Note: Descriptions are shown in the official language in which they were submitted.
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
ENHANCING FERMENTATION OF STARCH- AND SUGAR-BASED
FEEDSTOCKS
CROSS-REFERENCE
[0001] This application claims the benefit of U.S. Patent Application Serial
No. 13/731,633,
filed December 31, 2012, U.S. Provisional Application No. 61/597,347, filed
February 10,
2012, and U.S. Provisional Application No. 61/648,567, filed May 17, 2012,
each of which
application is incorporated herein by reference in its entirety.
BACKGROUND OF THE INVENTION
[0002] The demand for renewable fuels and chemical is growing significantly
and is required
to reduce reliance on petroleum-based products and to lower gas emissions. At
the same time,
interest in biofuels, such as ethanol, as an alternative to petroleum has
greatly increased, in
part due to the desire to promote domestic rural economics. Ethanol is the
most commonly
used biofuel, and current U.S. biofuel is almost exclusively derived from
corn. To meet some
of the higher ethanol production goals would require more corn than the United
States
currently produces. At the same time, a concern over the use of food crops for
fuel is an
obstacle to use of further corn for ethanol. Another obstacle to widespread
adoption of
biofuels and bio-products is the economic cost of producing the ethanol. A
major contributing
factor to this cost is transportation of biomass feedstock from the location
where feedstock is
grown to the location where it is processed into biofuels and chemicals. More
efficient use of
starch fermentation, supplemented with sugars from non-starch cellulosic
materials would
help defray these costs. Although the supplementation of cellulosic sugars
assists in starch
production, it would be helpful to optimize this process in a manner wherein
fermenting
organisms can utilize the maximum amount of sugar provided to them.
SUMMARY OF THE INVENTION
[0003] Disclosed herein are methods of producing one or more fermentation end-
products
comprising: (a) combining a first biomass comprising non-cellulosic
saccharides with a
saccharide stream comprising C6 monosaccharides to produce a blended feedstock
in a broth;
(b) contacting the blended feedstock with one or more biocatalysts; (c)
fermenting the non-
cellulosic saccharides of the first biomass and the C6 monosaccharides of the
saccharides
stream to produce one or more fermentation end-products comprising an alcohol
and a
polyol, wherein an alcohol yield is increased and a polyol yield is decreased
in comparison to
fermentation of the first biomass without the saccharide stream. In some
embodiments, the
alcohol is ethanol. In some embodiments, the polyol is glycerol. In some
embodiments, the
1
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
broth comprising the blended feedstock has a lower osmolarity than a broth
containing only
the first biomass at an equivalent total saccharide level in monosaccharide
equivalents. In
some embodiments, saccharide stream further comprises C5 monosaccharides. In
some
embodiments, the C6 monosaccharides comprise cellulosic-derived C6
monosaccharides,
non-cellulosic derived C6 monosaccharides, or a combination thereof. In some
embodiments,
the alcohol yield is increased by about 1% to about 100% relative to
fermentation of the first
biomass without the saccharide stream. In some embodiments, the alcohol is
produced at a
rate that is faster relative to fermentation of the first biomass without the
saccharide stream.
In some embodiments, the alcohol is produced at a rate that is about 1% to
about 100% faster
relative to fermentation of the first biomass without the saccharide stream.
In some
embodiments, the C6 monosaccharides of the saccharide stream are at a
concentration that
differs from a concentration of the non-cellulosic saccharides in the first
biomass by less than
+/- 50%, wherein the concentration of non-cellulosic saccharides in the first
biomass is in
monosaccharide equivalents. In some embodiments, the C6 monosaccharides of the
saccharide stream are at a concentration that differs from a concentration of
the non-
cellulosic saccharides in the first biomass by less than +/- 25%, wherein the
concentration of
non-cellulosic saccharides in the first biomass is in monosaccharide
equivalents. In some
embodiments, the method is a fed-batch fermentation wherein the saccharide
stream is added
over time. In some embodiments, the saccharide stream is added at a rate of
from about 0.01
mL/min/L of broth to about 5 mL/min/L of broth during the fermenting. In some
embodiments, the polyols yield is decreased by about 1% to about 100% relative
to the non-
fed batch fermentation. In some embodiments, the first biomass comprises
starch, sucrose, or
a combination thereof. In some embodiments, the first biomass comprises corn,
corn mash,
sugar cane, sugar beets, sugar palms, sweet sorghum, nypa palm, cassava, rice,
milo,
sorghum, sweet potatoes, wheat, molasses, tubers, roots, stems, whole grains,
barley, rye,
milo, sago, cassaya, tapioca, rice peas, beans, potatoes, beets, fruits, or a
combination thereof.
In some embodiments, the saccharide stream is produced from the pretreatment
and/or
hydrolysis of cellulose, hemicellulose, lignocellulose material, starch, or a
combination
thereof. In some embodiments, the saccharide stream is produced by the
pretreatment and/or
hydrolysis of a second biomass comprising cellulose, hemicellulose, and/or
lignocellulose. In
some embodiments, the second biomass comprises corn, corn syrup, corn stover,
corn cobs,
molasses, silage, grass, straw, grain hulls, bagasse, distiller's grains,
distiller's dried solubles,
distiller's dried grains, condensed distiller's solubles, distiller's wet
grains, distiller's dried
grains with solubles, wood, bark, sawdust, paper, poplars, willows,
switchgrass, alfalfa,
2
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
prairie bluestem, algae, fruit peels, pits, sorghum, sweet sorghum, sugar
cane, switch grass,
rice, rice straw, rice hulls, wheat, wheat straw, barley, barley straw,
bamboo, seeds, seed
hulls, oats, oat hulls, food waste, municipal sewage waste, or a combination
thereof. In some
embodiments, the one or more biocatalysts comprise one or more fermenting
microorganisms. In some embodiments, the one or more biocatalysts comprise one
or more
yeasts and/or one or more bacteria. In some embodiments, the one or more
biocatalysts
comprise one or more yeasts. In some embodiments, the one or more biocatalysts
comprise
one or more strains of Saccharomyces cerevisiae. In some embodiments, at least
one of the
one or more biocatalysts is a genetically-modified yeast that ferments C5 and
C6 saccharides.
In some embodiments, at least one of the one or more biocatalysts is a
bacteria that
hydrolyzes and/or ferments C5 and C6 saccharides. In some embodiments, at
least one of the
one or more biocatalysts is a hydrolytic enzyme. In some embodiments, the one
or more
biocatalysts comprise an endoglucanase, an exoglucanase, a cellobiohydrolase,
a cellulase, a
beta-glucosidase, a glycoside hydrolase, a glycosyltransferase, a lyase, an
esterase, a
glucamylase, or a combination thereof. In some embodiments, at least one of
the one or more
biocatalysts is an enzyme that hydrolyzes starch. In some embodiments, at
least one of the
one or more biocatalysts is an alpha-amylase, glucoamylase, beta-amylase, exo-
alpha-1,4-
glucanase, or pullulanase. In some embodiments, the one or more biocatalysts
comprise at
least one fermenting microorganism and at least one hydrolytic enzyme. In some
embodiments, the one or more biocatalysts comprise at least one fermenting
microorganism
that is a yeast or bacteria and at least one enzyme that hydrolyzes starch. In
some
embodiments, the blended feedstock comprises less than 100 g/L monosaccharides
prior to
contacting with the one or more biocatalysts. In some embodiments, the blended
feedstock
comprises from about 1 g/L to about 100 g/L monosaccharides prior to
contacting with the
one or more biocatalysts. In some embodiments, the first biomass and the
saccharide stream
are combined in a ratio of from about 50:50 to 99:1 (first biomass: saccharide
stream) by
volume or by weight:volume. In some embodiments, the first biomass and the
saccharide
stream are combined in a ratio of about 80:20 (first biomass: saccharide
stream) by volume or
by weight:volume. In some embodiments, the first biomass and the saccharide
stream are
combined in a ratio of about 90:10 (first biomass: saccharide stream) by
volume or by
weight:volume. In some embodiments, the blended feedstock comprises from about
10% to
about 50% solids from the first biomass. In some embodiments, the blended
feedstock
comprises from about 20% to about 40% solids from the first biomass. In some
embodiments,
the blended feedstock comprises from about 30% to about 36% solids from the
first biomass.
3
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
In some embodiments, the C6 monosaccharides of the saccharide stream are at a
concentration of from about 10% to about 70% w/v prior to combining with the
first biomass.
In some embodiments, the C6 monosaccharides of the saccharide stream are at a
concentration of from about 20% to about 50% w/v prior to combining with the
first biomass.
Also disclosed are fermentation end-products produced by these methods. In
some
embodiments, the fermentation end-product is ethanol.
[0004] Disclosed herein are methods of producing one or more fermentation end-
products
comprising: (a) combining a first biomass with one or more cellulosic-derived
C6
monosaccharides to produce a blended feedstock in a broth; (b) contacting the
blended
feedstock with one or more biocatalysts; and (c) fermenting the first biomass
and the one or
more cellulosic-derived C6 monosaccharides for sufficient time to produce one
or more
fermentation end-products from the blended feedstock, wherein a yield of at
least one of the
one or more fermentation end-products is increased relative to fermentation of
the first
biomass without the one or more cellulosic-derived C6 monosaccharides. In some
embodiments, the yield of the at least one of the one or more fermentation end-
products is
increased by about 1% to about 100% relative to fermentation of the first
biomass without the
one or more cellulosic-derived C6 monosaccharides. In some embodiments, at
least one of
the one or more fermentation end-products is produced at a rate that is faster
relative to
fermentation of the first biomass without the one or more cellulosic-derived
C6
monosaccharides. In some embodiments, at least one of the one or more
fermentation end-
products is produced at a rate that is about 1% to about 100% faster relative
to fermentation
of the first biomass without the one or more cellulosic-derived C6
monosaccharides. In some
embodiments, the one or more cellulosic-derived C6 monosaccharides are at a
concentration
that differs from a concentration of saccharides in the first biomass by less
than +/- 50%,
wherein the concentration of saccharides in the first biomass is in
monosaccharide
equivalents. In some embodiments, the one or more cellulosic-derived C6
monosaccharides
are at a concentration that differs from a concentration of saccharides in the
first biomass by
less than +/- 25%, wherein the concentration of saccharides in the first
biomass is in
monosaccharide equivalents. In some embodiments, the one or more fermentation
end-
products comprise one or more alcohols. In some embodiments, the one or more
fermentation
end-products comprise ethanol. In some embodiments, the method is a fed-batch
fermentation wherein the one or more cellulosic-derived C6 monosaccharides are
added over
time. In some embodiments, the one or more cellulosic-derived C6
monosaccharides are
added at a rate of from about 0.01 mL/min/L of broth to about 5 mL/min/L of
broth during
4
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
the fermenting. In some embodiments, a yield of one or more other products is
decreased
relative to a non-fed batch fermentation. In some embodiments, the yield of
the one or more
other products is decreased by about 1% to about 100% relative to the non-fed
batch
fermentation. In some embodiments, the one or more other products comprise one
or more
polyols or sugar alcohols. In some embodiments, the one or more other products
comprise
methanol, glycol, glycerol, erythritol, threitol, arabitol, xylitol, ribitol,
mannitol, sorbitol,
dulcitol, fucitol, iditol, inositol, volemitol, isomalt, maltitol, lactitol,
polyglycitol, or a
combination thereof In some embodiments, the one or more other products
comprise
glycerol. In some embodiments, the first biomass comprises non-cellulosic
sugars. In some
embodiments, the first biomass comprises non-cellulosic oligosaccharides. In
some
embodiments, the first biomass comprises starch. In some embodiments, the
first biomass
comprises corn, corn mash, sugar cane, sugar beets, sugar palms, sweet
sorghum, nypa palm,
cassava, rice, milo, sorghum, sweet potatoes, wheat, molasses, tubers, roots,
stems, whole
grains, barley, rye, milo, sago, cassaya, tapioca, rice peas, beans, potatoes,
beets, fruits, or a
combination thereof In some embodiments, the one or more cellulosic-derived C6
monosaccharides are produced from the pretreatment and/or hydrolysis of
cellulose,
hemicellulose, or lignocellulose material. In some embodiments, the one or
more cellulosic-
derived C6 monosaccharides are produced by the pretreatment and/or hydrolysis
of a second
biomass comprising cellulose, hemicellulose, or lignocellulose. In some
embodiments, the
second biomass comprises corn, corn syrup, corn stover, corn cobs, molasses,
silage, grass,
straw, grain hulls, bagasse, distiller's grains, distiller's dried solubles,
distiller's dried grains,
condensed distiller's solubles, distiller's wet grains, distiller's dried
grains with solubles,
wood, bark, sawdust, paper, poplars, willows, switchgrass, alfalfa, prairie
bluestem, algae,
fruit peels, pits, sorghum, sweet sorghum, sugar cane, switch grass, rice,
rice straw, rice hulls,
wheat, wheat straw, barley, barley straw, bamboo, seeds, seed hulls, oats, oat
hulls, food
waste, municipal sewage waste, or a combination thereof In some embodiments,
the one or
more biocatalysts comprise one or more fermenting microorganisms. In some
embodiments,
the one or more biocatalysts comprise one or more yeasts and/or one or more
bacteria. In
some embodiments, the one or more biocatalysts comprise one or more yeasts. In
some
embodiments, the one or more biocatalysts comprise one or more strains of
Saccharomyces
cerevisiae. In some embodiments, at least one of the one or more biocatalysts
is a genetically-
modified yeast that ferments C5 and C6 saccharides. In some embodiments, at
least one of
the one or more biocatalysts is a bacteria that hydrolyzes and/or ferments C5
and C6
saccharides. In some embodiments, at least one of the one or more biocatalysts
is a hydrolytic
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
enzyme. In some embodiments, the one or more biocatalysts comprise an
endoglucanase, an
exoglucanase, a cellobiohydrolase, a cellulase, a beta-glucosidase, a
glycoside hydrolase, a
glycosyltransferase, a lyase, an esterase, a glucamylase, or a combination
thereof. In some
embodiments, at least one of the one or more biocatalysts is an enzyme that
hydrolyzes
starch. In some embodiments, at least one of the one or more biocatalysts is
an alpha-
amylase, glucoamylase, beta-amylase, exo-alpha-1,4-glucanase, or pullulanase.
In some
embodiments, the one or more biocatalysts comprise at least one fermenting
microorganism
and at least one hydrolytic enzyme. In some embodiments, the one or more
biocatalysts
comprise at least one fermenting microorganism that is a yeast or bacteria and
at least one
enzyme that hydrolyzes starch. In some embodiments, the blended feedstock
comprises less
than 100 g/L monosaccharides prior to contacting with the one or more
biocatalysts. In some
embodiments, the blended feedstock comprises from about 1 g/L to about 100 g/L
monosaccharides prior to contacting with the one or more biocatalysts. In some
embodiments, the first biomass and the one or more cellulosic-derived C6
monosaccharides
are combined in a ratio of from about 50:50 to 99:1 (first biomass: cellulosic-
derived C6
monosaccharides) by volume or by weight:volume. In some embodiments, the first
biomass
and the one or more cellulosic-derived C6 monosaccharides are combined in a
ratio of about
80:20 (first biomass: cellulosic-derived C6 monosaccharides) by volume or by
weight:volume. In some embodiments, the first biomass and the one or more
cellulosic-
derived C6 monosaccharides are combined in a ratio of about 90:10 (first
biomass: cellulosic-
derived C6 monosaccharides) by volume or by weight:volume. In some
embodiments, the
blended feedstock comprises from about 10% to about 50% solids from the first
biomass. In
some embodiments, the blended feedstock comprises from about 20% to about 40%
solids
from the first biomass. In some embodiments, the blended feedstock comprises
from about
30% to about 36% solids from the first biomass. In some embodiments, the one
or more
cellulosic-derived C6 monosaccharides are at a concentration of from about 10%
to about
70% w/v prior to combining with the first biomass. In some embodiments, the
one or more
cellulosic-derived C6 monosaccharides are at a concentration of from about 20%
to about
50% w/v prior to combining with the first biomass.
[0005] Also provided are fermentation end-products produced by the methods
disclosed
herein. In some embodiments, the fermentation end-product is an alcohol. In
some
embodiments, the fermentation end-product is ethanol.
[0006] Also disclosed herein are methods of producing one or more fermentation
end-
products comprising: (a) combining a first biomass with one or more cellulosic-
derived C6
6
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
monosaccharides to produce a blended feedstock in a broth; (b) contacting the
blended
feedstock with one or more biocatalysts; and (c) fermenting the first biomass
and the one or
more cellulosic-derived C6 monosaccharides for sufficient time to produce one
or more
fermentation end-products from the blended feedstock, wherein at least one of
the one or
more fermentation end-products is produced at a rate that is faster relative
to fermentation of
the first biomass without the one or more cellulosic-derived C6
monosaccharides. In some
embodiments, a yield of at least one of the one or more fermentation end-
products is
increased relative to fermentation of the first biomass without the one or
more cellulosic-
derived C6 monosaccharides. In some embodiments, a yield of at least one of
the one or more
fermentation end-products is increased by about 1% to about 100% relative to
fermentation
of the first biomass without the one or more cellulosic-derived C6
monosaccharides. In some
embodiments, the rate that the at least one of the one or more fermentation
end-products is
produced is about 1% to about 100% faster relative to fermentation of the
first biomass
without the one or more cellulosic-derived C6 monosaccharides. In some
embodiments, the
one or more cellulosic-derived C6 monosaccharides are at a concentration that
differs from a
concentration of saccharides in the first biomass by less than +/- 50%,
wherein the
concentration of saccharides in the first biomass is in monosaccharide
equivalents. In some
embodiments, the one or more cellulosic-derived C6 monosaccharides are at a
concentration
that differs from a concentration of saccharides in the first biomass by less
than +/- 25%,
wherein the concentration of saccharides in the first biomass is in
monosaccharide
equivalents. In some embodiments, the one or more fermentation end-products
comprise one
or more alcohols. In some embodiments, the one or more fermentation end-
products comprise
ethanol. In some embodiments, the method is a fed-batch fermentation wherein
the one or
more cellulosic-derived C6 monosaccharides are added over time. In some
embodiments, the
one or more cellulosic-derived C6 monosaccharides are added at a rate of from
about 0.01
mL/min/L of broth to about 5 mL/min/L of broth during the fermenting. In some
embodiments, a yield of one or more other products is decreased relative to a
non-fed batch
fermentation. In some embodiments, the yield of the one or more other products
is decreased
by about 1% to about 100% relative to the non-fed batch fermentation. In some
embodiments,
the one or more other products comprise one or more polyols or sugar alcohols.
In some
embodiments, the one or more other products comprise methanol, glycol,
glycerol, erythritol,
threitol, arabitol, xylitol, ribitol, mannitol, sorbitol, dulcitol, fucitol,
iditol, inositol, volemitol,
isomalt, maltitol, lactitol, polyglycitol, or a combination thereof. In some
embodiments, the
one or more other products comprise glycerol. In some embodiments, the first
biomass
7
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
comprises non-cellulosic sugars. In some embodiments, the first biomass
comprises non-
cellulosic oligosaccharides. In some embodiments, the first biomass comprises
starch. In
some embodiments, the first biomass comprises corn, corn mash, sugar cane,
sugar beets,
sugar palms, sweet sorghum, nypa palm, cassava, rice, milo, sorghum, sweet
potatoes, wheat,
molasses, tubers, roots, stems, whole grains, barley, rye, milo, sago,
cassaya, tapioca, rice
peas, beans, potatoes, beets, fruits, or a combination thereof. In some
embodiments, the one
or more cellulosic-derived C6 monosaccharides are produced from the
pretreatment and/or
hydrolysis of cellulose, hemicellulose, or lignocellulose material. In some
embodiments, the
one or more cellulosic-derived C6 monosaccharides are produced by the
pretreatment and/or
hydrolysis of a second biomass comprising cellulose, hemicellulose, or
lignocellulose. In
some embodiments, the second biomass comprises corn, corn syrup, corn stover,
corn cobs,
molasses, silage, grass, straw, grain hulls, bagasse, distiller's grains,
distiller's dried solubles,
distiller's dried grains, condensed distiller's solubles, distiller's wet
grains, distiller's dried
grains with solubles, wood, bark, sawdust, paper, poplars, willows,
switchgrass, alfalfa,
prairie bluestem, algae, fruit peels, pits, sorghum, sweet sorghum, sugar
cane, switch grass,
rice, rice straw, rice hulls, wheat, wheat straw, barley, barley straw,
bamboo, seeds, seed
hulls, oats, oat hulls, food waste, municipal sewage waste, or a combination
thereof. In some
embodiments, the one or more biocatalysts comprise one or more fermenting
microorganisms. In some embodiments, the one or more biocatalysts comprise one
or more
yeasts and/or one or more bacteria. In some embodiments, the one or more
biocatalysts
comprise one or more yeasts. In some embodiments, the one or more biocatalysts
comprise
one or more strains of Saccharomyces cerevisiae. In some embodiments, at least
one of the
one or more biocatalysts is a genetically-modified yeast that ferments C5 and
C6 saccharides.
In some embodiments, at least one of the one or more biocatalysts is a
bacteria that
hydrolyzes and/or ferments C5 and C6 saccharides. In some embodiments, at
least one of the
one or more biocatalysts is a hydrolytic enzyme. In some embodiments, the one
or more
biocatalysts comprise an endoglucanase, an exoglucanase, a cellobiohydrolase,
a cellulase, a
beta-glucosidase, a glycoside hydrolase, a glycosyltransferase, a lyase, an
esterase, a
glucamylase, or a combination thereof. In some embodiments, at least one of
the one or more
biocatalysts is an enzyme that hydrolyzes starch. In some embodiments, at
least one of the
one or more biocatalysts is an alpha-amylase, glucoamylase, beta-amylase, exo-
alpha-1,4-
glucanase, or pullulanase. In some embodiments, the one or more biocatalysts
comprise at
least one fermenting microorganism and at least one hydrolytic enzyme. In some
embodiments, the one or more biocatalysts comprise at least one fermenting
microorganism
8
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
that is a yeast or bacteria and at least one enzyme that hydrolyzes starch. In
some
embodiments, the blended feedstock comprises less than 100 g/L monosaccharides
prior to
contacting with the one or more biocatalysts. In some embodiments, the blended
feedstock
comprises from about 1 g/L to about 100 g/L monosaccharides prior to
contacting with the
one or more biocatalysts. In some embodiments, the first biomass and the one
or more
cellulosic-derived C6 monosaccharides are combined in a ratio of from about
50:50 to 99:1
(first biomass: cellulosic-derived C6 monosaccharides) by volume or by
weight:volume. In
some embodiments, the first biomass and the one or more cellulosic-derived C6
monosaccharides are combined in a ratio of about 80:20 (first biomass:
cellulosic-derived C6
monosaccharides) by volume or by weight:volume. In some embodiments, the first
biomass
and the one or more cellulosic-derived C6 monosaccharides are combined in a
ratio of about
90:10 (first biomass: cellulosic-derived C6 monosaccharides) by volume or by
weight:volume. In some embodiments, the blended feedstock comprises from about
10% to
about 50% solids from the first biomass. In some embodiments, the blended
feedstock
comprises from about 20% to about 40% solids from the first biomass. In some
embodiments,
the blended feedstock comprises from about 30% to about 36% solids from the
first biomass.
In some embodiments, the one or more cellulosic-derived C6 monosaccharides are
at a
concentration of from about 10% to about 70% w/v prior to combining with the
first biomass.
In some embodiments, the one or more cellulosic-derived C6 monosaccharides are
at a
concentration of from about 20% to about 50% w/v prior to combining with the
first biomass.
[0007] Also disclosed herein are methods of producing one or more fermentation
end-
products comprising: (a) combining a first biomass with one or more cellulosic-
derived C6
monosaccharides to produce a blended feedstock in a broth; (b) contacting the
blended
feedstock with one or more biocatalysts; and (c) fermenting the first biomass
and the one or
more cellulosic-derived C6 monosaccharides for sufficient time to produce one
or more
fermentation end-products from the blended feedstock, wherein the one or more
cellulosic-
derived C6 monosaccharides are at a concentration that differs from a
concentration of
saccharides in the first biomass by less than +/- 50%, wherein the
concentration of
saccharides in the first biomass is in monosaccharide equivalents. In some
embodiments, a
yield of at least one of the one or more fermentation end-products is
increased relative to
fermentation of the first biomass without the one or more cellulosic-derived
C6
monosaccharides. In some embodiments, a yield of at least one of the one or
more
fermentation end-products is increased by about 1% to about 100% relative to
fermentation
of the first biomass without the one or more cellulosic-derived C6
monosaccharides. In some
9
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
embodiments, at least one of the one or more fermentation end-products is
produced at a rate
that is faster relative to fermentation of the first biomass without the one
or more cellulosic-
derived C6 monosaccharides. In some embodiments, at least one of the one or
more
fermentation end-products is produced at a rate that is about 1% to about 100%
faster relative
to fermentation of the first biomass without the one or more cellulosic-
derived C6
monosaccharides. In some embodiments, the concentration of the one or more
cellulosic-
derived C6 monosaccharides differs from the concentration of saccharides in
the first biomass
by less than +/- 25%, wherein the concentration of saccharides in the first
biomass is in
monosaccharide equivalents. In some embodiments, the one or more fermentation
end-
products comprise one or more alcohols. In some embodiments, the one or more
fermentation
end-products comprise ethanol. In some embodiments, the method is a fed-batch
fermentation wherein the one or more cellulosic-derived C6 monosaccharides are
added over
time. In some embodiments, the one or more cellulosic-derived C6
monosaccharides are
added at a rate of from about 0.01 mL/min/L of broth to about 5 mL/min/L of
broth during
the fermenting. In some embodiments, a yield of one or more other products is
decreased
relative to a non-fed batch fermentation. In some embodiments, the yield of
the one or more
other products is decreased by about 1% to about 100% relative to the non-fed
batch
fermentation. In some embodiments, the one or more other products comprise one
or more
polyols or sugar alcohols. In some embodiments, the one or more other products
comprise
methanol, glycol, glycerol, erythritol, threitol, arabitol, xylitol, ribitol,
mannitol, sorbitol,
dulcitol, fucitol, iditol, inositol, volemitol, isomalt, maltitol, lactitol,
polyglycitol, or a
combination thereof. In some embodiments, the one or more other products
comprise
glycerol. In some embodiments, the first biomass comprises non-cellulosic
sugars. In some
embodiments, the first biomass comprises non-cellulosic oligosaccharides. In
some
embodiments, the first biomass comprises starch. In some embodiments, the
first biomass
comprises corn, corn mash, sugar cane, sugar beets, sugar palms, sweet
sorghum, nypa palm,
cassava, rice, milo, sorghum, sweet potatoes, wheat, molasses, tubers, roots,
stems, whole
grains, barley, rye, milo, sago, cassaya, tapioca, rice peas, beans, potatoes,
beets, fruits, or a
combination thereof. In some embodiments, the one or more cellulosic-derived
C6
monosaccharides are produced from the pretreatment and/or hydrolysis of
cellulose,
hemicellulose, or lignocellulose material. In some embodiments, the one or
more cellulosic-
derived C6 monosaccharides are produced by the pretreatment and/or hydrolysis
of a second
biomass comprising cellulose, hemicellulose, or lignocellulose. In some
embodiments, the
second biomass comprises corn, corn syrup, corn stover, corn cobs, molasses,
silage, grass,
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
straw, grain hulls, bagasse, distiller's grains, distiller's dried solubles,
distiller's dried grains,
condensed distiller's solubles, distiller's wet grains, distiller's dried
grains with solubles,
wood, bark, sawdust, paper, poplars, willows, switchgrass, alfalfa, prairie
bluestem, algae,
fruit peels, pits, sorghum, sweet sorghum, sugar cane, switch grass, rice,
rice straw, rice hulls,
wheat, wheat straw, barley, barley straw, bamboo, seeds, seed hulls, oats, oat
hulls, food
waste, municipal sewage waste, or a combination thereof. In some embodiments,
the one or
more biocatalysts comprise one or more fermenting microorganisms. In some
embodiments,
the one or more biocatalysts comprise one or more yeasts and/or one or more
bacteria. In
some embodiments, the one or more biocatalysts comprise one or more yeasts. In
some
embodiments, the one or more biocatalysts comprise one or more strains of
Saccharomyces
cerevisiae. In some embodiments, at least one of the one or more biocatalysts
is a genetically-
modified yeast that ferments C5 and C6 saccharides. In some embodiments, at
least one of
the one or more biocatalysts is a bacteria that hydrolyzes and/or ferments C5
and C6
saccharides. In some embodiments, at least one of the one or more biocatalysts
is a hydrolytic
enzyme. In some embodiments, the one or more biocatalysts comprise an
endoglucanase, an
exoglucanase, a cellobiohydrolase, a cellulase, a beta-glucosidase, a
glycoside hydrolase, a
glycosyltransferase, a lyase, an esterase, a glucamylase, or a combination
thereof. In some
embodiments, at least one of the one or more biocatalysts is an enzyme that
hydrolyzes
starch. In some embodiments, at least one of the one or more biocatalysts is
an alpha-
amylase, glucoamylase, beta-amylase, exo-alpha-1,4-glucanase, or pullulanase.
In some
embodiments, the one or more biocatalysts comprise at least one fermenting
microorganism
and at least one hydrolytic enzyme. In some embodiments, the one or more
biocatalysts
comprise at least one fermenting microorganism that is a yeast or bacteria and
at least one
enzyme that hydrolyzes starch. In some embodiments, the blended feedstock
comprises less
than 100 g/L monosaccharides prior to contacting with the one or more
biocatalysts. In some
embodiments, the blended feedstock comprises from about 1 g/L to about 100 g/L
monosaccharides prior to contacting with the one or more biocatalysts. In some
embodiments, the first biomass and the one or more cellulosic-derived C6
monosaccharides
are combined in a ratio of from about 50:50 to 99:1 (first biomass: cellulosic-
derived C6
monosaccharides) by volume or by weight:volume. In some embodiments, the first
biomass
and the one or more cellulosic-derived C6 monosaccharides are combined in a
ratio of about
80:20 (first biomass: cellulosic-derived C6 monosaccharides) by volume or by
weight:volume. In some embodiments, the first biomass and the one or more
cellulosic-
derived C6 monosaccharides are combined in a ratio of about 90:10 (first
biomass: cellulosic-
11
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
derived C6 monosaccharides) by volume or by weight:volume. In some
embodiments, the
blended feedstock comprises from about 10% to about 50% solids from the first
biomass. In
some embodiments, the blended feedstock comprises from about 20% to about 40%
solids
from the first biomass. In some embodiments, the blended feedstock comprises
from about
30% to about 36% solids from the first biomass. In some embodiments, the one
or more
cellulosic-derived C6 monosaccharides are at a concentration of from about 10%
to about
70% w/v prior to combining with the first biomass. In some embodiments, the
one or more
cellulosic-derived C6 monosaccharides are at a concentration of from about 20%
to about
50% w/v prior to combining with the first biomass.
[0008] Disclosed herein are systems for producing one or more fermentation end-
products
comprising: (a) combining a fermentor comprising a broth; (b) a blended
feedstock
comprising a first biomass and one or more cellulosic-derived C6
monosaccharides in the
broth; and (c) one or more biocatalysts. In some embodiments, yield of at
least one of the
one or more fermentation end-products is increased relative to fermentation of
the first
biomass without the one or more cellulosic-derived C6 monosaccharides. In some
embodiments, the yield of the at least one of the one or more fermentation end-
products is
increased by about 1% to about 100% relative to fermentation of the first
biomass without the
one or more cellulosic-derived C6 monosaccharides. In some embodiments, at
least one of
the one or more fermentation end-products is produced at a rate that is faster
relative to
fermentation of the first biomass without the one or more cellulosic-derived
C6
monosaccharides. In some embodiments, at least one of the one or more
fermentation end-
products is produced at a rate that is about 1% to about 100% faster relative
to fermentation
of the first biomass without the one or more cellulosic-derived C6
monosaccharides. In some
embodiments, the one or more cellulosic-derived C6 monosaccharides are at a
concentration
that differs from a concentration of saccharides in the first biomass by less
than +/- 50%,
wherein the concentration of saccharides in the first biomass is in
monosaccharide
equivalents. In some embodiments, the one or more cellulosic-derived C6
monosaccharides
are at a concentration that differs from a concentration of saccharides in the
first biomass by
less than +/- 25%, wherein the concentration of saccharides in the first
biomass is in
monosaccharide equivalents. In some embodiments, the one or more fermentation
end-
products comprise one or more alcohols. In some embodiments, the one or more
fermentation
end-products comprise ethanol. In some embodiments, the system further
comprises a feeder
to add the one or more cellulosic-derived C6 monosaccharides to the broth over
time. In some
embodiments, the one or more cellulosic-derived C6 monosaccharides are added
at a rate of
12
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
from about 0.01 mL/min/L of broth to about 5 mL/min/L of broth. In some
embodiments, a
yield of one or more other products is decreased relative to a system that
does not contain the
feeder. In some embodiments, the yield of the one or more other products is
decreased by
about 1% to about 100% relative to the non-fed batch fermentation. In some
embodiments,
the one or more other products comprise one or more polyols or sugar alcohols.
In some
embodiments, the one or more other products comprise methanol, glycol,
glycerol, erythritol,
threitol, arabitol, xylitol, ribitol, mannitol, sorbitol, dulcitol, fucitol,
iditol, inositol, volemitol,
isomalt, maltitol, lactitol, polyglycitol, or a combination thereof In some
embodiments, the
one or more other products comprise glycerol. In some embodiments, the first
biomass
comprises non-cellulosic sugars. In some embodiments, the first biomass
comprises non-
cellulosic oligosaccharides. In some embodiments, the first biomass comprises
starch. In
some embodiments, the first biomass comprises corn, corn mash, sugar cane,
sugar beets,
sugar palms, sweet sorghum, nypa palm, cassava, rice, milo, sorghum, sweet
potatoes, wheat,
molasses, tubers, roots, stems, whole grains, barley, rye, milo, sago,
cassaya, tapioca, rice
peas, beans, potatoes, beets, fruits, or a combination thereof In some
embodiments, the one
or more cellulosic-derived C6 monosaccharides are produced from the
pretreatment and/or
hydrolysis of cellulose, hemicellulose, or lignocellulose material. In some
embodiments, the
one or more cellulosic-derived C6 monosaccharides are produced by the
pretreatment and/or
hydrolysis of a second biomass comprising cellulose, hemicellulose, or
lignocellulose. In
some embodiments, the second biomass comprises corn, corn syrup, corn stover,
corn cobs,
molasses, silage, grass, straw, grain hulls, bagasse, distiller's grains,
distiller's dried solubles,
distiller's dried grains, condensed distiller's solubles, distiller's wet
grains, distiller's dried
grains with solubles, wood, bark, sawdust, paper, poplars, willows,
switchgrass, alfalfa,
prairie bluestem, algae, fruit peels, pits, sorghum, sweet sorghum, sugar
cane, switch grass,
rice, rice straw, rice hulls, wheat, wheat straw, barley, barley straw,
bamboo, seeds, seed
hulls, oats, oat hulls, food waste, municipal sewage waste, or a combination
thereof In some
embodiments, the one or more biocatalysts comprise one or more fermenting
microorganisms. In some embodiments, the one or more biocatalysts comprise one
or more
yeasts and/or one or more bacteria. In some embodiments, the one or more
biocatalysts
comprise one or more yeasts. In some embodiments, the one or more biocatalysts
comprise
one or more strains of Saccharomyces cerevisiae. In some embodiments, at least
one of the
one or more biocatalysts is a genetically-modified yeast that ferments C5 and
C6 saccharides.
In some embodiments, at least one of the one or more biocatalysts is a
bacteria that
hydrolyzes and/or ferments C5 and C6 saccharides. In some embodiments, at
least one of the
13
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
one or more biocatalysts is a hydrolytic enzyme. In some embodiments, the one
or more
biocatalysts comprise an endoglucanase, an exoglucanase, a cellobiohydrolase,
a cellulase, a
beta-glucosidase, a glycoside hydrolase, a glycosyltransferase, a lyase, an
esterase, a
glucamylase, or a combination thereof. In some embodiments, at least one of
the one or more
biocatalysts is an enzyme that hydrolyzes starch. In some embodiments, at
least one of the
one or more biocatalysts is an alpha-amylase, glucoamylase, beta-amylase, exo-
alpha-1,4-
glucanase, or pullulanase. In some embodiments, the one or more biocatalysts
comprise at
least one fermenting microorganism and at least one hydrolytic enzyme. In some
embodiments, the one or more biocatalysts comprise at least one fermenting
microorganism
that is a yeast or bacteria and at least one enzyme that hydrolyzes starch. In
some
embodiments, the blended feedstock comprises less than 100 g/L
monosaccharides. In some
embodiments, the blended feedstock comprises from about 1 g/L to about 100 g/L
monosaccharides. In some embodiments, the first biomass and the one or more
cellulosic-
derived C6 monosaccharides are in a ratio of from about 50:50 to 99:1 (first
biomass:
cellulosic-derived C6 monosaccharides) by volume or by weight:volume. In some
embodiments, the first biomass and the one or more cellulosic-derived C6
monosaccharides
are in a ratio of about 80:20 (first biomass: cellulosic-derived C6
monosaccharides) by
volume or by weight:volume. In some embodiments, the first biomass and the one
or more
cellulosic-derived C6 monosaccharides are in a ratio of about 90:10 (first
biomass: cellulosic-
derived C6 monosaccharides) by volume or by weight:volume. In some
embodiments, the
blended feedstock comprises from about 10% to about 50% solids from the first
biomass. In
some embodiments, the blended feedstock comprises from about 20% to about 40%
solids
from the first biomass. In some embodiments, the blended feedstock comprises
from about
30% to about 36% solids from the first biomass. In some embodiments, the one
or more
cellulosic-derived C6 monosaccharides are at a concentration of from about 10%
to about
70% w/v prior to combining with the first biomass. In some embodiments, the
one or more
cellulosic-derived C6 monosaccharides are at a concentration of from about 20%
to about
50% w/v prior to combining with the first biomass. In some embodiments, the
system further
comprises a hydrolysis unit for producing the one or more cellulosic-derived
monosaccharides from a second biomass. In some embodiments, the system further
comprises a filter for separating solids from the one or more cellulosic
derived C6
monosaccharides.
[0009] Provided herein are methods of producing one or more fermentation end-
products
comprising: (a) combining a first biomass with one or more monosaccharides to
produce a
14
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
blended feedstock; (b) contacting the blended feedstock with one or more
biocatalysts; and
(c) fermenting the first biomass and the one or more monosaccharides for
sufficient time to
produce one or more fermentation end-products from the blended feedstock. In
some
embodiments, a yield of at least one of the one or more fermentation end-
products is
increased relative to fermentation of the first biomass without the one or
more
monosaccharides. In some embodiments, a yield of at least one of the one or
more
fermentation end-products is increased by about 1% to about 100% relative to
fermentation
of the first biomass without the one or more monosaccharides. In some
embodiments, a rate
of production for at least one of the one or more fermentation end-products is
increased
relative to fermentation of the first biomass without the one or more
monosaccharides. In
some embodiments, a rate of production for at least one of the one or more
fermentation end-
products is increased by about 1% to about 100% relative to fermentation of
the first biomass
without the one or more monosaccharides. In some embodiments, the one or more
fermentation end-products comprise one or more alcohols. In some embodiments,
the one or
more fermentation end-products comprise ethanol. In some embodiments, a yield
of one or
more other products is decreased relative to fermentation of the one or more
monosaccharides
without the first biomass. In some embodiments, a yield of one or more other
products is
decreased by about 1% to about 100% relative to fermentation of the one or
more
monosaccharides without the first biomass. In some embodiments, the one or
more other
products comprise one or more polyols or sugar alcohols. In some embodiments,
the one or
more other products comprise methanol, glycol, glycerol, erythritol, threitol,
arabitol, xylitol,
ribitol, mannitol, sorbitol, dulcitol, fucitol, iditol, inositol, volemitol,
isomalt, maltitol,
lactitol, polyglycitol, or a combination thereof In some embodiments, the one
or more other
products comprise glycerol In some embodiments, the first biomass comprises
non-cellulosic
sugars. In some embodiments, the first biomass comprises non-cellulosic
oligosaccharides. In
some embodiments, the first biomass comprises starch. In some embodiments, the
first
biomass comprises corn or corn mash, sugar cane, sugar beet, sugar palm, sweet
sorghum,
nypa palm, cassava, rice, milo, sorghum, sweet potato, wheat, molasses, or a
combination
thereof In some embodiments, the one or more monosaccharides are produced from
the
pretreatment and/or hydrolysis of cellulose, hemicellulose, or lignocellulose
material. In
some embodiments, the one or more monosaccharides are a C6-enriched
hydrolysate
produced by the pretreatment and/or hydrolysis of a second biomass comprising
cellulose,
hemicellulose, or lignocellulose. In some embodiments, the second biomass
comprises corn,
corn syrup, corn stover, corn cobs, molasses, silage, grass, straw, grain
hulls, bagasse,
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
distiller's grains, distiller's dried solubles, distiller's dried grains,
condensed distiller's
solubles, distiller's wet grains, distiller's dried grains with solubles,
wood, bark, sawdust,
paper, poplars, willows, switchgrass, alfalfa, prairie bluestem, algae, fruit
peels, pits,
sorghum, sweet sorghum, sugar cane, switch grass, rice, rice straw, rice
hulls, wheat, wheat
straw, barley, barley straw, bamboo, seeds, seed hulls, oats, oat hulls, food
waste, municipal
sewage waste, or a combination thereof. In some embodiments, the one or more
monosaccharides are at a concentration that differs from a concentration of
sugars in the first
biomass by less than about +/- 50%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the one or more
monosaccharides are at a concentration that differs from a concentration of
sugars in the first
biomass by less than about +/- 40%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the one or more
monosaccharides are at a concentration that differs from a concentration of
sugars in the first
biomass by less than about +/- 30%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the one or more
monosaccharides are at a concentration that differs from a concentration of
sugars in the first
biomass by less than about +/- 20%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the one or more
monosaccharides are at a concentration that differs from a concentration of
sugars in the first
biomass by less than about +/- 15%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the one or more
monosaccharides are at a concentration that differs from a concentration of
sugars in the first
biomass by less than about +/- 10%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the one or more
monosaccharides comprises less than about 50% C5 sugars. In some embodiments,
the one or
more monosaccharides comprises less than about 40% C5 sugars. In some
embodiments, the
one or more monosaccharides comprises less than about 30% C5 sugars. In some
embodiments, the one or more monosaccharides comprises less than about 20% C5
sugars. In
some embodiments, the one or more monosaccharides comprises less than about
10% C5
sugars. In some embodiments, the one or more monosaccharides comprises from
about 0.1%
to about 10% C5 sugars. In some embodiments, at least one of the one or more
biocatalysts is
a fermenting microorganism. In some embodiments, at least one of the one or
more
biocatalysts is a hydrolytic enzyme. In some embodiments, the one or more
biocatalysts
comprise at least one fermenting microorganism and at least one hydrolytic
enzyme. In some
16
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
embodiments, the blended feedstock comprises less than about 100 g/L
monosaccharides
prior to contacting with the one or more biocatalysts. In some embodiments,
the blended
feedstock comprises from about 1 g/L to about 100 g/L monosaccharides prior to
contacting
with the one or more biocatalysts. In some embodiments, the first biomass and
the one or
more sugars are combined in a ratio of from about 50:50 to 99:1 by volume or
by
weight:volume. In some embodiments, the first biomass and the one or more
sugars are
combined in a ratio of about 80:20 by volume or by weight:volume. In some
embodiments,
the first biomass and the one or more sugars are combined in a ratio of about
90:10 by
volume or by weight:volume. In some embodiments, the one or more biocatalysts
comprise
one or more yeast strains, one or more bacterial strains, or a combination
thereof. In some
embodiments, the one or more biocatalysts comprise Saccharomyces cerevisiae.
In some
embodiments, the one or more biocatalysts comprise an endoglucanase, an
exoglucanase, a
cellobiohydrolase, a cellulase, a beta-glucosidase, a glycoside hydrolase, a
glycosyltransferase, a lyase, an esterase, a glucamylase, or a combination
thereof.
[0010] Also provided herein are methods of producing one or more fermentation
end-
products comprising: (a) combining a first biomass with a C6-enriched
hydrolysate to
produce a blended feedstock; (b) contacting the blended feedstock with one or
more
hydrolytic enzymes and/or one or more fermenting microorganisms; and (c)
fermenting the
first biomass and the C6-enriched hydrolysate for a time sufficient to produce
one or more
fermentation end-products from the blended feedstock. In some embodiments, the
C6-
enriched hydrolysate comprises monosaccharides produced from the pretreatment
and/or
hydrolysis of a second biomass. In some embodiments, a yield of at least one
of the one or
more fermentation end-products is increased relative to fermentation of the
first biomass
without the C6-enriched hydrolysate. In some embodiments, a yield of at least
one of the one
or more fermentation end-products is increased by about 1% to about 100%
relative to
fermentation of the first biomass without the C6-enriched hydrolysate. In some
embodiments,
a rate of production for at least one of the one or more fermentation end-
products is increased
relative to fermentation of the first biomass without the C6-enriched
hydrolysate. In some
embodiments, a rate of production for at least one of the one or more
fermentation end-
products is increased by about 1% to about 100% relative to fermentation of
the first biomass
without the C6-enriched hydrolysate. In some embodiments, the one or more
fermentation
end-products comprise one or more alcohols. In some embodiments, the one or
more
fermentation end-products comprise ethanol. In some embodiments, a yield of
one or more
other products is decreased relative to fermentation of the C6-enriched
hydrolysate without
17
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
the first biomass. In some embodiments, a yield of one or more other products
is decreased
by about 1% to about 100% relative to fermentation of the C6-enriched
hydrolysate without
the first biomass. In some embodiments, the one or more other products
comprise one or
more polyols or sugar alcohols. In some embodiments, the one or more other
products
comprise methanol, glycol, glycerol, erythritol, threitol, arabitol, xylitol,
ribitol, mannitol,
sorbitol, dulcitol, fucitol, iditol, inositol, volemitol, isomalt, maltitol,
lactitol, polyglycitol, or
a combination thereof In some embodiments, the one or more other products
comprise
glycerol. In some embodiments, the first biomass comprises non-cellulosic
sugars. In some
embodiments, the first biomass comprises non-cellulosic oligosaccharides. In
some
embodiments, the first biomass comprises starch. In some embodiments, the
first biomass
comprises corn or corn mash, sugar cane, sugar beet, sugar palm, sweet
sorghum, nypa palm,
cassava, rice, milo, sorghum, sweet potato, wheat, molasses, or a combination
thereof In
some embodiments, the second biomass comprises cellulose, hemicellulose, or
lignocellulose. In some embodiments, the second biomass comprises corn, corn
syrup, corn
stover, corn cobs, molasses, silage, grass, straw, grain hulls, bagasse,
distiller's grains,
distiller's dried solubles, distiller's dried grains, condensed distiller's
solubles, distiller's wet
grains, distiller's dried grains with solubles, wood, bark, sawdust, paper,
poplars, willows,
switchgrass, alfalfa, prairie bluestem, algae, fruit peels, pits, sorghum,
sweet sorghum, sugar
cane, switch grass, rice, rice straw, rice hulls, wheat, wheat straw, barley,
barley straw,
bamboo, seeds, seed hulls, oats, oat hulls, food waste, municipal sewage
waste, or a
combination thereof In some embodiments, the C6-enriched hydrolysate comprises
monosaccharides at a concentration that differs from a concentration of sugars
in the first
biomass by less than about +/- 50%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the C6-enriched
hydrolysate comprises monosaccharides at a concentration that differs from a
concentration
of sugars in the first biomass by less than about +/- 40%, wherein the
concentration of sugars
in the first biomass assumes complete hydrolysis to monomers. In some
embodiments, the
C6-enriched hydrolysate comprises monosaccharides at a concentration that
differs from a
concentration of sugars in the first biomass by less than about +/- 30%,
wherein the
concentration of sugars in the first biomass assumes complete hydrolysis to
monomers. In
some embodiments, the C6-enriched hydrolysate comprises monosaccharides at a
concentration that differs from a concentration of sugars in the first biomass
by less than
about +/- 20%, wherein the concentration of sugars in the first biomass
assumes complete
hydrolysis to monomers. In some embodiments, the C6-enriched hydrolysate
comprises
18
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
monosaccharides at a concentration that differs from a concentration of sugars
in the first
biomass by less than about +/- 15%, wherein the concentration of sugars in the
first biomass
assumes complete hydrolysis to monomers. In some embodiments, the C6-enriched
hydrolysate comprises monosaccharides at a concentration that differs from a
concentration
of sugars in the first biomass by less than about +/- 10%, wherein the
concentration of sugars
in the first biomass assumes complete hydrolysis to monomers. In some
embodiments, the
C6-enriched hydrolysate comprises less than about 50% C5 sugars. In some
embodiments,
the C6-enriched hydrolysate comprises less than about 40% C5 sugars. In some
embodiments, the C6-enriched hydrolysate comprises less than about 30% C5
sugars. In
some embodiments, the C6-enriched hydrolysate comprises less than about 20% C5
sugars.
In some embodiments, the C6-enriched hydrolysate comprises less than about 10%
C5
sugars. In some embodiments, the C6-enriched hydrolysate comprises from about
0.1% to
about 10% C5 sugars. In some embodiments, the one or more fermenting
microorganisms
comprise one or more yeast strains, one or more bacterial strains, or a
combination thereof In
some embodiments, at least one of the one or more fermenting microorganisms is
Saccharomyces cerevisiae. In some embodiments, the one or more hydrolytic
enzymes
comprise an endoglucanase, an exoglucanase, a cellobiohydrolase, a cellulase,
a beta-
glucosidase, a glycoside hydrolase, a glycosyltransferase, a lyase, an
esterase, a glucamylase,
or a combination thereof In some embodiments, the blended feedstock comprises
less than
about 100 g/L monosaccharides prior to contacting with the one or more
fermenting
microorganisms and the one or more hydrolytic enzymes. In some embodiments,
the blended
feedstock comprises from about 1 g/L to about 100 g/L monosaccharides prior to
contacting
with the one or more fermenting microorganisms and the one or more hydrolytic
enzymes. In
some embodiments, the first biomass and the C6-enriched hydrolysate are
combined in a ratio
of from about 50:50 to 99:1 by volume or by weight:volume. In some
embodiments, the first
biomass and the C6-enriched hydrolysate are combined in a ratio of about 80:20
by volume
or by weight:volume. In some embodiments, the first biomass and the C6-
enriched
hydrolysate are combined in a ratio of about 90:10 by volume or by
weight:volume.
[0011] In another aspect, provided herein are methods of producing a C6-
enriched
hydrolysate comprising: (a) treating a biomass comprising cellulose and
hemicellulose and/or
lignin to solubilize the hemicellulose and/or lignin; (b) separating the
solubilized
hemicellulose and/or lignin from the cellulose; and (c) hydrolyzing the
cellulose, thereby
producing the C6-enriched hydrolysate. In some embodiments, the biomass
comprising
cellulose and hemicellulose and/or lignin comprises corn, corn syrup, corn
stover, corn cobs,
19
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
molasses, silage, grass, straw, grain hulls, bagasse, distiller's grains,
distiller's dried solubles,
distiller's dried grains, condensed distiller's solubles, distiller's wet
grains, distiller's dried
grains with solubles, wood, bark, sawdust, paper, poplars, willows,
switchgrass, alfalfa,
prairie bluestem, algae, fruit peels, pits, sorghum, sweet sorghum, sugar
cane, switch grass,
rice, rice straw, rice hulls, wheat, wheat straw, barley, barley straw,
bamboo, seeds, seed
hulls, oats, oat hulls, food waste, municipal sewage waste, or a combination
thereof. In some
embodiments, the biomass comprises corn stover. In some embodiments, the
treating
comprises stream treatment, hot water treatment, dilute acid treatment, dilute
base treatment,
steam explosion, acid-catalyzed steam explosion, or a combination thereof. In
some
embodiments, the treatment comprises steam treatment. In some embodiments, the
treating is
performed at a temperature of from about 175 to about 250 C. In some
embodiments, the
treating is performed at a temperature of about 205 C. In some embodiments,
the treating is
performed from about 1 minute to about 30 minutes. In some embodiments, the
treating is
performed for about 7.5 minutes. In some embodiments, the separating is
performed using a
filter press. In some embodiments, the separating step comprises washing the
biomass with
water. In some embodiments, the water is from about 25 to about 100 C. In
some
embodiments, the water is about 50 C. In some embodiments, the water is added
in an
amount from about 1 to about 5 L/kg of biomass (dry weight). In some
embodiments, the
water is added in an amount of about 3 L/kg of biomass (dry weight). In some
embodiments,
the hydrolyzing step comprises enzymatic hydrolysis with one or more enzymes.
In some
embodiments, the hydrolyzing step is performed at a pH of from about 3 to
about 7. In some
embodiments, the hydrolyzing step is performed at a pH of about 5. In some
embodiments,
the hydrolyzing step is performed in a slurry of from about 1 % to about 20%
wt/wt
biomass/water. In some embodiments, the hydrolyzing step is performed in a
slurry of about
8% wt/wt biomass/water. In some embodiments, the hydrolyzing step is performed
in a
jacketed reactor. Some embodiments further comprise concentrating the C6-
enriched
hydrolysate. In some embodiments, the C6-enriched hydrolysate is concentrated
by
evaporation. In some embodiments, the C6-enriched hydrolysate is concentrated
using a roto-
evaporator. In some embodiments, the C6-enriched hydrolysate is concentrated
to a C6 sugar
concentration of from about 100 g/L to about 500 g/L. In some embodiments, the
C6-
enriched hydrolysate is concentrated to a C6 sugar concentration of about 300
g/L. In some
embodiments, the C6-enriched hydrolysate comprises less than about 50% C5
sugars. In
some embodiments, the C6-enriched hydrolysate comprises less than about 40% C5
sugars.
In some embodiments, the C6-enriched hydrolysate comprises less than about 30%
C5
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
sugars. In some embodiments, the C6-enriched hydrolysate comprises less than
about 20%
C5 sugars. In some embodiments, the C6-enriched hydrolysate comprises less
than about
10% C5 sugars. In some embodiments, the C6-enriched hydrolysate comprises from
about
0.1% to about 10% C5 sugars.
[0012] Also provided are C6-enriched hydrolysates produced by any of the
methods
disclosed herein.
[0013] Provided herein are methods of producing ethanol comprising: (a)
combining a first
biomass comprising starch with a C6-enriched hydrolysate to produce a blended
feedstock,
(i) wherein the C6-enriched hydrolysate comprises monosaccharides produced
from the
pretreatment and/or hydrolysis of a second biomass, (ii) wherein the C6-
enriched hydrolysate
comprises monosaccharides at a concentration that differs from a concentration
of sugars in
the first biomass by less than about +/- 20%, wherein the concentration of
sugars in the first
biomass assumes complete hydrolysis to monomers, and (iii) wherein the blended
feedstock
comprises from about 1 g/L to about 100 g/L monosaccharides; (b) contacting
the blended
feedstock with one or more fermenting microorganisms and one or more
hydrolytic enzymes;
and (c) fermenting the first biomass and the C6-enriched hydrolysate to
produce ethanol from
the blended feedstock, wherein an increase rate of production and/or yield of
the ethanol is
achieved relative to the hydrolysis and fermentation of the first biomass
without the C6-
enriched hydrolysate. Also provided are methods of producing ethanol
comprising: (a)
combining a first biomass comprising starch with a C6-enriched hydrolysate to
produce a
blended feedstock, (i) wherein the C6-enriched hydrolysate comprises
monosaccharides
produced from the pretreatment and/or hydrolysis of a second biomass, (ii)
wherein the C6-
enriched hydrolysate comprises monosaccharides at a concentration that differs
from a
concentration of sugars in the first biomass by less than about +/- 20%,
wherein the
concentration of sugars in the first biomass assumes complete hydrolysis to
monomers, and
(iii) wherein the blended feedstock comprises from about 1 g/L to about 100
g/L
monosaccharides; (b) contacting the blended feedstock with one or more
fermenting
microorganisms and one or more hydrolytic enzymes; and (c) fermenting the
first biomass
and the C6-enriched hydrolysate to produce ethanol from the blended feedstock,
wherein a
yield of one or more polyols or sugar alcohols is decreased relative to
fermentation of the C6-
enriched hydrolysate alone.
[0014] Ethanol produced by any of the methods disclosed herein is also
provided.
21
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
INCORPORATION BY REFERENCE
[0015] All publications, patents, and patent applications mentioned in this
specification are
herein incorporated by reference to the same extent as if each individual
publication, patent,
or patent application was specifically and individually indicated to be
incorporated by
reference.
BRIEF DESCRIPTION OF THE DRAWINGS
[0016] The novel features of the invention are set forth with particularity in
the appended
claims. A better understanding of the features and advantages of the present
invention will be
obtained by reference to the following detailed description that sets forth
illustrative
embodiments, in which the principles of the invention are utilized, and the
accompanying
drawings of which:
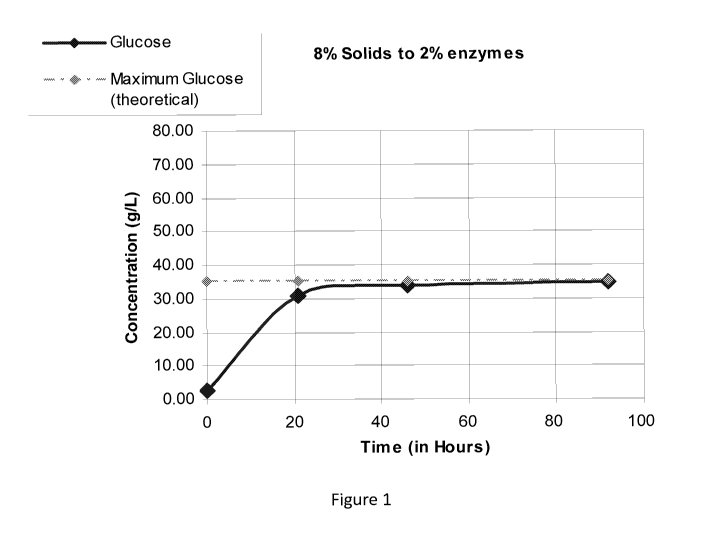
[0017] Figure 1 is a graph illustrating the trajectory of how biomass is
converted from solids
into liquefied C6 sugars during hydrolysis using cellulase enzymes.
[0018] Figure 2 is a graph illustrating the improvement in ethanol yield for
fermentation of
corn mash slurry spiked with various energy sorghum-derived sugar blends as
compared to
fermentation of corn mash slurry alone.
[0019] Figure 3 is a graph of glucose concentration over time for a corn mash
control
feedstock as compared to a feedstock spiked with sugar from energy sorghum.
[0020] Figure 4 is a graph comparing ethanol production for a corn mash
control against
corn mash spiked with energy sorghum sugars.
[0021] Figure 5 is a graph illustrating the improvement in ethanol yield for a
corn mash
slurry spiked with switchgrass-derived sugar as compared to corn mash slurry
alone.
[0022] Figure 6 is a graph comparing glucose uptake for a glucose control as
compared to a
glucose solution spiked with energy sorghum solids and a glucose solution
spiked with
switchgrass solids.
[0023] Figure 7 is a graph comparing ethanol production for a glucose control
as compared
to a glucose solution spiked with energy sorghum solids and a glucose solution
spiked with
switchgrass solids.
[0024] Figure 8 is a graph comparing ethanol production from corn mash to
ethanol
production from a fed batch fermentation of a 90:10 blend of corn mash and
cellulosic sugar
with C5 fermenting yeast.
[0025] Figure 9 is a graph comparing ethanol production from corn mash to
ethanol
production from a fed-batch fermentation of a 90:10 blend of corn mash and
wheat straw
cellulosic sugar at shake flask level.
22
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[0026] Figure 10 is a graph comparing ethanol production from corn mash to
ethanol
production from a fed batch fermentation of a 90:10 blend of corn mash and
cellulosic sugar
with a C5 fermenting yeast.
[0027] Figure 11 is a graph showing the increase in ethanol production in fed-
batch
fermentations compared to batch fermentations after 72 hours using a 90:10 and
a 80:20
blend of corn mash and cellulosic sugars.
[0028] Figure 12 (A&B) are progress curves showing mass loss (g) over time
during
fermentation of corn mash supplemented with varying levels of a C6 Saccharide
Stream
produced from corn stillage. The treatment IDs in the figure legends
correspond to Table 9.
[0029] Figure 13 is a chart showing the average mass loss due to carbon
dioxide after
fermentation of corn mash supplemented with varying levels of a C6 Saccharide
Stream
produced from corn stillage for 15.67 (dark grey bars) and 62 hours (light
grey bars). Bars
labeled with the same letter are not statistically different from one another
at either 15.67 or
62 hours. Error bars represent one standard deviation of nine independent
replicate
fermentation flasks at 15.67 hours and three independent replicate
fermentation flasks at 62
hours. The treatment IDs in the figure legend corresponds to Table 9.
[0030] Figure 14 is a comparison of the average final concentrations of
ethanol (left bars)
and unfermented sugars as glucose equivalent (right bars) after fermentation
of corn mash
dosed with varying levels of a C6 Saccharide Stream produced from corn
stillage for 62
hours as measured by HPLC. Error bars represent one standard deviation of
three
independent replicate fermentation flasks, except for QC which represents the
standard
deviation of six independent replicate flasks. Bars labeled with the same
letter in each group
are not statistically different from one another.
[0031] Figure 15 is a dose-response graph, representing the dose of C6
Saccharide Stream
produced from corn stillage versus glycerol concentration accumulated for all
treatments
identified in Table 9. The dashed lines represent the QC glycerol
concentration. Error bars
represent one standard deviation of three independent replicate fermentation
flasks, except for
QC which represents the standard deviation of six independent replicate
flasks.
[0032] Figure 16 shows the average final pH values after fermentation of corn
mash dosed
with varying levels of a C6 Saccharide Stream produced from corn stillage for
22 hours
(diamonds), 46 hours (squares) and 62 hours (triangles).
[0033] Figure 17 shows the average improvement in ethanol yield for corn mash
fermentations dosed with varying levels of a C6 Saccharide Stream produced
from corn
stillage.
23
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[0034] Figure 18 shows the average increase in ethanol yield (squares; left Y-
axis) and
residual sugars (triangles; right Y-axis) after fermentation of corn mash
dosed with varying
levels of a C6 Saccharide Stream produced from corn stillage.
[0035] Figure 19 shows the average mass loss for fermentations of 80:20 blends
of corn
mass and a C6 Saccharide Stream produced from corn stover (squares) or stock
sugar
(diamonds).
[0036] Figure 20 shows the average mass loss for fermentations of 80:20 blends
of corn
mass and a C6 Saccharide Stream produced from corn stover (squares) or stock
sugar
(diamonds).
[0037] Figure 21 shows the shows the ethanol production rate kinetics
throughout
fermentation a 70:30 blend of corn mash and C6 Saccharide Stream (cellulosic-
derived)
(square) compared to corn mash alone (diamond).
[0038] Figure 22 shows the shows the glycerol production rate kinetics
throughout
fermentation a 70:30 blend of corn mash and C6 Saccharide Stream (cellulosic-
derived)
(square) compared to corn mash alone (diamond).
DETAILED DESCRIPTION OF THE INVENTION
[0039] As used in the specification and the appended claims, the singular
forms "a," "an" and
"the" include plural referents unless the context clearly dictates otherwise.
Thus, for example,
reference to "a purified monomer" includes mixtures of two or more purified
monomers. The
term "comprising" as used herein is synonymous with "including," "containing,"
or
"characterized by," and is inclusive or open-ended and does not exclude
additional, unrecited
elements or method steps.
[0040] "About" means a referenced numeric indication plus or minus 10% of that
referenced
numeric indication. For example, the term about 4 would include a range of 3.6
to 4.4. All
numbers expressing quantities of ingredients, reaction conditions, and so
forth used in the
specification are to be understood as being modified in all instances by the
term "about."
Accordingly, unless indicated to the contrary, the numerical parameters set
forth herein are
approximations that can vary depending upon the desired properties sought to
be obtained.
At the very least, and not as an attempt to limit the application of the
doctrine of equivalents
to the scope of any claims in any application claiming priority to the present
application, each
numerical parameter should be construed in light of the number of significant
digits and
ordinary rounding approaches.
24
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[0041] Wherever the phrase "for example," "such as," "including" and the like
are used
herein, the phrase "and without limitation" is understood to follow unless
explicitly stated
otherwise. Therefore, "for example ethanol production" means "for example and
without
limitation ethanol production."
[0042] In this specification and in the claims that follow, reference will be
made to a number
of terms which shall be defined to have the following meanings.
[0043] Definitions
[0044] "Optional" or "optionally" means that the subsequently described event
or
circumstance may or may not occur, and that the description includes instances
where said
event or circumstance occurs and instances where it does not. For example, the
phrase "the
medium can optionally contain glucose" means that the medium may or may not
contain
glucose as an ingredient and that the description includes both media
containing glucose and
media not containing glucose.
[0045] Unless characterized otherwise, technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art.
[0046] " Fermentive end-product" and "fermentation end-product" are used
interchangeably
herein to include bio fuels, chemicals, compounds suitable as liquid fuels,
gaseous fuels,
triacylglycerols (TAGs), reagents, chemical feedstocks, chemical additives,
processing aids,
food additives, bioplastiks and precursors to bioplastiks, and other products.
Examples of
fermentive end-products include but are not limited to 1,4 diacids (succinic,
fumaric and
malic), 2,5 furan dicarboxylic acid, 3 hydroxy propionic acid, aspartic acid,
glucaric acid,
glutamic acid, itaconic acid, levulinic acid, 3-hydroxybutyrolactone,
glycerol, sorbitol,
xylitol/arabinitol, butanediol, butanol, methane, methanol, ethane, ethene,
ethanol, n-propane,
1-propene, 1-propanol, propanal, acetone, propionate, n-butane, 1-butene, 1-
butanol, butanal,
butanoate, isobutanal, isobutanol, 2-methylbutanal, 2-methylbutano1, 3-
methylbutanal, 3-
methylbutano1, 2-butene, 2-butanol, 2-butanone, 2,3-butanedio1, 3-hydroxy-2-
butanone, 2,3-
butanedione, ethylbenzene, ethenylbenzene, 2-phenylethano1,
phenylacetaldehyde, 1-
phenylbutane, 4-phenyl-1-butene, 4-phenyl-2-butene, 1-pheny1-2-butene, 1-
pheny1-2-butano1,
4-pheny1-2-butano1, 1-pheny1-2-butanone, 4-phenyl-2-butanone, 1-pheny1-2,3-
butandio1, 1-
pheny1-3-hydroxy-2-butanone, 4-phenyl-3-hydroxy-2-butanone, 1-pheny1-2,3-
butanedione,
n-pentane, ethylphenol, ethenylphenol, 2-(4-hydroxyphenyl)ethano1, 4-
hydroxyphenylacetaldehyde, 1-(4-hydroxyphenyl) butane, 4-(4-hydroxypheny1)-1-
butene, 4-
(4-hydroxypheny1)-2-butene, 1-(4-hydroxypheny1)-1-butene, 1-(4-hydroxypheny1)-
2-butano1,
4-(4-hydroxypheny1)-2-butano1, 1-(4-hydroxypheny1)-2-butanone, 4-(4-
hydroxypheny1)-2-
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
butanone, 1-(4-hydroxypheny1)-2,3-butandio1, 1-(4-hydroxypheny1)-3-hydroxy-2-
butanone,
4-(4-hydroxypheny1)-3-hydroxy-2-butanone, 1-(4-hydroxypheny1)-2,3-
butanonedione,
indo lylethane, indo lylethene, 2-(indole-3-)ethano1, n-pentane, 1-pentene, 1-
pentanol,
pentanal, pentanoate, 2-pentene, 2-pentanol, 3-pentanol, 2-pentanone, 3-
pentanone, 4-
methylpentanal, 4-methylpentano1, 2,3-pentanedio1, 2-hydroxy-3-pentanone, 3-
hydroxy-2-
pentanone, 2,3-pentanedione, 2-methylpentane, 4-methyl-l-pentene, 4-methyl-2-
pentene, 4-
methy1-3-pentene, 4-methyl-2-pentanol, 2-methy1-3-pentano1, 4-methyl-2-
pentanone, 2-
methy1-3-pentanone, 4-methy1-2,3-pentanedio1, 4-methyl-2-hydroxy-3-pentanone,
4-methyl-
3-hydroxy-2-pentanone, 4-methyl-2,3-pentanedione, 1-phenylpentane, 1-pheny1-1-
pentene,
1-pheny1-2-pentene, 1-pheny1-3-pentene, 1-pheny1-2-pentanol, 1-pheny1-3-
pentano1, 1-
pheny1-2-pentanone, 1-pheny1-3-pentanone, 1-pheny1-2,3-pentanedio1, 1-pheny1-2-
hydroxy-
3-pentanone, 1-pheny1-3-hydroxy-2-pentanone, 1-pheny1-2,3-pentanedione, 4-
methyl-l-
phenylpentane, 4-methyl- 1-phenyl- 1 -p entene, 4-methyl-1 -phenyl-2-p entene,
4-methyl-1 -
phenyl-3 -p entene, 4-methyl-1 -phenyl-3 -p entano 1, 4-methyl-1 -p heny1-2-p
entano 1, 4-methyl-1 -
phenyl-3 -p entanone, 4-methyl-1 -p heny1-2-p entanone, 4-methyl-1 -phenyl-2,3
-p entanedio 1, 4-
methyl-1 -phenyl-2,3 -p entanedione, 4-methyl-1 -phenyl-3 -hydro xy-2-p
entanone, 4-methyl-1 -
phenyl-2-hydro xy-3 -p entanone, 1-(4-hydroxyphenyl) pentane, 1-(4-
hydroxypheny1)-1-
pentene, 1-(4-hydroxypheny1)-2-pentene, 1-(4-hydroxypheny1)-3-pentene, 1-(4-
hydroxypheny1)-2-pentano1, 1-(4-hydroxypheny1)-3-pentano1, 1-(4-hydroxypheny1)-
2-
pentanone, 1-(4-hydroxypheny1)-3-pentanone, 1-(4-hydroxypheny1)-2,3-
pentanedio1, 1-(4-
hydroxypheny1)-2-hydroxy-3-pentanone, 1-(4-hydroxypheny1)-3-hydroxy-2-
pentanone, 1-(4-
hydro xyp heny1)-2,3 -p entanedione, 4-methyl-1 -(4-hydro xyp henyl) pentane,
4-methyl-1 -(4-
hydro xyp heny1)-2-p entene, 4-methyl-1 -(4-hydro xypheny1)-3 -p entene, 4-
methyl-1 -(4-
hydro xyp heny1)- 1 -p entene, 4-methyl-1 -(4-hydro xypheny1)-3 -p entano 1, 4-
methyl-1 -(4-
hydro xyp heny1)-2-p entano 1, 4-methyl-1 -(4-hydro xyp heny1)-3 -p entanone,
4-methyl-1 -(4-
hydro xyp heny1)-2-p entanone, 4-methyl-1 -(4-hydro xyp heny1)-2,3 -p
entanedio 1, 4-methyl-1 -(4-
hydroxypheny1)-2,3-pentanedione, 4-methyl-1-(4-hydroxypheny1)-3-hydroxy-2-
pentanone, 4-
methy1-1-(4-hydroxypheny1)-2-hydroxy-3-pentanone, 1-indole-3-pentane, 1-
(indole-3)-1-
pentene, 1-(indole-3)-2-pentene, 1-(indole-3)-3-pentene, 1-(indole-3)-2-
pentanol, 1-(indole-
3)-3-pentanol, 1-(indole-3)-2-pentanone, 1-(indole-3)-3-pentanone, 1-(indole-
3)-2,3-
pentanedio1, 1-(indole-3)-2-hydroxy-3-pentanone, 1-(indole-3)-3-hydroxy-2-
pentanone, 1-
(indo le-3)-2,3 -p entanedione, 4-methyl-1 -(indo le-3 -)pentane, 4-methyl-1 -
(indo le-3)-2-
p entene, 4-methyl-1 -(indo le-3)-3 -p entene, 4-methyl-1 -(indo le-3)- 1 -p
entene, 4-methyl-2-
(indo le-3)-3 -p entanol, 4-methyl-1 -(indo le-3)-2-p entano 1, 4-methyl-1 -
(indo le-3)-3 -p entanone,
26
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
4-methyl-1 -(indo le-3)-2-p entanone, 4-methyl-1 -(indo le-3)-2,3 -
pentanediol, 4-methyl- 1 -
(indo le-3)-2,3 -pentanedione, 4-methyl-1 -(indo le-3)-3 -hydro xy-2-p
entanone, 4-methyl-1 -
(indole-3)-2-hydroxy-3 -pentanone, n-hexane, 1 -hexene, 1 -hexanol, hexanal,
hexano ate, 2-
hexene, 3 -hexene, 2-hexanol, 3 -hexanol, 2-hexanone, 3 -hexanone, 2,3 -
hexanediol, 2,3 -
hexanedione, 3 ,4-hexanedio1, 3 ,4-hexanedione, 2-hydro xy-3 -hexanone, 3 -
hydro xy-2-
hexanone, 3 -hydroxy-4-hexanone, 4-hydroxy-3-hexanone, 2-methylhexane, 3 -
methylhexane,
2-methyl-2-hexene, 2-methyl-3 -hexene, 5-methyl-1 -hexene, 5 -methyl-2-hexene,
4-methyl-1 -
hexene, 4-methyl-2-hexene, 3 -methyl-3 -hexene, 3 -methyl-2-hexene, 3 -methyl-
1 -hexene, 2-
methyl-3 -hexanol, 5 -methyl-2-hexanol, 5 -methyl-3 -hexanol, 2-methyl-3 -
hexanone, 5-methyl-
2-hexanone, 5 -methyl-3 -hexanone, 2-methy1-3,4-hexanedio1, 2-methyl-3 ,4-
hexanedione, 5 -
methy1-2,3 -hexanediol, 5 -methyl-2,3 -hexanedione, 4-methy1-2,3-hexanedio1, 4-
methy1-2,3-
hexanedione, 2-methyl-3-hydroxy-4-hexanone, 2-methyl-4-hydroxy-3-hexanone, 5 -
methy1-2-
hydro xy-3 -hexanone, 5 -methyl-3 -hydro xy-2-hexanone, 4-methyl-2-hydroxy-3-
hexanone, 4-
methyl-3 -hydro xy-2-hexanone, 2,5 -dimethylhexane, 2,5-dimethy1-2-hexene, 2,5
-dimethy1-3 -
hexene, 2,5 -dimethy1-3 -hexanol, 2,5 -dimethy1-3 -hexanone, 2,5 -dimethy1-3
,4-hexanedio1, 2,5 -
dimethy1-3 ,4-hexanedione, 2,5 -dimethy1-3 -hydro xy-4-hexanone, 5 -methyl- 1 -
phenylhexane,
4-methyl-1 -phenylhexane, 5 -methyl- 1 -phenyl- 1 -hexene, 5 -methyl- 1 -
phenyl-2-hexene, 5 -
methyl- 1 -phenyl-3 -hexene, 4-methyl-1 -phenyl- 1 -hexene, 4-methyl-1 -phenyl-
2-hexene, 4-
methyl-1 -phenyl-3 -hexene, 5 -methyl- 1 -pheny1-2-hexano1, 5 -methyl- 1 -
phenyl-3 -hexanol, 4-
methyl-1 -pheny1-2-hexano1, 4-methyl-1 -phenyl-3 -hexanol, 5 -methyl- 1 -
phenyl-2-hexanone,
-methyl- 1 -phenyl-3 -hexanone, 4-methyl-1 -phenyl-2-hexanone, 4-methyl-1 -
phenyl-3 -
hexanone, 5 -methyl- 1 -phenyl-2,3 -hexanediol, 4-methyl-1 -phenyl-2,3 -
hexanediol, 5-methyl-
1 -phenyl-3 -hydro xy-2-hexanone, 5 -methyl- 1 -phenyl-2-hydroxy-3 -hexanone,
4-methyl-1 -
phenyl-3 -hydro xy-2-hexanone, 4-methyl-1 -phenyl-2-hydroxy-3 -hexanone, 5 -
methyl- 1 -
phenyl-2,3 -hexanedione, 4-methyl-1 -phenyl-2,3 -hexanedione, 4-methyl-1 -(4-
hydro xyp henyl)hexane, 5 -methyl- 1 -(4-hydro xyp heny1)- 1 -hexene, 5 -
methyl- 1 -(4-
hydro xyp heny1)-2-hexene, 5 -methyl- 1 -(4-hydro xypheny1)-3 -hexene, 4-
methyl-1 -(4-
hydro xyp heny1)- 1 -hexene, 4-methyl-1 -(4-hydro xypheny1)-2-hexene, 4-methyl-
1 -(4-
hydro xyp heny1)-3 -hexene, 5 -methyl- 1 -(4-hydro xypheny1)-2-hexanol, 5 -
methyl- 1 -(4-
hydro xyp heny1)-3 -hexanol, 4-methyl-1 -(4-hydro xyp heny1)-2-hexanol, 4-
methyl-1 -(4-
hydro xyp heny1)-3 -hexanol, 5 -methyl- 1 -(4-hydro xyp heny1)-2-hexanone, 5 -
methyl- 1 -(4-
hydro xyp heny1)-3 -hexanone, 4-methyl-1 -(4-hydro xyp heny1)-2-hexanone, 4-
methyl-1 -(4-
hydro xyp heny1)-3 -hexanone, 5 -methyl- 1 -(4-hydro xyp heny1)-2,3 -
hexanediol, 4-methyl-1 -(4-
hydro xyp heny1)-2,3 -hexanediol, 5 -methyl- 1 -(4-hydro xyp heny1)-3 -hydro
xy-2-hexanone, 5-
27
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
methyl-1 -(4-hydro xyp heny1)-2-hydro xy-3 -hexanone, 4-methyl-1 -(4-hydro xyp
heny1)-3 -
hydro xy-2-hexanone, 4-methyl-1 -(4-hydro xyp heny1)-2-hydro xy-3 -hexanone, 5-
methyl-1 -(4-
hydroxypheny1)-2,3-hexanedione, 4-methyl-1-(4-hydroxypheny1)-2,3-hexanedione,
4-methyl-
1 -(indo le-3 -)hexane, 5-methyl-1 -(indo le-3)- 1 -hexene, 5-methyl-1 -(indo
le-3)-2-hexene, 5 -
methyl- 1 -(indo le-3)-3 -hexene, 4-methyl-1 -(indo le-3)- 1 -hexene, 4-methyl-
1 -(indo le-3)-2-
hexene, 4-methyl-1 -(indo le-3)-3 -hexene, 5-methyl-1 -(indo le-3)-2-hexano1,
5-methyl-1 -
(indo le-3)-3 -hexanol, 4-methyl-1 -(indo le-3)-2-hexano1, 4-methyl-1 -(indo
le-3)-3 -hexanol, 5 -
methyl- 1 -(indo le-3)-2-hexanone, 5-methyl-1 -(indo le-3)-3 -hexanone, 4-
methyl-1 -(indo le-3)-
2-hexanone, 4-methyl-1 -(indo le-3)-3 -hexanone, 5-methyl-1 -(indo le-3)-2,3 -
hexanediol, 4-
methyl-1 -(indo le-3)-2,3 -hexanediol, 5-methyl-1 -(indo le-3)-3 -hydro xy-2-
hexanone, 5-methyl-
1 -(indo le-3)-2-hydroxy-3-hexanone, 4-methyl-1 -(indo le-3)-3 -hydro xy-2-
hexanone, 4-methyl-
1 -(indo le-3)-2-hydroxy-3-hexanone, 5-methyl-1 -(indo le-3)-2,3 -hexanedione,
4-methyl-1 -
(indo le-3)-2,3 -hexanedione, n-heptane, 1-heptene, 1-heptanol, heptanal,
heptanoate, 2-
heptene, 3-heptene, 2-heptanol, 3-heptanol, 4-heptanol, 2-heptanone, 3-
heptanone, 4-
heptanone, 2,3-heptanedio1, 2,3-heptanedione, 3,4-heptanedio1, 3,4-
heptanedione, 2-hydroxy-
3-heptanone, 3-hydroxy-2-heptanone, 3-hydroxy-4-heptanone, 4-hydroxy-3-
heptanone, 2-
methylheptane, 3-methylheptane, 6-methyl-2-heptene, 6-methyl-3-heptene, 2-
methy1-3-
heptene, 2-methyl-2-heptene, 5-methy1-2-heptene, 5-methy1-3-heptene, 3-methy1-
3-heptene,
2-methy1-3-heptano1, 2-methy1-4-heptano1, 6-methy1-3-heptano1, 5-methy1-3-
heptano1, 3-
methy1-4-heptano1, 2-methyl-3-heptanone, 2-methyl-4-heptanone, 6-methyl-3-
heptanone, 5-
methy1-3-heptanone, 3-methy1-4-heptanone, 2-methy1-3,4-heptanedio1, 2-methy1-
3,4-
heptanedione, 6-methy1-3,4-heptanedio1, 6-methyl-3,4-heptanedione, 5-methy1-
3,4-
heptanediol, 5-methy1-3,4-heptanedione, 2-methyl-3-hydroxy-4-heptanone, 2-
methy1-4-
hydroxy-3-heptanone, 6-methyl-3-hydroxy-4-heptanone, 6-methyl-4-hydroxy-3-
heptanone,
5-methy1-3-hydroxy-4-heptanone, 5-methy1-4-hydroxy-3-heptanone, 2,6-
dimethylheptane,
2,5-dimethylheptane, 2,6-dimethy1-2-heptene, 2,6-dimethy1-3-heptene, 2,5-
dimethy1-2-
heptene, 2,5-dimethy1-3-heptene, 3,6-dimethy1-3-heptene, 2,6-dimethy1-3-
heptano1, 2,6-
dimethy1-4-heptano1, 2,5-dimethy1-3-heptanol, 2,5-dimethy1-4-heptano1, 2,6-
dimethy1-3,4-
heptanedio1, 2,6-dimethy1-3,4-heptanedione, 2,5-dimethy1-3,4-heptanedio1, 2,5-
dimethy1-3,4-
heptanedione, 2,6-dimethy1-3-hydroxy-4-heptanone, 2,6-dimethy1-4-hydroxy-3-
heptanone,
2,5-dimethy1-3-hydroxy-4-heptanone, 2,5-dimethy1-4-hydroxy-3-heptanone, n-
octane, 1-
octene, 2-octene, 1-octanol, octanal, octanoate, 3-octene, 4-octene, 4-
octanol, 4-octanone,
4,5-octanedio1, 4,5-octanedione, 4-hydroxy-5-octanone, 2-methyloctane, 2-
methyl-3-octene,
2-methyl-4-octene, 7-methyl-3-octene, 3-methy1-3-octene, 3-methy1-4-octene, 6-
methyl-3 -
28
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
octene, 2-methyl-4-octanol, 7-methyl-4-octanol, 3-methy1-4-octanol, 6-methyl-4-
octanol, 2-
methy1-4-octanone, 7-methyl-4-octanone, 3-methy1-4-octanone, 6-methyl-4-
octanone, 2-
methy1-4,5-octanedio1, 2-methyl-4,5-octanedione, 3-methy1-4,5-octanedio1, 3-
methy1-4,5-
octanedione, 2-methyl-4-hydroxy-5-octanone, 2-methyl-5-hydroxy-4-octanone, 3-
methy1-4-
hydroxy-5-octanone, 3-methy1-5-hydroxy-4-octanone, 2,7-dimethyloctane, 2,7-
dimethy1-3-
octene, 2,7-dimethy1-4-octene, 2,7-dimethy1-4-octano1, 2,7-dimethy1-4-
octanone, 2,7-
dimethy1-4,5-octanedio1, 2,7-dimethy1-4,5-octanedione, 2,7-dimethy1-4-hydroxy-
5-octanone,
2,6-dimethyloctane, 2,6-dimethy1-3-octene, 2,6-dimethy1-4-octene, 3,7-dimethy1-
3-octene,
2,6-dimethy1-4-octanol, 3,7-dimethy1-4-octanol, 2,6-dimethy1-4-octanone, 3,7-
dimethy1-4-
octanone, 2,6-dimethy1-4,5-octanedio1, 2,6-dimethy1-4,5-octanedione, 2,6-
dimethy1-4-
hydroxy-5-octanone, 2,6-dimethy1-5-hydroxy-4-octanone, 3,6-dimethyloctane, 3,6-
dimethy1-
3-octene, 3,6-dimethy1-4-octene, 3,6-dimethy1-4-octanol, 3,6-dimethy1-4-
octanone, 3,6-
dimethy1-4,5-octanedio1, 3,6-dimethy1-4,5-octanedione, 3,6-dimethy1-4-hydroxy-
5-octanone,
n-nonane, 1-nonene, 1-nonano1, nonanal, nonanoate, 2-methylnonane, 2-methyl-4-
nonene, 2-
methy1-5-nonene, 8-methyl-4-nonene, 2-methyl-5-nonano1, 8-methy1-4-nonano1, 2-
methy1-5-
nonanone, 8-methyl-4-nonanone, 8-methyl-4,5-nonanedio1, 8-methyl-4,5-
nonanedione, 8-
methy1-4-hydroxy-5-nonanone, 8-methyl-5-hydroxy-4-nonanone, 2,8-
dimethylnonane, 2,8-
dimethy1-3-nonene, 2,8-dimethy1-4-nonene, 2,8-dimethy1-5-nonene, 2,8-dimethy1-
4-nonano1,
2,8-dimethy1-5-nonano1, 2,8-dimethy1-4-nonanone, 2,8-dimethy1-5-nonanone, 2,8-
dimethy1-
4,5-nonanedio1, 2,8-dimethy1-4,5-nonanedione, 2,8-dimethy1-4-hydroxy-5-
nonanone, 2,8-
dimethy1-5-hydroxy-4-nonanone, 2,7-dimethylnonane, 3,8-dimethy1-3-nonene, 3,8-
dimethy1-
4-nonene, 3,8-dimethy1-5-nonene, 3,8-dimethy1-4-nonano1, 3,8-dimethy1-5-
nonano1, 3,8-
dimethy1-4-nonanone, 3,8-dimethy1-5-nonanone, 3,8-dimethy1-4,5-nonanedio1, 3,8-
dimethy1-
4,5-nonanedione, 3,8-dimethy1-4-hydroxy-5-nonanone, 3,8-dimethy1-5-hydroxy-4-
nonanone,
n-decane, 1-decene, 1-decano1, decanoate, 2,9-dimethyldecane, 2,9-dimethy1-3-
decene, 2,9-
dimethy1-4-decene, 2,9-dimethy1-5-decano1, 2,9-dimethy1-5-decanone, 2,9-
dimethy1-5,6-
decanedio1, 2,9-dimethy1-6-hydroxy-5-decanone, 2,9-dimethy1-5,6-decanedionen-
undecane,
1-undecene, 1-undecanol, undecanal. undecanoate, n-dodecane, 1-dodecene, 1-
dodecanol,
dodecanal, dodecanoate, n-dodecane, 1-decadecene, n-tridecane, 1-tridecene, 1-
tridecanol,
tridecanal, tridecanoate, n-tetradecane, 1-tetradecene, 1-tetradecano1,
tetradecanal,
tetradecanoate, n-pentadecane, 1-pentadecene, 1-pentadecanol, pentadecanal,
pentadecanoate,
n-hexadecane, 1-hexadecene, 1-hexadecanol, hexadecanal, hexadecanoate, n-
heptadecane, 1-
heptadecene, 1-heptadecanol, heptadecanal, heptadecanoate, n-octadecane, 1-
octadecene, 1-
octadecanol, octadecanal, octadecanoate, n-nonadecane, 1-nonadecene, 1-
nonadecanol,
29
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
nonadecanal, nonadecanoate, eicosane, 1-eicosene, 1-eicosanol, eicosanal,
eicosanoate, 3-
hydroxy propanal, 1,3-propanediol, 4-hydroxybutanal, 1,4-butanediol, 3-hydroxy-
2-butanone,
2,3-butandiol, 1,5-pentane diol, homocitrate, homoisocitorate, b-hydroxy
adipate, glutarate,
glutarsemialdehyde, glutaraldehyde, 2-hydroxy-1-cyclopentanone, 1,2-
cyclopentanedio1,
cyclopentanone, cyclopentanol, (S)-2-acetolactate, (R)-2,3-Dihydroxy-
isovalerate, 2-
oxoisovalerate, isobutyryl-CoA, isobutyrate, isobutyraldehyde, 5-amino
pentaldehyde, 1,10-
diaminodecane, 1,10-diamino-5-decene, 1,10-diamino-5-hydroxydecane, 1,10-
diamino-5-
decanone, 1,10-diamino-5,6-decanedio1, 1,10-diamino-6-hydroxy-5-decanone,
phenylacetoaldehyde, 1,4-diphenylbutane, 1,4-dipheny1-1-butene, 1,4-dipheny1-2-
butene, 1,4-
dipheny1-2-butano1, 1,4-dipheny1-2-butanone, 1,4-dipheny1-2,3-butanedio1, 1,4-
dipheny1-3-
hydroxy-2-butanone, 1-(4-hydeoxypheny1)-4-phenylbutane, 1-(4-hydeoxypheny1)-4-
phenyl-
1-butene, 1-(4-hydeoxypheny1)-4-pheny1-2-butene, 1-(4-hydeoxypheny1)-4-pheny1-
2-butano1,
1-(4-hydeoxypheny1)-4-pheny1-2-butanone, 1-(4-hydeoxypheny1)-4-pheny1-2,3-
butanedio1, 1-
(4-hydeoxypheny1)-4-pheny1-3-hydroxy-2-butanone, 1-(indole-3)-4-phenylbutane,
1-(indole-
3)-4-pheny1-1-butene, 1-(indole-3)-4-pheny1-2-butene, 1-(indole-3)-4-pheny1-2-
butano1, 1-
(indole-3)-4-pheny1-2-butanone, 1-(indole-3)-4-pheny1-2,3-butanedio1, 1-
(indole-3)-4-
pheny1-3-hydroxy-2-butanone, 4-hydroxyphenylacetoaldehyde, 1,4-di(4-
hydroxyphenyl)butane, 1,4-di(4-hydroxypheny1)-1-butene, 1,4-di(4-
hydroxypheny1)-2-
butene, 1,4-di(4-hydroxypheny1)-2-butano1, 1,4-di(4-hydroxypheny1)-2-butanone,
1,4-di(4-
hydroxypheny1)-2,3-butanedio1, 1,4-di(4-hydroxypheny1)-3-hydroxy-2-butanone, 1-
(4-
hydroxypheny1)-4-(indole-3-)butane, 1-(4-hydroxypheny1)-4-(indole-3)-1-butene,
1-di(4-
hydroxypheny1)-4-(indole-3)-2-butene, 1-(4-hydroxypheny1)-4-(indole-3)-2-
butanol, 1-(4-
hydroxypheny1)-4-(indole-3)-2-butanone, 1-(4-hydroxypheny1)-4-(indole-3)-2,3-
butanedio1,
1-(4-hydroxypheny1-4-(indole-3)-3-hydroxy-2-butanone, indole-3-acetoaldehyde,
1,4-
di(indole-3-)butane, 1,4-di(indole-3)-1-butene, 1,4-di(indole-3)-2-butene, 1,4-
di(indole-3)-2-
butano1, 1,4-di(indole-3)-2-butanone, 1,4-di(indole-3)-2,3-butanedio1, 1,4-
di(indole-3)-3-
hydroxy-2-butanone, succinate semialdehyde, hexane-1,8-dicarboxylic acid, 3-
hexene-1,8-
dicarboxylic acid, 3-hydroxy-hexane-1,8-dicarboxylic acid, 3-hexanone-1,8-
dicarboxylic
acid, 3,4-hexanedio1-1,8-dicarboxylic acid, 4-hydroxy-3-hexanone-1,8-
dicarboxylic acid,
glycerol, fucoidan, iodine, chlorophyll, carotenoid, calcium, magnesium, iron,
sodium,
potassium, phosphate, lactic acid, acetic acid, formic acid, isoprenoids, and
polyisoprenes,
including rubber. Further, such products can include succinic acid, pyruvic
acid, enzymes
such as cellulases, polysaccharases, lipases, proteases, ligninases, and
hemicellulases and
may be present as a pure compound, a mixture, or an impure or diluted form.
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[0047] Fermentation end-products can include polyols or sugar alcohols; for
example,
methanol, glycol, glycerol, erythritol, threitol, arabitol, xylitol, ribitol,
mannitol, sorbitol,
dulcitol, fucitol, iditol, inositol, volemitol, isomalt, maltitol, lactitol,
and/or polyglycitol.
[0048] The term "fatty acid comprising material" as used herein has its
ordinary meaning as
known to those skilled in the art and can comprise one or more chemical
compounds that
include one or more fatty acid moieties as well as derivatives of these
compounds and
materials that comprise one or more of these compounds. Common examples of
compounds
that include one or more fatty acid moieties include triacylglycerides,
diacylglycerides,
monoacylglycerides, phospholipids, lysophospholipids, free fatty acids, fatty
acid salts,
soaps, fatty acid comprising amides, esters of fatty acids and monohydric
alcohols, esters of
fatty acids and polyhydric alcohols including glycols (e.g. ethylene glycol,
propylene glycol,
etc.), esters of fatty acids and polyethylene glycol, esters of fatty acids
and polyethers, esters
of fatty acids and polyglycol, esters of fatty acids and saccharides, esters
of fatty acids with
other hydroxyl-containing compounds, etc. A fatty acid comprising material can
be one or
more of these compounds in an isolated or purified form. It can be a material
that includes
one or more of these compounds that is combined or blended with other similar
or different
materials. It can be a material where the fatty acid comprising material
occurs with or is
provided with other similar or different materials, such as vegetable and
animal oils; mixtures
of vegetable and animal oils; vegetable and animal oil byproducts; mixtures of
vegetable and
animal oil byproducts; vegetable and animal wax esters; mixtures, derivatives
and byproducts
of vegetable and animal wax esters; seeds; processed seeds; seed byproducts;
nuts; processed
nuts; nut byproducts; animal matter; processed animal matter; byproducts of
animal matter;
corn; processed corn; corn byproducts; distiller's grains; beans; processed
beans; bean
byproducts; soy products; lipid containing plant, fish or animal matter;
processed lipid
containing plant or animal matter; byproducts of lipid containing plant, fish
or animal matter;
lipid containing microbial material; processed lipid containing microbial
material; and
byproducts of lipid containing microbial matter. Such materials can be
utilized in liquid or
solid forms. Solid forms include whole forms, such as cells, beans, and seeds;
ground,
chopped, slurried, extracted, flaked, milled, etc. The fatty acid portion of
the fatty acid
comprising compound can be a simple fatty acid, such as one that includes a
carboxyl group
attached to a substituted or un-substituted alkyl group. The substituted or
unsubstituted alkyl
group can be straight or branched, saturated or unsaturated. Substitutions on
the alkyl group
can include hydroxyls, phosphates, halogens, alkoxy, or aryl groups. The
substituted or
unsubstituted alkyl group can have 7 to 29 carbons and preferably 11 to 23
carbons (e.g., 8 to
31
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
30 carbons and preferably 12 to 24 carbons counting the carboxyl group)
arranged in a linear
chain with or without side chains and/or substitutions. Addition of the fatty
acid comprising
compound can be by way of adding a material comprising the fatty acid
comprising
compound.
[0049] The term "pH modifier" as used herein has its ordinary meaning as known
to those
skilled in the art and can include any material that will tend to increase,
decrease or hold
steady the pH of the broth or medium. A pH modifier can be an acid, a base, a
buffer, or a
material that reacts with other materials present to serve to raise, lower, or
hold steady the
pH. In one embodiment, more than one pH modifier can be used, such as more
than one acid,
more than one base, one or more acid with one or more bases, one or more acids
with one or
more buffers, one or more bases with one or more buffers, or one or more acids
with one or
more bases with one or more buffers. In one embodiment, a buffer can be
produced in the
broth or medium or separately and used as an ingredient by at least partially
reacting in acid
or base with a base or an acid, respectively. When more than one pH modifiers
are utilized,
they can be added at the same time or at different times. In one embodiment,
one or more
acids and one or more bases are combined, resulting in a buffer. In one
embodiment, media
components, such as a carbon source or a nitrogen source serve as a pH
modifier; suitable
media components include those with high or low pH or those with buffering
capacity.
Exemplary media components include acid- or base-hydrolyzed plant
polysaccharides having
residual acid or base, ammonia fiber explosion (AFEX) treated plant material
with residual
ammonia, lactic acid, corn steep solids or liquor.
[0050] "Growth phase" is used herein to describe the type of cellular growth
that occurs after
the "Initiation phase" and before the "Stationary phase" and the "Death
phase." The growth
phase is sometimes referred to as the exponential phase or log phase or
logarithmic phase.
[0051] The term "plant polysaccharide" as used herein has its ordinary meaning
as known to
those skilled in the art and can comprise one or more polymers of sugars and
sugar
derivatives as well as derivatives of sugar polymers and/or other polymeric
materials that
occur in plant matter. Exemplary plant polysaccharides include lignin,
cellulose, starch,
pectin, and hemicellulose. Others are chitin, sulfonated polysaccharides such
as alginic acid,
agarose, carrageenan, porphyran, furcelleran and funoran. Generally, the
polysaccharide can
have two or more sugar units or derivatives of sugar units. The sugar units
and/or derivatives
of sugar units can repeat in a regular pattern, or otherwise. The sugar units
can be hexose
units or pentose units, or combinations of these. The derivatives of sugar
units can be sugar
alcohols, sugar acids, amino sugars, etc. The polysaccharides can be linear,
branched, cross-
32
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
linked, or a mixture thereof. One type or class of polysaccharide can be cross-
linked to
another type or class of polysaccharide. The concentration of saccharides in a
biomass
containing plant polysaccharides such as cellulose, hemicellulose, starch, or
pectin can be
given in terms of monosaccharide equivalents. A monosaccharide equivalent
concentration is
the concentration of saccharides assuming complete hydrolysis of
polysaccharides to
monosaccharides.
[0052] The term "saccharification" as used herein has its ordinary meaning as
known to those
skilled in the art and can include conversion of plant polysaccharides to
lower molecular
weight species that can be utilized by the organism at hand. For some
organisms, this would
include conversion to monosaccharides, disaccharides, trisaccharides, and
oligosaccharides of
up to about seven monomer units, as well as similar sized chains of sugar
derivatives and
combinations of sugars and sugar derivatives.
[0053] The terms "SSF" and "SHF" are known to those skilled in the art; SSF
meaning
simultaneous saccharification and fermentation, or the conversion from
polysaccharides or
oligosaccharides into monosaccharides at the same time and in the same
fermentation vessel
wherein monosaccharides are converted to another chemical product such as
ethanol. "SHF"
indicates a physical separation of the polymer hydrolysis or saccharification
and fermentation
processes.
[0054] The term "biomass" as used herein has its ordinary meaning as known to
those skilled
in the art and can include one or more biological materials that can be
converted into a
biofuel, chemical or other product. Biomass as used herein is synonymous with
the term
"feedstock" and includes corn syrup, molasses, silage, agricultural residues
(corn stalks,
grass, straw, grain hulls, bagasse, etc.), animal waste (manure from cattle,
poultry, and hogs),
Distillers Dried Solubles (DDS), Distillers Dried Grains (DDG), Condensed
Distillers
Solubles (CDS), Distillers Wet Grains (DWG), Distillers Dried Grains with
Solubles
(DDGS), woody materials (wood or bark, sawdust, timber slash, and mill scrap),
municipal
waste (waste paper, recycled toilet papers, yard clippings, etc.), and energy
crops (poplars,
willows, switchgrass, alfalfa, prairie bluestem, algae, including macroalgae,
etc.). One
exemplary source of biomass is plant matter. Plant matter can be, for example,
woody plant
matter, non-woody plant matter, cellulosic material, lignocellulosic material,
hemicellulosic
material, carbohydrates, pectin, starch, inulin, fructans, glucans, corn,
sugar cane, grasses,
switchgrass, sorghum, high biomass sorghum, bamboo, algae and material derived
from
these. Plants can be in their natural state or genetically modified, e.g., to
increase the
cellulosic or hemicellulosic portion of the cell wall, or to produce
additional exogenous or
33
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
endogenous enzymes to increase the separation of cell wall components. Plant
matter can
also include plant cell culture or plant cell tissue culture. Plant matter can
be further
described by reference to the chemical species present, such as proteins,
polysaccharides and
oils. Polysaccharides include polymers of various monosaccharides and
derivatives of
monosaccharides including glucose, fructose, lactose, galacturonic acid,
rhamnose, etc. Plant
matter also includes agricultural waste byproducts or side streams such as
pomace, corn steep
liquor, corn steep solids, distillers grains, peels, pits, fermentation waste,
straw, lumber,
sewage, garbage and food leftovers. Peels can be citrus which include, but are
not limited to,
tangerine peel, grapefruit peel, orange peel, tangerine peel, lime peel and
lemon peel. These
materials can come from farms, forestry, industrial sources, households, etc.
Another non-
limiting example of biomass is animal matter, including, for example milk,
meat, fat, animal
processing waste, and animal waste. "Feedstock" is frequently used to refer to
biomass being
used for a process, such as those described herein.
[0055] "Broth" is used herein to refer to inoculated medium at any stage of
growth, including
the point immediately after inoculation and the period after any or all
cellular activity has
ceased and can include the material after post-fermentation processing. It
includes the entire
contents of the combination of soluble and insoluble matter, suspended matter,
cells and
medium, as appropriate.
[0056] The term "productivity" as used herein has its ordinary meaning as
known to those
skilled in the art and can include the mass of a material of interest produced
in a given time in
a given volume. Units can be, for example, grams per liter-hour, or some other
combination
of mass, volume, and time. In fermentation, productivity is frequently used to
characterize
how fast a product can be made within a given fermentation volume. The volume
can be
referenced to the total volume of the fermentation vessel, the working volume
of the
fermentation vessel, or the actual volume of broth being fermented. The
context of the
phrase will indicate the meaning intended to one of skill in the art.
Productivity is different
from "titer" in that productivity includes a time term, and titer is analogous
to concentration.
Titer and Productivity can generally be measured at any time during the
fermentation, such as
at the beginning, the end, or at some intermediate time, with titer relating
the amount of a
particular material present or produced at the point in time of interest and
the productivity
relating the amount of a particular material produced per liter in a given
amount of time. The
amount of time used in the productivity determination can be from the
beginning of the
fermentation or from some other time, and go to the end of the fermentation,
such as when no
additional material is produced or when harvest occurs, or some other time as
indicated by
34
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
the context of the use of the term. "Overall productivity" refers to the
productivity
determined by utilizing the final titer and the overall fermentation time.
[0057] "Titer" refers to the amount of a particular material present in a
fermentation broth. It
is similar to concentration and can refer to the amount of material made by
the organism in
the broth from all fermentation cycles, or the amount of material made in the
current
fermentation cycle or over a given period of time, or the amount of material
present from
whatever source, such as produced by the organism or added to the broth.
Frequently, the
titer of soluble species will be referenced to the liquid portion of the
broth, with insolubles
removed, and the titer of insoluble species will be referenced to the total
amount of broth
with insoluble species being present, however, the titer of soluble species
can be referenced
to the total broth volume and the titer of insoluble species can be referenced
to the liquid
portion, with the context indicating the which system is used with both
reference systems
intended in some cases. Frequently, the value determined referenced to one
system will be
the same or a sufficient approximation of the value referenced to the other.
[0058] "Concentration" when referring to material in the broth generally
refers to the amount
of a material present from all sources, whether made by the organism or added
to the broth.
Concentration can refer to soluble species or insoluble species, and is
referenced to either the
liquid portion of the broth or the total volume of the broth, as for "titer."
[0059] The term "biocatalyst" as used herein has its ordinary meaning as known
to those
skilled in the art and can include one or more enzymes and/or microorganisms,
including
solutions, suspensions, and mixtures of enzymes and microorganisms. In some
contexts this
word will refer to the possible use of either enzymes or microorganisms to
serve a particular
function, in other contexts the word will refer to the combined use of the
two, and in other
contexts the word will refer to only one of the two. The context of the phrase
will indicate
the meaning intended to one of skill in the art. For example, a biocatalyst
can be a fermenting
microorganism. The term biocatalyst includes fermenting microorganisms such as
yeast,
bacteria, or algae.
[0060] The terms "conversion efficiency" or "yield" as used herein have their
ordinary
meaning as known to those skilled in the art and can include the mass of
product made from a
mass of substrate. The term can be expressed as a percentage yield of the
product from a
starting mass of substrate. For the production of ethanol from glucose, the
net reaction is
generally accepted as:
C6111206 2 C2H5OH + 2 CO2
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
and the theoretical maximum conversion efficiency, or yield, is 51% (wt.).
Frequently, the
conversion efficiency will be referenced to the theoretical maximum, for
example, "80% of
the theoretical maximum." In the case of conversion of glucose to ethanol,
this statement
would indicate a conversion efficiency of 41% (wt.). The context of the phrase
will indicate
the substrate and product intended to one of skill in the art.
[0061] "Pretreatment" or "pretreated" is used herein to refer to any
mechanical, chemical,
thermal, biochemical process or combination of these processes whether in a
combined step
or performed sequentially, that achieves disruption or expansion of the
biomass so as to
render the biomass more susceptible to attack by enzymes and/or microbes. In
one
embodiment, pretreatment includes removal or disruption of lignin so as to
make the
cellulose and hemicellulose polymers in the plant biomass more available to
cellulolytic
enzymes and/or microbes, for example, by treatment with acid or base. In one
embodiment,
pretreatment includes disruption or expansion of cellulosic and/or hemicellulo
sic material.
Steam explosion, and ammonia fiber expansion (or explosion) (AFEX) are well
known
thermal/chemical techniques. Hydrolysis, including methods that utilize acids,
bases, and/or
enzymes can be used. Other thermal, chemical, biochemical, enzymatic
techniques can also
be used.
[0062] "Fed-batch" or "fed-batch fermentation" is used herein to include
methods of
culturing microorganisms where nutrients, other medium components, or
biocatalysts
(including, for example, enzymes, fresh organisms, extracellular broth,
genetically modified
plants and/or organisms, etc.) are supplied to the fermentor during
cultivation, but culture
broth is not harvested from the fermentor until the end of the fermentation,
although it can
also include "self seeding" or "partial harvest" techniques where a portion of
the fermentor
volume is harvested and then fresh medium is added to the remaining broth in
the fermentor,
with at least a portion of the inoculum being the broth that was left in the
fermentor. During
a fed-batch fermentation, the broth volume can increase, at least for a
period, by adding
medium or nutrients to the broth while fermentation organisms are present.
Suitable nutrients
which can be utilized include those that are soluble, insoluble, and partially
soluble, including
gasses, liquids and solids. In one embodiment, a fed-batch process is referred
to with a
phrase such as, "fed-batch with cell augmentation." This phrase can include an
operation
where nutrients and cells are added or one where cells with no substantial
amount of nutrients
are added. The more general phrase "fed-batch" encompasses these operations as
well. The
context where any of these phrases is used will indicate to one of skill in
the art the
techniques being considered.
36
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[0063] "Sugar compounds" or "sugar streams" is used herein to indicate mostly
monosaccharide sugars, dissolved, crystallized, evaporated, or partially
dissolved, including
but not limited to hexoses and pentoses; sugar alcohols; sugar acids; sugar
amines;
compounds containing two or more of these linked together directly or
indirectly through
covalent or ionic bonds; and mixtures thereof. Included within this
description are
disaccharides; trisaccharides; oligosaccharides; polysaccharides; and sugar
chains, branched
and/or linear, of any length. A sugar stream can consist of primarily or
substantially C6
sugars (e.g., a C6-rich stream), C5 sugars (e.g., a C5-rich stream), or
mixtures of both C6 and
C5 sugars in varying ratios of said sugars. C6 sugars have a six-carbon
molecular backbone
and C5 sugars have a five-carbon molecular backbone. Sugar compounds or sugar
streams
can be produced from the pretreatment and/or hydrolysis of biomass. The
biomass can
comprise cellulose, hemicellulose, lignocellulose, starch, or a combination
thereof. Sugars or
sugar streams produced from cellulose, hemicellulose, and/or lignocellulose
can be termed
"cellulosic-derived saccharides". Sugars or sugar streams produced from starch
can be termed
"non-cellulosic-derived saccharides" or "non-cellulosic derived saccharide
streams.
[0064] "C5-rich" composition means that one or more steps have been taken to
remove at
least some of the C6 sugars originally in the composition. For example, a C5-
rich
composition can include no more than about 50% C6 sugars, no more than about
40% C6
sugars, no more than about 30% C6 sugars, no more than about 20% C6 sugars, no
more than
about 10% C6 sugars, no more than about 5% C6 sugars, or it can include from
about 2% to
about 10% C6 sugars by weight. Likewise, a "C6-rich" composition is one in
which at least
some of the originally-present C5 sugars have been removed. For example, a C6-
rich
composition can include no more than about 50% C5 sugars, nor more than about
40% C5
sugars, no more than about 30% C5 sugars, no more than about 20% C5 sugars, no
more than
about 10% C5 sugars, no more than about 5% C5 sugars, or it can include from
about 2% to
about 10% C5 sugars by weight.
[0065] A "liquid" composition may contain solids and a "solids" composition
may contain
liquids. A liquid composition refers to a composition in which the material is
primarily liquid,
and a solids composition is one in which the material is primarily solid.
[0066] "Gentle Pretreatment" generally refers to the collection of processes
upstream of
hydrolysis, which result in composition that, when hydrolyzed, produces a
fermentable sugar
composition. The fermentable sugar composition can be used to enhance a non-
cellulosic
fermentation process, such as a corn mash fermentation process. In some
embodiments, the
gentle pretreatment process provides a fermentable sugar composition having a
favorable
37
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
nutrient balance (e.g. plant-derived extracted nutrients, which are part of
the composition as a
result of the pretreatment process) and/or an amount of toxic compounds (e.g.
phenolics and
sugar degradation products, organic acids and furans, which inhibit and/or
inactivate the
performance of enzymes and or fermentation organisms), which is limited such
that the
resultant fermentable sugar composition can enhance a non-cellulosic
fermentation process,
such as a corn mash fermentation process. For example, a gentle pretreatment
is one that
results in a sugar stream that is about 25% (w/v) C6 sugars or more, about 4
g/L
hydroxymethyl furfural or less, about 4 g/L furfural or less, about 10 g/L
acetic acid or less,
about 10 g/L formic acid or less for example as measured by typical HPLC
methods referred
to herein. ("About X amount of a substance or less" means the same as "no more
than about"
and includes zero¨i.e. includes the possibility that none of that substance is
present in the
composition.) "Gentle pretreatment" can include one or more of: pre-processing
biomass to
reduce size and/or create size uniformity; pretreatment itself (process for
making cellulose
more accessible to hydrolysis); and post-processing steps such as washing
steps.
[0067] The terms "non-cellulosic" and "sugar- or starch- based" are used
interchangeably and
have the same meaning. For example "non-cellulosic fermentation process" is
used
interchangeably and means the same thing as "sugar- and starch-based
fermentation process."
Starch is a carbohydrate consisting of consisting of a large number of glucose
units joined by
glycosidic bonds. The glycosidic bonds are typically the easily hydrolysable
alpha glycosidic
bonds. This polysaccharide can be produced by all green plants as an energy
store. There can
be two types of starch molecules: the linear and helical amylose and the
branched
amylopectin, although amylase can also contain branches.
[0068] A "first biomass" as used herein includes simple saccharides (e.g.,
mono and di-
saccharides, e.g., glucose, sucrose, lactose, etc.) and/or starch-containing
materials. For
example, a first biomass includes corn, corn mash, sugar cane, sugar beets,
sugar palms,
sweet sorghum, nypa palm, cassava, rice, milo, sorghum, sweet potatoes, wheat,
molasses,
tubers, roots, stems, whole grains, barley, rye, milo, sago, cassaya, tapioca,
rice peas, beans,
potatoes, beets, fruits, or any other sugar or starch containing materials, or
combination of
sugar or starch containing materials or sugar or starch containing biomasses.
The term
"simple saccharides and/or starch containing biomass" is also used
interchangeably herein
with a "first biomass."
Description
[0069] The following description and examples illustrate some exemplary
embodiments of
the disclosure in detail. Those of skill in the art will recognize that there
are numerous
38
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
variations and modifications of this disclosure that are encompassed by its
scope.
Accordingly, the description of a certain exemplary embodiment should not be
deemed to
limit the scope of the present disclosure.
[0070] Corn Fermentation
[0071] To overcome the cost of corn, and issues of glycerol production and
slow
fermentation, several entities have tried adding mixed cellulosic-derived
sugars either with or
without the pretreatment liquor. There has been little or no success using
such methods.
One reason is that the industrial yeasts used to ferment starch are primarily
C6-fermenting
species. In fact, almost all yeasts require C6 sugars as a feedstock and
cannot ferment
pentose (C5) sugar as rapidly as C6 sugar or cannot ferment C5 sugar at all.
Those that can
ferment C5 sugars do not tolerate high levels of ethanol, thus are not useful
in the industrial
production of bio fuels.
[0072] In the production of sugar-derived products through fermentation, it is
important to
carry out the fermentation as quickly as possible. The risk of contamination
increases as the
fermentation lengthens. Furthermore, the energy requirements increase the cost
of
fermentation, thus raising the price of the product.
[0073] Feedstock and Pretreatment of Feedstock
[0074] In one embodiment, the feedstock (biomass) contains cellulosic,
hemicellulosic,
and/or lignocellulosic material. The feedstock can be derived from
agricultural crops, crop
residues, trees, woodchips, sawdust, paper, cardboard, grasses, algae,
municipal waste and
other sources.
[0075] Cellulose is a linear polymer of glucose where the glucose units are
connected via
p(1 ¨>4) linkages. Hemicellulose is a branched polymer of a number of sugar
monomers
including glucose, xylose, mannose, galactose, rhamnose and arabinose, and can
have sugar
acids such as mannuronic acid and galacturonic acid present as well. Lignin is
a cross-linked,
racemic macromolecule of mostly p-coumaryl alcohol, conferyl alcohol and
sinapyl alcohol.
These three polymers occur together in lignocellulosic materials in plant
biomass. The
different characteristics of the three polymers can make hydrolysis of the
combination
difficult as each polymer tends to shield the others from enzymatic attack.
[0076] In one embodiment, methods are provided for the pretreatment of
feedstock used in
the fermentation and production of the biofuels and chemicals. The
pretreatment steps can
include mechanical, thermal, pressure, chemical, thermochemical, and/or
biochemical tests
pretreatment prior to being used in a bioprocess for the production of fuels
and chemicals, but
untreated biomass material can be used in the process as well. Mechanical
processes can
39
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
reduce the particle size of the biomass material so that it can be more
conveniently handled in
the bioprocess and can increase the surface area of the feedstock to
facilitate contact with
chemicals/biochemicals/biocatalysts. Mechanical processes can also separate
one type of
biomass material from another. The biomass material can also be subjected to
thermal and/or
chemical pretreatments to render plant polymers more accessible. Multiple
steps of treatment
can also be used.
[0077] Mechanical processes include, are not limited to, washing, soaking,
milling, size
reduction, screening, shearing, size classification and density classification
processes.
Chemical processes include, but are not limited to, bleaching, oxidation,
reduction, acid
treatment, base treatment, sulfite treatment, acid sulfite treatment, basic
sulfite treatment,
ammonia treatment, and hydrolysis. Thermal processes include, but are not
limited to,
sterilization, ammonia fiber expansion or explosion ("AFEX"), steam explosion,
holding at
elevated temperatures, pressurized or unpressurized, in the presence or
absence of water, and
freezing. Biochemical processes include, but are not limited to, treatment
with enzymes,
including enzymes produced by genetically-modified plants, and treatment with
microorganisms. Various enzymes that can be utilized include cellulase,
amylase, f3-
glucosidase, xylanase, gluconase, and other polysaccharases; lysozyme;
laccase, and other
lignin-modifying enzymes; lipoxygenase, peroxidase, and other oxidative
enzymes;
proteases; and lipases. One or more of the mechanical, chemical, thermal,
thermochemical,
and biochemical processes can be combined or used separately. Such combined
processes
can also include those used in the production of paper, cellulose products,
microcrystalline
cellulose, and cellulosics and can include pulping, kraft pulping, acidic
sulfite processing.
The feedstock can be a side stream or waste stream from a facility that
utilizes one or more of
these processes on a biomass material, such as cellulosic, hemicellulosic or
lignocellulosic
material. Examples include paper plants, cellulosics plants, distillation
plants, cotton
processing plants, and microcrystalline cellulose plants. The feedstock can
also include
cellulose-containing or cellulosic containing waste materials. The feedstock
can also be
biomass materials, such as wood, grasses, corn, starch, or sugar, produced or
harvested as an
intended feedstock for production of ethanol or other products such as by
biocatalysts.
[0078] In another embodiment, a method can utilize a pretreatment process
disclosed in U.S.
Patents and Patent Applications U520040152881, US20040171136, U520040168960,
U520080121359, U520060069244, U520060188980, U520080176301, 5693296, 6262313,
U520060024801, 5969189, 6043392, U520020038058, U55865898, U55865898,
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
US6478965, 5986133, or US20080280338, each of which is incorporated by
reference herein
in its entirety.
[0079] In another embodiment, the AFEX process is be used for pretreatment of
biomass. In
a preferred embodiment, the AFEX process is used in the preparation of
cellulosic,
hemicellulosic or lignocellulosic materials for fermentation to ethanol or
other products. The
process generally includes combining the feedstock with ammonia, heating under
pressure,
and suddenly releasing the pressure. Water can be present in various amounts.
The AFEX
process has been the subject of numerous patents and publications.
[0080] In another embodiment, the pretreatment of biomass comprises the
addition of
calcium hydroxide to a biomass to render the biomass susceptible to
degradation.
Pretreatment comprises the addition of calcium hydroxide and water to the
biomass to form a
mixture, and maintaining the mixture at a relatively high temperature.
Alternatively, an
oxidizing agent, selected from the group consisting of oxygen and oxygen-
containing gasses,
can be added under pressure to the mixture. Examples of carbon hydroxide
treatments are
disclosed in U.S. Patent No. 5865898 to Holtzapple and S. Kim and M. T.
Holzapple,
Bioresource Technology, 96, (2005) 1994, incorporated by reference herein in
its entirety.
[0081] In one embodiment, pretreatment of biomass comprises dilute acid
hydrolysis.
Example of dilute acid hydrolysis treatment are disclosed in T. A. Lloyd and
C. E Wyman,
Bioresource Technology, (2005) 96, 1967, incorporated by reference herein in
its entirety.
[0082] In another embodiment, pretreatment of biomass comprises pH controlled
liquid hot
water treatment. Examples of pH controlled liquid hot water treatments are
disclosed in N.
Mosier et at., Bioresource Technology, (2005) 96, 1986, incorporated by
reference herein in
its entirety.
[0083] In one embodiment, pretreatment of biomass comprises aqueous ammonia
recycle
process (ARP). Examples of aqueous ammonia recycle process are described in T.
H. Kim
and Y. Y. Lee, Bioresource Technology, (2005) 96, 2007, incorporated by
reference herein
in its entirety.
[0084] In one embodiment, the above mentioned methods have two steps: a
pretreatment step
that leads to a wash stream, and an enzymatic hydrolysis step of pretreated-
biomass that
produces a hydrolysate stream. In the above methods, the pretreatment step can
include acid
hydrolysis, hot water pretreatment, steam explosion or alkaline reagent based
methods
(AFEX, ARP, and lime pretreatments). Dilute acid and hot water treatment
methods can be
used to solubilize all or a portion of the hemicellulose. Methods employing
alkaline reagents
can be used remove all, most, or a portion of the lignin during the
pretreatment step. As a
41
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
result, the wash stream from the pretreatment step in the former methods
contains mostly
hemicellulose-based sugars, whereas this stream has mostly lignin for the high-
pH methods.
The subsequent enzymatic hydrolysis of the residual biomass leads to mixed
sugars (C5 and
C6) in the alkali based pretreatment methods, while glucose is the major
product in the
hydrolysate from the low and neutral pH methods. Such a hydrolysate can be
referred to as a
C6-enriched hydrolysate. In one embodiment, the treated material is
additionally treated with
catalase or another similar chemical, chelating agents, surfactants, and other
compounds to
remove impurities or toxic chemicals or further release polysaccharides.
[0085] In one embodiment, one or more monosaccharides are produced by
pretreating and/or
hydrolyzing a biomass comprising cellulose, hemicellulose, lignocellulose
and/or starch. The
biomass can be pretreated according to any of the methods disclosed herein;
for example, by
dilute acid, hot water treatment, stream explosion, or an alkaline
pretreatment. The biomass
can be pretreated using a combination of techniques; for example, the biomass
can be
pretreated using hot water or stream explosion followed by alkaline treatment.
The one or
more monosaccharides can include C6 and/or C5 monosaccharides. The one or more
monosaccharides can be in a C6-enriched hydrolysate (C6 Saccharide Stream).
The one or
more monosaccharides can be in a C5-enriched hydrolysate (C5 Saccharide
Stream). The one
or more monosaccharides can comprise both C5 and C6 saccharides (C5 + C6
Saccharide
Stream). The one or more monosaccharides can include cellulosic-derived
monosaccharides.
The one or more monosaccharides can include non-cellulosic-derived
monosaccharides (e.g.,
starch-derived monosaccharides). The one or more monosaccharides can include
glucose,
fructose, galactose, xylose, or any other saccharides.
[0086] A C6-enriched hydrolysate (C6 Saccharide Stream) is enriched for C6
sugars;
however, the C6-enriched hydrolysate can comprise C5 sugars. In one
embodiment, less than
about 50%, 40%, 30%, 20%, 10%, or 1% of the sugars in the C6-enriched
hydrolysate are C5
sugars. In another embodiment, about 0-50%, 0-40%, 0-30%, 0-20%, 0-10%, 0-1%,
0-0.1%,
0.1-50%, 0.1-40%, 0.1-30%, 0.1-20%, 0.1-10%, 0.1-1%, 1-50%, 1-40%, 1-30%, 1-
20%, 1-
10%, 10-50%, 10-40%, 10-30%, 10-20%, 20-50%, 20-40%, 20-30%, 30-50%, 30-40%,
of
40-50% of the sugars in a C6-enriched hydrolysate are C5 sugars. The C6-
enriched
hydrolysate can comprise one or more cellulosic-derived C6 monosaccharides
(e.g., glucose).
The C6-enriched hydrolysate can comprise one or more non-cellulosic derived
monosaccharides (e.g., starch-derived monosaccharides, e.g., glucose).
[0087] A hydrolyzate or saccharide stream comprising one or more cellulosic or
non-
cellulosic derived saccharides can further comprise residual solids (e.g.,
insoluble or
42
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
suspended solids). The residual solids can include unhydrolyzed cellulose,
hemicellulose,
lignin, or starch; proteins; fats; oils, or a combination thereof. The
hydrolyzate or sugar
stream can comprise from about 0 % to about 50% w/v residual solids; for
example, about 0-
50%, 0-25%, 0-15%, 0-10%, 0-5%, 0-1%, 1-50%, 1-25%, 1-15%, 1-10%, 1-5%, 5-50%,
5-
25%, 5-15%, 5-10%, 10-50%, 10-25%, 10-15%, 15-50%, 15-25%, 25-50%, 0%, 1%, 2%,
3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%,
20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%, 32%, 33%, 34%,
35%,
36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, or 50%
residual solids w/v.
[0088] Residual solids (e.g., insoluble or suspended solids) in a hydrolyzate
or saccharide
stream (e.g., C5 Saccharide Stream, C6 Saccharide Stream, C5 + C6 Saccharide
Stream, etc.)
can have, for example, particle sizes of from 10 ILIM to 2.5 mm or larger or
smaller. The
residual solids can have particles sizes of about 0.01-2.5 mm, 0.01-1 mm, 0.01-
0.5 mm, 0.01-
0.1 mm, 0.01-0.05 mm, 0.05-2.5 mm, 0.05-1 mm, 0.05-0.5 mm, 0.05-0.1 mm, 0.1-
2.5 mm,
0.1-1 mm, 0.1-0.5 mm, 0.5-2.5 mm, 0.5-1 mm, 1-2.5 mm, 0.01 mm, 0.02 mm, 0.03
mm, 0.04
mm, 0.05 mm, 0.06 mm, 0.07 mm, 0.08 mm, 0.09 mm, 0.1 mm, 0.11 mm, 0.12 mm,
0.13
mm, 0.14 mm, 0.15 mm, 0.16 mm, 0.17 mm, 0.18 mm, 0.19 mm, 0.2 mm, 0.25 mm, 0.3
mm,
0.35 mm, 0.4 mm, 0.45 mm, 0.5 mm, 0.55 mm, 0.6 mm, 0.65 mm, 0.7 mm, 0.75 mm,
0.8
mm, 0.85 mm, 0.9 mm, 0.95 mm, 1 mm, 1.05 mm, 1.1 mm, 1.15 mm, 1.2 mm, 1.25 mm,
1.3
mm, 1.35 mm, 1.4 mm, 1.45 mm, 1.5 mm, 1.6 mm, 1.7 mm, 1.8 mm, 1.9 mm, 2 mm,
2.1 mm,
2.2 mm, 2.3 mm, 2.4 mm, 2.5 mm.
[0089] Residual solids (e.g., insoluble or suspended solids) in a hydrolyzate
or saccharide
stream (e.g., C5 Saccharide Stream, C6 Saccharide Stream, C5 + C6 Saccharide
Stream, etc.)
can have, for example, particle sizes of from 10 1\43 to 2.5 mm3 or larger or
smaller. The
residual solids can have particles sizes of about 0.01-2.5 mm3, 0.01-1 mm3,
0.01-0.5 mm3,
0.01-0.1 mm3, 0.01-0.05 mm3, 0.05-2.5 mm3, 0.05-1 mm3, 0.05-0.5 mm3, 0.05-0.1
mm3, 0.1-
2.5 mm3, 0.1-1 mm3, 0.1-0.5 mm3, 0.5-2.5 mm3, 0.5-1 mm3, 1-2.5 mm3, 0.01 mm3,
0.02 mm3,
0.03 mm3, 0.04 mm3, 0.05 mm3, 0.06 mm3, 0.07 mm3, 0.08 mm3, 0.09 mm3, 0.1 mm3,
0.11
mm3, 0.12 mm3, 0.13 mm3, 0.14 mm3, 0.15 mm3, 0.16 mm3, 0.17 mm3, 0.18 mm3,
0.19 mm3,
0.2 mm3, 0.25 mm3, 0.3 mm3, 0.35 mm3, 0.4 mm3, 0.45 mm3, 0.5 mm3, 0.55 mm3,
0.6 mm3,
0.65 mm3, 0.7 mm3, 0.75 mm3, 0.8 mm3, 0.85 mm3, 0.9 mm3, 0.95 mm3, 1 mm3, 1.05
mm3,
1.1 mm3, 1.15 mm3, 1.2 mm3, 1.25 mm3, 1.3 mm3, 1.35 mm3, 1.4 mm3, 1.45 mm3,
1.5 mm3,
1.6 mm3, 1.7 mm3, 1.8 mm3, 1.9 mm3, 2 mm3, 2.1 mm3, 2.2 mm3, 2.3 mm3, 2.4 mm3,
2.5
mm3.
43
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[0090] In some embodiments, all or a portion of the residual solids (e.g.,
insoluble or
suspended solids) are sequestered and removed from a hydrolyzate or saccharide
stream (e.g.,
C5 Saccharide Stream, C6 Saccharide Stream, C5 + C6 Saccharide Stream, etc.).
The
sequestration and removal can be accomplished, for example, by flocculation,
filtration,
evaporation, centrifugation, or a combination thereof.
[0091] The level of residual solids (e.g., insoluble or suspended solids) in a
hydrolyzate or
saccharide stream (e.g., C5 Saccharide Stream, C6 Saccharide Stream, C5 + C6
Saccharide
Stream, etc.) can affect the rate and/or final titer of one or more
fermentation end-products in
a fermentation reaction. For example, decreasing the level of residual solids
can decrease the
rate and/or final titer of polyols and/or sugar alcohols. Without being
limited by theory, this
can be due to decreased osmotic stress upon the microbial biocatalyst(s) used
in the
fermentation reaction.
[0092] The level of residual solids (e.g., insoluble or suspended solids) in a
hydrolyzate or
saccharide stream (e.g., C5 Saccharide Stream, C6 Saccharide Stream, C5 + C6
Saccharide
Stream, etc.) can affect the growth rate of a biocatalyst microorganism in a
fermentation
reaction. The residual solids can contain nutrients (e.g., proteins, amino
acids, fats, oils, etc.)
or ions/trace metals that promote microorganism growth. Increased growth rates
can decrease
production of one or more fermentation end-products such as polyols or sugar
alcohols.
[0093] In one embodiment, pretreatment of biomass comprises ionic liquid (IL)
pretreatment.
Biomass can be pretreated by incubation with an ionic liquid, followed by IL
extraction with
a wash solvent such as alcohol or water. The treated biomass can then be
separated from the
ionic liquid/wash-solvent solution by centrifugation or filtration, and sent
to the
saccharification reactor or vessel. Examples of ionic liquid pretreatment are
disclosed in US
publication No. 2008/0227162, incorporated herein by reference in its
entirety.
[0094] In another embodiment, a method can utilize a pretreatment process
disclosed in U.S.
Patent No. 4600590 to Dale, U.S. Patent No. 4644060 to Chou, U.S. Patent No.
5037663 to
Dale. U.S. Patent No. 5171592 to Holtzapple, et at., et at., U.S. Patent No.
5939544 to
Karstens, et at., U.S. Patent No. 5473061 to Bredereck, et at., U.S. Patent
No. 6416621 to
Karstens., U.S. Patent No. 6106888 to Dale, et at., U.S. Patent No. 6176176 to
Dale, et at.,
PCT publication W02008/020901 to Dale, et at., Felix, A., et at., Anim. Prod.
51, 47-61
(1990)., Wais, A.C., Jr., et at., Journal of Animal Science, 35, No. 1,109-112
(1972), which
are incorporated herein by reference in their entireties.
[0095] Alteration of the pH of a pretreated feedstock can be accomplished by
washing the
feedstock (e.g., with water) one or more times to remove an alkaline or acidic
substance, or
44
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
other substance used or produced during pretreatment. Washing can comprise
exposing the
pretreated feedstock to an equal volume of water 2, 3, 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, 15,
16, 17, 18, 19, 20, 21, 22, 23, 24, 25 or more times. In another embodiment, a
pH modifier
can be added. For example, an acid, a buffer, or a material that reacts with
other materials
present can be added to modulate the pH of the feedstock. In one embodiment,
more than one
pH modifier can be used, such as one or more bases, one or more bases with one
or more
buffers, one or more acids, one or more acids with one or more buffers, or one
or more
buffers. When more than one pH modifiers are utilized, they can be added at
the same time or
at different times. Other non-limiting exemplary methods for neutralizing
feedstocks treated
with alkaline substances have been described, for example in U.S. Patent Nos.
4,048,341;
4,182,780; and 5,693,296.
[0096] In one embodiment, one or more acids can be combined, resulting in a
buffer.
Suitable acids and buffers that can be used as pH modifiers include any liquid
or gaseous acid
that is compatible with the microorganism. Non-limiting examples include
peroxyacetic acid,
sulfuric acid, sulfurous acid, lactic acid, citric acid, phosphoric acid, and
hydrochloric acid. In
some instances, the pH can be lowered to neutral pH or acidic pH, for example
a pH of 7.0,
6.5, 6.0, 5.5, 5.0, 4.5, 4.0, or lower. In some embodiments, the pH is lowered
and/or
maintained within a range of about pH 4.5 to about 7.1, or about 4.5 to about
6.9, or about pH
5.0 to about 6.3, or about pH 5.5 to about 6.3, or about pH 6.0 to about 6.5,
or about pH 5.5 to
about 6.9 or about pH 6.2 to about 6.7.
[0097] In another embodiment, biomass can be pre-treated at an elevated
temperature and/or
pressure. In one embodiment, biomass is pre treated at a temperature range of
20 C to
400 C. In another embodiment, biomass is pretreated at a temperature of about
20 C, 25 C,
30 C, 35 C, 40 C, 45 C, 50 C, 55 C, 60 C, 65 C, 80 C, 90 C, 100 C, 120 C, 150
C,
200 C, 250 C, 300 C, 350 C, 400 C or higher. In another embodiment, elevated
temperatures are provided by the use of steam, hot water, or hot gases. In one
embodiment,
steam can be injected into a biomass containing vessel. In another embodiment,
the steam,
hot water, or hot gas can be injected into a vessel jacket such that it heats,
but does not
directly contact the biomass.
[0098] In another embodiment, a biomass can be treated at an elevated
pressure. In one
embodiment, biomass is pre treated at a pressure range of about lpsi to about
30psi. In
another embodiment, biomass is pre treated at a pressure or about lpsi, 2psi,
3psi, 4psi, 5psi,
6psi, 7psi, 8psi, 9psi, lOpsi, 12psi, 15psi, 18psi, 20psi, 22psi, 24psi,
26psi, 28psi, 30psi or
more. In some embodiments, biomass can be treated with elevated pressures by
the injection
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
of steam into a biomass containing vessel. In one embodiment, the biomass can
be treated to
vacuum conditions prior or subsequent to alkaline or acid treatment or any
other treatment
methods provided herein.
[0099] In one embodiment, alkaline or acid pretreated biomass is washed (e.g.
with water
(hot or cold) or other solvent such as alcohol (e.g. ethanol)), pH neutralized
with an acid,
base, or buffering agent (e.g. phosphate, citrate, borate, or carbonate salt)
or dried prior to
fermentation. In one embodiment, the drying step can be performed under vacuum
to
increase the rate of evaporation of water or other solvents. Alternatively, or
additionally, the
drying step can be performed at elevated temperatures such as about 20 C, 25
C, 30 C, 35 C,
40 C, 45 C, 50 C, 55 C, 60 C, 65 C, 80 C, 90 C, 100 C, 120 C, 150 C, 200 C,
250 C,
300 C or more.
[00100] In
one embodiment, the pretreatment step includes a step of solids recovery.
The solids recovery step can be during or after pretreatment (e.g., acid or
alkali pretreatment),
or before the drying step. In one embodiment, the solids recovery step can
include the use of
a sieve, filter, screen, or a membrane for separating the liquid and solids
fractions. In one
embodiment, a suitable sieve pore diameter size ranges from about 0.001
microns to 8mm,
such as about 0.005microns to 3mm or about 0.01 microns to lmm. In one
embodiment, a
sieve pore size has a pore diameter of about 0.01microns, 0.02 microns, 0.05
microns, 0.1
microns, 0.5 microns, 1 micron, 2 microns, 4 microns, 5 microns, 10 microns,
20 microns, 25
microns, 50 microns, 75 microns, 100 microns, 125 microns, 150 microns, 200
microns, 250
microns, 300 microns, 400 microns, 500 microns, 750 microns, lmm or more. In
one
embodiment, biomass (e.g. corn stover) is processed or pretreated prior to
fermentation. In
one embodiment, a method of pre-treatment includes but is not limited to,
biomass particle
size reduction, such as for example shredding, milling, chipping, crushing,
grinding, or
pulverizing. In one embodiment, biomass particle size reduction can include
size separation
methods such as sieving, or other suitable methods known in the art to
separate materials
based on size. In one embodiment, size separation can provide for enhanced
yields. In one
embodiment, separation of finely shredded biomass (e.g. particles smaller than
about 8 mm in
diameter, such as, 8, 7.9, 7.7, 7.5, 7.3, 7, 6.9, 6.7, 6.5, 6.3, 6, 5.9, 5.7,
5.5, 5.3, 5, 4.9, 4.7, 4.5,
4.3, 4, 3.9, 3.7, 3.5, 3.3, 3, 2.9, 2.7, 2.5, 2.3, 2, 1.9, 1.7, 1.5, 1.3, 1,
0.9, 0.8, 0.7, 0.6, 0.5, 0.4,
0.3, 0.2, or 0.1 mm) from larger particles allows the recycling of the larger
particles back into
the size reduction process, thereby increasing the final yield of processed
biomass. In one
embodiment, a fermentative mixture is provided which comprises a pretreated
lignocellulosic
feedstock comprising less than about 50% of a lignin component present in the
feedstock
46
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
prior to pretreatment and comprising more than about 60% of a hemicellulose
component
present in the feedstock prior to pretreatment; and a microorganism capable of
fermenting a
five-carbon sugar, such as xylose, arabinose or a combination thereof, and a
six-carbon sugar,
such as glucose, galactose, mannose or a combination thereof. In some
instances,
pretreatment of the lignocellulosic feedstock comprises adding an alkaline
substance which
raises the pH to an alkaline level, for example NaOH. In one embodiment, NaOH
is added at
a concentration of about 0.5% to about 2% by weight of the feedstock. In one
embodiment,
pretreatment also comprises addition of a chelating agent.
[00101] Hydrolysis
[00102] In one embodiment, the biomass hydrolyzing unit provides useful
advantages
for the conversion of biomass to biofuels and chemical products. One advantage
of this unit
is its ability to produce monomeric sugars from multiple types of biomass,
including mixtures
of different biomass materials, and is capable of hydrolyzing polysaccharides
and higher
molecular weight saccharides to lower molecular weight saccharides. In one
embodiment,
the hydrolyzing unit utilizes a pretreatment process and a hydrolytic enzyme
which facilitates
the production of a sugar stream containing a concentration of a monomeric
sugar or several
monomeric sugars derived from cellulosic and/or hemicellulosic polymers.
Examples of
biomass material that can be pretreated and hydrolyzed to manufacture sugar
monomers
include, but are not limited to, cellulosic, hemicellulosic, lignocellulosic
materials; pectins;
starches; wood; paper; agricultural products; forest waste; tree waste; tree
bark; leaves;
grasses; sawgrass; woody plant matter; non-woody plant matter; carbohydrates;
starch;
inulin; fructans; glucans; corn; sugar cane; sorghum, other grasses; bamboo,
algae, and
material derived from these materials. This ability to use a very wide range
of pretreatment
methods and hydrolytic enzymes gives distinct advantages in biomass
fermentations.
Various pretreatment conditions and enzyme hydrolysis can enhance the
extraction of sugars
from biomass, resulting in higher yields, higher productivity, greater product
selectivity,
and/or greater conversion efficiency.
[00103] In one embodiment, the enzyme treatment is used to hydrolyze
various higher
saccharides (higher molecular weight) present in biomass to lower saccharides
(lower
molecular weight), such as in preparation for fermentation by biocatalysts
such as yeasts to
produce ethanol, hydrogen, or other chemicals such as organic acids including
succinic acid,
formic acid, acetic acid, and lactic acid. These enzymes and/or the
hydrolysate can be used
in fermentations to produce various products including fuels, and other
chemicals.
47
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
[00104] In one example, the process for converting biomass material into
ethanol
includes pretreating the biomass material (e.g., "feedstock"), hydrolyzing the
pretreated
biomass to convert polysaccharides to oligosaccharides, further hydrolyzing
the
oligosaccharides to monosaccharides, and converting the monosaccharides to
biofuels and
chemical products. Enzymes such as cellulases, polysaccharases, lipases,
proteases,
ligninases, and hemicellulases, help produce the monosaccharides can be used
in the
biosynthesis of fermentation end-products. Biomass material that can be
utilized includes
woody plant matter, non-woody plant matter, cellulosic material,
lignocellulosic material,
hemicellulosic material, carbohydrates, pectin, starch, inulin, fructans,
glucans, corn, algae,
sugarcane, other grasses, switchgrass, bagasse, wheat straw, barley straw,
rice straw,
corncobs, bamboo, citrus peels, sorghum, high biomass sorghum, seed hulls, and
material
derived from these. The final product can then be separated and/or purified,
as indicated by
the properties for the desired final product. In some instances, compounds
related to sugars
such as sugar alcohols or sugar acids can be utilized as well.
[00105] Chemicals that can be used in the methods disclosed herein can be
purchased
from a commercial supplier, such as Sigma-Aldrich. Additionally, commercial
enzyme
cocktails (e.g. AccelleraseTM 1000, CelluSeb-TL, CelluSeb-TS, CellicTM' CTec,
STARGENTM, MaxaligTM, Spezyme.RTM, Distillase.RTM, G-Zyme.RTM, Fermenzyme.RTM,
FermgenTM, GC 212, or OptimashTM) or any other commercial enzyme cocktail can
be
purchased from vendors such as Specialty Enzymes & Biochemicals Co., Genencor,
or
Novozymes. Alternatively, enzyme cocktails can be prepared by growing one or
more
organisms such as for example a fungi (e.g. a Trichoderma, a Saccharomyces, a
Pichia, a
White Rot Fungus etc.), a bacteria (e.g. a Clostridium, or a coliform
bacterium, a Zymomonas
bacterium, Sacharophagus degradans etc.) in a suitable medium and harvesting
enzymes
produced therefrom. In some embodiments, the harvesting can include one or
more steps of
purification of enzymes.
[00106] In one embodiment, treatment of biomass comprises enzyme
hydrolysis. In
one embodiment, a biomass is treated with an enzyme or a mixture of enzymes,
e.g.,
endoglucanases, exoglucanases, cellobiohydro lases, cellulase, beta-
glucosidases, glycoside
hydrolases, glycosyltransferases, lyases, esterases, amylases, glucoamylases,
and proteins
containing carbohydrate-binding modules. In one embodiment, the enzyme or
mixture of
enzymes is one or more individual enzymes with distinct activities. In another
embodiment,
the enzyme or mixture of enzymes can be enzyme domains with a particular
catalytic
activity. For example, an enzyme with multiple activities can have multiple
enzyme
48
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
domains, including for example glycoside hydrolases, glycosyltransferases,
lyases and/or
esterases catalytic domains.
[00107] In one embodiment, enzymes that degrade polysaccharides are used
for the
hydrolysis of biomass and can include enzymes that degrade cellulose, namely,
cellulases.
Examples of some cellulases include endocellulases and exo-cellulases that
hydrolyze beta-
1,4-glucosidic bonds.
[00108] In one embodiment, enzymes that degrade polysaccharides are used
for the
hydrolysis of biomass and can include enzymes that have the ability to degrade
hemicellulose, namely, hemicellulases. Hemicellulose can be a major component
of plant
biomass and can contain a mixture of pentoses and hexoses, for example, D-
xylopyranose, L-
arabinofuranose, D-mannopyranose, Dglucopyranose, D-galactopyranose, D-
glucopyranosyluronic acid and other sugars. In one embodiment, enzymes that
degrade
polysaccharides are used for the hydrolysis of biomass and can include enzymes
that have the
ability to degrade pectin, namely, pectinases. In plant cell walls, the cross-
linked cellulose
network can be embedded in a matrix of pectins that can be covalently cross-
linked to
xyloglucans and certain structural proteins. Pectin can comprise
homogalacturonan (HG) or
rhamnogalacturonan (RH).
[00109] In one embodiment, hydrolysis of biomass includes enzymes that can
hydrolyze starch. Enzymes that hydrolyze starch include alpha-amylase,
glucoamylase, beta-
amylase, exo-alpha-1,4-glucanase, and pullulanase.
[00110] In one embodiment, hydrolysis of biomass comprises hydrolases that
can
include enzymes that hydrolyze chitin. In another embodiment, hydrolases can
include
enzymes that hydrolyze lichen, namely, lichenase.
[00111] In one embodiment, after pretreatment and/or hydrolysis by any of
the above
methods the feedstock contains cellulose, hemicellulose, soluble oligomers,
simple sugars,
lignin, volatiles and ash. The parameters of the hydrolysis can be changed to
vary the
concentration of the components of the pretreated feedstock. For example, a
hydrolysis can
be chosen so that the concentration of soluble C5 saccharides is high and the
concentration of
lignin is low after hydrolysis. Examples of parameters of the hydrolysis
include temperature,
pressure, time, concentration, composition and pH.
[00112] In one embodiment, the parameters of the pretreatment and
hydrolysis are
changed to vary the concentration of the components of the pretreated
feedstock such that
concentration of the components in the pretreated and hydrolyzed feedstock is
optimal for
fermentation with a microbe such as a yeast or bacterium microbe.
49
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
[00113] In one embodiment, the parameters of the pretreatment are changed
to
encourage the release of the components of a genetically modified feedstock
such as enzymes
stored within a vacuole to increase or complement the enzymes synthesized by
biocatalyst to
produce optimal release of the fermentable components during hydrolysis and
fermentation.
[00114] In one embodiment, the parameters of the pretreatment and
hydrolysis are
changed such that concentration of accessible cellulose in the pretreated
feedstock is 1%, 5%,
10%, 12%, 13%, 14%, 15%, 16%, 17%, 19%, 20%, 30%, 40% or 50%. In one
embodiment,
the parameters of the pretreatment are changed such that concentration of
accessible cellulose
in the pretreated feedstock is 5% to 30%. In one embodiment, the parameters of
the
pretreatment are changed such that concentration of accessible cellulose in
the pretreated
feedstock is 10% to 20%.
[00115] In one embodiment, the parameters of the pretreatment are changed
such that
concentration of hemicellulose in the pretreated feedstock is 1%, 5%, 10%,
12%, 13%, 14%,
15%, 16%, 17%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 40%
or 50%. In one embodiment, the parameters of the pretreatment are changed such
that
concentration of hemicellulose in the pretreated feedstock is 5% to 40%. In
one embodiment,
the parameters of the pretreatment are changed such that concentration of
hemicellulose in
the pretreated feedstock is 10% to 30%.
[00116] In one embodiment, the parameters of the pretreatment and
hydrolysis are
changed such that concentration of soluble oligomers in the pretreated
feedstock is 1%, 10%,
15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%,
90%,
95%, or 99%. Examples of soluble oligomers include, but are not limited to,
cellobiose and
xylobiose. In one embodiment, the parameters of the pretreatment are changed
such that
concentration of soluble oligomers in the pretreated feedstock is 30% to 90%.
In one
embodiment, the parameters of the pretreatment and/or hydrolysis are changed
such that
concentration of soluble oligomers in the pretreated feedstock is 45% to 80%.
[00117] In one embodiment, the parameters of the pretreatment and
hydrolysis are
changed such that concentration of simple sugars in the pretreated feedstock
is 1%, 5%, 10%,
12%, 13%, 14%, 15%, 16%, 17%, 19%, 20%, 30%, 40% or 50%. In one embodiment,
the
parameters of the pretreatment and hydrolysis are changed such that
concentration of simple
sugars in the pretreated feedstock is 0% to 20%. In one embodiment, the
parameters of the
pretreatment and hydrolysis are changed such that concentration of simple
sugars in the
pretreated feedstock is 0% to 5%. Examples of simple sugars include, but are
not limited to,
C5 and C6 monomers and dimers.
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00118] In one embodiment, the parameters of the pretreatment are changed
such that
concentration of lignin in the pretreated and/or hydrolyzed feedstock is 1%,
5%, 10%, 12%,
13%, 14%, 15%, 16%, 17%, 19%, 20%, 30%, 40% or 50%. In one embodiment, the
parameters of the pretreatment and/or hydrolysis are changed such that
concentration of
lignin in the pretreated feedstock is 0% to 20%. In one embodiment, the
parameters of the
pretreatment and/or hydrolysis are changed such that concentration of lignin
in the pretreated
feedstock is 0% to 5%. In one embodiment, the parameters of the pretreatment
and
hydrolysis are changed such that concentration of lignin in the pretreated
and/or hydrolyzed
feedstock is less than 1% to 2%. In one embodiment, the parameters of the
pretreatment
and/or hydrolysis are changed such that the concentration of phenolics is
minimized.
[00119] In one embodiment, the parameters of the pretreatment and/or
hydrolysis are
changed such that concentration of furfural and low molecular weight lignin in
the pretreated
and/or hydrolyzed feedstock is less than 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%,
or 1%. In
one embodiment, the parameters of the pretreatment and/or hydrolysis are
changed such that
concentration of furfural and low molecular weight lignin in the pretreated
and/or hydrolyzed
feedstock is less than 1% to 2%.
[00120] In one embodiment, the parameters of the pretreatment and/or
hydrolysis are
changed such that the concentration of simple sugars is at least 75% to 85%,
and the
concentration of lignin is 0% to 5% and the concentration of furfural and low
molecular
weight lignin in the pretreated feedstock is less than 1% to 2%.
[00121] In one embodiment, the parameters of the pretreatment and/or
hydrolysis are
changed to obtain a high concentration of hemicellulose and a low
concentration of lignin. In
one embodiment, the parameters of the pretreatment and/or hydrolysis are
changed to obtain
a high concentration of hemicellulose and a low concentration of lignin such
that
concentration of the components in the pretreated stock is optimal for
fermentation with a
microbe such as biocatalyst.
[00122] In one embodiment, more than one of these steps can occur at any
given time.
For example, hydrolysis of the pretreated feedstock and hydrolysis of the
oligosaccharides
can occur simultaneously, and one or more of these can occur simultaneously to
the
conversion of monosaccharides to a fuel or chemical.
[00123] In another embodiment, an enzyme can directly convert the
polysaccharide to
monosaccharides. In some instances, an enzyme can hydrolyze the polysaccharide
to
oligosaccharides and the enzyme or another enzyme can hydrolyze the
oligosaccharides to
monosaccharides.
51
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
[00124] In another embodiment, the enzymes can be added to the
fermentation or they
can be produced by microorganisms present in the fermentation. In one
embodiment, the
microorganism present in the fermentation produces some enzymes. In another
embodiment,
enzymes are produced separately and added to the fermentation.
[00125] For the overall conversion of pretreated biomass to final product
to occur at
high rates, the enzymes for each conversion step can be present with
sufficiently high
activity. If one of these enzymes is missing or is present in insufficient
quantities, the
production rate of an end product can be reduced. The production rate can also
be reduced if
the microorganisms responsible for the conversion of monosaccharides to
product only
slowly take up monosaccharides and/or have only limited capability for
translocation of the
monosaccharides and intermediates produced during the conversion to end
product.
Additions of fractions obtained from pretreatment and/or pretreatment and
hydrolysis can
increase initial or overall growth rates. In another embodiment, oligomers are
taken up
slowly by a biocatalyst, necessitating an almost complete conversion of
polysaccharides and
oligomers to monomeric sugars.
[00126] In another embodiment, the enzymes of the method are produced by a
biocatalyst, including a range of hydrolytic enzymes suitable for the biomass
materials used
in the fermentation methods. In one embodiment, a biocatalyst is grown under
conditions
appropriate to induce and/or promote production of the enzymes needed for the
saccharification of the polysaccharide present. The production of these
enzymes can occur in
a separate vessel, such as a seed fermentation vessel or other fermentation
vessel, or in the
production fermentation vessel where ethanol production occurs. When the
enzymes are
produced in a separate vessel, they can, for example, be transferred to the
production
fermentation vessel along with the cells, or as a relatively cell free
solution liquid containing
the intercellular medium with the enzymes. When the enzymes are produced in a
separate
vessel, they can also be dried and/or purified prior to adding them to the
hydrolysis or the
production fermentation vessel. The conditions appropriate for production of
the enzymes
are frequently managed by growing the cells in a medium that includes the
biomass that the
cells will be expected to hydrolyze in subsequent fermentation steps.
Additional medium
components, such as salt supplements, growth factors, and cofactors including,
but not
limited to phytate, amino acids, and peptides can also assist in the
production of the enzymes
utilized by the microorganism in the production of the desired products.
[00127] Fermentation
52
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00128] Provided herein are methods and compositions for producing one or
more
fermentation end-products from blended feedstocks. The blended feedstocks can
comprise a
first biomass and one or more monosaccharides produced by pretreating and/or
hydrolyzing a
second biomass comprising cellulose, hemicellulose, lignocellulose and/or
starch. The one or
more monosaccharides can include C6 and/or C5 monosaccharides. The one or more
monosaccharides can be in a C6-enriched hydrolysate (C6 Saccharide Stream).
The one or
more monosaccharides can be in a C5-enriched hydrolysate (C5 Saccharide
Stream). The one
or more monosaccharides can comprise both C5 and C6 saccharides (C5 + C6
Saccharide
Stream). The one or more monosaccharides can include cellulosic-derived
monosaccharides.
The one or more monosaccharides can include non-cellulosic-derived
monosaccharides (e.g.,
starch-derived monosaccharides). The one or more monosaccharides can include
glucose,
fructose, galactose, xylose, or any other saccharides. The first biomass can
comprise non-
cellulosic polysaccharides (e.g., starch).
[00129] A blended feedstock can comprise from about 1% to about 50% solids
from a
first biomass. For example, the blended feedstock can comprise about 1-50%, 10-
50%, 20-
40%, 20-36%, 20-35%, 20-34%, 20-33%, 20-32%, 20-31%, 20-30%, 25-36%, 25-35%,
25-
34%, 25-33%, 25-32%, 25-31%, 25-30%, 30-36%, 30-35%, 30-34%, 30-33%, 30-32%,
or
30-31% solids from the first biomass. In one embodiment, the first biomass
comprises non-
cellulosic saccharides. In one embodiment, the non-cellulosic saccharides
comprise starch. In
another embodiment, the non-cellulosic saccharides comprise sucrose. In
another
embodiment, the non-cellulosic saccharides comprise starch and/or sucrose.
[00130] A blended feedstock can be produced by combining a first biomass
(e.g.,
containing non-cellulosic saccharides such as starch or sucrose) with one or
more cellulosic-
derived or non-cellulosic derived monosaccharides (e.g., C6 saccharides, C5
saccharides, or a
combination thereof). The one or more cellulosic-derived or non-cellulosic-
derived
monosaccharides can be at a concentration of from about 1% to about 70% w/v
prior to
combining with the first biomass. For example, the one or more monosaccharides
can be at a
concentration of about 1-70% w/v, 1-60% w/v, 1-55% w/v, 1-50% w/v, 1-40% w/v,
1-30%
w/v, 1-20% w/v, 1-10% w/v, 10-70% w/v, 10-60% w/v, 10-55% w/v, 10-50% w/v, 10-
40%
w/v, 10-30% w/v, 10-20% w/v, 20-70% w/v, 20-60% w/v, 20-55% w/v, 20-50% w/v,
20-
40% w/v, 20-30% w/v, 30-70% w/v, 30-60% w/v, 30-55% w/v, 30-50% w/v, 30-40%
w/v,
40-70% w/v, 40-60% w/v, 40-55% w/v, 40-50% w/v, 50-70% w/v, 50-60% w/v, 50-55%
w/v, 55-70% w/v, 55-60% w/v, or 60-70% w/v prior to combining with the first
biomass.
53
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00131] A blended feedstock can be produced by combining a first biomass
(e.g.,
containing non-cellulosic saccharides such as starch) with one or more
cellulosic-derived
monosaccharides. The one or more cellulosic-derived monosaccharides can be C6
saccharides and/or C5 saccharides. The one or more cellulosic-derived
monosaccharides can
be in a crude-lysate from the pretreatment and/or hydrolysis of cellulose,
hemicellulose,
and/or lignocellulosic material. The one or more cellulosic-derived
monosaccharides can
comprise less than 50%, 45%, 40%, 35%, 30%, 25%, 20%, 15%, 10%, 5%, 4%, 3%,
2%,
1.5%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1% or less w/v of
one or
more inhibitory compounds. The one or more inhibitory compounds can be one or
more
byproducts of the pretreatment and/or hydrolysis of cellulose, hemicellulose,
and/or
lignocellulose. The one or more inhibitory compounds can comprise one or more
organic
acids such as acetic acid, lactic acid, or formic acid, and/or one or more
furans such as
hydroxy methyl furfural or furfural, or a combination thereof.
[00132] Enhanced rates of fermentation can be achieved using blended
feedstocks
comprising a first biomass containing non-cellulosic saccharides (e.g.,
starch, sucrose, etc.)
and one or more cellulosic-derived monosaccharides (e.g., C6 monosaccharide,
C5
monosaccharides) in comparison to fermentation of the first biomass without
the one or more
cellulosic-derived monosaccharides. The enhanced rates of fermentation can be
from about
1% higher to about 100% higher; for example, about 1-100%, 1-75%, 1-50%, 1-
25%, 1-10%,
10-100%, 10-75%, 10-50%, 10-25%, 25-100%, 25-75%, 25-50%, 50-100%, 50-75%, 75-
100%, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%,
17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%,
32%,
33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%,
48%,
49%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 100% higher.
[00133] Increased yields of one or more fermentation end-products can be
achieved
using blended feedstocks comprising a first biomass containing non-cellulosic
saccharides
(e.g., starch, sucrose, etc.) and one or more cellulosic-derived
monosaccharides (e.g., C6
monosaccharide, C5 monosaccharides) in comparison to fermentation of the first
biomass
without the one or more cellulosic-derived monosaccharides. The increased
yields of one or
more fermentation end-products can be from about 1% higher to about 100%
higher; for
example, about 1-100%, 1-75%, 1-50%, 1-25%, 1-10%, 1-5%, 5-10%, 10-100%, 10-
75%,
10-50%, 10-25%, 25-100%, 25-75%, 25-50%, 50-100%, 50-75%, 75-100%, 1%, 1.1%,
1.2%,
1.3%, 1.4%, 1.5%, 1.6%, 1.7%, 1.8%, 1.9%, 2%, 2.1%, 2.2%, 2.3%, 2.4%, 2.5%,
2.6%,
2.7%, 2.8%, 2.9%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%,
16%,
54
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
17%, 18%, 19%, 20%, 21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%,
32%,
33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%,
48%,
49%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 100% higher.
[00134] A blended feedstock can be prepared by combining a first biomass
with a
saccharide stream comprising one or more cellulosic-derived monosaccharides,
one or more
non-cellulosic derived monosaccharides, or a combination thereof. The first
biomass can
comprise non-cellulosic saccharides such as starch or sucrose. The one or more
cellulosic-
derived monosaccharides or non-cellulosic derived monosaccharides can be
produced by the
pretreatment and/or hydrolysis of a second biomass. The second biomass can
comprise
cellulose, hemicellulose, lignocellulose, and/or starch. The pretreatment
and/or hydrolysis of
the second biomass can produce a C6-enriched hydrolysate (C6 Saccharide
Stream). The
pretreatment and/or hydrolysis of the second biomass can produce a CS-enriched
hydrolysate
(C5 Saccharide Stream). The pretreatment and/or hydrolysis of the second
biomass can
produce a hydrolysate containing both CS and C6 monosaccharides (C5 + C6
Saccharide
Stream). In one embodiment, the one or more cellulosic-derived monosaccharides
(e.g., from
a C6-enriched hydrolysate) are at a concentration that differs from the
concentration of
saccharides in the first biomass by less than about +/- 50%, 40%, 30%, 20%,
15%, 10%, 5%,
or 1%, wherein the concentration of saccharides in the first biomass assumes
complete
hydrolysis of the first biomass to monomers (e.g., is in monosaccharide
equivalents).
[00135] A blended feedstock can be prepared by combining a first biomass
with a
saccharide stream comprising one or more cellulosic-derived monosaccharides,
one or more
non-cellulosic derived monosaccharides, or a combination thereof. The first
biomass can
comprise non-cellulosic saccharides such as starch or sucrose. The one or more
monosaccharides can be produced by the pretreatment and/or hydrolysis of a
second biomass.
The second biomass can comprise cellulose, hemicellulose, lignocellulose,
and/or starch. The
pretreatment and/or hydrolysis of the second biomass can produce a C6-enriched
hydrolysate
(C6 Saccharide Stream), a CS-enriched hydrolyzate (C5 Saccharide Stream), or a
CS and C6
hydrolyzate (C5 + C6 Saccharide Stream). In one embodiment, the first biomass
and the
saccharides stream comprising the one or more monosaccharides are combined in
about a
50:50, 55:45, 60:40:, 65:35, 70:30, 75:35, 80:20, 85:15, 90:10, 95:5, or 99:1
ratio. The ratio
can be a weight to weight ratio, a weight to volume ratio, or a volume to
volume ratio.
[00136] Exposing microorganisms such as bacteria or yeast to hypertonic
solution can
cause an efflux of cellular water into the medium. In order to counteract the
outflow of water
molecules during growth, microorganisms can produce and accumulate one or more
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
osmoregulatory molecules such as polyhydroxy compounds. (e.g., see Nevoit and
Stahl
(1997) FEMS Microbiology Review 21:231-241 and Parekh and Pandey (1985)
Biotechnology and Bioengineering 27: 1089-1091, each of which is incorporated
by
reference in its entirety). During ethanolic fermentation of starch-containing
compounds,
microorganisms such as yeast can redirect part of the carbon released during
enzymatic
hydrolysis of starch to one or more other products such as polyols or sugar
alcohols (e.g.,
glycerol) instead of fermentation end-products such as ethanol. This can
occur, for example,
when glucose is overly abundant during the fermentation reaction, for example,
due to the
conversion of starch to glucose monomers or the addition of a saccharide
stream comprising
one or more monosaccharides (e.g., C5 monosaccharides, C6 monosaccharides, or
both).
Environmental factors affecting these pathways can include oxygen
availability, type of
nitrogen source, osmotic pressure, heat and pH. For example, when glucose is
overly
abundant, a high osmotic pressure can shift metabolism to the production of
glycerol.
Therefore, it may be possible to maintain high ethanol production using fed-
batch
fermentations. In one embodiment, one or more cellulosic-derived C6
monosaccharides are
added over time in a fed-batch fermentation reaction comprising a first
biomass containing
non-cellulosic saccharides such as starch.
[00137] Without being limited by theory, the high level of solids (e.g.,
insoluble solids
or suspended solids) in many fermentation reactions using biomass containing
non-cellulosic
saccharides (e.g., corn mash) can cause osmotic stress upon the biocatalyst
microorganisms
in the fermentation reaction (e.g., yeast, bacteria, etc.). The osmotic stress
can cause the
microorganisms to produce osmoregulatory compounds such as polyols (e.g.,
glycerol) or
sugar alcohols. By utilizing blended feedstocks comprising a first biomass
containing non-
cellulosic saccharides and a saccharide stream comprising one or more
monosaccharides
(e.g., cellulosic-derived or non-cellulosic derived monosaccharides), the
solids of the first
biomass can be diluted. This can reduce osmotic stress upon the microorganisms
and reduce
the production of fermentation end-products such as osmoregulatory compounds
(e.g.,
polyols or sugar alcohols). The reduced production of osmoregulatory compounds
can
increase the rate and/or yield of alcohols such as ethanol, methoanol,
butanol, etc.
[00138] Provided herein are methods and compositions for producing one or
more-
fermentation end-products wherein the production of one or more other products
(e.g.,
osmoregulatory molecules) such as polyols or sugar alcohols (e.g., glycerol)
is reduced.
Reduced production of polyols such as glycerol can be achieved using blended
feedstocks
comprising a first biomass and a saccharide stream comprising one or more
cellulosic-derived
56
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
monosaccharides, one or more non-cellulosic derived monosaccharides, or a
combination
thereof. The first biomass can comprise non-cellulosic saccharides such as
starch or sucrose.
The saccharide stream comprising the one or more monosaccharides can be
produced by the
pretreatment and/or hydrolysis of a second biomass. The second biomass can
comprise
cellulose, hemicellulose, lignocellulose, and/or starch. The saccharide stream
can be a C6-
enriched hydrolysate (C6 Saccharide Stream), a C5-enriched hydrolysate (C5
Saccharide
Stream), or a C5 and C6 containing hydrolyzate (C5 + C6 Saccharide Stream).
The amount of
one or more other products produced can be from about 1% to about 100% lower;
for
example, about 1-100%, 1-75%, 1-50%, 1-25%, 1-10%, 1-5%, 5-10%, 10-100%, 10-
75%,
10-50%, 10-25%, 25-100%, 25-75%, 25-50%, 50-100%, 50-75%, 75-100%, 1%, 2%, 3%,
4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%,
21%, 22%, 23%, 24%, 25%, 26%, 27%, 28%, 29%, 30%, 31%, 32%, 33%, 34%, 35%,
36%,
37%, 38%, 39%, 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 55%,
60%,
65%, 70%, 75%, 80%, 85%, 90%, 95%, or 100% lower. In one embodiment, the
saccharide
stream comprising the one or more monosaccharides are added in a fed batch
manner and the
reduced production of the one or more other products is in comparison to a non-
fed batch
fermentation reaction. In another embodiment, the saccharide stream comprising
the one or
more monosaccharides are added in a batch fermentation and the reduced
production of the
one or more other products is in comparison to a fermentation reaction
containing the first
biomass only.
[00139] Disclosed herein are fed-batch fermentation reactions in which a
first biomass
(e.g., containing non-cellulosic saccharides such as starch and/or sucrose)
and a saccharide
stream comprising one or more cellulosic-derived monosaccharides, one or more
non-
cellulosic derived monosaccharides, or a combination thereof are combined to
produce a
blended feed-stock in a broth. In a fed-batch reaction, the saccharide stream
comprising the
one or more monosaccharides (e.g., cellulosic-derived C6 monosaccharides) can
be added at
a rate of from about 0.001 mL/min/L of broth to about 50 mL/min/L of broth.
For example,
the saccharide stream comprising the one or more monosaccharides can be added
at a rate of
about 0.001-50 mL/min/L, 0.001-25 mL/min/L, 0.001-10 mL/min/L, 0.001-5
mL/min/L,
0.001-1 mL/min/L, 0.001-0.7 mL/min/L, 0.001-0.5 mL/min/L, 0.001-0.1 mL/min/L,
0.001-
0,01 mL/min/L, 0.01-50 mL/min/L, 0.01-25 mL/min/L, 0.01-10 mL/min/L, 0.01-5
mL/min/L, 0.01-1 mL/min/L, 0.01-0.7 mL/min/L, 0.01-0.5 mL/min/L, 0.01-0.1
mL/min/L,
0.1-50 mL/min/L, 0.1-25 mL/min/L, 0.1-10 mL/min/L, 0.1-5 mL/min/L, 0.1-1
mL/min/L,
0.1-0.7 mL/min/L, 0.1-0.5 mL/min/L, 0.5-50 mL/min/L, 0.5-25 mL/min/L, 0.5-10
mL/min/L,
57
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
0.5-5 mL/min/L, 0.5-1 mL/min/L, 0.5-0.7 mL/min/L, 0.7-50 mL/min/L, 0.7-25
mL/min/L,
0.7-10 mL/min/L, 0.7-5 mL/min/L, 0.7-1 mL/min/L, 1-50 mL/min/L, 1-25 mL/min/L,
1-10
mL/min/L, 1-5 mL/min/L, 5-50 mL/min/L, 5-25 mL/min/L, 5-10 mL/min/L, 10-50
mL/min/L, 10-25 mL/min/L, 25-50 mL/min/L, 0.001 mL/min/L, 0.002 mL/min/L,
0.003
mL/min/L, 0.004 mL/min/L, 0.005 mL/min/L, 0.006 mL/min/L, 0.007 mL/min/L,
0.008
mL/min/L, 0.009 mL/min/L, 0.01 mL/min/L, 0.02 mL/min/L, 0.03 mL/min/L, 0.04
mL/min/L, 0.05 mL/min/L, 0.06 mL/min/L, 0.07 mL/min/L, 0.08 mL/min/L, 0.09
mL/min/L,
0.1 mL/min/L, 0.2 mL/min/L, 0.3 mL/min/L, 0.4 mL/min/L, 0.5 mL/min/L, 0.6
mL/min/L,
0.7 mL/min/L, 0.8 mL/min/L, 0.9 mL/min/L, 1 mL/min/L, 1.1 mL/min/L, 1.2
mL/min/L, 1.3
mL/min/L, 1.4 mL/min/L, 1.5 mL/min/L, 1.6 mL/min/L, 1.7 mL/min/L, 1.8
mL/min/L, 1.9
mL/min/L, 2 mL/min/L, 2.5 mL/min/L, 3 mL/min/L, 3.5 mL/min/L, 4 mL/min/L, 4.5
mL/min/L, 5 mL/min/L, 5.5 mL/min/L, 6 mL/min/L, 6.5 mL/min/L, 7 mL/min/L, 7.5
mL/min/L, 8 mL/min/L, 8.5 mL/min/L, 9 mL/min/L, 9.5 mL/min/L, 10 mL/min/L, 11
mL/min/L, 12 mL/min/L, 13 mL/min/L, 14 mL/min/L, 15 mL/min/L, 16 mL/min/L, 17
mL/min/L, 18 mL/min/L, 19 mL/min/L, 20 mL/min/L, 21 mL/min/L, 22 mL/min/L, 23
mL/min/L, 24 mL/min/L, 25 mL/min/L, 26 mL/min/L, 27 mL/min/L, 28 mL/min/L, 29
mL/min/L, 30 mL/min/L, 31 mL/min/L, 32 mL/min/L, 33 mL/min/L, 34 mL/min/L, 35
mL/min/L, 36 mL/min/L, 37 mL/min/L, 38 mL/min/L, 39 mL/min/L, 40 mL/min/L, 41
mL/min/L, 42 mL/min/L, 43 mL/min/L, 44 mL/min/L, 45 mL/min/L, 46 mL/min/L, 47
mL/min/L, 48 mL/min/L, 49 mL/min/L, or 50 mL/min/L of broth.
[00140] In one embodiment, the concentration of monosaccharides in a
blended
feedstock prior to contacting with one or more biocatalysts (e.g., at the
start of a fermentation
or simultaneous saccharification and fermentation reaction) can be less than
about 100 g/L;
for example, less than about 100 g/L, 90 g/L, 80 g/L, 70 g/L, 60 g/L, 50 g/L,
40 g/L, 30 g/L,
25 g/L, 20 g/L, 15 g/L, 10 g/L, 9 g/L, 8 g/L, 7 g/L, 6 g/L, 5 g/L, 4 g/L, 3
g/L, 2 g/L, or 1 g/L.
In another embodiment, the concentration of monosaccharides in a blended
feedstock prior to
contacting with one or more biocatalysts (e.g., at the start of a fermentation
or simultaneous
saccharification and fermentation reaction) can be from about 1 g/L to about
100 g/L; for
example, about 1-100 g/L, 1-75 g/L, 1-50 g/L, 1-25 g/L, 1-10 g/L, 10-100 g/L,
10-75 g/L, 10-
50 g/L, 10-25 g/L, 25-100 g/L, 25-75 g/L, 25-50 g/L, 50-100 g/L, 50-75 g/L, or
75-100 g/L.
[00141] The present disclosure also provides a fermentative mixture
comprising: a
cellulosic feedstock pre-treated with an alkaline or acid substance and at a
temperature of
from about 80 C to about 120 C; subsequently hydrolyzed with an enzyme
mixture, and a
microorganism capable of fermenting a five-carbon sugar and/or a six-carbon
sugar. In one
58
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
embodiment, the five-carbon sugar is xylose, arabinose, or a combination
thereof. In one
embodiment, the six-carbon sugar is glucose, galactose, mannose, or a
combination thereof.
In one embodiment, the alkaline substance is NaOH. In some embodiments, NaOH
is added
at a concentration of about 0.5% to about 2% by weight of the feedstock. In
one embodiment,
the acid is equal to or less than 2% HC1 or H2SO4. In one embodiment, the
microorganism is
a Rhodococcus strain, a Clostridium strain, a Trichoderma strain, a
Saccharomyces strain, a
Zymomonas strain, or another microorganism suitable for fermentation of
biomass. In another
embodiment, the fermentation process comprises fermentation of the biomass
using a
microorganism that is Clostridium phytofermentans, Clostridium
algidixylanolyticum,
Clostridium xylanolyticum, Clostridium cellulovorans, Clostridium
cellulolyticum,
Clostridium thermocellum, Clostridium josui, Clostridium papyrosolvens,
Clostridium
cellobioparum, Clostridium hungatei, Clostridium cellulosi, Clostridium
stercorarium,
Clostridium termitidis, Clostridium thermocopriae, Clostridium celerecrescens,
Clostridium
polysaccharolyticum, Clostridium populeti, Clostridium lentocellum,
Clostridium
chartatabidum, Clostridium aldrichii, Clostridium herbivorans, Acetivibrio
cellulolyticus,
Bacteroides cellulosolvens, Caldicellulosiruptor saccharolyticum, Rhodococcus
opacus,
Ruminococcus albus, Ruminococcus flavefaciens, Fibrobacter succino genes,
Eubacterium
cellulosolvens, Butyrivibrio fibrisolvens, Anaerocellum thermophilum,
Halocella
cellulolytica, Thermoanaerobacterium thermosaccharolyticum, Sacharophagus
degradans,
or Thermoanaerobacterium saccharolyticum. In still another embodiment, the
microorganism
is genetically modified to enhance activity of one or more hydrolytic enzymes,
such as a
genetically-modified Saccaromyces cerevisae.
[00142] In one embodiment, a wild type or a genetically-improved
microorganism can
be used for chemical production by fermentation. Methods to produce a
genetically-
improved strain can include genetic modification, mutagenesis, and adaptive
processes, such
as directed evolution. For example, yeasts can be genetically-modified to
ferment C5 sugars.
Other useful yeasts are species of Candida, Cryptococcus, Debaryomyces,
Deddera,
Hanseniaspora, Kluyveromyces, Pichia, Schizosaccharomyces, and
Zygosaccharomyces.
Rhodococus strains, such as Rhodococcus opacus variants are a source of
triacylglycerols and
other storage lipids. (See, e.g., Waltermann, et at., Microbiology 146:1143-
1149 (2000)).
Other useful organisms for fermentation include, but are not limited to,
yeasts, especially
Saccaromyces strains and bacteria such as Clostridium phytofermentans,
Thermoanaerobacter ethanolicus, Clostridium thermocellum, Clostridium
beijerinickii,
Clostridium acetobutylicum, Clostridium tyrobutyricum, Clostridium
thermobutyricum,
59
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
Thermoanaerobacterium saccharolyticum, Thermoanaerobacter
thermohydrosulfuricus,
Clostridium acetobutylicum, Moorella ssp., Carboxydocella ssp., Zymomonas
mobilis,
recombinant E. Coli, Klebsiella oxytoca, Rhodococcus opacus and Clostridium
beijerickii.
[00143] An advantage of yeasts are their ability to grow under conditions
that include
elevated ethanol concentration, high sugar concentration, low sugar
concentration, and/or
operate under anaerobic conditions. These characteristics, in various
combinations, can be
used to achieve operation with long or short fermentation cycles and can be
used in
combination with batch fermentations, fed batch fermentations, self-
seeding/partial harvest
fermentations, and recycle of cells from the final fermentation as inoculum.
[00144] Examples of yeasts that can be used as a biocatalyst or fermentive
microorganism in the methods disclosed herein include but are not limited to,
species found
in the genus Ascoidea, Brettanomyces, Candida, Cephaloascus, Coccidiascus,
Dipodascus,
Eremothecium, Galactomyces, Kluyveromyces, Pichia, Saccharomyces,
Schizosaccharomyces, Sporopachydermia, Torulaspora, Yarrowia, or
Zygosaccharomyces;
for example, Ascoidea rebescens, Brettanomyces anomalus, Brettanomyces
bruxellensis,
Brettanomyces claussenii, Brettanomyces custersianus, Brettanomyces lambicus,
Brettanomyces naardenensis, Brettanomyces nanus, Candida albicans, Candida
ascalaphidarum, Candida amphixiae, Candida antarctica, Candida argentea,
Candida
atlantica, Candida atmosphaerica, Candida blattae, Candida carpophila, Candida
cerambycidarum, Candida chauliodes, Candida corydali, Candida dosseyi, Candida
dubliniensis, Candida ergatensis, Candida fructus, Candida glabrata, Candida
fermentati,
Candida guilliermondii, Candida haemulonii, Candida insectamens, Candida
insectorum,
Candida intermedia, Candida jeffresii, Candida kefyr, Candida krusei, Candida
lusitaniae,
Candida lyxosophila, Candida maltosa, Candida marina, Candida membranifaciens,
Candida milleri, Candida oleophila, Candida oregonensis, Candida parapsilosis,
Candida
quercitrusa, Candida rugosa, Candida sake, Candida shehatea, Candida
temnochilae,
Candida tenuis, Candida tropicalis, Candida tsuchiyae, Candida
sinolaborantium, Candida
sojae, Candida subhashii, Candida viswanathii, Candida utilis, Cephaloascus
fragrans,
Coccidiascus legeri, Dypodascus albidus, Eremothecium cymbalariae,
Galactomyces
candidum, Galactomyces geotrichum, Kluyveromyces aestuarii, Kluyveromyces
africanus,
Kluyveromyces bacillisporus, Kluyveromyces blattae, Kluyveromyces dobzhanskii,
Kluyveromyces hubeiensis, Kluyveromyces lactis, Kluyveromyces lodderae,
Kluyveromyces
marxianus, Kluyveromyces nonfermentans, Kluyveromyces piceae, Kluyveromyces
sinensis,
Kluyveromyces thermotolerans, Kluyveromyces waltii, Kluyveromyces wickerhamii,
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
Kluyveromyces yarrowii, Pichia anomola, Pichia heedii, Pichia guilliermondii,
Pichia
kluyveri, Pichia membranifaciens, Pichia norvegensis, Pichia ohmeri, Pichia
pastoris, Pichia
subpelliculosa, Saccharomyces bayanus, Saccharomyces boulardii, Saccharomyces
bulderi,
Saccharomyces cariocanus, Saccharomyces cariocus, Saccharomyces cerevisiae,
Saccharomyces chevalieri, Saccharomyces dairenensis, Saccharomyces
ellipsoideus,
Saccharomyces eubayanus, Saccharomyces exiguus, Saccharomyces florentinus,
Saccharomyces kluyveri, Saccharomyces martiniae, Saccharomyces monacensis,
Saccharomyces norbensis, Saccharomyces paradoxus, Saccharomyces pastorianus,
Saccharomyces spencerorum, Saccharomyces turicensis, Saccharomyces unisporus,
Saccharomyces uvarum, Saccharomyces zonatus, Schizosaccharomyces cryophilus,
Schizosaccharomyces japonicus, Schizosaccharomyces octosporus,
Schizosaccharomyces
pombe, Sporopachydermia cereana, Sporopachydermia lactativora,
Sporopachydermia
quercuum, Torulaspora delbrueckii, Torulaspora franciscae, Torulaspora
globosa,
Torulaspora pretoriensis, Yarrowia lipolytica, Zygosaccharomyces bailii,
Zygosaccharomyces bisporus, Zygosaccharomyces cidri, Zygosaccharomyces
fermentati,
Zygosaccharomyces florentinus, Zygosaccharomyces kombuchaensis,
Zygosaccharomyces
lentus, Zygosaccharomyces mellis, Zygosaccharomyces microellipsoides,
Zygosaccharomyces mrakii, Zygosaccharomyces pseudorouxii, or Zygosaccharomyces
rouxii, or a variant or genetically modified version thereof
[00145] Examples of bacteria that can be used as a biocatalyst or
fermentive
microorganism in the methods disclosed herein include but are not limited to
any bacterium
found in the genus of Butyrivibrio, Ruminococcus, Eubacterium, Bacteroides,
Acetivibrio,
Caldibacillus, Acidothermus, Cellulomonas, Curtobacterium, Micromonospora,
Actinoplanes, Streptomyces, Thermobifida, Thermomonospora, Microbispora,
Fibrobacter,
Sporocytophaga, Cytophaga, Flavobacterium, Achromobacter, Xanthomonas,
Cellvibrio,
Pseudomonas, Myxobacter, Escherichia, Klebsiella, Thermoanaerobacterium,
Thermoanaerobacter, Geobacillus, Saccharococcus, Paenibacillus, Bacillus,
Caldicellulosiruptor, Anaerocellum, Anoxybacillus, Zymomonas, Clostridium; for
example,
Butyrivibrio fibrisolvens, Ruminococcus flavefaciens, Ruminococcus succino
genes,
Ruminococcus albus, Eubacterium cellulolyticum, Bacteroides cellulosolvens,
Acetivibrio
cellulolyticus, Acetivibrio cellulosolvens, Caldibacillus cellulovorans,
Bacillus circulans,
Acidothermus cellulolyticus, Cellulomonas cartae, Cellulomonas cellasea,
Cellulomonas
cellulans, Cellulomonas fimi, Cellulomonas flavigena, Cellulomonas gelida,
Cellulomonas
iranensis, Cellulomonas persica, Cellulomonas uda, Curtobacterium
falcumfaciens,
61
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
Micromonospora melonosporea, Actinoplanes aurantiaca, Streptomyces reticuli,
Streptomyces alboguseolus, Streptomyces aureofaciens, Streptomyces
cellulolyticus,
Streptomyces flavogriseus, Streptomyces lividans, Streptomyces nitrosporeus,
Streptomyces
olivochromo genes, Streptomyces rochei, Streptomyces thermovulgaris,
Streptomyces
viridosporus, Thermobifida alba, Thermobifida fusca, Thermobifida
cellulolytica,
Thermomonospora curvata, Microbispora bispora, Fibrobacter succinogenes,
Sporocytophaga myxococcoides, Cytophaga sp., Flavobacterium johnsoniae,
Achromobacter
piechaudii, Xanthomonas sp., Cellvibrio vulgaris, Cellvibrio fulvus,
Cellvibrio gilvus,
Cellvibrio mixtus, Pseudomonas fluorescens, Pseudomonas mendocina, Myxobacter
sp. AL-
1, Escherichia albertii, Escherichia blattae, Escherichia coli, Escherichia
fergusonii,
Escherichia hermannii, Escherichia vulneris, Klebsiella granulomatis,
Klebsiella oxytoca,
Klebsiella pneumonia, Klebsiella terrigena, Thermoanaerobacterium
thermosulfurigenes,
Thermoanaerobacterium aotearoense, Thermoanaerobacterium polysaccharolyticum,
Thermoanaerobacterium zeae, Thermoanaerobacterium xylanolyticum,
Thermoanaerobacterium saccharolyticum, Thermoanaerobium brockii,
Thermoanaerobacterium thermosaccharolyticum, Thermoanaerobacter
thermohydrosulfuricus, Thermoanaerobacter ethanolicus, Thermoanaerobacter
brocki,
Geobacillus thermoglucosidasius, Geobacillus stearothermophilus,
Saccharococcus
caldoxylosilyticus, Saccharoccus thermophilus, Paenibacillus campinasensis,
Bacillus
flavothermus, Anoxybacillus kamchatkensis, Anoxybacillus gonensis,
Caldicellulosiruptor
acetigenus, Caldicellulosiruptor saccharolyticus, Caldicellulosiruptor
kristjanssonii,
Caldicellulosiruptor owensensis, Caldicellulosiruptor lactoaceticus,
Anaerocellum
thermophilum, Clostridium thermocellum, Clostridium cellulolyticum,
Clostridium
straminosolvens, Clostridium acetobutylicum, Clostridium aerotolerans,
Clostridium
beijerinckii, Clostridium bifermentans, Clostridium botulinum, Clostridium
butyricum,
Clostridium cadaveric, Clostridium chauvoei, Clostridium clostridioforme,
Clostridium
colicanis, Clostridium difficile, Clostridium fallax, Clostridium
formicaceticum, Clostridium
histolyticum, Clostridium innocuum, Clostridium ljungdahlii, Clostridium
laramie,
Clostridium lavalense, Clostridium novyi, Clostridium oedematiens, Clostridium
paraputrificum, Clostridium perfringens, Clostridium phytofermentans,
Clostridium
piliforme, Clostridium ramosum, Clostridium scatolo genes, Clostridium
septicum,
Clostridium sordellii, Clostridium sporo genes, Clostridium tertium,
Clostridium tetani,
Clostridium tyrobutyricum, Clostridium thermobutyricum, Zymomonas mobilis, or
a variant
or genetically modified version thereof.
62
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00146] In one embodiment, fed-batch fermentation is performed on the
treated
biomass to produce a fermentation end-product, such as alcohol, ethanol,
organic acid,
succinic acid, TAG, or hydrogen. In one embodiment, the fermentation process
comprises
simultaneous hydrolysis and fermentation (SSF) of the biomass using one or
more
microorganisms such as a Rhodococcus strain, a Clostridium strain, a
Trichoderma strain, a
Saccharomyces strain, a Zymomonas strain, or another microorganism suitable
for
fermentation of biomass. In another embodiment, the fermentation process
comprises
simultaneous hydrolysis and fermentation of the biomass using a microorganism
that is
Clostridium algidixylanolyticum, Clostridium xylanolyticum, Clostridium
cellulovorans,
Clostridium cellulolyticum, Clostridium thermocellum, Clostridium josui,
Clostridium
papyrosolvens, Clostridium cellobioparum, Clostridium hungatei, Clostridium
cellulosi,
Clostridium stercorarium, Clostridium termitidis, Clostridium thermocopriae,
Clostridium
celerecrescens, Clostridium polysaccharolyticum, Clostridium populeti,
Clostridium
lentocellum, Clostridium chartatabidum, Clostridium aldrichii, Clostridium
herbivorans,
Clostridium phytofermentans, Acetivibrio cellulolyticus, Bacteroides
cellulosolvens,
Caldicellulosiruptor saccharolyticum, Ruminococcus albus, Ruminococcus
flavefaciens,
Fibrobacter succino genes, Eubacterium cellulosolvens, Butyrivibrio
fibrisolvens,
Anaerocellum thermophilum, Halocella cellulolytica, Thermoanaerobacterium
thermosaccharolyticum, Sacharophagus degradans, or Thermoanaerobacterium
saccharolyticum.
[00147] In one embodiment, the fermentation process can include separate
hydrolysis
and fermentation (SHF) of a biomass with one or more enzymes, such as a
xylanases, endo-
1,4-beta-xylanases, xylosidases, beta-D-xylosidases, cellulases,
hemicellulases,
carbohydrases, glucanases, endoglucanases, endo-1,4-beta-glucanases,
exoglucanases,
glucosidases, beta-D-glucosidases, amylases, cellobiohydrolases,
exocellobiohydrolases,
phytases, proteases, peroxidase, pectate lyases, galacturonases, or laccases.
In one
embodiment, one or more enzymes used to treat a biomass is thermostable. In
another
embodiment, a biomass is treated with one or more enzymes, such as those
provided herein,
prior to fermentation. In another embodiment, a biomass is treated with one or
more
enzymes, such as those provided herein, during fermentation. In another
embodiment, a
biomass is treated with one or more enzymes, such as those provided herein,
prior to
fermentation and during fermentation. In another embodiment, an enzyme used
for hydrolysis
of a biomass is the same as those added during fermentation. In another
embodiment, an
enzyme used for hydrolysis of biomass is different from those added during
fermentation.
63
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
[00148] In some embodiments, fermentation can be performed in an apparatus
such as
bioreactor, a fermentation vessel, a stirred tank reactor, or a fluidized bed
reactor. In one
embodiment, the treated biomass can be supplemented with suitable chemicals to
facilitate
robust growth of the one or more fermenting organisms. In one embodiment, a
useful
supplement includes but is not limited to, a source of nitrogen and/or amino
acids such as
yeast extract, cysteine, or ammonium salts (e.g. nitrate, sulfate, phosphate
etc.); a source of
simple carbohydrates such as corn steep liquor, and malt syrup; a source of
vitamins such as
yeast extract; buffering agents such as salts (including but not limited to
citrate salts,
phosphate salts, or carbonate salts); or mineral nutrients such as salts of
magnesium, calcium,
or iron. In some embodiments redox modifiers are added to the fermentation
mixture
including but not limited to cysteine or mercaptoethanol.
[00149] In one embodiment, the titer and/or productivity of fermentation
end-product
production by a microorganism is improved by culturing the microorganism in a
medium
comprising one or more compounds comprising hexose and/or pentose sugars. In
one
embodiment, a process comprises conversion of a starting material (such as a
biomass) to a
biofuel, such as one or more alcohols. In one embodiment, methods can comprise
contacting
substrate comprising both hexose (e.g. glucose, cellobiose) and pentose (e.g.
xylose,
arabinose) saccharides with a microorganism that can hydrolyze C5 and C6
saccharides to
produce ethanol. In another embodiment, methods can comprise contacting
substrate
comprising both hexose (e.g. glucose, cellobiose) and pentose (e.g. xylose,
arabinose)
saccharides with R. opacus to produce TAG.
[00150] In some embodiments, batch fermentation with a microorganism of a
mixture
of hexose and pentose saccharides using the methods disclosed herein can
provide uptake
rates of about 0.1-8 g/L/h or more of hexose and about 0.1-8 g/L/h or more of
pentose
(xylose, arabinose, etc.). In some embodiments, batch fermentation with a
microorganism of
a mixture of hexose and pentose saccharides using the methods disclosed herein
can provide
uptake rates of about 0.1, 0.2, 0.4, 0.5, 0.6 0.7, 0.8, 1, 2, 3, 4, 5, or 6
g/L/h or more of hexose
and about 0.1, 0.2, 0.4, 0.5, 0.6 0.7, 0.8, 1, 2, 3, 4, 5, or 6 g/L/h or more
of pentose.
[00151] In one embodiment, a method for production of ethanol or another
alcohol
produces about 10 g/lto 120 gain 40 hours or less. In another embodiment, a
method for
production of ethanol produces about 10 g/l, 11 g/L, 12 g/L, 13 g/L, 14 g/L,
15 g/L, 16 g/L,
17 g/L, 18 g/L, 19 g/L, 20 g/L, 21 g/L, 22 g/L, 23 g/L, 24 g/L, 25 g/L, 26
g/L, 27 g/L, 28 g/L,
29 g/L, 30 g/L, 31 g/L, 32 g/L, 33 g/L, 34 g/L, 35 g/L, 36 g/L, 37 g/L, 38
g/L, 39 g/L, 40 g/L,
41 g/L, 42 g/L, 43 g/L, 44 g/L, 45 g/L, 46 g/L, 47 g/L, 48 g/L, 49 g/L, 50
g/L, 51 g/L, 52 g/L,
64
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
53 g/L, 54 g/L, 55 g/L, 56 g/L, 57 g/L, 58 g/L, 59 g/L, 60 g/L, 61 g/L, 62
g/L, 63 g/L, 64 g/L,
65 g/L, 66 g/L, 67 g/L, 68 g/L, 69 g/L, 70 g/L, 71 g/L, 72 g/L, 73 g/L, 74
g/L, 75 g/L, 76 g/L,
77 g/L, 78 g/L, 79 g/L, 80 g/L, 81 g/L, 82 g/L, 83 g/L, 84 g/L, 85 g/L, 86
g/L, 87 g/L, 88 g/L,
89 g/L, 90 g/L, 91 g/L, 92 g/L, 93 g/L, 94 g/L, 95 g/L, 96 g/L, 97 g/L, 98
g/L, 99 g/L, 100
g/L, 110 g/l, 120 g/l, or more alcohol in 40 hours by the fermentation of
biomass. In another
embodiment, alcohol is produced by a method comprising simultaneous
fermentation of
hexose and pentose saccharides. In another embodiment, alcohol is produced by
a
microorganism comprising simultaneous fermentation of hexose and pentose
saccharides.
[00152] In
another embodiment, the level of a medium component is maintained at a
desired level by adding additional medium component as the component is
consumed or
taken up by the organism. Examples of medium components included, but are not
limited to,
carbon substrate, nitrogen substrate, vitamins, minerals, growth factors,
cofactors, and
biocatalysts. The medium component can be added continuously or at regular or
irregular
intervals. In one embodiment, additional medium component is added prior to
the complete
depletion of the medium component in the medium. In one embodiment, complete
depletion
can effectively be used, for example to initiate different metabolic pathways,
to simplify
downstream operations, or for other reasons as well. In one embodiment, the
medium
component level is allowed to vary by about 10% around a midpoint, in one
embodiment, it is
allowed to vary by about 30% around a midpoint, and in one embodiment, it is
allowed to
vary by 60% or more around a midpoint. In one embodiment, the medium component
level
is maintained by allowing the medium component to be depleted to an
appropriate level,
followed by increasing the medium component level to another appropriate
level. In one
embodiment, a medium component, such as vitamin, is added at two different
time points
during fermentation process. For example, one-half of a total amount of
vitamin is added at
the beginning of fermentation and the other half is added at midpoint of
fermentation.
[00153] In
another embodiment, the nitrogen level is maintained at a desired level by
adding additional nitrogen-containing material as nitrogen is consumed or
taken up by the
organism. The nitrogen-containing material can be added continuously or at
regular or
irregular intervals. Useful nitrogen levels include levels of about 5 to about
10 g/L. In one
embodiment, levels of about 1 to about 12 g/L can also be usefully employed.
In another
embodiment, levels, such as about 0.5, 0.1 g/L or even lower, and higher
levels, such as about
20, 30 g/L or even higher are used. In another embodiment, a useful nitrogen
level is about
0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 7,
8,9, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21, 22 23, 24, 25, 26, 27, 28, 29 or 30 g/L. Nitrogen
can be supplied as
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
a simple nitrogen-containing material, such as an ammonium compounds (e.g.
ammonium
sulfate, ammonium hydroxide, ammonia, ammonium nitrate, or any other compound
or
mixture containing an ammonium moiety), nitrate or nitrite compounds (e.g.
potassium,
sodium, ammonium, calcium, or other compound or mixture containing a nitrate
or nitrite
moiety), or as a more complex nitrogen-containing material, such as amino
acids, proteins,
hydrolyzed protein, hydrolyzed yeast, yeast extract, dried brewer's yeast,
yeast hydrolysates,
distillers' grains, soy protein, hydrolyzed soy protein, fermentation
products, and processed
or corn steep powder or unprocessed protein-rich vegetable or animal matter,
including those
derived from bean, seeds, soy, legumes, nuts, mill(, pig, cattle, mammal,
fish, as well as other
parts of plants and other types of animals. Nitrogen-containing materials
useful in various
embodiments also include materials that contain a nitrogen-containing
material, including,
but not limited to mixtures of a simple or more complex nitrogen-containing
material mixed
with a carbon source, another nitrogen-containing material, or other nutrients
or non-
nutrients, and AFEX treated plant matter.
[00154] In another embodiment, the carbon level is maintained at a desired
level by
adding sugar compounds or material containing sugar compounds ("Sugar-
Containing
Material") as sugar is consumed or taken up by the organism. The sugar-
containing material
can be added continuously or at regular or irregular intervals. In one
embodiment, additional
sugar-containing material is added prior to the complete depletion of the
sugar compounds
available in the medium. In one embodiment, complete depletion can effectively
be used, for
example to initiate different metabolic pathways, to simplify downstream
operations, or for
other reasons as well. In one embodiment, the carbon level (as measured by the
grams of
sugar present in the sugar-containing material per liter of broth) is allowed
to vary by about
10% around a midpoint, in one embodiment, it is allowed to vary by about 30%
around a
midpoint, and in one embodiment, it is allowed to vary by 60% or more around a
midpoint.
In one embodiment, the carbon level is maintained by allowing the carbon to be
depleted to
an appropriate level, followed by increasing the carbon level to another
appropriate level. In
some embodiments, the carbon level can be maintained at a level of about 5 to
about 120 g/L.
However, levels of about 30 to about 100 g/L can also be usefully employed as
well as levels
of about 60 to about 80 g/L. In one embodiment, the carbon level is maintained
at greater
than 25 g/L for a portion of the culturing. In another embodiment, the carbon
level is
maintained at about 5 g/L, 6 g/L, 7 g/L, 8 g/L, 9 g/L, 10 g/L, 11 g/L, 12 g/L,
13 g/L, 14 g/L,
15 g/L, 16 g/L, 17 g/L, 18 g/L, 19 g/L, 20 g/L, 21 g/L, 22 g/L, 23 g/L, 24
g/L, 25 g/L, 26 g/L,
27 g/L, 28 g/L, 29 g/L, 30 g/L, 31 g/L, 32 g/L, 33 g/L, 34 g/L, 35 g/L, 36
g/L, 37 g/L, 38 g/L,
66
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
39 g/L, 40 g/L, 41 g/L, 42 g/L, 43 g/L, 44 g/L, 45 g/L, 46 g/L, 47 g/L, 48
g/L, 49 g/L, 50 g/L,
51 g/L, 52 g/L, 53 g/L, 54 g/L, 55 g/L, 56 g/L, 57 g/L, 58 g/L, 59 g/L, 60
g/L, 61 g/L, 62 g/L,
63 g/L, 64 g/L, 65 g/L, 66 g/L, 67 g/L, 68 g/L, 69 g/L, 70 g/L, 71 g/L, 72
g/L, 73 g/L, 74 g/L,
75 g/L, 76 g/L, 77 g/L, 78 g/L, 79 g/L, 80 g/L, 81 g/L, 82 g/L, 83 g/L, 84
g/L, 85 g/L, 86 g/L,
87 g/L, 88 g/L, 89 g/L, 90 g/L, 91 g/L, 92 g/L, 93 g/L, 94 g/L, 95 g/L, 96
g/L, 97 g/L, 98 g/L,
99 g/L, 100 g/L, 101 g/L, 102 g/L, 103 g/L, 104 g/L, 105 g/L, 106 g/L, 107
g/L, 108 g/L, 109
g/L, 110 g/L, 111 g/L, 112 g/L, 113 g/L, 114 g/L, 115 g/L, 116 g/L, 117 g/L,
118 g/L, 119
g/L, 120 g/L, 121 g/L, 122 g/L, 123 g/L, 124 g/L, 125 g/L, 126 g/L, 127 g/L,
128 g/L, 129
g/L, 130 g/L, 131 g/L, 132 g/L, 133 g/L, 134 g/L, 135 g/L, 136 g/L, 137 g/L,
138 g/L, 139
g/L, 140 g/L, 141 g/L, 142 g/L, 143 g/L, 144 g/L, 145 g/L, 146 g/L, 147 g/L,
148 g/L, 149
g/L, or 150 g/L.
[00155] The carbon substrate, like the nitrogen substrate, can be used for
cell
production and enzyme production, but unlike the nitrogen substrate, the
carbon substrate can
serve as the raw material for production of fermentation end-products.
Frequently, more
carbon substrate can lead to greater production of fermentation end-products.
In another
embodiment, it can be advantageous to operate with the carbon level and
nitrogen level
related to each other for at least a portion of the fermentation time. In one
embodiment, the
ratio of carbon to nitrogen is maintained within a range of about 30:1 to
about 10:1. In
another embodiment, the ratio of carbon nitrogen is maintained from about 20:1
to about 10:1
or more preferably from about 15:1 to about 10:1. In another embodiment, the
ratio of
carbon nitrogen is about 30:1, 29:1, 28:1, 27:1, 26:1, 25:1, 24:1, 23:1, 22:1,
21:1, 20:1, 19:1,
18:1, 17:1, 16:1, 15:1, 14:1, 13:1, 12:1, 11:1, 10:1, 9:1, 8:1, 7:1, 6:1, 5:1,
4:1, 3:1, 2:1, or 1:1.
[00156] Maintaining the ratio of carbon and nitrogen ratio within
particular ranges can
result in benefits to the operation such as the rate of metabolism of carbon
substrate, which
depends on the amount of carbon substrate and the amount and activity of
enzymes present,
being balanced to the rate of end product production. Balancing the carbon to
nitrogen ratio
can, for example, facilitate the sustained production of these enzymes such as
to replace those
which have lost activity.
[00157] In another embodiment, the amount and/or timing of carbon,
nitrogen, or other
medium component addition can be related to measurements taken during the
fermentation.
For example, the amount of monosaccharides present, the amount of insoluble
polysaccharide
present, the polysaccharase activity, the amount of product present, the
amount of cellular
material (for example, packed cell volume, dry cell weight, etc.) and/or the
amount of
nitrogen (for example, nitrate, nitrite, ammonia, urea, proteins, amino acids,
etc.) present can
67
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
be measured. The concentration of the particular species, the total amount of
the species
present in the fermentor, the number of hours the fermentation has been
running, and the
volume of the fermentor can be considered. In various embodiments, these
measurements
can be compared to each other and/or they can be compared to previous
measurements of the
same parameter previously taken from the same fermentation or another
fermentation.
Adjustments to the amount of a medium component can be accomplished such as by
changing the flow rate of a stream containing that component or by changing
the frequency
of the additions for that component. For example, the amount of saccharide can
be increased
when the cell production increases faster than the end product production. In
another
embodiment, the amount of nitrogen can be increased when the enzyme activity
level
decreases.
[00158] In another embodiment, a fed batch operation can be employed,
wherein
medium components and/or fresh cells are added during the fermentation without
removal of
a portion of the broth for harvest prior to the end of the fermentation. In
one embodiment, a
fed-batch process is based on feeding a growth limiting nutrient medium to a
culture of
microorganisms. In one embodiment, the feed medium is highly concentrated to
avoid
dilution of the bioreactor. In another embodiment, the controlled addition of
the nutrient
directly affects the growth rate of the culture and avoids overflow metabolism
such as the
formation of side metabolites. In one embodiment, the growth limiting nutrient
is a nitrogen
source or a saccharide source.
[00159] In various embodiments, particular medium components can have
beneficial
effects on the performance of the fermentation, such as increasing the titer
of desired
products, or increasing the rate that the desired products are produced.
Specific compounds
can be supplied as a specific, pure ingredient, such as a particular amino
acid, or it can be
supplied as a component of a more complex ingredient, such as using a
microbial, plant or
animal product as a medium ingredient to provide a particular amino acid,
promoter, cofactor,
or other beneficial compound. In some cases, the particular compound supplied
in the
medium ingredient can be combined with other compounds by the organism
resulting in a
fermentation-beneficial compound. One example of this situation would be where
a medium
ingredient provides a specific amino acid which the organism uses to make an
enzyme
beneficial to the fermentation. Other examples can include medium components
that are used
to generate growth or product promoters, etc. In such cases, it can be
possible to obtain a
fermentation-beneficial result by supplementing the enzyme, promoter, growth
factor, etc. or
68
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
by adding the precursor. In some situations, the specific mechanism whereby
the medium
component benefits the fermentation is not known, only that a beneficial
result is achieved.
[00160] In one embodiment, a fermentation to produce a fuel is performed
by culturing
a strain of R. opacus in a medium having a supplement of lignin component and
a
concentration of one or more carbon sources. The resulting production of end
product such as
TAG can be up to 1-fold, 2-fold, 3-fold, 4-fold, 5-fold, 6-fold, 7-fold, 8-
fold, 9-fold, and in
some cases up to 10-fold and higher in volumetric productivity than a process
using only the
addition of a relatively pure saccharide source, and can achieve a carbon
conversion
efficiency approaching the theoretical maximum. The theoretical maximum can
vary with
the substrate and product. For example, the generally accepted maximum
efficiency for
conversion of glucose to ethanol is 0.51 g ethanol/g glucose. In one
embodiment, a
biocatalyst can produce about 40-100% of a theoretical maximum yield of
ethanol. In
another embodiment, a biocatalyst can produce up to about 40%, 50%, 60%, 70%,
80%,
90%, 95% and even 100% of the theoretical maximum yield of ethanol. In one
embodiment,
a biocatalyst can produce up to about 1 %, 2 %, 3 %, 4 %, 5 %, 6 %, 7 %, 8 %,
9 %, 10%, 11
%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%, 20%, 21 %, 22%, 23%, 24%, 25%,
26%, 27%, 28%, 29%, 30%, 31 %, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40
%, 41 %, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51 %, 52%, 53%, 54%,
55 %, 56 %, 57 %, 58 %, 59 %, 60 %, 61 %, 62 %, 63 %, 64 %, 65 %, 66 %, 67 %,
68 %, 69
%, 70%, 71 %, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81 %, 82%, 83%,
84%, 85%, 86%, 87%, 88%, 89%, 90%, 91 %, 92%, 93%, 94%, 95%, 96%, 97%, 98
%, 99 %, 99.99 %, or100 % of a theoretical maximum yield of a fuel. It can be
possible to
obtain a fermentation-beneficial result by supplementing the medium with a
pretreatment or
hydrolysis component. In some situations, the specific mechanism whereby the
medium
component benefits the fermentation is not known, only that a beneficial
result is achieved.
[00161] Various embodiments offer benefits relating to improving the titer
and/or
productivity of fermentation end-product production by a biocatalyst by
culturing the
organism in a medium comprising one or more compounds comprising particular
fatty acid
moieties and/or culturing the organism under conditions of controlled pH.
[00162] In one embodiment, the pH of the medium is controlled at less than
about pH
7.2 for at least a portion of the fermentation. In one embodiment, the pH is
controlled within
a range of about pH 3.0 to about 7.1 or about pH 4.5 to about 7.1, or about pH
5.0 to about
6.3, or about pH 5.5 to about 6.3, or about pH 6.0 to about 6.5, or about pH
5.5 to about 6.9
or about pH 6.2 to about 6.7. The pH can be controlled by the addition of a pH
modifier. In
69
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
one embodiment, a pH modifier is an acid, a base, a buffer, or a material that
reacts with
other materials present to serve to raise of lower the pH. In one embodiment,
more than one
pH modifier can be used, such as more than one acid, more than one base, one
or more acid
with one or more bases, one or more acids with one or more buffers, one or
more bases with
one or more buffers, or one or more acids with one or more bases with one or
more buffers.
When more than one pH modifiers are utilized, they can be added at the same
time or at
different times. In one embodiment, one or more acids and one or more bases
can be
combined, resulting in a buffer. In one embodiment, media components, such as
a carbon
source or a nitrogen source can also serve as a pH modifier; suitable media
components
include those with high or low pH or those with buffering capacity. Exemplary
media
components include acid- or base-hydrolyzed plant polysaccharides having with
residual acid
or base, AFEX treated plant material with residual ammonia, lactic acid, corn
steep solids or
liquor.
[00163] In one embodiment, a constant pH can be utilized throughout the
fermentation.
In one embodiment, the timing and/or amount of pH reduction can be related to
the growth
conditions of the cells, such as in relation to the cell count, the end
product produced, the end
product present, or the rate of end product production. In one embodiment, the
pH reduction
can be made in relation to physical or chemical properties of the
fermentation, such as
viscosity, medium composition, gas production, off gas composition, etc.
[00164] Recovery of Fermentation End Products
[00165] In another aspect, methods are provided for the recovery of the
fermentive end
products, such as an alcohol (e.g. ethanol, propanol, methanol, butanol, etc.)
another biofuel
or chemical product. In one embodiment, broth will be harvested at some point
during of the
fermentation, and fermentive end product or products will be recovered. The
broth with end
product to be recovered will include both end product and impurities. The
impurities include
materials such as water, cell bodies, cellular debris, excess carbon
substrate, excess nitrogen
substrate, other remaining nutrients, other metabolites, and other medium
components or
digested medium components. During the course of processing the broth, the
broth can be
heated and/or reacted with various reagents, resulting in additional
impurities in the broth.
[00166] In one embodiment, the processing steps to recover end product
frequently
includes several separation steps, including, for example, distillation of a
high concentration
alcohol material from a less pure alcohol-containing material. In one
embodiment, the high
concentration alcohol material can be further concentrated to achieve very
high concentration
alcohol, such as 98% or 99% or 99.5% (wt.) or even higher. Other separation
steps, such as
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
filtration, centrifugation, extraction, adsorption, etc. can also be a part of
some recovery
processes for alcohol as a product or biofuel, or other biofuels or chemical
products.
[00167] In one embodiment, a process can be scaled to produce commercially
useful
biofuels. In another embodiment, biocatalyst is used to produce an alcohol,
e.g., ethanol,
butanol, propanol, methanol, or a fuel such as hydrocarbons hydrogen, TAG, and
hydroxy
compounds. In another embodiment, biocatalyst is used to produce a carbonyl
compound
such as an aldehyde or ketone (e.g. acetone, formaldehyde, 1-propanal, etc.),
an organic acid,
a derivative of an organic acid such as an ester (e.g. wax ester, glyceride,
etc.), 1, 2-
propanediol, 1, 3-propanediol, lactic acid, formic acid, acetic acid, succinic
acid, pyruvic
acid, or an enzyme such as a cellulase, polysaccharase, lipases, protease,
ligninase, and
hemicellulase.
[00168] In one embodiment, useful biochemicals can be produced from non-
food plant
biomass, with a steam or hot-water extraction technique that is carried out by
contacting a
charge of non-food plant pretreated biomass material such as corn stover or
sorhum with
water and/or acid (with or without additional process enhancing compounds or
materials), in
a pressurized vessel at an elevated temperature up to about 160 -220 C. and
at a pH below
about 7.0, to yield an aqueous (extract solution) mixture of useful sugars
including long-
chain saccharides (sugars), acetic acid, and lignin, while leaving the
structural (cellulose and
lignin) portion of the lignocellulosic material largely intact. In
combination, these potential
inhibitory chemicals especially sugar degradation products are low, and the
plant derived
nutrients that are naturally occurring lignocellulosic-based components are
also recovered
that are beneficial to a C5 and/or C6 fermenting organism. Toward this
objective, the aqueous
extract is concentrated (by centrifugation, filtration, solvent extraction,
flocculation,
evaporation), by producing a concentrated sugar stream, apart from the other
hemicellulose
(C5 rich) and cellulosic derived sugars (C6 rich) which are channeled into a
fermentable
stream.
[00169] Biofuel plant and process of producing biofuel:
[00170] Large Scale Fuel and Chemical Production from Biomass
[00171] Generally, there are several basic approaches to producing fuels
and chemical
end-products from biomass on a large scale utilizing of microbial cells. In
the one method,
one first pretreats and hydrolyzes a biomass material that includes high
molecular weight
carbohydrates to lower molecular weight carbohydrates, and then ferments the
lower
molecular weight carbohydrates utilizing of microbial cells to produce fuel or
other products.
In the second method, one treats the biomass material itself using mechanical,
chemical
71
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
and/or enzymatic methods. In all methods, depending on the type of biomass and
its physical
manifestation, one of the processes can comprise a milling of the carbonaceous
material, via
wet or dry milling, to reduce the material in size and increase the surface to
volume ratio
(physical modification).
[00172] In one embodiment, hydrolysis can be accomplished using acids,
e.g.,
Bronsted acids (e.g., sulfuric or hydrochloric acid), bases, e.g., sodium
hydroxide,
hydrothermal processes, ammonia fiber explosion processes ("AFEX"), lime
processes,
enzymes, or combination of these. Hydrogen, and other end products of the
fermentation can
be captured and purified if desired, or disposed of, e.g., by burning. For
example, the
hydrogen gas can be flared, or used as an energy source in the process, e.g.,
to drive a steam
boiler, e.g., by burning. Hydrolysis and/or steam treatment of the biomass
can, e.g., increase
porosity and/or surface area of the biomass, often leaving the cellulosic
materials more
exposed to the biocatalyst cells, which can increase fermentation rate and
yield. Removal of
lignin can, e.g., provide a combustible fuel for driving a boiler, and can
also, e.g., increase
porosity and/or surface area of the biomass, often increasing fermentation
rate and yield.
Generally, in any of the these embodiments, the initial concentration of the
carbohydrates in
the medium is greater than 20 mM, e.g., greater than 30 mM, 50 mM, 75 mM, 100
mM, 150
mM, 200 mM, or even greater than 500 mM.
[00173] Biomass processing plant and process of producing products from
biomass
[00174] In one aspect, a fuel or chemical plant that includes a
pretreatment unit to
prepare biomass for improved exposure and biopolymer separation, a hydrolysis
unit
configured to hydrolyze a biomass material that includes a high molecular
weight
carbohydrate, and one or more product recovery system(s) to isolate a product
or products
and associated by-products and co-products is provided. In another aspect,
methods of
purifying lower molecular weight carbohydrate from solid byproducts and/or
toxic impurities
is provided.
[00175] In another aspect, methods of making a product or products that
include
combining biocatalyst cells of a microorganism and a biomass feed in a medium
wherein the
biomass feed contains lower molecular weight carbohydrates and unseparated
solids and/or
other liquids from pretreatment and hydrolysis, and fermenting the biomass
material under
conditions and for a time sufficient to produce a biofuel, chemical product or
fermentive end-
products, e.g. ethanol, propanol, hydrogen, succinic acid, lignin, terpenoids,
and the like as
described above, is provided.
72
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00176] In another aspect, products made by any of the processes described
herein is
also provided herein.
[00177] One example is a method for producing chemical products from
biomass by
first treating biomass with an acid at elevated temperature and pressure in a
hydrolysis unit.
The biomass may first be heated by addition of hot water or steam. The biomass
may be
acidified by bubbling gaseous sulfur dioxide through the biomass that is
suspended in water,
or by adding a strong acid, e.g., sulfuric, hydrochloric, or nitric acid with
or without
preheating/presteaming/water addition. During the acidification, the pH is
maintained at a
low level, e.g., below about 5. The temperature and pressure may be elevated
after acid
addition. In addition to the acid already in the acidification unit,
optionally, a metal salt such
as ferrous sulfate, ferric sulfate, ferric chloride, aluminum sulfate,
aluminum chloride,
magnesium sulfate, or mixtures of these can be added to aid in the acid
hydrolysis of the
biomass. The acid-impregnated biomass is fed into the hydrolysis section of
the pretreatment
unit. Steam is injected into the hydrolysis portion of the pretreatment unit
to directly contact
and heat the biomass to the desired temperature. The temperature of the
biomass after steam
addition is, e.g., between about 130 C and 220 C. The acid hydrolysate is
then discharged
into the flash tank portion of the pretreatment unit, and is held in the tank
for a period of time
to further hydrolyze the biomass, e.g., into oligosaccharides and monomeric
sugars. Other
methods can also be used to further break down biomass. Alternatively, the
biomass can be
subject to discharge through a pressure lock for any high-pressure
pretreatment process.
Hydrolysate is then discharged from the pretreatment reactor, with or without
the addition of
water, e.g., at solids concentrations between about 10% and 60%.
[00178] After pretreatment, the biomass may be dewatered and/or washed
with a
quantity of water, e.g. by squeezing or by centrifugation, or by filtration
using, e.g. a
countercurrent extractor, wash press, filter press, pressure filter, a screw
conveyor extractor,
or a vacuum belt extractor to remove acidified fluid. Wash fluids can be
collected to
concentrate the C5 saccharides in the wash stream. The acidified fluid, with
or without
further treatment, e.g. addition of alkali (e.g. lime) and or ammonia (e.g.
ammonium
phosphate), can be re-used, e.g., in the acidification portion of the
pretreatment unit, or added
to the fermentation, or collected for other use/treatment. Products may be
derived from
treatment of the acidified fluid, e.g., gypsum or ammonium phosphate. Enzymes
or a mixture
of enzymes can be added during pretreatment to hydrolyze, e.g. endoglucanases,
exoglucanases, cellobiohydrolases (CBH), beta-glucosidases, glycoside
hydrolases,
glycosyltransferases, alphyamylases, chitinases, pectinases, lyases, and
esterases active
73
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
against components of cellulose, hemicelluloses, pectin, and starch, in the
hydrolysis of high
molecular weight components.
[00179] A fermentor, attached or at a separate site, can be fed with
hydrolyzed
biomass, any liquid fraction from biomass pretreatment, an active seed culture
of a
biocatalyst, such as a yeast, if desired a co-fermenting microbe, e.g.,
another yeast or E. coli,
and, if required, nutrients to promote growth of the biocatalyst or other
microbes.
Alternatively, the pretreated biomass or liquid fraction can be split into
multiple fermenters,
each containing a different strain of a biocatalyst and/or other microbes, and
each operating
under specific physical conditions. Fermentation is allowed to proceed for a
period of time,
e.g., between about 1 and 150 hours, while maintaining a temperature of, e.g.,
between about
25 C and 50 C. Gas produced during the fermentation is swept from fermentor
and is
discharged, collected, or flared with or without additional processing, e.g.
hydrogen gas may
be collected and used as a power source or purified as a co-product.
[00180] In another aspect, methods of making a fuel or fuels that include
combining
one or more biocatalyst and a lignocellulosic material (and/or other biomass
material) in a
medium, adding a lignin fraction from pretreatment, and fermenting the
lignocellulosic
material under conditions and for a time sufficient to produce a fuel or
fuels, e.g., ethanol,
propanol and/or hydrogen or another chemical compound is provided herein.
[00181] In another aspect, the products made by any of the processes
described herein
is provided.
EXAMPLES
[00182] The following examples serve to illustrate certain embodiments and
aspects
and are not to be construed as limiting the scope thereof.
[00183] Example 1. Maintenance of DDGS levels.
[00184] To supplement C6 with corn mash, a 20% C6 solution is added to an
80% corn
mash for an SSF fermentation. The 80% corn mash slurry contains 30% solids
which are
comprised of 72% starch, 12-15% glucan, oil and fiber about 7-10%. The oil and
fiber, after
the fermentation of the starch and glucan, constitutes what is called DDGS and
can be sold as
feed, primarily for cattle. The starch fermentation results in 21-22% sugars
which can be
converted to 10-11% ethanol.
[00185] If starch-derived sugar is blended with 26-30% C6 concentration in
the ratio
described supra (20:80), and then fermented, there can be an increase in sugar
yields. The
totals are 25-26% sugar which translates into a 12-13% ethanol yield. However,
20% of the
74
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
DDGS can be lost due to the dilution of the corn mash solids. The ethanol
yield can be
increased but there can be a loss of income from the DDGS yield.
[00186] The solution is to add a greater concentration of the solids (36%
solids instead
of 30% solids) to the 80% corn mash solution. Corn mash cannot normally be
fermented at a
high solids content (36%). However, because the solids are diluted in a C6-
supplemented
fermentation, DDGS yields can be maintained. Thus, in a 1000 gallon tank, 800
gallons of
corn mash containing 36% solids can be supplemented with 200 gallons of C6
solution
containing 26-30% C6 sugar, to prevent loss of DDGS. The corn mash solution,
that cannot
be fermented as 36% solids can be diluted to 30% solids. Thus there can be an
increase of
ethanol yield (or sugar yield) and production of the same amount of DDGS. The
3-4%
increase in sugar can lead to a 1-2% increase in ethanol
[00187] Example 2. Preparation of Cellulosic-Derived C6 Fermentable Sugars
(from sorghum and switchgrass)
[00188] Non-food cellulosic feedstock is received and pre-processed.
First, oversized
materials (example large chunks of wood) and contaminates (for example stones,
soil, etc.)
are selected and removed in a gross screening process. The undersized
particles, or fines,
including contaminates, such as sand, soil or the like, are separated and
removed in a fine
screening process. The remaining lignocellulosic material can be triturated
(e.g., by
chipping, tub grinding, hammer milling, or other available comminuting
procedure) to reduce
the feedstock to the preferred size and condition for further handling and
processing.
[00189] In one example, switch grass and ensiled energy sorghum was used.
Switch
grass was dried and hammer milled to reduce particle size. In the case of
ensiled energy
sorghum, the material was chopped and ensiled in a bunker. The water content
of each
biomass feedstock was determined and adjusted to a solids content of about 15%
(wt/v) solids
and moisture content of about 85% (wt/v) using a 24 hour soaking treatment.
[00190] The moisture-adjusted switch grass and energy sorghum feedstocks
were
separately prepared for an acid-catalyzed steam explosion pretreatment process
by
impregnating the feedstocks with 1 % H2504 (w/w, based on dry weight) and left
to soak
overnight. The impregnated raw material was then charged to a 60L pressurized
steam
explosion batch reactor at a temperature of about 200 C and a pH of about 2.9
for 7.5 min,
so that an aqueous extract (or liquor) containing solubilized components of
the lignocellulosic
material was obtained (C5 rich). The remaining lignocellulosic material (e.g.,
fibrous
material) was separated from the liquor or extract, and each was further
processed as
discussed infra.
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00191] Upon completion of pretreatment, the post-treated material,
comprising about
12% to about 14% solids [wt/v], was subjected to a water washing step to
separate solubilized
C5 sugar from C6 sugars. The washing was performed in two steps; in the first
step, the post-
treated feedstock was separated by centrifugation filter press which enables
the separation of
the solids from the liquid stream (the liquid stream being C5 rich, e.g.,
containing a high
proportion of C5 sugars). The solid material was further suspended in water to
recover
additional C5 sugar that may have remained in the separated solids portion. On
removing the
soluble hemicellulose sugars, the remaining solids contained the cellulose or
hexose rich C6
sugars. However, as a person of skill appreciates, no separation process is
perfect and the
solids portion may include some amount of C5 sugars, and the liquid stream may
include
some amount of C6 sugars. The liquid stream was then retained and maintained
separate
from the C6 stream. The separated solids were placed into a mixing tank. Once
inside the
mixing tank, the solids were adjusted to a pH of about 5 using 0.1 N NaOH. The
solids wee
then diluted to a dry solids content of about 8% solids. Enzymes (CELLIC CTech
2,
Novozymes North America, Franklinton, NC) were added to the solid slurry at 2%
loading
(v/wt) based on the dry weight of the solids.
[00192] Figure 1 is a sample sugar trajectory that illustrates how the
biomass is
converted from solids into liquefied stream of C6 sugars. Figure 1 was
generated by
collecting samples periodically for estimation (by HPLC) of cellulosic C6
sugars released by
enzymatic hydrolysis of solids. Once enzymatic hydrolysis was complete, the
liquid slurry is
separated by centrifugation or microfiltration; or, alternatively, the solids
can remain in the
broth. For the experiments herein, the solids were separated from the broth
using
evaporation. Approximately 30 L of the resultant C6-rich liquid slurry was
concentrated by
simple evaporation at a temperature of from about 70 C to about 80 C until the
sugar content
of the sorghum or switchgrass hydrolysate was raised from about 5% to about
20% w/v. The
resulting composition had a C6 sugar:C5 sugar ratio of about 90:10.
[00193] Example 3. Preparation and Fermentation of Corn Mash Glucose
Feedstock
[00194] Mash was derived from industrial dry milling operations (Western
New York
Energy). Large debris were removed from the standard corn by hand, and small
debris (<4
mm) were removed by passing through a No. 5 sieve before grinding using a
Wiley mill
fitted with a 2-mm screen. This produced particles of which 95% were smaller
than 1.5 mm.
The moisture content of the ground corn was 13.98 % (w/w, as received) and was
used to
76
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
determine the mass of corn needed to prepare mash at a dry solids
concentration of 25 %
(w/w).
[00195] A 0.13 g/ml working solution of the alpha amylase (Liquozyme SC
DS,
Novozymes) was added at a dose of 0.025 % (w/w) based on the wet weight of the
corn in the
slurry. The slurries were sealed and mixed at 50 rpm. Samples were liquefied
by incubating
at 83 C for 90 minutes, after which the samples were cooled to 40 and the
mass of mash was
calculated. The pH of the mash was adjusted to <5.2 by addition of 10 N
sulfuric acid. The
samples were shaken at 170 rpm at 32 C . Glucoamylase enzyme (starch breakdown
process
for corn mash) (Spirizyme Fuel, Novozymes) was prepared as a 0.25 g/mL
solution and
added at a dose of 0.66% (w/w, based on the wet weight of corn). Antibiotic,
Lactrol (Philbro,
Ridgefield Park, NH), was added to each flask to achieve a concentration of
0.5 ppm (w/w).
The resulting corn mash had a 30% glucose concentration (wt/vol).
[00196] A 0.1 g/ml suspension of yeast (Saccharomyces cerevisiae; Ethanol
Red;
Fermentis, Marcqen-Baroeul, France) was prepared in a sterile 250-ml flask and
incubated at
40 C for 20 minutes prior to inoculation into the fermentation containers
containing
saccharified corn mash feedstock. Nitrogen, as a 0.2 g/mL urea solution was
added to a total
concentration of nutrient nitrogen of 500 mg N/kg. The flasks were incubated
at 32 C at 170
rpm for 60-120 hours. A dextrose fermentation was run as a control.
[00197] Example 4. Blending of Cellulosic-Derived C6 Fermentable Sugars
and
Starch C6
[00198] Sugar compositions derived from ensiled energy sorghum and switch
grass
were prepared in accordance with Example 2. The cellulosic hydrolysate derived
from
sorghum and switchgrass comprise approximately 20% (wt/v) C6 sugars. Various
blending
ratios of corn mash (starch C6 with cellulosic C6) were prepared as follows:
For every 100 ml
of total whole fermentation broth, the amount of corn mash to cellulosic sugar
C6 sugars was
blended such that the final concentration of the sugars remained close to 20%.
Thus, for the
ratio of corn mash to sorghum of 90:10, 90 ml of 25% sugar slurry (wt/v) of
corn mash was
blended with 10 ml of cellulosic sorghum sugar slurry comprising 20% C6
sugars. In
accordance with this protocol, several blends of corn mash and sorghum were
prepared
wherein the final C6 sugars were close to 20% (wt/v) and final volume of the
broth was
adjusted, resulting in four samples having a ratio of about 80:20, 70:30,
60:40 and 50:50 corn
mash:C6 sugar. A similar process was followed to create fermentation
feedstock, which was
a blend of corn mash and switchgrass sugars.
77
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00199] Example 5. Ethanol Yield of Corn Mash blended with Cellulosic C6
sugars
[00200] Prior to fermentation, the sorghum sugar streams were
characterized for total
solids, total dissolved solids and HPLC analysis of sugar content. The
concentrations of total
dry solids and dissolved dry solids were measured using standard fermentation
procedures.
The sugar substrate concentration was analyzed by HPLC for cellobiose,
glucose, xylose,
galactose, arabinose and mannose. The concentrations of sugar and ethanol were
also
determined during fermentation by HPLC. Ethanol conversion rate was determined
by
finding the theoretical maximum of ethanol to be produced by a sample (total
glucose (g)
x0.55 = total ethanol (g)). By using the HPLC, the resulting ethanol after
fermentation was
compared to the theoretical maximum of ethanol to be generated by the starting
glucose level
of the sample as fermentation began: (Resulting ethanol)/(Theoretical max
Ethano1)=
Ethanol Conversion rate.
[00201] Table 1 and Figure 2 illustrate the approximate percent gain in
ethanol yield
over corn mash control in fermentation. The 80:20 corn mash blend with energy
sorghum
showed a 20.71% increase in ethanol conversion rate, the 70:30 corn mash blend
with energy
sorghum showed a 12.05% increase in ethanol conversion rate, the 60:40 corn
mash blend
with energy sorghum showed a 16.72% increase in ethanol conversion rate, and
the 50:50 corn
mash blend with energy sorghum showed a 11.99% increase in ethanol conversion
rate.
[00202] Table 1
% Ethanol
Improvement
Recovered in Ethanol
from Yield over
Initial Ethanol Theoretical Corn Mash
(g/L) sugar Yield Maximum control (%)
100% Corn Mash¨Control 318.4 112.6 68.4 ----
80:20 Corn Mash with Energy
265.7 117.5 82.6 20.7
Sorghum
70:30 Corn Mash with Energy
259.7 108.2 76.7 12.0
Sorghum
60:40 Corn Mash with Energy
238.1 103.5 79.9 16.7
Sorghum
78
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
50:50 Corn Mash with Energy
256.9 107.5 76.6 12.0
Sorghum
[00203] The results in Figures 3 and 4 demonstrate that corn mash
fermented with
energy-sorghum derived sugar compositions produce a higher efficiency of yeast
sugar
conversion to ethanol (approximately a 10-15% increase) as well as faster
conversion of
glucose sugars to ethanol (about 8 hours faster). Although the corn mash
feedstock initially
had more sugar than the corn mash and cellulosic sugar blends, all samples
resulted in
approximately the same final concentration of ethanol. Further, the
fermentation of 100%
corn mash took nearly ten hours longer to produce this final concentration.
[00204] Switchgrass cellulosic sugar was blended with corn mash to produce
80:20
and 50:50 corn mash:switchgrass ratio samples. Table 2 and Figure 5 illustrate
the percent
gain in ethanol yield over the corn mash control during yeast fermentation.
The 80:20 corn
mash/switchgrass blend produced a 3.14% rise in ethanol conversion rate, and
the 50:50 corn
mash blend with switchgrass demonstrated a 6.95% rise in ethanol conversion
rate.
[00205] Table 2
Improvement
in Ethanol
% Ethanol Recovered Yield
over
Initial Ethanol from Theoretical Corn
Mash
(g/L) sugars Yield Maximum
control (%)
100% Corn Mash -
Control 229.9 106.6 84.6 ----
80:20 Corn Mash with
Switch Grass 224.3 106.2 87.3 3.1
50:50 Corn Mash with
Switch Grass 219.3 108.5 90.5 7.0
[00206] Example 6. Impact of Sugar Compositions on Fermentation
[00207] Three 20% glucose solutions were prepared for fermentation. The
first was
used as a control. The second was spiked with 5g Switchgrass dry solids. The
third feedstock
was spiked with 5g energy sorghum dry solids. The three feedstocks were
adjusted to
comprise similar total sugar concentrations (see Table 3) and fermented with
yeast. The
79
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
Switchgrass and energy sorghum dry solids comprised the lignins, plant
proteins and oils
remaining after hydrolysis and removal of soluble C5 and C6 saccharides. The
dry solids were
filtered, washed, dried and weighed prior to mixing with the glucose.
[00208] Table 3
% Ethanol
Improvement in
Recovered from
Ethanol Yield over
Initial Ethanol Theoretical
Corn Mash control
(g/L) sugar Yield Maximum (%)
Glucose Control 196.3 84.4 78.2 ----
Glucose with 5g
Switchgrass Solids 191.6 96.8 91.9 17.5
Glucose with 5g Energy
Sorghum Solids 208.09 109.26 85.1 8.9
[00209] The results shown in Figures 6 and 7 and Table 3 demonstrate that
the addition
of cellulosic C6 solids to the fermentation broth or growth media stimulate
and enhance the
conversion of a C6 glucose carbohydrate and/or cellulosic glucose sugar
containing sources to
ethanol.
[00210] Not wishing to be bound by theory, it is believed that the level
of nutrient rich
ions present in the cellulosic hydrolysate stream enables the yeast to more
efficiently and
effectively consume glucose. Other mechanisms may also contribute to the
observed
beneficial effect: 1) appropriate levels of salt (ash) that alter membrane
permeability or
transport proteins, allowing more glucose into the cell and therefore more
ethanol production;
2) appropriate (low) levels of inhibitors may reduce protons on the exterior
of the
mitochondria in the yeast cell, stimulating processes that seek to maintain
the environmental
conditions which impacts cell energy generation (these processes would enhance
glucose
metabolism resulting in higher ethanol production); 3) appropriate levels of
trace ions and
salts like Zn, Mg, and K in the feedstock increase the pH environment around
and in the cell,
stimulating enzyme activity resulting in increased ethanol production.
[00211] Example 7: Preparation of Corn Stover used for Corn Mash Blending
[00212] Corn stover was processed through a steam pretreatment system
using only
steam and no additional catalysts to prepare a C6-enriched hydrolysate. The
pretreatment
temperature was 205 C and the resident time was about 7.5 minutes. Excess C5
sugars and
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
acetic acid that were generated during pretreatment were solubilized and
removed using a
filter press.
[00213] The stover was then added to a 55 gallon jacketed reactor and
total solids were
brought to about 8% (wt/wt) using water. The pH of the corn stover was
adjusted to about
5.0 using sodium hydroxide. The 8% slurry of corn stover contained
approximately 5 kg of
dry biomass. To this, 1 L of enzymes were added to the broth. This represented
approximately 4x the standard dosage (20% of total solids (v/wt)) in order to
ensure complete
hydrolysis. During enzymatic hydrolysis, the temperature was maintained at
approximately
50 C for about 72 hours with agitation. The total weight of the corn stover
plus water was
approximately 62.5 kg. The slurry was then centrifuged following hydrolysis to
remove all,
or substantially all, of the remaining solids from the hydrolyzed sugars and
the sugars were
then concentrated through evaporation until the C6 sugar level reached about
300g/L. The
sugar solution was sealed and kept at 4 C until fermentation.
[00214] Example 8: Corn Mash Blending with Cellulosic Sugar derived from
Corn Stover
[00215] Corn mash oligosaccharides (e.g., starch) containing between about
25% and
30% sugars was blended with monomeric C6 sugar under various fermentation
conditions.
The monomeric sugars comprised either a solution of pure glucose or a C6-
enriched
hydrolysate produced from corn stover (e.g., cellulosic sugars) according to
the procedure in
Example 7. Both sources of monomeric sugars comprised about 25% sugars. The
blended
feedstocks were then simultaneously saccharified and fermented using a
combination of
enzymes and yeast.
[00216] The corn mash control, undiluted, was saccharified and fermented
as is. For
the conditions with blended feedstocks, either 80 or 90 grams of corn mash
material was
weighed out and funneled into the appropriate flask. Following this step, 20
or 10 mL of
either a ¨25% sterile solution of glucose or ¨25% corn stover hydrolysate was
added to the
corn mash and mixed. Fermentations comprising only pure glucose or the corn
stover
hydrolysate, had 1 mL of Yeast Nutrient Media added. 1 mL of 5% Urea and 5%
Magnesium
sulfate solution was added to all samples prior to fermentation, and the pH of
each solution
was adjusted to 4.8 ¨ 5.2.
[00217] Yeast, propagated beforehand to exponential growth phase, was
added to each
sample. For each corn mash sample, 50 iut of glucoamylase was added at the
same time to
initiate the simultaneous saccharification of the corn mash and the yeast
fermentation.
81
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
Samples of the fermentation were analyzed at 2.5, 5, 24, and 96 hours for
glucose and
ethanol. The results are summarized in Table 4.
[00218] Control fermentations were carried out with pure glucose solution
or the C6-
enriched corn stover hydrolysate. The concentration of sugars in the
fermentation reactions
was approximately 20%. The glucose and the C6 hydrolysate produced similar
yields of
ethanol. The pure glucose fermentation produced an ethanol titer of 105.54 g/L
after 96
hours; the C6-enriched hydrolysate fermentation reaction produced an ethanol
titer of 104.03
g/L after 96 hours. This experiment shows that the C6-enriched hydrolysate
yields are similar
to a pure glucose solution.
[00219] Table 4: Summary of fermentation data
Time 0 2.5 5 24 96
(hrs)
Glucose
Glucose (g/L) 192.9 162.1 130 61.54 0
Control
(20%) Ethanol
0 7.98 22.94 72.15 105.54
(g/L)
Glucose
Corn Stover(g/L) 191.9 196 187.8 47.43 2.97
Hydrolysate
(20%C6) Ethanol 0
1.98 8.5 71.33 104.03
(g/L)
Glucose
12.7 107.8 151.28 54.91 15.74
SSF Corn (g/L)
Mash Control Ethanol 0 10.4 33.15 109.87 142.33
(g/L)
Glucose
SSF Corn (g/L) 65.9 125.8 151.49 47 4.32
Mash: Glucose
(80:20) Ethanol 0
8.4 32.35 112.82 153.85
(g/L)
SSF Corn Glucose
70.1 130.3 131.35 55.04 19.93
Mash:Corn (g/L)
Stover
Hydrolysate Ethanol
(80:20) (g/L) 0 9.1 31.23 114.11 137.79
Glucose
SSF Corn (IL) 28.1 132.3 153.22 45.76 8.65
Mash: Glucose
(90:10) Ethanol 0
9.6 32.88 117.46 153
(g/L)
82
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
SSF Corn Glucose
41 118 122.82 48.33 14.87
Mash:Corn (g/L)
Stover
Hydrolysate Ethanol
(90:10) (g/L) 0 12 33.37 112.53 150.71
[00220] Example 9. Fed Batch fermentation using Blended Cellulosic-derived
and
non-Cellulosic Sugars
[00221] Using varying proportions of cellulosic-derived C6 stream, the
average
ethanol yield of liquefied corn mash was compared to the average ethanol yield
of a control
containing only water and corn solids. Also, a glucose solution instead of a
C6 rich stream
was also used as a term of comparison for concentration of products, given the
high sugar
concentration of the amendments. Fed-batch strategy was also examined to
evaluate the
performance of the yeast when a C6 sugar rich stream was fed into the
fermenter as
controlled additions during SSF operations.
[00222] Most dry milling operations in the U.S. follow a particular series
of steps.
They mill the corn, adjust the solids and starch content, cook the corn mash
with alpha
amylase and, finally, to gain efficiency, carry out SSF with the addition of
glucoamylase
(GA) and a fermenting yeast in a single vessel. The success of this operation
can be
determined and driven by several factors such as the starch content in the
mash leading to
total fermentable sugars (e.g., the higher the sugar content, the higher the
amount of ethanol
produced); the kind of yeast used (sugar, ethanol and temperature tolerant);
the inoculum
level of the yeast and fermentation time; the residual sugars at the end of
fermentation (most
corn ethanol plants prefer to maximize sugar utilization and minimize the
presence of
residual sugars as it impacts the quality of DDGS on distillation);
temperature; the control of
the release and presence of monomeric C6 sugars and salts during fermentation;
the level of
fermentation inhibitors formed (e.g., acetic acid, lactic acid, formic acid,
HMF, furfural and
lignin produced); the presence of CS sugars and their concentration in the
mash (e.g., xylose
can interfere with fermentation as most fermenting yeasts are unable to
assimilate this sugar;
in this case, addition of a CS fermenting yeast can be used to achieve
complete sugar
conversion).
[00223] To gain efficiency and lower capital expenditures, most corn dry
milling
operation currently average about 48 hours of fermentation. A limiting factor,
affecting the
finishing time, can be the rate of the glucoamylase (GA) addition and the
conversion of
83
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
dextrin's to monomeric C6 sugars. Most operators try to keep glucose levels at
about 1% or
less at just after 18 hours of fermentation because the glucoamylase is
expensive, so it is
necessary to be as efficient as possible.
[00224] Further, in most of these operations, glucose levels are kept low
to reduce
osmotic stress on the yeast, especially past 18 -24 hours of fermentation when
other stressors
may begin to impact the yeast (e.g., ethanol, lactic acid, acetic acid, etc.).
Dosing GA at a
higher concentration typically results 3-5% glucose at 24 hours; however by
reducing the
GA, a reduction in the glycerol production (presumably from less osmotic
stress) is noticed.
To achieve optimum availability of monomeric C6 glucose without compromising
osmotic
stress effects during corn mash fermentation with yeast, a fed¨batch operation
was carried out
wherein cellulosic derived C6 sugar was controlled and 'spoon fed' or fed
continuously to the
corn mash to insure no excess build up of monomeric C6 sugars in the mash.
This strategy
enabled the microorganism to get optimum feeding-on-demand monomeric C6 sugar,
steering the yeast metabolic pathway away from glycerol while enabling rapid
conversion to
ethanol.
[00225] Corn mash slurry was prepared for the different fermentation
treatments
(e.g., corn mash only and blended with cellulosic-derived C6 monosaccharides).
Briefly, the
moisture content of the corn was used to determine the mass of corn needed to
prepare mash
at a dry-solids concentration of 25 % (w/w). The alpha-amylase enzyme
(Liquozyme SC DS,
Novozymes, U.S.A.) was diluted to ensure more precise delivery of enzyme to
each sample.
A 0.13-g/m1 working solution of the alpha amylase was added at a dose of 0.025
% (w/w)
based on the wet weight of the corn. The pH was adjusted to 5.7 ¨ 5.85 using
1N ammonium
hydroxide and the samples agitated while incubating at 83 C for 90 minutes.
Following
liquidation of the samples, they were cooled to 40 C.
[00226] Prior to fermentation, the cellulosic C6 rich stream derived from
various
feedstocks (e.g., corn stover, wheat straw, energy sorghum, switchgrass, etc.)
was prepared
using pretreatment procedures that maximize recovery of fermentable
saccharides. The
fermentations were performed with crude hydrolysates that were characterized
for total
solids, total dissolved solids and HPLC analysis of sugar content. The
concentrations of total
dry solids and dissolved dry solids were measured using standard NREL
procedures. The
sugar substrate concentration was analyzed by HPLC for C5 sugars and glucose
as C6.
[00227] Characterization of a C6 rich stream
[00228] A C6 rich stream was prepared from processing corn stover that was
pretreated in a commercial Biogasol unit capable of processing 100MT dry
biomass per
84
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
day with dilute H2SO4 (15 minutes at 170 C with 0.6%, H2SO4). The C5 sugars
were
separated and the C6 /lignin rich solids were enzyme hydrolyzed with Novozyme
Cellic Ctec3 cellulase enzymes. Post hydrolysis, the lignin was separated and
the C6
rich stream with a residual amount of C5 was further concentrated to 25%
solubilized
solids.
[00229] The contents of the cellulose hydrolysis stream (C6 rich stream)
is shown in
Table 5 below.
[00230] Table 5: Characterization of cellulosic C6 stream
pH Dissolved Acetic acid HMF C5 as Glucose
solids Xylose (%w/v)
(%w/w) (%w/v)
5.0 25.44 0 0 2.5 23.48
[00231] The mass of the cooled mash was calculated for each sample. The pH
of the
mash was adjusted to <5.2 by addition of 1-N sulfuric acid.
[00232] All enzymes, nutrients, and other amendments added to the
fermentation flasks
were freshly prepared before use. The total concentration of nutrient nitrogen
as urea was 500
mg N/kg to a final concentration of 500 ppm as nitrogen (w/w, based on the
total mass of
mash). The glucoamylase enzyme (Spirizyme Fuel, Novozymes) was prepared as a
0.25 g/ml
solution and added at a dose of 0.066% (w/w, based on the wet weight of corn).
Antibiotic,
FermGuard Xtreme (Ferm Solutions, Inc, Danville, KY), was added to each sample
as a dose
of 0.5 ppm (w/w). Yeast extract (0.16 g) was added to the glucose treatment
that fermented
with no corn solids.
[00233] A 0.1 g/ml suspension of yeast (Saccharomyces cerevisiae;
FermaxGreen and
FermaxGold Ethanol Red; Fermentis, Marcq-en-Baroeul, France) was prepared in a
sterile 1L
flask having the corn mash with glucoamylase. This suspension was incubated
and mixed for
6 hours at 34 C prior to inoculation of a 6.5% volume of the suspension into
a sample.
[00234] The mass of each sample was recorded after all additions were
made, and the
samples were incubated at 32 C with agitation for 62 hours.
[00235] Table 6: Summary design of cellulosic-C6 rich stream to water
ratio per
treatment for determination of effects on ethanol yield
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
TREATMENT TYPE OF SOLUTION SOLUTION: CORN MASH SLURRY
RATIO
=
10% Cellulosic¨C6 rich 10:90
20% Cellulosic¨C6 rich. 20:80
Glucose Control Glucose, 250 g/L:0
Fed batch Cellulosic¨C6 rich (46% 10:90
C6 solution)
L 1 L fed to 9 L corn
mash
[00236] Samples were collected at various time intervals and the combined
mass of the
mash and sample was measured. The samples were analyzed for concentrations of
substrates
(glucose, subunits, residual xylose) and products (ethanol, glycerol, lactic
acid, and acetic
acid) by HPLC. The remaining samples were collected at the end of fermentation
and
analyzed for pH and substrate/product concentrations. The final concentrations
of total dry
solids and dissolved dry solids were measured after incubation for 62 hours
[00237] Fed batch operation
[00238] Partially dextrinized corn mash was charged in a 30 L C-30
Sartorius stainless
steel Steam In Place (SIP) bioreactor, pH adjusted to 5 with 1 N KOH and the
temperature
maintained at 32 C. The yeast was propagated separately. Glucoamylase enzyme
(Spirizyme
Fuel, Novozymes) was prepared as a 0.25 g/ml solution and added at a dose of
0.066% (w/w,
based on the wet weight of corn). Antibiotic, FermGuard Xtreme, was added to
each tank to
achieve a dose of 0.5 ppm (w/w).
[00239] The yeast inoculum was prepared by adding 0.15 g of dried matrix
green and
matrix gold yeast to the corn mash, at pH 5 with KOH and propagated for 6 hr.
Seed was
inoculated into the fermenter at 6.5% v/w (T=0 hr). The fermenter operating
parameters were
as follows: temperature 34 C, RPM = 300, feed rate of cellulosic derived C6
rich stream (1 L
of 48% w/v sugar as C6 and 4.3% w/v C5 as xylan) was fed continuously and
controlled at a
feed rate of 0.7 ml/min. During the first 24 -36 h of the operation, 1L of the
C6 rich solution
was drained into the fermenter. Samples were drawn at time intervals and
analyzed for
residual sugars and progress of ethanol fermentations. In some samples, an
engineered yeast
that was capable of fermenting C6 glucose and C5 sugar (xylose) was used. The
intent was to
test and see if mixed cultures of C6 and an engineered C5 fermenting yeast
assist in
successful fermentation of sugar mixtures of glucose and xylose in the corn
mash.
86
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00240] Fed batch operation in 2 L (seed propagator) and 30 L automatic
fermenters
(Sartorius, PA)
[00241] Table 7
Sample HPLC DROP RESULTS (w/v) except Ethanol (v/v)
T Tot. Lact. Ac.
(h) Sug. DP4S DP3S DP2S Glu Fruc. Acid Glyc. Acid Et0H
Cont. 1 48 2.26 1.33 0.47 0.25 0.17 0.04 0.16
1.75 0.03 15.71
Cont. 2 48 2.54 1.52 0.48 0.26 0.24 0.04 0.21
1.75 0.02 15.44
Cont. 3 48 1.96 1.04 0.46 0.23 0.19 0.04 0.18
1.77 0.03 15.7
Test 4
(90:10) 48 2.88 1.15 0.71 0.36 0.25 0.41 0.15 1.45 0.03 16.32
Test 5
(90:10) 48 2.66 1 0.68 0.39 0.17 0.42 0.15 1.45 0.03 16.35
Test 6
(90:10) 48 2.69 1.03 0.69 0.36 0.2 0.41 0.13 1.43 0.04 16.2
Test 1
(80:20) 48 3.24 1.08 0.76 0.5 0.12 0.78 0.15 1.26 0.05 16.84
Test 2
(80:20) 48 3.27 1.1 0.76 0.49 0.14 0.78 0.15 1.25 0.04 16.71
Test 3
(80:20) 48 3.3 1.08 0.77 0.5 0.16 0.79 0.16 1.27 0.05 16.89
Cont.: Control (corn mash only); T (h): Time in hours; Tot. Sug.: Total
Sugars; DP4S:
saccharides with 4 degrees of polymerization; DP3S: saccharides with 3 degrees
of
polymerization; DP2S: saccharides with 2 degrees of polymerization; Glu.:
Glucose; Fruc.:
Fructose; Lact. Acid: Lactic Acid; Glyc.: Glycerol; Ac. Acid: Acetic Acid;
Et0H: Ethanol.
[00242] As shown in Table 7, fed-batch fermentations blending corn mash
with
cellulosic-derived C6 monosaccharides resulted in higher yields of ethanol and
lower yields
of glycerol after 48 hours as compared to control fermentations of corn mash
only.
[00243] Fed batch fermentations of cellulosic C6 rich sugar from wheat
straw (WS)
blended with corn mash at an 90:10 or 80:20 ratio (corn mash : wheat straw)
were performed
in an automated 30L system. The results of the fermentation were compared to
control batch
process fermentations of blended feedstocks at the same ratio. All
fermentations were
inoculated with three strains of industrial C6 fermenting yeasts and one
genetically
engineered yeast that can ferment both C5 and C6 sugars. The results are shown
in Table 8
and Figures 10 and 11.
87
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
[00244] Table 8. Fed-batch vs. batch fermentation.
Formic Acetic
Glucose Xylose Acid Acid Ethanol
Treatment (g/L) (g/L) (g/L) (g/L)
(g/L)
Corn Mash (CM) TO 21.15 4.55 0.4 3.54 3.35
30 L fed batch 90:10 CM:WS T5 172.9 1.55 0.3 8.17 22.8
30 L fed batch 90:10 CM:WS T18 36.65 3.1 0.6 17.82 99.1
30 L fed batch 90:10 CM:WS T36 10.55 5.7 0.85 16.76
124.2
30 L fed batch 90:10 CM:WS T48 3.65 6.4 0.9 18.97 143.85
Control Batch Process 90:10 TO 53.7 4.4 0.4 3.29 1.8
Control Batch Process 90:10 T5 146.65 0.75 0.25 3.06 3
Control Batch Process 90:10 T18 110.3 3.3 0.45 10.88
51.35
Control Batch Process 90:10 T36 2.15 0.85 0.5 11.92 105.15
Control Batch Process 90:10 T48 2.9 4.65 0.65 13.58 118.25
[00245] The
fed batch runs indicated initial significant improvement in ethanol volume
productivity in g/l/h. Combining cellulosic derived C6 sugar with corn mash as
a fed batch
process produces higher ethanol production in a shorter amount of time over
the first portion
of the fermentation in the 30L and flask samples. The fed batch process not
only has shown
an increase in ethanol production compared to corn mash on its own, it has
also shown to be
superior to batch process where all of the cellulosic sugar in the blend is
added initially in
fermentation. Further optimization with the mixed culture of C6 and C5
fermenting yeast in
the 30L reactor resulted in higher ethanol titers (25%) and reduced the amount
of residual
sugar in solution, especially in the remaining C5 portion.
[00246]
Figure 8 shows that by feeding a higher concentration of cellulosic sugar over
time, higher ethanol production can be achieved when cellulosic sugar is
blended with corn
mash after 48 hours and in a shorter amount of time. Residual sugar in both
fermentations
was under 0.13% v/v. The C5 portion of solution remained mostly unfermented
due to the
lack of C5 fermenting microbe.
[00247]
Figure 9 shows the amount of ethanol produced in a 90:10 blend of corn mash
and cellulosic sugar at the shake flask level vs. the ethanol production in
standard corn mash
from as is, at an industrial size scale. The addition of cellulosic sugar
throughout the
88
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
fermentation can account for the increase in ethanol production at the
beginning of the
fermentation.
[00248] Figure 10 illustrates the results of fed batch replacement of 10%
of the corn
mash with 10% concentrated cellulosic sugar solution (52% total C5 and C6
sugar). There
was an increase of 4.75% v/v ethanol by this method than with corn mash
without any
blending. The standard yeast combined with a C5 and C6 fermenting yeast strain
led to a
higher ethanol yield.
[00249] Figure 11 shows the percent increase in ethanol production between
the 90:10
and 80:20 blended flasks of corn mash with cellulosic sugar derived from wheat
straw. The
90:10 fed batch run had a 24.63% increase in ethanol production compared to
the batch run in
a 90:10 ratio. The 80:20 fed batch blend also saw positive improvement,
yielding 12% of an
ethanol increase compared to the batch run in the same blend.
[00250] Example 10
[00251] Summary
[00252] In this example, different proportions of a corn stillage
hydrolyzate containing
starch-derived C6 saccharides (C6 Saccharide Stream) was added (dosed) to corn
mash in
fermentation reactions. Fermentation reaction progress was measured by mass
loss, ethanol
concentration, and ethanol yield. The data show statistically significant
increases in mass
loss, ethanol concentrations and yield for doses above 10% C6 Saccharide
Stream. For the
higher doses, the total residual sugars concentration may indicate an
incomplete fermentation.
[00253] Objective
[00254] An objective of this example is to determine how different
proportions of a C6
Saccharide Stream added to corn mash affect the ethanol yield. The average
ethanol yield in
flask fermentations of liquefied corn mash, prepared using different
proportions of C6
Saccharide Stream, was compared to the average ethanol yield of a quality
control (QC)
treatment, containing only water and corn solids. A glucose control, utilizing
a glucose
solution instead of water or C6 Saccharide Stream was also used as a term of
comparison for
concentration of products, given the high sugars concentration of the
amendments.
[00255] Preparation of C6 Saccharide Stream
[00256] Hot water at about 190 F was added to corn stillage material
until the total
solids level was about 12%. The mixture was continuously agitated at about 185
F for about
90 min, then the pH was adjusted to about 5.8 with sodium hydroxide. The corn
stillage
material was treated with alpha amylase amylase enzymes and glucoamylase
enzymes. Solids
89
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
were sequestered by filter press and the liquid fraction was concentrated to
about 25%
glucose (w/v) by evaporation.
[00257] Preparation of Corn Mash
[00258] Mashes were prepared for ten different fermentation treatments
shown in
Table 9. Large debris were removed from the corn by hand, and small debris (<4
mm) were
removed by passing through a No. 5 sieve (Fisher Scientific Company) before
grinding using
a Wiley mill (Thomas Scientific, NJ) fitted with a 2-mm screen. This produces
flour for
which 95 % of the particles are smaller than 1.5 mm.
[00259] Table 9. Summary of experimental conditions.
treatment ID type of solution solution:water ratio in
mash
5% C6 Saccharide Stream 5:95
10% C6 Saccharide Stream 10:90
20% C6 Saccharide Stream 20:80
30% C6 Saccharide Stream 30:70
40% C6 Saccharide Stream 40:60
47.5% C6 Saccharide Stream 47.5:52.5
50% C6 Saccharide Stream 50:50
QC/0% QC 0:100
glucose control glucose, 250 g/L:0
[00260] The moisture of the ground corn was determined gravimetrically by
drying a
subsample of the flour in a forced-air oven at 105 C for 3 hours. The
moisture content for
corn used the first week was 14.04 % (w/w, as received) and for week two it
was 14.35 %
(w/w, as received). The moisture content of the corn was used to determine the
mass of corn
needed to prepare 160 g of mash at a dry-solids concentration of 25 % (w/w).
[00261] The mass of corn, total deionized (DI) water added, and enzyme
needed to
prepare 160 g of corn slurry at a total dry solids concentration of 25 % (w/w)
for each
replicate was determined using a mash calculator. For each treatment, nine
independent
replicate slurries were prepared. The required amount of DI water was
transferred into
labeled Labomat beakers, followed by addition of the required mass of corn
flour. The alpha-
amylase enzyme (Liquozyme SC DS, Novozymes) was diluted to ensure more precise
delivery of enzyme to each flask. A 0.13 g/mL working solution of the alpha
amylase was
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
used and added at a dose of 0.025 % (w/w) based on the wet weight of the corn.
The slurries
were hand swirled after all components were in the Labomat beakers. The pH was
adjusted
to 5.7 ¨ 5.85 using 6N ammonium hydroxide. Sealed beakers were attached to a
vertically
mounted wheel in the Labomat (Model BFL12 805, Mathis, Switzerland), which
rotated at 50
rpm during the incubation. The wheel was programmed to reverse direction every
50
seconds to improve the mixing efficiency. Samples were liquefied by incubating
at 83 C for
90 minutes, after which the samples were cooled to 40 C in the Labomat. Prior
to
fermentation, the C6 Saccharide Stream was characterized for total solids,
total dissolved
solids and HPLC analysis of sugar content. The concentrations of total dry
solids and
dissolved dry solids were measured using standard procedures. The sugar
substrate
concentration was analyzed with HPLC for oligosaccharides having a degree of
polymerization of four or more (DP4+), three (DP3), two (DP2), and one
(glucose).
[00262] Once the mash was cooled, the entire contents (approximately 160
g) of each
Labomat beaker were transferred to a sterile 250-ml Erlenmeyer flask. The
masses of the
mash and flasks were recorded, and the mass of mash transferred to the
fermentation flasks
was calculated. The pH of the mash was adjusted to <5.2 by addition of 150 1
of 10-N
sulfuric acid. The flasks were shaken at 170 rpm on an incubator/shaker
(Sartorius, Certomat
BS-1) at 32 C until preparation of all mashes was complete.
[00263] All enzymes, nutrients, and other amendments added to the
fermentation
flasks were freshly prepared before use. The total concentration of nutrient
nitrogen was 500
mg N/kg, urea was added as a sterile 0.2 g/ml solution to a final
concentration of 500 ppm as
nitrogen (w/w, based on the total mass of mash). The glucoamylase enzyme
(Spirizyme Fuel,
Novozymes) was prepared as a 0.25-g/mL solution and added at a dose of 0.066%
(w/w,
based on the wet weight of corn). Antibiotic, Lactrol (Phibro, Ridgefield
Park, NJ), was
added to each flask to achieve a dose of 0.5 ppm (w/w). Yeast extract (0.16 g)
was added to
the glucose treatment which fermented with no corn solids.
[00264] A 0.1-g/mL suspension of yeast (Saccharomyces cerevisiae; Ethanol
Red;
Fermentis, Marcq-en-Baroeul, France) was prepared in a sterile 250-ml flask.
This
suspension was incubated and mixed for 20 minutes at 40 C before inoculation
into the
fermentation flasks. Each fermentation flask was inoculated with 160 1 of the
yeast
suspension to attain an initial concentration of 1 x 107 yeast cells/mt.
[00265] The mass of each flask was recorded after all additions were made,
and the
sanitized fermentation traps were reinserted into each flask. The flasks were
incubated at 32
C with shaking at 170 rpm in an incubator/shaker (Sartorius, Certomat BS-1)
for 62 hours.
91
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00266] Samples were collected at 22, 46 and 62 hours. Mass measurements
were
taken twice a day; the combined mass of the mash and flask was measured for
each flask with
the traps in place. One-third of the experimental flasks were taken
sacrificially at 22 hours
and another third harvested at 46 hours, flask material at both of these
sample time points
were analyzed for pH and the concentrations of substrates (glucose, DP2, DP3,
and DP4+,
where "DPx" represent glucose oligomers with "x" subunits) and products
(ethanol, glycerol,
lactic acid, and acetic acid) using HPLC. The remaining flasks were collected
at the end of
fermentation and analyzed for pH and substrate/product concentrations. Samples
were
prepared for HPLC by centrifugation to remove large solids, followed by
filtration through
0.45 gm syringe filters, and acidification to pH of approximately 2 by
addition of sulfuric
acid to a final concentration of 0.01 N. The final concentrations of total dry
solids and
dissolved dry solids were measured after incubation for 62 hours, and the
density of the beer
filtrate was measured by densitometer (Anton Paar DMA 4500, Anton-Paar GmbH,
Graz,
Austria).
[00267] Results
[00268] Characterization of C6 Saccharide Stream
[00269] The average composition of four samples of the C6 Saccharide
Stream is
shown in Table 10. The original pH of the individual samples were: 1.319,
1.17, 1.39, and
1.195.
[00270] Table 10. Characterization of C6 Saccharide Stream
Average
total dissolved
pH DP4+ DP3
DP2 Glucose
solids solids
(4 (%w/v) (%w/v) (%w/v) (%w/v)
(%w/w) (%w/w)
samples)
1.27 25.08 24.44 6.36 1.51 1.75 21.48
[00271] Reaction Progress (Mass Loss)
[00272] Fermentation progress curves are shown in Figure 12 (A&B) and the
average
mass losses for all treatments at all time points are shown in Table 11. The
cumulative mass
losses after incubation for 15.67 and 62 hrs are shown in Figure 13.
Significant differences
in the average reaction progress after 15.67 and 62 hours were identified
among the eight
treatments using one-way ANOVA at the 95% confidence level. Further analysis
by
Dunnett's test against the QC shows that at 15.67 hours all treatments were
different than the
92
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
QC; the treatments with 5%, 10%, 20%, and 30% C6 Saccharide Stream lost more
mass than
the QC, while treatments with 40%, 47.5%, and 50% SWE A and the glucose
treatment lost
less mass than the QC. Further analysis by Dunnett's test shows that at 62
hours the
treatments with 5% and 10% of C6 Saccharide Stream were the only two
treatments which
had statistically identical mass loss to the QC sample. At 62 hours, Tukey's
test for paired
comparisons shows that the glucose treatment is lower than all other
treatments, the break
down for mass loss at the end of fermentation is as follows:
{5% = 10% = QC} <20% < {30% = 40% = 47.5% = 50%}
[00273] Table 11. Average mass loss in grams during fermentation
[00274] fermentation time (hours)
treatmen 15.67 22 39 46 62
t
5% 6.82 10.91 13.21 13.72
14.35
10% 6.97 10.90 13.16 13.70 14.52
20% 6.41 11.60 14.49 14.95 15.73
30% 5.75 11.08 15.42 15.94 16.97
40% 3.91 9.40 15.73 16.51 17.69
47.5% 3.26 8.30 15.26 16.14 17.00
50% 3.04 7.85 15.08 16.02 17.28
QC 5.20 10.05 13.24 13.64
14.23
Glucose 0.27 0.46 1.30 1.68 2.56
[00275] The fermentation progress was slower in the glucose control,
although that the
solution was supplemented with yeast nutrients. The data for the glucose
control is reported
in Tables 13-16.
[00276] Final Concentrations of Residual Sugars and Fermentation Products
[00277] Final concentrations of total dissolved sugars (Table 12, Figure
14) were less
than 2% (w/v, expressed as glucose equivalents) for treatments QC and C6
Saccharide
Stream at 5%, 10%, 20%, and 30%; the other treatments dosed with C6 Saccharide
Stream at
40%, 45.7%, and 50% had less than 4% (w/v); the glucose treatment had 21.13%
(w/v). A
concentration of total residual sugars less than 2% is expected for complete
fermentations of
corn mash. Significant differences among the final total dissolved sugars
concentrations
among the eight treatments were identified using one-way ANOVA at the 95%
confidence
93
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
level. Further analysis by Dunnett's test revealed the C6 Saccharide Stream
treatments in
proportions of 30%, 40%, 47.5%, and 50% had statistically higher residual
sugars
concentrations compared to the QC. For treatments that contained the C6
Saccharide Stream,
Tukey's test was used to identify statistically equivalent pairs; two groups
were found to have
statistically the same final total residual sugars concentrations: 5%, 10%,
20%, and 30% was
one group and 40% 47.5%, and 50% the other.
[00278] Table 12. Average final concentrations and standard deviations of
residual
sugars, glycerol, ethanol, and final yield
Treatment Residual sugars* Glycerol Ethanol Yield
(%, w/v) (%, w/v) (%, w/v) (g/g)
5% 0.586 0.010 1.087 0.024 10.426 0.069 0.350
+ 0.004
10% 1.001 0.014 1.188 0.002 10.562 0.111 0.355 +
0.004
20% 1.243 0.002 1.181 0.003 11.613 0.112 0.387 +
0.001
30% 1.814 0.014 1.174 0.011 12.630 0.071 0.417 +
0.004
40% 3.061 0.995 1.257 0.112 13.063 0.150 0.430 +
0.003
47.5% 3.801 1.544 1.325 0.136 12.718 0.326 0.414
+ 0.013
50% 3.141 0.154 1.292 0.044 12.888 0.411 0.421 +
0.015
QC 0.294 0.011 0.862 0.009 10.288 0.061 0.350
+ 0.003
*concentrations expressed as glucose equivalents
[00279] Total glycerol concentrations were also analyzed, as they can be a
reflection of
osmotic or other stress during fermentation. Yeast stress can affect ethanol
production
adversely. All treatments receiving the C6 Saccharide Stream had higher final
glycerol
concentrations than the QC; significant differences were identified using one-
way ANOVA
and by Dunnett's test against the control (QC). Figure 15 shows the glycerol
concentration as
a function of dose.
[00280] Significant differences among the final ethanol concentrations
among the eight
treatments were identified using ANOVA. Further analysis by Tukey's test for
paired
differences identified the data could be sorted into two groups; 5%, 10%, and
QC are
statistically the same as one another and lower than the group of 20%, 30%,
40%, 45.7% and
50%, which are statistically the same as one another (Figure 14).
[00281] The average pH for each treatment throughout fermentation is shown
in Figure
16. No correlations based on dose were identified when pairing residual
sugars, glycerol, and
ethanol concentrations with pH.
94
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00282] Ethanol Yield
[00283] The ethanol yield showed significant differences among the
treatments by one-
way ANOVA at the 95% confidence level; Dunnett's test against QC shows that
only
treatments with 5% and 10% SWE A were statistically the same as the control,
although in a
dose-response plot, the 10% treatment also trends higher than control. Of the
treated mashes,
C6 Saccharide Stream at 40% had the highest yield overall, but this treatment
had statistically
the same yield as 30%, 47.5%, and 50%, as analyzed by Tukey's test for paired
differences.
The normalized dose response graph (Figure 17) shows that overall the mashes
treated with
20% or higher C6 Saccharide Stream produced a statistically higher amount of
ethanol than
the control. In this graph, the y-axis represents the amount in excess of 100%
of ethanol
yield, compared to the QC samples which are noted as 0% in Figure 17.
[00284] Table 13 shows the final product yields and residual sugar levels
for each of
the replicate samples in the treatment categories. Table 14 shows the final
product yields and
residual sugar levels averaged over all replicate samples in the treatment
categories. Table 15
shows the mass loss at 15.67, 22, 39, 46, and 62 hours of fermentation for
each of the
replicates within the treatment conditions. Table 16 shows the pH for each of
three replicates
within each treatment condition at 22, 46, and 62 hours of fermentation.
[00285] Table 13. Final substrate and product concentrations
substrates (g/100 ml) Total products (g/100 ml)
sugars*
Treatmen (g gluc.
t eq./100 Lactic acetic
ID DP4+ DP3 DP2 glucose ml) acid glycerol acid ethanol
0.342 0.049 0.128 0.012 0.574 0.109 1.100 0.003 10.456
5% 0.359 0.047 0.129 0.013 0.594 0.109 1.103 0.004 10.475
0.353 0.052 0.127 0.013 0.590 0.101 1.060 0.004 10.347
0.642 0.066 0.171 0.032 0.988 0.134 1.187 0.006 10.610
10% 0.662 0.056 0.183 0.036 1.016 0.138 1.190 0.009 10.435
0.641 0.060 0.183 0.037 0.999 0.135 1.186 0.008 10.641
0.850 0.069 0.180 0.047 1.244 0.177 1.178 0.010 11.484
20% 0.846 0.072 0.176 0.048 1.241 0.177 1.182 0.012 11.678
0.846 0.072 0.179 0.049 1.244 0.178 1.183 0.011 11.677
30% 1.262
0.103 0.229 0.090 1.828 0.192 1.187 0.050 12.665
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
1.253 0.103 0.226 0.089 1.814 0.195 1.167 0.046 12.548
1.242 0.100 0.226 0.089 1.800 0.194 1.169 0.048 12.677
1.762 0.114 0.293 0.136 2.504 0.218 1.198 0.062 13.063
40% 2.827 0.261 0.382 0.420 4.210 0.262 1.386 0.108 13.214
1.727 0.123 0.286 0.138 2.469 0.223 1.187 0.061 12.913
3.484 0.357 0.448 0.894 5.578 0.303 1.480 0.136 12.783
47.5% 2.100 0.156 0.323 0.224 3.040 0.240 1.270 0.081 13.008
1.955 0.150 0.303 0.156 2.785 0.252 1.226 0.078 12.365
2.188 0.171 0.331 0.253 3.191 0.250 1.309 0.090 13.021
50% 2.087 0.163 0.309 0.175 2.969 0.259 1.243 0.084 12.427
2.221 0.167 0.347 0.279 3.264 0.254 1.326 0.092 13.218
QC
0.126 0.044 0.098 0.008 0.298 0.077 0.858 0.000 10.319
0.132 0.043 0.098 0.009 0.304 0.075 0.853 0.000 10.316
0.125 0.044 0.094 0.009 0.292 0.078 0.855 0.000 10.244
0.131 0.039 0.091 0.000 0.281 0.068 0.876 0.001 10.380
0.130 0.038 0.093 0.000 0.282 0.064 0.872 0.001 10.258
0.129 0.051 0.104 0.000 0.306 0.068 0.858 0.001 10.214
0.050 0.038 0.167 20.184 20.456 0.023 0.319 0.064 1.680
glucose 0.050 0.038 0.166 21.664 21.934 0.023 0.322 0.067 1.680
0.050 0.038 0.166 20.730 21.000 0.023 0.314 0.063 1.655
values in bold are below detection limits
*total sugars = XDP4+*CDP4+ + XDP3*CDP3 + XDP2*CDP2 + Cglucose
where: XDP4+ = 1.111 g glucose equivalents/g DP4+
XDP3 = 1.07 g glucose equivalents/g DP3
XDP2 = 1.05 g glucose equivalents/g DP2
Ci = concentration of compound "i"
[00286] Table 14. Summary of average final substrate and product
concentrations
Substrates (g/100 ml) Total Products (g/100 ml)
sugars*
(g gluc.
Treat.
glucos eq./100 lactic glycer acetic ethano
ID DP4+ DP3 DP2 e ml) acid ol acid 1
5% 0.351 0.049 0.128 0.013 0.586 0.106 1.087 0.004 10.426
96
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
0.009 0.002 0.001 0.001 0.01 0.005 0.024 0.0004 0.069
0.648 0.061 0.179 0.035 1.001 0.135 1.188 0.008 10.562
10%
0.012 0.005 0.007 0.003 0.014 0.002 0.002 0.002 0.111
0.847 0.071 0.178 0.048 1.243 0.177 1.181 0.011 11.613
20%
0.002 0.002 0.002 0.001 0.002 0.001 0.003 0.001 0.112
1.252 0.102 0.227 0.089 1.814 0.194 1.174 0.048 12.63
30% 0.01
0.002 0.001 0.001 0.014 0.001 0.011 0.002 0.071
2.105 0.166 0.32 0.231 3.061 0.234 1.257 0.077 13.063
40%
0.625 0.083 0.053 0.163 0.995 0.024 0.112 0.027 0.15
2.513 0.221 0.358 0.425 3.801 0.265 1.325 0.098 12.718
47.5%
0.844 0.118 0.079 0.407 1.544 0.034 0.136 0.032 0.326
2.165 0.167 0.329 0.236 3.141 0.254 1.292 0.088 12.888
50%
0.07 0.004 0.019 0.054 0.154 0.005 0.044 0.004 0.411
0.129 0.043 0.097 0.004 0.294 0.072 0.862 0.001 10.288
QC
0.003 0.005 0.005 0.005 0.011 0.006 0.009 0.001 0.061
0.05 0.038 0.166 20.86 21.13 0.023 0.319 0.065
1.671
glucose 0.000
1 0.0003 0.001 0.748 0.747 0.0002 0.004 0.002 0.015
*total sugars = XDP4+*CDP4+ + XDP3*CDP3 + XDP2*CDP2 + Cglucose
where: XDP4+ = 1.111 g glucose equivalents/g DP4+
XDP3 = 1.07 g glucose equivalents/g DP3
XDP2 = 1.05 g glucose equivalents/g DP2
Ci = concentration of compound "i"
[00287] Table 15. Mass loss (g) throughout fermentation
97
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
Fermentation time (hrs)
Treatment ID 15.67 22 39 46 62
6.36 10.77
6.74 10.90
6.90 10.93
7.04 10.95 13.18 13.64
5% 6.85 10.96 13.17 13.63
6.92 10.94 13.26 13.77
6.91 10.99 13.25 13.80 14.34
6.98 11.01 13.28 13.82 14.43
6.64 10.76 13.10 13.66 14.27
6.68 10.91 \
6.99 10.78
7.20 11.00
6.94 10.65 12.75 13.28
10% 6.99 11.01 13.27 13.77
7.03 11.08 13.39 13.86
7.16 11.04 13.36 13.87 14.54
6.91 10.64 12.89 13.48 14.34
6.86 11.00 13.32 13.94 14.67
6.07 11.40 \
\
6.59 11.76 \
6.42 11.60
6.31 11.52 14.55 14.98
20% 6.49 11.64 14.36 14.79
6.58 11.72 14.45 14.82
6.45 11.62 14.40 14.90 15.56
6.41 11.62 14.63 15.15 15.85
6.37 11.54 14.52 15.04 15.78
5.44 10.91
30% 5.65 11.00 \
5.70 11.07
98
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
5.85 11.17 15.51 1156..0720 1
6.03 11.28 15.21
5.80 11.07 14.78 15.18 k
5.76 11.13 15.77 16.36 17.06
5.69 11.00 15.58 16.13 16.86
5.83 11.12 15.68 16.25 16.98
3.96 9.58 \
4.36 10.17 \
\ \ N
4.14 9.68 L \ 1\
3.19 8.18 ' 15.45 16.6 \
40% 4.18 9.80 16.14 16.72
4.26 10.01 15.16 15.58 L ,
4.10 9.66 16.25 16.87 17.73
3.11 7.95 15.26 16.59 17.74
3.93 9.58 16.11 16.72 17.59
3.27 8.63 \ 1
3.47 8.65
2.45 6.59
3.59 8.83 16.03 17.14
47.5% 3.80 9.30 14.93
3.55 8.70 15.75 16.75 k
2.37 6.47 13.73 15.25 17.02
3.43 8.71 15.92 16.69 17.54
3.41 8.86 15.21 15.70 16.45
3.28 8.48
2.11 5.87
3.33 8.43
3.43 8.73 15.10 15.55
50%
3.28 8.25 15.65 16.73
2.17 5.90 13.02 14.57 k
3.39 8.49 15.92 16.76 17.63
3.26 8.40 15.11 15.59 16.34
99
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
3.14 8.14 15.69 16.9 17.87
5.02 10.03 1
5.26 10.11
5.19 10.15 k
5.38 10.04 13.08 13.48
,
5.27 10.23 13.09 13.49
5.29 10.18 13.15 13 56
= k '
5.38 10.25 13.15 13.64 14.17
5.38 10.24 13.11 13.59 14.18
5.32 10.20 12.99 13.48 14.07
QC
5.30 10.06 1
4.91 9.70 \
5.00 9.91 k
,
5.40 10.15 ' 13.24 13.59
5.08 9.91 13.48 13.84
5.15 9.95 13.43 13.77 L
5.12 9.95 13.42 13.78 ' 14.39
5.35 10.22 13.44 13.81 14.41
4.79 9.67 13.30 13.61 14.14
0.24 0.43 \
\ \ 1
0.25 0.44 \ \ \
0.26 0.44 L \ 1\
0.30 0.48 1.30 1.67 \
glucose
0.28 0.45 1.28 1.65
control
0.27 0.45 1.28 1.65 L '
0.30 0.49 1.35 1.73 2.60
0.29 0.48 1.33 1.72 2.56
0.28 0.46 1.28 1.64 2.52
100
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
[00288] Table 16 pH throughout fermentation
fermentation time (hrs)
Treatment ID 22 46 62
3.36 3.50 3.60
5% 3.31 3.47 3.55
3.29 3.38 3.46
2.92 3.18 3.39
10% 2.93 3.18 3.30
2.91 3.21 3.35
3.60 3.94 4.00
20% 3.59 3.90 4.06
3.51 3.91 4.04
4.11 4.41 4.49
30% 4.02 4.40 4.50
4.08 4.33 4.49
3.84 3.74 3.95
40% 3.76 3.81 3.91
3.75 3.80 3.93
3.73 3.85 3.80
47.5% 3.65 3.78 3.85
3.61 3.73 3.85
4.07 4.58 4.46
50% 4.05 4.41 4.59
4.04 4.37 4.41
4.15 4.51 4.64
4.20 4.45 4.44
4.53 4.38 4.45
QC
4.23 4.51 4.49
4.57 4.45 4.49
4.22 4.63 4.46
glucose 3.81 3.54 3.69
control 3.80 3.60 3.76
101
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
3.85 3.68 3.76
[00289] Conclusions
[00290] This example tested the effect of adding different proportions of
C6
Saccharide Stream to corn mash on ethanol production. The criteria evaluated
to determine
the effect of these additions were mass loss, ethanol concentration, and
ethanol yield.
Residual sugars and glycerol were also evaluated for each treatment in order
to get a clearer
picture of the overall process, especially in regards to optimization of the
C6 Saccharide
Stream amendment dose for corn-to-ethanol fermentations.
[00291] Statistically significant increases in mass loss, ethanol
concentrations and
yield were found for doses above 10% C6 Saccharide Stream. For the higher
doses of C6
Saccharide Stream (40%, 47.5% and 50%), the total residual sugars
concentration may
indicate an incomplete fermentation. In practical applications, a value much
above 2% of
total residual sugars can create processing problems during distillation.
Given the increase
ethanol yield however, it may be desirable to find an optimum dose whereby the
addition of
C6 Saccharide Stream would maximize the benefits (increased yield) and
minimize the
residual sugars.
[00292] Figure 9 shows a dose response plot of the increase in ethanol
yield and the
residual sugar levels for the treatment conditions tested. Taking into
consideration the
uncertainty on the measurements, these data suggest that a dose between 35%
and 45% of C6
Saccharide Stream may perform the best in a fuel ethanol fermentation.
[00293] If it is desirable to have a concentration of residual sugars
below 2%, a lower
dose may also be useful. These results were achieved by fermenting a mash that
contained
25% solids. An increase in ethanol yield of 5-20% is a major improvement over
ethanol
fermentations with no amendments.
[00294] Example 11
[00295] In this example, three different saccharide streams and two
different lignin
streams were subjected to free amino acids profiling (Table 17), trace metals
analysis (Table
18), and fatty acid profiling (Table 19). All of the samples were produced
from the hydrolysis
of pretreated corn stover.
[00296] C5 + C6 Saccharides Stream & C5 +C6 Lignin Stream
[00297] Pretreated corn stover containing about 30% solids was used to
produce the
`C5 + C6 Saccharide Stream' and the `C5 + C6 Lignin Stream'. The solids were
placed into
a jacketed kettle with an agitator. Water was added to the pretreated solids
to create an about
102
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
10% solids solution (wt/v). The temperature of the pretreated corn stover was
then brought
up to about 50 C. The pH was adjusted using ammonium hydroxide to about 5Ø
Once pH
and temperature are both set, cellulase enzymes were added to the solids at a
dosing of about
5% of total dry solids (wt/wt). The solution was kept at about 50 C, a pH of
about 5.0 and at
constant agitation for about 72 hours. The solids were then separated via
filtration from the
liquid stream to produce the C5 + C6 Lignin Stream at about 20% solids (w/w).
The liquid
stream at this point contains C5 and C6 monosaccharides . The liquid stream
was then
concentrated via evaporation to the desired monosaccharide levels to produce
the C5 + C6
Saccharides Stream. The C5 + C6 Saccharides stream contained about 18.7% C6
and about
6.8% C5 saccharides.
[00298] C5 Saccharides Stream
[00299] Pretreated corn stover containing about 30% solids was used to
create the C5
saccharides stream. To extract the monomeric C5 saccharides, hot water (at
about 50 C) was
mixed with the pretreated solids at a rate of about 1L of water for every 1 kg
of wet solids.
Once the water was mixed with the pretreated solids, the biomass and hot water
solution was
mixed for about 15 minutes at about 50 C. The solids were then filtered out
and the liquid
fraction was collected. The liquid fraction was then sequestered. The solids
were then re-
collected and re-washed with the same ratio of hot water (at about 50 C) and
mixed for about
15 minutes at about 50 C. The solids were then once again filtered out and the
liquid fraction
was collected and sequestered. The liquid fraction from the second wash was
then combined
with the liquid fraction from the first wash and the entire liquid fraction
was concentrated via
evaporation to the desired saccharide levels, yielding the C5 Saccharides
Stream. The C5
Saccharides Stream contained about 12.9% C5 saccharides and about 1.3 % C6
saccharides.
[00300] C6 Saccharides Stream & C6 Lignin Stream
[00301] The C6 Saccharides Stream and C6 Lignin Stream are produced from
the
solids sequestered during production of the C5 Saccharide Stream. The solids
were placed
into a jacketed kettle with an agitator. Water was added to the pretreated
solids to create an
about 10% solids solution (w/v). The temperature of the pretreated corn stover
was then
brought up to about 50 C. The pH was adjusted using ammonium hydroxide to
about 5Ø
Once pH and temperature were both set, cellulase enzymes (Celtech 3 cellulase
from
Novozyme) were added to the solids at a dosing of about 5% of total dry solids
(wt/wt). The
solution was kept at about 50 C, a pH of about 5.0 and at constant agitation
for about 72
hours. The solids were then separated from the liquid stream via filtration to
produce the C6
Lignin Stream at about 20% solids. The liquid stream at this point is enriched
for C6
103
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
monosaccharides. The liquid stream was then concentrated via evaporation and
vacuum to
the desired saccharide levels to produce the C6 Saccharide Stream. The C6
Saccharide
Stream contained about 25.1% C6 saccharides and about 2.6% C5 Sugars.
[00302] Table 17. Amino acids profiling
(% w/v)
C5 +C6
iiiii=ijcotigii=ijtim
MiOWMIC
iiiiiiii$4006.40.00iiiiiiiiiiiiiiii$.#00.04100iiiiiiiiiiiiiiii$4.00#010#$iiiiii
iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii=
Stfeatiiiiiiiiiiiiiiiiiiiiii
iiiiii
iiiiiiiiiiiiiiiiiiiiiSitektijiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiSt004fit
iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiSta4t6iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
Aspartic <0.01% <0.01% 1 <0.01% <0.01% 1
<0.01%
Threonine <0.01% <0.01% <0.01% <0.01% <0.01%
Serine <0.01% <0.01% <0.01% <0.01% <0.01%
Glutamic Acid <0.01% <0.01% <0.01% <0.01% <0.01%
Proline <0.01% <0.01% <0.01% <0.01% <0.01%
Glycine <0.01% <0.01% <0.01% <0.01% <0.01%
Alanine <0.01% <0.01% <0.01% <0.01% <0.01%
Cystine, free <0.01% <0.01% <0.01% <0.01% <0.01%
Valine <0.01% <0.01% <0.01% <0.01% <0.01%
Methionine <0.01% <0.01% <0.01% <0.01% <0.01%
Isoleucine <0.01% <0.01% <0.01% <0.01% <0.01%
Leucine <0.01% <0.01% <0.01% <0.01% <0.01%
Tyrosine <0.01% <0.01% <0.01% <0.01% <0.01%
Phenylalanine <0.01% <0.01% <0.01% <0.01% <0.01%
Lysine 0.01% 0.01% 0.01% <0.01% <0.01%
Histidine <0.01% <0.01% <0.01% <0.01% <0.01%
Arginine <0.01% <0.01% <0.01% <0.01% 0.05%
[00303] Table 18. Trace metal analysis (PPM; Parts Per Million)
iiiiiiiiiiiiiiiiiiiiiiiiiiiiiii43.6iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiieSiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
iiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
MifCr.1010 iiiiiiiiiiiiC6 tliOji0
MOtOIM Iiiiiiii$000###00$iiiiiii iiiiiiii$00Ø0fitio$iiiiiii
Iiiiiiii$000hoidookiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiii
iiim
S=te0:A.iiiMi iiiiiiiiiS.te**6:i.fit.W
iiiiiiiiiiiiiiiiiiiiStfeatiimEnuSt004iiimuMunSteatitmummumumumu mumunNEEM
Aluminum 6.4 <4.9 36 25 180 '
Antimony <0.50 <0.49 <0.49 <0.50 <0.50
Arsenic <0.25 <0.24 <0.24 <0.25 <0.25
104
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
Barium 0.26 0.15 0.10 5.4 6.2
Cadmium <0.099 <0.097 <0.097 <0.10 <0.099
Calcium 330 86 340 210 1100
Chromium 1.7 0.15 5.4 6.9 15
Cobalt <0.099 <0.097 0.29 <0.10 0.15
Copper 1.3 0.77 4.7 6.3 10
Iron 21 <1.2 170 89 260
Lead <0.19 <0.18 <0.18 0.34 0.45
Magnesium 71 25 180 32 48
Manganese 2.0 0.26 5.1 1.0 1.2
Nickel 1.2 <0.24 4.9 2.0 3.6
Phosphorus 150 95 170 57 34
Potassium 570 97 1500 200 420
Selenium <0.74 <0.73 <0.73 <0.75 <0.74
Silver <0.25 <0.24 <0.24 <0.25 0.29
Sodium 3000 480 460 1100 61
Tin <0.50 <0.49 <0.49 0.79 1.0
Vanadium <0.30 <0.49 <0.49 <0.50 0.51
Zinc 1.8 <0.49 8.2 2.3 1.7
[00304] Table 19. Fatty acid profiling (% w/w)
'ilC6:','L:,..1g0j#iiii
Fatty Acid .$.4Ø01.0i1.00.0 i$.4.00.1.04.00.4. $#Ø0k4i440$
iiiiiiiiiiiiiiit4Mitiiiiiiiiiiii
Sittomiiiiiiiiiiii.
reilloillgoilleinigniginiginigin milstioiiiim MStiaiiin MStitiiiiin
MiStitiiiin MUMUMMMA
...........................................................................,...
...............................................................
............................... .................................
...............................................................:
C4:0 Butyric 1 0.00 ' 0.00 0.00 0.00 0.00
C6:0 Hexanoic 0.00 0.00 0.00 0.00 0.00
C8:0 Octanoic 0.00 0.00 0.00 0.00 0.00
C10:0 Decanoic 0.00 0.00 0.00 0.00 0.00
C12:0 Laurie 0.00 0.00 0.00 0.00 0.00
C13:0 Tridecanoic 0.00 0.00 0.00 0.00 0.00
C14:0 Myristic 0.00 0.00 0.00 0.00 0.00
C15:0 Pentadecanoic 0.00 0.00 0.00 0.00 0.00
105
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
C16:0 Palmitic 0.00 0.00 0.00 0.00 0.04
C17:0 Heptadecanoic 0.00 0.00 0.00 0.00 0.00
C18:0 Stearic 0.00 0.00 0.00 0.02 0.02
C20:0 Arachidic 0.00 0.00 0.00 0.00 0.01
C21:0 Heneicosanoic 0.00 0.00 0.00 0.00 0.00
C22:0 Behenic 0.00 0.00 0.00 0.01 0.01
C23:0 Tricosanoic 0.00 0.00 0.00 0.00 0.00
C24:0 Lignoceric 0.00 0.00 0.00 0.01 0.01
C14:1 (cis-9) Myristoleic 0.00 0.00 0.00 0.00 0.00
C15:1 (cis-10) Pentadecinoic 0.00 0.00 0.00 0.00 0.04
C16:1 (cis-9) Palmitoleic 0.00 0.00 0.00 0.00 0.00
C17:1 (cis-10)
0.00 0.00 0.00 0.00 0.00
Heptadecenoate
C18:1 (cis-9) Oleic 0.00 0.00 0.00 0.05 0.06
C20:1 (cis-11) Eicosenoic 0.00 0.00 0.00 0.00 0.00
C22:1 (cis-13) Erucic 0.00 0.00 0.00 0.00 0.00
C24:1 (cis-15) Nervonic 0.00 0.00 0.00 0.00 0.00
C18:2 (cis-9, 12) Lonoleic 0.00 0.00 0.00 0.07 0.05
C18:3 (cis-6, 9, 12) y-
0.00 0.00 0.00 0.00 0.00
lino lenic
C18:3 (cis-9, 12,15)
0.00 0.00 0.00 0.02 0.00
Lino lenic
C20:2 (cis-11, 14)
0.00 0.00 0.00 0.00 0.00
Eicosadienoic
C20:3 (cis-8, 11,14)
0.00 0.00 0.00 0.00 0.00
Eicosatrienoic
C20:3 (cis-11, 14, 17)
0.00 0.00 0.00 0.00 0.00
Eicosatrienoic
C20:4 (cis-5, 8, 11, 14)
0.00 0.00 0.00 0.00 0.00
Arachidonic
C20:5 (cis-5, 8, 11, 14, 17)
0.00 0.00 0.00 0.00 0.00
Eicosapentanoic
106
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
C22:2 (cis-13, 16)
0.00 0.00 0.00 0.00 0.01
Docosadienoic
C22:6 (cis-4, 7, 10, 13, 16,
19) 0.00 0.00 0.00 0.00 0.00
Docosahexaenoic
C18:1 (trans-9) Methyl
0.00 0.00 0.00 0.01 0.00
Elaidate
C18:2 (trans-9, 12) Methyl
0.00 0.00 0.00 0.00 0.00
Linoelaidate
[00305] Example 12
[00306] In
this example, yeast fermentations are performed using 80:20 blends of corn
mash and a C6 Saccharide Stream produced from corn stover or an equivalent
amount of pure
sugars. The corn mash was prepared according as in Example 10 and had a
glucose
concentration of about 30% (w/v).
[00307] Preparation of C6 Saccharide Stream containing cellulosic-derived
saccharides
[00308] Upon
completion of pretreatment, the post-treated material, which comprised
about 25% to about 30% solids [wt/v], was subjected to a water washing step to
separate
solubilized C5 saccharides from C6 Saccharides (the C5 saccharide stream
includes some
amount of C6 from glycan hydrolysis "impurity"). The washing was performed in
two steps;
in the first step, the post- treated feedstock was separated by filter press
to separate the solids
from the liquid stream containing the C5 saccharides. The solid material was
further
suspended in water to recover additional C5 saccharides which may have
remained in the
separated solids portion. On removing the soluble hemicellulose sugars (C5
saccharides), the
remaining solids contained the cellulose (C6 saccharides). The liquid stream
was then
retained and kept separate from the solids.
[00309] The separated solids were placed into a mixing tank. Once inside
the mixing
tank, the solids were adjusted to a pH of about 5 using 0.1 N Sodium
Hydroxide. The solids
were then diluted with water to about 10% solids. An enzyme cocktail (CELLIC
CTech 2,
Novozymes) was then added to the solid slurry. The amount of the enzyme added
to the
mixture was 2% loading (v/wt) based on the dry weight of the solids. The
slurry was mixed
at 50 C continuously for 72 hours.
107
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
[00310] Once enzymatic hydrolysis was complete, the liquid slurry was
separated by
centrifugation or microfiltration; or, alternatively, the solids can remain in
the broth. For the
experiments herein, the solids were separated from the broth using
evaporation. A lab-scale
evaporation set up in Buchi R220SE evaporator was used. About 30 L of the
resultant C6-
rich liquid slurry was concentrated by simple evaporation at a temperature of
60 degrees C
until the sugar content of the Corn Stover hydrolysate (C6 Saccharide Stream)
was raised
from about 5% to about 25% w/v. The resulting composition had a C6 saccharide:
C5
saccharide ratio of about 90:10.
[00311] Fermentation Reaction
[00312] Six bench top fermentation flasks were set up for each condition.
Each flask
contained a total of 275 g of fermentation substrate made up of 220 g of corn
mash and 55 g
of the C6 Saccharide Stream containing 25.4% glucose & 4.3% xylose or a stock
solution of
the same sugar concentrations. Additional fermentation additives included 0.5
mL of a 40%
urea solution, 0.305 mL of a 1:10 dilution of Spirozym Ultra XHS delivered at
0.036% w/w,
0.245 mL of a 1:100 dilution of protease, 0.1 mL of a 1:100 dilution of
phytase, and a scoop
of V50.
[00313] Fermentation progress was monitored by weighing the flasks at 6
hour
intervals for a total of 66 hours. The mass loss for each flask containing an
80:20 blend of
corn mash and the C6 Saccharide Stream (Exp. 1-6) or an 80:20 blend of corn
mash and stock
sugars (Cont. 1-6) is shown in Table 20. The average mass loss for each
condition is graphed
in Figure 19.
[00314] Table 20. Fermentation progress
Total Weight Loss (g)
6 hr 12 hr 18 hr 24 hr 30 hr 36 hr 42 hr 48 hr 54 hr 60 hr 66 hr
Cont. 1 2.99 12.93 21.10 25.13 28.00 29.88 31.47
Cont. 2 -0.60 9.59 17.43 22.26 25.78 27.74 29.97 29.03 29.72 28.98 29.63
Cont. 3 1.96 12.13 20.01 25.01 28.19 30.24 31.15 31.35 31.28 31.53 31.64
Cont. 4 1.63 12.49 20.21 23.79 27.75 30.12 30.86
Cont. 5 2.11 11.03 19.56 23.34 27.91 29.26 29.53 28.88 29.43 30.15 29.25
Cont. 6 2.76 11.96 19.63 23.59 27.80 29.65 29.71 29.60 29.98 30.06 30.78
Cont.
1.81 11.69 19.66 23.85 27.57 29.48 30.45 29.72 30.10 30.18 30.33
Avg.
108
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
Exp. 1 1.27 12.43 19.36 22.86
25.64 27.13 28.14
Exp. 2
2.00 11.43 19.62 23.71 26.81 28.34 30.36 30.00 31.40 30.61 31.83
Exp. 3
1.45 11.82 19.64 24.11 27.44 29.52 30.36 30.69 30.69 30.85 31.44
Exp. 4 1.97 13.57 20.20 24.73
28.81 30.09 30.82
Exp. 5
0.66 11.19 18.70 23.17 26.81 28.55 29.62 29.69 29.72 30.39 29.87
Exp. 6
2.72 13.78 20.49 24.81 29.52 30.13 30.60 31.33 31.44 31.82 32.56
Exp.
1.68 12.37 19.67 23.90 27.51 28.96 29.98 30.43 30.81 30.92 31.43
Avg
[00315] Residual sugars (DP4, DP3, Maltose, and Glucose), and fermentation
products
(lactic acid, glycerol, acetic acid, and ethanol) were analyzed by HPLC. Two
flasks from
each condition were analyzed after 42 hours; the remaining four flasks for
each condition
were analyzed after 66 hours of fermentation. Tested flasks were discarded due
to exposure
to air and interruption of fermentation. The results are shown in Table 21.
[00316] Table 21. Residual sugars and total products
Residual Sugars (% w/v) Final Products (% w/v)
Lactic Acetic
[00317] DP4
DP3 ...Maltose Glucose... Acid _Glycerol_ Acid _Ethanol
Cont. 1
(42 hr) 0456 0049 0 772 0054 0 135
1 675 0097 13 129
Cont. 2
(66 hr) 0.448 0.045 0.706 0.057 0.131 1.617
0.112 13.293
Cont. 3
(66 hr) 0.446 0.045 0.736 0.056 0.144 1.665
0.108 13.239
Cont. 4
...............................................................................
...............................................................................
...........................................
(42 hr) 0445 0047 0
726ett0055 0 1441 1 646 0097 13 184
Cont. 5
(66 hr) 0.436 0.047 0.753 0.054 0.145 1.664
0.109 13.17
Cont. 6
(66 hr) 0.45 0.047 0.772 0.058 0.145 1.678
0.112 13.093
Cont. Avg
(66 hr) 0.445 0.046 0.742 0.0563 0.141 1.656
0.110 13.199
Exp. 1
(42 hr)g11111111111110.59W1111111111ATTP1111111111111111111M84W
0439 013 ......................... ......................
.......................... ..................................................
............................ .......................
...........................
...................... ....................... .....................
....................... 13251
.........................,
Exp. 2
(66 hr) 0.572 0.17 0.831 0.09 0.129 1.541
0.113 13.522
Exp. 3
(66 hr) 0.568 0.168 0.825 0.086 0.131 1.53
0.11 13.596,
Exp. 4
...............................................................................
...............................................................................
...........................................
...............................................................................
...............................................................................
...........................................
(42 hr) NimiiT52w
pg.1149&ii
109
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
Exp. 5
(66 hr) 0.565 0.169 0.804 0.081 0.132 1.539 0.113
13.481
Exp. 6
(66 hr) 0.565 0.167 0.815 0.067 0.132 1.532 0.11
13.628
Exp. Avg
(66 hr) 0.568 0.169 0.819 0.081 0.131 1.536 0.112
13.557
[00318] The fermentations dosed with the C6 Saccharide Stream showed a
2.7%
increase in ethanol yield and a 7.2% decrease in glycerol production in
comparison to the
fermentations dosed with stock sugars.
[00319] Example 13
[00320] In this example, yeast fermentations are performed using 80:20
blends of corn
mash C6 Saccharide Stream produced from corn stover or an equivalent amount of
pure
sugars. The corn mash and C6 Saccharide Streams were prepared as in Example
12.
[00321] Six bench top fermentation flasks were set up for each condition.
Each flask
contained a total of 275 g of fermentation substrate made up of 220 g of corn
mash and 55 g
of the C6 Saccharide Stream containing 25.4% glucose & 4.3% xylose or a stock
solution of
the same sugar concentrations. Additional fermentation additives included 0.5
mL of a 40%
urea solution, 0.305 mL of a 1:10 dilution of Spirozym Ultra XHS delivered at
0.036% w/w,
0.245 mL of a 1:100 dilution of protease, 0.1 mL of a 1:100 dilution of
phytase, and a scoop
of V50.
[00322] Fermentation progress was monitored by weighing the flasks at 6
hour
intervals for a total of 60 hours. The mass loss for each flask containing an
80:20 blend of
corn mash and the C6 Saccharide Stream (Exp. 1-6) or an 80:20 blend of corn
mash and stock
sugars (Cont. 1-6) is shown in Table 22. The average mass loss for each
condition is graphed
in Figure 20.
[00323] Table 22. Fermentation progress
Total Weight Loss (g)
6 hr 12 hr 18 hr 24 hr 30 hr 36 hr 42 hr 48 hr 54 hr 60 hr
Cont. 1 3.50 14.34 21.05 25.25 28.85 29.97
30.41 31.05 31.28 31.66
Cont. 2 2.88 13.74 21.00 26.85 30.16 30.53
30.08 31.26 30.10 31.20
Cont. 3 2.96 13.17 20.55 24.39 28.46 29.97
31.56 31.33 31.78 32.63
Cont. 4 3.90 14.78 21.35 24.94 28.50 30.50
31.17 31.06 32.77 31.04
Cont. 5 5.42 15.26 23.03 27.06 29.80 31.03
31.13 31.43 31.93 31.35
Cont. 6 1.96 13.11 19.48 23.12 27.20 28.37
29.16 29.01 29.11 29.16
110
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
Cont.
3.44 14.07 21.08 25.27 28.83 30.06 30.59 30.86 31.16 31.17
Avg.
Exp. 1 3.08 13.91 20.26 23.78 26.54 28.75
30.29 29.92 30.41 30.17
Exp. 2 4.07 15.25 21.13 25.50 28.34 31.13
32.12 32.09 31.70 31.95
Exp. 3 2.64 14.09 21.51 25.49 28.48 29.98
30.04 31.23 30.91 31.33
Exp. 4 3.29 15.69 22.82 26.16 30.59 33.07 33.24 34.53
35.70 35.34
Exp. 5 3.35 14.48 21.21 25.55 29.58 30.79
31.21 31.29 31.28 33.14
Exp. 6 2.59 14.01 20.64 24.92 28.46
29.56 29.83 30.65 30.58 31.48
Exp.
3.17 14.57 21.26 25.23 28.67 30.55 31.12 31.62 31.76 32.24
Avg
[00324]
Residual sugars (DP4, DP3, Maltose, and Glucose), and fermentation products
(lactic acid, glycerol, acetic acid, and ethanol) were analyzed by HPLC after
60 hours. The
results are shown in Table 21.
[00325] Table 21. Residual sugars and total products (% w/v)
Residual Sugars Final Products
Lactic Acetic
DP4 DP3 Maltose Glucose Acid Glycerol Acid Ethanol
Cont. 1 0.469 0.062 0.772 0.07 0.1 1.713 0.091
13.765
Cont. 2 0.458 0.064 0.78 0.069 0.102 1.737 0.089
13.649
Cont. 3 0.461 0.062 0.77 0.067 0.1 1.695
0.087 13.6
Cont. 4 0.459 0.064 0.818 0.066 0.102 1.762 0.085
13.702
Cont. 5 0.453 0.064 0.804 0.069 0.104 1.752 0.085
13.595
Cont. 6 0.459 0.064 0.822 0.068 0.136 1.894
13.503
Cont.
0.460 0.063 0.794 0.068 0.107 1.759 0.087 13.636
Avg
Exp. 1 0.657 0.171 0.9 0.098 1.588 0.096
13.602
Exp. 2 0.64 0.172 0.869 0.102 0.1 1.608 0.095
13.944
Exp. 3 0.666 0.174 0.888 0.101 0.104 1.634 0.099
13.85
Exp. 4 0.66 0.172 0.895 0.175 0.102 1.603 0.104
13.875
Exp. 5 0.638 0.169 0.849 0.085 0.102 1.603 0.1
14.009
111
CA 02864144 2014-08-08
WO 2013/120035
PCT/US2013/025457
Exp. 6 0.667 0.173 0.916 0.103 1.616 0.096 13.81
Exp.
0.655 0.172 0.886 0.116 0.102 1.609 0.098 13.848
Avg
Blank cells represent machine errors and are excluded from the average values.
[00326] The fermentations dosed with the C6 Saccharide Stream showed a
1.6%
increase in ethanol yield and an 8.5% decrease in glycerol production in
comparison to the
fermentations dosed with stock sugars at the same concentration.
[00327] Example 14
[00328] In this example, yeast fermentations are performed to compare a
corn mash
only fermentation with a 70:30 blend of corn mash and a C6 Saccharide Stream
produced
from corn stover. The corn mash and C6 Saccharide Stream were produced as in
Example 12.
[00329] Fermentation Reaction
[00330] 215 mL of corn mash was propagated in a 1 L flask. In addition, 65
mL of
distilled water, 16 mL of city water, 1 mL of a 40% urea solution and 0.03 g
antibiotic was
also added to the propagation flask. Solutions were warmed to 32 C before
propagation was
started. Once heated to 32 C, 0.15 g of yeast and 38 iut of glucoamylase was
added to the
flask and agitated at 150 RPM for 10 hours.
[00331] Fermentation was carried out in 750 mL solutions in 1L flasks for
96 hours.
The fermentation conditions are detailed in Table 22.
[00332] Table 22. Fermentation substrate and additives
C6
Corn Mash Yeast prop
Sample Urea Glucoamylase Saccharide
(ferm feed) drop
Stream
Corn mash
675 mL 0.975 mL 0.1725 mL 0 mL 75 mL
control
70:30 Corn
Mash: C6
450 mL 0.975 mL 0.1095 mL 225 mL 75 mL
Saccharide
Stream
[00333] The rate of ethanol production during fermentation of corn mash
only
(diamonds) and (70:30) corn mash: C6 Saccharide Stream (squares) is shown in
Figure 21.
The final ethanol titers were similar between the two conditions; however, the
70:30 blend
112
CA 02864144 2014-08-08
WO 2013/120035 PCT/US2013/025457
fermentation reaction reached the plateau level earlier. These data indicate
that the ethanol
production rate kinetics is improved by use of the blended feedstock.
[00334] The rate of glycerol production during fermentation of corn mash
only
(diamonds) and (70:30) corn mash: C6 Saccharide Stream (squares) is shown in
Figure 22.
The final glycerol levels and the rates of production of glycerol were both
higher in the corn
mash only fermentation.
[00335] The conversion efficiency of fermentation of corn mash only and
the 70:30
blend of corn mash and the C6 Saccharide Stream is shown in Table 23.
[00336] Table 23.
Sample Initial sugar Residual Sugar Ethanol % Sugar to
(g/L) sugar (g/L) utilization produced ethanol
(g/L) conversion
efficiency
Corn mash 231.5 9.25 96% 134.35 114%
control
70:30 blend 229.8 9.3 95.9% 130 115.6%
[00337] While preferred embodiments of the present invention have been
shown and
described herein, it will be obvious to those skilled in the art that such
embodiments are
provided by way of example only. Numerous variations, changes, and
substitutions will now
occur to those skilled in the art without departing from the invention. It
should be understood
that various alternatives to the embodiments of the invention described herein
may be
employed in practicing the invention. It is intended that the following claims
define the
scope of the invention and that methods and structures within the scope of
these claims and
their equivalents be covered thereby.
113