Note: Descriptions are shown in the official language in which they were submitted.
81781023
PERFUSION BIOREACTOR SYSTEMS COMPRISING A CELL AGGREGATE
TRAP AND METHODS OF OPERATING THE SAME
RELATED APPLICATIONS
[0001] The present application claims the benefit of and
priority to U.S. Provisional Patent Application No.
61/587,940 filed January 18, 2012, and entitled "PERFUSION
BIOREACTOR SYSTEMS AND METHODS OF OPERATING THE SAME"
(Attorney Docket No. BH-001/L).
BACKGROUND
[0002] Conventional perfusion bioreactor systems and
processes include a bioreactor that functions to culture
cells in a fluid medium such as a tissue culture fluid (TCF).
The cultured cells and TCF are removed from the bioreactor,
such as by pumping, and the cells are separated from the TCF
by a conventional cell retention unit. A harvest output
stream from the cell retention unit still containing some
cells, particles, and debris is then further processed.
Harvest output as used herein contains the TCF that is
further processed to obtain the desired product (e.g.,
coagulation factor). Filtration technologies, such as dead-
end depth filtration, membrane filtration, microfiltration,
and/or centrifugation can be used to further concentrate
and/or purify the harvest output from the cell retention
unit.
[0003] Another output stream of TCF exiting the cell
retention unit having a relatively high concentration of
cells is directly returned (e.g., recycled or re-circulated)
to the bioreactor. In such a continuous perfusion bioreactor
process, the harvest output stream and the recirculation
1
Date Recue/Date Received 2020-04-30
CA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
output stream are substantially continuous during the
cultivation period, which can be ten days or more. However,
using this type of conventional perfusion configuration can
lead to conditions where cell density within the bioreactor
may be relatively inadequately controlled.
[0004] Accordingly, there is a need for perfusion
bioreactor systems and methods that more effectively control
bioreactor cell density.
2
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
SUMMARY
[0005] In a first embodiment, a perfusion bioreactor
system is provided. The perfusion bioreactor system
comprises (1) a bioreactor configured to contain a tissue
culture fluid and cells to be cultured; (2) a cell retention
unit configured to receive tissue culture fluid containing
cells from the bioreactor, separate some cells from the
tissue culture fluid and provide harvest output of tissue
culture fluid and cells, and provide a recirculation output
of tissue culture fluid and cells; and (3) a cell aggregate
trap configured to receive the recirculation output of
tissue culture fluid and cells, separate cell aggregates
from the recirculation output of tissue culture fluid and
cells, and return the remaining tissue culture fluid and
cells to the bioreactor.
[0006] In another embodiment, a cell aggregate trap is
provided. The cell aggregate trap comprises (1) a
sedimentation chamber; (2) a trap inlet configured to
receive a recirculation output of tissue culture fluid and
cells; (3) a side flow chamber configured to return at least
some of the recirculation output of tissue culture fluid
containing cells to a bioreactor; and (4) a discard trap
outlet coupled to the sedimentation chamber configured to
output cell aggregates.
[0007] In another system embodiment, a perfusion
bioreactor system is provided. The perfusion bioreactor
system comprises (1) a bioreactor configured to contain a
tissue culture fluid and cells to be cultured; (2) a cell
retention unit configured to separate some cells from the
tissue culture fluid and provide harvest output; and (3) a
3
81781023
cell aggregate trap configured to separate cell aggregates from
the tissue culture fluid and cells and provide an output having
relatively lower amount of cell aggregates.
[0008] In a method embodiment, a method of operating a
perfusion bioreactor system is provided. The method comprises
(1) providing tissue culture fluid containing cells to a cell
retention unit from a bioreactor (2) separating in the cell
retention unit some cells from the tissue culture fluid to
provide a harvest output of tissue culture fluid and cells and
a recirculation output of tissue culture fluid and cells; and
(3) separating in a cell aggregate trap, cell aggregates from
the recirculation output of tissue culture fluid and cells. The
tissue culture fluid and cells can be returned to the
bioreactor having relatively lower amount of cell aggregates.
[0009] In another method embodiment, a method of operating a
perfusion bioreactor system is provided. The method comprises
(1) providing a flow of tissue culture fluid and cells from a
bioreactor; (2) separating in a cell retention unit some cells
from the tissue culture fluid to provide a harvest output; and
(3) separating in a cell aggregate trap, cell aggregates from
the tissue culture fluid and cells, so as to produce tissue
culture fluid having relatively lower amounts of cell
aggregates. The tissue culture fluid and cells can be returned
to the bioreactor having relatively lower amount of cell
aggregates.
[0009a] The present application as claimed relates to:
- a cell aggregate trap, comprising: a trap inlet for receiving
a recirculation output of tissue culture fluid and cells; a
sedimentation chamber to separate cell aggregates from the
4
Date Recue/Date Received 2020-04-30
81781023
tissue culture fluid containing cells; a side flow chamber
having a trap outlet to provide an output of tissue culture
fluid containing cells being in fluid communication with the
sedimentation chamber; and a discard trap outlet coupled to the
sedimentation chamber configured to output cell aggregates, the
sedimentation chamber comprising an upper region and a lower
region, the upper region being positioned above a centerline of
the side flow chamber, while the lower region being positioned
below the centerline of the side flow chamber;
- a perfusion bioreactor system, comprising: a bioreactor
having a bioreactor inlet, a bioreactor outlet and a culture
chamber to contain a tissue culture fluid and cells to be
cultured; a cell retention unit fluidly coupled to the
bioreactor outlet and having an inlet for receiving tissue
culture fluid containing cells from the bioreactor, a cell
separation technology to separate some cells from the tissue
culture fluid, the cell separation technology being disc
filters, spin filters, flat sheet filters, micro-porous hollow
fiber filters, cross-flow filters, vortex-flow filters,
continuous centrifuges, centrifugal bioreactors, gravity
settlers, ultrasonic wave devices, or hydrocyclones, a first
outlet to provide harvest output of tissue culture fluid and
cells, and a second outlet to provide a recirculation output of
tissue culture fluid and cells; and a cell aggregate trap as
described herein fluidly coupled to the second outlet;
- a perfusion bioreactor system, comprising: a bioreactor
having a bioreactor inlet, a bioreactor outlet and a culture
chamber to contain a tissue culture fluid and cells to be
cultured; a cell aggregate trap as described herein fluidly
coupled to the bioreactor outlet; and a cell retention unit
4a
Date Recue/Date Received 2020-04-30
81781023
fluidly coupled to the trap outlet and having an inlet for
receiving tissue culture fluid containing cells from cell
aggregate trap, a cell separation technology to separate some
cells from the tissue culture fluid, the cell separation
technology being disc filters, spin filters, flat sheet
filters, micro-porous hollow fiber filters, cross-flow filters,
vortex-flow filters, continuous centrifuges, centrifugal
bioreactors, gravity settlers, ultrasonic wave devices, or
hydrocyclones, a first outlet to provide harvest output of
tissue culture fluid and cells, and a second outlet to provide
a recirculation output of tissue culture fluid and cells to the
bioreactor; and
- a method of operating a perfusion bioreactor system as
described herein, comprising: providing tissue culture fluid
containing cells to the cell retention unit from the
bioreactor; separating in the cell retention unit some cells
from the tissue culture fluid to provide a harvest output of
tissue culture fluid and cells, and a recirculation output of
tissue culture fluid and cells; and separating in the cell
aggregate trap, cell aggregates from the recirculation output
of tissue culture fluid and cells.
[0010]
These and other features of the present teachings are
set forth herein.
4b
Date Recue/Date Received 2020-04-30
CA 02861270 2014-07-15
WO 2013/109520
PCT[US2013/021533
BRIEF DESCRIPTION OF THE DRAWINGS
[00].1] The skilled artisan will understand that the
drawings, described below, are for illustration purposes
only. The drawings are not intended to limit the scope of
the present teachings in any way.
[0012] FIG. 1 shows a block diagram of an embodiment of a
perfusion bioreactor system including a cell aggregate trap
according to the embodiments.
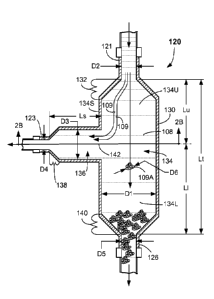
[0013] FIG. 2A shows a cross-sectioned side view of a
cell aggregate trap according to the embodiments.
[0014] FIG. 2B shows an upwardly looking cross-sectioned
end view of an embodiment of a cell aggregate trap taken
along section line 2B-2B of FIG. 2A.
[0015] FIG. 3 shows a flowchart illustrating a method of
operating a perfusion bioreactor system according to the
embodiments.
[0016] FIG. 4 shows another flowchart illustrating
another method of operating a perfusion bioreactor system
according to the embodiments.
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
DESCRIPTION OF VARIOUS EMBODIMENTS
[0017] Culturing of cells (including animal, plant, or
microbial cells) can be used to produce biologically-active
substances and pharmaceutically-active products. However, in
certain cell cultures, the cells can, to some extent, adhere
to one another and form relatively large cell agglomerates,
cell clumps, or aggregations (hereinafter referred to as
"cell aggregates"). When such cell aggregates are present,
they can cause certain processing problems in the perfusion
hioreactor process. In particular, the presence of cell
aggregates can cause a cell density within the bioreactor to
be relatively unstable, i.e., it is difficult to adequately
maintain, hold, or control within a desired cell density set
point range. It is also difficult to accurately measure the
cell concentration in the presence of cell aggregates. As a
result, from time-to-time, TCF and cells need to be
discarded by periodically operating a discard pump of the
conventional perfusion bioreactor system. Furthermore, it is
difficult to determine the amount of discarded TCF and cells.
Of course, this discard also discards valuable TCF
containing the desired product. Moreover, the cells in
bioreactor culture, particularly animal or plant cells are
generally very sensitive to imparted mechanical shear
forces. Accordingly, it is not only desired to minimize
discarded material, but it is desirable to minimize exposure
of the cells to possibly damaging shear forces. Furthermore,
it is desired to be able to precisely control cell density
within the bioreactor.
[0018] Therefore, according to embodiments of the present
invention, an improved perfusion bioreactor system is
provided. The improved perfusion bioreactor system comprises
6
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
a cell aggregate trap that is provided, configured and/or
adapted to operate in conjunction with a cell retention unit.
The cell aggregate trap is functionally based upon
sedimentation wherein cell aggregates settle out and can be
removed from a re-circulating flow stream. By the use of a
cell aggregate trap in conjunction with the cell retention
unit in the perfusion bioreactor system, a relatively high
majority of the cells can be returned to the bioreactor and
cell aggregates, that can be detrimental to the perfusion
process, can be removed and discarded.
[0019] According to other embodiments, the perfusion
bioreactor system comprises a bioreactor, a cell retention
unit coupled to the bioreactor, a cell retention unit
configured to receive TCF and cells from the bioreactor,
separate some cells from the TCF and provide a harvest
output, and a cell aggregate trap configured to receive a
recirculation output of TCF and cells from the cell
retention unit, separate cell aggregates from the TCF and
cells, and return the remaining TCF and cells to the
bioreactor.
[0020] In another embodiment, a method of operating a
perfusion bioreactor system is provided. The method
comprises providing TCF and some cells, received the TCF and
cells in a cell aggregate trap, and separating in the cell
aggregate trap cell aggregates from the TCF and cells. The
remaining TCF and cells can be returned to the bioreactor
having relatively low amount of cell aggregates.
[0021] In another embodiment, a method of operating a
perfusion bioreactor system is provided. The method
comprises providing a TCF containing cells to a cell
retention unit from a bioreactor, separating some cells from
the TCF to provide a harvest output, with the remaining re-
7
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
circulated TCF and cells being received by a cell aggregate
trap, and separating in the cell aggregate trap, cell
aggregates from the TCF and cells. Remaining TCF and cells
can be returned to the bioreactor having relatively lower
amount of cell aggregates. The methods, perfusion bioreactor
systems, and cell aggregate traps described herein can be
adapted for coagulation factor production and/or other
suitable processes for producing biological agents or
factors.
[0022] These and other embodiments of perfusion
bioreactor systems comprising cell aggregate traps, cell
aggregate traps, and methods of operating perfusion
bioreactor systems are described below with reference to
FIGs. 1-4. FIG. 1 illustrates a block diagram of an
embodiment of a perfusion bioreactor system 100. The
perfusion bioreactor system 100 comprises a bioreactor 102
having a bioreactor inlet 104 and a bioreactor outlet 106.
The bioreactor 102 comprises a culture chamber 105
configured to hold a tissue culture fluid (TCF) 108 and
cells 139 to be cultured. The perfusion bioreactor system
100 can be used for the production of biologics such as
coagulation factors. For example, the perfusion bioreactor
system 100 and methods can be used to manufacture
coagulation factors such as Factor VII, VIII, or Factor IX,
or other suitable factors or substances.
[0023] Example methods for production of Factor VIII are
described in US 6,338,964 entitled "Process and Medium For
Mammalian Cell Culture Under Low Dissolved Carbon Dioxide
Concentration," the disclosure of which is hereby
incorporated by reference in its entirety herein. For
example, a cell culture process can comprise culturing cells
in a TCF which contains a high concentration of a complexing
agent and a buffer which is low in added NaHCO3
8
GA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
concentration. The cell culture process can be carried out
in a culture chamber, such as culture chamber 105 in FIG. 1,
which can be a stirred tank fermenter with stirring
impellers in some embodiments. The fermenter can be provided
with a microsparger at a bottom of the culture chamber or a
membrane as an oxygenation system. The TCF can be a medium
composition based on a commercially available DMEM/F12
formulation manufactured by JRH (Lenexa, Kansas) or Life
Technologies (Grand Island, N.Y.) supplied with other
supplements such as iron, Piuronic F-68, or insulin, and can
be essentially free of other proteins. Compiexing agents
histidine (his) and iminodiacetic acid (IDA) can be used,
and organic buffers such as MOPS (3-[N-
Morpholino]propanesuifonic acid), TES (N-
tris[Hydroxymethyi]methy1-2-aminoethanesulfonic acid), BES
(N,N-bisL2-Hydroxyethyij-2-aminoethanesulfonic acid) and
TRIZMA (tris[Hydroxvmethvl]aminoethane) can be used; all of
which can be obtained from Sigma (Sigma, St. Louis, Mo.),
for example. In some embodiments, the TCF can be
supplemented with known concentrations of these complexing
agents and organic buffers individually or in combination.
The TCF can contain EDTA, e.g., 50 uM, as an iron chelating
agent. Other compositions, formulations, supplements,
complexing agents and/or buffers can be used.
[0024] Cell cultivation can be started by inoculating
with cells from previously-grown culture. Typical bioreactor
parameters can be maintained (e.g., automatically) under
stable conditions such as temperature at about 35 C to 37 C,
pH at about 6.8 to 7.0, dissolved. oxygen. (DO) at about 30%
to 70% of air saturation, stirring speed at about 30 rpm to
80 rpm, and approximately constant liquid volume. Other
bioreactor parameters can be used. DO and pH can be measured
on-line using commercially-available probes. The bioreactor
9
GA 02001270 2014-07-15
WO 2013/109520
PCT/US2013/021533
process can be started in batch mode for about. 1-2 days,
allowing the initial cell concentration to double. This can
be followed by a perfusion stage wherein the TOT is pumped
continuously into the bioreactor and the TOE' containing
cells (and possibly some cell aggregates) are pumped out. A
flow rate of TOE' can be controlled and increased
proportionally with the cell concentration. A steady state
or stable perfusion process can be attained when the cell
concentration reaches a target high level (e.g., about. 10
x106celis/mL to 20 x106ceils/mL) in the bioreactor and can
be controlled at this concentration. At this point, the flow
rate can be held constant. The cell density can be held.
between about 4 million to about 40 million cells per
milliliter in the perfusion bioreactor system. Other
biologics, coagulation factors, cell concentrations, cell
densities or the like can be employed.
[0025] Referring again to FIG. 1, the cells 109 can be
eukaryotic or prokaryotic such as animal, plant, or
microbial cells. For example, the cells 109 can be baby
hamster kidney cells (BHK cells), hybrid of kidney and B
cells (HKB cells), human embryonic kidney cells (HEK cells -
also referred to as HEK 293 or 293 cells), or the like. The
TCF 109 can be introduced into the culture chamber 105
through TCF inlet 105A, or elsewhere in the perfusion
bioreactor system 100. The cells 109 in the TCF 108 can, due
to their properties and processing, at times form cell
aggregates 109A, as shown in the enlarged view. "Cell
aggregates" as used herein means a cell agglomerate, cell
clump, or aggregation of cells that are connected and
adhered to each other to form a grouping of cells. "Cell
aggregates" that can be removed by using one or more of the
present embodiments can number about 10 or more cells, about
20 or more cells, or even about 40 or more cells. One or
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
more of the present embodiments can remove cell aggregates
in a range of from about 10 cells to about 50,000 cells, or
even in a range of from about 40 cells to about 300 cells.
More generally, cell aggregates 109A that can be removed by
using one or more of the various embodiments can include
cell agglomerates of a size and shape where at least some
internal cells in the agglomerate will tend to die off due
to lack of adequate oxygen and/or nutrients during the
perfusion process. Generally, cell aggregates are quite
large. For example, cell aggregates 109A having a minimum
dimension (across the cell aggregate) of about 60 microns or
more, or even 100 microns or more can be separated and
removed by using various embodiments of the invention. One
or more of the present embodiments can remove cell
aggregates having a minimum dimension in a range of about 60
microns to about 3,000 microns, or even in a range of about
100 microns to about 500 microns. Additionally or
alternatively, smaller cell aggregates can be separated and
removed. The presence of cell aggregates 109A in the
bioreactor 102 is generally undesirable, and the present
perfusion bioreactor system 100 and methods 300, 400
described herein can remove at least some, and in many cases,
most of the cell aggregates 109A therefrom. The depicted
perfusion bioreactor system 100 comprises a cell retention
unit 110 fluidly coupled to the bioreactor 102 and
configured to receive TCF 108 containing cells 109 in a
first cell concentration (Cl) (including possibly some cell
aggregates 109A) from the bioreactor 102. Example first cell
concentrations (Cl) can range from about 4 x 10"6 cells/mL
to about 40 x 10^6 cells/mL. Other cell concentration ranges
can be used. The TCF 108 containing cells 109 in the first
concentration (Cl), in the depicted embodiment, are expelled
from the bioreactor outlet 106 and received at a cell
11
GA 02001270 2014-07-15
WO 2013/109520 PCT/US2013/021533
retention unit inlet 112 by passing through a first conduit
113. The conduit 113 can couple to an optional heat
exchanger 113H that functions to cool the TCF 108 containing
cells 109 that are expelled from the bioreactor outlet 106.
The cell retention unit 110 is configured and operational,
and therefore functions to separate most of the cells 109
from the TCF 108 and provide a harvest output of TCF 108
containing only a small amount of cells 109 having a second
cell concentration (02) at a first retention unit outlet 114.
Accordingly, the second cell concentration (02) is less than
the first concentration (Cl), that is 02 < Cl, and in
particular, 02 << C1). Example second cell concentrations
(02) can range from about 0.1 x 10^6 cells/mL to about 2 x
10^6 cells/mL. Other cell concentration ranges can be used.
The so-called harvest output passes from the first retention
unit outlet 114, through second conduit 115, such as by a
pumping action of a harvest pump 117 coupled to the second
conduit 115. The harvest output can be further isolated
and/or purified in downstream isolation and purification
processes 118. These additional isolation and purification
processes 118 can be carried out in a continuous or batch
fashion. For example, these downstream isolation and
purification processes 118 can be carried out as described
in US Pub. No. 2008/0269468 entitled 'Devices And Methods
For Integrated Continuous Manufacturing Of Biological
Molecules," the disclosure of which is hereby incorporated
by reference herein in its entirely. In a batch mode, for
instance, once a specified volume of harvest has been
collected, which is typically after 1-4 days or more, one or
more harvest collection vessels can be disconnected from a
sterile fermentation vessel and the collected material can
be designated as one harvest batch. The next step is to
remove cells, debris, and particles. In industrial scale
12
GA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
this can be done usina centrifugation followed by dead-end.
membrane filtration, or by dead-end depth-filtration
followed by dead-end membrane filtration. Another technique
such as tangential flow (or "crossflow") microfiltration or
any other suitable filtration technique can be also used- In
any case, the product of the particle removal process is a
batch of clarified tissue culture fluid (cTCF). This cTCF
can be purified (concentrated) by any suitable process such
as crossflow ultrafiltration or by packed. bed chromatography.
[0026] In a continuous mode, a volume of the harvest
output can be purified by a continuous purification system
integrated, with the perfusion bioreactor system, which can
be maintained under sterile conditions. "Continuous" as used
herein means uninterrupted in time, sequence, and/or
operation for prolonged periods. For example, the cell
retention unit 110 can carry out initial cell retention and
produce a harvest output of clarified TCF 108 at first
retention unit outlet 114. The isolation and purification
process 118 can comprise further filtering (isolation) of
the harvest output provided by the second conduit. 115 by a
suitable filter system having, in some embodiments, a final
filter rating of about 3 microns or smaller, 0.45 microns or
smaller, or even 0.2 microns or smaller to provide cTCF.
Other filter ratings can be used
[0027] The filtering process can be followed by a
purification process comprising a continuous ultrafiltration
separation process, for example. in some embodiments, the
ultrafiltration can occur at a specific flow rate below the
transition point of the molecule of interest in the
pressure-dependent region of the flux versus TMP curve,
wherein the specific flow rate is maintained substantially
constant throughout the continuous ultrafiltration.
Relatively higher yields can be achievable using a
13
GA 02001270 2014-07-15
WO 2013/109520
PCT/US2013/021533
continuous isolation and purification. process 118. In some
embodiments, the cTOF is passed through an ultrafiltration
membrane having an area in square meters approximately equal
to between 0.1 to 2 times the volumetric flow rate of the
cTOF in liters/hour, or even approximately equal to between
0.3 to I times the volumetric flow rate of the cTOF in
liters/hour. Other membrane areas can be used.
[0028] Following separation within the cell retention
unit 1101 a recirculation output of TCF 108 and a relatively
higher concentration of cells 109 are provided in a third
cell concentration (C3) at a second retention unit outlet
119. The third cell concentration (C3) is generally
relatively higher than the first concentration (Cl), that is
C3 > Cl because although some small volume of cells 109 will
be lost in the harvest output stream, the volume of TCF 108
extracted at the first retention unit outlet 114 is greater.
Example third cell concentrations (C3) can range from about
6 x 10^6 cells/mL to about 60 x 10^6 cells/mL. Other cell
concentration ranges can be used. The cell retention unit
110 can be based upon any known cell separation technology,
such as disc filters, spin filters, flat sheet filters,
micro-porous hollow fiber filters, cross-flow filters,
vortex-flow filters, continuous centrifuges, centrifugal
bioreactors, gravity settlers, ultrasonic wave devices,
hydrocyclones, and the like. Any suitable type of cell
retention unit 110 can be used that is configured and
operational, and therefore functional to separate an
incoming first cell concentration (Cl) into outgoing cell
concentrations (02 and C3).
[0029] In the depicted embodiment, the perfusion
bioreactor system 100 comprises a cell aggregate trap 120.
The cell aggregate trap 120 is configured and operational,
and therefore functional to receive the recirculation output
14
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
of TCF 108 and cells 109 at the third cell concentration
(C3) at a trap inlet 121 from the cell retention unit 110.
Cell retention unit 110 can be fluidly coupled to the cell
aggregate trap 120 by a third conduit 122. In some
embodiments, the functions of cell retention unit 110 and
the cell aggregate trap 120 can be integrated into one
single unit. Accordingly, in such an embodiment, the conduit
122 can be eliminated and the output of the cell retention
unit 110 can be directly received by the trap input 121.
[0030] The cell aggregate trap 120 functions to separate
cell aggregates 109A from the recirculation output of TCF
108 and cells 109 at the third cell concentration (C3)
received at the cell aggregate trap 120. In one
implementation, the separation of cell aggregates 109A is
carried out continuously; that is the flow is continuous
from the cell retention unit 110 during operation. Generally,
when present in the flow stream, at least some, and
generally a relatively high percentage of the cell
aggregates 109A are removed by the cell aggregate trap 120
and a remaining TCF 108 and cells 109 in a fourth cell
concentration (C4) are returned to the inlet 104 of the
bioreactor 102. Example fourth cell concentrations (C4) can
range from about 5 x 10^6 cells/mL to about 50 x 10^6
cells/mL. Other cell concentration ranges can be used. In
some embodiments, the perfusion bioreactor system 100 and
methods including the cell aggregate trap 120 can remove
about 20 percent to about 80 percent of cell aggregates,
although other percentages of cell aggregates can be removed.
[0031] The TCF 108 and cells 109 can exit the trap outlet
123 and pass through fourth conduit 124 to the bioreactor
inlet 104. One or more recirculation pumps 125 can be
provided and operated to cause flow of the TCF 108 and cells
109. The one or more pumps 125 can be located at any
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
convenient location, such as in conduits 113, 122, or 124 or
other suitable locations. In the depicted embodiment, the
pump 125 is coupled to the fourth conduit 124.
[0032] Additionally, the cell aggregate trap 120 can
comprise any suitable trap discard outlet 126 that is
configured and operational, and therefore functional to
allow a small amount of TCF 108 and some cell aggregates
109A to be removed from the cell aggregate trap 120. A fifth
cell concentration (05) is provided in the trap discard
outlet 126. Example fifth cell concentration (05) can range
from about 12 x 10'6 cells/mL to about 90 x 10'6 cells/mL.
Other cell concentration ranges can be used. Because some
cell aggregates 109A have been removed from the process flow
stream by the cell aggregate trap 120, the cell
concentration (C4) is generally less than the cell
concentration (03), that is 04 < 03. The cell aggregates
109A and small volumes of TCF 109 can flow from the trap
discard outlet 126 to be discarded. A discard pump 127 can
be continuously or periodically operated to flow cell
aggregates 109A and a small amount of TCF 108 through the
discard conduit 128 to a discard, such as a flexible bag, or
other type of discard container.
[0033] The structure and operation of the cell aggregate
trap 120 will now be described with reference to FIGs. 2A
and 2B. The cell aggregate trap 120 comprises a trap body
130 that can be made out of a rigid material, such as
stainless steel, glass, or plastic. Other materials can be
uscd. The TCF 108 and colic 109 (possibly including some
cell aggregates 109A) are received at the trap inlet 121,
such as at a top of the trap body 130, for example. As
depicted, the TCF 108 and cells 109 and possibly cell
aggregates 109A can, during operation, flow directly into an
expansion zone 132 that can be formed at a location directly
16
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
adjacent to the trap inlet 121 and into a sedimentation
chamber 134 of the cell aggregate trap 120. The expansion
zone 132 can be made up of angled or curved walls that
gradually increase a cross-sectional area of the
sedimentation chamber 134 along a length of the
sedimentation chamber 134. In the depicted embodiment, the
expansion zone 132 is shown as a frustoconical region.
However, any generally smooth transition between the cross-
sectional area of the trap inlet 121 to the cross sectional
area of the sedimentation chamber 134 can be used. In
general, a transitional rate of increase in area exiting
from the inlet 121 can be less than about 8.4 cm2/cm, and in
some embodiments less than about 4.2 cm2/cm, in an attempt to
minimize shear forces imparted to the cells 109. Other
transitional rates can be used. However, in some embodiments,
an expansion zone 132 may not be present.
[0034] The cell aggregate trap 120 can comprise a side
flow chamber 136. The side flow chamber 136 is constructed,
configured, and operational in conjunction with the
sedimentation chamber 134 to allow TCF 108 and cells 109 to
exit the cell aggregate trap 120 through trap outlet 123,
while allowing the cell aggregates 109A to settle out under
the force of gravity within the sedimentation chamber 134.
In the depicted embodiment, the side flow chamber 136 is
generally cylindrical and extends horizontally from a side
134S of the sedimentation chamber 134. For example, the side
flow chamber 136 can extend generally perpendicular from the
sedimentation chamber 134. However, other shapes,
configurations and orientations other than perpendicular can
be used.
[0035] Similar to the expansion zone 132 of the
sedimentation chamber 134, the cell aggregate trap 120 can
include a contraction zone 138 at a location directly
17
CA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
adjacent to the trap outlet 123 from the side flow chamber
136. In some embodiments, the contraction zone 138 can have
a transitional rate of area contraction no greater than
about 8.4 cm2/cm, and in some embodiments, of about 4.2
cm2/cm or less. Larger or smaller transition rates can be
used. In some embodiments, a D3/D4 ratio can be provided
that can be greater than about 2, for example. Similarly, a
discard contraction zone 140 can be provided at the trap
discard outlet 126 at a bottom of the sedimentation chamber
134. The trap discard outlet 126 can have a maximum
transverse dimension D5 (e.g., an inside diameter). In one
or more embodiments, the discard contraction zone 140 can
have a transitional rate of area contraction no greater than
about 8.4 cm2/cm, and in some embodiments, of about 4.2
cm2/cm or less. Larger or smaller transition rates can be
used. In some embodiments, a D1/D5 ratio can be greater than
about 2, for example.
[0036] In more
detail, the sedimentation chamber 134 can,
in some embodiments, have a circular cross section having
transverse dimension (D1) (e.g., an inside diameter) of
between about 1.9 cm and about 6.4 cm, and in some
embodiments between about 2.5 cm and about 5.1 cm. A maximum
cross-sectional area of the sedimentation chamber 134 can be
between about 2.9 cm2 and about 32 cm2, and in some
embodiments between about 5.1 cm2 and about 20 cm2, for
example. The trap inlet 121 can have a circular cross
section having transverse dimension (D2) (e.g., an inside
diameter) of between about 0.48 cm and about 1.6 cm, and in
some embodiments between about 0.64 cm and about 1.3 cm, for
example. In some embodiments, a D1/D2 ratio can be greater
than about 2, or even greater than about 4, for example.
However, other suitable cross-sectional shapes and sizes can
be used.
18
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
[0037] Suitable dimensions of the cell aggregate trap 120
can be dependent on a capacity of the perfusion bioreactor
system 100 (e.g., a volumetric throughput thereof), and the
dimensions thereof can be enlarged or decreased based upon
the flow capacity. The dimensions of the cell aggregate trap
120 can also depend on other factors such as fluid density
or viscosity, or the like. The maximum cross-sectional area
of sedimentation chamber 134 can be equal to or larger than
the maximum cross-sectional area of trap inlet 121. In the
depicted embodiment, the maximum cross-sectional area of
sedimentation chamber 134 is larger than the maximum cross-
sectional area of trap inlet 121. In particular, in some
embodiments, the maximum cross-sectional area of
sedimentation chamber 134 can be about 4 times or larger,
about 10 times or larger, about 30 times or larger, or even
about 60 times or larger than the maximum cross-sectional
area of trap inlet 121.
[0038] The sedimentation chamber 134 comprises an upper
region 134U and a lower region 134L. The upper region 134U
is positioned above a centerline 142 of the side flow
chamber 136, while the lower region 134L Is positioned below
the dente/line 142 of the side flow chamber 136. In the
depicted embodiment, a total length (Lt) of the
sedimentation chamber 134 from an upper end of the expansion
zone 132 to a lower end of the contraction zone 140 can be
between about 9 cm and 37 cm, and in some embodiments
between about 14 cm and 28 cm. A length (Lu) of the upper
region 134U from an upper end of the contraction zone 132 to
the centerline 142 of the side flow chamber 136 can be
between about 5 cm and 18 cm, and in some embodiments
between about 7 cm and 14 cm. A length (L1) of the lower
region 134L from a lower end of the contraction zone 140 to
the centerline 142 of the side flow chamber 136 can be
19
CA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
between about 5 cm and 18 cm, and in some embodiments
between about 7 cm and 14 cm. Other dimensions can be used.
Generally, it is desired that a ratio of Ll/Lu > 0.5, and in
some embodiments Ll/Lu > 4, for example, can be employed.
Other ratios can be used.
[0039] During operation, a volumetric flow rate through
the cell aggregate trap 120 is generally held at between
about 0.0025 m3/min and about 0.0068 m3/min, and in some
embodiments between about 0.0030 m3/min and about 0.0045
m3/min. Other volumetric flow rates (e.g., capacities) can be
used. However, in some embodiments, it is desirable to keep
the liquid flow generally within a laminar Reynolds number
range within the sedimentation chamber 134. Reynolds numbers
within the sedimentation chamber 134 can be less than about
2300, less than about 1000, or even less than about 500 in
some embodiments, in order to minimize mixing and promote
adequate settling and separation of the cell aggregates 109A,
wherein the Reynolds Number is approximately defined by
Equation 1.
Re = pQ/p Equation 1.
where:
Q is the volumetric flow rate of the fluid (m3/s),
u is the dynamic viscosity of the fluid (kg/(ms), and
p is the density of the fluid (kg/m3).
[0040] However, to promote adequate retention of the
cells 109 in Lhe flow stream such LliaL Lhe TCF 108 and cells
109 can exit the cell aggregate trap 120 from the trap
outlet 123, and generally resist setting out in the
sedimentation chamber 134, the flow within the sedimentation
chamber 134 can have Reynolds numbers sufficient to avoid
settling of the cells 109, for example.
CA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
[0041] The side flow chamber 136 can have a circular
cross section having maximum transverse dimension (D3) (e.g.,
an inner diameter) of between about 1.9 cm and about 6.4 cm,
and in some embodiments between about 2.5 cm and about 5.1
cm. A maximum cross-sectional area of the side flow chamber
136 can between about 2.9 cm2 and about 32 cm2, and in some
embodiments between about 5.1 cm2 and about 20 cm2, for
example. However, other suitable cross-sectional shapes and
sizes can be used. A total length (Ls) of the side flow
chamber 136 from an entry into the side flow chamber 136 to
an exit end of the contraction zone 138 can be between about
4 cm and 15 cm, and in some embodiments between about 5 cm
and 11 cm. The maximum outlet dimension (D4) (e.g., an inner
diameter) of the outlet 123 from the side flow chamber 136
can be between about 0.48 cm and 1.6 cm, and in some
embodiments between about 0.64 cm and 1.3 cm. Other
dimensions can be used. Flow in the side flow chamber 136
can have a Reynolds number of greater than about 2300, or
even greater than about 4000, for example. Other Reynolds
number ranges can be used. The Reynolds numbers can be
selected to minimize setting of cells 109 in the side flow
chamber 136.
[0042] In some embodiments, a maximum cross-sectional
area (Asc) of the sedimentation chamber 134 is equal to or
larger than a maximum cross-sectional area (Asfc) of the
side flow chamber 134, that is Asc Asfc. In particular,
the maximum cross-sectional area (Asc) of the sedimentation
chamber 134 can be the same, or even 5 times or more larger
than a maximum cross-sectional area of the side flow chamber
136. Other Asc/Asfc ratios can be used. The difference in
cross-sectional areas can generally function to improve
sedimentation capacity. Representative dimensions D1-D5
described herein are directed towards an example embodiment
21
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
of a perfusion bioreactor system 100 having a capacity of
about 2000 to 3000 liters per day of flow in second conduit
115 (FIG. 1). Perfusion bioreactor systems 100 having
smaller or larger capacities can benefit by using an
embodiment of the invention, such as a perfusion bioreactor
system 100 having a capacity of about 100 to 200 liters per
day of flow second conduit 115.
[0043] In
operation, the cell aggregate trap 120, through
appropriate dimensioning and volumetric flow rates provided
in the sedimentation chamber 134 and side flow chamber 136,
as recited herein, is configured and operational, and, thus
adapted to remove cell aggregates 109A of greater than or
equal to about 10 aggregated cells, greater than or equal to
about 20 aggregated cells 109, or even greater than or equal
to about 40 aggregated cells 109. In some embodiments, a
smaller number of aggregated cells can be removed. Cells 109
and TCF 108 are allowed to exit the side flow chamber 136.
Thus, undesirable cell aggregates 109A are removed by
operation of various embodiments of the invention. In some
embodiments, the undesirable cell aggregates 109A removed by
the cell aggregate trap 120 can have a minimum cross-wise
dimension (D6) (See FIG. 2A) of greater than about 60
microns, greater than about 100 microns, or even larger.
Smaller size cell aggregates can be removed. One advantage
of the use of the cell aggregate trap 120 is that a rate of
discard of TCF 108 from the perfusion bioreactor system 100
can be reduced. In particular, a rate of discard of TCF 108
can be slowed such that a discard cell concentration (05)
from the cell aggregate trap 120 is greater than or equal to
about 3 times the first cell concentration (Cl) (wherein C5
3C1), or even greater than or equal to about 5 times
(Cl) (wherein 05 5C1).
22
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
[0044] The cell aggregate trap 120 in the depicted
embodiment is shown installed at the exit from the cell
retention unit 110. However, it should be understood that
the cell aggregate trap 120 can be placed elsewhere in the
perfusion bioreactor system 100. For example, a cell
aggregate trap like the cell aggregate trap 120 can be
provided in the location of the first conduit 113 (e.g.,
adjacent to the bioreactor outlet 106 or cell retention unit
inlet 112, or otherwise coupled to the first conduit 113).
In this embodiment, the recirculation output of TCF 108 and
cells 109 including possibly cell aggregates 109A passes
through the cell aggregate trap and then to a cell retention
unit 110. Thus, cell aggregates 109A can be removed from the
flow stream prior to entry into the retention unit 110.
Optionally, a cell aggregate trap can be integrated into the
bioreactor 102, such as at or near the bioreactor inlet 104.
[0045] Methods of operating various embodiments of the
perfusion bioreactor system 100 will now be described with
reference to FIG. 3. One method 300 of operating the
perfusion bioreactor system 100 comprises, in 302, providing
to a cell retention unit (e.g., cell retention unit 110)
from d Lioieactor (e.g., bioreactor 102), a tissue culture
fluid (e.g., TCF 108) containing cells (e.g., cells 109 and
possibly some cell aggregates 109A). The tissue culture
fluid containing cells can be in a first concentration (Cl)
Furthermore, the method 300 comprises, in 304, separating in
the cell retention unit some cells from the tissue culture
fluid to provide a harvest output (e.g., in second conduit
115) of tissue culture fluid and cells and a recirculation
output of tissue culture fluid and cells. The harvest output
can be in a second cell concentration (C2). The
recirculation output of tissue culture fluid and cells can
be in a third cell concentration (C3). Recirculation output
23
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
of tissue culture fluid and cells can be provided in third
conduit 122. In 306, separating, in a cell aggregate trap
(e.g., cell aggregate trap 120), cell aggregates (e.g.,
109A) from the recirculation output of tissue culture fluid
and cells takes place. Finally, in 308, returning the tissue
culture fluid and cells to the bioreactor having relatively
lower amount of cell aggregates can be accomplished (the
relatively lower amount is in comparison to the tissue
culture fluid and cells that would be returned to the
bioreactor 102 without the cell aggregate trap 120). The
returning tissue culture fluid and cells can have a fourth
cell concentration (C4). Periodically or continuously during
the separation process, cell aggregates 109A separated from
the cells 109 within the sedimentation chamber 134 can
settle to the bottom of the sedimentation chamber 134 and
can exit and be discarded from the cell aggregate trap (e.g.,
cell aggregate trap 120), such as from trap discard outlet
126.
[0046] Another example method of operating the perfusion
bioreactor system 100 will now be described with reference
to FIG. 4. The method 400 comprises, In 402, providing a
flow of tissue culture fluid (e.g., TCF 108) and cells (e.g.,
cells 109 and possibly some cell aggregates 109A) from a
bioreactor (e.g., bioreactor 102). Furthermore, the method
400 comprises, in 404, separating in a cell retention unit
(e.g., cell retention unit 110) some cells from the tissue
culture fluid to provide a harvest output (e.g., output in
conduit 115). In 406, separating, in a cell aggregate trap
(e.g., cell aggregate trap 120), cell aggregates from the
tissue culture fluid and cells takes place. In 408,
returning the tissue culture fluid and cells to the
bioreactor having relatively lower amount of cell aggregates
can be accomplished (the relatively lower amount is in
24
GA 02861270 2()14-07-15
WO 2013/109520
PCT/US2013/021533
comparison to the tissue culture fluid and cells that would
be returned to the bioreactor 102 without the cell aggregate
trap 120). As should be recognized from the above, the cell
aggregate trap (e.g., cell aggregate trap 120) can be placed
after or before the cell retention unit (e.g., cell
retention unit 110), or elsewhere in the perfusion
bioreactor system 100 where cell aggregates 109A can be
effectively removed from a recirculation flow stream thereof.
Furthermore, more than one cell aggregate trap can be
provided in the perfusion bioreactor system.
[0047] The methods according to embodiments are useful
for removing cell aggregates (e.g., 109A) having greater
than or equal to about 10 aggregated cells, greater than or
equal to about 20 aggregated cells (or even greater than or
equal to about 40 aggregated cells) that can be adhered
together as a clump or mass (although smaller cell
aggregates can be removed in some embodiments). Ranges of
cell aggregates (e.g., 109A) as disclosed above can be
removed, for example. As such, density within the bioreactor
102 during the operation of the perfusion process can be
relatively more tightly controlled. Furthermore, In another
advantage, discard volume of the TCF 108 can be reduced.
Accordingly, this has a significant benefit in that product
volume loss can be minimized. This advantage is significant
even in perfusion bioreactor systems 100 where only a
relatively small amount of cell aggregates 109A are formed.
[0048] The foregoing description discloses only example
embodiments of cell aggregate traps, perfusion biorcactor
systems including a cell aggregate trap, and methods of
operating the perfusion bioreactor systems. It is not
intended that the present teachings be limited to such
embodiments. On the contrary, the present teachings
encompass various alternatives, modifications, and
CA 02861270 2014-07-15
WO 2013/109520
PCT/US2013/021533
equivalents, as will be appreciated by those of skill in the
art. For example, other embodiments of the cell aggregate
trap can be used to remove relatively small cell aggregates
should their presence be undesirable to the performance of
the perfusion bioreactor system. The section headings used
herein are for organizational purposed only and are not to
be construed as limiting the subject matter described in any
way.
26