Note: Descriptions are shown in the official language in which they were submitted.
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
HALOALKENE COMPLEXES
[0001] This application claims the benefit of priority to U.S. patent
application number
13/774,751, filed on February 22, 2013, U.S. patent application number
13/774,838, filed on
February 22, 2013, U.S. patent application number 13/774,908, filed on
February 22, 2013,
U.S. patent application number 13/774,950, filed on February 22, 2013, each of
which are
continuations in part of U.S. patent application number 13/731,608, filed on
December 31,
2012, which claims priority to U.S. provisional application number 61/608954,
filed on
March 9, 2012. This and all other extrinsic materials discussed herein are
incorporated by
reference in their entirety. Where a definition or use of a term in an
incorporated reference is
inconsistent or contrary to the definition of that term provided herein, the
definition of that
term provided herein applies and the definition of that term in the reference
does not apply.
Field of the Invention
[0002] The field of the invention is haloalkene complexes, and more
specifically haloalkene
complexes for use as a refrigerant composition, and especially in
refrigeration systems.
Background
[0003] The background description includes information that may be useful in
understanding
the present invention. It is not an admission that any of the information
provided herein is
prior art or relevant to the presently claimed invention, or that any
publication specifically or
implicitly referenced is prior art.
[0004] Existing commercially available refrigerant compositions generally
comprise specific
chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and
hydrofluorocarbons
(HFCs), and can have some uses beyond refrigeration.
[0005] For example, U.S. Patent Application Publication No. 2011/0012052 to
Van Horn et
al. teaches a mixture of specific alkenes with HFCs, for use as a heat
transfer composition.
However, Van Horn's mixture is apparently not a complex. The alkene and HFC
are described
by Van Horn as being miscible, which means they mix in all proportions. There
is no
indication that Van Horn's mixture is sufficiently activated to be complexed
through Van der
Waals forces.
[0006] U.S. Patent Application Publication No. 2007/0092545 to Bale teaches an
aerosol
coolant spray comprising an essential oil, HFC, and diluent material, for
killing and removing
ticks. However, Bale apparently fails to teach a complex formed via activation
of the mixture.
1
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
Moreover, Van Horn and Bale each fail to address the global warming concerns
associated
with refrigerant compositions and do not appear to teach the user of a
catalyst in producing a
refrigerant composition.
[0007] The last few decades have seen a growing emphasis on reducing the
global warming
impacts caused by refrigerants and refrigerant compositions. Various studies
have been
conducted to address the pitfalls of existing refrigerants and refrigeration
systems (See e.g.,
"Real Zero ¨ Reducing refrigerant emissions & leakage ¨ feedback from the IOR
Project" by
Cowan et al. Available at http://www.epa.gov/greenchill/downloads
/IOR ReducingRefrigerantEmissions.pdf). Two ways in which global warming
impacts can
be reduced is through (a) increases in energy efficiency of heating and
cooling units (e.g.,
AT/P, wherein AT represents the change in temperature (T), and Pm represents
the electric
power represented in watts (W)) and (b) decreases in leak rates of
refrigerants.
[0008] Currently, HVAC (heating, ventilation, and air conditioning) and
refrigeration
accounts for much of the worldwide energy consumption. Unfortunately, it has
yet to be
appreciated that certain complexes can be used as a refrigerant to increase
energy efficiency
or decrease leak rates, thereby reducing the global warming impact caused by
existing
refrigerants. Thus, there is still a need for improved complexes and
refrigerant compositions.
Summary of The Invention
[0009] The inventive subject matter provides compositions, apparatus, systems
and methods
of haloalkene complexes that can be used for refrigeration, heating, air
conditioning, or any
other commercially suitable uses.
[0010] As used herein, the term "haloalkene complex" means two or more
molecules held
together through Van der Waals forces (also known as Van der Waals
interactions), wherein
at least one of the molecules is a haloalkene, or at least one molecule is a
haloalkane and at
least one molecule is an alkene. Preferably, the complex is formed upon
activation, in a
controlled environment, of at least one of the molecules. It is contemplated
that a haloalkene
complex's molecular arrangement can change when pressure is increased or
decreased.
[0011] In one aspect of the inventive subject matter, a composition comprises
a polar heat
transfer fluid, such as a hydrofluorocarbon, complexed with an organic oil
fatty acid. As used
herein, the term "organic oil fatty acid" can include a fatty acid of an
organic oil or a fatty
acid of an organic oil blend. Preferably, the heat transfer fluid is complexed
with at least
2
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
some of the organic oil fatty acid through Van der Waals forces, upon
activation of the fatty
acid under heat and pressure.
[0012] A polar heat transfer fluid can be complexed with at least 1, 5, or
even 10 or more %
of a first organic oil of a composition. This complexing can exist between
polar heat transfer
fluid molecules and fatty acids of the oil blend via Van der Waals forces. In
some
embodiments, the fatty acid molecules of an oil blend are activated in an open
or closed
vessel under heat and pressure. In some embodiments, a composition made by
this
complexing can comprise approximately 95-99 weight percent (wt%) of the polar
heat
transfer fluid, and approximately 1-5 wt% of the oil blend. Thus, the
composition can
comprise an oil blend to polar heat transfer fluid ratio of 1:99 or 5:95, or
any ratio in between.
Moreover, all commercially suitable ratios of polar heat transfer fluid to oil
blend is
contemplated, including for example: 0.1: 99.9; 10:90; 25:75; 50:50; 75:25; or
99:1 (e.g.,
where a small amount of polar heat transfer fluid is included with the oil
blend, in the vessel,
under heat and pressure, etc.), among others.
[0013] It should be appreciated that the oil complexes contemplated herein
include food and
other natural oils, as well as synthetic oils.
[0014] As used herein the term "fatty acid" refers to a substituted or non-
substituted,
saturated or unsaturated, carboxylic acid with a long aliphatic tail (chain).
This would
include, for example, a fatty acid ester, a fatty acid having no double bonds,
and a fatty acid
having multiple double bonds. As used herein a simple fatty acid is a non-
substituted,
saturated or unsaturated fatty acid. Oleic acid and linoleic acid are examples
of simple fatty
acids. It is contemplated that the inventive concepts herein, including those
embodied in the
originally filed claims, could apply to the more general type of fatty acid,
and to simple fatty
acids.
[0015] In some aspects of the inventive subject matter, at least .1 wt%, 1
wt%, 2 wt%, at least
3 wt%, at least 4 wt%, at least 5 wt%, at least 10 wt%, at least 15 wt%, at
least 20 wt%, at
least 50 wt%, or at least 95 wt% of the heat transfer fluid of a composition
is complexed with
an organic oil fatty acid. A heat transfer fluid can be complexed with at
least 1%, at least 5%,
at least 10%, at least 25%, at least 50%, or at least 80% of the fatty acid
composing the
composition.
[0016] Each of the organic oils or the oil blend as a whole can compose at
least 0.1 wt%, at
least 1 wt%, at least 2.5 wt%, at least 5 wt%, at least 10 wt%, at least 15
wt%, at least 20
wt%, at least 25 wt%, at least 50 wt%, or at least 95 wt% or more of the
composition. A heat
3
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
transfer fluid can be complexed with at least 1%, at least 5%, at least 10%,
at least 25%, at
least 50%, or at least 80% of the organic oil(s) composing the composition.
[0017] In some embodiments a first fatty acid (e.g., linoleic acid or oleic
acid, etc.) can
compose at least 0.1 wt%, at least 1 wt%, at least 2.5 wt%, at least 5 wt%, at
least 10 wt%, at
least 15 wt%, at least 20 wt%, or at least 25 wt% of a composition. In less
preferred
embodiments, the first fatty acid can compose less than 0.1 wt% of the
composition.
[0018] Contemplated compositions can comprise two or more different organic
oils, and each
organic oil can comprise one or more fatty acids having one, two, three, or
even more carbon-
to-carbon double bonds. In some embodiments, the fatty acid(s) compose at
least one food oil
of an oil blend, including for example, walnut, canola, sunflower or almond
oil.
[0019] The polar heat transfer fluid can comprise any commercially suitable
heat transfer
fluid, but is preferably a hydrofluorocarbon, and even more preferably a halo-
ethane such as a
tetrafluoroethane.
[0020] At least one of the organic oil(s), the fatty acid(s) and the polar
heat transfer fluid can
be activated in any suitable apparatus, including for example, a tube or pipe
or closed vessel
apparatus comprising at least one of a copper, nickel, palladium, zinc,
platinum, rhodium,
iridium, or an alloy thereof, or a copper mesh, a steel mesh, or Nylon scrub
pads. It is also
contemplated that the activation can occur under heat and pressure. As used
herein, the term
"under heat and pressure" means at least 15 C, and at least 1.25 atmosphere
(atm). Other
contemplated heating temperatures include at least any of 20 C, 30 C, 50 C,
100 C, 150 C,
or even 200 C or more. Other contemplated pressures include at least any of
1.5 atm, 5 atm,
atm, 25 atm, 100atm, or even 150 or more atm. Where an oil blend is activated
(e.g., in a
closed vessel having a catalyst), it is contemplated that the oil blend can be
a composition of
the inventive subject matter, even without the addition of a polar heat
transfer fluid.
[0021] In one aspect, a small amount of polar heat transfer fluid can be added
before or
during activation of an oil blend such that at least some of the polar heat
transfer fluid
molecules are complexed with a fatty acid molecule of the activated oil blend.
It is also
contemplated that a small amount of polar heat transfer fluid can be added
shortly after
activation (e.g., within one hour, within two hours, etc.). Still further, the
activated oil blend
and small amount of polar heat transfer fluid can then be injected into a
large quantity of the
polar heat transfer fluid for further complexing.
4
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
[0022] It is contemplated that a composition of the inventive subject matter
can have a
superior compressibility factor than existing refrigerants and refrigerant
compositions.
[0023] In some embodiments of the inventive subject matter, 0.1 to 95 wt% of
1,1,1,2-
tetrafluoroethane (also known as r-134a) is mixed with 27 to 99.9 wt% of one
or more
organic oil(s), and at least 0.1 % of the r-134a is complexed with some of the
organic oil(s)
via Van der Waals forces (e.g., the r-134a interacts with a hydrogen of a
carbonyl group of a
fatty acid of the organic oil, or a carbon-to-carbon double bond of the
organic oil). Without
wishing to be limited to any particular theory or mechanism of action, it is
contemplated that
an absorptive process can occur wherein the r-134a is complexed to the fatty
acid(s) of the
organic oil(s) via a Van der Waals force attraction to the carbon-to-carbon
double bonds, and
that such complexing can tend to inhibit oxidation or other deterioration of
the fatty acid.
[0024] The double carbon bond is a relatively stable zone, where the atoms on
either side
generally do not spin as rapidly about as with comparable singly bonded
carbons. This is
borne out in experimental data, where the complexing of an r-134a molecule
with a double
carbon bond of a fatty acid can create a unique signature that is detectable
with H-NMR and
x-ray diffraction. While not wishing to be limited by any particular mechanism
of action or
theory of operation, in this or other recitations of theory herein, it appears
that some type of
significant complexing is taking place when the activated oil blend is
dissolved in r-134a.
[0025] In some embodiments, the r-134a can be mixed with 27 to 99.9 wt% of at
least two
different organic oils. It is contemplated that the first and second organic
oils can be activated
in a tubing apparatus under a heat of 15 to 200 or more C and a pressure of 1
to 150 or more
atm for a period of time between one minute and twenty-four or more hours.
This activation
can occur prior to mixing and/or complexing with the r-134a, or can occur with
r-134a
already mixed with the first and second organic oils (e.g., the oils and at
least some of the r-
134a can be activated and complexed within the apparatus). It is also
contemplated that the
oils can be activated first, and mixed / complexed with r-134a at a later time
(ranging from
immediately after activation to days, months, or even years later).
[0026] Various objects, features, aspects and advantages of the inventive
subject matter will
become more apparent from the following detailed description of preferred
embodiments,
along with the accompanying drawing figures in which like numerals represent
like
components.
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
Brief Description of The Drawing
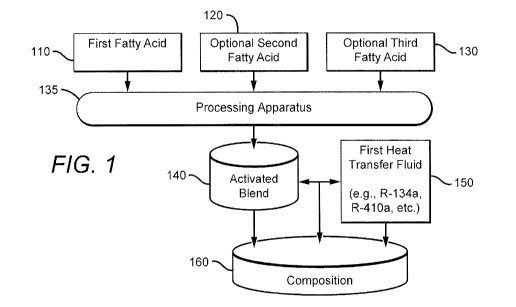
[0027] Fig. 1 is a schematic illustrating the production of a composition of
the inventive
subject matter.
[0028] Fig. 2A shows the chemical structure of a fatty acid molecule (oleic
acid).
[0029] Fig. 2B is a front perspective view of a r-134a molecule complexed with
an fatty acid
molecule (oleic acid).
[0030] Fig. 2C is a top perspective view of a r-134a molecule complexed with a
fatty acid
molecule (oleic acid).
[0031] Fig. 3 is a schematic of a r-134a molecule.
[0032] Fig. 4 is a chart showing a side by side comparison of 3 ton units
running
continuously using BluonTM TdX versus r-22 refrigerants.
[0033] Fig. 5 is a chart showing a side by side comparison of 3 ton units
running
continuously using BluonTM TdX versus r-410a refrigerants.
[0034] Fig. 6 is a schematic of a typical refrigeration cycle.
Detailed Description
[0035] The following discussion provides many example embodiments of the
inventive
subject matter. Although each embodiment represents a single combination of
inventive
elements, the inventive subject matter is considered to include all possible
combinations of
the disclosed elements. Thus if one embodiment comprises elements A, B, and C,
and a
second embodiment comprises elements B and D, then the inventive subject
matter is also
considered to include other remaining combinations of A, B, C, or D, even if
not explicitly
disclosed.
[0036] It should be noted that while the below description sometimes focuses
on a B1 TM oil
blend, and BluonTM TdXTm (B1 oil blend injected into a large quantity of r-
134a), the
inventive subject matter should be interpreted to include other combinations
of halo-alkene
complexes comprising a heat transfer fluid and a fatty acid.
[0037] The inventive subject matter should be interpreted to also include a
refrigerant
composition comprising a polar heat transfer fluid that is passed through a
catalyst in an open
or closed vessel.
6
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
[0038] Still further, the inventive subject matter should also be interpreted
to also include an
activated oil blend that can be used with various other fluids (e.g., non-heat
transfer fluids)
having molecules that are sufficiently polar to complex with a fatty acid of
the activated oil
blend. Examples include hydraulic oils, antifreeze or other suitable fluids
having a liquid or
gas composition that dissipates heat in mechanical environments.
[0039] Figure 1 is a schematic illustrating how a composition of the inventive
subject matter
could be made. First, second and third fatty acids (110, 120, 130) composing
at least one of
first, second and third organic oils are combined and processed under heat and
pressure, in
processing apparatus 135 having a controlled environment, to form an activated
blend 140 of
organic oils.
[0040] The controlled environment under which one or more of the fatty acids
are processed
can include, among other things, predetermined materials, temperatures,
pressures, or times.
One example of a predetermined material can comprise material that the
processing apparatus
composes (e.g., copper, iron, steel, wood, plastic, etc.), or a catalyst
inserted into the
processing apparatus. A predetermined temperature or pressure can be the
temperature/pressure or range of temperatures/pressures that the organic
oil(s) or fatty acid(s)
are exposed to during processing. A predetermined time can be the length of
time the organic
oil(s) or fatty acid(s) are processed, the length of time the organic oil(s)
or fatty acid(s) are
processed under a given temperature, the length of time the organic oil(s) or
fatty acid(s) are
processed under a given pressure, and so forth.
[0041] Examples of fatty acids include for example, oleic acid, linoleic acid,
linolenic acid,
myristoleic acid, palmitoleic acid, sapienic acid, elaidic acid, vaccenic
acid, arachidonic acid,
eicosapentaenoic acid, erucic acid, docosahexaenoic acid, and palmitic acid,
linolaidic acid,
and a-linolenic acid. In some embodiments, unsaturated fatty acids are
preferred. Each acid
can be derived from any suitable source, including for example, an organic oil
(e.g., a plant
oil, food oil, etc.).
[0042] As used herein, an "organic oil" is any oil produced by plants,
animals, and other
organisms through natural metabolic processes other than crude oil or
petroleum-based oils.
Contemplated food oils include walnut oil, almond oil, canola oil, flaxseed
oil, beech nut oil,
coconut oil, cottonseed oil, olive oil, palm oil, peanut oil, safflower oil,
sesame oil, soybean
oil, sunflower oil, cashew oil, hazelnut oil, macadamia oil, pecan oil, pine
nut oil, pistachio
oil, grapefruit seed oil, lemon oil, orange oil, pumpkin seed oil, watermelon
seed oil, or any
other suitable food based oil. It is contemplated that a composition having
only a single type
of fatty acid (or predominantly a single type of fatty acid) can comprise a
higher or lower
7
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
wt% of the fatty acid (or the organic oil(s) comprising the fatty acid)
depending on the type
used. For example, a composition having only (or predominantly) oleic acid can
have less
than, twice as many, or even three times or more fatty acids than a
composition having only
(or predominantly) linoleic acid, or some other acid.
[0043] It should also be noted that it may be possible to manufacture a wide
variety of
synthetic oils that can be activated and complexed with a polar heat transfer
agent. Such oils
could have an odd number of carbons, an even number of carbons, no double
carbon bonds,
two or more double bonds, etc.).
[0044] Once the fatty acid (or oil comprising the fatty acid) is processed and
activated, the
activated blend 140 can be infused, injected into, or otherwise combined with
first heat
transfer fluid 150 to produce composition 160 comprising a halo-alkene complex
having Van
der Waals interactions. As discussed above, a small amount of the heat
transfer fluid could
have been mixed with the fatty acids in the processing apparatus, and
complexed therein
upon activation of the fatty acids.
[0045] As used herein, the term "Van der Waals force" or "Van der Waals
interaction" means
the sum of the attractive or repulsive forces between molecules (or between
parts of the same
molecule), other than those due to covalent bonds, or the electrostatic
interaction of ions with
one another or with neutral molecules. It is true that some authorities use
the term more
narrowly to exclude hydrogen bonding, but as used herein the term can include
hydrogen
bonding, forces between two permanent dipoles (Keesom force), forces between a
permanent
dipole and a corresponding induced dipole (Debye force), and forces between
two
instantaneously induced dipoles (London dispersion force).
[0046] All commercially suitable heat transfer fluids are contemplated,
including for
example, methane-based (r-(000-099)) refrigerants, ethane-based (r-(100-199))
refrigerants,
propane-based (r-(200-299)) refrigerants, cyclic organic (r-(300-399))
refrigerants, zeotropes
(r-(400-499)), azeotropes (r-(500-599)), organic (r-(600-699)) refrigerants,
inorganic (r-(700-
709)) refrigerants, and unsaturated organic (r-(1000-1099)) refrigerants.
[0047] It is contemplated that a composition of the inventive subject matter
can be used in an
existing refrigeration system that is compatible with r-134a, r-407, r-410 or
r-22, or some
other refrigerants. However, some modifications, preferably minor, can be
required (e.g., a
small part change, addition, etc.). An inferior refrigerant can be completely
removed from the
system, and the system can be recharged with a composition of the inventive
subject matter.
Moreover, a composition of the inventive subject matter can be added to a
system without
8
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
complete removal of a prior refrigerant from the system. This is due to the
fact that the
compositions appears to be more energy efficient and self-sealing than
existing refrigerants,
even when combined with one or more contaminants (e.g., an inferior
refrigerant or
refrigerant composition, such as r-134a, r-407, r-410, r-22, etc.).
[0048] Moreover, a composition of the inventive subject matter could be used
in a novel unit
comprising a different ratio of compressor size to coil size. For example, as
compared to an
existing refrigeration unit having a compressor size to coil size ratio of
X:Y, a new unit can
have a ratio of X-Z:Y, X+Z:Y, X:Y-W, or X:Y+W, wherein Z is at least 10%, 20%,
30%,
50%, or even 75% or more of X, and wherein W is at least 10%, 20%, 30%, 50%,
or even
75% or more of Y. As another example, a new unit can have a greater number of,
or a
different configuration of, coils.
[0049] One possible composition of the inventive subject matter is the novel
BluonTM
TdXTm. Bluon TdX comprises a mixture of approximately 95-99 wt% of 1, 1, 1, 2-
Tetrafluoroethane (i.e., r-134a) at least partially complexed with
approximately 1-5 wt% of
B1 TM, a non-toxic oil blend comprising one or more organic oils, wherein the
oil blend has an
oleic acid to linoleic acid ratio of between 70:30 and 50:50. It can be
preferred that the ratio
of oleic acid to linoleic acid is approximately 60:40 wt%. In some other
embodiments, a
composition can comprise an oil blend comprising an oleic acid to linoleic
acid ratio of up to
approximately 0.1:1, 0.5:1, 2:1, or even 60:1 or more. The organic oils of B1
can include one
or more of a canola oil, a walnut oil, an almond oil, and a sunflower oil,
among others ("the
B1 oils"). One contemplated B1 blend comprises walnut, almond and canola oils
("CAW B1
blend"). Another contemplated B1 blend comprises canola and sunflower oil ("CS
B1
blend"), preferably at an approximate ratio of between 5:1 and 1:3, and even
more preferably
at an approximate ratio of between 5:1 and 2:1 (e.g., 3:1). Yet another
contemplated B1 blend
comprises walnut, almond and canola oils, and a small amount of r-134a.
[0050] A perspective view of a fatty acid molecule composing a preferred oil
blend of the
inventive subject matter (e.g., Bloil blend) is shown in Figure 2A.
Perspective views of a r-
134a molecule complexed with a fatty acid molecule are shown in Figures 2B-2C.
[0051] It appears that the complexing can occur in two steps. The first step
occurs when the
two positively charged hydrogen atoms of r-134 Van der Waals interact with an
exposed
negatively charged double carbon bond, to form a shared triad/quad. This is a
relatively weak
form of Van der Waals interaction and relies on surface reaction chemistry to
form. This
relatively weak interaction could explain an observed effervescence.
9
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
[0052] A second stage bonding apparently occurs when the extremely negatively
charged
fluoride, attached to the same carbon of the r-134a with the two hydrogens,
then bonds to the
two positively charged hydrogen atoms which are attached to the two carbons of
the double
carbon bond. A synergistic effect of the two oppositely charged/aligned triads
can have an
overall strengthening effect and could lock in this multi-interaction.
[0053] As the presence of r-134a bound to the B1 oils increases, the viscosity
of Bluon TdX
can also increase. As discussed above, any commercially suitable
refrigerant(s) can be
infused with any suitable oil or oil blend comprising a fatty acid to produce
a composition of
the inventive subject matter. Thus, the activated oils and specific complexes
discussed in
detail herein are only some of the possible compositions of the inventive
subject matter.
[0054] On the one hand, R-134a has been shown experimentally to provide the
most
significant improvement in refrigeration efficiency when mixed with the oils
of a B1 oil
blend, possibly due to its highly polar nature as compared with other
refrigerants. In
particular, a mixture comprising approximately 95-99 wt% of r-134a and
approximately 1-5
wt% of B1 (which can also include approximately 50% of an oleic acid and 33%
of a linoleic
acid) was found to be very efficient.
[0055] The B1 oils of one possible B1 blend comprising walnut oil, almond oil,
and canola
oil, the CAW blend, are quite similar in chemical composition, as shown in
Tables 1A-B
(below). The Oleic acid accounts for approximately 50% of the "fatty acids" in
the B1 blend
(comprising precursor/feedstock oils) and are an Alkene with an 18 long carbon
chain. Oleic
acid has one double carbon bond. Linoleic acid accounts for around 34% of the
fatty acids in
the blend and is also 18 carbons long, with two double carbon bonds. Linolenic
acid is around
9% of the fatty acids in the blend and is 18 carbons long, with three double
carbon bonds.
Palmitic acid is around 5% of the fatty acids in the blend and is 16 carbons
long.
Feedstock Oil Ratios
Walnut Oil Almond Oil Canola Oil
Oleic Acid 28% 69% 61%
Linoleic Acid 51% 17% 21%
Linolenic Acid 5% - 9%
Palmic Acid 11% 7% 4%
CA 02866711 2014-09-08
WO 2013/134603
PCT/US2013/029786
Blend for B1
Total Weighted by Carbon #
Carbon Type
Bonding Sites
Oleic Acid 49.30% 34.50% 18C Alkene
Linoleic Acid 33.30% 45.00% 18C Alkene
Linolenic Acid 8.67% 18.30% 18C Alkene
Palmic Acid 5.30% 0.00% 16C Alkane
[0056] These food oils predominantly consist of relatively long-chain carbon
molecules or
fatty acids bonded to a glycerol. Fatty acids in free form have a carboxyl
group (COOH) at
the first (Alpha) carbon on the carbon chain, making them carboxylic acids. In
plants, most
fatty acids are bonded in triplets to a glycerol molecule to form a
triglyceride. A triglyceride
can have different types of oils in various arrangements attached to it. Oleic
fatty acids in
some plants tend to be mostly bonded in di-glycerides, especially those
derived from
rapeseed oil (Canola Oil). Mono-glycerides are only present in significant
amounts in a few
plants, such as peanuts. In common practice, the tri, di or mono-glycerides
are ignored and
only the fatty acid or "oil" content is listed. This is due to the glyceride
fatty acid bond being
esterified before most kinds of chemical testing, allowing for the various
fractions of fatty
acids to be accurately measured.
[0057] One important discovery from an H-NMR application was the presence of
complexed
R -134a to the Bluon TdX oils (e.g., of the B1 oil blend) by inter-molecular
hydrogen
bonding and Van der Waals forces. The chemical complexing of the r-134a to the
oils leaves
a detectable signature, and is relatively stable and remains in tact even
after days in a
depressurized state. Surprisingly, the amount of tightly complexed r-134a to
the Bluon TdX
oils apparently increased over time when used in an air conditioning system,
thereby
inhibiting degradation of the oils.
[0058] A catalyst can be used to cause a reaction between the r-134a and a
fatty acid. When
r-134a is bubbled intensively through the oil, it is possible that no reaction
occurs, even at
300 degrees F and over long periods of time. This is likely due to the rapid
spinning along the
axis of the carbon to carbon single bonds on both the r-134a and fatty acid
molecules. In the
liquid oil, the singly bonded carbons can spin relative to each other many
thousands of times
11
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
a second. In the r-134a gas, the relative spin rate can be magnitudes faster,
and it is likely that
the two molecules simply bounce off each other.
[0059] When a catalyst is present (e.g., above the sparge, etc.), a rapid
reaction can occur,
even at room temperature. Pressure in the reaction chamber can rapidly drop
while
temperature rises, thereby evidencing an exothermic reaction. The type of
chemistry
occurring can include surface reaction chemistry. When inert gasses such as
Nitrogen were
run through the chamber with the catalysts, no reaction was observed.
[0060] When a fatty acid is at least partially immobilized on copper or other
activation
surface through Van der Waals forces, the carbon to carbon single bond
spinning is vastly
reduced. This reduction is also true for the r-134a when it reacts with the
surface. This allows
the Keeson and Debye forces to predominate, and Van der Waals absorption of
the r-134a
onto the oil occurs. This reaction occurs in an extremely short interval of
time, before the
product is swept off the surface into the mass of the oil blend. The source of
heat observed
during the reaction is likely from the heat released due to the phase change
of the r-134a from
a gas to a liquid.
[0061] In opening the reactor chamber and passing a copper mesh through the
freshly
absorbed complexes, effervescence can be observed. However, this phenomenon
goes away
over time without evidence of degassing into the reactor chamber. It appears
that the initial
absorption Van der Waals interaction / complexing changes to a different
stronger Van der
Waals complexing over time. This was evidenced by a strong r-134a signature
even in Bluon
TdX that was weeks old and suspending over boiling water in test tubes for
hours. Nor was a
weakening of the r-134a signature observed when the Bluon TdX was exposed to
the
atmosphere over a long period. No significant degassing was observed after
approximately
two weeks.
[0062] Over time the measurable signature of the r-134a in the oil measurably
increased
when used in an air conditioning unit. The signature appears to increase along
with the
repeated mixing of the oil and r-134a through normal machine operations. A
noticeable
increase in viscosity and change in color can also occur with an increasing r-
134a signature.
An end of r-134a that sticks out can apparently form ever shifting double
hydrogen bonds
with the numerous hydrogen atoms of other oil molecules, which increases
viscosity. The
complexing apparently does not remove or replace any atoms on either the r-
134a or fatty
acid molecules, as the signatures of both molecules remained.
12
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
[0063] In some testing, prior to use in a refrigeration system, Bluon TdX
shows a slight
presence of two quartets at 4.7 and 4.58 in the H-NMR, indicating r-134a
bonding. Bluon
TdX that was used for 120 days showed much more pronounced quartets at these
sites. This
shows more r-134a is binding to the oils over time, indicating that Bluon TdX
grows even
better with use, at least up to a certain point.
[0064] The molecular Van der Waals behavior of these new halo-alkenes also has
been
shown to change over time. The halo-alkene Bluon TdX recovered from unused
samples is a
clear yellow viscous liquid. This clear yellow color indicates whatever bonded
water existed
in the CAW B1 blend, has been expunged. The Bluon TdX liquid is also more
viscous than
the CAW Bl, flowing at a noticeably slower rate. The blue-green color of the
120 day used
Bluon TdX, indicates that as more r-134a binds to the oils, intra-molecular
(resonant
frequencies) rise, along with viscosity. In testing of the fresh Bluon TdX,
the oil was very
hydrophobic and would not mix with any amount of water.
[0065] A composition of the inventive subject matter can produce the same
amount of
heating or cooling in a system using less than 90%, less than 75%, less than
50%, or even less
than 33% of conventional refrigerants (e.g., r-134a, r-410, r-22, etc.). For
example, sensor
arrays and data streams recorded show that Bluon TdX can produce the same
amount of
cooling in a system for somewhere between 35% and 60% of the wattage compared
to some
conventional refrigerants. A composition of the inventive subject matter can
also keep a
space colder or hotter for longer periods of time than conventional
refrigerants. For example,
it has been found that Bluon TdX can keep a space colder or hotter for longer
periods of time
than existing refrigerants or refrigerant compositions. Thus, a system
utilizing Bluon TdX or
other composition of the inventive subject matter can provide the same cooling
or heating as
a system utilizing r-410, while running for approximately 10-30 minutes less
per hour.
Moreover, refrigeration units and systems charged with compositions of the
inventive subject
matter (e.g., Bluon TdX) can produce significantly less condensation off
evaporate coils. For
example, over an eight hour test run of two air conditioning systems, an r-
410a charged
system had an evaporator coil temperature of 55.2 degrees F and condensate of
5.75 gallons,
while a Bluon TdX charged system had an evaporator coil temperature of 51.4
degrees F and
condensate of 1 gallon. This phenomenon of reduced condensation was observed
in each
Bluon TdX charged air conditioning system. This highly unusual electron
resonant effect
appears to contribute in making Bluon TdX a novel and very unique halo-alkene.
The drop in
condensation is a contributing factor to the greatly increased efficiency of
Bluon TdX
charged systems.
13
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
[0066] R-134a is unique among the fluorocarbon refrigerants, in that it is
also used as a
solvent in the pharmaceutical industry. This solvent ability is due to the
polar nature of its
molecule as shown in Figure 3. One side of the molecule has the negatively
charged fluoride
atoms, while the other side has the positively charged hydrogen. The polar
nature of water
also makes it an excellent solvent.
[0067] It should also be noted that Debye or other Van der Waals forces can be
quite strong
between or among long chain oils (triglycerides). This attraction is why these
oils are liquid
over such a wide range of temperatures and have such a high vaporization point
(boiling
point). These characteristics are useful for frying and evidently
refrigeration.
[0068] Figure 4 is a series of charts representing a side by side comparison
of two 3 ton
units, one running continuously using Bluon TdX, and the other running
continuously r-22
refrigerant. The Bluon TdX comprises a CAW B1 oil blend. Figure 5 is a series
of charts
representing a side by side comparison of two 3 ton units, one running
continuously using
Bluon TdX, and the other running continuously r-410a refrigerant. Again, the
Bluon TdX
comprises a CAW B1 oil blend.
[0069] As shown in Figure 6, air conditioning systems generally utilize a
refrigerant cycle
having two main parts, the condenser cycle and the evaporator cycle. The
following
description is of a standard air conditioner system. The condenser cycle
starts at the
compressor, where the warmed gas from the evaporator cycle is compressed back
into a
semi-liquid. This semi-liquid is then pumped through condenser coils, where a
fan removes
the heat into the outer environment and the gas becomes fully liquefied. This
liquefied cooled
fluid then flows to the expansion valve, where it changes from a liquid into a
gas and
adiabatically cools. This cooled gas then flows into the evaporator coils,
were a fan blows
cooled air into the controlled environment and the gas is warmed.
[0070] Increased pumping efficiency in the compressor, is likely the most
significant cause
of the increased efficiencies of Bluon TdX and other compositions of the
inventive subject
matter. One reason for this increased efficiency is the highly viscous
characteristics of the oil
blends of the inventive subject matter (e.g., CAW Bl, CS Bl, etc.). The oil
blends (and thus
the Bluon TdX) can increase the sealing around the piston in a reciprocal
pump, the spinning
blades in a centrifugal pump or internals of a scroll pump, over commonly used
mineral oils.
Another minor reason, is it takes less energy to pump an incompressible
liquid, than it takes
to pump a compressible gas. The oil blend in the Bluon TdX is always or almost
always
going to be liquid, as the temperature of the oils will never come remotely
close to their
vaporization points. Some atomization likely occurs at the expansion valve,
but will quickly
14
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
re-liquefy onto the internal surface of the evaporator. The r-134a is driven
into a liquid at the
compressor and also likely dissolves more rapidly into the Bluon TdX oil
blend, than a
mineral oil. At this higher pressure, Van der Waals forces would likely
complex the r-134a to
the oils, in much the same manner the oils are bonded to each other in a
liquid state.
[0071] After leaving the condenser, the cooled liquid reaches the expansion
value and the r-
134a can begin its transition into a gas. The phase transition can be driven
to completion in
the evaporator coils. This is also likely where a secondary cause of increased
efficiencies of
compositions of the inventive subject matter (e.g., Bluon TdX) is found. It is
a unique
physical process likely dependent on r-134a's polar interaction with the
structure of the
particular oils. Some preparatory discussion is necessary to delve into this
unique process.
[0072] Polarity, solvent ability and heat capacity in molecules are closely
related. Due to the
unique structure of r-134a and the C=C/C=0 binding sites on the mixed oils, as
well as Van
der Waals dispersion forces, a sharing of heat capacity can occur during the
fully liquid
phase. In some preferred oil mixtures, the ratio of oleic acid to linoleic
acid is approximately
3:2. In other preferred oil mixtures, the ratio of oleic acid to linoleic acid
can be up to
approximately 60:1 or more. These two acids have quite different heat
capacities despite their
close chemical structure of 18 carbon units. This is due to the number of
double (C=C)
carbon bonds. Oleic acid has a heat capacity of 2.88 kJ/(kg=K) (kilojoules
perKilogramsK), to
linoleic acid's heat capacity of 0.37 kJ/(kg=K).
[0073] R-134a is only two carbon units long and its heat capacity is 1.34
kJ/(kg=K). Although
smaller than Oleic acid, the key to r-134's usefulness is its heat of
vaporization at
approximately -15.3 F (boiling point) at atmospheric pressure. It can
transform from a liquid
to a gas phase around the temperatures useful for cooling, allowing it to
efficiently shed heat.
This is a key to any good refrigerant. The fatty acid oils cannot do this, due
to their extremely
high heat of vaporization. Linolenic acid has a very low heat of vaporization
at 450 F.
[0074] On the other hand, these organic oil fatty acids can generally have
melting points
around the temperatures that air conditioning unit evaporators operate. Oleic
acid has a
melting point of approximately 55 F, while that of linoleic acid is
approximately 23 F and
linolenic acid is at approximately 12 F. For r-134a, the relevant value is
heat of vaporization
at approximately -15.3 F. The expansion valves on standard air conditioner
units are
generally adjusted to take the evaporator toward the freezing point of water,
but not so cold
that ice forms on the outer surface of the evaporator. Therefore, the r-134a
is not going to
reach its full potential cooling, but will vaporize above the melting points
of the high acid
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
oils. The oils in the Bluon TdX are generally almost always or always going to
be liquid,
although some atomization likely occurs at the expansion valve.
[0075] This is also supported by triglycerides generally having a lower
melting point than
their constituent fatty acids. In testing the Bluon TdX for behavior at 32 F
and even down to
14 F, the Bluon TdX flowed sluggishly, but did not freeze. This indicates the
Bluon TdX and
some other compositions of the inventive subject matter will not freeze in the
evaporator unit
of the air conditioner and will remain a liquid throughout the cycle. At high
pressure, r-134a
is a liquid, but evaporates from the oils at the expansion valve., R-134a will
preferentially
carry away much of the heat of the oils, allowing the oils to act as a
secondary "assistant"
refrigerant.
[0076] Another reason for the significant increase in refrigerant efficiency
can be attributed
to surface binding of the Bluon TdX, and other compositions of the inventive
subject matter,
to the metal of the refrigerant system. This is evident from the fact that
when a unit was
switched from Bluon TdX to r-410a, there was a temporary improvement in
efficiency, most
likely due to the Bluon TdX halo-alkene complexes closely binding to the
internal surfaces of
the cooling system, until it was removed by the various constituents of r-
410a. A smaller
amount of efficiency is also gained by this lubrication effect, due to the
smoother flow of gas
and oils through the system.
[0077] This surface binding feature is also apparently responsible for the
observed reduced
refrigerant composition leakage from the air conditioning units. Most air
conditioning system
components were designed to use the larger Freon 113 (C2C13F3), until it was
banned due to it
possibly damaging the ozone layer. The significant leakage problems with r-
410a or r-134a
are due to their smaller molecular geometry than the Freon 113 they were
designed to
replace. The fluorine atoms of r-410a and r-134a are much smaller than the
chlorine atoms of
Freon 113. Air conditioning systems charged with r-410a typically leak around
20% of the
coolant into the atmosphere annually, r-134a has a slightly lower leakage
rate. This is why
car air conditioners, almost exclusively use r-134a, and need to be recharged
every few years.
However, the leakage rate of r-134a is still significantly higher than the
leakage rate of Bluon
TdX and other compositions of the inventive subject matter.
[0078] The likely physical process by which this leakage is reduced, is
through the larger
halo-alkene complexes efficiently filling any small fissures between the
seals. Thus, the
Bluon TdX partially or substantially seals the system utilizing it, and
reduces the need to
recharge the system. Moreover, a system can be charged with approximately 35-
50% less
Bluon TdX (or other compositions of the inventive subject matter) than the
installed
16
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
refrigerant, such as r-22 or r-410a. The oils would stick to the rubber and
metals with even
stronger Van der Waals interactions than they stick to each other. As these
oil molecules are
held together by significant Van der Waals forces, they would greatly reduce
the passage of
any Bluon TdX out of the air conditioning system. This high Van der Waals
complexing
potential is apparently not applicable to the usual mineral oil lubricants
used in standard r-22,
r-410a or r-134a system.
[0079] The UN Montreal Protocols of 2009 call for phasing out various
refrigerants. In 2013,
the amount of some refrigerants produced is allegedly to be frozen. As
compositions of the
inventive subject matter can operate more efficiently and reduce leakage, they
can help
overcome these imposed production limitations. These complexes can help many
nations
achieve the goal of the Montreal Protocols faster.
[0080] Car manufacturers in Europe are reported to be in dire straits, since
they are mandated
to phase out r-134a in European cars, and they have no good alternatives. See
"Refrigerants
heat up in Europe" by Clay Boswell, found at
http://chemical.ihs.com/IHS/Public/NewsEventsArt/ PR
Articles/Feb08Refrigerants.pdf .
Bluon TdX and other compositions of the inventive subject matter have the
potential to help
solve their problem from several angles. An important factor to achieve this
is the inventive
subject matter's (e.g., Bluon TdX's) ability to significantly reduce leakage
in air conditioning
systems. Most automobile air conditioning systems will have several recharges
over their
lifetimes and Direct Emissions can be up to 40% of their Total Equivalent
Warming Impact
(TEWI). An average quality automobile air conditioning system will lose around
12% of its
refrigerant annually. From operating the test units using Bluon TdX, observed
leakage is
greatly reduced. If leakage could be reduced 90% by Bluon TdX, total TEWI in
automobile
air conditioning systems could be reduced by 35%.
[0081] Another more important factor is the increase in operating efficiencies
of the air
conditioning unit. The Indirect Emissions of automobile air conditioning units
are around
60% of the total TEWI in temperate regions and much more in the tropics.
Testing has shown
that only around 35% to 60% the wattage needed to run an air conditioning
system on some
other refrigerants is needed to run an air conditioning system on Bluon TdX.
This would
shave another 20% to 30+% off the TEWI. There is apparently not much that can
be done
about the Transportation Effect of the TEWI. In total, around 55% to 65+% of
TEWI could
be shaved off the standard r-134a automobile air conditioning system, if they
were converted
to Bluon TdX or another composition of the inventive subject matter. This
would reduce the
17
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
TEWI of a Bluon TdX system below that of a CO2 air conditioning system in most
parts of
the planet.
[0082] The amount of energy taken up by turning water vapor in the atmosphere
into a liquid
(enthalpy of condensation) is rather large, approximately 2.27 million J/kg
(joules per
kilogram). It is apparently more than ten times more enthalpy than any
refrigerant used inside
a system. There is a large energy drain as moisture or ice reduces air
interaction with the coils
of a refrigeration system, making them even less efficient at removing heat
from the air. A
significant benefit of compositions of the inventive subject matter (e.g.,
Bluon TdX) is that it
produces less than 1/3 of the condensation that standard air conditioning
systems produce,
thereby increasing a refrigeration system's efficiency and cooling efficacy.
Additional Disclosure
[0083] The inventive subject matter also provides compositions, apparatus,
systems and
method in which a refrigerant composition comprises a polar heat transfer
fluid, such as
1,1,1,2-tetrafluoroethane, which is passed through an open or closed vessel
containing a
catalyst.
[0084] The polar heat transfer fluid (or mixture described below) can be
passed through a
vessel under heat of at least 15 C, 20 C, 50 C, or even 100 C or more, or a
pressure of at
least 1.25 atm, 5 atm, 25 atm, or even 150 or more atm.
[0085] In some embodiments of the inventive subject matter, the catalyst can
comprise at
least one of a copper, a polyamide (e.g., Nylon, etc.), or a stainless steel.
A composition of
the inventive subject matter can comprise a mixture of a polar heat transfer
fluid with a long
chain fatty acid (i.e., fatty acid having more than 12 carbon molecules)
preferably activated
by the catalyst. Preferred long chain fatty acids are oleic and linoleic
acids, which can
advantageously be derived from or included in one or more food oils, including
for example
walnut oil, almond oil, sunflower oil, or canola oil.
[0086] Where polar heat transfer fluid is passed through a vessel as a mixture
with a long
chain fatty acid, it is contemplated that a catalyst in the vessel can
activate the fatty acid to
allow for complexing with polar heat transfer fluid molecules. Where a portion
of a long
chain fatty acid is complexed with a polar heat transfer fluid molecule, a
haloalkene complex
can result. Some contemplated haloalkene complexes can comprise a ketone or an
ester.
[0087] A mixture that is passed through a vessel can comprise any suitable
wt/wt ratio of the
food oil to polar heat transfer fluid, including for example, 10:1, 20:1,
50:1, 75:1, or even
99:1 or more. The polar heat transfer fluid can comprise 10, 5, 1, or even 0.1
or less wt
18
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
percent of the mixture passed through the vessel. The mixture, upon exiting a
vessel
comprising a catalyst, can be further mixed with any other suitable fluid,
including for
example, additional amounts of polar heat transfer fluid to thereby obtain a
composition of
the inventive subject matter.
[0088] The inventive subject matter also provides compositions, apparatus,
systems and
methods in which an activated oil blend having fatty acid molecules configured
to complex
with a molecule of another fluid is manufactured.
[0089] As used herein, the term an "oil blend" should be interpreted broadly
to include, for
example, a composition comprising two or more different types of oils, as well
as a
composition comprising one type of oil derived from different sources. Thus,
it is
contemplated that an oil blend can comprise only one type of oil (e.g., a
walnut oil) that is
extracted from two or more different sources (e.g., two different walnuts).
[0090] Activated oil blends of the inventive subject matter can be
manufactured by placing a
catalyst and one or more precursor oils in a vessel, and circulating the
precursor oil(s) in the
vessel with the catalyst. Some preferred catalysts include copper, a polyamide
such as Nylon,
and stainless steel. However, all catalysts effective to prepare a fatty acid
molecule for
complexing with another molecule are contemplated.
[0091] Contemplated oils for use in the claimed subject matter include long
chain fatty acids,
especially oleic and linoleic acids. Food oils containing long chain fatty
acids include, among
other things, canola oil, walnut oil, sunflower oil, flaxseed oil, and almond
oil. Where an oil
blend comprises oleic and linoleic acids, it is contemplated that the oleic
acid and linoleic
acid can be present in a weight to weight (wt/wt) ratio of between 80:20 and
20:80, a ratio of
between 70:30 and 50:50, or any other suitable ratio.
[0092] Some oils for use in the claimed subject matter can alternatively or
additionally
comprise other types of fatty acids, including for example, linolenic acid or
palmic acid. For
example, a contemplated oil blend can comprise: (1) at least 10 wt%, at least
20 wt% or even
at least 30 wt% or more of an oleic acid (2) at least 5 wt%, at least 10 wt%,
or even at least
20 wt% or more of a linoleic acid, (3) at least 1 wt%, at least 2 wt%, or even
at least 5 wt% or
more of a linolenic acid; (4) at least 1 wt%, at least 2 wt%, or even at least
5 wt% or more of
a palmic acid; or (5) any combination thereof
[0093] A refrigerant composition of the inventive subject matter can comprise
a mixture,
such as the ones described above, wherein at least some of the mixture is
blended in a vessel
19
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
containing a catalyst. Preferably, the refrigerant compositions comprise
haloalkene
complexes formed of molecules of a mixture of the inventive subject matter.
[0094] From a methods perspective, it is contemplated that a user can
manufacture, purchase
or otherwise obtain a refrigerant composition of the inventive subject matter,
and add a
sufficient amount of the refrigerant composition to a refrigeration system or
unit to improve
operation of the refrigeration system.
[0095] Where a user is adding a refrigerant composition of the inventive
subject matter to an
existing refrigeration system or unit, it is contemplated that the user can
optionally at least
partially remove a previously utilized commercial refrigerant from the unit
prior to adding the
novel refrigerant composition. As used herein, the term "at least partially
remove" should be
interpreted broadly to include, for example, complete removal, substantially
complete
removal, or even removal of at least 25%, at least 50%, or even at least 75%.
[0096] It is contemplated that the user can at least partially remove the
previously utilized
commercial refrigerant using any suitable method, including for example, vapor
recovery or
liquid recovery methods.
[0097] Operation of a refrigeration system can be improved by adding a novel
refrigerant
composition comprising a mixture of activated long chain fatty acids with a
polar heat
transfer fluid. Activation is preferably accomplished by blending a mixture of
the oils and
the heat transfer fluid in a vessel containing a catalyst. Contemplated
improvements can
include, among other things, a reduction in power consumption, a reduction in
evaporator coil
condensation, or a reduction in leakage of operating fluid.
[0098] When an operating fluid is run through a refrigeration unit, it is
contemplated that the
pressures and concentrations of the operating fluid can be such that the oil
blend is a liquid
upon exiting the condenser, and a gas when exiting the evaporator.
[0099] Depending at least in part on the specific refrigerant composition(s)
obtained and
added to a refrigeration system or unit, it is contemplated that a method of
the inventive
subject matter can improve operation of the refrigeration system in at least
one of the
following ways: (1) reducing power consumption of a refrigeration unit
relative to a given
load on a month over month basis (e.g., by at least 10%, by at least 25%, by
at least 45%, by
at least 50%, etc.); (2) reducing evaporator coil condensation rate of a
refrigeration unit
relative to a given load on a month over month basis (e.g., by at least 10%,
by at least 25%,
by at least 45%, by at least 50%, etc.); or (3) reducing leakage of operating
fluid from a
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
refrigeration unit relative to a given load on a month over month basis (e.g.,
by at least 10%,
by at least 25%, by at least 45%, by at least 50%, etc.).
[00100] Activated oil blends can optionally be manufactured to include a
hydrocarbon,
such that fatty acid molecules are activated and complexed with the
hydrocarbon in a vessel
having a catalyst. Preferred hydrocarbons can comprise hydro-halo-carbons such
as a
hydrofluorocarbon heat transfer fluid. One exemplary hydrofluorocarbon
includes r-134a,
chemically known as 1,1,1,2-tetrafluoroethane. In one aspect of the inventive
subject matter,
the hydro-halo-carbon can comprise at least 20 wt%, at least 50 wt%, at least
75 wt%, or even
at least 90 wt% or more of an operating fluid.
[00101] Regardless of whether or not an activated oil blend comprises a
hydrocarbon, it is
contemplated that oil blends of the inventive subject matter can be injected
into or otherwise
mixed with larger quantities of hydrocarbon. For example, an activated oil
blend can be
combined with r-134a molecules that are configured to complex with the
activated fatty acid
molecules of the oil blend to thereby produce a refrigerant composition.
[00102] The inventive subject matter also provides refrigeration systems in
which an
operating fluid is moved between a condenser and an evaporator, and the
operating fluid
preferably comprises an activated oil blend and a polar heat transfer fluid.
Refrigeration
systems are configured to utilize an operating fluid comprising an activated
oil blend. The
activated oil blend can comprise one or more precursor oils that are blended
in a closed vessel
containing a catalyst. Preferred operating fluids also comprise a polar heat
transfer fluid such
as r-134a, wherein at least some of the polar heat transfer molecules are
complexed to a
component of the activated oil blend.
[00103] It is contemplated that the ratio of one fatty acid to one heat
transfer fluid can
comprise any suitable ratio, including for example, 1:1000, 1:100, 1:10, 1:5
or even 100:1 or
more. It is also contemplated that the ratio of one food oil (from which at
least one fatty acid
is derived) to another food oil, of a mixture (non-activated) or activated
blend, can comprise
any suitable ratio including for example, 1:1, 1:2, 1:3, 1:4, or even 1:100 or
less. In some
embodiments, a chemical marker can also be included.
[00104] As used herein, and unless the context dictates otherwise, the term
"coupled to" is
intended to include both direct coupling (in which two elements that are
coupled to each
other contact each other) and indirect coupling (in which at least one element
is interposed
between the two elements). Therefore, the terms "coupled to" and "coupled
with" are used
synonymously.
21
CA 02866711 2014-09-08
WO 2013/134603 PCT/US2013/029786
[00105] It should be noted that one having ordinary skill in the art should
realize that all
numbers herein are approximates, regardless or whether or not the numbers are
preceded by
the word "approximately".
[00106] In some embodiments, the numbers expressing quantities of ingredients,
properties such as concentration, reaction conditions, and so forth, used to
describe and claim
certain embodiments of the invention are to be understood as being modified in
some
instances by the term "about." Accordingly, in some embodiments, the numerical
parameters
set forth in the written description and attached claims are approximations
that can vary
depending upon the desired properties sought to be obtained by a particular
embodiment. In
some embodiments, the numerical parameters should be construed in light of the
number of
reported significant digits and by applying ordinary rounding techniques.
Notwithstanding
that the numerical ranges and parameters setting forth the broad scope of some
embodiments
of the invention are approximations, the numerical values set forth in the
specific examples
are reported as precisely as practicable. The numerical values presented in
some
embodiments of the invention may contain certain errors necessarily resulting
from the
standard deviation found in their respective testing measurements.
[00107] As used in the description herein and throughout the claims that
follow, the
meaning of "a," "an," and "the" includes plural reference unless the context
clearly dictates
otherwise. Also, as used in the description herein, the meaning of "in"
includes "in" and
"on" unless the context clearly dictates otherwise.
[00108] Unless the context dictates the contrary, all ranges set forth herein
should be
interpreted as being inclusive of their endpoints, and open-ended ranges
should be interpreted
to include commercially practical values. Similarly, all lists of values
should be considered as
inclusive of intermediate values unless the context indicates the contrary.
All methods
described herein can be performed in any suitable order unless otherwise
indicated herein or
otherwise clearly contradicted by context. The use of any and all examples, or
exemplary
language (e.g. "such as") provided with respect to certain embodiments herein
is intended
merely to better illuminate the invention and does not pose a limitation on
the scope of the
invention otherwise claimed. No language in the specification should be
construed as
indicating any non-claimed element essential to the practice of the invention.
[00109] It should be apparent to those skilled in the art that many more
modifications
besides those already described are possible without departing from the
inventive concepts
herein. The inventive subject matter, therefore, is not to be restricted
except in the scope of
the appended claims. Moreover, in interpreting both the specification and the
claims, all
22
CA 02866711 2014-09-08
WO 2013/134603
PCT/US2013/029786
terms should be interpreted in the broadest possible manner consistent with
the context. In
particular, the terms "comprises" and "comprising" should be interpreted as
referring to
elements, components, or steps in a non-exclusive manner, indicating that the
referenced
elements, components, or steps may be present, or utilized, or combined with
other elements,
components, or steps that are not expressly referenced. Where the
specification claims refers
to at least one of something selected from the group consisting of A, B, C
.... and N, the text
should be interpreted as requiring only one element from the group, not A plus
N, or B plus
N, etc.
23