Note: Descriptions are shown in the official language in which they were submitted.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
METHODS AND APPARATUS FOR THE FABRICATION
AND USE OF GRAPHENE PETAL NANOSHEET STRUCTURES
CROSS REFERENCE TO RELATED APPLICATION
This application claims the benefit of priority to U.S. Provisional Patent
Application Serial No. 61/523,646, filed August 15, 2011, the disclosure of
which is
expressly incorporated herein by reference.
BACKGROUND OF THE INVENTION
Nanostructures have recently been utilized in a variety of biosensing
applications
due to their enhanced surface area, precise biomolecule-electrode connections,
and
enhanced delivery of application agents. In the realm of electrochemical
sensing,
conductive nanostructures immobilized on electrodes enhance electrocatalytic
behavior
due to quantum confinement and may exhibit properties including more favorable
Faradic-to-capacitive current ratios, higher current densities, and faster
mass transport
by convergent diffusion than their larger micro/macro electrode counterparts.
In order to
increase biosensor current output to measurable levels, large arrays of
nanostructures
(i.e., nanoelectrode arrays [NEAs]), have been immobilized on electrode
surfaces.
These NEA biosensors, fabricated with various nanostructures (e.g., nanowires,
nanotubes, and nanocrystals) have shown promising results, displaying high
sensitivities and fast response times.
Recently developed graphene petal nanosheets, with reactive edge planes
similar to
oriented pyrolytic graphite (HOPG) or vertically oriented CNTs, can be grown
directly on
1
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
a variety of surfaces without the need for metal catalysts¨creating a
nanostructured
surface well suited for integration into numerous electrochemical sensing
applications.
Various biofunctionalization techniques have been developed to immobilize
biorecognition agents onto electrode surfaces including covalent binding
through self-
assembled monolayers (SAMs), non-covalent membranes, and electrodeposition
with
conductive polymers. Each biofunctionalization technique has advantages. Self-
assembled monolayers provide a covalent link to the biorecognition agent and
electrode
surface. Non-covalent membranes can be rapidly assembled on electrode
surfaces.
Poly(3,4-ethylenedioxythiophene) (PEDOT and sometimes referred to as PEDT) is
an
electrically conductive polymeric material that can be utilized in biosensor
interfaces due
to its biocompatibility, stability, and high conductivity. Mixtures of the
monomer 3,4-
ethylenedioxythiophene (EDOT) and Poly(styrene-sulfonate) (PSS) are soluble in
aqueous environments and can be controllably electrodeposited onto conductive
surfaces. Furthermore PEDOT displays high stability with aqueous electrolytes.
This
high electrochemical stability, owing to inherent dioxyethylene bridging
groups, makes
PEDOT well suited for enzyme immobilization.
Water soluble molecules can also be incorporated into the PEDOT matrix during
electro-polymerization. PEDOT has been used as an enzyme immobilization matrix
for
use in glucose and cholesterol amperometric biosensing applications.
Carbon nanomaterials (e.g., carbon nanotubes, nanospheres, nanohorns,
nanoplates, nanoparticles) have attracted considerable research attention due
to their
unique properties and potential applications. Transition metals such as Fe and
Ni have
been traditionally viewed as important catalysts for sp2 carbon growth since
they enable
2
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
rapid dissociation of carbon-rich molecules to form metal¨carbon alloys that
precipitate
carbon through a vapor¨liquid¨solid mechanism. Two dimensional graphene in the
form
of single-layer graphene (SLG) or few layer graphene (FLG) has been the
particular
focus of much recent research because of its unique electronic properties.
In contrast to the production of conformal sheets of SLG or FLG, small
crystalline
graphitic petals (GPs), or carbon nanowalls (or nanosheets) containing a few
layers of
graphene have interesting industrial applications because they grow roughly
perpendicular to a substrate and dramatically increase the surface area from
which they
grow. The GPs are thin, containing only a few graphitic layers, and can be
catalyst free,
suggesting they might be a source of free-standing graphitic material. Various
methods
have been reported to grow GPs in the past decade, among which microwave
plasma-
enhanced chemical vapor deposition (MPCVD) is particularly common. GPs can be
used for field emission enhancement, hydrogen storage, sensors, nano-
composites and
as a growth template for nanostructures of different materials.
In order to satisfy the requirements of today's increasingly multifunctional
portable electronic devices, sustainable and renewable power sources, such as
supercapacitors and batteries, are designed and fabricated in the trend of
being small,
thin, lightweight, environmentally friendly and even flexible. Electrochemical
capacitors
(ECs), also known as supercapacitors or ultracapacitors, with the merits of
high power
density, fast power delivery or uptake and excellent cycle stability, have
become some
of the most promising candidates for next-generation high-performance power
devices.
Due to high theoretical capacities, electrically conducting polymers (ECPs),
such
as polyaniline (PANI), polypyrrole (PPy), and polythiophene (PTP), are
commonly used
3
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
as pseudo-capacitive materials to further increase the energy and power
density.
Among them, PANI gains particular interests in the past 30 years because of
its high
theoretical specific capacitance (2000 F/g), high degree of processability and
chemical
stability in air, as well as its fairly high conductivity and favorable
electrochemical cycling
characteristics. In addition, PANI can also be synthesized in different
morphologies
(e.g., films, nanofibers, arrays) on different substrates. Despite of the high
theoretical
specific capacitance, ref. indicates that the current experimental value is
far less than
the theoretical one, because of the limited mass transport rates of anions and
relatively
low PANI conductivities. Therefore, it is essential to coat PANI on templates
with a high
specific surface area to fully exploit its electrochemical capacitive
properties. Various
porous carbon materials (e.g., carbon cloth, activated carbon, mesoporous
carbon, and
carbon nanotubes) were used as conductive templates.
Graphene, a new member of carbon nanomaterials with unique properties, was
also combined with PANI to fabricate composites by in situ chemical or
electrochemical
polymerization, and self-assembly. In the most of the previous work, reduced
graphene
oxide was used as templates or supports for PANI nanostructures. Free-standing
chemically converted graphene/ PANI nanofiber paper-like composite was
synthesized
through vacuum filtration of suspensions of the two components. The composite
shows
a specific capacitance of 210 F/g and 160 F/cm3 but with a poor cycling life
(21`)/0 loss at
3 Ng after 800 cycles). Reduced graphene nanosheets/PANI composite was
synthesized using in situ polymerization in the graphene nanosheet suspension
and a
specific capacitance of 1046 F/g (based on GNS/PANI composite) was obtained at
a
scan rate of 1 mV/s. However, the specific capacitance shows a significant
loss at 100
4
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
mV/s (-50 %) compared with that at 1 mV/s in the presence of conducting agent
and
binding materials.
Graphene nanosheets (nanowalls), or graphitic petals (GPs), containing a few
layers of graphene and growing roughly perpendicularly to a substrate over a
large
surface area, are the ideal candidates for electrochemcial energy storage
applications,
due to high specific area and high electrical conductivity. They were
previously
synthesized on different substrates, such as Ni foil and carbon cloth, for
electrochemical
energy storage application. The unique sharp edges of GPs greatly increase
charge
storage as compared with that of designs that rely on basal plane surfaces.
Density
functional theory analysis shows the presence of these edges affects not only
the
reactivity of the carbon material toward the adsorption of Li atoms but also
their diffusion
properties. Up to date, utilization of this highly conductive and unique GP
structure as a
nanotemplate to further exploit the electrochemical properties of the
pseudocapacitive
materials (e.g., conducting polymer) has rarely been reported, not to mention
the
applications of these composite electrodes in flexible two-terminal devices.
While in the application level of supercapacitors, all-solid-state and
flexible
supercapacitor devices, based on polymer gel electrolyte, have recently
aroused
particular interests in this research field because of their obvious
advantages in
environmental friendliness, flexibility, cost and versatility in comparison
with many
currently employed counterparts. The advantages of paper-like supercapacitors
in
structure design over conventional supercapacitor device configuration (a
separator
sandwiched between two electrodes sealed in liquid electrolyte) have been well
5
CA 02845539 2014-02-14
WO 2013/066474 PCT/US2012/051008
addressed. However, the specific capacitance and high power properties of the
former
flexible solid-state devices still needs to be further improved.
6
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
SUMMARY OF THE INVENTION
One aspect of the present invention pertains to an apparatus including a
substrate
having a surface. Other embodiments include a plurality of carbon mounds
located on the
surface. Still other embodiments pertain to a plurality of graphitic petals,
each petal growing
from a corresponding one of said mounds.
Another aspect of the present invention pertains to a method for depositing
carbon on
a surface. Some embodiments include providing a substrate having an outer
surface. Yet
other embodiments include depositing carbon on a roughened outer surface.
Still other
embodiments include growing a graphitic petal from the carbon on the roughened
surface.
Yet another aspect of the present embodiment pertains to a method for
depositing
carbon on a surface. Some embodiments include providing a substrate having a
first layer
of a first material on top of a second layer of a second material, the first
layer having an
outer surface. Other embodiments include diffusing a third gaseous material
through the
first layer. Yet other embodiments exposing the substrate during said
diffusing to an
electrical field. Still other embodiments include depositing a fourth material
containing
carbon on the outer surface.
Another aspect of the present embodiment pertains to a biosensor. Some
embodiments include an electrode. Yet other embodiments include a silica based
wafer, multilayered petal nanosheets supported by the wafer, and platinum
nanoparticles supported by the nanosheets. Still other embodiments include an
enzyme and poly(3,4-ethylenedioxythiophene) electrodeposited on the electrode.
Still another aspect of the present invention pertains to a method of
producing a
biosensor. Some embodiments include providing an electrode comprising a silica
7
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
based wafer, petal nanosheets supported by the wafer, and electrodepositing
platinum
nanoparticles on the nanosheets. Still other embodiments include
electrodepositing an
enzyme and poly(3,4-ethylenedioxythiophene) on the electrode.
Yet another aspect of the present invention pertains to a supercapacitor. Some
embodiments include a carbon nanotube substrate. Yet other embodiments include
graphitic petal structure supported by the substrate, and manganese dioxide
supported
by the graphitic petal structure.
Factors influencing the formation and structure of graphitic petals grown by
microwave plasma-enhanced chemical vapor deposition on oxidized silicon
substrates
are investigated through process variation and materials analysis. Unlike the
spatially
homogeneous growth mechanisms reported elsewhere, some graphitic petals are
found
to grow at an accelerated rate, often growing ¨20 times faster than other
petals located
only a fraction of a micrometer away. Using scanning electron microscopy and
atomic
force microscopy, the rapid growth rate of these fast-growing petals is
attributed to the
formation of nanoscale cones. Electron energy loss spectroscopy reveals that
the
formation of these nanoscale cones is associated with a localized roughening
of the
oxidized silicon substrate. Raman spectroscopy and transmission electron
microscopy
are used to confirm the graphitic nature of the as-grown petals. Also, a
simple scribing
method can be used to control both the location and formation of petals on
flat Si
substrates.
It will be appreciated that the various apparatus and methods described in
this
summary section, as well as elsewhere in this application, can be expressed as
a large
number of different combinations and subcombinations. All such useful, novel,
and
8
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
inventive combinations and subcombinations are contemplated herein, it being
recognized that the explicit expression of each of these combinations is
unnecessary.
9
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
BRIEF DESCRIPTION OF THE DRAWINGS
Some of the figures shown herein may include dimensions. Further, some of the
figures shown herein may have been created from scaled drawings or from
photographs
that are scalable. It is understood that such dimensions, or the relative
scaling within a
figure, are by way of example, and not to be construed as limiting.
FIGs. 1-la through 1-1c illustrate characterization of the graphene petal
nanosheets (GPN) electrodes before and after exposure to an oxygen plasma
etch.
FIG. 1-la illustrates field emission scanning electron microscopy (FESEM)
micrographs of a GPN electrode grown by microwave plasma chemical vapor
deposition (MPCVD) on a Titanium (Ti) coated silicon substrate. Inset shows a
magnified view.
FIG. 1-lb discloses raman spectra of the GPNs displaying an intensity increase
in the D peak after 02 plasma etch.
FIG. 1-1c shows cyclic voltammograms created by scanning the potential
between -0.2 V and 0.6 Vat a scan rate of 100 mV/s versus a Ag/AgCI reference
electrode in 4 mM Fe(CN)63- and 1 M KNO3.
FIG. 1-1d displays a hydrogen peroxide (H202) calibration plot displaying
amperometric sensing of H202oxidation in 20 mL of phosphate buffered saline
(PBS)
(pH 7.4) via a 3-electrode electrochemical set-up with a working potential of
500 mV.
Incremental H202 concentration increases of 10 pM are injected into the test
vial while a
working potential of 500 mV is applied between the GPN and Pt auxiliary
electrodes.
Inset portrays a bar graph of H202 sensitivity of the GPN electrode before and
after the
oxygen plasma etch.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIGs. 1-2a through 1-2f illustrate characterization of the platinum
nanoparticle
modified graphene petal nanosheet (PtNP-GPN) electrodes before enzyme
immobilization.
FIGS. 1-2a through 1-2e illustrate field emission scanning electron microscopy
FIG. 1-2a illustrates FESEM micrographs of PtNPs electrodeposited on GPNs
with current pulses (500 ms) of 312 pA) used to electrodeposit Pt
nanoparticles of
distinct size and density onto the GPNs.
FIG. 1-2b illustrates FESEM micrographs of PtNPs electrodeposited on GPNs
FIG. 1-2c illustrates FESEM micrographs of PtNPs electrodeposited on GPNs
with current pulses (500 ms) of 1.25 mA) used to electrodeposit Pt
nanoparticles of
distinct size and density onto the GPNs.
15
FIG. 1-2d illustrates FESEM micrographs of PtNPs electrodeposited on GPNs
with current pulses (500 ms) of 2.5 mA used to electrodeposit Pt nanoparticles
of
distinct size and density onto the GPNs.
FIG. 1-2e illustrates FESEM micrographs of PtNPs electrodeposited on GPNs
with current pulses (500 ms) of 5.0 mA used to electrodeposit Pt nanoparticles
of
FIG. 1-2f shows a bar graph displaying the H202 sensitivity of the GPN
electrode
before and after oxygen plasma etch and the PtNP-GPN electrodes. Errors bars
show
standard deviation for 3 different experiments.
11
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 1-3a illustrates a tilted cross-sectional schematic illustrating the
G0x/PEDOT biofunctionalized PtNP-GPN glucose biosensor with adjacent magnified
view portrayal of GOx immobilized on a single PtNP. Glucose binds within the
GOx
enzymatic pocket producing H202 while consuming 02.
FIG. 1-3b displays glucose calibration plots of the Pt-GPN biosensors. Pt
electrodeposition current pulses of 312pA, 625 pA, 1.25 mA, 2.5 mA, and 5.0 mA
portray the dynamic current response for a glucose concentration range of 5 ¨
60 mM
by 5mM aliquots.
FIG. 1-3c shows linear glucose sensing range with linear regression analysis
and
coefficient of determination (R2) corresponding to FIG. 3b.
FIG. 1-3d discloses glucose calibration plots for a glucose concentration
range of
approximately 0.01 mM to approximately 26.65 mM: (1) by incremental glucose
concentration steps of 10 pM within the glucose concentration range of 10-50
pM , (2)
by incremental glucose concentration steps of 100 pM within the glucose
concentration
range of 100-500 pM, (3) by incremental glucose concentration steps of 1 mM
within the
glucose concentration range of 1-5 mM, (4) by an incremental glucose
concentration
step of 2.5 mM within the glucose concentration range of 5-7.5 mM, (5) by an
incremental glucose concentration step of 5 mM within the glucose
concentration range
of 7.5-17.5 mM, and (5) by a glucose concentration step of 10 mM above the
glucose
concentration of 17.5 mM.
FIG. 1-3e shows linear glucose sensing range and coefficient of determination
(R2) corresponding to FIG. 3d.
12
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 1-4a illustrates glucose sensing ranges of the Pt-GPN glucose biosensors.
Pt electrodeposition current pulses of 312pA, 625 pA, 1.25 mA, 2.5 mA, and 5.0
mA are
compared to glucose levels found in urine, blood, tears, and saliva.
FIG. 1-4b displays biosensor lifetime measurements where the glucose
sensitivity for each distinct Pt-GPN glucose biosensor was monitored over a
period of 5
weeks.
FIG. 1-4c discloses selectivity test demonstrating minimal interference from
100
pM aliquots of uric acid (UA), acetaminophen (AP), and ascorbic acid (AA) and
successful detection of glucose (5 mM) within the backdrop of said
electroactive,
interfering species for the Pt-MGPN glucose biosensor (Pt electrodeposition of
2.5 mA).
FIG. 2-1. Schematic diagram of the MPCVD chamber illustrating the approximate
location of the oxidized silicon substrate with respect to the plasma.
FIG. 2-2. Surface morphologies of etched 5i02/Si after the hydrogen plasma
etching before GP growth. (a) 5i02/Si boundary showing an advancing etch front
(arrow
indicated). (b) A magnified image of the etch front.
FIG. 2-3. AFM images of a micrometer-size EOS feature on the etched 5i02/Si
substrate. (a) Top-view (b) 3-dimensional, perspective view. Trenches or
fissures in the
EOS feature are apparent.
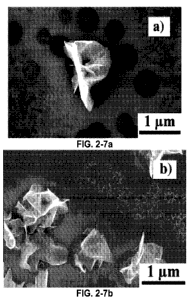
FIG. 2-7. Top-view FESEM images of GPs grown for 15min in a plasma power of
700 W. (a) Top-view of a cluster of ¨12 nanoscale cones. One nanocone supports
the
growth of a GP which resembles the letter 'P'. (b) A region of the substrate
where
smaller GPs are found in close proximity to cones where larger GPs emerge. (c)
and (d)
The nucleation and growth of GPs with a distribution of sizes from the same
nanocone.
13
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 2-8. Raman spectra of GPs prepared for 1 min, 5 min and 15 min.
FIG. 2-9. (a) A TEM image of an as-grown GP. (b) A high-resolution TEM image
of the GP.
FIG. 2-10. GP growth for different durations (a) 1 min of growth, when carbon
deposits form nanoislands across the surface of the substrate, (b) 7 min of
growth,
when large GPs emerge from nanoscale cones, (c) 10 min of growth, when the co-
existence of the smaller and larger GPs appears and (d) 20 min of growth, when
a full
coverage of GPs fills the surface of the substrate.
FIG. 2-11. Top-view FESEM images of GPs prepared on the oxidized silicon
substrates at (a) 700 W, (b) 300W both for 7 min.
FIG. 2-12. Controlled formation of nanocones and GPs by a simple scratch on a
silicon substrate with a 500-nm-thick oxide layer. (a) A low magnification
image
illustrating three scratched lines. (b) A top-view high magnification image of
the boxed
region of (a). The confined growth of GP is evident. (c) Evidence for nanocone
growth.
(d) A cross-sectional SEM image of a substrate cleaved perpendicular to a
scratched
line. The image shows a reduced oxide layer thickness, the presence of
nanocones,
and the growth of GPs.
FIG. 3-1. Schematic illustration of CC/GPs/PANI nanostructures as high-
performance EC electrodes. The synthesis process involves two steps: (I)
uniform and
large-area coverage of GPs on highly conductive CC substrate by MPCVD method;
(II)
Controlled and conformal PANI nanoscale thin layer coating on CC/GP substrates
by
electropolymerization method.
14
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 3-2. Structural characterization of CC/GPs/PANI hybrid composite. (A) SEM
images of pure carbon cloth (inset, lower magnification); (B) SEM images of a
fully GP
covered CC substrate. (inset, a high magnification of GPs) (C) A high
magnification of
conformal PANI coating on GP surfaces. (D) Raman characterization of pure CC,
CC/GPs and CC/GPs/PANI.
FIG. 3-3. Electrochemical performances of the CC/GPs/PANI electrodes. (A)
Both the mass specific capacitance and area-normalized specific capacitance as
a
function of electrochemical polymerization time at 2 mV/s for CC/GPs/PANI
electrode.
(B) CV curves of the hybrid CC/GPs/PANI composite electrode (5 min of PANI
electropolymerization) at different scan rates of 2, 5, 10, 20,50 and 100 mV/s
with
potential windows ranging from 0 to 0.8 V vs. Ag/ AgCI in 1 M H2504 aqueous
electrolyte. (C) The comparison of area-normalized specific capacitance of
Pure CC,
CC/GPs, CC/PANI and CC/GPs/PANI at different scan rates. (D) The comparison of
mass specific capacitance for both pure CC and CC/GP substrates.
FIG. 3-4. Galvanostatic constant-current charge/discharge performance of
CC/GPs/PANI hybrid composite electrode. (A) Galvanostatic constant-current
charge/discharge performances are evaluated for the CC/GPs/PANI hybrid
electrode at
different constant-current densities. (B) Specific capacitances of the
CC/GPs/PANI
hybrid electrode at different constant-current densities. (C) Ragone plot of
the estimated
specific energy and specific power at various charge/discharge rates (current
densities).
The dashed line region for electrochemical capacitors was cited from previous
references. (D) Charge/discharge cycling test at the current density of 10
mA/cm2,
showing ¨7% loss in capacitance after 2000 cycles.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 3-5. Electrochemcial performances of the two-terminal highly flexible
supercapacitors. (A) Schematic illustration of all-solid state highly flexible
CC/GPs/PANI
supercapacitors based on PVA-H2504 polymer gel electrolyte. (B) Galvanostatic
charge/discharge performances of as-prepared all-solid-state supercapacitors.
(C)
Comparison of the specific energy and power density (per cm3 of stack) of
typical
electrolytic capacitors, batteries, commercial supercapacitors and as-prepared
devices
in a Ragone plot. (D) Charge/discharge cycling test at the current density of
5 mA/cm2,
showing ¨10% loss after 1000 cycles. (E) CV curves at 5 mV/s for the
supercapacitor
group from 0 V to 2.5 V in both normal and bent conditions. The overlapping CV
curves
of the two situations indicate the excellent mechanical properties of the
device under
flexible testing conditions. (F) Digital pictures that show three highly
flexible devices in
series, wrapped around a glass rod (inset), to light a green light-emitting-
diode well.
FIG. 4-1. SEM images of (a) graphitic petals. (b) A magnified image of
graphitic
petals showing smooth surfaces. (c) Mn02 coated on graphitic petals. (d) A
magnified
image of uniform Mn02 coating on graphitic petals.
FIG. 4-2. (a) Cyclic voltammetry curves of the Mn02/GP/BP composites at
different scan rates in 1 M Na2504 aqueous electrolyte. (b) Cyclic voltammetry
curves of
BP, GP/BP, Mn02/BP, and Mn02/GP/BP at 10 mV/s. (c) Specific capacitances of
Mn02/GP/BP (black), Mn02/BP (red), GP/BP (dark cyan) and BP (blue) at
different scan
rates. (d) Charge/discharge curve of Mn02/GP/BP at different current
densities. (e)
Ragone plot of the estimated specific energy and specific power at various
current
densities. (f) Capacity retention of Mn02/GP/BP as a function of cycle number.
16
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 4-3. (a) Schematic diagram of Mn02 clusters and graphene (top view); (b)
Electronic density of states under compressive/tensile stresses (c) The
comparative
electronic density of states of graphene, Mn02 and Mn02/graphene; (d) Iso-
electronic
charge contour plot shown at a particular plane (indicated by the yellow line
from the top
view in (a), perpendicular to the graphene plane and along a zig-zag
direction) with
electronic charge distribution at Mn02/graphene interface.
FIG. 5-2 A uniform and large-area coverage of GPs on flexible CC substrates.
FIG. 5-3 SEM morphology of PANI coated on CC/GPs for different
electropolymerization time: (a) 5 min, (b) 10 min, and (c) 20 min.
FIG. 5-4 Current vs. time during PANI electropolymerization process for both
pure CC and CC/GP substrate.
FIG. 5-5 (A) Galvanostatic constant-current charge/discharge curves of the
CC/GPs/PANI electrode at higher current densities. (B) IR drop of the
CC/GPs/PANI
electrode in 1 M H2504 electrolyte.
FIG. 5-6 (A) CV curves at 5 mV/s of a single flexible supercapacitor device
base
on CC/GPs/PANI electrode with polymer gel as electrolyte; (B) normal (C) bent
and (D)
twisted conditions.
FIG. 6-3.1. FESEM images of nanoscale cones observed in the middle regions of
the substrate after a growth time of 15 min for a plasma power of 700 W. (a)
Side-view;
(b) top-view
FIG. 6-3.2. High resolution TEM and EELS characterization of a thin slice cut
from a nanocone. (a) A bright field TEM image of a thin slice taken across a
nanocone.
Elemental mapping shows the spatial distribution of mapped elements. (b) ¨ (d)
Silicon,
17
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
oxygen, and carbon maps, respectively. (e) HRTEM image of the Si02/C interface
of the
nanocone indicates the graphitic nature of the C layers with an interlayer
distance of
0.35 nm. The dark spots in the image correspond to a Pt protection layer
deposited
during sample preparation.
FIG. 6-3.3. Side-view FESEM images of GPs grown for 15 min in a plasma with a
power of 700 W. (a) Nanoscale cones at low magnification that illustrate the
localized,
rapid growth of a few GPs as well as smaller, surroundings GPs which grow at a
considerable slower rate. (b) An FESEM image of a large GP emerging radially
from a
single nanoscale cone. (c) A GP growing in the shape of a nano horn. (d) A
large, thin
GP emerging from a nanocone.
FIG. 6-3.6. SEM images of (a) graphitic petals. (b) A magnified image of
graphitic
petals showing smooth surfaces. (c) Mn02 coated on graphitic petals. (d) A
magnified
image of uniform Mn02 coating on graphitic petals.
FIG. 6-3.7. (a) Cyclic voltammetry curves of the Mn02/GP/BP composites at
different scan rates in 1 M Na2504 aqueous electrolyte. (b) Charge/discharge
curve of
Mn02/GP/BP at different current densities. (c) Specific capacitances of
Mn02/GP/BP
(black), Mn02/BP (red), GP/BP (dark cyan) and BP (blue) at different scan
rates. (d)
Ragone plot of the estimated specific energy and specific power at various
current
densities. (f) Capacity retention of Mn02/GP/BP as a function of cycle number.
FIG. 6-3.9. (A) SEM images of pure carbon cloth (inset, lower magnification);
(B)
SEM images of a fully GP covered CC substrate. (inset, a high magnification of
GPs)
(C) A high magnification of conformal PANI coating on GP surfaces. (D) Raman
characterization of pure CC, CC/GPs and CC/GPs/PANI.
18
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 6-3.10. (A) The comparison of area-normalized specific capacitance of
Pure
CC, CC/GPs, CC/PANI and CC/GPs/PANI at different scan rates. (B) The
comparison
of mass specific capacitance for both pure CC and CC/GP substrates. (C) Ragone
plot
of the estimated specific energy and specific power at various
charge/discharge rates
(current densities). The dashed line region for electrochemical capacitors was
cited from
previous references. (D) Charge/discharge cycling test at the current density
of 10
mA/cm2, showing approximately 7% loss after 2000 cycles.
FIG. 6-4.1. (A) SEM image of etched GP electrode patterns; (B) A higher
magnification of the GP electrodes coated with Ti/Au; (C) A low-magnification
SEM
image (side view) and a higher magnification of the GP patterned electrodes,
indicating
that the electrode has a uniform thickness of 4 micro-meter. (D) The
boundaries of GP
layers nesting on each other are marked (see red dots), demonstrating the 3D
structures and sharp edges.
FIG. 6-4.3. Electrochemical characterization of GP-based micro-
supercapacitors.
(A) Specific capacitances vs. scan rates before electrochemical oxidation. (B)
Specific
capacitances vs. scan rates after electrochemical oxidation for 30 min. (C)
Ragone plots
of Ragone plot energy density vs. power density for as-prepared micro-
supercapacitors
in aqueous electrolytes and the up-to-date reported values of different
electrode
materials in organic electrolytes. (D) Cyclic stability of as-prepared GP-
based micro-
supercapacitors.
FIG. 6-4.4. Schematic of growth process of CNT/GP patterns for micro-
supercapacitor application.
19
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 6-4.5. SEM characterization of CNT/GP structures on Si/Si02 substrates.
(A) SEM characterization of interdigitated CNT/GP patterned electrodes for
micro-
supercapacitors at a low magnification. (B) A tilted SEM image of a CNT/GP
electrode.
(C) A top view of CNT/GP electrode. (D) A side view of CNT/GP electrode.
FIG. 6-4.6. Electrochemical Characterization of CNT/GP micro-supercapacitors.
(A) shows the cyclic voltammetry curves of patterned CNT/GP electrodes at
different
scan rates. (B) charge/discharge curves of CNT/GP-CNT/GP electrodes at
different
current densities (C) Comparative CV curves of micro-supercapacitors based on
CNT-
CNT and CNT/GP-CNT/GP electrodes at a scan rate of 20 mV/s. (D Comparative CV
curves of CNT and CNT/GP electrodes at a scan rate of 20 mV/s in a three-
electrode
system.
FIG. 6-4.7. Free-standing GP foam after removing Ni foam. (a)-(c) optical
image
of free-standing GP foam. (d) and (e) SEM images of GP foam at a low and high
magnification.
FIG. 6-4.8. (a) CC micro-conduits on carbon cloth at a lower magnification.
(b) A
CNT micro-conduit at higher magnification.
FIG. 6-4.9. (a) CNT/GP micro-conduit on CC at a low magnification. (b) A
CNT/GP micro-conduit with a heart shape. (c) A CNT/GP micro-conduit at higher
magnification. (d) CNT/GP at a high magnification.
FIG. 6-4.10. Cyclic voltammetry characterization of CNT/GP micro-conduit
electrodes.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
DESCRIPTION OF THE PREFERRED EMBODIMENT
For the purposes of promoting an understanding of the principles of the
invention, reference will now be made to the embodiments illustrated in the
drawings
and specific language will be used to describe the same. It will nevertheless
be
understood that no limitation of the scope of the invention is thereby
intended, such
alterations and further modifications in the illustrated device, and such
further
applications of the principles of the invention as illustrated therein being
contemplated
as would normally occur to one skilled in the art to which the invention
relates. At least
one embodiment of the present invention will be described and shown, and this
application may show and/or describe other embodiments of the present
invention. It is
understood that any reference to "the invention" is a reference to an
embodiment of a
family of inventions, with no single embodiment including an apparatus,
process, or
composition that should be included in all embodiments, unless otherwise
stated.
Further, although there may be discussion with regards to "advantages"
provided by
some embodiments of the present invention, it is understood that yet other
embodiments may not include those same advantages, or may include yet
different
advantages. Any advantages described herein are not to be construed as
limiting to
any of the claims.
Although various specific quantities (spatial dimensions, temperatures,
pressures, times, force, resistance, current, voltage, concentrations,
wavelengths,
frequencies, heat transfer coefficients, dimensionless parameters, etc.) may
be stated
herein, such specific quantities are presented as examples only, and further,
unless
otherwise noted, are approximate values, and should be considered as if the
word
21
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
"about" prefaced each quantity. Further, with discussion pertaining to a
specific
composition of matter, that description is by example only, and does not limit
the
applicability of other species of that composition, nor does it limit the
applicability of
other compositions unrelated to the cited composition.
What will be shown and described herein, along with various embodiments of the
present invention, is discussion of one or more tests that were performed. It
is
understood that such examples are by way of examples only, and are not to be
construed as being limitations on any embodiment of the present invention.
One embodiment of the present invention pertains to a nanostructured biosensor
that addresses some of the limitations that nanoelectrode array biosensors
currently
face. In lieu of lithography/etch back fabrication techniques, porous
templates, or metal
catalyst driven carbon nanotube arrays, one embodiment includes the growth of
multilayered graphene petal nanosheets (GPNs) on a silicon wafer through a
chemical
vapor deposition technique. The GPNs act as a conductive template for
subsequent Pt
nanoparticle electrodeposition. An electrodeposition process is used to grow
platinum
nanoparticles (PtNPs) along the graphene petal edges and planes to enhance
electrochemical performance. The size and density of the PtNPs are manipulated
to
improve the biosensor sensitivity and dynamic sensing range. A sensor
biofunctionalization protocol is used to electrodeposit an enzyme with the
electrically
conductive polymer Poly(3,4-ethylenedioxythiophene) (PEDOT) onto the electrode
surface. In order to benchmark the performance against other biosensors, the
enzyme
glucose oxidase (G0x) (perhaps the most widely studied enzymatic biosensing
paradigm since its inception from Clark and Lyons in 1962), is encapsulated
within the
22
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
PEDOT matrix for subsequent amperometric glucose sensing. The optimized PtNP-
GPN glucose biosensor performance proves to be exemplary with strong glucose
sensitivity even after 5 weeks of use, minimal interference from endogenous
electroactive species (i.e., ascorbic acid, uric acid, and acetaminophen)
typically found
in human serum samples, and a low detection limit and wide linear sensing
range that
improves upon the performance of glucose biosensors previously reported in the
literature.
In one embodiment a monolithic layer of GPNs were grown across a Ti coated
silicon substrate through a microwave plasma chemical vapor deposition
technique. The
petals grow across the surface of the electrode¨protruding a distance of
approximately
500 nm from the surface (FIG. 1-1). The 1 ¨10 nm thickness of the petals (as
measured by a Veeco atomic force microscope) is consistent with previous
reported
morphologies corresponding to 5 ¨ 25 graphene layers. In an effort to increase
the
electroactive nature of the GPN electrodes and improve subsequent PtNP
deposition,
the GPN electrodes were exposed to a 30 second oxygen plasma etch. The effects
of
this etching process were characterized with Raman spectroscopy, ferricyanide
cyclic
voltammetry, and amperometric hydrogen peroxide (H202) sensing. Improvements
can
be attributed to the 02 plasma etch generating defects and oxygenated species
on the
superficial graphene layers. The generated defects render the graphene surface
more
electroactive than the untreated, superficial basal planes, while newly formed
oxygenated species alter the electrode nature from hydrophobic to hydrophilic¨
enhancing the ability of electrolyte to impregnate the carbon surface.
23
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
The Raman spectra of the GPNs before and after the oxygen plasma etch is
shown in FIG. 1-1b. The Raman spectra display a D band near 1350 cm-1, a G
band
near 1580 cm-1, and a 2D band near 2700 cm-1. The D peak, which is a disorder
induced peak, arises only in the presence of defects. The peak intensity ratio
(both ID/IG
and 12D/IG) and the shape and full width at half maximum (FWHM) of the 2D peak
have
been used to characterize single and few layer graphene. The relative
intensity of the G
peak and the 2D peak ( I2D/IG ¨ 0.5) and the FWHM of the 2D peak [FWHM(2D) =
64]
indicate that the petals are likely made up of only a few layers of graphene
sheets. The
oxygen plasma etch results in an increase in the ID/IG ratio, (from 0.17 to
0.48), thus
indicating increased defects in the plasma treated GPN. These defects created
through
plasma etching assist in subsequent nanoparticle deposition by serving as
nanoparticle
nucleation sites.
The heterogeneous electron transfer (ET) rate of carbon based electrodes is
highly dependent upon electrode surface structure. In the case of sp2
hybridized carbon
in graphene, the rate of ET is enhanced at exposed edge planes or defect sites
in lieu of
the basal plane surface. In order to quantify the ET rate of the GPN
electrodes,
ferricyanide cyclic voltammetry before and after the oxygen plasma etch was
performed
while the separation between the anodic and cathodic peak currents (EE) was
measured (FIG. 1-1c). As illustrated in FIG. 1-1c, cyclic voltammetry
measurements
were taken by immersing the electrodes in 4mM Fe(CN)63- and 1 M KNO3 and
scanning
the potential between -0.2 V and +0.6 V at a scan rate of 100 mV/s versus a
Ag/AgCI
reference electrode. The AEp values for these scan rates fall between 110 and
135 mV
which are a marked improvement to the electron transfer kinetics of
ferricyanide for
24
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
basal plane pyrolytic graphite electrodes (EEp = 360 - 596) that have been
exposed to
air for over 30 minutes and compare quite similarly to the AEp values reported
for edge
plane pyrolytic MWCNTs (EEp = 109 ¨ 137) held under similar conditions. Thus
the
GPN electrodes exhibit an ET rate that is well suited for electrochemical
sensing.
Furthermore, the peak anodic current ('Pa) more than doubles from 0.28 0.5
mA to
0.58 0.5 after the plasma 02 etch¨indicating an enhancement in the electro-
reactivity
of the electrode.
Finally the GPN electrodes were electrochemically characterized by testing
their
sensitivity to hydrogen peroxide (H202)¨the measurable electroactive species
byproduct of oxidase enzymes including G0x. Amperometric H202 testing was
first
performed via a 3 electrode set-up, were the GPN electrode was biased with 500
mV
against a Pt wire auxiliary in 20 mL of phosphate buffered saline (PBS: pH
7.4) while
Ag/AgCI acted as the reference electrode. H202 calibration plots are created
by adding
aliquots of H202 with increasing concentration into the test vial while the
solution is
continuously stirred (500 rpm) (FIG. 1-1d). The effect of the plasma etching
is clearly
noticeable as the sensitivity to H202 increases from 0.015 mA mM-1 cm-2 to
0.595 mA
mM-1 cm-2 before and after etching respectively.
In an effort to increase the electro-reactivity of the GPN electrodes, Pt
nanoparticles of varying size and density are electrodeposited onto the GPNs
(FIGs. 2a-
2e). Pt nanoparticles are electrodeposited through a current pulse technique
with a
similar 3-electrode set-up discussed below. Five distinct currents are used to
create
five Pt-GPN electrodes with Pt nanoparticles of differing size and density.
Current
pulses of 312 pA initiate nanoparticle growth along the GPN ridge lines with
an average
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
nanoparticle width of (46 5) nm. The ridgeline nanoparticles grow to (86
5) nm at
625 pA current pulses while nanoparticles (< 20 nm) began to form on each
petal face.
Ridgeline nanoparticles begin to coalesce at current pulses of 1250 pA with
average
widths of (100 10) nm while petal face nanoparticles (< 20 nm) begin to
become more
apparent. At 2500 pA current pulses the petal tips are generally coated with
Pt
nanoparticles (<10 nm in width) extending from ridgeline nanoparticles (width
of (100
25) nm) while all visible petal faces now contain an array of nanoparticles
(<20nm) . At
5000 pA current pulses the Pt ridgelines have now expanded in width to (300
50) nm
while the petal face nanoparticles have grown to (35 10) nm. Thus by
changing the
pulse deposition current the Pt nanoparticle size, density, and morphology can
be
altered. These distinct Pt nanoparticle characteristics have significant and
unique
impacts on subsequent H202 and glucose sensing.
As with the GPN electrodes, the 5 distinct Pt-GPN electrodes were
electrochemically characterized by testing their sensitivity to H202 (FIG. 1-
2f).
Amperometric H202 calibration plots were performed in the same manner as
mentioned previously with a working potential of 500 mV. The H202 sensitivity
of the
GPN electrode is enhanced with the introduction of Pt as the sensitivity jumps
from
0.595 mA mM-1 cm-2 (GPN electrode after oxygen plasma etch) to 9.71 mA mM-1 cm-
2,
an increase of more than 16 fold, after Pt electrodeposition with 312 pA
current pulses.
The H202 sensitivity continues to increase for higher Pt electrodeposition
current pulse
until a maximum sensitivity of 13.7 mA mM-1 cm-2 is reached for the Pt-GPN
biosensor
with 2.50 mA current pulses. The H202 sensitivity decreases to 12.9 mA mM-1 cm-
2 for
Pt-GPN biosensor with 5.0 mA current pulses. As a supplementary control
experiment,
26
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Pt was electrodeposited onto planar highly ordered pyrolytic graphite (HOPG)
at the
same conditions (2.5 mA current pulses, 250 cycles) as the optimized Pt-MGPN
electrode. The H202 sensitivity of the optimized Pt-MGPN was nearly 5 times as
great
as the Pt-HOPG electrode¨ illustrating the enhanced sensitivity of MGPNs over
conventional carbon-based substrates.
In order to convert the PtNP-GPN electrodes into enzymatic biosensors, the
enzyme GOx is mixed with the conductive polymer PEDOT and subsequently
electrodeposited onto the electrode surface. During electrochemical glucose
sensing,
glucose is broken down by GOx into hydrogen peroxide (H202) and is
subsequently
oxidized at the electrode surface, producing measurable current signal (Eq. 1
& 2). A
schematic portraying the biofunctionalized PtNP-GPN glucose biosensors as well
as the
enzymatic function of GOx is illustrated in FIG. 3a.
GOx
D ¨glucose + 02 + H,0 D ¨ gluconic acid + H,02 (1)
biO ¨) 2H + 2e¨
Amperometric glucose sensing is carried out in the same 3-electrode set-up and
working potential (500 mV) as the amperometric H202 testing. Amperometric
glucose
calibration plots for all 5 PtNp-GPN biosensors were created by adding
successive
aliquots of increasing concentrations of glucose and measuring the
corresponding
steady-state signal response, typically achieved within 5 seconds (FIGs. 3b-
3e). The
glucose sensitivity for the Pt-GPN biosensors and linear sensing range of the
PtNp-
GPN glucose biosensors follow similar trends found in the amperometric H202
testing
where values continue to increase for higher Pt electrodeposition current
pulses until a
27
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
maximum sensitivity (0.24 pA mM-1 cm-2) and linear sensing range (0.01 ¨ 50
mM) is
reached for the PtNp-GPN biosensor with 2.50 mA current pulses (FIGs. 3a-3e
and
FIG. 4). The glucose detection limit (S/N=3, signal-to-noise ratio of 3) and
linear
sensing range of the optimized Pt-GPN biosensor is listed and compared to
glucose
biosensors comprised of similar materials including graphene, carbon
nanotubes, and
PtNPs (Table 1).
Table 1. Electrochemical biosensor performance comparison of glucose
biosensors
based upon, graphene/graphite, carbon nanotubes, and metallic nanoparticles.
Biosensor Base Material Detection Sensing
Ref.
Limit Range
(PM) (mM)
G0x-PEDOT/PtNP/GPN5 Graphene/Graphite 0.3 0.01-50 this
discl
osur
e
G0x-Nafion-Pt-xGnPs Graphene/Graphite 1 1-20
52
Ppy-G0x-Gn Graphene/Graphite
3 .002-.040 53
G0x-CNx-MWCNT5 Carbon Nanotubes 10 .02-1.02
54
G0x-Fc-MWCNT5 Carbon Nanotubes 3 .012-3.8
55
G0x-HRP-Ppy-SWCNT Carbon Nanotubes 0.5 .012 ¨
56
3.8
G0x-Nafion/AuNP5-MWCNT5 Carbon Nanotubes 20
0.05 ¨ 22 57
G0x-PtNP5-SWCNT5 Carbon Nanotubes 0.5 0.005-5
58
G0x-PtNps-CNT5/Ti02 Carbon Nanotubes 5.7 0.006-1.5
59
G0x/aligned-SWCNT5 Carbon Nanotubes 80 up to 30
25
G0x/AuNW5-CS Metallic Nanoparticles 5 .01 ¨ 10
60
G0x-CS-IL/AuNP5 Metallic Nanoparticles 1.5 0.003-9
61
G0x-CS-Nafion/AuN Ps Metallic Nanoparticles 2.7 .005-2.4
62
(G0x) ¨ glucose oxidase, (xGnPs) ¨ exfoliated graphite nanoplatelets, (Gn) ¨
graphene,
(Ppy) ¨ polypyrrole, (MWCNT) ¨ multi-walled carbon nanotubes, (SWCNT) ¨ single-
28
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
walled carbon nanotube, (PtNPs) ¨ platinum nanoparticles, (AuNPs) ¨ gold
nanoparticles, (AuNWs) ¨ Au nanowires, (CNx-MWCNTs) ¨ nitrogen doped multi-
walled
carbon nanotubes, (Fc) ¨ ferrocenecarboxaldehyde, (HRP) ¨ horseradish
peroxidase,
(CS) ¨ chitosan, (IL) ¨ ionic liquid
The sensing range of the optimized Pt-GPN biosensor was wider respectively
then other nanostructured biosensors reported in the literature. Furthermore,
the linear
sensing range of the Pt-GPN biosensor not only enables glucose sensing within
the
physiological range for blood glucose found within healthy patients with blood
glucose
within the range of approximately 3.6 mM and approximately 7.5 mM (65 mg/dL ¨
135
mg/dL) and diabetic patients with blood glucose within the range of
approximately 1.1
mM and approximately 20.8 mM (20 mg/dL ¨ 350 mg/dL); it enables glucose
sensing in
saliva, tears, and urine as well¨opening the door for unique glucose sensing
paradigms were glucose levels from distinct human serums could be monitored
simultaneously (FIG. 4a).
The durability of G0x/PEDOT electrodeposition technique was validated by
performing glucose biosensing measurements over a 5 week period. Between
weekly
testing, the sensors were stored within a capped Petri dish with no
refrigeration¨
mimicking off-the-shelf storage typical of home blood glucose monitoring
systems. The
sensitivity of the optimized Pt-GPN biosensor retained more than 75% of its
sensitivity
even after 5 weeks of testing¨demonstrating the robust nature of the enzyme
immobilization protocol with cyclic testing and storage (FIG. 4b).
The glucose selectivity of the PtNP-GPN glucose biosensors was tested by
sensing glucose within three known electroactive species (uric acid (UA),
acetaminophen (AP), and ascorbic acid (AA)), commonly found in human serum
samples. A glucose concentration of 5 mM (which corresponds to a typical human
29
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
blood glucose level) electrochemically monitored after the addition of 100 pM
aliquots of
AP, UA, and AA exhibits minimal interference from endogenous electroactive
species
as illustrated by FIG. 4c.
A glucose concentration of 5mM was electrochemically monitored for all 5
sensors after physiological relevant concentrations (1pM) of UA, AP, and AA
were
added to the test vial according to previous inference testing protocols. The
percent
ratio of current response for interfering substance to glucose is presented in
Table 2.
The Pt-GPN glucose biosensors that were created with respective Pt current
pulses of
625 pA, 1.25 mA, and 2.5 mA maintain a minimal current response of UA, AP, and
AA.
Furthermore, the biosensor retains more than 75% of its sensitivity even after
5 weeks
of use and storage at room temperature (-25 C)
Various embodiments of the present invention pertain to the use of graphene
petal nanosheets (GPNs) in an electrochemical biosensing application. The
emergence
of GPN is in its infancy within the research literature, but initial research
has begun to
uncover favorable electrochemical properties stemming from the exposed petal
tips that
exhibit the fast ET rates typically found in graphitic edge planes. Various
embodiments
include the concept of using the GPNs as templates for Pt nanoparticle growth
to
enhance the electro-reactivity of the petals and in effect present a
nanoelectrode array
fabrication protocol that eliminates the complexity of traditional NEA design
that typically
includes anodic alumina or polycarbonate templates and/or multi-step
lithography steps.
These Pt nanoparticle GPNs outperform conventional planar Pt nanoparticle/
HOPG in
terms of H202 sensitivity ( ¨5:1 respectively), thus demonstrating the impact
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
nanostructured, three dimensionally arrayed MGPNs fused with Pt nanoparticles
can
exhibit in electrochemical sensing.
The link between electrode nanostructuring and enzymatic biosensing
sensitivity
remains relatively unexplored in the literature. Various embodiments of the
present
invention illustration how Pt nanoparticle size, morphology, and density can
modulated
to improve the linear sensing range and the detection limit of the enzymatic
biosensors.
Some embodiments widen the glucose sensing range into the physiological
concentration levels found in urine, tears, and saliva in addition to blood.
Furthermore,
the electrodeposition of GOx with PEDOT onto the PtNP modified GPNs enables
robust
glucose sensing with minimal interference for over one month from endogenous
electroactive species commonly found in human serum samples. The results of
the
selectivity experiments can be explained in part by the electrodeposited PEDOT
layer.
The electrodeposition of PEDOT at high concentrations ( > = 1 mA) as performed
in this
work can over-oxidize carbon atoms on the polymer backbone¨ transforming the
PEDOT polymer chain charge from positive to partially negative. Thus the
electrodeposited PEDOT tends to repel negatively-charged electrochemical
interferents
( e.g. , ascorbic and uric acid) due to electrostatic repulsion during
electrochemical
biosensing.
The bottom-up growth of GPNs on a silicon wafer, electrodeposition of Pt
nanoparticles, and electrodeposition of enzyme encapsulated within the
conductive
polymer PEDOT are all scalable fabrication techniques that can be potentially
integrated
into a wide array of electronic devices. This highly sensitivity biosensing
platform
should be quite versatile as the GOx can be interchanged with other enzymes
such as
31
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
glutamate oxidase, lactate oxidase, and alcohol oxidase for the advancement of
basic
research and in-field biosensing associated with neurological disorders,
patient trauma,
food quality, and next generation bio-ethanol fuel technologies. Furthermore,
various
inventive embodiments incorporate these fabrication protocols into lab-on-a-
chip
platforms where the GPNs, PtNPs, and respective enzymes can all be
electrodeposited
onto distinct microelectrodes for multi-plexed biosensing purposes.
Using immobilized glutamate oxidase allows the testing of glutamate levels for
the diagnosis and treatment of Alzheimer's disease, Parkinson's disease, and
epilepsy.
The technology can also be employed in a wide range of non-medical fields.
Examples
include incorporation of acetylcholinesterase enzyme to detect
organophosphorus
pesticides in agricultural applications, polyphenol oxidase to detect the
presence of
phenolic pollutants in environmental applications, and organophosphorus
hydrolase to
detect nerve-agents for national defense. The platform represents an enabling
technology for the detection of miniscule quantities of a wide variety of
analytes.
Various embodiments discussed above were fabricated using methods that will
now be described. A thin film of Ti (100 nm) is e-beam evaporated onto an
oxidized
silicon wafer [P <100> Si (5 pm), 5i02 (500 nm)] at a base pressure of 5.0 x
10-7 Torr.
The metalized wafer is diced with a diamond-blade dicing saw (Disco DAD-2H/6)
into
equally-sized electrodes (0.35cm2) after a thin film of AZ1518 photoresist is
spun and
hard baked (10 min at 120 C) unto the wafer to protect the surface during
cutting
operation. After wafer dicing, the electrodes are solvent cleaned with
acetone,
methanol, and isopropyl alcohol and subsequently dried under a gentle stream
of N2
gas to remove the photoresist and debris before GPN Synthesis.
32
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
The growth of the GPNs is carried out by microwave plasma chemical vapor
deposition (MPCVD) with a SEKI AX5200S MPCVD reactor. The Ti coated silicon
electrodes are elevated 6 mm above a 5.1 cm diameter molybdenum puck, placed
inside the MPCVD reactor chamber and heated to 700 C in a hydrogen ambient by
a
3.5 kW radio-frequency power supply at a pressure of 30 Torr. A hydrogen
plasma is
generated over the sample via a 5 kW ASTeX AX2100 microwave generator, while
methane (CH4) gas, the acting precursor for GPN growth, is pumped into the
chamber
for 10 minutes at a flow rate of 10 SCCM. The hydrogen plasma decomposes the
methane gas to permit monolithic GPN growth across the entire surface of the
electrode
(FIG. 1-1a).
In an effort to improve the electroactive nature of the GPNs, the GPN
electrode
was exposed to an 02 plasma etch within a Plasma Tech Reactive Ion Etch (RIE).
The
GPN electrode was placed inside the vacuum chamber of the reactor and pumped
down to a base pressure of 0.1 mTorr to eliminate/minimize contaminating
species that
may have been introduced into the chamber during loading. 02 was introduced
into the
chamber at a flow rate of 50 SCCM and the chamber pressure was adjusted to 60
mTorr. A 02 plasma was generated over the GPN electrode for 30 seconds by
setting
the RF generator to the 100W power setting.
A 3 electrode electrochemical set-up (BASi Epsilon Three-Electrode Cell Stand -
potentiostat) where the GPNs acted as the working electrode, Pt gauze as the
auxiliary
electrode, and Ag/AgGI as the reference electrode were dipped within a plating
bath
consisting of 4 mM H2PtC16.6H20 (Sigma Aldrich 206083) and 0.5 M Na2504 (Fluka
71960) to electrodeposit Pt nanoparticles onto the GPN electrodes. Current
pulses
33
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
(500 ms) of 312uA, 625 uA, 1.25 mA, 2.5 mA and 5.0 mA were utilized in cycles
of 250
to manipulate the size and density of Pt nanoparticles deposited on the GPNs.
Glucose oxidase is first mixed with Poly(3,4-ethylenedioxythiophene) (PEDOT)
before it is electrodeposited onto the PtNP-GPN electrodes. The G0x/PEDOT
solution
is created by first mixing 0.1M poly(styrenesulfonate) in H20. Next, 0.03 M
3,4-
ethylenedioxythiophene (Sigma Aldrich 483028) is added to the mixture while
the
solution is agitated. The enzyme glucose oxidase (G0x) (Sigma Aldrich G7141)
is next
added to the mixture in a concentration of 2 mg/ml. The subsequent PEDOT/G0x
solution is electrodeposited onto each Pt-GPN electrode via constant current
pulses of
lmA that are applied between the working electrode (Pt-GPN) and auxiliary
electrode
(Pt gauze) for 500 cycles.
Although PEDOT has been tested, shown, and described in various
embodiments of the present invention, other embodiments anticipate the use of
any
conductive polymers or, intrinsically conducting polymers, including any
organic
polymers that conduct electricity. Such compounds typically have metallic
conductivity
or can be semiconductors, and are organic materials. They can offer high
electrical
conductivity and preferably do not show similar mechanical properties to other
commercially available polymers. The electrical properties can be fine-tuned
using the
methods of organic synthesis and by dispersion techniques. In one embodiment
this
enzyme could be glucose oxidase and the conductive polymer could be PEDOT
which
is produced by mixing poly(styrenesulfonate) in H20 and then adding M 3,4-
ethylenedioxythiophene to the mixture while the solution is agitated. Then
enzyme is
added to this mixture before electrodeposition.
34
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
All electrochemical testing was performed in a 3 electrode set-up (BASi
Epsilon
Three-Electrode Cell Stand - potentiostat) where the GPN or PtNp-GPN
electrodes
acted as the working electrode, a Pt wire as the auxiliary electrode, and
Ag/AgCI as the
reference electrode. Amperometric hydrogen peroxide (H202) and glucose sensing
experiments were performed in phosphate buffered saline (PBS, 0.1 M pH 7.4) at
a
working potential of 500mV under constant stirring (500 rpm) with a 0.5 cm
(length)
magnetic stir bar while successive increasing concentration aliquots of said
target
analyte were pipetted into the testing vial. The Pt-GPN biosensors act as
small
electrochemical dipstick were the sensor region of the electrode (0.35cm2) is
submerged in the testing solution and the other end is electrically wired to
the
potentiostat.
Raman spectroscopy was performed using T64000 system by Horiba Scientific.
All the spectra were collected at room temperature using a laser excitation at
488 nm
wavelength. The laser power was 2 mW and a 50X objective lens was used.
A S-4800 Hitachi microscope was utilized at a power setting of 5.0 kV to
obtain
all field emission scanning electron microscopy (FESEM) micrographs. No
additional
processing steps were required before image analysis.
Taking advantage of the high aspect ratio of the GPs and by varying the growth
time, it is possible to identify a mechanism that explains the rapid growth of
GPs from
nanocones. There are at least two possible sources for C, leading to two
different
growth processes of the GPs: (i) a rapid growth mechanism from the top of a GP
which
is dominated by C species from the decomposition of CH4, and (ii) a slower
growth from
the side of a GP which is governed by the precipitation and diffusion of
carbon atoms
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
from the substrate, forming multiple layers and thus allowing the GPs to grow
in
thickness as well as vertical height. There is also an inherent self-limiting
aspect to the
growth process. When smaller petals merge to form a larger interconnected
network,
the resultant petal structure forms an electrostatic equipotential surface,
reducing the
effect of the inhomogeneous, local electric field distribution, leading to a
more uniform
growth of GPs.
It is possible to develop techniques to control and confine the growth of GPs
on
flat substrates. Such a processing step is useful if the desirable properties
of this high
surface area form of C are to be utilized in practical applications.
Controlling the rapid
growth of GPs can be achieved by producing localized rough regions on a flat
Si wafer.
One way to accomplish this goal is to create a pattern of lines scratched into
a 5i02
layer. In this study, these lines were produced using a simple scribing tool.
After
scratching the oxide layer and subjecting the substrate to MPCVD growth
conditions,
highly localized regions of GPs were in fact observed to grow in patterned
lines on the
flat substrate. Complex patterns can be generated at the nanoscale using a
diamond tip
mounted to a microcantilever rastered across a substrate by a controller
operating a
contact mode AFM. A simple patterning technique will allow the controlled
growth of
GPs on oxidized silicon substrates for many promising practical applications.
. The schematic diagram of the chamber is shown in FIG. 2-1. In brief, the
plasma source consists of a 2.45 GHz frequency microwave power supply with
variable
power. Oxidized silicon wafers (p-type <111>) with different thicknesses of
5i02 top
layers were used as substrates. Unless otherwise stated, the substrate
dimension in
these experiments is 1x1 cm2. The substrates, elevated 15 mm above a 55-mm-
36
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
diameter Mo puck by ceramic spacers, were subjected to MPCVD conditions of H2
(50
sccm) and CH4 (10 sccm) as the primary feed gases at 30 Torr total pressure.
The GP
growth time varied from 30s to 30 min to produce samples at different stages
of growth.
The substrates were initially exposed to hydrogen plasma for approximately 6
min,
during which the plasma power gradually increased from 300 W to 700 W. At a
plasma
power of 300 W, visible plumes appeared at each corner of the substrate
because of
the high localized electric field. When plasma power increased to 700 W, the
size of
plumes increased, and eventually they coalesced to cover the entire substrate.
This
plasma is sufficient to heat the samples from room temperature up to ¨1100 C,
as
measured by a dual-wavelength pyrometer (Williamson PRO 92). After introducing
CH4, the measured temperature decreases slightly to ¨1000 C. To better
understand
the formation of the nanoscale cones, a lower plasma power (300 W) was used
during
GP growth for some experiments.
A Hitachi S-4800 field emission scanning electron microscope (FESEM) operated
at 5 kV was used to study sample surface morphology. A FEI Titan 80-300
operated at
300 kV was utilized for a high-resolution transmission electron microscopy
(HRTEM) to
characterize structure of the as-grown GPs, as well as substrate/oxide and
oxide/GP
interfaces. The same instrument was equipped with Gatan imaging filter (GIF
Tridiem,
model 863), which allows acquisition of elemental mapping images via electron
energy
loss spectroscopy (EELS). TEM samples for GP structure analysis were prepared
by
scratching a sample surface with a razor blade to remove deposited material
into a vial
with acetone followed by ultrasonic bath treatment for several minutes, after
which a
drop of obtained suspension was put onto a lacey carbon 300 mesh copper TEM
grid.
37
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
For interface analysis, cross-sectional TEM samples were prepared by a focused
ion
beam (FIB) lift-out technique in a FEI Nova 200 dual beam SEM/FIB system
equipped
with a KlöckeTM nanomanipulator.
AFM imaging studies of bare SiO2/Si substrates after etching by the hydrogen
plasma were performed with a Veeco Dimension 3100 scanning probe microscope
(SPM) using a NS-IV controller in tapping mode with a Pt¨Ir coated Si tip
(spring
constant=1-5 N/m and resonant frequency=75 kHz). Raman characterization was
performed with an Xplora spectrometer (Horiba Jobin Yvon Inc.) with a fixed
laser
excitation wavelength of 532 nm, power of 2.5 mW, spot size of 600 nm, and
magnification of 100x.
Before GP growth, the effect of the hydrogen plasma on the substrates was
investigated in order to understand the role, if any, of hydrogen plasma
pretreatment
prior to GP growth. As-received Si/5i02 substrates with a 500-nm-thick 5i02
layer were
etched in hydrogen plasma for approximately 6 min without introducing CH4 into
the
chamber, corresponding to a null growth time. FIG. 2-2 shows FESEM images of
the
etched substrates. A gray-scale contrast boundary is evident in FIG. 2-2(a)
located at
the corner edge of the substrate and demarcates a region that has been
significantly
etched (darker region) as compared to the substrate's center (lighter region),
where
substrate etching occurs at a slower rate. FIG. 2-2(b) shows a close-up of the
boundary
between the dark and light regions. At the boundary, the lateral size of the
localized
etched oxidized silicon (EOS) features range from tens of nanometers to
several
micrometers. These EOS features were further studied using AFM imaging. FIG. 2-
3
shows the results of parallel AFM studies which reveal a local roughening of
the
38
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
substrate with identifiable nanoscale trenches or fissures emanating in a
roughly radial
direction from a central point. AFM images of several EOS features indicate
that the
overall height can be a few hundred nanometers above the surrounding
substrate.
The formation of these localized EOS features is directly attributed to
exposure to
the hydrogen plasma. The edge of the substrate couples to the plasma,
producing a
region with an enhanced electric field, causing the formation of a plasma
sheath. The
nature of the sheath depends on various parameters including the geometry of
the
substrate, its position inside the chamber, ionizing species in the plasma,
the
background pressure, and the plasma power. As shown in FIG. 2-1, the substrate
is
electrically isolated from ground, and hence acts as an electrically floating
object
immersed in the plasma. During hydrogen plasma etching, two processes occur in
parallel. The first is an erosion of material from the Si02/plasma interface,
causing a
gradual reduction of the Si02 thickness. The second process is the diffusion
of
hydrogen atoms from the plasma through the oxide layer, causing an aggregation
of H
at the Si/SiO2 interface. The localized trench-like surface morphologies
observed in
FIGS. 2-2 and 2-3 are assumed to result from reactions such as
+ + (Al2)0, rspx)
(1)
which describes a process in which hot SiO and H20 vapor escape from the 5i02
substrate, causing a localized swelling across the surface. This reaction
accelerates in
regions where the electric field is relatively high (e.g., the edge of an
oxidized silicon
substrate) and gradually consumes the substrate as the etch front moves away
from the
substrate edges. Because of the release of SiO and H20 vapor, radial-like
trenches or
fissures dominate the surface of EOS features. Because of the local electric
field
39
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
gradient from the edge to the center of the substrate, hydrogen plasma etching
will be
less prevalent in the middle regions of the substrate. As a result, EOS
features of
nanometer size with lower densities may predominate for short etch times. Upon
introduction of a carbon source (methane) to the plasma, carbonaceous material
will
deposit on the substrate and undergo subsequent surface diffusion. The
nanoscale
trenches in the EOS features will efficiently trap diffusing carbon atoms,
thus forming
preferential sites for carbon aggregation. Carbon aggregation, along with
direct carbon
deposition onto the EOS regions, becomes one process for nucleation of fast
growing
GPs.
Focusing on GPs grown in the middle region of the oxidized silicon substrate
enables a better understanding of the GP growth mechanism. A Si02/Si substrate
with a
500-nm-thick Si02 top layer was placed inside the MPCVD chamber, and the
plasma
power was slowly ramped from 300 W to 700 W before introducing CH4 at a flow
rate of
10 sccm to initiate GP growth for 15 min at a plasma power of 700 W. FIG. 3-
1shows
tilted side-view and top-view SEM images from the middle region of the
substrate
subjected to these conditions. The emergence of nanoscale cone-like features
with
diameters ranging from 50 nm to a few 100 nm is evident.
Elemental mapping and HRTEM characterization (see FIG. 3-2) elucidate the
chemical composition and structure of these nanocones. FIG. 3-2(a)¨(d)
contains a
bright field TEM image, silicon map, oxygen map and carbon map, respectively,
of a
thin slice cut from a representative nanocone. The silicon map in FIG. 3-2(b)
shows the
extent of the silicon substrate used in these experiments. The oxygen map in
FIG. 3-
2(c) shows the extent of the Si02 layer, indicating that the core of the
nanocone is Si02.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
The original Si02 layer thickness was 500 nm. After hydrogen plasma etching
and
roughening, the Si02 layer is reduced to roughly 150 nm in thickness. The
carbon map
in FIG. 3-2(d) shows a thin (-20 nm) carbon film conformally covering the
surface of the
sample. Interestingly, a bright region observed on the nanocone (marked by an
arrow)
indicates a locally enhanced concentration of carbon atoms. This bright region
indicates
where the rapid growth of a GP is likely to occur. Further HRTEM
characterization of the
Si02/C interface layer (FIG. 3-2(e)) confirms that the carbon film covering
the surface of
the nanocone is graphitic with interlayer spacing of 0.35 nm. The black
smudges seen
in FIG. 3-2(e) are Pt nanoparticles used to attach the sample during sample
preparation.
The chemical composition of nanocones caused by the plasma etching may be
based on the starting chemical composition of the substrate. Furthermore, a
¨20 nm
thick C layer conformally coats the Si02 nanocone and shows evidence for the
formation of graphitic layers. All these findings are consistent with the
conclusion that
nanocones form preferential sites for C aggregation and lead to the rapid
growth of GP
at later stages.
The growth of GPs has been studied on oxidized silicon substrates which
initially
have a 500-nm-thick Si02 layer. In what follows, the GPs that grow for 15 min
under a
plasma power of 700 W are studied in further detail. FIG. 3-3 shows side-view
FESEM
images of GPs emerging from nanoscale cones. The growth is non-uniform across
the
substrate since the GPs are found to emerge from select regions of a few
nanoscale
cones. Contributing factors to this highly inhomogeneous growth environment
are local
electric fields, varying carbon deposition rates, and microscopic cone
geometry. It
41
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
seems clear that once conditions are favorable for GP growth, rapid emergence
of a
localized GP can result.
FIG. 3-3(a) shows nanoscale cones and GPs at a relatively large scale. FIG. 3-
3(b)-(d) shows FESEM images of individual nanoscale cones from which GPs
emerge.
In FIG. 3-3(b), a GP grows along a radial direction from the cone axis. FIG. 3-
3(c)
shows a GP in the shape of a horn growing from a nanoscale cone decorated with
visible trenches or fissures. The conical horn GP has a subtended interior
cone angle of
approximately 600. The outer edge of the horn is not smooth, but faceted. FIG.
3-3(d)
shows that a GP can be thin enough (less than 1 nm) to be semi-transparent in
an
FESEM.
FIG. 2-7 shows top-view FESEM images of rapidly growing GPs emerging from
individual nanoscale cones. These images demonstrate that the rapidly growing
GPs
are confined to the cones. Slower growing GPs appear primarily in the flat
areas
between the nanocones. Some cones are decorated with a few large GPs, while
others
show a distribution of GP sizes.
Raman spectroscopy is often used to characterize the graphitic nature of the
GPs. Within this context, three particular Raman peaks are useful. The D band
at 1350
cm-1 is known to result from various types of defects and anomalies of
transverse
optical vibrations near the K-point. The G peak at 1580 cm-1 arises because of
the
doubly degenerate zone center E2g mode. The 2D band at 2700 cm-1 is due to
intervalley zone-boundary transverse optical phonon scattering. This peak
consists of
multiple sub-peaks and is difficult to analyze quantitatively if there are
more than 5
graphitic layers.
42
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 2-8 shows the Raman data from GPs for different growth times of 1 min, 5
min and 15 min. The ID/IG and I2D/ IG ratios calculated from FIG. 2-10 for 5
min of growth
are 0.38 and 0.65, respectively; those for 15 min of growth are 0.25 and 0.56,
respectively. The decreasing presence of defects with growth time suggests
that
increasingly graphitic GPs are produced over time.
The atomic structures of the GPs were also investigated using HRTEM. FIG. 2-
9(a) shows a representative TEM image of as-grown GPs. The left-most edge of
this
micrograph provides evidence for thin nanosheets. FIG. 2-9(b) shows a HRTEM
image
of a cross-section through a thin GP. The micrograph indicates that the petal
is
comprised of between 4 and 7 layers of graphene with a planar lattice spacing
of
approximately 0.35 nm.
In order to understand the nucleation and growth mechanism of GPs further,
identical substrates were studied after different growth times in 700 W plasma
(1, 7, 10,
min), keeping other parameters the same. Top-view FESEM images in FIG. 2-10
15 reveal the evolution of nanoscale comes and GPs throughout the growth
process. FIG.
2-10(a) shows the substrate after 1 min of growth, when carbon deposits form
nanoislands across the surface of the substrate; the same situation is
observed in the
case of growth for 30 s (not shown here). No GPs are observed for these short
times,
but the nanoislands are thought to be nucleation sites of GPs on Si
substrates. FIG. 2-
20 10(b) shows the emergence of GPs after 7 min of growth. Large GPs emerge
from
individual nanoscale cones. FIG. 2-10(c) shows the substrate after 10 min of
growth.
The co-existence of the smaller and larger GPs is now evident. FIG. 2-10(d)
shows the
43
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
substrate after 20 min of growth, when coverage of GPs fills the entire
surface of the
substrate.
These images support a mechanism whereby the oxidized silicon substrate is
roughened by plasma etching while carbon is deposited on the substrate. Carbon
diffuses across the substrate while preferential trapping occurs in localized
EOS
features. This leads to preferential GP growth from nanoscale cones during the
initial
stages of the growth process. As time increases, carbon diffusion across the
entire
substrate feeds the growth of GPs everywhere on the substrate, leading to a
dense
coverage of GPs.
To understand the influence of plasma power in the formation of GPs,
experiments were performed in which the plasma power was varied, while fixing
all
other growth conditions. FIG. 2-11 shows top-view FESEM images of the GPs
observed with two different plasma powers (300 W, 700 W) but the same growth
time (7
min). As expected, GPs decorage the nanoscale cones (see the marked boundaries
of
a nanocones in FIG. 2-11(a)) for a plasma power of 700 W. However, no
nanoscale
cones are observed on the substrates (even at the edge of the substrates) when
the
plasma power is reduced to 300 W (see FIG. 2-11(b)). The fact that no
nanoscale
cones were observed for low plasma power suggests that plasma power intensity
is an
important factor in the formation of the nanoscale cones.
The plasma power directly influences two growth parameters: i) the final
temperature of the substrate and ii) the intensity of electric field above the
substrate. A
low plasma power results in a lower temperature on the surface, which reduces
the
diffusion rate of the carbon atoms and thus reduces the growth rate of GPs.
Evidently,
44
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
the lower power also reduces the possibility of forming nanoscale cones. A low
electric
field leads reduces hydrogen plasma etching, which in turn hinders EOS
formation and
thus the formation of well-defined nanoscale cones. Eventually, GPs grow from
the
irregular and roughened Si02 surface due to the partial etching produced by
the low-
power hydrogen plasma, as shown in FIG. 2-11(b).
Similar experiments have been performed on different substrates such as Ti/Si,
Ni foil, and Cu foil in order to investigate whether similar nanocone
formation occurs.
Although all substrates produced GPs, no nanoscale cones were observed on
these
substrates (even for a high plasma power of 700 W) during early-stage GP
growth. We
conclude that the silicon dioxide layer and the high plasma power are
primarily
responsible for the formation of these nanoscale cone features.
The local growth of GPs is largely uncontrolled in the present process,
occurring
at random locations across a substrate. Different GPs grow at different rates,
even
though separated one from another by a fraction of a micrometer. To take
advantage of
the GP material properties, improved control of the growth process is needed.
To this
end, we highlight three processes that are all important to GP growth:
I. Carbon species that are directly adsorbed onto the outermost edge
of GPs are helpful for rapid GP growth. This process uses an enhanced electric
field in the plasma, due in part to the high aspect ratio of the GPs. Direct
deposition of carbon material onto a petal edge and incorporation into an
emerging petal allows for rapid GP vertical growth.
II. Carbon can also fall directly onto the substrate due to
decomposition of CH4 in the plasma. While the rate of carbon deposition may be
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
uneven due to a variety of factors (e.g., a shadowing effect produced by
larger
growing petals (see FIG. 2-7(b)), the deposited carbon will randomly diffuse
until
a GP nucleation site is encountered.
III. Carbon may be continually etched by the plasma, either
from the
uppermost edge of a GP or from the substrate itself. This point emphasizes the
diversity of phenomena caused by the presence of hydrogen: i) as an etchant to
remove amorphous carbon, ii) as a promoter of crystalline graphite by removing
secondary nuclei that might interfere with GP growth, and iii) by eliminating
cross-linking of carbon at free edges of growing GPs, thus preventing
excessive
edge thickening.
Expanding on Eq. (1), the following chemical reactions may occur on a
nanoscale cone at elevated local temperatures:
+ 4C 211 SiO (gag) Cars -4-
SiC(fInWriphOUS)
C(amorphaus.) C(reacted
(2)
In writing Eq. (2), subtle differences in C atoms present and distinguish
between
amorphous (unreacted) C and C that has reacted to form a variety of species
such as C
clusters and C nanoparticles. Analysis of the XPS spectrum collected as a
function of
deposition time clearly indicates a three-step process in which first a SiC
layer is formed
on the silicon, followed by an amorphous carbon layer which is then
subsequently
covered by few-layer graphene flakes. Throughout the process, the presence of
SiC is
important because it serves as a catalyst that facilitates the growth of
carbon, either
through precipitation and/or SiC decomposition.
46
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
GPs originate from the unreacted C species only, since once reacted to form C
clusters, the C will exhibit a reduced diffusion coefficient. The local
environment
encountered by unreacted C is capable (under the proper set of conditions) of
transforming amorphous carbon to ordered, graphitic-like carbon sheets that
take the
form of GPs. During the initial period of growth on an oxidized Si substrate,
GPs grow
rapidly from nanoscale cones.
Wherever nanoscale cones are formed, a very local enhanced decomposition of
hydrocarbons into deposited C results, possibly due to the local enhancement
of the
electric field. Because C accumulate more rapidly on the conical structures as
compared to flat regions of the substrate, the likelihood increases that
precipitated C
self-assembles on substrate features that promote multiple sets of graphitic
planes that
then rapidly emerge as vertically oriented GPs.
These insights suggest that by controlling the formation of nanopeaks, the
rapid
emergence of graphitic features can be patterned onto flat substrates in a
prescribed
way. One way to control the growth and formation of nanocones is to
intentionally
roughen a silicon substrate by inscribing a scratch in the 500-nm-thick oxide
layer. The
scratch will provide many sites that will seed the rapid growth of GPs.
FIG. 2-12 displays top-view and side-view FESEM images of a scratched
substrate in an attempt to produce the controlled growth of nanocones and GPs.
FIG. 2-
12(a) shows three lightly scribed scratches in a Si substrate at low
magnification after 6
min of growth at 700W plasma power. FIG. 2-12(b) shows a magnified top-view
image
of one scratched area in FIG. 2-12(a) (indicated by the rectangular box) where
preferential GP growth is evident. FIG. 2-12(c) is a magnified image of FIG. 2-
12(b)
47
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
showing that the GPs are seeded by the formation of nanocones (see arrow).
FIG. 2-
12(d) is a typical cross-sectional FESEM image of a wafer edge that was
produced by
cleaving the substrate perpendicular to a scratched line. FIG. 2-12(d)
indicates a
reduced oxide layer thickness in the scratched area of ¨250 nm after the
hydrogen
plasma etching during the growth process. Cross-sectional SEM images of other
scratched lines reveal reduced oxide layer thicknesses in the range of 150 to
300 nm.
The roughened surfaces in these cross-sectional images further corroborate the
contention that GPs grow from nanocones, in agreement with the top-view SEM
characterization in FIG. 2-12(c). Further experiments show that preferential
GP growth
is confined to all scratched lines we have tested to date.
Factors influencing the formation and structure of graphitic petals (GPs)
grown by
microwave plasma-enhanced chemical vapor deposition on oxidized silicon
substrates
are investigated through process variation and materials analysis. Some
graphitic petals
are found to grow at an accelerated rate, often growing approximately 20 times
faster
than other petals located only a fraction of a micrometer away. Using scanning
electron
microscopy and atomic force microscopy, the rapid growth rate of these fast-
growing
petals is attributed to the formation of nanoscale cones in the plasma etched
5i02 layer
(see FIG. 3.1). Electron energy loss spectroscopy reveals that the formation
of these
nanoscale cones is associated with a localized roughening of the oxidized
silicon
substrate¨a process that depends on plasma power. Raman spectroscopy and
transmission electron microscopy are used to confirm the graphitic nature of
the as-
grown petals. Insights gained into the growth mechanism of these graphitic
petals
suggest a simple scribing method can be used to control both the location and
formation
48
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
of petals on flat Si substrates. Experiments performed to test this hypothesis
show that
controlled petal growth can be achieved, a development that enables an
exploitation of
the graphitic petal properties in many practical applications.
Elemental mapping and high-resolution transmission electron microscopy
(HRTEM) characterization (see FIG. 3.2) elucidate the chemical composition and
structure of these nanocones. FIGs. 3.2a-d disclose a bright field TEM image,
silicon
map, oxygen map and carbon map, respectively, of a thin slice cut from a
representative nanocone. The silicon map in FIG. 3.2b shows the extent of the
silicon
substrate used in these experiments. The oxygen map in FIG. 3.2c shows the
extent of
the 5i02 layer, indicating that the core of the nanocone is 5i02. The original
5i02 layer
thickness was 500 nm. After hydrogen plasma etching and roughening, the 5i02
layer is
reduced to roughly 150 nm in thickness. The carbon map in FIG. 3.2d shows a
thin
(approximately 20 nm) carbon film conformally covering the surface of the
sample.
Interestingly, a bright region observed on the nanocone (marked by an arrow)
indicates
a locally enhanced concentration of carbon atoms. This bright region indicates
where
the rapid growth of a GP is likely to occur. Further HRTEM characterization of
the
Si02/C interface layer (FIG.. 3.2e) confirms that the carbon film covering the
surface of
the nanocone is graphitic with interlayer spacing of 0.35 nm. The black
smudges seen
in FIG. 3.2e are Pt nanoparticles used to attach the sample during sample
preparation.
FIG. 3.3 shows side-view FESEM images of GPs emerging from nanoscale
cones after 15 min of growth under a plasma power of 700 W. FIG. 3.3a shows
nanoscale cones and GPs at a relatively large scale. FIGs. 3.3b ¨ d show FESEM
images of individual nanoscale cones from which GPs emerge. In FIG. 3.3b, a GP
49
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
grows along a radial direction from the cone axis. FIG. 3.3c shows a GP in the
shape of
a horn growing from a nanoscale cone decorated with visible trenches or
fissures. The
conical horn GP has a subtended interior cone angle of approximately 600. The
outer
edge of the horn is not smooth, but clearly faceted. FIG. 3.3d shows that a GP
can be
thin enough (less than 1 nm) to be semi-transparent in an FESEM.
Yet another embodiment pertains to a hybrid manganese dioxide/graphitic petal
structure on carbon nanotube substrates to achieve high specific capacitance,
energy
density, power density, and long cycle life for flexible supercapacitor
application.
Vertical nanoscale graphitic petals were prepared by microwave plasma chemical
vapor
deposition on commercial carbon nanotube substrates and subsequently coated
with a
thin layer of Mn02. The thickness is controlled by the immersion time. An
immersion
timeof 40 minutes was arbitrarily chosen in our study. We think this gives a
Mn02
coating of 5-10 nanometers thick.
To make composites suitable for electrochemical electrodes, prior to Mn02
coating or electrochemical measurement, concentrated H2SO4 and HNO3 (volume
ratio
3:1) were used to functionalize the surface of GPs at 50 C for 2 hours in an
oven. The
samples were then washed by deionized water and dried at 100 C overnight. A
neutral
precursor solution (¨ pH 7) for the Mn02 coating process was prepared by
mixing 0.1 M
Na2SO4 (Alfa Aesar) and 0.1 M KMn04 (Alfa Aesar) solutions. The GPs grown on
BP
were immersed into the solution, which was kept at 80 C in an oven for 40 min.
The
loading amount can be easily controlled by adjusting the immersion time. The
sample
was then rinsed with deionized water and subsequently annealed at 200 C for 3
hours
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
using a hotplate in air. The mass of coated Mn02 was calculated from the
weight
difference before and after the coating process.
The graphitic petal/carbon nanotube architecture without any binder provides
an
efficient scaffold for maximizing the electrochemical performance of Mn02. A
specific
capacitance (based on the mass of Mn02) of 579 F/g is obtained at a scan rate
of 2
mV/s in 1 M Na2SO4 aqueous electrolyte. The energy density and power density
at 50
Ng are 28.2 Wh/kg and 24.5 kW/kg (with a maximum value of 114 kW/kg),
respectively.
In addition, the composite electrode shows long-term cyclic stability (less
than 10%
decrease of specific capacitance after 1000 cycles). Such behavior indicates
that the
Mn02/graphitic petal/carbon nanotube composite is a promising electrode
material for
high-performance supercapacitors. Density functional theory indicates that
coating of
Mn02 on the surface of GPs enhances the conduction path of the electron
transport
during the charge/discharge process.
SEM images of GPs synthesized by MPCVD are shown in FIG. 3.6a. The petals
extend approximately 500 nm out from the BP surface, and the typical span
width of a
single unwrinkled 2-D grain ranges from 100 nm to 500 nm. The thickness of a
GP layer
can reach several nanometers, corresponding to less than 50 graphene layers. A
magnified image of one petal marked by the rectangular box in FIG. 3.6a is
shown in
FIG. 3.6b, revealing the smooth surfaces of the GPs. These surfaces provide
accessible
sites for Mn02 coating. The crumpled structures of the vertical graphene
sheets with
both sides exposed to Mn02 precursor solution can provide large specific area
for
coating. FIG. 3.6c shows the morphology of Mn02 coated on GPs. FIG. 3.6d
contains a
51
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
magnified image of the area marked by the rectangular box in FIG. 3.6c,
showing a thin
uniform layer of Mn02 on the smooth GP surfaces, even on the smaller petals.
FIG 3.7a shows the cyclic voltammetry (CV) curves of the Mn02/GP/BP
composites at scan rates of 2, 5, 10, 20, 50, 100 mV/s in 1 M Na2SO4 aqueous
solution
with potential windows ranging from 0 to 0.8 V. The constant-current
charge/discharge
curves of the as-prepared Mn02/GP/BP hybrid structure at different current
densities
are shown in FIG. 3.7b. The charge/discharge curves display a symmetric shape,
indicating that the structure has a good electrochemical capacitive
characteristic. FIG.
3.7c shows comparative specific capacitances of BP, GP/BP, Mn02/BP and
Mn02/GP/BP calculated from CV curves at voltage scan rates from 2 to 100 mV/s.
At a
scan rate of 2 mV/s, the specific capacitance of the Mn02/GP/BP hybrid
composite
reaches 579 F/g (based on the mass of pristine Mn02). At a high scan rate of
100 mV/s,
the specific capacitance of Mn02/GP/BP still remains close to 320 F/g, which
is
comparable to the rate performance reported elsewhere. However, for the same
Mn02
coating time, the specific capacitance of Mn02/BP is only about 266 F/g (based
on
pristine Mn02) at 2 mV/s (see FIG. 3.7c). The superior rate capability of
Mn02/GP/BP
composites demonstrates the advantages of this new architecture of GP/BP as a
highly
conductive scaffold for maximizing the utilization of the practical
electrochemical
performance of Mn02. Since previous studies show that only a thin layer of
Mn02 is
involved in the charge storage process, the specific capacitance of Mn02
coated on the
supporting GP/BP can be further improved by optimizing the thickness of coated
Mn02
layer. FIG. 3.7d shows the Ragone plot for the Mn02/GP/BP structured electrode
at
different current densities. At a high current density of 50 Ng, the
calculated energy
52
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
density is 28.2 Wh/kg, and the average power density is 24.5 kW/kg. The
maximum
power density, calculated from V2/4RM, is found to be 114 kW/kg. These values
are
higher than the reported energy density (14.8 Wh/kg) and power density (2.5
kW/kg) of
electrodeposited Mn02 films on BP substrates, suggesting that as-prepared
composite
is a promising electrode material for supercapacitors.
Another embodiment pertains to the design and fabrication of a hybrid three-
dimensional nanoarchitecture by electropolymerizing aniline monomers into a
nanometre-thick conformal polyaniline (PANI) film on graphitic petals (GPs)
that are
directly grown on highly conductive carbon cloth (CC) through microwave plasma
enhanced chemical vapor deposition (MPCVD) for flexible supercapacitor
application.
The hybrid CC/GPs/PANI electrodes yield greatly improved capacitive
performance with
a high specific capacitance of approximately 2000 F/g (based on PANI mass),
close to
the theoretical capacitance, and a large area-normalized specific capacitance
of
approximately 2.5 F/cm2 (equivalent to a volumetric capacitance of
approximately 230
F/cm3) at 1 A/g. The hybrid electrodes also exhibit an rate capability with an
energy
density of 109.9 Wh/kg and a maximum power density of 265.1 kW/kg at a high
current
density of 100 A/g, respectively, and long-term cycling stability
(approximately 7% loss
of its initial capacitance after 2000 cycles), with a coulombic efficiency of
approximately
99.8%. Moreover, all-solid-state flexible supercapacitors based on the hybrid
CC/GPs/PANI electrodes are also fabricated, which show beneficial
electrochemical
properties, outperforming the reported all-solid-state flexible
supercapacitors up to date.
Such improved performance indicate that the hybrid nanocomposite electrodes
can be
used for supercapacitors.
53
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 3.9A shows the morphology and microstructure of pure carbon cloth at low
(FIG. 3.9A inset) and high magnifications. The diameter of a carbon fibre in
the carbon
cloth is approximately 9 microns. The surface of a carbon fibre is relatively
smooth. FIG.
3.9B displays the morphology and microstructure of GPs fully covering carbon
fibres at
low and high (FIG. 3.9B inset) magnifications. GPs are grown approximately 500
nm out
from the carbon fibre surface and the typical span width of a single
unwrinkled two-
dimensional (2-D) plane ranges from 100 nm to 500 nm. The thickness of the 2-D
GP
plane can reach several nanometers, corresponding to less than 50 graphene
layers.
TEM characterizations of such GPs on carbon fibers indicates that the fiber-
petal
transition is continuous which facilitates electron transport at the interface
between
carbon fibers and GPs. The diameter of a carbon fibre decorated with GPs does
not
change noticeably.
FIGs. 3.9B (inset) and 3.90 are comparative SEM images of GPs before and
after electrochemical polymerization. Apparently, before the electrochemical
polymerization, 2-D unwrinkled GP surfaces are thin and smooth; however, after
electrochemcial polymerization for 5 min, PANI are conformally coating GP
surfaces,
making them rougher and relatively thicker. The PANI mass can be easily
adjusted by
controlling the electrochemical polymerization time. FIG. 3.9D shows
comparative
Raman spectroscopy of CC, CC/GPs and CC/GPs/PANI. Apart from the D and G bands
in the Raman spectroscopy of CC/GPs/PANI hybrid materials, another two new
representative peaks (circle indicated), indexed at 1167 cm-1 and 1468 cm-1,
are due to
the presence of PANI structure, corresponding to C-H vibrations in
quinoid/phenyl
groups and semiquinone radical cation structure in PANI.
54
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 3.10A displays the comparison of area-normalized specific capacitance of
Pure CC, CC/GPs, CC/PANI and CC/GPs/PANI at different scan rates.
Electrochemical
polymerization time for both CC/PANI and CC/GPs/PANI electrodes are 5 min.
Pure CC
contributes negligible area-normalized specific capacitance to the electrodes
(0.01
F/cm2 at a scan rate of 2 mV/s). After decorating GPs on CC by PECVD method,
the
area-normalized specific capacitance of the composite electrode reaches 0.7
F/cm2 at a
scan rate of 2 mV/s and decreases slightly with increasing scan rate. In order
to
demonstrate the structures of GPs in the electrochemical charge storages in
some
embodiments, a pure CC is electrochemically coated with PANI for comparison
with the
hybrid composite electrode of CC/GPs/PANI with the same PANI
electropolymerization
time (5 min). At a scan rate of 2 mV/s, the area-normalized specific
capacitance of
CC/GPs/PANI reaches 1.84 F/cm2, approximately one order of magnitude higher
than
that of CC/PANI (0.19 F/cm2). At a scan rate of 100 mV/s, the area-normalized
capacitance remains 71 %, higher than the reported value in reduced graphene
oxide/PANI electrodes containing binder (approximately 50 % retention at 100
mV/s),
indicating beneficial rate capabilities of the hybrid CC/GPs/PANI electrode.
To find out how GP structures affect the efficiency of PANI in the
pseudocapacitive reactions with acidic electrolyte, FIG. 3.10B shows the
comparison of
mass specific capacitance based only on PANI for both pure CC and CC/GP
substrates.
Apparently, PANI coated on CC/GP substrates has much higher mass specific
capacitance than that on pure CC substrates. At a scan rate of 2 mV/s, the
mass
specific capacitance of PANI is approximately 3 times as high as that on pure
CC,
indicating that the unique GP structures play a synergetic role utilizing PANI
in
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
electrochemical reactions. FIG. 3.100 shows the Ragone plot for the CC/GPs/PAN
I
composite electrode at the potential window of 0.8 V in 1 M H2SO4 aqueous
electrolyte.
The energy density decreases from 202.2 to 109.9 Wh/ kg, while the maximum
power
density increases from 118.5 to 265.1 kW/kg, as the galvanostatic
charge/discharge
current increased from 1 to 100 Ng. FIG. 3.10D shows the specific capacitance
retention of the CC/GPs/PANI hybrid electrode as a function of
charge/discharge cycling
numbers. The composite electrode showed approximately 7% loss in the
capacitance
after 2000 charge-discharge cycles, indicating excellent long-term stability.
Coulombic
efficiency of the hybrid electrode is approximately 99.8%, indicating high
efficiency of
the rapid electron-transfer for charge storage and delivery.
FIG. 3-5A shows the schematic illustration of all-solid state highly flexible
CC/GPs/PANI supercapacitors based on PVA-H2SO4 polymer gel electrolyte.
Galvanostatic charge/discharge performances were carried out on an individual
flexible
device in FIG. 3-5B, which shows that charge/discharge curves of a CC/GPs/PANI
paper-like supercapacitor at different constant current densities ranging from
1 A/g to 50
Ng. Comparison of the specific energy and power density (per cm3 of stack) of
typical
electrolytic capacitors, supercapacitors and batteries in a Ragone plot is
shown in FIG.
3-5C. It compares the performance of our all-solid-state flexible device with
the current
various kinds of state-of-the-art commercial energy storage devices. The
CC/CPs/PANI
based supercapacitor exhibit energy densities of up to 3.38 mWh/cm3, a value
that
reaches the upper range of the lithium thin-film battery and almost
approximately 10
times as high as that of the commercial 3.5V/25-mF supercapacitor. The cycling
life
tests over 1000 cycles for the CC/GPs/PANI hybrid electrode at a current
density of 5
56
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
MA/CM2 were carried out using constant current galvanostatic charge/discharge
cycling
techniques in the potential windows from 0 to 0.8 V, as shown in FIG. 3-5D.
Approximately 10 % loss in capacitance after 1000 cycles and coulombic
efficiencies of
the hybrid electrode of approximately 99.5% were measured for the device,
indicating a
relatively good stability and high efficiency of the rapid electron-transfer
for charge
storage and delivery. To show the practical application of highly flexible and
all-solid-
state paper-like supercapacitors, we prepared three supercapacitor units (each
size
approximately 0.5 cm x approximately 2.0 cm) in series to light a green light-
emitting-
diode (LED, the lowest working potential is 1.5 V). The as-prepared
supercapacitor
group shows no performance degradation when in highly flexible conditions, as
shown
in FIG. 3-5E. CV curves of the supercapacitor group (scanning from 0 V to 2.5
V) in
both normal and bend conditions almost overlaps, indicating the highly
flexibility of the
device. FIG. 3-5F demonstrated that three highly flexible devices in series,
wrapped
around a glass rod (inset), were used to light a green LED well. After being
charged at
2.5 V for 15 min, the highly flexible device could light the LED very well for
more than 30
min.
Symmetric micro-supercapacitors can include several-micrometer-thick layer
graphitic petals, synthesized by micro-wave plasma enhanced chemical vapor
deposition and patterned by conventional optical lithography and reactive ion
etching
techniques on oxidized silicon substrate. High charge/discharge rates up to
100,000
mV/s, three orders of magnitude higher than conventional supercapacitors, have
been
measured for the microdevices in 1 M H2504 aqueous electrolyte. After
electrochemical
oxidation of the graphitic petals, a high volumetric capacitance of
approximately 270
57
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
F/cm3 (equivalent to an area-normalized capacitance of 108 mF/cm2) was
calculated at
a scan rate of 20 mV/s, two orders of magnitudes higher than before the
electrochemcial oxidation, while still maintaining high charge/discharge
rates. The
micro-meter sized device exhibited an energy density of 4 mWh/cm3
(approximately 1.6
pWh/cm2) and a power density of 48 W/cm3 (approximately 192 mW/cm2) at a
current of
100 pA and excellent cyclic stability (1% capacitance loss after 1,500
cycles).
FIG. 4.1A shows SEM images of the GP micropatterns with Ti/Au coated on the
top of the surfaces. The SEM images show that the width of the electrodes and
the gap
between are 30 pm and 35 pm, respectively. FIG. 4.1B displays a magnified SEM
image of micro-patterns, showing that Ti/Au metal films are coated on the
surface of the
GPs. FIG. 4.10 shows side-view SEM images of GP micropatterns at a low
magnification and a high magnification (inset). The thickness of the GP micro-
electrodes
is approximately 4 pm. The inset image shows the morphology of the GP
structures with
sharp edges. The thickness of the first layer of CNWs is typically limited to
several
micrometers generally < 3 pm because of the restricted intrinsic electric
field strength
across the sheath region. The thickness of the two dimensional (2D) carbon
nanowalls
(or the first carbon nanowalls layer) to reach approximately 2 pm. In order to
achieve 3D
GP growth, apart from the first 2D GP layer growth, a second growth was
carried out by
MPCVD after functionalizing the first 2D layer with ¨0- or ¨000- groups by
oxidizing
them in 2 M HNO3 at 95 C for 6 h. In this process, 3D GP networks can be
easily
synthesized by a one-step method because of the unique setup during the growth
process (elevated substrates). The thickness of the GP electrode can be
controlled by
58
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
the growth time and plasma power. 3D GP networks comprise many GP layers
nesting
on each other, demonstrated by the boundaries of the GP layers outlined in
FIG. 4.1D.
FIG. 4.3A shows specific capacitances vs. scan rates (as high as 100,000 mV/s)
of GP-based micro-supercapacitors before electrochemical oxidation. The
volumetric
capacitances calculated for GP electrodes in 1M H2SO4 electrolyte is higher
than the
reported values (1.3 F/cm3) for OLCs in 1 M Et4NBF4/anhydrous propylene
carbonate.
After electrochemical oxidation of the graphitic petals, a high volumetric
capacitance of
approximately 270 F/cm3 (equivalent to an area-normalized capacitance of 108
mF/cm2)
was calculated at a scan rate of 20 mV/s (see FIG. 4.36), two orders of
magnitudes
higher than before the electrochemcial oxidation, while still maintaining high
charge/discharge rates. At 10,000 mV/s, the volumetric capacitance of the
micro-device
remains 75 F/cm3. FIG. 4.30 shows the Ragone plot energy density versus power
density for as-prepared micro-supercapacitors and the up-to-date reported
values for
different materials- rGO in both sandwich (rGO-S) and planar (rGO-P)
structures, ACs,
OLCs and carbon micro-beads (CM65) in organic electrolytes. Noticeably, the
volumetric power and energy densities of GP-based micro-supercapacitors with
aqueous electrolytes are comparable to or even higher than up-to-date micro-
supercapacitors with organic electrolytes, indicating the potential of using
such
structures in electrodes for micro-power application. FIG. 4.3D shows the
micro-
supercapacitors exhibits good cyclic stability (1% capacitance loss after
1,500 cycles).
CNTs, particularly vertical aligned CNT arrays (VCNTs), exhibit usefullness as
supercapacitor electrode materials. . Therefore, new fabrication techniques
are still
needed to achieve high ordered CNT array electrodes with excellent horizontal
59
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
electronic properties and mechanical robustness. In this disclosure, GPs were
selectively grown on CNT patterns for micro-supercapacitor electrode
application. The
uniqueness of the GP strengthening CNT vertical arrays overcomes the problems:
(1)
GPs intercalate CNTs arrays, further reduces the contact resistance between
CNTs and
also improves contacts between CNTs and bottom metal layers. (2) GPs enhance
the
mechanical robustness of the VCNT arrays and the orientation of VCNT arrays
maintains when wetted by electrolytes, which facilitates ion diffusion during
charge and
discharge process. (3) GPs further increase surface area of the electrodes and
thus
increases specific capacitances. The schematic of fabrication process of
GP/CNTs are
showing in FIG. 4.4.
Before the growth, Ti/Al/Fe tri-layer catalysts (30/10/5 nm) were deposited on
Si/Si02 wafer by Vecco thermal evaporator at a base pressure of 1.0 x 10-
7Torr. Then
the substrates are loaded on a 55-mm-diameter Mo puck in the MPCVD chamber.
During the growth of the CNT micro-conduits, H2 (50 sccm) and CH4 (10 sccm)
were
introduced as gas sources, with a pressure of 10 Torr total pressure. The
plasma power
during the growth was 300 W and the substrates were heated to 800 C. The CNT
growth time in this work was 30 min.
For the selective growth of GPs on CNTs, as-prepared CNT patterns on Si/Si02
substrates, elevated 15 mm above a 55-mm-diameter Mo puck by ceramic spacers,
were subjected to the same MPCVD system with a condition of H2 (50 sccm) and
CH4
(10 sccm) as the primary feed gases at 30 Torr total pressure. The GP growth
time was
6 min. The plasma power is 500 W during the growth process. This plasma is
sufficient
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
to heat the samples from room temperature up to approximately 1100 C, as
measured
by a dual-wavelength pyrometer (Williamson PRO 92).
FIG. 4.5A shows SEM characterization of interdigitated CNT/GP patterned
electrodes for micro-supercapacitors at a low magnification. The spaces
between two
adjacent electrodes are 100 pm and GP-free zones. FIG. 4.5B shows a tilted SEM
image of a CNT/GP electrode. FIG. 4.50 and 4.5D are a top-view and side-view
SEM
images of CNT/GP electrodes, respectively.
FIG. 4.6 shows the electrochemical characterization of CNT/GP patterned
electrodes for micro-supercapacitors. FIG. 4.6A shows the cyclic voltammetry
curves of
patterned CNT/GP electrodes at different scan rates in 1 M H2SO4 aqueous
electrolyte.
FIG. 4.6B shows the charge/discharge curves of CNT/GP-CNT/GP electrodes at
different current densities. FIG. 4.60 displays comparative CV curves of micro-
supercapacitors based on CNT-CNT and CNT/GP-CNT/GP electrodes at a scan rate
of
mV/s. FIG. 4.6D displays comparative CV curves of CNT and CNT/GP electrodes at
15 a scan rate of 20 mV/s in a three-electrode system (Ag/AgCI as a
reference electrode).
Both FIGs. 4.60 and 4.6D show that decorations of GP on arrays significantly
reduces
the internal resistances, making the CV curves more rectangular.
Materials, facilities and experiments: Ni foam (MTI Corp., thickness: 1.6 mm,
purity> 99.99%, surface density: 350 30g/mA2 and porosity: 95`)/0) was used as
a 3-D
20 template to grow GPs in a MPCVD system. Before the growth, the Ni foam
was
compressed (700 LBs press force, Fairweather, model: HIP 143) from 1.6 mm to
110 pm
in order to couple well with hydrogen plasma. The Ni foam substrate, with a
diameter of
12 mm, was elevated 17 mm above a Mo puck by ceramic spacers. The sample was
61
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
subjected to MPCVD conditions of H2 (50 sccm) and CH4 (10 sccm) as the primary
feed
gases at 20 Torr total pressure. The plasma power was 500W. The growth time
was 30
min. The Ni foams covered with graphite petals were immersed in a PMMA
solution (4
wt% in ethyl lactate), and then baked at 1800 for 30 min. The samples were
then put
into a 3M HCI solution at 80 C for 5 h to completely dissolve the nickel to
obtain GP
foam-PMMA composite. Finally free-standing GP foams were obtained by
dissolving the
PMMA with hot acetone at 55 C. SEM images of free-standing GP foams (see FIGs.
4.7a-c) after removing Ni templates can be seen as in FIGs. 4.7d and 4.7e. GP
ligaments can be as thin as lpm with a hollow channel inside (See FIGs. 4.7e).
Ti/Al/Fe tri-layer catalysts (30/10/5 nm) were deposited on carbon cloth
substrates by Vecco thermal evaporator at a base pressure of 1.0 x 10-7 Torr.
Then the
substrates are loaded on a 55-mm-diameter Mo puck in the MPCVD chamber. During
the growth of the CNT micro-conduits, H2 (50 sccm) and CH4 (10 sccm) were
introduced
as gas sources, with a pressure of 10 Torr total pressure. The plasma power
during the
growth was 300 Wand the substrates were heated to 800 C. After 10 min of
growth,
CNTs are growing on the surface of carbon micro-fibers in the shape of
conduits, with
an outer diameter of 30 - 40 pm, as shown in FIGs. 4.8a and 4.8b.
For the growth of GPs on CNT micro-conduits, as-prepared CNT/CC substrates,
elevated 15 mm above a 55-mm-diameter Mo puck by ceramic spacers, were
subjected
to the same MPCVD system with a condition of H2 (50 sccm) and CH4 (10 sccm) as
the
primary feed gases at 30 Torr total pressure. The GP growth time was 25 min.
The
plasma power is 500 W during the growth process. This plasma is sufficient to
heat the
samples from room temperature up to approximately 1100 C, as measured by a
dual-
62
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
wavelength pyrometer (Williamson PRO 92). FIG. 4.9 displays the SEM images of
GPs
grown on surfaces of CNTs. FIG. 4.9a shows CNT/GP micro-conduits on CC
substrates
at a lower magnification. The shape of CNT/GP micro-conduit is similar to CNT
conduit
on CC. FIG. 4.9b displays a CNT/GP micro-conduit in a heart shape. FIGs. 4.9c
and
4.9d show GPs decorating CNT structures at a higher magnification. The typical
span
width of a single unwrinkled 2-D plane ranges from 100 nm to 200 nm. The
thickness of
the 2-D GP planes can reach several nanometers, corresponding to less than 50
graphene layers. CNT/GP micro-conduit consists of a lot of tree-branch
structures, in
which CNTs serves as a branchlet and GPs as leaves as illustrated in FIG.
4.9d.
There are several aspects of CNT micro-conduits decorated by GP structure: (1)
GPs increase the specific areas of the CNT structure. (2) GPs improve the
mechanical
properties of CNT micro-conduits by intercalating CNT tightly together. The
enhanced
mechanical robustness of these CNT/GP micro-conduits was demonstrated by using
concentrated acid to modify the CNT/GP micro-conduit surfaces. After
concentrated
acid treatment (H2SO4: HNO3 volume ratio = 3: 1) at 40 C for 3 hours, the
CNT/GP
micro-conduit structure still maintains. (3) GPs further improve the electron
conductivity
of the conduit and reduce contact resistance between CNTs.
Cyclic voltammetry characterization of CNT/GP micro-conduit electrodes with
different scan rates in a three-electrolyte system is shown in FIG. 4.10. A
specific
capacitance of as high as 0.8 F/cm2 at 2 mV/s was achieved, indicating that
the hybrid
electrodes are ideal candidates as electrodes for electrochemcial
supercapacitors and
lithium ion batteries.
63
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Hybrid manganese dioxide/graphitic petal structures grown on carbon nanotube
substrates are shown to achieve high specific capacitance, energy density,
power
density, and long cycle life for flexible supercapacitor applications.
Vertical nanoscale
graphitic petals were prepared by microwave plasma chemical vapour deposition
on
commercial carbon nanotube substrates and subsequently coated with a thin
layer of
Mn02. The graphitic petal/carbon nanotube architecture without any binder
provides an
efficient scaffold for maximizing the electrochemical performance of Mn02. A
specific
capacitance (based on the mass of Mn02) of 580 F/g is obtained at a scan rate
of 2
mV/s in 1 M Na2SO4 aqueous electrolyte. The energy density and power density
at 50
Ng are 28 Wh/kg and 25 kW/kg, respectively. In addition, the composite
electrode
shows long-term cyclic stability (less than 10% decrease in specific
capacitance after
1000 cycles) while maintaining a small internal resistance. Parallel density
functional
studies were performed to investigate the stability and electronic structure
of the
Mn02/graphene interface.
Electrochemical capacitors (ECs), known as supercapacitors or ultracapacitors,
with high power density, fast power delivery and long cycle life, promise to
complement
or even replace batteries in energy storage applications such as
uninterruptible back-up
power supplies, load-leveling, portable electronics, hybrid electronic
vehicles and
renewable energy systems. To achieve high power and high energy density,
suitable
electrode materials should undergo fast reversible redox reactions. Metal
oxides (e.g.,
Mn02, Ru02, VO, Fe203) offer high pseudocapacitance through fast and
reversible
redox reactions near the surface of active materials. Because of its high
specific
capacitance (720 F/g), Ru02 is one of the most promising candidates for ECs.
Mn02,
64
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
with low cost, low toxicity, and most importantly high theoretical specific
capacitance
(-1370 F/g) has attracted much attention as a pseudocapacitive electrode
material.
However, its poor electric conductivity (10-5-10-6 S/cm) and its tendency to
function
capacitively in thin surface layers create practical challenges to realizing
its high
theoretical capacitance.
Carbon materials (e.g., carbon nanotubes, carbon fibres, activated carbon,
graphene) are useful as supercapacitor electrodes due to high specific area,
high
conductivity and low mass density. Among these, vertical graphene nanosheets
or
graphitic petals (GPs) are useful as active electrode materials in ECs.
However, to date,
this highly conductive and two-dimensional (2-D) carbon nanosheet structure as
a
nanotemplate has not yet been systematically studied and optimized to exploit
the
electrochemical properties of the pseudocapacitive materials (e.g., metal
oxide).
Various embodiments of the present invention pertain to the EC performance of
vertical GPs grown by microwave plasma chemical vapor deposition (MPCVD) on
flexible commercial buckypaper (BP). The BP provides a light, flexible, and
mechanically robust substrate for GP growth. This substrate, when coated with
a thin
Mn02 layer, forms an architecture referred to as a Mn02/GP/BP composite
electrode.
The GP/BP architecture offers an effective scaffold for exploiting the
electrochemical
behavior of Mn02, realizing high energy and power density characteristics for
electrochemical supercapacitor applications.
The formation of petals in one embodiment usesa plasma environment. Briefly,
the plasma source consists of a 2.45 GHz frequency microwave power supply with
variable power. Commercial buckypaper (Nanocomp Technologies, Inc., USA),
washed
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
in 6 M HNO3 for 15 min to eliminate the residuals and surfactant before GP
growth, was
used as substrates to grow GPs. The substrates, elevated 9 mm above a Mo puck
by
ceramic spacers, were subjected to MPCVD conditions of H2 (50 sccm) and CH4
(10
sccm) as the primary feed gases at 30 Torr total pressure. The substrates were
initially
exposed to hydrogen plasma for approximately 2 min, during which the plasma
power
gradually increased from 300 W to 600 W. The GP growth duration was 20 min.
The
typical dimensions of the samples were 15 mm x 5 mm.
To make GP/BP composites suitable for electrochemical electrodes prior to
Mn02 coating or electrochemical measurement, concentrated H2SO4 and HNO3
(volume
ratio 3:1) were used to functionalize the surface of GPs at 50 C for 2 hours
in an oven.
The samples were then washed in deionized water and dried at 100 C overnight.
A
neutral precursor solution (pH 7) for the Mn02 coating process was prepared by
mixing
0.1 M Na2SO4 (Alfa Aesar) and 0.1 M KMn04 (Alfa Aesar) solutions. The GPs
grown on
BP were immersed into the solution, which was kept at 80 C in an oven for 40
min. The
loading amount was controlled by adjusting the immersion time. The sample was
then
rinsed with deionized water and subsequently annealed at 200 C for 3 hours
using a
hotplate in air. The mass of coated Mn02 was calculated from the weight
difference
before and after the coating process. The loading amount of Mn02 in this study
is
approximately 110 pg, measured using a microbalance with an accuracy of 1 pg.
The electrochemical performance of the Mn02/GP/BP hybrid structure was
evaluated using a BASi Epsilon electrochemical system (Bioanalytical Systems
Inc.,
Indiana, USA). The standard three-electrode cell consisted of Ag/AgCI as the
reference
electrode, Pt mesh as the counter electrode and the synthesized composite
sample as
66
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
the working electrode. A 1 M Na2SO4 solution served as the electrolyte at room
temperature. Scan rates of 2, 5, 10, 20, 50, and 100 mV/s were employed for
cyclic
voltammetry, and charge/discharge measurements were carried out at different
current
densities of 5, 10, 20, 30, 40 and 50 A/g. Long-term cyclic stability of the
composite
electrodes was evaluated repeatedly at 100 mV/s for 1000 cycles. A potential
window in
the range from 0 to 0.8 V was used in all measurements. A Hitachi S-4800 field
emission scanning electron microscope (FESEM) was used to image the surface
morphology of all the samples.
Throughout this study, multiple samples were prepared under identical
conditions
to test for reproducibility of the processing conditions. CV data acquired
from the
multiple samples could be reproduced to within 5%.
To understand the electronic structure of the Mn02/GP composite large clusters
of (4x2) Mn02 were simulated on a graphene supercell (6x6) using density
function
theory (DFT). Although in real cases the Mn02 structure displays diverse
conformations
with edge- and corner-sharing Mn06 possessing various pore sizes within the
range of
approximately 0.19 nm to approximately 0.46 nm, with a distribution of Mn
cations
among the network of oxygen atoms, we employed the simplest configuration.
Mn02
forms many different crystallographic structures. The different structures are
characterized by atomic-scale pores (also called tunnels) which penetrate
throughout
the material. Electronic structure calculations were carried out by DFT with
the plane-
wave self-consistent field (PWSCF) code. The generalized gradient
approximation
(GGA) was implemented to estimate the exchange correlation energy of
electrons.
Ultrasoft pseudopotentials were used to represent the interaction between
ionic cores
67
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
and valence electrons. Kohn-Sham wave functions were represented with a plane-
wave
basis using an energy cutoff of 40 Ry and charge density cutoff of 240 Ry. A
uniform
mesh of k points (5 x 5 x 1) was taken for integration over the Brillouin
zone.
SEM images of GPs synthesized by MPCVD are shown in FIG. 4-1a. The petals
extend approximately 500 nm from the BP surface, and the typical span width of
a
single unwrinkled 2-D petal ranges from 100 nm to 500 nm. The thickness of a
GP can
reach several nanometers, corresponding to less than 50 graphene layers. A
magnified
image of one petal marked by the rectangular box in FIG. 4-la is shown in FIG.
4-1b,
revealing the smooth surfaces of the GPs. These surfaces provide easily
accessible
sites for Mn02 coating. The crumpled structures of the vertical graphene
sheets with
both sides exposed to Mn02 precursor solution offer large specific area for
coating. FIG.
4-1c shows the morphology of Mn02 coated on GPs. FIG. 4-1d contains a
magnified
image of the area marked by the rectangular box in FIG. 4-1c, clearly showing
a thin
uniform layer of Mn02 on the smooth GP surfaces, even on the smaller petals.
Previous studies suggest that Mn04- ions can be reduced spontaneously to Mn02
on the surface of carbon nanotubes by oxidizing exterior carbon atoms via the
following
redox reaction:
4Mn04- + 30 + H20 ¨ 4 Mn02 + C032- + 2HCO3-
(1)
A similar mechanism applies here in the case of Mn02 coating on GP surfaces.
Reduction of permanganate ion (Mn04-) to Mn02 on carbon is pH-dependent.
Neutral
pH solution leads to thin films of Mn02, while acidic solution can result in
large
agglomerated Mn02 particles. Consequently, the thin film of Mn02 coated on GPs
can
be attributed to the neutral electrolyte used in this disclosure.
68
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
FIG. 4-2a shows cyclic voltammetry (CV) curves of the Mn02/GP/BP composites
at scan rates of 2, 5, 10, 20, 50, 100 mV/s in 1 M Na2SO4 aqueous solution
with
potential windows ranging from 0 to 0.8 V. The advantages of the unique
electrochemical behaviours of Mn02/GP/BP electrodes are apparent in FIG. 4-2b
which
shows a comparison of CV curves for BP, GP/BP, Mn02/BP and Mn02/GP/BP at a
fixed
scan rate of 10 mV/s. The shapes of these curves are quasi-rectangular,
indicating the
presence of electrical double-layer capacitance and pseudocapacitance. The
Mn02-
coated GP/BP architecture involves redox reactions in the cyclic voltammetry
tests as
Mn atoms are converted into higher/lower (IV/111) oxidation states. These
conversions
are induced by intercalation/extraction of H30+ or alkali cations (Na) to/from
the Mn02
outer layer. The mechanism of this reaction can be expressed as the following
reaction:
(Mn02) surface + X+ + e- (Mn00X) surface
(2)
(X+ = Na + or H30+)
The average specific capacitance from CV curves was determined by the
following formula:
1
C= _____________________________________________________ I(V)dV
(3)
where C is the specific capacitance in F/g, s is the scan rate in V/s, M is
the mass of the
added Mn02 to the electrodes in g, Vh and V, are high and low potential limits
of the CV
tests in V, I is the instantaneous current on CV curves, and V is the applied
voltage (V).
The specific capacitance of BP at a scan rate of 2 mV/s calculated from the CV
curves
is 27 F/g, which is comparable to reported values for CNTs. The specific
capacitance of
GP/BP (based on total mass of the two components) calculated at 2 mV/s is 47
F/g,
69
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
which is approximately 70% higher than that of bare BP. The same improvement
(-70%) was also observed in H2SO4 electrolyte, indicating an inherent
improvement in
specific capacitance after GP growth on BP. This result is attributed to an
increase in
the specific area after growing GPs on the BP substrate coupled with electric
field
enhancement introduced by the sharp edges of the GPs.
The specific capacitance of the Mn02/GP/BP composites was calculated based
on the mass of pristine Mn02 for the following reasons: (1) The surface of
carbon was
coated with Mn02; consequently, the carbon materials would participate weakly
in the
charge storing process as charge is primarily stored at the outer layer of
Mn02 through
a Faradic reaction. (2) The specific capacitances of BP and GP/BP are mainly
of the
electrostatic double-layer type and are far smaller than the specific
capacitance of Mn02
caused by Faradic redox reactions, making it reasonable to calculate the
specific
capacitance based on the mass of pristine Mn02.
FIG. 4-2c shows comparative specific capacitances of BP, GP/BP, Mn02/BP and
Mn02/GP/BP calculated from CV curves at voltage scan rates from 2 to 100 mV/s.
At a
scan rate of 2 mV/s, the specific capacitance of the Mn02/GP/BP hybrid
composite
reaches 580 F/g (based on the mass of pristine Mn02). At a high scan rate of
100 mV/s,
the specific capacitance of Mn02/GP/BP still remains close to 320 F/g, which
is
comparable to the rate performance reported by others. However, for the same
Mn02
coating time, the specific capacitance of Mn02/BP is only about 266 F/g (based
on
pristine Mn02) at 2 mV/s (see FIG. 4-2c). The superior rate capability of
Mn02/GP/BP
composites demonstrates the advantages of this new architecture of GP/BP as a
highly
conductive scaffold for maximizing the utilization of the practical
electrochemical
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
performance of Mn02. Since previous studies show that only a very thin layer
of Mn02
is involved in the charge storage process, the specific capacitance of Mn02
coated on
the supporting GP/BP can likely be further improved by optimizing the
thickness of
coated Mn02 layer.
Constant-current charge/discharge curves of the as-prepared Mn02/GP/BP
hybrid structure at different current densities are shown in FIG. 4--2d. The
charge/discharge curves display a symmetric shape, indicating that the
structure has a
good electrochemical capacitive characteristic. The specific capacitance
derived from
galvanostatic (GV) tests can be calculated from the following formula:
/
C = (4)
Mu
where Id is the discharge current in A, and u is the slope of the discharge
curve after
the initial potential drop associated with the cell internal resistance (IR
drop). The
specific capacitances derived from the discharge curves agree well with the
results
calculated from CV measurements. At 5 Ng, the calculated specific capacitance
is 493
F/g, which is almost identical to the specific capacitance 497 F/g calculated
at 10 mV/s,
corresponding to an average current density close to 5 A/g (see FIG. 4-2b).
The energy density E (in Wh/kg) and the power density P (in kW/kg) are
important parameters to characterize the electrochemical performance of
supercapacitors. In this disclosure, these quantities were calculated by:
E= CV2
2M (5)
E
P= _
At
(6)
71
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
where V is the applied voltage in volts and At is the discharge time in
seconds. FIG.
3.7d shows the Ragone plot for the Mn02/GP/BP structured electrode at
different
current densities. At a high current density of 50 Ng, the calculated energy
density is 28
Wh/kg, and the average power density is 25 kW/kg. These values are more
promising
than reported energy densities (14.8 Wh/kg) and power densities (2.5 kW/kg) of
electrodeposited Mn02 films on BP substrates. Various embodiments of the
present
invention include Mn02/GP/BP composites as electrode material in
supercapacitor
applications.
Cycle lifetime is a factors in supercapacitor applications. Typical issues
facing
Mn02-based electrodes in aqueous electrolyte include: mechanical expansion of
Mn02
during ion insertion/desertion processes, Mn02 film detachment from electrode
surfaces, and Mn dissolution into electrolyte. A cyclic stability test over
1000 cycles for
the Mn02/GP/BP structured electrode at a scan rate of 100 mV/s was carried out
in a
potential window ranging from 0 to 0.8 V. FIG. 3.7f shows the specific
capacitance
retention as a function of cycle number. The composite electrode shows less
than 10%
loss in specific capacitance after 1000 charge/discharge cycles, indicating
good
capacity retention.
DFT simulations can help to elucidate the fundamental properties at interfaces
between Mn02 and graphene, particularly in terms of lattice stability and
electronic
structure of the composite. A schematic diagram of Mn02 clusters and graphene
(top
view) is shown in FIG. 4-3a. In this configuration, constrained relaxation was
carried out
(only atomic positions of the Mn02 are allowed to relax, with initially
relaxed graphene).
The Mn02/GP composite is relaxed with energy converged to less than 2
kcal/mol. The
72
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
formation energy of the composite is calculated to be 128 kcal/mol, suggesting
covalent
bonding between Mn02 and graphene. During the charge/discharge process, we
expect
the composite to undergo compressive/tensile stresses. To mimic the phenomena,
we
have simulated the structure with various pressure values in the supercell.
From the
electronic density of states, the composite exhibits metallic behaviour
(finite density of
states at EF) in both cases, and this metallic state changes little with
different stresses
as shown in FIG. 4-3b, where different negative/positive pressures are used to
mimic
compressive/tensile stresses. The comparative electronic density of states of
graphene,
Mn02 and Mn02/graphene (the most stable structure) are shown in FIG. 4-3c.
The low interfacial resistance achieved in the Mn02/GP/BP electrode is a
matter
of interest, and we use the results of DFT calculations to provide further
insight into this
result. The iso-electronic charge contour plot drawn in FIG. 4-3(d) is a two-
dimensional
cut of the charge density in a vertical plane that contains the yellow line
drawn parallel
to the zig-zag direction as shown in FIG. 4-3(a). This vertical plane was
chosen to
highlight the redistribution of charge from the graphene layer toward the
oxygen atoms
in Mn02. Further iso-electronic contour plots in different planes are provided
in the
supplementary information. From these plots, we infer that charge transfer at
the
graphene-Mn02 interface is facilitated by the oxygen atoms in the Mn02
complex,
providing some understanding for the low interfacial resistance experienced by
electron
transport through the composite interface. Without this conduction channel,
charge
transfer would be reduced, making the composite less suitable for
supercapacitor
applications.
73
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
A new structure of Mn02/GP/BP has been demonstrated for flexible
supercapacitor electrodes, showing promising electrochemical behavior. The
GP/BP
architecture without any binder provides an efficient scaffold for maximizing
the practical
electrochemical performance of Mn02, realizing high specific capacitance, rate
capability and long-term cycle life, high energy density and high power
density. The
metallic nature of the Mn02/GP composite provides a facile conduction path for
electron
transport in the charge /discharge process. These results suggest that such a
Mn02/GP/BP architecture may be practically useful for next generation high-
performance supercapacitors.
Another embodiment of the present invention includes a hybrid three-
dimensional
nanoarchitecture by electropolymerizing aniline monomers into a nanometre-
thick
conformal polyaniline (PANI) film on graphitic petals (GPs) that are directly
grown on
highly conductive carbon cloth (CC) through microwave plasma enhanced chemical
vapor deposition (MPCVD) for flexible supercapacitor applications. The hybrid
CC/GPs/PANI electrodes yield greatly improved capacitive performance with a
high
specific capacitance of ¨2000 F/g (based on PANI mass), close to the
theoretical
capacitance, and a large area-normalized specific capacitance of ¨2.5
F/cm2(equivalent
to a volumetric capacitance of ¨230 F/cm3) at 1 Ng. The hybrid electrodes also
exhibit
an excellent rate capability with an energy density of 109.9 Wh/kg and a
maximum
power density of 265.1 kW/kg at a high current density of 100 A/g,
respectively, and an
outstanding long-term cycling stability (-7% loss in its initial capacitance
after 2000
cycles), with a coulombic efficiency of ¨99.8%. Moreover, all-solid-state
flexible
supercapacitors based on the hybrid CC/GPs/PANI electrodes are also
fabricated,
74
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
which show beneficial electrochemical properties, outperforming the reported
all-solid-
state flexible supercapacitors to date.
Some embodiments pertain to the fabrication of a novel hybrid nanoarchitecture
by electropolymerizing aniline monomers into a nanometre-thick PANI film and
conformally coating it on GPs that are directly grown on highly conductive
carbon cloth
through MPCVD method. Such unique 3D porous networks, without any binder, not
only allow large loading of active electrode materials but also facilitate
easy access of
electrolytes to the electrodes. In 1 M H2504 aqueous electrolyte, CC/GPs/PANI
electrodes yield greatly improved capacitive performance with a specific
capacitance of
1502 F/g (-2000 F/g at a current density of 1 Ng) at 2 mV/s (5 min of PANI
electropolymerization), ¨3 times as high as that of CC/PANI and an area-
normalized
specific capacitance of ¨2.5 F/cm2(equivalent to a volumetric capacitance of
¨230
F/cm3) at 1 Ng, ¨10 times as high as that of CC/PAN I, outperforming many
other
currently available carbon-based electrodes. Such rationally designed ECs also
exhibited ultrahigh energy and power densities and excellent cycling
performance. To
demonstrate their promising applications as flexible power sources, all-solid
state and
paper-like flexible supercapacitors based on CC/GPs/PANI were also fabricated,
exhibiting excellent electrochemical properties, and demonstrated to light a
LED. A
nanoscale electrode based on such highly conductive, porous and 3D frameworks
can
provide breakthroughs for designing future multifunctional ECs.
As-prepared GPs serve as highly graphitic and conductive templates, which
PANI films were subsequently coated conformally on by electropolymerization of
aniline
monomers. Schematic illustrations of such novel hybrid CC/GPs/PANI
nanostructures
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
are shown in FIG. 3-1. This strategy has several advantages: (1)
Macroscopically, 3D
carbon cloth would provide a flexible and highly conductive substrate and
create
channels for fast and effective electrolyte ion transport. (2) Highly
graphitic and
conductive GPs would largely increase the specific surface of the electrodes
and
provide a direct path for the electrons transport. The sharp edges of GPs
would not only
largely increase the charge storage but also speed up the ion diffusion due to
lower
energy barriers. (3) A nanoscale thin layer of PANI would enable a fast,
reversible
faradic reaction and provide a short ion diffusion path.
The novel 3D nanostructure was achieved in one embodiment by two-step
methods. First, carbon cloth substrates, elevated 9 mm above a Mo puck by
ceramic
spacers, are subjected to MPCVD conditions of H2 (50 sccm) and CH4 (10 sccm)
as the
primary feed gases at 30 Torr total pressure for 25 min.. SEM images in FIG. 3-
2A show
the morphology and microstructure of pure carbon cloth at low (inset) and high
magnifications. The diameter of a carbon fibre in the carbon cloth is
approximately 9
microns. The surface of a carbon fibre is relatively smooth. FIG. 3-2B display
the
morphology and microstructure of GPs fully covering carbon fibres at low and
high
(inset) magnifications. These GPs uniformly cover CC substrate in a large
scale,
providing a basis for large-scale fabrication process (see FIG. 5-2). GPs are
grown
approximately 500 nm out from the carbon fibre surface and the typical span
width of a
single unwrinkled two-dimensional (2-D) grain ranges from 100 nm to 500 nm.
The
thickness of the 2-D GP plane can reach several nanometers, corresponding to
less
than 50 graphene layers. TEM characterizations of such GPs on carbon fibers in
our
previous work indicates that the fiber-petal transition is continuous which
facilitates
76
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
electron transport at the interface between carbon fibers and GPs. The
diameter of a
carbon fibre decorated with GPs does not change noticeably. The thickness of
CC/GPs
sample grown in this condition is measured to be approximately 110 microns.
These
GPs on carbon cloth are highly hydrophobic and can benefit from further acid
treatment.
Second, electropolymerization process is carried out on a CC/GP sample
impregnated with 20 mL solution containing 0.05 M aniline monomers in 0.5 M
H2504 at
0.8 V versus Ag/AgCI reference electrode. After the polymerization, the as-
prepared
composite was washed in deionized water and then dried at 80 C over 2 hours.
The
adsorbed aniline monomers on the both surfaces of a vertical GP will be
20 Raman spectroscopy is often used to characterize graphene based
materials.
The D band at 1350 cm-1 is known to result from various types of defects and
anomalies
of transverse optical vibrations near the K-point. The G peak at 1580 cm-
larises
because of the doubly degenerate zone center E2g mode. FIG. 3-2D shows
77
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
comparative Raman spectroscopy of CC, CC/GPs and CC/GPs/PAN I. Apart from the
D
and G bands in the Raman spectroscopy of CC/GPs/PANI hybrid materials, another
two
new representative peaks (circle indicated), indexed at 1167 cm-1 and 1468 cm-
1, are
due to the presence of PANI structure, corresponding to C-H vibrations in
quinoid/phenyl groups and semiquinone radical cation structure in PANI.
Electrochemical polymerization time ranging from 0, 30s, 2 min, 5 min, 8 min,
10
min to 15 min is used to study the influence of PANI mass on comprehensive
electrochemical properties including specific capacitance based on active
material and
also the overall area-normalized capacitance of the nanocomposite electrode.
FIG. 3-3A shows both the mass specific capacitance and area-normalized
specific capacitance as a function of electrochemical polymerization time at 2
mV/s for
CC/GPs/PANI electrode. When the electropolymerization process only lasted for
30s,
aniline monomers polymerization on the graphene surfaces was not enough,
consequently only part of PANI covered the surface of GPs, giving high mass
specific
capacitance based on the mass of PANI, while in fact part of the capacitance
comes
from the electric double layer contribution. Meanwhile the area-normalized
specific
capacitance reaches 1.1 F/cm2, higher than that of CC/GP electrode (0.7
F/cm2). PANI
coated on mesoporous carbon monolith carbonized from mesophase pitch at 1000
C
can have a high specific capacitance of 2200 F/g (based on PANI mass), with a
significant contribution coming from electric double layer capacitance [9].
This may be
because of the hydrophobic properties of carbon monolith after carbonization
of
mesophase pitch at high temperature, giving rise to nonuniform coverage of
PANI on
the electrode surface. Moreover, inflexible carbon monolith substrate will
limit its
78
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
practical applications. PANI mass increases and the specific capacitance based
on
PANI significantly decreases as electropolymerization time prolongs. The
overall area-
normalized specific capacitance of the composite electrode gradually
increases,
reaches a saturation when electropolymerization time is 5 min and eventually
reaches
approximately to 2 F/cm2 at a scan rate of 2 mV/s when electropolymerization
time is 15
min.
As PANI polymerization time prolongs, more and more PANI will participate in
the electrochemical reactions, leading to more pseudocapacitance and
consequently
higher area-normalized specific capacitance. However, due to the limited ion
transport
in inner part of active material when PANI is thick and relatively low
electrical
conductivity, only the outer layer of PANI can be utilized in pseudocapacitive
reactions,
giving rise to relatively low mass specific capacitance and also the
saturation of overall
area-normalized specific capacitance. To make a balance between efficiency of
PANI
utilization and overall area-normalized specific capacitance, we choose
electropolymerization time as 5 min for the further discussions in the work.
FIG. 3-3B shows the cyclic voltammetry (CV) curves of the hybrid CC/GPs/PANI
composite electrode (5 min of PANI electropolymerization) at different scan
rates of 2,
5, 10, 20, 50 and 100 mV/s with potential windows ranging from 0 to 0.8 V vs.
Ag/ AgCI
in 1 M H2SO4 aqueous electrolyte. Redox peaks (C1/A1, C2/A2 and C3/A3) from
the CV
curves indicate the presence of pseudocapacitive PANI. Redox transitions
between a
semiconducting state (leucoemeraldine form) and a conducting state (polaronic
emeraldine form) are responsible for peaks C1/A1, and the Faradaic
transformation of
emeraldine pernigraniline initiates the redox peaks C2/ A2. Peaks C3/A3 have
been
79
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
attributed to the formation/reduction of bipolaronic pernigraniline. It is
also noted that the
cathodic peaks (02) shift positively and the anodic peaks (A2) shift
negatively with the
increment of potential sweep rates, which is because of the increment of the
resistance
in the electrodes.
FIG. 3-30 displays the comparison of area-normalized specific capacitance of
Pure CC, CC/GPs, CC/PANI and CC/GPs/PANI at different scan rates.
Electrochemical
polymerization time for both CC/PANI and CC/GPs/PANI electrodes are 5 min.
Pure CC
contributes negligible area-normalized specific capacitance to the electrodes
(0.01
F/cm2 at a scan rate of 2 mV/s). After decorating GPs on CC by PECVD method,
the
area-normalized specific capacitance of the composite electrode reaches 0.7
F/cm2 at a
scan rate of 2 mV/s and decreases slightly with increasing scan rate. In order
to
compare structures of GPs in the electrochemical charge storages, a pure CC is
electrochemically coated with PANI for comparison with the hybrid composite
electrode
of CC/GPs/PANI with the same PANI electropolymerization time (5 min). At a
scan rate
of 2 mV/s, the area-normalized specific capacitance of CC/GPs/PANI reaches
1.84
F/cm2, approximately one order of magnitude higher than that of CC/PANI (0.19
F/cm2).
At a scan rate of 100 mV/s, the area-normalized capacitance remains 71 %,
higher than
the reported value in reduced graphene oxide/PANI electrodes containing binder
(approximately 50% retention at 100 mV/s), indicating the rate capabilities of
the hybrid
CC/GPs/PANI electrode.
FIG. 3-3D shows the comparison of mass specific capacitance based only on
PANI for both pure CC and CC/GP substrates. Apparently, PANI coated on CC/GP
substrates has much higher mass specific capacitance than that on pure CC
substrates.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
At a scan rate of 2 mV/s, the mass specific capacitance of PANI is
approximately 3
times as high as that on pure CC, indicating that the unique GP structures
play a
synergetic role utilizing PANI in electrochemical reactions. This synergetic
role of GPs
can be attributed the following advantages: 1) vertical graphene nanosheets
allow PANI
to be coated on both sides of the GP surfaces, further increasing the specific
area. 2)
moreover, based on the prior work and analysis, we believe that the sharp
edges of GP
further enhance the local electric field, allowing more charges to be stored
along the
edges; sharp edges will also affect the ion diffusion paths on the GP/PANI
surfaces.
SEM images indicate that the edges maintained after coating with PANI.
Rate capability is one factor for evaluating the power applications of
supercapacitors. Galvanostatic constant-current charge/discharge performances
are
evaluated for CC/GPs/PANI hybrid electrode at different constant-current
densities,
ranging from 1A/g up to 100 Ng based on the mass of PANI. The charge/discharge
cycling curves have a symmetric nature, indicating that the composite has a
good
electrochemical capacitive characteristic and superior reversible redox
reaction. This
symmetric nature of the CC curves can be maintained even at a low density of 1
A/g, as
shown in FIG. 3-4A. Charge/discharge curve of the hybrid electrode at higher
current
densities can be seen from supporting information FIG. 5-5, in which the IR
drop at
higher current densities can be seen.
The mass specific capacitance and area-normalized specific capacitance derived
from the discharging curves at different charge/discharge rates (current
densities) are
shown in FIG. 3-4B. At a constant current density of 1 Ng, the calculated mass
specific
capacitance is 1998 F/g, close to the theoretical capacitance of PANI and the
area-
81
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
normalized specific capacitance is approximately 2.56 F/cm2, corresponding to
a
volumetric charge storage of ¨237 F/cm3, which is higher than those reported
values so
far. The highest area-normalized capacitance for PAN I/carbon based electrodes
is
reported to be 1.8 F/cm2. Reduced graphene oxide paper coated with PANI show a
volumetric charge storage of 135 F/cm3 and 160 F/cm3. PANI electropolymerized
on
stainless steel is reported with a area-normalized capacitance of 0.9 F/cm2 at
a deposit
charge of 2.35 C/cm2. Volumetric capacitances of PANI-based electrodes are
usually
higher than those of carbon nanotubes (<16 F/cm3), graphene paper (64 F/cm3),
carbide-derived carbon (61-90 F/cm3) and activated carbons (< 50 F/cm3). Both
mass
specific capacitance and area-normalized capacitance decrease relatively fast
at low
current densities and then stay stable at high current densities. At 100 Ng,
the mass
specific capacitance can still be as high as ¨1200 F/g and area-normalized
capacitance
1.5 F/cm2, which are consistent with the values calculated using CV curves.
Specific energy and power densities are the two factors for evaluating the
power
applications of electrochemical supercapacitors. An electrochemical
supercapacitor can
provide high energy density or high specific capacitance at high charging-
discharging
rates. The internal resistance which can be determined from the initial
voltage drop of
the discharge curves is also an important factor affecting the maximum power
of the
device. At a current density of 100 Ng, the VIR is approximately 0.12 V,
corresponding
to a low internal resistance of 2.5 0. This demonstrates the reduced charge-
transfer
resistance of the CC/GPs/PANI hybrid electrode. FIG. 3-4C shows the Ragone
plot for
the CC/GPs/PANI composite electrode at the potential window of 0.8 V in 1 M
H2504
aqueous electrolyte. The energy density decreases from 202.2 to 109.9 Wh/ kg,
while
82
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
the maximum power density increases from 118.5 to 265.1 kW/kg, as the
galvanostatic
charge/discharge current increased from 1 to 100 Ng.
Another aspect for supercapacitor applications is cycling capability or
cycling life.
The cycling life tests over 2000 cycles for the CC/GPs/PANI hybrid electrode
at a
current density of 10 mA/cm2 were carried out using constant current
galvanostatic
charge/discharge cycling techniques in the potential windows ranging from 0 to
0.8 V.
FIG. 3-4D shows the specific capacitance retention of the CC/GPs/PANI hybrid
electrode as a function of charge/discharge cycling numbers. The composite
electrode
showed ¨7% loss in the capacitance after 2000 charge-discharge cycles,
indicating
long-term stability. Coulombic efficiency of the hybrid electrode is ¨99.8%,
indicating
high efficiency of the rapid electron-transfer for charge storage and
delivery.
The novel hybrid 3D nanostructure electrode shows excellent electrochemcial
properties in a three-electrode testing system, according to one embodiment.
Furthermore, in the application level, the flexible composites also show
potential as
electrodes for advanced flexible all-solid-state supercapacitors with a two-
terminal
configuration. In another embodiment, paper-like CC/GPs/PANI supercapacitors
are
fabricated with improved supercapacitor performance. FIG. 3-5A shows the
schematic
illustration of all-solid state highly flexible CC/GPs/PANI supercapacitors
based on PVA-
H2SO4 polymer gel electrolyte. Macroscopically, as-fabricated devices possess
superior
mechanical properties and show no cracks or any performance degradation under
highly flexible conditions, even in highly bent (180 angle bending) and
twisted
conditions.
83
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Galvanostatic charge/discharge performances were carried out on an individual
flexible device in FIG. 3-5B, which shows that charge/discharge curves of a
CC/GPs/PANI paper-like supercapacitor at different constant current densities
ranging
from 1 Ng to 50 Ng. The charge/discharge curves maintain almost symmetric at
all
current densities. At a current density of 1 Ng, the calculated mass specific
capacitance
is ¨1200 F/g and an area-normalized specific capacitance of ¨1.5 F/cm2 based
on a
single electrode. The internal resistance calculated based on the IR drop
(0.0158 V) is
29 0 at 1 A/g, which is higher than that in aqueous electrolyte in three-
electrode system
due to the intrinsic lower ion conductivity of polymer electrolyte and the
inhomogeneity
of large area sample preparation during device fabrication process, which will
be further
optimized in the future.
Comparison of the specific energy and power density (per cm3 of stack) of
typical
electrolytic capacitors, supercapacitors and batteries in a Ragone plot is
shown in FIG.
3-5C. It compares the performance of our all-solid-state flexible device with
the current
various other energy storage devices. The CC/CPs/PANI based supercapacitor
exhibit
energy densities of up to 3.38 mWh/cm3, a value that reaches the upper range
of the
lithium thin-film battery and almost ¨10 times as high as that of the
commercial 3.5V/25-
mF supercapacitor. Additionally, the CC/CPs/PANI based supercapacitor is able
to
deliver a power density of 3 W/cm3, which is two-orders of magnitude higher
than that of
the Lithium thin-film battery. The cycling life tests over 1000 cycles for the
CC/GPs/PANI hybrid electrode at a current density of 5 mA/cm2 were carried out
using
constant current galvanostatic charge/discharge cycling techniques in the
potential
windows from 0 to 0.8 V, as shown in FIG. 3-5D. ¨10% loss in capacitance after
1000
84
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
cycles and coulombic efficiencies of the hybrid electrode of ¨99.5% were
measured for
the device, indicating a relatively good stability and high efficiency of the
rapid electron-
transfer for charge storage and delivery.
Three supercapacitor units were prepared (each size ¨0.5 cm x ¨2.0 cm) in
series to light a green light-emitting-diode (LED, the lowest working
potential is 1.5 V).
The as-prepared supercapacitor group shows no performance degradation when in
highly flexible conditions, as shown in FIG. 3-5E. CV curves of the
supercapacitor group
(scanning from 0 V to 2.5 V) in both normal and bend conditions almost
overlaps,
indicating the highly flexibility of the device. FIG. 3-5F demonstrated that
three highly
flexible devices in series, wrapped around a glass rod (inset), were used to
light a green
LED well. After being charged at 2.5 V for 15 min, the highly flexible device
could light
the LED very well for more than 30 min.
The ultrathin highly flexible and all-solid state supercapacitor device based
on
CC/GPs/PANI here has already demonstrated the improved flexibility and
electrochemical performances to the current commercial supercapacitor devices.
Many
parameters such as the thickness of the polymer gel, force applied to compress
two
electrodes together, concentration of acid in polymer gel and good electrical
contact
between two individual devices, et al., can be optimized in order to fabricate
highly
flexible devices with better electrochemcial properties.
One embodiment pertains to a novel 3D nanostructure based on CC/GPs/PANI
for highly flexible supercapacitor electrode. Systematic studies were carried
out to
optimized the amount of PANI mass in order to utilize PANI to the maximum
extent
while also maintain a high area-normalized capacitance of the electrode in a
three-
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
electrode testing system. It is found that the existence of GPs significantly
improves the
comprehensive electrochemcial properties of the hybrid electrode, due to the
large
specific surface area and unique sharp edge structures. The CC/GPs/PANI hybrid
electrode shows outstanding electrochemical performances, such as high
specific mass
capacitance as well as high area-normalized and volumetric capacitance, good
cycling
life and high energy and power densities. All-solid-state supercapacitor, with
two slightly
separated CC/GPs/PANI nanocomposite electrodes and PVA-H2SO4 bifunctional
polymer gel as solid-state electrolyte and separator were fabricated and
tested. The
flexible device shows excellent electrochemical performances in specific
capacitance,
energy and power density and cycling life. Features of one embodiment of the
present
invention were demonstrated to light a green LED out under highly flexible
(testing)
conditions to use of this lightweight, highly flexible and all-solid state
polymer based
supercapacitors.
Yet another embodiment of the present invention pertains to the use of
flexible,
conductive preferably carbon-based substrate. Commercial carbon cloth (CC,
Fuel Cell
Earth LLC), made of microfibers, were used directly as substrates without
further
processing for graphitic petal (GP) synthesis by microwave plasma enhanced
chemical
vapor deposition (MPCVD). The schematic diagram of the chamber for the growth
process is shown in FIG. 2-1. The plasma source consists of a 2.45 GHz
frequency
microwave power supply with variable power. Carbon cloth substrates, elevated
15 mm
above a 55-mm-diameter Mo puck by ceramic spacers, were subjected to MPCVD
conditions of H2 (50 sccm) and CH4 (10 sccm) as the primary feed gases at 30
Torr total
pressure. The GP growth time was 25 min. The plasma power is 700 W during the
86
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
growth process. This plasma is sufficient to heat the samples from room
temperature up
to ¨1100 C, as measured by a dual-wavelength pyrometer (Williamson PRO 92).
First, preparation of the H2SO4-polyvinyl alcohol (PVA) gel polymer
electrolyte
was prepared as follows: 6 g H2SO4 was mixed with 60 ml deionized water and
then 6
g PVA powder was added. The whole mixture was heated up steadily from room
temperature to ¨ 90 C under vigorous stirring until the solution became
clear. Then the
dilute polymer electrolyte solution was cooled down to room temperature.
Two pieces of the obtained CC/CPs/PANI nanocomposite sheets (each
geometrical size ¨ 0.5 cm x 2.0 cm, with the edge of one side glued with
silver paste for
a well electrical contact) was immersed in the dilute polymer electrolyte
solution (the
part glued with silver paste was kept out) for 30 min and picked out. The
dilute solution
soaked the inside network of the electrode well and formed a coating layer
around the
surface of the electrode. Then the electrodes with the electrolyte solution
coating on
were left in the fume hood at room temperature for 4 h to vaporize the excess
water.
After the H2SO4-PVA electrolyte became solidified, the two electrodes were
tightly
pressed together into one integrated unit, by sandwiching a thin layer of
viscous
polymer electrolyte between them as an adhesive.
A Hitachi S-4800 field emission scanning electron microscope (FESEM) was
used to image the surface morphology of the samples. A FEI Titan 80-300
operated at
300 kV was utilized for a high-resolution transmission electron microscopy
(HRTEM) to
characterize structure of the as-grown GPs. Raman characterization was
performed
with an Xplora spectrometer (Horiba Jobin Yvon Inc.) with a fixed laser
excitation
87
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
wavelength of 532 nm, power of 2.5 mW, spot size of 600 nm, and magnification
of
100X.
Cyclic voltammetry (CV) measurements of the CC/GPs/PANI hybrid structure
were carried out on a BASi Epsilon electrochemical system (Bioanalytical
Systems Inc.,
Indiana, USA) to evaluate the specific capacitance at different scan rates
from 2 mV/s to
100 mV/s. Galvanostatic charge/discharge measurements (Gamry Echem Testing
System, Gamry Instruments, Inc., USA) were used to evaluate the specific
capacitance
(Cs), internal resistance (IR), energy density (Es), power density (Ps),
coulombic
efficiency (q) and cycling life of the devices. Different current densities (-
1A/g to 100
Ng) were applied and a current density of 10 mA/cm2 was used for the cycling
life tests
for three-electrode configuration cell. The standard three-electrode cell
consisted of
Ag/AgCI as the reference electrode, Pt mesh as the counter electrode and the
synthesized composite sample as the working electrode, respectively. A 1 M
H2504
solution served as the electrolyte at room temperature. The potential was
between 0 to
0.8 V (0 to 2.4 V for the tests of three in-series supercapacitor group).
FIG. 5-2 displays a uniform and large area of GP coverage on CC substrates by
MPCVD method. This device is useful as an electrode.
MPCVD-grown GPs are graphitic and therefore highly hydrophobic. In order to
conformally coat GP surfaces with a thin layer of PANI film, prior to
electropolymerization process, the as-prepared samples were treated with
concentrated
acid H2504/HNO3 (3/1 v/v) at room temperature for 5 h to functionalize their
surfaces so
that they would be hydrophilic. The sample was thoroughly washed in deionized
water
until pH value is ¨7.
88
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
The three-electrode system for PANI electropolymerization was constructed with
a Pt mesh as a counter electrode, Ag/AgCI as a reference electrode and CC/PANI
directly as a working electrode. The electrolyte was 0.5 M H2SO4 and 0.05 M
aniline.
PANI was in situ electropolymerized on CC/PANI at a constant potential of 0.8
V versus
Ag/AgCI for different periods of time ranging from 30 s, 2 min, 5 min, 8 min,
10 min, 15
min to 20 min. FIG. 5-3 shows the SEM morphologies of PANI coated on GPs for 5
min
and 15 min for the electropolymerization process. The mass of PANI can be
controlled
by the electropolymerization time. After the polymerization process, the as-
prepared
composite film was washed in deionized water and then dried at 80 C over 2
hours.
FIG. 5-4 displays how current changes as a function of electropolymerization
time at 0.8 V vs. Ag/AgCI for both CC and CC/GP substrates. More aniline
monomers
will react with the CC/GPs substrate with higher specific area, leading to
higher current
and more change transfer than that of only CC.
The internal resistance can be determined from the initial voltage drop of the
discharge curves. FIG. 5-5 shows voltage drop (VIR) vs. discharge current
densities.
The voltage drop increases linearly with the increment of current densities.
At a high
current density of about 100 Ng, the VIR is approximately 0.12 V,
corresponding to a
low internal resistance of 2.5 0.
FIG. 5-6 shows the CV curves for a single flexible device from OV to 0.8 V at
different testing conditions (e.g., normal, bent and twisted). The CV curves
almost
overlaps, demonstrating the high flexibility of the device. The digital photos
of those
testing conditions are given in FIG. 5-5.
89
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Specific capacitances derived from cyclic voltammetry (CV) tests can be
calculated from the equation [2] [3]:
1
C = _________________________________________ I (V) dV
2sM (Vh vh-vi
(1)
where C is the specific capacitance in Fig, s is the scan rate in V/s, M is
the mass of
electrodes in g, Vh and V, are high and low potential limits of the CV tests
in V, / is the
instantaneous current on CV curves, and V is the applied voltage in V.
Specific capacitances derived from galvanostatic charge/discharge tests can be
calculated from the equation [4]:
C =1
Mu
(2)
where /d is the discharge current in A, and u is the slope of the discharge
curve after IR
drop.
The internal resistance R (in 0) was determined from the voltage drop at the
beginning of a discharge curve by [5, 6] :
R = AVIR I 2Id (3)
where AViR is the voltage dropped across the internal resistance in V.
Specific energy (E) and specific power (P) derived from galvanostatic
charge/discharge tests can be calculated from the following equations [5-7]:
2
E= CV
2M
(4)
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
r, E
(5)
f-=¨
At
V2
(6)
Pmax =
4RM
where V is the applied voltage in volts and At is the discharge time in
seconds. Pim, is
the maximum power density.
The coulombic efficiency (ri) of a battery is the ratio of the number of
charges that
was input into the battery during charging compared to the number that can be
extracted from the battery during discharging. The losses that reduce
coulombic
efficiency are primarily due to the loss in charge due to other redox
reactions in the
battery. It is calculated from the following equation [5]:
Qdisch arg e itdisch arg e tdisch arg e
=
(7)
ych arg e itch arg e tch arg e
Table 1.
Flexibility Electrode
Specific ED PD
of the
andElectrolyte Stability
capacitance (Wh/Kg) (kW/Kg)
substrate treatment
¨ 2000 F/g
¨7 %
PANI on (PANI)_ 47.7; 1 M
H2SO4 loss
1
Flexible CC/GP 2.5 Fice; 109.9 265.1 and
PVA- after
3
substrate 237 F/cm3 (max.)
H2SO4 gel 2000
at 1 A/g; cycles
X1. One
embodiment of the present invention pertains to an apparatus
comprising a substrate having a surface, a plurality of carbon mounds located
on the
surface, and a plurality of graphitic nanowalls, each nanowall growing from a
corresponding one of mounds.
91
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
X2. Yet another embodiment of the present invention pertains to a
method for
depositing carbon on a surface, comprising providing a substrate having an
outer
surface, roughening the outer surface, and depositing carbon on the roughened
outer
surface, and growing a graphitic petal from the carbon onthe roughened
surface.
X3. Yet another embodiment of the present invention pertains to a method for
depositing carbon on a surface, comprising providing a substrate having a
first layer of a
first material on top of a second layer of a second matrial, the first layer
having an outer
surface, diffusing a third material through the first layer, exposing the
substrate during
diffusing to an electrical field and depositing a fourth material containing
carbon on the
roughened outer surface.
X4. Yet another embodiment of the present invention pertains to a
biosensor
comprising an electrode comprising a wafer, multilayered petal nanosheets
supported
by the wafer, and platinum nanoparticles supported by the nanosheets, and
an enzyme electrodeposited on the electrode.
X5. Yet another embodiment of the present invention pertains to a method of
producing a biosensor, the method comprising providing an electrode comprising
a
substrate, petal nanosheets supported by the substrate, and electrodepositing
platinum
nanoparticles on the nanosheets, and electrodepositing an enzyme on the
electrode.
X6. Yet another embodiment of the present invention pertains to an
apparatus
comprising a carbon nanotube substrate, a graphitic petal structure supported
by the
substrate, and a metal oxide supported by the graphitic petal structure,
wherein the
metal oxide is from a neutral precursor solution
92
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
X7. Yet another embodiment of the present invention pertains to a method of
graphitic petal synthesis, the method comprising subjecting carbon cloth
substrate to
microwave plasma enhanced chemical vapor deposition.
X8. Yet another embodiment of the present invention pertains to a method of
coating of a graphitic petal surface, the method comprising providing a metal
mesh
counter electrode, a reference electrode and a working electrode, providing an
electrolyte including an acid and aniline, and electropolymerizing the aniline
to graphitic
petal surface.
X9. Yet another embodiment of the present invention pertains to a three
dimensional nanostructure comprising a carbon cloth substrate, graphitic petal
structure
supported by the substrate, and a film covering the graphitic petal structure.
Any of the preceding statements X1 through X9 wherein the mounds are
substantially conically shaped.
Any of the preceding statements X1 through X9 wherein the surface is
substantially coated with carbon. of the preceding statements X1 through X9
wherein
nanowalls grown substantially vertically from mounds.
Any of the preceding statements X1 through X9 em n the surface is coated with
a
layer of a carbide material.
Any of the preceding statements X1 through X9 wherein the material is silicone
carbide.
Any of the preceding statements X1 through X9 wherein the surface is
roughened prior to growth of nanowalls.
93
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein the surface is
roughened mechanically.
Any of the preceding statements X1 through X9 wherein the surface is
roughened by plasma etching.
Any of the preceding statements X1 through X9 wherein the surface is
roughened after gaseous diffusion through the surface.
Any of the preceding statements X1 through X9 wherein mounds are grown from
the surface.
Any of the preceding statements X1 through X9 wherein mounds have a base
diameter of less than about one micrometer.
Any of the preceding statements X1 through X9 wherein mounds have a base
diameter greater than about one hundred nanometers.
Any of the preceding statements X1 through X9 wherein roughening is by
mechanically etching the outer surface.
Any of the preceding statements X1 through X9 wherein the substrate includes a
layer of an oxide.
Any of the preceding statements X1 through X9 wherein roughening includes
diffusing hydrogen through the oxide.
Any of the preceding statements X1 through X9 which further comprises creating
nanocones on the outer surface during depositing.
Any of the preceding statements X1 through X9 wherein growing is from a
nanocone.
94
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein nanocones have a base
diameter of less than about one micrometer.
Any of the preceding statements X1 through X9 wherein nanocones have a base
diameter greater than about one hundred nanometers.
Any of the preceding statements X1 through X9 wherein growing is by exposing
the outer surface to a plasma containing a carbonaceous material.
Any of the preceding statements X1 through X9 wherein the carbonaceous
material is a hydrocarbon.
Any of the preceding statements X1 through X9 wherein growing is without using
a metal catalyst.
Any of the preceding statements X1 through X9 wherein roughening includes
creating a plurality of upwardly extending peaks.
Any of the preceding statements X1 through X9 wherein growing is from a peak.
Any of the preceding statements X1 through X9 which further comprises creating
a carbide layer on the outer surface.
Any of the preceding statements X1 through X9 wherein creating is before
growing.
Any of the preceding statements X1 through X9 wherein the carbide is a
catalyst
for growing.
Any of the preceding statements X1 through X9 wherein the roughened surface
includes a plurality of conically-shaped structures.
Any of the preceding statements X1 through X9 wherein after depositing the
outer surface includes a plurality of carbon-covered upwardly extending
shapes.
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein the shapes are
substantially conical.
Any of the preceding statements X1 through X9 wherein the base of the conical
shapes are greater than about one hundred nanometers in diameter.
Any of the preceding statements X1 through X9 wherein the first layer has a
thickness, and exposing includes reducing the thickness.
Any of the preceding statements X1 through X9 wherein the first layer has a
first
thickness before diffusing, and a second, lesser thickness before depositing.
Any of the preceding statements X1 through X9 which further comprises growing
a graphitic structure from the deposited carbon.
Any of the preceding statements X1 through X9 wherein the graphitic structure
is
a petal.
Any of the preceding statements X1 through X9 wherein the first material is an
oxide of the second material.
Any of the preceding statements X1 through X9 wherein the first material
includes a silica.
Any of the preceding statements X1 through X9 wherein the second material
includes silicon.
Any of the preceding statements X1 through X9 wherein the substrate is
electrically isolated from ground during exposing.
Any of the preceding statements X1 through X9 wherein the electrical field
comprises radio waves.
96
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein the radio waves have a
frequency greater than about one gigahertz.
Any of the preceding statements X1 through X9 wherein the radiated power of
the field is greater than about 300 watts.
Any of the preceding statements X1 through X9 wherein the radiated power of
the field is greater than about 500 watts.
Any of the preceding statements X1 through X9 wherein the electrical field
heats
the substrate to greater than about one thousand degrees Centigrade.
Any of the preceding statements X1 through X9 which further comprises heating
the substrate to greater than about one thousand degrees Centigrade.
Any of the preceding statements X1 through X9 which further comprises heating
the substrate during exposing.
Any of the preceding statements X1 through X9 wherein the third gaseous
material is inorganic.
Any of the preceding statements X1 through X9 wherein the third gaseous
material is hydrogen.
Any of the preceding statements X1 through X9 wherein the nanosheets are
grown on the wafer through chemical vapor deposition.
Any of the preceding statements X1 through X9 wherein the nanoparticles are
located along the edges of the nanosheets.
Any of the preceding statements X1 through X9 wherein the nanoparticles are
grown along the edges of the nanosheets.
97
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein the nanoparticles are
grown by an electrodeposition process.
Any of the preceding statements X1 through X9 wherein the electrodeposition
process includes current pulses of approximately 500 ms.
Any of the preceding statements X1 through X9 wherein the electrodeposition
process includes current within the range of approximately 312 pA to
approximately 5.0
mA.
Any of the preceding statements X1 through X9 wherein the electrodeposition
process includes current selected from the group consisting of approximately
312 pA,
approximately 625 pA, approximately 1.25 mA, approximately 2.5 mA, and
approximately 5.0 mA.
Any of the preceding statements X1 through X9 wherein the electrodeposition
process includes currently of approximately 2.5 mA.
Any of the preceding statements X1 through X9 wherein the enzyme is glucose
oxidase.
Any of the preceding statements X1 through X9 wherein the enzyme is
encapsulated within the poly(3,4-ethylenedioxythiophene).
Any of the preceding statements X1 through X9 wherein the electrode is
subjected to an oxygen plasma etch.
Any of the preceding statements X1 through X9 wherein electrodepositing
nanoparticles includes growing nanoparticles along edges and planes of the
nanosheets.
98
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein the enzyme is glucose
oxidase.
Any of the preceding statements X1 through X9 wherein the carbon nanotube
substrate is buckypaper.
Any of the preceding statements X1 through X9 wherein a layer of manganese
dioxide is coated on the graphitic petal structure, wherein the layer is
within the range of
approximately five to approximately ten nanometers in thickness.
Any of the preceding statements X1 through X9 wherein the microwave plasma
enhanced chemical vapor deposition conditions include primary feed gases at 30
torr
total pressure, a 2.45 GHz frequency microwave power supply, and 700 W plasma
power.
Any of the preceding statements X1 through X9 wherein the primary feed gases
include H2 and CH4.
Any of the preceding statements X1 through X9 wherein the H2 flow rate is 50
standard cubic centimeters per minute.
Any of the preceding statements X1 through X9 wherein the CH4 flow rate is 10
standard cubic centimeters per minute.
Any of the preceding statements X1 through X9 wherein the microwave plasma
enhanced chemical vapour deposition conditions include a 2.45 GHz frequency
microwave power supply.
Any of the preceding statements X1 through X9 wherein the microwave plasma
enhanced chemical vapor deposition conditions include a 700 W plasma power
rating.
99
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein subjecting carbon cloth
substrates to microwave plasma enhanced chemical vapor deposition occurs for
approximately 25 minutes.
Any of the preceding statements X1 through X9 wherein the carbon cloth
substrate is heated from room temperature to approximately 1100 C.
Any of the preceding statements X1 through X9 wherein the carbon cloth
substrate is made of microfibers.
Any of the preceding statements X1 through X9 wherein the substrate is
elevated
approximately 15 mm above the molybdenum puck.
Any of the preceding statements X1 through X9 wherein the molybdenum puck is
approximately 55 mm in diameter.
Any of the preceding statements X1 through X9 wherein the substrate is
elevated
by at least one ceramic spacer.
Any of the preceding statements X1 through X9 further comprising the step of
coating polyaniline (PANI) onto graphitic petals grown on carbon cloth.
Any of the preceding statements X1 through X9 further comprising the step of
treating the surface of the graphitic petals with a three to one by volume
mixture of
sulfuric acid and nitric acid for approximately five hours.
Any of the preceding statements X1 through X9 further comprising the step of
washing off the acid with deionized water until pH is approximately 7.
Any of the preceding statements X1 through X9 wherein the sulfuric acid and
aniline are at approximately equal molarity.
100
CA 02845539 2014-02-14
WO 2013/066474
PCT/US2012/051008
Any of the preceding statements X1 through X9 wherein the concentration of
each of sulfuric acid is approximately 0.5 M.
Any of the preceding statements X1 through X9 wherein electropolymerizing
occurs at a constant potential of approximately 0.8 V relative to the
reference electrode.
Any of the preceding statements X1 through X9 wherein the period of time for
electropolymerizing is within the range of approximately 30 seconds to
approximately
twenty minutes.
Any of the preceding statements X1 through X9 further comprising the steps of
washing the resultant composite film with deionized water and drying the
composite film
for approximately two hours at approximately eighty degrees Celsius.
Any of the preceding statements X1 through X9 wherein the graphitic petal
structure is directly grown on substrate by microwave plasma enhanced chemical
vapor
deposition.
Any of the preceding statements X1 through X9 wherein the substrate is
flexible.
Any of the preceding statements X1 through X9 wherein the polyaniline film is
coated on the structure by electropolymerization.
While the inventions have been illustrated and described in detail in the
drawings
and foregoing description, the same is to be considered as illustrative and
not restrictive
in character, it being understood that only certain embodiments have been
shown and
described and that all changes and modifications that come within the spirit
of the
invention are desired to be protected.
101