Note: Descriptions are shown in the official language in which they were submitted.
CA 02851786 2014-02-11
WO 2014/026217 - 1 - PCT/AU2013/000857
"Processing of Lithium Containing Material"
Field of the Invention
[0001] The present invention relates to the treatment of lithium containing
material.
[0002] More particularly, the present invention relates to a process for the
treatment of a lithium containing material and the production of lithium
hydroxide
and lithium carbonate. The process utilising the electrolysis of a lithium
chloride
solution obtained from either a spodumene ore or concentrate, or from brines.
In
one form, the process of the present invention is intended to provide a high
purity
or battery grade lithium hydroxide and lithium carbonate product.
[0003] The process of the present invention may further provide a hydrochloric
acid product. Still further, the process of the present invention, in one
form,
utilises precious metal containing mixed metal oxide (MMO) electrodes to
heighten the efficiency of an electrochemical portion of the process.
Background Art
[0004] Known processes for the production of lithium carbonate from lithium
containing ores or concentrates typically utilise the thermal treatment of an
alpha-
spodumene ore or concentrate. This thermal treatment can be referred as
decrepitation and transforms the alpha-spodumene to beta-spodumene which is
in turn able to be solubilised by acid. The step in which the beta-spodumene
is
solubilised in acid takes place in a kiln and produces soluble lithium salt.
The
lithium salt is passed to one or more tanks in which the lithium salt is
purified.
Leached crude lithium salt is subsequently passed to a step in which the pH of
the
slurry is adjusted, whereby certain impurities, including iron and magnesium
are
intended to be precipitated. Thus purified lithium salt is treated with soda
ash to
produce lithium carbonate. This lithium carbonate can be further treated with
hydrated lime to produce lithium hydroxide.
CA 02851786 2014-02-11
WO 2014/026217 - 2 - PCT/AU2013/000857
[0005] Processes for the production of lithium carbonate and lithium hydroxide
from brines typically involves the use of evaporation ponds to increase the
concentration of the salts contained therein before being passed to a series
of
steps aimed to reduce the impurities present.
[0006] The above described processes of the prior art are relatively
inefficient in
the removal of impurities remaining in the pregnant leach solution, which
results in
a relatively impure lithium hydroxide and lithium carbonate product. This is
particularly problematic when attempting to produce high quality or battery
grade
lithium hydroxide and lithium carbonate products.
[0007] The process of the present invention has as one object thereof to
overcome substantially one or more of the above mentioned problems associated
with prior art processes, or to at least provide a useful alternative thereto.
[0008] The preceding discussion of the background art is intended to
facilitate an
understanding of the present invention only. This discussion is not an
acknowledgement or admission that any of the material referred to is or was
part
of the common general knowledge as at the priority date of the application.
[0009] Throughout the specification and claims, unless the context requires
otherwise, the word "comprise" or variations such as "comprises" or
"comprising",
will be understood to imply the inclusion of a stated integer or group of
integers
but not the exclusion of any other integer or group of integers.
[0010] The term "battery grade lithium carbonate" refers to a product having a
purity of about 99.5% or higher. Similarly, the term "battery grade lithium
hydroxide" refers to a product having a purity of about 99% or higher.
Disclosure of the Invention
[0011] In accordance with the present invention there is provided a process
for the
treatment of a lithium containing material, the process comprising the steps
of:
(i) Preparing a process solution from the lithium containing
material;
CA 02851786 2014-02-12
= =
PCT/AU2013/000857
- 3 -
Receivec20/01/2014
(ii) Passing the process solution from step (i) to a series of impurity
removal steps thereby providing a substantially purified lithium chloride
solution;
(iii) Passing the purified lithium chloride solution of step (ii) to an
electrolysis step thereby producing a lithium hydroxide solution; and
(iv) Carbonating the lithium hydroxide solution produced in step (iii) by
passing compressed carbon dioxide through the solution, thereby
producing a lithium carbonate precipitate,
wherein the lithium containing material is an alpha-spodumene ore or ore
concentrate and the process further comprises a first step in which that alpha-
spodumene ore or ore concentrate is calcined to produce beta-spodumene.
[0012] In one form of the present invention, the process solution Of step (i)
is
prepared in the form of a pregnant leach solution. Preferably, the pregnant
leach
solution is formed by passing a lithium containing material to a leach step in
which
the material is leached with hydrochloric acid,
[0013] Preferably, the impurity removal step (ii) further comprises a
concentration
step wherein the pregnant leach solution is concentrated to near saturation of
lithium chloride.
[0014] The lithium hydroxide solution produced in step (iii) may be thickened
by
evaporation of water to provide lithium hydroxide monohydrate crystals.
[0015] In a further form of the present invention a first portion of the
lithium
hydroxide solution produced in step (iii) is thickened by
evaporation/crystallisation
to provide lithium hydroxide monohydrate crystals and a second portion thereof
is
carbonated by passing compressed carbon dioxide through the solution, thereby
producing a lithium carbonate precipitate.
[0016] Preferably, the impurity removal steps of step (ii) include one or more
of
hyrdropyrolysis of Al and Fe chlorides, pH increase to precipitate hydroxides
of Al,
AMENDED SHEET
IPEA/AU
CA 02851786 2014-02-12
=
PCT/AU2013/000857
- 4 -
Received20/01/2014
Fe, Mg and Mn, lithium carbonate precipitation for removal of Ca, and
fractional
crystalisation for the removal of Na and K.
[0017] Still preferably, the fractional crystallisation for the removal of Na
and K is
conducted immediately after the concentration step.
[0018] The impurity removal steps preferably further comprises an ion exchange
step. Preferably, the ion exchange step removes substantially all calcium, =
magnesium and other multivalent cations remaining in the pregnant leach
solution. Still preferably, such multivalent cations are removed to a level of
less ,
than about 10 ppm.
[0019] Still preferably, water evaporated from the
solution in
evaporation/crystallisation is recompressed, combined with make-up steam and
utilised in evaporation/crystallisation. The evaporation/crystallisation
step =
preferably utilises a vacuum evaporative crystalliser.
[0020] Preferably, the beta-spodumene is cooled and milled prior to the leach
step. The beta-spodumene is preferably milled to less than about 300 pm. Still
preferably, the beta-spodumene is milled to a P80 of about 75 pm.
[0021] Preferably, the leach step is conducted at elevated temperature.
[0022] The hydrochloric acid solution used in the leach step is preferably
about
20% NCI w/w.
[0023] Still preferably, the elevated temperature of the leach step is about
the
boiling point of the hydrochloric acid solution used in the leach step.
[0024] The leach step is preferably conducted at atmospheric pressure.
[0025] In one form of the present invention the leach step is conducted in a
chlorination kiln at about 108 C over a residence time of about 6 to 10 hours.
Preferably, the residence time is about 8 hours. =
= AMENDED SHEET
IPEA/AU
CA 02851786 2014-02-12
PCT/AU2013/000857
- 5 -
Receivec20/01/2014
Brief Description of the Drawings
(00261The process of the present invention will now be described, by way of
example only, with reference to one embodiment thereof and the accompanying
drawing, in which:-
Figure 1 is a schematic flow-sheet depicting a process for the treatment of
a lithium containing material in accordance with a first embodiment of the
present invention in which the lithium containing material is an alpha-
. spodumene concentrate.
Best Mode(s) for Carrying Out the Invention
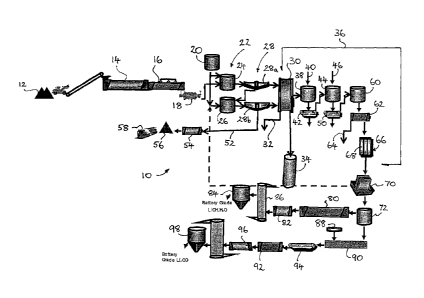
[0027] In Figure 1 there is shown a process 10 for the treatment of a lithium
containing material in accordance with a first embodiment of the present
invention
in which embodiment the lithium containing material is provided in the form of
an
alpha-spodumene concentrate.
[00281All of the unit operations embodied in the process 10 are intended to
operate continuously with full process instrumentation and control being
provided
for.
[0029] An alpha-spodumene concentrate 12 is passed to a calcining step in
which
the concentrate 12 is calcined in a calcining furnace 14 at a temperature of
between about 1050 C to 1100 C to convert the alpha-spodumene to leachable
beta-spodumene. Off-gases from the calciner are directed through a cyclone
(not
shown) and an electrostatic precipitator (not shown) specified to comply with
known environmental emissions limits. The resulting hot calcine is passed to a
cooler 16 and indirectly cooled to about 80 C. It is then dry-milled to less
than
300 pm, for example to a P80 of about 75 pm, in a mill, for example a closed
circuit
ball mill 18. =
[0030] After storage in a surge bin (not shown), the milled beta-spodumene is
mixed with at least a 40 to 300% stoichiometric excess of 20% hydrochloric
acid
w/w 20 in a slurrying step. The slurrying step feeds a leach step, for example
a
leach circuit 22, comprising a first leach stage 24 and a second leach stage
26.
AMENDED SHEET =
IPEA/AU
CA 02851786 2014-02-12
PCT/AU2013/000857
-6 - =
Receivec20/01/2014
[0031] The leach step is conducted at about 108 C, being the boiling point of
the
hydrochloric acid leach solution added in the slurrying step, for a period of
about 6
to 12 hours, for example about 8 hours, in continuous leach tanks. A pulp
density
of about 40% is used in the leach circuit 22 to maximise the leach
concentration
and to ensure that the solubility limit of lithium chloride during leaching is
not
exceeded. Off-gases are cleaned in a wet scrubber (pot shown). The leach step
= 22 produces a residue slurry and a process\ solution, for example a
pregnant
leach solution. The lithium and the aluminosilicate in the beta-spodumene
leaches into solution with other impurities to give a sub-saturated
concentration of
lithium chloride in the pregnant leach liquor.
[0032] The pregnant leach solution from the leach circuit 22 is passed to a
thickening circuit 28, preferably comprising two stages 28a and 28b aligned
with
the stages 24 and 26 of the leach circuit 22. An overflow from the thickening
circuit 28 is directed to a pyrohydrolysis step 30, operating at about 300oC,
and in
which chlorides of Al and Fe present in the pregnant leach solution are
converted
into their respective insoluble oxides 32. Any residual HCI is also recovered
in an
HCL removal step 34.
[0033] In addition to the Al and Fe described immediately 'above as being
recovered using the pyrohydrolysis step 30, remaining soluble iron, aluminium
and magnesium are removed In large part from the leach liquor through a series
of impurity removal steps, indicated in a broad sense by impurity removal
steps 36
in Figure 1. The impurity removal steps 36 further include a pH modification
step
38 through the addition of LiOH 40 to raise the pH to about 9. The product of
step
38 is passed to a belt filter 42 from which Al, Fe, Mn and Mg containing
precipitates are recovered. The impurity removal steps 36 further include a
calcium precipitation step 44 with the addition of either sodium carbonate
(soda
ash) or lithium carbonate 46, producing a calcium containing precipitate 48
from a
further belt filter 50.
[0034] A thickener underflow product 52 of the second thickening step 28b is
passed to a drying step 54 before passing to waste 56 and subsequent disposal
58.
AMENDED SHEET
IPEA/AU
CA 02851786 2014-02-12
PCT/AU2013/000857
- 7 -
Receivec20/01/2014
[0035] The liquid product of-the belt filter 50, being largely LiC1 solution,
is passed
to a concentration step 60 and in turn to a fractional crystallisation step
62. In the
, concentration step 60 the LiCI solution is concentrated to near
saturation point, for
example 35 to 40% LICI w/w, and is cooled to a sub zero temperature. In the
subsequent fractional crystallisation step 62 Na and K impurities 64 are
largely
removed, as NaCI and KCI crystals, respectively, by filtration,
[0036] After the removal of substantially all impurities as described above,
the
lithium chloride solution is passed through an ion exchange step 66,
comprising
an Ion Exchange (IX) column 68 by which substantially all of any residual
calcium,
magnesium and other multivalent cations are removed to a level of less than
about lOppm, for example loom.
[0037] The further purified lithium chloride solution is then heated to 90 C
and
pumped to an electrolysis step 70 comprising a number of electrolysers, for =
example 6 to 20 electrolysers, in which lithium chloride and water are
consumed
to produce lithium hydroxide, chlorine and hydrogen.
[0038] After, passing through the electrolysers, the weak or depleted lithium
chloride solution contains dissolved chlorine gas. Before this weak lithium
chloride solution is recycled to the slurrying step immediately prior to the
leach
circuit 22, the dissolved chlorine is removed in two stages. In a first stage
hydrochloric acid is added to the lithium chloride solution to reduce the pH
to <5
which forces some of the chlorine gas out of solution. The remaining dissolved
chlorine gas is then removed by air stripping the solution (not shown).
[0039] Chlorine and hydrogen produced as by-products are combined to produce
HCI acid which is used in the slurrying step and leaching circuit 22. =
[0040] The lithium hydroxide solution obtained from the electrolysis step 70
is
passed firstly to a holding tank 72, from which it can either be (i)
evaporated and
crystallised to produce lithium hydroxide monohydrate crystals, or (ii) sent
to
carbonation step to convert into lithium carbonate, as clearly shown in Figure
1.
[0041] In the first of these options, the lithium hydroxide in solution is
crystallised
AMENDED SHEET
PEA/AU
CA 02851786 2014-02-12
PCT/AU2013/000857
- 8 - Receive&
0/01/2014
in, for example, a vacuum evaporative crystalliser 80 (Oslo type) operating at
a
temperature of about 80 C and pressure of about 45 kPa(a). The residence time
is about 60 minutes so as to achieve a coarse crystal product. The resulting
water vapour is recompressed, combined with make-up steam and used as the
heating medium for the crystalliser 80.
[00421 Lithium hydroxide crystals are washed by cold water (not shown)
achieving
a wash efficiency of 99%. The resulting wash solution is recycled back to the
leach circuit 22 as noted above. Solids from the centrifuge are fed to an
indirect-
fired kiln or dryer 82,' operating at about 120 C, which dries the crystals.
The
crystal product, being battery grade Li0H.H20, is pneumatically conveyed to
product bins 84, and cooled to 50 C in a jacketed screw conveyer 86 as it is
conveyed ultimately to bagging stations (not shown).
[0043] In the second option noted above, lithium carbonate may be produced by
carbonation of lithium hydroxide solution by passing compressed carbon dioxide
gas 88 though the solution of lithium hydroxide in a carbonation vessel 90 in
which lithium carbonate is precipitated. This slurry is fed to a
washer/centrifuge
92 by way of a filter 94, after which wash water is recycled with any
remaining
lithium hydroxide solution or mother liquor to electrolysis 70. Wet lithium
carbonate crystals are fed to a dryer 96 in which hot air is used to dry the
crystals.
Medium pressure air is used to heat the air. After drying the battery grade
lithium
carbonate may be micronized to a particle size requested by a customer prior
to
passing to storage bins 98 and subsequent bagging (not shown).
[0044]Condensate throughout the process is used as make-up water for hot
process water, cold process water and cooling water. As the process does not
return condensate there is an overall positive water balance and about 1/10th
of
the process water is discharged to a sewerage system (not shown),
[0045] It is envisaged that tantalite and alumina may also be recovered using
the
process of the present invention. The filter cake from the thickening step may
be
discharged to a tantalite recovery plant (not shown). Discharge from the
tantalite
recovery plant may be fed onto a belt filter to remove water, which is
returned to
the tantalite recovery plant. The filter does not use washing and has a
filtration
= AMENDED SHEET
IPEA/AU
CA 02851786 2014-02-12
PCT/AU2013/000857
- 9- Receivec20/01/2014
are of 19 m2. The filter cake from the belt filter is dried in a direct-fired
kiln. The
dry alumina silicate is cooled to 50 C in a jacketed screw conveyor and then
pneumatically conveyed to a storage bin prior to dispatch.
(004611n accordance with a second embodiment of the present invention the
lithium containing material may be provided in the form of a lithium
containing
brine. Brines de not require the calcining, cooling, milling and leach steps
as
described for the first embodiment of the present invention but it is
envisaged that
the remainder of the process will be substantially similar to that of the
first
embodiment described above.
[0047] s can be seen from the above, the process of the present invention
provides a process by which a high purity or battery grade lithium hydroxide
and
lithium carbonate products may be obtained from an alpha-spodumene ore or
concentrate, or from a lithium containing brine, whilst also allowing the
production
of a hydrogen chloride gas product.
[0048] Modifications and variations such as would be apparent to the skilled
addressee are considered to fall within the scope of the present invention.
For
= example, it is envisaged that the leach circuit 22 may comprise only a
single leach
stage/operation without departing from the scope of the present invention.
=
AMENDED SHEET
IPEA/AU