Note: Descriptions are shown in the official language in which they were submitted.
CA 02837303 2013-11-25
TAPERED JOINT IMPLANT AND RELATED TOOLS
Background
Field
[0002] This application relates generally to anatomical implants, and
more
specifically, to hydrogel joint implants and various tools, devices, systems
and methods
related thereto.
Description of the Related Art
[0003] Implants are often used to replace deteriorated or otherwise
damaged
cartilage within a joint. Such devices can be used to treat osteoarthritis,
rheumatoid arthritis,
other inflammatory diseases, generalized joint pain and/or other joint
diseases. To ensure
proper function and long term effectiveness, such implants should be properly
secured within
a patient's bone or other implant site.
Summary
[0004] A method of treating a joint of a patient is described comprising
creating a
recess, hole or other opening in a bone located at or near a targeted joint,
wherein the recess
comprises a generally wedge, reverse tapered, truncated cone shape and/or
other shape in
which the bottom of the recess comprises a larger diameter or other cross-
sectional dimension
than a top of the recess. The recess or other opening in the bone comprises a
surface opening
along an outer surface of the bone, a bottom opening along the distal end of
the recess and
side walls that generally extend between the surface opening and the bottom
opening,
wherein a diameter or other cross-sectional dimension of the bottom opening is
larger than a
diameter or other cross-sectional dimension of the surface opening.
-1-
CA 2837303 2017-05-23
[0005] The method further involves at least partially radially
compressing a joint
implant having a wedge or truncated cone shape, wherein the joint implant
comprises a first
end and a second end and a body extending between the first end and the second
end. The
second end of the implant is generally opposite of the implant's first end.
When the joint
implant is in a radially uncompressed state, a diameter or other cross-
sectional dimension of
the first end is smaller than a diameter or other cross-sectional dimension of
the second end.
The method further involves inserting the joint implant within the recess,
while the joint
implant is in a radially compressed state, wherein the second end of the joint
implant is
inserted first within the recess. The second end of the joint implant is
adjacent the bottom
opening of the recess, and the first end of the joint implant is adjacent the
surface opening of
the recess when the joint implant is properly positioned within the recess.
The method may
further involves releasing the joint implant from a radially compressed state
to a less
compressed state, when the joint implant is properly positioned within the
recess, wherein,
when the joint implant is in a less compressed state, the diameter or other
cross-sectional
dimension of the second end of the joint implant is larger than the diameter
or other cross-
sectional dimension of the surface opening of the recess. When the joint
implant is in a
radially uncompressed state, the body of the joint implant imparts a radial
force at least
partially along the side walls of the recess, thereby securing the joint
implant within the
recess.
[0006] Creating the recess in a bone involves using a drill bit
comprising an
articulating cutter configured to selectively enlarge the recess near the
bottom opening along
the distal end of the recess. Creating the recess comprises moving a sleeve of
the drill bit so
as to radially expand the articulating cutter outwardly at or near the distal
end of the recess.
In an arrangement, the drill bit is cannulated, wherein the drill bit is
positioned over a guide
pin to place a working end of the drill bit near a targeted location of the
recess.
[0007] The joint implant may be radially compressed and inserted within
the
recess using an introducer. The joint implant may be urged through an interior
of the
introducer using a plunger or other pusher member. In some cases, the joint
implant is urged
through an interior of the introducer using a mechanically-assisted device.
The mechanically-
2
CA 02837303 2013-11-25
assisted device may comprise a handle and a clamp coupled to the handle,
wherein moving
the clamp relative to the handle urges a plunger within an introducer to
radially compress the
joint implant and insert the joint implant within the recess. The clamp may be
rotatably
coupled to the handle. An interior of the introducer may be polished to
further reduce
friction. Movement of the implant through an introducer is facilitated with
the use of a
vacuum source, a pressure source and/or any other pneumatic, mechanical,
electrical and/or
other device.
[0008] The joint implant may comprise a hydrogel, such as, for example,
polyvinyl alcohol (PVA), other polymeric materials and/or the like. A content
of PVA and/or
any other polymeric component of the hydrogel is approximately 20% to 60% by
weight
(e.g., about 20, 25, 30, 35, 40, 45, 50, 55, 60%, values between the foregoing
percentages,
etc.). A content of PVA and/or any other polymeric component of the hydrogel
is less than
approximately 20% or greater than approximately 60% by weight. A ratio of the
diameter or
other cross-sectional dimension of the second end of the joint implant to the
diameter or other
cross-sectional dimension of the first end of the joint implant may be
approximately between
approximately 1.05 and 1.3 (e.g., about 1.05, 1.1, 1.15, 1.2. 1.25, 1.3,
ratios between the
foregoing, etc.). In other arrangement, a ratio of the diameter or other cross-
sectional
dimension of the second end of the joint implant to the diameter or other
cross-sectional
dimension of the first end of the joint implant is less than approximately
1.05 or greater than
approximately 1.3. A ratio of the diameter or other cross-sectional dimension
of the second
end of the joint implant to the diameter or other cross-sectional dimension of
the first end of
the joint implant may be at least about 1.1
[0009] The diameter or other cross-sectional dimension of the second
end of the
implant may be approximately 5% to 25% larger (e.g., about 5, 10, 15, 20, 25%,
values
between the foregoing percentages, etc.) than the diameter or other cross-
sectional dimension
of the implant. The diameter or other cross-sectional dimension of the second
end of the
implant may be less than approximately 5% or greater than approximately 25% of
the
diameter or other cross-sectional dimension of the implant. The recess is
located within or
near at least one of a toe, finger, ankle, knee, shoulder, hip or any other
joint. The top end of
-3-
CA 2837303 2017-05-23
the joint implant may be approximately 5 mm to 20 mm (e.g., about 5, 6, 7, 8,
9, 10, 11, 12,
13, 14, 15, 16, 17, 18, 19, 20 mm, values between the foregoing, etc.) in
diameter or in other
cross-sectional dimension. The top end of the joint implant may be greater
than
approximately 20 mm or smaller than approximately 5 mm (e.g., about 1, 1.5, 2,
2.5, 3, 3.5,
4, 4.5, 4.9 mm, ranges between the foregoing, less than about 1 mm, etc.).
[0010] According to some embodiments, there is described a hydrogel
implant
configured for implantation within a joint of a patient, comprising: a top end
configured to
form an articulation surface when properly implanted within a joint; a bottom
end generally
opposite of the top end; a main hydrogel body extending between the top end
and the bottom
end and having a longitudinal centerline; wherein a diameter or a cross-
sectional dimension
of the bottom end is greater than a diameter or a cross-sectional dimension of
the top end;
and side walls generally extending between the top end and the bottom end,
said side walls
being generally sloped relative to the longitudinal centerline; wherein the
implant comprises a
tapered shape due to, at least in part, to a difference between the diameters
or cross-sectional
dimensions of the top end and the bottom end; and wherein the implant is
configured for
placement within an implant site having a similar reverse tapered shape,
thereby reducing the
likelihood of unintentional removal of the implant from the implant site
following
implantation.
[0011] According to some embodiments, the hydrogel comprises polyvinyl
alcohol (PVA) and/or any other polymeric material. In some embodiments, the
content of
PVA in the hydrogel is approximately 35% to 45% by weight (e.g., about 35, 36,
37, 38, 39,
40, 41, 42, 43, 44, 45%, values between the foregoing, etc.). In other
embodiments, the
content of PVA in the hydrogel is greater than approximately 45% by weight
(e.g., about 45,
50, 55, 60, 65, 70%, greater than about 70%, ranges between the foregoing
values, etc.) or
less than approximately 35% by weight (e.g., 5, 10, 15, 20, 25, 30, 35%,
ranges between the
foregoing values, less than about 5%, etc.). According to one embodiment, the
content of
PVA or other component in the hydrogel is approximately 40% by weight. In some
embodiments, the implant is load bearing and generally non-biodegradable. In
some
embodiments, the implant is configured for placement within at least one of a
toe, finger,
4
CA 2837303 2017-05-23
ankle, knee, shoulder, hip or any other joint. In some embodiments, a
transition between the
top end and the side walls is generally curved or otherwise smooth.
[0012] According to some embodiments, the top end of the implant is
approximately 5 mm to 20 mm in diameter or other cross-section dimension
(e.g., about 5,
10, 15, 20 mm, ranges between the foregoing values, etc.). In other
embodiments, the top
end of the implant is greater than about 20 mm (e.g., 25, 30, 35, 40 mm,
greater than 40 mm,
etc.) or smaller than about 5 mm (e.g., 1, 1.5, 2, 2.5, 3, 3.5, 4.5, 5 mm,
ranges between the
foregoing, less than about 1 mm, etc.). In some embodiments, a diameter of the
bottom end
is approximately 5% to 25% larger than a diameter of the top end (e.g., about
5, 6, 7, 8, 9, 10,
12, 14, 16, 18, 20, 25%, ranges between the foregoing, less than about 5 %,
greater than
about 25%, etc.). In some embodiments, a diameter of the bottom end is
approximately 10%
to 15% larger than a diameter of the top end (e.g., about 10, 11, 12, 13, 14,
15%, ranges
between the foregoing, less than about 10%, greater than about 15%, etc.).
[0013] According to some embodiments, a distance between the top end and
the
bottom end of the implant is approximately 4 mm to 16 mm (e.g., about 4, 5, 6,
7, 8, 9, 10,
11, 12, 13, 14, 15, 16 mm, values between the foregoing, etc.). In other
embodiments, a
distance between the top end and the bottom end of the implant is less than
approximately 4
mm (e.g., less than 1 mm, about 1 mm, about 2 mm, about 3 mm, about 4 mm,
ranges
between the foregoing, etc.) or greater than approximately 16 mm (e.g., about
16, 17, 18, 19,
20, 22, 24, 26, 28, 30, 35, 40, 45, 50 mm, greater than about 50 mm, etc.). In
some
embodiments, a ratio of the diameter or other cross-sectional dimension of the
bottom end of
the implant to the diameter or other cross-sectional dimension of the top end
of the implant is
approximately between 1.05 and 1.3 (e.g., about 1, 1.05, 1.1, 1.15, 1.2, 1.25.
1.3, ranges
between the foregoing, etc.). In some embodiments, a ratio of the diameter or
other cross-
sectional dimension of the bottom end of the implant to the diameter or other
cross-sectional
dimension of the top end of the implant is greater than about 1.3 (e.g., about
1.3, 1.35, 1.4,
1.45, 1.5, 1.6, 1.7, 1.8, 1.9, 2.0, greater than about 2.0, ranges between the
foregoing, etc.). In
some embodiments, a ratio of the diameter or other cross-sectional dimension
of the bottom
end of the implant to the diameter or other cross-sectional dimension of the
top end of the
implant is at least about 1.1.
[0014]
According to some embodiments, there is described a hydrogel implant
configured for implantation within a joint of a subject, comprising: a top
surface configured
to form an articulation surface when properly implanted within a joint; a
bottom surface
generally opposite of the top surface; a main hydrogel body extending between
the top
surface and the bottom surface, the main hydrogel body comprising a
longitudinal centerline;
wherein a diameter or other cross-section dimension of the bottom surface is
greater than a
diameter or other cross-sectional dimension of the top surface; and at least
one side wall
generally extending between the top surface and the bottom surface, wherein
the at least one
side wall is sloped relative to the longitudinal centerline; wherein the
implant comprises a
tapered shape due to, at least in part, to a difference between the diameters
or other cross-
sectional dimensions of the top surface and the bottom surface; and wherein
the implant is
configured for placement within an implant site having a similar reverse
tapered shape,
thereby reducing the likelihood of unintentional removal of the implant from
the implant site
following implantation.
6
CA 2837303 2018-10-01
[00015] There is also described a joint implant system comprising: an implant
as
described above; a mechanically-assisted delivery tool for delivering the
implant within a
wedge-shaped recess of a subject; and an introducer tube and a plunger
configured to move
within an inner lumen of the introducer tube, wherein the implant is
configured to be
positioned within the inner lumen, and wherein the plunger is configured to
move the implant
through the inner lumen; wherein movement of the implant within the inner
lumen of the
introducer tube radially compresses the implant and permits the implant to be
positioned into
the wedge-shaped recess.
[0016]
[0017]
[0018]
7
CA 2837303 2018-10-01
[0019] A
method of treating a joint of a patient is also described which comprises
creating a recess in a bone located at or near a targeted joint, wherein the
recess comprises a
generally wedge, truncated cone or reverse tapered shape. The recess in a bone
comprises a
surface opening along an outer surface of the bone, a bottom opening along the
distal end of
the recess and side walls generally extending between the surface opening and
the bottom
opening, wherein a diameter or other cross-sectional dimension of the bottom
opening is
larger than a diameter or other cross-sectional dimension of the surface
opening. In one
embodiment, the method comprises at least partially radially compressing a
joint implant
having wedge or truncated cone shape, wherein the joint implant includes a
first end and a
second end and body extending between the first end and the second end such
that the second
end is generally opposite of the first end. In some embodiments, when the
joint implant is in
a radially uncompressed state, a diameter or other cross-sectional dimension
of the first end is
smaller than a diameter or other cross-sectional dimension of the second end.
In some
embodiments, while the joint implant is in a radially compressed state, the
method
additionally comprises inserting the joint implant within the recess, wherein
the second end
of the joint implant is inserted first within the recess. In one embodiment,
the second end of
the joint implant is adjacent the bottom opening of the recess, and wherein
the first end of the
joint implant is adjacent the surface opening of the recess when the joint
implant is properly
positioned within the recess. In one embodiment, the method comprises
releasing the joint
implant from a radially compressed state to a less compressed state, when the
joint implant is
properly positioned within the recess. In one embodiment, when the joint
implant is in a less
compressed state, the diameter or other cross-sectional dimension of the
second end of the
joint implant is larger than the diameter or other cross-sectional dimension
of the surface
opening of the recess. In some embodiments, when the joint implant is in a
radially
uncompressed state, the body of the joint implant imparts a radial force at
least partially along
the side walls of the recess, thereby securing the joint implant within the
recess.
8
CA 2837303 2018-10-01
100201
According to some embodiments, creating the recess in a bone comprises
using a drill bit comprising an articulating cutter configured to selectively
enlarge the recess
near the bottom opening along the distal end of the recess. In one embodiment,
creating the
recess comprises moving a sleeve of the drill bit so as to radially expand the
articulating
cutter outwardly at or near the distal end of the recess. In some embodiments,
the drill bit is
cannulated. In one embodiment, the drill bit is positioned over a guide pin or
other guide or
positioning member to place a working end of the drill bit at or near a
targeted location of the
recess. In some embodiments, the joint implant is radially compressed and
inserted within
the recess using an introducer. In some embodiments, the joint implant is
urged through an
interior of the introducer using a plunger or other pusher member. In one
embodiment, the
joint implant comprises a hydrogel. In some embodiments, the hydrogel
comprises polyvinyl
alcohol (PVA). In one embodiment, a content of PVA and/or other component of
the
hydrogel is approximately 20% to 60% by weight. In some embodiments, the water
content
of the hydrogel is approximately 40% to 80% by weight.
9
CA 2837303 2018-10-01
[0021] According to some embodiments, a ratio of the diameter or other
cross-
sectional dimension of the second end of the joint implant to the diameter or
other cross-
sectional dimension of the first end of the joint implant is approximately
between 1.05 and
1.3. In some embodiments, a ratio of the diameter or other cross-sectional
dimension of the
second end of the joint implant to the diameter or other cross-sectional
dimension of the first
end of the joint implant is at least about 1.1. In one embodiment, the
diameter or other cross-
sectional dimension of the second end of the implant is approximately 5% to
25% larger than
the diameter or other cross-sectional dimension of the implant. In some
embodiments, the
recess is located within or near at least one of a toe, finger, ankle, knee,
shoulder, hip or other
joint. In some embodiments, the top end of the joint implant is approximately
5 mm to 20
mm in diameter.
[0022] According to some embodiments, a drill bit configured to be
used with a
bone drill to make a reverse taper or wedge recess within a bone along or near
a joint of a
patient comprises a main body comprising a proximal end and a distal end, such
that the
proximal end of the main body is configured to couple to a driving portion of
a bone drill in
order to selectively rotate said drill bit. According to one embodiment, the
drill bit further
comprises a flange located along the distal end of the main body. In some
embodiments, the
drill bit comprises one or more stationary cutters extending distally from the
flange, wherein
the stationary cutters are configured to create a generally cylindrical
opening within a bone.
The drill bit further comprises at least one articulating cutter extending
distally from the
flange, wherein the articulating cutter is configured to be selectively moved
between a stowed
position and a radially extended position. In one embodiment, the articulating
cutter is
configured to create a reverse taper or wedge shaped recess within a bone when
in the radially
extended position, wherein a diameter of a bottom opening of the recess is
larger than a
diameter of a surface opening of the recess.
CA 2837303 2018-10-01
[0023]
According to some embodiments, the drill bit comprising an articulating
cutter is inserted within a generally cylindrical recess created by a first
bit, such that a reverse
taper recess or wedge shape is created within the generally cylindrical recess
when the
articulating cutter is moved to the radially extended position. In some
embodiments, the
articulating cutter is coupled to the main body using a hinge or other pivot
point. In one
embodiment, the at least one articulating cutter is normally resiliently
biased in the stowed
position. In some embodiments, the drill bit is cannulated, allowing the drill
bit to be placed
over a guide pin or other positioning member in order to accurately position
the drill bit to or
near a targeted portion of a bone (e.g., joint). In one embodiment, the drill
bit further
comprises a sleeve or other movable member configured to be slid or otherwise
moved
relative to the main body, wherein retracting the sleeve or other member
radially causes the
articulating cutter to be moved from the stowed position and the radially
extended position.
11
CA 2837303 2018-10-01
[0024]
[0025]
[0026]
[0027]
[0028]
[0029]
According to some embodiments, a method of treating a joint of a patient
comprises creating a recess in a bone located at or near a targeted joint,
wherein the recess
includes a generally wedge or truncated cone shape. In one embodiment, the
recess in a bone
comprises a surface opening along an outer surface of the bone and a bottom
opening along
the distal end of the recess, such that a diameter of the surface opening is
generally smaller
than a diameter of the bottom opening. The method additionally comprises
providing a joint
implant having a wedge or truncated cone shape, wherein a diameter of a top
end of the joint
implant is generally smaller than a diameter of a bottom end of the joint
implant. The
method further includes inserting the joint implant within the recess so that
the bottom end of
the joint implant is adjacent to the bottom opening of the recess. In some
embodiments, the
diameter of the bottom end of the joint implant is larger than the diameter of
the surface
opening of the recess. In some embodiments, the size of the implant matches or
substantially
matches the size of the recess. In some embodiments, the size of the implant
is larger (e.g.,
nominally, significantly, etc.) than the size of the recess. Accordingly, in
such arrangements,
the implant remains at least partially radially compressed within after
implantation into the
target recess or other implant site. The amount of radial compression in the
implant after
implantation into the recess can vary from approximately 0% to about 20%
(e.g., about 1%,
2%, 3%, 4%, 5%, 10%, 15%, 20%, values between the foregoing percentages,
etc.). In one
embodiment, for example, the compression ratio of an implant is approximately
10%,
wherein the diameter (or other cross sectional dimension) of the recess base
is about 90% of
the base or bottom diameter of the implant.
12
CA 2837303 2018-10-01
[0030]
According to some embodiments, the step of creating a recess in a bone
comprises using a drill bit comprising an articulating cutter configured to
create the generally
wedge or truncated cone shape in the recess. In one embodiment, the joint
implant is inserted
within the recess using an introducer. In some embodiments, the joint implant
is urged
through an interior of the introducer using a plunger or other pusher member
(e.g., manually
13
1,
CA 2837303 2018-10-01
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
or with the assistance of mechanical, hydraulic, pneumatic or other externally
driven device).
In some embodiments, the implant comprises a hydrogel (e.g., PVA). In some
embodiments,
the recess is located within a toe, finger, ankle, knee, shoulder, hip or any
other joint.
Brief Description of the Drawings
[0031] These and other features, aspects and advantages of the present
application
are described with reference to drawings of certain embodiments, which are
intended to
illustrate, but not to limit, the various inventions disclosed herein. It is
to be understood that
the attached drawings are for the purpose of illustrating concepts and
embodiments of the
present application and may not he to scale.
[0032] FIG. 1 schematically illustrates a side view of a tapered implant
according
to one embodiment;
[0033] FIG. 2 schematically illustrates a side view of the implant of
FIG. 1
positioned within a corresponding implant site, according to one embodiment;
[0034] FIG. 3A illustrates a side view of a tapered implant according to
one
embodiment;
[0035] F1G. 3B illustrates a top view of the tapered implant of FIG. 3A;
[0036] FIG. 4 illustrates a top view of an open mold assembly for making
tapered
implants, according to one embodiment;
[0037] FIGS. 5 and 6 illustrate side views of the mold assembly of FIG.
4:
[0038] FIG. 7 illustrates a perspective view of a drill bit configured
for use with a
bone drill, according to one embodiment;
[0039] FIGS. 8A and 8B illustrate side views of the drill bit of FIG. 7;
[0040] FIG. 8C illustrates a distal end view of the drill bit of FIG. 7;
[0041] FIG. 8D illustrates a cross sectional view of the proximal shaft
portion of
the drill bit of FIG. 7;
[0042] FIG. 8E illustrates a detailed side view of the distal working
end of the
drill bit of FIG. 7;
[0043] FIG. 9A illustrates a side view of one embodiment of a drill bit
with an
articulating cutter in a stowed or retracted orientation;
-14-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0044] FIG. 9B a distal end view of the drill bit of FIG. 9A;
[0045] FIG. 9C illustrates a side view the drill bit of FIG. 9A with its
articulating
cutter in an extended or deployed orientation;
[0046] FIG. 9D a distal end view of the drill bit of FIG. 9C;
[0047] FIG. 10A illustrates a side view of one embodiment of a drill bit
with an
articulating cutter in a stowed or retracted orientation;
[0048] FIG. 10B a distal end view of the drill bit of FIG. 10A;
[0049] FIG. 10C illustrates a side view the drill bit of FIG. 10A with
its
articulating cutter in an extended or deployed orientation;
[0050] FIG. 10D a distal end view of the drill bit of FIG. 10C;
[0051] FIG. 11 illustrates a perspective view of an implant introducer
according
to one embodiment;
[0052] FIG. 12A illustrates a side view of the introducer of FIG. 11;
[0053] FIG. 12B illustrates a longitudinal cross-sectional view of the
introducer of
FIG. 11;
[0054] FIG. 13A illustrates a distal end view of the introducer of FIG.
11;
[0055] FIG. 13B illustrates a detailed view along the neck portion of
the
introducer depicted in FIG. 11;
[0056] FIG. 14 illustrates a longitudinal cross-sectional view of
another
embodiment of an implant introducer;
[0057] FIGS. 15A-15C illustrate time-sequential side views of an implant
being
inserted within an implant site using the introducer of FIG. 11;
[0058] FIG. 16A illustrates a perspective view of an assembled implant
delivery
tool according to one embodiment;
[0059] FIG. 16B illustrates an exploded view of the delivery tool of
FIG. 16A;
[0060] FIG. 16C illustrates a cross-sectional view of the delivery tool
of FIG.
16A;
[0061] FIG. 16D illustrates a perspective view of an assembled implant
delivery
tool according to one embodiment;
[0062] FIG. 16E illustrates an exploded view of the delivery tool of
FIG. 16D;
-15-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0063] FIG. 17A illustrates a perspective view of an introducer;
[0064] FIG. 17B illustrates a cross-sectional view of the introducer of
FIG. 17A;
[0065] FIG. 18 illustrates a side view of a plunger;
[0066] FIG 19A illustrates a perspective view of a handle;
[0067] FIG. 19B illustrates a top view of the handle of FIG. 19A;
[0068] FIG. 20A illustrates a side view of a clamp;
[0069] FIG. 20B illustrates another view of the clamp of FIG. 20A; and
[0070] FIGS. 21A-21C illustrate sequential views of an implant being
moved
through and deployed from a delivery tool.
Detailed Description
[0071] The discussion and the figures illustrated and referenced herein
describe
various embodiments of a cartilage implant, as well as various tools, systems
and methods
related thereto. A number of these devices and associated treatment methods
are particularly
well suited to replace deteriorated or otherwise damaged cartilage within a
joint. Such
implants are configured to remain within the patient's joint on a long-term
basis (e.g., for
most or all of the life of the patient), and as such, are configured, in some
embodiments, to
replace native cartilage. Thus, in some embodiments, the implants are
configured to be
substantially non-biodegradable and/or non-erodable. In some embodiments, for
example, an
implant is configured to remain within the patient's joint or other portion of
the anatomy for a
minimum of 20 to 100 years (e.g., about 20, 25, 30, 35, 40, 45, 50, 55, 60,
65, 70, 75, 80, 85,
90, 95, 100 years, durations between the foregoing values, etc.) without
losing its structural
and/or physical properties and/or without losing its ability to function as a
cartilage
replacement component or device. In other embodiments, the implants are
configured to
remain within the anatomy for greater than 100 years without losing its
structural and/or
physical properties and/or without losing its ability to function as a
cartilage replacement
component. Accordingly, such embodiments can be used to treat osteoarthritis,
rheumatoid
arthritis, other inflammatory diseases, generalized joint pain and/or other
joint diseases.
However, the various devices, systems, methods and other features of the
embodiments
-16-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
disclosed herein may be utilized or applied to other types of apparatuses,
systems, procedures
and/or methods, including arrangements that have non-medical benefits or
applications.
[0072] FIG. 1 schematically illustrates one embodiment of an implant 10
intended
for placement within or near a joint of a patient (e.g., toe, finger, ankle,
knee, hip, shoulder,
etc.). As shown, the implant 10 can include a generally tapered overall shape,
wherein its
base surface 14 is larger than the opposite, top surface 16. As discussed in
greater detail
below, the smaller, top surface 16 can comprise the articulation surface
(e.g., a surface that is
at least partially exposed to a joint), whereas the larger bottom or base
surface 14 is securely
retained within a corresponding opening specially created in the anatomy
(e.g., through bone,
cartilage, other native tissue, etc.). As a result of such a design, the sides
18 of the implant 10
can comprise a taper angle U (e.g., relative to generally vertical sides),
thereby giving the
implant a generally truncated cone or frustum-like shape. As discussed in
greater detail
herein, such a reverse-taper, wedge or truncated cone shape can help ensure
proper
securement of the implant 10 within a patient's anatomy.
[0073] FIG. 2 schematically illustrates an implant 10 similar to the one
depicted
in FIG. 1 snugly positioned within a corresponding recessed area R of a
patient's tissue T
(e.g., bone, cartilage, etc.). In some embodiments, such a recessed area R is
formed at or near
the patient's joint so that the implant 10 can be used to replace and/or
augment damaged
cartilage (e.g., on a long-term or permanent basis, as discussed above).
Alternatively,
however, the implant 10 can be positioned generally away from a joint or other
articulation
surface. Thus, any of the implant embodiments disclosed herein, or equivalents
thereof, can
be used in a human or animal anatomy for a variety of different indications or
other purposes,
such as, for example, joint therapy, reconstructive surgery, tissue
augmentation, cosmetic
surgery and/or the like. For any of the embodiments disclosed herein, or
equivalents thereof,
the implant 10 can be load bearing or non-load bearing, as desired or
required. In some
embodiments, once implanted within the anatomy, the implant 10 is configured
to be non-
biodegradable for at least the expected useful life of the implant 10. In some
embodiments,
the implant 10 is adapted to generally retain its general structure, shape,
structure, size,
strength, compressibility, function and/or other properties during the life of
the patient into
which the implant is inserted. For example, the implant 10 can be configured
to generally
-17-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
maintain its original physical, chemical, biocompatibility and/or
characteristics for at least
about 100 years. In some embodiments, the implant retains the same or
substantially the
same water content, resiliency, durability, strength, coefficient of friction
and/or any other
properties for the period of time that it is positioned within the anatomy of
the patient. In
other embodiments, the implant 10 is configured to generally maintain its
original physical,
chemical, biocompatibility and/or characteristics for less or more than about
100 years (e.g.,
about 50 years, 60 years. 70 years, 80 years, 90 years, 110 years, 120 years,
130 years, 150
years, 200 years, more than about 200 years, less than about 50 years, etc.),
as desired or
required. In some embodiments, the implant 10 is configured to resist or
substantially resist
biodegradation or mass reduction during such target time period.
[0074] With continued reference to FIG. 2, during delivery of the
implant 10
within the recess, the implant 10 can be compressed inwardly (e.g., as
schematically depicted
by the arrows 20). At least some methods of delivering such implants within an
appropriately
sized and shaped recess are discussed in greater detail herein. In some
embodiments, once
the implant 10 has been properly positioned within the recess R, the implant
10 is permitted
to expand outwardly, thereby filling in or otherwise encompassing all or
substantially all of
the volume of the recess R. In some embodiments, the diameter or other cross-
sectional
dimension of the base 14 of the implant 10 is greater than the corresponding
diameter or
other cross-sectional dimension of the recess R. This helps prevent the
implant 10 from
moving out of the recess after implantation. The reverse tapered shape of the
implant 10 and
the recess R into which it is placed can help ensure that implant 10 remains
securely within
the recess R following implantation. In some embodiments, the outwardly
directed forces of
the implant 10 in the direction of the adjacent interior surfaces of the
recess R assist in
maintaining the implant 10 within the recess R during use (e.g., after
implantation).
[0075] According to some embodiments, the base (or bottom) 14 and/or the
top
16 of the implant 10 is generally circular. Alternatively, the shape of the
ends 14, 16 can be
different than circular, such as, for example, oval, square, other
rectangular, other polygonal,
irregular and/or the like. Further, once securely implanted in a patient's
anatomy (e.g., within
a recess R), the top 16 of the implant 10 can be generally flush with the
adjacent tissue
surface. However, in other embodiments, the top 16 of the implant 10 extends
above the
-18-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
adjacent tissue T (e.g., as illustrated in FIG. 2) or below the adjacent
tissue T following
implantation. For example, in one embodiment, the top 16 of the implant is
slightly "proud"
or raised relative to the adjacent tissue (e.g., cartilage) in order to
reestablish a desired
contour of the damaged joint surface. In some embodiments, such a raised or
otherwise
protruding configuration can assist in creating a smoother transition between
the exposed
surface of the implant 10 and adjacent native cartilaginous surfaces of a
joint.
[0076] The top and/or bottom surfaces 16, 14 of the implant 10 can be
generally
flat or planar. In other embodiments, the surface 16, 14 can be non-planar
(e.g., curved,
domed, convex, concave, fluted, ridged, etc.), as desired or required. The
shape of the top
and/or bottom surfaces can be selected based on a patient's anatomy, the
location within the
patient's anatomy in which the implant will be placed and/or one or more other
factors or
considerations. For example, the implant can be configured to generally or
specifically match
the slopes, contours and/or other features of the patient's existing
cartilaginous and/or bone
tissue, the recess and/or the like. Accordingly, the function of a
rehabilitated joint or other
targeted anatomical region being treated can be improved.
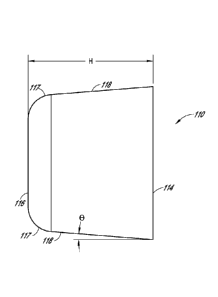
[0077] Another embodiment of a tapered implant 110 configured to replace
or
augment damaged cartilage within a patient is illustrated in FIGS. 3A and 3B.
As shown, the
implant 110 can comprise a bottom or base surface 114 and a top surface 116,
which is at
least partially exposed to adjacent anatomical tissues (e.g., other
cartilaginous surfaces, bone,
other portions that function as an articulating surface of a joint, etc.)
after implantation. As
with the implant of FIGS. 1 and 2, the depicted embodiment includes a base 114
that is
generally wider or otherwise larger than the top surface 116. For example, the
diameter or
other comparable cross-sectional dimension of the base can be larger than that
of the top.
Accordingly, the implant 110 can include generally sloped sides 118 that
terminate in a top
surface 116 of small diameter (or other cross sectional dimension) than that
of the base or
bottom surface 114. The sloped surfaces can be generally flat or curved, as
desired or
required. Further, as shown in FIG. 3A, the transition between the sides 118
and the top 116
can be rounded or otherwise smooth. However, the transition from the side
surfaces 118 to
the top 116 of the implant 110 can be more or less smooth than illustrated in
FIG. 3A. In
other words, in some embodiments, the radius of the curved corners is larger
or smaller than
-19-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
disclosed herein. For example, as schematically illustrated in FIG. 1, an
implant can
comprise generally sharp transitions between the top surface and the sides.
[0078] As discussed herein with reference to FIGS. 1 and 2, the top,
bottom
and/or side surfaces of the implant 110 can be generally planar (e.g., flat)
or non-planar (e.g.,
curved, concave, convex, undulating, fluted, etc.), as desired or required.
Further, although
not illustrated in FIG. 3A, the recess or other opening in which the implant
110 will be
positioned can include a similar reverse-tapered shape (e.g., having a wider
or large base and
a smaller top) to help ensure that the implant 110 remains securely in place
following
implantation. Additional details regarding reverse tapered openings within a
patient's
anatomy (e.g., bone), including details related to tools and methods that help
create such
openings, are provided below.
[0079] With continued reference to FIGS. 3A and 3B, an implant 110 can
include
a generally circular or oval cross-sectional shape. Thus, in some embodiments,
the implant
110 is generally shaped like a frustum, truncated cone, cylinder and/or the
like. However, the
overall shape of any of the implants disclosed herein can vary depending on
the specific
application or use. For example, the shape of the base (or bottom), top and/or
any other
cross-sectional area of an implant can be generally rectangular (e.g.,
square), other polygonal,
irregular and/or the like.
[0080] Regardless of its exact size and shape, the base portion can be
larger or
wider than the top of the implant in order to help ensure that the implant
remains securely
positioned within a targeted portion of a patient's anatomy (e.g., a joint)
following
implantation. For example, in some embodiments, the dimension (or area) of the
base or
bottom of the implant is approximately 10% to 15% (e.g., about 10%, 11%, 12%,
13%, 14%,
15%, ranges between such values, etc.) longer, wider or otherwise larger than
the top of the
implant. Thus, in embodiments having generally circular bottom and top
surfaces, such as,
for example, the implant 110 illustrated in FIGS. 3A and 3B, the diameter of
the base or
bottom 114 is approximately 10% to 15% (e.g., about 10%, 11%, 12%, 13%, 14%,
15%,
ranges between such values, etc.) larger than the diameter of the top 116. In
other
embodiments, the base 114 can be more than about 15% larger or less than about
10% larger
than the top 116, as desired or required. For example, in some embodiments,
the diameter (or
-20-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
other cross-sectional dimension) of the base 114 is larger than the diameter
(or other cross-
sectional diameter) of the top 116 by approximately 1%, 2%, 3%, 4%, 5%, 6%,
7%, 8%, 9%,
less than 1%, other values between the foregoing percentages and/or the like.
Alternatively,
the diameter (or other cross-sectional dimension) of the base 114 is larger
than the diameter
(or other cross-sectional diameter) of the top 116 by approximately 16%, 17%,
18%, 19%,
20%, 25%, 30%, 35%, 40%, 50%, 60%, more than 60% and/or the like. According to
some
embodiments, for any of the implant arrangements disclosed herein, the ratio
of the diameter
(or other cross-sectional dimension) of the base 114 to the diameter (or other
cross-sectional
dimension) of the top 116 of the implant is between about 1 and about 1.3
(e.g.,
approximately or more than 1.05, 1.06, 1.07, 1.08, 1.09, 1.1, 1.11, 1.12,
1.13, 1.14, 1.15,
1.16, 1.17, 1.18, 1.19, 1.2, 1.21, 1.22, 1.23, 1.24, 1.25, 1.26, 1.27, 1.28,
1.29, 1.3, values
between the foregoing ratios, etc.). In other embodiments, the ratio is
between about 1 and
1.05 (e.g., approximately or greater than 1.01, 1.02, 1.03, 1.04, 1.05), or
greater than about
1.3 (e.g., approximately or more than 1.3, 1.35, 1.4, 1.45, 1.5, 1.55, 1.6,
greater than 1.6,
etc.), as desired or required.
[0081] As discussed above with reference to the embodiments illustrated
in FIGS.
1-3B, an implant having a wedge or reverse tapered design (e.g., an implant
having a larger
base than top) can help prevent or reduce the likelihood of unintended
ejection or other
escape from the implant site after implantation. Thus, in some embodiments,
the push-out
force (e.g., the force necessary to eject or otherwise remove the implant from
the implant site)
is advantageously increased for wedge shaped implants relative to implants
that do not
include a wedge or reverse taper design (e.g., cylindrical implants, right
angle implants,
implants having generally vertical sides, etc.). As a result, the likelihood
of maintaining such
embodiments within a joint or other part of the anatomy after implantation is
advantageously
increased.
[0082] With continued reference to FIG. 2, the implant can be positioned
within a
recess or other opening formed within the patient's bone, cartilage or other
tissue. As shown,
in some embodiments, the implant 10 is sized, shaped and otherwise configured
to fill all or
most of the volume of the recess R once properly inserted therein. Further,
according to
some embodiments, the implant is radially oversized relative to the
corresponding implant
-21-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
site (e.g., recess, opening, etc.) into which it will be placed. For example,
an implant can be
radially oversized by approximately 5% to 15% (e.g., about 5%, 6%, 7%, 8%, 9%,
10%,
11%, 12%, 13%, 14%, 15%, other percentages between such values, etc.) relative
to the
implant site. In alternative embodiments, an implant can be radially oversized
by less than
about 5% or more than about 15%, as desired or required. In such oversized
embodiments,
once implanted, the implant can exert a radial or other outwardly directed
force on the
corresponding recess. In some embodiments, such a configuration can help
ensure that the
implant remains securely within the recess after implantation. In yet other
embodiments, the
implant comprises a similar or identical size as the implant site or is
generally radially
undersized relative to the implant site.
[0083] As a result of the shape of the implant and the corresponding
implant site
(e.g., recess, other opening, etc.), it may be necessary to radially compress
the implant (e.g.,
inwardly, as schematically illustrated by the arrows 20 in FIG. 2) in order to
insert the
implant within the implant site. Accordingly, one or more introducers or other
delivery tools
can be used to facilitate the placement of a tapered implant within an implant
site. Additional
inwardly-directed compressive forces on the tapered implant may be required
for implants
that are radially oversized relative to the target implant site, as discussed
above. The degree
to which an implant can be compressed (e.g., circumferentially, radially
inwardly, etc.) may
depend on one or more factors, properties, characteristics and/or other
considerations, such
as, for example, implant size, water content, ingredients and other
components, strength,
elasticity, surrounding temperature, method of manufacturing and/or the like.
[0084] According to some embodiments, radial compression of an implant
can
affect the implant's overall height, the shape or contours of its outer
surfaces (e.g., top or
articulating surface, base or bottom surface, sides, etc.) and/or one or more
other properties or
characteristics of the implant. By way of example, in some embodiments, radial
compression
of an implant causes the height of the implant to increase (e.g., relative to
the height of the
implant when it is not radially compressed). Consequently, careful
consideration may need to
be given to the design of the implant based on, among other things, the
expected level of
radial compression that may occur once the implant has been properly secured
within the
implant site. Therefore, the amount of radial compression, and thus its effect
on the
-22-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
implant's diameter, height, other dimensions, shape and/or other properties,
may need to be
carefully determined prior to implantation. Otherwise, upon implantation, an
implant may
not properly align with adjacent cartilage or other tissue surfaces in a joint
or other
anatomical location.
[0085] According to some embodiments, any of the implant embodiments
disclosed herein comprise polyvinyl alcohol (PVA) hydrogels. The implants can
comprise
one or more other materials, either in addition to or in lieu of PVA, such as,
for example,
other hydrogels, other polymeric materials, other additives and/or the like.
In some
embodiments, the PVA content of a hydrogel is approximately 40% by weight.
However, the
PVA content of an implant can he less or more than about 40% by weight (e.g.,
approximately 10%, 15%, 20%, 25%, 30%, 32%, 34%, 36%, 37%, 38%, 39%, 41%, 42%,
43%, 44%, 46%, 48%, 50%, 55%, 60%, 65%, 70% by weight, less than about 10% by
weight, more than about 70% weight, values between the foregoing ranges,
etc.), as desired
or required.
[0086] Further, the implants can comprise water, saline, other liquids,
combinations thereof and/or the like. In some embodiments, the use of saline
within a
hydrogel implant may be preferred over water, because, under certain
circumstances, saline
can help maintain osmotic balance with surrounding anatomical tissues
following
implantation. The exact composition of an implant (e.g., PVA or other hydrogel
materials,
water, saline or other liquids, other additives, etc.) can be selected so as
to provide the
resulting implant with the desired or required strength, load bearing
capacity, compressibility,
flexibility, longevity, durability, resilience, coefficient of friction and/or
other properties and
characteristics.
[0087] In several embodiments, the implants disclosed herein are
configured for
drug delivery and/or are seeded with growth factors and/or cells. In some
embodiments, the
implants comprise one or more of the following: chondrocytes, growth factors,
bone
morphogenetic proteins, collagen, hyaluronic acid, nucleic acids, and stem
cells. Such
factors and/or any other materials included in the implant and selectively
delivered to the
implant site can help facilitate and promote the long-term fixation of the
implant within the
joint or other target area of the anatomy.
-23-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0088] In some embodiments, the implants disclosed herein are configured
for
anchoring during implantation. The implant can comprise one or more anchor
sites (which
may comprise non-hydrogel portions or tabs) to facilitate anchoring (e.g.,
suturing, stapling,
etc.). In one embodiment, the implant is pre-coupled to one or more anchors.
Such anchors
can comprise removable and/or permanent fixtures. In some embodiments, the
anchors are
resorbable or otherwise dissolvable after implantation (e.g., following a
particular time
period, such as, for instance, 1-30 days, 2-30 weeks, 6-U months, 1-5 years,
greater than 5
years, less than 1 day, etc.). In one embodiment, the implant comprises at
least one abrasive
surface. In one embodiment, the implant comprises one or more adhesive
components. In
other embodiments, the tapered shape of the implant permits secure
implantation without the
need for any anchoring or other fixation. In some embodiments, for any of the
implants
disclosed herein, one or more implant surfaces can be configured to promote
bone adhesion
by one or more coatings, substances and/or the like and/or by using an
appropriate surface
texture along the surface(s). For example, the implant surface can be
roughened, can include
pores (e.g., superficial pores) and/or any other feature, as desired or
required.
[0089] In some embodiments, the implants disclosed herein are supported
or
reinforced by a rigid support frame, such as a ceramic or metallic frame. In
some
embodiments, the implants disclosed herein are supported or reinforced by a
flexible or rigid
mesh structure. In other embodiments, the implants do not contain any support
or
reinforcement structure.
[0090] Any of the implant embodiments disclosed herein, or equivalents
thereof,
can be manufactured using freeze/thaw cycling and/or any other production
method. For
example, a hydrogel formulation comprising water, saline, PVA (and/or other
hydrogel
materials), other polymeric materials, other additives and/or the like can be
heated and/or
otherwise treated as part of a freeze/thaw manufacturing process. In one
embodiment, a
hydrogel solution comprising saline and about 40% PVA by weight is heated to
approximately 121 C under elevated pressure conditions (e.g., to affect
dissolution of the
polymer). For example, such a solution can be autoclaved in order to
facilitate complete or
substantially complete dissolution of the PVA in the saline, water and/or
other liquid. Next,
the temperature and/or pressure of the solution can be lowered to permit
entrapped air and/or
-24-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
other gases to escape. In one embodiment, after the autoclaving or similar
step, the solution
is generally maintained at a temperature of approximately 95 C and
atmospheric pressure for
a predetermined time period.
[0091] The solution can then be transferred (e.g., pumped, poured, etc.)
into open
molds where, once set, will form the desired shape of the implants. One
embodiment of such
an open mold assembly 200 is illustrated in FIGS. 4-6. As shown, the open mold
assembly
200 can include a plurality of individual mold cavities 210, each of which is
configured to
receive a hydrogel solution. With specific reference to the cross sectional
views of FIGS. 5
and 6, in some embodiments, the hydrogel solution is configured to fill only a
lower portion
216 mold's assembly cavities 210. Alternatively, the cavities can be filled
with the desired
hydrogel solution to a level that is above the lower portion 216. Accordingly,
under such
circumstances, the resulting device that is formed therein will extend into
the upper portion
212 of the cavity 210. As described in greater detail below, any part of the
device that
extends above the lower portion 216 can be removed in order to produce an
implant having
generally sloped or contoured side walls and a reverse tapered design, in
accordance with
various implant arrangements disclosed herein.
[0092] With continued reference to FIGS. 4-6, the cavities 210 of the
mold
assembly 200 can be shaped, sized and otherwise configured so that the
implants formed
therein comprise a wedge, truncated cone or reverse taper design. For example,
in such
designs, the base ends of the implants are generally larger than the
corresponding, opposite
top ends. Once the implants have been molded, they can he removed from the
upper ends of
the assembly 200. The molded items can be removed either after initial
formation or after
they undergo additional treatment (e.g., freeze/thaw cycling, other heat
and/or pressure
treatment, etc.). As noted above, depending on how much hydrogel solution is
placed in the
cavities, the molded implants removed from the cavities 210 of the assembly
200 may need
to be cut, altered or otherwise processed. For example, in some embodiments,
any portion of
the implants formed by the generally cylindrical cavity section in the upper
portion 212 of the
cavities may need to be excised and discarded as part of a subsequent
reshaping step.
Accordingly, the remaining implants can generally resemble the shape of the
implant
-25-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
embodiment of FIGS. 3A and 3B or any other implant having a generally reverse
taper or
wedge design.
[0093] Due in part to the remaining production steps, accommodation of
any
changes in size (e.g., expansion, contraction, etc.) that may occur or are
likely to occur to the
implants can be considered during manufacturing by properly sizing and
otherwise designing
the mold assembly 200. The amount of contraction or expansion of the implants
can be
based on one or more factors or conditions, such as, for example, the number
of freeze/thaw
cycles to which the implants arc subjected, the temperature and/or pressure
ranges associated
with the remaining steps and/or the like.
[0094] Alternatively, the implants can be formed, at least in part,
using an
injection molding process and/or any other molding or casting procedure. In
such injection
or transfer molding techniques, once the hydrogel or other implant solution
has been
prepared, it can be loaded into an injection cylinder or other container of a
molding press.
The solution can then be forcibly transferred into a closed mold assembly
using a pneumatic
or hydraulic ram or any other electromechanical device, system or method. In
some
embodiments, the hydrogel and/or other solution or implant component is
injected into a
corresponding closed mold assembly through a standard runner and gate system.
Injection
molding of implants can provide one or more benefits relative to open mold
assemblies. For
instance, the devices formed as part of the injection molding techniques
typically do not
require additional cutting, reshaping, resizing and/or processing, as they are
essentially in
their final shape immediately after the injection molding step has been
completed.
[0095] Regardless of how the implants are molded or otherwise shaped or
manufactured, they can be subsequently subjected to one or more freeze/thaw
cycles, as
desired or required. In some embodiments, for example, the implants, while in
their
respective mold cavities, are cooled using a total of four freeze/thaw cycles
wherein the
temperature is sequentially varied between approximately -20 C and 20 C. In
other
embodiments, however, the number of freeze/thaw cycles, the temperature
fluctuation and/or
other details related to cooling the implants can be different than disclosed
herein, in
accordance with a specific production protocol or implant design.
-26-
CA 02837303 2013-11-25
[0096] Following freeze/thaw cycling, the implants can be removed from
their
respective mold cavities and placed in one or more saline and/or other fluid
(e.g., other
liquid) baths where they can be subjected to additional cooling and/or other
treatment
procedures (e.g., to further stabilize the physical properties of the
implants). According to
some embodiments, for instance, the implants undergo an additional eight
freeze/thaw cycles
while in saline. In other embodiments, such follow-up cooling procedures are
either different
(e.g., more or fewer freeze/thaw cycles, different type of bath, etc.) or
altogether eliminated
from the production process, as desired or required.
[0097] When the cooling (e.g., freeze/thaw cycling) and/or other
treatment steps
have been completed, the implants can be inspected to ensure that they do not
include any
manufacturing flaws or other defects. Further, at least some of the implants
can be subjected
to selective testing to ensure that they comprise the requisite physical and
other
characteristics, in accordance with the original design goals and target
parameters for the
implants. Further, it may be necessary to cut or otherwise process the
implants in order to
remove any excess portions. In some embodiments, the completed implants are
packaged in
hermetically sealed plastic trays (or other containers) comprising foil or
other types of lids or
covering members. A volume of saline and/or other liquid can be included
within such trays
or other containers to ensure proper hydration of the implants during storage
and/or any other
steps preceding actual use. In one embodiment, the implant trays or other
containers are
terminally sterilized using e-beam exposure between about 25 and 40 kGy.
Additional
details related to producing hydrogel implants can be found in U.S. Patent
Nos. 5,981,826
and 6,231,605.
[0098] According to some embodiments, the overall height (e.g., between
the
base or bottom surface and the top or articulating surface) of a tapered
implant is
approximately 10 mm. Further, the diameter or other cross-sectional dimension
along or near
the top surface of the implant can be about 10 mm. However, in other
embodiments, the
height, diameter and/or other dimensions of a wedge-type implant can vary, as
desired or
required. For example, implants adapted for use in larger joints (e.g., knee,
shoulder, hip,
etc.) can have a height and/or diameter larger than 10 mm (e.g., about 11 mm,
12 mm, 13
mm, 14 mm, 15 mm, 16 mm, 18 mm, 20 mm, greater than 20 mm, dimensions between
the
-27-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
foregoing values, etc.). Likewise, implants configured for use in smaller
joints (e.g., toes)
can be smaller than 10 mm in height (e.g., about 2 mm, 4 mm, 6 mm, 8 mm)
and/or 10 mm in
top diameter (e.g., about 2 mm, 4 mm, 6 mm, 8 mm).
[0099] As discussed above with reference to FIGS. 1 and 2, in order to
ensure that
the implant securely remains within a joint or other anatomical location
following
implantation, the implant can be positioned within an implant site that also
comprises a
similar reverse taper, wedge or truncated cone shape. Accordingly, several
embodiments of
making such a tapered recess or other opening within bone tissue arc described
in greater
detail below.
[0100] FIGS. 7-8B illustrate one embodiment of a drill bit 300 that can
be used to
create a reverse taper recess into which an implant may be positioned. As
shown, the drill bit
300 can comprise a main body portion 310 that extends at least partially along
the
longitudinal dimension of the drill bit 300. In the illustrated embodiment,
the proximal end
320 of the drill bit 300 comprises a shaft 322 that is sized, shaped and
otherwise configured
to selectively mate with a corresponding portion of a bone drill (not shown).
In the depicted
embodiment, the shaft 322 comprises a generally triangular cross-sectional
shape, as shown
in FIG. 8D. However, in alternative arrangements, the shape, size and/or other
details of the
shaft can vary. The shaft 322 can include a standard or non-standard
configuration. Bone
drills with which the various drill bit embodiments disclosed herein are used
can be either
manually operated or power driven (e.g., mechanically, pneumatically,
hydraulically, etc.).
[0101] With continued reference to FIGS. 7, 8A and 8B, and as shown in
the
detail view of FIG. 8E, a distal end 330 of the drill bit 300 can include a
flange 340 and one
or more abrading members or cutters 356 extending distally from the flange
340. As best
illustrated in the front view of FIG. 8C, the drill bit 300 can comprise a
total of three cutters
356 that are generally equally spaced apart (e.g., at angles of approximately
1200 relative to
one another). In other embodiments, however, the quantity, size, shape,
position, orientation,
spacing and/or other characteristics or properties of the cutters 356 can be
different than
illustrated herein. For example, in some arrangements, a drill bit can include
more or fewer
cutters (e.g., 1, 2, 4, 5, more than 5), as desired or required. Likewise, the
cutters can be
larger or smaller or can extend along different portions of the distal end of
the drill bit.
-28-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0102] According to some embodiments, a drill bit can be cannulated,
such that
one or more passages or openings 326 extend (e.g., longitudinally) through the
device. For
example, as illustrated in FIGS. 7 and 8A-8E, such a passage 326 can generally
extend from
the proximal end of the drill bit 300 to the distal end, terminating in an
opening 351 along the
distal hub 352 to which the cutters 356 are secured. As discussed in greater
detail below, the
inclusion of such passages or openings 326 can help ensure that the drill bit
is accurately
positioned within a patient's joint or other portion of the anatomy before
commencing a
drilling procedure.
[0103] As the drill hit 300 is rotated (e.g., either manually or using
one or more
external driving sources, etc.), sharp edges formed along the distal and/or
peripheral portions
of the cutters 356 can abrade and remove cartilage, bone and/or other tissue
that they engage
and contact. In some embodiments, the longitudinal distance D1 (FIG. 8A)
between the
distal face 341 of the flange member 340 and the distal ends of the cutters
356 can limit the
depth of the recess or opening that is created within the patient's bone or
other anatomical
area. Likewise, the peripheral surfaces of the cutters 356 can define a
diameter or other
cross-sectional dimension D2 (FIG. 8A) that effectively limits the diameter of
the resulting
recess or other openings in the patient's bone or other targeted tissue. Thus,
each drill bit 300
can be configured to create an implant site having specific dimensions (e.g.,
depth, diameter,
etc.). Consequently, in some arrangements, drill bits of varying size and
shape are available
to the surgeon or other clinician in order to accurately create a specific
desired implant site
within the patient. For any of the embodiments disclosed herein, the distal
edges and/or other
surfaces of the cutting blades or cutters can be generally flat and/or
otherwise contoured (e.g.,
to generally match and/or receive the base of the implant).
[0104] As the drill bit 300 is rotated and advanced into a targeted
region of the
patient's anatomy, abraded bone, cartilage and/or other tissue and/or other
debris will be
created at or near the distal end 330 of the device. Accordingly, in order to
permit such
debris to be removed from the treatment site, the flange 340 can include one
or more
openings 344. Thus, abraded materials can stay clear of and not interfere with
the working
end of the drill bit, allowing the cutters 356 to continue to function
normally. Once the distal
face 341 of the flange 340 abuts the top surface of the bone being drilled,
further
-29-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
advancement of the drill bit 300 can be prevented. This alerts the clinician
that the implant
site having the desired depth and diameter has been properly created.
[0105] With continued reference to the front view of FIG. 8C, the
cutters 356 can
be joined along a hub 352 along or near the center of the distal face 341 of
the flange 340. As
shown, the cutters 356 can extend at least radially outwardly from the central
hub 352,
toward the outer periphery of the flange 340. As noted above, the radial
length of the cutters
356 can help determine the diameter of the recess or opening that will be
created within a
patient's bone or other tissue. In the depicted embodiment, however, because
of the generally
vertical orientation of the peripheral edges 357 of the cutters 356, the
corresponding implant
opening that will be created by the drill bit 300 will he generally
cylindrical. Therefore,
additional implant site preparation is required in order to create an opening
having a reverse
taper shape.
[0106] Accordingly, a drill bit having an articulating cutter or a
movable cutting
arm can be used to create the necessary taper or slope along the side walls of
the recess or
opening in a bone or other targeted region of the anatomy. In some
embodiments, the
articulating cutter is configured to create a curved contour along the bottom
and/or side
surfaces of the recess. For example, such curved surfaces can include one or
more convex
and/or concave portions, as desired or required. One embodiment of a drill bit
400
configured to create such a reverse tapered implant site is illustrated in
FIGS. 9A-9D. Like
the arrangement discussed above with reference to FIG. 7, the depicted drill
bit 400 can
comprise a main body portion 410 that terminates at or near a distal flange
assembly 440.
Further, a proximal end of the drill bit 400 can comprise a shaft 420 that is
sized, shaped and
otherwise configured to engage and mate with a corresponding portion of a
drill (not shown).
In addition, a central hub 452 located along on near the distal face 441 of
the flange 440 can
help secure one or more stationary cutters 456 that are configured to abrade
bone, cartilage
and/or other tissue with which they come in contact. The arrangement
illustrated in FIGS.
9A-9D comprises two stationary cutters 456 that are spaced generally opposite
of each other
(e.g., 180 apart). However, in alternative embodiments, a drill bit comprises
more or fewer
stationary cutters, as desired or required.
-30-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0107] With continued reference to FIGS. 9A and 9B, the drill bit 400
can
additionally comprise one or more articulating cutters or movable cutting arms
460. As
discussed in greater detail herein, such a movable cutter 460 can be
selectively deployed (e.g.,
radially outwardly about a hinge or other pivot point) in order to create a
desired draft angle
along the side of the implant site. In FIGS. 9A and 9B, the articulating
cutter 460 is shown in
the stowed or radially contracted position. Thus, as the drill bit is rotated
and advanced into a
bone, a generally cylindrical bore or opening will be created by the
stationary cutters 456.
Once the drill bit 400 can been advanced sufficiently far into the targeted
bone or other site,
the distal face 441 of the flange 440 will contact and abut an exterior
surface of the bone or
other site being drilled. This can prohibit the continued advancement of the
cutters 456 and
advantageously limit the depth of the resulting implant site.
[0108] According to some embodiments, once the stationary cutters 456 of
the
drill bit 400 have created a generally cylindrical recess or opening within
the patient's
targeted bone or other site and the flange 440 contacts a corresponding
abutting surface, the
surgeon or other clinician can cause the articulating cutter 460 to be
deployed outwardly.
Thus, the desired reverse taper or wedge shape can be created along the sides
of the implant
site. As shown in FIG. 9C, in one embodiment, the articulating cutter 460 is
moved
outwardly by selectively retracting a sleeve 470 in the proximal direction
(e.g., away from the
flange 440 and the distal end of the drill bit, as generally represented in
FIG. 9C by arrow P).
In some embodiments, the sleeve 470 includes a contoured grip portion 472 to
allow the user
to more easily grasp and retract the sleeve 470. Consequently, the
articulating cutter 460 can
be rotated or otherwise moved in a manner generally represented by arrow A.
This permits
the peripheral edge of the articulating cutter 460 to contact and abrade
additional bone,
cartilage and/or other tissue, thereby creating the desired reverse taper or
truncated cone
shape within the recess R (FIG. 2).
[0109] In some embodiments, once released outwardly (e.g., by retraction
of the
sleeve 470), the articulating cutter 460 can assume a fully extended
orientation in order to
create the necessary taper to the adjacent side walls of the implant site.
Thus, a sufficiently
strong biasing or other type of force can be imparted on the articulating
cutter 460 to ensure
that it can reach the targeted fully deployed position. The articulating
cutter 460 can be
-31-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
biased radially outwardly using a spring or other resilient member.
Alternatively, any other
force imparting device or method can be used to ensure that the articulating
cutter 460 fully
extends when selectively deployed by the clinician. Once the necessary taper
along the sides
of the implant site has been created, the sleeve 470 can be returned to its
original orientation
(e.g., closer to the flange 440, as illustrated in FIG. 9A), causing the
articulating cutter 460 to
move to its stowed or radially retracted position.
[0110] According to some embodiments, the sleeve 470 is normally
resiliently
biased in the distal position (e.g., as illustrated in FIG. 9A). Thus, in such
configurations, as
discussed above, a surgeon or other user needs to retract the sleeve 470
proximally relative to
the main body portion 410 of the drill hit 400 in order to deploy the
articulating cutter 460.
In one embodiment, as illustrated in FIG. 10A, the sleeve 570 is normally
maintained in a
distal orientation with the assistance of a spring 516 or other resilient
member. In the
illustrated embodiment, the sleeve 570 includes a body portion 572 and an
enlarged grip
portion 574. A proximal end of the spring 516 can be coupled to a stop nut 512
coupled to or
integrally formed with the main body portion 510. The sleeve 570 contacts the
stop nut 512
to prevent further retraction once the articulating cutter 560 has been
deployed. However,
any other device or method can be used to normally maintain the position of
the sleeve 470,
570 relative to adjacent portions of the drill bit 400, 500 (e.g., the main
body portion 410,
510). For example, in some embodiments, the drill bit is configured to
automatically deploy
the articulating cutter in a outwardly oriented position once the flange of
the drill bit contact
the outer surface of the bone structure or other treatment site (e.g., once
the cylindrical recess
has been made to the desired depth). In such arrangements, the articulating
cutter can he
deployed in its expanded position as a result of automatic mechanical
actuation between the
flange, cutter and/or other portion of the drill bit relative to the implant
site.
[0111] In other embodiments, a reverse tapered recess can be created
using a two
or multi-step process. For example, as part of an initial step, a first drill
bit can be used to
create a generally cylindrical opening within a targeted bone. One embodiment
of a drill bit
that is configured to only create a generally cylindrical opening is
illustrated and discussed
herein with reference to FIGS. 7 and 8A-8C. Then, once the first drill bit has
been removed,
a second drill bit having an articulating arm, such as, for example, the bit
discussed herein
-32-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
with reference to FIGS. 9A-9D and 10A-10D, can be inserted into the generally
cylindrical
opening. By moving the articulating cutter to its extended position,
therefore, the desired
wedge or reverse tapered shape can be created within the recess or implant
site.
[0112] With reference to FIG. 10A, a drill bit 500 can comprise a total
of three
stationary cutters 556 and an articulating cutter 560. However, in other
arrangements, a drill
bit can comprise more or fewer stationary cutters 556 and/or articulating
cutters 560, as
desired or required by a particular application or use. Further, as depicted
in FIG. 10C, the
articulating cutter 560 can attach to the main body 510 and/or any other
portion of the drill bit
500 using a hinge 564 or other pivot point. Thus, as discussed in greater
detail above with
reference to FIGS. 9A-9D, the articulating cutter 560 can he selectively
rotated about such a
hinge 564 between stowed and extended orientations. Accordingly, the drill
bits 400, 500
illustrated in FIGS. 9A-9D and 10A-10D can be advantageously used to create an
implant
site having a desired reverse taper or wedge design. In other embodiments,
drill bits
comprising articulating cutters can be introduced into the implant site after
a generally
cylindrical recess or opening has been created by a drill bit that does not
include an
articulating cutter (e.g., the drill bit shown in FIG. 7), as part of a two-
step procedure.
[0113] According to some embodiments, the drill bit can be advanced to
the
targeted drill site of the patient bone or other anatomical location with the
assistance of a
guide pin. As discussed herein, any one of the drill bit arrangements
disclosed herein can
include a longitudinal lumen or other passage. Thus, a guide pin can be tamped
at least
partially into the surface of the hone to he drilled. The guide pin may be
advanced through
the patient's anatomy using a trocar or similar device. Next, a cannulated
drill bit, as
discussed herein, can be passed over the guide pin to ensure that the distal,
working end of
the drill bit is properly positioned relative to the treatment site (e.g.,
joint).
[0114] Once a reverse taper implant site has been created in the
targeted joint or
other portion of the patient (and, where applicable, the guide pin or other
member has been
removed), a clinician can deliver the implant to the implant site using an
introducer 600. As
illustrated in FIGS. 11-13B, an introducer 600 can include a generally
cylindrical introducer
tube 610 having an opening 620 through which the implant may be passed. In
some
embodiments, the distal end 606 of the introducer tube 610 can comprise a neck
or other
-33-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
narrowed portion 608. As shown in FIG. 13B, the neck portion 608 can include a
wall 612
having a rounded distal edge 613. In some embodiments, the neck portion 608
has a length
(labeled 614 in FIG. 12B) of about 0.155 inches to about 0.170 inches.
Further, as best
illustrated in the longitudinal cross-sectional view of FIG. 12B, the internal
diameter of the
introducer tube 610 can vary along its length. For example, in the depicted
embodiment, a
proximal portion 618 of the introducer 600 comprises a flared shape, wherein
the inside
diameter of the opening 620 is progressively reduced in the proximal to distal
direction.
Further, as shown, the opening 620 can maintain a generally constant inner
diameter along a
second, more distal portion 616 of the introducer tube 610. In other
embodiments, the inner
diameter, length, other dimension and/or other details or properties of the
introducer 600,
including its flared interior portion 628, its generally cylindrical interior
portion 626 of the
introducer tube 610, its neck portion 608 and/or the like can be different
than shown in FIGS.
11, 12A-12B and 13A-13B and described herein. By way of example, the
embodiment
illustrated in FIG. 14 comprises a longer flared interior portion 728 (e.g.,
relative to the
adjacent generally cylindrical portion 726) than the introducer 600 of FIG.
12B.
[0115] The neck portion 608 of the introducer tube 610 can be positioned
at least
partially within the opening or recess into which the implant will be secured.
In some
embodiments, the introducer can be sized, shaped and otherwise configured to
that the neck
portion 608 fits generally snugly within the implant site. With reference to
FIGS. 15A-15C,
an implant 10 can be placed within the opening 628 along the proximal end 602
of the
introducer 600. As shown, in some embodiments, the implant 10 is advanced into
the
interior of the introducer 600 with its base or bottom 14 end first.
[0116] As the implant 10 is urged deeper (e.g., more distally) into the
interior of
the introducer 600, the implant 10 may become radially compressed by the
adjacent interior
walls. If sufficient force is applied to the implant 10, the implant 10 passes
through the neck
portion 608 of the introducer and into the implant site R. As illustrated in
FIG. 15C, in such
an arrangement, the implant's base end 14 will be located along the bottom of
the implant
site. According to some embodiments, a plunger or other pusher member (not
shown) can be
inserted within the interior of the introducer to help push the implant
through the introducer
and into the implant site. Such a plunger or pusher member can be operated
manually and/or
-34-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
with the assistance of an external power-assist device (e.g., mechanically,
pneumatically,
hydraulically, etc.), as desired or required.
[0117] According to some embodiments, once a reverse taper site has been
created in the targeted joint or other portion of the patient (and, where
applicable, the guide
pin or other member has been removed), a clinician can deliver the implant to
the implant site
using a mechanically-assisted delivery tool or introducer 800. One embodiment
of such a
tool is illustrated in FIGS. 16A-16C. Another embodiment of such a tool is
illustrated in
FIGS. 16D-16E. As shown, the delivery tool or introducer 800 can comprise,
among other
things, an introducer tube 810, a plunger 820, a handle 830 and a clamp 840.
[0118] Such mechanically-assisted delivery devices can be helpful in
advancing
the implant through the interior of an introducer tube against a relatively
large resistance of
back-pressure. Such a resistive force can be particularly high when the
implant comprises a
relatively large taper angle 0. Accordingly, in some embodiments, the use of
such delivery
tools makes the delivery of reverse taper implants into corresponding implant
sites possible,
while allowing the clinician to safely and accurately guide the implant into a
targeted
anatomical implant site. In several embodiments, the delivery tool is capable
of overcoming
resistive forces of about 5 to about 20 pounds. In some embodiments, the
delivery tool
exerts a force about 5 to about 25. In some embodiments, the delivery device
is operated by
or with the assistance of one or more motors. For example, in some
embodiments, the clamp
is moved (e.g., rotated) relative to the handle using (or with the assistance
of) one or more
stepper motors and/or any other type of motor or actuator. In some
embodiments, delivery of
an implant through the introducer tube 810 is accomplished with at least some
assistance
from air or pneumatic pressure. For example, air or other fluid can be
injected into the
interior of the introducer tube once the implant is inserted therein. The
delivery of air can be
incorporated into a plunger member 820 (e.g., via one or more interior lumens)
so that the
implant can be advanced through the introducer tube 810 into the implant site
using
mechanical force (e.g., by moving the plunger 820 through the tube 810) and/or
by injecting
air and/or other fluids into the interior of the tube 810. The fluid openings
through the
plunger 820 and/or any other fluid passages can be placed in fluid
communication with a
compressor or other fluid generating device. Advancement of the implant
through the
-35-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
introducer tube 810 can be accomplished by applying a vacuum along or near the
distal end
of the tube 810 (e.g., through one or more vacuum ports along the introducer
tube 810). Such
vacuum ports or openings can be placed in fluid communication with a vacuum or
other
suction generating device.
[0119] According to some embodiments, the delivery tool comprises one or
more
depth stop features or components to ensure that the implant being delivered
to a target
implant site is properly delivered into the target implant site. In some
embodiments, the depth
stop features help protect the structural integrity of the implant as the
implant is being
inserted within the target anatomical implant site.
[0120] In some embodiments, the delivery device comprises and/or is
operatively
coupled to one or more pressure gauges or other pressure or force measuring
devices,
members or features. Such gauges or other measurement devices can help ensure
that a
maximum backpressure or force is not exceeded when operating the device. This
can help
protect the integrity of the implant (e.g., to ensure that the structural
integrity, water
composition and/or other properties of the implant are maintained), protect
the delivery
device, protect the user and/or the patient and/or provide one or more other
advantages or
benefits.
[0121] According to some embodiments, the introducer tube 810 of the
delivery
tool or device 800 comprises one or more viewing windows that permit the
implant to be
viewed as it is being advanced through the device 800 to the implant site. In
some
embodiments, the introducer tube 800 (and thus the longitudinal axis along
which the implant
is advanced through the delivery tool or device) is substantially
perpendicular with the
surface of the bone or other anatomical site into which the implant will be
delivered and/or
the handle 830 of the device 800.
[0122] According to some embodiments, at least a portion of the interior
of the
introducer tube 810 comprises and/or is otherwise coated or lined with one or
more
absorbable or lubricious layers, materials and/or other substances. Such
materials can help
preserve the moisture level of the implant as it is being advanced through the
introducer tube
810. The interior surface of the introducer tube can comprise a low
coefficient of friction to
facilitate the delivery of an implant through the delivery device or tool 800.
In some
-36-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
embodiments, the effective coefficient of friction along the interior of the
introducer tube can
be lowered polishing such surfaces. As noted herein, the introducer, including
its interior
surfaces, can comprise surgical grade stainless steel.
[0123] According to some embodiments, the delivery tool or device 800 is
incorporated into the drill bit configured to create a reverse tapered implant
site. For
example, such a combination device can be coupled to a drill or other
mechanical device to
first create the implant site. Then, the combination device can take advantage
of the
mechanical output generated by the drill and/or other mechanical or motorized
device to help
urge the implant through the introducer tube of the combination device.
[0124] As illustrated in FIGS. 17A and 17B, the introducer tube 810 of
the
mechanically-assisted delivery tool 800 can be hollow and generally
cylindrical in shape.
However, in other embodiments, the shape, general structure and/or other
characteristics of
the tube 810 can be different than disclosed herein. In some embodiments, the
introducer
tube 810 comprises an externally threaded portion 814, a proximal portion 812
extending
between a proximal end 802 and the externally threaded portion 814, and a
distal portion 816
extending between the externally threaded portion 814 and a distal end 804.
The distal end
804 of the introducer 810 can comprise a neck or other narrowed portion 806.
[0125] As best illustrated in the longitudinal cross-sectional view of
FIG. 17B, the
internal diameter of the introducer tube 810 can vary along at least a portion
of the tube's
length. For example, in the depicted embodiment, the proximal portion 812 of
the introducer
or introducer tube 810 has a generally constant, consistent or flat inner
diameter. In addition,
as shown, the distal portion 816 of the introducer tube 810 can comprise a
generally tapered
or sloped portion 816a, such that the inside diameter of the tube is
progressively reduced in
the proximal to distal direction. In some embodiments, the slope along the
interior surface of
the tube 810 can be generally linear. However, in other arrangements, the
slope of the
interior surface of the tube 810 is at least partially non-linear (e.g.,
curved, rounded, irregular,
etc.), either in addition to or in lieu of any generally linear and/or
constant portions, as
desired or required for a particular application or use. Further, in some
embodiments, as
illustrated in the cross-sectional view of FIG. 17B, a portion 816b proximate
the distal end
804 comprises a generally constant or flat (e.g., non-sloped) inner surface or
diameter.
-37-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
Further, in other embodiments, the inner diameter or surface, length, other
dimensions and/or
other details or properties of the introducer tube 810, including any internal
tapered or sloped
portions 816a, any generally cylindrical (e.g., constant, flat, non-sloped,
etc.) interior portions
816b, any neck portions 806 and/or the like can be different than shown in
FIGS. 17A-17B
and described herein.
[0126] According to some embodiments, the proximal portion 812 of the
introducer tube 810 includes one or more slits or other openings 818. As
shown, such a slit
818 can begin adjacent to or near the externally threaded portion 814 of the
tube 810 and can
extend to or near the proximal end 802 of the tube 810. In some embodiments,
the proximal
portion 812 of the introducer tube includes two (or more) slits 818 located
opposite each
other in the introducer 810 to form a channel through the proximal portion
812. In some
embodiments, for example as shown in FIGS. 16D-16E, the proximal portion 812
of the
introducer tube 810 comprises a flange 819 or other protruding or flared
portion extending
outwardly (e.g., radially outwardly in a continuous or intermittent manner)
from or near the
proximal end 802. In other embodiments, the flange or other protruding member
819 can be
located along one or more other longitudinal locations of the tube 810, as
desired or required.
The flange 819 can be substantially or generally flat and/or can include any
other shape (e.g.,
curved, fluted, etc.). The flange 819 can be integrally formed or attached to
the proximal
portion 812 of the tube 810. Alternatively, the flange 819 can be a separate
member that can
be selectively attached to or removed from the tube 810 and/or any other
portion of the tool
800.
[0127] With reference to FIG. 18, the plunger 820 of the tool 800 can be
generally
cylindrical in shape with an enlarged proximal head portion 822 that includes
a domed
proximal end 824. In some embodiments, in a properly assembled mechanically-
assisted
delivery tool 800, the plunger 820 is shaped, sized and otherwise configured
to slide within
the hollow interior passage of the introducer tube 810. Thus, as discussed in
greater detail
herein, by actuating the tool, a clinician or other user can selectively move
the plunger within
an interior portion of the introducer tube 810 in order to urge an implant
(e.g., a tapered
implant) through the distal end of the tube and into a targeted implant site
of a patient.
-38-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0128] With continued reference to FIG. 18, the main body 826 of the
plunger
820 can have a diameter approximately the same as and/or slightly smaller than
the inner
diameter of the neck portion 806 and distal portion 816b of the introducer
810. In some
embodiments, as illustrated in the embodiment of FIG. 16E, the head portion
822 of the
plunger 820 includes a motion limiter or depth stop 828. The motion limiter
828 can
comprise one or more knobs, protrusion members and/or other members or
features that
generally extend outwardly from the head portion 822 of the plunger. In some
embodiments,
such a motion limiter, depth stop member or feature and/or other protruding
member 828 is
configured to slide within the slit(s) 818 or other openings of the introducer
tube 810. These
features can help prevent or otherwise limit distal movement of the plunger
820 relative to
the introducer tube (e.g., when the motion limiter or depth stop 828 contacts
or abuts the base
of the slit(s) 818). Further, such a feature can help prevent or limit
rotation of the plunger
relative to the tube 810 during use. In some embodiments, the head portion 822
of the
plunger 820 comprises a diameter approximately the same as and/or slightly
smaller than the
inner diameter of the proximal portion 812 of the introducer tube 810.
Accordingly,
movement of the plunger 820 relative to the tube 810, beyond a particular
point, will
generally be prevented or limited when the head portion 822 contacts or abuts
the narrowing
inner diameter of the tapered portion 816a of the distal portion 816 of the
introducer tube.
Therefore, the corresponding abutting features of the plunger 820 and the
introducer tube 810
can advantageously help limit the depth to which an implant (e.g., tapered
implant) can be
delivered relative to an implant site of a patient. In some embodiments, this
can help
improve the safety and efficacy of the implant, the related tools and the
implant procedure.
[0129] According to some embodiments, as illustrated in FIG. 19A, the
handle
830 of the delivery tool 800 comprises a generally circular internally
threaded nut portion or
introducer tube receiving portion 834. As shown, the threaded nut portion or
introducer tube
receiving portion 834 can be interposed between an elongate proximal section
832 and an
elongate distal section 836. In the depicted arrangement, the introducer tube
receiving
portion 834 is located closer to the distal section 836 of the handle 830.
However, in other
embodiments, the portion 834 can be located along any other portion of the
handle 830, as
desired or required. Further, the introducer tube receiving portion 834 can
include one or
-39-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
more other engagement or connection features or devices (e.g., snap
connections, press-fit or
friction-fit connections, screws or other fasteners, adhesives, etc.), either
in lieu of or in
addition to a threaded connection.
[0130] With continued reference to the perspective view of the handle
illustrated
in FIG. 19A, the proximal portion or section 832 of the handle can be longer
than the distal
portion or section 836. In other words, as noted above, the introducer tube
receiving portion
834 can be positioned closer to the distal end than the proximal end of the
handle 830.
However, in other embodiments, the introducer tube receiving portion 834 is
located at or
near between the distal and proximal ends of the handle, or at, near or closer
to the proximal
end of the handle, as desired or required.
[0131] As shown in FIG. 19A, the proximal section 832 and distal section
836
can extend in generally opposite directions from the nut or introducer tube
receiving portion
834. However, in some embodiments, a longitudinal axis of the distal section
836 is slightly
offset from a longitudinal axis of the proximal section 832. Such a
configuration can assist
with the coupling of the clamp 840 as described herein. For example, in the
illustrated
embodiment (e.g., when viewed from the top as shown in FIG. 19B), a centerline
or
orientation of the distal section or portion 836 of the handle is generally
offset with respect to
the centerline or orientation of the proximal section 832. The introducer tube
receiving
portion 834 can be sized, shaped and otherwise configured so that the distal
section 816 of
the introducer tube 810 can pass through the opening of the introducer
receiving portion 834.
Further, the externally threaded portion 814 of the introducer tube 810 can
operatively engage
and mate with the internal threaded portion of the introducer tube receiving
portion 834. As
noted above, in other embodiments, the handle 830 can engage the introducer
tube 810 using
one or more other attachment methods, features or devices (e.g., fasteners,
snap-fit or
friction-fit connections, other mating connections or couplings, adhesives,
etc.) either in
addition to or in lieu of a threaded connection.
[0132] In some embodiments, the elongate proximal section or portion 832
of the
handle comprises a grasping portion 838 configured to be selectively gripped
and
manipulated by a user during use. The grasping portion 838 can be contoured,
shaped and/or
otherwise configured to improve the user's grip on the handle 830. In the
illustrated
-40-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
embodiment, the distal section or portion 836 of the handle comprises a
generally rectangular
cross-section. However, the distal portion and/or any other portion of the
handle 830 can
include any other shape (e.g., circular, oval, square, polygonal, etc.). When
the nut portion of
introducer receiving portion 834 is oriented horizontally, the distal section
836 of the handle
comprises a generally vertical shape so that it is taller than it is deep.
[0133] According to some embodiments, the distal section 836 of the
handle 830
comprises a keyhole 837 or other opening for coupling to the clamp 840 of the
device. The
keyhole 837 or other opening can be configured to allow the clamp 840 to be
quickly and
easily connected to and/or disconnected from the handle 830. In other
arrangements,
however, the clamp 840 can he permanently or substantially permanently
attached to the
handle 830. In other embodiments, the size, shape, orientation, and/or other
details or
properties of the handle 830 can be different than shown in FIGS. 19A-19B and
described
herein.
[0134] With reference to FIGS. 20A and 20B, the clamp 840 can comprise
an
elongate member having a slight curve. A proximal portion of the clamp 840 can
include a
handle or grasping portion 848 that a user can grip during use of the device.
A distal portion
846 of the clamp 840 is generally sized, shaped and otherwise configured such
that it can be
moved within the slit 818 of the introducer tube 810. In some embodiments, as
illustrated
herein, the distal end of the clamp 840 comprises a key 847 for insertion
within the keyhole
or other opening 837 of the handle 830 in order to couple the clamp to the
handle.
[0135] Therefore, the handle 830 and the clamp 840 can be connected to
one
another about a hinge or other rotatable point, thereby permitting the handle
to be selectively
rotated and/or otherwise moved relative to the clamp. As discussed in greater
detail herein,
such a relative rotation between the clamp and the handle can be used to
provide the
mechanical force necessary to move the plunger 820 within the introducer tube
810. This can
advantageously urge an implant (e.g., tapered hydrogel implant) through the
tube 810 and
into a target recess of an implant site. Accordingly, the forces created by
moving the clamp
relative to the handle can help move an implant against relatively high back-
forces (e.g.,
against relatively high friction and/or other resistive forces) within the
introducer tube. Such
movement of the implant can be particularly difficult for reverse tapered
implants where at
-41-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
least a portion of such implants experiences generally high radially
compressive forces while
being moved through an interior lumen or other opening of the introducer tube
810.
[0136] According to some embodiments, to assemble the delivery device
800 in
preparation for use, the user inserts the implant 10 (e.g., reverse tapered
implant, other joint
implant, etc.) into the introducer tube 810 via the proximal end 802. The
plunger 820 can
then be inserted into the proximal end 802 of the introducer tube 810 and used
to distally
advance the implant 10 within the introducer tube 810. Once the handle 830 is
coupled to the
introducer tube 810 (e.g., by threading the nut portion or introducer tube
receiving portion
834 onto the externally threaded portion 814 of the introducer tube 810), the
clamp 840 can
he coupled to the handle 830 by inserting the key 847 (or other protruding
portion or feature)
of the clamp 840 into the keyhole 837 (or other opening) of the handle 830.
When
assembled, e.g., as illustrated in FIGS. 16A, 16C, 16D and 21A-21C, the clamp
840 is
generally positioned and movable within the slit 818 of the introducer tube
810.
[0137] As discussed in greater detail herein, the clamp 840 can be
rotatably
attached to the handle 830 (e.g., at a hinge point), thereby allowing a user
to selectively rotate
or otherwise move the clamp relative to the handle (e.g., to move the clamp
840 toward or
away from the handle 830 within the slit, groove or other opening of the
introducer tube 810).
In some embodiments, an offset between the distal section 836 and proximal
section 832 of
the handle 830 permits the distal portion 846 of the clamp 840 to be aligned
with the slit 818
in the introducer tube so that the clamp can be selectively moved within the
slit 818 when the
clamp 840 and handle 830 are coupled to one another (e.g., via the key 847-
keyhole 837 joint
or a similar feature or mechanism). Therefore, in some embodiments, the
delivery device
800 is configured for quick, easy and convenient assembly and disassembly for
cleaning,
sterilization, repair, maintenance and/or any other reason or purpose.
[0138] According to some embodiments, the various components of the
mechanically-assisted delivery device 800 comprise one or more rigid and/or
semi-rigid
materials that are configured to withstand the forces, moments, chemicals
and/or other
substances, temperature fluctuations and/or other elements to which they may
be exposed.
For example, the components of the implant delivery device can comprise one or
more metals
(e.g., stainless steel, other surgical steel, other types of steel, etc.),
alloys, plastics and/or the
-42-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
like. Such materials can permit the device to be autoclaved, sterilized or
otherwise cleaned
during a specific disinfection protocol. In addition, the structural and other
physical
characteristics of the device can permit the user to exert the necessary
forces using the device
to deliver implants of various sizes, shapes and/or configurations through the
corresponding
introducer tube and into a target implant site of a patient.
[0139] In use, the distal neck portion 806 of the introducer tube 810
can be
positioned at least partially within the opening, recess or other implant site
into which the
implant 10 will be secured. In some embodiments, the introducer tube 810 is
sized, shaped
and otherwise configured to that the neck portion 806 fits generally snugly
within the implant
site. To deliver the implant 10 (e.g., reverse taper implant) through the
device 800 and into
the targeted implant site, the user can urge the clamp 840 toward the handle
830 of the device
(e.g., so that the clamp rotates or otherwise moves relative to the handle).
According to some
embodiments, as the distal portion 846 of the clamp 840 moves downwardly
through the slit,
slot or other opening 818 of the introducer tube 810, a portion of the clamp
840 (e.g., the
distal portion 846) contacts the plunger 820 (e.g., the domed proximal end
824), and urges
the plunger 820 distally within the introducer tube 810.
[0140] As illustrated in FIGS. 21A-21C, such a movement, in turn, urges
the
implant 10 distally within the introducer tube 810. As the implant 10 is urged
deeper (e.g.,
more distally) into the interior of the introducer tube 810, the implant 10
may become radially
compressed by the interior shape (e.g., tapered portion 816a) of the
introducer tube 810. If
sufficient force is applied to the implant 10 by moving the clamp relative to
the handle, the
implant 10 can pass through the neck portion 806 of the introducer tube and
into the implant
site. In some embodiments, the motion limiter 828 or similar feature of the
plunger 820 can
contact the distal end of the slit or similar opening 818 of the introducer
tube 810 when the
implant 10 has been released from the delivery device 800 into the implant
site. As depicted
in FIG. 21C, this can help prevent the plunger 820 from continuing to move
toward and into
the implant site and possibly damaging the implant site and/or the implant 10.
While the user
grasps the handle 830 and the clamp 840 with one hand, he or she can apply a
required force
on the flange 819 that extends outwardly from the proximal end 802 of the
introducer tube
810 with the other hand to stabilize and control the introducer 810.
-43-
CA 02837303 2013-11-25
WO 2012/162552 PCT/US2012/039452
[0141] Accordingly, the mechanically-assisted delivery devices disclosed
herein,
or equivalents thereof, can facilitate the compression and delivery of reverse
tapered implants
within a target implant site. In some embodiments, the mechanically-assisted
delivery device
can be configured to be operated at least partially with the assistance of a
mechanical motor,
a pneumatic device and/or another external device. For example, the clamp of
the device can
be moved relative to the handle by or with the assistance of one or more
motors (e.g.,
regulated by a user using a button, knob, dial and/or other controller). Such
embodiments
can further facilitate the delivery of implants within an implant site of a
patient.
[0142] To assist in the description of the disclosed embodiments, words
such as
upward, upper, bottom, downward, lower, rear, front, vertical, horizontal,
upstream,
downstream have been used above to describe different embodiments and/or the
accompanying figures. It will be appreciated, however, that the different
embodiments,
whether illustrated or not, can be located and oriented in a variety of
desired positions.
[0143] Although several embodiments and examples are disclosed herein,
the
present application extends beyond the specifically disclosed embodiments to
other
alternative embodiments and/or uses of the inventions and modifications and
equivalents
thereof. It is also contemplated that various combinations or subcombinations
of the specific
features and aspects of the embodiments may be made and still fall within the
scope of the
inventions. Accordingly, it should be understood that various features and
aspects of the
disclosed embodiments can be combine with or substituted for one another in
order to form
varying modes of the disclosed inventions. Thus, it is intended that the scope
of the present
inventions herein disclosed should not be limited by the particular disclosed
embodiments
described above, but should be determined only by a fair reading of the claims
that follow.
-44-