Note: Descriptions are shown in the official language in which they were submitted.
CA 02860813 2014-07-07
WO 2013/117805 PCT/F12013/050089
TITLE OF THE INVENTION
SYSTEM FOR POWER CONTROL IN CELLS FOR ELECTROLYTIC RE-
COVERY OF A METAL
BACKGROUND OF THE INVENTION
Field of the invention:
The invention relates to the electrolytic
processing of metals. Examples of electrolytic recov-
ery of metals are electrorefining and electrowinning.
The invention relates particularly to control and dis-
tribution of electrical power within a single electro-
lytic cell or distribution of electrical power between
multiple electrolytic cells.
BACKGROUND OF THE INVENTION
In electrolytic recovery of metals an elec-
tric current is passed between two electrodes immersed
in an electrolyte which is a solution containing the
metal in a dissolved ionic form. The electrical cur-
rent causes the metal to be deposited on the cathode.
Electrorefining (ER) is an electrolytic pro-
cess for purifying a metal. An impure metal anode is
dissolved and the pure metal is deposited at the cath-
ode. The anode is made electrically positive and the
cathode is made negative by application of an external
voltage, so that an electrical current passes through
the electrolyte between the anodes and cathodes. For
example, in the electrorefining of copper, the anode
is made of impure copper, the copper enters the elec-
trolyte as the anode dissolves anodically giving cop-
per (II) ions (Cu2+(aq)) historically referred to as
"cupric" ions. Typically, the electrolyte contains
copper as copper sulfate with sulfuric acid as a sup-
porting electrolyte. The copper (II) ions are trans-
ported through the electrolyte and reduced at the
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
2
cathode, where pure copper is deposited. Impurity ele-
ments from the anode can remain as solids and deposit
in the anode slimes in the cell, or they can dissolve
in the electrolyte. Impurity elements comprise, for
example, nickel or arsenic.
In copper electrorefining plants, the concen-
tration of impurity elements such as nickel and arse-
nic typically build up with time, and liberator cells
are typically used for purifying the tankhouse elec-
trolyte from the main production process. Elec-
trowinning (EW) is an electrolytic process for recov-
ering dissolved metals from an electrolyte. A number
of metals can be won from solution using electrolytic
methods. These metals include but are not limited to
copper, nickel, gold, silver, cobalt, zinc, chromium
and manganese.
In industrial electrowinning of copper, the
cell voltage is usually approximately 2.0V, the cur-
rent density can be in the range 200 to 400 Amperes
per square meter and the area of each electrode face
is usually about 1 square meter. The term cell is used
to describe a tank in which at least one anode and one
cathode are immersed in an electrolyte solution which
is usually aqueous. In the following description the
term tankhouse means an arrangement, wherein at least
one cell (or tank) and power source are present in a
building or enclosed structure, that is, a house. In a
typical configuration a tankhouse comprises a plurali-
ty of cells.
When using a plurality of cells in conven-
tional electrowinning, it is usual to have several
cells powered by the same rectifier, giving the same
current density at each cathode.
Liberator cells are similar to standard elec-
trowinning cells. During the process copper is plated
out on the cathodes and copper ion concentration in
the electrolyte decreases.
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
3
In certain technical solutions of the elec-
trolytic recovery of metals, the state of the electro-
lyte changes during the process. For example, in a pu-
rification process of the electrolyte from copper
electrorefining, it is desirable to remove most of the
copper ions along with harmful impurities from the
electrolyte. The properties of the electrolyte - espe-
cially the copper concentration - change with time
during the process.
In later stages of electrolytic recovery of
metals in liberator cells, where copper concentration
is low, if the current density is too high, the result
can be the undesired production of a powdery copper
deposit (or copper sludge) at the cathode. There is
also a risk of toxic arsine gas production at the
cathode surface.
In terms of electrode materials, liberator EW
cells are similar to standard electrowinning cells,
the anodes are insoluble. For copper liberators the
anodes are usually lead-based alloys; rolled lead-
calcium-tin alloy, or antimonial lead. Mixed metal ox-
ide (MMO) coated titanium anodes - also known by the
trademark name of Dimensionally Stable AnodeTM (DSA) -
may also be used.
The cathodes in liberator cells are usually
spent anodes from the electrorefining tankhouse, but
can also be permanent cathodes with stainless steel
blades. Older refineries may still use copper starter
sheet technology.
Copper cathodes deposited in the liberator
cells which contain impurities (such as arsenic and
antimony) are returned to the smelter to be melted and
cast into anodes for electrorefining.
Decopperised electrolyte can be sent to the
electrorefining cells, or further processed, for exam-
ple, for nickel removal.
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
4
In certain technical solutions of electrolyt-
ic recovery of metals, the state of the electrolyte
inevitably changes during the process. For example, in
a purification process of the electrolyte it is de-
sired to remove all copper together with harmful impu-
rities from the electrolyte. This means that proper-
ties of the electrolyte change with time during the
process.
Liberator cells are similar to normal elec-
trolytic cells, but they have lead anodes in place of
copper anodes. Copper in the solution is deposited on
copper starting sheets. As the copper in the solution
is depleted, the quality of the copper deposit is de-
graded. Liberator cathodes containing impurities such
as antimony are returned to the smelter to be melted
and cast into anodes. Purified electrolyte is recycled
to the electrolytic cells.
In a liberator tankhouse, the aim is to re-
move copper from the solution as a solid copper cath-
ode deposit. The current densities employed are typi-
cally lower than in standard copper electrowinning or
Electrorefining. As the process proceeds, the copper
concentration of the electrolyte gets down to lower
cation concentrations.
Reference is now made to Figure 1 which il-
lustrates a system for electrolytic recovery of metals
in prior art. In Figure 1 there is a voltage source
100 which provides a negative voltage to conductor 102
which is further connected to a cathode busbar 118.
Voltage source 100 provides a positive voltage to con-
ductor 104 which is further connected to anode busbar
129 of a cell 120. There are two electrolytic cells,
namely a cell 110 and cell 120. Cell 110 comprises a
cathode 114 and an anode 116. Cell 110 contains an
electrolyte solution. Cell 120 comprises a cathode 124
and an anode 126. Cell 120 contains an electrolyte so-
lution. Cathode busbar 118 is connected to cathodes in
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
cell 110 such as cathode 114. Cathode busbar 128 is
connected to cathodes in cell 120 such as cathode 124.
Anode busbar 119 is connected to anodes in cell 110
such as anode 116. Anode busbar 129 is connected to
5 anodes in cell 120 such as anode 126. Cell 110 and
cell 120 are connected electrically in series so that
anode busbar 119 is connected along its length to
cathode busbar 128.
During the liberation process the electrolyte
has initially high copper concentration. As the elec-
trolyte is processed the copper concentration decreas-
es and acid concentration increases. In prior art so-
lutions, the maximum current density which can be used
is dictated by the copper concentration in the lean
electrolyte, since all the cells are connected elec-
trically in series and carry approximately the same
current.
In prior art a cell contains a plurality of
anodes, all connected electrically in parallel and a
plurality of cathodes also connected electrically in
parallel. The voltage across a cell is therefore ap-
proximately equal to the voltage that would be experi-
enced between a single anode and a single cathode. By
way of example, this voltage would be approximately
1.7 to 2.8 Volts in the case of the electrowinning of
copper, depending on the current density employed.
It is difficult to convert electrical power
from mains voltage to a dc voltage of this magnitude
efficiently. For this reason it is common practice to
connect cells in series so that they all conduct the
same current, but the voltage across the series chain
of cells is equal to the sum of all the cell voltages.
By this means the voltage rating of the central dc
current source, commonly called a rectifier, is ele-
vated and high efficiency can be obtained.
The difficulty with this arrangement is that
the same current density is used in all cells. Cells
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
6
may operate at a much lower current density than the
copper concentration in the electrolyte would permit.
This causes that there are more cells compared to a
situation where the process would be run using optimum
current densities. Thus, a liberator tank house uses
more electrical power than in an optimum case.
SUMMARY OF THE INVENTION:
According to an aspect of the invention, the
invention is a system for electrolytic processing of a
metal comprising at least two electrolysis cells for
the metal and a rectifier, wherein the at least two
cells comprise at least three anodes and at least two
interleaved cathodes. For the system is characteristic
that the anodes or the cathodes of a first cell have
an electrical connection to a positive or a negative
terminal of the rectifier, respectively, via a first
electrical path having a first resistance; the anodes
or the cathodes of a second cell have an electrical
connection to a positive or a negative terminal of the
rectifier, respectively, via a second electrical path
having a second resistance; the second resistance is
configured to be higher than the first resistance; and
the system further comprises a channel for electrolyte
from the first cell to the second cell, the electro-
lyte containing the metal in a dissolved ionic form,
metal concentration in the first cell being higher
than in the second cell.
According to another aspect of the invention,
the invention is a system for electrolytic processing
of a metal comprising at least two electrolysis cells
for the metal and a rectifier, wherein a first cell
comprises a plurality of cathodes interleaved between
a plurality of anodes and a second cell comprises a
plurality of cathodes interleaved between a plurality
of anodes. For the system is characteristic that the
anodes of the first cell have an electrical connection
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
7
to a positive terminal of the rectifier via a first
electrical path having a first resistance; the anodes
of the second cell have an electrical connection to a
positive terminal of the rectifier via a second elec-
trical path having a second resistance; the number of
anodes and cathodes in the second cell is configured
to be higher than the number of anodes and cathodes in
the first cell to diminish a difference between the
first resistance and the second resistance; and the
system further comprises a channel for electrolyte
from the first cell to the second cell, the electro-
lyte containing the metal in a dissolved ionic form,
metal concentration in the first cell being higher
than in the second cell.
In one embodiment of the invention, the con-
figuration of anodes and cathodes in the first and se-
cond cells is such that a cathode plate is placed be-
tween two anode plates. The cathode and anode plates
may be substantially parallel in a cell. The plates
may be rectangular, for example, 1 meter by 1 meter.
The distances from cathodes to neighboring anodes, be-
tween which the cathodes are arranged, may be substan-
tially same. By substantially the same distances may
be meant a difference of less than 10 centimeters in
the distances. By substantially parallel may be meant
at most an angle of 10 degrees between plates.
In one embodiment of the invention, the at
least two cells comprise the at least three anodes and
the at least two interleaved cathodes, the cathodes
being interleaved between the anodes. Thus, between
two anodes there is a cathode.
In one embodiment of the invention, the an-
odes of a first cell have an electrical connection to
a positive terminal of the rectifier, respectively,
via a first electrical path having a first resistance,
the anodes of a second cell have an electrical connec-
tion to a positive terminal of the rectifier, respec-
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
8
tively, via a second electrical path which has the se-
cond resistance.
In one embodiment of the invention, the cath-
odes of a first cell have an electrical connection to
a negative terminal of the rectifier, respectively,
via a first electrical path having a first resistance,
and the cathodes of a second cell have an electrical
connection to a negative terminal of the rectifier,
respectively, via a second electrical path which has a
second resistance.
In one embodiment of the invention, the first
electrical path and the second electrical path com-
prise metal conductors.
In one embodiment of the invention, the first
electrical path consists of conducting material and
the second electrical path comprises at least one re-
sistor device in addition to at least one conductor.
In one embodiment of the invention, the first
electrical path comprises conducting material and the
second electrical path comprises at least one resistor
device.
In one embodiment of the invention, the se-
cond electrical path comprises a resistor and an anode
or a cathode bar in series, the anode or the cathode
bar being connected to each anode or cathode, respec-
tively, of the second cell.
In one embodiment of the invention, the se-
cond electrical path comprises a resistor and an anode
bar in series, the anode bar being connected to each
anode of the second cell.
In one embodiment of the invention, the se-
cond electrical path comprises a resistor and a cath-
ode bar in series, the cathode bar being connected to
each cathode of the second cell.
In one embodiment of the invention, the se-
cond electrical path comprises an anode bar to which
are connected electrical paths for each of the at
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
9
least three anodes of the second cell, the electrical
paths for each of the at least three anodes having re-
spective resistors.
In one embodiment of the invention, the se-
cond electrical path comprises an anode or a cathode
bar, respectively, of the first cell.
In one embodiment of the invention, the metal
is copper.
In one embodiment of the invention, the first
cell and the second cell are liberator cells.
In one embodiment of the invention, the sys-
tem further comprises an intermediate voltage supply
configured to supply local converters. The local con-
verters are connected to anode or cathode busbars of a
cell. There may be local converters for each cell in
the system. The local converters may be connected to a
number of cells.
In one embodiment of the invention, the elec-
trolytic process is electrowinning or electrorefining.
In one embodiment of the invention, high ca-
thodic current density is used in the first cell in
the process, where copper concentration is high and
lower cathodic current densities are used in the se-
cond cell where copper concentration is lower.
In one embodiment of the invention, there is
used a separate voltage supply, such as a local con-
verter, on every cathode that would make control of
current density in each individual cell, each individ-
ual cathode, group of cells or sections of rectifiers
much easier.
In one embodiment of the invention an exter-
nal resistance is used to control the distribution of
current between at least two cells connected in paral-
lel, in that way each cell would have a cathode with a
different current density.
In one embodiment of the invention, there is
a power management system for a tank house for elec-
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
trorefining and electrowinning. The system comprises a
plurality of cathode and anode pairs arranged into at
least one cell and a plurality of voltage supplies
coupled to each of the cells. The plurality of voltage
5 supplies are configured to supply voltage to said
cells as a response to the properties of the electro-
lyte at each cell. The properties include, for exam-
ple, copper concentration and acid concentration and
the properties may vary within a tankhouse comprising
10 a plurality of cells.
In one embodiment the voltage supplies are
local converters. Voltage supplies mentioned above are
configured to supply each of the pairs individually or
they may also be configured to supply a group of pairs
or a portion of a cell.
In one embodiment of the invention cells are
liberator cells. In one embodiment of the invention
the power management system further comprises an in-
termediate voltage supply configured to supply local
converters.
The embodiments of the invention described
hereinbefore may be used in any combination with each
other. Several of the embodiments may be combined to-
gether to form a further embodiment of the invention.
A system or an apparatus to which the invention is re-
lated may comprise at least one of the embodiments of
the invention described hereinbefore.
It is to be understood that any of the above
embodiments or modifications can be applied singly or
in combination to the respective aspects to which they
refer, unless they are explicitly stated as excluding
alternatives.
The benefit of the present invention is that
it is possible to use the best possible current densi-
ty in electrowinning and electrorefining when done in
a liberator cell or similar cell in which it is not
beneficial to maintain the same current density at
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
11
each of the cells. For example, when the concentration
of copper is low, high current densities cannot be
used because of the risk of producing a copper powder
deposit or arsine gas. The present invention achieves
different current densities in different cells in the
same tankhouse. A further benefit of the present in-
vention is that it provides better control of elec-
trowinning and electrorefining processes when the cur-
rent density can be chosen such that it will provide
best possible results at the given conditions.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are included
to provide a further understanding of the invention
and constitute a part of this specification, illus-
trate embodiments of the invention and together with
the description help to explain the principles of the
invention. In the drawings:
Fig. 1 is a block diagram of a system for
electrolytic recovery of metals in prior art;
Fig. 2 is a block diagram of a system for
electrolytic recovery of metals where anode and cath-
ode busbars for two cells are connected in parallel,
the anodes having individual resistors, in one embodi-
ment of the invention;
Fig. 3A illustrates a resistor in one embodi-
ment of the invention;
Fig. 3B illustrates a parallel combination of
a resistor and a transistor in one embodiment of the
invention;
Fig. 3C illustrates a transistor in one em-
bodiment of the invention;
Fig. 4 is a block diagram of a system for
electrolytic recovery of metals where anode and cath-
ode busbars for two cells are connected in parallel,
the anode busbar having a shared resistor, in one em-
bodiment of the invention;
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
12
Fig. 5 is a block diagram illustrating an al-
ternative system for resistive control in a liberator
arrangement, in one embodiment of the invention;
Fig. 6 is a block diagram showing a system
employing a central rectifier to produce two current
paths, in one embodiment of the invention;
Fig. 7 illustrates mounting of linear regula-
tors on anode hanger bars, in one embodiment of the
invention;
Fig. 8 shows a method of connecting cell sec-
tions such that several current densities are provid-
ed, in one embodiment of the invention;
Fig. 9 is a block diagram showing a system
where different current densities are provided for up-
stream and downstream cells with respect to the elec-
trolyte flow using serial connection of cells, in one
embodiment of the invention; and
Fig 10 is a block diagram showing an alterna-
tive method of connecting the cells or cell sections
in which the polarity of the current bars on either
side of the cells are swapped over, in one embodiment
of the invention.
DETAILED DESCRIPTION OF THE INVENTION
Reference will now be made in detail to the
embodiments of the present invention, examples of
which are illustrated in the accompanying drawings.
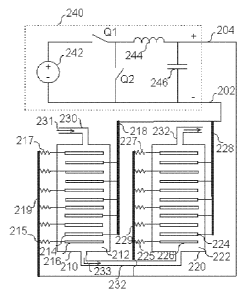
Figure 2 is a block diagram of a system for
electrolytic recovery of metals where anode and cath-
ode busbars for two cells are connected in parallel,
the anodes having individual resistors, in one embodi-
ment of the invention.
In Figure 2 there is a power supply 240, the
negative terminal of which is connected to conductor
202 which is further connected to a cathode busbar 218
of a cell 210 and a cathode busbar 228 of cell 220.
Thus, cathode busbars 218 and 228 are connected in
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
13
parallel. The positive terminal of power supply 240 is
connected to conductor 204, which is further connected
to an anode busbar 219 of cell 210 and an anode busbar
229 of cell 220. Thus, anode busbars 219 and 229 are
connected in parallel. A potential difference or volt-
age is applied between conductor 202 and conductor
204, such that there is a potential difference between
resistor 217 and cathode busbar 218. The same poten-
tial difference is applied, in parallel, between re-
sistor 227 and cathode busbar 228. In this way elec-
trodes connected to resistor 217 and resistor 227 are
held at an anodic potential. Cathode busbar 218 and
cathode busbar 228 are held at a cathodic potential.
Cell 210 comprises a number of cathodes such as a
cathode 214 and a number of anodes such as an anode
216. The number of anodes in cell 210 is one larger
than the number of cathodes. The anodes and cathodes
may be plates. Cell 210 contains electrolyte 212, when
the cell is used for electrolysis. Cell 220 comprises
a number of cathodes such as a cathode 224 and a num-
ber of anodes such as an anode 226. Cell 220 may con-
tain electrolyte 222. In cell 220 the number of anodes
is one larger than the number of cathodes. The anodes
and cathodes may be plates, for example, 1 meter by 1
meter plates, in cells 210 and 220. Cathode busbar 218
is connected to cathodes in cell 210 such as cathode
214. Cathode busbar 228 is connected to cathodes in
cell 220 such as cathode 224. Anode busbar 219 is con-
nected to anodes in cell 210 such as anode 216 via re-
sistors such as resistor 215 and resistor 217. The re-
sistance in resistors between anode busbar 219 and an-
odes in cell 210 may be low or the resistors are op-
tional. Anode busbar 229 is connected to anodes in
cell 220 such as anode 226 via resistors such as re-
sistor 225 and resistor 227. The resistances in resis-
tors 215 and 217, which are connected to the anodes at
the ends of cell 210, have a higher resistance com-
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
14
pared to the resistance in other resistors between an-
ode busbar 219 and the respective anodes. Similarly,
resistances in resistors 225 and 227, which are con-
nected to the anodes at the ends of cell 220, have a
higher resistance compared to the resistance in other
resistors between anode busbar 229 and the respective
anodes. This is due to the fact that the current to
anodes at the ends of cells 210 and 220 is lower since
these anodes face only one cathode, on one side. The
difference in the resistance between a resistor con-
nected to an anode at a cell end and a resistor con-
nected to an anode located between two cathodes is
proportional to the difference in current caused by an
anode plate facing only a single cathode plate instead
of two cathode plates. A pipe 230 provides electrolyte
to cell 210. Pipe 232 provides the electrolyte to from
cell 210 to cell 220. The processed solution exits
from pipe 234. The arrows 231 and 233 indicate the di-
rection of the electrolyte flow. As may be seen from
Figure 2, the solution flows in series through the
cells. Power supply 240 may be a step down Direct Cur-
rent (DC) to DC converter. Power supply 240 may com-
prise a direct current source 242, an inductor 244, a
capacitor 246, transistor Q1 and Q2. Transistors Q1
and Q2 are acting synchronously in anti phase. Power
supply 240 may incorporate a switching regulator in
order to be highly efficient in the conversion of
electrical power. The switching regulator may be a
buck circuit. Power supply 240 may also be a central
rectifier.
Electrically, cell 210 and 220 are not con-
nected in series but in parallel. In general, all an-
ode busbars are connected to the positive terminal of
the central rectifier, that is, power supply 240. All
the cathode busbars, namely, busbar 218 and 228 are
connected to the negative terminal of central rectifi-
er 240. Cathodes of cell 220 operate at a lower cur-
CA 02860813 2014-07-07
WO 2013/117805 PCT/F12013/050089
rent than those in the first cell 210 as a result of
having a resistance connected in series with each
cathode or anode. The current in the cathodes decreas-
es progressively in the cell chain, for example, from
5 600 Amps in the first tank to 200 Amps in the last
tank.
In one embodiment of the invention, cell 210
and cell 220 are liberator cells. Cell 210 represents
a first stage of a liberator cell circuit and cell 220
10 represents a second stage of a liberator cell circuit.
In the first stage the electrolyte is distributed in
cascade through at least cell 210. There may also be
at least one other cell in the first stage. In the se-
cond stage the electrolyte is distributed in cascade
15 through at least cell 220. There may also be at least
one other cell in the second stage. By the use of two
stages may be achieved a decrease in the copper con-
centration from an initial value of 40-60 g/dm3 in the
feed solution down to 10-15 g/dm3. In the second stage
copper is removed from 10-15 g/dm3 down to ca. 1
g/dm3. In the first stage copper may be removed from
the solution as solid copper, which is deposited on
the cathodes. The electrolyte is cascaded through the
liberator (EW) cells, and an electrical current is ap-
plied. The current densities employed are set to be
lower than in standard copper electrowinning or elec-
trorefining.
Thus, when the electrolyte is cascaded
through a plurality of anode-cathode pairs the current
density is preferably controlled in order to get the
best possible result.
As metallic copper is deposited on the cath-
ode surface, the copper concentration in the electro-
lyte solution is depleted and the quality of the cop-
per deposited at the cathode can decrease.
In one embodiment of the invention, the re-
sistors may be in series with the cathodes instead of
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
16
the anodes. For example, so that each of cathodes in
cell 220 are connected to cathode busbar 228 via their
own resistors.
The different currents are obtained by using
different values of series resistor. For better cur-
rent control, the resistor can be replaced by a resis-
tor and transistor, typically a power MOSFET, in par-
allel with the resistor operating as a controlled re-
sistor and providing fine control of the current. Al-
ternatively the resistor can be replaced entirely by a
transistor to give complete current control. These op-
tions are illustrated in Figure 3. Figure 3A shows a
resistor alone. Figure 3B shows a parallel combination
of resistor and transistor (power MOSFET, Metal-Oxide-
Semiconductor Field-Effect Transistor). Figure 3C
shows a transistor (power MOSFET) alone.
In the embodiment of the invention, an exter-
nal resistance is used to control the distribution of
current between two or more cells connected in paral-
lel. In that way each cell would have a cathode with a
different current density. The concept is to use ex-
ternal resistances to divide the current from a single
rectifier, such that different current densities can
be obtained in different cells (or cell sections) in
the process. The resistors in Figure 2 are just an ex-
ample of means for providing desired current to each
of the cells. A person skilled in the art understands
that this may be provided also by using different
means, such as local convertors. The
external re-
sistances would be of differing values and electrical-
ly connected before the anodes in the process in order
to control the distribution of current between cells
connected in parallel. The external resistance may al-
so be adjustable. In that way each cell (or section of
cells) would have cathodes with a current density
which is a function of the external resistance.
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
17
In a copper liberator cell house, wherein it
is desired to remove as much copper as possible from
the electrolyte solution, it should be possible to di-
vide current from a single power supply such that a
high current density (e.g. 300A/m2) can be applied in
the first cells where Cu concentration is high and a
lower current density in the last cells where Cu con-
centration is low (e.g. 100A/m2). In intermediate
cells 200 A/m2 might be used. In this way it will be
possible to gain good current efficiency in each set
of cells, so that use of electrical power is opti-
mized.
Figure 4 is a block diagram of a system for
electrolytic recovery of metals where anode and cath-
ode busbars for two cells are connected in parallel,
the anode busbar having a shared resistor, in one em-
bodiment of the invention.
In Figure 4 there is a power supply 240 as
disclosed in Figure 2. The power supply provides a
negative voltage to conductor 402 which is further in
parallel connected to a cathode busbar 418 of a cell
410 and a cathode busbar 428 of cell 420. Power supply
440 provides a positive voltage to conductor 404 which
is further connected to an anode busbar 419 of cell
410 and an anode busbar 429 of cell 420. Cell 410 com-
prises a number of cathodes such as a cathode 414 and
a number of anodes such as an anode 416. Cell 410 may
contain electrolyte 412. Cell 420 comprises a number
of cathodes such as a cathode 424 and a number of an-
odes such as an anode 426. Cell 420 may contain elec-
trolyte 422. In both cells the number of anodes is one
larger than the number of cathodes. Cathode busbar 418
is connected to cathodes in cell 410 such as cathode
414. Cathode busbar 428 is connected to cathodes in
cell 420 such as cathode 424. Anode busbar 419 is con-
nected to anodes in cell 410 such as anode 416. Anode
busbar 429 is connected to anodes in cell 420 such as
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
18
anode 426. Anode busbar 429 is connected to conductor
404 via a resistor 427. A pipe 430 provides electro-
lyte to cell 410. Pipe 432 provides the electrolyte to
from cell 410 to cell 420. The processed solution ex-
its from pipe 434. As may be seen from Figure 4, the
solution flows in series through the cells.
Figure 5 illustrates an alternative method
for incorporating resistive control in a liberator ar-
rangement, in one embodiment of the invention.
In the arrangements shown in Figures 2 and 4,
all cathodes are in parallel and all anodes are in
parallel. This requires a central rectifier of a low-
voltage, high-current output. The voltage is approxi-
mately that of a single cell. When the number of cath-
odes and anodes is large, the magnitude of the recti-
fier current may be inconveniently large. It is then
advantageous to use a series arrangement of cells so
that the central rectifier voltage becomes larger and
its current rating smaller for a given power output.
Figure 5 illustrates such an arrangement. In Figure 5
there is a rectifier 540 and cells 510, 511, 512, 513,
514 and 515. The rectifier has a positive terminal 204
and a negative terminal 202. The cells have been
formed from three larger tanks, such as a combination
of cells 510 and 515 would have been. The cells may
also be formed by dividing the tanks into two individ-
ual cells using barriers 540, 541 and 542. However,
cells 510 and 515 share anode busbar 520 and cathode
busbar 521. Similarly, cells 511 and 514 share anode
busbar 522 and cathode busbar 523. Similarly, cells
512 and 513 share anode busbar 524 and cathode busbar
525. Cathode busbar 521 and anode busbar 522 is con-
nected using conductor 550, whereas cathode busbar 523
and anode busbar 524 is connected using conductor 551.
Thus, the barrier separated cell pairs are connected
in series. The cell electrical current in cells 510
and 511 may be higher than in cells 512 and 513. Re-
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
19
spectively, the cell electrical current in cells 512
and 513 may be higher than in cells 514 and 515. The
flow of electrolyte is illustrated with arrows 530,
531, 532, 533, 534, 535 and 536. Electrical current
flows from positive terminal 204 of rectifier 540 to a
negative terminal 202 of rectifier 540.
In cells 515, 514 and 513 resistors (not
shown) are connected in series with the anodes or
cathodes to regulate the flow of current through these
cathodes. More accurate control over the current value
is obtained by the use of resistors with transistors
in parallel or by transistors alone as previously de-
scribed. The resistor values are chosen so that the
total current taken by each cell divides between the
upper and lower sections of the cell in the desired
ratio for each cell. The resistor values differ for
each cell so that there is a gradation of current den-
sity experienced by the electrolyte as it flows
through the series of cell sections. It will be appre-
ciated that, if required, the cells can be separated
electrically and not joined by an equalizer-type ar-
rangement incorporating the cathode and anode bars. In
that case a single resistor can be used for the upper
and lower sections of the tank in a similar manner to
that set out with respect to Figures 2 and 4.
In one embodiment of the invention, each tank
in Figure 5 is divided into two equal halves by a bar-
rier such as barriers 540, 541 and 542, which separate
the electrolyte in the two halves in the tanks. The
flow of electrolyte is illustrated with arrow 502.
However, the cathode busbars and anode busbars along
the side of the cells are continuous.
In one embodiment of the invention, instead
of two cell sections, two separate cells can be used
and the cathode bars and anode bars of these can be
electrically connected. An equalizer bar type arrange-
ment can be used to join cells in series. There will
CA 02860813 2014-07-07
WO 2013/117805 PCT/F12013/050089
be more longitudinal current flow along the equalizer
bars than is usual in prior art arrangements. Extra
anodes will be required at the ends of cell sections.
The electrolyte flows through the cells using one half
5 of the cell, for example, the upper halves in Figure
5, and flows in a contrary direction through the other
cell half sections, for example, the lower halves in
Figure 5. Current flows from a rectifier positive ter-
minal 204 to a rectifier negative terminal 202. The
10 cell voltages are additive.
Figure 6 shows a further embodiment of the
invention in which a rectifier 640 is employed to pro-
duce two parallel current paths, in one embodiment of
the invention. In place of rectifier 640 power source
15 240 of Figure 2 may also be used.
In Figure 6 the first current path goes via
cells 610 - 614 and the second current path via cells
620 - 624. In neighbouring cells, such as cells 610
and 611, the anode and cathode busbars are connected
20 via an electrical conductor. The cells are divided in
two equal sections as illustrated in Figure 6 by the
separation of cells in first cells 610 - 614 and se-
cond cells 620 - 624. The electrolyte flow is illus-
trated with arrows 650, 652 and 654. In this case,
however, the cathode bars and anode bars are not con-
tinuous along the length of two cell sections or two
cells, such as cells 610 and 620, but are also divid-
ed. The arrangement therefore may be described as a
two series of half-length cells. Resistors 632, 634
and 636 are employed to produce an exchange of current
between the first and the second current paths. As the
concentration of the target metal ion (e.g. copper) in
the electrolyte decreases in the upper series of cells
610 - 614, the current in this path is decreased by
diverting part of the current to the lower current
path. Similarly, current is added to the lower current
path where it meets electrolyte of with higher concen-
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
21
trations of the target metal ions. The cell sections
or half cells can be connected by two busbars 660 and
662. Busbar 662 connected the cathodes of cells 614
and 624 to negative terminal 202 of rectifier 640
while busbar 660 connected the anodes of cells 610 and
620 to the positive terminal 204 of rectifier 640.
There will be more longitudinal current flow than is
usual when equaliser bars are used in prior art ar-
rangements. Resistors, transistors or resistors in
parallel with transistors may be used as the current
diverters. Resistors 632, 634 and 636 could be a
switched-mode converter in which case the losses could
be less that when 632, 634 and 636 are resistors.
Figure 7 shows how transistors acting as cur-
rent-mode linear regulators may be mounted on the an-
ode or cathode hanger bars as an alternative to mount-
ing current-controlling elements on the sides of the
cells, in one embodiment of the invention.
The use of on-board linear regulators is par-
ticularly applicable to the cell arrangement shown in
Figures 2 and 5. Cathodes or anodes in which the cur-
rent is to be regulated (shown shaded in Figures 2 and
5) may be replaced by current regulated electrodes of
the design illustrated in Figure 7 in which current
passes between the hanger bar 713 and the electrode
blade 714 via transistors 715 - 719 (typically power
MOSFET transistors). These transistors operate in the
linear regime to control current flow between the
hanger bar and the electrode blade.
Figure 8 shows a method of connecting cell
sections such that several current densities are pro-
vided as might be advantageous in a liberator EW pro-
cess, in one embodiment of the invention. The numbers
1 - 30 in each cell in columns indicate the cathodes.
The accompanying numbers by the side of numbers 1 - 30
in columns indicate the current in amperes flowing
through each cathode. In this illustration, the series
CA 02860813 2014-07-07
WO 2013/117805 PCT/F12013/050089
22
connection of cells and cell sections draws from the
central rectifier positive terminal 808 a current of
6,000 Amps which is returned via the central rectifier
negative terminal 809. The electrical current paths
between cells are illustrated with arrows 811. The
number of cathodes in each section is adjusted accord-
ing to the current density to be employed in that sec-
tion. Extra anodes will be required at the ends of the
sections. Cells are divided into sections by dividers
810 as are the anode bars and cathode bars. Electro-
lyte flow 802 takes the electrolyte around these bar-
riers. Anode bars and cathode bars are connected in
sequence by busbars or cables.
Figure 9 shows a system where cells are sepa-
rated into three stages, in one embodiment of the in-
vention. In Figure 9 there is a rectifier 540. Recti-
fier 540 provides a negative voltage via negative ter-
minal 902 to a cathode bar 918 to which a cathode 914
is connected within a cell 910. Rectifier 540 provides
a positive voltage via positive terminal 904 to an an-
ode bar 966 to which anodes are connected within a
cell 960. An anode 912 of cell 910 is connected to an
anode bar 916. Anode bar 916 is connected to a cathode
bar 928 of cell 920. Anodes in cell 920 are connected
to anode bar 926. Electrolyte flows from cell 910 to
cell 960 via cell 920, cell 930, 940 and cell 950. The
cells are electrically connected in series. A series
connection of cells draws from a positive terminal 904
of rectifier 540 a current of, for example, 6000 Amps
which is returned via the negative terminal 902 of
rectifier 540. The number of cathodes in each cell is
adjusted according to the current density to be em-
ployed in that cell.
Figure 10 shows an alternative method of con-
necting the cells or cell sections in which the polar-
ity of the current bars on either side of the cells
are swapped over (anode bars are swapped with cathode
CA 02860813 2014-07-07
WO 2013/117805
PCT/F12013/050089
23
bars) to make for easier and shorter connections, in
one embodiment of the invention.
The embodiments of the invention described
hereinbefore may be used in any combination with each
other. Several of the embodiments may be combined to-
gether to form a further embodiment of the invention.
A system or an apparatus to which the invention is re-
lated may comprise at least one of the embodiments of
the invention described hereinbefore.
It is obvious to a person skilled in the art
that with the advancement of technology, the basic
idea of the invention may be implemented in various
ways. The invention and its embodiments are thus not
limited to the examples described above; instead they
may vary within the scope of the claims.