Note: Descriptions are shown in the official language in which they were submitted.
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
POROUS STRUCTURE WITH INDEPENDENTLY CONTROLLED SURFACE
PATTERNS
CROSS-REFERENCE TO RELATED PATENT APPLICATIONS
[0001] This application claims priority to Provisional U.S. Patent Application
61/606087,
filed March 2, 2012, which is incorporated herein by reference in its
entirety.
BACKGROUND OF THE DISCLOSURE
[0002] Reabsorptive transport in vivo occurs through natural barriers, formed
by a single
layer of polarized epithelial cells supported by a basement membrane (BM)
which governs
the transport. Solutes and molecules cross the epithelial barrier by
transcellular or
paracellular pathways to the interstitial space and surrounding blood vessels,
resulting in
reabsorption of essential water and solutes. Common examples of reabsorptive
or absorptive
barriers in the body include those of the respiratory, gastrointestinal, and
urinary tracts. Fluid
and solute transport across these barriers make them particularly susceptible
to injury by
circulating toxins, pathogenic antibodies or certain drugs.
SUMMARY OF THE DISCLOSURE
[0003] According to one aspect of the disclosure, a cell culture support
device includes a
first and second polymer layer, each with a flow chamber defined therethrough.
Additionally, the cell culture support device includes a surface (also
referred to as a cross
channel interface) between the first polymer layer and the second polymer
layer. The surface
separates the first flow chamber from the second flow chamber. The surface
includes a
plurality of pores configured to allow communication and transport between the
flow
chambers. Furthermore, the surface includes a pattern independent of the
geometry of the
plurality of pores.
[0004] In some implementations, the surface is a membrane and the flow
chambers are
configured to be cellular chambers. In some implementations, the top layer of
the device is
configured to allow imaging of the surface in some implementations.
[0005] In certain implementations, the pattern is a topographic pattern and/or
a chemical
pattern. The pattern can be selected to enhance the growth of specific cell
types. In some
1
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
implementations, more than one pattern is formed on the face of the surface
and/or flow
chambers.
[0006] In other implementations, the pores are selected to produce a specific
type of
interaction between the flow chambers. In some implementations, the size of
the pores is
selected to prevent cell migration between the flow chambers but to allow cell
nutrients and
cell signaling analytes to migrate between the flow chambers. The size of the
pores is
between about 3 gm and about 15 gm in some implementations. In other
implementations,
the pattern and/or the geometry of the pores is selected to elicit a
particular arrangement,
function, shape, or density of cellular growth.
[0007] In yet other implementations, at least one of the polymer layers and/or
the surface
includes a biodegradable polymer. In some implementations, the pattern is
selected to
influence a degradation rate of the surface and/or to facilitate cellular
attachment to particular
locations within the cell culture support device.
[0008] According to another aspect of the disclosure, a method for fabricating
a cell culture
support device includes forming a first flow chamber in a first polymer layer,
and forming a
second flow chamber in a second polymer layer. Additionally, pores of a
specific size are
formed through a surface. A selected pattern is then applied to the surface.
The selection of
the pattern is independent from the selection of the pore size and position.
The forming is
done such that the formation of the pattern on the surface preserves the
plurality of pores.
Additionally, the method includes coupling the first polymer layer and the
second polymer
layer such that the surface separates the first flow chamber from the second
flow chamber.
[0009] In some implementations, cells are seeded into at least one of the flow
chambers. In
certain implementations, the pores are formed such that they have a specific
pore density
along the surface. In other implementations, the pattern is a topographic
pattern and/or a
chemical pattern selected responsive to the type of cells to be grown on the
surface.
[0010] In some implementations, the method also includes selecting and forming
additional
patterns onto the surface and or walls of the flow chamber. The additional
patterns can be the
same as, or different than, the initially selected pattern. In some
implementations, the
2
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
patterns are selected to elicit a particular arrangement, function, shape, or
density of cells
grown on the surface.
[0011] In yet other implementations, the polymer layers and/or surface include
a
biodegradable polymer. The pattern is selected to influence a degradation rate
of the surface
in some implementations.
[0012] According to yet another aspect of the disclosure, a cell culture
support system
includes a first and second polymer layer each with flow chambers defined
therethrough and
a surface separating the flow chamber of the first polymer layer from the flow
chamber of the
second polymer layer. The surface includes a plurality of pores configured to
allow
communication and transport between the flow chambers. Additionally, at least
one face of
the surface is patterned. The pattern is independent of the geometry of the
pores and
preserves the size of the pores when formed. The system also includes an
imager configured
to image a face of the surface.
[0013] In certain implementations, the system includes a means for coupling
the surface
between the first and second polymer layers, a flow meter configured to
measure flow
through at least one of the flow chambers, a pressure sensor configured to
measure the
pressure at an inlet and/or an outlet of the flow chambers, a fluid pump
configured to flow
fluid through the flow chambers, and macro-molecule injector coupled to an
inlet of the flow
chambers.
BRIEF DESCRIPTION OF THE DRAWINGS
[0014] The skilled artisan will understand that the figures, described herein,
are for
illustration purposes only. It is to be understood that in some instances
various aspects of the
described implementations may be shown exaggerated or enlarged to facilitate
an
understanding of the described implementations. In the drawings, like
reference characters
generally refer to like features, functionally similar and/or structurally
similar elements
throughout the various drawings. The drawings are not necessarily to scale,
emphasis instead
being placed upon illustrating the principles of the teachings. The drawings
are not intended
to limit the scope of the present teachings in any way. The system and method
may be better
3
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
understood from the following illustrative description with reference to the
following
drawings in which:
[0015] Figure 1 is a block diagram of an example system in which a cell
culture support
device is employed.
[0016] Figure 2 is a solid model of an example cell culture support device, as
can be
employed in the system of Figure 1.
[0017] Figure 3 is a cross sectional view of an example cell culture support
device, in
which the flow chambers are seeded with cells.
[0018] Figures 4A-4C are a series of solid models illustrating example cross
channel
interface pore topographies.
[0019] Figure 5 is a flow chart of an example method for manufacturing a cell
culture
support device similar to the device of Figure 2.
[0020] Figure 6 is a flow chart of an example method for using a cell culture
support device
in a system similar to the system of Figure 1.
[0021] Figure 7A is a cross sectional schematic of an example cell culture
support device.
[0022] Figure 7B is an image of a cell culture support device manufactured
based on the
schematic shown in Figure 7A.
[0023] Figures 7C-7E are a series of scanning electron micrographs, at various
magnifications, of the cell culture support device shown in Figure 7B.
[0024] Figures 8A-8E are a series of scanning electron micrographs showing
topographies
of various example cross channel interfaces.
[0025] Figure 9A is a plot illustrating the relationship between the change in
pore diameter
and hot-embossing dwell time.
4
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
[0026] Figure 9B is a plot illustrating how pore diameter is affected by cross
channel
interface topography when hot embossed.
[0027] Figure 9C is a series of scanning electron micrographs of example cross
channel
interfaces with various topographies.
[0028] Figure 9D is a plot illustrating how pore elongation is affected by
cross channel
interface topography and pore diameter.
[0029] Figure 10A is a brightfield microscopy image of a cell culture support
device's flow
chamber seeded with HK-2 cells.
[0030] Figure 10B is a brightfield microscopy image of the same flow chamber
show in
Figure 10A, viewed under higher magnification.
[0031] Figure 10C is a series of confocal microscopy images of the cells
seeded in the flow
chamber shown in Figure 10A.
[0032] Figure 10D is a brightfield microscopy image of a cell culture support
device's flow
chamber seeded with primary renal proximal tubule epithelial cells.
[0033] Figure 10E is a brightfield microscopy image of the same flow chamber
shown in
Figure 10D viewed under higher magnification.
[0034] Figure 1OF is a series of confocal microscopy images of the cells
seeded in the flow
chamber shown in Figure 10D.
[0035] Figure 11A is a scanning electron micrograph of an example cross
channel interface
prior to cellular seeding.
[0036] Figure 11B is a scanning electron micrograph of an example cross
channel interface
after a cellular mat has formed on the cross channel interface.
DETAILED DESCRIPTION
[0037] The various concepts introduced above and discussed in greater detail
below may be
implemented in any of numerous ways, as the described concepts are not limited
to any
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
particular manner of implementation. Examples of specific implementations and
applications
are provided primarily for illustrative purposes.
[0038] Models of absorptive barriers in the respiratory, gastrointestinal, and
urinary tracts
would offer a platform to better understand the biology and function of
reabsorptive barriers,
to interrogate underlying disease mechanisms affecting those barriers, and to
provide rapid
screening of drugs for toxic effects to and excretion by organs containing
those barriers. In
particular, since the kidney is susceptible to drug toxicity and governs
excretion of drugs, its
renal epithelial structures provide valuable test cases for in vitro models of
reabsorptive
barriers. Disclosed herein is a systems and methods for the manufacture and
use of such
barriers in vitro. In some implementations, the systems and methods disclosed
herein are used
as a medical device to assist organ function.
[0039] Figure 1 illustrates a cell culture support system 100. The system 100
includes at
least one cell culture support device 101. The system 100 also includes at
least one pump
103 that pumps fluid from a first fluid reservoir 102 into an inlet of the
cell culture support
device 101. As the fluid passes from the pump 103 to the cell culture support
device 101, it
passes through a flow meter 104 and past a molecule injector 105. Upon exiting
the cell
culture support device 101 the fluid passes by a fluid sampler 108 and through
a second flow
meter 109 and is then deposited into a second fluid reservoir 110. A pressure
sensor 106
measures the pressure at, or near, the inlet and the outlet of the cell
culture support device
101. Additionally, the system 100 includes an imager 107, which is used to
view cells within
the cell culture support device 101.
[0040] As discussed above, the system 100 includes a number of components to
support the
cell culture support device 101. The pump 103 drives fluid from the first
fluid reservoir 102
through the cell culture support device 101. In some implementations, the pump
103 is a
peristaltic pump or a syringe pump. In implementations using a syringe pump,
the fluid
reservoir 102 is the barrel of a syringe. The pump 103 controls the fluid
flowing through the
cell culture support device 101. For example, the pump can control the fluid's
flow rate and
the duration of the flow through the cell culture support device 101. In some
implementations, the flow is continuous and in other implementations the flow
is pulsatile.
The pump 103 can be configured to control the shear stress the fluid exerts on
cells within the
6
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
cell culture support device 101. The fluids passed through the cell culture
support device 101
can include, but are not limited to, cell culture medium, cell nutrients,
reagents, test agents,
buffer fluids, reactant fluids, fixing agents, stains, simulated and real
biological fluids such as
blood filtrate, whole blood, blood serum, blood plasma, urine, dilute urine.
[0041] In some implementations, the above agents and/or other molecules are
added to the
fluid flowing into the cell culture support device 101 by the molecule
injector 105. In certain
implementations, the molecule injector 105 is a second syringe pump. In other
implementations, continuous delivery of nutrients by the fluid creates
favorable conditions
for long term cell culture within the cell culture support device 101. The
system 100 also
includes a fluid sampler 108. The fluid sampler 108 is positioned near the
outlet of the cell
culture support device 101. In some implementations, the fluid sampler 108 is
configured to
siphon off a small amount of the fluid exiting the cell culture support device
101. The
collected fluid may be tested for specific molecular markers, reagents, or
other such
molecules.
[0042] The system 100 further includes a flow meter 104 near the inlet of the
cell culture
support device 101, a flow meter 109 near the outlet of the cell culture
support device 101,
and a pressure sensor 106. The pressure sensor 106 measures the pressure of
the fluid as it
enters and exits the cell culture support device 101. In certain
implementations, the
measurements made by the flow meters 104 and 109 and the pressure sensor 106
are used to
calculate the shear stress imparted on cells within the cell culture support
device 101.
[0043] The imager 107 is used to observe cells within the cell culture support
device 101.
In some implementations, the cells are imaged while fluid is flowing through
the cell culture
support device 101. In other implementations, at the end of an experiment
fixing fluid is
passed through the cell culture support device 101 and the cells are imaged
upon completion
of experimentation. In other implementations, the imager 107 is configured to
monitor the
integrity of the cross channel interface. For example, the imager 107 can be
configured to
measure the degree to which the cross channel interface 202 has degraded.
[0044] Figure 2 is a solid model illustrating the cell culture support device
101 in greater
detail. As illustrated, the cell culture support device 101 is a multi-layered
polymer device.
7
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
The cell culture support device 101 includes a first polymer layer 203 with a
first flow
chamber 206 defined therethrough, and a second polymer layer 201 has a second
flow
chamber 205 defined therethrough. The first polymer layer 203 of the cell
culture support
device 101 also includes a cross channel interface 202. In some other
implementations, the
cross channel interface 202 is an additional polymer layer that is coupled
between the first
polymer layer 203 and second polymer layer 201. In certain implementations,
the cross
channel interface 202 is a membrane made of a thermoplastic, such as
polystyrene,
polycarbonate, polyimide, polysulfone, polyethersulfone; biodegradable
polyesters, such as
polycaprolactone (PCL); soft elastomers, such as polyglycerol sebacate (PGS);
or other
polymers such as polydimethylsiloxane (PDMS) and poly(N-isopropylacrylamide).
In yet
other implementations, the cross channel interface 202 is made from silicon,
glass, or silicon
nitride. The cross channel interface 202 is manufactured, in some
implementations, through
processing methods such as track-etching, electro-spinning, microfabrication,
micromolding,
gel deposition, phase separation, particle leaching, and solvent leaching. In
yet other
implementations, the cross channel interface 202 is a multilayered membrane
that includes
several layers of material. For example, the material can be a structural
backing, a skin layer,
a porous layer, a layer that serves as a permeable spacer, or allows lateral
flow.
[0045] As discussed above, in certain implementations, the interior of the
cell culture
support device 101 is imaged with the imager 107. Accordingly, in some
implementations,
the roof 204 of the first flow chamber 206 is configured to allow for visual
inspection of the
first flow chamber 206, cross channel interface 202, and/or second flow
chamber 205. In
other implementations, the roof 204 is a polymer layer manufactured out of a
material similar
to, or the same as, the polymer layers. In certain implementations, the cell
culture support
device 101 includes more than one flow chamber within a polymer layer.
Additionally, in
some implementations, the cell culture support device 101 includes more than
two polymer
layers with flow chambers. For example, the cell culture support device 101
can include
three or more polymer layers each separated from one another by a different
cross channel
interface 202. The polymer layers can include, but are not limited to, a
thermoplastic, such as
polystyrene, polycarbonate, polyimide; biodegradable polyesters, such as
polycaprolactone
(PCL); soft elastomers, such as polyglycerol sebacate (PGS); or other polymers
such as
polydimethylsiloxane (PDMS) and poly(N-isopropylacrylamide). In certain
implementations,
8
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
the polymer material is selected for its ability to be micro-machined and
support cell growth.
In some implementations, the length, width, and height of the flow chambers
are selected to
mimic kidney structures. In other implementations, the height of a flow
chamber is between
about 10 gm and about 100 gm, the width is between about 250 gm and about 2
mm, and the
length is between about 5 mm and about 10 mm.
[0046] Discussed in greater detail in relation to Figures 3 and 4A-4C, but
briefly, the cross
channel interface 202 enables communication between the first flow chamber 206
and the
second flow chamber 205. A plurality of pores 207 are defined through the
cross channel
interface 202 and at least one face of the cross channel interface 202
includes a topography
that is independent of the pores 207. A cross channel interface 202 with pores
207 that are
created independent of the topography generates a porous membrane that
facilitates basement
membrane (BM)-like architecture and enables better control of experimental
variables. In
some implementations, the topography is selected such that it has a specific
effect on the
pores 207. For example, the topography can be selected such that it alters the
pore share or
closes the pores in a specific area of the cross channel interface 202 or
reduces the size of the
pores 207 by a specific size.
[0047] In some implementations, the pores 207 of the cross channel interface
202 are
generated by track-etching. Track-etching creates highly uniform pores 207.
The pore sizes
range between about 3 gm and 15 gm wide. The cross channel interface 202 is
between
about 6 gm and 30 gm thick.
[0048] As indicated above, at least one face of the cross channel interface
202 is patterned
with a selected topography. In certain implementations, at least one wall of
the first flow
chamber 206 and/or second flow chamber 205 is also patterned with a selected
topography.
In some implementations, the patterned faces (e.g. a cross channel interface
202 face and a
first flow chamber 206 wall) are patterned with a different topographies. In
yet other
implementations, different sections of the same face are patterned with
different
topographies.
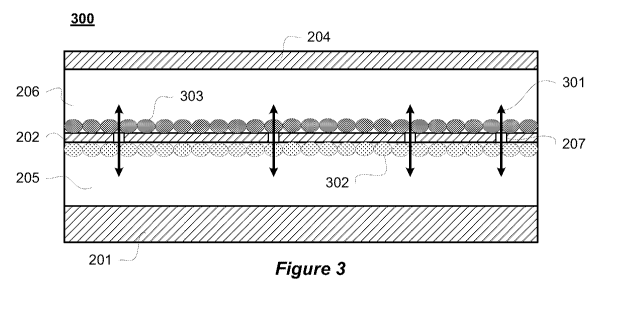
[0049] Figure 3 is a cross-sectional view of a cell culture support device 300
similar to the
cell culture support device 101 of Figure 2. The cell culture support device
300 includes a
9
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
first polymer layer in which a first flow chamber 206 is defined, and a second
polymer layer
201 in which a second flow chamber 205 is defined. A cross channel interface
202 forms the
roof of the second flow chamber 205 and the floor of the first flow chamber
206. A top
polymer layer 204 forms the roof of the first flow chamber 206. As previously
discussed, the
cross channel interface 202 includes a plurality of pores 207. As illustrated,
the cell culture
support device 300 includes a first plurality of cells 302 seeded in the first
flow chamber 206
and a second plurality of cells 303 seeded in the second flow chamber 205. In
some
implementations, cells are only seeded into one of the flow chambers 206 and
205. As
illustrated by the flow arrows 301, fluid communication occurs through the
pores 207. The
fluid communication can include, but is not limited to, the passive transport
of
macromolecules, nutrients, and test agents. In some implementations, the size
of the pores
207 allows for cells to migrate across the cross channel interface 202 and in
other
implementations the size and/or shape of the pores 207 inhibits trans-chamber
migration of
cells but allows for the migration of nutrients and cellular signaling
analytes between the
chambers. The pores 207 can be dispersed randomly or in an ordered fashion
throughout the
length of the cross channel interface 202, and in some implementations, the
pores 207 are
limited to specific regions of the cross channel interface 202. In some
implementations, the
arrangement, shape, and size is referred to as the pore geometry.
[0050] Figure 4A-4C are solid models illustrating topographical patterns that
can be
applied to a cross channel interface. For illustrative purposes the cross
channel interfaces
includes three pores 207; however, the is no requirement for similar pore
spacing, alignment,
or concentrations. The topography of a cross channel interface 202 is selected
based on the
sells that are to be seeded into the cell culture support device 101, and can
be selected to
effect a particular arrangement, function, shape and/or density of cells. In
some
implementations, the cross channel interface 202 is manufactured from a
biodegradable
polymer. In some of these implementations, the surface pattern is selected to
facilitate and/or
control the degradation of the cross channel interface 202. In certain
implementations, the
topographies are selected and manufactured such that they degrade at a
specific rate or when
exposed to a specific chemical agent. For example, the patterning topography
and cross
channel interface 202 may be configured such that the cross channel interface
202 completely
dissolves once a cellular mat has grown on the cross channel interface 202.
Thus, in such an
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
implementation, after cross channel interface 202 degradation, the first
polymer layer 203 and
second polymer layer 201 are separated only by the cellular mat.
[0051] Figure 4A illustrates a portion of cross channel interface 410 that is
smooth and does
not contain a topographical pattern. Figure 4B illustrates a cross channel
interface 420 with a
ridge and groove pattern. The ridge and groove pattern can be aligned
perpendicular to,
parallel with, or angled to the flow of fluid through a flow chamber. In other
implementations, the ridges are between about 0.5 gm and about 1.0 gm wide,
have a pitch
between about 1.0 gm and about 2 gm, and are between about .5 gm and 1.0 gm
tall. In
some implementations, the dimensions and spacing of the ridges is constant
along the entirety
of a patterned surface. In other implementations, one or more of the ridge
parameters is
varied along the length of the patterned surface. For example, the spacing
between the ridges
may start at 1.0 gm and transition to a 2.0 gm spacing at the flow chamber's
outlet. In
certain implementations, the ridges and/or grooves are rounded.
[0052] Figure 4C illustrates a cross channel interface 430 with a pit pattern.
As illustrated,
the pits are cylinderical, but in other implementations the pits can be
rectangular, square,
frustroconical, conical, and/or hemispherical. Additionally, the above pit
patterns can be
inversed to create posts. The pits and posts can be patterned in a regular,
ordered fashion or
randomly. For example, in the regular, ordered fashion, hemispherical posts
may be aligned
in ordered rows with 1.0 gm between each post of a row and 1.5 gm between each
row. In
some implementations, the cross channel interface 202 topographies includes a
plurality of
the above described topographies.
[0053] Figure 5 is a flow chart of a method 500 for manufacturing a cell
culture support
device, such as that of system 100. The method 500 includes forming a first
flow chamber in
a first polymer layer (step 501), and also forming a second flow chamber in a
second polymer
layer (step 502). Additionally, the method 500 includes forming pores in a
cross channel
interface (step 503). A topographical pattern is selected (step 504) and
formed into the cross
channel interface (step 505). In some implementations, the cross channel
interface is coupled
between the first polymer layer and the second polymer layer (step 506).
11
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
[0054] As set forth above, a first flow chamber is manufactured in a first
polymer layer
(step 501) and a second flow chamber is manufactured in a second polymer layer
(step 502).
The flow chambers can be manufactured by photolithographic techniques,
injection molding,
direct micromachining, deep RIE etching, hot embossing, or any combinations
thereof In
some implementations, as illustrated in Figure 2, the cross channel interface
202 is a
component of the first polymer layer 203. In other implementations, the cross
channel
interface 202 is a separate component that is separately manufactured and
subsequently
coupled between the first and second polymer layers.
[0055] The method 500 of manufacturing a cell culture support device continues
with the
formation of pores in the cross channel interface (step 503). In some
implementations, the
pores 207 of the cross channel interface 202 are manufactured by leaching
micro-particles
from the cross channel interface 202, phase separation micro-molding, track
etching, or any
combination thereof In certain implementations, the cross channel interface
202 is obtained
prefabricated with pores 207.
[0056] The method 500 continues with the selection of a topographical pattern
to apply to
the cross channel interface (step 504) and then the application of the
selected pattern to the
cross channel interface (step 505). As discussed above, the selection of the
pattern can be
dependent on the cells to be grown on the cross channel interface 202 and/or
the desired
arrangement, function, shape, and density of the seeded cells. Responsive to
selecting the
topographical pattern, the pattern is applied to the cross channel interface
202.
[0057] The cross channel interface 202, and any wall of a flow chamber to be
patterned,
can be patterned with hot-embossing. Hot-embossing can be accomplished as a
two step
molding process. First, a silicon mold is fabricated using photolithography
and reactive ion
etching. The first step of the process generates a positive of the selected
pattern. Next, a
negative is created from the positive. The negative is formed by
electroforming nickel to the
positive mold. The electroforming is accomplished by applying a voltage
difference between
a nickel source and the silicon mold. This causes the nickel to flow into the
silicon mold. In
some implementations, prior to embossing, the patterned face of the nickel
mold is soaked in
a 1 mM solution of hexadecanethiol (HDT), which forms a self-assembled
monolayer (SAM)
to decrease surface energy to aid in subsequent polymer release.
12
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
[0058] The second step of the hot-embossing method includes placing the cross
channel
interface 202 in contact with the topographically patterned face of the nickel
mold. The mold
and cross channel interface 202 are sandwiched between two Kapton polyimide
films and
silicone rubber sheets to decrease sticking and add compliance. The stack is
then placed in a
uniformly heated, temperature- and pressure-controlled automatic hydraulic
press. A light
load is applied to the stack while the temperature is set to about 150 C. The
load is applied
for a specified dwell time before being cooled to 60 C under constant
pressure. Upon
cooling, the newly patterned membrane is released from the nickel mold and
analyzed for
changes in pore size and geometry. The combination of the dwell time, pressure
and
temperature are selected such that the topographical pattern is fully created
in the cross
channel interface 202, but does not cause polymer material to flow into the
pores 207. In
some implementations, the dwell time was selected to be between about 10 and
about 20
minutes, under a pressure of between about 700 kPa and about 850 kPa, and at a
temperature
of about 125 C to about 175 C. For example, a dwell time of 15 minutes at
820 kPa and
150 C preserves pore architecture. In other implementations, alternative
embossing
parameters and processes may be employed.
[0059] In some implementations, the method 500 of manufacturing a cell culture
support
device includes coupling the cross channel interface between the first and
second polymer
layers (step 506). As discussed above, in some implementations, the cross
channel interface
202 is a component of the first or second polymer layers, and therefore, in
these
implementations, the first polymer layer 203 would be directly completed to
the second
polymer layer 201. In other implementations, the cross channel interface 202
is coupled
between the first polymer layer and the second polymer layer. In certain
implementations,
the components of the cell culture support device 101 are reversibly coupled
to one another.
For example, a clamp can be used to couple the components together during an
experiment
and allow for the cross channel interface 202 to be removed after an
experiment for further
analysis.
[0060] Figure 6 is a flow chart of a method 600 for using a cell culture
support device in a
system similar to the system 100. The method 600 begins with the provisioning
of a cell
culture support device (step 601). Then, cells are seeded into at least one
flow chamber of the
13
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
cell culture support device (step 602). The method 600 continues with the
flowing of fluid
through the flow chambers of the cell culture support device (step 603) and
the injection of a
molecule into the inlet of at least one of the flow chambers (step 604).
Responsive to flowing
fluid through the flow chambers, at least one flow parameter is measured (step
605) and the
concentration of the injected molecule is measured at an outlet of at least
one flow chamber
(step 606).
[0061] As set forth above, the method 600 begins by providing a cell culture
support device
(step 601), such as the cell culture support device 101 of Figure 2. Next,
cells are seeded into
the cell culture support device (step 602). In some implementations, the cell
culture support
device 101 is provided pre-assembled, and in other implementations, the cell
culture support
device 101 is provided unassembled. For example, cells can be seeded onto the
cross channel
interface 202 prior to assembly of the cell culture support device 101. The
cell seeded cross
channel interface 202 can then be cultured in an incubator until the cells
reach a maturity
level appropriate for experimentation. In other implementations, cells are
injected into the
cell culture support device 101 with a syringe and allowed to adhere to the
cross channel
interface 202 and/or other surfaces of the flow chambers. In certain
implementations, the cell
culture support device 101 is sterilized prior to cellular seeding. For
example, the cell culture
support device 101 can be sterilized with ethylene oxide and then rinsed with
70% ethanol.
Also prior to cellular seeding, in certain implementations, the cross channel
interface 202
and/or remaining components of the cell culture support device 101 are coated
with an agent,
such as an agent to inhibit or encourage cellular growth. For example,
surfaces of the cell
culture support device 101 exposed to cells can be coated with an
extracellular matrix,
collagen IV, collagen I, laminin, fibronectin, agrin, nephrin, or similar
proteins, Arginine-
Glycine-Aspartic acid or similar peptides, or adhesive motifs.
[0062] The method 600 continues with the flowing of fluid through the cell
culture support
device (step 603). As described above in relation to Figure 1, fluid flow
through the chambers
of the cell culture support device 101 is controlled with a pump 103. In some
implementations, fluid is only flowed through one of the flow chambers. For
example, a first
chamber can act as a cellular well without perfusion, such that the cells
within the camber are
not exposed to shear stress caused by flowing fluid. In this example,
nutrients or other agents
14
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
can be transported to the cellular well through the pores 207 in a cross
channel interface 202
that separate the cellular well from flowing fluid in a flow chamber beneath
the cellular well.
[0063] The method 600 also includes injecting a molecule into the inlet of at
least one flow
chamber (step 604). The molecule can be a cell culture medium, cell nutrient,
reagent, test
agent, buffer fluid, reactant fluid, fixing agent, and/or stain. In some
implementations, the
injection of the molecule and/or the pump 103 is computer controlled so that a
specific flow
rate and molecule concentration is achieved within the cell culture support
device 101.
Example molecules to be injected can include, but are not limited to, water,
sodium,
potassium, chlorine and other ions; urea creatinine, and other metabolic
products; oxygen,
carbon dioxide, nitrogen, and other gases; macromolecules of defined molecular
weights such
as inulin, ficoll, dextran, albumin and other proteins; pharmaceutical agents
and their
metabolically-modified forms; toxins; cells and subcellular biological
components such as
platelets and microparticles; large particles of solids include micro and nano
particles; lipid
and other vesicles either synthetic or naturally-derived; bubbles or other gas-
phase particles.
[0064] Responsive to flowing fluid through the cell culture support device, at
least one flow
parameter is measured (step 605). The measurement of the flow parameter can
include
parameters that are either directly measured, such as fluid flow rate and
fluid pressure,
derived measurements, such as shear stress measurements. In certain
implementations, the
measurements are made at the inlet, outlet and/or within the cell culture
support device. In
other implementations, cross channel permeability is measured. For example,
hydraulic
permeability, which measures the flux of a chemical, molecule or agent through
a membrane
at a given transmembrane pressure, can measured. In certain implementations,
the
transmembrane pressure is measured by direct measurements or by deriving the
measurement
based on pressure levels at channel inputs and outputs. The fluid flow rate
can be quantified
by measuring filling of a vessel of known size, measuring mass of
inputs/outputs over time,
flow visualization techniques, particle image velocimetry, and techniques
using tracer
elements or contrast agents.
[0065] Additionally, the method 600 includes measuring the concentration of
the injected
molecule at an outlet of at least one of the flow chambers (step 606). In some
implementations, transport through the cross channel interface 202 (and in
some
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
implementations, the layer of cells seeded on the cross channel interface 202)
is measured by
injecting a molecule into an inlet of a first flow channel and then measuring
the concentration
of the molecule at the outlet of a second flow channel.
[0066] In some implementations, the transport of specific species across the
membrane is
analyzed by evaluating the concentrations of solutes, particles and other
components of fluids
in a cellular flow chamber, at the inlet of a cellular flow chamber, and/or at
the outlet of a
cellular flow chamber. In certain implementations, the concentration of the
molecules is
measured with a sensor, a molecule selective dye, a soluble nanosensor, a
molecular label,
such as a radioactive label or tracer. In some implementations, the evaluation
of
concentration and flow can be configured such that a sieving coefficient of a
molecule or
component is quantified for the device, cross channel interface 202, and/or
the membrane-cell
construct. The sieving coefficient is the concentration of a specific analyte
in the fluid
passing through the membrane divided by the concentration of that same analyte
in the fluid
being fed to the membrane. The sieving coefficient can reflect the selectivity
of a porous
membrane.
EXAMPLES
I. Topographic Pattering
[0067] Figure 7A-7E illustrates a series of images, at different
magnification, of a cell
culture support device manufactured using the above described hot-embossing
method. In
this example, the cross channel interface was manufactured as an independent
topographically-patterned membrane assembled into a cell culture support
device. Figure 7A
is a schematic of the overall cross-sectional architecture of the cell culture
support device. As
in the cell culture support device 101 of Figure 2, the schematic illustrates
a top cell chamber
defined in a first polymer layer and a bottom cell chamber defined in a second
polymer layer.
A cross channel interface is coupled between the first polymer layer and
second polymer
layer, and a cover slide provides the roof for the top cell chamber. Figure 7B
illustrates an
assembled cell culture support device, of which Figures 7C-7E provide greater
detail.
[0068] Figure 7C is a scanning electron micrograph illustrating the cross
section of the
device in Figure 7B. The micrograph shows the porous nature of the cross
channel interface
separating the top and bottom chambers. Figures 7D and 7E are scanning
electron
16
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
micrographs magnifying the inserts of Figure 7C. Figures 7D and 7E illustrate
the well-
defined groove topography coexisting with the porous architecture.
II. Topographic Examples
[0069] Figures 8A¨E are a series of scanning electron micrographs showing
example
pattern topographies created with the hot-embossing method described above on
cross
channel interfaces with 8 gm diameter pores. Figure 8A is an image of a cross
channel
interface 202 including three pores and a smooth topography. Figures 8B-8D
illustrate the
ridge and groove topography discussed in relation to Figures 4A-4C. Figures 8B-
8D also
illustrate the effect of ridge width on topography. The width of the ridges in
Figure 8B is 0.5
gm, 0.75 gm in Figure 8C, and 1.0 gm Figure 8D. Similarly, Figure 8E
illustrates a cross
channel interface with evenly spaced 1.0 gm pits. In Figures 8B-8E, the
topographical
features are 0.75 m deep.
III. Hot-Embossing Parameters
[0070] As discussed above, the embossing of the topographical pattern onto the
cross
channel interface are done in a controlled manner as to not alter the pore
architecture. To
determine the appropriate embossing parameters, a series of cross channel
interfaces were
hot-embossed under a constant load of 820 kPa at 150 C. For the trials the
dwell time ranged
from 10 to 30 minutes. Figure 9A is a graph illustrating how pore diameter (y
axis) changes
with dwell time (x axis). The plot in Figure 9A shows that nominal pore size
did not
significantly change for dwell times less than 15 minutes when compared to the
pre-
embossed cross channel interface. However, as dwell time increased past 20
minutes, pore
diameter significantly decreased when compared to pre-embossing diameters.
[0071] Figure 9B is a series of bar charts that illustrate how different
topographies affect
pore diameter during the embossing process. For this experiment, cross channel
interfaces
were created to have 3 gm, 5 gm, 8 gm, or 12 gm diameter pores. The diameter
of the pores
on each cross channel interface were measured and averaged to serve as
controls for the
experiment. The cross channel interfaces were then hot-embossed with a dwell
time of 20
minutes under 820 kPa at 150 C. The resulting pore diameters were measured and
averaged.
[0072] Figure 9B shows that embossing reduces pore diameter for membranes with
large
pores, such as the 8 gm and the 12 gm diameter pores, but does not reduce pore
diameter for
17
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
pores with small pore diameters, such as the 3 gm and the 5 gm diameter pores.
The 8 gm
and 12 gm pore cross channel interfaces showed a decrease in pore diameter
over all
topographies after embossing. Also, while a pore diameter of 12 gm was desired
for the
fourth group (the 12 gm group), the actual pore diameter was measured to be
closer to 10 gm.
Thus, the decrease in pore diameter caused by the hot-embossing process in the
12 gm cross
channel interface, although statistically significant, was smaller than the
difference between
the desired control pore diameter and the actual measured 12 gm pore diameter.
[0073] Some decrease in pore diameter is expected after embossing due to the
flow of the
polymer under high temperature and pressure. Pore deformation was independent
of pattern
type. A dwell time of 10-15 minutes provided a good balance of pattern
transfer onto the
cross channel interfaces without significantly changing pore diameter.
[0074] In some experiments, pores were not perfect circles and the elongation
of the pores
was exacerbated during the hot-embossing process. Figure 9C is a series of
scanning electron
micrographs illustrating the differences in elongation of 3-12 gm pores
embossed with a
ridge and groove topography (a 10Grat topography corresponds to 1.0 gm wide
ridges spaced
2.0 gm apart). Figure 9D is a bar chart, with the same groupings represented
in Figure 9B,
and illustrates the amount of elongation, represented as a fraction, induced
by hot-embossing
with a dwell time of 20 minutes under 820 kPa at 150 C. The average pore in
the non-
embossed control cross channel interfaces exhibited a slightly elongated shape
with a fraction
of elongation ranging from 0.16 for 12 [tm pores to 0.34 for 3 [tm pores.
Figure 9D shows
how the fraction of elongation for pores after embossing was dependent on
initial pore
diameter and in some cases, the topography. Cross channel interfaces with
smaller pore
diameters, i.e. 3 gm and 5 gm, yielded significantly higher fractions of pore
elongation when
compared to larger pore sizes. Cross channel interfaces with a pore size of 3
gm exhibited
pores with an average elongation fraction of almost 0.5 when embossed with the
10Grat
pattern, a 49% increase from the control. On average, elongation of the 3 gm
pores increased
by 37% across all linear patterns. For larger pore sizes, i.e. 8 gm and 12 gm,
the change in
pore elongation became insignificant across most topographies when compared to
the control
pore geometry. The elongation of 8 gm pores increased by an average of only
15.3% among
linear patterns. Cross channel interfaces with 12 gm pores had the lowest
fraction of
elongation, with an average elongation of 0.23 among linear patterns.
Topographical features
18
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
in the form of 1 gm pits had the least affect on pore elongation for small and
large pore sized
membranes, with a range from 0.37 for 3 gm pores to 0.22 for 12 gm pores.
IV. Cells proliferate on and respond to porous membrane topography
[0075] A cell culture support device, similar to that of the device in Figures
7A-7E,
consisting of two channels separated by the patterned porous cross channel
interface (also
referred to as a membrane) served as a platform to characterize the response
of renal
epithelial cells cultured on the patterned porous membrane. The device's
performance was
evaluated in three ways. First, HK-2 response to unpatterned and patterned
membranes
outside the device was characterized by analyzing cellular alignment. Second,
the cell
culture support device was evaluated by scanning electron microscopy and
optical
microscopy to verify channel geometry and alignment. Third, immunofluorescent
techniques
were used to label markers indicative of a reabsorptive epithelial layer for
cells cultured in
the cell culture support device.
[0076] During the experiments, HK-2 cells and renal proximal tubule epithelial
cells
(RPTECs) proliferated from initial seeding to confluency within the cell
culture support
device over approximately 4 days. A uniform initial seeding density and
appropriate culture
time yielded complete confluency of both HK-2 and RPTECs over the cell culture
support
device channel area of 1.25 mm2 shown respectively in the brightfield
composites in Figure
10A and Figure 10D, with higher magnification views provided in Figures 10B
and 10F.
HK-2 and RPTEC monolayers expressed paxillin (Figures 10C(i) and 10F(i)), a
typical
epithelial marker of focal adhesions; ZO-tight junction complexes (Figures
10C(ii) and
10F(ii)); and acetylated tubulin, an indicator of primary cilia and
cytoplasmic microtubules
(Figures 10C(iii) and 10F(iii)). Paxillin expression in HK-2 samples signified
focal
adhesions that were less discrete with a weaker signal than RPTEC samples.
Both HK-2s and
RPTECs expressed ZO-1 in distinct borders outlining the perimeter of cells,
indicating initial
formation of a tight-junction-based sealed epithelial barrier. Acetylated
tubulin morphology
differed between the HK-2s and RPTECs. The HK-2s showed somewhat distributed
cytoplasmic microtubules, but distinct primary cilia were not expressed on the
apical surface.
The RPTECs expressed acetlylated tubulin in a single punctuate spot on the
apical surface of
each cell, indicating formation of a primary cilia.
19
CA 02865795 2014-08-27
WO 2013/131088 PCT/US2013/028879
[0077] Formation of the complete, confluent cellular monolayer within the
channel layer
allows interrogation of the layer for permeability, a requisite for a
reabsorptive barrier. As
the layer is confluent, the cell culture support device allows fluidic and/or
electrical access to
any point on the flow chamber and thus the direct measurement of cellular
transport.
Therefore, the cell culture support device allows for the quantification of
reabsorptive
properties. Formation of ZO-1 junctions indicate an epithelial barrier capable
of active
transport. The cellular junctions can be improved by conditioning the cells
with mechanical
and/or other stimuli. The HK-2 cells formed a more mature monolayer due to its
longer
culture time, causing subtle differences in paxillin expression. Focal
adhesions in highly
developed monolayers are not discrete and tend to have a weaker signal, which
was seen in
the HK-2 samples as compared to the RPTECs. The lack of primary cilia in HK-2
cells was
not abnormal. Primary cilia may not be fully expressed in HK-2 populations if
their
formation is not enhanced by serum starvation or shear stress. Cytoplasmic
tubulin was more
prevalent compared with RPTEC, with signs of a microtubule-organizing center
that may
nucleate cilia development. The presence of primary cilia in the RPTECs
indicated the cells
will be responsive to mechanical stimuli, such as shear stress, as the cilia
can serve to
transduce mechanical signals to chemical activity. Continuous flow of nutrient
rich fluid
through the cell culture support device delivers nutrients to the cell
populations, thereby
creating more favorable conditions for long term cell culture in a small
channel volume while
simultaneously mimicking the filtrate flow seen by proximal tubule cells in
vivo. Finally, as
shown by the scanning electron micrographs of Figures 11A and 11B, the cells
block the
pores of the membrane. Figure 11A is a scanning electron micrograph of a
membrane prior
to cellular seeding and Figure 11B is a membrane showing that the seeded cells
block the
pores of the membrane. Figure 11B indicates transport across the membrane-cell
layer
construct can be limited to transcellular transport if paracellular transport
is limited through
tight junction and other cell-cell junction formation. With the ability to
stimulate cells
mechanically; interrogate cells with imaging, electrical, and fluidic means;
and support
growth of an epithelial layer expressing indications of a mechanically-
responsive
reabsorptive barrier, the patterned porous membrane of the cell culture
support device allows
the quantification reabsorptive barrier function.