Note: Descriptions are shown in the official language in which they were submitted.
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
1
Title: Catalyst composition and method for use in selective
catalytic reduction of nitrogen oxides
The present invention relates to catalyst composition for
use in selective reduction of nitrogen oxides in off-gases
by reaction with ammonia or a precursor thereof.
Catalysts for NH3-SCR, i.e. selective reduction of nitrogen
oxides (N0x) by use of ammonia as reductant are well known
in the art. Those catalysts include zeolitic material, op-
tionally promoted with copper or iron
The problem to be solved by this invention is to provide a
catalyst composition and method for the reduction of nitro-
gen oxides with a DeN0x activity at reaction temperatures
between 150 and 550 C.
Off-gases from lean combustion engines contain in addition
to NOx, hydrocarbons, CO and soot particles which can be
reduced or removed by catalytic oxidation. Consequently,
the catalyst composition and method of this invention shall
further include soot and hydrocarbon oxidation activity si-
multaneously with the DeN0x activity.
Our recent studies revealed several examples of a pro-
nounced synergistic effect in composite catalysts prepared
by mechanical mixing of acidic zeolite or zeotype powder
and redox active metal compounds.
We have found that catalyst composition comprising a one or
more acidic zeolite or zeotype components physically ad-
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
2
mixed with one ore more redox active metal compounds shown
an improved activity in the selective reduction of nitrogen
oxides and oxidation of hydrocarbons, CO and soot contained
in off-gas.
The term "redox active metal compounds" as used herein re-
lates to metal compounds which reversibly can be oxidized
and reduced in terms of changes in oxidation number, or
oxidation state, of the metal atom or compound.
Pursuant to the above findings, the present invention pro-
vides a catalyst composition for selective reduction of ni-
trogen oxides and soot oxidation comprising one or more
acidic zeolite or zeotype components selected from the
group consisting of BEA, MFI, FAU, FER, CHA, MOR or mix-
tures thereof physically admixed with one ore more redox
active metal compounds selected from the group consisting
of Cu/A1203, Mn/A1203, Ce02-Zr02, Ce-Mn/A1203 and mixtures
thereof.
Catalyst compositions prepared by mechanical mixing of the
above mentioned zeolites or zeotype materials and redox
metal components mixing according to the invention exhibit
a pronounced synergistic effect. DeN0x activity of such
composite catalysts significantly exceeds activity of their
individual components.
The acidic zeolite or zeotype component can be used in pro-
tonic form or promoted with Fe.
Preferably, the weight ratio between the zeolite components
and the redox components is between 1:1 to 1:50
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
3
In an embodiment of the invention, the redox components are
dispersed on a support selected from the group consisting
of of A1203, h02, Si02, Ce02, Zr02 or mixtures thereof.
It is generally preferred that the mean molar ratio Si/A1
of the zeolite components according to the invention is
from 5 to 100.
The above described catalyst composition according to the
invention can be utilised as coating material or as coat on
structured bodies of metallic, ceramic, metal oxide, SiC or
silica materials or fibres.
Thus, the invention provides furthermore a monolithic
structured body being coated with a catalyst composition
according to anyone of the above disclosed embodiments of
the invention.
The monolithic structured body is preferably made from me-
tallic, ceramic, metal oxide, SiC or silica fiber materi-
als.
The monolithic structured body may be in form of a particle
filter, e.g. a honeycomb structured filter or a wall flow
filter.
In further an embodiment, the catalyst composition is
coated on the body in of two or several separate catalyst
layers in series or as two or several catalyst layers in
parallel and wherein the layers have different compositions
or layer thicknesses.
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
4
Specific advantages resulting from the invention are
1) Addition of Ce02-Zr02, Cu/A1203, Mn/A1203 or Ce-
Mn/A1203 to acidic zeolite or zeotype in protonic form or
promoted with iron markedly enhances DeN0x activity at 'react
< 250 C without increasing amount of zeolite component. In
this case, overall volume of the catalyst is increased by
the volume of redox component added.
2) Alternatively, amount of expensive zeolite/zeotype com-
ponent in the composite catalyst can be significantly re-
duced by its replacement with equivalent volume of redox
component. In this case overall volume of the catalyst re-
mains constant, but the amount of zeolite component can be
decreased by 2-5 times, without notable sacrificing DeN0x
performance. When Ce-Mn/A1203 component is used for the
catalyst preparation, notable improvement of NOx conversion
at 'react < 250 C is observed despite decreased amount of
zeolite component.
3) In addition to favourable DeN0x activity, [Ce02-Zr02 +
zeolites/zeotypes] or [Ce-Mn/A1203 +
zeolites/zeotypes]
compositions demonstrate significant soot oxidation activ-
ity, which makes them promising candidates for development
of integrated DeN0x-DeSoot catalytic systems.
4) In addition to favorable DeN0x activity, [Ce02-Zr02 +
zeolites/zeotypes] or [Ce-Mn/A1203 +
zeolites/zeotypes]
compositions demonstrate significantly lower ammonium slip
at high temperature due to selective oxidation of excess
ammonia.
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
The invention provides additionally a method for the selec-
tive reduction of nitrogen oxides and oxidation of soot
contained in an off-gas comprising the step of contacting
5 the off-gas in presence of ammonia with a catalyst composi-
tion comprising one or more acidic zeolite or zeotype com-
ponents selected from the group consisting of BEA, MFI,
FAU, FER, CHA, MOR or mixtures thereof physically admixed
with one ore more redox active metal compounds selected
from the group consisting of Cu/A1203, Mn/A1203, Ce02-Zr02,
Ce-Mn/A1203 and mixtures thereof.
The acidic zeolite or zeotype component can be used in pro-
tonic form or promoted with Fe
In an embodiment of the inventive method, the one or more
redox active metal compounds are dispersed on a support se-
lected from the group consisting of A1203, Ti02, Si02, Zr02
or mixtures thereof.
In still an embodiment of the inventive method, the cata-
lyst composition is contacted with the off-gas at a tem-
perature below 250 C.
In a further embodiment of the inventive method excess of
ammonia is selectively oxidized to nitrogen by contact with
the catalyst composition.
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
6
Examples
Example 1
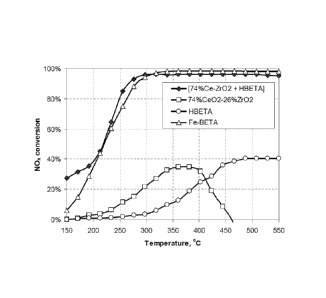
Synergistic effect in NH3-DeN0x over Ce02-Zr02 + H-Beta
zeolite catalyst compositions.
[Ce02-Zr02 + H-Beta zeolite] composite catalyst was pre-
pared by thorough mixing 74wt%Ce02-26wt%Zr02 powder with H-
Beta powder at a weight ratio of 10. This weight ratio re-
sults in volume ratio of components Ce02-Zr02/H-Beta = 3/1
due to difference in densities of these materials. The pow-
ders were thoroughly grinded in agate mortar for 10-15 min,
followed by pelletization. The pellets were crushed and
sieved collecting 0.2 - 0.4 mm fraction for catalytic test.
Similarly pelletized 74wt%Ce02-26wt%Zr02, H-Beta, and Fe-
Beta zeolite were used as reference samples.
The catalysts were tested in the NH3-DeN0x in the tempera-
ture range of 150-550 C. The test was performed under fol-
lowing conditions: decreasing reaction temperature with a
rate of 2 C/min, feed gas composition: 500 ppm NO, 540 ppm
NH3, 10 vol % 02, 6 vol% H20, balanced with N2 to obtain a
total flow of 300 mL/min.
Catalyst loading and resulted GHSV:
0.197g with 74wt%Ce02-Zr02 + 0.02g H-Beta zeolite, catalyst
volume 0.134 ml, GHSV = 135 000 h-1
Under these conditions Ce02-Zr02 + H-Beta zeolite composite
catalyst showed DeN0x activity, which substantially
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
7
exceeded activities of individual 74wt%Ce02-Zr02 (0.131g
Ce02-Zr02, catalyst volume 0.067 ml, GHSV = 270,000 h-1)
and H-Beta zeolite (0.04g, catalyst volume 0.067 ml, GHSV =
270 000 h-1), indicating pronounced synergistic effect be-
tween components of composite catalyst as shown in Fig. 1.
NO conversion over composite catalyst is similar to NOx
conversion over commercial Fe-Beta zeolite (Fe-Beta) at
230-550 C, and exceeds NO conversion over Fe-Beta zeolite
at 150-200 C.
Example 2
Enhanced DeN0x performance of [Ce02-Zr02 + Fe-Beta] compos-
ite catalyst at 'react < 250 C
Two samples of [Ce02-Zr02 + Fe-Beta zeolite] composite
catalyst were prepared by thorough grinding of 74wt%Ce02-
26wt%Zr02 and Fe-Beta zeolite powders.
A first sample was prepared by mixing 74wt%Ce02-26wt%Zr02
and Fe-Beta zeolite powders at a weight ratio of 3.3. This
weight ratio results in a volume ratio of 74wt%0e02-
26wt%Zr02/Fe-Beta components in composite catalyst = 1/1.
A second sample was prepared by mixing 74wt-Y5Ce02-26wt%Zr02
and Fe-Beta powders at a weight ratio of 10. For the second
sample volume ratio of 74wt%Ce02-26wt%Zr02/Fe-Beta zeolite
equals 3/1.
After grinding in agate mortar for 10-15 min, the resulted
mixtures were pelletized. The pellets were crushed and
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
8
sieved collecting 0.2 - 0.4 mm fraction for catalytic test.
Similarly pelletized Fe-Beta zeolite was used as reference.
Activities of the prepared samples were tested using the
following catalyst loading which kept constant amount of
Fe-Beta zeolite component in the reactor:
The first sample with 1/1 volume component ratio: [0.065g
74%Ce02-Zr02 + 0.02g Fe-Beta zeolite].
The second sample with 3/1 volume component ratio: [0.197g
74%Ce02-Zr02 + 0.02g Fe-Beta zeolite].
Reference sample: 0.02 g Fe-Beta zeolite.
The catalysts were tested in NH3-DeN0x within the tempera-
ture range of 150-550 C. The test was performed under fol-
lowing conditions: decreasing reaction temperature with a
rate of 2 C/min, feed gas composition: 500 ppm NO, 540 ppm
NH3, 10 vol % 02, 6 vol% H20, balanced with N2 to obtain a
total flow of 300 mL/min.
Catalyst loading and resulted GHSV:
[0.197g 74%Ce02-Zr02 + 0.02g Fe-Beta zeolite], catalyst
vol. = 0.134 ml, GHSV = 135 000 h-1;
[0.065g 74%Ce02-Zr02 + 0.02g Fe-Beta zeolite], catalyst
vol. = 0.067 ml, GHSV = 270 000 h-1;
0.02 Fe-Beta zeolite, catalyst vol. = 0.034 ml, GHSV =
540 000 h-1.
Under these test conditions [Ce02-Zr02 + Fe-Beta zeolite]
composite catalysts showed enhanced DeN0x activity within
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
9
low-temperature range (150-300 C), which significantly ex-
ceeded activity of individual Fe-Beta zeolite, as shown in
Fig. 2. It is important to note that the activity of [Ce02-
Zr02 + Fe-Beta zeolite] is improved when the amount of
Ce02-Zr02 component was increased.
Example3
Catalyst with reduced amount of zeolite component.
Three samples of [Ce02-Zr02 + Fe-Beta zeolite] composite
catalyst were prepared by thorough grinding of 74wt%Ce02-
26wt%Zr02 powder with Fe-Beta zeolite powder:
A first sample was prepared by mixing 74wt%0e02-26wt%Zr02
and Fe-Beta powders at a weight ratio of 3.3. In this case
volume ratio of 74wt% Ce02- 26wt% Zr02 /Fe-Beta zeolite
equals 1/1.
A second sample was prepared by mixing 74wt%Ce02-26wt%Zr02
and Fe-Beta zeolite powders at a weight ratio of 15.5. For
the second sample volume ratio of 74wt%Ce02-26wt%Zr02 and
Fe-Beta zeolite components equals 5/1.
A third sample was prepared by was prepared by mixing
74wt%Ce02-26wt%Zr02 and Fe-Beta zeolite powders at a weight
ratio of 30. For the second sample volume ratio of
74wt%Ce02-26wt%Zr02 and Fe-Beta zeolite components equals
10/1.
After grinding in agate mortar for 10-15 min, the resulted
mixtures were pelletized. The pellets were crushed and
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
sieved collecting 0.2 - 0.4 mm fraction for catalytic test.
Similarly pelletized Fe-Beta zeolite was used as reference.
Activities of the prepared samples were tested using the
5 following catalyst loading which kept constant volume of
the catalyst in the reactor. In all experiments described
below overall volume on the catalyst loaded was 0.067 ml,
which results in GHSW - 270 000 h-l:
10 First sample (1/1 vol component ratio): [0.065g 74wt%Ce02-
Zr02 + 0.02g Fe-Beta zeolite].
Second sample (5/1 vol component ratio): [0.109g 74wt%Ce02-
Zr02 + 0.007g Fe-Beta zeolite].
Third sample (10/1 vol component ratio): [0.119g 74wt%Ce02-
Zr02 + 0.0035g Fe-Beta zeolite].
Reference sample: 0.02 g Fe beta-zeolite.
Feed gas composition: 540 ppm NH3, 500 ppm NO, 10 % 02, 6 %
H20 balance with N2.
Under these conditions [Ce02-Zr02 + Fe-Beta zeolite] com-
posite catalysts showed DeN0x performances, which were es-
sentially identical to the performance of reference Fe-Beta
zeolite sample, despite significantly reduced amount of
zeolite catalyst (Fe-Beta zeolite) loaded into the reactor
as a part of composite [Ce02-Zr02 + Fe-Beta zeolite].
The data in Fig. 3 show that amount of zeolite can be re-
duced at least 10 times without sacrificing DeN0x perform-
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
11
ance of [Ce02-Zr02 + Fe-Beta zeolite] by its replacement
with corresponding volume of Ce02-Zr02.
Example 4
Enhanced DeN0x performance of [Ce-Mn/A1203 + Fe-Beta zeo-
lite] composite catalyst at 'react < 250 C.
[Ce-Mn/A1203 + Fe-Beta] composite catalysts were prepared
by thorough mixing 15wt%Ce-15wt%Mn/A1203 powder with Fe-
Beta powder at a weight ratio of 0,8:1; 1,7:1 and 3,4:1
keeping the same total volume of the catalyst constant.
These weight ratios result in volume ratio of components
Ce-Mn/A1203/Fe-Beta = 2/1; 1/1 and 1/2 due to difference in
densities of these materials. The powders were thoroughly
grinded in agate mortar for 10-15 min, followed by pelleti-
zation. The pellets were crushed and sieved collecting 0.2
- 0.4 mm fraction for catalytic test. Similarly pelletized
Fe-Beta was used as reference.
The catalysts were tested in the NH3-DeN0x in the tempera-
ture range of 150-550 C. The test was performed under fol-
lowing conditions: decreasing reaction temperature with a
rate of 2 C/min, feed gas composition: 500 ppm NO, 540 ppm
NH3, 10 vol % 02, 6 vol% H20, balanced with N2 to obtain a
total flow of 300 mL/min.
Catalyst load: 0.04g Fe-Beta and
[0.045g Ce-Mn/A1203 + 0.013g Fe-Beta] (2/1 ratio), [0.034g
Ce-Mn/A1203 + 0.02g Fe-Beta] (1/1 ratio), [0.022g Ce-
Mn/A1203 + 0.027g Fe-Beta] (1/2 ratio).
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
12
Under these conditions all [Ce-Mn/A1203+ Fe-Beta] composite
catalysts showed DeN0x activity, which radically exceeded
activities of individual Ce-Mn/A1203 and Fe-Beta at tem-
peratures below 350 C, indicating pronounced synergistic
effect between components of composite catalyst (Fig. 4).
Besides that, ammonia slip on composite catalysts was sig-
nificantly lower than for a reference Fe-Beta catalyst in-
dicating that those composite systems can be used as inte-
grated DeN0x-ASC.
Example 5
Enhanced DeN0x performance of [lOwt%Cu/A1203 + H-zeolite]
composite catalysts.
Three samples of [lOwt%Cu/A1203 + H-zeolite] composite
catalyst were prepared by thorough grinding of
lOwt%Cu/A1203 and H-Beta, H-ZSM-5, or H-ferrierite powder.
A first sample was prepared by mixing lOwt%Cu/A1203 and H-
Beta (Si/A1 = 20) powders at a weight ratio of 1/1.
A second sample was prepared by mixing lOwt%Cu/A1203 and H-
ZSM-5 powders (Si/A1 = 20) at a weight ratio of 1/1.
A third sample was prepared by mixing lOwt%Cu/A1203 and H-
ferrierite powders (Si/A1 = 32) at a weight ratio of 1/1.
After grinding in agate mortar for 10-15 min, the resulted
mixtures were pelletized. The pellets were crushed and
sieved collecting 0.2 - 0.4 mm fraction for catalytic test.
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
13
Similarly corresponding pelletized zeolites (H-Beta, H-ZSM-
5, and H-ferrierite) were used as reference.
Activities of the prepared samples were tested using the
following catalyst loading which kept constant amount of
zeolite component in the reactor:
The first sample with 1/1 weight component ratio: [0.040g
lOwt%Cu/A1203 + 0.040g H-Beta].
The second sample with 1/1 weight component ratio: [0.040g
lOwt%Cu/A1203 + 0.040g H-ZSM-5].
The third sample with 1/1 weight component ratio: [0.040g
lOwt%Cu/A1203 + 0.040g H-ferrierite].
Reference samples: 0.040 g H-Beta; 0.040g H-ZSM-5, or H-
ferrierite, or 0.040 g lOwt%Cu/A1203.
The catalysts were tested in NH3-DeN0x within the tempera-
ture range of 150-550 C. The test was performed under fol-
lowing conditions: decreasing reaction temperature with a
rate of 2 C/min, feed gas composition: 500 ppm NO, 540 ppm
NH3, 10 vol % 02, 6 vol% 1120, balanced with N2 to obtain a
total flow of 300 mL/min.
Catalyst loading and resulted GHSV:
[0.040g lOwt%Cu/A1203 + 0.040g H-Beta], catalyst vol. =
0.134 ml, GHSV = 135 000 h-1;
[0.040g lOwt%Cu/A1203 + 0.040g H-ZSM-5], catalyst vol. =
0.134 ml, GHSV = 135 000 h-1;
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
14
[0.040g 10wt%Ou/A1203 + 0.040g H-ferrierite], catalyst vol.
= 0.134 ml, GHSV = 135 000 h-1;
Reference catalysts
0.040g H-Beta, catalyst vol. = 0.067 ml,
GHSV = 270,000 h-1;
0.040g H-ZSM-5, catalyst vol. = 0.067 ml,
GHSV = 270,000 h-1;
0.040g H-ferrierite, catalyst vol. = 0.067 ml,
GHSV = 270,000 h-1;
0.040g Cu/A1203, catalyst vol. = 0.067 ml,
GHSV = 270,000 h-1.
Under these test conditions [10wt%Cu/A1203 + H-zeolite]
composite catalysts showed enhanced DeN0x within the whole
temperature range (150-550 C), which significantly exceeded
activity of individual components, as shown by comparing
Fig. 5 and Fig. 6.
Example 6
Catalyst with enhanced soot oxidation activity.
[Ce02-Zr02 + Fe-Beta] with 3/1 vol. component ratio was
prepared as described in Example 2. For testing soot oxida-
tion activity of [Ce02-Zr02 + Fe-Beta] a part of pelletized
sample was crushed, and the catalyst powder was mixed with
soot ("Printex U", Degussa) at a weight ratio catalyst/soot
= 1/10. Soot and catalyst were mixed by shaking in a glass
bottle for 5 min, thus establishing loose contact between
soot and the catalyst. Reference sample was prepared in a
similar manner using Fe-Beta powder.
CA 02853154 2014-04-22
WO 2013/060487 PCT/EP2012/058003
Soot oxidation was carried out at temperature ramp =
10 C/min in a flow of dried air. Profiles of soot oxidation
over [Ce02-Zr02 + Fe-Beta] and Fe-Beta are displayed in
Fig. 7. [Ce02-Zr02 + Fe-Beta] significantly higher activity
5 in soot oxidation then individual Fe-Beta, as evidenced by
a shift of soot oxidation maximum from - 600 C for (Fe-Beta
+ soot) to - 420 C for ([Ce02-Zr02 + Fe-Beta] + soot).