently preserved the size and density of platelets as judged by dot
plot analysis (top panels).
Corresponding flow cytometry histograms of platelet counts and beads
(reference) are also shown
(lower panels).
Fig. 31 is bar graphs depicting the cell density of S. marcescens grown for 48
h in different
media with or without 1 mM DANA in the wells of 96-well PVC plate (panel A).
Fig. 31 in panel B
depicts biofilm formation of S. marcescens, incubated for 48 h in different
media with or without 1 mM
DANA in the wells of 96-well PVC plate. Also shown in panel B, the biofilm in
each well was stained
with crystal violet, and the dye was recovered and measured at 595 nm. The
absorption at 595 nm
(A595nm) is proportional to the bacterial cells in the biofilm.
Fig. 32 is a bar graph showing the differences in terminal I3-galactose
content on fresh platelets
isolated from healthy subjects. Platelet surface terminal galactose exposure
was measured by flow
cytometry using the I3-galactose specific lectin ECL, as depicted in the
schematic drawing of lectin
binding to a glycan-structure.
CA 02835434 2013-11-07
WO 2012/158983 -14-
PCT/US2012/038462
Fig. 33 is a flow cytometry dot plot analysis and corresponding flow cytometry
(A) of mouse
platelets stored in 30% plasma and 70% PAS (referred to as INTERSOLTm
solution) by volume at RT
for 48 h in the absence of additive (InterSol), the presence of 1 mM DANA
(InterSol + DANA), 10
mM glucose (InterSol + Glucose), and 1 mM DANA plus 10 mM glucose (InterSol +
DANA+Glucose). Note, that the platelet population appears resting, as judged
by their forward and
side scatter characteristics. B is a bar graph showing the percent of acquired
events in the gated platelet
population for the InterSol solution with DANA, glucose or both.
Fig. 34 is a representative flow cytometry dot plot analysis of platelets
stored in the absence ((-)
DANA) or presence of 0.5 mM DANA ((+) DANA) (upper panel A). A corresponding
histogram of
platelet counts vs side scatter (SSC) is also shown (lower panel B). The table
represents the mean
fluorescence intensity (MFI) measured in the side scatter (SSC-H (MFI)) in the
absence or presence of
DANA.
Fig. 35, panel A, is a representative flow cytometry histogram analysis of
surface P-selectin
exposure after human platelet storage in plasma in the absence or presence of
DANA as described in
Fig. 34. P-selectin exposure was measured using a monocolonal FITC conjugated
antibody to P-
selectin (CD62P-FITC). Fig. 35, panel B, shows that quantification of P-
selectin positive platelets
defined in M2 (as indicated in Fig. 35, panel A) and the corresponding MFI.
Fig. 36 is a flow cytometry dot plot analysis of human platelets stored at RT
for 7 days in 30%
plasma and 70% PAS solution (by volume) (PASa, 7.15 mM Na2HPO4, 2.24 mM
NaH2PO4, 10 mM
sodium citrate, 30 mM sodium acetate, 79.2 mM NaC1, 5.0 mM KC1, and 1.5 mM
MgC12, pH 7.2) in
the presence of 0, 0.1 and 0.5 mM DANA. The platelets are defined in `G1'
while the platelet
microparticles are defined in `G2'. The gate statistics is shown for each dot
plot.
DETAILED DESCRIPTION OF THE INVENTION
A description of preferred embodiments of the invention follows.
Platelet Additive Solution (PAS)
After platelets are obtained from a donor, they can be suspended in fluid
referred to as Platelet
Additive Solution (PAS). Essentially, PAS replaces a portion of the plasma in
which the isolated
CA 02835434 2013-11-07
WO 2012/158983 -15-
PCT/US2012/038462
platelets are placed during apheresis. PAS is a medium that is generally a
physiologically compatible,
aqueous electrolyte solution. In addition to certain agents that can be
normally present in such
solutions in varying combinations and concentrations as described hereinafter,
the PAS solution of the
present invention includes one or more sialidase inhibitors, and optionally
one or more glycan
modifying agents.
Heretofore, PAS solutions are used because they are believed to reduce
allergic and febrile
transfusion reactions, facilitate ABO-incompatible platelet transfusions,
enable the use of pathogen
inactivation techniques, and make more plasma available for other purposes
(e.g., for fractionation).
One embodiment of the present invention includes a PAS solution having the
sialidase inhibitor
and optionally a glycan-modifying agent. More specifically, the present
invention includes a PAS
composition having a sialidase inhibitor and/or a glycan-modifying
composition, and one or more of
PAS components (e.g., salts, buffers, nutrients, and any combination thereof).
PAS of the present
invention can include a variety of components such as one or more salts (e.g.,
NaC1, KC1, CaC12,
MgC12, and MgSO4), one or more buffers (e.g., acetate, bicarbonate, citrate,
or phosphate), and
nutrients (e.g., Na acetate, Na gluconate, glucose, maltose, or mannitol).
The term "Platelet Additive Solution" or "PAS" of the present invention refers
to the solution or
medium having at least one or more sialidase inhibitors, one or more storage
medium components, and
optionally, one or more glycan modifying agents. The "inventive composition"
includes one or more
sialidase inhibitors and optionally one or more glycan modifying agents. The
phrase "platelet
composition" or "platelet storage composition" refers to the resulting storage
composition (prior to
transfusion into a recipient), which includes the PAS of the present
invention, the platelets, and
optionally, any associated plasma and/or anticoagulant.
The medium of the PAS of the present invention includes a physiologically
compatible,
aqueous electrolytic solution. Such solutions can contain ionic elements in
solution such as sources of
sodium, potassium, magnesium, calcium, chloride, and phosphate. The PAS of the
present invention
can also contain, e.g., sources of citrate that can be added in the form of
citric acid or sodium salt. The
solution of the present invention further includes, for example, carbon or
nutrient source, such as
acetate, glucose or gluconate, and can be present in combination with a salt.
A phosphate source, in an
embodiment, can be included to help maintain ATP production. These elements
can be present in the
solution of the present invention in an amount ranging from about 5 mM to
about 450 mM (e.g., 10, 20,
30, 40, 50, 60, 70, 80, 90, 100, 150, 200, 250, 300, 350, 400, 450 mM). The
solution is maintained at a
pH ranging from about 6.4 and about 7.6 (e.g., about 7.1 to about 7.4), and
preferably at pH of about
CA 02835434 2013-11-07
WO 2012/158983 -16-
PCT/US2012/038462
7.2.
In an embodiment, a source of sodium (Na) can be present in the PAS of the
present invention
in an amount between about 100 and 300 mM (e.g., between about 150 mM and
about 250mM). In a
particular embodiment, a source of sodium is present at about 190 mM. Sodium
can be present as a
salt or in combination as a buffer, or carbon source. For example, sodium can
be present in the form of
sodium chloride (NaC1), sodium citrate, sodium acetate, sodium phosphate or a
combination thereof
Other suitable sources of sodium can be used in the PAS of the present
invention including those
known in the art or later discovered.
A source of chloride (Cl) can also be present in the PAS of the present
invention in an amount
between about 40 mM and about 110 mM (e.g., between about 60 mM and about 90
mM. In a
particular aspect, the source of chloride is present at about 87.2 mM.
Chloride can be present in the
form of sodium chloride (NaC1), magnesium chloride (MgC12), potassium chloride
(KC1), or a
combination thereof Any source of chloride known in the art or later
discovered can be used with the
present invention so long as it is suitable for use with PAS of the present
invention. Na ' and Cl-,
mainly in the form of NaC1, are tonicity modifiers that contribute to the
isotonicity of platelet additive
solution.
A source of potassium, in an embodiment, can be present in the PAS of the
present invention.
It can be present in an amount ranging between about 0.5 mM and about 10 mM,
and for example,
between about 3 mM and about 8 mM. In a particular embodiment, potassium is
present in an amount
of about 5 mM. Potassium sources include potassium chloride, potassium
citrate, potassium acetate,
potassium phosphate, potassium sulfate or a combination thereof. Other sources
of potassium known
in the art or later discovered can be used with the present invention. The
presence of potassium ion in
the medium can assist, in certain aspects, in maintaining intracellular
magnesium ion concentration.
Potassium ion also could also be involved in the transport of pyruvate across
the mitochondria
membrane for oxidative phosphorylation in the citric acid cycle (TCA cycle).
In addition, I( plays
important roles in membrane stability by contributing to the electrical
continuity of lipids and proteins.
Magnesium is another salt that can be included in the PAS of the present
invention. A source of
magnesium can be present in an amount ranging between about 0.5 mM and about
2.5 mM, and in
particular, in an amount ranging between about 1 mM and 2 mM. In an
embodiment, magnesium is
present in the PAS of the present invention in about 1.5 mM. Sources of
magnesium include
magnesium chloride, magnesium citrate, magnesium sulfate and a combination
thereof. Sources of
magnesium known in the art or later discovered can be used. In one embodiment,
magnesium ion can
CA 02835434 2013-11-07
WO 2012/158983 -17-
PCT/US2012/038462
be present in the PAS of the present invention at concentrations close to
plasma levels, which will be
about 3 mEq/L (1.5 mM). Mg2 might be necessary to maintain membrane ATPase
activity. In an
aspect, magnesium ion in the medium should maintain the optimal intercellular
magnesium levels in
the platelets and may promote oxidative phosphorylation in the platelets and
in so doing help maintain
the pH of the medium. Furthermore, Mg2' plays important roles in membrane
stability by contributing
to the electrical continuity of lipids and proteins.
Calcium is another yet salt that can be included in the PAS of the present
invention. A source
of calcium can be present in an amount ranging between about 0.5 mM and about
2.5 mM (e.g.,
between about 1 mM and 2 mM). In a certain embodiment, calcium is present in
the PAS of the
present invention in about 1.5 mM. Sources of calcium include calcium
chloride, calcium acetate,
calcium citrate or a combination thereof Sources of calcium known in the art
or later discovered can
be used.
Citrate can be used to buffer the solution. A source of citrate is present in
the PAS of the
present invention in an amount ranging between about 2 mM and about 20 mM, and
for example, in an
amount 5 mM and about 15 mM. In an aspect, the PAS of the present invention
includes about 10 mM
of citrate. Examples of citrate sources that can be used in the present
invention include sodium citrate
(e.g., monosodium citrate, disodium citrate, trisodium citrate), citric acid,
potassium citrate, magnesium
citrate and a combination thereof Other sources of citrate can be used
including those known in the art
or later discovered so long as it is suitable for use with PAS of the present
invention. Citrate plays
multiple roles in PAS of the present invention as an anticoagulant, a carbon
source for the TCA cycle
and buffer.
Acetate is yet another component of the PAS of the present invention. Acetate
is a carbon
source used as a nutrient for the isolated platelets. A source of acetate can
be present in an amount
ranging between about 10 mM and about 50 mM, and for example, in an amount
ranging between
about 25 mM and about 45 mM. The PAS of the present invention includes about
30 mM of acetate.
Sources of acetate include sodium acetate, potassium acetate, magnesium
acetate, or a combination
thereof Other sources of acetate can be used including those known in the art
or later discovered so
long as it is suitable for use with PAS of the present invention. Acetate
serves as carbon and buffer.
In the PAS of the present invention, a nutrient source can be provided.
Acetate and other
carbohydrates such as glucose or sucrose, as well as citrate, can be used
individually or in various
combinations to provide a source of energy for platelets in storage by being a
source of intermediate
metabolites for the production of energy in the citric acid cycle. A
combination of a carbon source can
CA 02835434 2013-11-07
WO 2012/158983 -18-
PCT/US2012/038462
be used. In the case that glucose and/or sucrose is used, the concentration
can be present in an amount
ranging from about 0.5 mM to about 25 mM (e.g., about 2 mM to about 22 mM).
Other nutrients can be substituted for or included with the acetate of the PAS
of the present
invention. For example, oxaloacetate can be present in the PAS of the present
invention or can be
added to platelet suspension after the PAS of the present invention has been
added to a platelet rich
fraction. Oxaloacetate is a four-carbon molecule found in the mitochondria
that condenses with Acetyl
Co-A to form the first reaction of the TCA cycle (citric acid cycle).
Oxaloacetate can be supplied to
the stored platelets either directly or in the form of precursor amino acids
such as aspartate. In some
embodiments oxaloacetate can be present in the PAS of the present invention
from about 10 mM to
about 45 mM. More particularly, oxaloacetate can be present in the PAS of the
present invention from
about 20 mM to about 40 mM, or from about 24 mM to about 36 mM, or from about
28 mM to about
33 mM.
Phosphate (PO4) is another component that can be used in the PAS of the
present invention. A
source of phosphate can be present in the PAS of the present invention in an
amount ranging between
about 5 mM and about 50 mM (e.g., between about 20 and 40 mM). In a particular
embodiment, a
source of phosphate is present in about 28 mM. Forms of phosphate include
sodium monophosphate,
diphosphate, triphosphate, or a combination thereof. Other sources of
phosphate known in the art or
discovered in the future can be used.
Components such as acetate, citrate, and phosphate can be added in combination
with one or
more salts, such as the calcium, magnesium, potassium, or sodium salts or any
sub-combination of
these salts to balance the osmolarity of the buffered solution.
In an embodiment, the PAS of the present invention includes one or more
sialidase inhibitors,
and optionally, one or more glycan modifying agents, and the components
described in Table 1:
CA 02835434 2013-11-07
WO 2012/158983 -19-
PCT/US2012/038462
Table 1:
Range (mM) Amount
for a specific formulation (mM)
Low High PAS1 PAS2 PAS3 PAS4
Sodium [Na] 100.0 300.0 156.7 148.3 155.2 146.8
Chloride [Cl] 40.0 110.0 87.2 78.8 87.7 79.3
Citrate 2.0 20.0 10.0 10.0 10.0 10.0
Acetate 10.0 50.0 30.0 30.0 30.0 30.0
Phosphate [PO4] 5.0 50.0 9.4 9.4 9.4 9.4
Potassium [K] 0.5 10.0 5.0 5.0 5.0 5.0
Magnesium [Mg] 0.5 2.5 1.5 1.5 1.5 1.5
Calcium [Ca] 0.5 2.5 0.0 0.0 1.0 1.0
[Glucose] 5 25.0 0.0 16.8 0.0 16.8
[DANA] 0.1 10.0 1.0 1.0 1.0 1.0
Total (mM) 163.6 605.0 300.8 300.8 300.8 300.8
The PAS of the present invention as described herein can also be buffered, in
an embodiment,
by amino acids. The amino acids can be used as the primary buffering agents,
or can be used in
conjunction with other buffering agents such as phosphate. In one embodiment
the amino acid,
histidine, can be used to buffer the storage solution. Thus, the storage
solution can contain amino acids
from about 1 mM to about 7 mM, or from about 2 mM to about 5 mM.
In addition to or as an alternative to the foregoing, the PAS disclosed herein
can further include
other components that promote oxidative phosphorylation. An antioxidant can be
added to the PAS or
platelet composition of the present invention. Examples of antioxidants
include glutathione, selenium
and the like. In some embodiments the antioxidant can be present in the PAS of
the present invention in
an amount ranging between about 0.5 uM to about 3 mM (e.g., about 1.0 [tM to
about 2 mM). In some
embodiments, glutathione, or its precursor N-acetylcysteine, and/or selenium
alone or in combination
can be present in the PAS in an amount between about 0.5 uM to about 3 mM
(e.g., about 1.0 uM to
about 2 mM).
To further promote oxidative phosphorylation, the PAS of the present invention
can further
include components that assist in stabilizing membranes. For example, a
phospholipid or a mixture or
phospholipids can be included in the storage solution. In some embodiments,
phospholipids can be
present in the PAS of the present invention in an amount ranging from about
0.1 mg/mL to about 7.5
mg/mL (e.g., between about 0.25 mg/mL to about 5 mg/mL). More particularly, L-
alpha
phosphatidylcholine can be present in the PAS of the present invention in an
amount between about 0.1
mg/mL to about 7.5 mg/mL (e.g., about 0.25 mg/mL to about 5 mg/mL).
CA 02835434 2013-11-07
WO 2012/158983 -20-
PCT/US2012/038462
Additional components that can be included in the PAS of the present invention
are non-
essential amino acids. For example, non-essential amino acids in an amount
ranging from about 0.5
mM to about 14 mM can be present in the PAS (e.g., about 1.0 mM to about 10
mM). In an
embodiment, L-alanine can be included in an amount ranging from about 0.5 mM
to about 14 mM
(e.g., about 1.0 mM to about 10 mM).
Unsaturated free long chain fatty acids can further be included in the PAS of
the present
invention. The PAS described herein can contain an amount of unsaturated free
long fatty acid changes
ranging between about from about 0.05 mM to about 1.5 mM (e.g., about 0.1 mM
to about 1 mM). In
an embodiment, the PAS of the present invention can contain palmitic acid from
about 0.05 mM to
about 1.5 mM, or about 0.1 mM to about 1 mM.
United States Pharmacopeia (USP) water for injection (WFI) can be used as a
solvent to make
the buffer solution for the PAS of the present invention.
The phrase "platelet composition" (e.g., the PAS of the present invention and
isolated platelets)
refers to a composition whose total volume contains between about 1% to about
50% by volume of
plasma. The platelet composition, in one aspect, contains less than about 50%
(e.g., less than about
45%, 40%, 35%, 30%, 25%, 20%, 15%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%) by
volume
plasma. Conversely, the platelet storage composition of the present invention
has between about 50%
and about 99% (e.g., about 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%) by
volume of PAS of
the present invention, which contains one or more sialidase inhibitors,
electrolytic solution, phosphate
and/or buffering compounds, carbon source (s), and optionally, one or more
glycan modifying agents.
In certain embodiments, the platelet storage composition is essentially plasma
free having mostly the
PAS of the present invention and platelets. In an embodiment, the platelets
generally make up about
1% by volume of the total platelet composition.
In an embodiment, once the PAS of the present invention is added to the
isolated platelets, PAS
of the present invention constitutes about 70% and the plasma constitutes
about 30% of the isolated
platelet solution. The percentage of PAS of the present invention by volume
can vary depending on its
use, e.g., for transfusion into chronically anemic patients or acutely anemic
patients. Hypervolemia is a
concern especially in trauma patients suffering from acute anemia.
Accordingly, the percentage of
PAS can be modified to minimize or avoid hypervolemia.
The platelet composition in the PAS of the present invention can be assessed
at one or more
time points during storage. Assessment of the platelet content, platelet
morphology, metabolism,
bacterial proliferation, the extent of platelet activation, extent of lysis or
a combination thereof can be
CA 02835434 2013-11-07
WO 2012/158983 -21-
PCT/US2012/038462
performed. The assessment of platelets, their function, and bacterial
proliferation is further described
herein to assess the platelets' ability to be transplanted, survive in vivo
and maintain hemostasis after
transfusion. The PAS of the present invention allows platelets to be stored
longer, and have longer
circulation and maintain hemostasis after transfusion, as compared to
platelets not stored in the PAS of
the present invention. Storage times, circulation times, and hemostasis are
also further described
herein.
Metabolism of platelets can be assessed by measuring ATP and levels of
glucose, lactate and
lactate dehydrogenase (LDH). ATP measurements can be carried out using assays
known in the art
such as Bioluminescent assay kit (Sigma, Poole, Dorset, UK). Glucose, lactate
and LDH were can also
be measured using assays known in the art, such as Vitros DT60 11 chemistry
system (Shield,
Kimbolton, Cambridgeshire, UK). The platelets use a carbon source such as
acetate during metabolism
to maintain ATP, a major energy carrier. The PAS of the present invention can
maintain a pH within
the range of between about 6.4 and about 7.6, and preferably between about 7.1
to about 7.4.
Applicants have characterized several underlying mechanisms that account for
the high
susceptibility of platelets to irreversible intolerance by the recipients of
transfusions and the resulting
loss of platelet's in vivo hemostatic activity. Applicants' discoveries are
related to sialic acid and its
role in the viability of platelets.
Surprisingly, Applicants have found that the catalytic hydrolysis of sialic
acid residues from
platelet surface glycans by the platelet's own sialidase enzymes generally
contributes to the irreversible
intolerance of platelets. Applicants have further discovered that endogenous
sialidase enzyme surface
activity actually increases during platelet storage. Yet another surprising
discovery is that sialidase-
producing bacteria desialylate plasma and platelet sialioglycoconjugates to
obtain nutrients such as
sialic acid which supports bacterial growth and proliferation. See Fig. 1A.
Bacterial proliferation leads
to biofilm formation, platelet activation and aggregation. Desialylated
platelets enhance bacteria-
platelet interaction and eventually are cleared from circulation via lectin-
mediated mechanism
(Fig.1B). Accordingly, the addition of a sialidase inhibitor prevents sialic
acid from being cleaved
from the platelet surface, thereby preventing platelet clearance and
prolonging its survival.
Additionally, a sialidase inhibitor inhibits the proliferation of bacteria in
a platelet preparation (Fig.1C).
The dual sialidase inhibitor-function provides a superior platelet preparation
with longer survivals and
reduces the chance of causing bacteria-related sepsis when transfused into a
recipient at the point of
care.
With these counterintuitive and surprising results in hand, Applicants have
developed methods
CA 02835434 2013-11-07
WO 2012/158983 -22-
PCT/US2012/038462
to effectively treat platelets with inhibitors of sialidase after they are
harvested from donors and prior to
storage at or below room temperature. Treated with sialidase inhibitors, the
inventive platelet
compositions retain in vivo hemostatic activity for longer durations as
compared to untreated platelets.
The inventive platelet compositions treated with sialidase inhibitors can be
stored for prolonged periods
at or below room temperature as compared to untreated platelets. The storage
of platelets according to
the inventive methods extends the shelf life of platelets and helps increase
the supply of platelets that
remain viable for transfusion with inhibited bacterial proliferation.
As noted, Applicants' discoveries are related to sialic acid and its role in
the viability of
platelets. The hydrolysis of sialic acid from the outer membrane of platelets
is believed to contribute to
the unique and irreversible intolerance of platelets. Studies have reported
that platelets loose sialic acid
from membrane glycoproteins during aging and circulation, and that in vitro
desialylated platelets are
cleared rapidly. Loss of sialic acid exposes underlying immature glycans such
as 13-galactose.
Asialoglycoprotein (ASGP) receptors are known to mediate endocytosis of
proteins, cells, and particles
carrying exposed 13-galactose. Many cells, including hepatic macrophages and
hepatocytes, express
and present the (ASGP) receptor. Accordingly, it is believed that when
endogenous sialidase enzymes
cleave sialic acid residues from the platelet surface, penultimate sugars such
as 13-galactose are exposed
on the platelet surface and platelets undergo ASGP mediated ingestion.
While the loss of surface receptors (e.g., GPIb and GPV) on platelets has been
associated with
platelet survival, prior to the present invention the role of surface sialic
acid with respect to surface
receptors on platelets was unknown. Furthermore, the role of surface sialic
acid regarding the survival
of platelets was unclear. Applicants have used in vitro and in vivo studies to
characterize relationships
between surface sialic acid and platelet receptor loss. Accordingly,
Applicants' results have been
applied to the inventive methods described herein for prolonging the survival
of platelets. This
relationship between surface sialic acid and platelet receptor loss turns out
to be an important factor in
determining platelet survival. Applicants have found that inhibiting the loss
of surface sialic acid
prevents platelet surface receptor GPIb and GPV loss during storage in vitro
and rescues platelet
survival in vivo.
For example, mouse platelets stored at room temperature for 6 h lost surface
sialic acid, as
evidenced by flow cytometry data provided herein. See Exemplification. This
loss correlated with a
30-60% loss of surface receptors GPIb and GPV, but not GPIX and integrin
aIIb133. Furthermore,
treatment of mouse platelets with the neuraminidase (NA) substrate, fetuin,
partially decreases the loss
of GPIb and GPV to 10-20%. In vitro, sialic acid was cleaved from the platelet
surface by adding a2-
CA 02835434 2013-11-07
WO 2012/158983 -23-
PCT/US2012/038462
3,6,8-neuraminidase (NA; Vibrio cholerae) or a2-3,6,-NA (Clostridium
perfringens) to mouse
platelets. Removal of sialic acid correlated with the removal of 50-60% of
surface GPIba and GPV,
but not GPIX and integrin aIIb133. Addition of fetuin, or the more specific
sialidase inhibitor, the
sodium salt of 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA), completely
prevented this loss,
as determined by both flow cytometry and Western blot analysis, also provided
herein.
The clearance of platelets is exacerbated upon cooling. It has been discovered
that cooling of
human platelets causes clustering of the von Willebrand factor (vWf) receptor
complex a subunit
(GPIba) complexes on the platelet surface. The clustering of (GPIba) complexes
on the platelet
surface elicits recognition by macrophage complement type three receptors
(aM132, CR3) in vitro and
in vivo. CR3 receptors recognize N-linked sugars with terminal 13-G1cNAc on
the surface of platelets,
which have formed GPIba complexes, and phagocytose the platelets, clearing
them from the circulation
and resulting in a concomitant loss of hemostatic function. Although capping
the 13-G1cNAc moieties
by galactosylation prevents clearance of short-term¨cooled platelets, this
strategy is ineffective after
prolonged refrigeration (e.g., refrigeration of platelets longer than 5 days).
Prolonged refrigeration
further increased the density and concentration of exposed galactose residues
on platelets GPIba such
that hepatocytes, through Ashwell-Morell receptor (ASGP receptor or hepatic
lectin) binding, become
increasingly involved in platelet removal. Macrophages rapidly removed a large
fraction of transfused
platelets independent of their storage conditions. With prolonged platelet
chilling, hepatocyte-
dependent clearance further diminishes platelet recovery and survival after
transfusion. Inhibition of
chilled platelet clearance by both 132 integrin and Ashwell-Morell receptors
may afford a potentially
simple method for storing platelets in the cold.
As noted above, Applicants have discovered that sialidase enzyme activity is
platelet derived,
not plasma derived, and sialidase enzyme activity substantially increases
during the storage of platelets.
Specifically, Applicants have discovered that human platelets contain the
sialidases Neul and Neu3,
and release Neul into plasma at room temperature, and more so upon storage in
the cold, indicating
that released Neul is involved in the removal of surface sialic acid from
glycans on the surface of
platelets.
The present invention provides platelet compositions and methods for
prolonging in vivo
hemostatic activity and reducing platelet clearance, wherein the platelets are
obtained from a donor and
treated with a sialidase inhibitor to counteract the effects of endogenous
sialidase activity and inhibit
bacterial proliferation. Also provided are compositions and methods for
prolonging the storage of
CA 02835434 2013-11-07
WO 2012/158983 -24-
PCT/US2012/038462
viable platelets, such as mammalian platelets, particularly human platelets.
The invention also provides
methods for making improved platelet compositions.
The present invention, in certain aspects, provides platelet compositions that
have enhanced
circulation properties and that retain substantially normal in vivo hemostatic
activity. In certain
embodiments, the invention provides a novel platelet composition comprising
one or more sialidase
inhibitors. As noted, sialidase enzymes catalyze the hydrolysis of terminal
sialic acid residues from
host cell receptors. Thus, sialidase inhibitors are used in numerous aspects
of the present invention to
reduce sialidase enzyme activity, prevent the hydrolysis of terminal sialic
acid residues from platelet
surface glycans, inhibit bacterial proliferation and prolong the in vivo
hemostatic activity of platelets
for transfusion.
The present invention provides for platelet compositions and related methods
to prepare, store,
and preserve platelet compositions that enhance the platelet function and/or
allow platelets to retain
substantially normal in vivo hemostatic activity after platelets have been
stored at or below room
temperature. Certain underlying mechanisms have been discovered and contribute
to the high
susceptibility of platelets to undergo irreversible intolerance or loss of
platelet in vivo hemostatic
activity experienced by recipients of platelet transfusions. The hydrolysis of
sialic acid residues from
platelet surface glycans by sialidase enzymes contributes to the irreversible
intolerance of platelets.
"Irreversible intolerance" refers to a platelet's inability to retain or
return to normal platelet function
survival after being subjected to temperatures below that of room temperature.
"Platelet viability" is
defined as the platelet's ability to survive in vivo.
The present invention provides platelet compositions and methods of inhibiting
sialidase
enzyme activity in platelets isolated from a donor and stored at or below room
temperature. Thus, in
certain aspects, the invention provides compositions having one or more
sialidase inhibitors, and
optionally one or more glycan-modifying agents. The present invention, in
other aspects, provides
methods for increasing the circulation time of platelet compositions having
one or more sialidase
inhibitors. The present invention further provides platelet compositions and
methods for reduced
temperature storage of platelets, which increases the storage time of the
platelets, as well as methods
for reducing clearance of or increasing the circulation time of a population
of platelets in a mammal.
Also provided are platelet compositions and methods for the preservation of
platelets with preserved
hemostatic activity as well as methods for making platelet compositions and
pharmaceutical
compositions thereof containing the platelet compositions and for
administering the pharmaceutical
CA 02835434 2013-11-07
WO 2012/158983 -25-
PCT/US2012/038462
compositions to a mammal to mediate hemostasis. Also provided are kits for
treating a platelet
preparation for storage and containers for storing the same.
The Platelet and How It Is Isolated
The term "isolated" as used herein means separated away from its native
environment. As used
herein with respect to a population of platelets, isolated refers to removing
platelets from the blood of a
mammal.
Based on standard blood collection methods, there are generally two types of
donated platelets:
random donor platelets and single donor platelets. Random donor platelets are
platelets isolated from
whole blood donations by means of any one of several standard methods
practiced by those skilled in
the art, and two or more random donor platelets are subsequently pooled in a
quantity sufficient to
constitute a therapeutic dose prior to transfusion to a patient. A single
random donor platelet can also
be used without pooling for pediatric patients. Current standard methods
include isolating random
donor platelets from a buffy coat, a platelet button, platelet rich plasma and
the like. Single donor
platelets are platelets obtained from one donor by means of centrifugal
separation in an apheresis
machine in a quantity sufficient to constitute one or more therapeutic dose(s)
for subsequent transfusion
to a patient(s). Apheresis machines used currently for the collection of
single donor platelets are
manufactured by companies such as Terumo BCT (Terumo Corporation), Fenwal
Inc., and
Haemonetics Corporation. Current AABB (formerly the American Association of
Blood Banks)
Standards define a therapeutic dose of platelets as approximately? 3x1011
platelets.
To carry out the methods described herein, either random donor platelets or
single donor
platelets are isolated from a donor by means of standard techniques known to
one skilled in the art.
The isolated platelet preparation is treated with one or more sialidase
inhibitors and/or glycan-
modifying agents as described herein.
Random donor platelets are obtained from the whole blood donations. Whole
blood can be
obtained from a donor and prepared by a suitable method depending on the type
of blood components
desired. The present invention involves isolating platelets in the form of a
buffy coat, a platelet button,
platelet concentrate, platelet rich plasma and the like.
Whole blood is made up of a number of components including plasma, red blood
cells,
platelets, white blood cells and other components. Accordingly, in addition to
platelets, other
components from whole blood can be isolated and prepared (e.g., red blood
cells, plasma, etc.) when a
unit of blood is obtained from a donor. Whole blood is generally collected
from a donor by
CA 02835434 2013-11-07
WO 2012/158983 -26-
PCT/US2012/038462
venipuncture. The container (e.g., bag or tube) into which one deposits the
blood can contain an
anticoagulant such as a citrate or citrate dextrose based component, e.g.,
citrate phosphate dextrose
(CPD or CP2D), citrate phosphate dextrose adeninel (CPDA-1).
During routine blood collection, a 600 mL bag that contains 70 mL of
anticoagulant is used to
collect approximately 500 mL 10% of whole blood, or 63 mL of anticoagulant is
used to collect 450
mL 10% of whole blood. The whole blood collection bag often has satellite
bags attached thereto to
hold isolated components. At the time whole blood is collected, tubes of donor
blood samples are also
collected for use in performing certain required tests on each blood donation,
including ABO and Rh
determination, infection disease markers and the like.
Platelets are normally separated from whole blood and other blood components
by
centrifugation. Centrifuge technology allows separation of blood components by
their various
densities. Therefore, the liquid and cellular constituents of whole blood are
separated into distinct
layers as the result of centrifugation, ranging from red blood cells (RBC),
the most dense, to plasma,
the least dense. The time of centrifugation varies depending on the centrifuge
and the g-force provided
by the centrifuge. The amount of time of centrifugation can be determined by
one of skill in the art.
Companies such as Sorvall and Beckman manufacture centrifuges that can be used
for this process.
Appropriate centrifugation (e.g., a soft spin) results in a bag that contains
a mass of RBC at its
distal end and a mass of platelet rich plasma (PRP), a mixture of platelets
and plasma at its proximal
end, with a meniscus formed primarily by white cells in between the two
layers. By means of the use
of a plasma expressor or extractor (made by companies such as Fenwal, Inc. and
Terumo Corporation),
the PRP is expressed into a satellite bag, leaving the mass of RBC in the
original whole blood
collection bag.
The satellite bag containing the PRP is centrifuged again (e.g., hard spin) to
separate the plasma
from the platelets. Upon re-centrifugation, the platelets, because of their
greater density, form a loosely
aggregated cluster called a platelet button. By use of a plasma expresser or
extractor, the platelet poor
plasma (PPP) can then be expressed into a second satellite bag leaving the
platelet button and a small
volume of plasma (together, known as platelet concentrate) in the first
satellite bag. The platelet
concentrate consists of a volume of approximately 30 to 70 mL, and the PPP
consists of a fluid volume
of approximately 180 to 320 mL. Each of the separated blood components, i.e.,
RBC, PPP and platelet
concentrate, is known as a "unit", and each is transfused separately.
Generally, the bag of platelet concentrate contains a minimum of 5.5 x 109
platelets. Units of
platelet concentrate are stored at 20-24 C on mechanical rotators. Platelets
not treated with the
CA 02835434 2013-11-07
WO 2012/158983 -27-
PCT/US2012/038462
compositions of the present invention have a shelf life of about 5 days.
As generally practiced by those skilled in the art, 4-6 platelet concentrate
units are pooled to
obtain a single therapeutic dose for transfusion to a patient. The pooled
platelet concentrate has about
3.0 x 1011 platelets or greater. The pooled and non-pooled platelet
concentrate obtained from this
process comprise one form of "isolated platelets" that can be utilized in the
present invention or treated
with the inventive compositions described herein. In a particular embodiment,
the bag used for pooling
the platelet concentrate can have the inventive compositions described therein
(e.g., sialidase inhibitor
and/or glycan modifying agent), as further described herein. Alternatively,
the inventive composition
can be added to the platelet concentrate before, after or during pooling.
Random donor platelets may also be isolated by the "buffy coat" method
generally utilized in
Europe and Canada. Whole blood is obtained, as described herein, and undergoes
a hard spin
centrifugation. The hard spin results in a bag having plasma as the top
fraction, red blood cells as the
bottom fraction, and a middle layer containing platelets and leukocytes. This
middle layer is known as
the buffy coat.
For the purpose of producing buffy coat prepared platelets, buffy coats are
generally isolated
and pooled by one of two methods depending on the format of the bag in which
the whole blood was
collected. The first method is known as the "top and bottom drain method" in
which the bag into
which the whole blood was collected has a top and bottom drain with one or
more satellite containers
attached to each end. An extractor (e.g., Optipress0 Extractor from Fenwal)
presses the bag flat such
that the plasma layer is drained through the top drain and the red blood cells
are drained through the
bottom drain. The extractor is designed such that the buffy coat containing
primarily platelets and
leukocytes with a small volume of plasma and RBC, together comprising
approximately 30 to 60 mL
of fluid volume, is retained within the bag. Approximately 4-6 buffy coat
units are pooled to make a
therapeutic dose of platelets for transfusion to a patient. In pooling,
individual buffy coat units are
sterilely connected in a chain format often referred to as the "chain method"
(e.g., the bottom drain of a
bag is connected to the top drain of the next bag, and so on.). A platelet
additive solution or plasma can
be sterilely connected to the chain and used to help rinse individual buffy
coat containers as the buffy
coats are transferred to the bottom pooling bag along with the platelet
additive solution or plasma.
A second method for isolating and pooling buffy coat prepared platelets
utilizes a similar whole
blood collection bag as used with PRP prepared platelets. Following the
isolation of the buffy coat in
the whole blood as described previously, the buffy coat is separated from the
whole blood by first
removing the plasma into one of the attached satellite containers and
transferring the buffy coat into a
CA 02835434 2013-11-07
WO 2012/158983 -28-
PCT/US2012/038462
second attached satellite container, sometimes referred to as "milking the
buffy coat" leaving the RBC
in the original container. Approximately 4-6 buffy coat units are pooled to
make a therapeutic dose of
platelets for transfusion to a patient. In pooling, individual buffy coat
units are sterilely connected and
pooled into a pooling container along with a platelet additive solution or
plasma. In this method, the
pooling bag has multiple docks (e.g., like legs of a "spider") to which the
individual units are
connected. Each buffy coat unit is then transferred from the individual bag
into the pooling bag using
the platelet additive solution or plasma as a rinsing agent to help reduce
platelet loss in pooling. This
pooling method is sometimes referred to as the "spider method" and can also be
used with buffy coats
prepared by top and bottom separation.
Regardless of the method used to pool the individual units, the pooled bag
undergoes
centrifugation again. This centrifugation is a long, soft spin in which a
fraction containing platelets and
the plasma/platelet additive solution is formed at the top of the pooling bag
and the remaining red
blood cells and leukocytes become part of the bottom fraction. Using a plasma
expresser or extractor,
the top layer of platelets and plasma/platelet additive solution is
transferred to another bag resulting in a
therapeutic dose of platelets.
Single donor platelets are platelets obtained from one donor by means of
centrifugal separation
in an automated apheresis machine in a quantity sufficient to constitute one
or more therapeutic dose(s)
for subsequent transfusion to a patient(s). Platelets isolated by this method
are generally known as
single donor platelets because a therapeutic dose can be collected from a
single donor. In such a
procedure, the donor's blood flows from a point of venipuncture through a
sterile centrifuge in which
the platelets and a certain volume of plasma are centrifugally separated and
isolated, with the balance
of the donor's blood being returned to the donor through the initial
venipuncture or a second point of
venipuncture. Anticoagulant compositions, described herein, can be added to
the platelets or be present
in the bag into which the platelets are collected. Various automated apheresis
devices are
commercially available from companies such as Haemonetics Corporation
(Braintree, MA), Terumo
BCT (Lakewood, CO), Fenwal, Inc., Lake Zurich, IL and Fresenius Kabi,
Friedberg, Germany.
The collection of platelets by apheresis generally produces 2 platelet units,
wherein each unit
contains approximately 200 to 300 mL of plasma and approximately 3.5 x 1011
platelets. Single donor
platelets can be stored at 20-24 C for about 5 days.
Apheresis collection kits often include two platelet collection bags since
most apheresis
machines collect two units of platelets. The composition of the present
invention, as described herein,
can be included in the platelet collection bags for apheresis machines or can
be added to the bag before,
CA 02835434 2013-11-07
WO 2012/158983 -29-
PCT/US2012/038462
during or after collection of the platelets using a sterile connection
technique. Platelet collection bags
can be manufactured with the composition of the present invention and further
include additional
components such as anticoagulant compositions as described herein or known in
the art.
After platelets are collected by apheresis, they can be suspended in the PAS
of the present
invention, as described herein.
The compositions and methods present invention can be used with platelets
isolated by any
technique known in the art or developed in the future so long as a therapeutic
concentration of platelets
is obtained.
The present invention includes bags or containers including the sialidase
inhibitor and/or
glycan-modifying composition or the "inventive composition" as described
herein. Based on the
platelet isolation process, the inventive composition can be included or
manufactured with various
platelet collection bags. Platelet collection bags can be gas permeable or
made from a plastic material
such as PVC material. Platelet collections bags can be used in the random
donor collection process or
in the single donor collection process. With respect to the random donor
collection process, the
inventive composition can be placed into the collection bag in which the
platelet units are pooled;
therefore the present invention includes a pooled collection bag having the
inventive composition.
Similarly, in the single donor collection process, the inventive composition
can be included in
apheresis platelet collection bags. Along with the inventive composition, such
bags include other
components used in the apheresis process such as anticoagulant compositions.
Conventional platelet bags or packs are formed of materials that are designed
and constructed of
a sufficiently permeable material to maximize gas transport into and out of
the pack (02 in and CO2
out). The present invention allows for storage of platelets at temperatures
below room temperature or
at room temperature, as further described herein. The methods described herein
reduce or diminish the
amount of CO2 generated by the platelets during storage. Accordingly, in an
embodiment, the present
invention further provides platelet containers that are substantially non-
permeable to CO2 and/or 02,
which containers are useful particularly for cold storage of platelets. In
another embodiment, the
containers or bags include gas permeable containers.
With either collection process described above, the inventive compositions can
alternatively be
added to the isolated platelets using a sterile technique or connection. In
which case, the inventive
composition can be sold separately in a separate bag, container, syringe,
tube, or other similar blood
collection medium.
In one embodiment, the composition of the present invention having the
sialidase inhibitor
CA 02835434 2013-11-07
WO 2012/158983 -30-
PCT/US2012/038462
and/or glycan-modifying agent, as further described herein, is contacted with
the platelets in a closed
system, e.g., a sterile, sealed platelet pack so as to avoid microbial
contamination. Typically, a
venipuncture conduit is the only opening in the pack during platelet
procurement or transfusion.
Accordingly, to maintain a closed system during treatment of the platelets
with the composition of the
present invention, such composition is placed in a relatively small, sterile
container which is attached to
the platelet pack by a sterile connection tube (see e.g., U.S. Pat. No.
4,412,835, the contents of which
are incorporated herein by reference). The connection tube may be reversibly
sealed, or have a
breakable seal, as will be known to those of skill in the art. After the
platelets are isolated, the seal to
the container including the composition of the present invention is opened and
the composition is
introduced into the platelet bag. In one embodiment, the composition of the
present invention is
contained in a separate container having a separate resealable connection tube
to permit the sequential
addition of the composition to the platelets.
The Sialidase Inhibitor
Once the isolated platelets are obtained, platelets are treated with the
composition of the present
invention, which includes one or more sialidase inhibitors, and optionally one
or more storage
enhancing compositions such as glycan-modifying agents (e.g., monosaccharides
such as arabinose,
fructose, fucose, galactose, mannose, ribose, gluconic acid, galactosamine,
glucosamine, N-
acetylgalactosamine, muramic acid, sialic acid (N-acetylneuraminic acid), and
nucleotide sugars such
as cytidine monophospho-N-acetylneuraminic acid (CMP-sialic acid), uridine
diphosphate galactose
(UDP-galactose) and UDP-galactose precursors such as UDP-glucose). In some
preferred
embodiments, the glycan-modifying agent is UDP-galactose and/or CMP-sialic
acid. The composition
of the present invention includes a "cocktail" in which more than one or a
combination of these
constituents is included. The phrase, "composition" or "inventive composition"
refers to one or more
sialidase inhibitors, and optionally one or more glycan-modifying agents.
"Sialidase enzymes," "sialidases," also called "neuraminidases," as used
herein, are glycoside
hydrolase enzymes that cleave the glycosidic linkages of neuraminic acids.
Sialidase enzymes catalyze
the hydrolysis of terminal sialic acid residues from platelet surface glycans.
See Fig. 8. Thus, sialidase
inhibitors are used in several aspects of the present invention. Sialidase
inhibitors reduce sialidase
enzyme activity, prevent the hydrolysis of terminal sialic acid residues from
platelet surface glycans,
preserve the integrity of platelet surface glycans, and/or maintain the
function of platelets that are
stored prior to transfusion.
CA 02835434 2013-11-07
WO 2012/158983 -31-
PCT/US2012/038462
Sialidase/neuraminidase enzymes are a large family, found in a range of
organisms.
Neuraminidase enzymes are glycoside hydrolase enzymes (EC 3.2.1.18) that
cleave the glycosidic
linkages of neuraminic acids. A commonly known neuraminidase is a viral
neuraminidase, a drug
target for the prevention of influenza infection. Other homologs are found in
mammalian cells, and at
least four mammalian sialidase homologs have been described in the human
genome [e.g., Neul
(Uniprot accession numbers: Q5JQI0, Q99519), Neu2 (Q9Y3R4), Neu3 (Q9UQ49.1),
and Neu4
(A8K056, B3KR54, Q8WWR8).
As used herein, "sialidase inhibitor," or "neuraminidase inhibitor," can be
any compound, small
molecule, peptide, protein, aptamer, ribozyme, RNAi, or antisense
oligonucleotide and the like. As
used herein, "inhibit" means to interfere with the binding or activity of an
enzyme. Inhibition can be
partial or total, resulting in a reduction or modulation in the activity of
the enzyme as detected.
For example, a sialidase/neuraminidase inhibitor according to the invention
can be a protein,
such as an antibody (monoclonal, polyclonal, humanized, and the like), or a
binding fragment thereof,
directed against a neuraminidase protein. An antibody fragment can be a form
of an antibody other
than the full-length form and includes portions or components that exist
within full-length antibodies,
in addition to antibody fragments that have been engineered. Antibody
fragments can include, but are
not limited to, single chain Fv (scFv), diabodies, Fv, and (Fab')2,
triabodies, Fc, Fab, CDR1, CDR2,
CDR3, combinations of CDR' s, variable regions, tetrabodies, bifunctional
hybrid antibodies,
framework regions, constant regions, and the like (see, Maynard et at., (2000)
Ann. Rev. Biomed. Eng.
2:339-76; Hudson (1998) Curr. Opin. Biotechnol. 9:395-402). Antibodies can be
obtained
commercially, custom generated, or synthesized against an antigen of interest
according to methods
established in the art (Janeway et at., (2001) Immunobiology, 5th ed., Garland
Publishing).
Additionally, a sialidase/neuraminidase inhibitor can be a non-antibody
peptide or polypeptide
that binds a neuraminidase (e.g., a bacterial neuraminidase). A peptide or
polypeptide can be a portion
of a protein molecule of interest other than the full-length form, and
includes peptides that are smaller
constituents that exist within the full-length amino acid sequence of a
protein molecule of interest.
These peptides can be obtained commercially or synthesized via liquid phase or
solid phase synthesis
methods (Atherton et at., (1989) Solid Phase Peptide Synthesis: a Practical
Approach. IRL Press,
Oxford, England). The peptide or protein-related sialidase/neuraminidase
inhibitors can be isolated
from a natural source, genetically engineered or chemically prepared. These
methods are well known
in the art.
A sialidase/neuraminidase inhibitor can also be a small molecule that binds to
a neuraminidase
CA 02835434 2013-11-07
WO 2012/158983 -32-
PCT/US2012/038462
and disrupts its function. Small molecules are a diverse group of synthetic
and natural substances
generally having low molecular weights. They are isolated from natural sources
(for example, plants,
fungi, microbes and the like), are obtained commercially and/or available as
libraries or collections, or
synthesized. Candidate sialidase/neuramindase inhibitor small molecules can be
identified via in silico
screening or high-through-put (HTP) screening of combinatorial libraries. Most
conventional
pharmaceuticals, such as aspirin, penicillin, and many chemotherapeutics, are
small molecules, can be
obtained commercially, can be chemically synthesized, or can be obtained from
random or
combinatorial libraries as described below (Werner et at., (2006) Brief Funct.
Genomic Proteomic
5(1):32-6). In a preferred embodiment of the invention, a small-molecule
sialidase/neuramindase
inhibitor is the sodium salt of 2,3-dehydro-2-deoxy-N-acetylneuraminic acid
(DANA).
According to the present invention, the sialidase/neuraminidase inhibitor can
also be an FDA
approved viral sialidase/neuraminidase inhibitor, such as the viral
sialidase/neuraminidase inhibitor
oseltamivir also known as ethyl (3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-
yloxy)-cyclohex-1-ene-
1-carboxylate (Tamiflu, Genentech, Cambridge, Massachusetts), zanamivir also
known as ((2R,3R,45)-
4-guanidino-3-(prop-1-en-2-ylamino)-2-((1R,2R)-1,2,3-trihydroxypropyl)-3,4-
dihydro-2H-pyran-6-
carboxylic acid) (Relenza; Glaxo Smith Kline, Research Triangle Park, N.C.);
and Peramivir
((1S,2S,3S,4R)-3-[(1S)-1-acetamido-2-ethyl-buty1]-4-(diaminomethylideneamino)-
2-hydroxy-
cyclopentane- 1 -carboxylic acid) (BioCryst, Birmingham, Ala.), or a variant
thereof For example, the
viral sialidase/neuraminidase inhibitor, oseltamivir is an ethyl ester prodrug
that can be purchased from
Roche Laboratories (Nutley, N.J.). Amino acid sequences of FDA approved viral
sialidase/neuraminidase inhibitors may also be derivatized, for example,
bearing modifications other
than insertion, deletion, or substitution of amino acid residues, thus
resulting in a variation of the
original product (a variant). These modifications can be covalent in nature,
and include for example,
chemical bonding with lipids, other organic moieties, inorganic moieties, and
polymers. For reviews
on viral sialidase/neuraminidase inhibitors, please see "The war against
influenza: discovery and
development of sialidase inhibitors." Nature Reviews: Drug Discovery (2007) 6
(12): 967-74. Klumpp
et at., (2006) Curr. Top. Med. Chem. 6(5):423-34; Zhang et at., (2006) Mini
Rev. Med. Chem.
6(4):429-48; Jefferson et at., (2006) Lancet 367(9507):303-13; Alymova et at.,
(2005) Curr Drug
Targets Infect. Disord. 5(4):401-9; Moscona (2005) N. Engl. J. Med.
353(13):1363-73; De Clercq
(2004) J. Clin. Virol. 30(2):115-33; Stiver (2003) CMAJ 168(1):49-56; Oxford
et at., (2003) Expert
Rev. Anti. Infect. Ther. 1(2):337-42; Cheer et at., (2002) Am. J. Respir. Med.
1(2):147-52; Sidewell et
at., (2002) Expert Opin. Investig. Drugs. 11(6):859-69; Doucette et at.,
(2001) Expert Opin.
CA 02835434 2013-11-07
WO 2012/158983 -33-
PCT/US2012/038462
Pharmacother. 2(10):1671-83; Young et al., (2001) Philos. Trans. R. Soc. Lond.
B. Biol. Sci.
356(1416):1905-13; Lew et at., (2000) Curr. Med. Chem. 7(6):663-72); Taylor et
at., (1996) Curr.
Opin. Struct. Biol. 1996 6(6): 830-7 and published U.S. Patent Application.
Nos. 2009/0175805,
2006/0057658, 2008/0199845 and 2004/0062801, the entirety of each of which is
incorporated herein
by reference.
Accordingly, a "sialidase inhibitor" includes, but is not limited to one or
more of the following:
fetuin, 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA) or a
pharmaceutically acceptable salt
thereof; Oseltamivir (ethyl (3R,4R,55)-5-amino-4-acetamido-3-(pentan-3-yloxy)-
cyclohex-1-ene-1-
carboxylate); Zanamivir ((2R,3R,45)-4-guanidino-3-(prop-1-en-2-ylamino)-2-
((1R,2R)-1,2,3-
trihydroxypropy1)-3,4-dihydro-2H-pyran-6-carboxylic acid); Laninamivir
((4S,5R,6R)-5-acetamido-4-
carbamimidamido-6-[(1R,2R)-3-hydroxy-2-methoxypropy1]-5,6-dihydro-4H-pyran-2-
carboxylic acid);
and Peramivir ((1S,2S,3S,4R)-3-[(1S)-1-acetamido-2-ethyl-buty1]-4-
(diaminomethylideneamino)-2-
hydroxy-cyclopentane- 1 -carboxylic acid) or a pharmaceutically acceptable
salt thereof In a still
further preferred embodiment, the sialidase inhibitor is the sodium salt of
2,3-dehydro-2-deoxy-N-
acetylneuraminic acid or a combination thereof Sialidase inhibitors used with
the present invention
include those known in the art or those later developed.
As used herein, a "glycan" or "glycan residue" is a polysaccharide moiety on
the surface of the
platelet, exemplified by the GPIba polysaccharide. A "terminal" glycan residue
is the
monosaccharide/sugar residue at the terminus of the polysaccharide chain,
which typically is attached
to polypeptides on the platelet surface. A glycan-modifying agent includes an
agent that modifies
glycan residues on the platelet. The glycan-modifying agent repairs cleavage
that occurs on the glycan
residue. In an embodiment, the glycan-modifying agent alters the sugar
residues of the polysaccharide
chain of GPIba on the surface of the platelet.
Whereas sialidase inhibitors serve to preserve the integrity of the glycan
structures, and
specifically the glycan termini, the glycan-modifying agents serve to modify
or repair glycans by the
addition of monosaccharide(s) to the glycan. Thus, sialidase inhibitors and
glycan-modifying agents
serve distinct and complementary functions.
"Glycan-modifying agents," as described herein, include monosaccharides such
as arabinose,
fructose, fucose, galactose, mannose, ribose, gluconic acid, galactosamine,
glucosamine, N-
acetylgalactosamine, muramic acid, sialic acid (N-acetylneuraminic acid), and
nucleotide sugars such
as cytidine monophospho-N-acetylneuraminic acid (CMP-sialic acid), uridine
diphosphate galactose
(UDP-galactose) and UDP-galactose precursors such as UDP-glucose. Glycan-
modifying agents
CA 02835434 2013-11-07
WO 2012/158983 -34-
PCT/US2012/038462
include precursors of CMP-sialic acid or UDP-galactose. In some preferred
embodiments, the glycan-
modifying agent is UDP-galactose or CMP-sialic acid.
UDP-galactose is an intermediate in galactose metabolism, formed by the enzyme
UDP-
glucose-a-D-galactose-l-phosphate uridylyltransferase which catalyzes the
release of glucose-1-
phosphate from UDP-glucose in exchange for galactose-1-phosphate to make UDP-
galactose.
UDP-galactose and sialic acid are available from several commercial suppliers
such as Sigma. In
addition, methods for synthesis and production of UDP-galactose are known in
the art and described in
the literature (see for example, Liu et at., ChemBioChem 3, 348-355, 2002;
Heidlas et at., J. Org.
Chem. 57, 152-157; Butler et at., Nat. Biotechnol. 8, 281-284, 2000; Koizumi
et at., Carbohydr. Res.
316, 179-183, 1999; Endo et al., Appl. Microbiol., Biotechnol. 53, 257-261,
2000). UDP-galactose
precursors are molecules, compounds, or intermediate compounds that may be
converted (e.g.,
enzymatically or biochemically) to UDP-galactose. One non-limiting example of
a UDP-galactose
precursor is UDP-glucose. In certain embodiments, an enzyme that converts a
UDP-galactose
precursor to UDP-galactose is added to a reaction mixture (e.g., in a platelet
container).
In certain embodiments, the glycan-modifying agent is CMP-sialic acid or a CMP-
sialic acid
precursor. In further embodiments, the platelet compositions comprising a CMP-
sialic acid precursor
further comprise an enzyme that converts the CMP-sialic acid precursor to CMP-
sialic acid. In certain
embodiments, the glycan-modifying agent is CMP-sialic acid. In certain
embodiments, the glycan-
modifying agent is UDP-galactose. In certain embodiments, the glycan-modifying
agents are CMP-
sialic acid and UDP-galactose.
In certain embodiments, the sialidase inhibitor is a protein. In further
embodiments, the
sialidase inhibitor is an antibody directed against a neuraminidase protein
wherein the antibody is
monoclonal, polyclonal, humanized, or a binding fragment thereof In certain
embodiments, the
methods comprising a sialidase inhibitor that is a protein or an antibody
further comprise an effective
amount of at least one glycan-modifying agent. In certain embodiments, the
glycan-modifying agent is
CMP-sialic acid or a CMP-sialic acid precursor. In certain embodiments, the
CMP-sialic acid
precursor further comprises an enzyme that converts the CMP-sialic acid
precursor to CMP-sialic acid.
In certain embodiments, the glycan-modifying agent is UDP-galactose. In
certain embodiments, the
glycan-modifying agents are CMP-sialic acid and UDP-galactose.
Treating Platelets
The isolated platelets are treated by the composition of the present
invention. Briefly, the
CA 02835434 2013-11-07
WO 2012/158983 -35-
PCT/US2012/038462
overall process is described as follows. Within a time period of being
isolated, the composition of the
present invention is contacted with the isolated platelets to thereby obtain a
treated platelet composition
(e.g., referred to herein as a "platelet composition"). The platelet
composition can be stored either at
room temperature or in cold temperature and then warmed. The platelet
composition is transfused into
an individual in need of platelets and, as a result of the treatment with the
inventive compositions, the
transfused platelets exhibit reduced bacterial proliferation and in vivo
remain in circulation longer, and
maintain hemostasis longer, as compared to untreated platelets.
In an embodiment, the platelet composition includes one or more of the
sialidase inhibitors, as
described herein. In a certain embodiment, DANA is used as the sialidase
inhibitor. In an embodiment
in which a cocktail of the composition of the present invention is used, in
addition to the sialidase
inhibitor, the glycan-modifying agent, such as UDP-galactose and/or CMP-sialic
acid, can be added.
After isolation of the platelets, as described herein or using other methods
known in the art, the
platelets are treated with the composition of the present invention. The
composition of the present
invention is contacted with the isolated platelets in an amount that reduces
sialidase activity, inhibits
bacterial proliferation, allows platelets to maintain hemostasis, and/or
allows platelets to retain the
ability to activate and form a clot. In an embodiment, an effective amount of
either a sialidase inhibitor
or a sialidase inhibitor in combination with one or more glycan-modifying
agents is that amount of the
sialidase inhibitor or the sialidase inhibitor in combination with one or more
glycan-modifying agents
that preserves or alters a sufficient number of glycan residues on the surface
of platelets, such that
when introduced to a population of platelets, reduces sialidase activity,
inhibits bacterial proliferation,
and/or increases circulation time of platelets or reduces the clearance of the
population of platelets in a
mammal following transfusion of the platelets into the mammal.
For example, an "effective amount" of either a sialidase inhibitor and/or a
glycan-modifying
agent to contact with isolated platelets ranges from about 1 micromolar to
about 2,000 micromolar, and
most preferably about 200 micromolar to about 1.2 millimolar (e.g., between
about 1 and 10
micromolar, about 1 and about 100 micromolar, about 100 and about 500
micromolar, about 500
micromolar and about 1.0 millimolar, about 1.0 and about 1.5 millimolar, and
about 1.0 and about 2.0
millimolar). In another aspect, are in a range between about 10 micromolar to
about 1000 micromolar,
between about 100 micromolar to about 150 micromolar, or between about 200
micromolar to about
1200 micromolar.
When using the cocktail of the present invention, modification of platelets
with either a
sialidase inhibitor or a sialidase inhibitor in combination with one or more
glycan-modifying agents can
CA 02835434 2013-11-07
WO 2012/158983 -36-
PCT/US2012/038462
be performed as follows. The population of platelets is contacted with the
selected sialidase inhibitor
or sialidase inhibitor in combination with one or more glycan-modifying
agents. Multiple sialidase
inhibitors and/or glycan-modifying agents (e.g., two, three, four or more) can
be used simultaneously
or sequentially. If used sequentially in time, the sialidase inhibitors and/or
glycan-modifying agents are
provided close enough in time to confer the desired effect. In some
embodiments, 0.1-500 mU/mL
galactose transferase or sialyl transferase is added to the population of
platelets. Galactose transfer can
be monitored functionally using lectins such as FITC-ECL or sWGA binding. The
goal of the glycan
modification reaction is to reduce sWGA binding to resting room temperature
sWGA binding-levels.
Galactose transfer can be quantified using 14 C-UDP-galactose. UDP-galactose
is mixed with "C-
UDP-galactose to obtain appropriate galactose transfer. Platelets are
extensively washed, and the
incorporated radioactivity measured using a y-counter. The measured cpm
(counts per minute) permits
calculation of the incorporated galactose. Similar lectin-binding techniques
are applicable to
monitoring sialic acid transfer.
The isolated platelets can be treated with the platelet composition in a time
period before
significant hydrolysis of sialic acid occurs. The addition of the composition
to the platelets can occur
during the isolation process, shortly after the isolation process or within
another time period.
As single donor platelets are removed from the donor's circulation by
apheresis, as described
herein, the composition of the present invention can be added in a sterile
manner. For example, after
the blood is centrifuged by the apheresis machine and the platelets are
separated from the rest of the
blood components, the composition of the present invention can be added into
the bag containing
platelets. In another embodiment, the collection bag into which the platelets
are deposited after
centrifugation can already contain the composition of the present invention.
In another embodiment,
the composition of the present invention can be added to the bag into which
the platelets are being
collected simultaneously with the collection of the platelets. Once the
platelets come into contact with
the composition of the present invention, the components can be mixed or
agitated (e.g., bag turned
upside down and right side up) to ensure that the platelets come into contact
with inventive
composition. In this example, little or no time passes between the collection
of the platelets and their
treatment with the inventive composition. Accordingly, contact of the
inventive composition and the
isolated platelets can occur during platelet donation or soon after platelet
isolation (e.g., between 1
minute and about 120 minutes within platelet isolation).
In an embodiment, the composition of the present invention can be added to the
isolated
platelets "immediately" after donation, within a certain time period after
donation, or "simultaneously"
CA 02835434 2013-11-07
WO 2012/158983 -37-
PCT/US2012/038462
during donation. In an embodiment, the composition of the present invention is
contacted with the
platelets in a range between about 1 minute and about 6 hours (e.g., about 1,
2, 3, 4, 5, 6, 7, 8, 9, 10, 11,
12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30,
31, 32, 33, 34, 35, 36, 37, 38,
39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57,
58, 59, 60 min, 1 V2 h, 2, h, 2 1/2
h, 3 h, 3 1/2 h, 4 h, 4 1/2 h, 5 h, 5 1/2 h, 6 h).
When random donor platelets are isolated from multiple donors, the inventive
composition can
be added after the platelets are isolated from the whole blood. In an
embodiment, the addition of the
inventive composition to the platelets can occur when the platelets from the
donors are pooled. The
pooling bag that generally holds about 6 units of random donor platelets can
include the inventive
composition so that when the platelets are added to the pooling bag, the
isolated platelets come into
contact with the composition. Alternatively, the composition can be sterilely
connected to and
introduced into the pooling bag during or after the platelets are pooled. In
any event, the methods of
the present invention include contacting the isolated platelets within 1 hour
to about 8 hours (e.g.,
between 1 and about 3 hours). In an embodiment, contacting the inventive
composition with the
isolated platelets should occur before platelets are refrigerated.
According to still yet another aspect of the invention, a device for
collecting and processing
platelets is provided. The device has a container or bag for collecting
platelets, wherein the container
or bag includes the composition of the present invention. In another
embodiment, the device includes a
container or bag that contains the isolated platelets and at least one
satellite container or bag, wherein
the satellite container includes the composition of the present invention. The
bag containing the
platelets and the bag containing the composition of the present invention can
be in sterile
communication with one another.
The platelets, after being contacted with the inventive composition, can be
stored at room
temperature or be refrigerated. In certain aspects, platelets are refrigerated
to enable storage for longer
periods of time. However, as further described herein, sialidase inhibitors
inhibit bacterial proliferation
and allow platelets to be stored at room temperature.
In certain embodiments, the platelet compositions of the present invention
include an effective
amount of a sialidase inhibitor that is added to a population of platelets
after the platelets have been
obtained from a donor. In another embodiment, the novel platelet composition
comprises an effective
amount of a sialidase inhibitor that is added to a population of platelets
after the platelets have been
obtained from a donor and the resulting platelet composition is stored for a
period of time at room
temperature without a substantial loss of in vivo hemostatic activity and
inhibition of bacterial
CA 02835434 2013-11-07
WO 2012/158983 -38-
PCT/US2012/038462
proliferation. In another preferred embodiment, the novel platelet composition
comprises an effective
amount of a sialidase inhibitor that is added to a population of platelets
after the platelets have been
obtained from a donor; the resulting platelet composition is cooled to a
temperature below room
temperature; stored for a period of time at a temperature below room
temperature and rewarmed back
to room temperature without a substantial loss in vivo hemostatic activity.
The terms "cooling," "cold temperature," "temperature below room temperature,"
and
"temperature below ambient temperature," interchangeably refer to any
temperature between 28 C
and -100 C. In any of the embodiments of the invention described herein, the
temperature is
alternatively selected from the group of temperatures consisting of 27 C, 26
C, 25 C, 24 C, 23 C, 22 C,
21 C, 20 C, 19 C, 18 C, 17 C, 16 C, 15 C, 14 C, 13 C, 12 C, 11 C, 10 C, 9 C, 8
C, 7 C, 6 C, 5 C, 4 C,
3 C, 2 C, 1 C, 0 C, -1 C, -2 C, -3 C, -4 C, -5 C, -6 C, -7 C, -8 C, -9 C, and -
10 C. In some
embodiments, the platelet preparation is stored at a temperature of less than
about 15 C, preferably less
than 10 C, and more preferably less than 5 C. In some other embodiments, the
platelet preparation is
stored at room temperature. In other embodiments, the platelets are frozen,
e.g., 0 C, -20 C, or -80 C
or cooler.
As used in all of the aspects and embodiments of the invention herein, the
term "period of time"
refers to a duration of time during which platelets or platelet compositions
are stored at any given
temperature. The term "period of time" can range from seconds to minutes to
hours to days to weeks.
In preferred embodiments, the term "period of time" refers a number of hours
including about 3 to
about 120 hours, e.g., 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19, 20, 21, 22, 23, 24, 25, 26,
27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45,
46, 47, 48, 49, 50, 51, 52, 53,
54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72,
73, 74, 75, 76, 77, 78, 79, 80,
81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99,
100, 101, 102, 103, 104, 105,
106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, and 120
hours. In certain
embodiments the period of time for which treated platelets can be stored
include about 1 and about 30
days (e.g., about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19, 20, 21, 22, 23, 24, 25, 26,
27, 28, 29, and 30).
In an embodiment, treated platelets can be stored at room temperature for
about 1 to about 14
days (e.g., about 7 days). After 7 days, in an aspect, the platelets can be
refrigerated as described
herein.
In various other embodiments, the treated platelets are stored at room
temperature. Treatment
with one or more sialidase inhibitors, optionally, one or more glycan-
modifying agents
CA 02835434 2013-11-07
WO 2012/158983 -39-
PCT/US2012/038462
preserves/modifies the platelet population, i.e., preserves or improves the
hemostatic function of the
platelet population following transfusion into a mammal, and reduces the
incidence of storage lesions
in room temperature stored platelets, when compared to untreated platelet
samples over a period of
time following treatment. Treated platelet samples stored at or below room
temperature are thus
suitable for autologous or heterologous transfusion after extended periods of
storage time, in an
embodiment, for at least about 2 days, at least about 3 days, at least about 4
days, at least about 5 days,
at least about 6 days, at least about 7 days, at least about 8 days, at least
about 9 days, at least about 10
days, at least about 11 days, at least about 12 days, at least about 13 days,
at least about 14 days, at
least about 21 days, or at least about 28 days.
As used in all of the aspects and embodiments of the invention herein, the
term "warmed
slowly" refers a gradual rate of warming (e.g., 0.1, 0.2, 0.3, 0.4, 0.5, 0.6,
0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7,
8, 9, or 10 C per hour or per day). As described herein, any of the aspects or
embodiments of the
invention further comprises a step of warming the treated platelet preparation
above room temperature,
for example, by warming the platelets to 37 C. Warming can occur gradually or
by stepwise
temperature increases. It is preferable to warm either room-temperature-stored
or cold-stored and
treated platelet population by slow addition of heat, and with continuous
gentle agitation such as is
common with the rewarming of blood products. A blood warming device is
disclosed at
WO/2004/098675 and is suitable for rewarming a treated platelet population
from cold storage
conditions.
Inhibition of Bacterial Proliferation and Pathogen-Induced Platelet
Degradation
This invention provides a novel method to reduce pathogen-induced platelet
degradation and
inhibit pathogen growth/propagation by inhibiting pathogen sialidases.
Sialidase inhibitors exhibit anti-
microbial properties that prevent pathogenic proliferation.
The term "pathogen" as used herein, refers to one or more microorganisms or
the like that cause
infection as described in (Dodd, R. Y. New Engl. J. Med. 327:419-421 (1992);
Soland, E. M., et at. J.
Am. Med. Assoc. 274:1368-1373 (1995) and Schreiber, G. B., et al. New Engl. J.
Med. 334:1685-1690
(1996)). Exemplary pathogens include, but are not limited to a virus,
bacteria, parasite, protozoa or
fungus. Examples of virus include, but are not limited to Herpes simplex
virus, HIV, hepatitis, hepatitis
A, hepatitis B, hepatitis C, Respiratory syncycial virus, blue tongue virus,
and bovine diarrhea virus.
Virus also includes Cytomegalovirus, Epstein-Barr virus, Herpes Simplex type I
and II viruses, and
other viruses that circulate freely in the blood, as well as cell-associated
viruses. Fungus includes, but
CA 02835434 2013-11-07
WO 2012/158983 -40-
PCT/US2012/038462
is not limited to e.g., Aspergillus. And typical parasites include, but are
not limited to, for example:
Ameoba, Plasmodiunm, Leishmania, Mycosus profundus, Trypanosoma, Spirochete,
and Arbovius.
With respect to bacteria commonly associated with platelets and whose
proliferation is inhibited
by a sialidase inhibitor include, but is not limited to Aspergillus, Bacillus
sp, Bacteroides eggerthii,
Candida albicans, Citrobacter sp, Clostridium perfringens, Corynebacterium sp,
Diphtheroid,
Enterobacter aero genes, Enterobacter amnigenus, Enterobacter cloacae,
Enterococcus avium,
Enterococcus faecalis, Escherichia coli, Fusobacterium spp., Granulicatella
adiacens, Heliobacter
pylori, Klebsiella sp, (K. pneumonia, K. oxytoca), Lactobacillus sp, Listeria
sp, Micrococcus sp,
Peptostreptococcus, Proteus vulgaris, Pseudomonas sp, Pseudomys oralis,
Propionibacterium sp,
Salmonella sp, Serratia sp, Staplhylococcus sp (Coagulase-negative
Staphylococcus, Staphylococcus
epidermidis, Staphylococcus aureus), Streptococcus sp, (S. gallolyticus, S.
bovis, S. pyo genes, S.
viridans), Serratia marcescens and Yersinia enterocolitica.
The term "pathogen-induced platelet degradation" as used herein, refers to any
degree of
platelet degradation, decrease in hemostatic activity, or increase in the
clearance rate of platelets that is
caused by one or more pathogens.
The term "detrimental effect" as used herein, can refer to a detrimental
effect upon the viability
of platelets (e.g., an increase in platelet degradation, decrease in
hemostatic activity, or increase in the
clearance rate of platelets) that is caused by one or more pathogens. The term
"detrimental effect" as
used herein, can also refer to the detrimental effect upon the patient (e.g.,
the consequences of the
infection itself) that is caused by one or more pathogens such as sepsis.
The term "bacterial contamination" as used herein, refers to contamination by
any of the above-
described bacterial pathogens or by non-pathogenic bacteria that are capable
of producing bacteria-
derived sialidase. "Inhibiting bacterial proliferation" refers to reducing
and/or inhibiting the growth of
bacteria in a platelet preparation.
The term "bacteria-derived sialidase" as used herein, refers to sialidase that
is produced by
bacteria. The inhibition of "bacteria-derived sialidase" as used herein, can
optionally inhibit platelet-
derived sialidase and/or patient-derived sialidase in addition to the
inhibition of bacteria-derived
sialidase.
The invention, in other aspects, provides a novel method to inhibition of
bacterial proliferation
in a platelet preparation by obtaining a population of platelets from a donor
and contacting the platelets
with an effective amount of the inventive compositions e.g., a sialidase
inhibitor. In a preferred
embodiment, the method of the present invention further include storing the
treated platelet
CA 02835434 2013-11-07
WO 2012/158983 -41-
PCT/US2012/038462
composition for a period of time at room temperature without a substantial
loss in vivo hemostatic
activity. Alternatively, as described herein, the treated platelets can be
cooled the resulting platelet
composition to a temperature below room temperature; stored for a period of
time at a temperature
below room temperature and rewarmed back to room temperature without a
substantial loss in vivo
hemostatic activity.
Preferred embodiments of the inventive method to reduce pathogen growth in a
platelet
preparation, as described herein, include contacting platelets with an
effective amount of a sialidase
inhibitor, as described herein, and optionally with an effective amount of at
least one glycan-modifying
agent, as described herein.
The anti-proliferative inhibition of bacteria by the sialidase inhibitor
allows platelets to be
stored for longer with a reduced risk of bacterial contamination, and
certainly for the time period
described herein.
Bacterial contamination of platelets is a concern because it causes sepsis in
patients receiving
them. Bacterial contamination can be the result of non-sterile techniques in
obtaining blood and/or
platelets from the donor, or in poor handling of the platelets after donation.
Even despite good sterile
techniques in obtaining donated blood or platelets, bacteria can still persist
in the platelet preparation.
For example, even though a technician uses an antibacterial agent to clean the
skin at the site of
donation, bacteria can be embedded within the layers of the skin. So upon
penetration of the skin with a
needle, bacterial contamination of the platelet donation can occur. As a
result, bacterial testing at the
point of care (e.g., at the time the recipient receives the platelets) is
performed to reduce the risk of
sepsis.
Additionally, bacterial contamination can results in the formation of biofilm
on the interior
surfaces of blood containers/bags. The biofilm formation is the result of
bacteria attaching to the
interior surface of the bag and proliferating using the surface as a support.
As the bacterial
proliferation increases, the biofilm formation also increases.
Accordingly, contacting the platelet preparation with sialidase inhibitors
provides unexpected
anti-proliferative inhibition of bacteria and a reduction in biofilm formation
on the interior surface of
the platelet bag. Using the methods described herein the platelet preparation
is contacted with an
effective amount of one or more sialidase inhibitors which inhibits endogenous
platelet sialidase but
also bacterial sialidase. This treatment of platelets results in prolonged
storage of platelets with
reduced bacterial growth/proliferation, which provides platelets with an
increased survival and
hemostasis in vivo after transfusion into a recipient.
CA 02835434 2013-11-07
WO 2012/158983 -42-
PCT/US2012/038462
Encompassed in the method of the present invention is testing for bacterial
proliferation at one
or more time points to determine that bacterial proliferation is in fact
inhibited before being transfused
into the recipient. Bacterial testing can occur at a single time point (e.g.,
at the point of care) and the
results can be compared to a standard to determine if bacterial proliferation
has occurred in the treated
platelets to be transferred. Additionally, bacterial testing of the treated
platelets can occur at more than
one time point to assess if the particular sample has exhibited inhibition of
bacterial proliferation. An
increase in bacterial proliferation or the presence of bacterial proliferation
indicates that the treated
platelets are contaminated and cannot be used for transfusion. The absence of
bacterial proliferation
indicates that the treated platelets can be used for transfusion. The
sialidase inhibitor of the present
invention results in treated platelets that are suitable for transfusion.
A number of tests exist to determine the presence of bacterial contamination
in a treated platelet
preparation. Bacteria can be tested by the presence of a polypeptide or
protein that is common to
bacteria and not found in platelets, by culture techniques, Gram staining,
scanning techniques, the
presence of nucleic acid that is conserved in bacteria, scans, and the like.
A commonly used test in determining bacterial contamination of a platelet
preparation is the
Pan Genera Detection (PDG) (Verax Biomedical, Incorporated, Worcester
Massachusetts). The PGD
test can detect an array of bacteria in blood components. This broad detection
is based on the existence
of shared, or conserved, antigens that are common to the cell walls of the two
broad classes of bacteria,
lipoteichoic Acids on Gram-positive bacteria and lipopolysaccharides on Gram-
negative bacteria. The
test targets these conserved Gram-positive and Gram-negative antigens to test
biological samples for a
broad range of bacterial contaminants by using binding agents to directly bind
to these targets.
Although the level or presence of the specific bacteria is not determined by
this test, the test does
determine the presence of a number of bacteria in the platelet preparation.
Culture methods can be employed to determine the presence or absence of
bacterial
contamination and/or bacterial proliferation. One commercially available test
is referred to as the
BacT/ALERT test (bioMerieux, Inc., Durham, NC). Bacterial detection is based
on the evolution of
carbon dioxide by proliferating bacteria. A carbon-dioxide-sensitive liquid
emulsion sensor at the
bottom of the culture bottle changes color and is detected through alteration
of light reflected on the
sensor. BacT/ALERT test detects the presence of a number of bacteria, fungi,
and yeasts.
Another method for bacterial detection involves measuring the oxygen content
in a platelet
preparation sample. An example is the Pall eBDS test (Pall Corporation, Port
Washington, NY). The
approach to detection measures the oxygen content of air within the sample
pouch as a surrogate
CA 02835434 2013-11-07
WO 2012/158983 -43-
PCT/US2012/038462
marker for bacteria. An oxygen analyzer is used to measure the percent of
oxygen in the headspace gas
of the pouch or bag having the platelets. If bacteria are present in the
platelet sample collected, an
increasing amount of oxygen is consumed through the metabolic activity and
proliferation of the
bacteria in the sample during incubation, resulting in a measurable decrease
in oxygen content of the
plasma as well as the air within the sample pouch.
More conventional methods for determining the presence of bacterial
proliferation is a platelet
preparation is Gram staining. Gram staining allows one to differentiate
bacterial species into classes
(Gram-negative or Gram-positive) in an effort to begin to identify the
microorganism. The test detects
peptidoglycan, a glycan in the cell wall of the bacteria.
A sample from the treated platelet preparation can be obtained and cultured to
determine if any
bacteria are present. The growth media is inoculated or plated with the sample
and under controlled
conditions suitable for bacterial growth. Bacteria can be grown and
identified.
Other methods known in the art or developed in the future can be used to
determine bacterial
proliferation in the treated platelet preparation of the present invention.
The methods of the present invention involve reducing bacterial proliferation
and/or biofilm
formation by contacting the platelet preparation with an effective amount of
one or more sialidase
inhibitors. The bacterial proliferation is reduced, as compared to a standard
or to another assessment
taken at a different time point. The methods described herein reduce bacterial
proliferation and/or
biofilm formation by at least about 5% (e.g., by about 10%, 20%, 30%, 40%,
50%, 60%, 70%, 80%,
90%, or 100%). In an embodiment, the methods of the present invention
completely inhibit bacterial
proliferation and/or biofilm formation, as compared to that at the time of
treatment of the platelet
preparation with the sialidase inhibitor.
Storage of Platelets:
The invention embraces a method for increasing the storage time of platelets.
During storage
with the sialidase inhibitor described herein, platelets can be stored with
reduced sialidase activity,
inhibited bacterial proliferation and without substantial loss of platelet
function or hemostatic activity
such as the loss of the ability to circulate or without an increase in the
rate of platelet clearance.
The platelets are collected from blood by standard techniques known to those
of ordinary skill
in the art, as described herein. The storage composition includes at least one
sialidase inhibitor and
optionally at least one glycan-modifying agent in an amount sufficient to
reduce platelet clearance. In
CA 02835434 2013-11-07
WO 2012/158983 -44-
PCT/US2012/038462
some embodiments, the storage composition further comprises an enzyme that
catalyzes the
modification of a glycan moiety on the platelet.
The invention, in certain aspects, provides a novel method of storing a
platelet composition in
which the steps includes obtaining a population of platelets from a donor and
treating the platelets with
an effective amount of one or more sialidase inhibitors and optionally one or
more glycan modifying
agents. In an embodiment, the novel method of storing a platelet composition
involves obtaining a
population of platelets from a donor; adding an effective amount of a
sialidase inhibitor to the
population of platelets and storing the resulting platelet composition for a
period of time at room
temperature without a substantial loss in vivo hemostatic activity. In another
embodiment, the novel
method of storing a platelet composition encompasses obtaining a population of
platelets from a donor;
adding an effective amount of a sialidase inhibitor to the population of
platelets; cooling the resulting
platelet composition to a temperature below room temperature; storing the
platelet composition for a
period of time at a temperature below room temperature and rewarming the
platelet composition back
to room temperature without a substantial loss in vivo hemostatic activity. In
further embodiments, the
platelet composition is rewarmed slowly. In certain embodiments, the platelet
composition retains
substantially normal hemostatic activity when transfused into a mammal after
storage. In further
embodiments, the platelet composition when transfused into a mammal after
storage, has a circulation
half-life of about 5% or greater than the circulation half-life of untreated
platelets. In certain preferred
embodiments, the platelet composition is suitable for transfusion into a human
after storage.
In accordance with the invention, following treatment with a sialidase
inhibitor, the population
of treated platelets can be stored at room temperature or chilled without the
deleterious effects (cold-
induced platelet activation) experienced upon chilling of untreated platelets.
The preservation and/or
selective modification of glycan moieties reduce clearance, thus permitting
longer-term storage than is
presently possible. In one aspect, one or more sialidase inhibitors are added
to the population of
platelets that are kept between about room temperature (between about 20 C and
25 C) and 37 C. As
used herein, chilling refers to lowering the temperature of the population of
platelets to a temperature
that is less than about 25 C. In some embodiments, the platelets are chilled
to a temperature that is less
than about 15 C. In some preferred embodiments, the platelets are chilled to a
temperature ranging
from between about 0 C to about 4 C. Chilling also encompasses freezing the
platelet preparation, i.e.,
to temperatures less than 0 C, -20 C, -50 C, and -80 C or cooler. Processes
for the cryopreservation of
cells are well known in the art.
CA 02835434 2013-11-07
WO 2012/158983 _45_
PCT/US2012/038462
In some embodiments, the population of platelets is stored at room temperature
for at least 3
days. For example, the population of treated platelets is stored at room
temperature for at least 4, 5, 6,
7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26,
27, and 28 days or longer.
Additionally, in certain aspects, a population of treated platelets can be
stored chilled for at least
3 days. A population of treated platelets is stored chilled e.g., for at least
4, 5, 6, 7, 8, 9, 10, 11, 12, 13,
14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, and 28 days or longer.
Transfusion of Platelets into Mammals (e.g., Humans)
After storage, the present invention, in some aspects, provides a method of
transfusing a patient
with a treated platelet composition having one or more sialidase inhibitors,
wherein the platelet
composition was prepared according to the methods described herein. Similarly,
using these steps, the
present invention provides a novel method for mediating hemostasis in a
mammal.
Additionally, the present invention relates to methods for increasing the
circulation time of
platelets, or reducing the clearance of platelets. The circulation time of a
population of platelets is
defined as the time when one-half of the platelets in that population are no
longer circulating in a
mammal after transfusion into that mammal.
As used herein, "clearance" means removal of the treated platelets from the
blood circulation of
a mammal (such as but not limited to by macrophage phagocytosis). More
specifically, clearance of a
population of platelets refers to the removal of a population of platelets
from a unit volume of blood or
serum per unit of time. Reducing the clearance of a population of platelets
refers to preventing,
delaying, or reducing the clearance of the population of platelets or the rate
at which platelets clear.
Patients in need of platelet transfusion include those with e.g., anemia,
thrombocytopenia,
dysfunctional platelet disorders, active platelet-related bleeding, or serious
risk of bleeding (e.g.,
prophylactic use). Patients with the following medical conditions at times
require platelet transfusion:
leukemia, myelodysplasia, aplastic anemia, solid tumors, congenital or
acquired platelet dysfunction,
central nervous system trauma. Patients undergoing extracorporeal membrane
oxygenation or
cardiopulmonary bypass also receive platelet transfusions.
In one aspect of the invention, the method for increasing circulation time of
an isolated
population of platelets involves contacting an isolated population of
platelets with at least one sialidase
inhibitor in an amount effective to reduce the clearance of the population of
platelets. As used herein, a
population of platelets refers to a sample having one or more platelets.
Reducing the clearance of a platelet encompasses reducing the clearance of
platelets that results
after storage of the platelets at or below room temperature. Reducing the
clearance of a platelet can
CA 02835434 2013-11-07
WO 2012/158983 -46-
PCT/US2012/038462
result from reducing storage lesions obtained at or below room temperature, or
reducing "cold-induced
platelet activation," that occurs upon the cold storage of platelets. Cold-
induced platelet activation is a
term having a particular meaning to one of ordinary skill in the art. Cold-
induced platelet activation
can be manifested by changes in platelet morphology, some of which are similar
to the changes that
result following platelet activation. The structural changes indicative of
room-temperature-induced or
cold-induced platelet activation are most easily identified using techniques
such as light or electron
microscopy. On a molecular level, platelet activation results in actin bundle
formation and a
subsequent increase in the concentration of intracellular calcium. Actin-
bundle formation is detected
using, for example, electron microscopy. An increase in intracellular calcium
concentration is
determined, for example, by employing fluorescent intracellular calcium
chelators. Many of the above-
described chelators for inhibiting actin filament severing are also useful for
determining the
concentration of intracellular calcium (Tsien, R., 1980, supra.). Accordingly,
various techniques are
available to determine whether or not platelets have experienced room-
temperature-induced or cold-
induced activation.
The addition of a sialidase inhibitor to platelets prevents the hydrolysis of
sialic acid residues
from the termini of glycans and preserves the structures of glycan moieties on
platelets, resulting in
diminished clearance of treated platelets. This effect can be measured, for
example, using either an in
vitro system employing differentiated THP-1 cells or mouse macrophages,
isolated from the peritoneal
cavity after thioglycolate injection stimulation. The rate of clearance of
treated platelets compared to
untreated platelets can be determined. To test clearance rates, the treated
platelets are fed to the
macrophages and ingestion of the platelets by the macrophages is monitored.
Reduced ingestion of
treated platelets as compared to untreated platelets (1.2-fold or greater)
indicates successful
modification of the glycan moiety for the purposes described herein.
Also, the addition of a sialidase inhibitor to platelets inhibits bacterial
proliferation, which in
turn, reduces platelet clearance and prevents sepsis. Assessment of bacterial
proliferation is described
herein.
In certain embodiments of the invention, the circulation time of the
population of platelets is
increased by at least about 10%, 20%, 25%, 30%, or 40%. In yet some
embodiments, the circulation
time of the population of platelets is increased by at least about 50% to
about 100%. In still yet other
embodiments, the circulation time of the population of platelets is increased
by about 150% or greater.
CA 02835434 2013-11-07
WO 2012/158983 -47-
PCT/US2012/038462
Platelet Compositions:
After being subjected to the sialidase inhibitor, as described herein, the
platelets are treated and
are referred to herein as "platelet compositions" or "treated platelets." The
present invention includes a
novel platelet composition comprising one or more sialidase inhibitors, as
described herein. In another
embodiment, the novel platelet composition further comprises an effective
amount of at least one
glycan-modifying agent. The treated platelets have a plurality of intact
glycan molecules on the surface
of the platelet that would otherwise have been cleaved without sialidase
inhibitor treatment. The
glycan molecules of the platelet composition of the present invention include
those in which sialic acid
cleavage is presented and the glycan molecules remain intact. In the event
that sialic acid is cleaved,
then the glycan-modifying agents (e.g., CMP-sialic acid, or UDP-galactose, or
both) allow for sialic
acid additions to the terminal sugar residues, or galactosylation of the
terminal sugar residues, or both
sialylation and galactosylation of the terminal sugar residues. In some
embodiments, the modified
glycan moieties are GPIba molecules. The invention also encompasses a platelet
composition in a
storage medium. In some embodiments, the storage medium can be a
pharmaceutically acceptable
carrier.
In some embodiments, the terminal glycan molecules so modified, are GPIba
molecules. The
treated platelets include glycan structures with terminal GPIba molecules,
that following treatment
have terminal galactose or sialic acid attached to the GPba molecules. In
another aspect, the invention
provides a platelet composition comprising a plurality of treated platelets.
In some embodiments, the
platelet composition further comprises a storage medium. In some embodiments,
the platelet
composition further comprises a pharmaceutically acceptable carrier.
In some embodiments, the population of platelets treated according to the
inventive methods
described herein demonstrates inhibited bacterial proliferation and
substantially normal hemostatic
activity, preferably after transfusion into a mammal. In some embodiments, the
population of platelets
treated according to the inventive methods described herein demonstrates
reduced bacterial
proliferation and improved hemostatic activity, relative to a similarly stored
but untreated population of
platelets.
In a further preferred embodiment, the novel platelet composition, as
described above, provides
a stable platelet preparation. In certain embodiments, the stable platelet
preparation of the invention is
capable of being stored for at least 24-360 hours, and the platelet
preparation is suitable for
administration/transfusion to a human after storage without significant loss
of hemostatic function or
without a significant increase in platelet clearance in the human as compared
to untreated platelets. In
CA 02835434 2013-11-07
WO 2012/158983 -48-
PCT/US2012/038462
certain preferred embodiments, the stable platelet preparation is capable of
being cold-stored. In
certain other preferred embodiments, the platelets are capable of being stored
at room temperature
without substantial reduction in biological activity compared to non-treated
platelets.
The invention, in other aspects, provides compositions comprising a novel
platelet composition,
as described herein, and further comprising at least one pharmaceutically
acceptable excipient. A
"pharmaceutically acceptable excipient," as used herein, includes any and all
solvents, diluents, or
other liquid vehicle, dispersion or suspension aids, surface active agents,
isotonic agents, thickening or
emulsifying agents, preservatives, antioxidants, solid binders, lubricants,
and the like, as suited to the
particular dosage form desired. Remington 's Pharmaceutical Sciences,
Sixteenth Edition, E. W.
Martin (Mack Publishing Co., Easton, PA, 1980) discloses various excipients
used in formulating
pharmaceutical compositions and known techniques for the preparation thereof
Except insofar as any
conventional excipient medium is incompatible with the compounds of the
invention, such as by
producing any undesirable biological effect or otherwise interacting in a
deleterious manner with any
other component(s) of the pharmaceutical composition, its use is contemplated
to be within the scope
of this invention.
In certain embodiments, the platelet composition is suitable for transfusion
into a human patient
afflicted with a bleeding disorder or anemia. In preferred embodiments, the
platelet composition can
be stored for at least 5 days with inhibited bacterial proliferation prior to
administration to a human,
and wherein the composition can be transfused into a human after storage
without significant loss of
hemostatic function or without a significant increase in platelet clearance in
the human as compared to
untreated platelets.
The term "pharmaceutically acceptable" means a non-toxic material that does
not interfere with
the effectiveness of the biological activity of the platelets and that is a
non-toxic material that is
compatible with a biological system such as a cell, cell culture, tissue, or
organism. Pharmaceutically
acceptable carriers include diluents, fillers, salts, buffers, stabilizers,
solubilizers, and other materials
which are well known in the art, for example, a buffer that stabilizes the
platelet preparation to a pH of
7.3 - 7.4, the physiological pH of blood, is a pharmaceutically acceptable
composition suitable for use
with the present invention.
The invention further embraces a method for making a pharmaceutical
composition for
administration to a mammal. In a preferred embodiment, the novel
pharmaceutical composition
comprising platelets further comprises an effective amount of a sialidase
inhibitor that is added to a
population of platelets after the platelets have been obtained from a donor
and the resulting platelet
CA 02835434 2013-11-07
WO 2012/158983 -49-
PCT/US2012/038462
composition is stored for a period of time at room temperature without a
substantial loss in vivo
hemostatic activity. In another preferred embodiment, the novel pharmaceutical
composition
comprising platelets further comprises an effective amount of a sialidase
inhibitor that is added to a
population of platelets after the platelets have been obtained from a donor;
the resulting platelet
composition is cooled to a temperature below room temperature; stored for a
period of time at a
temperature below room temperature and rewarmed back to room temperature
without a substantial
loss in vivo hemostatic activity. In some embodiments, the method of preparing
the novel
pharmaceutical compositions comprising platelets comprises neutralizing,
removing or diluting the
sialidase inhibitors and/or glycan-modifying agent(s) and/or the enzyme(s)
that preserve and/or
catalyze the modification of the glycan moiety, and placing the treated
platelet preparation in a
pharmaceutically acceptable carrier. In one preferred embodiment, the
platelets are stored at room
temperature (about 22 C) prior to and during neutralization or dilution. In
another preferred
embodiment, the platelets are chilled, stored, and then warmed to room
temperature (about 22 C) prior
to neutralization or dilution. In some embodiments, the platelets are
contained in a pharmaceutically
acceptable carrier prior to contact with the sialidase inhibitors and/or
glycan-modifying agent(s) and/or
the enzyme(s) that preserve and/or catalyze the modification of the glycan
moiety and it is not
necessary to place the platelet preparation in a pharmaceutically acceptable
carrier following
neutralization or dilution.
As used herein, the terms "neutralize" or "neutralization" refer to a process
by which the
sialidase inhibitors and/or glycan-modifying agent(s) and/or the enzyme(s)
that preserve and/or
catalyze the modification of the glycan moiety are rendered substantially
incapable of glycan
modification of the glycan residues on the platelets, or their concentration
in the platelet solution is
lowered to levels that are not harmful to a mammal, for example, less that 50
micromolar of the glycan-
modifying agent. In some embodiments, the chilled platelets are neutralized by
dilution, e.g., with a
suspension of red blood cells. Alternatively, the treated platelets can be
infused into the recipient,
which is equivalent to dilution into a red blood cell suspension. This method
of neutralization
advantageously maintains a closed system and minimizes damage to the
platelets. In a preferred
embodiment, no neutralization is required.
An alternative method to reduce toxicity is by inserting a filter in the
infusion line, the filter
containing, e.g., activated charcoal or an immobilized antibody, to remove the
sialidase inhibitors
and/or glycan-modifying agent(s) and/or the enzyme(s) that preserve and/or
catalyze the modification
of the glycan moiety.
CA 02835434 2013-11-07
WO 2012/158983 -50-
PCT/US2012/038462
Either or all of the sialidase inhibitors and/or glycan-modifying agent(s)
and/or the enzyme(s)
that preserve and/or catalyze the modification of the glycan moiety also may
be removed or
substantially diluted by washing the treated platelets in accordance with
standard clinical cell washing
techniques.
The invention further provides a method for mediating hemostasis in a mammal.
The method
includes administering the above-described treated platelets or an above-
transfusionof the treated
platelets or pharmaceutical composition can be done in accordance with
standard methods known in the
art. According to one embodiment, a human patient is transfused with red blood
cells before, after or
during administration of the treated platelets. The red blood cell transfusion
serves to dilute the
administered, treated platelets, thereby neutralizing the sialidase inhibitors
and/or glycan-modifying
agent(s) and/or the enzyme(s) that preserve and/or catalyze the modification
of the glycan moiety.
The dosage regimen for mediating hemostasis using the treated platelets is
selected in
accordance with a variety of factors, including the type, age, weight, sex and
medical condition of the
subject, the severity of the disease, the route and frequency of
administration. An ordinarily skilled
physician or clinician can readily determine and prescribe the effective
amount of treated platelets
required to mediate hemostasis.
The dosage regimen can be determined, for example, by following the response
to the treatment
in terms clinical signs and laboratory tests. Examples of such clinical signs
and laboratory tests are
well known in the art and are described, see, HARRISON'S PRINCIPLES OF
INTERNAL MEDICINE, 15th
Ed., Fauci AS et at., eds., McGraw-Hill, New York, 2001.
For example, to determine the optimal concentrations and conditions for
preventing room-
temperature-induced activation or cold-induced activation of platelets by
treating them with one or
more sialidase inhibitors and optionally a glycan-modifying agent, increasing
amounts of these agents
are contacted with the platelets prior to storing platelets at room
temperature and/or exposing the
platelets to a chilling temperature. The optimal concentrations of the
sialidase inhibitors and/or glycan-
modifying agent(s) that prevent cleavage of the sialic acid and/or catalyze
the modification of the
glycan moiety are the minimal effective concentrations that preserve intact
platelet function as
determined by in vitro tests (e.g., observing morphological changes in
response to glass, thrombin,
cryopreservation temperatures; ADP-induced aggregation) followed by in vivo
tests indicative of
hemostatic function (e.g., recovery, survival and shortening of bleeding time
in a thrombocytopenic
animal or recovery and survival of51Cr-labeled platelets in human subjects).
CA 02835434 2013-11-07
WO 2012/158983 -51-
PCT/US2012/038462
Methods of Preparing Platelet Compositions:
The invention, in other aspects, provides a novel method of preparing a
platelet composition
involve obtaining a population of isolated platelets from a donor and treating
the platelets with an
effective amount of a sialidase inhibitor within a time period described
herein. In a preferred
embodiment, the novel method of preparing a platelet composition comprises
obtaining a population of
platelets from a donor; adding an effective amount of a sialidase inhibitor to
the population of platelets
and storing the resulting platelet composition for a period of time at room
temperature without a
substantial loss in vivo hemostatic activity. In another preferred embodiment,
the novel method of
preparing a platelet composition includes obtaining a population of platelets
from a donor; adding an
effective amount of a sialidase inhibitor to the population of platelets;
cooling the resulting platelet
composition to a temperature below room temperature; storing the platelet
composition for a period of
time at a temperature below room temperature and rewarming the platelet
composition back to room
temperature without a substantial loss in vivo hemostatic activity. In further
embodiments, the platelet
composition is rewarmed slowly. In certain embodiments, the population of
platelets retains
substantially normal hemostatic activity when transfused into a mammal. In
further embodiments, the
population of platelets when transfused into a mammal, has a circulation half-
life of about 5% or
greater (e.g., 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, or 150%)
than the circulation
half-life of untreated platelets. In certain preferred embodiments, the
treated platelet population is
suitable for transfusion into a human.
Preferred embodiments of the inventive methods of preparing a platelet
composition as
described herein encompass treating the population of platelets with an
effective amount of a sialidase
inhibitor, as described herein.
Further preferred embodiments of the inventive methods of preparing a platelet
composition, as
described herein, involve treating a population of platelets an effective
amount of a sialidase inhibitor,
and further treating the population of platelets with an effective amount of
at least one glycan-
modifying agent, as described herein.
In some embodiments the invention provides for the combination of the methods
of treating
platelet described herein with one or more other methods of platelet
preservation known in the art. For
example the methods of platelet modification provided in the present invention
are useful in
combination with the methods described in, e.g., but not limited to, the
following US Patent
Publication No.: 20090053198 Al, and US patents: 7,030,110; 7,029,654;
7,005,253; 6,900,231;
6,866,992; 6,730,783; 6,706,765; 6,706,021; 6,693,115; 6,638,931; 6,635,637;
6,566,379;
CA 02835434 2013-11-07
WO 2012/158983 -52-
PCT/US2012/038462
6,521,663; 6,518,310; 6,514,978; 6,497,823; 6,476,016; 6,472,399; 6,420,397;
6,417,161;
6,350,764; 6,344,486; 6,344,466; 6,326,492; 6,277,556; 6,245,763; 6,235,778;
6,221,669;
6,204,263; 6,037,356; 5,919,614; 5,763,156; 5,753,428; 5,660,825; 5,622,867;
5,582,821;
5,571,686; & 5,569,579; 5,550,108; 5,529,821; 5,474,891; 5,466,573; 5,399,268;
5,376,524;
5,344,752; 5,269,946; 5,256,559; 5,236,716; 5,234,808; and 5,198,357.
Kits
The present invention also provides kits that are used for platelet
collection, processing and
storage, further including suitable packaging materials and instructions for
using the kit contents. It is
preferred that all reagents and supplies in the kit be sterile, in accordance
with standard medical
practices involving the handling and storage of blood and blood products.
Methods for sterilizing the
kit contents are known in the art, for example, ethylene oxide gas, gamma
irradiation and the like. In
certain embodiments, the kit may include venipuncture supplies and/or blood
collection supplies, for
example a needle set, solution for sterilizing the skin of a platelet donor,
and a blood collection bag or
container. Preferably the container is "closed", i.e., substantially sealed
from the environment. Such
closed blood collection containers are well known in the art, and provide a
means of preventing
microbial contamination of the platelet preparation contained therein. Other
embodiments include kits
containing supplies for blood collection and platelet apheresis. The kits may
further include a quantity
of one or more sialidase inhibitors with or without the glycan-modifying
agent, sufficient to modify the
volume of platelets collected and stored in the container. In other
embodiments, the kit includes a
blood collection system having a blood storage container wherein the sialidase
inhibitor agent is
provided within the container in an amount sufficient to treat the volume of
blood or platelets held by
the container. The quantity of the sialidase inhibitor alone or the sialidase
inhibitor with the glycan-
modifying agent will depend, in part, on the volume of the container. It is
preferred the sialidase
inhibitor alone or the sialidase inhibitor with the glycan-modifying agent be
provided as a sterile non-
pyogenic solution, but it can also be supplied as a lyophilized powder. For
example, a blood bag is
provided having a capacity of 250 mL. Contained in the blood bag is a quantity
of sialidase inhibitor
such that when 250 mL of blood is added, the final concentration of the
sialidase inhibitor is
approximately 1200 micromolar. Other embodiments contain different
concentrations of sialidase
inhibitor alone or the sialidase inhibitor with the glycan-modifying agent,
for example but not limited
to quantities resulting in final concentrations of 10 micromolar to 10
millimolar, and preferably 100
micromolar to 1.2 millimolar of the sialidase inhibitor alone or the sialidase
inhibitor with the glycan-
CA 02835434 2013-11-07
WO 2012/158983 -53-
PCT/US2012/038462
modifying agent. Other embodiments use combinations of sialidase inhibitor or
the sialidase inhibitor
with the glycan-modifying agent, e.g., to effect sialyiation or
galactosylation of glycans on blood
products introduced into the container.
Platelet Function and Assessment of Treated Platelets
After treatment of platelets, the platelet functions can be assessed with
various in vitro methods.
The recovery and survival of the treated platelets can be further evaluated,
which are mostly performed
with radioactive-labeled platelets in healthy volunteers.
"Hemostatic activity," as described herein, refers to the ability of a
population of platelets to
mediate bleeding cessation (e.g., to form a clot). Normal hemostatic activity
refers to an amount of
hemostatic activity seen in the treated platelets, that is functionally
equivalent to or substantially
similar to the hemostatic activity of untreated platelets in vivo, in a
healthy (non-thrombocytopenic or
non-thrombopathic mammal) or functionally equivalent to or substantially
similar to the hemostatic
activity of a freshly isolated population of platelets in vitro.
After treatment of platelet, platelets can be assessed to determine if
platelets maintained their
function, e.g., their ability to activate and form a clot. Various assays are
available for determining
platelet hemostatic activity (Bennett, J. S. and Shattil, S. J., 1990,
"Platelet function," Hematology,
Williams, W. J., et at., Eds. McGraw Hill, pp 1233-12250). In an embodiment,
demonstration of
"hemostasis" or "hemostatic activity" can also include a demonstration that
platelets infused into a
thrombocytopenic or thrombopathic (i.e., non-functional platelets) animal or
human circulate and stop
natural or experimentally-induced bleeding. To determine hemostatic activity
of platelets, laboratories
use in vitro tests. These tests, which include assays of aggregation,
secretion, platelet morphology and
metabolic changes, measure platelet functional responses to activation. These
in vitro tests determine
in vivo hemostatic platelet function.
In an embodiment, platelets treated with compositions of the present invention
(e.g., sialidase
inhibitors) exhibit a level of platelet function similar to that of untreated
but freshly obtained/isolated
platelets.
A test that measures the platelets' ability to clot is an aggregation assay.
The platelet
aggregation test uses an aggregometer to measure the cloudiness or turbidity
of blood plasma.
Agonists to promote clotting are used in an aggregation assay. Examples of
agonists include adenosine
diphosphate (ADP), epinephrine (adrenaline), thrombin, collagen, TXA2, and
ristocetin. Since
agonists are added to the sample in order to perform the test, the results are
impacted if the donor of the
CA 02835434 2013-11-07
WO 2012/158983 -54-
PCT/US2012/038462
sample is taking an anticoagulant. The addition of an agonist to a plasma
sample causes the platelets to
clump together, making the fluid more transparent. The aggregometer then
measures the light
transmission through the specimen to determine the extent of the clotting by
the platelets in response to
the agonist. When an agonist is added the platelets aggregate and absorb less
light and so the
transmission increases and this is detected by the photocell in the
aggregometer. The normal time for
platelet aggregation varies somewhat depending on the laboratory, the
temperature, the shape of the
vial in which the test is performed, and the patient's response to different
agonists. Establishing
normal clot times and amounts of agonists for an aggregation assay can be
determined by one of skill in
the art. Exemplary amounts of agonist are as follows: ADP between 1 [tM to 10
uM, collagen
between 1 and 4 ug/mL, Ristocetin between 0.5 mg/mL and 1.5, 5 mg/mL,
adrenaline between 5 and
10 uM, arachadonic acid (precursor of TXA2) about 500 ug/mL, and thrombin
between 50 nmol/L and
100 nmol/L. For example, the difference between the response to ristocetin and
other products should
be noted because ristocetin triggers aggregation through a different mechanism
than other agonists.
Platelets that have about 65% or greater platelet aggregation in response to
adenosine diphosphate
(ADP), arachidonic acid, collagen, thrombin, TXA2, epinephrine and/or
ristocetin are considered
platelets with normal clotting function. Accordingly, platelets treated with
the sialidase inhibitors of
the present invention and exhibit about 65% or greater (e.g., about 65% to
about 100%) platelet
aggregation in an aggregation assay are considered to exhibit homeostatic
activity.
Another test that measures coagulation thrombelastography. Thrombelastography
is available,
for example, from Haemonetics Corporation (Braintree, MA) under the trade name
TEG. In
thrombelastography, a small sample of platelets (typically 0.36 mL) is placed
into a cuvette (cup)
which is rotated gently through 4 45' (cycle time 6/min) to imitate sluggish
venous flow and activate
coagulation. When a sensor shaft is inserted into the sample a clot forms
between the cup and the
sensor. The speed and strength of clot formation is measured in various ways,
and depends on the
activity of the plasmatic coagulation system, platelet function, fibrinolysis
and other factors which can
be affected by illness, environment and medications. Generally, four values
that represent clot
formation are determined by this test: the R value (or reaction time), the K
value, the angle and the MA
(maximum amplitude). The R value represents the time until the first evidence
of a clot is detected. The
K value is the time from the end of R until the clot reaches 20 mm and this
represents the speed of clot
formation. The angle is the tangent of the curve made as the K is reached and
offers similar information
to K. The MA is a reflection of clot strength. A mathematical formula
determined by the manufacturer
can be used to determine a Coagulation Index (CI) which takes into account the
relative contribution of
CA 02835434 2013-11-07
WO 2012/158983 -55-
PCT/US2012/038462
each of these 4 values into 1 equation. The treated platelets of the present
invention are able to form
clots, and maintain hemostasis.
Immunological Assessment of Platelet Markers/Function
Platelet function including its ability to activate before and/or after
treatment with the
composition and also after transfusion into an individual can be assessed.
Examples of platelet
activation markers include P-selectin, PAC-1, GPIIb, GPIIIa, GPIb and GPIIIa.
Soluble and membrane
bound markers can be assessed to determine the state of platelet activation
and assess homeostasis of
the treated platelet preparation. Methods that measure soluble and membrane
bound platelet markers
include several suitable assays. Suitable assays encompass immunological
methods, such as flow
cytometry, radioimmunoassay, enzyme-linked immunosorbent assays (ELISA),
chemiluminescence
assays, and assessment with a volumetric capillary cytometry system. Any
method known now or
developed later can be used for measuring such markers.
The inventive methods utilize antibodies reactive with platelet markers or
portions thereof In a
preferred embodiment, the antibodies specifically bind with membrane bound
and/or soluble platelet
makers or a portion thereof When the antibodies bind, they inhibit the
function of the protein or
marker to which the bind. The antibodies can be polyclonal or monoclonal, and
the term antibody is
intended to encompass polyclonal and monoclonal antibodies, and functional
fragments thereof The
terms polyclonal and monoclonal refer to the degree of homogeneity of an
antibody preparation, and
are not intended to be limited to particular methods of production.
In several of the preferred embodiments, immunological techniques detect
platelet marker
levels by means of an anti-platelet marker antibody (i.e., one or more
antibodies). Anti-platelet marker
antibody includes monoclonal and/or polyclonal antibodies, and mixtures
thereof. Labeling platelets
with antibodies directed against surface membrane glycoproteins and then
analyzing the binding by
flow cytometry is a rapid and sensitive technique for assessing homeostasis.
For example, GPIIb,
GPIIIa, GPIb and GPIIIa can be assessed using antibodies CD41, CD61, CD42b and
CD61,
respectively. Elevated levels of membrane bound or soluble P-selection can
indicate the extent of
platelet activation and can be detected using monoclonal antibodies, S12 or
W40. Antibodies for
detecting such markers can be purchased commercially or raise an appropriate
immunogen using
methods known in the art.
Any method known now or developed later can be used for measuring membrane
bound platelet
markers. One method for assessing membrane bound platelet marker levels which
the invention
CA 02835434 2013-11-07
WO 2012/158983 -56-
PCT/US2012/038462
utilizes is flow cytometry. Methods of flow cytometry for measuring platelet
or membrane bound
markers are well known in the art. (Shattil, Sanford J, et al. "Detection of
Activated Platelets in Whole
Blood using Activation-Dependant Monoclonal Antibodies and Flow Cytometry,"
Blood, Vol. 70, No
1 (July), 1987: pp307-315; Scharf, Rudiger E., et al., "Activation of
Platelets in Blood Perfusing
Angioplasty-damaged Coronary Arteries, Flow Cytometric Detection,"
Arteriosclerosis and
Thrombosis, Vol 12, No 12 (December), 1992: pp 1475-1487, the teachings of
which are incorporated
herein by reference in their entirety). For example, a sample comprising
platelets can be contacted with
an antibody having specificity for the marker under conditions suitable for
formation of a complex
between an antibody and marker expressed on platelets, and detecting or
measuring (directly or
indirectly) the formation of a complex. In an example, the level of membrane
bound markers can be
assessed by flow cytometry by obtaining a first and second sample comprising
platelets, contacting said
first sample, serving as a control, with a platelet activation agonist, such
as phorbol myristate acetate
(PMA), ADP (adenosine diphosphate), thrombin, collagen, and/or TRAP (thrombin
receptor activating
peptide), under conditions suitable for activation of platelets in said first
sample, preferably for a period
of time effective to maximally activate said platelets, and preferably while
maintaining the second
sample under conditions suitable for maintaining the endogenous platelet
activation level. The method
then involves contacting or staining the samples with a composition comprising
an anti-platelet marker
antibody, having a fluorescent label, preferably in an amount in excess of
that required to bind the
marker expressed on the platelets, under conditions suitable for the formation
of labeled complexes
between said antibody and activated platelets. Then one determines (detecting
or measuring) the
formation of complex in said samples, wherein the amount of complex detected
indicates the extent of
platelet activation in said second sample. In an embodiment, the amount of
platelet activation in
isolated platelets treated with the composition of the present invention and
stored is similar to the
amount of platelet activation from freshly obtained platelets from a donor.
In addition to using flow cytometry to measure membrane bound platelet
markers, a
radioimmunoassay can also be employed. Using a radioimmunoassay, endogenous
platelet activation
can be assessed by an immunobinding assay by obtaining a first and second
sample comprising
platelets, wherein each sample contains a preselected number of platelets;
contacting said first sample
with a platelet activation agonist, such as phorbol myristate acetate (PMA),
ADP (adenosine
diphosphate), thrombin, collagen, and/or TRAP (thrombin receptor activating
peptide), under
conditions suitable for activation of platelets in said first sample,
preferably for a period of time
effective to maximally activate said platelets, and preferably while
maintaining the second sample
CA 02835434 2013-11-07
WO 2012/158983 -57-
PCT/US2012/038462
under conditions suitable for maintaining the endogenous platelet activation
level. Then the samples
are contacted with an antibody composition that is specific to the marker
being assessed. The antibody
can have a radioactive label; or a binding site for a second antibody which
has the radioactive label.
The formation of the complex in the samples are detected, wherein the amount
of complex detected in
said second sample as compared to that detected in said first sample is
indicative of the extent of
platelet activation in said second sample.
Assaying for Detection of Soluble Platelet Markers
Any method known now or developed later can be used for measuring soluble
platelet markers.
In a preferred embodiment, soluble platelet marker is determined using an
ELISA assay or a sandwich
ELISA assay. For detection of a soluble platelet marker in a suitable sample,
a sample (e.g., blood) is
collected, and preferably platelets are removed (partially or completely) from
the sample, for example
by preparation of serum or plasma (e.g., isolation of platelet poor plasma).
Samples are preferably
processed to remove platelets within a time suitable to reduce artificial
increases in soluble platelet
marker, such as those due to secretion or proteolysis from platelets. Samples
can be further processed
as appropriate (e.g., by dilution with assay buffer (e.g., ELISA diluents)).
Additionally, the technician
can add a reagent that stabilizes and prevents in vitro platelet activations.
Examples of these stabilizing
reagents are apyrase and prostaglandin El (PGE1).
To determine a measurement for soluble platelet markers using an ELISA assay
in a suitable
sample such as serum, platelet poor plasma (PPP), the method involves
combining a suitable sample,
and a composition that includes an anti-platelet antibody as detector, such as
biotinylated anti-platelet
MAb and HRP-streptavidin, or HRP-conjugated anti-platelet Mab, and a solid
support, such as a
microtiter plate, having an anti-platelet marker capture antibody bound
(directly or indirectly) thereto.
The detector antibody binds to a different epitope from that recognized by the
capture antibody, under
conditions suitable for the formation of a complex between said anti-platelet
maker antibodies and
soluble platelet marker. The method involves determining the formation of
complex in the samples.
The solid support, such as a microtiter plate, dipstick, bead, or other
suitable support, can be
coated directly or indirectly with an anti-platelet maker antibody. For
example, an anti-platelet marker
antibody can coat a microtiter well, or a biotinylated anti-platelet marker
Mab can be added to a
streptavidin coated support. A variety of immobilizing or coating methods as
well as a number of solid
supports can be used, and can be selected according to the desired format.
CA 02835434 2013-11-07
WO 2012/158983 -58-
PCT/US2012/038462
In a particularly preferred embodiment, the sample (or standard) is combined
with the solid
support simultaneously with the detector antibody, and optionally with a one
or more reagents by
which detection is monitored.
A known amount of soluble platelet maker standard can be prepared and
processed as described
above for a suitable sample. This standard assists in quantifying the amount
of the maker detected by
comparing the level of platelet marker in the sample relative to that in the
standard. A physician,
technician, apparatus or a qualified person can compare the amount of detected
complex with a suitable
control to determine if the levels are elevated.
Typical assays for platelet markers are sequential assays in which a plate is
coated with first
antibody, plasma is added, the plate is washed, second tagged antibody is
added, and the plate is
washed and bound second antibody is quantified. However, binding kinetics
revealed that in a
simultaneous format, the off-rate of the second antibody was decreased and the
assay was more
sensitive. Thus, a simultaneous format in which the solid support is coated
with a capture antibody,
and plasma and detector antibody are added simultaneously, can achieve
enhanced sensitivity and is
preferred.
A technician, physician, qualified person or apparatus can compare the results
to a suitable
control such as a standard, levels of one or more platelet markers in normal
individuals, and baseline
levels of the platelet markers in a sample from the same donor. For example,
the assay can be
performed using a known amount of a platelet marker standard in lieu of a
sample, and a standard
curved established. One can relatively compare known amounts of the platelet
marker standard to the
amount of complex formed or detected.
Storage lesions can be assessed to determine the health of a platelet and its
ability to activate
and form a clot. Storage lesions include morphological and molecular changes
to platelets upon
storage at or below room temperature. One of the first visible effects of
platelet impairment is the
irreversible loss of the discoid morphology towards a spherical shape, and the
appearance of spiny
projections on the surface due to calcium-dependent gelsolin activation and
phosphoinositide-mediated
actin polymerization. Certain morphological changes induced in platelets can
be readily observed
under a microscope. A loss in shape is accelerated at low temperatures and
particularly when platelets
are exposed to temperature lower than 20 C. In addition to increased
modifications in shape, notable
increases occur in intracellular calcium levels and the degree of actin
polymerization. Moreover, stored
platelets secrete alpha granule and lysosomal contents, which can be assessed
immunologically, as
described herein, and reorganize the microtubule coil lying under the plasma
membrane through
CA 02835434 2013-11-07
WO 2012/158983 -59-
PCT/US2012/038462
depolymerization processes. Accordingly, storage lesions that occur at or
below room temperature can
readily be measured by methods known in the art and described herein to
quantify the effectiveness of
the inventive platelet compositions and related methods. The standard is to
compare the quality of the
platelet storage solution of the present invention to the quality of platelet
storage solutions without a
sialidase inhibitor. Accordingly, platelets treated with the composition of
the present invention
maintain shape and function that is at least similar to or better than
platelets not stored in the PAS of
the present invention (e.g., stored in a known platelet storage solution such
as InterSol (Fenwal) and
SSP+ (MacoPharma)).
EXEMPLIFICATION
Example 1: Human platelets: Prolonged storage at and below room temperature
resulted in sialic acid
loss and increased sialidase (neuraminidase) activity for human platelets
Loss of platelet sialic acid during prolonged storage under refrigeration
Platelets were stored at 4 C in the absence or presence of 1.2 mM nucleotide
sugars and the
total sialic acid was quantified. The platelets were centrifuged, thoroughly
washed, and resuspended in
140 mM NaC1, 3 mM KC1, 0.5 mM MgC12, 5 mM NaHCO3, 10 mM glucose and 10 mM
HEPES, pH
7.4. Aliquots of the resuspended platelets were lysed with RIPA buffer (Cell
Signaling Technology) for
protein quantification using Pierce BCA Protein Assay Kit, or processed to
quantify platelet sialic acid
using QuantiChromTM Sialic Acid Assay Kit per the manufacturer's instructions
(BioAssays Systems).
The assay kit uses an improved Warren method in which sialic acid is oxidized
to formylpyruvic acid
which reacts with thiobarbituric acid to form a pink colored product. The
absorbance at 549 nm is
directly proportional to sialic acid concentration, which in the test sample
can be calculated from a
linear standard curve obtained from sialic acid standards per the
manufacturer's instructions. Fresh
platelets contain ¨10 iLig of sialic acid per mg of platelet protein.
Prolonged storage under refrigeration
resulted in great loss of platelet sialic acid (Day 5_b, Donor A, ¨ 35%; Donor
B, ¨ 25%), compared
with fresh platelets (Day 5a), normalized to 100%. However, the loss of sialic
acid was slowed in
donor B platelets by the presence of CMP-sialic acid and UDP-Gal (B Day 5_b)
in the stored platelets,
the donor sugar required for resialylation (Fig. 2) . UDP-Gal alone had no
effect (Day 5_c). It is noted
that the platelet from Donor B with less sialic acid loss had less initial
sialidase surface activity than
that from Donor A (See below, Fig. 3B).
CA 02835434 2013-11-07
WO 2012/158983 -60-
PCT/US2012/038462
Sialidase activity during platelet storage.
Human platelets express surface-exposed sialidases. Sialidase activity is a
particular concern
since it is presumably responsible for the loss of platelet sialic acid during
storage. Therefore, in
addition to the direct analysis of sialic acid content, quantification of the
total platelet sialidase activity
and surface sialidase activity during storage is critical to understand the
mechanism of sialic acid loss.
Furthermore, sialidase activity may hinder an attempted resialylation
approach. A determination of the
nature of the sialidases in fresh and stored platelets is important. Shown
herein is a reliable and
sensitive fluorometric assay method for platelet sialidase activity using 4-
methylumbelliferyl-a-D-N-
acetylneuraminic acid (4-MU-NeuAc) as a substrate. Cleavage of the substrate
by sialidase released
sialic acid and methylumbelliferone (MU), upon termination with Na2CO3,
wherein the later was read
at Xex/em = 355/460 nm. Sialidase activity was measured in non-permeabilized
or permeabilized
platelets. Fig.3A shows that intact fresh platelets do not contain significant
surface sialidase activity. In
contrast, abundant sialidase activity, including both surface and
intracellular sialidase activities, was
measured in permeabilized fresh platelets. Further analysis indicates that
surface sialidase activity of
fresh platelets varies among donors (Fig. 3A, donor A and B), but increased
platelet sialidase activity
upon cold storage was observed in all cases including Donor A and B (Fig. 3C).
The detection of increased platelet sialidase activity upon refrigeration and
its absence in the
storage media (not shown) suggested that cool temperatures may increase the
surface exposure of
sialidase(s). To test this assumption, the sialidase exposure on platelets was
examined by
immunofluoresence. Four human sialidases have been identified, Neul, Neu2,
Neu3 and Neu4. Neul
is a lysosomal enzyme; Neu2 is a cytosolic sialidase; Neu3 is a plasma
membrane sialidase, wherein its
activity is specific for gangliosides; and Neu4 is a novel human luminal
lysosomal enzyme. Neul,
Neu2, Neu3 and Neu4 share high degrees of similarity and amino acid blocks of
highly conserved
residues. However, these sialidases are different from one another in terms of
subcellular localization,
substrate preference in vitro, and tissue distribution. Neul is a lysosomal
sialidase that is presumed to
have a narrow substrate specificity. The natural substrate for this enzyme is
unknown and activity has
thus only been reported on artificial substrates such as 4-MU-NeuAc and nitro-
phenyl-NeuAc, but not
on gangliosidases, fetuin, or sialyllactose. Neu2 is a cytosolic enzyme with
wide substrate specificity.
Neu3 is a plasma membrane-bound sialidase, originally described as ganglioside
sialidase. Neu3
preferentially hydrolyses gangliosides, although glycoproteins, 4-MU-NeuAc,
sialyllactose, etc. are
also hydrolysed. Lysosomal Neul and surface-bound Neu3 (antibodies are
commercially available)
were the focus of the current studies. As shown in Fig. 4, Neu3 can readily be
visualized on the surface
CA 02835434 2013-11-07
WO 2012/158983 -61-
PCT/US2012/038462
of fresh platelets and its expression is not affected by refrigeration. In
contrast, Neul only
demonstrated weak surface exposure on fresh platelets, consistent with its
subcellular localization in an
intracellular lysosomal granule. However, upon refrigeration for 48 h, its
surface exposure is greatly
increased. The data demonstrates that Neul is at least partially responsible
for the platelet surface
sialidase activity increase during refrigeration.
In summary, platelet storage under refrigeration promotes platelet surface
sialic acid loss and
increases platelet surface sialidase expression. Similar findings were also
made for RT-stored platelets
(not shown).
Example 2: Mouse platelets: Sialidase activity increases during cold storage
of mouse platelets and
the sialidase inhibitor DANA increases mouse platelet survival in vivo.
Mouse platelet sialidase activity increases following 48 h cold storage.
We have determined sialidase surface activity in isolated, intact, fresh mouse
platelets and
following cooling and rewarming using Amplex Red Neuraminidase (Sialidase)
Assay Kit (Molecular
probes, Eugene, OR, USA). Mouse platelets (2x109) maintained at room
temperature or refrigerated
for 48 h were isolated and suspended in the provided reaction buffer (0.5 M
Tris-HC1, pH 7.2 and 1
mM CaC12). Platelet derived sialidase activity was measured over 2.5 h at room
temperature. Fig. 5
shows that sialidase activity substantially increases following platelet
storage in the cold (4 C)
compared to fresh room temperature platelets (RT). Critically, sialidase
activity is not plasma derived,
as platelets were extensively washed proir to sialidase activity assays. As a
control, sialidase activity
(Clostridium perfringens (Component H)) was measured over the same time period
(inset). Neul
surface expression is increased by 3.5 fold on stored platelets as determined
by flow cytometry using
anti-Neul specific antibodies (not shown).
Fetuin as a competitive sialidase substrate during platelet storage.
Fetuin (1 mg/mL) was added to mouse platelet rich plasma prior to cold storage
or to fresh
platelets at room temperature and I3-galactose exposure measured by flow
cytometry using FITC
conjugated RCA-1-lectin, a lectin specific for exposed I3-galactose. Addition
of fetuin greatly inhibits
sialic acid hydrolysis during platelet storage, preventing RCA-1 binding.
Fetuin addition has no effect
on RCA-1 binding to fresh platelets (Fig. 6). These results show that
sialidase activity increases during
platelet cold storage, presumably mediating sialic acid hydrolysis.
CA 02835434 2013-11-07
WO 2012/158983 -62-
PCT/US2012/038462
The sialidase inhibitor DANA increases platelet life span in vivo.
The quantification of sialic acid was determined in freshly isolated platelets
and long-term
stored platelets using a Sialic Acid Quantification Kit (Sigma, St. Louis MI,
USA). The Sialic Acid
Quantification Kit determines total N-acetylneuraminic acid (sialic acid)
following the release from
glycoconjugates using a2-3,6,8,9-neuraminidase to cleave all sialic acid
linkages, including branched
sialic acid. Results show that 2x109 freshly isolated mouse platelets (-2.5 mg
protein) contain ¨3 gmol
sialic acid. Following long-term storage, platelets lose >50% of their sialic
acid content (not shown).
It had been previously postulated that sialic acid normally covers 13-
galactose residues and
permits platelet survival. These results show that normal platelet survival is
regulated by hepatocyte
ASGP receptor, independent of macrophages. Surface sialic acid is normally
hydrolyzed by sialidases.
These studies then addressed whether inhibition of sialidase activity in vivo
has an effect on platelet
survival. Mouse platelets have prolonged survival after injecting mice with
the specific sialidase
inhibitor, sodium salt of 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (DANA).
Mice were injected
with 100 mM DANA or PBS (phosphate buffered saline) as a control, after in
vivo platelet
biotinylation. Inhibition of sialidase activity with DANA increases the
survival of biotinylated platelets
(DANA) compared to biotinylated platelet survival in control mice (Control)
(Fig. 7). These results
indicate that inhibition of neuraminidase activity in vivo prolongs platelet
survival. However, the effect
may not be platelet specific. As shown, the recovery and survival of fresh
platelets are significantly
enhanced in Asgr-1 or Asgr-2 deficient mice (Sorensen et al., Blood, 2009,
Vol. 114, pgs 1645-1654)
revealing that the hepatocyte Ashwell-Morell receptors routinely survey the
platelet surface for 0-
galactose exposure. Taken together, these data indicate that platelets lose
sialic acid while circulating,
possibly due to sialidase activity, representing a new clearance mechanism for
senile platelets.
Example 3: The role of sialylation/desialylation in defining the circulatory
lifetimes of platelets.
Human platelets produce Neul and Neu3 and release Neul into plasma.
The studies herein address two novel mechanisms that contribute to increases
in the clearance
of platelets that occur upon storage. The first platelet clearance mechanism,
which is induced rapidly
by refrigeration in the absence of plasma, is mediated when GlcNAc residues on
the N-linked glycan of
GPIba become exposed and are recognized by the lectin domain of the aM132
receptor on liver
phagocytes. The second clearance mechanism, induced by long-term platelet
storage in plasma in the
cold, is of slow onset and occurs when GPIba is desialylated and recognized by
the ASGP receptors on
both liver hepatocytes and macrophages. Recent data unveils an unexpected role
for endogenous
CA 02835434 2013-11-07
WO 2012/158983 -63-
PCT/US2012/038462
sialidases and glycosyltransferases (GTs) in modulating the circulatory life
times of normal platelets.
In addition, as demonstrated herein, platelets express GTs and sialidases on
their surfaces and secrete
both. The convergence of these two mechanistic pathways strongly suggests that
platelets have an
inherent capacity to self-regulate survival in blood by renewing the glycans
of their surface
glycoproteins and that platelet lifetimes can be modulated in either positive
or negative directions by
carbohydrate addition or removal machinery, respectively. The glycan
structures promoting platelet
circulation or clearance are thus ideally suited for therapeutic manipulation
by sialidases or GT activity
(See Fig. 8).
Lysates of human platelets were subjected to SDS-PAGE and immunoblotting using
antibodies
specific for Neul and Neu3 (provided by Dr. N. Stamatos, Univ. of Maryland).
Fig. 9 shows that
human platelets contain both Neul and Neu3. Fig. 10 shows that human platelets
release Neul into
plasma after 24 hours of storage in the cold, indicating that released Neul
could mediate the removal of
surface sialic acid from platelet GPIba. As predicted from Fig. 10 and Fig. 4,
sialidase activity
associated with platelet surface increases with the time of cooling.
Human platelets express glycosyltransfereses and release them into plasma upon
activation.
Glycosyltransfereses (GTs) are expressed on platelets and packaged internally
into a secretory
compartment. Platelets have a surface associated 134gal-T (134Gal-T1) that
catalyzes the coupling of
Gal in a 01-4 linkage to exposed N-acetylglucosamine (G1cNAc) residues on the
N-linked glycans of
GPIba, improving short-term cooled mouse platelet circulation (Hoffmeister KM,
Josefsson EC, Isaac
NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of
chilled blood platelets.
Science. 2003 Sep 12;301(5639):1531-4). The nature of this glycosylation
machinery is becoming
increasingly evident from the data provided herein. For example, platelets
were paneled with
antibodies to determine which enzymes are expressed. Human platelet lysates
were displayed by SDS-
PAGE and immunoblotted against antibodies that recognize GTs. Cross-reactive
proteins are present
against 3 GalNAc-Ts, a Gal-T, and a Sial-T (Fig. 11A).
The presence of internal GT stores suggests that platelets might move GTs to
their surface upon
activation. The amount of each GT isoform associated with either resting
platelets or activated
platelets was assessed, as was the amount released into the corresponding
medium. Resting platelets
were maintained at 37 C or treated with 25 ILIM TRAP for 5 min. Maximal
release was observed after
1 minute. Enzymatic activity remaining in the 800 xg pelleted platelets (P) or
was released into the
media (M). The media was clarified at 100,000 xg for 90 min prior to activity
measurements. Fig. 11B
shows that ¨93% of the total GT activity associates with resting platelets, as
collected by centrifugation
CA 02835434 2013-11-07
WO 2012/158983 -64-
PCT/US2012/038462
(P), although a small portion of the activity is released into the bathing
medium (M). However,
following activation of platelets with 25 ILIM thrombin receptor activating
peptide (TRAP) for 5 min,
the amount of cell associated activity drops and ¨50% of the total GalNAc-,
Gal-, and Sial-T activities
are released into the medium. Ultracentrifugation did not remove enzymatic
activity from the
supernatant, excluding the possibility that the secreted activity resides in
platelet microparticles.
Hence, GTs are packaged within platelets in a secretory compartment. The
nature of this internal GT
compartment was also addressed. Immunofluorescent labeling of fixed and
permeabilized platelets
with antibodies directed towards certain selected GTs, or the well-
characterized Golgi matrix protein
GM130, revealed internal staining of 2-5 granular structures per platelet (not
shown). Hence, platelets
contain abundant amounts of GTs and sialidases and the ability of platelets to
circulate depends on
having GPIba in a maximally sialylated state.
Endogenous active platelet sialyltransferases incorporate sialic acid into
GPIba.
Endogenous resialylation was studied by following the fate of i.v. injected
fluorescent-CMP-
sialic acid (FITC-SA) in mouse platelets. After the injection, platelets were
isolated and analyzed for
the incorporation of fluorescence by flow cytometry (Fig. 12) and by
determining the extent to which
the fluorescent-tag was incorporated into mouse (not shown) and human GPIba
(Fig. 12) by SDS-
PAGE and immunoblotting analysis. Similar results were obtained using 14C CMP-
sialic acid, as
shown in Fig. 12. FITC labeled CMP-SA (FITC-SA) or FITC alone (FITC) were
injected into wild
type mice. After 1 hour, the mice were bled and FITC incorporation into
platelets was determined by
flow cytometry. Isolated human platelets were incubated with FITC (F), FITC-
SA, or left untreated (-).
Resting (Rest) and TRAP (TRAP) activated platelets were subjected to
immunoblotting using anti-
FITC (FITC), -GPIba, -aIIb or ¨von Willebrand factor (vWf) antibodies. Actin
is shown as a loading
control.
Proteolysis of GPIba and GPV by the metalloprotease TACE (ADAM] 7) is not
required to initiate
platelet clearance after desialylation.
During room temperature platelet storage or platelet storage under
refrigeration, the loss of
GPIba and GPV is observed. In contrast to other platelet receptors, such as
GPIX, GPIb 13, GPVI or 133
remain unchanged following platelet storage independent of the storage
temperature (Fig. 13). TACE
mediates proteolysis of GPIba and GPV during platelet refrigeration as shown
by inhibition of TACE
using the metalloprotease inhibitors GM6001 or platelets deficient for TACE
(Fig. 14). Surprisingly
preservation of receptor loss during platelet refrigerated storage does not
prevent refrigerated platelet
clearance (Fig. 14). Removal of sialic acid from TACE deficient platelets
diminishes platelet
CA 02835434 2013-11-07
WO 2012/158983 -65-
PCT/US2012/038462
circulatory lifetime (Fig. 15C). This demonstrates that proteolysis of GPIba
or GPV is not required to
initiate platelet clearance after desialylation. Platelets were isolated from
TACE activity deficient mice
and treated with sialidase for 15 min at 37 C (+Neu) or left untreated (-Neu).
Fluorescently (CMFDA)
labeled platelets (2 x108) were injected into wild type mice and their
circulation times were determined.
Importantly, no differences in surface vWf receptor expression were observed
in sialidase (Neu) treated
and untreated TACE-/- platelets when measured by flow cytometry (Fig. 15B). In
contrast, after
sialidase treatment, I3-galactose exposure increased by ¨5 fold as determined
using RCA I and ECL
lectins (Fig. 15A)).
Example 4: Surface sialic acid prevents loss of GPIba and GPV during platelet
storage and rescues in
vivo survival of mouse platelets.
Platelet processing and storage are associated with platelet lesion (e.g.,
shape change, activation,
release reaction, and apoptosis), which is partially due to loss of surface
receptors. Surface sialic acid is
considered to be a key determinant for the survival of circulating blood cells
and glycoproteins.
However, its role in platelet receptor loss and platelet survival is unclear.
In this study, the relationship
between surface sialic acid and platelet receptor loss was investigated in
vitro and in vivo.
Removal of sialic acid from platelet vWf receptor stimulates GPIba and GPV
shedding.
Incubation of mouse platelets with increasing concentrations of the broad
spectrum A. ureafaciens a2-
3,6,8-sialidase increased surface I3-galactose exposure, but not 13-G1cNAc, as
detected by lectin binding
assays in the flow cytometer (Fig. 16). Fig. 17 presents progressive loss of
surface of surface GPIba
and GPV in conjunction with decrease in sialic acid content (p < 0.05). GPIba
receptor expression was
followed with multiple anti-GPIba antibodies to exclude the possibility that
desialylation altered
antibody binding to GPIba. We detected a ¨6 fold increase of terminal f3-
galactose, but not f3-G1cNAc,
following treatment with 5 mU sialidase. B-galactose exposure was completely
inhibited by of the
competitive sialidase inhibitor DANA (Fig. 18). Sialidase treatment did not
affect the expression of
surface GPIX-receptor or integrin a11b133 (p> 0.05) (Fig. 19). Critically,
addition of the competitive
sialidase inhibitor DANA prevented all GPIba and GPV shedding (Fig. 19),
consistent with the
hypothesis that sialic acid loss primes GPIba and GPV for metalloprotease-
mediated shedding. Fig. 20
confirms the flow cytometry data shown in Fig. 19 by using immunoblot analysis
of total platelet
lysates, platelet supernatants and corresponding platelet pellets with or with
addition of neuraminidase
and DANA. In support of this notion, Fig. 21 shows that fresh platelets
treated with sialidase are
CA 02835434 2013-11-07
WO 2012/158983 -66-
PCT/US2012/038462
cleared rapidly from the circulation in a process prevented by DANA addition
to the storage buffer.
Importantly, addition of DANA preserved receptors expression of room
temperature stored mouse
platelets (Fig. 22) and platelet survival (not shown).
Desialylation is required for TACE-mediated GPIba and GPV shedding.
To confirm that desialylated GPIba and GPV are better TACE substrates than the
sialylated forms,
platelets were treated with recombinant TACE (rTACE) in the presence or
absence of DANA.
Platelets treated with rTACE released 47% 6 and 18% 12 of their GPIba and
GPV (p < 0.05),
respectively (Fig. 25), but negligible amounts of their GPIX and a11b133 (p >
0.05) (not shown). Receptor
shedding by rTACE, but not rTACE activity (not shown) was completely prevented
by DANA (Fig.
25). Addition of the MMP inhibitor GM6001 to sialidase-treated platelets did
not prevent I3-galactose
exposure, e.g. loss of sialic acid (Fig. 23), but inhibited receptor shedding
by rTACE (Fig. 24) (p <
0.05). I3-galactose exposure induced by sialidase increased 7-fold in the
presence of GM6001 and
rTACE (Fig. 23), showing that GM6001 has no effect on sialidase activity but
completely inhibits
rTACE and endogenous metalloprotease function. Hence, the data show that
desialylation of GPIba
and GPV is a likely prerequisite for TACE-mediated receptor shedding in the
cold and support the
concept that TACE cleavage of GPIba depends on prior sialidase activation.
Example 5: Bacterial contamination/proliferation in platelet concentrates
leads to formation of
excessive free sialic acid in the storage media.
In hospitals and blood centers, platelets are stored at room temperature. To
reduce the risk of
bacterial growth and iatrogenic infections after transfusion, platelet shelf
life is limited to 5 days in the
United States. Platelets cannot be stored in a similar manner to red blood
cells (RBC) under
refrigeration with less risk for bacterial growth and transfusion related
infections. Refrigerated
platelets are rapidly cleared from the recipient's circulation, despite
improved in vitro function.
Refrigeration of platelets irreversibly clusters the platelet glycoprotein Iba
(GPIba) complex, leading to
rapid platelet clearance when infused through lectin-mediated pathways.
Storage of platelets for transfusion at room temperature promotes bacterial
growth in bacterially
contaminated (unsterile) platelets. Many bacteria are able to interact with
platelets and induce platelet
aggregation by direct interaction between a bacterial surface protein and a
platelet receptor or an
indirect interaction where plasma proteins bind to the bacterial surface and
subsequently bind to a
CA 02835434 2013-11-07
WO 2012/158983 -67-
PCT/US2012/038462
platelet receptor. See Figs. 1A-C. Bacteria secrete a variety of biological
active substances into their
local milieu. Secreted proteins are particularly important in bacterial
pathogenesis. These proteins
have a range of biological functions ranging from host cell toxicity to more
subtle alterations of the
host cell for the benefit of the invader. In bacterial contaminated platelet
products, the bacterial-
derived products can be capable of triggering platelet activation or causing
damage to the platelets.
Many bacteria-secreted hydrolases such proteases and glycosidases (i.e
sectreted enzymes) contribute
to the bacterial virulence or are thought to play a role in promoting
bacterial growth as nutrients. In
platelet products, enzymes secreted by contaminating bacteria can truncate
platelet glycans and/or
accelerate platelet receptor shedding. Platelets are especially susceptible to
sialidase activity (sialic
acid hydrolysis) since they are heavily decorated with glycans terminated by
sialic acid. Sialidase-
mediated loss of sialic acid residues will result in clearance of the
desialylated platelets by the asialo-
glycoprotein receptor (ASGR) of liver hepatocytes upon transfusion. The
presence of sialidase-
producing bacteria in platelet product will be particularly detrimental to
platelets. In addition, after the
loss of sialic acid, asialoglycoconjugates may become substrates for the
additional bacterial
glycosidases. Subsequent release of underlying glycans will generate nutrients
that will enhance
bacterial proliferation and generate ligands for bacteria-platelet
interactions.
Although it is well-known that bacterial contamination in platelet products
can lead to
transfusion-related sepsis and platelet activation through bacteria-platelet
interactions, the presence of
sialidase-producing bacteria in platelet products and their potential impact
on platelet quality have
neither been recognized nor studied. It is expected that the presence of
sialidase-producing bacteria in
platelet products desialylates sialylglycoproteins on platelets and in plasma
and increase the free sialic
acid concentration in the storage media.
Materials and Methods: One bag of platelet concentrate (Research Blood
Components, Boston,
MA) was aseptically split to two 50-mL Falcon tubes. Prostaglandin El (PGE1,
Sigma-Aldrich) was
added to 1 [tg/mL and the samples were centrifuged for 20 min at 200 xg to
sediment contaminated red
cells. The supernatant (purified platelet concentrate, PC) was removed from
the contaminating RBC
and pooled in a new 50 mL falcon tube. The purified PC was then split,
providing identical products
for storage at 4 C and room temperature (RT), respectively. All steps were
executed under aseptic
conditions. On Days 0, 8 and 13 of storage, aliquots from each storage
condition were removed and
visually inspected for color change caused by bacterial growth at the time of
sampling. The samples
were centrifuged for 10 min at 1000 xg. The resultant supernatants (platelet
poor plasma, PPP) were
CA 02835434 2013-11-07
WO 2012/158983 -68-
PCT/US2012/038462
further centrifuged for 10 min at 10,000 xg, 4 C. The supernatants from the
second spin (platelet-free
plasma, PFP) were analyzed for free sialic acid using QuantiChrom Sialic Acid
Assay Kit (BioAssay
Systems, Hayward, CA) according to the manufacturer's instructions.
Results: Color change was readily visible on Day 8 and 13 in the PC sample
stored at room
temperature, suggesting the proliferation of "naturally" (as opposed to
spiked) occurring bacteria under
these conditions. No visible color change was noticed in the 4 C stored
samples.
The free sialic acid (FSA) in fresh PRP and PFP, and PFP recovered from
storage samples was
measured and the results are shown in Fig. 26. Although human plasma contains
high concentrations
of total sialic acid (1-2 mM), the amount of FSA in fresh PC or PFP is only ¨
4 M, accounting for less
than 0.5% of total sialic acid. The FSA level remains unchanged during 8-day
storage at 4 C and
increased by 1.4 fold during the second week (day 13) of 4 C storage (dashed
line). This data shows
that under condition that bacterial growth is retarded, the platelet sialic
acid loss due to the endogenous
platelet sialidase is minimal. In contrast, during storage at RT, FSA
increased by ¨ 3-fold on Day 8
and ¨ 9-fold on Day 13. The rapid increase of FSA in the RT-stored sample
cannot be solely attributed
to action of the endogenous platelet sialidase. It is likely the result of
exogenous sialidase released by
contaminating bacteria. The data also shows that the contaminating bacteria
are sialidase-producing
bacteria.
Conclusion: Sialidase-producing bacteria are potentially present in all
platelet products. The
bacterial sialidase can desialylate platelets, compromising their biological
functions.
Example 6: Bacterial proliferation in platelet product can be inhibited by
sialidase inhibitor
Sialidases play important role in pathogenicity and nutrition of sialidase-
producing bacteria.
Sialic acid occupies the terminal position within glycan molecules on the
surfaces of many vertebrate
cells, where it functions in diverse cellular processes such as intercellular
adhesion and cell signaling.
Pathogenic bacteria have evolved to use this molecule beneficially in at least
two different ways: 1)
they can coat themselves in sialic acid, providing resistance to components of
the host's innate immune
response, 2) or they can use it as a nutrient. Sialic acid itself is either
synthesized de novo by these
bacteria or scavenged directly from the host. Our discovery of the presence of
sialidase-producing
bacteria as contaminants in platelet product suggests a novel approach of
inhibiting bacterial growth in
CA 02835434 2013-11-07
WO 2012/158983 -69-
PCT/US2012/038462
platelet products by inhibiting sialidase activity with sialidase inhibitors.
Sialidase inhibitors are not new to the pharmaceutical industry. The influenza
virus medicines
Tamiflu and Relenza inhibit the influenza virus sialidase, which is required
for spreading of the virus
from infected cells. However, they have not been used in platelet products.
Conventionally, platelets are suspended in 100% plasma. Although plasma
(rather whole
blood) is the natural medium of platelets in vivo, it might have deleterious
effects on platelets during
storage, because plasma enzymes such as proteases can damage platelet
membranes. A storage
solution that can maintain platelet function as well or better than plasma is
desirable, in part to make
plasma available for other purposes, but especially to mitigate transfusion-
related adverse reactions,
such as TRALI. Therefore, much attention has been devoted to platelet additive
solutions with
satisfactory platelet preservation capacity with low residual plasma.
Platelet additive solutions (PASs) were first developed in the 1980s, and
continue to be
improved until today. The use of PASs as replacement for plasma has a number
of benefits, both for
the quality of the platelet concentrates and for the patients. The growth
kinetics of model bacteria in
platelets stored in a 35%:65% ratio of plasma to INTERSOLTm (30 mM sodium
phosphate, 10 mM
sodium citrate, 30 mM sodium acetate and 70 mM sodium chloride, pH 7.4) where
initial bacterial
concentrations are 0.5 to 1.6 CFUs/mL have been studied. The more rapid
initiation of log-phase
growth for bacteria within a PAS storage environment resulted in a bacterial
concentration up to 4 logs
higher in the PAS units compared to the plasma units at 24 hours. This may
present an early bacterial
detection advantage for PAS-stored platelets.
To increase the formation of planktonic bacteria, thereby improving the
sensitivity of the
bacterial detection, platelet storage studies were performed in a mixture of
PAS (InterSol) and plasma
(80:20). Many bacterial detection methods are available. We used SLP Reagent
Set (297-51501, Wako
Chemicals USA), containing silkworm larvae plasma (SLP) and 3,4-
dihydrophenylalanine (DOPA),
reconstituted according to manufacturer's instruction and stored as 100 iut
aliquots at -80 C. When a
sample is mixed with SLP reagent, peptidoglycan derived from the cell wall of
Gram-positive and
Gram-negative bacteria in the sample initiates a series of reactions including
activation of multiple
serine proteases called prophenoloxidase(proP0) cascade. The phenoloxidase
(PO) produced in the
cascade reactions oxidizes the substrate in the SLP reagent, 3,4-
dihydrophenylalanine (DOPA), to form
melain (dark blue). The bacteria concentration in the test sample is inversely
proportional to the onset
CA 02835434 2013-11-07
WO 2012/158983 -70-
PCT/US2012/038462
time of color development: shorter time = higher concentration of bacteria;
longer time = lower
concentration of bacteria.
Materials and Methods: One bag of a platelet concentrate was split to two 50-
mL Falcon tubes,
PGE1 was added. After centrifugation at 900 xg for 10 min, 80% of the
supernatant (PPP) (relative to
the total volume) was removed, and replaced with equal volume of platelet
additive solution. The
platelet was thoroughly re-suspended. The platelet suspensions were pooled and
split to 4 aliquots in
15-mL Falcon tubes. DANA (1 mM) in PBS was added to two tubes while only PBS
was added to the
other tubes. One pair of samples with and without DANA was stored at 4 C. The
second pair was
kept at RT (22 C - 24 C). Aliquots of 1.0 mL were removed on Day 0 and Day 9,
and immediately
pelleted (5 min, 15,800 x g). The supernatants were discarded, and the
pellets, containing platelets and
bacteria, were stored at -80 C until use. All experimental steps were carried
out under aseptic
conditions.
The pellet containing both platelets and bacteria, recovered from 1-mL
aliquots sampled at
different time points, was re-suspended in 100 iut of 0.1 M NaOH, and heated
for 10 min at 70 C.
After brief cooling, the solution was neutralized with 135 iut of 80 mM MES.
The reaction mixtures
were clarified by centrifugation (5 min at 15,800 xg). Aliquots of 10 iut of
the supernatant were mixed
with equal volumes of SLP reagent, reconstituted from the components in the
SLP kit following the
manufacturer's instructions. The samples were left on the bench, and color
development was
monitored. The time of color detection (TOCD) was recorded.
Results: The results are shown in Fig. 27. Selected photographs taken during
the analysis of
Day 9 samples are shown in Fig.27, panels A-C. Light, but visible, color
development was observed
after 15 min for RT-stored sample without DANA, suggesting the highest
bacterial concentration in
this sample. TOCD was extended to ¨ 34 min in the presence of DANA (#3, Fig.
27, panel B). Not
surprisingly, bacterial growth is greatly inhibited at low storage
temperatures, TOCD in 4 C-stored
samples (Fig.27, panel C) (<45 min) was increased compared to TOCD in RT-
stored sample in the
absence or presence of DANA. Its TOCD at 4 Cis further extended in the
presence of DANA (¨ 50
min, Fig. 27 panel C). Quantitative data is shown in Fig. 27, panel D.
Conclusion: Sialidase inhibitor DANA can effectively inhibit the bacterial
growth during
platelet storage. Although the nature of the bacteria is unknown, they are
likely sialidase-producing
CA 02835434 2013-11-07
WO 2012/158983 -71-
PCT/US2012/038462
bacteria. In addition, it was observed that the contaminating bacteria are not
completely dormant at
4 C.
Example 7: DANA inhibits bacterial proliferation in stored mouse platelets and
improves the survival
and recovery of mouse platelet in vivo.
Mouse platelets have a life-span of approximately 4-5 days, considerably
shorter than human
platelets (8 ¨ 10 days). They are also much less stable than human platelets
when stored at room
temperature or 4 C. The mechanism of the rapid deterioration in vitro of mouse
platelet is not well
understood, however it is possible that mouse platelet storage is affected by
bacterial contamination
due to a lack of aseptic platelet procurement protocol, in contrast to the
collection of human platelets.
To date, it remains unclear if potential bacterial contamination contributes
the rapid deterioration of
mouse platelets.
Materials and Methods: Mouse blood was obtained from anesthetized mice using
3.75 mg/g of
Avertin (Fluka Chemie, Steinheim, Germany) by retro-orbital eye bleeding into
0.1 volume of Aster-
Jandl anticoagulant and centrifuged at 300 x g for 8 min at RT to obtain
platelet rich plasma (PRP).
Platelets were separated from plasma by centrifugation at 1200 x g for 5 min
and washed twice in 140
mM NaC1, 5 mM KC1, 12 mM trisodium citrate, 10 mM glucose, and 12.5 mM
sucrose, 1 [tg/mL
PGE1, pH 6.0 (platelet wash buffer) by centrifugation. Washed platelets were
re-suspended at a
concentration of 1 x 109/mL in 140 mM NaC1, 3 mM KC1, 0.5 mM MgC12, 5 mM
NaHCO3, 10 mM
glucose and 10 mM HEPES, pH 7.4 (platelet resuspension buffer), labeled with 5
[iM 5-
chloromethylfluorescein diacetate (CMFDA) for 15 min at 37 C. Unincorporated
dye was removed by
centrifugation and platelets suspended in plasma. DANA, sialyllactose and
glucose (as a nutrient) were
added to final concentrations of 0.5, 0.5 and 8 mM, respectively, from their
corresponding PBS stock
solutions. Only PBS was added to the controls. The platelet suspensions were
stored at 4 C or RT for
48 h. After 48 h, the stored platelets were transfused by retro-orbital
injection of 3x108 platelets in 200
L. Following transfusion, blood was collected by retro-orbital eye bleeding at
time points of 5 min, 2
and 24 h. The percentage of CMFDA positive platelets in PRP was determined by
flow cytometry.
Results: All samples were visually inspected for evidence of bacterial
contamination. Severe
plasma color bleaching was observed for the room temperature-stored platelet
in the absence of
DANA, suggesting bacterial growth. No visible change was noted under all other
conditions. The
recovery and survival of mouse platelet, stored for 48 h at RT in the presence
of platelet preservatives
CA 02835434 2013-11-07
WO 2012/158983 -72-
PCT/US2012/038462
is greatly improved compared with stored platelets lacking preservatives (Fig.
28). Mouse platelets
deteriorate rapidly when stored at RT, which is clearly shown in Fig. 29.
Importantly, in the presence
of DANA, sialyllactose and glucose in the storage media approximately 5-fold
more platelets were
recovered (Fig. 29) compared to control samples.
Conclusion: Sialidase inhibitor DANA is capable of effectively preserving
mouse platelets from
deterioration during storage and to greatly improve the recovery and survival
of transfused platelets.
Example 8: Preservation of mouse platelets in the presence of different
concentrations of DANA
DANA is a potent, broad-spectrum sialidase inhibitor against viral, bacterial
and mammalian
sialidases with Ki in the low ILLM range. It is used routinely at 1 mM in all
our studies. It is expected
that its concentration can be dramatically lowered while maintaining its
efficacy against the bacteria-
caused deterioration of stored mouse platelets.
Materials and Methods: Mouse platelets were isolated as described in Example
3, re-suspended
in platelet resuspension buffer and split to four aliquots. Glucose was added
8 mM to all samples, and
DANA was added to final concentrations of 0, 0.1, 1.0 and 10 mM, respectively,
both from 100 mM
stock solutions in PBS. The samples were incubated for 30 min at 37 C,
centrifuged and supernatants
removed. The platelets re-suspended in plasma. DANA and glucose were restored
to their initial
concentrations. The platelet suspensions were stored at RT. After 48 h,
platelets under each storage
condition were counted by flow cytometry.
Results: Mouse platelets perish rapidly when stored at RT, which is clearly
shown in Fig. 30A.
However, in the presence of mere 0.1 mM DANA in the storage media,
approximately 5-fold more
platelets were recovered (Fig.30B).
Conclusion: Sialidase inhibitor DANA is capable of effectively preserving
mouse platelet from
deterioration, greatly improving the recovery and survival of the transfused
platelets.
Example 9: Inhibition of the proliferation and biofilm formation of Serratia
marcescens by sialidase
inhibitor DANA
Bacterial contamination of blood products is currently the most significant
transfusion-
associated infectious risk. Platelet concentrates (PCs) are the most likely
product to be contaminated
due to their storage conditions (22 C with agitation, neutral pH, and high
glucose content), which are
CA 02835434 2013-11-07
WO 2012/158983 -73-
PCT/US2012/038462
particularly amenable to bacterial growth. Although Gram-positive bacteria are
most commonly
recovered from contaminated PCs, Gram-negative bacteria are more frequently
associated with severe
illness and fatality. Gram-negative Serratia marcescens is a significant human
opportunistic pathogen,
which has been implicated in numerous adverse transfusion reactions (ATRs)
involving contaminated
PCs. The ability of this species to survive under unfavourable environmental
conditions, resist
disinfection and form surface-associated communities of micro-organisms
(biofilms) presents a
challenge for its elimination in the clinical environment. Recently, it has
been shown that the closely
related species Serratia liquefaciens forms biofilms under platelet storage
conditions, which is
associated with reduced detection by colony counting.
In order to proliferate in platelet products, the contaminated bacteria are
likely to have a
machinery to obtain and/or utilize sialic acid. Serratia marcescens is a Gram-
negative bacterium that
has been implicated in adverse transfusion reactions associated with
contaminated platelet concentrates.
It produces a range of extremely virulent products, including proteases,
nucleases, lipases, chitinases
and haemolysin; however, the presence of a secreatable sialidase has not yet
been described. Based on
the virulent characteristics of the secreted products by Serratia marcescens,
the presence of sialidases
is highly plausible. Therefore, this strain was chosen to test our sialidase-
inhibition strategy to inhibit
bacterial growth. The Serratia marcescens strain (ATCC # 43862) has previously
been used in studies
involving bacterial detection and growth in blood products.
Materials and Methods: Bacterial strain and growth conditions. Serratia
marcescens strain
(ATCC # 43862) was purchased from American Type Culture Collection (Manassas,
VA). Cells were
grown in brain-heart infusion broth (ATCC media 3) at 37 C and 250 rpm. Frozen
stocks were
prepared from overnight culture and stored at -80 C in brain-heart infusion
broth containing 15%
glycerol by volume.
Biofilm formation: To prepare the seed culture, the cryostock of Serratia
marcescens was
inoculated into 3 mL of brain-heart infusion broth with a cotton swab and
incubated at 37 C with
agitation at 250 rpm for 6 h. The cell density was determined at 600 nm on a
dual wavelength
spectrometer and diluted to 0.5 McFarland Standard (1.5 x 108 cells/mL) with
sterile PBS. Ten iut of
the diluted culture was inoculated into 140 iut of 30% plasma in PAS, 30% PC
by volume in PAS or
100% plasma, supplemented with or without 1 mM DANA, in the wells of 96-well
PVC plates
(Corning Biosciences). For each media, six replicates were performed. Ten iut
of PBS was inoculated
into the control wells. The microtiter plates were then sealed with sterile
porous film (VWR) and
CA 02835434 2013-11-07
WO 2012/158983 -74-
PCT/US2012/038462
placed on a platform shaker. The cultures were incubated for 48 h with gentle
shaking (¨ 100 rpm).
The cultures were gently mixed and transferred to a polystyrene plate for the
determination of
planktonic cell density at OD 595nm. The wells on the original microtiter
plates were washed with 3 x
200 iut of PBS, air dried, stained for 15 min with an aqueous solution of 0.1%
(wt/vol) crystal violet,
rinsed with water, and air dried for 1 hr. The crystal violet retained by the
biofilm was eluted with 200
iut of dimethyl sulfoxide (DMSO) or 30% acetic acid, and read at 595 nm.
Results: Under suboptimal growth conditions on the microtiter plate, lacking
of adequate
agitation and aeration, and low temperature, S. marcescens grew well in pure
plasma (Fig. 31A). The
cell growths were dramatically retarded in 30% plasma or 30% PC in PAS.
Remarkably, inclusion of 1
mM DANA in the growth media inhibited the bacterial growth under all
conditions. In parallel with
trends observed for bacterial growth, the formation of biofilm correlated well
with planktonic cell
density and negatively impacted by the presence of DANA in the growth media
(Fig. 31B). The
measurment of the A595nm of the biofilm formation for the bacteria grown in
plasma could not be
accurately interpreted due to the signal overflow, suggesting stronger biofilm
formation in pure plasma
than in PAS-based media.
Conclusion: Sialidase inhibitor DANA is capable of inhibiting the
proliferation and biofilm
formation of S. marcescens when analyzed with 96-well PVC plate. The data also
show that S.
marcescens contains a previously unreported machinery to obtain and/or utilize
sialic acid to
proliferate and/or form biofilms.
Example 10: Variations in platelet surface glycans among healthy volunteers
Platelets have the shortest shelf-life of all major blood components and are
the most difficult to
store; these limitations complicate platelet transfusion practices. Dr.
Slichter and colleagues (Puget
Sound Blood Center, Seattle, WA) have identified significant differences in
recovery and survival of
transfused fresh radiolabeled autologous platelets among healthy subjects. The
cause of the inter-
individual differences in platelet recovery and survival remains unclear. We
demonstrated that the loss
of sialic acid from the surfaces of cold-stored and transfused platelets
promotes their clearance by
hepatic Asialoglycoprotein receptors (Ashwell Morell receptors). The loss of
platelet surface sialic acid
correlates with increases in surface sialidase activity during platelet
storage. Here we investigated
whether fresh platelets from individual donors exhibit differences in surface
glycan exposure, which
may affect post-transfusion platelet recovery and survival.
CA 02835434 2013-11-07
WO 2012/158983 -75-
PCT/US2012/038462
Material and Methods: Venous blood was obtained from volunteers by
venipuncture into 0.1
volume of Aster Jandl citrate-based anticoagulant. Approval for blood drawing
was obtained from the
Institutional Review Board of Brigham and Women's Hospital, and informed
consent was approved
according to the Declaration of Helsinki. Platelet-rich plasma (PRP) was
prepared by centrifugation at
125 xg for 20 min and platelets were separated from the plasma proteins by gel-
filtration through a
small Sepharose 2B column. Isolated platelets were incubated for 20 min at
room temperature with 10
[tg/mL of the 13-galactose specific FITC-conjugated E. cristagalli lectin
(ECL). The samples were
diluted with 200 iut of PBS and immediately analyzed by flow cytometry on a
FACSCalibur flow
cytometer (Beckton Dickenson). The mean fluorescence intensity was determined
in gated platelet
population.
Results: The presence of a terminal galactose on surface glycoproteins (i.e.
glycans lacking of
SA) on freshly-isolated platelets varies considerably among healthy subjects
(three of five individuals
had low levels of exposed galactose (15.3 4.1, MFI), as expected. However,
two subjects exhibited
considerably higher (2-7.5-fold) levels of galactose exposure. These results
were confirmed using a
second galactose-specific lectin RCA I, and by repeated measurements of the
same individuals at two
different time points. Similarly, preliminary studies with platelet
concentrates demonstrated a
remarkable variation in platelet surface sialidase activity (Fig. 32), which
correlated with rates of sialic
loss during platelet storage and possibly during platelet circulation in vivo.
Our results show that fresh
platelets from healthy individuals vary in surface sialidase activity and
sialic acid content.
These results indicate that the surface sialic acid could represent a factor
that affects the
recovery and survival of the transfused fresh platelets.
Example 11: General procedure of preparing platelet additive solution
containing a sialidase inhibitor
The PAS of the present invention can be made as follows. The total volume of
the bag is 500
mL.
To prepare a platelet additive solution, the following components of USP grade
are obtained:
1) Electrolytes such as Na, Cl, K, Ca, and Mg.
2) An energy source such as glucose or citrate to sustain aerobic metabolism.
3) A buffer such as phosphate.
4) Water for injection (WFI).
CA 02835434 2013-11-07
WO 2012/158983 -76-
PCT/US2012/038462
5) A sialidase inhibitor.
Table 2 provides the concentrations and amount (grams) of components including
energy sources,
buffers and electrolytes required to prepare 1000 mL of platelet additive
solution. Water is added in an
amount of 1000 mL and the solution is buffered to maintain a pH of pH 7.2.
Sialidase inhibitor such as DANA can be added from sterile 0.1-1000 mM stock
solution in
water to the desired concentrations.
Table 2
PAS 1 PAS 2 PAS 3 PAS 4
g/1000 g/1000 g/1000 g/1000
Component
mM mL mM mL mM mL mM mL
Dibasic sodium phosphate, anhydrous
(Na2HPO4), USP 7.15 1.015 7.15 1.015 7.15
1.015 7.15 1.015
Mono basic phosphate, monohydrate
(NaH2PO4.1-120), USP 2.24 0.310 2.24 0.310 2.24
0.310 2.24 0.310
Sodium citrate, dihydrate
(C6H5Na307=2H20), USP 10.00 2.940 10.00 2.940 10.00
2.940 10.00 2.940
Sodium acetate, trihydrate
(CH3COONa), USP 29.98 4.080 29.98 4.080 29.98
4.080 29.98 4.080
Sodium chloride (NaC1), USP 79.20 4.629 70.80 4.138 77.70
4.541 69.30 4.050
Potassium chloride (KC1), USP 5.00 0.373 5.00 0.373 5.00
0.373 5.00 0.373
Magnesium chloride, hexahydrate
(MgC12=6H20), USP 1.50 0.305 1.50 0.305 1.50
0.305 1.50 0.305
Calcium chloride, dihydrate
(CaC12=2H20), USP 0.00 0.000 0.00 0.000 1.00
0.147 1.00 0.147
Glucose (C6H1206), USP 0.00 0.000 16.80 3.028 0.00
0.000 16.80 3.028
DANA, sodium salt (solid or stock
aqueous solution) 1.00 0.313 1.00 0.313 1.00
0.313 1.00 0.313
Water for injection, USP, to 1000 mL
Example 12: Preservation of mouse platelets in PAS containing a sialidase
inhibitor.
Mouse platelets have a life span of approximately 4-5 days, considerably
shorter than human
platelets (8-10 days). They are also much less stable than human platelets
when stored at room
temperature or 4 C. However, these shortcomings of mouse platelets can be
exploited to assess the
efficiency of platelet additive solutions for the preservation of platelets.
Materials and Methods: Mouse blood was obtained from anesthetized mice using
3.75 mg/g of
Avertin (Fluka Chemie, Steinheim, Germany) by retro-orbital eye bleeding into
0.1 volume of Aster-
Jandl anticoagulant and centrifuged at 200 xg for 8 min at RT. The
supernatant, containing platelet
rich plasma, buffy coat, and some RBC, was removed and centrifuged at 300 xg
for 6 min to obtain
CA 02835434 2013-11-07
WO 2012/158983 -77-
PCT/US2012/038462
platelet rich plasma (PRP). Four 150 iut aliquots of PRP were transferred to 4
x 1.5 mL Eppendorf
tubes, and centrifuged at 1000 x g for 5 min. About 70% of the supernatant
(105 L) was removed
from each tube, and replaced with equal volume of InterSol. DANA and/or
glucose (as a nutrient)
were added to final concentrations of 1.0 and 10 mM, respectively, from 100 mM
stock solutions in
PBS. The volumes in tubes lacking one or both additives were evened out with
PBS. The platelet
suspensions were stored at RT for 48 h on a shaker and analyzed by flow
cytometry.
Results: Not surprisingly, mouse platelets deteriorated rapidly in InterSol
when stored at RT
(Fig. 33, panel Aa). Only 57% platelets were gated (Fig. 33, panel Ba).
Remarkably, Over 80% of the
original platelet events were counted within the platelet gate when stored
with 1 mM sialidase inhibitor
DANA (Fig. 33, panels Ab and Bb). Addition of 10 mM glucose resulted in even
higher platelet
counts recovery after storage (Fig. 33, panels Ac and Bc). A combination of
both DANA and glucose
preserved all platelets (93% gated, Fig. 33, panels Ad and Bd). DANA alone or
a combination with
glucose results in a more resting platelet population as judged by their
forward and side scatter
characteristics (the population is "less elongated", i.e., formed less
platelet aggregates) than glucose
alone (Fig. 33, panels Ab and Ad, compare with Fig. 33, panel Ac). This data
suggests that DANA is
more effective than glucose in preserving platelets in a resting state and in
preserving platelet numbers
following platelet storage.
Conclusion: Together, the data indicate that the presence of DANA during
platelet storage
improves the quality of the stored platelets in at least 30% plasma in
platelet additive INTERSOLTm
solution.
Example 13: Improved in vitro quality of human platelets stored in plasma in
the presence of sialidase
inhibitor DANA.
The state of a "healthy" platelet is partially defined by its shape and size.
Platelet shape change
and aggregation are hallmarks of platelet activation. Once activated,
platelets change shape and secrete
their granular contents. Storage of platelets is accompanied by platelet
activation, i.e. platelet shape
change and granule release. Human platelets also increase surface sialidase
expression and lose surface
sialic acid during storage. Presumably, sialidases are stored in granules and
are released during storage
to the platelet surface. The results from Example 12 suggest that mouse
platelets may also lose sialic
acid during storage and this process can be effectively inhibited by the
presence of sialidase inhibitor
DANA in the storage, greatly improving the post-transfusion recovery and
survival of platelets. The
CA 02835434 2013-11-07
WO 2012/158983 -78-
PCT/US2012/038462
data further indicate that the quality of stored human platelets can be
improved by including a sialidase
inhibitor in the storage media.
Resting platelets have a discoid shape and produce different side-scatter
(SSC) signals in the
flow cytometer, depending on their relative orientation to the laser beam. A
resting platelet population
has a wide ("round") distribution in the SSC/FSC signal. Upon stimulation,
platelets form pseudopods
and become spherical (shape change) thereby producing a characteristic SSC
signal irrespective of their
relative orientation to the laser beam. Therefore, an activated platelet
population appears more
"condensed" on a FCS/SSC plot.
Based on these considerations, we investigated if DANA affects human platelet
activation (i.e.
shape change and granule release) during storage in plasma.
Materials and Methods: Venous blood was obtained from volunteers by
venipuncture into 0.1
volume of Aster Jandl citrate-based anticoagulant. Approval for blood drawing
was obtained from the
Institutional Review Board of Brigham and Women's Hospital, and informed
consent was approved
according to the Declaration of Helsinki. Platelet-rich plasma (PRP) was
prepared by centrifugation at
125 xg for 20 min and platelets were separated from PRP after adding PGE1 (1
g/mL) by
centrifugation for 5 min at 850 xg. The supernatant (platelet-poor plasma,
PPP) was saved. The
platelet pellet was resuspended in PPP, 1/2 volumes of original PRP, and
divided into aliquots. DANA
was added to 1.0 mM from 100 mM stock in PBS to half of the aliquots, only PBS
was added to the
controls. The samples were stored in the wells of a 96-well microtiter plate
covered with a gas-
permeable film with agitation on a shaker at room temperature. Platelet size
and density were
measured by forward (FSC) and side scatter (SSC) on a FACSCalibur flow
cytometer (BD). Platelets
were gated by their forward and side scatter characteristics. For the analysis
platelet degranulation, i.e.,
a-granule release, stored platelets were analyzed for P-selectin surface
expression by incubating with
0.1 g/mL of FTIC mouse anti-human CD62P (BD Pharmingen) antibody in 50 iut of
PBS for 30 min
at RT. The mixture was then diluted with 200 iut of PBS and immediately
analyzed by flow
cytometry. The percentage of P-selectin (FITC)¨positive cells was determined
in gated platelet
population.
Results: After 72 h storage at RT in plasma, human platelets displayed a
decrease in side and
forward scatter characteristics (Fig. 34, panel A, left side) compared with
fresh RT platelets (not
CA 02835434 2013-11-07
WO 2012/158983 -79-
PCT/US2012/038462
shown). The decrease in side and forward scatter characteristics is
characteristic for platelet activation.
In contrast, addition of 0.5 mM DANA during platelet storage led a visible
improvement of the platelet
shape (Fig. 34, panel B, right side). Comparisons of histograms of platelet
count/SSC showed that
platelets stored with DANA have increased mean fluorescence intensity (MFI),
(Fig. 34, panel B, left
side , note that the profile migrates slightly to the right side), suggesting
that platelets stored in the
presence of DANA have higher granularity or internal complexity and are less
activated. Similarly,
histograms of platelet count/FSC histograms showed that platelets stored with
DANA have higher side
scatter mean fluorescence intensity (Fig. 34, panel B, right side, note that
the profile migrates slightly
to the right side). These results show that platelets stored in the presence
of DANA are bigger and
retain a discoid, resting shape.
These results were confirmed by analyzing the P-selectin exposure of the
stored platelets with
FTIC mouse anti-human CD62P (P-selectin) antibody (Fig. 35). Inclusion of DANA
during storage
significantly prevented the exposure of P-selectin, and inhibited a-granule
release.
Together, the data indicate that the presence of DANA during platelet storage
improves the
quality of the stored platelets in 100% plasma.
Example 14: Improved in vitro quality of human platelets stored in PASs
containing sialidase inhibitor
DANA.
Data described in Example 12 demonstrated that sialidase inhibitor DANA can
effectively
preserve the quality of mouse platelets stored 30% plasma in platelet additive
solution referred to as
INTERSOL solution. Data described in Example 13 clearly showed that DANA is
also effective for
preserving the quality of human platelets in 100% plasma. In this Example, the
studies were extended
to human platelets stored in plasma/PAS in a ratio of 30:70, in the absence or
presence of DANA.
Materials and Methods: Human platelets were obtained as described in Example
13. The
platelet pellet was resuspended in PPP, 1/5 volumes of original PRP, and
aliquoted into wells of a 96-
well microtiter plate (60 iut per well). PAS (designated as PASa), containing
7.15 mM Na2HPO4, 2.24
mM NaH2PO4, 10 mM sodium citrate, 30 mM sodium acetate, 79.2 mM NaC1, 5.0 mM
KC1, and 1.5
mM MgC12, was added to corresponding wells at 140 iut per well, DANA was added
to 0, 0.1 and 0.5
mM from 10 or 100 mM stock in PBS to proper wells. The sample volumes in the
wells were evened
out with PBS. The plate was then covered with a gas-permeable film and placed
on a shaker. Platelet
CA 02835434 2013-11-07
WO 2012/158983 -80-
PCT/US2012/038462
size and density were measured by forward (FSC) and side scatter (SSC) on a
FACSCalibur flow
cytometer (BD) at Day 7, and pH was checked at Day 9.
Results: All storage samples maintained at pH 6.8 after 9 days, demonstrating
this PAS
formulation has enough buffer capacity for storing platelets for at least 9
days. In contrast, under
similar storage conditions in 100% plasma, the pH of the stored platelet
samples dropped below pH
6.5. Significant deterioration of human platelets was noted after 7 days of
storage at RT when stored in
30% plasma and 70% PAS solution. As shown in Fig. 36, panel A, only 55% of the
total acquired
events were gated in the gate defined for fresh platelets (G1) while more than
40% of the acquired total
events were platelet microparticles (platelet microparticles are considered as
a readout of platelet
deterioration) defined in G2. In contrast, when platelets were stored in the
presence of 0.1 mM DANA
over 70% of the total acquired events were gated as platelets (Fig.36, panel
B, G1). Accordingly, a
dramatic reduction of microparticle formation from 41.5% (Fig. 36, panel A,
G2) to 23.95% (Fig. 36,
panel B, G2) was observed. Increase of DANA concentration in the storage media
to 0.5 mM further
increased platelet counts (81.6% gated, Fig. 36, panel C, Gl) and reduced the
formation of
microparticles (13.52%, G2). Of particular note is that the platelet
population appears "resting" upon
addition of DANA to the storage solution, as judged by their side and forward
scatter characteristics.
Conclusion: Consistent with results described in Examples 12 and 13, DANA can
effectively
preserve the quality of human platelets in 30% plasma in a platelet additive
solution, i.e., reduce
platelet activation and microparticle formation, showing that a sialidase
inhibitor such as DANA can be
used as an important component in PAS formulations for platelet storage.
The relevant teachings of all the references, patents and/or patent
applications cited herein are
incorporated herein by reference in their entirety.
While this invention has been particularly shown and described with references
to preferred
embodiments thereof, it will be understood by those skilled in the art that
various changes in form and
details may be made therein without departing from the scope of the invention
encompassed by the
appended claims.