Note: Descriptions are shown in the official language in which they were submitted.
CA 02842649 2014-01-16
MI)2013M13a 1W PCT/EP2011/062673
MULTIVALENT ANTIGEN-BINDING FV MOLECULE
The invention relates to new tandem By diabodies and uses the-
reof.
BACKGROUND OF THE INVENTION
Various formats of multivalent recombinant antibody fragments
have been designed as alternatives to quadroma derived antibodies.
US 7,129,330, Kipriyanov et al. J. Mol. Biol. (1999) 293, 41- 56
and Kipriyanov Meth. Mol. Biol. (2009) 562, 177-193 describe the con-
struction and production of a particular format of multivalent antibo-
dy fragments which are named "tandem diabodies" (TandAlo), since their
design is based on intermolecular pairing of VH and V7 variable domains
of two different polypeptides as described for diabodies (Holliger et
al.,1993, Proc. Natl. Acad. Sci. USA, 90:6444-6448). The described an-
tibodies are bispecific for CD19 and CD3. In contrast to bivalent
scFv-scFv (scFv)2 tandems the tandem diabodies are tetravalent, because
they have four antigen-binding sites. Polypeptides with the domain or-
der VHA-VLB-VHB-VLA from the N-terminus to the C-terminus of the poly-
peptides forming the tandem diabodies are described. The orders of va-
riable domains and the linker peptides between them were designed such
that each domain associates with a complementary domain in another
identical molecule thereby forming the dimerized tetravalent tandem
diabodies. The tandem diabodies are devoid of immunoglobulin constant
domains. It was reported that the tandem diabodies have advantages
such as a high affinity, a higher avidity, lower clearance rates and
exhibit a favorable in vitro and in vivo efficiency.
Several additional tandem diabodies are known comprising antibody
specificities such as, for example, anti-CD16, anti-EpCAM and anti-
CD30. In all cases, however, the order of the four antibody domains
along the polypeptide chains of the tandem diabody from the N-terminus
to the C-terminus was always VHA-VLB-VHB-VLA, where VH and VL represent
the antibody heavy and light chain variable domains of antibodies with
specificities for antigens A and B, respectively.
Such bispecific tandem diabodies can make a bridge between a tumor
cell (e.g. B-CLL cell) and an effector cell of the human immune system
(NK cell, T cell, monocyte, macrophage or granulocyte) thus permitting
killing of the tumour cell. The tight binding of the tumor cell and
the cytotoxic cell induces the destruction of the tumor cell.While
such tandem diabodies have proved to be favorable for therapeutic ap-
plications, e.g. for therapeutic concepts for the treatment of tumors,
there remains a need for improved antigen-binding molecules.
SUMMARY OF THE INVENTION
In one aspect, the present invention provides a dimeric antigen-
binding molecule comprising a first and a second polypeptide chain,
each of the first and the second polypeptide chains comprising (a) a
CA 02842649 2014-01-16
W02013/013700 2 PCT/EP2011/062673
first domain VLA being a light chain variable domain specific for a
first antigen A; (b) a second domain VHB being a heavy chain variable
domain specific for a second antigen B; (c) a third domain VLB being a
light chain variable domain specific for the second antigen B; and (d)
a fourth domain VHA being a heavy chain variable domain specific for
the first antigen A, wherein said domains are arranged in each of said
first and second polypeptide chains in the order VLA-VHB-VLB-VHA from
the N-terminus to the C-terminus of said polypeptide chains, and the
first domain VA of the first polypeptide chain is in association with
the fourth domain VHA of the second polypeptide chain to form an anti-
gen binding site for the first antigen A; and the second domain VHB of
the first polypeptide chain is in association with the third domain VLB
of the second polypeptide chain to form an antigen binding site for
the second antigen B; and the third domain VLB of the first polypeptide
chain is in association with the second domain VHB of the second poly-
peptide chain to form an antigen binding site for the second antigen
B; and the fourth domain VHA of the first polypeptide chain is in asso-
ciation with the first domain VLA of the second polypeptide chain to
form an antigen binding site for the first antigen A.
In some embodiments, the antigen-binding molecule as described
herein is a homodimer and the first and the second polypeptide chains
have the same amino acid sequence. In some embodiments, the first and
the second polypeptide chains are non-covalently associated. In some
embodiments, the antigen-binding molecule is tetravalent. In some em-
bodiments, the antigen-binding molecule is bispecific. In some embodi-
ments, the domains are human domains or humanized domains. In some em-
bodiments, the antigen-binding molecule comprises at least one further
functional unit. In some embodiments, the antigen binding molecule is
specific for a B-cell, T-cell, natural killer (NK) cell myeloid cell
or phagocytotic cell. In some embodiments, the antigen-binding mole-
cule is bispecific, which antigen-binding molecule is further specific
for a tumor cell. In some embodiments, the first light chain variable
domain (VLA) and the first heavy chain variable domain (VHA) are spe-
cific for a tumor cell. In some embodiments, the antigen-binding mole-
cule is bispecific for albumin and CD3.
In another aspect, the present invention provides a polypeptide
chain comprising (a) a first domain VLA being a light chain variable
domain specific for a first antigen A; (b) a second domain VHB being a
heavy chain variable domain specific for a second antigen B; (c) a
third domain VLB being a light chain variable domain specific for the
second antigen B; and (d) a fourth domain VHA being a heavy chain va-
riable domain specific for the first antigen A; wherein the domains
are arranged in the polypeptide chain in the order VLA-VHB-VLB-VHA from
the N-terminus to the C-terminus of the polypeptide chains. In some
3
embodiments, the first domain VIA and the fourth domain VHA do not
associate to form an antigen binding site for the first antigen A and the
second domain VHB and the third domain VLB do not associate to form an
antigen binding site for the second antigen B. In some embodiments, the
first domain VIA and the second domain VAB, the second domain VHB and the
third domain VLB, and the third domain V113 and the fourth domain VHA are
separated by not more than about 12 amino acid residues. In some
embodiments the polypeptide chain comprises amino acid residues upstream
from the first domain VIA and/or downstream from the fourth domain VHA. In
some embodiments, the polypeptide chain is linked to a further functional
unit. In a particular embodiment the variable domains are specific for
albumin and CD3.
In another aspect, the present invention provides a dimeric antigen-
binding molecule comprising at least four antigen-binding sites, wherein
each antigen-binding site is formed by a heavy chain variable domain VH
and a light chain variable domain VL, the antigen binding molecule
consisting of a first and a second polypeptide chain, each of the first
and the second polypeptide chains containing a first domain VA being a
light chain variable domain specific for a first antigen A; a second
domain VHB being a heavy chain variable domain specific for a second
antigen B; a third domain VLB being a light chain variable domain
specific for the second antigen B; and a fourth domain VA being a heavy
chain variable domain specific for the first antigen A, wherein antigen A
or antigen B is CD3, the domains are arranged in each of the first and
second polypeptide chains in the order VLA-VHB-VLB-VHA from the N-terminus
to the C-terminus of the polypeptide chains, the first domain VIA is
linked with the second domain VHB by a first linker Li, the second domain
VHB is linked with the third domain VLB by a linker L2 and the third
domain VLB is linked with the fourth domain VA with a third linker L3,
and the linkers Ll, L2 and L3 consist of 12 or less amino acid residues.
In another aspect, the present invention provides a nucleic acid
molecule encoding a polypeptide chain as described herein.
CA 2842649 2018-11-14
3a
In another aspect, the present invention provides a composition
comprising the antigen-binding molecule according to the invention, and a
pharmaceutically acceptable carrier.
In yet another aspect, the present invention provides use of the
antigen-binding molecule or the composition according to the invention,
including use in the manufacture of a medicament, for immunosuppressive
treatment, or for the treatment of autoimmune disease, inflammatory
disease, infectious disease, allergy or cancer.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 illustrates the gene organization of a construct encoding an
antigen-molecule according to the invention, where VA represents a light
chain variable immunoglobulin domain specific for an antigen A, VHB
represents a heavy chain variable immunoglobulin domain specific for an
antigen B, VLB represents a light chain variable immunoglobulin domain
specific for the antigen B, VHA represents a heavy chain variable
immunoglobulin domain specific for the antigen A, Li a peptide linker or
a peptide bond connecting VTA and VHB, L2 a peptide linker or a peptide
bond connecting VHB and VLB, and L3 a peptide linker or a peptide bond
connecting VLB and VA.
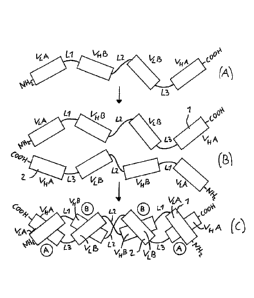
Fig. 2 illustrates the formation of a dimeric antigen-binding
molecule according to the invention from non-functional monomeric
polypeptide chains (A) by intra-molecular pairing of variable domains of
a first polypeptide chain 1 and a second polypeptide chain 2 with one
another (B) to a functional antigen-binding molecule according to the
inventions in the format of a tandem diabody, where "1- represents the
first polypeptide chain, "2" represents the second polypeptide chain, VIA
represents a light chain variable immunoglobulin domain specific for an
antigen A, VHB represents a heavy chain variable immunoglobulin domain
specific for an antigen B, VLB represents a light chain variable
immunoglobulin domain specific for the antigen B, VA represents a
CA 2842649 2018-11-14
CA 02842649 2014-01-16
4
W02013/013700 PCT/EP2011/062673
heavy chain variable immunoglobulin domain specific for the antigen A,
Li a peptide linker or a peptide bond connecting VLA and VHB, L2 a pep-
tide linker or a peptide bond connecting VHB and VLB, and L3 a peptide
linker or a peptide bond connecting VLB and VHA
Fig. 3 shows a comparison of CD19xCD3 tandem diabodies in a cyto-
toxicity assay. Option 0 = antibody Al with the domain order VHA-VLB-
VHB-VLA. Option 2 = antibody B with the domain order VLA-VHB-VLB-VHA ac-
cording to the invention. lx104 calcein-labelled Raji cells were incu-
bated with 5x105 PBMC in the presence of increasing concentrations of
the indicated CD19xCD3 tandem diabodies. PBMC were cultured overnight
in the presence of 25 U/mL human IL-2 before they were used as effec-
tor cells in the assay. After 4 h incubation fluorescent calcein in
the cell culture medium released from apoptotic target cells was meas-
ured at 520 nm and
specific lysis was calculated. =50 values were
analysed by non-linear regression using GraphPad software. The mean
and standard deviations of duplicates were plotted.
Fig. 4 shows a comparison of CD19xCD3 tandem diabodies in a cyto-
toxicity assay. Option 0 = antibody A2 with the domain order VHA-VLB-
VHB-VLA. Option 2 = antibody C with the domain order VLA-VHB-VLB-VHA ac-
cording to the invention. 1x104 calcein-labelled Raji cells were incu-
bated with 5x105 freshly isolated PBMC in the presence of increasing
concentrations of the indicated CD19xCD3 tandem diabodies. After 4 h
incubation fluorescent calcein in the cell culture medium released
from apoptotic target cells was measured at 520 nm and
specific ly-
sis was calculated. EC5D values were analysed by non-linear regression
using GraphPad software. The mean and standard deviations of dupli-
cates were plotted.
Fig. 5 shows the TCR modulation by HSAxCD3 TandAb antibodies of
Example 2 in the presence or absence of HSA. 0D3' Jurkat cells were
cultured for 2 h in the presence of increasing concentrations of the
HSAxCD3 TandAb option 0 (VHA-VLB-VHB-VLA; triangle) or option 2 (VLA-VHB-
VIB-VHA according to the invention; square) antibodies with (filled
symbols) or without (open symbols) the addition of 50 mg/mL HSA. After
washing, remaining TCR/CD3 complexes were measured by flow cytometry
using a P05-conjugated anti-TCRa/(3 antibody. Mean fluorescence values
were used for analysis by non-linear regression (experiment CAB-306).
Fig. 6 shows the vector map with the restriction sites of
pCDNA5FRT which encodes antibody B.VH and VL: variable domains of the
heavy and the light chains.
Fig. 7 shows the vector map with the restrictions sites of pSKK3
which encodes antibody C. VH and VL: variable domains of the heavy and
light chains.
CA 02842649 2014-01-16
W02013/013700 PCT/EP2011/062673
DETAILED DESCRIPTION OF THE INVENTION
In one aspect, the present invention provides a recombinant di-
meric and tetravalent antigen-binding molecule with four immunoglobu-
lin domains (two heavy chain variable domains and two light chain va-
riable domains) linked with one another in a polypeptide chain and ar-
ranged in the order VLA-VHB-VLB-VHA from the N-terminus to the C-
terminus of the polypeptide chain. Such an antigen-binding molecule of
the present invention triggers an enhanced biological activity, such
as, e.g., an enhanced immune response or enhanced immune suppression.
In one embodiment, it illustrates that a dimeric, bispecific an-
tigen-binding molecule of the tandem diabody format being specific for
CD3 and CD19 and having polypeptide chains with the domain order VLA-
VHB-VLB-VHA is more than 6o times more active in vitro, i.e. cytotoxic,
than a corresponding tandem diabody molecule with the same domains but
in the reverse domain order VHA-VLB-VHB-VLA.
In another embodiment, it illustrates that a dimeric, bispecific
antigen-binding molecule of the tandem diabody format being specific
for an albumin (HSA) and CD19 and having polypeptide chains with the
domain order VLA-VHB-V-,B-VHA has a significantly more effective T cell
receptor modulation activity in vitro, i.e. is more immunosuppressive,
than a corresponding tandem diabody molecule with the same domains but
in the reverse domain order VHA-VLB-VHB-VLA.
Thus, tandem diabodies with the domain order VLA-VHB-VIB-VHA from
the N-terminus to the C-terminus of the polypeptide chains have an in-
creased potential for immunotherapy. A further advantage of the en-
hanced biological activity is that the effective therapeutic dosages
for such tandem diabodies may be reduced. Moreover, side effects
caused by the administered antigen binding molecules may also be re-
duced due to the lower dosages. Without being bound by any theory, the
new domain order allows a modified crosslinking of the dimeric antigen
binding molecule between the antigen A and the antigen B compared with
the tandem diabodies of the art and, in certain aspects of the inven-
tion, this will enable the molecule to bind to target antigens, e.g.,
receptors, more efficiently than the dimeric antigen binding molecules
of the art.
Therefore, the biological activity of a dimeric, antigen-binding
molecule such as a tandem diabody can be enhanced, when the four vari-
able domains of each polypeptide chain which form the dimeric antigen-
binding molecule are arranged in the order VTA-VHB-VTB-VHA from the N-
terminus to the C-terminus of each polypeptide chain. The triggered
"biological activity" depends on the specificities of the antigen-
binding molecule and may encompass cytotoxicity, phagocytosis, antigen
presentation, cytokine release or immune suppression, for example an-
tibody dependent cell mediated cytotoxicity (ADCC), antibody dependent
CA 02842649 2014-01-16
W02013/013700 6 PCT/EP2011/062673
cell mediated phagocytosis (ADCP)and/or complement dependent cytotox-
icity (CDC).
In some embodiments, the present invention provides a dimeric an-
tigen-binding molecule comprising a first and a second polypeptide
chain, wherein each of the first and the second polypeptide chains
comprises a first domain VLA being a light chain variable domain spe-
cific for a first antigen A, a second domain VHB being a heavy chain
variable domain specific for a second antigen B, a third domain VLB
being a light chain variable domain specific for the second antigen B,
a fourth domain VHA. being a heavy chain variable domain specific for
the first antigen A, and said domains are arranged in each of said
first and second polypeptide chains in the order VLA-VHB-VLB-VHA from
the N-terminus to the C-terminus of said polypeptide chains.
In some embodiments, the first, second, third and fourth variable
domains are arranged in an orientation preventing intramolecular pair-
ing within the same polypeptide chain and the first polypeptide chain
is associated, i.e. dimerized, with the second polypeptide chain such
that the first domain VIA of the first polypeptide chain is in associa-
tion with the fourth domain VHA of the second polypeptide chain to form
an antigen binding site for the first antigen A, the second domain VHB
of the first polypeptide chain is in association with the third domain
VIB of the second polypeptide chain to form an antigen binding site for
the second antigen B, the third domain VLB of the first poll/peptide
chain is in association with the second domain VHB of the second poly-
peptide chain to form an antigen binding site for the second antigen B
and the fourth domain VHA of the first polypeptide chain is in associa-
tion with the first domain VLA of the second polypeptide chain to form
an antigen binding site for the first antigen A.
The term "antigen-binding molecule" refers to an immunoglobulin
derivative with multivalent antigen-binding properties, preferably
having at least four antigen-binding sites. Each antigen-binding site
is formed by a heavy chain variable domain VH and a light chain varia-
ble domain VL of the same antigen, i.e. epitope, specificity. Prefera-
bly the antigen-binding molecule according to the invention is devoid
of immunoglobulin constant domains or fragments of immunoglobulin con-
stant domains, but in certain cases described below a constant domain
or parts thereof may be linked to the antigen-binding molecule.
The antigen-binding molecule is "dimeric" which term refers to a
complex of two polypeptide monomers. These two polypeptide monomers
are the first and the second polypeptide chains. Preferably the anti-
gen-binding molecule is a "homodimer" which term means that the anti-
gen-binding molecule is composed of identical polypeptide monomers. In
a preferred homodimeric antigen-binding molecule according to the in-
vention the first and the second polypeptide chain may have the same
CA 02842649 2014-01-16
7
W02013/013700 PCT/EP2011/062673
amino acid sequence, i.e. the first and the second polypeptide chains
are identical and, thus, are encoded and expressed by the same single
polynucleotide. This is different in the case of so-called bispecific
diabodies, which are heterodimers that are encoded by two distinct po-
lynucleotides. In the former case each of the first and the second po-
lypeptide chains contain four variable domains, four binding sites are
formed and the antigen-binding molecule is tetravalent. Such tetrava-
lent homodimeric antigen-binding molecules have received some recogni-
tion in the art as tandem diabodies.
Preferably, in the antigen-binding molecule the first and the
second polypeptide chain are non-covalently associated with each oth-
er, in particular with the proviso that there is no covalent bound be-
tween the first and second polypeptide chain. However, if desired, the
two polypeptide chains may be additionally stabilized by at least one
covalent linkage, e.g. by a disulfide bridge between cysteine residues
of different polypeptide chains.
The term "polypeptide chain" refers to a polymer of amino acid
residues linked by amide bonds. The first and the second polypeptide
chains are, preferably, single chain fusion proteins which are not
branched. In each of the first and second polypeptide chains the four
domains are arranged such that the second domain VHB is C-terminal from
the first domain VLA, the third domain VLB is C-terminal from the
second domain VHB and the fourth domain VHA is C-terminal from the
third domain VB. The first and the second polypeptide chains may have
contiguous amino acid residues in addition N-terminal to the first do-
main VLA and/or C-terminal to the fourth domain VHA. For example, the
polypeptide chain may contain a Tag sequence, preferably at the C-
terminus which might be useful for the purification of the polypep-
tide. An example of a Tag sequence is a His-Tag, e.g. a His-Tag con-
sisting of six His-residues.
In some embodiments, the first, second, third and fourth domains
are covalently connected such that the domains of the same polypeptide
chain do not associate, i.e. pair, with each other. The domains may be
linked such that the first domain VLA is linked with the second domain
VHB by a first linker Li, the second domain VHB is linked with the
third domain VIB by a second linker L2 and the third domain VLB is
linked with the fourth domain VHA by a third linker L3, wherein the
first linker Li and the third linker L3 are distal to the central
linker L2 on each of the first and second polypeptide chains. Linker
Li, linker L2 and linker L3 can be each a peptide linker comprising at
least one amino acid residue or a peptide bound without any interven-
ing amino acid residue between the two adjacent domains.
In some embodiments, the length of each of the linkers Li, L2 and
L3 is such that the domains of the first polypeptide chain can asso-
CA 02842649 2014-01-16
W02013/013700 8 PCT/EP2011/062673
ciate with the domains of the second polypeptide chain to form the di-
meric antigen-binding molecule. The length of the linkers influences
the flexibility of the antigen-binding molecule. The desired flexibil-
ity of the antigen-binding molecule depends on the target antigen den-
sity and the acessibility of the target antigen, i.e. epitopes. Longer
linkers provide more flexible antigen-binding molecules with more
agile antigen-binding sites. The effect of linker length on the forma-
tion of dimeric antigen-binding molecules is described, for example,
in Todorovska et al., 2001 Journal of Immunological Methods 248:47-66;
Perisic et al., 1994 Structure 2:1217-1226; Le Gall et al., 2004, Pro-
tein Engineering 17:357-366 and WO 94/13804.
In certain preferred embodiments, the linkers Ll, L2 and/or L3
are "short", i.e. consist of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or
about 12 amino acid residues. Such short linkers favor the correct di-
merization of the first with the second polypeptide chain by binding
and forming antigen-binding sites between light chain variable domains
and heavy chain variable domains of different polypeptide chains. In
particular, the central linker L2 should be short such that it pre-
vents formation of a single chain Fv (scFv) antigen-binding unit with-
in the same polypeptide chain by the two adjacent domains VHB and VLB.
The central linker L2 influences the flexibility of the polypeptide
chain. If the central linker L2 is long, and flexible (in general con-
sisting of about 12 or more amino acid residues) the polypeptide chain
can fold head-to-tail and form a single-chain antigen-binding molecule
known in the art as a single chain diabody. If the central linker L2
is short and rigid the polypeptide chain cannot fold head-to-tail and
dimerizes with another polypeptide chain. The number of amino acid re-
sidues of a linker for preventing a head-to-tail folding also depends
on the kind of variable domains combined in the polypeptide. In gener-
al, shortening the linker to about 12 or less amino acid residues gen-
erally prevents adjacent domains of the same polypeptide chain from
interacting with each other. Therefore, the central linker L2 and the
distal linkers Ll and L3 should preferably consist of about 12 or less
amino acid residues to prevent pairing of adjacent domains of the same
polypeptide chain. In a preferred embodiment of the invention the
linkers Ll, L2 and/or L3 consist of about 3 to about 10 contiguous
amino acid residues. The linkers may consist of different numbers of
amino acid residues, but it is preferred that the distal linkers Ll
and L3 have the same number of amino acid residues or do not differ in
length by more than one or two amino acid residues. In a certain as-
pect of the invention at least one of the linkers Ll, L2 and/or L3
consists of nine amino acid residues. In a particular embodiment of
the invention all three linkers Ll, L2 and L3 consist of nine amino
CA 02842649 2014-01-16
9
W02013/013700 PCT/EP2011/062673
acid residues. In some embodiments, at least one of the linkers Li, L2
and/or L3 consists of less than between 10 to 3 amino acid residues.
Additional amino acid residues provide extra flexibility. In an
alternative aspect the central linker L2 may have about 12 or less
amino acid residues to prevent a head-to-tail folding of the polypep-
tide chain and at least one of the distal linkers Li and/or L3 may
have more than about 12 amino acid residues to provide extra flexibil-
ity. In another embodiment, two polypeptide chains having a central
linker L2 with more than 12 amino acid residues correctly dimerize
with one another to a tetravalent, dimeric antigen-binding molecule
(see for example Le Gall et al., 2004, Protein Engineering 17:357-
366). However, if longer linkers, e.g. consisting of about 13 or more,
in particular of about 15 or more, amino acid residues are utilized,
the dimeric antigen-binding molecule may be stabilized additionally by
at least one covalent bond between such two polypeptide chains.
Regarding the amino acid composition of the linkers, in some em-
bodiments, peptides are selected that do not interfere with the dime-
rization of the first and second polypeptide chains. For example,
linkers comprising glycine and serine residues generally provide flex-
ibility and protease resistance. The amino acid sequence of the link-
ers can be optimized, for example, by phage-display methods to Improve
the antigen binding and production yield of the molecules. In particu-
lar embodiments of the invention the linker may comprise the amino ac-
id sequence GGSGGSGGS.
The first domain VLA, the second domain VHB, the third domain VLB
and the fourth domain VFIA are light chain and heavy chain variable do-
mains of an immunoglobulin. The variable domains comprise the hyperva-
riable loops or complementary binding regions (CDRs) containing the
residues in contact with the antigen and the segments which contribute
to the correct folding and display of the CDRs. It is preferred that
each of the heavy chain and light chain variable domains comprises the
respective three CDRs. The domains may be derived from any immunoglo-
bulin class, e.g., IgA, IgD, IgE and IgM or a subclass thereof. The
immunoglobulin may be of animal, in particular mammal, origin. Each
domain may be a complete immunoglobulin heavy or light chain variable
domain, a mutant, fragment or derivative of a naturally occurring va-
riable domain, or a synthetic, e.g. recombinant domain which is genet-
ically engineered. A derivative is a variable domain which differs by
the deletion, substitution, addition or insertion of at least one ami-
no acid from the amino acid sequence of a naturally occurring variable
domain. Synthetic, e.g. recombinant domains, can be obtained, for ex-
ample, by well known reproducible methods from hybridoma-derived anti-
bodies or phage-display immunoglobulin libraries. For example phage
display methods can be used to obtain variable domains of human anti-
CA 02842649 2014-01-16
W02013/013700 PCT/EP2011/062673
bodies to an antigen by screening libraries from human immunoglobulin
sequences. The affinity of initially selected antibodies can be fur-
ther increased by affinity maturation, for example chain shuffling or
random mutagenesis. A person of ordinary skill in the art is familiar
with methods for obtaining domains from natural or recombinant antibo-
dies (for laboratory manuals see, for example, Antibody engineering:
methods and protocols / edited by Benny K.C. Lo; Benny K.C. II Series:
Methods in molecular biology (Totowa, N.J.)). Generally, any antibody
known in the art can be used as a source for the variable domains of
the invention.
In a certain aspect of the invention at least one, preferably
all, of the first domain VLA, the second domain VHB, the third domain
VIB and the fourth domain VHA are fully human, humanized or chimeric
domains. A humanized variable domain comprises a framework region sub-
stantially having the amino acid sequence of a human immunoglobulin
and a CDR of a non-human immunoglobulin. Humanized antibodies can be
produced by well-established methods such as, for example CDR-grafting
(see, for example, Antibody engineering: methods and protocols /
edited by Benny K.C. Lo; Benny K.C. II Series: Methods in molecular
biology (Totowa, N.J.)). Thus, a skilled person is readily able to
make a humanized or fully human version of antigen-binding molecules
and variable domains from non-human, e.g. murine, sources with the
standard molecular biological techniques known in the art for reducing
the immunogenicity and improving the efficiency of the antigen-binding
molecule in a human immune system. In a preferred embodiment of the
invention all domains (e.g. VLA, VHB, VLB and VHA) are humanized or ful-
ly human; most preferred, the dimeric antigen-binding molecule accord-
ing to the invention is humanized or fully human. The term "fully hu-
man" as used herein means that the amino acid sequences of the varia-
ble domains and the peptides linking the variable domains in the first
and second polypeptide chains originate or can be found in humans. In
certain embodiments of the invention the variable domains may be human
or humanized but not the peptides linking the variable domains.
In one embodiment the first domain VLA, the second domain VHB, the
third domain VLB and the fourth domain VHA are specific for the same
antigen such that antigen-binding sites formed by the domains bind ei-
ther to the same epitope or to different epitopes on the same antigen.
In this case the expressions "antigen A" and "antigen B" refer to the
same antigen. Such antigen-binding molecules are monospecific.
In another embodiment the first domain VLA, the second domain VHB,
the third domain VLB and the fourth domain VHA are specific for differ-
ent antigens such that VLA and VHA form an antigen-binding site for an
antigen A of a first specificity and VHB and VLB form an antigen-
binding site for an antigen B of a second specificity. The different
CA 02842649 2014-01-16
11
W02013/013700 PCT/EP2011/062673
antigens may be associated with different kind of cells or represent
different antigens of the same kind of cell. Such antigen-binding mo-
lecules according to the invention are bispecific.
In some embodiments, at least one antigen-binding site may be
specific for a bacterial substance, viral protein, autoimmune marker
or an antigen present on a particular cell such as a cell surface pro-
tein of a B-cell, T-cell, natural killer (NK) cell, myeloid cell, pha-
gocytic cell, tumor cell.
In an aspect of the invention the dimeric antigen-binding mole-
cule is bispecific comprising a first specificity for an effector cell
and a second specificity for a target cell different from the effector
cell. Such antigen-binding molecules are able to cross-link two cells
and can be used to direct effector cells to a specific target. In
another aspect of the invention the dimeric antigen-binding molecule
may be bispecific for a target cell and a molecule selected from the
group consisting of a drug, toxin, radionucleotide, enzyme, albumin
and lipoprotein, naturally occurring ligands such as cytokines or che-
mokines. If the target molecule is albumin, the albumin or serum albu-
min may be selected from the group of origins consisting of human, bo-
vine, rabbit, canine and mouse.
"Effector cells" typically refer to cells of the immune system
which can stimulate or trigger cytotoxicity, phagocytosis, antigen
presentation, cytokine release. Such effector cells are, for example
but not limited to, T cells, natural killer (NK) cells, granulocytes,
monocytes, macrophages, dendritic cells, erythrocytes and antigen-
presenting cells. Examples of suitable specificities for effector
cells include but are not limited to 0D2, CD3, 0D5, CD28 and other
components of the T-cell receptor (TCR) for T cells; CD16, CD38, CD44,
CD56, CD69, CD335 (NKp46), 0D336 (NKp44), CD337 (NKp30), NK1380, NKG2C
and NKG2D for NK cells; CD18, CD64 and CD89 for granulocytes; CD18,
CD64, CD89 and mannose receptor for monocytes and macrophages; CD64
and mannose receptor for dendritic cells; CD35 for erythrocytes. In
certain aspects of the invention those specificities, i.e. cell sur-
face molecules, of effector cells are suitable for mediating cell
killing upon binding of a bispecific antibody to such cell surface mo-
lecule and, thereby, inducing cytolysis or apoptosis.
"Target cells" typically refers to the sites to which the effec-
tor cells should be directed to induce or trigger the respective bio-
logical, e.g. immune, response. Examples of target cells may be tumor
cells or infectious agents such as viral or bacterial pathogens, for
example dengue virus, herpes simplex, influenza virus, HIV or cells
carrying autoimmune targets such as IL-2, an autoimmune marker or an
autoimmune antigen.
CA 02842649 2014-01-16
W02013/013700 12 PCT/EP2011/062673
In a preferred embodiment of the invention the dimeric antigen-
binding molecule is bispecific for a tumor cell and an effector cell,
in particular a T cell or a NK cell. Suitable specificities for tumor
cells may be tumor antigens and cell surface antigens on the respec-
tive tumor cell, for example specific tumor markers. Such a bispecific
dimeric antigen-binding molecule binds to both the tumor cell and the
immune effector cell thereby triggering the cytotoxic response Induced
by the T cell or the NK cell. The term "tumor antigen" as used herein
comprises tumor associated antigen (TAA) and tumor specific antigen
(TSA). A "tumor associated antigen" (TAA) as used herein refers to a
protein which Is present on tumor cells, and on normal cells during
fetal life (once-fetal antigens), and after birth in selected organs,
but at much lower concentration than on tumor cells. A TAA may also be
present in the stroma in the vicinity of the tumor cell but expressed
at lower amounts in the stroma elsewhere in the body. In contrast, the
term "tumor specific antigen" (TSA) refers to a protein expressed by
tumor cells. The term "cell surface antigen" refers to any antigen or
fragment thereof capable of being recognized by an antibody on the
surface of a cell.
Examples of specificities for tumor cells include but are not li-
mited to CD19, CD20, CD30, the laminin receptor precursor protein,
EGFR1, EGFR2, EGFR3, Ep-CAM, PLAP, Thomsen-Friedenreich (TF) antigen,
MUC-1 (mucin), IGFR, CD5, IL4-R alpha, IL13-R, FceRI and IgE as de-
scribed in the art.
In one embodiment the specificity for an effector cell may be CD3
or CD16 and the specificity for a tumor cell may be selected from
CD19, CD20, CD30, the laminin receptor precursor, Ep-CAM, EGFR1,
EGFR2, EGFR3, PLAP, Thomsen-Friedenreich (TF) antigen, MUC-1 (mucin),
IGFR, CD5, IL4-R alpha, IL13-R, FccRI and IgE. Particular examples of
such antigen binding molecules are bispecific for CD3 and CD19 or CD16
and CD30.
In a certain aspect of the invention the first domain VLA and the
fourth domain VHA have the specificity for a tumor cell and the other
two domains, namely the second domain VHB and the third domain VLB,
have the specificity for an effector cell, in particular T cell or NK
cell. In one embodiment the first domain VA and the fourth domain VHA
have the specificity for a tumor cell and the other two domains, name-
ly the second domain VHB and the third domain VLB, have the specificity
for CD3 or CD16. In a certain embodiment thereof the first domain VTA
and the fourth domain VHA have a specificity for CD19, CD20, the lami-
nin receptor precursor, Ep-CAM, EGFR1, EGFR2, EGFR3, PLAP, Thomsen-
Friedenreich (TF) antigen, MUC-1 (mucin), IGFR, CD5, IL4-R alpha,
IL13-R, FceRI and the other two domains, namely the second domain VHB
and the third domain VLB, have a specificity for CD3.
CA 02842649 2014-01-16
W02013/013700 13 PCT/EP2011/062673
In another aspect of the invention the first domain VLA and the
fourth domain VHA have the specificity for an effector cell, in partic-
ular T cell or NK cell, and the other two domains, namely the second
domain VHB and the third domain VLB, have the specificity for a tumor
cell. In one embodiment the first domain VTA and the fourth domain VHA
have the specificity for a CD3 or 0D16 and the other two domains,
namely the second domain VHB and the third domain VLB, have the speci-
ficity for a tumor cell. In a particular preferred embodiment the
first domain VLA and the fourth domain VHA have the specificity for a
CD3 and the other two domains, namely the second domain VHB and the
third domain VLB, have the specificity for a tumor cell selected from
the group consisting of CD19, CD20, CD30, the laminin receptor precur-
sor, Ep-CAM, EGFR1, EGFR2, EGFR3, PLAP, Thomsen-Friedenreich (TB) an-
tigen, MUC-1 (mucin), IGFR, 0D5, IL4-R alpha, IL13-R, FccRI and IgE.
CD3 antigen is associated with the T-cell receptor complex on T-
cells. In the case where specificity for an effector cell is 0D3, the
binding of the dimeric antigen-binding molecule according to the in-
vention to CD3 can trigger the cytotoHic activity of I-cells on target
cells. Namely, by bispecific binding of the dimeric antigen binding
molecule to CD3 and to a target cell, e.g. tumor cell, cell lysis of
the target cell may be induced. Dimeric antigen-binding molecules with
a specificity towards CD3 and their production are known in the art
(and described for example in Kipriyanov et al., 1999, Journal of Mo-
lecular Biology 293:41-56, Le Gall et al., 2004, Protein Engineering,
Design & Selection, 17/4:357-366).
Monospecific anti-CD3 antigen binding molecules are known for
their immunosuppressive properties by binding to and modulating the T
cell receptor (e.g. as described in W02004/024771). In one embodiment,
the antigen-binding molecule according to the present invention is
bispecific for CD3 and albumin for use as a immunosuppressive agent,
e.g. in transplantation.
The CD16 (FcyIIIA) antigen is a receptor expressed on the surface
of NK cells. NK cells possess an inherent cytoloytic activity and by
bispecific binding of the dimeric antigen-binding molecule according
to the invention to CD16 the cytotoxic activity of NK cell towards the
target cell can be triggered. An example of a bispecific antigen-
binding molecule having specificity towards CD16 is described, for ex-
ample, in Arndt et al., 1999, Blood, 94:2562-2568. In a particular em-
bodiment of the invention at least one of the heavy chain or light
chain variable domains are from an anti-CD16 antibody described in WO
2006/125668, in particular of antibodies which recognizes the CD16A
isoform, but not the CD16B isoform.
Dimeric antigen-binding molecules according to the invention,
wherein the tumor specificity is towards CD19 antigen may be used for
CA 02842649 2014-01-16
W02013/013700 14 PCT/EP2011/062673
immunotherapy of B-cell malignancies, because the CD19 antigen is ex-
pressed on virtually all B-lineage malignancies from lymphoblastic
leukemia (ALL) to non-Hodgkin's lymphoma (NHL). In particular for the
treatment of non-Hodgkin's lymphoma dimeric antigen-binding molecules
having specificity towards CD19 or CD20 can be used. Dimeric antigen-
binding molecules having specificity towards 0D19 and their production
are known in the art (and described, for example, in Cochlovius et
al., 2000, Cancer Research 60:4336-4341).
Dimeric antigen-binding molecules according to the invention,
wherein the tumor specificity is towards the laminin receptor or the
laminin receptor precursor may be used, for example but not limited,
for the treatment of B-cell chronic lymphocyte leukemia (B-CLL), non-
Hodgkin's lymphoma, Hodgkin's lymphoma, lung cancer, colon carcinoma,
mammary carcinoma, pancreatic carcinoma, prostate cancer, in particu-
lar in the condition of metastasizing cancer or minimal residual can-
cer. Antigen-binding molecules having specificity towards the laminin
receptor precursor are described, for example, in Zuber et al., 2008,
J. Mol. Biol., 378:530-539.
Dimeric antigen-binding molecules according to the invention
wherein the tumor specificity is towards EGFR1 may be of particular
use in the treatment of cancers wherein EGFR1 expression is up-
regulated or altered, for example in cancers of the breast, bladder,
head and neck, prostate, kidney, non-small cell lung cancer, colorec-
tal cancer and glioma.
Dimeric antigen-binding molecules according to the invention
wherein the tumor specificity is towards TB-antigen may be particular-
ly useful in treating breast or colon cancer and/or liver metastases.
Dimeric antigen-binding molecules wherein the tumor specificity
is towards CD30 may be particularly useful in treating Hodgkin's dis-
ease. Antigen-binding molecules having the specificity towards CD30
are described, for example, in Arndt et al., 1999, Blood, 94:2562-
2568.
Dimeric antigen-binding molecules wherein the tumor specificity
is towards the alpha chain of the IL4 receptor (IL4R alpha) may be
particularly useful in treating solid tumors, in particular carcinomas
of the breast, ovaries, renal system, head and neck, malignant melano-
ma and AIDS-related Kaposi's sarcoma. Dimeric antigen-binding mole-
cules wherein at least one additional specificity is towards
EGFR3/HER3 and/or EGFR2/neu may be particularly useful in treating
breast cancer. Dimeric antigen-binding molecules wherein the tumor
specificity is towards IGFR may be particularly useful in treating
prostate cancer, colorectal cancer, ovarian cancer or breast cancer.
CA 02842649 2014-01-16
W02013/013700 PCT/EP2011/062673
Dimeric antigen-binding molecules wherein the tumor specificity
is towards CD5 may be particularly useful in treating chronic lympho-
cytic leukaemia.
Dimeric antigen-binding molecules wherein the tumor specificity
is towards MUC-I may be particularly useful in the treatment of gas-
tric cancer and ovarian cancer.
Dimeric antigen-binding molecules wherein the tumor specificity is to-
wards EpCAM may be particularly useful in the treatment of carcinomas
of the colon, kidney, and breast.
Dimeric antigen-binding molecules wherein the tumor specificity
is towards PLAP may be of particular use in the treatment of ovarian
or testicular cancer.
Dimeric antigen-binding molecules wherein the tumor specificity
is towards OFA-iLR may be particularly useful in the treatment of me-
tastatic tumors.
In a certain aspect of the invention the antigen binding molecule
as described herein is dimeric and bispecific for CD3 and CD19 or the
antigen-binding molecule is dimeric and bispecific for CD16 and CD19.
In a particular embodiment thereof the first domain VLA and the fourth
domain VHA are specific for CD3 and CD16, respectively, while the
second domain VHB and the third domain VLB are specific for CD19. In
both cases the first and second polypeptide chains each have the do-
main order VLC1)3 _vHCD1 9_ vLCD19_ r vLCD1 _vHCD1 9_ vLCT 1 _vHCD16 from
the N-
terminus to the C-terminus of the polypeptide chains. In a preferred
embodiment the first, second, third and fourth domains are humanized
or fully human. In a most preferred embodiment the first and second
polypeptide chain as defined above is humanized or fully human. In
another aspect of the invention the dimeric antigen binding molecule
may be bispecific, for example, to EpCAM and CD3; albumin, such as,
e.g., HSA and CD3; or EGFR and CD3.
In a further aspect of the invention the antigen binding molecule
as described herein is specific for albumin, for example human serum
albumin (HSA), and another antigen different from albumin. Such anti-
gen-binding molecule binds to serum albumin, thereby increasing the
serum-half life in serum and in vivo. Thus, such antigen-binding mole-
cules are advantageous for medical or diagnostic uses and pharmaceuti-
cal compositions, wherein the polypeptide of such antigen-binding mo-
lecules comprise a light chain variable domain and a heavy chain vari-
able domain of a therapeutic or diagnostic antibody and a light chain
variable domain and a heavy chain variable domain specific for albu-
min. Known and/or commercially available therapeutic, diagnostic or
anti-albumin antibodies can be used as sources for the light chain va-
riable domains and heavy chain variable domains. Moreover, methods to
raise and generate antibodies or Fv fragments specific for albumin,
CA 02842649 2014-01-16
W02013/013700 16 PCT/EP2011/062673
e.g., HSA, are known in the art. In such antigen-binding molecules the
domains of the polypeptide chain are arranged in the order V-,A-VHB-VLB-
VFIA, wherein antigen A or antigen B is albumin. In a preferred embodi-
ment albumin is antigen A. In a certain aspect of the invention the
other antigen is CD3. In a particular embodiment antigen A is human
serum albumin (HSA) and the polypeptide of a HSAHCD3 antigen-binding
molecule has the domain order VLH SA_ vHCD3 _vLCD3_vHILSA as shown in Example
2.
For generating such antigen-binding molecule, for example, the varia-
ble domains of anti-HSA and anti-CD3 antibodies or antibody fragments
may be generated and inserted in the respective order, for example,
analogous as described for CD3xCD19 in Example 1 into the expression
plasmid shown in Fig. 7 by replacing the anti-CD3 and anti-CD19 do-
mains shown or any other suitable expression plasmid or expression
construct.
A further aspect of the invention provides a dimeric antigen-
binding molecule according to any one of the embodiments described
above which is linked with a further functional unit, e.g. a function-
al domain or agent, which independently mediates a biological func-
tion, in particular a biochemical event. The further functional unit
may be complexed with or covalently bound to at least one of the two
individual polypeptide chains of the dimeric antigen-binding molecule.
In one aspect, the further functional unit may be covalently bound to
only one of the individual polypeptide chains and in another aspect
the further functional unit may be covalently bound to both polypep-
tide chains of the dimeric antigen-binding molecule thereby linking
the two polypeptide chains. In a further aspect, each of the two poly-
peptide chains is covalently bound individually to a further function-
al unit. When the further functional unit is covalently bound to at
least one of the two polypeptide chains, the further functional unit
may be fused to at least one of the two polypeptide chains by a pep-
tide bond or a peptide linker. Alternatively, the further functional
unit may be linked by a chemical conjugation such as a disulfide
bridge, e.g. between a cysteine residue of at least one polypeptide
chain and a cysteine residue of the further functional unit, ester
linkage or by chemical crosslinking. In a certain aspect of the inven-
tion the further functional unit may be linked to the antigen binding
molecule by a cleavable linker such as, for example, a disulfide
bound.
The further functional unit may be linked to the N-terminus or C-
terminus of the first and/or second polypeptide chains. If one further
functional unit is linked to both, the first and second, polypeptide
chains, the further functional unit may be linked N-terminal to one
polypeptide chain and C-terminal to the other polypeptide chain.
CA 02842649 2014-01-16
W02013/013700 17 PCT/EP2011/062673
Homobifunctional and heterobifunctional reagents for chemical
crosslinking of a polypeptide chain with a further functional unit
such as a further polypeptide or an agent are well known in the art.
Examples include but are not limited to 5,5"-dithiobis(2-nitrobenzoic
acid) (DTNB), o-phenylenedimaleimide (o-PDM), succinimidyl 3-(2-
pyridyldithio)propionate (SPDP), N-succinimidyl S-acetylthio acetate
(SATA), succinimidyl 4-(N-maleimidomethyl)cyclohexane-l-carboxylate
(SMCC) or 4-(4-N-maleimidophenyl)butyric acid hydrazide (MPBH). Me-
thods for crosslinking of polypeptide chains comprising immunoglobulin
chains with a further polypeptide or a chemical agent are described
for example in Graziano et al., Methods in Molecular Biology, 2004,
vol. 283, 71-85 and Hermanson, G.T. "Bioconjugate Techniques" Academic
Press, London 1996.
In one aspect the further functional unit may be at least one
further variable immunoglobulin domain. The further variable immunog-
lobulin domain may be specific for the first antigen A or the second
antigen B for which the binding sites of the dimeric antigen-binding
molecule are specific or, alternatively, specific for a third antigen
C which is different from antigen A and antigen B. In a certain aspect
a further light chain variable domain VL and a further heavy chain va-
riable VH may be fused to each of the two polypeptide chains such that
one further domain, in particular Vri, is fused to the N-terminus and
the other further domain, in particular VL, is fused to the C-terminus
resulting in a polypeptide having six variable domains which will as-
sociate with another identical polypeptide to a dimeric antigen-
binding molecule having six antigen-binding sites. In another aspect
one further variable immunoglobulin domain may be fused to one of the
polypeptide chains of the antigen-binding molecule which then non-
covalently associates with a complementary variable immunoglobulin do-
main with the same specificity of a further third polypeptide thereby
forming a further antigen-binding site between the dimeric antigen-
binding molecule and the further third polypeptide. In another aspect
a further antigen-binding unit including a scFv or a diabody may be
linked as a further functional unit to the dimeric antigen-binding mo-
lecule.
In a certain aspect the further functional unit may be at least
one further dimeric antigen-binding molecule as described herein. Ac-
cordingly, two or more dimeric antigen-binding molecules according to
the invention may be linked with one another to increase the valency
and avidity of the antigen binding molecules.
In another aspect the further functional unit may be an effector
domain including Fc domain, CH2 domain, CH3 domain, hinge domain or a
fragment thereof. Such a unit may confer effector properties on the
antigen-binding molecule in the case of binding to Fc receptors. Such
CA 02842649 2014-01-16
W02013/013700 18 PCT/EP2011/062673
functional units may further be used to increase the serum-half life
of the antigen-binding molecule.
In another aspect the further functional unit may be an enzyme.
In the case where the enzyme is capable of converting a pro-drug to an
active drug, such an antigen-binding molecule may be used in antibody-
dependent enzyme prodrug therapy (ADEPT). For this the antigen-binding
molecule directs the enzyme to the tissue of Interest and when the an-
tigen-binding molecule binds to the tissue, the prodrug is activated
at that site. Further, the use of bispecific antigen-molecules for
targeting enzymes for cancer therapeutics is known in the art, for ex-
ample, but not limited to bispecific antigen-molecules having speci-
ficities for CD30 and alkaline phosphatase which catalyze the conver-
sion of mitomycin phosphate to mitomycin alcohol, or specifities for
placental alkaline phosphatase and P-lactamase which activate cepha-
losporin-based anti-cancer prodrugs. Suitable are also bispecific an-
tigen-binding molecules having specificity for fibrin and tissue plas-
minogen activator for fibrinolysis and the use of enzyme conjugated
antigen-binding molecules in enzyme-based immunoassays.
In another aspect the functional unit may be a drug, toxin, radioi-
sotope, lymphokine, chemokine or labeling molecule. Such an antigen-
binding molecule delivers the functional unit to the desired site of
action. For example a chemotherapeutic drug linked to an antigen-
binding molecule being specific for a tumor antigen can be delivered
to a tumor cell and toxins may be delivered to pathogens or tumor
cells. An antigen-binding molecule linked with a toxin may be used to
target NK cells or macrophages and are preferably specific for 0D16.
Examples of a toxin are but not limited to ribosyl transferase, serine
protease, guanyl cyclase activator, calmodulin dependent adenyl cyc-
lase, ribunuclease, DNA alkylating agent or mitosis inhibitor, e.g.
doxorubicin. The labeling molecule may be, for example, a fluorescent,
luminescent or radiolabel molecule, a metal chelate or an enzyme (e.g.
horse-radish peroxidase, alkaline phosphatase, 13-galactosidase, malate
dehydrogenase, glucose oxidase, urease, catalase etc.) which, in turn,
when later exposed to a substrate will react to the substrate in such
a manner as to produce a chemical moiety which can be detected and can
be used for in vivo imaging or immunoassays, when it is linked to the
antigen-binding molecule according to the invention. When used for an
immunoassay, the dimeric antigen-binding molecule can also be immobi-
lized on an insoluble carrier, e.g. glass, polystyrene, polypropylene,
polyethylene, dextran, nylon, natural and modified celluloses, poly-
acrylamides, agarose and magnetic beads.
For increasing serum-half life of the antigen-binding molecules
according to the invention in the body, the antigen-binding molecule,
if desired, may be fused to albumin or pegylated, sialylated or glyco-
CA 02842649 2014-01-16
19
W02013/013700 PCT/EP2011/062673
sylated (see, for example, Stork et al., 2009, J. Biol. Chem.,
283:7804-7812). Alternatively to a fusion of additional albumin to the
antigen-binding molecule according to the present invention, the anti-
gen-binding molecule itself may be specific for albumin and another
antigen as described here previously.
The dimeric antigen-binding molecule according to any one of the
embodiments described here previously may be produced by expressing
polynucleotides encoding the individual polypeptide chains which asso-
ciate with each other to form the dimeric antigen-binding molecule.
Therefore, a further embodiment of the invention are polynucleotides,
e.g. DNA or RNA, encoding the polypeptide chains of the dimeric anti-
gen-binding molecule as described herein above.
The polynucleotides may be constructed by methods known to the
skilled person, e.g. by combining the genes encoding the first domain
V:A, the second domain VHB, the third domain VLB and the fourth domain
VFIA either separated by peptide linkers or directly linked by a peptide
bound, into a single genetic construct operably linked to a suitable
promoter, and optionally a suitable transcription terminator, and ex-
pressing it in bacteria or other appropriate expression system. De-
pending on the vector system and host utilized, any number of suitable
transcription and translation elements, including constitutive and in-
ducible promoters, may be used. The promoter is selected such that it
drives the expression of the polynucleotide in the respective host
cell.
The polynucleotides may be codon optimized with the codon bias
being altered to suit the particular expression in the chosen host.
The polynucleotide may be inserted into vectors, preferably ex-
pression vectors, which represent a further embodiment of the inven-
tion. These recombinant vectors can be constructed according to meth-
ods well known to the person skilled in the art; see, e.g., Sambrook,
Molecular Cloning A Laboratory Manual, Cold Spring Harbor Laboratory
(1989) N.Y.
A variety of expression vector/host systems may be utilized to
contain and express the polynucleotides encoding the polypeptide
chains of the present invention. These include, but are not limited
to, microorganisms such as bacteria transformed with recombinant bac-
teriophage, plasmid, or cosmid DNA expression vectors, yeast trans-
formed with yeast expression vectors; insect cell systems infected
with virus expression vectors (e.g., baculovirus); plant cell systems
transformed with virus expression vectors (e.g., cauliflower mosaic
virus, CaMV; tobacco mosaic virus, TMV) or with bacterial expression
vectors (e.g., Ti or pBR322 plasmids); or animal cell systems, for
which, e.g., viral-based expression systems may be utilised.
CA 02842649 2014-01-16
W02013/013700 20 PCT/EP2011/062673
A particular preferred expression vector for expression in E.coli
is pSKK (LeGall et al., J Immunol Methods. (2004) 285(1):111-27) or
pcDNA5 (Invitrogen) for the expression in mammal cells.
Thus, the dimeric antigen-binding molecule as described herein may
be produced by introducing a polynucleotide or vector encoding the
polypeptide chain as described above into a host cell and culturing
said host cell under conditions whereby the polypeptide chain is ex-
pressed. The dimeric antigen-binding molecule obtained from the ex-
pressed polypeptide chains may be isolated and, optionally, further
purified. Conditions for the growth and maintenance of host cells, the
expression, isolation and purification of dimeric antigen-binding
molecules according to the invention from these host cells are fully
described in the art.
In a further embodiment of the invention compositions comprising a
dimeric antigen-binding molecule or a polynucleotide as described
herein above and at least one further component are provided. For use
in preventing or treating a disease or disorder the composition con-
taining the dimeric antigen-binding molecule or the polynucleic acid
molecule encoding the polypeptide chains forming the antigen-binding
molecule is preferably combined with a suitable pharmaceutically ac-
ceptable carrier. The term "pharmaceutically acceptable carrier" is
meant to encompass any carrier, which does not interfere with the ef-
fectiveness of the biological activity of the ingredients and that is
not toxic to the patient to whom it is administered. Examples of suit-
able pharmaceutical carriers are well known in the art and include
phosphate buffered saline solutions, water, emulsions, such as
oil/water emulsions, various types of wetting agents, sterile solu-
tions etc. Such carriers can be formulated by conventional methods and
can be administered to the subject at a suitable dose. Preferably, the
compositions are sterile. These compositions may also contain adju-
vants such as preservative, emulsifying agents and dispersing agents.
Prevention of the action of microorganisms may be ensured by the in-
clusion of various antibacterial and antifungal agents. Administration
of the suitable compositions may be effected by different ways, e.g.
by intravenous, intraperetoneal, subcutaneous, intramuscular, topical
or intradermal administration. The route of administration, of course,
depends on the kind of therapy and the kind of compound contained in
the pharmaceutical composition. The dosage regimen will be determined
by the attending physician and other clinical factors. As is well
known in the medical arts, dosages for any one patient depends on many
factors, including the patient's size, body surface area, age, sex,
the particular compound to be administered, time and route of admini-
stration, the kind of therapy, general health and other drugs being
administered concurrently.
CA 02842649 2014-01-16
W02013/013700 21 PCT/EP2011/062673
The invention further provides a medical use or a method wherein
the dimeric antigen-binding molecule as described herein above is ad-
ministered in an effective dose to a subject, e.g., patient, for immu-
nosuppressive treatment, e.g. in transplantation, the treatment of au-
toimmune disease, inflammatory disease, infectious disease, allergy or
cancer (e.g. non-Hodgkin's lymphoma; chronic lymphocytic leukemia;
Hodgkin's lymphoma; solid tumors e.g. those occurring in breast can-
cer, ovarian cancer, colon cancer, cancer of the kidney, or cancer of
the bile duct; minimal residual disease; metastatic tumors e.g. those
metastasizing in the lungs, bones, liver or brain). The antigen-
binding molecule can be used in prophylactic or therapeutic settings,
alone or in combination with current therapies.
The cancers that can be treated using the antigen-binding mole-
cule of the present invention include but are not limited to primary
and metastatic adrenal cortical cancer, anal cancer, aplastic anemia,
bile duct cancer, bladder cancer, bone cancer, bone metastasis, CNS
tumors, peripheral CNS cancer, breast cancer, Castleman's Disease,
cervical cancer, childhood Non-Hodgkin's lymphoma, colon and rectum
cancer, endometrial cancer, esophagus cancer, Ewing's family of tumors
(e.g. Ewing's sarcoma), eye cancer, gallbladder cancer, gastrointesti-
nal carcinoid tumors, gastrointestinal stromal tumors, gestational
trophoblastic disease, hairy cell leukemia, Hodgkin's disease, Ka-
posi's sarcoma, kidney cancer, laryngeal and hypopharyngeal cancer,
acute lymphocytic leukemia, acute myeloid leukemia, children's leuke-
mia, chronic lymphocytic leukemia, chronic myeloid leukemia, liver
cancer, lung cancer, lung carcinoid tumors, Non-Hodgkin's lymphoma,
male breast cancer, malignant mesothelioma, multiple myeloma, myelo-
dysplastic syndrome, myeloproliferative disorders, nasal cavity and
paranasal cancer, nasopharyngeal cancer, neuroblastoma, oral cavity
and oropharyngeal cancer, osteosarcoma, ovarian cancer, pancreatic
cancer, penile cancer, pituitary tumor, prostate cancer, retinoblas-
toma, rhabdomyosarcoma, salivary gland cancer, sarcoma (adult soft
tissue cancer), melanoma skin cancer, non-melanoma skin cancer, stom-
ach cancer, testicular cancer, thymus cancer, thyroid cancer, uterine
cancer (e.g. uterine sarcoma), vaginal cancer, vulvar cancer, and
Waldenstrom's macroglobulinemia.
An "effective dose" refers to amounts of the active ingredient that
are sufficient to affect the course and the severity of the disease,
leading to the reduction or remission of such pathology. An "effective
dose" useful for treating and/or preventing these diseases or disor-
ders may be determined using methods known to a skilled person (see
for example, Fingl et al., The Pharmacological Basis of Therapeutics,
Goddman and Gilman, eds. Macmillan Publishing Co., New York, pp. 1-46
(1975)).
CA 02842649 2014-01-16
W02013/013700 22 PCT/EP2011/062673
In another aspect of the invention the dimeric antigen-binding mole-
cule as described herein above is used in the manufacture of a immuno-
suppressive medicament or medicament for the treatment of autoimmune
disease, inflammatory disease, infectious disease, allergy or cancer
(e.g. non-Hodgkin's lymphoma; chronic lymphocytic leukaemia; Hodgkin's
lymphoma; solid tumours e.g. those occurring in breast cancer, ovarian
cancer, colon cancer, cancer of the kidney, or cancer of the bile
duct; minimal residual disease; metastatic tumours e.g. those metasta-
sizing the lungs, bones, liver or brain). Where specified, multispeci-
fic binding molecules have been described above as having a particular
utility in the treatment of a specified disease, these binding mole-
cules may also be used in the manufacture of a medicament for that
specified disease.
The methods for preparing pharmaceutical compositions, i.e. medica-
ments, and the clinical application of antigen binding molecules in
the prevention and/or treatment of diseases such as, for example, can-
cer are known to the skilled artisan.
In a particular aspect of the invention the dimeric antigen bind-
ing molecule is bispecific and used for cancer therapy, because such
antibodies can be used to retarget cytotoxic effector cells against
tumor cells. This therapeutic concept is well known in the art. For
example, clinical studies showed tumor regression in patients treated
with an anti-CD3 x antitumor bispecific antibody (e.g. Canevari, S. et
al., J. Natl. Cancer Inst., 87:1463-1469,1996) or patients treated
with an anti-CD16 x antitumor bispecific antibody (e.g. Hartmann et
al.; Clin Cancer Res. 2001;7(7):1873-81). Proof-of-concept has also
been shown for various recombinant bispecific antibody molecules com-
prising only variable domains (Fv) such as, for example, dimeric and
tetravalent CD3xCD19 antigen binding molecules having a domain order
VHA-VLB-VHB-VLA (Cochlovius et al.; Cancer Research, 2000, 60:4336-
4341)or recently in clinical studies with monomeric single-chain Fv
antibody molecules of the BiTE(10-format (two single-chain antibodies of
different specificities linked together; Micromet AG, Germany; Bargou
R. et al., Science, 2008, 321(5891):974-977; Baeuerle PA and Reinhardt
C., Cancer Res. 2009, 69(12):4941-4944). The dimeric antigen binding
molecules described herein can be used as medicaments and applied in
methods of treatment in a similar way as the bispecific antibodies of
the art, as they are capable of redirecting therapeutic, e.g. cytotox-
ic, mechanisms using the same combined antibody specificities. Fur-
ther, immunosuppressiv antibodies monospecific for CD3 such as Muromo-
nab-CD3 are known for the treatment of transplant rejection, acute re-
jection of renal transplants (allografts), hepatic and cardiac trans-
plants. Thus, antigen-binding molecules bispecific for albumin and CD3
may be used in the same methods of treatments as the known monospecif-
CA 02842649 2014-01-16
W02013/013700 23 PCT/EP2011/062673
ic anti-CD3 antibodies. Moreover, the antigen-binding molecules spe-
cific to albumin and a another antigen, i.e. therapeutic or diagnostic
target, as described herein may be used for the respective clinical
applications of the antigen specificity other than albumin.
The antigen-binding molecule and the compositions thereof can be
in the form of an oral, intravenous, intraperitoneal, or other pharma-
ceuticaly acceptable dosage form. In some embodiments, the composition
is administered orally and the dosage form is a tablet, capsule, cap-
let or other orally available form. In some embodiments, the composi-
tion is parenteral, e.g. intravenous, intraperitoneal, intramuscular,
or subcutaneous, and is administered by means of a solution containing
the antigen-binding molecule.
A skilled person will readily be able without undue burden to
construct and obtain the antigen-binding molecules described herein by
utilizing established techniques and standard methods known in the
art, see for example Sambrook, Molecular Cloning A Laboratory Manual,
Cold Spring Harbor Laboratory (1989) N.Y.; The Protein Protocols Hand-
book, edited by John M. Walker, Humana Press Inc. (2002); or Antibody
engineering: methods and protocols / edited by Benny K.C. Lo; Benny
K.C. II Series: Methods in molecular biology (Totowa, N.J.)). In addi-
tion, a skilled person will be able to make the antigen-binding mole-
cules described herein by utilizing standard methods known in the art
and modifying the methods described in US 7,129,330, Kipriyanov et al.
J. Mol. Biol. (1999) 293, 41- 56 or Le Gall et al., 2004, Protein En-
gineering 17:357-366 such that dimeric antigen-binding molecules as
described above comprising two polypeptide chains having the domain
order VLA- VHB-VLB-VHA from the N-terminus to the C-terminus of each po-
lypeptide chains are obtained.
The examples below further illustrate the invention without lim-
iting the scope of the invention.
Example 1:
To construct functional dimeric tandem diabodies (TandAb )using a
domain arrangement other than VHA-VLB-VHB-VLA, several such dimeric tan-
dem diabodies were constructed with the domain arrangement VIA-VHB-VLB-
VHA according to the invention using the two domains of a humanized
anti-CD19 single chain antibody and a humanized anti-CD3 single chain
antibody, respectively. The findings were confirmed by using two va-
riants of each antigen-binding molecule, representing the products of
different stages of an affinity maturation procedure that was carried
out for both the humanized anti-CD19 and humanized anti-CD3 antibo-
dies.
The murine monoclonal antibodies HD37 and UCHT directed against
CD19 and CD3, respectively, were the starting material for obtaining
humanized antibodies with relatively high affinities. In each case the
CA 02842649 2014-01-16
W02013/013700 24 PCT/EP2011/062673
VH domain was first combined with a library of human VL in an scFv pha-
gemid vector to select a suitable human VT_ chain by phage display. In a
second step the selected human VL chain was combined with a library of
VH domains in which the CDR3 region remained constant. This procedure
resulted in a humanized anti CD19 and anti 0D3, respectively, that on-
ly contained a short murine sequence in the VHCDR3 region. These
clones were subsequently affinity matured introducing point mutations
at residues thought to be involved in antigen binding. The best bind-
ing mutants were then selected by phage display. The clones chosen for
constructing the TandAb were M13 and M39 binding to CD19 and C4 and
LcHC21 binding to CD3.
The following antibodies were generated:
Antibody Al: CD19m39xCD3C4 ( option 0) VHCD3C4_ITLCD19M39_vHCD19M39_vLCD3C4
Antibody B: CD19m"xCD3C4 (option 2) VLCD3C4_vHCD19M39_vLCD19M39_vHCD3C4
Antibody A2: CD19m13xCD3LCHC21 ( option 0)
VHCD3LCHC21_vLCD19M13_vHCD19M13_vLCD3LCHC21
Antibody C: CD19m13xcD3LcHc21 (option 2 )CD3LCHC21 vHCD19M13_ CD19M13_
vHCD3LCHC21
The plasmids encoding the hybrid monomers VT,CD3C4_vHCD19M39_vT CD19M39_
vHCD3C4 of antibody B and V1CD3LCHC21_vH0D19M13_vL0D19M13_vHCD3LCHC21 of
antibody C
were generated by a DNA engineering and processing provider. The - ..
se-
quence backbone of the VLcD3c4-VHCD1 M39 -VLCD19M39 -VHCD3C1 monomer comprises
the
DNA sequences of two scFv antibodies, namely scEvCD19m" and scFvCD3c1,
I
respectively. The VI,CD3LCHC23-V11CD19M1 3_ vCD19T41 3 -VHCD3LCHC21 monomer
sequence com-
bines the variable domains of the single chain Fv 0D191'413 and single
chain By CD3LCHC21. All four scFv were obtained by phage display selec-
tion of single chain antibodies against the antigens CD19 and CD3. In
both cases the sequence information was used to construct the above
hybrid monomers. A 9 amino acid (G,S)3 linker was used to link the do-
mains with one another. The synthesized gene coding for VLCD3C4 THCD19M39_
v_CD19M39_vHCD3C4 was cloned into the mammalian expression vector pCDNA5FRT
(Invitrogen). The gene of VLCD3LCHC2l_vHCD19M13_v-LCD19M13_vHCD3LCHC21 was
also
cloned into an expression vector and amplified by PCR using a forward
primer introducing an NcoI cleaving site and a reverse primer intro-
ducing a NotI cleaving site. After analysis and isolation by agarose
gel, the PCR product was subsequently double digested by NcoI and NotI
and cloned into the NcoI and NotI linearised pSKK3 vector. The correct
cloning was confirmed by DNA sequencing.
The vector map of pCDNA5FRT encoding antibody B is shown in Fig. 6.
The vector map of pSKK3 encoding antibody C is shown in Fig. 7.
For high level production the vector containing the gene VT,cD3c4-
vliCD 19M39_ vLCD19M3 9_ vHCD3C4 was transiently transfected (using CaPO4)
into ad-
herent HEK293 cells. Protein fermentation was performed under growth
conditions well known in the art.
The recombinant protein was expressed as a His-Tag fusion protein with
a signal peptide. The protein was isolated from cell culture superna-
CA 02842649 2014-01-16
W02013/013700 25 PCT/EP2011/062673
tant by immobilized metal affinity chromatography (IMAC) as described
(Kipriyanov et al., 1999, J.Mol.Biol., 293, 41-56). The purified ma-
terial was subsequently analysed by SDS-PAGE. Coomassie staining of an
SDS PAGE gel and size-exclusion chromatography on a calibrated Super-
dex 200 HR10/30 column (Amersham Pharmacia, Freiburg, Germany) in so-
dium-phosphate buffer (30mM NaPO4, 0.75M arginine/HC1, pH6.0) revealed
a pure and correctly assembled recombinant protein (Antibody B).
For high level expression, the gene coding for the humanized
vLCD3LCHC2 _v-HOD19M13_vi CD191/13_vHCD3LCHC21 monomer followed by a 6x His-
Tag was
cloned into the pSKK3 plasmid containing the hok/sok gene cell suicide
system and a skp gene encoding the Skp/OmpH periplasmic factor (LeGall
et al., 2004, J. Immunol. Methods, 285, 111-127). The plasmid was was
transfected into an E.coli K12 strain (ATCC 31608TM)
The transformed bacteria were grown in shake flasks and induced
essentially as described previously (Cochlovius et al., 2000, J. Immu-
nol., 165, 888-895). The recombinant proteins were isolated from both
the soluble periplasmic fraction and the bacterial medium supernatant
by immobilized metal affinity chromatography (IMAC) as already de-
scribed (Kipriyanov et al., 1999, J.Mol.Biol., 293, 41-56).
The purified material was subsequently analysed by SDS-PAGE
stained by Coomassie blue and size-exclusion chromatography on a cali-
brated Superdex 200 HR10/30 column (Amersham Pharmacia, Freiburg, Ger-
many) in sodium-phosphate buffer (30mM NaPO4, 0.75M arginine/HC1,
pH6.0). The product appeared to be pure and correctly assembled.
The comparative antibodies Al and A2 were generated in the same
way as antibodies B and C, respectively, wherein the domain order of
antibodies Al and A2, respectively, were reversed in comparison to
that of antibodies B and C, respectively.
Cytotoxicity assays were performed essentially as described by T.
Dreier et al. (2002, Int J Cancer 100, 690-697). The PMBCs that were
used as effector cells were isolated from the peripheral blood of
healthy volunteers by density gradient centrifugation. In some cases,
the PBMC were cultured overnight in the presence of 25 U/mL human IL-2
before they were used as effector cells in the cytotoxicity assay.
Purity and antigen expression of the isolated PBMC was checked by flow
cytometry in each case (data not shown).
CD19-' JOK-1 or Raji target cells were cultured in RPMI 1640 me-
dium supplemented with 10 GFCS, 2 mM L-glutamine and 100 IU/mL penicil-
lin G sodium and 100 pg/mL streptomycin sulfate (herein referred to as
RPMI medium; all components from Invitrogen). For the cytotoxicity as-
say cells were labeled with 10 pM calcein AM
(Molecular
Probes/Invitrogen) for 30 min in RPMI medium without FCS at 37 C. Af-
ter gently washing the labeled cells were resuspended in RPMI medium
to a density of 1x105/mL. 1x104 target cells were then seeded together
CA 02842649 2014-01-16
W02013/013700 26 PCT/EP2011/062673
with 5x105 PBMC with the indicated antibodies in individual wells of a
round-bottom 96-well micro plate in a total volume of 200 pL/well. Af-
ter centrifugation for 2 min at 200 g the assay was incubated for 4
hours at 37 C in a humidified atmosphere with 5% CO,. 15 min prior to
the end of incubation 20 pL of 10% Triton X-100 in RPMI medium were
added to the wells with target cells only. 20 pL RPMI medium was added
to all other wells. 100 pL cell culture supernatant were harvested
from each well after an additional centrifugation for 5 min at 500 g,
and the fluorescence of the released calcein was measured at 520 nm
using a fluorescence plate reader (Victor 3, Perkin Elmer). On the ba-
sis of the measured counts, the specific cell lysis was calculated ac-
cording to the following formula: [fluorescence (sample) - fluores-
cence (spontaneous)] / [fluorescence (maximum) - fluorescence (sponta-
neous)] x 100%. Fluorescence (spontaneous) represents the fluorescent
counts from target cells in the absence of effector cells and antibo-
dies and fluorescence (maximum) represents the total cell lysis in-
duced by the addition of Triton X-100. Sigmoidal dose response curves
and EC50 values were calculated using the Prism software (GraphPad
Software).
Results:
The results of the cytotoxicity assays for tandem diabodies hav-
ing the following domain order starting at the N-terminus of VHA-VLB-
VHB-VLA (antibody A) and VLA-VHB-VLB-VHA (antibody B), respectively, us-
ing the anti 0D19 variant M39 and the anti CD3 variant 04 are shown In
Figure 3.
Surprisingly, there was a very large difference in the cytotoxic
activity of the two tandem diabodies. The tandem diabody having the
domain arrangement according to the invention designated as "antibody
B" was more than 60x more active than the tandem diabody designated
"antibody B" as determined by a comparison of their 5050 values under
the given conditions.
The superiority of the domain arrangement represented by the present
invention (antibody C) for better cytotoxicity was confirmed by using
two additional variants of the anti CD19 and anti CD3 antibodies (see
Figure 4).
The EC50 value of the tandem diabody with the domain order accord-
ing to the invention represented by option 2 is extremely low (0.1pM).
It is 27x more active than the TandAb represented by option 0 after
comparing the E050 values under the given conditions.
Example 2:
T cell receptor modulation by human serum albumin (HSA)xCD3 Tan-
dAb antibodies in vitro
To determine whether the HSAxCD3 TandAb antibodies with different
domain orders differ in efficacy in inducing T cell receptor (TCR)/CD3
CA 02842649 2014-01-16
W02013/013700 27 PCT/EP2011/062673
modulation on T cells in vitro CD3+ Jurkat cells were cultured in the
presence of increasing concentrations of the bispecific HSAxCD3 TandAb
antibodies and subsequently analyzed for remaining TCR. The modulation
assay was performed in the presence or absence of HSA to measure the
influence of HSA on the activity of the TandAbs.
In brief, 1x106 Jurkat cells were seeded in individual wells of a
round-bottom 96-well micro plate in RPMI 1640 medium supplemented with
2 mM L-glutamine and 100 IU/mL penicillin G sodium and 100 pg/mL
streptomycin sulfate (all components from Invitrogen). In a separate
micro plate Jurkat cells were seeded in RPMI medium as described be-
fore but with the addition of 50 mg/mL HSA (Sigma). After the addition
of the indicated antibodies, cells were incubated in a total volume of
200 pL/well at 37 C in a humidified incubator in the presence of 5%
CO2. As a control, cells were cultured in the absence of antibodies.
After washing with ice-cold phosphate buffered saline (PBS, Invitro-
gen, Karlsruhe, Germany) supplemented with 2% heat-inactivated FCS
(Invitrogen, Karlsruhe, Germany) and 0.1% sodium azide (Roth,
Karlsruhe, Germany) (referred to as a FAGS buffer) the cells were
stained with 10 pL PC5-conjugated anti-TCR a/13 antibody (Beckman-
Coulter) in a total volume of 100 pL in FAGS buffer for 45 on ice in
the dark. After washing twice with FACS buffer the fluorescence of 104
cells was measured at 675 nm with an FC500 MPL flow cytometer (Beck-
man-Coulter). Mean fluorescence values were determined using the CXP
software (Beckman-Coulter) and used for analysis by non-linear regres-
sion/4 parameter logistic fit using the GraphPad Prism version 3.03
for Windows, GraphPad Software, San Diego California USA.
The results obtained from the TCR modulation experiment CAB-306
depicted in Fig. 5 and summarized in Tab.1 demonstrate comparable TCR
modulation efficacy of both HSAxCD3 TandAb in domain order VHA-VLB-
VHB-VLA (=option 0) and VLA-VHB-VLB-VHA (=option 2) which demonstrates
the modulation efficacy when HSA is antigen B. However, in the pres-
ence of physiological concentrations of HSA the modulation efficacy in
case of the option 0 (VHA-VLB-VHB-VLA) TandAb is considerably decreased,
whereas the EC50 value for the TandAb in the option 2 (VLA-VHB-VLB-VHA)
orientation is only increased by factor 2.6.
These data clearly indicate the superior properties of the
HSAxCD3 TandAb in domain orientation option 2 (VLA-VHB-V-,B-VHA) when
compared with the HSAxCD3 TandAb option 0 (VHA-V1B-VHB-VLA).
TandAb antibody TandAb domain order [C50 w/o [C50
with fadincreasein
batch HSA HSA EC50
MST13.1 HSAxCD3 option 0 861 pM ¨140 000 > 100
VHA-VLB-VHB-VLA PM
MST13.3 HSAxCD3 option 2 726 pM 1 913 pM 2.6
VLA-VHB-VLB-VHA
CA 02842649 2014-01-16
W02013/013700 28 PCT/EP2011/062673
Table 1: Summary of the results from the TCR modulation experiment:
The EC50 values from the TCR modulation experiment with the two HSAHCD3
TandAb antibodies in the presence or absence of HSA (Fig. 5; experi-
ment CAB-306) were determined by non-linear regression/4 parameter lo-
gistic fit.
While preferred embodiments of the present invention have been
shown and described herein, it will be obvious to those skilled in the
art that such embodiments are provided by way of example only. Numer-
ous variations, changes, and substitutions will now occur to those
skilled in the art without departing from the invention. It should be
understood that various alternatives to the embodiments of the inven-
tion described herein may be employed in practicing the invention. It
is intended that the following claims define the scope of the inven-
tion and that methods and structures within the scope of these claims
and their equivalents be covered thereby.
, CA 02842649 2014-03-25
28a
SEQUENCE LISTING IN ELECTRONIC FORM
In accordance with section 111(1) of the Patent Rules, this
description contains a sequence listing in electronic form in
ASCII text format (file: 94022-19 seq 25-03-14 vl.txt).
A copy of the sequence listing in electronic form is available
from the Canadian Intellectual Property Office.
The sequences in the sequence listing in electronic form are
reproduced in the following table.
SEQUENCE TABLE
<110> AFFIMED THERAPEUTICS AG
<120> Multivalent Antigen-Binding Fv Molecule
<130> 94022-19
<140> 2,842,649
<141> 2011-07-22
<160> 1
<170> BiSSAP 1.0
<210> 1
<211> 9
<212> PRT
<213> artificial sequences
<220>
<221> SOURCE
<222> 1..9
<223> /mol_type="protein"
/note="peptide linker"
/organism-"artificial sequences"
<400> 1
Gly Gly Ser Gly Gly Ser Gly Gly Ser
1 5