Note: Descriptions are shown in the official language in which they were submitted.
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
METAL CATALYST COMPOSITION
[001] This disclosure relates to metal catalyst compositions, methods of
forming
metal catalyst compositions, and in particular metal catalyst compositions
that reduce
coke formation during dehydrogenation reactions that produce olefins.
[002] Dehydrogenation converts alkanes to alkenes (i.e., olefins) using
metal
catalysts, which increase the selectivity of the reaction. While metal
catalysts increase
the selectivity, they are vulnerable to carbon buildup, i.e., "coking." Coking
deactivates
the metal catalyst within minutes (e.g., 10 minutes (min)), after the
dehydrogenation
begins and blocks the flow of reactants, which results in higher pressure
drops in the
reactor.
[003] To remove coke, the metal catalysts undergo an oxidation treatment at
high temperatures (e.g, greater than 500 degrees Celsius ( C)). This adds cost
to the
olefin production, reduces carbon efficiency, and leads to sintering of metal
particles of
the metal catalyst, which causes catalyst degradation.
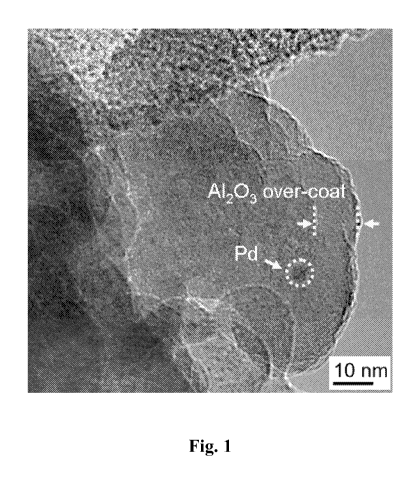
[004] Figure (Fig.) 1 illustrates a high resolution transmission electron
microscope,(TEM) image of the metal catalyst composition Example (Ex) 1.
[005] Fig. 2 illustrates diffuse reflectance infrared spectroscopy (DRIFTS)
spectra of carbon monoxide (CO) chemisorption at saturation coverage on the
metal
catalyst composition of Ex 1 treated at different temperatures and of the
uncoated metal
catalyst of Comparative Example (Corn Ex) 1.
[006] Fig. 3 illustrates thermal gravirnetric analysis (TGA) measurements
of
coke formation on the metal catalyst composition of Ex 1 and on the uncoated
metal
catalyst of Corn Ex 1.
[007] Fig. 4 illustrates TGA measurements of coke formation on the metal
catalyst composition of Ex 2 and on the uncoated metal catalyst of Corn Ex 2.
[008] Fig. 5 illustrates scanning transmission electron microscopy (STEM)
images for the over-coated catalyst of Ex 3.
[009] Fig. 6 illustrates STEM images for the uncoated catalyst in Corn Ex
1.
[010] Fig. 7 illustrates STEM images for the uncoated catalyst in Corn Ex
2.
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
MCC#70810-WO-PCT
1412.0090011
[011] Fig. 8 illustrates the dehydrogenation of ethane (DHE) reaction test
using
the metal catalyst composition of Ex 2.
[012] Fig. 9 illustrates the pore size distribution of the metal catalyst
composition of Ex 1 before and after activation.
[013] Fig. 10A illustrates the nitrogen adsorption-desorption isotherms of
the
uncoated metal catalyst of Corn Ex 1.
[014] Fig. 10B illustrates the nitrogen adsorption-desorption isotherms of
the
metal catalyst composition of Ex 1 prior to activation.
[015] Fig. 10C illustrates the nitrogen adsorption-desorption isotherms of
the
metal catalyst composition of Ex 1 after activation.
[016] Fig. 10D illustrates the pore size distribution calculated from the
adsorption branch of the isotherms for Fig. 10A-C.
[017] . "Over-coat" refers to a thin coating controlled to an atomic level
that is
deposited onto nano-size metal particles of a catalyst and the catalyst
support.
[018] "Alumina over-coat" refers to a coating of alumina controlled to an
atomic
level that is deposited onto nano-size metal particles of a catalyst and the
catalyst support.
[019] "Coking" refers to a solid carbonaceous buildup during the
dehydrogenation reaction.
[020] "Substrate" refers to a base and/or a support.
[021] "Layer" refers to a coating. A layer can be a substrate and/or can be
formed on a substrate or on another layer. A layer can be formed from the
compounds of
the present disclosure using an ALD process. Theoretically, an ALD cycle forms
a layer
that is uniformly one atom or molecule thick on an exposed surface. However,
the layer
may not occur on all portions of the exposed surface. Such a partial layer is
understood
to be a layer herein.
[022] "Deposition", "deposition process" and "vapor deposition process"
refer
to a process in which a layer is formed on one or more surfaces of a substrate
and/or a
layer.
[023] "Dehydrogenation" refers to a chemical reaction that involves the
elimination of hydrogen (H2) and can include dehydrogenation reactions that
are
oxidative or nonoxidative reaction.
2
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
[024] "Atomic layer deposition" (ALD) is also meant to include the related
terms "atomic layer epitaxy" (ALE), molecular beam epitaxy (MBE), gas source
MBE,
organometallic MBE, and chemical beam epitaxy when performed with alternating
pulses
of chemical components.
[025] "As-prepared" refers to the metal catalyst composition prior to
activation.
[026] "Moieties" refers to any discrete unit of material structure, where
the
discrete units are comprised of atoms, molecules, ions or combinations
thereof.
[027] "Ligand" refers to refers to a nonmetallic ion, molecule, or atom
that is
attached to a central atom of a coordination compound.
[028] A method of forming a metal catalyst composition includes performing
a
plurality of atomic layer deposition cycles to form an alumina over-coat on a
supported
metal catalyst, where one or more of the atomic layer deposition cycles
include sequential
steps: (a) contacting a supported metal catalyst surface with a ligand-
containing alumina
precursor for a predetermined contact time to form an intermediate layer
having a
plurality of aluminum moieties that chemically bond to the supported metal
catalyst
surface, (b) reacting the aluminum moieties with an oxidation reagent for a
predetermined reaction time to convert at least a portion of the ligands to
hydroxyl groups
thereby forming a layer of the alumina over-coat on the supported metal
catalyst surface
or a previously formed layer of the alumina over-coat, (c) prior to
replicating the
sequential steps (a) and (b) to form an additional layer of the alumina over-
coat,
contacting the layer of the alumina over-coat formed in step (b) with an inert
gas for a
predetermined final stripping time to remove unreacted oxidation reagents, and
( d)
activating the alumina over-coat, after forming a final layer of the alumina
over-coat, to
form a plurality of pores therein. For one or more embodiments, at least 80
percent (%)
of the plurality of pores have a diameter within a range of from 0.3 nanometer
(nm) to 5
nm. The metal catalyst composition reduces an amount of coke formed during a
dehydrogenation or an oxidative dehydrogenation reaction, as compared to same
supported metal catalysts without the alumina over-coat.
[029] The metal catalyst composition includes the supported metal catalyst
having the support with the plurality of metal particles, and an alumina over-
coat
deposited on a surface of the supported metal catalyst by atomic layer
deposition
3
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
followed by activating the alumina over-coat. Activating the alumina over-coat
forms
pores in the alumina over-coat, at least 80 percent (%) of the plurality of
pores have a
diameter within a range of from 0.3 nanometer (nm) to 5 nm, and where the
alumina
over-coat has a thickness within a range of from 1 nm to 100 nm. The disclosed
metal
catalyst compositions reduce an amount of coke formed during a dehydrogenation
reaction, as compared to metal catalysts without the alumina over-coat.
[030] ALD is a deposition technique in which vapors generated during each
cycle can be sequentially directed to and/or sequentially contacted with a
surface to form,
for example, an alumina containing layer on the surface. The supported metal
catalysts
can be commercially purchased or formed with ALD. Forming supported metal
catalysts
with ALD is discussed in U.S. Provisional Application serial number
61/276,260, titled
"METAL CONTAINING COMPOSITES", The Dow Chemical Company docket
number 68638, the disclosure which is incorporated herein by reference.
[031] Supported metal catalysts include a substrate which supports a
support,
e.g., an oxide support, and metal particles that are chemically bonded to the
support.
Metal particles provide an activation site for the catalytic reaction.
[032] Examples of the metal particles of the supported metal catalysts
include,
but are not limited to, palladium, platinum, rhodium, iridium, ruthenium,
rhenium, gold,
silver, copper, nickel, and combinations thereof The metal particle is
preferably
palladium.
[033] Examples of the support include, but are not limited to, aluminum
oxide,
titanium dioxide, silicon dioxide, niobium oxide, copper oxide, iron oxide,
zinc oxide,
cerium oxide, magnesium oxide, zirconium oxide, carbon aerogel, and
combinations
thereof The support is preferably aluminum oxide. Additionally, the support of
the
supported metal catalyst can be porous or can be non-porous.
[034] The substrate can have the same chemical composition or different
chemical composition as the support. Examples of the substrate include, but
are not
limited to, silicon, glass, metals, polymers, porous host matrices (aerogels,
silica gel,
mesoporous silica), nitride, sulfide, carbon, nanowires, oxides, and
combinations thereof.
[035] The alumina over-coat is formed of sequential layers of the ligand-
containing alumina precursors (also referred herein as "alumina precursors")
and
4
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
oxidizing reagents deposited via ALD. The deposition of each of the layers
provides a
consistently uniform layer growth rate for the alumina over-coat. There is an
essentially
constant growth rate of the alumina over-coat for each of the ALD cycles used
in forming
the alumina over-coat such that the thickness of the alumina over-coat is
controlled to the
atomic level.
[036] ALD is performed in a viscous flow reactor system as discussed in
J.W.
Elam, M. D. Groner and S.M. George, "Viscous Flow Reactor with Quartz Crystal
Microbalance for Thin Film Growth by Atomic Layer Deposition", Rev. Sci.
Instrum. 73,
2981-2987 (2002), which is herein incorporated by reference. Other ALD systems
are
also suitable for embodiments of this disclosure. The ALD system maintains a
temperature within a range of from 20 C to 600 C.
[037] The alumina over-coat can be formed on the supported metal catalyst
by
ALD, as described herein. For ALD, vapors used to form the alumina over-coat
are
sequentially pulsed into a reactor of the ALD system. "Vapors" include
volatile and/or
high vapor pressure liquids and/or solids.
[038] Each ALD cycle of the present disclosure includes the following
steps.
An ALD cycle includes contacting a supported metal catalyst surface with a
ligand-
containing alumina precursor for a predetermined contact time to form an
intermediate
layer having a plurality of aluminum moieties that chemically bond to the
supported
metal catalyst surface. The predetermined contact time is from 1 second (s) to
300 s,
preferably 60 s. Ligand-containing alumina precursors are selected from
compounds
having the formula Al(RI)3, where each Rl is independently methyl, ethyl,
propyl,
chloride, bromide, dialkylamino, or alkoxide.
[039] The ALD cycle includes contacting the intermediate layer with an
inert
gas for a predetermined intermediate stripping time to remove unreacted ligand-
containing alumina precursors. The predetermined intermediate stripping time
is from 1
s to 300 s, preferably 60 s. The inert gas is selected from the group
consisting of helium,
nitrogen, argon, neon, carbon dioxide and a combination thereof.
[040] The ALD cycle includes reacting the aluminum moieties with an
oxidation
reagent for a predetermined reaction time to convert at least a portion of the
ligands to
hydroxyl groups thereby forming a layer of the alumina over-coat on the
supported metal
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
catalyst surface or a previously formed layer of the alumina over-coat. The
predetermined reaction time is from 1 s to 300 s, preferably 60 s. Oxidizing
reagents are
selected from the group including water, ethylene glycol, formalin, ammonia,
hydrogen
sulfide, hydrogen peroxide, nitrous oxide, nitrogen dioxide, phosphine,
arsine, and
combinations thereof.
[041] Prior to forming an additional layer of the alumina over-coat, the
ALD
cycle includes contacting the layer of the alumina over-coat previously formed
with an
inert gas for a predetermined final stripping time to remove unreacted
oxidation reagents.
The predetermined final stripping time is from 1 s to 300 s, preferably 60 s.
[042] The predetermined times discussed herein are not limited to the
ranges
provided and other predetermined times are possible.
[043] Each ALD cycle can be performed two or more times. Different numbers
of ALD cycles can be performed to obtain an optimal thickness for various
applications.
While a number of ALD cycles can be performed, the accessibility of the metal
particles
of the supported metal catalyst may contribute to determining the number of
ALD cycles
for particular applications. For example, if the thickness of the alumina over-
coat is too
small (e.g., less than 1 nm) the alumina over-coat may not minimize coking and
sintering.
On the other hand, if the thickness of the alumina over-coat is too large
(e.g., greater than
15 nm) the metal particles may not be accessible by the reactants during the
dehydrogenation or oxidative dehydrogenation reactions; thereby rendering the
catalyst
ineffective.
[044] A thickness of the over-coat is dependent on the number of ALD cycles
that deposited the over-coat and is controlled to the nanometer. The alumina
over-coat
has a thickness from 1 nm to 100 nm, preferably 1 nm to 15 nm, more preferably
9 nm.
[045] The method includes activating the alumina over-coat. Activating
forms
pores in the alumina over-coat allowing the metal particles to become
accessible by the
reactants. During activation, the alumina over-coat undergoes a structural
transformation
from the surface of the alumina over-coat to the metal particles, and removes
carbon
residual that accumulated from the ALD process, which forms the pores and
exposes the
metal particle.
6
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
[046] At least 80 % of the pores in the alumina over-coat have a diameter
within
a range of from 0.3 to 5 nm, preferably within a range of from 0.5 nm to 3 nm.
In some
embodiments, at least 90% of the pores in the alumina over-coat have a
diameter within a
range of from 0.3 rim to 5 nm, preferably within a range of from 0.5 nm to 3
nm. The
small diameter minimizes an amount of produced olefins that can reach the
metal
particles and helps to prevent coke formation.
[047] Activating includes calcining and/or reducing. Calcining, also
referred to
as calcination, is the heating of a solid to a temperature below its melting
point that
brings about a thermal decomposition and/or dissociation. Calcining can occur
in the
presence of a non-reducing gas at a temperature within a range of from 30 C
to 1000 C
for a time interval within a range from 3 min to 5 days (i.e., 120 hours
(hrs)). For some
applications, calcining occurs at a temperature within a range of from 500 C
to 800 C
for 60 min to 10 hrs, and more preferably at 700 C for 2 hrs. Suitable non-
reducing
gases include, but are not limited to, helium, nitrogen, argon, neon, oxygen,
ozone, dry
air, and combinations thereof. Calcining can be performed in the ALD system or
in a
separate reactor, where the separate reactor produces and maintains the
calcination
conditions as discussed herein.
[048] Reduction can occur in the presence of hydrogen, hydrocarbons, carbon
monoxide, formalin, and combinations thereof. Reduction can occur in the
presence of a
non-oxidizing gas at a temperature within a range of from 30 C to 1000 C for
a time
interval within a range from 3 mm to 5 days. For some applications, reduction
occurs at
a temperature within a range of from 500 C to 800 C for 60 min to 10 hrs,
and more
preferably at 700 C for 2 hrs. Suitable non-oxidizing gases include helium,
nitrogen,
argon, neon, hydrogen, and combinations thereof
[049] A method for reducing coke formation during dehydrogenation or
oxidative dehydrogenation is disclosed. The method includes contacting an
alkane with
the metal catalyst composition in the presence of inert gas, oxygen, and
combinations
thereof at a temperature within a range of 300 C to 800 C, where the metal
catalyst=
composition is formed by performing a plurality of ALD cycles on a supported
metal
catalyst surface, as described herein. The alkanes can include, but are not
limited to,
7
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
C2 to C20 carbon atom alkanes, either alone or in mixtures, preferably having
two to
eight carbon atoms. Suitable alkanes include, but are not limited to, ethane,
propane, n-
butane isobutane, n-pentane, isoamylenes, n-hexane, isohexanes, n-heptane,
isoheptane,
octane and isooctanes. The alkanes may include both linear and branched
alkanes.
[0501 Problems that lead to coking include the migration of metal
particles and
produced olefins polymerizing on the metal particle. It is believed that the
alumina over-
coat inhibits the problems that lead to coking. For example, the over-coat
acts as a
barrier such that the migration of the metal particles is inhibited. Since
larger metal
particles are more likely to make coke than small metal particles, minimizing
the growth
of metal particles, by preventing migration, helps minimize coke formation.
Additionally, the alumina over-coat restricts the access of the produced
olefins to the
metal particles. By having pores with a diameter of 2 nm or less, the access
of produced
olefins is restricted to the metal particle and prevents the required
concentration of
produced olefins needed to polymerize from collecting on the metal particle.
EXAMPLES
[051] Materials include trimethylaluminum (TMA), (CH3)3A1, assay 97%,
Sigma-Aldrich 6; palladium on alumina (Pd/A1203)catalyst, Pd nanoparticles
with an
average size of 3.2 0.67 nm and loading of 1.88%, synthesized by wet
impregnation
onto gamma A1203.; formalin (37% HCOH and 15% CH301-lin aqueous solution),
Sigma-Aldrich 0; ultrapure water (Millipore TM); palladium(II)
hexafluoroacetylacetonate, Pd(C5HF602)2, (Sigma-Aldrich 6); silica gel
S10040M,
(SiliCycle 0); inert gas: nitrogen; Palladium nitrate (Pd(NO3)2), 10%
solution, Johnson
Matthey6; Citric acid; Gamma-alumina (180 m2/g, 0.6 cc/g), Sasole.
[052] Equipment includes viscous flow reactor system (J.W. Elam and as
discussed herein); mount quartz crystal microbalance (Maxtek Inc.), having
thickness
_ monitor model TM-400R and QCM sensors CCAT1BK-1007-000 (Colorado Crystal
Corporation), in Maxtek BSH-150 bakeable sensor (INFICON) housing and seal
with
Epotek P1011 epoxy (Epoxy Technology, Inc.). Operate the reactor at a pressure
of 1-2
TOIT with the nitrogen flow at 360 standard cubic centimeters per minute
(sccm).
Monitor pressure by a 10 Torr Baratron capacitance manometer to maintain flow
velocity
of 2.5 meters/second. High resolution transmission electron microscope (TEM),
(JEOL
8
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
JEM-2100F FAST TEM); Thermal gravirnetric analysis (TGA), (TA Instrument);
Diffuse reflectance Infrared (IR) spectroscopy (DRIFTS), (Thermo Nicolet Nexus
870
instrument with an MCT detector), and Nitrogen BET (ASAP 2020, Micromeritics
0).
[053] Ex 1: 45A1/Pd/Al2%
[054] Sequentially expose the Pd/A1203 catalyst to 45 ALD cycles including
TMA and ultrapure water at 200 C. Each ALD cycle includes a 60 s TMA
exposure,
and a 60 s ultrapure water exposure, follow each exposure by a 60 s inert gas
purge with
nitrogen. The resulting as-prepared metal catalyst composition has a loading
of 1.03 %.
TEM measurements showed that there is an approximately 9 nm alumina over-coat
on
the surface of the Pd/A1203 catalyst after the 45 ALD cycles, as seen in Fig.
1.
[055] Activate the alumina over-coat by calcining the as-prepared metal
catalyst
composition at 700 C for 2 hrs in 10 % oxygen in helium and then reducing in
5 %
, hydrogen in helium at 300 C for 30 mm.
[056] Ex 2: 45A1/Pd/A.12Q3-ALD.
[057] Synthesize a Pd/A1203 catalyst by ALD using Pd(C5HF602) and formalin
at 80 C for one ALD cycle. The particle size of the Pd nanoparticles was 1.1
0.5 nm
and has a loading of 0.41%. Sequentially expose the synthesized Pd/A1203
catalyst to 45
ALD cycles as described in Ex 1. The resulting as-prepared metal catalyst
composition
has a loading of 0.23%.
[058] Activate the alumina over-coat by calcining the as-prepared metal
catalyst
composition at 700 C for 2 hrs in 10 % oxygen in helium and then reducing in
5 %
hydrogen in helium at 300 C for 30 min.
[059] Ex 3: 30A1/Pd/A17%-ALD
[060] Repeat Ex 2, but with changes. Perform 30 ALD cycles instead of 45
cycles.
[061] Corn Ex 1: Pd/A17123_
[062] Dissolve 3.0 g of citric acid in 2 mL water. Add 7 mL of 10% Pd(NO3)2
solution to the citric acid to form a Pd-citric acid solution. The Pd-citric
acid solution
was added drop wise to 15 g of gamma-alumina and dried for 1 hr at
approximately 20
C and then overnight at 125 C to form the catalyst. Calcine 5 g of catalyst
in flowing
air at 300 C for 5 hrs. Reduce the calcined catalyst in flowing hydrogen (200
seem/min)
9
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
by heating to 100 C for 30 min then increasing the temperature to 175 C for
30 min and
finally 250 C for 30 min, purge with He and cool to approximately 20 C
[063] Corn Ex 2: Pd/A17123-ALD
[064] Synthesize a Pd/A1203 catalyst by ALD using Pd(C5HF602) and formalin
at 80 C for one ALD cycle.
[065] Accessibility of Ex 1 (45A1/Pd/A1703) at 200 C
[066] Determine the accessibility of the as-prepared 45A1/Pd/A1203 from Ex
1
using DRIFTS with CO as a probe molecule after reducing in 5% hydrogen at 200
C for
30 min. DRIFTS measurements of CO chemisorption were performed at 20 C using
a
Thermo Nicolet Nexus 870 instrument with an MCT detector. A cold trap at
approximately negative (-) 80 C was used in the gas inlet line to remove all
the iron
carbonyls. The background spectra was taken after purging the sample with
ultrahigh
purity (99.999%) helium for 30 min at a flow rate of approximately 80 seem.
Pure CO
(Airgas, research grade) at a flow rate of approximately 40 seem was then
introduced to
the DRFITS cell for 20 min to saturate the Pd surface. After the CO
saturation, another 5
min of helium purge at a flow rate of approximately 70 seem was performed to
remove
gas phase CO in the DRIFTS cell. The spectrum was recorded (512 scans, a
resolution of
4 cm-I) after the helium purge.) The DRIFT spectra of CO chemsorption for
45A1/Pd/A1203-200 C at saturation coverage is shown in Fig. 2.
[067] Accessibility of Ex 1 (45A1/Pd/A17Q3) at 500 C
[068] Activate the alumina over-coat by calcining the as-prepared
45A1/Pd/A1203 from Ex 1 at 500 C for 2 hrs in 10 % oxygen in helium and then
reduce
in 5 % hydrogen in helium at 300 C for 30 min. Repeat the accessibility of Ex
1
(45A1IPd/A1203) at 200 C with changes. The Ex 1 (45A1/Pd/A1203) at 500 C
replaces
the metal catalyst composition from of Ex 1 (45A1/Pd/A1203) at 200 C. The
DRIFT
spectra of CO chemsotption for 45AllPd/A1203-500 C at saturation coverage is
shown in
Fig. 2.
[069] Accessibility of Ex 1 (45A1/Pd/Al2Q3) at 700 C
[070] Activate the alumina over-coat by calcining the 45A1/Pd/A1203 from Ex
I
at 700 C for 2 hrs in 10 % oxygen in helium and then reduce in 5 % hydrogen
in helium
at 300 C for 30 min. Then repeat Accessibility of Ex 1 (45A1/13d/A1203) at
200 C with
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC470810-WO-PCT
1412.0090011
changes. The Ex 1 (45A1/Pd/A1203) at 700 C replaces the metal catalyst
composition
from of Ex 1 (45A1/Pd/A1203) at 200 C. The DRIFT spectra of CO chemsorption
for
45A1/Pd/A1203-700 C at saturation coverage is shown in Fig. 2.
[071] Accessibility of Corn Ex 1 (Pd/Al2Q3_)
[072] Determine the accessibility of the uncoated metal catalyst of Corn Ex
1.
Repeat Accessibility of Ex 1 (45A1/Pd/A1203) at 200 C with changes. The
uncoated
metal catalyst of Corn Ex 1 replaces the metal catalyst composition from of Ex
1
(45A1/Pd/A1203) at 200 C. The DRIFT spectra of CO chemsorption for Pd/A1203
at
saturation coverage is shown in Fig. 2.
[073] Accessibility Analysis
[074] As seen in Fig. 2, the results indicate that the 45A1/Pd/A1203-200 C
catalyst was completely covered by the alumina over-coat, thereby, minimizing
the
. accessibility of the CO molecule. The results for the 45M/Pd/A1203-500 C
and
45A1/Pd/A1203-700 C indicate that as the temperature during calcining
increases, the
accessibility of the CO molecule also increases. The capacity of the CO peak
of
45M/Pd/A1203-700 C is in the same order of the uncoated sample Pd/A1203,
indicating
that the alumina over-coat became porous to allow Pd nanoparticles to become
reaccessible after the calincation treatments.
[075] Reduction of Coke Formation: Using Ex 1 (45A1/Pd/A12123_)
[076] Perform in situ TGA to quantify the reduction of coke formation using
20
milligrams (mg) of the metal catalyst composition from Ex 1 during an
oxidative
dehydrogenation of ethane (ODHE) reaction at 650 C for 90 min. The flow rates
of
ethane, oxygen, and diluent helium are 10.5, 3.5, and 66.5 sccm, respectively.
Prior to
ODHE reaction, the sample was heated with a heating rate of 5 C/min in 10%
oxygen/helium (80 seem) to 700 C and then maintain isothermal for 2 hrs. Cool
the
sample to 650 C in helium (80 sccm) and then maintain isothermal for 1 hr.
After the
treatment, the mixture of reactants was introduced to the sample for 90 min at
650 C.
The result is illustrated in Fig. 3.
[077] Comparative Coke Formation: Using Corn Ex 1 (Pd/Al2,(23_)
11
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
[078] Repeat Reduction of Coke Formation: Using Ex 1 with changes. The
uncoated metal catalyst of Com Ex 1 replaces the metal catalyst composition
from Ex 1.
The result is illustrated in Fig. 3.
[079] Analysis
[080] As seen in Fig. 3, the TGA results illustrate that there was 11.83 mg
of
coke formation on the 20 mg uncoated metal catalyst of Corn Ex 1 after 60 min
of the
reaction at 650 C. For the metal catalyst composition of Ex 1, there was 0.40
mg of coke
formation on the 20 mg metal catalyst composition of Ex 1 after 1 hr of the
reaction at
650 C. The amount of coke formation on the metal catalyst composition from Ex
1 after
60 mm was approximately 6.2% of the coke formation on the uncoated catalyst of
Com
Ex 1. The amount of coke formation on the metal catalyst composition of Ex 1
slowly
increased during the reaction period of 90 minutes but is still significantly
lower than the
coke formation on the uncoated metal catalyst of Corn Ex 1.
[081] Reduction of Coke Formation: Using Ex 2 (45A1/Pd/A19Q2-ALD)
[082] Repeat Reduction of Coke Formation: Using Ex 1 with changes. The
metal catalyst composition from Ex 2 replaces the metal catalyst composition
from Ex 1.
The result is illustrated in Fig. 4.
[083] Comparative Coke Formation 1: Using Corn Ex 2 (Pd/Al2(21-ALD)
[084] Repeat Reduction of Coke Formation: Using Ex 1 with changes. The
uncoated metal catalyst of Com Ex 2 replaces the metal catalyst composition
from Ex 1.
The result is illustrated in Fig. 4.
[085] Analysis
[086] As seen in Fig. 4, the TGA results illustrate that there was 4.36 mg
of coke
formation on the 20 mg uncoated metal catalyst of Com Ex 2 after 1 hr of the
reaction at
650 C. For the metal catalyst composition of Ex 2, there was 0.05 mg of coke
formation
on the 20 mg metal catalyst composition of Ex 1 after 1 hr of the reaction at
650 C. The
amount of coke formation on the metal catalyst composition from Ex 2 was
approximately 2.0 % of the coke formation on the uncoated catalyst from Corn
Ex 2. The
amount of coke formation on the metal catalyst composition of Ex 2 slowly
increased
during the reaction period of 180 minutes but is still significantly lower
than the coke
formation on the uncoated metal catalyst of Com Ex 2.
12
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
[087] Thermal Stability: Using Ex 1 (45A1/Pd/A12123)
[088] Dilute the as-prepared metal catalyst composition of Ex 1 with fine
quartz
chips and slowly heat up with a heating rate of 2 C/min. Calcine in 10 %
oxygen at 700
C for 2 hrs. Perform an oxidative dehydrogenation of ethane (ODHE) at 675 C
for 28
hrs. The flow rates of ethane, oxygen, and diluent helium are 9, 3, and 48
sccm,
respectively. Use STEM measurements to determine any morphology changes of the
Pd
nanoparticles. No visible morphology changes of the Pd nanoparticles were
observed on
the used catalyst.
[089] Thermal Stability: Using Ex 2 (45A1/Pd/Al2O3.-ALD)
[090] Repeat Thermal Stability: Using Ex 1 with changes. The metal catalyst
composition of Ex 2 replaces the metal catalyst composition of Ex I. No
visible
morphology changes of the Pd nanoparticles were observed on the used catalyst.
[091] Thermal Stability: Using Ex 3 (30 AllPd/A19c3L-ALD)
[092] Repeat Thermal Stability: Using Ex 1 with changes. The metal catalyst
composition of Ex 3 replaces the metal catalyst composition of Ex 1. The
results are
illustrated in Table 1 and the STEM images are shown in Fig. 5. Considerable
morphology changes were observed, the STEM images are shown in Fig. 5.
[093] As seen in Fig. 5, (A) illustrates the 30A1/Pd/A1203-ALD catalyst
after the
ODHE reaction with low magnification and (B) illustrates the 30A1/Pd/A1203-ALD
catalyst after the ODHE reaction with high magnification.
[094] Thermal Stability: Using Com Ex 1 (Pd/A1,111;31)
[095] Repeat Thermal Stability: Using Ex 1 with changes. The uncoated metal
catalyst of Corn Ex 1 replaces the metal catalyst composition of Ex 1 and the
ODHE
reaction was run for 30 min because the coke formation blocked the reactor and
shut
down the reaction. STEM measurements demonstrated that there was nanowire
formation and considerable sintering on the used catalyst. The STEM images are
shown
in Fig. 6.
[096] As seen in Fig. 6, (A) illustrates fresh unused Pd/A1203 catalyst and
(B)
and (C) illustrate the used Pd/A1203 catalyst after the ODHE reaction with
different
magnification.
[097] Thermal Stability: Using Corn Ex 2(Pd/AI Q-ALD)
13
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
[098] Repeat Thermal Stability: Using Ex 1 with changes. The uncoated metal
catalyst of Corn Ex 2 replaces the metal catalyst composition of Ex 1 and the
ODHE
reaction was run for 30 min because of the coke formation. STEM measurements
demonstrated that there was nanowire formation and considerable sintering on
the used
catalyst. The STEM images are shown in Fig. 7.
[099] As seen in Fig. 7, (A) illustrates fresh unused Pd/A1203-ALD catalyst
and
(B) and (C) illustrate the used Pd/A1203-ALD catalyst after the ODHE reaction
with
different magnification.
[0100] Dehydrogenation of Ethane (DHE): Using Ex 2 (45A1/1341/A1,122-ALD)
[0101] Perform a DHE reaction with the metal catalyst composition at 675
C for
hrs with a flow of ethane at 9 sccm and N2 at 41 seem. The conversion of
ethane was
found to be 4.9 %, with an ethylene selectivity of 97.6 %, which is shown in
Fig. 8. There
was no visible decrease in activity in the period of 5 hrs during the DHE
reaction and the
selectivity of ethylene only slightly dropped from 100 % to 97.6 % after 2
hrs. This long
term of DHE reaction test suggests that the metal catalyst compositions
inhibit coke
formation in DHE reactions, as compared to reaction using catalysts that do
not have the
over-coat normally deactivate in a few minutes due to large coke deposition.
[0102] Porosity of ALD Applied Over-Coat
[0103] Use silicycle S10040M SiO2 having a surface area of approximately
100
meters squared per gram (m2/g), a primary particle size of 75-200 micrometers
(rim) as a
support. Load 500 mg of the support onto a powder holder and hold in the ALD
system
at 200 C. Sequentially expose the support to TMA and ultrapure water at 200
C for 45
ALD cycles. Each ALD cycle includes a 60 s TMA exposure, and a 60 s ultrapure
water
exposure, and follow each exposure by a 60 s inert gas purge with nitrogen.
Use nitrogen
BET to determine the pore size distribution of the alumina over-coat as-
prepared and
after calcination at 700 C in air for 2 his. The results are shown in Fig. 9.
[0104] As seen in Fig. 9, the pore size distribution of the as-prepared
over-coated
support and the over-coated support after calcinations illustrate that the
population of the
pores at the size of approximately 2 rim significantly increased after high
temperature
treatment, which was evidentially identical to the size of the pores formed on
the
45A1/Pd/A1203 catalysts after the same treatment. Therefore the formation of
the pores
14
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
within the ALD A1203 over-coats is most likely due to removal of carbon
residues from
the ALD process. Certainly, the structural changes in the amorphous A1203 over-
coats
could also contribute the pore formation by the thermal treatment.
[0105] Porosity of Over-Coated Metal Catalyst: Using Ex 1(45A1/Pd/A12123)
[0106] Determine the porosity of the alumina over-coat by nitrogen BET. In
order to address the changes in surface area and pore size distribution, the
quantity of
adsorbed nitrogen and surface area were normalized based on the weight of an
uncoated
catalyst (Pd/A1203). The BET surface area, as deduced from the adsorption-
desorption
isotherms of the uncoated catalyst (Pd/A1203), the as-prepared metal catalyst
composition
of Ex 1, and the metal catalyst composition of Ex 1 after activating the over-
coat are
shown in Figs 10A, 10B, and 10C, respectively. As seen in Figs 10A and 10B,
the
surface area decreased from 253 m2/gram to 30 m2/gram as a consequence of
applying 45
ALD cycles. The large hysteresis loop observed in Fig. 10B indicates that slit-
shaped
pores were formed after the ALD cycles.
[0107] However, after activating the alumina over-coat by calcining at 700
C for
2 hr under 10% oxygen in helium followed by reduction under 5% hydrogen in
helium at
300 C for 30 min (45A1/Pd/A1203-700C), the BET surface area returned to 213
m2/gram,
shown in Fig. 10C.
[0108] Fig. 10D illustrates the pore size distribution calculated from the
absorption branch of the isotherms of the samples in Figs 10A-10C. Fig. 10D
illustrates
the A1203 support of the uncoated Pd/A1203 catalyst was mesoporous with an
average
pore size of 6.6 urn. After 45 cycles of ALD A1203 over-coating, the mesopores
disappeared, revealing the dramatic BET surface area decrease. This is not
surprising,
since the thickness of ALD A1203 over-coat (apprximately 9 urn, as shown in
Fig. 1) is
greater than the characteristic diameter of the mesopores. After high
temperature
treatment, the mesopores with a diameter of 6.6 urn were restored, and new
pores were
formed at approximately 2 urn (Fig. 10D). The pores were formed by structural
changes
in the amorphous A1203 over-coat caused by the thermal treatment, removal of
carbon
residues from the ALD process, and dewetting of the A1203 over-coat from the
surface of
the Pd nanoparticles caused by the large lattice mismatch between palladium
and
CA 02837584 2013-11-27
WO 2012/166514
PCT/US2012/039343
TDCC#70810-WO-PCT
1412.0090011
alumina. These pores made it possible for the embedded Pd nanoparticles to
become
accessible while the overlayer imparts high thermal stability.
16