Note: Descriptions are shown in the official language in which they were submitted.
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
APPLICATION FOR PATENT
INVENTOR: GRAHAME NIGEL TAYLOR
TITLE: METHOD OF USING DITHIAZINES AND DERIVATIVES
THEREOF IN THE TREATMENT OF WELLS
SPECIFICATION
Field of the Invention
[0001] The invention relates to methods of inhibiting corrosion in
metallic
surfaces during treatment of a well by introducing into the well a dithiazine
or a
dithiazine derivative.
Background of the Invention
[0002] During the production life of an oil or gas well, the production
zone within
the well is typically subjected to numerous treatments. Corrosion of metallic
surfaces,
such as downhole tubulars, during such treatments is not uncommon and is
evidenced
by surface pitting, localized corrosion and loss of metal. Metallic surfaces
subject to
such corrosion are carbon steels, ferritic alloy steels, and high alloy steels
including
chrome steels, duplex steels, stainless steels, martensitic alloy steels,
austenitic
stainless steels, precipitation-hardened stainless steels and high nickel
content steels.
[0003] Further, aqueous fluids, such as those used in drilling and
completion,
have a high salt content which causes corrosion. Gases, such as carbon dioxide
and
hydrogen sulfide, also generate highly acidic environments to which metallic
surfaces
become exposed. For instance, corrosion effects from brine and hydrogen
sulfide are
seen in flow lines during the processing of gas streams. The presence of
methanol,
often added to such streams to prevent the formation of undesirable hydrates,
further
often increases the corrosion tendencies of metallic surfaces.
[0004] Further, naturally occurring and synthetic gases are often
conditioned by
treatment with absorbing acidic gases, carbon dioxide, hydrogen sulfide and
hydrogen
cyanide. Degradation of the absorbent and acidic components as well as the
generation of by-products (from reaction of the acidic components with the
absorbent)
results in corrosion of metallic surfaces.
1
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
[0005] The
use of corrosion inhibitors during well treatments to inhibit the rate of
corrosion on metal components and to protect wellbore tubular goods is well
known.
Commercial corrosion inhibitors are usually reaction mixtures or blends that
contain
at least one component selected from nitrogenous compounds, such as amines,
acetylenic alcohols, mutual solvents and/or alcohols, surfactants, heavy oil
derivatives
and inorganic and/or organic metal salts.
[0006] Many
conventional corrosion inhibitors used to reduce the rate of acid
attack on metallic surfaces and to protect the tubular goods of the wellbore
are
becoming unacceptable in oilfield treatment processes. For
instance, many
conventional corrosion inhibitors have become unacceptable due to
environmental
protections measures that have been undertaken. In some instances, such as in
stimulation processes requiring strong acids, high temperatures, long duration
jobs
and/or special alloys, the cost of corrosion inhibitors may be so high that it
becomes a
significant portion of total costs.
[0007]
Efforts have been undertaken to find alternative corrosion inhibitors which
are cost effective and which are capable of controlling, reducing or
inhibiting
corrosion.
Summary of the Invention
[0008]
Corrosion of metallic tubulars in a well may be inhibited from forming by
introducing into the well a dithiazine or dithiazine derivative of the
structural
formulae (I) ¨ (V):
s ss s s s
s s
x-
I il R1 R\ /
R2 R
(I) (II) (III)
2
CA 02849067 2014-03-18
WO 2013/043491
PCT/US2012/055424
s.-"=,,,s
s s [.,.,e J e
X
N
C) ...'
W4
-.................,,õ0
R140
0
(IV) (V)
Or
wherein R is selected from the group consisting of a Ci to C12 saturated or
unsaturated
hydrocarbyl group or a Ci to Cio w-hydroxy saturated or unsaturated
hydrocarbyl
group; R1 is selected from the group consisting of a Ci-C24 straight or
branched chain
alkyl group or a C1-C24 arylalkyl group; R2 is a straight or branched alkylene
group;
R14 is a C1-C24 straight chain or branched alkyl group; or a C6 -C 24 aryl or
arylalkyl
group; R15 is a C1-C6 alkyl or a C6-C30 aryl or alkylaryl group; and X is
chlorine,
bromine or iodine.
[0009] The compounds of formula (II), (III) and (V) are quaternized
derivatives of
the dithiazine of formula (I) and (IV).
[00010] The dithiazine of structure (I) may be isolated from a whole spent
fluid
(WSF) formed by reaction of hydrogen sulfide and a triazine, such as a
triazine of
formula (VI):
R-...õN,,R
(VI)
V
I
R
wherein R is defined above in a hydrogen sulfide scavenger gas scrubbing
operation.
Alternatively, the whole spent fluid containing the dithiazine of structure
(I) may be
introduced into the well without isolation of the dithiazine.
[00011] Synergistic effects on inhibition of corrosion have further been noted
when
the dithiazine or dithiazine derivatives of formulae (I) - (V) are formulated
with at
least one corrosion inhibitor component selected from alkyl, alkylaryl or
arylamine
quaternary salts; mono or polycyclic aromatic amine salts; imidazoline
derivatives; a
3
CA 02849067 2016-04-18
mono-, di- or trialkyl or alkylaryl or arylalkyl phosphate ester; a monomeric
or
oligomeric fatty acid or salt; an alkyl or alkenyl substituted pyridinium; a
polyamine
amide; a polyhydroxy or alkoxylated amine or amide; a pyrazine derivative or a
mercaptocarboxylic acid. Synergistic effects have further been noted when WSF
is
combined with a corrosion inhibiting formulation containing any one or more of
these
corrosion inhibitor components.
[00011a] In accordance with an aspect of the present invention there is
provided a
dithiazine of the formula:
x e
0
Ryej
RR
(IV) or (V)
wherein:
R14 is a C1-C24 straight chain or branched alkyl group; or a C6-C24 aryl or
arylalkyl
group;
R15 is a C1-C6 alkyl or a C6-C30 aryl or alkylaryl group; and
X is chlorine, bromine or iodine.
[00011b] In accordance with a further aspect of the present invention there
is provided a
method of inhibiting corrosion during the treatment of a subterranean
formation which
comprises introducing into a gas or oil well a corrosive inhibiting effective
amount of a
dithiazine of the formula:
(W)
S s
J
Or
(VVs
4
CA 02849067 2016-04-18
wherein: =
R14 is a C1-C24 straight chain or branched alkyl group; or a C6-C24 aryl or
arylalkyl
group;
R15 is a C1-C6 alkyl or a C6-C30 aryl or alkylaryl group; and
X is chlorine, bromine or iodine.
Brief Description of the Drawings
[00012] In order to more fully understand the drawings referred to in the
detailed
description of the present invention, a brief description of the drawings is
presented, in
which:
[00013] FIGs. 1-7 demonstrate the effectiveness as whole spent fluids
(WSF),
formulated with additives defined herein.
[00014] FIGs. 8-12 demonstrate the effectiveness of quaternized reaction
products
of dithiazine compared to non-quaternized dithiazines.
Detailed Description of the Preferred Embodiments
[00015] Corrosion is inhibited during the treatment of a subterranean
formation
which is penetrated by an oil, gas or geothermal well by introducing into the
well a
dithiazine of the formula (I):
(I)
wherein R is selected from the group consisting of a CI to C12 saturated or
unsaturated
hydrocarbyl group or a C1 to C10 w-hydroxy saturated or unsaturated
hydrocarbyl group;
In a preferred embodiment, R is either (i) -R3-0H, wherein R3 is an alkylene
group,
preferably R is a CI-C6-0H group, most preferably -CH2CH2-0H; or (ii) a Ci-C6
alkyl
group, more preferably methyl or ethyl, and most preferably methyl.
4a
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
[00016] The dithiazine of formula (I) is preferably that obtained from the
homogeneous fluid which is produced during a hydrogen sulfide scavenger gas
scrubbing operation. In such scrubbing operations, a scavenger is introduced
to a
stream of liquid hydrocarbons or natural gas (sour gas) which contains
hydrogen
sulfide. In addition, such gas scrubbing operations remove hydrogen sulfide
from oil
production streams as well as petroleum contained in storage tanks, vessels,
pipelines,
etc. The scavenger is most commonly a water soluble triazine such as those of
formula (VI):
R.R
(VI)
'K
R
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
wherein R is defined above. Typically, the triazine is 1,3,5-
tris(hydroxyethyl)-
hexahydro-s-triazine. The production of dithiazines during a scrubbing
operation
using a triazine as scavenger is reported in U.S. Patent No. 6,582,624 wherein
the
spent fluid is reported to contain amorphous dithiazine solids. Typically,
dithiazines
remain part of the whole spent fluid resulting from the scrubbing operation.
Whole
spent fluid is typically discarded.
[00017] In the method described herein, the dithiazine of formula (I)
resulting from
the scrubbing operation may be introduced into a gas, oil or geothermal well
where it
functions as a corrosion inhibitor.
[00018] The whole spent fluid containing the dithiazine may be introduced to
the
well or dithiazine. Alternatively, the dithiazine of formula (I) may be
isolated from
the whole spent fluid by processes known in the art.
[00019] In addition to the dithiazines of formula (I), excellent corrosion
inhibition
properties may be obtained by the use of dithiazine derivatives of either
formula (II),
formula (III), (IV) or (V):
S S S S S S
) x -
or ) e)
N N
\ /
R N 111 R
R2
(II) (III)
6
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
s s
N
R14
-...,.,.,0
0
(XIV)
ss
(IV) (V)
L,,:e..0=00,01
x e
N R1 5
C)
R14 'O
(XV)
wherein R and X are as described above; R1 is selected from the group
consisting of a
Ci-C24 straight chain or branched alkyl group or a C1-C24 arylalkyl group;
preferably a
C1-C22 alkyl group, more preferably a C1-C12 alkyl group, most preferably a Ci-
C6
alkyl group; R2 is a C1-C6 straight or branched chain alkylene group; R14 is a
C1-C24
straight chain or branched alkyl group; or a C6-C24 aryl or arylalkyl group;
R15 is a C1-
C6 alkyl or a C6-C30 aryl or alkylaryl group; and X is chlorine, bromine or
iodine. R1
may also be substituted with an aryl group including benzyl and naphthyl.
Alternatively, R1 may be a C1-C24 arylalkyl group, such as benzyl, wherein the
alkyl
portion of the arylalkyl group may be linear or branched and the aryl portion
of the
arylalkyl group may be substituted with a C1-C6 alkyl group, such as methyl.
In a
preferred embodiment, R2 is -CH2-CH2-CH2-CH2- or -CH2-CH2-CH2-CH2-CH2-. In a
preferred embodiment, R of the compound of formula (III) is either ¨CH2CH2OH
or ¨
CH3, R14 of the compound of formula (IV) is either methyl or phenyl; and R14
and R15
of the compound of formula (V) is ¨CH3 and benzyl, respectively.
7
CA 02849067 2014-03-18
WO 2013/043491
PCT/US2012/055424
[00020] The quaternized product of formula (II) may be prepared by reacting a
dithiazine of formula (I) with a halide of the formula RiX in approximately
equimolar
ratios. A representative quaternization reaction for the preparation of the
compounds
of formula (II) may be illustrated as follows:
ss ss
) R1¨x
X e (A)
N
I R1
R
(I) (1)
[00021] In a preferred embodiment, the RiX used in the quaternization reaction
is
benzyl chloride or chloromethyl naphthalene and R is either ¨CH2CH2OH or ¨CH3.
Most preferred are those reactants set forth in Table I below which is
followed by the
designated reaction schemes (B), (C), (D) and (E):
Table I
Reaction Scheme R RiX
(B) ¨CH2CH2OH Benzyl chloride
(C ¨CH3 Benzyl chloride
(D) ¨CH2CH2OH Chloromethyl naphthalene
(E) ¨CH3 Chloromethyl naphthalene
CI
ss
ss
) (VIII) I,.........4 )
N e (B)
N C1
S:"
HOX .OH
(VII)
(IX)
8
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
a
ss ss
) 0
ae (c)
N
I (VIII)
-Ip... 113C
CH3
9C)
I.
(XI)
CI
SS 00 0
e
r.1 N 0 (D)
(XII)
_),..
OH 00
(VII)
(xm)
a
ss ss
) 100 )
ae (E)
N
I (XII) c.N
H3
(X) SO
(XIV)
The quaternized product of formula (III) may be prepared by reacting a
dithiazine of
formula (I) with a dihalide of the formula X-R2-X wherein each R2 is a C1-C6
straight
9
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
or branched alkylene group, preferably -CH2-CH2-CH2-CH2- or -CH2-CH2-CH2-CH2-
CH2-; and wherein X is chlorine, bromine or iodine.
[00022] A representative quaternization reaction for the preparation of the
compounds of formula (III) may be illustrated by reaction scheme (F):
........--........ ........---.....õ .....õ-----
.....õ
s s s s s s
(F)
x-R2-x 1........ L...._ ::....
....j
, IV,
R"........ '........... R2--- e 6,--"R
R X X
(I) (u.)
where R is ¨R3-0H, the dithiazine of formula (I) may be derivatized to form
compounds of structural formula (IV) and (V). For instance, the compound of
formula (IV) may be prepared by reacting the dithiazine of formula (I) with a
stoichiometric amount of acid chloride, such as an alkyl, aryl or arylalkyl
acid
chloride or acid anhydride. In a preferred embodiment, the dithiazine is
reacted with
an acid chloride of the formula Ri4C0C1. Typically, an alkyl amine, such as
triethylamine, is used at the end of the reaction, especially when an acid
chloride is
used, in order to remove any halide salt. A representative reaction scheme for
the
production of the compound of formula (IV) wherein R14 is phenyl may be
represented by the following:
s s
s s
) )
N
N
Benzoyl Chloride
000
OH
(VII)
(XV)
[00023] The dithiazine derivative of formula (V) may be prepared by reacting
the
dithiazine of formula (I) with a stoichiometric amount of an alkyl, aryl or
arylalkyl
acid anhydride to form an ester of the dithiazine and then reacting the ester
of the
CA 02849067 2014-03-18
WO 2013/043491
PCT/US2012/055424
dithiazine with an alkyl, aryl or alkylaryl halide. A representative reaction
scheme for
the production of compounds of formula (V) where R14 is ¨CH3 and R15 is benzyl
may be illustrated as:
ss
ss
ss
Acetic Anhydride N Iõe e
ci
.........õ. N
Benzyl Chloride
H3C
OH ..........____õ.0 LC, 0
0 (XVI) H3C 0
(VII)
(xvie
[00024] The amount of dithiazine or dithiazine derivative introduced into the
well
is an amount sufficient to inhibit corrosion of the base materials, especially
iron, of
tubulars in the well. Typically, the amount of dithiazine or dithiazine
derivative
introduced into the well is in the range of from about 0.05% to about 5% by
volume
of the treatment fluid introduced.
[00025] In a preferred embodiment, the dithiazine of formula (I) or the
dithiazines
of formulae (II), (III), (IV) and (V) (all of which may be isolated or within
whole
spent fluid) may be formulated with at least one other component selected from
the
groups:
(0 an alkyl, hydroxyalkyl, alkylaryl arylalkyl or arylamine
quaternary
salt;
(ii) a mono or polycyclic aromatic amine salt;
(iii) an imidazoline derivative;
(iv) a mono-, di- or trialkyl or alkylaryl or arylalkyl phosphate ester;
(v) a monomeric or oligomeric fatty acid or salt thereof;
(vi) an alkyl or alkenyl substituted pyridinium;
(vii) a polyamine amide;
(viii) a polyhydroxy or alkoxylated amine or amide;
(ix) a pyrazine derivative; and
(x) mercaptocarboxylic acids.
[00026] In a more preferred embodiment, the dithiazine or dithiazine
derivatives
are formulated with the formulation component (a) through (x) such that the
volume
ratio of dithiazine to the additive component is typically between from about
1:0.5 to
11
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
about 1:2.0, more typically between from about 1:0.8 to about 1:1. Formulating
the
dithiazine (or quaternized dithiazine) with the additive component may be
effectuated
by adding the additive to the whole spent fluid or by adding the additive to
an alcohol
containing solvent (for example methanol) containing the dithiazine or
quaternized
dithiazine.
[00027] Exemplary of the alkyl, hydroxyalkyl, alkylaryl arylalkyl or arylamine
quaternary salts are those alkylaryl, arylalkyl and arylamine quaternary salts
of the
formula [N 'R5R6R7R8][X-] wherein R5, R6, R7 and R8 contain one to 18 carbon
atoms,
X is Cl, Br or I. In a preferred embodiment, any or all of the R5, R6, R7 and
R8 are a
C1-C6 alkyl group or a hydroxyalkyl group wherein the alkyl group is
preferably a Ci-
C6 alkyl or an alkyl aryl such as benzyl. The mono or polycyclic aromatic
amine salt
with an alkyl or alkylaryl halide include salts of the formula [N+R5R6R7R8][X1
wherein R5, R6, R7 and R8 contain one to 18 carbon atoms, X is Cl, Br or I.
[00028] Typical quaternary ammonium salts are tetramethyl ammonium chloride,
tetraethyl ammonium chloride, tetrapropyl ammonium chloride, tetrabutyl
ammonium
chloride, tetrahexyl ammonium chloride, tetraoctyl ammonium chloride,
benzyltrimethyl ammonium chloride, benzyltriethyl ammonium chloride,
phenyltrimethyl ammonium chloride, phenyltriethyl ammonium chloride, cetyl
benzyldimethyl ammonium chloride, and hexadecyl trimethyl ammonium chloride.
Preferred are alkylamine benzyl quaternary ammonium salts, benzyl
triethanolamine
quaternary ammonium salts and benzyl dimethylaminoethanolamine quaternary
ammonium salts.
[00029] In addition, the salt may be a quaternary ammonium or alkyl pyridinium
quaternary salt such as those represented by the general formula:
1 (xvill)
N
1
R9B-
wherein R9 is an alkyl group, an aryl group or an alkyl group having from 1 to
about
18 carbon atoms and B is chloride, bromide or iodide. Among these compounds
are
alkyl pyridinium salts and alkyl pyridinium benzyl quats. Exemplary compounds
include methyl pyridinium chloride, ethyl pyridinium chloride; propyl
pyridinium
12
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
chloride, butyl pyridinium chloride (such as N-butyl-4-methylpyridinium
chloride),
octyl pyridinium chloride, decyl pyridinium chloride, lauryl pyridinium
chloride, cetyl
pyridinium chloride, benzyl pyridinium, dodecylpyridinium chloride,
tetradecylpyridinium chloride, N-methylpyridinium chloride, N-methylpyridinium
bromide, N-ethylpyridinium chloride, N-ethylpyridinium bromide, 2-
vinylpyridinium
chloride, 2-vinylpyridinium bromide, 3-vinylpyridinium chloride, 3-
vinylpyridinium
bromide, 4-vinylpyridinium chloride and 4-vinylpyridinium bromide, and an
alkyl
benzyl pyridinium chloride, preferably wherein the alkyl is a C1-C6
hydrocarbyl
group, as well as mixtures thereof
[00030] The additive may further be an imidazoline derived from a diamine,
such
as ethylene diamine (EDA), diethylene triamine (DETA), triethylene tetraamine
(TETA) etc. and a long chain fatty acid such as tall oil fatty acid (TOFA).
Suitable
imidazolines include those of formula (IV):
RI)
1
R12
._..,..-N (XIX)
>_ RE)
R13 .--N
wherein R12 and R13 are independently a C1-C6 alkyl group more preferably
hydrido,
R" is hydrido and R1 a C1-C20 alkyl, a Ci-C20 alkoxyalkyl group. In a
preferred
embodiment, R", R12 and R13 are each hydrido and R1 is the alkyl mixture
typical in
tall oil fatty acid (TOFA).
[00031] Suitable mono-, di- and trialkyl as well as alkylaryl phosphate esters
and
phosphate esters of mono, di, and triethanolamine typically contain between
from 1 to
about 18 carbon atoms. Preferred mono-, di- and trialkyl phosphate esters,
alkylaryl
or arylalkyl phosphate esters are those prepared by reacting a C3_18 aliphatic
alcohol
with phosphorous pentoxide. The phosphate intermediate interchanges its ester
groups
with triethyl phosphate with triethylphosphate producing a more broad
distribution of
alkyl phosphate esters. Alternatively, the phosphate ester may be made by
admixing
with an alkyl diester, a mixture of low molecular weight alkyl alcohols or
diols. The
low molecular weight alkyl alcohols or diols preferably include C6 to C10
alcohols or
diols. Further, phosphate esters of polyols and their salts containing one or
more 2-
13
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
hydroxyethyl groups, and hydroxylamine phosphate esters obtained by reacting
polyphosphoric acid or phosphorus pentoxide with hydroxylamines such as
diethanolamine or triethanolamine are preferred.
[00032] The additive may further be a monomeric or oligomeric fatty acid.
Preferred are C14-C22 saturated and unsaturated fatty acids as well as dimer,
trimer and
oligomer products obtained by polymerizing one or more of such fatty acids.
[00033] The additive may also be a polyamine amide such as products from the
reaction of polyethyleneamines and carboxylic acids, such as
polymethylenepolyaminedipropionamides as well as acylated derivatives of amino
acids appended to a polyols, polyamine or hydroxylamine backbone.
[00034] Suitable polyhydroxy or alkoxylated amines or amides such as tallow-
CONH(CH2)3NHCH2CH2OH and tallow-NH(CH2)3NH2 ethoxylated with 3 moles of
ethylene oxide as well as deoxyglucityl derivatives of alkylamines.
[00035] Suitable pyrazine derivatives include methyl substituted derivatives
such
as 3,5-dimethylpyrazine, 2,3,5,6-tetramethylpyrazine and 2,4,6-
trimethylpyridine
reached with an aldehyde such as 1-dodecanal or ketone, such as 5,7-dimethy1-
3,5,9-
decatriene-2-one.
[00036] Further the additive may be a mercaptocarboxylic acid such as
thioglycolic
acid, 3,5 '-dithiopropionic acid or potassium dimethyldithio carbamate.
[00037] A water soluble fluid containing the dithiazine, dithiazine
derivative, or
whole spent fluid and, optionally, one or more formulation components, is
typically
introduced into the wellbore or subterranean formation. The fluid of the water
soluble
fluid may be a hydrophilic solvent such as water (including fresh water,
brackish
water and brine, such as sodium chloride, potassium chloride and ammonium
chloride
brine). The hydrophilic solvent may further be a hydrophilic organic solvent,
such as
methanol or ethylene glycol.
[00038] In a preferred embodiment, the dithiazine is not separated and the
whole
spent fluid (containing the dithiazine or dithiazine derivative) is then
combined with a
corrosion inhibiting formulation containing at least one corrosion inhibitor
of
component (a) through (x). The weight ratio of spent fluid to the corrosion
inhibiting
formulation may be from 99:1 to 1:99 weight percent and typically is between
from
about 25:75 to about 75:25, preferably approximately 50:50.
[00039] In this embodiment, the whole spent fluid typically acts as a diluent
to the
corrosion inhibiting formulation. In a preferred embodiment, the corrosion
inhibiting
14
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
formulation is a commercial formulation containing one or more corrosion
inhibitors.
The corrosion inhibiting formulation may further contain water, an organic
hydrophilic solvent, such as methanol. Suitable organic hydrophilic solvents
include
methanol and ethylene glycol, etc. Use of the whole spent fluid permits
dilution of
the corrosion inhibiting formulation by as much as 40 to 60 volume percent.
[00040] The whole spent fluid and corrosion inhibiting formulation exhibit a
synergistic effect in the resulting blended fluid. For instance, a three to
four fold
decrease in corrosion inhibition has been noted from the blended fluid
compared to
the amount of corrosion inhibition seen in the whole spent fluid and corrosion
inhibiting formulation individually. The synergism has been especially noted
where
the volume ratio of dithiazine (in the whole spent fluid) to corrosion
inhibitor(s) (in
the corrosion inhibiting formulation) is between from about 1:0.5 to about
1:2Ø
[00041] The use of a blend of whole spent fluid with corrosion inhibiting
formulation has great commercial implications since it is not necessary to
isolate the
dithiazine from the whole spent fluid. As such, the whole spent fluid is
combined
with the corrosion inhibiting formulation. Since whole spent fluid is
typically
regarded as a waste material, the blend defined herein lessens the
environmental
impact created by the generation of whole spent fluid in the oil and gas
service
industry. In so doing, an otherwise useless waste product is converted to a
product
demonstrating superior corrosion inhibiting properties.
[00042] Since the dithiazine, dithiazine derivatives and synergistic blends
dramatically reduce corrosion on metal, they may be used in a variety of
industrial
applications. Alternatively, the dithiazine or dithiazine derivatives may be
introduced
prior to or subsequent to, as well as during, a well treatment operation. For
instance,
the dithiazine or dithiazine derivatives as well as formulations containing
the
dithiazine or dithiazine derivative may be introduced into the well during
stimulation.
[00043] Other embodiments within the scope of the claims herein will be
apparent
to one skilled in the art from consideration of the description set forth
herein. It is
intended that the specification, together with the examples, be considered
exemplary
only, with the scope and spirit of the invention being indicated by the claims
which
follow.
[00044] The following examples are illustrative of some of the embodiments of
the
present invention.
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
[00045] All percentages set forth in the Examples are given in terms of weight
units except as may otherwise be indicated.
EXAMPLES
[00046] Example 1. The following are used to describe components of this
Example.
[00047] The dithiazine refers to 5-hydroxyethyl-hexahydro-1,3,5-dithiazine of
the
formula I wherein R is ¨CH2CH2-0H.
[00048] Unspent fluid ("UF") refers to the hexahydro-1,3,5-tri(hydroxyethyl)-s-
triazine of the formula II above wherein each R is ¨CH2CH2-0H prior to any
spending with hydrogen sulfide.
[00049] Whole spent fluid ("WSF") refers to the homogeneous fluid produced in
a
hydrogen sulfide scavenger gas scrubbing operation wherein the tower was
charged
with a triazine [1,3,5-tris(hydroxyethyl)-hexahydro-s-triazine] containing
fluid in
water and methanol. The fluid contains a high level of the dithiazine, it
still being in
the WSF.
[00050] Isolated dithiazine ("iDTZ") refers to dithiazine from the WSF that
has
been separated out of solution in its pure form.
[00051] Formulated products were paired using one of the following additives:
Methyl/Ethylpyridinium benzyl quat (APBQ);
Benzyldimethylcocoamine benzyl quat (ABQ);
TOFA DETA Imidazoline derivative ("TDID");
Benzyl triethanolaminium quat (BTEAQ); and
Benzyl dimethylaminoethanolaminium quat (BDMAEQ).
Formulated WSF refers to the product formed by dissolving the additive in the
WSF
at a concentration of 12.2 weight percent with methanol at 10 weight percent.
Formulated iDTZ refers to the product prepared by dissolving iDTZ in methanol
at a
concentration of 9.6 weight percent and then adding to the resultant the
additive at a
concentration of 19.2 weight percent. This product was then mixed for a brief
period
while heating to approximately 60 C.
[00052] Corrosion rate studies were performed using at ambient temperature a
Gamry G Series potentiostat and the conventional Linear Polarization
Resistance
(LPR) module within the DC1OSTM corrosion technique software package (Rp/Ec
16
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
trend). The instantaneous corrosion rate of the three electrode probe system
was
determined using voltage settings -0.2V to +0.02V versus open-circuit
potential, E0.
These studies were carried out during an approximately 20-24 hr run time. The
treat
rates of the corrosion inhibitors were between 50 and 200 ppm. A standard
carbon
dioxide saturated brine system comprised of 3 weight percent sodium chloride
and 0.3
weight percent calcium chloride in 2 liter corrosion cells sparged with carbon
dioxide
was employed. LPR scans are shown in FIGS. 1-8 wherein:
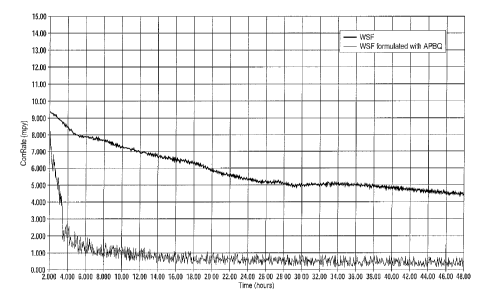
FIG. 1 contrasts the differences at a treatment rate of 500 ppm in
corrosion rates between WSF and formulated WSF containing the additive
APBQ. As shown, much higher corrosion rates are demonstrated with WSF
than formulated WSF;
FIG. 2 contrasts the differences at a treatment rate of 500 ppm in
corrosion rates between formulated WSF and UF both containing the additive
APBQ. As shown, formulated WSF is a better corrosion inhibitor than UF;
FIG. 3 contrasts the differences at a treatment rate of 430 ppm of WSF
and iDTZ. FIG. 3 shows that iDTZ is much more effective than WSF as a
corrosion inhibitor;
FIG. 4 contrasts the differences at a treatment rate of 430 ppm of
Formulated iDTZ (with the additive APBQ) and iDTZ alone. FIG. 4 shows
that Formulated iDTZ is a better corrosion inhibitor than iDTZ alone;
FIG. 5 contrasts the differences at a treatment rate of 430 ppm in
corrosion rates between iDTZ alone and Formulated iDTZ (with the additive
ABQ), Formulated iDTZ (with the additive TDID) and Formulated iDTZ
(with the additive APBQ). FIG. 5 shows the Formulated iDTZ (with APBQ)
to be the best corrosion inhibitor. Formulated iDTZ (with TDID) and
Formulated iDTZ (with ABQ) demonstrate similar results. All of the
formulated iDTZs demonstrated better corrosion inhibition than iDTZ alone;
FIG. 6 contrasts the differences at a treatment rate of 125 ppm in
corrosion rates between Formulated iDTZ (with BTEAQ) and Formulated
iDTZ (with APBQ). FIG. 6 demonstrates better corrosion results with
Formulated iDTZ (with APBQ); and
FIG. 7 contrasts the differences at a treatment rate of 125 ppm in
corrosion rates between Formulated iDTZ (with APBQ) and Formulated iDTZ
17
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
(with BDMAEQ). FIG. 7 demonstrates better corrosion results with
Formulated iDTZ (with APBQ).
[00053] Example 2. A dithiazine quaternization reaction product was prepared
in
accordance with the following synthetic pathway:
ss ss
) ________________ 1
N R1¨x
,...-
,....,:) xe
I N
R1
R
(I) 01)
by dissolving in 50 mls of methanol about 2.84 grams (17.2 mmols) of the
dithiazine
together with a 10% molar excess of the R1-X reagent (2.42 grams, 19.1 mmols).
The
solution was heated to 125 C and stirred for 6 hrs in a Parr reactor. Upon
cooling the
solution was recovered as a dark red.
[00054] Corrosion rate studies were conducted using 35 parts of active species
per
million by mass at ambient temperature on a Gamry G Series potentiostat and
the
conventional Linear Polarization Resistance (LPR) module within the DC105Tm
corrosion technique software package (Rp/Ec trend) in accordance with the
procedure
set forth in Example 1. The instantaneous corrosion rate of the three
electrode probe
system was determined using voltage settings -0.2V to +0.02V versus open-
circuit
potential, E0. These studies were carried out during an approximately 20-24 hr
run
time. The treat rates of the corrosion inhibitors were between 50 and 200 ppm.
A
standard carbon dioxide saturated brine system comprised of 3 weight percent
sodium
chloride and 0.3 weight percent calcium chloride in 2 liter corrosion cells
sparged
with carbon dioxide was employed. LPR scans are shown in FIGS. 8-10 wherein:
FIG. 8 contrasts the differences of compound (I) wherein R is methyl
at 35 parts per million (ppm) (by mass) [or 0.26 ppm (by moles)] and
compound (II) wherein R is methyl and Rl is benzyl at 35 parts per million
(ppm) (by mass) [or 0.13 ppm (by moles)].
FIG. 9 contrasts the differences of compound (I) wherein R is HO-
CH2-CH2- 35 parts per million (ppm) (by mass) [or 0.21 ppm (by moles)] and
18
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
compound (II) wherein R is HO-CH2-CH2- and Rl is benzyl at 35 parts per
million (ppm) (by mass) [or 0.12 ppm (by moles)].
FIG. 10 contrasts the differences of compound (I) wherein R is HO-
CH2-CH2- at 35 ppm [or 0.21 ppm (by moles)] and compound (B) wherein R
is HO-CH2-CH2- and Rl is naphthylmethyl at 35 parts per million (ppm) (by
mass) [or 0.10 ppm (by moles)].
As shown, much better corrosion rate inhibition is demonstrated with the
derivatized
dithiazines represented by compound (II) than compound (I) especially
considering
that lower molar quantities of the derivatized dithiazines were used.
[00055] Example 3. A dithiazine quaternization reaction product was prepared
in
accordance with the following synthetic pathway:
S s
x¨R2¨x
N R R
X X
(I) (no
by dissolving in 50 mls of methanol about 3grams (18.2mmols) of the dithiazine
together with the X-R2-X- reagent (9.1 mmols). The solution was heated to 125
C
and stirred for 6 hrs in a Parr reactor. Upon cooling the solution was
recovered as a
dark red.
[00056] Corrosion rate studies were conducted at ambient temperature on a
Gamry
G Series potentiostat and the conventional Linear Polarization Resistance
(LPR)
module within the DC1OSTM corrosion technique software package (Rp/Ec trend)
in
accordance with the procedure set forth in Example 1. The instantaneous
corrosion
rate of the three electrode probe system was determined using voltage settings
-0.2V
to +0.02V versus open-circuit potential, E0. These studies were carried out
during an
approximately 20-24 hr run time. The treat rates of the corrosion inhibitors
were
between 50 and 200 ppm. A standard carbon dioxide saturated brine system
comprised of 3 weight percent sodium chloride and 0.3 weight percent calcium
chloride in 2 liter corrosion cells sparged with carbon dioxide was employed.
LPR
scans are shown in FIGS. 11 and 12 wherein:
19
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
FIG. 11 contrasts the differences of the structure of formula (I) wherein
R is HO-CH2-CH2- at 0.21 ppm (by moles) and the structure of formula (III)
wherein R is HO-CH2-CH2- and R2 is C5H10 at 0.12 ppm (by moles).
FIG. 12 contrasts the differences of the structure of formula (I) wherein
R is HO-CH2-CH2- at 0.21 ppm (by moles) and the structure of formula (III)
wherein R is HO-CH2-CH2- and R2 is C4H8 at 0.12 ppm (by moles).
As shown, much better corrosion rate inhibition is demonstrated with the
derivatized
dithiazines represented by compound (III) than the dithiazines represented by
compound (I).
[00057] Example 4. 5 -(2-b enzoyloxyethyl)hexahydro-1,3 ,5 -
dithiazine was
prepared in accordance with the following synthetic pathway:
s s
)s s
) N
N
Benzoyl Chloride
000
OH
(IV)
(XV)
by dissolving in 1 ml of methylene chloride about 400 mg of 5-hydroxyethyl-
hexahydro-1,3,5-dithiazine and a stoichiometric amount of benzoyl chloride in
a
sealed ReactiVial vessel at 100 C and stirred for 8 hrs. About 400 mg of
triethylamine was added to the resulting product and triethylammonium chloride
salt
was removed.
[00058] Example 5. 5-(2-acetoxyethyl)hexahydro-1,3,5-dithiazine was prepared
in
accordance with the following synthetic pathway:
CA 02849067 2014-03-18
WO 2013/043491 PCT/US2012/055424
ss ss
ss
Acetic Anhydride
ci
......,,N
___________________ a- .,''. Benzyl Chloride
_)"...
C)
OH H3C.................õ.õ0
14111
(IV) 0 (XVI ) H 3C,,,...`,.
0
(xvie
by dissolving in 1 ml of methylene chloride about 400 mg of 5-hydroxyethyl-
hexahydro-1,3,5-dithiazine and a stoichiometric amount of acetic anhydride and
heating the mixture at 100 C for approximately 8 hours. The solvent was then
removed from the resulting mixture and the ester of formula (XVI) was
isolated.
Above 200 mg of the ester was then dissolved in methanol and a stoichiometric
amount of benzyl chloride was added to a sealed ReactiVial vessel. The
solution was
heated to approximately 120 C for about 6 hours.
Example 6. Corrosion inhibition tests were conducted on the product solutions
of
Example 4 and Example 5 by ambient pressure linear polarization resistance
(LPR)
which was performed at ambient temperature in 2000 mL glass resin kettles.
Corrosion rates were monitored using a linear polarization resistance
instrument with
3 electrode probes. Tests were 24-hour exposures of AISI 1018 (UNS G10180)
steel
electrodes to stirred solutions at room temperature using a brine (3% NaC1,
0.3%
CaC12 x 2H20) sparged with CO2. In each test, 50 ppm of compounds of formula
(I),
(II), (III) and (IV) in Examples 4 and 5 in the brine were evaluated.
Corrosion rates
after 23 hours were determined by weight loss of the reference electrode.
Electrodes
were cylindrical with a surface area of 9 cm2. Measured corrosion rates are
set forth
in Table I.
Table I
Compound Corrosion,
Mils per year
(n1PY)
(I) 1.60
(II) 0.60
(III) 0.18
(IV) 0.26
21
CA 02849067 2015-09-14
The compound of formula (II) exhibited more than 100% improved corrosion rate
over
the compound of formula (I). The corrosion rate of the compound of formula (I)
was just
over one tenth of the corrosion rate of the compound of formula (I). The
compound of
formula (IV) had better than 100% improvement in corrosion rate over the
compound of
formula (I).
[00059] Example 7. Whole spent fluid (WSF) produced in a hydrogen sulfide
scavenger gas scrubbing operation was combined with commercially available
corrosion
inhibitor formulations - Technihib 366 ("A") and Technihib 356 ("B"), both
products of
Baker Hughes Incorporated. Corrosion rates after 21 hours were determined as
set forth
in Example 6 and are set forth in Table II wherein 75 ppm of each of the
formulations
was tested:
Table II
Formulation Wt. Ratio Corrosion.
Mils per year
(m )
A MEM 3.54
INSFisi 5050 1.87
100 ,1,55
WSFB 5050 2 4.3
Table II illustrates that better results were obtained when a mixture of the
whole spent
fluid was used in combination with the commercial formulation than when the
commercial formulation was used by itself.
[00060] From the foregoing, it will be observed that numerous variations
and
modifications may be effected without departing from the scope of the
invention.
22