Note: Descriptions are shown in the official language in which they were submitted.
CA 02837611 2013-11-28
PCT/EP2012/058360 - 1
2011P11617W0AU
Reduction of metal oxides using a gas stream containing both
hydrocarbon and hydrogen
Technical field
The present invention relates to a process for reducing metal
oxides, preferably iron oxides, using a gas stream containing
both hydrocarbon and hydrogen. The invention also relates to a
device for carrying out such a process.
Prior art
Coke oven gas is formed when coke is generated in integrated
smelting works or stand-alone production plants and is used to
date, for example, for reinforcing the heating value of the
blast furnace top gases before use thereof in recuperators, as
fuel gas in slab reheating furnaces or roller hearth furnaces,
and for electricity generation in power plants. As main
components it contains not only hydrocarbon - for example one
or more hydrocarbons CnH2n+2, wherein n can be 1 or 2 or 3 or 4;
but chiefly methane, that is to say n = 1 - but also hydrogen.
In some integrated smelting works, coke oven gas is also used
for generating technically pure hydrogen, for example for use
in annealing furnaces. Typical coke oven gas compositions
formed in integrated smelting works are as follows
CA 02837611 2013-11-28
PCT/EP2012/058360 7 - 2 -
2011P11617W0AU
COG analysis (dry):
H2 [% by volume] 65 62.1
N2 [% by volume] 2.5 Included in remainder
CO [% by volume] 6 6.2
CH4 [% by volume] 22 21.4
CnH. [% by volume] 3 Included in remainder
CO2 [% by volume] 1.5 Included in remainder
H20 [% by volume] Saturated Included in remainder
H2S [g/Nm3 (S.T.P)] 0.35 n.a.
Tar [g/Nm3 (S.T.P)] 5 n.a.
Dust [g/Nm3 (S.T.P)] 5 n.a.
Remainder [% by volume] 10.3
Although the coke oven gas contains components such as hydrogen
and carbon monoxide which are readily usable for reducing metal
oxides in general, and iron oxides in particular, on account of
the hydrocarbon content, it can only be used with restrictions
for reducing metal oxides, especially iron oxides, in a
reducing unit, since, as a consequence of highly endothermic
reactions of the hydrocarbons proceeding on the introduction of
coke oven gas into the reducing unit,
for example hydrocarbon CH4
CH4 4 2 H2 C Cracking A H298 = + 74.86 [kJ/mol]
3 Fe + CH4 4 Fe3C + 2 H2 Carbonizing A H298 = + 99.7 [kJ/mol]
the reduction temperature would decrease too greatly, which in
turn would greatly restrict the productivity of the reducing unit.
Brief description of the invention
Technical object
It is an object of the present invention to provide a process
which permits the use of a gas stream containing hydrocarbon
and hydrogen
CA 02837611 2013-11-28
4
PCT/E22012/058360 ' - 3 -
2011P11617W0AU
for reducing metal oxides. It is likewise an object to provide
a device for carrying out such a process.
Technical solution
This object is achieved according to the invention by a process
for reducing metal oxides using a gas stream containing not
only hydrocarbon but also hydrogen,
which is characterized in that
the gas stream containing not only hydrocarbon but also
hydrogen
is separated
into a hydrogen-rich fraction
and a hydrocarbon-rich fraction,
and subsequently
at least a subquantity of the hydrocarbon-rich fraction
is subjected
to at least one operation of the group
- oxidation using technically pure oxygen,
- reformation using CO2 and H20,
and then it is introduced at least as a component of a reducing
gas into a reducing unit containing the metal oxides,
wherein the hydrocarbon content is adjusted by the at least one
operation of the aforementioned group,
in such a manner that the hydrocarbon content in the reducing
gas is, on entry into the reducing unit, less than 12% by
volume, preferably less than 10% by volume, particularly
preferably less than 8% by volume.
Metal oxides can be, for example, iron oxides, or oxides of
nickel, copper, lead, cobalt.
The reduction of the metal oxides preferably proceeds to form
extensively metalized metal - that is to say the degree of
metalization is greater than or equal to 90%, preferably
greater than or equal to 92%, for example sponge iron.
CA 02837611 2013-11-28
4
PCT/EP2012/058360 - 4 -
2011P11617W0AU
Advantageous effects of the invention
The gas stream containing not only hydrocarbon but also
hydrogen can contain one or two or more types of hydrocarbon.
For example, it contains relatively low-saturated hydrocarbons
CnH2n+2 r wherein n = 1, that is to say methane, or n = 2, that
is to say ethane, or n = 3, that is to say propane, or n = 4,
that is to say butane or isobutane. It can also contain
relatively low-monounsaturated or polyunsaturated hydrocarbons,
wherein, for example, CnH2fl applies, for example ethene. It can
also contain aromatic hydrocarbons, such as benzene or toluene.
In the gas stream containing not only hydrocarbon but also
hydrogen, one or more types of hydrocarbon having the general
formula CnHm can also be present, wherein m can be
m = n,
m = 2n,
m = 2n+2.
According to the invention, the gas stream containing not only
hydrocarbon but also hydrogen is separated into a hydrogen-rich
fraction and a hydrocarbon-rich fraction. In this case the
hydrocarbon-rich fraction contains not only hydrocarbons, but
also further components such as argon, nitrogen, carbon
monoxide, carbon dioxide and steam. The term hydrocarbon-rich
relates to the fact that this fraction, compared with the gas
stream containing not only hydrocarbon but also hydrogen, has a
higher content of hydrocarbon.
The hydrogen-rich fraction contains not only hydrogen.
The term hydrogen-rich relates to the fact that this fraction,
compared with the gas stream containing not only hydrocarbon
but also hydrogen, has a higher content of hydrogen.
Subsequently for the separation, at least a subquantity of the
hydrocarbon-rich fraction obtained in the separation is
subjected to at least one operation of the group
CA 02837611 2013-11-28
PCT/EP2012/058360 _ 5 _
2011P11617W0AU
- oxidation using technically pure oxygen,
- reforming using CO2 and H20.
It can also be subjected to a combination of these two
operations.
In the case of a combination, partial oxidation is preferably
performed first using technically pure oxygen for the purpose
of temperature elevation, and subsequently reforming is
performed using CO2 and H20, for example in an autothermal
reformer. In an autothermal reformer, the reformer does not
need to be fired, because no feed line of fuel gas to the
autothermal reformer is necessary. This saves expenditure on
construction and reduces the exhaust gases of the reformer.
In this case, in the oxidation, the total amount of
hydrocarbons is not oxidized, but only a part of the amount of
hydrocarbons - in the context of this application, this is also
termed partial oxidation.
In this case, in the reforming, the total amount of
hydrocarbons is not reformed, but a predominant part of the
amount of hydrocarbons.
Via said operations, alone or in combination, the content of
hydrocarbons decreases.
After at least a subquantity of the hydrocarbon-rich fraction
obtained in the separation has been subjected to at least one
operation of said group, it is introduced at least as a
component of a reducing gas into a reducing unit containing the
metal oxides - this means of course that the product obtained
in the operation or operations is introduced.
At least as a component of a reducing gas means that the
reducing gas can also contain other components which may
optionally be added before a mixture obtained in the addition
is introduced as reducing gas into the reducing unit.
According to the invention, the hydrocarbon content of the
subquantity is set by the at least one operation of said group
in such a manner that the hydrocarbon content in the reducing
gas, on entry into the
CA 02837611 2013-11-28
PCT/EP2012/058360 - 6 -
2011P11617W0AU
reducing unit, is less than 12% by volume, preferably less than
10% by volume, particularly preferably less than 8% by volume,
but greater than 1% by volume, preferably greater than 2% by
volume, particularly preferably greater than 3% by volume. Said
limits are comprised herein. The higher the hydrocarbon content
is in the reducing gas on entry into the reducing unit, the
higher the reduction temperature must be set - in reducing shafts
as reducing unit, also termed - gas temperature bustle or the
lower is the productivity of the plant. At a hydrocarbon content
set according to the invention, the reduction temperature owing
to a lower endothermic reactions of the hydrocarbons does not
fall so greatly that the productivity of the reducing unit
decreases below an economically acceptable level.
The lower limit of the hydrocarbon content is determined, for
example, in the reduction of iron oxides, by the required
carbon content - carbon bound as Fe3C or elemental carbon - in
the reduced product for the steelworks - there, for example, an
electric arc furnace. With increasing carbon content in the
reduced product, the energy requirement in the subsequent
treatment in the electric arc furnace decreases. A hydrocarbon
content in the reducing gas on entry into the reducing unit in
the range of the lower limit is used, for example, for
generating a minimum content of carbon in a sponge iron, in
particular in the form of Fe3C, or such a hydrocarbon content
is necessary optionally for controlling the temperature in the
reducing unit.
In addition, for example in the production of sponge iron, hot
briquetted iron (HBI) plants - as are customary in direct
reduction (DR) plants - also require certain minimum briquetting
temperatures - preferably > 650 C for avoidance of increased
maintenance costs and to achieve product densities > 5 g/cm3 -
which, in the event of excessive cooling of the DRI in the
reducing unit, cannot be achieved owing to endothermal reactions.
According to a preferred embodiment, the gas stream containing
not only hydrocarbon but also hydrogen is coke oven gas.
CA 02837611 2013-11-28
PCT/EP2012/058360 ' - 7 -
2011P11617W0AU
The latter embodiment is preferred because coke oven gas, in an
integrated smelting works, is usually formed in any case, or, in
a stand-alone coking plant, is only used for electricity
generation, or is flared off without being used. Using the
process according to the invention, it can be utilized for
efficient iron production; the material utilization thereof
achieved in this case has a higher efficiency than, for example,
utilization for electricity generation. An integrated smelting
works is taken to mean a steel generation route which consists,
inter alia, of coking plant, sintering plant and blast furnace.
The gas stream containing not only hydrocarbon but also hydrogen
can also be gas generated in a coal gasifier.
According to a preferred embodiment, the gas stream
containing not only hydrocarbon but also hydrogen
is separated
into a hydrogen-rich fraction
and a hydrocarbon-rich fraction
by at least one operation of the group
- pressure-swing adsorption,
- membrane separation.
The pressure-swing adsorption proceeds, for example, in a PSA or
VPSA plant, wherein PSA means Pressure Swing Adsorption and VPSA
means Vacuum Pressure Swing Adsorption. More preferably, a
prepurification of the gas stream proceeds before the pressure-
swing adsorption, for example in a prepurification appliance for
separating off tar and dust using tar filters made of fibers or
adsorption materials. Owing to the differing adsorption forces, a
gas stream containing not only hydrocarbon but also hydrogen, for
example coke oven gas, in the case of an appropriate design of the
plant size of pressure-swing adsorption plants and by operation
using correspondingly designed cycle times using a PSA plant or a
VPSA plant can be separated into a hydrogen-rich fraction and a
hydrocarbon-rich fraction. The hydrogen is formed on the product
side virtually without a significant pressure drop. The
hydrocarbon-rich fraction is foLmed at very low pressure or a
CA 02837611 2013-11-28
PCT/EP2012/058360 ' - 8 -
2011P11617W0AU
vacuum and is then compressed to the required pressure in the
subsequent process steps.
In the case of membrane separation, the separation proceeds on
the basis of the differing permeability of a membrane. Hydrogen
is produced in this case in the concentrated state on the low-
pressure side of the membrane.
According to a preferred embodiment, at
least a proportion
of the
at least one subquantity of the hydrocarbon-rich fraction which
was subjected to at least one operation of the group
- oxidation using technically pure oxygen,
- reforming using CO2 and H20,
is mixed
with an auxiliary reducing gas,
before the resultant mixture of these two components is
introduced
as reducing gas into the reducing unit containing the metal
oxides.
In this case the reducing gas introduced into the reducing unit
containing the metal oxides is generated by mixing two
components, wherein the one component is obtained by oxidizing
and/or reforming at least one subquantity of the hydrocarbon-
rich fraction.
In such a procedure, other gases having a reduction potential
can also be materially utilized for the reduction of metal
oxides by adding them as auxiliary reducing gas.
In a device for carrying out such a process according to the
invention, corresponding feed lines are present for introducing
auxiliary reducing gases to the proportion of the hydrocarbon-
rich fraction, or optionally to the total amount of the
hydrocarbon-rich fraction,
which has been subjected to at least one operation of the group
CA 02837611 2013-11-28
PCT/EP2012/058360- 9 -
2011P11617W0AU
- oxidation using technically pure oxygen,
- reforming using CO2 and H20.
According to a preferred embodiment, the mixing ratio of the
two components is set in dependence on a preset temperature for
the mixture. In this manner, it is ensured that the reducing
gas is in the temperature region which is favorable in terms of
the process and economics for reducing metal oxides. By setting
the temperature, the reaction rate in the reducing
reactor - kinetics, can be set optimally. In addition, the
efficiency of the reducing gas preheating can be optimized.
Corresponding devices for controlling the mixing ratio and also
temperature measuring devices for measuring the temperature of
the mixture and/or for measuring the temperatures of the
components are present in a device for carrying out the process
according to the invention.
According to a preferred embodiment, the two components are
mixed after the auxiliary reducing gas has been heated in a gas
furnace. This makes possible an improved temperature setting of
the reducing gas. The temperature of the reducing gas should
preferably be in the range 780 - 1050 C, according to the H2/C0
ratio in the reducing gas.
According to a preferred embodiment, top gas is taken off from
the reducing unit, and the auxiliary reducing gas is obtained
at least in part by mixing top gas that is dedusted and
substantially freed from CO2, and at least one further gas. In
this manner, the reductants (CO and H2) still present in the
top gas are utilized again for reducing the metal oxides.
Advantageously, the at least one further gas comprises the
hydrogen-rich fraction obtained in the separation of the gas
stream, preferably coke oven gas, containing not only
hydrocarbon but also hydrogen.
A CA 02837611 2013-11-28
PCT/EP2012/058360 - 10 -
2011P11617W0AU
In this manner, the reduction potential present in this
fraction is also utilized for reducing metal oxide; utilized,
especially in that the reduction rate - kinetics - is generally
more rapid via hydrogen:
3 Fe203+H2 3 2 Fe304+H20 A H298 = -2.72
[kJ/mol]
Fe304+H2 4 3Fe0+H20 A H298 = +59.83
[kJ/mol]
Fe0+3H2 4 Fe + 2H2+H20 A H298 = +29.60
[kJ/mol]
Advantageously, the gas furnace is operated with a fuel gas
which at least in part comprises at least one gas of the group
- tail gas formed in the removal of CO2 from the top gas,
- top gas,
- gas stream, preferably coke oven gas, containing not only
hydrocarbon but also hydrogen,
- hydrogen-rich fraction obtained by separation of the gas
stream, preferably coke oven gas, containing not only
hydrocarbon but also hydrogen,
- hydrocarbon-rich fraction obtained by separation of the gas
stream, preferably coke oven gas, containing not only
hydrocarbon but also hydrogen.
In this manner, these gases are utilized in the process for
reducing metal oxides, which increases the efficiency thereof.
When hydrogen-rich gases are used for firing the gas furnace
from below, the CO2 emission can be kept correspondingly low.
Single, a plurality of, or all of the corresponding fuel gas
feed line(s) to the gas furnace is/are present in a device for
carrying out the process according to the invention:
- a tail gas feed line for feeding tail gas produced in the
removal of CO2 from the top gas, which tail gas feed line exits
from the 002 removal plant.
CA 02837611 2013-11-28
PCT/EP2012/058360- 11 -
=
2011P11617W0AU
- A top gas feed line for feeding top gas, which top gas feed
line exits from a top gas outlet line withdrawing top gas from
the reducing unit.
- A fuel gas feed line for feeding gas stream containing not
only hydrocarbon but also hydrogen, which fuel gas feed line
exits from a feed line for a gas stream containing not only
hydrocarbon but also hydrogen and which itself opens out into a
device for separating a gas stream containing not only
hydrocarbon but also hydrogen into a hydrogen-rich fraction and
a hydrocarbon-rich fraction.
- A fuel gas feed line for feeding a hydrogen-rich fraction
obtained by separation of the gas stream, preferably coke oven
gas, containing not only hydrocarbon but also hydrogen,
which fuel gas feed line exits
from a device for separating a gas stream containing not
only hydrocarbon but also hydrogen into a hydrogen-rich
fraction and a hydrocarbon-rich fraction,
or from an outlet line for the hydrogen-rich fraction
which itself arises from
a device for separating a gas stream containing not
only hydrocarbon but also hydrogen into a hydrogen-rich
fraction and a hydrocarbon-rich fraction,
- a fuel gas feed line for feeding a hydrocarbon-rich,
hydrogen-rich fraction obtained by separation of the gas
stream, preferably coke oven gas, containing not only
hydrocarbon but also hydrogen, which fuel gas feed line exits
from a feed line for the hydrocarbon-rich, hydrogen-rich
fraction which itself
arises from a device for separating a gas stream
containing not only hydrocarbon but also hydrogen
into
CA 02837611 2013-11-28
PCT/EP2012/058360- 12 -
2011P11617W0AU
a hydrogen-rich fraction and a hydrocarbon-rich
fraction,
or a device for separating a gas stream containing not
only hydrocarbon but also hydrogen into a hydrogen-rich
fraction and a hydrocarbon-rich fraction.
Advantageously,
the reducing unit is a reducing shaft and
a first subquantity
of the hydrocarbon-rich fraction
is introduced directly into the reducing shaft,
and
a second subquantity
of the hydrocarbon-rich fraction
before introduction thereof into the reducing shaft
is subjected to at least one operation of the group
- oxidation using technically pure oxygen
- reforming using CO2 and H20,
and then is introduced at least as component of a reducing gas
into a reducing unit containing the metal oxides,
and the hydrocarbon content
is set by the at least one operation of said group, in such a
manner
that the hydrocarbon content in the reducing gas, on entry into
the reducing unit, is less than 12% by volume, preferably less
than 10% by volume, particularly preferably less than 8% by
volume.
The first subquantity can thus be utilized for carbonization of
the metal generated in the reducing unit; for example, it can
be utilized for carbonization of metallic iron.
CA 02837611 2013-11-28
PCT/EP2012/058360- 13 -
=
2011P11617W0AU
Advantageously, the at least one gas stream containing 002
and/or H20 is added to the hydrocarbon-rich fraction before
reforming using 002 and H20. In this process, this can be, for
example, steam, tail gas from a CO2 removal process - for
example from the removal of 002 from the top gas - top gas from
the reducing shaft, or converter gas. Water can also be added.
In this manner, these gases are utilized in the process for
reducing metal oxides, which increases the efficiency thereof,
and reduces the environmental emissions, since CO2 is converted
back to CO.
Corresponding feed lines for feeding one or more of these gases
which exit from devices producing such gases or lines bearing
such gases are present in a device for carrying out the process
according to the invention.
In the hydrocarbon-rich fraction, H2S is also enriched.
According to a preferred embodiment, therefore, desulfurization
of the hydrocarbon-rich fraction is carried out before it is
subjected to at least one operation of the group
- oxidation with technically pure oxygen,
- reforming using CO2 and H20 or.
The sulfur content can thereby be reduced in the largely
metalized metal.
In a device for carrying out a process according to the
invention, then, in a feed line for the hydrocarbon-rich
fraction 3, a desulfurization device is present, before - seen
in the direction of flow - the feed line opens out into a unit
for carrying out an operation of the group
- oxidation with technically pure oxygen,
- reforming using CO2 and H20.
CA 02837611 2013-11-28
PCT/EP2012/058360 = - 14 -
2011P11617W0AU
The process according to the invention has the following
advantages:
- efficient material utilization of coke oven gas for reducing
metal oxides, especially for reducing iron oxides for sponge
iron production - advantage in comparison with the thermal
utilization of coke oven gas proceeding to date according to
the prior art,
- in comparison with utilization of natural gas for reducing
metal oxides, especially for the reduction of iron oxides for
sponge iron production, high economic advantages in comparison
with natural gas, since the coke oven gas is produced at lower
costs
- very environmentally friendly process, in particular owing to
low CO2 and NO emissions, since firstly in some embodiments a
very hydrogen-rich gas can be used for the reduction and
secondly by utilization of low-carbon gases in the reformer
and/or gas furnace, emissions thereof can further be reduced.
- Furthermore, in the reformer, some of the CO2 emissions can
be converted back to CO and subsequently utilized for the
reduction.
The specific carbon emission factor in the case of coke oven
gas is 43.7 kg of CO2/GJ of fuel, while in the case of natural
gas it is 55.7 kg of CO2/GJ of fuel. The use of coke oven gas
is therefore considerably more environmentally friendly than
the use of natural gas.
A further subject matter of the present application is a device
for carrying out the process according to the invention
having a reducing unit for reducing metal oxides,
having a device for separating a gas stream containing not only
hydrocarbon but also hydrogen into a hydrogen-rich fraction and
a hydrocarbon-rich fraction,
having, arising therefrom, a
feed line for the hydrocarbon-rich fraction which
opens out
into a unit for
CA 02837611 2013-11-28
PCT/EP2012/058360 = - 14a -
2011P11617W0AU
carrying out an operation of the group
- oxidation using technically pure oxygen
- reforming using CO2 and H20,
and having one or more introduction lines for introducing at
least one gas stream from the group
CA 02837611 2013-11-28
=
PCT/EP2012/058360 = = - 15 -
2011P11617W0AU
- hydrocarbon-rich fraction,
- gas stream obtained in the unit for carrying out oxidation
using technically pure oxygen,
- gas stream obtained in the unit for carrying out reforming
using CO2 and H20,
into the reducing unit.
Preferably, the device for separating a gas stream containing
not only hydrocarbon but also hydrogen into a hydrogen-rich
fraction and a hydrocarbon-rich
hydrogen-rich fraction is
a device for separating coke oven gas into a hydrogen-rich
fraction and a hydrocarbon-rich fraction.
Preferably, the device for separating a gas stream containing
not only hydrocarbon but also hydrogen into a hydrogen-rich
fraction and a hydrocarbon-rich fraction is
a device of the group
- device for pressure-swing adsorption,
- device for membrane separation.
Preferably, the one or more introduction lines open out
into the reducing unit,
wherein
upstream of the opening of at least one of the introduction
lines into the
reducing unit, an
auxiliary reducing gas line for feeding auxiliary reducing gas
to the
reducing unit
opens out into this introduction line.
Preferably, upstream of the opening of the auxiliary reducing
gas line into the introduction line, a
gas furnace is present in the auxiliary reducing gas line.
CA 02837611 2013-11-28
PCT/EP2012/058360 = - 16 -
2011P11617W0AU
Preferably, x introduction lines are present, wherein x is
greater than 2 or is equal to 2, of the at most x-1 introduction
lines it is true that, upstream of the opening of at least one
of the introduction lines into the reducing unit, an auxiliary
reducing gas line, for feeding auxiliary reducing gas to the
reducing unit, opens out into this introduction line.
In this manner, at least one introduction line is present into
which no auxiliary reducing gas line opens out. Therefore, a
subquantity of the hydrocarbon-rich fraction can be introduced
directly into the reducing shaft without being mixed with
auxiliary reducing gas; this subquantity can be used, for
example, for carbonizing the metal generated in the reducing
unit; for example it can be used for carbonizing metallic iron.
According to one embodiment, the reducing unit is a reducing
shaft, for example a fixed-bed reducing shaft for carrying out
a MIDREXO or HYLO reduction process.
According to one embodiment, the reducing unit is a fluidized-
bed cascade.
Brief description of the drawings
With reference to the schematic and exemplary figures
hereinafter, the invention will be described with reference to
embodiments.
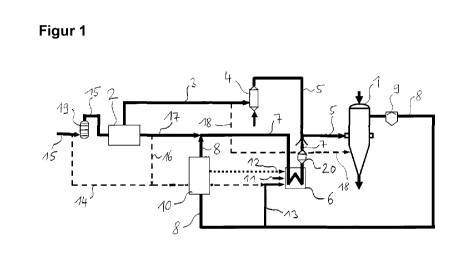
Figure 1 shows a device for carrying out a process according to
the invention, in which coke oven gas is separated into a
hydrogen-rich fraction and a hydrocarbon-rich fraction and the
latter is subjected to an oxidation before it is introduced
into a reducing shaft as part of a reducing gas.
Figure 2 shows a device and procedure similar to figure 1, with
the difference that the hydrocarbon-rich fraction is subjected
to
CA 02837611 2013-11-28
PCT/EP2012/058360 ' - 17 -
2011P11617W0AU
reforming using CO2 and H20 before it is introduced as part of
a reducing gas into a reducing shaft.
Figure 3 shows a device and procedure according to the invention
which chiefly differs from figure 1 in that a fluidized-bed
cascade is present as reducing unit, and the device present for
separating coke oven gas, instead of a device for pressure-swing
adsorption, is a device for membrane separation.
Figure 4 shows a device and procedure according to the
invention which chiefly differs from figure 1 in that a
fluidized-bed cascade is present as reducing unit, and the
device for separating coke oven gas, instead of a device for
pressure-swing adsorption, is a device for membrane separation.
Description of the embodiments
Figure 1 shows a device for carrying out a process according to
the invention. This comprises, as reducing unit for reducing
metal oxides, a reducing shaft 1 which contains iron ore, that
is to say iron oxides. It likewise comprises a device for
separating a gas stream containing not only hydrocarbon but
also hydrogen, in this case a PSA or a VPSA plant 2 using
pressure-swing adsorption, into a hydrogen-rich fraction and a
hydrocarbon-rich fraction. In the present example, the gas
stream containing not only hydrocarbon but also hydrogen is
coke oven gas. From the PSA or VPSA plant 2 there arises a feed
line for the hydrocarbon-rich fraction 3 which opens out into a
unit for carrying out an oxidation using technically pure
oxygen 4. In this unit for carrying out an oxidation using
technically pure oxygen 4, the hydrocarbon-rich fraction is
partially oxidized; that is, the entire amount of substance is
not oxidized, but only a part of the amount of substance of the
hydrocarbon-rich fraction. Via an introduction line 5 for
introducing the gas stream obtained in the unit for carrying
out oxidation using technically pure oxygen 4,
CA 02837611 2013-11-28
PCT/EP2012/058360= * - 18 -
2011P11617W0AU
this gas stream is introduced as a component of a reducing gas
into the reducing shaft 1. In the partial oxidation the
hydrocarbon content is set in such a manner that the
hydrocarbon content in the reducing gas is less than 12% by
volume on entry into the reducing shaft.
The gas stream obtained in the unit for carrying out oxidation
using technically pure oxygen 4 is mixed with an auxiliary
reducing gas, the resultant mixture is introduced as reducing
gas into the reducing shaft 1. The two components of the
reducing gas are mixed after the auxiliary reducing gas has
been heated in a gas furnace 6. The auxiliary reducing gas is
added via an auxiliary reducing gas line V for feeding
auxiliary reducing gas to the reducing unit 1, which reducing
gas line 7 opens out into the introduction line 5. Via the
introduction line 5, therefore, not only the gas stream
obtained in the unit for carrying out oxidation using
technically pure oxygen 4, but also the auxiliary reducing gas
is introduced into the reducing shaft 1, specifically as a
mixture termed reducing gas. The temperature preset of the
auxiliary reducing gas which is heated in the gas furnace 6 is
set in dependence on a temperature preset for the mixture. The
gas furnace 6 is arranged in the auxiliary reducing gas line 7.
From the reducing shaft 1, top gas is conducted away via a top
gas outlet line 8. The auxiliary reducing gas, in the example
shown, is formed by mixing dedusted - a gas scrubber 9 is present
in the top gas outlet line 8 - top gas that is largely freed from
CO2 - a CO2 removal plant 10 is present in the top gas outlet
line 8 - and a further gas. The further gas is the hydrogen-rich
fraction obtained in the separation of the coke oven gas.
The gas furnace 6 is operated using a fuel gas. The fuel gas is
burnt with feed of air through an air feed line 11 opening out
into the gas burner. The fuel gas consists of gases of the
group
- tail gas formed in the removal of CO2 from the top gas,
CA 02837611 2013-11-28
PCT/EP2012/058360 = - 19 -
2011P11617W0AU
- top gas,
- coke oven gas,
- hydrogen-rich fraction obtained by separation of coke oven
gas.
For feeding these gases into the gas burner 6, there are
present
- a tail gas feed line 12 for feeding tail gas formed in the
removal of 002 from the top gas which exits from the CO2
removal plant 10 and opens out into the gas burner,
- a top gas feed line 13 for feeding top gas which exits from
the top gas outlet line 8 conducting away top gas from the
reducing unit and opens out into the gas burner,
- a coke oven gas feed line 14 for feeding coke oven gas, which
exits from a feed line for coke oven gas 15 and opens out into
the top gas feed line 13,
- a hydrogen fraction feed line 16 which branches off from a
hydrogen fraction outlet line 17 exiting from the PSA or VPSA
plant 2 and opens out into the coke oven gas feed line 14.
In order that auxiliary reducing gas can be obtained by mixing
top gas that is dedusted and largely freed from CO2 and the
hydrogen-rich fraction obtained in the separation of the coke
oven gas, not only the hydrogen fraction outlet line 17 but
also the top gas outlet line 8 open out into the auxiliary
reducing gas line 7.
The feed line for coke oven gas 15 exits from a coke oven gas
source that is not shown and opens out into the PSA or VPSA
plant 2.
In the device shown in figure 1, two introduction lines opening
out into the reducing shaft 1 are present. The introduction
line 5, called first introduction line, has already been
described. A further introduction line, called second
introduction line 18, branches off from the feed line for the
hydrocarbon-rich fraction 3 and opens out into the reducing
shaft. Via this second introduction line 18, a subquantity of
the hydrocarbon-rich fraction can be introduced directly into
CA 02837611 2013-11-28
, .
'
PCT/EP2012/058360 - 19a -
2011P11617W0AU
the reducing shaft. This subquantity can thus be used for
carbonizing the metallic iron, in this case sponge iron,
generated in the reducing shaft 1. A cooling gas line for
feeding cooling gas into
= CA 02837611 2013-11-28
PCT/EP2012/058360 " - 20 -
2011P11617W0AU
the reducing shaft 1 is not shown for reasons of clarity; in
principle, for the purpose of carbonization, a subquantity of
the hydrocarbon-rich fraction could also be added to the
cooling gas via a corresponding branch from the feed line for
the hydrocarbon-rich fraction 3 which opens out into the
cooling gas line.
In the tar filter appliance 19 arranged in the feed line for
coke oven gas 15, tar is removed from the coke oven gas.
In the burner 20, the auxiliary reducing gas can be partially
oxidized with feed of technically pure oxygen, if this is
wanted for temperature elevation.
For reasons of clarity, depiction of device parts which are not
essential to the present invention has been dispensed with, for
example the depiction of diverse compressors, bypass lines, gas
holders, gas coolers, flare stacks.
In figure 2, in an otherwise similar device and procedure, the
hydrocarbon-rich fraction, instead of a partial oxidation, is
subjected to reforming using 002 and H20 before it is
introduced as part of a reducing gas into a reducing shaft.
Plant parts and process steps which are identical to figure 1
are not described again here for the most part, and the
reference signs for the same plant parts, for better clarity,
are not entered into the drawing. The reforming takes place in
a unit for carrying out reforming using 002 and H20, here a
reformer 21, into which the feed line for the hydrocarbon-rich
fraction 3 opens out. Off-gas from the reformer 21 is used via
a heat exchanger 22 for heating the hydrocarbon-rich fraction
before entering into the reformer 21.
Via a plurality of feed lines 23a, 23b, which open out into the
feed line for the hydrocarbon-rich fraction 3, before entry
into the reformer 21, a plurality of CO2-containing gas streams
are added to the hydrocarbon-rich fraction. Via feed line 23a,
tail gas from the 002
CA 02837611 2013-11-28
PCT/EP2012/058360 - 21 -
=
2011P11617W0AU
removal plant 10 is added; the feed line 23a arises from the
tail gas feed line 12. Via feed line 23b, top gas is added. Via
a water feed line 24 which opens out into the feed line for the
hydrocarbon-rich fraction 3, before entry into the reformer 21,
steam and/or water is added to the hydrocarbon-rich fraction.
The reformer 21 can be fired using top gas, coke oven gas or
with the hydrocarbon-rich fraction; corresponding lines opening
out into the reformer 21, for the sake of clarity, are not
shown.
Via a branch line 29 which branches off from the second
introduction line 18 and opens out into the first introduction
line 5, the hydrocarbon content in the reducing gas on entry
into the reducing shaft 1 can be influenced via the feed of
hydrocarbon-rich fraction.
In figure 3, the reducing unit is a fluidized-bed cascade 25,
from the last fluidized-bed reactor 26 of which, seen in the
direction of flow of the reducing gas, top gas is taken off;
the top gas line is given the reference sign 8, as is the top
gas line in figure 1. The introduction line 5, which in
figure 1 is shown opening out into the reducing shaft 1, is, in
figure 3, shown opening out into the first fluidized-bed
reactor 27, similarly seen in the direction of flow of the
reducing gas. As a device for separating coke oven
gas, - instead of, as in figure 1, a device for pressure-swing
adsorption - there is a device for membrane separation 28. Via
a branch from the feed line for the hydrocarbon-rich fraction
3, hydrocarbon-rich fraction can be fed into the first
introduction line 5, which offers a possibility for influencing
the hydrocarbon content in the reducing gas.
Figure 4 differs from figure 2 by the same modifications by
which figure 3 differs from figure 1. In addition, in figure 1,
in contrast to figure 2, no heat exchanger 22 is present.
CA 02837611 2013-11-28
PCT/EP2012/058360 - 22 -
=
2011P11617W0AU
List of reference signs
1 Reducing shaft
2 PSA or VPSA plant
3 Feed line for the hydrocarbon-rich
fraction
4 Unit for carrying out an oxidation
using technically pure oxygen
(First) introduction line
6 Gas furnace
7 Auxiliary reducing gas line for
feeding auxiliary reducing gas to
the reducing unit 1
8 Top gas outlet line
9 Gas scrubber
CO2 removal plant
11 Air feed line
12 Tail gas feed line
13 Top gas feed line
14 Coke oven gas feed line
Supply line for coke oven gas
16 Hydrogen fraction feed line
17 Hydrogen fraction outlet line
18 Second introduction line
19 Tar filter appliance
Burner
21 Reformer
22 Heat exchanger
23a, 23b Feed line
24 Water feed line
Fluidized-bed cascade
26 Last fluidized-bed reactor
. CA 02837611 2013-11-28
PCT/EP2012/058360= - 23 -
2011P11617W0AU
27 First fluidized-bed reactor
28 Device for membrane separation
29 Branch line