Note: Descriptions are shown in the official language in which they were submitted.
WO 2012/170531 PCT/US2e12/941099
HEPATOCYTE BASED INSULIN GENE THERAPY FOR DIABETES
Cross-Reference to Related Application
100011 This application claims priority to U.S. Provisional Application
Serial No.
61/494,134, filed June 7 2011.
Statement Regarding Federally Sponsored Research or Development
[0002] N/A
Background
[0003] This invention relates to treatment of diabetes using hepatocyte-
based
therapy, and specifically to a method of utilizing hepatocyte cells comprising
a genetic
construct that has a coding sequence for a proinsulin expressible in the cells
in response
to glucose levels. The proinsulin synthesized in the cells is further
processed into a
secretable, active insulin.
[0004] Insulin is normally produced in and secreted by the beta cells of
the islets of
Langerhans in the pancreas. Mature insulin is a protein having two pOtypeptide
chains, A
and B. held together by disulfide bonds. The glucose responsive release of
insulin from
the beta cells is a complex event including gene expression, posttransiational
modification and secretion. The initial protein product and insulin precursor
is
preproinsulin. a single polypeptide chain having an N-terminal signal sequence
and an
intervening sequence, the C-peptide. between the B and A chains. The signal
sequence
is cleaved during transport from the rough endoplasmic reticulum to form
proinsulin. The
proinsulin is packaged into secretory granules along with specific enzymes
required for
its processing. ProInsulin folds into a specific three-dimensional structure,
forming
disulfide bonds. Mature insulin results from removal of the C-peptide. In beta
cells, This
function is catalyzed by endopeptidases that recognize the specific amino acid
sequences at the junction of the B chain and the C peptide (B-C junction) and
at the
junction of the C chain and the A peptide (C-A junction). Mature insulin,
stored in
secretory granules, is released in response to elevated blood glucose levels.
The
detailed mechanism of insulin release is not completely understood, but the
process
CA 2836 921 2 0 1 8-0 7-13
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
involves migration to and fusion of the secretory granules with the plasma
membrane
prior to release.
[0005] In normally functioning beta cells, insulin production and release
is affected
by the glycolytic flux. Glucokinase and glucose transporter 2 (GLUT-2) are two
proteins
that are believed to be involved in sensing changes in glucose concentration
in beta
cells. A reduction in GLUT-2, which is involved in glucose transport, is
correlated with
decreased expression of insulin; loss of glucokinase activity causes a rapid
inhibition of
insulin expression.
[0006] Autoimmune destruction of pancreatic beta cells causes insulin-
dependent
diabetes mellitus or Type I diabetes. As a consequence of partial or complete
loss of
beta cells, little or no insulin is secreted by the pancreas. Most cells, with
the exception
of brain cells, require insulin for the uptake of glucose. Inadequate insulin
production
causes reduced glucose uptake and elevated blood glucose levels. Both reduced
glucose uptake and high blood glucose levels are associated with a number of
very
serious health problems. In fact, without proper treatment, diabetes can be
fatal.
[0007] One conventional treatment for diabetes involves periodic
administration of
injectable exogenous insulin. This method has extended the life expectancy of
millions of
people with the disease. However, blood glucose levels must be carefully
monitored to
ensure that the individual receives an appropriate amount of insulin. Too much
insulin
can cause blood glucose levels to drop to dangerously low levels. Too little
insulin will
result in elevated blood glucose levels. Even with careful monitoring of blood
glucose
levels, control of diet, and insulin injections, the health of the vast
majority of individuals
with diabetes is adversely impacted in some way. Replacement of beta cell
function is a
treatment modality that may have certain advantages over insulin
administration,
because insulin would be secreted by cells in response to glucose levels in
the
microenvironment. One way of replacing beta cell function is by pancreas
transplantation,
which has met with some success. However, the supply of donors is quite
limited, and
pancreas transplantation is very costly and too problematic to be made widely
available
to those in need of beta cell function.
[0008] There have been many other proposed alternatives for beta cell
replacement,
including replacing beta cell function with actual beta cells or other insulin-
secreting,
pancreas-derived cell lines (Lacy, et al., Ann. Rev. Med., 37:33, 1986).
Because the
immune system recognizes heterologous cells as foreign, the cells have to be
protected
from immunoactive cells (e.g., T-cells and macrophages mediating cytolytic
processes).
2
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
One approach to protect heterologous cells is physical immunoisolation;
however,
immunoisolation itself poses significant problems.
[0009] U.S. Pat. No. 5,427,940
issued to Newgard discloses another approach to
beta cell replacement. This patent describes an artificial beta cell produced
by
engineering endocrine cells of the At-T-20 ACTH secreting cells. A stably
transfected
cell. At-T-20, is obtained by introducing cDNA encoding human insulin and the
glucose
transporter gene, i.e. the GLUT-2 gene, driven by the constitutive CMV
promoter. The
cell line already expresses the correct isoform of glucokinase required for
glucose
responsive expression of the proinsulin gene. Although the cell line is
responsive to
glucose, it is secretagogue-regulated at concentrations below the normal
physiological
range. Therefore, use of these cells in an animal would likely cause chronic
hypoglycemia; furthermore, these cells are derived from a heterologous source
and bear
antigens foreign to the recipient host.
[0010] U.S. Pat. No. 5,534,404
issued to Laurance et al. discloses another approach
to obtaining a cell line in which insulin production is secretagogue-
regulated.
Subpopulations of beta-TC-6 cells having an increased internal calcium
concentration, a
property associated with insulin secretion, were selected using a cell sorter.
After
successive passages, a subpopulation of cells that produce insulin in response
to
glucose in the physiological range (4-10 mM) was selected, and the cells were
encapsulated for therapeutic use in alginate bounded by a PAN/PVC
permselective
hollow fiber membrane according to the method of Dionne (International Patent
application No. PC1IUS92/03327).
[0011] Valera, et al., FASEB
Journal, 8: 440 (1994) describes transgenic mouse
hepatocytes expressing insulin under the control of the phosphoenol puruvate
carboxy
kinase (PEPCK) promoter. The PEPCK promoter is sensitive to the
glucagon/insulin
ratio and is activated at elevated glucose levels. The PEPCK/ insulin chimeric
gene was
injected into fertilized mouse eggs and offspring were screened for
integration of the
transgene. In transgene positive mice, under conditions of severe islet
destruction by
streptozotocin (SZ), the production and secretion of intact insulin by the
liver
compensated for loss of islet function. Despite these prior art attempts,
there is a
continuing need for alternative methods to conventional insulin therapy for
the treatment
of diabetes.
3
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
Brief Summary of the Invention
[0012] In one embodiment, the present invention is a method for obtaining
glucose-
regulated expression of insulin ex vivo in hepatocyte cells, wherein the
method
comprises delivering a first, second or third genetic vector for glucose-
regulated
synthesis of insulin into an isolated hepatocyte cell
wherein glucose-regulated
expression of insulin occurs. The first vector comprises a promoter enhancer,
1-5
glucose inducible regulatory elements, a liver-specific promoter, a gene
encoding insulin
with modified peptidase sites and an albumin 3'UTR and lacks an HGH intron.
The
second vector comprises an HGH intron, glucose inducible regulatory elements,
a liver-
specific promoter, a gene encoding insulin with modified peptidase sites and
an albumin
3'UTR and lacks a promoter enhancer. The third vector comprises an HGH intron,
glucose inducible regulatory elements, a liver-specific promoter, a gene
encoding insulin
with modified peptidase sites, an albumin 3'UTR and a promoter enhancer.
[0013] In one embodiment, the invention comprises the step of transplanting
the
hepatocytes back into a mammal.
[0014] In one embodiment, the genetic vector is delivered by exposing the
cells to a
virus infective for the cells, wherein the virus comprises the genetic
construct, and
whereby at least a portion of the cells are infected by the virus under
suitable conditions
and at a sufficient multiplicity.
[0015] In one embodiment the mammal is human and the insulin is human
insulin.
[0016] In another embodiment, the invention is a vector, as described
above,
suitable for controlling blood glucose levels.
[0017] In another embodiment, the invention is a method of controlling
blood glucose
levels in a mammal, comprising the steps of treating a mammal with a first,
second or
third vector, as described above.
[0018] In one embodiment of the invention, the mammal's cholesterol level
decreases after treatment.
[0019] In one embodiment of the invention, the mammal's triglyceride level
decreases after treatment.
[0020] The method of claim 10 wherein the mammal is a cat or dog.
[0021] Other embodiments of the present invention are described in the
specification,
claims and drawings.
4
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
Brief Description Of The Drawings
[0022] Figure 1 is a diagram comparing fasting blood glucose versus time in
STZ-
treated diabetic rats treated with various levels of TA-1M.
[0023] Figure 2 is a graph comparing postprandial blood glucose levels
versus time
for TA-1 minicircle DNA treated STZ-treated diabetic rats.
[0024] Figure 3 is a graph comparing fasting body weight versus time in TA-
1
minicircle DNA treated STZ-treated diabetic rats.
[0025] Figure 4 is a diagram of the TA1 expression cassette.
[0026] Figure 5 is a diagram of the TA4 expression cassette.
[0027] Figure 6 is the DNA sequence of the TA1 expression cassette.
[0028] Figure 7 is the DNA sequence of the TA4 expression cassette.
[0029] Figure 8 is a diagram of glucose levels versus time versus insulin
levels
during an intraperitoneal glucose tolerance test of TA1-treated diabetic rats.
[0030] Figure 9 is a bar chart comparing ex vivo insulin production in
hepatocytes
treated with various insulin gene constructs.
[0031] Figure 10 is a diagram of the TA2 expression cassette.
[0032] Figure 11 is the DNA sequence of the TA2 expression cassette.
[0033] Figure 12 is a diagram of blood glucose versus days in rats, fasted
overnight
and treated with TA1 and Ta4 constructs.
[0034] Figure 13 is a diagram of body weight versus days in rats treated
with TAI
gene construct.
[0035] Figure 14 is a graph of body weight versus days on rats treated with
TAI and
TA4 gene construct.
[0036] Figure 15 is a table of human insulin levels in serum of diabetic
rats treated
with plasmid or minicircle DNA.
[0037] Figure 16 is a graph of blood glucose levels versus time for rats
experiencing
a second treatment of TA1 minicircle DNA.
[0038] Figure 17 is a table of glucose-dependent insulin production from
human
stem cells derived from hepatocytes.
[0039] Figure 18 is a graph of body weight versus days for diabetic rats
treated with
TA1M,TA2M and TA3M.
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
Description Of The Invention
In General
[0040] Due to a shortage of
donor pancreata and the limited long-term success of
islet transplants, alternatives for treating Type I diabetes (T1D) are needed.
We have
developed a gene therapy-based glucose regulated hepatic insulin production
therapy
that demonstrates great promise in treating T1D in experimental animals. Our
approach
is to apply insulin gene therapy to autologous native hepatocytes or stem cell-
derived
hepatocytes in an attempt to overcome the two critical shortcomings in
treating T1D,
which are the shortage of donor organs and the need for life-long use of
immunosuppression in transplantation patients.
[0041] As the Examples below
demonstrate, we examined novel DNA constructs for
the ability to improve insulin production. For example, a novel insulin
construct (TA1,
described below) which contains the human growth hormone (HGH) intron, a
translational enhancer, glucose inducible regulatory elements, albumin
promoter, human
insulin with modified peptidase sites, and the albumin 3'-UTR improved insulin
production in cultured hepatocytes and diabetic rats and mice. TA1 resulted in
a ¨25-fold
increase in insulin production from isolated rat hepatocytes compared to our
previously
published insulin construct [Alam & Sollinger, Transplantation. 2002 Dec
27;74(12):1781-7].
[0042] In one aspect, the
present invention is a novel DNA construct designed to
improve insulin production in hepatocytes. Another aspect of the present
invention is the
= creation of hepatocytes with improved insulin production. Another aspect
of the present
invention is a method of relieving the symptoms of Type I diabetes in a
mammalian
patient by modulating the production of insulin.
Constructs of the Present Invention
[0043] The Examples below
demonstrate four insulin constructions containing
various elements. The Examples demonstrate that three constructs (TA1, TA2 and
TA4)
were successful in providing an insulin gene therapy that provides tight
control of insulin
production. Therefore, the present invention encompasses three types of
vector. The
first vector type comprises a transcriptional enhancer, glucose inducible
regulatory
elements, a gene promoter, a translational enhancer, a gene encoding insulin
with
modified peptidase site and an albumin 3' UTR and lacks an HGH intron. The
second
vector type comprises an HGH intron, glucose inducible regulatory elements, a
gene
6
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
promoter, a translational enhancer, a gene encoding insulin with modified
peptidase site
and an albumin 3' UTR and lacks a transcriptional enhancer. The third vector
type
comprises all of the listed elements.
[0044] In another embodiment
of the present invention, the constructs of the present
invention consist essentially of the elements listed above. By "consist
essentially of' we
mean that a vector of the present invention will consist of the element
described above
and possibly other regulatory elements necessary for vector function. For
example,
plasmids and minicircle vectors may include sequences to facilitate the
addition or
removal of functional elements, such as restriction sites, or sequences
necessary for the
replication of the vector itself.
[0045] Applicants note that
SEQ ID NO: 1, SEQ ID NO: 2 and SEQ ID NO: 3 (Figs 6,
7 and 11) are the entire nucleotide sequence of the TA1, TA2 and TA4
expression
constructs, respectively. These listings do not include sequences that
correspond to
minicircle recombination sites, etc. For example, SEQ ID NOs: 1 and 2 do not
include
specific sequences used for recombination that are found within the commercial
minicircle parental plasmid that flank the expression cassettes. Because all
these
sequences are part of commercial plasmids and readily available, the sequences
are not
included in the provided information.
[0046] SEQ ID NO: 1, SEQ ID
NO: 2 and SEQ ID NO:3 include elements that are
necessary for the present invention (for example, the elements listed below)
and linking
and nonessential sequences that are useful for cloning but may be substituted
by many
other sequences with similar functions.
7
CA 02836921 2013-11-20
WO 2012/170531
PCT/US2012/041099
Residue Location in Residue Location in Residue
Location in
SEQ ID NO: 1 (TA1) SEQ ID NO: 2 (TA4) SEQ ID NO:3 (TA2)
Alpha fetoprotein (AFP)
Enhancer 15-265 15-265
Glucose Inducible
Regulatory Elements 279-369 46-136 279-369
Albumin Promoter
Sequence 380-706 147-474 380-707
HGH lntron 475-754 708-987
VEGF Translational
Enhancer 707-870 755-918 988-1151
Gene Encoding Human
Insulin with Modified B-C
and C-A Peptide Junctions
for Furin Compatibility 871-1203 919-1251 1152-1484
Albumin 3' UTR 1204-2077 1252-2125 1485-2358
[0047] More
specifically, the vectors of the present invention comprise the following
elements:
Promoter Enhancer
[0048] By "promoter
enhancer," we preferably mean the alpha-fetoprotein enhancer.
The Examples below disclose the use of the alpha-fetoprotein enhancer. This
element is
designed to enhance transcription of the functionally linked gene sequence
encoding a
protein in liver cells. Alpha-fetoprotein enhancer increases the effectiveness
of albumin
promoter and increases the binding of RNA polymerase complex, thereby
producing
more mRNA, ultimately leading to an increase in protein production. The
endogenous
factors present in liver cells interact with alpha-fetoprotein enhancer region
which
activates the albumin and alpha-fetoprotein promoters during liver development
and in
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
fully developed liver. Because the effect of AFP enhancer is extinguished in
fully
developed liver cells through repression of its activity, the region
associated with
repression is not included in our AFP enhancer sequence, which allows the
enhancer
activity to persist in fully developed liver cells.
[0049] A suitable form of
the AFP enhancer of the present invention is disclosed in
Jin et al., Developmental Biology 336 (2009) 294-300. A specific sequence of
the AFP
enhancer can be found at residues15-265 of SEQ ID NO: 1 (TA1 expression
cassette).
[0050] In another
embodiment of the invention, one would use other promoter
enhancers suitable for use with a liver-specific promoter. Many normal
promoters are
quite large in size and contain multiple regions that modulate transcriptional
activity as
required for the existing physiological needs at a given time. Therefore,
selection of an
appropriate promoter enhancer is context dependent. It must work with the
promoter in
question. If empirical determinations validate functional efficacy of
enhancers from other
promoters, in conjunction with the liver-specific promoter used, appropriate
modifications
in insulin expression cassettes can be made to achieve the desired results.
Currently,
according to the Cold Spring Harbor Laboratory database, there are
approximately 400
known regulatory regions and elements that function in liver cells.
Translational Enhancer
[0051] By "translational
enhancer," we preferably mean the VEGF translational
enhancer. The Examples below disclose the use of the VEGF translational
enhancer.
This element is designed to enhance translation of the functionally linked
protein
encoding sequence. The VEGF translational enhancer acts as a ribosomal entry
site; it
increases the effectiveness of the translation process. Thus, its presence
causes a
larger amount of insulin protein production from a given amount of insulin
mRNA.
[0052] A specific sequence
of the VEGF translational enhancer can be found at
residues 707-870 of SEQ ID NO: 1 (TA1 expression cassette) and residues 755-
918 of
SEQ ID NO: 2 (TA4 expression cassette).
Glucose Inducible Regulatory Elements (GIREs)
[0053] The vector of the
present invention requires 1-5 GIREs, preferably 2-4 GIREs,
most preferably 3 GIRES. One may find the sequence for suitable GIREs at
residues
279-369 in SEQ ID NO: 1 and 46-136 in SEQ ID NO: 2. Suitable GIREs are also
9
WO 2012/170531 PCT/US2012/041099
described below in the Examples and may also be found at US Patents 7,425,443
and
6,933,133,
[0054] As used herein, a
"glucose inducible regulatory element" (GIRE) refers to a
polynucleotide sequence containing at least one pair of perfect CACGTG motifs,
each
member of the pair separated from the other by a sequence of five base pairs.
A
"glucose responsive regulatory module" contains one or more GIREs. In one
example,
the regulatory elements were inserted 5' of the 5' untranslated region of the
human
proinsulin gene and then cloned into an adenovirus vector which was used to
transfect
hepatocytes. As the Examples below demonstrate, the GIREs provide
transcriptional
regulation of insulin mRNA in hepatocytes in response to physiologically
relevant
glucose concentrations.
Promoter Sequence
[0055] The constructs of
the present invention also involve the use of a gene
promoter, preferably an albumin promoter. The albumin promoter is a hepatocyte
(liver)
specific promoter and is used to ensure that production of insulin is
restricted only to
liver cells. Therefore, if some of the insulin gene construct ends up in
organs other than
liver, the construct will not be expressed. Additionally, various components
and
mechanisms necessary to confer glucose responsiveness to insulin expression
using
gene constructs of the present invention are endogenous to liver cells. As
illustrated in
Examples herein, the rat albumin promoter (184 bp), (Heard et at,. Determinant
of rat
albumin promoter tissue specificity analyzed by an improved transient
expression
system. Mol Cell Biol 1987: 7: 2425) was generated by PGR using rat genomic
DNA
template, as described previously (Alam et al., Glucose-regulated insulin
production In
hepatocytes. Transpantation 2002; 74:1781). The use of the rat albumin
promoter
sequence in the example Is provided for illustrative purposes only. Constructs
containing
an albumin promoter from other species, such as humans, are expected to confer
similar
properties to the constructs.
[0056] One may obtain an
albumin promoter by use of primers and PCR
amplification after examination of SEQ ID NOs: 1 and 2. The promoter sequence
is
found at residues 380-706 of SEQ ID NO: 1 and 147-474 of SEQ ID NO: 2 and 380-
707
of SEQ ID NO :3.
[0057] In principle, any
constitutively active liver cell specific promoter capable of
sustained moderate to high level transcription can be substituted for albumin
promoter.
to
CA 2836921 2018-07-13
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
An example of such a promoter is alpha 1-antitrypsin inhibitor (Hafenrichter
DG et al.
Blood 1994; 84, 3394-404). Currently, according to the Cold Spring Harbor
Laboratory
database there are approximately 300 known liver specific promoters.
Gene Encodina Insulin with Modified Peptidase Site
[0058] The vectors of the
present invention comprise a gene encoding insulin,
preferably human insulin or non-human mammalian insulin, with a modified
peptidase
site. The insulin genes of the present invention are also disclosed in SEQ ID
NO: 1 at
residue 871-1203 and SEQ ID NO: 2 at residue 919-1251.
[0059] In one aspect of the
invention, one may wish to treat non-human animals. To
ensure that no immune reaction to insulin occurs when diabetic animals, such
as cats or
dogs, are treated using insulin gene therapy, one would use species-specific
insulin in
minicircle DNA for treating animals. For example, one would use published
sequences of
insulin for cats and dogs (Kwok et al. 1983 J Biol Chem 258 2357-2363) to
generate 3'
and 5 primers to amplify the coding sequence of insulin from cDNA preparations
made
from isolated pancreatic RNA from respective species by standard molecular
biology
techniques. Alternatively, the coding sequence can also be chemically
synthesized.
Similarly one may wish to substitute insulin sequences from other animals when
treating
those animals. These sequences are readily available.
[0060] Human insulin cDNA was
modified at two junctions of proinsulin where
proteolytic processing and maturation of insulin occurs by specific enzymes
residing in
beta cells but absent in liver cells. The modification at the B and C peptide
of human
insulin from KTRR to RTKR and at the C and A peptide from LQKR to RQKR makes
the
two insulin junctions compatible with cleavage specificity of endogenous
protease, furin,
of liver cells. These modifications are described in the following
publications: Simonson
GD. Groskreutz CM, Gorman CM, et al. Synthesis and processing of genetically
modified human proinsulin by rat myoblast primary cultures. Hum Gene Ther
1996; 7:
71.: Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered pro-
insulin
constitutively processed and secreted as mature, active insulin. J Biol Chem
1994; 269:
6241.
[0061] Regarding the use of
non-human insulin, all modifications to the sequence of
preproinsulin will be similar in nature to that described for human insulin,
wherein the
recognition/processing sites for peptidases found in 11-cells (and
neuroendocrine tissue)
will be changed to sites that can be processed by commonly found proteases in
liver
11
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
(such as Turin) and other cells. There are some minor sequence differences in
insulin
from various species but the key point is to retain the authentic sequence of
mature
insulin for the given species after processing.
[0062] The purpose of the
specific mutation is to change the amino acid sequence in
such a way that proteolytic processing is possible by commonly found furin.
There are
multiple codons for several of the amino acids. Theoretically, one can alter
the DNA
sequence by using an alternative codon but still produce the same polypeptide.
[0063] These modifications
have been successfully used by Applicants in a
published report (Alam T, So!linger HW. Glucose-regulated insulin production
in
hepatocytes. Transplantation 2002; 74:1781). An unmodified insulin gene will
produce
unprocessed proinsulin because the specific enzymes necessary for the
maturation by
proteolytic processing are absent in liver cells. Proinsulin has minimal
biological activity
of approximately 100 fold less than the mature insulin.
[0064] One may obtain a
modified insulin gene by use of primers and PCR
amplification with knowledge of the insulin gene in SEQ ID NOs: 1, 2 and 3.
Albumin 3 UTR
[0065] The albumin 3' UTR is
known to contribute to longevity of the albumin mRNA
in hepatocytes. This sequence was obtained from an expression vector plasmid
from
Mirus (pMIR0375) but this sequence can also be amplified by PCR using reverse
transcribed mRNA from liver. The albumin 3' UTR sequence is disclosed in SEQ
ID NO:
1 at 1204-2077 and at SEQ ID NO: 2 at residues 1252-2125.
HGH Intron
[0066] Two of the constructs
of the present invention, TA4 and TA2, comprise the
HGH intron. The HGH intron is known to add to the efficiency of mRNA
processing and
helps in yielding quantitatively more mRNA. There are several other introns,
such as
beta-globin, that serve similar function to a varying degree. However, the HGH
intron is
known to function well and is preferred. The HGH intron may be amplified by
PCR from
the commercially available plasmid pAAV-LacZ [Stratagene, La Jolla, CA]. The
sequence can also be readily amplified by PCR using genomic DNA as the
template.
The sequence of the HGH intron is disclosed at residues 475-754 of SEQ ID NO:
2 and
708-987 of SEQ ID NO:3.
12
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
Minicircle Embodiment
[0067] Optionally, the vector of the present invention is in the
"minicircle DNA"
format. This is a vector that is virtually devoid of all DNA sequences that
are unrelated to
expression of insulin. The original minicircle DNA production vector was
obtained from
the laboratory of Mark Kay, described in the following publications: Chen, ZY,
He, CY,
Ehrhardt, A and Kay, MA (2003). Minicircle DNA vectors devoid of bacterial DNA
result
in persistent and high-level transgene expression in vivo. Mol Tiler 8: 495-
500, and
Chen ZY. He CY, Kay MA (2005) Improved production and purification of
minicircle DNA
vector free of plasmid bacterial sequence and capable of persistent transgene
expression in vivo. Human Gene Ther. 16:126. A newly revised method to easily
produce minicircle DNA was published recently (Kay MA, He C, Chen Z. A robust
system for production of minicircle DNA vectors. 2010. Nature Biotech
28,1287). The
vector and the E. coil needed to produce the minicircle are commercially
available from
System Biosciences (SBI), Mountain View, CA (systembio.com), and are currently
used
by us.
[0068] The present invention of insulin expression constructs conforms to
the
generally accepted and proven placement scheme of various elements in relation
to
each other. Thus, the gene expression constructs of the present invention are
comprised
of AFP enhancer ¨ conditional inducer - promoter - intron 1 - gene - intron 2
¨
termination/5' UTR. In our Examples, the GIREs are the conditional inducers
and there
is a translational enhancer inserted after the HGH intron and before the
modified insulin
gene. After the insulin gene, the second intron for efficient mRNA processing
is from
albumin followed by the 3' UTR of albumin.
[0069] Figs. 4, 5 and 10 disclose preferred placement of elements in gene
constructs of the present invention.
Method of the Present Invention
[0070] In one aspect, the present invention is a method of controlling
blood glucose
levels in a mammalian patient (preferably a human or non-human mammal),
comprising
the steps of treating a mammal with hepatocytes that have been modified with a
first,
second or third vector, as described above. The first vector comprises
promoter
enhancer, glucose inducible regulatory elements, a liver-specific promoter, a
gene
encoding insulin with modified peptidase site and an albumin 3' UTR and lacks
an HGH
intron. The second vector comprises an HGH intron, glucose inducible
regulatory
13
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
elements, a liver-specific promoter, a gene encoding insulin with modified
peptidase site
and the albumin 3' UTR and lacks promoter enhancer. The third vector comprises
an
HGH intron, glucose inducible regulatory elements, a liver-specific promoter,
a gene
encoding insulin with modified peptidase site, the albumin 3' UTR and promoter
enhancer.
[0071] One would observe the
mammal's blood glucose and insulin levels after
vector treatment and note that the mammal's blood glucose or insulin levels
are
controlled and normal.
[0072] To test our insulin
expression constructs ex vivo in hepatocytes, TAI, 1A2
and TA4 insulin constructs were cloned in adenovirus vector, as described
earlier (Alam
T, Sollinger HW. Glucose-regulated insulin production in hepatocytes.
Transplantation
2002; 74:1781.) Freshly isolated normal rat hepatocytes were plated on
collagen coated
cell culture plates and transfected with adenovirus containing the insulin
gene construct.
These cells were then exposed to low (3.5mM), normal (5.6mM) and high (27.5mM)
concentrations of glucose. Aliquots of medium were drawn at various time
intervals and
insulin present in the culture medium was quantitated by ELISA. Results showed
that
hepatocytes transfected with each insulin construct produced insulin in a
glucose
concentration dependent manner (Fig. 9). At the high concentration of glucose,
the
amount of insulin production was 4-10x higher than at the low concentration of
glucose.
[0073] By "controlled," we
mean that the method of the present invention is
preferably characterized by tight control of glucose regulation. The tight
control refers to
the empirical observation of glucose regulation itself. In non-diabetic
individuals, the
blood glucose returns to normal at 2hr post meal. Before the present
invention, one
would have anticipated that following the correction of hyperglycemia in a
mammal in
response to elevation in blood glucose levels, the preformed insulin mRNA
would remain
for a while and continue producing insulin. Depending on how long such a
condition
persists, one would expect that the mammal would then become hypoglycemic.
However, our results showed that the insulin levels in serum increased soon
after the
increase in blood glucose levels, as we had anticipated, but the insulin
levels did not
stay high for too long and followed the blood glucose level curve with a delay
of about
15-30min (See Fig. 8).
[0074] Typically, the present
invention provides that insulin levels will stay within
0.5uU-100uU/ml. (This is comparable to the maximum amount of insulin that is
released
from normal islets under hyperglycemic challenge of approximately 100uU/m1.).
14
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
[0075] Typically, blood
glucose concentration will stay within 80-150mg/dI after
treatment. The high end relates to a temporary rise soon after having a meal.
If glucose
concentration does rise above 150, the level does not stay at that level for
beyond a
short period (30-60min).
[0076] Poorly controlled
diabetes causes hyperlipidemia and the severity of
hyperlipidemia is dependent on the degree of hyperglycemia. Liver function
tests are
performed for two reasons. Severe diabetes is associated with a degree of
systemic
inflammatory responses, including elevation in serum levels of some liver
enzymes. Our
data provide evidence that following insulin gene therapy, a correction in
serum levels of
liver enzymes is apparent. Secondly, the hydrodynamic deliver procedure is
known to
cause a transient stress to liver but this damage is short-lived. Our data
support these
findings and assert that there is no long-term risk associated with gene-
therapy in the
context of liver function. In fact, the therapy normalizes the liver function,
as evidenced
by the albumin production.
[0077] In one embodiment of
the present invention, the lipid and/or liver enzyme
profile of the treated animal is corrected. In one embodiment of the present
invention,
the animal will have a lipid/enzymes panel wherein the plasma lipid or liver
enzymes
concentration is equivalent to or less than a normal control. By "normal
control" we mean
an animal who is not diabetic. By "lipid/enzymes panel" we mean either a
plasma
triglycerides measurement, an alanine transaminase( AST), an aspartate
transaminase
(ALT) or plasma albumin measurement. In another embodiment of the invention,
we
would expect to see a drop in cholesterol (mg/di) of at least 20% compared to
a diabetic
control. Based on a conservative estimate, approximately 1 week may be
sufficient for a
substantial correction or normalization of hyperlipidemia.
[0078] The method of the
present invention involves the treatment of a mammal,
preferably a human patient or non-human mammal, with the vectors of the
present
invention.
[0079] Introduction of our
insulin expression constructs into liver cells can be
achieved either in vivo or ex vivo. In the first method, the gene construct
will be
introduced either using a minicircle DNA by hydrodynamic method described
herein,
injecting the condensed minicircle DNA nanoparticles as such, or after coating
nanoparticles with compounds that are known to target liver cells.
Alternatively, liver
cells will be harvested from the patient through a biopsy, expanded in cell
culture, and
CA 02836921 2013-11-20
WO 2012/170531 PCT/US20121041099
transfected with a construct of the present invention using minicircle or safe
viral vectors
such as adeno-associated virus (AAV) (already used in many clinical trials).
[0080] The transfected cells will be tested for their ability to produce
insulin and
modulate the quantity of production of insulin in response to changes in
concentrations
of glucose. Appropriate number of cells to provide necessary amount of insulin
(assisted
with information from ex vivo measurements) will be transplanted into the
liver of the
mammal via radiological and ultrasound guidance. The ex vivo method allows for
lower
vector load as well as for targeted delivery of gene.
[0081] Incorporating scaffold/matrix-attachment regions (S/MARs) that serve
as
constitutively active anchors for the nuclear scaffold (Wong SP et at. Gene
Ther.
2011;18(1):82-7; Heng HH et al. J Cell Sci. 2004;117(Pt 7):999-1008; Lufino MM
et al.,
Nucleic Acids Res. 2007;35(15):e98. PMCID: 1976449) will likely increase the
survival of
TA1minicircle (TA1m)-containing S/MARs (TA1m-S/MAR) or other minicircles due
to the
association of SARs with the nuclear scaffold and may also reduce gene
silencing
(Wong SP et al. Gene Ther. 2011:18(1):82-7).
[0082] The Examples below describe the delivery of DNA into rat liver by
hydrodynamic procedures (Zhang G. et al., Methods Mol Biel 245:251-264, 2004;
Zhang
G. et at.. Hum Gene Ther 8:1763-1772, 1997). The delivery of the DNA into
mammalian
liver will typically not be by the hydrodynamic method described in the
Examples. Other
alternatives, such as nano-particles of condensed DNA that do not require a
large
volume and high pressure, will be employed. In a preferred embodiment,
suitable
glucose regulation will last at least 2-4 weeks after treatment with the
constructs.
[0083] Hydrodynamic venous delivery of naked plasmid DNA has led to
successful
gene uptake and transduction of liver cells (Sebestyen MG et at. Hum Gene
Ther.
2007;18(3):269-85. PMCID: 2268901; Wooddell CI et al. J Gene Med.
2008;10(5):551-
63; Wooddell Cl et al. Hum Gene Ther. 2011;22(7):889-903. PMCID: 3135275).
This
method relies on a rapid, high-volume intravenous injection (-10% of body
weight).
However, with hydrodynamic venous delivery, much of the DNA is absorbed in the
venous system, particularly the lungs, prior to its delivery to the liver. As
an alternative,
one may inject the vectors into the arterial system, such as the femoral
artery, which has
a systolic blood pressure of approximately 120 mm/Hg. The remaining vector
will reach
the liver via the portal vein at 10-12 cm.H20. (Blood pressure is usually
presented in
mm of Mercury (mm/Hg). Venous blood pressure is lower and sometimes a unit
based
16
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
on cm of water (cm/H20) is used.) Overall, intra-arterial injection should
greatly enhance
TA1m constructs uptake compared to hydrodynamic venous delivery.
[0084] Injection into the
femoral artery should dramatically increase vector liver
uptake and transduction of hepatocytes. We expect that treated animals will
maintain
normoglycemia for more than 1 month, even when fed ad libitum.
Examples
Example 1: Generation Of Constructs
[0085] Human insulin based
gene constructs containing various elements to
modulate expression were generated with the aim of producing biologically
active insulin
in response to changes in glucose levels.
Four insulin constructs designated TA1, TA2, TA3, and TA4 contain various
elements in
order shown below:
AFP-Enhancer 3GIREs Mb-Promoter HGH-
intron Human Insulin Alb 3'-UTR
TAI
TA2
TA3
TA4
AFP-Enhancer: Alphafetoprotein enhancer was used from Mirus vector pMIR0375.
3GIREs: 3 units of glucose inducible regulatory elements are connected in
tandem; the
sequences are based on S14. S14 is a glucose responsive transcriptional
enhancer. The
elements responsible for glucose-dependent transcriptional enhancement have
been
identified in published work: Shih H. Towle HC. Definition of the carbohydrate
response
element of the rat S14 gene: Context of the CACGTG motif determines the
specificity of
carbohydrate regulation. J Biol Chem 1994; 269: 9380.
Alb-Promoter: Albumin promoter (Albumin promoter was amplified by PCR from rat
genomic DNA, as described in US Patent 6,352,857, US Patent 6,933,133, US
Patent
7,425,443 and the following publication: Alam T, Sollinger HW. Glucose-
regulated insulin
production in hepatocytes. Transplantation 2002; 74:1781)
HGH-intron: Human growth hormone intron was amplified by PCR from a
commercially
available plasmid pAAV-LacZ (Stratagene).
17
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
Human Insulin: Human insulin cDNA sequence was modified at B-C and C-A
junction
for furin cleavage compatibility so that liver cells are able to process
preproinsulin to
functional insulin.
Alb 3'-UTR: Albumin 3'-untranslated region was used from Mirus vector
pMIR0375. It
can also be amplified by PCR using reverse transcribed mRNA from liver.
[0086] Insulin expression constructs described above were incorporated into
replication-defective adenovirus for transient expression for initial testing
purposes.
Insulin expression in rat hepatocytes, ex vivo, was glucose responsive and
each
construct yielded significantly higher amount of insulin (4-12 fold
improvement over the
previously described constructs), Alam & So!linger, Transplantation. 2002 Dec
27;74(12):1781-7.
[0087] TA2 and TA3 were also tested in ex vivo insulin production. (See
Fig. 9).
[0088] Initially we incorporated TA1 in an adenovirus vector to test its
ability to
control hyperglycemia in rats that were rendered diabetic by streptozotocin
(STZ)
treatment. Results showed that such a treatment fully corrected fasting
hyperglycemia,
restored the weight loss caused by diabetes to normal rate of weight gain and
significantly reduced postprandial hyperglycemia.
[0089] The adenovirus vectors containing TA1-TA4 were tested individually
for their
ability to correct diabetic hyperglycemia in STZ-rats. Results showed a full
correction of
fasting hyperglycemia and a partial correction of postprandial hyperglycemia.
The benefit
of a single gene therapy treatment on overall metabolism and preventing body
weight
loss lasted well beyond the time of full correction of fasting hyperglycemia.
During the
period of full correction of fasting hyperglycemia, the rate of weight gain in
diabetic rats
treated with our insulin gene constructs was indistinguishable from the normal
controls.
[0090] To improve efficacy of gene therapy through better gene expression
by
increasing levels and duration of insulin expression, minicircles of DNA
containing only
the gene expression constructs were produced. All of the above insulin gene
expression
constructs were cloned into a plasmid vector p24)C31 as previously described
(Chen et
al., 2003, Mol Ther, 8, 495-500; Chen et al., 2005, Human Gene Ther, 16, 126-
131).
This published method of Chen et al. was substantially modified to improve
purity of
minicircle DNA as described below.
[0091] Encouraged by results from transient expression afforded by
adenovirus
vector, we generated a plasmid vector, known as "minicircle DNA," that is
virtually
18
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
devoid of all DNA sequences that are unrelated to expression of insulin. TA1
minicircle
was introduced into the livers of rats via an established hydrodynamic
procedure (Zhang
G. et al., Methods Mol Biol 245:251-264, 2004; Zhang G. et al., Hum Gene Ther
8:1763-
1772, 1997). Results obtained from STZ-diabetic rats treated with TAI
minicircle DNA
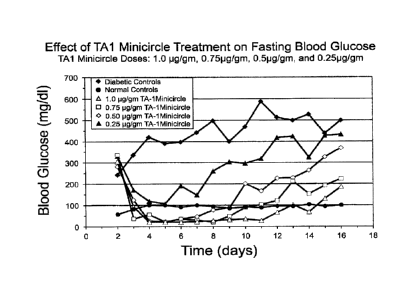
show a full correction of hyperglycemia among ad lib fed animals (Fig. 1) in
addition to
restoration of rate of weight gain to normal (Fig. 2) and correction of
fasting
hyperglycemia.
[0092] The TAI-minicircle-treated diabetic rats were subjected to a glucose
tolerance test by intraperitoneal injections of 4gm/kg glucose. Results from
these
experiments bore a marked similarity to observations from normal control rats.
The peak
of elevated blood glucose levels appeared at 15min post injection and
hyperglycemia
dissipated in about 60 min (Fig. 8). The time to correct hyperglycemia induced
by
4gm/kg glucose IP injection is similar to normal animals. An increased insulin
output in
response to elevated levels of glucose closely follows the rise in glucose
levels and
insulin production declines as the level of glucose progressively reduces.
[0093] To confirm that insulin production was glucose-dependent, we
measured
human insulin levels in plasma at 30min time intervals and found that the
human insulin
levels peaked at 30 min and declined relatively quickly, essentially following
the blood
glucose profile with a 15 min delay. Thus, there was an approximately 15 min
lag
between the profiles of blood glucose and insulin levels.
[0094] Given the nature of glucose-induced transcription of insulin mRNA
that gives
rise to circulating insulin, after achieving euglycemia, continued presence of
insulin
mRNA could have caused a sustained secretion of high levels of insulin until
the mRNA
was degraded. Reduction in insulin levels in only - 60min to the levels
observed in
fasting animals prior to glucose injections, is an unexpected, albeit very
desirable, result.
[0095] Modifications to the published minicircle DNA production method were
useful
and necessary to obtain pure minicircle DNA that was free of detectable
unprocessed or
partially processed minicircle DNA. These modifications involved elimination
of a 2hr
incubation step, claimed to be necessary for in vivo digestion of the DNA
circle that
consists of the unneeded sequences from the parental plasmid that were
eliminated
from the minicircle containing the gene of interest. In practice, this step
was only partially
effective.
[0096] In our procedure, elimination of this 2 hr incubation step caused no
perceptible change in quality or quantity of recovered DNA and the final
product was
19
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
comprised of a mixture of minicircle DNA and the parental unprocessed plasmid
DNA as
well as partially processed plasmid DNA. The mixture of DNA thus produced was
treated,
ex vivo, with a restriction enzyme that could cut the parental plasmid but not
the
minicircle DNA containing our insulin gene constructs. The product of this
reaction was
purified by CsCI equilibrium density gradient to separate the circular DNA
from linear
DNA.
[0097] The TA1 insulin minicircle DNA was tested for its ability to correct
diabetic
hyperglycemia in STZ-treated diabetic rats. Groups of rats were rendered
diabetic by
intravenous streptozotocin injections (100mg/kg). The TAI insulin minicircle
DNA was
injected via tail vein into diabetic rats according to a previously published
method
(hydrodynamic delivery method described by J. Wolff group, Zhang G. et al.,
Methods
Mol Blot 245:251-264, 2004; Zhang G. at al., Hum Gene Ther 8:1763-1772, 1997).
Four
groups of diabetic rats were injected with indicated amounts of TA1 minicircle
DNA (1.0
pg, 0.75 pg, 0.5 pg, and .025 pg per gm body weight). Results are shown in
Figs. 1, 2,
and 3.
[0098] This is the first time we have been able to fully correct blood
glucose levels in
diabetic rats fed ad libitum (Fig. 2) by insulin gene therapy. This treatment
fully restored
rate of weight gain in diabetic rats (Fig. 3).
Example 2: Creation Of Adenovirus Constructs
[0099] Referring to Fig 12, insulin constructs TAI and TA4 were created in
adenovirus and equal plaque forming units injected into rat livers, as
indicated. TAI and
TA4 were used under identical conditions. Both were able to fully correct
fasting
hyperglycemia, as shown in Fig 12. However, the vectors did not fully correct
blood
glucose levels in rats fed ad libitum.
[00100] Referring to Fig 13, TA1 in adenovirus was injected into rat livers
as
indicated. Note that the TAI treated rats regained the weight initially lost
due to diabetes
and maintained a higher weight than the STZ diabetic rats.
[00101] Referring to Fig 14, TAI or TA4 in adenovirus was injected into
livers of
diabetic rat groups, as indicated. Note that both TAI treated and TA4 treated
rats had
maintained higher body weight than STZ diabetic rats. Fig 14 shows a subset of
data
points from Fig 13, representing 0-48 days. The information on rate of weight
gain in
various groups of rats (shown in the inset box on the top left corner) is
derived from data
obtained 24 days post treatment (d8-d31). The rate of weight gain in diabetic
rats treated
CA 02836921 2013-11-20
WO 20121170531 PCT/US2012/041099
with TA1 in adenovirus is equal to normal control rats, whereas the diabetic
untreated
rats experienced a net loss of weight on a daily basis. Fig 13 has many more
data points,
and the early days occupy only a small portion of the area, and therefore the
degree of
correction in weight gain may be somewhat difficult to appreciate. Fig 14
shows this
aspect more clearly.
Example 3: Evaluation Of Human Insulin In Serum Of Diabetic Rats
[00102] Referring to Fig
15, a comparison of human insulin in rat serum shows that
animals treated with TAI minicircle DNA produce larger amounts of insulin
compared to
other plasmid vectors (pTED110, TA1/pENTR). The plasmid pENTR contains an
ampicillin resistance gene which has been replaced by a Kanamycin resistance
gene in
pTED110, and S/MAR has also been added to it; both have the TA1 insulin
construct.
The TAI minicircle containing S/MAR produced the most insulin in vivo. The
Ultrasensitive Human Insulin ELISA (Mercodia, Inc) has a detection limit of
0.15-20
mU/L, as advertised. The table in Fig 15 shows the relative in vivo
effectiveness of
various vectors used for insulin gene therapy. All insulin vectors contained
the TA1
expression cassette. pENTR is a commercial plasmid containing an ampicillin
resistance
gene. The pTED is our modified plasmid where we replaced the ampicillin
resistance
gene with kanamycin resistance gene to increase the in vivo survival of the
vector in
non-dividing cells.
[00103] We also added S/MAR
to pTED110 to increase survival of vector in dividing
cells and to some degree, increase the overall expression. The data are in
agreement
with our vector design expectations.
[00104] Finally, when
extraneous sequences were eliminated and insulin gene
constructs were used as minicircle DNA molecules, the expression levels of
insulin were
significantly increased, more so when S/MAR was included in the minicircle. In
all four
sets of experiments, the molar equivalence of TA1 was maintained at a constant
level.
[00105] The table (Fig 15)
in conjunction with data obtained from adenovirus
mediated transduction ex vivo (Fig 9) and in vivo (Fig. 12 and Fig. 13, Fig.
14. and Fig.
17) provides the proof that the insulin expression cassettes are able to
produce glucose-
dependent insulin, as intended. However, the magnitude and longevity of
insulin
expression does depend on the vector employed to deliver the insulin
expression
cassette(s). We expect that these insulin expression cassettes will be readily
adaptable
21
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
to take advantage of new vectors developed to have better characteristics for
gene
therapy, such as ease of delivery, expression level, and longevity of
expression
Example 4: Second Treatment With TA1M
[00106] Referring to Fig 16, a
second treatment with TA1 minicircle DNA corrects the
gradually elevating fasting blood glucose to normal level.
[00107] Fig 1 discloses data
that are relevant to the information presented in Fig 16.
As the information in two figures comes from different studies, individual
data points are
not identical, but the trend is the same. One should refer to the curve
corresponding to
use of 1.0 pg/gm TAI minicircle DNA in Fig 1 for comparison. Fig 16 shows that
it
appears possible to correct hyperglycemia by a second treatment as the effect
of the
first treatment diminishes.
Example 5: Hepatocytes Derived From Human Stem Cells
[00108] Referring to Fig 17,
hepatocytes were derived from human embryonic stem
(hES) cells and human adult induced pluripotent (iPS) cells as described in Si-
Tayeb et
al 2010. Hepatology 51(1):297-305:. PMCID: 2946078 and were transduced with
TA1 in
adenovirus, ex vivo. Three cell culture plates were kept in medium with low
glucose
(3.5mM), and three plates were kept in high glucose (27.5mM). Freshly prepared
normal
rat hepatocytes were treated similarly. Flasks containing stem cell derived
hepatocytes
were confluent with cells, whereas primary rat hepatocyte plates were ¨60%
confluent.
[00109] Results (Fig. 17) show
a robust production of glucose-dependent insulin in
embryonic and induced pluripotent-cell-derived hepatocytes.
Example 6: Examination Of Rat Weight After Treatment With Minicircle Vectors
[00110] Referring to Figure 18,
groups of diabetic rats (minimum number of rats in a
group = 5) were injected with the indicated insulin minicircle DNA, 1A1, TA2,
or TA3, via
tail veins. Fasting body weights (Mean- S.D) of rats are shown. Normalization
in rate of
weight gain is similar when TA1m or TA2m was used, whereas TA3m was less
effective.
Example 7: Evaluation Of Lipid Profiles
[00111] Two groups of rats were
rendered diabetic by intravenous streptozotocin
treatment. One group of diabetic rats (n=5) was treated with 1pg TA1
minicircle DNA/gm
body weight of animal. The second group of diabetic rats was used as an
untreated
22
CA 02836921 2013-11-20
WO 2012/170531 PCT/US2012/041099
control. A third group of normal rats was included as age matched healthy
controls.
Blood was drawn from each experimental animal after 10 days, and plasma was
analyzed for lipid contents and various markers of liver damage and hepatic
function, as
shown in the table below:
Aspartate Alanine Alkaline Plasma
Animal Triglycerides Cholesterol Transaminase Transaminase Posphatase Albumin
Groups (mg/di) (mg/dl) (U/L) (U/L) (U/L) (g/dl)
TA-1m
Treated 53 34 141 15 302 33 77 40 210 95 3.4
0.2
Diabetic 704 313 191 36 617 349 152 75 423 73 2.5
0.2
Normal
Control 100 14 129 6 504 100 106 16 172 55
3.4 0.1
[00112] TA1m treatment corrected all deficiencies caused by the
uncontrolled
diabetes. Thus, the levels of cholesterol and triglyceride in plasma of
treated rats were
reversed to normal levels. Likewise liver function markers showed an
improvement and
reduced levels of albumin in diabetic rats returned to normal.
23