Note: Descriptions are shown in the official language in which they were submitted.
CA 02866746 2014-10-06
TITLE OF INVENTION
RESECTOR BALLOON
SYSTEM
FIELD OF THE INVENTION
[0001] The present invention relates to systems and methods for the
resection of unwanted biological material, such as tissue growths and tumors,
in
bodily cavities. More specifically, the invention relates to a balloon
catheter with
a resecting surface that is operated in a pulsing fashion to resect the target
material with minimal trauma.
BACKGROUND OF THE INVENTION
[0002] The removal of unwanted and/or life threatening biological
material from interior portions of bodily cavities, such as organs, vessels,
articular joints and structures, sinuses, and various bodily lumens, is a very
common procedure in various medical specialties and disciplines, such as
pulmonology, cardiology, urology, gynecology, gastro-enterology, neurology,
otolaryngology, orthopedics, and general surgery. Accordingly, various
instruments and methods have been employed to perform these procedures,
which are generally well known in the art.
CA 02866746 2014-10-06
- 2 -
[0003] One of the most important complications in such procedures is
bleeding. The bleeding and resulting morbidity of tissue that occurs in many
of the currently known surgical procedures is the result of abrasive,
traumatic,
and invasive excising and removal techniques. Many of these techniques risk
perforation of the vessel or lumen in which the procedure is being performed,
resulting in grave complications for the surgeon and patient. In addition,
many patient maladies are simply not remedied by these procedures because no
interventional, minimally invasive treatment modality exists, the methods are
not
efficient, safe, and reproducible, and/or the instruments employed lack the
appropriate visualization, physiological measurement, and/or feedback
necessary
to ensure the safety, efficacy, and reproducibility of the procedure.
Accordingly, a
new type of treatment is required.
[0004] One instrument that is commonly used in various types of medical
procedures is an inflatable balloon catheter, of which many different types
exist,
which are utilized to perform various necessary functions. For example, these
inflatable balloons are often used to control or stop bleeding, to hold
instruments
in place, or to prevent or facilitate other flow or movement within the bodily
cavity. For example, many urological catheters are held in place via a balloon
that impacts the sidewalls of the urinary tract, many gynecological
instruments
are held in place via balloons that impact the sidewalls of the vaginal vault,
endovascular balloons are often used to control bleeding, inflatable balloons
are
sometimes used to control the backflow of radio-opaque agents injected into
the
CA 02866746 2014-10-06
- 3 -
cystic duct to detect the presence of gall stones during general surgical
cholecystectomy procedures, and, recently, balloon catheters have been
employed to release sinus congestion.
[0005] One particular application of such catheters is lung cancer. Among
all types of cancer, this has the lowest survival rate, as more than one third
of all
deaths due to cancer are caused by lung cancer. Over 1.5 million new cases are
diagnosed worldwide each year. The most frequent cause of death for lung
cancer patients is airway obstruction. In lung cancer patients, one third of
all
cases initially, and another third of the cases in the long term, present main
airway obstruction, which may cause asphyxia, hemorrhaging, and infection.
These complications are the most frequent causes of death in lung cancer
patients.
[0006] Use of interventional bronchoscopy for the treatment of lung
cancer and the resultant airway obstruction increases the quality of life and
survival rates of patients suffering from Chronic Obstructive Pulmonary
Disease
(COPD) and the obstructive co-morbidities associated with the cancer.
Accordingly, balloon catheters have been routinely used with various
endoscopes and with flexible and rigid bronchoscopes for dilation, as a
tamponade to stop bleeding, and as an interference fixation device to hold
instruments in place and prevent the retropulsion of those instruments under
backflow pressure.
[0007] In light of the aforementioned need for a new type of treatment
CA 02866746 2014-10-06
- 4 -
for removing undesirable biological material in bodily cavities, it has been
realized that inflatable balloon catheters may further be employed as
interventional tools for the excision and removal of such materials¨such as
endoluminal obstructions and tumors and endovascular occlusions¨in various
applications, such as the aforementioned interventional medical specialties of
pulmonology, cardiology, urology, gynecology, gastro- enterology, neurology,
otolaryngology, and general surgery. The use of balloon catheters in this way
has presented a method of treatment that is simple, safe, highly effective,
and
inexpensive compared to other types of methods and devices that are used,
such as mechanical, laser, electrocautery, cryotherapy, etc.
[0008] Accordingly, a new class of balloons has been suggested for this
purpose, such as that disclosed in European Patent Application No. EP 1
913 882 by Karakoca. This device employs a balloon catheter with a hardening
surface, which can be inserted into bodily cavities. After the device is
inserted,
the balloon is inflated, and the balloon is moved back and forth within the
cavity
such that the textured surface performs a shaving action on the unwanted
biological material. In this way, the targeted material is resected.
[0009] However, this particular instrument and method of using it suffers
from a number of disadvantages and shortcomings. One of the most significant
problems with this resector balloon is that unwanted biological material is
removed by shaving it with the hardened surface on the outside of the balloon¨
i.e., by moving the balloon back and forth and/or rotating it. This mechanism
of
CA 02866746 2014-10-06
- 5 -
action can be abrasive and traumatic. Moreover, the hardened surface coupled
with the shaving action can sometimes lack the precision necessary to prevent
complications such as bleeding and structural perforation of the affected
anatomical structure. Furthermore, the amount of torque and back and forth
force needed on the balloon may cause a device failure, particularly where the
balloon is attached to the catheter.
[0010] Another disadvantage of this resector balloon is that its hardened
surface is a separate membrane located on the outside of the balloon. This
membrane has different stretching characteristics than the balloon and effects
the
performance of the balloon catheter negatively. It may be required to pre-
exercise the balloon catheter outside the body before use. Additionally, it
may
break off under the frictional stresses of the procedure and further obstruct
or
compromise the bodily cavity in which the balloon is deployed.
[0011] Another problem with this resector balloon is that it further lacks
accuracy because it lacks the capability to precisely gauge the size of the
environment in which it is being used to provide physiological measurements
and
feedback that could aid treatment intervention and efficacy. For example,
there
is no way for the surgeon to know the diameter of the affected bodily cavity
itself,
proximal or distal to the obstruction therein. Similarly, there is no way for
the
surgeon to know the intra-lumen diameter where the unwanted tissue growth or
tumor resides, and further, no way to accurately adjust for changes in this
diameter over time as the growth or tumor is resected. Because it has no
CA 02866746 2014-10-06
- 6 -
mechanism for measuring the intra-lumen diameter at different points within
the
cavity, and particularly, how this changes over time, one is not able to be
properly
adjust the amount of pressure supplied to the balloon and thereby prevent
complications and expedite treatment.
[0012] A related problem with this device is that there is no way for a
physician to measure the intra-articular space between two articular
structures, endplates, or surfaces.
[0013] Yet another related problem with this device is that there is no
way for the surgeon to know the density of the bodily cavity proximal or
distal to
the obstruction, nor can the surgeon know the density of the growth or tumor
itself. Because there is no mechanism for measuring the density of the cavity
or
the obstruction, one is likewise unable to properly control the pressure in
the
balloon to aid surgical precision, minimize potential complications, and
expedite
the procedure.
[0014] Still another related problem with this device is that it does not
have a way of identifying the type of balloon catheter that is connected to
the
pump. As a result, the balloon may be accidentally over-inflated, and thus,
the balloon could burst.
[0015] Another disadvantage of this resector balloon is that it is
comprised of a single, unitary structure, which means that one is only able to
inflate the entire balloon as a whole. This results in several deficiencies,
CA 02866746 2014-10-06
- 7 -
including: the inability to measure the intra-lumen diameter at different
locations,
including both the bodily cavity itself (proximal/distal to obstruction) and
the
obstructive biological material; the inability to pinpoint the location(s)
requiring
the maximum pressure in order to precisely and methodically resect the
obstruction; the inability to tamponade specific areas in order to control
bleeding;
the inability to capture material that has been excised in order to extract it
from
the bodily cavity; and a tendency for the balloon to slip and migrate.
[0016] Yet another deficiency of this device is that it is not able to be
positioned as optimally as may be desired. For example, the overall diameter
of
this balloon catheter requires a rigid or flexible endoscope with a working
channel. In addition to the fact that such endoscopes may not be readily
available, they are single lumen devices. As a result, a guide wire cannot be
used to guide them into bodily cavities either through a rigid or flexible
endoscope
or alongside, in parallel to, a rigid or flexible endoscope. Likewise, this
device
does not have the ability to linearly translate the balloon along the catheter
construct, which would enable one to optimize balloon placement and
productivity. Finally, the device does not include material for externally
identifying
its position, such as a radio-opaque material. Therefore, one is not able to
easily
identify the position of the balloon via an external imaging modality, such as
radiographic or ultrasonic imaging. Each of these shortcomings contributes to
one's inability to position the balloon as precisely as may be desired.
CA 02866746 2014-10-06
- 8 -
[0017] Another disadvantage of this resector balloon is that there is no
way to provide the physician illuminated light, non-thermal illuminated light,
and
direct visual feedback of the area ahead of the balloon, ahead of the balloon
looking back towards the balloon, along the sides of the balloon or behind the
balloon to optimize treatment intervention and efficacy.
[0018] A further deficiency of this resector balloon stems from the fact
that it is a single lumen device where the proximal end is closed off. As a
result,
it does not allow for passage of fluid, such as air or blood, from the distal
end of
the catheter to the proximal end when the balloon is inflated.
This is particularly important in interventional pulmonology applications,
where
aspiration in the event of airway obstruction is critical. Likewise, this is
important
in interventional cardiology applications to permit the bypass of blood flow
during
the operation of a vessel segment.
[0019] Another deficiency of this device is that it does not have the
ability to deliver cryogenic agents or forms of energy that could assist in
the
resection of the undesirable biological material. As a result, one is unable
to
supply cryogenic agents or forms of energy such as radio-frequency,
ultrasonic,
and electrosurgical energy in order to perform ablation, desiccation,
cauterization, excision, decortications, and/or tissue modification in order
to
optimize hemostasis and resection.
CA 02866746 2014-10-06
- 9 -
[0020] A further deficiency of these balloon catheters is that there is no
way to provide localized delivery of drugs, stents, biologic materials, nano-
particulates, or related technologies to the surface of the balloon. Thus, one
is
unable to use the device to supply these means of providing medicinal,
therapeutic, and restorative treatments.
[0021] What is desired, therefore, is a resector balloon system for
removing undesirable biological materials that does not cause unnecessary
trauma to the affected bodily cavity as a result of a shaving action used to
resect
that material. What is also desired is a resector balloon system with
controllable
rates of inflation and deflation. What is further desired is a resector
balloon
system that does not require a separate membrane affixed to the exterior of
the
balloon. What is also desired is a resector balloon system that can be
administered either through an endoscope, alongside an endoscope, or via
radiographic or ultrasonic imaging. What is also desired is a resector balloon
system that is able to provide physiologic feedback to determine intra-lumen
diameters and densities where the unwanted biological material resides and at
locations proximal or distal to such material, the intra-articular space
between
two articular structures, and the type of balloon catheter connected. What is
also
desired is a resector balloon system that is able to provide dimensional and
performance metrics of the balloon catheter construct in vivo. What is further
desired is a resector balloon system that can be optimally positioned within
the
bodily cavity and can pinpoint specific areas at which to provide maximum
inflation. What is also desired is a resector balloon system that can supply
light
CA 02866746 2014-10-06
- 10 -
and visualization capabilities, cryogenic agents and various forms of energy
to
assist surgical techniques, and drugs and related materials to the anatomical
site.
What is further desired is a resector balloon system that allows for the
passage
of fluids from the proximal to the distal end of the catheter.
SUMMARY OF THE INVENTION
[0022] Accordingly, it is an object of the present invention to provide a
resector balloon system for removing undesirable biological material that does
not require a shaving mechanism of action.
[0023] It is a further object of the present invention to provide a resector
balloon system for removing undesirable biological material that does not
employ
a separate membrane affixed to the outside of the balloon.
[0024] It is yet another object of the present invention to provide a
resector balloon system for removing undesirable biological material that
provides physiological feedback from which the intra-lumen diameter where
the material resides, as well as the bodily cavity itself proximal and distal
to the
material, can be determined, and the pressure and flow supplied to the balloon
can be adjusted accordingly.
[0025] It is still another object of the present invention to provide a
resector balloon system for removing undesirable biological material that
provides physiological feedback from which the intra-articular space between
CA 02866746 2014-10-06
- 11 -
two articular structures, endplates, or surfaces can be determined, and the
pressure and flow supplied to the balloon can be adjusted accordingly.
[0026] It is yet another object of the present invention to provide a
resector balloon system for removing undesirable biological material that
provides physiological feedback from which the intra-lumen density where the
material resides, as well as the bodily cavity itself proximal and distal to
the
material, can be determined, and the pressure and flow supplied to the
balloon can be adjusted accordingly.
[0027] It is another object of the present invention to provide a resector
balloon system for removing undesirable biological material that can identify
the
type of balloon catheter that is connected to the pump.
[0028] It is still another object of the present invention to provide a
resector balloon system for removing undesirable biological material where the
balloon portion has different segments that can be inflated independently.
[0029] It is yet another object of the present invention to provide a
resector balloon system for removing undesirable biological material that has
at
least one additional passageway other than that used for the fluid that
inflates
the balloon.
CA 02866746 2014-10-06
- 12 -
[0030] It is another object of the present invention to provide a resector
balloon system for removing undesirable biological material that enables the
balloon to be translated along the catheter.
[0031] It is still another object of the present invention to provide a
resector balloon system for removing undesirable biological material that
facilitates exterior imaging.
[0032] It is yet another object of the present invention to provide a
resector balloon system for removing undesirable biological material that
provides visualization from within the bodily cavity.
[0033] It is another object of the present invention to provide a resector
balloon system for removing undesirable biological material that can deliver
energy to the target area.
[0034] It is yet another object of the present invention to provide a
resector balloon system for removing undesirable biological material that can
deliver cryogenic agents to the target area.
[0035] It is still another object of the present invention to provide a
resector balloon system for removing undesirable biological material that can
deliver drugs, stents, nano-particulates, and similar materials to the target
area.
CA 02866746 2014-10-06
- 13 -
[0036] In order to overcome the deficiencies of the prior art and to
achieve at least some of the objects and advantages listed, the invention
comprises a method of resecting biological material with a resector balloon
system, the method including inserting a catheter comprising at least one
balloon having an outer wall with a resecting surface into a bodily cavity
having biological material to be resected, inflating the balloon by supplying
fluid thereto such that the resecting surface of the balloon contacts the
biological material, and repeatedly deflating and inflating the balloon by
supplying fluid to the balloon in pulsed fashion such that the repeated
deflation and inflation causes the resecting surface to resect the biological
material.
[0037] In some of these embodiments, the step of inflating the balloon
includes supplying fluid to the balloon with an electro-pneumatic pump, and
the
step of repeatedly deflating and inflating the balloon is controlled by the
electro-
pneumatic pump based at least partially on an established volume change or
frequency. In some cases, the method further includes detecting a
balloon type for the catheter inserted into the bodily cavity, wherein the
step of
inflating the balloon is controlled based at least partially on the balloon
type
detected, and in certain cases, the step of repeatedly deflating and inflating
the balloon includes determining a density of the biological material or a
diameter within the biological cavity, and adjusting the amount of fluid
supplied
to the balloon based at least in part on the determined density or diameter.
CA 02866746 2014-10-06
=
- 14 -
[0038] In some embodiments, the at least one balloon includes a
plurality of balloon segments, and the step of inflating the balloon includes
inflating at least one of the balloon segments separately from at least one
other balloon segment.
[0039] The invention also comprises a resector balloon system,
including a catheter with at least one balloon having an outer wall, the outer
wall comprising a resecting surface for resecting biological material, and a
pump that inflates the balloon by supplying fluid thereto, wherein the pump
supplies fluid to the at least one balloon in pulsed fashion to repeatedly
deflate
and inflate the balloon.
[0040] In some embodiments, In certain advantageous embodiments,
the pump is an electro-pneumatic pump. In some embodiments, the pump
includes a processor that controls the pulsed supply of fluid based on an
established frequency, while in other embodiments, the pump includes a
processor that controls the pulsed supply of fluid based on an established
change of volume within the balloon.
[0041] In some embodiments, the invention further includes a connector
that connects the catheter to the pump, wherein the connector is a balloon
identification connector with which the pump identifies the balloon. In some
of
these embodiments, the connector includes a balloon identification plate and a
key that orients the identification plate when the catheter is connected to
the
CA 02866746 2014-10-06
- 15 -
pump such that the pump identifies the balloon using the identification plate.
In
some cases, the pump identifies the balloon from the identification plate
electro-
optically, while in other cases, the pump identifies the balloon from the
identification plate electro-mechanically. In certain embodiments, the pump
includes balloon profile data corresponding to the balloon and a processor
that
controls the supply of fluid to the balloon based at least partially on the
balloon
profile data. The balloon profile data may also include correction data for
different types of tissues.
[0042] In some embodiments, the pump includes at least one sensor for
making at least one measurement, and a processor that calculates a density of
the biological material in the biological cavity based at least partially on
the at
least one measurement and the balloon profile data. In some of these
embodiments, the at least one sensor includes a sensor that determines the
pressure of the fluid output to the balloon and a sensor that determines the
flow
of the fluid output to the balloon, and in some cases, the pump controls the
supply of fluid to the balloon at least partially based on the calculated
density.
[0043] Similarly, in some embodiments, the pump includes at least one
sensor for making at least one measurement, and a processor that calculates a
diameter in the biological cavity based at least partially on the at least one
measurement and the balloon profile data. In some of these embodiments, the
at least one sensor includes a sensor that determines the pressure of the
fluid
output to the balloon and a sensor that determines the flow of the fluid
output to
CA 02866746 2014-10-06
- 16 -
the balloon, and in some cases, the pump controls the supply of fluid to the
balloon at least partially based on the calculated diameter.
[0044] In certain embodiments, the system further includes a connector
that connects the catheter to the pump, wherein the connector is a balloon
identification connector with which the pump identifies the balloon, the pump
includes balloon profile data corresponding to the balloon the pump includes a
processor that determines a desired frequency or change in volume in the
balloon
based at least partially on the balloon profile data, and the pump controls
the
supply of fluid to the balloon based at least partially on the determined
frequency
of change in volume.
[0045] In some embodiments, the at least one balloon comprises a
plurality of balloon segments and the catheter includes a plurality of lumens
through which the pump supplies fluid to the balloon segments such that the
pump inflates at least one of the balloon segments separately from at least
one
other of the balloon segments.
[0046] In certain advantageous embodiments, the system further includes
at least one outer lumen for supplying fluid to the at least one balloon
segment
and an inner lumen. In some of these embodiments, the inner
lumen comprises an air or bodily fluid passage, while in some embodiments, at
least one guide wire is disposed in the inner lumen. In certain of these
embodiments, the system further includes at least one channel connecting the
CA 02866746 2014-10-06
- 17 -
inner lumen and the outer surface of the balloon for delivering a medicinal or
therapeutic agent to the biological cavity. In some of these embodiments, the
catheter includes an imaging device aperture, further comprising a fiber optic
bundle disposed in the catheter and exiting the hole for viewing the
biological
cavity. Some of the lumens can be used for multiple purposes. For example,
once the catheter is inserted into position with the aid of the guide wire,
the inner
lumen can then be used for visualization.
[0047] In certain embodiments, the pump includes a vacuum source
with which the pump evacuates resected material from the bodily cavity,
through a channel in the inner lumen. In some embodiments, the pump
includes a vacuum source that evacuates the fluid from the balloon.
[0048] In some embodiments, the system further includes an energy
source for supplying energy and at least one wire molded into the catheter for
conducting energy from the energy source to the biological cavity.
[0049] In certain advantageous embodiments, the fluid is a gas. In
some embodiments, the fluid is a cryogenic fluid.
[0050] In certain advantageous embodiments, the system includes a
mesh molded into the catheter, wherein the resecting surface comprises a
textured surface of the outer wall of the balloon produced by the mesh. In
other
embodiments, the outer wall of the balloon comprises a plurality of inflatable
CA 02866746 2014-10-06
,
- 18 -
cavities that provide the resecting surface. In still other embodiments, the
system further includes a plurality of spring wires mounted to the outer wall
of
the balloon, wherein the resecting surface comprises the spring wires, and in
some cases, the system also includes an energy source connected to the spring
wires for supplying energy thereto.
[0051] In some embodiments, the balloon has first and second ends,
and the system further includes at least one imaging marker mounted adjacent
at least one of the ends of the balloon, which in some cases, comprises a
radio-opaque ring.
BRIEF DESCRIPTION OF THE DRAWINGS
[0052] Figure 1 is a front, partially schematic view of a resector balloon
system in accordance with the invention.
[0053] Figure 2A is a front, partially schematic view of the balloon
catheter of the system of Figure 1.
[0054] Figure 2B is an end, partially cross-sectional view of the inflated
balloon of the system of Figure 2A.
[0055] Figure 2C is a partially cross-sectional view of the deflated
balloon of the system of Figure 2A.
CA 02866746 2014-10-06
- 19 -
[0056] Figure 3A is a front, partially schematic view of the balloon
catheter of Figure 2A employing multiple balloon segments.
[0057] Figure 3B is a side view of the balloon of the catheter of Figure
3A with the center balloon inflated.
[0058] Figure 3C is a side view of the balloon of the catheter of Figure
3A with the center balloon deflated.
[0059] Figure 3D is a partially cross-sectional view of the balloon
catheter of Figure 3A.
[0060] Figure 3E is a side view of the balloon of the catheter of Figure
3A with the balloon segments spatially separated.
[0061] Figure 3F is a partially cross-sectional view of the balloon
catheter of Figure 3E.
[0062] Figure 4A is a side, partially schematic view of the balloon
catheter of Figure 1 with an energy delivery assembly.
[0063] Figure 4B is a side, partially schematic view of the balloon
catheter of Figure 4A.
[0064] Figure 5 is a side view of the balloon catheter of Figure 1 with
spring wires mounted to the balloon.
CA 02866746 2014-10-06
- 20 -
[0065] Figure 6A is a side, partially schematic view of the balloon
catheter of Figure 1 with an imaging device.
[0066] Figure 66 is an end view of the imaging device of Figure 6A.
[0067] Figures 7A-F are side, partially cross-sectional views of the
balloon catheter of Figure 1 being operated in a bodily cavity.
[0068] Figure 8 is a block diagram illustrating the pneumatics of the
pump of Figure 1.
[0069] Figure 9 is a block diagram illustrating the electronics of the
pump of Figure 1.
[0070] Figure 10A illustrates a front panel of the pump of Figure 1.
[0071] Figure 10B illustrates a graphical display of the front panel of
Figure 10A.
[0072] Figure 100 illustrates a front panel of a remote for the pump of
Figure 10A.
[0073] Figure 11A illustrates a front panel of the pump of Figure 1.
[0074] Figure 11B illustrates a rear panel of the pump of Figure 11A.
CA 02866746 2014-10-06
- 21 -
[0075] Figure 11C illustrates a front panel of a remote for the pump of
Figure 11A.
[0076] Figure 12A-B is a flow diagram illustrating the operation of the
resector balloon system of Figure 1.
[0077] Figure 13 is an example of a typical plot of volume versus flow
time characteristics before and after correction.
DETAILED DESCRIPTION OF THE INVENTION
[0078] The basic components of one embodiment of a resector balloon
system in accordance with the invention are illustrated in Figure 1. As used
in
the description, the terms "top," "bottom," "above," "below," "over," "under,"
"above," "beneath," "on top," "underneath," "up," "down," "upper," "lower,"
"front,"
"rear," "back," "forward" and "backward" refer to the objects referenced when
in
the orientation illustrated in the drawings, which orientation is not
necessary for
achieving the objects of the invention.
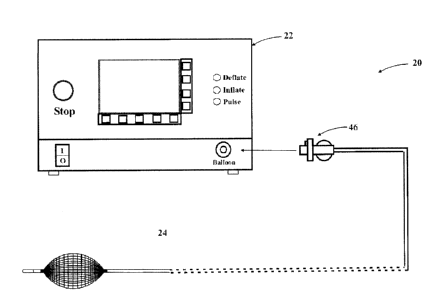
[0079] The system 20 includes a fluid source (22), such as an electro-
pneumatic pump having controls on the front thereof, from which a physician or
assistant can control the system (as well as a remote control unit), which is
further described below. A balloon catheter (24) is connected to the pump
(22),
to which the pump (22) supplies a fluid, such as a gas, liquid, or mixture
thereof.
CA 02866746 2014-10-06
- 22 -
In certain cases, a cryogenic fluid is supplied by the pump (22) in order to
further
aid a particular procedure, such as tumor desiccation.
[0080] As shown in Figures 2A-B, the balloon catheter (24) includes a
catheter (26) made of a polyethylene material and having an outer diameter of
1.8 mm and a length of about 1.2 to 3 meters. A bendable section (28) having a
length of about 5 to 10 mm at the distal end of the catheter (24) serves as a
safety tip. As a result, when the catheter (24) is inserted through the
available
opening of a bodily cavity, it will bend instead of puncturing the walls of
the cavity.
[0081] A balloon portion (30) made of latex or other suitable material is
located near the distal end of the catheter (24) or at an otherwise desirable,
predefined distance along the catheter (24). The balloon (30) comes in a
variety
of sizes and diameters, which can be selected to suit the particular
application for
which the device is being used. Typically, such balloons will have lengths of
5,
10, 15, 20, 30 or 50 mm and diameters of 2.5, 5, 10, 15, 20,
30 or 50 mm. This variety of available balloon sizes allows the balloon
catheter (24) to be used in bodily cavities of various diameters and
dimensions, such as large and small bronchial branches, sinuses, and vessels,
having different types of tumors and tissues to be treated. The pump (22)
supplies the air at a pressure of approximately 2 atmospheres in order to be
able
to inflate such balloons to full size, ranging from 2.5 mml to 50 mml.
CA 02866746 2014-10-06
s,
- 23 -
[0082] In certain advantageous embodiments, the balloon (30) includes
imaging markers (32), such as radio opaque rings, located at or near the ends
thereof. Such markers can be selected and appropriately positioned in order to
reflect the relevant waves of various imaging modalities (e.g., x-ray) in
order to allow the use of such modalities to assist with the precise
positioning of
the balloon (30).
[0083] Referring to Figure 2B, which shows a cross-section of the
balloon (30), the balloon is covered with a flexible resecting surface (34),
which
may, for example, comprise a fiber mesh affixed to the surface of the balloon
(30). In certain advantageous embodiments, the resecting surface (34)
comprises a textured surface approximately 0.2 mm thick that is an integral
part
of the balloon and which is incorporated therein during the molding process.
In
these cases, the resecting surface (34) is made by integrating into the
balloon
material a fine, fiber mesh, which can be made of lycra, polyurethane,
composite springs, or other appropriate material. The crossover point of the
mesh members produce outwardly-facing, small knots
or dimples, which create micro-impacts on the tumor tissue (or other
biological
material to be resected) during the inflation/deflation cycles further
described
below. In other embodiments, dimensional surface structures or inflatable
sinuses that are encapsulated in the surface substrate of the balloon (30) are
employed. Such impregnated structures within the surface substrate of the
balloon can mimic mesh-like structures, bumps, ridges, etc.
CA 02866746 2014-10-06
- 24 -
[0084] Referring back to Figure 2A, the balloon catheter (24) includes an
inner lumen breakout Y junction (40) to facilitate the introduction of a guide
wire,
air bypass, drug delivery, or visualization conduit. The proximal end of the
inner
lumen (42) after Y junction (40) is terminated with a luer connector (44). The
outer lumens are terminated at their proximal end with a keyed connector (46),
which includes a key (48) and a balloon identification plate (50).
[0085] The Y junction (40) serves several purposes. First, it brings out a
separate, inner lumen (42) of the catheter (24) to a suitable connector, such
as
the aforementioned luer connector (44), in order to provide an independent
passage, such as a two-way air passage between the distal and proximal
ends of the balloon catheter (24), which can be critical in certain
applications
(i.e., bronchoscopy) when the balloon is inflated. Additionally, the Y
junction
(40) also includes a shut-off valve (not shown) for stopping the balloon (30)
from deflating. This may be used, for example, when it is required to leave
the
inflated balloon in place for a lengthy period of time in order to treat
chronic
bleeding.
[0086] As noted above, the catheter (24) is terminated at the proximal
end with a keyed balloon identification plate (50). The purpose of this
connector
is to electronically detect the catheter (24) when it is inserted into the
pump (22)
and to identify the particular type of balloon catheter being used. The key
(48)
orients the connector (46) and the identification plate (50) in such a way
that the
CA 02866746 2014-10-06
- 25 -
balloon type can be identified by the pump (22) using electro-optical or
electro-
mechanical means.
[0087] Each type of balloon (30) that can be used with the pump (22) is
characterized, and balloon profile data is registered in lookup tables. By
identifying the type of balloon (30) that is connected the pump (22), the
appropriate profile data can be retrieved and used to ensure that the
appropriate
pressure, volume, flow, and timing adjustments can be made to safely and
effectively operate the balloon (30). The balloon profile data contained in
the
lookup table, along with appropriate pressure and flow measurements (as
further
discussed below), allows one to make tissue density approximations. This
balloon profile data and approximated lumen diameter and tissue density, as
well
as any user commands, are used to adjust the amount of gas the pump (22)
delivers to the balloon (30) in order to achieve the desired inflation and
deflation
amounts.
[0088] As shown in Figure 2C, which shows a cross-section of the
catheter (26) at the distal end where the deflated balloon (30) with the
resecting surface (34) is located. In certain embodiments, the inner lumen
(42) of the catheter (26) extends through the bendable section of the catheter
tip (28) and is open at the distal end. As noted above, in certain
applications,
such as bronchoscopy, this inner lumen (42) serves as a passageway that
allows the air to move freely in both directions from each end of the balloon
(30) when it is inflated. Additionally, the inner lumen (42) can be used as a
CA 02866746 2014-10-06
- 26 -
means for accurately positioning the balloon catheter (24), as it can be used
as a conduit for a guide wire (63) when inserting the deflated balloon
catheter
(24) into the bodily cavity. In other applications, such as in treating
coronary
artery disease, bypass holes (not shown) to the inner lumen may be provided
at an appropriate location after the proximal end of the balloon (30) and the
inner lumen (42) is thereafter blocked such that a breakout junction therefor
is
unnecessary.
[0089] The outer lumens (60) of the catheter (26) are used to inflate and
deflate the balloon (30) through the holes (62) provided in the catheter's
outer
walls (64). These outer lumens (60) are blocked at the distal end of the
balloon
(30) so that air intended for inflation and deflation will not escape.
[0090] In certain advantageous embodiments, as illustrated in Figures
3A-B, the balloon catheter (24) includes a multi-balloon construct (70) at its
distal end. This construct may include, for example, a proximal balloon
segment (72), a center balloon segment (74), and a distal balloon segment
(76). At the proximal end of the catheter (24), the Y junction (40) brings out
another lumen (78) that supplies fluid to the proximal balloon segment (72)
and the distal balloon segment (76) separately from the center balloon
segment (74). The additional lumen (78) is connected to another keyed
connector (80), similar to the keyed connected (46). In this way, the center
balloon segment (74) is inflated and deflated independently of the proximal
and distal balloons (72,76).
CA 02866746 2014-10-06
- 27 -
[0091] Employing separate proximal and distal balloon segments in this
way serves several purposes. First, one is able to inflate the proximal and
distal
balloon segments (72,76) to an amount appropriate to hold the catheter (24)
steady where the tissue to be removed is located while the center balloon (74)
is
cyclically inflated and deflated to resect the unwanted biological material,
as
illustrated in Figures 3B-C. By doing so, one can prevent the balloon (30)
from
slipping and migrating during the procedure, and possibly causing damage to
the
bodily cavity itself, which is particularly important in cavities subject to
significant
backflow pressures and in applications where balloon catheterization is
required
for an extended period of time. Additionally, by inflating the proximal and
distal
balloons (72,76), one can prevent the resected material from escaping into the
bodily cavity, and instead, can capture the loose tissue for easy removal.
Finally,
by employing multiple, independently inflatable bladders or sinuses in this
way,
one is able to more selectively and precisely tamponade different sections of
the
bodily cavity, measure their intra-lumen diameters and densities, and resect
obstructive tissue.
[0092] Figure 3D shows how the outer lumens are used to inflate and
deflate the three balloons (72, 74, 76). As noted above, the inner lumen (42)
is
used for air bypass and/or a guide wire conduit. The lower lumen (82) has
inflation/deflation hole (84) in the catheter walls only at the position along
the
length of the catheter (24) where the center balloon (74) is located, while
the
upper lumen (86) contains inflation/deflation holes (88) only at the position
CA 02866746 2014-10-06
- 28 -
along the length of the catheter where the proximal and distal balloons
(72,76)
are located. It should be noted that the proximal and distal balloon segments
(72,76) can also be inflated/deflated independently from each other by further
separating the outer lumen to include an additional lumen, and positioning the
inflation/deflation holes at the appropriate locations along the length of
catheter (24). Likewise, additional balloon segments could be added, which
could each similarly be inflated independently from the others by increasing
the
number of lumens and adding a separate termination at the proximal end at the
Y junction (40).
[0093] Though the balloon segments illustrated in Figures 3A-C are
shown adjacent one another, in other embodiments, as shown in Figure 3E, the
different balloon segments may be spatially separated from each other. The
balloon segments may be separated by, for example, a distance of about
1 cm, though this separation can be more or less depending on the particular
application. By separating the balloon segments in this way, holes (90) can be
provided to other lumens (92) in the catheter.
[0094] As shown in Figure 3F, the lumens (92) and holes (90) can be
used to deliver, for example, a medicinal drug. In this way, with the proximal
and distal balloons (72, 76) remaining inflated and the center balloon
resecting
the unwanted biological material (as further described below), the drug is
contained in the targeted site and evenly distributed. It should be noted
that,
however, that in other embodiments, such drugs, nano- particulates, etc. may
CA 02866746 2014-10-06
..
- 29 -
be dispersed through multiple distal tips or through orifices in the lateral
walls of
the balloon. Accordingly, such drugs can be released via a methodic and/or
timed release.
[0095] The lumens (92) and holes (90) can be used to deliver any
number of things to assist with opening the cavity, circulation, aspiration,
respiration, assisting the decomposition of an obstruction, or stimulating
healing
in the affected area, including air, aspirates, drugs, biologics, biogenetic
agents,
nano-particulates, solutions, stem cell and gene therapies, and stents and
scaffolds. Specifically, the device could be used for the deployment and
implantation of pro-generative vehicles and/or catalysts in the repair,
treatment,
and therapy of the targeted areas, including biologic, nano- particulate
materials
and/or biogenetic materials, structures, scaffolds, and similar devices and
vehicles, including, for example, bone morphogenetic proteins,
microcrystalline
nano-particulates, collagens, de-mineralized bone chips, calcium based
structures, poly glycolic acids, poly lactic acids, and hyaluronic acids. The
device
can likewise be used for the deployment and implantation of inert, inelastic,
and
semi-rigid materials, such as, for example, PEEK, ceramic, cobalt chrome,
titanium, and stainless steel, and for the implantation of reinforcing
constructs
within, along, and/or around anatomic structures, which may be deployed and
then impregnated, impacted, and otherwise filled, either prior to or after
insertion,
with inert materials including, for example, polymethyl meth-acrylate, bone
cements, polyethylene, polypropylene, latex, and PEEK.
CA 02866746 2014-10-06
- 30 -
[0096] Additionally, in some of these multiple-balloon embodiments, the
above-described imaging markers (e.g., radio opaque rings), can be located
at or near the ends of each balloon segment in order to facilitate the use of
certain imaging modalities to assist with the precise positioning of the
balloons.
[0097] As illustrated in Figure 4A, in certain advantageous embodiments,
a flexible catheter (100) with electrically conductive wires (103) and
electrodes
(104) is used to deliver energy to a desired biological material to be
treated. As
shown in Figure 4B, an access hole (106) is used to introduce the
electrocautery
electrodes (104) to the target site. The electrodes (104) are molded into the
flexible catheter (100), and are electrically connected to conductive wires
(103),
which are also molded into the catheter (100) and electrically insulated from
one
another. The distal ends of the wires (103) are, in turn, connected to an
energy
generating device for supplying the requisite energy (108), such as, for
example,
a suitable electro- surgical unit.
[0098] The electrodes (104) are made of suitable spring metals that are
straight inside the lumen of the catheter (26), but spring into their original
shape
when pushed out through the access hole (106). The electrodes are deployed by
pushing the catheter (100) in and out at the Y junction (40). The electrodes
(104)
are positioned in the desired position by rotating the balloon catheter (26)
and
incrementally inflating and deflating the balloon (30) as needed. It should be
noted that both monopolar (one of the electrodes is remotely connected) and
CA 02866746 2014-10-06
- 31 -
bipolar (both electrodes are localized) implementations may be employed. In
this
way, various forms and types of energy, such as radio-frequency and
electrosurgical energy, can be supplied in a 3600 fashion to perform ablation,
cauterization, excision, decortications, and/or tissue modification in order
to
optimize hemostasis and resection. A similar energy delivery system can be
constructed for delivery of ultrasound.
[0099] In certain advantageous embodiments, the invention also
includes insulating materials and insulation barriers along and within the
surfaces of the balloon construct to insulate the balloon from the thermal,
ultrasonic, and associated deleterious effects of the different forms energy
delivered by the above described balloon catheter (24). Accordingly, the
balloon (30) is protected against becoming deflated or otherwise comprised
under the stress of the energy delivery process(es).
[00100] As illustrated in Figure 5, in certain embodiments, straight, steel
spring wires (110) are mounted on the balloon (30) in a cylindrical fashion.
The
wire ends are fixed to the balloon catheter (24) at the proximal end (112) of
the
balloon (30) such that they do not move with respect to the catheter (26). At
the
distal end (114), the wires (110) are not fixed and extend far into channels
that
are provided in the balloon catheter (26). Accordingly, when the balloon (30)
is
inflated, the spring wires (110) are forced by the inflation to take the shape
of the
balloon (30). In this way, another means of providing a resecting surface for
the
balloon (30) is provided by insulating the tips of the spring wires (110) from
one
CA 02866746 2014-10-06
- 32 -
another and by providing conductive wire (103) out through the Y junction
(40),
which can also be used as to provide monopolar or bipolar electrodes for
electrocautery.
[00101] In some embodiments, as shown in Figure 6A, a fiber optic
image bundle (120) is introduced through an access hole (122) or (124) to
image
the surrounding area. At the proximal end of the balloon catheter (26) the Y
junction (40) provides access through ports (126) and/or (128). As illustrated
in
Figure 6B, the fiber optic image bundle (120) is made of an incoherent fiber
bundle (130) for illumination and a coherent imaging fiber bundle (132) at the
core, and a lens (not shown). Two separate bundles, one for illumination and
the other for image (not shown) can also be used. At the distal end of the
fiber
optic bundle (120), the imaging coherent fibers are separated from
illumination
fibers (not shown) and interfaced to an image sensor, such as CMOS or CCD,
through appropriate optics (not shown). Similarly, the illumination fibers are
interfaced to a light source (not shown). It should be noted, however, that
other
sources of illumination, such as light emitting diodes, may also be employed.
It
should also be noted that the image sensor (CCD or CMOS available today in
2mm size) can be located at the tip of the imaging catheter assembly (not
shown), eliminating the need for coherent imaging fiber bundle, thus
increasing
the image quality and reducing cost.
CA 02866746 2014-10-06
- 33 -
[00102] In this sort of way, the physician can be provided with
illuminated light, non-thermal illuminated light, and direct visual feedback
of the
area ahead of the balloon (30), along the sides of the balloon, and/or behind
the balloon. The imaging sensor and illumination optics possess the ability to
be translated linearly or rotationally through and/or around the balloon (30),
thereby allowing for 3600 visualization of the treatment area.
[00103] The operation of the balloon (30) can be generally described with
reference to Figures 7A-F. Referring first to Figure 7A, after a visual
inspection
via an endoscope, x-ray, and/or ultrasound, a balloon catheter is selected,
and
the deflated device is inserted into position in a bodily cavity. This may be
accomplished by using the working channel of an endoscope or, as previously
noted, along a guide wire that is previously inserted into the body and
inserting
the proximal end of the guide wire which is outside the body into the inner
lumen
of the catheter. The catheter is connected to a pump (the components and
operation of which are further described in detail below), at which time the
pump
determines the type of balloon catheter that has been inserted.
[00104] Referring next to Figure 7B, the balloon is inflated by the pump
(which knows the type of balloon to which it is connected) at an air pressure
of
approximately 2 atmospheres for a fixed amount of time, and the flow is
measured (after the physician presses an inflate button on the pump). The pump
than calculates the initial approximation of the tissue density and the size
of the
opening in the tumor tissue, and displays the results for confirmation by the
CA 02866746 2014-10-06
- 34 -
physician. As the pump is operated, this data is continuously updated and
displayed.
[00105] As shown in Figures 7C-D, when a pulse button on the pump is
pressed, the balloon is deflated and inflated in a cyclical fashion, based
either on
parameters that were entered by the user, or on default parameters selected by
the pump, which are based on the characteristics of the particular balloon
(which
has been identified as a result of the aforementioned balloon identification
plate)
and the diameter and/or density measurements made by the system. In this
way, the pulse mode of the pump causes the balloon to pulsate according to a
desired frequency or change in volume within the balloon, producing a
periodically recurring increase and decrease in balloon size.
[00106] Accordingly, the resecting surface of the balloon repeatedly
comes into contact with the tissue growth, tumor, or other unwanted
obstruction
to create micro-impacts thereon. As the balloon is deflated and inflated, the
resecting surface creates just enough interference fixation, concentrically,
along
with compressive force excitation and friction upon the unwanted biological
material, to promote compressive force exhaustion and abrasion to elicit the
decomposition and excision thereof, such that the targeted biological material
is
resected in a non-traumatic way. As the tissue is destroyed and removed, the
balloon is inflated to a larger starting diameter and these steps are repeated
until
all the unwanted tissue is resected.
CA 02866746 2014-10-06
- 35 -
[00107] Meanwhile, the pump continually monitors the balloon pressure
and gas flow, and it updates a graphical display accordingly, as is further
described below. This gives the physician an indication as to when to stop the
pulse mode and evacuate the loosened tissue.
[00108] Referring to Figure 7E, once the tumor and/or tissue is broken up,
the balloon is deflated (by pressing a deflate button on the pump), and the
balloon is inserted further distally into the bodily cavity, past the location
of
unwanted tissue.
[00109] A shown in Figure 7F, the balloon is then re-inflated (by
pressing the inflate button on the pump) and gently pulled towards the
proximal end, bringing with it the loose tissue and debris to a point where it
can be removed using forceps or suction. In a multi-balloon construct, the
debris can be removed through one of the available lumens.
[00110] For example, one particular application to optimize 3600 lumen
des-obstruction, des-occlusion, cleansing, and debris capture involves the use
of
four bladders in series. All four bladders are first inflated to des-obstruct
the lumen. Then, the distal bladder is inflated fully, while the middle distal
bladder is deflated completely and the middle proximal bladder is deflated
partially. As the balloon catheter is retracted, the middle proximal bladder
is
optimally inflated, rotation of the middle proximal bladder is initiated, and
the
debris is thus resected from the inner walls of the lumen. The debris is then
CA 02866746 2014-10-06
- 36 -
captured upon retraction upon the fully inflated distal bladder and contained
within the middle distal and proximal bladders.
[00111] These steps are repeated as many times as necessary until all of
the unwanted tissue is removed. Typically, the procedure will between 5-
45 minutes, depending on the density of the tumor or unwanted tissue.
[00112] A pump (22) that controls the operation of the resector balloon
described above will hereafter be described. Figure 8 represents a block
diagram of the pneumatic components and operation of the pump. The pump
includes an air compressor (232) and a pressure tank (233), such as a Festo
model CRVZS-0.1, which enable it to achieve up to 10 atmospheres of
continuous pressure. The air pressure in the tank (233) is continuously
monitored by a microcontroller (254), which is further described in connection
with the electronics of the pump (Figure 9) below. The microcontroller
initiates
the compressor (232) to operate via an electrical signal output (253) when the
tank pressure drops below 4-5 atmospheres. The size of the tank (233) is
selected such that at least one procedure can be completed without the
compressor operating. The microcontroller calculates and displays the amount
of air in the tank (233), which indicates to the user whether there is enough
air to
complete the procedure. A check valve (234), such as a Festo
model H-1/8-A/1, is located between the compressor (232) and the tank (233) in
order to prevent the pressured gas from flowing back into the compressor
(232).
In another variation of the pump (22), however, the above-referenced
CA 02866746 2014-10-06
- 37 -
compressor and pressure tank are not included, and the pressurized air or
carbon dioxide is instead provided from an external source, such as gas tank
or
the operating room walls commonly found in an operating room.
[00113] The pressurized gas from the air tank (233) first goes through a
pressure regulator (238), which is electronically controlled via an analog
electrical output (OV-10V) signal (246) generated by the microcontroller to
supply
air to the balloon at an exact pressure, which can be set and changed by the
physician. However, any pressures higher than the upper limit for the
particular
balloon being used will generate a warning signal. As explained above,
different
balloon catheters may be used depending on the application, which are
identifiable via key connectors. Therefore, pressure, volume, and flow
characteristics of different types of balloons are contained in lookup tables
in
order to optimize the operation of the balloons and to ensure their consistent
performance.
[00114] Accordingly, when the pressure is set higher than the balloon's
upper limit, the detection of gas flow will cause the pump to stop and produce
the
warning, and the physician must then take a specific action to override this
condition. Similarly, if there is no balloon pressure, the detection of gas
flow will
also generate a warning, as this may mean the balloon has ruptured. It should
further be noted that the pump will also not operate if a catheter is not
connected.
Additionally, a balloon's operation when first removed from the packaging may
vary from its normal operation, requiring that they are first exercised before
use in
CA 02866746 2014-10-06
,
- 38 -
the body. Therefore, the setup and preparation function of the pump allows for
this variance.
[00115] In certain advantageous embodiments, a vacuum source (239),
such as a Festo model VN-05-L-T3-PQ2-VQ2-R01-B, is also included in the
pump so that the balloon can be rapidly deflated in a consistent
manner. This component also aids in achieving higher frequencies during the
pulse mode of operation. The vacuum source (239) is turned on and off by
the microcontroller via an electrical output signal (247).
[00116] Two microprocessor-controlled solenoid valves¨a deflation
valve (240) and an inflation valve (241)¨are used to control the inflation and
deflation of the balloon. The appropriate balloon inflation size is achieved
by
keeping the gas pressure constant, using the balloon pressure, flow, and
volume
characteristics from the lookup table data, and timing the on/off activation
periods of the valves (240, 241). Deflation valve (240) and inflation valve
(241)
are controlled by a deflate electrical signal (248) and an inflate electrical
signal
(249), respectively, which are generated by the aforementioned
microcontroller.
[00117] The gas pressure is continuously monitored by the microcontroller
using pressure regulator (242) at the input from the tank (233), a pressure
regulator (243) at the output of the regulator (238), and pressure regulator
(244)
at the output to the balloon. These pressure regulators, which may be, for
CA 02866746 2014-10-06
- 39 -
example, Festo model SDET-22T-D1O-G14-U-M12, provide to the microcontroller
analog electrical signal (0V-10V) inputs (250, 251, 252) that vary
proportionally to
the pressure at the regulators (242, 243, 244). The gas passes through an
electronic flow meter (245), such as a Festo model SFET- F010-L-WQ6-B-K1,
and a filter (246), before being delivered to the balloon. The flow meter
(245)
provides an analog electrical signal input (254) to the microcontroller that
indicates the amount of gas flow to the balloon.
[00118] The pressure regulator (244) and flow meter (245), along with
the known dimensions of the balloon, provide the feedback necessary to
determine the tumor dimensions and resistance via circumferential force and
depth resistance, from which a determination is made as to the diameter of
the lumen and the density of the tumor. Using these parameters, the
microcontroller makes the appropriate pressure and timing adjustments
necessary to maximize the effectiveness of the balloon, provide the
physiologic
metrics of the affected and non-affected areas, and provide data points and
indicators related to the specific dimensional and density characteristics of
the
intra-lumen anatomy and pathology aid the physician in safely determining and
delivering treatment.
[00119] In this way, the gas pressure is strictly monitored and maintained
at 2 atmospheres in order to keep the balloon from bursting. The high gas
input
pressure (up to 10 atmospheres) is reduced to and regulated at 2 atmospheres
electronically and under software control. However, the pressure delivered to
the
CA 02866746 2014-10-06
- 40 -
balloon can be increased or decreased under certain conditions via operator
commands.
[00120] In some embodiments, one more temperature sensors are also
employed to take continuous physiologic temperature readings of the tissues,
tumors, membranes, or other intraluminal tissues and/or devices (whether
organic or inorganic) in vivo, before, during, and after the application of
cryogenic
and/or thermal treatment modalities. In some embodiments, the system takes
continuous temperature readings of a cryogenic or thermal treatment device, in
vivo, and concurrently assess the temperatures, rates of temperature changes,
and depth of energy penetration into the intraluminal tissues to facilitate
control
of the distribution and/or application of the cryogenic or thermal treatment
modality in order to optimize tissue modification and/or dissection.
[00121] Fig. 9 represents a block diagram of the components and
operation of the electronics of the pump (22). The microcontroller (254) is a
RISC processor and lies at the heart of the electronics. Connected to the
microcontroller (254) through appropriate electrical signals are the usual
static,
dynamic, and flash memory (255) for firmware and data, lookup table (256), and
an interface (257) for communication with external devices. This interface can
be used for programming, updating, diagnostics, and/or control through a
Universal Serial Bus (USB) (258). An interface to a remote control
CA 02866746 2014-10-06
- 41 -
hand held unit (278), further described below, can also be established through
the interface circuit (257). Additionally, the pump includes a real time date
time integrated circuit (not shown).
[00122] A digital-to-analog (D to A) converter (268) is used to control the
pressure regulator that supplies air pressure to the balloon. The D to A
converter (268) generates an analog electrical signal (269) from OV to 10V
that
is proportional to the desired pressure. A series of analog-to-digital (A to
D)
converters (270) allows the microcontroller (254) to read the pressure signal
(250) at the pressure air tank (233), the pressure signal (251) at the output
of
the pressure regulator (238), the pressure signal (252) at the output to the
balloon, and the air flow (254) to the balloon.
[00123] Another series of digital outputs with appropriate interface circuits
(275) allows the microcontroller (254) to control the compressor (232)
(ON/OFF)
with command signal (253), the vacuum source (239) (ON/OFF) with command
signal (247), the deflate solenoid valve (240) (Open/Close) with command
signal
(248), and the inflate solenoid valve (241) (Open/Close) with command signal
(249).
[00124] A series of input circuits (276) are connected to switches on
the front panel of the pump (22) in order to input user controls, which is
further
described below. Additionally, a display driver circuit (277) interfaces the
microcontroller (254) to the front panel LCD display, also described below.
CA 02866746 2014-10-06
,
- 42 -
[00125] As shown in Figure 10A, in certain embodiments, the pump (22)
includes user control buttons in the form of soft keys (263) along the bottom
and
side of a graphical LCD display panel (264). The functions of the control
buttons
(263) are displayed on the LCD panel (264) and change depending on the mode
of the pump. The buttons (263) can be used to enter a setup mode, display
settings, recall collected data, or increase/decrease frequency and pressure.
In
addition to the soft key functions, the graphical LCD display (264) may show
the
pump's settings, pressure, frequency, and flow values, warnings, other
information such as time, date, and lapse time, and any other information that
may be useful to the physician for conducting the procedure and for gathering
procedural data, as shown in Figure 10B.
[00126] The front panel of the pump (22) includes a deflate button (259),
an inflate button (260), and a pulse button (261) to change the mode in which
the
pump (22) is operating. The front panel also includes an On/Off switch (265),
as
well as an emergency stop button (266), which stops the airflow to the balloon
by
closing the inflate valve (241) and opening the deflate valve (240) and
starting the
vacuum source (239). Also included on the front panel of the pump (22) is one
or
more keyed receptacle(s) (267) for the aforementioned keyed connector(s) of
the
balloon catheter.
[00127] In certain embodiments, the front panel of the pump (22) also
includes an interface (210) for a handheld remote control (278), as previously
CA 02866746 2014-10-06
- 43 -
described. This handheld remote control (278), shown in Figure 100, can be
located in the sterile field, and can be hardwired or wirelessly connected to
the
pump (22) using readily available communication technologies, such as infrared
or radio frequency (i.e. Bluetooth). Just like the front panel of the pump
(22), the
remote control (278) has three push buttons (259, 260, 261) for deflation,
inflation, and pulse commands. The remote control (278) also has a ready light
(262) that indicates when it is ready to accept a command.
[00128] As shown in Figure 11A-C, in another variation of the pump (22),
the compressor, the pressurized air tank, and the vacuum source are not
included. Even though the balloon could deflate faster with a vacuum source,
the
elasticity of the fiber mesh and latex balloon will still generate sufficient
frequency
to make it useful. As shown in Figure 11A, the front panel of the device
includes
a pump On/Off switch (215), a balloon TYPE selector knob (216), a balloon
OUTLET connector (217), a balloon inflation/deflation RATE selection push
button switch (218), and rate L (low), M (medium), and H (high) indicator LEDs
(219). As shown in Figure 11B, the rear panel includes a VAC power inlet
(220),
PRESSURE control knob (221), pressurized gas INLET connector (222) and
REMOTE control connector (223). The balloon pressure gauge is located on top
of the unit.
[00129] The operation of the system will now be described with
reference to Figures 12A-B. An initialization step includes setting up and
running diagnostic testing on all internal components, including pressure
CA 02866746 2014-10-06
- 44 -
transducers, flow meters, solenoid valves, etc., and displaying any warnings
or,
if no problems are detected, displaying a system READY indication to the user
(step 300).
[00130] After initialization, the pump opens the deflate valve and closes
the inflate valve to insure that there is no air pressure and flow at the
outlet to
the balloon catheter (step 302). The system will then read the internal tank
pressure (step 304). If the pressure is too low (decision block
306), the system will display the amount of air available and wait for user
confirmation to start the compressor (step 308). Alternatively, if an internal
compressor is not available, the air pressure at the inlet will be read and a
warning will be displayed to connect external pressured air.
[00131] The system will then display a message and wait for a balloon
catheter to be connected. When the balloon is connected, it will be detected
through electro-optical or electro-mechanical means (step 310) and display a
message to the user to confirm the balloon type (step 312). If confirmed with
the
user (decision block 314), the system will then display a message to the user
to
confirm that the balloon should be tested (step 316) and, if confirmed by the
user
(decision block 318), the balloon will be tested and pre-exercised (step 320).
The system will then display a message to the user (step 322), and upon
receiving confirmation from the user (decision block 324), will scan for a
command from the front panel, the remote control, or a serial interface (step
326). During the operation of the system and while waiting for a command,
CA 02866746 2014-10-06
- 45 -
receipt of the emergency stop command will cause the rapid deflation of the
balloon.
[00132] Each "inflate" command (command 330) will inflate the balloon by
an incremental amount based on the type of balloon that is connected
(step 332). This incremental inflation is accomplished by opening the inflate
valve for a set amount of time while the deflate valve remains closed. In this
way, the balloon is inflated to the size desired by the user. Alternatively,
pressing and holding the inflate button will inflate the balloon in a
continuous
fashion.
[00133] While inflating, the flow of gas (ml/sec) is measured (step
332). After closing the inflate valve, the balloon pressure is measured, and
an
approximation of the volume V is made based on the ideal gas law (V=nRT/P)
and the lookup table, which contains balloon characteristics and universal
constants (step 334). Here, T is assumed constant at 310 K (body temperature
can be measured and entered into the equation as well), R is a gas law
constant,
n is moles of gas, which is proportional to the measured flow, and P is the
measured pressure. With each incremental inflation, V is recalculated, and the
relative volume change (V2-V1) is displayed (step 336). Knowing the shape of
the balloon from the balloon identification, and using
the data from the lookup table, the relative change in balloon diameter (D2-
D1)
is also calculated and displayed. As shown in Figure 13, typical volume versus
flow time characteristic data can be depicted in a graphical format. A typical
CA 02866746 2014-10-06
- 46 -
characteristic performance curve of the balloon (400) is translated to an
actual
linear performance (401).
[00134] Similarly, each "deflate" command (command 340)
incrementally deflates the balloon by opening the deflate valve for set period
of
time while the inflate valve remains closed (step 342).
[00135] When the pump receives a "pulse" command (command 350),
the balloon is inflated and deflated in a pulsed fashion based on set
parameters
(step 352, decision block 354, step 356, decision block 358), which include an
inflation priority. In the pulse mode, this aspect of the inflate/deflate
cycles can
be set as desired. The pump has a feature to
control this function based on change in volume (delta volume) or frequency
priority. Because the gas pressure is maintained at a constant value (i.e., 2
atmospheres), the time it will take to inflate the balloon to the desired size
will
vary due to the different sizes and volumes of the types of balloons.
Therefore,
in the delta volume priority, the maximum and minimum frequencies are
calculated and set for the particular balloon used in order to maximize the
delta
volume between the inflated and the deflated states. In the frequency
priority,
the maximum and minimum delta volumes are calculated and set for the
particular balloon in order to maximize the frequency of the inflate/deflate
cycles.
CA 02866746 2014-10-06
- 47 -
[00136] Delta volume and/or frequency is calculated for each
inflation/deflation cycle, and the display is updated accordingly. If the
"Inflate"
button is pressed during this pulse mode, the pulse mode is stopped with the
balloon in the inflated state. Likewise, if the "Deflate" button is pressed
during
the pulse mode, the pulse mode stops with the balloon in the deflated state.
[00137] If the user wishes to change the set frequency and/or delta
volume for the pulse mode, this can be done by pressing the Up/Down soft keys
located on the LCD display panel (command 360, steps 362-364). The user can
also press soft keys located on the display panel to enter the status and
setup
displays (command 370, steps 372-374). These include screens to set up and
enter initialization data into the system, and to displaying data accumulated
during the procedure.
[00138] It should be noted that, during all states of operation of the
pump, the vacuum source is turned on and off to achieve faster deflation and
higher inflation/deflation cycles.
[00139] It should be noted that, while the described embodiments have at
times been described with respect to use on tumors and tissue, the system may
also be employed in other applications. Similarly, while the present invention
has
been described with respect to the pulsation mechanism of action described
herein, such action is not exclusive. That is, other mechanisms of action may
be
employed in addition to pulsation as needed, such as linear translation of the
CA 02866746 2014-10-06
- 48 -
balloon along the catheter, as well as rotation. Such motion may be particular
useful in cases, such as, for example, plaque excision and mucosa resection in
ENT applications.
[00140] Another example in which the above-described system can be
usefully employed is to remedy the decompression of compressed articulations
in restoring articular joint spaces, heights, and functions in a minimally
invasive
fashion. The decompression balloon includes a wide variety of shapes and
dimensions to address and replicate the broad
anatomic joint dimensions found in human and other mammalian bodies,
including the spine, knee, shoulder, hip, ankle, elbow, wrist, hands, fingers,
feet,
toes jaw, ribs, clavicle, and related articulations. An application of this
art would
be as a minimally invasive method to deploy an interspinous process spacer
comprised of a unique geometric, dimensional balloon construct that possessed
the ability, when inflated, to decompress the interspinous process
articulation.
The balloon construct could be inserted under endoscopic, radiographic, and/or
ultrasound visualization via a small incision and/or via wire guidance. Then,
the
balloon spacer would be inflated to provide the requisite decompression of the
interspinous process. As a result, the stress shielding and failure modalities
often
witnessed using current materials and methods can be mitigated. This method is
widely applicable to the many articular joints in the human and mammalian
bodies.
CA 02866746 2014-10-06
- 49 -
[00141] The above-described system can be used for minimally invasive
interventional treatment for Facet Joint fusion. A unique dimensionally shaped
balloon that mimics the articular surfaces of the facet joint is deployed to
the facet
joint via wire guidance under endoscopic and/or fluoroscopic visualization and
then inflated. The abrasive mesh-like surface of the balloon is concentrically
and
radially pulsed to create micro-abrasions upon the articular cartilage, and
ablative
energy is then applied to the conductive ridges atop the exterior surface of
the
balloon, eliciting decomposition and decortication of the articular surface.
Any
bleeding is tamponaded by inflating the balloon to create compression and/or
via
application of electrosurgical energy that is transmitted via the conductive
ridges
atop the exterior surface of the balloon. The balloon is then rotated to
further
decorticate and widen the articular space. The balloon is then deflated, and
an
inert implant, bone dowel, or other osteo-conductive and osteo-promotive
biologic
implant is then inserted along the deflated catheter and/or guide wire and
into the
articular joint space to create an interference fit and promote fusion. An
iteration
of this procedure would also include the deployment of a facet joint
replacement
implant. This procedure has broad application across the broad spectrum of
articular joint fusion and articular joint replacement.
[00142] It should be understood that the foregoing is illustrative and not
limiting, and that obvious modifications may be made by those skilled in the
art.
The scope of the claims should not be limited by the preferred embodiments set
forth in the examples, but should be given the broadest interpretation
consistent
with the description as a whole.