Note: Descriptions are shown in the official language in which they were submitted.
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
TITLE
REDUCING SULFIDE IN PRODUCTION FLUIDS DURING OIL
RECOVERY
This application claims the benefit of United States National
Application 13/226,717, filed September 7, 2011, which is incorporated by
reference in its entirety.
FIELD OF THE INVENTION
This disclosure relates to the field of oil recovery. More specifically,
it relates to reducing sulfide in production fluids recovered from oil
reservoirs.
BACKGROUND OF THE INVENTION
Hydrogen sulfide (H25) is commonly found in oil reservoirs due to
its production by sulfate-reducing bacteria (SRB), which may be
indigenous to an oil reservoir and/or introduced such as during water
injection in water flooding secondary oil recovery methods. The
metabolism of these SRB converts sulfate that is typically present in
injection water to sulfide, which results in souring of a reservoir and the
oil
produced, thereby reducing the value of the recovered crude oil. In
addition sulfide in production water causes corrosion of equipment used
to recover oil including storage reservoirs, surface facilities, and
pipelines,
and it can cause plugging by the formation of iron sulfide, as well as
causing health and environmental hazards.
In oil reservoirs and in production and injection fluids either or both
of SRB and nitrate-reducing bacteria (NRB) may be present, either as
indigenous populations or through introduction. When both are present,
there may be competition for nutrients between SRB and nitrate-reducing
bacteria (NRB). The presence of SRB and NRB, the presence and types
of nutrients available, as well as the balance of sulfate, nitrate, and
nitrite
are all factors affecting levels of sulfide in the reservoirs and fluids.
1
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
One method used to reduce sulfide has been to add nitrate to
injection water that is administered field-wide to an oil reservoir through
multiple injection wells (Griroryan et al. (2009) J. Can. Petrol Technol.
48:58-61). Injection of water containing nitrate has been tested in
continuous or pulsed applications, and when introduced to a portion of a
limited section of a reservoir, using nitrate at 150 ppm to 40,000 ppm
(Voordouw et al. (2009) Environ. Sci. Technol. 43:9512-9518). Recently it
was shown that light components of oil, like toluene, were degraded when
nitrate was introduced into a reservoir via injection wells to prevent sulfide
formation, because the presence of nitrate in the reservoir for a long
period of time was sufficient to encourage growth of oil degrading nitrate
reducers (Agrawal, et al. (2011) Abstract Published in the 3rd International
Symposium on Applied Microbiology and Molecular Biology in Oil
Systems June 13-15, 2011, Calgary, Alberta, Canada). Biodegradation of
light oil components is undesirable because this causes increased oil
viscosity and the higher viscosity causes increased resistance to oil flow.
US 5,405,531 discloses removing H25 and preventing SRB
production of H25 in an aqueous system by introducing nitrite and nitrate
and/or molybdate ions in concentrations where denitrifying
microorganisms outcompete SRB for available nutrients. Generally less
than about 3000 ppm of total nitrate and nitrite ions is added to the
aqueous system that is then injected into an oil-bearing formation, more
particularly between about 25 and 500 ppm. US 7,833,551 discloses
inhibiting sulfide production by SRB by treating SRB with a non-oxidizing
biocide and a metabolic inhibitor, which requires lower concentrations of
biocide and inhibitor than when each is used alone.
There remains a need for additional effective methods to reduce
sulfide in production fluid.
SUMMARY OF THE INVENTION
The invention relates to methods that lead to reduced sulfide in
production fluid obtained from an oil reservoir. Accordingly, the invention
provides a method for treating an oil production well comprising:
2
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
a) providing an oil production well in an oil reservoir having a well
casing and a production pipe;
b) adding an aqueous solution comprising at least one inorganic
oxidizing agent to the well casing wherein said solution flows down
the well casing and contacts production fluid in the well bore below
the production pipe ; and
c) producing the production fluid through the production pipe;
wherein the sulfide concentration in the production fluid is reduced
as compared to the sulfide concentration in production fluid
obtained with omission of step (b).
In one embodiment the inorganic oxidizing agent is nitrate ions,
nitrite ions, or a mixture of nitrate and nitrite ions.
In another embodiment the inorganic oxidizing agent is selected
from permanganates, persulfates, inorganic peracids, chromates,
bromates, iodates, chlorates, perchlorates, chlorites, hypochlorites,
inorganic peroxides, and oxides.
BRIEF DESCRIPTION OF THE DRAWINGS
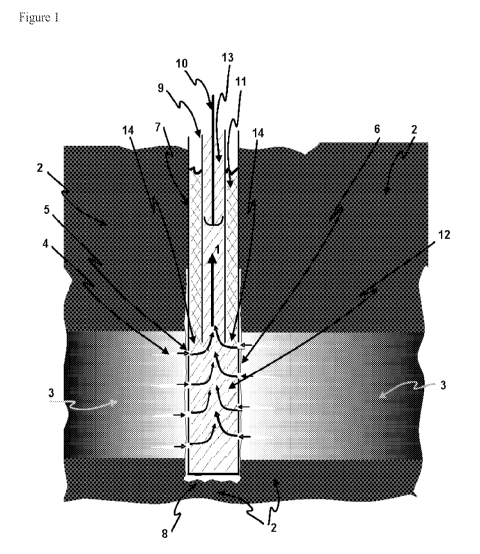
Figure 1 is a schematic representation of a production well, the
subterranean sites adjacent to the production well, and fluids in the well.
DETAILED DESCRIPTION
Applicants specifically incorporate the entire content of all cited
references in this disclosure. Unless stated otherwise, all percentages,
parts, ratios, etc., are by weight. Trademarks are shown in upper case.
Further, when an amount, concentration, or other value or parameter is
given as either a range, preferred range or a list of upper preferable values
and lower preferable values, this is to be understood as specifically
disclosing all ranges formed from any pair of any upper range limit or
preferred value and any lower range limit or preferred value, regardless of
whether ranges are separately disclosed. Where a range of numerical
values is recited herein, unless otherwise stated, the range is intended to
include the endpoints thereof, and all integers and fractions within the
3
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
range. It is not intended that the scope of the invention be limited to the
specific values recited when defining a range.
The following definitions are provided for the special terms and
abbreviations used in this application:
As used herein, the terms "comprises," "comprising," "includes,"
"including," "has," "having," "contains" or "containing," or any other
variation thereof, are intended to cover a non-exclusive inclusion. For
example, a composition, a mixture, process, method, article, or apparatus
that comprises a list of elements is not necessarily limited to only those
elements but may include other elements not expressly listed or inherent
to such composition, mixture, process, method, article, or apparatus.
Further, unless expressly stated to the contrary, "or" refers to an inclusive
or and not to an exclusive or. For example, a condition A or B is satisfied
by any one of the following: A is true (or present) and B is false (or not
present), A is false (or not present) and B is true (or present), and both A
and B are true (or present).
Also, the indefinite articles "a" and "an" preceding an element or
component of the invention are intended to be nonrestrictive regarding the
number of instances (i.e. occurrences) of the element or component.
Therefore "a" or "an" should be read to include one or at least one, and the
singular word form of the element or component also includes the plural
unless the number is obviously meant to be singular.
The term "invention" or "present invention" as used herein is a non-
limiting term and is not intended to refer to any single embodiment of the
particular invention but encompasses all possible embodiments as
described in the specification and the claims.
As used herein, the term "about" modifying the quantity of an
ingredient or reactant of the invention employed refers to variation in the
numerical quantity that can occur, for example, through typical measuring
and liquid handling procedures used for making concentrates or use
solutions in the real world; through inadvertent error in these procedures;
through differences in the manufacture, source, or purity of the ingredients
employed to make the compositions or carry out the methods; and the like.
4
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
The term "about" also encompasses amounts that differ due to different
equilibrium conditions for a composition resulting from a particular initial
mixture. Whether or not modified by the term "about", the claims include
equivalents to the quantities. In one embodiment, the term "about" means
within 10% of the reported numerical value, preferably within 5% of the
reported numerical value.
The terms "oil reservoir", and "oil-bearing stratum" may be used
herein interchangeably and refer to a subterranean or sub sea-bed
formation from which oil may be recovered. The formation is generally a
body of rocks and soil having sufficient porosity and permeability to store
and transmit oil.
The term "well bore" refers to a channel from the surface to an oil-
bearing stratum with enough size to allow for the pumping of fluids either
from the surface into the oil-bearing stratum, called an "injection well", or
from the oil-bearing stratum to the surface, called a "production well".
The terms "denitrifying" and "denitrification" mean reducing nitrate
for use in respiratory energy generation.
The term "water flooding" refers to injecting water through well
bores into an oil reservoir. Water flooding is performed to flush out oil from
an oil reservoir when the oil no longer flows on its own out of the reservoir.
The term "sweep efficiency" relates to the fraction of an oil-bearing
stratum that has seen fluid or water passing through it to move oil to
production wells during water flooding. One problem that can be
encountered with water flooding operations is the relatively poor sweep
efficiency of the water, i.e., the water can channel through certain portions
of a reservoir as it travels from injection well(s) to production well(s),
thereby bypassing other portions of the reservoir. Poor sweep efficiency
may be due, for example, to differences in the mobility of the water versus
that of the oil, and permeability variations within the reservoir which
encourage flow through some portions of the reservoir and not others.
The term "pure culture" means a culture derived from a single cell
isolate of a microbial species. The pure cultures specifically referred to
5
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
herein include those that are publicly available in a depository, and those
identified herein.
The term "electron acceptor" refers to a molecular compound that
receives or accepts an electron(s) during cellular respiration.
Microorganisms obtain energy to grow by transferring electrons from an
"electron donor" to an electron acceptor. During this process, the electron
acceptor is reduced and the electron donor is oxidized. Examples of
acceptors include oxygen, nitrate, fumarate, iron (III), manganese (IV),
sulfate or carbon dioxide. Sugars, low molecular weight organic acids,
carbohydrates, fatty acids, hydrogen and crude oil or its components such
as petroleum hydrocarbons or polycyclic aromatic hydrocarbons are
examples of compounds that can act as electron donors.
The term "biofilm" means a film or "biomass layer" of
microorganisms. Biofilms are often embedded in extracellular polymers,
which adhere to surfaces submerged in, or subjected to, aquatic
environments. Biofilms consist of a matrix of a compact mass of
microorganisms with structural heterogeneity, which may have genetic
diversity, complex community interactions, and an extracellular matrix of
polymeric substances.
The term "plugging biofilm" means a biofilm that is able to alter the
permeability of a porous material, and thus retard the movement of a fluid
through a porous material that is associated with the biofilm.
The term "simple nitrates" and "simple nitrites" refer to nitrate (NO3-)
and nitrite (NO2), respectively.
The term "bioplugging" refers to making permeable material less
permeable due to the biological activity, particlularly by a microorganism.
The term "injection water" refers to fliud injected into oil reservoirs
for secondary oil recovery. Injection water may be supplied from any
suitable source, and may include, for example, sea water, brine,
production water, water recovered from an underground aquifer, including
those aquifers in contact with the oil, or surface water from a stream, river,
pond or lake. As is known in the art, it may be necessary to remove
particulate matter including dust, bits of rock or sand and corrosion by-
6
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
products such as rust from the water prior to injection into the one or more
well bores. Methods to remove such particulate matter include filtration,
sedimentation and centrifugation.
The term "production water" means water recovered from
production fluids extracted from an oil reservoir. The production fluids
contain both water used in secondary oil recovery and crude oil produced
from the oil reservoir.
The term "inoculating an oil well" means injecting one or more
microorganisms or microbial populations or a consortium into an oil well or
oil reservoir such that microorganisms are delivered to the well or reservoir
without loss of viability.
The term "souring" refers to an increase in free sulfide
concentration with time, which can be measured by recording the H2S
concentration in the gas phase of a sample.
The present invention relates to methods for reducing sulfide in
production fluid that include adding a treatment solution that is an aqueous
solution containing nitrate ions or nitrite ions or a mixture of nitrate and
nitrite ions, where any of these compositions is herein called a
"nitrate/nitrite solution", to the well casing of an oil production well. In
addition, the present invention relates to methods for reducing sulfide in
production fluid that include adding a treatment solution that is an aqueous
solution containing another strong inorganic oxidizing agent to the well
casing of an oil production well. The treatment solution mixes with
production fluid containing oil and water whereby sulfide is removed by
oxidation. By adding the nitrate/nitrite or other inorganic oxidizing agent-
containing treatment solution to the well casing of a production well, a
greatly reduced volume of the solution is needed to reduce souring as
compared to when injecting a solution into an injection well where it flows
into an oil reservoir. In addition, removing sulfide occurs rapidly in the
production fluid in the well as compared to slow sulfide removal when
injecting a solution into an injection well where it flows into an oil
reservoir.
An additional benefit is limited biodegradation of oil components during the
short residence time in the well pipe.
7
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
Nitrate/nitrite or other inorganic oxidizing agent-containing solution and
treatment
In the present method an aqueous solution containing nitrate ions
and/or nitrite ions, or another inorganic oxidizing agent, is added to the
well casing of a production well. The total concentration of nitrate and/or
nitrite ions, or of other inorganic oxidizing agent, is sufficient to reduce
sulfide concentration in production fluid. The ions move, or the other
inorganic oxidizing agent moves, by mixing and diffusion into the
production fluid of oil and water as shown in one embodiment that is
diagrammed in Figure 1.
The nitrate/nitrite or other inorganic oxidizing agent-containing
treatment solution (11) is added into the water production well casing (7)
which is inside the well bore (6) drilled through rock layers (2 and 3). A
gap exists between the well casing (7) and the face of the rock layer made
by the well bore (6). Rock layer (2) represents impermeable rock above
and below a permeable rock layer (3) that holds or traps oil. Perforations
in the casing (5) allow oil containing production fluid to flow from fractures
(4) in the permeable rock (3) into the well casing that extends through the
permeable rock that is the oil reservoir (3) near the bottom of the well hole
(8). The nitrate/nitrite or other inorganic oxidizing agent-containing
solution
flows down the well casing outside of the production tubing or production
pipe (9) and contacts the oil and water production fluid from the oil
reservoir (12) below the production pipe in the well bore as both fluids
enter the lower part of the well (14). The volume of nitrate/nitrite or other
inorganic oxidizing agent-containing solution that is added is sufficient to
fill the well casing. With the height of the nitrate/nitrite or other
inorganic
oxidizing agent-containing solution in the well casing higher than the
natural level of production fluid in the well bore, the concentrated
nitrate/nitrite or other inorganic oxidizing agent-containing solution mixes
down into the production fluid towards the bottom of the well forming a
production fluid mixture containing nitrate ions, nitrite ions, or a mixture
of
nitrate and nitrite ions or a production fluid mixture containing the other
inorganic oxidizing agent. In production mode the production fluid mixed
8
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
with nitrate/nitrite or other inorganic oxidizing agent-containing solution
flows up (1) through the production tubing or production pipe (9) inside the
well casing (7) through action of the pump rod with check valves (10). The
nitrate/nitrite or other inorganic oxidizing agent-containing treatment
solution is thus in contact with the production fluid and removes sulfide
from the production fluid as the mixture flows up in the production pipe to
the surface and is recovered. Sulfide in the production fluid is removed
before it gets to the fluid processing unit on the surface.
Nitrite ions are either supplied in the nitrate/nitrite treatment solution
and/or are formed during contact with the production fluid as a product of
nitrate ion metabolism by nitrate-reducing bacteria (NRB) in the production
fluid. In one embodiment at least a portion of nitrate ions are reduced to
nitrite ions by NRB in the production fluid. Sulfide concentration is
reduced by direct chemical conversion of sulfide by nitrite (oxidation to
sulfur or sulfate). Sulfide concentration is also reduced by promoting
growth of sulfide oxidizing nitrate reducing bacteria (SONRB) by nitrate. In
addition, production of sulfide is reduced by promoting growth of NRB by
nitrate, resulting in reduced growth and therefore activity of sulfate-
reducing bacteria (SRB) which produce sulfide.
In mixing of the nitrate/nitrite solution with oil and water production
fluid from the reservoir, nitrate and/or nitrite ions diffuse into the
production
fluid and are diluted. If no nitrite is provided in the nitrate/nitrite
solution,
nitrite ions are generated by NRB in the well. In the mixture of oil and
water production fluid with nitrate/nitrite solution the concentration of
nitrite
ions (supplied or formed from nitrate) is sufficient to oxidize the majority
of
sulfide to remove it from the production fluid. In the mixture the
concentration of nitrate ions is sufficient to promote growth of nitrate
reducing bacteria (NRB) so that dissolved organic carbon (DOC) nutrients
are used by NRB instead of by sulfate-reducing bacteria (SRB) to reduce
new production of sulfide.
In one embodiment the nitrite concentration following mixing of the
nitrate/nitrite solution with oil and water production fluid from the oil
reservoir is at least about 5-fold greater than the concentration of sulfide
in
9
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
the production fluid in the well. Applicants have found that a ratio of at
least about 5:1 of nitrite ions:sulfide ions (NO2-:S2-) supports rapid
oxidation of the sulfide, as shown herein in Example 2. The concentration
of sulfide in the oil and water production fluid of an oil reservoir may be
readily measured by one skilled in the art, for example, by using a
colorimetric assay based on methylene blue formation (Cline (1969)
Limnol. Oceanogr. 14:454-458) or a paper strip assay such as Hydrogen
Sulfide Test strips (#481197-1, Industrial Test Systems, Inc., Rock Hill,
SC USA),.
Mixing of the nitrate/nitrite solution from the well casing with the oil
and water production fluid in the well below the production pipe will dilute
the nitrate/nitrite solution. The rate and amount of dilution will depend on
factors including the method of adding the solution to the well casing (such
as pulse, continuous, or single addition), and the density of the production
fluid in the bottom of the well. Typically dilution may be by about 1-fold to
about 5-fold or more. The concentration of nitrate and/or nitirite ions in the
solution added to the well casing may be adjusted to accommodate any
dilution factor. For example, with 5-fold dilution in order to have a final
5:1
ratio of NO2-:52-, a 25-fold higher molar concentration of nitrite than
sulfide
in the oil and water production fluid is needed in the nitrate/nitrite
solution.
The nitrite may be supplied in the nitrate/nitrite solution directly, or
formed
by reduction of nitrate by NRB. Thus for sulfide oxidation, the total molar
concentration of nitrate and/or nitrite ions in the nitrate/nitrite solution
is
25-fold greater than the molar concentration of sulfide in the production
fluid. For example, when treating an oil reservoir with production fluid
having a sulfide concentration of about 25 ppm or 0.78 mMoles per liter
and based on having a 5-fold excess of nitrite ions and a 5-fold dilution
factor, a nitrate/nitrite solution added to the well casing has a total
concentration of nitrate and/or nitrite ions of at least about 897 ppm or
19.5 mMoles per liter.
Additional nitrate may be included in the nitrate/nitrite solution to
promote growth of NRB so that available carbon source in production fluid
is used up by NRB thereby suppressing growth and production of sulfide
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
by SRB. For example, about 93 ppm nitrate would be used in metabolism
of about 50 ppm glucose, as calculated based on the assumption that all
glucose carbon is converted to carbon dioxide. Again assuming a 5-fold
dilution of the nitrate/nitrite solution in mixing with production fluid in
the
well below the production pipe, about 465 ppm of nitrate would support
growth of NRB to metabolize 50 ppm of available carbon source.
In addition, native or introduced populations of a specialized type of
sulfide oxidizing, nitrate reducing organisms (SONRB), which rely on
sulfide oxidation to generate energy for growth, rather than oxidation of
organic material, such as glucose used in the example above, may be
present in the treated zone of the well. Growth and metabolism of SONRB
are supported by the nitrate provided in the nitrate/nitrite solution. These
bacteria may contribute to reducing the amount of sulfide in production
fluid such that the concentration of nitrite needed to oxidize sulfide is
reduced. Thus a lower amount of nitrite ions, or nitrate ions that are
reduced to nitrite ions by NRB, is needed in the presence of SON RB.
Maximum concentrations of nitrate and/or nitrite ions used would be
determined by the desired treatment goal as determined by one skilled in
the art controlling well souring. In one embodiment to support both effects
of oxidizing sulfide already present and reducing new production of sulfide,
the nitrate/nitrite solution has a combined concentration of nitrate and/or
nitrite ions of at least about 700 ppm. Typically excess concentration is
used. The nitrate and/or nitrite combined concentration may be about 800,
900, 1000 ppm or more, up to a limit where toxic effects of the salts on
the desired microbial populations becomes an issue, which is
approximately 1500 ppm for nitrite and 3000 ppm for nitrate. In another
embodiment, to maximize sulfide oxidation capacity where toxicity to
microorganisms is not a concern, nitrite concentrations in excess of
100,000 ppm may be used as limited by concentrations that do not
adversely corrode metal parts of the system and/or cause problems in
down stream oil processing.
The nitrate/nitrite solution may be made using nitrate ions and/or
nitrite ions in any form that are released in solution, such as in any soluble
11
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
salt form such as calcium, sodium, potassium, ammonium, and any
combination mixtures of salts. Typically sodium salts of nitrate and/or
nitrite are used. These salts are dissolved in an aqueous solution from any
suitable source such as for example, sea water, brine, production water,
water recovered from an underground aquifer, including those aquifers in
contact with the oil, or surface water from a stream, river, pond or lake. As
is known in the art, it may be desired to remove particulate matter
including dust, bits of rock or sand and corrosion by-products such as rust
from the water prior to use in a treatment solution. Methods to remove
such particulate matter include filtration, sedimentation and centrifugation.
An aqueous solution of the present method may contain a different
inorganic oxidizing agent, other than nitrate/nitrite. The inorganic oxidizing
agent may be any water soluble strong inorganic oxidizing agent, with
strong meaning that the inorganic oxidizing agent has reaction standard
half-cell potential of greater than -0.478 volts. One of skill in the art will
be
familiar with, or can readily identify, a strong inorganic oxidizing agent,
examples of which include, but are not limited to, permanganates,
persulfates, inorganic peracids, chromates, bromates, iodates, chlorates,
perchlorates, chlorites, hypochlorites, inorganic peroxides, and certain
oxides. Some specific examples include ammonium dichromate,
ammonium perchlorate, ammonium permanganate, barium bromate,
barium chlorate, barium peroxide, cadmium chlorate, calcium chlorate,
calcium chromate, calcium perchlorate, chlorine dioxide, potassium
persulfate, and hydrogen peroxide. In one embodiment the inorganic
oxidizing agent is chlorine dioxide, hypochlorite, persulfate, or hydrogen
peroxide. At least one inorganic oxidizing agent is included in the aqueous
solution. Typically oxidizing agents are used separately.
In mixing of the inorganic oxidizing agent-containing solution with oil
and water production fluid from the reservoir, the agent diffuses into the
production fluid and is diluted. In the mixture of oil and water production
fluid with the inorganic oxidizing agent-containing solution, the
concentration of the agent is sufficient to oxidize the majority of sulfide to
12
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
remove it from the production fluid. Sulfide is directly oxidized by the agent
by direct chemical conversion of sulfide to sulfur or sulfate.
In one embodiment the inorganic oxidizing agent concentration
following mixing of the inorganic oxidizing agent-containing solution with
oil and water production fluid from the oil reservoir is at least about 1.5 to
6
times greater than the concentration of sulfide in the production fluid in the
well. The agent concentration may be at least about 1.5, 2, 2.5, 3, 3.5, 4,
4.5, 5, 5.5, or 6 times greater than the concentration of sulfide in the
production fluid in the well. The exact agent concentration needed to
oxidize the majority of the sulfide may be readily determined for a specific
agent by one skilled in the art, as shown in examples herein for chlorine
dioxide, potassium persulfate, and hydrogen peroxide. It is found herein
that ratios of 1.71:1, 5.3:1, and 3.76:1 are effective in causing rapid and
complete or almost complete oxidation of sulfide by these agents,
respectively. The concentration of sulfide in the oil and water production
fluid of an oil reservoir may be readily measured by one skilled in the art,
for example, by using a colorimetric assay based on methylene blue
formation (Cline (1969) Limnol. Oceanogr. 14:454-458) or a paper strip
assay such as Hydrogen Sulfide Test strips (#481197-1, Industrial Test
Systems, Inc., Rock Hill, SC).
Mixing of the inorganic oxidizing agent-containing solution from the
well casing with the oil and water production fluid in the well below the
production pipe will dilute the agent solution. The rate and amount of
dilution will depend on factors including the method of adding the solution
to the well casing (such as pulse, continuous, or single addition), and the
density of the production fluid in the bottom of the well. Typically, dilution
may be by about 1-fold to about 5-fold or more. The concentration of
inorganic oxidizing agent in the solution added to the well casing may be
adjusted to accommodate any dilution factor. For example, with 5-fold
dilution in order to have a final 1.5:1 ratio of chlorine dioxide to sulfide
(C102:52), a 7.55-fold higher molar concentration of agent than sulfide in
the oil and water production fluid is needed in the agent-containing
solution. Thus for sulfide oxidation, the total molar concentration of the
13
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
chlorine dioxide in the chlorine dioxide containing solution added is 7.5-
fold greater than the molar concentration of sulfide in the production fluid.
For example, when treating an oil production well containing production
fluid having a sulfide concentration of about 25 ppm or 0.78 mMoles per
liter and based on having a 1.5-fold excess of chlorine dioxide and a 5-fold
dilution factor, a chlorine dioxide-containing solution added to the well
casing has a total concentration of chlorine dioxide of at least about
394.29 ppm [7.5 x 0.78 = 5.85 mMoles 0102; 5.85 x 67.4 (Mwt of 0102) =
394.29 ppm] or 5.85 mMoles per liter.
The inorganic oxidizing agent-containing solution may be made
using any of the agents, as exemplified above, in a form that is soluble in
water. The agent is dissolved in an aqueous solution from any suitable
source such as for example, sea water, brine, production water, water
recovered from an underground aquifer, including those aquifers in contact
with the oil, or surface water from a stream, river, pond or lake. As is
known in the art, it may be desired to remove particulate matter including
dust, bits of rock or sand and corrosion by-products such as rust from the
water prior to use in a treatment solution. Methods to remove such
particulate matter include filtration, sedimentation and centrifugation.The
nitrate/nitrite or other inorganic oxidizing agent-containing solution is
first
added to the well casing prior to producing from the well. Typically the first
addition is just prior to producing from the well. Addition of the
nitrate/nitrite or other inorganic oxidizing agent-containing solution to the
well casing of a production well may be by any method typically used to
add fluids to the well casing such as by pumping. The nitrite/nitrate or
other inorganic oxidizing agent-containing solution may be added to the
well casing only once, or intermittently by periodic filling of the well
casing
before and during production from the well (pulsed). Alternatively the
nitrite/nitrate or other inorganic oxidizing agent-containing solution may be
added to the well casing continuously by continuous introduction of the
solution into the well casing at the top of the production well casing at the
surface, before and during production from the well.
14
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
In one embodiment there is a separate delivery tubing or pipe within
the well casing that is outside of the production tubing or production pipe.
This delivery tubing extends from the surface to the lower part of the well
to deliver the nitrate/nitrite or other inorganic oxidizing agent-containing
solution to the point where it mixes with the production fluid below the
production pipe. Though this system for addition requires a separate
tubing, it allows more specific control of the concentration of nitrate and/or
nitrite ions, and of another inorganic oxidizing agent, delivered to the
production fluid.
The nitrate/nitrite or other inorganic oxidizing agent-containing
solution may be added alone, or as a mixture with one or more other fluids
added to the well casing. When mixed with other fluids, the final nitrite and
nitrate ion concentrations, or other inorganic oxidizing agent concentration,
in the mixture are those described above. For example, nitrite and nitrate
ions or other inorganic oxidizing agent may be added to power water used
to drive a jet type production well pump. A more concentrated nitrate/nitrite
or other inorganic oxidizing agent-containing solution may be prepared
and diluted into another fluid to be added to the well casing.
The nitrate/nitrite or other inorganic oxidizing agent-containing
solution may be added to the casing of a production well that is a single
well oil recovery system or a production well in a multiple well oil recovery
system. In a single well oil recovery system the production well is
alternately the production well and the injection well. This type of well is
typically used in a "Huff and Puff' process. For this type of well and
process, the nitrate/nitrite or other inorganic oxidizing agent-containing
solution is typically added to the well casing after a well treatment is
injected or introduced to the well when the well is returned to production.
In a multiple well system, the nitrate/nitrite or other inorganic oxidizing
agent-containing solution is typically added to the production well casing
prior to and/or during the period when production fluids are being
recovered.
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
Combination of nitrate/nitrite or other inorganic oxidizing agent-containing
solution and MEOR treatments
The present method may be used in oil reservoirs where
microbially enhanced oil recovery (MEOR) methods (Brown, L. R., Vadie,
A. A,. Stephen, 0. J. SPE 59306, SPE/DOE Improved Oil Recovery
Symposium, Oklahoma, April 3-5, 2000) are practiced. MEOR methods
are used to improve oil recovery by the actions of microorganisms in an oil
reservoir, which may include releasing oil from substrates and/or plugging
highly permeable zones by formation of plugging biofilms. MEOR methods
include injecting oil reservoirs with nutrient solutions that support
microbial
growth, and also may include inoculation of oil reservoirs with one or more
microorganisms as disclosed for example in US 7,776,795, US 7,708,065,
and commonly owned and co-pending US Pat. Appl. Pub. #20090263887,
which are each incorporated herein by reference. Thus when using MEOR
the production fluid may contain relatively higher levels of one or more
carbon substrates to support growth of indigenous microorganisms.
Carbon substrates may be in excess in the oil reservoir, and in the oil and
water mixture that enters the well becoming production fluid. When
microorganisms are introduced in MEOR, there may be higher levels of
microorganisms, and/or different populations of microorganisms, than
without MEOR.
When higher levels of carbon substrates are present in production
fluid, and SRB are present, higher levels of sulfide may be present in
production fluid than encountered when not injecting a nutrient solution in
a MEOR process. It is thus of particular importance to remove sulfide and
reduce growth and production of sulfide by SRB that thrive on the injected
nutrients when a MEOR process is used in an oil reservoir. When using a
MEOR treatment, the nitrate/nitrite or other inorganic oxidizing agent-
containing solution is first added to the well casing of a production well
after the MEOR treatment to the production well or to an injection well in
the same oil reservoir and connected to the production well, but prior to
producing from the well.
16
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
Depending on the sulfide concentration in the production fluid, it
may be advised to use higher concentrations of nitrate and/or nitrite ions in
a nitrate/nitrite solution or other inorganic oxidizing agent in solution in
conjunction with a MEOR process. The concentration needed may be
determined by one skilled in the art by analysis of the concentration of
sulfide in production fluids, and following ratios described above.
EXAMPLES
The present invention is further defined in the following Examples.
It should be understood that these Examples, while indicating preferred
embodiments of the invention, are given by way of illustration only. From
the above discussion and these Examples, one skilled in the art may
ascertain the essential characteristics of this invention, and without
departing from the spirit and scope thereof, may make various changes
and modifications of the invention to adapt it to various usages and
conditions.
GENERAL METHODS
The meaning of abbreviations are used in this application are as
follows: "hr" means hour(s), "min" means minute(s), "day" means day(s),
"mL" means milliliters, "mg/mL" means milligram per milliliter, "L" means
liters, "4" means microliters, "mM" means millimolar, " M" means
micromolar, "nM" means nano molar, " g/L" means microgram per liter,
"pmol" means picomol(s), " C" means degrees Centigrade, " F" means
degrees Fahrenheit, "mm" means millimeter, "ppm" means part per million,
"g/L" means gram per liter, "mL/min" means milliliter per minute, "mUhr"
means milliliter per hour, "g" means gram, "mg/L" means milligram per
liter, "
Sulfide Analysis
Sulfide analysis was done using the methylene blue colorimetric
assay with optical density read at 670 nm as described in (Cline (1969)
Limnol. Oceanogr. 14:454-458) .
17
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
Example 1
NO2- reactivity with sulfide
A sodium nitrite solution was used to oxidize a sodium
sulfide solution at room temperature in a closed system in order to
look at the kinetics of the reaction and to prevent the volatilization of
sulfide. Based on a balanced redox reaction, 1 mole of nitrite
should be able to oxidize at least 0.5 mole of hydrogen sulfide. The
nitrite and sulfide solutions used in the experiment were made up in
artificial brine, which mimics the moderately high salinity of many oil
reservoirs. The brine had the following composition: CaC12.2H20,
6.75 g, NaCI, 26.1 g, Na2SO4, 0.015 g, MgC12.6H20 g, 4.45, KCI,
0.7 g plus enough water to make a total of 500 ml of brine solution.
The sulfide solution in brine was approximately 15 ppm S2-. The
nitrite solutions were approximately 50 ppm and 725 ppm NO2-.
Two different treatments were run. In treatment 1 (Table 1) the
nitrite:sulfide molar ratio was 29:1, which resulted in reaction
conditions where nitrite was approximately 14.5 (i.e. 29/2) fold in
excess of the nominal concentration needed to oxidize all sulfide in
the reaction vessel. In treatment 2 (Table 1) the nitrite/sulfide molar
ratio was 2.1:1, which resulted in reaction conditions where nitrite
was approximately 1.05 (i.e. 2.1/2) fold in excess of the nominal
concentration needed to oxidize all sulfide in the the 26 ml crimp-
cap-sealed glass reaction vessel. The sulfide remaining was
assayed as described in General Methods at 10 min and 120 min of
reaction time and the results are given in Table 1.
Although both treatments had enough nitrite to oxidize all of
the sulfide present based on the redox balanced equation, only
treatment 1 showed complete removal of sulfide at the 10 minute
and 2 hour observation time points (Table 1). These data show that
in order to have the oxidation reaction occur rapidly the
nitrite:sulfide ratio should be in excess of 2.1:1.
18
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
Table 1: Changes in sulfide concentrations with time following the
addition of nitrite.
Treat- NO2-:S2- Reaction ppm S2- Observed Expected
ment molar Time, added ppm S2- ppm S2- after
ratio in min to following NO2- addition
reactor reactor NO2
addition
1 29 10 19 0 0
120 19 0 0
2 2.1 10 19 15 0
120 19 15 0
Example 2
NO2- reactivity with sulfide: Titration of molar ratios needed for a
rapid reaction
A range of nitrite:sulfide ratios were tested to determine the
molar ratio needed to cause a rapid oxidation of sulfide. The nitrite
and sulfide solutions used in the experiments were made up in
artificial brine as described in Example 1. Experiments were
performed as described in Example 1 using eight different
treatments. The nitrite/sulfide molar ratios used were 2, 5, 10, 15,
20, 25, 30, and 35. Results given in Table 2 showed that the
reaction rate remained slow at a ratio of 2, as seen in the previous
Example, but at a ratio of 5:1 or higher the reaction occurred
rapidly with sulfide becoming undetectable in 10 minutes or less.
The ability to rapidly remove sulfide at lower nitrite:sulfide ratios
makes the process more economical
19
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
Table 2: Changes in sulfide concentrations with time following the
addition of nitrite at NO2-:S2- ratios from 2:1 to 35:1.
ppm S2- Observed ppm
NO2-:S2- molar Reaction
added S2- following
ratio in reactor Time, min
to reactor NO2- addition
2:1 10 26 8.9
120 26 3.8
5:1 10 26 0
120 26 0
10:1 10 26 0
120 26 0
15:1 10 26 0
120 26 0
20:1 10 26 0
120 26 0
25:1 10 26 0
120 26 0
30:1 10 26 0
120 26 0
35:1 10 26 0
120 26 0
Example 3 (prophetic)
Production well treatment using continuous addition of nitrate/nitrite
solution in well casing to mitigate sulfide present in production fluid
of soured wells
In this example, a producer well is continuously treated to
mitigate sulfide present in the production water using a nitrate/nitrite
mixture. Sulfide in the production fluids often results from well
souring, following the start of water injection for secondary oil
recovery. Rather than treating the injected water and all reaches of
the subterranean oil reservoir, as is the normal practice to mitigate
souring by sulfide formation, a process is used that treats the
smaller produced water volume, making it more economical, while
still sweetening the produced fluids by removing sulfide.
A nitrate/nitrite solution is produced by dissolving any
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
inexpensive nitrate and nitrite salts, such as Na NO2 or Na NO3, in
water. This solution is continuously pumped into the well casing of a
production well. The production fluid flow entrains the casing
solution of nitrate/nitrite into the production fluid moving up the well
pipe or, in the case of a jet pump, the nitrate/nitrite solution is
incorporated into the power water, which joins the produced fluid
flow after passing through the jet pump drive. The nitrate/nitrite
mixture contains nitrite (NO2-) at a molar ratio of approximately 25:1
with respect to the molar concentration of sulfide (S2-) in the
produced water and contains nitrate at a concentration of about 7.5
mMoles NO31L per 50 ppm of dissolved organic carbon (DOC) in
the production water. This fluid is pumped down the casing at a
rate such that the nitrate/nitrite solution is entrained into the
production fluids at a ratio of at most 5 parts production fluids per 1
part nitrate/nitrite solution.
Two production wells in a soured field are found to contain
approximately 25 ppm S2- and 50 ppm DOC.. A solution containing
900 ppm NO2- + 465 ppm NO3- is pumped into the well casing to
treat one of the wells as described above. After a week, S2-
concentration is observed to have dropped to 5 ppm, and a week
later sulfide is found to be undetectable in the treated well. The
neighboring, untreated production well, producing oil from the same
soured reservoir, is observed to still contain approximately 25 ppm
S2- in its produced water after the same two week period.
Example 4 (prophetic)
Nitrate/nitrite treatment combined with a MEOR process that utilizes
organic nutrients
In this example, a producer well is treated for microbial enhanced
oil recovery using an organic nutrient. A solution of 100 ppm yeast extract
plus 4000 ppm of disodium malate is fed batch wise to an oil reservoir
through a production well. This is accomplished by pumping this nutrient
solution down the casing of the well and into the oil reservoir. The intent
21
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
of this treatment is to improve oil recovery from this single well. After
pumping these nutrients into the reservoir, the oil well is shut in for a
period of 2 weeks while the microbial population in the well consumes the
malate carbon substrate. Analysis of water in the reservoir and of the
injection water pumped into the reservoir before and after the nutrient
treatment show that there are sulfate reducing bacteria present and that
there is 100 ppm sulfate in these waters. It is therefore anticipated that
some sulfide will be produced from the sulfate reducing bacteria
metabolizing the malate or metabolic byproducts of the malate. After the
14 day shut in and just before the well is put back onto production, a
solution of 1 wt% nitrate and 6.5 wt% nitrite is pumped into the well casing.
The volume of this solution is enough to fill the well casing. The well is
then put back on production. The nitrate/nitrite solution in the well casing
becomes mixed with the production fluids and analysis of the well effluent
shows no signs of sulfide in the produced water.
In contrast, an identical nearby production well producing oil from
the same reservoir is treated in the exact same fashion without the
nitrate/nitrite post treatment. It is observed that there is 50 ppm of sulfide
present in the water produced by this well when this well was put back on
production. Both wells show a substantial increase in oil production
amounting to an extra 30% increase in production rate for a period of a
month after the MEOR treatment.
Example 5
Chlorine dioxide removal of sulfide: Titration of molar ratios needed
for a rapid reaction
A range of chlorine dioxide:sulfide ratios was tested to
determine the molar ratio needed to cause a rapid oxidation and
removal of sulfide. The chlorine dioxide and sulfide solutions used
in the experiments were made up in artificial brine as described in
Example 1. Experiments were performed as described in Example
1 using five different treatments and testing for sulfide remaining
after 10 min of reaction time. The chlorine dioxide:sulfide molar
22
CA 02846805 2014-02-26
WO 2013/036316
PCT/US2012/044626
ratios (0102, M:S2-, M) used were appproximately 0.11:1, 0.29:1,
0.57:1, 1.7:1, and 3.42:1. Results given in Table 3 showed that
sulfide at 100 mg/L concentraton was rapidly and completely
removed with the 0102:S2- molar ratio of approximately 1.71:1. At
this ratio of reactants and above the sulfide concentration dropped
from 100 ppm to undetectable in 10 minutes or less.
Table 3 Changes in sulfide concentrations after al 0 minute reaction time
following the addition of chlorine dioxide at various molar ratios.
mg/L S2- Observed mg/L
0102:S2- molar Reaction
added S2- following
ratio in reactor Time, min
to reactor 0102 addition
0.11:1 10 100 79.6
0.29:1 10 100 56.4
0.57:1 10 100 21.4
1.71:1 10 100 0.0
3.42:1 10 100 0.0
Example 6
Persulfate removal of sulfide: Titration of molar ratios needed for a
rapid reaction
Using a solution of potassium persulfate, a range of
persulfate:sulfide ratios was tested to determine the molar ratio
needed to cause a rapid oxidation and removal of sulfide. The
potassium persulfate and sulfide solutions used in the experiments
were made up in artificial brine as described in Example 1.
Experiments were performed as described in Example 1 using
seven different treatments with replication and testing for sulfide
remaining after 10 min of reaction time. The persulfate:sulfide
(52082-:52-) molar ratios used were 0.1:1, 0.2:1, 0.3:1, 0.7:1, 1.3:1,
2.7:1, and 5.3:1. Results given in Table 4 showed that at the
highest ratio of oxidant (persulfate) to sulfide that was tested, 5.3:1,
the 100 mg/L sulfide concentraton was greatly reduced , but not
reduced to an undetectable concentration. This shows that a molar
23
CA 02846805 2014-02-26
WO 2013/036316 PCT/US2012/044626
ratio exceeding 5.3 is needed to rapidly and completely remove
sulfide present at a concentration of 100 mg/L.
Table 4 Changes in sulfide concentrations after al 0 minute
reaction time following the addition of a persulfate solution at
various molar ratios.
S208 :Smolar Reaction mg/L S2- Observed mg/L
added S2- following
ratio in reactor Time, min
to reactor 52082- addition
0.1:1 10 100 101.9
0.1:1 10 100 97.4
0.2:1 10 100 96.7
0.2:1 10 100 94.5
0.3:1 10 100 76.7
0.3:1 10 100 78.0
0.7:1 10 100 24.5
0.7:1 10 100 25.8
0.7:1 10 100 40.3
0.7:1 10 100 40.2
1.3:1 10 100 14.8
1.3:1 10 100 15.4
1.3:1 10 100 19.8
1.3:1 10 100 20.6
2.7:1 10 100 9.2
2.7:1 10 100 8.6
5.3:1 10 100 3.7
5.3:1 10 100 3.0
Example 7
Hydrogen peroxide removal of sulfide: Titration of molar ratios
needed for a rapid reaction
A range of hydrogen peroxide:sulfide ratios was tested to
determine the molar ratio needed to cause a rapid oxidation and
removal of sulfide. The hydrogen peroxide and sulfide solutions
used in the experiments were made up in artificial brine as
described in Example 1. Experiments were performed as described
24
CA 02846805 2014-02-26
WO 2013/036316 PCT/US2012/044626
in Example 1 using five different treatments and testing for sulfide
remaining after 10 min ofreaction time. The hydrogen
peroxide:sulfide (H202:S2-) molar ratios used were approximately
0.19:1, 0.47:1, 0.94:1, 1.88:1, and 3.76:1. Results given in Table 5
showed that sulfide at 100 mg/L concentraton was rapidly and
completely removed with the H202:S2- molar ratio of approximately
3.76:1. At this ratio of reactants the sulfide concentration dropped
from 100 ppm to undetectable in 10 minutes or less.
Table 5 Changes in sulfide concentrations after a 10 minute
reaction time following the addition of hydrogen peroxide at various
molar ratios.
mg/L S2- Observed mg/L
H202:S2- molar Reaction
added S2- following
ratio in reactor Time, min
to reactor H202 addition
0.19:1 10 100 72.2
0.47:! 10 100 42.8
0.94:1 10 100 23.4
1.88:1 10 100 2.5
3.76:1 10 100 0.0