Note: Descriptions are shown in the official language in which they were submitted.
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
TRANSMUCOSAL DRUG DELIVERY DEVICES
FOR USE IN CHRONIC PAIN RELIEF
RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application No.
61/578,755, filed
December 21, 2011. The entire contents of this application are incorporated
herein by
reference.
This application is related to U.S. Patent Application No. 08/734,519, filed
on
October 18, 1996, now U.S. Patent No. 5,800,832, issued September 1, 1998;
U.S. Patent
m Application No. 09/144,827, filed on September 1, 1998, now U.S. Patent
No. 6,159,498,
issued on December 12, 2000; U.S. Patent Application No. 11/069,089, filed on
March 1,
2005, now U.S. Patent No. 7,579,019, issued on August 25, 2009; U.S. Patent
Application
No. 11/639,408, filed on December 13, 2006; U.S. Patent Application No.
11/817,915, filed
on September 6, 2007; U.S. Patent Application No. 13/834,306, filed on July
15, 2011, now
U.S. Patent No. 8,147,866, issued on April 3, 2012; U.S. Patent Application
No. 13/590,094,
filed on August 20, 2012, the entire contents of which are incorporated herein
by reference.
BACKGROUND
Chronic pain is pain that persists beyond the expected healing time, if
resulting from
injury, and can progress from a bothersome nuisance to a profound affliction.
Chronic pain
can cause a marked alteration in behavior with depression and anxiety,
restriction in daily
activities and excessive use of medication and medical services in an
afflicted individual.
The treatment of chronic pain is difficult, often inadequate and associated
with high
economic and psychological cost.
Buprenorphine is a partial Lit-opiate receptor agonist, an ORL1/nociceptin
receptor
agonist with high affinity and a slow dissociation rate and a lc-opiate
receptor antagonist.
Buprenorphine is metabolized by the liver, via the CYP3A4 isozyme of the
cytochrome P450
enzyme system, into norbuprenorphine (by N-dealkylation) and other
metabolites.
Buprenorphine has a low oral bioavailability due to very high first-pass
metabolism.
Buprenorphine is an analgesic, available commercially as Temgesic 0.2 mg
sublingual tablets, and as Buprenex in a 0.3 mg/ml parenteral formulation.
Buprenorphine
1
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
is also available as a sublingual preparation (Subutex ) and as a sublingual
abuse-resistant
formulation with naloxone (Suboxone ). The FDA approved Suboxone/Subutex in
2002 as a
treatment of opioid dependence. Sublingual buprenorphine has been used for
opioid
detoxification and maintenance.
A recent open-label study used sublingual buprenorphine (Suboxone ) for the
treatment of chronic pain to those chronic opioid users (Malinoff et al.,
2005, American
Journal of Therapeutics 12, 379-384). Patients were treated with daily
buprenorphine doses
that ranged from 2-20 mg (mean 8 mg). The treatment lasted from 2.4 months to
16.6
months (mean 8.8 months). The article reports that patients experienced
improvement in
to their condition and reported a decrease in their sensation of pain.
Still, effective methods for treating chronic pain that are not associated
with adverse
effects are needed, especially to those opioid naive or opioid experienced
patients.
BRIEF DESCRIPTION OF THE DRAWINGS
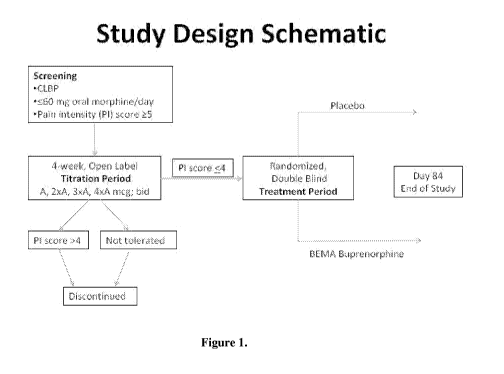
Figure 1 is a schematic representation of the design of a clinical study for
evaluating
the efficacy and safety of twice daily administration of BEMA buprenorphine in
subjects
with chronic low back pain.
Figure 2 is a schematic representation of disposition of subjects who
participated in
the clinical study to evaluate the efficacy and safety of twice daily
administration of BEMA
buprenorphine in subjects with chronic low back pain.
Figure 3 is a graph showing mean change from baseline in daily pain intensity
experienced by the subjects with chronic low back pain after twice daily
administration of
BEMA buprenorphine.
SUMMARY OF THE INVENTION
The present teachings provide methods for treating chronic pain by
administering low
doses of buprenorphine twice daily (or once daily) via a mucoadhesive
bioerodable drug
delivery device. The methods and devices efficiently treat chronic pain
without significant
side effects, for example, less than 15% (preferably less than 10%, more
preferably less than
5%) patients experience constipation.
2
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
The devices comprise about 100 j.tg to 0.9 mg buprenorphine, and provide
steady-
state C. of plasma buprenorphine concentration in a range between about 0.1
and about 1.2
ng/mL, such that the subject is treated for chronic pain.
In one embodiment, the buprenorphine delivery device comprises a bioerodable
mucoadhesive layer comprising a therapeutically effective amount of
buprenorphine disposed
in a buffered polymeric diffusion environment, wherein the polymeric diffusion
environment
is a buffered environment having a pH of between about 4 and about 6. In
another
embodiment, the buprenorphine delivery device further comprises a bather layer
comprising
a polymeric barrier environment disposed adjacent to the mucoadhesive layer to
provide a
to unidirectional gradient upon application to a mucosal surface for the
rapid and efficient
delivery of buprenorphine, wherein the unidirectional gradient delivers
buprenorphine across
the buffered polymeric diffusion environment upon application to the mucosal
surface. In
still another embodiment, the device comprises a mucoadhesive layer comprising
an effective
amount of buprenorphine buffered to a pH of between about 4.0 and about 6.0,
and a backing
layer buffered to a pH between about 4.0 and about 4.8.
In one embodiment, the device comprises about 120 jig to 0.9 mg buprenorphine.
The methods and devices disclosed therein can be used to treat a subject with
chronic
low back pain, such as moderate to severe chronic low back pain, or a subject
with
neuropathic pain or osteoarthritic pain.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides methods of treating chronic pain with low doses
of
buprenorphine. The present method of treating pain is also associated with
lack of significant
opioid adverse effects. For example, the subject is treated without
experiencing any severe
common opioid adverse effects. Or, the subject is treated experiencing mild or
moderate
common opioid adverse effects, or no common opioid adverse effects.
The present invention also provides effective chronic pain relief with twice
daily
administration of buprenorphine. The present invention is based, at least in
part, on the
surprising discovery that a transmucosal drug delivery device containing low
doses of
buprenorphine can be administered twice daily to opioid experienced subjects
for effective
management and relief of chronic pain, such as chronic low back pain. The
present invention
3
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
is also based on the discovery that this therapy does not result in
substantial side effects
associated with opioids, such as constipation and nausea.
Definitions
The following definitions are provided as guidance as to the meaning of
certain terms
used herein.
As used herein, the articles "a" and "an" mean "one or more" or "at least
one," unless
otherwise indicated. That is, reference to any element of the present
invention by the
indefinite article "a" or "an" does not exclude the possibility that more than
one of the
element is present.
As used herein, the term "acute pain" refers to pain characterized by a short
duration,
e.g., three to six months. Acute pain is typically associated with tissue
damage, and
manifests in ways that can be easily described and observed. It can, for
example, cause
sweating or increased heart rate. Acute pain can also increase over time,
and/or occur
intermittently.
As used herein, the term "bioavailability" is as defined in 21 CFR Section
320.1 and
refers to the rate and extent to which the active ingredient or active moiety
is absorbed from a
drug product and becomes available at the site of action. The term
"bioavailability",
"absolute bioavailability" or "total bioavailability" refers to the total
bioavailability including
amounts that are absorbed through the oral mucosal membrane (i.e.,
transmucosally) and
through the GI mucosa of the lower GI tract. In some embodiments, the
transmucosal drug
delivery devices of the present invention provide bioavailability of
buprenorphine of between
65% and 85%. In some embodiments, the bioavailability of buprenorphine is 80%.
As used herein, the term "bioequivalence" or "bioequivalent" is as defined in
21 CFR
Section 320.1, and means the absence of a significant difference in the rate
and extent to
which the active ingredient or active moiety in pharmaceutical equivalents or
pharmaceutical
alternatives becomes available at the site of drug action when administered at
the same molar
dose under similar conditions in an appropriately designed study. The
pharmacokinetic
parameters C. and AUC for bioequivalent actives fall within the 80%-125% range
of each
other.
4
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
As used herein, the term "chronic pain" refers to pain which persists beyond
the usual
recovery period for an injury or illness. In one embodiment, chronic pain is
the pain that lasts
longer than one week. Chronic pain can be constant or intermittent. Common
causes of
chronic pain include, but are not limited to, arthritis, cancer, Reflex
Sympathetic Dystrophy
Syndrome (RSDS), repetitive stress injuries, shingles, headaches,
fibromyalgia, and diabetic
neuropathy.
As used herein, the term "chronic low back pain" refers to a muscoskeletal
disorder,
wherein the subject experiences pain in the lumbar, or low back region for at
least 12 weeks.
In a specific embodiment, a subject experiences chronic low back pain for at
least 3 months.
As used herein, the term "moderate to severe chronic low back pain" refers to
the
chronic low back pain characterized, e.g., by pain intensity of? 5 on an 11-
point Numerical
Rating Scale (NRS, wherein 0 represents no pain and 10 represents the worst
pain
imaginable).
As used herein, the term "neuropathic pain" refers to a complex, chronic pain
that
usually is accompanied by tissue injury and results from lesions or diseases
affecting the
somatosensory system. With neuropathic pain, the nerve fibers themselves may
be damaged,
dysfunctional or injured. These damaged nerve fibers send incorrect signals to
other pain
centers. The impact of nerve fiber injury includes a change in nerve function
both at the site
of injury and areas around the injury.
As used herein, the term "osteoarthritic pain" refers to pain resulting from
osteoarthritis, a degenerative joint disease and the most common type of
arthritis. It is
associated with the degradation and loss of a cartilage that covers and
cushions the ends of
bones in normal joints. Osteoarthritis causes the cartilage in a joint to
become stiff and lose
its elasticity, making it more susceptible to damage. Over time, the cartilage
may wear away
in some areas, greatly decreasing its ability to act as a shock absorber. As
the cartilage wears
away, tendons and ligaments stretch, causing pain. If the condition worsens,
the bones could
rub against each other, causing even more pain and loss of movement.
As used herein, unless indicated otherwise, the term "buprenorphine", includes
any
pharmaceutically acceptable form of buprenorphine, including, but not limited
to, salts,
esters, and prodrugs thereof. As used herein, the term "buprenorphine
derivative" refers to
5
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
compounds having similar structure and function to buprenorphine. In some
embodiments,
buprenorphine derivatives include those of the following formula:
R3
CH3
"mullR4
R5
0\µ
R60 Oat
or pharmaceutically acceptable salts or esters thereof, wherein
\s"
is a double or single bond; R3 is selected from a -C14 alkyl group or a
cycloalkyl-
substituted-C14 alkyl group; R4 is selected from a alkyl; R5 is ¨OH, or
taken together,
R4 and R5 form a =0 group; and R6 is selected from ¨H or a -C14 alkyl group.
Buprenorphine derivatives include, but are not limited to, etorphine and
diprenorphine. General buprenorphine derivatives are described in
International Application
to Publication No. WO 2008/011194, which is hereby incorporated by
reference.
As used herein, unless indicated otherwise, the term "naloxone" includes any
pharmaceutically acceptable form of naloxone, including, but not limited to,
salts, esters, and
prodrugs thereof.
As used herein, "non-parenteral" refers to modes of administration other than
by
direct systemic delivery of the medicament. As such, "non-parenteral" excludes
the use of
intravenous (IV) injection, intramuscular (IM) injection, Intraperitoneal (IP)
injection,
subcutaneous (SC) injection, etc. for administration of the medicament and
includes
transdermal, oral transmucosal administration, and administration via the GI
tract, generally.
As used herein, the term "mucoadhesive layer" or "polymeric diffusion
environment"
refers to an environment capable of allowing flux of a medicament to a mucosal
surface upon
creation of a gradient by adhesion to a mucosal surface. The flux of a
transported
medicament is proportionally related to the diffusivity of the environment
which can be
6
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
manipulated by, e.g., adjusting the pH, taking into account the ionic nature
of the medicament
and/or the ionic nature of the polymer or polymers included in the
environment.
As used herein, the term "backing layer" or "barrier environment" or "non-
adhesive
polymeric environment" refers to an environment in the form of, e.g., a layer
or coating or
barrier layer, capable of slowing, or reducing flux of a medicament from the
mucoadhesive
layer into the oral cavity. In some embodiments, the backing layer may contain
a second
medicament intended for dissolution in the saliva. In such cases, the pH of
the backing layer
is adjusted, such that it impedes flux of the medicament toward the
mucoadhesive layer
where transmucosal absorption may occur. As used herein, the term
"unidirectional
to gradient" refers to a gradient which allows for the flux of a medicament
(e.g., buprenorphine)
through the device, e.g., through a polymeric diffusion environment, in
substantially one
direction, e.g., to the oral mucosa of a subject. For example, the polymeric
diffusion
environment may be a mucoadhesive polymeric diffusion environment in the form
of a layer
or film disposed adjacent to a backing layer or film. Upon oral mucosal
application, a
gradient is created between the mucoadhesive polymeric diffusion environment
and the
mucosa, and the medicament flows from the mucoadhesive polymeric diffusion
environment,
substantially in one direction towards the mucosa, until the backing layer
dissolves.
As used herein, "treating" or "treatment" of a subject includes the
administration of a
drug to a subject with the purpose of preventing, curing, healing,
alleviating, relieving,
altering, remedying, ameliorating, improving, stabilizing or affecting a
disease or disorder, or
a symptom of a disease or disorder (e.g., to alleviate pain).
The term "subject" refers to living organisms such as humans, dogs, cats, and
other
mammals. Administration of the medicaments included in the devices of the
present
invention can be carried out at dosages and for periods of time effective for
treatment of a
subject. In some embodiments, the subject is a human. In some embodiments, the
pharmacokinetic profiles of the devices of the present invention are similar
for male and
female subjects.
An "effective amount" of a drug necessary to achieve a therapeutic effect may
vary
according to factors such as the age, sex, and weight of the subject. Dosage
regimens can be
adjusted to provide the optimum therapeutic response. For example, the dosage
may be
administered once daily, or may be divided into two individual dosages for
twice daily
7
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
administration. The dose may also be proportionally reduced as indicated by
the exigencies
of the therapeutic situation.
The term "transmucosal," as used herein, refers to any route of administration
via a
mucosal membrane. Examples include, but are not limited to, buccal,
sublingual, nasal,
vaginal, and rectal. In one embodiment, the administration is buccal. In one
embodiment,
the administration is sublingual. As used herein, the term "direct
transmucosal" refers to
mucosal administration via the oral mucosa, e.g., buccal and/or sublingual.
As used herein, the term "water erodable" or "at least partially water
erodable" refers
to a substance that exhibits a water erodability ranging from negligible to
completely water
to erodable. The substance may readily dissolve in water or may only
partially dissolve in water
with difficulty over a long period of time. Furthermore, the substance may
exhibit a differing
erodability in body fluids compared with water because of the more complex
nature of body
fluids. For example, a substance that is negligibly erodable in water may show
an erodability
in body fluids that is slight to moderate. However, in other instances, the
erodability in water
and body fluid may be approximately the same.
As used herein, "addiction therapy" as related to a subject includes the
administration
of a drug to a subject with the purpose of reducing the cravings for the
addictive substance.
As used herein, the term "opioid tolerance" refers to the phenomenon in which
a
subject is less susceptible to the effect of an opioid drug as a consequence
of its prior
administration. "Acute tolerance" describes tolerance that develops very
rapidly following
either a single dose or a few doses of opioids given over a short period of
time. "Chronic
tolerance" describes the observation that opioid administration over a longer
period of time
produces reduced effects. Associative tolerance is best expressed with low
doses of opioids
at long interdose intervals and is readily modified by behavioral or
environmental
interventions. Nonassociative tolerance is best expressed with high doses of
drugs at short
interdose intervals and is not modified by behavioral or environmental
interventions.
As used herein, the term "opioid tolerant subject" refers to a subject
currently
receiving opioid therapy. In one embodiment, the subject is taking >60 mg oral
morphine/day or equianalgesic dose of another opioid for 1 week or longer, as
specified in
the Table 1 below.
8
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
Table 1.
Approximate
Opioid
Equianalgesic Oral Doses
Morphine 60 mg
Tramadol 300 mg
Hydromorphone 12 mg
Oxycodone 30 mg
Hydrocodone 30 mg
Oxymorphone 20 mg
Codeine 400 mg
As used herein, the term "opioid experienced subject" refers to a subject
currently
receiving opioid therapy. In one embodiment, the subject's daily use of
opioids does not
exceed the daily doses of opioids as specified in the Table 1 above.
As used herein, the term "opioid naive subject" refers to a subject who is not
currently
receiving opioid therapy. In one embodiment, the subject has not been exposed
to opioids for
1 week or longer.
As used herein, the term "abusive" or "abusive manner" refers to uses of the
devices
to beyond oral transmucosal administration such as by extracting the drug
and injecting or
snorting.
As used herein, the term "low dose of buprenorphine" refers to the daily dose
less
than 1.8 mg (e.g., about 200 jig to about 1800 jig, or about 240 jig to 1800
jig) of
buprenorphine.
As used herein, the term "steady-state plasma concentration" refers to the
state,
wherein the fluctuation in plasma drug concentrations are the same or similar
after each dose.
The term "steady-state C. of plasma buprenorphine concentration" refers to the
state,
wherein the post dose maximum plasma concentration of buprenorphine does not
differ from
one dose to another. The term "steady-state Cmir, of plasma buprenorphine
concentration"
refers to the state, wherein the post-dose minimum plasma concentration of
buprenorphine
does not differ from one dose to another. In one embodiment, the devices used
in the present
invention provide steady-state C. of plasma buprenorphine concentration in a
range
between about 0.1 and about 1.0 ng/mL. In another embodiment, the devices used
in the
9
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
present invention provide steady-state C. of plasma buprenorphine
concentration in a range
between about 0.1 and about 0.5 ng/mL.
As used herein, the term "common opioid adverse effects" refers to adverse
effects
commonly experienced by the subjects taking opioid analgesics. These common
opioid
The term "mild common opioid adverse effects" refers to adverse effects that
do not
require a special treatment and do not interfere with the subject's daily
activities. The term
15 The term "significant constipation" refers to chronic or severe
constipation associated
with the continuous use of morphine or other opioids.
The term "significant nausea" refers to a severe condition of nausea that is
commonly
known in the art. In some embodiments, the term "significant nausea" is
defined with a
visual analog scale (VAS) score of greater than or equal to 25 mm on a 0 to
100 mm scale.
20 As used herein, the term "disposed" refers to the uniform or non-uniform
distribution
of an element within another.
Pain Management
Certain aspects of the present invention include methods for providing pain
management and/or relief to a subject in need thereof. The pain can be any
pain known in the
25 art, caused by any disease, disorder, condition and/or circumstance and
can be chronic pain or
acute pain. Chronic pain can arise from many sources including, cancer, Reflex
Sympathetic
Dystrophy Syndrome (RSDS), and migraine. Acute pain is typically directly
related to tissue
damage, and lasts for a relatively short amount of time, e.g., hours to days,
or up to 7 days.
In other embodiments, the pain is breakthrough cancer pain.
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
In some aspects, the present invention provides methods of managing or
treating
chronic pain in a subject. In some embodiments, the subject is opioid
experienced, opioid
tolerant or opioid naive, as defined above. In a specific embodiment, the
subject is opioid
tolerant. In another embodiment, the subject has not responded to previous
treatment with
the maximal doses of non-steroidal anti-inflammatory drugs.
In some embodiments, the chronic pain is chronic lower back pain (CLBP). In
some
embodiments, the chronic lower back pain is moderate to severe chronic lower
back pain. In
other embodiments, the pain is neuropathic pain or osteoarthritic pain. In a
specific
embodiment, the subject to be treated for moderate to severe chronic low back
pain is an
to opioid experienced subject.
It has also been presently found that twice daily (or once daily)
administration of low
doses of buprenorphine via transmucosal drug delivery devices of the present
invention is
associated with low incidence or absence of common opioid adverse effects
associated with
opioid analgesics. In one embodiment, the adverse effect is nausea. In another
embodiment,
the adverse effect is constipation.
Administration and Dosing of Buprenorphine
In some embodiments, transmucosal drug delivery devices of the present
invention
(e.g., BEMA Buprenorphine) are administered once daily or twice daily. In some
embodiments, the total daily dose of buprenorphine administered is between 200
[tg and 1800
jig, e.g., 200 jig, 220 jig, 240 jig, 280 jig, 300 jig, 320 jig, 350 jig, 360
jig, 400 jig, 450 jig,
480 jig, 500 jig, 550 jig, 600 jig, 620 jig, 650 jig, 700 jig, 720 jig, 750
jig, 800 jig, 860 jig,
900 jig, 960 jig, 1000 jig, 1100 jig, 1200 jig, 1250 jig, 1300 jig, 1400 jig,
1500 jig, 1600
jig, and 1800 mg.
In some embodiments, the transmucosal drug delivery devices of the present
invention comprise low doses of buprenorphine. In one embodiment, the low dose
of
buprenorphine contained in the devices is defined as the dose of about 100 jig
to about 900
jig of buprenorphine. In some embodiments, the low dose of buprenorphine
comprised in the
mucoadhesive device of the invention is 100 jig, 110 jig, 120 jig, 140 jig,
150 jig, 160 jig,
175 jig, 180 jig, 200 jig, 225 jig, 240 jig, 250 jig, 275 jig, 300 jig, 310
jig, 325 jig, 350 jig,
360 jig, 375 jig, 400 jig, 430 jig, 450 jig, 480 jig, 500 jig, 550 jig, 600
jig, 625 jig, 650 jig,
11
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
700 [tg, 750 jig, 800 jig, 900 jig, 1000 jig, 1200 jig, 1250 jig, 1300 jig,
1400 jig, 1500 mg,
1600 jig, and 1800 mg.
Transmucosal Pharmaceutical Delivery Device
Preparation of transmucosal pharmaceutical delivery devices have been
previously
described, e.g., in U.S. Patent Application No. 08/734,519, filed on October
18, 1996, now
U.S. Patent No. 5,800,832, issued September 1, 1998; U.S. Patent Application
No.
09/144,827, filed on September 1, 1998, now U.S. Patent No. 6,159,498, issued
on December
12, 2000; U.S. Patent Application No. 11/069,089, filed on March 1, 2005, Now
U.S. Patent
No. 7,579,019, issued on August 25, 2009; U.S. Patent Application No.
11/639,408, filed on
m December 13, 2006, published as US 2007/0148097; U.S. Patent Application
No.
11/817,915, filed on September 6, 2007, published as US 2010/0015183; U.S.
Patent
Application No. 13/834,306, filed on July 15, 2011, now U.S. Patent No.
8,147,866, issued
on April 3, 2012; U.S. Patent Application No. 13/590,094, filed on August 20,
2012; U.S.
Patent Application No. 12/537,571, filed on August 7, 2009, published as US
2011/0033541;
and U.S. Patent Application No. 12/537,580, filed on August 7, 2009, published
as US
2011/0033542, the entire contents of which are incorporated herein by
reference.
i. Mucoadhesive layer
In some embodiments, the devices of the present invention adhere to a mucosal
surface of the subject within about 5 seconds following application. In some
embodiments,
the devices of the present invention comprise an opioid agonist. In some
embodiments, the
devices of the present invention include a bioerodable- or water-erodable
mucoadhesive
layer, and the opioid agonist is comprised in the mucoadhesive layer. In one
embodiment,
the opioid agonist is buprenorphine. The dose of buprenorphine that can be
incorporated into
the device of the present invention depends on the desired treatment dosage to
be
administered and can range from about 20 jig to about 20 mg, or from about 120
jig to about
2000 jig of buprenorphine.
ii. Backing layer
In some embodiments, the device further comprises at least one additional non-
adhesive polymeric environment, e.g., a backing layer. This layer is disposed
adjacent to the
mucoadhesive polymeric diffusion environment, e.g., a backing layer, functions
to facilitate
12
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
the delivery of the opioid agonist, such as buprenorphine, to the mucosa. This
additional
layer may comprise the same or a different combination of polymers as the
mucoadhesive
polymeric diffusion environment or the non-adhesive polymeric diffusion
environment.
In some embodiments, the backing layer includes an additional medicament, such
as
an opioid antagonist, to render the device of the invention abuse-resistant.
In some
embodiments, the opioid antagonist is naloxone. The dose of naloxone that can
be
incorporated into the backing layer of the device of the present invention can
range from
about 2.5 j.tg to about 5 mg of naloxone. In some embodiments, the amount of
buprenorphine
and the amount of naloxone disposed in the device are present in a ratio
chosen such that the
to effect of buprenorphine is negated by naloxone if the mixture is
injected or snorted. In some
embodiments, the amount of buprenorphine and the amount of naloxone disposed
in the
device are present in a 4:1 w/w ratio.
EXEMPLIFICATION OF THE INVENTION
The invention will be further understood by the following examples. However,
those
skilled in the art will readily appreciate that the specific experimental
details are only
illustrative and are not meant to limit the invention as described herein,
which is defined by
the claims which follow thereafter.
Example 1. Preparation of the devices of the invention
Transmucosal devices are configured in the form of a disc, rectangular in
shape with
round corners, yellow on one or both sides of the cheek). Buprenorphine is
present in the
mucoadhesive layer, and this side is to be placed in contact with the buccal
mucosa (inside
the cheek). The drug is delivered into and across the mucosa as the disc
erodes in the mouth.
The non-adhesive, backing layer controls the rate erosion of the disc, and
minimizes the
amount of buprenorphine dissolved in saliva and ultimately swallowed, a
pathway of lower
absorption due first pass metabolism. The mucoadhesive polymeric diffusion
layer and the
backing layer are bonded together and do not delaminate during or after
application.
The mucoadhesive layer for the transmucosal devices of the present invention
comprising the desired dosage of buprenorphine is prepared by mixing purified
water,
propylene glycol (about 4.6% total formulation, by dry weight), sodium
benzoate (about
0.5% total formulation, by dry weight), methylparaben (about 0.9% total
formulation, by dry
13
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
weight), propylparaben (about 0.2% total formulation, by dry weight), vitamin
E acetate
(about 0.06% total formulation, by dry weight), citric acid (about 0.5% total
formulation, by
dry weight), yellow iron oxide (about 0.5% total formulation, by dry weight),
monobasic
sodium phosphate (about 3.4% total formulation, by dry weight). The above
ingredients are
added sequentially to a mixing vessel. After the components are dissolved,
buprenorphine
HC1 (about 1.3% total formulation, by dry weight) is added, and the vessel was
heated to
120 F to 130 F. After dissolution, the polymer mixture (hydroxypropyl
cellulose (about
6.8% total formulation, by dry weight), hydroxyethyl cellulose (about 20.3%
total
formulation, by dry weight), polycarbophil (about 6.3% total formulation, by
dry weight),
to and carboxy methyl cellulose (about 54.3% total formulation, by dry
weight)) are added to
the vessel, and stirred until dispersed. Subsequently, heat is removed from
the mixing vessel.
As the last addition step, tribasic sodium phosphate and sodium hydroxide are
added to adjust
the blend to a desired pH. The blend is mixed under vacuum for a few hours.
Each prepared
mixture is stored in an air-sealed vessel until its use in the coating
operation.
The backing layer is prepared by adding purified water to a mixing vessel
followed by
sequential addition of sodium benzoate (about 0.5% total formulation, by dry
weight),
methylparaben (about 0.4% total formulation, by dry weight), propylparaben
(about 0.1%
total formulation, by dry weight), citric acid (about 0.5% total formulation,
by dry weight),
vitamin E acetate (about 0.05% total formulation, by dry weight), sodium
saccharin (about
0.5% total formulation, by dry weight). Subsequently, a mixture of the
polymers
hydroxypropyl cellulose (about 63% total formulation, by dry weight) and
hydroxyethyl
cellulose (about 32% total formulation, by dry weight) are added and stirred
at a temperature
between about 120 F and 130 F, until evenly dispersed. Upon cooling to room
temperature,
titanium dioxide (about 2.5% total formulation, by dry weight) and peppermint
oil (about
0.8% total formulation, by dry weight) are then added to the vessel and
stirred. The prepared
mixture is stored in an air-sealed vessel until it is ready for use in the
coating operation.
The layers are cast in series onto a St. Gobain polyester liner. First, the
backing layer
is cast using a knife-on-a-blade coating method. The backing layer is then
cured in a
continuous oven at about 65 C to 95 C and dried. After two coating and
drying iterations,
an approximately 8 mil (203 to 213 micrometers) thick backing layer is
obtained.
Subsequently, the mucoadhesive polymeric diffusion environment is cast onto
the backing
14
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
layer, cured in an oven at about 65 C to 95 C and dried. The devices are
then die-cut by
kiss-cut method and removed from the casting surface.
Example 2. Placebo-controlled, Double-blind Study to Evaluate the Efficacy of
BEMA
Buprenorphine in Subjects with Moderate to Severe Chronic Low Back Pain
A 12-week, placebo-controlled, double-blind randomized withdrawal study was
conducted to evaluate the efficacy and safety of buprenorphine delivered twice
daily via
transmucosal drug delivery device with enhanced uptake (BEMA buprenorphine) in
subjects
with moderate to severe chronic low back pain. The study was also designed to
define the
range of BEMA Buprenorphine doses effective for management of moderate to
severe
to chronic lower back pain.
i. Study Design
The study consisted of an open-label dose titration period lasting up to 4
weeks,
followed by a randomized, double-blind, placebo-controlled treatment period of
12 weeks.
The subjects continued on their current pain therapy during the initial
screening period (days
-14 to -1) and until 12 to 24 hours prior to Day 0/1 of the open-label dose
titration period.
Predose assessments were performed on open-label titration period Day 0 and
the first dose
of study drug was taken on open-label titration period Day 1.
During the open-label titration period, the subjects were administered BEMA
Buprenorphine approximately every 12 hours, and dose adjustments were
performed at
intervals over a period of up to 4 weeks until a stabilized dose was found
(i.e., the dose that
provided meaningful pain relief and was well tolerated). The titration
sequence of BEMA
Buprenorphine is illustrated in the Table 4 below. The subjects for whom a
stabilized dose
could not be found were discontinued from the study.
Table 4. BEMA Buprenorphne Titration Schedule
Study Days Titration Sequence - BEMA Buprenorphine
Low Dose (Q12 hours)
1 A
7 ( 3 days) 2xA
14 ( 3 days) 3xA
21 ( 3 days) 4xA
15
CA 02859859 2014-06-18
WO 2013/096811 PCT/US2012/071330
The subjects for whom a stabilized dose was identified and who had taken that
dose at
least 12 times over the last 7 days entered a 12-week, double-blind treatment
period, in which
half of subjects received BEMA Placebo and half continued receiving BEMA
Buprenorphine
at the stabilized dose. Each subject's participation in the entire study
lasted 19 weeks. The
schematic showing the study design is shown in Figure 1.
ii. Subject population used in the study
The subjects who were selected for the inclusion in the study were opioid
naive or
opioid experienced, as defined earlier in this application. Opioid experienced
subjects were
subjects taking <60 mg oral daily dose of morphine or equianalgesic dose of
another allowed
to opioid for 1 week or longer. Opioid naive subjects were not taking any
opioids for 1 week or
longer.
A total of 334 subjects entered the study, of which 332 subjects entered the 4-
week
open-label dose titration period. As 97 subjects discontinued intervention
during the open-
label titration period, a total of 235 subjects continued on to the 12-week
double-blind
treatment period. Of the 117 subjects who received BEMA Buprenorphine, 28
discontinued
intervention, and 89 subjects completed the study. Of the 118 subjects who
received placebo,
37 discontinued intervention, and 81 subjects completed the study. Subject
disposition
during the study is summarized in Figure 2, and the characteristics of the
population who
participated in the study are summarized in the Table 5 below:
Table 5. Study Population Characteristics
Open Label Double Blind Double
Blind
Titration Treatment Treatment
BEMA BEMA Placebo
Buprenorphine Buprenorphine
Number of subjects 332 117 118
Mean Age (yrs.) 51 51 51
Women, n (%) 55.5 53 56
Opioid Naive (%) 62.7 62.4 69.5
Mean Pain Intensity at 7
Screening
Mean Pain Intensity at NA 3.23 3.26
Baseline
16
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
iii. Analgesic Efficacy of BEMA Buprenorphine
Analgesic efficacy was assessed daily by having the subject record their
average pain
intensity over the past 24 hours on a scale of 0 to 10, where 0 represents no
pain and 10
represents the worst pain imaginable (11-point Numerical Rating Scale, NRS).
The mean
change in daily pain intensity from baseline (CBL) to final visit during
double blind treatment
period is presented in Tables 6-12 below. Tables 6-8 present the mean change
data for
different subject groups, and Tables 9-12 present the mean change data for the
subject groups
treated with different doses of buprenorphine.
Table 6. Average Daily Pain Intensity - All Subjects
Parameter BEMA Placebo
Buprenorphine
Number of subjects 117 118
Primary Analysis, mean
(SD)
Baseline 3.23(1.19) 3.26(1.22)
Final 3.59 (1.91) 3.77 (2.22)
Least Squares Mean 0.35 0.51
Difference
Treatment comparison of -0.16
Change from Baseline
(CBL)
Beta Buprenorphine (CBL
BB) minus placebo
p-value 0.53
to Table 7. Average daily pain intensity -opioid experienced subjects
Parameter BEMA Placebo
Buprenorphine
Number of subjects 44 36
Primary Analysis,
mean (SD)
Baseline 3.50 (1.14) 3.43 (0.87)
Final 4.05 (2.04) 4.86 (2.03)
Least Squares Mean 0.57 1.41
Difference
Treatment comparison -0.84
of CBL
BB minus placebo
p-value 0.067
17
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
Table 8. Average daily pain intensity in opioid naive subjects
Parameter BEMA Placebo
Buprenorphine
Number of subjects 73 82
Primary Analysis,
mean (SD)
Baseline 3.07 (1.20) 3.19 (1.33)
Final 3.31 (1.78) 3.29 (2.1)
Least Squares Mean 0.21 0.13
Difference
Treatment comparison 0.08
of CBL
BB minus placebo
p-value 0.78
As shown in Table 7, the change from baseline in average daily pain score on
BEMA
Buprenorphine compared to placebo is nearly statistically significant in the
opioid
experienced population.
Table 9. Average daily pain intensity in subjects treated with the daily dose
of A pg of
buprenorphine
Parameter BEMA Placebo
Buprenorphine
Dose A mcg
N 28 33
Primary Analysis, mean (SD)
Baseline 2.79 (1.51) 3.12 (1.38)
Final 3.58 (1.79) 2.88 (2.18)
Least Squares Mean Difference 0.72 -0.24
Treatment comparison of CBL 0.90
BB minus placebo
p-value 0.085
to
18
CA 02859859 2014-06-18
WO 2013/096811 PCT/US2012/071330
Table 10. Average daily pain intensity in subjects treated with the daily dose
of 2xA lig
of buprenorphine
Parameter BEMA Placebo
Buprenorphine
Dose 2xA mcg
N 31 33
Primary Analysis, mean (SD)
Baseline 3.25 (1.13) 3.33 (1.12)
Final 3.23 (1.73) 4.04 (2.27)
Least Squares Mean Difference -0.03 0.72
Treatment comparison of CBL -0.74
BB minus placebo
p-value 0.17
Table 11. Average daily pain intensity in subjects treated with the daily dose
of 3xA lig
of buprenorphine
Parameter BEMA Placebo
Buprenorphine
Dose 3xA mcg
N 22 31
Primary Analysis, mean (SD)
Baseline 3.43 (0.87) 3.48 (1.25)
Final 4.24 (2.48) 4.0 (2.23)
Least Squares Mean Difference 0.79 0.53
Treatment comparison of CBL 0.26
BB minus placebo
p-value 0.70
to
19
CA 02859859 2014-06-18
WO 2013/096811 PCT/US2012/071330
Table 12. Average daily pain intensity in subjects treated with the daily dose
of 4xA pg
of buprenorphine
Parameter BEMA Placebo
Buprenorphine
Dose 4xA mcg
36 21
Primary Analysis, mean (SD)
Baseline 3.45 (1.09) 3.04 (1.05)
Final 3.69 (1.75) 4.27 (1.92)
Least Squares Mean 0.29 1.13
Difference
Treatment comparison of CBL -0.84
BB minus placebo
p-value 0.11
Graphed in Figure 3 is the mean change from baseline in daily pain intensity
for all
subjects; all subjects receiving 2xA [tg, 3xA jig, or 4xA jig, BEMA
Buprenorphine; all
opioid experienced subjects; and all opioid experienced subjects receiving 2xA
jig, 3xA jig,
or 4xA p g BEMA Buprenorphine.
iv. Incidence of Adverse Events
Adverse events (AE) were recorded for all subjects in the study. AE was
defined as
to any untoward medical occurrence in a patient or clinical investigation
subject administered a
pharmaceutical product and which does not necessarily have a causal
relationship with this
treatment. The total number of adverse events recorded for both the open label
titration and
double blind treatment periods are listed in the Table 13 below.
20
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
Table 13. Total Treatment Emergent Adverse Events (TEAEs)
Open Label Double Blind
Titration Treatment
Adverse event profile BEMA BEMA Placebo
Buprenorphine, Buprenorphine n (%)
n(%) n(%)
Subjects with > 1 AE 219 (66) 73 (62) 68 (58)
Subjects with > 1 AE causing 6 (5) 3 (2)
discontinuation
Discontinued due to opioid
withdrawal
Subjects with > 1 SAE 1(1) 0
AEs reported in > 5% of
subjects
Nausea 108 (32) 11(9) 10 (8)
Vomiting 20 (6) 6 (5) 4 (3)
Constipation 36 (11) 7 (6) 3 (2)
Dizziness 30 (9) 4 (3) 1 (1)
Headache 39 (12) 12 (10) 5 (4)
The intensity of AEs was characterized as mild, moderate, or severe as
follows:
Mild: AEs that were transient, did not require a special treatment and did not
interfere
with the subject's daily activities.
Moderate: AEs that introduced a low level of inconvenience or concern to the
subject
and could interfere with daily activities, but were usually ameliorated by
simple therapeutic
measures.
Severe: AEs that interrupted a subject's usual daily activity and typically
required
systemic drug therapy or other treatment.
Table 14 below shows the number and percent of subjects who experienced TEAEs
during the open label titration period involving 330 subjects, with all TEAEs
characterized by
event intensity and relationship to the study drug. Table 15 below shows
analogous data for
the double blind treatment period and buprenorphine treatment group involving
117 subjects.
The maximum intensity ever recorded for each event and the drug relationship
at that
intensity were used to categorize adverse events. "Drug-related" category is
listed as "R" and
21
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
includes adverse events with investigator-assessed relation to drug of
"probably" or
"possibly". "Non drug-related" category is listed as "NR".
Table 14. TEAEs by Event Intensity and Drug Relationship During Open Label
Titration Period.
AE Intensity and Drug Relationship
AE Mild Moderate Severe Not Overall
Profile Reported
R NR NR NR
R NR R NR R R
n n n n
n(%) n(%) n(%) n(%) n(%) n(%)
No. of
49 52 96
subject 63 34 12 10 124
(14.8 (15.8 0 0 (29.1
with > 1 (19.1) (10.3) (3.6) (3.0)
(37.6)
) ) )
TEAE
Constipati 23 4 5 1 3 0 0 0 31 5
on (7.0) (1.2) (1.5) (0.3) (0.9) (9.4)
(1.5)
Nausea 50 9 42 1 5 1 0 0 97 11
(15.2 (2.7) (12.7) (0.3) (1.5) (0.3)
(29.4) (3.3)
)
Vomiting 3 6 7 0 2 1 0 0 12 7
(0.9) (1.8) (2.1) (0.6) (0.3) (3.6)
(2.1)
Dizziness 13 3 10 1 3 0 0 0 26 4
(3.9) (0.9) (3.0) (0.3) (0.9) (7.9)
(1.2)
Headache 15 9 8 3 3 1 0 0 26 13
(4.5) (2.7) (2.4) (0.9) (0.9) (0.3) (7.9)
(3.9)
10
22
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
Table 15. TEAEs by Event Intensity and Drug Relationship During Double Blind
Treatment Period.
AE Intensity and Drug Relationship
AE Mild Moderate Severe Not Overall
Profile Reported
R NR NR NR
R NR R NR R R
n n n n
n(%) n(%) n(%) n(%) n(%) n(%)
(%) (%) (%) (%)
No. of
24 52
subject 9 11 23 1 5 21
(20.5 0 0
(44.4
with? 1 (7.7) (9.94) (19.7) (0.9) (4.3) (17.9)
) )
TEAE
Constipati 3 0 4 0 0 0 0 0 7 0
on (2.6) (3.4) (6.0)
Nausea 5 3 3 0 0 0 0 0 8 3
(4.3) (2.6) (2.6) (6.8) (2.6)
Vomiting 0 2 2 1 1 0 0 0 3 3
(1.7) (1.7) (0.9) (0.9) (2.6) (2.6)
Dizziness 1 0 2 0 1 0 0 0 4 0
(0.9) (1.7) (0.9) (3.4)
Headache 1 7 2 1 0 1 0 0 3 9
(0.9) (0.6) (1.7) (0.9) (0.9) (2.6) (7.7)
Example 3. Pharmacokinetic profiles for BEMA Buprenorphine
Pharmacokinetic parameters for the BEMA Buprenorphine doses used in the
treatment of chronic pain were determined in a separate, multiple dose study.
BEMA
Buprenorphine contained buprenorphine doses of 2xA mg, and 4xA ?lg. Each dose
was
administered twice daily for 3 days with serial blood samples collected.
Selected
pharmacokinetic parameters are shown in the Table 16 below.
Table 16. Selected Pharmacokinetic Parameters for BEMA Buprenorphine Buccal
Films comprising lxA g, 2xA g, 3xA g and 4xA g buprenorphine
Pharmacokinetic Parameters (Mean values) lxA 2xA 3xA lig 4xA lig
lig lig
T. (hr) 2.90 2.61 2.00 2.20
C. (ng/mL) 0.0766 0.156 0.216 0.364
Cmin (ng/mL) 0.0157 0.0371 0.0558 0.0862
Can (ng/mL) 0.0409 0.0805 0.113 0.195
AUC0_, (hr*ng/mL) 0.4903 0.9658 1.358 2.343
AUCiasi (hr*ng/mL) 0.4085 0.7902 1.111 5.033
23
CA 02859859 2014-06-18
WO 2013/096811
PCT/US2012/071330
T refers to the time to reach the steady-state Cõ of plasma buprenorphine
concentration.
C refers to the maximum concentration in plasma in steady-state.
Cram refers to the minimum concentration in plasma in steady-state.
Ca.õ refers to the average concentration in plasma in steady-state.
AUC0, refers to the area under the plasma concentration time curve from time-
zero through the dosing interval
AUCast refers to the area under the concentration-time curve from time-zero to
the time of the last quantifiable
concentration.
24