Note: Descriptions are shown in the official language in which they were submitted.
CA 02863264 2014-09-12
SYSTEM AND METHOD FOR DELIVERY OF REGIONAL CITRATE
ANTICOAGULATION TO EXTRACORPOREAL BLOOD CIRCUITS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a divisional of co-pending application serial No.
2,643,140 filed on August 21, 2008.
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates to a system and method for the delivery of regional
citrate anticoagulation (RCA) to extracorporeal blood circuits.
2. Background Art
Continuous renal replacement therapy (CRRT) is a form of extracorporeal
blood treatment (EBT) that is performed in the intensive care unit (ICU) for
patients with acute renal failure (ARF) or end-stage renal disease (ESRD), who
are often hemodynamically unstable with multiple co-morbidities. In a specific
form of CRRT, continuous veno-venous hemofiltration (CVVH) (FIG. 1), blood is
pumped through a hemofilter and uremic toxin-laden plasma ultrafiltrate is
discarded at a rate of 1-10 liters per hour (convective removal of solutes).
An
equal amount of sterile crystalloid solution (replacement fluid, CRRT fluid)
with
physiological electrolyte and base concentrations is simultaneously infused
into
the blood circuit either before the hemofilter (pre-dilution) or after the
hemofilter
(post-dilution) to avoid volume depletion and hemodynamic collapse. From a
theoretical and
- 1 -
CA 02863264 2014-09-12
physiological point of view, when run continuously for 24 hours per day, CVVH
is the closest of all available renal replacement therapy (RRT) modalities
today to
replicating the function of the native kidneys. Most experts in the field
believe that
it should be the preferred treatment modality for unstable patients with renal
failure.
Nevertheless, 90% of RRT in the ICU is performed as intermittent hemodialysis
(IHD), sustained low efficiency dialysis (SLED), or sometimes as continuous
veno-
venous hemo-diafiltration (CVVHDF). Common to all of these latter methods of
RRT is that the removal of most solutes is predominantly by the process of
diffusion
from blood plasma through the membrane of the hemofilter into the dialysis
fluid.
Diffusion is less efficient in the removal of larger solutes than convection
and
therefore, from a theoretical standpoint, CVVH is a superior method of RRT.
The most important reason for the limited use of CVVH in the ICU
is that anticoagulation is mandatory to prevent clotting of the extracorporeal
circuit
in 24-hour treatments. Systemic anticoagulation has an unacceptable rate of
major
bleeding complications and cannot be done safely. Similarly, extracorporeal
blood
treatments including plasmapheresis, plasma adsorption on specialized columns,
blood banking procedures, lipid apheresis systems, plasma adsorption-based
endotoxin removal, treatment with a bioartificial kidney device that contains
live
renal tubular cells, or with a liver replacement therapy circuit also require
powerful
regional anticoagulation. Regional citrate anticoagulation has emerged as a
possible
solution to the clinical problem of circuit clotting.
Citrate (or the quickly buffered citric acid) is present in the human
plasma as the trivalent negative citrate anion. This ion chelates ionized
calcium in
the plasma resulting in a single negative Ca-citrate complex and in low free
ionized
calcium levels. Since the coagulation cascade requires free ionized calcium
for
optimal function, blood clotting in the extracorporeal blood circuit (EBC) can
be
completely prevented by an infusion of citrate into the arterial (incoming)
limb of
the EBC. When the blood is passed through the extracorporeal processing unit,
the
anticoagulant effect can be fully reversed by the local infusion of free
ionized
calcium into the venous (return) limb of the EBC. Therefore, theoretically,
regional
-2-
CA 02863264 2014-09-12
citrate anticoagulation can be both very powerful and fully reversible without
systemic (intra-patient) bleeding tendencies.
Regional citrate anticoagulation has been performed for more than 20
years. Nevertheless, all currently described regional citrate anticoagulation
methods
are labor intensive and complex with the ICU nurse administering several
potentially
very dangerous IV infusions in the circuit and/or in central venous lines with
frequent laboratory measurements and prescription adjustments. Physician
errors
in prescription and nursing errors in administration can quickly lead to major
complications, and even to death. Due to its well-documented dangers, regional
citrate anticoagulation has not gained wide use in clinical practice. The
recognized
dangers of RCA include hypernatremia; metabolic alkalosis; metabolic acidosis;
hypocalcemia 1 (due to net calcium loss from the patient); hypocalcemia 2 (due
to
systemic citrate accumulation); rebound hypercalcernia (due to release of
calcium
from citrate after CVVH is stopped); hypophosphatemia; fluctuating levels of
anticoagulation; nursing and physician errors; ionized hypomagnesemia;
declining
filter performance; trace metal depletion; access disconnection; wrong
connection
of citrate, calcium infusions, and/or of the blood circuit to the patient; and
accidental
disconnection of the citrate or calcium infusion.
BRIEF DESCRIPTION OF THE DRAWINGS
FIGURE 1 illustrates a prior art system for continuous veno-venous
hemofiltration (CVVH) or CVVH with dialysis (CVVHDF);
FIGURE 2 illustrates a system according to the present invention for
using citrate in the pre-dilution solution and infusion of a post-dilution
solution to
enhance removal of citrate in the hemofilter;
FIGURE 3 illustrates use of a regional citrate anticoagulation (RCA)
system according to the present invention to anticoagulate the extracorporeal
circuit
of applications other than CRRT;
-3-
CA 02863264 2014-09-12
FIGURES 4a-4b illustrate a continuous renal replacement therapy
(CRRT) circuit based on pre- and post-dilution hemofiltration with an
integrated
online sensor system (OSS) and hematocrit sensors according to the present
invention;
FIGURE 5a illustrates a hemodialysis system which may be used for
24-hour sustained low efficiency dialysis (SLED) or 4-5 hour intermittent
hemodialysis (IHD) with RCA according to the present invention;
FIGURE 5b illustrates a conductivity-based online clearance monitor
(OCM) according to the present invention for 24-hour SLED or IHD with online-
generated dialysis fluid and automated RCA;
FIGURE 6a illustrates a hemodialysis system which may be used for
continuous veno-venous hemodialysis with pre-dilution hemofiltration (CVVHDF
or c-SLEDF) with RCA according to the present invention;
FIGURE 6b illustrates a conductivity-based OCM according to the
present invention for pre-dilution CVVHDF with online-generated therapy fluid
and
automated RCA;
FIGURE 7a illustrates a hemodialysis system which may be used for
4-5-hour post-dilution hemodiafiltration (intermittent post-HDF) with RCA
according to the present invention;
FIGURE 7b illustrates a conductivity-based OCM according to the
present invention for post-dilution hemodiafiltration (HDF) with online-
generated
therapy fluid and automated RCA;
FIGURE 8a illustrates a hemodialysis system which may be used for
simultaneous pre- and post-dilution continuous veno-venous hemofiltration
(CWH)
or 4-6 hour intermittent high volume hemofiltration (HVHF) with RCA according
to the present invention;
-4-
CA 02863264 2014-09-12
FIGURE 8b illustrates a conductivity-based OCM according to the
present invention for pre- and post-dilution CVVH or HVHF with online-
generated
replacement fluid and automated RCA;
FIGURES 9a and 9b illustrate a triple lumen venous catheter with an
infusion pathway according to the present invention;
FIGURES 10a and 10b illustrate a quadruple lumen catheter with an
infusion pathway according to the present invention;
FIGURE 10c illustrates a quadruple lumen vascular access catheter
according to another aspect of the present invention with connection lines of
different lengths and colors;
FIGURE 10d illustrates a quadruple lumen vascular access catheter
according to another aspect of the present invention with the male and female
line
connectors reversed and of different colors;
FIGURE 1 la illustrates connectors according to the present invention
used to attach standard dialysis blood lines (independent arterial and venous
blood
circuit ends) for dialysis using separate arterial and venous needles;
FIGURE llb illustrates connectors according to the present invention
used to attach a citrate-dedicated dialysis blood tubing (different arterial
and venous
blood circuit ends) for dialysis using separate arterial and venous needles;
FIGURE 12a illustrates an arterial infusion line connector according
to the present invention which may be used to attach a citrate-dedicated
dialysis
arterial blood line using separate arterial and venous needles;
FIGURE 12b illustrates a venous infusion line connector according
to the present invention which may be used to attach a standard or citrate-
dedicated
dialysis venous blood line using separate arterial and venous needles;
-5-
CA 02863264 2014-09-12
FIGURE 13 illustrates citrate-dedicated blood circuit tubing with
integrated arterial and venous medication infusion line connectors according
to the
present invention which may be used to connect the extracorporeal circuit to
the
patient using separate arterial and venous access needles or a double lumen
hemodialysis catheter;
FIGURES 14a-14b illustrates a triple lumen vascular access catheter
according to the present invention for use with single needle dialysis
operational
mode;
FIGURES 14c-14d illustrates a triple lumen vascular access catheter
according to the present invention for use with single needle dialysis
operational
mode that accommodates citrate-dedicated blood tubing and medication infusion
lines with different arterial and venous connectors;
FIGURE 15a illustrates a connector according to the present
invention for circuit priming and for attachment to a single vascular access
needle
from a dialysis blood line set and medication infusion lines for use with
single
needle dialysis operational mode;
FIGURE 15b illustrates a connector according to the present
invention for circuit priming and for attachment to a single vascular access
needle
from a dialysis blood line set for use with single needle dialysis operational
mode;
FIGURES 15c and 15d illustrate a connector according to the present
invention for circuit priming and for attachment to a single vascular access
needle
from a citrate-dedicated dialysis blood line for use with single needle
dialysis
operational mode;
FIGURE 16a illustrates a connector according to the present
invention for attachment to a single vascular access needle or to a single
lumen
catheter from a dialysis blood line for use with single needle dialysis
operational
mode;
-6-
CA 02863264 2014-09-12
FIGURE 16b illustrates a connector according to the present
invention for attachment to a single vascular access needle or to a single
lumen
catheter from a citrate-dedicated dialysis blood line for use with single
needle
dialysis operational mode;
FIGURE 17a illustrates a hemodialysis system which may be used for
24-hour sustained low efficiency dialysis (SLED) or 4-5 hour intermittent
hemodialysis (IHD) with RCA according to the present invention;
FIGURE 17b illustrates a hemodialysis system which may be used for
simultaneous pre- and post-dilution continuous veno-venous hemofiltration
(CVVH)
or 4-6 hour intermittent high volume hemofiltration (HVHF) with RCA according
to the present invention;
FIGURE 17c illustrates a hemodialysis system with sensors and
online generation of fluid for continuous SLED with RCA according to the
present
invention;
FIGURE 17d illustrates a hemodialysis system with sensors and
online generation of fluid for pre-dilution CWH with RCA according to the
present
invention;
FIGURE 18 depicts a calculation according to the present invention
of the maximum possible systemic citrate level during RCA;
FIGURE 19 depicts a calculation according to the present invention
of the conductivity of plasma (Cp.) in the arterial limb of the extracorporeal
circuit
entering the hemodialyzer;
FIGURE 20a illustrates an OCM in accordance with the present
invention;
-7-
CA 02863264 2014-09-12
FIGURE 20b illustrates an OCM in accordance with another aspect
of the present invention;
FIGURE 21 depicts a comparison according to the present invention
of the effects of permanent access recirculation on the fresh dialysis fluid
conductivity bolus-based online dialysance measurement (D) versus the circuit
arterial limb blood conductivity bolus-based online dialysance measurement
(D8);
FIGURE 22 illustrates a basic hemofiltration circuit according to the
present invention which may be used to extract a small amount of ultrafiltrate
for
chemical analysis;
FIGURE 23 illustrates a complete hemofiltration circuit according to
the present invention which may be used to extract a small amount of
ultrafiltrate
for chemical analysis;
FIGURE 24 illustrates a hemofiltration circuit according to the
present invention which may be used for priming and initial testing of pumps
and
pressure transducers;
FIGURE 25 illustrates a complete hemofiltration circuit according to
the present invention which may used to extract a small amount of
ultrafiltrate for
chemical analysis;
FIGURE 26 illustrates a hemofiltration circuit according to the
FIGURE 27a illustrates an air gap backflow prevention device which
may be used to isolate ultrafiltrate from the patient circuit according to the
present
invention;
-8-
CA 02863264 2014-09-12
FIGURE 27b illustrates a backflow prevention device comprising a
series of one way valves which may be used to isolate ultrafiltrate from the
patient
circuit according to the present invention;
FIGURE 27c illustrates a reduced pressure zone backflow prevention
device which may be used to isolate ultrafiltrate from the patient circuit
according
to the present invention;
FIGURE 28a illustrates a hemofiltration circuit according to the
present invention showing a possible location for a reduced pressure zone
backflow
prevention device;
FIGURE 28b illustrates a hemofiltration circuit according to the
present invention showing a possible location for an air gap backflow
prevention
device;
FIGURE 29a depicts a configuration according to the present
invention for deriving the patient systemic solute level (Csys) by measuring
the
ultrafiltrate solute concentration CuF and dividing by the hemofilter sieving
coefficient S for the specific solute;
FIGURE 29b depicts a configuration according to the present
invention for deriving the patient systemic citrate level Csy, by measuring
the
ultrafiltrate citrate concentration CuF;
FIGURE 29c depicts a configuration according to the present
invention for deriving the patient systemic citrate level Csys by measuring
the
ultrafiltrate citrate concentration CuF.
FIGURE 30a is a schematic illustration of a citrate, calcium and
magnesium sensor according to the present invention for use in a continuously
flowing fluid circuit;
-9-
CA 02863264 2014-09-12
FIGURE 30b is a schematic illustration of a citrate sensor according
to the present invention for use in a continuously flowing fluid circuit;
FIGURE 31 is a schematic illustration of systemic citrate kinetics
during citrate anticoagulation including citrate generation, citrate body
clearance and
citrate filter clearance in accordance with the present invention;
FIGURE 32 is a schematic illustration of solute fluxes in the
extracorporeal circuit during CRRT according to the present invention using
citrate
as a small solute example;
FIGURE 33 is a graph depicting plasma citrate concentration in the
patient during RCA in accordance with the present invention;
FIGURE 34a is a graph depicting citrate concentration measured by
a citrate sensor in the drain circuit of a renal replacement therapy machine
utilizing
RCA with fixed CRRT prescription settings according to the present invention
that
result in the development of a citrate steady state determined by the CRRT
settings
and the patient's citrate metabolism;
FIGURE 34b is a graph depicting citrate concentration measured by
a citrate sensor in the drain circuit of a dialysis machine utilizing RCA
according to
the present invention; and
FIGURE 34c is a graph depicting the effluent citrate concentration
as measured by an online filter clearance and patency monitor according to the
present invention.
DETAILED DESCRIPTION OF THE INVENTION
As required, detailed embodiments of the present invention are
disclosed herein; however, it is to be understood that the disclosed
embodiments are
merely exemplary of the invention that may be embodied in various and
alternative
-10-
CA 02863264 2014-09-12
forms. The figures are not necessarily to scale, some features may be
exaggerated
or minimized to show details of particular components. Therefore, specific
structural and functional details disclosed herein are not to be interpreted
as limiting,
but merely as a representative basis for teaching one skilled in the art to
variously
employ the present invention.
The present invention includes a comprehensive, two replacement
fluid system and method for the delivery of regional citrate anticoagulation
(RCA)
to extracorporeal blood circuits, wherein the system may include an online
clearance
monitor (OCM) and a circuit effluent online sensor system (OSS) for the
continuous
determination of patient plasma content of ultrafilterable solutes. It is
understood
that components described for one system according to the present invention
can be
implemented within other systems according to the present invention as well.
The system and method according to the present invention is capable
of delivering RCA to an extracorporeal system requiring anticoagulation. The
system addresses the difficulties and risks to patients associated with
extracorporeal
anticoagulation methods and CRRT devices currently in use for continuous veno-
venous hemoflltration (CVVH). The system may include a combination of various
CRRT and dialysis machine hardware components, a software control module, and
a sensor module to measure citrate or other solute levels online to ensure the
maximum accuracy and safety of treatment prescriptions, and the use of
replacement
fluids designed to fully exploit the design of the system according to the
present
invention.
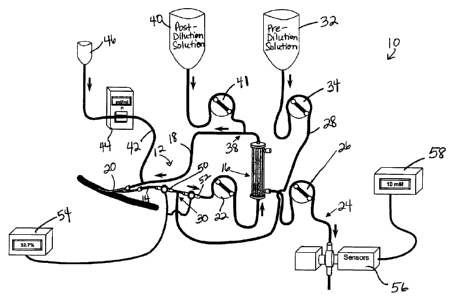
With reference first to FIG. 2, a system for CRRT according to the
present invention is illustrated and designated generally by reference numeral
10.
System 10 includes a CRRT circuit 12 including an arterial blood line 14, a
hemofflter 16 in fluid communication with arterial blood line 14, and a venous
blood
line 18 in fluid communication with hemofilter 16. Arterial and venous blood
lines
14, 18 are arranged to be connected to an access catheter 20 in order to
withdraw
blood from and return blood to a patient. A blood pump 22 is operably
connected
to arterial blood line 14 in order to facilitate movement of blood from access
-11-
CA 02863264 2014-09-12
catheter 20 through CRRT circuit 12. According to one aspect of the present
invention, blood pump 22 may be precise, with pumping speeds which may be
adjustable in 5 ml/min or fmer increments. An effluent line 24 is also in
fluid
communication with hemofilter 16 for carrying effluent fluid to a drain to be
discarded. An ultrafiltration pump 26 may be operably connected to effluent
line
24 to facilitate this process, wherein ultrafiltration pump 26 may be an
overall
ultrafiltration pump that may be non-volumetric in a scale-based system, or a
net
ultrafiltration pump which may be volumetric.
While CRRT circuit 12 is shown and described, it is understood that
the system according to the present invention may comprise any extracorporeal
circuit, either wholly or only partially outside the body. Furthermore, it is
understood that "patient" as used herein is not limited to human beings, but
may
comprise other species as well.
With continuing reference to FIG. 2, system 10 further comprises a
pre-filter infusion line 28 having a pre-dilution connection 30 to arterial
blood line
14 upstream from hemofilter 16. Pre-filter infusion line 28 may supply a pre-
dilution solution, such as a citrate-containing anticoagulation solution as
described
below, from a pre-filter source (e.g., bag 32). A pre-filter replacement fluid
pump
34 may be operably connected to pre-filter infusion line 28 to facilitate
infusion of
the pre-dilution solution, wherein pre-filter pump 34 may be implemented as a
volumetric pump. A non-volumetric pump may be acceptable with scale-based
balancing. Hemofilter 16 may then be used to remove the citrate anticoagulant
(and
the bound calcium) from the blood before it is returned to the patient. System
10
may also include a post-filter infusion line 36 having a post-dilution
connection 38
to venous blood line 18 downstream from hemofilter 16 for restoring the so
processed anticoagulated blood to normal volume. Post-filter infusion line 36
may
supply a post-dilution solution, such as an essentially calcium-free,
bicarbonate
solution as described below, from a post-filter source (e.g., bag 40). A post-
filter
replacement fluid pump 41 may be operably connected to post-filter infusion
line 36
to facilitate infusion of the post-dilution solution, wherein post-filter pump
41 may
-12-
CA 02863264 2014-09-12
be implemented as a volumetric pump, although a non-volumetric pump may be
acceptable with scale-based balancing.
In accordance with the present invention, an additional IV infusion
line 42 and associated IV infusion pump 44 may be utilized for an IV solution
infusion into venous blood line 18 downstream from post-dilution connection
38.
In particular, IV infusion pump 44 may be used to administer a pre-mixed
calcium
and magnesium-containing infusion from an IV infusion source (e.g., bag 46) in
coordination with the CVVH prescription (described below) and patient
chemistry
values. Patients will differ in their need for calcium supplementation to
reverse the
citrate anticoagulation as they will have different albumin and steady state
citrate
levels. There may also be differences in calcium release from or uptake into
the
bones. Finally, one may have to administer extra calcium and magnesium in the
initial few-hour "loading" phase of RCA to saturate the expanding systemic
citrate
pool until the steady state is reached. As depicted in FIG. 3, the
anticoagulated
blood restored to normal volume with the post-filter replacement fluid
infusion can
be perfused into any secondary extracorporeal blood treatment (EBT) device 48.
FIG. 4a illustrates additional components which may be included in
system 10 according to the present invention. System 10 may integrate online
(e.g.,
optical) hematocrit sensors 50 and/or 52 operably connected to arterial blood
line
14 to determine the dilution of the incoming blood and in communication with
an
associated display 54. Hematocrit sensors 50, 52 may be deployed in duplicate,
one
before (sensor 50) and one after (sensor 52) pre-dilution connection 30. First
hematocrit sensor 50 may be used to determine arterial plasma flow in real
time.
Second hematocrit sensor 52 may allow for checking the reliability of the two
sensors 50, 52 against each other when the pre-dilution fluid is not running.
When
the pre-dilution fluid is running at a known (machine settings and volumetric
pump
defined) rate, the readout from hematocrit sensors 50, 52 may allow for the
determination of the degree of hemodilution with the pre-filter infusion, and
thereby
for the calculation of the delivered blood flow to the dialyzer 16. Online
hematocrit
sensors 50 and/or 52 allow minute-to-minute calculation of the plasma volume
in the
blood flowing into the dialyzer 16. This ensures the most accurate and
possibly
-13-
CA 02863264 2014-09-12
continuously-adjusted dosing of citrate-containing pre-filter fluid to achieve
the
target citrate to plasma flow ratio. Hematocrit sensors 50 and/or 52 can also
be
used to detect access recirculation. Finally, the readout from first
hematocrit sensor
50 (before the pre-dilution infusion) allows for monitoring the patient's
blood
volume and will detect excessive net ultrafiltration leading to intravascular
volume
depletion with concomitant hemoconcentration in the patient before hemodynamic
compromise could result. Doppler based fluid flow, hematocrit monitors, and
volumetric fluid pumps may be used on arterial and venous blood lines 14, 18
as
well as the replacement fluid lines 28, 36 and effluent fluid line 24 for
maximal
precision in ensuring that the set blood flow rate on blood pump 22 matches
the
actual blood flow delivered by the action of blood pump 22, and that all other
fluid
flows (pre-filter fluid flow, effluent flow, venous blood flow and net
ultrafiltration
amount) are all the same as defined by the machine settings.
As shown in FIG. 4b, a total of four hematocrit sensors 50, 51, 52,
53 may be used to determine the dilution of the blood hemoglobin in the
arterial
limb 14 as well as the venous limb 18 of the extracorporeal circuit 12. FIG.
4b
depicts a comprehensive battery of four online hematocrit sensors 50-53
deployed
in close physical proximity to each other, at strategic points of the
extracorporeal
blood circuit 12 for a single modular implementation integrated into system 10
according to the present invention. Such integration is fully possible and is
contemplated in all other systems described herein. In addition to sensors 50,
52
described above, sensors 51, 53 may be deployed in duplicate, one before and
one
after the post-dilution connection 38. The venous limb hemoglobin
concentration,
which may be determined using sensor 51, may be temporarily increased by
increased ultrafiltration on the hemofilter 16 with or without a simultaneous
decrease
in the rate of infusion of one or more of the crystalloid fluids used by the
system.
Conversely, the circuit venous limb hemoglobin concentration (sensor 51) can
be
temporarily decreased by faster infusion of one or more of the crystalloid
fluids used
by the system with or without a simultaneous decrease in ultrafiltration. The
effect
on the arterial limb hemoglobin concentration (sensor 50) of such programmed,
intermittent, temporary changes in the venous limb hemoglobin concentration
allow
-14-
CA 02863264 2014-09-12
the precise, automated, intermittent calculation of access recirculation, R as
apparent
to those skilled in the art.
System 10 may further include an integrated online sensor system
(OSS) comprising a solute sensor or sensor array 56 operably connected to
effluent
fluid line 24 for determining the solute concentration of the ultraffltrate,
and in
communication with an associated display 58. In one embodiment, solute sensor
56
may comprise an online citrate sensor which may be used to eliminate the risk
of
undetected citrate accumulation and may double as an online delivered
clearance and
liver function monitor. Solute sensor 56 may also function as an online
calcium and
magnesium sensor. The current clinical practice of monitoring laboratory
parameters every six hours to detect citrate accumulation is not applicable to
the new
treatment protocols with higher clearance goals and a concomitant more rapid
citrate
accumulation that would occur with a sudden decline in liver function. More
frequent laboratory testing is clinically not practical. Solute sensor 56
according to
the present invention allows for the derivation of the citrate, calcium and
magnesium
level in the patient's systemic plasma. Under such monitoring, RCA may be
performed with complete safety. The post-filter fluid summary bicarbonate
content
could also be adjusted and the liver function monitored in real time through
observing the metabolism of citrate. Solute sensor 56 may also serve as an
online
clearance module.
All of these elements may be coordinated and monitored by a control
program, which may be utilized to determine the optimal ratio of pre- and post-
dilution fluids and the fluid flow rates required to reach treatment goals
while
minimizing citrate load into the patient.
Disposable, sterile fluid circuits may be utilized according to the
present invention. System 10 may work with, but is not limited to, blood flows
in
the range of 50 - 450m1/min with flows optimally around the 75 to 200 ml/min
range (for 24-hour CVVH versus high volume hemofiltration (HVHF) operational
mode). This is a benefit, as even the least optimally performing catheter
access will
deliver such flows. According to one aspect of the present invention,
hemofilter 16
-15-
CA 02863264 2014-09-12
may be removable from system 10, so that an appropriate size filter could be
used
for the prescribed blood flow and hourly ultrafiltration goals, and also so
that
elective filter changes could be performed every 24 hours because of
predictable
protein fouling even in the absence of clotting. More frequent filter changes
may
also be needed for the clinical application (e.g. cytokine removal).
Since only convective clearance may be used according to the present
invention (no diffusive or dialytic component is required), the
anticoagulation
achieved remains uniform along the axis of hemofilter 16, promising superior
results
when compared with other protocols using CVVH with simultaneous dialysis
(CVVHDF). The amount of middle molecular weight urernic toxin clearance
including inflammatory cytokines will also be predictably greater than in any
prior
CRRT implementations. System 10 according to the present invention running on
a CVVH machine or a dedicated device with the necessary pumps and controls may
be used to safely provide citrate anticoagulation to any extracorporeal blood
circuit,
wherein the maximum operational blood flow may be, but is not limited to, 450
ml/minute.
The RCA system according to the present invention eliminates the
risks associated with a separate concentrated citrate infusion for
anticoagulation in
CVVH and other extracorporeal circuits. Citrate removal by hemoffiter 16 is
important for safe operation of a CVVH system using citrate anticoagulation.
If
hemofiltration is stopped and blood continues to flow through the circuit 12
to
prevent coagulation, the separate infusion of citrate has to be stopped
immediately
or the patient will receive an excess amount of citrate which could be life
threatening. In RCA system 10, if for any reason hemofiltration stops and
blood
continues to flow through circuit 12 to prevent coagulation (e.g., replacement
solution bags 32, 40 are empty), the delivery of citrate with the pre-dilution
fluid
and also the delivery of calcium with the post-dilution fluid are immediately
aborted
to protect the patient from an infusion of excess citrate and calcium.
The RCA system according to the present invention markedly reduces
the need for health care personnel to monitor and adjust CRRT based on
-16-
CA 02863264 2014-09-12
hemofiltration. The use of the post-filter fluid provides for enhanced
clearance and
variability in the treatment prescription with the varying potassium and
alkali
content depending on the fluid selected as described below. Finally, the RCA
system according to the present invention greatly reduces the risk of citrate
1) Hypematremia: Only isonatric solutions may be used including the calcium
2) Metabolic alkalosis: The sum of bicarbonate and anions metabolizable to
bicarbonate (in mEq) may be kept between 25-50 InEq bicarbonate equivalents
per
liter of replacement fluid. This is in keeping with fluid alkali content per
liter
3) Metabolic acidosis: With the above flexibility in fluid alkali content, it
could
only develop if citrate were not metabolized. Even so, if the post-dilution
fluid is
bicarbonate based, life-threatening wash out of bicarbonate could not occur
with
-17-
CA 02863264 2014-09-12
generation (analogous to the course diabetic ketoacidosis in a Type 1 diabetic
ESRD
patient). In the near anhepatic patient, bicarbonate lost through
ultraffitration will
not be regenerated by citrate metabolism. However, even such patients can
continue
on RCA with CVVH, provided that the citrate extraction is > =60%, 50
4) Hypocalcemia 1 (due to net calcium loss from the patient): The ultratrate
total
calcium and magnesium losses are easily calculable in the RCA system according
5) Hypocalcemia 2 (due to citrate accumulation): Citrate will be given in the
pre-
-18-
CA 02863264 2014-09-12
citrate volume of distribution in the patient. Finally the patient's citrate
clearance
in L/minute may be calculated and subsequently used to generate the expected
citrate
accumulation curve and guide calcium and magnesium replacement to saturate the
retained citrate. In citrate non-metabolizers (patients with liver failure),
RCA with
CVVH will be either terminated or carefully continued with special
consideration
of the risk of rebound hypercalcemia at the cessation of RCA and metabolic
acidosis
from bicarbonate wash-out.
6) Rebound hypercalcemia (due to release of calcium from citrate after CVVH is
stopped): The RCA prescription will ensure that systemic citrate levels stay <
=3-5
mM corresponding to about maximum 0.6-1 mM chelated calcium that could be
released after RCA is stopped in all patients who can metabolize citrate. Most
patients will have 1 mM citrate and about 0.25 mM Ca chelated by citrate in
the
steady state. The RCA system and method according to the present invention may
be designed to keep systemic ionized Ca levels around 1-1.25 and therefore the
highest calcium level after RCA is stopped will be <=1.6-1.85 mM and most
patients will rebound to <=1.5 mM Ca levels after treatment. If a patient with
liver failure is treated with RCA for CVVH, the prescription may be modified
so
that the steady state citrate level does not exceed 4 mM and the ionized
calcium will
be maintained at 1Ø A 35 ml/kg/hour treatment goal may still be achieved for
any
patient size. Total magnesium will be kept at 50 % of total calcium (mM/mM).
This will require large doses of the additional calcium and magnesium
infusions, as
there will be more calcium and magnesium in the ultrafiltrate. If the liver
function
improves the values will gradually normalize with ongoing CVVH and a reduction
in the calcium and magnesium infusion without rebound hypercalcemia. If the
liver
does not improve, rebound hypercalcemia will not occur as the citrate will not
be
metabolized. Finally, prior to a liver transplant, high volume hemofiltration
without
citrate anticoagulation can be rendered for a few hours to wash out all
citrate and
chelated extra calcium and magnesium before the new liver (with good metabolic
function) is put in. This way even the anhepatic patient will be able to
receive high
dose RCA for CVVH.
-19-
CA 02863264 2014-09-12
7) Hypophosphatemia: Because of the lack of calcium or magnesium, the pre-
filter
and post-filter solutions both can also be supplemented with phosphate by the
manufacturer without the risk of calcium- or magnesium-phosphate
precipitation.
The phosphate-containing fluids can be used even when the serum phosphorus is
high as the large filtration goals will allow significant net phosphate
removal.
Conversely, the fluids may also serve to correct hypophosphatemia towards
normal
when needed.
8) Fluctuating levels of anticoagulation: The fixed composition of the pre-
filter
fluid and the blood plasma flow to pre-filter fluid ratio that is kept fixed
during a
treatment ensures predictable citrate levels and very effective
anticoagulation in the
circuit as well as a clearly defined hourly citrate load into the patient.
Since only
convective clearance is used, the concentrations of ionized calcium and
citrate
remain unchanged and uniform along the axis of hemofilter 16, quite different
from
other protocols using CVVHDF. The consideration of the patient's hemoglobin,
and total plasma protein level allows for maximizing the post-dilution
ultrafiltration
without inducing excessive hemoconcentration.
9) Nursing and physician errors: These are near completely eliminated by the
system and method according to the present invention, as the nurse's role is
mainly
to obtain blood samples for total and ionized calcium at specified intervals
and notify
nephrology of the results. The nurse may also make the needed changes to the
mixed calcium and magnesium infusion based on the dosing program (may be
provided as a web application or integrated into the RCA for CVVH system
according to the present invention). Since the control program may write the
entire
prescription and continuously monitor the machine settings, physician errors
are
eliminated. Citrate sensor 56 may obviate the need for any laboratory
monitoring.
10) Ionized hypomagnesemia: Since clinical monitoring of ionized magnesium is
usually not possible, the protocol will aim to maintain a 2:1 mM/mM ratio
between
total plasma calcium and total plasma magnesium. To achieve this, the rnM
ratio
of calcium and magnesium may be fixed at 2:1 in the regulated
calcium/magnesium
-20-
CA 02863264 2014-09-12
infusion. Such dosing ensures that total and ionized magnesium levels will be
appropriate for the steady state plasma citrate levels.
11) Declining filter performance: Due to the purely convective nature of small
solute removal, this is not expected to be a problem before transmembrane
pressure
alarms are generated. Elective filter changes every 24 hours may be
recommended
due to the predictable protein fouling of the filters even in the absence of
clotting.
12) Trace metal depletion: Cationic trace metal supplementation may be
provided
with the calcium infusion to restore precise mass balance for these trace
solutes.
Should any trace metal be incompatible with chloride as an anion, it can be
provided
in a higher concentration in the pre-filter solution.
13) Access disconnection: Most patients treated will have catheter access with
a
low risk of accidental disconnection.
14) Wrong connection of citrate, calcium, or blood circuit to patient: These
errors
are prevented by the hardware and disposable tubing set design of the system
as
explained herein.
15) Disconnection of the citrate, post-filter or calcium infusion: This can be
completely prevented by appropriate circuit tubing design (contiguous
connection
to the blood line, air in-line detection plus scale based monitoring).
The various solutions and fluids which may be utilized according to
-21-
CA 02863264 2014-09-12
The description of a single material, compound or constituent or a
group or class of materials, compounds or constituents as suitable for a given
purpose in connection with the present invention implies that mixtures of any
two
or more single materials, compounds or constituents and/or groups or classes
of
materials, compounds or constituents are also suitable. Also, unless expressly
stated
to the contrary, percent, "parts of," and ratio values are by weight.
Description of
constituents in chemical terms refers to the constituents at the time of
addition to any
combination specified in the description, and does not necessarily preclude
chemical
interactions among constituents of the mixture once mixed.
The replacement solutions that may be used by the system according
to the present invention include solutions which are referred to below as
"CitrateEasy" and "BicarbFasy" solutions for CVVH and which may be provided
in two formulations each, described in detail below. Using the system and
method
according to the present invention, the citrate solution may be introduced
into
extracorporeal circuit 12 before the blood enters hemofilter 16. The system
and
method of the present invention may utilize a combination of pre--dilution and
post-
dilution hemofiltration, wherein the pre-dilution solution may be CitrateEasy
and the
post-dilution fluid may be BicarbEasy.
CitrateEasy is a near isonatric (to physiologic human plasma) and
isoalkalic (to other commercial CRRT fluids and in terms of metabolizable
bicarbonate equivalent anions per liter) citrate anticoagulant-containing
hemofiltration solution. BicarbEasy is a bicarbonate-based hemofiltration
fluid that
may be essentially calcium and magnesium free and contains phosphate.
BicarbEasy
may be manufactured in a single chamber bag 40, allowing for ease of use and
significant cost savings in the process. The post-dilution ultrafiltration
provides for
maximal fractional extraction of the citrate load from extracorporeal circuit
12 and
for maximal uremic clearance achieved for a given rate of extracorporeal
circuit
blood flow. Since CitrateEasy and BicarbEasy are essentially free of calcium
and
magnesium, phosphate can be added to both for physiologic phosphate balance.
The
composition of both the pre-filter and post-filter fluids and the control
algorithm of
the system and method according to the present invention allows for high blood
-22-
CA 02863264 2014-09-12
flows and high per hour clearance rates to be accomplished with the special
requirements of twelve hour daily CVVH and high volume hemofiltration (HVHF),
without overloading the patient with citrate or inducing undue acid-base or
electrolyte changes.
The use of the CitrateEasy fluid with the system of the present
invention eliminates the need for and all the associated risks of a separate
concentrated citrate infusion. Citrate removal by hemofilter 16 is important
for safe
operation of a CVVH system using citrate anticoagulation. The separate
infusion
of citrate in a traditional set-up will have to be stopped immediately when
solute
clearance is aborted or the patient will receive an excess amount of citrate
which
could be life threatening. In the system using CitrateEasy, if for any reason
hemofiltration stops, the delivery of citrate with the pre-dilution fluid is
immediately
aborted.
Further, while calcium and magnesium are essentially completely
eliminated from the replacement fluids, the net balance of these divalent
cations in
the CVVH circuit may be kept zero in the individual patient by careful and
strictly
machine-regulated and coordinated dosing of a combined calcium and magnesium
supplement infusion. Nursing errors with the calcium and magnesium infusion
may
be eliminated by physically integrating this infusion pump 44 with system 10
for the
delivery of additional mixed calcium and magnesium into venous blood line 18
of
circuit 12, ensuring maintenance of physiologic ionized calcium and free
magnesium
levels in the patient. The system according to the present invention may
monitor the
rate settings of this pump 44 and may alert the operator if the value detected
is
unusual in the light of other treatment and patient parameters. Finally, the
mandatory addition of phosphate to the pre-filter and post-filter replacement
fluid
by the manufacturer will eliminate the need for separate intravenous phosphate
administration to prevent hypophosphatemia due to removal by CVVH. The pre-
filter phosphate may yield a further (minor) Ca chelation and anticoagulation
as
well.
-23-
CA 02863264 2014-09-12
Pre-Filter CitrateEasy fluids:
It is understood that the fluids may be provided in a lx, 5X, 10X,
50X, or any other concentrated or diluted ratio of the fluid components
described
herein. In addition, citrate could be replaced by isocitrate or another non-
toxic,
metabolizable calcium chelator. Any such variations of the following fluids
are fully
contemplated.
mmol/L piEq/L
Sodium (Na) 135-150 135-150
Potassium (IC") 0-4 0-4
Citrate (Cie-) 8-16.67 24-50
Acid citrate (CitH3) 0-10 0-30
Chloride (co 95-120 95-120
Calcium (Ca') 0-4.0 0-8.0
Magnesium (Mg') 0-2.0 0-4.0
Dextrose 5.5-11.0 5.5-11.0
Phosphate 0.0-5.0 0.0-5.0
Inulin 0-few mM 0-few mM
PAH 0-few mM 0-few mM
Trace metals Only if incompatible with the Ca infusion
Inulin and PAH may be introduced in their usual, fluoroprobe-, or
biotin-labeled form here to allow online monitoring of glomerular filtration
rate and
renal tubular secretory function as described with reference to the online
sensor
system. In addition, the above solution may be provided consisting essentially
of
all components except for inulin, PAH, and trace metals.
Pre-Filter Solution 1: " CitrateEasyl6CaOIC2/4P1"
This is a high citrate fluid with phosphorus added, wherein one
preferred mode of operation is simultaneous pre- and post dilution CVVH. This
solution may not be advised for patients with liver failure and inability to
attain
> =66% citrate extraction and/or preexisting severe metabolic acidosis. This
solution works with BicarbEasy25/50Ca0K2/4P1.
-24-
CA 02863264 2014-09-12
mmol/L mEq/L
Sodium (Nat) 140-145 140-145
Potassium (K) 2 or 4 2 or 4
Citrate (Cie-) 14 42
Acid citrate 2 6
Chloride (Cr) 105 or 107 105 or 107
Calcium (Ca') 0 0
Magnesium (Mg') 0 0
Phosphoric acid (H3PO4) 1.25 1.25
Dextrose 5.5 5.5
The removal of both calcium and magnesium is important to the
maximal anticoagulant effect and for the safe addition of phosphate to the
fluids.
The addition of phosphate is possible as there are no divalent ions that could
precipitate it. The addition of acid citrate in this ratio is also novel.
Finally, the
sodium is slightly higher than in most commercial replacement fluids.
The CitrateEasy 16 fluid is the most likely to yield completely normal
plasma electrolyte values with high volume treatments. The lack of calcium and
magnesium and the acid citrate and basic citrate values in combination with
phosphate make this a unique fluid. Adding additional solutes to published
fluids
at the point of use with multiple additives would likely be too cumbersome and
error
prone to be an alternative.
Pre-Filter Solution 1: "CitrateEasyl6Ca0K2/4P4"
This is a variation for a pre- and post-dilution system without IV
infusion pump 44. This is a high citrate fluid with more phosphorus added,
wherein
one preferred mode of operation is simultaneous pre- and post dilution CVVH.
This
solution may not be advised for patients with liver failure and inability to
attain
> =66% citrate extraction and/or preexisting severe metabolic acidosis. This
solution works with BicarbEasy25/50Ca3.5/1C2/4P0.
-25-
CA 02863264 2014-09-12
mmol/L
Sodium (Na) 145 145
Potassium (K+) 2 or 4 2 or 4
Citrate (Cit3-) 15.0 45
Acid citrate 1 3
Chloride (co 102 or 104 102 or 104
Calcium (Ca2+) 0 0
Magnesium (Me+) 0 0
Phosphoric acid (H3PO4) 4 4
Dextrose 5.5 5.5
The removal of both calcium and magnesium is important to the
maximal anticoagulant effect and for the safe addition of phosphate to the
fluids.
More phosphate is added, as the post-filter fluid will have calcium and
therefore
cannot have phosphate. The acid citrate is reduced because of the phosphoric
acid.
Pre-Filter Solution 1: " CitrateEasyl6Ca2 .5K2/4P1"
This is a variation for an isolated pre-dilution system without pump
44. The fluid has calcium added, wherein one preferred mode of operation is
isolated pre-dilution CVVH. This solution may not be advised for patients with
impaired liver function. The low 33% citrate extraction due to the absence of
post-
filtration may recommend the use of an online citrate sensor for safe
treatments.
mmol/L inEq/L
Sodium (Na) 145 145
Potassium (K+) 2 or 4 2 or 4
Citrate (Cie-) 14 42
Acid citrate 2 6
Chloride (a) 112.5 or 114.5 112.5 or 114.5
Calcium (Ce+) 2.5 5
Magnesium (Mg2+) 1.25 2.5
Phosphoric acid (H3PO4) 1.25 1.25
Dextrose 5.5 5.5
-26-
CA 02863264 2014-09-12
The addition of both calcium and magnesium ensures mass balance
for these ions. The anticoagulant effect may be reduced but still good due to
the
excess amount of citrate. Similarly, the very low ionized calcium levels and
acidic
pH in the fluid bags allows the safe addition of phosphate by the manufacturer
as
well.
Pre-Filter Solution 2: "CitrateEasy8Ca0P1"
This less acidic citrate fluid with phosphorus added can be used for
patients with liver failure and an inability to attain >66% citrate extraction
(indefinite use) and/or preexisting severe metabolic acidosis (initial use).
This
solution works with BicarbEasy25/50Ca0K2/4P1.
mmol/L mEq/L
Sodium (Na) 145 145
Potassium (K+) 2 or 4 2 or 4
Citrate (Cie) 7 21
Acid citrate 1 3
Chloride (Cl-) 124.75 or 126.75 124.75 or 126.75
Calcium (Ca") 0 0
Magnesium (Mg") 0 0
Phosphate (H2PO4-) 1.25 1.25
Dextrose 5.5 5.5
The safety of the phosphate-containing CVVH fluid is predicted based
on inorganic fluid chemistry principles: sodium and potassium do not
precipitate
with phosphate. The addition of phosphate will eliminate hypophosphatemia, a
relatively less acute but clinically still significant complication of CVVH
seen
particularly often when high clearance goals are targeted and achieved.
Finally,
CitrateEasy should come with at least two different potassium concentrations
(2 and
4 mM) to allow flexibility in potassium mass balance handling.
-27-
CA 02863264 2014-09-12
Pre-Filter Solution 2: "CitrateEasy8CaO1C2/4P4"
This is a variation for a pre- and post-dilution system without pump
44. This less acidic citrate fluid with more phosphate added can be used for
patients
with liver failure and an inability to attain >66% citrate extraction
(indefinite use)
and/or preexisting severe metabolic acidosis (initial use). This solution
works with
BicarbEasy25/50Ca3 51C2/4P0
nunol/L inEct/L
Sodium (Na) 145 145
Potassium (K+) 2 or 4 2 or 4
Citrate (Cit3-) 7 21
Acid citrate 1 3
Chloride (Cr) 120 or 122 120 or 122
Calcium (Ca') 0 0
Magnesium (Mg') 0 0
Phosphate (H2PO4) 4 4
Dextrose 5.5 5.5
The safety of the phosphate-containing CWH fluid is predicted based
on inorganic fluid chemistry principles: sodium and potassium do not
precipitate
with phosphate. The addition of more phosphate will eliminate
hypophosphatemia,
even with a calcium-containing, and therefore phosphate-free, post-filter
bicarbonate
fluid. The overall acid content of the fluid is nearly unchanged.
Post-Filter BicarbEasy Fluids:
It is understood that the fluids may be provided in a 1X, 5X, 10X,
50X, or any other concentrated or diluted ratio of the fluid components
described
herein.
nunol/L mEq/L
Sodium (Na) 135-150 135-150
Potassium (K+) 0-4 0-4
Bicarbonate 20-60 20-60
-28-
CA 02863264 2014-09-12
Chloride (a) 85-120 85-120
Calcium (Ce+) 0-4 0-8
Magnesium (Me+) 0-2.0 0-4.0
Phosphate (P043-) 0-5 0-15
Dextrose 5.5-11.0 5.5-11.0
Post-Filter Solution 3 and 4 (BicarbEasy25Ca0K2/4P1 and
BicarbEasy50Ca0K2/4P1):
BicarbEasy25 and BicarbEasy50 are designed to complement the
CitrateEasyl6 and CitrateEasy8 fluids, and they are provided with variable
potassium content. BicarbEasy50 with CitrateEasy8 may be advised for patients
who have severe preexisting metabolic acidosis and/or liver failure. These
patients
will have systemic bicarbonate levels around 15 or less, and for them the use
of the
CitrateEasyl6 fluid could possibly lead to dangerous circuit acidification to
pH near
6.0 or less. The amount of bicarbonate in the BicarbEasy50 solution is much
more
than in the BicarbEasy25 fluid and will provide more bicarbonate through the
CVVH circuit when the patient has liver failure, and thus will correct
metabolic
acidosis faster in other patients who can metabolize citrate.
The addition of phosphate may be mandatory by the manufacturer and
safe as divalent cations (magnesium and calcium) have been essentially removed
from the fluids. The phosphate may be provided as a pH-adjusted mix of the tri-
basic and di-basic salt in the BicarbEasy solutions to avoid CO2 gas
generation when
mixed with bicarbonate in a single bag, or simply as the tri-basic salt. In
the latter
case, upon entering the blood, some additional bicarbonate generation (about
2.5
mEq per liter of post-filter fluid) will occur as the phosphate picks up
hydrogen ions
from carbonic acid dissolved in the plasma. Finally, BicarbEasy should come
with
at least two different potassium concentrations (2 and 4 mM) to allow
flexibility in
potassium mass balance handling. A major
advantage is that the
BicarbEasy25/50Ca0 fluids can be manufactured in a single compartment sterile
bag
40 as opposed to current bicarbonate formulations that have to separate the
bicarbonate in a dedicated second compartment because of the risk of Ca-
carbonate
and Mg-carbonate precipitation.
-29-
CA 02863264 2014-09-12
Post-Filter Solution 3: "BicarbEasy25CaO1C2/4P1"
May be preferred in combination with CitrateEasyl6Ca0K2/4P1 for
patients with no evidence of liver failure or severe preexisting metabolic
acidosis.
nunol/L mEq/L
Sodium (Nat) 140 140
Potassium (Kt) 2 or 4 2 or 4
Bicarbonate 25 25
Chloride (co 113.25 or 115.25 113.25 or 115.25
Calcium (Ca2+) 0 0
Magnesium (Mg') 0 0
Phosphate (P043") 1.25 about 3.75
Dextrose 5.5 5.5
The removal of calcium and magnesium and the addition of tri-basic
phosphate provides a novel solution according to the present invention. The
phosphate may also be pH-adjusted between the tri-basic and di-basic salt form
to
be compatible with the bicarbonate in the fluid without CO2 generation. The
exact
bicarbonate concentration will depend on the clinical protocol. Higher
treatment
goals allow (and require) the use of lower bicarbonate concentrations in the
post-
filter fluid as long as citrate metabolism is not impaired, to avoid metabolic
alkalosis.
Post-Filter Solution 3: "BicarbEasy25Ca3.5K2/4P0"
This is a variation for a pre-post-dilution system without pump 44,
which may be preferred in combination with CitrateEasy 16Ca01(2/4P4 for
patients
with no evidence of liver, failure or severe preexisting metabolic acidosis.
rrunol/L rnEq/L
Sodium (Nat) 140 140
Potassium (Kt) 2 or 4 2 or 4
Bicarbonate 29 29
Chloride (a) 128.5 or 130.5 128.5 or 130.5
-30-
CA 02863264 2014-09-12
Calcium (Ca2+) 3.5 7
Magnesium (Me) 1.75 3.5
Phosphate (P043-) 0 0
Lactic acid with Ca 4 4
Dextrose 5.5 5.5
The addition of a very high calcium and magnesium is a novel
solution according to the present invention. The phosphate is removed, and the
bicarbonate should be separated from the calcium, magnesium and lactic acid,
such
as in a traditional two-chamber bag. The exact bicarbonate concentration will
depend on the clinical protocol. Higher treatment goals allow (and require)
the use
of lower bicarbonate concentrations in the post-filter fluid as long as
citrate
metabolism is not impaired, to avoid metabolic alkalosis. The lactic acid may
be
added to ensure an acid pH after the mixing of the contents at the point of
use, to
lessen the risk of carbonate precipitation. The bicarbonate content is before
mixing
with the lactic acid; after mixing it will be 25.
Post-Filter Solution 4: "BicarbEasy50CaO1C2/4P1"
This solution may be preferred in combination with
CitrateEasy8Ca0K2/4P1 for patients with liver failure or until severe
metabolic
acidosis is corrected.
rnmol/L
Sodium (Na) 140 140
Potassium (K+) 2 or 4 2 or 4
Bicarbonate 50 50
Chloride (Cr) 88.25 or 90.25 88.25 or 90.25
Calcium (Ce+) 0 0
Magnesium (Mg') 0 0
Phosphate (P043-) 1.25 about 3.75
Dextrose 5.5 5.5
-31-
CA 02863264 2014-09-12
The removal of calcium and magnesium and the addition of a
phosphate is a novel solution according to the present invention. Most
importantly,
the bicarbonate is very high to compensate for the bicarbonate lost in the
ultraffitrate
through the circuit and for the lack of liver conversion of citrate into
bicarbonate in
a liver failure patient. The phosphate may be pH adjusted between the tri-
basic and
di-basic salt form to be compatible with the bicarbonate in the fluid without
CO2
generation and to avoid carbonate formation.
Post-Filter Solution 4: "BicarbEasy50Ca3.5K2/4P0"
This is a variation for a pre- and post-dilution system without pump
44, which may be preferred in combination with CitrateFasy8Ca0K2/4P4 for
patients with evidence of liver failure or severe preexisting metabolic
acidosis.
nunol/L mEq/L
Sodium (Na) 140 140
Potassium (K) 2 or 4 2 or 4
Bicarbonate 54 54
Chloride (CO 98.5 or 100.5 98.5 or 100.5
Calcium (Ca2) 3.5 7
Magnesium (Mg24) 1.75 3.5
Phosphate (P043-) 0 0
Lactic acid with Ca 4 4
Dextrose 5.5 5.5
The addition of a very high calcium and magnesium is a novel
solution according to the present invention. The phosphate is removed, and the
bicarbonate should be separated from the calcium, magnesium and lactic acid,
such
as in a traditional two-chamber bag. The high bicarbonate concentration may be
needed in the absence of citrate metabolism. The lactic acid is added to
ensure an
acid pH after the mixing of the contents at the point of use, to lessen the
risk of
carbonate precipitation. The bicarbonate content is before mixing with the
lactic
acid; after mixing it will be 50.
-32-
CA 02863264 2014-09-12
Solution 5:
Concentrated calcium and magnesium chloride infusion (0.5X,
2X, 4X, 20X or other concentrated or diluted formulations) with a 2:1 to 4:1
(preferred 2.5: 1) Ca:Mg molar ratio.
mmol/L mEq/L
Calcium 50 100
Magnesium 25 50
Sodium 150 150
Chloride 300 300
Trace metals may be added in a molar ratio to calcium that is the
same as in the ultrafiltrate during CVVH with RCA at a time point when the
systemic blood plasma has normal trace metal and total calcium content. This
fluid
may be infused into venous blood line 18 of circuit 12 as close to the venous
port
of access catheter 20 as possible. A dedicated IV infusion pump 44 integrated
into
the system according to the present invention may drive the fluid flow. The
amount
infused may be set by the operator and monitored for safety by a calcium
dosing
program to ensure full coordination with the patient's chemistry values that
are
updated regularly, the patient's estimated volume of distribution for calcium,
as well
as the RCA for CVVH prescription parameters and citrate sensor data. A typical
prescription will result in a flow rate of 100-140 ml/hour with the above
fluid
composition. This allows for precise pumping and 10% dosing steps with the PBP
pump in use on one commercial device (e.g., Prismaflex). It is expected that
the
rate of the infusion will be steady and unchanged after the first few hours of
treatment with the system of the present invention and no significant changes
to the
calcium infusion rate will be needed.
Finally a circuit priming solution may also be utilized for calibration
of the OSS according to the present invention:
mmol/L mEq/L
Sodium (Na) 130-150 130-150
-33-
CA 02863264 2014-09-12
Citrate (Cie) 1-20 3-60
Chloride (a) 100-140 100-140
Calcium (Ca") 0.5-10 1-20
Magnesium (Me+) 0.25-5 = 0.5-10
According to one non-limiting aspect of the present invention, a preferred
composition may be:
mmol/L mEq/L
Sodium (Nat) 140 140
Citrate (Cie) 7 21
Chloride (Cl') 124.1 124.1
Calcium (Ca") 1.7 3.4
Magnesium (Mg") 0.85 1.7
This solution may be used to prime the circuit at the start of the
procedure and will allow the OSS to test the accurate functioning of the
safety
sensors 56 for citrate, calcium and magnesium.
The rationale behind the CitrateEasy and BicarbEasy fluid designs
according to the present invention is explained below. First, the sodium
content
may be 140-145 mEq/L, whereas all commercial fluids use a 140 sodium solution.
It is of note that patients treated with such fluids often stay or become
hyponarremic
to around 136 serum values. The explanation may be that the strength of the
Gibbs-
Dolman effect is slightly different when the same fluid is infused pre-filter
or post-
filter (the negatively charged proteins are diluted in the pre-filter infusion
mode).
The solutions according to the present invention may use the industry standard
sodium of 140 for the post-filter fluid and 145 for the pre-filter fluid. The
additional
5 mM sodium above usual fluid sodium content may result in serum sodium levels
around 140-142 in most patients.
The potassium content may be 2.0-4.0 mEq/L. Manipulation of
potassium mass balance may be achieved by selecting 2.0 or 4.0 K CitrateEasy
and
-34-
CA 02863264 2014-09-12
BicarbEasy fluids. Two bags of each fluid may be hung and used at any given
time.
The ratio of 2 and 4 K bags therefore can change from 0:4 to 4:0. This will
make
the overall K content of the summary replacement fluids adjustable in 0.5 mEq
increments, satisfactory for all K mass balance purposes. Finally, when only
pre-
dilution hemofiltration is performed, the pre-dilution CitrateEasy fluids will
have
at least a 2.0 and 4.0 K formulation with phosphate.
The pre-filter fluid may have an alkali equivalent content of 20 and
40 mEq/L. Current hemofiltration fluids usually contain 40-47 mEq/L lactate
(1/1
bicarbonate equivalent) or 13.3-14 mmol/L or 40-42 mEq/L trisodium-citrate
(3/1
bicarbonate equivalent). Even with high clearances achieved with such high
alkali
equivalent containing fluids in some protocols, serum bicarbonate stabilizes
around
24-28 values and severe alkalosis does not occur. The exact explanation is
unclear,
but may be explained by the unstable patient losing bicarbonate through body
metabolism as well as ultrafiltration of bicarbonate and the metabolizable
anions
citrate and lactate, the sum of which could easily equal 30-40 mEq/L. Whatever
the
mechanism, it seems prudent to design the fluid to deliver at least 40 mEq net
bicarbonate equivalent citrate per liter in patients who can metabolize
citrate. The
net alkali content for the pre-filter fluids may be fine-tuned with clinical
data
between 35 and 45 mEq/L. These calculations do not apply to the CitrateEasy8
fluids which are designed assuming impaired citrate metabolism and rely on the
high
bicarbonate BicarbEasy50 fluids for alkali mass balance. Variable ratio of
similar
CitrateEasy 8 and 16 bags (2:0, 1:1 and 0:2) can also be used for citrate
dosing
flexibility.
The citrate and acid citrate combined content may be mEq/L (24 or
48): The total citrate content will be 8-16 mmol, while the net alkali
equivalent
citrate will be only 7-14 mmol or 21-42 mEq, and the acid citrate content will
be 3-8
mEq. Due to the different pKas of the three carboxyl groups on the citrate
molecule, the mixture of the above will yield about equal amounts of
citrateNa3 and
citrateNa2H. Since the ratio of the salt and acid form is near 1/1, the fluid
pH will
be around the pKa3 = 6.3. This will have the added benefit of being protective
from bacterial growth in the fluid. When the fluid reaches the patient's
blood, the
-35-
CA 02863264 2014-09-12
citrateHNa2 will react with the bicarbonate in the blood to generate
citrateNa3 and
H20 plus CO2. Assuming a mixing ratio of 2 liters of plasma to 1 liter of pre-
filter
fluid and ignoring RBC buffering, the new bicarbonate will be (67% of systemic
serum values after dilution) - 3. For example, if systemic bicarbonate is 24,
the
circuit bicarbonate after the pre-filter fluid infusion will be 13. However,
there will
also be a 14 mEq/L added alkali equivalent citrate in the fluid for a total
alkali
content of at least 27. The generated CO2 will also contribute to the
acidification
of the circuit and will ultimately be eliminated through the circuit and by
pulmonary
gas exchange. The amount of CO2 added to the patient's blood is not clinically
significant based on calculations as well as the outcomes of clinical trials
of CVVH
using concentrated acid citrate dextrose as anticoagulant (ACD-A, Baxter).
However, the circuit acidification with the high local citrate levels will
ensure that
nearly all calcium in the plasma will be removed from albumin and other
proteins
and will be freely ultrafilterable. This will make calcium mass balance
calculations
in the CVVH circuit very reliable. Bicarbonate levels will be restored by
citrate
metabolism in the patient as well as by the alkalinizing effects of the post-
dilution
step where citrate will be exchanged for bicarbonate. The circuit
acidification may
possibly further interfere with blood clotting.
CRRT fluid calcium and magnesium has essentially zero content.
The massive amount of citrate in the pre-filter fluid strips calcium and
magnesium
from albumin. Total ultrafilterable calcium will be nearly equal to total
calcium in
circuit blood due to this "stripping" of calcium from albumin by citrate as
well as
with significant acidification of the circuit with the pre-filter fluid. The
cumulative
ultrafilterable calcium content is predicted at 0.25 mM/mM citrate (in
systemic
blood), 0.2 mM/g stripped from albmin, and 1.25 mM targeted systemic ionized
calcium for a total filterable calcium of 1.5-3.0 mmol/liter filtrate after
adjusting for
the pre-dilution effect, depending on citrate accumulation, albumin level and
systemic ionized calcium. Individual patients who may markedly differ in their
serum albumin and citrate and therefore total plasma calcium levels cannot be
kept
in ideal balance without a dedicated Ca and Mg infusion. Therefore, the
present
invention replaces all of these losses with a dedicated calcium and magnesium
=
infusion which may be strictly coordinated with the operation of the machine.
This
-36-
CA 02863264 2014-09-12
allows for both pre-filter and post-filter CRRT fluids with physiological
phosphate
concentrations, the ratio of which can be varied freely, in good agreement
with the
physiologic and symmetric fluid concepts according to the pre-post dilution
method
of the present invention. The lack of calcium and magnesium allows for single-
chamber bicarbonate-based fluid formulation, a major manufacturing advantage
over
currently existing formulations.
Calcium and magnesium replacement may include trace metals. This
is coordinated strictly with calcium dosing by using a single mixed infusion
of these
two cations (and possibly trace metals that are also chelated by citrate) to
account
for the filtered losses of calcium, magnesium and trace metals through the
CVVH
circuit.
Dextrose content may be 5.5 mmol/L. To match the physiologic
plasma glucose concentration, as CVVH is not meant to be a form of nutrition.
Recent publications on the improved clinical outcomes with strict glycemic
control
in the ICU may also warrant the use of hemofiltration fluid with physiologic
glucose
content, lower than what was used in the past. The impact of potentially
substantial
glucose removal from the diabetic patient with suboptimal blood sugar control
and
high clearance goal CVVH will need to be recognized by the ICU team and proper
blood sugar control will need to be maintained.
Phosphate may be about 1.25 mmol/L. The absence of calcium and
magnesium allows the mixing of phosphate in all CRRT fluid bags without the
risk
of Ca3(PO4)2 or Mg3(PO4)2 precipitation. The addition of phosphate to a
commercial
single chamber bicarbonate based CRRT fluid is also a reality for the first
time and
represents a major improvement over currently available bicarbonate based
solutions. Hypophosphatemia or hyperphosphatemia cannot occur with these fluid
designs. Finally, pre-filter phosphate itself may act as an additional
anticoagulant
by also chelating calcium to a minor degree.
Citrate content may be 8 or 16 mmol/L and bicarbonate content may
be 25 or 50 nunol/L. The scales of a Prismaflex machine, for example, can hold
-37-
CA 02863264 2014-09-12
2 fluid bags each or a total of 10 liters per scale. Flexibility in citrate
dosing (when
the plasma flow to pre-filter fluid flow ratio is kept constant at 2:1) may be
achieved
by varying the ratio of the 8- and 16-mmol citrate bags from 0:2 to 1:1 to
2:0.
Flexibility in bicarbonate dosing may be achieved by varying the ratio of the
25- and
50-mmol bicarbonate bags from 0:2 to 1:1 to 2:0. Also, the post-filter fluids
can
be supplemented with half ampoule (25 mEq) bicarbonate per bag if needed for
further flexibility.
For reference, pKa values for acids relevant to RCA at 25 C are as follows:
Citratel: 3 . 13
Citrate2: 4.76
Citrate3 : 6.40
Carbonic acid 1: 6.37
Carbonic acid2: 10.33
Phosphoric acid 1: 2.12
Phosphoric acid2: 7.2
Phosphoric acid3: 12.67
The present invention includes a control program for determining the
optimal ultrafiltration, pre- and post dilution fluid, and blood flow rates
required to
reach treatment goals while minimizing citrate load into the patient. The
control
program also estimates supplemental calcium and magnesium infusion rates and
can
monitor the settings of integrated single calcium plus magnesium infusion pump
44
for added safety. The control program can also calculate bicarbonate balance
using
either citrate sensor 56 or clinical laboratory data to inform clinical care
decisions
on replacement fluid selection for the patient. This control program may be
incorporated into the software of the system used for delivering the fluids
according
to the present invention. The control program simplifies the use of the system
and
allows for exact calculation of the prescribed treatment variables including
blood
flow, pre-filter fluid flow and post-filter fluid flow, net ultrafiltration,
as well as rate
-38-
CA 02863264 2014-09-12
of calcium and magnesium supplement infusion.
The physician may select the duration of the treatment, the hourly
treatment goal, and indicate the presence of severe liver dysfunction and or
acidosis.
The systemic hemoglobin and albumin concentration may also be needed. The
control program may then calculate the most effective, safe the prescription
that can
be delivered without dangerous citrate accumulation in the systemic plasma of
the
patient. All patients (including those with liver failure) can safely reach
the 35
ml/kg/hr treatment goal for 24-hour CVVH. The clearance goal is expressed
corrected for the degree of pre-dilution. Unique kinetic modeling modules and
citrate sensor 56 may be provided to predict citrate accumulation, bicarbonate
wash-
out or accumulation, and the development of hypo- or hypercalcemia with any
particular prescription before these complications could occur providing a
chance
for the operator (or the automated dosing program) to make corrective changes
to
the treatment parameters.
Principles of the control algorithm include:
1) Operational mode of simultaneous pre- and post-dilution CVVH with two
different fluids to maximize single pass citrate extraction on hemofilter 16.
The
novel addition of a maximal amount of ultrafiltration possible for a given
blood flow
with simultaneous post-dilution (citrate-free) fluid replacement allows
enhancing the
fractional removal of the citrate load to 50-75% in a single pass through
hemofilter
16. This means that the twice as high pre-filter fluid rates can be reached by
use of
the system and method according to the present invention with the same
obligatory
citrate load into the patient as with prior RCA protocols. The ultrafiltration
may be
further doubled by the post-dilution step. The summary effect is a 3 to 4-fold
increase in uremic clearance for the same citrate load. In clinical practice,
this will
allow the treatment of almost all patients to the most aggressive pre-dilution
adjusted
clearance goal of 35 ml/kg/hr with markedly enhanced safety.
2) Sufficient plasma total calcium to citrate ratio must be achieved for
effective
anticoagulation. The total Ca (mM) to citrate (mM) ratio will range between 2
to
4 in extracorporeal circuit 12. Part of the citrate may be provided as acid
citrate in
-39-
CA 02863264 2014-09-12
the pre-filter fluid (to further enhance anti-coagulation through
acidification of
thrombin and other coagulation cascade proteins). The plasma flow may be
monitored online with a hematocrit and blood flow sensor module 50, 52. This
will
allow the calculation of the delivered calcium load into circuit 12 and will
define the
necessary anticoagulant infusion rate. Both calcium and citrate do not
distribute into
the RBC volume.
3) The prescription should eliminate the possibility of citrate accumulation
even in
the complete absence of liver metabolism (liver failure). This may be achieved
by
keeping the citrate single pass plasma extraction above 66% when the
CitrateFasy16
fluids are used in a 2:1 plasma to fluid ratio and above 50% when the
CitrateEasy8
fluids are used in a 2:1 plasma to fluid ratio. This will limit the systemic
plasma
citrate to 3.75-5 mM or less, regardless of liver function.
4) The target plasma total calcium level should be defined (usually 2-2.5
mmol/L,
depending on the serum albumin concentration and the achieved citrate
extraction
ratio) by the operator. This will have an indirect impact on the systemic
plasma
ionized Ca content in steady state. The systemic citrate level will have a
modest
impact, even in ICU patients with liver failure, because citrate accumulation
beyond
3-5 inM levels cannot occur when filter performance is maintained at the
specified
fluid flow rates.
5) Providing prescriptions and therapy fluid compositions that allow exact
mass
balance calculations for citrate, calcium and magnesium, sodium and
bicarbonate
(and trace metal minerals).
6) Varying the ratio of the different pre-filter fluid bags and post-filter
fluid bags
for greater flexibility in citrate and bicarbonate dosing.
In the following description, a glossary of the abbreviations used is
as follows:
Csys: calculated steady state systemic plasma citrate concentration in a
patient with
zero citrate metabolism (liver failure; worst case scenario in RCA)
-40-
CA 02863264 2014-09-12
E: apparent circuit post-anticoagulant infusion arterial plasma citrate to
therapy
fluid citrate concentration difference reduction ratio during a single filter
pass
("plasma citrate extraction ratio")
DCit: apparent citrate plasma dialysance when expressed for QP)
QB: the total blood flow here
QP: The arterial blood plasma flow (effective blood water flow for citrate)
Cinf: The increase in the arterial plasma citrate concentration as a result of
the pre-
filter replacement fluid infusion with the pre-dilution effect removed
Hgb: hemoglobin concentration in the arterial blood
C8, Cl6Cit: citrate concentration (mM) in the citrate pre-filter fluid
B25, B50: bicarbonate concentration (mM) in the post-filter fluid
Quf: net ultrafiltration negative fluid balance goal
QCa/Mg: calcium plus magnesium infusion rate
Qpre: pre-filter citrate based replacement fluid flow rate
Qpost: post-filter bicarbonate based replacement fluid flow rate
DCit: the calculated citrate dialysance (DCit* when expressed for the adjusted
QBCit during calculations and DCit when expressed for the unadjusted QP)
f: correction factor to obtain the ultrafilterable fraction of Ca from total
plasma Ca
S: sieving coefficient; SCond; SCit)
The control algorithm according to the present invention may include,
but is not limited to, the following flow steps:
1) Start machine in pre- and post-CVVH mode.
2) a) Machine advises filter, tubing, citrate pre-filter, bicarbonate post-
filter
and calcium solutions.
b) Confirm all disposables are as advised by the machine.
C) Connect tubing to dialyzer (if not pre-connected) and fluid bags.
d) Load tubing onto infusion pumps.
e) Prime system with priming solution.
f) Test system integrity (current machine protocol).
-41-
CA 02863264 2014-09-12
3) RCA priming checks: performed with the circuit arterial and venous ends
connected in recirculation mode.
a) Confirm accuracy of the OSS by filtering the circuit priming solution (a
standard for Ca, Mg and citrate).
b) Alarm: the values returned by the OSS are not accurate, confirm correct
priming solution, check OSS.
c) Confirm citrate replacement fluid loading onto pre-dilution pump 34 by
turning on the pump 34 and measuring the increase in citrate concentration in
the
drain circuit 24 of the hemofilter 16 (if OSS available).
d) Alarm: it is not the citrate infusion solution that is loaded onto the
citrate
pump 34 based on effluent citrate changes.
e) Confirm calcium infusion loading onto the calcium pump 44 by turning
on the calcium pump 44 and measuring the increase in calcium and magnesium in
the drain circuit 24 of the hemofilter 16 (if OSS available).
0 Alarm: it is not the calcium infusion solution that is loaded onto the
Ca2+-pump 44 based on effluent calcium changes.
4) Input Patient Information.
a) Sex, height, age, weight (if Watson volume and VE calculations are
desired; minimum data is weight).
b) Minimum laboratory data is hemoglobin, serum albumin, and serum
bicarbonate concentration.
5) Treatment Information advised by software based on prior selections.
a) Input: Dialyzer type (determines expected KoACit, SCit).
b) Input: Maximum hemoconcentration allowed in the circuit (may define
as 60%).
c) Input: Daily maximum replacement fluid amount (may be about 80-100
liters).
d) Input: Total pre-dilution adjusted plasma clearance goal for CVVH (may
be about 40+ liters).
e) Input: Total net ultrafiltration desired per treatment (or over 24 hours).
f) Input: Set CRRT machine alarm parameters.
-42-
CA 02863264 2014-09-12
g) Input: Estimated liver plasma citrate clearance: normal 0.5, poor 0.25,
none 0 (all L/min).
h) Input: Type of calcium solution (ICU versus OPD, likely uniform).
i) Input: Maximum citrate level in systemic blood allowed (may be about
4.0 mM).
6) Connect the patient.
7) Safety checks after initial patient connection in CVVH mode.
a) Start treatment, confirm citrate is infusing in the arterial limb 14 by
watching the effluent citrate level (if available).
b) Measure access recirculation with online hemodilution technique (if
available).
8) Display Confirmation Alarms.
a) Alarm if more than 10-15% recirculation is detected. The treatment will
still be safe, but less effective for uremic clearance.
b) Measure Hgb concentration with the online sensor (alarm if more than
20% different from initially provided value).
c) Alarm if citrate-containing pre-dilution fluid is not on arterial limb 14
of
circuit 12.
9) Analyze input data.
a) Determine highest post-filtration flow possible as % of QB with set
hemoconcentration limit.
b) If plasma fraction of blood is <=0.66, then Program Qpost for the
above maximum post-filtration, minus (QCa/Mg+Quf) for maximum citrate
clearance with a given QB and total Qtf. Otherwise, maximum post-filtration is
50% of QB.
c) The Qpre is always 50% of QP (QP:Qpre =2:1).
d) For citrate single pass fractional extraction, E is
(Qpre+Qpost+QCa/Mg +Quf)/(Qpre +QP).
e) The pre-dilution bag C16:C8 ratio is: If E=0.66 2:0, if between 0.66-
-43-
CA 02863264 2014-09-12
0.6 1:1, if 0.60-.50 0:2.
0 If E> =0.5 cannot be achieved with CVVH because of limited postUF,
use of SLED or CVVHDF may be advised.
g) The post-dilution bag B25 :B50 ratio is: Initially 1:1, adjust 2:0 (usual),
or 0:2 (liver failure).
h) Aim for 10 liters of pre-dilution fluid use every 6 hours so that bag
changes are predictable.
10) Determine prescription and machine settings.
a) Display QB, Qpre and Qpost, Quf, QCa/Mg.
b) Display expected maximum Csys ( < = 4 mM citrate).
c) Display expected circuit Ca loss (mmol/hour) before replacement infusion
(prescriptions can have uniform QP and DCit versus weight adjusted).
d) Operator selects K content (2K and 4K bags of each fluid type, use ratio
2:0, 1;1, 0:2). The 2:0, 1:1, 0:2 ratios of different bags may be used for
flexibility
in K, citrate and bicarbonate dosing in a system where each scale can hold two
5 L
bags at a time.
11) Calcium Dosing.
a) ECit is essentially equal to ECa * f, where f is the correction for
ultrafilterable fraction (f will be about 0.95 when 2:0, 0.9 when 1:1 and 0.8
when
0:2 ratio C16:C8 pre-dilution fluids are used. f may also have to be corrected
for
albumin levels and circuit pH.).
b) Target systemic plasma total Ca (mM) is defined: Use Csys (0.25 mM
Ca/lmM citrate), systemic albumin (0.2 mM Ca/1 g/dL) and target systemic
ionized
Ca (target Cai = 1.00 mM when systemic citrate is assumed to be equal to Csys
=
3).
c) Circuit Ca loss in steady state is equal to QP (L/hour) * Target systemic
total Ca (mM) * ECit * f.
d) QCa/Mg is easily calculated from the circuit Ca loss and Ca concentration
of the Ca infusion solution.
e) At the start, the operator may have to give 1-4 amps of Ca-gluconate over
1-2 hours to bring the systemic ionized Ca close to 1.25-1.5.
-44-
CA 02863264 2014-09-12
0 Ca dosing may be completely automated with the OSS integrated into
effluent line 24.
12) Continuous safety check.
a) Citrate solution is properly on citrate pump 34 and arterial limb 14 is
arterial (expected constant step-up in effluent citrate from baseline) (with
OSS).
Alarm if citrate bag changed to calcium or saline or access connection
reversed
during operation based on effluent citrate and calcium monitoring with all the
above
IV fluids having different ingredients.
b) Input: Set access blood flow rate: current (QB) (Alarm: when QB is
changed because of access issues recalculate all pump speeds and fluid flows).
c) Input: Measured hemoglobin concentration (Alarm: when changed by
more than 10% alert operator to possible bleeding or over-ultrafiltration;
recalculate
prescription, recommend CBC check, net ultrafiltration target revision).
13) 6 Hourly safety check: input data.
a) Input: measured venous blood gas (VBG) and ionized Ca on the arterial
limb before citrate or on the venous limb 18 of the blood circuit 12 after the
Ca
infusion (Systemic arterial blood gas (ABG) or VBG with ionized Ca also
acceptable).
b) Systemic total and ionized Ca if indicated only.
c) Hemoglobin every 12 hours (or online with sensor).
d) Albumin once daily or if receiving albumin/plasma products.
e) Hourly net UF goal if changed.
0 Test OSS with zero QB and with filtering the standard priming solution.
14) Recalculation of the prescription.
a) Re-calculate: maximum post-filtration, maximum ECit, bicarbonate flux
then adjust.
b) Pre-filter fluid C16:C8 ratio.
c) Post-filter fluid B25 :B50 ratio.
d) Supplement either B25 or B50 with 1/2 amp NaHCO3 per 5 L bag if
needed.
-45-
CA 02863264 2014-09-12
e) Adjust rate of QCa/Mg infusion.
0 Adjust QB (to adjust QP) and Qpre to use about 10 L/6 hours pre-filter
fluid (keep QP:Qpre 2:1).
15) Other alarms.
a) Change filter electively every 24-48 hours to prevent protein fouling even
in the absence of clotting.
b) Change entire circuit every 72 hours.
c) Replace OSS sensors as needed regularly.
System 10 according to the present invention may contain an OSS for
measuring calcium, magnesium and citrate in the ultrafiltrate. As explained
herein,
the calcium, magnesium and citrate values measured from the ultrafiltrate by
the
OSS can be used to back-calculate the values in the patient's plasma. As also
explained, the kinetic curve of systemic plasma citrate concentration can be
used to
derive the exact value of the liver clearance of citrate as well as the volume
of
distribution of citrate, VE. Using the above parameters, systemic citrate
levels can
be accurately predicted at any future T time point. The calcium pump 44 and
citrate
pump 34 as well as the entire prescription including the therapy fluid
bicarbonate
concentration (when flexible) can then be completely controlled by the machine
software according to the present invention.
Filter performance can be monitored by online citrate clearance
measurements. The direct citrate clearance measurements again enable complete
precision in calcium and citrate dosing. Since calcium exits through
hemofilter 16
almost entirely as Ca-citrate complex, the measured citrate dialysance will be
nearly
equal to the total calcium dialysance. The slightly lower Ca-dialysance will
be due
to the Gibbs-Donnan effect and the minimal albumin-bound Ca in circuit 12
(about
5-20% depending on the amount of citrate infused in the arterial limb 14 of
the
circuit 12, the acidity of the citrate infusion and the plasma albumin level).
In further accordance with the present invention, an RCA system is
provided which may include an online clearance monitor (OCM) and can safely
-46-
CA 02863264 2014-09-12
provide fully automated RCA with any online fluid generation-based modality of
RRT currently in clinical use. This embodiment of the RCA system is designated
generally by reference numeral 110 and is illustrated in FIGS. 5-8, wherein
components similar to those described for system 10 are given like reference
numerals except for the addition of a "1" prefix. System 110 is capable of
simultaneous pre- and post-dilution hemoffltration for the greatest therapy
fluid
efficiency. The online fluid generation system of RCA system 110 may follow
the
traditional two- (acid and base) concentrate component design, thus allowing
the
greatest variability in the final sodium and bicarbonate concentration to best
suit the
needs of indiviaual patients. Finally, system 110 also incorporates a dialysis
machine module to measure conductivity of the fresh online therapy fluid as
well as
the filter effluent fluid. These measurements are obtained in conjunction with
alterations of the citrate anticoagulant solution infusion rate and are
analyzed using
calculations markedly different from prior art. The method according to the
present
invention allows precise online clearance measurements even in CRRT
operational
modes which is not possible with the prior art, and thus allows the continuous
monitoring of the filter performance (clearance). This, in turn, ensures the
maintenance of the efficacy and safety of the treatment prescription.
RCA system 110 can safely provide therapy to critically ill patients
even if they have acute liver failure with inability to metabolize citrate.
The system
design prevents citrate accumulation in the patient, while maintaining highly
efficient anticoagulation of the extracorporeal circuit. System 110 can also
provide
fully regionally anticoagulated blood to any extracorporeal blood circuit,
such as up
to a maximum operational blood flow of 500 ml/minute. System 110 is thus
suitable
to accommodate the emerging hybrid therapies that combine uremic solute
clearance
with plasmapheresis or plasma adsorption by running the anticoagulated blood
through specialized adsorption columns or plasma separation devices. Following
citrate removal in the dialyzer, the anticoagulated blood could also be
perfused
through a bioartificial kidney device that contains live renal tubular cells
or through
a MARS liver replacement therapy circuit before the reversal of
anticoagulation by
the calcium infusion. RCA system 110 achieves these goals with minimal input
from the operator and delivers the treatment without any need for intervention
in all
-47-
CA 02863264 2014-09-12
modes of operation. This will broaden the settings in which a 12 to 24-hour
CRRT
procedure can be performed and will likely increase its utilization. System
110 can
also be used to provide highly effective and safe RCA for any modality of RRT
including in-center intermittent hemodialysis or hemodiafiltration and home
quotidian or nocturnal hemodialysis, making it applicable to the far greater
market
of outpatient RRT sessions where heparin anticoagulation is not preferred.
As will be described in greater detail below, system 110 according
to the present invention also utilizes novel therapy fluid concentrates, a
novel citrate
anticoagulant and novel single premixed calcium plus magnesium infusions that
have
been designed to fully exploit the system's capabilities. A control algorithm
is
provided which derives a safe treatment prescription according to the
treatment
goals selected by the operator. Special access catheters and/or special
circuit tubing
connectors allow system 110 to provide RCA as soon as the blood enters the
catheter tip (or the circuit tubing from the access needle). The single needle
operational mode eliminates the concerns about access disconnection.
The following describes a comprehensive system and method
according to the present invention for providing highly effective and
completely safe
RCA for a hemodialysis machine 160 designed for CRRT. With reference to FIGS.
5a, 6a, 7a, and 8a, system 110 includes a CRRT circuit 112 which includes
arterial
blood line 114, hemofilter 116, and venous blood line 118. System 110 includes
a
blood pump 122 which should ensure as accurate as possible agreement between
the
set and delivered blood flow. System 110 may also include volumetric balancing
chambers 162 for coordinating total ultrafiltration and CRRT replacement fluid
infusion volumes, obviating the need for the machine operator to rely on a
scale-
based system with frequent measurement and exchange of various fluid bags.
Volumetric balancing chambers 162 include a replacement fluid
pump(e.g., volumetric) that diverts a portion of the online therapy fluid for
pre- or
post-dilution hemodiafiltration (FIG. 6a). Fluid removed by this pump
subtracts
from the total fresh therapy fluid delivered to the hemofilter 116. The action
of the
balancing chamber 162 ensures that all fresh therapy fluid delivered to the
-48-
CA 02863264 2014-09-12
extracorporeal circuit as a pre-dilution replacement infusion, post-dilution
replacement infusion, or dialysis fluid is precisely equal to the total
circuit effluent
minus a small portion of the effluent that is diverted before such balancing
by the
net ultrafiltration pump. This volumetric pump may infuse about 75% of the
therapy fluid either as pre-dilution replacement fluid (simultaneous pre-post-
dilution
CVVH) or as dialysate in pre- or post-dilution hemodiafiltration. Finally,
this pump
may pump 100% of the therapy fluid as dialysis fluid in pure hemodialysis.
Additional pump(s) 164 (e.g. volumetric) may be provided to divert a portion
of the
online therapy fluid for pre-dilution hemodiafiltration (FIG. 6a), post-
dilution
hemodiafiltration (FIG. 7a), or simultaneous pre- and post-dilution
hemodiafiltration
(FIG. 8a). Another volumetric pump may divert a small portion of the effluent
fluid
as net ultrafiltrate before the bulk of the effluent enters the volumetric
balancing
chamber. Still another optional pump is an additional blood pump that is only
needed if the single-needle dialysis mode is used. The benefit of this
operational
mode is that the machine immediately detects accidental access disconnection.
This
is of great clinical value when a permanent access (fistula or graft) is used
for CRRT
in the ICU or for nocturnal hemodialysis in-center or at home.
System 110 according to the present invention may include a
volumetric, precise IV infusion pump 134 for the infusion of concentrated
citrate
anticoagulant into the arterial limb 114 of the extracorporeal circuit 112.
Pump 134
may operate in the 0.1-20 rill/min flow rate range and may be precise to 3%
of the
prescribed rate. Also, for essentially continuous flow of the pumped liquid,
the
volume per single pumping cycle may be in the 0.05-0.2 nil/cycle range. In one
implementation, this pump 134 may have a dedicated air detector controlling a
line
clamp (not shown). A volumetric, precise IV infusion pump 144 may be provided
for the infusion of concentrated calcium and magnesium chloride into the
venous
limb 188 of the extracorporeal circuit 112 to restore calcium and magnesium
mass
balance. The same pump specifications would apply here as for the citrate pump
134. In one implementation, this pump 144 may also have a dedicated air
detector
controlling a line clamp (not shown). All of the above-described pumps may be
operated and monitored for safety by a control algorithm built into the
hemodialysis
machine software program.
-49-
CA 02863264 2014-09-12
FIG. 5a depicts a system with pumps and fluid connections suitable
for sustained low efficiency dialysis (SLED) or 4-5 hour intermittent
hemodialysis
(IHD). FIG. 6a depicts a system with pumps and fluid connections suitable for
continuous veno-venous hemodialysis with pre-dilution ultrafiltration
(CVVHDF).
FIG. 7a depicts a system with pumps and fluid connections suitable for post-
dilution
hemodiafiltration (HDF). FIG. 8a depicts a system with pumps and fluid
connections suitable for continuous simultaneous pre- and post-dilution veno-
venous
hemofiltration (CVVH) or 4-6 hour intermittent high volume hemoffltration
(HVHF).
RCA system 110 may include a conductivity-based online clearance
monitor (OCM) 170 that provides precise measurement of the delivered small
solute
clearance in any operational mode. FIGS. 5b, 6b, 7b, and 8b illustrate a
conductivity-based OCM according to the present invention with online-
generated
dialysis fluid and automated RCA corresponding to the different treatment
scenarios
depicted in FIGS. 5a, 6a, 7a, and 8a, respectively, wherein all of the
parameters are
known or measured except Cp and C. OCM 170 according to the present
invention may include conductivity sensors 172, 174 operably connected to line
176
carrying filtered sterile pyrogen-free online therapy fluid and line 178
carrying
effluent fluid, respectively. Precise dosing of RRT based on conductivity
dialysance
will provide pharmacists with invaluable data for medication dosing and will
aid
clinical research in CRRT.
Automated self-check methods for proper circuit fluid connections
according to the present invention may be provided to provide safety
monitoring of
the RRT circuit connections. At startup, before the patient is connected to
the
extracorporeal circuit 112, the machine 160 may automatically fill the blood
circuit
with priming solution and may remove air from all infusion lines as well. The
machine 160 may then run a few-minute mock treatment session with the priming
solution instead of blood recirculating in the blood circuit. During this
time, loading
of the calcium pump 144 and the citrate pump 134 with the appropriate infusion
solution may be confirmed by giving a bolus from each pump and confirming the
expected change in the filter effluent conductivity appropriate for the pumped
-50-
CA 02863264 2014-09-12
medication infusion. This startup method will utilize the fact that the
conductivity
of the citrate anticoagulant and the calcium infusion is markedly different.
During
this startup period, the baseline filter conductivity dialysance may also be
obtained
with priming solution in the circuit and compared with the value expected for
the
filter and the prescription fluid flow rates. Significant differences may
trigger a
filter alarm. After the proper loading of the medication pumps is confirmed
with
the effluent conductivity method, the patient may be connected and the blood
circuit
tubing filled with blood.
The machine 160 may then give a small priming solution bolus in the
blood circuit and check for access recirculation by looking for any
hemodilution in
the arterial limb 114 of the blood circuit using the online hematocrit sensor
150. If
recirculation is detected, the machine 160 may prompt the operator to review
the
access connections and/or the access itself. After assessing for
recirculation, the
machine 160 may deliver a citrate bolus into the arterial limb of the blood
circuit
connected to the citrate pump 134 and may compare the imaged change in filter
effluent fluid conductivity with what is expected. If the citrate pump 134 is
infusing
into the venous limb 118 due to wrong connection, the bolus will not be seen
in the
filter effluent and the machine 160 will halt the citrate infusion and alert
the operator
to the wrong connection. During this initial citrate bolus, the baseline
filter
conductivity dialysance may also be obtained, now with blood in the circuit,
and
compared with the expected value for the filter and the prescription fluid
flow rates.
Significant differences may trigger a filter alarm. Finally, if during a
treatment
interruption the patient is removed from the machine, and the blood circuit is
subsequently wrongly reconnected leading to venous infusion of the citrate
anticoagulant, the resultant marked change (drop) in the filter effluent
conductivity
may be immediately detected and may cause a machine alarm and cessation of the
citrate infusion and RRT delivery until the connections are reviewed by the
operator.
The RCA system 110 eliminates the risks associated with the nurses
dosing a concentrated citrate and or calcium infusion for anticoagulation
during a
CRRT or other extracorporeal blood treatment procedure that uses RCA. Citrate
-51-
.
CA 02863264 2014-09-12
removal by the hemofilter 116 is important for safe operation of a CRRT system
using citrate anticoagulation. If solute removal is stopped and blood
continues to
flow through the extracorporeal circuit to prevent coagulation, the infusion
of the
anticoagulant solution has to be stopped immediately or the patient will
receive an
excess amount of citrate which could be life threatening. In RCA system 110,
if for
any reason solute removal stops and blood continues to flow through the
extracorporeal circuit to prevent coagulation (for example: when the machine
has
a dialysate/replacement fluid conductivity alarm), the delivery of citrate as
well as
any calcium plus magnesium replacement infusion is immediately aborted to
protect
the patient from receiving an excessive amount of citrate and or calcium plus
magnesium.
The automated RCA system 110 according to the present invention
markedly reduces the need for health care personnel to monitor and adjust the
CRRT. Significant modifications to the software running the hemodialysis
machine
160 are necessary to provide online conductivity dialysance measurements
during
CRRT and support the various operational modes with RCA according to the
present
invention. The control program (described below) allows tailoring of the
prescription to the specific treatment objectives and the individual patient's
condition
with scientific accuracy by defining only a few variables. Calcium infusion
dosing
is predictive and automated. Finally, the RCA system 110 eliminates the risk
of
citrate accumulation in the patient associated with RCA during hemofiltration
or any
other extracorporeal blood processing intervention, such as up to blood flow
rates
of 500 ml/min. This is expected to finally bring this treatment modality from
highly
specialized academic health care institutions to a broad group of patients and
to
allow the safe operation of the procedure by less experienced health care
personnel
in most (not-academic) health care settings.
The RCA system 110 eliminates the dangers of prior RCA protocols
in CRRT as discussed below:
1) Hypernatremia: The coordinated and carefully calculated pre-filter infusion
of
the anticoagulant citrate and use of the online generated therapy fluid
solution
-52-
CA 02863264 2014-09-12
always ensures the adherence to an operator-selected final sodium
concentration in
the summary fluids that come into contact with the patients' blood in the
range of
135 to 145 as well as attaining a selected (usually 140 mM) sodium
concentration
in the venous blood returning to the patient.
2) Metabolic alkalosis: The sum of bicarbonate and anions metabolizable to
bicarbonate (in mEq) may be kept between 25-40 mEq bicarbonate equivalents per
liter of summary therapy fluid. This is in keeping with fluid alkali content
per liter
prescribed in most high dose CRRT protocols in the literature. Since the
therapy
fluid bicarbonate concentration can be freely adjusted in the range of 25-40
mM, this
complication will be eliminated or easily corrected.
3) Metabolic acidosis: The system does not depend on citrate metabolism to
provide bicarbonate to the patient. The prescriptions will keep the systemic
citrate
level in a narrow range (0-3 mM) regardless of liver function. Therefore
metabolic
acidosis will not develop even in the patient with severe liver failure and no
significant citrate metabolism and even anhepatic patients can continue on
high dose
CRRT with RCA system 110 without the need for separate bicarbonate
supplementation.
4) Hypocalcemia 1 (due to net calcium loss from the patient): The
ultrafiltrate total
calcium and magnesium losses are precisely calculable in system 110 that
measures
conductivity dialysance directly and calculates Ca and Mg dialysance
indirectly.
The combined calcium plus magnesium infusion regulated by the machine 160 will
be dosed by the control program also taking into account any initial citrate
accumulation predicted by kinetic modeling. It is expected that the system 110
will
be fully automated and that no changes to the infusion rate will be needed
during
therapy. Such predictive dosing will also enable the operator writing the CRRT
prescription to specify or "dial in" the target systemic plasma total calcium
level that
corresponds to a target (normal) ionized calcium level in the given patient.
Clinical
prudence will likely mandate that the patient's systemic total and ionized
calcium
levels continue to be measured every 6 hours with adjustments made to the
infusion
as required (but such adjustments are not expected). Magnesium will be dosed
to
-53-
CA 02863264 2014-09-12
maintain a total plasma Ca:Mg = 2:1 to 2:0.8 molar ratio (as ionized magnesium
measurements are not routinely available and all calcium chelators (albumin,
citrate
etc also chelate magnesium).
5) Hypocalcemia 2 (due to citrate accumulation): Citrate will be given by a
machine controlled IV pump 134. This eliminates the risk of nursing errors
with
a separate citrate infusion. The default mode of RCA system 110 provides for
75%
or higher citrate extraction on the hemofilter 116 in a single pass during
CRRT.
This eliminates the possibility of marked systemic citrate accumulation even
in the
absence of liver metabolism. Appropriate calcium infusion dosing will prevent
the
initial mild hypocalcemia due to a limited systemic citrate buildup. This will
be
accomplished by using the estimated systemic plasma levels of citrate as
predicted
by a kinetic modeling program. The kinetic program analyzes the CRRT
prescription (fluid compositions and flow rates as well as blood flow rate).
It also
utilizes anthropomorphic data to predict the citrate volume of distribution in
the
patient. Finally, for safety the patient's citrate clearance in L/minute will
be
estimated as zero, to generate the expected citrate accumulation curve and
guide
calcium and magnesium replacement to saturate the retained citrate in the
first few
hours of the treatment. In all patients, RCA system 110 will always be run in
the
safest mode with no possibility of citrate accumulation or significant
metabolic
acidosis.
6) Rebound hypercalcemia (due to release of calcium from citrate after CRRT
with
RCA is stopped): System 110 may not allow treatment prescriptions that could
result in systemic citrate levels in excess of about 3 mmol/L. This will
ensure that
systemic citrate levels stay < =3 mM corresponding to about maximum 0.6 mM
chelated calcium that could be released after RCA is stopped in all patients
who can
metabolize citrate. (Most patients will have 1 mM plasma citrate and about
0.25
mM Ca chelated by citrate in the steady state). The RCA protocol according to
the
present invention is designed to keep systemic ionized Ca levels around 1-1.25
and
therefore the highest calcium level after RCA is stopped will be <=1.5-1.75 mM
and most patients will rebound to 1.5 mM Ca levels after treatment.
-54-
CA 02863264 2014-09-12
7) Hypophosphatemia: In all operational modes except short outpatient dialysis
or
HVHF, the online generated pre-filter therapy fluid can be supplemented with
physiologic amounts of phosphate by the manufacturer of the concentrate
without
the risk of calcium- or magnesium-phosphate precipitation. The phosphate-
containing fluid can be used even when the serum phosphorus is high, as the
large
clearance goals will allow significant net phosphate removal while the
hyperphosphatemia is present. Conversely, the pre-filter fluid will also serve
to
correct hypophosphatemia towards normal when needed.
8) Fluctuating levels of anticoagulation: The high citrate to calcium ratio
maintained in the circuit 112 (and the marked pre-dilution in some operational
modes) ensures predictable citrate levels and very effective anticoagulation
in the
circuit 112 as well as a clearly defined hourly citrate load into the patient.
9) Nursing errors: The RCA system 110 is designed so that the nurses or other
operators only need to ensure timely supply of the fluids used by the system
110 and
regular laboratory monitoring for total and ionized calcium as clinical
prudence
dictates. Therefore, nursing errors are near completely eliminated by the
system
design, as the nurse's role is mainly to obtain blood samples at specified
intervals
and notify the operating physician of the results as well as possibly manually
enter/confirm treatment prescriptions as specified.
10) Rare: Ionized hypomagnesemia: Since clinical monitoring of ionized
magnesium is usually not possible, the method according to the present
invention
may aim to maintain a 2:1 molar ratio between total plasma calcium and total
plasma
magnesium. To achieve this, the molar ratio of calcium and magnesium may be
fixed at 2:1 in the RCA system-regulated calcium plus magnesium infusion as
well
as in some lx therapy fluids (dialysate). Such dosing ensures that total and
ionized
magnesium levels will be appropriate for the steady state plasma citrate
levels.
11) Declining filter performance: The novel conductivity-based online
clearance
monitor will detect this complication and alert the operator that the filter
needs to
be replaced. The optical hematocrit sensors 150 can detect access
recirculation and
-55-
CA 02863264 2014-09-12
can enable the correction of blood bolus-based clearance measurements as well
as
derived systemic citrate and calcium levels for this phenomenon.
12) Trace metal depletion: Cationic trace metal supplementation may be
provided
with the calcium infusion to restore precise mass balance for these trace
solutes.
Any trace metal incompatible with the calcium infusion can be provided in the
citrate anticoagulant infusion in an adjusted concentration.
13) Access disconnection: Needle disconnection can be safely detected if a
single
needle operational mode is used in combination with the novel circuit tubing
connector to access a permanent access for CRRT or daily nocturnal dialysis.
14) Wrong connection of citrate, calcium or acid concentrate or blood circuit
to
patient: These errors are prevented by the hardware design of the system 110
as
well as through conductivity monitoring based safety checks.
15) Disconnection of the calcium and or citrate infusion: This can be
completely
prevented by appropriate circuit tubing design (non-disconnectable, physically
continuous infusion to blood line connection). The disconnection of the
citrate
infusion can also be detected by monitoring the circuit effluent conductivity
and or
citrate concentration.
For use with RCA system 110, an anticoagulant citrate solution may
be provided according to the present invention with 5.33:0.66 molar ratio of
tri-
sodium citrate and acid citrate and a total concentration in the 100 to 500
mmol/L
range. At a plasma flow of 100 rnl/min, a 150 mM solution will be infused
around
240 ml/hour. The acid citrate content was reduced to increase the conductivity
and
allow safe (from the standpoint of circuit acidification) intermittent
bolusing for
online clearance measurements. The citrate concentration will be the highest
allowed by the FDA that still allows precision in delivering exact amounts of
sodium
citrate boluses during the clearance measurements. If the solution according
to the
present invention is not available, a commercially available tri-sodium
citrate can
be used (139 =non) at about 260 ml/min at 100 ml/min plasma flow. In one
-56-
CA 02863264 2014-09-12
modification specifically contemplated herein,, trace metal minerals that are
incompatible with the calcium infusion may be added to the above citrate
solutions
in a concentration sufficient to restore circuit mass balance for the specific
trace
metal mineral. Finally, the concentration of the citrate solution may also be
correlated with the calcium infusion to make sure these two fluids have
markedly
different conductivities.
In addition, the anticoagulant citrate solution may contain sodium
chloride in the 0-4000 nunol/L concentration range to increase the
conductivity of
the solution. The fluid may contain NaCl at about 150 inM. This will increase
the
accuracy of the novel conductivity-based clearance monitor without requiring
the use
of highly concentrated sodium citrate solutions. The higher sodium and
chloride
content of the anticoagulant is easily compensated for by reducing the sodium
and
chloride content of the online dialysis and/or replacement fluid if needed.
The
addition of any concentrated electrolyte solution (including the other
specific
example of sodium bicarbonate in the 0-2000 mmol/L concentration range when
only basic citrate anticoagulant is used) to the citrate anticoagulant
solution to
increase its conductivity for the purposes of online clearance monitoring
through
conductivity measurements and to identify the solution through its measured
conductivity is fully contemplated in accordance with the present invention.
Novel acid concentrates may be designed according to the concentrate
proportioning systems of the hemodialysis machine 160. The final 1X therapy
fluid
concentrations are defined for all operational modes (the 34X acid and base
concentrate compositions follow from the 1X values as apparent to those
skilled in
the art). The acid concentrates in one implementation will have essentially
zero Ca,
Mg and citrate content and some will have (in the case of the CRRT
concentrates)
phosphate in them. The unique acid concentrates may be diluted and mixed with
the
standard bicarbonate concentrate. However, in a variation of all the unique
CRRT
acid concentrates, the phosphate will not be added to the acid concentrate but
rather
it will be in the base concentrate as an approximately 20:1 mixture of di- and
mono-
sodium phosphate salt to be pH compatible with the bicarbonate. The purely
diffusive and convective operational modes in CRRT may perform well with a
single
-57-
CA 02863264 2014-09-12
fluid design. This acid concentrate design is presented for procedural
simplicity and
flexibility for all CRRT. The same single acid concentrate without phosphate
is
suitable for all intense, 4-5 hour outpatient HVHF, HDF or IHD operational
modes.
A novel calcium plus magnesium chloride mixed infusion with a
Ca:Mg molar ratio of 2:1 (range 4:1 to 2:1) and in one implementation a total
Ca
about 200 mmol/L and total Mg about 80 mmol/L is contemplated with the
possible
simultaneous use of a traditional, lower conductivity citrate anticoagulant.
At a
plasma flow of 100 ml/min, this will result in a 40-70 nil/hour calcium
infusion rate.
The dilution of the solution will be selected to ensure the precision of
dosing (a
reasonably concentrated solution will be used as allowed by the pumping
precision
of the IV pump). In another application, the solution will be more dilute with
a total
Ca about 50 mmol/L and total Mg 25 mmol/L with the possible simultaneous use
of a high conductivity novel citrate anticoagulant. In one modification
contemplated
herein, trace metal minerals may be added to the above solutions with each
specific
trace metal having a specific predefmed molar ratio to calcium (similar to the
concept for magnesium). This molar ratio (for each specific trace metal
species)
will be the same as the molar ratio of total calcium to the total specific
trace in the
RRT circuit effluent during RCA. (This ratio, in turn, may be about the same
as
their respective total molar concentration ratio in human plasma during RCA.)
The
ratio for each trace metal will be refined based on results of clinical mass
balance
studies.
Finally, in one embodiment, all calcium replacement solutions may
be supplemented with sodium chloride to a final concentration of 150 mmol/L
for
easier sodium mass balance calculations and also to modulate the final
conductivity
of the fluid. The addition of any concentrated electrolyte solution (including
the
specific example of sodium chloride in the 0-2000 mmol/L concentration range)
to
the calcium replacement solution for the purposes of easier mass balance
calculations
and to increase its conductivity to help identify the solution through its
measured
(directly, or indirectly through its effects on the filter effluent)
conductivity is fully
contemplated in accordance with the present invention.
-58-
CA 02863264 2014-09-12
The novel fluids which may be used by RCA system 110 according
to the present invention are detailed below. Common to all 1X final dialysate
compositions is the fact that they are generated by diluting and mixing an
acid and
a base concentrate. The shown separation of the components into the acid and
base
concentrates was chosen to best accommodate the online fluid generation system
of
the dialysis machine (Fresenius 2008) used for initial testing. However, all
permutations of separations of the components of the final dialysates in all
concentrated and diluted formulation including, but not limited to, a 0.25X -
50X
concentration range that by mixing would result in the same 1X online fluid
are fully
contemplated. Also, all concentrates can be provided as dry powders as well to
be
dissolved and diluted with water. For online therapy fluids, the complete 1X
fluid,
as well as the portion of the individual solute components coming from the
acid
concentrate are defined herein.
Citrate anticoagulant solutions for RCA system 110:
For all patients receiving CRRT (pre-post-dilution CVVH, pre-
dilution 24-hour CVVHDF, or 24-hour SLEDD), the usually used anticoagulant
solution is a 5.33:0.66 molar mixture of basic and acid citrate:
1. Acid Citrate Anticoagulant 1 for CRRT:
Acid Citrate Anticoagulant 1 for CRRT:
about 4% w/v total citrate; a mixture of
basic and acid citrate in a 8:1 molar ratio =ion
Sodium chloride 150 150
Total Citrate 150 450
Trisodium (Basic) Citrate 133.33 400
Citric Acid 16.67 50
The hypertonic sodium content makes online clearance measurements
possible and more accurate with the novel method described earlier. The
accuracy
is greatest when the fluid sodium concentration is highest, limited by taking
into
account the precision of the sodium citrate pump 134. The conductivity
increment
of the anticoagulant over normal plasma is also significantly (150%) different
from
the calcium infusion to detect an accidental mix-up of the infusates. The acid
-59-
CA 02863264 2014-09-12
component is included for its antibacterial effects during storage and also as
it
contributes to predictable circuit calcium-albumin dissociation as well as
anticoagulation by a separate circuit acidification effect. This solution is
around the
4% weight per volume concentration (w/v) limit for citrate recommended by the
FDA for direct infusion.
2. Acid Citrate Anticoagulant 2 for short, intense IHD, HDF or pre-post HVHF:
Acid Citrate Anticoagulant 2 for short,
intense IHD, HDF or pre-post HVHF:
about 4% w/v total citrate; a mixture of
basic and acid citrate in a 2:1 molar ratio mmol/L mEq/L
Sodium Chloride 250 250
Total Citrate 150 450
Trisodium (Basic) Citrate 100 300
Citric Acid 50 150
These acid citrate anticoagulants are different from the prior art (e.g.,
the ACD-A Solution of Baxter) as they contain no dextrose and have a higher
total
citrate and sodium content. The acidity of the anticoagulant is very important
and
provides for further disruption of the coagulation cascade beyond the
chelation of
calcium. In solution 2, the proportion of the acid is increased as the total
amount
of citrate mixed with a liter of plasma is reduced in shorter, more intensive
renal
replacement therapy sessions. (The need for intense anticoagulation is less
here as
filter clotting only needs to be averted for 4-5 hours as opposed to days in
CRRT).
The sodium concentration is highest to allow precise online clearance
measurements,
and more importantly, to allow the use of a low sodium content in the special
acid
concentrates used for RCA, making it possible for the system to detect these
concentrates through the lower conductivity of the final therapy fluid
generated at
standard dilution ratios with their use. This is important to avoid the
accidental use
of a low or zero calcium acid concentrate meant for RCA during an RRT session
without RCA and the combination of moderately lower sodium acid concentrates
and
final dialysis fluids with higher sodium anticoagulant infusions is
specifically
contemplated according to the present invention.
-60-
CA 02863264 2014-09-12
The two anticoagulant fluids described above have identical total
molar citrate content to eliminate the chance of a severe citrate dosing error
if one
solution is inadvertently used instead of the other and also have identical
sodium
(and conductivity) content to allow uniformity during the online clearance
measurements. FDA recommendations on maximum citrate content of infusates
may mandate the use of fluids with total citrate content limited to 4% w/v.
However, this may not be necessary as these fluids are part of the
extracorporeal
circuit and are immediately diluted there. The strictly machine-controlled
administration of these infusates ensures that no concentrated citrate can
enter the
patient's body. Anticoagulant solutions with basic to acid citrate ratio 2:1
to 8:1,
and total millimolar citrate content 50 to 1000 mmol/L are contemplated
according
to the present invention, along with total citrate content around 4% w/v.
Anticoagulant solutions with only basic citrate (50-1200 mmol/liter citrate)
and
additional sodium bicarbonate or sodium chloride either or both in the range
of 0-
2000 mmol/L to increase the conductivity are also contemplated. Finally,
anticoagulant infusions with similar designs but the citrate molecules
replaced by
other chelators of calcium that are safe for human infusion in large amounts
(for
example isocitrate) are also fully contemplated in accordance with the present
invention.
Novel calcium plus magnesium premixed single replacement solution:
A concentrated calcium and magnesium chloride infusion having a
0.25X-4X continuous range diluted/concentrated formulations with a 2:1 (range
1:1
to 4:1) molar ratio of calcium and magnesium are provided according to the
present
invention. All other possible formulations with similar Ca and Mg content and
with
any anion accompanying these cations that can be used for human IV infusion
are
also fully contemplated including, but not limited to, lactate, acetate or
gluconate.
1. CaC12 and MgCl2 infusion in the venous blood circuit limb near the access
catheter or needle:
-61-
CA 02863264 2014-09-12
A. CaC12 and MgC12
infusion in venous
limb near catheter mmol/L mEq/L
Calcium 50 100
Magnesium 25 50
Sodium 150 150
Chloride 300 300
Trace metals see text see text
B. CaC12 and MgC12
infusion in venous
limb near catheter nunol/L mEq/L
Calcium 200 400
Magnesium 80 160
Sodium 150 150
Chloride 710 710
Trace metals see text see text
The above are the most likely formulations of the infusion and are
based on the novel concept that under any operating conditions during RCA for
CRRT, calcium and magnesium is lost from the extracorporeal circuit in the
effluent
fluid in a roughly 2:1 to 2:0.8 molar ratio (depending on the steady sate
citrate level
in the patient's plasma), corresponding to the molar ratio of these ions in
human
plasma under normal physiologic conditions (about 2.4:1) as altered by the
accumulated modest systemic citrate levels. Therefore, the calcium plus
magnesium
infusion that restores the normal total calcium and magnesium content of blood
in
the venous limb of the circuit should also contain these ions in a 2:1 to
2:0:8 molar
ratio. Such a solution may be important to the optimal performance of RCA with
CRRT. With a plasma flow of about 100 ml/min and corresponding calcium and
magnesium losses in the circuit, the above more dilute (A) fluid will provide
convenient flow rates of 200-300 ml/hour. More dilute (A; such as for CRRT)
and
concentrated (B; such as for outpatient HD) forms of the above solution with
calcium to magnesium molar ratio in the range of 1:1 to 4:1 are also
contemplated.
Selection of the proper calcium content will be guided by the need for
precise pumping (more dilute fluid preferred) and the need for limited volume
to be
infused and conductivity to be different from that of the citrate
anticoagulant
-62-
CA 02863264 2014-09-12
solutions. The idea to use online conductivity measurement of either the
citrate and
calcium infusion fluids directly (such as with a non-contact, sterile method)
or the
changes in filter effluent fluid conductivity in response to a presumed (if
the infusion
bags are connected appropriately) citrate anticoagulant and/or calcium
infusion bolus
to detect accidental mix-up of the citrate and calcium infusions is novel
according
to the present invention.
Trace cationic metal element supplementation with the calcium infusion:
In their cationic form, trace elements like chromium, copper,
manganese, molybdenum, selenium, zinc and iron are chelated by citrate. It is
expected that citrate will strip many or all of these trace metals from their
carrier
proteins in the plasma and will remove them from the patient's body through
the
extracorporeal circuit. Similar to the concept of proportional magnesium
removal
detailed above, it is expected that the removal of the trace metals will be
proportional to the removal of calcium, according to their individual renal
replacement therapy circuit effluent molar concentration ratios to the
effluent
calcium. Therefore, the present invention provides a calcium plus magnesium
infusion that is supplemented by the cationic trace metals present in human
plasma,
in a Ivied molar ratio to the calcium in the infusion as defined by their
total calcium
to total trace metal molar concentration ratios in the circuit effluent during
RCA,
plus or minus 100% range in the molar concentration ratio. The anion
accompanying the Ca2+' Mg2+ and cationic trace metals will have to be
compatible
with all cations without precipitation and will have to be safe for IV
infusion. The
likely candidates include, but are not limited to, chloride, lactate,
gluconate or
acetate. All possible formulations with any suitable anion of this calcium
plus
magnesium and multiple trace element infusion that satisfies the above molar
ratio
requirements are fully contemplated in accordance with the present invention.
In
a separate approach, it is also possible to provide the trace element
replacement with
the citrate anticoagulant, the dialysis fluid or with the pre- or post-
dilution
replacement fluid infusion, therefore the present invention also contemplates
supplementing these fluids with trace metal elements to restore mass balance
for
these metals during regional citrate anticoagulation.
-63-
CA 02863264 2014-09-12
Finally, all calcium replacement solutions may be supplemented with
sodium chloride to a final concentration of 150 mmol/L for easier sodium mass
balance calculations and also to modulate the final conductivity of the fluid.
The
addition of any concentrated electrolyte solution (including the specific
example of
sodium chloride in the 0-4000 mmol/L concentration range) to the calcium
replacement solution for the purposes of easier mass balance calculations and
to
increase its conductivity to help identify the solution through its measured
(directly,
or indirectly through its effects on the filter effluent) conductivity is
fully
contemplated according to the present invention.
Bicarbonate with phosphate for any treatment modality:
In one embodiment of the novel 1X dialysate formulations, all novel
electrolyte features are provided by the unique composition of the acid
concentrates.
In this manner, the standard base (bicarbonate) concentrates currently in use
with
commercial dialysis machines can be used without alterations. However, in one
possible embodiment, the phosphate could be provided as part of the base
concentrate, to eliminate concerns about incompatibility with Ca2+ and Mg2+
ions
in the acid concentrate.
1. Base concentrate with phosphate:
The most important design feature here is the need to provide the
phosphate as a mixture of its disodium and monosodium salts in a ratio that
results
in the same buffered pH as the pH of a solution prepared by dissolving just
sodium-
bicarbonate in water. The target pH value is defined as pH = (plCal +plCa2)/2,
where pKal and pKa2 are the acid dissociation constants of carbonic acid at 25
C
and the ionic strength of the concentrate (expected about 6.4 and 10.3 with
the target
pH around 8.4). The ratio of the sodium phosphate salts can be derived from
the
equation pH = pKa2 + log (salt/acid), where pKa2 is now the second acid
dissociation constant of phosphoric acid, about 7.1 at 25 C. Therefore, the
ratio of
the salt (disodium-phosphate) to acid (monosodium phosphate) will be about
20:1.
The exact ratio may be different slightly (the pKas may be slightly different
at the
ionic strength of the concentrate) and can be easily determined
experimentally. Such
fluid design ensures that excessive CO2 gas, or conversely CO32- ion
generation does
-64-
CA 02863264 2014-09-12
not occur in the bicarbonate/phosphate combined concentrate. The concentrate
will
be provided so that the 1X bicarbonate can vary between 20 to 40 mmol/L
depending on the dilution. The phosphate will be 1.25 mmol when the
bicarbonate
is 30 and will vary from 0.8 to about 1.7 mmol/L with the dilution of the
concentrate.
Base concentrate with phosphate:
Base concentrate with
phosphate contribution after 1X base fluid 1Xbase fluid
mixing with the acid component component
concentrate and water to lx mmol/L ME:a
Sodium 32.44 32.44
HCO3- 30 30
H2PO4(-):HPO4(2-) in 1:20
ratio 1.25 *2.44
Base concentrate without phosphate:
Base concentrate after 1X base fluid 1Xbase fluid
mixing with the acid component component
concentrate and water to 1X mmol/L mEq/L
Sodium 30 30
11CO3- 30 30
Acid concentrates with phosphate dedicated to the various operational modes:
The most important novel features are the low sodium, calcium and
magnesium and the added citrate and phosphate content (where applicable).
These
fluids also assume the use of the sodium chloride supplemented citrate
anticoagulant
solutions. About 25% +/- range is also contemplated for all of these novel
component concentrations. The 1X sodium concentration is approximate and will
be
clinically variable as allowed by the sodium-modeling program (standard
feature of
modern dialysis machines) to suit the individual patient and the selected
treatment
modality. The final lx therapy fluids could also be theoretically provided as
bagged
sterile fluids and the compositions for such use are also contemplated herein.
-65-
CA 02863264 2014-09-12
Acid concentrate with phosphate dedicated to simultaneous pre-and post-
dilution
C'VVH:
Replacement fluid acid
concentrate with phosphate
components after mixing 1X final fluid 1X acid fluid
with the base concentrate composition; component;
and water to lx mEq/L
Sodium 136 106
Potassium 4.0 4.0
Chloride 110 110
Bicarbonate 30 0
Calcium 0 0
Magnesium 0 0
Phosphoric acid 1.25 1.25
Dextrose 5.5 5.5
Acid concentrate with phosphate dedicated to 12-24-hour SLEDD: (only if near
complete removal of calcium and citrate from the circuit blood is found to be
clinically detrimental)
Dialysis fluid acid
concentrate with phosphate
components after mixing IX final fluid IX acid fluid
with the base concentrate composition; component;
and water to lx inmol/L
Sodium 139 111
Potassium 4.0 4.0
Acid and basic citrate 1:2 0.9 2.7
Chloride 114.1 114.1
Bicarbonate 28 0
Calcium 0.3 0.6
Magnesium 0.15 0.3
Phosphoric acid 1.25 1.25
Dextrose 5.5 5.5
The calcium can range from 0.0 mM to 0.8 mM and magnesium from
0.0 mM to 0.4 mM (magnesium is about 40-50% of calcium usually). Acid citrate
can vary from 0.0 mM to 1.5 mM and total citrate from 0.5 to 3.0 mM. All such
variations of the above fluid are fully contemplated according to the present
invention. All other ion concentrations can change by about +40% and all such
-66-
CA 02863264 2014-09-12
variations are also contemplated herein.
Single, compromise acid concentrate with phosphate for all online CRRT:
Therapy fluid acid
concentrate with phosphate
components after mixing 1X final fluid 1X acid fluid
with the base concentrate composition; component;
and water to 1X mmol/L MEcVL
Sodium 138 108
Potassium 4.0 4.0
Chloride 114 114
Bicarbonate 28 0
Calcium 0 0
Magnesium 0 0
Phosphoric acid 1.25 1.25
Dextrose 5.5 5.5
The calcium can range from 0.0 mM to 0.8 mM and magnesium from
0.0 mM to 0.4 mM (magnesium is about 40-50% of calcium usually). Acid citrate
can vary from 0.0 mM to 1.5 mM and total citrate from 0.5 to 3.0 mM. All such
variations of the above fluid are fully contemplated according to the present
invention. All other ion concentrations can change by about +-10% and all such
variations are also contemplated herein.
Single, compromise acid concentrate without phosphate for all outpatient
intensive
blood purification therapies including pre-and post-dilution HVHF and regular
HD
and post dilution HDF:
Dialysis fluid acid
concentrate components lx final fluid
after mixing with the base composition; lx acid fluid
concentrate and water to lx mmol/L component; mEq/L
Sodium 136 99
Potassium 2.0 or 3.0 or 4.0 2.0 or 3.0 or 4.0
Acetic acid 3.0 3.0
Chloride 101 or 102 or 103 101 or 102 or 102
Bicarbonate 37 0
Calcium 0.0 0.0
Magnesium 0.0 0.0
-67-
CA 02863264 2014-09-12
Dextrose 5.5 5.5
The greatest concern with high blood flows is systemic citrate
accumulation. Therefore, there is no citrate in the above fluids and acetate
is used
for acidification (to prevent bacterial growth). The acetate content is
comparable to
standard acid concentrates in clinical use but could be reduced markedly at lx
dilution if desired for a nearly acetate free therapy fluid and such
alterations are fully
contemplated according to the present invention. At blood flows above 300 ml,
current filter technology will limit the plasma citrate and calcium extraction
to 60-80
% in a single pass. In alternative embodiments, the calcium can range from 0.0
mM
to 1.0 mM and magnesium from 0.0 mM to 0.5 mM (magnesium is about 40-50%
of calcium usually). Acid citrate can vary from 0.0 mM to 1.5 mM and total
citrate
from 0.5 to 3.0 mM. All such variations of the above fluid are contemplated
herein.
All other ion concentrations can change by about +-10% and all such variations
are
also fully contemplated. Finally, potassium (K) concentration can be 2, 3, or
4 mM
in any of the above 1X therapy fluids.
Specifically, the sodium at the standard 34X dilution may be targeted
to about 130 mM by providing the low or zero calcium and magnesium acid
concentrates with about 3-5% less electrolyte content (with preserving the
above
molar ratios) for safety monitoring purposes (particularly when hypertonic
sodium
is present in the modified citrate anticoagulant) and this method is provided
according
to the present invention. The final conductivity of the dialysate at usual
dilution
ratios then would be about 12.6, about 10 % less than the usual 14.0 due to
the lower
sodium and absent calcium and magnesium, allowing the machine to detect
through
fresh dialysate conductivity monitoring (done routinely on all dialysis
machines) that
a calcium and magnesium free acid concentrate is being used. When the operator
confirms the use of the special acid concentrate for RCA, the acid concentrate
dilution ratio could be automatically adjusted to yield a final fluid with
about 134-138
mM sodium as required by the treatment prescription. The only drawback to this
method is that high sodium profiling may be mildly limited with the use of
such acid
concentrates.
-68-
CA 02863264 2014-09-12
The 1X fluid compositions are provided above. These values may still
be slightly modified based on clinical experience. The machine will vary the
dilution
of the concentrates depending on the treatment prescription to best suit the
individual
patient. This will result in a range of concentrations of the electrolytes in
the final
ready to use online generated fluid.
When needed, the phosphate can be provided in the acid concentrate
instead as well in an acid form to hinder bacterial growth. Phosphate may be
omitted
from the acid and base concentrates specifically designed for short, intense,
3-6-hour,
3-times-per-week therapy. When phosphoric acid is not used, acidity of the
acid
concentrate is ensured by the inclusion of citric acid or acetic acid. In the
absence
of calcium and magnesium, salt fouling of the fluid circuits is very unlikely
and
acidification mainly serves to prevent bacterial growth in the acid
concentrate. The
citrate and sodium content is correlated with the operational mode and the
expected
composition and rate of infusion of the anticoagulant solution. The lower
sodium,
calcium and magnesium content results in lower conductivity at standard
dilution
ratios, allowing the machine to detect the presence of the unusual acid
concentrate
for RCA, an important safety feature.
When a predominantly diffusive mode of blood purification is
employed during CRRT, (pre-dilution HDF or SLED), calcium and magnesium may
have to be present in the fresh therapy fluid (albeit at reduced
concentrations), to
avoid the complete decalcification of the blood that might have untoward
physiologic
consequences (this possible untoward effect is speculative as no clinical
protocols to
date have achieved such high fractional citrate and calcium extraction in the
extracorporeal circuit and is in fact not expected to occur).
Concentrations shown are the contributions to the final 1X combined
concentrate from Part 1 (Acid) and Part 2 (Base). Depending on the relative
flow of
fluids from the concentrate Part 1 and Part 2 (machine and online fluid
generation
system design dependent), the exact composition design of the Part 1 and Part
2
concentrates can naturally be defined exactly to yield the desired final
diluted
summary lx product. Such calculations and final concentrate compositions are
-69-
CA 02863264 2014-09-12
apparent to those skilled in the art from the usual practice of online fluid
generation
and from the target concentration ranges to be reached in the final 1X fluid
as
described above, and are contemplated according to the present invention.
The physical design of RCA system 110 and the fluid compositions
(anticoagulant, calcium plus magnesium infusion, separate acid and base
concentrates) according to the present invention allow for the independent and
flexible selection of anticoagulation intensity (the amount of citrate infused
into a liter
of plasma), calcium and magnesium infusion rate, therapy fluid sodium and
potassium concentration and therapy fluid bicarbonate concentration. Detailed
knowledge of the movement of the key small solutes in the patient's body and
in the
extracorporeal circuit during RCA allows automatic, precise mass balance
calculations for all solutes during the use of any treatment operational mode.
This
permits the selection of fluid flow rates and therapy fluid composition best
suited for
the individual prescription. The solute fluxes may be inferred from the
prescription
and fluid compositions as well as verified/adjusted based on the online
clearance
measurements.
Online hematocrit sensor 150 and OCM 170 provide for continuous
safety monitoring of the performance of system 110. The OCM 170 allows for
mathematical precision in clearance dosing, in calcium dosing, in predicting
citrate
accumulation and in calculating the diffusive versus convective component of
the
blood purification important for medication dosing and research purposes. The
hematocrit sensor 150 may also detect access recirculation. Finally,
subsequent
measurement of online clearance with the anticoagulant infusion bolus based
method
and the traditional dialysate conductivity modeling based method, when
correlated
with the measured access recirculation, may allow the online monitoring of the
patients cardiac output with clinically useful accuracy when a permanent
(arterial)
access is used.
The software control module according to the present invention may
include elements to verify proper circuit tubing connections and may guide the
selection of safe citrate prescriptions by the operator. As a safety measure,
-70-
CA 02863264 2014-09-12
prescriptions that entail the possibility of citrate accumulation or other
complications
may not be allowed. This is described in detail below in the flow steps for
RCA
system 110.
Operational modes that may be supported include: 1) Purely
convective RRT with simultaneous pre-dilution and post-dilution hemofiltration
for
both 24-hour CVVH and intensive 4-5 hour HVHF therapy (FIGS. 8a and 8b); 2)
Purely diffusive RRT with only net ultrafiltration for both 24-hour SLED and
conventional 4-5 hour 1HD (FIGS. 5a and 5b); 3) Post-dilution hemofiltration
(online
post-HDF) for outpatient 4-5 hour therapy with high blood flows and a desire
to
maximize clearance and control cost (FIGS. 7a and 7b); 4) Pre-dilution
hemofiltration (online pre-HDF or CVVHDF) for 24-hour CRRT with a desire to
deliver both convective and diffusive clearance and minimize clotting (FIGS.
6a and
6b); 5) Optional single needle operational mode for all extended therapy (CRRT
or
nocturnal therapy) modalities. The greatest benefit of this mode is that it
ensures that
blood withdrawal from the patient is immediately halted if an access needle
disconnection occurs. In contrast, when two needles are used, in the case of a
venous access disconnection, there is a potential for a catastrophic bleed as
the
machine may keep aspirating blood through the arterial needle.
For each of these modalities with appropriate prescriptions, the plasma
small solute clearance can be calculated and verified periodically with a
novel online
conductivity dialysance method according to the present invention. Assuming
access
recirculation is monitored and measured by the hematocrit sensors 150, 152,
the
whole blood clearance for solutes like urea can also be inferred from the
data. This
will provide the clinician with unprecedented flexibility and precision in the
selection
of the small solute hourly clearance goal as well as the degree of convective
versus
diffusive blood purification. Control programs deriving the prescriptions for
each
operational mode are developed allowing for complete automation of the
prescription
writing. The total therapy fluid flow will usually not exceed 250% of the
total plasma
flow or about 160% of the total blood flow regardless of the purification
method
used. Such fluid efficiency is fully comparable with what is achieved with
current
traditional clinical dialysis prescriptions.
-71-
CA 02863264 2014-09-12
Fundamentals of the RCA prescription according to the present
invention are as follows. Sufficient plasma total calcium to citrate ratio
must be
achieved for effective anticoagulation. The total Ca (mM) to citrate (mM)
ratio may
range between 2 to 4 in the extracorporeal circuit. Part of the citrate may be
provided as acid citrate in the anticoagulant infusion (to further enhance
anti-
coagulation through acidification of thrombin and other coagulation cascade
proteins
and increase the ultrafilterable fraction of calcium by disrupting its binding
to
albumin). The plasma flow may be monitored online with a hematocrit and blood
flow sensor module 150, 152. This will allow the calculation of the delivered
calcium load into the circuit and will define the necessary anticoagulant
infusion rate.
Access recirculation may also be monitored by the hematocrit sensor 150, 152.
The prescription should eliminate the possibility of citrate
accumulation even in the complete absence of liver metabolism (liver failure).
This
may be achieved by keeping the citrate plasma dialysance above 60-80% of the
plasma flow in the extracorporeal circuit and correlating it with the amount
of citrate
infused into a liter of circuit plasma and the citrate concentration in the
therapy fluid
used. The target plasma total calcium level should be defined (usually 2-2.5
mmol/L
depending on the serum albumin concentration) by the operator. This will have
an
indirect impact on the systemic plasma ionized Ca content in steady state. The
ultrafilterable and dialyzable fraction of total calcium should be selected
(this will
range from 0.7 to .95 depending of the calcium to citrate ratio, albumin level
and pH
in the circuit). The plasma albumin level may be further considered as it will
impact
the systemic ionized Ca level at the targeted total systemic plasma Ca level.
The
systemic citrate level will have minimal impact, even in ICU patients with
liver
failure, because citrate accumulation beyond 2 to 3 mM levels cannot occur
when
filter performance is maintained at the specified fluid flow rates.
Prescriptions and
therapy fluid compositions may be provided that allow exact mass balance
calculations for citrate, calcium and magnesium, sodium and bicarbonate (and
trace
metal minerals).
In the following description, a glossary of the abbreviations used is as
follows:
-72-
CA 02863264 2014-09-12
Csys: calculated steady state systemic plasma citrate concentration in a
patient with
zero citrate metabolism (liver failure; worst case scenario in RCA)
E: apparent circuit post-anticoagulant infusion arterial plasma citrate to
therapy fluid
citrate concentration difference reduction ratio during a single filter pass
("plasma
citrate extraction ratio"); (ECit, ECa)
DCit: apparent citrate plasma dialysance (DCit* when expressed for the
adjusted
QBCit during calculations and DCit when expressed for the unadjusted QP)
DCond: apparent "summary conductivity solute" whole blood dialysance. This
value
may be predicted from filter KoACond, Qb, Qd, and Quf and/or determined by the
sodium citrate bolus based measurement or by the traditional online
conductivity
dialysance measurement method (for high blood flow treatment sessions; this
latter
method is not discussed here being prior art and not applicable in SLED)
QB: the effective arterial blood water flow for the solute analyzed; QBCond is
closely equal to the arterial whole blood water flow for conductivity and
QBCit is
closely equal to arterial blood plasma water flow for citrate. In the case of
citrate, for
the calculation of "E" the plasma water volume is adjusted for the free water
shifts
between the RBCs and the plasma space in response to the hypertonic citrate
anticoagulant and DCit* is calculated with these adjustments. Once E =
DCit*/QBCit (= DCit/QP) is derived, the unadjusted QP and DCit can be used to
simplify the subsequent calculation of Csys.
QP: The arterial blood plasma flow without adjustment for the effects of the
hypertonic anticoagulant infusion (These shifts are accounted for during the
calculation of E).
Cinf: The increase in the arterial plasma citrate concentration as a result of
the
anticoagulant infusion, before any pre-filter replacement fluid infusion or
adjustment
for water shifts between blood fluid compartments. (These shifts are accounted
for
during the calculation of E).
Hgb: hemoglobin concentration in the arterial blood
Qpre: pre-filter replacement fluid flow rate
Qpost: post filter replacement fluid flow rate
Qd: dialysis fluid flow rate
Quf: net ultrafiltration (negative fluid balance goal plus the citrate and Ca
infusion
rates)
-73-
CA 02863264 2014-09-12
QCa/Mg: the flow rate of the calcium and magnesium infusion
Qtf: total therapy fluid flow rate ( = Qd in SLED)
DCond: "conductivity solute" dialysance determined by the sodium citrate bolus
based measurement
DCit: the calculated citrate dialysance (DCit* when expressed for the adjusted
QBCit
during calculations and DCit when expressed for the unadjusted QP)
Ddiff : the calculated diffusive component of the measured total dialysance
(DdiffCond, DdiffCit); in SLED the diffusive dialysance is equal to the total
dialy sauce
KoA: mass transfer area coefficient; measure of filter performance specific to
solute
(KoACond, KoACit)
a and S: solute diffusivity and sieving coefficients; aCond; aCit, SCond;
SCit,
f: correction factor to derive the dialyzable/filterable fraction of the total
plasma Ca
For the control program for RCA system 110, the flow steps may
include:
1) Start Machine in RCA Mode
2) a) Select Treatment Type: Sustained Low-Efficiency Dialysis (SLED),
Hemodiafiltration (pre-HDF or post-HDF), or Pure hemofiltration (pre-CVVH or
Pre +post-CVVH).
b) Select Treatment Duration: 10-hour or 24-hour.
c) Select Access Connection: Regular versus Single-Needle.
3) a) Machine advises filter, tubing, anticoagulant and calcium
solutions and
RCA acid concentrate.
b) Confirm all disposables are as advised by the machine.
c) Connect tubing to dialyzer.
d) Connect infusion pumps.
e) Prime system with priming solution.
f) Test system integrity (current machine protocol).
-74-
CA 02863264 2014-09-12
4) RCA priming checks: performed with the circuit arterial and venous ends
connected in recirculation mode.
a) Confirm conductivity of therapy fluid is at target with RCA Mode specific
lesser dilution of the acid concentrate.
b) Alarm if conductivity is abnormal: inappropriate acid concentrate for
RCA treatment.
c) Confirm citrate infusion loading onto the citrate pump by turning on the
citrate pump and measuring the increase in conductivity in the drain circuit
of the
dialyzer.
d) Alarm: it is not the citrate infusion solution that is loaded onto the
citrate
pump based on effluent conductivity changes.
e) Confirm calcium infusion loading onto the calcium pump by turning on the
calcium pump and measuring the increase in conductivity in the drain circuit
of the
dialyzer.
f) Alarm: it is not the calcium infusion solution that is loaded onto the Ca2+-
pump based on effluent conductivity changes.
5) Input Patient Information.
a) Sex, height, age, weight (minimum data is weight) (if Watson volume and
VE calculations are desired).
b) Minimum data is systemic hemoglobin and albumin concentration.
6) Select SLED, HDF, or HF
For SLED:
7) Treatment information advised by software based on prior selections.
a) Confirm: Filter type (determines expected KoACond, KoACit, SCond,
b) Input: Maximal access blood flow rate expected (QB).
c) Confirm: Dialysate fluid flow rate (HD and HDF 200% QB; CVVH
200% QP).
d) Input: Total net ultrafiltration desired per treatment (during 10 or 24
hours).
-75-
CA 02863264 2014-09-12
e) Confirm: Set dialysis machine alarm parameters.
Confirm: Type of citrate anticoagulant solution (ICU versus OPD; likely
uniform).
g) Confirm: Type of calcium solution (ICU versus OPD, likely uniform).
h) Confirm: Maximum citrate level in systemic blood allowed (2.0 - 4.0
mM).
i) Confirm: Dialysis acid and base concentrates used.
8) Connect the patient
9) Safety checks after initial patient connection in isolated HD mode.
a) Start treatment, confirm citrate infusing in the arterial limb by watching
the effluent conductivity.
b) Measure access recirculation with automated online hemodilution or
temperature technique.
c) Measure baseline in vivo KoACond at QB 150-300 and QD 300-600
(ml/min) in 12-hour SLED.
d) Compare with expected value for selected specific filter; alert operator if
significant difference.
e) Calculate baseline in vivo KoACit from the above measurement
1) Measure baseline in vivo KoACond at QB (priming solution) 75-150 and
QD 150-300 (ml/min) in 24-hour SLED.
g) Compare with expected value for selected specific filter; alert operator if
significant difference.
h) Calculate baseline in vivo KoACit from the above measurement (in the 12-
hour mode both dialysate bolus based and blood bolus based DCond will be
measured).
10) Display Confirmation Alarms.
a) Alarm if more than 10-15% recirculation is detected; the treatment will
still be safe, but less effective for uremic clearance.
b) Measure Hgb concentration with the online sensor (Alarm if more than
20% different from initially provided value).
-76-
CA 02863264 2014-09-12
c) Alarm if citrate not on arterial limb of circuit (confirm during bolus).
d) Alarm if filter Dcond more than 10-20% different from expected in vivo
value and possibly refuse the filter.
e) Alarm if the expected and the detected replacement fluid conductivity
values at the RCA Mode dilution of the hyponatric RCA acid concentrate do not
match.
11) Analyze input data.
a) Determine prescription and machine settings with in vivo DCond.
b) Display machine generated QB, Cinf, Qd Quf, QCitl, QCa/Mg.
c) Display expected DCond (ml/min)(if using weight-adjusted prescribing).
d) Display expected maximum Csys.
e) Display expected Ca replacement infusion dose (mmol/hour) for circuit
Ca losses (prescriptions can have uniform QB and DCond versus weight
adjusted).
FOR HDF:
7) Treatment Information advised by software based on prior selections.
a) Input: Dialyzer type (determines expected KoACond, KoACit, SCond,
SCit).
b) Input: Maximum hemoconcentration allowed in the circuit (define in the
range 50-60%).
c) Input: Therapy fluid summary flow rate (200% of QB).
d) Input: Total clearance goal for CRRT (DCond based Kt/V or just Kt).
d) Input: Total net ultrafiltration desired per treatment (or over 24 hours).
0 Input: Set dialysis machine alarm parameters.
g) Input: Type of citrate solution (ICU versus OPD; likely uniform).
h) Input: Type of calcium solution (ICU versus OPD, likely uniform).
i) Input: Maximum citrate level in systemic blood allowed (2.0 - 4.0 mM).
j) Input: Dialysis acid and base concentrates used.
8) Connect the patient
9) Safety checks after initial patient connection in isolated HD mode.
-77-
CA 02863264 2014-09-12
a) Start treatment, confirm citrate infusing in the arterial limb by watching
the effluent conductivity.
b) Measure access recirculation with online hemodilution technique.
c) Measure baseline in vivo KoACond at QB 150-300 and QD 300-600
(ml/min) in 12-hour SLED.
d) Compare with expected value for selected specific filter; alert operator if
significant difference.
e) Calculate baseline in vivo KoACit from the above measurement.
0 Measure baseline in vivo KoACond at QB (priming solution) 75-150 and
QD 150-300 (ml/min) in 24-hour SLED.
g) Compare with expected value for selected specific filter; alert operator if
significant difference.
h) Calculate baseline in vivo KoACit from the above measurement (in the 12-
hour mode both dialysate bolus based and blood bolus based DCond will be
measured).
10) Display Confirmation Alarms.
a) Alarm if more than 10-15% recirculation is detected; the treatment will
still be safe, but less effective for uremic clearance.
b) Measure Hgb concentration with the online sensor (Alarm if more than
20% different from initially provided value).
c) Alarm if citrate not on arterial limb of circuit (confirm during bolus).
d) Alarm if filter Dcond more than 10-20 % different from expected in vivo
value (and possibly refuse the filter).
e) Alarm if the expected and the detected replacement fluid conductivity
values at the RCA Mode dilution of the hyponatric RCA acid concentrate do not
match.
11) Analyze input data and change to HDF operational mode.
a) Determine post-dilution possible as % of QB with set hemoconcentration
limit.
b) If CRRT, always use pre-HDF, Qpre 30% of QB and the rest of the
therapy fluid as QD.
-78-
CA 02863264 2014-09-12
c) If short therapy, use post-HDF with Qpost 20% of QB if
hemoconcentration limit allows.
d) Otherwise, use pre-HDF for short therapy as well with Qpre 30% of QB.
e) Determine prescription and machine settings based on treatment goals,
patient data and the blood bolus based DCond and if available the dialysate
bolus
based DCond values.
f) Display QB, Cinf, Qpre (pre-HDF) or Qpost (post-HDF), Quf, QCitl ,
QCa/Mg.
g) Display expected total DCond (ml/min).
h) Display expected maximum Csys.
i) Display expected circuit Ca loss (mmol/hour) before replacement infusion
(prescriptions can have uniform QB and DCond versus weight adjusted).
FOR HF:
7) Treatment Information advised by software based on prior selections.
a) Input: Dialyzer type (determines expected KoACond, KoACit, SCond,
SCit).
b) Input: Maximum hemoconcentration allowed in the circuit (define in the
range 50-60%).
c) Input: Therapy fluid sununary flow rate (150% of QB).
d) Input: Total clearance goal for CVVH (DCond based Kt/V or just Kt).
e) Input: Total net ultraffltration desired per treatment (or over 24 hours).
f) Input: Set dialysis machine alarm parameters.
g) Input: Type of citrate solution (ICU versus OPD; likely uniform).
13.) Input: Type of calcium solution (ICU versus OPD, likely uniform).
i) Input: Maximum citrate level in systemic blood allowed (2.0 - 3.0 mM).
j) Input: Dialysis acid and base concentrates used.
8) Connect the patient
9) Safety checks after initial patient connection in isolated HD mode.
a) Start treatment, confirm citrate infusing in the arterial limb by watching
the effluent conductivity.
-79-
CA 02863264 2014-09-12
b) Measure access recirculation with online hemodilution technique.
c) Measure baseline in vivo KoACond at QB 150-300 and QD 300-600
=
(ml/min) in 12-hour SLED.
d) Compare with expected value for selected specific filter; alert operator if
significant difference.
e) Calculate baseline in vivo KoACit from the above measurement.
0 Measure baseline in vivo KoACond at QB (priming solution) 75-150 and
QD 150-300 (ml/min) in 24-hour SLED.
g) Compare with expected value for selected specific filter; alert operator if
significant difference.
h) Calculate baseline in vivo KoACit from the above measurement (in the 12-
hour mode both dialysate bolus based and blood bolus based DCond will be
measured).
10) Display Confirmation Alarms.
a) Alarm if more than 10-15% recirculation is detected; the treatment will
still be safe, but less effective for uremic clearance.
b) Measure Hgb concentration with the online sensor (Alarm if more than
20% different from initially provided value).
c) Alarm if citrate not on arterial limb of circuit (confirm during bolus).
d) Alarm if filter Dcond more than 10-20 % different from expected in vivo
value (and possibly refuse the filter).
e) Alarm if the expected and the detected replacement fluid conductivity
values at the RCA Mode dilution of the hyponatric RCA acid concentrate do not
match.
a) Determine post-dilution possible as % of QB with set hemoconcentration
limit.
b) Determine prescription and machine settings based on treatment goals,
patient data and the blood bolus based DCond and if available the dialysate
bolus
c) Program Qpost for the above maximum post-filtration, minus
-80-
CA 02863264 2014-09-12
(Qcitl +QCa/Mg +Quf) for maximum citrate clearance with a given QI3 and total
Qtf. The Qpre is Qtf (150 % of QB) - Qpost.
d) Determine prescription and machine settings.
e) Display QB, Cinf, Qpre and Qpost, Quf, Qcitl, QCa/Mg.
f) Display expected DCond (ml/min) and expected maximum Csys.
g) Display expected circuit Ca loss (mmol/hour) before replacement infusion
(prescriptions can have uniform QB and DCond versus weight adjusted).
For ALL OPERATIONAL MODES:
12) Calcium Dosing.
a) DCit is essentially equal to DCa * f correction for dialyzable fraction
(0.95
to 0.8 depending on albumin level and Cinf).
b) Target systemic plasma total Ca (mM) is defined: Use Csys (0.25 mM
Ca/lmM citrate), systemic albumin (0.2 mM Ca/1 g/dL) and target systemic
ionized
Ca (target Cai = 1.00 mM when systemic citrate is assumed to be equal to Csys
=
3).
c) Circuit Ca loss in steady state is equal to DCa * (Target systemic total Ca
¨ Cad), where Catf is the calcium concentration in the fresh therapy fluid
(mM).
d) QCa/Mg is easily calculated from the circuit Ca loss and Ca concentration
of the Ca infusion solution.
e) At start, the operator may have to give 1-4 amps of Ca-gluconate over 1-2
hours to bring the systemic ionized Ca close to 1.25-1.5.
13) Continuous safety check.
a) Citrate solution is properly on the citrate pump and arterial limb is
arterial
(expected constant step-up in effluent conductivity from baseline Ctf
conductivity)
(Alarm if citrate bag changed to calcium or saline or access connection
reversed
during operation based on effluent conductivity monitoring with all the above
IV
fluids having different conductivity).
b) Input Ctf constant in RCA Mode when proper, unique RCA acid and
standard base concentrates are used (Alarm if non-RCA acid concentrate is
being
supplied at any time).
c) Input: Online measured total Dcond from standard operation and estimated
-81-
CA 02863264 2014-09-12
Cp (Alarm if filter performance is declining to prompt bolus clearance
interrogation
and/or filter change).
d) Input: Measured access blood flow rate: current (QB) (Alarm: when QB
is changed because of access issues recalculate all pump speeds and fluid
flows).
e) Input: Measured hemoglobin concentration (Alarm: when changed by
more than 10% alert operator to possible bleeding or over-ultrafiltration;
recalculate
prescription, recommend to operator CBC check, net ultrafiltration target
revision).
14) Hourly safety check: input data.
a) Input: online measured total Dcond (blood bolus based and when possible
dialysate bolus based methods both.
b) Input: Measured circuit blood flow rate: current (QB).
C) Input: Set therapy fluid flow rate (usually 150-200 % of QB).
d) Input: Measured hemoglobin concentration.
e) Input: Set total net ultrafiltration.
15) Recalculation of the prescription.
a) Calculate; DdiffCond, KoACond, KoACit, DdiffCit, Total DCit.
b) Calculate the maximum possible citrate in systemic blood (Csys; 2.0 - 4.0
mM).
c) Alarm if Csys more than 3 mM and address as follows: Change filter if
d) Display current clearance after all changes: DCond (in nil/min).
e) Adjust QB, QD, Qcit, QCa/Mg, NetUf, and Qpre and or Qpost as
applicable.
16) Other alarms.
a) Citrate bag is about to run out: (if the machine measures bag weight or
knows bag volume and logs new bag setups).
b) Calcium bag is about to run out: (if the machine measures bag weight or
-82-
CA 02863264 2014-09-12
c) RCA acid concentrate is about to run out: (if the machine measures the
acid concentrate reservoir weight).
d) The treatment goal (total time or total clearance or total net UF has been
reached): in 12-hour treatments.
RCA system 110 may contain an online sensor system (OSS) for
measuring calcium, magnesium and citrate in the ultrafiltrate. The same flow
steps
detailed above apply to such a system, except that data from the OSS may be
used to
adjust the calcium infusion according to systemic citrate and calcium levels.
As
explained herein, the calcium, magnesium and citrate values measured from the
ultraflltrate by the OSS can be used to back-calculate the values in the
patient's
plasma. As also explained, the kinetic curve of systemic plasma citrate
concentration
can be used to derive the exact value of the liver clearance of citrate as
well as the
volume of distribution of citrate, VE. Using the above parameters, systemic
citrate
levels can be accurately predicted at any future T time point. The calcium and
citrate
pump as well as the entire prescription including the therapy fluid
bicarbonate
concentration (when flexible) can then be completely controlled by the machine
software.
Filter performance can be monitored both by conductivity as well as
citrate clearance measurements. The direct citrate clearance measurements
again
enable complete precision in calcium and citrate dosing. Since calcium exits
through
the hemofilter almost entirely as Ca-citrate complex, the measured citrate
dialysance
will be nearly equal to the total calcium dialysance. The slightly lower Ca-
dialysance
will be due to the Gibbs-Donnan effect and the minimal albumin-bound Ca in the
circuit (about 5-20% depending on the amount of citrate infused in the
arterial limb
of the circuit, the acidity of the citrate infusion and the plasma albumin
level). At
any point where blood bolus based conductivity dialysance is measured, blood
bolus
based citrate dialysance will also be measured simultaneously with the OSS
when
available on the machine.
Turning now to another aspect of the present invention, home
nocturnal dialysis is a re-discovered, expanding method of RRT. Most experts
-83-
CA 02863264 2014-09-12
believe that it is the best method of RRT, resulting in excellent uremic and
blood
pressure control, freedom from most dietary restrictions otherwise mandatory
for
ESRD patients on 3 times-per-week dialysis, and resulting in fewer
hospitalizations,
lesser use of phosphate binders and most importantly better quality of life.
Nevertheless, only a minute fraction of ESRD patients are currently on
nocturnal
dialysis.
The most important reasons for the limited use of nocturnal dialysis
include the following. Highly effective anticoagulation is mandatory during 8-
12-
hour treatments to prevent clotting of the extracorporeal circuit and
associated alarms
and sleep disruption. The only agent in common use, heparin, has significant
side
effects and a systemic bleeding risk that increases with higher doses. In
addition,
single needle operational mode is preferred to lessen the risk of major
bleeding in the
event of permanent access disconnection. This again
requires powerful
anticoagulation. Complex online dialysis fluid generation systems are
expensive to
deploy and maintain in the home, and online clearance measurements that could
be
used to monitor efficacy and compliance have not been widely adapted to slow
nocturnal dialysis. Furthermore, RCA has not been developed for home
treatments.
Still further, biofilm formation and bacterial contamination of components of
the
dialysis system is a major concern, and costs must not exceed markedly the
overall
costs of 3 times weekly in-center dialysis.
According to the present invention, an RCA home system 210 (FIGS.
17a-17d) may be designed as an RRT device that also doubles to deliver
automated
RCA for home nocturnal dialysis. One purpose of the present invention is to
provide
a device that can deliver previously unprecedented high convective or
diffusive
clearances and can be operated by laypersons in home settings without the need
for
highly complex treatment protocols. RCA home system 210 is a modified version
of the RCA system 110 that is specifically re-designed for the unique
challenges of
home RRT. Therefore, components of system 210 that are similar to components
of
system 110 are identified with like reference numerals except for the
substitution of
a "2" prefix.
-84-
CA 02863264 2014-09-12
RCA home system 210 according to the present invention may include
a combination of various CRRT and dialysis machine hardware components
arranged
in a unique design, two special modes of operation of the device (simultaneous
pre-
and post-dilution hemofiltration and continuous sustained low efficiency
dialysis (c-
SLED)), and a software control module. System 210 may also include a sensor
module 256 to measure citrate, calcium and magnesium levels online to ensure
the
maximum accuracy, fluid efficiency and safety of treatment prescriptions.
System
210 may use a novel replacement fluid concentrate, a novel citrate
anticoagulant, and
a novel single premixed calcium plus magnesium infusion which were designed to
fully exploit the system's capabilities. RCA home system 210 may resemble a
traditional hemodialysis machine and can be constructed from hemodialysis
machine
components except for online citrate sensor 256 as described below. Most
elements
have been discussed above with reference to the RCA system 110 of the present
invention.
RCA home system 210 can safely provide at least up to 12 liters per
hour of convective clearance to patients without relying on the liver to
metabolize
citrate. The system design prevents citrate accumulation in the patient, while
maintaining highly efficient anticoagulation of the extracorporeal circuit
212. A
control program may be used to derive a safe treatment prescription according
to
treatment goals selected by the operator. An online citrate sensor 256 may be
used
to eliminate the risk of citrate accumulation (that may occur only with
declining filter
performance in SLED mode) and doubles as an online delivered clearance and
liver
metabolic function monitor.
System 210 according to the present invention is shown in FIGS. 17a
and 17b for a machine capable of pre- and post-dilution CVVH for maximal fluid
efficiency and in FIGS. 17c and 17d for an even simpler machine that performs
only
pre-dilution CVVH or SLED depending on the tubing connection. The common
features with RCA system 110 are either not repeated or repeated only briefly
herein.
The most important differences and novel elements are detailed below.
-85-
CA 02863264 2014-09-12
RCA home system 210 may include a single, sterile bag 280 (e.g., 5
liter plastic) that may contain a novel, single component, about 30-50X
electrolyte
concentrate. The hemofiltration replacement fluid may be diluted from this
concentrate by mixing it with ultrapure water generated by a water treatment
module
of the RCA online system 210. The online fluid generation follows well-
established
design from currently existing dialysis machines. However, instead of the
traditional
two bags, system 210 according to the present invention requires only one
concentrate chamber or bag 280 that contains all electrolytes necessary except
calcium and magnesium. This is a major departure from current fluid mixing
systems. The single concentrate reduces complexity of the fluid circuit and
makes
the dilution procedure very precise and safe with conductivity monitoring of
the
ready-to-use replacement fluid as the established safety check for the degree
of
dilution. Since day-to-day flexibility in therapy fluid sodium and bicarbonate
= concentration is not needed in home nocturnal dialysis programs, this
simplicity of
fluid generation has no significant clinical drawbacks. Individual
prescriptions can
still be attained if the manufacturer provides several individual single-
component
concentrates with moderately variable final potassium, bicarbonate, and
possibly
phosphate contents. The appropriate concentrate can be selected for the
patient about
once monthly, similar to the selection of peritoneal dialysis fluid
composition and
prescription for patients on peritoneal dialysis.
=
RCA home system 210 may include two highly precise volumetric
infusion pumps 234, 244 which may have dedicated air in line detectors and
line
clamps (not shown), optionally color-coded and with special tubing to deliver
the
citrate anticoagulant and the calcium plus magnesium infusions as described
below
with reference to FIGS. 9-16. Infusion lines 228, 242 may have special end
connections that will only attach to the appropriate solution bags 232, 246
and at the
other end will be welded to the entry points in extracorporeal circuit 212 to
minimi7e
the risk of disconnection from the circuit 212 and wrong connection of
infusate bags
232, 246. Pumps 234, 244 may be designed to accept only the right type of
infusate
tubing and may be fully coordinated with the operation of blood pump 222 and
other
fluid pumps. This again prevents accidental connection of the wrong infusate
in the
wrong place and also ensures that citrate and calcium plus magnesium infusions
are
-86-
CA 02863264 2014-09-12
stopped when the machine blood pump 222 and/or replacement fluid pump 264 are
not operating. In addition, the fluid bags 232, 246 may be manufactured to be
significantly different in weight and size as well as in the color of the
plastic and/or
legend to further reduce the chance of accidental wrong connection.
RCA home system 210 may utilize Doppler-based fluid flow and
hematocrit monitors or alternatively optical hematocrit sensors 250, 252 on
the
arterial and venous blood lines 214, 218 as well as possibly on the
replacement fluid
line 228 and effluent fluid line 224 for maximal precision in ensuring that
the set
blood flow rate on blood pump 222 matches the actual blood flow delivered by
the
action of the blood pump 222 and all other fluid flows (pre-filter fluid flow,
effluent
flow, venous blood flow and net ultrafiltration amount) are all the same as
defined
by the machine settings. All crystalloid fluid pumps may be volumetric for
precise
control of fluid flow rates.
An online citrate, calcium and magnesium sensor 256 may be provided
in the effluent fluid line 224. This sensor array 256 allows for the
derivation of the
citrate, calcium and magnesium level in the patient's systemic blood. In one
safe
operational mode of RCA home system 210 (more than 66% citrate extraction),
citrate accumulation can only occur if the filter performance declines.
Laboratory
testing is not available in the home setting. For maximum safety, the indirect
data
from the online conductivity clearance monitor may not be sufficient in the
home
setting. However, the online citrate and calcium sensor 256 may warn of any
change
in systemic citrate and calcium levels in real time and prompt the patient and
or the
remote monitoring personnel to review and adjust the treatment settings to
ensure the
safe continuation of the RRT treatment. Sensor 256 may also serve as an online
clearance module, may provide information for the fine-tuning of the calcium
plus
magnesium dosing and monitor the metabolic function of the liver.
RCA home system 210 may include disposable, sterile fluid circuits
which may include the replacement fluid and effluent fluid balancing chambers
262
of the RRT machine 260. While the ultrapure dialysate generation module is not
sterile, starting with a sterile concentrate will greatly reduce the risk of
bacterial
-87-
CA 02863264 2014-09-12
contamination in the final dialysate. Water from this module and also the
generated
replacement fluid may pass through low flux sterilizing filters 282 with pore
size
small enough to prevent the passage of whole bacteria or endotoxins and
pyrogens
derived from bacteria. If the implementation of the disposable sterile
balancing
chamber 262 is too costly, the fresh online replacement fluid may be filter-
sterilized
after passing through the usual, non-disposable, fixed balancing chamber. The
filter
sterilization may be necessary to allow direct infusion of the online
replacement fluid
into the RRT circuit blood space. These concerns are less pronounced in the
nocturnal SLED diffusive operational mode of the device, where the online
fluid
remains separated from the blood space by the membrane of hemofilter 216.
Specially designed dialysis catheters, access needles, circuit tubing and
connectors
may also be utilized as described elsewhere herein. Single needle operational
mode
is as previously discussed for RCA system 110.
The elements of the CRRT machine 260 include, but are not limited
to, hemofilter 216, usual fluid and blood circuit tubes, conductivity
monitors, fluid
heating element, blood leak detector, and air detectors as used on
conventional RRT
machines. RCA home system 210 according to the present invention further
includes
an operational mode of pre- and post-dilution CVVH, marked isolated pre-
dilution
CVVH or SLED with a single, online generated calcium and magnesium free fluid
to maximize single pass citrate (and coincident calcium) extraction on the
filter 216.
Initially, the RCA online system-controlled concentrated citrate infusion may
reduce
ionized calcium in the systemic blood entering the arterial limb 214 of the
extracorporeal circuit 212. This blood may then be diluted with the
essentially
calcium-free pre-filter fluid. The original hematocrit, blood volume and
electrolyte
composition may then be restored by ultrafiltration on the hemofilter 216
except that
the blood leaving the filter 216 will have a 50-75% reduced total calcium and
magnesium as well as uremic solute content (the actual reduction is precisely
determined by the treatment settings) and a low ionized calcium level
preventing
blood clotting. Finally, before the blood is returned to the patient, the RCA
online
system-controlled calcium plus magnesium infusion restores normal total
calcium and
magnesium levels. This procedure will usually be performed with blood flows in
the
range of 150-300 mi./minute during 8-12-hour nocturnal CVVH or SLED.
-88-
CA 02863264 2014-09-12
As described above, RCA home system 210 may utilize an essentially
calcium and magnesium free pre-filter online-generated replacement fluid. The
online fluid generation is simpler and safer since all the remaining
electrolytes
including phosphate and bicarbonate can now be combined into a single
concentrate
bag 280 making the fluid generation system safer and simpler. An integrated IV
pump 244 may be provided to administer a premixed calcium plus magnesium
containing infusion. System 210 may control this pump 244 to deliver the
supplemental calcium and magnesium in a fixed ratio in coordination with the
RRT
prescription and monthly patient chemistry values. A novel dosing program may
be
used to drive the pump 244. The online calcium and citrate sensor 256 may
alarm
if a machine failure or calcium plus magnesium line disconnection was to cause
hypocalcemia (or hypercalcemia if too much infusion is given).
In accordance with the present invention, a combination of ti-sodium
citrate and acid citrate in the pre-dilution fluid may be implemented with the
fluid
conductivity further manipulated by the addition of sodium chloride for safety
monitoring purposes. The present invention further contemplates a mandatory
addition of phosphate to the pre-filter replacement fluid (or dialysis fluid)
concentrates. This eliminates the need for monitoring serum phosphate levels
and
for separate intravenous phosphate administration. Phosphate losses can be
very
large and can quickly lead to severe hypophosphatemia with high daily
(nocturnal)
clearance goals unless the phosphate is provided in the replacement fluid.
Since
calcium and magnesium are essentially not present in the RRT fluid
concentrate,
phosphate can be added commercially, preserving physiologic phosphate levels
in the
therapy fluid and consequently in the patient. Finally, phosphate is also a
calcium
chelator and may result in a further minor reduction in the ionized calcium
level in
the circuit. If stored in a single compartment with bicarbonate, phosphate may
be
provided in a pH-adjusted buffered form to avoid the possibility of CO2 gas or
carbonate generation by reacting with bicarbonate.
Integrated online hematocrit sensors 250, 252 may be provided
straddling the pre-dilution fluid connection 230 on the arterial limb 214 of
the blood
circuit 212. The online hematocrit sensors 250, 252 allow minute-to-minute
-89-
CA 02863264 2014-09-12
calculation of the plasma volume in the blood flowing into the circuit 212.
This
ensures the most accurate and possibly continuously adjusted dosing of citrate
to
achieve the target citrate to plasma calcium ratio. Another benefit of the
hematocrit
sensor 250, 252 is that it can be utilized for periodic automated monitoring
for
catheter recirculation using an induced hemodilution-based technique. This
allows
the correction of measured clearances for access recirculation when this
phenomenon
is present. Detecting recirculation in the access early is important to ensure
full
exposure of the circuit to uremic blood from the patient and in correctly
performing
clearance calculations using the OSS. Further, in a method according to the
present
invention, the described online hematocrit sensor pair 250, 252 can also be
used to
derive the delivered blood flow in the arterial limb 214 of the circuit 212 by
analyzing the hemodilution induced by the infusion of a known amount of pre-
filter
replacement fluid. The pre-filter fluid may be delivered by existing highly
accurate
volumetric pumping technology. The observed hemodilution in response to a
known
amount of pre-filter fluid infusion will allow the precise back calculation of
the
delivered blood flow that was diluted in this fashion. Finally, the hematocrit
sensor
250, 252 as a blood volume monitor may detect blood volume contraction in the
patient due to excessive ultrafiltration and may alert the patient and stop
the net fluid
removal before resultant hemodynamic compromise could develop.
RCA home system 210 may further include integrated Doppler sensors
to monitor fluid flow rates in the arterial blood line 214, venous blood line
218, pre-
filter fluid line 228, and effluent fluid line 224. These fluid flows are
predetermined
by the settings of the machine. With modern machine technology using precise
volumetric pumps on the crystalloid fluid lines (but using a non-occlusive
roller pump
as usual on the blood line to avoid hemolysis) and the generally lower flow
rates
utilized during CRRT, clinically significant, more than 10% deviations from
the
preset flow rates are unlikely. The machine 260 has multiple safeguards
against
deviations from the prescribed fluid flow rates. These include the balancing
chamber
262 for correlating the effluent and the replacement fluid flows, the
duplicate
hematocrit sensors 250, 252 to monitor delivered blood flow as well as the
ratio of
delivered blood flow to pre-filter fluid flow, and finally the Doppler sensor
system.
The simultaneous use of all of these measures ensure the safe operation of RCA
home
-90-
CA 02863264 2014-09-12
system 210 according to the present invention that utilizes a strict
coordination of the
flow rates of the various fluids it utilizes. Finally, continuous, precise
monitoring
of the patient's systemic citrate and calcium levels through the composition
of the
effluent fluid will provide yet another, ultimate level of safety for the
procedure.
Effluent line 224 of RCA home system 210 may contain an OSS that
can indirectly monitor the systemic concentration of citrate, calcium and
magnesium.
This module can analyze the ultrafiltrate and derive the patient's plasma
citrate and
total calcium and magnesium level continuously with mathematical precision and
display it in real time. The OSS may alarm when dangerously rising citrate
levels
or abnormal (low or high) total calcium levels are detected. Measuring citrate
may
also serve as a basis for a novel online clearance module, filter patency
monitor and
liver function monitor. The concepts used to implement the citrate sensor 256
are
also applicable to other ultrafilterable solutes. Monitoring of sodium,
glucose, pH,
bicarbonate and CO2 as well as any ultrafflterable small solute level is also
possible.
The design, fluids, and control program of the RCA home system 210
eliminate all of the risks of RCA as described below. RCA home system 210 may
include all of the safety features of RCA System 110 as discussed herein. The
modifications of home system 210, most notably the single-chamber concentrate
280
and the OSS will address additional risks unique to the home treatment
environment
1) Metabolic alkalosis: The baseline acid-base chemistry is expected to be
normal
in stable home patients. The therapy fluid bicarbonate of 25-40 may be
selected
about once monthly and will depend on the weekly equivalent clearance
delivered,
baseline liver function and endogenous acid generation rate (protein
nutrition). The
single chamber concentrate 280 will reduce complexity and will prevent
erroneous
bicarbonate or sodium settings by the operator as these will be largely fixed
with a
single concentrate.
2) Metabolic acidosis: see above for metabolic alkalosis.
-91-
CA 02863264 2014-09-12
3) Hypocalcemia 1 (due to net calcium loss from the patient): The
ultrafiltrate total
calcium and magnesium losses are precisely calculable in the RCA home system
210.
The online total calcium sensor module 256 may be necessary for catastrophic
system
failures (for example, disconnection or leakage of the calcium plus magnesium
replacement infusion) in the home. This sensor module will remove all concerns
related to calcium, magnesium and citrate levels in the patient's plasma. This
module
will eliminate the need for laboratory monitoring of the patient's systemic
total and
ionized calcium and magnesium levels during RCA. The fundamental principle of
the sensor 256 is simultaneous determination of free ionized calcium, free
ionized
magnesium and free ionized citrate levels in the effluent fluid of the
circuit. This
allows for the mathematical derivation of the total calcium content of the
effluent
fluid with clinically sufficient accuracy.
4) Hypocalcemia 2 (due to citrate accumulation): Safe prescriptions prevent
citrate
accumulation even in the absence of liver metabolism by providing for a 66-75%
citrate extraction on the hemofilter in a single pass. The OSS will derive the
systemic citrate level in real time and will detect a rise in citrate levels
accurately
before the systemic ionized calcium level could drop by more than 0.25 mmol/L.
A
kinetic program may analyze the RRT prescription (fluid compositions and flow
rates
as well as blood flow rate). It also may utilize anthropometric data to
predict the
citrate volume of distribution in the patient. Data from the OCM allows filter
clearance calculations. Finally, an estimate of the patient's citrate
clearance in
L/minute may also be derived from the measured systemic citrate curve. This
will
allow the prediction of the citrate curve after a prescription change.
5) Rebound hypercalcemia (due to release of calcium from citrate after CVVH is
stopped): The RCA home system 210 may not allow home treatment prescriptions
to continue without modification if the patient's detected systemic citrate
level
exceeds 3.0 mmol/L. This will ensure that systemic citrate levels stay < =3.0
mM
corresponding to about maximum 0.75 mM chelated calcium that could be released
after RCA is stopped in all patients who can metabolize citrate. (Most
patients will
have 1 mM plasma citrate and about 0.25 mM Ca chelated by citrate in the
steady
state). The RCA protocol may be designed to keep systemic ionized Ca levels
-92-
CA 02863264 2014-09-12
around 1-1.25 and therefore the highest calcium level after RCA is stopped
will be
<=1.75 mM and most patients will rebound to 1.25-1.5 mM Ca levels after
treatment. Utilizing the OSS, the system 210 can also provide a lower citrate
and or
calcium infusion rate in the last few hours of the treatment to lower the
total systemic
citrate and calcium levels prior to stopping the RRT.
6) Hypophosphatemia: Depending on the achieved equivalent weeldy clearance and
dietary habits, the single bag concentrate 280 may have varying amount of
phosphate,
to suit the individual patient.
7) Nursing errors: The RCA home system 210 may be designed to be fully
automated and provide home nocturnal RRT with citrate anticoagulation without
any
intervention from nurses or other health care personnel.
8) Rare: Ionized hypomagnesemia: Magnesium dosing may be fully coordinated
with calcium. The only variable, the molar ratio of calcium to magnesium may
be
fine tuned in the range of 2:1 to 2:0.5 with more clinical experience in the
future.
Similar to the current clinical practice of having several acid concentrates
with
different calcium to magnesium molar ratios, it is likely that the calcium and
magnesium infusion according to the present invention will have to be
formulated as
two or three distinct varieties with slightly different Ca:Mg molar ratios in
the above
range to accommodate the individual patient.
9) Declining filter performance: The conductivity-based OCM as well as the OSS
monitoring the citrate bolus-based online clearance can detect this
complication and
alert the operator that the filter needs to be replaced.
10) Trace metal depletion: Cationic trace metal supplementation may be
provided
with the calcium infusion to restore precise mass balance for these trace
solutes. Any
trace metal incompatible with the calcium infusion can be provided in the
citrate
anticoagulant infusion in an adjusted concentration.
-93-
CA 02863264 2014-09-12
11) Access disconnection: Needle disconnection can be safely detected if a
single
needle operational mode is used in combination with a novel circuit tubing
connector
to access a permanent access for daily nocturnal dialysis.
12) Wrong connection of citrate, calcium or acid concentrate or blood circuit
to
patient: These errors may be prevented by the hardware design of the system
210
as well as through conductivity monitoring-based safety checks.
13) Disconnection of the calcium and or citrate infusion: This can be
completely
prevented by appropriate circuit tubing design (non-disconnectable, physically
continuous infusion to blood line connection). The disconnection of the
citrate
infusion can also be detected by monitoring the circuit effluent conductivity
and or
citrate concentration. As a major improvement, disconnection of the calcium
infusion can now be detected with the OSS through detecting decreasing
systemic
calcium levels despite normal functioning of the rest of the RCA home system
210.
The optical hematocrit sensors 250, 252 can detect access recirculation and
can
enable the correction of blood bolus-based clearance measurements as well as
the
correction of derived systemic citrate and calcium levels for this phenomenon.
The novel therapy fluid used by the RCA home system 210 is
described below. All concentrations and dilutions including, but not limited
to, 1X,
5X 10X, and 50X formulations are fully contemplated in accordance with the
present
invention.
Novel single pre- and post-filter replacement fluid (or dialysate in nocturnal-
SLED
mode):
Pre-filter fluid (with 37X lx fluid 1X fluid
dilution used) fnmol/L
Sodium 138 138
Potassium 4 4
HCO3- *27 27
Chloride 112.3 112.3
Calcium 0 0
Magnesium 0 0
-94-
CA 02863264 2014-09-12
Phosphate (HPO4--:
H2PO4- =20:1) *1.35 *2.7
Dextrose 5.5 5.5
The most likely concentrate composition is provided above, wherein
values denoted with an * may be slightly modified based on clinical
experience. The
manufacturer may modestly vary the potassium, sodium and bicarbonate content
of
the concentrate to best suit the individual patient. This will result in a
range of
combinations of the electrolytes in the final ready to use online generated
fluid
similar to several compositions of peritoneal dialysis bags being available to
patients
on peritoneal dialysis.
The ranges of possibilities in the 1X therapy fluid composition are provided
below:
Therapy fluid 1X (mmol/L)
Sodium 130-150
Potassium 2-4
HCO3- 20-40
Chloride 90-135
Calcium 0-0
Magnesium 0-0
Phosphate (HPO4--: H2PO4- =20:1) 0-1.5
Dextrose 5.5-11
The provided concentrate is an important component of RCA home
system 210 of the present invention. The lower potassium and higher
bicarbonate
concentrates are proposed for the few patients who want only every other day
nocturnal therapy. The phosphate may be provided as a tri-basic and di-basic
salt,
pH-adjusted to be compatible with bicarbonate and to avoid CO2 gas generation
by
virtue of being in the same concentrate container. (The zero range for
phosphate may
only be needed when 3x weekly brief 3-6 hours outpatient treatments are done
with
the RCA home system 210 and fluids).
-95-
CA 02863264 2014-09-12
A novel control program that monitors all sensor data and ensures a
safe prescription based on treatment goals, mode of operation (pre- and post-
dilution
CVVH versus c-SLED as selected by the operator), and possibly patient
variables
input from the sensor devices (OSS) may be utilized according to the present
invention. The control module has the capability to completely automate the
safe
functioning of the RCA home system 210 but is proposed in the default
operational
mode primarily as a safety and alarm tool with no authority to automatically
change
treatment settings (other than stop the machine if needed during an alarm).
The control program that may be used by the RCA home system 210
may be essentially identical to the control program of RCA system 110, wherein
data
from the OSS may be used to adjust the calcium infusion according to systemic
citrate and calcium levels. When the RCA home system 210 is implemented as
shown in FIGS. 17a-17b, the operational modes of pre- and post-dilution CVVH
and
SLED can be used as discussed for system 110. For the implementations in FIGS.
17c-17d, the SLED mode is unchanged; however, CVVH may only be performed in
isolated marked (66%) pre-dilution mode. Modified calculations from the pre-
and
post-u1trafiltration mode as discussed for RCA system 110 with post-infusion
being
zero can still be used. The program simplifies the use of the device and
allows for
exact and automated calculation of the prescribed treatment variables
including blood
flow, citrate anticoagulant infusion rate, pre filter fluid flow, and degree
of dilution
of the pre-filter fluid during online generation as well as the rate of the
calcium plus
magnesium supplemental infusion. Once or a few times monthly, the physician
may
program the treatment modality, the duration and the frequency of the
treatments and
the hourly clearance goals and can provide data on measured hemoglobin and
albumin levels as well as the patient's liver function (usual liver clearance)
as
determined from prior treatments.
In the default mode, the program will generate a prescription based on
a markedly high pre-dilution (with or without post-dilution depending on the
system
design) with a pre-filter fluid flow to plasma flow ratio of 2:1 that will not
allow
dangerous citrate accumulation in the systemic plasma of the patient even in
the
absence of liver metabolism. All patients can safely reach up to 100-200
ml/kg/hr
-96-
CA 02863264 2014-09-12
treatment goals with such a prescription. The clearance goal is expressed
corrected
for the degree of pre-dilution. More fluid efficient prescriptions that
utilize lesser
amounts of pre-dilution of the patient's blood in the arterial limb 214 of the
circuit
212 would rely on the liver to clear some of the systemic citrate. If such
prescriptions
are allowed, should a sudden and unexpected reduction in liver function occur,
the
provided citrate and calcium sensor 256 may detect citrate accumulation and
the
resulting danger of ionized hypocalcemia before this complication could
develop to
a clinically significant degree. The generated alarm may contact the remote
monitoring center to warn about the liver function and will trigger the
machine to
default to safe treatment parameters.
During the operation of RCA systems 10, 110, 210 according to the
present invention, the arterial and venous blood flow, as well as the citrate
and
calcium infusions are precisely controlled by the system without any
intervention
from the health care personnel. This design affords the safe use of special
catheter
or circuit tubing connector designs as shown in FIGS. 9-16. These accessories
may
replace or connect to standard blood circuit tubing in current clinical use.
The
special blood circuits, access catheters or circuit tubing connectors may
introduce the
citrate anticoagulant as early as possible into the arterial blood pathway and
reverse
the anticoagulant effect by the calcium infusion into the venous blood pathway
as late
as possible. These designs are possible as the blood flow as well as the
citrate and
calcium infusion flows are now precisely controlled and monitored by the
dialysis
machine instead of the human operator. The new blood circuits can come with
special end connectors or can be completely integrated with the citrate and
calcium
delivery systems.
FIGS. 9a and 9b illustrate a triple lumen access catheter 300 having
a first lumen 302 representing an arterial blood withdrawal path, a second
lumen 304
representing a venous blood return path, and a third lumen 306 representing an
arterial infusion path. Third lumen 306 may be in fluid communication with
first
lumen 302 via an opening 308 in the lumen wall that allows for injection of an
infusion solution. According to one aspect of the present invention, opening
308 may
be provided near the entrance 310 of first lumen 302 used to withdraw blood
from
-97-
CA 02863264 2014-09-12
the patient, wherein third lumen 306 may have a cap 312 or other closure at
that end.
The infusion solution may contain the citrate (or other) anticoagulant, and
the
infusion solution line (not shown) may have an air detector. Catheter 300
allows the
introduction of citrate anticoagulant into the arterial blood as early as
possible.
FIGS. 10a and 10b illustrate a quadruple lumen catheter 314 having
a first lumen 316 representing an arterial blood withdrawal path, a second
lumen 318
representing a venous blood return path, and a third lumen 320 representing an
arterial infusion path, and a fourth lumen 322 representing a venous infusion
path.
Third lumen 320 may be in fluid communication with first lumen 302 via an
opening
324 in the lumen wall that allows for injection of an infusion solution, such
as citrate
anticoagulant. Fourth lumen 322 may be in fluid communication with second
lumen
318 via an opening 326 in the lumen wall that allows for injection of an
infusion
solution, such a calcium solution. According to one aspect of the present
invention,
opening 324 may be provided near the entrance 328 of first lumen 316 used to
withdraw blood from the patient, wherein third lumen 320 may have a cap 330 or
other closure at that end. Likewise, opening 326 may be provided near the exit
332
of second lumen 318 used to return blood to the patient, wherein fourth lumen
322
may have a cap 334 or other closure at that end. Therefore, using catheter 314
citrate may be infused into the arterial line to immediately provide
anticoagulation
of the blood entering the extracorporeal circuit. In order to provide
anticoagulation
throughout the entire circuit, calcium which reverses citrate anticoagulation,
may be
infused in the venous return line at the last possible location before blood
is returned
to the patient. The infusion solution lines (not shown) may have air
detectors.
FIG. 10c illustrates a quadruple lumen vascular access catheter 314
according to another aspect of the present invention which connect to arterial
blood
line 14, 114, venous blood line 18, 118, citrate infusion line 28, 128 and
calcium
infusion line 42, 142 which may have different lengths and/or colors and which
may
be fused at a fixed point so that the circuit 12, 112 may only be connected
together
in the correct position. This arrangement ensures that the anticoagulant is
always
infused into the arterial line 14, 114 and the venous infusion solution is
always
delivered into the venous blood returned to the patient. The infusion solution
lines
-98-
CA 02863264 2014-09-12
28, 128, 42, 142 may have air detectors (not shown).
As illustrated in FIG. 10d, quadruple lumen vascular access catheter
314 may include connectors 340 of different configurations, such as with the
male
and female line connectors reversed and of different colors. The catheter
connection
ends correspond to connection ends of the complementary type for the dialysis
arterial and venous blood tubing 14, 114, 18, 118 as well as the anticoagulant
and
calcium infusion lines 28, 128, 42, 142. Therefore, the arterial and venous
blood
ports as well as the medication infusion ports may all be color-coded and
mutually
incompatible to prevent errors stemming from line reversal or other
misconnection.
This ensures that the circuit 12, 112 can only be connected with the catheter
314 in
the correct configuration and that the anticoagulant is always infused into
the arterial
line and the venous infusion solution is always delivered into the venous
blood
returned to the patient. The infusion solution lines may have air detectors.
The
citrate and calcium ports on catheters 300 and 314 may have safety valve
mechanisms
to prevent air aspiration if one or both of the infusion lines disconnect, and
the blood
pump continues to run. Catheter 314 may be designed for short (3-5 hours long)
DID sessions with RCA where achieving high blood flows and hourly clearance
goals
is necessary.
A triple lumen catheter 350 with a single blood path (FIGS. 14a-14d)
may be used for clinical applications where a high blood flow is not mandatory
and
a smaller diameter catheter (possibly even in a peripheral vein) may be
acceptable.
In this catheter 350, blood flow direction is alternating in a single lumen. A
central
lumen 352 may be used to withdraw blood from the patient during a first,
arterial
pump cycle, then on the next, venous pump cycle infuse blood back into the
patient.
A second lumen 354 representing an arterial cycle infusion pathway in
communication with central lumen 352 may be used to infuse citrate
anticoagulant
or another solution into the incoming blood during the arterial pump cycle.
During
the venous cycle, a third lumen 356 representing a venous cycle infusion
pathway in
communication with central lumen 352 may be used to infuse calcium or another
infusion into the blood before reentry into the circulation. The calcium
infusion line
may be clamped during the arterial pump cycle, and the anticoagulant infusion
line
-99-
CA 02863264 2014-09-12
may be clamped during the venous pump cycle.
As with catheters 300 and 314, the anticoagulant may be introduced
into central lumen 352 via an opening 358 in the lumen wall at the tip of the
catheter
350, such that the blood receives anticoagulant at the exact point where it
enters the
extracorporeal circuit. In order to provide anticoagulation throughout the
entire
circuit, calcium which reverses citrate anticoagulation, may be introduced
into central
lumen 352 via an opening 360 in the lumen wall at the tip of the catheter 350.
Also
as above, second lumen 354 and third lumen 356 may be provided with a cap 362
or
364, respectively, or other closure. As shown in FIGS. 14b and 14d, catheter
350
according to the present invention may accommodate blood tubing and infusion
lines
with different arterial and venous connectors.
This smaller catheter 350 may be particularly suited for heart failure
patients who could benefit from 12-24-hour ultrafiltration with RCA using a
peripheral vein access, and in whom placement of a large dialysis catheter for
conventional access is difficult to justify because of the associated risk of
complications. Catheter 350 requires a dialysis machine that is capable of the
single
needle dialysis operational mode (this is an optional module on modern
dialysis
machines). An additional benefit of this symmetrical design is that mixed up
connection of the arterial and venous blood lines and/or the citrate and
calcium
infusion lines cannot result in any clinical complication as long as the
temporal
coordination between the blood pumping cycles and the citrate and calcium
pumping
cycles is preserved. The asymmetrical connector designs of FIG. 14d may only
be
needed if a dedicated RCA blood circuit tubing is used with asymmetrical blood
and
infusion line end designs.
Permanent accesses (arterio-venous fistulas and grafts) are very rarely
utilized for CRRT because of fears of unnoticed venous access needle
disconnection
and subsequent catastrophic blood loss in the ICU. Similar concerns surround
the
use of permanent accesses in home nocturnal dialysis programs. In a two-needle
dialysis session, when the venous needle disconnects (slips out of the
access), the
machine may not alarm and can cause massive blood loss with continued arterial
-100-
CA 02863264 2014-09-12
pumping. As a solution, the catheter described above (FIGS. 14a-14d) can also
be
implemented as a circuit tubing connector that attaches to a single needle
that is
inserted into a permanent vascular access (single needle dialysis operational
mode is
required), which may be embodied as a quintuple lumen circuit connector 366
with
a single blood path (FIGS. 15a - 15d).
FIGS. 15a-15d illustrate a connector 366 (e.g., plastic) according to
the present invention for circuit priming and to attach to a single vascular
access
needle from a standard dialysis blood line set and standard medication
infusion lines
for use with single needle dialysis operational mode. The central lumen 368
may be
used to withdraw blood from the patient during an arterial pump cycle, then on
the
next, venous pump cycle infuse blood back into the patient. A needle
connection 370
may be disposed on one end of central lumen 368. Connector 366 includes an
arterial blood port 372 in fluid communication with central lumen 368 and
arranged
to be connected to an arterial blood line, a venous blood port 374 in fluid
communication with central lumen 368 and arranged to be connected to a venous
blood line, an arterial cycle infusion port 376 in fluid communication with
central
lumen 368 and arranged to be connected to an arterial infusion line for
injection an
infusion (e.g., citrate anticoagulant) during the arterial pump cycle, and a
venous
cycle infusion port 378 in fluid communication with central lumen 368 and
arranged
to be connected to a venous infusion line for injection of an infusion (e.g.,
calcium)
during the venous pump cycle. Arterial and venous blood ports 372, 374 and
arterial
and venous infusion ports 376, 378 may branch outwardly from central lumen 368
as shown. In addition, needle connection 370 may be capped for circuit
priming.
According to one aspect of the present invention, arterial and venous
infusion ports 376, 378 may be closer to needle connection 370 than are
arterial and
venous blood ports 372, 374. With this configuration, the blood may receive
anticoagulant as it enters the extracorporeal circuit, and may receive calcium
as it
leaves the circuit to be returned to the patient. As above, the venous
infusion pump
may be turned off during the arterial pump cycle, and the citrate infusion
pump may
be turned off during the venous pump cycle.
-101-
CA 02863264 2014-09-12
The design of connector 366 has the same benefits as far as mixed up
connection of blood and/or medication lines are concerned as single lumen
catheter
350 described above. The most important added benefit is that the connector
366
allows single needle dialysis to be performed on a permanent access. This
operational mode is particularly suited for extended therapy sessions (e.g.,
nocturnal
dialysis and CRRT) where a high blood flow is not needed, but the risk of a
catastrophic bleed from access needle disconnection is greater. In the single
needle
mode, if the needle disconnects, the system may sense air in the arterial limb
of the
circuit with the next arterial (or intake) cycle and may alarm immediately,
essentially
eliminating the risk of a major unnoticed bleeding in the event of needle
disconnection.
The connectors depicted in FIGS. 15a-15b connect to blood lines with
symmetrical ends with the connector of FIG. 15a accommodating infusion lines
with
identical ends and the connector of FIG. 15b accommodating infusion lines with
asymmetrical ends. The connectors depicted in FIGS. 15c-15d accommodate a
citrate-dedicated blood circuit and connect to blood lines with asymmetrical
ends,
with the connector of FIG. 15c accommodating infusion lines with identical
ends and
the connector of FIG. 15d accommodating infusion lines with asymmetrical ends.
Finally, these devices may be very useful during the initial circuit priming
and safety
check step. All lines can be connected, and the needle connection can be
attached to
a priming solution line to prime and test the system. After testing is
complete, the
priming line may be removed and the needle connected.
Special blood circuits, blood circuit connectors, and medication
infusion lines designed for two-needle or conventional double lumen dialysis
catheter
access treatments with RCA according to the present invention are shown in
FIGS.
1 la-11b, 12a-12b, 13, and 16a-16b.
FIG. 1 la illustrates connectors 380, 382 (e.g., plastic) according to
the present invention which may be used as a kit to attach standard dialysis
blood
lines (independent arterial and venous blood circuit ends) for dialysis using
separate
arterial and venous needles. Connector 380 may be an arterial connector which
-102-
CA 02863264 2014-09-12
=
includes a central lumen 384, a needle connection 386, and an arterial
infusion port
388 for the infusion of citrate or other anticoagulant at the point where
blood enters
the extracorporeal circuit. A similar connector 382, with the male and female
connectors reversed, may be a venous connector which includes a central lumen
390,
a needle connection 392, and a venous infusion port 394 for the infusion of
calcium
at the point where blood is returned to the patient. The orientation of the
male and
female connectors may be maintained from the beginning to the end of each
infusion
line. As above, the location of the arterial and venous infusion ports 388,
394
provides anticoagulation throughout the entire circuit. FIG. 11a depicts a
configuration where the blood ports 396, 398 are the same but the infusion
ports 388,
394 are different. FIG. 11b depicts both the blood ports 396, 398 and infusion
ports
388, 394 having different configurations, which may be used to attach a
citrate-
dedicated dialysis blood tubing (different arterial and venous blood circuit
ends).
FIG. 12a illustrates an arterial infusion line connector 500 according
to an aspect of the present invention which may be used to attach a citrate-
dedicated
dialysis arterial blood line using separate arterial and venous needles.
Connector 500
includes a central lumen 502, a needle connection 504, an arterial blood port
506,
and an arterial infusion port 508. As shown, arterial infusion port 508 and
citrate
infusion line 28, 128, 228 may be integrated into one unit, preventing
accidental
anticoagulant infusion disconnection. Citrate infusion line 28, 128, 228 may
have
a specific key segment 510 configured to be received by citrate pump 34, 134,
234
(and not calcium pump), as well as a specific bag connector 512 configured to
be
received by the citrate bag (and not calcium bag). This arrangement provides
anticoagulation throughout the entire circuit and ensures that the citrate can
only be
infused into the arterial limb of the blood circuit.
FIG. 12b illustrates a venous infusion line connector 514 according
to an aspect of the present invention which may be used to attach a standard
or
citrate-dedicated dialysis venous blood line using separate arterial and
venous
needles. Connector 514 includes a central lumen 516, a needle connection 518,
a
venous blood port 520, and a venous infusion port 522. As shown, venous
infusion
port 522 and calcium infusion line 42, 142, 242 may be integrated into one
unit,
-103-
CA 02863264 2014-09-12
preventing accidental calcium infusion disconnection. Calcium infusion line
42, 142,
242 may have a specific key segment 524 configured to be received by calcium
pump
44, 144, 244 (and not citrate pump), as well as a specific bag connector 526
configured to be received by the calcium bag (and not citrate bag). If a
citrate
dedicated blood tubing is used, the calcium can only be infused into the
venous limb
of the blood circuit.
FIG. 13 illustrates citrate-dedicated blood circuit tubing with
integrated arterial and venous medication infusion line connectors 530, 532
according
to the present invention which is used to connect the extracorporeal circuit
to the
patient using separate arterial and venous access needles or a traditional
double lumen
hemodialysis catheter. The advantages to this configuration are that the
connectors
530, 532, blood lines 14, 114, 214 and 18, 118, 218, and infusion lines 28,
128, 228
and 42, 142, 242 are integrated into one unit, preventing accidental citrate
or calcium
infusion disconnection. Integration of the citrate infusion line 28, 128, 228
with the
arterial connector 530 and the calcium infusion line 42, 142, 242 with the
venous
connector 532 ensures that anticoagulant only enters the blood circuit in the
arterial
limb and calcium only enters the venous limb.
FIGS. 16a-16b illustrate a connector 534 (e.g., plastic) according to
the present invention to attach to a single vascular access needle or to a
single lumen
catheter from a standard dialysis blood line for use with single needle
dialysis
operational mode. Connector 534 includes a central lumen 536, needle
connection
538, arterial blood port 540, venous blood port 542, arterial infusion port
544, and
venous infusion port 546. Arterial infusion port 54-4 may be integrated with
arterial
infusion line 28, 128, 228, and venous infusion port 546 may be integrated
with
venous infusion line 42, 142, 242, eliminating the chance of infusion line
disconnection. FIG. 16a depicts arterial and venous blood ports 540, 542
having the
same configuration, and FIG. 16b depicts arterial and venous blood ports 540,
542
having different configurations, such as for accommodating citrate-dedicated
blood
circuit ends.
-104-
CA 02863264 2014-09-12
In the above embodiments, the medication infusion lines may be made
of rigid plastic material that minimizes changes in the filling volume of the
lines with
the pumping cycles to guarantee the greatest accuracy of infusate delivery.
The
connection to the citrate or calcium infusion solution bag should be above the
pumping segment and air detector portions of the IV infusion pumps, so that
accidental disconnection from or emptying of the bag would be detected
immediately
by detecting air in the line.
The catheters and connectors according to the present invention which
may be used for RCA may maximize anticoagulation efficiency and (in the case
of
the single needle tubing connector) will allow safe use of permanent vascular
accesses
for 12 to 24 hour CRRT or home nocturnal hemodialysis. The single lumen
catheter
for RCA may allow the more common use of a peripheral vein for isolated UF
with
RCA for example for volume overloaded heart failure patients in whom placement
of a traditional access catheter may be deemed too aggressive. When high blood
flows and hourly clearances are not needed but accidental venous access
disconnection could be fatal as in CRRT and nocturnal dialysis, a triple lumen
RCA
catheter or a single needle plastic adapter, each with a single blood pathway
and
symmetrical or asymmetrical (to accommodate asymmetrical infusion line ends)
citrate- and calcium infusion connections may be used. Proper coordination of
the
arterial (aspiration) blood pump cycle with activation of the citrate infusion
pump and
the venous (re-infusion) blood pump cycle with the activation of the calcium
infusion
pump ensures proper circuit anticoagulation as well as the reversal of the
anticoagulation just when the processed blood is returned into the patient. In
the
event of access disconnection, the machine alarms when a pressure change is
detected
and/or air is aspirated into the blood line in the arterial (aspiration)
cycles following
the disconnection, preventing clinically significant blood loss.
The citrate and the calcium bags may have different and mutually
incompatible connection locking mechanisms to completely prevent inadvertent
wrong connection of the bags. In addition, the total conductivity of the
citrate and
calcium infusions will be substantially different. This will help detect wrong
connection of the bags through the online clearance-monitoring tool or by
direct
-105-
CA 02863264 2014-09-12
conductivity measurements of the infusates themselves. Conductivity monitoring
of
the anticoagulant infusion line and the calcium plus magnesium infusion line
by any
method to detect the presence of inappropriate fluid conductivity and hence
inappropriate fluid flowing in these tubing segments is fully contemplated
according
to the present invention.
The conductivity-based online clearance monitor according to the
present invention is now discussed in further detail.
Traditional safety monitoring with laboratory measurements of total
and ionized calcium and Lytes 7 with anion gap every 6 hours (as in the
current
clinical protocols in use) is not sufficient for treatments with high hourly
clearance
goals. While traditional laboratory monitoring is insufficient to ensure the
safety of
RCA with high hourly clearance goals, such goals are becoming the standard of
care
and are easily achieved with online fluid generation at a reasonable cost.
However,
if the prescription is carefully written and the various fluid flows and
compositions
are defmed appropriately, RCA with high clearance goals will keep all major
electrolyte values in the normal range. The only variable in the system 10,
110, 210
that could often be a cause of complications is the possibly declining filter
performance, for example with progressive protein fouling of the membrane
and/or
clotting of the fiber bundle. Therefore, in the absence of a commercially
available
online citrate and/or ionized calcium sensor, online filter clearance
(performance)
monitoring in conjunction with safe prescriptions may be utilized for patient
safety
in the implementation of online safety monitoring for the RCA system 10, 110,
210
according to the present invention.
In order to write a safe prescription that prevents systemic citrate
accumulation even in shock-liver (anhepatic) patients, the present invention
includes
a calculation method whereby the maximal systemic citrate concentration that
can
develop in the absence of citrate metabolism is calculated for any RRT
prescription.
The principles of writing a safe prescription are explained below. In essence,
the
maximum possible systemic citrate level during RCA with a given prescription
needs
to be calculated. The abbreviations used are as follows:
-106-
CA 02863264 2014-09-12
Cat: the concentration of citrate in the anticoagulant fluid
Qat: the flow rate of the anticoagulant fluid
Cvs: steady state systemic plasma citrate concentration in a patient with zero
citrate
metabolism
Cven: the plasma citrate concentration in the blood circuit venous limb before
the
blood is returned to the patient
E: apparent circuit post-anticoagulant infusion arterial plasma citrate to
therapy fluid
citrate concentration difference reduction ratio during a single filter pass
("plasma
citrate extraction ratio")
Dcit: apparent citrate plasma dialysance (Dck* when expressed for the adjusted
QBcit
during calculations and Do, when expressed for the unadjusted Qp)
Dcond: apparent "summary conductivity solute" whole blood dialysance (this
value
may be predicted from filter KoAcond, Qb, Qd, Qpre, Qpos, and Quf and/or
determined
by the sodium citrate bolus based measurement or by the traditional online
conductivity dialysance measurement method (for high blood flow treatment
sessions))
QB: the effective blood flow for the solute analyzed; QBccei is closely equal
to the
arterial whole blood water flow for conductivity and QBat is closely equal to
arterial
blood plasma water flow for citrate. In the case of citrate, for the
calculation of "E"
the plasma water volume is adjusted for the free water shifts between the RBCs
and
the plasma space in response to the hypertonic citrate anticoagulant and the
mildly
hypotonic pre-filter online therapy fluid infusion (when applicable) and Da,*
is also
calculated with these adjustments. Once E = i
D /0i ( is derived,
the
¨ Ct*- .03Ct DCit/Q P)
unadjusted Qp and Du can be used to simplify the subsequent calculation of
Csys.
Qp: The arterial blood plasma flow without adjustment for the effects of the
hypertonic anticoagulant infusion (These shifts are accounted for during the
calculation of E).
Ca: Citrate concentration in the therapy fluid
Cmf: The increase in the arterial plasma citrate concentration as a result of
the
anticoagulant infusion, before any pre-filter replacement fluid infusion or
adjustment
for water shifts between blood fluid compartments. (These shifts are accounted
for
during the calculation of E). Cinf = CCit /(QHCit/QCit) =
-107-
CA 02863264 2014-09-12
In the anhepatic patient, there is no systemic citrate metabolism and
citrate clearance is solely through the extracorporeal circuit, where FIG. 18
illustrates an explanation of the calculation of the maximum possible systemic
citrate
level during RCA. In the anhepatic patient, the steady state is reached when
Csys =
C. = If the variable E is defined as: E = Cinf/((Csys Cm f)-Ctf) then ((Csys
+Cinf)-
C,f)*(1-E) +Cif = Cõ, will be true. Rearrangement yields Csys = Cinf*(1-E)/E +
C.
In the steady state, the anticoagulant load Qp*Cinf = DCit ((C8 , the
plasma water citrate dialysance multiplied by the citrate concentration
gradient
between the anticoagulated arterial plasma and the therapy fluid.
Rearrangement
yields Dcii/Qp = (Cinf/(Cs),s+C,õ rC,f) = E. Do, can be calculated from Dcoõd;
the
DcondB (whole blood conductivity dialysance) is measured by the online
clearance
module. The flows QB and Qp are known. Finally, cy, is calculated from E, Ctf
and
Cmf=
Therefore, the steady state is reached when the new citrate loaded into
the combined patient and CRRT circuit space is equal to the net citrate
removed from
the patient and CRRT circuit combined in the circuit effluent as shown in
Equation
1:
1: Citrate load = Qp * Cid = Dcit * ((C,"+Cind-Cd = Citrate removal
The citrate removal is by definition the apparent plasma citrate dialysance
multiplied
by the citrate concentration gradient. For simplicity, after calculating E we
use Qp
and the apparent plasma Da, (instead of QBCit and DCit*) without adjustment
for
water shifts between blood fluid compartments as these adjustments were made
during the calculation of E.
Rearrangement yields Equation 1*:
1*: (Cinf/((Csys + Cinf)-C,f) =Dcit/Qp = E
Dcit/Qp = Dck*/QBcit can be calculated from the measured total Dom(' (see
below); the
Dcoõd (apparent whole blood conductivity dialysance) is measured by the online
-108-
CA 02863264 2014-09-12
dialysance module and QBcoõd and QBat are known. When calculating Dcit* from
Dcond, the differences in the summary sieving and diffusivity coefficients for
the
negatively charged citrate and citrate-Ca or citrate-Mg complexes (probably
slightly
above 1) as compared to the summary sieving and diffusivity coefficients for
conductivity (equal to 1) are considered. In general, a low estimate for E can
usually
be calculated online if the online conductivity dialysance can be measured
during
RRT with RCA with clinically acceptable accuracy (method described below).
Using the definition of E as: E = (Cwt. / ((C,),,+Cinf)-Ctf) and some
rearrangement, Equation 2 will then be true:
2: ((Csys + Cinf)-Ctf)*(1-E) + Cif = Cys
Alternatively, in the anhepatic patient, the steady state also means that C,y,
= Cven,
in other words the venous blood plasma citrate concentration returning to the
patient
will be equal to the arterial (systemic) plasma citrate concentration before
the infusion
of the fresh anticoagulant (we ignore the effects of the minimal net
ultrafiltration).
Therefore, Equation 2 again follows with a different logic using the initial
definition
of E:
2: Cven = C,, = ((C,y, Cif) ¨ * (1-E) +
ct,
Finally, solving Equation 2 for C,, yields Equation 3:
3: Co, = C1nf*(1-E)/E + Cif
A few examples for calculating Cos with Equation 3 are given below.
During pre-post-dilution CVVH for CRRT, the maximal practical E will be about
0.75. If the therapy fluid has no citrate in it, (C11 = 0), then even with
very strong
anticoagulation with Cinf = 7.5 mM the C,),, will be 2.5 mM or less (less if
there is
liver metabolism of citrate). If the therapy fluid has citrate with Cif = 1.2,
then with
lesser, but still strong, anticoagulation with Cia = 6 the C,y, will be 3.2 mM
or less
(less if there is liver metabolism of citrate). When using the RCA system 10,
110,
210 according to the present invention, the maximal practical E may be about
0.66.
-109-
CA 02863264 2014-09-12
The C111 can be calculated according to its definition and will be 8 mM with
the
Citrateasyl6 fluid and a 2 liter plasma to 1 liter CitrateEasy 16 fluid flow
ratio.
Both the pre-dilution fluid and the post-dilution fluid then can be thought of
as
replacement fluids with Ctf = 0. The maximum Cm will be 4 mM or less (if there
is liver metabolism).
During pre-dilution CVVHDF or pure SLED for CRRT, the maximal
practical E will be about 0.85. Even if the therapy fluid has citrate in it,
(C,1 = 1.2)
and even with very strong anticoagulation with Chit = 7.5 the Csys will be 2.7
mM or
less (less if there is liver metabolism of citrate). If the therapy fluid has
no citrate
with Ctf = 0 and even with very strong anticoagulation with Chif = 7.5 the
C.)f. will
be 1.5 mM or less (less if there is liver metabolism of citrate).
During high volume pre-post hemofiltration (HVHF), intermittent
hemodialysis (IHD) or postHDF with high blood and therapy fluid flow rates,
the
maximal attainable E can be as low as 0.6-0.7. Under these circumstances, the
anticoagulation intensity, Cid must be reduced to about 4-5 mM and Ctf should
be
preferably zero or maximum 0.8 mM. Also, a filter with the highest surface
area,
flux and resultant citrateKoA may be utilized. All of these alterations ensure
that Csys
remains in the 2-3 range even in the absence of liver function (not mentioning
the fact
that it is unlikely that a patient with no liver metabolism of citrate would
be
encountered in the outpatient setting).
In summary, in Equation 3 control over all the variables is possible.
By selecting the appropriate citrate pump speed for a given arterial blood
plasma
flow, Cid may be defined. By carefully designing the therapy fluid
concentrate, Ca
may be selected. By using an appropriate filter and blood flow and therapy
fluid
flow rates and a database of Dcond and Do, values predicted from these
variables, E
can be programmed to the target 0.6-0.9 range, as long as the filter
performance
remains unchanged from baseline. This last prerequisite is important to the
continued
safety of the RCA after the start of the procedure. Finally, a low estimate
for E can
be monitored online by monitoring Dcond online and calculating E. The need for
online filter performance monitoring may require that the RCA system 10, 110,
210
-110-
CA 02863264 2014-09-12
according to the present invention have an online clearance module that works
at all
blood and therapy fluid flows, including the low flows typically used for
CRRT. The
only possible exception may be in the operational mode of pre- and post-
dilution
hemofiltration as here the all-convective citrate clearance is highly reliable
and is
easily calculated.
The present invention provides a novel online conductivity dialysance
monitor (OCM) for RRT with RCA. A commercially available module essentially
determines conductivity dialysance by altering the conductivity of the fresh
dialysis
fluid and measuring the subsequent conductivity change of the spent dialysate
(see
U.S. Patent Nos. 6,702,774 and 6,939,471). Common to all previous
implementations is the concept of varying the sodium concentration (and
conductivity) of the fresh dialysate by about 10% and measuring the reflection
of
these programmed variations in the conductivity of the spent dialysate. This
approach is not feasible with the low therapy fluid flow rates of CRRT.
Using the currently available methods, changing the composition of
the fresh dialysate takes a very long time at the low dialysate flow rates
typically used
for CRRT. The rate of change is related to the ratio of the dialysate flow
rate (Qd)
and the volume of the concentrate mixing chamber and dialysate tubing V.,
ratio =
QdN.. At the low dialysate flow rates used in CRRT, the pumping of the
dialysate
also becomes intermittent, causing further difficulties in the measurement.
Finally
the effects of access-, cardiopulmonary- and systemic recirculation may all
become
more pronounced. At the very high fractional plasma clearance rate (K) needed
for
the safe removal of citrate (K > =80% of blood circuit plasma flow (Qp)), even
large
changes in the fresh dialysate conductivity will cause only modest ( < =30% of
the
change in the fresh fluid), and therefore difficult to precisely measure
changes in the
effluent fluid conductivity. Finally, theoretically the KoA (mass transfer
area
coefficient; a standardized measure of membrane performance) of the membrane
could be determined at a conveniently higher Qd, using techniques of the
current art.
However, this KoA would not be the same as the KoA present at the low Qd
values
of CRRT because of fluid layering and channeling effects that develop at those
low
flow rates.
-111-
CA 02863264 2014-09-12
The current art does not utilize the possibility of introducing a bolus
of concentrated sodium citrate or other conductive solution into the arterial
limb 14,
114, 214 of the blood circuit 12, 112, 212. Hemodialysis machines in current
clinical use do not have integrated sodium pumps on the blood circuit.
However, the
citrate pump 34, 134, 234 necessary for anticoagulant administration in the
RCA
system 10, 110, 210 according to the present invention may in essence be a
concentrated sodium solution pump and is eminently suited for the purpose of
online
conductivity dialysance monitoring. Therefore, in a fundamental departure from
the
practiced art, the present invention includes a novel modification of
conductivity-
based hemofilter clearance monitoring in which the conductivity dialysance may
be
determined by using the concentrated sodium citrate pump 34, 134, 234 to
modulate
the incoming blood sodium citrate content (and thereby conductivity) by means
of a
small bolus of trisodium citrate infusion (as opposed to modulating the fresh
therapy
fluid sodium concentration, which will be kept constant). The effects of such
modulation are a precise and immediate change in the arterial blood plasma
sodium
citrate concentration and conductivity, as shown in FIG. 19 for the
calculation of the
effect of the sodium citrate bolus, and an almost immediate change in the
filter
effluent fluid sodium content and conductivity, as shown in FIGS. 20a-20b.
In particular, FIG. 19 illustrates a calculation of the conductivity of
plasma (Con) in the arterial limb of the extracorporeal circuit entering the
hemodialyzer. All parameters are known or measured values except Cp and hence
Cpin. FIG. 20a illustrates an online clearance monitor in accordance with the
present
invention. A conductivity sensor Cl can be placed in the therapy fluid line
before
the fluid is infused into the filter (into the dialysate and/or the blood
compartment).
A second conductivity sensor C2 can be placed in the effluent line of the
dialysis
machine. Increasing the concentration of sodium citrate (and possibly sodium
chloride or sodium bicarbonate) and hence the conductivity of the blood plasma
(Cpo)
entering the dialyzer for a short period of time (TB = 12- t1; bolus method)
produces
a corresponding response in the sodium concentration and hence the
conductivity
measured in the effluent, C2(t). Data from the transient increase in effluent
conductivity can be used to determine the dialyzer conductivity dialysance
online.
The C2(t) - C1(t) (inter-bolus) persistent difference can also be used for
less accurate
-112-
CA 02863264 2014-09-12
but truly continuous monitoring of conductivity dialysance and hence filter
performance in between boluses. (Differences in CI(t), C2(t) and C1(t) are not
to
scale). During the positive bolus method, QB may be reduced to keep (QB +
Qcit)
unchanged.
FIG. 20b also illustrates an online clearance monitor in accordance
with the present invention. Decreasing the concentration of the anticoagulant
sodium
citrate (and sodium chloride or sodium bicarbonate possibly with it) and hence
the
conductivity of the blood plasma (C..) entering the dialyzer for a short
period of time
(TB = t2 - t1; "negative bolus" method) produces a corresponding response in
the
sodium concentration and hence the conductivity measured in the effluent,
C2(t).
Data from the transient decrease in effluent conductivity can be used to
determine the
dialyzer conductivity dialysance online. The c2(t) - cp difference can also be
used
for less accurate but truly continuous monitoring of filter performance in
between
boluses. (Differences in Cl(t), C2(t) and Cob(t) are not to scale). Also, both
AB and
ACeffB are negative values as expected for the negative bolus method. During
the
negative bolus method, QB may be increased to keep (QB + Qcit) unchanged.
The rate of change of the effluent conductivity will be related to the
ratio QbNf, where Qb is the arterial blood flow rate and Vf is the blood
filling volume
of the filter. This ratio is much larger than the Qd/Vm mentioned earlier in
the
description of prior art, and ensures that the method will be practical for
the low fluid
flow rates prevalent in CRRT prescriptions. The magnitude of the change will
be
related to the ratio of Qb/Qd and usually will be about 50-80% of the change
in the
plasma conductivity, allowing precise measurements. Due to the 90-100%
fractional
extraction of the conductivity bolus in the CRRT operational mode,
cardiopulmonary
and systemic recirculation is predicted to have an insignificant effect on the
measurement, particularly if a high-low bolus technique is used. Access
recirculation
may have a more marked effect; however, this can be corrected for by measuring
the
degree of recirculation with the hematocrit sensor. Overall, the apparent
conductivity dialysance measured by the blood side bolus and dialysate side
sensor
method will give a comparably accurate indirect tool to monitor conductivity
(and
indirectly citrate and urea) dialysance as the prior art. This method,
however, will
-113-
CA 02863264 2014-09-12
work at very low QB values and will not result in salt loading of the patient,
both
improvements over prior art.
The following equations show the calculation of the apparent
conductivity dialysance for all treatment modalities as measured from effluent
conductivity changes and the calculation of the target safety variable true
filter Dat
from the true filter Dcond which, in turn, can be calculated from the measured
D.
These calculations are performed assuming no access, cardiopulmonary or
systemic
recirculation during the novel conductivity dialysance measurement procedure.
These conductivity dialysance calculations may be expressed for total
blood water flow, since following the infusion of hypertonic citrate into the
plasma
space, water and urea will quickly cross red blood cell (RBC) membranes to
continuously equilibrate tonicity, osmolality and conductivity between the
plasma and
RBC space in the extracorporeal circuit. The dialysance calculations for
citrate
should be expressed with plasma water flow and plasma water dialysance, also
accounting for the temporary water shifts between the RBC space and the plasma
space in response to the hypertonic citrate infusion and the hypotonic pre-
filter
replacement fluid infusion when used. QB in this regard is always the
effective blood
water flow for the specific solute being investigated. Such prerequisites are
apparent
to those skilled in the art and such modified calculations, while not shown
for all
possible variations, are also fully contemplated according to the present
invention.
In the first step, the total true filter Dcond is obtained. (The effect of
access- and cardiopulmonary recirculation on the measurement of conductivity
dialysance and the calculation of the true filter Dam is discussed later.)
Next, the
diffusive dialysance component of the total apparent dialysance is calculated
(where
applicable). Once the diffusive dialysance is known, the KoAc.d of the filter
can be
calculated. The KoAcond is converted to KoAat based on the known constant
diffusivity constants for conductivity and citrate. Using the Koko QBat (as
adjusted
for water shifts between the fluid spaces of whole blood and the pre-filter
replacement fluid flow) and QD, the diffusive component of the total apparent
Dot*
is calculated. Finally, the total Dut* is derived by adding the convective
dialysance
-114-
CA 02863264 2014-09-12
component (when applicable) to the diffusive component calculated earlier.
Once
Dcõ* is known, Eat and maximum Cv, can be determined as shown herein regarding
writing a safe prescription for RCA.
The terms used in the equations are defined below:
QB: effective arterial blood water flow (Qgcmo Qgco adjusted arterial blood
plasma
water flow for citrate)
Hgb: hemoglobin concentration in the arterial blood
Qp: arterial plasma flow
Cp: "conductivity solute" concentration in the plasma water entering the
filter
without citrate infusion
Cp1n(1): "conductivity solute" concentration in the plasma entering the filter
with
normal citrate infusion rate
Co(t): "conductivity solute" concentration in the plasma entering the filter
during
the citrate bolus at "t" time point
Cop(B): "conductivity solute" concentration in the plasma entering the filter
during
the citrate bolus
Cat: "conductivity solute" concentration in the citrate anticoagulant
Qci,(1): flow rate of the citrate anticoagulant during normal conditions
Qa,(B): flow rate of the citrate anticoagulant during the temporary sodium
citrate
bolus
Qpre: pre-filter substitution fluid flow rate
Qp.õ: post-filter substitution fluid flow rate
Qd: dialysis fluid flow rate
Q5: total substitution fluid flow rate
Qur: net ultraffltration (negative fluid balance goal plus the citrate and Ca
infusion
rates)
Q: total therapy fluid flow rate (= Qd Q)
Co(l): "conductivity solute" concentration of the effluent fluid during
baseline
citrate anticoagulation conditions
-115-
CA 02863264 2014-09-12
AB: the total amount of increased "conductivity or solute" appearing in the
effluent
in response to the sodium citrate bolus delivered by the anticoagulant pump
TB: the exact duration of the citrate pump running faster to deliver the
citrate bolus
DCeff(B): the time averaged effluent "conductivity solute" concentration
increase
during the citrate bolus
C(B): the time averaged effluent "conductivity solute" concentration during
the
citrate bolus
Ca: "conductivity solute" concentration of the fresh therapy fluid
Dcond: "conductivity solute" dialysance determined by the sodium citrate bolus
based
measurement
Dot: the calculated citrate dialysance (Dat* when expressed for the adjusted
QBcd
during calculations and Do, when expressed for the unadjusted Q)
Ddiff the calculated diffusive component of the measured total dialysance
(Ddiffcond,
DdiffCi)
KoA: mass transfer area coefficient; measure of filter performance specific to
solute
(KoAcond, KoAck)
S: summary solute sieving coefficient Sun& Sat
a: summary solute diffusivity coefficient (Gibbs-Dorman factor; a,d; ack)
The summary conductivity of the blood and the fresh and spent
therapy fluids is essentially provided by sodium ions with their accompanying
small
solute (chloride, bicarbonate, phosphate and citrate) anions. Equation (1)
defines the
apparent conductivity dialysance under baseline operating conditions (modified
from
published art):
Deddd = (Qd + Qs + Quf)(Ceffl (1)
a cc,õ (Cpinl - Ctf)
To largely reduce the effect of the unknown Cp (affecting Cpinl) in the
calculation,
the bolus method may be used. For greatest accuracy (as allowed by the
precision
of the blood pump), during the citrate bolus the total filter blood water
flow, Qb
Qcd(B) is kept constant by temporarily decreasing the QB by the bolus to
baseline
-116-
CA 02863264 2014-09-12
baseline %depending on how concentrated the anticoagulant solution is) and the
Qtr
is kept unchanged. Under such conditions, the Dome' will remain practically
constant
during the bolus.
When the citrate bolus is given, the effluent conductivity as a function
of time, Ceff(t) will first rise and then fall as shown in FIG. 20a. (A
negative bolus
method implemented by reducing the citrate infusion as shown in FIG. 20b is
also
possible). Integrating the change in conductivity from baseline (Ceff(t)- crop
by dt
from the time point, ti at the start of the bolus to the time point, t3 after
the bolus
when the effluent conductivity returns to baseline, and then dividing it by
the
duration of the citrate bolus infusion, TB yields the time averaged increase
of effluent
conductivity over baseline corresponding to the bolus, DCeffB. Adding this
value to
Ceff(I) yields the time averaged effluent conductivity during the citrate
bolus, CemB).
By defining Ceff(B) in this manner, Equation (2) is true after all data is
collected from the bolus:
(CeffB - Ctf)
D wad = (Qd + Qs + Quf) a cond (CpinB - Cpinl) (2)
It is known that acond is equal to 1 when the "hypothetical summary
solute" conductivity is being studied. Equations (1) and (2) can be rearranged
and
combined to eliminate cf., the result is Equation (3):
(CeffB - Ceff1)
D., = (Qd + Qs + Quf)(3)
a cond (CpinB - Cpin1)
In Equation (3), all variables are either set on the machine (Qd, Q. and
Quf) or are measured and calculated (CeffB, Ceff1). The denominator (Cpiõ13-
Cpiõ1) can
be calculated (ignoring the temporary and fully reversing osmotic water shifts
between the red blood cell volume and the plasma volume of the blood in
response
to the hypertonic sodium citrate anticoagulant infusion and subsequent
hypotonic
therapy fluid exposure) as follows from Equations (4), (5) and (6) ( see FIGS.
19,
20a, and 20b):
-117-
CA 02863264 2014-09-12
(Qb= Cp)+ (QcitleCcit)
Cpinl - (4)
(Qb+ Qcitl )
Similarly, Equation (5) when lowering QB during the bolus as discussed above:
(Qb - QCitB + Qcit 1 ).Cp)+ (QcitB=Ccit)
CpinB - (5)
(Qb+ Qcitl)
Using Equations (4) and (5) to express (Cor,B - C9n1), after rearrangement
yields
Equation (6):
(QcitB- Qcit1)(Ccit- Cp)
(CpinB- Cpinl = (6)
(Qb+ Qcitl)
In Equation (6), all variables are known except C. However, since
the electrolyte composition and therefore the conductivity of the human plasma
is
strictly regulated, in one approach Cp is assumed approximately equal to 14 mS
+-
1.5 mS. The relative error range this assumption introduces into the
calculation will
depend on the value of C. If the Cat value is very large (highly concentrated
citrate
solution with additional sodium chloride or sodium bicarbonate (550 mM or
higher
sodium content), the error introduced by the estimated Cp will be 1-3 % at
most.
Such errors will be further reduced as the treatment returns the patient's
plasma
electrolyte composition towards normal in a few hours and Cp approximates the
normal 14 mS. In a second solution to the problem of Cp being unknown, Dcond
is
first calculated using the assumed value of Cp as described above in Equation
(6) and
then (3). The calculated Dcond is then inserted into Equation (1) and Equation
(1) is
solved for Cpinl . Subsequently, Cpinl is inserted into Equation (4) and
Equation (4)
is solved for Cp. The so derived Cp is then re-inserted into Equation (6) and
Equation
(6) is re-solved for (CpiaB - C91n1). This value is then re-inserted into
Equation (3)
to recalculate the Dcond. These steps may be performed recursively by a
computing
module until the individual fmal values for Dcond and Cp are approximated
within
0.1% . Finally, a third variation of the technique is contemplated, during
which
conductivity is also measured on the arterial and venous blood lines without
direct
-118-
CA 02863264 2014-09-12
physical contact with the blood or compromising sterility. This allows for
maximal
precision of the clearance measurements, but requires some novel detection
elements.
Using the value obtained from Equation (6) it is now possible to solve
Equation (3) and derive the value of D. The Dcoõd value obtained for
conductivity
dialysance can be converted into blood clearance values for urea and other
small
solutes, taking into account how the Gibbs-Donnan effect may influence the
movement of negative versus positive ions as compared to the neutral, non-
ionic
solute urea or the summary charge neutral "hypothetical sununnry conductivity
solute". Once the apparent or total, Dcond conductivity dialysance is known,
it is
possible to calculate the diffusive dialysance, Ddiffcand component as
described in prior
art and published in the literature (however, the effect of any access
recirculation if
present must be removed first, as discussed below).
For any solute during intermittent hemodialysis with some net
ultrafiltration (Qui) and during post-dilution hemodiafiltration with Qp.,
replacement
fluid rate and Qui net ultrafiltration, Ddiff is derived by using Equation (7)
(where we
assume QB is the effective blood water flow for the specific solute and the
solute
specific sieving coefficient, S is used):
Ddiff (postHDF). (Qb) -S(Qpost+ Quf)
Qb-S(Qpost+ Quf) (7)
For any solute during pre-dilution hemofiltration with Q9,.. replacement
fluid rate and Q, net ultrafiltration, Dõ is derived by using Equation (8)
(where we
again assume QB is the effective blood water flow for the specific solute
corrected
for the effects of water shifts between the RBC and plasma space and the
infusion of
the pre-filter replacement fluid and we use the solute specific sieving
coefficient, S:
(Qb+ Qpre)
-S(Qpre+Quf))
Qb
D diff preHDF= (Qb+ Qpre) (8)
(Qb+ Qpre-S(Qpre+ Quf)
-119-
CA 02863264 2014-09-12
Equation (8) can be deduced from Equation (7) if one considers pre-dilution
hemofiltration to be a special case of simple dialysis with net
ultrafiltration where the
new Qb* is equal to Qb Qiu.u, the pre-dilution corrected aroud* is equal
to
Droud=((Q, + Qpre)/Qp) and net ultrafiltration becomes Qpre + Q. In the
special case
of simultaneous pre-and post-dilution hemofiltration, when Qd = 0 and the
solute
sieving coefficient is S, Equation (3) still applies:
arca = (Qp,, + Q08 + Quf) = ((CeffB - Ceff1)/(S = (Cp1,13 Cpb,1)))
Since this operational mode involves no dialysis, Ddiff is zero and is not
calculated,
and Dm., can also be expressed as:
Draw= S = (Q pre Qp., + Quf) = (Qb /(Q + (2p))
From this:
D101 = DTotalConcl*(SCit/SCond)*(QP/QB)*((QB QPre)/(QP+QPre))
From these equations, it is apparent that a decline of the small solute
apparent dialysance is unlikely in pure convective renal replacement therapy
as long
as the target total ultrafiltration of Qpre + Qpost + Quf is achieved, unless
S changes
markedly, which is not probable due to the highly predictable nature of small
solute
movement with purely convective blood purification. Dialysance for individual
solutes is calculated by knowing their S sieving coefficients and the total
ultrafiltration rate. (The apparent S value may change modestly for
electrically
charged solutes depending on the ratio of the pre-dilution (Qpre) and post-
dilution
(Q) replacement fluid flow rates and this may have to be considered in the
electrolyte mass balance calculations when selecting the therapy fluid
composition for
various ratios of Qpre and Qpos, fluid flows.)
Once the Ddiffcond has been determined from the total Dc0õd measured
at a given (effective) Qb and Qd, the KoACond of the filter membrane can be
calculated as published in the literature, (see Equations (9.1) and (9.2)) and
can be
-120-
CA 02863264 2014-09-12
compared to the expected value provided by the filter manufacturer and/or
established in vivo by local user experience.
In pure dialysis and post-dilution HDF, Equation (9.1):
Qb = Qd
Qd(Qb - Ddiff)
KoACond=
(Qb - Qd)) 111( (Qb)(Qd - Ddift) (9.1)
In pre-dilution HDF, Equation (9.2):
KoACond- (Qb Qpre) Qd Qd(Qb+ Qpre- Ddiff)
(Qb+ Qpre - Qd)) = In( (Qb+ Qpre)(Qd - Ddiff) (9.2)
The KoACond changes measured as a function of time while keeping the therapy
parameters unchanged for any given filter will allow the detection of
declining filter
performance and impending clotting. The KoACond is converted to KoACit as
shown in Equation (9.3):
KoACit= KoACond = acit
(9.3)
a cond
Knowing the KoACit allows the calculation of the DdiffCit from the effective
Qi3cit
for citrate and the Qpre and Qd fluid flow rates prescribed, as shown in
Equations
(10.1) and (10.2). In pure dialysis and post dilution HDF, Equation (10.1) is
used
where QB now denotes QBci, and KoA denotes KoACit:
e (Q"(6) -1
Ddiff Cit=QBCit ______________________ (Qd_Qb) (10.1)
e KoA Qd.c" Qb
Qd
In pre-dilution HDF, Equation (10.2) is used where QB now denotes Q3cit and
KoA
denotes KoACit:
KoA
Qd=mp.Qpno _ 1
DdiffCit = (Qb + Qpre) ________ (10.2)
e KoA (24-(Qb QPre) (Qb+ Qpre)
Q0=021,442pro Qd
-121-
CA 02863264 2014-09-12
Finally, the obtained DdiffCit can be inserted into Equation (7) or (8)
with the Qpõ or Qpes, and Quf values as appropriate, and the equations can be
solved
to derive the total Do*. Here the S sieving coefficient will now be specific
for
citrate, So. The obtained total Do* is used to derive E= D /OB
cit* cit= The
obtained
E allows the calculation of the safety parameter, Csys as described herein
regarding
safe prescriptions for RCA.
In summary, in the novel arterial circuit limb blood-bolus method
according to the present invention, conductivity dialysance may be measured by
inducing a precise, calculated change in the input plasma conductivity
entering the
hemofilter and measuring the response in the filter effluent fluid.
Conductivity
dialysance may then be converted into citrate dialysance as shown in the above
equations. The feasibility of such conversion depends on the in vivo ratio of
the
diffusivity coefficients for conductivity and citrate. This ratio is estimated
to be
about 3, and in the range of 2 to 4. The citrate dialysance, in turn, defmes
the
maximum possible systemic citrate level with a given treatment prescription,
which
is the safety parameter that is desired to be maintained and monitored. In a
variation
of the technique, in the event that it is not desirable to increase the input
solute
concentration, conductivity dialysance can be measured by introducing a
calculated
decrease in the input solute concentration and measuring the change in
conductivity
of the filter effluent fluid. All of the equations remain unchanged because
both (CeffB
- Ceffl) and (CpinB - Cpthl) in Equation (3) will be negative when the input
concentration is decreased (and QB is appropriately increased) (FIG. 20b).
Any method that induces a known change to the dialyzer input blood
composition, which results in a measurable change in the composition of the
effluent
fluid of the hemofilter can be used to measure the dialysance of the
hemofilter and
then calculate citrate dialysance and is fully contemplated in accordance with
the
present invention. The present invention also contemplates methods that induce
a
change in the input blood composition and measure the effects on the filter
output
blood composition.
-122-
CA 02863264 2014-09-12
The effect of access recirculation on the online conductivity dialysance
may also be measured by the method according to the present invention. Below,
the
differential impact of access recirculation on the online conductivity
dialysance based
filter clearance measurements is reviewed when performed with the traditional,
fresh
dialysis fluid conductivity bolus method versus the circuit arterial limb
blood-bolus
method of the present invention (see FIG. 21). The following will be presumed:
1) The dialysis fluid bolus method, when possible to execute with the high/low
step
functions as described in Kidney International, Vol. 66, Supplement 89 (2004),
pp.
S3-S24, will be considered equivalent to the effective dialysance of the
circuit that
includes the effects of the possibly present access as well as cardiopulmonary
recirculation (De) and systemic conductivity recirculation will be assumed
negligible.
2) The access recirculation will be measured with a hematocrit sensor
(hemodilution)
or thermal sensor (thermodilution) based method prior to any online clearance
measurement.
3) When venous catheter access is used, only access recirculation may be
present
(Dem). Both methods will allow the calculation of the true filter dialysance,
DFilia.
before it is altered by any recirculation.
4) When a permanent access is used, at least with circuit blood flows up to
300
ml/min, our blood bolus based method will measure DBolus. Assuming that
significant
systemic and cardiopulmonary recirculation is not present with our method
(this is
reasonable with single pass conductivity extraction > =80% with QB <= 300
ml/min) and measuring access recirculation will allow the derivation of the
true filter
dialysance, Der as well as the effective dialysance altered by access
recirculation
only, De. This is the data needed for citrate kinetics calculations.
5) In a permanent access, the dialysate bolus method will measure Dem. Even if
the
access recirculation R and the permanent access blood flow QAc is measured
(for
instance with the use of the reverse blood line connector device and the
hemodilution
or thermodilution technique) in the absence of the cardiac output CO, Dm..
cannot
be calculated.
-123-
CA 02863264 2014-09-12
The terms used in the equations are defined below, wherein the calculations
are
depicted in FIG. 21:
QB: effective circuit arterial whole blood water flow for conductivity
adjusted arterial blood plasma water flow for citrate, (Qach)
QAc: effective access whole blood water flow for conductivity
(Qaccond), adjusted
arterial blood plasma water flow for citrate, (QAcett)
CAC: "conductivity solute" concentration in the plasma water in the access
CAR: "conductivity solute" concentration in the plasma water entering the
filter
(pre-dilution removed), as modified by recirculation
CAnRs: "conductivity solute" concentration from the access arterial blood in
the
plasma water entering the filter (pre-dilution removed), as modified by
recirculation
CA: "conductivity solute" concentration from the arterial limb bolus infusion
in
the plasma water entering the filter (pre-dilution removed), as modified by
recirculation
Cven: "conductivity solute" concentration in the plasma water exiting the
filter
CB: "conductivity solute" concentration step-up in the plasma entering the
filter over
, plasma entering the arterial limb of the blood circuit during the citrate
bolus
Cat: "conductivity solute" concentration in the citrate anticoagulant
Qat(B): flow rate of the citrate anticoagulant during the temporary sodium
citrate
bolus
Qpre: pre-filter substitution fluid flow rate
Quf: net ultrafiltration
QR: the recirculating circuit venous limb blood
R: the recirculation ratio defined as R = QR/Q3
DFllier: true filter "conductivity solute" dialysance
DE: effective "conductivity solute" dialysance determined affected by access
and
cardiopulmonary recirculation and determined by the dialysate bolus based
measurement
DB.1õ: measured "conductivity" dialysance affected by access but not
cardiopulmonary recirculation and determined by the blood bolus based
measurement
DEffi: effective "conductivity" dialysance determined from DEolui by
correcting for
access recirculation only
-124-
CA 02863264 2014-09-12
FIG. 21 is a comparison of the effects of permanent access (depicted;
however, the calculations are also fully applicable to catheter access)
recirculation
on the fresh dialysis fluid conductivity bolus based online dialysance
measurement
(Deffe,..õ) versus the circuit arterial limb blood conductivity bolus based
online
dialysance measurement (DB4,10). DFilter is the intrinsic filter dialysance
with the
effects or access recirculation removed. Cardiopulmonary and systemic
recirculation
is ignored. R is equal to QR/QB and can be measured online by hemodilution or
thermodilution methods.
Equation 1:
DFilter = (1 - R)
DEffective-
Q
1- R=' 3
- DFilter)
QB QUF
This describes the relationship between Deffl and Dfilter with R access
recirculation.
Deffl is measured by the dialysis fluid bolus method if the cardiopulmonary
recirculation is negligible or absent as is the case with venous catheter
access. It was
derived as follows:
1.1) Deffective * CAc = CArtRS * Dfilter
1.2) Cven = CArtRS * (Qb-Dfilter)/(QB-QUF)
1.3) CArtRS = R * Cven + (1-R) * CAc
1.4) CArtRS CAc * (1-R)/(1-R((Qb-Dfilter)/(QB-QUF))
Equation 2:
DFilter
DBolus -
1- R = Q. - DFilter)
Qs - QuF
This describes the relationship between Dbolus and Dfilter with R access
recirculation. Dbolus is measured by the circuit arterial limb bolus method if
the
cardiopulmonary recirculation is negligible or absent as is the case with
venous
catheter access and in general with this method when the QB is <= 300 and QD
is
-125-
CA 02863264 2014-09-12
150-200% of QB with a large surface area, high flux filter. R is measured
online with
the hemodilution or thermodilution technique. This Equation 2 is novel
according
to the present invention and is derived as follows:
2.1) Dbolus * CB = CArtRB * Miter
2.2) Cven = CArt * (Qb-Dfilter)/(QB-QUF)
2.3) CArtRB = R * Cven + (1-R) * CAc + CB
2.4) CAc = 0 (reasonable when single pass bolus extraction is > =80% or if we
examine the recirculation effects on the bolus in isolation)
2.5) CArtRB = CB/(1-ROQb-Dfilter)/(QB-QUF))
Combining Equations 2.1 and 2.5 and rearranging yields Equation 2.
Equation 3:
QuF
- R) - ¨
Qs
Dfilter = Dbolus =
1_ R=1 Dbolus) QuF
QB QB
This equation is derived from Equation 2 by simple rearrangement and solution
for
Dfilter. The Dfilter conductivity dialysance can be separated into diffusive
and
convective component, the diffusive component converted into citrate diffusive
dialysance and finally summary citrate dialysance calculated as described
earlier.
Subsequently, Dbolus citrate and Deffl citrate can be calculated using
effective QB
as plasma water flow for citrate.
Equation 4:
Deffectiv Dbolus .(1 - R)
-126-
CA 02863264 2014-09-12
This equation follows from Equations 1 and 2. As discussed above, Dbolus is
measured by the novel blood bolus method according to the present invention.
Deffective (Dem) can then be calculated using the measured R value. If a
venous
catheter access is used, Deffl will be equal to the effective urea clearance.
De m must
be converted into Ddr2, the effective urea clearance including a correction
for cardiac
output when a permanent (arterial) access is used:
Equation 5:
Dem = (1/(1 +Dem/(CO-QAc))) * Deffl
As mentioned, in a permanent access, the dialysate bolus method will measure
Den.
The blood bolus method according to the present invention will measure DB01
and
will allow the calculation of Dem if the access recirculation R is measured.
Finally,
if the permanent access blood flow QAc is measured (for instance with the use
of the
reverse blood line connector device and the hemodilution or thermodilution
technique), Equation 5 allows the calculation of the cardiac output CO with
some
simple rearrangements.
Equation 6:
CO = ((Dem * Dem) / (DD)) + QAC
The method according to the present invention may allow the
measurement of the cardiac output with clinically useful accuracy. The present
invention contemplates use of this method with a dialysis machine with the
appropriate sensor equipment, access connection reversal device and the
cannulation
of a permanent access to measure the cardiac output of a patient during
treatment.
The online sensor system (OSS) according to the present invention will
now be farther described.
-127-
CA 02863264 2014-09-12
In recent years there has been expansive growth in the field of sensor
technology. There are a multitude of new sensors that measure various
substances
including glucose, electrolytes, and macromolecules. Most of these sensors can
be
produced to scale to work with very small fluid samples. The greatest hurdle
for
The sensors placed into the OSS do not come in direct contact with
human blood; instead they analyze a fluid sample obtained by ultrafiltration
of
circulating blood. An illustration of a basic hemofiltration circuit for OSS
400 which
may be used to extract a small amount of ultrafiltrate for chemical analysis
is shown
-128-
CA 02863264 2014-09-12
sufficient anticoagulant and ultrafiltrate to provide 1-2 ml of ultrafiltrate
hourly
during a 24-hour period of intermittent, hourly operation. Such sample size is
more
than adequate for the novel sensor technologies.
One advantage of OSS 400 according to the present invention is that
it is very safe because the ultrafiltrate is discarded after the measurements
and
therefore possible contaminants or allergens in the sensor array 56, 156, 256
part of
the circuit cannot come into contact with the patient. Devices for detecting
an analyte
in blood have been developed, however those devices bring the sensors into
direct
contact with blood in vivo by coupling the device with a venous flow device,
such as
an extracorporeal membrane oxygenator or hemodialysis machine. A sensor in
contact with human blood will require sterilization and adherence to safety
procedures to minimize risks to patients. Contact with human blood will result
in
biofouling of the sensor, which will possibly reduce sensor performance.
Coatings
added to sensor surfaces to limit degradation and improve performance have the
added risk of possible adverse patient reactions. It will be mandatory for
blood
contact sensors to go through FDA testing to ensure that they do not cause
anaphylaxis in patients. Since the OSS 400 according to the present invention
uses
an ultrafiltrate of the blood, large molecules such as proteins remain in the
blood and
are not available to foul sensor surfaces and thereby reduce performance. The
OSS
400 eliminates the need for anaphylaxis testing because once the ultrafiltrate
passes
the sensor 56, 156, 256 it may be completely discarded. The use of OSS 400 can
markedly accelerate the time from development of a specific sensor to
transitioning
to human clinical use either in the testing and development phase or for
routine
patient care.
OSS 400 according to the present invention may have different
implementations. In one embodiment, the OSS 400 may be provided as a compact
device (e.g., 2 x 3 inch-size) for ease of use and immediate applicability for
hospitalized patients (and possibly even for outpatients for 24-48 hours; as a
"chemical Hotter monitor"). This form of the OSS 400 only requires a small
peripheral IV for access to the patient's venous blood and is designed to
serve as a
safe plasma-sampling device. In another embodiment, the OSS 400 may be
provided
-129-
CA 02863264 2014-09-12
as a full size CRRT machine OSS. The effluent fluid line 24, 124, 224 in such
a
circuit may be used to provide samples for the sensor array 56, 156, 256 of
the OSS
400. Importantly, in this implementation, more complex assessment of the
patient's
condition beyond simple plasma concentration measurements is possible,
including
measuring renal and liver clearances of various substances and thereby
monitoring
renal and liver function online, in real time.
The implementation of the OSS 400 as a small ultrafiltration circuit
attached to a peripheral (venous) IV line may be used for patients not
receiving
CRRT therapy. This embodiment of the OSS 400 includes a small hemofiltration
device that may extract only a few milliliters of ultrafiltrate per hour from
a
miniature extracorporeal circuit. A basic hemofiltration circuit which may be
used
to extract a small amount of ultrafiltrate for chemical analysis is shown in
FIG. 22.
Catheter 20, 120, 220 may comprise a small, double lumen intravenous catheter
which may be placed in a suitable vein. Blood may be removed from the patient
using the arterial pump 22, 122, 222 at a few milliliters per minute. At the
same
time, the infusion pump 34, 134, 234 may add anticoagulation solution to the
blood
at an appropriate rate to prevent clotting. Anticoagulated blood from the
arterial limb
14, 114, 214 of the circuit may be pumped through a miniature hemofilter 16,
116,
216 and ultrafiltrate may be extracted from the blood by the ultrafiltration
pump 26,
126, 226. Sensors 56, 156, 256 in the ultrafiltration circuit may analyze the
ultrafiltrate for the selected analytes.
FIG. 23 shows a more complete hemofiltration circuit according to the
present invention which may be used to extract a small amount of ultrafiltrate
for
chemical analysis. Arterial and venous pressure sensors 402, air-in-fluid
detectors
404, a blood in circuit detector 406 and line clamps 408 may be added to
provide
patient safety. FIG. 24 shows a hemofiltration circuit which may be used for
priming
and initial pressure testing of pumps and pressure transducers. All three
pumps may
have very precise flow rates which allows for accurate calculation of blood
analyte
concentrations.
-130-
CA 02863264 2014-09-12
The OSS 400 according to the present invention can operate is a
continuous mode or in an intermittent mode in which samples are collected at
pre-
selected intervals. In intermittent mode, the entire circuit can be refilled
with the
anticoagulant solution. If the infusion pump 34, 134, 234 is run at a slightly
higher
rate than the arterial pump 22, 122, 222, the entire extracorporeal circuit
12, 112,
212 will be filled with anticoagulation solution. Running the infusion pump
34, 134,
234 for a short period of time after the blood pump 22, 122, 222 is stopped
and the
blood line is clamped will direct fluid into the access catheter, filling it
with
anticoagulation solution. Only a minuscule volume of infusion fluid is needed
for
anticoagulation of the circuit, which avoids the risk of infusing an excess
amount of
anticoagulant into the patient. When acid citrate anticoagulant is used, the
approximately 5.4 pH of the anticoagulant-filled circuit will also prevent
bacterial
growth in the event of a contamination.
During continuous operation, the section of the circuit between point
A and point B in FIG. 25 is not exposed to the infusion solution containing an
anticoagulant. To address this situation, a triple lumen venous catheter with
an
infusion port at the tip of the withdrawal lumen may be utilized, such as
catheter 300
depicted in FIG. 9). This triple lumen catheter 300 allows an anticoagulant
solution
to be infused through a hole in the lumen wall directly into the entrance of
the arterial
blood withdrawal path. Since the venous return path contains anticoagulant,
the
entire triple lumen catheter 300 is continuously exposed to anticoagulant.
FIG. 26
shows triple lumen catheter 300 in the OSS hemofiltration circuit according to
the
present invention.
For a case where a sensor requires complete isolation of the
ultrafiltrate because the testing procedure requires reagents that are very
hazardous
to the patient, one of the backflow prevention devices 410, 412, 414 in FIG.
27 can
be used such that the ultrafiltrate extracted from the blood the fluid can be
isolated
from the patient circuit. In one implementation (FIG. 27a), an air gap device
410
may be used where the input fluid enters a chamber 416 from the top and falls
through an air space. If for any reason the sensor system 56, 156, 256 causes
a
backflow to occur, the ultrafiltrate will flow harmlessly out of an opening
418 to the
-131-
CA 02863264 2014-09-12
air. This solution may be applicable when the OSS 400 is used in a fixed
orientation
to gravity, for instance as part of a large CRRT circuit. For the compact size
OSS
which may be attached to the patient's body and not have a fixed orientation
to
gravity, one of the two valve-system-based backflow prevention devices can be
used
(FIGS. 27b-27c). In FIG. 27b, a device 412 including a series of two or more
one
way valves 420 can be used, such that if the first valve fails, the second
valve must
also fail before backflow can occur.
In FIG. 27c, a reduced pressure zone device 414 is illustrated, wherein
the input pressure must exceed a set pressure P to open valve 422, and fluid
in a
reduced pressure zone 424 is approximately P-0.19P because a pressure of P-
0.2P
is required to open valve 426. Any initial backflow is stopped by valve 426.
If valve
426 fails, backflow is prevented by valve 422 and any increase in pressure
above P-
0.15P opens valve 428 and the backflow is diverted out of the device 414. The
use
of one of these devices 410, 412, 414 ensures that if for any reason the OSS
400
causes a backflow to occur, the ultrafiltrate will flow harmlessly out through
the
backflow opening where it is collected and sent to the drain. FIGS. 28a and
28b
show possible locations for backflow prevention devices 414 and 410,
respectively.
The compact size OSS 400 according to the present invention may be
easily connected to a peripheral vein of the patient and can be transported
with the
patient if needed. It is very safe to use because sensors 56, 156, 256 do not
come in
direct contact with human blood and after analyte measurements are made the
ultrafiltrate is sent to the drain. The data obtained may be stored for later
retrieval
and or may be transmitted by a wireless connection.
The OSS 400 according to the present invention may also be
implemented as part of an extracorporeal blood circuit 12, 112, 212 used to
provide
CRRT in the ICU. The OSS 400 can be implemented with any existing device that
extracts an ultrafiltrate from body fluids. Specific application of a CRRT
circuit as
an OSS to measure patient plasma levels of glucose, citrate, calcium,
magnesium,
inulin and para-aminohippuric acid (PAH) is described herein. Importantly,
when
the OSS 400 is implemented as part of a CRRT circuit, truly online, continuous
-132-
CA 02863264 2014-09-12
measurement of the plasma concentration of any filterable solute for which a
specific
sensor is available becomes possible. Thus, kinetic analysis of the solute
concentration curve as a function of time becomes clinically feasible without
the need
for onerous frequent blood sampling. The kinetic data provides a wealth of new
information, ensures monitoring of the liver metabolic function, and may
possibly
allow measuring the glomerular filtration rate and renal plasma flow in real
time.
Such methods are not currently available and are needed clinically.
This implementation of the OSS 400 according to the present invention
in an RRT circuit is clinically immediately available, by minor modification
of
existing RRT devices and by placing the sensor array 56, 156, 256 into the
effluent
line of an RRT device. The OSS 400 may be best implemented integrated into the
RCA systems according to the present invention and described herein which were
either designed solely or have the option to deliver purely convection-based,
high
dose RRT with fully effective RCA. FIGS. 29a-29c show the OSS 400 integrated
into the RCA system according to the present invention running in either
isolated pre-
dilution or simultaneous pre- and post-dilution hemofiltration mode (only the
pre-
dilution flow relevant to the OSS application is shown). The sensor 56, 156,
256 is
placed into the effluent fluid line carrying ultrafiltrate (and or dialysate)
away from
the hemofilter.
In particular, FIG. 29a illustrates a configuration for deriving the
patient systemic solute level (Cso) by measuring the ultrafiltrate solute
concentration
CuF and dividing by the hemofilter sieving coefficient S for the specific
solute. All
other parameters are known values and the C,y, is calculated according to the
formulas shown. Access recirculation is not present. FIG. 29b illustrates a
configuration for deriving the patient's systemic citrate level Cso by
measuring the
ultrafiltrate citrate concentration Cu. Cs), can be calculated knowing the
arterial
plasma flow QpAn rate, citrate infusion flow %hat rate, citrate concentration
of the
infusion solution CFluidiand the filter sieving coefficient for citrate S. The
hematocrit
sensors 50, 150, 250 and 52, 152, 252 allow the calculation of the plasma
citrate
concentration by contributing to the measurement of the delivered arterial
plasma
flow and by measuring access recirculation (assumed not present here). FIG.
29c
-133-
CA 02863264 2014-09-12
Illustrates a configuration for deriving the patient's systemic citrate level
Csys by
measuring the ultrafiltrate citrate concentration CuF when the increase in
citrate
concentration from the anticoagulant infusion in the arterial limb plasma with
the pre-
dilution effect removed is Cif, access recirculation is R = QpR/Qp and filter
citrate
dialysance with recirculation effects removed is DF, plasma citrate bolus
dialysance
with recirculation is DB and filter systemic citrate effective clearance with
recirculation is DE where DE = DB * (1-R). All variables are known or can be
measured and or calculated as shown before. The calculations can be applied
for any
solute for which the above parameters are known, measured and or calculated.
The effluent fluid contains a wealth of information on the patient's
plasma solute composition, but in current clinical practice it is discarded
without any
further analysis. This fluid is a clear crystalloid with a small amount of
albumin,
small peptides, and cytokines also present. The transparency and minimal
viscosity
of the effluent fluid provide for an ideal environment for an optical- and/or
chemical
sensor array. The OSS 400 according to the present invention may operate in a
manner such that solute concentrations are converted to light (optical)
signals by
solute-specific, possibly disposable, chemical-optical transducer systems
(chips or
optrodes) that are exposed to the effluent flow, such as in a possibly
disposable, light
transparent flow-through chamber. Readout of the optical signals may be done
through the light-transparent wall of the chamber or through the optical
filament part
of the optrode by a fixed, excitation light generating (if needed) and optical
signal
capturing and analyzing module. Multiple light wavelengths may be used
simultaneously for both excitation and readout on an unlimited number of
sufficiently
small emitting, capturing and analyzing modules.
In a modification of this method, Raman scatter spectroscopy may be
used on the effluent line and the specific solutes may be identified by their
unique
Raman spectra. Quantification may be possible by measuring the signal
intensity of
specific spectral peaks. The advantage of this method is that solute specific
chemical-
optical probes are not needed as specificity is provided by the unique Raman
spectra
of the target solute. Citrate will be in a large molar excess compared to most
other
molecules in the effluent and it may be possible to quantitate it with Raman
scatter
-134-
CA 02863264 2014-09-12
spectroscopy, possibly even differentiating free citrate, Ca-citrate and Mg-
citrate.
The present invention contemplates the use of Raman scatter spectroscopy to
monitor
systemic solute levels through monitoring the RRT circuit effluent fluid, with
the
specific example of measuring all species of citrate in the effluent.
Finally, the fluid here is waste fluid and will not be exposed to the
patient's blood again, eliminating any chance of any elements of the sensor
getting
into direct or indirect contact with the patient. This is completely
ascertained when
a back-flow prevention safety device 410, 412, 414 (as shown in FIG. 27) is
added
before the effluent is exposed to the sensors 56, 156, 256. Finally, the
effluent
tubing 24, 124, 224 can easily be modified to allow the connection of the OSS
400
in this segment of the CRRT circuit.
The calculation of systemic solute levels from solute levels measured
in the ultrafiltrate including corrections for the effects of access
recirculation when
present is described below. Once real-time measurement of a solute is provided
in
the effluent, a special software calculator may be used to determine the
contribution
of solute entering the extracorporeal circuit from the systemic circulation of
the
patient (the systemic plasma solute level) and the contribution of solute
freshly
infused into the CRRT circuit pre-filter (if the solute is contained in the
pre-filter
infusion(s), as may be the case for glucose, citrate, inulin and PAH). This
calculation requires that the extracorporeal circuit plasma flow to summary
pre-filter
fluid infusion ratio remain constant for the time of the calculation and is
very reliable
when only convective clearance is used, as in the RCA systems according to the
present invention.
In the RCA system, plasma flow may be monitored in real time by the
online hematocrit sensors and possibly by a Doppler-based system as well as
shown
in FIGS. 29a-29b. The pre-filter fluid infusion rate is also known in real-
time, as the
pre-filter fluid pump 34, 134, 234 of the machine delivers it and it also may
be
monitored by the function of the Doppler and hematocrit sensors 50, 150, 250
and
52, 152, 252. Therefore, the contribution of solute freshly infused into the
circuit
blood plasma can be calculated in real time. The calculation also relies on
the sieving
-135-
CA 02863264 2014-09-12
coefficient of the solute being known. Such information has been published for
glucose and citrate in the literature and can easily be measured for most
small solutes
including inulin and PAH. The sieving characteristics of a given solute on the
specific filter used are not likely to change as long as effective
anticoagulation is
used, and can also be monitored by the OCM for conductivity or citrate. Thus,
in
the RCA system, under steady operational parameters, the solute concentration
measured by the OSS in the ultrafiltrate can be immediately used to provide
the solute
level present in the patient's systemic blood. The exact calculations for any
filterable
solute are shown in FIG. 29a and for the specific example of citrate in FIG.
29b.
The calculations can be provided for CVVHD, CVVHDF and c-SLED as well, as
long as measurements are done for the given filter type at fixed dialysate,
ultrafiltrate
and blood flow and pre-filter fluid infusion rates assuming that the solute
transfer
properties of such RRT circuits are defined and monitored by a precise online
clearance monitor. However, purely convective clearance may be preferred in
this
method for greater reliability of solute transport.
The OSS 400 can be integrated with the RRT device to send an alarm
to the operator when a critical (high or low) threshold of systemic plasma
solute
concentration is breached and possibly to automatically adjust treatment
settings to
correct the solute level abnormality. A clinical assessment of the patient
with full
laboratory parameters may also follow. Finally, falsely abnormal solute levels
in the
blood entering the extracorporeal circuit due to recirculation at the catheter
tip can
be detected by the recirculation detection feature of the online hematocrit
sensors 50,
150, 250 and 52, 152, 252 which may be integrated into the RCA system, and
corrections in the calculations are possible, eliminating false solute level
alarms with
or without an intervention on the recirculating access as indicated (FIG. 29c
and as
explained below).
The terms used in the equations are defined below, and the physical
layout of the OSS 400 with the key calculations is shown in FIGS. 29a-29b and
for
recirculation in FIG. 29c:
QAc: effective access blood water flow specific for the solute measured
-136-
CA 02863264 2014-09-12
QB: effective circuit arterial blood water flow specific for the solute
measured;
arterial blood plasma water flow for citrate, (QpA,i)
Csy, (same as CAC): solute concentration in the effective blood water in the
arterial
limb of the access
CAnR: solute concentration in the plasma water entering the filter (pre-
dilution
removed), as modified by recirculation
CAnRsys: solute concentration portion from the access arterial blood in the
plasma
water entering the filter (pre-dilution removed), as modified by recirculation
CAnRinf: solute concentration portion from the arterial limb solute (citrate)
infusion
in the plasma water entering the filter (pre-dilution removed), as modified by
recirculation
Ch,paR: The solute concentration in the plasma water entering the filter; this
is CAnR
adjusted for pre-dilution
Cu: The solute concentration in the ultrafiltrate exiting the filter
Cinf: solute concentration step-up in the effective blood water of the blood
entering
the filter over the blood entering the arterial limb of the blood circuit
during citrate
infusion, with pre-dilution removed
Cõi: summary solute concentration in the pre-filter fluids
Qmuid: summary flow rate of the pre-filter fluids
QR: the recirculating circuit venous limb blood effective water flow (solute
specific)
R: the recirculation ratio defined as R = QR/QB; (measured by hemodilution or
thermodilution)
Dpjiter: true filter solute dialysance (calculated)
DBolus: measured solute dialysance affected by access but not cardiopulmonary
recirculation and determined by the blood bolus based measurement for
conductivity
(OCM) or citrate (citrate sensor)
DEBI: effective solute dialysance determined from DB,1 by correcting for
access
recirculation only
S: summary solute sieving coefficient (Scow, Sat, S501)
These calculations assume:
1) All the equations presented and or used in the section on access
recirculation
effects on conductivity dialysance based online clearance measurements are
-137-
CA 02863264 2014-09-12
referenced here as needed
2) Access recirculation, R, is measured online.
3) While the general term D, (dialysance) is used, all clearance is convective
for
greater predictability of small to medium size solute movement. However, this
method limitation is not mandatory.
4) DB.1* is measured for conductivity and or citrate and Dm: is calculated.
5) DFuter*/QB > = 0.8 and QB is <= 300 ml/min so that cardiopulmonary and
systemic recirculation can be neglected.
6) The sieving coefficient is known for both the solute used to measure Dme,*
as
well as the solute for which the systemic concentration needs to be
determined.
7) DF;Iter* is converted to Dater using the sieving coefficient of the
"solute" used for
measuring DBot: and the sieving coefficient of the target solute to be
measured as
well as knowing the effective blood water flows for both solutes. (These
calculations
are discussed with reference to the OCM where in a specific example
Dconductivuy is
converted into Da).
8) The target solute Dame and R is used to calculate the target solute Dew.
and
Deffectivel =
Specific equations used in the calculations are as follows:
Equation 1:
¨ S = sieving
C,
coefficient
CinPu'R S
The Cõ is measured by the OSS and S is known for the target solute.
Equation 2:
Q PArt Q Pluidl
C mat¨ C Biped?.
Q PArt
CArtit is derived by adjusting Cinpum for the effects of the pre-dilution with
()Fluid .
-138-
CA 02863264 2014-09-12
Equation 3 (by defmition):
C Fluid Q Fluid
C ¨
PAri
The recirculating solute fluxes originating from the systemic circulation
(csys) and
from the blood bolus infusion can be conceptually separated (if the post-
dilution fluid
and or the dialysis fluid target solute concentration is zero as it is for
citrate, calcium
and magnesium) and
Equation 4 follows by definition:
* C1f Den * Csys CAnRSys * Didier CAnRInf * Dfilter CArtR
* DFilter
In Equation 4, CAR is derived from measuring CuF , and Dgolu,, Dem , Dfitter
is either
directly measured or calculated. Using Dem = DBoius * (1-R) and solving
Equation
4 for Csy, (equivalent to CAC or "arterial" access solute concentration)
yields
Equation 5:
D (C Q )
r, * _ B Fluid Fluid
ArtR F
Q PA's
Csys ¨
D5(1¨ R)
In one implementation, the physical design of the access device used
for the OSS 400 and the low QB/QAc ratio is expected to eliminate access
recirculation. However, the above novel calculations are provided when the OSS
400
is implemented as part of a (convective) RRT circuit where recirculation,
although
rare, may occur and its elimination may not be clinically immediately
possible.
Specific examples for the clinical use of OSS 400 integrated into a CRRT
circuit for
the simple measurement of systemic blood glucose and citrate levels are
provided
below.
For systemic blood glucose monitoring, patients with ARF in the ICU
often have diabetes and/or various degrees of liver dysfunction. In such
patients,
tight glycemic protocols for blood sugar control are often difficult to
administer
-139-
CA 02863264 2014-09-12
safely and can have a high rate of hypoglycemic complications. Since these
patients
will often have baseline mental status changes as well and may be sedated and
their
liver's ability to respond to hypoglycemia may be compromised, a real risk of
catastrophic hypoglycemic events is apparent. Frequent blood sugar monitoring
by
standard clinical methods is costly, labor intensive and may be inconvenient
to the
patient. Reliable glucose sensors have been developed by several companies in
the
quest for creating the "artificial endocrine pancreas" and are currently in
preclinical
or clinical trials. They all have to satisfy FDA safety regulations delineated
for
devices that come into direct contact with human blood or body fluids inside
the
body. However, such safety concerns would not apply if the sensors were
deployed
in the OSS allowing immediate human clinical trials of the clinical
feasibility and
value. These sensors could be immediately placed in the effluent line of a
CRRT
circuit.
For systemic blood citrate (and calcium) level monitoring, RCA during
the delivery of CRRT (and possibly home nocturnal dialysis in the future) is
emerging in the literature as the anticoagulation method of choice. In all
applications
of RCA in any form of RRT, there is a significant amount of citrate infused
into the
extracorporeal circuit. A portion of the citrate infused into the circuit
ultimately
enters the patient and is converted into bicarbonate by the metabolic action
of the
liver. When the liver function is markedly compromised, citrate is not
converted,
with consequent systemic citrate accumulation and hypocalcemia, hypomagnesemia
and metabolic acidosis. In-coordinate prescriptions can also lead to net
calcium gain
or loss in the circuit, leading to further complications. In current clinical
practice,
laboratory values including Lytes 7 and total and ionized calcium are measured
every
6 hours to detect a lack of citrate metabolism and abnormalities of calcium
homeostasis. This increases the cost of RCA and does not provide complete
safety
as citrate accumulation can occur in 1-2 hours with sudden, marked changes in
liver
function with the current RRT prescriptions targeting higher treatment goals
and fluid
flows than in the past. Laboratory monitoring is obviously not an option in
the home
setting.
-140-
CA 02863264 2014-09-12
The online citrate, calcium and magnesium sensor according to the
present invention may detect systemic citrate, calcium and magnesium level
changes
in real time before clinically significant derangements could occur,
completely
eliminating concerns about these solutes. This is likely to increase physician
use of
RCA in RRT in the ICU and may allow deployment of RCA in the home setting with
the RCA home system described herein. The online citrate, calcium and
magnesium
sensor can easily provide online clearance measurements. Finally, the dosing
of the
replacement calcium plus magnesium infusion in RCA for CVVH is in part
determined by the systemic citrate level, and the online citrate sensor can
provide this
information continuously.
The online citrate sensor is an implementation of the OSS with a
specific optical citrate sensor that is placed into the CRRT circuit effluent
fluid line
carrying ultrafiltrate and or dialysate away from the hemofflter (FIG. 29b).
Citrate
present in the ultraffltrate and/or dialysate fluid will be in the 0 to 15 rnM
range
under normal operating conditions. In one example, the citrate sensor may
utilize
= luminescence from a complex of citrate with a europium-based ligand
(e.g., Chemical
Communications 2005, pages 3141-3143: Parker et al, "A pH-insensitive,
ratiometric
chemosensor for citrate using europium luminescence"). This sensor technology
is
based on allowing citrate to reversibly associate with a europium ion-based
complex.
During spectrophotometry, the citrate-europium complex is exposed to an
excitation
light source and luminescence is measured at different wavelengths. The
citrate
concentration in the sample is determined by ratiometric analysis, calculating
the
ratio of the luminescence intensities at the different wavelengths. This
citrate sensor
technology has no interference from phosphates, lactate or bicarbonate, has a
response time in the millisecond range and is not affected by pH changes in
the range
of 4.8-8Ø In accordance with the present invention, the abundant, clear
crystalloid
CRRT effluent fluid is eminently suitable for spectrophotometry analysis
online. As
the detection relies on luminescence changes with europium and citrate
association
and dissociation, there is no consumption of reagents (if the europium ligand
is
immobilized in the flow-through detection chamber) or fading as with electrode
or
enzyme based methods of citrate detection published by others in the past. The
published optical system was fine-tuned to the 0-3 mM citrate concentration;
however
-141-
CA 02863264 2014-09-12
this may be adjustable by changing the amount and chemical design of the
europium
complex used.
In accordance with the present invention, the sensor 56, 156, 256 may
be created by either diverting a small amount of the CRRT effluent 600 ( < 1
ml/minute to conserve reagents) for mixing with the europium-ligand contAining
detection reagent 602 in a flow-through light transparent chamber 604 (FIG.
30a) or
by covalently immobilizing the detector europium complex onto the wall of a
flow-
through light transparent chamber 604 in the fluid pathway of the entire
effluent
(FIG. 30b). FIG. 30a illustrates a citrate, calcium and magnesium sensor 56,
156,
256 according to the present invention for use in a continuously flowing fluid
circuit.
The system mixes the opto-chemical probes 602 and the drain fluid 600
containing
citrate, calcium and magnesium before making optical measurements. A light
source
606, prism 608, and mirrors 610 create a measurement optical path 612 and a
control
optical path 614. Probe binding results in changes in light absorption and or
emission at specific wavelengths, where changes in light intensity may
detected by
optical detectors 616 (e.g., charge coupled devices) and converted into
electronic
signals. The total citrate, calcium and magnesium concentrations may be
determined
by a processing unit using calculations based on the obtained data. FIG. 30b
illustrates a citrate, calcium and magnesium sensor 56, 156, 256 for use in a
continuously flowing fluid circuit which utilizes chemical probes 618
immobilized,
such as in a hydrophilic polymer film that coats surfaces, in a light
transparent
cuvette 604. The probes 618 bind citrate, calcium and magnesium which freely
diffuse between the drain fluid 600 and the cuvette 604, where probe binding
results
in changes in light intensity that may be used to determine the total citrate,
calcium
and magnesium concentrations as above.
Due to the tight coordination of the europium ion into the complex
covalent bond chemical structure of the ligand (akin to the coordination of
the iron
(Fe) ion in the heme group), a sensor 56, 156, 256 based on the europium
ligand-
coated chamber 604 should be very stable for days of continuous operation. The
flow through chamber 604 may be locked into a spectrophotometer module on the
machine that provides excitation light 606 (e.g., at 384 nm wavelength) and
-142-
CA 02863264 2014-09-12
luminescence detection 616 (e.g., at 579 um and 616 tun wavelengths). For
patient
safety and increased accuracy, two citrate sensors may be used. Sensor values
may
be compared and, if they deviate by a predefined value, the system will signal
an
alarm to prompt corrective measures. One light transparent chamber 604 with
two
optical paths 612, 614 could be used for the system as shown (FIGS. 30a and
30b).
There are multiple other chemical-optical sensing technologies which
may also be used for citrate (see, for example, Anslyn et al, Tetrahedron,
Volume
59, Number 50, 8 December 2003, pp. 10089-10092(4)) and may form the basis of
the optical-chemical transducer part of the online citrate sensor according to
the
present invention. However, it is understood that the application of
simultaneous
monitoring of citrate, calcium and possibly magnesium levels in effluent
fluids of
extracorporeal blood treatment devices is fully contemplated according to the
present
invention, regardless of the specific sensing technology used. One physical
implementation includes a combination of possibly disposable, optical-chemical
transducers and possibly fixed, non-disposable optical excitation, readout and
analysis modules, wherein the latter may be separated from the effluent fluid
by a
light-transparent, sterile/fluid barrier (flow through chamber). Once real-
time
measurement of citrate, calcium and magnesium is provided in the effluent, a
software module may determine the systemic citrate concentration based on the
methodology described herein for any filterable solute in general. Specific
use of the
data obtained are described below.
For detection of systemic citrate accumulation due to lack of liver
metabolism, the machine can send an alarm to the operator when a critical
threshold
of systemic plasma citrate concentration is exceeded. A clinical assessment of
the
patient and the CRRT treatment with full laboratory parameters can then follow
with
appropriate changes to the care of the patient. Increasing citrate levels in
the blood
entering the extracorporeal circuit due to recirculation at the catheter tip
can be
detected by the recirculation detection feature of the online hematocrit
sensor which
may be integrated into the RCA system of the present invention, thereby
eliminating
false citrate alarms and allowing for exchange of the dysfunctional access
catheter in
a timely manner.
-143-
CA 02863264 2014-09-12
The online citrate sensor may be used to guide the calcium plus
magnesium infusion dosing. During the operation of the RCA system according to
the present invention (and in other CRRT systems with RCA), the ultrafiltrate
calcium content and magnesium concentration is equal to the total patient
systemic
plasma calcium or magnesium concentration adjusted for the degree of pre-
dilution
with the calcium and magnesium free pre-filter fluid, respectively. The high
clearance goals achieved with the RCA System ensure that only chloride,
albumin,
lactate, bicarbonate and citrate can persist as anions in high concentrations
in the
patient's plasma. Other anions including phosphate will be quickly reduced to
physiologic levels by the effects of the CVVH procedure, and systemic pH will
also
approximate the normal 7.4. The anion beta-hydroxybutirate can be eliminated
by
administering glucose and insulin if needed. Under these conditions, the
patient's
systemic total and ionized calcium and magnesium levels can be programmed as
long
as the only clinically significant variable, the current systemic plasma
citrate level is
known, which may be provided by the online citrate and calcium sensor
according
to the present invention. (Lactate, the other patient-specific clinically-
variable anion
does not seem to affect ionized Ca levels sufficiently to be of clinical
concern). Since
the systemic blood citrate level is derived by the online citrate sensor and
the plasma
albumin concentration of the patient is known from laboratory studies (and is
unlikely
to fluctuate quickly), a desirable total systemic plasma calcium and magnesium
concentration can be targeted (a constant fraction of which, in turn, will
appear in the
ultrafiltrate as net loss from the patient) to keep the ionized calcium (and
magnesium)
in the physiologic range. Using this programming of calcium and magnesium
replacement, all patients may be at target ionized calcium values with much
lesser
need for frequent monitoring of their laboratory parameters. It is also of
importance
that the sieving coefficient of calcium and magnesium in the RCA system may be
near 1.0 (different from 0.6 in regular CVVH without citrate) due to the
unique RCA
fluid design of the present invention and the fact that only convective
clearance may
be used.
Next, an online filter clearance (performance) and patency monitor is
described. Accuracy of the online citrate and calcium sensor according to the
present
invention can be easily tested by deploying the sensor in duplicate and by
varying the
-144-
CA 02863264 2014-09-12
circuit plasma flow to citrate anticoagulant infusion ratio by changing the
blood flow
rate and pre-filter fluid flow rate ratio (citrate blood bolus based method).
This will
result in an immediate and predictable change in the ultrafiltrate citrate
level. The
changes measured by the sensor can be compared with the predicted changes to
monitor filter patency and sensor accuracy. Ideally, the sensor is checked
initially
at the time of the priming of the circuit with a modified saline solution that
contains
Ca, Mg and citrate at the start of the CRRT procedure. Subsequent measurements
should match the initial filter and sensor performance. Once the citrate
clearance is
known (particularly in a purely convection based RRT treatment as delivered
with the
RCA systems), the clearance of any solute with a known sieving coefficient on
the
specific type of hemofilter used can be easily calculated. This will be of
great value
to pharmacists with the increasingly widespread use of high clearance targets
in RRT
protocols and concomitant very effective removal of various medications. The
signal
to baseline ratio of the citrate based online clearance monitor can be as high
as 1:1
(by doubling the citrate infusion rate temporarily), possibly ensuring more
accurate
measurements than existing technology can provide (depending on the resolving
ability of the citrate and calcium sensors as well). The current gold standard
online
clearance method relies on varying the sodium concentration of the dialysate
and
detecting the changes by monitoring the conductivity of the circuit effluent.
The data
obtained mainly reflects the movement of the small solute sodium and may be of
lesser value when the clearance of middle to large size molecules is
investigated. In
contrast, when OSS technology of the present invention for high molecular
weight
inulin is used (see below), the filter online clearance of middle and large
molecular
weight solutes can also be monitored, which cannot be accomplished by other
devices
currently in clinical use. Monitoring such clearance may become important in
the
future to follow the efficacy of the removal of cytokines and antibiotics
through the
filter with high volume CVVH.
Finally, it should be noted that in the event of a citrate sensor
malfunction, the RCA system according to the present invention will still
continue
to operate and provide RCA for RRT in a safe default mode relying on their
safe
prescription algorithms and OCM modules for the monitoring of filter
performance
and hence, indirectly, citrate clearance.
-145-
CA 02863264 2014-09-12
The clinical use of the OSS according to the present invention
integrated into a CRRT circuit to obtain systemic solute concentration kinetic
curves
as a function of time to calculate and monitor liver metabolic function,
glomerular
filtration rate and renal plasma flow with specific examples for citrate,
inulin and
PAH monitoring is now described. Despite significant advances in ICU therapy,
the
mortality of acute renal failure (ARF) requiring renal replacement therapy has
remained essentially unchanged in the past decades at very high levels,
particularly
when ARF is caused by acute tubular necrosis (ATN) in the setting of the
systemic
inflammatory syndrome (SIRS) with or without multi-organ dysfunction syndrome
(MODS). Emerging data suggests that the very early (within 0-24 hours of the
start
of the acute kidney injury (AKI), initiation of high dose convective CRRT may
have
a favorable impact on patient survival and recovery of renal function. It is
expected
that in the future, high dose CVVH will be started earlier for a broader group
of
patients as long as difficulties of the procedure are overcome (as described
with
reference to the RCA system according to the present invention).
However, the new treatment approach will create new clinical
dilemmas as well. Many patients will be non-oliguric, and with the high
clearance
goals, traditional markers of renal function including blood urea nitrogen
(BUN) and
creatinine will be in the normal or near normal range. Furthermore, the levels
of
these solutes are also influenced by a multitude of factors other then renal
function
including, but not limited to, the amount of muscle mass and muscle breakdown
for
creatinine, and tissue catabolism, the use of corticosteroids, and the
presence or
absence of gastrointestinal bleeding for BUN. Precise information on renal
function
will be indispensable for proper medication and CRRT dosing and to know when
renal recovery has occurred to the degree that the CRRT could be stopped. As a
result, the development of new, clinically feasible methods to assess
glomerular
filtration rate and renal function will be necessary.
Similarly, currently there is no reliable, inexpensive clinical method
to follow liver parenchymal function in critically ill patients. Laboratory
tools in
current use provide only indirect assessment and may take 12-24 hours to
reflect
marked changes in liver function. The only alternate to routine chemistry
testing, the
-146-
CA 02863264 2014-09-12
ICG-Pulsion technology and device, is fairly costly, does not provide
continuous
data, and has failed to gain wide-spread use to date. More accurate and real-
time
assessment of liver function is needed for the safety of RCA that usually
depends on
primarily the liver to clear the bulk of the citrate entering the patient's
body. Timely
information on the metabolic function of the liver would also be helpful in
the
management of acutely ill liver failure patients being evaluated for liver
transplantation and in the post-liver transplantation period.
The present invention provides a method of kinetic analysis of
systemic concentration curves obtained by the OSS as a function of time for
various
solutes that can be used to determine liver metabolic function, glomerular
filtration
rate, and renal tubular secretory function in real time, online. The
description below
reviews the theoretical principle of solute kinetic modeling as relevant to
the clinical
tool according to the present invention, utilizing the specific example of
citrate. The
use of two additional specific substances are then briefly reviewed that could
be of
immediate interest in clinical practice.
An explanation of the theoretical principle of single pool kinetic
modeling of solute (e.g. citrate) loading into the apparent solute volume of
distribution of the patient treated with CRRT and clearance of the solute
(e.g. citrate)
from the patient by the CRRT circuit and body clearance mechanisms (metabolism
and/or elimination by the liver and/or kidneys) is described below. While
citrate is
used in this example, the equations are applicable to any water-soluble
substance.
Citrate loading (generation) into the patient in CRRT occurs through
the infusion of new anticoagulant into the circuit. The systemic citrate
kinetics
during citrate anticoagulation are shown including citrate generation, citrate
body
clearance and citrate filter clearance, including the citrate mass balance
fluxes in the
patient and the extracorporeal circuit, are shown in FIG. 31. These concepts
can be
generalized to any solute that enters the body and/or is produced in the body
at a
steady rate and is cleared from the body through a concentration dependent
mechanism by metabolism and/or elimination by filtration and/or secretion by
the
liver, kidney and/or the CRRT circuit as applicable. FIG. 32 is an explanation
of
-147-
CA 02863264 2014-09-12
solute fluxes in the extracorporeal circuit during CRRT using citrate as a
small solute
example, wherein the citrate load in the circuit is the fraction of the newly
infused
anticoagulant that is not cleared on the filter in a single pass (further
correction is
needed when access recirculation is present). When the blood flow,
anticoagulant
flow, net ultrafiltration amount, and replacement fluid flows as well as the
filter
performance are constant, this amount is also constant. Citrate removal from
the
patient is the summary of the citrate cleared from the systemic blood of the
patient
on the hemofilter and the citrate cleared by the patient's body, predominantly
by
metabolism in the liver. These mass fluxes of citrate can be described by
equations
as shown below. The definitions of the parameters used in the calculations are
as
follows:
C(s) (mmol/L): the patient's systemic plasma citrate concentration at "t" time
point
after CVVH started
C(0) (nunol/L): the plasma citrate concentration at the start of CRRT with
RCA,
defined as zero
C(Steady) (mmol/L): the plasma citrate concentration when the steady state is
reached
T(90%), (minutes): the time it takes to build up the plasma citrate level to
90% of the
steady state value
V(d) (L): the patient's volume of citrate distribution (predicted to be equal
to the
extracellular fluid volume)
G (mmol/min): the net citrate load into the patient from the pre-filter fluid
after
passing through the filter
K (L/min): the total clearance of citrate from the patient's body that is the
summary
of:
Ko,)(L/min): the body clearance or metabolism of citrate (the equivalent of Kr
in the
urea equation)
(L/min): filter clearance of systemic citrate
B (L/min): the net change in V per minute (net ultrafiltration rate)
QB: effective circuit arterial blood water flow specific for the solute
measured;
arterial blood plasma water flow for citrate, (QM)
CB,f: citrate concentration step-up in the effective blood water entering the
filter
during baseline anticoagulation over the effective blood water citrate
concentration
-148-
CA 02863264 2014-09-12
entering the arterial limb of the blood circuit with pre-dilution removed
CBolus: citrate concentration step-up in the effective blood water entering
the filter
during the citrate bolus over the effective blood water citrate concentration
entering
the filter with baseline anticoagulation, with pre-dilution removed
QR: the recirculating circuit venous limb blood effective water flow (solute
specific)
R: the recirculation ratio defmed as R = QR/QB; (measured by hemodilution or
thermodilution)
Dimus: measured solute dialysance affected by access but not cardiopulmonary
recirculation and determined by the blood bolus based measurement for
conductivity
(OCM) or citrate (citrate sensor)
Dun: effective solute dialysance determined from DRA by correcting for access
recirculation only
R is measured as discussed previously herein. Using the citrate,
calcium and magnesium sensor features of the OSS according to the present
invention
as well as the novel citrate blood bolus-based online clearance method
described
herein for conductivity and fully applicable for citrate (after making the
adjustments
for the effective QB being QP for citrate), citrate DBolus is measured and
Deffectivel is calculated. The following will be true:
1.G = Cinf * (QBCit-DBolus)
2.K(f) = Deffectivel = DBolus * (1-R)
The change in the systemic concentration of citrate as a function of time will
be
determined by the difference in the positive citrate flux into the patient (G)
that is
constant and the negative citrate removal flux (filter and body clearance
multiplied
by the systemic citrate concentration), which negative flux is variable and is
determined by the changing systemic citrate level. The mathematical formula is
shown in equation 1.
1) d(CV)/dt=G-(K.0,)+1C(0)*C (single pool, variable volume citrate
equation)
-149-
CA 02863264 2014-09-12
This equation will be clearly familiar to nephrologists. This is, in fact, the
well-
known formula for the single pool kinetic modeling of urea removal during
hemodialysis. The following differences should be noted:
a. G is body generation of urea whereas it is a steady patient load of citrate
here
b. Urea distributes in total body water whereas citrate distributes only in
the
extracellular volume (ECV)
c. Urea clearance is defined as whole blood volume per minute and citrate
as plasma volume per minute (following from their respective volumes of
distribution)
d. The relative importance of G and Ko) is much greater for citrate during
RCA for RRT than for urea during traditional hemodialysis
However, none of these differences will affect the applicability of the
equation or its
solution. Single pool modeling can be reliably used as the rate of solute
transfer per
hour is fairly low in CRRT and the citrate volume of distribution is the ECV
with
rapid equilibration from the intravascular space (intracellular levels are
kept low
mandatorily by metabolism to prevent interference with intracellular calcium
signaling and probably by the lack of high capacity transmembrane carriers in
most
tissues except the liver and to a lesser degree skeletal muscle.) The
mathematical
solution developed for urea single pool kinetic modeling will therefore be
applicable
to predicting systemic citrate levels at any time point of the RCA for CVVH
treatment. The solution of Equation 1 yields Equation 2:
2) C =C(0) ((V-B*0/V)expy(K(b) +K(0 +B)/B)+(G/(K(b)+K(0 +B)))*(1-
((V-B*t)/V)exp((K0,)+K(0+ B)/B)
Fortuitously, the net ultrafiltration per hour (B) in CRRT is relatively
negligible
when compared to the ECV of the patient and the equation can be simplified by
eliminating B while preserving clinically adequate accuracy to give the
solution for
a single pool, fixed volume citrate kinetic model (Equation 3):
-150-
CA 02863264 2014-09-12
3) C =C(0) e-exp((1CO3)+1C(0)*t/V)+(G/(1CO3)+K(0))*(1-e-
exp(Kb)+K(0)*tN)
FIG. 33 illustrates plasma citrate concentration in the patient during RCA.
The
systemic plasma citrate concentration kinetic curve predicted by Equation 3
can be
obtained by the OSS of the present invention when implemented as a citrate
sensor
in the effluent line of a CRRT circuit. In the clinical setting, all variables
that
determine the shape of the kinetic curve of the individual patient can be
exactly
defined by the treatment operational mode and the CRRT fluid composition(s) as
well
as circuit blood and CRRT fluid flow rates and/or measured by the online blood
bolus dialysance method described herein. The parameters are constant (C(0),
K(o and
G) or near constant (V(d)) at fixed circuit plasma and CRRT fluid flow rates.
The Vol)
can be estimated fairly accurately from anthropometric data. Therefore, for a
given
patient, the Kb value can be mathematically derived from the systemic citrate
concentration curve imaged by the online citrate sensor according to the
present
invention. Any subsequent change in the K(b) will result in predictable
changes in the
systemic plasma citrate concentration and will be detected in real time by
monitoring
this variable, C(s), by the OSS. Clinically important predictions of Equation
3 are as
described below.
In steady state when the systemic citrate concentration is constant, the
citrate load is equal to citrate removal, Equation 4:
4) G =
It follows that C(õõdy) is defined only by the CRRT treatment settings
defining G and
IC0) and the patient's citrate metabolism K.0,) and is not influenced by the
citrate
volume of distribution, Equation 5:
5) C(...dy)=G/a1C(I,)+K(0)
It is then shown that if a CRRT prescription is provided that achieves more
than 70%
single pass clearance of the anticoagulant citrate infusion on the filter,
proportionally
keeping G low and Ka) high, the queady) cannot exceed 2 mmol/L even if K(1,)
is zero
-151-
CA 02863264 2014-09-12
(consistent with no metabolism of citrate by the patient's liver), regardless
of the
magnitude of prescribed clearance goals (if Cinf is around 5-6 mM). Such a
prescription is important to the safety of RCA in RRT when the liver function
is
either unknown or is known to be severely compromised. Such prescriptions in
current clinical practice are limited to the mainly diffusive treatments of
CWHDF
with high dialysate flow rates and c-SLED. Safe prescriptions based on purely
convective clearance accomplishing high treatment goals are provided for the
first
time by the dosing programs of the RCA system and method according to the
present
invention.
When a CRRT prescription is used that allows for dangerous citrate
accumulation if the patient's liver is not metabolizing citrate (such
prescriptions are
more fluid and cost efficient and could be used for about 90% of patients who
do not
have liver failure), it is important to know clinically how long an individual
patient
needs to be monitored closely after the initiation of RCA for CRRT to reliably
determine whether he or she can metabolize the infused citrate, particularly
if the
online citrate sensor of the present invention is not used. Assuming the
systemic
citrate concentration at the start of the RCA, Co) is zero, the time to reach
90% of
the predicted Cdy) based on the assumed liver function, T(90%) can be
calculated, as
in Equation 6:
6) T(90%)= (V00*ln(10))/(K00+ K(0)
This equation shows that a patient with liver failure with K00 zero, large ECV
(V(d))
and a CRRT prescription with a low clearance goal (and resultant low G and KO
may take up to 5-10 hours to reach toxic citrate levels, but nevertheless will
reach
these levels eventually. Monitoring should cover this period and adequacy of
liver
metabolism should not be concluded based on relatively low citrate levels in
the first
few hours of treatment. Using these concepts, all patients with insufficient
liver
function at start can be correctly identified in the first few hours of RCA
with CRRT,
particularly when the OSS is used to monitor citrate levels and Ku,) in real
time.
-152-
CA 02863264 2014-09-12
An example of imaged systemic citrate plasma concentration curves
as a function of time are provided in FIG. 34a (normal operation of CRRT with
RCA). FIG. 34a illustrates citrate concentration measured by a citrate sensor
in the
drain circuit of an RRT machine utilizing RCA with fixed CRRT prescription
settings
that result in the development of a citrate steady state determined by the
CRRT
settings and the patient's citrate metabolism. FIG. 34b
illustrates citrate
concentration measured by a citrate sensor in the drain circuit of a dialysis
machine
utilizing RCA. When the patient experiences hepatic failure and no longer
metabolizes citrate, the steady state is disrupted and the plasma and
ultrafiltrate
citrate concentration will increase until another markedly higher steady-state
citrate
level is reached. The magnitude of change in the citrate level will depend on
the
CRRT settings. In FIG. 34b, when after a period of normal operation of RCA
with
CRRT the liver function of the patient changes (deteriorates) suddenly (for
instance,
a previously stable patient tolerating RCA for CRRT well may develop liver
failure
after a cardiac arrest and resuscitation and subsequent citrate accumulation
in as little
as one to two hours if the RCA for CRRT is continued). Such a complication
would
not be detected in time with only routine every-six-hour monitoring of total
and
ionized calcium levels, as is the current clinical practice. This is where the
unique
value of the online citrate sensor according to the present invention as a
safety device
may be fully realized and demonstrated.
Finally, knowing the value of Ko,) in real time has other benefits as
well. For example, it also means that citrate conversion into bicarbonate in
the
patient's body can be calculated exactly, allowing accurate determinations of
bicarbonate mass balance during RCA for CRRT (the greatest precision is
provided
by the RCA system where all solute movement is convection based and all fluid
flows
are provided flexibly, but in tight coordination and are continuously
monitored by the
hematocrit and Doppler sensors and the volumetric pumps of the system). This
allows for exact calculation of net bicarbonate gained or lost through the RCA
for
CRRT procedure with its implications for the patient's acid-base balance.
The use of the OSS according to the present invention to monitor liver
function through citrate levels, glomerular filtration rate through inulin
levels and
-153-
CA 02863264 2014-09-12
renal tubular secretory function through PAH levels is now described. For all
of
these applications, the present invention provides a method of keeping the
single pass
filter extraction of the measured solute below 50% to increase the sensitivity
of the
method.
For monitoring liver metabolic function with the OSS, the OSS will
obtain a kinetic curve of systemic plasma citrate levels as described above
and once
a steady state is reached, will continuously display the C(,teady) value of
systemic
citrate. As shown in Equation 5:
5) C(steadY)CO) +1S0)
Icb) is the liver clearance of citrate and has been measured to be between 0.2-
0.5
L/min in ICU patients. The value of ISo will be 0.03-0.07 L/min with CRRT
clearance goals in the range of 20-35 ml/kg/hour as current clinical practice.
Since
K(1,) is almost 10-fold greater than Km, even small percent changes in IC(b)
will be
sensitively reflected in the C(teady) value if the single pass citrate
extraction on the
filter is 50% or less. This makes the systemic steady state citrate level an
excellent
marker of liver perfusion and metabolic function. Sudden decreases in liver
function
will be reflected in the imaged systemic citrate level almost immediately
(FIG. 34b),
alerting the health care team to this complication hours before derangements
of blood
clotting (INR) or alterations in other liver function tests could be expected.
The only
currently available, distantly similar clinical method to image the liver
metabolic
function, the ICG-Pulsion device is based on a bolus IV injection and
subsequent
selective liver clearance of a fluorescent label conjugated ICG molecule with
the
transcutaneous imaging of the washout of the fluorescent label from the
patient's
circulation. This application is costly, it has caused adverse reactions, and
it does
not provide continuous 24-hour monitoring and so far has failed to gain a wide
user
base.
Monitoring renal function with the OSS with inulin and para-amino-
hippuric acid (PAH) can also be accomplished according to the present
invention.
Traditionally, inulin has been the "gold standard solute" used to monitor
glomerular
-154-
CA 02863264 2014-09-12
filtration rate in human renal research protocols. Inulin is an inert
polysaccharide of
varying molecular size that is non-toxic, not metabolized by the human body
and is
eliminated solely by glomerular filtration without any tubular secretion or
reabsorption. According to the present invention, inulin may be introduced
into the
CRRT circuit (and the patient) with the anticoagulant infusion pre-filter and
the exact
same kinetic modeling used as provided for citrate to describe its
accumulation and
elimination; therefore according to Equation 5:
5) C(8(cadY)= Gi(((b) + K(0)
The order of magnitude of the targeted C(,y) value will be defined by the
sensitivity
of the inulin sensor in the effluent line of the CRRT circuit and can be
achieved by
carefully correlating the concentration of inulin in the anticoagulant
infusion with the
infusion rate. If a simple, sensitive inulin sensor is not available, inulin
can also be
provided conjugated with a non-toxic fluorescent or another chemical label for
convenient optical detection. One difficulty may be that the K(b) value of
interest for
inulin (the glomerular filtration rate of the patient in acute renal failure
on CRRT)
will fall in the range of 0.000 to 0.050 L/min. Obviously, most patients' GFR
will
be close to zero initially with the values increasing when recovery of renal
function
is occurring. At the same time, the IS0 will be around 0.03-0.07 L/min with
CRRT
clearance goals in the range of 20-35 ml/kg/hour as mentioned above. Since the
monitored parameter IC(,) is smaller than Ko) (a fixed value with a fixed CRRT
prescription), the C(steady) inulin will be a less sensitive marker of GFR and
recovery
of renal function than citrate levels are of liver function. One way to
improve the
sensitivity of the method is by using inulin enriched in larger inulin
polymers (up to
molecular weight of 10-60 kiloDaltons) that may have a significant sieving
phenomenon on the hemofilter but not in the glomerulus, and correspondingly
may
have a markedly reduced Km when compared to standard inulin with mostly
smaller
polysaccharide oligomers. As an added benefit, the detection of large
molecular
weight inulin can be used as an online clearance-monitoring tool for middle
and large
molecular weight solutes (for instance to predict the continued effectiveness
of
convective cytolcine removal in sepsis). Such monitoring technology does not
exist
in current clinical practice.
-155-
CA 02863264 2014-09-12
While these maneuvers to improve the sensitivity of the inulin-based
method to monitor the recovery of renal function may work, the simultaneous
use of
para-amino-hippuric acid (PAH) or another, water soluble and ultrafilterable,
non-
toxic small solute undergoing extensive tubular secretion in the kidney, may
be
5) C(,,eady)= G/(IC(b) + Km)
PAH is a small organic acid solute that is non-toxic, cleared exclusively by
the
kidneys and has been extensively used in renal research protocols. Its IS0
will be
around 0.03-0.07 L/min with CRRT clearance goals in the range of 20-35
nil/kg/hour, similar to citrate. However, PAH is cleared by the kidneys by
both
-156-
CA 02863264 2014-09-12
The OSS according to the present invention may also be implemented
as a comprehensive safety module to provide online, truly continuous display
of the
systemic plasma total calcium, magnesium and citrate levels during any
implementation of extracorporeal blood purification using regional citrate
anticoagulation. Several RRT systems have been described herein that can
provide
RRT with RCA safely. In these systems, appropriately designed fluid
compositions
and carefully programmed fluid flows ensure a predictable and neutral calcium
and
magnesium mass balance, and in default operational modes preclude the
development
of citrate accumulation even if the patient has liver failure. However, more
replacement fluid efficient (and thereby more economic) prescriptions can be
used for
about 90% of patients who can metabolize citrate. The clinical problem is that
patients can deteriorate and stop metabolizing citrate at any time during
their
treatment course. Online citrate level monitoring is therefore necessary with
such
prescriptions and can be implemented as described herein. Stable filter
performance
is important to the safety of diffusion based RRT with RCA prescriptions. The
OCM
according to the present invention may be used; however, it provides only
indirect
information on citrate clearance that may not suffice for the higher safety
prerequisites of home RCA protocols. Finally, calcium and magnesium levels are
maintained through the maintenance of mass balance in the RRT circuit.
However,
even with the best design, a catastrophic system failure may occur, one
example
being the puncture of the calcium plus magnesium replacement infusion line
with
subsequent failure to infuse these ions into the patient as needed. When high
blood
flows are utilized, such a system failure could lead to life threatening
hypocalcemia
within 10-20 minutes. Routine laboratory monitoring every 6 hours as done in
current clinical practice will not be able to detect such a problem in a
timely manner.
Therefore, real time (online) monitoring of calcium and magnesium levels in
the
systemic plasma of the patient is needed.
The present invention provides a novel, mathematically exacting,
continuous monitoring method to address the above problem. The method utilizes
the knowledge that the composition of the patient's systemic plasma can be
back-
calculated from the composition of the ultrafiltrate, knowing exactly what
composition fluids and in what amounts were infused into the systemic blood in
the
-157-
CA 02863264 2014-09-12
arterial limb of the circuit before ultrafiltration and the sieving of
individual solutes
on the filter. This data is readily available in a given treatment. These
calculations
have been described previously herein, including corrections for access
recirculation,
when present. The method also utilizes the simultaneous measurement of ionized
citrate and ionized calcium and/or ionized magnesium and/or any of their
complexes
with citrate. The final method and detections used may differ slightly from
what is
described herein based on future clinical experience, but the method according
to the
present invention includes the simultaneous measurement of the relevant
interacting
cations and anions. The method also utilizes the application of chemical and
mathematical principles governing the interactions of these ions in the
ultrafiltrate
(these interactions have been elucidated in detail in the literature) with the
specific
purpose to derive the total calcium and total citrate levels in the
ultrafiltrate in real
time for safety monitoring of the RCA for CRRT procedure without any need to
interrupt or modify the citrate anticoagulation.
The back-calculation of plasma concentration from ultrafiltrate
concentration may be accomplished in CVVH and the calculations used are
displayed
in FIGS. 29a-29c for a solute that distributes only into the plasma volume and
not
into the red blood cells. Calcium and citrate both distribute in this way. The
calculations can also be performed when diffusion-based clearance is used
(dialysis)
or when a mixture of dialysis and convection is used (hemodiafiltration), and
are not
discussed here as they are known to those skilled in the art using the general
concept
of dialysance. The use of the online clearance function of the citrate sensor
according to the present invention will verify through the measured D&A, (and
by
using the separately measured R) the accuracy of predictions of solute
movement on
the filter based on theoretical calculations under these more complex
circumstances.
Ionized calcium in the ultrafiltrate can be measured with a calcium
selective electrode. Such electrodes are in routine clinical use today and
could be
easily adapted to be inserted into the CRRT circuit effluent line.
Unfortunately, these
electrodes can be fairly error prone, require regular calibration and testing
for
accuracy and, with prolonged use, the electrode solutions will get depleted
requiring
maintenance or replacement of the electrode. While a calcium electrode may be
-158-
CA 02863264 2014-09-12
used, the present invention also contemplates the use of no-maintenance,
possibly
disposable optical calcium sensors (optrodes or chemical-optical chips) for
the
calcium sensor. Such optrodes have been described in the literature and have
many
advantages over traditional calcium electrodes. Magnesium movement in the CRRT
circuit parallels calcium movement. Magnesium replacement is also completely
coordinated with calcium by virtue of being in the same replacement infusion
solution. Therefore, only one of the two ions needs to be monitored during
CRRT
as there is not even any theoretical possibility of only one ion level
becoming
abnormal separate from the other as a consequence of the RRT procedure (it
could,
however, occur as a consequence of rare clinical situations and stemming from
derangements in the patient's physiology). Nevertheless, if clinically
desirable,
duplicate monitoring could be done with a magnesium selective electrode or
preferably with a magnesium selective optrode or chemical-optical chip.
One problem inherent to the measurement of calcium or magnesium
by any method online (electrode or optrode or other) is that these methods
detect the
ionized Ca 2+ or Me+ species. Unfortunately, in the citrate-rich CRRT
effluent, 80-
90% of calcium is bound by citrate and is not available for measurement as the
ionized form. To circumvent this, periodic cessation of citrate infusion into
the
circuit could be considered but is not especially feasible as it involves the
risk of
clotting. It would also have to be done every 5-10 minutes at the highest
blood flows
targeted by the RCA system. The neutralization of the chelating effect of
citrate by
either eliminating it on an anion exchange resin or by acidifying the effluent
to about
pH 2 are both cumbersome and predictably prone to errors. Fortuitously, the
detailed understanding of the chemical interactions of various ions in the
ultrafiltrate
affords a convenient and precise solution to this problem without the above
undesirable maneuvers.
The solution requires the additional measurement of the free, 3-valent
negatively charged citrate anion in the ultrafiltrate. This may be
accomplished most
conveniently by the method discussed earlier herein regarding the citrate
sensor (see
Parker et al. above). In that publication, the effect of competing divalent
metal ions
on citrate binding to the detecting complex were not investigated, but it is
highly
-159-
CA 02863264 2014-09-12
likely that the europium-ligand complex will only bind the free, trivalent
negative
citrate anion with high affinity and therefore will be eminently suitable for
its
selective detection in the CRRT effluent. In addition, the present invention
also
envisions the possible use of other citrate detection methods. The most
promising
alternative may be the method described by Anslyn et al. to detect calcium and
citrate
simultaneously and or building citrate optrodes where the citrate anion is
bound by
a specific receptor protein or antibody that was engineered to act as a
molecular
switch to transmit a fluoroprobe generated optical signal upon binding with
citrate.
The receptor peptide may have to be modified with molecular mutagenesis to
optimize its specificity for the target trivalent citrate anion and increase
pH
independence of the binding in the range 6.5-7.5. Such molecular engineering
is
certainly feasible with currently available biotechnology. In general, all
possible
technologies that could be adapted for simple and inexpensive measurement of
citrate
in the effluent are fully contemplated for use with the method according to
the present
invention. It may also be possible to engineer receptor peptides that
selectively bind
the Mg-citrate and or Ca-citrate complex enabling their independent
measurement.
Finally, one or more different citrate sensors could be deployed
simultaneously.
The CRRT effluent fluid contains a multitude of positively and
negatively charged anions, many of which will interact and form complexes with
each other. For the purpose of safety monitoring of the RCA for CRRT
procedure,
the quantitatively most important positive ions are sodium, calcium and
magnesium
and the quantitatively most important anions are chloride, bicarbonate,
citrate,
phosphate and lactate. The chemical principles governing the interactions of
these
anions in human plasma and ultrafiltrate were described in a series of classic
physiological experiments (see Walser, J. Phys. Chem. 1961, 65, 159; Walser,
Journal of Cellular & Comparative Physiology. 55:245-50, 1960 Jun; Walser, J
Clin
Invest. 1961 April; 40(4): 723-730). The scientists used ultrafiltration of
plasma as
a research tool; extracorporeal blood purification for renal replacement
therapy was
in its infancy at the time. The implications of the published science for RCA
seem
to have not been recognized to date. Following from the published work, the
measurement of total calcium in the ultrafiltrate is accomplished as follows
in
-160-
CA 02863264 2014-09-12
Equation 7:
7)KCaCit ((Ca2+)free * (Cit3')frõ.)/(CaCir)
-
Where:
KCaCit- = the dissociation constant of the ionic calcium-citrate complex
(constant);
(Ca2+)free = the free ionized calcium concentration (measured by the calcium
sensor)
(Cit3-)f = the free ionized trivalent citrate concentration (measured by the
citrate
sensor)
(CaCir) = the calcium-citrate ionic complex with a single negative charge
The dissociation constant has a fixed value at a given temperature and ionic
strength
of the solution. Since the human plasma has a very narrow range of acceptable
(compatible with life) ion concentrations for all major ionic species and
since the pre-
filter fluids also have a near physiological composition (except for the
presence of
citrate), the ionic strength of the CRRT effluent can be considered constant
and
eliminated as a variable. Also, the warming of the replacement fluid ensures
that the
temperature of the ultraffltrate is kept constant near 37 C. Therefore, the
Kaicit.
dissociation constant will be a fixed value under the operating conditions of
CRRT.
This allows us to rearrange Equation 7 to express the amount of calcium
complexed
with citrate in Equation 7*:
7*) (CaCir) = ((Ca2+)f,,. *
(Ce)free)/Kcacit-
It is of note that all variables on the right side above are measured or
constant,
therefore (CaCir) can be expressed continuously in real time. The effluent Ca
also
exists in complex with phosphate, lactate and bicarbonate. Complex formation
with
chloride does not occur. However, complex formation with phosphate will be
minimized by keeping the effluent pH around 6.6-7.0 (by using acid citrate
anticoagulant) at which pH most phosphate will be in the H2PO4- form that does
not
bind calcium in a significant manner. The amount of calcium bound to
bicarbonate
and lactate is minimal and constant, and at worst can be accounted for by a
constant
correction factor in the equation (designated Fa.). Finally, the impact of
high
-161-
CA 02863264 2014-09-12
clearance CRRT will serve to normalize and standardize bicarbonate, phosphate
and
all other organic anion and possibly even lactate concentrations in the plasma
and
ultrafiltrate after a few hours of operation. Therefore, the target variable,
the total
calcium concentration in the effluent can be expressed as follows in Equation
8:
8) (Ca)1õte = (Ca2+)fi, + (CaCir) + Fa
This can be rearranged using Equation 7* to yield Equation 8*:
8*) (Ca)õ = (Ca2+)f * (1 + ((Cie-)free/Kcacit-)) + Fa
(The Fa, is a minor constant factor to account for calcium bound to other
anions
(bicarbonate, lactate, phosphate, others) that is included for sake of
completeness but
is likely to be clinically not relevant.)
Similar determinations can be done for magnesium that behaves similarly to
calcium
except that it may bind with citrate with about 2.5 times as high affinity as
shown in
Equation 9:
9) (M8)tota1 = (Mg2+)1ree * (1 + ((C1t3)frae/Kmgcit.)) FMS
The variables denote the same as for calcium except that magnesium is used as
the
metal ion.
The Kcaci,_ and Kmsci, dissociation constants were previously determined at a
sodium
concentration of 140 mmol/L and at 25 Celsius temperature (see Am J Kidney
Dis.
2005 Mar;45(3):557-64; Curr Opin Nephrol Hypertens. 1999 Nov;8(6):701-7).
Minor adjustments will be needed as the effluent temperature will be around 37
C in
clinical practice. This depends on the heat loss from the effluent fluid
before
contacting the sensor. A heater element on the effluent fluid line may be
deployed
to ensure standard measurement conditions.
-162-
CA 02863264 2014-09-12
Finally, with the total effluent calcium and/or magnesium
concentration determined with clinically satisfactory accuracy, the back-
calculation
to the systemic plasma value can be performed immediately as described in
FIGS.29a-29c and as will be apparent to those skilled in the art. (If both
calcium and
As far as the measurement of total citrate in the CRRT effluent is
considered, similar principles can be used to derive this value. For the
complete and
detailed explanation of the calculations, see Walser et al as described above.
The
10) (Cit)õ = (Cit3-)free * (1 + ((Nelf _
=roc_J -K
-NaCit2) OCa2+)free/Kcici0
((Mg2+)free/KMgCit-))
Where the variables are:
(Cit)tota, is the total citrate concentration of the effluent;
(Na*) free = the free ionized sodium concentration of the effluent (after a
few hours
of operation of the CRRT procedure this will be normalized to a constant at
140
mmol/L and can also be derived if necessary by measuring the conductivity of
the
KNaCit2- = the dissociation constant of the ionic sodium-citrate complex
(constant);
Kcac,t. = the dissociation constant of the ionic calcium-citrate complex
(constant);
Kmgcit- = the dissociation constant of the ionic magnesium-citrate complex
(constant);
-163-
CA 02863264 2014-09-12
In the clinical setting, the contribution of sodium will be constant and
will likely not need to be measured, just expressed with a constant correction
factor
(may be denoted as an optional Fa). The most scientifically accurate
determination
of the total citrate level in the effluent requires the simultaneous
measurement of the
free ionized calcium, free ionized magnesium and the free trivalent ionized
citrate.
However, as discussed previously herein, the movement of magnesium is
completely
coordinated with calcium in the CRRT circuit according to the present
invention.
Therefore, as long as the magnesium supplement is exclusively provided as a
combined, fixed ratio infusion with calcium, the contribution of magnesium
bound
citrate can be derived from measuring the calcium only, assuming that the
ratio of
total effluent calcium to total effluent magnesium will be equal to the ratio
of calcium
to magnesium in the combined replacement infusion. This is explained below.
Equation 11 (valid under steady state and without gross perturbations of body
calcium or magnesium balance):
11) (Ca)tota1i(M8)1ou1 = 'Ca/Mg
By rearranging the above, we get Equation 11*:
11*) (M8)1ota1 = (Ca)toud/Rums
Where the new variable is:
Rcamg = the fixed molar ratio of calcium and magnesium in the replacement
fluid
(around 2-2.5; the exact value will be chosen after extensive clinical
testing)
8*) (Ca)t.õõ = (Ce+)free * (1 + ((Cie)f,,/Kcacit.)) +
9) (Mg)tote = (Me+)free * (1 + ((Cithf /K + F
'ree ¨ Mg
Therefore, the solution in Equation 12 yields:
12) (mg2+)free = goca2+),ree * + ((c1t3)froccac,0) + FcjiRoilmd - Fmgyo
+
((Cie)free/Kmgc;,_))
-164-
CA 02863264 2014-09-12
The so derived (Me)free than can be inserted into Equation 10 (in lieu of a
measured
value) to determine the total citrate concentration. An alternative is the
measurement
of free ionized magnesium and the derivation of calcium along the same
principles,
as in Equation 13:
13) (Ce = ((((Me) free
* (1 + ((Cie)f/Kmscit.)) + Fmg)/Rmsta,) - Fa,)/(1
+ Cielfree/Kcacit_
Where Rmsica = the fixed molar ratio of magnesium and calcium in the
replacement
fluid and naturally:
14) Rcemg = 1/Rmgica
However, since in rare clinical conditions, for example in hypercalcemia of
malignancy or hungry bone syndrome, the mass balance of calcium and magnesium
can become dissociated inside the patient's body, and also because of the
vital
importance of systemic ionized calcium, ionized calcium monitoring may be
always
performed with or without ionized magnesium monitoring).
In Equations 12 and 13, the principle was used that when two variables out of
the
three variables of interest (ionized Ca2+, ionized Mg2+ and ionized trivalent
Cit3-)
are measured, the third one can be calculated using the added information from
equation 11. Using this principle, the free trivalent citrate can also be
calculated,
when the ionized Ca and ionized Mg is measured as long as Equation 11 applies
as
true (this will be the case in most clinical situations). The solution of
Equations 11,
8* and 9 for the free trivalent citrate concentration yields Equation 15:
15) (Cit3-)free = ORcaims * ((/182+)free + Fmg)) - ((Ca2+)five Fca))
(( Ca2+)free
Kmgcli-) - ()ums * (M82+)free * Kcacit.))/(Kcacit- * Kmgcit))
Equation 15 can be used when a specific citrate sensor is not yet clinically
available
and as long as accurate (Ce+)free and (mg2+)õ, measurements are available with
ion
specific electrodes. Equation 15 further assumes that Equation 11 is true.
This will
-165-
CA 02863264 2014-09-12
be the case when there is no pathophysiologic process present in the patient's
body
that would result in the body absorption or release of calcium or magnesium in
a ratio
different from the ratio of these ions in the calcium plus magnesium
replacement
infusion fluid. As long as the systemic plasma ionized calcium is maintained
around
the physiologic 1.25 mmol/L, this will be true for the large majority of
patients.
Overall, as stated earlier, the simultaneous measurement of the free
ionized calcium, free ionized magnesium and the free trivalent ionized citrate
may
provide the best method of monitoring. However, Equation 15 can be used with
commercially available calcium and magnesium electrodes and is a better
solution to
the problem of citrate monitoring than anything existing in current practice
until an
ionized citrate sensor becomes commercially available. The method according to
the
present invention includes the application of Equations 12, 13 and 15 or
variations
of these equations based on the same principles to continuously compare and
verify
the data provided by the proposed three different sensors. As long all of the
sensors
perform precisely, the measured values of (Ca2+)fiee, (Mg2+)free and (Cit3-)f
should
fulfill the above equations.
Finally, with the total effluent citrate concentration determined with
clinically satisfactory accuracy, the back-calculation to the systemic plasma
value can
be performed immediately as described in FIGS. 29a-29c and as is apparent to
those
skilled in the art.
The above calculations are obviously not meant to be performed by the
clinician at the bedside. However, when the OSS according to the present
invention
delivers the above measured values in real time, a small computer integrated
into the
OSS can easily be programmed to process the data as above and display the
effluent
values in real time. The calculation of the systemic plasma values then
requires the
OSS to have information about the treatment settings (fluid flows and
composition).
This data can be provided by integrating the OSS into the CRRT device or could
be
entered manually (as these variables typically do not need frequent changes
during
the RCA for CRRT procedure) if the OSS is implemented as a stand-alone citrate
and
calcium sensing safety device.
-166-
CA 02863264 2014-09-12
The OSS according to the present invention may improve the safety
of RCA for RRT as follows. The generalized concept of the OSS is outlined for
the
safe monitoring of any water soluble, filterable substance in the effluent
line of the
OSS circuit that is either normally present in the body or is introduced by IV
infusion
through the fluid infusion pathway of the CRRT circuit or by other means. One
immediately feasible specific example is the online monitoring of tight
glycemic
protocols. The OSS as a citrate sensor may be designed as a safety monitor for
real-
time, online detection of citrate accumulation in the patient who is receiving
RCA
during the delivery of CRRT whether in the form of continuous veno-venous
hemofiltration (CVVH), continuous veno-venous hemodialysis (CVVHD) or
continuous veno-venous hemodiafiltration (CVVHDF). The sensor eliminates the
need for frequent laboratory testing to detect this complication and is
equally
adaptable to intermittent hemodialysis (IHD) and continuous sustained low
efficiency
dialysis (c-SLED) performed with RCA as well.
The OSS as a citrate sensor may be designed to provide real-time,
online clearance measurements (FIG. 34c) for any type of blood purification
based
renal replacement therapy that utilizes RCA including CVVH, CVVHDF, CVVHD,
and SLED as well as ND. FIG. 34c illustrates an online filter clearance and
patency
monitor according to the present invention, where the citrate concentration is
measured by a citrate sensor in the drain circuit of an RRT machine utilizing
RCA.
Increasing the infusion of citrate into the blood entering the CRRT circuit
for a short
period of time produces a corresponding response in the citrate measured in
the drain
circuit. Data from the transient increase in citrate concentration can then be
used to
determine the dialyzer citrate clearance online. Accuracy may be superior to
the
existing online clearance monitoring technology based on conductivity
measurements,
depending on the resolving accuracy of the citrate sensor implementation.
The OSS as a citrate plus calcium (and magnesium) sensor may be
designed to provide data that allows accurate, real-time display of the
patient's
systemic calcium, magnesium and citrate levels for safety and the dosing of
calcium
and magnesium replacement infusions appropriate for the losses of these ions
through
the CRRT circuit, thereby reducing and likely obviating the need for frequent
-167-
CA 02863264 2014-09-12
calcium and magnesium monitoring during the CRRT procedure. It also allows for
the mathematically exact derivation of the individual patient's rate of
citrate
metabolism, which in turn allows the selection of the most appropriate RCA
fluid
compositions for the patient to maintain acid-base balance.
The OSS according to the present invention may be designed to
monitor organ function in real time with specific examples of monitoring liver
function through citrate metabolism and monitoring glomerular filtration rate
and
renal tubular function through the detection of inulin and PAH levels in the
effluent
of CRRT circuits. The ability to monitor liver metabolic function in real time
can
be of great benefit in acute- or chronic liver failure and pre- and post-liver
transplantation. The ability to monitor glomerular filtration rate and or
renal tubular
secretory function will be of great importance in the future, when assessing
when to
stop CRRT because of recovery of renal function will be a clinical challenge
in non-
oliguric ARF patients. These patients will all have normal serum chemistries
on
CRRT as a result of the high CRRT clearance goals gradually becoming the
standard
of practice. The OSS can monitor any organ, including the heart as long as a
water-
soluble, filterable compound is identified that is cleared exclusively by the
target
organ at a rate in excess of its clearance through the extracorporeal blood
circuit.
The OSS of the present invention as a citrate, calcium and magnesium
sensor will allow RCA for RRT to be delivered safely with possibly no
intervention
and monitoring from health care personnel. The OSS as part of the RCA home
system with OCM and OSS with single needle access will represent a major
safety
improvement over any home RRT machine and will allow RCA (that is more
powerful than heparin and has no systemic bleeding effects) to enter the home
setting
for nocturnal renal replacement therapies.
While embodiments of the invention have been illustrated and
described, it is not intended that these embodiments illustrate and describe
all
possible forms of the invention. The scope of the claims should not be
limited by particular embodiments set forth herein, but should be construed
in a manner consistent with the specification as a whole.
-168-