Note: Descriptions are shown in the official language in which they were submitted.
CA 02861419 2014-07-15
DESCRIPTION
METHOD OF RECOVERING GOLD AND METHOD OF MANUFACTURING GOLD
USING THE SAME
Technical Field
[0001]
The present invention relates to a method of
recovering gold, and a method of manufacturing gold using
the method.
Background Art
[0002]
Gold is one of metals of great value and is present
as fine metal particles in natural veins. As a method of
smelting the gold, a method of leaching with cyanide and a
method of recovering as mercury amalgam are known.
[0003]
In a method of leaching gold with cyanide, the gold
is dissolved as a cyano complex in a solution. It is known
that the gold cyano complex is even more stable than other
complex ions of gold. Generally, the leached gold is
adsorbed on activated carbon and eluted with an aqueous
solution including sodium hydroxide as a main component.
Thereafter, the gold is recovered from the eluent including
the gold through an electrowinning method.
1
CA 02861419 2014-07-15
[0004]
Since the gold is adsorbed as a cyano complex on the
activated carbon, elution only with sodium hydroxide is
also possible. However, generally, a slight amount of
cyanide is added to the aqueous sodium hydroxide solution
in order to improve the result of elution of the gold from
the activated carbon.
[0005]
In addition, an elution method using a solution
obtained by mixing sodium sulfide with an aqueous sodium
hydroxide solution is known as a method of preferentially
eluting gold from activated carbon to which both of gold
and silver of a cyanide solution including both of the gold
and silver are adsorbed (Patent Literature 1).
[0006]
In many cases, gold is not only included in gold
veins, but also included in a small amount as a byproduct
in pyrite, chalcopyrite, and other metal sulfide ores. The
gold is separated in smelting of main components thereof
and smelted to separate metal gold.
[0007]
In the case of gold included as a byproduct in metal
sulfide ore, for example, chalcopyrite, the gold generally
moves to an anode in a pyrometallurgical process and is
then condensed into the slime in an electrolytic refining
2
CA 02861419 2014-07-15
process. The gold in the electrolytic slime is recovered
as metal gold through a hydrometallurgical method (Patent
Literatures 2 and 3) or a pyrometallurgical method.
[0008]
Recently, smelting techniques have been studied to
treat concentrates through hydrometallurgical method
without using pyrometallurgical method in consideration of
environmental burden and impurities in the concentrates,
and the gold leaching method using strong acid with an
sufficient oxidation potential for melting noble metal is
proposed (Patent Literature 4). The method disclosed in
Patent Literature 4 discloses that, when gold is leached by
the acidic halide solution, the halide forms a stable
complex with noble metal such as gold, but it interacts
weaker than cyanide (Paragraph [0017] of the specification
in Patent Literature 4). In addition, the method discloses
that in the solution containing the noble metal, the noble
metal can be adsorbed on activated carbon and recovered,
and further discloses that the activated carbon is
incinerated or eluted with a cyanide solution to use an
eluent thereof in electrowinning to thereby recover the
noble metal (Paragraph [0019] of the specification in
Patent Literature 4). In addition, an adsorbent including
a lignin derivative as a raw material is also known as an
adsorbent of gold (Patent Literature 5).
3
CA 02861419 2015-12-10
Citation List
Patent Literature
[0009]
Patent Literature 1: US 2579531
Patent Literature 2: JP 9-316561 A
Patent Literature 3: JP 2001-316735 A
Patent Literature 4: JP 2006-512484 A
Patent Literature 5: JP 2005-305329 A
Summary of Invention
[0010]
The gold leached using a halide solution forms a
halide complex, but it is less stable than a cyanide
complex. Accordingly, when the gold is adsorbed on
activated carbon, the gold is reducted and presents as
metal gold on the activated carbon. Therefore, the gold
cannot be eluted only with sodium hydroxide, and it is
necessary to perform the elution with cyanide solution.
[0011]
In many cases the usage of cyanide solution for the
gold leaching is restricted due to the toxicity. Therefore,
a method for leaching gold with high efficiency without
using cyanide is desirable. As an example thereof, in the
4
CA 02861419 2015-12-10
case of a leaching method using acid, a strong oxidant is
required due to the stability of gold, and the leaching
entails a lot of costs. In addition, in order to leach the
gold in gold-bearing sulfide ore, it is necessary to leach
sufficiently metal sulfide ore which is a main component,
thereby sufficiently gold and the leachate contact with
each other. Even when the gold in primary copper sulfide
ore or pyrite is dissolved in this manner, the
concentration of the gold leached into the solution is
quite low as compared with that in the case using cyanide.
[0012]
Therefore, even when leaching is performed with acid,
it is necessary to further condense the gold through an
adsorption method or a solvent extraction method in the
subsequent process. In the adsorption method, activated
carbon is known as an adsorbent, but it is necessary to use
cyanide in the elution of gold which is adsorbed as
metallic gold. When cyanide is not used, the gold is
recovered by incinerating activated carbon and thus the
cost increases as compared with the case of elution. In
addition, in the case of the adsorbent as described in
Patent Literature 5, the cost increases or a problem occurs
in that the adsorbent cannot be used repeatedly, whereby it
is not yet commercialized.
[0013]
CA 02861419 2015-12-10
In the case of solvent extraction, extraction,
settling, and back extraction facilities are necessary. And
the extracting selectivity for gold becomes a problem t.
because the gold concentration is significantly low
compared with the impurities concentration so that an
adsorption method which may be more simply operated is
preferred.
[0014]
The present inventors have constantly conducted
studies for resolving the above-described problems, and as
a result, found that when metal sulfide leaching is
performed using a halogen bath to leach gold together with
main metal components, the resulting gold leachate is
treated by activated carbon, and then elution is performed
with sodium hydroxide to prepare the concentrated gold
solution, the gold included in the metal sulfide ore may be
efficiently recovered at low cost.
[0015]
According to an aspect of the invention that has been
completed based on such research findings, a method of
recovering gold includes: leaching gold using acidic
leachate which includes chloride ions and/or bromide ions
as anions and copper and iron as cations from gold-bearing
6
CA 02861419 2014-07-15
sulfide ore to the acidic leachate by heating; adsorbing
the gold in the acidic leachate on activated carbon; and
eluting the gold adsorbed on the activated carbon with an
alkali solution to obtain a concentrated gold solution.
[0016]
In an embodiment of the method of recovering gold
according to the invention, the gold-bearing sulfide is
concentrate including at least one selected from the group
consisting of chalcocite, bornite, covellite, chalcopyrite,
pyrite, enargite, and arsenopyrite.
[0017]
In another embodiment of the method of recovering
gold according to the invention, the gold-bearing sulfide
is the residue including gold obtained by leaching copper,
iron, or arsenic, which are main metal components, by 80%
or greater from the concentrate using the acidic leachate,
and then by performing solid-liquid separation.
[0018]
In a further embodiment of the method of recovering
gold according to the invention, in the acidic leachate, 40
to 200 g/L of chloride ions, 20 to 100 g/L of bromide ions,
to 25 g/L of copper, and 0.01 to 10 g/L of iron are
contained, and a pH is 0 to 1.9.
[0019]
In a still further embodiment of the method of
7
CA 02861419 2015-12-10
recovering gold according to the invention, the leaching by
heating is performed at 60 to 100 C.
[0020]
In a still further embodiment of the method of
recovering gold according to the invention, the alkali
solution includes sodium hydroxide of 0.05 to 1 M.
[0021]
In a still further embodiment of the method of
recovering gold according to the invention, the alkali
solution includes sodium hydroxide, and sodium sulfide of
0.1 to 10 mol times greater than that of the sodium
hydroxide.
[0022]
In a still further embodiment of the method of
recovering gold according to the invention, the elution is
performed under the atmospheric pressure.
[0023]
According to another aspect of the invention, a
method of manufacturing gold includes: manufacturing or
preparing metallic gold by reduction from a concentrated
gold solution obtained by the method of recovering gold
according to the invention.
[0024]
8
CA 02861419 2014-07-15
According to the invention, it is possible to
efficiently recover gold included in metal sulfide ore at
low cost.
Brief Description of Drawings
[0025]
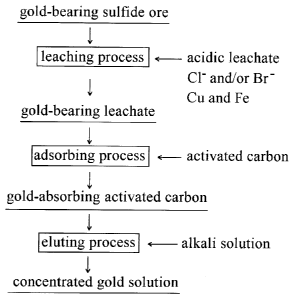
Fig. 1 is a flowchart illustrating a method of
recovering gold according to an embodiment of the invention.
Description of Embodiments
[0026]
Fig. 1 illustrates a flowchart schematically
illustrating a method of recovering gold according to an
embodiment of the invention.
In many cases, a slight amount of gold is included as
fine metal particle in metal sulfide ores such as
chalcocite, bornite, covellite, pyrite, enargite, and
arsenopyrite. Therefore, in order to recover gold, first,
it is preferable that the metal sulfide ore are crushed and
then made into concentrate by a flotation method for
condensation. In addition, if copper, iron, or arsenic,
which are main metal components, are leached by 80% or
greater from the concentrate using acidic leachate and
solid-liquid separation is then performed, it is also
possible to further condense the gold in leached residue
9
CA 02861419 2014-07-15
and the treatment efficiency becomes favorable.
[0027]
As a method of leaching gold included in the metal
sulfide ore or gold preferably condensed by the above-
described concentrate or the residue after leaching of the
main metal component, a leaching method using strong
oxidizing acid such as aqua regia, a leaching method using
cyanide, and the like are known, but any of them has some
problems in environmental burden and safety. Particularly,
the leaching using cyanide is a method which should be
avoided since its usage is usually restricted due to the
high toxicity
[0028]
In the case of leaching by strong oxidizing acid,
there is no appropriate method for further concentration of
dissolved gold. And when the gold is adsorbed on activated
carbon which is a well-known adsorbent or an adsorbent such
as functional resins, the stripping efficiency is not
enough whereby when incineration is performed for every
adsorbent, the cost significantly increases. It is said
why the stripping is not properly performed is that gold
halide complex formed after strong acid leaching is
reducted up to crude metal on the activated carbon.
[0029]
However, the following Non Patent Literature 1
CA 02861419 2014-07-15
discloses that when gold is leached under special
conditions, it forms a polysulfide complex.
(Non Patent Literature 1) M. E. Berndt, T.
Buttram, D. Earley III, W. E. Seyfried Jr., Geochimica et
Cosmochimica Acta, 58,(2), 587-594, 1994.
The gold polysulfide complex is more stable than the
halide complex, and is not easily reducted up to metallic
gold even when it adsorbed on an adsorbent.
[0030]
On the contrary, in this invention, gold is dissolved
as a polysulfide complex which is easily adsorbed onto
activated carbon without the special conditions as
disclosed in Non Patent Literature 1, and the gold adsorbed
on the activated carbon is easily eluted with sodium
hydroxide and recovered.
[0031]
In the invention, first, using acidic leachate which
contains chloride ions and/or bromide ions as anions and
copper and iron as cations, gold is leached from gold-
bearing sulfide ore to the acidic leachate by heating. The
leaching temperature is preferably 60 to 100 C. The pH of
the acidic leachate is preferably 0 to 1.9. The leaching
of gold is more favorably performed if the leaching
temperature and the pH of the leachate are adjusted in the
above ranges, respectively.
11
CA 02861419 2014-07-15
[0032]
In the acidic leachate, chloride ions and bromide
ions are each preferably contained in an amount of 20 to
200 g/L, and copper and iron are each preferably included
in an amount of 0.01 to 30 g/L. Furthermore, in the acidic
leachate, 40 to 200 g/L of chloride ions, 20 to 100 g/L of
bromide ions, 5 to 25 g/L of copper and 0.01 to 10 g/L of
iron are more preferably contained. By defining the
composition of the acidic leachate in this manner,
chalcopyrite, enargite, and the like which are unlikely to
dissolve in acid may be favorably dissolved. In addition,
when bromine is included, there is an effect of stabilizing
the dissolved gold as Au (I).
[0033]
Valuable metals are leached by dissolving the metal
sulfide in the acidic leachate through the above-described
heating leaching process. The slightly included gold is
leached together with main metals. If necessary, after
solid-liquid separation, the gold included in residue is
leached in the same manner with an acidic solution having
this composition.
[0034]
Next, the gold in the acidic leachate is brought into
contact with and adsorbed on activated carbon. The gold
may be brought into contact with activated carbon in a
12
CA 02861419 2014-07-15
batch-type manner, or by continuously passing the acidic
leachate through an adsorption tower filled with activated
carbon.
[0035]
After the gold has been adsorbed on the activated
carbon, it is necessary to keep the gold a polysulfide
complex to elute easily, but for this, it is essential that
S (-II) is present at the time of leaching. In the case of
the invention, various metal sulfides correspond thereto.
[0036]
The gold adsorbed on the activated carbon is eluted
with an alkali solution, and preferably NaOH or a mixed
solution of NaOH and Na2S. Here, when the alkali
concentration is low, it is difficult to elute the gold,
and when the alkali concentration is high, there is a
danger that unexpected heat may be generated during the
preparation. From such a viewpoint, when NaOH is used, the
concentration is preferably 0.05 to 1 M, and more
preferably 0.1 to 0.5 M. The amount of Na2S is preferably
small due to the cost and difficulty in handling. However,
the lower the concentration of Na2S, the less the gold
eluting effect. In addition, when the Na2S concentration
is too high, the effect is saturated and burden on the
treatment of excess Na2S also increases. From such a
viewpoint, when a mixed solution of NaOH and Na2S is used,
13
CA 02861419 2014-07-15
the amount of Na2S is preferably 0.1 to 10 mol times, and
more preferably 0.5 to 1.5 mol times greater than that of
NaOH.
[0037]
When gold included in metal sulfide ore is leached
through the method of the invention, the gold in the
solution is present as a polysulfide complex. Even when it
is adsorbed on activated carbon, the complex is not
reducted and does not transform into stable metallic gold.
[0038]
It is thought that the form of the gold polysulfide
complex which is adsorbed on activated carbon is gold
sulfide or the following form.
Au(HSnH).X
(wherein, X represents halogen, m represents an
integer of 1 to 4, and n represents an integer of 1 to 9)
In the case of the former form (gold sulfide), it
reacts with S2- and is dissolved and thus eluted (Non
Patent Literature 2). In the case of the latter form, the
complex is negatively charged by reaction with H of
hydrogen polysulfide coordinated with NaOH and thus eluted.
(Non Patent Literature 2) Seishi Takagi,
Qualitative Analytical Chemistry, Vol. II, Ion Reaction,
Nankodo Co., Ltd.
[0039]
14
CA 02861419 2014-07-15
When gold is leached using the method disclosed in
Patent Literature 4, or a strong oxidant such as acid mixed
hydrogen peroxide and hydrochloric acid, sulfur is securely
oxidized up to S(0) and the gold is not eluted as a
polysulfide complex, but a di-halogen complex or a
tetrahalogen complex. In this case, the complex is
reducted to the metallic gold when it is adsorbed on
activated carbon, and the elution may not be achieved by
NaOH solution.
[0040]
The elution may adopt a batch type or a continuous
water-passage type. However, the sulfide ion is oxidized
with oxygen and thus loses its negative charge, and the
eluted neutral gold complex is re-adsorbed on activated
carbon or deposite on the reactor. To prevent such
phenomenon, it is preferable that stirring is not
vigorously performed when the elution is performed in a
batch-type manner. When it is necessary to perform
stirring, the stirring is performed under the inert gas.
Otherwise, an excess sodium sulfide addition is set or
sodium sulfide is added at an appropriate time. In
addition, it is preferable that the elution be performed
under the atmospheric pressure.
[0041]
A concentrated gold solution may be obtained by
CA 02861419 2014-07-15
elution from the activated carbon. Here, the "concentrated
gold solution" means a solution including 50 to 5000 mg/L
of gold. As gold recovering methods by reduction from the
concentrated solution, reduction using sodium oxalate or
sulfur dioxide, or a solvent extraction-electrowinning
method is known and it is possible to obtain the metallic
gold using any means.
Examples
[0042]
Hereinafter, Examples of the invention will be
described. However, these Examples are provided in order
to understand the invention and advantages thereof better
and there is no intent to limit the invention.
[0043]
(Example 1)
Metal sulfide concentrate including gold (Cu: 17
mass%, Fe: 27 mass%, S: 25 mass%, Au: 90 ppm, chalcopyrite
and pyrrhotite Fel-xS as main ores) was weighed to 35 g/L
with respect to leachate. The leachate contained 180 g/L
of Cl, 20 g/L of Br, 18 g/L of Cu, and 2 g/L of Fe, and a
pH thereof was 1.5. The leachate was heated to 85 C and
stirring was performed with air blown at 0.1 L/min. The
resulting leachate with a gold concentration of 2 mg/L or
greater obtained in this manner was allowed to pass through
16
CA 02861419 2014-07-15
a column filled with activated carbon derived from coconut
shell (Activated Carbon MC manufactured by Taihei Chemical
Industrial Co., Ltd.) to adsorb the gold onto the activated
carbon. The gold concentration in the leachate after
passing through the column was less than 0.1 mg/L.
The activated carbon was taken out from the column
when the gold concentration of the activated carbon in the
column accomplished about 7000 g/ton. The amount of the
adsorbed gold was determined by cupellation and ICP-AES,
and the result was 7500 g/ton.
The activated carbon, to which the gold was adsorbed,
was soaked in an eluent at a ratio of 20 g/L to perform the
elution under the atmospheric pressure (first step). A
NaOH solution of 0.1 M at 85 C was used as the eluent.
Next, the eluent was replaced with fresh one and the
elution was repeated under the same conditions (second
step). The test results are listed in Table 1.
[0044]
[Table 1]
Results of Elution of Gold in Example 1
Time Elapsed (h) 1 2 3
Gold Concentration
First Step 31 43 51
(mg/L)
17
CA 02861419 2014-07-15
Gold Concentration
Second Step 13 16 19
(mg/L)
[0045]
It was found that the gold adsorbed on the activated
carbon after leaching through the above-described method
could be eluted with NaOH solely. In addition, the total
elution ratio was improved by repeating the elution.
[0046]
(Example 2)
Using an eluent in which Na2S was contained in a NaOH
solution of 0.1 M in an equimolar amount, gold was eluted
from the activated carbon which had been used in Example 1
under the atmospheric pressure. The treatment was
performed at room temperature and the gold concentration in
the solution was determined by ICP-AES every a specified
time. The test results are listed in Table 2.
[0047]
[Table 2]
Results of Elution of Gold in Example 2
Time Elapsed
1 24 48 72 96
(h)
Gold
Concentration 11 94 140 170 180
(mg/L)
[0048]
18
CA 02861419 2014-07-15
From Table 2, it was found that Na2S addition brought
the gold concentration increase in the elution. It was
thought that the reason for this was that S of Na2S allowed
the eluted gold to be stably present in the solution after
eluted as a polysulfide complex.
[0049]
(Example 3)
Using an eluent in which Na2S was contained in a NaOH
solution of 0.1 M in an equimolar amount, gold was eluted
from activated carbon to which had been used in Example 1
under the atmospheric pressure. The treatment was
performed at room temperature, and differently from Example
2, the elution was continued while stirring was performed.
The gold concentration in the solution was determined by
ICP-AES every a specified time. The results are listed in
Table 3.
[0050]
[Table 3]
Results of Elution of Gold in Example 3
Time Elapsed (h) 1 24 48 72
Gold Concentration
29 150 92 16
(mg/L)
[0051]
From Table 3, it was found that when elution of gold
was performed with stirring, the gold was favorably eluted,
19
CA 02861419 2014-07-15
but the gold was re-adsorbed onto the activated carbon or
precipitated with the course of time and the gold
concentration in the eluent was reduced, as compared with
Example 2 in which stirring was not performed. It was
thought that the reason for this was that the polysulfide
complex was oxidized with air and thus lost its charge. So
it could be prevented by some measures such as execution in
a sealed container or under the inert gas such as nitrogen,
addition of Na2S during the elution, or addition of excess
Na2S at the initial time. It was not necessary to add a
specific reagent.
[0052]
(Comparative Example 1)
Gold was leached as a dihalogen complex or a
tetrahalogen complex using mixed acid of hydrogen peroxide
and hydrochloric acid, and then the composition of the
leachate was adjusted using cupric chloride, copper bromide,
ferric chloride, and sodium chloride so that 180 g/L of Cl,
20 g/L of Br, 18 g/L of Cu, and 2 g/L of Fe were contained.
The gold concentration of the leachate after the adjustment
was about 5 mg/L. The leachate was allowed to pass through
a column filled with activated carbon derived from coconut
shell (Activated Carbon MC manufactured by Taihei Chemical
Industrial Co., Ltd.) to adsorb the gold onto the activated
carbon.
CA 02861419 2014-07-15
The amount of the adsorbed gold was determined by
cupellation and ICP-AES, and the result was 42000 g/ton.
The activated carbon to which the gold was adsorbed
was soaked in 0.1 M NaOH solution at a ratio of 20 g/L and
the temperature was maintained at 85 C to perform the
elution under the atmospheric pressure. The gold
concentration in the solution was determined by ICP-AES
every a specified time. The results are listed in Table 4.
[0053]
[Table 4]
Results of Elution of Gold in Comparative Example 1
Time Elapsed
1 2 3 4 5
(h)
Gold
Concentration <1 <1 <1 <1 <1
(mg/L)
[0054] From Table 4, it was obvious that the gold
leached as a halogen complex was adsorbed on activated
carbon, but the elution could not be carried out by the
NaOH solution.
21