Note: Descriptions are shown in the official language in which they were submitted.
CA 02836643 2016-03-15
DRY POWDER VANCOMYCIN COMPOSITIONS AND ASSOCIATED METHODS
BACKGROUND
Vancomycin is a glycopeptide antibiotic used in the prophylaxis and treatment
of
infections caused by Gram-positive bacteria. Vancomycin is the international
nonproprietary
name (INN) corresponding to the compound with the following formula:
NH 0 0
( 0
HO
HN'441).H"µ'
0H0 so: 0
so 'OH
0 0
Cf a
HO.
OH ro
OH
It has been proposed that vancomycin acts by inhibiting proper cell wall
synthesis in
Gram-positive bacteria. More specifically, it is believed that vancomycin
prevents incorporation
of N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG) glycan subunits
into the
peptidoglycan matrix; which forms the major structural component of Gram-
positive cell walls.
The binding of vancomycin to the terminal D-alanyl-D-alanine moieties of the
NAM/NAG-
peptides prevents their incorporation into the peptidoglycan matrix.
Vancomycin has been administered intravenously for systemic therapy, as well
as orally
in the treatment of pseudomembranous colitis. Vancomycin has also been used
off-label in a
nebulized aerosol form for the treatment of various infections of the upper
and lower respiratory
tract. However, the use of an approved drug in an off-label manner may put a
patient at risk as
the safety and efficacy have not been studied and/or the appropriate dose may
not be given.
Furthermore, delivery by nebulization can take up to 20 minutes, which is a
significant burden to
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
2
patients. Currently, there are no known commercially available dry powder
inhalable forms of
vancomycin.
SUMMARY
The present disclosure generally relates to dry powder compositions and
methods for
administering and preparing such compositions.
In one embodiment, the present disclosure provides a composition comprising
vancomycin or a pharmaceutically acceptable salt thereof, wherein the
composition is a dry
powder.
In another embodiment, the present disclosure also provides a method
comprising
administering a dry powder composition comprising vancomycin or a
pharmaceutically
acceptable salt thereof to a subject via pulmonary administration.
In yet another embodiment, the present disclosure provides a method comprising
spray
drying an aqueous composition comprising vancomycin or a pharmaceutically
acceptable salt
thereof and a hydrophobic amino acid so as to form a dry powder composition.
The features and advantages of the present invention will be apparent to those
skilled in
the art. While numerous changes may be made by those skilled in the art, such
changes are
within the spirit of the invention.
DRAWINGS
Some specific example embodiments of the disclosure may be understood by
referring, in
part, to the following description and the accompanying drawings.
Figure 1 is a graph depicting the aerodynamic particle size distribution of a
dry powder
vancomycin composition (Lot SA010) as measured using the Andersen Cascade
Impactor and
Monodose RS01 Model 7 inhaler.
Figure 2 is a graph depicting the effect of leucine content on the delivery
efficiency of
dry powder vancomycin compositions (Lots SA002, SA006, SA007, 5A008 and SA009)
as
measured using the fast screening impactor and Monodose RS01 Model 7 inhaler.
Emitted dose
(%) is the amount of drug in the dry powder that exits the inhaler as a
percentage of the initial
amount of drug in the dry powder present in the capsule. Fine particle
fraction (%) is the amount
of drug in the dry powder having an aerodynamic size less than 5 1.1m as a
percentage of the
emitted dose. Delivery efficiency (%) is the amount of drug in the dry powder
having an
aerodynamic diameter less than 5 wn as a percentage of the initial amount of
drug in the capsule.
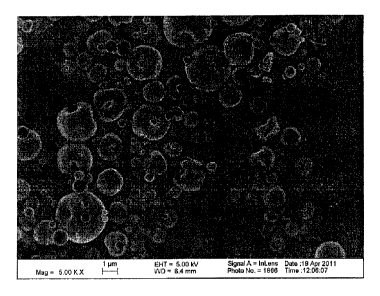
Figure 3 is an image of lab-scale dry powder vancomycin particles (Lot SA010)
obtained
using a scanning electron microscope.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
3
Figure 4 is a graph depicting the aerodynamic particle size distribution of a
dry powder
vancomycin composition (Lot SA010) stored at 25 C and 60% relative humidity
(RH) for a
period up to 6 months as measured using the Andersen Cascade Impactor and
Monodose RS01
Model 7 inhaler.
Figure 5 is a graph depicting the emitted dose content uniformity under 3
different
stability conditions for a period up to 6 months of 10 consecutive actuations
through a Monodose
RS01 Model 7 inhaler compared to FDA draft guidance specification limits of
20% (Lot
SA010).
Figure 6 is a graph depicting the aerodynamic particle size distribution of a
dry powder
vancomycin composition of the present disclosure (Lot G-11-26-1) as measured
from three
Monodose RS01 Model 7 inhalers tested at 100 L/min for 2.4 s (equivalent to 4
L) using a Next
Generation Impactor.
Figure 7 is a graph depicting the emitted dose content uniformity across ten
Monodose
RS01 Model 7 inhalers tested at 100 L/min for 2.4 s (equivalent to 4 L) for
lot G-11-26-1. The
20% and 25% represent the FDA draft guidance limits.
Figure 8 is a graph depicting the effect of trehalose concentration on the
chemical
stability of a dry powder vancomycin composition of the present disclosure at
50 C for 4 weeks.
Figure 9 is a graph depicting a comparison of two lots of dry powder
vancomycin
compositions containing 10% leucine. One contains no API or process
modifications (Lot
SA010) and the other has a more pure API source, was processed under nitrogen,
precautions
were taken to protect from light and the water content in the powder
composition was lower (Lot
G-11-026-1).
Figure 10 is an image of pilot-scale dry powder vancomycin particles (Lot
19SA01.HQ00005) obtained using a scanning electron microscope.
Figure 11 is a graph depicting plasma concentrations as a function of time
(semi-log)
after a single dose administration of a vancomycin dry powder composition (Lot
G-11-026-1) or
intravenous administration of vancomycin (inhaled vancomycin dose 16 mg,
circle; inhaled
vancomycin dose 32 mg, triangle; inhaled vancomycin dose 80 mg, square;
Intravenous infusion
250 mg over 60 min, circle with line).
While the present disclosure is susceptible to various modifications and
alternative forms,
specific example embodiments have been shown in the figures and are herein
described in more
detail. It should be understood, however, that the description of specific
example embodiments is
not intended to limit the invention to the particular forms disclosed, but on
the contrary, this
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
4
disclosure is to cover all modifications and equivalents as illustrated, in
part, by the appended
claims.
DESCRIPTION
The present disclosure generally relates to dry powder vancomycin compositions
and
methods for administering and preparing such compositions. In some
embodiments, a
composition of the present disclosure may be administered to a subject via
pulmonary
administration in an amount effective to treat and/or prevent a bacterial
infection in the subject.
Administration of an effective amount of a composition of the present
disclosure may be
particularly useful in treating a gram-positive bacterial infection in a
subject suffering from
pneumonia, cystic fibrosis, bronchiectasis, or other chronic lung disease with
a bacterial
infection of the subject's airway and/or lung.
In one embodiment, a composition of the present disclosure is a dry powder
comprising
vancomycin or a pharmaceutically acceptable salt thereof. As used herein, the
term vancomycin
includes analogues and derivatives of vancomycin. As used herein, the term
"dry" means that the
composition has a moisture content such that the particles are readily
dispersible in an inhalation
device to folin an aerosol. In some embodiments, this moisture content may be
below about 10%
by weight water, below about 7% by weight water, below about 5% by weight
water or below
about 3% by weight water. Furthermore, as used herein, the term "powder" means
a composition
that consists of finely dispersed solid particles that are capable of being
readily dispersed in an
inhalation device and subsequently inhaled by a subject so that the particles
reach the lungs to
pennit penetration into the upper and lower airways. Thus, the powder is said
to be "respirable."
In some embodiments, a dry powder composition of the present disclosure may
have a tap
density greater than about 0.4 g/cm3, greater than about 0.45 g/cm3 or greater
than about 0.5
g/cm3. While vancomycin may be predominately used in the descriptions
contained in this
disclosure, it should be understood that the present disclosure may be
practiced with any other
glycopeptide antibiotics (e.g. vancomycin, teicoplanin, telavancin, bleomycin,
ramoplanin, and
decaplanin), and derivatives and analogues thereof, among other things, to
treat certain gram
positive infections.
In certain specific embodiments, a dry powder composition of the present
disclosure
comprises vancomycin, or a pharmaceutically acceptable salt thereof, present
in an amount of
about 90% by weight of the composition, the powder having a tap density of
greater than about
0.4 g/cm3, and further comprises leucine in an amount of about 10% by weight
of the
composition.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
In some embodiments, a dry powder composition of the present disclosure may
comprise
particles having an average particle size of less than or equal to about 10
microns (pm) in
diameter as defined by the mass median aerodynamic diameter (MMAD) (measured
using
cascade impaction). In some embodiments, at least 95% of the particles have a
MMAD of less
5 than about 10 [un. In some embodiments, the diameter may be less than or
equal to about 7 1..un.
In other embodiments, the diameter may be less than or equal to about 5 m. In
certain specific
embodiments, the diameter may be between about 0.5 pm and about 5 pm in
diameter,
particularly about 1 um to about 3 pm. Dry powder compositions of the present
disclosure
comprising particles having an average particle size of less than or equal to
about 10 pm in
diameter may be particularly useful for delivery via an oral inhalation
device.
In other embodiments, a dry powder composition of the present disclosure may
comprise
particles having an average particle size of greater than or equal to about 10
pm in diameter as
defined by MMAD (measured using cascade impaction). In some embodiments, at
least 95% of
the particles have a MMAD of greater than about 10 um. In certain specific
embodiments, the
particle size may be between about 10 um and about 50 um in diameter,
particularly about 20
pm to about 40 p.m. Dry powder compositions of the present disclosure
comprising particles
having an average particle size of greater than or equal to about 10 p.m in
diameter may be
particularly useful for nasal delivery.
In some embodiments, the particles may be hollow. In some embodiments, the
particles
may be porous. In some embodiments, the particles may have a spheroidal shape
distribution,
which may be relatively uniform. In some embodiments, the vancomycin potency
as measured
by microbial activity is effectively unchanged when compared to the
unformulated drug (i.e.
within 5% of the drug).
Vancomycin or a pharmaceutically acceptable salt thereof suitable for use in
the present
disclosure is generally available through various commercial vendors. Examples
of suitable
pharmaceutically acceptable salts of vancomycin include, but are not limited
to, vancomycin
hydrochloride, vancomycin sulfate, etc.
In some embodiments, in addition to vancomycin or a pharmaceutically
acceptable salt
thereof, a composition of the present disclosure may further comprise one or
more additives. One
example of a suitable additive includes a hydrophobic amino acid. Such
hydrophobic amino
acids may include, but are not limited to, tryptophan, tyrosine, leucine,
trileucine and
phenylalanine. In some embodiments, it may be desirable to include a
hydrophobic amino acid in
a composition of the present disclosure so as to improve the physical
stability and/or
dispersibility of the composition, improve the chemical stability of
vancomycin or a
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
6
pharmaceutically acceptable salt thereof, and/or to alter the taste of the
composition by masking
the bitter taste of vancomycin and its salts, and/or to alter the rate the
composition is absorbed
into the systemic circulation from the lung (e.g., increase or slow the rate).
While not wishing to
be bound to any particular theory, it is currently believed that the
hydrophobic amino acid
additive remains on the surface of the particles and protects them from
moisture and light,
thereby increasing the stability of the formulation.
Another example of a suitable additive includes a carbohydrate bulking agent.
Such
carbohydrate bulking agents may include, but are not limited to, lactose,
mannitol, trehalose,
raffinose, and maltodextrins. In some embodiments, it may be desirable to
include a
carbohydrate bulking agent in a composition of the present disclosure so as to
improve the
physical stability of the composition. Furthermore, in some embodiments, the
carbohydrate
bulking agent may also improve the chemical stability of vancomycin or a
pharmaceutically
acceptable salt thereof. Other additives known to those of ordinary skill in
the art may also be
included.
Generally, additives suitable for use in the compositions of the present
disclosure may be
included in an amount of about 50% or less by weight of the composition, 30%
or less by weight
of the composition, or 10% or less by weight of the composition. In other
embodiments,
additives suitable for use in the compositions of the present disclosure may
be included in an
amount of from about 10% to about 30% by weight of the composition. In other
embodiments,
additives suitable for use in the compositions of the present disclosure may
be included in an
amount of from about 10% to about 20% by weight of the composition.
The compositions of the present disclosure may further comprise
pharmaceutically
acceptable auxiliary substances or adjuvants, including, without limitation,
pH adjusting and
buffering agents and/or tonicity adjusting agents, such as, for example,
sodium acetate, sodium
lactate, sodium chloride, potassium chloride, calcium chloride, etc. Leucine
has the dual benefit
of also modifying the pH. Similarly, the compositions of the present
disclosure may contain
pharmaceutically acceptable carriers and excipients including microspheres,
microcapsules,
nanoparticles or the like.
In certain embodiments, the dry powder composition may be reconstituted and
the
resulting reconstituted powder may have a pH greater than 3.0, preferably
greater than 3.5 and
most preferably greater than 4Ø
As previously mentioned, administration of an effective amount of a dry powder
vancomycin composition may be particularly useful in alleviating symptoms
and/or treating
subjects suffering from conditions including, but not limited to pneumonia and
cystic fibrosis
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
7
with gram-positive bacterial infections, and/or colonization of the airways-
and/or lung
parenchyma by bacteria or other pathogens susceptible to vancomycin or its
derivatives. Other
conditions may include, but are not limited to, bronchitis, bronchiectasis,
diffuse
panbronchiolitis, bronchiolitis, bronchiolitis obliterans, bronchiolitis
obliterans organizing
pneumonia (BOOP), pneumonia of any cause, including but not limited to
community acquired
pneumonia, nosocomial pneumonia and ventilator associated pneumonia (VAP).
Examples of
gram-positive bacterial infections may include, but are not limited to,
bacterial infections by
Streptococcus pneumoniae, and Staphylococcus aureus, including methicillin-
resistant
Staphylococcus aureus.
As will be recognized by one of ordinary skill in the art, the effective
amount needed to
treat a particular condition or disease state will depend on the pathogen,
individual, the
condition, length of treatment, the regularity of treatment, the type of
vancomycin used, and
other factors, but can be readily determined by one of ordinary skill. The
patient can achieve a
desired dosage by inhaling an appropriate amount of the composition.
A dry powder composition of the present disclosure may be delivered to a
subject by any
means so long as the solid particles of the dry powder composition are capable
of being inhaled
by a subject so that the particles reach the lungs to permit penetration into
the upper and lower
airways. In certain embodiments, a dry powder composition of the present
disclosure may be
delivered to a subject by placing the dry powder within a suitable dosage
receptacle in a
sufficient amount. Suitable dosage receptacles include those used in reservoir
devices (e.g.,
devices that contain more than one dose in which the device itself meters the
dose) or factory-
metered dose devices (e.g., devices in which each dose is contained in either
a single unit or
multiple units). In one example, a suitable reservoir device may have a dosage
receptacle that fits
within a suitable inhalation device to allow for the aerosolization of the dry
powder composition
by dispersion into a gas stream to form an aerosol and then delivering the
aerosol so produced
from a mouthpiece attached for subsequent inhalation by a subject in need of
treatment. Such a
dosage receptacle includes any container enclosing the composition known in
the art such as
gelatin, hydroxypropyl methyl cellulose or plastic capsules with a removable
portion or body
that can be cut or pierced that allows dispersal of the dry powder composition
(e.g., via a gas
stream directed into the container and via centrifugal force). Such containers
are exemplified by
those shown in U.S. Pat. No. 4,227,522 issued Oct. 14, 1980; U.S. Pat. No.
4,192,309 issued
Mar. 11, 1980; and U.S. Pat. No. 4,105,027 issued Aug. 8, 1978. Suitable
containers also include
those used in conjunction with GlaxoSmithKline's Ventolin Rotahaler brand
powder inhaler or
Fisons' Spinhaler brand powder inhaler. Another suitable unit-dose container
which provides a
CA 02836643 2016-03-15
8
superior moisture barrier is formed from an aluminum foil plastic laminate.
The powder
composition is filled by weight or by volume into the depression in the
formable foil and
hermetically sealed with a covering foil-plastic laminate. Such a container
for use with a powder
inhalation device is described in U.S. Pat. No. 4,778,054 and is used with
GlaxoSmithKline's
Diskhaler (U.S. Pat. Nos. 4,627,432; 4,811,731; and 5,035,237).
In other embodiments, a dry powder composition of the present
disclosure may be delivered to a subject via a tracheal tube.
In some embodiments, compositions of the present disclosure may be prepared by
spray
drying an aqueous mixture of vancomycin or a salt thereof and a
pharmaceutically acceptable
carrier under conditions sufficient to provide a respirable dry powder
composition. In some
embodiments, the dry powder composition is substantially amorphous.
Generally speaking, spray drying is a process in which a homogeneous aqueous
mixture
of vancomycin or a salt thereof and the carrier is introduced via a nozzle
(e.g., a two fluid
nozzle), spinning disc or an equivalent device into a hot gas stream to
atomize the solution
thereby forming fine droplets which subsequently mix into a hot gas stream.
The aqueous
mixture may be a solution, suspension, slurry, or the like, but should be
homogeneous to ensure
uniform distribution of the components in the mixture and ultimately the
powdered composition.
Preferably the aqueous mixture is a solution. In some embodiments, the aqueous
mixture may
have a solids content of at least 1% by weight water. In other embodiments,
the aqueous mixture
may have a solids content of at least 2% by weight water. In other
embodiments, the aqueous
mixture may have a solids content of at least 4% by weight water. The solvent,
generally water,
rapidly evaporates from the droplets producing a fine dry powder having
particles 1 to 5 pm in
diameter.
In some embodiments, the spray drying is done under conditions that result in
a
substantially amorphous powder of homogeneous constitution having a particle
size that is
respirable, a low moisture content and characteristics that allow for ready
aerosolization. In some
embodiments, the particle size of the resulting powder is such that more than
about 95% of the
mass is in particles having a diameter of about 10 gm or less.
For the spraying process, such spraying methods as rotary atomization,
pressure
atomization and two-fluid atomization can be used. Examples of suitable
devices are disclosed in
U.S. Patent No. 6,372,258.
Alternatively, dry powder compositions may be prepared by other processes such
as
lyophilization and jet milling as disclosed in WO 91/16038.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
9
A number of formulation and processing strategies may be useful to improve,
among
other things, the storage and stability properties of the dry powder
vancomycin compositions of
the present disclosure. In certain embodiments, the vancomycin used may be
chosen to be of a
higher purity. In other embodiments, steps may be taken to avoid oxidation of
vancomycin. For
example, processing and packaging of the composition may be performed under
nitrogen.
Similarly, in some embodiments the processing and packaging may be performed
to minimize
exposure to direct light. Such steps may reduce light-mediated degradation of
vancomycin. In
some embodiments, steps may be taken to reduce the amount of moisture in the
composition.
Such steps may be useful to avoid hydrolysis, deamidation, and oxidation of
vancomycin. As
mentioned above, a carbohydrate bulking agent may be added to the composition,
which also
may improve chemical stability.
Furthermore, while the dry powder vancomycin compositions of the present
disclosure
have many advantageous properties, in certain embodiments, one particularly
advantageous
property is that the compositions may have a phannacokinetic profile that is
favorable for
antibacterial efficacy of a time dependent bactericidal antibiotic, such as
vancomycin, in that it
may provide a prolonged high concentration of vancomycin in the lung, and
thereby increase the
time during which the minimum inhibitory concentrations of target pathogens
are exceeded.
For example, in certain embodiments, upon administration of a dry powder
vancomycin
composition to a subject, the median amount of time necessary for the blood
plasma levels of a
subject to reach their maximum concentration of vancomycin (Tmax) may be
greater than or equal
to about 30 minutes, greater than or equal to about one hour, or less than or
equal to about six
hours. In some embodiments, Tmax may be between about one hour and three
hours. Similarly, in
certain embodiments, the median amount of time necessary for blood plasma
levels of a subject
to decrease to one half of the total maximum concentration of vancomycin
(t172) may be greater
than six hours. In some embodiments, t1/2 may be about eight hours.
In certain embodiments, upon administration of a dry powder vancomycin
composition to
a subject, the mean maximum blood plasma concentration of vancomycin (Cmax)
may be within
the range of about 50% to about 150% of about n x 620 ng/mL, wherein n
represents a factor to
be multiplied and may be a value from 0.01 to 10 and when n =1 the dose is 80
mg.
In one particular embodiment, wherein the vancomycin or the pharmaceutically
acceptable salt thereof is present in an amount of about 80 mg, the dry powder
composition may
provide a mean maximum blood plasma concentration of vancomycin within the
range of about
50% to about 150% of about 620 ng/mL, a mean AUC0_2411 value within the range
of about 50%
to about 150% of about 6,250 nghr/mL, and a median Tim\ value within the range
of about 0.75
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
hours to 3 hours, as measured following a single pulmonary administration. As
would be
recognized by one of skill in the art, for dry powder compositions containing
lower or higher
concentrations of vancomycin than 80 mg, the above ranges may be adjusted
directly
proportionally by the dose. Accordingly, in certain embodiments the present
disclosure also
5 provides compositions wherein the vancomycin or the pharmaceutically
acceptable salt thereof is
present in the dry powder composition in an amount of about n x 80 mg and
wherein the dry
powder composition provides: a mean maximum blood plasma concentration of
vancomycin
(Cmax) within the range of about 50% to about 150% of about n x 620 ng/mL; an
mean AUC0_24h
value within the range of about 50% to about 150% of about n x 6,250 nghr/mL;
and a median
10 Tmax value in the range of about 0.5 hours to about 6 hours, wherein the
maximum blood plasma
concentration of vancomycin, the AUC0_24h value, and the Tmax value are
measured following a
single pulmonary administration of the dry powder composition; wherein n
represents a factor to
be multiplied and may be a value from 0.01 to 10.
In some embodiments, the dry powder vancomycin compositions of the present
disclosure may provide a delivery efficiency of 40% or more. In other
embodiments, the dry
powder vancomycin compositions of the present disclosure may provide a
delivery efficiency of
60% or more. Delivery efficiency (%) is the amount of the dry powder having an
aerodynamic
diameter less than 5 [tm as a percentage of the initial amount of dry powder
in the capsule.
Delivery efficiency is the emitted dose (%) (i.e., the amount of the dry
powder that exits the
inhaler as a percentage of the initial amount of the dry powder present in the
capsule) multiplied
by the fine particle fraction (%) (i.e., respirable amount or the amount of
the dry powder having a
mass median aerodynamic diameter (MMAD) of 5 [im or less as a percentage of
the emitted
dose).
In some embodiments, the dry powder vancomycin compositions of the present
disclosure may provide an absolute bioavailability of 40% or more. Absolute
bioavailability is
calculated as AUCo-inr of vancomycin after the vancomycin composition
administration divided
by AUCo-inr of vancomycin after intravenous administration and adjusted for
dose.
To facilitate a better understanding of the present invention, the following
examples of
certain aspects of some embodiments are given. In no way should the following
examples be
read to limit, or define, the entire scope of the invention.
EXAMPLE 1
Dry powder vancomycin compositions were produced on a Buchi lab scale spray
dryer at
high yield (75-95%) and at two different batch sizes (1 g and 20 g) with no
loss in purity. These
powders exhibited very high delivery efficiencies and consistency across lots.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
11
The full aerodynamic particle size distribution for the 20 g batch is depicted
in Figure 1
showing that the vast majority of the particle size distribution is less than
5 j.tm and that a
significant proportion (approx. 59%) is within the ultra-fine particle
fraction (3 Jim) predictive of
deep lung delivery.
Initial studies have shown that the emitted dose content uniformity of
vancomycin
powders easily meet the FDA's draft guidance specifications for aerosol dose
content uniformity.
This has been one of the greatest challenges facing the pulmonary drug
delivery industry in the
last 10 years. The specifications state that no more than 1 out of 10
actuations should be outside
20% of the label claim with no actuation outside 25% of the label claim.
In some embodiments, the delivery efficiency of dry powder vancomycin
compositions
may be improved through the addition of small amounts of leucine (Figure 2).
The addition of
leucine to vancomycin powders significantly reduces the water content of the
powder and the
mass median aerodynamic diameter. In addition, the addition of leucine results
in powders with
more physiologically acceptable pH values. See Table 1 below for the effect of
leucine
concentration on powder water content, particle size and reconstituted pH.
TABLE 1
Attribute API
5% Leucine 10% Leucine 20% Leucine
(Lot SA008) (Lot SA006) (Lot SA007)
Water content by Karl Fischer (%) n/a 7.9 6.4
5.8
MMAD by AeroSizer (i.tm) n/a 1.82 1.71
1.57
Reconstituted pH 3.0 4.0 4.2
4.4
Protocol for making one example of a dry powder vancomycin composition
Solution Preparation
An example batch formula is included in Table 2. The target mass of leucine is
weighed
into a beaker. The required mass of de-ionized water is added and mixed using
a magnetic stir
bar. Vancomycin hydrochloride is then added and the solution is stirred for
1.0-1.5 hours until
visibly clear. The feedstock solution is prepared immediately before being
spray dried. The
solution concentration is approximately 4% w/w total dissolved solids.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
12
TABLE 2
Batch formula for a nominal 90% vancomycin HC1, 10% leucine formulation (Lot
SA010)
Ingredient Mass (g)
Vancomycin hydrochloride 17.1
Leucine 1.9
Water 475.2
Processing Procedure
A benchtop spray drying system (Buchi model 191) with high efficiency cyclone
is used
to generate and collect the powder. For 19 g batch sizes, the powder is more
conveniently
recovered from the process stream across 4 cyclone powder collection events.
The equilibrium
drying condition is established using de-ionized water. When stable operation
is obtained, the
nozzle input is switched to feedstock solution. The solution is fed to the
dryer until a quarter of
the solution has been utilized and then the nozzle is switched back to water
for approximately 5
minutes to clear the system. The dryer is then shutdown momentarily to allow
for collector
change-out, restarted immediately and lined-out on water prior to resuming
feed solution.
The filled collector is rapidly capped on removal to minimize exposure to room
humidity.
Each collector is then transferred into individual glass sample vials within a
low humidity dry
glove box and the complete set of vials packaged into an aluminum pouch with
desiccant and
heat-sealed.
Figure 3 depicts a scanning electron microscope (SEM) image of a vancomycin-
leucine
powder formulation from Lot SA010.
Stability of dry powder vancomycin compositions
Results from a 6-month stability study conducted on Lot SA010 show that the
aerosol
particle size distribution does not change with time (Figure 4). Similarly,
the emitted dose does
not change and remains within FDA draft guidance limits of 20% of the mean
(Figure 5). The
chemical stability from this initial study predicted a composition that would
require refrigerated
storage.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
13
EXAMPLE 2
As previously mentioned, in some embodiments, dry powder vancomycin
compositions
of the present disclosure may be prepared using spray drying methods. Such
methods have
proven to be very efficient and exhibit excellent batch to batch consistency.
Table 3 below shows yield, primary particle size and water content data for
batch sizes
ranging from 25 g to 100 g, which were prepared using spray drying methods.
X10, X50 and X90
are the mass diameter of particles to which 10%, 50% and 90% of the
distribution is smaller,
respectively. ND = Not determined.
Additionally, the full aerodynamic particle size distribution for Lot G-11-26-
1 is included
in Figure 6 and shows that the vast majority of the particle size
distribution is less than 5 pin
(85 %) and that a significant proportion (approx. 70%) is within the ultra-
fine particle fraction
(<3 tun) predictive of deep lung delivery.
TABLE 3
Lot # Lot Size Yield X10 X50 X90
Water
(g) (0/0) (11m) (11m) (11m)
Content
(%)
G-11-18 25 86 0.51 1.20 2.55 5.77
G-11-19 25 89 0.54 1.25 2.49 5.10
D-11-039 50 86 0.47 1.33 2.53 3.99
D-11-040 100 92 0.46 1.34 2.47 3.78
D-11-041 50 ND 0.47 1.31 2.46 4.10
D-11-042 50 90 0.48 1.31 2.47 3.97
D-11-043 100 ND 0.49 1.32 2.50 3.72
D-11-044 75 92 0.52 1.42 3.61 3.90
D-11-045 75 91 0.49 1.33 2.87 4.34
D-12-001 50 87 0.44 1.24 2.59 4.39
D-12-002 50 84 0.42 1.25 2.59 4.81
D-12-003 50 87 0.44 1.26 2.55 4.35
D-12-004 75 88 0.46 1.27 2.57 4.55
D-12-006 25 91 0.45 1.28 2.63 4.70
Figure 7 shows that the emitted dose content uniformity for Lot G-11-26-1
meets the
FDA's draft guidance
specifications for aerosol dose content uniformity (i.e., no more than 1 out
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
14
of 10 actuations should be outside 20% of the label claim with no actuation
outside 25% of
the label claim).
Stability
A summary of the 6-month stability study for Lot G-11-026-1 is included in
Table 4.
TABLE 4
Attribute Specification Months
0 1 3 6
Appearance White powder Pass Pass Pass
Pass
Assay (mg) 13.3 - 18.0 15.9 15.8 15.9
15.3
Purity (%) > 85.0 95.6 95.5 95.8
95.5
Emitted Dose (mg) 9.1 -13.6 12.0 11.9 12.2
11.9
Fine Particle Dose (mg) 6.3 - 11.7 8.9 8.9 9.2
9.1
Water Content (1)/0) 3.0 - 8.0 4.5 4.6 4.1
4.1
EXAMPLE 3
A carbohydrate bulking agent was included in a vancomycin-leucine formulation.
The
chemical stability of this formulation was studied at 50 C for 4 weeks. Figure
8 shows that the
addition of a carbohydrate bulking agent (e.g., trehalose) can improve the
chemical stability of
dry-powder vancomycin compositions of the present disclosure.
Two lots of a vancomycin composition containing 10 % leucine were compared.
One of
the lots contained no process modifications (Lot SA010) and the other, was
processed under
nitrogen, protected from light, and protected from moisture (Lot G-11-026-1).
As shown in
Figure 9, these modifications significantly retarded the degradation of
vancomycin. Indeed,
extrapolation of the data suggests the composition to be room temperature
stable for at least 2
years.
EXAMPLE 4
The powder production process has been successfully scaled-up from lab to
pilot-scale
equipment. The process can produce up to 1,000 g of powder per day at high
yield (75%) and
with no loss in purity.
Figure 10 depicts a SEM image of particles from a 1,000 g lot (Lot
19SA01.HQ00005)
manufactured on a Niro Mobile Minor "2000" spray dryer.
Table 5 below shows powder testing data from two scaled-up lots. Table 6 shows
the
corresponding final product test results for Lot 19SA01.HQ00002.
CA 02836643 2013-11-18
WO 2012/159103
PCT/US2012/038775
TABLE 5
Lot # Lot Size Yield X10 X50 X90 Water
(g) (%) (Pm) (pm)
Content
(0/0)
19SA01.HQ00002 1 000 75 0.49 1.83 3.92
4.7
19SA01.HQ00005 1 000 75 0.90 1.81 3.52
5.4
TABLE 6
Attribute Specification Results
Appearance White powder Pass
Assay (mg) 13.3 ¨ 18.0 16.5
Purity (%) > 85.0 96.0
Emitted Dose (mg) 9.1 ¨ 13.6 12.4
Fine Particle Dose (mg) 6.3 ¨ 11.7 8.0
Water Content (%) 3.0 ¨ 8.0 4.7
5
EXAMPLE 5
Following a single administration of a dry powder vancomycin composition (Lot
G-11-
062-1) in eighteen healthy volunteers, a slow absorption phase followed by an
elimination phase
were observed. The main pharmacokinetic parameters after single dose
administration of the
10 inhaled vancomycin composition and intravenous vancomycin are shown in
Table 7. The
corresponding mean plasma concentration curves over 24 h are shown in Figure
11. AUCo-t
refers to the area under the plasma concentration-time curve to the last
measurable time point (24
h) calculated by the linear trapezoidal rule. AUC0_f refers to the area under
the concentration-
time curve to infinity. Cmax refers to the maximum blood plasma concentration
of vancomycin,
15 and 'max refers to the amount of time necessary to reach maximum blood
plasma levels of
vancomycin, and tin refers to the elimination half-life associated with the
terminal slope (KO of
the semilogarithmic drug concentration-time curve, calculated as 0.693/Kei.
Table 7
ti12 Tmax Cmax AUC(04) AUC(0-inf)
CA 02836643 2013-11-18
WO 2012/159103 PCT/US2012/038775
16
Dose (h) (h) (n gim I) ( It *ng/m1) (h*ng/rn1)
Inhaled vancomycin
16 mg Mean 8.45 2.08 108.8 1,209.6 1,461.4
SD 2.02 0.80 33.2 237.7 257.2
32 mg Mean 8.65 1.83 231.5 2,379.8 3,051.1
SD 0.53 0.61 89.6 975.4 959.1
80 mg Mean 8.04 1.33 617.8 6,257.9 7,135.7
SD 1.30 0.41 230.0 1506.9 1,457.9
IV vancomycin
250 mg Mean 7.23 0.92 10,028.3 41,027.8 44,356.3
SD 1.13 0.20 1767.7 2,696.0
3,623.4
The median Tn value was more than one hour with all doses, suggesting a slow
absorption of vancomycin from the lungs. There was a slight trend towards a
shorter Tn..), with
increasing doses (16 mg: 2 h (range 1-3 h); 32 mg: 1.5 h (range 1-3 h); 80 mg:
1.25 h (range 1-
2 h). The observed Tma, values were considerably higher than expected based on
previously
published results with inhaled antibiotic powder (TOBI Podhaler prescribing
information, Tmax
of 1 h with all tested doses ranging from 28 mg to 112 mg). The Cm ax of
vancomycin was very
closely dose proportional between 16 mg and 80 mg (R > 0.95). Likewise, very
good dose
linearity was observed in the AUC values between the different inhaled
vancomycin doses (R>
0.95). The absolute bioavailability of vancomycin after the administration of
the vancomycin
composition, based on the results from those subjects within each cohort who
received both the
vancomycin composition and IV infusion, was on average 49% 8% (calculated as
AUC01 of
vancomycin after the vancomycin composition administration divided by AUC0f of
vancomycin after intravenous administration and adjusted for dose).
The t112 with the different vancomycin dose levels was very consistent,
approximately
8 h, and longer than the tin observed following intravenous infusion. The
apparent prolongation
of tin further suggests prolonged pulmonary absorption that continued to feed
vancomycin into
the systemic circulation during the elimination phase of the concentration
curve.
This pharmacokinetic profile is very favorable for antibacterial efficacy of a
time
dependent (and concentration-independent at concentrations exceeding
approximately 1 gimp
bactericidal antibiotic, such as vancomycin, in that it provides a more
prolonged high
CA 02836643 2016-03-15
17
concentration of vancomycin in the lung, and thereby increases the time during
which the
minimum inhibitory concentrations of target pathogens are exceeded.
Therefore, the present invention is well adapted to attain the ends and
advantages
mentioned as well as those that are inherent therein. The particular
embodiments disclosed above
are illustrative only, as the present invention may be modified and practiced
in different but
equivalent manners apparent to those skilled in the art having the benefit of
the teachings herein.
Furthermore, no limitations are intended to the details of construction or
design herein shown,
other than as described in the claims below. It is therefore evident that the
particular illustrative
embodiments disclosed above may be altered or modified. The scope of the
claims should not
be limited by the preferred embodiments or the examples but should be given
the broadest
interpratation consistent with the description as a whole. While compositions
and methods are
described in terms of "comprising," "containing," or "including" various
components or steps,
the compositions and methods can also "consist essentially of' or "consist of'
the various
components and steps. All numbers and ranges disclosed above may vary by some
amount.
Whenever a numerical range with a lower limit and an upper limit is disclosed,
any number and
any included range falling within the range is specifically disclosed. In
particular, every range of
values (of the form, "from about a to about b," or, equivalently, "from
approximately a to b," or,
equivalently, "from approximately a-b") disclosed herein is to be understood
to set forth every
number and range encompassed within the broader range of values. Also, the
terms in the claims
have their plain, ordinary meaning unless otherwise explicitly and clearly
defined by the
patentee. Moreover, the indefinite articles "a" or "an," as used in the
claims, are defined herein to
mean one or more than one of the element that it introduces. If there is any
conflict in the usages
of a word or term in this specification and one or more patent or other
documents cited herein,
be adopted.