Note: Descriptions are shown in the official language in which they were submitted.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
METHOD OF FORMING DEXTRAN AND THROMBIN SHEETS
FIELD OF THE INVENTION
[0001] The invention relates generally to products having hemostatic
characteristics.
More particularly, the invention relates to a method of forming dextran and
thrombin sheets
that are suitable for use in hemostatic applications.
BACKGROUND OF THE INVENTION
[0002] The body's natural response to stem bleeding from a wound is to
initiate blood
clotting via a complex process known as the coagulation cascade. The cascade
involves two
pathways that ultimately lead to the production of the enzyme thrombin, which
catalyzes the
conversion of fibrinogen to fibrin.
[0003] Fibrin is then cross-linked to Ruin a clot, resulting in hemostasis.
For wounds
that are not severe, and in individuals that have no countervening conditions,
the body is
usually able to carry out this process efficiently in a manner that prevents
excessive loss of
blood from the wound. However, in the case of severe wounds, or in indiv-
iduals in whom the
clotting mechanism is compromised, this may not be the case.
[0004] For such individuals, it is however possible to administer
components of the
coagulation cascade, especially thrombin and fibrinogen, directly to the wound
to bring about
hemostasis. Bandaging of bleeding wounds is also a usual practice, in part to
isolate and
protect the wounded area, and also to provide a means to exert pressure on the
wound, which
can also assist in controlling bleeding.
[0005] While these methods may be carried out satisfactorily in cases of
mild trauma
or under conditions of "controlled" wounding (e.g. surgery), many situations
in which such
treatments are most needed are also those in which it is the most difficult to
provide them.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
Examples of such wounds include, for example, those inflicted during combat,
or
unanticipated wounds that occur as the result of accidents. In such
circumstances, survival of
the wounded individual may depend on stopping blood loss from the wound and
achieving
hemostasis during the first few minutes after injury. Unfortunately, given the
circumstances
of such injuries, appropriate medical intervention may not be immediately
available.
[0006] In particular, the treatment of penetrating wounds such as bullet
wounds or
some wounds from shrapnel is problematic. This is due to the difficulty in
placing a bandage
and/or therapeutic agents at the actual site of injury, which includes an area
that is well below
the body surface and difficult or impossible to access using conventional
techniques.
[0007] Jiang et al. in Biomacromolecules, v. 5, p. 326-333 (2004) teaches
electrospun
dextran fibers. Agents associated with the fibers (e.g. BSA, lysozyme) are
directly
electrospun into the fibers. The fibers may also include other polymers
electrospun with the
dextran.
[0008] Jiang et al. in Journal of Biomedical Materials Research Part B:
Applied
Biomaterials, p. 50-57 (2006) discloses electrospun fibers that are a
composite of
poly(c-caprolactone) as a shell and dextran as a core. These fibers provide
the slow release
of agents (bovine serum albumin, BSA) that are also electrospun into the
fibers.
[0009] Smith et at., U.S. Patent No. 6,753,454, discloses electrospun
fibers
comprising a substantially homogeneous mixture of a hydrophilic polymer and a
polymer that
is at least weakly hydrophobic, which may be used to form a bandage. The
bandage may
comprise active agents (e.g. dextran). However, the disclosed fibers are not
readily soluble in
liquid.
[0010] MacPhee et al., U.S. Patent No. 6,762,336, teaches a hemostatic
multilayer
bandage that comprises a thrombin layer between two fibrinogen layers. The
bandage may
contain other resorbable materials such as glycolic acid or lactic acid based
polymers or
2
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
copolymers. Neither electrospun fibers nor dextran fibers are taught as
components of the
bandage.
[0011] Smith et al., U.S. Patent No. 6,821,479, teaches a method of
preserving a
biological material in a dry protective matrix, the matrix comprising fibers
such as
electrospun fibers. One component of the fibers may be dextran, but
homogeneous dextran
fibers are not described.
[0012] Cochrum et al., U.S. Patent No. 7,101,862, teaches hemostatic
compositions
and methods for controlling bleeding. The compositions comprise a cellulose
containing
article (e.g. gauze) to which a polysaccharide is covalently or ionically
crosslinked. The
crosslinked polysaccharide may be dextran. However, the compositions are not
electrospun
and exogenous clotting agents are not included in the compositions.
[0013] Wnek et al., U.S. Patent Publication No. 2004/0018226, discloses
fibers
produced by an electroprocessing technique such as electrospinning. The fibers
comprise
enclosures within the fibers for containing substances that are not miscible
with the fibers.
Dextran is not taught as a fiber component.
[0014] Fisher et al., U.S. Patent Publication No. 2007/0160653, teaches a
hemostatic
textile comprising hemostatic factors (e.g. thrombin, fibrinogen) but the
fibers are formed
from electrospun glass plus a secondary fiber (e.g. silk, ceramic, bamboo,
jute, rayon, etc.)
[0015] Carpenter et al., U.S. Patent Publication No. 2008/0020015, teaches
wound
dressing comprised of various biodegradable polymers and hydrogels having
allogenic or
autologous precursor cells (e.g. stem cells) dispersed within the polymers.
The polymers may
be prepared by electrospinning, and one polymer component may be dextran.
However, the
polymers cannot be immediately soluble upon contact with liquid, as they must
provide a
scaffolding for delivery of the cells over time, even though the polymers
eventually
biodegrade in situ.
3
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0016] Li et al., U.S. Patent Publication No. 2008/0265469, describes
electrospun
nanofibers that may include dextran. However, the nanofibers are not described
as readily
soluble in liquids.
[0017] Eskridge et al., U.S. Patent Publication No. 2009/0053288, teaches a
woven
hemostatic fabric comprised of about 65% fiberglass yam and about 35% bamboo
yam. The
fiberglass component may be electrospun, and hemostatic factors such as
thrombin may be
associated with the fabric, e.g. by soaking the material in a solution of
thrombin. This
document indicates that dextran may be added as a hygroscopic agent.
[0018] There is an ongoing need to provide improved methods and means to
initiate
blood clotting in wounds to stop or at least slow blood loss. In particular,
there is an ongoing
need to improve the capability to readily promote hemostasis in severe wounds
in a facile
manner, especially under circumstances where immediate treatment by medical
personnel is
limited or unavailable.
[0019] Bowlin et al., U.S. Patent Publication No. 2011/0150973, discloses a
method
of delivering one or more agents of interest to a location of interest. The
method includes
applying or delivering to a location of interest a hemostatic hemostatic
product. The
hemostatic hemostatic product includes electrospun dextran fibers that
dissolve upon contact
with liquid. The hemostatic hemostatic product also includes one or more
agents of interest
associated with said electrospun dextran fibers. Applying or delivering
results in dissolution
of the electrospun dextran fibers in liquid at the location of interest to
thereby release the one
or more agents of interest into the liquid.
SUMMARY OF THE TNVENTTON
[0020] An embodiment of the invention is directed to a method of forming
hemostatic
sheets. Dextran and water are mixed to form a dextran-water mixture. The
dextran-water
4
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
mixture is fowled into a first support layer. Fibrinogen and thrombin are
mixed to form a
fibrinogen and thrombin mixture. The fibrinogen and thrombin mixture is
dispersed on the
first support layer to foini a hemostatic sheet.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] The accompanying drawings are included to provide a further
understanding
of embodiments and are incorporated in and constitute a part of this
specification. The
drawings illustrate embodiments and together with the description serve to
explain principles
of embodiments. Other embodiments and many of the intended advantages of
embodiments
will be readily appreciated as they become better understood by reference to
the following
detailed description. The elements of the drawings are not necessarily to
scale relative to
each other. Like reference numerals designate corresponding similar parts.
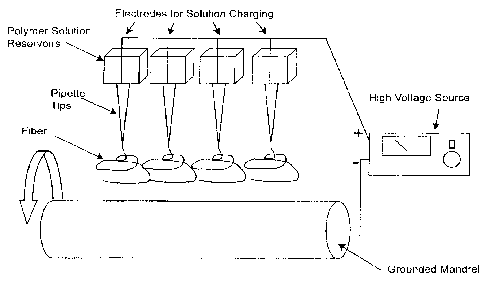
[0022] FIG. I. Schematic of the electrospinning apparatus. The key elements
of the
electrospinning system include a high voltage power supply, a source reservoir
for the
polymer and a grounded mandrel. This system utilizes a cylindrical target
mandrel; however
the electrospinning process can be adapted to produce much more complex
shapes. Single
and/or multiple polymers can be independently or simultaneously delivered to
the electric
field from one or more source reservoirs. Electro spinning distinct and unique
polymers from
separate sources in a temporal sequence can be used to produce a laminated
structure.
[0023] FIGS. 2 A and B. A, schematic of air brush based dextran processing;
B,
dextran fibers produced by electroaerosol processing. The amount of material
depicted is
probably enough material for about two hemostatic products. Note the loft of
the material.
An electric field was used to target the dextran to the mandrel.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0024] FIG. 3. Scanning electron micrograph of electrospun dextran fibers.
The
nominal average cross sectional diameter of the individual fibers was 1
micron, providing a
large surface area.
[0025] FIG. 4A-E. Schematic representations of exemplary hemostatic
products
formed form electrospun dextran fibers. A, hemostatic product with non-
peimeable support
material as a backing; B, hemostatic product with net-like support material;
C, hemostatic
product with non-permeable backing and a net-like support material holding the
electrospun
fibers in place on the backing; D, hemostatic product for delivery of
therapeutics to a deep
wound; E, alternative embodiment of a hemostatic product for delivery of
therapeutics to a
deep wound.
[0026] FIGS. SA and B. Changes in cytokine levels in animals exposed to the
salmon
fibrinogen/thrombin hemostatic product. (A) Levels of IL-1 ft 1L-6, TNF-a, IFN-
y, IL-4 and
IL-10 are shown as the log ratio of the cytokine level determined in blood
drawn at the initial
surgery to implant the vascular port compared to peak levels following
exposure. Changes
were seen in both pro-inflammatory responses (IL-113, IL-6, TNIT-a, IFN-y) and
humoral
responses (IL-4 and IL-10). (B) Changes in the eytokines within an individual
animal show
that initial exposure (first arrow) and the subsequent intravenous infusion of
proteins (second
arrow) elicited a response that could be detected in samples taken at the next
blood draw.
[0027] FIG. 6A-F. Qualitative assessment of immunoglobulin production by
swine in
response to salmon proteins by Western blotting. (A) PAGE of salmon (Sal),
human (Hu)
and swine (Sw) fibrinogen preparations and corresponding Western blots with
serum from
two animals (B and C). Serum from pre-exposure and final euthanasia blood
draws are
presented in these panels. IgG isotypes present in the serum were visualized
by specific HRP
anti-swine IgG second antibodies and are detected as binding to the proteins
in the gel
samples. Arrows indicate the positions of the IgG heavy and light chains
components in the
6
CA 02854294 2014-05-01
WO 2013/059341
PCT/US2012/060643
swine protein lanes which are also recognized by the 2nd antibody. Molecular
weights are
show to the left (kDalx10-3). (D) PAGE of salmon (Sal), human (Hu) and swine
(Sw)
thrombin preparations and corresponding Western blots with serum from the same
animals
shown in (C and D). In these animals, thrombin was not recognized in E, but
there is a faint
reaction in the salmon protein lane in F (arrow). The camera in the detection
system detected
the heavy swine thrombin protein on the membrane as a white band in F.
[0028] FIG. 7A-D. Time course of antibody development in animals exposed to
salmon thrombini'fibrinogen hemostatic products through the demial patch
protocol. ELISAs
were performed using anti-IgG reagents. The following antigens were used as
the targets in
the ELISAs: (A) salmon fibrinogen, (B) salmon thrombin, (C) human fibrinogen
and (D)
human thrombin. The increases in absorbance observed at the later samples
panels A, B, C
occurred following intravenous infusion of salmon proteins. Each curve
represents data from
a different animal.
[0029] FIG. 8A-D. Time course of antibody development in animals exposed to
salmon thrombin/fibrinogen hemostatic products through the abdominal patch
protocol.
ELISAs were performed using anti-IgG reagents. The following antigens were
used as the
targets in the ELISAs: (A) salmon fibrinogen, (B) salmon thrombin, (C) human
fibrinogen
and (D) human thrombin.
[0030] FIG. 9A-D. Progression of demial healing following full-thickness
wound.
Images from samples taken at 7 days from control (A) and salmon hemostatic
product-treated
(B) injuries show a fibrinonecrotic coagulum filling the wound defect (*) and
an epithelial
cell projection towards wound center in both cases as wound healing progresses
following
initial clotting. (H&E staining, bars=100 urn). Samples taken at 28 days from
control (C) and
salmon hemostatic product-treated (D) injuries show complete re-
epithelialization by a
hyperplastic and hyperkeratotic epideimis. (H & E staining, bars=100 um).
7
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0031] FIG. 10. Schematic of the coagulation cascade.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0032] An embodiment of the invention is directed to a process of forming a
hemostatic product from a plurality of sheets hemostatic material. As an
initial step in
forming the hemostatic product, the sheets of hemostatic material are
prepared.
[0033] The sheets have a relatively uniform thickness and appearance.
Fonning the
sheets with a relatively uniform thickness and appearance enhances the ability
to accurately
use the hemostatic products to treat the particular injury.
[0034] When the hemostatic product is applied to the injury site, the
materials used to
fabricate the hemostatic product dissolve to thereby release the materials to
the injury site and
provide the hemostatic effect.
[0035] In one embodiment, the site of action is a wound bed, and the active
agents
that are delivered by the hemostatic product are factors or agents that
participate in the
coagulation cascade such as thrombin and fibrinogen. Application of the
hemostatic product
to a wound results in dissolution of the dextran fibers in blood within the
wound bed, which
in turn results in release or delivery of the active agents at or into the
site.
[0036] Thrombin and fibrinogen that are associated with the hemostatic
product are in
fofins that are biologically active when they come into contact with blood.
Hence, upon
dissolution, the thrombin acts on the fibrinogen, converting it to fibrin,
which then foul's a
clot within the wound, staunching the flow of blood.
[0037] This invention uses layers that provide a product that is easier to
commercialize and provides a more uniform dispersion of the components in the
hemostatic
product.
8
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0038] The invention provides dextran fibers, especially electrospun
dextran fibers.
The electrospun dextran fibers may be formed into a variety of hemostatic
products for a
variety of purposes. Generally, one or more substances of interest are
associated with the
electrospun dextran fibers in the hemostatic product such as for the purpose
of delivering the
one or more substances of interest to a liquid of interest. Upon contact with
the liquid, the
electrospun dextran fibers dissolve in a relatively short period of time and
the associated
substances are released into the liquid milieu.
[0039] In one embodiment of the invention, the electrospun dextran fibers
are formed
into a hemostatic product. The hemostatic product generally includes active
agents
associated with the electrospun dextran fibers, the active agents being
delivered to a site of
action (e.g. a wound) via application of the hemostatic product to the site.
[0040] The site of action contains or will contain a liquid, and when the
hemostatic
product is applied to the site of action, the electrospun dextran fibers in
the hemostatic
product dissolve in the liquid, and the active agents associated with or
sequestered in or
around the mat of dextran fibers are released into the liquid.
[0041] In one embodiment, the site of action is a wound bed, and the active
agents
that arc delivered by the hemostatic product are factors or agents that
participate in the
coagulation cascade such as thrombin and fibrinogen. Application of an
electrospun dextran
fiber hemostatic product to a wound results in dissolution of the dextran
fibers in blood
within the wound bed, which in turn results in release or delivery of the
active agents at or
into the site.
[0042] Thrombin and fibrinogen that are associated with the hemostatic
product are in
forms that are biologically active when they come into contact with blood.
Hence, upon
dissolution, the thrombin acts on the fibrinogen, converting it to fibrin,
which then forms a
clot within the wound to thereby staunch the flow of blood.
9
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0043] In some embodiments of the invention, only spun dextran fibers are
utilized
and thus after clot foimation, there is no need to disturb the clot to remove
hemostatic
product components, since none remain at the site. In other embodiments, as
described
below, the hemostatic product may include other materials such as support or
backing
material, which, after initial rapid application of the hemostatic product,
may later be
removed for further treatment of the wound by conventional methods.
[0044] Electrospinning is a non-mechanical processing strategy and can be
scaled to
accommodate the large volumes necessary to meet the needs of commercial
processing. A
schematic representation of one type of set-up for electrospinning is provided
in FIG. 1. In
this process a polymer solution, or melt, is injected with current to create a
charge imbalance.
The charged solution is then placed in proximity to a grounded target (in FIG.
1, a grounded
mandrel).
[0045] At a critical voltage the charge imbalance begins to overcome the
surface
tension of the polymer source, forming an electrically charged jet. Within the
electric field,
the jet is directed towards the grounded target and the carrier solvent
evaporates. Depending
upon reaction conditions, and the polymers used in the process,
electrospinning can be
utilized to produce a fine aerosol of material or a continuous non-woven mat
of fibrillar
material, as shown in FIG. 1.
[0046] For many polymers, the nature of the electrospinning process
intrinsically
provides a high degree of control over the diameter of the resulting fibers.
Micron to
nanoscalc diameters can be selectively achieved simply by regulating the
starting
concentrations of the polymers present in the electrospinning solutions. By
controlling the
motion of the ground target with respect to the source solution, fibrils may
be deposited into a
random matrix or into aligned arrays that are oriented along a defined axis.
[0047] A second schematic of an electrospinning apparatus is shown in
FIG. 2A. The
key elements of the electrospinning system include a high voltage power
supply, a source
reservoir for the polymer and a grounded target mandrel. The system that is
depicted utilizes a
cylindrical target mandrel; however the electrospinning process can be adapted
to produce much
more complex shapes.
[0048] Single and/or multiple polymers can be independently or
simultaneously
delivered to the electric field from one or more source reservoirs. In
addition, electrospinning
distinct and unique polymers from separate sources in a temporal sequence can
be used to
produce a laminated structure. FIG. 2B shows the result of electrospinning
about 10 grams of
dextran dissolved in deionized water onto a round mandrel target, as described
in detail in the
Example 1 below. FIG. 3 shows a scanning electron micrograph of electrospun
dextran fibers
in which the average cross sectional diameter of the individual fibers is
about 1 micron.
[0049] Those of skill in the art will recognize that electrospinning is
not the only way
to make dextran fibers. Such fibers may be produced by other methods of
aerosolization.
However, the electric field helps in the efficient collection of the fibers,
and electrospinning
may yield more unifoiiu fibers. Other technologies which might also be
employed for
spinning dextran fibers, including those described in Luo et al., U.S. Patent
No. 7,067,444;
Bogue et al., U.S. Patent No. 6,116,880; and Fuisz etal., U.S. Patent No.
5,447,423.
[0050] In particular, so-called "cotton-candy machines" (with or without
applied
electrostatic force) may be suitable for use in fabricating the dextran fibers
of the invention. More
detailed descriptions of methods of preparing the dextran fibers of the
invention are provided in
Example 2 below.
11
CA 2854294 2018-01-30
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0051] Other methods include compressing a dextran solution between two
plates or
other flat surfaces and drawing the plates or surfaces away from each other,
usually
repeatedly. Dextran fibers faun between the two surfaces.
[0052] In some embodiments, substances other than dextran are used to form
fibers
for use in the hemostatic products of the invention, especially (but not
exclusively) when a
cotton-candy machine is employed. Examples of such substances include but arc
not limited
to sugars such as dextrose, sucrose, etc.
[0053] The commercially available dextran that is used to produce the
electrospun
fibers of the invention is synthesized from sucrose by enzymes on the cell
surface of certain
lactic acid bacteria, the best-known being Leuconostoc mesenteroides and
Streptococcus
mutans. Dextran is a complex, branched glucan (a polysaccharide made of many d-
glucose
molecules) composed of chains of varying lengths (e.g. from 10 to 200
kilodaltons). The
straight chain consists of a-1,6 glycosidic linkages between glucose
molecules, while
branches begin from a-1,4 linkages (and in some cases, a-1,2 and ct-1,3
linkages as well).
[0054] Dextrans are commercially available in a wide range of molecular
weights e.g.
from about 10 kilodaltons (kDa) to about 200 kDa. Commercial preparations are
mixtures of
dextrans of varying molecular weights, usually in narrower weight ranges and
may be
provided, for example, as "low" or "high" molecular weight dextrans. For
example,
"Dextran 40" has an average molecular weight of 40 kDa, "Dextrans 75" has an
average
molecular weight of 75 kDa, etc.
[0055] In the practice of the invention, the dextrans used for
electrospinning are
typically in a molecular weight range of from about 10 to about 200 kDa, or
from about 25 to
about 200 kDa, or from about 50 to about 200 kDa, or from about 75 to 200 kDa,
and usually
from about 60 to 90 kDa, or from about 100 to about 200 kDa.
12
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0056] Further, as would be understood by those of skill in the art, the
median size of
the dextran molecules in a dextran preparation also has an effect in that if
the median weight
is high in a particular lot, less dextran may be used to faun the desired
amount of fibers.
[0057] In general, the conditions for electrospinning dextran are as
follows: an
ambient temperature of from about 60 to about 75 F, a relative humidity of
from about 30%
to about 40%, and typically at least about 20%. The resulting fibers are
typically in the
nanometer or millimeter range of cross-sectional diameter, usually from about
0.75 microns
to about 1.25 microns.
[0058] The electrospun fibers are "dry" and should be protected from
exposure to
moisture to prevent premature dissolution. However, some water is associated
with the fibers
and fiber compositions can contain from about 7 to about 8% water, but must be
less than
about 5% when the fibers are sterilized by x-ray irradiation.
[0059] The hemostatic products of the invention are usually foimed of
substantially
homogeneous spun dextran. The amount of dextran per hemostatic product can
vary widely,
depending on the size of hemostatic product that is being manufactured, with
typical
hemostatic product formulations using from about 5-10 grams of dextran
(usually 100,000-
200,000 Mr) per hemostatic product.
[0060] However, the range can be extended widely, e.g. from as low as about
0.5
grams or less (for small hemostatic products) to as high as 100 or more grams
per hemostatic
product, for large hemostatic products. In some embodiments of the invention,
it may be
helpful to use lesser amounts of dextran (e.g. about 0.1 to about 0.5 grams of
dextran per
hemostatic product) to concentrate the active agents that are delivered by the
hemostatic
product into a smaller volume.
[0061] Of more consequence is the concentration of dextran in the solution
from
which the fibers are spun. Generally, a solution of dextran for
electrospinning will be of a
13
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
concentration in the range of from about 0.1 to about 10 gams per ml of
solvent, or from
about 0.5 to about 5 grams per ml, and usually such a solution is at a
concentration of about 1
gram per ml, about 0.15 mg. A preferred range would be from about 0.9 to
about 1.1 grams
of dextran per ml of solution that is to be spun.
[0062] Those of skill in the art will recognize that, due to the
variability of molecular
weight ranges in dextran preparations, and due to inherent variability from
batch to batch of
commercially available preparations purporting to be of a particular molecular
weight range,
it is typically necessary to test each batch of dextran with respect to
electrospinning
properties. Such tests are well within the purview of one of skill in the art,
and usually
involve trials of electrospinning a range of concentrations of dextran
dissolved in a suitable
solvent, to ascertain which concentration(s) result(s) in the most desirable
fiber
characteristics, e.g. stability (e.g. to heat, humidity, etc.), uniformity,
cross-sectional
diameter, etc.
[0063] Those of skill in the art will recognize that critical indicators of
success are
very obvious when trying a new batch of dextran. Too little dextran in the
spinning solution
results in "spitting" from the needle, whereas too much dextran results in the
production of
dried droplets, or failure to spin at all.
[0064] Likewise, when the humidity is too low, similar results can occur,
i.e. fibers
fail to faun and in some cases fail to target efficiently to the ground. These
characteristics
can be assessed according to methods that are well known to those of skill in
the art,
including hut not limited to visual observation, testing of fiber strength and
flexibility,
observation via electron microscopy, solubility testing, resistance to heat
and/or irradiation,
color and tendency to discoloration, etc. As would be understood by those of
skill in the art,
all such testing may be carried out under varying conditions of heat,
humidity, etc.
Formulations may also be assessed using animal testing.
14
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0065] The area (length and width) of a hemostatic product of the invention
can vary
widely and can be adjusted by adjusting spinning parameters. In addition, the
mats of dextran
fibers can be cut to a desired size after spinning. Generally, a hemostatic
product will be
from about 0.5 centimeters or less to about 30 centimeters or more in length
and/or width, but
larger or smaller sizes are also contemplated.
[0066] The height or thickness of the hemostatic product can likewise vary
considerably depending on the intended use of the hemostatic product. In
certain
embodiments, the hemostatic product has a thickness of between about 1
millimeter and
about 5 centimeters.
[0067] The thickness of the hemostatic product (which is related to the
volume) may
impact the rate of dissolution of the dextran upon contact with liquid. For
example, a thin
hemostatic product (e.g. about 2 millimeters), will dissolve more rapidly than
a hemostatic
product that is thicker, providing the loft of the fibers is comparable.
[0068] In most embodiments, dissolution of the dextran fibers is extremely
rapid, e.g.
about 5 minutes or less after exposure to liquid, or about 4 minutes or less,
or about 3 minutes
or less, or about 2 minutes or less, or about 1 minute or less, e.g. the
hemostatic product
typically takes only a few seconds to dissolve (e.g. from about 1 to about 20
seconds to
dissolve.
[0069] This rapid dissolution may be referred to herein as "instantaneous"
or
"immediate" dissolution. Compression of an electrospun dextran mat may be used
to
modulate the rate of dissolution, with greater levels of compression inversely
impacting the
rate, i.e. generally, the greater the degree of compression, the slower the
rate of dissolution.
The rapid rate of dissolution is advantageous, particularly when delivering
biologically active
agents (e.g. hemostatic agents) to a site of action such as a wound. Rapid
dissolution of the
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
carrier dextran fibers provides extremely rapid delivery of the hemostatic
agents to the wound
upon deployment of the hemostatic product.
[0070] Those of skill in the art will recognize that a plethora of liquid
solvents exist in
which it is possible to dissolve dextran. However, superior results for
electrospinning
dextran are generally achieved when the solvent is water, especially deionized
or distilled or
deionized, distilled (ddH20) or other forms of relatively pure water. In
addition, there is far
less environmental impact associated with the use of water,
[0071] It has been found that, generally, high concentrations of salt in
the solvent
should be avoided. Whereas salt is often used to facilitate the spinning of
some electrospun
polymers, this is not the case for dextran. The concentration of salts in the
spinning solution
should be kept at a minimum to successfully faun dextran fibers.
[0072] The one or more active agents that are associated with the dextran
fibers of the
hemostatic product may be any active agent that it is desirable or
advantageous to deliver to
the site where the electrospun dextran fiber device is to be used or applied.
In one
embodiment of the invention, the electrospun dextran fiber device is a
hemostatic product and
is used to deliver beneficial agents, for example, to a wound.
[0073] Such wounds include wounds and breaches of body or tissue integrity
that
occur as a result of trauma (e.g. accidental trauma, trauma resulting from
conflicts such as
gunshot wounds, knives, etc.), as well as wounds which are purposefully
incurred, such as
surgical incisions, body piercings, etc.
[0074] Usually the agents are bioactive agents that have a beneficial or
therapeutic
effect at the wound site. In one embodiment, the site is a bleeding wound at
which it is
desired to form a blood clot to stop or slow the bleeding. In this embodiment,
the therapeutic
substances of interest may include, for example, thrombin and fibrinogen,
although other
16
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
agents active in promoting hemostasis, including but not limited to capscian,
may also be
included.
[0075] In addition, electrospun or non-electrospun collagen, agents that
absorb water,
various dry salts that would tend to absorb fluids when placed in contact with
e.g. blood;
engineered thrombin or thrombin mimics; engineered fibrinogen; agents that
cause
vasospasm (e.g. ADP, 5-hydroxytryptaminc, 5-HT and thromboxane, (TXA-2) to
help
contract and seal a bleeding vessel, etc. may also be included.
[0076] In addition, other components of the clotting cascade may be added
to the
hemostatic product, for example: tissue factors that are normally only
expressed on the
surface of damaged cells and which start the normal clotting cascade;
serotonin which
enhances platelet clumping and promotes vessel constriction; and other agents
that are used
to replace missing components of the clotting cascade in hemophilia, for
example, factor 7
(which activates the so called external extrinsic coagulation cascade) and
crude extracts of
platelets.
[0077] These agents essentially work to "jump start" clotting by initiating
the cascade
further down the reaction network, as illustrated in FIG. 10. In FIG. 10, the
various factors
(and their alternative nomenclature and/or characteristics and/or activities)
are as follows:
= Factor VII (Proconvertin): serine protease, Vitamin K dependent synthesis
in
the liver;
= Factor VIII: Glycoprotein binds vWF, produced by endothelium and liver;
= Factor TX (Christmas-Eve Factor): serine protease;
= Factor X (Stuart-Prowler Factor, Clotting Factor X): serine
endopeptidase,
converts prothrombin to thrombin; and
= Factor XI (Plasma thromboplastin antecedent): serine protease, plasma
protein;
17
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
= Factor XII (Hageman factor): serine protease, plasma protein binds
collagen;
= Factor XIII (Fibrin stabilizing Enzyme): stabilizes fibrin polymer.
plasma
protein, also present in platelets and monocyte image.
[0078] In FIG. 10, italic pathways denote inhibition and the central role
of thrombin
in the activation of coagulation and inactivation of coagulation processes is
shown, where:
= VI = Cofactor for Xa in the conversion of prothrombin to thrombin;
= APC = Activated Protein C, an extracellular signal molecule, inhibits FVI
(equivalent to FVa, a cofactor of XA in the conversion of prothrombin to
thrombin) and FVIITa through a proteolytic event; and
= TAFI = Thrombin Activatable Fibrinolysis Inhibitor, an inhibitor of clot
lysis.
[0079] In addition, active agents that function to promote late stages of
wound
healing may also be included to, for example, facilitate cell migration and
remodeling. The
incorporation of collagen is an example of such an active agent.
[0080] One or more of any of these active agents may be used in the
practice of the
present invention. The therapeutic agents must be amenable to drying and are
associated with
the other components of the hemostatic product in the dry state, since liquid
may negatively
affect at least one of the components used in the hemostatic product. For
example, the active
agents may be desiccated or lyophilized, or water may be removed by some other
means.
[0081] Generally, the amount of water that is present in the substances
when they are
associated with the electrospun dextran fibers is less than about 5%, and
preferably less that
about 2%. These substances retain full or partial activity when rehydrated,
e.g. in blood.
Generally therapeutic substances associated with the devices of the invention
retain, upon
contact with liquid, at least about 25%, or about 50%, or even about 75 to
100% of their
activity before drying or desiccation, as compared to standard preparations of
the substance
using standard assays that are known to those of skill in the art.
18
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0082] In some embodiments, thrombin or fibrinogen, or both, are associated
with the
hemostatic product. In some embodiments, the thrombin and fibrinogen are
salmon thrombin
and fibrinogen_ Advantages of using salmon as a source of these materials
include but are
not limited to the lack of concern about transmission of etiologic agents
(e.g. viruses) that
may occur when human and other mammalian sources of thrombin or fibrinogen
(e.g.
bovine) are used.
[0083] Salmon thrombin and fibrinogen are highly efficacious and have no
deleterious side effects, when used in the pig model, which is a recognized
animal model that
is considered to be indicative of results in humans.
[0084] The quantity of fibrinogen added to the hemostatic product is
generally in the
range of from about 10 milligrams to about 3 grams. In certain embodiments,
the amount of
fibrinogen in each of the hemostatic products is between about 20 milligrams
to about 1
gram.
[0085] The quantity of thrombin added to each of the hemostatic products is
generally
between about 10 and 10,000 NIB Units. In certain embodiments, the amount of
thrombin in
each of the hemostatic products is between about 20 and 6,000 NIH Units.
[0086] In some embodiments, the therapeutic agents may themselves be
electrospun.
For example, the therapeutic agents are dissolved in and spun from a solution.
In some
embodiments, the therapeutic agents may be electrospun into fibers. In other
embodiments,
the active agents may be electrospun into other forms such as droplets, beads,
etc.
[0087] In some applications, active agents such as thrombin may be
electrosprayed
with sucrose to form sugar droplets, which tends to stabilize thrombin and can
also "trap"
other substances of interest for delivery to the hemostatic product.
[0088] For thrombin and fibrinogen, in most embodiments, these (or other)
active
agents are in a finely dispersed dry, particulate or granular form e.g. as a
fine powder or dust,
19
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
as electrospinning may tend to decrease their activity. In other words, the
active agents are
not electrospun either by themselves.
[0089] The provision of the substances in the form of a fine powder
provides a large
surface area of contact for dissolution when the materials come into contact
with fluid.
Generally, such particles will have average diameters of between about 1 and
10,000
microns, and, in certain embodiments, between about 10 and 1,000 microns.
[0090] Such dry solid particles may be formed by any of several means,
including but
not limited to grinding, pulverizing, crushing, etc. However, those of skill
in the art will
recognize that other forms of these active agents may also be included in the
hemostatic
product, e.g. flakes, films, sheets, strings, etc. Further, in some
embodiments, thrombin and
fibrinogen are in the form of electrospun droplets when associated with an
excipient or
carrier.
[0091] Association of substances of interest with the excipient or carrier
may be
accomplished by any of many suitable techniques that are known to those of
skill in the art,
and will depend in part on the precise form of the substance and the means at
hand. For
example, for powdered, particulate thrombin and fibrinogen, association may be
carried out
by sprinkling, shaking, blowing, etc. the agents onto a layer of the excipient
or carrier.
[0092] Depending on the density of the fiber mat, the substances of
interest may
become relatively evenly dispersed throughout the woven mat of fibers or may
be largely
confmed to the topmost section of the fiber mat. If no backing is present, the
latter
embodiment is preferable, to prevent the particulate substance of interest
from falling through
and out of the mat.
[0093] The density of the fibrous mat can be adjusted (e.g. increased), for
example,
by adjusting its thickness and/or by compressing the mat under pressure so
that the fibers are
closer together. Other techniques for association also exist, e.g. the
placement of dry but
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
liquid soluble sheets or strips of material onto or between layers of a
carrier, electrospinning
the added materials as a discrete layer or in discrete layers, etc., and any
such technique may
be employed.
[0094] The techniques for assembling the hemostatic products of the
invention may
be carried out manually or may be mechanized, or a combination of manual
manipulation and
mechanization may be used. For thrombin in particular, 5,000 N1I-1 Units of
thrombin is a
relatively small volume of powder. Therefore, inert carriers or bulking agents
such as
dextrose may be added to insure more complete dispersal of active agents in
the hemostatic
product.
[0095] The association of substances of interest with the excipient may be
carried out
according to many different arrangements. For example, a first layer of
excipient may be
formed, and one or more of the substances may he associated with the first
layer. Then
another second layer of excipient may be folined on top of the substance(s) of
interest, and
the same or other substances of interest may be associated with the second
layer, and so on.
[0096] A final or outennost layer of excipient may be added to prevent the
dislodgement of substances of interest from the layer(s) below. The number of
layers of
excipient that are used in a hemostatic product of the invention may vary
widely, from as few
as 1-2 to as many as several dozen, or even several hundred, depending on the
desired
characteristics of the hemostatic product.
[0097] Typically, a hemostatic product will contain 1-2 layers. In other
embodiments
the hemostatic product may include between 2-20 layers. The very slight amount
of moisture
that is present in a prepared hemostatic product may help to trap and retain
the thrombin and
fibrinogen on the surface of the hemostatic product.
21
CA 02854294 2014-05-01
WO 2013/059341
PCT/US2012/060643
[0098] In some embodiments of the invention, the hemostatic products also
include
one or more support structures or support materials incorporated therein. For
example, a
backing may be incorporated into the hemostatic product.
[0099] The support material may be formed from various electrospun
materials such
as polyglycolic acid (PGA), polylactic acid (PLA), and their copolymers
(PLGAs); charged
nylon, etc. In one embodiment, the support material is compressed electrospun
dextran
fibers. By "compressed electrospun dextran fibers" we mean that electrospun
dextran fibers
are compressed together under pressure.
[0100] Compression of electrospun dextran fibers is carried out, for
example, under
pressure between two plates (e.g, a vice), and can compress a mat of fibers
with a height
(thickness) of about 3 inches to a sheet with a height of about 0.5 inches or
even less (e.g.
about 0.1 to about 0.4 inches). In some embodiments, the electrospun dextran
fibers are
electrospun directly onto a previously electrospun support material, while in
other
embodiments, the support material and the electrospun dextran fibers are
associated after
electrospirming of each, e.g. by joining of one or more layers of each.
[0101] In other embodiments, the support material is not an electrospun
material but
is some other (usually lightweight) material. Examples of such materials
include but are not
limited to gauze; various plastics; hydrogels and other absorbent materials
that can facilitate
absorption of blood and therefore clot formation; etc.
[0102] The support material may or may not be soluble in liquid, or may be
slowly
soluble in liquid, and may or may not be permeable to liquid. Slowly soluble
materials
include those from which absorbable or dissolving (biodegradable) stitches or
sutures are
farmed, included PGA, polylactic and caprolactone polymers.
[0103] Tn certain embodiments, the support material may dissolve relatively
quickly
such as less than about 1 hour. In other embodiments, the support material may
dissolve
22
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
within from about 10 days to 8 weeks. In either case, the support material
provides the
advantage of not having to remove the hemostatic product and risk disrupting
the clot.
[0104] However, in any case, the support material should not interfere with
the
immediate dissolution of the excipients and delivery of the active agents
associated therewith
into the liquid that dissolves the excipients. Thus, the support material
might be only on one
side of the electrospun dextran fiber device, so that when the device is, for
example, a
hemostatic product, and is applied to a wound, the hemostatic product is
oriented so that the
excipients come into direct contact with the blood in the wound bed and the
support material
does not, i.e. the support material is the "top" or outermost surface of the
hemostatic product
when placed on the wound.
[0105] This embodiment is illustrated, for example, in FIG. 4A, in which
electrospun
dextran fibers 10 are shown as deposited onto non-porous, liquid impermeable
support
material 20. When applied to a wound, excipient 10 would face downward into
the wound,
and non-porous support material 20 would face away from the wound.
[0106] This arrangement could provide an advantage in that pressure could
be applied
to the wound through the support material, to facilitate the stoppage of
bleeding.
Alternatively, the support material may contain pores, openings or spaces that
allow liquid to
access the excipients in the hemostatic product even when the support material
is present.
For example, the support material may be a net or web of material that is
insoluble (or slowly
soluble) but that permits liquid to freely access the excipients and
associated substances of
interest.
[0107] This embodiment is illustrated schematically in FIG. 4B, which shows
electrospun dextran fibers 10 deposited on (or possibly under, or on and
under, or woven
throughout) netting 40, which is shown partially in phantom where covered by
electrospun
23
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
dextran fibers 10. In yet other embodiments, both a "backing" or "top" support
material and a
second web-like support material may be present in the devise.
[0108] This embodiment is illustrated schematically in FIG. 4C, which shows
electrospun dextran fibers 10 deposited on non-porous support material 50 and
overlaid with
net-like material 60, i.e. electrospun dextran fibers 10 are "sandwiched-
between non-porous
support material 50 and net-like material 60.
[0109] One of skill in the art will be able to envision many other
combinations and
shapes of excipient layers and support materials that would provide advantages
in particular
scenarios. For example, excipients might be wrapped or wound around an
elongated support
such as a filament or string, or wrapped around a particular form with the
shape of a cavity in
which the hemostatic product is likely to be placed, such as a bullet hole,
etc.
[0110] In one such embodiment, the fibrinogen is provided in a powder and
that
powder is dispensed on the surface of one of the layers of the hemostatic
product. In other
embodiments, the fibrinogen is mixed with thrombin and then the fibrinogen-
thrombin
mixture is spread on one of the layers in the hemostatic product. The
fibrinogen may be
provided on the surface of each hemostatic product at a concentration of up to
about 10 grams
per hemostatic product.
[0111] In another embodiment of the invention that is directed to
fabricating the
hemostatic product, dextran is mixed with water until a substantially
homogeneous mixture is
prepared. The dextran may be provided in a powder having a relatively fine
particle
granulation. The dextran and water may be selected to have a relatively high
purity such as is
typically used in medical applications. An example of one such suitable water
is distilled
water.
[0112] In one such configuration, there are between about 3 grams and about
9 grams
of dextran with about 6 milliliters of water. In other embodiments, there are
about 6 grams of
24
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
dextran is mixed with about 6 milliliters of water. A person of skill in the
art will appreciate
that a variety of techniques may be used to mix together the dextran and water
to produce the
substantially homogeneous mixture from dextran and water. A non-limiting
example of a
technique that may be used to mix together the dextran and water is
electrospinning.
[0113] The duration of mixing that is needed to prepare the substantially
homogeneous mixture of dextran and water may depend on a variety of factors
such as the
type of equipment that is used to perform the mixing. In certain
configurations, this mixing is
performed for greater than about 30 minutes. The mixing may be performed at a
temperature
of between about 40 F and about 70 F.
[0114] Next, thrombin is mixed with the dcxtran-water mixture. The thrombin
is
added to the dextran-water mixture to provide the hemostatic product therefrom
with a
concentration of thrombin that is between about 20 and 6,000 NIH Units.
[0115] Thrombin may be provided in a powder having a relatively fine
particle
granulation. The thrombin may be selected to have a relatively high purity
such as is
typically used in medical applications.
[0116] Similar to the process used to prepare the dextran-water mixture,
electrospinning may be used when mixing the thrombin with the dextran-water
mixture. The
mixing of the thrombin with the dextran-water mixture may be performed at a
temperature of
between about 40 F and about 70 F.
[0117] The duration of mixing that is needed to prepare the substantially
homogeneous mixture of thrombin, dextran and water may depend on a variety of
factors
such as the type of equipment that is used to perform the mixing. In certain
configurations,
the mixing is performed for between about 10 and 30 minutes. In other
configurations, the
mixing is performed for about 1 hour.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0118] Once the mixing is completed, the mixture is introduced into an
electrospinning machine. The electrospinning machine is configured to produce
a sheet
having a width of up to about 1 meter. However, shorter widths may also be
used depending
on the desired dimensions for the hemostatic product.
[0119] A release sheet is placed beneath the electrospinning machine onto
which the
fibers are placed. The release sheet provides support for the fibers during
the processing and
cutting. The release sheet should be selected to not interact with the
components being used
in the fabricating the hemostatic product. The release sheet may be configured
to be
separated from the product after the cutting and/or other processing is
completed.
[0120] Because of factors such as challenges associated with incorporating
the
fibrinogen with the other components utilized in forming the hemostatic
product, the
fibrinogen may be provided on a surface of one of the layers in the hemostatic
product as
opposed to being incorporated into one or more of the layers.
[0121] The crux of the problem at the site of a penetrating injury is that
the wounded
tissue is relatively inaccessible. For example, for a bullet wound (e.g. in
the leg or thigh)
bleeding does not occur as much at the surface but deeper within the tissue,
within a cavity
formed by the bullet, where it cannot be easily treated by a hemostatic
product that is simply
spread over the external site of the injury (e.g. the point of entry of the
bullet, knife, shrapnel,
sword, bayonet, etc., or other cause of injury).
[0122] This aspect of the invention solves the problems associated with
penetrating
injuries, which can cause extensive bleeding in the deep tissues, and takes
advantage of the
highly soluble nature of the dextran hemostatic product. A complicating factor
in this type of
injury concerns the ability to deliver hemostatic materials that are highly
soluble to such a
site.
26
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0123] There may be bleeding and other fluids evident at the entry site of
the wound
and the application of a hemostatic product to this superficial site may
result in the complete
dissolution of the hemostatic product at the surface-without the delivery of
the active
materials to the underlying source of the bleeding within the wound cavity.
The invention
circumvents this occurrence by providing delivery of active agents deep into
the wound. Prior
art hemostatic products have failed to adequately address this problem.
[0124] The present invention solves this problem by providing a hemostatic
product,
the shape and application of that can be adapted to use with such wounds. For
example, an
elongated cylindrical "cigar-shaped" hemostatic product that contains thrombin
and
fibrinogen, and which may contain support material, is provided.
[0125] The hemostatic product may be stored within a protective covering or
packaging or tube. This tube protects the hemostatic product from the ambient
environment.
Both the hemostatic product and the tube are preferably sterile. These
components may be
further enclosed in an outer wrapper of e.g. paper, polymer, blister pack,
similar to that used
for disposable syringes, to prevent loss of sterility.
[0126] When used, the outer wrapping is torn open and the sterile tube
containing the
hemostatic product is accessed. In some embodiments, one end of the tube is
removed and
placed over the outeiniost accessible portion of the injury. The tube may also
comprise a
"plunger" or similar means which enables the user to expel the hemostatic
product from the
tube and into the wound, in effect "injecting" the hemostatic product into the
wound.
[0127] Means such as those that are used for the vaginal delivery of, for
example,
tampons, (i.e. a "cylinder within a cylinder") may be employed, or a syringe-
like means of
delivery may be used. The hemostatic product can thereby be introduced deep
into the tissue
along the wound track and the therapeutic agents in the hemostatic product are
delivered to
where they are most needed, i.e. to the interior of the wound.
27
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0128] In other embodiments, a plunger per se is not included, but the tube
is
fashioned so that both ends can be opened, and the hemostatic can be pushed
into the wound
from one open end by exerting pressure on the opposite open end of the tube
using any object
that fits at least partially into the tube, sufficiently to push the
hemostatic product out of the
tube and into the wound. Examples of such objects include a finger and a
stick.
[0129] Such an object may be included with the hemostatic product of the
invention.
Those of skill in the art will recognize that, due to the relatively high
malleability of some
configurations, this embodiment of the hemostatic product may include support
material
around or within the hemostatic product (e.g. biologically compatible netting,
rod, etc. that
will disintegrate via biodegradation) to render the hemostatic product more
robust and less
flexible as it is shunted down into the wound.
[0130] Further, the outeimost end of the hemostatic product, that end on
which
pressure is exerted (e.g. with a plunger) to expel the hemostatic product from
the tube into the
wound, may be reinforced with support material so that the plunger or other
object used to
push on the hemostatic product can deliver sufficient force to remove the
hemostatic product
from the tube.
[0131] An exemplary schematic depiction of this embodiment of the invention
is
provided in FIG. 4D, where hemostatic product 100, comprised of spun dextran
fibers 110
and (optional) support material 120, and having a first end 130 and second end
140 is
illustrated as enclosed within tube 200.
[0132] Hemostatic product 100 is enclosed within tube 200 but is not shown
in
phantom for the sake of clarity. Tube 200 has openings 210 and 220, both of
which may be
capped prior to use (caps not shown) or may be left open, especially if the
entire apparatus is
packaged in sterile packaging 400. Sterile packaging 400 is removed or
breached to provide
28
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
access the apparatus prior to use. To use the apparatus, openings 210 and 220
of the tube
must be open.
[0133] To deliver hemostatic product 10 to a penetrating wound, an object
such as
plunger 300 in inserted into end 210 of the tube. Pressure is exerted on
hemostatic product
100 as plunger 300 contacts device end 130, and hemostatic product 100 is
consequently
pushed out of tube 200 via opening 220 (in the direction indicated by the
arrows) and into the
penetrating wound (not shown). A second schematic representation of such a
hemostatic
product is provided in FIG. 4E.
[0134] In this depiction, support material is not included and the dry,
sterile
hemostatic product material (e.g. dextran fibers) with associated therapeutic
agents is located
or positioned within a small, sealed cylinder with a cap at one end and a
plunger at the other.
Upon deployment, the cap is discarded, the open end of the cylinder is placed
over the mouth
of the wound and may be inserted into the wound, and the plunger is depressed,
displacing or
injecting the hemostatic product material deeply into the wound.
[0135] Similar designs may be used to deliver the hemostatic product to
orifices or
channels such as the nasal passages, the ear canal, the vagina, the anus, into
blood vessels,
etc. The components that are used in such an application will be formed into a
shape that is
on the order of about 1 to about 6 inches in length, and from about IA inch to
1 inch in
diameter, i.e. the dimensions of the hemostatic product will be suitable for
insertion through
the external opening and deep into an orifice or a wound cavity.
[0136] All such arrangements, shapes, and embodiments of carrier layers and
support
materials as described herein are intended to be encompassed by the invention.
[0137] The hemostatic product may be sterilized prior to use, generally by
using
electromagnetic radiation, for example, X-rays, gamma rays, ultraviolet light,
etc. If thrombin
is included in the hemostatic product, it may be desirable to reduce the
moisture content of
29
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
the hemostatic product (e.g. a bandage or gauze) to less than about 5%, to
preserve thrombin
activity during sterilization.
[0138] This
moisture content reduction can be achieved by drying the fabricated
hemostatic product, e.g., under a vacuum, or by using a fabrication method
that reduces
moisture content from the beginning. Typically, the hemostatic products are
sterilized using
X-rays in a dose of between about 5 and 25 kilograys (kGray). Any method that
does not
destroy the carrier or the activity of substances associated with the fibers
may be used to
sterilize the hemostatic products of the invention.
[0139] When
the hemostatic product is a bandage, the substances of interest that are
associated with the fibers of the hemostatic product may include thrombin and
fibrinogen,
and the hemostatic product may be used to staunch bleeding. However, the range
of active
ingredients may vary with the specific application of the hemostatic product.
[0140] For
example, hemostatic products comprised of only thrombin might be used
_ for small injuries or in combination with other interventions. In
addition, other
therapeutically beneficial substances may also be associated with the
hemostatic product,
including but not limited to: antibiotics, medicaments that alleviate pain,
growth factors, bone
morphogenic protein, vasoactive materials (e.g. substances that cause
vasospasms), steroids
to reduce inflammation and combinations thereof
[0141] In
other embodiments, the devices of the invention do not contain agents that
promote clotting. Those of skill in the art will recognize that the devices of
the invention are
highly suitable for delivering many substances of interest to a desired liquid
environment or
location. For example, the devices may be designed for delivery of therapeutic
or beneficial
substances to any moist environment of the body, where there is sufficient
liquid to dissolve
the electrospun dextran fibers and release the active substance, and where
dissolved dextran
is not problematic.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0142] Examples include but are not limited to oral, nasal, tracheal, anal,
lung, and
vaginal delivery of substances such as anti-microbial agents, analgesic
agents, nutritional
agents, etc. Oral applications include the delivery of substances useful for
dental treatments,
e.g. antibiotics, pain medications, whitening agents, etc. Examples of
therapeutic or
beneficial substances that can be used in conjunction with the devices include
antibiotics,
medicaments that alleviate pain, growth factors, bone morphogenic proteins,
vasoactive
materials (e.g. substances that cause vasospasms), inflammation reduction
steroids and
combinations thereof.
[0143] However, in some embodiments, no bodily fluid is present (or if
insufficient
body fluid is present) and the applied hemostatic product can be "activated"
by wetting, e.g.
by spraying, or by otherwise applying a source of moisture (e.g. by exposing
the hemostatic
product to a moist material such as a sponge), or dropping hemostatic products
into a liquid
(e.g. a body of water), to cause release of the agents of interest associated
with the dextran
fibers.
[0144] Due to the small footprint and light-weight characteristics of the
hemostatic
products, they are ideal for situations where space and weight of supplies are
at a premium.
Examples of such situations include but arc not limited to: military
operations where the
weight and size of the components of a soldier's gear are an issue; in first
aid kits; for
emergency care during travel (e.g. during space flight, camping, etc.); etc.
[0145] The hemostatic products may be used in a variety of situations and
for a
variety of purposes in which space and weight are not considerations. For
example, the
hemostatic products of the invention provide a convenient means to administer
thrombin and
fibrinogen to surgical wounds in a conventional operating theater.
[0146] The hemostatic products of the invention may also be advantageously
utilized
whenever it is desired to package and eventually release one or more dried
substances, but
31
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
where it is unfeasible or undesirable to handle the dried substances directly,
e.g. where the
quantity is extremely small, or the substance is toxic.
[0147] In such cases, the electrospun dextran fibers of the invention may
serve as a
"scaffolding" or carrier for containing, storing and/or transporting the
substance(s) until use,
i.e. until contacted with liquid that dissolves the electrospun dextran
fibers, concomitantly
releasing the substances into the liquid. Such substances may include, for
example, enzymes
or their precursors (e.g. pro-enzymes or zymogens) and their substrates,
substances that
activate a protein or enzyme (e.g. proteases, cofactors, etc.), and the like.
[0148] The invention also relates to the use of stabilizers that resist the
premature
degradation of the components utilized in the hemostatic product. The
stabilizer also
enhances the usable shelf life of the hemostatic product. In certain
embodiments, the
stabilizer provides the hemostatic product with a shelf life of at least about
2 years. In other
embodiments, the hemostatic product exhibits a shelf life of at least 3 years.
[0149] As used herein, the term usable shelf life means that the hemostatic
product
does not exhibit noticeable degradation when viewed without magnification or
with
magnification such as a magnifying glass or microscope.
[0150] One such stabilizer is adapted for use in conjunction with thrombin.
It is
believed that the thrombin stabilizer gets into the structure of the thrombin
and thereby
reduces the rate at which the thrombin breaks down. The at least one thrombin
stabilizer may
be mixed with the thrombin before the thrombin is mixed with the other
components used to
fabricate the hemostatic product.
[0151] In one embodiment, the thrombin stabilizer contains a sugar such as
sucrose.
In certain embodiments, the sucrose is used in the thrombin stabilizer at a
concentration of up
to about 5 percent by weight of the thrombin. In other embodiments, the
sucrose
concentration is about 1 percent by weight of the thrombin.
32
[0152] Prior to mixing the thrombin stabilizer with the thrombin, the
thrombin stabilizer
may be mixed with dextran. It is believed that the dextran enhances the
ability of the sucrose to
enter into the structure of the thrombin.
[0153] In certain embodiments, the dextran is used in the thrombin
stabilizer at a
concentration of up to about 5 percent by weight of the thrombin. In other
embodiments, the
dextran concentration is about 1 percent by weight of the thrombin.
[0154] Similarly, a stabilizer may be used in conjunction with the
fibrinogen. Prior to
applying the fibrinogen to the other components of the hemostatic bandage, the
fibrinogen
stabilizer may be mixed with the fibrinogen. It is believed that the
fibrinogen stabilizer gets
into the structure of the fibrinogen and thereby reduces the rate at which the
fibrinogen
breaks down.
[0155] In one embodiment, the fibrinogen stabilizer contains a sugar
such as sucrose.
In certain embodiments, the sucrose is used in the fibrinogen stabilizer at a
concentration of up
to about 5 percent by weight of the fibrinogen. In other embodiments, the
sucrose
concentration is between about 2 and 3 percent by weight of the fibrinogen. In
still other
embodiments, the sucrose concentration is about 1 percent by weight of the
fibrinogen.
[0156] Prior to mixing the fibrinogen stabilizer with the fibrinogen,
the fibrinogen
stabilizer may be mixed with a solubility enhancing agent. It is believed that
the solubility
enhancing agent enhances the ability of the sucrose to enter into the
structure of the
fibrinogen. In certain embodiments, the solubility enhancing agent is a
detergent. In other
embodiments, the solubility enhancing agent is PluronicTm.
[0157] In certain embodiments, the solubility enhancing agent is used in
the fibrinogen
stabilizer at a concentration of up to about 1 percent by weight of the
fibrinogen. In other
embodiments, the solubility enhancing agent concentration is about 0.002
percent by weight of
the fibrinogen.
33
CA 2854294 2018-01-30
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0158] In another embodiment of the invention, the fibrinogen and thrombin
are
placed on the surface of and/or integrated into the matrix of a dissolving
film. Using the
fibrinogen and thrombin in such a configuration enables the hemostatic product
to be
positioned over the position on the person's body where the blood is being
emitted and, as
such, where hemostasis is desired.
[0159] The dissolvable film may be configured to dissolve relatively
quickly when
exposed to liquid such as blood. In certain embodiments, the film dissolves in
less than about
30 seconds. In other embodiments, the film dissolves in less than about 5
seconds. An
example of one suitable dissolving film is marketed by Hughes Medical Corp.
[0160] An example of another dissolving film is a dissolving paper that is
fabricated
from materials that do not pose a health hazard to the patient after the
dissolving paper has
dissolved. In certain embodiments, the dissolving paper may be fabricated from
a material
that enhances the ability of at least one of the fibrinogen and thrombin to
achieve hemostasis.
An example of one such dissolving paper is marketed by Daymark Technologies.
[0161] In another embodiment, the fibrinogen and thrombin are provided
between
two layers of a dissolvable material. An example of one such suitable
dissolving film is
marketed by Hughes Medical Corp. and which is discussed above.
[0162] The fibrinogen and thrombin may be provided in a variety of
configurations
using the concepts of the invention. In one such configuration, at least one
of the fibrinogen
and the thrombin are provided in a powder that is retained between the layers
of the
dissolvable material.
[0163] The dissolvable material should have sufficient structural integrity
to retain the
fibrinogen and thrombin therebetween while resisting interaction with the
fibrinogen and
thrombin. The dissolvable material should also dissolve relatively quickly
when exposed to
liquids such as blood such that the fibrinogen and thrombin are released
therefrom.
34
CA 02854294 2014-05-01
WO 2013/059341
PCT/US2012/060643
[0164] As used herein, "quickly dissolving" means that the dissolvable
material
breaks down to a sufficient extent such that a significant portion of the
fibrinogen and
thrombin are in contact with the blood in less than about 30 seconds. In other
embodiments,
the dissolvable material breaks down in less than about 10 seconds.
[0165] The dissolvable material should also facilitate readily bonding such
that two
layers of the dissolvable material can be attached together around the edges
thereof to thereby
form an enclosure that is adapted to retain the fibrinogen and thrombin
therein.
[0166] An example of one suitable technique for attaching the dissolvable
materials
to each other is applying a small amount of liquid to at least one of the
pieces of material that
are intended to be bonded together. The water causes a slight breakdown of the
dissolvable
materials such that when two layers of the dissolvable material are placed
adjacent to each
other, the layers of the dissolvable material bond together.
[0167] The dissolvable material may be fabricated from a variety of
materials. The
dissolvable material should not negatively impact the stability of the
fibrinogen and
thrombin. The material used to fabricate the dissolvable layer should also be
selected to not
have any adverse health effects on the person or animal on which the product
is intended to
be used.
[0168] In certain embodiments, the material used to fabricate the
hemostatic product
may alone or in conjunction with the fibrinogen or thrombin enhance the rate
of hemostasis.
Examples of components that may be used for the dissolvable material include
cellulose
derived materials.
[0169] In one configuration, a separate device is used to maintain the
hemostatic
product in a desired position with respect to the patient. An example of one
such device is
gauze that is wrapped around the portion of the body that is bleeding.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0170] In another configuration, at least a portion of the hemostatic
product is
covered with an adhesive. The adhesive may be positioned around at least a
portion of an
edge of the hemostatic product. In an alternative configuration, the adhesive
covers a
substantial portion of an inner surface of the hemostatic product.
[01711 In such a configuration where the adhesive is likely to come into
contact with
the portion of the patient's body that is bleeding, the adhesive should be
selected to be
biocompatible to minimize the potential of the patient experiencing
complications caused by
contact between the adhesive and the tissue that is bleeding.
[0172] In another embodiment, the fibrinogen and thrombin may be placed
inside of
an enclosure and/or on the surface of an enclosure that does not dissolve when
exposed to
liquids such as blood. Such a configuration may facilitate forming a blood
clot thereon such
that the blood clot could be removed from the patient.
[0173] Such a configuration may be similar to a conventional tea bag in
that the
enclosure may be attached to a string that is used in conjunction with at
least one of
positioning the product proximate an area where hemostasis is desired or
removing the
product and the associated blood clot from the patient.
[0174] The string may be fabricated from a material that is sufficiently
strong such
that the string does not break either when positioning the product or when
removing the
product from the patient. The string should also be fabricated from a material
that is not
likely to produce adverse biological interactions.
[0175] The enclosure may be configured to discharge the fibrinogen or
thrombin over
a selected period of time. The rate at which the fibrinogen and thrombin are
discharged may
be adjustable based upon the rate and/or volume of blood that is being
discharged from the
patient.
36
CA 02854294 2014-05-01
WO 2013/059341
PCT/US2012/060643
[0176] In an alternative configuration, the enclosure may be configured to
breakdown
over an extended period of time. As the enclosure breaks down, the fibrinogen
and thrombin
may be discharged from the product.
[0177] By controlling the rate at which the fibrinogen and thrombin arc
discharged
from the product and/or the rate at which the enclosure degrades, the product
minimizes the
folmation of a clot having a relatively large size but rather may facilitate
the formation of a
plurality of clots having a smaller size. Such smaller clots may be more
readily broken down
within the body than if relatively large clots were caused to be formed.
[0178] This configuration of the hemostasis product may be particularly
suited for use
in conjunction with bleeding in patients that while being within a bodily
cavity such a bodily
cavity is accessible from outside of the body. Examples of surgical techniques
with which
the hemostasis product may be used in conjunction include sinus and tonsil
surgery.
[0179] In another embodiment, the fibrinogen and thrombin are compressed
into a
tablet. In addition to the fibrinogen and thrombin, the tablet may also
include at least one
excipient. The excipient should facilitate not only holding together the
fibrinogen and
thrombin as well as promoting relatively quickly dissolving of the tablet.
[0180] As used herein, the term "relatively quickly" means that the tablets
dissolve
when placed in a liquid in less than about 30 seconds. In other
configurations, the tablets
dissolve in less than about 10 seconds. Quickly dissolving the tablets enables
the fibrinogen
and the thrombin to be quickly released from the tablets such that these
materials may
provide rapid hemostasis.
[0181] Additionally, in certain embodiments, the excipients that are used
in
formulating the tablets should not decrease the stability and/or solubility of
the fibrinogen
and the thrombin. In certain embodiments, the excipients used in formulating
the tablets
should increase the stability of the fibrinogen and thrombin.
37
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0182] An example of one such excipient is sorbitol, which has been foimed
into
small particles such as by using spray-drying. In one such configuration, the
particles have a
generally spherical shape and have a generally uniform size.
[0183] The spray-dried sorbitol particles not only provide advantageous
fiowability
characteristics but also exhibit desirable compactability characteristics when
forming the
tablets using a direct compression technique.
[0184] Additionally, the spray-dried sorbitol particles provide good
solubility for
release of the fibrinogen and thrombin from the tablets. An example of one
such spray-dried
sorbitol particle is marketed by SPI Pharma under the designation SORBITA_13
SD 250.
[0185] Another excipient that may be used in fabricating the tablets is
mannitol,
which has been formed into small particles such as be using spray drying. The
particles may
be formed with a narrow particle size distribution, which reduces the
potential of the
components segregating while the tablets are being formed.
[0186] An advantage of the mannitol is that this material is non-
hydroscopic such that
the mannitol does not add moisture to the other components used in the tablets
or contribute
to moisture pickup either during the process of forming the tablets or after
the tablets have
been Ruined. The mannitol thereby protects the water-sensitive fibrinogen and
thrombin.
[0187] The spray-dried mannitol particles not only provide advantageous
flowability
characteristics but also exhibit desirable compactability characteristics when
forming the
tablets using a direct compression technique.
[0188] The spray-dried mannitol particles also promote rapid
disintegration or
dissolvability of the tablets such that the fibrinogen and thrombin can be
quickly released
from the tablets. An example of one such spray-dried mannitol particle is
marketed by SPI
Pharma under the designation MANNOGEM EZ.
38
CA 02854294 2014-05-01
WO 2013/059341
PCT/US2012/060643
[0189] Other materials that may be used as excipients when preparing the
tablets
include fructose and maltose. Similar to the other excipients that are
discussed above, the
preceding excipients may be foimed into small particles before being used
mixed with the
other components that are used in the tablets.
[0190] Another excipient that may be used in conjunction with fibrinogen
and
thrombin is a quick dissolving platform that is marketed under the designation
PHARMABURST 500 by SPI Pharma. This material provides the tablets with the
ability to
be rapidly dissolved while also providing desirable characteristics for
compaction and
friability.
[0191] Depending on the excipient that is used in the tablet, it may also
be desirable
to use a lubricant when preparing thc tablet. The lubricant may enhance the
physical
properties of the tablets. Examples of such physical properties include
brittleness, friability
and hardness. An example of one such lubricant is sodium stearyl fumarate,
which is
available from SPI Pharma under the designation LUBRIPHARM.
[0192] The concentration of the lubricant that is used in fabricating the
tablets may
depend on a variety of factors such as the types of excipients that are used.
In certain
embodiments, the concentration of the lubricant is up to about 5 percent by
weight. In other
embodiments, the concentration of the lubricant is between about 2 and 3
percent by weight.
In still other embodiments, the concentration of the lubricant is about 2.5
percent by weight.
[0193] Once the components are mixed together, the mixture is subjected to
compression, which thereby causes the components to faun the tablets. In
certain
embodiments, the compressive force is at least 5,000 psi. In other
embodiments, the
compressive force is between about 10,000 psi and about 12,000 psi.
39
[0194] When preparing the tablets using the preceding process, it may not
be necessary
to include dextran. Even though dextran may not be required, it is possible to
use
dextran along with the other components that are used to formulate the
tablets.
[0195] In another embodiment of the invention, the fibrinogen and
thrombin may be
incorporated into a fast dissolving tablet such as by using technology
marketed by Catalent
Corporation under the designation Zydis TM.
[0196] The fast dissolving tablets dissolve in less than 30 seconds and,
in some
configurations, dissolve in less than about 5 seconds. Quickly dissolving the
tablets is important
because at the tablets dissolve, the fibrinogen and thrombin contained therein
is released and
can thereby produce hemostasis.
[0197] The amount of the fibrinogen and thrombin used in the tablet may
be selected
based upon the volume of bleeding. In certain embodiments, there is up to
about 1 gram of
fibrinogen and thrombin in each of the tablets. In other embodiments, there is
about 500
micrograms of fibrinogen and thrombin in each of the tablets.
[0198] In another embodiment of the invention, the fibrinogen and
thrombin are applied
to a surface of or incorporated into an applicator. Such an applicator enables
the fibrinogen and
thrombin to be accurately delivered to an area where hemostasis is desired.
[0199] In one such configuration, the applicator has an elongated portion
that may be
grasped by a person who is using the hemostatic product. The applicator may
have a
configuration that is similar to a swab. This configuration of the hemostatic
product is
particularly suited for locations that are difficult to directly reach. An
example of one such
condition that this hemostatic product may be used to treat is epistaxis.
[0200] At least one of the fibrinogen and thrombin may be electrospun
either alone or
with another component such as dextran. The fibers produced using such a
process may be
wrapped around a distal end of the applicator.
CA 2854294 2018-01-30
[0201] The applicator may be configured to release the fibrinogen and
thrombin once
the hemostatic product encounters blood. Using such a process, the fibrinogen
and thrombin
would cause clots to form. The clots could be removed from the patient. If the
clots are
sufficiently small, the clots may be allowed to remain in the patient such
that the clots could
eventually be broken down.
[0202] In another configuration of this hemostatic product, at least one
of the
fibrinogen and thrombin may be configured to remain relatively close to or be
confined to the
applicator such that when the fibrinogen and thrombin cause at least one clot
to form, such
clots remain attached to the applicator. This configuration facilitates
removal of the clots from
the patient and may be desirable where the clots are likely to be sufficiently
large to make it
undesirable for the clots to remain in the body.
[0203] To facilitate the fibrinogen and thrombin not being released from
the applicator,
the fibrinogen and thrombin may be incorporated into a material that is
attached to an end of the
applicator. An example of one such material is foam. The foam may be either
open cell foam or
closed cell foam. The foam should have pores that are sufficiently large to
receive the
fibrinogen and thrombin. The foam should not have a strong affinity for either
fibrinogen or
thrombin so that when the fibrinogen and thrombin are exposed to water, these
components are
released from the foam.
[0204] In another embodiment of the invention, the fibrinogen and
thrombin are
incorporated into foam. An example of one such suitable foam is an absorbable
gelatin sponge
such as is available under the designation VETSPONTm from Novartis.
[0205] Depending on the application at which it is desired to use the
hemostatic sponge,
it may be desirable to pre-wet the hemostatic sponge prior to the hemostatic
sponge being
applied to the region where hemostasis is desired.
41
CA 2854294 2018-01-30
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0206] Another advantage of using the foam is that the foam may be
configured to be
bendable so that the hemostatic foam can be bent into a configuration that
conforms to the
shape of the region in which the hemostasis is desired. Once the hemostatic
foam is bent into
the desired configuration, it may remain in that configuration even without a
fastening device
being used to hold the hemostatic tham in the desired shape and/or position.
[0207] Similar to the foam that is described above, the foam may be either
open cell
foam or closed cell foam. The foam should not have a strong affinity for
either fibrinogen or
thrombin so that when the fibrinogen and thrombin are exposed to water, these
components
are released from the foam.
[0208] The fibrinogen and thrombin may be incorporated into the components
that
are used to fabricate the foam such that rather than the fibrinogen and
thrombin being applied
to a surface of the foam, the fibrinogen and thrombin are dispersed through
the matrix of the
foam.
[0209] Such a configuration facilitates ongoing release of the fibrinogen
and thrombin
from the foam and may be particularly beneficial when it is desired to form a
clot in a region
of the body that is likely to experience rebleeding.
[0210] The quick dissolving tablets are suited for use in a variety of
applications. An
example of one such application is oral bleeding. If the product is intended
for use in
conjunction with oral bleeding, the tablet may be flavored.
[0211] In conjunction with various surgical techniques, it is necessary to
foi n an
incision in the patient. Once the surgery is complete, it is necessary for
sutures or staples to
be used to close up the incision. While the sutures or staples are effective
at holding together
the tissue, these closure mechanisms are not always effective to stop blood
from flowing out
through the incision.
42
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0212] The hemostatic product may be placed over at least a portion of the
suture line
through which blood is passing. The fibrinogen and thrombin contact such blood
and thereby
provide hemostasis.
[0213] Vascular access devices are used in conjunction with a variety of
medical
treatments such as delivering chemotherapy drugs to a patient. In one
configuration, the
vascular access devices arc surgically implanted in a large vein that is near
the patient's heart.
The vascular access devices may be left in place for an extended period of
time such as more
than a year.
[0214] One challenge of surgically implanting the vascular access devices
is stopping
the bleeding around the vascular access device. For example, the blood may
leak out of the
suture lines or around the conduit. In some situations where it is not
possible to stop the leak,
it is necessary to bring the patient back to the operating room in an effort
to stop the leak.
[0215] The hemostatic product may be placed around the vascular access
device to
thereby cause hemostasis. Depending on the shape of the vascular access
device, it is
possible for the hemostatic product to have a variety of configurations.
[0216] In other situations, the conduit associated with the vascular access
device may
be porous. The hemostatic product may be used to provide hemostasis and
thereby prevent
blood from passing through the porous conduit.
[0217] One or more of the preceding configurations of the hemostatic
product may be
suited for use to stop bleeding from a patient such as when a catheter is
removed from a
femoral artery in the patient. In such a configuration, the hemostatic product
may include an
adhesive, which holds the hemostatic product in place while hemostasis is
occurring.
[0218] Aortic root surgery is used to treat a dilation or enlargement of
the aorta.
Because of the nature of the aorta, hemostasis plays an important role in the
success of the
procedure. One of the configurations of the hemostatic product that are
discussed above may
43
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
be used to provide the hemostasis without causing constriction of the aorta,
which frequently
results from the prior art agents that are used to provide hernostasis in
conjunction with this
type of surgery.
[0219] For wounds that are deeper into the patient, it may be desirable to
use the
hemostatic product in the form of a pledget that is placed at least partially
into the wound and
which thereby causes hemostasis within the wound.
[0220] In yet another configuration, the hemostatic product may be
provided in an
elongated configuration such as a tampon-type shape. The hemostatic product
could also be
folioed in the shape of a worm or rope. Such configurations could be used for
hemostasis
after vaginal surgeries. These configurations could also be used in
conjunction with an
elongated hole in the patient such as may be caused by a gunshot wound.
[0221] The cylindrical configuration of the hemostatic product is also
suitable for use
in situations where there is a large opening in tissue. In such situations,
multiple cylindrical
hemostatic products may be inserted into the large opening to thereby fill a
portion of such
opening.
[0222] The facilitate administering the elongated hemostatic product, it
may be
desirable for the hemostatic product to be stored in an applicator such as a
plunger. The
plunger could be used to insert the elongated hemostatic product into the
wound in the
patient.
[0223] In another configuration of the hemostatic product, each of the
components of
the hemostatic product are package separately such as in a syringe. Such a
configuration
enables the components to be dispensed at different rates so that the
hemostasis may be
customized to the particular patient or for a particular type of wound. The
applicator may
allow each of the components to be delivered alternatively or simultaneously.
44
CA 02854294 2014-05-01
WO 2013/059341
PCT/US2012/060643
[0224] The hemostatic product may consist substantially of the fibrinogen,
thrombin
and a carrier that are adapted to be bioabsorbed. Alternatively, the
fibrinogen and thrombin
may be placed on an outer surface of the hemostatic product such that the
hemostatic product
would be removed from within the patient after hemostasis is completed.
[0225] In yet another configuration, the fibrinogen and thrombin are
delivered via an
aerosol. The fibrinogen and thrombin may be provide in the form of
mierospheres that are
capable of being dispensed using an aerosol container.
[0226] It is also possible to use the hemostatic product in conjunction
with robotic
surgical procedures. While robotics provide the ability for surgical
procedures to be
performed by a surgeon who is at a location that is remote to where the
patient is located, the
robotics have certain limitations. The hemostatic product may be used in
conjunction with
the robotic surgical procedures to provide hemostasis and thereby overcome
such limitations.
[0227] In addition to being used to produce hemostasis in humans, the
concepts of the
invention may be adapted for use in conjunction with other animals. Examples
of such
animals on which the invention can be used include dogs and cats.
[0228] In another embodiment of the invention, an effective amount of water
is mixed
with dextran to foini an aqueous dextran solution. Thereafter, the aqueous
dextran solution is
electrospun to faun a dextran sheet.
[0229] The dextran sheet may be stored until it is desired to fabricate the
hemostatic
product. In one such configuration, the dextran sheet is rolled. Rolling of
the dextran sheet
not only reduces the area taken up by the dextran sheet while the dextran
sheet is being stored
but also reduces the potential that the dextran sheet will be damaged prior to
fabricating the
hemostatic product.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0230] When the dextran sheet is being rolled, care should be exercised to
not roll the
dextran sheet too tightly because such a process would increase the density of
the dextran
sheet. Alternatively, tightly rolling the dextran sheet may be desired to
increase the density
of the dextran sheet prior to fabricating the hemostatic product.
[0231] The thrombin and fibrinogen are mixed together at the ratio
discussed in the
other portions of this patent application just before it is desired to
fabricate the hemostatic
product. The mixing should provide a relatively uniform dispersion of the
thrombin and
fibrinogen in the mixture.
[0232] In certain embodiments, the thrombin is dispersed on the dextran
sheet to
provide a thrombin concentration of between about 2 and 200 N1H Units of
thrombin per
square centimeter of the dextran sheet.
[0233] In certain embodiments, the fibrinogen is dispersed on the dextran
sheet to
provide a fibrinogen concentration of between about 20 and 60 grams of
fibrinogen per
square centimeter of the dextran sheet.
[0234] The dextran sheet is unrolled and the thrombin and fibrinogen
mixture is
dispersed over the surface of the dextran sheet. In certain embodiments, the
thrombin and
fibrinogen mixture is dispersed in a substantially uniform manner over the
surface of the
dextran sheet. This even dispersion is desired because it enables each portion
of the
hemostatic product to have a substantially hemostatic activity.
[0235] This process is repeated and the sheets are stacked until the
hemostatic product
exhibits a desired amount of hemostatic activity. In certain embodiments, the
hemostatic
product includes between about 2 and 20 dextran layers.
[0236] The thrombin and fibrinogen mixture is not placed on the uppellnost
layer of
the dextran sheet. Using this configuration, the thrombin and fibrinogen are
located at an
interior location in the hemostatic product. Fabricating the hemostatic
product in this manner
46
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
enhances the ability to retain thrombin and fibrinogen inside of the
hemostatic product even
though the thrombin and fibrinogen are sprinkled on the surface of the dextran
sheet.
[0237] While it is possible to put thrombin and fibrinogen on the outside
of the
hemostatic product, a portion of the thrombin and fibrinogen may become
dissociated from
the hemostatic product prior to use. In view of the cost of thrombin and
fibrinogen, it is
desirable for substantially all of the thrombin and fibrinogen to remain
associated with the
hemostatic product until it is desired to use the hemostatic product to
maximize the efficacy
of the hemostatic product.
[0238] Even though the thrombin and fibrinogen are mixed together prior to
placing
the thrombin and fibrinogen on the dextran sheet, the thrombin and fibrinogen
are sufficiently
dispersed on the dextran sheet so that the thrombin and fibrinogen do not
react prior to
placing the hemostatic product at the location where hemostasis is desired.
[0239] The hemostatic product is then cut into pieces. In certain
embodiments, the
pieces may be formed in a generally square shape. The size of the pieces may
be selected
based upon the intended use of the hemostatic product. For example, when the
hemostatic
product is intended for surgical applications, the pieces may have a smaller
size than if the
hemostatic product is intended for trauma applications.
[0240] A cutter may be used to cut the hemostatic product into the desired
size. In
addition to cutting the hemostatic product into pieces, the cutter may cause
the layers of the
dextran sheets that are adjacent to the cutter to be compressed together. This
compression
causes the dextran layers to stay together.
[0241] In certain embodiments, the pieces of the hemostatic product are
vacuum
packaged. In addition to maintaining the hemostatic product sterile, the
vacuum packaging
also compresses the layers in the hemostatic product, which enhances the
ability of the layers
to resist separation after the hemostatic product is removed from the package
prior to use.
47
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0242] This process thereby enhances the ability to use the hemostatic
product
because the layers in the hemostatic product resist coming apart. An advantage
of using this
process is that no additional steps are necessary to retain the layers
together. Additionally, it
does not require the use of additional components and/or additional processing
steps, which
could affect the efficacy of the hemostatic product.
Examples
102431 Dextran is mixed with an effective amount of water to foint an
aqueous
dextran solution. The aqueous dextran solution is electrospun to form an
electrospun dextran
sheet.
[0244] Thrombin and fibrinogen were mixed together and then dispersed on
the
electrospun dextran sheet. The thrombin was dispensed at a rate of between
about 1.3 and
2.7 NH Units per square centimeter of the electrospun dextran sheet. The
fibrinogen was
dispensed at a rate of between about 3.6 and 7.4 milligrams per square
centimeter of the
electrospun dextran sheet
[0245] This process was repeated until there were 8 layers of the
electrospun dextran
sheet in a stacked configuration. The thrombin and fibrinogen mixture was not
dispersed on
the surface of the uppermost layer. The electrospun dextran sheet has a
thickness of between
about 1 and 3 millimeters.
[0246] A cutter was then used to cut the hemostatic product into pieces
having a
width of about 4.8 centimeters and a length of about 4.8 centimeters. In
addition to fottning
pieces of a desired size, the cutting causes the electrospun dextran layers to
be pushed
together. This process caused the electrospun dextran layers to resist
separation. The pieces
of the hemostatic product were vacuum packaged for storage until use. In
addition to
48
preventing contamination of the hemostatic product, the vacuum packaging
caused the layers
of the electrospun dextran to be urged together.
[0247] A commercially available absorbable fibrin sealant product
marketed under the
designation TACHOSILTI4 (Nycomed) was used to compare with the performance of
the
hemostatic product described in this patent application.
[0248] The TACHOSILTm product contains an equine collagen sponge that
is coated on
one side thereof with the thrombin and fibrinogen. The TACHOSILTm product
contains
between 3.6 and 7.4 milligrams of human fibrinogen per square centimeter of
the product and
between 1.3 and 2.7 N111 Units of human thrombin per square centimeter of the
product.
' [0249] Utilizing the preceding information regarding the
concentration of thrombin
and fibrinogen on the TACHOSILIm product, the TACHOSILTm product was cut into
square
pieces to provide an amount of thrombin and fibrinogen on each piece of the
TACHOSILTm
product that was approximately equal to the amount of thrombin and fibrinogen
on the
hemostatic product that was prepared above.
[0250] The hemostatic efficacy of the hemostatic product of this
patent application and
the TACHOSILTm product was evaluated using the swine liver injury model, which
has
previously been used to evaluate hemostatic efficacy. The swine liver injury
model enables
multiple tests to be conducted on a single animal
[025 1] The tests were conducted using adult, domestic, breed-
indifferent, female pigs
having a weight of between about 50 and 70 kilograms. Each animal was
subjected to an
initial examination to verify the animal was in good health. Prior to the
procedure, the
animals were quarantined for three days during which the animals were provided
food and
water ad libitum.
[0252] Prior to initiating the procedure, anesthesia was administered
to the animal.
During the procedure, anesthesia and fluid maintenance was administered to the
animal. A
49
CA 2854294 2018-01-30
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
heating pad was placed under the animal during the procedure to assist in
maintaining the
animal's body temperature during the procedure.
[0253] A midlinc laparotomy was performed to provide access to the
abdominal
organs where the hepatic biopsies were to be performed. A biopsy punch having
a diameter
of approximately 8 millimeters was used to create each hepatic biopsy. The
hepatic biopsies
each have a width of about 8 millimeters and a depth of about 4 millimeters.
The cylindrical
hepatic biopsy was surgically removed with a scissor.
[0254] The presence of continuous free bleeding at the biopsy site was
observed for
about 20-25 seconds. Thereafter, the hemostatic product was applied to the
biopsy site. The
hemostatic hemostatic product was held in place with manual pressure for about
20 seconds.
[0255] The manual pressure was then removed while leaving the hemostatic
product
on the biopsy site. The biopsy site was observed for about 2 minutes for
visible signs of
active bleeding.
[0256] If bleeding persisted, an additional hemostatic product was placed
over the
biopsy site. The hemostatic product was held in place with manual pressure for
about 20
seconds. Thereafter, the manual pressure was removed while leaving the
hemostatic product
on the biopsy site. The biopsy site was observed for about 2 minutes for
visible signs of
active bleeding. This process was repeated for up to 4 times if the bleeding
had not
previously stopped.
[0257] As a control, the biopsy punch was used to create a hepatic biopsy
having a
width of about 8 millimeters and a depth of about 4 millimeters. The biopsy
was observed
for about 2 minutes. During this time period, the biopsy did not stop
bleeding.
[0258] Tests using the hemostatic product of this patent application, the
TACHOSIL
product and the control were perfotined in a random order to minimize the
potential effect
caused by the order in which the hemostatic product was used during the
evaluation.
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0259] The order in which the evaluation of the hemostatic agents was
performed and
the results obtained from such evaluations are set forth in Table 1 below.
Table 1
Hemostasis agent Number of compressions to Time to achieve
hemostasis
achieve hemostasis (minutes)
Tachosil 2 4
This invention 1 2
This invention 2 4
Control nia n/a
Tachosil 4 8
Control n/a n/a
This invention 1 2
Tachosil 2 4
[0260] The evaluation was repeated using 5 additional animals. The order in
which
the hemostatic products were used on the 5 additional animals was also
randomized.
[0261] The results from the first evaluation set forth in Table 1 were
combined with
the results from the 5 additional animals. There were a total of 18 samples
for each the
hemostatic product from this patent application and the TACHOSIL product.
[0262] For these 18 samples, the mean time for the hemostatic product from
this
patent application to cause hemostasis was 2.9 minutes with a standard
deviation of 1.7
minutes. For these 18 samples, the mean time for the TACHOSIL product to cause
hemostasis was 4.7 minutes with a standard deviation of 1.4 minutes. The
hemostatic
product from this patent application thereby provided a reduction in time to
achieve
hemostasis of nearly two minutes.
51
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
[0263] The animal blood was evaluated prior to the biopsy and after
hemostasis was
achieved for potential changes in the hematology. There were no significant
differences in
the hematology results for the hemostatic product from this patent application
and the
TACHOSIL product.
[0264] The clots provided by the hemostatic product of this patent
application were
compared to the clots provided by the TACHOSIL product using pathology. Fibrin
was
identifiable in all of the biopsies treated with the hemostatic product of
this patent
application. On the other hand, fibrin was not identifiable in 5 out of 18
biopsies treated with
the TACHOSIL product.
[0265] The total number of biopsies needing additional compressions for the
hemostatic product of this patent application was 5. The total number of
biopsies needing
additional compressions for the TACHOSIL product was 17. The hemostatic
product of this
patent application thereby represents a reduction of biopsies needing
additional compressions
of over 70% as compared to the TACHOSIL product.
[0266] The total number of additional compressions needed to provide
hemostasis
with the hemostatic product of this patent application was 8. The total number
of additional
compressions needed to provide hemostasis for the TACHOSIL product was 24. The
hemostatic product of this patent application thereby represents a reduction
of additional
compressions of over 60% as compared to the TACHOSIL product.
[0267] The "basketweave" configuration of the hemostatic product of this
patent
application allowed entrapment of erythrocytes dispersed throughout the biopsy
site. On the
other hand, the TACHOSIL product resulted in trapping of sheets of
erythrocytes at the base
of the biopsy site or between areas of amorphous implant material.
[0268] Because the thrombin and fibrinogen used in the TACHOSIL product are
obtained from human plasma, there is a risk that the TACHOSIL product may
contain
52
CA 02854294 2014-05-01
WO 2013/059341 PCT/US2012/060643
infectious agents. Another advantage of the hemostatic product of the current
invention over
the TACHOSIL is that the hemostatic product of the current invention appears
to remain
stable at a higher temperature than the TACHOSIL product.
[0269] While a direct comparison was not conducted, the electrospun dextran
base
used in the hemostatic product produced according to this invention rapidly
dissolves upon
contact with fluids. The dextran is absorbed into the body in a relatively
short time frame
that ranges from minutes to a few days. The rapid depletion of the dextran
carrier can
provide beneficial results in conjunction with reducing the risk of
inflammation and scaring.
[0270] In contrast, the collagen sponge used in the TACHOSIL product breaks
down
over a much longer period of time such as up to about 4 months. This aspect of
the
TACHOSIL product is indicated by the manufacturer to be a feature because it
is indicated
that the collagen sponge holds fibrin clots to the wound surface to achieve
hemostasis. The
slow absorption of collagen can be associated with increased risk of
inflammation and
scaring.
[0271] The electrospun dextran used in the hemostatic product provides this
hemostatic product with a higher level of flexibility compared to the collagen
sponge used in
the TACHOSIL product. This enhanced flexibility enables the hemostatic product
described
in this patent application to more readily conform to the surface of the wound
than the
TACHOSIL product.
[0272] In the preceding detailed description, reference is made to the
accompanying
drawings, which four' a part hereof, and in which is shown by way of
illustration specific
embodiments in which the invention may be practiced. In this regard,
directional
terminology, such as "top," "bottom," "front," "back," "leading," "trailing,"
etc., is used with
reference to the orientation of the Figure(s) being described. Because
components of
embodiments can bc positioned in a number of different orientations, the
directional
53
terminology is used for purposes of illustration and is in no way limiting. It
is to be
understood that other embodiments may be utilized and structural or logical
changes may be
made without departing from the scope of the present invention. The preceding
detailed
description, therefore, is not to be taken in a limiting sense, and the scope
of the present
invention is defined by the appended claims.
[0273] It is
contemplated that features disclosed in this application, as well as those
described in the above applications, can be mixed and matched to suit
particular circumstances.
Various other modifications and changes will be apparent to those of ordinary
skill.
54
CA 2854294 2018-01-30