Note: Descriptions are shown in the official language in which they were submitted.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
RECOVERY OF LEAD AND INDIUM FROM GLASS, PRIMARILY FROM
ELECTRONIC WASTE MATERIAL
DESCRIPTION
TECHNICAL FIELD
The present invention relates to a process for recovering lead and/or indium
from glass
containing Pb0 and/or indium oxide, primarily from electronic waste material.
BACKGROUND ART
Lead is used in radiation shielding glasses in order to absorb gamma radiation
and X-
rays, e.g. in the cathode ray tubes, CRTs, used in computer screens and
television sets,
where lowering the exposure of the viewers to soft X-rays is of concern.
Modern CRTs have a front panel made of essentially lead-free glass, there
behind a
funnel made from leaded glass, and at the far end a neck of highly leaded
glass. These
CRTs represent an environmental hazard if disposed improperly. In October
2001, the
United States Environmental Protection Agency created rules stating that CRTs
must be
brought to special recycling facilities. In November 2002, the EPA began
fining
companies that disposed of CRTs through landfills or incineration. Regulatory
agencies,
local and statewide, monitor the disposal of CRTs and other computer
equipment. In
Europe, disposal of CRT televisions and monitors is covered by the WEEE
Directive
2002/96/EC.
CRT monitors make up about 6 % of the electronic waste in Europe, and
approximately
450,000 tons of cathode ray tube monitors are disposed of in Europe every
year. 70 %
of all the CRT monitors are dumped in landfills or exported to the foreign
countries,
since no effective recycling systems are available. As indicated, CRT screens
are
becoming a bigger problem especially in countries that manufactures these
monitors,
such as Japan, USA and Taiwan. Many countries handle the CRT waste by sending
it to
lesser developed countries in exchange for money to avoid regulation
protocols.
However, this is a temporary solution, as new techniques are being developed
to find
more effective ways to extract the lead and other hazardous components to be
separated
from the glass. The rest of said 70 % of CRT monitors contain 33,300 tons of
lead
oxides. Calculated as pure lead, about 31,000 tons of lead is being dumped in
landfills
annually in Europe.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
2
The main concern with the dumping of CRT screens is the leaching of lead from
the
glasses. The leached lead is dangerous both for human beings and environment.
Dumping of CRT screens mostly occurs in developing countries. This is a big
problem,
since it is very common for homeless people, both adults and children, to live
in, and
around refuse dumps. These people are therefore very exposed to the lead that
is
leached from screens by the rainwater. Many people who live around refuse
dumps
therefore become lead poisoned by constant exposure. The most common way for
the
exposed people to become lead poisoned is that they are drinking water with
high
content of lead. Lead can also be spread to lands through the air, by mining
and by
direct discharges in water and lands. It takes a very long time for lead to
form harmless
compounds in the nature. A big problem is when lead is spread to farms and
arable
lands. When this happens, people are exposed to lead poisoning from eating the
fruits
and vegetables that are grown on these lands. Adults and children can also be
affected
when they eat meat from animals that has grazed on grounds exposed to lead,
and also
when they eat fish, which is affected by lead that comes from discharges from
industries
in seas and lakes, leachate from refuse dumps and landfills and outflow from
sewages.
No safe threshold for lead exposure has been discovered ¨ i.e., there is no
known
amount of lead that is too small to cause the body harm.
Studies of shredding cathode ray tube glass into cullet (small glass pieces)
and reusing
them for cathode ray tubes have been made by Association for Electric Home
Appliances. Of these studies, a system of extracting a cathode ray tube from a
television
main body and shredding the cathode ray tube into glass cullet has been
developed (see
"Electrotechnology", January, 1997, for example).
A method of recovering glass as cullet is disclosed in, e.g., Japanese Laid-
Open Patent
Application No. 61-50688. There is also known a method of shredding cathode
ray tube
glass into cullet (small glass pieces) and reusing them for cathode ray tubes
(e.g.,
Japanese Laid-Open Patent Application No. 9-193762). A method of separating a
cathode ray tube into a face plate and funnel in accordance with materials,
and
shredding them into cullet is disclosed in, e.g., Japanese Laid-Open Patent
Application
No. 05-185064. Further, a method of separating a cathode ray tube into a face
plate and
funnel, peeling fluorescent substances and a black mask from the face plate,
and
recycling the face place is disclosed in Japanese Laid-Open Patent Application
No. 7-
037509.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
3
To reuse cathode ray tube glass, the glass must be separated into panel glass
and lead-
containing funnel glass. This is because, if lead is mixed in panel glass by a
predetermined amount or more, a browning phenomenon occurs, and the lead-
containing glass cannot be reused as a raw material of the panel glass. For
this reason, a
cathode ray tube is separated into a panel and funnel. For this purpose, there
are
proposed a method of defining a position to cut a cathode ray tube (Japanese
Laid-Open
Patent Application No. 9-115449), and a method of melting frit glass, which
joins a
panel and funnel, thereby separating the panel and funnel (Japanese Laid-Open
Patent
Application No. 7-45198).
WO 2009/139715 Al discloses a process for chlorinating ore, slag, mill scale,
scrap,
dust and other resources containing recoverable metals from the groups 4¨ 6, 8-
12, and
14 in the periodic table. It is well known that metal values can be recovered
from many
sources such as scrap, ores and sea nodules by chlorination. The formed metal
chlorides
can subsequently be separated and extracted by fractional distillation and
condensation,
electrolysis of the salt or by hydrometallurgical processing. However, to get
a
considerably higher reaction rate and yield of valuable metals than what is
possible
when ferric chloride and /or cupric chloride are used as chlorine donors,
aluminum
chloride is substituted for said chlorides.
Further, US 4,853,094 discloses a process for the production of metal Me (e.g.
Sn) or an
alloy containing metal Me from a metal halide MeX. (e.g. Sn C14) by
electrolysis in a
cell having an anode, a liquid metal cathode comprising one or more metals M
(e.g.
liquid Zn) and a liquid electrolyte comprising a salt melt of one or more
alkali metal or
alkaline earth metal halides (e.g. LiC1/KC1 mixture). The process comprises
introducing
metal halide MeX., in which Me represents a metal selected from the groups 2b,
3b
(including the lanthanide series and the actinide series), 7b and 8 of the
periodic system
and Cr, Cu, Au, Ga, Sn, Pb and Bi, X represents halogen and n represents the
valence of
the metal Me, into the liquid metal cathode and isolating Me or an alloy
containing Me
from the metal cathode material.
In Journal of Hazardous Materials 161(1109-1113) Issus 2/3 2009 a method of
recovering lead through a pyro vacuum process was suggested. However, as far
as we
know, no method that has been commercially accepted has been proposed that
solves
the problem of rendering glass from scrapped CRT funnels harmless by
recovering lead
therefrom.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
4
Another metal oxide that is desirable to recover from glass is Indium. Indium
tin oxide
(ITO) is one of the most widely used transparent conducting oxides and is e.g.
used in
flat panel displays such as LCD, LED, OLED, PDP etc, antistatic coatings, in
light
emitting diodes and in various sources. Indium may also be present as indium
doped
zinc oxide. The price of indium is high and the supply is low. Hence there is
a need of
cost effective recycling of indium from glass present in electronic waste
material.
DESCRIPTION OF THE INVENTION
A main object of the present invention is to recover metal oxide, in
particular lead or
indium, from glass containing Pb0 and/or indium oxide, primarily from
electronic
waste material.
This object is achieved in accordance with the invention in that the process
comprises:
a) crushing the glass to produce a cullet,
b) forming a mixture consisting of by weight % of the mixture;
- 60-95 of chloride salt composition consisting of at least two metal
chlorides selected from the group consisting of alkali metal chlorides and
alkaline earth metal chlorides,
- 5- 30 of AlC13, and
- optionally 0-10 of halides, additional chlorides, sulfides and/or oxides,
c) heating said mixture to form a salt melt,
d) dissolving the cullet in the salt melt,
e) recovering at least one of lead and indium from the salt melt.
Thereby valuable metals can be recovered from glass as well as making the
residue after
the process essentially harmless. In regard to lead, it is possible to achieve
a lead
extraction ratio of more than 95 %, which makes the residue after the process
essentially
harmless.
Preferably the amount of A1C13 is in the range of 5-20 % by weight of the
mixture, more
preferably 7-15 wt%, most preferably 8-13 wt%.
Preferably, the recovery of at least one of lead and indium from the melt
includes:
- electrolyzing the melt; and
- selectively electrodepositing at least one of lead and indium.
By selective electro-deposition it is possible to recover most of the oxides
present in the
glass.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
The temperature of the salt melt should preferably not exceed 1000 C during
the
dissolution of the cullet, more preferably the temperature should not exceed
900 C, the
suitable temperature being of the order of at least 500 C during the
dissolution of the
cullet and the electrolysis of the melt. The upper limit is set by the fact
that, at higher
5 temperature some chlorides start evaporating from the melt. The lower
limit is decided
by the liquidus temperature of the salt bath. It is preferable to have the
temperature of
operation at least 100 C above the liquidus temperature of the salt bath.
Graphite rods are suitably used for anode and cathode during the electrolysis
due to
their inertness and low cost. When recovering lead, it is suitable that a
pervious cathode
diaphragm is provided around the cathode for collecting liquid lead. This is
mainly to
avoid the contamination of the residue, which is nearly pure silica by liquid
lead.
The cathode diaphragm suitably is made from alumina and has a plurality of
holes so
that the liquid molten salt electrolyte can permeate through these holes while
the solid
residues can not pass through.
Preferably, a voltage of 2 to 3 V, more preferably 2.3-2.7 V, is used for
extracting lead
by electrolysis, as it was found to be favorable in the preliminary trials.
This is in
conformity with cyclic voltameric studies as well with graphite electrodes.
The melt is electrolyzed for a time suitably on the order of 2 to 8 hours,
preferably 3-6
hours.
Preferably, the weight ratio between flux and cullet is on the order of 0.25
up to 1.5,
more preferably 0.3-1Ø For lead containing cullet most preferably 0.35-0.45.
An
increase of flux content and flux/cullet ratio seems not to contribute of
extraction ratio
of lead oxide since low ratios of flux content and flux/cullet already show
very high
extraction ratio.
Preferably, the temperature is held for a time on the order of for 4-8 hours
during the
dissolution step. Then the cullet will be softened and the lead and/or indium
value is
extracted into the salt melt.
It is possible to vaporize metal chlorides from the melt and condensing them
for
subsequent recovery of the metals of the condensed chlorides. The metal
chlorides from
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
6
the melt may also be recovered by leaching in water and extracted to recover
the metals
as hydroxides by a hydrometallurgical method.
After recovering the lead and/or indium and optionally other metals present in
the cullet,
the chloride salts of the melt may be recycled.
After recovering lead and indium and possibly other metals from glass, a
processing
residue consisting essentially of A1203 and Si02 remains, which is useful for
landfill,
building construction, or as a raw material for refractory industry.
Lead containing glass primarily comes from a funnel-shaped part and/or a neck
part of a
cathode ray tube of a computer screen or a television set.
Alternatively, the at least one of lead and indium can be recovered by a
process using a
liquid aluminum anode. In this process A1C13 is generated by an in situ
formation during
the electrolysis. This process includes the steps of:
a) crushing the glass to produce a cullet,
b) providing a crucible containing a chloride salt melt, at least one cathode
and
an anode connected to the salt melt, heating means for heating the salt melt,
and an aluminum melt present at the bottom of the crucible, said aluminum
melt forming the anode or a part of the anode,
c) providing an initiating chlorine donor to the salt melt for starting the
reactions in the salt melt, said initiating chloride donor being aluminum
chloride and/ or at least one metal chloride that can be electrolyzed in step
g)
to form aluminum chloride,
d) holding the temperature of the salt melt and the temperature of the
aluminum
melt at a temperature where both are in liquid phase,
e) introducing said cullet into the liquid salt melt,
f) reacting the aluminum chloride as a chlorine donor with the cullet to
form at
least one of lead chloride and indium chloride being dissolved in the salt
melt,
g) electrolyzing the salt melt and selectively depositing at least one of lead
and
indium at the cathode, optionally using a cathode bag, and in situ forming
aluminum chloride at the contact surface between the aluminum melt and the
salt melt,
h) recovering at least one of lead and indium from the salt melt.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
7
Cullet
The cullet is prepared by crushing glass containing Pb0 and/or indium oxide.
Pb0
containing glass primarily comes from a funnel-shaped part and/or a neck part
of a
cathode ray tube of a computer screen or a television set. Indium oxide
containing glass
primarily comes from flat panel displays coated with indium tin oxide.
Preferably, glass
containing Pb0 and glass containing indium oxide are separated, so that the
cullet
contains lead or indium. However, indium and lead from a mixed cullet can be
recovered by selective electrodepositing.
Salt composition
Preferably the salt composition consists of at least two of the salts selected
from the
group: NaC1, KC1, LiC1, and CaC12, preferably at least three of the salts
selected from
the group: NaC1, KC1, LiC1, and CaC12. Preferably the composition is selected
so that
the salt composition has a liquidus temperature below 700 C, preferably below
600 C,
more preferably below 500 C. For a given combination of salts, the composition
is
preferably chosen to be within 10 % by weight from the lowest eutectic point
of the salt
combination, more preferably within 5% by weight, most preferably within 1 %
by
weight. However, other contents may be used as long as the liquidus
temperature of the
salt combination is at least 50 C lower than the operating temperature during
electrolyzing; preferably 100 C lower than the operating temperature.
In a preferred embodiment the salt composition essentially consists of by
weight % of
the salt composition, 3-20 NaC1, 30-70 KC1, 20-60 LiC1, preferably 5-15 NaCL,
40-60
KC1, 30-50 LiC1, more preferably 7-12 NaC1, 45-55 KC1, 35-45 LiC1. Such salt
composition can provide low liquidus temperatures (eutectic temperature around
350
C), good electrical conductivity to a comparably low cost.
In an alternative embodiment the salt composition essentially consists of by
weight % of
the salt composition, 10-50 NaC1, 2-20 KC1, 50-80 CaC12 preferably 25-35 NaC1,
3-10
KC1, 60-75 CaC12.
In an another alternative embodiment the salt composition essentially consists
of by
weight % of the salt composition, 5-20 NaC1, 20-40 LiC1, 40-70 CaC12
preferably 7-15
NaC1, 25-35 LiC1, 50-60 CaC12.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
8
In an another alternative embodiment the salt composition essentially consists
of by
weight % of the salt composition, 35-65 KC1, 20-50 LiC1, 5-20 CaC12 preferably
45-55
KC1, 30-40 LiC1, 10-15 CaC12.
Dissolving
To dissolve the cullet, a mixture is preferably provided, which consists of A)
60-95 %
by weight of the mixture of a chloride salt composition consisting of at least
two metal
chlorides selected from the group consisting of alkali metal chlorides and
alkaline earth
metal chlorides, B) 5- 30 % by weight of the mixture of A1C13, and C)
optionally 0-10
% by weight of the mixture of halides, additional chlorides, sulfides and/or
oxides, for
instance but not limited to: Cao, Li20, Na0, MgC12, BaC12. In the most
preferred
embodiment the salt composition consist of NaC1, KC1, and LiC1 and having a
composition around the lowest eutectic point for the NaC1-KC1- LiC1 system.
The
mixture is heated to form a salt melt. The cullet can be added before heating
the mixture
or after the salt melt has been formed. This mixture is heated in a refractory
container
under protective atmosphere, suitably argon, to form a melt. The atmosphere
may also
be nitrogen. Furthermore, chlorine gas may bed admixed to the nitrogen or
argon
atmosphere. To dissolve the cullet, the melt is kept at high temperature
usually for a
time between about 4 and about 8 hours. As a rule, the amount of cullet is
preferably
such that a weight ratio flux/cullet is between about 0.25 and about 1.5, more
preferably
0.30-1.0, most preferably 0.35-0.45. The temperature should be lower than 1000
C,
more preferably lower than 900 C. Otherwise lead chloride may evaporate from
the
melt. For optimal economy the temperature is preferably in the range of 550-
700 C
during the dissolution of the cullet and the electrolysis of the melt, more
preferably 580-
650 C.
For Pb0 the dissolving reaction is:
3Pb0+2A1C13¨> 3PbC12 + A1203 (la)
A flux/cullet ratio of 0.37 appears to be sufficient to dissolve lead oxide in
the salt melt.
For indium the dissolving reaction is:
In203 + 2A1C13 2InC13 + A1203 (lb)
Since the aluminum chloride is difficult to recover after the extraction
process, it is
desirable that there is no excessive addition of aluminum chloride.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
9
To recover at least one of lead and indium from the melt, various processes
that are
known per se may be used. It is interesting to note that the salt melt used
for extraction
can be recycled. The lead and/or indium as well as other metals can be
selectively
electro-deposited from the salt melt. However, it is also possible to use
vaporization of
the metal chlorides and condensing them, or leach the salt phase in water and
extracting
the metals as hydroxides by hydrometallurgy method. The process can be
designed to
be continuous by combining the two steps. The anode off-gas from a subsequent
electrolysis, C12, can be reused for accentuating the dissolution of
slag/ores. The residue
after processing glass consists essentially of A1203 and Si02 and can be used
for
landfill, building construction or as a raw material for the refractory
industry.
Electrolysis using conventional anode/s and cathode/s
The electrolysis preferably is carried out in the refractory container that
holds the salt
melt with the dissolved cullet, and the lead and/or indium and possibly other
metal is
recovered as a cathode deposit.
In one embodiment lead is recovered from Pb0 containing glass. Two electrodes
of
graphite are immersed into the salt melt containing dissolved cullet and
connectable to a
DC source, which can deliver a DC current at a voltage of 2 to 3 V, preferably
2.3-
2.7 V, so that the following reactions occur with respect to lead.
2C1--> C12 (g) + 2e- ........................................................
(2)
Pb2+ + 2e--> Pb (1) ........................................................
(3)
2A13+ + 302--> A1203 (s), ..................................................
(4)
This is around 1 volt higher than the theoretical decomposition voltage for
PbC12 to
compensate for polarization and other effects. The voltage chosen is low
enough to
avoid decomposition of A1C13. The decomposition of A1C13 in the electrolysis
process
also need an over-voltage in regards of the theoretical decomposition voltage
of A1C13.
In this case, 2.4+1=3.4 V. Thus a voltage lower than 3 V avoids electrolytic
decomposition of A1C13.
Indium can be co-deposited with Sn when the indium oxide is indium tin oxide
or thay
may be selectively electrodeposited.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
Preferably the batch of melt is electrolyzed for 2 to 8 hours. During the
electrolysis, the
temperature is preferably held above 500 C, more preferably about 600 C.
For collecting liquid lead, a pervious cathode bag may be provided around the
cathode.
5 The cathode bag suitably is made from alumina and has a plurality of
holes, through
which the ions can pass. The holes may be cuts extending in the
circumferential
direction.
After recovering lead and/or indium and possibly other metals, the chloride
salts of the
10 melt may be recycled. Then, a processing residue consisting essentially
of A1203 and
Si02 remains, which is useful for landfill, building construction, or as a raw
material for
refractory industry. The lead containing glass processed in accordance with
the
invention primarily comes from a funnel-shaped part and/or a neck part of a
cathode ray
tube of a computer screen or a television set.
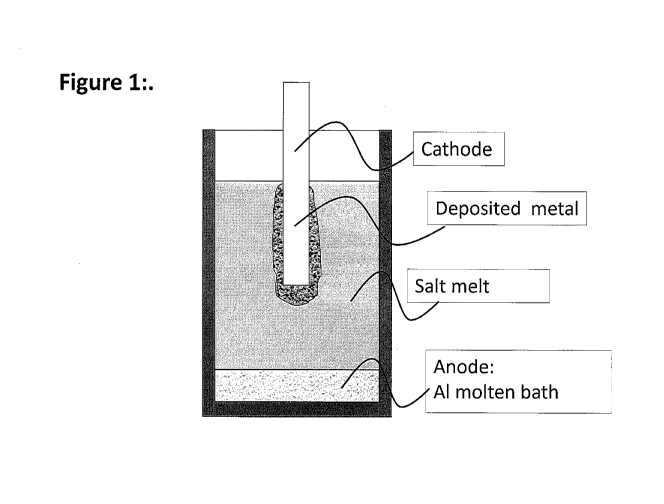
Dissolving and electrolysis using aluminium anode
In an alternative embodiment, an aluminium melt form the anode or a part of
the anode,
for instance by immersing an electrode, e.g. a graphite electrode, in the
aluminium melt
and connecting it to positive polarity during electrolysis. Alternatively, the
crucible is at
least partly made in a conductive material being in contact with the aluminium
melt, and
connecting the crucible positive polarity during the electrolysis. Thereby,
the crucible
and the molten aluminium operate as an anode. Of course at least one cathode
is still
required during electrolysis, e.g. one or more graphite electrode/s submerged
in the salt
melt. voltage
When using an aluminium melt at the bottom of the crucible as the anode or
part of the
anode, the salt melt and the aluminium are heated to a temperature where both
are in
liquid phase. To improve viscosity of the salt melt, the temperature of the
salt melt is
preferably at least 50 C above the liquidus temperature of the salt melt,
more
preferably at least 100 C above the liquidus temperature of the salt melt. The
temperature should be at least 660 C and not more than 1000 C, preferably
the
temperature is in the range of 700-900 C.
During the electrolysis metals/s from metal chloride/s is deposited at the
cathode. At the
contact surface between the salt melt and the aluminium melt chloride ions are
reacting
with aluminium, thereby forming A1C13. This means that during steady state the
salt
melt can be wholly or partly self-supporting in regards of A1C13 and also that
emission
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
11
of chlorine gas is reduced. Lesser amounts of chlorine gas may form even when
using
an aluminium melt as the anode or part of the anode. This gas may be
recovered.
At the cathode metal/s are deposited in solid or liquid state for metal/s with
lower
melting point than the temperature of the salt melt. For collecting liquid
metal, e.g. lead,
a pervious cathode bag may be provided around the cathode. The cathode bag
suitably
is made from alumina and has a plurality of holes, through which the ions can
pass. The
holes may be cuts extending in the circumferential direction.
The composition of the salt melt is preferably the same as when using a
conventional
graphite anode/s.
An initiating chloride donor is provided to start the reactions in the salt
melt. The
initiating chloride donor may be aluminium chloride and/or at least one metal
chloride
that can be electrolyzed, i.e. so that chloride ions forms A1C13 at the
contact surface
between the salt melt and the aluminium melt.
In one embodiment, the initiating chloride donor includes a metal chloride of
the same
type as provided in the chloride salt composition, e.g. at least one metal
chloride
selected from the group consisting of chlorides of Li, Na, K, Rb, Cs, Fr, Be,
Mg, Ca, Sr,
Ba, and Ra.
In a preferred embodiment, the initiating chloride donor includes aluminium
chloride
added to the mixture before heating it or to the salt melt, said aluminium
chloride being
added up to 20 % by weight of the salt mixture, preferably 1-15 % by weight,
more
preferably 5-10% by weight.
When using aluminium melt as the anode or part of the anode the steps
dissolving and
recovering by electrolysis are expedited simultaneously, preferably for at
least 2 hours.
As the indium and/or lead is deposited, additional cullet can be stepwise or
continuously
added to the salt melt. The electrolysis and dissolving operation can for
instance be
performed for 2-8 hours; where after metals deposited at the cathode/s is
collected, and
the electrolysis can be restarted. To avoid interrupts of the electrolysis,
another "clean"
electrode can be submerged. Alternatively, the salt melt may have a plurality
of
electrodes which one after the other is activated as a cathode and while the
former is
deactivated. Thereby the metals can be selectively deposited at individual
electrodes.
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
12
The residue after processing contains A1203 and other stable oxides such as
Si02.
EXAMPLE OF RECOVERY OF LEAD FROM CRT MONITOR
A sample of CRT glass was supplied by East Japan Recycling Systems Corporation
(EJRS). The CRT monitors were taken apart, and some of the parts were
recycled. The
treatment of CRT monitors by EJRS is described in the following.
The monitors were taken apart by hand, and the CRTs were separated. Printed
boards,
deflecting yokes, interconnections and speakers were also taken and recycled.
The
separated CRTs were polished by a brush cleaning equipment, and excrescences
on the
surface of CRT were removed. Then the panel and funnel were separated by a P/F
divider. Since the glass compositions of the panel and funnel are different,
they have to
be recycled separately. The funnel glass was washed and dry-scrubbed to remove
the
paint of carbon and iron oxide on the surface of the glass, and then the
funnel cullet was
produced. This cullet is the raw material of CRT glass.
As indicated above, the glass used in a CRT is of different kinds in the
panel, the funnel
and the neck of a CRT. The panel contains a barium-strontium glass, the weight
of
which is about two-thirds of the CRT monitor's total weight. The funnel is
made of
leaded glass. The weight of it is about one-third of the CRT monitor's total
weight. The
neck is made out of a high lead content glass, which surrounds the electron
gun.
Two different analyses were made of the funnel cullet supplied were made. The
result
of the analyses is shown in Table 1, where the two different analyses are
designated
CRT 1 and CRT 2.
CA 02844047 2014-02-03
WO 2013/025169
PCT/SE2012/050885
13
Table 1, Composition of the funnel glass
(Weight %)
Component Reference CRT 1 CRT 2
Na20 5-7 10.685 7.000
MgO 1-3 1.469 1.363
A1203 3-5 2.177 2.094
Si02 50-53 59.250 60.950
1(20 7-9 10.324 8.490
CaO 3-5 1.721 2.076
FeO 0-0.1 0.156 0.156
Sr0 0-1 0.459 0.445
Zr02 0-1 0.022 0.020
Sb203 0.1-0.5 0.057 0.057
BaO 0-1 0.580 0.564
Pb0 21-24 20.316 18.787
Li20 0-1 0.017 0.017
TiO2 0.3-0.5 0.018 0.017
ZnO 0-1 0.237 0.240
Total 107.485 102.275
The metal chlorides were supplied by Sigma-Aldrich, USA, and the refractory
container
was an alumina crucible (99.5 %, 40 mm outer diameter, 36 mm inner diameter,
60 mm
height, from KERANOVA AB, Sweden) having a lid. The chloride salts (8% by
weight
of NaCL, 53 % by weight of KCL and 39 % by weight of LiC1) and the cullet were
mixed evenly, placed in the crucible and held for a predetermined time in an
oven.
Argon gas (ICP5.0, AGA gas AB, Sweden) was introduced from the bottom of the
furnace as protective gas. Graphite rods (diameter of 6 mm, Lorraine, Paris)
were used
as electrodes and iron rods (6 mm diameter) as lead wires.
During the dissolution process, the electrodes were held 2-4 cm above the salt
melt.
After the salt mixture was melted and held for dissolution at a fixed time,
the electrodes
were immersed in the salt melt, and electrolysis started, supplied by DC power
(HP
Hewlett 6632A). After electrolysis, the electrodes were taken out from the
salt melt,
held above the salt melt and cooled down under the protection of argon gas.
The sample
in the crucible was dissolved in the distilled water completely and filtrated.
The residue
was prepared for chemical analysis. ICP-AES analysis was applied for the
elements Al,
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
14
Fe, Sr, Zr, Sb, Ba, Zn, Pb, Li and Ti. Atomic absorption analysis was applied
for the
elements Na, Mg, Ca and K. The deposition products on the cathode were
analyzed by
Scanning Electron Microscope (SEM) (JSM-840, JEOL) equipped with Energy-
Dispersive X-ray Spectrometry analysis (EDS link, Oxford).
Four factors, viz, holding temperature, holding time, flux content (mole ratio
to NaC1-
KC1-LiC1 mixture) and flux/cullet (weight ratio), were investigated. The
parameters
varied during the tests were temperature (600, 800 and 1000 C), holding time
(4 and 8
hours), flux content (11, 15, 19 and 29 wt%) and flux/cullet (0.37, 0.5, 0.7,
0.9 and 1.5)
were applied. The extraction ratio of various metal oxides in the CRT glass in
the salt
melt is shown in Table 2. In this table, the extraction ratio is defined as
the ratio of
remained oxides in residue versus the amount of corresponding oxides in the
initial
CRT glass.
0
Table 2 Extraction ratio of metal oxides in NaCl-KCl-LiCl system
No. Extraction ratio of metal oxides (%)
Conditions
Na20 MgO A1203 K20 CaO FeO Sr0 Sb203 BaO Pb0 Li20 ZnO Temp
Time Flux Flux/CRT
1 97.7 99.1 -623 94.2 99.6 77.7 96.1 73.2 95.3 96.4 -1242 97.1 0.37
2 98.5 98.8 -122 95.5 99.6 67.7 95.7 84.0
95.6 95.0 -2471 91.2 11 wt% 0.7
3 97.7 99.1 -849 95.5 99.8 73.4 96.7 62.9 96.2 95.1 -1204 79.3 0.9
4 98.5 99.1 -219 93.6 99.8 49.4 93.8 76.8 93.8 94.9 -1755 91.7 0.37
98.5 99.1 -768 96.2 99.4 70.0 95.8 67.0
95.5 96.0 -1724 95.1 15 wt% 0.7
4h
co
6 97.7 99.1 -623 94.2 99.6 77.7 96.1 73.2 95.3 96.4 -1242 97.1 0.9
0
7 98.5 98.8 -122 95.5 99.6 67.7 95.7 84.0
95.6 95.0 -2471 91.2 600 C 0.37
CA
\ )
8 97.7 99.1 -849 95.5 99.8 73.4 96.7 62.9
96.2 95.1 -1204 79.3 19 wt% 0.7 0
9 98.5 97.7 -449 78.8 74.0 73.4 94.4 67.0 94.8 94.6 -1306 98.3 0.9 0
87.0 94.2 -238 51.2 86.7 62.5 91.9 68.3
92.2 93.4 -9537 93.5 29 wt % 0.5 0
UJ
11 98.1 97.5 -352 95.4 94.2 28.1 92.2 66.9
92.5 93.3 -2459 89.0 11 wt%
12 98.5 98.6 -673
97.9 96.9 64.0 96.9 66.6 96.7 96.8 -972 96.6 8 h 19 wt% 0.7
13 98.5 98.5 -584 97.9 96.2 69.2 96.4 67.8
96.5 96.3 -1136 97.3 29 wt %
14 96.7 55.5 -868 85.7 98.1 81.7 72.0 14.4 69.8 60.8 -8018 90.6 0.9 1-d
19 wt%
95.6 63.1 -1015 82.1 95.7 77.0 -4.3 9.4 71.6 64.9 -
7340 92.8 4 h
800 C
1.5
16 97.3 37.4 -439 91.0 94.5 58.9 43.7 -111
42.5 51.9 -8398 88.7 29 wt%
17 96.6 42.0 -799 85.0 86.1 11.4 69.7
-4.0 66.5 53.3 -8322 90.1 8 h
19 wt%
0.9
18 94.4 76.0 -880 73.2 99.2 48.4 95.5 43.5
94.3 90.9 -5238 94.4 1000 C 4 h
cio
cio
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
16
Two methods were used to estimate the amount of extracted oxides. First, the
residue
after dissolution was filtrated, and then subjected to two chemical analyses,
viz. ICP-
AES and atomic absorption analysis. It should be mentioned that this
extraction ratio is
not always equal to the solubility in the salt melt. Some of the oxides can be
evaporated
as chlorides. Extraction ratio of aluminum and lithium oxide shows a negative
value,
which means that the amount of the oxide increased. Almost all of the oxides
show very
high extraction ratio, except iron and antimony oxide. However, iron and
antimony
oxide show an extraction ratio of approximately 70 %. Concerning lead oxide,
the ratio
is about 50-60 % at 800 C, and 91 % at 1000 C. These values are lower than
that
obtained at 600 C. In addition, increases of flux content and flux/cullet
ratio appear not
to contribute to increasing the extraction ratio of lead oxide, since already
low ratios of
flux content and flux/cullet show very high extraction ratios. In the present
experimental
tests, a flux/cullet ratio of 0.37 appears to be sufficient to dissolve lead
oxide in the salt
melt. This value is the theoretical amount to complete reaction (1) above.
The variation of lead oxide extraction ratio due to different process
parameters was
investigated. The extraction ratio of lead oxide at 600 C was approximately
96 % and
did not depend on the holding time. Concerning variation with temperature, the
extraction ratio was about 95, 60 and 91 % at 600, 800 and 1000 C,
respectively. The
ratio seems to be random. However, it can be said that the high temperature
did not
contribute to a rise of the extraction ratio. Further, high flux content did
not give a
significant change in the extraction ratio. Similarly, a change in the
flux/cullet ratio did
not result in any significant change of the extraction ratio of lead oxide
that was
approximately 95 %. Consequently, higher temperature, flux content and
flux/CRT
ratio, longer holding times are not needed. As mentioned above, already lower
values of
the parameters give high extraction ratios, and therefore an increase of
parameter values
does not result in any a rise of the extraction ratio. Based on the results,
the optimized
process parameters were chosen as 600 C of temperature, 0.37 of flux/cullet,
4 hours of
holding time, and 11 % by weight of flux content. In the test at 1000 C the
evaporation
of A1C13 was high. It is therefore preferred to keep the temperature lower
than 1000 C,
preferably lower than 900 C.
The dissolution test proves that the metal values in CRT glass can be
extracted into the
salt phase. Under different cell potentials, various metals or alloys can be
deposited on
the cathode surface. All of the metal chlorides in the melt have a theoretical
decomposition voltage. The decomposition voltages of silicon chloride and lead
chloride are close to each other, which means that it is expected to be
difficult to depose
CA 02844047 2014-02-03
WO 2013/025169 PCT/SE2012/050885
17
the two separated from each other. However, it is very difficult to dissolve
silicon oxide
in the salt melt, and thus silicon tetrachloride does not practically exist in
the melt.
Consequently, it is possible to deposit lead selectively. It should be noted
that the
electro-deposition of metals can be affected by a number of factors even in
pure molten
salt system, as for example, over voltage, current density, current
efficiency, electro
bath conductivity, the nature and surface of the cathode material and distance
between
electrodes, etc.
In the experimental electrolysis process, 600 C of temperature, 0.9 of
flux/cullet, 4
hours of holding time and 19 wt% of flux content were selected because of the
small
size of the crucible. Then, the amount of CRT glass was 9.3 g, which contained
approximately 1.8 g of lead oxide. If 100 % of the lead oxide is dissolved in
the salt
melt and all of the lead is deposited, approximately 1.7 g of lead is
recovered. This
amount of lead is the available maximum value for a small crucible.
As demonstrated above, a salt extraction process has been developed for the
recovery of
lead and other metals from CRT glass. According to the analysis of CRT glass,
the glass
contains approximately 19 % of lead oxide, and approximately 96 % of lead
oxide was
extracted by the dissolution in the salt melt step. The optimized process
parameters in
the dissolution step were (a) a temperature of 600 C, (b) a holding time of 4
hours, (c)
a flux/cullet ratio of 0.37, and (d) a flux content of 11 wt%. Several
electrolysis tests
were performed with the molten salts and CRT glass. A voltage of 2.3 V was
used to
electro-deposit lead. A liquid phase of lead was deposited and dropped from
the cathode
into a cathode bag, where it was collected.
INDUSTRIAL APPLICABILITY
The main concern with the dumping of cathode ray tube (CRT) screens is the
leaching
of lead from the glasses. Lead is used in radiation shielding glasses in order
to absorb
gamma radiation and X-rays, e.g. in the cathode ray tubes used in computer
screens and
television sets, where lowering the exposure of the viewers to soft X-rays is
of concern.
The leached lead is dangerous both for human beings and environment. The
present
invention solves the problem of rendering glass from scrapped CRT funnels
harmless
by recovering lead therefrom. Lead and other metals are present as oxides in
the glass
and are dissolved in a chloride salt melt, from which they are recovered by
electrolysis,
for example. The residue after processing, which consists essentially of A1203
and 5i02,
can be used for landfill, building construction or as a raw material for the
refractory
industry.
CA 02844047 2014-02-03
WO 2013/025169
PCT/SE2012/050885
18
Similarly, indium can be recovered from indium tin oxide coated glass