Note: Descriptions are shown in the official language in which they were submitted.
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
INSTRUMENTS AND DEVICES FOR SUBCHONDRAL JOINT REPAIR
TECHNICAL FIELD
The present invention relates to instruments and devices for the surgical
treatment of joints, and more particularly to instruments and devices for the
subchondral repair and treatment of bone tissue at these joints, and
associated
methods of use.
BACKGROUND ART
Human joints, in particular the knee, hip and spine, are susceptible to
degeneration from disease, trauma, and long-term repetitive use that
eventually lead
to pain. Knee pain, for example, is the impetus for a wide majority of medical
treatments and associated medical costs. The most popular theory arising from
the
medical community is that knee pain results from bone-on-bone contact or
inadequate
cartilage cushioning. These conditions are believed to frequently result from
the
progression of osteoarthritis, which is measured in terms of narrowing of the
joint
space. Therefore, the severity of osteoarthritis is believed to be an
indicator or
precursor to joint pain. Most surgeons and medical practitioners thus base
their
treatments for pain relief on this theory. For example, the typical treatment
is to
administer pain medication, or more drastically, to perform some type of joint
resurfacing or joint replacement surgery.
However, the severity of osteoarthritis, especially in joints such as the knee
and ankle, has been found to correlate poorly with the incidence and magnitude
of
knee pain. Because of this, surgeons and medical practitioners have struggled
to
deliver consistent, reliable pain relief to patients especially if
preservation of the joint
is desired.
Whether by external physical force, disease, or the natural aging process,
structural damage to bone can cause injury, trauma, degeneration or erosion of
otherwise healthy tissue. The resultant damage can be characterized as a bone
defect
1
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
that can take the form of a fissure, fracture, microfracture, lesion, edema,
tumor, or
sclerotic hardening, for example. Particularly in joints, the damage may not
be
limited to a bone defect, and may also include cartilage loss (especially
articular
cartilage), tendon damage, and inflammation in the surrounding area.
Patients most often seek treatment because of pain and deterioration of
quality
of life attributed to the osteoarthritis. The goal of surgical and non-
surgical
treatments for osteoarthritis is to reduce or eliminate pain and restore joint
function.
Both non-surgical and surgical treatments are currently available for joint
repair.
Non-surgical treatments include weight loss (for the overweight patient),
activity modification (low impact exercise), quadriceps strengthening,
patellar taping,
analgesic and anti-inflammatory medications, and with corticosteroid and/or
viscosupplements. Typically, non-surgical treatments, usually involving
pharmacological intervention such as the administration of non-steroidal anti-
inflammatory drugs or injection of hyaluronic acid-based products, are
initially
administered to patients experiencing relatively less severe pain or joint
complications. However, when non-surgical treatments prove ineffective, or for
patients with severe pain or bone injury, surgical intervention is often
necessary.
Surgical options include arthroscopic partial meniscectomy and loose body
removal. Most surgical treatments conventionally employ mechanical fixation
devices such as screws, plates, staples, rods, sutures, and the like are
commonly used
to repair damaged bone. These fixation devices can be implanted at, or around,
the
damaged region to stabilize or immobilize the weakened area, in order to
promote
healing and provide support. Injectable or fillable hardening materials such
as bone
cements, bone void fillers, or bone substitute materials are also commonly
used to
stabilize bone defects.
High tibial osteotomy (HTO) or total knee arthroplasty (TKA) is often
recommended for patients with severe pain associated with osteoarthritis,
especially
when other non-invasive options have failed. Both procedures have been shown
to be
effective in treating knee pain associated with osteoarthritis.
However, patients only elect HTO or TKA with reluctance. Both HTO and
TKA are major surgical interventions and may be associated with severe
2
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
complications. HTO is a painful procedure that may require a long recovery.
TKA
patients often also report the replaced knee lacks a "natural feel" and have
functional
limitations. Moreover, both HTO and TKA have limited durability. Accordingly,
it
would be desirable to provide a medical procedure that addresses the pain
associated
with osteoarthritis and provides an alternative to a HTO or TKA procedure.
One of the difficulties of currently available surgical access devices and
insertion tools is the ability to target a specific area of the bone to be
treated, in a fast,
accurate, easy and controlled manner. Presently, in order to treat or repair a
bone
defect at a joint, the surgeon often has to take multiple steps using multiple
surgical
tools in order to access, locate, and treat the target defect site. Even so,
the surgeon
does not have a reliable instrument or system that would allow him to easily
and
quickly target an area such as the subchondral region of a joint, and either
deliver or
remove material to that target region. In order to perform repeated or
multiple
procedures in the same defect location with the currently available tools,
additional
and unnecessary time in the operating room would be required, as well as an
increased risk for complications since numerous instruments and maneuvers are
at
play.
Accordingly, it is desirable to provide instruments that allow fast, easy, and
controllable surgical access to the target site, or the bone defect, to be
treated. It is
further desirable to provide instruments that enable the user to easily and
quickly
deliver or remove material at the target site for treatment.
3
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
SUMMARY OF INVENTION
The present disclosure provides instruments and associated methods for the
surgical repair and treatment of bone tissue, particularly of bone tissue at
joints. More
specifically, the present disclosure provides instruments that allow fast,
easy, precise,
and controllable delivery or removal of materials subchondrally to a bone
joint being
treated.
In one embodiment, a delivery instrument is provided. The instrument may
take the form of an injection needle. The injection needle may be configured
to
deliver material to a subchondral area of a bone joint to be treated. The
injection
needle may include a sharp tip, elongate shaft, and tool attachment end. In
use, the
injection needle may be threaded into the bone to be treated. The injection
needle
may be used with a stabilization instrument. The tool attachment end may be
threaded for threaded connection to other instruments.
In another embodiment, a delivery instrument is provided. The instrument
may be an injection needle configured to deliver material to a subchondral
area of a
bone joint to be treated. The injection needle may comprise a sharp tip, a
tool
attachment end, and an elongate shaft extending therebetween. The needle may
be
partially or wholly cannulated. It may also be fenestrated with helical
grooves or
spiral cutouts inside of which can reside injection ports or holes. These
cutouts may
serve as surface features that enable the injection needle to be threaded into
bone
tissue while the holes may allow the delivery of a material to the subchondral
area of
the bone. The cutouts may also direct or guide the flow of material out of the
holes.
Optionally, threads may be provided. The threads allow the needle to be
threaded
into the bone tissue during use.
In still another embodiment, a delivery instrument in the form of a semi-
cannulated pin is provided. The semi-cannulated pin can be drilled into bone
to
deliver material. The pin may have etchings or indicia along the shaft that
indicate
depth or distance from the tip or relative distance to another marker. The pin
may
also have a marker, such as a visual or tactile marker, that indicates
directionality. A
secondary pin may also be placed inside the first pin and rotated to control
the
4
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
opening and closing of the hole or fenestration, and thereby the delivery of
material
out of the system.
In yet another embodiment, a delivery system is provided. The system may
comprise a fenestrated cannula attached to a handle. The system may further
include
a stabilizer. The stabilizer may include a pin configured to slide into the
cannula to
stabilize it as it is being inserted into the subchondral region of the bone.
After
insertion, an injectable material may be deployed through the cannula. The pin
may
be driven down into the cannula forcing the material into the bone. The
cannula may
also be retracted so that the pin would force more material into the bone,
with the
injection rate being proportional to the retraction rate.
In still yet another embodiment, a delivery instrument is provided. The
instrument may comprise a tip, an elongate shaft, a device attachment end, and
a
removable and slidable sleeve. The instrument may attach to a fenestrated
cannula at
the tip, while the device attachment end may attach to an injection system.
The sleeve
may comprise a split ring attached to a tether that connects to a band around
the
device attachment end. The sleeve is configured to slide over the elongate
shaft of the
instrument and the cannula, covering the fenestrations of the cannula.
In even still another embodiment, a gauge is provided. The gauge may be
used with the cannulas or pins to provide volumetric and pressure readings
while also
a mechanical assist. The gauge allows the user even greater control over the
amount
of material injected into the bone as well as the volume and pressure of the
material.
In another embodiment, two or more fenestrated cannulas can be used to allow
both injection and removal of material from a bone to be treated. A bone plug
may be
provided to prevent injected material from exiting through the cannula at its
tip
instead of through the sides via the fenestrations. The plug may also be used
to plug
up any access holes created during the surgery.
In still another embodiment, a bone restricter or plug may be provided to
restrict the flow of materials. The plug may be delivered into the subchondral
space
and allowed to expand or at the same time or before the material is injected.
The plug
may be provided to prevent injected material from exiting through the cannula
at its
tip instead of through the sides via the fenestrations.
5
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
In yet another embodiment, a fenestrated cannula may be provided with a one-
way valve. The valve may allow the passage of a pin or wire, while restricting
any
backflow of material or bone substance.
In another embodiment, highly porous implants are provided. These implants
may be housed internally within the cannula or other delivery instrument. The
pores
allow for the flow of material out of the cannula but also redirect or induce
dispersion
of the material during injection.
In still another embodiment, an injection material delivery system is
provided.
The system may comprise two components: an outer cannula with an open tip and
an
inner rod configured to slide inside the outer cannula, the inner rod having a
closed
end. Each of the outer cannula and inner rod have openings that can be aligned
together to open the orifices, or misaligned to close the orifices. A locking
mechanism can be provided to maintain the two components together. The system
can be used with a plunger that is configured to be inserted through the outer
cannula
after the inner rod has been removed. A plug, such as for instance an
allograft plug,
may be used with the system to further prevent backflow of material out of the
bone
to be treated.
It is to be understood that both the foregoing general description and the
following detailed description are exemplary and explanatory only and are not
restrictive of the disclosure. Additional features of the disclosure will be
set forth in
part in the description which follows or may be learned by practice of the
disclosure.
6
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part
of this specification, illustrate several embodiments of the disclosure and
together
with the description, serve to explain the principles of the disclosure.
FIG. 1 shows a perspective view of an injection needle of the present
disclosure.
FIG. 2A shows the injection needle of FIG. 1 in use with a stabilization
instrument.
FIG. 2B shows the injection needle of FIG. 1 in use with an instrument
connection.
FIG. 3A shows a perspective view of another injection needle of the present
disclosure.
FIG. 3B shows an enlarged view of the tip of the injection needle of FIG. 3A.
FIG. 4A shows a perspective view of yet another injection needle of the
present disclosure.
FIG. 4B shows an enlarged view of the tip of the injection needle of FIG. 4A.
FIG. 5A shows a fenestrated pin.
FIG. 5B shows a delivery system comprising the fenestrated pin of FIG. 5A
with an associated internal pin.
FIGS. 6A and 6B show a system comprising a stabilization instrument in use
with a cannula of the present disclosure.
FIG. 6C is a cross-sectional view of the system of FIG. 6A during use.
FIG. 6D is a cross-sectional view of the system of FIG. 6A after use.
FIG. 7A shows a perspective view of another delivery instrument of the
present disclosure having a fenestration cover.
7
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
FIG. 7B shows another perspective view of the instrument of FIG. 7A with the
fenestrations closed.
FIG. 7C shows a system comprising the instrument and fenestration cover of
FIG. 7A with an injection needle and stabilization instrument.
FIGS. 7D and 7E illustrate a method of using the system of FIG. 7C.
FIG. 8 shows a perspective view of a gauge and mechanical assist mechanism
of the present disclosure.
FIG. 9 shows a method of delivering and removing a material from an area of
a bone joint to be treated.
FIG. 10 shows a cannula with an attached bone plug.
FIG. 11A shows a cannula with an artificial plug.
FIG. 11B shows an enlarged view of the artificial plug of FIG. 11A.
FIG. 12 shows a partial exposed view of a cannula with a valve.
FIG. 13 shows a perspective view of a highly porous implant of the present
disclosure.
FIG. 14 shows a perspective view of a highly porous implant of the present
disclosure.
FIG. 15A shows a first component of still yet another exemplary embodiment
of an injection material delivery system of the present disclosure.
FIG. 15B shows an enlarged view of the first component of FIG. 15A.
FIG. 16A illustrates another component of the injection material delivery
system of FIG. 15A.
FIG. 16B shows an enlarged view of the second component of FIG. 16A.
FIG. 17A shows an exploded view of the system of FIG. 15A.
8
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
FIG. 17B shows an enlarged view of the system of FIG. 15A together.
FIG. 18 shows a locking mechanism of the system of FIG. 15A.
FIG. 19A shows the system of FIG. 15A in use with a plunger.
FIG. 19B shows an exploded view of FIG. 19B.
9
CA 02838816 2016-10-20
WO 2012/170805 PCT/US2012/041534
DETAILED DESCRIPTION OF THE EMBODIMENTS
The present disclosure provides methodologies, devices and instruments for
diagnosing and treating joint pain to restore natural joint function and
preserving, as
much as possible, the joint's articular and cartilage surface. Treatments
through the
joint that violate the articular and cartilage surface often weaken the bone
and have
unpredictable results. Rather than focusing on treatment of pain through the
joint,
alternative treatments that diagnose and treat pain at its source in the
subchondral
region Of a bone of a joint to relieve the pain are provided. Pain associated
with
joints, especially osteuarthritic joint.s, can be correlated to bone defects
or changes at
the subchondral level rather than, for example, the severity of osteoarthritic
progression or defects at the articular surface level. In particular, hone
defects, such
as hone marrow lesions, edema, fissures, fractures, hardened bone, etc. near
the joint
surface lead to a mechanical disadvantage and abnormal stress distribution in
the
periarticular bone, which may cause inflammation and generate pain. By
altering the
makeup of the periarticular bone (Which may or may not be sclerotic) in
relation to
the surrounding region, it is possible to change the structural integrity of
the affected
hone and restore normal healing function, thus leading to a resolution of the
inflammation surrounding the defect.
Treatment of the bone by mechanical and biological means to restore the
normal physiologic stress distribution, and restore the healing balance of the
bone
tissue at the subchondral level, is a more effect way of treating pain than
conventional
techniques. That is, treatment can be effectively achieved by mechanically
strengthening or stabilizing the defect, and biologically initiating or
stimulating a
healing response to the defect. Methods, devices, and systems for a
subchondral
procedure that achieve these goals are disclosed in co-owned U.S. Patent No.
8,062,364 entitled "OSTEOARTHRITIS TREATMENT AND DEVICE" as well as
in co-owned and co-pending U.S. Patent Application Publication Nos.
2011/0125156
entitled "MFI1101) FOR TREATING JOINT PAIN AND ASSOCIATED
INSTRUMINIS" and 2011/0125157 entitled "SUBCHONDRAI, TREATMNNT OF
JOINT PAIN," both of which were tiled on November 19, 2010.
This suhchondral procedure,
and its associated devices, instruments, etc. are also marketed under the
registered
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
trademark name of SUB CHONDROPLASTY(TM). The
SUBCHONDROPLASTY(TM) procedure is a response to a desire for an alternative
to patients facing partial or total knee replacement.
In general, the SUBCHONDROPLASTY(TM) or SCP(TM) technique is
intended to both strengthen the bone and stimulate the bone. In SCP(TM), bone
fractures or non-unions are stabilized, integrated or healed, which results in
reduction
of a bone defect, such as a bone marrow lesion or edema. In addition, SCP(TM)
restores or alters the distribution of forces in a joint to thereby relieve
pain. SCP(TM)
can be performed arthroscopically or percutaneously to treat pain by
stabilizing
chronic stress fracture, resolving any chronic bone marrow lesion or edema,
and
preserving, as much as possible, the articular surfaces of the joint.
SUBCHONDROPLASTY(TM) generally comprises evaluating a joint, for example,
by taking an image of the joint, detecting the presence of one or more
subchondral
defects, diagnosing, which of these subchondral defects is the source of pain,
and
determining an extent of treatment for the subchondral defect. The technique
is
particularly suited for treating chronic defects or injuries, where the
patient's natural
healing response has not resolved the defect. It should be noted, however,
that the
technique is equally applicable to treatment of defects in the subchondral
region of
bone where the defect is due to an acute injury or from other violations.
Several
exemplary treatment modalities for SCP(TM) for the different extents of
treatment
needed can be employed. Accordingly, a medical practitioner may elect to use
the
techniques and devices described herein to subchondrally treat any number of
bone
defects, as he deems appropriate.
Detection and identification of the relevant bone marrow lesion or bone
marrow edema (BML or BME) can be achieved by imaging, e.g., magnetic resonance
imaging (MRI), X-ray, bone scans, manual palpation, chemical or biological
assay,
and the like. A Ti-weighted MRI can be used to detect sclerotic bone, for
example.
Another example is that a T2-weighted MRI can be used to detect lesions,
edemas,
and cysts. X-ray imaging may be suitable for early-stage as well as end-stage
arthritis. From the imaging, certain defects may be identified as the source
of pain.
In general, defects that are associated with chronic injury and chronic
deficit of
healing are differentiated from defects that result, e.g., from diminished
bone density.
11
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
SCP(TM) treatments are appropriate for a BML or BME that may be characterized
as
a bone defect that is chronically unable to heal (or remodel) itself, which
may cause a
non-union of the bone, stress or insufficiency fractures, and perceptible
pain. Factors
considered may include, among other things, the nature of the defect, size of
the
defect, location of the defect, etc. For example, bone defects at the edge
near the
articular surface of periphery of a joint may be often considered eligible for
treatment
due to edge-loading effects as well as the likelihood of bone hardening at
these
locations. A bone defect caused by an acute injury would generally be able to
heal
itself through the patient's own natural healing process. However, in such
situations
where the bone defect is due to an acute injury and either the defect does not
heal on
its own, or the medical practitioner decides that the present technique is
appropriate,
SCP(TM) treatment can be administered on acute stress fractures, BML or BME,
or
other subchondral defects, as previously mentioned.
The SCP(TM) treatment may continue after surgery. In particular, the patient
may be monitored for a change in pain scores, or positive change in function.
For
example, patients are also checked to see when they are able to perform full
weight-
bearing activity and when they can return to normal activity. Of note, the
SCP(TM)
procedure can be revised and thus allows for optional further treatment in the
event
that a patient requires or desires a joint replacement or other type of
procedure. The
procedure does not exclude a future joint repair or replacement treatment to
be
applied, and thus may also be performed in conjunction with other procedures,
such
as cartilage resurfacing, regeneration or replacement, if desired. In those
instances
where additional treatment is desired, the SCP(TM) treated area may remain
undisturbed while the additional treatment is performed, such as where
cartilage
resurfacing is desired. Alternatively, the SCP(TM) treated area can be
removed, and
not create an obstacle to the additional treatment, such as where a partial or
total joint
replacement is desired. Advantageously, the SCP(TM) treatment may be provided
as
a first or initial treatment, reserving for the future and possibly
forestalling until a
later date than otherwise might be the case more invasive treatments such as
partial or
total joint replacement.
A number of treatment modalities, and associated devices, instruments and
related methods of use for performing SUB CHONDROPLASTY(TM) are disclosed
12
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
in the aforementioned publications. These treatment modalities may be used
alone or
in combination.
In one treatment modality, the subchondral bone in the region of the bone
marrow lesion or defect can be strengthened by introduction of a hardening
material,
such as a bone substitute, at the site. The bone substitute may be an
injectable
calcium phosphate ensconced in an optimized carrier material. In SCP(TM), the
injected material may also serve as a bone stimulator that reinvigorates the
desired
acute bone healing activity.
For example, polymethylmethacrylate (PMMA) or calcium phosphate (CaP)
cement injections can be made at the defect site. PMMA injection may increase
the
mechanical strength of the bone, allowing it to withstand greater mechanical
stresses.
CaP cement injection may also increase the mechanical strength of the bone,
while
also stimulating the localized region for bone fracture repair. In one
embodiment, the
injection can be made parallel to the joint surface. In another embodiment,
the
injection can be made at an angle to the joint surface. In yet another
embodiment, the
injection can be made below a bone marrow lesion. Preferably, the injection is
made
without disrupting the joint surface.
In another treatment modality, the subchondral bone region can be stimulated
to trigger or improve the body's natural healing process. For example, in one
embodiment of this treatment modality, one or more small holes may be drilled
at the
region of the defect to increase stimulation (e.g., blood flow, cellular
turnover, etc.)
and initiate a healing response leading to bone repair. In another embodiment,
after
holes are drilled an osteogenic, osteoinductive, or osteoconductive agent may
be
introduced to the site. Bone graft material, for example, may be used to fill
the hole.
This treatment modality may create a better load-supporting environment
leading to
long term healing. Electrical or heat stimulation may also be employed to
stimulate
the healing process of a chronically injured bone. Chemical, biochemical
and/or
biological stimulation may also be employed in SCP(TM). For instance,
stimulation
of bone tissue in SCP(TM) may be enhanced via the use of cytokines and other
cell
signaling agents to trigger osteogenesis, chondrogenesis, and/or angiogenesis
to
perhaps reverse progression of osteoarthritis.
13
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
In yet another treatment modality, an implantable device may be implanted
into the subchondral bone to provide mechanical support to the damaged or
affected
bone region, such as where an insufficiency fracture or stress fracture has
occurred.
The implant may help create a better load distribution in the subchondral
region. In
the knees, the implant may support tibio-femoral compressive loads. In
addition, the
implant may mechanically integrate sclerotic bone with the surrounding healthy
bone
tissue. The implants may be place in cancellous bone, through sclerotic bone,
or
under sclerotic bone at the affected bone region. The implant may also be
configured
as a bi-cortical bone implant. In one embodiment, one side of the implant can
be
anchored to the peripheral cortex to create a cantilever beam support (i.e., a
portion of
the implant is inserted into bone but the second end stays outside or near the
outer
surface of the bone). The implant may be inserted using a guide wire. In one
example, the implant may be inserted over a guide wire. In another example,
the
implant may be delivered through a guide instrument.
The implant may further be augmented with a PMMA or CaP cement
injection, other biologic agent, or an osteoconductive, osteoinductive and/or
osteogenic agent. The augmentation material may be introduced through the
implant,
around the implant, and/or apart from the implant but at the affected bone
region,
such as into the lower region of a bone marrow lesion or below the lesion. For
example, the implant may act as a portal to inject the augmentation material
into the
subchondral bone region.
While each of the above-mentioned treatment modalities may be administered
independent of one another, it is contemplated that any combination of these
modalities may be applied together and in any order so desired, depending on
the
severity or stage of development of the bone defect(s). Suitable implantable
fixation
devices for the surgical treatment of these altered bone regions or bone
defects,
especially at the subchondral level, are disclosed in co-pending and co-owned
U.S.
Patent Application Publication No. 2011/0125265 entitled "IMPLANTABLE
DEVICES FOR SUBCHONDRAL TREATMENT OF JOINT PAIN," U.S. Patent
Application Publication No. 2011/0125264 entitled "IMPLANTABLE DEVICES
FOR SUBCHONDRAL TREATMENT OF JOINT PAIN," and U.S. Patent
Application Publication No. 2011/0125272 entitled "BONE-DERIVED
14
CA 02838816 2016-10-20
WO 2012/170805 PCT/1JS2012/041534
IMPLANTABLE DEVICES FOR SIIIICHONDRAL TREATMENT OF JOINT
PAIN," all of which were filed on November 19, 2010.
These devices and instruments can
he use in combination with cements or hardening materials commonly used to
repair
damaged bone by their introduction into or near the site of damage, either to
create a
binding agent, cellular scaffold or mechanical scaffold for immobilization,
regeneration or remodeling of the bone tissue. As previously stated, treatment
of the
bone defect at the subchondral level preferably is performed without
disrupting the
joint surface.
In general, the present disclosure provides embodiments related to instruments
and associated methods for the surgical treatment of a joint, and particularly
Lo a bone
defect at that joint region. More specifically, the embodiments relate to
instruments
for navigating and positioning devices into an area sufficiently near a defect
(a' the
joint. Even more specifically, the instruments and associated methods for use
are
suitable for the repair Of a femoral bone of a knee joint. These instruments
amid
devices may be used in a manner consistent with the subchondral procedures
previously described.
In a healthy joint such as a tibio-lemoral joint, the compressive load between
the contact bones (i.e., the femur and the tibia) is properly distributed,
thus keeping
the contact stresses in the cartilage to a reasonably low level. As the
cartilage starts to
wear out or degenerate locally, the tibio-femoml contact area reduces and
starts to get
localized at the site of the cartilage defect. The localization of the
stresses may also
occur clue to vams or valgus deformity. Sometimes, the condition may occur
because
of osteoporosis, where bone becomes weak and is no longer able to support
normal
loads. This condition leads to higher localized contact stresses in the
cartilage, and
the subchondral region below the cartilage. Once the stresses reach beyond a
certain
threshold level, it leads to defects like bone marrow lesions and edema, and
perhaps
generates knee pain. 11' the problem persists, the high contact stresses can
lead to
sclerotic bone fonnation as well. The presence of sclerotic bone can
compromise
vascularization of the local area, and also create a mechanical mismatch in
the bone
tissue. This mismatch may start to expedite degeneration of all pans of the
joint
leading to increased levels of osteoarthritis.
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
Pain associated with osteoarthritic joints can be correlated to bone defects
or
changes at the subchondral level. In particular, bone defects such as bone
marrow
lesions, edema, fissures, fractures, etc. near the joint surface lead to
abnormal stress
distribution in the periarticular bone, which may or may not cause
inflammation and
generate pain. By altering the makeup of the periarticular bone (which may or
may
not be sclerotic) in relation to the surrounding region, it is possible to
change the
structural integrity of the affected bone, leading to a resolution of the
inflammation.
Treatment of the bone in an effort to alter the structural makeup of the
affected
periarticular bone leads to reduced inflammation and pain has proven to be
successful.
Over time, restoration of normal physiologic stress distribution can be
achieved in
load bearing joints such as the hip and knee, and mechanical congruity
restored,
thereby resulting in healing of the inflammation and reduction or elimination
of pain.
As previously mentioned, there is a need for surgical instruments that will
facilitate the application of the methodologies described above at the target
site, or the
bone defect, to be treated. Applicants have discovered instruments that are
particularly suitable for accessing certain areas of the bone within the range
of about
2-15 mm from the bone surface, and more commonly about 5-10 mm from the bone
surface, such as the articular surface or the subchondral bone area, and
therefore
require more precise defect location features. These instruments are also
particularly
suited to deliver bone substitute material, devices, implants, etc. without
disrupting
the joint surface. Accordingly, the present disclosure provides suitable
instruments
and associated methods for the surgical treatment of these bone defects,
especially at
the subchondral level near sclerotic bone.
In general, the embodiments relate to instruments and associated methods for
the surgical treatment of a joint, and particularly to a subchondral bone
defect at that
joint region. More specifically, the embodiments relate to instruments that
allow fast,
easy, precise, and controllable delivery or removal of materials subchondrally
to a
bone joint being treated.
Turning now to the drawings, FIG. 1 shows an exemplary embodiment of a
delivery instrument of the present disclosure. The delivery instrument may
take the
form of an injection needle 100 that is configured to deliver a material to a
subchondral area of a bone joint to be treated. The injection needle 100 may
include a
16
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
trocar tip 102, an elongate shaft 106 and an attachment end 104. The
attachment end
104 may be threaded for threaded connection to other delivery instruments or
tools,
such as a syringe. The trocar tip 102 may be closed (as shown), though the
elongate
shaft 106 may be cannulated. The tip 102 may be a drill point or cutting
blade, if so
desired, instead of the trocar tip. As further shown, the region near the tip
102 may
also include one or more holes 108 for delivery of a material out of the
needle 100
(i.e., the needle 100 is fenestrated). The elongate shaft 106 may also include
surface
features that would allow the user to thread the injection needle 102 into
bone tissue
during use. These surface features may comprise helically cut grooves 110 on
the
outer surface of the elongate shaft 106 and are recessed below the outer
surface.
Alternatively, the surface features may comprise threads on the outer surface
of the
elongate shaft 106 and are raised above the outer surface.
In use, the injection needle 100 may be threaded into the bone to be treated.
The tip 102 may be configured to pierce hardened, sclerotic bone. The theading
of
the injection needle 102 allows the user some level of control and stability
during the
injection of a material to the subchondral area to be treated. For example,
the threads
110 may serve as a seal, preventing the backflow of material out of the
insertion
portal. In addition, the threads 110 may also provide control, preventing the
user
from going too deep into the bone tissue. It is contemplated that the threads
110
would be employed in the cortical bone region of the bone to be treated.
FIG. 2A illustrates the injection needle 100 of FIG. 1 in use with a
stabilization instrument that, in the present example, may take the form of a
handle
120. The handle 120 may comprise a main body 122 having gripping portions 124
and a central channel 106 for receiving the injection needle 100. The gripping
portions 124 may include a gripping surface 128 to facilitate manually
controlling the
threading of the injection needle 100 into bone tissue.
FIG. 2B illustrates the injection needle 100 of FIG. 1 in use with an
additional
component, an instrument connection 140. This instrument connection 140 may be
configured to slide over and secure onto the threaded end 104 of the injection
needle
100, or it may be configured to attach to the injection needle 100 at the base
of the
connection 140. In the present example, the instrument connection 140 may be a
drill
17
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
adapter that is configured to allow attachment of the injection needle 100 to
a drill or
power driver.
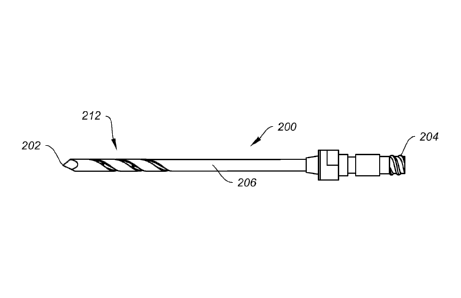
FIG. 3A shows another exemplary embodiment of an injection needle 200 of
the present disclosure. The injection needle 200 shares similar features with
injection
needle 100, and has a sharp tip 202, an elongate shaft or body 206 and a tool
attachment end 204. The closed end, sharp tip 202 may be a trocar tip, drill
point or
cutting blade, as desired. The elongate body 206 may be cannulated, and the
attachment end 204 may be threaded to allow threaded connection to other
instruments. Like injection needle 100, the present injection needle 200 may
also be
fenestrated, with a helix port region 212 comprising a helical groove or
spiral cutout
214 inside of which can reside injection ports or holes 216, as shown in
greater detail
in FIG. 3B. The helical cutout 214 may serve as threads or other surface
enhancement, enabling the injection needle to be threaded into bone tissue
while the
holes 216 may allow the delivery of a material to the subchondral area of the
bone to
be treated. It is contemplated that the helical cutout 214 may also serve an
additional
function of directing or guiding the flow of material out of the holes 216.
FIGS. 4A and 4B show still another exemplary embodiment of a delivery
instrument in which the injection needle 200 of FIGS. 3A and 3B includes
threads
210. In this embodiment, the injection needle 200 may be threaded into the
bone
tissue during use. For example, the injection needle 200 may be threaded into
the
outer cortical bone for additional control or stability, as previously
discussed.
FIG. 5A shows yet another exemplary embodiment of a delivery instrument of
the present disclosure that may take the form of a semi-cannulated pin 300
that can be
drilled into bone and deliver material in a controlled manner. The pin 300 may
include a sharp cutting tip 302 that can be a trocar tip, drill tip or cutting
blade. The
tip 302 extends into an elongate body 306 and terminates into an instrument
attachment end 304. As with the previous delivery instruments, the pin 300 may
be
fenestrated and include a hole 308 near the tip 302. Along the shaft 306 may
be
etchings or indicia 310 that indicate depth or the distance from the tip, or
relative
distance to another marker. The etchings 310 could also correspond to the
holes 308
or fenestrations. Another visual marker or window 312 may also be provided on
the
shaft 306, as well as a tactile marker 314 that may correspond with the
fenestration(s)
18
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
and indicate directionality to the user. This tactile marker 314 could be a
unidirectional protrusion that corresponds to the orientation of the hole 308
to help the
user align the hole 308 during use.
It is contemplated that the pin 300 would include a tip 302 sharp enough to
cut
through bone tissue. The fenestration(s) or holes 308 would feed to the back
of the
pin 300, with the etchings 310 facilitating the control of delivery. Further,
the
attachment end 304 would allow connection to another instrument such as a
drill or
syringe. For example, the attachment end 304 could cooperate with a Luer lock
adapter or other similar adapters.
FIG. 5B shows a system 330 comprising the pin 300 of FIG. 5A along with a
secondary pin 320 having an elongate shaft 322 and a spring-type tong 324 or
other
blocking mechanism that may block the hole or fenestration 308 of pin 300 when
the
secondary pin 320 is inside. This secondary pin 320 may be placed inside the
first pin
300 and rotated about so as to control the opening and closing of the hole or
fenestration 308, and thereby the delivery of material out of the system 330.
Additionally, the secondary pin 320 also serves other functions. In one
example, the
secondary pin 320 may be inserted into primary pin 300 and the tong 324
adjusted so
as to block the fenestration 308 prior to insertion. Blockage of the
fenestration 308
prevents the hole from getting clogged as it is being drilled inside the body,
as
material may become trapped in the hole if it is not covered. In another
example, the
secondary pin 320 may additionally be used to remove any remaining material
inside
the primary pin 300 after extrusion of material out of the hole or
fenestration 308,
acting as a plunger to rid the primary pin 300 of remaining material.
During use, the primary pin 300 may be drilled into bone with the internal or
secondary pin 320 in place and configured as to cover the hole 308. The
external
etchings 310, window 312 and tactile marker 314 would be used to control depth
and
orientation of the pin 300. After the drill is taken off the pin 300, which
may or may
not have an AO connection, the secondary pin 320 would be removed by pulling
out
to leave a cannulation to the fenestrated hole 308. In this case, the tip 302
of the pin
is solid and sharp, not cannulated. The cannulation is contemplated as
reaching the
hole or fenestration 308. Once the primary pin 300 has reached its final
destination,
the secondary pin 320 is removed and the primary pin 300 would be oriented by
19
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
twisting it axially to direct the injectable material to be delivered. The
hole 308 may
be oriented by using the tactile and visual markings provided on the pin 300.
A
syringe could be connected to the attachment end 304 of the pin to inject the
material
to the desired bone area. After injection, the pin 300 may be removed from the
bone.
FIGS. 6A-6D show another exemplary delivery system that allows for
injection with retraction. As shown in FIGS. 6A and 6B, the system 400
comprises a
fenestrated cannula 410 having one or more fenestrations or holes 412 for
delivering a
material to a target bone site. The cannula 410 may be attached to a handle
420 as
shown. The system may further include a stabilizer 430 that is configured to
cooperate with the cannula 410. The stabilizer 430 may include a pair of
bumpers
436 extending from a pair of arms 432 of the main body 434. Between the arms
432
and extending from the main body 434 may be a pin 438 that is configured to
slide
into the cannula 410, thereby stabilizing the cannula 410 as it is being
inserted into
the subchondral region of the bone 2 to be treated.
The pin 438 may be configured to have a tight fit with the cannula 410 in
order to minimize backflow. The stabilizer 430 can be configured to rest
against the
patient's body, bone, muscle, fat, etc. with the tip of the pin 410 relatively
close to the
stabilizing surface, or the edge of the bumpers 436. For example, the bumpers
436
may comprise shaped portions that complement the surface of the patient's
anatomy
and allow the bumpers 436 to rest against the surface of bone. Additionally,
the
bumpers 436 may be movable or pivotable relative to the main body 434 to allow
adjustment to the patient's anatomy. The stabilizer 430 is configured to allow
the
cannula 410 to be fully retracted to a state where the pin 438 is proud.
FIGS. 6C and 6D show in greater detail a method of using the system 400 of
the present disclosure. After insertion of the cannula 410 into the bone 2, an
injectable material 10 may be deployed through the cannula 410. As shown, the
stabilizer 430 can then be used. The pin 438 may be driven down into the
cannula
410 forcing the injectable material 10 into the bone 2 until the bumpers 436
rest
against the patient's body. At this point, the cannula 410 may be pulled back
with
respect to the stabilizer 430, which remains in place with respect to the
patient. As
the cannula 410 is retracted, the pin 438 would force more of the injectable
material
10 into the bone 2, with the injection rate being proportional to the
retraction rate. It
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
is contemplated that the injectable material 10 will continue to eject so long
as the
cannula 410 continues to be pulled back. When the tip of the pin 438 is
outside of the
bone 2, the cannula 410 should be entirely retracted while the injected
material 10 is
left inside in the cavity left behind by the cannula 410.
FIGS. 7A-7E illustrate still another exemplary embodiment of a delivery
system of the present disclosure. FIGS. 7A and 7B illustrate an auxiliary
delivery
instrument 500 having a removable and slidable cover or sleeve 520 configured
to
cooperatively work with an injection needle, such as injection needle 100. The
plunging device or delivery instrument 500 may be configured with a tip 502,
elongate shaft 506 and device attachment end 504. The tip 502 may be
configured for
engagement with the injection needle, as shown in FIGS. 7C-7E, while the
elongate
shaft 506 may be cannulated. A syringe or injector system may be attached to
the
device attachment end 504 for delivery of material therethrough. The
attachment end
504 may further include a band 512 from which extends a tether 514 that ends
in the
removable sleeve. In this exemplary embodiment, the removable sleeve may be a
split ring 516, configured for sliding engagement with the tip 502 and shaft
506 of the
instrument 500. The split ring 516 can snap over, and slide along, the shaft
506 to the
tip 502, as well as along the shaft 106 of the injection needle 100 and over
the
fenestrations 108, in order to prevent the backflow of material out of the
injection site.
The tether 514 has a length sufficient to allow the split ring 516 to extend
the length
of the shafts of the instrument and injection needle when both are connected,
as
shown in FIGS. 7C-7E. The length of the split ring and tether could be such
that the
instrument 500 acts as a depth control stop or index, or provide needle depth
control,
when the needle is inserted into the bone 2.
FIGS. 7C-7E show the auxiliary delivery instrument 500 attached to a needle
100 and in use with the stabilizing instrument 120 of the present disclosure.
This
embodiment has a relatively smaller body contact area compared with previous
embodiments. In a method of implementing the instrument 500, the needle 100 is
first inserted into the bone 2 to be treated. An injectable material may then
be
injected through the needle 100, through instrument 500, and into the bone 2.
As
shown in FIG. 7C, the split ring 516 may slide over the elongate shaft of
injection
needle 100 and against the bone 2, covering the fenestrations 108. During use,
the
21
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
split ring 516 of the instrument 500 is pushed against the side of the bone 2,
covering
the fenestrations 108, to keep the injected material inside the bone 2 during
the
withdrawal of the needle 100. After the procedure has been completed, the
split ring
516 may be snapped off the instrument 500 entirely, as shown in FIG. 7E.
In addition, the auxiliary delivery instrument 500 may also serve as a
plunging
device. In this example, the instrument 500 may be inserted within the
injection
needle 100 and used to plunge the injection needle 100 as it is retracted from
the bone
2. This allows the user to back fill the cavity or void in the bone 2 in a
continuous,
smooth motion. The split ring position can be indexed and fixed to the plunger
device
500 such that the tip of the device 500 is held in a fixed position relative
to the bone 2
when the split ring 516 is placed against the bone 2. For example, the plunger
tip 502
can be positioned at the bone cortex such that the injectable material is
evacuated
from the needle tip up to the cortex, but not beyond the cortex. When the
needle is
removed, the injectable material remains in the cavity up to the boundary of
the
cortex. In other words, the split ring 516 can be referenced to the end of the
plunger
tip 502 that pushes the cement through to the end of the needle 100. When the
split
ring 516 is indexed against the bone 2 on the outside of the needle 100, the
inner
plunger or elongate shaft 506 is positioned at the bone at the same area.
As previously described, the methods of treatment of the present disclosure
focus on the subchondral region of the bone joint. Accordingly, devices that
can help
determine the ideal range of pressure within a subchondral region are desired.
This
determination would eliminate variances in user subjectivity and render more
predictable and repeatable results. FIG. 8 shows an embodiment of a gauge 600
that
may be used with any one of the cannulas or pins of the present disclosure for
this
purpose. The gauge 600 would allow the user to accurately identify a desired
range
of pressure to achieve optimal patient results.
As shown, the gauge 600 of FIG 8 may provide volumetric and pressure
readings while also a mechanical assist. As shown, the gauge 600 may include
an
attachment end 602 that may be configured as a Luer lock connection, for
instance.
The attachment end 602 thus connects to any number of injectable material
delivery
instruments such as the cannulas and fenestrated pins disclosed. The gauge 600
also
includes a mechanical pressure gauge 604, an electrical pressure gauge 606,
and
22
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
volumetric markers 608 that represent relative readings or represent remaining
volume. The gauge 600 may comprise a transparent body or tube 610 that allows
the
user to visualize the contents of the gauge 600. In addition, a mechanical
assist
mechanism 612 may also be included. This mechanical assist mechanism 612 may
be
screw based, for example, as shown. Each half turn could be configured to
represent
a 1 cc volume, for example. Other mechanisms may of course be employed.
The gauge 600 allows the user even greater control over the amount of
material injected into the bone 2 being treated, without losing the tactile
pressure
response normally experienced. This gauge 600 allows some pressure measurement
outputs that could be similar to a pop-up timer or tire gauge, and could be
either
electrical or mechanical. For instance, the pressure readings could be
mechanical and
provide a go or no go signal via a blow out valve, and gauged to give a read
out.
Another example of an electrical mechanism is to have a constant read out from
the
gauge 600. Such a gauge 600 is intended to allow the user to control the
volume and
pressure of the material injected and still be able to exceed a digital
pressure reading,
if that was so desired.
FIG. 9 illustrates a method of using two or more cannulas 410 of the present
disclosure to allow both injection and removal of material from a bone 2 to be
treated.
The cannulas 410 may be open ended or optionally they may include
fenestrations. In
the example shown, the cannulas 410 may be used with a stabilizing instrument
120
similar to those previously described. One cannula 410 may be inserted into
the bone
2 and toward the subchondral space, to allow an injectable material 10 to be
delivered. Another cannula 410 may be inserted so that the ends of the
cannulas 410
are within the subchondral space. As material 10 is injected into the first
cannula 410,
the second cannula 410 may be utilized to remove any edema at the same site.
The
second cannula provides a port to the bone defect, such as an edema, such that
during
injection through the first cannula, fluid from the edema can escape through
the
second cannula. With this method, the risks or hazards of high pressure
injection into
the bone at the defect or edema can be averted.
FIG. 10 shows the same cannula 410 and stabilizing instrument 120 along
with a fresh bone plug 20 created during the procedure. In one exemplary
embodiment of using the fenestrated cannula 410 of the present invention, a
pin may
23
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
be inserted into bone, and the cannula 410 placed over the pin and driven into
the
bone to the end of the pin. The pin may be removed, while the cannula 410
pushed
further into the bone tissue. The user could then tamp the fresh bone to the
end to
create a bone plug 20 at the end of the cannula 410. Once attached to the end
of the
cannula 410, the bone plug 410 would be able to block injected material from
coming
out of the tip end, instead of the intended fenestrations along the side or
shaft of the
cannula. The bone plug 410 could be used later to plug up the access portal
created
during drilling, if so desired. Additionally, visual markers can be provided
on the pin
to ascertain the insertion depth of the cannula placed over the pin, such that
the
cannula end is indexed to a position beyond the tip of the pin to capture bone
material
in the cannula. This bone material at the end of the cannula can then be
impacted and
used to create a bone plug at the end of the cannula.
FIG. 11 shows still another method of treating a bone joint similar to the one
previously described in FIG. 10, but now with a bone restricter or plug 30.
For
instance, the tip of the cannula 410 may include a bone restricter or plug 30
that acts
to restrict the flow of materials. The plug 30 may comprise a main body 32
formed of
a plurality of flanges 34 attached to a central stem 36, as shown in FIG. 11B.
The
plug 30 may be formed of resorbable or absorbable or degradable material. The
plug
may further be formed of a flexible material such as PLGA, for example.
Initially the
plug 30 may be retained in the cannula 410 whereupon it can be delivered into
the
subchondral space of the bone joint to be treated, and allowed to expand. This
plug
may be delivered at the same time the injectable material is injected, or
before the
material is injected, as desired. During injection, the plug 30 would serve to
prevent
material from being ejected out the tip instead of the intended fenestrations
on the
25 side of the cannula 410. The plug 30 could also be inserted into the
access portal
created for the insertion of the cannula 410 after injection, in order to
prevent any
backout of material.
FIG. 12 illustrates an embodiment of the fenestrated cannula 410 with a one-
way valve 40 that can be attached at one end of the cannula 410 to allow the
passage
30 of a pin or wire, but would otherwise restrict the flow of any backflow
of material or
bone substance through the cannula 410. The valve 40 could be configured to
attach
to the tip end of the cannula 410, allow for the pin to slide over but closes
once the
24
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
pin has been removed. Use of the valve 40 would force bone substance to flow
through the fenestrations of the cannula 410.
FIGS. 13 and 14 represent highly porous implants 50, 60 that may be housed
internally within the cannula or other delivery instrument. These implants 50,
60
would have a highly porous geometry and allow the flow of material out of the
cannula but also redirect or induce dispersion of the material during
injection. Similar
to the plugs 20, 30 described above, these implants may also serve to prevent
flow of
material out through the tip of the cannula, instead of the sides through
fenestrations.
The implants 50, 60 may be formed of a resorbable, absorbable or degradable
material, such as calcium phosphate or collagen, for example. As shown in FIG.
13,
the highly porous implant 50 may have a body 52 formed as a generally
cylindrical
plug. In FIG. 14, the highly porous implant 60 may comprise a more structured
body
62. The structured body 62 may have a mesh-like or lattice-like pattern, with
interconnected struts 64 and voids or interstices 66, in between.
FIG. 15A shows the first component of an injectable material delivery system
700 comprising an outer cannula 710 with an attachment end 714, shaft 716, and
open-ended tip 712 that allows the outer cannula 710 to be used over a
guidewire.
Fenestrations or holes 718 are provided on the shaft 716 for the delivery of
an
injectable material, as shown in detail in FIG. 15B. As shown in FIG. 15A, the
cannula 712 may be used with a handle 120 similar to the one previously
described.
FIG. 16A shows the second component of the injectable material delivery
system 700 comprising an inner rod 740 having a first, closed tip 742, a shaft
744, and
an attachment end 746. An end cap 748 can be provided on the attachment end
that is
configured to attach to a Luer lock, for example, for connection to a syringe.
Near the
closed tip 742 the rod 740 may have a slot or opening 750 that corresponds to
the
fenestrations, such that the slot or opening 750 aligns with the holes 718 of
the outer
cannula 710 when the inner rod 740 is inserted inside.
As can be seen in FIGS. 17A and 17B, in which both the first and second
components 710, 740 of the injectable material delivery system 700 are shown,
the
inner rod 740 is configured to slide into the outer cannula 710. When the
inner rod
740 is inserted to the end of the tip 712 of the cannula 710, the slot or
opening 750 of
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
the inner rod 740 can be rotated so that the opening 750 aligns with the
fenestrations
718, thereby opening up the holes 718 and allowing material to escape, or not
align
with the fenestrations 718, thereby closing off the holes 718 and preventing
material
from escaping.
In one exemplary method of use, the outer cannula 710 may be inserted into
bone with the use of a guide wire, after which the guide wire is removed. The
second
component, the inner rod 740 is then inserted into the outer cannula 710. The
inner
rod 740 may form a very tight seal with the outer cannula 710, mating
perfectly with
it to prevent inadvertent injection material extrusion between the spaces. In
one
embodiment, the inner rod 740 may have a trocar tip so that the combined outer
cannula 710 and inner rod 740 can be inserted together over a guide wire. The
inner
rod 740 can then be adjusted to align the opening 750 of the inner rod 740
with the
fenestrations 718 of the outer cannula 710 (in the "open" position.)
Once the inner rod 740 is in position, the two components can be locked
together. FIG. 18 shows an exemplary locking mechanism 760 comprising a cutout
or hook on the end cap 748, and knob or peg on a handle component similar to
those
previously described and attachable to the outer cannula 710, on the
respective
components such that one component can be twisted relative to the other to
enable the
hook to latch onto the knob. For example, the inner rod 740 may be
rotationally
keyed or locked to the outer cannula 710 to ensure that the openings are
aligned.
Alternatively, the inner rod end cap 748 may be rotated or indexed to a
specific
position relative to the outer cannula 710 such that the opening 750 of the
inner rod
740 is aligned to only some of the fenestrations 718 in one section or
quadrant of the
outer cannula 710, thereby allowing directional control of the ejection of
material.
After the two components are secured together, the end cap 748 may be
attached to an injection device such as a syringe, for example. Injectable
material
may then be injected through the inner rod 740. The material will follow the
path of
least resistance, and therefore exit at the end of the system 700 through the
orifice
created by the aligned hole 750 and the fenestrations 718.
To clear the inner rod 740 of all of the injectable material, a plunger 780
may
be inserted through the inner rod 740, as shown in FIG. 19A. The plunger 780
may
26
CA 02838816 2013-12-09
WO 2012/170805
PCT/US2012/041534
comprise an elongate rod extending from a handle, and should make a close fit
with
the inner rod 740 to effectively clear all residual material inside the rod
740. The
plunger 780 and inner rod 740 may then be removed, leaving the outer cannula
710
behind. Next, a plug such as an allograft plug, for example, may be inserted
into the
outer cannula 710 and pushed into the bone using the plunger 780, like in FIG.
19B.
It is contemplated that the back pressure on the injected material would
diffuse around
the plug and fill up any voids.
In an alternative method, after the injection of material and the removal of
the
plunger 780 and inner rod 740, a cannulated plug may be inserted down through
the
cannula, and a cannulated plunger or other pushing device used to push down
the
cannulated plug, which may be an allograft plug, for example. In some
embodiments,
the guide wire may be one having a very small diameter to allow for a
cannulated
plug with a small opening.
In still another method, after the injection of material and the removal of
the
plunger 780 and inner rod 740, a guide wire can be reinserted through the
remaining
outer cannula 710. The outer cannula 710 can then be removed, leaving just the
guide
wire in place. A plug can then be slid down the guide wire and pushed into
place to
cover the opening or void.
Other embodiments will be apparent to those skilled in the art from
consideration of the specification and practice of the embodiment disclosed
herein. It
is intended that the specification and examples be considered as exemplary
only, with
a true scope and spirit of the embodiment being indicated by the following
claims.
27