Note: Descriptions are shown in the official language in which they were submitted.
CA 02852177 2014-05-23
DEMANDES OU BREVETS VOLUMINEUX
. LA PRESENTE PARTIE DE CETIE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME ________________________ DE 9.N.
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadian des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OFAa =
NOTE: For additional volumes please contact the Canadian, Patent Office.
CA 02852177 2014-05-23
64005-1372D2
GLUCAGON/GLP-1 RECEPTOR CO-AGONISTS
This is a division of Canadian Patent Application Serial No. 2,728,284 filed
on
June 16, 2009.
It is to be understood that the expression "the present invention" or the like
used in this specification encompasses not only the subject matter of this
divisional
application but that of the parent also.
CROSS REFERENCE TO RELATED APPLICATIONS
= This application claims priority to the following: U.S. Provisional
Patent
Application No. 61/073,269 filed on June 17, 2008, U.S. Provisional Patent
Application
No. 61/078,168 filed July 3, 2008, U.S. Provisional Patent Application No.
61/090,412 filed
on August 20, 2008, and U.S. Provisional Patent Application No. 61/177,476
filed on
May 12, 2009. The disclosure of each application is hereby expressly
incorporated by
reference in its entirety.
1
CA 02852177 2014-05-23
64005-1372D2
=
BACKGROUND
Pre-proglucagon is a 158 amino acid precursor polypeptide that is processed in
different tissues to form a number of different proglucagon-derived peptides,
including glucagon, glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2
(GLP-
, . 2) and oxyntomodulin (0)CM), that are involved in a wide variety
of physiological
functions, including glucose homeostasis, insulin secretion, gastric emptying,
and
15 intestinal growth, as well as the regulation of food intake.
Glucagon is a 29-amino.
acid peptide that corresponds to amino acids 33 through 61 of pre-proglucagon,
while
GLP-1 is produced as a 37-amino acid peptide that corresponds to amino acids
72
through 108 of pre-proglucagon. GLP-1(7-36) amide or GLP-1(7-37) acid are
biologically potent forms of GLP-1, that demonstrate essentially equivalent
activity at
20 the GLP-1 receptor.
Hypoglycemia occurs when blood glucose levels drops too low to provide
enough energy for the body's activities. In adults or children older than 10
years,
= hypoglycemia is uncommon except as a side effect of diabetes treatment,
but it can
result from other medications or diseases, hormone or enzyme deficiencies, or
tumors.
25 When blood glucose begins to fall, glucagon, a hormone produced
by the pancreas,
signals the liver to break down glycogen and release glucose, causing blood
glucose
levels to rise toward a normal level. Thus, glucagon's most recognized role in
glucose regulation is to counteract the action of insulin and maintain blood
glucose
levels. However for diabetics, this glucagon response to hypoglycemia may be
=
30 impaired, making it harder for glucose levels to return to the
normal range.
Hypoglycemia is a life threatening event that requires immediate medical
attention. The administration of glucagon is an established medication for
treating
acute hypoglycemia and it can restore normal levels of glucose within minutes
of
=
=
=
=
la
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
administration. When glucagon is used in the acute medical treatment of
hypoglycemia, a crystalline form of glucagon is solubilized with a dilute acid
buffer
and the solution is injected intramuscularly. While this treatment is
effective, the
methodology is cumbersome and dangerous for someone that is semi-conscious.
Accordingly, there is a need for a glucagon analog that maintains or exceeds
the
biological performance of the parent molecule but is sufficiently soluble and
stable,
under relevant physiological conditions, that it can be pre-formulated as a
solution,
ready for injection.
Additionally, diabetics are encouraged to maintain near normal blood glucose
tO levels to delay or prevent microvascular complications. Achievement of
this goal
usually requires intensive insulin therapy. In striving to achieve this goal,
physicians
have encountered a substantial increase in the frequency and severity of
hypoglycemia in their diabetic patients. Accordingly, improved pharmaceuticals
and
methodologies are needed for treating diabetes that are less likely to induce
is hypoglycemia than current insulin therapies.
GLP-1 has different biological activities compared to glucagon. Its actions
include stimulation of insulin synthesis and secretion, inhibition of glucagon
secretion, and inhibition of food intake. GLP-1 has been shown to reduce
hyperglycemia (elevated glucose levels) in diabetics. Exendin-4, a peptide
from
20 lizard venom that shares about 50% amino acid identity with GLP-1,
activates the
GLP-I receptor and likewise has been shown to reduce hyperglycemia in
diabetics.
There is also evidence that GLP-1 and exendin-4 may reduce food intake and
promote weight loss, an effect that would be beneficial not only for diabetics
but also
for patients suffering from obesity. Patients with obesity have a higher risk
of
25 diabetes, hypertension, hyperlipidemia, cardiovascular disease, and
musculoskeletal
diseases.
Accordingly, there remains a need for alternative and preferably improved
methods for treating diabetes and obesity.
30 SUMMARY
As described herein, high potency glucagon agonists analogs are provided that
also exhibit increased activity at the glucagon receptor, and in further
embodiments
exhibit enhanced biophysical stability and/or aqueous solubility. In addition,
in
accordance with another aspect of the invention, glucagon agonist analogs are
2
CA 02852177 2014-05-23
64005-1372D2
=
=
provided that have lost native glucagon's selectivity for the glucagon
receptor verses
the GLP-I receptor, and thus represent co-agonists of those two receptors.
Selected
amino acid modifications within the glucagon analogs can control the relative
activity
of the analog at the GLP-I receptor verses the glucagon receptor. Thus, yet
another
aspect of the invention provides glucagon co-agonist analogs that have higher
activity
at the glucagon receptor versus the GLP-I receptor, glucagon co-agonist
analogs that
have approximately equivalent activity at both receptors, and glucagon co-
agonist
analogs that have higher activity at the GLP-I receptor versus the glucagon
receptor.
The latter category of co-agonist can be engineered to exhibit little or no
activity at
the glucagon receptor, and yet retain ability to activate the GLP-1 receptor
with the
same or better potency than native GLP-1. Any of these analogs may also
include
modifications that confer enhanced biophysical stability and/or aqueous
solubility.
= = .. =
=
3
CA 02852177 2014-05-23
64005-1372D2
In a particular embodiment, the invention relates to a pegylated glucagon
peptide of the following structure:
Peptid e
N S 0 -0/ \_.CH3
-Thr
0
wherein "Peptide" comprises the following sequence:
X1SQGT FTSDY SKYLD ERRAK DFVC*W LMNTa SEQ ID NO: 588
wherein: "Xl" is alpha, alpha-dimethyl imidazole acetic acid, "C" is a
Cysteine residue on
the glucagon peptide having a thiol which is connected to a polyethylene
glycol of
about 20 kD or about 40 kD average weight; and "a" is a C-terminal amide; and
further
wherein there is a lactam bridge between amino acids 16 and 20 of the Peptide;
or a
pharmaceutically acceptable salt thereof.
In another particular embodiment, the invention relates to a pegylated
glucagon
peptide of the following structure:
Peptide
rN
CH3
LS"Th
0
wherein "Peptide" comprises the following sequence:
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa SEQ ID NO: 319
wherein: "X2" is aminoisobutyric acid, "C*" is a Cysteine residue on the
glucagon peptide
having a thiol which is connected to a polyethylene glycol of about 20 kD or
about 40 kD
average weight; and "a" is a C-terminal amide; or a pharmaceutically
acceptable salt thereof.
3a
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
activity at the respective glucagon and GLP-1 receptors. For example,
modifications
can be made to each peptide to produce a glucagon peptide having anywhere from
at
least about 1% (including at least about 1.5%, 2%, 5%, 7%, 10%, 20%, 30%, 40%,
50%, 60%, 75%, 100%, 125%, 150%, 175%) to about 200% or higher activity at the
GLP-1 receptor relative to native GLP-1 and anywhere from at least about 1%
(including about 1.5%, 2%, 5%, 7%, 10%, 20%, 30%, 40%, 50%, 60%, 75%, 100%,
125%, 150%, 175%, 200%, 250%, 300%, 350%, 400%, 450%) to about 500% or
higher activity at the glucagon receptor relative to native glucagon. In some
embodiments, the glucagon peptides described herein exhibit no more than about
to 100%, 1000%, 10,000%, 100,000%, or 1,000,000% of the activity of native
glucagon
at the glucagon receptor. In some embodiments, the glucagon peptides described
herein exhibit no more than about 100%, 1000%, 10,000%, 100,000%, or
1,000,000%
of the activity of native GLP-1 at the GLP-1 receptor. The amino acid sequence
of
native glucagon is SEQ ID NO: 1, the amino acid sequence of GLP-1(7-36)amide
is
SEQ ID NO: 52, and the amino acid sequence of GLP-1(7-37)acid is SEQ ID NO:
50.
In exemplary embodiments, a glucagon peptide may exhibit at least 10% of the
activity of native glucagon at the glucagon receptor and at least 50% of the
activity of
native GLP-1 at the GLP-1 receptor, or at least 40% of the activity of native
glucagon
at the glucagon receptor and at least 40% of the activity of native GLP-I at
the GLP-
1 receptor, or at least 60% of the activity of native glucagon at the glucagon
receptor
and at least 60% of the activity of native GLP-1 at the GLP-1 receptor.
Selectivity of a glucagon peptide for the glucagon receptor versus the GLP-1
receptor can be described as the relative ratio of glucagon/GLP-1 activity
(the
peptide's activity at the glucagon receptor relative to native glucagon,
divided by the
peptide's activity at the GLP-1 receptor relative to native GLP-1). For
example, a
glucagon peptide that exhibits 60% of the activity of native glucagon at the
glucagon
receptor and 60% of the activity of native GLP-1 at the GLP-1 receptor has a
1:1 ratio
of glucagon/GLP-1 activity. Exemplary ratios of glucagon/GLP-1 activity
include
about 1:1, 1.5:1,2:1, 3:1,4:1, 5:1,6:1, 7:1, 8:1, 9:1 or 10:1, or about
1:10,1:9, 1:8,
1:7, 1:6, 1:5, 1:4, 1:3, 1:2, or 1:1.5. As an example, a glucagon/GLP-1
activity ratio
of 10:1 indicates a 10-fold selectivity for the glucagon receptor versus the
GLP-1
receptor. Similarly, a GLP-1/glucagon activity ratio of 10:1 indicates a 10-
fold
selectivity for the GLP-1 receptor versus the glucagon receptor.
4
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831 =
In accordance with one embodiment, analogs of glucagon are provided that
have enhanced potency and optionally improved solubility and stability. In one
embodiment, enhanced glucagon potency is provided by an amino acid
modification
at position 16 of native glucagon (SEQ ID NO: 1). By way of nonlimiting
example,
such enhanced potency can be provided by substituting the naturally occurring
serine
at position 16 with glutamic acid or with another negatively charged amino
acid
having a side chain with a length of 4 atoms, or alternatively with any one of
glutamine, homoglutamic acid, or homocysteic acid, or a charged amino acid
having a
side chain containing at least one heteroatom, (e.g. N, 0, S, P) and with a
side chain
length of about 4 (or 3-5) atoms. In one embodiment the enhaneed potency
glucagon
agonist comprises a peptide of SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ
ID NO: 5, SEQ ID NO: 6, SEQ ID NO: 7 or a glucagon agonist analog of SEQ ID
NO: 5. In accordance with one embodiment a glucagon analog protein having
enhanced potency at the glucagon receptor relative to wild type glucagon is
provided
wherein the peptide comprises the sequence of SEQ ID NO: 7, SEQ ID NO: 8, SEQ
ID NO: 9 or SEQ ID NO: 10, wherein the glucagon peptide retains its
selectivity for
the glucagon receptor relative to the GLP-I receptors.
Glucagon receptor activity can be reduced, maintained, or enhanced by an
amino acid modification at position 3, e.g. substitution of the naturally
occurring
glutamine at position 3. In one embodiment, substitution of the amino acid at
position
3 with an acidic, basic, or hydrophobic amino acid (glutamic acid, omithine,
norleucine) has been shown to substantially reduce or destroy glucagon
receptor
activity. The analogs that are substituted with, for example, glutamic acid,
omithine,
or norleucine have about 10% or less of the activity of native glucagon at the
glucagon receptor, e.g. about 1-10%, or about 0.1-10%, or greater than about
0.1%
but less than about 10%, while exhibiting at least 20% of the activity of GLP-
1 at the
GLP-1 receptor. For example, exemplary analogs described herein have about
0.5%,
about 1% or about 7% of the activity of native glucagon, while exhibiting at
least 20%
of the activity of GLP-1 at the GLP-1 receptor.
In another embodiment, the naturally occurring glutamine at position 3 of the
glucagon peptide can be substituted with a glutamine analog without a
substantial loss
of activity at the glucagon receptor, and in some cases, with an enhancement
of
glucagon receptor activity. For example, a glucagon peptide comprising a
glutamine
analog at position 3 may exhibit about 5%, about 10%, about 20%, about 50%, or
5
CA 0 2 852 17 7 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
about 85% or greater the activity of native glucagon (e.g. SEQ ID NO: 1) at
the
glucagon receptor. In some embodiments a glucagon peptide comprising a
glutamine
analog at position 3 may exhibit about 20%, about 50%, about 75%, about 100%,
about 200% or about 500% or greater the activity of a corresponding glucagon
peptide having the same amino acid sequence as the peptide comprising the
glutamine
analog, except for the modified amino acid at position 3 (e.g. SEQ ID NO: 601
or
SEQ ID NO: 602) at the glucagon receptor. In some embodiments, a glucagon
peptide comprising a glutamine analog at position 3 exhibits enhanced activity
at the
glucagon receptor, but the enhanced activity is no more than 1000%, 10,000%,
100,000%, or 1,000,000% of the activity of native glucagon or of a
corresponding
glucagon peptide having the same amino acid sequence as the peptide comprising
the
glutamine analog, except for the modified amino acid at position 3.
= In some embodiments, the glutamine analog is a naturally occurring or a
non-
naturally occurring amino acid comprising a side chain of Structure I, II or
III:
0
fR1¨CH2¨X-11¨R2
Structure I
=
+-R1¨CH2--11--Y
Structure II
0
1--R1¨CH2¨S¨CH2¨R4
Structure III
wherein RI is Co_3 alkyl or C0-3 heteroalkyl; R2 is NHR4 or CI.3 alkyl; R3 is
C1
alkyl; R4 is H or C1_3 alkyl; X is NH, 0, or S; and Y is NHR4, SR3, or OR3. In
some
embodiments, X is NH or Y is NHR4. In some embodiments, RI is C0-2 alkyl or CI
heteroallcyl. In some embodiments, R2 is NHR4 or C1 alkyl. In some
embodiments,
R4 is H or C' alkyl. In exemplary embodiments, an amino acid comprising a side
chain of Structure I is provided where, RI is CH2-S, X is NH, and R2 is CH3
(acetamidomethyl-cysteine, C(Acm)); R' is CH2, X is NH, and R2 is CH3
(acetyldiaminobutanoic acid, Dab(Ac)); RI is Co alkyl, X is NH, R2 is NHR4,
and R4
is H (carbamoyldiaminopropanoic acid, Dap(urea)); or RI is CH2-CH2, X is NH,
and
R2 is CH3(acetylornithine, Orn(Ac)). In exemplary embodiments, an amino acid
6
CA 02852177 2014-05-23
WO 2009/155258
PCT/1JS2009/047438
29920-208831
comprising a side chain of Structure II is provide where, RI is CH2, Y is
NHR4, and
R4 is CH3 (methylglutamine, Q(Me)); In exemplary embodiments, an amino acid
comprising a side chain of Structure III is provided where, RI is CH2 and R4
is H
(methionine-sulfoxide, M(0)); In specific embodiments, the amino acid at
position 3
is substituted with Dab(Ac) For example, glucagon agonists can comprise the
amino
acid sequence of SEQ ID NO: 595, SEQ ID NO: 601 SEQ ID NO: 603, SEQ ID NO:
604, SEQ ID NO: 605, and SEQ ID NO: 606.
In another embodiment analogs of glucagon are provided that have enhanced
or retained potency at the glucagon receptor relative to the native glucagon
peptide,
but also have greatly enhanced activity at the GLP-1 receptor. Glucagon
normally has
about 1% of the activity of native-GLP-1 at the GLP-1 receptor, while GLP-I
normally has less than about 0.01% of the activity of native glucagon at the
glucagon
receptor. Enhanced activity at the GLP-1 receptor is provided by replacing the
carboxylic acid of the C-terminal amino acid with a charge-neutral group, such
as an
is amide or ester. In one embodiment, these glucagon analogs comprise a
sequence of
SEQ ID NO: 20 wherein the carboxy terminal amino acid has an amide group in
place
of the carboxylic acid group found on the native amino acid. These glucagon
analogs
have strong activity at both the glucagon and GLP-1 receptors and thus act as
co-
agonists at both receptors. In accordance with one embodiment a glucagon and
GLP-
1 receptor co-agonist is provided wherein the peptide comprises the sequence
of SEQ
ID NO: 20, wherein the amino acid at position 28 is Asn or Lys and the amino
acid at
= position 29 is Thr-amide.
Enhanced activity at the GLP-I receptor is also provided by stabilizing the
alpha-helix structure in the C-terminal portion of glucagon (around amino
acids 12- =
29), through formation of an intramolecular bridge between the side chains of
two.
amino acids that are separated by three intervening amino acids, i.e., an
amino acid at
position "i" and an amino acid at position "i+4", wherein i is any integer
between 12
and 25, by two intervening amino acids, i.e., an amino acid at position "j"
and an
amino acid at position "j+3," whereinj is any integer between 12 and 27, or by
six
intervening amino acids, i.e., an amino acid at position "k" and an amino acid
at
position "k+7," wherein k is any integer between 12 and 22. In exemplary
embodiments, the bridge or linker is about 8 (or about 7-9) atoms in length
and forms
between side chains of amino acids at positions 12 and 16, or at positions 16
and 20,
or at positions 20 and 24, or at positions 24 and 28. The side chains of these
amino
= 7
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
acids can be linked to one another through non-covalent bonds, e.g., hydrogen-
bonding or ionic interactions, such as the formation of salt bridges, or by
covalent
bonds.
In accordance with one embodiment a glucagon agonist is provided
comprising a glucagon peptide of SEQ ID NO: 20, wherein a lactam ring is
formed
between the side chains of a lysine residue, located at position 12, 20 or 28,
and a
glutamic acid residue, located at position 16 or 24, wherein the two amino
aids of the
glucagon peptide whose side chains participate in forming the lactam ring are
spaced
from one another by three intervening amino acids. In accordance with one
embodiment the lactam bearing glucagon analog comprises an amino acid sequence
selected from the group consisting of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO:
13, SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17 and SEQ ID
NO: 18. In one embodiment the carboxy terminal amino acid of the lactam
bearing
peptide comprises an amide group or an ester group in place of the terminal
carboxylic acid. In one embodiment a glucagon peptide of SEQ ID NO: 11, SEQ ID
NO: 12, SEQ ID NO: 13, and SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16,
SEQ ID NO: 17 and SEQ ID NO: 18 further comprises an additional amino acid
covalently bound to the carboxy terminus of SEQ ID NO: 11, SEQ ID NO: 12, SEQ
ID NO: 13, SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17 or
SEQ ID NO: 18. In a further embodiment a glucagon peptide is provided
comprising
a sequence selected from the group consisting of SEQ ID NO: 66, SEQ ID NO: 67,
SEQ ID NO: 68 and SEQ ID NO: 69 further comprises an additional amino acid
covalently bound to the carboxy terminus of SEQ ID NO: 66, SEQ ID NO: 67, SEQ
ID NO: 68 and SEQ ID NO: 69. In one embodiment the amino acid at position 28
is
asparagine or lysine and the amino acid at position 29 is threonine.
In some specific embodiments, stabilization of the alpha helix structure in
the
C-terminal portion of the glucagon agonist peptide is achieved through the
formation
of a covalent intramolecular bridge other than a lactam bridge. For example,
suitable
= covalent bonding methods (i.e., means of forming a covalent
intramolecular bridge)
include any one or more of olefin metathesis, lanthionine-based cyclization,
disulfide
= bridge or modified sulfur-containing bridge formation, the use of a, co-
diaminoalkane
tethers, the formation of metal-atom bridges, and other means of peptide
cyclization
are used to stabilize the alpha helix.
8
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Enhanced activity at the GLP-1 receptor is also provided by stabilizing the
alpha-helix structure in the C-terminal portion of the glucagon peptide
(around amino
acids 12-29) through introduction of one or more a, a-disubstituted amino
acids at
positions that retain the desired activity. In some aspects, stabilization of
the alpha-
helix is accomplished in this manner without purposeful introduction of an
intramolecular bridge such as a salt bridge or covalent bond. Such peptides
may be
considered herein as a peptide lacking an intramolecular bridge. In specific
aspects,
stabilization of the alpha-helix is accomplished by introducing one or more a,
a-
disubstituted amino acids without introduction of a covalent intramolecular
bridge,
e.g., a lactam bridge, a disulfide bridge. Such peptides may be considered
herein as a
peptide lacking a covalent intramolecular bridge. In some embodiments, one,
two,
three, four or more of positions 16, 17, 18, 19, 20, 21, 24 or 29 of a
glucagon peptide
is substituted with an a, a-disubstituted amino acid. For example,
substitution of
position 16 of the glucagon peptide with amino iso-butyric acid (AIB) enhances
GLP-
1 activity, in the absence of a salt bridge or lactam. In some embodiments,
one, two,
three or more of positions 16, 20, 21 or 24 are substituted with AIB.
Enhanced activity at the GLP-1 and glucagon receptors for glucagon analog
peptides lacking an intramolecular bridge (e.g., a covalent intramolecular
bridge) is
provided by the addition of an acyl or alkyl group to the side chain of the
amino acid
at position 10 of the peptide. In some aspects, the acyl or alkyl group is not
naturally-
occurring on an amino acid. In specific aspects, the acyl or alkyl group is
non-native
to any naturally-occuring amino acid. In some embodiments, the acyl group is a
fatty
acyl group, e.g., a C4 to C30 fatty acyl group. For example, provided herein
is a
glucagon analog peptide lacking a covalent intramolecular bridge comprising
AIB at
position 16 and a C14, C16, or C18 fatty acyl group covalently attached to a
Lys
residue at position 10. Also provided is a glucagon analog peptide lacking an
intramolecular bridge (e.g., a covalent intramolecular bridge) comprising AIB
at
positions 2 and 16 and a C14, C16, or CI8 fatty acyl group covalently attached
to a
Lys residue at position 10. Such acylated glucagon analog peptides lacking an
intramolecular bridge (e.g., a covalent intramolecular bridge) may be
pegylated as
further described herein.
A further enhancement in GLP-1 activity and glucagon activity for acylated
glucagon analog peptides lacking an intramolecular bridge (e.g., an
intramolecular
bridge) may be achieved by incorporating a spacer between the acyl or alkyl
group
9
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
=
and the side chain of the amino acid at position 10. In accordance with some
embodiments, the spacer (e.g., an amino acid, a dipeptide, a tripeptide, a
hydrophilic
bifunctional spacer, or a hydrophobic bifunctional spacer) is 3 to 10 atoms
(e.g., 6 to
atoms) in length. In accordance with certain specific embodiments, the total
length
5 of the spacer and acyl or alkyl group is 14 to 28 atoms, e.g., 17 to 28,
19 to 26 atoms,
19 to 21 atoms. Suitable spacers for purposes of enhancing GLP-1 activity and
glucagon activity for acylated or alkylated peptides lacking an intramolecular
bridge
(e.g., a covalent intramolecular bridge) are further described herein.
For example, provided herein is a non-native glucagon peptide that differs
Enhanced activity at the GLP-1 receptor is also provided by an amino acid
modification at position 20. In one embodiment, the glutamine at position 20
is
replaced with another hydrophilic amino acid having a side chain that is
either
Any of the modifications described above which increase or decrease
glucagon receptor activity and which increase GLP-1 receptor activity can be
applied
individually or in combination. Combinations of the modifications that
increase GLP-
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
I receptor activity may provide higher GLP-1 activity than any of such
modifications
taken alone. For example, the invention provides glucagon analogs that
comprise
modifications at position 16, at position 20, and at the C-terminal carboxylic
acid
group, optionally with a covalent bond between the amino acids at positions 16
and
20; glucagon analogs that comprise modifications at position 16 and at the C-
terminal
carboxylic acid group; glucagon analogs that comprise modifications at
positions 16
. and 20, optionally with a covalent bond between the amino acids at positions
16 and
20; and glucagon analogs that comprise modifications at position 20 and at the
C-
terminal carboxylic acid group; optionally with the proviso that the amino
acid at
position 12 is not Arg; or optionally with the proviso that the amino acid at
position 9
is not Glu.
Other modifications at position 1 or 2, as described herein, can increase the
peptide's resistance to dipeptidyl peptidase IV (DPP IV) cleavage. For
example, the
amino acid at position 2 may be substituted with D-serine, D-alanine, valine,
glycine,
N-methyl serine, N-methyl alanine, or amino isobutyric acid. Alternatively, or
in
addition, the amino acid at position I may be substituted with D-histidine,
desaminohistidine, hydroxyl-histidine, acetyl-histidine, homo-histidine, N-
methyl
histidine, alpha-methyl histidine, imidazole acetic acid, or alpha, alpha-
dimethyl
imidiazole acetic acid (DMIA).
It was observed that modifications at position 2 (e.g. AIB at position 2) and
in
some cases modifications at position .I may reduce glucagon activity,
sometimes
significantly; surprisingly, this reduction in glucagon activity can be
restored by
stabilizing the alpha-helix in the C-terminal portion of glucagon, e.g.
through a
covalent bond between amino acids at positions "i" and "i+4", e.g., 12 and 16,
16 and
20, or 20 and 24. In some embodiments, this covalent bond is a lactam bridge
between a glutamic acid at position 16 and a lysine at position 20. In some
embodiments, this covalent bond is an intramolecular bridge other than a
lactam
bridge. For example, suitable covalent bonding methods include any one or more
of
olefin metathesis, lanthionine-based cyclization, disulfide bridge or modified
sulfur-
containing bridge formation, the use of a, co-diaminoalkane tethers, the
formation of
metal-atom bridges, and other means of peptide cyclization.
Glucagon peptides with GLP-1 activity that contain a non-conservative
substitution of His at position 1 with a large, aromatic amino acid (e.g.,
Tyr) can
retain GLP-1 activity provided that the alpha-helix is stabilized via an
intramolecular
11
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831 =
bridge, e.g. through a covalent bond between amino acids at positions "i" and
"1+4",
e.g., 12 and 16, 16 and 20, or 20 and 24. In some embodiments, this covalent
bond is
a lactam bridge between a glutamic acid at position 16 and a lysine at
position 20. In
= some embodiments, this covalent bond is an intramolecular bridge other
than a lactam
bridge. For example, suitable covalent bonding methods include any one or more
of
olefin metathesis, lanthionine-based cyclization, disulfide bridge or modified
sulfur-
containing bridge formation, the use of a, co-diaminoalkane tethers, the
formation of
metal-atom bridges, and other means of peptide cyclization.
In yet further exemplary embodiments, any of the foregoing compounds can
be further modified to improve stability by modifying the amino acid at
position 15 of
SEQ ID NO: 1 to reduce degradation of the peptide over time, especially in
acidic or
alkaline buffers.
In another embodiment the solubility of the glucagon peptides disclosed herein
are enhanced by the covalent linkage of a hydrophilic moiety to the peptide.
In one
embodiment the hydrophilic moiety is a polyethylene glycol (PEG) chain,
optionally
linked to the peptide at one or more of positions 16, 17, 21, 24, 29, within a
C-
.
terminal extension, or at the C-terminal amino acid. In some embodiments, the
native
amino acid at that position is substituted with an amino acid having a side
chain
suitable for crosslinking with hydrophilic moieties, to facilitate linkage of
the
hydrophilic moiety to the peptide. In other embodiments, an amino acid
modified to
comprise a hydrophilic group is added to the peptide at the C-terminal amino
acid. In
one embodiment the peptide co-agonist comprises a sequence selected from the
group
consisting of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14,
SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18 and SEQ ID NO:
19 wherein the side chain of an amino acid residue at one of position 16,
17,21 or 24
of said glucagon peptide further comprises a polyethylene glycol chain, having
a
molecular weight selected from the range of about 500 to about 40,000 Daltons.
In
one embodiment the polyethylene glycol chain has a molecular weight selected
from
. the range of about 500 to about 5,000 Daltons. In another embodiment the
polyethylene glycol chain has a molecular weight of about 10,000 to about
20,000
Daltons. In yet other exemplary embodiments the polyethylene glycol chain has
a
molecular weight of about 20,000 to about 40,000 Daltons.
In another embodiment the solubility of any of the preceding glucagon analogs
can be improved by amino acid substitutions and/or additions that introduce a
charged
12
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
amino acid into the C-terminal portion of the peptide, preferably at a
position C-
terminal to position 27 of SEQ ID NO: 1. Optionally, one, two or three charged
amino acids may be introduced within the C-terminal portion, preferably C-
terminal
to position 27. In accordance with one embodiment the native amino acid(s) at
positions 28 and/or 29 are substituted with a charged amino acids, and/or in a
further
embodiment one to three charged amino acids are also added to the C-terminus
of the
peptide. In exemplary embodiments, one, two or all of the charged amino acids
are
negatively charged. Additional modifications, e.g. conservative substitutions,
may be
made to the glucagon peptide that still-allow it to retain glucagon activity.
In one
embodiment an analog of the peptide of SEQ ID NO: 20 is provided wherein the
analog differs from SEQ ID NO: 20 by 1 to 2 amino acid substitutions at
positions 17-
26, and in one embodiment the analog differs from the peptide of SEQ ID NO: 20
by
an amino acid substitution at position 20.
In accordance with some embodiments, the glucagon peptides disclosed herein
are modified by truncation of the C-terminus by one or two amino acid
residues.
Such modified glucagon peptides, as shown herein, retain similar activity and
potency
at the glucagon receptor and GLP-1 receptor. In this regard, the glucagon
peptides
can comprise amino acids 1-27 or 1-28 of the native glucagon peptide (SEQ ID
NO:
1), optionally with any of the additional modifications described herein.
In accordance with one embodiment the glucagon peptides disclosed herein
are modified by the addition of a second peptide to the carboxy terminus of
the
glucagon peptide, for example, SEQ ID NO: 26, SEQ ID NO: 27 or SEQ ID NO: 28.
In one embodiment a glucagon peptide having a peptide sequence selected from
the
group consisting of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO:
14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18, SEQ ID NO:
19, SEQ ID NO: 66, SEQ ID NO: 67, SEQ ID NO: 68, and SEQ ID NO: 69 is
covalently bound through a peptide bond to a second peptide, wherein the
second
peptide comprises a sequence selected from the group consisting of SEQ ID NO:
26,
SEQ ID NO: 27 and SEQ ID NO: 28. In a further embodiment, in glucagon peptides
which comprise the C-terminal extension, the threonine at position 29 of the
native
glucagon peptide is replaced with a glycine. A glucagon analog having a
glycine
substitution for threonine at position 29 and comprising the carboxy terminal
extension of SEQ ID NO: 26 is four times as potent at the GLP- I receptor as
native
glucagon modified to comprise the carboxy terminal extension of SEQ ID NO: 26.
13 =
=
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
Potency at the GLP-1 receptor can be further enhanced by an alanine
substitution for
the native arginine at position 18.
Any of the glucagon peptides disclosed herein can be modified to comprise an
acyl group or alkyl group, e.g., a C4 to C30 acyl or alkyl group. In some
aspects, the
acyl group or alkyl group is non-native to any naturally-occurring amino acid.
Acylation or alkylation can increase the half-life of the glucagon peptides in
circulation. Acylation or alkylation can advantageously delay the onset of
action
and/or extend the duration of action at the glucagon and/or GLP-1 receptors
and/or
improve resistance to proteases such as DPP-IV. As shown herein, the activity
at the
glucagon receptor and GLP-1 receptor of the glucagon peptide is maintained, if
not
substantially enhanced, after acylation. Further, the potency of the acylated
glucagon
peptides were comparable to the unacylated versions of the glucagon peptides,
if not
substantially enhanced. Glucagon peptides may be acylated or allcylated at the
same
amino acid position where a hydrophilic moiety is linked, or at a different
amino acid
position. In some embodiments, the invention provides a glucagon peptide
modified
to comprise an acyl group or alkyl group covalently linked to the amino acid
at
position 10 of the glucagon peptide. The glucagon peptide may further comprise
a
spacer between the amino acid at position 10 of the glucagon peptide and the
acyl
group or alkyl group. In some embodiments, the acyl group is a fatty acid or
bile acid, =
or salt thereof, e.g. a C4 to C30 fatty acid, a C8 to C24 fatty acid, cholic
acid, a C4 to
C30 alkyl, a C8 to C24 alkyl, or an alkyl comprising a steroid moiety of a
bile acid.
The spacer is any moiety with suitable reactive groups for attaching acyl or
alkyl
groups. In exemplary embodiments, the spacer comprises an amino acid, a
dipeptide,
a tripeptide, a hydrophilic bifunctional spacer, or a hydrophobic bifunctional
spacer.
In some embodiments, the spacer is selected from the group consisting of: Trp,
Glu,
Asp, Cys and a spacer comprising NH2(CH2CH20)n(CH2)n,COOH, wherein m is any
integer from 1 to 6 and n is any integer from 2 to 12. Such acylated or
alkylated
glucagon peptides may also further comprise a hydrophilic moiety, optionally a
polyethylene glycol. Any of the foregoing glucagon peptides may comprise two
acyl
groups or two alkyl groups, or a combination thereof.
Thus, as disclosed herein high potency glucagon analogs or glucagon co-
.
agonist analogs are provided that also exhibit improved solubility and/or
stability. An
exemplary high potency glucagon analog exhibits at least about 200% of the
activity
of native glucagon at the glucagon receptor, and optionally is soluble at a
14
CA 02852177 2014-05-23
=
WO 2009/155258
PCT/US2009/047438
29920-208831
concentration of at least 1 mg/mL at a pH between 6 and 8, or between 6 and 9,
or
between 7 and 9 (e.g. pH 7), and optionally retains at least 95% of the
original peptide
(e.g. 5% or less of the original peptide is degraded or cleaved) after 24
hours at 25 C.
As another example, an exemplary glucagon co-agonist analog exhibits greater
than
about 40% or greater than about 60% activity at both the glucagon and the GLP-
1
receptors (at a ratio between about 1:3 and 3:1, or between about 1:2 and
2:1), is
optionally soluble at a concentration of at least 1 mg/mL at a pH between 6
and 8 or
between 6'and 9, or between 7 and 9 (e.g. pH 7), and optionally retains at
least 95% of
the original peptide after 24 hours at 25 C. Another exemplary glucagon co-
agonist
io analog exhibits about 175% or more of the activity of native
glucagon at the glucagon
receptor and about 20% or less of the activity of native GLP-1 at the GLP-1
receptor,
is optionally soluble at a concentration of at least 1 mg/mL at a pH between 6
and 8 or
between 6 and 9, or between 7 and 9 (e.g. pH 7), and optionally retains at
least 95% of
the original peptide after 24 hours at 25 C. Yet another exemplary glucagon co-
is agonist analog exhibits about 10% or less of the activity of
native glucagon at the
glucagon receptor and at least about 20% of the activity of native GLP-1 at
the GLP-1
receptor, is optionally soluble at a concentration of at least 1 mg/mL at a pH
between
6 and 8 or between 6 and 9, or between 7 and 9 (e.g. pH 7), and optionally
retains at
least 95% of the original peptide after 24 hours at 25 C. Yet another
exemplary
20 glucagon co-agonist analog exhibits about 10% or less but
above 0.1% , 0.5% or 1%
of the activity of native glucagon at the glucagon receptor and at least about
50%,
60%, 70%, 80%, 90% or 100% or more of the activity of native GLP-I at the GLP-
1
receptor, is optionally soluble at a concentration of at least 1 mg/mL at a pH
between
6 and 8 or between 6 and 9, or between 7 and 9 (e.g. pH 7), and optionally
retains at
25 least 95% of the original peptide after 24 hours at 25 C. In
some embodiments, the
glucagon peptides exhibit no more than about 100%, 1000%, 10,000%, 100,000%,
or
1,000,000% of the activity of native GLP-1 at the GLP-1 receptor. In some
embodiments, such glucagon analogs retain at least 22, 23, 24, 25, 26, 27 or
28 of the
naturally occurring amino acids at the corresponding positions in native
glucagon
30 (e.g. have 1-7, 1-5 or 1-3 modifications relative to
naturally occurring glucagon).
Any one of the following peptides is excluded from the compounds of the
invention, although any of the following peptides comprising one or more
further
modifications thereto as described herein exhibiting the desired GLP-1 or co-
agonist
activity, pharmaceutical compositions, kits, and treatment methods using such
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
compounds may be included in the invention: The peptide of SEQ ID NO: 1 with
an
[Arg12] substitution and with a C-terminal amide; The peptide of SEQ ID NO: 1
with
[Arg12,Lys20] substitutions and with a C-terminal amide; The peptide of SEQ ID
NO: 1 with [Arg12,Lys24] substitutions and with a C-terminal amide; The
peptide of
SEQ ID NO: 1 with [Arg12,Lys29] substitutions and with a C-terminal amide; The
peptide of SEQ ID NO: 1 with a [G1u9] substitution; The peptide of SEQ ID NO:
1
missing His I, with [G1u9, Glu16, Lys29] substitutions and C-terminal amide;
The
peptide of SEQ ID NO: 1 with [G1u9, Glut 6, Lys29] substitutions and with a C-
terminal amide; The peptide of SEQ ID NO: 1 with [Lys13, Glu 1 7]
substitutions
0 linked via lactam bridge and with a C-terminal amide; The peptide of SEQ
ID NO: 1
with [Lys17, G1u21] substitutions linked via lactam bridge and with a C-
terminal
amide; The peptide of SEQ ID NO: 1 missing His 1, with [G1u20, Lys24]
substitutions
linked via lactam bridge.
In accordance with one embodiment a pharmaceutical composition is provided
16
CA 0 2 852 17 7 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
= 29920-208831
part of a kit that includes a disposable device for administering the
composition to a
patient. The containers or kits may be labeled for storage at ambient room
temperature
or at refrigerated temperature.
In accordance with one embodiment a method of rapidly increasing glucose
level or treating hypoglycemia using a pre-formulated aqueous composition of
glucagon peptides of the invention is provided. The method comprises the step
of
administering an effective amount of an aqueous solution comprising a novel
modified glucagon peptide of the present disclosure. In one embodiment the
glucagon peptide is pegylated at position 21 or 24 of the glucagon peptide and
the =
PEG chain has a molecular weight of about 500 to about 5,000 Daltons. In one
embodiment the modified glucagon solution is prepackaged in a device that is
used to
administer the composition to the patient suffering from hypoglycemia.
In accordance with one embodiment an improved method of regulating blood
glucose levels in insulin dependent patients is provided. The method comprises
the
steps of administering insulin in an amount therapeutically effective for the
control of
diabetes and administering a novel modified glucagon peptide of the present
disclosure in an amount therapeutically effective for the prevention of
hypoglycemia,
wherein said administering steps are conducted within twelve hours of each
other. In
one embodiment the glucagon peptide and the insulin are co-administered as a
single
composition, wherein the glucagon peptide is pegylated with a PEG chain having
a
molecular weight selected from the range of about 5,000 to about 40,000
Daltons
In another embodiment a method is provided for inducing the temporary
paralysis of the intestinal tract. The method comprises the step of
administering one
or more of the glucagon peptides disclosed herein to a patient.
Metabolic Syndrome, also known as metabolic syndrome X, insulin resistance
syndrome or Reaven's syndrome, is a disorder that affects over 50 million
Americans.
Metabolic Syndrome is typically characterized by a clustering of at least
three or more
of the following risk factors: (1) abdominal obesity (excessive fat tissue in
and around
the abdomen), (2) atherogenic dyslipidemia (blood fat disorders including high
triglycerides, low HDL cholesterol and high LDL cholesterol that enhance the
accumulation of plaque in the artery walls), (3) elevated blood pressure, (4)
insulin
resistance or glucose intolerance, (5) prothrombotic state (e.g. high
fibrinogen or
plasminogen activator inhibitor-1 in blood), and (6) pro-inflammatory state
(e.g.
17
=
CA 02852177 2014-05-23
=
WO 2009/155258 PCT/US2009/047438
29920-208831
elevated C-reactive protein in blood). Other risk factors may include aging,
hormonal
imbalance and genetic predisposition.
Metabolic Syndrome is associated with an increased the risk of coronary heart
disease and other disorders related to the accumulation of vascular plaque,
such as
5 stroke and peripheral vascular disease, referred to as atherosclerotic
cardiovascular
disease (ASCVD). Patients with Metabolic Syndrome may progress from an insulin
resistant state in its early stages to full blown type II diabetes with
further increasing
risk of ASCVD. Without intending to be bound by any particular theory, the
relationship between insulin resistance, Metabolic Syndrome and vascular
disease =
10 may involve one or more concurrent pathogenic mechanisms including
impaired
insulin-stimulated vasodilation, insulin resistance-associated reduction in NO
availability due to enhanced oxidative stress, and abnormalities in adipocyte-
derived
hormones such as adiponectin (Lteif and Mather, Can. J. Cardiol. 20 (suppl.
B):66B-
76B (2004)).
15 According to the 2001 National Cholesterol Education Program Adult
Treatment Panel (ATP III), any three of the following traits in the same
individual
meet the criteria for Metabolic Syndrome: (a) abdominal obesity (a waist
circumference over 102 cm in men and over 88 cm in women); (b) serum
triglycerides
(150 mg,/d1 or above); (c) HDL cholesterol (40 mg,/d1 or lower in men and 50
mg/di or
20 lower in women); (d) blood pressure (130/85 or more); and (e) fasting
blood glucose
(110 mg/di or above). According to the World Health Organization (WHO), an
individual having high insulin levels (an elevated fasting blood glucose or an
elevated
post meal glucose alone) with at least two of the following criteria meets the
criteria
for Metabolic Syndrome: (a) abdominal obesity (waist to hip ratio of greater
than 0.9,
25 a body mass index of at least 30 kg/m2, or a waist measurement over 37
inches); (b)
cholesterol panel showing a triglyceride level of at least 150 mg/di or an HDL
cholesterol lower than 35 mg/di; (c) blood pressure of 140/90 or more, or on
treatment
for high blood pressure). (Mathur, Ruchi, "Metabolic Syndrome," ed. Shiel,
Jr.,
William C., MedicineNet.com, May 11, 2009).
30 For purposes herein, if an individual meets the criteria of either or
both of the
criteria set forth by the 2001 National Cholesterol Education Program Adult
Treatment Panel or the WHO, that individual is considered as afflicted with
Metabolic
Syndrome.
18
CA 02852177 2014-05-23
WO 2009/155258
PCT/1JS2009/047438
29920-208831
Without being bound to any particular theory, glucagon peptides described
herein are useful for treating Metabolic Syndrome. Accordingly, the invention
provides a method of preventing or treating Metabolic Syndrome, or reducing
one,
two, three or more risk factors thereof, in a subject, comprising
administering to the
subject a glucagon peptide described herein in an amount effective to prevent
or treat
Metabolic Syndrome, or the risk factor thereof
Nonalcoholic fatty liver disease (NAFLD) refers to a wide spectrum of liver
disease ranging from simple fatty liver (steatosis), to nonalcoholic
steatohepatitis
(NASH), to cirrhosis (irreversible, advanced scarring of the liver). All of
the stages of
NAFLD have in common the accumulation of fat (fatty infiltration) in the liver
cells
(hepatocytes). Simple fatty liver is the abnormal accumulation of a certain
type of fat,
triglyceride, in the liver cells with no inflammation or scarring. In NASH,
the fat
accumulation is associated with varying degrees of inflammation (hepatitis)
and
scarring (fibrosis) of the liver. The inflammatory cells can destroy the liver
cells
(hepatocellular necrosis). In the terms "steatohepatitis" and
"steatonecrosis", steak)
refers to fatty infiltration, hepatitis refers to inflammation in the liver,
and necrosis
refers to destroyed liver cells. NASH can ultimately lead to scarring of the
liver
(fibrosis) and then irreversible, advanced scarring (cirrhosis). Cirrhosis
that is caused
by NASH is the last and most severe stage in the NAFLD spectrum. (Mendler,
Michel, "Fatty Liver: Nonalcoholic Fatty Liver Disease (NAFLD) and
Nonalcoholic
Steatohepatitis (NASH)," ed. Schoenfield, Leslie J., MedicineNet.com, August
29,
2005).
Alcoholic Liver Disease, or Alcohol-Induced Liver Disease, encompasses
three pathologically distinct liver diseases related to or caused by the
excessive
consumption of alcohol: fatty liver (steatosis), chronic or acute hepatitis,
and
cirrhosis. Alcoholic hepatitis can range from a mild hepatitis, with abnormal
laboratory tests being the only indication of disease, to severe liver
dysfunction with
complications such as jaundice (yellow skin caused by bilirubin retention),
hepatic
encephalopathy (neurological dysfunction caused by liver failure), ascites
(fluid
accumulation in the abdomen), bleeding esophageal varices (varicose veins in
the
esophagus), abnormal blood clotting and coma. Histologically, alcoholic
hepatitis has
a characteristic appearance with ballooning degeneration of hepatocytes,
inflammation with neutrophils and sometimes Mallory bodies (abnormal
aggregations
of cellular intermediate filament proteins). Cirrhosis is characterized
anatomically by
19
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
widespread nodules in the liver combined with fibrosis. (Worman, Howard J.,
"Alcoholic Liver Disease", Columbia University Medical Center website).
Without being bound to any particular theory, glucagon peptides described
herein are useful for the treatment of Alcoholic Liver Disease, NAFLD, or any
stage
thereof, including, for example, steatosis, steatohepatitis, hepatitis,
hepatic
inflammation, NASH, cirrhosis, or complications thereof. Accordingly, the
invention provides a method of preventing or treating Alcoholic Liver Disease,
NAFLD, or any stage thereof, in a subject comprising administering to a
subject a
glucagon peptide described herein in an amount effective to prevent or treat
Alcoholic
Liver Disease, NAFLD, or the stage thereof. Such treatment methods include
reduction in one, two, three or more of the following: liver fat content,
incidence or
progression of cirrhosis, incidence of hepatocellular carcinoma, signs of
inflammation, e.g. abnormal hepatic enzyme levels (e.g., aspartate
aminotransferase
AST and/or alanine aminotransferase ALT, or LDH), elevated serum ferritin,
elevated serum bilirubin, and/or signs of fibrosis, e.g. elevated TGF-beta
levels. In
preferred embodiments, the glucagon peptides are used treat patients who have
progressed beyond simple fatty liver (steatosis) and exhibit signs of
inflammation or
hepatitis. Such methods may result, for example, in reduction of AST and/or
ALT
levels.
In yet another embodiment a method of treating hyperglycemia, or a method
of reducing weight gain or inducing weight loss is provided, which involves
administering an effective amount of an aqueous solution comprising a glucagon
peptide of the invention. In one embodiment either method comprises
administering
an effective amount of a composition comprising a glucagon agonist selected
from the
group consisting of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO:
14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18 and SEQ ID
NO: 19. In another embodiment, the method comprises administering an effective
amount of a composition comprising a glucagon agonist, wherein the glucagon
agonist comprising a glucagon peptide selected from the group consisting of
SEQ ID
NO: I 1, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID
NO: 16, SEQ ID NO: 17, SEQ ID NO: 18, SEQ ID NO: 19, SEQ ID NO: 66, SEQ ID
NO: 67, SEQ ID NO: 68, and SEQ ID NO: 69, wherein amino acid 29 of the
glucagon
peptide is bound to a second peptide through a peptide bond, and said second
peptide
comprises the sequence of SEQ ID NO: 26, SEQ ID NO: 27 or SEQ ID NO: 28. In
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
further embodiments, methods of treating diabetes involving co-administering a
conventional dose or a reduced dose of insulin and a glucagon peptide of the
invention are provided. Methods of treating diabetes with a glucagon peptide
of the
invention, without co-administering insulin are also provided.
In yet another aspect, the invention provides novel methods for treating
hyperglycemia and novel methods for decreasing appetite or promoting body
weight =
loss that involve administration of a glucagon/GLP-1 co-agonist molecule
(including
pharmaceutically acceptable salts thereof) that activates both the glucagon
receptor
and the GLP-1 receptor. Agonism, i.e., activation, of both the glucagon and
GLP-1
receptors provides an unexpected improvement compared to GLP-1 agonism alone
in
treating hyperglycemia. Thus, the addition of glucagon agonism provides an
unexpected additive or synergistic effect, or other unexpected clinical
benefit(s).
Administration with a conventional dose of insulin, a reduced dose of insulin,
or
without insulin is contemplated according to such methods. Agonism of the
glucagon
receptor also has an unexpected beneficial effect compared to GLP-1 agonism
alone
in promoting weight loss or preventing weight gain.
Exemplary glucagon/GLP-1 co-agonist molecules include glucagon peptides
of the invention, GLP-1 analogs that activate both GLP-1 and glucagon
receptors,
fusions of glucagon and GLP-1, or fusions of glucagon analogs and GLP-1
analogs,
= 20 or chemically modified derivatives thereof. Alternatively, a
compound that activates
the glucagon receptor can be co-administered with a compound that activates
the
GLP-1 receptor (such as a GLP-1 analog, an exendin-4 analog, or derivatives
thereof).
The invention also contemplates co-administration of a glucagon agonist analog
with =
a GLP-1 agonist analog.
Such methods for treating hyperglycemia and/or for decreasing appetite or
promoting body weight loss include administration of a glucagon analog with a
modification at position 12 (e.g. Arg12), optionally in combination with
modifications
at position 16 and/or 20. The methods of the invention also include
administration of
glucagon analogs comprising an intramolecular bridge between the side chains
of two
amino acids within the region of amino acids 12 and 29 that are separated by
three
intervening amino acids, e.g. positions 12 and 16, positions 13 and 17 (e.g,.
Lys13
Glul7 or Glul3 Lys17), positions 16 and 20, positions 17 and 21 (e.g. Lys17
Glu 21
or Glul7 Lys 21), positions 20 and 24, or positions 24 and 28, with the
optional
21
CA 02852177 2014-05-23
=
WO 2009/155258
PCT/US2009/047438
29920-208831
proviso that the amino acid at position 9 is not Glu, and optionally including
a C-
terminal amide or ester.
In accordance with one embodiment excluded from such glucagon/GLP-1 co-
agonist molecules are any glucagon analogs or GLP-1 analogs in the prior art
known
to be useful in such a method. In another embodiment peptides described in
U.S.
Patent No. 6,864,069 as acting as both a GLP-1 agonist and a glucagon
antagonist for
treating diabetes are also excluded as glucagon/GLP-1 co-agonist molecules. In
another embodiment, excluded is the use of glucagon antagonists to treat
diabetes,
such as the antagonists described in Unson et al., J. Biol. Chem., 264:789-794
(1989),
Ahn et al., J. Med. Chem., 44:3109-3116 (2001), and Sapse et al., Mol. Med.,
8(5):251-262 (2002). In a further embodiment oxyntomodulin or a glucagon
analog =
that contains the 8 C-terminal amino acids of oxyntomodulin (SEQ ID NO: 27)
are
also excluded as glucagon/GLP-1 co-agonist molecules.
Such methods for treating hyperglycemia are expected to be useful for a
variety of types of hyperglycemia, including diabetes, diabetes mellitus type
I,
diabetes mellitus type II, or gestational diabetes, either insulin-dependent
or non-
insulin-dependent, and reducing complications of diabetes including
nephropathy,
retinopathy and vascular disease. Such methods for reducing appetite or
promoting
loss of body weight are expected to be useful in reducing body weight,
preventing
weight gain, or treating obesity of various causes, including drug-induced
obesity, and
reducing complications associated with obesity including vascular disease
(coronary
artery disease, stroke, peripheral vascular disease, ischemia reperfusion,
etc.),
hypertension, onset of diabetes type II, hyperlipidemja and musculoskeletal
diseases.
All therapeutic methods, pharmaceutical compositions, kits and other similar
embodiments described herein contemplate that the use of the term glucagon
analogs
includes all pharmaceutically acceptable salts or esters thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
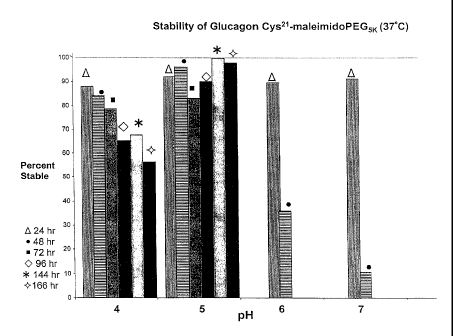
Fig. 1 is a bar graph representing the stability of Glucagon
Cys21maleimidoPEG5K at 37 C incubated for 24, 48, 72, 96, 144 and 166 hours,
respectively.
= Fig. 2 represents data generated from HPLC analysis of Glucagon
Cys2ImaleimidoPEG5K at pH 5 incubated at 37 C for 24, 72 or 144 hours,
respectively.
22
CA 02852177 2014-05-23
WO 2009/155258
=PCT/1JS2009/047438
29920-208831
Fig. 3 represents data showing receptor mediated cAMP induction by
glucagon analogs. More particularly, Fig. 3A compares induction of the
glucagon
receptor by glucagon analogs E16, K20 41, E15, E16 A, E16, K20 7, EIS, E16 4,
E16 10. and Gluc-NH2 =
Fig. 4A and 4B represents data showing receptor mediated cAMP induction by
glucagon analogs. More particularly, Fig. 4A compares induction of the
glucagon
receptor by glucagon analogs Gluc-NH2 11, El 6Gluc-NH2 A, E3, E16 Gluc-NH2 V,
0m3, El 6 Gluc-NH2 4 and N1e3, E16 Gluc-NH2, IPI= relative to native glucagon
whereas Fig. 48 compares induction of the GLP-1 receptor by glucagon analogs
Gluc-NH2 E16 Glue-NH2 A, E3, El6Gluc-NH2 7, 0m3, El6 Gluc-NH2 4 and
Nle3, E16 Gluc-NH2,0. relative to native GLP-1 M.
Fig. 5A and 5B represents data showing receptor mediated cAMP induction by
glucagon analogs. More particularly, Fig. 5A compares induction of the
glucagon
receptor by glucagon analogs (E16, K20 Gluc-NH2 11(522M, stock solution), EIS,
E16
Glue-NH2 .11.(5nM, stock solution), E16, K20 Gluc-NH2 V(10nM, stock solution),
EIS, E16 Gluc-NH2 4 (10W, stock solution) and E16 Glue-NH210.) relative to
glucagon-NH2 (111), whereas Fig. 5B compares induction of the GLP-I receptor
by
glucagon analogs (E16, K20 Gluc-NH2 41, EIS, E16 Glue-NH2 A, and E16 Gluc-
NH2, 10) relative to GLP-1 (II) and glucagon-NH2 (0).
= 20 Fig. 6A and 6B represents data showing receptor mediated cAMP
induction by
glucagon analogs. More particularly, Fig. 6A compares induction of the
glucagon
receptor by glucagon analogs (Gluc-NH2 K12E16-NH2 lactam A, E161(20-N1-12
lactam V, K20E24-NH2 lactam 4 and E24K28-NH2 lactam lo.) relative to glucagon
(U), whereas Fig. 6B compares induction of the GLP-1 receptor by glucagon
analogs
= 25 (Gluc-NH2 111, KI2E16-NH2 lactam A, E16K20-NH2 lactam 7, K20E24-NH2
lactam
4 and .E24K28-NH2 lactam 11.) relative to GLP-1 (II).
Fig. 7A and 7B represents data showing receptor mediated cAMP induction by
glucagon analogs. More particularly, Fig. 7A compares induction of the
glucagon
receptor by glucagon analogs (Gluc-NH2 El6 Gluc-
NH2, A, KU, E16 Glue-NF12
30 lactam V, E16, K20 Gluc-NH2 4 and E16, K20 Gluc-NH2 lactam 0.)
relative to
glucagon (N), whereas Fig. 7B compares induction of the GLP-1 receptor by
glucagon analogs (Gluc-NH2 /1, E16 Glue-NH2, A, K12, El6 Gluc-NH2 lactam V,
E16, K20 Glue-NH2 4 and E16, K20 Glue-NH2 lactam 0.) relative to GLP-1 (II).
23
=
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Figs. 8A-8F represent data showing receptor mediated cAMP induction by
glucagon analogs at the glucagon receptor (Figs. 8A, 8C and 8E) or the GLP-1
receptor (Figs. 8B, 8C and 8F) wherein hE = homoglutamic acid and hC =
homocysteic acid.
Fig. 9A and 9B: represent data showing receptor mediated cAMP induction
by GLP (17-26) glucagon analogs, wherein amino acid positions 17-26 of native
glucagon (SEQ ID NO: 1) have been substituted with the amino acids of
positions 17-
26 of native GLP-1 (SEQ ID NO: 50). More particularly, Fig. 9A compares
induction
of the glucagon receptor by the designated GLP (17-26) glucagon analogs, and
Fig.
9B compares induction of the GLP-1 receptor by the designated GLP (17-26)
glucagon analogs.
Figs. 10A-E: are graphs providing in vivo data demonstrating the ability of
the
glucagon peptides of the Present invention to induce weight loss in mice
injected
subcutaneously with the indicated amounts of the respective compounds.
Sequence
Identifiers for the glucagon peptide listed in Figs WA -10E are as follows,
for Fig.
10A: Chimera 2 Aib2 C24 40K PEG (SEQ ID NO: 486), Aib2 C24 Chimera 2 40K
lactam (SEQ ID NO: 504) and Aib2 E16 IC20 Gluc-NH2 Lac 40K (SEQ ID NO: 528);
Fig. 10B: Aib2 C24 Chi 2 lactam 40K (SEQ ID NO: 504), DMIA1 C24 Chi 2 Lactam
40K (SEQ ID NO: 505), Chimera 2 DMIA1 C24 40K (SEQ ID NO: 519), and
Chimera 2 Aib2 C24 40K (SEQ ID NO: 486); wherein the number at the end of the
sequence designates the dosage used, either 70 or 350 nmol/lcg; Fig. 10C: AIB2
w/
lactam C24 40K (SEQ ID NO: 504), AIB2 E16 1(20 w/ lactam C24 40K (SEQ ID
NO: 528), DMIA1 El 6 1(20w! lactam C24 40K (SEQ ID NO: 510), DMIA1 E16
1(20w! lactam CEX 40K (SEQ ID NO: 513) and DMIA1 E16 K20 w/o lactam CEX
40K (SEQ ID NO: 529); Fig. IOD: AIB2 w lactam C24 40K (SEQ ID NO: 504),
AIB2 E16 K20 w lactam C24 40K (SEQ ID NO: 528), DMIA1 E16 K20 w lactam
C24 40K (SEQ ID NO: 510) and DMIA1 E16 K20 w lactam/Cex C24 40K (SEQ ID
NO: 513), wherein the number at the end of the sequence designates the dosage
used,
either 14 or 70 nmol/kg/wk; Fig. 10E: AIB2 w/o lactam C24 40K (SEQ ID NO:
486),
Chi 2 AIB2 C24 CEX 40K (SEQ ID NO: 533), AIB2 El 6 Al 8 K20 C24 40K (SEQ
ID NO: 492), AIB2 w/o lactam CEX G29 C40 40K (SEQ ID NO: 488), AIB2 w/o
lactam CEX C40 C41-2 (SEQ ID NO: 532), AIB2 w/o lactam CEX C24 C40- 2 (SEQ
ID NO: 531) and AIB2 w/o lactam C24 60K (SEQ ID NO: 498), wherein the
24
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
designation 40K or 60K represents the molecular weight of the polyethylene
chain
attached to the glucagon peptide.
Figures 11-13 are graphs providing in vivo data demonstrating the ability of
acylated glucagon peptides to induce weight loss (Figure 11), reduce food
intake
(Figure 12), and reduce blood glucose levels (Figure 13) in mice injected
subcutaneously with the indicated amounts of the compounds.
Figures 14A and 14B represent data showing glucagon and GLP-1 receptor
mediated cAMP induction, respectively, by glucagon analogs.
Figure 15 represents a graph of blood glucose (mg/dL) as a function of time
Figure 16 represents a graph of blood glucose (mg/dL) as a function of time
Figure 17 represents a graph of blood glucose (mg/dL) as a function of time
Figure 18 represents a graph of blood glucose (mg/dL) as a function of time
Chimera-2 AIB2, Cys24-40kD PEG (open triangles), Chimera-2 AIB2,
K10-
C16 Cys24-40kD PEG (diamonds), or Chimera-2 AIB2, Cys24-40kD PEG (open
squares) followed by glucose challenge 24 hours after administration of the
peptide.
Figure .19 represents a graph of the change in body weight (%) as a function
of
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
triangle with solid line); Chimera-2 AIB2, Km-C16 Cys24-40kD Peg (15nmoVkg,
inverted triangle with dotted line; 70 nmol/kg; inverted triangle with solid
line).
Figure 20 represents a graph of the total change in body weight (%) in mice 14
days after QW injections of 10, 20, 40, or 80 nmol/kg Peptide A K10-C14 or 20
nmol/kg Chimera-2 AIB2 Km-C8 Cys24-40kD or a vehicle control
Figure 21 represents a graph of the blood glucose levels (mg/dL) in response
to a glucose injection of mice injected with 10, 20, 40, or 80 nmoUlcg Peptide
A K' -
C14 or 20 nmol/kg Chimera-2 AIB2K10-C8 Cys24-4010 or a vehicle control 24
hours
prior to the glucose injection.
Figure 22 represents a graph of the total change in body weight (%)of mice
injected with vehicle control, Liraglutide, (C16) Glucagon Amide, 7E-yE-C16
Glucagon Amide, AA-C16 Glucagon Amide, ori3AftA-C16 Glucagon Amide at the
indicated dose.
Figure 23 represents a graph of the fat mass (g) as measured on Day 7 of the
study of mice injected with vehicle control, Liraglutide, (C16) Glucagon
Amide, yE-
yE-C16 Glucagon Amide, AA-C16 Glucagon Amide, or flAA-C16 Glucagon Amide
at the indicated dose.
Figure 24 represents a graph of the change in blood glucose (mg/dL; Day 7
levels minus Day 0 levels) of mice injected with vehicle control, Liraglutide,
(C16)
Glucagon Amide, yE-?E -CI6 Glucagon Amide, AA-C16 Glucagon Amide, or 3Al3A-
C16 Glucagon Amide at the indicated dose.
Figure 25 represents represents a graph of the mean residue ellipticity as a
function of wavelength (nm) for Peptide X-PEG or Peptide Y-PEG in 10 mM
Phosphate (pH 5.9) either with or without 10% TFE.
Figure 26 represents a graph of the % cAMP produced in response to
Glucagon, GLP-1, Peptide X, Peptide X-PEG, Peptide Y, or Peptide Y-PEG binding
to either the glucagon receptor (left) or GLP-1 receptor (right) as a function
of peptide
concentration (nM).
Figure 27 represents a collection of graphs which demonstrate the in vivo
effects on A) body weight, B) fat mass, C) food intake, and D) fasting blood
glucose
levels in diet induced obese mice treated for one week with vehicle control,
Peptide
X-PEG, or Peptide Y-PEG. More specifically, Figure 27A represents a graph of
the
% change in body weight (BW) as a function of time (days), Figure 27 B
represents a
graph of the % change in fat mass as measured on Day 7 (as compared to initial
fat
26
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
mass measurements), Figure 27C represents a graph of the total food intake (g)
over
the course of the study as measured on Day 7, and Figure 27D represents a
graph of
the change in blood glucose (mg/dL) as measured on Day 7 (in comparison to
initial
blood glucose levels).
Figure 28 represents a collection of graphs which demonstrate the in vivo
effects on body weight (Figures 28A and 28C) and fasting blood glucose levels
(Figures 28B and 28D) in mice treated with either Peptide X-PEG (Figures 28A
and
28B) or Peptide Y-PEG (Figures 28C and 28D) at varying doses (nmol/kg/week).
Figure 29 represents a collection of graphs showing the in vivo effects on A)
body weight (BW), B) body fat mass, C) overall food intake, D) energy
expenditure,
E) respiratory quotient, F) locomotor activity, G) fasting blood glucose, H)
glucose
tolerance, and I) total plasma insulin levels in diet induced obese mice
treated for one
month with a vehicle control, Peptide X-PEG, or Peptide Y-PEG.
Figure 30 represents a collection of graphs showing the in vivo Week 3 effects
on calorimetric measurements of A) food intake, B) total energy expenditure,
C) total
respiratory quotient, D) locomotor activity, E) total locomotor activity, F)
area under
the curve ipGTT, G) plasma C-peptide levels, H) PEPCK/HPRT fold expression,
and
I) G6P/HPRT fold expression levels in diet induced obese mice treated for one
month
with a vehicle control, Peptide X-PEG, or Peptide Y-PEG.
Figure 31 represents a collection of graphs demonstrating the in vivo effects
on
plasma A) cholesterol, B) cholesterol FPLC, C) triglycerides, D) leptin., E)
resistin,
and F) adiponectin in diet induced obese mice treated for one month with a
vehicle
control, Peptide X-PEG, or Peptide Y-PEG.
Figure 32 represents a collection of graphs demonstrating the in vivo effects
on
A) BAT UCP-1 expression levels and B) white adipose tissue as reflected by
phosphorylation of hormone sensitive lipase (pHSL) in mice treated with a
vehicle
control, Peptide X-PEG, or Peptide Y-PEG.
Figure 33 represents a collection of graphs demonstrating the in vivo effects
of
a vehicle control, Peptide X-PEG, or Peptide Y-PEG in DIO rats on A) body
weight
and B) fat mass. Figure 33C represents a graph of the relative expression of
CD68 to
TFIIB as quantitatively assessed by real-time RT-PCR in epidiymal adipose
tissue
isolated from mice treated for two weeks with Peptide Y-PEG, Peptide X-PEGõ or
vehicle. Data are presented as relative CD68 mRNA expression normalized to TF
I IB
mRNA expression and expressed as mean SEM.
27
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Figures 34A to 34F represent a collection of graphs demonstrating the in vivo
effects on body weight (BW; 34A and 34B), fat mass (34C), food intake (34D),
and
blood glucose levels (34E and 34F) in GLP-1-R knock out mice treated with a
vehicle
control, Peptide X-PEG, or Peptide Y-PEG
Figures 35A to 35C represent a series of graphs demonstrating the in vivo
effects on body weight (35A), blood glucose (35B), and fat mass (35C) in DIO
mice
treated with vehicle control, Peptide V, or Peptide U. .
DETAILED DESCRIPTION
DEFINITIONS
In describing and claiming the invention, the following terminology will be
used in accordance with the definitions set forth below.
As used herein, the term "pharmaceutically acceptable carrier" includes any of
the standard pharmaceutical carriers, such as a phosphate buffered saline
solution,
water, emulsions such as an oil/water or water/oil emulsion, and various types
of
wetting agents. The term also encompasses any of the agents approved by a
regulatory agency of the US Federal government or listed in the US
Pharmacopeia for
use in animals, including humans.
As used herein the term "pharmaceutically acceptable salt" refers to salts of
compounds that retain the biological activity of the parent compound, and
which are
not biologically or otherwise undesirable. Many of the compounds disclosed
herein
are capable of forming acid and/or base salts by virtue of the presence of
amino
and/or carboxyl groups or groups similar thereto.
Pharmaceutically acceptable base addition salts can be prepared from
= 25 inorganic and organic bases. Salts derived from inorganic bases,
include by way of
example only, sodium, potassium, lithium, ammonium, calcium and magnesium
salts.
Salts derived from organic bases include, but are not limited to, salts of
primary, =
secondary and tertiary amines.
Pharmaceutically acceptable acid addition salts may be prepared from
inorganic and organic acids. Salts derived from inorganic acids include
hydrochloric
acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the
like. Salts
derived from organic acids include acetic acid, propionic acid, glycolic acid,
pyruvic
acid, oxalic acid, malic acid, malonic acid, succinic acid, maleic acid,
fumaric acid,
28
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
= tartaric acid, citric acid, benzoic acid, cinnamic acid, mandelic acid,
methanesulfonic
= acid, ethanesulfonic acid, p-toluene-sulfonic acid, salicylic acid, and
the like.
As used herein, the term "treating" includes prophylaxis of the specific
disorder or condition, or alleviation of the symptoms associated with a
specific
disorder or condition and/or preventing or eliminating said symptoms. For
example,
as used herein the term "treating diabetes" will refer in general to altering
glucose
blood levels in the direction of normal levels and may include increasing or
decreasing blood glucose levels depending on a given situation.
As used herein an "effective" amount or a "therapeutically effective amount"
of a glucagon peptide refers to a nontoxic but sufficient amount of the
peptide to
provide the desired effect. For example one desired effect would be the
prevention or
treatment of hypoglycemia, as measured, for example, by an increase in blood
glucose
level. An alternative desired effect for the co-agonist analogs of the present
disclosure would include treating hyperglycemia, e.g., as measured by a change
in
blood glucose level closer to normal, or inducing weight loss/preventing
weight gain,
e.g., as measured by reduction in body weight, or preventing or reducing an
increase
in body weight, or normalizing body fat distribution. The amount that is
"effective"
will vary from subject to subject, depending on the age and general condition
of the
individual, mode of administration, and the like. Thus, it is not always
possible to
specify an exact "effective amount." However, an appropriate "effective"
amount in
any individual case may be determined by one of ordinary skill in the art
using routine
experimentation.
The term, "parenteral" means not through the alimentary canal but by some
other route such as subcutaneous, intramuscular, intraspinal, or intravenous.
As used herein, the term "purified" and like terms relate to the isolation of
a
molecule or compound in a form that is substantially free of contaminants
normally
associated with the molecule or compound in a native or natural environment.
As used herein, the term "purified" does not require absolute purity; rather,
it is
intended as a relative definition. The term "purified polypeptide" is used
herein to
describe a polypeptide which has been separated from other compounds
including, but
not limited to nucleic acid molecules, lipids and carbohydrates.
The term "isolated" requires that the referenced material be removed from its
original environment (e.g., the natural environment if it is naturally
occurring). For
= example, a naturally-occurring polynucleotide present in a living animal
is not
29
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
isolated, but the same polynucleotide, separated from some or all of the
coexisting
materials in the natural system, is isolated.
As used herein, the term "peptide" encompasses a sequence of 3 or more
amino acids and typically less than 50 amino acids, wherein the amino acids
are
naturally occurring or non-naturally occurring amino acids. Non-naturally
occurring
amino acids refer to amino acids that do not naturally occur in vivo but
which,
nevertheless, can be incorporated into the peptide structures described
herein.
As used herein, the terms "polypeptide" and "protein" are terms that are used
interchangeably to refer to a polymer of amino acids, without regard to the
length of =
the polymer. Typically, polypeptides and proteins have a polymer length that
is
greater than that of "peptides."
A "glucagon peptide" as used herein includes any peptide comprising, either
the amino acid sequence of SEQ ID NO: I, or any analog of the amino acid
sequence
of SEQ ID NO: 1, including amino acid substitutions, additions, deletions or
post
=
translational modifications (e.g., methylation, acylation, ubiquitination,
intramolecular
covalent bonding such as lactam bridge formation, PEGylation, and the like) of
the
peptide, wherein the analog stimulates glucagon or GLP-1 receptor activity,
e.g., as
measured by cAMP production using the assay described in Example 14.
The term "glucagon agonist" refers to a complex comprising a glucagon
peptide that stimulates glucagon receptor activity, e.g., as measured by cAMP
production using the assay described in Example 14.
As used herein a "glucagon agonist analog" is a glucagon peptide comprising a
sequence selected from the group consisting of SEQ ID NO: 10, SEQ ID NO: 11,
SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14 and SEQ ID NO: 15, or an analog
of such a sequence that has been modified to include one or more conservative
amino
acid substitutions at one or more of positions 2, 5, 7, 10, 11, 12, 13, 14,
17, 18, 19, 20,
21, 24, 27, 28 or 29.
As used herein an amino acid "modification" refers to a substitution, addition
or deletion of an amino acid, and includes substitution with or addition of
any of the
20 amino acids commonly found in human proteins, as well as atypical or non-
naturally occurring amino acids. Throughout the application, all references to
a
particular amino acid position by number (e.g. position 28) refer to the amino
acid at
that position in native glucagon (SEQ ID NO:1) or the corresponding amino acid
position in any analogs thereof. For example, a reference herein to "position
28"
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
would mean the corresponding position 27 for a glucagon analog in which the
first
amino acid of SEQ ID NO: 1 has been deleted. Similarly, a reference herein to
"position 28" would mean the corresponding position 29 for a glucagon analog
in
which one amino acid has been added before the N-terminus of SEQ ID NO: I.
Commercial sources of atypical amino acids include Sigma-Aldrich (Milwaukee,
WI),
ChemPep Inc. (Miami, FL), and Genzyme Pharmaceuticals (Cambridge, MA).
Atypical amino acids may be purchased from commercial suppliers, synthesized
de
novo, or chemically modified or derivatized from other amino acids.
As used herein a "glucagon co-agonist" is a glucagon peptide that exhibits
to activity at the glucagon receptor of at least about 10% to about 500% or
more relative
to native glucagon and also exhibits activity at the GLP-1 receptor of about
at least =
10% to about 200% or more relative to native GLP-1.
As used herein a "glucagon/GLP-1 co-agonist molecule" is a molecule that
exhibits activity at the glucagon receptor of at least about 10% relative to
native
glucagon and also exhibits activity at the GLP-1 receptor of at least about
10%
relative to native GLP-1.
As used herein the term "native glucagon" refers to a peptide consisting of
the
sequence of SEQ ID NO: 1, and the term "native GLP-1" is a generic term that
designates GLP-1(7-36)amide (consisting of the sequence of SEQ ID NO: 52), GLP-
1(7-37)acid (consisting of the sequence of SEQ ID NO: 50) or a mixture of
those two
compounds. As used herein, a general reference to "glucagon" or "GLP-1" in the
absence of any further designation is intended to mean native glucagon or
native
GLP-1, respectively.
As used herein an amino acid "substitution" refers to the replacement of one
amino-acid residue by a different amino acid residue.
As used herein, the term "conservative amino acid substitution" is defined
herein as exchanges within one of the following five groups:
I. Small aliphatic, nonpolar or slightly polar residues:
Ala, Ser, Thr, Pro, Gly;
II. Polar, negatively charged residues and their amides and esters:
Asp, Asn, Glu, Gin, cysteic acid and homocysteic acid;
III. Polar, positively charged residues:
His, Arg, Lys; Ornithine (Orn)
31
CA 02852177 2014-05-23
WO 2009/155258 =
PCT/US2009/047438
29920-208831
IV. Large, aliphatic, nonpolar residues:
Met, Leu, Ile, Val, Cys, Norleucine (Nle), homocysteine
V. Large, aromatic residues:
Phe, Tyr, Tip, acetyl phenylalanine
As used herein the general term "polyethylene glycol chain" or "PEG chain",
= refers to mixtures of condensation polymers of ethylene oxide and water,
in a
branched or straight chain, represented by the general formula H(OCH2CH2)0H,
wherein n is at least 9. Absent any further characterization, the term is
intended to
include polymers of ethylene glycol with an average total molecular weight
selected
from the range of 500 to 40,000 Daltons. "polyethylene glycol chain" or "PEG
chain"
is used in combination with a numeric suffix to indicate the approximate
average
molecular weight thereof. For example, PEG-5,000 refers to polyethylene glycol
chain having a total molecular weight average of about 5,000.
As used herein the term "pegylated" and like terms refers to a compound that
has been modified from its native state by linking a polyethylene glycol chain
to the
compound. A "pegylated glucagon peptide" is a glucagon peptide that has a PEG
chain covalently bound to the glucagon peptide.
As used herein a general reference to a peptide is intended to encompass
peptides that have modified amino and carboxy termini. For example, an amino
acid
chain comprising an amide group in place of the terminal carboxylic acid is
intended
to be encompassed by an amino acid sequence designating the standard amino
acids. '
As used herein a "linker" is a bond, molecule or group of molecules that binds
two separate entities to one another. Linkers may provide for optimal spacing
of the
two entities or may further supply a labile linkage that allows the two
entities to be
separated from each other. Labile linkages include photocleavable groups, acid-
labile
moieties, base-labile moieties and enzyme-cleavable groups.
As used herein a "dimer" is a complex comprising two subunits covalently
bound to one another via a linker. The term dimer, when used absent any
qualifying
language, encompasses both homodimers and heterodimers. A homodimer comprises
two identical subunits, whereas a heterodimer comprises two subunits that
differ,
although the two subunits are substantially similar to one another.
As used herein the term "charged amino acid" refers to an amino acid that
comprises a side chain that is negatively charged (i.e., de-protonated) or
positively
32
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
charged (i.e., protonated) in aqueous solution at physiological pH. For
example
negatively charged amino acids include aspartic acid, glutamic acid, cysteic
acid,
homocysteic acid, and homoglutamic acid, whereas positively charged amino
acids
include arginine, lysine and histidine. Charged amino acids include the
charged
amino acids among the 20 amino-acids commonly found in human proteins, as well
as
atypical or non-naturally occurring amino acids.
As used herein the term "acidic amino acid" refers to an amino acid that
comprises a second acidic moiety, including for example, a carboxylic acid or
sulfonic acid group.
The term "alkyl" refers to a linear or branched hydrocarbon containing the
indicated number of carbon atoms. Exemplary alkyls include methyl, ethyl, and
linear propyl groups.
The term "heteroallcyl" refers to a linear or branched hydrocarbon containing
the indicated number of carbon atoms and at least one heteroatom in the
backbone of
the structure. Suitable heteroatoms for purposes herein include but are not
limited to
N, S, and 0.
EMBODIMENTS
The invention provides glucagon peptides with increased or decreased activity
at the glucagon receptor, or the GLP-1 receptor, or at both receptors. The
invention
also provides glucagon peptides with altered selectivity for the glucagon
receptor
versus the GLP-1 receptor.
Increased activity at the glucagon receptor is provided by an amino acid
modification at position 16 of native glucagon (SEQ ID NO: I) as described
herein.
Maintained or increased activity at the glucagon receptor is also provided by
an amino acid modification at position 3 of native glucagon with a glutamine
analog
(e.g. (Dab(Ac)).
Reduced activity at the glucagon receptor is provided, e.g., by substitution
of
the amino acid at position 3 with an acidic., basic, or hydrophobic amino acid
as
described herein.
Increased activity at the GLP- I receptor is provided by replacing the
carboxylic acid of the C-terminal amino acid with a charge-neutral group, such
as an
amide or ester.
33
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Increased activity at the GLP-1 receptor is provided by modifications that
stabilize the alpha helix in the C-terminal portion of glucagon (e.g. around
residues
12-29). In some embodiments, such modifications permit formation of an
intramolecular bridge between the side chains of two amino acids that are
separated
by three intervening amino acids, for example, positions 12 and 16, or 16 and
20, or
20 and 24, as described herein. In other embodiments, such modifications
include
insertion or substitution modifications that introduce one or more a, a-
disubstituted
amino acids, e.g. AIB at one or more of positions 16, 20, 21 or 24.
Increased activity at the GLP-1 and glucagon receptors for peptides lacking an
to intramolecular bridge, e.g., a covalent intramolecular brige, is
provided by covalently
attaching an acyl or alkyl group to to the side chain of the amino acid at
position 10 of
the peptide, wherein the acyl or alkyl group is non-native to the amino acid
at position
10. Further increased activity at the GLP-1 and glucagon receptors for such
peptides
lacking an intramolecular bridge, e.g., a covalent intramolecular bridge, may
be
achieved by incorporating a spacer between the acyl or alkyl group and the
side chain
of the amino acid at position 10. Suitable spacers are described herein and
include, "
but not limited to spacers that are 3. to 10 atoms in length.
Increased activity at the GLP-1 receptor is provided by an amino acid
modification at position 20 as described herein.
Increased activity at the GLP-1 receptor is provided in glucagon analogs
comprising the C-terminal extension of SEQ ID NO: 26. GLP-1 activity in such
analogs comprising SEQ ID NO: 26 can be further increased by modifying the
amino
acid at position 18, 28 or 29, or at position 18 and 29, as described herein.
Restoration of glucagon activity which has been reduced by amino acid
modifications at positions 1 and 2 is provided by a covalent bond between the
side
chains of two amino acids that are separated by three intervening amino acids,
for
example, positions 12 and 16, or 16 and 20, or 20 and 24, as described herein.
A further modest increase in GLP-1 potency is provided by modifying the
amino acid at position 10 to be Trp.
Any of the modifications described above which increase or decrease
glucagon receptor activity and which increase GLP- I receptor activity can be
applied
individually or in combination. Any of the modifications described above can
also be
= combined with other modifications that confer other desirable properties,
such as
increased solubility and/or stability and/or duration of action.
Alternatively, any of
34
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
the modifications described above can be combined with other modifications
that do
not substantially affect solubility or stability or activity. Exemplary
modifications
include but are not limited to:
(A) Improving solubility, for example, by introducing one, two, three or more
charged amino acid(s) to the C-terminal portion of native glucagon, preferably
at a
position C-terminal to position 27. Such a charged amino acid can be
introduced by
substituting a native amino acid with a charged amino acid, e.g. at positions
28 or 29,
or alternatively by adding a charged amino acid, e.g. after position 27, 28 or
29. In
exemplary embodiments, one, two, three or all of the charged amino acids are
negatively charged. In other embodiments, one, two, three or all of the
charged amino
acids are positively charged. Such modifications increase solubility, e.g.
provide at
least 2-fold, 5-fold, 10-fold, 15-fold, 25-fold, 30-fold or greater solubility
relative to
native glucagon at a given pH between about 5.5 and 8, e.g., pH 7, when
measured
after 24 hours at 25 C.
(B) Increasing solubility and duration of action or half-life in circulation
by
addition of a hydrophilic moiety such as a polyethylene glycol chain, as
described
herein, e.g. at position 16, 17, 20, 21, 24 or 29, or at the C-terminal amino
acid of the
peptide.
(C) Increasing , by modification of the aspartic acid at position 15, for
example, by deletion or substitution with glutamic acid, homoglutamic acid,
cysteic
acid or homocysteic acid. Such modifications can reduce degradation or
cleavage at a
pH within the range of 5.5 to 8, for example, retaining at least 75%, 80%,
90%,95%,
96%, 97%, 98% or 99% of the original peptide after 24 hours at 25 C.
(D) Increasing stability by modification of the methionine at position 27, for
example, by substitution with leucine or norleucine. Such modifications can
reduce
oxidative degradation. Stability can also be increased by modification of the
Gln at
position 20 or 24, e.g. by substitution with Ala, Ser, Thr, or AIB. Such
modifications
can reduce degradation that occurs through deamidation of Gin. Stability can
be
increased by modification of Asp at position 21, e.g. by substitution with
Glu. Such
modifications can reduce degradation that occurs through dehydration of Asp to
form
a cyclic succinimide intermediate followed by isomerization to iso-aspartate.
(E) Increasing resistance to dipeptidyl peptidase IV (DPP IV) cleavage by
modification of the amino acid at position 1 or 2 as described herein.
CA 02 85217 7 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
(F) Conservative or non-conservative substitutions, additions or deletions
that
do not affect activity, for example, conservative substitutions at one or more
of
positions 2, 5, 7, 10, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 24, 27, 28 or
29; deletions
at one or more of positions 27, 28 or 29; or a deletion of amino acid 29
optionally
combined with a C-terminal amide or ester in place of the C-terminal
carboxylic acid
group;
(G) Adding C-terminal extensions as described herein;
(H) Increasing half-life in circulation and/or extending the duration of
action
and/or delaying the onset of action, for example, through acylation or
alkylation of the
(I) Homodimerization or heterodimerization as described herein.
In exemplary embodiments, the glucagon peptide may comprise a total of 1,
up to 2, up to 3, up to 4, up to 5, up to 6, up to 7, up to 8, up to 9, or up
to 10 amino
acid modifications relative to the native glucagon sequence.
Other modifications include substitution of His at position 1 with a large,
aromatic amino acid (e.g., Tyr, Phe, Trp or amino-Phe);
Ser at position 2 with Ala;
substitution of Tyr at position 10 with Val or Phe;
substitution of Lys at position 12 with Arg;
substitution of Asp at position 15 with Glu;
substitution of Ser at position 16 with Thr or MB.
One embodiment disclosed herein is directed to a glucagon agonist that has
been modified relative to the wild type peptide of His-Ser-Gln-Gly-Thr-Phe-
Thr-Ser-
Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser- Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-
36
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In accordance with one embodiment the serine residue at position 16 of native
glucagon is substituted with an amino acid selected from the group consisting
of
glutamic acid, glutamine, homoglutamic acid, homocysteic acid, threonine or
glycine.
In accordance with one embodiment the serine residue at position 16 of native
glucagon is substituted with an amino acid selected from the group consisting
of
glutamic acid, glutamine, homoglutamic acid and homocysteic acid, and in one
embodiment the serine residue is substituted with glutamic acid. In one
embodiment
the glucagon peptide having enhanced specificity for the glucagon receptor
comprises
the peptide of SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 10 or a glucagon agonist
to analog thereof, wherein the carboxy terminal amino acid retains its
native carboxylic -
acid group. In accordance with one embodiment a glucagon agonist comprising
the
sequence of NH2-His-Ser-Gln-Gly:Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-
= Glu-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr-COOH (SEQ ID NO:
10) is provided, wherein the peptide exhibits approximately fivefold enhanced
potency at the glucagon receptor, relative to native glucagon as measured by
the in
vitro cAMP assay of Example 14.
Hydrophilic moieties
The glucagon peptides of the present invention can be further modified to
improve the peptide's solubility and stability in aqueous solutions at
physiological pH,
while retaining the high biological activity relative to native glucagon.
Hydrophilic
moieties such as PEG groups can be attached to the glucagon peptides under any
suitable conditions used to react a protein with an activated polymer
molecule. Any
means known in the art can be used, including via acylation, reductive
alkylation,
Michael addition, thiol allcylation or other chemoselective
conjugation/ligation
methods through a reactive group on the PEG moiety (e.g., an aldehyde, amino,
ester,
thiol, a-haloacetyl, maleimido or hydrazino group) to a reactive group on the
target
compound (e.g., an aldehyde, amino, ester, thiol, a-haloacetyl, maleimido or
hydrazino group). Activating groups which can be used to link the water
soluble
polymer to one or more proteins include without limitation sulfone, maleimide,
sulfhydryl, thiol, triflate, tresylate, azidirine, oxirane, 5-pyridyl, and
alpha-
halogenated acyl group (e.g., alpha-iodo acetic acid, alpha-bromoacetic acid,
alpha-
chloroacetic acid). If attached to the peptide by reductive allcylation, the
polymer
selected should have a single reactive aldehyde so that the degree of
polymerization is
controlled. See, for example, Kinstler et al., Adv. Drug. Delivery Rev. 54:
477-485
37
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
(2002); Roberts et al., Adv. Drug Delivery Rev. 54: 459-476 (2002); and
Zalipsky et
al., Adv. Drug Delivery Rev. 16: 157-182 (1995).
In a specific aspect of the invention, an amino acid residue on the glucagon
peptide having a thiol is modified with a hydrophilic moiety such as PEG. In
some
embodiments, the thiol is modified with maleimide-activated PEG in a Michael
addition reaction to result in a PEGylated peptide comprising the thioether
linkage
shown below:
Pebde 0
cs_cr
0),
nCH3
0 0
=
In some embodiments, the thiol is modified with a haloacetyl-activated PEG in
a
i0 nucleophilic substitution reaction to result in a PEGylated peptide
comprising the
= thioether linkage shown below:
Peptide
H3
1/44 n
0
=
Suitable hydrophilic moieties include polyethylene glycol (PEG),
polypropylene glycol, polyoxyethylated polyols (e.g., POG), polyoxyethylated
sorbitol, polyoxyethylated glucose, polyoxyethylated glycerol (POG),
polyoxyallcylenes, polyethylene glycol propionaldehyde, copolymers of ethylene
glycol/propylene glycol, monomethoxy-polyethylene glycol, mono-(C1-C 10)
alkoxy-
or aryloxy-polyethylene glycol, carboxymethylcellulose, polyacetals, polyvinyl
alcohol (PVA), polyvinyl pyrrolidone, poly-1, 3-dioxolane, poly-1,3,6-
trioxane,
ethylene/maleic anhydride copolymer, poly (.beta.-amino acids) (either
homopolymers or random copolymers), poly(n-vinyl pyrrolidone)polyethylene
glycol,
propropylene glycol homopolymers (PPG) and other polyakylene oxides,
polypropylene oxide/ethylene oxide copolymers, colonic acids or other
polysaccharide polymers, Ficoll or dextran and mixtures thereof. Dextrans are
polysaccharide polymers of glucose subunits, predominantly linked by al -6
linkages.
Dextran is available in many molecular weight ranges, e.g., about 1 IcD to
about 100
IcD, or from about 5, 10, 15 or 20 IcD to about 20, 30, 40, 50, 60, 70, 80 or
90 kD.
Linear or branched polymers are contemplated. Resulting preparations of
conjugates
38
CA 02 852 17 7 2 0 14-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
may be essentially monodisperse or polydisperse, and may have about 0.5, 0.7,
1, 1.2,
1.5 or 2 polymer moieties per peptide.
In accordance with one embodiment, introduction of hydrophilic groups at
positions 17, 21, and 24 of the peptide of SEQ ID NO: 9 or SEQ ID NO: 10 are
anticipated to improve the solubility and stability of the high potency
glucagon analog
in solutions having a physiological pH. Introduction of such groups also
increases
duration of action, e.g. as measured by a prolonged half-life in circulation.
Suitable
hydrophilic moieties include any water soluble polymers known in the art,
including
PEG, homo- or co-polymers of PEG, a monomethyl-substituted polymer of PEG
Conjugates
The present disclosure also encompasses other conjugates in which glucagon
peptides of the invention are linked, optionally via covalent bonding and
optionally
via a linker, to a conjugate moiety. Linkage can be accomplished by covalent
The peptide can be linked to conjugate moieties via direct covalent linkage by
reacting targeted amino acid residues of the peptide with an organic
derivatizing agent
that is capable of reacting with selected side chains or the N- or C-terminal
residues of
these targeted amino acids. Reactive groups on the peptide or conjugate moiety
39
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
intermediate carriers, such as polysaccharide or polypeptide carriers.
Examples of
polysaccharide carriers include aminodextran. Examples of suitable polypeptide
carriers include polylysine, polyglutamic acid, polyaspartic acid, co-polymers
thereof,
and mixed polymers of these amino acids and others, e.g., serines, to confer
desirable
solubility properties on the resultant loaded carrier.
Cysteinyl residues are most commonly are reacted with a-haloacetates (and
corresponding amines), such as chloroacetic acid, chloroacetamide to give
carboxymethyl or carboxyamidomethyl derivatives. Cysteinyl residues also are
derivatized by reaction with bromotrifluoroacetone, alpha-bromo-11-(5-
imidozoyl)propionic acid, chloroacetyl phosphate, N-alkylmaleimides, 3-nitro-2-
pyridyl disulfide, methyl 2-pyridyl disulfide, rochloromercuribenzoate, 2-
chloromercuri-4-nitrophenol, or chloro-7-nitrobenzo-2-oxa-1,3-diazole.
Histidyl residues are derivatized by reaction with diethylpyrocarbonate at pH
5.5-7.0 because this agent is relatively specific for the histidyl side chain.
Para-
bromophenacyl bromide also is useful; the reaction is preferably performed in
0.1 M
sodium cacodylate at pH 6Ø
Lysinyl and amino-terminal residues are reacted with succinic or other
carboxylic acid anhydrides. Derivatization with these agents has the effect of
reversing the charge of the lysinyl residues. Other suitable reagents for
derivatizing
alpha-amino-containing residues include imidoesters such as methyl
picolinimidate,
pyridoxal phosphate, pyridoxal, chloroborohydride, trinitrobenzenesulfonic
acid, 0-
methylisourea, 2,4-pentanedione, and transaminase-catalyzed reaction with
glyoxylate.
Arginyl residues are modified by reaction with one or several conventional
reagents, among them phenylglyoxal, 2,3-butanedione, 1,2-cyclohexanedione, and
ninhydrin. Derivatization of arginine residues requires that the reaction be
performed
in alkaline conditions because of the high plc of the guanidine functional
group.
Furthermore, these reagents may react with the groups of lysine as well as the
arginine epsilon-amino group.
The specific modification of tyrosyl residues may be made, with particular
interest in introducing spectral labels into tyrosyl residues by reaction with
aromatic
diazonium compounds or tetranitromethane. Most commonly, N-acetylimidizole and
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
tetranitromethane are used to form 0-acetyl tyrosyl species and 3-nitro
derivatives,
respectively.
Carboxyl side groups (aspartyl or glutamyl) are selectively modified by
reaction with carbodiimides (R-N=C=N-R'), where R and R' are different alkyl
groups, such as 1-cyclohexy1-3-(2-morpholiny1-4-ethyl) carbodiimide or 1-ethy1-
3-(4-
azonia-4,4-dimethylpentyl) carbodiimide. Furthermore, aspartyl and glutamyl
residues are converted to asparaginyl and glutaminyl residues by reaction with
ammonium ions.
Other modifications include hydroxylation of proline and lysine,
phosphorylation of hydroxyl groups of seryl or threonyl residues, methylation
of the
alpha-amino groups of lysine, arginine, and histidine side chains (T. E.
Creighton,
= Proteins: Structure and Molecular Properties, W.H. Freeman & Co., San
Francisco,
pp. 79-86 (1983)), deamidation of asparagine or glutamine, acetylation of the
N-
terminal amine, and/or amidation or esterification of the C-terminal
carboxylic acid
group.
Another type of covalent modification involves chemically or enzymatically
coupling glycosides to the peptide. Sugar(s) may be attached to (a) arginine
and
histidine, (b) free carboxyl groups, (c) free sulfhydryl groups such as those
of
cysteine, (d) free hydroxyl groups such as those of serine, threonine, or
hydroxyproline, (e) aromatic residues such as those of tyrosine, or
tryptophan, or (f)
the amide group of glutamine. These methods are described in W087/05330
published 11 Sep. 1987, and in Aplin and Wriston, CRC Crit. Rev. Biochem., pp.
259-306 (1981).
Exemplary conjugate moieties that can be linked to any of the glucagon
peptides described herein include but are not limited to a heterologous
peptide or
polypeptide (including for example, a plasma protein), a targeting agent, an
immunoglobulin or portion thereof (e.g. variable region, CDR, or Fc region), a
diagnostic label such as a radioisotope, fluorophore or enzymatic label, a
polymer
including water soluble polymers, or other therapeutic or diagnostic agents.
In one
embodiment a conjugate is provided comprising a glucagon peptide of the
present
invention and a plasma protein, wherein the plasma protein is selected form
the group
consisting of albumin, transferin, fibrinogen and globulins. In one embodiment
the
plasma protein moiety of the conjugate is albumin or transferin.
41
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In some embodiments, the linker comprises a chain of atoms from Ito about
60, or I to 30 atoms or longer, 2 to 5 atoms, 2 to 10 atoms, 5 to 10 atoms, or
10 to 20
= atoms long. In some embodiments, the chain atoms are all carbon atoms. In
some
embodiments, the chain atoms in the backbone of the linker are selected from
the
group consisting of C, 0, N, and S. Chain atoms and linkers may be selected
according to their expected solubility (hydrophilicity) so as to provide a
more soluble
conjugate. In some embodiments, the linker provides a functional group that is
= subject to cleavage by an enzyme or other catalyst or hydrolytic
conditions found in
the target tissue or organ or cell. In some embodiments, the length of the
linker is.
long enough to reduce the potential for steric hindrance. If the linker is a
covalent
bond or a peptidyl bond and the conjugate is a polypeptide, the entire
conjugate can
be a fusion protein. Such peptidyl linkers may be any length. Exemplary
linkers are
from about 1 to 50 amino acids in length, 5 to 50, 3 to 5, 5 to 10, 5 to 15,
or 10 to 30
amino acids in length. Such fusion proteins may alternatively be produced by
recombinant genetic engineering methods known to one of ordinary skill in the
art.
As noted above, in some embodiments, the glucagon peptides are conjugated,
e.g., fused to an immunoglobulin or portion thereof (e.g. variable region,
CDR, or Fc
region). Known types of immunoglobulins (Ig) include IgG, IgA, IgE, IgD or
IgM.
The Fc region is a C-terminal region of an Ig heavy chain, which is
responsible for
binding to Fc receptors that carry out activities such as recycling (which
results in
prolonged half-life), antibody dependent cell-mediated cytotoxicity (ADCC),
and =
complement dependent cytotoxicity (CDC).
For example, according to some definitions the human IgG heavy chain Fc
region stretches from Cys226 to the C-terminus of the heavy chain. The "hinge
region" generally extends from Glu216 to Pro230 of human IgG1 (hinge regions
of
other IgG isotypes may be aligned with the IgG1 sequence by aligning the
cysteines
involved in cysteine bonding). The Fc region of an IgG includes two constant
domains, CH2 and CH3. The CH2 domain of a human IgG Fc region usually extends
from amino acids 231 to amino acid 341. The CH3 domain of a human IgG Fc
region
usually extends from amino acids 342 to 447. References made to amino acid
numbering of immunoglobulins or immunoglobulin fragments, or regions, are all
based on Kabat et al. 1991, Sequences of Proteins of Immunological Interest,
U.S.
Department of Public Health, Bethesda, Md. In a related embodiments, the Fc
region
may comprise one or more native or modified constant regions from an
42
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
immunoglobulin heavy chain, other than CHI, for example, the CH2 and CH3
regions
of IgG and IgA, or the CH3 and CH4 regions of IgE.
Suitable conjugate moieties include portions of immunoglobulin sequence that
include the FcRn binding site. FcRn, a salvage receptor, is responsible for
recycling
immunoglobulins and returning them to circulation in blood. The region of the
Fc
portion of IgG that binds to the FcRn receptor has been described based on X-
ray
crystallography (Burmeister et al. 1994, Nature 372:379). The major contact
area of
the Fc with the FcRn is near the junction of the CH2 and CH3 domains. Fc-FcRn
contacts are all within a single Ig heavy chain. The major contact sites
include amino
domain and amino acid residues 385-387, 428, and 433-436 of the CH3 domain.
Some conjugate moieties may or may not include FcyR binding site(s). FcyR
are responsible for ADCC and CDC. Examples of positions within the Fc region
that
make a direct contact with FcyR are amino acids 234-239 (lower hinge region),
amino
immunoglobulin. Such variant Fc regions comprise at least one amino acid
modification in the CH3 domain of the Fe region (residues 342-447) and/or at
least
one amino acid modification in the. CH2 domain of the Fc region (residues 231-
341).
43
CA 02 852 17 7 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831 =
Ghetie 1995, Therapeutic Immunology 2:77 and Armour et al. 1999, Eur. J.
Immunol.
29:2613). Some exemplary amino acid substitutions are described in US Patents
7,355,008 and 7,381,408, each incorporated by reference herein in its
entirety.
Fusion protein and Terminal extension
The present disclosure also encompasses -glucagon fusion peptides or proteins
wherein a second peptide or polypeptide has been fused to a terminus, e.g.,
the
carboxy terminus of the glucagon peptide. More particularly, the fusion
glucagon
peptide may comprise a glucagon agonist of SEQ ID NO: 55, SEQ ID NO: 9 or SEQ
ID NO: 10 further comprising an amino acid sequence of SEQ ID NO: 26
44
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Charge neutral C-terminus
In accordance with one embodiment, an additional chemical modification of
the glucagon peptide of SEQ ID NO: 10 bestows increased GLP-1 receptor potency
to
a point where the relative activity at the glucagon and GLP-1 receptors is
virtually
equivalent. Accordingly, in one embodiment a glucagon/GLP-1 receptor co-
agonist is
provided wherein the terminal amino acid of the glucagon peptides of the
present
invention have an amide group in place of the carboxylic acid group that is
present on
the native amino acid. The relative activity of the glucagon analog at the
respective
glucagon and GLP-1 receptors can be adjusted by further modifications to the
glucagon peptide to produce analogs demonstrating about 40% to about 500% or
more of the activity of native glucagon at the glucagon receptor and about 20%
to
about 200% or more of the activity of native GLP-1 at the GLP-1 receptor, e.g.
50-
fold, 100-fold or more increase relative to the normal activity of glucagon at
the GLP-
1 receptor. In some embodiments, the glucagon peptides described herein
exhibit up
to about 100%, 1000%, 10,000%, 100,000%, or 1,000,000% of the activity of
native
glucagon at the glucagon receptor. In some embodiments, the glucagon peptides
described herein exhibit up to about 100%, 1000%, 10,000%, 100,000%, or
1,000,000% of the activity of native GLP-1 at the GLP-1 receptor.
Stabilization of the alpha helix/Intramolecular bridges
In a further embodiment glucagon analogs are provided that exhibit enhanced
GLP-1 receptor agonist activity wherein an intramolecular bridge is formed
between
two amino acid side chains to stabilize the three dimensional structure of the
carboxy
terminus of the peptide. The two amino acid side chains can be linked to one
another
through non-covalent bonds, e.g., hydrogen-bonding, ionic interactions, such
as the
formation of salt bridges, or by covalent bonds. When the two amino acid side
chains
are linked to one another through one or more covalent bonds, the peptide may
be
considered herein as comprising a covlent intramolecular bridge. When the two
amino acid side chains are linked to one another through non-covalent bonds,
e.g.,
hydrogen bonds, ionic interactions, the peptide may be considered herein as
comprising a non-covalent intramolecular bridge.
In some embodiments, the intramolecular bridge is formed between two amino
acids that are 3 amino acids apart, e.g., amino acids at positions i and i+4,
wherein i is
any integer between 12 and 25 (e.g., 12, 13, 14, 15, 16, 17, 18, 19, 20, 21,
22, 23, 24,
and 25). More particularly, the side chains of the amino acid pairs 12 and 16,
16 and
CA 02 852 17 7 2 0 1 4-05-2 3
=
WO 2009/155258
PCT/US2009/047438
29920-208831
20, 20 and 24 or 24 and 28 (amino acid pairs in which i = 12, 16, 20, or 24)
are linked
to one another and thus stabilize the glucagon alpha helix. Alternatively, i
can be 17.
In some specific embodiments, wherein the amino acids at positions i and i+4
are joined by an intramolecular bridge, the size of the linker is about 8
atoms, or about
5 7-9 atoms.
In other embodiments, the intramolecular bridge is formed between two amino
acids that are two amino acids apart, e.g., amino acids at positions j and
j+3, wherein j
is any integer between 12 and 26 (e.g., 12, 13, 14, 15, 16, 17, 18, 19,20, 21,
22, 23,
24, 25, and 26). In some specific embodiments, j is 17.
10 In some specific embodiments, wherein amino acids at positions j and
j+3 are
joined by an intramolecular bridge, the size of the linker is about 6 atoms,
or about 5
to 7 atoms.
In yet other embodiments, the intramolecular bridge is formed between two
amino acids that are 6 amino acids apart, e.g., amino acids at positions k and
k+7,
15 wherein k is any integer between 12 and 22 (e.g., 12, 13, 14, 15, 16,
17, 18, 19, 20,
21, and 22). In some specific embodiments, k is 12, 13, or 17. In an exemplary
embodiment, k is 17.
Examples of amino acid pairings that are capable of covalently bonding to
form a six-atom linking bridge include Om and Asp, Glu and an amino acid of
20 Formula I, wherein n is 2, and homoglutamic acid and an amino acid of
Formula I,
=
wherein n is 1, wherein Formula I is:
H2N-C-COOH
(CH2)õ
1
NH2
wherein n = I to 4
[Formula I]
Examples of amino acid pairing that are capable of covalently bonding to form
a seven-atom linking bridge include Orn-Glu (lactam ring); Lys-Asp (lactam);
or
Homoser-Homoglu (lactone). Examples of amino acid pairings that may form an
eight-atom linker include Lys-Glu (lactam); Homolys-Asp (lactam); Om-Homoglu
30 (lactam); 4-aminoPhe-Asp (lactam); or Tyr-Asp (lactone). Examples of
amino acid
pairings that may form a nine-atom linker include Homolys-Glu (lactam); Lys-
46
CA 02852177 2014-05-23
=
WO 2009/155258
PCT/US2009/047438
29920-208831
Homoglu (lactam); 4-aminoPhe-Glu (lactam); or Tyr-Glu (lactone). Any of the
side
chains on these amino acids may additionally be substituted with additional
chemical
groups, so long as the three-dimensional structure of the alpha-helix is not
disrupted.
= One of ordinary skill in the art can envision alternative pairings or
alternative amino
5 acid analogs, including chemically modified derivatives, that would
create a
stabilizing structure of similar size and desired effect. For example, a
homocysteine-
homocysteine disulfide bridge is 6 atoms in length and may be further modified
to
provide the desired effect. Even without covalent linkage, the amino acid
pairings
described above or similar pairings that one of ordinary skill in the art can
envision
to may also provide added stability to the alpha-helix through non-covalent
bonds, for
example, through formation of salt bridges or hydrogen-bonding interactions.
Further exemplary embodiments include the following pairings, optionally
with a lactam bridge: Glu at position 12 with Lys at position 16; native Lys
at position
12 with Glu at position 16; Glu at position 16 with Lys at position 20; Lys at
position
15 16 with Glu at position 20; Glu at position 20 with Lys at position 24;
Lys at position
20 with Glu at position 24; Glu at position 24 with Lys at position 28; Lys at
position
24 with Glu at position 28.
In accordance with one embodiment a glucagon analog is provided that
exhibits glucagon/GLP-1 receptor co-agonist activity wherein the analog
comprises
20 an amino acid sequence selected from the group consisting of SEQ ID NO:
11, 47, 48
and 49. In one embodiment the side chains are covalently bound to one another,
and
in one embodiment the two amino acids are bouncIto one another to form a
lactam
ring. The size of the lactam ring can vary depending on the length of the
amino acid
side chains, and in one embodiment the lactam is formed by linking the side
chains of
25 a lysine amino acid to a glutamic acid side chain.
The order of the amide bond in the lactam ring can be reversed (e.g., a lactam
.
ring can be formed between the side chains of a Lys12 and a Glul 6 or
alternatively
between a Glu 12 and a Lys16). In accordance with one embodiment a glucagon
analog of SEQ ID NO: 45 is provided wherein at least one lactam ring is formed
30 between the side chains of an amino acid pair selected from the group
consisting of
amino acid pairs 12 and 16, 16 and 20 , 20 and 24 or 24 and 28. In one
embodiment a
.glucagon/GLP-1 receptor co-agonist is provided wherein the co-agonist
comprises a
glucagon peptide analog of SEQ ID NO: 20 wherein the peptide comprises an
intramolecular lactam bridge formed between amino acid positions 12 and 16 or
47
CA 02852177 2014-05-23
=
= WO 2009/155258
PCT/US2009/047438
29920-208831
between amino acid positions 16 and 20. In one embodiment a glucagon/GLP-I
receptor co-agonist is provided comprising the sequence of SEQ ID NO: 20,
wherein
an intramolecular lactam bridge is formed between amino acid positions 12 and
16,
between amino acid positions 16 and 20, or between amino acid positions 20 and
24
5 and the amino acid at position 29 is glycine, wherein the sequence of SEQ
ID NO: 29
is linked to the C-terminal amino acid of SEQ ID NO: 20. In a further
embodiment
the amino acid at position 28 is aspartic acid.
Intramolecular bridges other than a lactam bridge can be used to stabilize the
alpha helix of the glucagon analog peptides. In one embodiment, the
intramolecular
10 bridge is a hydrophobic bridge. In this instance, the intramolecular
bridge optionally
is between the side chains of two amino acids that are part of the hydrophobic
face of
the alpha helix of the glucagon analog peptide. For example, one of the amino
acids
joined by the hydrophobic bridge can be the amino acid at position 10, 14, and
18.
In one specific aspect, olefin metathesis is used to cross-link one or two
turns
15 of the alpha helix of the glucagon peptide using an all-hydrocarbon
cross-linking
system. The glucagon peptide in this instance can comprise a-methylated amino
acids
bearing olefinic side chains of varying length and configured with either R or
S
stereochemistry at the i and i+4 or 1+7 positions. For example, the olefinic
side can
can comprise (CH2)n, wherein n is any integer between 1 to 0. In one
embodiment, n
20 is 3 for a cross-link length of 8 atoms. Suitable methods of forming
such
intramolecular bridges are described in the art. See, for example,
Schafmeister et al.,
J. Am. Chem. Soc. 122: 5891-5892 (2000) and Walensky et al., Science 305: 1466-
1470 (2004). Alternatively, the glucagon peptide can comprise 0-ally1 Ser
residues
located on adjacent helical turns, which are bridged together via ruthenium-
catalyzed
25 ring closing metathesis. Such procedures of cross-linking are described
in, for
example, Blackwell et al., Angew, Chem., Int. Ed. 37: 3281-3284 (1998).
In another specific aspect, use of the unnatural thio-dialanine amino acid,
=
lanthionine, which has been widely adopted as a peptidomimetic of cystine, is
used to
cross-link one turn of the alpha helix. Suitable methods of lanthionine-based
30 cyclization are known in the art. See, for instance, Matteucci et al.,
Tetrahedron
Letters 45: 1399-1401 (2004); Mayer et al., J. Peptide Res. 51: 432-436
(1998);
Polinslcy et al., J. Med. Chem. 35: 4185-4194 (1992); Osapay et al., J. Med.
Chem. 40:
2241-2251 (1997); Fukase et al., Bull. Chem. Soc. Jpn. 65: 2227-2240 (1992);
Harpp
et at., J. Org. Chem. 36: 73-80 (1971); Goodman and Shao, Pure App!. Chem. 68:
48
CA 0 2 852 17 7 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
1303-1308 (1996); and Osapay and Goodman, J. Chem. Soc. Chem. Commun. 1599-
1600 (1993). =
In some embodiments, a, co-diaminoalkane tethers, e.g., 1,4-diaminopropane
and I ,5-diaminopentane) between two Glu residues at positions i and i+7 are
used to
stabilize the alpha helix of the glucagon peptide.. Such tethers lead to the
formation of
a bridge 9-atoms or more in length, depending on the length of the
diaminoalkane
tether. Suitable methods of producing peptides cross-linked with such tethers
are
described in the art. See, for example, Phelan et Am. Chem. Soc. 119: 455-
460
(1997).
In yet another embodiment of the invention, a disulfide bridge is used to
cross-
link one or two turns of the alpha helix of the glucagon peptide.
Alternatively, a
modified disulfide bridge in which one or both sulfur atoms are replaced by a
methylene group resulting in an isosteric macrocyclization is used to
stabilize the
alpha helix of the glucagon peptide. Suitable methods of modifying peptides
with
disulfide bridges or sulfur-based cyclization are described in, for example,
Jackson et
al., J. Am. Chem. Soc. 113: 9391-9392 (1991) and Rudinger and Jost,
Experientia 20:
570-571 (1964).
In yet another embodiment, the alpha helix of the glucagon peptide is
stabilized via the binding of metal atom by two His residues or a His and Cys
pair
positioned at i and i+4. The metal atom can be, for example, Ru(III), Cu(II),
Zn(II),
or Cd(11). Such methods of metal binding-based alpha helix stabilization are
known
in the art. See, for example, Andrews and Tabor, Tetrahedron 55: 11711-11743
(1999); Ghadiri et al., J. Am. Chem. Soc. 112: 1630-1632 (1990); and Ghadiri
et al., J.
Am. Chem. Soc. 119: 9063-9064 (1997).
The alpha helix of the glucagon peptide can alternatively be stabilized
through other means of peptide cyclizing, which means are reviewed in Davies,
J.
Peptide. Sci. 9: 471-501 (2003). The alpha helix can be stabilized via the
formation
of an amide bridge, thioether bridge, thioester bridge, urea bridge, carbamate
bridge,
= sulfonamide bridge, and the like. For example, a thioester bridge can be
formed
between the C-terminus and the side chain of a Cys residue. Alternatively, a
thioester
can be formed via side chains of amino acids having a thiol (Cys) and a
carboxylic
acid (e.g., Asp, Glu). In another method, a cross-linking agent, such as a
dicarboxylic
acid, e.g. suberic acid (octanedioic acid), etc. can introduce a link between
two
49
CA 02 852 177 2 0 14-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
functional groups of an amino acid side chain, such as a free amino, hydroxyl,
thiol
group, and combinations thereof.
In accordance with one embodiment, the alpha helix of the glucagon peptide is
stabilized through the incorporation of hydrophobic amino acids at positions i
and
i+4. For instance, i can be Tyr and i+4 can be either Val or Leu; i can be Phe
and i+4
can be Cys or Met; I can be Cys and 1+4 can be Met; or i can be Phe and i+4
can be
Ile. It should be understood that, for purposes herein, the above amino acid
pairings
can be reversed, such that the indicated amino acid at position i could
alternatively be
located at 1+4, while the i+4 amino acid can be located at the i position.
In accordance with yet another embodiment of the invention, the glucagon
peptide with enhanced GLP-1 activity comprises (a) one or more substitutions
within
amino acid positions 12-29 with an a, a-disubstituted amino acid and
optionally, (b) a
C-terminal amide. In some aspects, it is to be appreciated that such glucagon
peptides
specifically lack an intramolecular bridge, e.g., a covalent intramolecular
bridge, that
stabilizes the alpha-helix in the C-terminal portion of glucagon (around
positions 12-
29). In some embodiments, one, two, three, four or more of positions 16, 17,
18, 19,
20, 21, 24 or 29 of glucagon is substituted with an a, a-disubstituted amino
acid, e.g.,
amino iso-butyric acid (MB), an amino acid disubstituted with the same or a
different
group selected from methyl, ethyl, propyl, and n-butyl, or with a cyclooctane
or
cycloheptane (e.g., 1-aminocyclooctane-l-carboxylic acid). For example,
substitution
of position 16 with AIB enhances GLP-1 activity, in the absence of an
intramolecular
bridge, e.g., a non-covalent intramolecular bridge (e.g., a salt bridge) or a
covalent
intramolecular bridge (e.g., a lactam). In some embodiments, one, two, three
or more
of positions 16, 20, 21 or 24 are substituted with AIB. Such a glucagon
peptide may
further comprise one or more of the other modifications described herein,
including,
but not limited to, acylation, allcylation, pegylation, deletion of 1-2 amino
acids at the
C-terminus, addition of and/or substitution with charged amino acids at the C-
terminus, replacement of the C-terminal carboxylate with an amide, addition of
a C-
terminal extension, and conservative and/or non-conservative amino acid
substitutions, such as substitution of Met at position 27 with Leu or Nle,
substitution
of Asp at position 15 with Glu (or like amino acid), substitution at position
1 and/or 2
with amino acids which achieve DPP-IV protease resistance, substitution of Ser
at
position 2 with Ala, substitution of Tyr at position 10 with Val or Phe,
substitution of
Lys at position 12 with Arg, substitution of Ser at position 16 with Thr or
AIB,
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
substitution of Gin at position 20 and/or 24 with Asp, Glu, or AIB,
substitution of Ser =
at position 16 with Glu or Thr, Arg at position 18 with Ala, Gin at position
20 with
Lys, Asp at position 21 with Glu, and Gin at position 24 with Asn or Cys. In
some
embodiments, the foregoing glucagon peptide comprises a Gin or Gly at position
29
or addition of a C-terminal extension, e.g., GGPSSGAPPPS (SEQ ID NO: 26) C-
terminal to the amino acid at position 28. In a specific aspect, the glucagon
peptide
comprises one or more of an amide group in place of the C-terminal
carboxylate, an
acyl group, e.g., a C16 fatty acid, and a hydrophilic moiety, e.g., a
polyethylene
glycol (PEG).
Also, in another specific aspect, the glucagon peptide comprises the amino
acid sequence of any of SEQ ID NOs: 1-25, 30-64, and 66-555 comprising no more
than ten modifications relative to SEQ ID NO: I and comprising one or more
amino
acid substitutions with AIB at positions 16, 20, 21, and/or 24, wherein the
peptide
lacks an intramolecular bridge, e.g., a covalent intramolecular bridge,
between the
side chains of two amino acids of the peptide. Accordingly, in a more specific
aspect,
the glucagon peptide comprises the amino acid sequence of any of SEQ ID NOs:
556-
561.
In accordance with some embodiments, the glucagon peptide lacking an
intramolecular bridge comprises one or more substitutions within amino acid
positions 12-29 with an a, a-disubstituted amino acid and an acyl or alkyl
group
. covalently attached to the side chain of the amino acid at position 10 of
the glucagon
peptide. In specific embodiments, the acyl or alkyl group is not naturally
occurring
on an amino acid. In certain aspects, the acyl or alkyl group is non-native to
the
amion acid at position 10. In exemplary embodiments, the glucagon peptide
lacking
an intramolecular bridge comprises the amino acid sequence of any of SEQ ID
NOs:
556-561 and an acyl or alkyl group covalently attached to the side chain of
the amino
acid at position 10 of the glucagon peptide. Such acylated or allcylated
glucagon
peptides lacking an intramolecular bridge exhibit enhanced activity at the GLP-
1 and
glucagon receptors as compared to the non-acylated counterpart peptides.
Further
enhancement in activity at the GLP-1 and glucagon reeeptors can be achieved by
the
acylated glucagon peptides lacking an intramolecular bridge by incorporating a
spacer
between the acyl or alkyl group and the side chain of the amino acid at
position 10 of
the peptide. Acylation and allcylation, with or without incorporating spacers,
are
further described herein.
51
CA 02852177 2014-05-23
WO 2009/155258
PCT/U52009/047438
29920-208831
Modification at position 1
In accordance with one embodiment of the invention, the glucagon peptide
= with enhanced GLP-1 activity comprises (a) an amino acid substitution of
His at
position I with a large, aromatic amino acid and (b) an intramolecular bridge
that
stabilizes that alpha-helix in the C-terminal portion of the molecule (e.g.
around
positions 12-29). In a specific embodiment, the amino acid at position I is
Tyr, Phe,
Trp, amino-Phe, nitro-Phe, chloro-Phe, sulfo-Phe, 4-pyridyl-Ala, methyl-Tyr,
or 3-
amino Tyr. In a specific aspect, the intramolecular bridge is between the side
chains
of two amino acids that are separated by three intervening amino acids, i.e.,
between
to the side chains of amino acids i and 1+4. In some embodiments, the
intramolecular
bridge is a lactam bridge. In a more specific embodiment of the invention, the
glucagon peptide comprises a large, aromatic amino acid at position 1 and a
lactam
bridge between the amino acids at positions 16 and 20 of the peptide. Such a
glucagon peptide may further comprise one or more (e.g., two, three, four,
five or
mote) of the other modifications described herein. For example, the glucagon
peptide
can comprise an amide in place of the C-terminal carboxylate. Accordingly, in
one
embodiment, the glucagon peptide comprises that amino acid sequence of SEQ ID
NO: 555.
Acylation
In accordance with one embodiment, the glucagon peptide comprises an acyl
group, e.g., an acyl group which is non-native to a naturally-occurring amino
acid.
The acyl group causes the peptide to have one or more of (i) a prolonged half-
life in
circulation, (ii) a delayed onset of action, (iii) an extended duration of
action, (iv) an
improved resistance to proteases, such as DPP-IV, and (v) increased potency at
the
In accordance with one embodiment, the glucagon peptide is modified to
comprise an acyl group which is attached to the glucagon peptide via an ester,
thioester, or amide linkage for purposes of prolonging half-life in
circulation and/or
52
CA 02852177 2014-05-23
=
WO 2009/155258
PCT/US2009/047438
29920-208831
delaying the onset of and/or extending the duration of action and/or improving
resistance to proteases such as DPP-IV.
. Acylation can be carried out at any position within the glucagon
peptide,
including any of positions 1-29, a position within a C-terminal extension, or
the C-
terminal amino acid, provided that glucagon and/or GLP-1 activity is retained,
if not
enhanced. Nonlimiting examples include positions 5, 7, 10, 11, 12, 13, 14, 16,
17, 18,
19, 20, 21, 24, 27, 28, or 29. In specific embodiments, acylation occurs at
position 10
of the glucagon peptide and the glucagon peptide lacks an intramolecular
bridge, e.g.,
a covalent intramolecular bridge (e.g., a lactam bridge). Such acylated
peptides
lacking an intramolecular bridge demonstrate enhanced activity at the GLP-1
and
glucagon receptors as compared to the corresponding non-acylated peptides
lacking a
covalent intramolecular bridge and in comparison to the corresponding peptides
lacking an intramolecular bridge acylated at a position other than position
10. As
shown herein, acylation at position 10 can even transform a glucagon analog
having
little activity at the glucagon receptor to a glucagon analog having activity
at both the
glucagon and GLP-1 receptors. Accordingly, the position at which acylation
occurs
can alter the overall activity profile of the glucagon analog.
Glucagon peptides may be acylated at the same amino acid position where a
hydrophilic moiety is linked, or at a different amino acid position.
Nonlimiting
examples include acylation at position 10 and pegylation at one or more
positions in
the C-terminal portion of the glucagon peptide, e.g., position 24,28 or 29,
within a C-
terminal extension, or at the C-terminus (e.g., through adding a C-terminal
Cys).
The acyl group can be covalently linked directly to an amino acid of the
glucagon peptide, or indirectly to an amino acid of the glucagon peptide via a
spacer,
wherein the spacer is positioned between the amino acid of the glucagon
peptide and
the acyl group.
= In a specific aspect of the invention, the glucagon peptide is modified
to
comprise an acyl group by direct acylation of an amine, hydroxyl, or thiol of
a side
chain of an amino acid of the glucagon peptide. In some embodiments, the
glucagon
peptide is directly acylated through the side chain amine, hydroxyl, or thiol
of an
amino acid. In some embodiments, acylation is at position 10, 20, 24, or 29.
In this
regard, the acylated glucagon peptide can comprise the amino acid sequence of
SEQ
ID NO: 1, or a modified amino acid sequence thereof comprising one or more of
the
amino acid modifications described herein, with at least one of the amino
acids at
53
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
=
positions 10, 20, 24, and 29 modified to any amino acid comprising a side
chain
amine, hydroxyl, or thiol. In some specific embodiments of the invention, the
direct
acylation of the glucagon peptide occurs through the side chain amine,
hydroxyl, or
thiol of the amino acid at position 10.
In some embodiments, the amino acid comprising a side chain amine is an
amino acid of Formula I:
H2N¨C¨COOH
(CI-)õ
NH2
wherein n = 1 to 4
[Formula I]
In some exemplary embodiments, the amino acid of Formula I, is the amino acid
wherein n is 4 (Lys) or n is 3 (Om).
In other embodiments, the amino acid comprising a side chain hydroxyl is an
amino acid of Formula II:
H2N¨C¨COOH
(CH2),
OH
wherein n = 1 to 4
[Formula II]
In some exemplary embodiments, the amino acid of Formula H is the amino acid
wherein n is 1 (Ser).
In yet other embodiments, the amino acid comprising a side chain thiol is an
amino acid of Formula III:
H2N¨C¨COOH
(CH2)õ
SH
wherein n = 1 to 4
=
[Formula III]
54
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In some exemplary embodiments, the amino acid of Formula III is the amino acid
wherein n is 1 (Cys).
In yet other embodiments, the amino acid comprising a side chain amine,
hydroxyl, or thiol is a disubstituted amino acid comprising the same structure
of
Formula I, Formula II, or Formula III, except that the hydrogen bonded to the
alpha
carbon of the amino acid of Formula I, Formula II, or Formula III is replaced
with a
second side chain.
In one embodiment of the invention, the acylated glucagon peptide comprises -
a spacer between the peptide and the acyl group. In some embodiments, the
glucagon
peptide is covalently bound to the spacer, which is covalently bound to the
acyl group.
In some embodiments, the spacer is an amino acid comprising a side chain
amine, hydroxyl, or thiol, or a dipeptide or tripeptide comprising an amino
acid
comprising a side chain amine, hydroxyl, or thiol. The amino acid to which the
spacer is attached can be any amino acid (e.g., a singly or doubly a-
substituted amino
acid) comprising a moiety which permits linkage to the spacer. For example, an
amino acid comprising a side chain NH2, ¨OH, or ¨COOH (e.g., Lys, Orn, Ser,
Asp,
or Glu) is suitable. In this respect, the acylated glucagon peptide can
comprise the
amino acid sequence of SEQ ID NO: 1, or a modified amino acid sequence thereof
comprising one or more of the amino acid modifications described herein, with
at
least one of the amino acids at positions 10, 20, 24, and 29 modified to any
amino
acid comprising a side chain amine, hydroxyl, or carboxylate.
In some embodiments, the spacer is an amino acid comprising a side chain
amine, hydroxyl, or thiol, or a dipeptide or tripeptide comprising an amino
acid
comprising a side chain amine, hydroxyl, or thiol.
When acylation occurs through an amine group of a spacer, the acylation can
occur through the alpha amine of the amino acid or a side chain amine. In the
instance in which the alpha amine is acylated, the amino acid of the spacer
can be any
amino acid. For example, the amino acid of the spacer can be a hydrophobic
amino
acid, e.g., Gly, Ala, Val, Leu, Ile, Trp, Met, Phe, Tyr, 6-amino hexanoic
acid, 5-
aminovaleric acid, 7-aminoheptanoic acid, and 8-aminooctanoic acid.
Alternatively,
the amino acid of the spacer can be an acidic residue, e.g., Asp and Glu.
In the instance in which the side chain amine of the amino acid of the spacer
is
acylated, the amino acid of the spacer is an amino acid comprising a side
chain amine,
e.g., an amino acid of Formula I (e.g., Lys or Om). In this instance, it is
possible for
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
both the alpha amine and the side chain amine of the amino acid of the spacer
to be
acylated, such that the glucagon peptide is diacylated. Embodiments of the
invention
include such diacylated molecules.
When acylation occurs through a hydroxyl group of a spacer, the amino acid
or one of the amino acids of the dipeptide or tripeptide can be an amino acid
of
Formula H. In a specific exemplary embodiment, the amino acid is Ser.
When acylation occurs through a thiol group of a spacer, the amino acid or
one of the amino acids of the dipeptide or tripeptide can be an amino acid of
Formula
III. In a specific exemplary embodiment, the amino acid is Cys.
In some embodiments, the spacer is a hydrophilic bifunctional spacer. In
certain embodiments, the hydrophilic bifunctional spacer comprises two or more
reactive groups, e.g., an amine, a hydroxyl, a thiol, and a carboxyl group or
any
combinations thereof. In certain embodiments, the hydrophilic bifunctional
spacer
comprises a hydroxyl group and a carboxylate. In other embodiments, the
hydrophilic
bifunctional spacer comprises an amine group and a carboxylate. In other
embodiments, the hydrophilic bifunctional spacer comprises a thiol group and a
carboxylate. In a specific embodiment, the spacer comprises an amino
poly(alkyloxy)carboxylate. In this regard, the spacer can comprise, for
example,
NH2(CH2CH20)n(CH2)mCOOH, wherein m is any integer from 1 to 6 and n is any
integer from 2 to 12, such as, e.g., 8-amino-3,6-dioxaoctanoic acid, which is
commercially available from Peptides International, Inc. (Louisville, KY).
In some embodiments, the spacer is a hydrophobic bifunctional spacer.
Hydrophobic bifunctional spacers are known in the art. See, e.g., Bioconjugate
Techniques, G. T. Hermanson (Academic Press, San Diego, CA, 1996), which is
incorporated by reference in its entirety. In certain embodiments, the
hydrophobic
bifunctional spacer comprises two or more reactive groups, e.g., an amine, a
hydroxyl,
a thiol, and a carboxyl group or any combinations thereof. In certain
embodiments,
the hydrophobic bifunctional spacer comprises a hydroxyl group and a
carboxylate.
In other embodiments, the hydrophobic bifunctional spacer comprises an amine
group
and a carboxylate. In other embodiments, the hydrophobic bifunctional spacer
comprises a thiol group and a carboxylate. Suitable hydrophobic bifunctional
spacers
comprising a carboxylate and a hydroxyl group or a thiol group are known in
the art
and include, for example, 8-hydroxyoctanoic acid and 8-mercaptooctanoic acid.
56
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831 =
In some embodiments, the bifunctional spacer is not a dicarboxylic acid
comprising an unbranched, methylene of 1-7 carbon atoms between the
carboxylate
groups. In some embodiments, the bifunctional spacer is a dicarboxylic acid
comprising an unbranched, methylene of 1-7 carbon atoms between the
carboxylate
groups.
The spacer (e.g., amino acid, dipeptide, tripeptide, hydrophilic bifunctional
spacer, or hydrophobic bifunctional spacer) in specific embodiments is 3 to 10
atoms
(e.g., 6 to 10 atoms, (e.g., 6, 7, 8, 9, or 10 atoms) in length. In more
specific.
embodiments, the spacer is about 3 to 10 atoms (e.g., 6 to 10 atoms) in length
and the
to acyl group is a Cl2 to C18 fatty acyl group, e.g., C14 fatty acyl group,
C16 fatty acyl
group, such that the total length of the spacer and acyl group is 14 to 28
atoms, e.g.,
about 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, or 28 atoms. In
some
embodiments, the length of the spacer and acyl group is 17 to 28 (e.g., 19 to
26, 19 to
21) atoms.
In accordance with certain foregoing embodiments, the bifunctional spacer can
be a synthetic or naturally occurring amino acid (including, but not limited
to, any of
those described herein) comprising an amino acid backbone that is 3 to 10
atoms in
length (e.g., 6-amino hexanoic acid, 5-aminovaleric acid, 7-aminoheptanoic
acid, and
8-aminooctanoic acid). Alternatively, the spacer can be a dipeptide or
tripeptide
spacer having a peptide backbone that is 3 to 10 atoms (e.g., 6 to 10 atoms)
in length.
Each amino acid of the dipeptide or tripeptide spacer can be the same as or
different
from the other amino acid(s) of the dipeptide or tripeptide and can be
independently
selected from the group consisting of: naturally-occurring and/or non-
naturally
occurring amino acids, including, for example, any of the D or L isomers of
the
naturally-occurring amino acids (Ala, Cys, Asp, Glu, Phe, Gly, His, Ile, Lys,
Leu,
Met, Asn, Pro, Arg, Ser, Thr, Val, Tip, Tyr), or any D or L isomers of the non-
naturally occurring amino acids selected from the group consisting of: 13-
alanine (0-
Ala), N-a-methyl-alanine (Me-Ala), aminobutyric acid (Abu), y-aminobutyric
acid (y-
Abu), aminohexanoic acid (a-Ahx), aminoisobutyric acid (Aib),
aminomethylpyrrole
carboxylic acid, aminopiperidinecarboxylic acid, aminoserine (Ams),
aminotetrahydropyran-4-carboxylic acid, arginine N-methoxy-N-methyl amide, 13-
aspartic acid (13-Asp), azetidine carboxylic acid, 3-(2-
benzothiazolyl)alanine, a-tert-
butylglycine, 2-amino-5-ureido-n-valeric acid (citrulline, Cit),13-
Cyclohexylalanine
(Cha), acetamidomethyl-cysteine, diaminobutanoic acid (Dab), diaminopropionic
acid
57
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
(Dpr), dihydroxyphenylalanine (DOPA), dimethylthiazolidine (DMTA), y-Glutamic
acid (y-Glu), homoserine (Hse), hydroxyproline (Hyp), isoleucine N-methoxy-N-
methyl amide, methyl-isoleucine (Melle), isonipecotic acid (Isn), methyl-
leucine
(MeLeu), methyl-lysine, dimethyl-lysine, trimethyl-lysine, methanoproline,
methionine-sulfoxide (Met(0)), methionine-sulfone (Met(02)), norleucine (Nle),
methyl-norleucine (Me-Nle), norvaline (Nva), ornithine (Om), para-aminobenzoic
acid (PABA), penicillamine (Pen), methylphenylalanine (MePhe), 4-
Chlorophenylalanine (Phe(4-C1)), 4-fluorophenylalanine (Phe(4-F)), 4-
ni trophenylalanine (Phe(4-NO2)), 4-cyanophenylalanine ((Phe(4-CN)),
phenylglycine
(Phg), piperidinylalanine, piperidinylglycine, 3,4-dehydroproline,
pyrrolidinylalanine,
sarcosine (Sar), selenocysteine (Sec), O-Benzyl-phosphoserine, 4-amino-3-
hydroxy-
.
6-methylheptanoic acid (Sta), 4-amino-5-cyclohexy1-3-hydroxypentanoic acid
(ACHPA), 4-amino-3-hydroxy-5-phenylpentanoic acid (AHPPA), 1,2,3,4,-tetrahydro-
isoquinoline-3-carboxylic acid (Tic), tetrahydropyranglycine, thienylalanine
(Thi) , 0-
benzyl-phosphotyrosine, 0-Phosphotyrosine, methoxytyrosine, ethoxytyrosine, 0-
(bis-dimethylamino-phosphono)-tyrosine, tyrosine sulfate tetrabutylamine,
methyl-
valine (MeVal), and alkylated 3-mercaptopropionic acid.
In some embodiments, the spacer comprises an overall negative charge, e.g.,
comprises one or two negatively charged amino acids. In some embodiments, the
dipeptide is not any of the dipeptides of general structure A-B, wherein A is
selected
from the group consisting of Gly, Gin, Ala, Arg, Asp, Asn, Ile, Leu, Val, Phe,
and
Pro, wherein B is selected from the group consisting of Lys, His, Trp. In some
embodiments, the dipeptide spacer is selected from the group consisting of:
Ala-Ala,
3-Ala- P-Ala, Leu-Leu, Pro-Pro, y-aminobutyric acid- y-aminobutyric acid, and
y-
Glu- y-Glu.
In some exemplary embodiments, the glucagon peptide is modified to
comprise an acyl group by acylation of an amine, hydroxyl, or thiol of a
spacer, which
spacer is attached to a side chain of an amino acid at position 10, 20, 24, or
29, or at
the C-terminal amino acid of the glucagon peptide.
In yet more specific embodiments, the acyl group is attached to the amino acid
at position 10 of the glucagon peptide and the length of the spacer and acyl
group is
14 to 28 atoms. The amino acid at position 10, in some aspects, is an amino
acid of
Formula I, e.g., Lys, or a disubstituted amino acid related to Formula I. In
more
specific embodiments, the glucagon peptide lacks an intramolecular bridge,
e.g., a
58
CA 02852177 2014-05-23
WO 2009/155258
PCT/U52009/047438
29920-208831
covalent intramolecular bridge. The glucagon peptide, for example, can be a
peptide
comprising one or more alpha, alpha-disubstituted amino acids, e.g., AIB, for
stabilizing the alpha helix of the peptide. Accordingly, the acylated glucagon
peptide
can comprise the amino acid sequence of any of SEQ ID NOs: 555-561 and 610-
612,
the AIB-containing peptides of Tables 20 and 28. As shown herein, such
peptides
comprising an acylated spacer covalently attached to the side chain of the
amino acid
at position 10 exhibit enhanced potency at both the GLP-1 and glucagon
receptors.
Suitable methods of peptide acYlation via amines, hydroxyls, and thiols are
known in the art. See, for example, Example 19 (for methods of acylating
through an
amine), Miller, Biochem Biophys Res Commun 218: 377-382 (1996); Shimohigashi
and Stammer, Int J Pept Protein Res 19: 54-62 (1982); and Previero et al.,
Biochim
Biophys Acta 263: 7-13 (1972) (for methods of acylating through a hydroxyl);
and
San and Silvius, J Pept Res 66: 169-180 (2005) (for methods of acylating
through a
thiol); Bioconjugate Chem. "Chemical Modifications of Proteins: History and
Applications" pages 1, 2-12 (1990); Hashimoto et al., Pharmacuetical Res.
"Synthesis
of Palmitoyl Derivatives of Insulin and their Biological Activity" Vol. 6, No:
2 =
pp.171-176 (1989).
The acyl group of the acylated glucagon peptide can be of any size, e.g., any
length carbon chain, and can be linear or branched. In some specific
embodiments of
the invention, the acyl group is a C4 to C30 fatty acid. For example, the acyl
group
can be any of a C4 fatty acid, C6 fatty acid, C8 fatty acid; CIO fatty acid,
C12 fatty
acid, C14 fatty acid, C16 fatty acid, C18 fatty acid, C20 fatty acid, C22
fatty acid,
C24 fatty acid, C26 fatty acid, C28 fatty acid, or a C30 fatty acid. In some
embodiments, the acyl group is a C8 to C20 fatty acid, e.g., a C14 fatty acid
or a C16
fatty acid.
In an alternative embodiment, the acyl group is a bile acid. The bile acid can
be any suitable bile acid, including, but not limited to, cholic acid,
chenodeoxycholic
acid, deoxycholic acid, lithocholic acid, taurocholic acid, glycocholic acid,
and
cholesterol acid.
=
In some embodiments of the invention, the glucagon peptide is modified to
comprise an acyl group by acylation of a long chain alkane by the glucagon
peptide.
In specific aspects, the long chain alkane comprises an amine, hydroxyl, or
thiol
group (e.g. octadecylamine, tetradecanol, and hexadecanethiol) which reacts
with a
carboxyl group, or activated form thereof, of the glucagon peptide. The
carboxyl =
59
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
group, or activated form thereof, of the glucagon peptide can be part of a
side chain of
an amino acid (e.g., glutamic acid, aspartic acid) of the glucagon peptide or
can be
part of the peptide backbone.
In certain embodiments, the glucagon peptide is modified to comprise an acyl
group by acylation of the long chain alkane by a spacer which is attached to
the
glucagon peptide. In specific aspects, the long chain alkane comprises an
amine,
hydroxyl, or thiol group which reacts with a carboxyl group, or activated form
thereof, of the spacer. Suitable spacers comprising a carboxyl group, or
activated
form thereof, are described herein and include, for example, bifunctional
spacers, e.g.,
As used herein, the term "activated form of a carboxyl group" refers to a
carboxyl group with the general formula R(C=0)X, wherein X is a leaving group
and
R is the glucagon peptide or the spacer. For example, activated forms of a
carboxyl
With regard to these aspects of the invention, in which a long chain alkane is
acylated by the glucagon peptide or the spacer, the long chain alkane may be
of any
= CIO alkane, C12 alkane, C14 alkane, C16 alkane, C18 alkane, C20 alkane,
C22
alkane, C24 alkane, C26 alkane, C28 alkane, or a C30 alkane. In some
embodiments,
Also, in some embodiments, an amine, hydroxyl, or thiol group of the
glucagon peptide is acylated with a cholesterol acid. In a specific
embodiment, the
glucagon peptide is linked to the cholesterol acid through an alkylated des-
amino Cys
= glycol moiety. In one embodiment, the glucagon peptide comprises the
structure:
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
0
HN,S
--"bi H
0 0
The acylated glucagon peptides described herein can be further modified to
comprise a hydrophilic moiety. In some specific embodiments the hydrophilic
moiety
can comprise a polyethylene glycol (PEG) chain. The incorporation of a
hydrophilic
moiety can be accomplished through any suitable means, such as any of the
methods
described herein. In this regard, the acylated glucagon peptide can comprise
SEQ ID
NO: 1, including any of the modifications described herein, in which at least
one of
the amino acids at position 10, 20, 24, and 29 comprise an acyl group and at
least one
of the amino acids at position 16, 17, 21, 24, or 29, a position within a C-
terminal
extension, or the C-terminal amino acid are modified to a Cys, Lys, Om, homo-
Cys,
or Ac-Phe, and the side chain of the amino acid is covalently bonded to a
hydrophilic
moiety (e.g., PEG). In some embodiments, the acyl group is attached to
position 10,
optionally via a spacer comprising Cys, Lys, Om, homo-Cys, or Ac-Phe, and the
hydrophilic moiety is incorporated at a Cys residue at position 24.
Alternatively, the acylated glucagon peptide can comprise a spacer, wherein
the spacer is both acylated and modified to comprise the hydrophilic moiety.
Nonlimiting examples of suitable spacers include a spacer comprising one or
more
amino acids selected from the group consisting of Cys, Lys, Om, homo-Cys, and
Ac-
Phe.
In a specific aspect of the invention, the acylated glucagon peptide comprises
the amino acid sequence of any of SEQ ID NOs: 534-544 and 546-549.
Alkylation
In accordance with some embodiments, the glucagon peptide is modified to
comprise an alkyl group, e.g., an alkyl group which is not naturally-occuring
on an
amino acid (e.g., an alkyl group which is non-native to a naturally-occurring
amino
acid). Without being held to any particular theory, it is believed that
alkylation of
61
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
glucagon peptides will achieve similar, if not the same, effects as acylation
of the
glucagon peptides, e.g., a prolonged half-life in circulation, a delayed onset
of action,
an extended duration of action, an improved resistance to proteases, such as
DPP-IV,
and increased potency at the GLP-1 and glucagon receptors.
Allcylation can be carried out at any positions within the glucagon peptide,
including any of positions 1-29, a position within a C-terminal extension, or
the C-
terminal amino acid, provided that the glucagon activity is retained.
Nonlimiting
examples include positions 5, 7, 10, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21,
24, 27, 28,
or 29. The alkyl group can be covalently linked directly to an amino acid of
the
Nonlimiting examples include alkylation at position 10 and pegylation at one
or more
In a specific aspect of the invention, the glucagon peptide is modified to
comprise an alkyl group by direct alkylation of an amine, hydroxyl, or thiol
of a side
embodiments of the invention, the direct alkylation of the glucagon peptide
occurs
through the side chain amine, hydroxyl, or thiol of the amino acid at position
10.
In some embodiments, the amino acid comprising a side chain amine is an
amino acid of Formula I. In some exemplary embodiments, the amino acid of
In other embodiments, the amino acid comprising a side chain hydroxyl is an
amino acid of Formula H. In some exemplary embodiments, the amino acid of
Formula H is the amino acid wherein n is I (Ser).
62
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In yet other embodiments, the amino acid comprising a side chain thiol is an
amino acid of Formula III. In some exemplary embodiments, the amino acid of
Formula III is the amino acid wherein n is 1 (Cys).
In yet other embodiments, the amino acid comprising a side chain amine,
hydroxyl, or thiol is a disubstituted amino acid comprising the same structure
of
Formula I, Formula II, or Formula III, except that the hydrogen bonded to the
alpha
carbon of the amino acid of Formula I, Formula II, or Formula III is replaced
with a
second side chain.
In one embodiment of the invention, the alkylated glucagon peptide comprises
a spacer between the peptide and the alkyl group. In some embodiments, the
glucagon peptide is covalently bound to the spacer, which is covalently bound
to the
= alkyl group. In some exemplary embodiments, the glucagon peptide is
modified to
comprise an alkyl group by alkylation of an amine, hydroxyl, or thiol of a
spacer,
which spacer is attached to a side chain of an amino acid at position 10, 20,
24, or 29
of the glucagon peptide. The amino acid to which the spacer is attached can be
any
amino acid comprising a moiety which permits linkage to the spacer. For
example, an
amino acid comprising a side chain NH2, ¨OH, or ¨COOH (e.g., Lys, Orn, Ser,
Asp,
or Glu) is suitable. In this respect, the alkylated glucagon peptide can
comprise the
amino acid sequence of SEQ ID NO: 1, or a modified amino acid sequence thereof
comprising one or more of the amino acid modifications described herein, with
at
least one of the amino acids at positions 10, 20, 24, and 29 modified to any
amino
acid comprising a side chain amine, hydroxyl, or carboxylate.
In some embodiments, the spacer is an amino acid comprising a side chain
amine, hydroxyl, or thiol or a dipeptide or tripeptide comprising an amino
acid
comprising a side chain amine, hydroxyl, or thiol.
When alkylation occurs through an amine group of a spacer, the alkylation can
occur through the alpha amine of an amino acid or a side chain amine. In the
instance
in which the alpha amine is alkylated, the amino acid of the spacer can be any
amino
acid. For example, the amino acid of the spacer can be a hydrophobic amino
acid,
e.g., Gly, Ala, Val, Leu, Ile, Trp, Met, Phe, Tyr, 6-amino hexanoic acid, 5-
aminovaleric acid, 7-aminoheptanoic acid, and 8-aminooctanoic acid.
Alternatively,
the amino acid of the spacer can be an acidic residue, e.g., Asp and Glu,
provided that
the alkylation occurs on the alpha amine of the acidic residue. In the
instance in
which the side chain amine of the amino acid of the spacer is alkylated, the
amino
63
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
acid of the spacer is an amino acid comprising a side chain amine, e.g., an
amino acid
of Formula I (e.g., Lys or Om). In this instance, it is possible for both the
alpha
amine and the side chain amine of the amino acid of the spacer to be
allcylated, such
that the glucagon peptide is diallcylated. Embodiments of the invention
include such
diallcylated molecules.
When allcylation occurs through a hydroxyl group of a spacer, the amino acid
or one of the amino acids of the dipeptide or tripeptide can be an amino acid
of
Formula II. In a specific exemplary embodiment, the amino acid is Ser.
When allcylation occurs through a thiol group of spacer, the amino acid or one
of the amino acids of the dipeptide or tripeptide can be an amino acid of
Formula III.
In a specific exemplary embodiment, the amino acid is Cys.
In some embodiments, the spacer is a hydrophilic bifunctional spacer. In
certain embodiments, the hydrophilic bifunctional spacer comprises two or more
reactive groups, e.g., an amine, a hydroxyl, a thiol, and a carboxyl group or
any
combinations thereof. In certain embodiments, the hydrophilic bifunctional
spacer is
comprises a hydroxyl group and a carboxylate. In other embodiments, the
hydrophilic
bifunctional spacer comprises an amine group and a carboxylate. In other
embodiments, the hydrophilic bifunctional spacer comprises a thiol group and a
carboxylate. In a specific embodiment, the spacer comprises an amino
poly(alkyloxy)carboxylate. In this regard, the spacer can comprise, for
example,
NH2(CH2CH20).(CH2)õõCOOH, wherein m is any integer from 1 to 6 and n is any
integer from 2 to 12, such as, e.g., 8-amino-3,6-dioxaoctanoic acid, which is
commercially available from Peptides International, Inc. (Louisville, KY).
In some embodiments, the spacer is a hydrophobic bifunctional spacer. In
certain embodiments, the hydrophobic bifunctional spacer comprises two or more
reactive groups, e.g., an amine, a hydroxyl, a thiol, and a carboxyl group or
any
combinations thereof. In certain embodiments, the hydrophobic bifunctional
spacer
comprises a hydroxyl group and a carboxylate. In other embodiments, the
hydropholic bifunctional spacer comprises an amine group and a carboxylate. In
other embodiments, the hydropholic bifunctional spacer comprises a thiol group
and a
carboxylate. Suitable hydrophobic bifunctional spacers comprising a
carboxylate and
a hydroxyl group or a thiol group are known in the art and include, for
example, 8-
hydroxyoctanoic acid and 8-mercaptooctanoic acid.
64
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
The spacer (e.g., amino acid, dipeptide, tripeptide, hydrophilic bifunctional
spacer, or hydrophobic bifunctional spacer) in specific embodiments is 3 to 10
atoms
(e.g., 6 to 10 atoms, (e.g., 6, 7, 8, 9, or 10 atoms)) in length. In more
specific
embodiments; the spacer is about 3 to 10 atoms (e.g., 6 to 10 atoms) in length
and the
alkyl is a C12 to C18 alkyl group, e.g., C14 alkyl group, C16 alkyl group,
such that
the total length of the spacer and alkyl group is 14 to 28 atoms, e.g., about
14, 15, 16,
17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, or 28 atoms. In some embodiments,
the
length of the spacer and alkyl is 17 to 28 (e.g., 19 to 26, 19 to 21) atoms.
In accordance with certain foregoing embodiments, the bifunctional spacer can
be a synthetic or non-naturally occurring amino acid comprising an amino acid
backbone that is 3 to 10 atoms in length (e.g., 6-amino hexanoic acid, 5-
aminovaleric
acid, 7-aminoheptanoic acid, and 8-aminooctanoic acid). Alternatively, the
spacer
can be a dipeptide or tripeptide spacer having a peptide backbone that is 3 to
10 atoms
(e.g., 6 to 10 atoms) in length. The dipeptide or tripeptide spacer can be
composed of
naturally-occurring and/or non-naturally occurring amino acids, including, for
example, any of the amino acids taught herein. In some embodiments, the spacer
comprises an overall negative charge, e.g., comprises one or two negatively
charged
amino acids. In some embodiments, the dipeptide spacer is selected from the
group
consisting of: Ala-Ala, 13-Ala- 13-A1a, Leu-Leu, Pro-Pro, y-aminobutyric acid-
y-
aminobutyric acid, and y-Glu- y-Glu.
Suitable methods of peptide allcylation via amines, hydroxyls, and thiols are
known in the art. For example, a Williamson ether synthesis can be used to
form an
ether linkage between a hydroxyl group of the glucagon peptide and the alkyl
group.
Also, a nucleophilic substitution reaction of the peptide with an alkyl halide
can result
in any of an ether, thioether, or amino linkage.
The alkyl group of the allcylated glucagon peptide can be of any size, e.g.,
any
length carbon chain, and can be linear or branched. In some embodiments of the
invention, the alkyl group is a C4 to C30 alkyl. For example, the alkyl group
can be
any of a C4 alkyl, C6 alkyl, C8 alkyl, CIO alkyl, C12 alkyl, C14 alkyl, C16
alkyl, C18
alkyl, C20 alkyl, C22 alkyl, C24 alkyl, C26 alkyl, C28 alkyl, or a C30 alkyl.
In some
embodiments, the alkyl group is a C8 to C20 alkyl, e.g., a C14 alkyl or a C16
alkyl.
In some specific embodiments, the alkyl group comprises a steroid moiety of a
bile acid, e.g., cholic acid, chenodeoxycholic acid, deoxycholic acid,
lithocholic acid,
taurocholic acid, glycocholic acid, and cholesterol acid.
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In some embodiments of the invention, the glucagon peptide is modified to
comprise an alkyl group by reacting a nucleophilic, long chain alkane with the
glucagon peptide, wherein the glucagon peptide comprises a leaving group
suitable
for nucleophilic substitution. In specific aspects, the nucleophilic group of
the long
chain alkane comprises an amine, hydroxyl, or thiol group (e.g.
octadecylamine,
tetradecanol, and hexadecanethiol). The leaving group of the glucagon peptide
can be
part of a side chain of an amino acid or can be part of the peptide backbone.
Suitable
leaving groups include, for example, N-hydroxysuccinimide, halogens, and
sulfonate
esters.
In certain embodiments, the glucagon peptide is modified to comprise an alkyl
group by reacting the nucleophilic, long chain alkane with a spacer which is
attached
to the glucagon peptide, wherein the spacer comprises the leaving group. In
specific
aspects, the long chain alkane comprises an amine, hydroxyl, or thiol group.
In
certain embodiments, the spacer comprising the leaving group can be any spacer
discussed herein, e.g., amino acids, dipeptides, tripeptides, hydrophilic
bifunctional
spacers and hydrophobic bifunctional spacers further comprising a suitable
leaving
group. =
With regard to these aspects of the invention, in which a long chain alkane is
alkylated by the glucagon peptide or the spacer, the long chain alkane may be
of any
size and can comprise any length of carbon chain. The long chain alkane can be
linear or branched. In certain aspects, the long chain alkane is a C4 to C30
alkane.
For example, the long chain alkane can be any of a C4 alkane, C6 alkane, C8
alkane,
CIO alkane, C12 alkane, C14 alkane, C16 alkane, C18 alkane, C20 alkane, C22
alkane, C24 alkane, C26 alkane, C28 alkane, or a C30 alkane. In some
embodiments,
the long chain alkane comprises a C8 to C20 alkane, e.g., a C14 alkane, C16
alkane,
or a C18 alkane.
Also, in some embodiments, allcylation can occur between the glucagon
peptide and a cholesterol moiety. For example, the hydroxyl group of
cholesterol can
displace a leaving group on the long chain alkane to form a cholesterol-
glucagon -
peptide product.
The alkylated glucagon peptides described herein can be further modified to
comprise a hydrophilic moiety. In some specific embodiments the hydrophilic
moiety
can comprise a polyethylene glycol (PEG) chain. The incorporation of a
hydrophilic
moiety can be accomplished through any suitable means, such as any of the
methods
66
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
described herein. In this regard, the allcylated glucagon peptide can comprise
SEQ ID
NO: 1 or a modified amino acid sequence thereof comprising one or more of the
amino acid modifications described herein, in which at least one of the amino
acids at
. position 10,20, 24, and 29 comprise an alkyl group and at least one of
the amino
acids at position 16, 17, 21, 24, and 29, a position within a C-terminal
extension or the
C-terminal amino acid are modified to a Cys, Lys, Om, homo-Cys, or Ac-Phe, and
the
side chain of the amino acid is covalently bonded to a hydrophilic moiety
(e.g., PEG).
In some embodiments, the alkyl group is attached to position 10, optionally
via a
spacer comprising Cys, Lys, Om, homo-Cys, or Ac-Phe, and the hydrophilic
moiety
to is incorporated at a Cys residue at position 24.
Alternatively, the allcylated glucagon peptide can comprise a spacer, wherein
the spacer is both allcylated and modified to comprise the hydrophilic moiety.
Nonlimiting examples of suitable spacers include a spacer comprising one or
more
amino acids selected from the group consisting of Cys, Lys, Om, homo-Cys, and
Ac-
Phe.
C-terminal truncation =
In some embodiments, the glucagon peptides described herein are further
modified by truncation or deletion of one or two amino acids of the C-terminus
of the
glucagon peptide (i.e., position 29 and/or 28) without affecting activity
and/or potency
at the glucagon and GLP-1 receptors. In this regard, the glucagon peptide can
comprise amino acids 1-27 or 1-28 of the native glucagon peptide (SEQ ID NO:
1),
optionally with one or more modifications described herein.
In one embodiment, the truncated glucagon agonist peptide comprises SEQ ID
NO: 550 or SEQ ID NO: 551. In another embodiment, the truncated glucagon
agonist
peptide comprises SEQ ID NO: 552 or SEQ ID NO: 553.
Charged C-terminal residues
The solubility of the glucagon peptide of SEQ ID NO: 20 can be further
improved, for example, by introducing one, two, three or more charged amino
acid(s)
to the C-terminal portion of glucagon peptide of SEQ ID NO: 20, preferably at
a
position C-terminal to position 27. Such a charged amino acid can be
introduced by
substituting a native amino acid with a charged amino acid, e.g. at positions
28 or 29,
or alternatively by adding a charged amino acid, e.g. after position 27, 28 or
29. In
exemplary embodiments, one, two, three or all of the charged amino acids are
67
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
negatively charged. Alternatively, solubility can also be enhanced by
covalently
linking hydrophilic moieties, such as polyethylene glycol, to the peptide.
Exemplary Embodiments
In accordance with one embodiment, a glucagon analog is provided
comprising the sequence of SEQ ID NO: 55, wherein said analog differs from SEQ
ID NO: 55 by Ito 3 amino acids, selected from positions 1, 2, 3, 5, 7, 10, 11,
13, 14,
17, 18, 19, 21, 24, 27, 28, and 29, wherein said glucagon peptide exhibits at
least 20%
of the activity of native GLP-1 at the GLP- I receptor.
In accordance with one embodiment a glucagon/GLP-1 receptor co-agonist is
in provided comprising the sequence:
= NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Xaa-Xaa-Arg-
Arg-Ala-Xaa-Asp-Phe-Val-Xaa-Trp-Leu-Met-Xaa-Xaa-R (SEQ ID NO: 33) wherein
the Xaa at position 15 is selected from the group of amino acids consisting of
Asp,
Glu, cysteic acid, homoglutamic acid and homocysteic acid, Xaa at position 16
is
selected from the group of amino acids consisting of Ser, Glu, Gln,
homoglutamic
acid and homocysteic acid, the Xaa at position 20 is Gin or Lys, the Xaa at
position
24 is Gin or Glu, the Xaa at position 28 is Asn, Lys or an acidic amino acid,
the Xaa
at position 29 is Thr, Gly or an acidic amino acid, and R is COOH or CONH2,
with
the proviso that when position 16 is serine, position 20 is Lys, or
alternatively when
position 16 is serine the position 24 is Glu and either position 20 or
position 28 is Lys.
In one embodiment the glucagon/GLP-1 receptor co-agonist comprises the
sequence
of SEQ ID NO: 33 wherein the amino acid at position 28 is aspartic acid and
the
amino acid at position 29 is glutamic acid. In another embodiment the amino
acid at
position 28 is the native asparagine, the amino acid at position 29 is glycine
and the
amino acid sequence of SEQ ID NO: 29 or SEQ ID NO: 65 is covalently linked to
the
carboxy terminus of SEQ ID NO: 33.
In one embodiment a co-agonist is provided comprising the sequence of SEQ
ID NO: 33 wherein an additional acidic amino acid added to the carboxy
terminus of
the peptide. In a further embodiment the carboxy terminal amino acid of the
glucagon
analog has an amide in place of the carboxylic acid group of the natural amino
acid.
In one embodiment the glucagon analog comprises a sequence selected from the
group consisting of SEQ ID NO: 40, SEQ ID NO: 41, SEQ ID NO: 42, SEQ ID NO:
43 and SEQ ID NO: 44.
68
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In accordance with one embodiment a glucagon peptide analog of SEQ ID
NO: 33 is provided, wherein said analog differs from SEQ ID NO: 33 by 1 to 3
amino
acids, selected from positions 1,2, 3, 5, 7, 10, 11, 13, 14, 17, 18, 19, 21
and 27, with
the proviso that when the amino acid at position 16 is serine, either position
20 is
lysine, or a lactam bridge is formed between the amino acid at position 24 and
either
the amino acid at position 20 or position 28. In accordance with one
embodiment the
analog differs from SEQ ID NO: 33 by 1 to 3 amino acids selected from
positions 1,
2, 3, 21 and 27. In one embodiment the glucagon peptide analog of SEQ ID NO:
33
differs from that sequence by 1 to 2 amino acids, or in one embodiment by a
single
to amino acid, selected form positions 1, 2, 3, 5, 7, 10, 11, 13, 14, 17,
18, 19, 21 and 27,
with the proviso that when the amino acid at position 16 is serine, either
position 20 is
lysine, or a lactam bridge is formed between the amino acid at position 24 and
either
the amino acid at position 20 or position 28.
. In accordance with another embodiment a relatively selective GLP-1 receptor
agonist is provided comprising the sequence NH2-His-Ser-Xaa-Gly-Thr-Phe- Thr-
Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Xaa-Xaa-Arg-Arg-Ala-Xaa-Asp-Phe-Val-Xaa-Trp-
Leu-Met-Xaa-Xaa-R (SEQ ID NO: 53) wherein the Xaa at position 3 is selected
from
the group of amino acids consisting of Glu, Om or Nle, the Xaa at position 15
is
selected from the group of amino acids consisting of Asp, Glu, cysteic acid,
homoglutamic acid and homocysteic acid, Xaa at position 16 is selected from
the
group of amino acids consisting of Ser, Glu, Gin, homoglutamic acid and
homocysteic acid, the Xaa at position 20 is Gin or Lys, the Xaa at position 24
is Gin
or Glu, the Xaa at position 28 is Asn, Lys or an acidic amino acid, the Xaa at
position
29 is Thr, Gly or an acidic amino acid, and R is COOH, CONH2, SEQ ID NO: 26 or
SEQ ID NO: 29, with the proviso that when position 16 is serine, position 20
is Lys,
or alternatively when position 16 is serine the position 24 is Glu and either
position 20
or position 28 is Lys. In one embodiment the amino acid at position 3 is
glutamic
acid. In one embodiment the acidic amino acid substituted at position 28
and/or 29 is
aspartic acid or glutamic acid. In one embodiment the glucagon peptide,
including a
co-agonist peptide, comprises the sequence of SEQ ID NO: 33 further comprising
an
additional acidic amino acid added to the carboxy terminus of the peptide. In
a
further embodiment the carboxy terminal amino acid of the glucagon analog has
an
amide in place of the carboxylic acid group of the natural amino acid.
69
CA 02852177 2014-05-23
WO 2009/155258
PCT/1JS2009/047,138
29920-208831
In accordance with one embodiment a glucagon/GLP-1 receptor co-agonist is
provided comprising a modified glucagon peptide selected from the group
consisting
of:
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Xaa-Xaa-Arg-
Arg-Ala-Xaa-Asp-Phe-Val-Xaa-Trp-Leu-Met-Xaa-Xaa-R (SEQ ID NO: 34), wherein
the Xaa at position 15 is selected from the group of amino acids consisting of
Asp,
Glu, cysteic acid, homoglutamic acid and homocysteic acid, Xaa at position 16
is
selected from the group of amino acids consisting of Ser, Glu, Gin,
homoglutamic
acid and homocysteic acid, the Xaa at position 20 is Gin or Lys, the Xaa at
position
to 24 is Gin or Glu and the Xaa at position 28 is Asn, Asp or Lys, R is
COOH or
CONH2, the Xaa at position 29 is Thr or Gly, and R is COOH, CONH2, SEQ ID NO:
26 or SEQ ID NO: 29, with the proviso that when position 16 is serine,
position 20 is
Lys, or alternatively when position 16 is serine the position 24 is Glu and
either
position 20 or position 28 is Lys. In one embodiment R is CONH2, the Xaa at
position 15 is Asp, the Xaa at position 16 is selected from the group of amino
acids
consisting of Glu, Gin, homoglutamic acid and homocysteic acid, the Xaas at
positions 20 and 24 are each Gin the Xaa at position 28 is Asn or Asp and the
Xaa at
position 29 is Thr. In one embodiment the Xaas at positions 15 and 16 are each
Glu,
the Xaas at positions 20 and 24 are each Gin, the Xaa at position 28 is Asn or
Asp, the
Xaa at position 29 is Thr and R is CONH2.
It has been reported that certain positions of the native glucagon peptide can
be modified while retaining at least some of the activity of the parent
peptide.
Accordingly, applicants anticipate that one or more of the amino acids located
at
positions at positions 2, 5, 7, 10, 11, 12, 13, 14, 17, 18, 19, 20, 21, 24,
27,28 or 29 of
the peptide of SEQ ID NO: II can be substituted with an amino acid different
from
that present in the native glucagon peptide, and still retain activity at the
glucagon
receptor. In one embodiment the methionine residue present at position 27 of
the
native peptide is changed to leucine or norleucine to prevent oxidative
degradation of
the peptide. In another embodiment the amino acid at position 20 is
substituted with
Lys, Arg, Om or Citrullene and/or position 21 is substituted with Glu,
homoglutamic
acid or homocysteic acid.
In one embodiment a glucagon analog of SEQ ID NO: 20 is provided wherein
Ito 6 amino acids, selected from positions 1, 2, 5, 7, 10, 11, 13, 14, 17, 18,
19, 21, 27,
28 or 29 of the analog differ from the corresponding amino acid of SEQ ID NO:
1,
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
with the proviso that when the amino acid at position 16 is serine, position
20 is Lys,
or alternatively when position 16 is serine the position 24 is Glu and either
position 20
or position 28 is Lys. In accordance with another embodiment a glucagon analog
of
SEQ ID NO: 20 is provided wherein 1 to 3 amino acids selected from positions
1, 2,
5, 7, 10, 11, 13, 14, 17, 18, 19, 20, 21, 27, 28 or 29 of the analog differ
from the
corresponding amino acid of SEQ ID NO: 1. In another embodiment, a glucagon
analog of SEQ ID NO: 8, SEQ ID NO: 9 or SEQ ID NO: 11 is provided.wherein 1 to
2 amino acids selected from positions 1,2, 5, 7, 10, 11, 13, 14, 17, 18, 19,20
or 21 of
the analog differ from the corresponding amino acid of SEQ ID NO: 1, and in a
further embodiment the one to two differing amino acids represent conservative
amino acid substitutions relative to the amino acid present in the native
glucagon
sequence (SEQ ID NO: 1). In one embodiment a glucagon peptide of SEQ ID NO:
12, SEQ ID NO: 13, SEQ ID NO: 14 or SEQ ID NO: 15 is provided wherein the
glucagon peptide further comprises one, two or three amino acid substitutions
at
positions selected from positions 2, 5, 7, 10, 11, 13, 14, 17, 18, 19, 20, 21,
27 or 29.
In one embodiment the substitutions at positions 2, 5, 7, 10, 11, 13, 14, 16,
17, 18, 19,
20, 21, 27 or 29 are conservative amino acid substitutions.
In accordance with one embodiment a glucagon/GLP-1 receptor co-agonist is
provided comprising a variant of the sequence of SEQ ID NO 33, wherein Ito 10
amino acids selected from positions 16, 17, 18, 20, 21, 23, 24, 27, 28 and 29,
respectively, of the variant differ from the corresponding amino acid of SEQ
ID NO:
1. In accordance with one embodiment a variant of the sequence of SEQ ID NO 33
is
provided wherein the variant differs from SEQ ID NO: 33 by one or more amino
acid
substitutions selected from the group consisting of Gln17, Ala18, Glu21,
11e23, A1a24,
Va127 and G1y29. In accordance with one embodiment a glucagon/GLP-1 receptor
co-agonist is provided comprising variants of the sequence of SEQ ID NO 33,
wherein 1 to 2 amino acids selected from positions 17-26 of the variant differ
from
the corresponding amino acid of SEQ ID NO: 1. In accordance with one
embodiment
a variant of the sequence of SEQ ID NO 33 is provided wherein the variant
differs
from SEQ ID NO: 33 by an amino acid substitution selected from the group
consisting of G1n17, A1a18, G1u21, 11e23 and A1a24. In accordance with one
embodiment a variant of the sequence of SEQ ID NO 33 is provided wherein the
variant differs from SEQ ID NO: 33 by an amino acid substitution at position
18
wherein the substituted amino acid is selected from the group consisting of
Ala, Ser,
71
CA 02 85217 7 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
Thr, and Gly. In accordance with one embodiment a variant of the sequence of
SEQ
ID NO 33 is provided wherein the variant differs from SEQ ID NO: 33 by an
amino
acid substitution of Ala at position 18. Such variations are encompassed by
SEQ ID
NO: 55. In another embodiment a glucagon/GLP-1 receptor co-agonist is provided
comprising variants of the sequence of SEQ ID NO 33, wherein 1 to 2 amino
acids
selected from positions 17-22 of the variant differ from the corresponding
amino acid
of SEQ ID NO: 1, and in a further embodiment a variant of SEQ ID NO 33 is
provided wherein the variant differs from SEQ ID NO: 33 by lor 2 amino acid
substitutions at positions 20 and 21. In accordance with one embodiment a
glucagon/GLP-1 receptor co-agonist is provided comprising the sequence:
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Xaa-Xaa-Arg-
Arg-Ala-Xaa-Xaa-Phe-Val-Xaa-Trp-Leu-Met-Xaa-Xaa-R (SEQ ID NO: 51), wherein
the Xaa at position 15 is Asp, Glu, cysteic acid, homoglutamic acid or
homocysteic
acid, the Xaa at position 16 is Ser, Glu, Gin, homoglutamic acid or
homocysteic acid,
the Xaa at position 20 is Gin, Lys, Arg, Om or citrulline, the Xaa at position
21 is
Asp, Glu, homoglutamic acid or homocysteic acid, the Xaa at position 24 is Gin
or
Glu, the Xaa at position 28 is Asn, Lys or an acidic amino acid, the Xaa at
position 29
is Thr or an acid amino acid and R is COOH or CONH2. In one embodiment R is
CONH2. In accordance with one embodiment a glucagon/GLP-1 receptor co-agonist
is provided comprising a variant of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO:
13, SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 47, SEQ ID NO: 48 or SEQ ID
NO: 49, wherein the variant differs from said sequence by an amino acid
substitution
at position 20. In one embodiment the amino acid substitution is selected form
the
group consisting of Lys, Arg, Om or citrulline for position 20.
In one embodiment a glucagon agonist is provided comprising an analog
peptide of SEQ ID NO: 34 wherein the analog differs from SEQ ID NO: 34 by
having
= an amino acid other than serine at position 2. In one embodiment the
serine residue is
substituted with aminoisobutyric acid, D-alanine, and in one embodiment the
serine
residue is substituted with aminoisobutyric acid. Such modifications
suppresses
cleavage by dipeptidyl peptidase IV while retaining the inherent potency of
the parent
compound (e.g. at least 75, 80, 85; 90, 95% or more of the potentcy of the
parent
compound). In one embodiment the solubility of the analog is increased, for
example,
by introducing One, two, three or more charged amino acid(s) to the C-terminal
portion of native glucagon, preferably at a position C-terminal to position
27. In
72
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
exemplary embodiments, one, two, three or all of the charged amino acids are
negatively charged. In another embodiment the analog further comprises an
acidic
amino acid substituted for the native amino acid at position 28 or 29 or an
acidic
amino acid added to the carboxy terminus of the peptide of SEQ ID NO: 34.
In one embodiment the glucagon analogs disclosed herein are further modified
at position 1 or 2 to reduce susceptibility to cleavage by dipeptidyl
peptidase IV. In
one embodiment a glucagon analog of SEQ ID NO: 9, SEQ ID NO: 11, SEQ ID NO:
= 12, SEQ ID NO: 13, SEQ ID NO: 14 or SEQ ID NO: 15 is provided wherein the
analog differs from the parent molecule by a substitution at position 2 and
exhibits
reduced susceptibility (i.e., resistance) to cleavage by dipeptidyl peptidase
IV. More
= particularly, in one embodiment position 2 of the analog peptide is
substituted with an
amino acid selected from the group consisting of D-serine, D-alanine, valine,
amino
n-butyric acid, glycine, N-methyl serine and aminoisobutyric acid. In one
embodiment position 2 of the analog peptide is substituted with an amino acid
= is selected from the group consisting of D-serine, D-alanine,
glycine, N-methyl serine
and aminoisobutyric acid. In another embodiment position 2 of the analog
peptide is
substituted with an amino acid selected from the group consisting of D-serine,
glycine, N-methyl serine and aminoisobutyric acid. In one embodiment the
glucagon
peptide comprises the sequence of SEQ ID NO: 21 or SEQ ID NO: 22.
In one embodiment a glucagon analog of SEQ ID NO: 9, SEQ ID NO: 11,
SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14 or SEQ ID NO: 15 is provided
wherein the analog differs from the parent molecule by a substitution at
position 1 and
exhibits reduced susceptibility (i.e., resistance) to cleavage by dipeptidyl
peptidase
IV. More particularly, position 1 of the analog peptide is substituted with an
amino
acid selected from the group consisting of D-histidine, alpha, alpha-dimethyl
=
imidiazole acetic acid (DMIA), N-methyl histidine, alpha-methyl histidine,
imidazole
acetic acid, desaminohistidine, hydroxyl-histidine, acetyl-histidine and homo-
histidine. In another embodiment a glucagon agonist is provided comprising an
analog peptide of SEQ ID NO: 34 wherein the analog differs from SEQ ID NO: 34
by
having an amino acid other than histidine at position I. In one embodiment the
solubility of the analog is increased, for example, by introducing one, two,
three or
more charged amino acid(s) to the C-terminal portion of native glucagon,
preferably
at a position C-terminal to position 27. In exemplary embodiments, one, two,
three or
all of the charged amino acids are negatively charged. In another embodiment
the
73
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
analog further comprises an acidic amino acid substituted for the native amino
acid at
position 28 or 29 or an acidic amino acid added to the carboxy terminus of the
peptide
of SEQ ID NO: 34. In one embodiment the acidic amino acid is aspartic acid or
glutamic acid.
In one embodiment the glucagon/GLP-1 receptor co-agonist comprises a
sequence of SEQ ID NO: 20 further comprising an additional carboxy terminal
extension of one amino acid or a peptide selected from the group consisting of
SEQ
ID NO: 26, SEQ ID NO: 27 and SEQ ID NO: 28. In the embodiment wherein a
single amino acid is added to the carboxy terminus of SEQ ID NO: 20, the amino
acid
is typically selected from one of the 20 common amino acids, and in one
embodiment
the additional carboxy terminus amino acid has an amide group in place of the
carboxylic acid of the native amino acid. In one embodiment the additional
amino
acid is selected from the group consisting of glutamic acid, aspartic acid and
glycine.
In an alternative embodiment a glucagon/GLP-1 receptor co-agonist is
provided wherein the peptide comprises at least one lactam ring formed between
the
side chain of a glutamic acid residue and a lysine residue, wherein the
glutamic acid =
residue and a lysine residue are separated by three amino acids. In one
embodiment
the carboxy terminal amino acid of the lactam bearing glucagon peptide has an
amide
group in place of the carboxylic acid of the native amino acid. More
particularly, in
one embodiment a glucagon and GLP-1 co-agonist is provided comprising a
modified
glucagon peptide selected from the group consisting of:
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Glu-
Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu- Met-Xaa-Xaa-R (SEQ ID NO: 66)
NH2-His-Ser-Gln-Gly-Thr-Phe- Thi--Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Glu-
Arg-Arg-Ala-Lys-Asp-Phe-Val-Gln-Trp-Leu- Met-Xaa-Xaa-R (SEQ ID NO: 67)
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-
Arg-Arg-Ala-Lys-Asp-Phe-Val-Glu-Trp-Leu- Met-Xaa-Xaa-R (SEQ ID NO: 68)
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-
Arg-Arg-Ala-Gln-Asp-Phe-Val-Glu-Trp-Leu- Met-Lys-Xaa-R (SEQ ID NO: 69)
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Glu-
Arg-Arg-Ala-Lys-Asp-Phe-Val-Glu-Trp-Leu- Met-Asn-Thr-R (SEQ ID NO: .16)
NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Glu-
Arg-Arg-Ala-Gln-Asp-Phe-Val-Glu-Trp-Leu- Met-Lys-Thr-R (SEQ ID NO: 17)
=
74
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
NI-12-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Glu-
Arg-Arg-Ala-Lys-Asp-Phe-Val-Glu-Trp-Leu- Met-Lys-Thr-R (SEQ ID NO: 18)
wherein Xaa at position 28 = Asp, or Asn, the Xaa at position 29 is Thr or
Gly, R is
selected from the group consisting of COOH, CONH2, glutamic acid, aspartic
acid,
glycine, SEQ ID NO: 26, SEQ ID NO: 27 and SEQ ID NO: 28, and a lactam bridge
is
formed between Lys at position 12 and Glu at position 16 for SEQ ID NO: 66,
between Glu at position 16 and Lys at position 20 for SEQ ID NO: 67, between
Lys at
position 20 and Glu at position 24 for SEQ ID NO: 68, between Glu at position
24
and Lys at position 28 for SEQ ID NO: 69, between Lys at position 12 and Glu
at
position 16 and between Lys at position 20 and Glu at position 24 for SEQ ID
NO:
16, between Lys at position 12 and Glu at position 16 and between Glu at
position 24
and Lys at position 28 for SEQ ID NO: 17 and between Glu at position 16 and
Lys at
position 20 and between Glu at position 24 and Lys at position 28 for SEQ ID
NO:
18. In one embodiment R is selected from the group consisting of COOH, CONF12,
glutamic acid, aspartic acid, glycine, the amino acid at position 28 is Asn,
and the
amino acid at position 29 is threonine. In one embodiment R is CONH2, the
amino
acid at position 28 is Asn and the amino acid at position 29 is threonine. In
another
embodiment R. is selected from the group consisting of SEQ ID NO: 26, SEQ ID
NO:
29 and SEQ ID NO: 65 and the amino acid at position 29 is glycine.
In a further embodiment the glucagon/GLP-1 receptor co-agonist is selected
from the group consisting of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ
ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17 and SEQ ID NO: 18,
wherein the peptide further comprises an additional carboxy terminal extension
of one
amino acid or a peptide selected from the group consisting of SEQ ID NO: 26,
SEQ
ID NO: 27 and SEQ ID NO: 28. In one embodiment the terminal extension
comprises
the sequence of SEQ ID NO: 26, SEQ ID NO: 29 or SEQ ID NO: 65 and the
glucagon peptide comprises the sequence of SEQ ID NO: 55. In one embodiment
the
glucagon/GLP-1 receptor co-agonist comprises the sequence of SEQ ID NO: 33
wherein the amino acid at position 16 is glutamic acid, the amino acid at
position 20
is lysine, the amino acid at position 28 is asparagine and the amino acid
sequence of
SEQ ID No: 26 or SEQ ID NO: 29 is linked to the carboxy terminus of SEQ ID NO:
33.
In the embodiment wherein a single amino acid is added to the carboxy
terminus of SEQ ID NO: 20, the amino acid is typically selected from one of
the 20
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
common amino acids, and in one embodiment the amino acid has an amide group in
place of the carboxylic acid of the native amino acid. In one embodiment the
additional amino acid is selected from the group consisting of glutamic acid
and
aspartic acid and glycine. In the embodiments wherein the glucagon agonist
analog
further comprises a carboxy terminal extension, the carboxy terminal amino
acid of
the extension, in one embodiment, ends in an amide group or an ester group
rather
= than a carboxylic acid.
In another embodiment the glucagon/GLP-1 receptor co-..agonist comprises the
sequence: NH2-His-Ser-Gln-Gly-Thr-Phe- Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-
Glu-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr-Xaa-CONH2 (SEQ
ID NO: 19), wherein the Xaa at position 30 represents any amino acid. In one
embodiment Xaa is selected from one of the 20 common amino acids, and in one
embodiment the amino acid is glutamic acid, aspartic acid or glycine. The
solubility
of this peptide can be further improved by covalently linking a PEG chain to
the side
chain of amino acid at position 17, 21, 24 or 30 of SEQ ID NO: 19. In a
further
embodiment the peptide comprises an additional carboxy terminal extension of a
peptide selected from the group consisting of SEQ ID NO: 26, SEQ ID NO: 27 and
SEQ ID NO: 28. In accordance with one embodiment the glucagon/GLP-1 receptor
co-agonist comprises the sequence of SEQ ID NO: 30, SEQ ID NO: 31 and SEQ ID
NO: 32.
Additional site specific modifications internal to the glucagon sequence of
SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14, SEQ ID NO: 15,
SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18, SEQ ID NO: 19 and SEQ ID NO:
64 can be made to yield a set of glucagon agonists that possess .variable
degrees of
GLP-1 agonism. Accordingly, peptides that possess virtually identical in vitro
potency at each receptor have been prepared and characterized. Similarly,
peptides
with tenfold selectively enhanced potency at each of the two receptors have
been
identified and characterized.. As noted above substitution of the serine
residue at
position 16 with glutamic acid enhances the potency of native glucagon at both
the
Glucagon and GLP-1 receptors, but maintains approximately a tenfold
selectivity for
the glucagon receptor. In addition by substituting the native glutamine at
position 3
with glutamic acid (SEQ ID NO: 22) generates a glucagon analog that exhibits
approximately a tenfold selectivity for the GLP-1 receptor.
76
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
The solubility of the glucagon/GLP-1 co-agonist peptides can be further
enhanced in aqueous solutions at physiological pH, while retaining the high
biological
activity relative to native glucagon by the introduction of hydrophilic groups
at
positions 16, 17, 21, and 24 of the peptide, or by the addition of a single
modified
amino acid (i.e., an amino acid modified to comprise a hydrophilic group) at
the
carboxy terminus of the glucagon/GLP-1 co-agonist peptide. In accordance with
one
embodiment the hydrophilic group comprises a polyethylene (PEG) chain. More
particularly, in one embodiment the glucagon peptide comprises the sequence of
SEQ
ID NO: 10, SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14, SEQ
ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17 or SEQ ID NO: 18 wherein a PEG chain
is covalently linked to the side chain of an amino acids at position 16, 17,
21, 24, 29
or the C-terminal amino acid of the glucagon peptide, with the proviso that
when the
peptide comprises SEQ ID NO: 10, SEQ ID NO: 11, SEQ ID NO: 12 or SEQ ID NO:
13 the polyethylene glycol chain is covalently bound to an amino acid residue
at
position 17,21 or 24, when the peptide comprises SEQ ID NO: 14 or SEQ ID NO:
15
the polyethylene glycol chain is covalently bound to an amino acid residue at
position
16, 17 or 21, and when the peptide comprises SEQ ID NO: 16, SEQ ID NO: 17 or
SEQ ID NO: 18 the polyethylene glycol chain is covalently bound to an amino
acid
residue at position 17 or 21.
In one embodiment the glucagon peptide comprises the sequence of SEQ ID
NO: 11, SEQ ID NO: 12 or SEQ ID NO: 13, wherein a PEG chain is covalently
linked to the side chain of an amino acids at position 17, 21, 24, or the C-
terminal
amino acid of the glucagon peptide, and the carboxy terminal amino acid of the
peptide has an amide group in place of the carboxylic acid group of the native
amino
acid. In one embodiment the glucagoxi/GLP-1 receptor co-agonist peptide
comprises
a sequence selected from the group consisting of SEQ ID NO: 12, SEQ ID NO: 13,
SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18
and SEQ ID NO: 19, wherein a PEG chain is covalently linked to the side chain
of an
amino acid at position 17,21 or 24 of SEQ ID NO: 12, SEQ ID NO: 13 and SEQ ID
NO: 19, or at position 16, 17 or 21 of SEQ ID NO: 14 and SEQ ID NO: 15 or at
position 17 or 21 of SEQ ID NO: 16, SEQ ID NO: 17 and SEQ ID NO: 18 of the
glucagon peptide. In another embodiment the glucagon/GLP-1 receptor co-agonist
peptide comprises the sequence of SEQ ID NO: 11 or SEQ ID NO: 19, wherein a
77
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
PEG chain is covalently linked to the side chain of an amino acids at position
17, 21
or 24 or the C-terminal amino acid of the glucagon peptide.
In accordance with one embodiment, and subject to the proviso limitations
described in the preceding paragraphs, the glucagon co-agonist peptide is
modified to
contain one or more amino acid substitution at positions 16, 17, 21, 24, or 29
or the C-
terminal amino acid, wherein the native amino acid is substituted with an
amino acid
having a side chain suitable for crosslinlcing with hydrophilic moieties,
including for
example, PEG. The native peptide can be substituted with a naturally occurring
amino acid or a synthetic (non-naturally occurring) amino acid. Synthetic or
non-
naturally occurring amino acids refer to amino acids that do not naturally
occur in
vivo but which, nevertheless, can be incorporated into the peptide structures
described
herein. Alternatively, the amino acid having a side chain suitable for
crosslinking
with hydrophilic moieties, including for example, PEG, can be added to the
carboxy
terminus of any of the glucagon analogs disclosed herein. In accordance with
one
embodiment an amino acid substitution is made in the glucagon/GLP-1 receptor
co-
agonist peptide at a position selected from the group consisting of 16, 17,
21, 24, or
29 replacing the native amino acid with an amino acid selected from the group
consisting of lysine, cysteine, omithine, homocysteine and acetyl
phenylalanine,
wherein the substituting amino acid further comprises a PEG chain covalently
bound
to the side chain of the amino acid. In one embodiment a glucagon peptide
selected
form the group consisting of SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14, SEQ
ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18, and SEQ ID NO: 19 is
further modified to comprise a PEG chain is covalently linked to the side
chain of an
amino acid at position 17 or 21 of the glucagon peptide. In one embodiment the
pegylated glucagon/GLP-1 receptor co-agonist further comprises the sequence of
SEQ ID NO: 26, SEQ ID NO: 27 or SEQ ID NO: 29.
In another embodiment the glucagon peptide comprises the sequence of SEQ
ID NO: 55 or SEQ ID NO: 56, further comprising a C-terminal extension of SEQ
ID
NO: 26, SEQ ID NO: 29 or SEQ ID NO: 65 linked to the C-terminal amino acid of
SEQ ID NO: 55 or SEQ ID NO: 56, and optionally further comprising a PEG chain
covalently linked to the side chain of an amino acids at position 17, 18,
21,24 or 29
or the C-terminal amino acid of the peptide. In another embodiment the
glucagon
peptide comprises the sequence of SEQ ID NO: 55 or SEQ ID NO: 56, wherein a
PEG chain is covalently linked to the side chain of an amino acids at position
21 or 24
78
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
of the glucagon peptide and the peptide further comprises a C-terminal
extension of
SEQ ID NO: 26, or SEQ ID NO: 29.
In another embodiment the glucagon peptide comprises the sequence of SEQ
ID NO: 55, or SEQ ID NO: 33 or SEQ ID NO: 34, wherein an additional amino acid
is added to the carboxy terminus of SEQ ID NO: 33 or SEQ ID NO: 34, and a PEG
chain is covalently linked to the side chain of the added amino acid. In a
further
embodiment, the pegylated glucagon analog further comprises a C-terminal
extension
of SEQ ID NO: 26 or SEQ ID NO: 29 linked to the C-terminal amino acid of SEQ
ID
NO: 33 or SEQ ID NO: 34. In another embodiment the glucagon peptide comprises
the sequence of SEQ ID NO: 19, wherein a PEG chain is covalently linked to the
side
chain of the amino acid at position 30 of the glucagon peptide and the peptide
further
comprises a C-terminal extension of SEQ ID NO: 26 or SEQ ID NO: 29 linked to
the
C-terminal amino acid of SEQ ID NO: 19. =
The polyethylene glycol chain may be in the form of a straight chain or it may
be branched. In accordance with one embodiment the polyethylene glycol chain
has
an average molecular weight selected from the range of about 500 to about
10,000
Daltons. In one embodiment the polyethylene glycol chain has an average
molecular
weight selected from the range of about 1,000 to about 5,000 Daltons. In an
alternative embodiment the polyethylene glycol chain has an average molecular
weight selected from the range of about 10,000 to about 20,000 Daltons. In
accordance with one embodiment the pegylated glucagon peptide comprises two or
more polyethylene chains covalently bound to the glucagon peptide wherein the
total
molecular weight of the glucagon chains is about 1,000 to about 5,000 Daltons.
In
one embodiment the pegylated glucagon agonist comprises a peptide consisting
of
SEQ ID NO: 5 or a glucagon agonist analog of SEQ ID NO: 5, wherein a PEG chain
is covalently linked to the amino acid residue at position 21 and at position
24, and
wherein the combined molecular weight of the two PEG chains is about 1,000 to
about 5,000 Daltons.
In certain exemplary embodiments, the glucagon peptide comprises the amino
acid sequence of SEQ ID NO: 1 with up to ten amino acid modifications and
comprises an amino acid at position 10 which is acylated or alkylated. In some
embodiments, the amino acid at position 10 is acylated or allcylated with a C4
to C30
fatty acid: In certain aspects, the amino acid at position 10 comprises an
acyl group or
an alkyl group which is non-native to a naturally-occurring amino acid.
79
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In certain embodiments, the glucagon peptide comprising an amino acid at
position 10 which is acylated or allcylated comprises a stabilized alpha
helix.
Accordingly, in certain aspects, the glucagon peptide comprises an acyl or
alkyl group
as described herein and an intramolecular bridge, e.g., a covalent
intramolecular
bridge (e.g., a Iactam bridge) between the side chains of an amino acid at
position i
and an amino acid at position i+4, wherein i is 12, 16, 20, or 24.
Alternatively or
additionally, the glucagon peptide comprises an acyl or alkyl group as
described
herein and one, two, three or more of positions 16, 20, 21 and/or 24 of the
glucagon
peptide are substituted with an a, a-disubstituted amino acid, e.g., MB. In
some
103 instances, the non-native glucagon peptide comprises Glu at position 16
and Lys at
position 20, wherein optionally a lactam bridge hikes the Glu and the Lys,
and,
optionally, the glucagon peptide further comprises one or more modifications
selected
from the group consisting of: Gin at position 17, Ala at position 18, Glu at
position
21, Ile at position 23, and Ala at position 24.
Also, in any of the embodiments, wherein the glucagon peptide comprises an
amino acid at position 10 which is acylated or allcylated, the glucagon
peptide can
further comprise a C-terminal amide in lieu of the C-terminal alpha
carboxylate.
In some embodiments, the glucagon peptide comprising an acyl or alkyl group
as described herein further comprises an amino acid substitution at position
1, at
position 2, or at positions 1 and 2, wherein the amino acid substitution(s)
achieve
DPP-IV protease resistance. For example, the His at position 1 may be
substituted
with an amino acid selected from the group consisting of: D-histidine, alpha,
alpha-
dimethyl imidiazole acetic acid (DMIA), N-methyl histidine, alpha-methyl
histidine,
imidazole acetic acid, desaminohistidine, hydroxyl-histidine, acetyl-histidine
and
homo-histidine. Alternatively or additionally, the Ser at position 2 may be
substituted
with an amino acid selected from the group consisting of: D-serine, alanine, D-
alanine, valine, glycine, N-methyl serine, N-methyl alanine, and amino
isobutyric
acid.
The glucagon peptide comprising the amino acid at position 10 which is
acylated or alkylated as described herein can comprise any amino acid sequence
which is substantially related to SEQ ID NO: I. For instance, the glucagon
peptide
comprises SEQ ID NO: 1 with up to 10 amino acid modifications (e.g., 0, 1, 2,
3, 4, 5,
6, 7, 8, 9, 10 modifications). In certain embodiments, the amino acid sequence
of the
acylated or allcylated glucagon peptide is greater than 25% identical to SEQ
ID NO: 1
CA 02852177 2014-05-23
= WO 2009/155258
PCT/US2009/047438
29920-208831
(e.g., greater than 30%, 35%, 40%, 50%, 60%, 70% 75%, 80%, 85%, 90%, 95%,
96%, 97%, 98%, 99%, or nearly 100% identical to SEQ ID NO: 1). In certain
specific embodiments, the glucagon peptide is one which comprises SEQ ID NOs:
55
with an amino acid at position 10 acylated or allcylated as described herein.
The
5 glucagon peptide can be any of SEQ ID NOs: 55, 55 with 1 or 2 amino acid
modifications, 2-4, 9-18, 20, 23-25, 33, 40-44, 53, 56, 61,62, 64,66-514, and
534.
The acyl or alkyl group of these embodiments may be any acyl or alkyl group
described herein. For example, the acyl group may be a C4 to C30 (e.g., C8 to
C24)
fatty acyl group and the alkyl group may be a C4 to C30 (e.g., C8 to C24)
alkyl group.
10 The amino acid to which the acyl or alkyl group is attached may be any
of the
amino acids described herein, e.g., an amino acid of any of Formula I (e.g.,
Lys),
Formula II, and Formula HI.
In some embodiments, the acyl group or alkyl group is directly attached to the
amino acid at position 10. In'some embodiments, the acyl or alkyl group is
attaChed
15 to the amino acid at position 10 via a spacer, such as, for example, a
spacer which is 3
to 10 atoms in length, e.g., an amino acid or dipeptide. Suitable spacers for
purposes
of attaching an acyl or alkyl group are described herein.
Uses
As described in detail in the Examples, the glucagon agonists of the present
20 invention have enhanced biophysical stability and aqueous solubility
while
demonstrating enhanced bioactivity relative to the native peptide.
Accordingly, the
glucagon agonists of the present invention are believed to be suitable for any
use that
has previously been described for the native glucagon peptide. Accordingly,
the
modified glucagon peptides described herein can be used to treat hypoglycemia
or to
25 increase blood glucose level, to induce temporary paralysis of the gut
for radiological
uses, or treat other metabolic diseases that result from low blood levels of
glucagon.
The glucagon peptides described herein also are expected to be used to reduce
or
maintain body weight, or to treat hyperglycemia, or to reduce blood glucose
level, or
to normalize blood glucose level.
30 The glucagon peptides of the invention may be administered alone or in
combination with other anti-diabetic or anti-obesity agents. Anti-diabetic
agents
known in the art or under investigation include insulin, sulfonylureas, such
as
tolbutamide (Orinase), acetohexamide (Dymelor), tolazamide (Tolinase),
chlorpropamide (Diabinese), glipizide (Glucotrol), glyburide (Diabeta,
Micronase,
8)
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831 =
Glynase), glimepiride (Amaryl), or gliclazide (Diamicron); meglitinides, such
as
repaglinide (Prandin) or nateglinide (Starlix); biguanides such as metformin
(Glucophage) or phenformin; thiazolidinediones such as rosiglitazone
(Avandia),
.pioglitazone (Actos), or troglitazone (Rezulin), or other PPART inhibitors;
alpha
glucosidase inhibitors that inhibit carbohydrate digestion, such as miglitol
(Glyset),
acarbose (Precose/Glucobay); exenatide (Byetta) or pramlintide; Dipeptidyl
peptidase-4 (DPP-4) inhibitors such as vildagliptin or sitagliptin; SGLT
(sodium-
dependent glucose transporter 1) inhibitors; glucokinase activators (GKA);
glucagon
receptor antagonists ((IRA); or FBPase (fructose 1,6-bisphosphatase)
inhibitors.
Anti-obesity agents known in the art or under investigation include appetite
suppressants, including phenethylamine type stimulants, phentermine
(optionally with
fenfluramine or dexfenfluramine), diethylpropion (Tenuate10), phendimetrazine
(Prelu-20, Bontrile), benzphetamine (Didrexe), sibutramine (Meridia , Reductil
);
rimonabant (Acompliae), other cannabinoid receptor antagonists; oxyntomodulin;
fluoxetine hydrochloride (Prozac); Qnexa (topiramate and phentermine), Excalia
(bupropion and zonisamide) or Contrave (bupropion and naltrexone); or lipase
inhibitors, similar to XENICAL (Orlistat) or Cetilistat (also known as ATL-
962), or
GT 389-255.
One aspect of the present disclosure is directed to a pre-formulated aqueous'
solution of the presently disclosed glucagon agonist for use in treating
hypoglycemia.
The improved stability and solubility of the agonist compositions described
herein
allow for the preparation of pre-formulated aqueous solutions of glucagon for
rapid
administration and treatment of hypoglycemia. In one embodiment a solution
comprising a pegylated glucagon agonist is provided for administration to a
patient
suffering from hypoglycemia, wherein the total molecular weight of the PEG
chains
linked to the pegylated glucagon agonist is between about 500 to about 5,000
Daltons.
In one embodiment the pegylated glucagon agonist comprises a peptide selected
from
the group consisting of SEQ ID NO: 23, SEQ ID NO: 24, and SEQ ID NO: 25, and
glucagon agonist analogs of SEQ ID NO: 23, SEQ ID NO: 24, and SEQ ID NO: 25,
or a pegylated lactam derivative of glucagon comprising the sequence of SEQ ID
NO:
20, wherein the side chain of an amino acid residue of said glucagon peptide
is
covalently bound to the polyethylene glycol chain.
The treatment methods in accordance with the present invention, including but
not limited to treatment of hypoglycemia, may comprise the steps of
administering the
82
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
presently disclosed glucagon agonists to a patient using any standard route of
administration, including parenterally, such as intravenously,
intraperitoneally,
subcutaneously or intramuscularly, intrathecally, transdermally, rectally,
orally,
nasally or by inhalation. In one embodiment the composition is administered
subcutaneously or intramuscularly. In one embodiment, the composition is
administered parenterally and the glucagon composition is prepackaged in a
syringe.
In another embodiment, the composition is prepackaged in an inhaler or other
aerosolized drug delivery device.
Surprisingly, applicants have discovered that pegylated glucagon peptides can
be prepared that retain the parent peptide's bioactivity and specificity.
However,
increasing the length of the PEG chain, or attaching multiple PEG chains to
the
peptide, such that the total molecular weight of the linked PEG is greater
than 5,000
Daltons, begins to delay the time action of the modified glucagon. In
accordance with
one embodiment, a glucagon peptide of SEQ ID NO: 23, SEQ ID NO: 24, and SEQ
ID NO: 25, or a glucagon agonist analog thereof, or a pegylated lactam
derivative of
glucagon comprising the sequence of SEQ ID NO: 20 is provided wherein the
peptide
comprises one or more polyethylene glycol chains, wherein the total molecular
weight
of the linked PEG is greater than 5,000 Daltons, and in one embodiment is
greater
= than 10,000 Daltons, but less than 40,000 Daltons. Such modified glimagon
peptides
have a delayed or prolonged time of activity but without loss of the
bioactivity.
= Accordingly, such compounds can be administered to extend the effect of
the
administered glucagon peptide.
Glucagon peptides that have been modified to be covalently bound to a PEG
chain having a molecular weight of greater than 10,000 Daltons can be
administered
in conjunction with insulin to buffer the actions of insulin and help to
maintain stable
blood glucose levels in diabetics. The modified glucagon peptides of the
present
disclosure can be co-administered with insulin as a single composition,
simultaneously administered as separate solutions, or alternatively, the
insulin and the
modified glucagon peptide can be administered at different time relative to
one
another. In one embodiment the composition comprising insulin and the
composition
comprising the modified glucagon peptide are administered within 12 hours of
one
another. The exact ratio of the modified glucagon peptide relative to the
administered
insulin will be dependent in part on determining the glucagon levels of the
patient,
and can be determined through routine experimentation.
83
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
In accordance with one embodiment a composition is provided comprising =
insulin and a modified glucagon peptide selected from the group consisting of
SEQ
ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5 and glucagon agonist
analogs thereof, wherein the modified glucagon peptide further comprises a
polyethylene glycol chain covalently bound to an amino acid side chain at
position 17,
21, 24 or 21 and 24. In one embodiment the composition is an aqueous solution
comprising insulin and the glucagon analog. In embodiments where the glucagon
peptide comprises the sequence of SEQ ID NO: 24 or SEQ ID NO: 25 the PEG chain
is covalently bound at position 21 or 24 of the glucagon peptide. In one
embodiment
the polyethylene glycol chain has a molecular weight of about 10,000 to about
40,000.
In accordance with one embodiment the modified glucagon peptides disclosed
herein are used to induce temporary paralysis of the intestinal tract. This
method has
utility for radiological purposes and comprises the step of administering an
effective
amount of a pharmaceutical composition comprising a pegylated glucagon
peptide, a
glucagon peptide comprising a c-terminal extension or a dimer of such
peptides. In
one embodiment the glucagon peptide comprises a sequence selected from the
group
consisting of SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID
NO: 6, SEQ ID NO: 7; SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 10, SEQ ID NO:
zo 11, SEQ ID NO: 12, SEQ ID NO: 13 SEQ ID NO: 14 and SEQ ID NO: 15. In one
embodiment the glucagon peptide further comprises a PEG chain, of about 1,000
to
40,000 Daltons is covalently bound to an amino acid residue at position 21 or
24. In
one embodiment the glucagon peptide is selected from the group consisting of
SEQ
ID NO: 10, SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13, SEQ ID NO: 14 and
SEQ ID NO: 15. In one embodiment the PEG chain has a molecular weight of about
500 to about 5,000 Daltons.
In a further embodiment the composition used to induce temporary paralysis
of the intestinal tract comprises a first modified glucagon peptide and a
second
modified glucagon peptide. The first modified peptide comprises a sequence
selected =
from the group consisting of SEQ ID NO: 23, SEQ ID NO: 24 and SEQ ID NO: 25,
optionally linked to a PEG chain of about 500 to about 5,000 Daltons, and the
second
peptide comprises a sequence selected from the group consisting of SEQ ID NO:
23,
SEQ ID NO: 24 and SEQ ID NO: 25, covalently linked to a PEG chain of about
10,000 to about 40,000 Daltons. In this embodiment the PEG chain of each
peptide is
84
CA 02852177 2014-05-23
= WO 2009/155258
PCT/US2009/047438
29920-208831
covalently bound to an amino acid residue at either position 17, 21 or 24 of
the
respective peptide, and independent of one another.
Oxyntomodulin, a naturally occurring digestive hormone found in the small
intestine, has been reported to cause weight loss when administered to rats or
humans
5 (see Diabetes 2005;54:2390-2395). Oxyntomodulin is a 37 amino acid
peptide that
contains the 29 amino acid sequence of glucagon (i.e., SEQ ID NO: I) followed
by an
8 amino acid carboxy terminal extension of SEQ ID NO: 27 (ICRNRNNIA).
Accordingly, applicants believe that the bioactivity of oxyntomodulin can be
retained
(i.e., appetite suppression and induced weight loss/weight maintenance), while
10 improving the solubility and stability of the compound and improving the
pharmacokinetics, by substituting the glucagon peptide portion of
oxyntomodulin
with the modified glucagon peptides disclosed herein. In addition applicants
also
believe that a truncated Oxyntomodulin molecule comprising a glucagon peptide
of
the invention, having the terminal four amino acids of oxyntomodulin removed
will
15 also be effective in suppressing appetite and inducing weight
loss/weight
maintenance.
Accordingly, the present invention also encompasses the modified glucagon
peptides of the present invention that have a carboxy terminal extension of
SEQ ID
NO: 27 (ICRNRNNIA) or SEQ ID NO: 28. These compounds can be administered to
20 individuals to induce weight loss or prevent weight gain. In accordance
with one
embodiment a glucagon agonist analog of SEQ ID NO: 33 or SEQ ID NO: 20,
further
comprising the amino acid sequence of SEQ ID NO: 27 (KRNRNNIA) or SEQ ID
NO: 28 linked to amino acid 29 of the glucagon peptide, is administered to
individuals to induce weight loss or prevent weight gain. More particularly,
the
25 glucagon peptide comprises a sequence selected from the group consisting
of SEQ ID
NO: 10, SEQ ID NO: 12, SEQ ID NO: 13 SEQ ID NO: 14 and SEQ ID NO: 15,
further comprising the amino acid sequence of SEQ ID NO: 27 (ICRNIINNIA) or
SEQ ID NO: 28 linked to amino acid 29 of the glucagon peptide.
Exendin-4, is a peptide made up of 39 amino acids. It is a powerful stimulator
30 of a receptor known as GLP-1. This peptide has also been reported to
suppress.
appetite and induce weight loss. Applicants have found that the terminal
sequence of
Exendin-4 when added at the carboxy terminus of glucagon improves the
solubility
and stability of glucagon without compromising the bioactivity of glucagon. In
one
embodiment the terminal ten amino acids of Exendin-4 (i.e., the sequence of
SEQ ID
CA 02852177 2014-05-23
WO 2009/155258
PCT/1JS2009/047438
29920-208831
NO: 26 (GPSSGAPPPS)) are linked to the carboxy terminus of a glucagon peptide
of
the present disclosure. These fusion proteins are anticipated to have
pharmacological
activity for suppressing appetite and inducing weight loss/weight maintenance.
In
accordance with one embodiment a glucagon agonist analog of SEQ ID NO: 33 or
SEQ ID NO: 20, further comprising the amino acid sequence of SEQ ID NO: 26
(GPSSGAPPPS) or SEQ ID NO: 29 linked to amino acid 29 of the glucagon peptide,
is administered to individuals to induce weight loss or prevent weight gain.
More
particularly, the glucagon peptide comprises a sequence selected from the
group
consisting of SEQ ID NO: 10, SEQ ID NO: 12, SEQ ID NO: 13 SEQ ID NO: 14,
SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 18, SEQ ID NO: 66,
SEQ ID NO: 67, SEQ ID NO: 68, SEQ ID NO: 69, SEQ ID NO: 55 and SEQ ID NO:
56 further comprising the amino acid sequence of SEQ ID NO: 26 (GPSSGAPPPS) or
SEQ ID NO: 29 linked to amino acid 29 of the glucagon peptide. In one
embodiment
the administered glucagon peptide analog comprises the sequence of SEQ ID NO:
64.
Multimers
The present disclosure also encompasses multimers of the modified glucagon
peptides disclosed herein. Two or more of the modified glucagon peptides can
be
linked together using standard linking agents and procedures known to those
skilled in
the art. For example, dimers can be formed between two modified glucagon
peptides
through the use of bifunctional thiol crosslinkers and bi-functional amine
crosslinkers,
particularly for the glucagon peptides that have been substituted with
cysteine, lysine
omithine, homocysteine or acetyl phenylalanine residues (e.g. SEQ ID NO: 3 and
SEQ ID NO: 4). The dimer can be a homodimer or alternatively can be a
heterodimer. In certain embodiments, the linker connecting the two (or more)
glucagon peptides is PEG, e.g., a 5 IcDa PEG, 20 kDa PEG. In some embodiments,
the linker is a disulfide bond. For example, each monomer of the dimer may
comprise a Cys residue (e.g., a terminal or internally positioned Cys) and the
sulfur
atom of each Cys residue participates in the formation of the disulfide bond.
In some
aspects of the invention, the monomers are connected via terminal amino acids
(e.g.,
N-terminal or C-terminal), via internal amino acids, or via a terminal amino
acid of at
least one monomer and an internal amino acid of at least one other monomer. In
specific aspects, the monomers are not connected via an N-terminal amino acid.
In
some aspects, the monomers of the multimer are attached together in a "tail-to-
tail"
=
86
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
orientation in which the C-terminal amino acids of each monomer are attached
together.
In one embodiment the dimer comprises a homodimer of a glucagon fusion
peptide wherein the glucagon peptide portion comprises SEQ ID NO: 11 or SEQ ID
NO: 20 and an amino acid sequence of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID
NO: 27 (KRNRNNIA) or SEQ ID NO: 28 (KRNR) linked to amino acid 29 of the
glucagon peptide. In another embodiment the dimer comprises a homodimer of a
glucagon agonist analog of SEQ ID NO: 11, wherein the glucagon peptide further
comprises a polyethylene glycol chain covalently bound to position 21 or 24 of
the
glucagon peptide.
In accordance with one embodiment a dimer is provided comprising a first
glucagon peptide bound to a second glucagon peptide via a linker, wherein the
first
glucagon peptide comprises a peptide selected from the group consisting of SEQ
ID
NO: 8, SEQ ID NO: 9, SEQ ID NO: 10 and SEQ ID NO: 11 and the second glucagon
peptide comprises SEQ ID NO: 20. In accordance with another embodiment a dimer
is provided comprising a first glucagon peptide bound to a second glucagon
peptide
via a linker, wherein said first glucagon peptide comprises a sequence
selected from
the group consisting of SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO:
5, SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 10, SEQ ID NO: 11 and the second
glucagon peptide comprise SEQ ID NO: 11, and pharmaceutically acceptable salts
of
said glucagon polypeptides. In accordance with another embodiment a dimer is
=
provided comprising a first glucagon peptide bound to a second glucagon
peptide via
a linker, wherein said first glucagon peptide is selected from the group
consisting of
SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO: 13 SEQ ID NO: 14, SEQ ID NO: 15,
SEQ ID NO: 16, SEQ ID NO: 17 and SEQ ID NO: 18 and the second glucagon
peptide is independently selected from the group consisting of SEQ ID NO: 11,
SEQ
ID NO: 12, SEQ ID NO: 13 SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ
ID NO: 17 and SEQ ID NO: 18, and pharmaceutically acceptable salts of said
glucagon polypeptides. In one embodiment the first glucagon peptide is
selected from
the group consisting of SEQ ID NO: 20 and the second glucagon peptide is
independently selected from the group consisting of SEQ ID NO: 8, SEQ ID NO: 9
and SEQ ID NO: 11. In one embodiment the dimer is formed between two peptides
wherein each peptide comprises the amino acid sequence of SEQ ID NO: 11.
87
=
CA 02852177 2014-05-23
= WO 2009/155258
PCT/US2009/047438
29920-208831
Kits
The modified glucagon peptides of the present invention can be provided in
accordance with one embodiment as part of a kit. In one embodiment a kit for
administering a glucagon agonist to a patient in need thereof is provided
wherein the
5 kit comprises a modified glucagon peptide selected from the group
consisting of 1) a
glucagon peptide comprising the sequence of SEQ ID NO: 20, SEQ ID NO: 9, SEQ
ID NO: 10 or SEQ ID NO:11; 2) a glucagon fusion peptide comprising a glucagon
agonist analog of SEQ ID NO: 11, SEQ ID NO: 20 or SEQ ID NO: 55, and an amino
acid sequence of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID NO: 27 (KRNRNNIA) or
10 SEQ ID NO: 28 (KRNR) linked to amino acid 29 of the glucagon peptide;
and 3) a
pegylated glucagon.peptide of SEQ ID NO: 11 or SEQ ID NO: 51, further
comprising
an amino acid sequence of SEQ ID NO: 26 (GPSSGAPPPS), SEQ ID NO: 27
(KRNRNNIA) or SEQ ID NO: 28 (KRNR) linked to amino acid 29 of the glucagon
peptide, wherein the PEG chain covalently bound to position 17, 21 or 24 has a
15 molecular weight of about 500 to about 40,000 Daltons. In one embodiment
the kit
comprise a glucagon/GLP- I co-agonist wherein the peptide comprises a sequence
selected from the group consisting of SEQ ID NO: 11, SEQ ID NO: 12, SEQ ID NO:
13 SEQ ID NO: 14, SEQ ID NO: 15, SEQ ID NO: 16, SEQ ID NO: 17 and SEQ ID
NO: 18.
20 In one embodiment the kit is provided with a device for administering
the
glucagon composition to a patient, e.g. syringe needle, pen device, jet
injector or other
needle-free injector. The kit may alternatively or in addition include one or
more
containers, e.g., vials, tubes, bottles, single or multi-chambered pre-filled
syringes,
cartridges, infusion pumps (external or implantable), jet injectors, pre-
filled pen
25 devices and the like, optionally containing the glucagon peptide in a
lyophilized form
or in an aqueous solution. Preferably, the kits will also include instructions
for use.
In accordance with one embodiment the device of the kit is an aerosol
dispensing
device, wherein the composition is prepackaged within the aerosol device. In
another
embodiment the kit comprises a syringe and a needle, and in one embodiment the
30 sterile glucagon composition is prepackaged within the syringe.
Pharmaceutical Formulations
In accordance with one embodiment a pharmaceutical composition is provided
wherein the composition comprises a glucadon peptide of the present
disclosure, or
pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable
carrier.
88
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
=
29920-208831
The pharmaceutical composition can comprise any pharmaceutically acceptable
ingredient, including, for example, acidifying agents, additives, adsorbents,
aerosol
propellants, air displacement agents, alkalizing agents, anticaking agents,
anticoagulants, antimicrobial preservatives, antioxidants, antiseptics, bases,
binders,
buffering agents, chelating agents, coating agents, coloring agents,
desiccants,
detergents, diluents, disinfectants, disintegrants, dispersing agents,
dissolution
enhancing agents, dyes, emollients, emulsifying agents, emulsion stabilizers,
fillers,
film forming agents, flavor enhancers, flavoring agents, flow enhancers,
gelling
agents, granulating agents, humectants, lubricants, mucoadhesives, ointment
bases,
o ointments, oleaginous vehicles, organic bases, pastille bases, pigments,
plasticizers,
polishing agents, preservatives, sequestering agents, skin penetrants,
solubilizing
agents, solvents, stabilizing agents, suppository bases, surface active
agents,
surfactants, suspending agents, sweetening agents, therapeutic agents,
thickening
agents, tonicity agents, toxicity agents, viscosity-increasing agents, water-
absorbing
agents, water-miscible cosolvents, water softeners, or wetting agents.
In some embodiments, the pharmaceutical composition comprises any one or a
combination of the following components: acacia, acesulfame potassium,
acetyltributyl citrate, acetyltriethyl citrate, agar, albumin, alcohol,
dehydrated alcohol,
denatured alcohol, dilute alcohol, aleuritic acid, alginic acid, aliphatic
polyesters,
alumina, aluminum hydroxide, aluminum stearate, amylopectin, a-amylose,
ascorbic
acid, ascorbyl palmitate, aspartame, bacteriostatic water for injection,
bentonite,
bentonite magma, benzalkonium chloride, benzethonium chloride, benzoic acid,
benzyl alcohol, benzyl benzoate, bronopol, butylated hydroxyanisole, butylated
hydroxytoluene, butylparaben, butylparaben sodium, calcium alginate, calcium
ascorbate, calcium carbonate, calcium cyclamate, dibasic anhydrous calcium
phosphate, dibasic dehydrate calcium phosphate, tribasic calcium phosphate,
calcium
propionate, calcium silicate, calcium sorbate, calcium stearate, calcium
sulfate,
calcium sulfate hemihydrate, canola oil, carbomer, carbon dioxide,
carboxymethyl
cellulose calcium, carboxymethyl cellulose sodium, 13-carotene, carrageenan,
castor
oil, hydrogenated castor oil, cationic emulsifying wax, cellulose acetate,
cellulose
acetate phthalate, ethyl cellulose, microcrystalline cellulose, powdered
cellulose,
silicified microcrystalline cellulose, sodium carboxymethyl cellulose,
cetostearyl
alcohol, cetrimide, cetyl alcohol, chlorhexidine, chlorobutanol, chlorocresol,
cholesterol, chlorhexidine acetate, chlorhexidine gluconate, chlorhexidine
89
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
hydrochloride, chlorodifluoroethane (HCFC), chlorodifluoromethane,
chlorofluorocarbons (CFC)chlorophenoxyethanol, chloroxylenol, corn syrup
solids,
anhydrous citric acid, citric acid monohydrate, cocoa butter, coloring agents,
corn oil,
cottonseed oil, cresol, m-cresol, o-cresol, p-cresol, croscarmellose sodium,
crospovidone, cyclamic acid, cyclodextrins, dextrates, dextrin, dextrose,
dextrose
anhydrous, diazolidinyl urea, dibutyl phthalate, dibutyl sebacate,
diethanolamine,
diethyl phthalate, difluoroethane (HFC), dimethyl-13-cyclodextrin,
cyclodextrin-type
compounds such as Captisol , dimethyl ether, dimethyl phthalate, dipotassium
edentate, disodium edentate, disodium hydrogen phosphate, docusate calcium,
docusate potassium, docusate sodium, dodecyl gallate, dodecyltrimethylammonium
bromide, edentate calcium disodium, edtic acid, eglumine, ethyl alcohol,
ethylcellulose, ethyl gallate, ethyl laurate, ethyl maltol, ethyl oleate,
ethylparaben,
ethylparaben potassium, ethylparaben sodium, ethyl vanillin, fructose,
fructose liquid,
fructose milled, fructose pyrogen-free, powdered fructose, fumaric acid,
gelatin,
glucose, liquid glucose, glyceride mixtures of saturated vegetable fatty
acids, glycerin,
glyceryl behenate, glyceryl monooleate, glyceryl monostearate, self-
emulsifying
glyceryl monostearate, glyceryl palmitostearate, glycine, glycols, glycofurol,
guar
gum, heptafluoropropane (HFC), hexadecyltrimethylammonium bromide, high
fructose syrup, human serum albumin, hydrocarbons (HC), dilute hydrochloric
acid,
hydrogenated vegetable oil, type II, hydroxyethyl cellulose, 2-hydroxyethyl-f3-
cyclodextrin, hydroxypropyl cellulose, low-substituted hydroxypropyl
cellulose, 2-
hydroxypropy1-13-cyclodextrin, hydroxypropyl methylcellulose, hydroxypropyl
methylcellulose phthalate, imidurea, indigo carmine, ion exchangers, iron
oxides,
isopropyl alcohol, isopropyl myristate, isopropyl palmitate, isotonic saline,
kaolin,
lactic acid, lactitol, lactose, lanolin, lanolin alcohols, anhydrous lanolin,
lecithin,
magnesium aluminum silicate, magnesium carbonate, normal magnesium carbonate,
magnesium carbonate anhydrous, magnesium carbonate hydroxide, magnesium
hydroxide, magnesium lauryl sulfate, magnesium oxide, magnesium silicate,
magnesium stearate, magnesium trisilicate, magnesium trisilicate anhydrous,
malic
acid, malt, maltitol, maltitol solution, maltodextrin, maltol, maltose,
mannitol,
medium chain triglycerides, meglumine, menthol, methylcellulose, methyl
methacrylate, methyl oleate, methylparaben, methylparaben potassium,
methylparaben sodium, microcrystalline cellulose and carboxymethylcellulose
sodium, mineral oil, light mineral oil, mineral oil and lanolin alcohols, oil,
olive oil,
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
monoethanolamine, montmorillonite, octyl gallate, oleic acid, palmitic acid,
paraffin,
peanut oil, petrolatum, petrolatum and lanolin alcohols, pharmaceutical glaze,
phenol,
liquified phenol, phenoxyethanol, phenoxypropanol, phenylethyl alcohol,
phenylmercuric acetate, phenylmercuric borate, phenylmercuric nitrate,
polacrilin,
polacrilin potassium, poloxamer, polydextrose, polyethylene glycol,
polyethylene
oxide, polyacrylates, polyethylene-polyoxypropylene-block polymers,
polymethacrylates, polyoxyethylene alkyl ethers, polyoxyethylene castor oil
derivatives, polyoxyethylene sorbitol fatty acid esters, polyoxyethylene
stearates,
polyvinyl alcohol, polyvinyl pyrrolidone, potassium alginate, potassium
benzoate,
potassium bicarbonate, potassium bisulfite, potassium chloride, postassium
citrate,
potassium citrate anhydrous, potassium hydrogen phosphate, potassium
metabisulfite,
monobasic potassium phosphate, potassium propionate, potassium sorbate,
povidone,
propanol, propionic acid, propylene carbonate, propylene glycol, propylene
glycol
alginate, propyl gallate, propylparaben, propylparaben potassium,
propylparaben
sodium, protamine sulfate, rapeseed oil, Ringer's solution, saccharin,
saccharin
ammonium, saccharin calcium, saccharin sodium, safflower oil, saponite, serum
proteins, sesame oil, colloidal silica, colloidal silicon dioxide, sodium
alginate,
sodium ascorbate, sodium benzoate, sodium bicarbonate, sodium bisulfite,
sodium
chloride, anhydrous sodium citrate, sodium citrate dehydrate, sodium chloride,
sodium cyclamate, sodium edentate, sodium dodecyl sulfate, sodium lauryl
sulfate,
sodium metabisulfite, sodium phosphate, dibasic, sodium phosphate, monobasic,
sodium phosphate, tribasic, anhydrous sodium propionate, sodium propionate,
sodium
sorbate, sodium starch glycolate, sodium stearyl fumarate, sodium sulfite,
sorbic acid,
sorbitan esters (sorbitan fatty esters), sorbitol, sorbitol solution 70%,
soybean oil,
spermaceti wax, starch, corn starch, potato starch, pregelatinized starch,
sterilizable
maize starch, stearic acid, purified stearic acid, stearyl alcohol, sucrose,
sugars,
compressible sugar, confectioner's sugar, sugar spheres, invert sugar, Su.
gartab,
Sunset Yellow FCF, synthetic paraffin, talc, tartaric acid, tartrazine,
tetrafluoroethane
(HFC), theobroma oil, thimerosal, titanium dioxide, alpha tocopherol,
tocopheryl
acetate, alpha tocopheryl acid succinate, beta-tocopherol, delta-tocopherol,
gamma-
tocopherol, tragacanth, triacetin, tributyl citrate, triethanolamine, triethyl
citrate,
trimethyl-P-cyclodextrin, trimethyltetradecylammonium bromide, tris buffer,
trisodium edentate, vanillin, type I hydrogenated vegetable oil, water, soft
water, hard
water, carbon dioxide-free water, pyrogen-free water, water for injection,
sterile water
91
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
for inhalation, sterile water for injection, sterile water for irrigation,
waxes, anionic
emulsifying wax, camauba wax, cationic emulsifying wax, cetyl ester wax,
microcrystalline wax, nonionic emulsifying wax, suppository wax, white wax,
yellow
wax, white petrolatum, wool fat, xanthan gum, xylitol, zein, zinc propionate,
zinc
salts, zinc stearate, or any excipient in the Handbook of Pharmaceutical
Excipients,
Third Edition, A. H. Kibbe (Pharmaceutical Press, London, UK, 2000), which is
incorporated by reference in its entirety. Remington 's Pharmaceutical
Sciences,
Sixteenth Edition, E. W. Martin (Mack Publishing Co., Easton, Pa., 1980),
which is
incorporated by reference in its entirety, discloses various components used
in
formulating pharmaceutically acceptable compositions and known techniques for
the
preparation thereof. Except insofar as any conventional agent is incompatible
with
the pharmaceutical compositions, its use in pharmaceutical compositions is
contemplated. Supplementary active ingredients also can be incorporated into
the
compositions.
The pharmaceutical formulations disclosed herein may be designed to be
short-acting, fast-releasing, long-acting, or sustained-releasing as described
below.
The pharmaceutical formulations may also be formulated for immediate release,
controlled release or for slow release. The instant compositions may further
comprise, for example, micelles or liposomes, or some other encapsulated form,
or
may be administered in an extended release form to provide a prolonged storage
and/or delivery effect. The disclosed pharmaceutical formulations may be
administered according to any regime including, for example, daily (1 time per
day, 2
times per day, 3 times per day, 4 times per day, 5 times per day, 6 times per
day),
every two days, every three days, every four days, every five days, every six
days,
weekly, bi-weekly, every 'three weeks, monthly, or bi-monthly.
= In some embodiments, the foregoing component(s) may be present in the
pharmaceutical composition at any concentration, such as, for example, at
least A,
wherein A is 0.0001% w/v, 0.001% w/v, 0.01% w/v, 0.1% w/v, 1% w/v, 2% w/v, 5%
w/v, 10% w/v, 20% w/v, 30% w/v, 40% w/v, 50% w/v, 60% w/v, 70% w/v, 80% w/v,
or 90% w/v. In some embodiments, the foregoing component(s) may be present in
the pharmaceutical composition at any concentration, such as, for example, at
most B,
wherein B is 90% w/v, 80% w/v, 70% w/v, 60% w/v, 50% w/v, 40% w/v, 30% w/v,
20% w/v, 10% w/v, 5% w/v, 2% w/v, I% w/v, 0.1% w/v, 0.001% w/v, or 0.0001%.
In other embodiments, the foregoing component(s) may be present in the
92
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
pharmaceutical composition at any concentration range, such as, for example
from
about A to about B. In some embodiments, A is 0.0001% and B is 90%.
The pharmaceutical compositions may be formulated to.achieve a
physiologically compatible pH. In some embodiments, the pH of the
pharmaceutical
composition may be at least 5, at least 5.5, at least 6, at least 6.5, at
least 7, at least
7.5, at least 8, at least 8.5, at least 9, at least 9.5, at least 10, or at
least 10.5 up to and
including pH 11, depending on the formulation and route of administration. In
certain
embodiments, the pharmaceutical compositions may comprise buffering agents to
achieve a physiological compatible pH. The buffering agents may include any
20 Position 3 Modification
Any of the glucagon peptides, including glucagon analogs, glucagon agonist
analogs, glucagon co-agonists, and glucagon/GLP-1 co-agonist molecules,
described
herein may be modified to contain a modification at position 3, e.g., Gin
substituted
with Glu, to produce a peptide with high selectivity, e.g., tenfold
selectivity, for the
Any of the glucagon peptides, including glucagon analogs, glucagon agonist
analogs, glucagon co-agonists, and glucagon/GLP-1 co-agonist molecules,
described
herein may be modified to contain a modification at position 3, e.g.,Gln
substituted
with a glutamine analog (e.g. Dab(Ac)), without a substantial loss of activity
at the
Preparation Methods
The compounds of this invention may be prepared by standard synthetic
methods, recombinant DNA techniques, or any other methods of preparing
peptides
93
=
=
CA 02 8 5 2 17 7 2 0 1 4-0 5-2 3
=
= WO 2009/155258
PCT/US2009/047438
29920-208831
and fusion proteins. Although certain non-natural amino acids cannot be
expressed
by standard recombinant DNA techniques, techniques for their preparation are
known
in the art. Compounds of this invention that encompass non-peptide portions
may be
synthesized by standard organic chemistry reactions, in addition to standard
peptide
5 chemistry reactions when applicable.
EXAMPLES =
General Synthesis Protocol:
Glucagon analogs were synthesized using HBTU-activated "Fast Boc" single
10 coupling starting from 0.2mmole of Boc Thr(OBz1)Pam resin on a modified
Applied
Biosystem 430 A peptide synthesizer. Boc amino acids and HBTU were obtained
from Midwest Biotech (Fishers, IN). Side chain protecting groups used were:
Arg(Tos), Asn(Xan), Asp(OcHex), Cys(pMeBzI), His(Bom), Lys(2CI-Z), Ser(OBz1),
Thr(OBz1), Tyr(2Br-Z), and Trp(CH0). The side-chain protecting group on the N-
15 terminal His was Boc.
Each completed peptidyl resin was treated with a solution of 20% piperdine in
dimethylformamide to remove the formyl group from the tryptophan. Liquid
hydrogen fluoride cleavages were performed in the presence of p-cresol and
dimethyl
sulfide. The cleavage was run for 1 hour in an ice bath using an HF apparatus
20 (Penninsula Labs). After evaporation of the HF, the residue was
suspended in diethyl
ether and the solid materials were filtered. Each peptide was'extracted into
30-70m1
aqueous acetic acid and a diluted aliquot was analyzed by HPLC [Beckman System
Gold, 0.46x5cm Zorbax C8, lmUmin, 45C, 214nm, A buffer =0.1%TFA,
B=0.1%TFA/90%acetonitrile, gradient of 10% to 80%B over 10min].
25 Purification was done on a FPLC over a 2.2 x 25 cm Kromasil CI8 column
while monitoring the UV at 214nm and 'collecting 5 minute fractions. The
homogeneous fractions were combined and lyophilized to give a product purity
of
>95%. The correct molecular mass and purity were confirmed using MALDI-mass
spectral analysis.
94
CA 02852177 2014-05-23
=
WO 2009/155258
PCT/US2009/047438
29920-208831
General Pegylation Protocol: (Cys-maleimido)
Typically, the glucagon Cys analog is dissolved in phosphate buffered saline
(5-10mg/m1) and 0.01M ethylenediamine tetraacetic acid is added (10-15% of
total
volume). Excess (2-fold) maleimido methoxyPEG reagent (Nektar) is added and
the
reaction stirred at room temp while monitoring reaction progress by HPLC.
After 8-
24hrs, the reaction mixture, is acidified and loaded onto a preparative
reverse phase
column for purification using 0.1%TFA/acetonitrile gradient. The appropriate
fractions were combined and lyophilized to give the desired pegylated analogs.
EXAMPLE 1
Synthesis of Glucagon Cys17(1-29) and Similar MonoCys Analogs
= 0.2mmole Boc Thr(OBz1) Pam resin (SynChem Inc) in a 60M' reaction vessel
and the following sequence was entered and run on a modified Applied
Biosystems
430A Peptide Synthesizer using FastBoc HBTU-activated single couplings.
HSQGTFTSDYSKYLDSCRAQDFVQWLMNT (SEQ ID NO: 35)
The following side chain protecting groups were used: Arg(Tos), Asp(OcHex),
Asn(Xan), Cys(pMeBz1), Glu(OcHex), His(Boc), Lys(2C1-Z), Ser(Bz1), Thr(Bz1),
Trp(CH0), and Tyr(Br-Z). The completed peptidyl resin was treated with 20%
piperidine/dimethylformamide to remove the Trp formyl protection then
transferred to
=an HF reaction vessel and dried in vacuo. 1.0m1 p-cresol and 0.5 ml dimehyl
sulfide
were added along with a magnetic stir bar. The vessel was attached to the HF
apparatus (Pennisula Labs), cooled in a dry ice/methanol bath, evacuated, and
aprox.
10m1 liquid hydrogen fluoride was condensed in. The reaction was stirred in an
ice
bath for I hr then the HF was removed in vacuo. The residue was suspended in
ethyl
ether; the solids were filtered, washed with ether, and the peptide extracted
into 50 ml
aqueous acetic acid. An analytical HPLC was run [0.46 x 5 cm Zorbax C8, 1
ml/min,
45C, 214nm, A buffer of 0.1%TFA, B buffer of 0.1%TFA/90%ACN, gradient=1 0%B
to 80%B over 10mind with a small sample of the cleavage extract. The remaining
=
extract was loaded onto a 2.2 x 25cm Kromasil C18 preparative reverse phase
column
and an acetonitrile gradient was run using a Pharmacia FPLC system. 5min
fractions
were collected while monitoring the UV at 214nm (2.0A). A=0.1%TFA,
B=0.1%TFA/50%acetonitrile. Gradient = 30%B to 100%B over 450min.
The fractions containing the purest product (48-52) were combined frozen,
and lyophilized to give 30.1mg. An HPLC analysis of the product demonstrated a
=
CA 02852177 2014-05-23
=
=
WO 2009/155258
PCT/US2009/047438
29920-208831
purity of >90% and MALDI mass spectral analysis demonstrated the desired mass
of
3429.7. Glucagon Cys21, Glucagon Cys24, and Glucagon Cys29 were similarly
prepared.
5 EXAMPLE 2
Synthesis of Glucagon-Cex and Other C-Terminal Extended Analogs.
285mg (0.2mmole) methoxybenzhydrylamine resin (Midwest Biotech) was
placed in a 60m1 reaction vessel and the following sequence was entered and
run on a
modified Applied Biosystems 430A peptide synthesizer using FastBoc HBTU-
10 activated single couplings.
HSQGTFTSDYSKYLDSRRAQDFVQWLMNTGPSSGAPPPS (SEQ ID NO:
36)
The following side chain protecting groups were used: Arg(Tos), Asp(OcHex),
Asn(Xan), Cys(pMeBz1), Glu(OcHex), His(Boc), Lys(2CI-Z), Ser(Bz1), Thr(Bz1),
15 Trp(CH0), and Tyr(Br-Z). The completed peptidyl resin was treated with
20%
piperidine/dimethylformamide to remove the Trp formyl protection then
transferred to
HF reaction vessel and dried in vacuo. 1.0m1 p-cresol and 0.5 ml dimehyl
sulfide
were added along with a magnetic stir bar. The vessel was attached to the HF
apparatus (Pennisula Labs), cooled in a dry ice/methanol bath, evacuated, and
aprox.
20 10m1 liquid hydrogen fluoride was condensed in. The reaction was stirred
in an ice
bath for lhr then the HF was removed in vacuo. The residue was suspended in
ethyl
ether; the solids were filtered, washed with ether, and the peptide extracted
into 50 ml
aqueous acetic acid. An analytical HPLC was run [0.46 x 5 cm Zorbax C8, 1
ml/min,
45C, 214nm, A buffer of 0.1%TFA, B buffer of 0.1%TFA/90%ACN, gradient=10%B
25 to 80%B over 10min.] on an aliquot of the cleavage extract. The extract
was loaded
onto a 2.2 x 25cm Kromasil C18 preparative reverse phase column and an
acetonitrile
gradient was run for elution using a Pharmacia FPLC system. 5min fractions
were
collected while monitoring the UV at 214nm (2.0A). A=0.1%TFA,
B=0.1%TFA/50%acetonitrile. Gradient = 30%B to I00%B over 450min. Fractions
30 58-65 were combined, frozen and lyophilized to give 198.1mg.
HPLC analysis of the product showed a purity of greater than 95%. MALDI
mass spectral analysis showed the presence of the desired theoretical mass of
4316.7
with the product as a C-terminal amide. Oxyntomodulin and oxyntomodulin-KRNR
96
CA 02852177 2014-05-23
=
WO 2009/155258
PCT/US2009/047438
29920-208831
were similarly prepared as the C-terminal carboxylic acids starting with the
appropriately loaded PAM-resin.
EXAMPLE 3
Glucagon Cys17 Mal-PEG-5K
15.1mg of Glucagon Cys17(1-29) and 27.3mg methoxy poly(ethyleneglycol)
maleimide avg. M.W.5000 (mPEG-Mal-5000,Nektar Therapeutics) were dissolved in
3.5m1 phosphate buffered saline (PBS) and 0.5ml 0.01M ethylenediamine
tetraacetic
acid (EDTA) was added. The reaction was stirred at room temperature and the
progress of the reaction was monitored by HPLC analysis [0.46 x 5 cm Zorbax
C8,
Imlimin,45C, 214nm (0.5A), A=0.1%TFA, B=0.1%TFA/90%ACN, gradient=10%B
to 80%B over 10min.].
After 5 hours, the reaction mixture was loaded onto 2.2 x 25 cm ICromasil C18
preparastive reverse phase column. An acetonitrile gradient was run on a
Pharmacia
FPLC while monitoring the UV wavelength at 214nm and collecting 5 min
fractions.
A=0.1%TFA, B=0.1%TFA/50% acetonitrile, gradient= 30%B to 100%B over 450
min. The fractions corresponding to the product were combined, frozen and
lyophilized to give 25.9 mg.
This product was analyzed on HPLC [0.46 x 5 cm Zorbax C8, 1 ml/min, 45C,
214nm (0.5A), A=0.1%TFA, B=0.1%TFA/90%ACN, gradient=10%B to 80%B over
10min.] which showed a purity of aprox. 90%. MALDI (matrix assisted laser
desorption ionization) mass spectral analysis showed a broad mass range
(typical of
PEG derivatives) of 8700 to 9500. This shows an addition to the mass of the
starting
glucagon peptide (3429) of approximately 5,000 a.m.u.
EXAMPLE 4
Glucagon Cys2I Mal-PEG-5K
21.6mg of Glucagon Cys2I(1-29) and 24mg mPEG-MAL-5000 (Nektar
Therapeutics) were dissolved in 3.5m1 phosphate buffered saline (PBS) and
0.5m1
0.01M ethylene diamine tetraacetic acid (EDTA) was added. The reaction was
stirred
at room temp. After 2hrs, another 12.7 mg of mPEG-MAL-5000 was added. After
8hrs, the reaction mixture was loaded onto a 2.2 x 25cm Vydac C18 preparative
reverse phase column and an acetonitrile gradient was run on a Pharmacia FPLC
at 4
97
CA 02852177 2014-05-23
= WO 2009/155258
PCT/US2009/047438
29920-208831
ml/min while collecting 5min fractions. A=0.1%TFA, B=0.1%TFA/50%ACN.
Gradient= 20% to 80%B over 450min.
The fractions corresponding to the appearance of product were combined
frozen and lyophilized to give 34 mg. Analysis of the product by analytical
HPLC
5 [0.46 x 5 cm Zorbax C8, 1 ml/min, 45C, 214nm (0.5A), A=0.1%TFA,
B=0.1%TFA/90%ACN, gradient=10%B to 80%B over 10min.] showed a
homogeneous product that was different than starting glucagon peptide. MALDI
(matrix assisted laser desorption ionization) mass spectral analysis showed a
broad
mass range (typical of PEG analogs) of 8700 to 9700. This shows an addition to
the
10 mass of the starting glucagon peptide (3470) of approximately 5,000
a.m.u.
EXAMPLE 5
Glucagon Cys24 Mal-PEG-5K
20.1mg Glucagon C24(1-29) and 39.5mg mPEG-Mal-5000 (Nektar
15 Therapeutics) were dissolved in 3.5m1 PBS with stirring and 0.5 ml 0.01M
EDTA was
added. The reaction was stirred at room temp for 7 hrs, then another 40 mg of
mPEG-
Mal-5000 was added. After approximately 15 hr, the reaction mixture was loaded
onto a 2.2 x 25 cm Vydac C18 preparative reverse phase column and an
acetontrile
gradient was run using a Pharmacia FPLC. 5 min. fractions were collected while
20 monitoring the UV at 214nm (2.0A). A buffer = 0.1%TFA, B buffer =
0.1%TFA/50%ACN, gradient = 30%B to 100%B over 450min. The fractions
corresponding to product were combined, frozen and lyophilized to give 45.8mg.
MALDI mass spectral analysis showed a typical PEG broad signal with a maximum
at 9175.2 which is approximately 5,000 a.m.u. more than Glucagon C24 (3457.8).
EXAMPLE 6
Glucagon Cys24 Mal-PEG-20K =
25.7mg of Glucagon Cys24(1-29) and 40.7mg rnPEG-Mal-20K (Nektar
Therapeutics) were dissolved in 3.5m1 PBS with stirring at room temp. and 0.5
ml
30 0.01M EDTA was added. After 6hrs, the ratio of starting material to
product was
aprox. 60:40 as determined by HPLC. Another 25.1mg of mPEG-Mal-20K was
added and the reaction allowed to stir another 16hrs. The product ratio had
not
significantly improved, so the reaction mixture was loaded onto a 2.2 x 25 cm
Kromasil C18 preparative reverse phase column and purified on a Pharmacia FPLC
98
CA 02852177 2014-05-23
a
= WO 2009/155258
PCT/1JS2009/047438
29920-208831
using a gradient of 30%B to 100%B over 450min. A buffer =0.1%TFA, B buffer =
0.1%TFA/50%ACN, flow = 4m1/min, and 5 min fractions were collected while
monitoring the UV at 214nm (2.0A). The fractions containing homogeneous
product
were combined, frozen and lyophilized to give 25.7 mg. Purity as determined by
5 analytical HPLC was ¨90%. A MALDI mass spectral analysis showed a broad
peak
from 23,000 to 27,000 which is approximately 20,000 a.m.u. more than starting
Glucagon C24 (3457.8).
EXAMPLE 7
10 Glucagon Cys29 Mal-PEG-5K
20.0mg of Glucagon Cys29(1-29) and 24.7 mg mPEG-Mal-5000 (Nektar
Therapeutics) were dissolved in 3.5 ml PBS with stirring at room temperature
and 0.5
ml 0.01M EDTA was added. After 4 hr, another 15.6 mg of mPEG-Mal-5000 was
added to drive the reaction to completion. After 8 hrs, the reaction mixture
was
15 loaded onto a 2.2 x 25 cm Vydac C18 preparative reverse phase column and
an
acetonitrile gradient was run on a Pharmacia FPLC system. 5 min fractions were
collected while monitoring the UV at 214nm (2.0A). A=0.1%TFA,
B=0.1%TFA/50%ACN: Fractions 75-97 were combined frozen and lyophilized to
give 40.0 mg of product that is different than recovered starting material on
HPLC
20 (fractions 58-63). Analysis of the product by analytical HPLC [0.46 x 5
cm Zorbax
C8, 1 ml/min, 45C, 214nm (0.5A), A=0.1%TFA, B=0.1%TFA/90%ACN,
gradient=10%B to 80%B over 10min.] showed a purity greater than 95%. MALDI
mass spectral analysis showed the presence of a PEG component with a mass
range of
8,000 to 10,000 (maximum at 9025.3) which is 5,540 a.m.u. greater than
starting
25 material (3484.8).
EXAMPLE 8
Glucagon Cys24 (2-butyrolactone)
30 To 24.7mg of Glucagon Cys24(1-29) was added 4m1 0.05M ammonium
bicarbonate/50%acetonitrile and 5.5 ul of a solution of 2-bromo-4-
hydroxybutyric
acid-y-lactone (100u1 in 900u1 acetonitrile). After 3hrs of stirring at room
temperature, another 105 ul of lactone solution was added to the reaction
mixture
which was stirred another 15hrs. The reaction mixture was diluted to 10m1 with
10%
35 aqueous acetic acid and was loaded onto a 2.2 x 25 cm Kromasil C18
preparative
99
CA 02852177 2014-05-23
=
WO 2009/155258 PCT/US2009/047438
=
29920-208831
reverse phase column. An acetonitrile gradient (20%B to 80%B over 450min) was
run on a Pharmacia FPLC while collecting 5min fractions and monitoring the UV
at
214nm (2.0A). Flow =4m1/min, A=0.1%TFA, B=0.1%TFA/50%ACN. Fractions 74-
77 were combined frozen and lyophilized to give 7.5mg. HPLC analysis showed a
5 purity of 95% and MALDI mass spect analysis showed a mass of 3540.7 or 84
mass
units more than starting material. This result is consistent with the addition
of a single
butyrolactone moiety.
EXAMPLE 9
10 Glucagon Cys24(S-carboxymethyl)
18.1mg of Glucagon Cys24(l -29) was dissolved in 9.4m1 0.1M sodium
phosphate buffer (pH=9.2) and 0.6m1 bromoacetic acid solution (1.3mg/m1 in
acetonitrile) was added. The reaction was stirred at room temperature and the
15 reaction progress was followed by analytical HPLC. After lhr another
0.1m1
bromoacetic acid solution was added. The reaction was stirred another 60min.
then
acidified with aqueous acetic acid and was loaded onto a 2.2 x 25cm ICromasil
C18
preparative reverse phase column for purification. An acetonitrile gradient
was run
on a Pharmacia FPLC (flow = 4m1/min) while collecting 5min fractions and
20 monitoring the UV at 214nm (2.0A). A=0.1%TFA, BA).1%TFA/50%ACN.
Fractions 26-29 were combined frozen and lyophilized to give
several mg of product. Analytical HPLC showed a purity of 90% and MALDI mass
spectral analysis confirmed a mass of 3515 for the desired product.
xis7f0
S GT F TSDYSKY LDSRRAODF V-N r
WLMNT¨coo.
Molecular Weight =3515.87 . SEQ ID NO: 38
Exact Mass =3512
Molecular Formula =C153H224N42050S2
EXAMPLE 10
Glucagon Cys24 maleimido,PEG-3.4K-dimer
16mg Glucagon Cys24 and 1.02mg Mal-PEG-Mal-3400,
30 poly(ethyleneglycol)-bis-maleimide avg. M.W. 3400, (Nektar Therpeutics)
were
dissolved in 3.5 phosphate buffered saline and 0.5m1 0.01M EDTA and the
reaction
was stirred at room temperature. After 16hrs, another 16mg of Glucagon Cys24
was
added and the stirring continued. After approximately 40hrs, the reaction
mixture was
100
CA 02 8 5 2 17 7 2 0 1 4-0 5-2 3
=
WO 2009/155258
PCT/US2009/047438
29920-208831
loaded onto a Pharmcia PepRPC 16/10 column and an acetonitrile gradient was
run
on a Pharmacia FPLC while collecting 2min fractions and monitoring the UV at
214nm (2.0A). Flow=2m1/min, A=0.1%TFA, B=0.1%TFA/50%ACN. Fractions 69-
74 were combined frozen and lyophilized to give 10.4mg. Analytical HPLC showed
5 a purity of 90% and MALDI mass spectral analysis shows a component in the
9500-
11,000 range which is consistent with the desired dimer.
GlucagonCys24(1-29)
GlucagonCys24(1-29)
3457.80
3457.80 0 y__S
3572.00
10487.60
0 PEG3400
0
10 EXAMPLE 11
Synthesis of Glucagon Lactams
285 mg (0.2 mmole) methoxybenzhydrylamine resin (Midwest Biotech) was
added to a 60 mL reaction vessels and the following sequence was assembled on
a
modified Applied Biosystems 430A peptide synthesizer using Boc DEPBT-activated
15 single couplings.
HSQGTFTSDYSKYLDERRAQDFVQWLMNT-NH2 (12-16 Lactam; SEQ
ID NO: 12)
20 The following side chain protecting groups were used: Asg(Tos),
Asp(OcHx),
Asn(Xan), Glu(OFm), His(BOM), Lys(Fmoc), Ser(BzI), Thr(Bz1), Trp(CH0),
Tyr(Br-Z). Lys(C1-Z) was used at position 12 if lactams were constructed from
16-
20, 20-24, or 24-28. The completed peptidyl resin was treated with 20%
piperidine/dimethylformamide for one hour with rotation to remove the Trp
formyl
25 group as well as the Fmoc and OFm protection from Lys12 and G1u16. Upon
confirmation of removal by a positive ninhydrin test, the resin was washed
with
dimethylformamide, followed by dichloromethane and than again with
dimethylformamide. The resin was treated with 520 mg (1 mmole) Benzotriazole-1-
yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBOP) in
30 dimethylformamide and diisopropylethylamine (DIEA). The reaction
proceeded for
101
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
8-10 hours and the cyclization was confirmed by a negative ninhydrin reaction.
The
resin was washed with dimethylformam.ide, followed by dichloromethane and
subsequently treated with trifluoroacetic acid for 10 minutes. The removal of
the Boc
group was confirmed by a positive ninhydrin reaction. The resin was washed
with
dimethylformamide and dichloromethane and dried before being transferred to a
hydrofluoric acid (HF) reaction vessel. 500 p.L p-cresol was added along with
a
magnetic stir bar. The vessel was attached to the FIF apparatus (Peninsula
Labs),
cooled in a dry ice/methanol bath, evacuated, and approximately 10 mL of
liquid
hydrofluoric acid was condensed into the vessel. The reaction was stirred for
1 hour
to in an ice bath and the HF was subsequently removed in vacuo. The residue
was
suspended in ethyl ether; the solids were filtered, washed with ether, and the
peptide
was solubilized with 150 mL 20% acetonitrile/1% acetic acid.
An analytical HPLC analysis of the crude solubilized peptide was conducted
under the following conditions [4.6 X 30 mm Xterra C8, 1.50 mUmin, 220 nm, A
buffer 0.1% TFA/10% ACN, B buffer 0.1% TFA/100% ACN, gradient 5-95%B over
15 minutes]. The extract was diluted twofold with water and loaded onto a 2.2
X 25
cm Vydac C4 preparative reverse phase column and eluted using an acetonitrile
gradient on a Waters HPLC system (A buffer of 0.1% TFA/10% ACN, B buffer of
0.1% TFA/10% CAN and a gradient of 0-100% B over 120 minutes at a flow of
15.00
ml/min. HPLC analysis of the purified peptide demonstrated greater than 95%
purity
and electrospray ionization mass spectral analysis confirmed a mass of 3506 Da
for
the 12-16 lactam. Lactams from 16-20, 20-24, and 24-28 were prepared
similarly.
EXAMPLE 12
Glucagon Solubility Assays: =
A solution (I mg/ml or 3mg/m1) of glucagon (or an analog) is prepared in
0.01N HC1. 100u1 of stock solution is diluted to lml with 0.0IN HC1 and the UV
absorbance (276nm) is determined. The pH of the remaining stock solution is
adjusted to pH7 using
200-250u1 0.1M Na2HPO4 (pH9.2). The solution is allowed to stand overnight at
4 C
then centrifuged. 100u1 of supernatant is then diluted to lml with 0.01N HCI,
and the
UV absorbance is determined (in duplicate).
The initial absorbance reading is compensated for the increase in volume and
the following calculation is used to establish percent solubility:
102
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
=
Final Absorbance
X 100 = percent soluble
Initial Absorbance
Results are shown in Table I wherein Glucagon-Cex represents wild type
glucagon
(SEQ ID NO: 1) plus a carboxy terminal addition of SEQ ID NO: 26 and Glucagon-
Cex R12 represents SEQ ID NO: 39.
Table 1 Solubility date for glucagon analogs
Analog Percent Soluble
Glucagon 16
Glucagon-Cex, R12 104
Glucagon-Cex 87
Oxyntomodulin 104 =
Glucagon, Cysl7PEG5K 94
Glucagon, Cys2IPEGSK 105
Glucagon, Cys24PEG5K 133.
to EXAMPLE 13
Glucagon Receptor Binding Assay =
The affinity of peptides to the glucagon receptor was measured in a
competition binding assay utilizing scintillation proximity assay technology.
Serial 3-
fold dilutions of the peptides made in scintillation proximity assay buffer
(0.05 M
Tris-HCI, pH 7.5, 0.15 M NaC1, 0.1% w/v bovine serum albumin) were mixed in 96
well white/clear bottom plate (Corning Inc., Acton, MA) with 0.05 tiM (31125Th
iodotyrosyl) Tyrl 0 glucagon (Amersham Biosciences, Piscataway, NJ), 1-6
micrograms per well, plasma membrane fragments prepared from cells over-
expressing human glucagon receptor, and 1 mg/well polyethyleneimine-treated
wheat
germ agglutinin type A scintillation proximity assay beads (Amersham
Biosciences,
Piscataway, NJ). Upon 5 min shaking at 800 rpm on a rotary shaker, the plate
was
incubated 12h at room temperature and then read on MicroBeta1450 liquid
scintillation counter (Perkin-Ehner, Wellesley, MA). Non-specifically bound
(NSB)
radioactivity was measured in the wells with 4 times grater concentration of
"cold"
native ligand than the highest concentration in test samples and total bound
radioactivity was detected in the wells with no competitor. Percent specific
binding
was calculated as following: % Specific Binding = ((Bound-NSB)/(Total bound-
NSB)) X 100. IC50 values were determined by using Origin software (OriginLab,
Northampton, MA).
103
CA 02852177 2014-05-23
WO 2009/155258
PCT/U52009/047438
.29920-208831
EXAMPLE 14
Functional Assay- cAMP Synthesis
The ability of glucagon analogs to induce cAMP was measured in a firefly
luciferase-based reporter assay. HEK293 cells co-transfected with either
glucagon- or
GLP-1 receptor and luciferase gene linked to cAMP responsive element were
serum
deprived by culturing 16h in DMEM (Invitrogen, Carlsbad, CA) supplemented with
0.25% Bovine Growth Serum (HyClone, Logan, UT) and then incubated with serial
dilutions of either glucagon, GLP-1 or novel glucagon analogs for 5 h at 37 C,
5%
CO2 in 96 well poly-D-Lysine-coated "Biocoat".plates (BD Biosciences, San
Jose,
CA). At the end of the incubation 100 microliters of LucLite luminescence
substrate
reagent (Perkin-Elmer, Wellesley, MA) were added to each well. The plate was
1
shaken briefly, incubated 10 min in the dark and light output was measured on
MicroBeta-1450 liquid scintillation counter (Perkin-Elmer, Wellesley, MA).
Effective 50% concentrations were calculated by using Origin software
(OriginLab,
Northampton, MA. Results are shown in Figs. 3-9 and in Tables 2 through 10.
Table 2
cAMP Induction by Glucagon Analogs with C-Terminus Extension
cAMP Induction
Peptide Glucagon Receptor GLP-1 Receptor
EC50, nM N* EC50, nM
Glucagon 0.22 0.09 14 3.85 1.64
10
GLP-1 2214.00 182.43 2 0.04 0.01 14
Glucagon Cex 0.25 0.15 6 2.75 2.03
7
Oxyntomodulin 3.25 1.65 5 2.53 1.74
5
Oxyntomodulin KRNR 2.77 1.74 4 3.21 0.49
2
Glucagon R12 0.41 0.17 6 0.48 1 0.11
5
Glucagon R12 Cex 0.35 0.23 10 1.25 1 0.63
10
Glucagon R12 1(20 0.84 0.40 5 0.82 0.49
5
Glucagon R12 K24 1.00 0.39 4 1.25 0.97
5
Glucagon R12 K29 0.81 0.49 5 0.41 0.24
6
Glucagon Amide 0.26 0.15 3 1.90 0.35
2
Oxyntomodulin C24 2.54 0.63 2 5.27 0.26
2
Oxyntomodulin C24 PEG 20K 0.97 0.04 1 1.29 0.11
1
104
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
*- number of experiments
Table 3
cAMP Induction by Pegylated Glucagon Analogs
cAMP Induction
Peptide Glucagon Receptor GLP-1 Receptor
EC50, nM N* EC50, nM
Glucagon 0.33 0.23 18 12.71 3.74
2
Glucagon C17 PEG 5K 0.82 0.15 4 55.86 1.13
2
Glucagon C21 PEG 5K 0.37 0.16 6 11.52 3.68
2
Glucagon C24 PEG 5K 0.22 0.10 12 13.65 2.95
4
Glucagon C29 PEG 5K 0.96 0.07 2 12.71 1 3.74
2
Glucagon C24 PEG 20K 0.08 1 0.05 3 Not
determined
Glucagon C24 Dimer 0.10 0.05 3 Not
determined
GLP-1 >1000 0.05 0.02 4
= - number of experiments
Table 4
to cAMP Induction by E16 Glucagon Analogs
Percent Potency Relative to Native Ligand
P.eptide GRec GLP-1Rec
E16 Gluc-NH2 187.2 17.8
Glucagon 100.0 0.8
Gluc-NH2 43.2 4.0
NLeu3, E16 Gluc-NH2 7.6 20.6
E3, El6 Gluc-NH2 1.6 28.8
0m3, E16 Gluc-NH2 0.5 0.1
GLP-1 <0.1 100
Table 5
cAMP Induction by E16 Glucagon Analogs
Percent Potency Relative to Native Ligand
Peptide GRec GLP-1Rec
El6 Gluc-NH2 187.2 17.8
tE15, E16 Gluc-NH2 147.0 9.2
E16, K20 Gluc-NH2 130.1 41.5
Gluc-NH2 43.2 4.0
105
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Table 6
EC50 values for cAMP Induction by E16 Glucagon Analogs
Glucagon GLP-1
Receptor Receptor
Peptide EC50 (nM) StDev n EC50 (nM)
StDev n
Glucagon 0.28 0.14 10 4.51 N/A 1
Glucagon-NH2 0.53 0.33 8 1.82 0.96 5
E16 Gluc-NH2 0.07 0.07 10 0.16 0.14 10
E16, G30 Gluc-NH2 0.41 0.36 5 0.24 0.10 5
E16, G30 Gluc-Cex 0.51 0.46 5 1.19 0.86 5
,GLP-1 2214 N/A 1 0.03 0.02 9
Table 7
EC50 values for cAMP Induction by E16 Glucagon Analogs
Glucagon GLP-1
Receptor Receptor
Peptide EC50 (nM) StDev n EC50 (nM)
StDev n
E16 Glucagon NH2 0.07 0.07 10 0.16 0.14 10
hCS0316 Glucagon-NH2 0.25 0.12 2 0.19 0.02 2
hE16 Glucagon-NH2 0.17 0.08 2 0.25 0.03 2
= 1116 Glucagon-NH2 0.45 0.3 2 0.38
0.11 2
Q16 Glucagon-NH2 0.22 0.1 2 0.39 0.08 2
016 Glucagon-NH2 0.56 0.15 2 0.93 0.28 2
(S16) Glucagon-NH2 0.53 0.33 8 1.82 0.96 5
106
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
Table 8
EC50 values for cAMP Induction by E16 Glucagon Analogs
Glucagon GLP-1
Receptor Receptor
Peptide EC50 (nM) StD n EC50 (nM) StDev
n
E16 Glucagon NH2 0.07 0.07 10 0.16 0.14 10
T16 Glucagon NH2 0.10 0.02 3 1.99 0.48 3
G16 Glucagon NH2 0.10 0.01 3 2.46 0.60 3
Glucagon NH2 0.53 0.33 4 1.82 0.96 5
GLP-1 2214 N/A 1 0.03 0.02 9
E16 Gluc NH2 was 4-fold more potent at the glucagon receptor relative to G16-
COOH and T16 Gluc NH2,when the compounds were tested side by side.
Table 9
cAMP Induction by E16/Lactam Glucagon Analogs
Percent Potency Relative to Native Ligand
Peptide GRec GLP-1Rec
E24IC28 Gluc-NH2 Lac 196.4 12.5
E16K20 Gluc-NH2 Lac 180.8 63.0
K12E16 Gluc-NH2 Lac 154.2 63.3
K20E24 Gluc-NH2 Lac 120.2 8.1
E16 Gluc-NH2 187.2 17.8 .
E16, K20 Gluc-NH2 130.1 41.5
Glucagon 100.0 0.8
Gluc-NH2 43.2 4.0
Table 10
cAMP Induction by GLP-1 17-26 Glucagon Analogs
Glucagon GLP-1
Receptor Receptor
Peptide EC50(nM) StD EC50(nM) StD
GLP-1 0.023 0.002
Gluc-NH2 0.159 0.023
E16 GLP-I 0.009 0.000
E16 Glucagon-NH2 0.072 0.007
E16 GLP(17-26)Glu(27-29)-NH2 0.076 0.004 0.014 0.001
E16 GLP(17-29)-NH2 0.46 0.023 0.010 0.000
E16 GLP(17-29)-NH2 E24, K28 0.23 0.020 0.007
E16 GLP(17-29)-NH2 E24, K28 Lactam 0.16 0.017 0.007 0.000
107
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
EXAMPLE 15
Stability Assay for glucagon Cys-maleimido PEG analogs
Each glucagon analog was dissolved in water or PBS and an initial HPLC
analysis was conducted. After adjusting the pH ( 4, 5, 6, 7), the samples were
incubated over a specified time period at 37 C and re-analyzed by HPLC to
determine the integrity of the peptide. The concentration of the specific
peptide of
interest was determined and the percent remaining intact was calculated
relative to the
initial analysis. Results for Glucagon Cys2I-maleimidoPEG5K are shown in Figs.
1
and 2.
EXAMPLE 16
The following glucagon peptides are constructed generally as described above
in Examples 1-11:
= 15 In all of the following sequences, "a" means a C-terminal
amide.
HSQGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 70)
HSQGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 71)
HSQGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
72)
HSQGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
73)
HSQGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
74)
= HSQGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
75)
HSQGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 76)
HSQGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
77)
HSQGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
78)
HSQGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
79)
108
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
HSQGT FTSDY SKYLD ICRAAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
80)
HSQGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 81)
HSQGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 12-16; SEQ ID NO:
82)
HSQGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 16-20; SEQ ID NO:
83)
HSQGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 84)
HSQGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 12-16; SEQ ID NO:
85)
HSQGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 16-20; SEQ ID NO:
86)
X I SQGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 87)
X1SQGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 88)
XI SQGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam 16-20; SEQ ID NO: 89)
- X I SQGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam 12-16; SEQ ID NO: 90)
X I SQGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam 1 12-16; SEQ ID NO: 91)
X I SQGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam 16-20; SEQ ID NO: 92)
X I SQGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 93)
X ISQGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam 16-20; SEQ ID NO: 94)
XISQGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 95)
X I SQGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam 12-16; SEQ ID NO: 96)
XISQGT FTSDY SKYLD KRAAE DFVQW LMNTa (lactam 16-20; SEQ ID NO: 97)
X ISQGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 98)
X1 SQGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 12-16; SEQ ID NO: 99)
XISQGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam 16-20; SEQ ID NO: 100)
X I SQGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 101)
X I SQGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam 12-16; SEQ ID NO: 102)
X1SQGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam 16-20; SEQ ID NO: 103)
Wherein in the preceding sequences, X1 = (Des-amino)His =
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 104)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 105)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam (4) 16-20; SEQ ID NO: 106)
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 107)
109
CA 02 852 17 7 2 0 1 4-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 108)
HX2QGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 109)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 110)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 111)
HX2QGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 112)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 113)
HX2QGT FTSDY SKYLD KRAAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 114)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 115)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 12-16; SEQ ID NO: 116)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 16-20; SEQ ID NO: 117)
HX2QGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 118)
HX2QGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam 12-16; SEQ ID NO: 119)
HX2QGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 16-20; SEQ ID NO: 120)
Wherein in the preceding sequences X2 = Aminoisobutyric acid
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 121)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 122)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 123)
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 124)
HX2QGT FTSDY SKYLD ERRAK DFVQW LIVINTa (lactam @ 12-16; SEQ ID NO: 125)
HX2QGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 126)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 127)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 128)
HX2QGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 129)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 130)
HX2QGT FTSDY SKYLD KRAAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 131)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 132)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 12-16; SEQ ID NO: 133)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 16-20; SEQ ID NO: 134)
HX2QGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 135)
HX2QGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 12-16; SEQ ID NO: 136)
HX2QGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 16-20; SEQ ID NO: 137)
Wherein in the preceding sequences X2 = (D-Ala)
HSEGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 138)
=
HSEGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 139)
110
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
HSEGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
140)
HSEGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam 12-16; SEQ ID NO:
141)
HSEGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
142)
HSEGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
143)
HSEGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 144)
HSEGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
145)
HSEGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
146)
HSEGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO:
147)
HSEGT FTSDY SKYLD KRAAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO:
148)
HSEGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 149)
HSEGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 12-16; SEQ ID NO:
150)
HSEGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 16-20; SEQ ID NO:
151)
HSEGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 152)
HSEGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 12-16; SEQ ID NO:
153)
HSEGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 16-20; SEQ ID NO:
154)
X I SEGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 155)
XISEGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 156)
X I SEGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 157)
X I SEGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam 12-16; SEQ ID NO: 158)
X I SEGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 159)
X I SEGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 160)
X I SEGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 161)
XI SEGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 162)
111
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
XISEGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam 12-16; SEQ ID NO: 163)
XISEGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam 12-16; SEQ ID NO: 164)
XISEGT FTSDY SKYLD KRAAE DFVQW LMNTa (lactam 16-20; SEQ ID NO: 165)
XISEGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 166)
XISEGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam 12-16; SEQ ID NO: 167)
XISEGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 16-20; SEQ ID NO: 168)
XISEGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 169)
XISEGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 12-16; SEQ ID NO: 170)
XISEGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 16-20; SEQ ID NO: 171)
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 172)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 173)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam 16-20; SEQ ID NO: 174)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam 16-20; SEQ ID NO: 179)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam 12-16; SEQ ID NO: 184)
HX2EGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam @ 12-16; SEQ ID NO: 187)
HX2EGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam 16-20; SEQ ID NO: 188)
Wherein in the preceding sequences X2 = Aminoisobutyric acid
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMNTa (SEQ ID NO: 189)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMNTa (SEQ ID NO: 190)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 191)
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMNTa (lactam 12-16; SEQ ID NO: 192)
HX2EGT FTSDY SKYLD KRRAE DFVQW LMNTa (lactam @ 16-20; SEQ ID NO: 194)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMNTa (SEQ ID NO: 195)
112
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
HX2EGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam 16-20; SEQ ID NO: 196)
HX2EGT FTSDY SKYLD ERAAQ DFVQW LMNTa (lactam @ 12-16; SEQ ID NO: 197)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMNTa (lactam 12-16; SEQ ID NO: 198)
HX2EGT FTSDY SKYLD KRAAE DFVQW LMNTa (lactam 16-20; SEQ ID NO: 199)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMNTa (SEQ ID NO: 200)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam 12-16; SEQ ID NO: 201)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMNTa (lactam @ 16-20; SEQ ID NO: 202)
HX2EGT FTSDY SKYLD EQAAK EFIAW LVKGa (SEQ ID NO: 203)
HX2EGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam (4) 12-16; SEQ ID NO: 204)
HX2EGT FTSDY SKYLD EQAAK EFIAW LVKGa (lactam 16-20; SEQ ID NO: 205)
Wherein in the preceding sequences X2 = (D-Ala)
HSQGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 206)
= HSQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 207)
HSQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 208)
HSQGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 209)
HSQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 210)
HSQGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 211)
HSQGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO:.212)
HSQGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 213)
HSQGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 214)
HSQGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 215)
HSQGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 216)
HSQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 217)
HSQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 12-16; SEQ ID NO: 218)
HSQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 219)
HSQGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 220)
HSQGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam 12-16; SEQ ID NO: 221)
HSQGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 16-20; SEQ ID NO: 222)
X1SQGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 223)
XISQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 224)
XISQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 225)
XISQGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 226)
XISQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 227)
XISQGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 228)
X I SQGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 229)
113
CA 02852177 2014-05-23
WO 2009/155258
PCT/1JS2009/047438
29920-208831
X 1 SQGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 230)
XISQGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 231)
XISQGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 232)
X I SQGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 233)
XISQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 234)
XISQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam 12-16; SEQ ID NO: 235)
XISQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 236)
XISQGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 237)
XISQGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 12-16; SEQ ID NO: 238)
XISQGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 16-20; SEQ ID NO: 239)
Wherein in the preceding sequences XI = (Des-amino)His; and wherein the C* is
a
Cys, or a Cys attached to a hydrophilic polymer, or alternatively the C* is a
Cys
attached to a polyethylene glycol of about 20 kD average weight, or
alternatively the
C* is a Cys attached to a polyethylene glycol of about 40 kD average weight.
HX2QGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 240)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 241)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 242)
HX2QGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 243)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 244)
HX2QGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 245)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 246)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 247)
. HX2QGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 248)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 249)
HX2QGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 250)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 251)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 12-16; SEQ ID NO: 252)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 253)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 254)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 12-16; SEQ ID NO: 255)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 16-20; SEQ ID NO: 256)
Wherein in the preceding sequences X2 = Aminoisobutyric acid; and wherein the
C*
is a Cys, or a Cys attached to a hydrophilic polymer, or alternatively the C*
is a Cys
attached to a polyethylene glycol of about 20 kD average weight, or
alternatively the
0' is a Cys attached to a polyethylene glycol of about 40 kD average weight.
114
=
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
HX2QGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 257)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 258)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 259)
HX2QGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 260)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 261)
HX2QGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 262)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 263)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 264)
HX2QGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 265)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 266)
HX2QGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 267)
1-1X2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 268)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 12-16; SEQ ID NO: 269)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 270)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 271)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 12-16; SEQ ID NO: 272)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 16-20; SEQ ID NO: 273)
= Wherein in the preceding sequences X2 = (D-Ala); and wherein the C* is a
Cys, or a
Cys attached to a hydrophilic polymer, or alternatively the C* is a Cys
attached to a
polyethylene glycol of about 20 IcD average weight, or alternatively the C* is
a Cys
attached to a polyethylene glycol of about 40 IcD average weight.
HSEGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 274)
HSEGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 275)
HSEGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO:
276)
HSEGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO:
277)
HSEGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO:
278)
HSEGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO:
279)
HSEGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 280)
HSEGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 281)
115
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
HSEGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 282)
HSEGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 283)
HSEGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 284)
HSEGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO:.285)
HSEGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam 12-16; SEQ ID NO: 286)
HSEGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam 16-20; SEQ ID NO: 287)
HSEGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 288)
HSEGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam 12-16; SEQ ID NO: 289)
HSEGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam 16-20; SEQ ID NO: 290)
X1SEGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 291)
X1SEGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 292)
X1SEGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 293)
X1SEGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 294)
X1SEGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 295)
X1SEGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 296)
X I SEGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 297)
X I SEGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactarn 16-20; SEQ ID NO: 298)
X I SEGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 299)
X1SEGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 300)
X1SEGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 301)
X1SEGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 302)
X1SEGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam 12-16; SEQ ID NO: 303)
X I SEGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 304)
X1SEGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 305)
X1SEGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 12-16; SEQ ID NO: 306)
X1SEGT FTSDY SKYLD EQAAK EF1C*W LVKGa (lactam @ 16-20; SEQ ID NO: 307)
Wherein in the preceding sequences X1 = (Des-amino)His; and wherein the C* is
a
Cys, or a Cys attached to a hydrophilic polymer, or alternatively the C* is a
Cys
attached to a polyethylene glycol of about 20 kD average weight, or
alternatively the
C* is a Cys attached to a polyethylene glycol of about 40 IcD average weight.
HX2EGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 308)
HX2EGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 309)
HX2EGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam 16-20; SEQ ID NO: 310)
HX2EGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 311)
HX2EGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 312)
116
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
HX2EGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 313)
HX2EGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 314)
HX2EGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 315)
HX2EGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam 12-16; SEQ ID NO: 316)
HX2EGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 317)
HX2EGT FTSDY SKYLD lUtAAE DEVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 318)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 319)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 12-16; SEQ ID NO: 320)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 321)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 322)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 12-16; SEQ ID NO: 323)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 16-20; SEQ ID NO: 324)
Wherein in the preceding sequences X2 = Aminoisobutyric acid; and wherein the
C*
is a Cys, or a Cys attached to a hydrophilic polymer, or alternatively the C*
is a Cys
= 15 attached to a polyethylene glycol of about 20 kD average weight, or
alternatively the
C* is a Cys attached to a polyethylene glycol of about 40 kD average weight.
HX2EGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (SEQ ID NO: 325)
HX2EGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 326)
HX2EGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 327)
HX2EGT FTSDY SKYLD ERRAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 328)
HX2EGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 329)
HX2EGT FTSDY SKYLD KRRAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 330)
HX2EGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 331)
HX2EGT FTSDY SKYLD ERAAK DFVC*W LMNTa (lactam @ 1620; SEQ ID NO: 332)
HX2EGT FTSDY SKYLD ERAAQ DFVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 333)
HX2EGT FTSDY SKYLD ERAAK DEVC*W LMNTa (lactam @ 12-16; SEQ ID NO: 334)
HX2EGT FTSDY SKYLD KRAAE DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 335)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 336)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 12-16; SEQ ID NO: 337) =
HX2EGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 338)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LVKGa (SEQ ID NO: 339)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 12-16; SEQ ID NO: 340)
HX2EGT FTSDY SKYLD EQAAK EFIC*W LVKGa (lactam @ 16-20; SEQ ID NO: 341)
Wherein in the preceding sequences X2 = (D-Ala); and wherein the C* is a Cys,
or a
Cys attached to a hydrophilic polymer, or alternatively the C* is a Cys
attached to a
117
CA 02 852 17 7 2 0 1 4-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
polyethylene glycol of about 20 kD average weight, or alternatively the C* is
a Cys =
attached to a polyethylene glycol of about 40 kD average weight.
HSQGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ 1D NO: 342)
HSQGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 343)
HSQGT FTSDY SKYLD. C*QAAK EFIAW LMNTa (SEQ ID NO: 344)
HSQGT FTSDY SKYLD C*QAAK.EFIAW LVKGa (SEQ ID NO: 345)
X1SQGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 346)
X1SQGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 347)
X1 SQGT FTSDY SKYLD C*QAAK EFIAW LMNTa (SEQ ID NO: 348)
X I SQGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 349)
Wherein X1 = (Des-amino)His; and wherein the C* is a Cys, or a Cys attached to
a
hydrophilic polymer, or alternatively the C* is a Cys attached to a
polyethylene glycol
of about 20 kD average weight, or alternatively the C* is a Cys attached to a
polyethylene glycol of about 40 kD average weight.
1-1X2QGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 350)
HX2QGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 351)
HX2QGT FTSDY SKYLD C*QAAK EFIAW LMNTa (SEQ ID NO: 352)
HX2QGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 353)
Wherein X2 = Aminoisobutyric acid; and wherein the C* is a Cys, or a Cys
attached
to a hydrophilic polymer, or alternatively the C* is a Cys attached to a
polyethylene
= glycol of about 20 kD average weight, or alternatively the C* is a Cys
attached to a
polyethylene glycol of about 40 kD average weight.
=
HX2QGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 354)
HX2QGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 355)
HX2QGT FTSDY SKYLD C*QAAK EFIAW LMNTa (SEQ ID NO: 356)
HX2QGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 357)
Wherein X2 = (D-Ala); and wherein the C* is a Cys, or a Cys attached to a
hydrophilic polymer, or alternatively the C* is a Cys attached to a
polyethylene glycol
of about 20 kD average weight, or alternatively the C* is a Cys attached to a
polyethylene glycol of about 40 kD average weight.
HSEGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 358)
HSEGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 359)
118
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
=
29920-208831
HSEGT FTSDY SKYLD C*QAAK EFIAW LMNTa (SEQ ID NO: 360)
HSEGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 361)
X ISEGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 362)
X I SEGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 363)
X I SEGT FTSDY SKYLD C*QAAK EF1AW LMNTa (SEQ ID NO: 364)
X I SEGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 365)
Wherein XI = (Des-amino)His; and wherein the C* is a Cys, or a Cys attached to
a
hydrophilic polymer, or alternatively the C* is a Cys attached to a
polyethylene glycol
of about 20 kD average weight, or alternatively the C* is a Cys attached to a
polyethylene glycol of about 40 kD average weight.
HX2EGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 366) =
HX2EGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 367)
HX2EGT FTSDY SKYLD C*QAAK EFIAW LMNTa (SEQ ID NO: 368)
HX2EGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 369)
Wherein X2 = (D-Ala) ; and wherein the C* is a Cys, or a Cys attached to a
-hydrophilic polymer, or alternatively the C* is a Cys attached to a
polyethylene glycol
of about 20 kD average weight, or alternatively the C* is a Cys attached to a
polyethylene glycol of about 40 kD average weight.
HX2EGT FTSDY SKYLD C*RRAK DFVQW LMNTa (SEQ ID NO: 370)
HX2EGT FTSDY SKYLD C*RAAK DFVQW LMNTa (SEQ ID NO: 371)
HX2EGT FTSDY SKYLD C*QAAK EFIAW LMNTa (SEQ ID NO: 372)
HX2EGT FTSDY SKYLD C*QAAK EFIAW LVKGa (SEQ ID NO: 373)
Wherein X2 = (D-Ala) ; and wherein the C* is a Cys, or a Cys attached to a
hydrophilic polymer, or alternatively the C* is a Cys attached to a
polyethylene glycol
of about 20 kD average weight, or alternatively the C* is a Cys attached to a
polyethylene glycol of about 40 kD average weight.
HSQGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 374)
HSQGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 375)
HSQGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam (4) 16-20; SEQ ID NO: 376)
HSQGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam (4) 12-16; SEQ ID NO: 377)
HSQGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 12-16; SEQ ID NO: 378)
HSQGT FTSDY SKYLD ICRRAE DFVQW LMDTa (lactam 16-20; SEQ ID NO: 379)
119
=
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
HSQGT FTSDY SKYLD ERAAK DFVQW LMDTa (SEQ ID NO: 380)
HSQGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 381)
HSQGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam 12-16; SEQ ID NO: 382)
HSQGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam (4/ 12-16; SEQ ID NO: 383)
HSQGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 12-16; SEQ ID NO: 386)
HSQGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 16-20; SEQ ID NO: 387)
XI SQGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 389)
XISQGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 390)
XISQGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam 12-16; SEQ ID NO: 391)
XI SQGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 12-16; SEQ ID NO: 392)
XISQGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 395)
XISQGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam 12-16; SEQ ID NO: 396)
XI SQGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam 12-16; SEQ ID NO: 397)
XISQGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 12-16; SEQ ID NO: 400)
X1 SQGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 16-20; SEQ ID NO: 401)
Wherein in the preceding sequences X1 = (Des-amino)His
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 402)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 403)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 404)
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 405)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 409)
HX2QGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 410)
120
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
11X2QGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 12-16; SEQ ID NO: 414)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 16-20; SEQ ID NO: 415)
Wherein in the preceding sequences X2 = Aminoisobutyric acid
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 416)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 417)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 418)
HX2QGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 419)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 420)
HX2QGT FTSDY SKYLD KRRAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 421)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMDTa (SEQ ID NO: 422)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 423)
HX2QGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 424)
. HX2QGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam 12-16; SEQ ID NO: 425)
HX2QGT FTSDY SKYLD KRAAE DFVQW LMDTa (lactam 16-20; SEQ ID NO: 426)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMDTa (SEQ ID NO: 427)
=
HX2QGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 12-16; SEQ ID NO: 428)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 16-20; SEQ ID NO: 429)
Wherein in the preceding sequences X2 = (D-Ala)
liSEGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 430)
HSEGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 431)
HSEGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 432)
HSEGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 433)
HSEGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 434)
HSEGT FTSDY SKYLD KRRAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 435)
HSEGT FTSDY SKYLD ERAAK DFVQW LMDTa (SEQ 1D NO: 436)
HSEGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 437)
HSEGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 438)
HSEGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 439)
HSEGT FTSDY SKYLD KRAAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 440)
HSEGT FTSDY SKYLD EQAAK EFIAW LMDTa (SEQ ID NO: 441)
HSEGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 12-16; SEQ ID NO: 442)
HSEGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 16-20; SEQ ID NO: 443)
X I SEGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 444)
121
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
XISEGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 445)
XISEGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 446)
XISEGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 447)
XISEGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 12-16; SEQ ID NO: 448)
XISEGT FTSDY SKYLD KRRAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 449)
=
XISEGT FTSDY SKYLD ERAAK DFVQW LMDTa (SEQ ID NO: 450)
XISEGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 451)
XISEGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 452)
XISEGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 453)
XISEGT FTSDY SKYLD KRAAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 454)
XISEGT FTSDY SKYLD EQAAK EFIAW LMDTa (SEQ ID NO: 455)
XISEGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 12-16; SEQ ID NO: 456)
XISEGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 16-20; SEQ ID NO: 457)
Wherein in the preceding sequences X1 = (Des-amino)His
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 458)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 459)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 460)
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 461)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 462)
HX2EGT FTSDY SKYLD KRRAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 463)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMDTa (SEQ ID NO: 464)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 465)
HX2EGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 466)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 467)
HX2EGT FTSDY SKYLD KRAAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 468)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMDTa (SEQ ID NO: 469)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 12-16; SEQ ID NO: 470)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 16-20; SEQ ID NO: 471)
Wherein in the preceding sequences X2 = Aminoisobutyric acid
=
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMDTa (SEQ ID NO: 472)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMDTa (SEQ ID NO: 473)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 474)
HX2EGT FTSDY SKYLD ERRAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 475)
HX2EGT FTSDY SKYLD ERRAK DFVQW LMDTa (lactam 12-16; SEQ ID NO: 476)
122
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
HX2EGT FTSDY SKYLD KRRAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 477)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMDTa (SEQ ID NO: 478)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam 16-20; SEQ ID NO: 479)
HX2EGT FTSDY SKYLD ERAAQ DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 480)
HX2EGT FTSDY SKYLD ERAAK DFVQW LMDTa (lactam @ 12-16; SEQ ID NO: 481)
HX2EGT FTSDY SKYLD KRAAE DFVQW LMDTa (lactam @ 16-20; SEQ ID NO: 482)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMDTa (SEQ ID NO: 483)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam 12-16; SEQ ID NO: 484)
HX2EGT FTSDY SKYLD EQAAK EFIAW LMDTa (lactam @ 16-20; SEQ ID NO: 485)
Wherein in the preceding sequences X2 = (D-Ala)
The following glucagon peptides with a GLP-1/glucagon activity ratio of about
5 or
more are also constructed generally as described above in Examples 1-11.
Generally,
in these peptides, AIB at position 2 provides DPP IV resistance but also
significantly
Is reduces glucagon activity.
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 486)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNC*a (SEQ ID NO: 487)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNGG PSSGA PPPSC*a (SEQ ID NO: 488)
HX2QGT FTSDY SKYLD EQAAK EFIAW LMNGG PSSGA PPPSC*a (lactam @ 16-20;
SEQ ID NO: 489)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNGG PSSGA PPPSa (SEQ ID NO: 490)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNGG PSSGA PPPSa (lactam @ 16-20;
SEQ ID NO: 491)
Wherein in the preceding sequences X2=AIB, and wherein the C* is a Cys, or a
Cys
attached to a hydrophilic polymer, or alternatively the C* is a Cys attached
to a
polyethylene glycol of about 20 IcD average weight, or alternatively the C* is
a Cys
attached to a polyethylene glycol of about 40 IcD average weight.
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNTa (SEQ ID NO: 492)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNC*a (SEQ ID NO: 493)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNGG PSSGA PPPSC*a (SEQ ID NO:
494)
HX2QGT FTSDY SKYLD ERAAK DFVQW LMNGG PSSGA PPPSC*a (lactam @ 16-20;
SEQ ID NO: 495)
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNGG PSSGA PPPSa (SEQ ID NO: 496)
123
CA 02852177 2014-05-23
WO 2009/155258
PCT/IJS2009/047438
29920-208831
HX2QGT FTSDY SKYLD ERAAK DFVC*W LMNGG PSSGA PPPSa (lactam @ 16-20;
SEQ ID NO: 497)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 498)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNC*a (SEQ ID NO: 499)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNGG PSSGA PPPSC*a (SEQ ID NO: 500)
HX2QGT FTSDY SKYLD ERRAK DFVQW LMNGG PSSGA PPPSC*a (lactam @ 16-20;
SEQ ID NO: 501)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNGG PSSGA PPPSa (SEQ ID NO: 502)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNGG PSSGA PPPSa (lactam @ 16-20;
SEQ ID NO: 503)
Wherein in the preceding sequences X2=AIB, and wherein the C* is a Cys, or a
Cys
attached to a hydrophilic polymer, or alternatively the C* is a Cys attached
to a
polyethylene glycol of about 20 kD average weight, or alternatively the C* is
a Cys
attached to a polyethylene glycol of about 40 kD average weight.
The following glucagon peptides which are GLF-1/glucagon co-agonists are also
constructed generally as described above in Examples 1-11. Formation of a
lactam
bridge between amino acids 16 and 20 restores the reduction in glucagon
activity
caused by the substitution at position 2.
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam @ 16-20; SEQ ID NO: 504)
Wherein in the preceding sequence X2=AIB, and wherein the C* is a Cys, or a
Cys
attached to a hydrophilic polymer, or alternatively the C* is a Cys attached
to a
polyethylene glycol of about 20 kD average weight, or alternatively the C* is
a Cys
attached to a polyethylene glycol of about 40 kD average weight.
X I SQGT FTSDY SKYLD EQAAK EFIC*W LMNTa (lactam 16-20; SEQ ID NO: 505)
X I SQGT FTSDY SKYLD EQAAK EFIAW LMNC*a (lactam @ 16-20; SEQ ID NO: 506)
X I SQGT FTSDY SKYLD EQAAK EFIAW LMNGG PSSGA PPPSC*a (lactam @ 16-20;
SEQ ID NO: 507)
XISQGT FTSDY SKYLD ERRAK DFVQW LMNGG PSSGA PPPSC*a (lactam @ 16-20;
SEQ ID NO: 508)
X I SQGT FTSDY SKYLD EQAAK EFIC*W LMNGG PSSGA PPPSa (lactam @ 16-20;
SEQ ID NO: 509)
X I SQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 510)
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 511)
124
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
X1 SQGT FTSDY SKYLD ERRAK DFVQW LMNC*a (lactam @ 16-20; SEQ ID NO: 512)
X I SQGT FTSDY SKYLD ERRAK DFVC*W LMNGG PSSGA PPPSa (lactam (4) 16-20;
SEQ ID NO: 513)
Wherein in the preceding sequences X I =DMIA (alpha, alpha-dimethyl imidiazole
ID NO: 514)
Wherein the C* is a Cys, or a Cys attached to a hydrophilic polymer, or
alternatively
the C* is a Cys attached to a polyethylene glycol of about 20 kD average
weight, or
alternatively the C* is a Cys attached to a polyethylene glycol of about 40 kD
average
15 weight.
HX2QGT FTSDY SKYLD ERRAK DFVC*W LMNTa (lactam @ 16-20; SEQ ID NO: 517)
HX2QGT FTSDY SKYLD ERRAK DFVCW LMNTa (lactam 16-20; SEQ ID NO: 528)
HX2QGT FTSDY SKYLD ERRAK EFIC*W LMNGG PSSGA PPPSC'a (SEQ ID NO: 531)
532)
HX2QGT FTSDY SKYLD EQAAK EFIC*W LMNGG PSSGA PPPSa (SEQ ID NO: 533)
Wherein in the preceding sequence X2=AIB, and wherein the C* is a Cys, or a
Cys
attached to a hydrophilic polymer, or alternatively the C* is a Cys attached
to a .
HSQGT FTSDYSKYLD EQAAK EF1C*W LMNTa (SEQ ID NO: 518)
X I SQGT FTSDYSKYLD EQAAK EFIC*W LMNTa (SEQ ID NO: 519)
XI SQGT FTSDY SKYLD ERRAK DFVC*W LMNGG PSSGA PPPSa (SEQ ID NO: 529)
X ISQGT FTSDY SKYLD ERRAK DFVC*W LMNTa (SEQ ID NO: 530)
Wherein in the preceding sequences X1=DMIA (alpha, alpha-dimethyl imidiazole
125
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
average weight, or alternatively the C* is a Cys attached to a polyethylene
glycol of.
about 40 kD average weight.
HSQGT FTSDYSKYLD SRRAQ DFVQW LMNTGPSSGAPPPSa (SEQ ID NO: 521)
HSQGT FTSDYSKYLD SRRAQ DFVQW LMNGGPSSGAPPPSa (SEQ ID NO: 522)
HSQGT FTSDYSKYLD SRRAQ DFVQW LMKGGPSSGAPPPSa (SEQ ID NO: 523)
HSQGT FTSDYSKYLD SRRAQ DFVQW LVKGGPSSGAPPPSa (SEQ ID NO: 524)
HSQGT FTSDYSKYLD SRRAQ DFVQW LMDGGPSSGAPPPSa (SEQ ID NO: 525)
HSQGT FTSDYSKYLD ERRAK DFVQW LMDGGPSSGAPPPSa (SEQ ID NO: 526)
HAEGT FTSDV SSYLE GQAAK EFIAW LVKGGa (SEQ ID NO: 527)
X1X2QGT FTSDY SKYLD ERX5AK DFVX3W LMNX4 (SEQ ID NO: 61)
wherein
X1 =His, D-histidine, desaminohistidine, hydroxyl-histidine, acetyl-histidine,
homo-
histidine or alpha, alpha-dimethyl imidiazole acetic acid (DMIA) N-methyl
histidine,
alpha-methyl histidine, or imidazole acetic acid,
X2=Ser, D-serine, Ala, Val, glycine, N-methyl serine or aminoisobutyric acid
(AIB),
N-methyl alanine and D-alanine.
X3=Ala, Gin or Cys-PEG
X4=Thr-CONH2 or Cys-PEG or GGPSSGAPPPS (SEQ ID NO: 515) or
GGPSSGAPPPSC-PEG (SEQ ID NO: 516)
Provided that when X3 is Cys-PEG, X4 is not Cys-PEG or GGPSSGAPPPSC-PEG
(SEQ ID NO: 516), and when X2=Ser, X1 is not His.
X5=Ala or Arg
X1X2QGT FTSDY SKYLD EQ X5AK EFI X3W LMNX4 (SEQ ID NO: 62)
wherein
X1 =His, D-histidine, desaminohistidine, hydroxyl-histidine, acetyl-histidine,
homo-
histidine or alpha, alpha-dimethyl imidiazole acetic acid (DMIA), N-methyl
histidine,
alpha-methyl histidine, or imidazole acetic acid
X2=Ser, D-serine, Ala, Val, glycine, N-methyl serine or aminoisobutyric acid
(AIB),
N-methyl alanine and D-alanine.
X3=Ala, Gin or Cys-PEG
X4=Thr-CONH2 or Cys-PEG or GGPSSGAPPPS (SEQ ID NO: 515) or
GGPSSGAPPPSC-PEG (SEQ ID NO: 516)
126
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Provided that when X3 is Cys-PEG, X4 is not Cys-PEG or GGPSSGAPPPSC-PEG
(SEQ ID NO: 516), and when X2=Ser, X1 is not His.
X5=Ala or Arg
HSEGT FTSDY SKYLD EQAAK EFIAW LXNTa (SEQ ID NO: 554), wherein X at
Any of the preceding sequences can include additional modifications, e.g., 1,
2, 3, 4 or 5 modifications that do not destroy activity, including but not
limited to
W10 or R20 substitutions that can be used to enhance potency. Any of the
preceding
2. In addition, any of the preceding compounds may optionally be linked to a
conjugate, such as a heterologous polypeptide, an inununoglobulin or a portion
thereof (e.g. Fc region), a targeting agent, a diagnostic label, or a
diagnostic or
EXAMPLE 17
The following glucagon peptides modified to comprise the c-terminal
extension of SEQ ID NO: 26 linked to the carboxy terminus of the glucagon
peptide
activity at the GLP-I and glucagon receptors using the in vitro assay
described in
Example 14.
Table 11 represents the activity of various glucagon analogs at the glucagon
and GLP-1 receptors. The data shows that for glucagon analogs comprising the c-
127
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
=
29920-208831
Table 11
Glucagon-Cex
Structure Activity Relationship
Glucagon Peptide L Glucagon Receptor I I GLP-1
ReceRt9r
EC50 Relative ECM (nM) Relative
(nM) Potency (%) Potency (%)
-MNT29 (SEQ ID NO: 1) 0.086 100
-MNTG3 PSSGAPPPS 0.14 61 1.19 2
(SEQ ID NO: 521)
-MNGG3 PSSGAPPPS 0.28 30 0.31 8
(SEQ ID NO: 522)
-MKGG3 PSSGAPPPS 0.61 14 0.80 3
(SEQ ID NO: 523)
-VIWG3 PSSGAPPPS 1.16 7 0.21 12
(SEQ ID NO: 524)
-MDGG3 PSSGAPPPS 0.12 72 0.13 19
(SEQ ID NO: 525)
E16K20-MDGG3 PSSGAPPPS 0.22 39 0.020 125
(SEQ ID NO: 526)
GLP-1-VKGG3 0.025 100
(SEQ ID NO: 527)
EXAMPLE 18
Table 12 represents in vitro data accumulated for various glucagon peptides
comparing their relative activities at the glucagon and GLP-I receptors.
128
CA 02852 177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831 =
Table 12: COMPARISON OF AGONISTS AND CO-AGONISTS w/ and w/o PEG
% Potency Relative to Native
CONTROLS GR GL-1R
Glucagon 100 0.78
GLP-I <0.01 100
Parent w/o PEG Parent w/PEG
% Potency Relative to % Potency Relative to
Native Native .
AGONISTS = GR GLP-IR GR GLP-1R
= = Chimera AIB2, Cys24 (SEQ ID NO: 486)
15.4 160.6 2.6 82.5
Chimera AI82, Cys29 (SEQ ID NO: 487) 20.1 124.6 5.6 . 54.3
Chimera AIB2, G1y29,30 Cys40 Cex (SEQ ID NO: . 2.2 359.1 0.3 68.8
488)
Chimera AU32, Gly29,30 Cys40 Cex Lactam (SEQ ID 14.2 169.6 3.2
63.6
NO: 489) .
Chimera AIB2, G1y29,30 Cys24 Cex (SEQ ID NO: 2.5 457.8 0.2 95.4
490)
Chimera AI132, G1y29,30 Cys24 Cex Lactam (SEQ ID 25.2 ' 381.5 1.4
96.4
NO: 491)
E16, K20A1132, Al8 Cys24 (SEQ ID NO: 492) -- -- 1.1 73.5
E16, K20A1B2, A18 G1y29,30 Cys24 Cex (SEQ ID -- -- 0.1 88.5
NO: 496) =
CO-AGONISTS GR GLP-1R GR GLP-1R
Chimera DM1A I, Cys24 Lactam (SEQ ID NO: 505) 160.7 = 82.5 19.1
12.5
Chimera A1132, Cys24 Lactam (SEQ ID NO: 504) 114.2 230.4 9.2 38.0
Chimera DMIA I, Cys29 Lactam (SEQ ID NO: 506) _ -- -- -- -
Chimera DMIA1, Gly29,30 Cys40 Cex Lactam (SEQ -- -- -- --
ID NO: 507)
E16, K20 DMIA I , G1y29,30 Cys40 Cex -- -- -- --
Lactam (SEQ ID NO: 508)
Chimera DMIA I , G1y29,30 Cys24 Cex Lactam (SEQ -- -- -- --
ID NO: 509)
E16, K20 DMIA I, Cys24 Lactam (SEQ ID NO: 510) -- -- 64.1 9.3
E16, K20 AI82, Cys24 Lactam (SEQ ID NO: 517) 108.3 96.9 15.8 31.0
Chimera Cys24 (SEQ ID NO: 518) -- -- 19.8 _ 29.3
E16, K20 DMIA I , Gly29,30 Cys24 Cex 116.0 78.3 = 12.6 11.3
Lactam (SEQ ID NO: 513)
Chimera DMIA I , Cys29 (SEQ ID NO: 520) -- -- 5.3 27.3
Chimera DMIA I, Cys24 (SEQ ID NO: 519) 28.9 64.5 6.9 19.3
129
=
CA 02852177 2014-05-23
WO 2009/155258
PCT/IJS2009/047438
29920-208831
EXAMPLE 19
Acylated and/or PEGylated peptides were prepared as follows. Peptides were
synthesized on a solid support resin using either a CS Bio 4886 Peptide
Synthesizer or
Applied Biosystems 430A Peptide Synthesizer. In situ neutralization chemistry
was
used as described by Schnolzer et al., Int. J. Peptide Protein Res. 40: 180-
193 (1992).
For acylated peptides, the target amino acid residue to be acylated (e.g.,
position ten)
was substituted with an N e -FMOC lysine residue. Treatment of the completed N-
terminally BOC protected peptide with 20% piperidine in DMF for 30 minutes
to removed FMOC/formyl groups. Coupling to the free e-amino Lys residue was
achieved by coupling a ten-fold molar excess of either an FMOC-protected
spacer
amino acid (ex. FMOC-(N-BOC)-Tryptophan-OH) or acyl chain (ex. Cl 7-COOH)
and PyBOP or DEPBT coupling reagent in DMF/DIEA. Subsequent removal of the
spacer amino acid's FMOC group is followed by repetition of coupling with an
acyl
i5 chain. Final treatment with 100% TFA resulted in removal of any side
chain
protecting groups and the N-terminal BOC group. Peptide resins were
neutralized
with 5% DIEA/DMF, dried, and then cleaved from the support using HF/p-cresol,
95:5, at 0 C for one hour. Following ether extraction, a 5% HOAc solution was
used
to solvate the crude peptide. A sample of the solution was then verified to
contain the
20 correct molecular weight peptide by ESI-MS. Correct peptides were
purified by RP-
HPLC using a linear gradient of 10% CH3CN/0.1% TFA to 0.1% TFA in 100%
CH3CN. A Vydac C18 22 mm x 250 mm protein column was used for the
purification. Acylated peptide analogs generally completed elution by a buffer
ratio
of 20:80. Portions were pooled together and checked for purity on an
analytical RP-
25 ITPLC. Pure fractions were lyophilized yielding white, solid peptides.
Yields
typically ranged from 10 mg to 100 mg depending on the synthesis.
If a peptide comprises a lactam bridge and target residues to be acylated,
acylation is carried out as described above upon addition of that amino acid
to the
peptide backbone.
30 For peptide pegylation, 40 kDa methoxy poly(ethylene glycol) maleimido-
propionamide (Chirotech Technology Ltd.) was reacted with a molar equivalent
of
peptide in 7M Urea, 50mM Tris-HC1 buffer using the minimal amount of solvent
needed to dissolve both peptide and PEG into a clear solution (generally less
than 2
mL for a reaction using 2-3 mg peptide). Vigorous stirring at room temperature
130
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
commenced for 4-6 hours and the reaction analyzed by analytical RP-I-IPLC.
PEGylated products appeared distinctly from the starting material with
decreased
retention times. Purification was performed on a Vydac C4 column with
conditions
similar to those used for the initial peptide purification. Elution occurred
around
buffer ratios of 50:50. Fractions of pure PEGylated peptide were found and
lyophilized. Yields were above 50%, varying per reaction.
Peptides were assayed for biological activity, by co-tranfecting HEIC293 cells
with either the glucagon receptor (GLUR) or GLP-1 receptor (GLP-1R) and a
luciferase gene linked to a cAMP responsive element. The transfected cells
were
to serum deprived by culturing for 16 hours in DMEM supplemented with
0.25%
Bovine Growth Serum and then incubated for 5 hours with serial dilutions of
the
selected analogs and either Glucagon or GLP-1 as standards, respectively.
Peptide
absorbance readings were obtained from UV Absorbance measurements at 280 nm on
a Genesys 6 Spectrophotometer (Thermo Electron Corporation). Beer's Law was
used to calculate solution concentrations based on the number of tryptophan
and
tyrosine residues in each analog. At the end of the incubation, 100 LtL
LucLite
luminescence substrate reagent was added to each well, the plate sealed and
shaken,
and placed into a Wallac Trilux luminescence counter for cAMP detection.
Effective
50% concentrations (EC50) were calculated using Origin software (OriginLab,
Northampton, MA).
Acylated glucagon-based co-agonist peptides were prepared. In vitro
results for a selection of these peptides are shown in Table 13. Although the
unacylated peptide, like native glucagon, was insoluble in phosphate-buffered
saline
solutions at 1 mg/mL concentrations, acylation was observed to enhance
solubility of
the peptide at neutral pH.
TABLE 13
Receptor Activation Curves and nM EC50 values for Acylated Peptides
Peptide GLP-1 Receptor N Glucagon
Receptor
GLP-1 0.04 15 >100 3
El 6 K20-glucagon-NH2 0.21 9 0.18 10
E16 K20-glucagon-NH2 with Kw-C16 0.09 8 0.40 8
E16 K20-glucagon-NH2 with K1u-W-C16 0.05 8 0.14 8
E16 K20-glucagon-NH2 with K'-Cig 0.03 8 0.12 8
E16 K20-glucagon-NH2 with K' -W-C3 0.04 11 0.05 12
Glucagon 7.42 6 0.07 17
131
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-20883I
All four acylated peptides exhibited increased potency at the GLP-1 receptor.
Inclusion of the tryptophan spacer provided better potency at the glucagon
receptor.
An acyl chaidlength of C18 is slightly preferred.
While acylation can extend the half-life of a peptide to hours or more,
PEGylation with repeats in tens of IcDa ranges can do even more. Peptides
comprising both types of modifications were prepared. These peptides are
expected
to exhibit extended half-life in circulation, as well as resistance to DPP-IV
and other
proteases. In vitro results for a selection of these peptides are shown in
Table 14.
TABLE 14
Receptor Activation Curves and nM EC50 values for Acylated, PEGylated
Peptides
Peptide GLP-1 N Glucagon
N
Receptor Receptor
GLP-1 0.04 15 >100 3
E16 K20-glucagon-NH2 (SEQ ID NO: 545) 0.21 9 0.18 10
E16 K20-glucagon-NH2 with Kw-W-C16 and 0.23 13 0.52 13
C24-40K PEG (SEQ ID NO: 546)
E 1 6 K20-glucagon-N112 with K"-C18 and C24- 0.15 12 0.84 13
40K PEG (SEQ ID NO: 547) =
E16 K20-glucagon-NH2 with Klu-W-Cis and 1.64 3 1.30 5
C24-40K PEG (SEQ ID NO: 548)
. Glucagon (SEQ ID NO: 1) 7.42 6 0.07 17
=
Two of the three peptides retained their high potency at both the GLP-1 and
glucagon receptors, with an EC50 of less than 1nM. The Km-W-C18 acylated and
PEGylated peptide exhibited about ten-fold potency losses at both receptors.
This
series of peptides shows that the position ten acylation is compatible with a
PEGylation in the C-terminal portion of the glucagon peptide, e.g. position
24, 28 or.
29, within a C-terminal extension, or at the C-terminus (e.g., through adding
a C-
terminal Cys).
EXAMPLE 20
Various acylated glucagon co-agonist peptides were made as essentially
described in Example 19 and tested for in vivo activity. Specifically, Peptide
A (SEQ
132
CA 0 2 852 17 7 2 0 14-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
ID NO:1 modified to contain AIB at position 2, Glu at position 16, Gin at
position 17,
Ala at position 18, Lys at position 20, Glu at position 21, Ile at position
23, Cys at
position 24, which Cys is bonded to a 40K PEG, and C-terminal amide) was
further
modified to comprise a Lys at position 10. The Lys10 was acylated with a C8
fatty
acid chain, a CI4 fatty acid chain, a C16 fatty acid chain, or a C18 fatty
acid chain.
Activity at the GLP-1 receptor of each of the acylated peptides was assayed as
described in Example 14 and compared to the activity of GLP-1(7-37)acid (SEQ
ID
NO: 50) as a control. The EC50 of each of the acylated peptides at the GLP-1
receptor
shown in Table 15 is similar to the EC50 of the GLP-1 peptide.
TABLE 15
GLP-1 Receptor
Activation Potency
EC50 (nM)
GLP-1 0.0222 0.0002
Peptide A Km-C8 0.0174 * 0.0004
Peptide A 0-Ci4 0.0168 0.0004
Peptide A Kiu-Cif, 0.0127 0.0003
Peptide A le-Cig 0.0118 0.0002
The peptides were then tested in .vivo by subcutaneously injecting diet-
induced
obesity (DIO) mice with various acylated and non-acylated peptides, or vehicle
alone,
QW (70 nmolfIcg/week) for 2 weeks. 6 mice per group with initial average body
weight of 44 g were tested. Body weight, body composition, food intake, and
blood
glucose levels were determined periodically.
As shown in Figure 11, the acylated peptides are able to cause weight loss to
a
similar extent than the non-acylated peptide. As shown in Figure 11, between
about 7
and 12% weight loss is achieved within the first 3 days of treatment with the
acylated
peptides. As shown in Figure 12, the acylated peptides caused a decrease in
food
intake. Furthermore, as shown in Figure 13, the ad libitum blood glucose
levels of the
acylated peptides were reduced after 1 day of treatment.
EXAMPLE 21
The following acylated glucagon co-agonist peptides were made as essentially
described in Example 19.
133
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
(A) "Chimera-2 Aib2 Lysl 0-C18 Cys24(40K)": native glucagon amino acid
sequence
(SEQ ID NO: 1) comprising the following modifications: Glu at position 16, Gin
at
position 17, Ala at position 18, Lys at position 20, Glu at position 21, He at
position
23, and Ala at position 24, and a C-terminal amide ("Chimera 2"), further
modified
with AIB at position 2, a Lys10 acylated with a C18 fatty acid and a Cys at
position
24 pegylated with a 40K PEG group; =
(B) "Chimera-2 Aib2 Lys10-C16 Cys24(40K)": Chimera 2 further modified with AIB
at position 2, a Lys10 acylated with a C16 fatty acid and a Cys24 pegylated
with a
40K PEG group
(C) "Glucagon Lys10-C18 E16 1(20 Cys24(40K)": native glucagon amino acid
sequence (SEQ ID NO: 1) comprising the following modifications: Glu at
position 16,
Lys at position 20, and C-terminal amide ("El 6K20-glucagon-NH2") was further
modified with a Lys10 acylated with a C18 fatty acid and a Cys24 pegylated
with a
40K PEG group;
(D) "Glucagon Lys10-TrpC16 E16 K20 Cys24(40K)": E16K20-glucagon-NH2 was
further modified with Lys10 linked to a Trp spacer which was acylated with a
C16
fatty acid;
(E) "Glucagon Lys10-TrpC18 E I 6 1(20 Cys24(40K)": E16K20-glucagon-NH2 was
further modified with Lys10 linked to a Trp spacer which was acylated with a
C18
fatty acid.
The acylated glucagon co-agonist peptides.were tested for their activities at
the Glucagon and GLP-1 receptors generally as described in Example 14. The
EC50
at each of the glucagon receptor and the GLP-1 receptor in comparison to
controls
(GLP-1 (7-37) OH (amino acids 7-37 of GLP-1), Glucagon (1-29)0H (SEQ ID NO:
1), and Chimera 2 Cys24 (40K) (Chimera 2 with a 40K PEG on Cys 24)) are as
shown in Table 16.
= 134
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
TABLE 16
EC50 at EC50 at
Glucagon GLP-1
Receptor Receptor
(nM) (nM)
GLP-1 (7-37) OH >1000.00 0.04
Glucagon (1-29) OH 0.07 7.5
Chimera 2 Cys24 (40K) 2.83 0.04
Chimera-2 Aib2 Lysl 0-C18 Cys24(40K) 8.55 0.14
Chimera-2 Aib2 Lys10-C16 Cys24(40K) 17.41 0.05
Glucagon Lys10-C18 E16 K20 Cys24(40K) 0.84 0.15
Glucagon Lys10-TrpC16 E16 K20 Cys24(40K) 0.54 0.23
Glucagon Lys10-TrpC18 E16 K20 Cys24(40K) 1.29 1.64
EXAMPLE 22
The following acylated glucagon co-agonist peptides were made as essentially
described in Example 19:
(A) Peptide A: native glucagon amino acid sequence (SEQ ID NO: -1) comprising
the
following modifications: Glu at position 16, Lys at position 20, and C-
terminal amide
("E16K20-glucagon-NH2");
(B) Peptide B: El 6K20-glucagon-NH2 further comprising a Lys10 acylated with a
CI6 fatty acid;
(C) Peptide C: El 6K20-glucagon-NH2 further comprising a Lysl 0 acylated with
a
C18 fatty acid;
(D) Peptide D: El 6K20-glucagon-NH2 further comprising a Lys10 linked to a Glu
(a
spacer residue) acylated with a CI6 fatty acid;
(E) Peptide E: El 6K20-glucagon-NH2 further comprising a Lys10 linked to.a Trp
(a
spacer residue) acylated with a C18 fatty acid.
135
CA 02852177 2014-05-23
WO 2009/155258
PCT/1JS2009/047438
29920-208831
The activity of the peptides were assayed generally according to Example 14
and the EC50 at each of the glucagon receptor and the GLP-1 receptor are shown
in
Table 17.
TABLE 17
EC50 at EC50 at
Glucagon GLP-1
Receptor Receptor
(nM) (nM)
GLP-1 OH >1000 0.037
Glucagon (1-29) OH (SEQ ID NO: 1) 0.098 10
Peptide A 0.203 0.188
Peptide B 0.236 0.125
Peptide C 0.086 0.032
Peptide D 0.062 0.056
Peptide E 0.044 0.031
EXAMPLE 23
A glucagon co-agonist peptide was made comprising the amino acid sequence
of SEQ ID NO: 1 with the following modifications: Glu at position 16, Gin at
position
17, Ala at position 18, Lys at position 20, Glu at position 21, Ile at
position 23, Ala at
position 24, Val at position 27, Lys at position 28 and C-terminal amide
("Chimera
1"). C-terminally truncated versions of Chimera I were made by deleting the
amino
acid at position 29 of Chimera I ("Chi 1 (1-28)"), or by deleting amino acids
at both
positions 28 and 29 of Chimera I ("Chi 1(1-27)").
A glucagon peptide comprising the amino acid sequence of SEQ ID NO: 1
with the following modifications: Glu at position 16, C-terminal amide ("E16
Glue-
NH2") was also C-terminally truncated, by deleting either the amino acid at
position
136
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
29 ("E16 GlucNH2 (1-28)") or by deleting amino acids at both positions 28 and
29
("E16 GlucNH2 (1-27)").
The activity at the glucagon receptor and the GLP-1 receptor of the truncated
peptides, as well as the non-truncated peptides, were assayed for functional
activity
generally according to Example 14. Deletion of amino acids at positions 28 and
29 of
the E16 GlucNH2 peptide or the Chimera 1 peptide did not significantly impact
the
activity of the peptide at the glucagon receptor. Deletion of amino acids at
positions
28 and 29 of E16 Gluc1\11-12 did not appreciably change the potency of the
peptide at
the GLP-1 receptor. Deletion of amino acids at positions 28 and 29 of Chimera
1 did
not impact its activity at the GLP-1 receptor. =
Deletion of the amino acid at position 29 of either the Chimera 1 peptide or
the E16 GlucNH2 peptide did not significantly impact the activity at either
the
glucagon receptor or the GLP-1 receptor.
Is EXAMPLE 24
Diet-induced obesity (DIO) mice were injected intraperitoneally at the -15 min
time point with 0.2, 2, 20, or 70 nmol/kg of one of the following:
(A) vehicle only,
(B) native glucagon amino acid sequence (SEQ ID NO: .1) comprising the
following modifications: Glu at position 16, Gln at position 17, Ala at
position 18,
Lys at position 20, Glu at position 21, Ile at position 23, and Ala at
position 24, and a
C-terminal amide ("Chimera 2") further modified to comprise AIB at position 2
and
Cys at position 24, which Cys is pegylated with a 40K PEG ("Chimera-2-A132
Cys24-
4010"),
(C) Chimera 2 further modified to comprise AIB at position 2, Lys at
position 10, which Lys is acylated with a C8 fatty acid, and Cys at position
24, which
Cys is pegylated with a 40K PEG ("Chimera-2 A1B20-C8 Cys24-401cD"), or
(D) Chimera 2 further modified to comprise AIB at position 2, Lys at
position 10, which Lys is acylated with a C16 fatty acid, and Cys at position
24,
which Cys is pegylated with a 40K PEG ("Chimera-2 AlB2 K' -C16 Cys24-401cD").
A saline solution comprising 25% (v/v) glucose was injected at a dose of 1.5
g/kg of body weight at the 0 mm time point. Blood glucose levels were measured
at
the -15, 0, 15, 30, 60, and 120 min time points.
137
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Figures 15-17 show the blood glucose levels (mg/dL) of mice injected with 2,
20, and 70 nmol/kg, respectively, at the indicated time points. For all doses
tested,
Chimera-2 AIB2 Km-C8 Cys24-40IcD demonstrated the greatest ability to lower
blood
glucose in the mice. As shown in Figure 17, this peptide had similar activity
as
Chimera-2-AIB2 Cys24-40k.D.
EXAMPLE 25
DIO mice were injected intraperitoneally at the -24 hr time point with 70
tO nmol/kg of one of the following:
(A) vehicle only,
(B) Chimera-2-AIB2 Cys24-40kD, as described above in Example 24,
(C) Chimera-2 AIB2 Km-C8 Cys24-401cD, as described above in Example
24, or
(D) Chimera-2 AIB2 Km-C16 Cys24-40IcD, as described above in Example
24.
A saline solution comprising 25% (v/v) glucose was injected at a dose of 1.5
= g/kg of body weight at the 0 min time point. Blood glucose levels were
measured at
the 0, 15, 30, 60, and 120 min time points.
Figure 18 demonstrates the blood glucose levels (mg/dL) of the mice at the
= indicated time points. All three peptides demonstrate significant
activity at lowering
blood glucose in the mice.
EXAMPLE 26
DIO mice were injected intraperitoneally with vehicle only or 15 or 70
runol/kg of one of the following:
(A) Chimera-2-AIB2 Cys24-40kD, as described above in Example 24,
(B) Chimera-2 AIB2 Km-C8 Cys24-40.10, as described above in Example
24, or
(C) Chimera-2 AIB2 Km-C16 Cys24-40IcD, as described above in Example
24.
Body weight was measured before injection and at 1, 3, 5, and 7 days post-
injection.
138
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Figure 19 demonstrates the % change of body weight for each group of mice.
At both doses tested, Chimera-2 AIB2Km-C8 Cys24-40IcD and Chimera-2-AIB2
Cys24-40IcD demonstrate comparable ability to lower body weight. At the higher
dose
tested, Chimera-2 AIB2Km-C16 Cys24-401cD demonstrates significant ability to
lower
body weight
EXAMPLE 27
A peptide of SEQ ID NO: 555, comprising a Tyrosine at position 1 and a
lactam bridge between E16 and K20, (and an amide in place of the C-terminal
carboxylate) was synthesized as essentially described above and tested in
vitro for
activity at GLP-1 and glucagon receptors by Example 14. The EC50 of the
peptide at
each receptor is shown in Table 18.
TABLE 18
Receptor EC50 (nM) Std. Dev Relative Activity
Glucagon 0.044 0.151 343.18%
GLP-1 0.062 0.062 100.00%
Relative activity is activity relative to the native hormone of the indicated
=
receptor.
Based on these data, it was determined that the peptide of SEQ ID NOs: 555
was an exemplary glucagon/GLP-1 co-agonist peptide.
EXAMPLE 28
A peptide of SEQ ID NO: 1 (Glucagon(1-29)), a peptide of SEQ ID NO: 1
with an amide replacing the C-terminal carboxylate (Glucagon (1-29a)), and a
peptide
of SEQ ID NO: 1 with AIB at each of positions 2 and 16 and an amide replacing
the
C-terminal carboxylate (Glucagon(1-29a) Aib2 Aibl6) were synthesized as
essentially
described above. These peptides were then tested in vitro for activity at the
GLP-1
receptor and glucagon receptors by the methods described in Example 14. The
EC50
of each peptide are shown in Table 19.
139
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
TABLE 19
Glucagon Receptor GLP-1 Receptor
Peptide EC50 (nM) SD EC50 (nM) SD
Glucagon( I -29) 0.04 0.01 3.65 0.21
Glucagon (1-29a) Aib2
0.09 0.02 0.10 0.01
Aibl6
Glucagon(1-29a) ND ND 0.50 0.05
GLP-1(1-31)0H ND ND . 0.03 0.00
SD = standard deviation
EXAMPLE 29
The following peptides were synthesized as essentially described above:
(1) Glucagon(1-29), as described in Example 28,
(2) Glucagon(1-29a) Aib2 Aib16 (as described in Example 28) with a Cys
to at position 24 and a Lys at position 10 covalently bonded to a Trp
comprising a Cl 6
fatty acid ("Glucagon (I-29a) Aib2 Lys1 -Trp-C16 Aib16 Cys24")
(3) Glucagon (1-29a) Aib2 Lysi -Trp-C16 Aibl6 Cys24 in which the Cys
comprises a 40 kD PEG group ("Glucagon (1-29a) Aib2 Lyslo_Trp -C16 Aibi6 Cys24-
4010"),
(4) Glucagon (1-29a) Aib2 Lysw-Trp-C16 Aibl6 Cys24 comprising Aib at
position 20 ("Glucagon (1-29a) Aib2 Lys l -Trp-C16 Aibi6 Aib2 Cys24), and
(5) Glucagon (1-29a) Aib2 Lys1 -Trp-C16 Aibl6 Aib2 Cys24 in which
the
Cys comprises a 40 10 PEG group ("Glucagon (1-29a) Aib2 Lysw-Trp-C16 Aibl6
Aib2 Cys24-401cD). =
These peptides were then tested in vitro for activity at the GLP-I receptor
and
glucagon receptors by the methods of Example 14. The EC50 of each peptide are
shown in Table 20.
140
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
TABLE 20
Glucagon Receptor GLP-1 Receptor
Peptide EC50 (nM) SD EC50 SD
Glucagon(1-29) 0.04 0.01
Glucagon (1-29a) Aib2
Lys")-Trp-C16 Aibl6 0.25 0.02 0.24 0.03
Cys24
Glucagon (1-29a) Aib2
Lysm-Trp-C16 Aibl6 0.29 0.03 0.19 0.02
= Cys24-40K
Glucagon (1-29a) Aib2 -
Lysi -Trp-C16 Aibl6 2.06 0.02 1.15 0.19
Aib20 c.ys24
Glucagon (1-29a) Aib2
Lysm-Trp-C16 Aib16 2.37 0.24 0.60 0.06
Ait)2 Cys24-401(
GLP-1(1-31)01-1 0.02 0.01
EXAMPLE 30
The in vivo effects of acylated and pegylated glucagon peptides were tested in
DIO mice. Specifically, 6 groups of DIO mice (8 mice per group), each group
having
an average initial body weight of 58 g, were injected intrapefitoneally with
10, 20,40,
or 80 nmol/kg of an acylated and pegylated glucagon peptide or a vehicle
control once
a week for 2 weeks. The acylated and pegylated glucagon peptides used in the
study
were Chimera-2 AIB2 Kw-C8 Cys24-40kD (as described in Example 26) and Peptide
A K' -C14 (as described in Example 20).
Changes in body weight of and food intake by the mice were measured 0, 1, 3,
=
5, 7, 8, 10, 12, and 14 days after injection. Blood glucose levels of the mice
were
monitored throughout the 14 days. Glucose tolerance tests were performed by
injecting a 25% glucose in saline solution 1 hour or 24 hours after
administration of
the acylated or pegylated peptide and measuring blood glucose levels at -60,
0, 15, 30,
60, or 120 min after the glucose injection.
141
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
As shown in Figure 20, the total body weight of mice injected with 40 or 80
nmol/kg of acylated and pegylated Peptide A K10-C14 was reduced as compared to
mice injected with the vehicle control.
As shown in Figure 21, the blood glucose levels of mice injected with 20, 40,
or 80 nmol/kg Peptide A 1(1 -C14 or with 20 nmol/Icg Chimera-2 AIB2 Km-C8
Cys24-
401cD in response to a glucose injection are lowered in comparison to vehicle
control.
EXAMPLE 31
Acylated glucagon analog peptides comprising or lacking a covalent
intramolecular bridge were made by solid-phase synthesis and tested for in
vitro
activity at the glucagon and GLP-I receptors. The EC50 (nM) at each receptor
and
the % activity of the peptide relative to the native peptide at the
corresponding
receptor is shown in Table 21.
142
CA 0 2 852 17 7 2 0 14-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
TABLE 21
EC50 at the EC50 at the
GLP-1 Activity Glucagon Activity
Peptide Name SEQ ID NO:
receptor of receptor of
(nM) GLP-1 (nM) Glucagon
DMIA I,
KIO(C14),
607 0.050 30% 0.027 203.7%
[E16/K20)-
Glue Amide
DMIAI,
KIO(C16),
608 0.015 100% 0.014 392%
[E16/K20]-
Glue Amide
DMIAI,
KIO(C18),
609 0.011 136% 0.13 42.3%
[E16/K20)-
Glue Amide
AIB2, A1B16,
KI0(C14) Glue 610 0.024 33.3% 0.044 77.3%
Amide
= AIB2, A1B16,
KI0(C16) Glue 611 0.011 72.3% 0.020 170%
Amide
AIB2, AIB16,
K I 0(C I 8) Glue 612 0.009 88.9% 0.016 212.5%
Amide
dS2, E16/K20,
K10(C14) Gluc 613 0.128 6.3% 0.155 21.9%
Amide
dS2, E16/K20,
K I 0(C16) Glue 614 0.041 19.5% 0.076 44.7%
Amide
dS2, E16/K20,
K I 0(C18) Glue 615 0.025 60% 0.028 196%
Amide
=
143
CA 02 852 177 2 0 14-05-2 3
WO 2009/155258 PCT/US2009/04 7438
29920-208831
Several glucagon analogs lacking a covalent intramolecular bridge and
comprising an AIB at position 2, an MB at position 16, and a fatly acyl group
attached via a spacer to a Lys residue at position 10 were made as essentially
described herein. The acylated glucagon analogs differed by the type of
spacer, the
presence or absence of pegylation, and/or by the size of the acyl group. The
acylated
glucagon analogs were tested for in vitro activity at the glucagon receptor
and the
= GLP-1 receptor as essentially described in Example 14. A summary of the
structure
and in vitro activity at the glucagon and GLP-1 receptors of each peptide is
shown in
Tables.22 and 23.
TABLE 22
Glucagon analog backbone amino acid sequence:
HXQGTFTSDKSKYLDXRRAQDFVQWLMNT-NH2
wherein X = MB
(SEQ ID NO: 562)
Size of EC50 at Glucagon EC50 at GLP-1
Peptide SEQ ID
Spacer Fatty Acyl Receptor Receptor
Name NO:
Group (nM) (nM)
wt
1 n/a n/a 0.031 0.014
glucagon
wt GLP-1 n/a n/a 0.036 + 0.010
26 637 None None 0.653 0.285 0.475 0.046
50 563 None C16 0.572 0.084 0.291 0.060
82 564 Ala-Ala C16 0.024 0.001 0.108 0.018
83 565 y-Glu- y-Glu C16 0.014 0.002 0.043
0.005
84 566 C16 0.011 0.004
6-amino-
85 567C16 0.010 0.005
hexanoic acid
86 568 Leu-Leu C16 0.011 0.006
= 87 569 Pro-Pro C16 0.017 0.009
77* 570 None C14 21.94 14.47 1.458 0.132
78* 571 y-Glu- y-Glu CI4 0.319 0.091 0.103 1
0.023
81* 573 Ala-Ala C14 0.597 + 0.175 0.271 0.019
79* 575 Ala-Ala C16 0.102 0.011 0.055 1 0.001
80* 576 y-Glu- y-Glu C16 0.108 + 0.028 0.042
0.008
* indicates that the peptide comprised a Cys residue at position 24 (in place
of Gin)
which Cys was covalently attached to a 40 kDa PEG group.
144
CA 02 852 177 2 014-05-2 3
WO 2009/155258 PCT/US2009/047438
29920-208831
TABLE 23
Glucagon analog backbone amino acid sequence:
HXQGTFTSDKSKYLDXRRAQDFVWLMNT-NH2
wherein X = AIR
(SEQ ID NO: 562)
ECso at ECso at
Size of
Peptide SEQ ID Glucagon G LP- I
Spacer Fatty Acyl
Name NO: Receptor Receptor
Group
(nM) (nM)
wt 0.008 *
1 n/a n/a
glucagon 0.003
0.004
wt GLP-1 n/a n/a
0.001
0.144* 0.063*
77** 616 none C14
0.029 0.012
0.009* 0.008
78** 617 y-Glu C14
0.001 0.001
0.027* 0.018
811* 618 Ala-Ala C14
0.006 0.001
0.006* 0.008
80** 619 y-Glu- y-Glu C16
0.001 0.001
0.010* (wog
79" 620 Ala-Ala C16
0.001 0.001
** peptide comprising Cys at position 24 (in place of Gin) which Cys was not
covalently attached to a PEG molecule
As shown in Tables 22 and 23, the peptides comprising a fatty acyl group
attached via a spacer significantly increased their potency as compared to
peptides
comprising a fatty acyl group attached directly to the peptide backbone.
EXAMPLE 32
DIO mice (8 mice per group), each with an average bodyweight of 48.7 g,
were subcutaneously injected daily for seven days with vehicle only, with 30
nmol/kg
or 100 nmoUkg of an acylated glucagon analog peptide, or with the long-acting
GLP-
1 analog, Liraglutide (Novo Nordisk, Denmark). The acylated glucagon analogs
were
as follows:
"(C16) Glucagon Amide" comprised the amino acid sequence of wild-type
glucagon (SEQ ID NO: 1) with the Tyr at position 10 modified to an acylated
Lys
145
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
residue, wherein the acylated Lys comprised a C16 fatty acyl group, and the C-
terminal carboxylate replaced with an amide group;
"yE-yE-C16 Glucagon Amide" comprised the same structure of C16 Glucagon
Amide, except that the C16 fatty acyl group was attached to the Lys at
position 10
through a gamma-Glu-gamma-Glu dipeptide spacer (see structure of acylated Lys
below);
0
11, Lys
HN 0
yE
HO
NH YE
0 OH
C16
CH3
0
HO 0
"AA-C16 Glucagon Amide" comprised the same structure of C16 Glucagon
Amide, except that the C16 fatty acyl group was attached to the Lys at
position 10
through an Ala-Ala dipeptide spacer; and
"f3APA-C16 Glucagon Amide" comprised the same structure of C16 Glucagon
Amide, except that the C16 fatty acyl group was attached to the Lys at
position 10
through an 13-Ala-f3-Ala dipeptide spacer.
The body weight of the mice was monitored daily and the total change in body
weight (%) is shown in Figure 22. As shown in Figure 22, most of the acylated
glucagon peptides at each dose caused a reduction in body weight. While
Liraglutide
demonstrated an approximate 12% decrease in body weight, the glucagon analog
peptide yE-yE-CI6 Glucagon Amide exhibited the greatest ability to cause
weight loss
in mice at the matched dose. Even the lower dose of yE-yE -C16 Glucagon Amide
caused a substantial decrease in body weight
The fat mass of the mice was measured on Day 7 of the study. As shown in
Figure 23, the mice which were administered 100 nmoUlcg yE-yE-C16 Glucagon
Amide exhibited the lowest fat mass.
146
CA 02 852 177 2 0 14-05-2 3
WO 2009/155258 PCUUS2009/047438
29920-208831
Blood glucose levels of the mice were also monitored during the course of the
assay. As shown in Figure 24, the glucagon analog peptide yE-yE-C16 Glucagon
Amide at the higher dose worked as well as Liraglutide to decrease blood
glucose
levels in mice.
EXAMPLE 33
Acylation of a glucagon analog peptide having GLP-1 activity was evalulated
as follows. A non-acylated glucagon analog peptide comprising the structure of
Chimera 2 with MB at position 2 and Cys at position 24 (comprising a 40 kDa
PEG
molecule) was modified to comprise an acylated Lys residue at position 10. The
non-
acylated glucagon analog peptide comprised the amino acid sequence of SEQ ID
NO:
580. The Lys at position 10 was acylated with a C8, C14, C16, or C18 fatty
acyl
group and the acylated peptides comprised the structures of SEQ ID NOs: 534-
537,
respectively. The in vitro activity at the GLP-1 receptor of the non-acylated
peptide
and acylated versions thereof were tested as essentially described herein. The
EC50
at the GLP-1 receptor of each peptide is shown in Table 24.
TABLE 24
Glucagon analog peptide sequence
=
HXQGTFTSDYSKYLDEQAAKEFICWLMNT-NH2,
wherein X = AIB
(SEQ ID NO: 580)
EC50 (nM) SD .
GLP-1 0.026 0.003
Non-acylated Glucagon analog peptide (SEQ ID NO: 580J 0.095 0.015
Cs acylated Glucagon analog peptide (SEQ ID NO: 534) 9.058 0.002
C14 acylated Glucagon analog peptide (SEQ ID NO: 535) 0.044 0.005
=
C16 acylated Glucagon analog peptide (SEQ ID NO: 536) 0.033 0.005
C18 acylated Glucagon analog peptide (SEQ ID NO: 537) 0.011 0.001
EXAMPLE 34
147
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Glucagon analog peptides were made by solid-phase peptide synthesis as
described herein and were acylated at either position 10 or 30 of the peptide.
The
peptides and their structure were as follows:
"Peptide dS2E161C20K30-C14 Glue Amide" comprised the amino acid
sequence HXQGTFTSDYSKYLDERRAICDFVQWLMNTK-amide (SEQ ID NO:
581), wherein the X at position 2 is d-Ser, wherein the Lys at position 30 is
acylated
with a C14 fatty acyl group, and the C-terminal carboxylate is replaced with
an
amide;
"Peptide dS2K10(C14)E16K20-Gluc Amide" comprised the amino acid
sequence HXQGTFTSDKSKYLDERRA1CDFVQWLMNT-amide (SEQ ID NO:
582); wherein the X at position 2 is d-Ser, wherein the Lys at position 10 is
acylated
with a C14 fatty acyl group, and the C-terminal carboxylate is replaced with
an
amide;
"Peptide dS2E16K20K3O-C16 Glue Amide" comprised the amino acid
sequence HXQGTFTSDYSKYLDERRAKDFVQWLMNTK-amide (SEQ ID NO:
583), wherein the X at position 2 is d-Ser, wherein the Lys at position 30 is
acylated
with a C16 fatty acyl group, and the C-terminal carboxylate is replaced with
an
amide;
"Peptide dS2K10(C16)E16K20-Gluc Amide" comprised the amino acid
sequence HXQGTFTSDKSKYLDERRAKDFVQWLMNT-amide (SEQ ID NO:
584); wherein the X at position 2 is d-Ser, wherein the Lys at position 10 is
acylated
with a C16 fatty acyl group, and the C-terminal carboxylate is replaced with
an
amide;
"Peptide Chimera 2-AIB2-K10-acylated" comprised the amino acid sequence
HXQGTFTSDKSKYLDEQAAICEFICWLMNT-amide (SEQ ID NO: 585); wherein
the X at position 2 is AIB, the K at position 10 is acylated with a C18 fatty
acyl
group, Cys at position 24 comprises a 40 kDa PEG molecule, and the C-terminal
carboxylate is replaced with an amide; and
"Peptide Chimera 2-AIB2-K30-acylated" comprised the amino acid sequence
HXQGTFTSDYSKYLDEQAAICEFICWLMNTK-amide (SEQ ID NO: 586), wherein
the X at position 2 is AIB, the K at position 10 is acylated with a C18 fatty
acyl
group, Cys at position 24 comprises a 40 kDa PEG molecule, and the C-terminal
carboxylate is replaced with an amide.
148
CA 02 852 177 2 014-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
The in vitro activity at the GLP-1 receptor and glucagon receptor of each
peptide was tested as essentially described in Example 14. The results are
shown in
Table 25.
TABLE 25
Position at EC50 at the ECM at the
Peptide Name which acyl glucagon GLP-1
receptor
group is found receptor (nM) (nM)
Peptide dS2E16K20K3O-C14 Glue Amide 30 3.53 0.84
Peptide dS2K10(C14)E16K20-Gluc Amide 10 0.155 0.041
Peptide dS2E16K20K3O-C16 Glue Amide 30 4.89 3.05
dS2K10(C16)E16K20-Gluc Amide 10 0.076 0.041
Peptide Chimera 2-AlB2-K10-acylated 30 N/A 0.465
IPeptide Chimera 2-A1B2-K30-acylated 10 N/A 0.007
EXAMPLE 35
Solid-phase peptide synthesis was employed for the assembly of the sequence,
XSQGTFTSDYSKYLDERRA1CDFVCWLMNT-NH2, wherein X=DM1A (SEQ ID
NO: 587). After selective deprotection of the Glu at position 16 and the Lys
at
position 20, the peptide was cylized via a lactam bridge on resin. The crude
peptide
after cleavage was then purified by preparative RP-HPLC and characterized by
MS
(calc. for [M+H]: 3479.9 ; found 3480.9). PEGylation was conducted by mixing
the
peptide precursor and iodoacetyl-functioned 40k Da PEG (NOF)(1:1) in 7 M urea/
50
mM Tris buffer, pH 8.5, at room temperature for 45 minutes to form a covalent,
thioether bond between the PEG and a Cys of the peptide, as shown below
Peptide
LSNOOYCH3
0
The PEGylated peptide was purified by preparative HPLC and the desired
fractions
were collected and lyophilized to yield a off-white powder. The product was
confirmed by MALDI-TOF-MS (44000-46000, broad peak).
The in vitro activity at the GLP-1 receptor and glucagon receptor were tested
as essentially described in Example 14. The EC5Os at the GLP-I receptor and
glucagon receptor were 0.327 nM and 0.042 nM, respectively.
149
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
EXAMPLE 36
Solid-phase peptide synthesis was employed for the preparation of the peptide
precursor, HXEGTFTSDYSKYLDEQAAKEFICWLMNT-NH2, wherein X = AIB
(SEQ ID NO: 589). The crude peptide was then purified by preparative RP-HPLC
and characterized by MS (calc. for [M+H]: 3412.8 ; found 3413.9). PEGylation
was
conducted by mixing the peptide precursor and iodoacetyl-functioned 40k Da PEG
(NOF)(1:1) in 7 M urea/ 50 mM Tris buffer, pH 8.5, at room temperature for 45
minutes to form a covalent, thioether bond between the PEG and a Cys of the
peptide,
as shown below
Peptide
0
. The PEGylated peptide was purified by preparative HPLC and the desired
fractions
were collected and lyophilized to yield a off-white powder. The product was
confirmed by MALDI-TOF-MS (44000-46000, broad peak).
The in.vitro activity at the GLP-1 receptor and glucagon receptor were tested
as essentially described in Example 14. The EC5Os at the GLP-1 receptor and
glucagon receptor were 0.027 nM and 33 nM, respectively.
EXAMPLE 37
The following glucagon analog peptides comprising a backbone of Peptide J
HS-X-GTFTSDYSKYLDTRRAAEFVAWL(Nle)DE
(SEQ ID NO: 591)
or Peptide K
HS-X-GTFTSDYSKYLD(Aib)RRAADFVAWLMDE
(SEQ ID NO: 592)
with additional modification at position 3 were made by solid-phase peptide
synthesis
as essentially described herein. The peptides were tested for in vitro
activity at the
glucagon receptor as essentially described in Example 14. The EC50 (nM) of
each
peptide is shown in Table 26.
150
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
=
29920-208831
TABLE 26
EC50 at %
activity*
Peptide
Amino Acid at Position 3 SEQ ID NO: Glucagon
Backbone
Receptor (nM)
593 0.24 25%
C(Acm) 594 0.18 .
33%
Dab(Ac) 595 0.31 19%
Dap(urea) 596 0.48 13%
Q(Me) 597 0.48 13%
M(0) 598 0.91 7%
Om(Ac) 599 0.92 7%
600 0.39 15%
Dab(Ac) 601 0.07 86%
Q(Me) 602 0.11 55%
Q = glutamine; C(Acm) = acetamidomethyl-cysteine; Dab(Ac) =
acetyldiaminobutanoic acid; Dap(urea) = carbamoyldiaminopropanoic acid ;Q(Me)
methylglutamine; M(0) = methionine-sulfoxide; Orn(Ac) = acetylomithine.
As shown in Table 26, multiple amino acids could be placed at position 3
without a substantial loss of activity at the glucagon receptor, and, in some
cases, the
modification actually increased the activity, e.g., Dab(Ac) and Q(Me) on the
Peptide
K backbone.
EXAMPLE 38
Glucagon analog peptides comprising Dab(Ac) at position 3 on various
glucagon analog backbones were made as essentially described herein and the in
vitro
activity at the glucagon receptor was tested. The structures and activities of
each
peptide are shown in Table 27.
151
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/0417438
29920-208831
=
TABLE 27
EC50
SEQ
(nm) at
Amino acid sequence ID activi
G1ucagon
NO: tY*
Receptor
Wildtype Glucagon 1 0.026 100
HSQGTFTSDYSKYLDSRRAQDFVQWLMDT 642 0.015 173
HSDab(Ac)GTFTSDYSKYLDAibRRAADFVAWLLDE 603 0.069 37
HSDab(Ac)GTFTSDYSKYLDAibRRAADFVAWLLDTGPSSGAPP
604 0.023 113
PS amide
HSDab(Ac)GTFTSDYSKYLDAibRRASDFVSWLLDE 605 0.048 54
HSDab(Ac)GTFTSDYSKYLDAibRRATDFVTWLLDE 606 0.057 46
EXAMPLE 39
A first glucagon analog peptide (AIB2, AIB16, K10(C16) Glue Amide)
comprising SEQ ID NO: 1 with AIB at positions 2 and 16, Lys at position 10,
wherein
the Lys at position 10 was covalently attached to a C16 fatty acyl group, and
an amide
in place of the C-terminal carboxylate was made as essentially described
herein. A
second glucagon analog peptide (AIB2, AIB 16, KIO(C16), K30 Glue Amide) having
the same structure as the first glucagon analog peptide, except that a Lys was
added to
the C-terminus. The in vitro activity of the peptides was tested as
essentially
described in Example 14 and was additionally tested in a solution comprising
20%
human plasma. The EC50 (nM) at each receptor for the peptides is shown in
Table
28.
152
29920-208831
JI
rji
00
TABLE 28
EC50 (nm) EC50 (nm) EC50 (nm)
at
at GLP-1 at GLP-1
EC50 (nm)
Glucagon
Receptor Receptor
SEQ at
Amino acid sequence
Receptor (20%
ID NO: Glucagon
4:1
(20%
human
Receptor
human
plasma)
plasma)
co
Glucagon 1 0.026
0.046
'Ji
GLP-1
0.022 0.028
AlB2, AlB16, KI0(C16) Glucagon Amide 563 0.052
0.023 0.026 0.014
AlB2, A11316, KI0(C16), K30 Glucagon Amide 622 0.761
0.313 0.031 0.017
cr)
r.)
C13
ra.
00
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
EXAMPLE 40
The discovery of leptin documented the existence of an endocrine system that
regulates energy balance and body adiposity. It also recruited interest and
investment
in obesity research as a means to identify environmental and pharmacologic
approaches to manage what has become a global epidemic of disease.
Sufficiently
efficacious and safe pharmacologic treatment for obesity has yet to emerge and
surgery constitutes the only proven option to sustained weight loss. It is
reported
herein that the combinatorial efficacy of receptor agonism at two endocrine
hormonal
receptors to achieve potent satiety inducing and lipolytic effects in a single
peptide of
sustained duration of action. Two specific glucagon analogs with activity at
the
GLP1-R comparable to native GLP-1, but differing from each other in their
level of
glucagon receptor agonism were studied pharmacologically in rodent obesity
models.
Once weekly administration of these pegylated peptides selected from a series
of high
potency analogs with differential glucagon and GLP-1 activity normalized
adiposity
and glucose tolerance levels in diet induced obese mice (average body weight
ca. 50g)
within a month. Body weight loss was a consequence of body fat loss resulting
from
decreased food intake and increased energy expenditure, which increased with
the
level of glucagon receptor agonism. These co-agonist compounds also normalized
glucose and lipid metabolism including liver steatosis. Effects were dose
dependent
and successfully repeated in diet induced obese rats. These preclinical
studies
indicate that when full GLP-1 agonism is enhanced with an appropriate degree
of
glucagon receptor activation, body fat reduction can be substantially and
safely
accelerated. The findings shown herein establish a basis for clinical testing
and
suggest an attractive novel treatment option for the metabolic syndrome.
EXAMPLE 41
The following materials and methods pertain to the experiments described in
Examples 42 to 51.
Boc peptide synthesis and cleavage.
Peptide syntheses were performed using 0.2 mmol 4-methylbenzhydrylamine
(MBHA) resin (Midwest Biotech, Fishers, Indiana) on a modified Applied
Biosystems 430A peptide synthesizer. Solid-phase peptide syntheses utilized in
situ
neutralization for Boc-chemistry (Schnolzer, M. et al., International Journal
of
154
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Peptide Research and Therapeutics, 13:31-44 (2007)). Completed peptidyl-resins
were treated with HF/p-cresol (10:0.5 v/v) at 0 C for 1 h. HF was removed in
vacuo
and the deprotected peptide was precipitated and washed in diethyl ether. The
peptide
was dissolved in 20% acetonitrile/1% acetic acid and lyophilized. Most
peptides were
prepared by Boc chemistry. The following side chain protecting groups were
used for
Boc-amino acids (Midwest Biotech): Arg(Tos), Asp(OcHex), Asn(Xan), Glu(OcHex),
His(BOM), Lys(2-C1-Z), Ser(Bz1), Thr(Bz1), Trp(CH0), Tyr(Br-Z). Peptide
molecular weights were confirmed by electrospray ionization or MALDI-TOF mass
spectrometry and purified as described elsewhere.
Lactam Synthesis.
Cyclized peptides with i to i + 4 lactam formation were synthesized on resin.
Glu(OFm)-OH gamma ester (Peptides International, Louisville, Kentucky) and
Lys(Fmoc)-OH (Peptides International) were substituted for Glu(OcHex) and
Lys(2-
CI-Z) at positions involved in lactam formation. The fully protected peptidyl-
resin
=
was treated with 20% piperidisie in DMF for 45 minutes to remove Fmoc and OFm
protecting groups. On resin, lactam formation was achieved after treatment
with 5
equivalents of benzotriazole-1-yloxy-tris-pyrrolidino-phosphonium
hexafluorophosphate (PyBOP) (Fluka) in DMF/DIEA for 5 h. Lactam formation was
confirmed by ninhydrin analysis and mass reduction of 18 relative to the open
form of
the peptide.
Peptide Purification.
Following cleavage from the resin, crude peptide extracts were analyzed by
analytical reverse-phase FIPLC. Analytical separations were conducted in 0.1%
TFA
with an acetonitrile gradient on a Zorbax C8 column (0.46 X 5 cm). After
analytical
analysis, the crude extract was purified by semi-preparative chromatography in
0.1%
TFA with an acetonitrile gradient on a Vydac C4 or C18 column (2.2 X 25 cm).
Pegylated peptides were purifed using the same conditions. Preparative
fractions
were analyzed for purity (> 95%) by analytical reverse-phase HPLC utilizing
the
conditions listed for analytical separations. Peptide masses and purity were
confirmed
by electrospray ionization mass spectrometry (ESI-MS) or matrix-assisted laser
desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Pegylated
155
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
peptides showed a broad mass range spanning 43400 by MALDI-TOF. Purified
peptides were lyophilized and stored at 4 C.
Pegylation of Peptides.
Purified peptides were mixed at a 1:1 molar ratio with methoxy poly(ethylene
glycol) maleimido-propionamide-40K (Chirotech Technology Ltd, Cambridge) in 7M
urea/50mM Iris, pH 8Ø Reaction progress was monitored by analytical reverse-
phase HPLC and free peptide was consumed within 30 minutes. The reaction was
quenched in 0.1% TFA, purified and characterized as described elsewhere.
Glucagon and GLP-I Receptor-Mediated cAMP Synthesis.
Each peptide analog was tested for its ability to stimulate cAMP production
through the glucagon (Gcg) and GLP-1 receptors. HEK293 cells were co-
transfected
with the GcgR or GLP-1R cDNAs and a luciferase reporter gene-linked to a cAMP
response element (CRE). Cells were serum deprived for 16 h by culturing in
DMEM
(Invitrogen, Carlsbad, CA) and supplemented with 0.25% Bovine Growth Serum
(HyClone, Logan, UT). Serial dilutions of Glucagon and GLP-I analogs were
added
to 96-well poly-D-Lysine-coated plates (BD Biosciences, San Jose, CA)
containing
co-transfected 11EK293 cells, and plates were incubated for 5 h at 37 C, 5%
CO2.
Following incubation, an equivalent volume (100 pl) of LucLite luminescence
substrate reagent (Perkin-Elmer, Wellesley, MA) was added to each well and the
plate
was shaken for 3 min at 800 rpm. The plate was incubated for 10 min in the
dark and
light output was quantified on a MicroBeta1450 liquid scintillation counter
(Perkin-
Elmer, Wellesley, MA). Effective 50% concentrations (EC50) were calculated by
Origin software (OriginLab, Northampton, MA).
Circular dichroism measurements.
Peptides were dissolved in 10 mM phosphate buffer pH 5.9 with increasing
concentrations of TFE, and peptide concentrations were quantified. Each sample
was
diluted to 10 for CD measurements. CD data were collected on a JASCO J-715
circular dichroism spectropolarimeter with constant nitrogen stream and
temperature
control of the 1 mm path length cell set at 25 C. Spectral data were
accumulated for
scans from 270-190 rim with a scan speed of 100 tun/min and 1 nm wavelength
step. Solvent signal was subtracted and data were smoothed (Savitzlcy and
Golay,
156
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
Anal. Chem. 36:1627 (1964)); in the JASCO Spectra Manager software.
Millidegree
values obtained were converted to mean residue ellipticity with units of
degcmldmol'I. Calculated mean residue ellipticity values were input into
DICHRO WEB (Whitmore and Wallace, Biopolymers 89:392- 400 (2008); Whitmore
and Wallace, Nucleic Acids Research 32:W668-W673 (2004) to obtain percent
helicity values.
Animals.
C57BI/6 mice were obtained from Jackson Laboratories and fed a
diabetogenic diet from Research Diets, a high sucrose diet with 58% kcal from
fat.
Mice were single or group-housed on a 12:12-h light-dark cycle at 22 C with
free
access to food and water. All studies were approved by and performed according
to
the guidelines of the Institutional Animal Care and Use Committee of the
University
of Cincinnati.
Body Composition Measurements.
Whole body composition (fat and lean mass) was measured using NMR
technology (EchoMRI, Houston, TX).
Energy Balance Physiology Measurements.
Energy intake and expenditure, as well as home-cage activity, were assessed
by using a combined indirect calorimetry system (TSE Systems, Bad Homburg,
Germany). Oxygen consumption and CO2 production were measured every 45 min
for a total of 120 h (including 12 h of adaptation) to determine the
respiratory quotient
and energy expenditure. Food and water intake and meal patterns were
determined -
continuously for 120 h at the same time as the indirect calorimetry
assessments by
integration of scales into the sealed cage environment. Meals were defined as
food
intake events with a minimum duration of 60 s, and a break of 300 s between
food
intake events. Home-cage locomotor activity was determined using a
multidimensional infrared light beam system with beams scanning the bottom and
top
levels of the cage, and activity being expressed as beam breaks. Stationary
motor
activity (fidgeting) was defined as consecutive breaks of one single light
beam at
cage-bottom level, ambulatory movement as breaks of any two different light
beams
157
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
at cage-bottom level, and rearing as simultaneous breaks of light beams on
both cage-
bottom and the top level.
Blood Parameters.
Blood was collected after a 6-h fast from tail veins using EDTA-coated
Microvette tubes (Sarstedt, Nuremberg, Germany) and immediately chilled on
ice.
After 15 min of centrifugation at 3,000 g and 4 C, plasma was stored at -80 C.
Plasma insulin was quantified by a radioimmunoassay from Linea (Sensitive Rat
Insulin RIA; Linco Research, St. Charles, MO). Plasma TGs and cholesterol
levels
were measured by enzymatic assay kits (Thermo Electron, Waltham, MA). Samples
were analyzed individually with the exception that pooled samples (0.25 ml)
from 5
animals/group were subjected to fast-performance liquid chromatography (FPLC)
gel
filtration on two Superose 6 columns connected in series for lipoprotein
separation.
All assays were performed according to the manufacturer's instructions.
= =
Glucose tolerance test.
For the determination of glucose tolerance, mice were subjected to 6 h of
fasting and injected intraperitoneally (i.p.) with 2 g glucose/kg body wt (50%
D-
glucose (Sigma) in 0.9% saline) for the glucose tolerance test (GTT). Tail
blood
=
glucose levels (mg,/dly were measured by using a hand-held glucometer
(TheraSense
Freestyle) before (0 min) and at 15, 30, 60, 90, and 120 min after injection.
=
Western blot of WAT HSI.
Adipose tissue was placed in a 1.5-ml microfuge tube and lysed in ice cold
R1PA buffer (1X PBS, 1% Nonidet P40,0.5% sodium doxycholate, 0.1% SDS with
50 mM NaF, 0.5 M phenylmethylsulfonyl fluoride, 0.1 mM Na Vanadate, 20 .g/ml
Aprotinin, 10 fig/m1 Leupeptin) using a tissue lyser (Retsch, Inc Newtown, PA
Cat. #
85210) at 30 hz for 3 min. Samples were spun at 12,000 rpm for 15 min (4 C)
at
which time the intematant was removed to a new tube and sonicated for 15 sec
on ice.
Samples were spun at 14,000 rpm for 10 min (4 C) and the intematant was
collected
to a new tube. Samples were again spun at 19,000 rpm for 10 min (4 C) and the
intematant collected to a new tube. An aliquot of sample was then taken for
protein
=
assay. Samples were then boiled in 4x SDS/DTT buffer for 2 min. 50 ug of
protein
from cell lysate were subjected to SDS/PAGE on 9% (w/v) acrylamide resolving
gels
=
158
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
and transferred to Hybond ECL nictrocellulose membranes. Membranes were
blocked and probed with primary antibodies of interest (HSL (4107)) from Cell
Signaling; Phospho-HSL (ser 660) (4126) from Cell Signaling). After washing,
primary antibody detection was performed using either HRP-conjugated anti-
(rabbit
IgG) or anti-(mouse IgG) (1RP-conjugated anti-rabbit and anti-mouse secondary
antibodies were purchased from Bio-Rad (170-6515 & 170-6516)) and detected
using
enhanced chemiluminescence (Amersham Biosciences) and exposed to CL-Xposure
film (Pierce).
Immunohistochemistty.
Paraffin embedded sections of white epididyrnal adipose tissue (5 pm) were
stained with hematoxylin/eosin as described (Ogden, C. L. et al. JAMA 295:1549-
1555 (2006)). For each individual mouse tissue block, adipocyte size of 100
cells
from each of three different high-power fields was quantified as areal
measurement
using Image Pro Plus 5.1 software (Media Cybernetics, Bethesda, MD, USA).
Oil Red Staining.
To visualize lipid accumulation in liver tissue, 4-8 mm cross-sections of the
livers that were harvested at sacrifice were stained with Oil Red 0 dye.
Images at
both 20X and 40X magnification were acquired using a [compound-lens]
microscope.
Quantitative RT-PCR procedure.
Animals were sacrificed by decapitation in the fed state (1-4 h after the
morning feeding) and various tissues were sampled, freeze-clamped, and stored
at -
80 C for subsequent measurement of mRNA expression of PEPCK, G6P, and HPRT
(housekeeping) by real-time quantitative PCR (icycler, BioRad).
Total RNA was extracted from frozen tissue samples using a RNeasy Lipid
Tissue Kit (Qiagen, Ca# 74804) using the standard protocol. RNA concentrations
and
purity were determined by spectrophotometry using the Nanodrop. cDNA templates
for RT-PCR were obtained using 2 jig of total RNA. Reverse transcription
reaction
was performed with 10X DNase I Reaction Buffer, DNase I, Amp Grade, 1 U/ 1,
=
depc-H20, 25 mM EDTA, 10 mM dNTP Mix, oligo(dT)20 (50 M), 5X First-Strand
Buffer, 0.1 M DTT, RNaseOUT, and SuperScript III (Invitrogen).
159
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
The synthesized cDNAs were further amplified by PCR using the fluorescent
dye SYBR green (BioRad, CO 1708882) containing a final concentration of 0.5
tiM
of forward and reverse primers. Product purity was confirmed by dissociation
curves.
No-template controls were included in all assays, yielding no consistent
amplification.
A standard curve was used to obtain the relative concentration of PEPCK or
G6P, and
the results were corrected according to the concentration of HPRT, used as
housekeeping genes. The results are expressed as percent of vehicle, setting
the mean
of the vehicle group at 100% and then calculating each individual value of the
3
groups of animals studied.
Primer Sequences.
Primer sequences for PEPCK, G6P, and HPRT were taken from the NIH
website and primers were generated by IDT DNA.
Reverse Transcription and Quantitative Real-Time RT-PCR.
CD68 mRNA expression was quantified by real-time RT-PCR as described
(Nomiyama, T. et al. Journal of Clinical Investigation 117:2877-2888 (2007)).
Briefly, upon sacrifice, 100 mg epididymal adipose tissue was homogenized in
TRIZOL and total mRNA was reverse transcribed into cDNA. PCR reactions were
performed using an iCycler (Bio-Rad) and SYBR Green I system (Bio-Rad). Each
sample was analyzed in triplicate and normalized to values for TFI1B mRNA
expression. Mouse primer sequences used were as follows:
CD68, 5'-CAAGGTCCAGGGAGGTTGTG-3' (forward) (SEQ ID NO: 638),
5'-CCAAAGGTAAGCTGTCCATAAGGA-3' (reverse) (SEQ ID NO: 639);
and TFIIB, 5'-CTCTCCCAAGAGTCACATGTCC (SEQ ID NO: 640),
5'-CAATAACTCGGTCCCCTACAAC-3' (reverse) (SEQ ID NO: 641).
Statistical Analyses.
Unless indicated otherwise, all statistical analyses were performed using
GraphPad Prism one-way ANOVAs and column statistics. Stated P values are for
one-way analysis of variance. All results are presented as means SE.
(Receptor
activation data is S.D.). -
160
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
EXAMPLE 42
Two glucagon peptides, Peptides X and Y, comprising the amino acid
sequence of SEQ ID NO: 1 with amino acid modifications were made as described
herein. Both peptides comprised AIB at position 2, Glu at position 16, Gin at
position
17, Ala at position 18, Lys at position 20, Glu at position 21, Ile at
position 23, and
Cys at position 24. Site-specific 40-kd pegylation was achieved at Cys at
position 24
through reaction with a maleimide-functionalized linear peg to yield Peptide X-
PEG
and Y-PEG. Peptides Y and Y-PEG differed from Peptides X and X-PEG,
=
respectively, in that a single side-chain lactam bridge was introduced in the
middle of
Peptide Y or Peptide Y-PEG to stabilize the secondary structure and enhance
glucagon agonism. The two side chains of Glu at position 16 and Lys at
position 20
were covalently coupled in the course of peptide assembly as a side-chain
amide.
This macrocyclization of the peptide represents a 21-atom lactam. Peptides X-
PEG
and Y-PEG were tested for solubility and were found to be soluble in
physiological
buffers at concentrations that exceed 25 mg/ml, and Peptides X-PEG and Y-PEG
proved completely resistant to ex vivo incubation with plasma for periods of
one
week.
EXAMPLE 43
The secondary conformation of peptides when solubilized in various
concentrations of aqueous trifluoroethanol (TFE) was analyzed by circular
dichroism
(Figure 25). Glucagon was the least helical peptide tested, and had calculated
helicity
of 10, 15 and 33% in TFE solutions of 0, 10 and 20%, respectively (Table 29).
Under
the same experimental conditions, GLP-1 had enhanced helicity of 14, 29 and
55%,
demonstrating that these two peptides differ in primary as well as secondary
structure.
There were no significant changes in the helicity of Peptides X and Y when
pegylated, despite the fact that the pegylated portions represent more than
90% of the
molecule by mass (Table 29). In contrast, the apparent helicity of Peptide Y
in
phosphate buffer in the absence of TFE was approximately double that of
Peptide X,
from 17% to 36%. Consequently, the pegylated forms of these two chimeric
peptides
(Peptide X-PEG and Peptide Y-PEG) differed appreciably in secondary structure
(Figure 25) and the differences in biological properties are likely a function
of these
secondary structural differences.
161
CA 02 852 177 2 014-05-2 3
WO 2009/155258 PCT/US2009/047438
29920-208831 =
TABLE 29
Percent Helicity
Peptide
0% TFE 10% TFE 20% TFE
Glucagon 10 15 33
GLP-1 14 29 55
=
Peptide X 17 34 60
Peptide
12 31
X-PEG '
Peptide Y 36 35 64
Peptide
37 51
Y-PEG
EXAMPLE 44
The two peptides (Peptides X and Y) and their 40-kd pegylated derivatives
(Peptides X-PEG and Y-PEG) were assessed for their ability to stimulate cAMP
synthesis in cell-based CRE-luciferase reporter assays (Figure 26). As shown
in
162
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
Table 30, native glucagon activated glucagon receptors half-maximally at an
effective
concentration (EC50) of 0.055 0.014 nM and the GLP-1 receptor (GLP- I R) at
a
much higher concentration, EC50 of 3.29 0.39 nM. In contrast, GLP-1
activated its
receptor with an EC50 of 0.028 0.009 nM and proved highly specific in that
interaction at the glucagon receptor (GcgR) occurred at an EC50 exceeding 1
M.
The dynamic range in specificity exhibited for the native ligands at their
receptors is
in excess of a million. The potency of Peptide X-PEG at GLP-1R was twice that
of
native GLP-1 and even more enhanced at GcgR in a relative sense. However, the
GcgR activity was only approximately 10% that of native glucagon. The
introduction
of the lactam restored full glucagon agonism without a change at GLP-I R.
Consequently, Peptide Y-PEG is a fully potent, nearly balanced co-agonist
relative to
the native ligands at the two respective receptors. PEGylation of each peptide
=
reduced potency by as much as ten-fold at GcgR and five-fold at GLP-1R. The
slightly enhanced loss in activity at GcgR may be a function of the greater
relative
importance of the C-terminal sequence to glucagon receptor interaction. The
pegylated peptides (Peptides X-PEG and Y-PEG) were slightly less potent at GLP-
1R
than native GLP-1 but still had a subnanomolar EC50. Peptide X-PEG is seven-
fold
more selective than the lactam version of this peptide, i.e., Peptide Y-PEG,
at the
GLP-1R. Therefore, these two DPP-4-resistant peptides are suitable for
sustained in
vivo time-action experiments and well-matched for GLP-1R agonism, but differ
in
glucagon agonism.
163
29920-208831
0
TABLE 30
()I
b.)
(11
Glucagon Receptor GLP-1 Receptor
Peptide
Selectivity*
EC50 Standard % activity of native EC50 Standard
% activity of native
(nM) Deviation glucagon (P-M) Deviation GLP-1
0
co
Glucagon 0.055 0.014 100.00 3.293 0.389 0.86
0.009
0
0
Ul
GLP-1 >1000 <0.008 0.028 0.009 100.00
>12500
Peptide X 0.585 0.125 9.38 0.014 0.002 202.12
21.5
(9
00
29920-208831
0
Peptide X-
2.895 0.963 1.90 0.036 0.014 78.41 41.4
PEG
(11
00
Peptide Y 0.055 0.011 99.51 0.013 0.005 219.78
2.21
ci
co
(xi
Peptide Y-
0.667 0.264 8.22 0.059 0.029 47.61 5.79
PEG
0
0
(xi
41.
00
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
EXAMPLE 45
The 40-kd pegylated peptides Peptides X-PEG and Y-PEG were used as single
weekly subcutaneous (s.c) injections in diet-induced obese (D10) C57B6 mice. A
single injection of 325 nmol/kg of Peptide Y-PEG decreased body weight over
one
week by 25.8%, from 50.9 1.4 g to 37.8 0.8 g (p <0.0001, n = 8/group).
Comparable administration of Peptide X-PEG was effective but considerably less
potent, as the decrease in body weight was 9% (49.1 1.51 g to 44.68 1.38
g).
Saline-injected control mice did not change their body weight (before: 50.61
1.32
g, after: 50.87 1.46 g; Figure 27A). The body weight changes were a result
of a
decrease in fat mass (41.9% for the lactam peptide, 22.2% for open form, 2.3%
for
controls, p < 0.001; Figure 27B) and were paralleled by a significant decrease
in
average daily food intake (Peptide Y-PEG: 0.40 0.29 g/day, Peptide X-PEG:
1.83
0.81 g/day, saline: 2.70 0.78 g/day, p <0.0001, Figure 27C). Blood glucose
was
significantly decreased for both peptides when compared to control, and
slightly more
so in Peptide Y-PEG (Peptide Y-PEG: -90.1 mg/dL, Peptide X-PEG: -79.6 mg/dL,
control: -23.9 g,/dL, p=0.0433; Figure 27D). The relative difference between
the two
peptides (Peptide X-PEG and Peptide Y-PEG) was not statistically significant.
EXAMPLE 46
In a separate experiment, single s.c. injections of six different doses (0, 7,
14,
35, 70, 140 and 350 nmol/kg) of Peptide Y-PEG and Peptide X-PEG demonstrated
linearly responsive, dose-dependent decreases in body weight and blood glucose
(Figure 28A, 28B, 28C and 28D). This suggests that the observed effects are
pharmacologically relevant with no apparent toxicity, other than the indirect
effects of
rapid, excessive loss in body weight. The magnitude of the effect was more
prominent with Peptide Y-PEG and indicates that the additional element of
glucagon
agonism improves the potency of the peptide.
EXAMPLE 47
In a separate experiment, weekly s.c. injections of 70 nmol/kg of Peptide Y-
PEG or Peptide X-PEG decreased body weight of DIO mice by 28.1 % and 20.1 %,
respectively (p < 0.0001, n= 7-8/group; Figure 29A). The body weight changes
were -
associated with a decrease in fat mass (-62.9% for Peptide Y-PEG, -52.2% for
Peptide
X-PEG, and 5.1% for controls, p <0.0001; Figure 29B). Long-term effects of
these
166
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
lower doses on food intake (p = 0.95; Figure 29C) were less impressive than
short-
term effects with a higher dose (Figure 27C). Energy expenditure was increased
with
Peptide Y-PEG (14.60 0.69 kcal/[kg*h)) and Peptide X-PEG (17.19 1.49
kcal/[kg*h]) compared to vehicle (12.71 0.45 kcal/[kg*h]), p = 0.0187),
whereas the
respiratory quotient tended to be decreased (Figure 29D and 29E; 0.719 0.01
for
Peptide Y-PEG, 0.725 0.01 for Peptide X-PEG, and 0.755 0.01 for vehicle, p
=
0.1028), indicating that increased thermogenesis and altered nutrient
partitioning may
explain the overall negative energy balance. Increased energy expenditure was
not
associated with a change in spontaneous physical activity induced
thermogenesis
(NEAT) since locomotor activity did not differ between treatment groups and
controls
(p = 0.4281; Figure 29F). Neither automated online monitoring of acute feeding
nor
chronic monitoring of food intake revealed any differences in caloric intake
(automated p = 0.667, chronic p = 0.9484; Figure 30A).
Blood glucose levels were markedly decreased over the treatment period
starting at Day 3 after the first injection (mean decrease: Peptide Y-PEG -
32%,
Peptide X-PEG -24.5%, controls: -2.7%, p < 0.0001; Figure 29G). In response to
an
intraperitoneally (i.p.) glucose challenge on Day 3, blood glucose peaks
(Figure 29H)
and profiles (AUC) (Figure 30F) were markedly lower in the two treated groups
(Peptide Y-PEG 14183 1072, Peptide X-PEG 13794 824.1) compared to the
vehicle-treated controls (34125 3142, p < 0.0001). After one month of
treatment
=
with Peptide Y-PEG or Peptide X-PEG, plasma insulin was lower in the treatment
groups (1194 pg/ml, 1034 pg/ml , p=0.0244) compared to controls (2675 pg/ml),
suggesting improved insulin sensitivity (Figure 291). Plasma C- peptide levels
tended
to be decreased after one month of treatment with Peptide Y-PEG or Peptide X-
PEG
(738.8 pg/ml, 624.7 pg/ml) versus vehicle (1077 pg/ ml) (p = 0.108) ( Figure
30G).
To determine if the principal phenomenon generalizes across species, both
compounds were administered to diet-induced obese rats (mean weight 777.4 +/-
2.1
g, dose 70 nmol/kg/weelc, once-a-week injection, 3-week treatment). Peptide Y-
PEG
and Peptide X-PEG each decreased body weight (Peptide X-PEG: -11.15 +/- 0.88%;
Peptide Y-PEG: -20.58 +/- 2.26%, vehicle: 1.09 +/- 0.56%) (p <0.0001) and fat
mass of the DIO rats (Peptide X-PEG: -19.17 +/- 2.03%; Peptide Y-PEG: - 33.76
+/-
4.76%, vehicle: 0.65 +/- 1.20%; p <0.0001), confirming a species-independent
applicability of this anti-obesity treatment approach.
167
CA 02852177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
EXAMPLE 48
= =
Chronic s.c. treatment over 27 days with Peptide X-PEG and Peptide Y-PEG
decreased total cholesterol in DIO mice (106.9 6.3 mg/dL and 200.8 29.58
mg/dL,
respectively) relative to vehicle (254.0 25.33 mg/dL, p = 0.0441; Figure 31A).
In a
separate experiment, DIO mice received 70 nmol/kg s.c. of Peptide X-PEG,
Peptide
Y-PEG or vehicle on Days 0 and 7 and were evaluated on Day 9. Peptide Y-PEG
decreased plasma triglycerides, LDL cholesterol and total cholesterol (total
cholesterol 63.0 2.49 mg/dL compared to vehicle 177.7 11.8 mg/dL) (p
<0.0001),
while potentially causing a switch from LDL to HDL cholesterol (Figure 31.13).
=
Peptide X-PEG decreased both LDL and HDL cholesterol but had no significant
effect on triglycerides (Figure 31C). There was a significant decrease in
leptin (3343
723.3 pg/ml for Peptide Y-PEG; 7308 2927 for Peptide X-PEG, and 18,642
6124 for vehicle; p=0.0426; Figure 31D, 31E, 31F). Chronic treatment for 27
days
also normalized liver lipid content while control DIO mice maintained
significant
liver steatosis (data not shown).
EXAMPLE 49
One month treatment with Peptide X-PEG or Peptide Y-PEG resulted in
increased phosphorylation of hormone sensitive lipase (HSL) in white adipose
tissue
(WAT) of DIO mice (Peptide X-PEG: 1.135 0.315; Peptide Y-PEG: 1.625
0.149; vehicle: 0.597 0.204; p = 0.0369; Figure 32B), implying a glucagon-
specific
direct effect on WAT lipolysis. Concomitant with the decrease in fat mass of
mice
treated for two weeks at a dose of 35 nmol/lcg/week with the Peptide Y-PEG and
=
Peptide X-PEG, there was a significant reduction of adipocyte size in
epiclidymal
adipose tissues when compared to control mice (data not shown). However,
despite
having decreased fat mass and smaller adipocytes, this short term treatment of
two
weeks with the Peptide Y-PEG and Peptide X-PEG was not associated with a
significant reduction of adipose tissue macrophage content as quantified by
real-time
RT-PCR for CD68 (Figure 33C). Uncoupling protein I (UCPI) levels in brown
adipose tissue (BAT) were increased by Peptide X-PEG, but not by Peptide Y-PEG
treatment (Peptide X-PEG 2.167 0.429, Peptide Y-PEG 1.287 0.1558, and
vehicle
1.0 0.118; p = 0.0264; Figure 32A), consistent with a GLP-1-specific action
on
BAT resting thermogenesis. Hepatic gene expression reflective of hepatic
.gluconeogenesis was not affected by either Peptide X-PEG or Peptide Y-PEG
(Figure
168
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
30H and 301). Histology indicated that pancreatic islets tended to be smaller
following Peptide X-PEG treatment (data not shown).
EXAMPLE 50
In order to dissect the contributions of the GLP-1R and the GcgR agonist
components of Peptides X-PEG and Y-PEG, each was administered for one month to
GLP-1 receptor knock out (GLP-1R -/-) mice maintained on high-fat diet.
Peptide X-
PEG caused a reduction of body weight (p > 0.05; Figures 34A and 34B) and fat
mass (p> 0.05; Figure 34C) compared to saline. Peptide Y-PEG caused a
significant
decrease in body weight (p = 0.0025) and fat mass (p = 0.0025) in the GLP-1R -
/-
mice (Figures 34A- 34C). Peptide X-PEG had no effect on food intake in GLP-1R -
/-
mice, while Peptide Y-PEG suppressed food intake significantly (p = 0.017)
(Figure
34D). Peptide Y-PEG (but not Peptide X-PEG) had a tendency to increase blood
glucose in a glucose tolerance test in the absence of a functional GLP-1R (p =
0.03)
(Figures 34E.and 34F), implying that the GLP-1 component of the co-agonist is
needed to protect against glucagon-induced hyperglycemia.
=
EXAMPLE 51
As an independent assessment of the effect of Peptides X-PEG and Y-PEG
that can be attributable to glucagon agonism, two additional peptide agonists
with
comparable GLP-1R potency but markedly different GcgR activity were studied.
The
two peptides (Peptides U and V) are related to the Peptides X-PEG and Y-PEG.
Peptides U and V comprised the amino acid sequence of SEQ ID NO: 1 with the
following modifications: Glu at position 16, Gln at position 17, Ala at
position 18,
Lys at position 20, Glu at position 21, Ile at position 23, and Cys at
position 24, but
comprised a 20-kd pegylation at the Cys at position 24 and did not comprise
AIB at
position 2. Peptide V additionally comprised a substitution of Gln3 with Glu
which
selectively reduced glucagon agonism by more than ten-fold. Neither Peptide U
nor
Peptide V comprised a lactam bridge. Treatment of DIO mice each day for one
week
at 50 nmol/kg s.c. with Peptide V revealed a reduced effect on body weight
lowering
relative to the Peptide U (-9.09 0.80 vs. -13.71 0.92 g, respectively (p
<0.0001;
Figure 35A).
169
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
EXAMPLE 52
Glucagon peptides comprising a C16 fatty acyl group attached to a Lys residue
via a y-Glu spacer or a y-Glu-y-Glu dipeptide spacer, wherein the Lys residue
is
located at position 10 or at the C-terminus (at position 29), were made as
essentially
described herein. The peptides were tested for in vitro activities at the
glucagon and
GLP-1 receptors as described herein. The results are shown in Table 31.
=
170
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
TABLE 31
Peptide EC50 (nM) at
SEQ ID EC50 at GLP-1
Acylated Glucagon
Spacer NO: Receptor
= AA Receptor
Chi-2, d-Ser2 Lys10 yE 643 0.011 0.0014
Chi-2, d-Ser2 Lys10 yE-yE 644 0.008 0.003
Chi-2, AIB2 Lys10 yE 645 0.025 0.0014
Chi-2, AIB2 Lys10 yE-yE 646 0.014 0.0018
Chi-2, A1132, E3 Lys10 None 647 46.084 0.005
Chi-2, AIB2, E3 Lys10 yE-yE 648 2.922 0.004
Chi-2, AIB2, 17 Lys10 yE 649 0.014 0.024 (0.044*)
Chi-2, AIB2, 17 Lys10 yE-yE 650 0.007 0.010
DMIA1,
Lys10 yE 651 0.019 0.006 .
E16/1(20 lactam
DM1A1,
Lys10 yE-yE 652 0.014 0.004
E16/K20 lactam
DMIA1,
Lys29 yE 653 0.107 0.075
E16/K20 lactam
DMIA1,
Lys29 yE-yE 654 0.025 0.070
E16/K20 lactam
AIB2, AIB16,
Lys10 yE-yE 655 0.003 0.004
A18, D28
AIB2, AlB16,
Lys10 yE . 656 0.006 0.004
A18, D28
171
CA 02852 177 20 14¨ 05-23
WO 2009/155258 PCT/US2009/047438
=
=
2992o-208831
EXAMPLE 53
The peptides shown in Table 32 were made as essentially described herein:
TABLE 32
SEQ
Peptide Name ID = Sequence
NO:
Chimera-2 A ib2C24Ma140KPEG 624 H(Aib)QGTFTSDYSKYLDEQAAKEFICWLMNT-amide
Chimera-2
Aib2E16K2OlactamC24Ma140KPEG 625 H(Aib)QGTFTSDYSKYLDEQAAKEFICWLMNT-amide
Glucagon H(Aib)QGTFTSDYSKYLDEFtRAKDFVCWLMNT-amide
A ib2E16K2OlactamC24amideMa140KPEG 626 lactam
Glucagon (Dmia)SQGTFTSDYSKYLDERRAKDFVCWLMNT-amide
DmialE16K2OlactamC24Ma140KPEG 628 lactam
Glucagon (Dmia)SQGTFTSDYSKYLDERRAKDFVCWLMNT-OH
DmialE I 6K2OlactamC24Ma140KPEG 629 lactam
Glucagon (Dmia)SQGTFTSDYSKYLDERRAKDFVCWLMNT-amide
DmialE 1 6K2OlactamC24thioether4OKPEG 630 lactam
Chimera2 Aib2E3C24-Thioether40K PEG 631 H(Aib)EGTFTSDYSKYLDEQAAKEFICWLMNT-
amide
Glucagon DMIA I , E3, E15, E16, K20, (Dmia)SEGTFTS DYS
KYLEERRAKDFVC(PEG40K)WL MNT-
632 amide
C24-Peg
Glucagon Aib2Aibl6C24K10(rErE- H(Aib)QGTFTSDK(rErE-
C14)C24PEG4OK TE)amide 633 C14)SKYLDAibRRAQDFVC(PEG4OK TE)WLMNT-amide
Glucagon Aib2Aib 1 6K 10(AA- H(Aib)QGTFTSDK(AA-
C14)C24PEG4OK TE amide 634 C14)SKYLDAibRRAQDFVC(PEG4OK TE)WLMNT-amide
Glucagon A ib2A ibl6K10(A A-C16) amide 635 CH sb (( )yGLTDF AT iSb DR RKA(
AQAD-F v
QWLMNT amide
Glucagon Aib2Aib 1 6K10(rErE-C16) H(Aib)QGTFTSD K(rErE=
amide 636 Cl 6)SKYLDAibRRAQDFVQWLMNT amide
172
CA 02 852 177 2 0 14-05-2 3
WO 2009/155258
PCT/US2009/047438
29920-208831
All peptides of Table 32 demonstrated potent in vitro activities at both the
glucagon and GLP-1 receptors, except for the peptides of SEQ ID NOs: 624, 631,
and
632
Peptides of Set A comprising the amino acid sequence of native glucagon
(SEQ ID NO: 1) except for the changes outlined in Table 33 are made as
essentially
described herein.
=
TABLE 33
DPP-IVC-Terminal
Alpha Helix Stabilization Position 3 Backbone*
Protection Amide?
DM1A at
AIB at position 16 Gln (wild-type) Wild-type yes
position 1
AIB at
ALB at position 16 Gin (wild-type) Wild-type yes
position 2
d-Ser at
MB at position 16 Gln (wild-type) Wild-type yes
position 2
DMIA at
AIB at position 16 Glu Wild-type yes
position 1
A1B at
AID at position 16 Glu Wild-type yes
position 2
d-Ser at
AIB at position 16 Glu Wild-type yes
position 2
DM1A at AID at positions 16 and
Gln (wild-type) Wild-type yes
position 1 20
AIB at AIB at positions 16 and
Gln (wild-type) Wild-type yes
position 2 20
d-Ser at AIR at positions 16 and
Gln (wild-type) Wild-type yes
position 2 20
DM1A at AIR at positions 16 and
Glu Wild-type yes
position 1 20
173
CA 02852 177 2014-05-23
WO 2009/155258
PCT/US2009/047438
=
29920-208831
DPP-IV C-Terminal
Alpha Helix Stabilization Position 3 Backbone'
Protection Amide?
MB at AIB at positions 16 and
Glu Wild-type
es
position 2 20
d-Ser at AIB at positions 16 and
Glu Wild-type yes
position 2 20
DMIA at Glu at position 16 and
Gin (wild-type) Wild-type yes
position 1 Lys at position 20
AIB at Glu at position 16 and
Gin (wild-type) Wild-type yes
position 2 Lys at position 20
d-Ser at Glu at position 16 and
Gin (wild-type) Wild-type yes
position 2 Lys at position 20
Lactam bridge between
DMIA at side chains of Glu at
Gin (wild-type) Wild-type yes
position 1 position 16 and Lys at
position 20
Lactam bridge between
AIB at side chains of Glu at
Gin (wild-type) Wild-type
es
position 2 position 16 and Lys at
position 20
= Lactam bridge between
d-Ser at side chains of Glu at
Gin (wild-type) Wild-type yes
position 2 position 16 and Lys at
position 20
-DMIA at Glu at position 16 and
Glu Wild-type yes
position 1 Lys at position 20
AIB at Glu at position 16 and
Glu Wild-type yes
position 2 Lys at position 20
d-Ser at Glu at position 16 and
Glu Wild-type yes
position 2 Lys at position 20
Lactam bridge between
DMIA at side chains of Glu at
Glu Wild-type yes
position 1 position 16 and Lys at
position 20
174
CA 02852 177 2014-05-23
WO 2009/155258 PCT/US2009/047438
29920-208831
DPP-IV C-Terminal
Alpha Helix Stabilization Position 3 Backbone'
Protection Amide?
Lactam bridge between =
AIB at side chains of Glu at
position 2 position 16 and Lys at Glu Wild-type yes
position 20
Lactam bridge between
d-Ser at side chains of Glu at
Glu Wild-type yes
position 2 position 16 and Lys at
position 20
DMIA at Glu at position 16 and
Gin (wild-type) Chimera 2 yes
position 1 Lys at position 20
AIB at Glu at position 16 and
Gin (wild-type) Chimera 2 yes =
position 2 Lys at position 20
d-Ser at Glu at position 16 and
Gin (wild-type) Chimera 2 yes
position 2 Lys at position 20
Lactam bridge between
DMIA at side chains of Gin at
Gin (wild-type) Chimera 2 yes
position I position 16 and Lys at
position 20
Lactam bridge between
AIB at side chains of Glu at
Gin (wild-type) Chimera 2 yes
position 2 position 16 and Lys at
position 20
Lactam bridge between
d-Ser at side chains of Glu at
Gin (wild-type) Chimera 2 yes
position 2 position 16 and Lys at
position 20
DMIA at Glu at position 16 and
Glu Chimera 2 yes
position 1 Lys at position 20
AIB at Gin at position 16 and
Glu Chimera 2 yes
position 2 Lys at position 20
d-Ser at Glu at position 16 and
Glu Chimera 2 yes
position 2 Lys at position 20
175
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
DPP-IV C-Terminal
Alpha Helix Stabilization Position 3 Backbone*
Protection Amide?
Lactam bridge between
DMIA at side chains of Glu at
Glu Chimera 2 yes
position 1 position 16 and Lys at
position 20
Lactam bridge between
A1B at side chains of Glu at
Glu Chimera 2 yes
position 2 position 16 and Lys at
position 20
Lactam bridge between
d-Ser at side chains of Glu at
Glu Chimera 2 yes
= position 2 position 16 and Lys at
position 20
* indicates amino acids at positions 17, 28, 21, and 23 as wild-type or as
Chimera 2
(Gin at position 17, Ala at position 18, Glu at position 21, and Ile at
position 23).
Peptides having the same structure as the peptides of Set A, except that the
Met at position 27 is replaced with a Norleucine, are made as essentially
described
herein. These modified peptides are the peptides of Set B.
Peptides having the same structure as the peptides of Sets A and B, except
that
the Gin at position 24 is replaced with a Cys covalently attached to a 40 kDa
PEG, are
= made as essentially described herein. These pegylated peptides form the
peptides of
Set C.
Peptides having the same structure as the peptides of Set A, B, or C, except
that the Tyr at position 10 is replaced with a Lys covalently attached to a
C8, C12,
C14, C16, or C18 fatty acyl group, are made as essentially described herein.
The
peptides acylated with a C8 fatty acyl group form the peptides of Set D. The
peptides
acylated with a C12 fatty acyl group form the peptides of Set E. The peptides
acylated with a CI4 fatty acyl group form the peptides of Set F. The peptides
acylated with a C16 fatty acyl group form the peptides of Set G. The peptides
acylated with a C18 fatty acyl group form the peptides of Set H.
Peptides having the same structure as the peptides of Sets D through H, except
that the fatty acyl group is attached to the Lys at position 10 via a spacer,
are made as
essentially described herein. The peptides comprising a y-Glu- y-Glu spacer
form the
peptides of Set I. The peptides comprising a y-Glu spacer form the peptides of
Set J.
176
=
CA 02852177 2014-05-23
WO 2009/155258
PCT/US2009/047438
29920-208831
The peptides comprising an Ala-Ala spacer form the peptides of Set K. The
peptides
comprising a 13-Ala- j3-Ala spacer form the peptides of Set L.
All references, including publications, patent applications, and patents,
cited
herein are hereby incorporated by reference to the same extent as if each
reference
were individually and specifically indicated to be incorporated by reference
and were
set forth in its entirety herein.
The use of the terms "a" and "an" and "the" and similar referents in the
context
of describing the invention (especially in the context of the following
claims) are to be
construed to cover both the singular and the plural, unless otherwise
indicated herein
or clearly contradicted by context. The terms "comprising," "having,"
"including," and
"containing" are to be construed as open-ended terms (i.e., meaning
"including, but
not limited to,") unless otherwise noted.
Recitation of ranges of values herein are merely intended to serve as a
shorthand method of referring individually to each separate value falling
within the
range and each endpoint, unless otherwise indicated herein, and each separate
value
and endpoint is incorporated into the specification as if it were individually
recited
herein.
All methods described herein can be performed in any suitable order unless
otherwise indicated herein or otherwise clearly contradicted by context. The
use of
any and all examples, or exemplary language (e.g., "such as") provided herein,
is
intended merely to better illuminate the invention and does not pose a
limitation on
the scope of the invention unless otherwise claimed. No language in the
specification
should be construed as indicating any non-claimed element as essential to the
practice
of the invention.
Preferred embodiments of this invention are described herein, including the
best mode known to the inventors for carrying out the invention. Variations of
those
preferred embodiments may become apparent to those of ordinary skill in the
art upon
reading the foregoing description. The inventors expect skilled artisans to
employ
such variations as appropriate, and the inventors intend for the invention to
be
practiced otherwise than as specifically described herein. Accordingly, this
invention
includes all modifications and equivalents of the subject matter recited in
the claims
appended hereto as permitted by applicable law. Moreover, any combination of
the
above-described elements in all possible variations thereof is encompassed by
the
177
CA 02852177 2014-05-23
invention unless otherwise indicated herein or otherwise clearly contradicted
by
context.
SEQUENCE LISTING IN ELECTRONIC FORM
In accordance with Section 111(1) of the Patent Rules, this
description contains a sequence listing in electronic form in ASCII
text format (file: 64005-1372D2 Seq 14-MAY-14 vl.txt).
A copy of the sequence listing in electronic form is available from
the Canadian Intellectual Property Office.
The sequences in the sequence listing in electronic form are
reproduced in the following table.
SEQUENCE TABLE
<110> Indiana University Research and Technology Corporation
<120> PEGYLATED GLUCAGON/GLP-1 RECEPTOR CO-AGONISTS
<130> 64005-1372D2
<140> Division of CA 2,728,284
<141> 2009-06-16
<150> 61/073,269
<151> 2008-06-17
<150> 61/078,168
<151> 2008-07-03
<150> 61/090,412
<151> 2008-08-20
<150> 61/177,476
<151> 2009-05-12
<160> 656
<170> PatentIn version 3.5
<210> 1
<211> 29
<212> PRT
<213> Homo sapiens
178
CA 02852177 2014-05-23
<400> 1
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Net Asn Thr
20 25
<210> 2
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16)..(16)
<223> Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (17)..(17)
<223> Arg, Cys, Orn, homocysteine or acetyl phenylalanine
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 2
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Xaa Arg Ala Gin Asp Phe Val Gin Trp Leu Xaa Asn Thr
20 25
<210> 3
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16)..(16)
<223> Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (21)..(21)
<223> Asp, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
179
CA 02852177 2014-05-23
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 3
His Ser Gln Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Gln Xaa Phe Val Gln Trp Leu Xaa Asn Thr
20 25
<210> 4
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16)..(16)
<223> Glu, Gln, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (24) ..(24)
<223> Gln, Cys, Orn, homocysteine or acetyl phenylalanine
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 4
His Ser Gln Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Gln Asp Phe Val Xaa Trp Leu Xaa Asn Thr
20 25
<210> 5
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16)..(16)
<223> Glu, Gln, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (21)..(21)
<223> Asp, Cys, Orn, homocysteine or acetyl phenyalanine
180
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 5
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Gin Xaa Phe Val Xaa Trp Leu Xaa Asn Thr
20 25
<210> 6
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (21)..(21)
<223> Asp, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (27) ..(27)
<223> Met, Leu or Nle
<400> 6
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Xaa Phe Val Gin Trp Leu Xaa Asn Thr
20 25
<210> 7
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gln, Cys, Orn, homocysteine or acetyl phenylalanine
<220>
<221> MOD RES
181
CA 02852177 2014-05-23
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 7
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Xaa Trp Leu Xaa Asn Thr
20 25
<210> 8
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16)..(16)
<223> Glu, Gin, homoglutamic acid or homocysteic acid
<400> 8
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 9
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (27)..(27)
<223> Net, Leu or Nle
<400> 9
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gln Trp Leu Xaa Asn Thr
20 25
<210> 10
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
182
CA 02852177 2014-05-23
<400> 10
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 11
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation
<400> 11
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 12
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at position 12 and 16
<400> 12
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 13
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
183
CA 02852177 2014-05-23
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 13
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 14
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (22)7.(24)
<223> Lactam ring between side chains at positions 22 and 24
<400> 14
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Glu Trp Leu Met Asn Thr
20 25
<210> 15
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (24)7.(28)
<223> Lactam ring formed between side chains at positions 24 and 28
<400> 15
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Glu Trp Leu Met Lys Thr
20 25
<210> 16
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
184
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<220>
<221> MISC_FEATURE
<222> (20)..(24)
<223> Lactam ring between side chains at positions 20 and 24
<400> 16
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Glu Trp Leu Met Asn Thr
20 25
<210> 17
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<220>
<221> MISC FEATURE
<222> (24)..(28)
<223> Lactam ring between side chains at positions 24 and 28
<400> 17
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Asp Asp Phe Val Glu Trp Leu Met Lys Thr
20 25
<210> 18
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
185
CA 02852177 2014-05-23
<400> 18
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Glu Trp Leu Met Lys Thr
20 25
<210> 19
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (30)..(30)
<223> Variable amino acid
<400> 19
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr Xaa
20 25 30
<210> 20
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> mop RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin, Lys, Arg, Orn or Citrulline
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin or Glu
<220>
<221> MOD RES
186
CA 02852177 2014-05-23
<222> (28)..(28)
<223> Asn, Asp or Lys
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr or Gly
<400> 20
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Xaa Xaa
20 25
<210> 21
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (2)..(2)
<223> D-Ser, Ala, Gly, N-methyl Ser or aminoisobutyric acid
<400> 21
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 22
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (2)..(2)
<223> Aminoisobutyric acid
107
CA 02852177 2014-05-23
<400> 22
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 23
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (17)..(17)
<223> Cys-PEG
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 23
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Cys Arg Ala Gin Asp Phe Val Gin Trp Leu Xaa Asn Thr
20 25
<210> 24
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (21)..(21)
<223> Cys-PEG
<220>
<221> MOD_RES
<222> (27)..(27)
<223> Met, Leu or Nle
188
CA 02852177 2014-05-23
<400> 24
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Cys Phe Val Gin Trp Leu Xaa Asn Thr
20 25
<210> 25
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (24)..(24)
<223> Cys-PEG
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 25
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Cys Trp Leu Xaa Asn Thr
20 25
<210> 26
<211> 10
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide fragment representing the carboxy terminal 10
amino acids Exendin-4
<400> 26
Gly Pro Ser Ser Gly Ala Pro Pro Pro Ser
1 5 10
<210> 27
<211> 8
<212> PRT
<213> Artificial Sequence
189
CA 02852177 2014-05-23
<220>
<223> Synthetic peptide fragment representing the carboxy terminal 8
amino acids of oxyntomodulin
<400> 27
Lys Arg Asn Arg Asn Asn Ile Ala
1 5
<210> 28
<211> 4
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<400> 28
Lys Arg Asn Arg
1
<210> 29
<211> 10
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide fragment representing the carboxy terminal 10
amino acids of Exendin-4
<220>
<221> MISC_FEATURE
<223> c-term amidation
<400> 29
Gly Pro Ser Ser Gly Ala Pro Pro Pro Ser
1 5 10
<210> 30
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 30
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
190
CA 02852177 2014-05-23
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Xaa Asn Thr Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
<210> 31
<211> 37
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 31
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Xaa Asn Thr Lys Arg Asn
20 25 30
Arg Asn Asn Ile Ala
<210> 32
<211> 33
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 32
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Xaa Asn Thr Lys Arg Asn
20 25 30
Arg
<210> 33
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
191
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, homoglutamic acid, cysteic acid or homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin or Lys
<220>
<221> MOD RES
<222> (24) ..(24)
<223> Gin or Glu
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or an acidic amino acid
<220>
<221> MOD RES
<222> (29)-..(29)
<223> Thr, Gly or an acidic amino acid
<400> 33
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Xaa Xaa
20 25
<210> 34
<211> 29
<212> PRT
<213> Artificial sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (15) ..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD _RES
192
CA 02852177 2014-05-23
<222> (20)..(20)
<223> Gin or Lys
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin or Glu
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Asp or Lys
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr or Gly
<400> 34
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Xaa Xaa
20 25
<210> 35
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<400> 35
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Cys Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 36
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<400> 36
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
193
CA 02852177 2014-05-23
<210> 37
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (24)..(24)
<223> 2-butyrolactone bound through thiol group of Cys
<400> 37
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Cys Trp Leu Met Asn Thr
20 25
<210> 38
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (24)-..(24)
<223> Carboxymethyl group bound through thiol group of Cys
<400> 38
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Cys Trp Leu Met Asn Thr
20 25
<210> 39
<211> 39
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<400> 39
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Arg Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser
194
CA 02852177 2014-05-23
<210> 40
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (15)..(15)
<223> Glu or Asp
<220>
<221> MOD RES
<222> (28)..(28)
<223> Glu or Asp
<400> 40
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Xaa Thr
20 25
<210> 41
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation; lactam ring between side chains at positions 12
and 16
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<220>
<221> MOD RES
<222> (15)..(15)
<223> Glu or Asp
195
CA 02852177 2014-05-23
=
<220>
<221> MOD RES
<222> (28)..(28)
<223> Glu or Asp
<400> 41
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Xaa Thr
20 25
<210> 42
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation; lactam ring between side chains at positions 16
and 20
<220>
<221> MISC FEATURE
<223> c-teLm amidation
<220>
<221> MOD RES
<222> (15)..(15)
<223> Glu or Asp
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<220>
<221> MOD RES
<222> (28)..(28)
<223> Glu or Asp
<400> 42
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Xaa Thr
20 25
<210> 43
<211> 29
<212> PRT
<213> Artificial Sequence
196
CA 02852177 2014-05-23
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (15)..(15)
<223> Glu or Asp
<220>
<221> MISC FEATURE
<222> (20)..(24)
<223> Lactam ring between side chains at positions 20 and 24
<220>
<221> MOD_RES
<222> (28)..(28)
<223> Glu or Asp
<400> 43
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Ser
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Glu Trp Leu Met Xaa Thr
20 25
<210> 44
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (15)..(15)
<223> Glu or Asp
<220>
<221> MISC FEATURE
<222> (24)..(28)
<223> Lactam ring between side chains at positions 24 and 28
<220>
<221> MOD_RES
<222> (29)..(29)
<223> Glu or Thr
197
CA 02852177 2014-05-23
<400> 44
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Glu Trp Leu Met Lys Xaa
20 25
<210> 45
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (12) ..(12)
<223> Lys or Glu
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, homoglutamic acid, cysteic acid or homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Gin, Glu, Lys, homoglutamic acid, cysteic acid or
homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin, Glu or Lys
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin, Lys or Glu
<220>
<221> MOD RES
<222> (28) ..(28)
<223> Asn, Lys or an acidic amino acid
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly or an acidic amino acid
<400> 45
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Xaa Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Xaa Xaa
20 25
198
CA 02852177 2014-05-23
<210> 46
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16) ..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin or Lys
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin or Glu
<400> 46
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Asn Thr
20 25
<210> 47
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<400> 47
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gln Trp Leu Met Asn Thr
20 25
<210> 48
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
199
CA 02852177 2014-05-23
<400> 48
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Lys Asp She Val Glu Trp Leu Met Asn Thr
20 25
<210> 49
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<400> 49
His Ser Gin Gly Thr She Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Glu Trp Leu Met Lys Thr
20 25
<210> 50
<211> 31
<212> PRT
<213> Homo sapiens
<400> 50
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gin Ala Ala Lys Glu She Ile Ala Trp Leu Val Lys Gly Arg Gly
20 25 30
<210> 51
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, homoglutamic acid, cysteic acid or homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin, Lys, Arg, Orn, or Citrulline
200
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (21)..(21)
<223> Asp, Glu, homoglutamic acid, or homocysteic acid
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin or Glu
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or an acidic amino acid
<220>
<221> MOD RES
<222> (29) ..(29)
<223> Thr, Gly or an acidic amino acid
<400> 51
His Ser Gin Gly Thr She Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Xaa She Val Xaa Trp Leu Met Xaa Xaa
20 25
<210> 52
<211> 30
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<400> 52
His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Ser Tyr Leu Glu Gly
1 5 10 15
Gin Ala Ala Lys Glu She Ile Ala Trp Leu Val Lys Gly Arg
20 25 30
<210> 53
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
201
CA 02852177 2014-05-23
<222> (3)..(3)
<223> Glu, Orn or Nle
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, homoglutamic acid, cysteic acid or homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin or Lys
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin or Glu
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or an acidic amino acid
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr or an acidic amino acid
<400> 53
His Ser Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Xaa Xaa
20 25
<210> 54
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (17)..(17)
<223> Arg or Gin
<220>
<221> MOD RES
<222> (18)..(18)
<223> Arg or Ala
202
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (21)..(21)
<223> Asp or Glu
<220>
<221> MOD RES
<222> (23)..(23)
<223> Val or Ile
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin or Ala
<400> 54
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Xaa Xaa Ala Lys Xaa She Xaa Xaa Trp Leu Met Asn Thr
20 25
<210> 55
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (1)..(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His or
alpha, alpha-dimethyl imidiazole acetic acid (DMIA), N-methyl
His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
<222> (2)..(2)
<223> Ser, D-Ser, Ala, D-Ala, Val, Gly, N-methyl Ser, aminoisobutyric
acid (AIB) or N-methyl Ala
<220>
<221> MOD RES
<222> (3)..(3)
<223> Gin, Glu, Orn or Nle
<220>
<221> MOD RES
<222> (10)..(10)
<223> Tyr or Trp
<220>
<221> MOD RES
<222> (12)..(12)
<223> Lys, Citrulline, Orn or Arg
203
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid and homocysteic acid
<220>
<221> MOD RES
<222> (16) ..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (17)..(17)
<223> Arg, Gin, Lys, Cys, Orn, homocysteine or acetyl phenylalanine
<220>
<221> MOD RES
<222> (18) ..(18)
<223> Arg, Ala, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin, Lys, Arg, Orn or Citrulline
<220>
<221> MOD RES
<222> (21)..(21)
<223> Gin, Glu, Asp, Lys, Cys, Orn, homocystein or acetyl phenyalanine
<220>
<221> MOD RES
<222> (23) ..(23)
<223> Val or Ile
<220>
<221> MOD RES
<222> (24)..(24)
<223> Ala, Gin, Glu, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (27) ..(27)
<223> Met, Leu or Nle
<220>
<221> MOD RES
<222> (28) ..(28)
<223> Asn, Arg, Citrulline, Orn, Lys or Asp
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly, Lys, Cys, Orn, homocycsteine or acetyl phenyalanine
204
CA 02852177 2014-05-23
<400> 55
Xaa Xaa Xaa Gly Thr Phe Thr Ser Asp Xaa Ser Xaa Tyr Leu Xaa Xaa
1 5 10 15
Xaa Xaa Ala Xaa Xaa Phe Xaa Xaa Trp Leu Xaa Xaa Xaa
20 25
<210> 56
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (1)..(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
<222> (2)..(2)
<223> Ser, D-Ser, Ala, D-Ala, Val, Gly, N-methyl Ser, AIB or N-methyl
Ala
<220>
<221> MOD RES
<222> (3)..(3)
<223> Gln, Glu, Orn or Nle
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid and homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gln, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gln, Lys, Arg, Orn or Citrulline
<220>
<221> MOD RES
<222> (21)..(21)
<223> Gln, Glu, Asp, Cys, Orn, homocycstein or acetyl phenyalanine
<220>
<221> MOD RES
<222> (23)..(23)
<223> Val or Ile
205
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (24)..(24)
<223> Ala, Gln, Glu, Cys, Orn, homocycsteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or Asp
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly, Lys, Cys, Orn, homocycsteine or acetyl phenyalanine
<400> 56
Xaa Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Xaa Phe Xaa Xaa Trp Leu Xaa Xaa Xaa
20 25
<210> 57
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (1)..(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl- His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
<222> (2)..(2)
<223> Ser, D-Ser, Ala, D-Ala, Val, Gly, N-methyl Ser, AIB or N-methyl
Ala
<220>
<221> MOD RES
<222> (3)..(3)
<223> Gln, Glu, Orn or Nle
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side changes at position 12 and 16
206
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid and homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin, Lys, Arg, Orn or Citrulline
<220>
<221> MOD RES
<222> (21)..(21)
<223> Gin, Glu, Asp, Cys, Orn, homocycsteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (23) ..(23)
<223> Val or Ile
<220>
<221> MOD _RES
<222> (24)..(24)
<223> Ala, Gin, Glu, Lys, Cys, Orn, homocysteine or acetyl Phe
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or Asp
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<400> 57
Xaa Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Glu
1 5 10 15
Arg Arg Ala Xaa Xaa Phe Xaa Xaa Trp Leu Xaa Xaa Xaa
20 25
<210> 58
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
207
CA 02852177 2014-05-23
<222> (1)..(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
<222> (2)..(2)
<223> Ser, D-Ser, Ala, D-Ala, Val, Gly, N-methyl Ser, AIB or N-methyl
Ala
<220>
<221> MOD RES
<222> (3)..(3)
<223> Gin, Glu, Orn or Nle
<220>
<221> NOD RES
<222> (15)..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid or homocysteic acid
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<220>
<221> MOD RES
<222> (21)..(21)
<223> Gin, Glu, Asp, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (23)..(23)
<223> Val or Ile
<220>
<221> MOD RES
<222> (24)..(24)
<223> Ala, Gin, Glu, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or Asp
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
208
CA 02852177 2014-05-23
<400> 58
Xaa Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Glu
1 5 10 15
Arg Arg Ala Lys Xaa Phe Xaa Xaa Trp Leu Xaa Xaa Xaa
20 25
<210> 59
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (1)..(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
<222> (2)..(2)
<223> Ser, D-Ser, Ala, D-Ala, Val, Gly, N-methyl Ser, AIB, or N-methyl
Ala
<220>
<221> MOD RES
<222> (3)..(3)
<223> Gin, Glu, Orn or Nle
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid and homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MISC FEATURE
<222> (20)..(24)
<223> Lactam ring between side chains at position 20 and 24
<220>
<221> MOD RES
<222> (21)..(21)
<223> Gin, Glu, Asp, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (23)..(23)
<223> Val or Ile
209
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asn, Lys or Asp
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly, Lys, Cys, Orn, homocysteine or acetyl pheylalanine
<400> 59
Xaa Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Lys Xaa Phe Xaa Glu Trp Leu Xaa Xaa Xaa
20 25
<210> 60
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (1)..(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Ser, D-Ser, Ala, D-Ala, Val, Gly, N-methyl Ser, AIB or N-methyl
Ala
<220>
<221> MOD RES
<222> (3)..(3)
<223> Gin, Glu, Orn or Nle
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, cysteic acid, homoglutamic acid and homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
210
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin, Lys, Arg, Orn, or Citrulline
<220>
<221> MOD RES
<222> (21) ..(21)
<223> Gin, Glu, Asp, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<220>
<221> MOD RES
<222> (23)..(23)
<223> Val or Ile
<220>
<221> MISC FEATURE
<222> (24)..(28)
<223> Lactam ring between side chains at position 24 and 28
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr, Gly, Lys, Cys, Orn, homocysteine or acetyl phenyalanine
<400> 60
Xaa Xaa Xaa Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Xaa Phe Xaa Glu Trp Leu Xaa Lys Xaa
20 25
<210> 61
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> positions 30 to 40 are present only if position 29 is Gly; see
specification as filed for detailed description of substitutions
and preferred embodiments
<220>
<221> MOD RES
<222> (1)..(1)
<223> His, 0-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
211
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (2)..(2)
<223> Ser, D-Ser, Ala, Val, Gly, N-methyl Ser, Aib, N-methyl, Ala or
D-Ala
<220>
<221> MOD RES
<222> (18)..(18)
<223> Ala or Arg
<220>
<221> MOD RES
<222> (24) ..(24)
<223> Ala, Gin or Cys-PEG
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr-CONH2, Cys-PEG, or Gly
<220>
<221> MOD RES
<222> (40)..(40)
<223> Cys-PEG or not present
<400> 61
Xaa Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Xaa Ala Lys Asp Phe Val Xaa Trp Leu Met Asn Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 62
<211> 40
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<223> positions 30 to 40 are present only if position 29 is Gly; see
specification as filed for detailed description of substitutions
and preferred embodiments
<220>
<221> MOD RES
<222> (1)7.(1)
<223> His, D-His, (Des-amino)His, hydroxyl-His, acetyl-His, homo-His,
DMIA, N-methyl His, alpha-methyl His, or imidazole acetic acid
<220>
<221> MOD RES
212
CA 02852177 2014-05-23
<222> (2)..(2)
<223> Ser, D-Ser, Ala, Val, Gly, N-methyl Ser, AIBO, N-methyl Ala, or
D-Ala
<220>
<221> MOD RES
<222> (18)..(18)
<223> Ala or Arg
<220>
<221> MOD RES
<222> (24)..(24)
<223> Ala, Gin or Cys-PEG
<220>
<221> MOD RES
<222> (29) ..(29)
<223> Thr-CONH2, Cys-PEG, or Gly
<220>
<221> MOD RES
<222> (40)..(40)
<223> Cys-PEG or not present
<400> 62
Xaa Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Xaa Ala Lys Glu Phe Ile Xaa Trp Leu Met Asn Xaa Gly Pro Ser
20 25 30
Ser Gly Ala Pro Pro Pro Ser Cys
35 40
<210> 63
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin or Lys
<220>
<221> MOD RES
<222> (21)..(21)
<223> Asp, Lys, Cys, Orn, homocysteine or acetyl phealanine
213
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (24)..(24)
<223> Gin, Lys, Cys, Orn, homocysteine or acetyl phealanine
<220>
<221> MOD RES
<222> (27)..(27)
<223> Met, Leu or Nle
<400> 63
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser. Lys Tyr Leu Asp Xaa
1 5 10 15
Arg Arg Ala Xaa Xaa Phe Val Xaa Trp Leu Xaa Asn Thr
20 25
<210> 64
<211> 38
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MOD RES
<222> (15)..(15)
<223> Asp, Glu, homoglutamic acid, cysteic acid or homocysteic acid
<220>
<221> MOD RES
<222> (16)..(16)
<223> Ser, Glu, Gin, homoglutamic acid or homocysteic acid
<220>
<221> MOD RES
<222> (20)..(20)
<223> Gin or Lys
<220>
<221> MOD RES
<222> (24) ..(24)
<223> Gin or Glu
<220>
<221> MOD RES
<222> (28) ..(28)
<223> Asn, Lys or Asp
<400> 64
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Xaa Xaa
1 5 10 15
Arg Arg Ala Xaa Asp Phe Val Xaa Trp Leu Met Xaa Gly Gly Pro Ser
20 25 30
Ser Gly Pro Pro Pro Ser
214
CA 02852177 2014-05-23
<210> 65
<211> 11
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide fragment representing the carboxy terminal 10
amino acids of Exdendin-4
<220>
<221> MOD RES
<222> (11) ..(11)
<223> Cys-PEG
<400> 65
Gly Pro Ser Ser Gly Ala Pro Pro Pro Ser Cys
1 5 10
<210> 66
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asp or Asn
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr or Gly
<400> 66
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Xaa Xaa
20 25
<210> 67
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
215
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asp or Asn
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr or Gly
<400> 67
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Xaa Xaa
20 25
<210> 68
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Glucagon Analogue
<220>
<221> MISC FEATURE
<222> (20)..(24)
<223> Lactam ring between side chains at positions 20 and 24
<220>
<221> MOD RES
<222> (28)..(28)
<223> Asp or Asn
<220>
<221> MOD RES
<222> (29) ..(29)
<223> Thr or Gly
<400> 68
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Glu Trp Leu Met Xaa Xaa
20 25
<210> 69
<211> 29
<212> PRT
<213> Artificial Sequence
216
CA 02852177 2014-05-23
<220>
<223> glucagon analogue
<220>
<221> MISC FEATURE
<222> (24)7.(28)
<223> Lactam ring between side chains at positions 24 and 28
<220>
<221> MOD RES
<222> (29)..(29)
<223> Thr or Gly
<400> 69
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Ser
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Glu Trp Leu Met Lys Xaa
20 25
<210> 70
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<400> 70
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 71
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<400> 71
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
217
CA 02852177 2014-05-23
<210> 72
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 72
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 73
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 73
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 74
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
218
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 74
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 75
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 75
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Arg Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 76
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic peptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<400> 76
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
219
CA 02852177 2014-05-23
<210> 77
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MISC_FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 77
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 78
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 78
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 79
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
220
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 79
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 80
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 80
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Ala Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 81
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<400> 81
His Ser Gln Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
221
CA 02852177 2014-05-23
<210> 82
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MISC_FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 82
His Ser Gln Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 83
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-teLm amidation
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 83
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 84
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
222
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<223> c-term amidation
<400> 84
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 85
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 85
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 86
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 86
His Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
223
CA 02852177 2014-05-23
<210> 87
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<400> 87
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 88
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)T.(1)
<223> (Des-amino)His
<400> 88
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 89
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
224
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 89
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 90
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 90
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 91
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
225
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 91
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 92
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 92
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Arg Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 93
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
226
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC_FEATURE
<222> (29)..(29)
<223> c-term amidation
<400> 93
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 94
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)7.(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 94
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 95
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
227
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 95
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Gin Asp Phe Val Gln Trp Leu Met Asn Thr
20 25
<210> 96
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-telm amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 96
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 97
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-teLm amidation
228
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 97
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Ala Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 98
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)7.(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 98
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 99
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
229
CA 02852177 2014-05-23
<220>
<221> MOD_RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 99
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 100
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 100
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 101
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
230
CA 02852177 2014-05-23
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<400> 101
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 102
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-teLm amidation
<220>
<221> MOD RES
<222> (1)..(1)
<223> (Des-amino)His
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 102
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 103
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (1)7.(1)
<223> (Des-amino)His
231
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 103
Xaa Ser Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 104
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutyric acid
<220>
<221> MISC FEATURE
<222> (29)..(29)
<223> c-term amidation
<400> 104
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 105
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutyric acid
<220>
<221> MISC FEATURE
232
CA 02852177 2014-05-23
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 105
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 106
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutyric acid
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 106
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 107
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutyric acid
233
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 107
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 108
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-telm amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisebutyric acid
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 108
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 109
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-teLm amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutyric acid
234
CA 02852177 2014-05-23
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 109
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Arg Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 110
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutyric acid
<400> 110
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gln Trp Leu Met Asn Thr
20 25
<210> 111
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutyric acid
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
235
CA 02852177 2014-05-23
<400> 111
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 112
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> Synthetic Polypeptide
<220>
<221> MISC FEATURE
<223> c-teLm amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutryic acid
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 112
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 113
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutryic acid
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
236
CA 02852177 2014-05-23
<400> 113
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 114
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutryic acid
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 114
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Ala Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 115
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-telm amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutyric acid
237
CA 02852177 2014-05-23
<400> 115
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 116
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutryic acid
<220>
<221> MISC_FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 116
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 117
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> Aminoisobutryic acid
<220>
<221> MISC_FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
238
CA 02852177 2014-05-23
<400> 117
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 118
<211> 29
<212> PET
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutyric acid
<400> 118
His Xaa Gln Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 119
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> Aminoisobutyric acid
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
239
CA 02852177 2014-05-23
4
<400> 119
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 120
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (2)..(2)
<223> Aminoisobutyric acid
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 120
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 121
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (2)..(2)
<223> D-Ala
240
CA 02852177 2014-05-23
<400> 121
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 122
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<400> 122
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 123
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
241
CA 02852177 2014-05-23
<400> 123
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 124
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC_FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 124
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 125
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
242
CA 02852177 2014-05-23
<400> 125
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Arg Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 126
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)7.(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 126
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Arg Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 127
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-teLm amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
243
CA 02852177 2014-05-23
<400> 127
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 128
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 128
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 129
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-teLm amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (12)7.(16)
<223> Lactam ring between side chains at positions 12 and 16
244
CA 02852177 2014-05-23
<400> 129
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Gin Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 130
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-telm amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 130
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Arg Ala Ala Lys Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 131
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-teim amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
245
CA 02852177 2014-05-23
<400> 131
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Lys
1 5 10 15
Arg Ala Ala Glu Asp Phe Val Gin Trp Leu Met Asn Thr
20 25
<210> 132
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<400> 132
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 133
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
246
CA 02852177 2014-05-23
=
<400> 133
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 134
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC FEATURE
<222> (16)7.(20)
<223> Lactam ring between side chains at positions 16 and 20
<400> 134
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Met Asn Thr
20 25
<210> 135
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
247
CA 02852177 2014-05-23
<400> 135
His Xaa Gin Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 136
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC FEATURE
<223> c-term amidation
<220>
<221> MOD_RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC_FEATURE
<222> (12)..(16)
<223> Lactam ring between side chains at positions 12 and 16
<400> 136
His Xaa Gln Gly Thr Phe Thr Ser Asp Tyr Ser Lys Tyr Leu Asp Glu
1 5 10 15
Gin Ala Ala Lys Glu Phe Ile Ala Trp Leu Val Lys Gly
20 25
<210> 137
<211> 29
<212> PRT
<213> Artificial Sequence
<220>
<223> glucagon analog
<220>
<221> MISC_FEATURE
<223> c-term amidation
<220>
<221> MOD RES
<222> (2)..(2)
<223> D-Ala
<220>
<221> MISC_FEATURE
<222> (16)..(20)
<223> Lactam ring between side chains at positions 16 and 20
248
CA 02852177 2014-05-23
DEMANDES OU BREVETS VOLU1VIINEUX
. LA PItESENTE PARTIE DE CETTE DE1VLANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
cEa EST LE TOIKE I DE
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
=
=
.TUMBO APPLICATIONS / PATENTS
THIS SECTION OF l'HE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF'___
NOTE: For additional volumes please contact the Canadian Patent OfEce.