Note: Descriptions are shown in the official language in which they were submitted.
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
METHOD AND SYSTEM FOR PROVIDING VERSATILE NIRS SENSORS
STATEMENT REGARDING RELATED APPLICATIONS AND PRIORITY CLAIMS
This application is a continuation-in-part of U.S. Non-Provisional Patent
Application Serial No. 12/773,312 filed on May 4, 2010 entitled, "METHOD AND
SYSTEM FOR MONITORING OXYGENATION LEVELS OF COMPARTMENTS
AND TISSUE." This application claims priority to this Non-Provisional patent
application under 35 U.S.C. 120. This application also claims priority under
35
U.S.C. 119(e) to U.S. Provisional Patent Application Serial No. 61/556,871
filed on
November 8, 2011 entitled, "METHOD AND SYSTEM FOR PROVIDING
VERSATILE NIRS SENSORS." The entire contents of both the provisional patent
application and non-provisional patent application are hereby incorporated by
reference.
FIELD OF INVENTION
The invention relates to a coordinated, continual and real-time method and
system for monitoring oxygenation levels of a compartment and other tissue.
More
particularly, the invention relates to an orchestrated method and system that
monitors oxygenation levels of compartments as well as other tissue such as
traumatized tissue.
BACKGROUND OF THE INVENTION
Compartment syndrome is a medical condition where the pressure inside a
compartment, which is a muscle group surrounded by fascia or a thin, inelastic
film,
increases until the blood circulation inside the volume defined by the fascia
or thin
film is cut off. The most common site, in humans, occurs in the lower leg, and
more
specifically, in regions adjacent to the tibia and fibula. There are four
compartments
in the lower, human leg: the anterior (front), lateral (side next to the
fibula) and the
deep and superficial posterior (back).
These four compartments surround the tibia and fibula. Anyone of these four
compartments can yield a compartment syndrome when bleeding or swelling occurs
within the compartment. Compartment syndrome usually occurs after some trauma
or injury to the tissues, such as muscles or bones or vessel (or all three),
contained
within the compartment. Bleeding or swelling within a compartment can cause an
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
increase in pressure within that compartment. The fascia does not expand, so
as
pressure rises, the tissue and vessels begin to be compressed within the
compartment.
This compression of tissue, such as muscle, due to intra-compartmental
pressure can restrict and often times stop blood flow from entering the
compartment
that is destined for any tissues contained within the compartment. This
condition is
termed ischemia. Without blood flow to tissues, such as muscle, the tissues
will
eventually die. This condition is termed necrosis.
A simple working definition for a compartment syndrome is an increased
pressure within a closed space which reduces the capillary blood perfusion
below a
level necessary for tissue viability. As noted above, this situation May be
produced
by two conditions. The first condition can include an increase in volume
within a
closed space, and the second condition is a decrease in size of the space.
An increase in volume occurs in a clinical setting of hemorrhage, post
ischemic swelling, re-perfusion, and arterial-venous fistula. A decrease in
size
results from a cast that is too tight, constrictive dressings, pneumatic anti-
shock
garments, and closure of fascial defects. As the pressure increases in tissue,
it
exceeds the low intramuscular arteriolar pressure causing decreased blood in
the
capillary anastomosis and subsequent shunting of blood flow from the
compartment.
The clinical conditions that may be associated with compartment syndrome
include the management of fractures, soft tissue injuries, arterial injuries,
drug
overdoses, limb compression situations, burns, post-ischemic swelling,
constrictive
dressings, aggressive fluid resuscitation and tight casts.
Referring now to the FIGs., FIG. 1 illustrates an X-ray view of a human leg
100 with fractured bones of the tibia 105 and fibula 110 that lead to one or
more
compartment syndromes in the muscles 115 surrounding the bones of the human
leg
100. The tibia 105 and fibula 110 usually bleed in regions proximate to the
physical
break regions 120. This bleeding can form a large pool of stagnant blood
referred to
as a hematoma. The hematoma can start pressing upon muscles 115 that may be
proximate to the break 120. This pressure caused by the hematoma can severely
restrict or stop blood flow into the muscles 115 of a compartment, which is
the
diagnosis of a compartment syndrome.
-2-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Traditional Methods for Diagnosing Compartment Syndromes
Referring now to FIG. 2, this Figure is a side view of a human leg 100 in
which
compartment pressures are being measured with a large bore needle 200, having
a
gauge size such as 14 or 16 (which is the largest needle in the hospital
available to
clinicians), according to a conventional method known in the prior art. While
compartment pressures can be measured with this conventional method, the
method
is highly invasive procedure which can cause tremendous pain to the patient.
Needles with large gauge sizes of 14 or 16 are analogous to sticking a patient
with
an object as large as a nail or a pen.
In addition to causing tremendous pain to the patient, there are several more
problems associated with the conventional needle measuring method. First, it
is
very challenging for a medical practitioner to actually measure or read
pressure of a
compartment since the needle must be positioned at least within the interior
of a
compartment. To enter the interior of a compartment, the needle 200 must
penetrate
through several layers of skin and muscle. And it is very difficult for the
medical
practitioner to know if the needle has penetrated adequately through the
intermediate layers to enter into the compartment. This challenge
significantly
increases if the patient being measure is obese and has significant amounts of
subcutaneous fat in which to penetrate with the needle.
Often, the medical practitioner may not have a needle accurately positioned
inside a compartment which = can yield a reading of the tissue adjacent to the
compartment, such as muscle or skin. Such a reading of muscle or skin instead
of
the compartment of interest can provide the medical practitioner with elevated
or
depressed pressure readings that do not reflect the actual pressure contained
within
the compartment of interest. Pressure readings inside a compartment have been
shown to vary (increase) based on the depth of the reading as well as the
proximity
to the fracture site.
Because of the challenge medical practitioners face with precisely positioning
a needle within a compartment of interest and because of the numerous law
suits
associated with the diagnosis of compartment syndrome, many medical schools do
not provide any formal training for medical practitioners to learn how to
properly
place a needle within a compartment of interest for reading a compartment's
pressure. Therefore, many medical practitioners are not equipped with the
skills or
-3-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
experience to accurately measure compartment pressures with the needle
measuring method.
Currently, intra-compartmental pressures are the only objective diagnostic
tool. Due to the legal climate regarding this condition, clinicians are forced
to treat
an elevated value for compartment pressures or expose themselves to legal
ramifications with any complications. As
described later, the treatment of
compartment syndrome can cause significant morbidity and increase the risk for
infection. Therefore inaccurate and elevated pressure readings are a very
difficult
and potential dangerous pitfall.
Another problem associated with the training and experience required for the
needle measuring method is that, as noted above, compartment syndromes usually
occur when tissues within the compartment are experiencing unusual levels of
swelling and pressure. With this swelling and pressure, the tissues do not
have their
normal size. Therefore, any training of a medical practitioner must be made
with a
patient suffering under these conditions. A normal patient without any
swelling
would not provide a medical practitioner with the skills to accurately assess
a size of
a compartment when using the needle measuring method for determining
compartment pressure. Put another way, due to the trauma associated with the
injury, normal anatomy is not always present when attempting to measure
compartment pressures.
In addition to the problem of entering a compartment that may have an
abnormal size or anatomy, the needle measuring method has the problem of
providing only a snap-shot of data at an instant of time. When the
conventional
needle measuring method is used, it provides the medical practitioner with
pressure
data for a single instant of time. In other words, the needle pressure method
only
provides the medical practitioner with one data point for a particular time.
Once
pressure is read by the medical practitioner, he or she usually removes the
needle
from the patient. The data obtained from a single measurement in time gives no
information concerning the pressure trend, and the direction the intra-
compartmental
pressure is moving.
This collection of single data points over long periods of time is usually not
very helpful because pressures within a compartment as well as the patient's
blood
pressure can change abruptly, on the order of minutes. Also, because of the
pain
associated with the needle measuring method noted above, the medical
practitioner
-4-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
will seldom or rarely take pressure readings with within a few minutes of each
other
using a needle.
A further problem of the needle measuring method is that for certain regions
of the body, such as the lower leg, there are four compartments to measure.
This
means that a patient's leg must be stuck with the large bore needle at least
four
times in order for a medical practitioner to rule out that a compartment
syndrome
exists for the lower leg. In the lower leg of the human body, one compartment
is
located under a neighboring compartment such that a needle measurement may be
needed in at least two locations that are very close together, but in which
the medical
practitioner must penetrate tissues at a shallow depth at a first location to
reach the
first compartment; and for reaching the second compartment that is underneath
the
first compartment, a large depth must be penetrated by the needle, often with
the
needle piercing the first compartment and then the second compartment.
Another problem, besides pain that is associated with the needle pressure
measuring method, is that there is a lack of consensus among medical
practitioners
over the compartment pressure ranges which are believed to indicate that a
compartment syndrome may exist for a particular patient. Normal compartment
pressure in the human body usually approaches 4 mmHg in the recumbent
position.
Meanwhile, scientists have found that an absolute pressure measurement of 30
mmHg in a compartment may indicate presence of compartment syndrome.
However, there are other scientists who believe that patients with
intracompartmental pressures of 45 mmHg or greater should be identified as
having
true compartment syndromes. But other studies have shown patients with intra-
compartmental pressures above these limits with no clinical signs of
compartment
syndrome. Additional studies have shown that a pressure gradient based on
perfusion pressure (diastolic blood pressure minus intra-compartmental
pressure) is
the more important variable. Studies have shown in a laboratory setting that
once
the perfusion pressure drops to 10 mm Hg tissue necrosis starts to occur.
Other subjective methods for diagnosing compartment syndromes instead of
the needle measuring method exist, however, they may have less accuracy than
the
needle measuring method because they rely on clinical symptoms of a patient.
Some clinical symptoms of a patient used to help diagnose compartment
syndromes
include pulselessness (absence of a pulse), lack of muscle power, pain,
parastesias,
and if the flesh is cold to touch. Pain out or proportion and with passive
stretch are
-5-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
considered the earliest and most sensitive, but both are very low specificity.
One of
the major drawbacks of these symptoms is that for many of them the patient
must be
conscious and must be able to respond to the medical practitioner. This is
true for
the muscle power and pain assessment. For any inebriated patients or patients
who
are unconscious, the pain assessment and muscle power assessment cannot be
used accurately by the medical practitioner. In the setting of high energy
trauma
which is associated with compartment syndrome, many patients are not capable
of
cooperating with a good physical exam due to any number of causes including
head
trauma, medical treatment (including intubation), drug or alcohol ingestion,
neurovascular compromise or critical and life threatening injuries to other
body
systems.
For the pain assessment, if a lower leg compartment syndrome exists in a
patient, then the range of motion for a patient's foot or toes will be
extremely limited
and very painful when the patient's foot or toes are actively or passively
moved. The
pain from a compartment syndrome can be very immense because the muscles are
deprived of oxygen because of the compartment syndrome.
Another drawback using pain to assess the likelihood of a compartment
syndrome is that every human has a different threshold for pain. This means
that
even if someone should be experiencing a high level of pain, he or she may
have a
high threshold for pain and therefore, not provide the medical practitioner
with a
normal reaction for the current level of pain. Another problem with using pain
to
assess the likelihood of the existence of a compartment syndrome is that if
the
patient is experiencing trauma to other parts of their body, he or she may not
feel the
pain of a compartment syndrome as significantly, especially if the trauma to
the other
parts of the patient's body is more severe. This condition is termed a
distracting
injury. On the other hand, trauma causes the initial injury that precipitates
a
compartment syndrome. That initial trauma by definition will cause a baseline
amount of pain that is often very difficult to separate from a potential
compartment
syndrome pain. These initial injuries by themselves cause significant pain, so
a
patient that does not tolerate pain well may present similar to a compartment
syndrome without having any increased pressures simply because they react
vehemently to painful conditions.
-6-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Conventional Non-Invasive Techniques for Measuring Oxygenation Levels of a
Compartment
Non-invasive measuring of compartment syndromes using near infrared
sensors, such as spectrophotometric sensors, to measure oxygenation levels
within
a compartment has been suggested by the conventional art. However, these
conventional techniques have encountered the problem of a medical practitioner
locating compartments of interest and accurately and precisely positioning a
sensor
over a compartment of interest. Often the orientation of the scan and the
depth of
the scan produced by a near infrared sensor as well as the orientation of a
compartment can be challenging for a medical practitioner to determine because
conventional sensors are not marked with any instructions or visual aids.
Another
problem faced by the medical practitioner with conventional non-invasive
techniques
is determining how to assess the oxygenation level of compartments that lie
underneath a particular neighboring compartment, such as with the deep
posterior
compartment of the human leg.
In trauma settings, near infrared sensors often do not work when they are
placed over regions of the body that have hematomas or pools of blood. In such
conditions, a medical practitioner usually guesses at what regions of the
human body
do not contain any hematomas that could block compartment measurements. Also,
conventional near infrared sensors typically are not sterilized and cannot be
used in
surgical or operating environments.
Near infrared sensors (NIRS) in their current form are limited to a single
sensor with a single sensor depth. They also can be affected by skin
pigmentation
that is not accounted for in the current technology. Placement of the sensor
can be
difficult since an expanding hematoma can block a previously acceptable
placement.
Additionally, the only system as of this writing is a single monitor system.
There is
no product available at this time which will allow for multiple areas to be
monitored in
close proximity to one another without the potential for interference from
other
sensor light sources.
Treatment for Compartment Syndrome
Referring now to FIG. 3, this figure is a side view of a human leg 100 in
which
a surgical procedure, known as a fasciotomy, was performed in order to release
the
pressures in one or more compartments surrounding the bones of the leg
according
-7-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
to a technique known in the art in order to alleviate a compartment syndrome
that
was diagnosed. This surgical procedure includes an incision 300 that is made
along
the length of the leg 100 and is generally as long as the compartments
contained
within the leg 100. While a single incision 300 is illustrated in FIG. 3, a
second
incision is made on the opposing side of the leg so that a patient will have
two
incisions on each side of his leg 100. These incisions typically extend from
near the
knee to near the ankle on each side of the leg.
This procedure is very invasive and it often leaves the patient with severe
scars and venous congestion once healed. Also the procedure increases a
patient's
chances of receiving an air-borne infection because the incisions made on
either
side of the leg are usually left open for several days in order to allow for
the swelling
and excess bleeding to subside. Fasciotomies transform a closed fracture (one
in
which the skin is intact and minimal risk of infection) to an open fracture.
Open
fractures have a much higher risk of bone infections which requires multiple
surgical
debridements and ultimately amputation in some cases in ordered to
appropriately
treat. Additionally, some wound cannot be closed and require skin transfers,
or skin
grafts, from other parts of the body, usually from the anterior thigh.
Therefore, it is quite apparent that accurately diagnosing compartment
syndrome is critical because any misdiagnosis can lead to significant
morbidity. A
missed compartment syndrome can result in an insensate and contracted leg and
foot. A fasciotomy which is highly invasive procedure and which exposes a
patient
to many additional health risks should not be performed in the absence of a
compartment syndrome.
Additionally, time is an important factor in the evaluation of these patients.
Ischemic muscle begins to undergo irreversible changes after about six hours
of
decreased perfusion. Once irreversible changes or necrosis occur, a fasciotomy
should not be performed. Fasciotomies in the setting of dead muscle only
increase
the risk for severe infections and other complications. Late fasciotomies have
been
shown to have approximately a 50-75% risk of complication. Therefore,
fasciotomies
need to be performed early but judiciously in patients that are often
unresponsive or
uncooperative in order to prevent severe morbidity.
Accordingly, there is a need in the art for a non-invasive, real time method
and system that monitors oxygenation levels of a compartment and that is
provided
with sensors which can be precisely positioned over a compartment of interest
in
-8-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
order to assist in assessing conditions associated with a compartment
syndrome. A
further need exists in the art for a non-invasive method that monitors
oxygenation
levels of a compartment over long periods of time at frequent time intervals
and that
can monitor different compartments that may be in close proximity with one
another.
Summary of the Invention
A system and method is provided in which a wireless near-infrared
spectrometry sensor includes a light source for emitting near-infrared energy
into
tissue and a light receiver for receiving the near-infrared energy after it
exits the
The sensor may further include a substrate for supporting the light source,
battery or capacitor may have a size which is substantially smaller than the
substrate. The substrate may be part of a sterile bandage. The substrate of
the
wireless sensor may include absorbent materials to absorb any moisture or
liquid
adjacent to the light source or the light receiver. The wireless transceiver
may
-9-
=
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
include at least one of a radio-frequency transceiver, an optical transceiver,
an
acoustical transceiver, and a magnetic transceiver. The wireless sensor may
include
memory for storing the readings in connection with the light source. The
wireless
sensor may have multiple modes of operation.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 illustrates an X-ray view of a human leg with fractured bones of the
tibia and fibula that lead to one or more compartment syndromes in muscles
surrounding the bones of the human leg.
FIG. 2 is a side view of a human leg in which compartment pressures are
being measured with a large bore needle according to a conventional method
known
in the prior art.
FIG. 3 is a side view of a human leg in which a surgical procedure, known as
a fasciotomy, was performed in order to release the pressures in one or more
compartments surrounding the bones of the leg according to a technique known
in
the art.
FIG. 4 illustrates oxygen levels of compartments of a human leg being
measured by compartment sensors that include compartment alignment
mechanisms and central scan depth markers according to one exemplary
embodiment of the invention.
FIG. 5A illustrates a bottom view of two pairs of compartment sensors with
each sensor having a compartment alignment mechanism and a central scan marker
in addition to a separating device according to one exemplary embodiment of
the
invention.
FIG. 5B illustrates a bottom view of the four compartment sensors of FIG. 5A
but with the individual sensors divided from one another through using the
separating device, such as the perforations, according to one exemplary
embodiment of the invention.
- FIG.
6A illustrates a bottom view of a three sensor embodiment in which one
sensor of the three compartment sensors can scan at two or more depths
according
to one exemplary embodiment of the invention.
FIG. 6B, this figure illustrates the compartment sensor of FIG. 6A that can
scan at two or more depths in order to measure deeper compartments of an
animal
body according to one exemplary embodiment of the invention.
-10-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
FIG. 6C illustrates a bottom view of a wireless sensor comprising a substrate,
a light source, and optical receivers according to one exemplary embodiment of
the
invention;
FIG. 60 illustrates sensors that may be used for muscle training/endurance
applications according to one exemplary embodiment of the invention
FIG. 6E illustrates how wireless sensors may be grouped according to control
sites and injured-tissue sites according to one exemplary embodiment of the
invention
FIG 6F illustrates a first exemplary embodiment of how a wireless sensor may
be provided with a mechanism to wick moisture/fluids away from the sensor
elements such as the light source and the light receivers.
FIG 6G illustrates a second exemplary embodiment of how a wireless sensor
may be provided with a mechanism to wick moisture/fluids away from the sensor
elements such as the light source and the light receivers.
FIG. 6H is a logical flow chart illustrating a method for providing enhanced
and efficient wireless sensors according to one exemplary embodiment of the
invention.
FIG. 7 illustrates a near light detector and a far light detector that are
positioned within substrate material at predetermined distances from the
optical
transmitter of a compartment sensor according to one exemplary embodiment of
the
invention.
FIG. 8A illustrates a linear array of compartment sensors assembled as a
single mechanical unit that can provide scans at various depths according to
one
exemplary embodiment of the invention.
FIG. 8B illustrates a linear compartment sensor array that can include optical
transmitters that are shared among pairs of optical receivers according to one
exemplary embodiment of the invention.
FIG. 8C is a functional block diagram of compartment sensor that illustrates
multiple optical receivers that may positioned on opposite sides of a single
optical
transmitter and that may be simultaneously activated to produce their scans at
the
same time according to one exemplary embodiment of the invention.
-11-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
FIG. 9A illustrates a cross-sectional view of a left-sided human leg that has
the four major compartments which can be measured by the compartment sensors
according to one exemplary embodiment of the invention.
FIG. 9B illustrates a cross-sectional view of a right-sided human leg and
possible interference between light rays of simultaneous oxygenation scans
made by
the compartment sensors into respective compartments of interest according to
one
exemplary embodiment of the invention.
FIG. 9C illustrates a position of a compartment sensor in relation to the knee
for the deep posterior compartment of a right sided human leg according to one
exemplary embodiment of the invention.
FIG. 10 illustrates an exemplary display of numeric oxygenation values as
well as graphical plots for at least two compartments of an animal according
to one
exemplary embodiment of the invention.
FIG. 11 illustrates single compartment sensors with alignment mechanisms
and central scan depth markers that can be used to properly orient each sensor
with
a longitudinal axis of a compartment of an animal body according to one
exemplary
embodiment of the invention.
FIG. 12 illustrates compartment sensor arrays with alignment mechanisms
that can be used to properly orient each array with a longitudinal axis of a
compartment of an animal body according to one exemplary embodiment of the
invention.
FIG. 13A illustrates various locations for single compartment sensors that can
be positioned on a front side of animal body, such as a human, to measure
oxygenation levels of various compartments according to one exemplary
embodiment of the invention.
FIG. 13B illustrates various locations for single compartment sensors that can
be positioned on a rear side of animal body, such as a human, to measure
oxygenation levels of various compartments according to one exemplary
embodiment of the invention.
FIG. 14A illustrates various locations for compartment sensor arrays that can
be positioned over compartments on a front side of an animal body, such as a
human, to measure oxygenation levels of the various compartments according to
one exemplary embodiment of the invention.
-12-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
FIG. 14B illustrates various locations for compartment sensor arrays that can
be positioned over compartments on a rear side of an animal body, such as a
human, to measure oxygenations levels of the various compartments according to
one exemplary embodiment of the invention.
FIG. 14C illustrates an exemplary display and controls for the display device
that lists data for eight single compartment sensors according to one
exemplary
embodiment of the invention.
FIG. 140 illustrates an exemplary display of providing users with guidance for
properly orienting a single compartment sensor over a compartment of an
animal,
such as a human leg, according to one exemplary embodiment of the invention.
FIG. 15A illustrates a front view of lower limbs, such as two lower legs of a
human body, that are being monitored by four compartment sensor arrays
according
to an exemplary embodiment of the invention.
FIG. 15B illustrates a display of the display device that can be used to
monitor
hematomas and/or blood flow according to one exemplary embodiment of the
invention.
FIG. 16 illustrates a display of the display device for an instant of time
after
the display of FIG. 15B and which can be used to monitor hematomas and/or
blood
flow according to one exemplary embodiment of the invention.
FIG. 17 illustrates a sensor design for measuring the optical density of skin
according to one exemplary embodiment of the invention.
FIG. 18A illustrates a sensor that can penetrate two layers of skin to obtain
optical density values according to one exemplary embodiment of the invention.
FIG. 18B illustrates a sensor that can penetrate one layer of skin according
to
one exemplary embodiment of the invention.
FIG. 18C illustrates a modified compartment monitoring system that can
correlate skin pigmentation values with skin optical density values in order
to provide
offset values for oxygenation levels across different subjects who have
different skin
pigmentation according to one exemplary embodiment of the invention.
FIG. 19 is a functional block diagram of the major components of a
compartment monitoring system that can monitor a relationship between blood
pressure and oxygenation values according to one exemplary embodiment of the
invention.
-13-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
FIG. 20 is an exemplary display that can be provided on the display device
and which provides current blood pressure values and oxygenation levels of a
compartment of interest according to one exemplary embodiment of the
invention.
FIG. 21 is a functional block diagram that illustrates sterilized material
options
for a compartment sensor according to one exemplary embodiment of the
invention.
FIG. 22 illustrates an exemplary clinical environment of a compartment sensor
where the sensor can be positioned within or between a dressing and the skin
of a
patient according to one exemplary embodiment of the invention.
FIG. 23 is a graph of perfusion pressure plotted against oxygenation levels of
a study conducted to determine the sensitivity and responsiveness of the
inventive
compartment monitoring system according to one exemplary embodiment of the
invention.
FIG. 24 is a graph of perfusion pressure plotted against a change in the
oxygenation levels from a baseline for each subject of the study conducted to
determine the sensitivity and responsiveness of the inventive compartment
monitoring system according to one exemplary embodiment of the invention.
FIG. 25 is a logic flow diagram illustrating an exemplary method for
monitoring
oxygenation levels of a compartment according to one exemplary embodiment of
the
invention.
FIG. 26 is a functional block diagram illustrating additional applications of
and
Oxygenation Sensing System of FIG. 19 such as in Wound
Management/Monitoring/Healing according to one exemplary embodiment of the
invention.
FIG. 27 is a functional block diagram of an intensive care unit (ICU) central
controller and analyzer according to one exemplary embodiment of the
invention.
FIG. 28 is a logic flow diagram illustrating an exemplary method for
positioning sensors on a leg of an animal body, such as a human, for
monitoring
conditions for ACS according to one exemplary embodiment of the invention.
FIGs. 29A-G illustrate various locations for single compartment sensors that
can be positioned on a leg of an animal body, such as a human, to measure
oxygenation levels of various compartments according to exemplary embodiments
of
the invention.
-14-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
FIG. 30 is a logic flow diagram illustrating an exemplary method for
positioning sensors on an arm of an animal body, such as a human, for
monitoring
conditions for ACS according to one exemplary embodiment of the invention.
FIGs. 31A-H illustrate various locations for single compartment sensors that
can be positioned on an arm of an animal body, such as a human, to measure
oxygenation levels of various compartments according to exemplary embodiments
of
the invention.
FIG. 32 is a logic flow diagram illustrating an exemplary method for assessing
tissue conditions to help medical practitioners determine an amputation "line"
or
"level" according to one exemplary embodiment of the invention.
FIGs. 33A-33B provide a logic flow diagram illustrating an exemplary method
for assessing monitored conditions to help medical practitioners determine if
a
patient is experiencing anemia and/or shock according to one exemplary
embodiment of the invention.
FIG. 34 illustrates various sizes for sensors according to one exemplary
embodiment of the invention.
FIG. 35 illustrates a sensor having a predetermined geometric shape that
mirrors the geometric shape of a particular portion of a human anatomy
according to
one exemplary embodiment of the invention.
FIG. 36 illustrates various physical features that may be provided for a
sensor
according to one exemplary embodiment of the invention.
FIG. 37 illustrates sensors positioned along a length of a sleeve according to
one exemplary embodiment of the invention.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
A method and system for monitoring oxygenation levels in compartments of
an animal limb, such as in a human leg or a human thigh or a forearm, can be
used
to assist in the diagnosis of a compartment syndrome. The method and system
can
include one or more near infrared compartment sensors in which each sensor can
be
provided with a compartment alignment mechanism and a central scan depth
marker
so that each sensor may be precisely positioned over a compartment of a human
leg
or human thigh or forearm. The method and system can include a device for
displaying oxygenation levels corresponding to respective compartment sensors
that
are measuring oxygenation levels of a compartment of interest.
-15-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Referring now to the drawings, in which like reference numerals designate like
elements, FIG. 4 illustrates oxygen levels 402A, 402B of compartments of a
human
leg 100 being measured by a near-infrared spectroscopy (NIRS) sensors 405A,
405B that include a compartment alignment mechanisms 410A, 410B and central
scan depth markers 415A, 415B according to one exemplary embodiment of the
invention.
The alignment mechanism 410 of a compartment sensor 405 can include a
linear marking on a surface of the compartment sensor 405 that is opposite to
the
side which produces a light scan used to detect oxygenation levels. The linear
marking can be used by a medical practitioner to align a compartment sensor
405
with the longitudinal axis 450 of a compartment of interest. The invention is
not
limited to a solid line on the sensor 405. Other alignment mechanisms 410
within the
scope of the invention include, but are not limited to, tick marks, dashed
lines,
notches cut in the substrate of the compartment sensor 405 to provide a
geometric
reference for the medical practitioner, and other like visual orienting
alignment
mechanisms 405.
The central scan depth marker 415 can include a linear marking positioned on
a surface of a compartment sensor 405 that intersects the alignment mechanism
410
at a location along the alignment mechanism 410 that denotes the deepest
region of
a light scan produced by the compartment sensor 405. The depth of measurement
can be displayed in numeric form over the central scan depth marker 415 as a
guide
to aid medical practitioner since scan depth can vary based on the compartment
sensor's light source and receptor separation. The central scan depth marker
415
can insure that a medical practitioner properly aligns the compartment sensor
405 at
a location that will measure a compartment of interest. Similar to the
alignment
mechanism 410 noted above, the invention is not limited to a solid line on the
compartment sensor 405. Other central scan depth markers 415 within the scope
of
the invention include, but are not limited to, tick marks, dashed lines,
notches cut in
the substrate of the compartment sensor to provide a geometric reference for
the
medical practitioner, and other like visual orienting central depth markers
415.
Once the proper position for a compartment sensor 405 is determined by the
medical practitioner with the compartment alignment mechanism 410 and the
central
scan depth marker 415, the medical practitioner can apply the compartment
sensor
-16-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
405 on the patient by using an adhesive that is already part of the
compartment
sensor 405.
FIG. 4 illustrates three compartment sensors 405A, 405B, and 405C of a
system 400 for monitoring three different compartments of the lower human leg
100.
A fourth compartment sensor 405D not illustrated can be positioned on a side
of the
leg not illustrated and which monitors the fourth compartment of the lower
human leg
100. The compartment sensors 405 illustrated in FIG. 4 and discussed
throughout
this document can be of the type described in U.S. Pat. No. 6,615,065 issued
in the
name of Barrett et al. (the "065 Patent"), the entire contents of which are
hereby
incorporated by reference. The compartment sensors 405 can include those made
and distributed by Somanetics, Troy, MI. However, other conventional near
infrared
compartment sensors 405 can be used without departing from the scope and
spirit of
the invention.
The compartment sensors 405 can generally provide spectrophotometric in
vivo monitoring of blood metabolites such as hemoglobin oxygen concentration
in
any type of compartment and on a continuing and substantially instantaneous
basis.
The compartment sensors 405 are coupled to a processor and display unit
420 which displays the two oxygen levels 402A, 402B comprising the values of
seventy-three. The processor and display unit 420 can display all four oxygen
levels
of four compartments of the human leg 100 when at least four compartment
sensors
405 are deployed. The invention is not limited to four compartment sensor
embodiments. The invention can include any number of compartment sensors for
the accurate detection of conditions that may be associated with compartment
syndrome. For example, another exemplary embodiment illustrated in FIG. 14C
allows for eight sensor readings so that concomitant monitoring of the
contralateral
uninjured leg can be performed.
The processor of the display unit 420 can be a conventional central
processing unit (CPU) known to one of ordinary skill in the art. It may have
other
components too similar to those found in a general purpose computer, such as,
but
not limited to, memory like RAM, ROM, EEPROM, Programming Logic Units (PLUs),
firmware, and the like. Alternatively, the processor and display unit 420 can
be a
general purpose computer without departing from the invention.
The processor and display unit 420 can operate in a networked computer
environment using logical connections to one or more other remote computers.
The
-17-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
computers described herein may be personal computers, such as hand-held
computers, a server, a client such as web browser, a router, a network PC, a
peer
device, or a common network node. The logical connections depicted in the
Figures
can include additional local area networks (LANs) and a wide area networks
(WANs)
not shown. The processor and display unit 420 illustrated in FIG. 4 and the
remaining Figures may be coupled to a LAN through a network interface or
adaptor.
When used in a WAN network environment, the computers may typically include a
modem or other means for establishing direct communication lines over the WAN.
In a networked environment, program modules may be stored in remote
memory storage devices. It will be appreciated that the network connections
shown
are exemplary and other means of establishing a communications link between
computers other than depicted may be used.
Moreover, those skilled in the art will appreciate that the present invention
may be implemented in other computer system configurations, including other
hand-
held devices besides hand-held computers, multiprocessor systems,
microprocessor
based or programmable consumer electronics, networked personal computers,
minicomputers, mainframe computers, and the like.
The invention may be practiced in a distributed computing environment where
tasks may be performed by remote processing devices that are linked through a
communications network. In a distributed computing environment, program
modules
may be located in both local and remote storage devices.
The processor and display unit 420 can comprise any general purpose
computer capable of running software applications and that is portable for
mobile
applications or emergency applications.
The communications between the processor and display unit 420 and the
sensors 405 can be wire or wireless, depending upon the application. Typical
wireless links include a radio frequency type in which the processor and
display unit
420 can communicate with other devices using radio frequency (RF)
electromagnetic
waves. Other wireless links that are not beyond the scope of the invention can
include, but are not limited to, magnetic, optical, acoustic, and other
similar wireless
types of links.
In the exemplary embodiment illustrated in FIG. 4, the compartment sensors
405 are coupled to the processor and display unit 420 with cables 430A, 430B
which
can include electrical conductors for providing operating power to the light
sources of
-18-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
the compartment sensors 405 and for carrying output signals from the detectors
of
the sensors 405 to the display unit 420. The cables 430 may be coupled to a
quad-
channel coupler, a preamplifier 425A, 425B, and an integrated, multiple
conductor
cable 435. Alternatively, all wires could be packaged or merged into a single
unit or
cord or plug (not illustrated) for insertion into the monitor for ease of
management for
the clinician and to prevent misplacement of wire plugs into wrong sockets.
In addition to tracking compartment oxygen levels, the processor and display
unit 420 may receive data from a blood pressure monitor 445. The blood
pressure
monitor 445 may be coupled to a probe 440 that takes pressure readings from
the
patient at one or more locations, such as, but not limited to, an arm with a
cuff, a
needle in the volar wrist, the brachium (arm) via a sphygmomanometer, or
arterial
line. The probe 440 can be any one of a number of devices that can take blood
pressure readings, such as, but not limited to, automated inflating pressure
cuffs
(sphygmomanometer), arterial lines, and the like. Similarly, other types of
blood
pressure monitors 445 are not beyond the scope of the invention. Further
details of
the relationship between blood pressure and oxygen levels in the human body
will be
discussed and described more fully below in connection with FIGs. 19-20.
The display and processing unit 420 can display values at any one time for all
compartment sensors 405 being used. While the display and processing unit 420
only displays two oxygen levels for the embodiment illustrated in FIG. 4, the
display
and processing unit 420 could easily display all four values from the four
compartment sensors 405 that are being used to monitor the four compartments
of
the lower leg 100.
Referring now to FIG. 5A, this figure illustrates a bottom view of two pairs
of
compartment sensors 405 with each sensor 405 having a compartment alignment
mechanism 410 and a central scan marker 415 in addition to a separating device
505 according to one exemplary embodiment of the invention. The substrate 530
of
each compartment sensor 405 can comprise a foam or plastic material that may
have a soft and comfortable outer layer. The separating device 505 is
illustrated with
a dashed line in FIG. 5A.
According to one exemplary embodiment the separating device 505 can
comprise a perforation in the substrate 530. A perforation is a series of cuts
or
removed portions positioned along a line which can be perforated or separated.
This
means, for the exemplary embodiment illustrated in FIG. 5A, the first
compartment
-19-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
sensor 405A can be physically separated from the second compartment sensor
405B. The separating device 505 is not limited to perforations and it can
include
other types of devices. For example, the separating device 505 can comprise a
zipper, a plastic seal line, hook and loop fasteners and other like devices
that would
permit the rapid and accurate expansion of compartment sensors 405 when used
in
a trauma setting.
As noted above, the compartment sensors 405 can include alignment
mechanisms 410 and a central scan depth marker 415 in order to accurately
position
the compartment sensors 405 over compartments of interest. The alignment
mechanisms 410 and central depth markers 415 are illustrated with dashed or
dotted
lines because they are "hidden" relative to the bottom view of the compartment
sensors 405 which are illustrated in FIG. 5A.
Each compartment sensor 405 may comprise an optical transmitter 510 and
an optical receiver 515. The optical transmitter 510 may comprise an
electrically
actuated light source for emitting a selected examination spectra.
Specifically, the
optical transmitter 510 may comprise two or more narrow-bandwidth LEDs whose
center output wavelengths correspond to the selected examination spectra. Each
optical receiver 515 may comprise two or more light detectors, such as
photodiodes.
In the embodiment illustrated in FIG. 5A, the optical receiver 515 has a total
of four
photodiodes in which pairs of photodiodes work together to provide a "near"
detector
and a "far" detector. Each photo diode must have two receptors to receive
light at
two separate wavelengths to allow for calculations of oxy-hemoglobin and deoxy-
hemoglobin concentrations. Using two pairs of receptors allows for a deep and
shallow set to enable isolation of only the deep tissue oxygenation (see FIG.
7).
Referring briefly now to FIG. 7, the "near" light detector 702B and the "far"
light detector 702A are positioned within the substrate material 530 at
predetermined
distances from the optical transmitter 510. The "near" detector 702B formed by
the
two photodiodes that are closest to the optical transmitter 510 have a light
mean
path length 710B which is primarily confined to "shallow" layers 705 of a
compartment of interest. Meanwhile, the "far" detector 702A formed by the pair
of
photodiodes that are farthest from the optical transmitter 510 have a light
mean path
710A that is longer than that of the "near" detector and is primarily confined
to "deep"
layers of a compartment of interest in a leg 100.
-20-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
By appropriately differentiating the information from the "near" or "shallow"
detector 702B (which may produce a first data set) from the "far" or "deep"
detector
702A (which may produce a second data set), a resultant value for the tissue
optical
density may be obtained that characterizes the conditions within a compartment
of
interest without the effects that are attributable to the overlying tissue 705
which is
adjacent to the compartment of interest.
This enables the compartment monitoring system 400 (illustrated in FIG. 4) to
obtain metabolic information on a selective basis for particular regions
within the
patient and by spectral analysis of the metabolic information and by using
appropriate extinction coefficients, a numerical value or relative quantified
value such
as 402 of FIG. 4 may be obtained which can characterize metabolites or other
metabolite data, such as the hemoglobin oxygen saturation, within the
particular
region of interest. This region of interest is defined by the curved light
mean path
710A extending from the optical transmitter 510 to the "far" or "deep"
detector 702A
and between this path 710A and the outer periphery of the patient but
excluding the
region or zone defined by the curved light mean path 710B extending from the
optical transmitter 510 to the "near" or "shallow" detector 26. Further
details of the
compartment sensors 405 are described in U.S. Pat. No. 6,615,065, issued in
the
name of Barrett et al., which is hereby incorporated by reference.
Referring back now to FIG. 5A, each compartment sensor 405 has its own
cable 430 that provides power to the optical transmitter 405A and that
receives data
from the optical receiver 515. Each compartment sensor 405 may also include a
label 555 which may comprise a name and an anatomical location to position the
compartment sensor 405 on a patient. This label may be placed on the bottom of
the sensor 405 that contacts the patient as well as on the side that is
opposite to the
side which contacts the patient. For example, the first sensor 405A can have a
first
label 555A that comprises the phrase, "Lateral" to describe the name of the
compartment that this compartment sensor 405A that is designed to assess. The
numerical depth can also be displayed on the label, but is not limited to a
single
depth.
The first pair of compartment sensors 405A, 405B may be coupled to the
second pair of compartment sensors 405C, 405D with an expansion device 535.
The expansion device 535 may comprise an elastic material that stretches. The
expansion device 535 allows the pair of compartment sensors 405 to be
positioned
-21-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
on appropriate parts of a patient to monitor any compartments of interest. The
four
compartment sensor exemplary embodiment illustrated in FIG. 5A is designed for
the
four compartments of a human lower leg 100.
The expansion device 435 is not limited to elastic material. The expansion
device can include other mechanisms which allow for an adjustable separation
between the pairs of compartment sensors 405 so that the compartment sensors
405 may be precisely and appropriately positioned over specific compartments
of
interest. The expansion device 435 may include, but is not limited to,
springs, tape,
hook and loop fasteners, gauze, and other like materials.
Referring now to FIG. 5B, this FIG. illustrates a bottom view of the four
compartment sensors 405 of FIG. 5A but with the individual sensors 405 divided
from one another through using the separating device 505, such as the
perforations,
according to one exemplary embodiment of the invention. Specifically, the
first
compartment sensor 405A of the first pair. of sensors 405A, 4056 is physically
located away from the second compartment sensor 405B. Similarly, the third
compartment sensor 405C of the second pair of sensors 405B, 405C is physically
located away from the fourth compartment sensor 405C. The separating device
505,
the expansion device 535 in combination with the alignment mechanism 410 and
central scan depth marker 415 can allow the compartment sensors 405 to be
accurately and precisely positioned over compartments of interest, such as the
four
compartments of a human leg 100. In order to accurately monitor the
appropriate
compartment, a right 'and left configuration can be provided since compartment
alignment would be reversed based on which leg is examined by the medical
practitioner. Each configuration would be labeled as right or left. The
configuration
illustrated in FIGs. 5A and 5B are designed for human left leg 100 where the
expansion device would be positioned over the tibia.
Referring now to FIG. 6A, this figure illustrates a bottom view of a three
sensor embodiment in which one sensor 605 of the three compartment sensors
405A, 405B, 605 can scan at two or more depths according to one exemplary
embodiment of the invention. Specifically, a compartment sensor 605 may
include
an optical transmitter 510C that works with at least two different optical
receivers
515C1 and 515C2. As noted above, each optical receiver 515 may comprise two or
more light detectors, such as photodiodes. In the embodiment illustrated in
FIG. 6A,
each optical receiver 515C1 and 515C2 has a total of four photodiodes in which
-22-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
pairs of photodiodes work together to provide "near" detector and "far"
detectors for a
respective receiver 515C1, 515C2. This combination allows the compartment
sensor 605 to scan at least two different depths. And because of the
capability to
scan at two different depths, the compartment sensor 605 is provided with two
different central scan depth markers 415C1, 415C2.
Referring now to FIG. 6B, this figure illustrates the compartment sensor 605
of
FIG. 6A that can scan at two or more depths in order to measure deeper
compartments of an animal body according to one exemplary embodiment of the
invention. The twp optical receivers 515 of FIG. 6B work in principal in an
identical
manner relative to the optical receiver described in connection with FIG. 7
discussed
above. This means that the combination of the optical transmitter 510b and
optical
receiver 515C1 can provide an oxygenation level for a first scan depth 620B of
a
patient. Meanwhile, the combination of the optical transmitter 510C and the
optical
receiver 515C2 can provide an oxygenation level for a second scan depth 620A
of a
patient.
Therefore, this stacked compartment sensor 605 can be used to measure the
oxygenation level of a first compartment that maybe positioned underneath a
second
compartment, such as for the deep posterior compartment of a lower leg 100 of
a
human body which is positioned beneath the superficial posterior compartment
of the
leg 100. This stacked compartment sensor 605 can allow the display and
processing unit 420 to subtract the oxygenation level found at the first scan
depth
620B of the first compartment, such as the superficial posterior compartment,
from
the oxygenation level at the second scan depth 620A of the second compartment,
such as the deep posterior compartment.
The invention is not limited to the two stacked optical receiver embodiment
605 illustrated FIGs. 6A and 66, and can include any number of optical
receivers 515
positioned in the substrate material 530 so that various scan depths can be
made to
determine oxygenation levels within multiple compartments that may be stacked
on
or positioned adjacent to one another in a sequential or layered, shallow to
deep
arrangement.
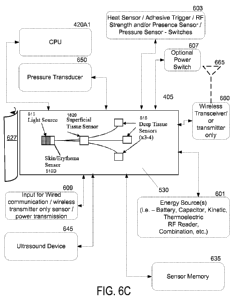
Referring now to FIG. 6C, this figure illustrates a bottom view of a wireless
sensor 405 comprising a substrate 530, a light source 510, and optical
receivers
515, similar to those described above according to one exemplary embodiment of
the invention. The sensor 405 also has a superficial tissue sensor or skin
sensor
-23-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
1820. Further details of the skin sensor 1820 will be described below in
connection
with FIG. 18.
The sensor 405 may be used to monitor tissue perfusion and/or other
modalities such as pH, temperature, blood pressure, intra-compartmental
pressure,
etc. According to this exemplary embodiment, the sensor 405 may be wireless.
This
means that the sensor 405 may have its own power or energy source 601 and it
may
communicate with the monitor 420 in a wireless manner using an antenna 665 as
will
be described in more detail below. The energy source 601 may comprise a
battery,
a capacitor, or any combination thereof. The size of the energy source 601 may
be
made to be very small, such as a size used for a conventional watch battery or
microcapacitor that are used in microelectronics as understood by one of
ordinary
skill in the art.
The life of the energy source 601, such as a battery or a capacitor, could be
designed to last as long as needed for a particular application: from a couple
hours
to a couple of days once activated. For example, in accordance with specific
medical conditions/applications, the sensor 405 may only need to monitor
tissue for
approximately 72 hrs to track possible conditions that may indicate an acute
compartment syndrome (ACS). The energy source 601 may be re-chargeable/re-
usable or disposable. A sensor 405 may be designed specifically for a type of
tissue
and a specific duration. As noted above, this means that sensors 405 may be
provided with predetermined scan depths depending on the type of tissue being
monitored. Scan depths of sensors 405 are further described below. The sensors
405 may also have different energy levels which are dependent on the type of
tissue
and the frequency at which readings may occur with that type of tissue.
According to one exemplary embodiment, the energy source 601 could be
heat from tissue of a body. Specifically, such an energy source 601 may
comprise a
thermoelectric generator that is based on the Seebeck coefficient as
understood by
one of ordinary skill in the art. This type of energy source 601 may be
similar to
watches that run off body heat.
For wireless sensors 405 used in exercise or training applications, motion or
movement of the body could be used to supply power to the re-chargeable energy
source 601. Such an energy source 601 may comprise a kinetic energy device. A
kinetic energy device may comprise oscillating weights that are turned by the
movements of the patient on which the sensor 405 is worn. These movements by a
-24-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
patient may create a magnetic charge in the sensor 405, which is turned into
the
electricity that is needed to power the watch. Specifically, the kinetic
energy device
may comprise weights, coils, a capacitor, and/or a battery as understood by
one of
ordinary skill in the art. The energy source 601 may use any one or a
combination of
the energy sources described above such as combining a thermoelectric energy
source with a kinetic energy source.
In a radio-frequency identification (RFID) embodiment, a radio-frequency
reader coupled to a monitor may provide energy to the wireless sensor 405 in
the
form of radio-frequency energy which is converted into electrical energy by
the
wireless sensor 405 as understood by one of ordinary skill in the art.
Additional low
energy signaling mechanism can be utilized such as low energy blue tooth. This
means that in addition to requesting data from the wireless sensor 405 using
RF, a
reader coupled to the monitor 420 may also supply power to the wireless sensor
405
with its RF energy.
According to this RFID exemplary embodiment, if the patient moved away
from wireless range of the monitor 420, then the sensor 405 may
stop/discontinue its
recordings to conserve energy for its energy source 601 or the sensor 405 may
decrease the frequency of its readings/measurements that are stored in memory
635. Once back in range of monitor 420, the sensor 405 may upload its data to
the
monitor 420 through wireless transceiver 660 and it may resume "normal"
readings.
If the sensor moves outside of the range of the monitor 420, an alarm may be
activated to notify the medical practitioner that the sensor cannot
effectively
communicate with the monitor 420. This alarm may be present in the monitor 420
that is activated by the CPU 4201A1 of the sensor 405. This alarm may comprise
an
audio and/or visual indicator such as a light emitting diode (LED), a message
on a
display, and/or a buzzer from a speaker.
Additionally, the frequency of signal capture for each reading made by a
sensor 405 may be increased based on clinically concerning values and/or they
may
be reduced in frequency by the CPU 420A1 if the values are stable and not
concerning to save battery life. Additionally, a socket/input 609 or
receptacle may be
provided in each sensor 405 where a cable may attach to each sensor 405. This
cable may provide both power and data to the sensor 405. The power provided
through the cable may re-charge the energy source 601 of the sensor 405.
-25-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
In those exemplary embodiments of the sensor 405 which may have the
socket/input 609, instead of using a wireless transceiver 660 which can
transmit or
receive data, the sensor 405 may comprise only a transmitter 660 so that
energy is
conserved by the sensor 405 from a reception perspective. In other words,
power
may be consumed by a sensor 405 when it receives data in a wireless manner. If
the sensor 405 is provided only with a transmitter 660 instead of a
transceiver 660,
then the sensor 405 could conserve some power relative to an embodiment which
has the transceiver 660. In an exemplary embodiment which only has a
transmitter
660, the sensor 405 may be programmed and/or receive control signals from the
monitor 420 when the sensor 405 has a cable coupled to it via the input/socket
609.
The light source 510 and the optical receivers 515 can be controlled over time
(and may be referred to in the art as time related reflectance) to adjust for
the
reflectivity and penetration depth of optical light which leaves the light
source 510
and enters layers of skin 1805 (See FIG. 18). The optical receivers 515 can be
controlled so that they are instructed to wait for measuring optical light
that has
penetrated deeper into the skin 1805 and other tissue layers. The light
reflectance
can be used to measure depth of fat subcutaneously in order to allow for
different
size people. For example, this light reflectance can be used and scaled for
people
with various thicknesses in skin and fat tissue.
Three to four or more receivers 515 can be positioned in an arch around the
same light source 510 at between about thirty and about forty-five degrees or
more.
One of ordinary skill in the art recognizes that any number of receivers 515
and light
sources 510 can be employed at various different positions without departing
from
the scope of the invention.
In other exemplary embodiments (not illustrated), the receivers 515 can be
positioned in a circle or at about 360 degrees around the single light source
510.
Each of the optical receivers 515 can be independently or separately
controlled.
The optical receivers 515 and/or the skin sensor 1820 can be used to
measure any initial reflectance over a short time period such that skin
pigment and
erythema can be assessed. Erythema is a condition of skin and tissue after
they
have been injured. Usually, with this condition, fair or white pigmented skin
individuals usually have red colored, swollen skin near tissue in an
Erythematic
condition. If the light source 510 uses red colored light to assess red
colored skin
-26-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
then false readings may occur in this situation. Therefore, the light source
510 can
be provided with a skin sensor 510D that is used under Erythema conditions.
Additional wavelengths can be added to the light source 510 outside of the
near infrared range in order to determine a measure of erythema of the
subcutaneous skin. By using wavelengths in the red and green spectrum, an
algorithm can be formulated to measure erythema (A dermaspectrum ll was used
by
the inventor in a study in which the inventor looked at pigment using green &
red
light to measure erythema). However, one of ordinary skill in the art
recognizes that
other colors or optical wavelengths, above or below the green colored
spectrum, can
be produced by the skin sensor 510D without departing from the invention.
The skin sensor 510 can scan at exemplary depths of about between four and
seven millimeters. However, other depths higher or lower than those
specifically
described are not beyond the scope of the invention.
The skin sensor 510D can be calibrated to measure both a pigmentation
index as well as an erythema index. The pigmentation index would be
incorporated
in all measurements. The erythema index would allow the sensor to determine if
the
tissue being measured was traumatized or not. Different calibrations could be
used
in different circumstances. If the tissue 1805 being measured is traumatized,
the
erythema index would be elevated and a hyperemic effect would be expected. If
the
erythema index is elevated and hyperemia is not present an alarm would be
triggered for concern about poor perfusion. Control, uninjured tissues could
be
recognized by lower erythema indices. The following are indices that can be
used by
the dermaspectrometer for measuring pigment (red light only) and erythema (red
&
green light):
Melanin index (100 log 1/ ired)
Erythema index (100 log ired / igreen)
If the skin layer 1805 is missing, then the skin sensor 510D can be shut off
while the light source 510 continues to illuminate a tissue area of interest.
This
ability will be important in traumatized tissue or wounds since in many cases
trauma"
results in loss of skin. When skin is missing the superficial recording will
be turned
off in order to account for lack of pigmentation and erythema. A separate
calibration
will be used in these cases where measurements are taken directly over tissue
which does not have skin, such as over muscle.
-27-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Additionally, a sterile sensor 405 with a sufficiently long sterilized cord
will be
required to allow for sterile technique to be maintained in an operating room
setting.
According to one exemplary embodiment, the light source 510 can be provided
with
at least two different light sources 510 that illuminate in different optical
wavelengths,
such as in the wavelengths for the color red and green. In this embodiment,
these
two different light sources can be used to detect an erythema condition in
which the
skin layers 1805 may be red in color.
The sensor 405 can be designed to account for different thickness or level of
fat layers present in a particular patient. Sensors 405 can be sized to
measure
different sized individuals. For example based on the circumference of a leg,
or
extremity/body part, different size devices can be fabricated with different
depths of
tissue monitoring (spread of light source and sensor) in order to maximize the
tissue
sampling in a correct location. A large, medium and small size can be designed
to
read tissues customized to different anatomic variations in different sized
people.
An ultrasound device 645 can be incorporated into the sensor 405 for
monitoring and for determining fat depth. The ultrasound device 645 and other
devices in FIG. 6C are illustrated with dashed lines to indicate that such
hardware/software may be optional for the sensor 405. Also, any combinations
of
this hardware/software can be included in the sensor 405 without departing
from the
invention.
A pressure transducer 650 can be incorporated into the sensor 405 in order to
determine if the dressings for a wound have been applied too tight.
Additionally, in
trauma settings, dressings are applied initially and swelling continues after
the
dressing application. If a pressure transducer 650 determines increasing
pressure
on the tissue 1805 from external forces (dressings, splints, casts), an alarm
can
sound to warn the clinician. If external pressures increase while and
oxygenation
values decrease, then the alarm will sound to release the dressing or loosen
the
restrictive dressing.
The sensors 405 can be used to take multiple readings of similar areas of
interest. Subcutaneous vessels can cause erroneous values. Subcutaneous vessel
effects can be removed if single sensor 405 is aberrant. Additionally, in
traumatized
tissue, readings can be difficult to obtain due to hematomas (collections of
blood).
Each sensor 405 may also account for small vessel abnormalities. A weighted
average of values from the scans can be taken which should yield better
sampling of
-28-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
tissue that is of interest. It should also allow for higher ability of
obtaining a reading
and maintaining a reading after placement since hematomas would have to block
multiple sensors to lose a signal completely. Abnormally high or low values
(deviated by a predetermined value such as ten or more percentage points) can
be
thrown out.
The sensor 405 may further comprise a memory device 635. The memory
device 635 may comprise volatile or non-volatile memory or a combination of
both.
The memory device 635 can comprise any type of machine readable medium. Any
machine readable medium can include, but is not limited to, floppy diskettes,
optical
disks, CD ROMs, magneto optical disk ROMs, RAMs, EPROMs, EEPROMs,
magnetic or optical cards, flash memory, or any other 'type of media/machine
readable medium suitable for storing records and/or electronic instructions
for a CPU
420A1.
The CPU/microcontroller 420A1 can run or execute programs for activating
the various components of the sensor 405 to take readings and storing them in
the
memory device 635. Alternatively, the CPU 420A1 may comprise firmware and/or
hardwired circuitry without departing from the scope of this disclosure. The
CPU
420A1 may compute values, running averages, store raw data and/or time stamps
in
sensor memory 635, and monitor capacity of the energy source 601 and send
warning signals/messages when the energy source 601 is running low. An alarm
can
sound if the signal is lost due to the battery life or if the sensor 405 moves
outside of
the range of the monitor 420, as described above. Additionally, if the sensor
405 is
removed or discarded, it can be permanently turned off or deactivated in order
to
prevent unneeded noise.
Additionally, the communication between the sensor 405 and the monitor 420
(comprising a computer) may be bi-directional. The sensor 405 can send data to
the
computer/monitor 420, but the computer 420 can signal changes in the sensor
405.
It can send a signal to make sure the sensor 405 is responsive and in range.
It can
also signal the sensor 405 to increase its frequency or decrease its frequency
of
readings based on collected data.
The CPU 420A1 of the sensor 405 may also be coupled to a wireless
transceiver 660 that uses an antenna 665. The wireless transceiver 660 can
employ
any one of a number of wireless media such as radio-frequency communications,
optical communications, magnetic/inductive
communications, acoustic
-29-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
communications, and other like wireless media, as described above in
connection
with FIG. 4. The wireless transceiver 660 can relay the data produced and
recorded
by the sensor 405 to a remote monitoring apparatus, such as a monitor 420.
Additionally, the data could be sent via satellite or other means to a central
monitoring station or even a clinicians phone, pager or other mobile device
for
distance monitoring or access.
In this way, the sensor 405 may become a portable unit that can be used by
the patient and/or monitored by a medical practitioner located at a distance
from the
patient. With the memory device 635, continuous data can be stored on the
sensor
405 without a need to record on a central device such as a processor and
display
unit 420. This ability to record data on the sensor 405 will allow a sensor to
couple
with different, remote monitors 420 to allow continual data collection and
display of
the data. This feature will allow for interchangeability between sensors 405
and
remote monitors 420, irrespective of their manufacturer. The data stored in
the
memory device 635 may be provided with time stamps so that the data can be
mapped over time.
According to an alternate exemplary embodiment, each sensor 405 may be
designed to take sample measurements in a periodic manner instead of making
continuous/constant measurements over time. One goal or objective in such an
exemplary embodiment would be to take quick pulses of measurements over long
periods of time instead of continuous measurements in order to conserve energy
from a limited energy source 601. Conventional sensors 405 may provide new
information about 1.0 to about 6.0 seconds continuously.
And such continuous measurements usually require larger energy sources
601 and usually require the sensors 405 to be wired to the energy sources.
When
monitoring brain tissue, such continuous measurements may be required.
However,
when monitoring other tissues, like muscle of a compartment, such an amount
and/or frequency of this information is not needed. Spot checks or a series of
reading over a short period of time can be used to monitor tissue without
continuous
readings to preserve battery life.
For example, human extremities, such as arms and legs, may be put under
tourniquet times of about 120 to about 150 minutes without permanent damage.
This means that when wireless sensors 405 are monitoring such tissues, then
their
sampling time may be adjusted to about once every sixty seconds or
significantly
-30-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
longer period to provide adequate information for monitoring a leg or arm for
the
development of a compartment syndrome.
This. time frequency and duration can be preprogrammed into the sensor 405
in which different tissue and thus, tissue specific and tissue assigned
sensors 405
can have preset settings for different frequencies and alarms. In other words,
this
tissue specific sensor system may establish tissue specific parameters for
specific
types of tissue in the body. Specific controls for each sensor 405 can be
established
for specific tissues that are to be monitored with a particular sensor 405.
Data for
injured tissue can be interpreted by different control sites. Multiple
controls can be
used for different tissue types in order to interpret data from multiple
sensors 405.
Referring briefly now to FIG. 6E, a wireless sensor 405 monitoring injured or
at risk muscle can be achieved with wireless sensors 405 programmed for muscle
tissue monitoring at injured tissue sites 689, while other wireless sensors
405 at
control sensor sites 688 may be designed for monitoring brain tissue and non-
injured
muscle tissues. Control sensor sites 688 can be used to monitor
global/systemic
perfusion. For example, one of the sensors 405 for a control site 688A may
monitor
brain tissue, while other control sites 68813 may monitor the an internal
organ which
includes, but is not limited to, the kidneys, liver, etc.
Usually, one preferred and exemplary control/injured monitoring scheme is as
follows: For each injured tissue site 689 being monitored which has a single
sensor
405, there will usually be two non-injured or control sites 689 being
monitored with
two other sensors 405, such as a sensor 405 for the brain and a sensor 405 for
tissue that is similar to the non-injured tissue. So if the injured tissue
site 689
comprises leg tissue, then the control sites 689 may comprise one sensor on
the
brain 405 and an sensor 405 on corresponding tissue in a healthy leg of the
patient.
According to one exemplary control vs. injured monitoring scheme, the
system may determine what is possible normal perfusion changes, versus
hypotension (i.e. when muscle oxygenation levels go down significantly, while
the
brain oxygenation levels starts to go down at the same time ¨ this unhealthy
condition may comprise hypotension). Meanwhile, if only injured tissue was
being
monitored with a sensor 405 or set of sensors 405, then a medical practitioner
may
not be sure if low oxygenation levels for the injured tissue mean hypotension
or
muscle activation.
-31-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
If the brain is injured, the traumatic brain tissue can be monitored while
muscle tissue can be monitored with two specific and separate control sites
688 as
illustrated in FIG. 6E. A control site 688 usually comprises un-injured
tissue.
Therefore, one of ordinary skill in the art recognizes that control sites 688
and injured
tissue sites 689 can easily be switched or swapped/interchanged depending upon
what tissue is injured and is monitored with sensors 405.
The relative comparison in oxygenation levels between control sensor sites
688 and injured tissue sensor sites 689 can be important for determining a
perfusion
state of the entire body which has the injured tissue. One of ordinary skill
the art will
recognize that the brain is considered privileged tissue in the human body and
will be
perfused at the expense of all other tissues. Therefore, monitoring the brain
as a
control sensor site 688 can help identify global perfusion and systemic whole
body
health in the setting of trauma. An additional control in an uninjured
extremity can
help to identify local changes in non-privileged areas of the body. By
monitoring
control sensor site areas 688, other areas that are at risk can be
interpreted.
Information on global perfusion (hypotension & adequate resuscitation) can be
determined in the setting of injured and traumatized tissue.
By having multiple control sites 688 and knowing that certain tissue will
decrease in perfusion prior to other tissues, an estimate of global perfusion
can be
determined and monitored over time by seeing which tissues are being well
perfused
and which are not, based on the oxygenation readings from the sensors 405.
This
ability to determine systemic volume (whole body blood volume) and perfusion
status
(I.E. - cardiac output, vasospasm, blood volume, oxygen saturation, etc...)
based on
oxygenation readings from sensors 405 is important in the traumatized setting
as
monitoring sites 688, 689 can change based on local factors such as increased
tissue pressure (compartment syndrome) or systemic factors (i.e. - anemia,
bleeding,
hypotension, hypoxia, etc...).
For example, in a traumatized patient, on initial evaluation, the patient may
not show signs of active bleeding but may have internal bleeding that is not
appreciated. As the patient continues to lose blood, less privileged sites
(i.e. ¨ non-
brain regions) will begin to show hypoperfusion indicated by low oxygenation
levels
from sensors 405. These less privileged sites would be early warning signs. As
more blood is lost, more privileged sites will have oxygenation values that
begin to
decrease. Once brain perfusion oxygenation values begin to fall (the brain
being
-32-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
one of the most privileged sites in the human body as explained above), severe
hypotension has been reached and an emergent state is present requiring blood
transfusions and diagnosis and correction of blood loss.
In another example, in a severe hypotension situation, blood flow to the
entire
body may slow down to an unhealthy state in which nonessential tissue, i.e.
areas
outside of the brain, heart, and kidneys may not receive an adequate supply of
blood. Such a condition may be more readily detectable with a control sensor
site
688A that is monitoring the perfusion of brain tissue. If such a condition is
detected
by a sensor 405, the medical practitioner may then focus on injured tissue at
the
injured tissue sensor sites 689. If the readings at the injured tissue sensor
sites 689
steadily decline or rapidly decline in combination with lower readings taken
at control
sensor sites 688A monitoring brain tissue, then the medical practitioner may
act
quickly to preserve the injured tissue if additional severe trauma is assessed
such as
the development of a compartment syndrome as described above and below.
According to one exemplary embodiment, the wireless sensor 405 may have
several modes of operation. These modes of operation may be manually selected
or
automatically selected. For example, a medical practitioner may select a
continuous
mode of operation such that the wireless sensor 405 takes continuous readings,
such as on the order of about one reading every second. In another example,
the
medical practitioner may select a periodic mode of operation where the medical
practitioner can select the length of time between measurements taken by
wireless
sensor 405. Exemplary lengths of time that may be selected include, but are
not
limited to, about every five, ten, fifteen, twenty, thirty, and sixty seconds.
Other
lengths may include every other two to ten minute intervals and the like as
understood by one of ordinary skill in the art.
Additionally, the reading or output of a wireless sensor 405 can be given as a
specific instantaneous number or a running average or area under the curve or
other
type of measure to show trends versus instantaneous numbers which can vary
widely over time and make setting alarms more difficult. The CPU 420A1 of each
wireless sensor 405 may use various mathematical tools and/or functions to
filter out
any signal noise produced and/or detected by a particular 405. Running
averages
as described above for readings taken by a 405 may be one way or tool to
assist
with the reduction and/or filtering out of noise and other similar false
readings/inaccurate data. Noise, movement, and/or other physiologic factors
can
-33-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
cause normal and non-dangerous changes which can be identified using trends
and
controls with the sensors 405, and particularly the CPU 420A1 of each sensor
405 in
order to prevent false alarms.
In other embodiments, the wireless sensor 405 may be programmed to
automatically adjust its measuring intervals. This means that if the wireless
sensor
405 does not detect any change in a condition, such as in an oxygenation
level, then
the wireless sensor 405 may maintain its current measuring interval or
increase the
length of the downtime between measuring in order to preserve power. However,
if
the wireless sensor 405 detects a change in a condition/reading that may be of
concern, then the wireless sensor 405 may increase the frequency at which its
measurements are taken, thereby decreasing the length between
measurements/readings. The wireless sensor 405 may be programmed to allow
manual overrides as desired by medical practitioners. The programming and
these
different modes of operation for each wireless sensor 405 may be supported by
a
CPU/microcontroller 420A as understood by one of ordinary skill in the art.
The activation of each wireless sensor 405 may be controlled: each sensor
405 may not be activated until it is placed on live tissue. This objective or
feature
can be achieved through one or more different activation sensors 603. One
activation sensor 603 may comprise a covering with an adhesive surface. This
activation sensor 603 may be a trigger which turns on the sensor 405 on once
the
sticker activation sensor 603 is peeled off. A circuit can be connected or
deactivated
with a thin piece of metal on the adhesive cover that would activate the
sensor when
it is removed. Additionally, a body heat activation sensor 603 may be used to
activate the wireless sensor 405 to take readings. This feature will prevent
the
battery or other energy source 601 (such as a capacitor) from being used up
before
it is placed on a body to monitor it. Other options to activate the wireless
sensor 405
include an on/off button/switch 607.
Additionally, a pressure sensor/actuator 603 may be used to activate the
wireless sensor 405 when placing the wireless sensor 405 on the body to obtain
the
appropriate placement of the sensor 405 on the body. The use of the pressure
sensor 603 may be beneficial since a medical practitioner may "test" the
sensor 405
using the pressure sensor 603 to get a read before removing any seals on the
adhesive materials 719 (described below in connection with FIGs. 6F-6G).
-34-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
For example, when placing the sensor 405 on a patient, typically the sensor
405 is placed over potential sites before an adhesive cover/seal 627 is
removed to
allow a sample read of tissue to ensure an appropriate reading and placement.
-
Without removing the adhesive cover/seal 627 to activate the wireless sensor
405,
the wireless sensor 405 may be placed on the body and pressure sensed by the
pressure actuator 603 may temporarily activate the sensor 405 to read the
tissue
proximate to the sensor 405 as understood by one of ordinary skill in the art.
The pressure sensed by the pressure actuator 603 may be in the form of a
medical practitioner placing their fingers on top of the sensor 405 and
holding it in
place over the tissue of interest to insure a good seal/tissue interface ¨
while not
removing the cover 627 of the adhesive 719. Once pressure is removed and no
longer sensed by the pressure actuator/sensor 603, the wireless sensor 405
returns
to an inactive mode until permanent placement is desired and the adhesive
cover
627 is removed.
When the adhesive cover 627 is removed from the adhesive materials 719
(see FIGs. 6F, 6G), the removal may activate a switch 603 and/or a heat sensor
603
may be exposed directly to the tissue of the patient. Either the adhesive
cover
switch 603 and/or heat sensor 603 may activate the wireless sensor 405 so that
it
enters into a full, operative state. The heat sensor 603 may also deactivate
the
wireless sensor 405 once the wireless sensor 405 is removed from a patient and
body heat is no longer detected by the heat sensor 603 as understood by one of
ordinary skill in the art.
Additionally, interchangeability between different sensors 405 for different
functions (such as a leg sensor 405 or an arm sensor 405 or a cerebral sensor
405
and a tissue transfer sensor 405) would be recognized by the system 1900 and a
set
series of alarms or readings would be recorded based on the sensor 405
inserted
into the monitor 420 or coupled to the monitor 420 in a wireless manner.
Therefore,
the same monitor 420 would be able to identify the type of sensor 405 couple
to the
monitor 429. A single monitor 420 could be compatible with multiple different
sensors 405 and display appropriate data and alarms based on the sensor 405
collecting data about the tissue/areas of interest.
Each wireless sensor 405 may have an assigned identity through a unique
identifier so that multiple sensors 405 can be placed around and on different
parts of
the body as described above. The unique identifier may comprise an
alphanumeric
-35-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
code having a predetermined number of digits that could be assigned during
manufacture of the sensor 405. The unique identifier may allow a
monitor/computer
420 to differentiate between sensors 405 placed on a single patient as well as
sensors 405 placed on other patients which may be in proximity for a single
monitor
420.
In other words, a computer/monitor 420 may use the unique identifier
assigned to each sensor 405 in order to track the sensors 405 assigned to each
particular patient. In this way, a monitor 420 may not accept or use readings
from
different wireless sensors 405 on different patients which may be in proximity
(within
RF range relative) to a desired patient. Each sensor 405 may be provided with
two
unique identifiers in which one is permanent and assigned at manufacture while
a
second unique identifier may comprise a patient identifier that can be shared
across
multiple sensors 405 assigned to a single patient. A patient identifier may be
assigned by the medical practitioner and can be changed after each use.
Each wireless sensor 405 may be marked (like 410 and 415 of FIG. 5B) prior
to placement so the sensor 405 could be designated for a specific location in
the
body and can be tracked against other areas of the body (See FIG. 6E). For
example, a sensor 405 may be assigned to a specific compartment of an
extremity
or as a control (uninjured) versus an injured area of the body (See FIG. 6E).
As
described above, sensors 405 can be preset to measure different types of
tissue and
or locations of the body. These sensors 405 would be site/tissue specific
sensors.
All sensors 405 can have the same set of algorithms, but then can be set at
time of placement for specific tissue. In other words, a first algorithm may
be specific
for brain tissue. A second algorithm may be specific for muscle tissue. Each
sensor
405 may have these two algorithms but each algorithm may be specifically
activated
or selected when the sensor 405 is positioned over the particular tissue of
interest.
This selection or activation of a particular algorithm may be completed at the
sensor
or computer level. In other words, a medical practitioner may select the
algorithm
when the sensor 405 is placed on tissue and/or a computer/monitor 420 may
automatically select the algorithm after it determines the type of tissue that
is being
monitored.
The memory device 635 may have a significant amount of capacity and it may
allow for notes to be added to patient history. In addition to the reflectance
values
recorded by the optical receivers 515, the memory device 635 may be able to
store
-36-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
other measured parameters taken by other sensors and/or tests. For example,
the
memory device 635 may also store a patient's blood pressure, temperature,
respiratory status (rate, supplemental oxygenation, pulse oximetry values,
etc.) over
time. Further, any lab work such as lactic acid levels, CBC count, and blood
chemistry may be provided and stored in memory device 635.
The sensor 405 can be incorporated into a wound dressing in order to monitor
physiological conditions during transportation of injured subjects. This
capability
would be built into a dressing that can be attached to vacuum devices for
management of extremity wound with gross contamination or skin loss. Each
sensor
may be flat in shape (See FIGs. 21 and 22 below) so it can fit under a
dressing.
Each sensor 405 may be sterile (FIG. 21) so it can be placed in the operating
room
(OR). Sensor 405 is only a sticker- couple millimeters thick so can fit under
dressings 2205.
FIG. 6D illustrates sensors 405 that may be used for muscle
training/endurance applications according to one exemplary embodiment of the
invention. This wireless sensor 405 may be reusable and a non-disposable
sensor.
Again, it may or may not have computing components 420A1. It could have a
larger
energy source 601, such as a double AA or triple AAA sized battery cell. It
could be
larger in size and allow for communication with a wrist watch type reader 667
that
reads the data being transmitted to provide real-time data. In this exemplary
embodiment, sampling in which measurements are taken over longer windows of
time such as on the order every minute, every other minute, or every five
minutes
could be used to minimize extra data while maximizing battery life of the
portable,
wireless sensor 405.
The reader 667 may indicate if the sensor 405 is transmitting a signal or not
transmitting a signal. So lack of signal originating from a sensor 405 by the
reader
667 may be recognized and detected as a condition for the reader 667 to
activate an
alarm (audio or visual or both). If a signal from a wireless sensor 405 is
lost or the
sensor 405 stops transmitting, the reader 667 may indicated this condition and
display a message that states that data is old and is not current.
The reader 667 may relay data from sensors 405 to a computer/base station
669. This computer/base station 669 may interpret and compute the data in
order to
provide a final NIRS value for display on another monitor display device 420.
This
display device 420 (See FIG. 10, 1300 of FIG. 14C) could be portable with a
larger
-37-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
battery or have an AC plug. This device 420, 1300 could be attached to a
patient
bed/stretcher/IV pole or it could be a hand-held unit.
As illustrated in FIG. 6D, the wireless sensor 405 may be held in position
with
an elastic bandage/wrap 688. The wrap 688 may include hook and loop type
fasteners, such as VELCRO(TM). The wrap 688 may be constructed from elastic
materials like SPANDEX(TM), etc. The wrap 688/sensor 405 combination may allow
for reuse for muscle training and/or exercising. While a single wireless
sensor 405 is
illustrated, multiple sensors 405 may be supported by the wrap 688.
Readings from the sensor 405 may be recorded in the wrist watch reader 667
that can be worn on an arm 255 of a subject 250. The wrist watch reader 667
may
utilize a wire or it may be use a wireless coupling with the sensor 405. Data
may
then be transferred to computer/base station 669 from the wrist watch reader
667 by
USB, blue tooth, other wireless means. A global positioning system (GPS) may
be
part of the wrist watch reader 667 and the GPS may help the reader 667 to
track
speed, location, distance, altitude, and/or time of an exercise regime.
Referring briefly now to FIGs. 6F and 6G, each wireless sensor 405 may be
provided with a mechanism to wick moisture/fluids away from the sensor
elements
such as the light source 510 and the light receivers 515. When a sensor 405 is
placed on tissue for more than several minutes, sweat generated by sweat
glands
can accumulate under the sensor 405 causing the sensor 405 to lift and
potentially
lose signal. Additionally, with exercise, sweat can accumulate near or at the
sensor
site depending on the duration of the exercise.
A wicking system or mechanism may comprise absorbent materials 721 which
are provided with predefined shapes to encompass or circumnavigate the sensor
elements which may include light sources 510 and light receivers 515. The
absorbent materials 721may comprise any one or a combination of materials,
such
as, but not limited to, super absorbent polymers (SAP), gauze, gauze
impregnated
with an agent designed to help sterility or to speed healing like boracic
lint, films,
gels, foams, hydrocolloids, alginates, hydrogels and polysaccharide pastes,
granules, beads, gauze with a nonstick film, over the absorbent gauze to
prevent the
wound from adhering to the dressing, and the like.
The shapes of the absorbent materials 721 may be varied and may be custom
made for particular body portions. In the exemplary embodiment illustrated in
FIG.
6F, absorbent reservoirs 721A,B,C which may comprise finger-like projections
may
-38-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
be provided. Alternatively, the reservoirs 721A,B,C may be made entirely
symmetrical as illustrated in FIG. 6G. The reservoirs may surround,
circumnavigate,
and/or enclose the adhesive materials 719 in any fashion as understood by one
of
ordinary skill in the art.
The absorbent materials 721 may be exchangeable to allow for exchange
after use and replaced with new ones or after being dried out. The absorbent
materials 721 may form channels that remove fluid from around the sensor's
working
components (i.e. the light source 510 and light receivers 515A, 515B). The
absorbent materials 721 may form strips or radial lines with larger reservoirs
on the
perimeter of a sensor 405 in order to pull moisture away and out from the
light
source 510 and light receivers 515. The amount of moisture that may be
collected
will likely be small so that the reservoirs 721A, 721B, 721C formed by the
absorbent
materials 721 need only to be a couple of millimeters in width and depth. A
suction/vacuum device providing a hose or conduit (not illustrated) may be
attached
to this absorbent material 721 if desired to pull the fluid into the conduit
and out from
the absorbent material 721.
Adhesive materials 719 may surround and may be positioned within the
reservoirs 721A, 7216, and 721C of the absorbent materials 721. The adhesive
materials 719 may fasten/attach a sensor 405 to the patient. The adhesive
materials
719 may include, but are not limited to, acrylic-based, hydrocolloid,
hydrogel, rubber-
based, polyurethane, soft silicone, cyanoacrylate types of adhesives, and/or
any
combination thereof.
FIG. 6H is a logical flow chart illustrating a method 711 for providing
enhanced and efficient wireless sensors 405 according to one exemplary
embodiment of the invention. The steps of this method 711 correspond to the
structures discussed above in connection with the wireless sensors 405
described
above in connection with FIGs. 6A-6D.
Block 712 is the first step of method 711. In block 712, each wireless sensor
405 may have its size is adjusted for its specific application. This means
that the
dimensions of each wireless sensor 405 may be adjusted to suit a particular
application. For example, in a wound dressing environment, a wireless sensor
405
may be made very thin (with a reduced thickness) so that it may fit within a
layer or
stack of layers of a bandage. In another example, such as illustrated in FIG.
6D, the
wireless sensor 405 may be made more rugged for multiple uses such as in
exercise
-39-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
environment. Also, each wireless sensor 405 may be provided with a range of
programs for different applications.
For example, each sensor 405 may be provided with a first algorithm that is
designed specifically for brain tissue. Each sensor 405 may also be provided
with a
second algorithm that is designed specifically for muscle tissue. These
algorithms
may be selected by the medical practitioner or automatically by a
monitor/computer
421 is determined the type of tissue being monitored by the wireless sensor
405.
Next, in block 714, each wireless sensor 405 may be provided with a unique a
numeric identifier during its manufacture. As described above in connection
with
FIG. 6C, each sensor 405 may have been assigned an identity so that multiple
sensors 405 can be placed around and on different parts of the body. The
unique
identifier may comprise an alpha numeric code that has a predetermined number
of
digits as understood by one of ordinary skill in the art. In
this way, a
computer/monitor 420 may assign specific unique identifiers to specific
portions of a
single patient while also assigning these specific identifiers to a particular
patient so
that signals originating from other wireless sensors 405 of other patients
will not be
misread or incorrectly grouped with a particular patient.
In block 716, each wireless sensor 405 may be provided with a compact,
reusable energy source 601. As noted previously, the energy source 601 may
comprise a battery, a capacitor, or any combination thereof. The size of the
energy
source 601 may be made to be very small, such as a size used for a
conventional
watch battery or a microchip. The energy source 601 may be re-chargeable/re-
usable. According to one exemplary embodiment, the energy source 601 could be
heat from tissue of a body.
This type of energy source 601 may be similar to watches that run off body
heat (thermoelectric) as understood by one of ordinary skill in the art. For
wireless
sensors 405 used in exercise or training applications, motion or movement of
the
body could be used to supply power to the re-chargeable energy source 601. The
energy source 601 may use any one or a combination of the energies described
above such as combining thermoelectric with a battery and/or capacitor. In a
radio-
frequency identification (RFID) embodiment, a radio-frequency reader coupled
to a
monitor may provide energy to the wireless sensor 405 in the form of radio-
frequency energy, such as low energy blue tooth, which is converted into
electrical
energy by the wireless sensor 405 as understood by one of ordinary skill in
the art.
-40-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Next, in block 718, each wireless sensor 405 may be provided with one or
more activation sensors 603 and one or more on/off switches 607. One
activation
sensor 603 may comprise a covering with an adhesive surface. This activation
sensor 603 may be a trigger which turns on the sensor 405 on once the sticker
activation sensor 603 is peeled off. Additionally, a body heat activation
sensor 603
may be used to activate the wireless sensor 405 to take readings. This feature
will
prevent the battery or other energy source 601 (such as a capacitor) from
being
used up before it is placed on a body to monitor it.
In block 720, each wireless sensor 405 may be provided with selectable
modes and/or automatic and/or manual selectable modes of operation. These
modes of operation may be manually selected or automatically selected. For
example, a medical practitioner may select a continuous mode of operation such
that
the wireless sensor 405 takes continuous readings, such as on the order of
about
one reading every second. In another example, the medical practitioner may
select
a periodic mode of operation where the medical practitioner can select the
length of
time between measurements taken by wireless sensor 405.
Exemplary lengths of time that may be selected include, but are not limited
to,
about every five, ten, fifteen, twenty, thirty, and sixty seconds. Other
lengths may
include every other two to ten minute intervals and the like as understood by
one of
ordinary skill in the art. In other embodiments, the wireless sensor 405 may
be
programmed to automatically adjust its measuring intervals when certain
conditions
are detected as described previously in connection with FIG. 6C.
Subsequently, in block 722, each wireless sensor 405 may compute values
and running averages with its CPU/microcontroller 420A1. In block 724,
multiple
control sites 688 such as illustrated in FIG. 6E may be monitored and compared
to
injured tissue censor sites 689. Such monitoring of different sites 688, 689
on a
human body may allow the system to interpret conditions of perfusion for the
entire
human body as understood by one of ordinary skill the art.
Next, in block 726, each wireless sensor 405, specifically the
CPU/microcontroller 420A1, may monitor the capacity of the one or more energy
sources 601 in order to determine the current energy level or state of the
energy
sources 601. And lastly in block 728, each wireless sensor 405 may generate
and
send warning messages if certain conditions are detected. For example, if a
wireless sensor 405 has detected that it is out of range or is about to get
out of range
-41-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
relative to its monitor 420, it may generate a warning signal/message. Other
conditions include, but are not limited to, low levels detected with respect
to the
energy source 601. The method 711 then returns back to block 712.
Referring now to FIG. 8A, this figure illustrates a linear array 805 of
compartment sensors 405 assembled as a single mechanical unit that can provide
scans at various depths 620A, 620B, 6200, and 620D. The compartment sensors
405 can be simultaneously activated to produce their scans of various depths
620 at
the same time when optical filters are used as will be described more fully
below in
connection with FIG. 8C. Alternatively, the sensors 405 of the linear array
805 can
produce their scans of various depths 620 by controlling a phase or timing of
the
activation of the sensors 405 so that no two sensors 405 are activated at the
same
time in order to reduce any potential of optical interference between the
sensors 405.
This phasing of the sensors can be controlled by the display and control unit
420 of
FIG. 4.
The first compartment sensor 405A can provide a first scan depth 620A that is
shorter or more shallow than a second scan depth 620B produced by the second
compartment sensor 405B. The scan depths 620 can increase in this manner along
its longitudinal axis which corresponds with its alignment mechanism 410 so
that the
linear array 805 matches the one or more depths of a single compartment of
interest.
As noted above in connection with FIG. 6B, the scan depth 620 of a compartment
sensor 405 is function of the separation distance between the optical
transmitter 510
and optical receiver 515. For example, a scan depth 620 of a compartment
sensor
405 can be decreased as the optical receiver 515 is moved closer along the
body of
the sensor 405 towards the optical transmitter 510C.
One of ordinary skill in the art recognizes that many of the compartments of
the human body have various different geometries and resulting depths relative
to
the outside skin of a patient. For
example, the compartments of the lower
human leg 100 tend to have a greater depth or volume adjacent to the knee and
generally taper or decrease in depth towards the ankle or foot. Therefore,
linear
arrays 805 of compartment sensors 405 can be designed to have depths that
match
a particular geometry of a compartment of interest. To achieve these different
scan
depths 620, each compartment sensor 405 can have an optical transmitter 510
and
an optical receiver 515 that is spaced or separated from each other by an
appropriate distance to achieve the desired scan depth 620. If a compartment
of
-42-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
interest has a substantially "flat" or "linear" depth relative to the skin
surface of a
patient, the linear array 805 can be designed such that each compartment
sensor
405 produces scans with uniform depths (not illustrated) to match a
compartment
with such a linear or flat geometry.
Like the single sensor embodiments described above in FIGs. 4-6A which are
designed to measure individual compartments, the compartment sensor array 805
may comprise an alignment mechanism 410 that can be positioned so that it
corresponds with the longitudinal axis 450 of a particular compartment. The
compartment sensor array 805 of FIG. 8A is not provided with any central depth
markers 415 like those of the single sensor embodiments since the depth
markers
415 may not be needed by the medical practitioner since he or she will be
assessing
the entire length of a particular compartment with the entire compartment
sensor
array 805 which is unlike that of the single sensor embodiments.
Alternatively,
multiple crosshatches and numerical depths (not illustrated) can be positioned
over
each light source/receptor set to locate where each measurement is obtained
for
identifying sites of a hematoma, which will be described in more detail in
connection
with FIGs. 15-16 below. Additionally, these positions could be used to locate
appropriate amputation level for diabetics or peripheral vascular disease,
which is
also described in more detail in connection with FIGs. 15-16 below.
Referring now to FIG. 8B, this figure illustrates a linear compartment sensor
array 805 that can include optical transmitters 510 that are shared among
pairs of
light receptors 515. For example, a single optical transmitter 510A1 can
produce
light rays 820A, 820B that can be used by two optical receivers 515A1, 515A2
that
are disposed at angles of one-hundred eighty degrees relative to each other
and the
optical transmitter 510A1 along the longitudinal axis and alignment mechanism
410A
of the compartment sensor array 805A. As described previously, the light
source
and receptor separation can be varied to best match the topography of the
compartment in the leg or other body part. Larger separation would allow for
deeper
sampling in the proximal leg versus more shallow depth closer to the ankle.
As discussed above in connection with the single sensor array 805 of FIG. 8A,
the sensors 405 of each compartment sensor array 805 illustrated in FIG. 8B
can be
simultaneously activated to produce their scans at the same time when optical
filters
(not illustrated in FIG. 8B) are used as will be described more fully below in
connection with FIG. 8C. Alternatively, the sensors 405 of each linear
compartment
-43-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
sensor array 805 can produce their scans by controlling a phase or timing of
the
activation of the sensors 405 so that no two sensors 405 are activated at the
same
time in order to reduce any potential for optical interference between the
sensors
405. This phasing of the sensors can be controlled by the display and control
unit
420 of FIG. 4.
Like the single sensor embodiment illustrated in FIG. 5A, the compartment
sensor array 805 of FIG. 86 can comprise an alignment mechanism 410 for
aligning
the structure with the longitudinal axis 450 of a compartment as well as a
separation
device 505A that can be used to divide the physical structure of the paired
array
805A, 80513 into two separate linear compartment sensor arrays 805A, 80513.
The
compartment sensor arrays 805 of FIG. 86 may also include labels 555 and an
expansion device 535, like those of FIG. 5A. The labels can be positioned on
the
front and back sides of each compartment sensor array 805. While the optical
transmitters 510 and receivers 515 of FIG. 86 are illustrated in functional
block form,
it is noted that these elements as well as other numbered elements, which
correspond to the numbered elements of FIGs. 4-7, work similar to the
embodiments
described and illustrated in FIGs. 4-7.
Referring now to FIG. 8C, this figure is a functional block diagram of
compartment sensor 405 that illustrates multiple optical receivers 515 that
may
positioned on opposite sides of a single optical transmitter 510 and that may
be
simultaneously activated to produce their scans at the same time. This
exemplary
embodiment can produce scans at the same time by using light with different
wavelengths.
Using light with different wavelengths can help reduce and
substantially eliminate any optical interference that can occur between
multiple light
rays that may be received by the multiple optical receivers 515. While the
optical
receivers 515 of FIG. 8C are illustrated in functional block form, it is noted
that these
receivers 515 as well as other numbered elements, which correspond to the
elements of FIGs. 4-7, work similar to the embodiments described and
illustrated in
FIGs. 4-7.
The two optical receivers 515A1, 515A2 of FIG. 8C may be simultaneously
activated when two optical filters 810A, 81013 having different wavelengths
are used.
The first optical filter 810A may have a first wavelength of lambda-one (Al)
which is
different than a second wavelength of lambda-two (A2) that is the wavelength
of the
second optical filter 810E31. The optical transmitter 510 can be designed to
produce
-44-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
light having wavelengths of the first and second wavelengths which correspond
with
the first and second optical filters 810A, 810B.
Light 820A with a first wavelength can be produced by the optical transmitter
510 propagating its light through a first optical filter 810A1 that is
designed to only let
the first wavelength pass through it. Similarly, Light 820B with a second
wavelength
can be produced by the optical transmitter 510 propagating its light through a
second
optical filter 810B1 that is designed to only let the second wavelength pass
through
it. A third optical filter 810A2 corresponding with the first optical filter
810A1 can be
designed to only pass the first wavelength such that the optical receiver
515A1 only
detects light of the first wavelength. Similarly, a fourth -optical filter
81062
corresponding with the second optical filter 810B1 can be designed to only
pass the
second wavelength such that the optical receiver 515A2 only detects light of
the
second wavelength.
In this way, simultaneous different compartment scans can be produced at the
same time with light having the first wavelength of lambda-one (Al) and light
having
the second wavelength of lambda-two (A2), in which the two optical receivers
515A1
and 515A2 share the same optical transmitter 510. This principal of optical
receivers
515 sharing the same optical transmitter 510 is also illustrated in FIG. 8B
which
provides the compartment sensor arrays 805 discussed above. Specifically, any
optical transmitter 510 / optical receiver 515 group that is positioned along
a single
alignment mechanism 410 and longitudinal axis 450 can be designed to have a
unique wavelength relative to its neighbors along the same line. So this means
that
each optical transmitter 510 / optical receiver 515 group of a particular
compartment
sensor array 805, such as first array 805A, can be designed to have unique
wavelengths relative to each other for illuminating the same compartment.
Meanwhile, a neighboring compartment sensor array 805, such as second array
805B, may have the same wavelength arrangement as the first array 805A.
One of ordinary skill in the art recognizes that each light optical
transmitter
and optical receiver design uses two separate wave lengths to solve for oxy-
hemoglobin and deoxy-hemoglobin concentrations, as illustrated in FIG. 7.
Therefore, the two optical wavelength design described for FIG. 8C above may
translate into four or more wavelengths for each optical transmitter 510 and
pair of
optical receivers 515. The two wavelength design for FIG. 8C was described
above
-45-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
for simplicity and to illustrate how groups of optical transmitters and
optical receivers
can operate at different wavelengths relative to the groupings.
The invention is not limited to only two optical receivers 515 that share the
same optical transmitter 510. The invention could include embodiments where a
single optical transmitter 510 is shared by a plurality of optical receivers
515 greater
than two relative to the exemplary embodiment illustrated in FIG. 8C.
Referring now to FIG. 9A, this figure illustrates a cross-sectional view of a
left-
sided human leg 100 that has the four major compartments 905 which can be
measured by the compartment sensors 405 according to one exemplary
embodiment of the invention. A first compartment 905B (also noted with a Roman
Numeral One) of the lower human leg 100 comprises the anterior compartment
that
is adjacent to the Tibia 910 and Fibula 915. A first compartment sensor 405B
is
positioned adjacent to the anterior compartment 905B and provides a first
oxygenation Scan having a depth of 620B.
A second compartment 905A (also noted with a Roman Numeral Two) of the
lower human leg 100 comprises the lateral compartment that is adjacent to the
Fibula 910. A second compartment sensor 405A is positioned adjacent to the
lateral
compartment 905A and provides a second oxygenation scan having a depth of
620A. ,
A third compartment 905D (also noted with a Roman Numeral Three) of the
lower human leg 100 comprises the superficial posterior compartment that is
behind
the Tibia 910 and Fibula 915. A third compartment sensor 405D is positioned
adjacent to the posterior compartment 905D and provides a third oxygenation
scan
having a depth of 620D.
A fourth compartment 905C (also noted with a Roman Numeral Four) of the
lower human leg 100 comprises the deep posterior compartment that is within a
central region of the human leg 100. A fourth compartment sensor 405C is
positioned adjacent to the deep posterior compartment 905C and provides a
fourth
oxygenation scan having a depth of 620C.
Referring now to FIG. 9B, this figure illustrates a cross-sectional view of a
right-sided human leg 100 and possible interference between light rays 820 of
simultaneous oxygenation scans made by the compartment sensors 405 into
respective compartments of interest according to one exemplary embodiment of
the
invention. This figure illustrates how light rays 820 produced by respective
-46-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
compartment sensors 405 can interfere with one another. To resolve this
potential
problem, the activation and hence, production of light rays 820, by the
compartment
sensors 405 can be phased so that light rays 820 produced by one compartment
sensor 405A are not received and processed by a neighboring compartment sensor
405B, 405C. When light is emitted from the compartment sensors 405 through
tissue, the light does not travel in a straight line. It is reflected and
spreads
throughout the whole tissue. Therefore, light interference or noise would be a
significant concern for multiple light sources placed in close proximity to
each other.
Alternatively, and as noted above, each compartment sensor 405 can produce
optical wavelengths that are independent of one another in order to reduce any
chances of optical interference.
Referring now to FIG. 9C, this figure illustrates a position 930 of a
compartment sensor 405C in relation to the knee 927 for the deep posterior
compartment 905C of a right sided human leg 100 according to one exemplary
embodiment of the invention. As illustrated in FIGs. 9A and 9B discussed
above, the
deep posterior compartment sensor 405C can be positioned such that the sensor
405C can directly sense the oxygenation levels of this compartment 905C
without
penetrating or going through another compartment. With respect to FIG. 9C, the
deep posterior compartment 905C can be accessed by placing the sensor along
the
posteromedial aspect of the medial tibia. In other words, palpation of the
shin bone
will allow the location of the tibia. The sensor 405 should be placed just
behind the
bone on the inside of the leg along the longitudinal axis 450C of the
compartment
905C (not illustrated in this Figure). The compartment sensor 405C can be
aligned
with the longitudinal axis 450C of the deep posterior compartment 905C through
using the alignment mechanism 410C. The compartment sensor 405C can
positioned at any point along the longitudinal axis 450C. The location of this
deep
posterior compartment sensor 405C on the lower leg 100 may be one inventive
aspect of the technology since it allows a direct scan of the deep posterior
compartment 905C.
Referring now to FIG. 10, this figure illustrates an exemplary display 1000 of
numeric oxygenation values 402 as well as graphical plots 1005 for at least
two
compartments of an animal according to one exemplary embodiment of the
invention. The
graphical plots 1005 can display the current instantaneous
oxygenation level for each compartment as a point as well as historical data
-47-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
displayed as other points along a line that plots the history for a particular
compartment sensor 405. In other words, the X-axis of the plots 1005 can
denote
time in any increments while the Y-axis of the plots can denote oxygenation
levels
monitored by a particular sensor 405.
While only two plots are illustrated, multiple plots can be displayed for each
respective sensor 405. In compartment sensor array 805 deployments, the
graphical
plot 1005 can represent an "average" of oxygenation levels measured by the
multiple
sensors of a particular linear compartment sensor array 805. The display
device 420
can include controls 1015 that allow for the selection of one or more
compartment
sensors 405 or one or more compartment sensor arrays 805 for displaying on the
display device 420. The display of historical oxygenation levels of a
compartment
905 over time through the plots 1005 is a significant improvement over
conventional
methods of direct pressure readings of compartments 905 which usually would
only
allow periodic measurements of compartments 905 on the order of every fifteen
or
thirty minutes compared to minutes or seconds now measured with the
compartment
sensors 405 described in this document.
Referring now to FIG. 11, this figure illustrates single compartment sensors
405 with alignment mechanisms 410 and central scan depth markers 415 that can
be used to properly orient each sensor 405 with a longitudinal axis 450 of a
compartment 905 of an animal body according to one exemplary embodiment of the
invention. While the longitudinal axis 450 of a compartment (shown with broken
lines) cannot actually be seen on the external surface of a lower human leg
100 by a
medical practitioner, a medical practitioner can envision this invisible axis
450 based
on the anatomy of the leg, such as looking at the knee 927 and comparing its
orientation with the ankle and foot of the leg 100. As described above, the
compartment extends from the knee to ankle and the sensor can be placed over a
portion or all of the compartment being measured. With these single
compartment
sensor 405 embodiments, each sensor 405 can be positioned along the length of
the
longitudinal axis 450 to obtain an oxygenation level for a particular
compartment 905
of interest.
Referring now to FIG. 12, this figure illustrates compartment sensor arrays
805 with alignment mechanisms 410 that can be used to properly orient each
array
805 with a longitudinal axis 450 of a compartment 905 of an animal body
according
to one exemplary embodiment of the invention. Since compartment sensor arrays
-48-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
805 will typically occupy close to the entire length of any given longitudinal
axis 450
of a compartment 905 of interest, the individual sensors 405 of the
compartment
sensor array 805 are usually not provided with central scan depth markers 415.
In
the sensor array embodiments, the arrays 805 are usually provided only with
the
alignment mechanism 410. However, the central scan depth markers 415 could be
provided if desired for a particular application or medical practitioner (or
both).
Referring now to FIG. 13A, this Figure illustrates various locations for
single
compartment sensors 405 that can be positioned on a front side of animal body,
such as a human, to measure oxygenation levels of various compartments 905
according to one exemplary embodiment of the invention. FIG. 13A illustrates
that
the invention is not limited to compartment sensors 405 that only measure
lower legs
100 of the human body. The compartment sensors 405 can measure various
different compartments 905 such as, but not limited to, compartments 905 of
the
arm, thighs, and abdomen.
Referring now to FIG. 13B, this Figure illustrates various locations for
single
compartment sensors 405 that can be positioned on a rear side of animal body,
such
as a human, to measure oxygenation levels of various compartments 905
according
to one exemplary embodiment of the invention. Similar to FIG. 13A above, the
compartment sensors 405 shown in this Figure can measure various different
compartments 905 such as, but not limited to, compartments 905 of the arm,
thighs,
and abdomen. Also, while grouped compartment sensors 405 that are coupled
together with expansion devices 535 are not illustrated here (such as those
described in connection with FIG. 5A above), one of ordinary skill recognizes
that
such grouped compartment sensors can be substituted anywhere were the single
compartment sensors 405 are shown.
Referring now to FIG. 14A, this Figure illustrates various locations for
compartment sensor arrays 805 that can be positioned over compartments 905 on
a
front side of an animal body, such as a human, to measure oxygenation levels
of the
various compartments 905 according to one exemplary embodiment of the
invention.
Like the single compartment sensor embodiments of FIGs. 13A-13B described
above, the compartment sensor arrays 805 can measure various different
compartments 905 such as, but not limited to, compartments 905 of the arm,
thighs,
and abdomen.
-49-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Referring now to FIG. 14B, this Figure illustrates various locations for
compartment sensor arrays 805 that can be positioned over compartments 905 on
a
rear side of an animal body, such as a human, to measure oxygenations levels
of the
various compartments 905 according to one exemplary embodiment of the
invention.
Also, while grouped compartment sensor arrays 805 that are coupled together
with
expansion devices 535 are not illustrated here (such as those described in
connection with FIG. 8B above), one of ordinary skill recognizes that such
grouped
compartment sensor arrays 805 can be substituted anywhere were the individual
compartment array sensors 805 are shown.
Referring now to FIG. 14C, this Figure illustrates an exemplary display 1300
and controls for the display device 420 that lists data for eight single
compartment
sensors 405 according to one exemplary embodiment of the invention. The eight
single compartment sensors 405 may be monitoring compartments of two limbs of
an animal, such as two lower legs of a human patient. One limb is usually
uninjured
while the other limb is typically injured, though the system is not limited to
unilateral
injuries.
The display 1300 may provide up to eight different plots or graphs 1335A,
1330A, 1325A, 1320B, 1335B, 1330B, 1325B, 1320B of data that are taken from
the
eight different sensors 405 or sensor arrays 805. The first pair of right and
left leg
sensors may monitor the anterior compartment 905B of FIG. 9A which is
displayed
with the letter "A" for the first row 1335 of data. The second pair of right
and left leg
sensors may monitor the lateral compartment 905A of FIG. 9A which is displayed
with the letter "L" for the second row 1335 of data. The third pair of right
and left leg
sensors may monitor the deep posterior compartment 905C which is displayed
with
the letters "DP" for the third row 1330 of data. The first pair of right and
left leg
sensors may monitor the superficial posterior compartment 905D which is
displayed
with the letters "SP" for the fourth row 1320 of data.
The display 1300 may also provide a measure of a difference 1340 in
oxygenation levels between the injured limb or region and the uninjured limb
or
region. This difference may be displayed by listing the two oxygenation levels
of
each respective limb separated by a slash "I" line. Underneath the two
oxygenation
levels for a respective pair of sensors for the injured and uninjured limbs, a
value
which is the difference between the oxygenation levels displayed above it may
be
listed. For example, for the first oxygenation difference value of 1340A, the
-50-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
oxygenation level for the right leg sensor is the value of forty-four while
the value for
the left leg sensor is the value of sixty-five. In this exemplary embodiment,
the right
leg is injured while the left leg is uninjured. The difference value displayed
under the
two oxygenation levels for the first data set 1340A is twenty-one.
Initial data from patients with extremity injuries measured by the inventor
have
shown that muscular skeletal injuries cause hyperemia (increased blood flow
and
oxygen) in the injured extremity. If a compartment syndrome develops, the
oxygenation drops from an elevated state to an equal and then lower level with
comparison to the uninjured limb. Therefore when comparing injured and
uninjured
extremities, the injured limb should show increased oxygenation levels. If
levels
begin to drop in the injured limb compared to the uninjured limb, an alarm or
alert
can be triggered to warn the medical practitioner. This alarm can be visual or
audible (or both).
With the display 1300, a medical practitioner can modify how data is displayed
by pressing the "mode" button 1305 on the display 1300 (which may comprise a
"touch-screen" type of display). The mode button 1305 permits the medical
practitioner to change the display of the screen. This function would allow
for
selection between multiple different settings to allow for data downloading,
changing
the time frame for which data is displayed, etc. With the time mark "button"
1310,
the medical practitioner can mark or "flag" certain data points being measured
for
later review. With the select "button" 1315, the medical practitioner can
select
between the multiple options that can be accessed through the mode button.
While the above description of FIG. 14C mentioned that eight single
compartment sensors 405 produced the data of the display 1300 of FIG. 14C, the
single compartment sensors 405 can be easily substituted by compartment sensor
arrays 805. In such a scenario in which compartment sensor arrays 805 are used
to
produce the data of display 1300, the displayed values can be an "average" of
the
values taken from a given array 805. This "average" can be calculated by the
processor of the display device 420.
Referring now to FIG. 14D, this Figure illustrates an exemplary display 1302
of providing users with guidance for properly orienting a single compartment
sensor
405 over a compartment of an animal, such as a human leg, according to one
exemplary embodiment of the invention. The display 1302 can be generated by
display device 420 so that a medical practitioner is provided with
instructions and
-51-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
graphical information on how to mount and operate the compartment sensors 405
of
the system. The display may provide an illustration of the body part having
the
compartment of interest. In
the exemplary embodiment of FIG. 14D, the
compartment of interest is located within the lower human leg 100.
An illustration of the lower human leg 100 is provided in display 1302. On the
body part having the compartment of interest, the display device 420 can
identify the
longitudinal axis 450 by marking or flagging this axis 450 with a text box
label 1309.
The display 1302 can also identify an illustration of the compartment sensor
405A by
marking or flagging this illustration with another text box label 1311. The
display
1302 can also identify a general region for a compartment of interest by
encapsulating the region with a geometric outline such as an ellipse and
marking this
ellipse with another text box label 1307.
The display 1302 can also include a miniaturized view 1301 of a cross-section
of the compartment of interest, similar to the views illustrated in FIGs. 9A
and 9B for
this exemplary embodiment that is assessing a lower leg compartment 905. The
display 1302 may also allow the user to expand the cross-sectional view 1301
of the
compartment of interest by allowing the user to double-click or touch the
actual
display of the cross-section. Multiple sections including an axial, coronal
and/or
sagittal view may be included in the on-screen instructions for placement.
Upon
such action by the user, the display device 420 may enlarge the cross-
sectional view
1301 to a size comparable or equivalent to that illustrated in FIG. 9A. Once
the
medical practitioner has positioned the sensor 405 on the patient over the
desired
compartment of interest, the display 1302 can be refreshed to include the next
compartment of interest.
Referring now to FIG. 15A, this Figure illustrates a front view of lower
limbs,
such as two lower legs of a human body, that are being monitored by four
compartment sensor arrays 805 according to an exemplary embodiment of the
invention. The four sensor arrays 805 can be positioned along compartments of
interest by orienting the alignment mechanism 410 along the longitudinal axis
of a
respective compartment. Multiple central scan depth markers 415 and numerical
depths (not illustrated in FIG. 15A) can be positioned over each light
source/receptor
set of a sensor array 805 to locate where each measurement is obtained for
identifying sites of a hematoma, which will be described in more detail in
connection
with FIGs. 15B-16 below.
-52-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Referring now to FIG. 15B, this Figure illustrates a display 1505 of the
display
device 420 that can be used to monitor hematomas and/or blood flow according
to
one exemplary embodiment of the invention. The display 1505 can include an
average oxygenation level 1515 of thirty-six at an instant of time that is
determined
from the two compartment sensor arrays 805A1, 805B1 of a patient's right leg
100A
which is injured in this exemplary case. Meanwhile, the display 1505 can also
include an average oxygenation level 1510 of fifty-three at the same instant
of time
that is determined from the two compartment sensor arrays 805A2, 805132 of a
patient's left leg 100B which is uninjured in this exemplary case.
The display 1505 can also provide oxygenation values that it is receiving from
each of the individual sensors 405 in a first sensor array 805 not
illustrated. For the
injured right leg 100A illustrated in the display, the oxygenation levels vary
between
thirty-two and forty-four. However, in the exemplary embodiment illustrated in
FIG.
15B, there are three individual sensors 405 (not illustrated in this Figure)
of the
sensor array 805A1 that are not producing any oxygenation values which have
been
provided with the letter "H" to denote a possible hematoma. For the uninjured
leg
100B, the individual compartment sensors 405 (not illustrated) of the two
sensor
arrays 805A2, 805E12 have provided oxygenation levels that range between 50
and
54 which are believed to be in the normal range for normal blood flow. Also,
While
individual sensors 405 that are not illustrated here (such as those described
in
connection with FIG. 4A above), one of ordinary skill recognizes that such
individual
compartment sensors 405 can be substituted anywhere were the compartment array
sensors 805 are shown.
Referring now to FIG. 16, this Figure illustrates a display 1600 of the
display
device 420 for an instant of time after the display of FIG. 15B and which can
be used
to monitor hematomas and/or blood flow according to one exemplary embodiment
of
the invention. The display 1600 illustrates that the hematoma or absence of
healthy
blood flow condition being tracked by sensor arrays 805A1, 805B1 (of FIG. 15A)
is
expanding. The display 1600 can include a warning message 1605 such as
"WARNING - HEMATOMA EXPANDING!" to alert the medical practitioner of the
changing conditions of the compartments 905 of interest in the injured or
traumatized
area. In FIG. 16, the average oxygenation level 1515 of the injured leg 100A
decreased in value from thirty-six to twenty-four. Further, the number of
individual
sensors 405 (not illustrated but values shown) detecting a hematoma or lack of
-53-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
healthy blood flow condition increased from two sensors detecting the
condition in
FIG. 15B to seven sensors detecting the condition in FIG. 16 as indicated by
the "H"
values on display 1505. Meanwhile, the average oxygenation level 1515 of the
uninjured left leg 100B changed slightly from fifty-three to fifty-two.
With the display 1600 that provides the compartment sensors 405 with "H"
values in combination with the central scan depth markers 415 provided on the
sensor arrays 805, the medical practitioner can easily locate the physical
sites on the
leg 100 that contain the hematoma or lack of healthy blood flow. These
positions
can also be used by the medical practitioner to locate appropriate amputation
level
for diabetics or peripheral vascular disease, since peripheral vascular
disease is
typically worse distally (closer to the toes) and gradually improves closer to
the knee.
The compartment sensor 405 or more specifically the array system 805 can be
used
to aid a clinician or surgeon in determining the level of amputation for
peripheral
vascular disease and or diabetes mellitus. By obtaining multiple readings at
different
levels from the knee to the ankle, the surgeon can determine the appropriate
level
for amputation. The level of amputation is important since if the tissue is
not well
perfused, the surgical wound will not heal and require revision surgery and
more of
the patient's leg must be removed.
Referring now to FIG. 17, this Figure illustrates a sensor design for
measuring
the optical density of skin according to one exemplary embodiment of the
invention.
The depth of tissue measurement using NIRS is based on separation of the
optical
transmitter 510 and the optical receiver (see FIGs. 18A-B). In order to obtain
readings of only the skin (very shallow depths), the separation between the
optical
transmitter 510 and optical receiver 515 would have to be very small and which
may
not be feasible. In this exemplary embodiment, the sensor 405 can comprise a
material 1705 of known optical density that can be positioned between the
substrate
530 and the skin 1710. In this way, the light mean paths 710A, 710B will only
penetrate upper layers of the leg 100, such as the skin layers 1710. The
thickness
of the known material 1705 can be varied to adjust for different desired scan
depths
made by the light mean paths 710A, 710B. Since the optical density of the
material
1705 is known, then any near infrared light absorption will be attributable to
the
layers of tissue of interest. And in this case, the optical density of the
skin 1710 can
be determined. According to a further exemplary embodiment, one of the
photoreceptors 702A, 702B can be removed from the optical receiver 515 in
order to
-54-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
decrease the depth of the scan. For example, if the second photoreceptor 702A
was
removed, the depth of the scan would only extend as deep as the light mean
path
710B for the photoreceptor 702B.
The inventor has recognized that skin pigmentation can affect the oxygenation
values of a patient that uses near-infrared compartment sensors 405. This
effect on
oxygenation levels is also acknowledged in the art. See an article published
by
Wassenar et al. in 2005 on near-infrared system (NIRS) values. As with solar
light,
skin pigmentation caused by the biochemical melanin is a major factor in light
absorption. In the inventor's research, skin pigmentation has been
demonstrated to
be a significant factor in measuring oxygenation levels among patients. The
inventor
has discovered that there was approximately a ten point difference when
comparing
low pigmentation subjects (Caucasians, Hispanics & Asians) with higher
pigmented
subjects (African American). The pigmented subjects had average scores of
approximately ten points lower when compared to non-pigmented subjects. See
Table 1 below that lists data on the difference between measured oxygenation
levels
of uninjured patients due to skin pigmentation.
Table #1: Difference in measured oxygenation levels between White and Dark
Pigmentation Skinned Subjects
Avg White Dark Diff p value
Anterior 60 51 9 <0.0001
Lateral 61 52 9 <0.0001
Deep
Post 66 53 13 <0.0001
Sup
Post 66 52 14 <0.0001
= N = 10 (White) and 17 (Dark) (This study compared 10 white
subjects to 17 darker pigmented subjects)
= Statistics used a non paired, two tail student t-test for p-values
= P values show very statistically significant differences between
white (Caucasian, Asian & Hispanic) vs. Dark (African American)
subjects
-55-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
The p-value can be described as the chance that these findings were due to
chance alone. In all four compartments, the chance of finding the difference
(9-14)
in average value between the two groups (dark and white) was less than 0.01%
or
less than 1 out of 10,000. In other words the likelihood of these findings
occurring by
chance alone is very unlikely. By convention, statistically significant
findings are
considered to be less than 5% or a p-value of <0.05 in comparison. See
APPENDIX
A for the raw data that supports this data.
Conventional studies (Wassenar et al., 2005 and Kim et al., 2000) have
showed that when subjects increase their activity, dark pigmented people tend
to
have higher rates of loss of signal.
There have been no attempts as of this writing to account for skin
pigmentation, or optical density, in oxygenation levels detected with sensors
like the
compartment sensor 405 discussed above. Therefore, the design illustrated in
FIGs.
17-18 have been developed by the inventor to account for pigmentation optical
density. With the embodiments of FIGs. 17-18, skin pigmentation influences can
be
calibrated and accounted for when measuring oxygenation levels with sensors
405
that use near infrared light absorption principles. In this way, true or more
accurate
oxygenation levels of subcutaneous tissue such as muscle, cerebral matter or
organ
tissue may be obtained. This calibration or pigmentation accounting would also
allow for comparison of values between different patients, since each
individual will
likely have different skin pigmentation values.
Referring now to FIG. 18A, this Figure illustrates a sensor 405 that can
penetrate two layers of skin 1805A, 1805B to obtain optical density values
according
to one exemplary embodiment of the invention. The distance D1 between the
optical
transmitter 510 and optical receiver 515 can be predetermined based on the
scan
depth 620A that is desired.
Referring now to FIG. 18B, this Figure illustrates a sensor 405 that can
penetrate one layer of skin 1805A according to one exemplary embodiment of the
invention. This figure demonstrates how the depth of measurement for
oxygenation
levels using the sensors 405 that operate according to near infrared light
absorption
principles is usually directly proportional to the optical transmitter and
optical receiver
separation distance D. In FIG. 18B, the separation distance D2 is smaller than
that
-56-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
of the separation distance D1 of FIG. 18A. Accordingly, the central scan depth
620B of FIG. 18B is also shorter than the central scan depth 620A of FIG. 18A.
According to one exemplary embodiment of the invention, the separation D1
and D2 between the optical transmitter 510 and optical receiver 515 can range
between approximately five millimeters to two centimeters. This separation
distance
D can be optimized to obtain an accurate reading of only the skin in the
particular
area of interest. One of ordinary skill in the art recognizes that skin is not
a constant
depth or thickness throughout a human body. Therefore, the depth 620 of the
scan
of a sensor 405 for which it is designed (ie. the leg for compartment
syndromes) may
preferably be designed to vary to obtain an accurate optical density value for
skin in
that specific body location.
Referring now to FIG. 18C, this figure illustrates a modified compartment
monitoring system 1800 that can correlate skin pigmentation values with skin
optical
density values in order to provide offset values for oxygenation levels
(derived from
near infrared light absorption principles) across different subjects who have
different
skin pigmentation according to one exemplary embodiment of the invention. The
system 1800 can comprise a central processing unit of the display device 420
or any
general purpose computer. The CPU of the display device 420 can be coupled to
a
compartment sensor 405' that has been modified to include a skin pigment
sensor
1820.
The skin pigment sensor 1820 may be provided with a known reflectance and
that can be used to calibrate the compartment sensor 405' based on relative
reflectance of skin pigment which can affect data generated from oxygenation
scans.
For example, the skin sensor 1820 can comprise a narrow-band simple
reflectance
device, a tristimulus colorimetric device, or scanning reflectance
spectrophotometer.
Conventional skin sensors available as of this writing include mexameter-18
(CK-
electronic, Koln, Germany), chromameters, and DermaSpectrometers. Other
devices appropriate and well suited for the skin sensor 1820 are found in U.S.
Pat.
Nos. 6,070,092 issued in the name of Kazama et at; 6,308,088 issued in the
name of
MacFarlane et al; and 7,221,970 issued in the name of Parker, the entire
contents of
these patents are hereby incorporated by reference.
The skin sensor 1820 can determine a standardized value for skin
pigmentation of a patient by evaluating the melanin and hemoglobin in the
patient's
skin. Once the skin melanin or pigment value is determined it can be
correlated to
-57-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
its calculated absorption or reflectance (effect) on the oxygenation levels
using a
predetermined calibration system, such as the skin pigment table 1825
illustrated in
FIG. 18C. From the skin pigment table 1825, the CPU 420 can identify or
calculate
an oxygenation offset value that can be incorporated in tissue hemoglobin
concentration calculations for deep tissue oxygenation scans. Accounting for
skin
pigmentation will usually allow for information or values to be compared
across
different subjects with different skin pigmentation as well as using the
number as an
absolute value instead of monitoring simple changes in value over time.
Referring now to FIG. 19, this figure is a functional block diagram of the
major
components of a compartment or oxygenation monitoring system 1900 that can
monitor a relationship between blood pressure and oxygenation values according
to
one exemplary embodiment of the invention. The compartment monitoring system
1900 can include a CPU 420A of a display device 420B that is coupled to
compartment sensors 405, a blood pressure probe 440, and a blood pressure
monitor 445. The CPU 420A may also be coupled to a voice synthesizer 1905 and
a
speaker 1907 for providing status information and alarms to a medical
practitioner.
The CPU 420A can receive data from the blood pressure monitor 445 in order
to correlate oxygenation levels with blood pressure. The CPU 420A can activate
an
alarm, such as an audible or visual alarm (or both) with the voice synthesizer
1905
and speaker or displaying a warning message on the display device 420B when
the
diastolic pressure of a patient drops. It has been discovered by the inventor
that
perfusion can be significantly lowered or stopped at low diastolic pressures
and
when compartment pressures are greater than the diastolic pressure. According
to
one exemplary embodiment, in addition to activating an alarm, the CPU 420A of
the
compartment monitoring system 1900 can increase a frequency of data collection
for
oxygenation levels and/or blood pressure readings when a low blood pressure
condition is detected by the oxygenation sensing system 1900.
Referring now to FIG. 20, this figure is an exemplary display 2005 that can be
provided on the display device 420 and which provides current blood pressure
values 2020 and oxygenation levels 2025 of a compartment of interest according
to
one exemplary embodiment of the invention. Display 2005 can be accessed by
activation of the mode switch 1305 of FIG. 14.
In addition to displaying current blood pressure values 2020 and oxygenation
levels 2025, the display 2005 can further include graphs that plot a blood
pressure
-58-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
curve 2035 and an oxygenation level curve 2040. The blood pressure curve 2035
can represent blood pressure data taken over time that is plotted against the
time
axis 2030 (X-axis) and the blood pressure axis 2010 (first Y-axis values). The
oxygenation level curve 2040 can represent oxygenation levels taken over time
that
is plotted against the time axis 2030 (X-axis) and the oxygenation level axis
2010
(second Y-axis values).
In this way, the relationship between blood pressure and potential
compartment pressure based on the oxygenation levels can be directly tracked
and
monitored by a medical practitioner. As noted above, it has been discovered by
the
inventor that perfusion can be significantly lowered or stopped at low
diastolic
pressures and when compartment pressures are greater than the diastolic
pressure.
So when the blood pressure of a patient starts to drop and if the oxygenation
levels
of a compartment being tracked also start to drop, the CPU 420A can sound an
audible alarm and display a warning message 2035 to the medical practitioner
to
alert him or her of this changing condition. This correlation between
hemoglobin
concentration (oxygenation levels) and diastolic pressure can be used to
estimate
intra-compartmental pressures without having to use invasive, conventional
needle
measurements.
Additionally, a running average of oxygenation values over a certain time
period can be calculated and displayed. The time period could be altered by
the m
between multiple time periods from seconds to minutes to even hours. The
purpose
of the running average would be to limit the amount of variability of the
oxygenation
values displayed on the screen. The current instantaneous value that is
displayed in
existing models is very labile. By using a running average, the trends can be
monitored and the instantaneous changes can be smoothed out. This ability to
decrease volatility would be important to prevent continual alert triggering
if an alarm
value was set by the medical practitioner.
In addition, with blood pressure input as described above, the diastolic,
systolic and/or mean arterial pressure (MAP) can be displayed (not
illustrated)
against time on the same graph. Using the two data series of oxygenation and
diastolic blood pressure, an estimate of perfusion pressure (diastolic
pressure minus
intra-compartmental pressure) can also be estimated by the CPU 420A.
Referring now to FIG. 21, this figure is a functional block diagram that
illustrates material options for a compartment sensor 405 according to one
-59-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
exemplary embodiment of the invention. Functional block 2105 indicates that
the
structure of the compartment sensor can be made with sterile materials. For
example, the substrate 530 (not illustrated) of the sensor 405 may be made of
anyone or combination of the following materials: various polymers such as the
polyurethanes, polyethylenes, polyesters, and polyethers or the like may be
used.
Alternatively, each compartment sensor can be made with a sterile coating 2110
that
encapsulates the compartment sensor 405. The sterile coating can be applied
during manufacturing of the sensor 405 or it can be applied after
manufacturing and
provided as a container or sealable volume.
Additionally, once the unit is
constructed and finished, the device can be sterilized using one or more off
multiple
processes including but not limited to chemical, heat, gas or irradiation
sterilization.
Referring now to FIG. 22, this figure illustrates an exemplary clinical
environment of a compartment sensor 405 where the sensor 405 can be positioned
within or between a dressing 2205 and the skin 1805 of a patient according to
one
exemplary embodiment of the invention. Since the inventive compartment sensor
405 can be made with or enclosed by sterile materials as noted in FIG. 21
above, the
compartment sensor 405 or an sensor array 805 can be positioned between a
dressing 2205 and a skin layer 1805 of a patient intra-operatively. In this
way, a
medical practitioner can monitor a compartment 905 of interest without the
need to
remove the dressing 2205 or adjust the position of the compartment sensor 405.
Case Studies Using Compartment Sensors 405 and Conventional Pressure
Measuring Methods
Case I
In 2007, a 44 year old Caucasian male fell 20 feet sustaining an isolated
closed proximal tibia fracture with extension into the knee. Initial treatment
included
external fixation for stabilization on the day of injury.
During surgery the
compartments were firm but compressible. At post operative check revealed that
the
compartments were more firm. There was mild pain with passive stretch, though
the
patient was diffusely painful throughout both lower extremities. Intra-
compartmental
pressures were measured for all four compartments using a conventional needle
method with a Striker device (Stryker Surgical, Kalamazoo, MI). The anterior
and
lateral pressures measured 50 mm Hg and the superficial and deep posterior
-60-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
compartments were 48 mm Hg. The diastolic pressure was 90 mm Hg resulting in a
40 mm Hg perfusion pressure.
Tissue oxygenation (St02) or oxygenation levels were evaluated using two
compartment sensors 405. The oxygenation levels were approximately 80% in all
four compartments. The compartment sensors 405 were placed on the lateral and
deep posterior compartments for continual monitoring, which maintained
oxygenation values near 80%. Higher percentage oxygenation levels indicate
more
perfusion and higher oxy-hemoglobin concentrations.
All clinical decisions were based of the clinical symptoms and pressure
measurements and not on the oxygenation levels. Two hours passed and
compartment pressures were repeated. The anterior and lateral compartments
remained at 50 mm Hg. The superficial and deep posterior compartments rose to
50
mm Hg as well. The patient's diastolic pressure remained at 90 mm Hg
maintaining
40 mm Hg of perfusion pressure. The oxygenation values remained near 80% for
both the lateral and deep posterior compartments. Clinical symptoms were
monitored closely throughout the night.
Approximately 24 hours after the initial injury, the patient became more
symptomatic and began requiring more pain medication. Intra-compartmental
measurements were repeated. The anterior and lateral compartments remained at
51 mm Hg. The superficial and deep posterior compartments measured 61 mm Hg
and 63 mm Hg respectively. However, the diastolic pressure dropped to 74 mm Hg
decreasing the perfusion pressure to 11 mm Hg. Based
on the pressure
measurements and clinical symptoms, the patient underwent fasciotomy and was
found to have no gross evidence of muscle necrosis or neuromuscular sequelae
at
late follow up.
Throughout the monitoring period, the lateral compartment maintained an
oxygenation level of approximately 80%. The oxygenation levels in the deep
posterior compartment began in the eighties and started to drop approximately
three
hours after the second compartment pressure measurement. At time of
fasciotomy,
the oxygenation level for the deep posterior compartment was 58%. The gradual
decline in muscle oxygenation mirrored the decrease in perfusion pressure over
an
extended period of time.
This first case suggests that the compartment sensors 405 can be used to
continually monitor an injured extremity. Initially, the patient had elevated
intra-
-61-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
compartmental pressures, but the perfusion pressure was greater than 30 mm Hg.
The ensuing increase in clinical symptoms and decrease in perfusion pressure
correlated with the gradual decrease in oxygenation levels. Impaired perfusion
was
reflected in a decline in the oxygenation levels. These results are consistent
with a
previous study by Garr et al. who showed a strong correlation between
oxygenation
levels and perfusion pressures in a pig model. This case also demonstrates the
ability of compartment sensors 405 to differentiate between compartments in
the leg
since the oxygenation levels in the lateral compartment remained elevated
while the
deep posterior values declined.
Case II
Also in 2007, a 32 year old Hispanic male sustained an isolated, closed
Schatzker VI tibial plateau fracture after falling from a scaffold. On initial
evaluation,
the patient had tight compartments, but there were no clinical symptoms of
compartment syndrome. Active and passive range of motion resulted in no
significant pain. Based on the concerns for the tense leg, intra-compartmental
pressure measurements were obtained using a Stryker device.
All compartments were greater than 110 mm Hg. The patient's blood
pressure was 170/112 mm Hg. The decision to perform a four compartment
fasciotomy was made. The compartment sensors 405 were placed on the deep
posterior compartment as well as the lateral compartment for continual
monitoring.
The lateral compartment was unable to give a consistent reading due to
hematoma
interference. The initial reading for the deep posterior was an oxygenation
level of
65%. The deep posterior tissue oxygenation level steadily declined from 65% to
55% over the hour of preoperative preparation.
Upon intubation, a sharp drop in the oxygenation levels from 55% to 43% was
observed. The anesthesia record showed a concomitant drop in blood pressure at
the time of induction from 171/120 mm Hg to 90/51 mm Hg. The patient underwent
an uneventful fasciotomy and external fixation. Tissue examination showed no
gross
signs of muscle necrosis and at nine months follow-up there were no signs of
sequelea. The oxygenation level monitoring of the compartment was acutely
responsive and showed real time changes to a decline in perfusion pressure in
an
injured extremity.
-62-
=
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
The responsiveness of the compartment sensors 405 to intra-compartmental
perfusion pressure is demonstrated by this second case study. This patient was
initially asymptomatic even though his compartments were over 110 mm Hg in all
compartments. The oxygenation levels from the compartment sensors 405 were
able to detect gradual perfusion declines over the hour prior to fasciotomy.
Prior to
induction of anesthesia, the patient was able to maintain some tissue
oxygenation by
maintaining a high diastolic blood pressure. Once the patient was anesthetized
during intubation, the diastolic pressure was significantly reduced. The
oxygenation
= levels of the compartments dropped within thirty seconds of induction
because the
slight perfusion gradient was completely abolished by the induced hypotension.
Case III
In 2007, a 62 year old Asian male suffered a closed midshaft tibia fracture in
a
motor vehicle crash. The patient was unresponsive and hypotensive at the scene
of
the accident and intubated prior to arrival. Upon presentation, the patient
was
hypotensive with a blood pressure of 90/55 mm Hg. The injured leg was
clinically
tight on examination.
Oxygenation levels were measured for all four compartments. The
oxygenation levels were approximately at 50% for the anterior and lateral
compartments while the two posterior compartments were approximately at 80%.
The compartment sensors 405 were placed on the anterior and superficial
posterior
compartments for continued monitoring. Intra-compartmental pressures were
measured at 50 mm Hg and 52 mm Hg in the anterior and lateral compartments
respectively using the conventional Striker device (needle pressure measuring
method). The superficial and deep compartment pressures were 19 mm Hg and 20
mm Hg respectively. After the patient was stabilized by the trauma team, he
underwent fasciotomy. There were no gross signs of muscle necrosis and no
complications at 7 months follow-up. Muscle oxygenation was able to
differentiate
between compartments with hypoperfusion and adequate perfusion in a
hypotensive
and intubated patient.
This third case is evidence that the compartment sensors 405 are useful in
assessing established or existing compartment syndromes. The compartment
sensors 405 can provide useful information in patients that are unable to give
feedback during a clinical examination such as this patient who was intubated
and
-63-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
hypotensive upon examination. These findings correlate with the findings by
Arbabi
et al. who demonstrated oxygenation levels to be responsive in hypotensive and
hypoxic pigs in a laboratory setting. The compartment sensors 405 can
distinguish
between different compartments and their respective perfusions. Clinically, in
this
case, the whole leg was tense, but intra-compartmental pressures were only
elevated in the anterior and lateral compartments. The oxygenation levels
measured
by the compartment sensors 405 were proportional to the perfusion pressure
with
low values in the anterior and lateral compartments, but elevated values in
the two
posterior compartments.
Conclusion for Three Case Studies:
These three cases suggest that compartment sensors 405 are responsive and
proportional to perfusion pressures within the injured extremity. These
findings
support previous studies documenting the importance of perfusion pressure and
not
an absolute value in the diagnosis of compartment syndrome. The compartment
sensors can distinguish between compartments and is useful in the
unresponsive,
intubated and hypotensive patient. Lastly, the compartment sensors 404 have
the
potential to offer a continual, noninvasive and real time monitoring system
that is
sensitive in the early compartment syndrome setting. In all three cases, a
difference
in oxygenation levels was demonstrated prior to any irreversible tissue
injury.
Case IV
A 60 year old Middle Eastern male was shot in the right thigh. Initially the
thigh was swollen but the patient was comfortable. After approximately 12
hours
after the initial injury the patient began to complain of increasing pain and
required
more pain medication. The thigh was more tense upon clinical exam. The patient
was taken to the OR for fracture fixation and potential fasciotomy of the
thigh.
NIRS sensors were placed on the anterior, posterior and medial (adductors)
compartments of the thigh. Values for the injured side were similar or
decrease
when compared to the uninjured side. As previously described, injured tissue
should
show increased values due to hyperemia. The injured side anterior, posterior
and
medial values were 54, 53 and 63 respectively. The uninjured values for the
anterior, posterior and medial were 51, 55 and 63 respectively.
-64-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
The compartment pressures were measured in all three compartments. The
intra-compartmental pressures for the anterior, posterior and adductors were
44, 59
and 30 respectively. Once the patient was induced for anesthesia and the
patient's
blood pressure dropped from 159/90 to 90/61, the patients NIRS values dropped
within in 30 seconds of the his blood pressure drop. Once the blood pressure
was
dropped and the perfusion pressure was eliminated, the new values for the
anterior,
posterior and medial compartments were 29, 40 and 35.
Study: Sphygmomanometer Model & Invention's Sensitivity & Responsiveness
A study was conducted to determine the sensitivity and responsiveness of the
inventive compartment monitoring system 400. Specifically, the purpose of the
study
was to evaluate the invention over the anterior compartment with a cuff around
the
thigh at different pressures (simulating a compartment Syndrome) to show
responsiveness to increasing pressures in the leg.
The inventor's hypothesis was that the inventive compartment monitoring
,system 400 will show normal oxygenation at levels below pressures equivalent
to
compartment syndrome. Once pressures become equal to the diastolic blood
pressure, it was believed the inventive system 400 would show significant
deoxygenation because the capillary perfusion pressure will be passed.
Continued
monitoring will be obtained until a plateau or nadir is obtained.
Materials & Methods:
Thigh Cuff Pressures: 0 mmHg: Baseline;
Increase cuff by 10 mmHg and hold for 10 minutes;
At the end of each ten minute period blood pressure and NIRS values were
obtained;
Repeat incremental increases until obtain decreased oxygenation level
readings;
and
Observe post release response & time to return to baseline
Outcomes:
It was confirmed that the compartment monitoring system 400 is sensitive to
changing pressures. A correlation with decreased perfusion was discovered once
the pressure approaches diastolic pressure. The inventive system 400 does not
-65-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
reflect complete vascular compromise until tourniquet pressure supersedes
systolic
blood pressure because of venous congestion. These findings are consistent
with
previously described studies.
Statistical Analysis:
A significant difference is observed once tourniquet pressure equals the
diastolic pressure (Perfusion pressure of zero). The
venous congestion
phenomenon which has been described with the tourniquet model for compartment
syndromes maintains some flow until cuff pressure is raised to above systolic
pressure (no flow). Venous congestion is the phenomenon when the higher
systolic
blood pressure is able to overcome the tourniquet pressure applied to the leg
during
that burst of pressure created by the heart's contraction when the tourniquet
compression is above diastolic pressure but below systolic pressure.
Referring now to FIG. 23, this figure is a graph 2300 of perfusion pressure
plotted against oxygenation levels (02) of the study conducted to determine
the
sensitivity and responsiveness of the inventive compartment monitoring system
400.
The section between points A and B show the combined points of all subjects
studied during the study when the tourniquet pressure was below the diastolic
pressure. As shown in the graph, the grouping is mostly flat and does not show
any
decrease as the tourniquet pressure is increased. After point B between point
B and
C, the tourniquet pressure is above the diastolic pressure and the perfusion
pressure
becomes zero or negative. During this section of the graph, there is a
significant
drop in muscle oxygenation. The data points in FIG. 23 use the actual
compartment
monitoring values, which as described above, can vary based on skin
pigmentation.
Therefore, there is a wider range of values in oxygenation numbers and a wider
spread of data points. See APPENDIX B for the raw data that supports this
graph
2300.
Referring now to FIG. 24, this figure is a graph 2400 of perfusion pressure
plotted against a change in the oxygenation levels (02) from a baseline for
each
subject of the study conducted to determine the sensitivity and responsiveness
of the
inventive compartment monitoring system 400.
In the FIG. 24, the change from baseline was used instead of the absolute
number presented by the compartment sensor. The effects of pigment were
-66-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
removed when change from baseline values was used. Baseline was defined as the
value before the tourniquet was placed. The spread between data points is much
less. As shown again between points A and B, there is a very small and gradual
decrease in tissue oxygenation until point B (moving from high perfusion
pressures
to lower perfusion pressures or from right to left). Once the perfusion
pressure,
becomes zero or negative, the change from baseline was much larger and more
rapid. Both graphs show how the tissue oxygenation is highly sensitive to
perfusion
pressure and the critical point is when the perfusion pressure changes from
positive
to negative. As described above, the diagnosis of compartment syndrome is
based
on the perfusion pressure (diastolic pressure minus compartment pressure).
Therefore, the compartment monitoring system 400 has the capability to show
real-
time changes in perfusion prior to any irreversible tissue damage. See
APPENDIX
B for the raw data that supports this graph 2300.
This study supports the theory that oxygenation levels measure with the
compartment sensors 405 decrease as perfusion pressure also decreases
(Perfusion pressure = diastolic ¨ cuff pressure). The study also indicates
that there
are no significant changes in measured oxygenation levels until there is
increase
above the diastolic pressure. The findings of this study as illustrated in
FIGs. 23 and
24 correlate with previous studies using other determinants of flow (Xenon
clearance; Clayton, 1977; Dahn, 1967; Heppenstall, 1986; Matava, 1994).
Study of Established Acute Compartment Syndromes:
Based on the clinical evaluation in established acute compartment syndrome
patients the diagnosis of compartment syndrome was made. Its purpose was to
evaluate the ability of the inventive compartment monitoring system 400 to
detect
hypoperfusion in the different compartments of the lower leg. This evaluation
was
made to demonstrate the invention's sensitivity to increased pressures versus
uninjured legs.
,
Hypothesis:
There will be a significant difference between the injured and uninjured
values
of the compartment monitoring system 400. There will also be an inverse
relationship between compartment pressures and measured oxygenation levels by
-67-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
the sensors 405. In other words, the oxygenation values would be directly
proportional to perfusion pressures.
Material & Methods:
Oxygenation levels and pressure measurements for each compartment in
established compartment syndromes were obtained. Readings for both legs were
compared for each compartment.
Unknowns:
How will thick subcutaneous fat affect the compartment sensors 405?
What values will we obtain for the posterior compartments?
Preliminary Results:
Hyperemia (increased oxygenation levels) for fractures without any
compartment syndrome symptoms has been demonstrated by the inventors studies
(Table #3 and #4). In early compartment syndromes, the oxygenation values were
equal between the two different legs. Once the compartment syndrome became
advanced, and the perfusion pressure was decreased or eliminated, the
oxygenation
values in the injured leg dropped below the uninjured leg. There was some
difficultly
in obtaining oxygenation levels over a hematoma. Therefore, when oxygenation
values between the two legs become equal, there should be concern for a
compartment syndrome and fasciotomy should be considered. Once the injured
levels drop below the uninjured leg, a fasciotomy should be performed.
Oxygenation levels are extremely responsive to changes in perfusion in
regards to pressure changes. Compartment sensors 405 can differentiate between
compartments. Oxygenation levels can work and are accurate in intubated
patients.
Oxygenation levels do respond over extended time periods and over very short
periods of time and rapid changes in intra-compartmental pressures.
Oxygenation levels and hyperemia are maintained at least two to three days
post injury or surgery. Post-operative values are also high in the operated on
leg-
-69-72 (Standard deviation of 9-12) with an average difference of 15-17%. The
compartment sensors 405 work as a noninvasive tool. Oxygenation levels can be
monitored by sensors 405 over extended periods of time. Compartment sensors
405
-68-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
do respond to changes in perfusion both gradual and sudden. The sensors 405
can
differentiate between different compartments.
Table #2: Comparison of Oxygenation Levels between Injured Limb and Non-
injured
Limb
=
Avg Injured Uninjured Diff value
Anterior 46 54 -6 0.07
Lateral 45 54 -9 0.01
Deep
Post 54 68 -14 0.05
Sup
Post 50 60 -10 0.04
Significant difference using one tailed, paired student t-test was used for
statistical
analysis.
In three out of four compartments, the p-value showed statistical significance
(p-value < 0.05). The one compartment that was not less than 0.05, the
anterior
compartment, the p-value was 0.07 which is very close to 0.05. As described
below,
the normal situation should be the opposite. The injured side should be and is
shown to be significantly higher when compared to the uninjured side. The p-
value
can be described as the chance that these findings were due to chance alone.
By
convention, statistically significant findings are considered to be less than
5% or a p-
value of <0.05 in comparison. This means that there is a 5% chance that these
findings are due to chance alone and that there is no difference between the
two
groups. See APPENDIX A for the raw data that supports this data.
Study of Fracture Hyperemia with Inventive Compartment Monitoring System 400
A study of fracture hyperemia with the inventive compartment monitoring
system 400 was made. The purpose of this study was to examine non compartment
syndrome patients with fractures of the lower leg.
-69-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Hypothesis:
The injured leg will show a hyperemic response to injury and have elevated
blood flow causing an increase in oxygenation values.
Materials & Methods:
Compare uninjured leg to injured leg to see if there is a statistical and
reproducible increase at time of injury. The data is important to describe
normal
fracture response to compare with compartment syndrome response.
Results:
Patients have approximately 15 pts higher on the injured side compared to the
uninjured side. Time of measurement was approximately 16 hours post injury
(range
2,52).
Table #3 Oxygenation Values for Injured versus Uninjured Lower Leg
Measurements.
Avg Injured Uninjured Diff p value
Anterior 69 55 14 <0.0001
Lateral 70 55 15 <0.0001
Deep
Post 74 57 17 <0.0001
Sup
Post 70 56 14 <0.0001
N = 26 (there were 26 subjects examined in this study.)
Statistical analysis calculated p-values using a two tailed, paired student t-
test.
In normal lower leg fracture situations without vascular injury or compartment
syndrome, comparison between injured and uninjured legs show that the injured
leg
should be significantly higher with and average elevation of between 14 and 17
points. This finding is consistent with the hyperemia associated with injury.
This
effect is a long lasting effect that lasts over 48 hours after injury and
surgery as seen
by these results. The p-value can be described as the chance that these
findings
were due to chance alone. In all four compartments, the chance of finding the
-70-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
difference (14-17) in average value between the two groups (injured and
uninjured)
was less than 0.01% or less than 1 out of 10,000. In other words the
likelihood of
these findings occurring by chance alone is very unlikely. By convention,
statistically
significant findings are considered to be less than 5% or a p-value of <0.05
in
comparison. See APPENDIX A for the raw data that supports this data.
Table #4 - Oxygenation Values for Injured versus Uninjured Lower Leg
Measurements 2 Days After Surgery.
Avg Injured Uninjured Diff p value
Anterior 71 55 16 <0.0001
Lateral 70 54 16 <0.0001
Deep
Post 73 58 15 <0.0001
Sup
Post 73 56 17 <0.0001
N = 17 (This study included 17 patients)
Average time of measurement was 71 hours after injury and 44 hours after
operation
The p-value can be described as the chance that these findings were due to
chance alone. In all four compartments, the chance of finding the difference
(15-17)
in average value between the two groups (injured and uninjured) was less than
0.01% or less than 1 out of 10,000. In other words the likelihood of these
findings
occurring by chance alone is very unlikely. By convention, statistically
significant
findings are considered to be less than 5% or a p-value of <0.05 in
comparison. See
APPENDIX A for the raw data that supports this data.
=
-71-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Table #5- Uninjured Controls Comparing Right and Left Leg Differences.
Avg
Avg Right Left Diff Val
Anterior 55 54 1 55
Lateral 56 54 2 56
Deep
Post 60 58 2 59
Sup
Post 59 58 1 58
N = to 25 (There were 25 patients included in this study.)
No difference was found between right and left sides.
These findings are important for two different reasons. First, the difference
between the two legs was very small (on average between 1 or 2 points).
Therefore,
the other findings that show significant differences between legs cannot be
explained
as normal variance. Uninjured patients have oxygenation values between the two
legs that are typically very similar (within 1-5 points of each other).
Second, normal
oxygenation values for uninjured subjects were in the high 50's. This value
varied
based on pigmentation of the skin as showed above. See APPENDIX A for the raw
data that supports this data.
Exemplary Method for Monitoring Oxygenation Levels of a Compartment
Referring now to FIG. 25, this figure is logic flow diagram illustrating an
exemplary method 2500 for monitoring oxygenation levels of a compartment
according to one exemplary embodiment of the invention. The processes and
operations of the inventive compartment monitoring system 400 described below
with respect to the logic flow diagram may include the manipulation of signals
by a
processor and the maintenance of these signals within data structures resident
in
one or more memory storage devices. For the purposes of this discussion, a
process can be generally conceived to be a sequence of computer-executed steps
leading to a desired result.
These steps usually require physical manipulations of physical quantities.
Usually, though not necessarily, these quantities take the form of electrical,
-72-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
magnetic, or optical signals capable of being stored, transferred, combined,
compared, or otherwise manipulated. It is convention for those skilled in the
art to
refer to representations of these signals as bits, bytes, words, information,
elements,
symbols, characters, numbers, points, data, entries, objects, images, files,
or the
like. It should be kept in mind, however, that these and similar terms are
associated
with appropriate physical quantities for computer operations, and that these
terms
are merely conventional labels applied to physical quantities that exist
within and
during operation of the computer.
It should also be understood that manipulations within the computer are often
referred to in terms such as listing, creating, adding, calculating,
comparing, moving,
receiving, determining, configuring, identifying, populating, loading,
performing,
executing, storing etc. that are often associated with manual operations
performed
by a human operator. The operations described herein can be machine operations
performed in conjunction with various input provided by a human operator or
user
that interacts with the computer.
In addition, it should be understood that the programs, processes, methods,
etc. described herein are not related or limited to any particular computer or
apparatus. Rather, various types of general purpose machines may be used with
the following process in accordance with the teachings described herein.
The present invention may comprise a computer program or hardware or a
combination thereof which embodies the functions described herein and
illustrated in
the appended flow charts. However, it should be apparent that there could be
many
different ways of implementing the invention in computer programming or
hardware
design, and the invention should not be construed as limited to any one set of
computer program instructions.
Further, a skilled programmer would be able to write such a computer
program or identify the appropriate hardware circuits to implement the
disclosed
invention without difficulty based on the flow charts and associated
description in the
application text, for example. Therefore, disclosure of a particular set of
program
code instructions or detailed hardware devices is not considered necessary for
an
adequate understanding of how to make and use the invention. The inventive
functionality of the claimed computer implemented processes will be explained
in
more detail in the following description.
-73-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Further, certain steps in the processes or process flow described in the logic
flow diagram must naturally precede others for the present invention to
function as
described. However, the present invention is not limited to the order of the
steps
described if such order or sequence does not alter the functionality of the
present
invention. That is, it is recognized that some steps may be performed before,
after,
or in parallel other steps without departing from the scope and spirit of the
present
invention.
Referring again to FIG. 25, Step 2501 is the first step in the process 2500
for
monitoring oxygenation levels of a compartment according to one exemplary
embodiment of the invention. In step 2501, a compartment sensor 405 may be
manufactured from sterile materials as described above in connection with FIG.
21.
Alternatively, a compartment sensor 405 can be encapsulated with sterile
materials
so that it can be used in a surgical environment or so that it can be place
adjacent to
wounds (or both).
In step 2503, a central scan depth marker 415 can be provided on a
compartment sensor 405. In step 2506, an alignment mechanism 410 can also be
provided on the compartment sensor 405 to allow a medical practitioner to
orient a
sensor 405 along a longitudinal axis of a compartment of interest.
In step 2509, an expansion device 535 may be provided between two or more
grouped compartment sensors 405 as illustrated in FIG. 5A. In step 2512, the
processor and display device 420 may receive input from a user on the type of
compartment that is to be monitored by the inventive system 400.
In step 2515 and in response to the input of step 2512, the display device 420
can display a location of the selected compartment of interest such as
illustrated in
FIG. 14D. The display device 420 can also display the longitudinal axis 450 of
the
compartment of interest. Next, in step 2518, the display device 420 may
display an
ideal or optimal position for the compartment sensor 405 along the
longitudinal axis
of the compartment of interest as illustrated in FIG. 14D.
In step 2521, with the information from steps 2515-2518, the medical
practitioner can identify a proper position of the compartment sensor on a
patient
through orienting the alignment mechanism 410 with the longitudinal axis of
the
compartment and by using the central scan depth marker 415.
In step 2527, the compartment sensor 405 can be placed on the patient. In
step 2530, the compartment sensor can obtain a skin pigment value of the
patient's
-74-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
skin through using a skin sensor 1820 as illustrated in FIG. 18C or thorough
using a
shallow sensor 405 as illustrated in FIG. 17. In step 2533, the processor 420A
can
determine an oxygenation offset value based on the skin pigment value obtained
in
step 2530.
Next, in step 2536, the offset value from step 2533 can be used during
oxygenation level monitoring. In step 2539, the blood pressure of the patient
can be
monitored with a probe 440 and blood pressure monitor as illustrated in FIGs.
4 and
19. In step 2542, the system 400 can monitor the oxygenation levels of one or
more
compartments of interest over time. In step 2545, the system 400 can also
monitor
the oxygenation levels of healthy compartments to obtain a baseline while
monitoring
the compartments adjacent to an injury or trauma as illustrated in FIG. 15B.
In step 2547, the oxygenation levels of compartments of interest can be
displayed on the display device 420 as illustrated in FIGs. 10, 14C, 15B-C,
16, and
20. In step 2550, the blood pressure of the patient can also be displayed on
the
display device as illustrated in FIG. 20. In step 2553, the display device 420
and its
processor can monitor the relationship between the blood pressure values and
oxygenation levels as illustrated in FIG. 20.
In step 2556, the display device 420 can activate an alarm in the form of an
audible or visual message (or both), when the oxygenation levels .drop below a
predetermined value or if a significant change in the levels is detected as
illustrated
in FIG. 20. In step 2559, the display device can also activate an alarm in the
form of
an audible or visual message (or both), when both the oxygenation levels and
blood
pressure drop simultaneously or if one of them falls below a predetermined
threshold
value as described in connection with FIG. 20.
In step 2562, the display device 420 and its processor can increase a
frequency of data collection for oxygenation levels and blood pressure values
if both
values drop. The exemplary process then ends.
Alternative Exemplary Embodiments:
The inventive compartment monitoring system 400 could also be used for free
flap as well as tissue transfer monitoring. Currently skin color and capillary
refill are
used to evaluate flap viability. This practice requires repeated examinations
and
subjective criteria. The conventional method requires leaving skin exposed or
taking
down dressings which can be very labor intensive. As a solution to the
conventional
-75-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
approach, a sensor 405 can be sterilized and it can record average oxygenation
levels over time. The sensor 405 can be placed on the flap (free or
transferred).
The compartment sensor 405 can also be used to monitor oxygenation of
tissue transferred for vascular patency. Specifically, for hand or any upper
extremity
surgery, the compartment sensor can be used to monitor the progress of
revascularization of fingers, hands and arms based on measured oxygenation
levels.
The sensor 405 can be applied to the injured extremity once vascular repair
has
been performed in order to continue monitoring of vascular repair. A baseline
of a
corresponding uninjured or healthy extremity can be made once repair to the
injured
extremity is done -- before closure -- in order to get a baseline value while
looking at
the repair. Sensors 405 for this application will also need to be sterilized
and be able
to conduct scans with depths of at least 0.5 centimeters.
Referring now to FIG. 26, this Figure is a functional block diagram
illustrating
additional applications of and Oxygenation Sensing System 1900 of FIG. 19 such
as
in Wound Management/Monitoring/Healing according to one exemplary embodiment
of the system.
Other applications of the Oxygenation Sensing System 1900 include, but are
not limited to, the following:
Traumatized Tissue 1805A1, 1805B1 Management/Monitoring/Healing of Fig. 26
In this exemplary application of the oxygenation sensing system 1900, the
properties of traumatized tissue 1805A1,1805B1 will be taken into account by
the
oxygenation sensing system 1900. One of ordinary skill the art recognizes that
traumatized tissue 1805A1, 1805B1 does not have the same qualities as
uninjured
tissue: Injured tissue may become hyperperfused along with an elevated
temperature. Bleeding may be present as well as other physiological
alterations.
One of ordinary skill the art recognizes that the physiological state of
tissue is very
different in injured tissue or diseased tissue. Since the body cannot
differentiate
between unplanned trauma (an accident) versus planned trauma (surgical
intervention), this concept includes post surgical tissue.
Injured tissue often becomes a "Privileged" area relative to other healthy
body
parts in that the body will typically maintain increased perfusion over other
areas that
are not injured even in times of poor global perfusion (hypotension). The
oxygenation
-76-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
sensing system 1900 may be designed to accommodate or to account for the
different physiological states of injured or traumatized tissue 1805A1,
180561.
Specifically, predetermined or pre-programmed algorithms in the oxygenation
sensing system 1900 may be used in the setting and monitoring of traumatized
or
wounded tissue. The oxygenation sensing system 1900 may track an Erythema
index, temperature, or other modalities or any combination of factors, which
can help
differentiate traumatized tissue versus non-traumatized tissue. The
oxygenation
sensing system 1900 may be designed to anticipate certain characteristics for
tissue
monitoring depending upon the state of the tissue such as whether the tissue
has
been injured or has not been injured (traumatized / non-traumatized tissue).
Alarms
1907 of the oxygenation sensing system 1900 may be set based on the type of
tissue being monitored.
The sensors 405 of the oxygenation sensing system 1900 may be sterilized in
order for use during evaluation of open wounds, such as illustrated in FIG.
21. The
oxygenation sensing system 1900 may be used to conduct an initial evaluation
in
perfused tissue (that may include, but is not limited to, muscle, skin, soft
tissue,
and/or organs). The oxygenation sensing system 1900 can aid in determining
what
should be debrided (dead tissue- not perfused) versus what is viable tissue.
The oxygenation sensing system 1900 can also help identify tissue with
adequate microcirculation (Capillaries, arterioles & veinules). This is
especially
beneficial in the evaluation of mangled extremities and for determining if an
extremity
is salvageable. The oxygenation sensing system 1900 may also help with
determining if amputations are indicated or if attempted salvage should be
considered. The oxygenation sensing system 1900 may assist in the assessment
of
vascularity of the extremity large vessels and small vessel perfusion. The
oxygenation sensing system 1900 may detect devitalized tissue, which when
allowed
to persist, may become a nidus for infection. One of ordinary skill in the art
recognizes that it is important to debride all dead/devitalized tissue to
prevent or
minimize the risk of infection.
The readings from oxygenation sensing system 1900 may indicate whether a
limb and/or tissue is perfused and has the ability to heal. One of ordinary
skill in the
art recognizes that healing is generally based on the ability of the tissue to
obtain
nutrients from the blood that is perfused throughout any given tissue.
-77-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Transplanted Tissue/Flaps Management/Monitoring/Healing 1805A2, 1805B2
For Transplanted Tissue/Flaps Management/Monitoring/Healing, each sensor
405 of the oxygenation sensing system 1900 usually must be sterile to allow -
placement during a surgical procedure. Each sensor 405 may be placed over the
anastomosis (connection of two or more ends of a vessel as in a repair of an
artery
or vein) in order to get a direct read on tissue around the connection of the
arteries &
veins that supply the transplanted tissue.
The placement of a sensor 405 at distal tips of tissue allows for monitoring
of
the most sensitive tissue for tracking poor perfusion and/or necrosis. These
areas
are call "watershed" areas where the tissue is at most risk for compromised
perfusion. The oxygenation sensing system 1900 may allow for early warnings of
decreased perfusion. Each sensor 405 of the oxygenation sensing system 1900
may be placed on tissue during a procedure once vessels are anastomosed. An
initial reading from each sensor 405 may be determined in the operating room
in
order to obtain a baseline reading. Since the body cannot differentiate
traumatized
tissue from "surgical trauma" performed by a surgeon, the tissue
characteristics are
similar to traumatized tissue and would be expected to have similar findings
and
values. Post surgical tissue should have a "privileged" state of increased
perfusion
to promote healing.
Usually, each sensor 405 of the oxygenation sensing system 1900 is
maintained in the same position over the transplanted tissue in order to
monitor the
vascular flow to the transplanted tissue. If the NIRS values detected by each
sensor
405 start to decrease past a certain threshold, an alarm, such as the audible
alarm
1907, may be activated in order to warn the medical practitioner that the flap
or
transplanted tissue is threatened.
If a signal of a sensor 405 from the oxygenation sensing system 1900 is lost
(such as during hematoma formation) an alarm, such as the audible alarm 1907,
is
signaled. The oxygenation sensing system 1900 can help detect an anastomosis
rupture or thrombosis (blockage). Early detection can allow for early
intervention
such as anastomosis repair or canulation to prevent flap/graft failure due to
extended
lack of perfusion/ischemia. One of ordinary skill in the art recognizes that
an
Anastomosis rupture can cause death of the transferred tissue as well as
patient
death through exsanguination if not diagnosed and treated early and
accurately. A
sentinel bleed is a small bleed at the site of anastomosis that typically is
small in
-78-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
nature but is indicative of vessel rupture or disruption. If the vessel is
large and the
patient is on anticoagulation (typical), the blood loss can be significant and
ultimately
lead to exsanguination. Bleeding that may occur below the skin may be detected
with oxygenation sensing system 1900 and is usually not detectable by medical
practitioners in a timely manner. Additionally, hematoma detection through
signal
loss could play a role in early detection.
The oxygenation sensing system 1900 may detect three common ways
transplanted tissue 1805A2, 180562 fails: A) Arterial clot/rupture. Such an
event
usually causes decreased oxygenation due to lack of new blood with oxygen
being
brought into the transplanted tissue. B) Venous occlusion/clot. Poor outflow
from
transplanted tissue may usually result in venous engorgement and an overall
drop in
tissue oxygenation due to increased venous blood (deoxygenated) present in the
tissue. C) Hematoma development (which is usually due to a vessel rupture) can
potentially cause a loss of signal, which may be an event for oxygenation
sensing
system 1900 to activate an alarm, such as audible alarm 1907.
Another application for oxygenation sensing system 1900 includes the
monitoring of the transfer of tram flaps and tissue 1805A2, 180562. For these
applications, the sensors 405 should be sterilized so that they can be
positioned on
the site of interest very early after the procedure to obtain an initial
reading on the
operating room (OR) table. The oxygenation sensing system 1900 may provide
data
that quantitatively measures oxygenation of transferred tissues.
The oxygenation sensing system 1900 may sense conditions for organ
transplant 2605 monitoring. Typically, a host versus graft reaction will
generally
cause an immune response in a patient to vascular supply affecting the
vascular flow
to the organ. Poor perfusion which can possibly be combated with additional
immune suppression may also be detected by oxygenation sensing system 1900.
The oxygenation sensing system 1900 may detect conditions that allow for early
surgical intervention for revisions if needed/possible.
The Oxygenation sensing system 1900 may also be used to revascularize
tissue due to chronic vascular insufficiency 2610. The oxygenation sensing
system
1900 may be able to manage/monitor/and/or promote healing of revascularized
tissue. The oxygenation sensing system 1900 may detect conditions related to
= bypass graft patency. With bypass graft patency, chronic poor tissue
perfusion of
distal extremities (typically the lower extremity) can be treated by bypassing
poor
-79-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
vasculature with a biological or synthetic graft to restore blood flow to
distal tissue.
Specifically, poor large vessel perfusion 2610 is bypassed to allow for an
adequate
supply of blood to distal tissue as understood by one of ordinary skill in the
art. Any
anastomosis can be monitored with a sensor 405 to insure the vessel connection
is
intact and functioning correctly and remaining open.
The oxygenation sensing system 1900 may promote extremity healing in
chronic disease conditions. For example, diabetes usually causes peripheral
vessel
disease resulting in the need for extremity (typically foot/leg) amputation.
Other
autoimmune and vasculitis are other medical conditions which can also cause
poor
perfusion in extremities. One of ordinary skill in the art recognizes that
wounds do
not heal if they are poorly perfused. Each sensor 405 can be used to determine
the
ability of the tissue to heal in order to determine the level of amputation.
This
application is similar to the use in the traumatized/mangled extremity and
determining what injured tissue to debride versus what to save.
The oxygenation sensing system 1900 may also monitor tissue replantation
1805A2, 1805132. Specifically, the system may monitor the status of
reattaching
tissue that has been traumatically amputated. For example, such tissue may
include, but is not limited to, fingers, hands, arms, feet or legs. The
oxygenation
sensing system 1900 may help a medical practitioner monitor anastomosis
integrity
and distal flow. Specifically, the oxygenation sensing system 1900 may help a
medical practitioner monitor anastomosis of both arterial inflow and venous
outflow.
One of ordinary skill in the art recognizes that replants can fail if not
provided with
adequate outflow. Replantation typically requires two veins to one artery to
allow for
adequate blood flow in and out of the replant area.
The oxygenation sensing system 1900 may be useful in monitoring vessel
injuries and/or vessel repairs 2610. Such injuries may include, but are not
limited, to
a laceration to a major arterial supply and/or extremity/organ. One of
ordinary skill in
the art recognizes that reperfusion can cause tissue death or compartment
syndrome due swelling once blood flow is restored to the tissue. The
oxygenation
sensing system 1900 may provide data that allows for post operative/repair
monitoring similar to the transplant of tissue as discussed above and the
system can
insure the vessel repair is adequate and remains open. Each sensor 405 of the
oxygenation sensing system 1900 can also monitor tissue pressure to diagnose
acute compartment syndrome due to reperfusion injury.
-80-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
The oxygenation sensing system 1900 can also assist with collecting data to
help a medical practitioner to decide what is the appropriate treatment for a
given
tissue region. The oxygenation sensing system 1900 may also assist the medical
practitioner with determining if a selected procedure has been completed
successfully. For example, with a fasciotomy, uninformed or unfamiliar medical
practitioners can fail to release all compartments. Meanwhile, one of ordinary
skill in
the art recognizes that a complete release of all compartments allows for
restoration
of hyperemia and return of blood flow. An inadequate release from a fasciotomy
can
occur if the fascia is not released proximal and distally enough or if skin is
not
released in some cases. A percutaneous release may lead to incomplete release.
During and after the fasciotomy, the oxygenation sensing system 1900 may be
able
to detect hyperemia. If the treated region does not become hyperemic, then
such a
condition may be a sign of incomplete release or lack of release (missed the
compartment) from the fasciotomy. Additionally, if the fasciotomy is performed
too
late, the tissue may be dead already. In this case, a lack of return of blood
flow
would indicate to the surgeon the need for debridement to prevent infection.
Another procedure in which oxygenation sensing system 1900 may also
assist the medical practitioner to determine if a selected procedure has been
completed successfully is revascularization 2610. The oxygenation sensing
system
1900 may detect if restoration of blood flow has been achieved for a
particular site.
Hyperemia is expected once blood flow is restored due to a reperfusion effect.
The
= oxygenation sensing system 1900, as noted above, can detect hyperemia for
a
particular region. Additionally, the system could be used to determine if the
bypass
is sufficient to restore flow or if additional measures need to be taken.
The oxygenation sensing system 1900 may also help a medical practitioner to
determine the success of a bypass surgery. The oxygenation sensing system 1900
may detect if a bypass anastomosis is present and if adequate blood flow
downstream relative to the bypass region has been restored. The oxygenation
sensing system 1900 may determine if the operated vessel is open and if any
related
extremity is receiving adequate blood flow.
The oxygenation sensing system 1900 may also help a medical practitioner to
quantitatively measure the success of operations related to: reperfusion
injury; ACS;
transplanted tissue 1805A2, 180562; replantation 1805A2, 1805B2; and the like.
For transplanted tissue 1805A2, 180562, the oxygenation sensing system 1900
may
-81-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
detect anastomosis and provide data that indicates whether the transplanted
tissue
is healing and not dying. For operations related to replantation 1805A2,
1805B2, the
oxygenation sensing system 1900 may provide data to indicate whether blood
flow is
restored to a severed limb. The oxygenation sensing system 1900 may also
provide
data to indicate whether a vessel injury and its related repair have been
successful.
Specifically, the sensors 405 can measure oxygenation of replanted tissue.
Exemplary depths in which the sensors 405 can scan include, but are not
limited to,
depths of about one centimeter or even less (such as for fingers).
For reperfusion injuries, the oxygenation sensing system 1900 can provide
reperfusion monitoring. The oxygenation sensing system 1900 can monitor
initial
hyperperfusion and may provide data to indicate if perfusion has increased or
decreased for a particular tissue region.
The oxygenation sensing system 1900 may be used to detect other conditions
such as Exertional Compartment Syndrome (Chronic) caused by exercise or other
types of physical activities. For detecting this condition, the sensors 405
can be the
same as those used to detect ACS in injured tissue. For exertional compartment
syndrome, the regions of interest will most typically be in the legs or
forearms of the
patient. The oxygenation sensing system 1900 may be used to detect conditions
in
highly trained and conditioned athletes. The oxygenation sensing system 1900
may
be provided with a base algorithm that compares highly trained athletes
compared to
recreational athletes and untrained individuals.
The base algorithm for the oxygenation sensing system 1900 for detecting
exertional compartment system should account for the different physiological
conditions associated with exercising. The oxygenation sensing system 1900 may
require readings for pre-exercise, intra-exercise, and post-exercise to
properly
calibrate the algorithm. The algorithm may take into account that unlike ACS,
exertional compartment syndrome may be present in an environment in which
muscles swell with blood and have increased metabolites. These differences
between ACS and exertional compartment syndrome may usually be accounted for
in the base algorithm for oxygenation sensing system 1900.
The sensors 405 for detecting exertional compartment syndrome may have a
placement similar to the placement used to detect ACS. However, the sensors
405
may be provided with additional mechanical features such as, but not limited
to,
additional cord length, spring biased cords, etc. to allow for activities like
jogging
-82-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
and/or marching by the patient. Other mechanical features may include, but are
not
limited to, additional adhesive or straps positioned on sensors 405 to insure
adequate sensor skin connection. Additionally, the sensors could be attached
to a
mobile unit that allows for monitoring while covering large distances.
Further, the
sensors 405 may be positioned within a sleeve 3710 for accurate sensor
placement
that will not allow for movement of the sensors 405 relative to the region of
interest,
as illustrated in FIG. 37. The sensors 405 and their mechanical features may
be
designed to function properly in the presence of exercise by-products such as
sweat
from the human body during the physical exertion of the patient being
monitored with
the oxygenation sensing system 1900. Additionally, the sleeve 3710 or
attachment
device should not cause changes in flow due to excessive compression.
Sensors could be used in a wireless fashion or only record data to be
analyzed once plugged into a monitor system after training is complete. A
memory
device can record data then be retrieved later in order to limit the size and
weight of
the monitoring system.
This concept can be carried over into athletic or military training to guide
optimization of physical training without exceeding the tolerance of the
muscle. A
mobile unit or one placed in a backpack or other carrying device could guide
intensity of workouts and training,
For detecting exertional compartment syndrome, the oxygenation sensing
system 1900 may be provided with features for reducing or eliminating noise
from
the movement of the sensors 405. For example, the oxygenation sensing system
1900 may be provided with software and/or hardware to implement noise
reduction
algorithms caused by physical movement of the sensors 405. In the exertional
compartment syndrome monitoring scenario erythema/pigment monitoring may not
be evaluated since it is unlikely that the tissue of interest is traumatized.
However,
other chemical and environmental factors (such as but not limited to pH and
temperature) may affect the local tissue.
The oxygenation sensing system 1900 may provide data that helps a medical
practitioner to assess tissue viability. The oxygenation sensing system 1900
may be
used to monitor superficial trauma on patients. The oxygenation sensing system
1900 may replace the prior art black lamps which have been used in the past
for
evaluating tissue perfusion. Prior art devices are typically intrusive and
bulky and are
not passive solutions for monitoring and testing perfusion.
-83-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Oxygenation sensing system 1900 may also provide a guide to a medical
practitioner for amputation procedures. The oxygenation sensing system 1900
may
be useful for patients with diabetes and who may need amputation of a limb due
to
complications arising from this disease. The oxygenation sensing system 1900
may
determine a level of amputation for adequate blood flow in lower extremity
ulcers/infected regions. In other words, the oxygenation sensing system 1900
can
help the medical practitioner determine a level or the "line" to draw for
amputating a
limb. The oxygenation sensing system 1900 allows a medical practitioner to
determine at what level of a limb will heal due to the detection of adequate
blood flow
by the sensors 405 positioned on the limb of interest. In this monitoring for
detecting
the level of amputation, the position of the sensors 405 can be similar to
those used
for detecting compartment syndrome to evaluate muscle. Sensors 405 designed
for
shallow scans may be used to monitor skin values.
Referring to FIG. 32, this figure is logic flow diagram illustrating an
exemplary
method 3200 for assessing tissue conditions to assist medical practitioners in
determining an amputation "line" according to one exemplary embodiment of the
invention. This method describes steps that can be used with either the
oxygenation
sensing system 1900 or the combined system 2700 as discussed below.
Step 3205 is the first step in the process 3200 in which baseline oxygenation
values for a cross-section of the population of patients with a common medical
trait
and/or condition are identified. For example, baseline oxygenation values may
be
established for patients having diabetes. Oxygenation values may be taken from
several patients having diabetes and having a need for amputation of an
extremity.
A minimum oxygenation value may be determined from this population of
patients that indicates healthy tissue compared to tissue that may need to be
the
debrided or amputated. One of ordinary skill in the art recognizes that the
invention
is not limited to patients having diabetes. The invention may address any
population
of patients having a common medical trait and/or condition such as a disease
so that
a baseline level of oxygenation values may be established for the patients
having a
common medical trait and/or condition and who need amputation of an extremity.
Next, in step 3210, the oxygenation sensors 405 may be applied along a
length of an extremity that may require amputation. The sensors 405 should be
positioned along tissue which is healthy as well as along tissue which will
likely need
amputation.
-84-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
In step 3215, the oxygenation sensing system 1900 monitors all the sensors
405 positioned along the length of the extremity. Subsequently, in step 3220,
the
oxygenation sensing system 1900 displays NIRS values for the sensors 405
positioned along the length of the extremity.
In step 3225, the oxygenation sensing system 1900 compares NIRS values
between the respective sensors 405 positioned along the length of the
extremity.
Next, in step 3230, the oxygenation sensing system 1900 identifies sensors
having
relatively low values based upon the comparison made among the line of sensors
405. In step 3235, the oxygenation sensing system 1900 compares the values
from
the sensors 405 to the baseline established for the patients having a common
medical trait and/or condition.
In step 3240, the oxygenation sensing system identifies the sensors 405
which have NIRS values that correspond to the baseline set of values that
indicate
amputation may be needed for an area of tissue. Next, in step 3245, the
oxygenation
sensing system 1900 displays visuals identifying sensors 405 that may
correspond
to the amputation "level" or "line" on the extremity of the patient. The
process then
ends.
The oxygenation sensing system 1900 may also be used to monitor organ
perfusion 2605. Such monitoring is beneficial for operations relating to organ
transplantation. The oxygenation sensing system 1900 may be used to help a
medical practitioner assess acute grafts and to determine if the host body has
rejected the transplantation. The oxygenation sensing system 1900 may also
monitor
abdominal compartment syndrome as well as the perfusion of internal organs
such
as, but not limited to, the kidneys, liver, spleen, and bowel (small & large
intestines).
The oxygenation sensing system 1900, especially the sensors 405, may be
adapted or designed for specific body types, such as obese individuals having
layers
of fatty tissue. Problems could arise if sensors 405 with normal scan depths
designed for normal body types (having nominal fatty layers) are used for
monitoring
obese individuals. Different scaled sensors 405 having deeper scan depths may
be
provided for obese individuals due to excess layers of skin and fat tissue
that are
normally present with obese body types.
The oxygenation sensing system 1900 may help determine if skin ready for
surgery 1805A1 such as for a pilon fracture. A pilon fracture is a comminuted
fracture of the distal tibia. The fracture usually includes a long oblique
fracture
-85-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
extending medial to lateral as well as a fracture extending to the tibiotalar
articular
surface. It results from an axial loading injury, with impaction of the talus
upon the
tibial plafond.
Current methods prior to treating a pilon fracture require a medical
practitioner
_ 5 to estimate if swelling has decreased enough to allow for healing
(i.e., such as if the
skin has wrinkles). This prior art subjective judgment of the medical
practitioner can
now be replaced with sensors 405 which may help determine if skin is well
perfused
and not stretched too tight. The oxygenation sensing system 1900 may be used
in
the operating room (OR) to determine if skin closure is too tight and if it is
not
allowing adequate blood flow to tissue to allow for healing. This data from
the
oxygenation sensing system 1900 will allow the medical practitioner to
quantitatively
determine if the wound should be left open to heal.
The oxygenation sensing system 1900 can generally help a medical
practitioner to monitor skin perfusion. The oxygenation sensing system 1900
may be
used at the time of an attempted closure and it may help prevent skin/wound
complications and dehiscence. The oxygenation sensing system 1900 may help to
detect if good blood flow is present for wound healing. Current, conventional
methods for assessing good blood flow require human observations to determine
if
skin is wrinkling.
The oxygenation sensing system 1900 may be used to help a medical
practitioner determine if a patient is experiencing hypotension and/or Shock
2605 or
anemia. The oxygenation sensing system 1900 may be used in an emergency room,
an intensive care unit (ICU), or an operating room (OR). If the oxygenation
system
1900 detects decreasing NIRS values, then the combined system 2700 may
determine that these decreasing NIRS values are likely due to lower serial
hemoglobins (Hgb) or hematocrits (Hct). Table 6 provided and discussed below
provides just one example of how certain conditions of a patient may indicate
the
presence or existence of hemorrhaghic shock or anemia.
The oxygenation sensing system 1900 may be designed to allow for
comparable values across a variety of patients by utilizing adjusted values.
For
example, short monitoring of tissue may sometimes provide raw values from the
oxygenation sensing system 1900 that may be very erratic and vary widely.
However, continual monitoring with the oxygenation system 1900 may provide a
baseline and allow a medical practitioner to adjust for values after the
baseline is
-86-
CA 02854663 2014-05-05
WO 2013/070864 PCT/US2012/064078
established. The oxygenation system 1900 may have off-set values that may
adjust
readings for variations of skin pigment, erythema, age, vasculature status,
respiratory status, demographics, tobacco use, and cardiac/health risks.
In summary, the oxygenation sensing system 1900 may comprise one or
more algorithm(s) to account for different variations in person being
monitored in
order to get an "adjusted value" that can be compared across individuals of
different
skin color, age, weight, sex, and/or injury status. The oxygenation system
1900 may
also establish predetermined threshold values for normal perfusion,
hypoperfusion,
as well as values that assess viable tissue versus nonviable tissue.
Additionally, the
assessment of the patient such as but not limited to the American Society of
Anesthesiology (ASA) physical status classification could be built into the
algorithm
to allow for warnings earlier in higher risk individuals.
Referring now to FIG. 27, this Figure is a functional block diagram of an
intensive care unit (ICU) central controller and analyzer 420C1 according to
one
exemplary embodiment of the inventive system. According to this exemplary
embodiment, the oxygenation sensing system 1900 described in FIG. 19 can be
made to be compatible with an ICU central controller 42001 which may be
coupled
to other sensors and systems.
For example the ICU central controller 420C1 may be coupled to a respiration
sensor 2705, a pH level sensor 2710, a temperature sensor 2715, a pulse
oxygenation sensor 2720, a heart rate sensor 2725, a ventilation sensor 2730,
an
ultrasound sensor 2735, and an altitude sensor 2740 as well as a monitor to
determine pressure on the injured area under the dressings. The ICU central
controller 42001 can provide directions on use of its system components. The
ICU
central controller 420C1 may have analyzing hardware or software or both for
providing recommendations for clinical interventions based on the data it
collects
from the various sensors as well as data 2702 that may be entered via keyboard
2765 by an operator that is specific to a patient. The ICU central controller
42001
=
may provide its directions, data, and recommendations on a display device
420C.
In another exemplary embodiment, the ICU central controller 42001 can be
part of an existing sensor system such as a heart rate and/or respiratory
monitoring
system. According to such an exemplary embodiment, the oxygenation sensing
system 1900 would be designed to be compatible with the ICU central controller
-87-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
420C1 with the appropriate hardware and/or software (i.e. compatible
connector,
compatible application programming interfaces ¨ APIs, etc.).
The system 2700 may provide a complete ACS smart device that combines
monitoring, diagnosing, and treating up to fasciotomy. The system 2700 may
intelligently combine all known technology and data to provide medical
practitioners
with guidelines. The system 2700, and particularly its oxygenation sensing
system
1900, may obtain NIRS values and interpret them based on trends in values.
The central controller 420C1 may function as a data collection device which
obtains data from ICU devices. The central controller 420C1 may obtain
directly
blood pressure (BP), pulse ox, lab values, demographic data and may replace an
ICU monitor.
The combined system 2700 may include an intramuscular pressure device
2750 for monitoring muscle pressure and for filtering exudates. The
intramuscular
pressure device 2750 may comprise an ultrafiltration catheter for pressure
reading
and a fluid removal system.
The combined system 2700 may further comprise a medicine delivery system
2745 that may operate in a manner similar to a drip line setup found in an ICU
that
would automatically administer medication to affect cardiovascular status. The
medicine delivery system may administer drugs such as, but not limited to,
pressors
to increase blood pressure to allow for elevated perfusion pressures. The
system
2700, via medicine delivery system 2745, may administer medicines
automatically
based on monitored conditions. For example, if the system 2700 via the
oxygenation
sensing system 1900 detects decreasing NIRS values, then the central
controller
420C1 could issue a command to the medical delivery system 2745 to administer
a
pressor. The medical delivery system 2745 may comprise a small pump for moving
liquid medicines into a patient. This system could deliver medicines or other
entities
such as but not limited to intravenous fluid boluses or blood products.
The combined system 2700 may also comprise a tissue firmness device 2755
to measure how firm an extremity is and to determine how tense the extremity
is.
The ability to compress the tissue is a subjective measurement clinicians use
to
assess injured extremities. As noted previously, the combined system 2700 may
also include an ultrasound sensor 2735. The ultrasound sensor 2735 may measure
the vibration or wave characteristics of the fascia. The data from each of the
sensors of the combined system 2700 may be time stamped by the central
controller
-88-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
420C1 for review and archival purposes. The data may be stored in the memory
device 2760 which can comprise volatile or non-volatile memory (or both).
Other Uses of a Combined System 2700 for Patient Monitoring and Medical
Management Indications
The combined system 2700 may incorporate the vital signs and NIRS values
in order to give recommendations for clinical intervention. The combined
system
2700 may also provide various levels of alarms for the medical practitioner.
For
example, the combined system 2700 may have a series of color-coded visual
indicators to provide a relative status of different conditions for patient.
According to
one exemplary embodiment, the combined system 2700 may display a red color
coded alarm display 420CI, a yellow color-coded alarm display 420CII, and a
green
color-coded alarm display 420C111.
According to one exemplary embodiment, the red color-coded alarm display
420CI may indicate a danger condition such as low perfusion, or that NIRS and
blood pressure are in phase or that NIRS values are demonstrating a decreasing
trend. A yellow color-coded alarm display 420CII may indicate a moderate drop
or
downward trend with perfusion existing at minimal levels. This yellow alarm
may
also indicate that blood pressure and the NIRS values are beginning to become
in
phase. The combined system 2700, and particularly the central controller
420C1,
may recommend considering other modalities to evaluate perfusion such as
intracompartmental pressure measurements, pressors, and/or transfusions.
Meanwhile, the green color-coded alarm display 420CIII may indicate that there
are
no signs of poor or below-normal perfusion levels.
The oxygenation sensing system 1900 of the combined system 2700 may
activate an audible alarm 1907 or anyone of the visual alarms 420C1-CIII (any
combination thereof) to indicate low blood pressure and decreasing NIRS
values.
The combined system 2700 may recommend transfusions, intravenous fluids,
and/or
pressors. In non-traumatized or traumatized patients the oxygenation sensing
system 1900 could be placed on the leg 100 to monitor patient perfusion
status.
Poor cardiac function of a patient will usually cause decreased levels of
perfusion.
So the combined system 2700 may utilize the heart rate sensor 2725 in such
situations for monitoring heart failure patients.
-89-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
The respiration sensor 2705 may be used by the combined system 2700 to
detect increased respirations as well as pH levels (elevated lactic acid
typically is
associated with lower respiratory alkalosis). The combined system 2700 by
monitoring the respiration sensor 2705 in combination with the oxygenation
sensing
system 1900 may signal an alarm, such as the audible alarm 1907 or any one of
the
visual alarms 420C1-CIII (or any combination thereof), to increase oxygen
supplementation or to recommend intubatation for a patient.
The combined system 2700 may use the pH level sensor 2710 to detect
changes in pH levels of the patient. A change in pH level of the patient in
combination with a decrease in NIRS values detected by the oxygenation sensing
system 1900 may cause the combined system 2702 signal alarm such as the audio
alarm 1907 or a visual alarm 420CI (or both) to indicate the patient has poor
resuscitation. Such a condition may typically be found in patients who have
had a
replacement of blood products after a large amount of blood loss due to an
injury or
because of surgery.
The intensive care unit 420C1 of the combined system 2700 may record vital
signs detected by the heart rate sensor 2725 and the pulse/oxygenation sensor
2720
in a memory device 2760 coupled to the intensive care unit 42001. The
oxygenation
sensing system 1900 may be designed so that it is compatible with programs of
common intensive care units 420C1. If the oxygenation sensing system 1900
detects decreasing NIRS values in combination with changes in vital signs such
as
heart rate, blood pressure, and/or respiration, then the intensive care unit
420C1
indicate that tissue perfusion may be reaching a vulnerable point. When this
vulnerable point is conveyed by the intensive care unit 42001, then a medical
practitioner may be required to intervene with the patient.
The combined system 2700 may also monitor and calculate if blood products
are needed by a patient. Similar to serial hemoglobins (Hgb) or hematocrits
(Hct),
the NIRS values detected by the oxygenation sensing system 1900 may be used to
monitor perfusion during or after surgery, such as after hip or knee
replacements or
other surgeries or trauma. If the oxygenation sensing system 1900 detects
decreasing NIRS values, then the combined system 2700 may determine that these
decreasing NIRS values are likely due to lower Hgb or Hct. The combined system
2700 may be designed to track transfusions given to the patient so that it may
correlate if a particular transfusion increases the Hgb or Hct levels in a
patient.
-90-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
According to this exemplary embodiment, the combined system 2700 becomes a
non-invasive means of monitoring blood transport of oxygen in an intra or post-
operation (post-op) setting.
During surgery or after surgery due to continued bleeding either under the
skin or through a wound, a patient may lose a significant amount of blood that
the
patient becomes anemic. Typically, this condition is followed through serial
blood
draws that examine factors such as hemoglobin concentrations or hematocrits.
Additional things such as lactic acid levels and other factors can also be
examined.
All these blood draws require needle sticks and can be difficult in sicker
patients that
have poor vascular systems. The ability of the oxygenation system would be to
follow the values through a noninvasive means.
Opposite to traumatized tissue, non-traumatized tissue is not "privileged" and
will have shunting of blood flow away from it to more vital organs such s the
brain,
heart and other vital organs. As a patient becomes anemic, the oxygenation in
distal
extremities will fall indicating a stressed condition. By correlating
decreasing
oxygenation values in the extremity or other areas of the body with decreasing
Hct or
Hgb a standardization and guideline can be determined which would alleviate
the
need for repeated lab draws. The reverse can also be said regarding the
monitoring
of oxygenation levels as blood products are replaced. An increase in
oxygenation
values would be expected as blood is replaced. The combined system 2700
may use predetermined tables such as Table 6 provided below in order to help
make
assessments about a patient. Table 6 provided below provides just one example
of
how certain conditions of a patient may indicate the presence or existence of
Hemorrhagic shock and/or Anemia.
Shock is a state of inadequate perfusion, which does not sustain the
physiologic needs of organ tissues. Many conditions, including blood loss but
also
including nonhemorrhagic states such as dehydration, sepsis, impaired
autoregulation, obstruction, decreased myocardial function, and loss of
autonomic
tone, may produce shock or shocklike states. Hemorrhagic shock is a condition
in
which blood loss exceeds the body's ability to compensate and provide adequate
tissue perfusion and oxygenation. This frequently is due to trauma, but it may
be
caused by spontaneous hemorrhage (e.g., gastro intestinal bleeding,
childbirth),
surgery, and other causes. Most frequently, clinical hemorrhagic shock is
caused by
-91-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
an acute bleeding episode with a discrete precipitating event. Less commonly,
hemorrhagic shock may be seen in chronic conditions with subacute blood loss.
In addition to helping a medical practitioner to determine shock in a patient,
the combined system may also assist the medical practitioner with determining
the
existence of anemia. Anemia is a condition of the blood where there is not
enough
oxygen carried to the body's cells. Anemia usually occurs over a longer period
of
time compared to the suddenness of hemorrhagic shock. Shock occurs can occur
within seconds or minutes while anemia generally occurs over hours and days
and is
systemic. Oxygen is mostly transported on hemoglobin molecules in red blood
cells.
Anemia is present when amounts of red blood cells and/or hemoglobin are below
normal. The most common sign of anemia is fatigue. A patient may also feel
weak,
dizzy, or just not well. An anemic patient may become pale, feel cold and
easily short
of breath. The patient's blood pressure may become low and heart rate may
become
rapid.
Table 6 provides exemplary values, therefore, one of ordinary skill the art
recognizes that other values/ranges within this table may be adjusted
depending
upon the subjective conditions of a particular patient and/or adjustments
provided by
one or more medical studies in the field.
By utilizing the values in Table 6, the combined system 2700 may help a
medical practitioner formulate a proper diagnosis of the presence or existence
of
hemorrhagic shock or intra/post operative anemia.
-92-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
TABLE 6- CLASSIFICATION OF HEMORRHAGIC SHOCK/ ANEMIA
Compensated Mild Moderate
Severe
(Anemia) (Anemia) (Shock)
(Shock)
Blood Loss (mL) 51000 1000-1500 1500-2000
>2000
Heart rate (bpm) <100 >100 >120 >140
Blood pressure Normal Orthostatic change Marked fall
Profound fall
Capillary refill Normal May be delayed Usually delayed
Always delayed
Respiration Normal Mild increase Moderate tachypnea
Marked tachypnea:
respiratory collapse
Urinary output (mUh) >30 20-30 5-20
Anuria
Mental status Normal or agitated Agitated Confused
Lethargic, obtunded
In addition to the conditions listed in Table 6 provided above, the combined
system 2700 may include NIRS values detected by the oxygenation sensing system
1900 as part of a shock assessment. As apparent to one of ordinary skill the
art and
as illustrated in the several figures, the location of measurement for the
shock
assessment can vary. For example, the location of measurement may include, but
is
not limited to, the arm, leg, foot, hand, torso, abdomen, buttock, and/or
multiple sites,
such as illustrated in FIGs. 13A, 13B so that the medical practitioner may
have
different options based on the local effects of trauma to the tissue at a
particular site
on the patient.
One of ordinary skill in the art will appreciate that injury to tissue causes
the
injured tissue to become "privileged" with increased blood flow to the injured
area at
the expense of non-injured areas. This means that the medical practitioner may
have
a need to monitor multiple locations of the patient since the injured area
being
monitored may have abnormally high readings and may not be responsive to
global
body perfusion changes. For example, an injury or operation to the leg may
cause
increased values in the injured area (leg) which may be resistant to global
changes
since the normal response is to shunt blood to the injured area to promote
healing.
Therefore, a different location than the injured site is required to monitor
the global
hematologic status of the body, such as perhaps the arm or contralateral leg.
The
monitoring site needs to reflect the changes in the body's hematologic status,
which
would not be the case in a "preferred" location such as a site of injury.
-93-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Referring to FIG. 33A, this figure is logic flow diagram illustrating an
exemplary method 3300 for assessing conditions in a patient to help medical
practitioners determine if a patient is experiencing anemia and/or shock
according to
one exemplary embodiment of the invention. The method 3300 also provides a non-
invasive way to determine if a patient needs additional blood products and/or
transfusions. This method 3300 describes steps that can be used with the
combined
system 2700 as discussed above.
Step 3305 is the first step in the process 3300 the oxygenation sensors 405
are applied to a patient prior to a surgical procedure. In step 3307, which is
similar to
steps 2860 and 3060, the medical practitioner may identify which sensors 405
are
monitoring healthy or "non-traumatized" tissue and which sensors 405 are
monitoring
traumatized tissue. As noted above with respect to FIG. 26, injured tissue
often
becomes a "Privileged" area relative to other healthy body parts in that the
body will
typically maintain increased perfusion over other areas that are not injured
even in
times of poor global perfusion (hypotension). The oxygenation sensing system
1900
and/or combined system 2700 may be designed to accommodate or to account for
the different physiological states of injured or traumatized tissue 1805A1,
180561.
Either system 1900 or .2700 may adjust its one or more monitoring algorithms
depending upon the state of the tissue.
Also, in this step 3307, either system 1900 or 2700 may automatically identify
which
tissue is traumatized and which is not. The systems 1900 and 2700 may make
these
determinations based on detected tissue characteristics (such as temperature,
erythema,
etc.). They systems 1900 and 2700 may then use non-traumatized tissue as a
control
relative to the monitored traumatized tissue as discussed above and below.
Next, in step 3310, the combined system 2700 obtain this preoperative
baseline NIRS values for the patient from the sensors 405. In step 3315, the
combined system 2700 may also receive a baseline set of values from
traditional
blood tests that have been applied to the patient prior to surgery. In this
step 3315,
this baseline set of values from traditional blood tests may be entered via
the
keyboard 2765 or these values may be transferred from another computer system
to
the intensive care unit 420C1 of the combined system 2700. The traditional
blood
tests may include, but are not limited to, those which establish levels of
hemoglobin
(Hgb) and hemocrit (Hct) within the blood of the patient.
-94-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
In step 3317, the sensors 405 may be removed from the patient or they may
be turned "off' while the patient undergoes surgery. In step 3319, which is
similar to
steps 2860, 3060, and step 3307, the medical practitioner may identify which
sensors 405 are monitoring healthy or "non-traumatized" tissue and which
sensors
405 are monitoring traumatized tissue. As noted above with respect to Figure
26,
injured tissue often becomes a "Privileged" area relative to other healthy
body parts
in that the body will typically maintain increased perfusion over other areas
that are
not injured even in times of poor global perfusion (hypotension). The
oxygenation
sensing system 1900 and/or combined system 2700 may be designed to
accommodate or to account for the different physiological states of injured or
traumatized tissue 1805A1, 180561. Either system 1900 or 2700 may adjust its
one
or more monitoring algorithms depending upon the state of the tissue.
Also, in this step 3319, either system 1900 or 2700 may automatically identify
which tissue is traumatized and which is not. The systems 1900 and 2700 may
make these determinations based on detected tissue characteristics (such as
temperature, erythema, etc.). They systems 1900 and 2700 may then use non-
traumatized tissue as a control relative to the monitored traumatized tissue
as
discussed above and below.
Next, in step 3320, the oxygenation sensors 405 are again applied to the
patient after the surgery and in the same relative locations prior to surgery.
In step
3325, the combined system 2700 monitors the NIRS values for the post operative
patient from the sensors 405.
In step 3330, the combined system 2700 may receive blood test values for the
post operative patient. Similar to step 3315, these values from traditional
blood tests
may be entered via the keyboard 2765 of these values may be transferred from
another computer system to the intensive care unit 420C1. In step 3335, the
combined system 2700 may automatically correlate the NIRS values from the
sensors 405 against the values from the traditional blood tests. In this step
3335, the
combined system 2700 may determine what type of blood products as well as what
to a volume of blood transfusions may be necessary to restore "normal" levels
of Hct
and/or Hgb in the patient.
In step 3340, the combined system 2700 may display its recommendations
regarding blood products and/or blood transfusion levels based on its
assessment of
-95-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
the NIRS values taken from the sensors 405. In step 3345, the combined system
2700 may continue monitoring the NIRS values provided by the sensors 405.
In step 3350, the combined system 2700 may monitor the heart rate sensor
2725. In step 3355, the combined system 2700 may also monitor the blood
pressure
sensor 440 within the oxygenation sensing system 1900. In step 3360, the
combined system 2700 may also monitor the respiration sensor 2705. The process
then continues to step 3365 in FIG. 33B.
In step 3365 of FIG. 33B, the combined system 2700 may compare the
monitored data from each of the sensors to a predetermined table or a set of
tables
such as Table 6 discussed above which lists values for one or more medical
conditions. Next, in decision step 3370, the combined system 2700 A.
determines if
the data corresponds to the certain medical conditions outlined in the
predetermined
table(s) such as Table 6. This means that in decision step 3370, with the
specific
exemplary table of Table 6, the combined system 2700 may determine if the
patient
is experiencing an anemic condition and/or a shock condition based on the
values in
the table. One of ordinary skill the art recognizes that the invention is not
limited to
table 6 that outlines conditions and/or properties of anemia and shock. The
invention may address any one of a variety of medical conditions based on the
values listed for each sensor which are provided in the one or more
predetermined
tables.
If the inquiry to decision step 3370 is positive, then the "YES" branch is
followed to step 3375. If the inquiry to decision step 3370 is negative, then
the "NO"
branch is followed and the process returns back to step 3325.
In step 3375, the combined system 2700 may activate an alarm and display
the medical conditions on the display device 420C that appear to correspond
with
the values presented in the one or more predetermined tables. The alarm may
comprise an audio or visual alarm (or both).
The combined system 2700 may also take into account the presence of
pressors, also known as medication that may be used to increase the blood
pressure
and to potentially allow for increased perfusion in areas with elevated tissue
pressure. Pressors are typically used to increase the cardiac output in order
to
maintain body perfusion. Pressors may be prescribed by the medical
practitioner as
a way to help prevent acute compartment syndrome by increasing the blood
pressure so that it can over come increases in intracompartmental pressures.
If a
-96-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
particular patient happens to be on a pressor, then the combined system 2700
may
be able to account for the use of this medication in an algorithm by accessing
predetermined tables that have been derived from patients who have been on
pressors while being monitored by the combined system 2700. Alternatively or
in
addition to, the medical practitioner may be warmed by the combined system
2700
then the NIRS values detected by the oxygenation sensing system 1900 may be
altered by these types of medications. In a similar manner, the combined
system
2700 may also take into account other drugs that impact other sensors of the
combined system 2700 such as drugs that may impact respiration which may
impact
readings by the respiration sensor 2705 as well as drugs that may affect the
heart
which may impact the heart rate sensor 2725.
The blood pressure monitor for 440 may provide diastolic values as well as
Mean arterial pressure (MAP) values. The combined system 2700 may use these
values to assess artier vascular function as understood by one of ordinary
skill the
art. The combined system 2700 may detect or sense a presence of shock when it
detects that the blood pressure values have decreased. An arterial line may be
provided with the blood pressure monitor 442 help monitor the blood pressure
of a
patient.
The temperature sensor 2710 of the combined system 2700 may be designed
to detect key temperature changes which may affect ability of enzymes to
perform
efficiently. The performance of enzymes may have an impact on blood clotting,
oxygenation transportation (hemoglobin), and other functions known to one of
ordinary skill the art.
The pulse-ox sensor 2710 may be used to monitor lung capacity for oxygen
exchange. The data from this sensor 2710 may allow for insight into the whole
body
oxygen transport.
The altitude sensor 2740 may be useful in situations in which the patient is
transported through various different altitudes such as during military
operations in
which a patient is transported by helicopter or flight evacuation planes to a
medical
facility. The altitude sensor 2740 may be able to assess and determine causes
of
changes in pressure within closed compartments such as, but not limited to,
the
fascia of extremities, organs, intracranial pressures, and the like.
The heart rate sensor 2725 may be able to detect circulatory capabilities of
the subject and may help detect the presence of shock. The combined system
2700
-97-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
may monitor an increased heart rate with the heart rate sensor 2725 and
decreased
NIRS values with the oxygenation sensing system 1900 which may cause the
combined system to sound the audible alarm 1907 and/or one of the visual
alarms
420C1-CIII to suggest to the medical practitioner that a transfusion may be
needed
by the patient.
The respiration sensor 2705 may be able to indicate whether a patient has
poor oxygenation are not. The combined system 2700 may use the signals from
the
respiration center 2705 and the NIRS values from the oxygenation sensing
system
1900 to determine whether a patient has poor ability to oxygenate, and if this
condition exists, the combined system 2700 may activate an alarm to suggest
increasing oxygen supplementation or intubation of the patient.
The pH sensor 2710 may indicate poor resuscitation if the combined system
2700 also detects changes in NIRS values from the oxygenation sensing system
1900.
The demographic data input 2702 may help the combined system 2700
determine if a patient has certain diseases which may affect perfusion, such
as
diabetes, peripheral neuropathy, and small vessel disease. The demographic
data
input 2702 may also help the combined system to determine if a patient may
have
lung disease, conditions associated with smoking/ Et0H (Alcohol), and
conditioned
associated with old age. The demographic data input 2702 may also allow for a
medical practitioner to input a sex of the patient as well as the ability to
assign
pigment values based on pigment type. If pigment data is provided by the
medical
practitioner, then the combined system 2700 may access predetermined charts
such
as the pigment chart developed by Taylor et al. 2006.
The NIRS sensors 405 of the combined system 2700, and particularly of the
oxygenation sensing system 1900, may be provided with different sizes to
correspond with patients having various sizes. For example, the NIRS sensors
405
may be provided in at least three different sizes such as small, medium, and
large as
illustrated in FIG. 34. These variations in sizes for the sensors 405 may
allow for the
monitoring of various different body sizes such as for fat, average, and
skinny people
due to the different scan depths that may be achieved through varying the
distance
"d" between the light source 510 and the light receiver 515 for a particular
sensor
405. The sizes of the sensors 405 may also be tailored for the specific body
part
being monitored, such as for the leg, forearm, thigh, and/or foot as
illustrated in FIG.
-98-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
35. In FIG. 35, the sensor 405 has a predetermined shape that corresponds to a
finger of a human. The variations in sizes for the sensors 405 may allow for
the
isolation of tissue at specific depths based on the body part being monitored.
The
sensors 405 may be provided with predetermined scan depths based on a MRI
study
in which limbs of the human body have been scanned to determine the average
depths for each compartment of the human body. Scan depths for each sensor 405
can be achieved by the type of light source provided on each sensor 405 as
well as
the geometry of the sensor 405 and arrangement in a sensor array 805 for the
oxygenation sensing system 1900 in order to best fit the compartment being
monitored.
The system 2700 may communicate with multiple sensor types that are
coupled to the system 2700 which includes the oxygenation sensing system 1900.
Other sensor types may include, but are not limited to, a cerebral monitor, an
organ
monitor such as the heart rate sensor 2725 and the respiration sensor 2705, a
spine
monitor, and other like monitors. The system 2700 may provide and display
directions for use of a sensor, such as each sensor 405 and the
initiation/calibration
of each sensor 405 as it establishes communications with system 2700.
The system 2700 can provide sensor directions in order to educate medical
practitioners on how to use/place sensors 405 at start up when either the
system
2700 is powered on or when a new sensor 405 establishes communications with
the
system 2700. The system 2700 may also allow a medical practitioner to bypass
the
instructions/directions on the system 2700 if the medical practitioners
familiar with
the device/application of the system 2700.
The system 2700 may have multiple different sockets or inputs from multiple
different pad/monitoring input sources based on the function of a particular
sensor
405. Different sockets or inputs may allow for different uses of a particular
sensor
405. The sockets or inputs may have labels 3605 which match labels positioned
on
each sensor 405 so that correct sockets and inputs are utilized in so that
medical
practitioners are not confused when attaching the sensors 405 to the system
2700
as illustrated in FIG. 36. Each socket can be made to only fit certain sensors
that will
be labeled specifically for that use. In other words, each socket 3610 of a
sensor
405 or sockets 3610 for a set of sensors 405 may be provided with unique
geometric
shapes as illustrated in FIG. 36. Additionally, based on what socket is used,
different
-99-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
algorithms can initiated based on what function the sensor 405 for which it is
to be
used.
A disconnect mechanism 3615 as illustrated in FIG. 36 can be incorporated
into the system 2700 that allows the sensors 405 to be disconnected quickly
and
easily for patient transfer, etc. This mechanism 3615 will allow for easy
disconnection but also easy reconnection, so sensors 405 are not reconnected
incorrectly. Color coding 3620 or different connection mechanisms for each
line can
insure appropriate reconnection to the accurate site. By using different
fitting
connection/locking mechanisms 3615 for each line, it insures that only the
correct
two ends of a line can be attached together.
As noted previously, sensors 405 to may store a unique identifier for the
system 2700 to recognize. Therefore, if the sensors 405 are unplugged then
reattached, the information will be retrieved from previous measurements. In
this
way, if a change from previous readings occurred while the sensor 405 was
unplugged (i.e., the patient was taken for a diagnostic test) the change would
be
recognized by the system 2700 once the sensor 405 was reattached.
Additionally, a memory device and mobile monitor may be provided that can
be temporarily attached to sensors 405 in order to retain data while the
sensors are
unplugged from the combined system 2700. As noted above, the sensor 405 itself
could retain data for processing after it removal or if the sensor was
switched
between different intensive care units 420C1.
The system 2700 may be provided with a keyboard/keypad 2765 so that
patient information, such as demographic data 2702, may be entered by a
medical
practitioner. The keyboard/keypad 2760 5A be coupled to the system 2700 by a
wired or wireless link.
System 2700 may take advantage of controls also referred to as uninjured or
areas of anatomy which are not of interest to the system 2700. The system 2700
may take baseline measurements of the controls in order to compare them with
the
areas of anatomy which are of interest to the system 2700. For bilateral lower
extremity injuries, system 2700 may use one or more forearms as a control. The
upper extremity of the patient should usually be shunted if it is uninjured
relative to
the bilateral lower extremity injuries. The system 2700 may also take into
account
that injured tissue may become hyperemic and adjust its measurements
accordingly.
-100-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
The system 2700 may help the medical practitioner locate the compartments
of a human leg. Specifically, the system may help the medical practitioner
locate the
Anterior, Lateral & Superficial Compartments (these compartments can be
accessed
over any area due to the superficial nature), and Deep posterior (which is
usually
more difficult to get a reading). The system 2700 can help the medical
practitioner
locate the most superficial portion of the Deep posterior which may be
generally
found at the posterior and medial boarder of tibia and is often the best place
to take
a reading with a sensor 405. As noted previously, the system 2700 may provide
an
initiation of monitoring directions to medical practitioners not familiar with
ACS to
insure appropriate placement of pads.
Referring to FIG. 28, this figure is logic flow diagram illustrating an
exemplary
method 2800 for positioning sensors 405 on a leg and for monitoring conditions
for
ACS according to one exemplary embodiment of the invention. This method 2800
describes steps that can be used with either the oxygenation sensing system
1900
or the combined system 2700 as discussed above. The method 2800 may also be
part of method 2500 of FIG. 25 in that the steps of method 2800 could be part
of
Step 2521 of FIG. 25 in which proper positions for sensors 405 are identified.
Step 2805 is the first step in the process 2800 in which instructions are
displayed on the display device 4208 / 420C for entering new patient
information into
the combined system 2700 or into the oxygenation sensing system 1900. New
patient information made include, but is not limited to, name, address, unique
identifiers assigned by a medical facility, insurance information, and the
like. This
information can be entered in using a keyboard or keypad 2765.
Next, in step 2810 the combined system 2700 or the oxygenation sensing
system 1900 may receive the new patient information and store it in memory,
such
as memory storage 2760 of FIG. 27. Next, in step 2815, one or more visual(s)
are
displayed on the display device 4208 / 420C which illustrate how to reduce
fractures
of the leg 100, a line the leg 100, and palpate the tibial ridge of the leg
100. One
exemplary visual for step 2815 is provided in FIG. 29A.
Referring briefly to FIG. 29A, this figure illustrates the right leg 100 of a
patient
and how a medical practitioner may provisionally reduce any fractures to align
the
leg 100. Referring back to FIG. 28, in step 2820 one or more visuals may be
displayed on the display device 4208 / 420C that illustrate how the sensors
405
should be positioned at the mid-tibial level or just proximal (towards the
knee), as
-101-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
provided in FIG. 29B. Visuals may include, but are not limited to, graphical
computer-generated still images that may comprise digital photographs and
text, as
well as video which comprises moving images. The visuals may also comprise
computer-generated images that provide illustrations instead of digital
photographs,
or any combination thereof. The visuals may or may not be accompanied by audio
information such as a narrator describing proper placement of the sensors 405.
In step 2825, one or more visuals may be displayed to illustrate how to
position the anterior (A) sensor 405 lateral to the tibial ridge over the
muscle. In
most cases, this position will be about or approximately two centimeters off
the ridge,
as provided in FIG. 29C. Next, in step 2830, one or more visuals may be
displayed
on the display device 4208 / 420C to illustrate how to position a lateral (L)
sensor
405 over the fibula on the lateral aspect of the leg 100, as provided in FIG.
29D.
In step 2835, one or more visuals may be displayed on the display device
4208 / 420C to illustrate how the mid-tibial level may be measured, as
provided in
FIG. 29E. In step 2840, one or more visuals may be displayed on the display
device
4208 / 420C illustrating how to position the deep posterior (DP) sensor 405
just
behind to the inner aspect of the shin over the flexor digitorum longus, as
provided in
. FIG. 29F.
Next in step 2845, one or more visuals illustrating how to position the
superficial posterior (SP) sensor 405 on the back part of the leg 100 may be
provided on the display device 4208 / 420C, as provided in FIG. 29G.
Subsequently,
in step 2050, the system 2700 or oxygenation sensing system 1900 may receive
confirmation from each of the sensors 405 to indicate that they are properly
connected to the CPU 420A. In step 2855, the system 2700 may initiate readings
from the sensors 405.
In step 2860, the medical practitioner may identify which sensors 405 are
monitoring healthy or "non-traumatized" tissue and which sensors 405 are
monitoring
traumatized tissue. As noted above with respect to FIG. 26, injured tissue
often
becomes a "Privileged" area relative to other healthy body parts in that the
body will
typically maintain increased perfusion over other areas that are not injured
even in
times of poor global perfusion (hypotension). The oxygenation sensing system
1900
and/or combined system 2700 may be designed to accommodate or to account for
the different physiological states of injured or traumatized tissue 1805A1,
180561.
Either system 1900 or 2700 may adjust its one or more monitoring algorithms
-102-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
depending upon the state of the tissue. Also, in this step 2860, either system
1900
or 2700 may automatically identify which tissue is traumatized and which is
not. The
systems 1900 and 2700 may make these determinations based on detected tissue
characteristics (such as temperature, erythema, etc.). They systems 1900 and
2700
may then use non-traumatized tissue as a control relative to the monitored
traumatized tissue as discussed above and below. The process then continues,
and
it may continue with Steps 2539 through 2562 of FIG. 25.
In addition to helping the medical practitioner locate the appropriate
positions
for sensors that monitor the compartments of the leg 100, the system 2700 may
easily measure and monitor conditions for acute compartment syndrome (ACS) in
a
forearm which is known to one of ordinary skill the art as the second most
common
area for ACS relative to the legs. The system 2700 may easily monitor the four
different compartments of a forearm which include the following: A) mobile wad
(extensor carpi radilis longus & brevis and the brachioradilis); B) Deep
flexors- flexor
digitorum profundus; C) Superficial flexors- flexor digitorum superficialis,
other
flexors (wrist) & pronator teres; and D)Extensors- wrist & finger extensors &
supinator.
The system 2700 may provide specific placement instructions including
illustrations or video for positioning sensors 405 for measuring and
monitoring ACS
in a forearm. The system 2700 may help the medical practitioner locate the
muscles
in the forearm. The system 2700 may help the medical practitioner identify
these
muscles by indicating that the muscles are often found more in the proximal
than the
distal one half of the forearm when the muscle belly of the forearm is
typically
located. The system 2700 may help the medical practitioner place the sensors
405
in the proximal one half of the forearm and over the distal one half of the
forearm as
possible.
The system 2700 may help the medical practitioner to account for the rotation
of a forearm to ensure appropriate monitoring and placement of the sensors
405.
The system 2700 may prompt the medical practitioner to position the sensors
405
when the forearm is in neutral rotation in which the thumb of the patient is
pointing
forward or ventral. The system 2700 may also monitor conditions in any
location of
the distal portion of the arm, such as but not limited to, a hand, finger,
palm thenar,
hypothenar eminence, wrist, etc.
-103-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Referring to FIG. 30, this figure is logic flow diagram illustrating an
exemplary
method 3000 for positioning sensors 405 on an arm 3100 and for monitoring
conditions for ACS according to one exemplary embodiment of the invention.
This
method describes steps that can be used with either the oxygenation sensing
system
1900 or the combined system 2700 as discussed above. The method 3000 may
also be part of method 2500 of FIG. 25 in that the steps of method 3000 could
be
part of Step 2521 of FIG. 25 in which proper positions for sensors 405 are
identified.
Step 3005 is the first step in the process 3000 in which instructions are
displayed on the display device 4208 / 420C for entering new patient
information into
the combined system 2700 or into the oxygenation sensing system 1900. New
patient information made include, but is not limited to, name, address, unique
identifiers assigned by a medical facility, insurance information, and the
like. This
information can be entered in using a keyboard or keypad 2765.
Next, in step 3010 the combined system 2700 or the oxygenation sensing
system 1900 may receive the new patient information and store it in memory,
such
as memory storage 2760 of FIG. 27. Next, in step 3015, one or more visuals
illustrating how to reduce forearm fracture and how to align the arm in the
fully
supinated position may be provided on the display device 4208 / 420C, as
provided
in FIG. 31A. Visuals may include, but are not limited to, graphical computer-
generated still images that may comprise digital photographs and text, as well
as
video which comprises moving images. The visuals may also comprise computer-
generated images that provide illustrations instead of digital photographs, or
any
combination thereof. The visuals may or may not be accompanied by audio
information such as a narrator describing proper placement of the sensors 405.
Next, in step 3020 one or more visuals illustrating how sensors 405 are
placed roughly 1/3 down the forearm 3100 closer to the elbow then the wrist,
may be
provided on display device 4208 / 420C, as set forth in FIG. 31B. In step
3025, one
or more visuals illustrating how to palpate the ulna of the forearm 3100 may
be
provided on the display device 4208 / 420C, as provided in FIG. 31C.
In step 3030, one or more visuals illustrating where to place a first sensor
405
just volar to the ulna, as provided in FIG. 310, may prove provided on the
display
device 4020. In step 3035, one or more visuals illustrating where to place a
second
sensor 405 in the mid-aspect of the volar surface of the forearm 3100 may be
provided on the display device 4020, as illustrated in FIG. 31E. Subsequently,
in
-104-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
step 3040, one or more visuals illustrating where to place a third sensor 405
in line
with the thumb and lateral epicondyle may be provided on the display device
420, as
set forth in FIGs. 31F-G.
Next, in step 3045, one or more visuals may be displayed on the display
device 4020 illustrating where to place a fourth sensor just dorsal to the
ulna, as set
forth in FIG. 31H. In step 3050, the system 2700 or oxygenation sensing system
1900 may receive confirmation from each of the sensors 405 to indicate that
they are
properly connected to the CPU 420A. In step 3055, the system 2700 may initiate
readings from the sensors 405.
In step 3060, which is similar to step 2860, the medical practitioner may
identify which sensors 405 are monitoring healthy or "non-traumatized" tissue
and
which sensors 405 are monitoring traumatized tissue. As noted above with
respect
to FIG. 26, injured tissue often becomes a "Privileged" area relative to other
healthy
body parts in that the body will typically maintain increased perfusion over
other
areas that are not injured even in times of poor global perfusion
(hypotension). The
oxygenation sensing system 1900 and/or combined system 2700 may be designed
to accommodate or to account for the different physiological states of injured
or
traumatized tissue 1805A1, 180561. Either system 1900 or 2700 may adjust its
one
or more monitoring algorithms depending upon the state of the tissue.
Also, in this step 3060, either system 1900 or 2700 may automatically identify
which tissue is traumatized and which is not. The systems 1900 and 2700 may
make these determinations based on detected tissue characteristics (such as
temperature, erythema, etc.). They systems 1900 and 2700 may then use non-
traumatized tissue as a control relative to the monitored traumatized tissue
as
discussed above and below. The process then continues, and it may continue
with
Steps 2539 through 2562 of FIG. 25.
The combined system 2700 may execute algorithms that are designed for
specific medical conditions. For example, the combined system 2700 may execute
an algorithm that is specifically for traumatized tissue. According to such a
traumatized tissue algorithm, the system 2700 could record NIRS values from
the
oxygenation system 1900 on the order of minutes instead of smaller increments
like
seconds or milliseconds since conditions for traumatized tissue do not change
that
rapidly relative to seconds or milliseconds. However, changes may be detected
across a scale of minutes such as on the order of every five minutes or so.
One of
-105-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
ordinary skill in the art will appreciate that the invention is not limited to
taking
readings every five minutes and can include other magnitudes depending on the
tissue / patient being monitored. With monitoring traumatized tissue, one of
ordinary
skill in the art recognizes that a medical practitioner usually only needs to
know
trends conveyed by the collected data to assess healing progress or any
complications.
For this specific yet exemplary application, the system 2700 in monitoring
traumatized tissue may use delays to determine if changes are maintained or if
they
are artifact (such as changes detected due to patient movement). The system
2700
may be smooth out data by using these delays. The system 2700 may signal an
audio or visual alarm (or both) if a trend is maintained for predetermined
period of
time that can be adjusted by the medical practitioner. For example, the
medical
practitioner could request the system 2700 to activate an alarm if a trend of
data is
constant over a period of two minutes, five minutes, or thirty minutes, just
to name a
few. These periods set by the medical practitioner can be set to any length as
desired by the medical practitioner.
The system 2700 may also delay or stop readings for a predetermined period
of time in response to other devices acting on a patient. For example,
oxygenation
system sensing 1900 may include a blood pressure cuff in addition to its blood
pressure probe 440. The system 2700 may cease readings made by the
oxygenation sensing system 1900 every time the blood pressure cuff cycles,
since
perfusion will be decreased when the blood pressure cuff is expanded on the
patient.
The system 2700 should not activate an alarm every time the blood pressure
cuff is
inflated.
- The system 2700 may have the function/feature of accessing stored data
from
previous readings. Such a function/feature is beneficial for when patients
need to be
disconnected to go to bathroom, to get tests done, or undergo surgery. Each
sensor
405 may be provided with a unique serial number or microchip so that the
central
controller 420C1 may recognize previous data from a particular sensor 405 when
it
reviews its memory 2760. In some exemplary embodiments, each sensor 405 may
be provided with local memory storage 635 (See FIG. 6C) on the sensor 405
itself.
Each sensor 405 may be provided with labels to provide a user with
information on what compartment the sensor 405 should be placed on (A or Ant
or
Anterior for the Anterior compartment or a number on it which then is used in
the set
-106-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
up instruction). The combined system 2700 may permit set up instructions to be
accessed from any screen provided on the display 4208 / 420C to assist the
medical
practitioner with correct placement of the sensors 405 on the tissue of
interest.
The system 2700 may generate printed labels for each compartment that can
The central controller 420C1 may be provided with one or more algorithms to
determine .if the tissue being monitored is a control or if it is the injured
tissue based
With control tissue, the system 2700 may determine that if control readings
are going down or if a downward trend is detected, then the system 2700 may
alert
the medical practitioner that a systemic problem, such as a hypotensive
condition,
may be present. The system 2700 may also account for body positions of the
Hardware components specific for ACS
The sensors 405 may be formed as a horse tail sensor that comprises one
-107-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
such as four sensors 405 grouped together, may be plugged in as one unit so
each
sensor does not plug in individually and allow for them to be switched, such
as
illustrated in FIG. 5B.
Each sensor 405 may be provided with physical markings such as with
permanent letters and/or numbers to allow accurate placement of sensors 405,
such
as illustrated in FIG. 6A. These permanent physical markings cannot be removed
or
switched (permanent at time of manufacturing). Additionally, for some sensors
405,
a right or left (R/L) designation may also be provided if a particular sensor
405 is
sized and shaped for a particular side of an extremity or body part, as
illustrated in
FIG. 35. If each sensor 405 is provided with a unique identifier readable by
the
central controller 420C1, then the central controller can alert the medical
practitioner
that a particular sensor has been inadvertently relocated by comparing present
readings with current readings.
Each sensor 405 may be provided with mechanical features, such as plugs
with geometries that are easily gripped and matched appropriately with a
corresponding socket so that they are easy for medical practitioners to plug
in and to
remove without inappropriately/inadvertently switching sensors 405, as
illustrated in
FIG. 36.
The system 2700 may provide visual instructions on the display device 4208 /
420C on how to place sensors 405 at start up. These instructions may be
accessible
at any time for reattachment of the sensors 405 to the CPU 420A. Each sensor
405
may be provided with batteries having a life of at least several hours to
allow a
patient to be transported. However, other battery life sizes are possible and
within
the scope of the invention.
A system where the sensors 405 may be detached from the monitoring
system 2700 to allow for a smaller mobile device may be provided. This smaller
system would still record data, but not have the display capabilities,
interpretational
functions, and/or an alarm system. However, it would have a battery, sensors
405,and memory to allow for mobile monitoring.
The system 2700 may comprise algorithms that are specific or tailored for
tissue/regions of interest. For example, the system 2700 may have algorithms
specific to a forearm 3100, in which proximal sensors 405 are evaluated or
weighted
secondary to tendon sensors 405 placed distally. The algorithm may adjust or
take
into account any rotation of the arm. Sensors 405 for the arm can be
positioned
-108-
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
distally such as on, but not limited to, the fingers, palm, thenar, and
hypothenar
eminence, just to name a few. For the leg, distal regions for sensors 405 may
include, but are not limited to, a plantar surface, toes, and the ankle. For
torso
regions, sensors 405 may be positioned on the abdomen as well for abdominal
compartment syndrome or any other area of the body, like the spinal cord,
brain
injury, hand, foot, thigh, buttocks, etc.
It should be understood that the foregoing relates only to illustrate the
embodiments of the invention, and that numerous changes may be made therein
without departing from the scope and spirit of the invention as defined by the
following claims.
-109-
=
CA 02854663 2014-05-05
WO 2013/070864 PCT/US2012/064078
Appendix A
Compartment Syndrome Patients
Anterior Lateral Deep Posterior Superficial Posterior
Pt Iniur Unin Diff 1CP PP InJur Unin Dill ICP PP Injur Unin
Dill ICP PP !Nur Unin DID 1CP PP
1 66 58 8 78 -13 58 65 -7 79 -14 76 -11 58 57 1 8,4
-19
2 35 50 -15 170 -70 41 49 -8 176 -76 116 , -
16 43 67 -24 115 -15
3 15 41 -26 107 -37 15 40 -25 104 -34 104 -34
47 45 2 99 -29
4 46 44 2 , 72 4 34 49 -15 82 -6 57 19
32 56 -24 71 5
05 47 -2 71 18 53 53 0 71 18 56 33 56 55 1 61 28
6 56 64 -6 59 -4 55 61 -6 57 -2 46 67 -
21 , 63 -8 55 64 -9 62 -7
7 56 81 -5 61 10 62 59 3 59
12
8 58 66 -8 142 -44 51 64 -13 142 -44 59 75 -
16 135 -37 50 77 ' -27 110 -12
9 SO 51 -1 57 1 53 54 -1 55 3 '
Avg 46.4 52.6 -694.5-18.1 45 54.4 -9.38 95.8 -19.4 53.7 67.7 -
14 83.5 -5.5 50.4 60 -9.63 82.6 -4.63
Std D 15.8 9.16 10.6 41.6 29.5 14.5 8.58 8.16 42.9 30.3 6.81 7.02
8.19 30.6 24.7 9.64 9.49 13.3 22.8 16.5
pVal 0.07 0.01 0.05 0.04
Rang 66.15 41.66 8,-26 15.58 40,65 0.-25 46.59 61.75 -5.-
21 ' 32,62 45,77 3,27 .
114
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix A
Non Compartment Syndrome
Initial Injury Study
Anterior Lateral Deep Post Sup Posterior
Injure Uninj Injure Uninj Injure Uninj Injure Uninj
Pt d ured DID d ured DID d ured DIft d urod DM
1 70 65 5 , 70 58 12 77 , 88 9
2 64 54 10 61 56 5 72 57 15
3 58 47 11 49 44 5 49 43 6
4 77 63 14 82 66 16 83 61 22 79 66 , 13
82 58 24 78 , 59 , 19 72 63 9 73 56 17
6 62 54 8 61 53 8 66 49 17 63 51 12
7 64 48 16 68 46 22 76 52 24 64 44 20
8 70 62 8 73 57 16 80 59 21 , 87 64 23
9 62 47 15 71 53 18 , 74 52 22 66 53 13
73 60 13 75 52 23 71 52 19 72 56 16
11 73 58 15 88 51 37 88 82 26 80 52 28
12 63 53 10 61 50 11 72 57 15 73 61 12
13 78 73 5 82 75 7 86 70 16 81 71 10
14 67 57 10 70 62 8 74 64 10 71 62 9
71 46 25 113 43 20 71 43 28 69 46 23
16 77 47 30 66 55 11 66 54 12 62 57 5
17 55 46 9 62 48 14 62 51 11 63 45 18
18 82 58 24 79 64 15 90 75 15 79 74 , 5
19 54 49 5 60 47 13 54 52 2 57 50 , 7
79 71 ' 8 90 81 9 87 72 15 86 69 17
21 70 49 21 65 47 18 64 45 19 81 52 9
22 78 44 34 78 43 33 82 54 28 69 48 21
23 74 , 65 9 76 63 , 13 78 81 17 73 , 85 8
24 68 53 15 68 56 , 10 , 77 58 19 70 55 15
62 51 11 68 53 15 60 51 9 60 50 10
26 68 53 15 66 56 10 77 58 _ 19,_ 70 55 15
Avg 69.3 55 14.2 70.2 55.3 14.9 74.3 57.2 17.2 70.2 56.5 13.7
Med 70 53.5 12 69 I 54 13.5 74 57 17 70.5 55.5 13
Std D 7.93 7.94 7.75 9.471 9.27 7.69 9.48 8.14 6.5 8.99 8.72 6.03
pVal 0.00Car, 3,C 0IICCCO3 , 0 0C3CC3
115
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix A
Non Compartment Syndrome 2 Days Post-Op
Anterior Lateral Deeo Post Sup Posterior
Injure Uninj Injure Unlnj Injure Uninj Injure Unlnj
Pt d ured Din d ured Din d ured Din d ured Diff
1 79 51 28 , 75 54 21 69 47 22 67 43 24
2 56 47 9 51 45 , 6 58 46 12 56 48 8
3 82 52 30 78 47 31 82 59 23 88 50 38
4 63 55 8 65 54 11 74 58 16 70 58 12
62 50 12 63 47 16 63 58 5 62 54 8
6 61 50 11 62 54 8 68 60 8 81 55 26
7 69 61 8 71 64 7 , 79 69 10 75 68 7 ,
8 68 42 26 , 65 39 26 75 41 34 62 44 18
9 85 73 12 79 62 17 95 75 20 93 73 20
71 62 9 72 58 14 66 61 5 74 61 13
11 83 70 13 82 67 16 87 71 16 , 88 72 16
12 77 47 30 66 55 11 66 54 12 82 57 5
13 64 58 8 62 50 12 67 56 11 63 51 12
14 70 49 21 80 55 25 73 63 10 65 54 11
84 64 20 90 63 27 88 64 24 68 63 25
16 60 47 13 63 51 12 64 51 13 76 43 33
17 71 63 8 61 67 4 87 62 25 63 60 3
Aug 70.9 55.2 15.6 69.7 54.2 15.5 74.2 58.5 186 72.5 56.1 16.4
Med 70 52 12 66 54 14 73 59 13 70 SS 13
Sid D 9.3 8.84 8.31 9.85 7.37 8 10.5 8.97 7.95 11.5 9.35
10
pVal snare o Doom cocoas o mom
116
CA 02854663 2014-05-05
WO 2013/070864 PCT/US2012/064078
Appendix A
Uninjured Subjects (Normal NIRS Values)
Anterior Lateral Deep Post Sup Posterior
PI R L Diff AV R L Diff AV R L OW AV R L DIff AV
1 50 46 4 4 46 48 -2 2 48 49 -1 I 40 40 0 0
2 58 56 2 2 60 60 0 0 63 60 3 3 64 57 7 7
3 49 47 2 2 48 50 -2 2 47 44 3 3 47 44 3 3
4 59 60 -1 1 60 57 3 3 58 61 -3 , 3 58
61 -3 3
54 51 3 3 51 48 3 3 55 51 4 4 55 52 3 3
6 55 52 3 3 58 53 5 5 68 65 3 3 69 63 6 6
7 56 57 -1 1 52 58 -6 6 52 62 -10 10 64 67 -3 3
8 54 57 -3 3 56 57 -1 1 61 61 0 , 0 55
60 -5 5
9 49 42 7 7 50 43 7 7 55 49 6 6 52 52 0 0
48 44 4 4 51 46 5 5 72 60 12 12 51 46 5 5
11 71 65 6 6 73 68 5 5 75 76 -1 1 69 75 -6 6
12 48 50 -2 2 48 53 -5 5 49 54 -5 5 4/ 09 -2 2
13 45 50 -5 5 46 51 -5 5 47 46 1 1 54 53 1 1
14 59 51 8 8 60 52 8 8 59 55 4 4 61 57 4 4
48 513 -8 8 50 55 -5 5 56 58 -2 2 49 55 -6 6
16 42 43 -1 1 44 43 1 1 43 42 1 1 46 37 9 9
17 54 56 -2 2 55 60 -5 5 64 60 4 4 67 62 5 5
18 54 54 0 0 51 47 4 4 52 54 -2 2 59 53 6 6
19 42 43 -1 1 48 42 6 6 52 47 5 5 42 48 -6 6
68 65 3 3 70 67 3 3 73 68 5 5 74 71 3 3
21 62 61 1 1 61 55 6 6 68 67 1 I 68 70 -2 2
22 49 46 3 3 52 48 4 4 66 55 11 11 64 66 -2 2
23 67 62 5 5 70 65 5 5 88 81 7 7 77 70 7 7
24 74 68 6 6 74 69 5 5 68 65 3 3 76 67 9 9
66 64 2 2 74 71 3 3 70 71 -1 1 71 68 5 5
Avg 55.2 53.8 1.4 3.32 56.3 54.6 1.68 4.16 60.4 58.4 1.92 3.92 59.2 57.6 1.52
4.32
T evg 54.5 55.5 59.4 58.4
Std D 8.81 7.73 3.83 2.29 9.4 8.5 4.35 1.95 10.9 9.83 4.74 3.21 10.8
10.2 4.81 2.48
117
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix A
White vs. Dark Pigmented Skin Comparison
Affrican American
Anterior Lateral Deep Post Sup Posterior
Pt R L Diff AV R L Diff AV R L DM AV R L Diff
AV
1 50 46 4 4 46 48 -2 2 48 49 -1 1 40 40 0 0
3 49 47 2 2 48 50 -2 2 47 44 3 3 47 44 3 3
7 56 57 -1 1 52 58 -6 6 52 62 -10 10 64 67 -3 3
8 54 57 -3 3 56 57 -1 1 61 61 0 0 55 60 -5 5
9 49 42 7 7 50 43 7 7 55 49 6 6 52 52 0 0
48 44 4 4 51 46 5 5 72 60 12 12 51 46 5 5
11 71 65 6 6 73 68 5 5 75 76 -1 1 69 75 -6 6
12 48 50 -2 2 48 53 -5 5 49 54 -5 5 47 49 -2 2
13 45 50 -5 5 46 51 -5 5 47 , 46 1 1 54
53 1 1
14 59 51 8 8 60 52 8 8 59 55 4 4 61 57 4 4
48 56 -8 8 50 55 -5 5 56 58 , -2 2 49 55
-6 6
16 42 43 -1 1 44 43 1 1 43 42 1 1 40 37 9 9
17 54 56 -2 2 55 60 -5 5 64 60 4 4 67 62 5 5
18 54 54 0 0 51 47 4 4 52 54 -2 2 59 53 6 6
19 42 43 -1 1 48 42 6 6 62 47 5 5 42 48 -6 6
26 49 46 3 3 49 48 1 1 42 41 1 I 44 42 2 2
27 60 53 7 7 52 54 -2 2 42 48 -6 6 46 46 0 0
Avg 51.3 50.7 0.53 51.9 51.5 0.33 55.5 54.5 1 53.5 53.2
0.33
51 51.7 55 53.4
White
Anterior Lateral Deep Post Sup Posterior
Pt R L Diff AV R I. 01ff AV R I. 010 AV R L
Diff AV
2 58 56 2 2 60 60 0 0 63 60 3 3 64 57 7 7
4 59 60 -1 1 60 57 3 3 58 61 -3 3 58 61 -3 3
5 54 51 3 3 51 48 3 3 55 51 4 4 55 52 3 3
6 55 52 3 3 59 53 5 5 68 65 3 3 69 4 63 6
6
68 85 3 3 70 67 3 3 73 68 5 5 74 71 3 3
21 62 61 1 1 61 55 6 6 68 67 1 1 68 70 -2 2
22 49 46 3 3 52 48 4 , 4 66 55 11 11, 64
66 , -2 2
23 67 62 5 5 70 , 65 5 5 88 81 7 7 77
70 7 7
24 74 68 6 6 74 69 5 5 68 65 3 3 76 67 9 9
66 64 2 2 74 71 3 3 70 71 -1 1 71 66 5 5
Avg 612 511.5 2.7 2.9 63 59.3 3.7 3.7 67.7 64.4 3.3 4.1 67.6 64.3 3.3 4.7
59.9 61.2 66.1 66
Dirt 8.85 9.45 11.1 12.6
Test _a =cr.: 3 MD 1 =cafe 0.03035
118
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
-
Appendix A
Compartment Syndrome Patients Demographics
PI last Early Late Fe Locatior Mach re p Injt HI WI BMI
Side Sex Race Age
1 65 1 TIE P MVC 6 66 165 26.629 L M B 15
2 100 , 1 , T/F M PvA 8 68 210 31.927 R M B 22
,
3 70 1 TIE P GSW 5 73 170 22.426 L M B 19
4 76 1 TIE M PvA 10 71 165 23.01
R M 8 59
89 1 , P 6 MVC 4 74 220 28.243 L M B 59
6 55 1 P 6 MVC 10 69 210 31.008 L M B 23
7 71 1 P 6 Fall 28 67 155 24.274 L M W 44
8 98 1 P 6 Fall 6 68 200 30.407 L M H 32
9 58 1 _ TT M MVC 13 66 150 24.208 L M A
62 .
Avg 75.8 10 69.111 182.78 26.904 37.222
Std D 16.5 7.3314 2.9345 26.939 3.6333 19.025
119
CA 02854663 2014-05-05
WO 2013/070864 PCT/US2012/064078
Appendix A
Non Compartment Syndrome Demographics
Initial Injury Study
Time Time p Locatio
PI Mitch Ht Wt B MI Side Sox Race Ago %02 blur op
Syst Mast Fx Open Tech n
1 MVC 71 185 25.799 R M B 18 0 , 12 TIP 1 Id
2 MVC 80 165 32.221 R F B 45 0 52 TT 2 SI
3 MVC 68 202 30.711 R F a 35 o 7 123 67 TT 3A
3 AI
4 MVC 67 230 38.019 R M W 45 0 10 125 56 T/F
2 Id
IVNC 67 130 20.359 L M B 18 0 31 , 134 79 T/F ,
1 M
6 Fall 72 205 27.8 R M W 60 0 15 120 69 SchVI 2 P
7 Fall 69 161 23.773 L M B 26 0 16 129 76 Pilon 2
D
8 PvA 71 170 23.708 R M W 31 0 11 141 59 T/F 1 M
9 Fall 67 215 33.67 L r4 e 45 0 18 151 95 Pdon 2 D
Fall , 66 , 195 31,47, R M B 30 , 0 , 5 117 58
Pilon , 2 D
11 MVC 67 140 21.925 R M , El 52 0 48 TT 3 P
12 MVC 72 210 28.478 L M B 56 0 9 183 113 TT 1 2
D
13 MVC 66 175 28.243 R F W 48 0 12 106 58 TT 3A
2 AI
14 MVC 73 260 36.938 R M B 27 0 17 129 65 T/F
2 id
Fall 67 155 24.274 L M W 44 0 .14 133 76 T/F 3 P
16 Fall 73 205 27.044 L M B 28 0 2 114 60 TT 2 2
M
17 MVC 72 175 23.732 L M W 21 100 8 80 24 T/F 1
M
18 MVC 75 345 43.117 R M W 42 0 14 131 85 SchV
2 P
19 MVC 68 175 26.606 R 'F B 42 0 9 163 88 Pilon 2
1 D
MVC 70 190 27.259 R M W 22 0 21 153 93 Pilon 2
2 D
21 PvA 70 170 24.39 R M B 54 100 32 140 , 75, TT
3A 3 Id
22 PvA 68 168 25.542 L M B 38 0 2 131 78 T/F 3
SI
23 Blcnv 71 195 27.194 I. M H 29 0 19 137 , 67
TT 1 M
24 MVC, 68 165 25.065 L M B 26 0 13 148 87
Sch V 2 P
MVC 64 210 36.042, R F B 23 0 5 123 49 T/F 1
M
26 MVC 68 165 25.085 1 M B 26 0 13 148 87
Sch V 2 P
Avg 68.8 191.58 28.326 10-1 5 8 35.692 2 ,
15.962. 133 72.348 3-Plat 7 7-1's 5-P
Med 15-48 20 17 17-TT 13-2's 14-1,41
Std D 3.21 43.706 5.3403 12.315 12.389 20.596 18.527 5-
p/Ion 5-3'5 6-0
pVal
120
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix A
Non Compartment Syndrome 2 Days Post-Op Demographics
Surger Time Time p Localle
Pt Mach Ht WI B MI Side Sex Race Age y lnjur op
Syst Blast Fe Open Tech n
1 GSW, 71 212 29.565 L M B 32 IMN 60 36 . 148 84 , TT
3 M õ
2 PvA 69 175 25.84 R , M H 34 IMN 130 119 116 65 TT
3A 3 M
3 MVC 67 130 20.359 I. M B 18 IMN 75 43 135 73 TT
1 M
4 Fall 72 205 27.8 R M W 60 Ex F 48 35 101
57 SkVI 2 P
MVC 74 200 25.676, R M 13 48 ORIF 96 45 148 103 F 2 1
13
-
6 Fall 69 , 161 23.773 L M B 26 ORIF 133 40
134 77 Pilon 2 0 ,
7 MVC 62 115 21.031 L F W 22 ORIF 90 44 107 67
Piton 1 D
8 PvA 75 170 21.246 R M B 47 IMN 43 31 131 86
T/F 2 2 , D
9 Fall, 64 170 29.177 L F W 51 ExF 47 41
- 144 , 79 , Ron 2 D
PvA 71 170 23.708 R M W 31 IMN 82 40 148 84
TT 1 Id
11 MVC 66 175 28.243 R F W 46 IMN 60 42 112 61 TT 3A
3 M
12 Fall 73 205 27.044 L M B 28 IMN 42 32 135 71
TT 2 3 M
13 MVC 88 175 26.606 R F B42 ExF 64 37 122 79 Pilon 1
0
..
14 NA., 70 170 ,. 24.39 R M B 54 ExF 56 33 119
55 TT 3A 3 3.1
MVC 70 190 27.259 Ft M W 22 ExF 58 46 105 68 TT
2 3 0
16 PvA 68 168 25.542 L M 8 38 IMN 53 42 115 65
T/F 3 m
17 MVC 68 165 26.086 I. M B 26 Ex F 87 27 137
85 Sch V 2 P
Avg 69.2 173.88 25.432 36.765 72 43.118 126.88 74.059
Med
Std D 3.4 25.085 2.7582 12.612 27.868 20.285 15.898 12.351
pVal
121
=
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
=
Appendix A
Uninjured Subjects (Normal NIRS Values) Demographics
Time p
Ht Wt OW Sex Race Age Injury Inj Syst Olast
Pt wk
1 84 130 22.312 F B 18 none 108 67
2 72 175 23.732 , M W 28 none 117 65
3 69 180 26.578 M B 33 ring Fx 10 139 87
4 67 185 28.972 FA W 36 none 124 78
60 110 21.481 F H 28 norbe 124 78
6 70 175 25.107 M W 32 none 125 71 ,
7 66 200 32.277 M B 29 , none , 144 92
8 68 200 30.407 M B 32 none 125 65
9 68 188 28.582 N B 58 none 150 81
69 192 28.35 M B 48 Clay fx 6 149 51
11 66 200 32277 F B 58 none 143 81
12 71 180 25.102 M B 28 none 125 55
13 66 142 22.917 F B 34 rone 126 89
14 82 196 35.645 F B 24 110110 124 75
69 1130 27.464 F B 42 none 132 90
16 63 138 24.443 M B 39 none 119 70
17 57 132 28.561 F B 43 none 151 91
18 71 175 24.405 M B 21 none 123 68
19 69 160 23.625 M B 21 none 133 78
76 265 32.253 M W 26 none 144 ' 87
21 87 160 25.057 M W 25 none 148 70
22 63 117 20.723 F W 51 flOrla 120 73
,
23 74 230 29.527 M W 32 none 123 = 69
24 72 195 26.444 ki W 33 none 118 77
72 205 27.8 M W 23 none _ 124 98
Avg 67.6 176.64 26.97 33.68 8 130.24 77.44
T EiVil
Sid D 4.43 35.222 3.7986 11.022 2.8284 12.011 10.863
122
..
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
=
..
Appendix A
White vs. Dark Pigmented Skin Comparison Demographics
Affrican American
1 64 130 22.312 F s 18 none 108 67
3 69 180 26.578 M 8 33 ling Fx 10 139 87
7 66 200 32.277 M s 29 none 144 92
a 68 200 30.407 M s 32 031113 125 65
9 88 188 28.582 ., M 8 ,. 58 none 150 81
69 192 28.35 M 13 48 Clay lx 6 149 51
11 66 200 32.277 F s 58 none 143 61
12 71 180 25.102 M 13 28 none 125 , 85 ,
13 66 142 22.917 F 8 34 none 126 89
14 62 196 35.845 F 8 24 none 124 75
69 186 27.464 F 8 42 none , 132 90
16 133 138 24.443 M s 39 none 119 70
17 57 132 28.561 F 8 43 none 151 91
18 71 175 24.405 M B 21 none 123 68
19 69 160 23.626 M s 21 none 133 78
s 69 150 22.149 M H 24 ROH am 2 120 80
8 63 98 17.358 F B 25 MC Ix 2 120 81
Avg 66.5 173.27 27.543 132.73 78
. .
White
2 72 175 23.732 NI W 28 none 117 65
4 67 185 28.972 M W 38 none 124 78
5 60 110 21.481 F H 28 none 124 78
6 70 175 25.107 M W 32 none 125 71
76 265 32.253 M 1V 26 none 144 87
21 67 160 25.057 M W 25 none 148 70
22 63 117 20.723 F W 51 none 120 73
23 74 230 29.527 M , W 32 none 123 69
24 72 195 26.444 M W 33 ilOne 116 77
72 205 27.6 M W 23 none 124 98
Avg 69.3 181.7 26.11 125.5 76.6
Diff
Test
123
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix B
APPENDIX B: Tourniquet Study Data
INVOS Diff
Perfusion from Baseline
Subject INVOS pressure (PP)
1 67 71 0
69 65 2
63 56 -4
62 36 -5
61 26 -6
59 17 -8
56 5 -11
55 -1 -12
31 -11 -36
16 -21 -51
15 -35 -52
2 65 88 0
66 75 1
64 63 -1
63 58 -2
57 48 -8
58 43 -7
60 35 -5
60 15 -5
58 4 -7
53 -13 -12
28 0 -37
20 -34 -45
3 63 74 0
66 59 3
67 49 4
65 42 2
66 27 3
65 15 2
64 10 1
61 2 -2
58 -11 -5
52 -6 -11
33 -28 -30
20 -34 -43
4 72 71 0
79 60 7
74 48 2
71 41 -1
71 32 -1
73 21 1
71 20 -1
70 5 -2
68 -9 -4
124
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix B
INVOS Diff
Perfusion from Baseline
Subject INVOS pressure (PP)
65 -13 -7
63 -26 -9
47 -32 -25
39 -46 -33
5 69 86 0
70 73 1
72 59 3
69 55 0
69 43 0
66 32 -3
66 20 -3
64 13 -5
62 -1 -7
59 -7 -10
35 -20 -34
22 -31 -47
6 58 72 0
60 59 2
58 49 0
61 39 3
58 27 0
58 19 0
57 6 -1
53 -4 -5
48 -14 -10
23 -25 -35
15 -31 -43
7 67 73 0
68 64 1
68 64 1
68 44 1
68 28 1
66 27 -1
65 9 -2
63 4 -4
61 -7 -6
46 -18 -21
15 -25 -52
8 65 54 0
65 40 0
64 26 -1
63 15 -2
62 4 -3
59 1 -6
41 -10 -24
24 -19 -41
16 -26 -49
9 68 67 0
125
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix B
INVOS Diff
Perfusion from Baseline
Subject INVOS pressure (PP)
71 59 3
70 47 2
67 41 -1
65 27 -3
64 19 -4
64 10 -4
63 -4 -5
61 -8 -7
51 -18 -17
24 -23 -44
15 -40 -53
10 69 69 0
66 57 -3
62 46 -7
64 39 -5
64 27 -5
63 17 -6
61 5 -8
62 -1 -7
57 -13 -12
53 -23 -16
39 -33 -30
33 -38 -36
11 62 67 0
60 55 -2
60 45 -2
58 33 -4
58 29 -4
53 15 -9
52 4 -10
43 -5 -19
20 -12 -42
15 -21 -47
12 71 66 0
69 57 -2
68 49 -3
64 42 -7
65 29 -6
62 28 -9
61 12 -10
58 -1 -13
50 -9 -21
40 -17 -31
28 -26 -43
24 -37 -47
13 67 69 0
69 59 2
67 48 0
126
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
Appendix B
INVOS Diff
Perfusion from Baseline
Subject INVOS pressure (PP)
68 41 1
65 28 -2
64 21 -3
63 8 -4
63 5 -4
57 -9 -10
50 -21 -17
23 -26 -44
15 -37 -52
14 67 68 0
, 68 53 1
65 45 -2
64 35 -3
63 21 -4
63 16 -4
61 5 -6
59 -3 -8
54 -13 -13
40 -23 -27
34 -35 -33
33 -38 -34
15 70 52 0
70 43 0
70 29 0
69 19 -1
68 10 -2
65 2 -5
59 -10 -11
42 -16 -28
36 -27 -34
34 -35 -36
33 -35 -37
16 72 77 0
71 63 -1
68 49 -4
68 45 -4
66 27 -6
66 24 -6
63 9 -9
60 -3 -12
53 -7 -19
45 -25 -27
40 -28 -32
38 -34 -34
17 , 61 76 0
59 66 -2
58 59 -3
61 _ 50 0
127
CA 02854663 2014-05-05
WO 2013/070864
PCT/US2012/064078
REPLACEMENT SHEET
Appendix B
.INVOS Diff
Perfusion from.Baseltne
Subject INVOS pressure (PP).
59 37 -2
58 29 -3
58 19 -3
59 12 -2
55 10 -6
45 , -9 -16
24 -19 -37
18 74 69 0
74 61 0
73 48 -1
, 73 39 -1
73 28 -1
71 17 -3
69 12 = -5
67 3 -7
64 -7 -10
45 -21 -29
39 -28 -35
36 -37 -38
19 72 67 0
73 54 1
74 45 2
74 37 2
73 29 1
71 16 -1
69 7 -3
67 -3 -5
66 -15 -6
60 -21 , -12
52 -33 -20
49 -43 , -23
48 -44 -24
20 70 78 0
70 70 , 0
70 54 0
69 45 -1
68 28 -2
66 24 -4
62 16 -8
60 3 -10
59 -8 -11
53 2 -17
32 -16 -38
25 -35 -45
128