Note: Descriptions are shown in the official language in which they were submitted.
1
DEVICE AND METHOD FOR UPGRADING PETROLEUM
FEEDSTOCKS USING AN ALKALI METAL CONDUCTIVE
MEMBRANE
TECHNICAL FIELD
[0002] The present disclosure relates to a process for removing nitrogen,
sulfur,
heavy metals, and acid protons from sulfur-, nitrogen-, and metal-bearing
shale oil,
bitumen, heavy oil and petroleum refinery streams so that these materials may
be
used as a hydrocarbon fuel. More specifically, the present disclosure relates
to
removing nitrogen, sulfur, heavy metals and acid protons from shale oil,
bitumen,
heavy oil, or petroleum refinery streams while at the same time, upgrading
these
materials to have a higher hydrogen-to-carbon ratio.
BACKGROUND
[0003] U.S. Patent Application Serial No. 12/916,984 has been published as
United
States Patent Application Publication No. 2011/0100874. The reader is presumed
to
be familiar with the disclosure of this published application. This published
application
will be referred to herein as the "874 application."
[0004] The demand for energy (and the hydrocarbons from which that energy is
derived) is continually rising. However, hydrocarbon raw materials used to
provide
this energy often contain difficult-to-remove sulfur and metals. For example,
sulfur
CA 2855966 2017-08-11
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
2
can cause air pollution and can poison catalysts designed to remove
hydrocarbons
and nitrogen oxide from motor vehicle exhaust, necessitating the need for
expensive
processes used to remove the sulfur from the hydrocarbon raw materials before
it is
allowed to be used as a fuel. Further, metals (such as heavy metals) are often
found
in the hydrocarbon raw materials. These heavy metals can poison catalysts that
are
typically utilized to remove the sulfur from hydrocarbons. To remove these
metals,
further processing of the hydrocarbons is required, thereby further increasing
expenses.
[0005] Currently,
there is an on-going search for new energy sources in order to
reduce the United States' dependence on foreign oil. It has been hypothesized
that
extensive reserves of shale oil, which constitutes oil retorted from oil shale
minerals,
will play an increasingly significant role in meeting this country's future
energy needs.
In the U.S., over 1 trillion barrels of usable, reserve shale oil are found in
a relatively
small area known as the Green River Formation located in Colorado, Utah, and
Wyoming. As the price of crude oil rises, these shale oil resources become
more
attractive as an alternative energy source. In order to utilize this resource,
specific
technical issues must be solved in order to allow such shale oil reserves to
be used,
in a cost effective manner, as hydrocarbon fuel. One issue associated with
these
materials is that they contain a relatively high level of nitrogen, sulfur and
metals,
which must be removed in order to allow this shale oil to function properly as
a
hydrocarbon fuel.
[0006] Other
examples of potential hydrocarbon fuels that likewise require a
removal of sulfur, nitrogen, or heavy metals are bitumen (which exists in
ample
quantities in Alberta, Canada) and heavy oils (such as are found in
Venezuela).
[0007] The high
level of nitrogen, sulfur, and heavy metals in shale oil, bitumen
and heavy oil (which may collectively or individually be referred to as "oil
feedstock")
makes processing these materials difficult. Typically, these oil feedstock
materials
are refined to remove the sulfur, nitrogen and heavy metals through a process
known as "hydro-treating."
[0008] Hydro-
treating may be performed by treating the material with hydrogen
gas at an elevated temperature and an elevated pressure using catalysts such
as
Co-Mo/A1203 or Ni-Mo/A1203.
[0009] In the
present invention, the oil feedstock is mixed with an alkali metal
(such as sodium) and hydrogen gas. This mixture is reacted under modest
pressure
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
3
(and usually at an elevated temperature). The sulfur and nitrogen atoms are
chemically bonded to carbon atoms in the oil feedstocks. The sulfur and
nitrogen
heteroatoms are reduced by the alkali metals to form ionic salts (such as
Na2S,
Na3N, Li2S, etc.). To prevent coking (e.g., a formation of a coal-like
product), the
reaction occurs in the presence of hydrogen gas that can form bonds with the
carbon
atoms of the oil feedstock previously bonded to the heteroatoms. The hydrogen
atom bonds to the carbon atoms that were previously bonded to the heteroatoms,
thereby increasing the hydrogen-to-carbon ratio of the oil feedstock and
decreasing
the heteroatom to carbon ratio of the resulting organic feedstock. After the
hydro-
treating reaction, the organic phase (oil feedstock) is less viscous and may
be sent
for further refining into a hydrocarbon fuel material.
[0010] The ionic
salts formed in the hydro-treating process may be removed from
the organic products by filtering, or first mixing the treated feedstock with
hydrogen
sulfide to form an alkali hydrosulfide, which forms a separate phase from the
organic
phase (oil feedstock). This reaction is shown below with sodium (Na) being the
alkali
metal, although other alkali metals may also be used:
Na2S + H2S 2NaHS (which is a liquid at 375 C)
Na3N + 3H2S 3NaHS + NH3
The nitrogen product is removed in the form of ammonia gas (NH3) which may be
vented and recovered, whereas the sulfur product is removed in the form of an
alkali
hydro sulfide, NaHS, which is separated for further processing. Any heavy
metals
will also be separated out from the organic hydrocarbons by gravimetric
separation
techniques.
[0011] As part of
the process, alkali metals are used. An advantage of using
alkali metals such as sodium or lithium instead of hydrogen to reduce the
heteroatoms is alkali metals offer a greater reduction strength. In other
words, the
alkali metals are better able to reduce the heteroatoms and form alkali metal
nitrides
or alkali metal sulfides. Further, by using alkali metals, there is less need
to saturate
rings with hydrogen to destabilize them so that the heteroatoms can be
reduced,
making it possible to remove heteroatoms with significantly less hydrogen.
[0012] It should be
noted that the alkali metal treatment process is known in the
industry and is described, for example, in U.S. Patent No. 3,787,315, U.S.
Patent
Application Publication No. 2009/0134040 and U.S. Patent Application
Publication
4
No. 2005/0161340.
[0013] A disadvantage of using the hydro-treating process is that hydrogen gas
is a
necessary reactant needed for the hydro-treating process. However, hydrogen
gas
can be expensive. Typically, hydrogen gas is formed by reacting hydrocarbon
molecules with water. For example, in the United States, 95% of the hydrogen
is
formed using the Steam-Methane Reforming Process from natural gas. In the
first
step known as the reforming step, methane (CH4) in natural gas is reacted with
steam
(H20) at 750 C - 800 C to produce synthesis gas (syngas). Syngas is a
mixture
primarily comprised of hydrogen gas (H2) and carbon monoxide (CO). In the next
step, known as the water gas shift reaction, the carbon monoxide produced in
the first
reaction is reacted with steam (H20) over a catalyst to form hydrogen gas (H2)
and
carbon dioxide (CO2). This second process (e.g., the water gas shift reaction)
occurs
in two stages: the first stage occurring around 350 C and the second stage
occurring
at about 200 C.
[0014] The overall reaction for the Steam-Methane Reforming Process is as
follows:
CH + 2H20¨> 4H2 + CO2
Thus for every (theoretical) mole of hydrogen gas produced, 0.25 moles methane
and
0.5 moles of water are required. Also, for every mole of hydrogen gas
produced, 0.25
moles of carbon dioxide are produced and released to the atmosphere. It should
be
noted that the Steam-Methane Reforming Process is typically only 65-75%
efficient.
Thus at 70% efficiency, the Steam-Methane Reforming Process will actually
utilizes
0.36 moles of methane and 0.71 moles of water while releasing 0.36 moles of
carbon
dioxide for every mole of hydrogen produced.
[0015] This production of carbon dioxide during the hydro-treating process is
considered problematic by many environmentalists due to rising concern over
carbon
dioxide emissions and the impact such emissions may have on the environment.
[0016] An additional problem in many regions is the scarcity of water
resources. For
example, in the region of Western Colorado and Eastern Utah where parts of the
Green River Formation of shale oil is located, the climate is arid and the use
of water
in forming hydrogen gas can be expensive.
[0017] Alternatively, some industrialists have used an electrolysis process to
provide
the hydrogen gas supply needed for their hydro-treating process. This
CA 2855966 2017-08-11
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
electrolysis reaction involves the electrolytic decomposition of water. In
this
electrolytic reaction, water is split to form hydrogen at a cathode and oxygen
at an
anode: H20 ¨> H2 + 1/2 02
In this reaction, electrical energy is used to split the water. If the cell
runs at 90%
efficiency and runs at about 1.4 Volts, then the electrical energy required is
about 72
kcal per mole of created hydrogen. For every mole of hydrogen produced in this
electrolysis reaction, one mole of water is consumed. Because one mole of
water is
consumed to produce hydrogen in this method, more water is required to produce
the hydrogen gas via electrolysis than is required to produce the hydrogen
using the
Steam-Methane Reforming Process (which requires 0.71 moles water). Thus, in
arid
climates where the cost of water is high, using an electrolysis process to
produce
hydrogen may not be economically feasible.
[0018] While conventional hydro-treating processes are known, they are
expensive and require large capital investments in order to obtain a
functioning
hydro-treating plant. There is a need in the industry for a new process that
may be
used to remove heteroatoms such as sulfur and nitrogen from oil feedstocks,
but that
is less expensive than hydro-treating. Such a process is disclosed herein.
[0019]
Additionally, naphthenic acids must be removed from many organic
streams that are produced by refineries. Naphthenic acids ("NAPs") are
carboxylic
acids present in petroleum crude or various refinery streams. These acids are
responsible for corrosion in refineries. A common measure of acidity of
petroleum is
called the Total Acid Number ("TAN") value and is defined as the milligrams
(mg) of
potassium hydroxide needed to neutralize the acid in one gram of the petroleum
material. (Other acids found in the oil feedstock may also contribute to the
TAN
value). All petroleum streams with TAN >1 are called high TAN. NAPs are a
mixture
of many different compounds and cannot be separated via distillation. Moreover
high
TAN crudes are discounted over Brent Crude prices. For example, Doba crude
with
a TAN of 4.7 is discounted by $19 per barrel on a base price of $80 for Brent
crude.
[0020] NAPs boil in
the same range as that of kerosene/jet fuel. (However,
kerosene/jet fuels have very stringent TAN specifications.) Attempting to
neutralize
these acids using aqueous caustic or other bases form salts. These salts in
presence of water, lead to formation of stable emulsions. Additional
methodologies
of NAP reduction include hydrotreating or decarboxylation that are both
destructive
methodologies and the NAPs cannot be recovered using these methods. Solvent
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
6
extraction or adsorption methodologies lead to high costs and energy usage for
sorbent regeneration or solvent boiling. A new method for NAPs removal with
lower
energy consumption wherein NAPs can be recovered and processed as commercial
products is required. Accordingly, a new method of neutralizing and/or
removing
NAPs is needed. Such a method and device is disclosed herein.
SUMMARY
[0021] The present
embodiments include a method of upgrading an oil feedstock
with the benefit of a strong alkali metal agent without directly being
required to
handle, store, or transport the alkali metal. The method comprises obtaining a
quantity of an oil feedstock, the oil feedstock comprising at least one carbon
atom
and a heteroatom and/or one or more heavy metals. The quantity of the oil
feedstock is reacted with an alkali metal generated on an electrode within the
reactor. The reaction with the alkali metal may also include using an
upgradant
hydrocarbon such as hydrogen gas or a hydrocarbon.
[0022] In order to
implement these embodiments, a reactor may be utilized with at
least two chambers separated in part by a membrane conductive to alkali metal
ions.
This membrane conducts alkali metal ions from an alkali metal ion source
material
(such as a liquid comprised of sodium salts or sodium metal). A positive
charged
electrode (anode) is in communication with the alkali metal ion source. The
opposite
chamber of the reactor (called the feedstock chamber) includes a feedstock
stream
(comprised of the organic oil feedstock) and a negatively charged electrode
(cathode). The alkali metal enters the feedstock chamber and reacts with the
heteroatom and/or the heavy metals in the feedstock to form one or more
inorganic
products, wherein the upgradant hydrocarbon reacts with the oil feedstock to
produce an upgraded oil feedstock. The reaction with the upgradent hydrocarbon
operates such that the number of carbon atoms in the upgraded oil feedstock
may
be greater than the number of carbon atoms in the original oil feedstock. The
inorganic products are then separated from the upgraded oil feedstock. (The
reaction of the oil feedstock, the alkali metal, and the upgradant hydrocarbon
molecule may be implemented with or without using hydrogen gas. If hydrogen
gas
is utilized, the amount of hydrogen gas needed is much less than would be
required
using conventional hydrotreating.)
[0023] In some
embodiments, the alkali metal comprises sodium, lithium, or
alloys of lithium and sodium. The upgradant hydrogen source may comprise
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
7
hydrogen, natural gas, shale gas and/or mixtures thereof. In other
embodiments, the
upgradant hydrocarbon comprises methane, ethane, propane, butane, pentane,
their
isomers, ethene, propene, butene, pentene, dienes, and/or mixtures thereof.
(Oil
retort gas, which is a mixture of gases that is produced in a refinery process
may
also be used as the upgradant hydrocarbon.)
[0024] The process
of reacting the feedstock with the alkali metal may consist of
two steps. A first step involves having alkali metal ions be transferred
across the
membrane and reduced to metal at the membrane surface at a negatively charged
electrode (which may be directly fixed to the membrane surface). A second step
involves having the formed alkali metal react directly with the constituents
in the oil
feedstock (or carried away with the oil to react downstream). The electrode
where
the alkali metal is formed may be porous, or comprised of a mesh. In another
embodiment, the electrode may be a film of metallic alkali metal connected to
an
electrical lead (or current collector). To maintain continuity, a screen or
mesh may
provide a divider to separate a zone where the alkali metal is reduced from
the oil
feedstock. This screen allows the alkali metal to pass through as it is
formed.
[0025] The reaction
between the alkali metal and the oil feedstock may occur at a
pressure that is between barometric and about 2500 psi and/or at a temperature
that
is between about 100 C temperature and 450 C. In other embodiments, the
reaction between the alkali metal and the oil feedstock occurs at a
temperature that
is above the melting point of the alkali metal but is lower than 450 C.
Further
embodiments may utilize a catalyst in the reaction. The catalyst may comprise
molybdenum, nickel, cobalt or alloys thereof, molybdenum oxide, nickel oxide
or
cobalt oxides and/or combinations thereof.
[0026] As the
reaction between the alkali metal and the oil feedstock produces
inorganic products, a separation step may be needed. The separation used in
the
process may occur in a separator, wherein the inorganic products form a phase
that
is separable from an organic phase that comprises the upgraded oil feedstock
and/or
unreacted oil feedstock. To facilitate this separation, a flux may be added to
the
separator. After separation, the alkali metal from the inorganic products may
be
regenerated and reused.
[0027] The upgraded
oil feedstock produced in the reaction may have a greater
hydrogen-to-carbon ratio than the oil feedstock. The upgraded oil feedstock
produced in the reaction may also have a greater energy value than the oil
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
8
feedstock. Further, the heteroatom-to-carbon ratio of the upgraded oil
feedstock
may be less than the heteroatom-to-carbon ratio of the oil feedstock.
[0028] Additional
embodiments may be designed in which an alkali metal is
added to the oil feedstock in order to reduce the TAN value of the oil
feedstock.
Specifically, the alkali metal may react with the oil feedstock to remove the
acidic
components, thereby lowering the TAN value. In some embodiments, the original
(unreacted) oil feedstock may have a TAN value of greater than or equal to 1,
but
after reaction with the alkali metal, may have a TAN value of less than or
equal to 1.
BRIEF DESCRIPTION OF THE DRAWINGS
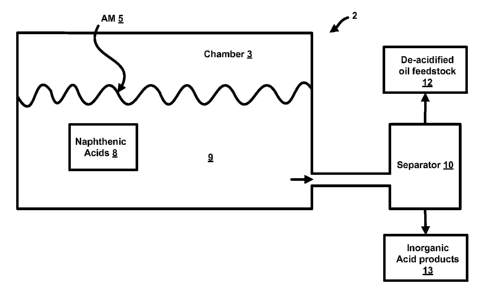
[0029] Figure 1
shows a schematic drawing of a device that may be used to de-
acidify a quantity of an oil feedstock;
[0030] Figure 2
shows a schematic drawing of a device that may be used to
upgrade a quantity of an oil feedstock;
[0031] Figure 3
shows a schematic drawing of a device that may be used to de-
acidify a quantity of an oil feedstock;
[0032] Figure 4
shows a schematic drawing of another embodiment of a device
that may be used to de-acidify a quantity of an oil feedstock;
[0033] Figure 5
shows a schematic drawing of another embodiment of a device
that may be used to upgrade a quantity of an oil feedstock;
[0034] Figure 6 is
a flow diagram of a method for upgrading a quantity of an oil
feedstock; and
[0035] Figure 7 a
schematic drawing of another embodiment of a system that
may be used to upgrade a quantity of an oil feedstock.
DETAILED DESCRIPTION
[0036] The present embodiments relate to a method to de-acidify feedstocks and
refinery streams. Such de-acidification is beneficial as it may operate to
reduce
piping corrosion and may convert naphthenic acids to a salt form. The present
embodiments involve the addition of alkali metals (such as sodium or lithium
metal)
to the feedstocks as a means of reacting with the naphthenic acids, thereby de-
acidifying these acids. When this reaction occurs, the naphthenic acids may be
converted into the corresponding sodium or lithium salts (or other inorganic
products). Hydrogen gas is also formed in this reaction. This
reaction is
summarized as follows:
[0037] R-COOH + Na (R-000-)Na+ + 1/2 H2
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
9
[0038] Alternatively, the sodium may further react with oxygen atoms to
eliminate
the carboxyl group as shown in the following formula:
[0039] R-COOH + 4Na + H2 ->R-CH3 + 2Na20
[0040] The reaction with NAPs in this manner may be desirable and may result
in
a reduction of Total Acid Number ("TAN") associated with the oil feedstock.
There
are multiple different ways in which the alkali metal may be added to the
feedstock.
In one embodiment, the sodium or lithium metal is directly added to the
stream.
Once this occurs, the inorganic products may then be filtered from the oil
stream.
Other embodiments may also be designed (as described herein) to provide other
mechanisms for adding the alkali metal to the stream of oil feedstock (such
as, for
example, by forming the alkali metal in situ).
[0041] It should be
noted that, in addition to reacting with the acids (such as
naphthenic acids), the alkali metals that are added to the feedstock may also
react to
remove sulfur, nitrogen, metals (such as heavy metals), etc. This process for
removing these metals/heteroatoms is discussed in the '874 application. Thus,
by
adding alkali metals to the oil feedstock, the problems associated with
metals/heteroatoms in the stream, as well as problems with acids in the
stream, may
be overcome.
[0042] It should be noted that many in the oil processing industry are
uncomfortable handling metallic sodium or lithium because of its reactive
nature. In
other words, these practitioners are uncomfortable using sodium/lithium and
are
uncomfortable adding these reagents directly to their oil feedstock streams.
Accordingly, the present embodiments also provide methods and devices which
operate to electrochemically produce alkali metals within an oil feedstock
chamber
(e.g., in situ), thereby bringing an alkali metal such as sodium in direct
contact with
the feedstock. Once this alkali metal is produced in the chamber, it is
consumed by
reacting with the heavy metals/heteroatoms and/or the acids in the feedstock.
These
embodiments may be desirable in that they provide the strong reducing power
and
reactivity associated with alkali metals without ever having an appreciable
amount of
the metal present. In other words, the present embodiments upgrade an oil
feedstock using the alkali metal (e.g., a strong agent) without the
practitioner being
required to handle, store, or transport the alkali metal.
[0043] Referring
now to Figure 1, a device 2 is illustrated that may be used to de-
acidify a quantity of a first oil feedstock 9. As shown in Figure 1, the oil
feedstock 9
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
is a liquid that is placed within a chamber 3. The chamber 3 may be a reaction
vessel, a chamber of an electrolysis cell (as will be described herein), etc.
Those
skilled in the art will appreciate what vessels, containers, etc., may be used
as the
chamber 3.
[0044] The oil feedstock 9 comprises a quantity of naphthenic acids 8. As
described above, naphthenic acids 8 comprise carboxylic acids present in
petroleum
crude or various refinery streams. Naphthenic acids 8 are a mixture of many
different compounds and cannot be separated out via distillation. In order to
eliminate the naphthenic acids 8 from the oil feedstock 9, a quantity of an
alkali metal
5 is added to the chamber 3. (The alkali metal is abbreviated as "AM.") In
some
embodiments, the alkali metal may be sodium, lithium or alloys of sodium and
lithium. The chamber 3 may be kept at a temperature that is above the melting
point
of the alkali metal 5 such that the liquid alkali metal 5 may easily be added
to the
liquid oil feedstock. In some embodiments, the reaction occurs at a
temperature that
is above the melting point of the alkali metal (or above a temperature of
about 100
C). In other embodiments, the temperature of the reaction is less than about
450
C.
[0045] When added to the chamber 3, the alkali metal 5 may react with the oil
feedstock 9. More specifically, the alkali metal 5 reacts with the quantity of
the
naphthenic acids 8 to form a de-acidified feedstock 12. As inorganic acid
products
13 may also be formed from this reaction, a product separator 10 may be used
to
separate the de-acidified oil feedstock 12 from the inorganic acid products.
Those
skilled in the art will appreciate how this separation may occur. Moreover,
those
skilled in the art will appreciate the structures (such as a settling chamber,
etc.) that
may be used as the product separator 10. The product separator 10 may be
integral
with the chamber 3 or may be a separate structure, as shown in Figure 1.
[0046] As explained herein, the reaction between the alkali metal 5 and the
naphthenic acids 8 operates to eliminate the naphthenic acids 8 from the oil
feedstock 9. Thus, the TAN value of the de-acidified oil feedstock 12 will be
lower
than the TAN value of the original (unreacted) first oil feedstock 9. For
example, in
some embodiments, the TAN value of the original (unreacted) oil feedstock 9
may be
greater than or equal to 1 (such as, for example, 3, 4, 5, etc.) whereas the
TAN value
of the de-acidified oil feedstock 12 is a lower value, such as less than or
equal to 1.
As noted above, other acids in the oil feedstock 9 may contribute to the TAN
value of
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
11
the feedstock 9. These acids may also react with the alkali metal in a similar
manner, further reducing the TAN value.
[0047] This reduction in TAN value may provide a significant financial benefit
to
the owner of the oil feedstock. As noted above, prices per barrel of oil
products that
are considered to be high TAN (e.g., with a TAN value greater than 1) are
often
discounted significantly with respect to barrels of oil products that are low
TAN.
Thus, by reducing the TAN value in the oil feedstock, the value of the oil
feedstock
may be significantly increased.
[0048] Referring now to Figure 2, another embodiment of the device 2a is
illustrated. As noted above, the device 2a is similar to the device 2 shown in
Figure
1. The device 2a may be designed to de-acidify the oil feedstock 9. At the
same
time, the device 2a may also be designed to further upgrade the first oil
feedstock 9
by removing heavy metals 14 and/or one or more heteroatoms 11 that are present
in
the oil feedstock 9.
[0049] As described above, heavy metals 14 (such as nickel, vanadium, iron,
arsenic, etc.) are often found in samples of oil feedstock materials 9. In
some
embodiments, it may be desirable to remove these heavy metals 14, as such
metals
can poison catalysts that are typically utilized in hydrocarbon processing.
However,
as shown in Figure 2, the device 2a may be designed such that the alkali metal
5
may react with the heavy metals 14 in the oil feedstock 9. More specifically,
in
addition to the alkali metal 5 reacting with the napthenic acids 8 to de-
acidify the
feedstock (as described above), the quantity of the alkali metal 5 may further
react
with the heavy metals 14, thereby reducing the heavy metals into their
metallic
states. This reaction may also occur in the chamber 3.
[0050] As shown in Figure 2, these heavy metals 16 may then be separated and
recovered (using the product separator 10). It should be noted that the heavy
metals
16, in their metallic state, are inorganic materials and thus may separate out
from the
organic oil feedstock materials. Accordingly, the product separator 10 may use
this
property as a means of separating out the heavy metals 16. Those skilled in
the art
will appreciate that other separation techniques may also be used to separate
out
the heavy metals 16. Once the metals 16 have been separated, they may be
recovered, sold, used in further processing, etc. As these metals are
generally
expensive commodities, the fact that such metals may be collected (and
used/sold)
may provide a significant commercial advantage for the owner of the feedstock.
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
12
[0051] In addition to removing heavy metals, the alkali metal 5 may also react
with
one or more heteroatoms 11 (such as N, S) that are present in the oil
feedstock 9.
These N, S atoms may be bonded to the carbon/hydrogen atoms in the organic oil
feedstock 9. However, as noted herein, the alkali metal 5 may react with these
one
or more heteroatoms 11 to form inorganic sulfur/nitrogen products 17. For
example,
if the alkali metal 5 is sodium, then the reaction with the heteroatoms 11
forms
inorganic sulfur/nitrogen products 17 such as Na2S, Na3N and/or other
inorganic
products. (Again, a product separator 10 may be used to separate out the
inorganic
sulfur/nitrogen products 17 from the oil feedstock). Once the
inorganic
sulfur/nitrogen products 17 have been removed, the heteroatom to carbon ratio
of
the resulting oil feedstock is less than the heteroatom to carbon ratio of the
original
(unreacted) oil feedstock 9.
[0052] It should be
noted that after the oil feedstock 9 has been de-acidified,
demetalized, de-sulfurized and/or de-nitrogenized, then this oil feedstock is
referred
to as an "upgraded" oil feedstock 12a in that this material is better suited
for further
refining, commercialization, etc.
[0053] It should be noted that in the embodiment shown in Figure 2, a single
product separator 10 is shown as separating out the heavy metals 16, the
inorganic
acid products 13 and the inorganic sulfur/nitrogen products 17, thereby
removing
these materials from the upgraded oil feedstock 12a. However, those skilled in
the
art will appreciate that multiple product separators 10 and/or separation
techniques
may be used to accomplish such separations. Further, there also may be a
sequential separation of the various materials from the upgraded oil feedstock
12a.
[0054] Likewise, it should be noted that in the embodiment of Figure 2, a
single
chamber 3 is used to react the oil feedstock 9 with the alkali metal 5 (and
thus
remove the naphthenic acids 8, heavy metals 14 and heteroatoms 11 from the
organic feedstock). Those skilled in the art will appreciate that such
reactions could
also occur in different chambers. In other words, embodiments may be designed
in
which a first chamber is used to react the alkali metal 5 with the heavy
metals 14
(and the heavy metals 14 are subsequently separated out), a second chamber is
used to react the alkali metal 5 with the naphthenic acid 8 (and the acid
products 13
are subsequently separated out) and then a third chamber used to react the
alkali
metal 5 with the heteroatoms 11 (and the sulfur/nitrogen products 17 are
subsequently separated out). Of course, if different chambers were used for
each of
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
13
these reactions, the reaction conditions such as pressure, temperature, flow
rates,
etc., could be adjusted/tailored to optimize each specific reaction.
[0055] In the embodiments shown in Figures 1 and 2, the alkali metal 5 is
shown
being added to the chamber 3. Those skilled in the art will appreciate that
there are
a variety of different ways by which the alkali metal 5 may be added in order
to
induce a reaction. For example, a sample of the alkali metal 5 may simply be
added
to the chamber 3. However, many in the oil processing industry are
uncomfortable
handling metallic sodium (or other metallic alkali metals) because of their
reactive
nature. Thus, other embodiments may be designed in which the alkali metal 5 is
formed in situ within the chamber 3 from alkali metals ions. In other words,
alkali
metal ions are added to the chamber 3 (which are safe and easy to handle) and
then
such ions are reduced back to the metallic state via an electrochemical
reduction
reaction. Once these alkali metal ions have been reduced in situ to form the
metallic
alkali metal 5, these formed alkali metals 5 immediately react with the oil
feedstock 9
(in the manner outlined herein) and are thus consumed almost instantaneously
after
formation. The embodiments that electrochemically form the alkali metal in
situ can
be advantageous in that they provide the strong reducing power and reactivity
of
alkali metal to the oil feedstock without ever having an appreciable amount of
the
metal present.
[0056] Referring now to Figure 3, an embodiment of a device 100 that may be
used to de-acidify oil feedstocks, as well as remove the heteroatoms/heavy
metals
and/or upgrade the feedstock is illustrated. Specifically, the device 100
consists of at
least two chambers, namely a feedstock chamber 20 and an alkali metal source
chamber 30. The feedstock chamber 20 has an outer wall 21 and may have an
inlet
22 and outlet 23.
[0057] The feedstock chamber 20 may be separated from the alkali metal source
chamber 30 by an alkali metal ion conductive separator 25. The ion separator
25
may be comprised of ceramic materials generally known as Nasicon, sodium beta
alumina, sodium beta prime alumina or sodium ion conductive glass if the
alkali
metal is sodium; or Lisicon, lithium beta alumina, lithium beta prime alumina
or
lithium ion conductive glass if the alkali metal is lithium. The materials
used to
construct the ion separator 25 are commercially available from Ceramatec,
Inc., of
Salt Lake City, Utah.
14
[0058] A cathode 26 which is negatively charged and connected to a power
source 40
(via wires 42) may be, at least partially, housed within the feedstock chamber
20.
Preferably the cathode 26 is located in close proximity to the ion separator
25 to
minimize ionic resistance. The cathode 26 may be contacting the ion separator
25 (as
shown in Figure 3) or screen printed on the ion separator 25. In other
embodiments,
the cathode 26 may be integrated with the ion separator 25 as disclosed in
U.S.
Patent Publication 2010/0297537 entitled "ELECTROCHEMICAL CELL
COMPRISING IONICALLY CONDUCTIVE MEMBRANE AND POROUS
MULTIPHASE ELECTRODE". By placing the cathode 26 on or near the ion
separator 25, the oil feedstock does not necessarily have to be ionically
conducting in
order to transfer ions/charges.
[0059] The alkali metal source chamber 30 has an outer wall 31 and may have an
inlet 32 and outlet 33. An anode 36 (which is positively charged) and
connected to the
power source 40 (via wires 42) may be, at least partially, housed within the
source
chamber 30. Suitable materials for the cathode 26 include materials
comprising,
carbon, graphite, nickel, iron which are electronically conductive. Suitable
materials
for the anode 36 include materials comprising titanium, platinized titanium,
carbon,
graphite. In the embodiment shown in Figure 3, the cathode 26 and the anode 36
are
connected to the same power supply 40. Further, Figure 3 shows the wires 42
exiting
the chambers 20, 30 via inlets 22, 32. Such depictions are made for clarity
and are
not limiting. Those skilled in the art will appreciate how the power source
40/wires 42
may be otherwise arranged in order to connect to the cathode 26 and/or the
anode
36. Likewise, those skilled in the art will appreciate that the cathode 26 and
the anode
36 may be connected to power supplies in various manners, etc.
[0060] A mode of operation for the device 100 will now be described.
Specifically, a
first oil feedstock 50 may enter the feedstock chamber 20 (such as, for
example, by
flowing through the inlet 22). Concurrently, a dissolved solution of alkali
metals 51 will
flow through the alkali metal source chamber 30. This solution of alkali
metals may
be, for example, a solution of sodium sulfide, lithium sulfide, sodium
chloride, sodium
hydroxide, etc. A voltage is then applied to the anode 36 and cathode 26 from
the
source 40. This voltage causes chemical reactions to occur. These reactions
cause
alkali metal ions 52 (abbreviated "AM ions" 52") to pass
CA 2855966 2017-08-11
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
through the ion separator 25. In other words, the alkali metal ions 52 flow
from the
alkali metal source chamber 30, through the ion separator 25, into the
feedstock
chamber 20.
[0061] Once the alkali metal ions 52 (such as, for example, sodium ions or
lithium
ions) pass through the ion separator 25, the ions 52 are reduced to the alkali
metal
state 55 (e.g., into sodium metal or lithium metal) at the cathode 26. Once
formed,
the alkali metal 55 intermixes with the feedstock 50 (as shown by arrow 58).
As
described herein, the reaction between the oil feedstock 50 and the alkali
metal 55
may involve a reaction between the acids (such as naphthenic acid) in the oil
feedstock 50. Thus, the reaction with the alkali metal 55, which was formed in
situ
within the chamber 20, operates to reduce the acid content in the oil
feedstock 50,
thereby reducing the TAN value of the oil feedstock 50.
[0062] Additionally and/or alternatively, the reaction between the oil
feedstock 50
and the alkali metal 55 formed within the chamber 20 may cause a reaction with
the
sulfur or nitrogen moieties within the oil feedstock 50. This reaction may
also reduce
heavy metals, such as vanadium and nickel in the feedstock 50. Further, as
explained in the '874 application, at an elevated temperature and elevated
pressure,
the reaction between alkali metals 55 and the heteroatoms (S, N) forces the
sulfur
and nitrogen heteroatoms to be reduced by the alkali metals into ionic salts
(such as
Na2S, Na3N, Li2S, etc.). These ionic salts may then be removed from the oil
feedstock 50. As such, the content of sulfur and nitrogen within the oil
feedstock 50
may be significantly reduced by the reaction of the alkali metal 55 formed
within the
chamber 20. In other words, the heteroatom-to-carbon ratio of the upgraded oil
feedstock may be less than the heteroatom-to-carbon ratio of the original
(unreacted)
oil feedstock. Also, the amount of heavy metals in the feedstock may further
be
reduced. Thus, the ratio of carbon to heavy metals in the upgraded (reacted)
feedstock is less than the ratio of carbon to heavy metals in the original
(unreacted)
feedstock.
[0063] Further, in addition to the oil feedstock 50, the chamber 20 may also
include a quantity of an upgradant hydrocarbon 60 that reacts with the oil
feedstock
50 (as shown by arrow 74). Specifically, as taught by the '874 application,
when the
sulfur/nitrogen moieties of the oil feedstock 50 react with the alkali metals
55, radical
species are formed that may react with the upgradant hydrocarbon 60. In some
embodiments, the upgradant hydrocarbon 60 may be hydrogen gas, including the
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
16
hydrogen gas formed by the reaction with naphthenic acid. (It should be noted
that if
hydrogen is used as the hydrocarbon 60, the amount of hydrogen needed is less
than the amount of hydrogen that would be required if a typical hydrotreatment
process were utilized). In other embodiments, the upgradant hydrocarbon 60
comprises natural gas, shale gas and/or mixtures thereof, methane, ethane,
propane, butane, pentane, their isomers, ethene, propene, butene, pentene,
dienes,
and/or mixtures thereof. As explained in the '874 application, this reaction
with the
upgradant hydrocarbon 60 may operate to produce an upgraded hydrocarbon that
has a greater hydrogen-to-carbon ratio than the original oil feedstock 60. The
upgraded oil feedstock produced in the reaction may also have a greater energy
value than the original oil feedstock 60. Typically the presence of upgradant
hydrocarbon 60 may result in a reduction of formation of insoluble solids
during the
reaction. It is believed that these solids are large organic polymers that are
formed
as part of the radical reactions. However, by using the upgradant hydrocarbon
60,
this hydrocarbon 60 acts as a "capping" species that prevents the formation of
these
solid, organic polymers. Thus, by using the hydrocarbon 60, the subsequent
yield of
the liquid oil feedstock (e.g., the desired product) may be increased.
[0064] The reactions described in Figure 3 may be conducted at elevated
temperatures. For example, the reactions may occur at temperatures above the
melting temperature of sodium or at higher temperatures found effective for
the
particular feedstock. The mode of operation of the device 100 may further
consist of
using molten sodium as the sodium source 51 in the alkali metal source chamber
30
or lithium metal as the lithium source. The reactions may further be conducted
at
elevated pressure, for example in the 300 ¨ 2000 pounds per square inch range.
[0065] In some embodiments, the oil feedstock 50 may be passed through the
device 100 (as the solution of sodium sulfide also passed through). Once
passed
through the device 100, the oil feedstock may flow into another vessel
operated at a
different temperature and pressure (e.g., temperatures and pressures more
conducive to the reactions desired and where the residence time of the
feedstock in
the second vessel size is matched to the reaction kinetics and flow rates).
[0066] As described herein, various solids, inorganic compounds, etc., may be
formed when performing the reactions outlined herein. These inorganic products
may comprise Na2S, NaN3, heavy metals and solid organic polymers that are
formed
by the radical reactions. In order to deal with these inorganic compounds, the
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
17
process used in conjunction with the device of Figure 3 may further involve
filtering,
or separating by centrifugal forces the feedstock after it has been exposed to
the
sodium for sufficient time to remove solids from the liquids. This separation
may
involve the use of a separator 80, as described below.
[0067] The oil feedstock 50, alkali metal solution 51 and other components of
the
device 100 may be dissolved in a polar solvent such as Formamide, Methyl
formamide, Dimethyl formamide, Acetamide, Methyl acetamide, Dimethyl
acetamide,
Triethylamine, Diethyl acetamide, Ethylene glycol, Diethylene glycol,
Triethylene
glycol, Tetraethylene glycol, Ethylene Carbonate, Propylene Carbonate,
Dimethylpropyleneurea, Butylene Carbonate, Cyclohexanol, 1,3-Cyclohexanediol,
1,2 Ethanediol, 1,2-Propanediol, Ethanolamine, Methyl sulfoxide, Dimethyl
sulfoxide,
Tetramethylene sulfoxide, Sulfolane, Gamma-butyrolactone, Nitrobenzene,
Acetonitrile, Pyridine, quinoline, ammonia, ionic liquids or molten fused
salts. For
example, the alkali metal solution 51 may be dissolved in one or more of these
solvents and then be allowed to flow into the alkali metal source chamber 30.
(The
salts that are used for the alkali metal solution 51 may be alkali metal
chlorides,
hydroxides, phosphates, carbonates, sulfides and the like.) Similarly, such
solvents
may be used with the oil feedstock 50 and/or the hydrocarbon 60 and then the
mixture may be allowed to flow into the chamber 20.
[0068] Depending on
the alkali metal source (e.g., the alkali metal solution 51), the
anode reaction in the alkali metal source chamber 30 may vary. For example
sulfides may form polysulfides and or elemental sulfur, chlorides may form
chlorine
gas, hydroxides may form oxygen gas, carbonates may form oxygen gas and evolve
carbon dioxide and the like. If the alkali metal source is an alkali metal,
metal ions
will simply form. These variations constitute different embodiments. Gas
handling
and recovery may be a part of the overall process.
[0069] As shown in Figure 3, the products formed in the oil feedstock chamber
20
may be sent to a product separator 80 (as shown by arrow 82). In this product
separator 80, the inorganic products may form a phase that is separable from
an
organic phase that comprises the upgraded oil feedstock and/or unreacted oil
feedstock. To facilitate this separation, a flux may be added to the product
separator. (Those skilled in the art are familiar with the materials that may
be used
as the flux that will facilitate separation between the organic feedstock
materials and
the inorganic products.) After separation, the alkali metal from the inorganic
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
18
products may be regenerated and reused. In some embodiments, the product
separator 80 may be a settling chamber or other similar structure.
[0070] Figure 4 is a schematic that includes another embodiment of the device
100. Much of the structures/elements depicted in Figure 4 are similar to that
which
was described in Figure 3. Accordingly, for purposes of brevity, the
discussion of
many of these structures/elements is omitted.
[0071] Figure 4 depicts a schematic embodiment similar to the depiction in
Figure
3 except a porous partition 101 resides between the ion separator 25 and the
feedstock 50. This partition 101 may be a metal mesh or perforated metal
sheet, or
glass fiber mesh, carbon fiber mesh or other material with holes or pores that
will
allow alkali metal to flow through. Alkali metal 102 is formed at the ion
separator 25
and may serve as the cathode with a negatively charged current collector 103
in
contact with the alkali metal 102.
(Alternatively, the porous partition 101, if
electronically conductive, may be negatively charged and serve as the current
collector.) Once the alkali metal 102 is formed, it may flow through the
porous
partition 101 and may then react with the oil feedstock 50 in the manner
described
above.
[0072] Thus, as indicated herein, there are at least three different processes
as
part of this invention for de-acidifying these streams:
1) Add sodium or lithium directly to the stream to form the acid salt and
hydrogen, then filter out the acid salts from the stream;
2) Use the novel device in a mode where a sodium or lithium source in alkali
metal source chamber and the feedstock is in the feedstock chamber, and
where sodium metal forms at the cathode located with the feedstock chamber,
where the alkali metal reacts with the feedstock to convert the acids to the
sodium or lithium salt of the acid and hydrogen evolves as a byproduct;
3) Use the novel device in a mode where a sodium or lithium source in alkali
metal source chamber and the feedstock is in the feedstock chamber, and
where alkali ions transport across the ion separator dividing the two chambers
under a potential gradient, and hydrogen evolves at the cathode located with
the feedstock chamber, where the alkali metal ions combine with the organic
acid anions to form a salt, in this case using a cathode material with low
hydrogen overpotential (such as platinum or other materials) may be
preferred.
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
19
[0073] In the case of acid removal, there may not be any reason to add an
upgradant hydrocarbon (gas) 60 to the feedstock chamber with the cathode since
hydrogen gas may be a byproduct. As such, this formed hydrogen may act as the
upgradant hydrocarbon 60. If heteroatoms such as sulfur, or nitrogen are
present,
an upgradent gas 60 such as hydrogen, natural gas, shale gas and/or mixtures
thereof, may be needed.
[0074] It should
also be noted that the addition of the alkali metal 102 may not
simply neutralize the acidic hydrogen in the napthenic acid. Specifically,
naphthenic
acid has the structure: R¨COOH. In some embodiments, the alkali metal 102 may
react with the oxygen atoms (in addition to the hydrogen atoms) such that the
remaining hydrocarbon after the alkali metal addition has the structure R¨CH3,
R¨
H, etc. (The reason for this is that the alkali metal 102 may also reduce the
oxygen
moiety as well as the hydrogen moiety.) The formed inorganic products may thus
include NaOH, Na20, etc. As noted above, after reaction with the alkali metal
103,
the TAN value of the feedstock 50 is reduced. However, given the above-recited
reactions with the oxygen moieties, the TAN value may not be increased (or
returned
to its original state) by simply reacting the de-acidified oil feedstock with
base (such
as NaOH). Rather, as described herein, the reduction of the TAN value may also
operate to convert the napthenic or other acid groups into pure hydrocarbon
functional groups (such as is R¨CH3, R¨H, etc.).
[0075] Referring now to Figure 5, another embodiment of a device 100 is
illustrated. Specifically, the device 100 may be used to upgrade an oil
feedstock 50.
More specifically, the feedstock 50 may be upgraded by having the feedstock 50
be
de-acidified, desulfirized, demetalized and denitrogenized. In other
words, the
device 100 is operable to remove sulfur, heavy metals, acids (such as
napthenic
acid) and nitrogen from the oil feedstock 50.
[0076] The embodiment of the device 100 that is shown in Figure 5 is similar
to
that which is shown and described in Figure 3. For purposes of brevity, much
of this
discussion will be omitted. However, for clarity, the wires 42 and the power
source
40 are not shown in Figure 5. However, those skilled in the art will
appreciate that
such structures are indeed present and may be necessary in order to conduct
the
electrolytic reactions associated with the device 100.
[0077] As described herein, the oil feedstock 50 shown in Figure 5 may include
quantities of heavy metals, napthenic acid and at least one heteroatom (e.g.,
= CA 02855966 2015-06-04
nitrogen and sulfur). Accordingly, such materials may be removed from the oil
feedstock 50
using the methods outlined herein. Specifically, the oil feedstock 50 is
contacted with quantities
of alkali metals 55a, 55b, 55c. (The arrows 58 are designed to represent the
reactions between
the alkali metals 55a, 55b, 55c and the oil feedstock 50.) More specifically,
the feedstock 50
may be contacted with a first quantity of an alkali metal 55a. The reaction
between the first
quantity of the alkali metal 55a and the feedstock 50 is such that the alkali
metal 55a reacts with
the heavy metals that are in the feedstock 50. This reacted feedstock may then
exit the chamber
20 and may pass through a product separator 80. The purpose of the product
separator 80 is to
remove the heavy metals from the oil feedstock. These heavy metals may then be
recovered,
sold, etc.
[0078] As shown by Figure 5, after passing through the product
separator 80, the
feedstock 50 (minus the heavy metals which were previously removed) may be
brought back into
the chamber 20. This chamber 20 may be the same chamber that was previously
used to remove
the heavy metals, or it may be a chamber 20 of a different device 100 that is
positioned
downstream from the product separator 80.
[0079] Once in the chamber 20, the oil feedstock 50 (which has had
the heavy metals
removed) may then be reacted with a second quantity of the alkali metal 55b.
This time, the
alkali metal 55b reacts with the napthenic acid to form a de-acidified oil
feedstock, wherein a
TAN value of the unreacted oil feedstock is greater than a TAN value of the de-
acidified oil
feedstock. Again, after the acids have been reacted, the reacted oil feedstock
50 may be sent to
the product separator 80 which may operate to remove the inorganic materials
that were formed
during the reaction with the second quantity of alkali metals 55b. This
separation of inorganic
materials may occur within the same product separator 80 that was used to
remove the heavy
metals or may be conducted in a different separator product 80.
[0080] After passing through the product separator 80, the feedstock
50 (minus the heavy
metals and the napthenic acids which were previously removed) may be brought
back into the
chamber 20. This chamber 20 may be the same chamber that was previously used
to remove the
heavy metals/napthenic acids, or it may be a chamber 20 of a different device
100 that is
positioned downstream from the product separator 80. Once in the chamber 20,
the oil feedstock
50 (which has had the heavy metals/napthenic acids removed) may then be
reacted with a third
quantity of the alkali metal 55c. This reaction with the third quantity of the
alkali metal 55c
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
21
removes at least one heteroatom (e.g., N, S) from the feedstock 50 to form an
upgraded oil feedstock. The heteroatom to carbon ratio of the upgraded oil
feedstock is less than a heteroatom to carbon ratio of the oil feedstock. Once
again,
this product may then pass through a product separator 80 to remove the
inorganic
materials and/or N, S moieties from the oil feedstock, thereby resulting in an
upgraded oil feedstock.
[0081] It should be
noted that the alkali metal quantities 55a, 55b, 55c in Figure 5
were introduced using the method of the device 100--e.g., by having alkali
metal ions
pass through the ion separator 25 and then be reduced to the metallic state in
situ
within the chamber 20. Of course, other embodiments may be designed in which
one or more of the alkali metal quantities 55a, 55b, 55c are introduced
directly into
the oil feedstock 50 (e.g., without having the metal be formed via a reduction
reaction). The different quantities of the alkali metals 55a, 55b, 55c may be
the
same alkali metal or may be different alkali metals.
[0082] Referring now to Figure 6, a flow diagram of a method 190 that may be
used to upgrade a quantity of a first oil feedstock 50a is shown.
Specifically, the
quantity of the oil feedstock 50 may be obtained. This oil feedstock 50 may
include
quantities of heavy metals, acids (such as napthenlic acid), and/or one or
more
heteroatoms (such as sulfur and nitrogen moieties). In order to upgrade the
oil
feedstock 50a, these metals/heteroatoms/acids may be removed from the oil
feedstock 50a. Specifically, the quantity of the oil feedstock 50a may be
added to a
chamber 110a. This chamber 110a may be referred to as a "de-metalization"
chamber in that the heavy metals are removed from the oil feedstock 50a in
this
chamber 110a. In some embodiments, the chamber 110a may be an oil feedstock
chamber 20 of the type described above. However, in other embodiments, the
chamber 110a may simply be another type of vessel that is designed to remove
metals from the oil feedstock 50a. In order to remove the metals from the oil
feedstock 50a, a quantity of alkali metals (such as alkali metals 55a shown in
Figure
5) may be added to the feedstock 50a. Once the reaction has occurred, the
products may be placed within a product separator 80a. Those skilled in the
art will
appreciate the types of devices (such as a settling chamber) that may be used
as the
product separator 80a. In this product separator 80a, heavy metals 125 are
separated out, leaving only a quantity of "de-metalized" oil feedstock 50b.
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
22
[0083] This oil feedstock 50b may then be added to a chamber 110b. The
chamber 110b may be the same chamber as the chamber 110a (e.g., the oil
feedstock material is re-inserted into the chamber 110a) or it may be a
different
vessel. The chamber 110b may be referred to as a "de-acidification" chamber in
that
the oil feedstock 50b may be de-acidified in this chamber 110b. In order to
conduct
this reaction, the oil feedstock 50b is reacted with a quantity of an alkali
metal (such
as second quantity of the alkali metal 55b shown in Figure 5). This reaction
with the
alkali metal 55b reacts with the napthenic acid in the feedstock 50b. More
specifically, the alkali metal 55b eliminates the naphtenic acid such that the
reacted
oil feedstock has a TAN value that is less than the TAN value of the
(unreacted) oil
feedstock 50a.
[0084] Once the reaction has occurred, the products may be placed within a
product separator 80b. Those skilled in the art will appreciate the types of
devices
(such as a settling chamber) that may be used as the product separator 80b.
The
product separator 80b may be the same structure as the product separator 80a
or
may be a different element. In this product separator 80b, inorganic acid
products
127 are separated out, leaving only a quantity of "de-acidified" oil feedstock
50c.
[0085] This de-acidified oil feedstock 50c (which has also been de-metalized)
may
then be added to a chamber 110c. This chamber 110c may be the same as either
or
both of the chambers 110a, 110b, or in other embodiments, the chamber 110c may
be a different chamber than the chambers 110a, 110b. This chamber 110c may be
referred to as a "de-sulfurization" chamber in that sulfur moieties from the
oil
feedstock may be removed. More specifically, an alkali metal (such as a third
quantity of the alkali metal 55c) may be added to react with the oil
feedstock. More
specifically, this reaction involves reacting the alkali metal with a
heteroatom, such
as sulfur. (This reaction is described above). Once reacted, the products may
be
added to a product separator 80c which operates to remove inorganic sulfur
products 129 from the oil feedstock, thereby producing de-sulfurized feedstock
50d.
[0086] This feedstock 50d may further be added to a chamber 110d. This
chamber 110d may be the same as or different than the chambers 110a, 110b,
110c.
In this chamber, heteroatoms such as nitrogen are removed from the oil
feedstock by
reacting the feedstock with an alkali metal quantity (such as, for example,
alkali
metal 55c of Figure 5). After this reaction has occurred, the inorganic
nitrogen
products 131 may be removed via product separator 80d (which may be the same
CA 02855966 2014-05-14
WO 2013/075021
PCT/US2012/065670
23
as, or a different structure than, the product separators 80a, 80b, 80c). The
resulting
oil feedstock, after all of these products have been removed, may be
classified as an
"upgraded" oil feedstock 50e.
[0087] In the embodiment shown in Figure 6, the reactions with the sulfur
moieties
and the nitrogen moieties (e.g., heteroatoms) are shown as different steps.
Those
skilled in the art will recognize that other embodiments may involve a single
step
(e.g., a single addition of a third quantity of an alkali metal) to eliminate
all of the S
and N heteroatoms. If the sulfur and nitrogen are eliminated together via a
single
addition of alkali metal, embodiments may be designed in which up to 80% of
the
sulfur may be removed from the oil feedstock before the nitrogen moieties
begin to
react with the alkali metal. It is also understood that depending on the
actual
operating conditions and nature of the feedstock, the order in which the
various
species are removed may differ from the order illustrated in Figure 6.
[0088] Referring now to Figure 7, another embodiment of a system 200 for
upgrading an oil feedstock is shown. It should be noted that the system 200
includes
many of the same features that are associated with the device 100 of Figure 3.
For
purposes of brevity, much of this discussion will be omitted. However, for
clarity, the
wires 42 and the power source 40 are not shown in Figure 7. However, those
skilled
in the art will appreciate that such structures are indeed present and may be
necessary in order to conduct the electrolytic reactions associated with the
system
200.
[0089] In the
embodiment of Figure 7, a quantity of a first oil feedstock 150a is
added to a TAN reduction chamber 205. This chamber 205 is a chamber into which
an alkali metal (in its metallic form) may be added. This addition of the
alkali metal
to the feedstock 150a operates to eliminate naphthenic acid in the feedstock
150a.
Accordingly, the TAN value of the feedstock 150a after it has been reacted in
the
TAN reduction vessel 205 is significantly reduced. A separator (which is not
shown
in Figure 7) may be used to remove the formed inorganic materials from the
feedstock. The feedstock leaving this chamber 205 may be referred to as de-
acidified oil feedstock 150b.
[0090] The de-acidified feedstock 150b may be added to a chamber 20 so that it
may be exposed to alkali metal 155b, thereby eliminating the heteroatoms
and/or the
heavy metals in the feedstock 150b. Thus, Figure 7 shows an embodiment in
which
the chamber 205 used to reduce the TAN value is separate from the chamber 20
24
that is used to de-nitrogenize/de-sulfurize the feedstock. Thus, as shown by
Figure 7,
the temperature and pressure and flow rate for optimal TAN reduction may be
used in
the TAN vessel 205, and then different temperatures/pressures/flow rates, etc.
may
be used in the chamber 20 for the other chemical reactions. These different
temperatures/pressures/flow rates may be matched to the reaction kinetics of
the
specific reactions.
[0091] The embodiment shown in Figure 7 illustrates that there is a
significant amount
of flexibility associated with the present embodiments. For example, as shown
in
Figure 7, there may be a TAN reduction chamber 205 that is designed to reduce
the
TAN value of the oil feedstock. Once this TAN value has been reduced (for
example
to a value that is less than or equal to 1 ), then other processes may be used
to
eliminate the heteroatoms, heavy metals, etc. associated with the oil
feedstock. Thus,
the owner of the oil feedstock can design a system that will be appropriate
for
processing their particular sample of hydrocarbon material.
[0092] Once the heteroatoms/heavy metals have been removed by the chamber 20,
the oil feedstock 150c may flow out of the chamber 20. This oil feedstock will
be
referred to as "upgraded" oil feedstock 150c.
[0093] Further, those skilled in the art will appreciate that the amount of
alkali metal
that is needed to reduce the TAN value below 1 , to remove the heteroatoms, to
react
with the heavy metals, etc., will depend upon the particular sample of oil
feedstock/hydrocarbon material. Accordingly, by performing testing on the
sample oil
feedstock, a skilled artisan can determine how much alkali metal may be needed
to
upgrade the oil feedstock.
CA 2855966 2017-08-11