Note: Descriptions are shown in the official language in which they were submitted.
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
PROCESS TO PREPARE LEVULINIC ACID
CROSS REFERENCE TO RELATED APPLICATIONS
[001] This application claims priority to U.S. Provisional Patent
Application No.
61/563,276, filed November 23, 2011, U.S. Provisional Patent Application No.
61/576,818,
filed December 16, 2011, U.S. Provisional Patent Application No. 61/581,006,
filed
December 28, 2011, and U.S. Provisional Patent Application No. 61/722,766,
filed
November 5, 2012, all entitled "PROCESS TO PREPARE LEVULINIC ACID", the
contents
of which are incorporated herein in their entirety for all purposes.
FIELD OF THE INVENTION
[002] The invention relates generally to the preparation and purification
of levulinic
acid.
BACKGROUND OF THE INVENTION
[003] Levulinic acid can be used to make resins, plasticizers, specialty
chemicals,
herbicides and as a flavor substance. Levulinic acid is useful as a solvent,
and as a starting
material in the preparation of a variety of industrial and pharmaceutical
compounds such as
diphenolic acid (useful as a component of protective and decorative finishes),
calcium
levulinate (a form of calcium for intravenous injection used for calcium
replenishment and
for treating hypocalcemia,. The use of the sodium salt of levulinic acid as a
replacement for
ethylene glycols as an antifreeze has also been proposed.
[004] Esters of levulinic acid are known to be useful as plasticizers and
solvents, and
have been suggested as fuel additives. Acid catalyzed dehydration of levulinic
acid yields
alpha-angelica lactone.
[005] Levulinic acid has been synthesized by a variety of chemical methods.
But
levulinic acid has not attained much commercial significance due in part to
the high cost of
the raw materials needed for synthesis. Another reason is the low yields of
levulinic acid
obtained from most synthetic methods. Yet, another reason is the formation of
a formic acid
byproduct during synthesis and its separation from the levulinic acid.
Therefore, the
production of levulinic acid has had high associated equipment costs. Despite
the inherent
-1-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
problems in the production of levulinic acid, however, the reactive nature of
levulinic acid
makes it an ideal intermediate leading to the production of numerous useful
derivatives.
[006] Cellulose-based biomass, which is an inexpensive feedstock, can be
used as a
raw material for making levulinic acid. The supply of sugars from cellulose-
containing plant
biomass is immense and replenishable. Most plants contain cellulose in their
cell walls. For
example, cotton comprises 90% cellulose. Furthermore, it has been estimated
that roughly
75% of the approximate 24 million tons of biomass generated on cultivated
lands and
grasslands are waste. The cellulose derived from plant biomass can be a
suitable source of
sugars to be used in the process of obtaining levulinic acid. Thus, the
conversion of such
waste material into a useful chemical, such as levulinic acid, is desirable.
BRIEF SUMMARY OF THE INVENTION
[007] A major issue in producing levulinic acid is the separation of pure
levulinic
acid from the byproducts, especially from formic acid and char. Current
processes generally
require high temperature reaction conditions, generally long digestion periods
of biomass,
specialized equipment to withstand hydrolysis conditions, and as a result, the
yield of the
levulinic acid is quite low, generally in yields of 10 percent or less.
[008] Therefore, a need exists for a new approach that overcomes one or
more of the
current disadvantages noted above.
[009] The present invention surprisingly provides novel approaches to more
efficiently prepare levulinic acid in commercial quantities with high yields
and high purities.
Additionally, the production of hydroxymethylfurfural is also described, which
is an
important intermediate to the product of levulinic acid.
[010] In one aspect, the use of a water insoluble cosolvent in the
processes improves
the yields of the hydroxymethylfurfural or levulinic acid and helps to reduce
undesired
byproducts. In another aspect, the use of high concentration of acid, e.g.,
about 20-50 weight
percent based on the total weight of reaction components and low reaction
temperature
(approximately 50 - 100 C) helps to improve yield of desired products with
reduction of
undesired byproducts.
[011] In one aspect, HMF can be prepared first followed by a second step to
prepare
the levulinic acid.
[012] While multiple embodiments are disclosed, still other embodiments of
the
present invention will become apparent to those skilled in the art from the
following detailed
description. As will be apparent, the invention is capable of modifications in
various obvious
-2-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
aspects, all without departing from the spirit and scope of the present
invention.
Accordingly, the detailed descriptions are to be regarded as illustrative in
nature and not
restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
[013] Figure 1 a is a flow diagram of one embodiment for a process to
prepare and/or
purify levulinic acid.
[014] Figure lb is a flow diagram of another embodiment for a process to
prepare
and/or purify levulinic acid.
[015]
[016] Figures 2a through 2e provide information regarding recovery of
levulinic acid
from Char; soluble and insoluble fractions. It was surprisingly found that
extraction of the
char provided levulinic acid almost exclusively, helping to further improve
the production of
levulinic acid.
[017] Figure 3 provides an aspen flowsheet diagram depicting various
reactor
configurations.
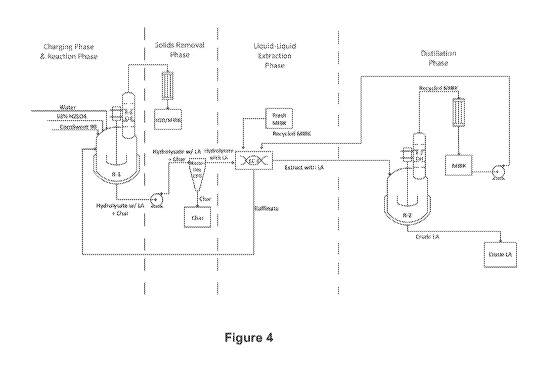
[018] Figure 4 depicts an industrial scale process to produce levulinic
acid.
[019] Figures 5a through 5c are pictures showing reactor components after
production of levulinic acid in accordance with the present invention.
[020] Figures 5d through 5g are pictures showing reactor components after
production of levulinic acid in accordance with the prior art.
DETAILED DESCRIPTION
[021] In the specification and in the claims, the terms "including" and
"comprising"
are open-ended terms and should be interpreted to mean "including, but not
limited to. . .
These terms encompass the more restrictive terms "consisting essentially of"
and "consisting
of"
[022] It must be noted that as used herein and in the appended claims, the
singular
forms "a", "an", and "the" include plural reference unless the context clearly
dictates
otherwise. As well, the terms "a" (or "an"), "one or more" and "at least one"
can be used
interchangeably herein. It is also to be noted that the terms "comprising",
"including",
"characterized by" and "having" can be used interchangeably.
[023] Unless defined otherwise, all technical and scientific terms used
herein have
the same meanings as commonly understood by one of ordinary skill in the art
to which this
-3-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
invention belongs. All publications and patents specifically mentioned
herein are
incorporated by reference in their entirety for all purposes including
describing and disclosing
the chemicals, instruments, statistical analyses and methodologies which are
reported in the
publications which might be used in connection with the invention. All
references cited in
this specification are to be taken as indicative of the level of skill in the
art. Nothing herein is
to be construed as an admission that the invention is not entitled to antedate
such disclosure
by virtue of prior invention.
[024] The present invention provides various advantages in the preparation
of
levulinic acid, hydroxymethyl furfural and/or formic acid. The following list
of advantages is
not meant to be limiting but highlights some of the discoveries contained
herein.
[025] First, a biomass material can be used as the initial feedstock to
prepare the
levulinic acid, hydroxymethyl furfural and/or formic acid. This ability
provides great
flexibility in obtaining a constant source of starting material and is not
limiting.
[026] Second, the biomass can be a refined material, such as fructose,
glucose,
sucrose, mixtures of those materials and the like. As such, there is a
plentiful supply of
materials that can be converted into the ultimate product(s). For example,
sugar beets or
sugar cane can be used as one source. Fructose-corn syrup is another readily
available
material. Use of such materials thus helps to reduce the costs to prepare the
desired products.
[027] Third, it has been discovered that use of high concentrations of
acid(s),
generally about 20 weight percent or more (based on the total mass of the
reaction medium)
provides a cleaner reaction product with less char and unwanted byproducts. It
has also been
found that use of high concentrations of acid(s), generally up to 75 weight
percent or more,
(based on the total mass of the reaction medium) provides faster reaction
times than lower
acid concentrations used with the same reaction conditions.
[028] Fourth, it has also been discovered that with the use of higher
concentrations
of acid, the reaction conditions can be conducted at much lower temperatures
than are
currently utilized in the literature. Again, this lessens the amount of char
and byproducts
from the reaction(s) that take place and increases the yield of the desired
product(s).
[029] Fifth, it has also been discovered that with the methods of the
present
invention, the char that is created is much easier to remove from the reactor.
For example,
Figures 5a, 5b and 5c depict internal PARR reactor components after carrying
out methods
according to the present invention with no additional cleaning. As can be seen
in the
photographs, there is little to no char accumulated on the reactor components.
In comparison,
Figures 5d through 5g depict internal PARR reactor components after carrying
out methods
-4-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
according to the prior art with no additional cleaning. As can be seen, there
is significant
char build up on the reactor components requiring large cleanup efforts.
[030] Sixth, it has been advantageously found to treat the biomass
material(s) in an
aqueous environment with a water immiscible solvent. Not to be limited by
theory, it is
believed that the partitioning of the starting materials from the product(s)
between the
aqueous and non-aqueous layers provides for one or more of: increased yield,
reduced
charring and/or by-products, faster reaction times and reduced reaction
temperatures.
[031] Seventh, it has also been found that the advantages of the new
process
conditions, including continuous addition of the biomass over a period of time
during the
reaction can be incorporated into existing processes to improve yield, reduce
costs, improve
efficiency and improve purity of product(s).
[032] Eighth, the processes described herein can be performed via CSTR or
continuous batch process conditions.
[033] In one embodiment, This process uses a high concentration of sulfuric
acid,
which has several distinct advantages. For one, the reactions can be run at
lower
temperatures compared to low acid processes and still hydrolyze the sugars in
a reasonable
time frame. It has been discovered that under these high acid, low-temperature
reaction
conditions (e.g., 80 C-110 C), the char byproduct that is formed is in the
form of suspended
particles that are easier to remove from the reactor and that can be filtered
from the liquid
hydrolysate product stream. In contrast, with low acid conditions, high
temperature is
required to effectively hydrolyze the sugar in a reasonable time frame and
those conditions
produce a char byproduct that coats the reactor components in such a manner
that it is
difficult to remove, and for the most part does not stay suspended in the
reaction mixture.
This high-acid reaction strategy, however, makes it difficult to isolate the
organic acid
products (levulinic acid and formic acid) from the inorganic acid reagent.
When small
amounts of sulfuric acid are used, as is typical in the prior art, the strong
inorganic acid can
effectively be neutralized to its salt form by careful addition of
stoichiometric amounts of
base. At the high acid contents used here, however, the quantity of salt
produced would be
excessive. Likewise, the use of an ion exchange column is impractical because
the large
quantity of inorganic acid would quickly fill the capacity of the column.
[034] Solvent extraction techniques, where the organic acids are preferably
extracted
into an organic solvent, are preferred. Even here, the high mineral acid
content poses
challenges. The organic solvent should be insoluble in the aqueous phase, but
in some cases,
the sulfuric acid can drive compatibility of the organic solvent and the
aqueous phase. When
-5-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
this happens, a portion of the organic solvent becomes soluble in the
concentrated sulfuric
acid aqueous phase and the risk of solvent loss to side reactions increases.
Even if the
organic solvent is stable in the aqueous sulfuric acid phase, the organic
solvent must be
recovered from the aqueous stream for recycling to the extraction unit for
optimized
economics. High mineral acid concentration also carries with it the potential
for higher
mineral acid concentrations in the organic phase. When this happens, there is
the risk of
solvent loss to side reactions with the mineral acid, particularly in the case
when the organic
stream is heated to distill the organic solvent. Therefore, solvent extraction
of the organic
acid products should ideally have at least some of the following
characteristics:
[035] little to no miscibility with water;
[036] little to no miscibility with the mineral acid;
[037] selectively partition the organic acids into the organic solvent
phase;
[038] have low partitioning of the mineral acid into the organic solvent
phase;
[039] have low reactivity between the organic extraction solvent and the
mineral
acid;
[040] have low reactivity between the organic extraction solvent & the
organic acid
products;
[041] have the ability to remove or reduce any mineral acid that partitions
into the
organic phase;
[042] easy to remove from organic acid, such as by backwashing or
distillation;
[043] allow the neutralization the organic acids.
[044] In one embodiment, the partition coefficient of the extraction
solvent for
levulinic acid is at least 0.3, more specifically, at least 0.5, more
specifically, at least 0.7,
more specifically, at least 1.0, more specifically at least 1.3, more
specifically, at least 1.5
more specifically, at least 1.7, and more specifically at least 2Ø In one
embodiment, the
partition coefficient of the extraction solvent for formic acid is at least
0.3, more specifically,
at least 0.5, more specifically, at least 0.7, more specifically, at least
1.0, more specifically at
least 1.3, more specifically, at least 1.5 more specifically, at least 1.7,
and more specifically at
least 2.0, more specifically, at least 2.3, more specifically, at least 2.5,
more specifically, at
least 3.0, more specifically, at least 3.5, more specifically, at least 4.0,
more specifically, at
least 5.0 more specifically, at least 6.0, more specifically, at least 7.0,
more specifically, at
least 8.0, and more specifically, at least 9Ø
[045] In one embodiment, to conduct a CSTR reaction with a given "residence
time"
t (in this case, t = typically 30 min to 1 hour) the volume of the reactor is
selected such that
-6-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
the typical "residence time" of the reactants is the designed target. The mass
of material held
in the reactor is designed to be the product of the mass flow rate into the
reactor and the
residence time. Longer residence time = larger quantity of material held in
the reactor.
Slower feed rate = smaller quantity of material held in the reactor. In
operation, it is
desirable for the feed to be a constant flow rate and composition; also the
exit stream is a
constant flow rate and composition, and the sum of the flow rates of all exit
streams equals
the flow rate of the feeds (on a mass basis).
[046] Typically, the reactor goes through a start-up phase until the
reactor achieves
"steady state" wherein the reactor contents, temperature, and pressure only
varies within a
controlled range. After steady state is achieved, the reactor is continuously
operated as long
as desired (days, weeks, months, years). During operation, the feed is steady,
and the exit
stream is steady. The reactor contents are steady. But the average residence
time of the
reactor contents is designed and held constant. The reactor content
composition is equal to
the composition of the exit streams.
[047] During the startup phase, many strategies can be used to reach steady
state as
quickly as possible. For example, the reactor contents may be started as 100%
water, or fed
with the desired steady state composition of the reactor contents. The
composition of the
feed streams can be allowed to vary, and the flow rate of the exit stream may
be varied to
achieve steady state (anywhere from zero to equal to the feed rate).
[048] It has been observed that the production of HMF could potentially
lead to
large amounts of undesirable char build up. For example, a CSTR design which
is
inadvertently designed so as to run at conditions which give a high HMF yield,
could be
expected to yield high char and discouraging results.
[049] It is thus, one technical advantage of one embodiment of the
invention to
provide a continuous reaction system in such a way to minimize the HMF
concentration.
[050] It has been observed in a batch reaction wherein the HMF
concentration starts
out at zero , builds to a peak, and then declines again to very low levels. In
a simple batch
reaction, such a profile is difficult to avoid. Likewise, a single,
continuous, plug-flow reactor
could experience a similar HMF concentration along the length of the tube. The
inventors
have found that in one embodiment, a carefully designed reaction system (for
example, an
initial CSTR followed by a plug flow reactor) could avoid having a high HMF
concentration
and still achieve high conversion.
-7-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[051] The following paragraphs provide for various aspects of the present
invention.
In one embodiment, in a first paragraph (1), the present invention provides a
process to
prepare levulinic acid comprising the steps:
[052] a) heating an aqueous solution of a mineral acid to about 60 C to
about
110 C in a reactor; and
[053] b) adding high fructose corn syrup, a mixture of at least two
different
sugars, sucrose, an aqueous mixture comprising fructose, an aqueous mixture
comprising
fructose and glucose, an aqueous mixture comprising hydroxymethylfurfural, an
aqueous
solution of fructose and hydroxymethylfurfural, an aqueous mixture of glucose,
an aqueous
mixture of maltose, an aqueous mixture of inulin, an aqueous mixture of
polysaccharides, or
mixtures thereof to the heated aqueous acid in the reactor over a period of
time to form a
reaction mixture including levulinic acid. In one embodiment, the high
fructose corn syrup, a
mixture of at least two different sugars, sucrose, an aqueous mixture
comprising fructose, an
aqueous mixture comprising fructose and glucose, an aqueous mixture comprising
hydroxymethylfurfural, an aqueous solution of fructose and
hydroxymethylfurfural, an
aqueous mixture of glucose, an aqueous mixture of maltose, an aqueous mixture
of inulin, an
aqueous mixture of polysaccharides, or mixtures thereof that are added to the
reaction
mixture over time comprises from about 0.1 to about 25, more specifically,
from about 1 to
about 20 and even more specifically from about 4 to about 15 percent by weight
of the final
mass of the reaction mixture. It is understood that as the sugar streams are
added to the
reactor, the sugar will continuously react with the mineral acid to form
levulinic acid and
other materials. Thus, the final reaction mixture may contain less than the
described ranges
of sugars. In another embodiment, the steady state concentration of the high
fructose corn
syrup, a mixture of at least two different sugars, sucrose, an aqueous mixture
comprising
fructose, an aqueous mixture comprising fructose and glucose, an aqueous
mixture
comprising hydroxymethylfurfural, an aqueous solution of fructose and
hydroxymethylfurfural, an aqueous mixture of glucose, an aqueous mixture of
maltose, an
aqueous mixture of inulin, an aqueous mixture of polysaccharides, or mixtures
thereof in the
reaction mixture is from about 0.1 to about 25, more specifically, from about
1 to about 20
and even more specifically from about 4 to about 15 percent by weight.
[054] 2. The process of paragraph 1, wherein the mineral acid is
sulfuric acid
(H2SO4), hydrochloric acid (HC1), hydrobromic acid (HBr) or hydroiodic acid
(HI).
[055] 3. The process of paragraphs 1 or 2, wherein the mineral acid
percentage
by weight is from about 5 to about 80 percent of the reaction mixture.
-8-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[056] 4. The process of paragraphs 1 or 2, wherein the mineral acid
percentage by
weight is from about 20 to about 80 percent of the reaction mixture.
[057] 5. The process of paragraphs 1 or 2, wherein the mineral acid
percentage by
weight is from about 20 to about 50 percent of the reaction mixture.
[058] 6. The process of any of paragraphs 1 through 5, wherein the high
fructose corn syrup is present between about 1 and about 99 weight percent of
fructose and
from about 99 to about 1 weight percent glucose with the remainder water,
wherein the sugar
content is between about 1 and about 99% by weight.
[059] 7. The process of any of paragraphs 1 through 6, wherein the high
fructose corn syrup is added over a period of from about 0.1 to about 40
hours.
[060] 8. The process of either of paragraphs 1 through 5, wherein the
mixture
of at least two different sugars is between about 1 and about 99 weight
percent of fructose
and from about 99 to about 1 weight percent glucose with the remainder water,
wherein the
sugar content is between about 20 and about 90% by weight.
[061] 9. The process of any of paragraphs 1 through 5 or 7, wherein the
mixture
of at least two different sugars is added over a period of from about 0.1 to
about 40 hours.
[062] 10. The process of any of paragraphs 1 through 5, wherein the
aqueous
mixture of fructose and glucose is between about 1 and about 99 weight percent
of fructose
and from about 99 to about 1 weight percent glucose with the remainder water,
wherein the
sugar content is between about 30 and about 85% by weight.
[063] 11. The process of any of paragraphs 1 through 5 or 10, wherein
the
aqueous mixture of fructose and glucose is added over a period of from about
0.1 to about 40
hours.
[064] 12. The process of either of paragraphs 1 through 5, wherein the
aqueous
solution of fructose contains from about 1 to about 100 percent fructose by
weight.
[065] 13. The process of any of paragraphs 1 through 5 or 12, wherein
the
aqueous solution of fructose is added over a period of from about 0.1 to about
40 hours.
[066] 14. The process of either of paragraphs 1 through 5, wherein the
aqueous
solution of hydroxymethylfurfural contains from about 0.1 to about 100 percent
hydroxymethylfurfural by weight.
[067] 15. The process of any of paragraphs 1 through 5 or 14, wherein
the
aqueous solution of hydroxymethylfurfural is added over a period of from about
0.1 to about
40 hours.
-9-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[068] 16. The process of any of paragraphs 1 through 5, wherein the
aqueous
mixture of fructose and HMF contains from about 0.1 to about 99.9 parts
fructose, from about
99.9 to about 0.1 parts hydroxymethylfurfural and from about 10 to about 99.8
parts water by
weight.
[069] 17. The process of any of paragraphs 1 through 5 or 16, wherein
the
aqueous mixture of fructose and hydroxymethylfurfural is added over a period
of from about
0.1 to about 40 hours.
[070] 18. The process of any of paragraphs 1 through 5, wherein the
aqueous
mixture of glucose contains from about 0.1 to about 99.9 parts glucose and
from about 0.1 to
about 99.9 parts water by weight.
[071] 19. The process of any of paragraphs 1 through 5 or 18, wherein
the
aqueous mixture of glucose is added over a period of from about 0.1 to about
40 hours.
[072] 20. The process of any of paragraphs 1 through 5, wherein the
aqueous
mixture of maltose contains from about 0.1 to about 99.9 parts maltose and
from about 0.1 to
about 99.9 parts water by weight.
[073] 21. The process of any of paragraphs 1 through 5 or 20, wherein
the
aqueous mixture of maltose is added over a period of from about 0.1 to about
40 hours.
[074] 22. The process of any of paragraphs 1 through 5, wherein the
aqueous
mixture of inulin contains from about 0.1 to about 99.9 parts inulin and from
about 0.1 to
about 99.9 parts water by weight.
[075] 23. The process of any of paragraphs 1 through 5 or 22, wherein
the
aqueous mixture of inulin is added over a period of from about 0.1 to about 40
hours.
[076] 24. The process of any of paragraphs 1 through 5, wherein the
aqueous
mixture of polysaccharides contains from about 0.1 to about 99.9 parts
polysaccharides and
from about 0.1 to about 99.9 parts water by weight.
[077] 25. The process of any of paragraphs 1 through 5 or 24, wherein
the
aqueous mixture of polysaccharides is added over a period of from about 0.1 to
about 40
hours.
[078] 26. The process of any of paragraphs 1 through 25, wherein the
aqueous
solution of mineral acid is stirred.
[079] 27. The process of any of paragraphs 1 through 26, wherein the
mixture is
heated for an additional period of time from about 0.1 hour to about 20 hours
at a temperature
range of from about 25 C to about 110 C.
-10-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[080] 28. The process of any of paragraphs 1 through 27, wherein the
mixture is
optionally cooled to ambient temperature.
[081] 29. The process of any of paragraph 1 through 28, further
comprising the
step of heating the mixture to a temperature of from about 25 C to about 160 C
to reduce any
residual glucose levels.
[082] 30. The process of either paragraphs 1 through 29, wherein the
aqueous
mixture comprises fructose and the levulinic acid is produced in greater than
about 65%
molar yield, optionally greater than about 75%, optionally greater than about
80%, optionally
greater than 85%, optionally greater than 90%.
[083] 31. The process of either paragraphs 1 through 29, wherein the
aqueous
mixture comprises glucose and the levulinic acid is produced in greater than
about 45% molar
yield, optionally greater than about 50%, optionally greater than about 55%,
optionally
greater than 60%, optionally greater than 65%.
[084] 32. The process of any of paragraphs 1 through 31, wherein any
remaining
fructose is not detected by liquid chromatography.
[085] 33. The process of any of paragraphs 1 through 32, wherein any
remaining
hydroxymethylfurfural is present at less than 0.5 weight percent in the
levulinic acid product.
[086] 34. The process of any of paragraphs 1 through 33, wherein ratio
of the
mass of levulinic acid to the mass of dry solids is greater than 1:1.
[087] 35. The process of any of paragraphs 1 through 34, wherein less
than 5
weight percent of dry char is produced relative to the entire weight of the
mixture.
[088] 36. The process of any of paragraphs 1 through 35, further comprising
filtering out solids from the mixture including levulinic acid to provide a
first filtrate. In one
embodiment, the filter is a candle filter, a Neutche filter, a basket
centrifuge, membrane
filters, or a cartridge filter.
[089] 37. The process of paragraph 36, wherein filtering is carried out
with filter
media having pore size less than 30 microns.
[090] 38. The process of paragraph 36, wherein filtering is carried out
with filter
media having pore size less than 20 microns.
[091] 39. The process of any of paragraphs 1 through 38, further comprising
combining the mixture comprising levulinic acid with an extraction solvent to
create an
extraction phase and a raffinate phase.
-11-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[092] 40. The process of paragraph 39, wherein the extraction solvent has a
partition coefficient for levulinic acid from water of at least 0.3,
optionally at least 0.5,
optionally at least 1.0, optionally at least 1.5, and optionally at least 2Ø
[093] 41. The process of paragraph 39 wherein the extraction solvent has a
partition
coefficient for formic acid from water of at least 0.3, optionally at least
0.5, optionally at least
1.0, optionally at least 1.5, optionally at least 2.0, optionally at least
5.0, optionally at least
7.0 and optionally at least 9Ø
[094] 42. The process of any of paragraphs 39 through 41 wherein the
extraction
solvent is selected from the group consisting of methyl iosamyl ketone, methyl
isobutyl
ketone, diisobutyl ketone, acetophenone, cyclohexanone, isophorone, neopentyl
alcohol,
isoamyl alcohol, n-hexanol, n-heptanol, 2-ethyl hexanol, n-octanol, 1-nonanol,
1-undecanol,
phenol, 4-methoxyphenol, guaiacol, 2-sec butyl phenol, nonyl phenol, methylene
chloride,
methyl isobutyl carbinol, anisol, ethylene glycol di-n-butyl ether, castor
oil, m-cresol, p-
cresol, o- cresol, cresol mixtures, 60/40 m-cresol/p-cresol, 75/25 m-cresol/p-
cresol, diethyl
carbonate, methyl salicylate, 2,4-dimethyl phenol and mixtures thereof
[095] 43. The process of any of paragraphs 39 through 42, further
comprising
recycling the raffinate phase to the reactor.
[096] 44. The process of paragraph 43, further comprising heating the
raffinate
phase from 120-180 C. In one embodiment, the method further comprises removing
any
additional solids that are formed, preferably by filtration.
[097] 45. The process of paragraph 44, further comprising cooling the
raffinate
phase to less than 110 C. In one embodiment, the method further comprises
removing any
additional solids that are formed, preferably by filtration.
[098] 46. The process of paragraph 45, further comprising adding high
fructose corn
syrup, a mixture of at least two different sugars, sucrose, an aqueous mixture
comprising
fructose, an aqueous mixture comprising fructose and glucose, an aqueous
mixture
comprising hydroxymethylfurfural, an aqueous solution of fructose and
hydroxymethylfurfural, an aqueous mixture of glucose, an aqueous mixture of
maltose, an
aqueous mixture of inulin, an aqueous mixture of polysaccharides, or mixtures
thereof to the
raffinate phase in the reactor over a period of time to form a mixture
including levulinic acid.
[099] 47. The process of any of paragraphs 39 through 46, further
comprising
separating levulinic acid, formic acid, or both from the extraction solvent.
[0100] 48. The process of any of paragraphs 36 through 47, wherein
the solids are
washed with water to provide a second filtrate and the first and second
filtrates are combined
-12-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
to form a final filtrate. In other embodiments the solids are washed with
water to provide a
second filtrate and the first and second filtrates are not combined.
[0101] 49. The process of any of paragraphs 1 through 48, wherein the
reactor is a
batch reactor.
[0102] 50. The process of any of paragraphs 1 through 48, wherein the
reactor is one
or more CSTRs.
[0103] 51. A process to prepare levulinic acid comprising the steps:
[0104] a) heating an aqueous solution of a mineral acid to about 60 C
to about
110 C; b) adding a first aqueous mixture comprising fructose and
glucose to the
heated aqueous mineral acid over a period of time to form a mixture including
levulinic acid;
[0105] c) optionally cooling the mixture to room temperature; and
[0106] d) heating the mixture, optionally in a sealed reactor, from
about 25 C to
about 160 C under pressure of 75 psi or below;
[0107] e) optionally cooling the heated mixture of step d) to room
temperature;
and
[0108] f) filtering the mixture to provide a first filtrate and
solids. In one
embodiment, the aqueous mixture comprising fructose and glucose are added to
the reaction
mixture over time comprise from about 0.1 to about 25, more specifically, from
about 1 to
about 20 and even more specifically from about 4 to about 15 percent by weight
of the final
mass of the reaction mixture. It is understood that as the sugar streams are
added to the
reactor, the sugar will continuously react with the mineral acid to form
levulinic acid and
other materials. Thus, the final reaction mixture may contain less than the
described ranges
of sugars. In another embodiment, the steady state concentration of the
aqueous mixture
comprising fructose and glucose in the reaction mixture is from about 0.1 to
about 25, more
specifically, from about 1 to about 20 and even more specifically from about 4
to about 15
percent by weight.
[0109] 52. The process of paragraph 51, wherein the mineral acid is
acid is
sulfuric acid (H2SO4), hydrochloric acid (HC1), hydrobromic acid (HBr) or
hydroiodic acid
(HI).
[0110] 53. The process of either paragraphs 51 or 52, wherein the
mineral acid
percentage by weight is from about 5 to about 80 percent of the mixture
including levulinic
acid.
-13-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0111] 54. The process of any of paragraphs 51 through 52, wherein the
solids can
be washed more than once to provide additional filtrates to be combined with
the first filtrate
to form a final filtrate.
[0112] 55. The process of any of paragraph 50 through 52, wherein the
final
filtrate is treated with a water immiscible solvent to form a water immiscible
layer and a
raffinate.
[0113] 56. The process of paragraph 55, wherein the water immiscible
layer is
separated from the aqueous layer and subjected to distillation.
[0114] 57. The process of any of paragraphs 55 or 56, wherein the
water
immiscible solvent is selected from the group consisting of methyl iosamyl
ketone, methyl
isobutyl ketone, diisobutyl ketone, acetophenone, cyclohexanone, isophorone,
neopentyl
alcohol, isoamyl alcohol, n-hexanol, n-heptanol, 2-ethyl hexanol, n-octanol, 1-
nonanol, 1-
undecanol, phenol, 4-methoxyphenol, guaiacol, 2-sec butyl phenol, nonyl
phenol, methylene
chloride, methyl isobutyl carbinol, anisol, ethylene glycol di-n-butyl ether,
castor oil, m-
cresol, p-cresol, o- cresol, cresol mixtures, 60/40 m-cresol/p-cresol, 75/25 m-
cresol/p-cresol,
diethyl carbonate, methyl salicylate, 2,4-dimethyl phenol and mixtures thereof
[0115] 58. The process of either of paragraphs 56 or 57, wherein the
distillation is
performed under vacuum to afford a levulinic acid product.
[0116] 59. The process of any of paragraphs 55 through 58, further
comprising the
steps:
[0117] a) combining the raffinate, and optionally a mineral acid with
water to
form a mixture comprising from about 5 to about 80% mineral acid;
[0118] b) heating the mixture to about 80 C to about 110 C;
[0119] c) adding a second aqueous solution of aqueous mixture of
fructose and
glucose to the mixture over a period of from about 0.1 to about 40 hours.
[0120] 60. The process of paragraph 59, wherein any of paragraphs 51
through 59
are repeated one or more times.
[0121] 61. An industrial process to process to prepare levulinic acid
comprising
the integrated steps of reaction, solids filtration, extraction, distillation,
and recycling of any
of paragraphs 1 through 60.
[0122] 62. The process of paragraph 51 further comprising the step of
adding a
filter aid to the reaction mixture prior to solids removal by filtration or
centrifugation.
[0123] 63. The process of any of paragraphs 51 through 62, wherein the
mixture
including levulinic acid is filtered through a 0.1 micron filter to about a 30
micron filter.
-14-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0124] 64. The process of any of paragraphs 51 through 63, wherein the
mixture
comprising levulinic acid is subjected to process conditions, wherein the
water, mineral acid,
the water immiscible solvent and optionally levulinic acid is recycled.
[0125] 65. The process of any of paragraphs 51 through 63 are carried out
in a batch
reactor.
[0126] 66. The process of any of paragraphs 51 through 63 are carried out
in a
CSTR.
[0127] 67. A process to prepare levulinic acid comprising the steps:
[0128] a) heating an aqueous solution of a mineral acid to about 60 C
to about
110 C;
[0129] b) adding high fructose corn syrup, a mixture of at least two
different
sugars, sucrose, an aqueous mixture comprising fructose, an aqueous mixture
comprising
fructose and glucose, an aqueous mixture comprising hydroxymethylfurfural, an
aqueous
solution of fructose and hydroxymethylfurfural, an aqueous mixture of glucose,
an aqueous
mixture of maltose, an aqueous mixture of inulin, an aqueous mixture of
polysaccharides, or
mixtures thereof to the heated aqueous mineral acid over a period of time to
form a reaction
mixture in a reactor to form a mixture including levulinic acid and solids;
[0130] c) filtering the solids from the mixture, optionally after
cooling;
[0131] d) adding a water immiscible liquid to the mixture so that the
mixture
forms first and second layers, wherein greater than 90% of the mineral acid is
in the first
layer and greater than 90% of the water immiscible liquid is in the second
layer;
[0132] e) recovering levulinic acid and optionally formic acid from
the second
layer; and
[0133] f) recycling the first layer back to the reactor. In one
embodiment, the
high fructose corn syrup, a mixture of at least two different sugars, sucrose,
an aqueous
mixture comprising fructose, an aqueous mixture comprising fructose and
glucose, an
aqueous mixture comprising hydroxymethylfurfural, an aqueous solution of
fructose and
hydroxymethylfurfural, an aqueous mixture of glucose, an aqueous mixture of
maltose, an
aqueous mixture of inulin, an aqueous mixture of polysaccharides, or mixtures
thereof are
added to the reaction mixture over time comprise from about 0.1 to about 25,
more
specifically, from about 1 to about 20 and even more specifically from about 4
to about 15
percent by weight of the final mass of the reaction mixture. It is understood
that as the sugar
streams are added to the reactor, the sugar will continuously react with the
mineral acid to
form levulinic acid and other materials. Thus, the final reaction mixture may
contain less
-15-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
than the described ranges of sugars. In another embodiment, the steady state
concentration of
the high fructose corn syrup, a mixture of at least two different sugars,
sucrose, an aqueous
mixture comprising fructose, an aqueous mixture comprising fructose and
glucose, an
aqueous mixture comprising hydroxymethylfurfural, an aqueous solution of
fructose and
hydroxymethylfurfural, an aqueous mixture of glucose, an aqueous mixture of
maltose, an
aqueous mixture of inulin, an aqueous mixture of polysaccharides, or mixtures
thereof in the
reaction mixture is from about 0.1 to about 25, more specifically, from about
1 to about 20
and even more specifically from about 4 to about 15 percent by weight
[0134] 68. The process of paragraph 67, further comprising heating the
first layer
from about 120 C to about 180 C for a period of time.
[0135] 69. The process of paragraph 68, further comprising cooling the
first layer to
below 100 C.
[0136] 70. The process of any of paragraphs 67 through 69, wherein the
mineral
acid is selected from the group consisting of sulfuric acid, hydrochloric
acid, hydrobromic
acid, hydroiodide and combinations thereof.
[0137] 71. The process of paragraph 70, wherein the mineral acid is
sulfuric acid.
[0138] 72. The process of any of paragraphs 67 through 71, wherein the
mineral acid
percentage by weight is from about 5 to about 80 percent of the reaction
mixture.
[0139] 73. The process of paragraph 72, wherein the mineral acid
percentage by
weight is from about 20 to about 80 percent of the reaction mixture.
[0140] 74. The process of paragraph 72, wherein the mineral acid
percentage by
weight is from about 20 to about 50 percent of the reaction mixture.
[0141] 75. The process of paragraph 71, wherein the mineral acid
percentage by
weight is from about 40 to about 80 percent of the reaction mixture.
[0142] 76. The process of any of paragraphs 67 through 75, wherein the
first layer is
heated for a period of time sufficient to convert greater than 90% of any
glucose into
levulinic acid.
[0143] 77. The process of any of paragraphs 67 through 76, wherein the
water
immiscible liquid is selected from the group consisting of methyl iosamyl
ketone, methyl
isobutyl ketone, diisobutyl ketone, acetophenone, cyclohexanone, isophorone,
neopentyl
alcohol, isoamyl alcohol, n-hexanol, n-heptanol, 2-ethyl hexanol, n-octanol, 1-
nonanol, 1-
undecanol, phenol, 4-methoxyphenol, guaiacol, 2-sec butyl phenol, nonyl
phenol, methylene
chloride, methyl isobutyl carbinol, anisol, ethylene glycol di-n-butyl ether,
castor oil, m-
-16-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
cresol, p-cresol, o- cresol, cresol mixtures, 60/40 m-cresol/p-cresol, 75/25 m-
cresol/p-cresol,
diethyl carbonate, methyl salicylate, 2,4-dimethyl phenol and mixtures thereof
[0144] 78. The process of any of paragraphs 67 through 77, wherein the
high
fructose corn syrup, a mixture of at least two different sugars, sucrose, an
aqueous mixture
comprising fructose, an aqueous mixture comprising fructose and glucose, an
aqueous
mixture comprising hydroxymethylfurfural, an aqueous solution of fructose and
hydroxymethylfurfural, an aqueous mixture of glucose, an aqueous mixture of
maltose, an
aqueous mixture of inulin, an aqueous mixture of polysaccharides, or mixtures
added over a
period of from about 0.1 to about 40 hours.
[0145] 79. The process of any of paragraphs 67 through 78, wherein the
mixture
is heated for an additional period of time from about 0.1 hour to about 20
hours at a
temperature range of from about 25 C to about 110 C.
[0146] 80. The process of either paragraphs 67 through 79, wherein the
mixture
comprises fructose and the levulinic acid is produced in greater than about
65% molar yield,
optionally greater than about 75%, optionally greater than about 80%,
optionally greater than
85%, optionally greater than 90%.
[0147] 81. The process of either paragraphs 67 through 79, wherein the
aqueous
mixture comprises glucose and the levulinic acid is produced in greater than
about 45% molar
yield, optionally greater than about 50%, optionally greater than about 55%,
optionally
greater than 60%, optionally greater than 65%.
[0148] 82. The process of any of paragraphs 67 through 81, wherein the
mass of
levulinic acid to the mass of solids ratio is greater than 1:1 .
[0149] 83. The process of any of paragraphs 67 through 82, wherein
less than 5
weight percent of dry char is produced relative to the entire weight of the
mixture.
[0150] 84. The process of any of paragraphs 67 through 83, wherein the
solids
that are formed do not adhere to glass, Teflon or metal surfaces.
[0151] 85. The process of paragraph 84, wherein the metal surface is a
hastelloy
metal surface, alloy 20 metal surface, alloy 2205 metal surface, AL6XN metal
surface or
zirconium metal surface.
[0152] 86. The process of any of paragraphs 67 through 85, wherein the
reactor is
batch reactor.
[0153] 87. The process of any of paragraphs 67 through 85, wherein the
reactor is a
CSTR.
-17-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0154] The following paragraphs also provide for various aspects of the
present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
process to prepare levulinic acid or 5-(hydroxylmethyl)furfural, comprising
the steps:
mixing biomass with an aqueous portion, a water immiscible portion, and an
acid to form a mixture; and
heating the mixture to a temperature of from about 50 C to about 280 C to
provide levulinic acid or 5-(hydroxymethyl)furfural in the water immiscible
portion.
[0155] la. The process of paragraph 1, wherein the mixture is heated
from about
80 C to about 250 C.
[0156] lb. The process of paragraph 1, wherein the mixture is heated
from about
100 C to about 220 C.
[0157] lc. The process of paragraph 1, wherein the mixture is heated from
about
50 C to about 100 C.
[0158] ld. The process of paragraph 1, wherein the mixture is heated from
about
50 C to about 90 C.
[0159] le. The process of paragraph 1, wherein the mixture is heated from
about
50 C to about 80 C.
[0160] lf. The process of paragraph 1, wherein the mixture is heated from
about
60 C to about 80 C.
[0161] 2. The process of paragraph 1, wherein the mixture is heated to
reflux
conditions.
[0162] 3. The process of any of paragraphs 1 through 2, wherein the
mixture is
heated under pressure, wherein the pressure range is from about 10 psi to
about 1000 psi.
[0163] 3a. The process of paragraph 3, wherein the pressure range of
from about
30 to about 500 psi.
[0164] 3b. The process of paragraph 3, wherein the pressure range if
from about
50 to about 200 psi.
[0165] 4. The process of any of paragraphs 1 through 3b, further
comprising the
step of mixing the mixture.
[0166] 5. The process of any of paragraphs 1 through 4, further
comprising the
step of cooling the mixture after the mixture is heated.
[0167] 6. The process of any of paragraphs 1 through 5, further
comprising the
step of separating the water immiscible portion containing the levulinic acid
or the 5-
(hydroxymethyl)furfural from the aqueous portion.
-18-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0168] 7. The process of paragraph 6, further comprising the step of
removing
the water immiscible portion from the levulinic acid or 5-
(hydroxymethyl)furfural.
[0169] 8. The process of paragraph 7, wherein the water immiscible
portion is
removed by distillation to provide a reaction material containing the
levulinic acid or 5-
(hydroxymethyl)furfural.
[0170] 9. The process of paragraph 8, further comprising the step of
treating the
reaction material with a solid sorbent.
[0171] 9a. The process of paragraph 9, wherein the solid sorbent
is/are pieces of
wood, an ion exchange resin, optionally with a solvent, molecular sieves,
optionally with a
solvent, or activated carbon, optionally with a solvent.
[0172] 10. The process of either paragraph 9 or 9a, further comprising
the steps of
removing the levulinic acid or 5-(hydroxymethyl)furfural from the solid
sorbent by heat,
pressure, or by rinsing with water, aqueous base or a polar solvent.
[0173] 11. The process of any of paragraphs 1 through 10, wherein the
biomass
comprises sludges from paper manufacturing process; agricultural residues;
bagasse pity;
bagasse; molasses; aqueous oak wood extracts; rice hull; oats residues; wood
sugar slops; fir
sawdust; naphtha; corncob furfural residue; cotton balls; raw wood flour;
rice; straw; soybean
skin; soybean oil residue; corn husks; cotton stems; cottonseed hulls; starch;
potatoes; sweet
potatoes; lactose; sunflower seed husks; sugar; corn syrup; hemp; waste paper;
wastepaper
fibers; sawdust; wood; residue from agriculture or forestry; organic
components of municipal
and industrial wastes; waste plant materials from hard wood or beech bark;
fiberboard
industry waste water; post-fermentation liquor; furfural still residues; and
combinations
thereof, a C5 sugar, a C6 sugar, a lignocelluloses, cellulose, starch, a
polysaccharide, a
disaccharide, a monosaccharide or mixtures thereof.
[0174] 12. The process of any of paragraph 11, wherein the biomass is
fructose,
sucrose, glucose or a mixture thereof
[0175] 13. The process of any of paragraphs 1 through 12, wherein the
acid is a
mineral acid.
[0176] 14. The process of paragraph 13, wherein the mineral acid is
sulfuric acid,
phosphoric acid, hydrochloric acid or mixtures thereof
[0177] 15. The process of either paragraph 13 or 14, wherein the
concentration of
mineral acid is from about 1 percent to about 75 percent by weight of the
mixture.
-19-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0178] 16. The process of paragraph 15, wherein the concentration of
the mineral
acid is from about 5 percent to about 60 percent by weight of the mixture,
more particularly
from about 20 weight percent to about 50 weight percent.
[0179] 17. The process of any of paragraphs 1 through 12, wherein the
acid is an
organic sulfonic acid.
[0180] 18. The process of paragraph 17, wherein the organic acid is
para-toluene
sulfonic acid, naphthalene sulfonic acid, camphor sulfonic acid or n-
dodecylbenzene sulfonic
acid.
[0181] 19. The process of any of paragraphs 1 through 18, further
comprising
adding a phase transfer catalyst to the mixture.
[0182] 20. The process of paragraph 19, wherein the phase transfer
catalyst is an
ammonium salt, a heterocyclic ammonium salt or a phosphonium salt.
[0183] 21. The process of any of paragraphs 1 through 20, wherein the
water
immiscible portion is methyl isobutyl ketone, ethyl levulinate, butyl
levulinate,
cyclohexanone, toluene, methyl-THF, methyl-tertiary butyl ether, methyl
isoamyl ketone,
hexane, cyclohexane, chloro-benzene, methylene chloride, dichloroethane, ortho-
dichlorobenzene, diisobutyl ketone, 2,6-dimethyl cyclohexanone,
tetrahydrofuran or mixtures
thereof
[0184] 22. The process of any of paragraphs 1 through 21, wherein the
process is
conducted in a continuously-stirred tank reactor (CSTR) or a plug-flow reactor
(PFR).
[0185] 23. The process of paragraph 22, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to biomass is added to the
reactor over a
period of 1 hour and an equivalent weight amount is removed during the same
time period.
[0186] 24. The process of paragraph 23, wherein the biomass is
fructose.
[0187] 25. The process of either paragraphs 23 or 24, wherein a ratio
of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of 1 hour and an
equivalent weight amount is removed during the same time period.
[0188] 26. The process of paragraph 22, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to biomass is added to the
reactor over a
period of time t and an equivalent weight amount is removed during the same
time period t.
[0189] 27. The process of paragraph 26, wherein the biomass is
fructose.
[0190] 28. The process of either paragraphs 26 or 27, wherein a ratio
of about
10:1 to about 15:1 water to mineral acid is added to the reactor over the
period of time t and
an equivalent weight amount is removed during the same time period.
-20-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0191] The process of any of paragraphs 1 through 21, wherein the process
is
conducted in a continuous addition batch reactor.
[0192] 30. The process of paragraph 29, wherein the continuous
addition batch
process is conducted wherein a ratio of about 2:1 to about 5:1 water to
biomass is added to
the reactor over a period of 1 hour.
[0193] 31. The process of paragraph 30, wherein the biomass is
fructose.
[0194] 32. The process of either paragraphs 30 or 31, wherein a ratio
of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of 1 hour.
[0195] 33. The process of paragraph 29, wherein the continuous addition
batch
process is conducted wherein a ratio of about 2:1 to about 5:1 water to
biomass is added to
the reactor over a period of time t.
[0196] 34. The process of paragraph 33, wherein the biomass is
fructose.
[0197] 35. The process of either paragraphs 33 or 34, wherein a ratio
of about
10:1 to about 15:1 water to mineral acid is added to the reactor over the
period of time t.
[0198] The following paragraphs provide for additional aspects of the
present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
process to prepare levulinic acid or formic acid, comprising the steps:
mixing up to 50 weight percent of a fructose containing material comprising
fructan, fructooligosaccharide, inulin, fructose, fructose-glucose blended
corn syrup, sucrose
or mixtures thereof, up to 75 weight percent of an acid catalyst and at least
20 weight percent
water to equal 100 weight percent to form a mixture; and
heating the mixture to a temperature of from about 50 C to about 280 C to
provide levulinic acid or formic acid.
[0199] la. The process of paragraph 1, wherein the mixture is heated
from about
80 C to about 250 C.
[0200] lb. The process of paragraph 1, wherein the mixture is heated
from about
100 C to about 220 C.
[0201] lc. The process of paragraph 1, wherein the mixture is heated
from about
50 C to about 100 C.
[0202] ld. The process of paragraph 1, wherein the mixture is heated
from about
50 C to about 90 C.
[0203] le. The process of paragraph 1, wherein the mixture is heated
from about
50 C to about 80 C.
-21-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0204] lf The process of paragraph 1, wherein the mixture is heated
from about
60 C to about 80 C.
[0205] 2. The process of any of paragraphs 1 through 1 f, wherein the
mixture is
heated under pressure, wherein the pressure range is from about 10 psi to
about 1000 psi.
[0206] 2a. The process of paragraph 2, wherein the pressure range of
from about
30 to about 500 psi.
[0207] 2b. The process of paragraph 2, wherein the pressure range if
from about
50 to about 200 psi.
[0208] 3. The process of any of paragraphs 1 through 2b, wherein the
acid is
present from about 10 weight percent to about 40 weight percent.
[0209] 3a. The process of any of paragraphs 1 through 3, wherein the
acid is
present from about 20 weight percent to about 30 weight percent.
[0210] 4. The process of any of paragraphs 1 through 3a, wherein the
mixture is
heated for 60 minutes or less.
[0211] 5. The process of paragraph 4, wherein the mixture is heated
for 30
minutes or less.
[0212] 6. The process of any of paragraphs 1 through 5, further
comprising the
step of mixing the mixture.
[0213] 7. The process of any of paragraphs 1 through 6, further
comprising the
step of cooling the mixture after the mixture is heated.
[0214] 8. The process of any of paragraphs 1 through 7, further
comprising the
step of isolating the levulinic acid or the formic acid from solid humin by-
product.
[0215] 9. The process of paragraph 8, further comprising the step of
treating the
humin by-product with a solvent to provide a filtrate.
[0216] 10. The process of paragraph 9, wherein the solvent is water,
methylisobutyl ketone, methyl-THF, cyclohexanone, acetonitrile, acetone,
methanol, ethanol,
butanol, MTBE or mixtures thereof
[0217] 11. The process of either paragraph 9 or 10, wherein the
isolated levulinic
acid or formic acid and the filtrate are combined to provide a final filtrate.
[0218] 12. The process of paragraph 11, wherein the molar yield of
levulinic acid
is from about 50% to about 90%.
[0219] 13. The process of paragraph 11, wherein the molar yield of
formic acid is
from about 50% to about 90%.
-22-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0220] 14. The process of any of paragraphs 1 through 13, further
comprising the
step of treating the mixture with a solid sorbent.
[0221] 14a. The process of paragraph 14, wherein the solid sorbent is/are
pieces of
wood, an ion exchange resin, optionally with a solvent, molecular sieves,
optionally with a
solvent, or activated carbon, optionally with a solvent.
[0222] 15. The process of either paragraph 14 or 14a, further
comprising the steps
of removing the levulinic acid or 5-(hydroxymethyl)furfural from the solid
sorbent by heat,
pressure, or by rinsing with water, aqueous base or a polar solvent.
[0223] 16. The process of any of paragraphs 1 through 15, wherein the
acid is a
mineral acid.
[0224] 17. The process of paragraph 16, wherein the mineral acid is
sulfuric acid,
phosphoric acid, hydrochloric acid or mixtures thereof
[0225] 18. The process of any of paragraphs 1 through 17, wherein the
process is
conducted in a continuously-stirred tank reactor (CSTR) or a plug-flow reactor
(PFR).
[0226] 19. The process of paragraph 18, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to fructose or sugar is added
to the reactor
over a period of 1 hour and an equivalent weight amount is removed during the
same time
period.
[0227] 20. The process of paragraph 19, wherein a ratio of about 10:1
to about
15:1 water to mineral acid is added to the reactor over a period of 1 hour and
an equivalent
weight amount is removed during the same time period.
[0228] 21. The process of paragraph 18, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to fructose or sugar is added
to the reactor
over a period of time t and an equivalent weight amount is removed during the
same time
period.
[0229] 22. The process of paragraph 21, wherein a ratio of about 10:1
to about
15:1 water to mineral acid is added to the reactor over the period of time t
and an equivalent
weight amount is removed during the same time period.
[0230] The following paragraphs provide for additional aspects of the
present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
process to prepare levulinic acid or formic acid, comprising the steps:
mixing a biomass material with an acid catalyst or supercritical water to form
a first mixture, wherein the biomass is converted to provide glucose;
-23-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
treating the glucose with an isomerization catalyst or a base catalyst to form
a
second mixture, wherein the glucose is converted into fructose;
mixing the fructose containing mixture with an acid and water form a third
mixture; and
heating the third mixture to a temperature of from about 50 C to about 280 C
to provide levulinic acid or formic acid.
[0231] la. The process of paragraph 1, wherein the biomass comprises
sludges
from paper manufacturing process; agricultural residues; bagasse pity;
bagasse; molasses;
aqueous oak wood extracts; rice hull; oats residues; wood sugar slops; fir
sawdust; naphtha;
corncob furfural residue; cotton balls; raw wood flour; rice; straw; soybean
skin; soybean oil
residue; corn husks; cotton stems; cottonseed hulls; starch; potatoes; sweet
potatoes; lactose;
sunflower seed husks; sugar; corn syrup; hemp; waste paper; wastepaper fibers;
sawdust;
wood; residue from agriculture or forestry; organic components of municipal
and industrial
wastes; waste plant materials from hard wood or beech bark; fiberboard
industry waste water;
post-fermentation liquor; furfural still residues; and combinations thereof, a
C5 sugar, a C6
sugar, a lignocelluloses, cellulose, starch, a polysaccharide, a disaccharide,
a monosaccharide
or mixtures thereof.
[0232] lb. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 80 C to about 250 C.
[0233] lc. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 100 C to about 220 C.
[0234] ld. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 100 C.
[0235] le. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 90 C.
[0236] lf. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 80 C.
[0237] lg. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 60 C to about 80 C.
[0238] 2. The process of any of paragraphs 1 through lg, wherein the
mixture is
heated under pressure, wherein the pressure range is from about 10 psi to
about 1000 psi.
[0239] 2a. The process of paragraph 2, wherein the pressure range of
from about
30 to about 500 psi.
-24-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0240] 2b. The process of paragraph 2, wherein the pressure range if
from about
50 to about 200 psi.
[0241] 3. The process of any of paragraphs 1 through 2b, wherein the
biomass
converting catalyst is hydrochloric acid, sulfuric acid, triflic acid,
trifluoroacetic acid or
mixtures thereof
[0242] 4. The process of any of paragraphs 1 through 3, wherein the
glucose
isomerization catalyst is glucoisomerase.
[0243] 5. The process of any of paragraphs 1 through 4, wherein the
glucose
converting base catalyst is a basic alkali or alkaline earth metal hydroxide
or carbonate.
[0244] 6. The process of any of paragraphs 1 through 5, wherein the
third
mixture contains about 0.1 to about 30 weight percent of a fructose containing
material.
[0245] 7. The process of paragraph 6, wherein the fructose containing
material
comprises fructan, fructooligosaccharide, inulin, fructose, fructose corn
syrup or mixtures
thereof
[0246] 8. The process of paragraph 7, wherein the fructose containing
material is
present from about 1 to about 99 weight percent.
[0247] 9. The process of paragraph 1, wherein the third mixture
contains up to
50 weight percent of the acid.
[0248] 10. The process of paragraph 9, wherein the acid is present
from about 2 to
about 40 weight percent.
[0249] 11. The process of paragraph 10, wherein the acid is a mineral
acid.
[0250] 12. The process of paragraph 11, wherein the mineral acid is
sulfuric acid,
phosphoric acid, hydrochloric acid or mixtures thereof
[0251] 13. The process of any of paragraphs 1 through 12, wherein the
third
mixture is heated for 60 minutes or less.
[0252] 14. The process of paragraph 13, wherein the mixture is heated
for 30
minutes or less.
[0253] 15. The process of any of paragraphs 1 through 14, further
comprising the
step of mixing one or more of the mixtures.
[0254] 16. The process of any of paragraphs 1 through 15, further
comprising the
step of cooling the third mixture after the mixture is heated.
[0255] 17. The process of any of paragraphs 1 through 16, further
comprising the
step of isolating the levulinic acid or the formic acid from solid humin by-
product.
-25-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0256] 18. The process of paragraph 17, further comprising the step
of treating the
humin by-product with a solvent to provide a filtrate.
[0257] 19. The process of paragraph 18, wherein the solvent is
water,
methylisobutyl ketone, methyl-THF, cyclohexanone, acetonitrile, acetone,
methanol, ethanol,
butanol, MTBE or mixtures thereof
[0258] 20. The process of either paragraph 18 or 19, wherein the
isolated levulinic
acid or formic acid and the filtrate are combined to provide a final filtrate.
[0259] 21. The process of paragraph 20, wherein the molar yield of
levulinic acid
is from about 50% to about 90%.
[0260] 22. The process of paragraph 20, wherein the molar yield of
formic acid is
from about 50% to about 90%.
[0261] 23. The process of any of paragraphs 1 through 22, wherein
the process is
conducted in a continuously-stirred tank reactor (CSTR) or a plug-flow reactor
(PFR).
[0262] 24. The process of paragraph 23, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to biomass is added to the
reactor over a
period of 1 hour and an equivalent weight amount is removed during the same
time period.
[0263] 25. The process of paragraph 24, wherein the biomass
comprises fructose.
[0264] 26. The process of either paragraphs 24 or 25, wherein a
ratio of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of 1 hour and an
equivalent weight amount is removed during the same time period.
[0265] 27. The process of paragraph 23, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to biomass is added to the
reactor over a
period of time t and an equivalent weight amount is removed during the same
time period.
[0266] 28. The process of paragraph 27, wherein the biomass
comprises fructose.
[0267] 29. The process of either paragraphs 26 or 27, wherein a
ratio of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of time t and an
equivalent weight amount is removed during the same time period.
[0268] 30. The process of any of paragraphs 1 through 29, further
comprising the
step of treating the final filtrate with a solid sorbent.
[0269] 31. The process of paragraph 30, wherein the solid sorbent
is/are pieces of
wood, an ion exchange resin, optionally with a solvent, molecular sieves,
optionally with a
solvent, or activated carbon, optionally with a solvent.
-26-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0270] 32. The process of either paragraph 30 or 31, further
comprising the steps
of removing the levulinic acid or formic acid from the solid sorbent by heat,
pressure, or by
rinsing with water, aqueous base or a polar solvent.
[0271] The following paragraphs provide for additional aspects of the
present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
continuous process for producing levulinic acid from a biomass using a first
reactor having an
entrance and an exit and a second reactor having an entrance and an exit said
process
comprising,
continuously supplying a sample containing said biomass to said first reactor
through
said entrance to said first reactor,
hydrolyzing said biomass in said first reactor at between 210 C and 230 C for
between 10 seconds and 100 seconds in the presence of a water immiscible
liquid and
mineral acid comprising between 1% and 5% by weight of said sample to produce
hydroxymethylfurfural and other reaction intermediates,
continuously removing an intermediate sample containing said
hydroxymethylfurfural
and other reaction intermediates from said first reactor through said exit of
said first reactor
in such a manner that substantially no axial mixing occurs in said first
reactor,
continuously supplying the intermediate sample that has been removed from said
first
reactor to said second reactor through said entrance to said second reactor,
hydrolyzing said hydroxymethylfurfural and other reaction intermediates in
said
intermediate sample in said second reactor at between 195 C and 215 C for
between 15
minutes and 30 minutes in the presence of, optionally a water immiscible
liquid, and a
mineral acid comprising between 3% and 7.5% by weight of said intermediate
sample to
produce levulinic acid, and
continuously removing levulinic acid from said second reactor through said
exit of
said second reactor, wherein the yield of levulinic acid removed from said
second reactor
comprises at least 60% of the theoretical yield.
[0272] 2. The process of paragraph 1, wherein the biomass comprises
sludges
from paper manufacturing process; agricultural residues; bagasse pity;
bagasse; molasses;
aqueous oak wood extracts; rice hull; oats residues; wood sugar slops; fir
sawdust; naphtha;
corncob furfural residue; cotton balls; raw wood flour; rice; straw; soybean
skin; soybean oil
residue; corn husks; cotton stems; cottonseed hulls; starch; potatoes; sweet
potatoes; lactose;
sunflower seed husks; sugar; corn syrup; hemp; waste paper; wastepaper fibers;
sawdust;
wood; residue from agriculture or forestry; organic components of municipal
and industrial
-27-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
wastes; waste plant materials from hard wood or beech bark; fiberboard
industry waste water;
post-fermentation liquor; furfural still residues; and combinations thereof, a
C5 sugar, a C6
sugar, a lignocelluloses, cellulose, starch, a polysaccharide, a disaccharide,
a monosaccharide
or mixtures thereof.
[0273] 3. The process of any of paragraphs 1 through 20, wherein the
water
immiscible liquid is methyl isobutyl ketone, ethyl levulinate, butyl
levulinate, cyclohexanone,
toluene, methyl-THF, methyl-tertiary butyl ether, methyl isoamyl ketone,
hexane,
cyclohexane, chloro-benzene, methylene chloride, dichloroethane, ortho-
dichlorobenzene,
diisobutyl ketone, 2,6-dimethyl cyclohexanone, tetrahydrofuran or mixtures
thereof
[0274] 4. The process of any of paragraphs 1 through 3, further
comprising the
step of treating the hydroxymethylfurfural from the first reactor or the
levulinic acid from the
second reactor with a solid sorbent.
[0275] 5. The process of paragraph 4, wherein the solid sorbent is/are
pieces of
wood, an ion exchange resin, optionally with a solvent, molecular sieves,
optionally with a
solvent, or activated carbon, optionally with a solvent.
[0276] 6. The process of either paragraph 4 or 5, further comprising
the steps of
removing the levulinic acid or formic acid from the solid sorbent by heat,
pressure, or by
rinsing with water, aqueous base or a polar solvent.
[0277] The following paragraphs provide for still an additional aspect of
the present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
process for producing formic acid from a carbohydrate-containing material, the
process
comprising: introducing a carbohydrate-containing material to a first reactor;
hydrolyzing the
carbohydrate-containing material in the first reactor in the presence of a
water immiscible
liquid and a mineral acid for a first time period at a first temperature and a
first pressure
effective to form an intermediate hydrolysate comprising one or more sugars;
transferring the
intermediate hydrolysate from the first reactor to a second reactor;
hydrolyzing the
intermediate hydrolysate in the second reactor for a second time period at a
second
temperature less than 195 degrees C and a second pressure effective to form a
hydrolysate
product comprising formic acid; and isolating the formic acid in a vapor from
the hydrolysate
product.
[0278] The following paragraphs provide for still additional aspects of
the present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
process to prepare levulinic acid or formic acid, comprising the steps:
-28-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
mixing a biomass material with an acid catalyst or supercritical water to form
a first mixture, wherein the biomass is converted to provide glucose;
treating the glucose with an isomerization catalyst or a base catalyst to form
a
second mixture, wherein the glucose is converted into fructose;
mixing the fructose containing mixture with an acid and water form a third
mixture;
heating the third mixture at a temperature of from about 50 C to about 280 C;
cooling the third mixture; and
treating the third mixture with an water immiscible solvent to form an aqueous
layer and a water immiscible layer, providing levulinic acid or formic acid in
the water
immiscible layer.
[0279] la. The process of paragraph 1, wherein the biomass comprises
sludges
from paper manufacturing process; agricultural residues; bagasse pity;
bagasse; molasses;
aqueous oak wood extracts; rice hull; oats residues; wood sugar slops; fir
sawdust; naphtha;
corncob furfural residue; cotton balls; raw wood flour; rice; straw; soybean
skin; soybean oil
residue; corn husks; cotton stems; cottonseed hulls; starch; potatoes; sweet
potatoes; lactose;
sunflower seed husks; sugar; corn syrup; hemp; waste paper; wastepaper fibers;
sawdust;
wood; residue from agriculture or forestry; organic components of municipal
and industrial
wastes; waste plant materials from hard wood or beech bark; fiberboard
industry waste water;
post-fermentation liquor; furfural still residues; and combinations thereof, a
C5 sugar, a C6
sugar, a lignocelluloses, cellulose, starch, a polysaccharide, a disaccharide,
a monosaccharide
or mixtures thereof.
[0280] lb. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 80 C to about 250 C.
[0281] lc. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 100 C to about 220 C.
[0282] ld. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 100 C.
[0283] le. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 90 C.
[0284] 1 f. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 80 C.
[0285] lg. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 60 C to about 80 C.
-29-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0286] 2. The process of any of paragraphs 1 through lg, wherein the
mixture is
heated under pressure, wherein the pressure range is from about 10 psi to
about 1000 psi.
[0287] 2a. The process of paragraph 2, wherein the pressure range of
from about
30 to about 500 psi.
[0288] 2b. The process of paragraph 2, wherein the pressure range if
from about
50 to about 200 psi.
[0289] 3. The process of any of paragraphs 1 through 2b, wherein the
biomass
converting catalyst is hydrochloric acid, sulfuric acid, triflic acid,
trifluoroacetic acid or
mixtures thereof
[0290] 4. The process of paragraph 1, wherein glucose converting
isomerization
catalyst is glucoisomerase.
[0291] 5. The process of paragraph 1, wherein the glucose converting
catalyst is
a basic alkali or alkaline earth metal hydroxide or carbonate.
[0292] 6. The process of paragraph 1, wherein the third mixture
contains about
0.1 weight percent to about 30 weight percent of a fructose containing
material.
[0293] 7. The process of paragraph 6, wherein the fructose containing
material
comprises fructan, fructooligosaccharide, inulin, fructose, fructose corn
syrup or mixtures
thereof
[0294] 8. The process of paragraph 7, wherein the fructose containing
material is
present from about 1 to about 99 weight percent.
[0295] 9. The process of paragraph 1, wherein the third mixture
contains up to
50 weight percent of the acid.
[0296] 10. The process of paragraph 9, wherein the acid is present
from about 2 to
about 40 weight percent.
[0297] 11. The process of paragraph 10, wherein the acid is a mineral
acid.
[0298] 12. The process of paragraph 11, wherein the mineral acid is
sulfuric acid,
phosphoric acid, hydrochloric acid or mixtures thereof
[0299] 13. The process of any of paragraphs 1 through 12, wherein the
third
mixture is heated for 60 minutes or less.
[0300] 14. The process of paragraph 13, wherein the mixture is heated
for 30
minutes or less.
[0301] 15. The process of any of paragraphs 1 through 14, further
comprising the
step of mixing one or more of the mixtures.
-30-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0302] 16. The process of any of paragraphs 1 through 15, further
comprising the
step of isolating the levulinic acid or the formic acid from solid humin by-
product.
[0303] 17. The process of paragraph 16, wherein the isolation step
is filtration.
[0304] 18. The process of paragraph 17, further comprising the step
of treating the
humin by-product with a solvent to provide a filtrate.
[0305] 19. The process of paragraph 18, wherein the solvent is
water,
methylisobutyl ketone, methyl-THF, cyclohexanone, acetonitrile, acetone,
methanol, ethanol,
butanol, MTBE or mixtures thereof
[0306] 20. The process of either paragraph 18 or 19, wherein the
isolated levulinic
acid or formic acid and the filtrate are combined to provide a final filtrate.
[0307] 21. The process of paragraph 20, wherein the molar yield of
levulinic acid
is from about 50% to about 90%.
[0308] 22. The process of paragraph 20, wherein the molar yield of
formic acid is
from about 50% to about 90%.
[0309] 23. The process of any of paragraph 1 through 22, wherein the
water
immiscible solvent is methyl isobutyl ketone, ethyl levulinate, butyl
levulinate,
cyclohexanone, toluene, methyl-THF, methyl-tertiary butyl ether, methyl
isoamyl ketone,
hexane, cyclohexane, chloro-benzene, methylene chloride, dichloroethane, ortho-
dichlorobenzene, diisobutyl ketone, 2,6-dimethyl cyclohexanone,
tetrahydrofuran or mixtures
thereof
[0310] 24. The process of any of paragraphs 1 through 23, wherein
the aqueous
layer or final filtrate and water immiscible layers are separated.
[0311] 25. The method of any of paragraphs 1 through 24, further
comprising the
step of concentrating the water immiscible layer containing the levulinic acid
or formic acid
to provide a concentrate.
[0312] 26. The method of paragraph 25, wherein the concentration
step is
conducted under reduced pressure.
[0313] 27. The method of paragraph 26, wherein the concentration
step is
conducted at an elevated temperature.
[0314] 28. The method of paragraphs 25 or 26, wherein the water
immiscible
layer is agitated.
[0315] 29. The method of any of paragraphs 26 through 28, wherein
the reduced
pressure is from about 10 to about 700 torr.
-31-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0316] 30. The method of any of paragraphs 26 through 29, wherein the
water
immiscible layer was heated to about 20 to about 140 C.
[0317] 35. The process of any of paragraphs 25 through 30, further
comprising the
step of subjecting the concentrate to wipe film evaporation to provide
purified levulinic acid.
[0318] 36. The process of paragraph 35, wherein the levulinic acid had
a purity of
at least 95%.
[0319] 37. The process of any of paragraphs 25 through 30, further
comprising the
step of treating the concentrate with a solid sorbent.
[0320] 38. The process of paragraph 37, wherein the solid sorbent
is/are pieces of
wood, an ion exchange resin, optionally with a solvent, molecular sieves,
optionally with a
solvent, or activated carbon, optionally with a solvent.
[0321] 39. The process of either paragraph 37 or 38, further
comprising the steps
of removing the levulinic acid or formic acid from the solid sorbent by heat,
pressure, or by
rinsing with water, aqueous base or a polar solvent.
[0322] 40. The process of any of paragraphs 1 through 24, wherein the
process is
conducted in a continuously-stirred tank reactor (CSTR) or a plug-flow reactor
(PFR).
[0323] 41. The process of paragraph 40, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to biomass is added to the
reactor over a
period of 1 hour and an equivalent weight amount is removed during the same
time period.
[0324] 42. The process of paragraph 41, wherein the biomass is
fructose.
[0325] 43. The process of either paragraphs 41 or 42, wherein a ratio
of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of 1 hour and an
equivalent weight amount is removed during the same time period.
[0326] 44. The process of paragraph 40, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to biomass is added to the
reactor over a
period of time t and an equivalent weight amount is removed during the same
time period.
[0327] 45. The process of paragraph 44, wherein the biomass is
fructose.
[0328] 46. The process of either paragraphs 44 or 46, wherein a ratio
of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of time t and an
equivalent weight amount is removed during the same time period.
[0329] The following paragraphs provide for still further additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), the present
invention provides
a process to prepare levulinic acid or formic acid, comprising the steps:
-32-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
mixing sucrose, glucose, fructose containing material or mixtures thereof with
and water to form a mixture,
heating the mixture at a temperature of from about 50 C to about 280 C;
cooling the mixture to provide an aqueous portion and solids;
isolating the aqueous portion from the solids; and
treating the aqueous portion with an water immiscible solvent to form an
aqueous layer and a water immiscible layer, providing levulinic acid or formic
acid in the
water immiscible layer.
[0330] la. The process of paragraph 1, wherein the mixture is heated
from about
80 C to about 250 C.
[0331] lb. The process of paragraph 1, wherein the mixture is heated
from about
100 C to about 220 C.
[0332] 1 c. The process of paragraph 1, wherein the mixture is heated
from about
50 C to about 100 C.
[0333] ld. The process of paragraph 1, wherein the mixture is heated
from about
50 C to about 90 C.
[0334] le. The process of paragraph 1, wherein the mixture is heated
from about
50 C to about 80 C.
[0335] lf The process of paragraph 1, wherein the mixture is heated
from about
60 C to about 80 C.
[0336] lg. The process of any of paragraphs 1 through lf, wherein the
mixture is
heated under pressure, wherein the pressure range is from about 10 psi to
about 1000 psi.
[0337] lh. The process of paragraph lg, wherein the pressure range of
from about
30 to about 500 psi.
[0338] li. The process of paragraph lg, wherein the pressure range if
from about
50 to about 200 psi.
[0339] 2. The process of paragraph 1, wherein the mixture contains
from about 1
to about 50 weight percent of sucrose, glucose, fructose containing material
or mixtures
thereof
[0340] 3. The process of paragraph 2, wherein the fructose containing
material
comprises fructan, fructooligosaccharide, inulin, fructose, fructose corn
syrup or mixtures
thereof
[0341] 4. The process of paragraph 2, wherein the sucrose, glucose,
fructose
containing material or mixtures thereof is present from about 5 to about 30
weight percent.
-33-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0342] 5. The process of paragraph 1, wherein the acid is present from
about 1 to
about 50 weight percent.
[0343] 6. The process of any of paragraphs 1 through 5, wherein the
mixture is
heated for 60 minutes or less.
[0344] 7. The process of paragraph 6, wherein the mixture is heated
for 30
minutes or less.
[0345] 8. The process of any of paragraphs 1 through 7, further
comprising the
step of mixing the mixture.
[0346] 9. The process of any of paragraphs 1 through 8, wherein the
isolation
step is filtration.
[0347] 10. The process of paragraph 9, further comprising the step of
treating the
solids with a solvent to provide a filtrate.
[0348] 11. The process of paragraph 10, wherein the solvent is water.
[0349] 12. The process of either paragraph 10 or 11, wherein the
aqueous portion
and the filtrate are combined to provide a final filtrate.
[0350] 13. The process of any of paragraph 1 through 12, wherein the
water
immiscible solvent is methyl isobutyl ketone, ethyl levulinate, butyl
levulinate,
cyclohexanone, toluene, methyl-THF, methyl-tertiary butyl ether, methyl
isoamyl ketone,
hexane, cyclohexane, chloro-benzene, methylene chloride, dichloroethane, ortho-
dichlorobenzene, diisobutyl ketone, 2,6-dimethyl cyclohexanone,
tetrahydrofuran or mixtures
thereof
[0351] 14. The process of any of paragraphs 1 through 13, wherein the
filtrate and
water immiscible layers are separated.
[0352] 15. The method of any of paragraphs 1 through 14, further
comprising the
step of concentrating the water immiscible layer containing the levulinic acid
or formic acid
to provide a concentrate.
[0353] 16. The method of paragraph 15, wherein the concentration step
is
conducted under reduced pressure.
[0354] 17. The method of paragraph 16, wherein the concentration step
is
conducted at an elevated temperature.
[0355] 18. The method of paragraphs 15 or 16, wherein the water
immiscible
layer is agitated.
[0356] 19. The method of any of paragraphs 16 through 18, wherein the
reduced
pressure is from about 10 to about 700 torr.
-34-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0357] 20. The method of any of paragraphs 16 through 19, wherein
the water
immiscible layer was heated to about 20 to about 140 C.
[0358] 21. The process of any of paragraphs 15 through 20, further
comprising the
step of subjecting the concentrate to wipe film evaporation to provide
purified levulinic acid.
[0359] 22. The process of paragraph 21, wherein the levulinic acid
had a purity of
at least 95%.
[0360] 23. The process of any of paragraphs 15 through 20, further
comprising the
step of treating the concentrate with a solid sorbent.
[0361] 24. The process of paragraph 23, wherein the solid sorbent
is/are pieces of
wood, an ion exchange resin, optionally with a solvent, molecular sieves,
optionally with a
solvent, or activated carbon, optionally with a solvent.
[0362] 25. The process of either paragraph 23 or 24, further
comprising the steps
of removing the levulinic acid or formic acid from the solid sorbent by heat,
pressure, or by
rinsing with water, aqueous base or a polar solvent.
[0363] 26. The process of any of paragraphs 1 through 15, wherein
the process is
a conducted in a continuously-stirred tank reactor (CSTR) or a plug-flow
reactor (PFR).
[0364] 27. The process of paragraph 26, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to sucrose, glucose, or
fructose containing
material is added to the reactor over a period of 1 hour and an equivalent
weight amount is
removed during the same time period.
[0365] 28. The process of paragraph 27, wherein the biomass
comprises fructose.
[0366] 29. The process of either paragraphs 27 or 28, wherein a
ratio of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of 1 hour and an
equivalent weight amount is removed during the same time period.
[0367] 30. The process of paragraph 26, wherein the CSTR process is
conducted
wherein a ratio of about 2:1 to about 5:1 water to sucrose, glucose, or
fructose containing
material is added to the reactor over a period of time t and an equivalent
weight amount is
removed during the same time period.
[0368] 31. The process of paragraph 30, wherein the biomass
comprises fructose.
[0369] 32. The process of either paragraphs 30 or 31, wherein a
ratio of about
10:1 to about 15:1 water to mineral acid is added to the reactor over a period
of time t and an
equivalent weight amount is removed during the same time period.
-35-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0370] The following paragraphs provide for yet still further additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), the present
invention provides
a method to purify levulinic acid comprising the steps:
dissolving levulinic acid in a solvent to provide a levulinic acid solution;
contacting the levulinic acid solution with molecular sieves or a period of
time;
separating the molecular sieves from the levulinic acid solution; and
heating the sieves or applying reduced pressure to the sieves to release
purified
levulinic acid or treating the sieves with water, aqueous base, or a polar
solvent to rinse the
levulinic acid from the sieves.
[0371] 2. The method of paragraph 1, wherein the molecular sieve size
range is
from about 2 angstroms to about 15 angstroms.
[0372] 3. The method of either paragraph 1 or 2, wherein the weight
ratio of
molecular sieves to solvent is about 1:10 to 10:1.
[0373] 4. The method of any of paragraphs 1 through 3, wherein the
concentration of levulinic acid in the levulinic acid solution is about 1 to
about 20 weight
percent, more particularly from about 2 to about 15 weight percent.
[0374] 5. The method of any of paragraphs 1 through 4, wherein the
solvent is
cyclohexanone, methyl-tetrahydrofuran, toluene or methyl isobutyl ketone.
[0375] 6. The method of any of paragraphs 1 through 5, wherein the
purified
levulinic acid has a purity of at least 95%.
[0376] 7. The method of any of paragraphs 1 through 6, wherein the
color index
(YI) of the purified levulinic acid has a color index of below 50 as measured
by ASTM
method E313.
[0377] The following paragraphs provide for further additional aspects of
the present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
method to purify levulinic acid comprising the steps:
dissolving from about 10 to about 50 weight percent levulinic acid in a
solvent
to provide a levulinic acid solution;
cooling the levulinic acid solution to about less than 15 C to induce
precipitation of levulinic acid; and
collecting the precipitated levulinic acid.
-36-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0378] 2. The method of paragraph 1, wherein the solvent is methyl
isobutyl
ketone, cyclohexanone, or toluene.
[0379] 3. The method of either of paragraphs 1 or 2, wherein the
precipitated
levulinic acid has a purity of at least 95%.
[0380] 4. The method of any of paragraphs 1 through 3, wherein the
precipitated
levulinic acid has a color index of less than 50 as measured by ASTM method
E313.
[0381] The following paragraphs provide for further additional aspects of
the present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
method to purify levulinic acid comprising the steps:
dissolving up to about 50 weight percent of levulinic acid in a solvent with
the
proviso that solvent is not water to provide a levulinic acid solution; and
adding an aqueous base solution to the levulinic acid solution to provide a
levulinic acid salt precipitate.
[0382] 2. The method of paragraph 1, wherein the base is an alkali
metal or an
alkaline earth metal hydroxide or carbonate.
[0383] 3. The method of paragraphs 1 or 2, wherein the weight
percentage of
base is from about 0.5 to about 5 equivalents based on the moles of levulinic
acid.
[0384] 4. The method of any of paragraphs 1 through 3, wherein the
solvent is
methyl isobutyl ketone, cyclohexanone, toluene or mixtures thereof
[0385] 5. The method of any of paragraphs 1 through 4, further
comprising the
step of isolating the levulinic acid salt precipitate.
[0386] The following paragraphs also provide for further additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), the present
invention provides
a method to prepare levulinic acid comprising the steps of:
combining levulinic acid, a biomass material, a mineral acid and less than 10
weight percent water to form a mixture, wherein the components equal 100
weight percent;
heating the mixture to a range of about 50 C to about 280 C to provide a
hydrolyzed mixture;
cooling the hydrolyzed mixture;
isolating solids from liquids; and
cooling the liquids to form precipitated levulinic acid.
[0387] la. The process of paragraph 1, wherein the biomass comprises
sludges
from paper manufacturing process; agricultural residues; bagasse pity;
bagasse; molasses;
aqueous oak wood extracts; rice hull; oats residues; wood sugar slops; fir
sawdust; naphtha;
-37-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
corncob furfural residue; cotton balls; raw wood flour; rice; straw; soybean
skin; soybean oil
residue; corn husks; cotton stems; cottonseed hulls; starch; potatoes; sweet
potatoes; lactose;
sunflower seed husks; sugar; corn syrup; hemp; waste paper; wastepaper fibers;
sawdust;
wood; residue from agriculture or forestry; organic components of municipal
and industrial
wastes; waste plant materials from hard wood or beech bark; fiberboard
industry waste water;
post-fermentation liquor; furfural still residues; and combinations thereof, a
C5 sugar, a C6
sugar, a lignocelluloses, cellulose, starch, a polysaccharide, a disaccharide,
a monosaccharide
or mixtures thereof.
[0388] lb. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 80 C to about 250 C.
[0389] lc. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 100 C to about 220 C.
[0390] 1 d. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 100 C.
[0391] le. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 90 C.
[0392] lf. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 50 C to about 80 C.
[0393] lg. The process of either paragraph 1 or la, wherein the
mixture is heated
from about 60 C to about 80 C.
[0394] lh. The process of any of paragraphs 1 through lg, wherein the
mixture is
heated under pressure, wherein the pressure range is from about 10 psi to
about 1000 psi.
[0395] li. The process of paragraph lh, wherein the pressure range of
from about
30 to about 500 psi.
[0396] 1j. The process of paragraph li, wherein the pressure range if
from about
50 to about 200 psi.
[0397] 2. The method of any of paragraphs 1 through 1 f, wherein the
weight
percentage of levulinic acid is from about 50 to about 90.
[0398] 3. The method of any of paragraphs 1 or 2, wherein the weight
percentage of biomass is from about 5 to about 30.
[0399] 4. The method of any of paragraphs 1 through 3, wherein the
mineral acid
weight percentage is from about 1 to about 20.
[0400] 5. The method of any of paragraphs 1 through 4, wherein the
weight
percentage of water is less than 8 percent.
-38-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0401] 6. The method of any of paragraphs 1 through 5, wherein the
hydrolyzed
mixture is cooled to range of below 20 C.
[0402] 7. The method of any of paragraphs 1 through 6, wherein the
liquids are
cooled to range of from about 60 C to about 10 C.
[0403] 8. The method of any of paragraphs 1 through 7, wherein the
biomass
comprises a C5 sugar, sucrose, a C6 sugar, a lignocelluloses, cellulose,
starch, a
polysaccharide, a disaccharide, a monosaccharide, a hard wood a soft wood, or
mixtures
thereof
[0404] 9. The process of any of paragraph 8, wherein the biomass is
sucrose,
fructose or glucose.
[0405] 10. The process of any of paragraphs 1 through 9, wherein the
mineral acid
is sulfuric acid, phosphoric acid, hydrochloric acid or mixtures thereof
[0406] The following paragraphs also provide for further additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), the present
invention provides
a method to prepare levulinic acid comprising the steps of:
combining levulinic acid, a mineral acid and less than 10 weight percent water
to form a mixture, wherein the components equal 100 weight percent;
mixing the mixture for a period of time at a temperature range of from about
50 C to about 280 C;
cooling the mixture to a temperature range of from about -30 C to about 5 C;
and
isolating solids from liquids to provide levulinic acid.
[0407] 2. The method of paragraph 1, wherein the weight percentage of
levulinic
acid is from about 70 percent to about 95 percent.
[0408] 3. The method of either paragraphs 1 or 2, wherein the mineral
acid
weight percentage is from about 5 percent to about 10 percent.
[0409] 4. The method of any of paragraphs 1 through 3, wherein the
weight
percentage of water from about 3 percent to about 8 percent.
[0410] 5. The method of any of paragraphs 1 through 4, wherein the
hydrolyzed
mixture is cooled to range of from about -25 C to about 10 C.
[0411] 7. The method of any of paragraphs 1 through 5, wherein the
mixture is
cooled to range of from about -20 C to about 5 C.
[0412] 8. The process of any of paragraphs 1 through 7, wherein the
mineral acid
is sulfuric acid, phosphoric acid, hydrochloric acid or mixtures thereof
-39-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0413] The following paragraphs also provide for further additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), the present
invention provides
a method to prepare levulinic acid or formic acid, comprising the steps:
mixing up to 30
weight percent of a fructose containing material comprising fructan,
fructooligosaccharide,
inulin, fructose, fructose-glucose blended corn syrup, sucrose or mixtures
thereof, up to 75
weight percent of an acid catalyst and at least 20 weight percent water to
equal 100 weight
percent to form a mixture; and heating the mixture to a temperature of from
about 50 C to
about 100 C to provide levulinic acid or formic acid.
[0414] 2. The process of paragraph 1, wherein the mixture comprises 40-
75% of
an acid catalyst.
[0415] 3. The process of paragraph 1, wherein the mixture comprises 50-
70% of
an acid catalyst.
[0416] 4. The process of any of paragraphs 1-3, wherein the acid
catalyst is
sulfuric acid
[0417] 5. The process of any of paragraphs 1-4 wherein the reaction is
run for
less than 480 minutes.
[0418] 6. The process of any of paragraphs 1-4 wherein the reaction is
run for
less than 360 minutes.
[0419] 7. The process of any of paragraphs 1-4 wherein the reaction is
run for
less than 120 minutes.
[0420] 8. The process of any of paragraphs 1-4 wherein the reaction is
run for
less than 60 minutes.
[0421] 9. The process of any of paragraphs 1-4 wherein the reaction is
run for
less than 30 minutes.
[0422] 10. The process of any of paragraphs 1-4 wherein the reaction
is run for
less than 15 minutes.
[0423] 11. The process of any of paragraphs 1-10 wherein the reaction
is run at a
temperature from about 50 C to about 90 C.
[0424] 12. The process of any of paragraphs 1-10 wherein the reaction
is run at a
temperature from about 50 C to about 80 C.
[0425] 13. The process of any of paragraphs 1-10 wherein the reaction
is run at a
temperature from about 60 C to about 80 C.
-40-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0426] The following paragraph provide for additional aspects of the
present
invention. In one embodiment, in a first paragraph (1), the present invention
provides a
process to prepare levulinic acid or formic acid, comprising the steps:
mixing up to 50 weight percent of a fructose containing material comprising
fructan, fructooligosaccharide, inulin, fructose, fructose-glucose blended
corn syrup, sucrose
or mixtures thereof, up to 75 weight percent of an acid catalyst and at least
20 weight percent
water to equal 100 weight percent to form a mixture; and
[0427] heating the mixture to a temperature of from about 50 C to about
280 C to
provide levulinic acid or formic acid.
[0428] The following paragraphs also provide for further still additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), applicable to
any of the above
noted paragraphs (noted as [051] through [0427], the process is conducted in a
continuous
addition batch reactor.
[0429] 2. The process of paragraph 1, wherein the continuous addition
batch
process is conducted wherein a ratio of about 2:1 to about 5:1 water to
biomass is added to
the reactor over a period of 1 hour.
[0430] 3. The process of paragraph 2, wherein the biomass is fructose.
[0431] 4. The process of either paragraphs 1 or 2, wherein a ratio of
about 10:1
to about 15:1 water to mineral acid is added to the reactor over a period of 1
hour.
[0432] 5. The process of paragraph 1, wherein the continuous addition
batch process
is conducted wherein a ratio of about 2:1 to about 5:1 water to biomass is
added to the reactor
over a period of time t.
[0433] 6. The process of paragraph 5, wherein the biomass is fructose.
[0434] 7. The process of either paragraphs 4 or 5, wherein a ratio of
about 10:1
to about 15:1 water to mineral acid is added to the reactor over the period of
time t.
[0435] The following paragraphs also provide for further still additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), applicable to
any of the above
noted paragraphs (noted as [051] through [0427], the biomass is added over a
period of from
about 0.1 to about 40 hours, more specifically, 0.25 to 20 hours, more
specifically, 0.5 to 10
hours, and even more specifically, 0.75 to 5 hours.
[0436] The following paragraphs also provide for further still additional
aspects of the
present invention. In one embodiment, in a first paragraph (1), applicable to
any of the above
noted paragraphs (noted as [051] through [0427], the formic acid and levulinic
acid are
extracted together using a first extraction solvent, or are extracted
separately, using a first and
-41-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
a second extraction solvent. In another embodiment, the formic acid is removed
from the
reaction mixture by distillation, steam stripping or extraction prior to
extracting the levulinic
acid.
[0437] The invention will be further described with reference to the
following non-
limiting Examples. It will be apparent to those skilled in the art that many
changes can be
made in the embodiments described without departing from the scope of the
present
invention. Thus the scope of the present invention should not be limited to
the embodiments
described in this application, but only by embodiments described by the
language of the
claims and the equivalents of those embodiments. Unless otherwise indicated,
all
percentages are by weight.
[0438] In one aspect, the invention is directed to a process to make
crystallizable
levulinic acid ("LA") from sugar solutions.
[0439] Hydrolysis of a 1-3 Molar solution of sucrose, glucose, fructose,
or blends of
the aforementioned, specifically fructose and sucrose, occurs in a batch or
continuous reactor,
specifically a continuous reactor. In one embodiment the method includes the
following steps
following hydrolysis of a 1-3 Molar solution of sucrose, glucose, fructose, or
blends of the
aforementioned:
[0440] (a) Filtration of solids from hydrolysate mixture.
[0441] (b) Water or extraction solvent wash of solids (optional).
[0442] (c)Extraction of LA and formic acid from aqueous hydrolysate into
an
extraction solvent.
[0443] (d) Removal of extraction solvent by distillation.
[0444] (e) Thin-film evaporation of LA.
[0445] (f) Crystallization of LA
[0446] (g) recovery of formic acid.
[0447] The process allows fast reaction time, easy to handle char
byproduct, good
yields, no neutralization step (optional), efficient extraction and
distillation to afford a
crystallizable LA product.
[0448] A few processes are known to make LA from sugar, but little is
known on how
to remove the LA and formic acid from the reactor and purify it from the
hydrolysate. The
disclosed process produces approximately 97% purity LA that crystallizes.
-42-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0449] Unless otherwise noted, the concentration of sulfuric acid used is
96-98%.
[0450] Example 1
[0451] Into a 1L hastelloy parr reactor 135.12g fructose (94% purity,
0.75mo1),
500mL DI water, and 38.17g sulfuric acid were charged. The reactor was sealed
and the
reaction mixture was heated up to a temperature of 150 C while stirring at 52
RPM. Once the
reaction mixture reached a temperature of 150 C, the mixture was held at that
temperature for
1 hour. After the 1 hour reaction time the heat was turned off and the heating
mantle was
lowered and the reactor was cooled using an ice water bath. Once the reactor
was cooled it
was dismantled and the reaction mixture was filtered through a 0.45 m filter
using vacuum
filtration in order to remove the char from the liquid. The liquid was
analyzed by HPLC and
found to contain 9.9 wt% levulinic acid. The liquid is referred to as
"hydrolysate".
[0452] Into a 1L separatory funnel 300.10g hydrolysate and 300.07g Methyl
isobutyl
ketone (MIBK) were charged. The separatory funnel was shaken in order to mix
everything
together and then the mixture was allowed to phase separate. The bottom
aqueous layer was
drained out of the bottom of the separatory funnel and collected in a beaker.
The top layer
was poured out of the top of the separatory funnel and into a 2-neck 1L round
bottom flask.
The bottom layer was then placed back into the separatory funnel and another
300.36g MIBK
were used for the second extraction. The mixture was shaken and allowed to
phase separate
again. The bottom layer was drained and discarded. The top layer was poured
out of the top
of the separatory funnel into the 2-neck 1L round bottom flask containing the
previous
MIBK-extract.
[0453] The 2-neck 1L round bottom flask (RBF) was situated into a heating
mantle
and equipped with a magnetic stir bar, thermocouple, vigreux column, short
path condenser,
and a 1L collection flask. The mixture was stirred at 600 rpm and the vacuum
was kept
between 15-30 torr. Over the course of the distillation the temperature of the
pot was slowly
increased until a maximum temperature of 70 C was reached. The MIBK was
distilled away
from the mixture, the crude levulinic acid (LA) mixture left in the pot was a
very dark brown
color.
[0454] The crude LA was purified by wipe film evaporation (WFE). The
crude LA
was placed into a reservoir and degassed. The heater was turned on and set to
70 C and the
vacuum was set to 0.25-0.3Torr. Once a temperature of 70 C was reached the
blades were
turned on and the crude LA was slowly fed into the WFE. Dark black material
was collected
in the heavy fraction and light yellow material was collected in the light
fraction. Once all of
-43-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
the material had passed through the WFE, the vacuum, heat and blades were shut
off and the
light fraction, LA, was analyzed by GC-FID. The GC-FID results showed that the
LA was
95% pure. A small sample of this LA was put into a scintillation vial and
cooled to 5 C, and
it crystallized. This light yellow LA was redistilled by WFE a second time.
This time the
temperature was set to 65 C and the vacuum was still at 0.25-0.3Torr. Again,
the dark
material was collected in the heavy fraction and a faint red material was
collected in the light
fraction. Once all of the LA had gone through the WFE it was shut down and the
light
fraction was analyzed by GC-FID. The GC-FID results of the LA after going
through the
WFE a second time showed that the LA was 97% pure. This LA was cooled to 5 C.
The
entire sample crystallized, indicating good quality levulinic acid.
[0455] Use of fructose as a feedstock for the production of levulinic
acid is known in
the art. HC1 has been used as a catalyst to make levulinic acid. HC1 is a very
corrosive
catalyst and creates the possibility of generating chlorinated organic
compounds, so this is not
a good option.
[0456] Zeolites have been used as catalysts for the production of LA. The
zeolites are
typically used in high concentrations, and presumably would foul due to the
formation of
solid humin substances during the conversion of fructose to LA. This catalyst
cost would not
be economically viable for the production of LA. Also, US Patent 7,317,116
describes the use
of fructose or high fructose corn syrup to make levulinic acid using
heterogeneous cation
exchange resin catalysts and polyethylene glycol solvents. The use of
heterogeneous
catalysts to produce LA from biomass or sugars would also have the problem of
fouling by
the formation of soluble polymeric and insoluble polymeric substances, known
as humins.
Additionally, the time of the reaction required to convert fructose to LA as
described in US
Patent 7,317,116 was 4-18h, which would be much too long for an industrial
continuous
process.
[0457] Herein, a new method is described for the conversion of fructose
or fructose-
containing feedstocks into levulinic acid and formic acid. The process allows
up to 30 wt%
feedstock and from about 4 to about 60 wt% mineral acid, such as sulfuric
acid, to be used in
an aqueous reaction mixture, while producing > 50 mol% LA in less than 60
minutes of
reaction time, preferably less than 30 minutes of reaction time, and more
preferably less than
20 minutes of reaction time.
[0458] In another embodiment, this process can be backwards integrated
into a
cellulose or ligno-cellulose producer or bio-refinery.
-44-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0459] In another embodiment, the use of washing the produced humin
substances
with a solvent or water, or a combination of both, is an added beneficial
method to produce a
higher mass recovery of LA and formic acid.
[0460] Example 2. 1 Mol/L D-Fructose (15 mL)was prepared by diluting
2.44 g of
crystalline D-Fructose (93.5% purity, 6.5% moisture, Aldrich) up to 15.0 mL
with DI water.
The 15.0 mL was transferred to a 3 oz. empty high pressure, high temperature
reaction vessel,
and concentrated sulfuric acid (4074) was added. The reaction vessel was
capped using a
Teflon sleeve, an o-ring, rubber washer and a stainless steel plug. The
reactor was securely
closed with stainless steel couplings. The reaction vessel was placed into a
180 C hot oil bath
to reach an internal temperature of around 160 C. After a specified reaction
time, the reaction
vessel was then removed from the hot oil and placed in a room temperature
water bath for 1
minute to begin cooling. Following the room temperature water bath, the
reactor was placed
in an ice water bath to quench the reaction. Once the reactor vessel had
cooled, it was opened,
and the contents were filtered, weighed, and then analyzed by HPLC. The humin
solids that
formed during the reaction were extracted with DI water and the LA in the
"wash" sample
was recovered and analyzed by HPLC and weighed separately to obtain the yield.
The two
yields of LA were added together to obtain the final mol% yield of LA relative
to the initial
moles of fructose charged in the feed. The final results are displayed in
Table I.
[0461] Examples 3-4. The procedure outlined in Example 2 was repeated,
except that
the feed concentration of fructose and acid catalyst was varied, as well as,
the temperature of
the reaction.
[0462] Table I.
Wt% LA
Yield
Wt% Molar Molar
Wt% Fructose Improvement
Sulfuric Max. Time Yield Yield
Example Fructose Conversion after
acid in Temperature (min) of LA of FA
in Feed (%) Washing
feed (%) (%)
Solid
Humins
2 13.6 4.7 143 C 50 100 54 66 16
3 18.9 5.5 156 C 30 100 63 76.9 12
4 23.5 5.5 160.1 C 30 100 62 79 15
-45-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0463] As can be seen from Table I, fructose can be hydrolyzed completely
in less
than 60 minutes of reaction time to afford up to 63 mol% yield and 79 mol%
yield of formic
acid (FA). Also, extracting LA from the solid humin material resulted in > 10
wt% yield
improvement of LA in all of the examples.
[0464] Utilizing High Fructose Corn Syrup, inulin, oligomeric fructan
polymers, and
the like are also be useful in this invention.
[0465] Another process of this invention involves the pretreatment of
glucose to
obtain > 70 % conversion to fructose directly before the fructose is
hydrolyzed to LA and
formic acid (FA). The process involves glucose conversion to fructose (without
crystallization of the fructose), the fructose then feeds into a solution with
water and sulfuric
acid catalyst to form LA and FA in less than 60 minutes of reaction time. This
pre-treatment
of the glucose or sugar feedstock may be enzymatically catalyzed or chemically
catalyzed to
afford > 70% conversion of the glucose or "sugar" to fructose. Methods of
glucose to
fructose conversion are generally known in the art.
[0466] The glucose and "sugar" polymer mixture may be obtained by the
enzymatic
degradation of starch, maltose, or the like, or alternatively, by the
hydrolytic or catalytic
degradation of cellulose to glucose. The glucose obtained from these reactions
may also be
obtained from a ligno-cellulosic feedstock.
[0467] Also, this process can be attached to a bio-refinery, which
depolymerizes
cellulose or ligno-cellulose into glucose for ethanol production, but instead
of producing
100% ethanol, some of the process streams containing crude or purified glucose
are
subsequently converted into fructose and then to LA and FA.
[0468] In a typical biomass process, biomass is converted into levulinic
acid (LA) and
formic acid (FA) by a strong-acid catalyst in a dilute, aqueous system. The LA
and FA are
then first extracted into a solvent phase to remove the LA and FA from the
aqueous phase
containing the strong-acid catalyst. The solvent may be, for example, methyl-
isobutyl ketone
(MIBK), methyl isoamyl ketone (MIAK), cyclohexanone, o, m, and para-cresol,
substituted
phenols, for example, 2-sec butyl phenol, C4-C18 alcohols, such as n-pentanol,
isoamyl
alcohol, n-heptanol, 2-ethyl hexanol, n-octanol, 1-nonanol, cyclohexanol,
methylene chloride,
1,2-dibutoxy-ethylene glycol, acetophenone, isophorone, o-methoxy-phenol,
methyl-
tetrahydrofuran, tri-alkylphosphine oxides (C4-C18) and ortho-dichlorobenzene
and mixtures
thereof Once the LA and FA is extracted into the solvent, the usual way of
purification of
-46-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
the LA and FA is by removing the solvent through energy-intensive distillation
followed by
distillation of the LA, another energy intensive step which may lead to side-
products and
yield losses.
[0469] One novel way of purifying the LA from the extractive solvent is
to remove
the LA by the use of an adsorbents, like molecular sieves, basic alumina,
silica gel, or the
like.
[0470] Example 5 A 10.22 gram solution of levulinic acid (4.8 wt%) in
cyclohexanone was weighed in a 125 mL Erlenmeyer Flask. 10.05 of 3A Molecular
Sieves
was added to the flask. The flask was sealed with parafilm to prevent
evaporation of the
solvent. The mixture was aged overnight (> 12h) at room temperature. A sample
of the liquid
was withdrawn from the flask and analyzed by HPLC. The amount of LA in the
final liquid
was found to be 3.7 wt%, indicating that approximately 0.11 g of LA had been
adsorbed by
the molecular sieves.
-47-
SEGT.P0013W0
[0471] Table 2
0
t..)
o
[0472] Examples 6-16 were repeated as described in Example 5, except that
different solvents and different sizes of molecular sieves
(...)
O-
-I
were used according to Table 2.
cio
(...)
,z
,-,
% LA
Size of
% LA in removed
Molecular g of Mol
solvent
Example Sieves Sieves g of solvent Solvent Type
(Initial) (Final)
3A 10.05 10.22 cyclohexanone 4.8 23
P
6 4A 10 10.01 cyclohexanone
4.8 10
21
.
7 5A 10 10.06 cyclohexanone
4.8
,
8 3A 10.03 10.03 methyl-THF
4.4 11 .
,
9 4A 10.06 10.04 methyl-THF
4.4
5A 10.1 10.02 methyl-THF 4.4 14
11 3A 10.02 10.04 toluene
5.2 42
37
12 4A 10.02 10.11 toluene
5.2
13 5A 10.04 10.13 toluene
5.2 40 1-d
n
14 3A 10.02 10.03 MIBK
4.0
cp
17.5
t..)
o
15 4A 10.01 10.06 MIBK
4.0 ,-,
t..)
32.5
O-
16 5A 10.09 10.02 MIBK
4.0 o,
o,
(...)
o,
cio
-48-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0473] As can be seen from the data, LA may be removed from typical
hydrolysate
extraction solvents by molecular sieves. The 3A and 5A size molecular sieves
seem to
provide a more selective removal of levulinic acid in virtually all solvent
systems. This
provides a unique and alternative pathway to remove levulinic acid in a
biomass-type
hydrolysis system involving an extraction solvent.
[0474] In other examples, basic alumina, silica gel, activated carbon,
biomass char,
zeolites, activated clays, anion exchange resins, and ion exchange resins, may
be used to
adsorb levulinic acid from an extraction solvents.
[0475] In a typical biomass process, biomass is converted into levulinic
acid (LA) and
FA by a strong-acid catalyst in a dilute, aqueous system. However, instead of
using water as
the solvent, one embodiment, it would be beneficial if the solvent was
actually one of the
products, for example, levulinic acid or formic acid.
[0476] This portion of the invention describes how the hydrolysis of
biomass may be
conducted in formic or levulinic acid. If the hydrolysis of biomass is
conducted in levulinic
acid, then once the reaction is finished, filtered to remove char, and cooled
to room
temperature, levulinic acid may form a crystalline solid. This solid form of
levulinic acid
offers a unique advantage of purification of the LA from biomass.
[0477] In a typical hydrolysis of biomass, 2-20 wt% sulfuric acid is used
as the
catalyst, and the amount of water used in the hydrolysis is between 60-95 wt%.
In this
invention, the majority of the water is removed and replaced with levulinic
acid, which
enables crystallization of the final LA product by cooling the hydrolysate
solution comprising
water, LA, and sulfuric acid.
[0478] Experimental-Crystallization of LA in a Hydrolysis mixture
[0479] Example 17 A mixture containing 10 wt% sulfuric acid, 87 wt%
levulinic
acid, and 3 wt% water was made in a 20 mL scintillation vial. The vial was
cooled in a
refrigerator at 5 C overnight. After 24h, crystals had formed in the vial,
indicating that the
levulinic acid had crystallized out of solution.
[0480] Examples 18-23 were repeated as described in Example 17, except
that
different amounts of LA, sulfuric acid, and water were used in the
experiments.
[0481] Table 3.
-49-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
DI Water Crystals
Sulfuric Acid
Example LA (wt%) (wt%) present after
(wt%)
cooling (4d)
17 10 87 3 yes
18 7.5 87 5.5 yes
19 5 87 8 no
20 5 92 3 yes
21 10 77 13 no
22 10 67 23 no
[0482] As can be seen from the data, LA may be crystallized out from
cooling a
solution of LA, water, and a strong acid catalyst. This could be very
advantageous for
enabling a process to produce and purify LA from the strong acid catalyzed
degradation of
furfuryl alcohol, sugars, or ligno-cellulosic biomass.
[0483] Example 23. The reaction is carried out by adding 640 g of
levulinic acid and
50 g of sulfuric acid (96+%, Aldrich), 100 g fructose (crystalline, 93+%
purity, Aldrich), and
6 g of DI water to a 1L Hastelloy autoclave equipped with a magnetically
couple overhead
stirrer. The contents are purged with nitrogen and heated to 160 C for lh.
The contents are
cooled to 40 C and filtered. Then, the contents are cooled below 10 C and
allowed to
crystallize. The crystalline product is filtered and subsequently purified.
[0484] Example 24. The reaction is carried out by adding 640 g of
levulinic acid and
50 g of sulfuric acid (96+%, Aldrich), 100 g furfuryl alcohol (crystalline,
93+% purity,
Aldrich), and 25 g of DI water to a 1L Hastelloy autoclave equipped with a
magnetically
couple overhead stirrer. The contents are purged with nitrogen and heated to
160 C for lh.
The contents are cooled to 40 C and filtered. Then, the contents are cooled
below 10 C and
allowed to crystallize. The crystalline product is filtered and subsequently
purified.
[0485] Example 25. The reaction is carried out by adding 640 g of
levulinic acid and
50 g of sulfuric acid (96+%, Aldrich), 100 g sucrose (crystalline, 97+%
purity, Aldrich), and
8 g of DI water to a 1L Hastelloy autoclave equipped with a magnetically
couple overhead
stirrer. The contents are purged with nitrogen and heated to 160 C for 1.5h.
The contents
are cooled to 40 C and filtered. Then, the contents are cooled below 10 C
and allowed to
crystallize. The crystalline product is filtered and subsequently purified.
[0486] Example 26. The reaction is carried out by adding 640 g of
levulinic acid and
50 g of sulfuric acid (96+%, Aldrich), 100 g glucose (crystalline, 98+%
purity, Aldrich), and
6 g of DI water to a 1L Hastelloy autoclave equipped with a magnetically
couple overhead
stirrer. The contents are purged with nitrogen and heated to 160 C for 2.5h.
The contents
-50-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
are cooled to 40 C and filtered. Then, the contents are cooled below 10 C
and allowed to
crystallize. The crystalline product is filtered and subsequently purified.
[0487] Example 27. The reaction is carried out by adding 640 g of
levulinic acid and
50 g of sulfuric acid (96+%, Aldrich), 100 g soft wood (pine, Home Depot), and
20 g of DI
water to a 1L Hastelloy autoclave equipped with a magnetically couple overhead
stirrer. The
contents are purged with nitrogen and heated to 160 C for 1 h. The contents
are cooled to 40
C and filtered. Then, the contents are cooled below 10 C and allowed to
crystallize. The
crystalline product is filtered and subsequently purified.
[0488] In a typical biomass process, biomass is converted into levulinic
acid (LA) and
FA by a strong-acid catalyst in a dilute, aqueous system. The levulinic acid
and optionally
the formic acid, is then first extracted into a solvent phase to remove the
levulinic acid and/or
the formic acid from the aqueous phase containing the strong-acid catalyst.
The solvent may
be, for example, methyl-isobutyl ketone (MIBK), methyl isoamyl ketone (MIAK),
cyclohexanone, o, m, and para-cresol, substituted phenols, for example, 2-sec
butyl phenol,
C4-C18 alcohols, such as n-pentanol, isoamyl alcohol, n-heptanol, 2-ethyl
hexanol, n-octanol,
1-nonanol, cyclohexanol, methylene chloride, 1,2-dibutoxy-ethylene glycol,
acetophenone,
isophorone, o-methoxy-phenol, methyl-tetrahydrofuran, tri-alkylphosphine
oxides (C4-C18)
and ortho-dichlorobenzene and mixtures thereof or the like, more specifically,
methyl
isoamyl ketone (MIAK), o, m, and para-cresol, phenol, isoamyl alcohol, n-
hexanol, n-
heptanol, 2-ethyl hexanol, o-methoxy-phenol, 2-4 dimethyl phenol, methyl
isobutyl carbinol,
and mixtures thereof or the like, and even more specifically, o, m, and para-
cresol, isoamyl
alcohol, neopentyl alcohol, methyl isobutyl carbinol, and mixtures thereof or
the like. Once
the LA is extracted into the solvent, the usual way of purification of the LA
is by removing
the solvent through energy-intensive distillation followed by distillation of
the LA, another
energy intensive step which may lead to side-products and yield losses.
[0489] One novel way of purifying the LA from the extractive solvent is
to distill off
a portion of the solvent, then allow the LA to crystallize out of the solvent
by cooling. LA is
usually diluted to about 1-20 wt% in the hydrolysate prior to extraction, and
after extraction,
the concentration of LA in the solvent can be from 0.5-50 wt%, preferably from
1-45 wt%,
and more preferably, from 2-40 wt%. The solvent may be distilled away from LA
in order to
concentrate the LA. The following examples describe the invention.
-51-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0490] Example 28 A 10% solution of levulinic acid in MIBK was made in a
20 mL
scintillation vial. The vial was sealed and put into a freezer at-15 C. The
solution remained
clear and homogenous indicating that no crystallization took place.
[0491] Examples 29-39 were repeated as described in Example 28, except
that
different solvents and different concentrations of LA were used according to
Table 4.
[0492] Table 4.
LA
Crystallization
Example Solvent Concentration
a -15 C
(wt%)
29 MIBK 20 no
30 MIBK 50 yes
31 cyclohexanone 10 no
32 cyclohexanone 20 no
33 cyclohexanone 50 no
34 toluene 10 yes
35 toluene 20 yes
36 toluene 50 yes
37 methyl THF 10 no
38 methyl THF 20 no
39 methyl THF 50 no
[0493] As can be seen from the data, LA may be crystallized out from
cooling a
solution of MIBK that contains > 20% LA. Also, Examples 34-36 demonstrate that
LA may
be crystallized from a solution of toluene that is cooled.
[0494] Another way to purify levulinic acid in an extraction solvent is
by adding a
base, for example, sodium hydroxide to form the metal salt, which would
precipitate from the
extraction solvent.
[0495] Example 40 2.52 g (0.02 mol) of levulinic acid and 47.57g methyl
isobutyl
ketone (MIBK) were added to a 250 mL beaker and mixed thoroughly until
homogeneous.
To this mixture, 1.75g of a 50/50 wt% sodium hydroxide solution was added. As
soon as the
sodium hydroxide was added, a white precipitate formed. A magnetic stir bar
was placed into
the beaker and put onto a stir plate to stir for a few minutes. With stirring,
it appeared as
though more precipitate formed. The precipitate was then filtered out using
vacuum filtration
and a 0.45 m filter. A small amount of the precipitate was put into a GC vial
and dissolved
with water and then run on the HPLC to be analyzed. The analysis showed that
the sodium
salt of levulinic acid was synthesized.
-52-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0496]
Example 41 A small portion of the 5% levulinic acid solution in MIBK made
in Example 40 was added into a saturated solution of calcium hydroxide in
water. Two liquid
phases formed that were cloudy at first, and then became transparent upon
stirring at room
temperature in a 250 mL beaker. No precipitate had formed.
[0497]
Example 42 An MIBK solution containing 4% levulinic acid, 1% formic
acid, 0.05% H2SO4, and 1% water was placed into a 250 mL beaker. A 50-50 wt%
solution
of sodium hydroxide in water was added to neutralize the acid species. Upon
addition, a gel-
like substance formed at the bottom of the flask. No precipitate formed.
[0498]
Example 43 An MIBK solution containing 4% levulinic acid, 1% formic
acid, and 0.05% H2SO4 was placed into a 250 mL beaker. A 50-50 wt% solution of
sodium
hydroxide in water was added to neutralize the acid species. Upon addition,
white precipitate
formed indicating that the sodium salt of levulinic acid had formed.
[0499]
Example 44 Approximately 1% water was added to Example 16, and the
precipitate turned into a gel-like substance. Thus, having less than 1% water
in the entire
crude mixture is advantageous for the formation of solid sodium levulinate in
a typical
hydrolysate solution of 4% LA in MIBK solvent.
[0500] In
another aspect, the present invention is directed to methods including the
use of organic or inorganic, hydrophobic co-solvents for the preparation of LA
from the
hydrolysis of biomass. In one embodiment, the invention includes charging a co-
solvent and
optionally, a co-catalyst, for the purposes of improving the overall yield of
levulinic acid
from biomass. The biomass may be lignocellulosic, cellulosic, starch-based, or
sugar-based
(monomeric, dimeric, or oligomeric sugars). The
process has the advantage of
simultaneously making and extracting HMF and or levulinic acid from biomass.
[0501]
Example 45 The reaction was carried out by adding 300 g of water and 15.02
g of sulfuric acid (96+%, Aldrich), 54.07 g fructose (crystalline, 93+%
purity, Aldrich), and
300 g of methyl-THF to a 1L three-neck flask that was equipped with a magnetic
stirrer and a
reflux condenser. The contents were purged with nitrogen continuously and
allowed to reflux
for 6h. Aliquots were removed from the flask as a function of time to measure
the
composition in both layers. Analysis of the reaction mixture showed formation
of HMF and
the absence of levulinic acid.
[0502]
Example 46 Example 45 is repeated except that 13 g of para-toluene sulfonic
acid was added to the mixture.
-53-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0503] Example 47 Example 45 is repeated except that methyl isobutyl
ketone was
used instead of methyl-THF.
[0504] Example 48 Example 47 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0505] Example 49 Example 45 is repeated except that cyclohexanone was
used
instead of methyl-THF.
[0506] Example 50 Example 49 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0507] Example 51 Example 46 is repeated except that toluene was used
instead of
methyl-THF.
[0508] Example 52 Example 51 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0509] Example 53 Example 45 is repeated except that 4-sec-butyl phenol
was used
instead of methyl-THF.
[0510] Example 54 Example 53 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0511] Example 55 Example 45 is repeated except that 1,2-dichloro-benzene
was
used instead of methyl-THF.
[0512] Example 56 Example 55 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0513] Example 57 Example 45 is repeated except that m-cresol was used
instead of
methyl-THF.
[0514] Example 58 Example 57 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0515] Example 59 Example 45 is repeated except that tri-octyl phosphine
oxide was
used instead of methyl-THF.
[0516] Example 60 Example 59 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0517] Example 61 Example 45 is repeated except that tri-butyl phosphate
was used
instead of methyl-THF.
[0518] Example 62 Example 61 is repeated except that 13 g of para-toluene
sulfonic
acid was added to the mixture.
[0519] Example 63 Example 45 is repeated except that sucrose was used
instead of
fructose.
-54-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0520] Example 64 Example 45 is repeated except that 20 g of naphthalene
sulfonic
acid was added to the mixture.
[0521] Example 65 Example 45 is repeated except that 20 g of camphor
sulfonic
acid was added to the mixture.
[0522] Example 66 Example 45 is repeated except that 10 g of benzene
sulfonic acid
was added to the mixture.
[0523] Any of examples 45-66 could be repeated at higher pressure in a
Hastelloy,
Zirconium, or glass-lined steel autoclave.
[0524] Any of the examples 45-66 could be repeated using glucose, soft
wood, hard
wood, starch, or cellulose.
[0525] Any of the examples 45-66 could be repeated using furfuryl alcohol
or
hydroxymethyl furfural.
[0526] Triflic acid, hydrochloric acid, hydrobromic acid, hydroiodic
acid, nitric acid,
phosphoric acid, boric acid, hydrofluoric acid, perchloric acid and mixtures
thereof may be
used instead of sulfuric acid if desired.
[0527] Example 67 After a sufficient time of reaction, the organic
solvent layer is
removed from the aqueous layer by a decanter or centrifuge. Then, a certain
quantity of
aqueous sodium hydroxide is added to the organic mixture until a precipitate
forms. The
precipitate is filtered, acidified, and crystallized to afford > 95% purity
levulinic acid. The
organic solvent is re-used in the process after distillation.
[0528] Example 68 After a sufficient time of reaction, the organic
solvent layer is
removed from the aqueous layer by a decanter or centrifuge. Then, the solvent
is cooled
down to < 10 C. The precipitate is filtered and crystallized to afford > 95%
purity levulinic
acid. The organic solvent is re-used in the process after distillation.
[0529] Continuously Stirred Tank Reactor (CSTR) Operations:
[0530] A stirred, 300 ml Parr Autoclave, of Hastelloy construction, was
used to
investigate a continuous process for the acid-catalyzed production of
levulinic and formic
acids from carbohydrate sugars. Variable feed flow rates, coupled with
controlled reactor
volumes, were used to obtain various residence times in the autoclave. This
reaction
produces insoluble by-products in addition to formic and levulinic acids.
Therefore, control
of product flow from the reactor was unsuccessful using standard laboratory
techniques due
to rapid plugging of regulators and other flow-restricting devices. This was
overcome by
using the pressure in the autoclave to periodically blow a controlled quantity
of reactor
-55-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
contents out of the autoclave and into a receiver through a two-way valve
connected to a dip
tube of a prescribed length (depth) inside the autoclave. The depth of the dip
tube controlled
the reactor volume around 180 ml. Continuously feeding reactants into the
autoclave at 3.0
ml/minute and rapidly pulsing the two-way valve full-open and full-closed
every 6.6 minutes
removed approximately 20 g of sample, giving a residence time in the reactor
of
approximately 60 minutes. After liquid is removed down to the control volume,
some gas is
allowed to also escape, thereby "blowing" the lines clear of liquid. The
diameter of the dip
tube is selected to allow removal of both the liquid and solid components of
the reaction
mixture without plugging the lines. 1/4 Inch lines proved sufficient for this
purpose. All
outlet lines require insulation/heating to maintain them at the same
temperature as the reactor.
This prevents premature precipitation of solids from the samples which can
cause plugging.
Reactants are fed into the autoclave at controlled flow rates using an Eldex
pump (A-120
VS).
[0531] Example 69 CSTR Run Using Corn Sweet 90:
[0532] Corn Sweet 90 is a high-fructose syrup (90% fructose, 8.5%
glucose, 1.5%
oligiomeric sugars) supplied by ADM. It contains 77% solids. 360g of this
syrup was
dissolved in 1.0 liter of 0.5M sulfuric acid and used as feed to the reactor.
The autoclave was
filled with 200m1 of distilled water and heated to 160 C. Internal pressure
reached
approximately 80-85 psig. After reaching reaction temperature, volume control
was initiated
by pulsing the valve and removing approximately 20 g of water. The pressure
drops
approximately 5-8 psig during sampling. Continuous feed was then initiated at
3 ml/minute
with sampling occurring every 6.6 minutes. The weight of the samples through
the run
averaged approximately 20 g. The samples were dark reddish-brown in appearance
and a
small amount of solids precipitated out of solution as the samples cooled.
Analysis of a
sample taken 3 hours after initiation of Corn Sweet feed was analyzed using
Liquid
Chromatography (Agilent HPLC; Restek Ultra C18 Column-15 cm; 98% 2.5 pH
Phosphate
Buffer/2% acetonitrile; 0.5 ml/minute Eluent flow at room temperature; UV/RI
detector).
The sample contained 4.7% formic acid, 9.6% levulinic acid and 0.12%
hydroxymethylfurfural (HMF). The low concentration of HMF is an excellent
indicator of
reaction completion. When the reaction was terminated, the autoclave was
opened and 27.49
g of black solids were removed.
[0533] CSTR Runs Using Sucrose and Sucrose/NORIT Activated Carbon:
-56-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0534] Example 70 An attempt was made to execute a continuous run using
1.M
sucrose in 0.5M sulfuric acid at 160 C. This reaction with sucrose proved
difficult to
execute. Outlet sample lines plugged quickly and insufficient time occurred to
achieve
steady state in a continuous mode. Sucrose feed was terminated and the
reaction was allowed
to run to completion (1 hour) in batch mode. After the end of the run, the
reactor was cooled
and opened to find it full of solids. The solids adhered strongly to all the
stainless steel
internals inside the reactor (agitator, thermo well, dip tubes but not to the
Hastelloy surfaces
of the reactor body.) It appeared that the solids were nucleating and then
growing on all the
stainless steel surfaces of the reactor internals.
[0535] Example 71 A second run was initiated under the same conditions as
those
described in Example 75 except 5 weight % of NORIT Activated Carbon (PAC-200;
BA#M-
1620) was added to the autoclave to begin with. This was an attempt to give
the solids
something else on which to nucleate and adhere. A one hour batch run was
completed and
sampled using the two-way blow-down valve. This time, in contrast to the
"NORIT-free"
run, the sample was easily removed from the reactor. As the sample cooled, no
separate
solids were observed coming out of solution. The NORIT that exited from the
reactor in the
sample settled to the bottom of the sample receiver and it appeared that the
solids that usually
precipitate from solution upon cooling were adsorbed on the NORIT. When the
autoclave
was cooled and opened, the amount of solids usually found adhering to all the
reactor
internals were markedly reduced. It, again, appeared that the NORIT had
allowed the
reaction solids to adsorb/adhere to the activated carbon. This will clearly
improve the
operability of the reaction, particularly when using sugars that are more
prone to form solids
in this reaction.
[0536] Example 72 Into a three neck 250 mL round bottom flask charged
130.01g
deionized water, 23.52g (0.13 mol) D-fructose, and 38.30g (0.39 mol) sulfuric
acid. The
round bottom flask was equipped with a magnetic stir bar, thermocouple,
condenser, and
glass stopper. The stir plate was set to stir at a rate of 550 RPM and the
fructose quickly
dissolved. The mixture changed from clear and colorless to clear and a peach
color. The heat
was turned on and set to a temperature of 60 C. The reaction was left to react
for two hours
and samples were taken and analyzed by HPLC. After the two hours, the reaction
was shut
down.
-57-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
Time (minutes) Temperature C % HMF (HPLC)
120 59.8 0.308
[0537] Example 73 Into a three neck 250 mL round bottom flask charged
13.08g
deionized water, 23.48g (0.13 mol) D-fructose, and 31.23g (0.39 mol)
polyphosphoric acid.
The round bottom flask was equipped with a magnetic stir bar, thermocouple,
condenser, and
glass stopper. The stir plate was set to stir at a rate of 550 RPM and the
fructose quickly
dissolved. The heat was turned on and set to a temperature of 60 C. The
reaction was held at
60 C for two hours and then the temperature was increased to 80 C. The
reaction was held at
80 C for one hour and a half and then the temperature was increased to 100 C.
The reaction
was held at 100 C for two hours and then the reaction was shut down. Samples
were taken
throughout the entire reaction and analyzed by HPLC.
Time (minutes) Temperature C % HMF (HPLC)
410 99.7 1.747
[0538] Example 74 Into a three neck 250 mL round bottom flask charged
130.12g
deionized water and 23.49g (0.13 mol) D-fructose. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
38.29g (0.39 mol) sulfuric acid was added into the flask. The heat was turned
on and set to a
temperature of 80 C. The reaction was held at 80 C for two hours and then the
temperature
was increased to 100 C. The reaction was held at 100 C for four hours and
fifteen minutes
and then the reaction was shut down. Samples were taken throughout the entire
reaction and
analyzed by HPLC.
Time (minutes) Temperature C % HMF %LA %FA
(HPLC) (HPLC) (HPLC)
385 100.0 0.007 6.28 2.607
[0539] Sugar Solution Preparation
[0540] Sugar-solution I is a mixture of 90 wt% fructose, 8.5 wt% glucose,
and 1.5
wt% sucrose. This mixture was dissolved in water to obtain a homogeneous
solution that was
1.5 Moles of Sugar-solution I/Liter.
-58-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0541] Example 75 1.5 Molar Sugar-solution I (715g) and concentrated
Sulfuric
Acid (35.75g) was added to a 1L Hastelloy reactor (Parr Model 4530 Reactor).
The Parr
reactor was assembled and securely closed. Mixing in the reactor began and set
to 100 rpm.
The initial time, temperature, and pressure of the reactor was noted. An
electrical heating
mantel was then placed around the reactor and set to 160C. Once the
temperature inside of
the reactor reached 160C, it was held for lhr. After lhr, an ice-water bath
was placed around
the Parr reactor to immediately begin cooling. When the temperature of the
Parr reactor was
below 30C, it was opened and the contents of the reactor were removed and
analyzed by
HPLC. Any solids that formed during the reaction were filtered from the
reaction mixture,
rinsed with water and dried in a vacuum oven to obtain the weight of total
solids.
[0542] The HPLC results showed 6.7% of Levulinic acid and 2.9% Formic
acid
formed. The percent solids were 4.5%.
[0543] Examples 76-78 Further reactions were performed under the same
procedure
as Example 75. Table 5 outlines the reactions, and HPLC results.
-59-
SEGT.P0013W0
Table 5
0
t..)
o
Liquid
lwt% Para- Liquid
Molar Corn Corn 95% Sulfiric Acid
Hydrolysate Top Bottom Dry Solids LA wt% -6-4
Solvent toluene
Sulfonic Hydrolysate
Sweet 90 (g)
Recovered+W Layer (g) Layer (g) Recovered
(g) (HPLC)
Acid (g) Recovered
(g)
ater (g)
1.0 50% Water 50%MIBK 25.05 4.52 516.34 575.33
195.26 380.79 12.59 3.617
same as above
1.0 50% Water 50%MIBK 25.09 0 530.89 573.3
183.95 389.35 14.07 3.726
same as above
6.191
50% Water
1.0 25.17 4.87 578.18 600.18
325.8 274.38 0.00 p
50%Cyclohexanone
,9
09
same as above
..'
2
.
,
L2
.
1 V
n
1-i
cp
t..)
=
,-,
t..)
'a
oe
-60-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0544] Examples 79-84 Further reactions were performed under the same
procedure
as Example 75. Changes were made to the concentration of sugar, solvent
mixture, and an
additional acid catalyst. Also the Parr reactor was purged with nitrogen
before and after the
reaction. The Parr reactor mixing was also increased to 400rpm. Table 6
outlines the
reactions, conditions, and HPLC results.
-6 1 -
SEGT.P00 13 WO
Table 6
0
k....)
o
1¨
Liquid Liquid
FA HMF L..)
Mole/L Mole/L
Top Bottom Dry Solids Furfury o
PTSA Hydrolysate Hydrolysate LA wt% wt% wt% LA
Example Sugar Solvent Sulfuric
Layer Layer Recovered 1 wt% % Solids oe
(wt%) Recovered Recovered + (HPLC) (HPLC (HPL (g)
L..)
Solution II Acid (g) (g) (g)
(HPLC) ,.0
(g) Water (g)
i C)
15=:( top kyeel :l:.:.:::0:.:.:.:= 55(0160.ØmW1a13teKr .: ::
.=.::=:=: .= :== :=:: I=::== :..: V= .6. :14::::
:5:=:=:::7=:::5=:=::: :1:=::9:=:::5=:=::.:..2 6.=::::::.: :::.: ..3.:...
8.1.1:=:..: 79 =1.:=:=:2=:=:=6::=:=:=:::=:::= =:3=:=::.:0=:=:=:::1=:=7
0P. 4:.= :::::: =0.::.=:.=:. = 0.0 7.06 I.....4 ..4...
.i
. -=.=.=.=.=.=.=.=.=.=.=.=.=.=.=.=:= -
........
==== =
===============================================================================
=========== ..
============================================================================
50% Water
6 (top layer) 1.0 50%MIBK 0.5 0 530.89 573.3 183.95
389.35 14.07 3.726 0.832 0 0.057 6.85 2.65
6 (Bottom Layer)_ same as above
_ 6.191 2.802 _ 0 0.05 24.10 NA ,
¨
...
.......:.4i¨i::::::"'.......: 5(r)
=
4. .: :.: .:
:.:
::
::
::
:
::
:t060iy iV co t S7SAS
00AV 3218 2738 0A0
000
vyclolex100
P
...
.....
..
:::
.....
.. .
¨ . . . ...
. . . .
. . .
.
...
. .
.
.
:
...
.: :.:
.....
...
.
.
.. . .=
. ne = =
. . ..
.=
. .
: : = =
. . = =
. . = =
=
...
= =
_
:...
" =
..... cn
o
Iv
_
Iv
o
/
al.
1
o
ul
1
Iv
o
.0
r)
CP
k.)
0
I,
k.)
0
01
01
CA)
01
00
-62-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0545] The solids in Example 79 did not stick to the sides of the reactor
or the stirrer
blades, while in Exs. 75-78 the solids were stuck to the sides of the reactor,
the bottom of the
reactor and the stir shaft. They were difficult to remove.
-63-
SEGT.P0013W0
o
Molar Liquid
w
=
Reaction Sugar 95% water
Dry Solids LA FA
Corn Hydrolysate
LA % '...,
Notebook Example Solution Solvent
Sulfuric 'a
rinse Recovered wt% wt%
Sweet Recovered
# 90 l (g) Acid (g) (9)
(9) (9) (HPLC) (HPLC) (g) Solids ,..4
100%
SMS163- Water
79 1.5 635 25.01 511.72 210.93 27.65
5.794 2.835 41.87 5.40
60 with 5%
lignin
P
.
.
,r,
.
.
,
,
.
,r,
,
.
Iv
n
,-i
cp
w
=
w
'a
c.,
c.,
,..4
c.,
oe
-64-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0546] Referring now to Figures la and 1 b. Figure la provides a general
process
description for one embodiment for the production of levulinic acid. Water,
mineral acid and
biomass are added to a reactor under reaction conditions to convert the
biomass into various
products, including levulinic acid and formic acid as well as solids char. The
solids are then
removed from the reaction mixture. The reaction mixture is then combined with
an
extraction solvent, which extracts a majority of the levulinic acid and formic
acid from the
water and sulfuric acid. In one embodiment, the formic acid is removed from
the
hydrolysate, or reaction mixture, either before or after the solids removal
step but prior to
adding the extraction solvent for levulinic acid. This can be accomplished by
methods
known in the art, such as distillation, steam stripping or extraction. In
other embodiments,
the formic acid can be extracted out of the reaction mixture after the
extraction of levulinic
acid utilizing a different extraction solvent than that used for levulinic
acid. In still another
embodiment, the formic acid and levulinic acid are both extracted using the
same extraction
solvent. The water and sulfuric acid is then optionally recycled back to the
reactor and the
formic acid and levulinic acid are separated from the extraction solvent,
after which the
extraction solvent can be recycled back to be re-used in the extraction step.
[0547] The reactor can be a batch reactor, a CSTR or a plug reactor. The
mineral acid
is sulfuric acid (H2SO4), hydrochloric acid (HC1), hydrobromic acid (HBr) or
hydroiodic acid
(HI), preferably sulfuric acid. The biomass comprises sludges from paper
manufacturing
process; agricultural residues; bagasse pity; bagasse; molasses; aqueous oak
wood extracts;
rice hull; oats residues; wood sugar slops; fir sawdust; naphtha; corncob
furfural residue;
cotton balls; raw wood flour; rice; straw; soybean skin; soybean oil residue;
corn husks;
cotton stems; cottonseed hulls; starch; potatoes; sweet potatoes; lactose;
sunflower seed
husks; sugar; corn syrup; hemp; waste paper; wastepaper fibers; sawdust; wood;
residue from
agriculture or forestry; organic components of municipal and industrial
wastes; waste plant
materials from hard wood or beech bark; fiberboard industry waste water; post-
fermentation
liquor; furfural still residues; and combinations thereof, a C5 sugar, a C6
sugar, a
lignocelluloses, cellulose, starch, a polysaccharide, a disaccharide, a
monosaccharide or
mixtures thereof Preferably the biomass is high fructose corn syrup, a mixture
of at least
two different sugars, sucrose, an aqueous mixture comprising fructose, an
aqueous mixture
comprising fructose and glucose, an aqueous mixture comprising
hydroxymethylfurfural, an
aqueous solution of fructose and hydroxymethylfurfural, an aqueous mixture of
glucose, an
aqueous mixture of maltose, an aqueous mixture of inulin, an aqueous mixture
of
-65-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
polysaccharides, or mixtures thereof, and more preferably, the biomass
comprises fructose,
glucose or a combination thereof
[0548] Figure lb provides a more specific process description for one
embodiment
for the production of levulinic acid.
[0549] Feeds
[0550] Concentration of feeds are controlled to maintain desired reaction
stoichiometry. "Make-up" stream flows are controlled based on the composition
and flow
rate of the recycle stream.
[0551] Reactors
[0552] One, optionally two, reactors are used to convert fructose to the
desired
products. The reactors are optionally vented to maintain an internal pressure;
the vent stream
is optionally collected to recover steam and formic acid product; the vent
stream can all be
returned to the reactor as a reflux. If there are two reactors in series, the
first reactor is
optionally controlled at a different temperature and at a high concentration
of acid in order to
achieve desired conversion and selectivity. The first reactor would generally
be controlled at
a lower temperature than the second. Optionally, a process step between the
two reactors
may be used to separate "tar" solids and/or to preferentially extract the
reaction products
(away from the aqueous feed) to feed into the second reactor.
[0553] The reactors may be operated in a batch-wise (wherein the
reactants are fed to
the reactor and the reaction continues until the desired degree of conversion,
and the products
are then emptied from the reactor) or in a continuous fashion (wherein
reactants are fed
continuously and the products are removed continuously). In one embodiment,
the reactors
are run in a continuous fashion with products removed in a steady fashion or
the reactants are
removed in a pulsed fashion. In another embodiment, the reactors are run in a
batch mode,
with the biomass preferably being added to the reactor over a period of time
t.
[0554] The agitation in the reactors should be adequate to prevent
agglomeration of
solid co-products which may be formed during the reaction. Specifically, the
reactors should
be designed with sufficient axial flow (from the center of the reactor to the
outer diameter and
back).
[0555] Flash
-66-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0556] The reaction products may be optionally cooled in a "flash"
process. The
flash step rapidly cools the reaction products by maintaining a pressure low
enough to
evaporate a significant fraction of the products. This pressure may be at or
below
atmospheric pressure. The evaporated product stream may be refluxed through
stages of a
distillation column to minimize the loss of desired reaction products,
specifically levulinic
acid, and to ensure recovery of formic acid reaction products and solvent.
Recovered solvent
may be recycled back to reactor 1 or 2.
[0557] The "bottoms" or less volatile stream from the flash step is
advanced to the
solids separation stage.
[0558] Solids separation
[0559] In the solids separation stage of the process, the solvent and
desired reaction
products, specifically levulinic acid and formic acid, are separated from any
solids which
may have formed during the reaction phase. The solids may be separated through
a
combination of centrifuge, filtration, and settling steps (ref Perrys Chemical
Engineering
Handbook, Solids Separation). The separated solids may be optionally washed
with water
and solvents to recover desired reaction products or solvent which may be
entrained in or
adsorbed to the solids. It has been found that in some embodiments, such as
those reactions
employing high levels of mineral acid (greater than 20%) that are reacted at
lower
temperatures, such as between 60-110 C, the solids may have density properties
similar to the
liquid hydrolysate which effectively allows the solids to be suspended in
solution. In these
embodiments, certain separation techniques such as centrifugation are not as
effective. In
these embodiments filtration utilizing filter media having a pore size less
than about 20
microns has been found to effectively remove solids from the mixture. When
removing
solids from the system a solid "cake" is formed. It is desirable that the cake
be up to 50%
solids. Thus any separation technique that obtains a cake having a higher
amount of solids is
preferred. A certain amount of LA and mineral acid will be present in the cake
and it may be
desirable to wash the cake with an extraction solvent or water to recover LA.
[0560] It has also been surprisingly found that the solid particles in
the high mineral
acid and lower temperature embodiments are easily filtered and do not inhibit
flow as the
cake is formed. It is believed that the properties of the char formed under
these process
conditions are such that any cake remains porous enough that a small filter
size (less than 20
microns) can be utilized while maintaining a high flow rate through the
medium.
-67-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0561] Referring now to Figures 2a through 2e, solid, black char was
isolated from a
fructose hydrolysate reaction mixture by filtration. The char was rinsed with
water 2 times to
recover additional levulinic acid and formic acid, and then, the char was
dried at 50-60 C and
30 Torr for at least 12 h. The dried char was subjected to solvent extraction
according to
Figure 2b. A considerable amount of material was extracted from the char.
Proton NMR was
used to analyze the soluble extract fraction, and it was found to contain
mostly levulinic acid
and formic acid. Thus, this solvent extraction method is surprisingly
advantageous for further
recovery of levulinic acid from the reaction mixture.
[0562] The isolated solids may be incinerated to generate power or
disposed.
[0563] The liquid stream, comprising (but not limited to) water, acid,
solvent,
levulinic acid, formic acid, and some "soluble tars" are advanced to the
extraction stage of
the process.
[0564] Extraction
[0565] In the extraction stage of the process, the liquid stream is mixed
with an
extraction solvent stream. The preferred extraction solvent dissolves
levulinic acid more
effectively than the other products in the liquid stream. The preferred
solvent does not
dissolve significantly into the water phase. Extraction configurations are
preferably multi-
stage and continuous, as described in Perry's Chemical Engineering Handbook.
[0566] The aqueous raffinate is recycled to the reactor phase, after
optional
distillation or purification steps to adjust the relative concentrations of
solvent, water, and
acid in the raffinate.
[0567] The extract solvent phase contains levulinic acid and formic acid
and is
progressed to the solvent removal stage of the process.
[0568] Suitable solvents to extract LA include, for example, polar water-
insoluble
solvents such as MIBK, MIAK, cyclohexanone, o, m, and para-cresol, substituted
phenols,
for example, 2-sec butyl phenol, C4-C18 alcohols, such as n-pentanol, isoamyl
alcohol, n-
heptanol, 2-ethyl hexanol, n-octanol, 1-nonanol, cyclohexanol, methylene
chloride, 1,2-
dibutoxy-ethylene glycol, acetophenone, isophorone, o-methoxy-phenol, methyl-
tetrahydrofuran, tri-alkylphosphine oxides (C4-C18) and ortho-dichlorobenzene
and mixtures
thereof Such solvents are used generally at room temperature so as not to
serve as potential
reaction component.
[0569] Solvent removal
-68-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0570] Levulinic acid may be separated from the solvent phase by
evaporating or
distilling the solvent. Alternatively, the levulinic acid may be crystallized
from the solvent
phase in a crystallization process. The solvent removal process may be a
combination of
distillation and crystallization. The recovered solvent may be recycled to the
extraction step
or to the reactor step.
[0571] The resulting stream of highly concentrated levulinic acid may be
advanced
for further chemical derivatization or may be further purified in another
distillation step such
as high vacuum wipe-film-evaporation or falling film evaporation. Preferably
the levulinic
acid stream is kept at a low temperature throughout the solvent removal steps
to inhibit the
formation of angelica lactone.
[0572] Mineral Acids
[0573] Suitable acids used to convert the biomass materials described
herein,
including sugars, include mineral acids, such as but not limited, to sulfuric
acid, hydrochloric
acid, hydrobromic acid, hydroiodic acid, nitric acid, phosphoric acid, boric
acid, hydrofluoric
acid, perchloric acid and mixtures thereof
[0574] Example 80
[0575] Into a three neck 250 mL round bottom flask 130.01g deionized
water and
23.51g (0.13 mol, 0.72M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
63.78g (0.65 mol, 3.60M) sulfuric acid was added into the flask. The heat was
turned on and
set to a temperature of 80 C. Samples were taken as the reaction mixture was
heated up and
analyzed by HPLC. The reaction was held at 80 C for four hours and then the
reaction was
shut down. The solids that were formed during the reaction were filtered and
then dried in a
vacuum oven overnight.
[0576] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
-69-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
Grams of Solids 3.44
% Solids based on Fructose 14.63
% Solids based on Total Reaction Weight 1.58
Grams of LA 8.28
% LA based on Fructose 35.20
% LA based on Total Reaction Weight 3.81
[0577] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0578] Example 81
[0579] Into a three neck 250 mL round bottom flask 133.12g deionized water
and
23.49g (0.13 mol, 0.71M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
63.75g (0.65 mol, 3.54M) sulfuric acid was added into the flask. The heat was
turned on and
set to a temperature of 90 C. Samples were taken as the reaction mixture
heated up and were
analyzed by HPLC. The reaction was held at 90 C for four hours and then the
reaction was
shut down. Samples were taken throughout the entire reaction and analyzed by
HPLC. The
solids that were formed during the reaction were filtered and then dried in a
vacuum oven
overnight.
[0580] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Solids 3.77
% Solids based on Fructose 16.05
% Solids based on Total Reaction 1.71
Weight
-70-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
Grams of LA 10.55
% LA based on Fructose 44.90
% LA based on Total Reaction Weight 4.79
[0581] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0582] Example 82
[0583] Into a three neck 250 mL round bottom flask 130.02g deionized
water and
23.42g (0.13 mol, 0.72M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
63.78g (0.65 mol, 3.60M) sulfuric acid was added into the flask. The heat was
turned on and
set to a temperature of 90 C. The reaction was held at 90 C for two hours and
twenty minutes
and then the reaction was shut down.
[0584] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Solids 5.19
% Solids based on Fructose 22.16
% Solids based on Total Reaction Weight 2.39
Grams of LA 8.25
% LA based on Fructose 35.22
% LA based on Total Reaction Weight 3.80
[0585] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0586] Example 83
[0587] Into a three neck 250 mL round bottom flask 65.04g deionized water
and
11.71g (0.065 mol, 0.60M) D-fructose were charged. The round bottom flask was
equipped
-71-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
63.80g (0.65 mol, 6.04M) sulfuric acid was added into the flask slowly. Once
all of the
sulfuric acid was added to the reaction mixture the heat was turned on and set
to a
temperature of 80 C. The reaction was held at 80 C for two hours and then the
reaction was
shut down. The solids that were formed during the reaction were filtered and
then dried in a
vacuum oven overnight.
[0588] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Solids 2.90
% Solids based on Fructose 24.77
% Solids based on Total Reaction Weight 2.06
Grams of LA 5.84
% LA based on Fructose 49.84
% LA based on Total Reaction Weight 4.15
[0589] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0590] Example 84
[0591] Into a three neck 250 mL round bottom flask 60.06g deionized water
and
10.88g (0.06 mol, 0.61M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
an ice water bath was placed beneath the round bottom flask in order to cool
the reaction
mixture. The ice water bath was used to prevent the reaction mixture from
getting too hot
when the sulfuric acid was added. Once the reaction mixture was cold, 58.96g
(0.60 mol,
6.04M) sulfuric acid was added into the flask making sure to keep the reaction
mixture below
45 C. Once all of the sulfuric acid was added to the reaction mixture the ice
water bath was
removed and the heating mantle was situated under the flask. The heat was
turned on and set
to a temperature of 90 C. The reaction was held at 90 C for thirty minutes and
then the
-72-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
reaction was shut down, the heating mantle was removed and an ice water bath
was used to
cool the mixture. The solids that were formed during the reaction were
filtered and then dried
in a vacuum oven overnight.
[0592] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Solids 5.49
% Solids based on Fructose 50.46
% Solids based on Total Reaction Weight 4.23
Grams of LA 4.08
% LA based on Fructose 37.51
% LA based on Total Reaction Weight 3.14
[0593] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0594] Example 85
[0595] Into a three neck 250 mL round bottom flask 60.04g deionized water
and
10.91g (0.06 mol, 0.46M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
an ice water bath was placed beneath the round bottom flask in order to cool
the reaction
mixture. The ice water bath was used to prevent the reaction mixture from
getting too hot
when the sulfuric acid was added. Once the reaction mixture was cold, 117.73g
(1.2 mol,
9.13M) sulfuric acid was added into the flask making sure to keep the reaction
mixture below
30 C. Once all of the sulfuric acid was added to the reaction mixture the ice
water bath was
removed and a heating mantle was situated under the flask. The heat was turned
on and set to
a temperature of 50 C. The reaction was held at 50 C for thirty minutes and
then the reaction
was shut down, the heating mantle was removed and cooled with the ice water
bath. Once the
reaction mixture was cooled it was filtered in order to obtain any solids that
were formed, the
surprising thing was that no solids were observed. The reaction mixture was
placed back into
the round bottom flask and set up again in order to continue the reaction. The
heat was turned
on and set back to 50 C. The reaction was left to run for another 433 minutes
and then was
-73-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
shut down. The reaction mixture was filtered again and this time solids were
observed. The
solids were put into a vacuum oven to dry overnight.
[0596] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Solids 8.68
% Solids based on Fructose 79.56
% Solids based on Total Reaction Weight 4.60
Grams of LA 3.85
% LA based on Fructose 35.31
% LA based on Total Reaction Weight 2.04
[0597] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0598] Example 86
[0599] Into a three neck 250 mL round bottom flask 40.04g deionized water
and
7.21g (0.04 mol, 0.37M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
an ice water bath was placed beneath the round bottom flask in order to cool
the reaction
mixture. The ice water bath was used to prevent the reaction mixture from
getting too hot
when the sulfuric acid was added. Once the reaction mixture was cold, 117.78g
(1.2 mol,
11.02M) sulfuric acid was added into the flask making sure to keep the
reaction mixture
below 30 C. Once all of the sulfuric acid was added to the reaction mixture
the ice water bath
was removed and a heating mantle was situated under the flask. The heat was
turned on and
set to a temperature of 50 C. The reaction was held at 50 C for forty five
minutes and then
the reaction was shut down, the heating mantle was removed and cooled with the
ice water
bath. In order to form more product, levulinic acid and formic acid, the
reaction mixture was
heated back up to 50 C and left to react for another thirty minutes. After the
thirty minutes
the reaction was shut down and cooled with an ice water bath. The reaction
mixture was
filtered but no solids were observed.
-74-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
Grams of LA 2.43
% LA based on Fructose 33.65
% LA based on Total Reaction Weight 1.47
[0600] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0601] Example 87
[0602] Into a three neck 250 mL round bottom flask 40.07g deionized water
and
7.35g (0.04 mol, 0.37M) D-fructose were charged. The round bottom flask was
equipped
with a magnetic stir bar, thermocouple, condenser, and glass stopper. The stir
plate was set to
stir at a rate of 550 RPM and the fructose quickly dissolved. Once the
fructose was dissolved,
an ice water bath was placed beneath the round bottom flask in order to cool
the reaction
mixture. The ice water bath was used to prevent the reaction mixture from
getting too hot
when the sulfuric acid was added. Once the reaction mixture was cold, 117.76g
(1.2 mol,
11.00M) sulfuric acid was added into the flask making sure to keep the
reaction mixture
below 30 C. Once all of the sulfuric acid was added to the reaction mixture
the ice water bath
was removed and a heating mantle was situated under the flask. The heat was
turned on and
set to a temperature of 50 C. The reaction was held at 50 C for two hours and
then the
reaction was shut down, the heating mantle was removed and cooled with the ice
water bath.
The reaction mixture was filtered and the solids were placed into a vacuum
oven to dry.
[0603] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Solids 3.00
% Solids based on Fructose 40.82
% Solids based on Total Reaction Weight 1.82
Grams of LA 2.55
% LA based on Fructose 34.64
% LA based on Total Reaction Weight 1.54
-75-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0604] It was observed that reduced char was present at filtration as
compared to
reactions at higher temperatures and lower acid levels and little to no char
was accumulated
on the reactor components.
[0605] Examples 80-87 demonstrate that running the reactions at reduced
temperatures (less than 100 C) improves the selectivity for levulinic acid,
and increased
levels of mineral acid (such as 40-72% of sulfuric acid) lead to faster
reaction times.
Combining these 2 features results in faster reactions that are highly
selective to levulinic
acid with significantly less char.
[0606] Reactor Modeling
[0607] A combination of experimental and modeling research has been
conducted in
order to analyze and recommend continuous reactor designs for the production
of levulinic
acid from fructose. The main focus of this example is a description of kinetic
and reactor
modeling methods, model validation, and recommended reactor configurations
that maximize
the yield of the desired product while minimizing undesirable byproducts, such
as HMF and
char.
[0608] A validated kinetic model has been developed by adapting an acid-
catalyzed
glucose decomposition mechanism from the thesis of Girsuta to describe batch
reactor data
for the conversion of fructose to levulinic acid. Kinetic parameters in the
model were
adjusted using regression analysis to fit the model to the data. The model has
been
implemented for two types of ideal continuous reactors: a continuous stirred
tank reactor
(CSTR) and a plug flow reactor (PFR). The CSTR model predictions compared
favorably
with a single data set from a continuous flow reactor experiment. The
experimental and
modeling results illustrate that byproduct formation is minimized using higher
catalyst
(H2SO4) concentrations (e.g. 5 mole/liter) and lower temperatures (50 to 100
C) than
employed in the thesis.
[0609] The validated model was implemented in an Aspen Plus flowsheet to
study the
effect of multiple reactor configurations, residence times and reactor
temperatures on the
yields of the desired product (levulinic acid) and the undesired byproduct
(humins, or char).
More than fifty configurations were run and the resulting yield and conversion
predictions
were analyzed to recommend a reactor configuration for experimental study. The
cases
studied all used a feed with catalyst (H2504) concentration of 5 mole/liter
and fructose
concentration of 1 mole/liter.
-76-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0610] Examples 88-102
[0611] Model Predictions for Multiple Reactor Configurations
[0612] The reaction rate mechanism that was implemented and verified in
Aspen for
fructose decomposition was used to study the performance of networks of CSTR
and PFR
reactors. Several networks were studied at the same time to simplify
comparison of
performance. The Aspen flowsheet diagram is shown in Figure 3. Five
configurations were
studied, as described in the figure.
[0613] Case A: Two CSTR reactors in series: large, then small
[0614] Case B: A small PFR followed by a large CSTR
[0615] Case C: A single CSTR
[0616] Case D: A large CSTR followed by a small PFR
[0617] Case E: Three CSTR reactors in series
[0618] The flowsheet simulation was run many times using an acid
concentration of 5
mole/liter and a sugar concentration of 1 mole/liter. The
total residence time was
constrained to be 180 minutes for all cases, to provide a consistent basis for
comparison. The
temperature range described in this report ranged from 100 to 120 C. Other
simulations
were done at temperatures ranging from 90 to 100 C, but they had lower
conversion and are
not described in this report. The individual residence times for the reactors
and the reactor
temperatures were varied for the study.
[0619] The results from three interesting sets of cases are shown in
Table 7.
Examples 88-102 are described in Table 7. In set 1, all temperatures were set
to 100 C, and
the reactor residence times were at their base value. In set 2, temperatures
were also 100 C,
but the residence time for the first reactor in each sequence was increased.
This modification
reduced the yield to the undesirable humin product. In set 3, the temperature
for the second
(or third) reactor was increased. In case 3D, the CSTR residence time was also
increased.
This modification increased the yield of desirable levulinic acid but did not
significantly
change the yield to the undesirable humins product. Cases 3D and 3E have very
similar
performance predictions.
[0620] Configurations 3D and 3E both had a large CSTR reactor followed by
one or
two small reactors at higher temperature. In case 3D, the second reactor is a
PFR, while in
case 3E, the second and third reactors are small CSTRs. These configurations
both had
fructose conversion greater than 99%, soluble Levulinic Acid yield above 63%,
Humins yield
of 1.23%, and HMF yield below 0.1%. Total yield of Levulinic Acid (soluble &
insoluble)
was predicted to be greater than 94% for these configurations.
-77-
SEGT.P0013W0
Table 7: Results summary for Sets 1-3 (Examples 88-102).
..
a v:j= Ii 1:: : :::::::: :E: ;...:
::: !!:=r: .9.g ::E* -'4. H x' : O
T_''. 9' ,7('f_x-:974:: 0 a 100 101 N
taT 6J
Description . ::=4 Case ;; Case L'asc
Case Case case: 2.t: kr';c c., c. Ca Ca c k-Z
Case 'i cO.,..9:,,,,iiiiiili::ic 4,9.:,,,10::,::' la i:,i
2..A.: iii ).J3 "le' 215.: : :: : :vi ,,. (ac
:::: asc : , sc s
1::A :.::: ::::
::::::: : :::::::::
yield LA 60.07 61.47 55.08 62.70 61.56 59.19
60.33 55.08 61.03 61.21t)
62.96 62.26 55.08 63.57 63.49
Soluble
fructose 95.88 99.30
89.49 98.95 98.07 94.75 97.81 89.49 96.94 97.20 99.04 99.74
89.49 99.82 99.74
conversion
Yield 1.23 1.84 1.05 1.31 1.40 1.14 1.75
1.05 1.23 1.23 1.14 1.66 1.05 1.23 1.23
Humins
Ratio LA/ 49.00 33.43 52.42 47.73 43.94 52.00
34.45 52.42 49.79 49.93 55.31 37.42 52.42 51.86
51.79
humins
P
Yield Lev.A 89.67 91.77 82.22 93.52 91.86 88.35 90.02
82.22 91.07 91.33 93.96 92.91 82.22 94.83 94.75
.32
Total..'
Yield of 1.40 0.18 3.15 0.26 0.61 1.84 0.88
3.15 1.05 0.96 0.44 0.09 3.15 0.09 0.09
r.,
HMF
Res. Time 120 180 120 60 150 180 150
120 150 180 170 120 1
u.,
Res. Time 60 120 60 30 150
30 30 150 30
CSTR 2
Res. Time 60
30 30
CSTR 3
Res. Time 61.84 61.89 31 31
30 10
PFR
Total Res. 180 182 180 182 180 180 181 180 181
180 180 180 180 180 180 Iv
n
Time (min)
CSTR1
Temp 100 100 100 100 100 100 100
100 100 100 100 100 cp
t..)
,-,
t..)
Temp 100 100 100 100 100
100 120 120 110
CSTR 2
Temp
100 100 120
oe
-78-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
0
cc)
,¨i
c>
c>
P-I
N
C.,
4.=
C/)
0
0
-,
0
0
-,
0
0
0
,--,
0
0
,--,
C4
f:4 0-1
=
ci) E
Cm) C14
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0621] Examples with continuous feed aspects or simple batch processes
[0622] HLPC Method
[0623] The instrument used was a WATERS 2695 LC system with a WATERS 2998
PDA detector. A Hamilton PRP-X300 column (71Am 250 x 4.1 mm) was used with 5
1AL
injections. The column temperature was maintained at 50 C. There are two
mobile phases
used. "Solvent A" is 20 mM of Phosphoric Acid in DI H20. "Solvent B" is
Methanol (HPLC
Grade). An isocratic flow of 2 mL/min is used with a (80% Solvent A/20%
Solvent B)
mobile phase mixture. Sample data is analyzed by extracting a chromatogram at
210 nm
wavelength.
[0624] LC-RI method The instrument used was a WATERS 1515 LC pump with a
WATERS 717 autosampler and WATERS 2410 RI detector. A Supelcosil-LC-NH2 (250mm
x 4.6mm x 5[Lm) was used with 10 1AL injections. The column temperature was
maintained at
50 C. The mobile phase was 75% Acetonitrile/25% Nanopure H20. An isocratic
flow of 1
mL/min was used. Samples were filtered and diluted 5-10x with Nanopure H20
before
analysis.
[0625] Example 103
[0626] 122.01g deionized water and 108.03g (96-98%) sulfuric acid was
charged into
a 500mL 4-neck round bottom flask. 40.08g HFCS 55 (high fructose corn syrup;
ADM, Inc.
55% Fructose) was charged into a 60mL syringe. The round bottom flask was
situated in a
heating mantle and equipped with a magnetic stir bar, thermocouple, condenser,
glass stopper
and the syringe pump inlet tube. The water and sulfuric acid solution was
stirred at 650 RPM
and heated up to a temperature of 90 C. The HFCS 55 was added using a syringe
pump over
a course of two hours at a rate of 15mL/hr. After all of the HFCS 55 had been
added into the
round bottom flask, the reaction was held at temperature for one hour. After a
total reaction
time of three hours a sample was taken to be analyzed by LC-UV and LC-RI and
then the
reaction was shut down and allowed to cool to ambient temperature. Once the
reaction
mixture was cool, the solids were filtered out and then washed with water and
acetone. The
solids were then measured using a moisture analyzer.
-80-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
% % % % % g g g g g g
FA LA HMF Fructose Glucose FA LA HMF Fructose Glucose Char
1.45 3.19 0.01 0.00 2.79 3.58 7.88 0.02 0.00 6.89 2.49
[0627] Example 104
[0628] 30g Fructose, 37.02g Glucose was dissolved into 33.09g deionized
water.
Then 40.02g of the sugar solution was placed into a 60mL syringe. 122.02g
deionized water
and 108.11g sulfuric acid (96-98%) was charged into a 500mL 4-neck round
bottom flask.
The round bottom flask was situated in a heating mantle and equipped with a
magnetic stir
bar, thermocouple, condenser, glass stopper and the syringe pump tube inlet.
The water and
sulfuric acid solution was stirred at 650 RPM and heated up to a temperature
of 90 C. The
sugar solution was added using a syringe pump over a course of two hours at a
rate of
15mL/hr. After all of the sugar solution had been added into the round bottom
flask, the
reaction was held at temperature for one hour. After a total reaction time of
three hours a
sample was taken and analyzed by LC-UV and LC-RI and then the reaction was
shut down
and allowed to cool to ambient temperature. Once the reaction mixture was
cool, the solids
were filtered out and then washed with water and acetone. The solids were then
measured
using a moisture analyzer.
% % % % % g g g g g g
FA LA HMF Fructose Glucose FA LA HMF Fructose Glucose Char
1.17 2.82 0.00 0.00 4.77 2.89 6.97 0.00 0.00 11.78 1.211
[0629] Examples 105, 106 and 107
[0630] 15mL of the resulting solution from Example 1 was added to an
empty 3 oz.
high pressure, high temperature reaction vessel equipped with a thermocouple
and pressure
gauge for monitoring the internal temperature and pressure (Example 4a). A
second reaction
vessel was also charged with 15mL of the resulting solution from Example 2
(Example 4b).
After proper assembly, the reaction vessels were then placed into a 140C hot
oil bath to reach
an internal temperature of around 130C. After 2 hours the reaction vessels
were removed
from the hot oil and placed in a room temperature water bath for 1 minute to
begin cooling.
Following the room temperature water bath, the reactors were placed in an ice
water bath to
quench the reactions. Once the reactions had cooled completely, the reactor
vessels were
opened and the mixtures were analyzed individually by HPLC. Any solids formed
during the
-81-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
reaction were also washed with DI water and weighed. The solids water wash was
also
analyzed by HPLC and included in the final product calculations.
[0631] The HPLC results for Example 105 show the glucose completely
converted to
products. The levulinic acid to solids mass ratio was 1.8. (Weight of LA to
weight to solids.)
For Example 106 the HPLC results show the glucose reacting to about 88%
conversion. The
levulinic acid to solids mass ratio was 1.74.
[0632] A third 3 oz. high pressure, high temperature reaction vessel
equipped with a
thermocouple and pressure gauge for monitoring the internal temperature and
pressure was
charged with 15mL of the resulting solution from Example 104 (Example 107).
After proper
assembly, the reaction vessel was placed into a 120C hot oil bath to reach an
internal
temperature of around 110C. After 3 hours the reaction vessel was removed from
the hot oil
and placed in a room temperature water bath for 1 minute to begin cooling.
Following the
room temperature water bath, the reactor was placed in an ice water bath to
quench the
reaction. Once the reaction had cooled completely, the reactor vessel was
opened and the
mixture was analyzed by HPLC. Any solids formed during the reaction were also
washed
with DI water and weighed. The solids water wash was also analyzed by HPLC and
included
in the final product calculations.
[0633] The HPLC results for Example 107 show the glucose conversion to be
87%.
The levulinic acid to solids mass ratio was 2.32.
[0634] Example 108
[0635] 47.95g deionized water and 99.68g sulfuric acid (96-98%) were
charged into a
250mL 3-neck round bottom flask. 2.40g Fructose and 10.02g deionized water was
charged
into a small beaker and placed on a stir plate to dissolve the fructose. The
round bottom flask
was situated in a heating mantle and equipped with a magnetic stir bar,
thermocouple,
condenser and glass stopper. The water and sulfuric acid was stirred at a rate
of 650 RPM and
heated up to a temperature of 90 C. The fructose solution was injected all at
once into the
reaction mixture and allowed to react for one hour. After a reaction time of
one hour a sample
was pulled to be analyzed by LC-UV and LC-RI then the reaction was shut down.
Once the
reaction mixture was at ambient temperature it was filtered and no solids were
observed.
% FA % LA % HMF % Fructose g FA g LA g HMF g Fructose g Char
0.29 0.64 0.00 0.00 0.46 1.02 0.00 0.00 0.00
-82-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0636] Example 109
[0637] 84.04g deionized water and 63.79g sulfuric acid (96-98%) was
charged into a
250mL 3-neck round bottom flask. 1.7108g HMF and 10.02g deionized water was
charged
into a scintillation vial and placed on a stir plate to dissolve the HMF. The
round bottom flask
was situated in a heating mantle and equipped with a magnetic stir bar,
thermocouple,
condenser and glass stopper. The water and sulfuric acid mixture was stirred
at 650 RPM and
heated up to a temperature of 90 C. The HMF solution was then injected all at
once into the
round bottom flask and allowed to react for one hour. The reaction was shut
down after a
reaction time of one hour and a sample was taken at the end and analyzed by LC-
UV. Once
the reaction mixture was at ambient temperature it was filtered and no solids
were observed.
% FA % LA % HMF g FA g LA g HMF g Char
0.44 0.97 0.00 0.70 1.55 0.00 0.00
[0638] Example 110
[0639] 1.6658g HMF and 10.0437g deionized water was charged into a
scintillation
vial and set on a stir plate to dissolve the HMF. 77.06g deionized water and
76.55g sulfuric
acid (96-98%) was charged into a 250mL 3-neck round bottom flask. The round
bottom flask
was situated in a heating mantle and equipped with a magnetic stir bar,
thermocouple,
condenser, and glass stopper. The water and sulfuric acid was heated up to 90
C while
stirring at 650 RPM. Once the HMF was all dissolved it was injected all at
once into the
water and sulfuric acid mixture. The reaction was shut down after a reaction
time of 30
minutes and a sample was taken at the end and analyzed by LC-UV. Once the
reaction
mixture was at ambient temperature it was filtered and no solids were
observed.
% FA % LA % HMF g FA g LA g HMF g Char
0.40 0.99 0.00 0.65 1.62 0.00 0.00
[0640] Example 111
[0641] 3.785g Fructose, 2.657g HMF and 10.014g deionized water was
charged into
a beaker then placed on a stir plate to dissolve the fructose and HMF. 139.35g
deionized
water and 103.03g sulfuric acid (96-98%) was charged into a 500mL 4-neck round
bottom
flask. The round bottom flask was situated in heating mantle and equipped with
a magnetic
-83-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
stir bar, thermocouple, condenser, and two glass stoppers. The water and
sulfuric acid were
stirred at 650 RPM and heated to 90 C. The fructose and HMF solution was then
injected
into the round bottom flask all at once and allowed to react for one hour.
After a reaction time
of one hour a sample was taken to be analyzed by LC-UV and LC-RI and then shut
down.
Once the reaction mixture was at ambient temperature it was filtered and no
solids were
observed.
% FA % LA % HMF % Fructose g FA g LA g HMF g Fructose g Char
0.45 1.11 0.23 0.279 1.05 2.59 0.54 0.65 0.00
[0642] Example 112
[0643] 13.24g HMF and 30.05g deionized water was charged into a beaker
then
placed the beaker on a stir plate to dissolve the HMF. 113.35g deionized water
and 103.05g
(96-98%) sulfuric acid was charged into a 500mL 4-neck round bottom flask. The
round
bottom flask was situated in a heating mantle and equipped with a magnetic
stir bar,
thermocouple, condenser, glass stopper and the syringe pump inlet. The water
and sulfuric
acid was stirred at 650 RPM and heated to a temperature of 90 C. The HMF
solution was
added using a syringe pump over a course of five hours at a rate of 7.4mL/hr.
After all of the
HMF had been added into the round bottom flask, the reaction was held at
temperature for
one hour. After a total reaction time of six hours a sample was taken and
analyzed by LC-UV
and then the reaction was shut down and allowed to cool to ambient
temperature. Once the
reaction mixture was cool, the solids were filtered out and then washed with
water and
methylene chloride and then the char was left to dry overnight. The char was
then put into a
scintillation vial which was then placed in a vacuum oven to dry until a
constant weight was
obtained.
% FA % LA % HMF g FA g LA g HMF g Char
2.21 4.71 0.00 5.37 11.44 0.00 0.745
[0644] Example 113
[0645] A 250mL Erlenmeyer flask was charged with 114.95g of 64% Sulfuric
Acid,
and 64.27g de-ionized water. The acidic water mixture was placed in an ice
bath and allowed
to cool. After the solution was cool, 3.78g Fructose and 2.65g
Hydroxymethylfurfural (HMF)
were also added to the Erlenmeyer flask. The mixture was mixed well until
completely
-84-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
dissolved. The resulting molarities calculate to 0.14M Fructose, 0.14M HMF and
5M Sulfuric
acid.
[0646] 15mL of the prepared solution was added to an empty 3 oz. high
pressure,
high temperature reaction vessel equipped with a thermocouple and pressure
gauge for
monitoring the internal temperature and pressure. After proper assembly, the
reaction vessel
was then placed into a 100C hot oil bath to reach an internal temperature of
around 90C.
After 60min the reaction vessel was removed from the hot oil and placed in a
room
temperature water bath for 1 minute to begin cooling. Following the room
temperature water
bath, the reactor was placed in an ice water bath to quench the reaction. Once
the reaction had
cooled completely, the reactor vessel was opened and the mixture was analyzed
by HPLC.
Any solids formed during the reaction were washed with DI water and weighed.
The solids
water wash was also analyzed by HPLC and included in the final product
calculations.
[0647] The HPLC results for Example 113 show the HMF conversion equal to
99%
conversion and the fructose completely reacting away after 60 min. The molar
percent yield
of levulinic acid (LA) was 96%. Also the LA to solids mass ratio was 2.95.
[0648] Example 114
[0649] A 250mL Erlenmeyer flask was charged with 114.94g of 64% Sulfuric
Acid,
and 63.14g de-ionized water. The acidic water mixture was placed in an ice
bath and allowed
to cool. After the solution was cool, 3.79g Fructose and 3.98g
Hydroxymethylfurfural (HMF)
were also added to the Erlenmeyer flask. The mixture was mixed well until
completely
dissolved. The resulting molarities calculate to 0.14M Fructose, 0.21M HMF and
5M Sulfuric
acid.
[0650] 15mL of the prepared solution was added to an empty 3 oz. high
pressure,
high temperature reaction vessel equipped with a thermocouple and pressure
gauge for
monitoring the internal temperature and pressure. After proper assembly, the
reaction vessel
was then placed into a 100C hot oil bath to reach an internal temperature of
around 90C.
After 60min, the reaction vessel was removed from the hot oil and placed in a
room
temperature water bath for 1 minute to begin cooling. Following the room
temperature water
bath, the reactor was placed in an ice water bath to quench the reaction. Once
the reaction had
cooled completely, the reactor vessel was opened and the mixtures were
analyzed
individually by HPLC. Any solids formed during the reaction were also washed
with DI
water and weighed. The solids water wash was also analyzed by HPLC and
included in the
final product calculations.
-85-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0651] The HPLC results for Example 114 show the HMF reacting to 99%
conversion and the fructose completely reacting away after 60min. The molar
percent yield of
levulinic acid (LA) was 96.71%. Also the LA to solids mass ratio was 3.95.
[0652] Examples with continuous feed and/or recycling aspects:
[0653] Example 115: Synthesis of LA + FA with a mixed sugar solution by
continuous feeding.
[0654] To a 3 neck, 1L round bottom flask equipped with condenser and
thermocouple magnetic stirring was charged 126.45 g H20 and 311.88 g of 64%
(wt) H2SO4.
The reaction mixture was heated to 90 C at which point 40.5 g of a sugar
solution containing
69.3 % fructose, 23 % water, 6.16 % glucose, and 1.54 % others was injected
over a 5 hour
period using a syringe pump. When all of the sugar solution had been added,
the reaction
mixture was cooled to room temperature and transferred to a 1L Hastelloy C
Parr reactor
kettle. The reactor was sealed and heated to 120 C for 90 minutes to fully
convert any
remaining reactant or intermediates to products. During this final step, the
pressure of the
reactor remained below 25 psi.
[0655] Table 8 HPLC analytical results of hydrolysis samples taken at
various times
during the reaction
Time of
reaction Temperature % Formic % Levulinic
(min) ( C) Acid Acid % HMF
305 89.4 1.539 3.222 0.072
Non-
90 120 1.526 3.391 Detectable
[0656] The above reaction mixture was cooled to room temperature using an
ice bath.
435 gm of this mixture was poured into a 150 ml Buchner funnel with a glass
frit (4 - 5
micron filter size), that was placed on top of 1000 mL filter flask connected
to a Teflon
-86-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
vacuum pump. Vacuum was used to aid the filtration (<250 mm), filtrate was
allowed to
drain for 5 ¨ 10 minutes before the Teflon vacuum pump was turned off. 413 gm
of filtrate
and 22.11 gm of wet solids were obtained (9 for composition details for the
filtrate). The
solid was washed with 4 X 50 mL DI water. Another 10 mL was added and the
filtrate of the
mL wash was tested using a pH probe (pH = 2.04). The solids were washed with
48 gm of
acetone and air dried overnight to give 5.0 gm of dry char (1.04 wt% based on
total initial
charge). The char was powdery in nature, and was not sticky. It flowed easily
before
filtration, and it did not stick to reactor components.
[0657] The 413 gm filtrate was poured into a 1000 mL cell culture spinner
flask,
followed by addition of 828 gm of methyl isobutyl ketone (99.8%, Macron
chemicals,
Philipsburg, NJ). The solution was stirred at 150 rpm for 30 minutes and the
two layers were
poured into a 2000 mL cylindrical separatory funnel. The two layers were
allowed to phase
separate over 30 mins. The bottom layer was drained into a 1000 mL three neck
round
bottom flask and the top layer (OEX) to a 2000 mL two neck round bottom flask
(see Table 9
for composition details for each layer).
[0658] The 2000 mL two neck round bottom flask containing the organic
extract
(OEX) was setup for short path distillation using a magnetic stirrer and
heating mantle
connected to a variable transformer. The short path distillation head was
connected to a
Teflon vacuum pump and a chiller (set at 10 C). Temperature of the organic
extract and the
distillate vapor was measured using a J-type thermocouple. The vacuum was
controlled to 50
mm using a digivac vacuum controller. The 2000 mL flask was subjected to 50 mm
vacuum
before the heating mantle was turned. Once the temperature in the round bottom
flask
reached 37 C the methyl isobutyl ketone started distilling over (distillate
vapor temperature
¨ 37 C). Distillation was stopped when 80% of the methyl isobutyl ketone was
distilled. The
levulinic acid in the bottom of the reactor vessel was isolated as a crude
solution in methyl
isobutyl ketone (See Table 9 for details).
[0659] The 1000 mL three neck round bottom flask containing the bottom
layer of the
extraction (raffinate) mixture was also setup for distillation. Setup for
distillation included a
distillation adapter, condenser connected to a chiller, J-type thermocouple
for the round
bottom flask and distillate vapor, Teflon vacuum pump and an oil bath with a
hotplate/stirrer
for heating. The pressure was controlled using a J-Kem scientific vacuum
controller. The
round bottom flask containing the raffinate was subjected to 50 mm vacuum
before it was
heated. Once the temperature in the round bottom flask reached 40 C the water
methyl iso
butyl ketone azeotrope started distilling over. Distillation was continued
till all the methyl
-87-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
isobutyl ketone was distilled over (distillation receiver shows only increase
in water layer, top
layer remains constant). The raffinate, after distillation, was used to make
the next batch. (See
Table 9 for composition details.
[0660] Table 9 Composition for filtration, extraction and distillation
streams
Sample stream Mass %Levulinic % Formic % Sulfuric
acid acid acid
Filtrate 413 3.39 1.53 Not
determined
Raffinate, 401 1.31 0.38 39.65
before
distillation
Raffinate, after 355.3 1.57 0.33 44.23
distillation
Organic 827 0.84 0.43 0.12
extract
Final crude 142.8 3.58 0.69 0.85
product
[0661] Example 116: Synthesis of LA and FA with Recycled Raffinate from
Example 1
[0662] To a 3 neck flask equipped with magnetic stirring, a chilled
condenser, and
thermocouple was charged 348 g of the recycled raffinate from Example 115.
This raffinate
contained approximately 157 g H2504, 5.6 g levulinic acid, and 1.2 g formic
acid. To the
raffinate charge was added 67 g of fresh water to bring the acid concentration
in the aqueous
phase to approximately 40 %. The aqueous phase was heated to 90 C before the
addition of
40.35 g of a sugar solution of identical composition to that used in Example
115 was added
over 5 hours. After the sugar addition was complete, a 120 C post cook
identical to that of
Example 115 was used to fully convert any unreacted reagents.
-88-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0663] Table 10 HPLC analytical results of composition.
Temperature % Formic % Levulinic
Time (min) ( C) Acid Acid % HMF
305 90 1.876 4.362 0.073
Non-
90 120 1.958 4.386 Detectable
[0664] The above reaction mixture was cooled to room temperature using an
ice bath.
398.8 gm of this mixture was poured into a 150 ml Buchner funnel with a glass
frit (4 - 5
micron filter size), that was placed on top of 1000 mL filter flask connected
to a Teflon
vacuum pump. The solids flowed easily out of the reactor and were not sticky
in nature.
Vacuum was used to aid the filtration (<250 mm), filtrate was allowed to drain
for 5 ¨ 10
minutes before the Teflon vacuum pump was turned off 379.2 gm of filtrate and
19.6 gm of
wet solids were obtained. The solid was washed with 10 X 100 mL DI water.
Another 80 mL
was added and the filtrate of the 80 mL wash was tested using a pH probe (pH =
1.96). The
solids were washed with 68 gm of acetone and air dried overnight to give 5.43
gm of dry char
(1.21 wt% based on total initial charge)
[0665] The extraction and purification procedure was repeated as
described in
Example 115 to afford a second recycled raffinate stream, Recycled Raffinate
Stream from
Example 116.
[0666] Example 117: Synthesis of LA and FA with Recycled Raffinate Stream
from
Example 116.
[0667] To a 3 neck flask equipped with magnetic stirring, a chilled
condenser, and
thermocouple was charged 250 g of the recycled raffinate from Example 116.
This raffinate
contained approximately 126 g H2504, 4.2 g levulinic acid, and 1.9 g formic
acid. To the
raffinate charge were added 82 g of 64% fresh H2504 and 106 g of fresh water
to bring the
acid concentration in the aqueous phase to approximately 40 %. The aqueous
phase was
heated to 90 C before the addition of 40.85 g of a sugar solution of
identical composition to
that used in Example 115 was added over 5 hours. After the sugar addition was
complete, a
120 C post cook identical to that of Example 115 was used to fully convert
any unreacted
-89-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
reagents. Again, analyses of the hydrolysis mixture at various times are
presented in Table
11.
[0668] Table 11 HPLC analytical results of composition.
Temperature % Formic % Levulinic
Time (min) ( C) Acid Acid % HMF
300 89.8 1.778 4.092 0.079
Non-
90 120 1.716 4.080 Detectable
[0669] The above reaction mixture was cooled to room temperature using an
ice bath.
447 gm of this mixture was poured into a 150 ml Buchner funnel with a glass
frit (4 - 5
micron filter size), that was placed on top of 1000 mL filter flask connected
to a Teflon
vacuum pump. The solid char was not sticky and did not adhere to reactor
components. It
flowed easily in the liquid mixture. Vacuum was used to aid the filtration
(<250 mm), filtrate
was allowed to drain for 5 ¨ 10 minutes before the Teflon vacuum pump was
turned off.
426.1 gm of filtrate and 20.95 gm of wet solids were obtained. The solid was
washed with 9
X 100 mL DI water. Another 80 mL was added and the filtrate of the 80 mL wash
was tested
using a pH probe (pH = 2.33). The solids were washed with 68 gm of acetone and
air dried
overnight to give 5.0 gm of dry char (1.04 wt% based on total initial charge)
[0670] The extraction and purification procedure was repeated as
described in
Example 115 to afford a third recycled raffinate stream, Recycled Raffinate
Stream from
Example 117.
[0671] Example 118: Synthesis of LA and FA with Recycled Raffinate Stream
from
Example 117.
[0672] To a 3 neck flask equipped with magnetic stirring, a chilled
condenser, and
thermocouple was charged 371 g of the recycled raffinate from Example 117.
This raffinate
contained approximately 177.5 g H2504, 7.1 g levulinic acid, and 2.9 g formic
acid. To the
raffinate were added 27 g of fresh water and 40.42 g of 64 % H2504 to bring
the acid
concentration in the aqueous phase to approximately 40 %. The aqueous phase
was heated to
90 C before the addition of 42.7 g of a sugar solution of identical
composition to that used in
-90-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
Example 115 was added over 5 hours. After the sugar addition was complete, a
120 C post
cook identical to that of Example 1 was used to fully convert any unreacted
reagents. Again,
analyses of the hydrolysis mixture at various times are presented in Table 12.
[0673] Table 12 HPLC analytical results of composition.
Temperature % Formic % Levulinic
Time (min) ( C) Acid Acid % HMF
305 90.1 2.174 4.626 0.085
Non-
90 120 2.124 4.380 Detectable
[0674] The above reaction mixture was cooled to room temperature using an
ice bath.
431 gm of this mixture was poured into a 150 ml Buchner funnel with a glass
frit (4 - 5
micron filter size), that was placed on top of 1000 mL filter flask connected
to a Teflon
vacuum pump. The solid char was not sticky and did not adhere to reactor
components. It
flowed readily in the liquid mixture. Vacuum was used to aid the filtration
(<250 mm),
filtrate was allowed to drain for 5 ¨ 10 minutes before the Teflon vacuum pump
was turned
off. 402.8 gm of filtrate and 28.3 gm of wet solids were obtained. The solid
was washed with
9 X 100 mL DI water. Another 50 mL was added and the filtrate of the 80 mL
wash was
tested using a pH probe (pH = 3.3). The solids were washed with 68 gm of
acetone and air
dried overnight to give 5.43 gm of dry char (1.13 wt% based on total initial
charge)
[0675] Example 119: Synthesis of LA and FA from Sugar solution with
higher
glucose content.
[0676] To a 3 neck flask equipped with magnetic stirring, a chilled
condenser, and
thermocouple was charged 200.14 g of 64 % H2SO4 and 122.53 g fresh water. The
aqueous
phase was heated to 90 C before 41.60 g of a sugar solution containing 64.6 %
fructose, 24.0
% water, 9.9 % glucose, and 1.5 % others was added over 5 hours. After the
sugar addition
was complete, the reaction was cooled to room temperature and filtered through
a fine glass
fritted glass filter to remove approximately 2 wt % insoluble humins that were
not sticky in
nature. The solids flowed quite easily in the reactor and did not stick to
reactor components.
-91-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0677] Table 13 HPLC analytical results of hydrolysis sample.
Temperature % Formic % Levulinic
Time (min) ( C) Acid Acid % HMF
300 90.2 2.431 4.290 0.187
[0678] After filtration, 341.57 g of hydrolysate was recovered and
extracted with 677
g MIBK. The MIBK was added on top of the hydrolysate, allowed to mix for 30
minutes and
settle for 30 minutes before separating the aqueous and organic layers. The
residual MIBK in
the aqueous layer was removed by vacuum distillation before the recycled
raffinate was used
in Example 120.
[0679] Example 120: Synthesis of LA + FA from Recycled raffinate from
Example
119
[0680] To a 3 neck flask equipped with magnetic stirring, a chilled
condenser, and
thermocouple was charged 295 g of the recycled raffinate from Example 119.
This raffinate
contained 124 g H2SO4, 2.7 g levulinic acid and 1.3 g formic acid, as well as
a small amount
of un-reacted glucose. The raffinate was augmented with 21 g fresh water and
86 g of a 64 %
H2SO4 solution to bring the acid concentration of the aqueous mixture to
approximately 40%.
The aqueous charge was then heated to 90 C before the addition of 63.8 g of a
sugar solution
with the same composition as the sugar solution in Example 115. The sugar
solution was
added via syringe pump over 5 hours, at which point the reaction was cooled to
room
temperature and filtered through a fine glass fritted glass filter to remove
approximately 2 wt
% insoluble humins. Again, analyses of the hydrolysis mixture at various times
are presented
in Table 14.
[0681] Table 14 HPLC analytical results of hydrolysis samples.
Temperature % Formic % Levulinic
Time (min) ( C) Acid Acid % HMF
300 90.0 2.325 5.764 0.138
-92-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0682] Following cooling, the reaction mixture was re-heated to 90 C and
held for 60
minutes to more completely convert starting materials or stable intermediates
to products.
[0683] Table 15 HPLC analytical results of hydrolysis samples during 90
C post
cook.
Temperature % Levulinic
Time (min) ( C) % Formic Acid Acid % HMF
Initial 25 3.218 5.914 0.041
0 90 3.252 6.105 0.041
60 90 3.139 6.275 Non-
Detectable
[0684] Example 121 for large scale production.
[0685] Figure 4 provides a process flow diagram for an embodiment of
Sugar to
Levulinic Acid conversion/scale up. The following provides an explanation of
the scale up
procedure.
[0686] The reaction was performed in a 2000 gallon glass lined reactor
(R1) and the
solids that were formed were to be removed in the Hastelloy centrifuge (CFG)
using the 8000
gallon poly tank for temporary storage of the hydrolysate. The centrifuged
hydrolysate was
then sent to 600 gallon settling tank (EC-1)for extraction with methyl
isobutyl ketone
(MIBK). The organic extract (OEX) was sent to another 2000 gallon glass line
reactor (R2)
for concentration (distillation of excess MIBK) and the hydrolysate was sent
back to the 2000
gallon reaction vessel (R1) for the next reaction. (See Figure 4.)
[0687] To a 2000 gallon glass line reactor (R1) equipped with condenser
and
thermocouple was charged 5540 lb water and 5380 lb of 93.3% (wt) sulfuric
acid. The reactor
was vented to a portable caustic scrubber (pH = 12.0) pulling at 685 Torr. The
reaction
mixture was heated to 90 C using pressurized steam. The CS90 (23% water,
69.3% fructose,
6.2 % glucose, 1.5% other sugars) was added at 310 lbs/hour using a diaphragm
pump. After
all the C590 had been added, the reaction mixture was maintained a 90 C for
an additional
-93-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
90 minutes The reactor was cooled to 40 C before subjecting the hydrolysate
to
centrifugation.
[0688] The reaction mixture was cooled to 44 C in 255 minutes at which
point it was
fed to the Hastelloy centrifuge (CFG). The hydrolysate was fed to the
centrifuge at ¨1600
lbs/hr and the centrifuge was spinning at 800 rpm. The liquid flowing through
the centrifuge
basket was fed to an 8000 gallon poly tank. Analysis of the first 2000 lbs of
sample in poly
tank showed 1.25% solids, which was not a significant reduction in solids.
Celite (filter aid)
slurried in water was fed to the centrifuge to coat the filter cloth followed
by addition of
hydrolysate from 2000 gallon reaction vessel (R1). ¨8000 lbs of hydrosylate
was centrifuged
and fed to the poly tank. The % solids in the poly tank was around 0.8% and
the 4000 lbs of
hydrolysate in reaction vessel (R1) had 1.4% solids. The hydrolysate from the
poly tank was
transferred to reaction vessel (R1) and the composite had 1.1% solids. The
hydrolysate was
then filtered using a sock filter (100 micron) housed in a stainless steel
canister. The filtered
sample showed 0.74% solids. Filtration was continued using the same filter
sock till the back
pressure changed from 10 ¨ 15 psig to around 40 psig. The filter sock were
changed in the
following sequence:
[0689] 100 micron ¨ 2 different socks
[0690] 25 micron ¨ 1 sock
[0691] 10 micron ¨ 1 sock
[0692] 1 micron ¨ 2 socks
[0693] The final percent solids after multiple sock filtrations were
0.8%. 50 lbs of
Celite was added to the hydrolysate that was transferred back to GL5 and the
hydrolysate was
subjected to centrifugation. The centrifuged hydrolysate had 0.4% solids.
[0694] The 6000 gallon settling tank (EC-1) was first filled with 23000
lbs of MIBK
followed by addition of hydrolysate from poly tank, the agitator was running
at 117 rpm
during the addition of hydrolysate. Agitator was turned off after 30 minutes
and the top layer
was sampled twice for analysis.
Time after % Levulinic % Formic Acid % Sulfuric acid % Water
-94-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
agitator was off Acid
(minutes)
15 0.94 0.45 0.5 1.19
30 0.95 0.45 0.68 1.28
[0695] Table 16: Analysis of Organic extract from settling tank (EC-1)
[0696] The bottom layer (raffinate) was carefully transferred back to the
reaction
vessel (R1) using a diaphragm pump connected to a sock filter canister with a
100 micron
filter sock. 10580 lbs of raffinate was transferred to the reaction vessel
(R1).
[0697] 12000 lbs organic extract (OEX) was transferred to another 2000
gallon glass
line reactor (R2) for concentration of the final product. The MIBK in the OEX
was distilled
at 100 Torr maintaining the vent temperature below 70 C. More material was
transferred
once the level in reactor (R2) was concentrated to 2000 lbs. After 28.5 hrs
4500 lbs of final
product was isolated with the following composition:
[0698] MIBK = 92.3%, Levulinic acid = 4.69%, Water = 0.03%
[0699] The raffinate was also subjected to distillation to remove any
MIBK. The
distillation was performed at 100 Torr so as to maintain the vent temperature
below 70 C.
After 3730 lbs of water/MIBK mixture was distilled the raffinate was sampled
for analysis.
[0700] Water = 62.18%, Sulfuric acid = 34.83%, Levulinic acid = 1.58%,
MIBK =
0.58% Solids = 0.15%
[0701] Example 122: 1st Recycle raffinate batch with CS90
[0702] To the 2000 gallon reaction vessel (R1) containing 8800 lbs of
raffinate (15'
recycle) was charged 451 lb water and 1690 lb of 93.3% (wt) sulfuric acid. The
reactor was
vented to a portable caustic scrubber (pH = 12.0) pulling at 685 Torr. The
reaction mixture
was heated to 90 C using pressurized steam. The C590 (23% water, 69.3%
fructose, 6.2 %
glucose, 1.5% other sugars) was added at 310 lbs/hour using a diaphragm pump.
After all the
-95-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
CS90 had been added, the reaction mixture was maintained a 90 C for an
additional 90
minutes. The reactor was cooled to 40 C before subjecting the hydrolysate to
centrifugation.
[0703] The reaction mixture was cooled to 45 C in 165 minutes at which
point it was
fed to the Hastelloy centrifuge. The hydrolysate was fed to the centrifuge at
¨2000 lbs/hr and
the centrifuge was spinning at 800 rpm. The liquid flowing through the
centrifuge basket was
fed to a 8000 gallon poly tank. Analysis of the sample in poly tank showed
0.8% solids.
[0704] 6000 gallon settling tank (EC-1) was first filled with 23000 lbs
of recycle
MIBK followed by addition of 12500 lbs hydrolysate from poly tank, the
agitator was
running at 117 rpm during the addition of hydrolysate. Agitator was turned off
after 30
minutes and the top layer was sampled four times for analysis.
Time after % Levulinic % Formic Acid % Sulfuric acid % Water
agitator was off Acid
(minutes)
15 1.36 0.74 0.13 1.1
50 1.35 0.74 0.15 1.08
65 1.37 0.74 0.15 1.06
80 1.45 0.75 0.16 1.07
[0705] Table 17: Analysis of Organic extract from EC-1
[0706] The bottom layer (raffinate) was carefully transferred back to the
reaction
vessel (R1) using a diaphragm pump. 15000 lbs of raffinate was transferred to
the reactor
(R1). Analysis of reactor contents (R1) showed high level of MIBK, so the
raffinate was sent
back to DC-1 for settling. 60 minutes later 12560 lbs raffinate was
transferred to the 2000
gallon reaction vessel (R1) for distillation of MIBK.
[0707] 12000 lbs organic extract (OEX) was transferred to another 2000
gallon glass
lined reactor (R2) for concentration of the final product. The MIBK in the OEX
was distilled
at 100 Torr maintaining the vent temperature below 70 C. More material was
transferred
once the level in reactor (R2) was concentrated to 2000 lbs. During couple of
the transfers
raffinate layer was observed in the OEX that was drained in to a 250 gallon
poly tote. (Total
-96-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
of 1000 lbs of raffinate drained in to the tote) After 24 hrs 2000 lbs of
crude product was
isolated with the following composition:
[0708] MIBK =86.55%, Levulinic acid = 9.17%, Sulfuric acid = 6.47%,
Formic acid
= 1.46%, Solids = 0.13%
[0709] The raffinate was also subjected to distillation to remove any
MIBK. The
distillation was performed at 100 Ton so as to maintain the vent temperature
below 70 C.
After 6645 lbs of water/MIBK mixture was distilled the raffinate was sampled
for analysis.
[0710] Water = 54.88%, Sulfuric acid = 42.96%, Levulinic acid = 3.55%,
MIBK =
0.06% Solids = 0.14%
[0711] Example 123: 2nd Recycle raffinate batch with CS90
[0712] To the 2000 gallon reaction vessel (R1) containing 6000 lbs of
raffinate (2nd
recycle) was charged 2090 lb water and 2430 lb of 93.3% (wt) sulfuric acid.
The reactor was
vented to a portable caustic scrubber (pH = 12.0) pulling at 685 Torr. The
reaction mixture
was heated to 90 C using pressurized steam. The C590 (23% water, 69.3%
fructose, 6.2 %
glucose, 1.5% other sugars) was added at 310 lbs/hour using a diaphragm pump.
After all the
C590 had been added, the reaction mixture was maintained a 90 C for an
additional 90
minutes The reactor was cooled to 40 C before subjecting the hydrolysate to
centrifugation.
[0713] The hydrolysate was fed to the centrifuge at ¨2000 lbs/hr and the
centrifuge
was spinning at 800 rpm. The liquid flowing through the centrifuge basket was
fed to an 8000
gallon poly tank. Analysis of the sample in poly tank showed 1.01% solids.
[0714] The 6000 gallon settling tank (EC-1) was first filled with 21905
lbs of recycle
MIBK followed by addition of 10700 lbs hydrolysate from poly tank, the
agitator was
running at 117 rpm during the addition of hydrolysate. Agitator was turned off
after 30
minutes and the top layer was sampled four times for analysis.
Time after % Levulinic % Formic Acid % Sulfuric acid % Water
agitator was off Acid
-97-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
(minutes)
30 1.35 0.94 1.77 1.47
60 1.4 0.99 1.78 Not determined
90 1.42 1.0 Not determined Not determined
120 1.41 0.96 Not determined Not determined
[0715] Table 18: Analysis of Organic extract from EC-1
[0716] The bottom layer (raffinate) was carefully transferred back to the
reaction
vessel (R1) using a diaphragm pump. 15750 lbs of raffinate was transferred to
the reactor
(R1).
[0717] 12000 lbs organic extract (OEX) was transferred to another 2000
gallon glass
line reactor (R2) for concentration of the final product. The MIBK in the OEX
was distilled
at 100 Torr maintaining the vent temperature below 70 C. More material was
transferred
once the level in reactor was concentrated to 2000 lbs. During transfers
raffinate layer was
observed in the OEX that was drained in to a 250 gallon poly tote. (Total of
4000 lbs of
raffinate drained in to the tote) After 30 hrs 2100 lbs of crude product was
isolated with the
following composition:
[0718] MIBK =83.1%, Levulinic acid = 7.04%, Formic acid = 2.12%
[0719] The raffinate was also subjected to distillation to remove any
MIBK. The
distillation was performed at 100 Torr so as to maintain the vent temperature
below 70 C.
[0720] Example 124
[0721] Into a 500 mL four neck round bottom flask was charged 102.57g
deionized
water and 103.04g of 98% sulfuric acid. The round bottom flask was placed in a
heating
mantle and equipped with a magnetic stir bar, thermocouple, condenser, glass
stopper and a
rubber stopper that held the outlet tube of the syringe pump. In a beaker
38.03g fructose and
25.60g deionized water were charged. The solution was mixed until the fructose
dissolved,
and it was transferred into a plastic syringe situated on a syringe pump. The
acid and water
mixture in the 500 mL round-bottom flask was heated to 90 C and then, the
fructose and
-98-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
water mixture was added via the syringe pump. The fructose was added over a
period of 1.25
hours so the rate on the syringe pump was set to 37.6 mL/hr. After a reaction
time of 1.25
hours, all of the fructose had been added into the flask. The reaction was
left to react for an
additional hour in order to react all of the fructose. The reaction was then
shut down and
allowed to cool down. Samples were taken throughout the entire reaction and
analyzed by
HPLC. Once the reaction mixture was cool it was filtered through a fritted
funnel and the
solids were washed with deionized water and acetone. The solids that were in
the funnel were
placed in a jar and put into a vacuum oven to dry. The final yield numbers and
composition
data are listed below.
Reaction Reaction Mol/L Mol/L Mol/L Mol/L
Time(min) T emp C FA LA HMF
Fructose
135 90.0 0.95 0.81 0.00 0.00
[0722] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Char 5.63
LA to Char Ratio 3.5
LA Molar %Yield on reacted sugar and HMF 81.4
FA Molar %Yield on reacted sugar and HMF 95.0
[0723] Into a 500 mL four neck round bottom flask was charged 102.09g
deionized
water and 103.04g of 98% sulfuric acid. The round bottom flask was placed on a
heating
mantle and equipped with a magnetic stir bar, thermocouple, condenser, glass
stopper and a
rubber stopper that held the outlet tube of the syringe pump. In a beaker was
charged 37.89g
fructose and 26.07g of deionized water. The solution was mixed until the
fructose dissolved,
and it was transferred into a plastic syringe situated on a syringe pump. The
sulfuric acid and
water mixture was heated to 90 C and then the fructose and water mixture was
added via the
syringe pump. The fructose was to be added over a period of 1.25 hours so the
rate on the
syringe pump was set to 38.4 mL/hr. After a reaction time of 1.25 hours, all
of the fructose
had been added into the flask. The reaction was left to react for an
additional hour in order to
react all of the fructose. The reaction was then shut down and allowed to cool
down. Samples
were taken throughout the entire reaction and analyzed by HPLC. Once the
reaction mixture
was cool it was filtered through a fritted funnel and the solids were washed
with deionized
-99-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
water and acetone. The solids that were in the funnel were placed in a jar and
put into a
vacuum oven to dry. The final yield numbers and composition data are listed
below.
Reaction Reaction Mol/L Mol/L Mol/L Mol/L
Time(min) T emp C FA LA HMF
Fructose
135 90.2 0.94 0.82 0.04 0.00
[0724] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Char 5.9
LA to Char Ratio 3.3
LA Molar %Yield on reacted sugar and 85.5
HMF
FA Molar %Yield on reacted sugar and 97.5
HMF
[0725] Into a 500 mL four neck round bottom flask was charged 103.09g
deionized
water and 103.03g of 98% sulfuric acid. The round bottom flask was placed on a
heating
mantle and equipped with a magnetic stir bar, thermocouple, condenser, glass
stopper and a
rubber stopper that held the outlet tube of the syringe pump. In a separate
beaker was charged
37.89g fructose and 25.06g deionized water. The solution was mixed until the
fructose
dissolved, and it was transferred into a plastic syringe situated on a syringe
pump. The
sulfuric acid and water mixture in the round bottom flask was heated to 90 C
and then the
fructose and water mixture was added via the syringe pump. The fructose was
added over a
period of 2.5 hours so the rate on the syringe pump was set to 18.8 mL/hr.
After a reaction
time of 2.5 hours, all of the fructose had been added into the flask. The
reaction was left to
react for an additional hour in order to react all of the fructose. The
reaction was then shut
down and allowed to cool down. Samples were taken throughout the entire
reaction and
analyzed by HPLC. Once the reaction mixture was cool it was filtered through a
fritted funnel
and the solids were washed with deionized water and acetone. The solids that
were in the
funnel were placed in a jar and put into a vacuum oven to dry. The final yield
numbers and
composition data are listed below.
-100-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
Reaction Reaction Mol/L Mol/L Mol/L Mol/L
Time(min) Temp C FA LA HMF
Fructose
210 90.3 1.00 0.89 0.04 0.00
[0726] Once the solids were dried, they were removed from the vacuum oven
and
weighed.
Grams of Char 5.6
LA to Char Ratio 3.8
LA Molar %Yield on reacted sugar and 92.9
HMF
FA Molar %Yield on reacted sugar and Near 100
HMF
[0727] Formic Acid Neutralization in MIBK
[0728] Example 125 A 5wt% solution of formic acid (.3g) in methyl
isobutyl ketone,
MIBK (5.2g) was made. An equal molar solution of sodium hydroxide (.2g) in
water (1.9g)
was prepared. The two mixtures were combined in a vial and mixed well. Two
layers formed
in the vial and they were both tested by HPLC for % formic acid. The HPLC
results show the
MIBK solution dropped from 4.8% to 0.2% formic acid.
[0729] Example 126-127 Additional experiments were completed under the
same
procedure as example 125. Changes in the initial scale of the experiment along
with a test
using less water were also performed. The results are summarized in Table 19.
[0730] Example 128 A 5wt% solution of formic acid (.3g) in MIBK (5.1g)
was
made. Sodium hydroxide powder (.4g) was added to the solution which is equal
to twice the
moles of formic acid. The mixture was mixed well for 1 hour. After mixing, the
MIBK was
tested by HPLC for % formic acid. The HPLC results show the MIBK solution
dropped from
4.7% to 0% formic acid.
[0731] Examples 129-130 Further experiments were carried out under the
same
procedure as example 128 along with changes in the base used. The results are
summarized in
Table 19.
-101-
SEGT.P0013W0
[0732] Table 11
Initial0
i
i
FA MIBK Water NaOH CaCO3 CaH202 %FA %FAn %FAn g FA g FA Total g
EX.
MIBK Water in in FA in
MIBK
(HPLC) (HPLC) MIBK Water solution
cio
125 0.27 5.20 1.88 0.23 NEMEN 4.85
0.22 6.51 0.011 0.123 0.134
128 0.25 5.08 NA 0.43 4.70 0.00
NA 0.000 NA 0.000
129 0.27 5.01 NA 0.60 5.14 4.84
NA 0.243 NA 0.243
130 0.27 5.08 NA 0.46 4.98
2.34 NA 0.119 NA 0.119
126 0.27 5.03 0.25 0.25 5.03 0.00
NA 0.000 ND 0.000
127 6.38 125.07 5.54 5.54 4.85 0.00
17.95 0.000 0.994 0.994
o
O
o
o
o
O
o
cio
-102-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0733] Table 19 shows that sodium hydroxide worked best at removing the
formic
acid from the MIBK compared to calcium carbonate and calcium hydroxide.
Calcium
carbonate does not show much promise in reducing the formic acid in MIBK.
However
calcium hydroxide does reduce the formic acid with equal molar ratios and may
remove more
if the ratio is increased.
[0734] Example 131: Isoamyl alcohol
[0735] Aqueous sulfuric acid stock solutions were prepared at various
concentrations
and mixed with isoamyl alcohol to yield the compositions (in weight %) below.
Phase
behavior (1 phase vs. phase separated) was determined visually. The data show
that the
solubility of the isoamyl organic solvent increases slightly as the amount of
sulfuric acid in
the mixture increases (#2 vs. #16). At the appropriate composition ratio, the
solubility of
sulfuric acid in isoamyl alcohol can be high (#15).
%
sulfuric % organic % water Visual Observations
acid
1 49.0% 2.0% 49.0% 1 phase
2 48.5% 2.9% 48.5% 1 phase
3 48.2% 3.6% 48.2% 2 phases
4 50.0% 0.0% 50.0% 1 phase
15.0% 69.9% 15.0% 1 phase
6 15.4% 69.2% 15.4% 2 phases
7 20.0% 0.0% 80.0% 1 phase
8 19.6% 2.0% 78.4% 2 phases
9 1.6% 92.1% 6.3% 1 phase
1.8% 90.8% 7.4% 2 phases
11 10.0% 0.0% 90.0% 1 phase
12 9.8% 2.1% 88.1% 2 phases
13 0.7% 92.8% 6.5% 1 phase
-103-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
14 0.9% 91.3% 7.9% 2 phases
15 0.0% 0.0% 100.0% 1 phase
16 0.0% 2.2% 97.8% 2 phases
17 0.0% 92.1% 7.9% 1 phase
18 0.0% 90.7% 9.3% 2 phases
[0736] Example 132: m-cresol
[0737] Aqueous sulfuric acid stock solutions were prepared at various
concentrations
and mixed with m-cresol to yield the compositions (in weight %) below. Phase
behavior (1
phase vs. phase separated) was determined visually. The data show that the
solubility of the
m-cresol organic solvent in the in sulfuric aqueous phase is low (#2, #6),
even at high
sulfuric acid concentration (#13). The compatibility of sulfuric acid with the
m-cresol
organic solvent is low (#8, #12, #15)
% sulfuric acid % organic % water Visual Observations
1 0.0% 1.3% 98.7% 1 phase
2 0.0% 1.9% 98.1% 2 phases
3 0.0% 87.7% 12.3% 1 phase
4 0.0% 86.0% 14.0% 2 phases
9.9% 0.8% 89.3% 1 phase
6 9.9% 1.4% 88.8% 2 phases
7 0.2% 98.2% 1.6% 1 phase
8 0.3% 97.5% 2.3% 2 phases
9 19.9% 0.6% 79.5% 1 phase
19.8% 0.8% 79.4% 2 phases
11 0.2% 99.2% 0.6% 1 phase
12 0.3% 98.5% 1.2% 2 phases
13 49.8% 0.5% 49.8% 2 phases
14 0.5% 99.1% 0.5% 1 phase
0.9% 98.3% 0.9% 2 phases
[0738] Example 133: 2-ethyl hexanol
-104-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0739] Aqueous sulfuric acid stock solutions were prepared at various
concentrations
and mixed with to yield the compositions (in weight %) below. Phase behavior
(1 phase vs.
phase separated) was determined visually. The data show that the solubility of
the 2-ethyl
hexanol organic solvent in the in sulfuric aqueous 2-ethyl hexanol phase is
low (#1), even at
high sulfuric acid concentration (#6). The compatibility of sulfuric acid with
the 2-ethyl
hexanol organic solvent is low when the organic solvent content is very high
(#10, #12, #14).
When both the organic solvent content and the sulfuric acid content are high,
there is a region
of compatibility (#15).
% SA % organic % water Visual Observations
1 0.0% 0.4% 99.6% 2-phase
2 10.0% 0.4% 89.6% 2-phase
3 19.9% 0.4% 79.7% 2-phase
4 49.8% 0.3% 49.8% 2-phase
5 79.4% 0.8% 19.8% 1 phase
6 78.4% 2.0% 19.6% 2-phase
7 0.0% 99.2% 0.8% 1 phase
8 0.0% 97.3% 2.7% 2-phase
9 0.1% 99.2% 0.7% 1 phase
10 0.2% 98.1% 1.7% 2-phase
11 0.2% 98.8% 0.9% 1 phase
12 0.4% 97.8% 1.8% 2-phase
13 1.9% 96.2% 1.9% 1 phase
14 2.3% 95.5% 2.3% 2-phase
15 34.3% 57.1% 8.6% 1 phase
16 35.6% 55.6% 8.9% 2-phase
[0740] Examples 134-136: Backwash with water to remove sulfuric acid from
mixed
cresols
[0741] To a vial were added 5 g of CSTR hydrolysate material that had
been filtered
through 1 um glass fiber filter disc, spiked with LA (1.9% formic acid, 8 wt%
levulinic acid,
50 wt. % sulfuric acid, and 40.1 wt. % water) and 5 g of mixed cresols from
Aldrich. The
-105-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
vial was capped and the mixture was shaken mechanically for 0.5 minutes. The
layers were
separated by centrifugation for 5 minutes and each layer was isolated for
weight
determination. The sulfuric acid in the organic layer was determined by
potentiometric auto-
titration with potassium hydroxide/methanol as titrant.
[0742] The organic layer from the hydrolysate partition experiment was
then washed
with the amount of DI water given in the table below. The layers were then
separated by
centrifugation for 5 minutes and each layer was isolated for weight
determination. The
sulfuric acid in the organic layer was determined by potentiometric auto-
titration with
potassium hydroxide/methanol as titrant. These experiments show a water wash
can reduce
the amount of sulfuric acid in the organic extraction phase.
Example Wt. % sulfuric acid in Mass of DI water Wt% sulfuric
acid
organic after initial wash (% by weight in organic layer
partition experiment relative to the mass after backwash
of organic phase) with DI water
134 0.64 10 0.08
135 0.73 50 0.01
136 0.70 100 0.01
[0743] Aldrich MSDS indicates an 80% mixture of cresol isomers and 20%
phenol.
[0744] GC/MS indicates the mixture to be 80% cresol isomers and 20% 2,4-
dimethylphenol.
[0745] Example 137: neutralization to remove sulfuric acid from mixed
cresols
[0746] To a vial were added 5 g of CSTR hydrolysate material that had
been filtered
through 1 um glass fiber filter disc, spiked with LA (1.9% formic acid, 8 wt%
levulinic acid,
50 wt. % sulfuric acid, and 40.1 wt. % water) and 5 g of a m-cresol/p-cresol
blend (60/40
blend ratio by weight). The vial was capped and the mixture was shaken
mechanically for 0.5
minutes. The layers were separated by centrifugation for 5 minutes and each
layer was
isolated for weight determination. The sulfuric acid in the organic layer was
determined by
potentiometric auto-titration with potassium hydroxide/methanol as titrant to
be 0.7% by
weight.
-106-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0747] The organic layer from the hydrolysate partition experiment was
then washed
with a saturated aqueous solution of 20% (by weight) of sodium bicarbonate .
The layers
were separated by centrifugation for 5 minutes and each layer was isolated for
weight
determination. The sulfuric acid in the organic layer was determined by
potentiometric auto-
titration with potassium hydroxide/methanol as titrant to be non-detectable.
[0748] Acros Organics 99% m-cresol and Alfa Aesar 99% p-cresol were used
in the
above example.
[0749] Examples 138 and 139: Backwash with water to remove sulfuric acid
from
isoamyl alcohol
[0750] To a vial were added 5 g of CSTR hydrolysate material that had
been filtered
through lum glass fiber filter disc, spiked with LA (1.9% formic acid, 8 wt%
levulinic acid,
50 wt. % sulfuric acid, and 40.1 wt. % water) and 5 g of isoamyl alcohol from
Aldrich. The
vial was capped and the mixture was shaken mechanically for 0.5 minutes. The
layers were
separated by centrifugation for 5 minutes and each layer was isolated for
weight
determination. The sulfuric acid in the organic layer was determined by
potentiometric auto-
titration with potassium hydroxide/methanol as titrant.
[0751] The organic layer from the hydrolysate partition experiment was
then washed
with the amount of DI water given in the table below. The layers were then
separated by
centrifugation for 5 minutes and each layer was isolated for weight
determination. The
sulfuric acid in the organic layer was determined by potentiometric auto-
titration with
potassium hydroxide/methanol as titrant. The resulting organic layer was then
washed again
with 100 weight % water, further lowering the sulfuric acid content. These
experiments show
a water wash can reduce the amount of sulfuric acid in the organic extraction
phase.
Example Wt. % sulfuric acid in Mass of DI water Wt% sulfuric
acid
organic after initial wash (% by weight in organic layer
partition experiment relative to the mass after backwash
of organic phase) with DI water
138 19.0 50 3.72
139 3.72 100 0.94
-107-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0752]
Example 140: Distillation of Formic acid from a mixture of formic acid,
levulinic acid, sulfuric acid, water, and unknown impurities
[0753] To
a 3 neck round bottom flask equipped with a magnetic stir bar was charged
255.60 g of a solution containing 11.12 g levulinic acid, 5.44 g formic acid,
99.43 g sulfuric
acid, 139.61 g H20, and trace amounts of several unknown impurities The flask
was
equipped with a thermocouple and a short path distillation apparatus with a
condenser chilled
to 1 C with recirculating coolant. The distillation system was evacuated down
to 40 Torr and
before the kettle was heated to 45 C. The distillate was exhibited a head
temperature
between 31-33 C. Distillate was allowed to come overhead until the head
temperature
dropped below 28 C, at which point the distillation kettle was cooled to 25
C, the pressure
increased to atmospheric pressure, and samples were taken from the kettle as
well as
distillation recovery flask. After sampling, the kettle was re-evacuated to 40
Torr and heated
this time to 55 C. The procedure of distilling till the head temp falls,
sampling, and
redistilling at an elevated temperature was repeated until no more formic acid
could be
observed in the distillation kettle.
[0754]
Table 20. Analysis of distillate and kettle samples taken during the
distillation
described in Example 140.
Kettle Mass g
% FA of % LA of
Cut Sample % LA % FA g LA g FA
Temp (g) H2SO4
H2SO4 Charge Charge
Pigfittdfeig87.42MM4W74I4Ggg4OOFMi4.ggna9OMNAigiMin7T87MiMOMOM
1 65 C ggggggggggggggggggggggggggggggggggggggggggggggggMMMMMMMMMMMM
Kettle 168.18 6.00 0.48 55.0 10.09 0.81 92.50 16.16 89.73
Pit):igiitlitiiiiiiVk4;.iriniVgri4438i&MIWEVROiiAt4FMMAgiMMINRAWMMA:Wign
2 75 C MggggggggggggggngMnnggnggggggN'gggggggggMMMMMMMMMMMM'MM
Kettle 159.20 6.49 0.28 59.3 10.33 0.45 91.73 8.97 91.86
Pisti1Iate400AfVVten4Stm*,AOAO3U437tmo19iLtRimmV9fem
3 80 C MaggggggggggggggggggnMWMggggggMaggEgnMgggggggnMEMOggggggn
Kettle 155.14 6.68 0.23 59.7 10.36 0.36 89.83 7.19 92.16
DigiltateAO2V7ZmgrWmiWiS%m,mfttOMi4AZkmfttmoi9:21tmmiOi9Z=
4 85 C
Kettle 152.88 6.59 0.25 60.6 10.07 0.39 89.78 7.69 89.60
90 C monomonomumumonummmonommmumonommonomonommoomo
Kettle 146.76 6.96 0.00 63.5 9.87 0.00 90.04 0.00 87.77
-108-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0755] Example 141: Vacuum distillation of Formic acid from a mixture of
formic
acid, levulinic acid, sulfuric acid, water, and unknown impurities with
continuous addition of
H20
[0756] To a 500 mL 4 neck round bottom flask equipped with a magnetic
stir bar was
charged 249.27 g of a solution containing 10.87 g levulinic acid, 5.31 g
formic acid, 97.13 g
sulfuric acid, 136.38 g H20, and trace amounts of several unknown impurities.
The flask was
equipped with a thermocouple, an addition funnel charged with 124.28 g DI H20,
and a short
path distillation apparatus with a condenser cooled to 1C with recirculating
coolant. The
pressure of the system was reduced to 40 Torr before a heating mantle set to
45 C was
activated. When the solution in the flask reached approximately 42 C,
distillate was
observed. The head temperature fluctuated around 31-32 C during distillation.
When the
distillate began to drip into the collection flask, H20 from the addition
funnel was added
dropwise at roughly the same rate as the distillate was being removed. When
all the H20
from the addition funnel had been added, the pressure of the system was raised
to
atmospheric pressure and the system was cooled. Samples of the reaction flask
mixture and
distillate were taken, and the addition funnel was charged with more H20. The
process of
distilling with dropwise addition of H20 was continued until formic acid was
no longer
detected in the distillation flask.
-109-
SEGT.P0013W0
[0757] Table 21: Analyses of distillate and kettle samples throughout
distillation described in Example 141.
Mass
0
% FA % LA t..)
H20 Mass % /0 % g g
Cut Sample g LA
,-,
(...)
Added (g) LA FA H2Sat
of of FA H2SO4 Charge O-
Charge -1
(g)
w
(...)
,z
1 124.28
15igiiiiiii6EVAISS11,1:17:11"15..õ.
4).:.6.1Mõ.4:::.õ..,,,:::.:,:..,,,,,,,,,,,,,,,,, .
-:-------::::::------::::::::::::::::::::::::----------------------------------
-----------------------------------------
Kettle 276.48 3.80 0.95 54.1 10.51 2.63 149.58 49.43 96.69
A-14-1.---111-17-------44:5-.7.61"""":"O'W"1""t"Mt0t5ki-i-i-i-i-i-i-iii.:-:-
ii:-:-
iIiiiiiiiiiiiiiiiiiiiiiiii$4::,'16:2.,:iiiiiiiiiiiiiiiiiiiiiiii,tittiiiiiiiiiii
i:1,'
,,,--st-----xjOo,----,,.M,-g-A.-..-.K.'m,m-:,:A,m-
e,,,,,:,,,,,,,,,,,,,...:,,,,,,,,,,,,,,,,,..-_____-____________.
2 48.62 -""----------------------------------------
-------------------------------
Kettle 269.46 3.72 0.67 49.1 10.41 1.81 132.30 34.08 95.74
31 14 )itillat 466 U02 079 001 037 1 54
006
Kettle 263.48 3.66
0.52.. .....3.1......30,...37_..1:38
,....._,,,,,3,,:"....,2,.....5:9, ?..,,,,,,,,,,,,,95,..34
4 47.25
bigtilldtaiiiiiiiiii4411iiiiiiiii9i9iiiiiiiiiiiit
Oiiiiiiiiiiiiiiiii199ctiiiiiiiiiVgZiiiiiiiiiiiiiii35ii",:iii:1:1:1:1:1:1:1::i:1
:1:1::Pi.'-:-.:-94.,iiiiiiiiiiiiii,'
-:Kettle- 279.62 3.74 0.44 42.9 11.48 1.22
119.96 22.89 105.57 P
547.86 IDISIII141.91iiiiiiii5ORZ #iiiiiiilMiiiiiiip40,1,1,1,1,1,1,1,1i,-
,,i,"ilililililililili"?pgililim:9 q,p1
,4i:3iiiiiiiiiiiiiiiiiiii!!iiiiiiiiiiiiiiiiiiiip...:.:0.:.:.:;ip_.9.J.:_,.:_,.:
_,.:_,.:_,.:_,.:_,.:_,.:_,.:_,.:_,.:..:
--"----------------------------------------------------------------------------
----------- 09
Kettle 244.88 3.72 0.34 44.0 10.34 0.84 107.75 15.85 95.08
............................................ ...1.
,,,,,,,,,,,,,,,,,,.
.,,,,,,,,,,,,,,,,,,,,,:l.............1..,.........................,..,..,......
...,:,...c.,,,.,,,.,,,.,,,.,,,.,,,.,,,.
6
4bigtillairlI210100itiiiiiii0A9iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii0...
.Al!...]:::Vi.1:2!!!!!!!!!!!!ir',',:,-t,$!!!!!!!!!i!i!:'!:'!:'!.-
1Eig..:4:':...i:=:;i;i;i;i;iiiiiiiii.-1.-1.-1.-'07..:.,-.,-.,-.,-,:-::-::-:
o
,
Kettle 246.85 3.47 0.25
.45.9 .. 10.13 .Ø61.,,,,l.12.40
.....11.43 ,,,,,,,,,,93.19,,,,,,, ,
:.:.:.:.:.:....,...:.:.:.:.:.:.:.õ.,.,.,.....,__. ,
7 101.4
bi$-
Oittaltiliiiiiiiiii1Q14iiiiiiiiiii9MOiiiiiiiiiii0g,iiiiiiiiiii*Iiiiiiiiiiiiiiii
iiiiiiii:9,'99iiiiiiiiiii:19;2.g.liiiiiiiiiiiiiiitrb0Ø.....hinin9..;91M
-.Kettle-- 236.03 3.55 0.15 40.7 10.19 0.35
99.67 6.57. 93.70
014a
-------"- --------
A 9682
rgifilait*W.StmC-6-WtEA4:4,u=val,,,A.-
,.:...:.,,0....:::24.ii::::::::':!:!:!:!:!:!:!:;995:.::.::.::.::.::.::.:.:
, I'..-'....,.., ,,ff,,,,,,,.,,,,,,,,,,% .,
=
8
Kettle 239.65 3.38 0.09 43.1 10.08 0.22 105.54 4.06
92.68
................----''
997.74
b......:.:.:Ig....i:.j.#:.4.:.:fe......:iiiiiiiiiiiiiiqPiiiiiiiiiiiiiiP.W.Pg.M.
giIiiiiiiiiiiiiiiii**iiiiiiiiiiiiiiiiiiiiiiiii099iiiiiiiiiiiiiiiigiAg.:Iiiiiiii
iiiiiii344,,,,,,,iiiiiiiiiiiiiiiiiiiiii #I ,' PZ iiiiiiiiiginct;#V.
Kettle 229.68 3.51 0.00 44.8 10.23 0.00 109.71 0.00
94.10
od
n
1-i
cp
w
=
,-,
w
'a,-
c,
c,
(...,
c,
00
-110-
CA 02856402 2014-05-20
WO 2013/078391 PCT/US2012/066368
[0758] Levulinic Acid Partition Coefficients
[0759] Examples 142-174: To a vial were added 5 g of CSTR hydrolysate
material
that had been filtered through lum glass fiber filter disc, spiked with LA
(1.9% formic acid, 8
wt% levulinic acid, 50 wt. % sulfuric acid, and 40.1 wt. % water) and 5 g of
organic solvent.
The vial was capped and the mixture was shaken mechanically for 0.5 minutes.
The layers
were separated by centrifugation for 5 minutes and each layer was isolated for
weight
determination. The sulfuric acid in the organic layer was determined by
potentiometric auto-
titration with potassium hydroxide/methanol as titrant.
[0760] The partition coefficient of levulinic acid in this system was
calculated
according to:
mLA/ms
[0761] Partition Coefficient =
m LA/ma
[0762] where mLA,a is the mass of levulinic acid in the organic solvent
phase, ma is
the total mass of the organic solvent phase, mLA,a is the mass of the
levulinic acid in the
aqueous phase, and ma is the total mass of the aqueous phase. The aqueous
phase is pipette
out of the mixture is weight. Ms is then calculated by difference. mLA,a is
measure by HPLC
and mLA,a is calculated by difference. The partition coefficient of formic
acid was calculated
in a similar fashion.
-111-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
Formic
% Sulfuric
LA Partition Acid
Organic Solvent acid in NOTES
Coefficient Partition
organic phase
Coefficient
142 Methyl isoamyl 0.63
0.38 0.447
ketone
143 Methyl isobutyl 0.82
0.67 NR
ketone
144 Diisobutyl 0.15
0.1 NR
ketone
145 Acetophenone 0.65 1.03 4.2
146 3.05
Required an
additional
2.44 g of
Cyclohexanone 2.26 3.01
water to
induce layer
separation
147 Isophorone 1.91 1.95 16.9
148 9.61
Required an
additional
Neopentyl 0.45 g
of
2.01 21.2
alcohol water to
induce layer
separation
149 6.91
Required an
additional 6
Isoamyl alcohol 2.33 19 wt%
water to
induce layer
separation
150 n-hexanol 0.93 7.23 19.9
151 n-heptanol 0.78 8.00 22.9
-112-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
152 2-ethyl hexanol 0.5 12.3 12.8
153 n-octanol 0.59 3.79 NR
154 1-nonanol 0.59 4.62 NR
155 1-undecanol 0.55 7.94 16
156 Phenol 3.94 0.57 1.02
157 4-methoxyphenol 1.77 6.1
158 Guaiacol 0.72 0.31 0.11
159 2-sec butyl 0.25
1.88 0.042
phenol
160 Nonyl phenol 0.26 0.14 NR
161 Methylene 0.14
0.23 NR
chloride
162 Methyl isobutyl 2.73
1.04 20.7
carbinol
163 Anisole 0.19 3.20 NR
164 Ethylene glycol 0.71
0.2 0.22
di-n-butyl ether
165 Castor oil 0.04 0.37 0.75
166 m-cresol 1.14 4.27 0.51
167 p-cresol 1.06 3.86 0.9
168 o-cresol 0.9 3.98 0.28
169 Cresol mix from 0.40
2.17 0.64
Aldrich*
170 60/40m- 0.45
2.38 0.7
cresol/p-cresol
171 75/25m- 0.41
2.29 0.57
cresol/p-cresol
172 Diethyl 0.62
0.26 0.04
carbonate
173 0.13 Below
Methyl salicylate 0.08 detection
limit
174 2,4- 1.97 0.39 0.22
-113-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
dimethylphenol
[0763] NR = Not reported
[0764] * Aldrich MSDS indicates an 80% mixture of cresol isomers and 20%
phenol.
GC/MS indicates the mixture to be 80% cresol isomers and 20% 2,4-
dimethylphenol.
[0765] Example 175: FORMIC ACID SEPARATION FROM MIAK BY
DISTILLATION
[0766] To a 1L round bottom flask equipped with variac-controlled electric
heating
mantle, thermocouple, magnetic stir bar, pressure sensor, 1-inch X 18-inch
vacuum-jacketed
glass column packed with wire gauze packing, and magnetic bucket-type reflux
control head
was added 76.0 g of formic acid and 76.0 g MIAK. The still was controlled at
200 torr for a
duration of 100 minutes and a reflux ratio of 6:1 reflux:collect. Bottom flask
temperature
ranged from 77.1 C to 101.5 C while the overhead temperature ranged from
60.1 C to 61.1
C. Three fractions were collected: Fraction 1, 13.8 g, 89.187% formic acid by
HPLC,
Fraction 2, 18.2 g, 88.842% formic acid by HPLC, Fraction 3, 26.4 g, 88.944%
formic acid
by HPLC, Residual bottoms, 76.7 g, 3.261% formic acid by HPLC.
[0767] Example 176: FORMIC ACID SEPARATION FROM MIBK BY
DISTILLATION
[0768] To a 1L round bottom flask equipped with variac-controlled electric
heating
mantle, thermocouple, magnetic stir bar, pressure sensor, 1-inch X 18-inch
vacuum-jacketed
glass column packed with wire gauze packing, and magnetic bucket-type reflux
control head
was added 63.47 g of formic acid and 641.55 g MIBK. The still was operated at
763 torr for a
duration of 260 minutes and a reflux ratio of 6:1 reflux:collect. Bottom flask
temperature
ranged from 115.3 C to 116.5 C while the overhead temperature ranged from
97.1 C to
114.7 C. Several fractions were collected:
% FA by
Fraction Mass (g) HPLC
1 14.72 13.925
2 33.38 12.949
-114-
CA 02856402 2014-05-20
WO 2013/078391
PCT/US2012/066368
3 38.06 12.267
4 74.97 11.097
44.87 10.152
6 103.8 8.889
7 68.06 7.64
8 15.47 6.755
Bottoms 300.77 5.267
[0769] Although the present invention has been described with reference to
preferred
embodiments, persons skilled in the art will recognize that changes may be
made in form and
detail without departing from the spirit and scope of the invention. All
references cited
throughout the specification, including those in the background, are
incorporated herein in
their entirety. Those skilled in the art will recognize, or be able to
ascertain, using no more
than routine experimentation, many equivalents to specific embodiments of the
invention
described specifically herein. Such equivalents are intended to be encompassed
in the scope
of the following claims.
-115-