Note: Descriptions are shown in the official language in which they were submitted.
1
NATURAL GAS LIQUEFACTION PROCESS
[0001] This paragraph has been intentionally left blank
BACKGROUND OF THE INVENTION
[0002] This section is intended to introduce various aspects of the art,
which may be
associated with exemplary embodiments of the present disclosure. This
discussion is
believed to assist in providing a framework to facilitate a better
understanding of particular
aspects of the present disclosure. Accordingly, it should be understood that
this section
should be read in this light, and not necessarily as admissions of prior art.
FIELD OF THE INVENTION
[0003] The present invention relates to the processing of gaseous fluids.
More
specifically, the present invention relates to the liquefaction of natural
gas, particularly
hydrocarbon gases produced in remote locations.
DISCUSSION OF TECHNOLOGY
[0004] As the world's demand for fossil fuels increases, energy companies
find
themselves pursuing hydrocarbon resources located in more remote and hostile
areas of the
world, both onshore and offshore. This includes the pursuit of natural gas.
[0005] Because of its clean burning qualities, natural gas has become
widely used in
recent years. However, many sources of natural gas are located in geographical
areas that are
great distances from commercial markets. In some instances, a pipeline is
available or may
be constructed for transporting produced natural gas to a commercial market.
However,
when a pipeline is not available for transportation, produced natural gas is
often transported
via large ocean-going vessels.
[0006] To maximize gas volumes for transportation, the gas is frequently
taken through a
liquefaction process. The liquefied natural gas ("LNG") is formed by chilling
very light
hydrocarbons, e.g., gases containing methane, to approximately -160 C. The
liquefied gas
may be stored at ambient pressure in special, cryogenic tanks disposed on
large ships.
Alternatively, LNG may be liquefied at an increased pressure and at a warmer
temperature,
CA 2840723 2018-11-07
CA 02840723 2013-12-30
WO 2013/022529 PCT/US2012/045005
2
i.e., above -160 C, in which case it is known as Pressurized LNG ("PLNG").
For purposes
of the present disclosure, PLNG and LNG may be referred to collectively as
"LNG."
[0007] As currently developed, gas is taken through a liquefaction
process at a location
proximate the point of production. This means that a large gathering and
liquefaction center
is erected in the producing country. Alternatively, the liquefaction process
may take place
offshore on a platform or vessel, such as a floating production, storage and
offloading (FPSO)
vessel. Currently, large liquefaction facilities exist in Qatar, Russia
(Sakhalin Island),
Indonesia, and other countries. Several significant LNG terminals are either
under
construction in or are presently planned for Australia.
[0008] After natural gas is chilled to a liquid state, the hydrocarbon
product is loaded
onto marine transport vessels. Such vessels are known as LNG tankers. The
chilling of
natural gas into a liquefied state enables the transport of much larger
volumes of gas.
[0009] In the design of an LNG plant, one of the most important
considerations is the
process for converting the natural gas feed stream into LNG. Currently, the
most common
liquefaction processes use some form of refrigeration system. Although many
refrigeration
cycles have been used to liquefy natural gas, there are three types of
refrigeration systems
most commonly used in LNG plants.
[0010] The first type of system is known as a "cascade cycle." A cascade
cycle uses
multiple, single-component refrigerants in heat exchangers arranged
progressively to reduce
the temperature of the gas to a liquefaction temperature. The second type of
refrigeration
system is the "multi-component refrigeration cycle." This system uses a multi-
component
refrigerant in specially designed exchangers. The third type of system is the
"expander
cycle." The expander cycle system expands gas from feed gas pressure to a low
pressure,
producing a corresponding reduction in temperature under Boyle's Law. Most
natural gas
liquefaction cycles use variations or combinations of these three basic types.
[0011] A recent variant of the expander cycle is the High Pressure
Expander Cycle. This
system provides a liquefaction process that is more efficient and compact than
the cycles
described above. As a result, it has become an attractive option for remote or
offshore
applications.
[0012] A limitation to the use of any liquefaction system is the presence
of contaminants
in the natural gas stream. Raw natural gas produced from subsurface reservoirs
typically
contains components that are undesirable in the LNG process. Such components
include
water, carbon dioxide and hydrogen sulfide. Water and CO2 should be removed
because they
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
3
will freeze at liquefaction temperatures and plug the liquefaction equipment.
H2S should be
removed as it may have adverse safety impacts or may adversely affect the LNG
product
specifications. Therefore, natural gas production is typically treated before
liquefaction to
remove the undesirable components or contaminants.
[0013] When H2S and CO2 are produced as part of a hydrocarbon gas stream
(such as
methane or ethane), the raw gas stream is sometimes referred to as "sour gas."
The H2S and
CO2 are often referred to together as "acid gases." Processes have been
devised to remove
acid gases from a raw natural gas stream. In some instances, cryogenic gas
processing is
used. This involves chilling the gas stream in a large cryogenic vessel so
that CO2 and H2S
components drop out as solids. The hydrocarbon components are distilled out of
the vessel.
This process typically requires that the raw gas stream undergo dehydration
before cryogenic
separation.
[0014] As an alternative, the hydrocarbon gas stream may be treated with
a solvent.
Solvents may include chemical solvents such as amines. Examples of amines used
in sour
gas treatment include monoethanol amine (MBA), diethanol amine (DEA), and
methyl
diethanol amine (MDEA). Physical solvents are sometimes used in lieu of amine
solvents.
Examples of physical solvents include Selexol and RectisolTM. In some
instances hybrid
solvents, meaning mixtures of physical and chemical solvents, have been used.
An example
is Sulfinol . However, the use of amine-based acid gas removal solvents is
most common.
In any instance, solvent extraction is typically accomplished using a large,
thick-walled
counter-current contacting tower.
[0015] The solvent extraction method uses a water-based solvent to absorb
the
undesirable species. As a consequence, the treated gas retains water that
again must be
removed to avoid subsequent freezing and plugging of the liquefaction
equipment.
[0016] Whether water is removed before or after acid gas separation, the
water removal
process is typically done in several stages to meet the extremely low water
content
requirement on the gas to be liquefied. A process under development is based
on a glycol
dehydration system for bulk water removal, followed by several molecular-sieve
beds as
polishing stages. Thus, several pieces of large and heavy equipment which are
sensitive to
motion are required for the solvent extraction step. Such equipment is
unattractive for
offshore applications where space and weight are a premium and wave motions
are
unavoidable.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
4
[0017] In addition to water, nitrogen may also be removed from the gas
stream. Nitrogen
should be removed as it contains no heating value and, accordingly, adversely
affects the fuel
quality. Nitrogen is typically removed after both acid gas removal and
liquefaction have
occurred. Nitrogen is removed using a distillation column known as a nitrogen
rejection unit,
or NRU. The NRU is sensitive to wave motions. Further, a NRU typically
involves several
large and heavy items of heat exchange equipment which are not particularly
suitable for
offshore applications.
[0018] Other adverse impacts exist from the presence of nitrogen in a raw
gas stream.
For example, removing the nitrogen after, rather than before, the liquefaction
step increases
the liquefaction power requirement for the gas. In this respect, nitrogen
increases the amount
of gas that must be liquefied. Further, the presence of nitrogen lowers the
liquefaction
temperature of the mixture since nitrogen has a lower boiling temperature than
methane.
[0019] Because of the stringent specifications for the LNG, feed
pretreatment facilities
are large, heavy, and costly. For example, in one floating LNG concept with
nominal levels
of contaminants (e.g., water saturation, 1% CO2, 4% N2) in the inlet gas,
facilities to remove
those contaminants represent approximately 20% of the total topside facilities
weight. For
developments with high levels of inlet gas contaminants (e.g., water
saturation plus 50% to
70% CO2 and H2S content), the contaminant removal facilities can represent
greater than
50% of topside facilities weight. Furthermore, the large vertical pressure
vessels or towers
that are typically used for contaminant removal may have an undesirable affect
on the
stability of a floating structure.
[0020] Therefore, a need exists for an improved facility for processing
natural gas for
liquefaction that is less sensitive to wave motions and that has little affect
on the stability of a
floating structure. Further, a need exists for a more compact, lightweight,
and lower-
horsepower LNG system that may be employed on an offshore platform. Still
further, a need
exists for a method of efficiently processing natural gas for liquefaction
that is compatible
with a high pressure expander cycle refrigeration system.
BRIEF SUMMARY OF THE INVENTION
[0021] A gas processing facility for the liquefaction of a natural gas
feed stream is first
provided. The facility is designed to be more compact and more efficient than
conventional
LNG facilities. Therefore, the facility offered herein is ideally suited for
LNG facilities that
are offshore or are located in remote locations. For example, the gas
processing facility may
be located on a floating platform or a gravity-based platform offshore.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
[0022] The
facility first comprises a gas separation unit having has at least one
fractionation vessel. The fractionation vessel serves to separate contaminants
from methane
gas. To this end, each vessel has a gas inlet for receiving a natural gas
mixture. Further, in
one embodiment each vessel includes an adsorbent material that has a kinetic
selectivity for
5 contaminants over methane greater than 5. In this way, the contaminants
become kinetically
adsorbed within the adsorbent material. Further, each vessel includes a gas
outlet. The gas
outlet releases a methane-rich gas stream.
[0023] The
vessel employs one or more adsorbent beds for adsorptive kinetic separation.
The adsorbent beds release the methane-rich gas feed stream. In one aspect, a
single vessel
having a plurality of adsorbent beds in series is used. For example, the at
least one
fractionation vessel in the gas separation unit may be a vessel containing a
plurality of
adsorbent beds in series, such that:
a first adsorption bed is designed to primarily remove water and other liquid
components from the dehydrated natural gas feed stream;
a second adsorption bed is designed to primarily remove a desiccant from the
dehydrated natural gas feed stream; and
a third vessel comprises an adsorption bed primarily for the removal of a sour
gas
component from the dehydrated natural gas feed stream.
Additional vessels may be added to adsorb and separate nitrogen and different
sour gases.
[0024] In another aspect, multiple vessels in series are employed, with
each vessel
releasing a progressively sweeter methane gas stream. For example,
a first vessel uses an adsorption bed designed for the removal of water
remaining in a
dehydrated natural gas feed stream;
a second vessel uses an adsorption bed designed for the removal of a desiccant
from
the dehydrated natural gas feed stream; and
a third vessel use an adsorption bed designed for the removal of a sour gas
component
from the dehydrated natural gas feed stream.
The sour gas component may be one or more sulfurous components. Alternatively,
the sour
gas component may be carbon dioxide.
[0025] The at least one fractionation vessel in the gas separation unit
operates on pressure
swing adsorption (PSA) or on rapid cycle pressure swing adsorption (RCPSA).
The at least
one fractionation vessel may further operate on temperature swing adsorption
(TSA) or rapid
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
6
cycle temperature swing adsorption (RCTSA). In any arrangement, the
fractionation vessels
are configured to adsorb CO2, H2S, H20, heavy hydrocarbons, VOC's, mercaptans,
or
combinations thereof.
[0026] The facility also includes a high-pressure expander cycle
refrigeration system.
The refrigeration system includes a first compression unit. The first
compression unit is
configured to receive a substantial portion of the methane-rich gas stream
from the gas
separation unit, and to compress the methane-rich gas stream to greater than
about 1,000 psia
(6,895 Oa). In this way, a compressed gas feed stream is provided.
[0027] The refrigeration system also chills the methane-rich gas feed
stream in one or
more coolers, and then expands the chilled gas feed stream to form a liquefied
product
stream. To this end, the system includes a first cooler configured to cool the
compressed gas
feed stream to form a compressed, cooled gaseous feed stream, and a first
expander
configured to expand the cooled, compressed, gaseous feed stream to form a
product stream.
[0028] The product stream has a liquid fraction and a small remaining
vapor fraction.
Preferably, the gas processing facility also includes a liquid separation
vessel. The separation
vessel is configured to separate the liquid fraction and the remaining vapor
fraction. The
vapor fraction is still very cold and may be captured as a flash gas and
circulated as part of a
first refrigeration loop. The first refrigeration loop will have at least one
heat exchanger that
serves as the first cooler. The first cooler will receive the vapor fraction
from the first
expander, and release (i) the compressed, cooled gaseous feed stream and (ii)
a partially-
warmed vapor stream after heat-exchanging with the compressed gas feed stream.
[0029] The high-pressure expander cycle refrigeration system may include
a separate heat
exchanger that is configured to further cool the compressed gas feed stream.
This is done at
least partially by indirect heat exchange between a refrigerant stream (along
with a portion of
the vapor stream) and the compressed, methane-rich gas feed stream. The
separate heat
exchanger is a second cooler. The refrigeration system will then also include
a second
refrigeration loop having (i) a second compression unit configured to re-
compress the
refrigerant stream after the refrigerant stream passes through the second
cooler, and (ii) a
second expander configured to receive the compressed refrigerant stream from
the second
cooler, and expand the compressed refrigerant stream prior to returning it to
the second
cooler.
[0030] The second cooler may sub-cool the chilled gas feed stream after
the chilled gas
feed stream leaves the first cooler. Alternatively and more preferably, the
second cooler pre-
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
7
cools the compressed gas feed stream before the compressed gas feed stream
enters the first
cooler. To do this, the second cooler receives the partially-warmed vapor
stream from the
first cooler for further heat-exchanging with the compressed gas feed stream,
and releases a
warmed vapor product stream to a third compression unit to complete the first
refrigeration
.. loop.
[0031] In any event, the first refrigeration loop preferably cycles the
vapor portion of the
product back to the first compression unit. To do this, the first
refrigeration loop may
include a third compression unit for compressing the partially-warmed vapor
stream after
heat-exchanging with the compressed gas feed stream, and a line for merging
the
compressed, partially-warmed vapor stream with the compressed methane-rich gas
feed
stream. This completes the first refrigeration loop.
[0032] The gas processing facility preferably further comprises a
dehydration vessel. The
dehydration vessel is configured to receive the natural gas feed stream and
remove a
substantial portion of water from the natural gas feed stream. The dehydration
unit then
.. releases a dehydrated natural gas feed stream to the gas separation unit.
[0033] A process for liquefying a natural gas feed stream is also
provided herein. The
process employs adsorptive kinetic separation to produce a methane-rich gas
stream. The
process then further utilizes a high-pressure expander cycle refrigeration
system to chill the
methane and to provide an LNG product. The LNG product is preferably generated
on a
floating platform or a gravity-based platform offshore.
[0034] The process first includes receiving the natural gas feed stream
at a gas separation
unit. The gas separation unit has at least one fractionation vessel. The
fractionation vessels
are designed in accordance with the fraction vessel described above in its
various
embodiments. The fractionation vessels preferably operate on pressure swing
adsorption
.. (PSA) or rapid cycle pressure swing adsorption (RCPSA) to regenerate a
series of adsorption
beds. The adsorption beds are designed to adsorb CO2, H2S, H20, heavy
hydrocarbons,
VOC' s, mercaptans, nitrogen, or combinations thereof
[0035] The process also includes substantially separating methane from
contaminants
within the natural gas feed stream. This is done through the use of adsorption
beds in the one
or more fractionation vessels. As a result, the process also includes
releasing a methane-rich
gas stream from the gas separation unit. In one aspect, separating methane
from
contaminants is conducted through the gas separation unit at a pressure of at
least about 500
pounds per square inch absolute (psia).
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
8
[0036] The process next comprises directing the methane-rich gas stream
into a high-
pressure expander cycle refrigeration system. The refrigeration system is
generally designed
in accordance with the refrigeration system described above in its various
embodiments.
Thus, the refrigeration system preferably includes a first refrigeration loop
for cycling the
vapor portion of the product for use as a coolant in a first cooler, and a
second refrigeration
loop for cycling a nitrogen-containing gas as a refrigerant in a second
cooler.
[0037] The process also includes compressing the methane-rich gas stream.
The gas
stream is compressed to a pressure that is greater than about 1,000 psia
(6,895 kPa) in order
to form a compressed gas feed stream. The process then comprises cooling the
compressed
gas feed stream through the second and first coolers to form a compressed,
cooled gaseous
feed stream.
[0038] The process also includes expanding the cooled, compressed,
gaseous feed stream.
This forms the LNG product stream having a liquid fraction and a remaining
vapor fraction.
[0039] The high-pressure expander cycle refrigeration system preferably
includes a liquid
separation vessel. The process then further comprises separating the liquid
fraction and the
remaining vapor fraction.
[0040] A method for liquefying a natural gas feed stream is also provided
herein. As
with the process described above, the method employs adsorptive kinetic
separation to
produce a methane-rich gas stream. The method then further utilizes a high-
pressure
expander cycle refrigeration system to chill the methane and to provide an LNG
product. The
LNG product is preferably generated on a floating platform or a gravity-based
platform
offshore.
[0041] The method first includes receiving the natural gas feed stream at
a gas processing
facility. The gas processing facility will include a dehydration vessel. The
method then
includes passing the natural gas feed stream through a dehydration vessel.
This serves to
remove a substantial portion of water from the natural gas feed stream. A
dehydrated natural
gas feed stream is then released to a gas separation unit as a dehydrated
natural gas feed
stream.
[0042] The gas separation unit has at least one fractionation vessel. The
fractionation
vessels are designed in accordance with the fraction vessel described above in
its various
embodiments. The fractionation vessels preferably operate on pressure swing
adsorption
(PSA) or rapid cycle pressure swing adsorption (RCPSA) to regenerate a series
of adsorption
beds.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
9
[0043] The method next comprises passing the dehydrated natural gas feed
stream
through the series of adsorbent beds. This serves to separate methane gas from
contaminants
in the dehydrated natural gas feed stream. The beds use adsorptive kinetic
separation. The
adsorption beds are designed to adsorb CO2, R,S, H20, heavy hydrocarbons,
VOC's,
mercaptans, nitrogen, or combinations thereof.
[0044] In one aspect, a single vessel having a plurality of adsorbent
beds aligned in series
is used.
[0045] In another aspect, multiple vessels in series are employed, with
the vessels being
aligned in series with the flow of the dehydrated natural gas feed stream.
Each vessel
releases a progressively sweeter methane gas stream.
[0046] As a result of passing the dehydrated natural gas feed stream
through the
adsorbent beds, a methane-rich gas stream is produced. The method comprises
releasing the
methane-rich gas stream from the gas separation unit. The methane-rich gas
stream is then
directed into a high-pressure expander cycle refrigeration system.
[0047] The refrigeration system is generally designed in accordance with
the refrigeration
system described above in its various embodiments. Thus, the refrigeration
system
preferably includes a first refrigeration loop for cycling the vapor portion
of the product for
use as a coolant in a first cooler, and a second refrigeration loop for
cycling a nitrogen-
containing gas as a refrigerant in a second cooler.
[0048] The method also includes compressing the methane-rich gas stream.
The gas
stream is compressed to a pressure that is greater than about 1,000 psia
(6,895 kPa) in order
to form a compressed gas feed stream. The process then comprises cooling the
compressed
gas feed stream to form a compressed, cooled gaseous feed stream.
[0049] The method further includes expanding the cooled, compressed,
gaseous feed
stream. This forms the LNG product stream having a liquid fraction and a small
remaining
vapor fraction. In one aspect, expanding the cooled, compressed, gaseous feed
stream
comprises reducing the pressure of the cooled, compressed, gaseous feed stream
to a pressure
between about 50 psia (345 kPa) and 450 psia (3,103 kPa).
BRIEF DESCRIPTION OF THE DRAWINGS
[0050] So that the present inventions can be better understood, certain
illustrations, charts
and/or flow charts are appended hereto. It is to be noted, however, that the
drawings
illustrate only selected embodiments of the inventions and are therefore not
to be considered
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
limiting of scope, for the inventions may admit to other equally effective
embodiments and
applications.
[0051] Figure 1 is a schematic flow diagram of a facility for producing
LNG, in
accordance with one embodiment herein. The facility includes a gas separation
unit that
5 produces a methane-rich gas stream, and a high-pressure expander cycle
refrigeration system
for generating an LNG product.
[0052] Figure 2 is a perspective view of a pressure swing adsorption
vessel as may be
used in the facility of Figure 1, in one embodiment. The vessel also
represents a kinetic
fractionator of the present inventions, in one embodiment.
10 [0053] Figure 3A is a perspective view of the adsorbent bed and
flow channels for the
pressure swing adsorption vessel of Figure 2, in one embodiment. Major flow
channels are
seen between adsorbent rods along a major axis of the adsorbent bed.
[0054] Figure 3B provides an exploded view of the adsorbent bed of Figure
3A. Figure
3B provides an exposed view of the optional second gas outlet. A transverse
flow channel is
shown extending into the vessel, serving as a minor flow channel.
[0055] Figure 3C is a longitudinal, cross-sectional view of the adsorbent
bed of Figure
3A, in an alternate embodiment. The view is taken across line C-C of Figure
3A. Here, a
series of stepped surfaces are seen along the adsorbent rods, which serve as
minor flow
channels.
[0056] Figure 4 is a perspective view of the adsorbent bed and flow
channels for the
pressure swing adsorption vessel of Figure 2, in a modified arrangement. Major
flow
channels are seen between adsorbent rods along a major axis of the adsorbent
bed.
Transverse flow channels are seen in exploded-away portions of the adsorbent
bed, which
serve as minor flow channels.
[0057] Figure 5 is a schematic flow diagram of a high-pressure expander
cycle
refrigeration system, in one embodiment. The refrigeration system receives a
methane-rich
gas stream, and generates an LNG product. The illustrative refrigeration
system employs a
secondary cooling loop that is a closed loop using nitrogen gas, or a nitrogen-
rich gas, or a
portion of the methane-rich gas stream from the gas separation unit.
[0058] Figure 6 is a flow chart showing steps for liquefying a raw natural
gas stream.
[0059] Figure 7 is a flowchart showing steps for separating contaminants
from the raw
natural gas stream using adsorptive kinetic separation.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
11
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
Definitions
[0060] As used herein, the term "hydrocarbon" refers to an organic
compound that
includes primarily, if not exclusively, the elements hydrogen and carbon.
Hydrocarbons
.. generally fall into two classes: aliphatic, or straight chain hydrocarbons,
and cyclic, or closed
ring hydrocarbons, including cyclic teipenes. Examples of hydrocarbon-
containing materials
include any form of natural gas, oil, coal, and bitumen that can be used as a
fuel or upgraded
into a fuel.
[0061] As used herein, the term "fluid" refers to gases, liquids, and
combinations of gases
and liquids, as well as to combinations of gases and solids, and combinations
of liquids and
solids.
[0062] As used herein, the term "hydrocarbon fluids" refers to a
hydrocarbon or mixtures
of hydrocarbons that are gases or liquids. For example, hydrocarbon fluids may
include a
hydrocarbon or mixtures of hydrocarbons that are gases or liquids at formation
conditions, at
.. processing conditions, or at ambient conditions (15 C and 1 atm pressure).
Hydrocarbon
fluids may include, for example, oil, natural gas, coal bed methane, shale
oil, pyrolysis oil,
pyrolysis gas, a pyrolysis product of coal, and other hydrocarbons that are in
a gaseous or
liquid state.
[0063] As used herein, an "acid gas" means any gas that dissolves in
water producing an
acidic solution. Non-limiting examples of acid gases include hydrogen sulfide
(H2S), carbon
dioxide (CO?), sulfur dioxide (SO2), carbon disulfide (CS?), carbonyl sulfide
(COS),
mercaptans, or mixtures thereof
[0064] As used herein, the term "subsurface" refers to geologic strata
occurring below the
earth's surface.
[0065] The term "seabed" refers to the floor of a marine environment. The
marine
environment may be an ocean or sea or any other body of water that experiences
waves,
winds, and/or currents.
[0066] The term "marine environment" refers to any offshore location. The
offshore
location may be in shallow waters or in deep waters. The marine environment
may be an
.. ocean body, a bay, a large lake, an estuary, a sea, or a channel.
[0067] The term "about" is intended to allow some leeway in mathematical
exactness to
account for tolerances that are acceptable in the trade. Accordingly, any
small deviations
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
12
upward or downward from the value modified by the term "about" should be
considered to be
explicitly within the scope of the stated value.
[0068] The term "swing adsorption process" includes processes such as
pressure swing
adsorption (PSA), thermal swing adsorption (TSA), and partial pressure swing
or
displacement purge adsorption (PPSA), including combinations of these
processes. These
swing adsorption processes can be conducted with rapid cycles, in which case
they are
referred to as rapid cycle pressure swing adsorption (RCPSA), rapid cycle
thermal swing
adsorption (RCTSA), and rapid cycle partial pressure swing or displacement
purge adsorption
(RCPPSA). The term swing adsorption also includes these rapid cycle processes.
[0069] As used herein, the term "pressure swing adsorption" shall be taken
to include all
of the processes, i.e., PSA, PPSA, RCPSA, and RCPPSA, including combinations
of these
processes, that employ a change in pressure for a purge cycle.
[0070] As used herein, the term "wellbore" refers to a hole in the
subsurface made by
drilling or insertion of a conduit into the subsurface. A wellbore may have a
substantially
circular cross section, or other cross-sectional shapes. As used herein, the
term "well," when
referring to an opening in the formation, may be used interchangeably with the
term
"wellbore."
[0071] The term "platform" means any platform or surface dimensioned and
configured
to receive fluid processing equipment.
DESCRIVIION OF SPECIFIC EMBODIMENI S
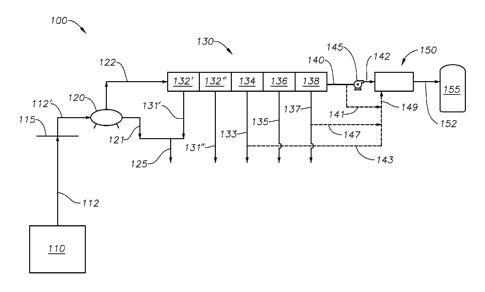
[0072] Figure 1 is a schematic diagram of a gas processing facility 100
for producing
LNG in accordance with one embodiment herein. The "LNG" is natural gas that
has been
liquefied through a cooling process. The gas processing facility 100 operates
to receive raw
natural gas, remove certain undesirable components in order to produce a
"sweetened" gas
stream that meets established specifications, and then chill the sweetened
("methane-rich")
gas stream into a substantially liquid phase for ready transport.
[0073] In the illustrative arrangement of Figure 1, the facility 100
receives production
fluids from a reservoir. A reservoir is shown schematically at 110. The
reservoir 110
represents a subsurface formation that contains hydrocarbon fluids in
commercially
acceptable quantities. The hydrocarbon fluids exist in situ in primarily a
gaseous phase.
[0074] The production fluids are produced through a plurality of
wellbores. A single
illustrative wellbore 112 is indicated in Figure 1. However, it is understood
that numerous
wellbores 112 will be drilled through the earth surface and into the
subsurface reservoir 110.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
13
The present inventions are not limited by the number of wellbores or the
manner in which
wellbore completions are made.
[0075] The wellbore 112 transports hydrocarbon fluids from the reservoir
110 and to an
earth surface 115. The earth surface 115 may be on land. More preferably for
the present
inventions, the earth surface 115 is a seabed. In this latter instance,
wellheads (not shown)
are placed along the bottom of a marine environment. Subsea jumpers and/or
flowlines will
direct production fluids to a manifold (not shown), which then delivers fluids
to an ocean
surface via one or more production risers.
[0076] In Figure 1, line 112' is shown transporting hydrocarbon fluids.
Line 112' may
be a flow line on land. More preferably, line 112' is representative of a
production riser
within a marine environment. In either instance, the production fluids are
delivered to a
separator 120.
[0077] Upon arrival at the separator 120, the production fluids represent
a raw natural gas
mixture. The production fluids contain methane, or natural gas. The production
fluids may
also contain so-called "heavy hydrocarbons," representing ethane and,
possibly, propane.
Most likely, the production fluids will also contain water (or brine), along
with nitrogen.
Also, the production fluids may contain hydrogen sulfide, carbon dioxide, and
other so-called
"sour gas" components. Finally, the production fluids may contain benzene,
toluene, or other
organic compounds.
[0078] The separator 120 provides a general separation of liquids from
gases. This is
typically done at production pressure. The separator 120 may be a gravity
separator having
thick steel walls. The separator 120 serves to filter out impurities such as
brine and drilling
fluids. It may also remove at least a portion of any condensed hydrocarbons.
Some particle
filtration may also take place.
[0079] More preferably, the separator 120 serves as a dehydration vessel.
The
dehydration vessel uses a desiccant such as ethylene glycol in order to absorb
water and
release gas-phase fluids. Liquids are released from the bottom of the
separator 120, while
gases are released at the top.
[0080] In Figure 1, line 121 represents a liquid line. The fluids in line
121 are primarily
water, with possibly some heavy hydrocarbons. The heavy hydrocarbons in line
121 will be
a small amount of ethane and, perhaps, a bit of propane and butane. Additional
separation
may take place through gravity separation, heat treatment, or other means
known in the art to
capture the valuable liquid hydrocarbons.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
14
[0081] Line 122 represents a gas line. The fluids in line 122 are
primarily methane, with
some ethane and other "heavy hydrocarbons" as well. In addition, the fluids in
line 122 will
have contaminants. These may include "sour" components such as hydrogen
sulfide and
carbon dioxide. These may also include water in vapor form. Further, the
contaminants may
include nitrogen. Certain metal contaminants may be suspended in the vapor,
such as
arsenic, cobalt, molybdenum, mercury, or nickel. Finally, trace organic
compounds such as
benzene, toluene, or xylene, may be present.
[0082] It is desirable to separate the various components so that a fluid
stream comprising
substantially methane is produced. For international sales, LNG specifications
may require
that natural gas have the following content:
Component Feed Specification
CO, < 50 ppmv
H20 < 0.5 ppmv
H2S < 3.5 ppm
Total S <20 mg/Nm-
Hg < pg/Nm3
C5+ < 0.1 M01. %
C6H6 <1 ppmv
Table 1
Pretreatment LNG Specifications
[0083] In order to achieve the LNG specifications of Table 1, gas
treatment must take
place. In Figure 1, a gas separation unit 130 is schematically shown. The gas
separation unit
130 may also be referred to as a Selective Component Removal System, or
"SCRS." The gas
separation unit 130 utilizes a series of adsorbent beds using Adsorptive
Kinetic Separation, or
"AKS."
[0084] AKS is a process that employs a relatively new class of solid
adsorbents that rely
upon the rate at which certain species are adsorbed onto a structured material
relative to other
species. The structured material is sometimes referred to as an absorbent bed.
Adsorbent
beds operate on the principle that different molecules can have different
affinities for
adsorption. This provides a mechanism for the adsorbent to discriminate
between different
gasses and, therefore, to provide separation.
[0085] In order to effectuate the separation, adsorbent beds employ a
highly porous
microstructure. Selected gas molecules become attached to the surface area
provided along
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
the pores. The gas adsorbed onto the interior surfaces of the micro-porous
material may
consist of a layer only a few molecules in thickness. The micro-porous
material may have
also surface areas of several hundred square meters per gram. Such
specifications enable the
adsorption of a significant portion of the adsorbent's weight in gas.
5 [0086] Different types of adsorbent beds are known. Typical
adsorbents include
activated carbons, silica gels, aluminas, and zeolites. In some cases, a
polymeric material can
be used as the adsorbent material. In any instance, the adsorbent bed
preferentially adsorbs a
more readily adsorbed component (known as the "heavy" gas) relative to a less
readily
adsorbed component (known as the "light" gas) of the gas mixture.
10 [0087] In addition to their affinity for different gases, zeolites
and some types of activated
carbons, called carbon molecular sieves, may utilize their molecular sieve
characteristics to
exclude or slow the diffusion of some gas molecules into their structure. This
provides a
mechanism for selective adsorption based on the size of the molecules. In this
instance, the
adsorbent bed restricts the ability of larger molecules to be adsorbed, thus
allowing the gas to
15 selectively fill the micro-porous structure of an adsorbent material
with one or more species
from a multi-component gas mixture.
[0088] Different adsorption techniques for gas separation are known. One
adsorption
technique is pressure swing adsorption, or "PSA." PSA processes rely on the
fact that, under
pressure, gaseous contaminants tend to be adsorbed within the pore structure
of an adsorbent
material, or within the free volume of a polymeric material, to different
extents. The higher
the pressure in the adsorption vessel, the more gas is adsorbed. In the case
of natural gas, the
natural gas mixture may be passed under pressure through an adsorption vessel.
The pores of
the polymeric or micro-porous adsorbent become filled with hydrogen sulfide
and carbon
dioxide to a greater extent than with methane. Thus, most or even all of the
H2S and CO2
will stay in the sorbent bed, while the gas coming out of the vessel will be
enriched in
methane. Any remaining water and possibly some heavy hydrocarbons will also be
retained
as adsorbents. In addition, any benzene, toluene, or other volatile organic
compounds will be
retained as adsorbents.
[0089] The pressure swing adsorption system may be a rapid cycle pressure
swing
adsorption system. In the so-called "rapid cycle" processes, cycle times can
be as small as a
few seconds. A rapid cycle PSA ("RCPSA") unit can be particularly
advantageous, as such a
unit is compact relative to normal PSA devices. Further, RCPSA contactors can
enable a
significant increase in process intensification (e.g., higher operating
frequencies and gas flow
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
16
velocities) when compared to conventional PSA.
[0090] When the adsorbent bed reaches the end of its capacity to adsorb
contaminants, it
can be regenerated by reducing the pressure. This causes the vessel to release
the adsorbed
components. A concentrated contaminant stream is thus released separate from
the methane
gas stream. In this way, the adsorption bed may be regenerated for subsequent
re-use.
[0091] In most PSA cases, reducing the pressure in the pressurized
chamber down to
ambient pressure will cause a majority of the hydrogen sulfide and other
contaminants to be
released from the adsorbent bed. In some cases, the pressure swing adsorption
system may
be aided by the use of a vacuum chamber to apply sub-ambient pressure to the
concentrated
contaminant stream. In the presence of lower pressure, sulfurous components,
carbon
dioxide, and heavy hydrocarbons will more completely desorb from the solid
matrix making
up the adsorbent bed.
[0092] A related gas separation technique is temperature swing
adsorption, or "TSA."
TSA processes also rely on the fact that, under pressure, gases tend to be
adsorbed within the
pore structure of micro-porous adsorbent materials or within the free volume
of a polymeric
material, to different extents. When the temperature of the adsorbent bed in
the vessel is
increased, the adsorbed gas molecules are released, or de-sorbed. This is done
in a
regeneration heater that employs a heated dry gas. The dry gas comprises
primarily methane,
but may also include nitrogen and helium. By cyclically swinging the
temperature of
adsorbent beds within a vessel, TSA processes can be used to separate gases in
a mixture.
[0093] When a TSA process is used, a set of valves may be provided to
pulse the flow of
heating or cooling fluids that enter and leave the vessel. An electric heating
or cooling jacket
may also be used to produce temperature swings. Optionally, the swing
adsorption unit uses
a partial pressure purge displacement process. In this case, a valve or set of
valves is
provided to pulse the flow of the purge displacement stream into the
adsorption bed. The
adsorption bed is contained within a pressure vessel. Optionally, this vessel
and the
associated valving is contained within a secondary pressure vessel. This
secondary pressure
vessel is designed to mitigate the significance of leaks through seals in the
valves inside the
swing adsorption unit. This can be especially important when rotary valves are
used.
[0094] A combination of pressure swing regeneration and thermal swing
regeneration
may be employed. In either instance, the gas treating facility 130 employs a
series of
absorbent beds, each designed to retain one or more components while releasing
the
remainder of the gas stream.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
17
[0095] The adsorptive material or "bed" is maintained in a pressure
vessel. Figure 2
provides a perspective view of an illustrative pressure swing adsorption
vessel 200. The
vessel 200 operates for the purpose of receiving a natural gas mixture, and
separating the
mixture into at least two components.
[0096] The vessel 200 defines an elongated, pressure-containing body. The
vessel 200
includes a housing 205. Preferably, the housing 205 is fabricated from iron or
steel. In the
arrangement of Figure 2, the vessel 200 rests along a surface 201 in a
substantially horizontal
orientation. However, the vessel 200 may alternatively be operated in a
vertical orientation.
In either instance, the vessel 200 may include various supporting legs or pads
215.
[0097] The vessel 200 is able to operate at high pressures so as to
accommodate the inlet
pressures experienced with the processing of natural gas. For example, such
inlet pressures
may exceed 200 psig, and more frequently may be greater than about 1,000 psig.
This allows
the vessel 200 to operate at or close to reservoir pressure. To monitor
internal pressure, the
vessel 200 includes gauges or other pressure-monitoring devices. A
representative gauge is
shown at 250 in Figure 2. Of course, it is understood that modern pressure-
monitoring
devices operate primarily as digital systems that interact with valves,
clocks, and operational
control software.
[0098] The vessel 200 has a first end shown at 202, and a second end
shown at 204. A
gas inlet 210 is provided at the first end 202, while a first gas outlet 230
is provided at the
second end 204. Optionally, a second gas outlet 220 is provided intermediate
the first end
202 and the second end 204, or intermediate the gas inlet 210 and the first
gas outlet 230.
[0099] In operation, the vessel 200 serves as a kinetic fractionator, or
adsorbent
contactor. A natural gas mixture, or feed stream, is introduced into the
vessel 200 through
the gas inlet 210. Arrow "/" indicates the flow of fluid into the vessel 200.
The natural gas is
contacted within the vessel 200 by an adsorbent bed (not shown in Figure 2).
The adsorbent
bed uses kinetic adsorption to capture contaminants. At the same time, the
adsorbent bed
releases a methane-rich gas stream through the first gas outlet 230. Flow of
the methane-rich
gas stream from the vessel 200 is indicated at arrow 01.
[0100] It is understood that the vessel 200 is part of the larger gas
separation unit 130.
The gas separation unit 130 includes valving, vessels, and gauges as needed to
carry out
regeneration of the adsorbent bed within the vessel 200 and the capture of the
separated gas
components. Further, where rapid cycle PSA is employed, the vessel will
include rotary
valving with a rotating manifold for rapidly cycling a natural gas mixture. In
this respect,
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
18
rapid cycle pressure swing adsorption (RCPSA) vessels can be constructed with
a rotary
valving system to facilitate the flow of gas through a rotary adsorber module
that contains a
number of separate adsorbent bed compartments or "tubes," each of which is
successively
cycled through the sorption and desorption steps as the rotary module
completes the cycles of
operation.
[0101] A rotary adsorber module is normally comprised of multiple tubes
held between
two seal plates on either end of the rotary adsorber module wherein the seal
plates are in
contact with a stator comprised of separate manifolds. The inlet gas is
conducted to the
RCPSA tubes and the processed purified product gas and the tail retentate gas
exiting the
RCPSA tubes are conducted away from the rotary adsorber module. By suitable
arrangement
of the seal plates and manifolds, a number of individual compartments or tubes
may pass
through the characteristic steps of the complete cycle at any given time. In
contrast, with
conventional PSA, the flow and pressure variations, required for the RCPSA
sorption/desorption cycle, changes in a number of separate increments on the
order of
seconds per cycle, which smoothes out the pressure and flow rate pulsations
encountered by
the compression and valving machinery. In this form, the RCPSA module includes
valving
elements angularly spaced around the circular path taken by the rotating
sorption module so
that each compartment is successively passed to a gas flow path in the
appropriate direction
and pressure to achieve one of the incremental pressure/flow direction steps
in the complete
RCP SA cycle.
[0102] In any arrangement, the vessel 200 utilizes an adsorbent bed to
capture
contaminants on the surface of a micro-porous adsorbent material and along the
pore spaces
therein. Figure 3A is a perspective view of an adsorbent bed 300, in one
embodiment. Here,
the illustrative adsorbent bed 300 has an annular adsorbent ring 305. The
adsorbent ring 305
is dimensioned to fit along an inner diameter of the housing 205 of the vessel
200.
[0103] Within the adsorbent ring 305 is a plurality of adsorbent rods 315.
The adsorbent
rods 315 run substantially along the length of the adsorbent bed 300. This
means that the
rods 315 run essentially from the first end 302 to the second end 304 of the
vessel 300. The
adsorbent ring 305 and the adsorbent rods 315 are fabricated from a material
that
preferentially adsorbs an undesirable gas. The undesirable gas may be water
vapor, CO2,
H2S, mercaptans, heavy hydrocarbons in gaseous phase, or combinations thereof.
[0104] The adsorbent material is preferably selected from the 8-ring
zeolites having a
Si:Al ratio from about 1:1 to about 1000:1, or preferably from about 10:1 to
about 500:1, or
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
19
more preferably from about 50:1 to about 300:1. The term "Si:Al ratio" as used
herein means
the molar ratio of silica to alumina of the zeolite structure. The more
preferred 8-ring zeolites
for the capture of sour gas include DDR, Sigma-1 and ZSM-58. Zeolite materials
having
appropriate pore sizes for the removal of heavy hydrocarbons include MFI,
faujasite, MCM-
41, and Beta. It is preferred that the Si:Al ratio of zeolites utilized for
heavy hydrocarbon
removal be from about 20:1 to about 1,000:1, and preferably from about 200:1
to about
1,000:1 in order to prevent excessive fouling of the adsorbent.
[0105] The zeolite may be present in the adsorbent ring 305 and the
adsorbent rods 315 in
any suitable form. For example, zeolite material may be in the form of beads
that are packed
to form the adsorbent material. Adsorbent beads, or aggregates, for swing
adsorption
processes are known in the art and can be of any suitable shape, including
spherical or
irregular. Adsorbent aggregates may be formed by adhering micro-porous zeolite
crystals
together with binder materials. The micro-pores exist due to the crystalline
structure of the
zeolite, in this case, preferably 8-ring zeolites. The binder material is
typically a dense
material that does not have adsorptive properties, but which is used to bind
the zeolite
crystals. In order to function effectively, the size of binder particles must
be smaller than the
size of the individual zeolite crystals.
[0106] In one embodiment of the adsorbent bed 300, a magnetic material may
be
incorporated into the adsorbent rods 315. For example, each rod 315 may have
an inner bore,
and a magnetic material may be placed along the inner bore. The rods 315 may
then be
subjected to a magnetic or an electromagnetic field during packing. The
magnetic field
causes the rods 315 to repel one another, thereby assuring uniform spacing
between the rods
315. Uniform packing of rods 315 is particularly important for kinetic and
fast cycled
adsorption processes so that gas components are not preferentially driven
through one flow
channel 310 over another. Application of the magnetic field may further
provide for a
homogeneous orientation of the zeolite material. Optionally, the magnetic
field may be
applied during the cycles themselves.
[0107] Referring again to Figure 3, within the annular adsorbent ring 305
and between
the adsorbent rods 315 is a plurality of flow channels. The flow channels are
seen at 310.
The flow channels 310 define major flow channels that flow along a major axis
of the
adsorbent bed 300.
[0108] The flow channels 310 create a type of structured adsorbent
contactor referred to
as a "parallel channel contactor." Parallel channel contactors are a subset of
adsorbent
20
contactors comprising structured (engineered) adsorbents in which
substantially parallel flow
channels are incorporated into the adsorbent structure. The flow channels 310
may be
formed by a variety of means, some of which are described in U.S. Pat. Publ.
No.
2008/0282887 titled "Removal of CO2, N2, and H2S from Gas Mixtures Containing
Same".
[0109] The adsorbent material forming the annular ring 305 and the rods
315 has a
"kinetic selectivity" for two or more gas components. As used herein, the term
"kinetic
selectivity" is defined as the ratio of single component diffusion
coefficients, D (in m2/sec),
for two different species. The single component diffusion coefficients are
also known as the
Stefan-Maxwell transport diffusion coefficients that are measured for a given
adsorbent for a
given pure gas component. Therefore, for example, the kinetic selectivity for
a particular
adsorbent for a component A with respect to a component B would be equal to
DA/ DB.
[0110] The single component diffusion coefficients for a material can be
determined by
tests known in the adsorptive materials art. The preferred way to measure the
kinetic
diffusion coefficient is with a frequency response technique described by
Reyes, et al. in
"Frequency Modulation Methods for Diffusion and Adsorption Measurements in
Porous
Solids," J. Phys. Chem. B. 101, pages 614-622 (1997).
In the kinetically controlled separation for the vessel 200, it is preferred
that
kinetic selectivity (i.e., DA / DB) of the selected adsorbent for the first
component (e.g., CO2)
with respect to the second component (e.g., methane) be greater than 5.
[0111] The term "selectivity" as used herein is based on a binary
comparison of the molar
concentration of components in the feed stream and the total number of moles
of these
components adsorbed by the particular adsorbent during the adsorption step of
the process
cycle under the specific system operating conditions and feed stream
composition. For a feed
gas stream containing a component A, a component B, and optionally additional
components,
an adsorbent that has a greater "selectivity" for component A than component B
will have at
the end of the adsorption step of the swing adsorption process cycle a ratio:
UA = (total moles of A in the adsorbent) / (molar concentration of
A in the feed)
that is greater than the ratio:
UB = (total moles of B in the adsorbent) / (molar concentration of
B in the feed)
where: UA is the "Adsorption Uptake of component A," and
UB is the "Adsorption Uptake of component B."
CA 2840723 2018-11-07
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
21
Therefore, for an adsorbent having a selectivity for component A over
component B that is
greater than one:
Selectivity = UA UB (where UA > Us).
[0112] Amongst a comparison of different components in a natural gas feed
stream, the
component with the smallest ratio of the total moles picked up in the
adsorbent to its molar
concentration in the feed stream is the lightest component in the swing
adsorption process.
The light component is taken to be the species, or molecular component, that
is not
preferentially taken up by the adsorbent in the adsorption process. This means
that the molar
concentration of the lightest component in the stream coming out during the
adsorption step
is greater than the molar concentration of that lightest component in the feed
stream. In the
present disclosure, the adsorbent contactor 200 has a selectivity for a first
component (e.g.,
CO2) over a second component (e.g., methane) of at least 5, more preferably a
selectivity for
a first component over a second component of at least 10, and most preferably
a selectivity
for a first component over a second component of at least 25.
[0113] Note that it is possible to remove two or more contaminants
simultaneously;
however, for convenience the component or components that are to be removed by
selective
adsorption may be referred to herein as a single contaminant or a heavy
component.
[0114] Recovery of the light component may also be characterized by
relative flow rate.
Thus, recovery of methane may be defined as the time averaged molar flow rate
of the
methane in the product stream (shown at 01 in the first outlet 230) divided by
the time
averaged molar flow rate of the methane in the feed stream (depicted as gas
inlet 210).
Similarly, recovery of the carbon dioxide and other heavy components is
defined as the time
averaged molar flow rate of the heavy components in the contaminant stream
(shown at 02 in
the second gas outlet 220) divided by the time averaged molar flow rate of the
heavy
component in the feed stream (depicted as gas inlet 210).
[0115] Additional technical information concerning component diffusion
coefficients and
kinetic selectivity is provided in co-owned U.S. Pat. Publ. No. 2008/0282887,
referenced
above.
[0116] In order to enhance the efficiency of the gas separation process,
minor flow
channels may also be provided in the bed 300. The minor flow channels increase
the surface
area exposure of the adsorbent material along the rods 315.
[0117] Figure 3B provides an exploded view of the adsorbent bed 300 of
Figure 3A.
The adsorbent bed 300 is cut across the optional second gas outlet 220. The
major flow
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
22
channels 310 running through the adsorbent bed 300 are again seen. In
addition, a transverse
flow channel is seen at 320. The transverse flow channel 320 serves as a minor
flow channel.
The flow channel 320 is seen partially extending into the adsorbent bed 300.
However, the
transverse flow channel 320 may optionally extend most of the way around the
circumference
of the annular adsorbent ring 305.
[0118] In the arrangement of Figure 3B, only a single minor flow channel
320 is shown.
However, the adsorbent bed 300 may have a plurality of minor flow channels
320. These
may optionally be manifolded together with flow converging on the second gas
outlet 220.
[0119] Figure 3C is a longitudinal, cross-sectional view of the adsorbent
bed 300 of
Figure 3A. The view is cut through line C-C of Figure 3A. Longitudinal
adsorbent rods
315 are seen in Figure 3C. In addition, major flow channels 310 are visible
between the rods
315.
[0120] A series of stepped surfaces 325 are seen along the adsorbent rods
315. The
stepped surfaces 325 also serve as minor flow channels. In lieu of stepped
surfaces 325, the
surfaces 325 may be helical or spiraled surfaces. In any arrangement, the
stepped surfaces
325 may be used in addition to or in lieu of the transverse channel 320 to
increase surface
area and improve kinetic selectivity without need of large and expensive heat
transfer units.
[0121] The major 310 and minor 320, 325 flow channels provide paths in the
fractionator
300 through which gas may flow. Generally, the flow channels 310, 320, 325
provide for
relatively low fluid resistance coupled with relatively high surface area.
Flow channel length
should be sufficient to provide the desired mass transfer zone, which is, at
least, a function of
the fluid velocity and the ratio of surface area to channel volume.
[0122] The flow channels 310, 320, 325 are preferably configured to
minimize pressure
drop in the vessel 200. Thus, tortuous flow paths are minimized or avoided. If
too much
pressure drop occurs across the bed 300, then higher cycle frequencies, such
as on the order
of greater than 100 cpm, are not readily achieved. In addition, and as noted
above, it is
preferred that the rods 315 be equidistantly spaced so as to create a degree
of channel
uniformity.
[0123] In one aspect, the flow channels 310 are generally divided so that
there is little or
no cross-flow. In this instance, a fluid flow fraction entering a channel 310
at the first end
302 of the fractionator 200 does not significantly communicate with any other
fluid fraction
entering another channel 310 at the first end 302 until the fractions
recombine upon exiting at
the second end 304. In this arrangement, the volumes of the major flow
channels 310 will be
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
23
substantially equal to ensure that all of the channels 310 are being fully
utilized, and that the
mass transfer zone defined by the interior volume of the vessel 200 is
substantially equally
contained.
[0124] The dimensions of the flow channels 310 can be computed from
considerations of
pressure drop along the contactor vessel 200. It is preferred that the flow
channels 310 have a
channel gap from about 5 to about 1,000 microns, preferably from about 50 to
about 250
microns. As utilized herein, the "channel gap" of a flow channel 310 is
defined as the length
of a line across the minimum dimension of the flow channel 310 as viewed
orthogonal to the
flow path. For instance, if the flow channel 310 is circular in cross-section,
then the channel
gap is the internal diameter of the circle. However, if the channel gap is
rectangular in cross-
section, the flow gap is the distance of a line bisecting the flow gap from
corner to corner.
[0125] It should be noted that the major flow channels 310 can be of any
cross-sectional
configuration or geometric profile. In Figures 3A and 3B, the major flow
channels 310 are
star-shaped. Regardless of the shape, it is preferred that the ratio of the
volume of adsorbent
material to the flow channel volume in the adsorbent contactor 200 be from
about 0.5:1 to
about 100:1, and more preferably from about 1:1 to about 50:1.
[0126] In some pressure swing applications, particularly with RCPSA
applications, the
flow channels are formed when adsorbent sheets are laminated together. The
flow channels
within the sheets will contain a spacer or mesh that acts as a spacer.
However, the spacers
take up much-needed space so the use of laminated sheets is not preferred.
[0127] In lieu of laminated sheets, a plurality of small, transverse minor
flow channels
may be machined through the adsorbent rods. Figure 4 provides a perspective
view of an
adsorbent bed 400 for the pressure swing adsorption vessel of Figure 2, in a
modified
arrangement. The adsorbent bed 400 has an outer surface 405. The outer surface
405 is
dimensioned to fit along an inner diameter of the housing 205 of the vessel
200 of Figure 2.
[0128] Major flow channels 410 are provided within a monolithic adsorbent
material 415.
The major flow channels 410 are formed along a major axis of the adsorbent bed
400.
However, to further increase surface area along the adsorbent rods, small
transverse channels
420 are formed through the monolithic material 415. These channels serve as
minor flow
channels 420.
[0129] The minor flow channels 420 may be very small tubular channels,
having a
diameter of less than about 25 microns, for example. The minor flow channels
420 are not so
large as to completely sever an adsorbent rod 415. In this way, the need for
supporting
24
spacers is avoided.
[0130] The optional minor flow channels 420 facilitate pressure balancing
between the
major flow channels 410. Both productivity and gas purity may suffer if there
is excessive
channel inconsistency. In this respect, if one flow channel is larger than an
adjacent flow
channel or receives more gas stream than another, premature product break-
through may
occur. This, in turn, leads to a reduction in the purity of the product gas to
unacceptable
purity levels. Moreover, devices operating at cycle frequencies greater than
about 50 cycles
per minute (cpm) require greater flow channel uniformity and less pressure
drop than those
operating at lower cycles per minute.
[0131] Returning now to Figures 2 and 3, the vessel 200 in Figure 2 is
shown as a
cylinder, and the adsorbent rods 315 therein are shown as tubular members.
However, other
shapes may be employed that are suitable for use in swing adsorption process
equipment.
Non-limiting examples of vessel arrangements include various shaped monoliths
having a
plurality of substantially parallel channels extending from one end of the
monolith to the
other; a plurality of tubular members; stacked layers of adsorbent sheets with
spacers
between each sheet; multi-layered spiral rolls or bundles of hollow fibers, as
well as bundles
of substantially parallel solid fibers.
[0132] In addition, other embodiments for a parallel channel contactor
may be employed.
Such embodiments include the contactors shown in and described in connection
with Figures
1 through 9 of U.S. Pat. Publ. No. 2008/0282887.
[0133] Returning to Figure 1, four illustrative separation stages are
shown. These are
stage 132'432", stage 134, stage 136, and stage 138. Each stage represents an
adsorbent
bed, with the stages 132/132", 134, 136, 138 being placed in series. The
adsorbent beds
preferably each reside within their own pressure vessel, such as vessel 200 of
Figure 2.
However, it is within the scope of the present application for at least some
of the beds to
reside within the same pressure vessel while remaining in series.
[0134] First, stage 132' represents the removal of water vapor from the
gas in line 122.
Thus, a first adsorbent bed is provided at stage 132' wherein the adsorbent
material is
designed to adsorb water vapor. Once the adsorbent material is saturated, the
bed in stage
132' is de-sorbed and water vapor is released through line 131'. Optionally,
the water vapor
is merged with the liquids line 121 from the separator 120, as indicated at
line 125.
[0135] The liquids in line 125 will be predominantly water. These liquids
may be
CA 2840723 2018-11-07
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
reinjected into the reservoir as part of a water flooding operation.
Alternatively, the water
may be treated and disposed of in a surrounding marine environment.
Alternatively still, the
water may be treated and taken through a desalinization process for use in
irrigation or
industrial use on-shore. Alternatively still, and as noted above, the liquids
in line 125 may
undergo further separation to capture any hydrocarbons.
[0136] It is
preferred that the first stage 132' simply be a "polishing" stage. This means
that most water has already been removed or "knocked out" by a previous
dehydration vessel
(such as vessel 120, and the adsorbent bed in stage 132' is simply removing
remaining water
vapor.
[0137] Where a
dehydration vessel is used, the fluids in line 122 will include a desiccant
such as ethylene glycol. Therefore, an ancillary first stage 132" is provided
for desiccant
removal. In Figure 1, desiccant is removed from the gas separation unit 130
through a
separate adsorption bed. Once the bed has become saturated, the desiccant is
released
through line 131". The desiccant may be recycled for use in the dehydration
vessel 120.
[0138] Figure 1
also shows a second stage of contaminant removal at 134. The
illustrative second stage 134 is for the removal of heavy hydrocarbons. As
noted, heavy
hydrocarbons will primarily include any ethane from the original gas stream.
Some propane
and butane may also be adsorbed. The heavy hydrocarbons are adsorbed onto the
adsorbent
bed, while sour gas and lighter hydrocarbons are released.
[0139] It is
possible that if the heavy hydrocarbon composition is very small, such
components will be adsorbed in the first 132'432" removal stage. This is also
dependent on
the composition of the adsorbent beds in the first 132'432" removal stage.
However, if the
heavy hydrocarbon content is large, such as greater than 3 to 5 percent, then
a separate,
dedicated adsorption stage 134 is desirable. Upon saturation, heavy
hydrocarbons are
released through line 133.
[0140] It is
preferred that the adsorbent bed in stage 134 be a zeolite material. Non-
limiting examples of zeolites having appropriate pore sizes for the removal of
heavy
hydrocarbons include MFI, faujasite, MCM-41 and Beta. It is preferred that the
Si/A1 ratio of
zeolites utilized in an embodiment of a process of the present invention for
heavy
hydrocarbon removal be from about 20 to about 1,000, preferably from about 200
to about
1,000 in order to prevent excessive fouling of the adsorbent.
[0141] Molecular
sieve beds fabricated from zeolite may be most effective at removing
C2 to C4 components, while silica gel beds may be most effective at removing
heavy
26
hydrocarbons. Additional technical information about the use of adsorptive
kinetic
separation for the separation of hydrocarbon gas components is provided in
U.S. Pat. Pub!.
No. 2008/0282884 entitled "Removal of Heavy Hydrocarbons From Gas Mixtures
Containing Heavy Hydrocarbons and Methane."
[0142] As noted, the separated heavy hydrocarbons will be released
through line 133.
The heavy hydrocarbons can be sold as a commercial fuel product.
Alternatively, the heavy
hydrocarbons may undergo some cooling to condense out the heavier components
and to
reclaim any methane vapor.
[0143] The gas stream next moves to the third stage 136. The third stage
136 provides
for the removal of sulfurous components. Sulfurous components may include
hydrogen
sulfide, sulfur. dioxide, and mercaptans. The sour gas components are adsorbed
onto the
adsorbent bed, while methane is passed on to an optional fourth stage 138.
Upon saturation,
the sulfurous components are released through line 135.
[0144] Where a dehydrated gas stream contains hydrogen sulfide, it may be
advantageous
to formulate the adsorbent with stannosilicates. Specifically, 8-ring zeolites
may be
fabricated with stannosilicates. The kinetic selectivity of this class of 8-
ring materials allows
H2S to be rapidly transmitted into zeolite crystals. Upon saturation, the bed
is purged. It is
understood that the sulfurous components will preferably be taken through a
subsequent
sulfur recovery process.
[0145] An optional fourth stage 138 is also provided in the gas
separation unit 130. The
fourth stage 138 provides for the removal of carbon dioxide and nitrogen from
the gas stream.
CO2 and N2 are adsorbed onto the adsorbent bed of stage 138, while a sweetened
gas stream
is released. Upon purging, CO2 and N2 exit the gas separation unit 130 through
exit line 137.
At the same time, the sweetened gas stream is released through line 140.
[0146] It is understood that the gas separation unit 130 may have fewer
or more than four
stages. The number of AKS stages is dependent on the composition of the raw
gas stream
entering through gas line 122. For example, if the raw gas stream in gas line
122 has less
than 0.5 ppm by volume H2S, then an adsorption stage for sulfurous components
removal
likely will not be required. Reciprocally, if the raw gas stream in gas line
122 has metal
contaminants such as mercury, then a separate AKS stage will be added for such
separation.
[0147] As noted, each stage 132'/132", 134, 136, 138 will employ an
adsorbent bed.
Each adsorbent bed may represent an adsorbent bed system that relies on a
plurality of beds
CA 2840723 2018-11-07
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
27
in parallel. These beds may be packed, for example, with activated carbons or
molecular
sieves. A first bed in each system is used for adsorption. This is known as a
service bed. A
second bed undergoes regeneration, such as through pressure reduction while
the first bed is
in service. Yet a third bed has already been regenerated and is held in
reserve for use in the
adsorption system when the first bed becomes substantially saturated. Thus, a
minimum of
three beds may be used in parallel for a more efficient operation.
[0148] In each stage 1327132", 134, 136, 138, the service bed may be in its
own
dedicated vessel, with the vessels of each stage being in series.
Alternatively, the service
beds may by aligned in series within one or more combined vessels. It is also
noted that the
beds may be fabricated from materials that will adsorb more than one component
at a time.
For example, a single bed may be designed to preferentially remove both
sulfurous
components and carbon dioxide. Alternatively, two separate vessels may be
provided in
series that are designed to remove substantially the same component. For
example, if the raw
gas stream in gas line 122 has a high CO2 content, then two beds may be
provided in
sequential vessels for preferential removal of the CO2.
[0149] A combination of different types of adsorbent beds may be used from
stage to
stage. Using a combination of adsorbent beds helps to prevent heavy
hydrocarbons from
remaining in the gas phase and ultimately ending up with the methane-rich gas
stream 140.
In any arrangement, a methane-rich gas 140 is released from the gas separation
unit 130.
[0150] The gas processing facility 100 also provides for the liquefaction
of the natural
gas. In the present context, this means that the sweetened, methane-rich gas
stream 140 will
be chilled. In Figure 1, a liquefaction facility is shown at 150.
[0151] Before entering the liquefaction facility 150, the methane-rich gas
stream 140 may
undergo modest compression. This is particularly true where there is a
distance between the
gas separation unit 130 and the liquefaction facility 150. In the facility 100
of Figure 1, an
optional compressor is shown at 145. The compressor 145 releases a compressed
methane-
rich gas stream 142 that feeds into the liquefaction facility 150.
[0152] In the present inventions, the liquefaction facility 150 is a high-
pressure,
expander-based facility. Figure 5 presents a schematic flow diagram of the
high-pressure
expander cycle refrigeration system 150, in one embodiment.
[0153] The refrigeration system 150 first includes a first compression unit
515. Upon
entering the liquefaction facility 150, the sweetened methane-rich gas stream
140 (or 142) is
passed through the first compression unit 515. The first compression unit 515
may be, for
CA 02840723 2013-12-30
WO 2013/022529 PCT/US2012/045005
28
example, a high pressure centrifugal dry seal compressor. The first
compression unit 515 will
increase the pressure of the methane-rich gas stream 140 to a pressure greater
than 1,000 psia
(6,895 kPa). In this way, a compressed gas feed stream 517 is created.
[0154] The liquefaction facility 150 also includes one or more compact heat
exchangers
for cooling the sweetened and compressed gas stream 517. In the arrangement of
Figure 5,
first 525 and second 535 heat exchangers are shown. The liquefaction facility
150 also
employs one or more high pressure expanders for further cooling. In Figure 5,
an expander
is shown at 540.
[0155] The expander 540 may be of several types. For example, a Joule-
Thompson valve
may be used. Alternatively, a turbo-expander may be provided. A turbo-expander
is a
centrifugal or axial flow turbine through which a high pressure gas is
expanded. Turbo-
expanders are typically used to produce work that may be used, for example, to
drive a
compressor. In this respect, turbo-expanders create a source of shaft work for
processes like
compression or refrigeration. In any embodiment, a liquefied natural gas, or
LNG stream, is
produced. An LNG stream is shown at line 542.
[0156] As noted, the liquefaction facility 150 includes a first heat
exchanger 525. The
heat exchanger 525 is part of a first refrigeration loop 520, and may be
referred to as a first
cooler. The first cooler 525 receives the compressed gas feed stream 517 from
the first
compression unit 515. The first cooler 525 then chills the compressed gas feed
stream 517
down to a substantially chilled temperature. For example, the temperature may
be as low as
¨100 C (-148 F).
[0157] The first cooler 525 releases a compressed, cooled gaseous feed
stream 522. The
compressed, cooled gaseous feed stream 522 is directed into the first expander
540. This
serves to further cool the compressed gas feed stream 517 down to a
temperature at which
substantial liquefaction of methane takes place. Thus, a liquefied product
stream 542 that is
at least about ¨162 C (-260 F) is released.
[0158] The product stream 542 will have a large liquid fraction and a
remaining small
vapor fraction. Therefore, it is preferred that the liquefaction facility 150
also include a
liquid separation vessel 550. The liquid separation vessel 550 is configured
to separate the
liquid fraction and the remaining vapor fraction. Thus, a liquid methane
stream 152 is
released in one line as the LNG commercial product, and a separate cold vapor
stream 552 is
released overhead.
[0159] The cold vapor stream 552 may be used as a coolant for the first
cooler 525 in the
CA 02840723 2013-12-30
WO 2013/022529 PCT/US2012/045005
29
first refrigeration loop 520. It can be seen in Figure 5 that the cold vapor
stream 552 enters
the first cooler 525 where heat exchange takes place with the compressed gas
feed stream
517. A partially-warmed product stream 554 is then released.
[0160] The partially-warmed product stream 554 is directed back to the
beginning of the
first refrigeration loop 520. This means that the partially-warmed product
stream 554 is
merged back with the methane-rich gas stream 140 (or 142). To accomplish this,
a third
compression unit 555 is provided. The third compression unit 555 releases a
compressed,
partially-warmed product stream 557. The compressed, partially-warmed product
stream 557
is preferably taken through the first compression unit 515 with the methane-
rich gas stream
142.
[0161] It is preferred that the gas liquefaction facility 150 include a
second heat
exchanger. The second heat exchanger is shown at 535, and represents a second
cooler. The
second heat exchanger 535 may optionally be placed in line in the first
refrigeration loop 520
after the first cooler 525. In this way, the second heat exchanger 535 would
provide sub-
cooling to the compressed, cooled gaseous feed stream 522. However, it is
preferred that the
second heat exchanger 535 be placed in line in the first refrigeration loop
520 before the first
cooler 525. This is the arrangement shown in Figure 5.
[0162] In Figure 5, the second heat exchanger 535, or second cooler,
receives the
partially-warmed product stream 554 from the first cooler 525. Indirect heat
exchange then
takes place between the partially-warmed product stream 554 and the compressed
gas feed
stream 517. The heat exchanger 535 pre-cools the compressed gas feed 517
stream before
the compressed gas feed stream 517 enters the first cooler 525. The second
heat exchanger
535 thus releases a pre-cooled compressed gas feed stream 532 into the first
cooler 525.
[0163] The heat exchanger 535 also releases a warmed product stream 556. In
this
arrangement, the warmed product stream 556 enters the third compression unit
555, and is
released as the compressed and partially-warmed product stream 557 that is
merged with the
methane-rich gas stream 142.
[0164] In order to provide effective pre-cooling in the second cooler 535,
it is desirable to
employ a coolant in addition to the partially-warmed product stream 554.
Therefore, a
second refrigeration loop 530 is also provided. The second refrigeration loop
530 employs a
refrigerant, indicated at line 534. The refrigerant in line 534 is preferably
a nitrogen gas, or a
nitrogen-containing gas. The use of nitrogen in the refrigerant expands the
pre-cooling
temperature regime.
CA 02840723 2013-12-30
WO 2013/022529 PCT/US2012/045005
[0165] Referring back to Figure 1, it can be seen that a portion of the
contaminant from
stage 138 is intercepted. This represents N2 and, perhaps, some CO2 in line
137. The
nitrogen is taken via line 147 to the gas liquefaction facility 150. In
addition, the operator
may take a portion of the methane-rich gas stream 140 to use as the
refrigerant 534. This is
done via line 141. Alternatively or in addition, the operator may take a
portion of the heavy
hydrocarbons separated from stage 136. The ethane (or other heavy
hydrocarbons) is taken
from line 133 via line 143. Lines 141, 143, and 147 are shown as dashed lines,
indicating
optional fluid interceptions.
[0166] The gas components in lines 141, 143, and 147 are selectively and
optionally
taken from the gas separation unit 130 and merged into line 149. This is shown
in Figure 1.
The components from lines 141, 143, and/or 147 are then directed through line
149 into the
second refrigeration loop 530. This is shown in Figure 5. Valve 501 is seen
for controlling
the flow of components from lines 141, 143, and/or 147 through line 149 into
the second
refrigeration loop 530. Valve 501 may also be used to divert a portion of the
components
from lines 141, 143, 147 for burning in a gas turbine to generate electricity
or for
regenerating a bed in connection with TSA.
[0167] It is, of course, understood that additional valving (not shown)
will control the
relative volumes of the components in lines 141, 143, 147 taken into line 149.
It is further
understood that the operator may draw from a dedicated tank of nitrogen (not
shown) for the
refrigerant in line 534. In any arrangement, the refrigerant from line 534
leaves the heat
exchanger 535 in a warmed state. The refrigerant moves through a second
compression unit
536 for pressure boosting, and is then taken through an expander 538 for re-
cooling. The
refrigerant in line 534 then re-enters the heat exchanger 535. A small chiller
(not shown)
may be added to the second refrigeration loop 530 after the expander 538 to
further cool the
refrigerant in line 534.
[0168] It is noted that the heat exchanger 535 may serve as the only cooler
for the gas
liquefaction facility 150. In this arrangement, the first cooler 525 would not
be used.
Further, the vapor portion 552 would preferably then be used as at least a
portion of the
refrigerant for line 534. However, the use of the heat exchanger 535 with the
expander 538
in the second refrigeration loop 530 improves the overall cooling efficiency
of the first
expander loop 520. In any instance, the present invention is not limited by
the specific
arrangement of coolers or refrigeration loops unless so expressly stated in
the claims.
[0169] Figure 6 is a flow chart showing steps for a process 600 for
liquefying a raw
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
31
natural gas stream. The process 600 employs adsorptive kinetic separation to
produce a
methane-rich gas stream. The process 600 then further utilizes a high-pressure
expander
cycle refrigeration system to chill the methane and to provide an LNG product.
The LNG
product is preferably generated on a floating platform or a gravity-based
platform offshore.
[0170] The process 600 first includes receiving the natural gas feed stream
at a gas
separation unit. The gas separation unit has one or more fractionation
vessels. The
fractionation vessels are designed in accordance with the fraction vessels
described above in
their various embodiments. The fractionation vessels preferably operate on
pressure swing
adsorption (PSA) or rapid cycle pressure swing adsorption (RCPSA) to
regenerate a series of
adsorption beds. The adsorption beds are designed to adsorb CO2, H2S, H20,
heavy
hydrocarbons, VOC 's, mercaptans, nitrogen, or combinations thereof.
[0171] The process 600 also includes substantially separating methane from
contaminants
within the natural gas feed stream. As a first separation step, the raw
natural gas feed stream
is optionally taken through a dehydration vessel. This serves to remove a
substantial portion
of water and other liquid phase components from the natural gas stream. The
step of
separating liquid-phase components (primarily water) from gas phase components
is shown
in Box 620. A dehydrated natural gas feed stream is then released as a
dehydrated natural gas
feed stream.
[0172] Next, gas-phase contaminants are removed from the dehydrated raw gas
stream.
The step of separating methane from gas-phase contaminants within the natural
gas feed
stream is shown at Box 630. This step is done through the use of adsorption
beds in the one
or more fractionation vessels. In one aspect, separating methane from
contaminants is
conducted through the gas separation unit at a pressure of at least about 500
pounds per
square inch absolute (psia).
[0173] Figure 7 is a flow chart showing steps 700 for separating
contaminants from the
raw natural gas stream. The steps use adsorptive kinetic separation to create
the methane-rich
gas stream.
[0174] First, water is adsorbed from the natural gas feed stream. In this
respect, an
adsorptive bed having water-retentive properties is employed. This step is
shown in Box 710.
As noted above, it is preferred that the water removal stage simply be a
"polishing" stage.
This means that most water has already been removed or "knocked out" by a
previous
dehydration vessel (per the step of Box 620).
[0175] Where a dehydration vessel is used, the contaminants in the gas
stream will
CA 02840723 2013-12-30
WO 2013/022529 PCT/US2012/045005
32
include a desiccant such as ethylene glycol. Accordingly, a next stage in
separating
components involves the adsorption of the desiccant. This is provided in Box
720.
[0176] As shown in Figure 7, various additional adsorptive stages may be
undertaken for
the removal of contaminants. These may include the removal of sulfurous
components (Box
730), the removal of carbon dioxide and/or nitrogen (Box 740), the removal of
mercury or
other metallic elements (Box 750), and the removal of heavy hydrocarbons (Box
760).
Depending on how the adsorbent beds are designed, some of these components may
be
removed in a single combined stage. Further, the order of contaminant removal
as provided
in the steps of Boxes 710 through 760 may be changed, although it is highly
preferred that
water be removed first as shown in Box 710. Thus, the process 600 is not
limited by the
order in which contaminants are removed in the steps 700 unless so stated in
the claims
herein.
[0177] In one aspect, a single vessel having a plurality of adsorbent beds
aligned in series
is used. For example, the at least one fractionation vessel in the gas
separation unit may
comprise a vessel containing a plurality of adsorbent beds in series, such
that:
a first adsorption bed is designed to primarily remove water and other liquid
components from the dehydrated natural gas feed stream;
a second adsorption bed is designed to primarily remove a desiccant from the
dehydrated natural gas feed stream; and
a third vessel comprises an adsorption bed designed primarily for the removal
of a
sour gas component from the dehydrated natural gas feed stream.
Additional vessels may be added to adsorb and separate different sour gases.
[0178] In another aspect, multiple vessels in series are employed, with the
vessels being
aligned with the flow of the dehydrated natural gas feed stream. Each vessel
releases a
progressively sweeter methane gas stream. For example,
a first vessel uses an adsorption bed designed for the removal of water
remaining in
the dehydrated natural gas feed stream;
a second vessel uses an adsorption bed designed for the removal of a desiccant
from
the dehydrated natural gas feed stream; and
a third vessel uses an adsorption bed designed for the removal of a sour gas
component from the dehydrated natural gas feed stream.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
33
The sour gas component may be one or more sulfurous components. Alternatively,
the sour
gas component may be carbon dioxide.
[0179] As a result of the adsorption steps 700 in Figure 7, a methane-rich
gas stream is
generated. This stream is released from the gas separation unit as a
dehydrated natural gas
feed stream. Accordingly, the process 600 next includes releasing a
dehydrated, methane-
rich gas stream from the gas separation unit. This is indicated at Box 640.
[0180] The methane-rich gas stream is directed to the high-pressure
expander cycle
refrigeration system. This is seen at Box 650. The refrigeration system is in
accordance with
the refrigeration system 150 shown above in Figure 5, and as described in any
of its various
embodiments. Thus, the refrigeration system preferably includes a first
refrigeration loop for
cycling the vapor portion of the product for use as a coolant in a first
cooler, and a second
refrigeration loop for cycling a nitrogen-containing gas as a refrigerant in a
second cooler.
The second cooler may utilize both the nitrogen-based refrigerant and a
partially-warmed
methane gas from the first cooler as working fluids.
[0181] The process 600 also includes compressing the methane-rich gas
stream. This is
provided at Box 660. The gas stream is compressed to a pressure that is
greater than 1,000
psia (6,895 kPa) in order to form a compressed gas feed stream.
[0182] The process 600 next comprises cooling the compressed gas feed
stream to form a
compressed, cooled gaseous feed stream. This is seen at Box 670. The cooling
step of Box
670 preferably involves taking the compressed gas feed stream through at least
one heat
exchanger within a first refrigeration loop 520. For example, the compressed
gas feed stream
may be pre-chilled using the heat exchanger 535 (second cooler) of Figure 5,
and then
further cooled using the first cooler 525 of Figure 5. Optionally, the heat
exchanger 535
(second cooler) may be placed in the first refrigeration loop 520 after the
first cooler 525. In
this way, the heat exchanger 535 sub-cools the compressed gas feed stream 517
after the
compressed gas feed stream 517 has passed through the first cooler 525.
[0183] The first refrigeration loop 520 cycles coolant through at least one
heat exchanger
(such as cooler 525), and then directs the used (warmed) coolant (554 and/or
556) to a
compression unit 555. The compression unit compresses the warmed coolant to
about 1,500
to 3,500 psia (10,342 to 24,132 kPa). More preferably, the compression unit
compresses the
warmed product stream to about 2,500 to 3,000 psia (17,237 to 20,684 kPa).
[0184] The second cooler 535 is preferably part of a second refrigeration
loop 530. The
second cooler 535 is configured to cool the compressed gas feed stream 517 at
least partially
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
34
by indirect heat exchange between a refrigerant stream 534 and the compressed,
gas feed
stream. The second refrigeration loop 530 may also include a compression unit
536. The
compression unit 536 is configured to re-compress the refrigerant stream after
the refrigerant
stream passes through the second cooler 535. The second refrigeration loop
will also then
include an expander. The expander receives the re-compressed, cooled
refrigerant stream,
and expands the compressed, cooled refrigerant stream prior to returning it to
the second
cooler 535.
[0185] The process 600 also includes expanding the cooled, compressed,
gaseous feed
stream 522. This is provided at Box 680. In one aspect, expanding the cooled,
compressed,
gaseous feed stream 522 comprises reducing the pressure of the cooled,
compressed, gaseous
feed stream to a pressure between about 50 psia (345 kPa) and 450 psia (3103
kPa).
Expansion of the cooled gaseous feed stream 522 forms the LNG product stream
542. The
product stream has a liquid fraction and a remaining vapor fraction.
[0186] The high-pressure expander cycle refrigeration system preferably
includes a liquid
separation vessel. The process then further comprises separating the liquid
fraction and the
remaining vapor fraction. The liquid portion may then be loaded into a
transport vessel. This
is indicated at Box 690 of Figure 6.
[0187] In order to demonstrate the utility of the process 600, and
particularly the step of
removing nitrogen using an AKS system, certain data has been generated. This
date is
presented in Tables in connection with certain examples, below.
EXAMPLES
[0188] The Tables below depict comparisons developed using an Aspen HYSYS
(version 2006) process simulator, a computer-aided design program from Aspen
Technology,
Inc., of Cambridge, Massachusetts. In connection with the Tables, the term
"SCRS" is used.
This term is an acronym for "Selective Component Removal System," and in this
context
refers to an AKS adsorptive system.
[0189] First, Table 2 illustrates the effect of removing nitrogen from the
natural gas
stream prior to liquefaction. The comparison is with the conventional approach
where the
nitrogen is removed after the nitrogen-containing natural gas is liquefied
using a distillation
column. The power saving (greater than 7%) achieved results in a reduction of
power
generation equipment. This, in turn, translates into space and weight
reductions, thereby
enabling offshore LNG production.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
Feed Molar LNG Product Specification
Composition w/o SCRS w/ SCRS
Nitrogen 0.0431 0.0101 0.0053
Methane 0.9559 0.9888 0.9936
Ethane 0.0010 0.0011 0.0011
Liquefaction Power (%) 100 93.3
Table 2
Effect of N2 Removal with SCRS on Liquefaction Horsepower
Note that the required specifications on the LNG are handily achieved
[0190] Next, Table 3 is provided to illustrate the benefit of using a high-
pressure
expander cycle refrigeration system on process performance. The thermal energy
required to
produce the LNG from an ambient temperature of 100 F is reduced as the feed
pressure is
increased, up to 17% for a pressure of 4,000 psia. Conventional gas
conditioning methods
reduce the feed gas pressure below 1,000 psia. Therefore compression equipment
and the
associated compression horsepower are required to boost the feed pressure in
order to
capitalize on the benefit of the reduced thermal energy at elevated pressures.
This offsets the
liquefaction horsepower reduction benefits.
Refrigeration
Feed Gas Duty Refrigeration Duty
Pressure (psia) (normalized) % Reduction
4,000 83.0 17.0
3,000 86.4 13.6
2,000 91.5 8.5
1,000 98.4 1.6
800 100.0 0.0
Table 3
Effect of Elevated Feed Gas Pressure on Refrigeration Duty Requirement
It has been discovered that operating the AKS-based gas separation unit at an
elevated
pressure preserves and even enhances these benefits.
[0191] Table 4 highlights the performance improvement using the inventive
separation
process. In the conventional approach, the benefit of the elevated feed gas
pressure is
achieved by adding feed gas compression: the energy associated with the
pressure reduction
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
36
from the wellhead dictated by the conventional solvent extraction gas treating
method is
typically wasted. The SCRS unit may be configured to preserve the wellhead
pressure and
thereby avoid the wasted energy resulting from the conventional approach.
Liquefaction Power ')/0 Incremental
Feed Gas
Reduction
Pressure
w/ Feed Gas Improvement
(psia) w/o SCRS
Compression w/ SCRS
5,000 10.9 38.7 27.9
4,500 12.0 37.4 25.3
4,000 13.1 35.9 22.8
3,500 14.1 33.9 19.8
3,000 15.0 32.0 17.0
2,500 15.0 28.8 13.8
2,000 14.3 24.3 10.0
1,500 10.5 16.0 5.5
1,250 7.6 10.5 2.9
1,000 0.0 0.0 0.0
Table 4
Effect of Elevated Feed Gas Pressure on Liquefaction Power
[0192] It is
believed that by using small, light-weight AKS separators to form the gas
separation unit, and by using a high-pressure expander cycle refrigeration
system, the
equipment footprint and weight of the gas conditioning or treating facilities
are reduced by
75%. This may translate into a 21% reduction in space and weight on a FLNG
barge.
Alternatively, the available space and weight may be used to increase the
capacity of the
FLNG barge. The reduction therefore improves the economic viability of the gas
commercialization project.
[0193] As can be
seen, an improved gas processing facility for the liquefaction of a
natural gas stream is provided. In one aspect, the facility comprises:
1. a gas
separation unit, the gas separation unit having at least one fractionation
vessel
comprised of:
a gas inlet for receiving a natural gas mixture comprising methane,
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
37
an adsorbent material that has a kinetic selectivity for contaminants over
methane
greater than 5, such that the contaminants become kinetically adsorbed within
the adsorbent
material, and
a gas outlet for releasing a methane-rich gas stream; and
a high-pressure expander cycle refrigeration system comprised of:
a first compression unit configured to receive a substantial portion of the
methane-rich gas stream and to compress the methane-rich gas stream to greater
than
about 1,000 psia (6,895 kPa), thereby providing a compressed gas feed stream;
a first cooler configured to cool the compressed gas feed stream to form a
compressed, cooled gaseous feed stream; and
a first expander configured to expand the cooled, compressed, gaseous feed
stream to form a product stream having a liquid fraction and a remaining vapor
fraction.
2. The gas processing facility of paragraph 1, wherein:
the first cooler is configured to receive a portion of the product stream from
the first
expander, and use the portion of the product stream to cool the compressed gas
feed stream
through heat exchange.
3. The gas processing facility of paragraph 1, wherein:
the first cooler is configured to use an external refrigerant stream to cool
the
compressed gas feed stream through heat exchange.
4. The gas processing facility of paragraph 1, wherein the high-pressure
expander cycle
refrigeration system further comprises:
a liquid separation vessel configured to separate the liquid fraction and the
remaining
vapor fraction from the first expander.
5. The gas processing facility of paragraph 4, wherein:
the first cooler receives at least a portion of the vapor fraction, and uses
the vapor
fraction to cool the compressed gas feed stream through heat exchange as part
of a first
refrigeration loop;
the first cooler releases (i) a chilled gas feed stream, and (ii) a partially-
warmed
product stream after heat-exchanging with the compressed gas feed stream; and
the high-pressure expander cycle refrigeration system further comprises:
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
38
a second cooler configured to further cool the compressed gas feed stream at
least partially by indirect heat exchange with a refrigerant stream and the
vapor
fraction; and
a second refrigeration loop having (i) a second compression unit configured to
re-compress the refrigerant stream after the refrigerant stream passes through
the
second cooler, and (ii) a second expander configured to receive the re-
compressed
refrigerant stream, and expand the re-compressed refrigerant stream prior to
returning
it to the second cooler.
6. The gas processing facility of paragraph 5, wherein the high-pressure
expander cycle
refrigeration system further comprises:
a third compression unit in the first refrigeration loop for compressing the
partially-
warmed product stream after heat-exchanging with the compressed gas feed
stream; and
a line for merging the compressed, partially-warmed product stream with the
gas feed
stream to complete the first refrigeration loop.
7. The gas processing facility of paragraph 5, wherein the second cooler
sub-cools the
chilled gas feed stream after the chilled gas feed stream leaves the first
cooler.
8. The gas processing facility of paragraph 5, wherein the second cooler
pre-cools the
compressed gas feed stream before the compressed gas feed stream enters the
first cooler.
9. The gas processing facility of paragraph 8, wherein:
the second cooler receives the partially-warmed product stream from the first
cooler
for further heat-exchanging with the compressed gas feed stream; and
releases a warmed product stream to a third compression unit to complete the
first
refrigeration loop.
10. The gas processing facility of paragraph 1, wherein the facility is
located on (i) a
floating platform, (ii) a gravity-based platform, or (iii) a ship-shaped
vessel offshore.
11. The gas processing facility of paragraph 1, wherein the at least one
fractionation
vessel in the gas separation unit operates on pressure swing adsorption (PSA)
or rapid cycle
pressure swing adsorption (RCPSA).
12. The gas processing facility of paragraph 11, wherein the at least one
fractionation
vessel is configured to adsorb CO2, H2S, H20, heavy hydrocarbons, VOC's,
mercaptans, or
combinations thereof.
13. The gas processing facility of paragraph 12, further comprising:
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
39
a dehydration vessel configured to receive the natural gas feed stream and
remove a
substantial portion of water from the natural gas feed stream, and release a
dehydrated natural
gas feed stream to the at least one fractionation vessel.
14. A process for liquefying a natural gas feed stream, comprising:
receiving the natural gas feed stream at a gas separation unit, the gas
separation unit
having at least one fractionation vessel comprised of:
a gas inlet for receiving a natural gas mixture comprising methane,
an adsorbent material that has a kinetic selectivity for contaminants over
methane greater than 5, such that the contaminants become kinetically adsorbed
within the adsorbent material, and
a gas outlet configured to release a methane-rich gas stream;
substantially separating methane from contaminants within the natural gas
feed stream;
releasing a methane-rich gas stream from the gas separation unit;
directing the methane-rich gas stream into a high-pressure expander cycle
refrigeration system;
compressing the methane-rich gas stream to a pressure that is greater than
1,000 psia (6,895 kPa) in order to form a compressed gas feed stream;
cooling the compressed gas feed stream to form a compressed, cooled gaseous
feed stream;
expanding the cooled, compressed, gaseous feed stream to form a product
stream having a liquid fraction and a remaining vapor fraction; and
separating the vapor fraction from the liquid fraction.
15. The process of paragraph 14, wherein the high-pressure expander cycle
refrigeration
system comprises:
a first compression unit configured to receive a substantial portion of the
methane-rich
gas stream and to generate the compressed gas feed stream;
a first cooler configured to cool the compressed gas feed stream to form the
compressed, cooled gaseous feed stream; and
a first expander configured to expand the cooled, compressed, gaseous feed
stream to
form the product stream.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
16. The process of paragraph 15, wherein cooling the compressed gas feed
stream
comprises:
delivering at least a portion of the vapor fraction from the product stream to
the first
cooler as part of a first refrigeration loop; and
heat-exchanging the vapor fraction of the product stream with the compressed
gas
feed stream to cool the compressed gas feed stream.
17. The process of paragraph 16, wherein:
the high-pressure expander cycle refrigeration system further comprises a
liquid
separation vessel; and
separating the vapor fraction from the liquid fraction is done using the
liquid
separation vessel.
18. The process of paragraph 17, further comprising:
releasing from the first cooler (i) a chilled gas feed stream as the product
stream, and
(ii) a partially-warmed product stream as a working fluid;
directing the partially-warmed product stream to a third compression unit; and
merging the compressed, partially-warmed product stream from the third
compression
unit with the methane-rich gas stream to complete the first refrigeration
loop.
19. The process of paragraph 18, wherein the high-pressure expander cycle
refrigeration
system further comprises:
a second cooler configured to further cool the compressed gas feed stream at
least
partially by indirect heat exchange between a refrigerant stream and the vapor
fraction; and
a second refrigeration loop having (i) a second compression unit configured to
re-
compress the refrigerant stream after the refrigerant stream passes through
the second cooler,
and (ii) a second expander configured to receive the compressed refrigerant
stream, and
expand the compressed refrigerant stream prior to returning it to the second
cooler.
20. The process of paragraph 19, wherein the second cooler sub-cools the
chilled gas feed
stream after the chilled gas feed stream leaves the first cooler.
21. The process of paragraph 19, wherein the second cooler pre-cools the
compressed gas
feed stream before the compressed gas feed stream enters the first cooler.
22. The process of paragraph 1, wherein the facility is located on (i) a
floating platform,
(ii) a gravity-based platform, or (iii) a ship-shaped vessel offshore.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
41
23. The process of paragraph 22, wherein the at least one fractionation
vessel in the gas
separation unit operates on pressure swing adsorption (PSA) or rapid cycle
pressure swing
adsorption (RCP SA).
24. The process of paragraph 23, wherein the at least one fractionation
vessel is
configured to adsorb CO2, I-17S, H2O, heavy hydrocarbons, VOC's, mercaptans,
or
combinations thereof.
25. The process of paragraph 22, further comprising:
passing the natural gas feed stream through a dehydration vessel in order to
remove a
substantial portion of water from the natural gas feed stream; and
release a dehydrated natural gas feed stream to the at least one fractionation
vessel for
contaminant removal.
26. A method for liquefying a natural gas feed stream, comprising:
receiving the natural gas feed stream at a gas processing facility;
passing the natural gas feed stream through a dehydration vessel in order to
remove a
substantial portion of water from the natural gas feed stream;
releasing a dehydrated natural gas feed stream to a gas separation unit as a
dehydrated
natural gas feed stream;
in the gas separation unit, passing the dehydrated natural gas feed stream
through a
series of adsorbent beds in order to separate methane gas from contaminants in
the
dehydrated natural gas feed stream using adsorptive kinetic separation;
releasing a methane-rich gas stream from the gas separation unit;
directing the methane-rich gas stream into a high-pressure expander cycle
refrigeration system;
compressing the methane-rich gas stream to a pressure that is greater than
1,000 psia
(6,895 kPa) in order to form a compressed gas feed stream;
cooling the compressed gas feed stream to form a compressed, cooled gaseous
feed
stream;
expanding the cooled, compressed, gaseous feed stream to form a product stream
having a liquid fraction and a remaining vapor fraction.
27. The method of paragraph 26, wherein the series of adsorbent beds
comprises:
a first adsorption bed for the removal of water remaining in the dehydrated
natural gas
feed stream;
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
42
a second adsorption bed designed primarily for the removal of a desiccant from
the
dehydrated natural gas feed stream; and
a third adsorption bed designed primarily for the removal of a sour gas
component
from the dehydrated natural gas feed stream.
28. The method
of paragraph 27, wherein each of the adsorbent beds has associated with
it two additional adsorbent beds to form three adsorbent beds, with:
a first of the three adsorbent beds being in service for adsorbing a selected
contaminant;
a second of the three adsorbent beds undergoing regeneration; and
a third of the adsorbent beds being held in reserve to replace the first of
the three
adsorbent beds; and
wherein the regeneration is part of a pressure-swing adsorption process.
[0194] As can be
seen, another enhanced gas processing facility for the liquefaction of a
natural gas stream is provided. In one aspect, the facility comprises:
IA. A gas
processing facility for the liquefaction of a natural gas feed stream, the
facility
comprising:
a gas separation unit, the gas separation unit having at least one
fractionation vessel
comprised of:
a gas inlet for receiving a natural gas mixture comprising methane,
an adsorbent material that has a kinetic selectivity for contaminants over
methane
greater than 5, such that the contaminants become kinetically adsorbed within
the adsorbent
material, and
a gas outlet for releasing a methane-rich gas stream; and
a high-pressure expander cycle refrigeration system comprised of:
a first compression unit configured to receive a substantial portion of the
methane-rich
gas stream and to compress the methane-rich gas stream to greater than about
1,000 psia
(6,895 kPa), thereby providing a compressed gas feed stream;
a first cooler configured to cool the compressed gas feed stream to form a
compressed, cooled gaseous feed stream; and
a first expander configured to expand the cooled, compressed, gaseous feed
stream to
form a product stream having a liquid fraction and a remaining vapor fraction.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
43
2A. The gas processing facility of paragraph 1A, wherein:
the first cooler is configured to receive a portion of the product stream from
the first
expander, and use the portion of the product stream to cool the compressed gas
feed stream
through heat exchange.
3A. The gas processing facility of paragraph 1A, wherein:
the first cooler is configured to use an external refrigerant stream to cool
the compressed gas
feed stream through heat exchange.
4A. The gas processing facility of paragraph 1A, wherein the high-pressure
expander
cycle refrigeration system further comprises:
a liquid separation vessel configured to separate the liquid fraction and the
remaining
vapor fraction from the first expander.
5A. The gas processing facility of paragraph 4A, wherein:
the first cooler receives at least a portion of the vapor fraction, and uses
the vapor
fraction to cool the compressed gas feed stream through heat exchange as part
of a first
refrigeration loop;
the first cooler releases (i) a chilled gas feed stream, and (ii) a partially-
warmed
product stream after heat-exchanging with the compressed gas feed stream; and
the high-pressure expander cycle refrigeration system further comprises:
a second cooler configured to further cool the compressed gas feed stream at
least
partially by indirect heat exchange with a refrigerant stream and the vapor
fraction; and
a second refrigeration loop having (i) a second compression unit configured to
re-
compress the refrigerant stream after the refrigerant stream passes through
the second cooler,
and (ii) a second expander configured to receive the re-compressed refrigerant
stream, and
expand the re-compressed refrigerant stream prior to returning it to the
second cooler.
6A. The gas processing facility of paragraph 5A, wherein the high-pressure
expander
cycle refrigeration system further comprises:
a third compression unit in the first refrigeration loop for compressing the
partially-
warmed product stream after heat-exchanging with the compressed gas feed
stream; and
a line for merging the compressed, partially-warmed product stream with the
gas feed
stream to complete the first refrigeration loop.
7A. The gas processing facility of paragraph 5A, wherein the second cooler
sub-cools the
chilled gas feed stream after the chilled gas feed stream leaves the first
cooler.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
44
8A. The gas processing facility of paragraph 5A, wherein the second cooler
pre-cools the
compressed gas feed stream before the compressed gas feed stream enters the
first cooler.
9A. The gas processing facility of paragraph 8A, wherein:
the second cooler receives the partially-warmed product stream from the first
cooler
for further heat-exchanging with the compressed gas feed stream; and
releases a warmed product stream to a third compression unit to complete the
first
refrigeration loop.
10A. The gas processing facility of paragraph 9A, wherein the third
compression unit
compresses the warmed product stream to about 1,500 to 3,500 psia (10,342 to
24,132 kPa).
11A. The gas processing facility of paragraph 1A, wherein the facility is
located on (i) a
floating platform, (ii) a gravity-based platform, or (iii) a ship-shaped
vessel offshore.
12A. The gas processing facility of paragraph 5A, wherein:
the refrigerant stream comprises a gas selected from the group consisting of:
nitrogen
gas, nitrogen-containing gas, a side stream from the methane-rich gas stream,
and the
remaining vapor fraction, and combinations thereof; and
the refrigerant stream in the second refrigeration loop flows in a closed
loop.
13A. The gas processing facility of paragraph 1A, wherein the at least one
fractionation
vessel in the gas separation unit operates on pressure swing adsorption (PSA)
or rapid cycle
pressure swing adsorption (RCPSA).
14A. The gas processing facility of paragraph 13A, wherein the at least one
fractionation
vessel in the gas separation unit further operates on temperature swing
adsorption (TSA) or
rapid cycle temperature swing adsorption (RCTSA).
15A. The gas processing facility of paragraph 13A, wherein the at least one
fractionation
vessel is configured to adsorb CO2, H2S, H20, heavy hydrocarbons, VOC's,
mercaptans, or
combinations thereof.
16A. The gas processing facility of paragraph 13A, wherein each of the at
least one
fractionation vessel cooperates with other fractionation vessels to form a
pressure swing
adsorption system comprising:
at least one service bed providing adsorption,
at least one bed in regeneration undergoing pressure reduction, and
at least one regenerated bed held in reserve for use in the adsorption system
when the
at least one service bed becomes substantially saturated.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
17A. The gas processing facility of paragraph 13A, further comprising:
a dehydration vessel configured to receive the natural gas feed stream and
remove a
substantial portion of water from the natural gas feed stream, and release a
dehydrated natural
gas feed stream to the at least one fractionation vessel.
18A. The gas processing facility of paragraph 17A, wherein the at least one
fractionation
vessel in the gas separation unit comprises a plurality of vessels in series,
such that:
a first vessel comprises an adsorption bed for the removal of water remaining
in the
dehydrated natural gas feed stream;
a second vessel comprises an adsorption bed designed primarily for the removal
of a
desiccant from the dehydrated natural gas feed stream; and
a third vessel comprises an adsorption bed designed primarily for the removal
of a
sour gas component from the dehydrated natural gas feed stream.
19A. The gas processing facility of paragraph 17A, wherein the at least one
fractionation
vessel in the gas separation unit comprises a vessel containing a plurality of
adsorbent beds in
series, such that:
a first adsorption bed is designed to primarily remove water and other liquid
components from the dehydrated natural gas feed stream;
a second adsorption bed is designed to primarily remove a desiccant from the
dehydrated natural gas feed stream; and
a third vessel comprises an adsorption bed primarily for the removal of a sour
gas
component from the dehydrated natural gas feed stream.
20A. A process for liquefying a natural gas feed stream, comprising:
receiving the natural gas feed stream at a gas separation unit, the gas
separation unit
having at least one fractionation vessel comprised of:
a gas inlet for receiving a natural gas mixture comprising methane,
an adsorbent material that has a kinetic selectivity for contaminants over
methane
greater than 5, such that the contaminants become kinetically adsorbed within
the adsorbent
material, and
a gas outlet configured to release a methane-rich gas stream;
substantially separating methane from contaminants within the natural gas feed
stream;
releasing a methane-rich gas stream from the gas separation unit;
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
46
directing the methane-rich gas stream into a high-pressure expander cycle
refrigeration system;
compressing the methane-rich gas stream to a pressure that is greater than
1,000 psia
(6,895 kPa) in order to form a compressed gas feed stream;
cooling the compressed gas feed stream to form a compressed, cooled gaseous
feed
stream;
expanding the cooled, compressed, gaseous feed stream to form a product stream
having a liquid fraction and a remaining vapor fraction; and
separating the vapor fraction from the liquid fraction.
21A. The process of paragraph 20A, wherein the high-pressure expander cycle
refrigeration
system comprises:
a first compression unit configured to receive a substantial portion of the
methane-rich
gas stream and to generate the compressed gas feed stream;
a first cooler configured to cool the compressed gas feed stream to form the
compressed, cooled gaseous feed stream; and
a first expander configured to expand the cooled, compressed, gaseous feed
stream to
form the product stream.
22A. The process of paragraph 21A, wherein cooling the compressed gas feed
stream
comprises:
delivering at least a portion of the vapor fraction from the product stream to
the first
cooler as part of a first refrigeration loop; and
heat-exchanging the vapor fraction of the product stream with the compressed
gas
feed stream to cool the compressed gas feed stream.
23A. The process of paragraph 22A, wherein:
the high-pressure expander cycle refrigeration system further comprises a
liquid
separation vessel; and
separating the vapor fraction from the liquid fraction is done using the
liquid
separation vessel.
24A. The process of paragraph 23A, further comprising:
releasing from the first cooler (i) a chilled gas feed stream as the product
stream, and
(ii) a partially-warmed product stream as a working fluid;
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
47
directing the partially-warmed product stream to a third compression unit; and
merging the compressed, partially-warmed product stream from the third
compression
unit with the methane-rich gas stream to complete the first refrigeration
loop.
25A. The process of paragraph 24A, wherein the high-pressure expander cycle
refrigeration
system further comprises:
a second cooler configured to further cool the compressed gas feed stream at
least
partially by indirect heat exchange between a refrigerant stream and the vapor
fraction; and
a second refrigeration loop having (i) a second compression unit configured to
re-
compress the refrigerant stream after the refrigerant stream passes through
the second cooler,
and (ii) a second expander configured to receive the compressed refrigerant
stream, and
expand the compressed refrigerant stream prior to returning it to the second
cooler.
26A. The process of paragraph 25A, wherein the second cooler sub-cools the
chilled gas
feed stream after the chilled gas feed stream leaves the first cooler.
27A. The process of paragraph 25A, wherein the second cooler pre-cools the
compressed
gas feed stream before the compressed gas feed stream enters the first cooler.
28A. The process of paragraph 23A, wherein the facility is located on (i) a
floating
platform, (ii) a gravity-based platform, or (iii) a ship-shaped vessel
offshore.
29A. The process of paragraph 23A, wherein the at least one fractionation
vessel in the gas
separation unit operates on pressure swing adsorption (PSA) or rapid cycle
pressure swing
adsorption (RCP SA).
30A. The process of paragraph 23A, wherein the at least one fractionation
vessel in the gas
separation unit further operates on temperature swing adsorption (TSA) or
rapid cycle
temperature swing adsorption (RCTSA).
31A. The process of paragraph 30A, wherein the at least one fractionation
vessel is
configured to adsorb CO2, 1-17S, 1-170, heavy hydrocarbons, VOC's, mercaptans,
or
combinations thereof.
32A. The process of paragraph 31A, further comprising:
passing the natural gas feed stream through a dehydration vessel in order to
remove a
substantial portion of water from the natural gas feed stream; and
release a dehydrated natural gas feed stream to the at least one fractionation
vessel for
contaminant removal.
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
48
33A. The process of paragraph 32A, wherein the at least one fractionation
vessel in the gas
separation unit comprises a plurality of vessels in series, such that:
a first vessel comprises an adsorption bed for the removal of water remaining
in the
dehydrated natural gas feed stream;
a second vessel comprises an adsorption bed designed primarily for the removal
of a
desiccant from the dehydrated natural gas feed stream; and
a third vessel comprises an adsorption bed designed primarily for the removal
of a
sour gas component from the dehydrated natural gas feed stream.
34A. The process of paragraph 32A, wherein the at least one fractionation
vessel in the gas
separation unit comprises a vessel containing a plurality of adsorbent beds in
series, such
that:
a first adsorption bed is designed to primarily remove water and other liquid
components from the dehydrated natural gas feed stream;
a second adsorption bed is designed to primarily remove a desiccant from the
dehydrated natural gas feed stream; and
a third vessel comprises an adsorption bed designed primarily for the removal
of a
sour gas component from the dehydrated natural gas feed stream.
35A. A method for liquefying a natural gas feed stream, comprising:
receiving the natural gas feed stream at a gas processing facility;
passing the natural gas feed stream through a dehydration vessel in order to
remove a
substantial portion of water from the natural gas feed stream;
releasing a dehydrated natural gas feed stream to a gas separation unit as a
dehydrated
natural gas feed stream;
in the gas separation unit, passing the dehydrated natural gas feed stream
through a
series of adsorbent beds in order to separate methane gas from contaminants in
the
dehydrated natural gas feed stream using adsorptive kinetic separation;
releasing a methane-rich gas stream from the gas separation unit;
directing the methane-rich gas stream into a high-pressure expander cycle
refrigeration system;
compressing the methane-rich gas stream to a pressure that is greater than
1,000 psia
(6,895 kPa) in order to form a compressed gas feed stream;
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
49
cooling the compressed gas feed stream to form a compressed, cooled gaseous
feed
stream;
expanding the cooled, compressed, gaseous feed stream to form a product stream
having a liquid fraction and a remaining vapor fraction.
36A. The method of paragraph 35A, wherein the series of adsorbent beds
comprises:
a first adsorption bed for the removal of water remaining in the dehydrated
natural gas
feed stream;
a second adsorption bed designed primarily for the removal of a desiccant from
the
dehydrated natural gas feed stream; and
a third adsorption bed designed primarily for the removal of a sour gas
component
from the dehydrated natural gas feed stream.
37A. The method of paragraph 36A, wherein the first, second, and third
adsorption beds are
aligned in series with flow of the dehydrated natural gas feed stream in a
single pressure
vessel.
38A. The method of paragraph 36A, wherein the first, second, and third
adsorption beds
reside in separate pressure vessels that are aligned in series with the flow
of the dehydrated
natural gas feed stream.
39A. The method of paragraph 36A, wherein each of the adsorbent beds comprises
a solid
adsorbent bed fabricated from a zeolite material.
40A. The method of paragraph 37A, wherein each of the adsorbent beds has
associated
with it two additional adsorbent beds to form three adsorbent beds, with:
a first of the three adsorbent beds being in service for adsorbing a selected
contaminant;
a second of the three adsorbent beds undergoing regeneration; and
a third of the adsorbent beds being held in reserve to replace the first of
the three
adsorbent beds; and wherein
the regeneration is part of a pressure-swing adsorption process.
41A. The method of paragraph 36A, wherein cooling the compressed gas feed
stream
comprises:
passing the compressed gas feed stream through a first heat exchanger in order
to
provide heat exchange with a cooled refrigerant stream, thereby forming a sub-
cooled gas
feed stream; and
CA 02840723 2013-12-30
WO 2013/022529 PCMJS2012/045005
passing the sub-cooled gas feed stream through a second heat exchanger in
order to
provide heat exchange with a cooling gas stream, thereby forming the
compressed, cooled
gaseous feed stream.
42A. The method of paragraph 41A, further comprising:
withdrawing a portion of the remaining vapor fraction from the product stream;
reducing the pressure of the withdrawn portion of the remaining vapor fraction
down
to a pressure of about 30 to 200 psia (207 to 1,379 kPa) to produce a reduced
pressure gas
stream;
passing the reduced pressure gas stream through the second heat exchanger as
the
cooling gas stream; and
releasing the reduced pressure gas stream from the second heat exchanger as a
partially-warmed gas stream.
43A. The method of paragraph 42A, further comprising:
passing the partially-warmed gas stream through the first heat exchanger as a
cooling
gas stream; and
returning the partially-warmed gas stream to the dehydrated natural gas feed
stream
for compressing with the methane-rich gas stream.
44A. The method of paragraph 36A, wherein:
compressing the methane-rich gas stream comprises compressing the methane-rich
gas stream to a pressure that is between about 1,200 psia (8,274 kPa) to 4,500
psia (31,026
kPa); and
expanding the cooled, compressed, gaseous feed stream comprises reducing the
pressure of the cooled, compressed, gaseous feed stream to a pressure between
about 50 psia
(345 kPa) and 450 psia (3,103 kPa).
[0195] As can be seen, processes, systems and methods for liquefying a
natural gas feed
stream using AKS and a high-pressure expander cycle refrigeration system are
provided.
Such processes, systems and methods allow for the formation of LNG using a
facility having
less weight than conventional facilities. The processes, systems and methods
also permit
rapid tool-up for offshore production operations. The inventions described
herein are not
restricted to the specific embodiment disclosed herein, but are governed by
the claims, which
follow. While it will be apparent that the inventions herein described are
well calculated to
achieve the benefits and advantages set forth above, it will be appreciated
that the inventions
CA 02840723 2013-12-30
WO 2013/022529 PCT/1JS2012/045005
51
are susceptible to modification, variation and change without departing from
the spirit
thereof.