Note: Descriptions are shown in the official language in which they were submitted.
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
COMPOSITIONS AND METHODS FOR USING CSF1R INHIBITORS
FIELD OF THE INVENTION
The present invention relates to compositions and methods for using CSF1-R
pathway
inhibitors, including anti-IL-34 antibodies, bispecific IL-34/CSF1 antibodies
and CSF1R
antibodies.
BACKGROUND
Interleukin-34, also known as C16orf77 or UNQ20374 (Clark et al., Genome Res
13: 2265-
2270 (2003)), was recently identified as a second and high-affinity ligand for
CSF-1R in a
human monocyte proliferation screening (Lin et al., Science 320: 807-811
(2008)). This
discovery has long been foreshadowed by the more severe phenotype in CSF-1R
null mice,
than CSF-1-deficient CSF-1 P/CSF-1 P mice (Dai et al., Blood 99: 111-120
(2002)). Like
CSF-1 (also known as M-CSF), the better-characterized ligand for CSF-1R, IL-34
stimulates
phosphorylation of ERK1/2 in human monocytes and promotes the formation of the
granulocyte-macrophage progenitor (CFU-GM) and megakaryocyte progenitor (CFU-
M) in
human bone marrow cultures (Lin et al., Science 320: 807-811 (2008)). Mediated
by the
common receptor CSF-1R, the transcript of the proto-oncogene c-fins, IL-34 and
CSF-1 serve
as the key regulators of the differentiation, proliferation, and survival of
the mononuclear
phagocyte lineage cell such as monocytes, macrophages and osteoclasts (Droin
et al., Journal
of leukocyte biology 87: 745-747 (2010)).
The function of IL-34 bears strong resemblance to that of CSF-1, but with
several notable
differences. Both cytokines support cell growth and survival in cell cultures
studies
equivalently (Chihara et al., Cell death and differentiation 17: 1917-1927
(2010); Wei et al.,
Journal of leukocyte biology 88: 495-505 (2010)). The IL-34 gene, when
expressed under the
control of the CSF-1 promoter, could rescue the phenotype of CSF-1-nullizygous
CSF-
1 P/CSF-1 P mice (Wei et al., Journal of leukocyte biology 88: 495-505
(2010)). IL-34 can
also substitute for CSF-1 to support RANKL-induced osteoclastogenesis
(Baud'huin et al.,
The Journal of pathology 221: 77-86 (2010)). However, the two factors appear
different in
their ability to induce the production of chemokines such as MCP-1 and eotaxin-
2 in primary
macrophages, the morphological change in TF-1-fms cells and the migration of
J774A.1 cells
(Chihara et al., Cell death and differentiation 17: 1917-1927 (2010)). IL-34
has been shown
1
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
to induce a stronger, but transient tyrosine phosphorylation of CSF-1R and
downstream
effectors, and rapidly downregulates CSF-1R expression (Chihara et al., Cell
death and
differentiation 17: 1917-1927 (2010)). Moreover, IL-34 and CSF-1 exhibit
differential
spatiotemporal patterns of expression in both embryonic and adult tissues,
which leads to the
complementary activation of the CSF-1R (Wei et al., Journal of leukocyte
biology 88: 495-
505 (2010)). Most strikingly, IL-34 but not CSF-1 messenger RNA is detected
together with
CSF-1R in embryonic brain which could explain why microglia develop in CSF-1
deficient
but not CSF-1R deficient mice (Ginhoux et al., Science 330: 841-845 (2010);
Mizuno et al.,
The American journal of pathology 179: 2016-2027 (2011)). Thus, while IL-34
and CSF-1
resemble each other, they are not necessarily identical in their developmental
roles, biological
activity, and signal activation kinetics or strength.
Despite a lack of appreciable sequence similarity with other proteins, IL-34
was proposed by
fold recognition methods to be a short-chain helical cytokine belonging to the
same family as
CSF-1, SCF, and F1t3L (Garceau et al., Journal of leukocyte biology 87: 753-
764 (2010)).
These latter three dimeric hematopoietic cytokines are unique among helical
cytokines in that
they have membrane-bound forms (Bazan, Cell 65: 9-10 (1991a); Hannum et al.,
Nature 368:
643-648 (1994)); IL-34 differs importantly in that it lacks a hydrophobic
transmembrane
segment. In addition, CSF-1, SCF and F1t3L cytokine dimers bind to the PDGFR
subfamily
(type III/V) of the receptor tyrosine kinase (RTK) family (Rosnet et al.,
Critical reviews in
oncogenesis 4: 595-613 (1993)) instead of hematopoietic cytokine receptors
(Bazan,
Immunology today 11: 350-354 (1990)). The CSF-1, SCF and F1t3L cytokine dimers
functionally mimic the PDGF and VEGF cystine knot growth factor dimers that
are the
activating ligands of the RTK family (Savvides et al., Nature structural
biology 7: 486-491
(2000); Wiesmann et al., Nature structural biology 7: 440-442 (2000)). All
members of this
RTK family share a similar overall architecture comprised of multiple Ig-like
domains in their
extracellular regions, a single transmembrane segment, and a cytoplasmic
tyrosine kinase
domain with a large insertion. Upon stimulation, CSF-1R dimerizes and
autophosphorylates
certain tyrosine residues in its intracellular domain, which serve as docking
sites for 5H2-
containing effector proteins, which contribute to macrophage differentiation
(Pixley et al.,
Trends in cell biology 14: 628-638 (2004)).
The structure of dimeric CSF-1 in complex with CSF-1R reveals a non-
symmetrical 2:1
complex, in which one CSF-1 protomer approaches its receptor at the cleft
between D2 and
D3, while the second CSF-1 protomer remains unoccupied (Chen et al.,
Proceedings of the
2
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
National Academy of Sciences of the United States of America 105: 18267-18272
(2008)).
Yet, the molecular basis whereby CSF-1R is also able to recognize IL-34, a
distantly-related
ligand with scant sequence identity, has remained elusive until described
herein. IL-34 can
function independently, but does not synergize with CSF-1 (Lin et al., Science
320: 807-811
(2008)). Indeed, CSF-1 competes with IL-34 for binding to CSF-1R (Wei et al.,
Journal of
leukocyte biology 88: 495-505 (2010)), suggesting a common ligand-binding site
on CSF-1R.
In contrast however, a recent comparative sequence study between CSF-1R and
its two
ligands suggested the CD loop of IL-34, and the junction between D3 and D4 of
CSF-1R
share strong sequence conservation correlation coefficients during evolution,
and therefore
may represent a unique binding mode that is distinct from the binding mode
employed by the
CSF-1/CSF-1R complex (Garceau et al., Journal of leukocyte biology 87: 753-764
(2010)).
Interestingly, an anti-CSF-1R antibody MAb 12-2D6 was reported to block the
binding of
both IL-34 and CSF-1 to CSF-1R, yet another antibody MAb 2-4A5 blocked only
CSF-
1/CSF-1R binding (Chihara et al., Cell death and differentiation 17: 1917-1927
(2010)).
These results strongly suggest that IL-34 and CSF-1 have overlapping, but not
identical
binding sites on the surface of CSF-1R.
All references cited herein, including patent applications and publications,
are hereby
incorporated by reference in their entirety.
SUMMARY
The invention provides anti-IL-34 antibodies, bispecific antibodies that bind
to IL-34 and
CSF-1, and anti-CSF-1R antibodies, and methods of using the same. In one
embodiment, the
antibodies of this invention have reduced antibody-dependent cell-mediated
cytotoxicity
(ADCC) and/or complement dependent cytotoxicity (CDC) activity. In one
specific
embodiment, the antibodies of this invention have reduced ADCC activity by
comprising at
least an Fc region substitution at one or more of the following residues 238,
265, 269, 270,
297, 327 and 329 (EU numbering). In one specific embodiment, the Fc region
substitution to
reduce ADCC activity is at residue 297. In another embodiment, the Fc region
substitution to
reduce ADCC activity is N297G or N297A. In another embodiment, the Fc
substitution to
reduce ADCC is D265A. In yet another embodiment, the Fc substitutions to
reduce ADCC
activit y are the substitution of residues 265 and 297 to alanine. In one
embodiment, the
bispecific anti-IL-34/anti-CSF-1 antibody is a knob-into-hole bispecific
antibody.
3
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Provided herein are isolated antibodies that bind to human IL-34, which bind
to an epitope
comprising at least one of amino acid residues G1u103, Leu109, G1n106, Asn150,
Leu127,
Asn128, Ser184, Leu186, Asn187, Lys44, G1u121, Asp107, Glul 1 1, Ser104,
G1n120,
Trp116, and Asn6lof a human IL-34, where the position of the amino acid
residues is based
on the position in SEQ ID NO:1, and which inhibit the binding between human IL-
34 and
human CSF-1R.
Provided herein are isolated antibodies that binds to human IL-34, which bind
to an epitope
comprising at least one of amino acid residues from G1u103 to Asn150 of a
human IL-34,
where the position of the amino acid residues is based on SEQ ID NO:1, and
which inhibit
the binding between human IL-34 and human CSF-1R.
In some embodiments, the antibody binds to an epitope comprising at least one
of amino acid
residues G1u103, Leu109, G1n106, and Asn150 of the human IL-34, where the
position of the
amino acid residues is based on the position in SEQ ID NO: 1. In some
embodiments, the
epitope further comprises at least one of amino acid residues Ser100, G1u123,
Trp116,
Thr124, Leu127, Asn128, G1n131, and Thr134 of the human IL-34, where the
position of the
amino acid residues is based on the position in SEQ ID NO: 1. In some
embodiments, the
antibody binds to amino acids within positions 100-108, 116-134, 109 and 150
of the human
IL-34, where the position of the amino acid residues is based on the position
in SEQ ID
NO:l.
In some embodiments, the antibody binds to an epitope comprising at least one
of amino acid
residues Asn128, 5er184, Leu186, Asn187, Lys44, and G1u121 of the human IL-34,
where
the position of the amino acid residues is based on the position in SEQ ID NO:
1. In some
embodiments, the epitope further comprises at least one of amino acid residues
Phe40,
Asp43, Leu125, G1n189, Thr36, and Va1185 of the human IL-34, where the
position of the
amino acid residues is based on the position in SEQ ID NO: 1. In some
embodiments, the
antibody binds to amino acids within positions 36-44, 121-128, and 184-187 of
the human IL-
34, where the position of the amino acid residues is based on the position in
SEQ ID NO: 1.
In some embodiments, the antibody binds to an epitope comprising at least one
of amino acid
residues from G1u103-Leu127 of the human IL-34, where the position of the
amino acid
residues is based on the position in SEQ ID NO: 1. In some embodiments, the
antibody binds
to an epitope comprising at least one of amino acid residues Asp107, Glulll,
Ser104,
G1n120, G1u103, Leu109, Trp116, and Asn61 of the human IL-34, where the
position of the
amino acid residues is based on the position in SEQ ID NO: 1. In some
embodiments, the
4
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
epitope further comprises at least one of amino acid residues Pro152, Va1108,
Leu110,
G1n106, G1u123, Leu127, Lys117, 11e60 and Lys55 of the human IL-34, where the
position of
the amino acid residues is based on the position in SEQ ID NO: 1. In some
embodiments, the
antibody binds to amino acids within positions 55-61, 100-108, 109, 111-127
and 152 of the
human IL-34, where the position of the amino acid residues is based on the
position in SEQ
ID NO:l.
In some embodiments, the antibody comprises a heavy chain variable region
sequence of at
least 90% sequence identity to the amino acid sequence of SEQ ID NO:3 and/or a
light chain
variable region sequence of at least 90% sequence identity to the amino acid
sequence of SEQ
ID NO:4. In some embodiments, the antibody comprises a heavy chain variable
region
sequence of the amino acid sequence of SEQ ID NO:3 and/or a light chain
variable region
sequence of the amino acid sequence of SEQ ID NO:4. In some embodiments, the
antibody
comprises (a) a HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY (SEQ
ID NO: 33); (b) a HVR-L3 comprising an amino acid sequence QQSFYFPNT (SEQ ID
NO:
39); and (c) a HVR-H2 comprising an amino acid sequence RISPYYYYSDYADSVKG (SEQ
ID NO: 52). In some embodiments, the antibody comprises (a) a HVR-H1
comprising an
amino acid sequence STWIH (SEQ ID NO: 59), (b) a HVR-H2 comprising an amino
acid
sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52); and (c) a HVR-H3 comprising an
amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33). In some embodiments, the
antibody comprises (a) a HVR-L1 comprising an amino acid sequence RASQDVSTAVA
(SEQ ID NO: 50); (b) a HVR-L2 comprising an amino acid sequence SASFLYS (SEQ
ID
NO: 53); and (c) a HVR-L3 comprising an amino acid sequence QQSFYFPNT (SEQ ID
NO:
39).
In some embodiments, the antibody comprises (a) a HVR-H3 comprising an amino
acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33) or GINQGSKRGAMDY (SEQ ID NO:
32); (b) a HVR-L3 comprising an amino acid sequence QQSFYFPNT (SEQ ID NO: 39)
or
QQSYTTPPT (SEQ ID NO: 43) or QQYTALPYT (SEQ ID NO: 49) or QQYSDLPYT (SEQ
ID NO: 45) or QQYSDVPYT (SEQ ID NO: 47) or QQSRTARPT (SEQ ID NO: 41); and (c)
a HVR-H2 comprising an amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52)
or RISPYSGYTNYADSVKG (SEQ ID NO: 51). In some embodiments, the antibody
comprises (a) a HVR-H1 comprising an amino acid sequence STWIH (SEQ ID NO:
59); (b) a
HVR-H2 comprising an amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52)
or RISPYSGYTNYADSVKG (SEQ ID NO: 51); and (c) a HVR-H3 comprising an amino
5
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33) or GINQGSKRGAMDY (SEQ ID
NO: 32). In some embodiments, the antibody comprises (a) a HVR-L1 comprising
an amino
acid sequence RASQDVSTAVA (SEQ ID NO: 50); (b) a HVR-L2 comprising an amino
acid
sequence SASFLYS (SEQ ID NO: 53); and (c) a HVR-L3 comprising an amino acid
sequence QQSFYFPNT (SEQ ID NO: 39) or QQSYTTPPT (SEQ ID NO: 43) or
QQYTALPYT (SEQ ID NO: 49) or QQYSDLPYT (SEQ ID NO: 45) or QQYSDVPYT
(SEQ ID NO: 47) or QQSRTARPT (SEQ ID NO: 41) or QQSFYFPN (SEQ ID NO: 38) or
QQSYTTPP (SEQ ID NO: 42) or QQYTALPY (SEQ ID NO: 48) or QQYSDLPY (SEQ ID
NO: 44) or QQYSDVPY (SEQ ID NO: 46) or QQSRTARP (SEQ ID NO: 40).
In some embodiments, the antibody comprises (a) a HVR-H3 comprising an amino
acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (b) a HVR-L3 comprising an amino acid
sequence QQYSDLPYT (SEQ ID NO: 45); and (c) a HVR-H2 comprising an amino acid
sequence RISPYSGYTNYADSVKG (SEQ ID NO: 51). In some embodiments, the antibody
comprises (a) a HVR-H1 comprising an amino acid sequence of STWIH (SEQ ID NO:
59);
(b) a HVR-H2 comprising an amino acid sequence RISPYSGYTNYADSVKG (SEQ ID NO:
51); and (c) a HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID
NO: 33). In some embodiments, the antibody comprises (a) a HVR-L1 comprising
an amino
acid sequence of RASQDVSTAVA (SEQ ID NO: 50); (b) a HVR-L2 comprising an amino
acid sequence SASFLYS (SEQ ID NO: 53); and (c) a HVR-L3 comprising an amino
acid
sequence QQYSDLPYT (SEQ ID NO: 45).
In some embodiments, the antibody comprises a heavy chain variable region
sequence of at
least 90% sequence identity to the amino acid sequence of SEQ ID NO:5 and/or a
light chain
variable region sequence of at least 90% sequence identity to the amino acid
sequence of SEQ
ID NO:6. In some embodiments, the antibody comprises a heavy chain variable
region
sequence of the amino acid sequence of SEQ ID NO:5 and/or a light chain
variable region
sequence of the amino acid sequence of SEQ ID NO:6. In some embodiments, the
antibody
comprises a heavy chain variable region sequence of at least 90% sequence
identity to the
amino acid sequence of SEQ ID NO:7 and/or a light chain variable region
sequence of at least
90% sequence identity to the amino acid sequence of SEQ ID NO:8. In some
embodiments,
the antibody comprises a heavy chain variable region sequence of the amino
acid sequence of
SEQ ID NO:7 and/or a light chain variable region sequence of the amino acid
sequence of
SEQ ID NO:8. In some embodiments, the antibody comprises a heavy chain
variable region
sequence of at least 90% sequence identity to the amino acid sequence of SEQ
ID NO:9
6
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
and/or a light chain variable region sequence of at least 90% sequence
identity to the amino
acid sequence of SEQ ID NO:10. In some embodiments, the antibody comprises a
heavy
chain variable region sequence of the amino acid sequence of SEQ ID NO:9
and/or a light
chain variable region sequence of the amino acid sequence of SEQ ID NO:10. In
some
embodiments, the antibody comprises a heavy chain variable region sequence of
at least 90%
sequence identity to the amino acid sequence of SEQ ID NO:11 and/or a light
chain variable
region sequence of at least 90% sequence identity to the amino acid sequence
of SEQ ID
NO:12. In some embodiments, the antibody comprises a heavy chain variable
region
sequence of the amino acid sequence of SEQ ID NO:11 and/or a light chain
variable region
sequence of the amino acid sequence of SEQ ID NO:12. In some embodiments, the
antibody
comprises a heavy chain variable region sequence of at least 90% sequence
identity to the
amino acid sequence of SEQ ID NO:13 and/or a light chain variable region
sequence of at
least 90% sequence identity to the amino acid sequence of SEQ ID NO:14. In
some
embodiments, the antibody comprises a heavy chain variable region sequence of
the amino
acid sequence of SEQ ID NO:13 and/or a light chain variable region sequence of
the amino
acid sequence of SEQ ID NO:14.
In some embodiments, the antibody comprises (a) a HVR-H3 comprising an amino
acid
sequence SRGAYRFAY (SEQ ID NO: 56); (b) a HVR-L3 comprising an amino acid
sequence QQSYTTPPT (SEQ ID NO: 43); and (c) a HVR-H2 comprising an amino acid
sequence SITPASGDTDYADSVKG (SEQ ID NO: 54). In some embodiments, the antibody
comprises (a) a HVR-H1 comprising an amino acid sequence SNYIH (SEQ ID NO:
55), (b) a
HVR-H2 comprising an amino acid sequence SITPASGDTDYADSVKG (SEQ ID NO: 54);
and (c) a HVR-H3 comprising an amino acid sequence SRGAYRFAY (SEQ ID NO: 56).
In
some embodiments, the antibody comprises (a) a HVR-L1 comprising an amino acid
sequence RASQDVSTAVA (SEQ ID NO: 50); (b) a HVR-L2 comprising an amino acid
sequence SASFLYS (SEQ ID NO: 53); and (c) a HVR-L3 comprising an amino acid
sequence QQSYTTPPT (SEQ ID NO: 43). In some embodiments, the antibody
comprises a
heavy chain variable region sequence of at least 90% sequence identity to the
amino acid
sequence of SEQ ID NO:15 and/or a light chain variable region sequence of at
least 90%
sequence identity to the amino acid sequence of SEQ ID NO:16. In some
embodiments, the
antibody comprises a heavy chain variable region sequence of the amino acid
sequence of
SEQ ID NO:15 and/or a light chain variable region sequence of the amino acid
sequence of
7
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
SEQ ID NO:16. In some embodiments, the antibody does not inhibit the binding
between
human CSF-1 and human CSF-1R.
In some embodiments, the anti-IL-34 antibody described herein binds to a dimer
of the IL-34.
In some embodiments, the anti-IL-34 antibody described herein binds to an
epitope that spans
over both protomers of the IL-34 dimer. In some embodiments, the anti-IL-34
antibody
described herein neutralizes IL-34 activity. In some embodiments, the anti-IL-
34 antibody
binds to human IL-34, inhibit the binding between human IL-34 and human CSF-
1R, and/or
neutralize IL-34 activity.
In some embodiments, the anti-IL-34 antibody described herein is a monoclonal
antibody. In
some embodiments, the anti-IL-34 antibody described herein a human, humanized
or
chimeric antibody. In some embodiments, the antibody is a bispecific antibody.
In some
embodiments, the bispecific antibody comprises a second binding specificity to
human CSF-
1.
Provided herein are bispecific antibodies comprising a first binding
specificity to human IL-
34 and a second binding specificity to human CSF-1 and their use in treating
myeloid
pathogenic immunological diseases and cancers. In some embodiments, the
antibody inhibits
binding of human IL-34 to human CSF-1R and inhibits binding of human CSF-1 to
human
CSF-1R. In another embodiment, provided herein are two polypeptides comprising
binding
specificity to human IL-34 and the binding specificity to human CSF-1,
respectively, each has
a heteromultimerization domain that is capable is heterodimerizing with each
other.
In some embodiments, the antibody described above is an antibody fragment that
binds
human IL-34. In some embodiments, the fragment is a Fab, Fab', Fab'-SH,
F(ab')2, Fv or
scFv fragment.
In some embodiments, the antibody described herein is a one-armed antibody. In
some
embodiments, the antibody described herein is a linear antibody. In some
embodiments, the
antibody described herein is a full length IgG1 or an IgG4 antibody.
Further provided herein are isolated antibodies that bind human CSF-1R, which
bind to an
epitope comprising at least one of amino acid residues Arg144, G1n248, G1n249,
5er250,
Phe252, and Asn254 of human CSF-1R, where the position of amino acid residue
is based on
the position in SEQ ID NO:2, and which inhibit the binding between human IL-34
and human
CSF-1R.
In some embodiments, the antibody binds to an epitope comprising amino acid
residue
Arg144 of CSF-1R, where the position of amino acid residue is based on the
position in SEQ
8
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
ID NO:2. In some embodiments, the antibody binds to an epitope comprising at
least one of
amino acid residues Arg144, Arg142, Arg146, and Arg150 of human CSF-1R, where
the
position of amino acid residues is based on the position in SEQ ID NO:2. In
some
embodiments, the epitope further comprises at least one of amino acid residues
Ser172 and
Arg192 of human CSF-1R, where the position of amino acid residues is based on
the position
in SEQ ID NO:2. In some embodiments, the epitope further comprises at least
one of amino
acid residues Arg146, Met149, Arg150, Phe169, 11e170, and G1n173 of human CSF-
1R,
where the position of amino acid residues is based on the position in SEQ ID
NO:2. In some
embodiments, the antibody binds to amino acids within positions 142-150 and
169-173,
where the position of amino acid residues is based on the position in SEQ ID
NO:2.
In some embodiments, the antibody binds to an epitope comprising at least one
of amino acid
residues Arg144, G1n248, G1n249, 5er250, Phe252, and Asn254 of human CSF-1R,
where
the position of amino acid residue is based on the position in SEQ ID NO:2. In
some
embodiments, the antibody binds to an epitope comprising at least one of amino
acid residues
Tyr257, G1n248, G1n249, Ser250, Phe252, and Asn254 of human CSF-1R, where the
position
of amino acid residues is based on the position in SEQ ID NO:2. In some
embodiments, the
epitope further comprises at least one of amino acid residues Pro247, G1n258,
and Lys259õ
where the position of amino acid residues is based on the position in SEQ ID
NO:2. In some
embodiments, the epitope further comprises at least one of amino acid residues
Va1231,
Asp251, and Tyr257 of human CSF-1R, where the position of amino acid residue
is based on
the position in SEQ ID NO:2. In some embodiments, the antibody binds to amino
acid
residues within positions 231, 248-252, and 254, where the position of amino
acid residues is
based on the position in SEQ ID NO:2.
Further provided herein are isolated nucleic acids encoding any of the
antibodies described
herein. Also provided herein are vectors comprising the nucleic acid of any of
the nucleic
acids provided herein. Also provided herein are host cells comprising the
nucleic acid
provided herein.
Further provided herein are methods of producing an antibody, comprising
culturing any of
the host cells provided herein, so that the antibody is produced. In some
embodiments, the
method further comprises recovering the antibody produced by the host cell.
Also provided herein are pharmaceutical compositions comprising any of the
antibodies
provided herein and a pharmaceutically acceptable carrier.
9
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Provided herein are antibodies described herein for use as a medicament.
Provided herein are
the antibodies described herein for use in treating a myeloid pathogenic
immunological
disease. Provided herein are the antibodies described herein for use in
inhibiting binding
between human IL-34 and human CSF-1R.
Provided herein is also use of any of the antibodies described herein in the
manufacture of a
medicament. In some embodiments, the medicament is for treating a myeloid
pathogenic
immunological disease. In some embodiments, the medicament is for inhibiting
binding
between human IL-34 and human CSF-1R.
Provided herein are methods of treating an individual having an inflammatory
disease and/or
an autoimmune disease with a myeloid pathogenic component ("myeloid pathogenic
immunological disease") comprising administering to the individual an
effective amount of
any one of the antibodies provided herein. Also provided herein are methods of
treating an
individual having an inflammatory disease and/or an autoimmune disease
comprising
administering to the individual an effective amount of any one of the
antibodies or
combination therapies provided herein. In some embodiments, the antibody is a
bispecific
antibody which inhibits the activity of human IL-34 and human CSF-1. In some
embodiments, the method comprises administering an effective amount of any of
the anti-IL-
34 antibodies provided herein in conjunction with an antibody that binds to
human CSF-1. In
some embodiments, the activity of human IL-34 and human CSF-1 is inhibited by
a bispecific
anti-IL-34 and anti-CSF1 antibody. In some embodiments, the inhibition of
activity is by
inhibiting the binding of human IL-34 to human CSF-1R, and inhibiting the
binding of
human CSF-1 and human C SF-1R.
In some embodiments of any of the methods above, the myeloid pathogenic
immunological
disease is rheumatoid arthritis (RA), inflammatory bowel disease (e.g.,
Crohn's, ulcerative
colitis), multiple sclerosis, systemic lupus erythematosus, lupus nephritis,
asthma,
osteoporosis, Paget's disease, atherosclerosis, metabolic syndrome, type II
diabetes,
macrophage activated syndrome (MAS), vasculitis (giant cell artheritis, ANCA
associated
vasculitis), discoid lupus, sarcoidosis, graft versus host disease, LSDs
(lysosomal storage
diseases like but not limited to Cytostinosis, Salic acid storage disorder,
Gaucher disease),
Histyocytosis including but not limited to Rosai-Dorfman disease, Faisalabad
histiocytosis, H
syndrome, pigmented hypertrichosis with insulin dependent diabetes (PHID)s,
vasculitis,
myocardial infarction and graft versus host disease. In one embodiment, the
vasculitis is
microscopic polyarteritis, CNS vasculitis, necrotizing, cutaneous, or
hypersensitivity
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
vasculitis, systemic necrotizing vasculitis, or ANCA-associated vasculitis,
such as Churg-
Strauss vasculitis or syndrome (CSS)). In another embodiment, the vasculitis
is large vessel
vasculitis or medium vessel vasculitis. In one embodiment the large vessel
vasculitis is
polymyalgia rheumatica or giant cell arteritis or Takayasu's arteritis. In one
embodiment, the
medium vessel vasculitis is Kawasaki's disease of polyarteritis nodosa. In one
specific
embodiment, an antibody of this invention (e.g., IL-34, bispecific IL-34/CSF1
antibody or
CSF1R antibody) is used to treat RA patients who inadequately respond to a
disease-
modifying antirheumatic drug (DMARD) therapy (DMARD-IR). In one further
embodiment,
the DMARD-IR patient has not been previously treated with an anti-TNF agent
("TNF
naïve"). In one embodiment, the DMARD is methotrexate. In one specific
embodiment, an
antibody of this invention (e.g., IL-34 antibody, bispecific IL-34/CSF1
antibody or CSF1R
antibody) is used to treat RA patients who inadequately respond to a disease-
modifying
antirheumatic drug (DMARD) therapy (DMARD-IR). In one further embodiment, the
DMARD-IR patient has not been previously treated with an anti-TNF agent ("TNF
naïve").
In one specific embodiment, an antibody of this invention (e.g., IL-34,
bispecific IL-34/CSF1
antibody or CSF1R antibody) is used to treat RA patients who inadequately
respond to anti-
TNF therapies (e.g., TNFR-Fc or anti-TNF antibodies). "). In one specific
embodiment, an
antibody of this invention (e.g., IL-34, bispecific IL-34/CSF1 antibody or
CSF1R antibody) is
used to treat patients suffering from a myeloid pathogenic immunological
disease who
inadequately responds to anti-TNF therapies (e.g., including, but not limited
to, TNFR-Fc,
anti-TNF antibodies and small molecule inhibitors of TNF or a TNF receptor).
In another embodiment of this invention, the RA patient to be treated with a
CSF1-R pathway
inhibitor of this invention has a Myeloid subtype and/or Fibroid subtype of
RA. In one
embodiment, the invention provides a method of treating rheumatoid arthritis
in an individual
suffering therefrom comprising administering a CSF1-R pathway inhibitor to a
patient who
has been determined to have a myeloid subtype and/or a fibroid subtype of RA.
In one
embodiment, the Myeloid or Fibroid subtype is determined by measuring the gene
expression
level or protein expression level of a myeloid subtype or fibroid subtype gene
and
determining whether the RA individual has a myeloid or a fibroid subtype of
RA, wherein a
determination that an RA individual has a myeloid or a fibroid subtype of RA
indicates that
the RA individual is more likely to respond to a CSF1-R pathway inhibitor.
In one embodiment of this invention, the pharmocodynamic effect of an antibody
of this
invention could be measured by monitoring the reduction in the levels of
nonclassical
11
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
(CD14+CD16++) monocytes and/or intermediate (CD14++CD16+) monocytes in the
blood
of a patient after treatment with the antibody.
Further provided herein are methods of inhibiting binding between human IL-34
and human
CSF-1R in an individual comprising administering to the individual an
effective amount of
any of the antibodies provided herein.
Further provided herein are articles of manufacture comprising any of the
antibodies provided
herein. In some embodiments, the article of manufacture further comprises
instructions for
administering an effective amount of the antibody to an individual for
treating a myeloid
pathogenic immunological disease in the individual. Also provided herein are
articles of
manufacture comprising any of the anti-IL-34 antibodies provided herein and
further
comprising an antibody that binds to human CSF-1. In some embodiments, the
article of
manufacture further comprises instructions for administering an effective
amount of the anti-
IL-34 antibody and the antibody that binds to human CSF-1 to an individual for
treating a
myeloid pathogenic immunological disease in the individual. In some
embodiments, the
myeloid pathogenic immunological disease is rheumatoid arthritis, inflammatory
bowel
disease, multiple sclerosis, systemic lupus erythematosus, lupus nephritis,
asthma,
osteoporosis, Paget's disease, atherosclerosis, metabolic syndrome, type II
diabetes, LSDs
(lysosomal storage diseases like but not limited to Cytostinosis, Salic acid
storage disorder,
Gaucher disease), Histyocytosis including but not limited to Rosai-Dorfinan
disease,
Faisalabad histiocytosis, H syndrome, pigmented hypertrichosis with insulin
dependent
diabetes (PHID).
This invention provides a method for diagnosing an RA patient to be treated
with a CSF1-R
pathway inhibitor comprising the step of measuring the gene expression level
or protein
expression level of a myeloid subtype or fibroid subtype gene and determining
whether the
RA individual has a myeloid or a fibroid subtype of RA, wherein a
determination that an RA
individual has a myeloid or a fibroid subtype of RA indicates that the RA
individual is more
likely to respond to a CSF1-R pathway inhibitor. In one embodiment, method
further
comprises the step of measuring the gene or protein expression level of IL-34
and/or CSF-1 in
the patient. In one embodiment, the CSF-1 level is measured in a biological
sample from the
sera or synovial fluid of an RA patient. In another embodiment, the IL-34
level is measured
in the sera, synovial fluid or tissue biopsy of an RA patient.
Also provided herein is a polypeptide comprising the first three IgG domains
(i.e., the first,
second, and third IgG from the N-terminus) of a CSF-1R, wherein the
polypeptide does not
12
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
comprise other IgG domains from the CSF-1R. In some embodiments, the
polypeptide
further comprises a linker between the IgG domains. In some embodiments, the
polypeptide
further comprises one or more fusion partners (e.g., an Fc sequence). Also
provided herein
are a nucleic acid encoding the polypeptide, a vector comprising the nucleic
acid, and a host
described herein may be combined to form other embodiments of the present
invention.
These and other aspects of the invention will become apparent to one of skill
in the art.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 shows the structure of the functional core of human IL-34. (A)
Schematic
Figure 2 shows the biophysical characterization of hIL-34s and hCSF-1
interactions with two
different hCSF-1Rs containing domains D1-D3 and D1-D5. (A) Analytical size
exclusion
chromatography analyses of hIL-34s, C SF-1R D1-D3 and D1-D5, and their
corresponding
complexes. Chromatograms are shown overlaid from independent runs as described
in the
Gibbs free energy change (AG), binding affinity (KD) and stoichiometry (n)
derived from
13
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
analyses of the three ITC titration experiments shown in panels B and C. N.D.
indicates not
determinable due to steepness of the curve.
Figure 3 shows a comparison of the Site 1 and 2 interfaces for CSF-1R in
complex with IL-34
and CSF-1. (A¨D) Close-up views of site 1 and site 2 of the IL-34/CSF-1R (A,
C) or CSF-
1/CSF-1R (B, D) interfaces. Key cytokine receptor interacting residues are
shown as sticks,
hydrogen bonds are drawn as dashed lines, and secondary structure elements are
marked on
the ribbons and strands.
Figure 4A shows inhibition of IL-34 biological activity by YW404.33.56 Fab in
the monocyte
viability assay. Figure 4B shows a close up view of interactions of CDR-loops
(H1-H3, L3) of
YW404.33.56 Fab with hIL-34s (cartoon representation). Critical residues
involved in the
interface interactions are highlighted in stick models.
Figure 5 shows receptor contacting residues mapped onto the secondary
structure of IL-34
(A) and CSF-1 (B). The Sitel and Site2 interfacial residues are highlighted by
dotted oval.
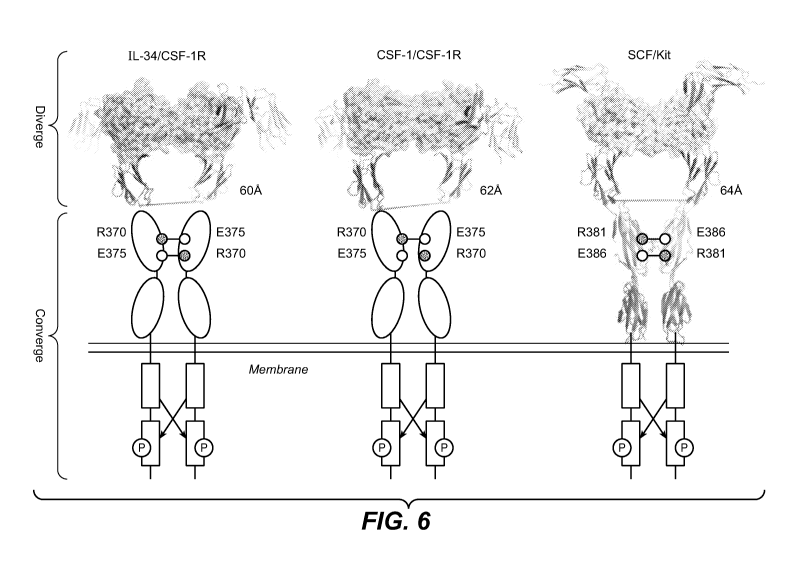
Figure 6 shows a comparison of the human IL-34/CSF-1R (left), murine CSF-1/CSF-
1R
(middle, PDB 3EJJ) and SCF/Kit (right, PDB 2E9W) signaling complex structures.
The
dimeric four-helical bundle cytokines are shown as cartoons and
semitransparent surfaces.
Receptor ectodomains are rendered as ribbon representation or shown as ovals
for CSF-1R
D4 and D5. The ionic pairs those have been implicated in the receptor
homotypic contacts of
CSF-1R and Kit are shown as circle and annotated.
Figure 7. Sequence alignment of selected IL-34 mammalian homologs (Homo
sapiens (SEQ
ID NO:68); Macaca mulatta (SEQ ID NO:69); Canis lupus familiaris (SEQ ID
NO:70);
Ailuropoda melanoleuca (SEQ ID NO:71); Equus caballus (SEQ ID NO:72); Bos
taurus
(SEQ ID NO:73); Mus musculus (SEQ ID NO:74); Rattus norvegicus (SEQ ID NO:75);
Consensus Sequence (SEQ ID NO:76)). Numbering and secondary structure is
according to
the human IL-34 (SEQ ID NO:68). Strictly conserved residues are shaded in dark
grey and
conserved residues in most of the sequences, as calculated by a similarity
score, are boxed.
IL-34 residues at site 1, site 2 and IL-34 dimerization interface are denoted
by solid circles,
circles and stars at the bottom, respectively. Triangles indicate the
disulfide bond pairing and
glycosylation site. The alignment figures were made using program ESPRIT
(WorldWide
Web at esprit.ibcp.fr/ESPript/ESPript).
Figure 8 shows neutralizing activity of anti-IL-34 Ab YW404.33 in the monocyte
proliferation assay.
14
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Figure 9 shows neutralizing activity of anti-IL-34 Abs YW404.1, YW404.6,
YW404.33,
YW405.1, YW405.3, YW406.1, YW406.93 (A) and Abs YW404.33, YW404.33.12 and
YW404.33.56 at a concentration of mIL-34 of 100 ng/ml (B) in the monocyte
proliferation
assay.
Figure 10: Variable heavy (A) and light (B) chain sequences of anti-IL-34 Abs
YW404.1,
YW404.3, YW404.33, YW404.33.10, YW404.33.12, YW404.33.11, YW404.33.56, and
YW404.33 .93. Amino acid residues targeted for affinity-maturation for these
antibodies are
surrounded by a box. Figure 10A shows the VH amino acid sequences for 404.1
(SEQ ID
NO:15), 404.6 (SEQ ID NO: 77), 405.3 (SEQ ID NO:25), 404.33 (SEQ ID NO:5),
404.33.10
(SEQ ID NO:7), 404.33.12 (SEQ ID NO:11), 404.33.11 (SEQ ID NO:9), 404.33.56
(SEQ ID
NO:3), and 404.33.93 (SEQ ID NO:13). Figure 10B shows the VL amino acid
sequences for
404.1 (SEQ ID NO:16), 404.6 (SEQ ID NO: 78), 405.3 (SEQ ID NO:26), 404.33 (SEQ
ID
NO:6), 404.33.10 (SEQ ID NO:8), 404.33.12 (SEQ ID NO:12), 404.33.11 (SEQ ID
NO:10),
404.33.56 (SEQ ID NO:4), and 404.33.93 (SEQ ID NO:14). The heavy chain
framework
region sequences between Kabat HVRs are FR1 sequence (SEQ ID NO:17), FR2
sequence
(SEQ ID NO:18), FR3 (SEQ ID NO:19), and FR4 (SEQ ID NO:20) shown in Figure
10A.
The light chain framework region sequences between Kabat HVRs are FR1 sequence
(SEQ
ID NO:21), FR2 sequence (SEQ ID NO:22), FR3 sequence (SEQ ID NO:23), and FR4
sequence (SEQ ID NO:24) shown in Figure 10B.
Figure 11 shows the histology score of Balb/c mice with dextran sulfate sodium
(DSS) ¨
induced inflammatory bowel disease (IBD) treated with either control antibody
(anti-
ragweed, a-RW), cyclosporine (CSA), anti-CSF-1 antibody (a-CSF-1), anti-IL-34
antibody (a-
IL-34) or a combination of anti-CSF-1 antibody and anti-IL-34 antibody.
Figure 12 shows that serum levels of IL-34 and CSF-1 were elevated in Balb/c
mice with
DSS-induced IBD treated with control antibody (a-RW) compared to control mice.
Figure 13 shows CSF-1 and IL-34 are expressed in serum, synovial fluid and
tissue from
rheumatoid arthritis patients.
Figure 14 shows that CSF1/IL34 pathway is present in primary and secondary TNF-
NR RA
patients.
Figure 15 shows that the treatment o fa combination of aCSF1+aIL34 matches
TNFRII-Fc
inflammation inhibition and is superior in protecting bone erosions in mouse
CIA (myeloid
drivers)
Figure 16 shows the dual blockade of CSF1 and IL-34 inhibits DSS colitis in a
model.
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Figure 17 shows that IL-34 is expressed in IBD colon but low/undetectable in
serum
Figure 18 shows that there is no correlation of IL-34/CSF-1 and TNFa
expression in synovial
fluid from rheumatoid arthritis and osteoarthritis patients.
Figure 19 shows the shows the reduction of mouse myeloid cells (Mf and
monotyes)
infiltrating joint synovia after only 7 days of anti-CSF1/IL-34 combination
treatment.
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
Without being bound by theory, the combinatorial approach of inhibiting both
IL-34 and
CSF-1 directly to treat myeloid pathogenic immunological diseases is believed
to be superior
to directly targeting their receptor or either IL-34 and CSF-1 alone.
Advantages to this
approach are predicted to include, but are not limited to, any one or
combination of the
following, better pharmacokinetic properties, better safety profiles, better
efficacy, better
potency and a better therapeutic window based on the safety and efficacy
considerations
above.
DEFINITIONS
The terms "anti-IL-34 antibody" and "an antibody that binds to IL-34" refer to
an antibody
that is capable of binding IL-34 with sufficient affinity such that the
antibody is useful as a
diagnostic and/or therapeutic agent in targeting IL-34. In some embodiments,
the extent of
binding of an anti-IL-34 antibody to an unrelated, non-IL-34 protein is less
than about 10% of
the binding of the antibody to IL-34 as measured, e.g., by a BIACORE assay or
a BLI assay.
In some embodiments, an antibody that binds to IL-34 has a dissociation
constant (Kd) of
< liAM, < 500 nM, < 250 nM, < 100 nM, < 10 nM, < 1 nM, < 0.1 nM, < 0.01 nM, or
< 0.001
nM (e.g., 10-8M or less, e.g., from 10-8M to 10-13M, e.g., from 10-9M to 10-13
M). In some
embodiments, an anti-IL-34 antibody binds to an epitope of IL-34 that is
conserved among
IL-34 from different species.
The term "IL-34," as used herein, refers to any native IL-34 from any
vertebrate source,
including mammals such as primates (e.g., humans) and rodents (e.g., mice and
rats), unless
otherwise indicated. The term encompasses "full-length," unprocessed IL-34 as
well as any
form of IL-34 that results from processing in the cell. The term also
encompasses naturally
occurring variants of IL-34, e.g., splice variants or allelic variants. The
amino acid sequence
of an exemplary human IL-34 is shown in SEQ ID NO: 1. In some embodiments, the
human
IL-34 comprises the amino acid sequence shown in SEQ ID NO:1, wherein amino
acid Q at
position 81 is deleted.
16
CA 02861122 2014-07-14
W02013/119716
PCT/US2013/024998
1 MPRGFTWLRY LGIFLGVALG NEPLEMWPLT QNEECTVTGF LRDKLQYRSR
LQYMKHYFPI
61 NYKISVPYEG VFRIANVTRL QRAQVSEREL RYLWVLVSLS ATESVQDVLL
EGHPSWKYLQ
121 EVETLLLNVQ QGLTDVEVSP KVESVLSLLN APGPNLKLVR PKALLDNCFR
VMELLYCSCC
181 KQSSVLNWQD CEVPSPQSCS PEPSLQYAAT QLYPPPPWSP SSPPHSTGSV
RPVRAQGEGL
241 LP (SEQ ID NO:1).
The terms "anti- CSF-1 antibody" and "an antibody that binds to CSF-1" refer
to an antibody
that is capable of binding CSF-1 with sufficient affinity such that the
antibody is useful as a
diagnostic and/or therapeutic agent in targeting CSF-1. In some embodiments,
the extent of
binding of an anti- CSF-1 antibody to an unrelated, non- CSF-1 protein is less
than about
10% of the binding of the antibody to CSF-1 as measured, e.g., by a BIACORE
assay or a
BLI assay. In some embodiments, an antibody that binds to CSF-1 has a
dissociation constant
(Kd) of < 104, < 500 nM, < 250 nM, < 100 nM, < 10 nM, < 1 nM, < 0.1 nM, < 0.01
nM, or
< 0.001 nM (e.g., 10-8M or less, e.g., from 10-8M to 10-13M, e.g., from 10-9M
to 10-13 M). In
some embodiments, an anti-CSF-1 antibody binds to an epitope of CSF-1 that is
conserved
among CSF-1 from different species.
The term "CSF-1," as used herein, refers to any native CSF-1 from any
vertebrate source,
including mammals such as primates (e.g., humans) and rodents (e.g., mice and
rats), unless
otherwise indicated. The term encompasses "full-length," unprocessed CSF-1 as
well as any
form of CSF-1 that results from processing in the cell. The term also
encompasses naturally
occurring variants of CSF-1, e.g., splice variants or allelic variants. An
exemplary human
CSF-1 is described in Takahashi et al., Biochem. Biophys. Res. Commun. 161
(2), 892-901
(1989).
The terms "anti- CSF-1R antibody" and "an antibody that binds to CSF-1R" refer
to an
antibody that is capable of binding CSF-1R with sufficient affinity such that
the antibody is
useful as a diagnostic and/or therapeutic agent in targeting CSF-1R. In some
embodiments,
the extent of binding of an anti- CSF-1R antibody to an unrelated, non- CSF-1R
protein is
less than about 10% of the binding of the antibody to CSF-1R as measured,
e.g., by a
BIACORE assay or a BLI assay. In some embodiments, an antibody that binds to
CSF-1R
17
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
has a dissociation constant (Kd) of < 1[LM, < 500 nM, < 250 nM, < 100 nM, < 10
nM, < 1
nM, < 0.1 nM, < 0.01 nM, or < 0.001 nM (e.g., 10-8M or less, e.g., from 10-8M
to 10-13M,
e.g., from 10-9M to 10-13 M). In some embodiments, an anti- CSF-1R antibody
binds to an
epitope of CSF-1R that is conserved among IL-34 from different species.
The term "CSF-1R" or "CSF1R" as used herein, refers to any native CSF-1R from
any
vertebrate source, including mammals such as primates (e.g., humans) and
rodents (e.g., mice
and rats), unless otherwise indicated. The term encompasses "full-length,"
unprocessed CSF-
1R as well as any form of CSF-1R that results from processing in the cell. The
term also
encompasses naturally occurring variants of CSF-1R, e.g., splice variants or
allelic variants.
The amino acid sequence of an exemplary human CSF-1R is shown in SEQ ID NO:2.
MGPGVLLLLL VATAWHGQGI PVIEPSVPEL VVKPGATVTL RCVGNGSVEW
DGPPSPHWTL
YSDGSSSILS TNNATFQNTG TYRCTEPGDP LGGSAAIHLY VKDPARPWNV
LAQEVVVFED
QDALLPCLLT DPVLEAGVSL VRVRGRPLMR HTNYSFSPWH GFTIHRAKFI
QSQDYQCSAL
MGGRKVMSIS IRLKVQKVIP GPPALTLVPA ELVRIRGEAA QIVCSASSVD
VNFDVFLQHN
NTKLAIPQQS DFHNNRYQKV LTLNLDQVDF QHAGNYSCVA SNVQGKHSTS
MFFRVVE SAY
LNLSSEQNLI QEVTVGEGLN LKVMVEAYPG LQGFNWTYLG PFSDHQPEPK
LANATTKDTY
RHTFTLSLPR LKPSEAGRYS FLARNPGGWR ALTFELTLRY PPEVSVIWTF
INGSGTLLCA
ASGYPQPNVT WLQCSGHTDR CDEAQVLQVW DDPYPEVLSQ EPFHKVTVQS
LLTVETLEHN
QTYECRAHNS VGSGSWAFIP ISAGAHTHPP DEFLFTPVVV ACMSIMALLL
LLLLLLLYKY
KQKPKYQVRW KIIESYEGNS YTFIDPTQLP YNEKWEFPRN NLQFGKTLGA
GAFGKVVEAT
AFGLGKEDAV LKVAVKMLKS TAHADEKEAL MSELKIMSHL GQHENIVNLL
GACTHGGPVL
VITEYCCYGD LLNFLRRKAE AMLGPSLSPG QDPEGGVDYK NIHLEKKYVR
RDSGFSSQGV
18
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
DTYVEMRPVS TSSNDSFSEQ DLDKEDGRPL ELRDLLHFSS QVAQGMAFLA
SKNCIHRDVA
ARNVLLTNGH VAKIGDFGLA RDIMNDSNYI VKGNARLPVK WMAPESIFDC
VYTVQSDVWS
YGILLWEIFS LGLNPYPGIL VNSKFYKLVK DGYQMAQPAF APKNIYSIMQ
ACWALEPTHR
PTFQQICSFL QEQAQEDRRE RDYTNLPSSS RSGGSGSSSS ELEEESSSEH
LTCCEQGDIA
QPLLQPNNYQ FC (SEQ ID NO:2)
A therapeutic agent according to this invention includes an agent that can
bind to the target
identified herein above, such as a polypeptide(s) (e.g., an antibody, an
immunoadhesin or a
peptibody), an aptamer or a small molecule that can bind to a protein or a
nucleic acid
molecule that can bind to a nucleic acid molecule encoding a target identified
herein (i.e.,
siRNA).
The term "CSF1-R pathway inhibitor" refers to a therapeutic agent that
inhibits CSF1-R
signaling. In one embodiment, the CSF1-R pathway inhibitor binds to CSF-1, IL-
34, CSF1-R
or CSF-1 and IL-34. In one embodiment, the agent that binds CSF-1, IL-34 or
CSF-1 and IL-
34 inhibits the binding of such protein(s) to CSF1-R. In another embodiment,
the agent that
binds CSF1-R inhibits the binding of CSF1-R to IL-34 and CSF-1. In one
embodiment, a
reduction in kinase activity of CSF1-R indicates a reduction in CSF-1R
signalling. In one
embodiment, the CSF1-R pathway inhibitor is an antibody of this invention. In
another
embodiment, the CSF-1R pathway inhibitor is a small molecule inhibitor of CSF1-
R. In
another embodiment, the CSF1-R pathway inhibitor is a CSF1-R extracellular
domain fused
to an Fc.
The term "antibody" herein is used in the broadest sense and encompasses
various antibody
structures, including but not limited to monoclonal antibodies, polyclonal
antibodies,
multispecific antibodies (e.g., bispecific antibodies), and antibody fragments
so long as they
exhibit the desired antigen-binding activity.
The term "variable region" or "variable domain" refers to the domain of an
antibody heavy or
light chain that is involved in binding the antibody to antigen. The variable
domains of the
heavy chain and light chain (VH and VL, respectively) of a native antibody
generally have
similar structures, with each domain comprising four conserved framework
regions (FRs) and
three hypervariable regions (HVRs). (See, e.g., Kindt et al., Kuby Immunology,
6th ed., W.H.
Freeman and Co., page 91 (2007).) A single VH or VL domain may be sufficient
to confer
19
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
antigen-binding specificity. Furthermore, antibodies that bind a particular
antigen may be
isolated using a VH or VL domain from an antibody that binds the antigen to
screen a library
of complementary VL or VH domains, respectively. See, e.g., Portolano et al.,
J. Immunol.
150:880-887 (1993); Clarkson et al., Nature 352:624-628 (1991).
The term "hypervariable region" or "HVR," as used herein, refers to each of
the regions of an
antibody variable domain which are hypervariable in sequence and/or form
structurally
defined loops ("hypervariable loops"). Generally, native four-chain antibodies
comprise six
HVRs; three in the VH (H1, H2, H3), and three in the VL (L1, L2, L3). HVRs
generally
comprise amino acid residues from the hypervariable loops and/or from the
"complementarity
determining regions" (CDRs), the latter being of highest sequence variability
and/or involved
in antigen recognition. An HVR as used herein can comprise residues located
within
positions 24-36 (for L1), 46-56 (for L2), 89-97 (for L3), 26-35B (for H1), 47-
65 (for H2), and
93-102 (for H3). For example, an HVR can include residues in positions
described
previously:
A) 24-34 (L1), 50-56 (L2), 89-97 (L3), 26-32 (H1), 52-56 (H2), and 95-102 (H3)
(Chothia and Lesk, J. Mol. Biol. 196:901-917 (1987);
B) 24-34 of Ll, 50-56 of L2, 89-97 of L3, 31-35B of H1, 50-65 of H2, and 95-
102
of H3 (Kabat et al., Sequences of Proteins of Immunological Interest, 5th Ed.
Public Health
Service, National Institutes of Health, Bethesda, MD (1991); and
C) 30-36 (L1), 46-55 (L2), 89-96 (L3), 30-35 (H1), 47-58 (H2), 93-101 (H3)
(MacCallum et al. J. Mol. Biol. 262:732-745 (1996).
Unless otherwise indicated, HVR residues and other residues in the variable
domain
(e.g., FR residues) are numbered herein according to Kabat et al., supra.
Unless otherwise indicated, HVR residues and other residues in the variable
domain (e.g., FR
residues) are numbered herein according to Kabat et al., supra.
With the exception of CDR1 in VH, CDRs generally comprise the amino acid
residues that
form the hypervariable loops. CDRs also comprise "specificity determining
residues," or
"SDRs," which are residues that contact antigen. SDRs are contained within
regions of the
CDRs called abbreviated-CDRs, or a-CDRs. Exemplary a-CDRs (a-CDR-L1, a-CDR-L2,
a-
CDR-L3, a-CDR-H1, a-CDR-H2, and a-CDR-H3) occur at amino acid residues 31-34
of Ll,
50-55 of L2, 89-96 of L3, 31-35B of H1, 50-58 of H2, and 95-102 of H3. (See
Almagro and
Fransson, Front. Biosci. 13:1619-1633 (2008).) Unless otherwise indicated, HVR
residues
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
and other residues in the variable domain (e.g., FR residues) are numbered
herein according
to Kabat et al., supra.
"Framework" or "FR" refers to variable domain residues other than
hypervariable region
(HVR) residues. The FR of a variable domain generally consists of four FR
domains: FR1,
FR2, FR3, and FR4. Accordingly, the HVR and FR sequences generally appear in
the
following sequence in VH (or VL): FR1-H1(L1)-FR2-H2(L2)-FR3-H3(L3)-FR4.
A "human consensus framework" is a framework which represents the most
commonly
occurring amino acid residues in a selection of human immunoglobulin VL or VH
framework
sequences. Generally, the selection of human immunoglobulin VL or VH sequences
is from a
subgroup of variable domain sequences. Generally, the subgroup of sequences is
a subgroup
as in Kabat et al., Sequences of Proteins of Immunological Interest, Fifth
Edition, NIH
Publication 91-3242, Bethesda MD (1991), vols. 1-3. In some embodiments, for
the VL, the
subgroup is subgroup kappa I as in Kabat et al., supra. In some embodiments,
for the VH, the
subgroup is subgroup III as in Kabat et al., supra.
An "acceptor human framework" for the purposes herein is a framework
comprising the
amino acid sequence of a light chain variable domain (VL) framework or a heavy
chain
variable domain (VH) framework derived from a human immunoglobulin framework
or a
human consensus framework, as defined below. An acceptor human framework
"derived
from" a human immunoglobulin framework or a human consensus framework may
comprise
the same amino acid sequence thereof, or it may contain amino acid sequence
changes. In
some embodiments, the number of amino acid changes are 10 or less, 9 or less,
8 or less, 7 or
less, 6 or less, 5 or less, 4 or less, 3 or less, or 2 or less. In some
embodiments, the VL
acceptor human framework is identical in sequence to the VL human
immunoglobulin
framework sequence or human consensus framework sequence.
The "class" of an antibody refers to the type of constant domain or constant
region possessed
by its heavy chain. There are five major classes of antibodies: IgA, IgD, IgE,
IgG, and IgM,
and several of these may be further divided into subclasses (isotypes), e.g.,
IgGi, IgG2, IgG3,
Igat, IgAi, and IgA2. The heavy chain constant domains that correspond to the
different
classes of immunoglobulins are called a, 6, 8, y, and it, respectively.
The term "Fc region" herein is used to define a C-terminal region of an
immunoglobulin
heavy chain that contains at least a portion of the constant region. The term
includes native
sequence Fc regions and variant Fc regions. In some embodiments, a human IgG
heavy chain
Fc region extends from Cys226, or from Pro230, to the carboxyl-terminus of the
heavy
21
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
chain. However, the C-terminal lysine (Lys447) of the Fc region may or may not
be present.
Unless otherwise specified herein, numbering of amino acid residues in the Fc
region or
constant region is according to the EU numbering system, also called the EU
index, as
described in Kabat et al., Sequences of Proteins of Immunological Interest,
5th Ed. Public
Health Service, National Institutes of Health, Bethesda, MD, 1991.
"Native antibodies" refer to naturally occurring immunoglobulin molecules with
varying
structures. For example, native IgG antibodies are heterotetrameric
glycoproteins of about
150,000 daltons, composed of two identical light chains and two identical
heavy chains that
are disulfide-bonded. From N- to C-terminus, each heavy chain has a variable
region (VH),
also called a variable heavy domain or a heavy chain variable domain, followed
by three
constant domains (CH1, CH2, and CH3). Similarly, from N- to C-terminus, each
light chain
has a variable region (VL), also called a variable light domain or a light
chain variable
domain, followed by a constant light (CL) domain. The light chain of an
antibody may be
assigned to one of two types, called kappa (x) and lambda (4 based on the
amino acid
sequence of its constant domain.
The term "monoclonal antibody" as used herein refers to an antibody obtained
from a
population of substantially homogeneous antibodies, i.e., the individual
antibodies
comprising the population are identical and/or bind the same epitope, except
for possible
variant antibodies, e.g., containing naturally occurring mutations or arising
during production
of a monoclonal antibody preparation, such variants generally being present in
minor
amounts. In contrast to polyclonal antibody preparations, which typically
include different
antibodies directed against different determinants (epitopes), each monoclonal
antibody of a
monoclonal antibody preparation is directed against a single determinant on an
antigen.
Thus, the modifier "monoclonal" indicates the character of the antibody as
being obtained
from a substantially homogeneous population of antibodies, and is not to be
construed as
requiring production of the antibody by any particular method. For example,
the monoclonal
antibodies to be used in accordance with the present invention may be made by
a variety of
techniques, including but not limited to the hybridoma method, recombinant DNA
methods,
phage-display methods, and methods utilizing transgenic animals containing all
or part of the
human immunoglobulin loci, such methods and other exemplary methods for making
monoclonal antibodies being described herein.
22
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
The term "chimeric" antibody refers to an antibody in which a portion of the
heavy and/or
light chain is derived from a particular source or species, while the
remainder of the heavy
and/or light chain is derived from a different source or species.
A "humanized" antibody refers to a chimeric antibody comprising amino acid
residues from
non-human HVRs and amino acid residues from human FRs. In some embodiments, a
humanized antibody will comprise substantially all of at least one, and
typically two, variable
domains, in which all or substantially all of the HVRs (e.g., CDRs) correspond
to those of a
non-human antibody, and all or substantially all of the FRs correspond to
those of a human
antibody. A humanized antibody optionally may comprise at least a portion of
an antibody
constant region derived from a human antibody. A "humanized form" of an
antibody, e.g., a
non-human antibody, refers to an antibody that has undergone humanization.
A "human antibody" is one which possesses an amino acid sequence which
corresponds to
that of an antibody produced by a human or a human cell or derived from a non-
human source
that utilizes human antibody repertoires or other human antibody-encoding
sequences. This
definition of a human antibody specifically excludes a humanized antibody
comprising non-
human antigen-binding residues.
An "antibody fragment" refers to a molecule other than an intact antibody that
comprises a
portion of an intact antibody that binds the antigen to which the intact
antibody binds.
Examples of antibody fragments include but are not limited to Fv, Fab, Fab',
Fab'-SH,
F(a02; diabodies; linear antibodies; single-chain antibody molecules (e.g.,
scFv); and
multispecific antibodies formed from antibody fragments.
The terms "full length antibody," "intact antibody," and "whole antibody" are
used herein
interchangeably to refer to an antibody having a structure substantially
similar to a native
antibody structure or having heavy chains that contain an Fc region as defined
herein.
An "isolated" antibody is one which has been separated from a component of its
natural
environment. In some embodiments, an antibody is purified to greater than 95%
or 99%
purity as determined by, for example, electrophoretic (e.g., SDS-PAGE,
isoelectric focusing
(IEF), capillary electrophoresis) or chromatographic (e.g., ion exchange or
reverse phase
HPLC). For review of methods for assessment of antibody purity, see, e.g.,
Flatman et al., J.
Chromatogr. B 848:79-87 (2007).
An "affinity matured" antibody refers to an antibody with one or more
alterations in one or
more hypervariable regions (HVRs), compared to a parent antibody which does
not possess
23
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
such alterations, such alterations resulting in an improvement in the affinity
of the antibody
for antigen.
"Affinity" refers to the strength of the sum total of noncovalent interactions
between a single
binding site of a molecule (e.g., an antibody) and its binding partner (e.g.,
an antigen). Unless
An "antibody that binds to the same epitope" as a reference antibody refers to
an antibody that
blocks binding of the reference antibody to its antigen in a competition assay
by 50% or more,
and conversely, the reference antibody blocks binding of the antibody to its
antigen in a
competition assay by 50% or more. An exemplary competition assay is provided
herein.
An "isolated" nucleic acid refers to a nucleic acid molecule that has been
separated from a
component of its natural environment. An isolated nucleic acid includes a
nucleic acid
"Isolated nucleic acid encoding an anti-IL-34 antibody" refers to one or more
nucleic acid
molecules encoding antibody heavy and light chains (or fragments thereof),
including such
"Percent (%) amino acid sequence identity" with respect to a reference
polypeptide sequence
is defined as the percentage of amino acid residues in a candidate sequence
that are identical
24
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
with the amino acid residues in the reference polypeptide sequence, after
aligning the
sequences and introducing gaps, if necessary, to achieve the maximum percent
sequence
identity, and not considering any conservative substitutions as part of the
sequence identity.
Alignment for purposes of determining percent amino acid sequence identity can
be achieved
in various ways that are within the skill in the art, for instance, using
publicly available
computer software such as BLAST, BLAST-2, ALIGN or Megalign (DNASTAR)
software.
Those skilled in the art can determine appropriate parameters for aligning
sequences,
including any algorithms needed to achieve maximal alignment over the full
length of the
sequences being compared. For purposes herein, however, % amino acid sequence
identity
values are generated using the sequence comparison computer program ALIGN-2.
The
ALIGN-2 sequence comparison computer program was authored by Genentech, Inc.,
and the
source code has been filed with user documentation in the U.S. Copyright
Office, Washington
D.C., 20559, where it is registered under U.S. Copyright Registration No.
TXU510087. The
ALIGN-2 program is publicly available from Genentech, Inc., South San
Francisco,
California, or may be compiled from the source code. The ALIGN-2 program
should be
compiled for use on a UNIX operating system, including digital UNIX V4.0D. All
sequence
comparison parameters are set by the ALIGN-2 program and do not vary.
In situations where ALIGN-2 is employed for amino acid sequence comparisons,
the %
amino acid sequence identity of a given amino acid sequence A to, with, or
against a given
amino acid sequence B (which can alternatively be phrased as a given amino
acid sequence A
that has or comprises a certain % amino acid sequence identity to, with, or
against a given
amino acid sequence B) is calculated as follows:
100 times the fraction X/Y
where X is the number of amino acid residues scored as identical matches by
the sequence
alignment program ALIGN-2 in that program's alignment of A and B, and where Y
is the
total number of amino acid residues in B. It will be appreciated that where
the length of
amino acid sequence A is not equal to the length of amino acid sequence B, the
% amino acid
sequence identity of A to B will not equal the % amino acid sequence identity
of B to A.
Unless specifically stated otherwise, all % amino acid sequence identity
values used herein
are obtained as described in the immediately preceding paragraph using the
ALIGN-2
computer program.
The term "vector," as used herein, refers to a nucleic acid molecule capable
of propagating
another nucleic acid to which it is linked. The term includes the vector as a
self-replicating
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
nucleic acid structure as well as the vector incorporated into the genome of a
host cell into
which it has been introduced. Certain vectors are capable of directing the
expression of
nucleic acids to which they are operatively linked. Such vectors are referred
to herein as
"expression vectors."
The terms "host cell," "host cell line," and "host cell culture" are used
interchangeably and
refer to cells into which exogenous nucleic acid has been introduced,
including the progeny of
such cells. Host cells include "transformants" and "transformed cells," which
include the
primary transformed cell and progeny derived therefrom without regard to the
number of
passages. Progeny may not be completely identical in nucleic acid content to a
parent cell,
but may contain mutations. Mutant progeny that have the same function or
biological activity
as screened or selected for in the originally transformed cell are included
herein.
As used herein, "treatment" (and grammatical variations thereof such as
"treat" or "treating")
refers to clinical intervention in an attempt to alter the natural course of
the individual being
treated, and can be performed either for prophylaxis or during the course of
clinical
pathology. Desirable effects of treatment include, but are not limited to,
preventing
occurrence or recurrence of disease, alleviation of symptoms, diminishment of
any direct or
indirect pathological consequences of the disease, preventing metastasis,
decreasing the rate
of disease progression, amelioration or palliation of the disease state, and
remission or
improved prognosis. In some embodiments, antibodies of the invention are used
to delay
development of a disease or to slow the progression of a disease.
An "individual" or "subject" is a mammal. Mammals include, but are not limited
to,
domesticated animals (e.g., cows, sheep, cats, dogs, and horses), primates
(e.g., humans and
non-human primates such as monkeys), rabbits, and rodents (e.g., mice and
rats). In some
embodiments, the individual or subject is a human.
The term "pharmaceutical formulation" refers to a preparation which is in such
form as to
permit the biological activity of an active ingredient contained therein to be
effective, and
which contains no additional components which are unacceptably toxic to a
subject to which
the formulation would be administered.
A "pharmaceutically acceptable carrier" refers to an ingredient in a
pharmaceutical
formulation, other than an active ingredient, which is nontoxic to a subject.,
A
pharmaceutically acceptable carrier includes, but is not limited to, a buffer,
excipient,
stabilizer, or preservative.
26
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
An "effective amount" of an agent, e.g., a pharmaceutical formulation, refers
to an amount
effective, at dosages and for periods of time necessary, to achieve the
desired therapeutic or
prophylactic result.
As is understood in the clinical context, an effective amount of a therapeutic
agent (e.g., an
antibody provided herein), drug, compound, or pharmaceutical composition may
or may not
be achieved in conjunction with another drug, compound, or pharmaceutical
composition.
Thus, an "effective amount" may be considered in the context of administering
one or more
therapeutic agents, and a single agent may be considered to be given in an
effective amount if,
in conjunction with one or more other agents, a desirable result may be or is
achieved.
As used herein, "in conjunction with" refers to administration of one
treatment modality in
addition to another treatment modality. As such, "in conjunction with" refers
to
administration of one treatment modality before, during or after
administration of the other
treatment modality to the individual.
The term "package insert" is used to refer to instructions customarily
included in commercial
packages of therapeutic products, that contain information about the
indications, usage,
dosage, administration, combination therapy, contraindications and/or warnings
concerning
the use of such therapeutic products.
"Inflammatory bowel disease" or "IBD" refers to the group of disorders that
cause the
intestines to become inflamed, generally manifested with symptoms including
abdominal
cramps and pain, diarrhea, weight loss and intestinal bleeding. The main forms
of IBD are
ulcerative colitis (UC) and Crohn's disease.
As used herein, "myeloid pathogenic immunological disease" refers to an
inflammatory
disease and/or an autoimmune disease with a myeloid pathogenic component.
As used herein, "DMARD" refers to a disease-modifying antirheumatic drug.
Examples of
DMARDs include adalimumab, cloroquine, hydroxychloroquine, sulfasalazine,
methotrexate,
leflunomide, azathioprine, D-penicillamine, gold salts (sodium aurothiomalate,
auraofin),
Gold (oral), Gold (intramuscular), minocycline, cyclosporine, etanercept,
golimumab,
infliximab, minocycline and ritixumab.
As used herein, "Fl" refers to fibroblast-rich type 1 subtype, "F2" refers to
fibroblast-rich
type 2 subtype, "L" refers to lymphoid-rich subtype or lymphoid subtype, and
"M" refers to
myeloid-rich subtype or myeloid subtype. Collectively, these subtypes identify
four
molecular subtypes of rheumatoid arthritis patients based on gene expression
analysis.
Collectively, Fl and F2 subtypes are referred to as the fibroid or "F"
subtype. The L subtype
27
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
of RA patients generally have a gene expression pattern characteristic of B
cell, plasma cell, T
cell, and macrophage involvement and evidence of B and T cell activation,
isotype switching,
Ig secretion, and cytokine production. The Myeloid subtype of RA patients
generally have a
gene expression pattern characteristic of monocyte, macrophage, neutrophil and
lymphocyte
involvement and evidence of macrophage activation, phagocytosis, respiratory
burst, T cell
activation and cytokine production. The Fibroid subtype of RA patients
generally have a
gene expression pattern characteristic of fibroblast and osteoblast
involvement and evidence
of bone formation, growth and differentiation and vasculogenesis.
As used herein and in the appended claims, the singular forms "a," "an," and
"the" include
plural reference unless the context clearly indicates otherwise. For example,
reference to an
"antibody" is a reference to from one to many antibodies, such as molar
amounts, and
includes equivalents thereof known to those skilled in the art, and so forth.
Reference to "about" a value or parameter herein includes (and describes)
embodiments that
are directed to that value or parameter per se. For example, description
referring to "about X"
includes description of "X."
It is understood that aspect and variations of the invention described herein
include
"consisting" and/or "consisting essentially of" aspects and variations.
COMPOSITIONS AND METHODS
The invention provides antibodies that bind to IL-34, bispecific antibodies
with a first binding
specificity for IL-34 and a second binding specificity for CSF-1 (further
referred to hereine as
bispecific anti-IL-34/CSF-1 antibodies), and antibodies that bind to CSF-1R.
Antibodies of
the invention are useful, e.g., for the diagnosis or treatment of myeloid
pathogenic
immunological diseases, including, but not limited to inflammatory bowel
disease,
rheumatoid arthritis and multiple sclerosis. In some embodiments, the anti-IL-
34 antibodies
bind to mammalian (e.g., human) IL-34. In some embodiments, the bispecific
anti-IL-
34/CSF-1 antibodies comprise a first binding specificity to a mammalian (e.g.,
human) IL-34
and a second binding specificity to a mammalian (e.g., human) CSF-1. In some
embodiments,
the anti-C SF-1R antibodies bind to mammalian (e.g., human) C SF-1R.
Exemplary Anti-IL-34 and CSF-1R Antibodies
Anti-IL-34 antibodies
In one aspect, the invention provides isolated antibodies that bind to IL-34
(e.g., human IL-
34). The anti-IL-34 antibodies described herein may have one or more of the
following
28
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
characteristics: (i) inhibition of binding of IL-34 (e.g., human IL-34) to CSF-
1R (e.g., human
CSF-1R); (ii) neutralization of IL-34 activity (e.g., human IL-34 activity);
(iii) inhibition of
IL-34 induced proliferation of peripheral blood mononuclear cells; (iv)
binding to a dimer of
IL-34 (e.g., human IL-34); (v) binding to an epitope that spans over both
protomers of IL-34
(e.g., human IL-34); (vi) no inhibition of binding of CSF-1 (e.g., human CSF-
1) to CSF-1R
(e.g., human CSF-1R). In some embodiments, the extent of binding of an anti-IL-
34 antibody
to an unrelated, non-IL-34 protein is less than about 10% of the binding of
the antibody to IL-
34 as measured, e.g., by a BIACORE assay or a biolayer interferometry (BLI)
assay. In some
embodiments, the antibody that binds to IL-34 has a dissociation constant (Kd)
of < 104,
< 500 nM, < 250 nM, < 100 nM, < 10 nM, < 1 nM, < 0.1 nM, < 0.01 nM, or < 0.001
nM (e.g.,
10-8M or less, e.g.,from 10-8M to 10-13M, e.g., from 10-9M to 10-13 M). In
some
embodiments, the anti-IL-34 antibody has a Kd value of less than about 500 nM.
In some
embodiments, the anti-IL-34 antibody has a Kd value of less than about 100 nM
or 10 nM. In
some embodiments, the anti-IL-34 antibody has a Kd value of less than about 1
nM. In some
embodiments, the IL-34 antibody has a Kd value of less than about 100 pM. In
some
embodiments, an anti-IL-34 antibody has a Kd of about 100-200 pM, about 100-
500 pM,
about 100 pM-1 nM, or of about 1nM-50nM. In some embodiments, an anti-IL-34
antibody
has a Kd of about 17 nM. In some embodiments, an anti-IL-34 antibody has a Kd
of about
120 nM. In some embodiments, the anti-IL-34 antibody binds to an epitope of IL-
34 that is
conserved among IL-34 from different species.
In one aspect, provided herein is an anti-IL-34 antibody, which binds to an
epitope
comprising at least any one of one, two, three, four, five, six, seven, eight,
nine, ten, eleven,
twelve, thirteen, fourteen, fifteen, or sixteen, or seventeen of amino acid
residues G1u103,
Leu109, G1n106, Asn150, Leu127, Asn128, 5er184, Leu186, Asn187, Lys44, G1u121,
Asp107, Glul 1 1, Ser104, G1n120, Trp116, and Asn61 of a human IL-34. In one
aspect,
provided herein is an anti-IL-34 antibody, which binds to an epitope
comprising at least one
of amino acid residues from G1u103 to Asn150 of a human IL-34. In one aspect,
provided
herein is an anti-IL-34 antibody, which binds to an epitope comprising at
least any one of one,
two, or three, or four of amino acid residues G1u103, Leu109, G1n106, and
Asn150 of a
human IL-34. In any of the aspects above, the anti-IL-34 antibody may bind to
an epitope
further comprising at least any one of one, two, three, four, five, six, or
seven, or eight of
amino acid residues Ser100, G1u123, Trp116, Thr124, Leu127, Asn128, G1n131,
and Thr134
of a human IL-34. In some embodiments, the anti-IL-34 antibody binds to amino
acids within
29
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
positions 100-108, 116-134, 109 and 150 of a human IL-34. In some embodiments,
the anti-
IL-34 antibody inhibits binding between human IL-34 and human CSF-1R. In some
embodiments, the anti-IL-34 antibody neutralizes human IL-34 activity. In some
embodiments, the anti-IL-34 antibody binds to a dimer of human IL-34. In some
embodiments, the anti-IL-34 antibody binds to an epitope that spans both
protomers of human
IL-34. In some embodiments, the anti-IL-34 antibody is a monoclonal antibody.
In some
embodiments, the anti-IL-34 antibody is a human, humanized, or chimeric
antibody. In some
embodiments, the anti-IL-34 antibody is an antibody fragment that binds to
human IL-34. As
used herein, the residue position herein corresponds to the residue position
in SEQ ID NO: 1.
In one aspect, provided herein is an anti-IL-34 antibody, which binds to an
epitope
comprising at least any one of one, two, three, four, five, six, seven, eight,
nine, ten, eleven,
twelve, thirteen, fourteen, fifteen, or sixteen, or seventeen of amino acid
residues G1u103,
Leu109, G1n106, Asn150, Leu127, Asn128, Ser184, Leu186, Asn187, Lys44, G1u121,
Asp107, Glulll, Ser104, G1n120, Trp116, and Asn6lof a human IL-34. In one
aspect,
provided herein is an anti-IL-34 antibody, which binds to an epitope
comprising at least any
one of one, two, three, four, or five, or six of amino acid residues Asn128,
5er184, Leu186,
Asn187, Lys44, and G1u121 of a human IL-34. In any of the aspects above, the
anti-IL-34
antibody may bind to an epitope further comprising at least any one of one,
two, three, four,
or five, or six of amino acid residues Phe40, Asp43, Leu125, G1n189, Thr36,
and Va1185 of a
human IL-34. In some embodiments, the anti-IL-34 antibody binds to amino acids
within
positions 36-44, 121-128, and 184-187 of a human IL-34. In some embodiments,
the anti-IL-
34 antibody inhibits binding between human IL-34 and human CSF-1R. In some
embodiments, the anti-IL-34 antibody neutralizes human IL-34 activity. In some
embodiments, the anti-IL-34 antibody binds to a dimer of human IL-34. In some
embodiments, the anti-IL-34 antibody binds to an epitope that spans both
protomers of human
IL-34. In some embodiments, the anti-IL-34 antibody is a monoclonal antibody.
In some
embodiments, the anti-IL-34 antibody is a human, humanized, or chimeric
antibody. In some
embodiments, the anti-IL-34 antibody is an antibody fragment that binds to
human IL-34. As
used herein, the residue position herein corresponds to the residue position
in SEQ ID NO: 1.
In one aspect, provided herein is an anti-IL-34 antibody that binds to an
epitope comprising at
least one of amino acid residues from G1u103-Leu127 of a human IL-34. In one
aspect,
provided herein is an anti-IL-34 antibody that binds to an epitope comprising
at least any one
of one, two, three, four, five, six, or seven, or eight of amino acid residues
Asp107, Glul 1 1,
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Ser104, G1n120, G1u103, Leu109, Trp116, and Asn61 of a human IL-34. In any of
the aspects
provided above, the antibody may bind to an epitope which further comprises at
least any one
of one, two, three, four, five, six, seven, or eight, or nine of amino acid
residues Pro152,
Va1108, Leu110, G1n106, G1u123, Leu127, Lys117, 11e60 and Lys55 of a human IL-
34. In
some embodiments, the antibody binds to amino acids within positions 55-61,
100-108, 109,
111-127 and 152 of a human IL-34. In some embodiments, the anti-IL-34 antibody
inhibits
binding between human IL-34 and human CSF-1R. In some embodiments, the anti-IL-
34
antibody neutralizes human IL-34 activity. In some embodiments, the anti-IL-34
antibody
binds to a dimer of human IL-34. In some embodiments, the anti-IL-34 antibody
binds to an
epitope that spans both protomers of human IL-34. In some embodiments, the
anti-IL-34
antibody is a monoclonal antibody. In some embodiments, the anti-IL-34
antibody is a
human, humanized, or chimeric antibody. In some embodiments, the anti-IL-34
antibody is an
antibody fragment that binds to human IL-34. As used herein, the residue
position herein
corresponds to the residue position in SEQ ID NO: 1.
In one aspect, the invention provides an anti-IL-34 antibody comprising at
least any one of
one, two, three, four, or five, or six HVRs in any combination as shown in
Figures 10A and
10B. In some embodiments, the anti-IL-34 antibody comprises at least any one
of one, two,
three, four, or five, or six HVRs selected from (a) HVR-H1 comprising an amino
acid
sequence of STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising an amino acid
sequence
RISPYYYYSDYADSVKG (SEQ ID NO: 52); (c) HVR-H3 comprising an amino acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (d) HVR-L1 comprising an amino acid
sequence RASQDVSTAVA (SEQ ID NO: 50); (e) HVR-L2 comprising an amino acid
sequence SASFLYS (SEQ ID NO: 53); and (f) HVR-L3 comprising an amino acid
sequence
QQSFYFPNT (SEQ ID NO: 39). In some embodiments, the anti-IL-34 antibody
comprises at
least any one of one, two, three, four, or five, or six HVRs selected from (a)
HVR-H1
comprising an amino acid sequence STWIH (SEQ ID NO: 59) or GFTFSST (SEQ ID NO:
30) or SSTWIH (SEQ ID NO: 57), (b) HVR-H2 comprising an amino acid sequence
RISPYYYYSDYADSVKG (SEQ ID NO: 52) or PYYYY (SEQ ID NO: 37) or
WVARISPYYYYSD (SEQ ID NO: 62); (c) HVR-H3 comprising an amino acid sequence
GLGKGSKRGAMDY (SEQ ID NO: 33) or ARGLGKGSKRGAMD (SEQ ID NO: 28); (d)
HVR-L1 comprising an amino acid sequence RASQDVSTAVA (SEQ ID NO: 50) or
STAVAWY (SEQ ID NO: 58); (e) HVR-L2 comprising an amino acid sequence SASFLYS
31
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
(SEQ ID NO: 53) or LLIYSASFLY (SEQ ID NO: 34); and (f) HVR-L3 comprising an
amino
acid sequence of QQSFYFPNT (SEQ ID NO: 39) or QQSFYFPN (SEQ ID NO: 38).
In some embodiments, the anti-IL-34 antibody comprises at least any one of
one, two, three,
four, or five, or six HVRs selected from (a) HVR-H1 comprising an amino acid
sequence of
STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising an amino acid sequence
RISPYSGYTNYADSVKG (SEQ ID NO: 51); (c) HVR-H3 comprising an amino acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (d) HVR-L1 comprising an amino acid
sequence of RASQDVSTAVA (SEQ ID NO: 50); (e) HVR-L2 comprising an amino acid
sequence SASFLYS (SEQ ID NO: 53); and (f) HVR-L3 comprising an amino acid
sequence
QQYSDLPYT (SEQ ID NO: 45). In some embodiments, the anti-IL-34 antibody
comprises at
least any one of one, two, three, four, or five, or six HVRs selected from (a)
HVR-H1
comprising an amino acid sequence STWIH (SEQ ID NO: 59) or GFTFSST (SEQ ID NO:
30) or SSTWIH (SEQ ID NO: 57)(b) HVR-H2 comprising an amino acid sequence
RISPYSGYTNYADSVKG (SEQ ID NO: 51) or PYSGY (SEQ ID NO: 36) or
WVARISPYSGYTN (SEQ ID NO: 61); (c) HVR-H3 comprising an amino acid sequence
GLGKGSKRGAMDY (SEQ ID NO: 33) or ARGLGKGSKRGAMD (SEQ ID NO: 28); (d)
HVR-L1 comprising an amino acid sequence of RASQDVSTAVA (SEQ ID NO: 50) or
STAVAWY (SEQ ID NO: 58); (e) HVR-L2 comprising the amino acid sequence of
SASFLYS (SEQ ID NO: 53) or LLIYSASFLY (SEQ ID NO: 34); and (f) HVR-L3
comprising an amino acid sequence QQYSDLPYT (SEQ ID NO: 45) or QQYSDLPY (SEQ
ID NO: 44).
In some embodiments, the anti-IL-34 antibody comprises at least any one of
one, two, three,
four, five, or six HVRs selected from (a) a HVR-H1 comprising an amino acid
sequence
STWIH (SEQ ID NO: 59); (b) a HVR-H2 comprising an amino acid sequence
RISPYYYYSDYADSVKG (SEQ ID NO: 52) or RISPYSGYTNYADSVKG (SEQ ID NO:
51); (c) a HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO:
33) or GINQGSKRGAMDY (SEQ ID NO: 32); (d) a HVR-L1 comprising an amino acid
sequence RASQDVSTAVA (SEQ ID NO: 50); (e) a HVR-L2 comprising an amino acid
sequence SASFLYS (SEQ ID NO: 53); and (f) a HVR-L3 comprising an amino acid
sequence QQSFYFPNT (SEQ ID NO: 39) or QQSYTTPPT (SEQ ID NO: 43) or
QQYTALPYT (SEQ ID NO: 49) or QQYSDLPYT (SEQ ID NO: 45) or QQYSDVPYT
(SEQ ID NO: 47) or QQSRTARPT (SEQ ID NO: 41). In some embodiments, the anti-IL-
34
antibody comprises (a) a HVR-H3 comprising an amino acid sequence
32
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
GLGKGSKRGAMDY (SEQ ID NO: 33) or GINQGSKRGAMDY (SEQ ID NO: 32); (b) a
HVR-L3 comprising an amino acid sequence QQSFYFPNT (SEQ ID NO: 39) or
QQSYTTPPT (SEQ ID NO: 43) or QQYTALPYT (SEQ ID NO: 49) or QQYSDLPYT (SEQ
ID NO: 45) or QQYSDVPYT (SEQ ID NO: 47) or QQSRTARPT (SEQ ID NO: 41); and (c)
a HVR-H2 comprising an amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52)
or RISPYSGYTNYADSVKG (SEQ ID NO: 51). In some embodiments, the anti-IL-34
antibody comprises at least any one of one, two, three, four, or five, or six
HVRs selected
from (a) HVR-H1 comprising an amino acid sequence STWIH (SEQ ID NO: 59) or
GFTFSST (SEQ ID NO: 30) or SSTWIH (SEQ ID NO: 57); (b) HVR-H2 comprising an
amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52) or
RISPYSGYTNYADSVKG (SEQ ID NO: 51) or PYYYY (SEQ ID NO: 37) or PYSGY (SEQ
ID NO: 36) or WVARISPYYYYSD (SEQ ID NO: 62) or WVARISPYSGYTN (SEQ ID NO:
61); and (c) HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID
NO: 33) or GINQGSKRGAMDY (SEQ ID NO: 32) or ARGLGKGSKRGAMD (SEQ ID
NO: 28) or ARGINQGSKRGAMD (SEQ ID NO: 27); (d) HVR-L1 comprising an amino acid
sequence RASQDVSTAVA (SEQ ID NO: 50) or STAVAWY (SEQ ID NO: 58); (e) HVR-
L2 comprising an amino acid sequence SASFLYS (SEQ ID NO: 53) or LLIYSASFLY
(SEQ
ID NO: 34); and (f) HVR-L3 comprising an amino acid sequence QQSFYFPNT (SEQ ID
NO: 39) or QQSYTTPPT (SEQ ID NO: 43) or QQYTALPYT (SEQ ID NO: 49) or
QQYSDLPYT (SEQ ID NO: 45) or QQYSDVPYT (SEQ ID NO: 47) or QQSRTARPT (SEQ
ID NO: 41) or QQSFYFPN (SEQ ID NO: 38) or QQSYTTPP (SEQ ID NO: 42) or
QQYTALPY (SEQ ID NO: 48) or QQYSDLPY (SEQ ID NO: 44) or QQYSDVPY (SEQ ID
NO: 46) or QQSRTARP (SEQ ID NO: 40).
In some embodiments, the anti-IL-34 antibody comprises at least any one of
one, two, three,
four, or five, or six HVRs selected from (a) a HVR-H1 comprising an amino acid
sequence
SNYIH (SEQ ID NO: 55); (b) a HVR-H2 comprising an amino acid sequence
SITPASGDTDYADSVKG (SEQ ID NO: 54); (c) a HVR-H3 comprising an amino acid
sequence SRGAYRFAY (SEQ ID NO: 56); (d) a HVR-L1 comprising an amino acid
sequence RASQDVSTAVA (SEQ ID NO: 50); (e) a HVR-L2 comprising an amino acid
sequence SASFLYS (SEQ ID NO: 53); and (f) a HVR-L3 comprising an amino acid
sequence QQSYTTPPT (SEQ ID NO: 43). In some embodiments, the anti-IL-34
antibody
comprises (a) a HVR-H3 comprising an amino acid sequence SRGAYRFAY (SEQ ID NO:
56); (b) a HVR-L3 comprising an amino acid sequence QQSYTTPPT (SEQ ID NO: 43);
and
33
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
(c) a HVR-H2 comprising an amino acid sequence SITPASGDTDYADSVKG (SEQ ID NO:
54). In some embodiments, the anti-IL-34 antibody comprises at least any one
of one, two,
three, four, or five, or six HVRs selected from (a) HVR-H1 comprising an amino
acid
sequence SNYIH (SEQ ID NO: 55) or GFTFTSN (SEQ ID NO: 31) or TSNYIH (SEQ ID
NO: 60); (b) HVR-H2 comprising an amino acid sequence SITPASGDTDYADSVKG (SEQ
ID NO: 54) or PASGD (SEQ ID NO: 35) or WVASITPASGDTD (SEQ ID NO: 63); (c)
HVR-H3 comprising an amino acid sequence SRGAYRFAY (SEQ ID NO: 56) or
ARSRGAYRFA (SEQ ID NO: 29); (d) HVR-L1 comprising an amino acid sequence
RASQDVSTAVA (SEQ ID NO: 50) or STAVAWY (SEQ ID NO: 58); (e) HVR-L2
comprising an amino acid sequence SASFLYS (SEQ ID NO: 53) or LLIYSASFLY (SEQ
ID
NO: 34); and (f) HVR-L3 comprising an amino acid sequence QQSYTTPPT (SEQ ID
NO:
43) or QQSYTTPP (SEQ ID NO: 42).
In one aspect, the invention provides an anti-IL-34 antibody comprising at
least one, at least
two, or all three VH HVR sequences selected from (a) HVR-H1 comprising an
amino acid
sequence STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising an amino acid sequence
RISPYYYYSDYADSVKG (SEQ ID NO: 52); (c) HVR-H3 comprising an amino acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33). In some embodiments, the anti-IL-34
antibody comprises HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY
(SEQ ID NO: 33). In some embodiments, the anti-IL-34 antibody comprises (a)
HVR-H3
comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33), and (b) a
HVR-L3 comprising an amino acid sequence QQSFYFPNT (SEQ ID NO: 39). In some
embodiments, the anti-IL-34 antibody comprises (a) HVR-H3 comprising an amino
acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (b) HVR-L3 comprising an amino acid
sequence QQSFYFPNT (SEQ ID NO: 39); and (c) HVR-H2 comprising an amino acid
sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52). In some embodiments, the antibody
comprises (a) HVR-H1 comprising an amino acid sequence STWIH (SEQ ID NO: 59);
(b)
HVR-H2 comprising an amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52);
and (c) HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO:
33).
In another aspect, the invention provides an anti-IL-34 antibody comprising at
least one, at
least two, or all three VL HVR sequences selected from (a) HVR-L1 comprising
an amino
acid sequence RASQDVSTAVA (SEQ ID NO: 50); (b) HVR-L2 comprising an amino acid
sequence SASFLYS (SEQ ID NO: 53); and (c) HVR-L3 comprising an amino acid
sequence
34
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
QQSFYFPNT (SEQ ID NO: 39). In some embodiments, the antibody comprises (a) HVR-
L1
comprising an amino acid sequence RASQDVSTAVA (SEQ ID NO: 50); (b) HVR-L2
comprising an amino acid sequence SASFLYS (SEQ ID NO: 53); and (c) HVR-L3
comprising an amino acid sequence QQSFYFPNT (SEQ ID NO: 39).
In another aspect, an anti-IL-34 antibody of the invention comprises (a) a VH
domain
comprising at least one, at least two, or all three VH HVR sequences selected
from (i) HVR-
H1 comprising an amino acid sequence STWIH (SEQ ID NO: 59), (ii) HVR-H2
comprising
an amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52), and (iii) HVR-H3
comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33); and (b) a VL
domain comprising at least one, at least two, or all three VL HVR sequences
selected from (i)
HVR-L1 comprising an amino acid sequence RASQDVSTAVA (SEQ ID NO: 50), (ii) HVR-
L2 comprising an amino acid sequence SASFLYS (SEQ ID NO: 53), and (iii) HVR-L3
comprising an amino acid sequence QQSFYFPNT (SEQ ID NO: 39).
In another aspect, the invention provides an anti-IL-34 antibody comprising
(a) HVR-H1
comprising an amino acid sequence of STWIH (SEQ ID NO: 59); (b) HVR-H2
comprising an
amino acid sequence RISPYYYYSDYADSVKG (SEQ ID NO: 52); (c) HVR-H3 comprising
an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (d) HVR-L1 comprising
an amino acid sequence RASQDVSTAVA (SEQ ID NO: 50); (e) HVR-L2 comprising an
amino acid sequence SASFLYS (SEQ ID NO: 53); and (f) HVR-L3 comprising an
amino
acid sequence QQSFYFPNT (SEQ ID NO: 39).
In one aspect, the invention provides an anti-IL-34 antibody comprising at
least one, at least
two, or all three VH HVR sequences selected from (a) HVR-H1 comprising an
amino acid
sequence STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising an amino acid sequence
RISPYSGYTNYADSVKG (SEQ ID NO: 51); (c) HVR-H3 comprising an amino acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33). In some embodiments, the anti-IL-34
antibody comprises HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY
(SEQ ID NO: 33). In some embodiments, the anti-IL-34 antibody comprises (a)
HVR-H3
comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33), and (b) a
HVR-L3 comprising an amino acid sequence QQYSDLPYT (SEQ ID NO: 45). In some
embodiments, the anti-IL-34 antibody comprises (a) HVR-H3 comprising an amino
acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (b) HVR-L3 comprising an amino acid
sequence QQYSDLPYT (SEQ ID NO: 45); and (c) HVR-H2 comprising an amino acid
sequence RISPYSGYTNYADSVKG (SEQ ID NO: 51). In some embodiments, the antibody
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
comprises (a) HVR-H1 comprising an amino acid sequence STWIH (SEQ ID NO: 59);
(b)
HVR-H2 comprising an amino acid sequence RISPYSGYTNYADSVKG (SEQ ID NO: 51);
and (c) HVR-H3 comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO:
33).
In another aspect, the invention provides an anti-IL-34 antibody comprising at
least one, at
least two, or all three VL HVR sequences selected from (a) HVR-L1 comprising
an amino
acid sequence of RASQDVSTAVA (SEQ ID NO: 50); (b) HVR-L2 comprising an amino
acid sequence SASFLYS (SEQ ID NO: 53); and (c) HVR-L3 comprising an amino acid
sequence QQYSDLPYT (SEQ ID NO: 45). In some embodiments, the antibody
comprises
(a) HVR-L1 comprising an amino acid sequence RASQDVSTAVA (SEQ ID NO: 50); (b)
HVR-L2 comprising an amino acid sequence SASFLYS (SEQ ID NO: 53); and (c) HVR-
L3
comprising an amino acid sequence QQYSDLPYT (SEQ ID NO: 45).
In another aspect, an anti-IL-34 antibody of the invention comprises (a) a VH
domain
comprising at least one, at least two, or all three VH HVR sequences selected
from (i) HVR-
H1 comprising an amino acid sequence STWIH (SEQ ID NO: 59), (ii) HVR-H2
comprising
an amino acid sequence RISPYSGYTNYADSVKG (SEQ ID NO: 51), (iii) HVR-H3
comprising an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33); and (b) a VL
domain comprising at least one, at least two, or all three VL HVR sequences
selected from (i)
HVR-L1 comprising an amino acid sequence of RASQDVSTAVA (SEQ ID NO: 50); (ii)
HVR-L2 comprising an amino acid sequence SASFLYS (SEQ ID NO: 53); and (iii)
HVR-L3
comprising an amino acid sequence QQYSDLPYT (SEQ ID NO: 45).
In another aspect, the invention provides an anti-IL-34 antibody comprising
(a) HVR-H1
comprising an amino acid sequence STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising
an
amino acid sequence RISPYSGYTNYADSVKG (SEQ ID NO: 51); (c) HVR-H3 comprising
an amino acid sequence GLGKGSKRGAMDY (SEQ ID NO: 33); (d) HVR-L1 comprising
an amino acid sequence of RASQDVSTAVA (SEQ ID NO: 50); (e) HVR-L2 comprising
an
amino acid sequence SASFLYS (SEQ ID NO: 53); and (f) HVR-L3 comprising an
amino
acid sequence QQYSDLPYT (SEQ ID NO: 45).
In another aspect, the invention provides an anti-IL-34 antibody comprising
(a) HVR-H1
comprising an amino acid sequence SNWIH (SEQ ID NO:79), (b) HVR-H2 comprising
an
amino acid sequence RISPNSGYTDYADSVKG (SEQ ID NO: 80); (c) HVR-H3 comprising
an amino acid sequence SMRARRGFDY (SEQ ID NO: 81); (d) HVR-L1 comprising an
amino acid sequence of RASQDVSTAVA (SEQ ID NO: 50); (e) HVR-L2 comprising an
36
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
amino acid sequence SASFLYS (SEQ ID NO: 53); and (f) HVR-L3 comprising an
amino
acid sequence QQSYTTPPT (SEQ ID NO: 43).
In another aspect, the invention provides an anti-IL-34 antibody derived from
an anti-IL-34
antibody exemplified herein.
In some embodiments, the anti-IL-34 antibody comprises any one or any
combination of two,
three, four, five, or six of the following HVRs:
HVR-H1: SX1X2IH, wherein X1 is N or T, and X2 is Y or W (SEQ ID NO: 64);
HVR-H2: X1IX2PX3X4X5X6X7X8YADSVKG, wherein X1 is S or R; and X2 is T or
S; X3 is A or Y; X4 is S or Y; X5 is G or Y; X6 is D or Y; X7 is T or S; and
X8 is D or N (SEQ
ID NO: 65);
HVR-H3: SRGAYRFAY (SEQ ID NO: 56), or GX1X2X3GSKRGAMDY, wherein
X1 is L or I; X2 is G or N; X3 is K or Q (SEQ ID NO: 66);
HVR-L1: RASQDVSTAVA (SEQ ID NO: 50);
HVR-L2: SASFLYS (SEQ ID NO: 53);
HVR-L3: QQ X11X2PX3X4X5X6T, wherein the X1 is S or Y; and X2 is Y, T, S, F, or
R; X3 is T, A, D, or Y; X4 is T, L, V, F, or A; X5 is P or R; X6 is P, Y, or N
(SEQ ID NO: 67).
In some embodiments, one or more amino acid residues in HVRs may be
substituted. In
some embodiments, the substitutions are conservative substitutions, as
provided herein.
In any of the above embodiments, an anti-IL-34 antibody is humanized. In some
embodiments, an anti-IL-34 antibody comprises HVRs as in any of the above
embodiments,
and further comprises an acceptor human framework, e.g., a human
immunoglobulin
framework or a human consensus framework. In another embodiment, an anti-IL-34
antibody
comprises HVRs as in any of the above embodiments, and further comprises a VH
comprising an FR1 sequence of SEQ ID NO:17, an FR2 sequence of SEQ ID NO:18,
an FR3
sequence of SEQ ID NO:19, a FR4 sequence of SEQ ID NO:20 and/or a VL
comprising an
FR1 sequence of SEQ ID NO:21, an FR2 sequence of SEQ ID NO:22, an FR3 sequence
of
SEQ ID NO:23, a FR4 sequence of SEQ ID NO:24.
In another aspect, an anti-IL-34 antibody comprises a heavy chain variable
domain (VH)
sequence having at least any one of 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%, or
99%, or 100% sequence identity to the amino acid sequence of SEQ ID NO:3 (VH
amino
acid sequence of antibody 404.33.56 shown Figure 10A). In some embodiments, a
VH
sequence having at least any one of 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, or
98%, or
99% identity contains substitutions (e.g., conservative substitutions),
insertions, or deletions
37
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
relative to the reference sequence, but an anti-IL-34 antibody comprising that
sequence
retains the ability to bind to IL-34. In some embodiments, a total of 1 to 10
amino acids have
been substituted, inserted and/or deleted in SEQ ID NO:3. In some embodiments,
substitutions, insertions, or deletions occur in regions outside the HVRs
(i.e., in the FR).
Optionally, the anti-IL-34 antibody comprises the VH sequence in SEQ ID NO:3,
including
post-translational modifications of that sequence. In a particular embodiment,
the VH
comprises one, two or three HVRs selected from: (a) HVR-H1 comprising an amino
acid
sequence STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising an amino acid sequence
RISPYYYYSDYADSVKG (SEQ ID NO: 52); (c) HVR-H3 comprising an amino acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33).
In another aspect, an anti-IL-34 antibody is provided, wherein the antibody
comprises a light
chain variable domain (VL) having at least any one of 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, 98%, or 99%, or 100% sequence identity to the amino acid sequence of
SEQ ID
NO:4 (VL amino acid sequence of antibody 404.33.56 shown Figure 10B). In some
embodiments, a VL sequence having at least any one of 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, or 98%, or 99% identity contains substitutions (e.g., conservative
substitutions),
insertions, or deletions relative to the reference sequence, but an anti-IL-34
antibody
comprising that sequence retains the ability to bind to IL-34. In some
embodiments, a total of
1 to 10 amino acids have been substituted, inserted and/or deleted in SEQ ID
NO:4. In some
embodiments, the substitutions, insertions, or deletions occur in regions
outside the HVRs
(i.e., in the FRs). Optionally, the anti-IL-34 antibody comprises the VL
sequence in SEQ ID
NO:4, including post-translational modifications of that sequence. In a
particular
embodiment, the VL comprises one, two or three HVRs selected from (a) HVR-L1
comprising an amino acid sequence RASQDVSTAVA (SEQ ID NO: 50); (b) HVR-L2
comprising an amino acid sequence SASFLYS (SEQ ID NO: 53); and (c) HVR-L3
comprising an amino acid sequence QQSFYFPNT (SEQ ID NO: 39).
In another aspect, an anti-IL-34 antibody comprises a heavy chain variable
domain (VH)
sequence having at least any one of 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%, or
99%, or 100% sequence identity to the amino acid sequence of SEQ ID NO:11 (VH
amino
acid sequence of antibody 404.33.12 shown Figure 10A). In some embodiments, a
VH
sequence having at least any one of 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, or
98%, or
99% identity contains substitutions (e.g., conservative substitutions),
insertions, or deletions
relative to the reference sequence, but an anti-IL-34 antibody comprising that
sequence
38
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
retains the ability to bind to IL-34. In some embodiments, a total of 1 to 10
amino acids have
been substituted, inserted and/or deleted in SEQ ID NO:11. In some
embodiments,
substitutions, insertions, or deletions occur in regions outside the HVRs
(i.e., in the FR).
Optionally, the anti-IL-34 antibody comprises the VH sequence in SEQ ID NO:11,
including
post-translational modifications of that sequence. In a particular embodiment,
the VH
comprises one, two or three HVRs selected from: (a) HVR-H1 comprising an amino
acid
sequence of STWIH (SEQ ID NO: 59); (b) HVR-H2 comprising an amino acid
sequence
RISPYSGYTNYADSVKG (SEQ ID NO: 51); (c) HVR-H3 comprising an amino acid
sequence GLGKGSKRGAMDY (SEQ ID NO: 33).
In another aspect, an anti-IL-34 antibody is provided, wherein the antibody
comprises a light
chain variable domain (VL) having at least any one of 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, 98%, or 99%, or 100% sequence identity to the amino acid sequence of
SEQ ID
NO:12 (VL amino acid sequence of antibody 404.33.12 shown Figure 10B). In some
embodiments, a VL sequence having at least any one of 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, or 98%, or 99% identity contains substitutions (e.g., conservative
substitutions),
insertions, or deletions relative to the reference sequence, but an anti-IL-34
antibody
comprising that sequence retains the ability to bind to IL-34. In some
embodiments, a total of
1 to 10 amino acids have been substituted, inserted and/or deleted in SEQ ID
NO:12. In some
embodiments, the substitutions, insertions, or deletions occur in regions
outside the HVRs
(i.e., in the FRs). Optionally, the anti-IL-34 antibody comprises the VL
sequence in SEQ ID
NO:12, including post-translational modifications of that sequence. In a
particular
embodiment, the VL comprises one, two or three HVRs selected from (a) HVR-L1
comprising an amino acid sequence of RASQDVSTAVA (SEQ ID NO: 50); (b) HVR-L2
comprising an amino acid sequence SASFLYS (SEQ ID NO: 53); and (c) HVR-L3
comprising an amino acid sequence QQYSDLPYT (SEQ ID NO: 45).
In another aspect, an anti-IL-34 antibody is provided, wherein the antibody
comprises a VH
as in any of the embodiments provided above, and a VL as in any of the
embodiments
provided above. In some embodiments, the antibody comprises the VH and VL
sequences in
SEQ ID NO:3 and SEQ ID NO:4, respectively, including post-translational
modifications of
those sequences. In some embodiments, the antibody comprises the VH and VL
sequences in
SEQ ID NO: 11 and SEQ ID NO:12, respectively, including post-translational
modifications
of those sequences. In some embodiments, the antibody comprises the VH and VL
sequences
in SEQ ID NO:5 and SEQ ID NO:6, respectively, including post-translational
modifications
39
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
of those sequences. In some embodiments, the antibody comprises the VH and VL
sequences
in SEQ ID NO: 7 and SEQ ID NO:8, respectively, including post-translational
modifications
of those sequences. In some embodiments, the antibody comprises the VH and VL
sequences
in SEQ ID NO: 9 and SEQ ID NO:10, respectively, including post-translational
modifications
of those sequences. In some embodiments, the antibody comprises the VH and VL
sequences
in SEQ ID NO:13 and SEQ ID NO:14, respectively, including post-translational
modifications of those sequences. In some embodiments, the antibody comprises
the VH and
VL sequences in SEQ ID NO:15 and SEQ ID NO:16, respectively, including post-
translational modifications of those sequences. In some embodiments, the
antibody
comprises the VH and VL sequences in SEQ ID NO:77 and SEQ ID NO:78,
respectively,
including post-translational modifications of those sequences.
In a further aspect, the invention provides an antibody that binds to the same
epitope as an
anti-IL-34 antibody provided herein. For example, in some embodiments, an
antibody is
provided that binds to the same epitope as an anti-IL-34 antibody selected
from the of an anti-
IL-34 antibody comprising a VH sequence of SEQ ID NO:3 and a VL sequence of
SEQ ID
NO:4, an anti-IL-34 antibody comprising a VH sequence of SEQ ID NO:11 and a VL
sequence of SEQ ID NO:12, an anti-IL-34 antibody comprising a VH sequence of
SEQ ID
NO:5 and a VL sequence of SEQ ID NO:6, an anti-IL-34 antibody comprising a VH
sequence
of SEQ ID NO:7 and a VL sequence of SEQ ID NO:8, an anti-IL-34 antibody
comprising a
VH sequence of SEQ ID NO:9 and a VL sequence of SEQ ID NO:10, an anti-IL-34
antibody
comprising a VH sequence of SEQ ID NO:13 and a VL sequence of SEQ ID NO:14, or
an
anti-IL-34 antibody comprising a VH sequence of SEQ ID NO:15 and a VL sequence
of SEQ
ID NO:16. In some embodiments, the anti-IL-34 antibody binds to the same
epitope as an
anti-IL-34 antibody comprising a VH sequence of SEQ ID NO:3 and a VL sequence
of SEQ
ID NO:4. In some embodiments, the anti-IL-34 antibody binds to the same
epitope as an anti-
IL-34 antibody comprising a VH sequence of SEQ ID NO:11 and a VL sequence of
SEQ ID
NO:12. In some embodiments, the epitope is a conformational epitope In some
embodiments, the anti-IL-34 antibody binds to the same epitope as an anti-IL-
34 antibody
comprising a VH sequence of SEQ ID NO:77 and a VL sequence of SEQ ID NO:78. In
some
embodiments, the epitope is a conformational epitope. In some embodiments, the
epitope is a
linear epitope.
In a further aspect of the invention, an anti-IL-34 antibody according to any
of the above
embodiments is a monoclonal antibody, including a chimeric, humanized or human
antibody.
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
In some embodiments, an anti-IL-34 antibody is an antibody fragment, e.g., a
Fv, Fab, Fab',
scFv, diabody, or F(ab')2 fragment. In another embodiment, the antibody is a
full length
antibody, e.g., an intact IgG1 or IgG4 antibody or other antibody class or
isotype as defined
herein.
In a further aspect, an anti-IL-34 antibody according to any of the above
embodiments may
incorporate any of the features, singly or in combination, as described in
Sections 1-7 below:
Anti-CSF-1R antibodies
In another aspect, the invention provides isolated antibodies that bind to CSF-
1R (e.g., human
CSF-1R). In one aspect, provided herein is an anti-CSF-1R antibody, which
binds to an
epitope comprising at least any one of one, two, three, four, or five, or six
of amino acid
residues Arg144, G1n248, G1n249, 5er250, Phe252, and Asn254 of human CSF-1R.
In one
aspect, provided herein is an anti-CSF-1R antibody, which binds to an epitope
comprising
amino acid residue Arg144 of human CSF-1R. In one aspect, provided herein is
an anti-CSF-
1R antibody, which binds to an epitope comprising at least any one of one,
two, or three, or
four of amino acid residues Arg144, Arg142, Arg146, and Arg250 of human CSF-
1R. The
anti-CSF-1R antibody of any of the aspects above may bind to an epitope
further comprising
at least one, or two of amino acid residues 5er172 and Arg192 of human CSF-1R.
The anti-
CSF-1R antibody of any of the aspects above may bind to an epitope further
comprising at
least any one of one, two, three, four, or five, or six of amino acid residues
Arg146, Met149,
Arg150, Phe169, I1e170, and G1n173 of human CSF-1R. In some embodiments, the
anti-CSF-
1R antibody binds to amino acids within positions 142-150 and 169-172 of CSF-
1R. As used
herein, the residue position herein corresponds to the residue position in SEQ
ID NO:2. In
some embodiments, the anti-CSF-1R antibody inhibits binding between human IL-
34 and/or
human CSF-1 to human CSF-1R.
In another aspect, provided herein is an anti-CSF-1R antibody, which binds to
an epitope
comprising at least any one of one, two, three, four, or five, or six of amino
acid residues
Arg144, G1n248, G1n249, Ser250, Phe252, and Asn 254 of human CSF-1R. In one
aspect,
provided herein is an anti-CSF-1R antibody, which binds to an epitope
comprising at least
any one of one, two, three, or four, or five of amino acid residues G1n248,
G1n249, Ser250,
Phe252, and Asn254 of human CSF-1R. In one aspect, provided herein is an anti-
CSF-1R
antibody, which binds to an epitope comprising at least any one of one, two,
three, four, or
five, or six of amino acid residues G1n248, G1n249, Ser250, Phe252, Asn254,
and Tyr257 of
41
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
human CSF-1R. The anti-CSF-1R antibody of any of the aspects above may bind to
an
epitope further comprising at least one, at least two, or three of amino acid
residues Pro247,
G1n258, and Lys259 of human CSF-1R. The anti-CSF-1R antibody of any of the
aspects
above may bind to an epitope further comprising at least one, at least two, or
three of amino
acid residues Va1231, Asp251, and Tyr257 of human CSF-1R. In some embodiments,
the
anti-CSF-1R antibody binds to amino acids within positions 231, 248-252 and
254 of CSF-
1R. As used herein, the residue position herein corresponds to the residue
position in SEQ ID
NO:2. In some embodiments, the anti-CSF-1R antibody inhibits binding between
human IL-
34 and/or human CSF-1 to human CSF-1R.
In a further aspect of the invention, an anti-CSF1R antibody according to any
of the above
embodiments is a monoclonal antibody, including a chimeric, humanized or human
antibody.
In some embodiments, anti-CSF-1R antibody is an antibody fragment, e.g., a Fv,
Fab, Fab',
scFv, diabody, or F(ab')2 fragment. In another embodiment, the antibody is a
full length
antibody, e.g., an intact IgG1 or IgG4 antibody or other antibody class or
isotype as defined
herein.
In a further aspect, an anti-CSF-1R antibody according to any of the above
embodiments may
incorporate any of the features, singly or in combination, as described in
Sections 1-7 below:
Antibody Affinity
In some embodiments, an antibody provided herein has a dissociation constant
(Kd) of
< liAM, < 100 nM, < 10 nM, < 1 nM, < 0.1 nM, < 0.01 nM, or < 0.001 nM (e.g.,
10-8M or
less, e.g.,from 10-8M to 10-13M, e.g., from 10-9M to 10-13 M).
In some embodiments, Kd is measured by a radiolabeled antigen binding assay
(RIA)
performed with the Fab version of an antibody of interest and its antigen as
described by the
following assay. Solution binding affinity of Fabs for antigen is measured by
equilibrating
Fab with a minimal concentration of (125I)-labeled antigen in the presence of
a titration series
of unlabeled antigen, then capturing bound antigen with an anti-Fab antibody-
coated plate
(see, e.g., Chen et al., J. Mol. Biol. 293:865-881(1999)). To establish
conditions for the
assay, MICROTITER multi-well plates (Thermo Scientific) are coated overnight
with 5
[tg/ml of a capturing anti-Fab antibody (Cappel Labs) in 50 mM sodium
carbonate (pH 9.6),
and subsequently blocked with 2% (w/v) bovine serum albumin in PBS for two to
five hours
at room temperature (approximately 23 C). In a non-adsorbent plate (Nunc
#269620), 100
pM or 26 pM
[1251]-antigen are mixed with serial dilutions of a Fab of interest (e.g.,
consistent
42
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
with assessment of the anti-VEGF antibody, Fab-12, in Presta et al., Cancer
Res. 57:4593-
4599 (1997)). The Fab of interest is then incubated overnight; however, the
incubation may
continue for a longer period (e.g., about 65 hours) to ensure that equilibrium
is reached.
Thereafter, the mixtures are transferred to the capture plate for incubation
at room
temperature (e.g., for one hour). The solution is then removed and the plate
washed eight
times with 0.1% polysorbate 20 (TWEEN-20 ) in PBS. When the plates have dried,
150
pi/well of scintillant (MICROSCINT-20 TM; Packard) is added, and the plates
are counted on
a TOPCOUNT TM gamma counter (Packard) for ten minutes. Concentrations of each
Fab that
give less than or equal to 20% of maximal binding are chosen for use in
competitive binding
assays.
According to another embodiment, Kd is measured using surface plasmon
resonance assays
using a BIACORE -2000 or a BIACORE c)-3000 (BIAcore, Inc., Piscataway, NJ) at
25 C
with immobilized antigen CM5 chips at ¨10 response units (RU). Briefly,
carboxymethylated
dextran biosensor chips (CM5, BIACORE, Inc.) are activated with N-ethyl-N'- (3-
dimethylaminopropy1)-carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide
(NHS)
according to the supplier's instructions. Antigen is diluted with 10 mM sodium
acetate, pH
4.8, to 5 [tg/ml (-0.2 [tM) before injection at a flow rate of 5 pi/minute to
achieve
approximately 10 response units (RU) of coupled protein. Following the
injection of antigen,
1 M ethanolamine is injected to block unreacted groups. For kinetics
measurements, two-fold
serial dilutions of Fab (0.78 nM to 500 nM) are injected in PBS with 0.05%
polysorbate 20
(TWEEN-20Tm) surfactant (PBST) at 25 C at a flow rate of approximately 25
pi/min.
Association rates (kon) and dissociation rates (koff) are calculated using a
simple one-to-one
Langmuir binding model (BIACORE Evaluation Software version 3.2) by
simultaneously
fitting the association and dissociation sensorgrams. The equilibrium
dissociation constant
(Kd) is calculated as the ratio koff/kon. See, e.g., Chen et al., J. Mol.
Biol. 293:865-881
(1999). If the on-rate exceeds 106 M-1 s-1 by the surface plasmon resonance
assay above,
then the on-rate can be determined by using a fluorescent quenching technique
that measures
the increase or decrease in fluorescence emission intensity (excitation = 295
nm; emission =
340 nm, 16 nm band-pass) at 250C of a 20 nM anti-antigen antibody (Fab form)
in PBS, pH
7.2, in the presence of increasing concentrations of antigen as measured in a
spectrometer,
such as a stop-flow equipped spectrophometer (Aviv Instruments) or a 8000-
series SLM-
AMINCOTm spectrophotometer (ThermoSpectronic) with a stirred cuvette.
43
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
According to another embodiment, the Kd is measured using a BLI assay, for
example, as
described herein.
Antibody Fragments
In some embodiments, an antibody provided herein is an antibody fragment.
Antibody
fragments include, but are not limited to, Fab, Fab', Fab'-SH, F(a1302, Fv,
and scFv
fragments, and other fragments described below. For a review of certain
antibody fragments,
see Hudson et al. Nat. Med. 9:129-134 (2003). For a review of scFy fragments,
see, e.g.,
Pluckthiin, in The Pharmacology of Monoclonal Antibodies, vol. 113, Rosenburg
and Moore
eds., (Springer-Verlag, New York), pp. 269-315 (1994); see also WO 93/16185;
and U.S.
Patent Nos. 5,571,894 and 5,587,458. For discussion of Fab and F(ab)2
fragments
comprising salvage receptor binding epitope residues and having increased in
vivo half-life,
see U.S. Patent No. 5,869,046.
Diabodies are antibody fragments with two antigen-binding sites that may be
bivalent or
bispecific. See, for example, EP 404,097; WO 1993/01161; Hudson et al., Nat.
Med. 9:129-
134 (2003); and Hollinger et al., Proc. Natl. Acad. Sci. USA 90: 6444-6448
(1993).
Triabodies and tetrabodies are also described in Hudson et al., Nat. Med.
9:129-134 (2003).
Single-domain antibodies are antibody fragments comprising all or a portion of
the heavy
chain variable domain or all or a portion of the light chain variable domain
of an antibody. In
some embodiments, a single-domain antibody is a human single-domain antibody
(Domantis,
Inc., Waltham, MA; see, e.g., U.S. Patent No. 6,248,516 B1).
In some embodiments, an antibody fragment is a monovalent antibody that has an
in vivo half
life substantially similar to an intact antibody. For example, such an
antibody fragment may
comprise one antigen binding arm linked to an Fc sequence capable of
conferring in vivo
stability to the fragment. In one embodiment, an antibody of the invention is
a one-armed
antibody as described in W02005/063816. In one embodiment, the one-armed
antibody
comprises Fc mutations constituting "knobs" and "holes" as described in
W02005/063816.
The antibody fragment may also be a "linear antibody", e.g., as described in
U.S. Pat. No.
5,641,870. Such linear antibody fragments may be monospecific or bispecific.
Antibody fragments can be made by various techniques, including but not
limited to
proteolytic digestion of an intact antibody as well as production by
recombinant host cells
(e.g., E. coli or phage), as described herein.
44
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Chimeric and Humanized Antibodies
In some embodiments, an antibody provided herein is a chimeric antibody.
Certain chimeric
antibodies are described, e.g., in U.S. Patent No. 4,816,567; and Morrison et
al., Proc. Natl.
Acad. Sci. USA, 81:6851-6855 (1984)). In one example, a chimeric antibody
comprises a
non-human variable region (e.g., a variable region derived from a mouse, rat,
hamster, rabbit,
or non-human primate, such as a monkey) and a human constant region. In a
further
example, a chimeric antibody is a "class switched" antibody in which the class
or subclass has
been changed from that of the parent antibody. Chimeric antibodies include
antigen-binding
fragments thereof
In some embodiments, a chimeric antibody is a humanized antibody. Typically, a
non-human
antibody is humanized to reduce immunogenicity to humans, while retaining the
specificity
and affinity of the parental non-human antibody. Generally, a humanized
antibody comprises
one or more variable domains in which HVRs, e.g., CDRs, (or portions thereof)
are derived
from a non-human antibody, and FRs (or portions thereof) are derived from
human antibody
sequences. A humanized antibody optionally will also comprise at least a
portion of a human
constant region. In some embodiments, some FR residues in a humanized antibody
are
substituted with corresponding residues from a non-human antibody (e.g., the
antibody from
which the HVR residues are derived), e.g., to restore or improve antibody
specificity or
affinity.
Humanized antibodies and methods of making them are reviewed, e.g., in Almagro
and
Fransson, Front. Biosci. 13:1619-1633 (2008), and are further described, e.g.,
in Riechmann
et al., Nature 332:323-329 (1988); Queen et al., Proc. Nat'l Acad. Sci. USA
86:10029-10033
(1989); US Patent Nos. 5, 821,337, 7,527,791, 6,982,321, and 7,087,409;
Kashmiri et al.,
Methods 36:25-34 (2005) (describing SDR (a-CDR) grafting); Padlan, Mol.
Immunol.
28:489-498 (1991) (describing "resurfacing"); Dall'Acqua et al., Methods 36:43-
60 (2005)
(describing "FR shuffling"); and Osbourn et al., Methods 36:61-68 (2005) and
Klimka et al.,
Br. J. Cancer, 83:252-260 (2000) (describing the "guided selection" approach
to FR
shuffling).
Human framework regions that may be used for humanization include but are not
limited to:
framework regions selected using the "best-fit" method (see, e.g., Sims et
al., J. Immunol.
151:2296 (1993)); framework regions derived from the consensus sequence of
human
antibodies of a particular subgroup of light or heavy chain variable regions
(see, e.g., Carter et
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
al., Proc. Natl. Acad. Sci. USA, 89:4285 (1992); and Presta et al., J.
Immunol., 151:2623
(1993)); human mature (somatically mutated) framework regions or human
germline
framework regions (see, e.g., Almagro and Fransson, Front. Biosci. 13:1619-
1633 (2008));
and framework regions derived from screening FR libraries (see, e.g., Baca et
al., J. Biol.
Chem. 272:10678-10684 (1997) and Rosok et al., J. Biol. Chem. 271:22611-22618
(1996)).
Human Antibodies
In some embodiments, an antibody provided herein is a human antibody. Human
antibodies
can be produced using various techniques known in the art. Human antibodies
are described
generally in van Dijk and van de Winkel, Curr. Opin. Pharmacol. 5: 368-74
(2001) and
Lonberg, Curr. Opin. Immunol. 20:450-459 (2008).
Human antibodies may be prepared by administering an immunogen to a transgenic
animal
that has been modified to produce intact human antibodies or intact antibodies
with human
variable regions in response to antigenic challenge. Such animals typically
contain all or a
portion of the human immunoglobulin loci, which replace the endogenous
immunoglobulin
loci, or which are present extrachromosomally or integrated randomly into the
animal's
chromosomes. In such transgenic mice, the endogenous immunoglobulin loci have
generally
been inactivated. For review of methods for obtaining human antibodies from
transgenic
animals, see Lonberg, Nat. Biotech. 23:1117-1125 (2005). See also, e.g., U.S.
Patent Nos.
6,075,181 and 6,150,584 describing XENOMOUSETm technology; U.S. Patent No.
5,770,429
describing HuMARO technology; U.S. Patent No. 7,041,870 describing K-M MOUSE
technology, and U.S. Patent Application Publication No. US 2007/0061900,
describing
VELociMousEO technology). Human variable regions from intact antibodies
generated by
such animals may be further modified, e.g., by combining with a different
human constant
region.
Human antibodies can also be made by hybridoma-based methods. Human myeloma
and
mouse-human heteromyeloma cell lines for the production of human monoclonal
antibodies
have been described. (See, e.g., Kozbor J., Immunol., 133: 3001 (1984);
Brodeur et al.,
Monoclonal Antibody Production Techniques and Applications, pp. 51-63 (Marcel
Dekker,
Inc., New York, 1987); and Boerner et al., J. Immunol., 147: 86 (1991).)
HUITiall antibodies
generated via human hybridoina tc.sehnology are also described in Li et
al., Proc. Natl.
Acad, Sci. USA. I 03:3557-3562 (2000, Additional methods include those
described, for
example, in U.S. Patent No. 7,189,826 (describing production of monoclonal
human IgM
46
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
antibodies from hybridoma cell lines) and Ni, Xiandai Mianyixue, 26(4):265-268
(2006)
(describing human-human hybridomas). Human hybridoma technology (Trioma
technology)
is also described in Vollmers and Brandlein, Histology and Histopathology,
20(3):927-937
(2005) and Vollmers and Brandlein, Methods and Findings in Experimental and
Clinical
Pharmacology, 27(3):185-91 (2005).
Human antibodies may also be generated by isolating Fv clone variable domain
sequences
selected from human-derived phage display libraries. Such variable domain
sequences may
then be combined with a desired human constant domain. Techniques for
selecting human
antibodies from antibody libraries are described below.
Library-Derived Antibodies
Antibodies of the invention may be isolated by screening combinatorial
libraries for
antibodies with the desired activity or activities. For example, a variety of
methods are
known in the art for generating phage display libraries and screening such
libraries for
antibodies possessing the desired binding characteristics. Such methods are
reviewed, e.g., in
Hoogenboom et al. in Methods in Molecular Biology 178:1-37 (O'Brien et al.,
ed., Human
Press, Totowa, NJ, 2001) and further described, e.g., in the McCafferty et
al., Nature
348:552-554; Clackson et al., Nature 352: 624-628 (1991); Marks et al., J.
Mol. Biol. 222:
581-597 (1992); Marks and Bradbury, in Methods in Molecular Biology 248:161-
175 (Lo,
ed., Human Press, Totowa, NJ, 2003); Sidhu et al., J. Mol. Biol. 338(2): 299-
310 (2004); Lee
et al., J. Mol. Biol. 340(5): 1073-1093 (2004); Fellouse, Proc. Natl. Acad.
Sci. USA 101(34):
12467-12472 (2004); and Lee et al., J. Immunol. Methods 284(1-2): 119-132
(2004).
In certain phage display methods, repertoires of VH and VL genes are
separately cloned by
polymerase chain reaction (PCR) and recombined randomly in phage libraries,
which can
then be screened for antigen-binding phage as described in Winter et al., Ann.
Rev. Immunol.,
12: 433-455 (1994). Phage typically display antibody fragments, either as
single-chain Fv
(scFv) fragments or as Fab fragments. Libraries from immunized sources provide
high-
affinity antibodies to the immunogen without the requirement of constructing
hybridomas.
Alternatively, the naive repertoire can be cloned (e.g., from human) to
provide a single source
of antibodies to a wide range of non-self and also self antigens without any
immunization as
described by Griffiths et al., EMBO J, 12: 725-734 (1993). Finally, naive
libraries can also be
made synthetically by cloning unrearranged V-gene segments from stem cells,
and using PCR
primers containing random sequence to encode the highly variable CDR3 regions
and to
47
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
accomplish rearrangement in vitro, as described by Hoogenboom and Winter, J.
Mol. Biol.,
227: 381-388 (1992). Patent publications describing human antibody phage
libraries include,
for example: US Patent No. 5,750,373, and US Patent Publication Nos.
2005/0079574,
2005/0119455, 2005/0266000, 2007/0117126, 2007/0160598, 2007/0237764,
2007/0292936,
and 2009/0002360.
Antibodies or antibody fragments isolated from human antibody libraries are
considered
human antibodies or human antibody fragments herein.
6. Multispecific Antibodies
Bispecific Antibodies
Bispecific antibodies are monoclonal antibodies that have binding
specificities for two
different antigens. In some embodiments, bispecific antibodies are human or
humanized
antibodies. In some embodiments, one of the binding specificities is for IL-34
(e.g., human
IL-34) and the other is for any other antigen. In some embodiments, bispecific
antibodies may
bind to two different epitopes of IL-34 (e.g., human IL-34). In some
embodiments, bispecific
antibodies comprise a first binding specificity to IL-34 (e.g., human IL-34)
and a second
binding specificity to CSF-1 (e.g., human CSF-1). In some embodiments,
bispecific
antibodies bind to the same epitope on IL-34 as any of the anti-IL-34
antibodies described
herein. In some embodiments, bispecific antibodies comprise at least any one
of one, two,
three, four, or five or six HVRs of any one of the anti-IL-34 antibodies
described herein.
Bispecific antibodies can be prepared as full length antibodies or antibody
fragments (e.g.,
F(a1302 bispecific antibodies).
Methods for making bispecific antibodies are known in the art. Traditionally,
the recombinant
production of bispecific antibodies is based on the co-expression of two
immunoglobulin
heavy chain-light chain pairs, where the two heavy chains have different
specificities
(Milstein and Cuello, Nature 305: 537 (1983)). Because of the random
assortment of
immunoglobulin heavy and light chains, these hybridomas (quadromas) produce a
potential
mixture of 10 different antibody molecules, of which only one has the correct
bispecific
structure. The purification of the correct molecule, which is usually done by
affinity
chromatography steps, is rather cumbersome, and the product yields are low.
Similar
procedures are disclosed in WO 93/08829 published May 13, 1993, and in
Traunecker et al.,
EMBOJ., 10: 3655 (1991).
48
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
According to a different approach, antibody variable domains with the desired
binding
specificities (antibody-antigen combining sites) are fused to immunoglobulin
constant domain
sequences. The fusion, for example, is with an immunoglobulin heavy chain
constant domain,
comprising at least part of the hinge, CH2, and CH3 regions. In some
embodiments, the first
heavy-chain constant region (CH1), containing the site necessary for light
chain binding, is
present in at least one of the fusions. DNAs encoding the immunoglobulin heavy
chain
fusions and, if desired, the immunoglobulin light chain, are inserted into
separate expression
vectors, and are co-transfected into a suitable host organism. This provides
for great
flexibility in adjusting the mutual proportions of the three polypeptide
fragments in
embodiments when unequal ratios of the three polypeptide chains used in the
construction
provide the optimum yields. It is, however, possible to insert the coding
sequences for two or
all three polypeptide chains in one expression vector when the expression of
at least two
polypeptide chains in equal ratios results in high yields or when the ratios
are of no particular
significance.
In some embodiments of this approach, the bispecific antibodies are composed
of a hybrid
immunoglobulin heavy chain with a first binding specificity in one arm, and a
hybrid
immunoglobulin heavy chain-light chain pair (providing a second binding
specificity) in the
other arm. It was found that this asymmetric structure facilitates the
separation of the desired
bispecific compound from unwanted immunoglobulin chain combinations, as the
presence of
an immunoglobulin light chain in only one half of the bispecific molecule
provides for a
facile way of separation. This approach is disclosed in WO 94/04690. For
further details of
generating bispecific antibodies see, for example, Suresh et al., Methods in
Enzymology,
121:210 (1986).
According to another approach, the interface between a pair of antibody
molecules can be
engineered to maximize the percentage of heterodimers which are recovered from
recombinant cell culture. The interface comprises at least a part of the CH3
domain of an
antibody constant domain. In this method, one or more small amino acid side
chains from the
interface of the first antibody molecule are replaced with larger side chains
(e.g., tyrosine or
tryptophan). Compensatory "cavities" of identical or similar size to the large
side chain(s) are
created on the interface of the second antibody molecule by replacing large
amino acid side
chains with smaller ones (e.g., alanine or threonine). This provides a
mechanism for
increasing the yield of the heterodimer over other unwanted end-products such
as
homodimers.
49
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Bispecific antibodies include cross-linked or "heteroconjugate" antibodies.
For example, one
of the antibodies in the heteroconjugate can be coupled to avidin, the other
to biotin. Such
antibodies have, for example, been proposed to target immune system cells to
unwanted cells
(U.S. Patent No. 4,676,980), and for treatment of HIV infection (WO 91/00360,
WO
92/00373, and EP 03089). Heteroconjugate antibodies may be made using any
convenient
cross-linking method. Suitable cross-linking agents are well known in the art,
and are
disclosed in U.S. Patent No. 4,676,980, along with a number of cross-linking
techniques.
Techniques for generating bispecific antibodies from antibody fragments have
also been
described in the literature. For example, bispecific antibodies can be
prepared using chemical
linkage. Brennan et al., Science 229: 81 (1985) describe a procedure wherein
intact antibodies
are proteolytically cleaved to generate F(ab')2' fragments. These fragments
are reduced in the
presence of the dithiol complexing agent sodium arsenite to stabilize vicinal
dithiols and
prevent intermolecular disulfide formation. The Fab' fragments generated are
then converted
to thionitrobenzoate (TNB) derivatives. One of the Fab '-TNB derivatives is
then reconverted
to the Fab'-thiol by reduction with mercaptoethylamine and is mixed with an
equimolar
amount of the other Fab'-TNB derivative to form the bispecific antibody. The
bispecific
antibodies produced can be used as agents for the selective immobilization of
enzymes.
Recent progress has facilitated the direct recovery of Fab'-SH fragments from
E. coli, which
can be chemically coupled to form bispecific antibodies. Shalaby et al., J.
Exp. Med.,175:
217-225 (1992) describe the production of a fully humanized bispecific
antibody F(ab')2
molecule. Each Fab' fragment was separately secreted from E. coli and
subjected to directed
chemical coupling in vitro to form the bispecific antibody. The bispecific
antibody thus
formed was able to bind to cells overexpressing the HER2 receptor and normal
human T
cells, as well as trigger the lytic activity of human cytotoxic lymphocytes
against human
breast tumor targets.
Various techniques for making and isolating bispecific antibody fragments
directly from
recombinant cell culture have also been described. For example, bispecific
antibodies have
been produced using leucine zippers. Kostelny et al., J. Immunol., 148(5):1547-
1553 (1992).
The leucine zipper peptides from the Fos and Jun proteins were linked to the
Fab' portions of
two different antibodies by gene fusion. The antibody homodimers were reduced
at the hinge
region to form monomers and then re-oxidized to form the antibody
heterodimers. This
method can also be utilized for the production of antibody homodimers. The
"diabody"
technology described by Hollinger et al., Proc. Natl. Acad. Sci. USA, 90:6444-
6448 (1993)
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
has provided an alternative mechanism for making bispecific antibody
fragments. The
fragments comprise a heavy-chain variable domain (VH) connected to a light-
chain variable
domain (VL) by a linker which is too short to allow pairing between the two
domains on the
same chain. Accordingly, the VH and VL domains of one fragment are forced to
pair with the
complementary VL and VH domains of another fragment, thereby forming two
antigen-
binding sites. Another strategy for making bispecific antibody fragments by
the use of single-
chain Fv (sFv) dimers has also been reported. See Gruber et al., J. Immunol.,
152:5368
(1994).
According to one embodiment, one polypeptide comprising an antigen binding
domain of this
invention comprises a heterodimerization domain. As used herein,
"heteromultimerization
domain" refers to alterations or additions to a biological molecule so as to
promote
heteromultimer formation and hinder homomultimer formation. Any
heterodimerization
domain having a strong preference for forming heterodimers over homodimers is
within the
scope of the invention. Illustrative examples include but are not limited to,
for example, US
Patent Application 20030078385 (Arathoon et al.; describing knob-into-holes);
W02007147901 (Kjxrgaard et al.; describing ionic interactions); WO 2009089004
(Kannan
et al.; describing electrostatic steering effects); W02011/034605 (Christensen
et al.;
describing coiled coils). See also, for example, Pack, P. & Plueckthun, A.,
Biochemistry 31,
1579-1584 (1992) describing leucine zipper or Pack et al., Bio/Technology 11,
1271-1277
(1993) describing the helix-turn-helix motif The phrase "heteromultimerization
domain" and
"heterodimerization domain" are used interchangeably herein.
The term "knob-into-hole" or "KnH" technology as mentioned herein refers to
the technology
directing the pairing of two polypeptides together in vitro or in vivo by
introducing a
pertuberance (knob) into one polypeptide and a cavity (hole) into the other
polypeptide at an
interface in which they interact. For example, KnHs have been introduced in
the Fc:Fc
binding interfaces, CL:CH1 interfaces or VH/VL interfaces of antibodies (e.g.,
U52007/0178552, WO 96/027011, WO 98/050431and Zhu et al. (1997) Protein
Science
6:781-788).
Further techniques for making multispecific, e.g., bispecific, antibodies
include, but are not
limited to, "knob-in-hole" engineering (see, e.g., U.S. Patent No. 5,731,168),
engineering
using electrostatic steering effects for making antibody Fc-heterodimeric
molecules
(WO 2009/089004A1).
51
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Antibodies with more than two valencies are contemplated. For example,
trispecific
antibodies can be prepared. Tutt et al., J. Immunol. 147: 60 (1991).
Engineered antibodies with three or more functional antigen binding sites,
including
"Octopus antibodies," are also included herein (see, e.g., US 2006/0025576A1).
The antibody or fragment herein also includes a "Dual Acting FAb" or "DAF"
comprising an
antigen binding site that binds to IL-34 as well as another, different antigen
(e.g., CSF-1) (see,
US2008/0069820, for example).
7. Antibody Variants
In some embodiments, amino acid sequence variants of the antibodies provided
herein are
contemplated. For example, it may be desirable to improve the binding affinity
and/or other
biological properties of the antibody. Amino acid sequence variants of an
antibody may be
prepared by introducing appropriate modifications into the nucleotide sequence
encoding the
antibody, or by peptide synthesis. Such modifications include, for example,
deletions from,
and/or insertions into and/or substitutions of residues within the amino acid
sequences of the
antibody. Any combination of deletion, insertion, and substitution can be made
to arrive at
the final construct, provided that the final construct possesses the desired
characteristics, e.g.,
antigen-binding.
Substitution, Insertion, and Deletion Variants
In some embodiments, antibody variants having one or more amino acid
substitutions are
provided. Sites of interest for substitutional mutagenesis include the HVRs
and FRs.
Conservative substitutions are shown in Table 1 under the heading of
"conservative
substitutions." More substantial changes are provided in Table 1 under the
heading of
"exemplary substitutions," and as further described below in reference to
amino acid side
chain classes. Amino acid substitutions may be introduced into an antibody of
interest and
the products screened for a desired activity, e.g., retained/improved antigen
binding,
decreased immunogenicity, or improved ADCC or CDC.
TABLE 1
Original Exemplary
Preferred
Residue Substitutions
Substitutions
Ala (A) Val; Leu; Ile Val
Arg (R) Lys; Gln; Asn Lys
Asn (N) Gln; His; Asp, Lys; Arg Gln
52
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Original Exemplary
Preferred
Residue Substitutions
Substitutions
Asp (D) Glu; Asn Glu
Cys (C) Ser; Ala Ser
Gln (Q) Asn; Glu Asn
Glu (E) Asp; Gln Asp
Gly (G) Ala Ala
His (H) Asn; Gln; Lys; Arg Arg
Ile (I) Leu; Val; Met; Ala; Phe; Norleucine Leu
Leu (L) Norleucine; Ile; Val; Met; Ala; Phe Ile
Lys (K) Arg; Gln; Asn Arg
Met (M) Leu; Phe; Ile Leu
Phe (F) Trp; Leu; Val; Ile; Ala; Tyr Tyr
Pro (P) Ala Ala
Ser (S) Thr Thr
Thr (T) Val; Ser Ser
Trp (W) Tyr; Phe Tyr
Tyr (Y) Trp; Phe; Thr; Ser Phe
Val (V) Ile; Leu; Met; Phe; Ala; Norleucine Leu
Amino acids may be grouped according to common side-chain properties:
(1) hydrophobic: Norleucine, Met, Ala, Val, Leu, Ile;
(2) neutral hydrophilic: Cys, Ser, Thr, Asn, Gln;
(3) acidic: Asp, Glu;
(4) basic: His, Lys, Arg;
(5) residues that influence chain orientation: Gly, Pro;
(6) aromatic: Trp, Tyr, Phe.
Non-conservative substitutions will entail exchanging a member of one of these
classes for
another class.
One type of substitutional variant involves substituting one or more
hypervariable region
residues of a parent antibody (e.g., a humanized or human antibody).
Generally, the resulting
variant(s) selected for further study will have modifications (e.g.,
improvements) in certain
biological properties (e.g., increased affinity, reduced immunogenicity)
relative to the parent
antibody and/or will have substantially retained certain biological properties
of the parent
53
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
antibody. An exemplary substitutional variant is an affinity matured antibody,
which may be
conveniently generated, e.g., using phage display-based affinity maturation
techniques such as
those described herein. Briefly, one or more HVR residues are mutated and the
variant
antibodies displayed on phage and screened for a particular biological
activity (e.g., binding
affinity).
Alterations (e.g., substitutions) may be made in HVRs, e.g., to improve
antibody affinity.
Such alterations may be made in HVR "hotspots," i.e., residues encoded by
codons that
undergo mutation at high frequency during the somatic maturation process (see,
e.g.,
Chowdhury, Methods Mol. Biol. 207:179-196 (2008)), and/or SDRs (a-CDRs), with
the
resulting variant VH or VL being tested for binding affinity. Affinity
maturation by
constructing and reselecting from secondary libraries has been described,
e.g., in
Hoogenboom et al. in Methods in Molecular Biology 178:1-37 (O'Brien et al.,
ed., Human
Press, Totowa, NJ, (2001).) In some embodiments of affinity maturation,
diversity is
introduced into the variable genes chosen for maturation by any of a variety
of methods (e.g.,
error-prone PCR, chain shuffling, or oligonucleotide-directed mutagenesis). A
secondary
library is then created. The library is then screened to identify any antibody
variants with the
desired affinity. Another method to introduce diversity involves HVR-directed
approaches,
in which several HVR residues (e.g., 4-6 residues at a time) are randomized.
HVR residues
involved in antigen binding may be specifically identified, e.g., using
alanine scanning
mutagenesis or modeling. CDR-H3 and CDR-L3 in particular are often targeted.
In some embodiments, substitutions, insertions, or deletions may occur within
one or more
HVRs so long as such alterations do not substantially reduce the ability of
the antibody to
bind antigen. For example, conservative alterations (e.g., conservative
substitutions as
provided herein) that do not substantially reduce binding affinity may be made
in HVRs.
Such alterations may be outside of HVR "hotspots" or SDRs. In some embodiments
of the
variant VH and VL sequences provided above, each HVR either is unaltered, or
contains no
more than one, two or three amino acid substitutions.
A useful method for identification of residues or regions of an antibody that
may be targeted
for mutagenesis is called "alanine scanning mutagenesis" as described by
Cunningham and
Wells (1989) Science, 244:1081-1085. In this method, a residue or group of
target residues
(e.g., charged residues such as arg, asp, his, lys, and glu) are identified
and replaced by a
neutral or negatively charged amino acid (e.g., alanine or polyalanine) to
determine whether
the interaction of the antibody with antigen is affected. Further
substitutions may be
54
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
introduced at the amino acid locations demonstrating functional sensitivity to
the initial
substitutions. Alternatively, or additionally, a crystal structure of an
antigen-antibody
complex to identify contact points between the antibody and antigen. Such
contact residues
and neighboring residues may be targeted or eliminated as candidates for
substitution.
Variants may be screened to determine whether they contain the desired
properties.
Amino acid sequence insertions include amino- and/or carboxyl-terminal fusions
ranging in
length from one residue to polypeptides containing a hundred or more residues,
as well as
intrasequence insertions of single or multiple amino acid residues. Examples
of terminal
insertions include an antibody with an N-terminal methionyl residue. Other
insertional
variants of the antibody molecule include the fusion to the N- or C-terminus
of the antibody
to an enzyme (e.g., for ADEPT) or a polypeptide which increases the serum half-
life of the
antibody.
Glycosylation variants
In some embodiments, an antibody provided herein is altered to increase or
decrease the
extent to which the antibody is glycosylated. Addition or deletion of
glycosylation sites to an
antibody may be conveniently accomplished by altering the amino acid sequence
such that
one or more glycosylation sites is created or removed.
Where the antibody comprises an Fc region, the carbohydrate attached thereto
may be altered.
Native antibodies produced by mammalian cells typically comprise a branched,
biantennary
oligosaccharide that is generally attached by an N-linkage to Asn297 of the
CH2 domain of
the Fc region. See, e.g., Wright et al., TIBTECH 15:26-32 (1997). The
oligosaccharide may
include various carbohydrates, e.g., mannose, N-acetyl glucosamine (G1cNAc),
galactose, and
sialic acid, as well as a fucose attached to a GlcNAc in the "stem" of the
biantennary
oligosaccharide structure. In some embodiments, modifications of the
oligosaccharide in an
antibody of the invention may be made in order to create antibody variants
with certain
improved properties.
In some embodiments, antibody variants are provided having a carbohydrate
structure that
lacks fucose attached (directly or indirectly) to an Fc region. For example,
the amount of
fucose in such antibody may be from 1% to 80%, from 1% to 65%, from 5% to 65%
or from
20% to 40%. The amount of fucose is determined by calculating the average
amount of
fucose within the sugar chain at Asn297, relative to the sum of all
glycostructures attached to
Asn 297 (e.g., complex, hybrid and high mannose structures) as measured by
MALDI-TOF
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
mass spectrometry, as described in WO 2008/077546, for example. Asn297 refers
to the
asparagine residue located at about position 297 in the Fc region (Eu
numbering of Fc region
residues); however, Asn297 may also be located about 3 amino acids upstream
or
downstream of position 297, i.e., between positions 294 and 300, due to minor
sequence
variations in antibodies. Such fucosylation variants may have improved ADCC
function. See,
e.g., U.S. Patent Publication Nos. US 2003/0157108 (Presta, L.); U.S.
2004/0093621 (Kyowa
Hakko Kogyo Co., Ltd). Examples of publications related to "defucosylated" or
"fucose-
deficient" antibody variants include: US 2003/0157108; WO 2000/61739; WO
2001/29246;
US 2003/0115614; US 2002/0164328; US 2004/0093621; US 2004/0132140; US
2004/0110704; US 2004/0110282; US 2004/0109865; WO 2003/085119; WO
2003/084570;
WO 2005/035586; WO 2005/035778; W02005/053742; W02002/031140; Okazaki et al.,
J.
Mol. Biol. 336:1239-1249 (2004); Yamane-Ohnuki et al., Biotech. Bioeng. 87:
614 (2004).
Examples of cell lines capable of producing defucosylated antibodies include
Lec13 CHO
cells deficient in protein fucosylation (Ripka et al., Arch. Biochem. Biophys.
249:533-545
(1986); U.S. Pat Appl No US 2003/0157108 Al, Presta, L; and WO 2004/056312 Al,
Adams
et al., Acta crystallographica Section D, Biological crystallography 66: 213-
221 (2010),
especially at Example 11), and knockout cell lines, such as alpha-1,6-
fucosyltransferase gene,
FUT8, knockout CHO cells (see, e.g., Yamane-Ohnuki et al., Biotech. Bioeng.
87: 614
(2004); Kanda, Y. et al., Biotechnol. Bioeng., 94(4):680-688 (2006); and
W02003/085107).
Antibodies variants are further provided with bisected oligosaccharides, e.g.,
in which a
biantennary oligosaccharide attached to the Fc region of the antibody is
bisected by GlcNAc.
Such antibody variants may have reduced fucosylation and/or improved ADCC
function.
Examples of such antibody variants are described, e.g., in WO 2003/011878
(Jean-Mairet et
al.); U.S. Patent No. 6,602,684 (Umana et al.); and US 2005/0123546 (Umana et
al.).
Antibody variants with at least one galactose residue in the oligosaccharide
attached to the Fc
region are also provided. Such antibody variants may have improved CDC
function. Such
antibody variants are described, e.g., in WO 1997/30087 (Patel et al.); WO
1998/58964
(Raju, S.); and WO 1999/22764 (Raju, S.).
Fc region variants
In some embodiments, one or more amino acid modifications may be introduced
into the Fc
region of an antibody provided herein, thereby generating an Fc region
variant. The Fc region
variant may comprise a human Fc region sequence (e.g., a human IgGl, IgG2,
IgG3 or IgG4
56
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Fc region) comprising an amino acid modification (e.g., a substitution) at one
or more amino
acid positions.
In some embodiments, the invention contemplates an antibody variant that
possesses some
but not all effector functions, which make it a desirable candidate for
applications in which
the half-life of the antibody in vivo is important yet certain effector
functions (such as
complement and ADCC) are unnecessary or deleterious. In vitro and/or in vivo
cytotoxicity
assays can be conducted to confirm the reduction/depletion of CDC and/or ADCC
activities.
For example, Fc receptor (FcR) binding assays can be conducted to ensure that
the antibody
lacks FcyR binding (hence likely lacking ADCC activity), but retains FcRn
binding ability.
The primary cells for mediating ADCC, NK cells, express FcyRIII only, whereas
monocytes
express FcyRI, FcyRII and FcyRIII. FcR expression on hematopoietic cells is
summarized in
Table 3 on page 464 of Ravetch and Kinet, Annu. Rev. Immunol. 9:457-492
(1991). Non-
limiting examples of in vitro assays to assess ADCC activity of a molecule of
interest is
described in U.S. Patent No. 5,500,362 (see, e.g., Hellstrom, I. et al., Proc.
Nat'l Acad. Sci.
USA 83:7059-7063 (1986)) and Hellstrom, I et al., Proc. Nat'l Acad. Sci. USA
82:1499-1502
(1985); 5,821,337 (see Bruggemann, M. et al., J. Exp. Med. 166:1351-1361
(1987)).
Alternatively, non-radioactive assays methods may be employed (see, for
example, ACTITm
non-radioactive cytotoxicity assay for flow cytometry (CellTechnology, Inc.
Mountain View,
CA; and CytoTox 96 non-radioactive cytotoxicity assay (Promega, Madison, WI).
Useful
effector cells for such assays include peripheral blood mononuclear cells
(PBMC) and Natural
Killer (NK) cells. Alternatively, or additionally, ADCC activity of the
molecule of interest
may be assessed in vivo, e.g., in an animal model such as that disclosed in
Clynes et al., Proc.
Nat'l Acad. Sci. USA 95:652-656 (1998). Clq binding assays may also be carried
out to
confirm that the antibody is unable to bind Clq and hence lacks CDC activity.
See, e.g., Clq
and C3c binding ELISA in WO 2006/029879 and WO 2005/100402. To assess
complement
activation, a CDC assay may be performed (see, for example, Gazzano-Santoro et
al., J.
Immunol. Methods 202:163 (1996); Cragg, M.S. et al., Blood 101:1045-1052
(2003); and
Cragg, M.S. and M.J. Glennie, Blood 103:2738-2743 (2004)). FcRn binding and in
vivo
clearance/half-life determinations can also be performed using methods known
in the art (see,
e.g., Petkova, S.B. et al., Int'l. Immunol. 18(12):1759-1769 (2006)).
Antibodies with reduced effector function include those with substitution of
one or more of
Fc region residues 238, 265, 269, 270, 297, 327 and 329 (U.S. Patent No.
6,737,056). Such
Fc mutants include Fc mutants with substitutions at two or more of amino acid
positions 265,
57
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
269, 270, 297 and 327, including the so-called "DANA" Fc mutant with
substitution of
residues 265 and 297 to alanine (US Patent No. 7,332,581).
Certain antibody variants with improved or diminished binding to FcRs are
described. (See,
e.g., U.S. Patent No. 6,737,056; WO 2004/056312, and Shields et al., J. Biol.
Chem. 9(2):
6591-6604 (2001).)
In some embodiments, alterations are made in the Fc region that result in
altered (i.e., either
improved or diminished) Clq binding and/or Complement Dependent Cytotoxicity
(CDC),
e.g., as described in U.S. Patent No. 6,194,551, WO 99/51642, and Idusogie et
al., J.
Immunol. 164: 4178-4184 (2000).
Antibodies with increased half-lives and improved binding to the neonatal Fc
receptor
(FcRn), which is responsible for the transfer of maternal IgGs to the fetus
(Guyer et al., J.
Immunol. 117:587 (1976) and Kim et al., J. Immunol. 24:249 (1994)), are
described in
U52005/0014934A1 (Hinton et al.). Those antibodies comprise an Fc region with
one or
more substitutions therein which improve binding of the Fc region to FcRn.
Such Fc variants
include those with substitutions at one or more of Fc region residues: 238,
256, 265, 272, 286,
303, 305, 307, 311, 312, 317, 340, 356, 360, 362, 376, 378, 380, 382, 413, 424
or 434, e.g.,
substitution of Fc region residue 434 (U.S. Patent No. 7,371,826).
See also Duncan et al., Nature 322:738-40 (1988); U.S. Patent No. 5,648,260;
U.S. Patent
No. 5,624,821; and WO 94/29351 concerning other examples of Fc region
variants.
Cysteine engineered antibody variants
In some embodiments, it may be desirable to create cysteine engineered
antibodies, e.g.,
"thioMAbs," in which one or more residues of an antibody are substituted with
cysteine
residues. In particular embodiments, the substituted residues occur at
accessible sites of the
antibody. By substituting those residues with cysteine, reactive thiol groups
are thereby
positioned at accessible sites of the antibody and may be used to conjugate
the antibody to
other moieties, such as drug moieties or linker-drug moieties, to create an
immunoconjugate,
as described further herein. In some embodiments, any one or more of the
following residues
may be substituted with cysteine: V205 (Kabat numbering) of the light chain;
A118 (EU
numbering) of the heavy chain; and S400 (EU numbering) of the heavy chain Fc
region.
Cysteine engineered antibodies may be generated as described, e.g., in U.S.
Patent No.
7,521,541.
58
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Antibody Derivatives
In some embodiments, an antibody provided herein may be further modified to
contain
additional nonproteinaceous moieties that are known in the art and readily
available. The
moieties suitable for derivatization of the antibody include but are not
limited to water soluble
polymers. Non-limiting examples of water soluble polymers include, but are not
limited to,
polyethylene glycol (PEG), copolymers of ethylene glycol/propylene glycol,
carboxymethylcellulose, dextran, polyvinyl alcohol, polyvinyl pyrrolidone,
poly-1, 3-
dioxolane, poly-1,3,6-trioxane, ethylene/maleic anhydride copolymer,
polyaminoacids (either
homopolymers or random copolymers), and dextran or poly(n-vinyl
pyrrolidone)polyethylene
glycol, propropylene glycol homopolymers, prolypropylene oxide/ethylene oxide
co-
polymers, polyoxyethylated polyols (e.g., glycerol), polyvinyl alcohol, and
mixtures thereof
Polyethylene glycol propionaldehyde may have advantages in manufacturing due
to its
stability in water. The polymer may be of any molecular weight, and may be
branched or
unbranched. The number of polymers attached to the antibody may vary, and if
more than
one polymer are attached, they can be the same or different molecules. In
general, the number
and/or type of polymers used for derivatization can be determined based on
considerations
including, but not limited to, the particular properties or functions of the
antibody to be
improved, whether the antibody derivative will be used in a therapy under
defined conditions,
etc.
In another embodiment, conjugates of an antibody and nonproteinaceous moiety
that may be
selectively heated by exposure to radiation are provided. In some embodiments,
the
nonproteinaceous moiety is a carbon nanotube (Kam et al., Proc. Natl. Acad.
Sci. USA 102:
11600-11605 (2005)). The radiation may be of any wavelength, and includes, but
is not
limited to, wavelengths that do not harm ordinary cells, but which heat the
nonproteinaceous
moiety to a temperature at which cells proximal to the antibody-
nonproteinaceous moiety are
killed.
Recombinant Methods and Compositions
Antibodies may be produced using recombinant methods and compositions, e.g.,
as described
in U.S. Patent No. 4,816,567. In some embodiments, isolated nucleic acid
encoding an anti-
IL-34 antibody, a bispecific anti-IL-34/CSF-1 antibody or an anti-CSF-1R
antibody described
herein is provided. Such nucleic acid may encode an amino acid sequence
comprising the VL
and/or an amino acid sequence comprising the VH of the antibody (e.g., the
light and/or
59
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
heavy chains of the antibody). In some embodiments, one or more vectors (e.g.,
expression
vectors) comprising such nucleic acid are provided. In some embodiments, a
host cell
comprising such nucleic acid is provided. In some embodiments, a host cell
comprises (e.g.,
has been transformed with): (1) a vector comprising a nucleic acid that
encodes an amino acid
sequence comprising the VL of the antibody and an amino acid sequence
comprising the VH
of the antibody, or (2) a first vector comprising a nucleic acid that encodes
an amino acid
sequence comprising the VL of the antibody and a second vector comprising a
nucleic acid
that encodes an amino acid sequence comprising the VH of the antibody. In some
embodiments, the host cell is eukaryotic, e.g., a Chinese Hamster Ovary (CHO)
cell or
lymphoid cell (e.g., YO, NSO, Sp20 cell). In some embodiments, a method of
making an anti-
IL-34 antibody, a bispecific anti-IL-34/CSF-1 antibody or an anti-CSF-1R
antibody is
provided, wherein the method comprises culturing a host cell comprising a
nucleic acid
encoding the antibody, as provided above, under conditions suitable for
expression of the
antibody, and optionally recovering the antibody from the host cell (or host
cell culture
medium).
For recombinant production of an anti-IL-34 antibody, a bispecific anti-IL-
34/CSF-1 antibody
or an anti-CSF-1R antibody, nucleic acid encoding an antibody, e.g., as
described above, is
isolated and inserted into one or more vectors for further cloning and/or
expression in a host
cell. Such nucleic acid may be readily isolated and sequenced using
conventional procedures
(e.g., by using oligonucleotide probes that are capable of binding
specifically to genes
encoding the heavy and light chains of the antibody).
Suitable host cells for cloning or expression of antibody-encoding vectors
include prokaryotic
or eukaryotic cells described herein. For example, antibodies may be produced
in bacteria, in
particular when glycosylation and Fc effector function are not needed. For
expression of
antibody fragments and polypeptides in bacteria, see, e.g., U.S. Patent Nos.
5,648,237,
5,789,199, and 5,840,523. (See also Charlton, Methods in Molecular Biology,
Vol. 248
(B.K.C. Lo, ed., Humana Press, Totowa, NJ, 2003), pp. 245-254, describing
expression of
antibody fragments in E. coli.) After expression, the antibody may be isolated
from the
bacterial cell paste in a soluble fraction and can be further purified.
In addition to prokaryotes, eukaryotic microbes such as filamentous fungi or
yeast are suitable
cloning or expression hosts for antibody-encoding vectors, including fungi and
yeast strains
whose glycosylation pathways have been "humanized," resulting in the
production of an
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
antibody with a partially or fully human glycosylation pattern. See Gerngross,
Nat. Biotech.
22:1409-1414 (2004), and Li et al., Nat. Biotech. 24:210-215 (2006).
Suitable host cells for the expression of glycosylated antibody are also
derived from
multicellular organisms (invertebrates and vertebrates). Examples of
invertebrate cells
include plant and insect cells. Numerous baculoviral strains have been
identified which may
be used in conjunction with insect cells, particularly for transfection of
Spodoptera
frugiperda cells.
Plant cell cultures can also be utilized as hosts. See, e.g., U.S. Patent Nos.
5,959,177,
6,040,498, 6,420,548, 7,125,978, and 6,417,429 (describing PLANTIBODIESTm
technology
for producing antibodies in transgenic plants).
Vertebrate cells may also be used as hosts. For example, mammalian cell lines
that are
adapted to grow in suspension may be useful. Other examples of useful
mammalian host cell
lines are monkey kidney CV1 line transformed by 5V40 (COS-7); human embryonic
kidney
line (293 or 293 cells as described, e.g., in Graham et al., J. Gen Virol.
36:59 (1977)); baby
hamster kidney cells (BHK); mouse sertoli cells (TM4 cells as described, e.g.,
in Mather,
Biol. Reprod. 23:243-251 (1980)); monkey kidney cells (CV1); African green
monkey kidney
cells (VERO-76); human cervical carcinoma cells (HELA); canine kidney cells
(MDCK;
buffalo rat liver cells (BRL 3A); human lung cells (W138); human liver cells
(Hep G2);
mouse mammary tumor (MMT 060562); TRI cells, as described, e.g., in Mather et
al., Annals
N.Y. Acad. Sci. 383:44-68 (1982); MRC 5 cells; and F54 cells. Other useful
mammalian host
cell lines include Chinese hamster ovary (CHO) cells, including DHFR- CHO
cells (Urlaub et
al., Proc. Natl. Acad. Sci. USA 77:4216 (1980)); and myeloma cell lines such
as YO, NSO and
5p2/0. For a review of certain mammalian host cell lines suitable for antibody
production,
see, e.g., Yazaki and Wu, Methods in Molecular Biology, Vol. 248 (B.K.C. Lo,
ed., Humana
Press, Totowa, NJ), pp. 255-268 (2003).
Assays
Anti-IL-34 antibodies, bispecific anti-IL-34/CSF-1 antibodies and anti-CSF-1R
antibodies
provided herein may be identified, screened for, or characterized for their
physical/chemical
properties and/or biological activities by various assays known in the art.
Binding assays and other assays
In one aspect, an antibody of the invention is tested for its antigen binding
activity, e.g., by
known methods such as ELISA, Western blot, etc.
61
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
In another aspect, competition assays may be used to identify an anti-IL-34
antibody or a
bispecific anti-IL-34/CSF-1 antibody that competes with, for example, an anti-
IL-34 antibody
described herein. For example, antibodies that compete with an anti-IL-34
antibody
comprising aVH sequence of SEQ ID NO:5 and a VL sequence of SEQ ID NO:6 for
binding
to IL-34. In some embodiments, such a competing antibody binds to the same
epitope (e.g., a
linear or a conformational epitope) that is bound by, for example, an anti-IL-
34 antibody
comprising aVH sequence of SEQ ID NO:5 and a VL sequence of SEQ ID NO:6.
Detailed
exemplary methods for mapping an epitope to which an antibody binds are
provided in
Morris (1996) "Epitope Mapping Protocols," in Methods in Molecular Biology
vol. 66
(Humana Press, Totowa, NJ).
In an exemplary competition assay, immobilized IL-34 is incubated in a
solution comprising a
first labeled antibody that binds to IL-34 (e.g., an anti-IL-34 antibody
comprising aVH
sequence of SEQ ID NO:5 and a VL sequence of SEQ ID NO:6) and a second
unlabeled
antibody that is being tested for its ability to compete with the first
antibody for binding to IL-
34. The second antibody may be present in a hybridoma supernatant. As a
control,
immobilized IL-34 is incubated in a solution comprising the first labeled
antibody but not the
second unlabeled antibody. After incubation under conditions permissive for
binding of the
first antibody to IL-34, excess unbound antibody is removed, and the amount of
label
associated with immobilized IL-34 is measured. If the amount of label
associated with
immobilized IL-34 is substantially reduced in the test sample relative to the
control sample,
then that indicates that the second antibody is competing with the first
antibody for binding to
IL-34. See Harlow and Lane (1988) Antibodies: A Laboratory Manual ch.14 (Cold
Spring
Harbor Laboratory, Cold Spring Harbor, NY).
In one aspect, assays are provided for identifying anti-IL-34 antibodies,
bispecific anti-IL-
34/CSF-1 antibodies or anti-CSF1R antibodies having biological activities.
Biological
activity may include, e.g., inhibition of proliferation of human peripheral
blood mononuclear
cells (PBMCs), inhibition of binding of IL-34 to CSF-1R, or inhibition of
binding of CSF-1
to CSF-1R . Antibodies having such biological activity in vivo and/or in vitro
are also
provided.
In some embodiments, an antibody of the invention is tested for such
biological activity. For
example, the neutralizing activity of an anti-IL-34 antibody, a bispecific
anti-IL-34/CSF-1
antibody or anti-CSF-1R antibody can be measured using a cell proliferation
assay by
CellTiter-Glo. hIL-34 or mIL-34 is combined with serial dilutions of anti-IL-
34 mAbs,
62
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
bispecific anti-IL-34/CSF-1 antibodies or anti-CSF1 antibodies before adding
onto cells, such
as peripheral blood mononuclear cells (PBMCs). The antibody inhibition
activity is obtained
by measuring RLU after incubating the plates at 37 C for 72 hours. The Half
Maximal
Inhibitory Concentration (IC50), defined as the concentration of antibody
required to yield
half maximal inhibition of IL-34 activity on cells, when IL-34 is present at a
concentration to
elicit 70-80% proliferation response, can be calculated with KaleidaGraph.
Inhibition of binding of IL-34 or CSF-1 to CSF-1R by an antibody provided
herein may be
tested in ELISA assays using immobilized IL-34 or CSF-1 and soluble CSF-1R in
the
presence of serial dilution of the antibody, e.g., an anti-IL-34 antibody,
bispecific IL-34/CSF-
1 antibody or anti-CSF-1 antibody.
Methods and Compositions for Diagnostics and Detection
In some embodiments, any of the anti-IL-34 antibodies and anti-CSF-1R provided
herein is
useful for detecting the presence of IL-34 or CSF-1R in a biological sample.
The term
"detecting" as used herein encompasses quantitative or qualitative detection.
In some
embodiments, a biological sample comprises a cell or tissue.
In some embodiments, anti-IL-34 antibodies for use in a method of diagnosis or
detection is
provided. In a further aspect, a method of detecting the presence of IL-34 in
a biological
sample is provided. In some embodiments, the method comprises contacting the
biological
sample with an anti- IL-34 antibody as described herein under conditions
permissive for
binding of the anti- IL-34 antibody to IL-34, and detecting whether a complex
is formed
between the anti- IL-34 antibody and IL-34. Such method may be an in vitro or
in vivo
method. In some embodiments, an anti- IL-34 antibody is used to select
subjects eligible for
therapy with an anti-IL-34 antibody, e.g., where IL-34 is a biomarker for
selection of patients.
In some embodiments, anti-CSF-1R antibodies for use in a method of diagnosis
or detection
is provided. In a further aspect, a method of detecting the presence of CSF-1R
in a biological
sample is provided. In some embodiments, the method comprises contacting the
biological
sample with an anti- CSF-1R antibody as described herein under conditions
permissive for
binding of the anti-CSF-1R antibody to CSF-1R, and detecting whether a complex
is formed
between the anti-CSF-1R antibody and CSF-1R. Such method may be an in vitro or
in vivo
method. In some embodiments, an anti-CSF-1R antibody is used to select
subjects eligible
for therapy with an anti-CSF-1R antibody, e.g., where CSF-1R is a biomarker
for selection of
patients.
63
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Exemplary disorders that may be diagnosed using an antibody of the invention
include
myeloid pathogenic immunological diseases such as rheumatoid arthritis,
inflammatory bowel
disease, or multiple slerosis.
In some embodiments, labeled anti-IL-34 antibodies and anti-CSF-1R are
provided. Labels
include, but are not limited to, labels or moieties that are detected directly
(such as
fluorescent, chromophoric, electron-dense, chemiluminescent, and radioactive
labels), as well
as moieties, such as enzymes or ligands, that are detected indirectly, e.g.,
through an
enzymatic reaction or molecular interaction. Exemplary labels include, but are
not limited to,
the radioisotopes 32P, 14C, 125-% 1 3H, and 1311, fluorophores such as rare
earth chelates or
fluorescein and its derivatives, rhodamine and its derivatives, dansyl,
umbelliferone,
luceriferases, e.g., firefly luciferase and bacterial luciferase (U.S. Patent
No. 4,737,456),
luciferin, 2,3-dihydrophthalazinediones, horseradish peroxidase (HRP),
alkaline phosphatase,
13-ga1actosidase, glucoamylase, lysozyme, saccharide oxidases, e.g., glucose
oxidase,
galactose oxidase, and glucose-6-phosphate dehydrogenase, heterocyclic
oxidases such as
uricase and xanthine oxidase, coupled with an enzyme that employs hydrogen
peroxide to
oxidize a dye precursor such as HRP, lactoperoxidase, or microperoxidase,
biotin/avidin, spin
labels, bacteriophage labels, stable free radicals, and the like.
Biomarkers for Myeloid and Fibroid Subtypes
In one embodiment, RA patients to be treated with a CSF1-R pathway inhibitor
are those
patients that correspond to the Myeloid or Fibroid subtypes (F1 and/or F2) of
RA. In certain
embodiments, a M subtype therapeutic target is selected from one or a
combination of genes
listed in Table 6 of W02011/028945. In certain embodiments, a M subtype
therapeutic target
is selected from one or a combination of genes listed in Table 2 of
W02011/028945. In
certain embodiments, a M subtype therapeutic target is selected from one or a
combination of
genes listed in Table 11 of W02011/028945. In certain embodiments, a M subtype
therapeutic target is selected from one or a combination of proteins encoded
by one or a
combination of genes listed in Table 6 of W02011/028945. In certain
embodiments, a M
subtype therapeutic target is selected from one or a combination of proteins
encoded by one
or a combination of genes listed in Table 2 of W02011/028945. In certain
embodiments, a M
subtype therapeutic target is selected from one or a combination of proteins
encoded by one
or a combination of genes listed in Table 11 of W02011/028945. In certain
embodiments, a
therapeutic target of M subtype of RA is selected from one or more of CLEC5A,
CLEC7A,
ALCAM, IL1RAP, IRAK1, NRP2, TREM1, and VEGF.
64
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
In another aspect, methods of diagnosing a certain subtype of RA, described
herein as the M
subtype, comprise measuring the gene expression of one or a combination of
genes listed in
Table 6 of W02011/028945, or measuring the amount of protein expressed by one
or a
combination of genes listed in Table 6 of W02011/028945. In certain
embodiments, one or
more of the genes identified in Table 6 of W02011/028945, or proteins encoded
by said
genes, are biomarkers of the M subtype. In certain embodiments, methods of
diagnosing M
subtype RA comprise measuring the gene expression of one or a combination of
genes listed
in Table 2 of W02011/028945, or measuring the protein expressed by one or a
combination
of genes listed in Table 2 of W02011/028945. In certain embodiments, one or
more of the
genes identified in Table 2 of W02011/028945, or proteins encoded by said
genes, are
biomarkers of the M subtype. In certain embodiments, methods of diagnosing M
subtype RA
comprise measuring the gene expression of one or a combination of genes listed
in Table 11
of W02011/028945, or measuring the protein expressed by one or a combination
of genes
listed in Table 11 of W02011/028945. In certain embodiments, one or more of
the genes
identified in Table 11 of W02011/028945, or proteins encoded by said genes,
are biomarkers
of the M subtype. In certain embodiments, methods of diagnosing M subtype of
RA
comprise measuring the gene expression or protein expression of one or more of
ADAM8,
CTSB, CXCL3, ICAM1, IL18BP, IL1B, IL8, MMP12, CCL2, VEGFA, and S100A11.
In certain embodiments, a F2 subtype therapeutic target is selected from one
or a combination
of genes listed in Table 7 of W02011/028945. In certain embodiments, a F2
subtype
therapeutic target is selected from one or a combination of genes listed in
Table 3 of
W02011/028945. In certain embodiments, a F2 subtype therapeutic target is
selected from
one or a combination of genes listed in Table 12 of W02011/028945. In certain
embodiments, a F2 subtype therapeutic target is selected from one or a
combination of
proteins encoded by one or a combination of genes listed in Table 7 of
W02011/028945. In
certain embodiments, a F2 subtype therapeutic target is selected from one or a
combination of
proteins encoded by one or a combination of genes listed in Table 3 of
W02011/028945. In
certain embodiments, a F2 subtype therapeutic target is selected from one or a
combination of
proteins encoded by one or a combination of genes listed in Table 12 of
W02011/028945. In
certain embodiments, a therapeutic target of F2 subtype of RA is selected from
one or more
of IL17D, IL17RC, TIMP3, and TNFRSF11B.
In another aspect, methods of diagnosing a certain subtype of RA, described
herein as the F2
subtype, comprise measuring the gene expression of one or a combination of
genes listed in
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Table 7 of W02011/028945, or measuring the protein expressed by one or a
combination of
genes listed in Table 7 of W02011/028945. In certain embodiments, one or more
of the
genes identified in Table 7 of W02011/028945, or proteins encoded by said
genes, are
biomarkers of the F2 subtype. In certain embodiments, methods of diagnosing F2
subtype
RA comprise measuring the gene expression of one or a combination of genes
listed in Table
3 of W02011/028945, or measuring the protein expressed by one or a combination
of genes
listed in Table 3. In certain embodiments, one or more of the genes identified
in Table 3 of
W02011/028945, or proteins encoded by said genes, are biomarkers of the F2
subtype. In
certain embodiments, methods of diagnosing F2 subtype RA comprise measuring
the gene
expression of one or a combination of genes listed in Table 12 of
W02011/028945, or
measuring the protein expressed by one or a combination of genes listed in
Table 12 of
W02011/028945. In certain embodiments, one or more of the genes identified in
Table 12 of
W02011/028945, or proteins encoded by said genes, are biomarkers of the F2
subtype. In
certain embodiments, methods of diagnosing F2 subtype of RA comprise measuring
the gene
expression or protein expression of one or more of FGF10, FGF18, FGF2, LRP6,
TGFbeta2,
WNT11, BMP6, BTC,CLU, CRLF1, TIMP3, FZD10, FZD7, FZD8, and IL17D.
In certain embodiments, a Fl subtype therapeutic target is selected from one
or a combination
of genes listed in Table 8 of W02011/028945. In certain embodiments, a Fl
subtype
therapeutic target is selected from one or a combination of genes listed in
Table 4 of
W02011/028945. In certain embodiments, a Fl subtype therapeutic target is
selected from
one or a combination of genes listed in Table 13 of W02011/028945. In certain
embodiments, a Fl subtype therapeutic target is selected from one or a
combination of
proteins encoded by one or a combination of genes listed in Table 8 of
W02011/028945. In
certain embodiments, a Fl subtype therapeutic target is selected from one or a
combination of
proteins encoded by one or a combination of genes listed in Table 4 of
W02011/028945. In
certain embodiments, a Fl subtype therapeutic target is selected from one or a
combination of
proteins encoded by one or a combination of genes listed in Table 13 of
W02011/028945. In
certain embodiments, a therapeutic target of Fl subtype of RA is selected from
one or more
of CDH11, ITGAll, and CLEC11A.
In another aspect, methods of diagnosing a certain subtype of RA, described
herein as the Fl
subtype, comprise measuring the gene expression of one or a combination of
genes listed in
Table 8 of W02011/028945, or measuring the protein expressed by one or a
combination of
genes listed in Table 8 of W02011/028945. In certain embodiments, one or more
of the
66
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
genes identified in Table 8 of W02011/028945, or proteins encoded by said
genes, are
biomarkers of the Fl subtype. In certain embodiments, methods of diagnosing Fl
subtype
RA comprises measuring the gene expression of one or a combination of genes
listed in Table
4 of W02011/028945, or measuring the protein expressed by one or a combination
of genes
listed in Table 4 of W02011/028945. In certain embodiments, one or more of the
genes
identified in Table 4 of W02011/028945, or proteins encoded by said genes, are
biomarkers
of the F1 subtype. In certain embodiments, methods of diagnosing Fl subtype RA
comprises
measuring the gene expression of one or a combination of genes listed in Table
13 of
W02011/028945, or measuring the protein expressed by one or a combination of
genes listed
in Table 13 of W02011/028945. In certain embodiments, one or more of the genes
identified
in Table 13, or proteins encoded by said genes, are biomarkers of the Fl
subtype. In certain
embodiments, methods of diagnosing Fl subtype of RA comprise measuring the
gene
expression or protein expression of one or more of ITGAll, MMP11, MMP13,
MMP16,
MMP28, ADAM12, ADAM22, CTSK, CTHRC1, ENPEP, POSTN, ANGPT2, SFRP2, TIE1,
and VWF.
Pharmaceutical Formulations
Pharmaceutical formulations of an anti-IL-34 antibody, a bispecific anti-IL-
34/CSF-1
antibody, or an anti-CSF-1R antibody as described herein are prepared by
mixing such
antibody having the desired degree of purity with one or more optional
pharmaceutically
acceptable carriers (Remington's Pharmaceutical Sciences 16th edition, Osol,
A. Ed. (1980)),
in the form of lyophilized formulations or aqueous solutions. Pharmaceutically
acceptable
carriers are generally nontoxic to recipients at the dosages and
concentrations employed, and
include, but are not limited to: buffers such as phosphate, citrate, and other
organic acids;
antioxidants including ascorbic acid and methionine; preservatives (such as
octadecyldimethylbenzyl ammonium chloride; hexamethonium chloride;
benzalkonium
chloride; benzethonium chloride; phenol, butyl or benzyl alcohol; alkyl
parabens such as
methyl or propyl paraben; catechol; resorcinol; cyclohexanol; 3-pentanol; and
m-cresol); low
molecular weight (less than about 10 residues) polypeptides; proteins, such as
serum albumin,
gelatin, or immunoglobulins; hydrophilic polymers such as
polyvinylpyrrolidone; amino acids
such as glycine, glutamine, asparagine, histidine, arginine, or lysine;
monosaccharides,
disaccharides, and other carbohydrates including glucose, mannose, or
dextrins; chelating
agents such as EDTA; sugars such as sucrose, mannitol, trehalose or sorbitol;
salt-forming
67
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
counter-ions such as sodium; metal complexes (e.g., Zn-protein complexes);
and/or non-ionic
surfactants such as polyethylene glycol (PEG). Exemplary pharmaceutically
acceptable
carriers herein further include insterstitial drug dispersion agents such as
soluble neutral-
active hyaluronidase glycoproteins (sHASEGP), for example, human soluble PH-20
hyaluronidase glycoproteins, such as rHuPH20 (HYLENEX , Baxter International,
Inc.).
Certain exemplary sHASEGPs and methods of use, including rHuPH20, are
described in US
Patent Publication Nos. 2005/0260186 and 2006/0104968. In one aspect, a
sHASEGP is
combined with one or more additional glycosaminoglycanases such as
chondroitinases.
Exemplary lyophilized antibody formulations are described in US Patent No.
6,267,958.
Aqueous antibody formulations include those described in US Patent No.
6,171,586 and
W02006/044908, the latter formulations including a histidine-acetate buffer.
The formulation herein may also contain more than one active ingredients as
necessary for the
particular indication being treated, preferably those with complementary
activities that do not
adversely affect each other. For example, it may be desirable to further
provide an anti-CSF-
1 antibody. Such active ingredients are suitably present in combination in
amounts that are
effective for the purpose intended.
Active ingredients may be entrapped in microcapsules prepared, for example, by
coacervation
techniques or by interfacial polymerization, for example,
hydroxymethylcellulose or gelatin-
microcapsules and poly-(methylmethacylate) microcapsules, respectively, in
colloidal drug
delivery systems (for example, liposomes, albumin microspheres,
microemulsions, nano-
particles and nanocapsules) or in macroemulsions. Such techniques are
disclosed in
Remington's Pharmaceutical Sciences 16th edition, Osol, A. Ed. (1980).
Sustained-release preparations may be prepared. Suitable examples of sustained-
release
preparations include semipermeable matrices of solid hydrophobic polymers
containing the
antibody, which matrices are in the form of shaped articles, e.g., films, or
microcapsules.
The formulations to be used for in vivo administration are generally sterile.
Sterility may be
readily accomplished, e.g., by filtration through sterile filtration
membranes.
Therapeutic Methods and Compositions
Any of the anti-IL-34 antibodies, bispecific anti-IL-34/CSF-1 antibodies or
anti CSF-1R
antibodies provided herein may be used in therapeytic methods.
In one aspect, an anti-IL-34 antibody or an anti-CSF-1R antibody for use as a
medicament is
provided. In further aspects, an anti-IL-34 antibody or an anti-CSF-1R
antibody for use in
68
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
treating myeloid pathogenic immunological diseases is provided. In some
embodiments, the
myeloid pathogenic immunological disease is rheumatoid arthritis, inflammatory
bowel
disease, multiple sclerosis, systemic lupus erythematosus, lupus nephritis,
asthma,
osteoporosis, Paget's disease, atherosclerosis, metabolic syndrome, type II
diabetes, LSDs
(lysosomal storage diseases like but not limited to Cytostinosis, Salic acid
storage disorder,
Gaucher disease), Histyocytosis including but not limited to Rosai-Dorfman
disease,
Faisalabad histiocytosis, H syndrome, pigmented hypertrichosis with insulin
dependent
diabetes (PHID). In some embodiments, an anti-IL-34 antibody or an anti-CSF-1R
antibody
for use in a method of treatment is provided. In some embodiments, the
invention provides
an anti-IL-34 antibody or an anti-CSF-1R antibody for use in a method of
treating an
individual having a myeloid pathogenic immunological disease (e.g., rheumatoid
arthritis,
inflammatory bowel disease or multiple sclerosis) comprising administering to
the individual
an effective amount of the anti-IL-34 antibody or anti-CSF-1R antibody. In
some
embodiments, the method further comprises administering to the individual an
effective
amount of at least one additional therapeutic agent, e.g., as described below.
In some
embodiments, the invention provides an anti-IL-34 antibody or an anti-CSF-1R
antibody for
use in inhibiting binding of IL-34 to CSF-1R. In some embodiments, the
invention provides
an anti-IL-34 antibody or an anti-CSF-1R antibody for use in a method of
inhibiting binding
of IL-34 to CSF-1R in an individual comprising administering to the individual
an effective
amount of the anti-IL-34 antibody or anti-CSF-1R antibody to inhibit binding
of IL-34 to
CSF-1R. In some embodiments, the invention provides an anti-IL-34 antibody or
an anti-
CSF-1R antibody for use in neutralizing activity of IL-34. In some
embodiments, the
invention provides an anti-IL-34 antibody or an anti-CSF-1R antibody for use
in a method of
neutralizing activity of IL-34 in an individual comprising administering to
the individual an
effective amount of the anti-IL-34 antibody or anti-CSF-1R antibody to
neutralize activity of
IL-34. An "individual" according to any of the above embodiments is preferably
a human.
In a further aspect, the invention provides for the use of an anti-IL-34
antibody or an anti-
CSF-1R antibody in the manufacture or preparation of a medicament. In some
embodiments,
the medicament is for treatment of myeloid pathogenic immunological disease.
In some
embodiments, the myeloid pathogenic immunological disease is rheumatoid
arthritis,
inflammatory bowel disease, multiple sclerosis, systemic lupus erythematosus,
lupus
nephritis, asthma, osteoporosis, Paget's disease, atherosclerosis, metabolic
syndrome, type II
diabetes, LSDs (lysosomal storage diseases like but not limited to
Cytostinosis, Salic acid
69
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
storage disorder, Gaucher disease), Histyocytosis including but not limited to
Rosai-Dorfman
disease, Faisalabad histiocytosis, H syndrome, pigmented hypertrichosis with
insulin
dependent diabetes (PHID). In some embodiments, the medicament is for use in a
method of
treating a myeloid pathogenic immunological disease (e.g., rheumatoid
arthritis, inflammatory
bowel disease or multiple sclerosis) comprising administering to an individual
having a
myeloid pathogenic immunological disease (e.g., rheumatoid arthritis,
inflammatory bowel
disease or multiple sclerosis) an effective amount of the medicament. In some
embodiments,
the method further comprises administering to the individual an effective
amount of at least
one additional therapeutic agent, e.g., as described below. In some
embodiments, the
medicament is for inhibiting binding of IL-34 to CSF-1R in an individual. In
some
embodiments, the medicament is for use in a method of inhibiting binding of IL-
34 to CSF-
1R in an individual comprising administering to the individual an effective
amount of the
medicament to inhibit binding of IL-34 to CSF-1R in an individual. In some
embodiments,
the medicament is for neutralizing the activity of IL-34 in an individual. In
some
embodiments, the medicament is for use in a method of neutralizing the
activity of IL-34 in
an individual comprising administering to the individual an effective amount
of the
medicament to neutralize the activity of IL-34 in an individual. An
"individual" according to
any of the above embodiments may be a human.
In a further aspect, the invention provides a method for treating a myeloid
pathogenic
immunological disease. In some embodiments, the myeloid pathogenic
immunological
disease is rheumatoid arthritis, inflammatory bowel disease, multiple
sclerosis, systemic lupus
erythematosus, lupus nephritis, asthma, osteoporosis, Paget's disease,
atherosclerosis,
metabolic syndrome, type II diabetes, LSDs (lysosomal storage diseases like
but not limited
to Cytostinosis, Salic acid storage disorder, Gaucher disease), Histyocytosis
including but not
limited to Rosai-Dorfman disease, Faisalabad histiocytosis, H syndrome,
pigmented
hypertrichosis with insulin dependent diabetes (PHID). In some embodiments,
the method
comprises administering to an individual having a myeloid pathogenic
immunological disease
(e.g., rheumatoid arthritis, inflammatory bowel disease or multiple sclerosis)
an effective
amount of the anti-IL-34 antibody or anti-CSF-1R antibody. In some
embodiments, the
method further comprises administering to the individual an effective amount
of at least one
additional therapeutic agent, as described below. An "individual" according to
any of the
above embodiments may be a human.
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
In a further aspect, the invention provides a method for inhibiting binding of
IL-34 to CSF-1R
in an individual. In some embodiments, the method comprises administering to
the
individual an effective amount of an anti-IL-34 antibody or an anti CSF-1R
antibody to
inhibit binding of IL-34 to CSF-1R in an individual. In a further aspect, the
invention
provides a method for neutralizing activity of IL-34 in an individual. In some
embodiments,
the method comprises administering to the individual an effective amount of an
anti-IL-34
antibody or an anti CSF-1R antibody to neutralize activity of IL-34 in an
individual. In some
embodiments, an "individual" is a human.
In one aspect, a bispecific anti-IL-34/CSF-1 antibody for use as a medicament
is provided. In
further aspects, a bispecific anti-IL-34/CSF-1 antibody for use in treating
myeloid pathogenic
immunological disease is provided. In some embodiments, the myeloid pathogenic
immunological disease is rheumatoid arthritis, inflammatory bowel disease,
multiple
sclerosis, systemic lupus erythematosus, lupus nephritis, asthma,
osteoporosis, Paget's
disease, atherosclerosis, metabolic syndrome, type II diabetes, LSDs
(lysosomal storage
diseases like but not limited to Cytostinosis, Salic acid storage disorder,
Gaucher disease),
Histyocytosis including but not limited to Rosai-Dorfman disease, Faisalabad
histiocytosis, H
syndrome, pigmented hypertrichosis with insulin dependent diabetes (PHID). In
some
embodiments, a bispecific anti-IL-34/CSF-1 antibody for use in a method of
treatment is
provided. In some embodiments, the invention provides a bispecific anti-IL-
34/CSF-1
antibody for use in a method of treating an individual having myeloid
pathogenic
immunological disease (e.g., rheumatoid arthritis, inflammatory bowel disease
or multiple
sclerosis) comprising administering to the individual an effective amount of
the bispecific
anti-IL-34/CSF-1 antibody. In some embodiments, the invention provides a
bispecific anti-
IL-34/CSF-1 antibody for use in inhibiting binding of IL-34 to CSF-1R and
binding of CSF-1
to CSF-1R. In some embodiments, the invention provides a bispecific anti-IL-
34/CSF-1
antibody for use in a method of inhibiting binding of IL-34 to CSF-1R and
binding of CSF-1
to CSF-1R in an individual comprising administering to the individual an
effective amount of
the bispecific anti-IL-34/CSF-1 antibody to inhibit binding of IL-34 to CSF-1R
and binding
of CSF-1 to CSF-1R. In some embodiments, the invention provides a bispecific
anti-IL-
34/CSF-1 antibody for use in neutralizing activity of IL-34 and/or CSF-1. In
some
embodiments, the invention provides a bispecific anti-IL-34/CSF-1 antibody for
use in a
method of neutralizing activity of IL-34 and/or CSF-1 in an individual
comprising
administering to the individual an effective amount of the bispecific anti-IL-
34/CSF-1
71
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
antibody to neutralize activity of IL-34 and/or CSF-1. An "individual"
according to any of the
above embodiments is preferably a human.
In a further aspect, the invention provides for the use of a bispecific anti-
IL-34/CSF-1
antibody in the manufacture or preparation of a medicament. In some
embodiments, the
medicament is for treatment of myeloid pathogenic immunological disease. In
some
embodiments, the myeloid pathogenic immunological disease is rheumatoid
arthritis,
inflammatory bowel disease, multiple sclerosis, systemic lupus erythematosus,
lupus
nephritis, asthma, osteoporosis, Paget's disease, atherosclerosis, metabolic
syndrome, type II
diabetes, LSDs (lysosomal storage diseases like but not limited to
Cytostinosis, Salic acid
storage disorder, Gaucher disease), Histyocytosis including but not limited to
Rosai-Dorfman
disease, Faisalabad histiocytosis, H syndrome, pigmented hypertrichosis with
insulin
dependent diabetes (PHID). In some embodiments, the medicament is for use in a
method of
treating myeloid pathogenic immunological disease (e.g., rheumatoid arthritis,
inflammatory
bowel disease or multiple sclerosis) comprising administering to an individual
having
myeloid pathogenic immunological disease (e.g., rheumatoid arthritis,
inflammatory bowel
disease or multiple sclerosis) an effective amount of the medicament. In some
embodiments,
the medicament is for inhibiting binding of IL-34 to CSF-1R and binding of CSF-
1 to CSF-
1R in an individual. In some embodiments, the medicament is for use in a
method of
inhibiting binding of IL-34 to CSF-1R and binding of CSF-1 to CSF-1R in an
individual
comprising administering to the individual an effective amount of the
medicament to inhibit
binding of IL-34 to CSF-1R and binding of CSF-1 to CSF-1R in an individual. In
some
embodiments, the medicament is for neutralizing the activity of IL-34 and/or
CSF-1 in an
individual. In some embodiments, the medicament is for use in a method of
neutralizing the
activity of IL-34 and/or CSF-1 in an individual comprising administering to
the individual an
effective amount of the medicament to neutralize the activity of IL-34 and/or
CSF-1 in an
individual. An "individual" according to any of the above embodiments may be a
human.
In a further aspect, the invention provides a method for treating a myeloid
pathogenic
immunological disease. In some embodiments, the myeloid pathogenic
immunological
disease is rheumatoid arthritis, inflammatory bowel disease, multiple
sclerosis, systemic lupus
erythematosus, lupus nephritis, asthma, osteoporosis, Paget's disease,
atherosclerosis,
metabolic syndrome, type II diabetes, LSDs (lysosomal storage diseases like
but not limited
to Cytostinosis, Salic acid storage disorder, Gaucher disease), Histyocytosis
including but not
limited to Rosai-Dorfman disease, Faisalabad histiocytosis, H syndrome,
pigmented
72
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
hypertrichosis with insulin dependent diabetes (PHID). In some embodiments,
the method
comprises administering to an individual having a myeloid pathogenic
immunological disease
(e.g., rheumatoid arthritis, inflammatory bowel disease or multiple sclerosis)
an effective
amount of the a bispecific anti-IL-34/CSF-1 antibody. An "individual"
according to any of
the above embodiments may be a human.
In a further aspect, the invention provides a method for inhibiting binding of
IL-34 to CSF-1
and binding of CSF-1 to CSF-1R in an individual. In some embodiments, the
method
comprises administering to the individual an effective amount of a bispecific
anti-IL-34/CSF-
1 antibody to inhibit binding of IL-34 to CSF-1R and binding of CSF-1 to CSF-
1R in an
individual. In a further aspect, the invention provides a method for
neutralizing activity of IL-
34 and/or CSF-1 in an individual. In some embodiments, the method comprises
administering to the individual an effective amount of a bispecific anti-IL-
34/CSF-1 antibody
to neutralize activity of IL-34 and/or IL-34 in an individual. In some
embodiments, an
"individual" is a human.
In a further aspect, the invention provides pharmaceutical formulations
comprising any of the
anti-IL-34 antibodies, bispecific anti-IL-34/CSF-1 antibodies, or anti-CSF-1R
antibodies
provided herein, e.g., for use in any of the above therapeutic methods. In
some embodiments,
a pharmaceutical formulation comprises any of the anti-IL-34 antibodies,
bispecific anti-IL-
34/CSF-1 antibodies, or anti-CSF-1R antibodies provided herein and a
pharmaceutically
acceptable carrier. In another embodiment, a pharmaceutical formulation
comprises any of
the anti-IL-34 antibodies, bispecific anti-IL-34/CSF-1 antibodies, or anti-CSF-
1R antibodies
provided herein and at least one additional therapeutic agent, e.g., as
described below.
Antibodies of the invention can be used either alone or in combination with
other agents in a
therapy. For instance, an antibody of the invention may be co-administered
with at least one
additional therapeutic agent. In some embodiments, an additional therapeutic
agent is an anti-
CSF1-antibody.
Such combination therapies noted above encompass combined administration
(where two or
more therapeutic agents are included in the same or separate formulations),
and separate
administration, in which case, administration of the antibody of the invention
can occur prior
to, simultaneously, and/or following, administration of the additional
therapeutic agent and/or
adjuvant.
In further aspects, an anti-IL-34 antibody and an anti-CSF-1 antibody for use
in treating
myeloid pathogenic immunological disease are provided. In some embodiments,
the myeloid
73
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
pathogenic immunological disease is rheumatoid arthritis, inflammatory bowel
disease,
multiple sclerosis, systemic lupus erythematosus, lupus nephritis, asthma,
osteoporosis,
Paget's disease, atherosclerosis, metabolic syndrome, type II diabetes, LSDs
(lysosomal
storage diseases like but not limited to Cytostinosis, Salic acid storage
disorder, Gaucher
disease), Histyocytosis including but not limited to Rosai-Dorfman disease,
Faisalabad
histiocytosis, H syndrome, pigmented hypertrichosis with insulin dependent
diabetes (PHID).
In some embodiments, an anti-IL-34 antibody and an anti-CSF-1 antibody for use
in a method
of treatment are provided. In some embodiments, the invention provides an anti-
IL-34
antibody and an anti-CSF-1 antibody for use in a method of treating an
individual having
myeloid pathogenic immunological disease (e.g., rheumatoid arthritis,
inflammatory bowel
disease or multiple sclerosis) comprising administering to the individual an
effective amount
of the anti-IL-34 antibody in conjunction with an anti-CSF-1 antibody. In some
embodiments, the invention provides an anti-IL-34 antibody and an anti-CSF-1
antibody for
use in inhibiting binding of IL-34 to CSF-1R and binding of CSF-1 to CSF-1R.
In some
embodiments, the invention provides an anti-IL-34 antibody and an anti-CSF-1
antibody for
use in a method of inhibiting binding of IL-34 to CSF-1R and binding of CSF-1
to CSF-1R in
an individual comprising administering to the individual an effective amount
of the anti-IL-34
antibody in conjunction with an anti-CSF-1 antibody to inhibit binding of IL-
34 to CSF-1R
and binding of CSF-1 to CSF-1R. In some embodiments, the invention provides an
anti-IL-
34 antibody and an anti-CSF-1 antibody for use in neutralizing activity of IL-
34 and/or CSF-
1. In some embodiments, the invention provides an anti-IL-34 antibody and an
anti-CSF-1
antibody for use in a method of neutralizing activity of IL-34 and/or CSF-1 in
an individual
comprising administering to the individual an effective amount of the anti-IL-
34 antibody in
conjunction with an anti-CSF-1 antibody to neutralize activity of IL-34 and/or
CSF-1. An
"individual" according to any of the above embodiments is preferably a human.
In a further aspect, the invention provides a method for treating a myeloid
pathogenic
immunological disease. In some embodiments, the myeloid pathogenic
immunological
disease is rheumatoid arthritis, inflammatory bowel disease, multiple
sclerosis, systemic lupus
erythematosus, lupus nephritis, asthma, osteoporosis, Paget's disease,
atherosclerosis,
metabolic syndrome, type II diabetes, LSDs (lysosomal storage diseases like
but not limited
to Cytostinosis, Salic acid storage disorder, Gaucher disease), Histyocytosis
including but not
limited to Rosai-Dorfman disease, Faisalabad histiocytosis, H syndrome,
pigmented
hypertrichosis with insulin dependent diabetes (PHID). In some embodiments,
the method
74
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
comprises administering to an individual having a myeloid pathogenic
immunological disease
(e.g., rheumatoid arthritis, inflammatory bowel disease or multiple sclerosis)
an effective
amount of an anti-IL-34 antibody in conjunction with an anti-CSF-1 antibody.
An
"individual" according to any of the above embodiments may be a human.
In a further aspect, the invention provides a method for inhibiting binding of
IL-34 to CSF-1R
and binding of CSF-1 to CSF-1R in an individual. In some embodiments, the
method
comprises administering to the individual an effective amount of an anti-IL-34
antibody in
conjunction with an anti CSF-1 antibody to inhibit binding of IL-34 to CSF-1R
and binding
of CSF-1 to CSF-1R in an individual. In some embodiments, an "individual" is a
human.
In a further aspect, the invention provides a method for neutralizing activity
of IL-34 and/or
CSF-1 in an individual. In some embodiments, the method comprises
administering to the
individual an effective amount of an anti-IL-34 antibody in conjunction with
an anti CSF-1
antibody to neutralize activity of IL-34 and/or CSF-1 in an individual. In
some embodiments,
an "individual" is a human.
The anti-IL-34 antibody, anti-CSF-1R antibody, and bispecific anti-IL-34/CSF-1
antibody
described herein may be used for targeting the myeloid stroma of a tumor. In
some
embodiments, the antibody is used for treating cancer (such as colon, lung,
breast, prostate
and uterine cancer, etc.). In some embodiments, the antibody is administered
to an individual
in conjunction with another anti-cancer therapy for treating the cancer in the
individual. In
some embodiments, the anti-cancer therapy is a treatment with Herceptin ,
Avastin , or
Tarceva .
The anti-IL-34 antibody, anti-CSF-1R antibody, and bispecific anti-IL-34/CSF-1
antibody
described herein may be also used for treating lysosomal storage disease
(LSD), and
autoimmune disease including but is not limited to rheumatoid arthritis,
inflammatory bowel
disease (IBD, e.g., Crohn's, ulcerative colitis), multiple sclerosis, vascular
diseases including
but not limited to atherosclerosis, myocardial infarction and angina,
osteoporosis, Alzheimer's
disease, diabetes mellitus (Type 1 and/or Type 2), infectious diseases, and
cancer.
Lysosomal Storage Disease (LSD) is a metabolic disorder that results from
defects in
lysosomal function. Lysosomal storage diseases result when a specific
organelle in the body's
cells ¨ the lysosome ¨ malfunctions. Examples of LSDs include, but are not
limited to,
diseases caused by a protein that is deficient/defective such as,
defective/deficient lysosomal
hydrolases (e.g., sphingolipidoses like gangliosidosis, Gaucher and various
Niemann-Pick
diseases), posttranslationally modified sulfatases (e.g., Multiple sulfatase
deficiency),
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
defective/deficient membrane transport proteins such as nucleotide/nucleoside
transporters or
N-acetylglucosamine-l-phosphate transferase (e.g., mucolipidosis type II and
IIIA),
defective/deficient enzyme protecting proteins such as cathepsin A (e.g., GM2-
AP deficiency,
variant AB, Niemann-Pick disease, type C2, SAP deficiency),
defective/deficient
transmembrane proteins such as M-CSFR, ENT3, NPC 1 and sialin (e.g., Niemann-
Pick
disease, type C 1 , Salla disease). Categories of LSDs include lipid storage
disorders (e.g.,
sphingolipidoses), gangliosidosis (e.g, Tay-Sachs disease), leukodystrophies,
mucopolysaccharidoses (including Hunter syndrome and Hurler disease),
glycoprotein storage
disorders (e.g., Pompe disease) and mucolipidoses.
More common LSDs are known as the following: Activator Deficiency/GM2
Gangliosidosis,
Alpha-mannosidosis, Aspartylglucosaminuria, Cholesteryl ester storage disease,
Chronic
Hexosaminidase A Deficiency, Cystinosis, Danon disease, Fabry disease, Farber
disease,
Fucosidosis, Galactosialidosis, Gaucher Disease (Type I, Type II, Type III),
GM 1
gangliosidosis, (Infantile, Late infantile/Juvenile , Adult/Chronic), I-Cell
1 5 disease/Mucolipidosis II, Infantile Free Sialic Acid Storage
Disease/ISSD, Juvenile
Hexosaminidase A Deficiency, Krabbe disease (Infantile Onset, Late Onset),
Metachromatic
Leukodystrophy, Mucopolysaccharidoses disorders (e.g., Pseudo-Hurler
polydystrophy/Mucolipidosis IIIA, MPSI Hurler Syndrome, MPSI Scheie Syndrome,
MPS I
Hurler-Scheie Syndrome, MPS II Hunter syndrome, Sanfilippo syndrome Type A/MPS
III A,
Sanfilippo syndrome Type B/MPS III B, Sanfilippo syndrome Type C/MPS III C ,
Sanfilippo
syndrome Type D/MPS III D, Morquio Type A/MPS IVA, Morquio Type B/MPS IVB, MPS
IX Hyaluronidase Deficiency, MPS VI Maroteaux-Lamy, MPS VII Sly Syndrome,
Mucolipidosis I/Sialidosis, Mucolipidosis IIIC, Mucolipidosis type IV),
Multiple sulfatase
deficiency, Niemann-Pick Disease (Type A, Type B, Type C), Neuronal Ceroid
Lipofuscinoses (e.g., CLN6 disease - Atypical Late Infantile, Late Onset
variant, Early
Juvenile, Batten-Spielmeyer-Vogt/Juvenile NCL/CLN3 disease, Finnish Variant
Late
Infantile CLN5, Jansky-Bielschowsky disease/Late infantile CLN2/TPP 1 Disease,
Kufs/Adult-onset NCL/CLN4 disease, Northern Epilepsy/variant late infantile
CLN8,
Santavuori-Haltia/Infantile CLN1/PPT disease, Beta-mannosidosis), Pompe
disease/Glycogen
storage disease type II, Pycnodysostosis, Sandhoff disease/Adult Onset/GM2
Gangliosidosis.
Sandhoff disease/GM2 gangliosidosis ¨ Infantile, Sandhoff disease/GM2
gangliosidosis ¨
Juvenile, Schindler disease, Salla disease/Sialic Acid Storage Disease, Tay-
Sachs/GM2
gangliosidosis, and Wolman disease.
76
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
An "autoimmune disease" described herein is a disease or disorder arising from
and directed
against an individual's own tissues or a co-segregate or manifestation thereof
or resulting
condition therefrom. Examples of autoimmune diseases or disorders include, but
are not
limited to arthritis (rheumatoid arthritis such as acute arthritis, chronic
rheumatoid arthritis,
gouty arthritis, acute gouty arthritis, chronic inflammatory arthritis,
degenerative arthritis,
infectious arthritis, Lyme arthritis, proliferative arthritis, psoriatic
arthritis, vertebral arthritis,
and juvenile-onset rheumatoid arthritis, osteoarthritis, arthritis chronica
progrediente, arthritis
deformans, polyarthritis chronica primaria, reactive arthritis, and ankylosing
spondylitis),
inflammatory hyperproliferative skin diseases, psoriasis such as plaque
psoriasis, gutatte
psoriasis, pustular psoriasis, and psoriasis of the nails, dermatitis
including contact dermatitis,
chronic contact dermatitis, allergic dermatitis, allergic contact dermatitis,
dermatitis
herpetiformis, and atopic dermatitis, x-linked hyper IgM syndrome, urticaria
such as chronic
allergic urticaria and chronic idiopathic urticaria, including chronic
autoimmune urticaria,
polymyositis/dermatomyositis, juvenile dermatomyositis, toxic epidermal
necrolysis,
scleroderma (including systemic scleroderma), sclerosis such as systemic
sclerosis, multiple
sclerosis (MS) such as spino-optical MS, primary progressive MS (PPMS), and
relapsing
remitting MS (RRMS), progressive systemic sclerosis, atherosclerosis,
arteriosclerosis,
sclerosis disseminata, and ataxic sclerosis, inflammatory bowel disease (IBD)
(for example,
Crohn's disease, autoimmune-mediated gastrointestinal diseases, colitis such
as ulcerative
colitis, colitis ulcerosa, microscopic colitis, collagenous colitis, colitis
polyposa, necrotizing
enterocolitis, and transmural colitis, and autoimmune inflammatory bowel
disease), pyoderma
gangrenosum, erythema nodosum, primary sclerosing cholangitis, episcleritis),
respiratory
distress syndrome, including adult or acute respiratory distress syndrome
(ARDS), meningitis,
inflammation of all or part of the uvea, iritis, choroiditis, an autoimmune
hematological
disorder, rheumatoid spondylitis, sudden hearing loss, IgE-mediated diseases
such as
anaphylaxis and allergic and atopic rhinitis, encephalitis such as Rasmussen's
encephalitis and
limbic and/or brainstem encephalitis, uveitis, such as anterior uveitis, acute
anterior uveitis,
granulomatous uveitis, nongranulomatous uveitis, phacoantigenic uveitis,
posterior uveitis, or
autoimmune uveitis, glomerulonephritis (GN) with and without nephrotic
syndrome such as
chronic or acute glomerulonephritis such as primary GN, immune-mediated GN,
membranous GN (membranous nephropathy), idiopathic membranous GN or idiopathic
membranous nephropathy, membrano- or membranous proliferative GN (MPGN),
including
Type I and Type II, and rapidly progressive GN, allergic conditions, allergic
reaction, eczema
77
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
including allergic or atopic eczema, asthma such as asthma bronchiale,
bronchial asthma, and
auto-immune asthma, conditions involving infiltration of T cells and chronic
inflammatory
responses, chronic pulmonary inflammatory disease, autoimmune myocarditis,
leukocyte
adhesion deficiency, systemic lupus erythematosus (SLE) or systemic lupus
erythematodes
such as cutaneous SLE, subacute cutaneous lupus erythematosus, neonatal lupus
syndrome
(NLE), lupus erythematosus disseminatus, lupus (including nephritis,
cerebritis, pediatric,
non-renal, extra-renal, discoid, alopecia), juvenile onset (Type I) diabetes
mellitus, including
pediatric insulin-dependent diabetes mellitus (IDDM), adult onset diabetes
mellitus (Type II
diabetes), autoimmune diabetes, idiopathic diabetes insipidus, immune
responses associated
with acute and delayed hypersensitivity mediated by cytokines and T-
lymphocytes,
tuberculosis, sarcoidosis, granulomatosis including lymphomatoid
granulomatosis, Wegener's
granulomatosis, agranulocytosis, vasculitides, including vasculitis (including
large vessel
vasculitis (including polymyalgia rheumatica and giant cell arteritis or
Takayasu's arteritis),
medium vessel vasculitis (including Kawasaki's disease and polyarteritis
nodosa),
microscopic polyarteritis, CNS vasculitis, necrotizing, cutaneous, or
hypersensitivity
vasculitis, systemic necrotizing vasculitis, and ANCA-associated vasculitis,
such as Churg-
Strauss vasculitis or syndrome (CSS)), temporal arteritis, aplastic anemia,
autoimmune
aplastic anemia, Coombs positive anemia, Diamond Blackfan anemia, hemolytic
anemia or
immune hemolytic anemia including autoimmune hemolytic anemia (AIHA),
pernicious
anemia (anemia perniciosa), Addison's disease, pure red cell anemia or aplasia
(PRCA),
Factor VIII deficiency, hemophilia A, autoimmune neutropenia, pancytopenia,
leukopenia,
diseases involving leukocyte diapedesis, CNS inflammatory disorders, multiple
organ injury
syndrome such as those secondary to septicemia, trauma or hemorrhage, antigen-
antibody
complex- mediated diseases, anti-glomerular basement membrane disease, anti-
phospholipid
antibody syndrome, allergic neuritis, Bechet's or Behcet's disease,
Castleman's syndrome,
Goodpasture's syndrome, Reynaud's syndrome, Sjogren's syndrome, Stevens-
Johnson
syndrome, pemphigoid such as pemphigoid bullous and skin pemphigoid, pemphigus
(including pemphigus vulgaris, pemphigus foliaceus, pemphigus mucus-membrane
pemphigoid, and pemphigus erythematosus), autoimmune polyendocrinopathies,
Reiter's
disease or syndrome, immune complex nephritis, antibody-mediated nephritis,
neuromyelitis
optica, polyneuropathies, chronic neuropathy such as IgM polyneuropathies or
IgM-mediated
neuropathy, thrombocytopenia (as developed by myocardial infarction patients,
for example),
including thrombotic thrombocytopenic purpura (TTP) and autoimmune or immune-
mediated
78
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
thrombocytopenia such as idiopathic thrombocytopenic purpura (ITP) including
chronic or
acute ITP, autoimmune disease of the testis and ovary including autoimune
orchitis and
oophoritis, primary hypothyroidism, hypoparathyroidism, autoimmune endocrine
diseases
including thyroiditis such as autoimmune thyroiditis, Hashimoto's disease,
chronic thyroiditis
(Hashimoto's thyroiditis), or subacute thyroiditis, autoimmune thyroid
disease, idiopathic
hypothyroidism, Grave's disease, polyglandular syndromes such as autoimmune
polyglandular syndromes (or polyglandular endocrinopathy syndromes),
paraneoplastic
syndromes, including neurologic paraneoplastic syndromes such as Lambert-Eaton
myasthenic syndrome or Eaton-Lambert syndrome, stiff-man or stiff-person
syndrome,
encephalomyelitis such as allergic encephalomyelitis or encephalomyelitis
allergica and
experimental allergic encephalomyelitis (EAE), myasthenia gravis such as
thymoma-
associated myasthenia gravis, cerebellar degeneration, neuromyotonia,
opsoclonus or
opsoclonus myoclonus syndrome (OMS), and sensory neuropathy, multifocal motor
neuropathy, Sheehan's syndrome, autoimmune hepatitis, chronic hepatitis,
lupoid hepatitis,
giant cell hepatitis, chronic active hepatitis or autoimmune chronic active
hepatitis, lymphoid
interstitial pneumonitis, bronchiolitis obliterans (non-transplant) vs NSIP,
Guillain-Barre
syndrome, Berger's disease (IgA nephropathy), idiopathic IgA nephropathy,
linear IgA
dermatosis, primary biliary cirrhosis, pneumonocirrhosis, autoimmune
enteropathy syndrome,
Celiac disease, Coeliac disease, celiac sprue (gluten enteropathy), refractory
sprue, idiopathic
sprue, cryoglobulinemia, amylotrophic lateral sclerosis (ALS; Lou Gehrig's
disease), coronary
artery disease, autoimmune ear disease such as autoimmune inner ear disease
(AIED),
autoimmune hearing loss, opsoclonus myoclonus syndrome (OMS), polychondritis
such as
refractory or relapsed polychondritis, pulmonary alveolar proteinosis,
amyloidosis, scleritis, a
non-cancerous lymphocytosis, a primary lymphocytosis, which includes
monoclonal B cell
lymphocytosis (e.g., benign monoclonal gammopathy and monoclonal garnmopathy
of
undetermined significance, MGUS), peripheral neuropathy, paraneoplastic
syndrome,
channelopathies such as epilepsy, migraine, arrhythmia, muscular disorders,
deathess,
blindness, periodic paralysis, and channelopathies of the CNS, autism,
inflammatory
myopathy, focal segmental glomerulosclerosis (FSGS), endocrine ophthalmopathy,
uveoretinitis, chorioretinitis, autoimmune hepatological disorder,
flbromyalgia, multiple
endocrine failure, Schmidt's syndrome, adrenalitis, gastric atrophy, presenile
dementia,
demyelinating diseases such as autoimmune demyelinating diseases, diabetic
nephropathy,
Dressler's syndrome, alopecia areata, CREST syndrome (calcinosis, Raynaud's
phenomenon,
79
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
esophageal dysmotility, sclerodactyly, and telangiectasia), male and female
autoimmune
infertility, mixed connective tissue disease, Chagas' disease, rheumatic
fever, recurrent
abortion, farmer's lung, erythema multiforme, post-cardiotomy syndrome,
Cushing's
syndrome, bird-fancier's lung, allergic granulomatous angiitis, benign
lymphocytic angiitis,
Alport's syndrome, alveolitis such as allergic alveolitis and fibrosing
alveolitis, interstitial
lung disease, transfusion reaction, leprosy, malaria, leishmaniasis,
kypanosomiasis,
schistosomiasis, ascariasis, aspergillosis, Sampter's syndrome, Caplan's
syndrome, dengue,
endocarditis, endomyocardial fibrosis, diffuse interstitial pulmonary
fibrosis, interstitial lung
fibrosis, idiopathic pulmonary fibrosis, cystic fibrosis, endophthalmitis,
erythema elevatum et
diutinum, erythroblastosis fetalis, eosinophilic faciitis, Shulman's syndrome,
Felty's
syndrome, flariasis, cyclitis such as chronic cyclitis, heterochronic
cyclitis, iridocyclitis, or
Fuch's cyclitis, Henoch-Schonlein purpura, human immunodeficiency virus (HIV)
infection,
echovirus infection, cardiomyopathy, Alzheimer's disease, parvovirus
infection, rubella virus
infection, post-vaccination syndromes, congenital rubella infection, Epstein-
Barr virus
infection, mumps, Evan's syndrome, autoimmune gonadal failure, Sydenham's
chorea, post-
streptococcal nephritis, thromboangitis ubiterans, thyrotoxicosis, tabes
dorsalis, chorioiditis,
giant cell polymyalgia, endocrine ophthamopathy, chronic hypersensitivity
pneumonitis,
keratoconjunctivitis sicca, epidemic keratoconjunctivitis, idiopathic
nephritic syndrome,
minimal change nephropathy, benign familial and ischemia-reperfusion injury,
retinal
autoimmunity, joint inflammation, bronchitis, chronic obstructive airway
disease, silicosis,
aphthae, aphthous stomatitis, arteriosclerotic disorders, aspermiogenese,
autoimmune
hemolysis, Boeck's disease, cryoglobulinemia, Dupuytren's contracture,
endophthalmia
phacoanaphylactica, enteritis allergica, erythema nodosum leprosum, idiopathic
facial
paralysis, chronic fatigue syndrome, febris rheumatica, Hamman-Rich's disease,
sensoneural
hearing loss, haemoglobinuria paroxysmatica, hypogonadism, ileitis regionalis,
leucopenia,
mononucleosis infectiosa, traverse myelitis, primary idiopathic myxedema,
nephrosis,
ophthalmia symphatica, orchitis granulomatosa, pancreatitis, polyradiculitis
acuta, pyoderma
gangrenosum, Quervain's thyreoiditis, acquired spenic atrophy, infertility due
to
antispermatozoan antobodies, non-malignant thymoma, vitiligo, SCID and Epstein-
Barr
virus- associated diseases, acquired immune deficiency syndrome (AIDS),
parasitic diseases
such as Lesihmania, toxic-shock syndrome, food poisoning, conditions involving
infiltration
of T cells, leukocyte-adhesion deficiency, immune responses associated with
acute and
delayed hypersensitivity mediated by cytokines and T-lymphocytes, diseases
involving
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
leukocyte diapedesis, multiple organ injury syndrome, antigen-antibody complex-
mediated
diseases, antiglomerular basement membrane disease, allergic neuritis,
autoimmune
polyendocrinopathies, oophoritis, primary myxedema, autoimmune atrophic
gastritis,
sympathetic ophthalmia, rheumatic diseases, mixed connective tissue disease,
nephrotic
syndrome, insulitis, polyendocrine failure, peripheral neuropathy, autoimmune
polyglandular
syndrome type I, adult-onset idiopathic hypoparathyroidism (AOIH), alopecia
totalis, dilated
cardiomyopathy, epidermolisis bullosa acquisita (EBA), hemochromatosis,
myocarditis,
nephrotic syndrome, primary sclerosing cholangitis, purulent or nonpurulent
sinusitis, acute
or chronic sinusitis, ethmoid, frontal, maxillary, or sphenoid sinusitis, an
eosinophil-related
disorder such as eosinophilia, pulmonary infiltration eosinophilia,
eosinophilia-myalgia
syndrome, Loffler's syndrome, chronic eosinophilic pneumonia, tropical
pulmonary
eosinophilia, bronchopneumonic aspergillosis, aspergilloma, or granulomas
containing
eosinophils, anaphylaxis, seronegative spondyloarthritides, polyendocrine
autoimmune
disease, sclerosing cholangitis, sclera, episclera, chronic mucocutaneous
candidiasis, Bruton's
syndrome, transient hypogammaglobulinemia of infancy, Wiskott-Aldrich
syndrome, ataxia
telangiectasia, autoimmune disorders associated with collagen disease,
rheumatism,
neurological disease, ischemic re-perfusion disorder, reduction in blood
pressure response,
vascular dysfunction, antgiectasis, tissue injury, cardiovascular ischemia,
hyperalgesia,
cerebral ischemia, and disease accompanying vascularization, allergic
hypersensitivity
disorders, glomerulonephritides, reperfusion injury, reperfusion injury of
myocardial or other
tissues, dermatoses with acute inflammatory components, acute purulent
meningitis or other
central nervous system inflammatory disorders, ocular and orbital inflammatory
disorders,
granulocyte transfusion-associated syndromes, cytokine-induced toxicity, acute
serious
inflammation, chronic intractable inflammation, pyelitis, pneumonocirrhosis,
diabetic
retinopathy, diabetic large-artery disorder, endarterial hyperplasia, peptic
ulcer, valvulitis, and
endometriosis.
An antibody of the invention (and any additional therapeutic agent) can be
administered by
any suitable means, including parenteral, intrapulmonary, and intranasal, and,
if desired for
local treatment, intralesional administration. Parenteral infusions include
intramuscular,
intravenous, intraarterial, intraperitoneal, or subcutaneous administration.
Dosing can be by
any suitable route, e.g., by injections, such as intravenous or subcutaneous
injections,
depending in part on whether the administration is brief or chronic. Various
dosing schedules
81
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
including but not limited to single or multiple administrations over various
time-points, bolus
administration, and pulse infusion are contemplated herein.
Antibodies of the invention would be formulated, dosed, and administered in a
fashion
consistent with good medical practice. Factors for consideration in this
context include the
particular disorder being treated, the particular mammal being treated, the
clinical condition
of the individual patient, the cause of the disorder, the site of delivery of
the agent, the
method of administration, the scheduling of administration, and other factors
known to
medical practitioners. The antibody need not be, but is optionally formulated
with one or
more agents currently used to prevent or treat the disorder in question. The
effective amount
of such other agents depends on the amount of antibody present in the
formulation, the type
of disorder or treatment, and other factors discussed above. These are
generally used in the
same dosages and with administration routes as described herein, or about from
1 to 99% of
the dosages described herein, or in any dosage and by any route that is
empirically/clinically
determined to be appropriate.
For the prevention or treatment of disease, the appropriate dosage of an
antibody of the
invention (when used alone or in combination with one or more other additional
therapeutic
agents) will depend on the type of disease to be treated, the type of
antibody, the severity and
course of the disease, whether the antibody is administered for preventive or
therapeutic
purposes, previous therapy, the patient's clinical history and response to the
antibody, and the
discretion of the attending physician. The antibody is suitably administered
to the patient at
one time or over a series of treatments. Depending on the type and severity of
the disease,
about 1 ig/kg to 15 mg/kg (e.g., 0.1mg/kg-10mg/kg) of antibody can be an
initial candidate
dosage for administration to the patient, whether, for example, by one or more
separate
administrations, or by continuous infusion. One typical daily dosage might
range from about
1 ig/kg to 100 mg/kg or more, depending on the factors mentioned above. For
repeated
administrations over several days or longer, depending on the condition, the
treatment would
generally be sustained until a desired suppression of disease symptoms occurs.
One
exemplary dosage of the antibody would be in the range from about 0.05 mg/kg
to about 10
mg/kg. Thus, one or more doses of about 0.5 mg/kg, 2.0 mg/kg, 4.0 mg/kg or 10
mg/kg (or
any combination thereof) may be administered to the patient. Such doses may be
administered intermittently, e.g., every week or every three weeks (e.g., such
that the patient
receives from about two to about twenty, or e.g., about six doses of the
antibody). An initial
higher loading dose, followed by one or more lower doses may be administered.
An
82
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
exemplary dosing regimen comprises administering. However, other dosage
regimens may
be useful. The progress of this therapy is easily monitored by conventional
techniques and
assays.
Articles of Manufacture or Kit
In another aspect of the invention, an article of manufacture or kit
containing materials useful
for the treatment, prevention and/or diagnosis of the disorders described
above is provided.
The article of manufacture comprises a container and a label or package insert
on or
associated with the container. Suitable containers include, for example,
bottles, vials,
syringes, IV solution bags, etc. The containers may be formed from a variety
of materials
such as glass or plastic. The container holds a composition which is by itself
or combined
with another composition effective for treating, preventing and/or diagnosing
the condition
and may have a sterile access port (for example the container may be an
intravenous solution
bag or a vial having a stopper pierceable by a hypodermic injection needle).
At least one
active agent in the composition is an antibody of the invention. The label or
package insert
indicates that the composition is used for treating the condition of choice.
Moreover, the
article of manufacture may comprise (a) a first container with a composition
contained
therein, wherein the composition comprises an antibody of the invention; and
(b) a second
container with a composition contained therein, wherein the composition
comprises a further
cytotoxic or otherwise therapeutic agent. The article of manufacture in this
embodiment of
the invention may further comprise a package insert indicating that the
compositions can be
used to treat a particular condition. In some embodiments, the article of
manufacture
comprises an anti-IL-34 antibody as described herein. In some embodiments, the
article of
manufacture comprises a bispecific IL-34/CSF-1 antibody as described herein.
In some
embodiments, the article of manufacture comprises an anti-CSF-1R antibody as
described
herein. In some embodiments, the article of manufacture comprises an anti-I-34
as described
herein and an anti-CSF-1 antibody. Alternatively, or additionally, the article
of manufacture
may further comprise a second (or third) container comprising a
pharmaceutically-acceptable
buffer, such as bacteriostatic water for injection (BWFI), phosphate-buffered
saline, Ringer's
solution and dextrose solution. It may further include other materials
desirable from
a commercial and user standpoint, including other buffers, diluents, filters,
needles, and
syringes.
83
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
EXAMPLES
The following are examples of methods and compositions of the invention. It is
understood
that various other embodiments may be practiced, given the general description
provided
above.
Example 1: Structures of IL-34, IL-34/CSF-1R complexes and IL-34/antibody
complexes
Methods
Protein Expression and Purification
The coding sequences of human IL-34 active core (residues N21¨V193 for hIL-34s
and
residues N21-P242 for hIL-34fl) fused with a C-terminal Flag-tag or His6-tag,
and the
extracellular domains of human CSF-1R (residues 20-299 for domains D1¨D3 and
residues
20-512 for domains Dl¨D5) attached with a C-terminal His6-tag, were cloned
into the
pAcGP67 vector (BD Biosciences). Recombinant baculovirus was generated by co-
transecting sf9 cells with the pAcGP67A constructs and linearized baculovirus
DNA in ESF
921 media (Expression Systems LLC, Woodland, CA) using the BaculoGoldTM
Expression
System according to manufacturer's instructions (Pharmingen). Virus was
generated through
three rounds of amplification and 4 ml of the round-3 stock was used to infect
1 liter of
Tni.PRO cells at a density of 2 x 106 cells/ml. Cells were grown 48 h at 27 C
and removed
from the media by centrifugation. Supernatant was removed from cells and
supplemented
with 1 mM NiC12, 5 mM CaC12, in 50 mM Tris-HC1 pH 7.5. The proteins in the
supernatant
were captured by Ni-NTA column (Qiagen) using gravity flow, washed with 30 ml
wash
buffer (50 mM Tris-HC1 pH 7.5, 300 mM NaC1, 10 mM imidazole), and eluted off
the
column with elution buffer (50 mM Tris-HC1 pH 7.5, 300 mM NaC1, 300 mM
imidazole).
Protein was concentrated and was further purified over a size exclusion column
(HiLoad
16/60 Superdex 200, GE Biosciences) equilibrated with 5 mM Tris-HC1 pH 7.5 and
100 mM
NaCl. Fractions containing proteins of interest were analyzed by SDS-PAGE,
pooled, and
concentration was determined by the absorbance at 280 nm.
Analytical Gel Filtration
500 1 protein samples at 1 mg/mL concentration were injected sequentially
into a Superdex
200 10/300 GL column (GE Biosciences) equilibrated with PBS. The same column
was
calibrated by a mixture of protein molecular weight standards (Bio-Rad), and
the elution
84
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
volumes of the proteins of interest were used to estimate their corresponding
molecular
weight.
Isothermal Titration Calorimetty
All protein samples were buffer-exchanged into PBS buffer using a Superdex 200
10/300 GL
column prior to ITC experiments to minimize buffer dilution effects.
Calorimetric titrations
were carried out on a VP-ITC 200 calorimeter (MicroCal, Northampton, MA), and
the data
were processed with MicroCal Origin 7.0 software. The IL-34 or CSF-1 protein,
the injectant,
was added to CSF-1R D1-D3, or CSF-1R D1-D5, over the course of a number of
injections
until the receptors were fully saturated. The CSF-1R D1-D3 or D-D5, the
injectant, was
added to cells containing IL-34, CSF-1, or IL-34/CSF-1R D1-D3 complex over the
course of
a number of injections until the receptors were fully saturated.
Affinity measurements using Bio-Layer Interferometry (BLI)
IL-34 or CSF-1 binding to CSF-1RD1-D3
Binding assays were conducted using an Octet RED384TM BLI instrument
(forteBio).
Biotinylated IL-34 or CSF-1 were immobilized on streptavidin-coated biosensor,
washed, and
transferred into reaction buffer (lx Kinetics assay buffer, forteBio #18-5032)
containing four-
fold serial dilutions of CSF-1RD1-D3 (1600 nM to 25 nM for IL-34 and 3200 nM
to 50 nM
for CSF-1). Association reactions were monitored until the binding reached
steady-state.
Subsequently, the bound materials were transferred into reaction buffer and
dissociation
reactions were monitored to ensure reversible binding between cytokines and
CSF-1RD1-D3.
Association rates (kon) and dissociation rates (koff) were calculated using a
simple single-site
binding equation. The equilibrium dissociation constant (Kd) was calculated as
the ratio koff /
kon. Binding kinetics was measured at 30 C and samples were agitated @ 1000
rpm.
Antibody affinities were also evaluated using BIACORE 3000. The affinity was
generally in
line with the BLI measurement. Anti-IL-34 human IgGs were captured by mouse
anti-human
IgG coated on the CMS sensor chip to achieve approximately 250 response units
(RU). For
kinetics measurements, two-fold serial dilutions of human and mouse IL-34
(3.9nM to
500nM) were injected in PBT buffer (PBS with 0.05% Tween 20) at 25 C with a
flow rate of
30m1/min. Association rates (kon) and dissociation rates (koff) were
calculated using a simple
one-to-one Langmuir binding model (BIAcore Evaluation Software version 3.2).
The
equilibrium dissociation constant (KD) was calculated as the ratio koff/kon.
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Crytallization and Structure Determination
hIL-34s CFlag/YW405.3 Fab
Equal molar ratios of hIL-34s CFlag and YW405.3 Fab were mixed and subjected
to a final
gel filtration step using HiLoad 16/60 Superdex 200 column, and concentrated
to 30 mg/ml
before crystallization trials. Orthorhombic crystals of the hIL-34s
CFlag/YW405.3 Fab
complex were grown at 19 C from Hampton Research Index HT H02 (86) containing
0.2 M
Potassium sodium tartrate tetrahydrate, 20% w/v Polyethylene glycol 3,350.
hIL-34s CFlag
Two dimensional plates of hIL-34s CFlag were obtained at 19 C by mixing equal
volumes
of the protein at 10 mg/ml and reservoir solution containing 0.1 M Hepes pH
7.5, 10% w/v
Polyethylene glycol 6,000, 5% v/v (+/-)-2-Methyl-2,4-pentanediol (Hampton
Research
Crystal Screen HT G06 (78)).
hIL-34s CHis/hCSF-1R D1-D3 CHis
hIL-34s CHis and hCSF-1R Dl-D3 CHis were co-expressed in insect cells and
purified by
nickel-affinity and size exclusion chromatography using HiLoad 16/60 Superdex
200 column.
The complex was concentrated to 20 mg/ml and screened with the sitting-drop
vapor-
diffusion technique. Spindle-shaped crystals of hIL-34s CHis/hCSF-1R D1-D3
CHis
complex grew at 19 C from Qiagen Protein Complex Screen A02 (2) containing
0.1M
CaAcet, 0.1M Mes pH 6, 15% PEG 400.
hIL-34s CFlag/YW404.33.56 Fab
The Fab fragment of YW404.33.56 was mixed with hIL-34s CFlag at a 1:1 molar
ratio and
further purified by a HiLoad 16/60 Superdex 200 column and concentrated to 20
mg/ml. A
single hIL-34s CFlag/YW404.33.56 Fab complex crystal grew after one month at
19 C over
a reservoir solution of 0.2 M Ammonium phosphate monobasic, 0.1 M Tris pH 8.5,
50% AT/AT
(+/-)-2-Methyl-2,4-pentanediol.
Data Collection
All cryostabilized crystals were flash frozen in liquid nitrogen prior to data
collection. All
datasets were collected at ALS BL 5Ø2, using ADSC Q315 CCD detectors. The
diffraction
images were indexed, integrated and scaled using HKL2000 (Otwinowski et al.,
Methods in
86
CA 02861122 2014-07-14
WO 2013/119716 PCT/US2013/024998
Enzymology 276: 307-326 (1997)). A summary of all statistics for data
collection, phasing,
and crystallographic refinement is given in Table 2.
Table 2. X-ray data collection, phasing and refinement statistics
Data collection hIL-34s hIL-34s/hCSF- hIL-34s/YW405.3 hIL-
1R Fab
34s/YW404.33.56
Fab
Wavelength (A)
Space group P21 P3121 C2 P212121
Cell dimension a=57.694A a=b=101.361A a=117.917A a=105.549A
b=77.866A c=175.252A b=181.822A b=122.465A
c=58.083A a=0=90 c=79.254A c=149.155A
a=y=90 y=120.000 a=y=90 a=0=y=90
0=103.245 0=118.268
Resolution (A) 50.0-1.85 (1.92- 50.0-3.0 (3.11- 50.0-2.6
(2.69- 50.0-3.0 (3.11-
Total observations 1.85) 3.00) 2.60) 3.00)
Unique 154925 164357 243871 208888
observations 42509 21502 44009 38972
I/SigI 11.3 (2.0) 23.5 (2.8) 11.0 (2.3) 16.7 (2.2)
Redundancy 3.6 (3.2) 7.6 (7.0) 5.5 (5.4) 5.4 (5.0)
Completeness (%) 99.8 (99.0) 99.8 (99.8) 99.3 (96.9) 99.6 (99.9)
Rsyma (%) 9.3 (51.9) 6.1 (62.7) 11.1 (60.9) 8.6 (62.3)
Refinement
Resolution (A) 50.0-1.85 50.0-3.0 50.0-2.6 50.0-3.0
No. of protein 2614 4724 8729 8948
atoms 78 134 122 111
No. of sugar atoms 486 16 126 50
No of waters 17.4% 28.4% 22.8% 23.5%
Rwork 20.3% 31.3% 25.9% 27.3%
iv
-n0 freeb 0.015 0.004 0.006 0.006
rmsd bond lengths 1.402 0.881 0.979 0.927
rmsd bond angles
Ramachandran
plot
Most favored (%) 93.9 88.2 89.6 90.5
Additional allowed 6.1 10.6 9.9 8.8
(%) 0.0 0.4 0.1 0.7
Generously 0.0 0.8 0.4 0.0
allowed (%)
Disallowed (%)
Values in parentheses represent the highest resolution shell
aRsym(I) = IhklIi l Ii(1i11) - <011(0> l /Ihk1IiIi(hk1) where the summations
are over i
observations of each reflections and all hkl. <I(hk1)> is the average
intensity of the i
observations. Rwork =1F(obs) - F(ca1c)1/F(obs)
bRfree is calculated for 5% of randomly selected reflections not used in the
refinement
87
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Structure Determination and Refinement
Complete datasets to 1.85 A, 3.0 A, 2.6 A, and 3.0 A were collected from
single crystals of h-
IL-34s, hIL-34s/CSF-1R D1-D3 complex, hIL-34s/YW405.3 Fab complex, and hIL-
34s/YW404.33.56 Fab complex, respectively (Table 2).
hIL-34s CFlag/YW405.3 Fab and hIL-34s CFlag
The hIL-34s CFlag/YW405.3 Fab complex structure was solved by molecular
replacement
using the program PHASER (Storoni et al., Acta crystallographica Section D,
Biological
crystallography 60: 432-438 (2004)) with search models generated from a single
Fab (Protein
Data Bank (PDB) accession number 2QQN) by searching for the Fv and Fc region
of the Fab
sequentially. A single unambiguous solution including 2 Fabs was found
revealing very
limited electron density for IL-34 outside the CDR regions of the Fab. The
phases from this
initial solution were drastically improved by imposing solvent flattening, 2-
fold NCS
averaging using prime-and-switch map plot routine in PHENIX AutoBuild (Adams
et al.,
Acta crystallographica Section D, Biological crystallography 66: 213-221
(2010)). The
resulting map was of sufficient high quality to allow manual tracing of the
entire backbone of
IL-34 in Coot. This partial IL-34 model was fed into the 1.85 A resolution
dataset of hIL-
34s CFlag by molecular replacement and subject to model rebuilding using Coot
(Emsley et
al., Acta crystallographica Section D, Biological crystallography 66: 486-501
(2010)), and
refinement using Refmac (Murshudov et al., Acta crystallographica Section D,
Biological
crystallography 53: 240-255 (1997)) iteratively. In the final rounds of
refinement 4 N-acetyl-
glucosamine groups and 4 mannose moieties were added to residues Asn75 of IL-
34 and 486
water molecules were included in the final model. The completed IL-34 model
was then used
for rebuilding and refining the hIL-34s CFlag/YW405.3 Fab complex structure to
convergence at 2.6 A resolution.
hIL-34s CHis/hCSF-1R D1-D3 CHis
The individual components of the hIL-34s CHis/hCSF-1R D1-D3 CHis complex were
sequentially located using the molecular replacement program PHASER, with hIL-
34s,
murine CSF-1R D1-D2 and murine CSF-1R D3 (PDB ID 3EJJ) as the search models.
CSF-
1R D1-D3 was rebuilt and refined according to the human amino acid sequence
and the final
hIL-34s CHis/hCSF-1R D1-D3 CHis model was completed by interactive refinement
in
Refmac and model-building in Coot.
88
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
hIL-34s CFlag/YW404.33.56 Fab
The hIL-34s CFlag in complex with the blocking Fab YW405.33.56 was determined
by the
molecular replacement method implemented in the program PHASER with the
structure of
hIL-34s and the Fv and Fc region of a Fab with similar framework, followed by
manual
fitting of the CDR regions of the YW404.33.56 Fab. Several rounds of model
rebuilding and
structure refinement were carried out using the 3.0 A dataset until
convergence was reached.
A summary of the refinement statistics and the stereochemistry analysis of all
four structures
are given in Table 2. The program MolProbity (Chen et al., Acta
crystallographica Section D,
Biological crystallography 66: 12-21 (2010)) was used to inspect the quality
of the final
models. Coordinates have been deposited with the Protein Data Bank
(www.rcsb.org) with
accession codes xxx, yyy, zzz, www for hIL-34s, hIL-34s/hCSF-1R D1-D3, hIL-
34s/YW404.33.56 Fab and hIL-34s/YW405.3, respectively. Figures were prepared
using the
program PyMol (WorldWideWeb at pymol.sourceforge.net)
Human mononuclear cells and the cell viability / proliferation assay
Human peripheral blood mononuclear cells (PBMC) were isolated by gradient
centrifugation
over Ficoll. In the cell viability / proliferation assay, 1x104 freshly
isolated PBMC per well in
96-well plate were stimulated with IL-34 (full length and short version) or
CSF-1 in serial
dilutions. After incubation at 37 C for 72 hours, ATP levels in cells were
measured by
CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, cat. #G7571) for
determining
cell viability / proliferation. The proliferation was shown as Relative
Luminescent Unit
(RLU).
Neutralization of human IL-34 bioactivity
The optimal concentration of the antibody required to neutralize hIL-34
activity is dependent
on the cytokine amount, cell type and the type of assay. To measure the
ability of the antibody
to neutralize the bioactivity of hIL-34 or mIL-34 on PBMC, we used a cell
proliferation assay
by CellTiter-Glo. Based on cell response to serial dilutions of IL-34, 100
ng/ml of IL-34
amount was selected for determining the antibody neutralizing activity. The
Half Maximal
Inhibitory Concentration (IC50) is defined as the concentration of antibody
required to yield
half maximal inhibition of IL-34 activity on cells, when IL-34 is present at a
concentration to
elicit 70-80% proliferation response. 100 ng/ml hIL-34 or mIL-34 was combined
with serial
dilutions of anti-IL-34 mAbs or anti-CSF1 antibodies before adding onto cells
in a total
89
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
volume of 100 ul. The antibody inhibition activity was obtained by measuring
RLU after
incubating the plates at 37 C for 72 hours. IC50 values were calculated with
KaleidaGraph.
Antibody generation by phage display
Library Sorting and Screening to Identifj; Anti-IL-34 Antibodies
Murine IL-34 (PR0307278) was used as antigen for library sorting. Nunc 96 well
Maxisorp
immunoplates were coated overnight at 4oC with target antigen (10m/m1) and
were blocked
for 1 hour at room temperature with phage blocking buffer PBST (phosphate-
buffered saline
(PBS) and 1% (w/v) bovine serum albumin (BSA) and 0.05% (v/v) tween-20). Human
synthetic antibody phage libraries VH (see, e.g., Lee et al., J. Immunol.
Meth. 284:119-132,
2004) and VH/VL (see Liang et al., Journal of molecular biology 366: 815-829
(2007)) with
synthetic diversities in the selected CDRs were added to antigen plates
separately and
incubated overnight at room temperature. The following day antigen-coated
plates were
washed ten times with PBT (PBS with 0.05% Tween-20), and bound phage were
eluted with
50mM HC1 and 500mM NaC1 for 30 minutes and neutralized with an equal volume of
1 M
Tris base (pH7.5). Recovered phages were amplified in E. coli XL-1 Blue cells.
During the
subsequent selection rounds, incubation of antibody phage with the antigen-
coated plates was
reduced to 2-3 hours, and the stringency of plate washing was gradually
increased.
After 4 rounds of panning, significant enrichment was observed. 96 clones were
picked each
from VH and VH/VL library sorting to determine whether they were human/murine
IL-34
cross or murine IL-34 specific binders. The variable regions of these clones
were PCR
sequenced to identify unique sequence clones. These unique phage clones were
subsequently
reformatted into IgGs by cloning VL and VH regions of individual clones into
the LPG3 and
LPG4 vector respectively (Carter et al., Proc. Natl. Acad. Sci. USA, 89: 4285-
4289 (1992)),
transiently expressing in mammalian CHO cells, and purifying with a protein A
column.
Construct Libraries for Affinity Improvement of YW404.33
Phagemid pW0703 (derived from phagemid pV0350-2b (Lee, 2004) displaying
monovalent
Fab on the surface of M13 bacteriophage) served as the library template for
grafting light
(VL) and heavy (VH) chain variable domains of YW404.33 for affinity
maturation. Stop
codons were then incorporated in CDR-L3 of the library template. Soft
randomization
strategy was used for affinity maturation, which introduced a mutation rate of
approximately
50% at the selected positions by using a poisoned oligonucleotide strategy
with 70-10-10-10
mixtures of bases favoring the wild type nucleotides (Gallop et al., Journal
of medicinal
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
chemistry 37: 1233-1251 (1994)). Specifically residues at positions 28-32 of
CDR-L1, 50
and 53-55 of CDR-L2, 91-94 and 96 of CDR-L3, 28-35 of CDR-H1, 50-58 of CDR-H2,
95-
100 of CDR-H3, were targeted. And three different libraries with combinations
of soft-
randomized CDR loops, L1/L2/L3, L3/H1/H2 and L3/H3, were constructed.
Phage Sorting Strategy to Generate Affinity Improvement
For affinity improvement selection, phage libraries were subjected to plate
sorting for the first
round, followed by four rounds of solution sorting. At the first round of
plate sorting, three
libraries were sorted against murine IL-34 coated plate (NUNC Maxisorp plate)
for 2 hours at
room temperature. Next, four more rounds of solution sorting were carried out
together with
two methods of increasing selection stringency. The first of which is for on-
rate selection by
decreasing biotinylated target protein concentration from 50 nM to 0.5 nM, and
the second of
which is for off-rate selection by adding excess amounts of non-biotinylated
target protein
(100-500 fold more) to compete off weaker binders at room temperature. Each
phage library
was also incubated with non-biotinylated mIL-34 to serve as background phage
binding for
estimating the enrichment of each round of panning.
High Throughput Affinity Screening ELISA (Single Spot Competition)
After five rounds of panning, a high-throughput single-point competitive phage
ELISA was
used to rapidly screen for high-affinity clones as described (Sidu, 2004).
Clones with low
binding ratio to immobilized mIL-34 in the presence of 5nM mIL-34 verse in the
absence of
mIL-34 were chosen for further characterization.
Affinity Measurement
An Octet QK equipped with streptavidin (SA) biosensor tips (forteBio, Menlo
park, CA,
USA) was used for affinity measurement. SA biosensor tips were equilibrated in
assay buffer
(lx Kinetics assay buffer, forteBio) for 10min prior to analysis. Binding
kinetics were
measured at 300C and samples were agitated @ 1000 rpm. SA tips were saturated
with
5ug/m1 of biotinylated human IL-34 (R&D, Cat #5265-IL-010/CF) for 200s, which
resulted in
capture levels of 0.22 0.02 nm within a column of six tips. Three fold Serial
dilutions of
anti-IL-34 antibodies (100nM, 33.3nM, 11.1nM, 3.7nM, 1.2nM), as well as buffer
blank were
prepared. Both association and dissociation were monitored for 300s. All
reactions and
measurements were performed at room temperature. Data were analyzed using
Octet data
analysis software 6.4 (forteBio).
91
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Results
Herein the first crystal structure of human IL-34 alone and in complex with
CSF-1R is
presented, and their binding properties in solution were characterized. It is
shown that IL-34
indeed belongs to the short-chain helical cytokine family, with the smallest
dimerization
interface among the family members. The structure of the IL-34/CSF-1R complex
showed a
similar overall ligand/receptor assembly in terms of the domains involved, as
was seen for the
CSF-1 complex. However, the receptor domains involved undergo an unexpected
rearrangement, resulting in different interface compositions. A careful
comparison between
these two cytokine signaling complexes provided an understanding of the
molecular
determinants that allow CSF-1R to promiscuously bind two structurally-related,
yet
evolutionarily-distant ligands IL-34 and CSF-1. Furthermore, co-crystal
structures of IL-34 in
complex with Fab fragments of two phage-derived anti-IL-34 monoclonal
antibodies
provided a structural rationale for their respective non-blocking and
neutralizing activities,
and offer novel insights into therapeutic development.
Structure of the active core of human IL-34
The mature full-length human IL-34 is comprised of 222 amino acids, but its
last ¨50
residues are predicted to be largely disordered with little discernable
secondary structure by
Jpred 3(Cole et al., Nucleic acids research 36: W197-201 (2008)) and PsiPRED
(McGuffin et
al., Bioinformatics 16: 404-405 (2000)). Moreover, constructs of IL-34
containing the first
202 or 182 residues were indistinguishable from the full-length protein in
activating the
growth of TF-1-fms cells, while constructs encompassing only the first 162
residues showed
significantly diminished activity (Chihara et al., Cell death and
differentiation 17: 1917-1927
(2010)). In addition, seven cysteine residues (35, 168, 177, 179, 180, 191,
and 199) were
found in human IL-34 and six of them (all but C199) are well-conserved across
species
(Figure 1A, Figure 7). Therefore, a truncated IL-34 construct comprised of
residues N21¨
V193 of the mature polypeptide, herein referred to as hIL-34s, was chosen for
the subsequent
studies. hIL-34s was fused to a C-terminal flag-tag, expressed recombinantly
in insect cells,
purified as described in the methods, and used in all subsequent studies,
unless otherwise
noted. hIL-34s was as active as CSF-1 and slightly more active than
recombinant full-length
IL-34 in its ability to promote human monocyte viability (Figure 1B). The
human IL-34 gene
contains no recognizable membrane-associating regions, while the mouse
ortholog exists
92
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
naturally as GPI-anchored and soluble isoforms as a result of alternative RNA
splicing and
proteolytic processing (Figure 1A).
The structure of the active core domain of human IL-34 was determined by
molecular
replacement from its complex with a non-blocking antibody YW405.3 Fab fragment
(Table
6) at 2.6 A resolution, and refined using a 1.85 A resolution dataset
collected from a single
hIL-34s crystal (Table 2). Not surprisingly, structure analysis indicated that
IL-34 has the
distinctive antiparallel four-helix bundle cytokine fold consisting of aA, aB,
aC, and aD
(Sprang et al., Curr Opin Struc Biol 3: 815-827 (1993)), but the structure
contains a number
of notable features outside this core portion (Figure 1C). The crossing 13-
strands 1 and 2 are
much shortened and partially substituted by three short helices (al, a2, and
a3) compared to
CSF-1 (Chen et al., Proceedings of the National Academy of Sciences of the
United States of
America 105: 18267-18272 (2008); Pandit et al., Science 258: 1358-1362 (1992))
(Figure
1D). Furthermore, two intramolecular disulfide pairs located at the pole of
each protomer, and
away from the dimer interface, share no structural similarities with disulfide
bonds found in
the structures of the related dimeric "short-chain" helical cytokines CSF-1,
SCF, and F1t3L
(Jiang et al., The EMBO journal 19: 3192-3203 (2000); Savvides et al., Nature
structural
biology 7: 486-491 (2000); Zhang et al., Proceedings of the National Academy
of Sciences of
the United States of America 97: 7732-7737 (2000)). The first disulfide bond
(between C35
and C180) connects helices aA and aD, while the other disulfide bond (between
C177 and
C191) connects aD to the C-terminal helix a4. Consequently, the C-terminal
tail including a4
is inverted, compared with that of CSF-1, and packs onto the surface of aA and
aD. The other
two cysteines C168 and C179 remain unpaired and consequently are not essential
for the
proper folding of IL-34. Overall, as judged by PDBeFOLD structural
superposition (Krissinel
et al., Acta crystallographica Section D, Biological crystallography 60: 2256-
2268 (2004)),
IL-34 was shown to be structurally most similar to SCF (root-mean-square
deviation (rmsd:
2.6 A), yet more divergent from the functionally realted CSF-1 (rmsd: 3.2 A).
The results of this structure analysis also showed that the two protomers of
IL-34 in the
asymmetric unit further assemble into a non-covalent dimer in a manner similar
to that seen
for CSF-1, SCF, and F1t3L. In forming the IL-34 dimer, each subunit buries 656
A2, with the
aA-131 loop and aB-aC loop from one monomer interlocking with the reciprocal
segments of
the other monomer. A compact and relatively flat hydrophobic patch centered on
P58/P58' is
formed by packing the side chains of H56, Y57, F58, P59, and Y62 of one
protomer against
residues P114', H113', L110', L109', V108', Y62', P59', and F58' from the
neighboring
93
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
monomer. These interactions may confer obligate IL-34 dimer formation despite
the smaller
buried surface area (1310 A2) compared with the related non-covalent SCF and
F1t3L dimers,
comprised of 1690 A2 and 1640 A2, respectively. The residues at the IL-34
dimer interface
are highly conserved among orthologs from other species (Figure 7). The
dimeric
organization of IL-34 observed in hIL-34s crystals is likely to be
representative of the
organization of the protein in solution as the same "head-to-head" dimeric
arrangement was
also present in the three IL-34 complex structures discussed herein. One
predicted N-linked
glycosylation site in mature IL-34 located at N76 was included in the
construct that was
crystallized, and electron density for (G1cNAc)2Man was observed attached to
the side chain.
The results indicated that orientation of the carbohydrate is fixed by
stacking interactions
between the aromatic ring of Y68 and the hydrophobic face of the second GlcNAc
residue,
together with polar interactions mediated by three conserved residues in IL-
34, E69, R79, and
K157, suggesting that this sugar moiety is an integral part of the IL-34
structure. The
analogous glycosylation site is conserved in rodent IL-34, which also contains
an additional
predicted N-linked glycosylation site at a position equivalent to human S100,
which is solvent
accessible in the hIL-34s structure.
Biophysical characterization of the IL-34/CSF-1R complex
To probe the oligomeric state of hIL-34s in solution, analytical size
exclusion
chromatography was used (Figure 2A). The theoretical molecular mass for the
hIL-34s
monomer is 22 kDa, yet the protein eluted earlier than the 44kDa reference,
indicating hIL-
34s forms dimers in solution, in agreement with the two-fold non-
crystallographic symmetric
dimer structure observed in the hIL-34s crystal. In order to assess the
molecular size of IL-34
ligand/receptor complexes, a construct containing nearly the entire
extracellular domain
(ECD) including immunoglobulin domains D1 through D5 of the receptor (hCSF-1R
D1-D5)
was mixed with IL-34 at 1:1 protomer molar ratios and analyzed by the same
technique. The
apparent size of this complex was consistent with the size calculated for a
2:2 complex
(Figure 2A). Similarly, in order to narrow down the minimal receptor construct
necessary for
complex formation, a mixture of hIL-34s with a receptor construct containing
only the first
three immunoglobulin domains (hCSF-1R D1-D3) was subject to size exclusion
analysis,
revealing an apparent size which is larger than 158 kDa, in agreement with a
2:2 complex
(Figure 2A).
94
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
To accurately determine the stoichiometry and energetics of IL-34 binding to
CSF-1R and
compare them with CSF-1 binding, the thermodynamic parameters for binding were
assessed
by isothermal titration calorimetry (ITC) (Figure 2B, D). Titrating CSF-1R
receptor into the
IL-34 cytokine confirmed that both the full-length and truncated forms of the
receptor, CSF-
1R D1-D5 and D1-D3, were saturated by equal molar amounts of IL-34 monomer.
This, in
conjunction with the apparent sizes determined by analytical size exclusion
chromatography,
strongly supported a 2:2 cytokine/receptor stoichiometry in solution for the
complex (Figure
2D). Since the high affinity of the IL-34/CSF-1R D1-D5 interaction yielded a
steep titration
transition that precluded determining the dissociation constant with high
accuracy (Figure 2B,
left panel), displacement ITC was carried out by titrating CSF-1R D1-D5 into a
mixture of
IL-34 with CSF-1R D1-D3 (Figure 2B, right panel). The binding isotherms
determined herein
indicated IL-34 binding to its receptor is driven mainly by enthalpic terms.
The exothermic
nature of IL-34 binding to both CSF-1R D1-D3 and D1-D5 indicated that IL-
34/CSF-1R
complex formation likely involves key polar interactions. Inclusion of the
membrane-
proximal domains CSF-1R D4-D5 lead to a large increase in affinity (1.6 and 94
nM Kd for
D1-D5 and D1-D3, respectively), which is significantly more favorable
enthalpically,
although slightly disfavored entropically. These results suggested that
although hCSF-1R D1-
D3 is sufficient to confer high-affinity ligand-binding, CSF-1R D4-D5 likely
contains
additional homotypic receptor interaction sites upon formation of the entire
cytokine/receptor
signaling complex, as seen in the complex structure of SCF bound to the full
ectodomains of
a KIT receptor dimer (Yuzawa et al., Cell 130: 323-334 (2007)). Surprisingly,
the better
characterized ligand CSF-1 exhibited 9.5 and 13-fold lower affinity compared
to IL-34 for
binding to hCSF-1R D1-D3 and D1-D5, respectively (Figure 2C, D). Compared to
IL-34, the
results indicated that CSF-1 complexes are driven by both favorable enthalpy
and entropy.
Similar to IL-34, the human CSF-1 titrations also suggested 2:2
ligand/receptor
stoichiometries for both forms of the receptors. Although consistent with an
earlier report
(Guilbert et al., The Journal of biological chemistry 261: 4024-4032 (1986)),
this represents a
key difference between human and murine CSF-1. In the absence of D4 and D5,
murine CSF-
1 was reported to form a 2:1 partial complex with murine CSF-1R leaving half
of the receptor
binding surface on murine CSF-1 unoccupied in solution (Chen et al.,
Proceedings of the
National Academy of Sciences of the United States of America 105: 18267-18272
(2008)).
To further understand the kinetics of cytokine/receptor binding in this
system, biotinylated IL-
34 or CSF-1 were immobilized on streptavidin-coated biosensor tips in order to
measure rates
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
of association and dissociation with serial receptor dilutions using the Bio-
Layer
Interferometry (BLI) technique. The results from this orthogonal method also
confirm that IL-
34 (kon = 2.9 x 105 s-1M-1, koff = 3.5 x 10-2 s-1, Kd = 120 nM) binds CSF-1R
more tightly than
CSF-1 (koõ = 7.7 x 104 s-1M-1, koff = 5.4 x 10-2 s-, Ka = 720 nM) primarily
due to a 3.8-fold
faster kon coupled to a slightly slower koff=
Structure of the IL-34/CSF-1R D1-D3 complex
The 3.0 A resolution structure of hIL-34s bound to hCSF-1I D1-D3 was solved by
molecular
replacement using structures of hIL-34s and murine CSF-1R (Chen et al.,
Proceedings of the
National Academy of Sciences of the United States of America 105: 18267-18272
(2008)) as
search probes (Table 2). Although all biophysical methods tested in this study
indicated that
hCSF-1R D1-D3 forms a 2:2 complex with hIL-34s in solution, the crystal
structure
unambiguously revealed one CSF-1R D1-D3 bound to one hIL-34s homodimer in the
asymmetric unit. Structure analysis suggested that the analogous receptor
binding site on the
adjacent protomer is involved in crystal packing, prohibiting formation of the
expected 2:2
complex in this crystal structure form. However, this structure nicely
revealed the IL-
34/receptor interace. It showed that IL-34 is bound to a concave surface
formed by D2 and D3
of CSF-1R in a configuration resembling that employed by CSF-1 when bound to
the CSF-1R
receptor. A similar two-site binding mode seen in the CSF-1 cytokine/receptor
complex is
also preserved in the IL-34/CSF-1R interface. These results indicated that
Site 1 involved
interactions between IL-34 and Ig domain D2 of the receptor, and Site 2
between IL-34 and
receptor domain D3. Each site provides 1280 A2 and 1160 A2 total buried
surface area,
respectively. The linker between D2 and D3, although fully ordered in the
structure, is spared
from the interaction with IL-34, which effectively parses the IL-34/CSF-1R
interface into two
spatially separated sites.
IL-34 Ligand/receptor Binding Site/
Structure analysis revealed that Site 1 is formed mainly by receptor D2 domain
residues
comprising the CD and EF loops (residues 142-150 and 169-173, respectively)
which dock
onto the rugged surface provided by IL-34 helices aB (residues 100-108), aC
(116-134), the
intervening loop (residue 109), and a3 (residue 150) (Figure 3A).
Complimentary
electrostatic interactions between the negatively-charged surface of IL-34 and
the positively-
charged surface on CSF-1R in this region appeared important in mediating IL-34
binding at
this site. In particular, centrally located salt bridges are formed between
the basic amino acids
96
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
R142, R146 of CSF-1R with the acidic E103 on IL-34. R144 and R150 of CSF-1R at
the
periphery of the CD loop engage in hydrogen-bonding interactions with the side
chain oxygen
of N150 and the backbone carbonyl oxygen of Q106 and L109, respectively. A
small
hydrophobic patch formed by the aliphatic side chain of L127 in IL-34 fits
snugly between
F169 and 1170 of CSF-1R. While this site appeared dominated by polar
interactions, a
number of intermolecular van der Waals contacts also line the interface as
detailed in Table 3.
IL-34 Ligand/receptor Binding Site 2
The results further indicated that the slightly smaller Site 2 is formed
mainly by receptor D3
residues from the BC and DE loops (residues 231 and 254, respectively) and
strand D
(residues 248-252), packed against the surface generated by portions of IL-34
helices aA, aC,
and a4 (residues 36-44, 121-128, 184-187, respectively) (Figure 3C). This
interface can be
further divided into two polar interaction regions, separated by a hydrophobic
area formed by
IL-34 residues F40 and L125, and CSF-1R residues V231, Y257, and F252. The
first region
is formed by a combination of backbone and side chain hydrogen-bonding
interactions
between the beginning of the CSF-1R D3 D-strand and IL-34 a4. The side chain
amide
hydrogen and oxygen of N187 of IL-34 participates in up to three hydrogen
bonds with the
receptor involving the side chain amide of Q249, and the backbone nitrogen and
carbonyl
oxygen of S248 of CSF-1R. Additionally, two backbone-backbone hydrogen bonds
are
formed between CSF-1R Q248 and IL-34 S184 and L186. Deletion of residues
comprising
the a4 region in IL-34 results in drastic reduction in protein expression
levels, and
significantly weaker activity in terms of the ability to stimulate the growth
of TF-1-fins cells
(Chihara et al., Cell death and differentiation 17: 1917-1927 (2010)). The
results reported
here confirmed that residues encompassing a4 are an integral part of the
active core domain
of IL-34, and are therefore important for its activity. The second polar
region in Site 2
encompasses three side-chain-mediated hydrogen bonds between the terminal side
chain
atoms of IL-34 N128, K44 and E121, and the hydroxyl group of Y257, the
carbonyl oxygen
of F252 and the backbone amide of N254 of CSF-1R. Additional interactions
mediated by
van der Waals contacts are listed in Table 3.
Table 3. IL-34/CSF-1R and CSF-1/CSF-1R interactions.
CSF-1R D2 IL-34 Distance CSF-1 Distance
(A) (A)
Hydrogen bonds and salt bridges (Shared)
Arg142 N111 G1n58 081 3.4
97
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
N112 G1u103 0E1 3.1
N112 G1u103 0E2 3.2
Arg146 N111 G1n58 081 2.4
N112 G1u103 0E1 2.6 Asp59 061
3.4
Arg150 Nril Leu109 0 3.2
N112 G1n106 0 3.5 Asp62 0 3.0
N Asp62 061 3.4
N Asp62 062 3.2
Hydrogen bonds and salt bridges (Unique)
Arg144 N111 Asn150 061 2.5
Lys151 Nc G1u78 081 3.9
Lys168 Nc G1u78 081 3.6
Lys168 Nc G1u78 082 3.7
Leu170 0 Asn85 N62 3.1
van der Waals contacts (Shared)
Arg146 Ser100, Asn150 Phe55
Met149 (Leu149) G1u123 Asp62, Gln81
Arg150 Trp116 Arg66
Phe169 (Va1169) Thr124, Leu127, Asn128, G1u82
Gln131
I1e170 (Leu170) G1u123, Thr124, Leu127 G1u78
G1n173 (Asn173) Leu127 G1u78
van der Waals contacts (Unique)
Lys151 Asp62
Ser172 Leu127
Arg192 Thr134
CSF-1R D3 IL-34 Distance CSF-1 Distance
(A) (A)
Hydrogen bonds and salt bridges (Shared)
Tyr257 OH Asn128 N62 2.7 Met10 0 2.6
Hydrogen bonds and salt bridges (Unique)
G1n248 N 5er184 0 2.7
0 Leu186 N 2.8
G1n249 N82 Asn187 061 3.1
Ser250 N Asn187 061 3.0
0 Asn187 N62 3.5
Asp251 061 Asn13 N 3.0
062 G1y14 N 2.9
Phe252 0 Lys44 Nc 2.6
Asn254 N G1u121 082 3.8
van der Waals contacts (Shared)
Va1231 Phe40 His6, Met10
Ser250 Phe40, Asp43 His9
Asp251 Phe40, Asp43 G1y12
Phe252 Leu125 G1y14, His15, Arg79
Tyr257 Phe40, Leu125 His9, His15
van der Waals contacts (Unique)
98
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
G1y232 His6
Phe233 His6
Pro247 Ser184, G1n189
G1n248 Thr36, Va1185, Asn187
G1n249 Leu186
Asn255 Arg79
G1n258 Phe40
Lys259 Thr36
Structural basis for IL-34 antagonism by mAb YW404.33.56
To dissect the discrete functions of IL-34 and CSF-1 and explore the
therapeutic benefit from
blocking IL-34 signaling, phage display technology was used to generate
antibodies that
specifically antagonize both human and murine IL-34, enabling the
interrogation of IL-34
function in both human patients and rodent models. YW404.33 was identified by
screening of
VH and VH/VL phage display libraries and selected out of a panel of 96 clones
based on its
high binding affinity (IQ = 17 nM, Table 4) and its blocking activity for
human IL-34.
YW404.33 was subsequently affinity-matured using a soft randomization strategy
as
described in the Methods. YW404.33.56 shows a 140-fold improvement in binding
affinity
(IQ = 120 pM) as measured by BLI experiments when compared with the parental
clone. In
human monocyte viability (Figure 4A) and proliferation (Figure 8) assays, the
biological
activity of IL-34 was completely blocked by this antibody, similar to the
receptor Fc fusion,
but not by an anti-CSF-1 antibody.
Table 4. Octet measurement of binding kinetics for the non-neutralizing
antibody
YW405.3/hIL-34, and neutralizing antibodies YW404.33/hIL-34, YW404.33.56/hIL-
34.
Anti-IL-34 Mabs k011(s-1M-1) koff (s-1) Ka (M)
YW405.3 1.45E+06 4.52E-03 3.12E-09
YW404.33 3.58E+05 6.11E-03 1.71E-08
YW404.33.56 3.38E+06 4.19E-04 1.24E-10
To better understand the mechanism whereby YW404.33.56 mAb blocks IL-34
biological
activity, the structure of hIL-34s in complex with the Fab fragment of
YW404.33.56 was
solved at 3.0 A resolution (Table 2). Crystals contained one hIL-34s dimer
bound to two
YW404.33.56 Fabs in the asymmetric unit. Interestingly, structure analysis
showed that each
YW404.33.56 Fab recognizes a largely continuous area at the junction of two IL-
34
protomers. On the IL-34 dimer side, the majority of the interface (786 A2 or
79%) is
contributed by helices aB, aC, and their intervening loop, from one protomer,
whereas a
99
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
smaller fraction (215 A2 or 21%) is contributed by the aB-I31 loop of the
other IL-34
protomer (Figure 4B). On the YW404.33.56 Fab side, the bulk of the
interactions are
mediated by the heavy chain CDR-H1 (55 A2), CDR-H2 (267 A2), and CDR-H3 (316
A2)
with smaller contributions from the light-chain CDRs (303 A2). Four salt
bridges
(R50yw4o4.33.56_E11 11L-345 R100bYW404.33.56_E 1 11L-345 R100bYW404.33.5643
071L-345
and
K100aYW404.33.56_E 03IL-34) form an "electrostatic zipper" along the groove
between aB and
aC of IL-34. This strong electrostatic complementarity between IL-34 and
YW404.33.56 Fab
was reminiscent of the Site 1 charge-charge interactions in the IL-34/CSF-1R
complex
described above. Additionally, w33YW404.33.565 y54YW404.33.565 K98YW404.33.565
and
S100Yw40433=56 form side chain specific hydrogen bonds with D1071L-345 D10711-
345 S10411-34,
and Q12011-34, respectively. In addition, other YW404.33.56 backbone carbonyl
groups
mediate hydrogen bonds, and several other van der Waals interactions as
detailed in Table 5.
The carbohydrate chain of the glycosylated N76 of IL-34 extends away from the
interaction
interface, and has no direct contact with the YW404.33.56 Fab. Unexpectedly,
hydrophobic
packing across the interface, which often mediates high-affinity protein-
protein interactions,
is absent in this antibody/antigen system. The shape complementarity between
the Fab
paratope and IL-34 epitope was 0.61, which is slightly smaller than the
average value (0.64-
0.68) for other antibody/antigen complexes (Lawrence et al., Journal of
molecular biology
234: 946-950 (1993)), suggesting the paratope of YW404.33.56 mAb could in
theory be
further optimized to achieve higher shape and chemical complementarities for
tighter binding
to IL-34.
Superposition of the IL-34 molecules in the IL-34/YW404.33.56 and IL-34/CSF-1R
complexes showed that both the heavy and light chains of the Fab clash with
CSF-1R. These
results indicated that YW404.33.56 binds to an epitope that overlaps largely
with binding
epitopes responsible for Site 1, CSF-1R D2-binding, and includes residues from
helices aB
and aC from one IL-34 protomer. 6 out of 11 residues or 220 A2 out of 600 A2
of the buried
solvent-accessible surface areas from IL-34 at the IL-34/CSF-1R binding Site 1
are occupied
by interactions with the YW404.33.56 in the IL-34/Fab complex. Therefore, the
binding of
YW404.33.56 to IL-34 would compete directly for association with the receptor
CSF-1R,
which explains the molecular mechanism of the inhibition of IL-34 signaling by
YW404.33.56.
100
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Table 5. IL-34/YW404.33.56 Fab interactions.
YW404.33.56 H chain IL-34 mol A Distance (A)
Hydrogen bonds and salt bridges
Trp33 N81 Asp107 061 3.5
N81 Asp107 062 3.4
Arg50 N111 Glulll 082 3.0
Arg50 Nfl2 Glulll 081 3.0
Tyr54 OH Asp107 062 3.0
Lys98 Nc Ser104 Oy 3.5
G1y99 N Asp107 061 2.4
Ser100 Oy G1n120 081 3.0
Lys100A Nc G1u103 081 2.8
Arg100B NE Glulll 082 3.7
Arg100B Nfll Asp107 061 3.4
Arg100B Nfll Asp107 0 2.9
Arg100B Nfl2 Leu109 0 2.8
Arg100B Nfl2 Glulll 081 3.9
Arg100B Nfl2 Glulll 082 3.2
van der Waals contacts
Thr28 Pro152
Tyr54 Va1108
Tyr56 Leull0
Lys98 G1u103, Asp107
G1y99 G1n106, Trp116
Ser100 G1n106, Trp116
Lys100A G1u123
Arg100B Leu109, Leu110, Trp116
YW404.33.56 L chain IL-34 mol A Distance (A)
Hydrogen bonds and salt bridges
Phe92 0 Trp116 N81 3.3
van der Waals contacts
Phe53 Leu127
Ser91 Trp116
Phe92 Glulll, Lys117, G1n120
Phe94 Glulll
YW404.33.56 H chain IL-34 mol B Distance (A)
Hydrogen bonds and salt bridges
Tyr54 0 Asn61 N62 2.7
Tyr55 0 Asn61 N62 3.4
van der Waals contacts
Trp33 11e60
Tyr54 11e60
Try56 Lys55, 11e60
101
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Table 6. IL-34/YW405.3 interactions
YW405.3 H chain IL-34 mol A Distance
(A)
Hydrogen bonds and salt bridges
Trp52A 0 Arg170 Nfl2 3.2
Tyr53 0 Arg170 NE 2.7
OH Asn167 061 2.3
G1y54 0 Lys162 Nc 2.8
G1y55 0 Tyr47 OH 2.7
Asp56 061 Lys162 Nc 3.8
061 Tyr47 OH 3.7
062 Lys55 Nc 3.6
Asp98 061 Lys63 Nc 2.7
061 Arg160 Nfll 2.6
062 Lys63 Nc 3.7
062 Arg160 Nfll 3.2
van der Waals contacts
Tyr53 Arg73, 11e74
G1y54 Tyr47, Asp166
Tyr99 Arg73
YW405.3 L chain IL-34 mol A Distance
(A)
Hydrogen bonds and salt bridges
Tyr32 OH Leu158 0 3.5
Trp92 0 Arg160 Nfll 3.8
G1u94 N Asn61 061 3.5
van der Waals contacts
Tyr32 Lys63
Trp92 Asn61, Lys63, Leu158
Ser93 Asn61
YW405.3 L chain IL-34 mol B Distance
(A)
Hydrogen bonds and salt bridges
Ser26 0 Asn155 N 2.9
van der Waals contacts
A1a25 Pro154
Ser26 Pro154
Discussion
Conformational change in IL-34 upon binding to the receptor
Herein the first three-dimensional structures of IL-34, solved both on its own
and in complex
with its receptor CSF-1R are reported. Comparison of the IL-34 protomer
structure in its
102
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
unbound and CSF-1R-bound forms revealed that the structure of the cytokine is
nearly
unchanged upon binding to its receptor. Even the side chain rotamer
conformations adopted
by the receptor interfacial residues showed surprisingly little difference
between free and
bound states, indicating the unbound IL-34 promoter is highly compatible with,
and primed
for receptor-binding. The apparent rigidity of the IL-34 protomer structure
based on
comparisons of the four structures presented herein was consistent with the
recently
determined F1t3L structure (Verstraete et al., Blood 118: 60-68 (2011)), but
quite different
from the more plastic CSF-1 (Chen et al., Proceedings of the National Academy
of Sciences
of the United States of America 105: 18267-18272 (2008)) or SCF (Liu et al.,
The EMBO
journal 26: 891-901 (2007); Yuzawa et al., Cell 130: 323-334 (2007))
structures, which must
undergo significant local structural changes to accommodate receptor-binding.
Nevertheless,
significant changes in the orientation of one IL-34 protomer relative to the
other protomer
about the dimer interface upon complex formation were observed. When described
by the tilt
angle of aC, receptor-binding or YW404.33.56-binding induced a 6.6 or 4.6
increase in the
angles between IL-34 protomers, respectively. Similar hinge-like rigid-body
movements were
reported previously in the CSF-1/CSF-1R, SCF/Kit and F1t3L/F1t3 systems.
Unexpectedly,
another antibody YW405.3 triggered a similar rotation, but along the reverse
direction,
resulting in 6.4 decrease in the tilt angle. Such high degree of plasticity
is unprecedented in
other dimeric four helical bundle cytokines, and can likely be attributed to
the smaller, and
very hydrophobic IL-34 dimerization interface.
The dual recognition of IL-34 and CSF-1 by CSF-1R
While IL-34 and CSF-1 are sparsely similar at the primary sequence level, it
was found that
they indeed adopt a similar four helical bundle core fold and related
dimerization and receptor
binding interfaces, with differences apportioning to extra-core loops and
structural
embellishments - a recurrent finding in the comparison of helical cytokine
structures (Bazan,
Neuron 7: 197-208 (1991b); Hill et al., Journal of molecular biology 322: 205-
233 (2002);
Rozwarski et al., Structure 2: 159-173 (1994)). In fact, a telling remnant of
helical cytokine
ancestry (aside from the three-dimensional fold relationship) is the
similarity in exon/intron
structures of their respective genes (Bazan, Cell 65: 9-10 (1991a); Betts et
al., The EMBO
journal 20: 5354-5360 (2001)); in this respect, the structure of the IL-34
gene is homologous
to the CSF-1, SCF and F1t3L genes.
103
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Both IL-34 and CSF-1 bound the CSF-1R receptor with high-affinity and induced
similar, if
not identical, biological activity (Chihara et al., Cell death and
differentiation 17: 1917-1927
(2010); Lin et al., Science 320: 807-811 (2008)). This striking phenomenon
lead to the direct
comparison of the structure of the human IL-34/CSF-1R complex to that of the
murine CSF-
1/CSF-1R complex to determine the molecular mechanisms governing receptor
sharing by
these structurally similar but evolutionally divergent ligands. This analysis
indicated that,
CSF-1R utilizes a common "dual interface mode" for its interactions with both
cytokines. The
total solvent-accessible surface area buried at the interface between IL-34
and CSF-1R is
approximately 2400 A2, significantly larger than the 1700 A2 buried in the CSF-
1/CSF-1R
interface. Notably, the regions of CSF-1R that interact with IL-34 and CSF-1
largely overlap,
yet are not identical. Consistent with this result, the anti-CSF-1R mAb clone
12-2D6 that
blocked signaling by both cytokines (Chihara et al., Cell death and
differentiation 17: 1917-
1927 (2010)), recognizes an epitope between residues 1-308 within the first
three domains of
the CSF-1R receptor. 12-2D6 most likely functions by binding to a site on the
receptor
overlapping the IL-34/CSF-1 binding sites, and therefore abrogates receptor
signaling
irrespective of the ligands. In contrast, mAb clone 2-4A5 blocked CSF-1, but
not IL-34
binding to TF-1-fms cells by recognizing an epitope residing between residues
349-512
(Chihara et al., Cell death and differentiation 17: 1917-1927 (2010)).
Considering this
epitope is remote from the ligand-binding sites of both cytokines, the ability
of 2-4A5 to
distinguish CSF-1 from IL-34 remains an enigma. Nevertheless, steric hindrance
could be
created by the specific geometry of this antibody/CSF-1R complex, which
affects only CSF-1
binding.
Further analysis revealed substantial differences in both the numbers and
types of interactions
between the two CSF-1R signaling complexes. The sequences of human and mouse
CSF-1R
are more than 70% identical with no gaps within Dl-D3; therefore allowing
direct structural
comparison of CSF-1R in the human IL-34 complex to that of the murine CSF-1
complex
(Chen et al., Proceedings of the National Academy of Sciences of the United
States of
America 105: 18267-18272 (2008)). The Site 1 interfaces share a number of
common
features. Most of the polar interactions between the CSF-1R CD loop and helix
aB of both
cytokines are mediated by the same group of basic residues on the receptor
(R142, R146, and
R150), which dictate the shared charge complementarity in this region (Figure
3A, B).
Interestingly, these three residues on CSF-1R remain invariant while other
shared interfacial
contacts are more divergent among CSF-1R orthologs in other eukaryotic
species. A centrally
104
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
located acidic residue (E103 in IL-34 and D59 in CSF-1) at the second turn of
helix aB
participates in salt bridges in both structures. The hydrogen-bonding
interactions between the
EF loop of CSF-1R and N85 in CSF-1 are substituted by a nearby hydrophobic
patch in the
IL-34/CSF-1R complex. The most prominent differences are located at the edges
of the CD
and EF loops of CSF-1R. The salt bridges located at the lower edge of the two
connecting
loops between K151CSF-1R, K168CSF-1R and E78csF-1
are absent in IL-34/CSF-1R. Instead a
unique hydrogen bond bridging N150"34 and R144cS1-1R bring
IL-34 into close contact with
the upper edge of the CD loop. The differences between the Site 2 interfaces
are even more
striking. Wherein an extensive hydrogen-bonding network between the CD loop, D
strand
and DE loops of CSF-1R and helices aA, aC, and a4 in IL-34 forms the core of
the interface
at Site 2. Yet, such interactions are completely absent in the CSF-1/CSF-1R
complex,
resulting in a much smaller Site 2 interface. However, hydrophobic
interactions involving
V231 on the receptor were observed in both complexes.
Large rearrangement in the CSF-1R receptor D2-D3 domain orientation in the IL-
34 versus
CSF-1 cytokine/receptor complexes
The orientation of receptor domains D1 and D2 are conserved in both
cytokine/receptor
complexes; however the orientation of receptor domain D3 relative to Dl-D2
showed a
significant 27 change when comparing the two complexes. Engagement by IL-34
triggered a
rotation between D2 and D3 of CSF-1R, producing an elongated, nearly linear
pose that is
significantly different from the kinked configuration of the CSF-1 bound form.
This overall
reorientation of CSF-1R could be attributed, at least in part, to the distinct
IL-34 molecular
surface, which would sterically clash with CSF-1R without inducing the new
orientation
observed in the structure reported herein. The receptor-contacting residues,
when mapped
onto the secondary structure of both cytokines clearly showed that both
interaction sites are
more spread out on IL-34 than CSF-1 (Figure 5A, B). This is mainly due to the
distinct
structural features outside the four-helix bundle core, namely, a3 and a4.
Hence, a flatter
interface is created on IL-34, requiring a more extended conformation of the
receptor, while
CSF-1 protrudes more into a cleft between CSF-1R D2 and D3 created from their
more
"bent" orientation. The interface between neighboring domains D2 and D3 is
minimal in the
CSF-1/receptor complex, yet the only salt bridge between D3 E230 and D2 K194
in that
complex is broken in the IL-34/receptor complex, allowing D2 and D3 to adopt a
more
extended, almost linear arrangement upon IL-34 binding. The receptor D2-D3
hinge sequence
105
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
196IN.,
KVIPGP202 completely reconfigures itself, using K197 and G201 as the two pivot
points,
while leaving D1-D2 ending with N196 and D3 starting with P202 with minimal
structural
perturbation. Thus, the substantial elbow flexibility between D2 and D3
domains allows the
C SF-1R molecule to adapt to the distinct binding surfaces provided by IL-34
and CSF-1
(Figure 5A, B). In contrast, the receptor D1-D2-D3 region of KIT appeared to
behave as a
rigid body upon SCF binding, where D4 and D5 realign and mediate receptor-
receptor
interactions between the two SCF-bound KIT molecules (Yuzawa et al., Cell 130:
323-334
(2007)). In Kit, there is a significant hydrophobic interface between domains
D2-D3, which is
absent in CSF-1R. Inter-domain flexibility has been used as an adaptive
mechanism by which
multiple viral proteins utilize the same host cell receptor for entry. Two
structurally unrelated
proteins, the measles virus hemagglutinin and adenovirus type 1 1/2 1 knobs
share the first two
SCR domains of CD46, the linker between which is quite structurally plastic
(Cupelli et al.,
Journal of virology 84: 3189-3200 (2010); Persson et al., Nature structural &
molecular
biology 14: 164-166 (2007); Santiago et al., Nature structural & molecular
biology 17: 124-
129 (2010)). Moreover, perturbation of receptor domain orientations can lead
to pronounced
functional consequences, as demonstrated by a 14 rotation in erythropoietin
receptor (EPOR)
in complex with synthetic agonist and antagonist peptide (Livnah et al.,
Nature structural
biology 5: 993-1004 (1998)). IL-34 was reported to induce a stronger but more
transient
activation of CSF-1R, and more rapidly downregulate CSF-1R levels compared to
CSF-1
(Chihara et al., Cell death and differentiation 17: 1917-1927 (2010)).
Possibly this
reorientation of CSF-1R receptor domains could modulate its signaling potency
in
combination with the different affinity to these two cytokines.
Equally spaced CSF-1R D3-D4 junctions in the cytokine/CSF-1R signaling
complexes prime
D4, D5 for degenerative signaling
Receptor-mediated homotypic interactions have been proposed to play an
indispensible role
in the activation of type III RTKs. Such interactions have been either
captured structurally in
the case of KIT D4 (Yuzawa et al., Cell 130: 323-334 (2007)) and VEGFR2 D7
(Yang et al.,
Proceedings of the National Academy of Sciences of the United States of
America 107: 1906-
1911 (2010)), or characterized biochemically in the case of PDGFRI3 (Shim et
al.,
Proceedings of the National Academy of Sciences of the United States of
America 107:
11307-11312 (2010)). The nature of these interactions is a pair of reciprocal
salt bridges in
the EF loop between the two receptor KIT D4 domains (D7 in VEGFR or D4 in
PDGFR).
106
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Due to the relatively high sequence identity between CSF-1R and KIT,
punctuated by this
conserved ionic pair in CSF-1R D4, similar receptor homotypic interactions
probably also
drive CSF-1R dimerization in relaying the ligand-binding signal across the
membrane. Given
the biophysical analyses described herein indicated a 2:2 stoichiometry of IL-
34/CSF-1R
complexes in solution, the 2:1 IL-34/CSF-1R complex observed in the crystal
structure was
most likely influenced by crystal packing forces. Thus, the expected full 2:2
ligand/receptor
signaling complex was modeled by applying the 2-fold symmetry between two IL-
34
protomers to CSF-1R. When the absent copy of CSF-1R was added to the
unoccupied site on
the IL-34 dimer, the distance (60 A) between the two C termini of CSF-1R D3 in
this 2:2 IL-
34/CSF-1R model is very similar to that in the 2:2 CSF-1/CSF-1R model (62 A),
and to that
in the 2:2 SCF/Kit structure (64 A) (Figure 6). Taken together, although the
hinge between
CSF-1R D2-D3 can adopt very different conformations to adapt to distinct
surface
topographies of the two cytokines, the reorientation of D2-D3 nonetheless
results in the D3-
D4 junction being spaced equivalently in the two cytokine/receptor signaling
complexes,
presenting CSF-1 D4 as a convergent point for homotypic interactions to elicit
degenerative
responses downstream.
Ligand-binding promiscuity in CSF-1R utilizes distinct mechanisms from shared
hematopoietic cytokine receptors
The molecular mechanism of cytokine-binding promiscuity have been deciphered
for a
number of shared cytokine receptors such as the common gamma chain (yc), gp130
and
interferon a receptors (IFNAR1/2), since they all have been captured
structurally in at least
two different ligand-bound states (Thomas et al., Cell 146: 621-632 (2011);
Wang et al.,
Annual review of immunology 27: 29-60 (2009)). The ectodomains of both yc and
gp130
contain one cytokine binding homology region (CHR) consisting of two Ig-like
fibronectin
type-III (FNIII) domains (Wang et al., Annual review of immunology 27: 29-60
(2009)).
Although the cytokine-binding site is housed at the interdomain junction in
both shared class
I cytokine receptors, no significant elbow movements have been observed by
comparing the
IL-2/yc quaternary complex with IL-4/yc ternary complex (LaPorte et al., Cell
132: 259-272
(2008); Wang et al., Science 310: 1 159-1 163 (2005)), or the unliganded gp130
with three
gp130 family cytokine complexes (Boulanger et al., Molecular cell 12: 577-589
(2003a);
Boulanger et al., Science 300: 2101-2104 (2003b); Bravo et al., The EMBO
journal 17: 1665-
1674 (1998); Chow et al., Science 291: 2150-2155 (2001)). Even the rotameric
states of the
107
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
interfacial residues in yc and gp130 remain largely unchanged upon binding to
different
cytokines (Wang et al., Annual review of immunology 27: 29-60 (2009)). yc and
gp130 use
"chemically inert complementary surfaces", with a shared hydrophobic core
region
surrounded by peripheral polar patches, to recognize short-chain and long-
chain cytokines,
respectively (Boulanger et al., Molecular cell 12: 577-589 (2003a); Wang et
al., Annual
review of immunology 27: 29-60 (2009)). The recently determined type I IFN
receptor
complexes revealed that paralogs IFNa2 and IFN03 are sandwiched between the
first three
FNIII domains of IFNAR1 (SD1-SD3) and the two FNIII-like domains of the more
compact
IFNAR2 (D1, D2) (Thomas et al., Cell 146: 621-632 (2011)). Interestingly,
although the N-
terminal SD1 domain rotates relative to the 5D2-5D3 segment of IFNAR1 upon IFN-
binding,
once bound to the IFN, IFNAR1 exhibits nearly identical conformations
regardless of the
identity of the bound cytokine. Both IFNAR1 and IFNAR2 rely on a few conserved
"anchor
point" residues on the surface of type I IFNs for cross-reactivity (Thomas et
al., Cell 146:
621-632 (2011)). However a number of less conserved amino acids are
interspersed across
the constellation of the conserved IFNs binding surface for fine-tuning their
individual
binding affinity towards the receptor.
By comparison, the structural basis of CSF-1R promiscuity is quite different
from the
aforementioned shared cytokine receptors. The core and peripheral binding
interface
architecture on C SF-1R is somewhat similar to the class I cytokine/receptor
recognition
paradigm, although C SF-1R uses mainly polar interactions in Site 1 as its
core, instead of
hydrophobic interactions as observed in yc and gp130. CSF-1R clearly relies to
a greater
extent on its conformational plasticity to enable cross-reactivity, a
structural adaptation not
seen in other shared cytokine receptors. This is perhaps not surprising,
considering IL-34
shares merely 11% sequence identity with CSF-1 and has escaped the recognition
by
predictive bioinformatics routines (Conklin et al., Bioinformatics 21: 1776-
1781 (2005)),
though more sensitive fold recognition methods fare better (JFB, unpublished).
CSF-1R
manages to keep impressive affinity towards IL-34 and CSF-1 during evolution
by a
combination of inter-domain structural plasticity and compositional changes of
interfacial
residues. This provides a very efficient though not the only mechanism, as
witnessed in other
cytokine systems, of degenerate recognition of distantly-related helical
cytokines.
Example 2: Characterization of human anti-IL-34 antibodies
Methods
Epitope mapping by competition ELISA
108
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
ELISA plates were coated with 1H muIL-34flag (1 ug/ml) in PBS at 4 C overnight
or at room
temperature for 2 hours and blocked using PBS with 1%BSA and 0.15% Tween20.
Biotinylated YW404.33 antibody at a concentration of 30 nmolar was added to
seven serial
dilutions of anti-IL-34 antibody to be tested starting at a concentration of
300 nmolar. The
antibody mixtures were briefly preincubated at room temperature. The mixtures
were then
added to the coated ELISA plates and incubated for about 30 minutes to about 1
hour at room
temperature. Plates were washed and bound biotinylated antibody was detected
with SA-
HRP. Anti-muIL-34 and Herceptin were used as controls in this assay.
Results
Human anti-IL-34 antibodies were generated using phage display technology and
tested for
their ability to neutralize IL-34 activity in cell based assays as described
in Example 1. The
neutralizing activity of anti IL-34 antibody YW404.1, YW404.6 and YW404.33,
for example,
is shown in Figure 9A. The specificity of these antibodies for human versus
murine IL-34 as
well as their blocking activity, and binding affinity were compared (Table 7).
Furthermore,
these antibodies were tested in a competition ELISA to determine if they bind
to overlapping
epitopes on IL-34 (Table 7).
Table 7: Comparison of YW404.1, YW404.6, YW404.33
Blocking Binding
h/m Specificity Epitope
Activity Affinity
YW404.1 Cross ++ +++ distinct
overlapped
YW404.6 murine ++ + with
YW404.33
overlapped
YW404.33 Cross +++ +++
with YW404.6
Several human anti-IL-34 antibodies were then affinity-matured using soft
randomization
strategy and their affinity was measured as described in Example 1. Affinity-
matured
antibodies were further tested in cell-based neutralization assays as
described in Example 1.
As shown in Figure 9B, affinity improvement correlated with better cell
blocking activity for
anti IL-34 Abs YW404.33, YW404.33.12 and YW404.33.56.
Furthermore, IC50 values were determined based on the ability of the antibody
to neutralize
the bioactivity of IL-34 on mononuclear cells MNFS60. To measure the ability
of the
antibody to neutralize the bioactivity of flag-tagged mIL-34 on MNFS50, a cell
proliferation
assay by CellTiter-Glo0 was used. Based on cell response to serial dilutions
of IL-34, 50
ng/ml of IL-34 amount was selected for determining the antibody neutralizing
activity. The
109
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Half Maximal Inhibitory Concentration (IC50) is defined as the concentration
of antibody
required to yield half maximal inhibition of IL-34 activity on cells, when IL-
34 is present at a
concentration to elicit 70-80% proliferation response.
50 ng/ml hIL-34 was combined with serial dilutions of anti-1L34 mAbs before
adding onto
cells in a total volume of 100 ul. The antibody inhibition activity was
obtained by measuring
RLU after incubating the plates at 37 C for 72 hours. IC50 was calculated
with
KaleidaGraph. For example, anti IL-34 Abs YW404.33.56, 404.33 and YW 404.33.93
had
IC50 values of 20.21 ng/ml, 77.42 ng/ml and 31.62 ng/ml, respectively.
The sequences of the variable heavy and light chain and the CDR regions of
these antibodies
as well as YW404.1, YW404.6, YW405.3, YW404.33.10 YW404.33.12 and YW404.33.11
are shown in Figures 10A and B.
Example 3: Inhibition of DSS-induced inflammatory bowel disease in mice using
a
combination of anti-CSF-1 antibody and anti-IL-34 antibody
Methods
Balb/c mice were pre-weighed and randomized prior to treatment and treated as
follows.
Group 1 (n=8 per group) received normal drinking water without dextran sulfate
sodium
(DSS) throughout the experiment. Groups 2 ¨ 6 were kept on drinking water with
3% DSS
(dextran suldium sulfate 3g/100 ml (3%)) from day 0 to day 6 of the
experiment. After day 6,
groups 2 ¨ 6 received normal drinking water without DSS until sacrificed.
Group 2 was
treated with 400 ug anti-ragweed antibody (a-RW-mIgG2 a) every other day
starting one day
before day 0 until day 8 as negative control. Group 3 was treated daily,
starting one day
before day 0 until day 8, with 25 mg/kg cyclosporine A in 0.9% sodium chloride
(CSA),
intraperitoneally. Group 4 was treated with 200 ug of an anti-CSF-1 antibody
(rat antibody of
ATCC #CRL-2702, clone 5A1) and 200 ug a-RW antibody every other day starting
one day
before day 0 until day 8. Group 5 was treated with 200 ug anti-IL-34 antibody
(YW
404.33.12) and 200 ug a-RW antibody every other day starting one day before
day 0 until day
8. Group 6 was treated with 200 ug anti-CSF-1 antibody rat antibody of ATCC
#CRL-2702,
clone 5A1) and 200 ug anti-IL-34 antibody (YW 404.33.12) every other day
starting one day
before day 0 until day 8. All mice were sacrificed on day 8 and their colitis
severity score was
determined based on parameters such as cryptloss and infiltration of
inflammatory cells
including T-cells, B-cells and macrophages.
110
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
Results
Mice with DSS-induced IBD that were treated with either the anti-IL-34
antibody or the anti-
CSF-1 antibody showed a reduced colitis severity score as compared to mice
treated with the
control a-RW antibody. The inhibition of both, CSF-1 and IL-34 using a
combination of the
anti-CSF-1 antibody and the anti-IL-34 antibody was even more effective in
this IBD model
(Figure 11).
The involvement of IL-34 and CSF-1 in IBD was further supported by measuring
serum
levels of IL-34 and CSF-1 in control (no DSS) mice and mice with DSS-induced
IBD treated
with control antibody (a-RW). The levels of CSF-1 and IL-34 were determined
using
ELISAs. Mice with DSS-induced IBD treated with control antibody showed
elevated IL-34
and CSF-1 levels compared to control mice (Figure 12).
Example 4 ¨ IL-34 and CSF-1 in human RA patients
Expression of CSF-1, IL-34 and TNFalpha protein in the serum, synovial fluid
and tissue
from rheumatoid arthritis patients is shown in Figure 13. IL-34 is expressed
at lower levels
than CSF-1 and TNFalpha in those fluids. IHC staining of synovium tissue from
RA patients
with anti-1L34 antibody indicates that IL-34 is likely enriched in the
tissue/extracellular
matrix (ECM).
Microaray results measuring gene expression of Myeloid subtype genes in the
joints of RA
patients who (A) responded to TNF blockade (TNF-R) or did not respond to a TNF
blockade
(TNF-NR) or (B) who were treated with rituximab after non-responsive treatment
with a TNF
blockade are shown in Figure 14. Rituximab non-responders are "Rituximab-NR"
and
rituximab responders are "Rituximab-R." The Myeloid subtype enriches for anti-
TNF
responders (TNF-R). The results also indicate that the CSF1/IL34 pathway is
involved in
primary and secondary TNF-NR RA patients. As the transcripts of IL34 and CSF1
are
present at high levels in TNF-NR and Rituximab-NR it is likely that the
IL34/CSF1 pathway
significantly contributes to pathogenicity in these patients not responding to
anti-TNFa or
Rituximab therapy. Blocking both cytokines in these patients is likely to be
required for
clinical benefit.
Example 5 ¨ Inhibition of both IL-34 and CSF-1 in CIA models
The treatment combination of anti-CSF1 antibody with anti-1L34 antibody was
compared
against TNFRII-Fc in a mouse CIA model (Figure 15). DBA-1J mice were injected
with
111
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
bovine collagen type II in CFA at day 0 and 21 by i.d. The arthritis score was
then monitored
daily. The antibody treatment started at either day 24 or day 31 (arthritis
established, clinical
score = 4) for 7 weeks before termination. The antibody treatments were: a-
ragweed (antibody
mIgG2a isotype control), TNFRII-Ig (mIgG2a), anti-muCSF1 (in-house, mIgG2a),
anti-1L34
(YW404.33.12, mIgG2a), anti-mCSF1+anti-1L34 (combination of 3 &4), anti-mCSF1
(DANA)+anti-1L34 (YW404.33.12 DANA), All animals (n=10/group) were treated
with
200ug/animal, 3 times/week of the above reagents by ip. for 7 weeks before
termination. The
endpoint PD including longitudinal clinical score, histopathology (paw and
related tissues),
FACS (tissue monotyes subsets and Mf) and bone volume (uCT) analysis.
The study shows the comparable or trending better inhibition of clinical
scores and histology
scores (inflammation, fibroplasia, cartilage) with the combination of anti-
CSF1 antibody and
anti-IL-34 antibody over TNFRII-Fc, especially with the antibodies that have
reduced ADCC
activity (i.e., DANA mutation is D265A, N297A in the Fc region). Furthermore,
the
treatment with a combination of aCSF1+aIL34 is clearly superior compared to
TNFRII-Fc in
protecting against bone erosions, and furthermore, treatment with the
combination of anti-
CSF1 and anti-IL-34 antibodies is superior to either one alone.
Example 6 ¨ Inhibition of both IL-34 and CSF-1 in additional IBD models
In another model for IBD, DSS colitis was induced in C57BL/6J Female, 6-8 wk
old mice
(Figure 16). C57B6 mice were orally administered 3% DSS for 5 days to induce
acute colitis,
characterized by epithelial damage, reversible weight loss and neutrophilic
infiltration in large
intestine. The antibody treatment started at -1 days with 4 doses total. The
study was
terminated at day 8 for analysis. The treatment groups (n=10/group) : 3% DSS +
a-ragweed
(mIgG2a control, 400 ug/mouse. ip), 3% DSS + a-mCSF1 Ab (in house,
200ug/mouse, ip),
3% DSS + a-1L34 Ab (YW404.33.12, 200ug/mouse ip.), 3% DSS + a-mCSF1/a-1L34
(200ug/mouse a-CSF1 and 200ug/mouse a-1L34, ip.). Dexamethasone was
administered at
0.5 mg/kg, QD, ip. The naïve mice were not treated with DSS. Anti-ragweed
antibody was
used as a negative control. After termination the colon histology score was
readout for this
model.
Analysis of TNFAARE colitis mice showed that there is higher CSF-1/IL-34 and
CSF-1R
protein and mRNA expression when there is higher myeloid cell proliferation
and activation
(data not shown). Mf and monocytes were significantly proliferated and
activated in
TNFAARE mice spleen. TNFAARE mice produced higher CSF-land IL-34 in gut tissue
112
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
compared to wild-type (wt) mice. Higher CSF-1R, CSF1 & IL-34 mRNA expression
levels
were observed in TNFAARE mice ileum compared to wild-type mice.
Example 7 ¨ IL-34 and CSF-1 in human Crohn's and UC patients
An analysis of CSF-1 and IL-34 protein from serum and tissue of human patients
suffering
from Crohn's disease or ulcerative colitis showed that IL-34 is expressed at
very low levels in
the sera, but is expressed at higher levels in tissue. CSF-1, on the other
hand, is expressed
well in the sera and in tissue (Figure 17). This tissue data further supports
the belief that
inhibition of both IL-34 and CSF1 in inflammatory bowel disease, including
ulcerative colitis
and Crohn's disease, will be superior to treating IBD patients with inhibitors
of either alone.
In RA patients, IL-34 protein expression was lower tha CSF1, but easily
detectable in serum
and synovial fluid. In those same patients, CSF-1 protein expression was
highly expressed in
both the serum and the synovial fluid (Figure 13).
An analysis of IL-34, CSF-1 and TNFalpha protein in the synovial fluid of RA
and
osteoarthritis patients showed that there is no correlation between expression
levels of IL-
34/CSF-1 and TNFa in those fluids in rheumatoid arthritis and osteoarthritis
patients (Figure
18). Therefore, the data indicates that are patients suffering from RA and
osteoarthritis that
have distinct molecular profiles, i.e., TNFalpha epression versus CSF-1/IL-34
expression,
despite their similar phenotypes.
Example 8 ¨ Inhibition of IL-34 and CSF-1 reduces the infiltration of myeloid
cells
Mouse CIA was set up as mentioned previously. The animal joint synovial
cell/tissue were
collected from the mice treated by a-CSF1+a-1L34 combination Abs or anti-
ragweed for twice
at beginning on day 39 (established CIA, clinical score = 4) =in 7 days period
(n=3 / sample).
Animal were euthanized after 7 days treatment. The joint synovial tissue/cells
were collected
and collagenase digested for single cell suspension. Cells were stained with
labeled anti-
CD1 lb, Ly6C, Ly6G and F4/80 antibodies (purchase from BD Biosystem). Labeled
cells
were analyzed by flow cytometer to defined the myeloid subsets (Mf:
CD11b+/F480+,
inflammatory monocytes: CD11b+/Ly6C+/Ly6Glow and residential monocytes:
CD11b+/Ly6G+/Ly6C low).
This data shows the reduction of mouse myeloid cells (Mf and monotyes)
infiltrating joint
synovia after only 7 days of anti-CSF1/IL-34 combination treatment of already
arthritic
animals before any clinical benefit can be observed. This shows that a major
mechanism for
113
CA 02861122 2014-07-14
WO 2013/119716
PCT/US2013/024998
myeloid inhibition as the result of combo therapy inhibition is the reduction
of myeloid cell
migration, infiltration and or expansion in the synovium.
Although the foregoing invention has been described in some detail by way of
illustration and
example for purposes of clarity of understanding, the descriptions and
examples should not be
construed as limiting the scope of the invention. The disclosures of all
patent and scientific
literature cited herein are expressly incorporated in their entirety by
reference. These
disclosures include the publication cited as Ma et al., (2012) Structure
20:676-687 and
United States Provisional Application No. 61,595,658, filed February 6, 2012
and United
States Provisional Application No. 61/680,674, filed August 7, 2012, from
which this
application claims benefit, all of which are incorporated by reference in
their entirety.
114