Note: Descriptions are shown in the official language in which they were submitted.
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
VAPOR PERMEABLE FABRIC CONSTRUCTS WITH STATIC OR DYNAMIC
ANTIMICROBIAL COMPOSITIONS
INTRODUCTION
[0001] Protecting valuable
military and aerospace assets, such as military
ground vehicles, weapon systems and other equipment on naval ships and support
equipment at airfields from corrosion, mold and mildew is a highly important
task for the
armed services. Equipment being used in the field has to be ready for use at
all times.
This need for readiness requires armed forces to spend a tremendous amount of
money,
time and manpower on the upkeep of equipment.
[0002] Common methods to
protect these assets vary from shelters with
controlled environments to shrink wrap films to heavy-duty-tarps. However all
of these
methods tend to have a shortcoming for day-to-day use and protection of this
valuable
equipment. Shelters cannot be built at all locations and they require a lot of
capital to
build. Shrink wrapping this kind of equipment with shrink film might provide
protection
for short periods of time, however most shrink film create a barrier where
condensation
cannot escape over time, thus creating a corrosive environment inside the
wrap. Heavy
duty covers, such as tarps, can be used on smaller items, however when it
comes to
larger equipment such as ground equipment or tanks, they add too much weight,
eliminating ease of handling ¨ taking away readiness.
[0003] One main issue with
armored military vehicles such as Bradley or
Abram Tanks is that their surface is made up of complex materials such as
"Chobham
Armour". Chobham Armour often uses a mixture of several ceramic material or
metal
matrix composites that combine metals, plastics and ceramic. Materials most
often used
on armor production include boron carbide, silicon carbide, aluminum oxide,
aluminum
nitride, titanium boride and synthetic diamond composite. By using these
materials,
armor manufacturers focus on creating the hardest possible surfaces at
lightest weight.
However, these hard surfaces most often work against a cover that is designed
to protect
the tank against environmental hazards, such as corrosion. The process of
dragging a
cover over a tank's armored surface eventually weakens most fabric and
shortens the
lifecycle of that cover.
1
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[0004] Oil and gas
exploration and pipeline industries also have problems
with environmental corrosion. According to NACE International corrosion is one
of the
leading causes of storage tank and piping failures. Corrosion related costs
for
transmission pipelines estimated at $ 7 billion annually in the U.S. alone and
another $ 5
billion for gas distribution. Protective covers are being used to prevent
corrosion on
flanges, valves and welded joints. A light weight post-formable fabric with
high water
vapor transmission rate can help prevent corrosion due to condensation and
other
environmental elements.
[0005] Automobiles and
motorcycles also need covers for transport or storage
in order to protect against paint damage and corrosion. As these vehicles get
transported,
it is important to provide sufficient protection against weather elements and
possible
road hazards such as stones.
[0006] Airport ground-
support equipment, such as fueling vehicles need
protection from environmental hazards as well. However this equipment and what
can
be used as a cover are highly regulated by aviation rules, due to fire hazard
that can be
caused by static electricity discharge.
[0007] A protective cover
system containing a moisture absorbing layer is
described in US 7183230, US 2005/0059306, US 2007/0228599, and US
2011/0027523.
[0008] There is a need for a
fabric construct that combines breathability,
flexibility, and durability against rough surfaces being covered, yet provide
a soft-touch
to a sensitive finished surface and that can be converted into protective
covers for
everyday use.
SUMMARY
[0009] The fabric constructs
of the present invention include a porous fabric
and breathable (i.e. vapor permeable) polymer coating applied to the porous
fabric layer,
where the end product yields a fabric composite that is water proof, flexible
and
breathable. The construct has a preferred water vapor transmission rate of at
least 100
grams / sq meter / day. Under certain embodiments, the fabric construct will
also include
a flocked layer applied to the porous fabric layer on the side opposite the
breathable
polymer coating. The flocked layer employs a combination of materials, size
spacing
2
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
and application parameters to create an energy dissipating fiber network which
is
resilient yet soft to the touch.
[0010] In other embodiments
the side of the construct opposite the breathable
polymer coating includes, instead of a flocked layer, a non-woven fabric that
likewise
provides a soft non-abrasive surface suitable for contacting an object to be
protected
without scratching. Covers formed from the fabric constructs - by either
custom sewing
or thermoforming to a certain shape by way of non-limiting example - are
highly
effective in use.
[0011] Whichever of a
flocked layer and a non-woven layer is present, it is
generally used in association with an adhesive package to assist in retaining
the flocking
or the non-woven on the host porous fabric. The adhesive is hydrophilic and is
preferably modified to be hydrophilic by incorporating hydrophilic titanates
and/or
hydrophilic zirconates as components. The hydrophilic nature of the adhesive
helps to
concentrate the water molecules closer to the highly permeable polymer layer.
Also. the
adhesive can be used as a carrier for a vapor corrosion inhibitor chemistry so
that the
fabric construct can be turned into corrosion preventative covers for military
vehicles
and equipment, aerospace parts and equipment, automobiles, boats, oil and
pipeline
equipment and other high value items that needs protection from environmental
hazards
such as corrosion, mold and mildew. Especially when flocked fibers are
present, the
adhesive preferably includes a foamed adhesive. In preferred embodiments, the
adhesive
contains a dynamic or a static antimicrobial composition.
[0012] Covers made from the
fabric constructs described herein will provide
protection from the environment similar to a tarp or shrink wrap film, but
also provide a
controlled micro-environment by means of high rates of breathability (100
grams / day /
sq meter or higher) to retard corrosion especially working in synergy with
vapor
corrosion inhibitors (VCI' s).
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] FIG.1 is a partially
separated perspective view showing the different
layers that make up the fabric construct of the present invention;
3
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[0014] FIG.2 is a cross
sectional view of a construct of the invention, Fig. 2a
showing separated components and Fig. 2b showing the integration of different
layers
with each other;
[0015] FIG. 3 is a
demonstrative view showing the fabric construct applied to
an object that is prone to corrosion;
[0016] FIG. 4 is a partially
separated perspective view showing the different
layers of an alternative fabric construct according to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
[0017] It is to be
understood that references to vapor and the like refer to
water vapor, i.e. water in the gaseous state. Vapor permeable materials allow
the passage
of water vapor. Waterproof materials resist the flow of liquid water.
[0018] Fabric constructs are
useful for being formed into protective covers
that protect a covered object from corrosion and other damage due to moisture.
The
constructs, and the covers made from them, allow water vapor to pass through
in a
preferred direction, while being waterproof and keeping liquid water out.
[0019] The constructs are
made by applying various polymeric compositions
as adhesive layers or as vapor permeable layers onto a porous fabric, and by
applying
flocked fibers or a soft non-woven fabric to the porous fabric by means of the
adhesives.
As used in the description and the claims, the fabrics, fibers, and
compositions that go
into making the constructs become part of the corresponding layers of the
construct,
which for ease or reference are referred to as layers of the construct. Thus,
when a
polymer composition is applied onto a fabric, the composition forms a layer on
the
fabric. This will apply to adhesive layers and to vapor permeable composition
layers.
When a porous fabric is incorporated into the construct, it will form a porous
fabric
layer. When flocked fibers are applied by means of (preferably foamed)
adhesive, they
form a flocked fiber layer of the construct, and so on. Manufacture of the
constructs is
described by the steps of applying various compositions to fabrics, or by
applying fibers
to an adhesive and the like. These applying steps result in incorporation of
all the
components of the thing applied into the final construct, with the exception
of volatiles
and the like that are removed during further processing. An example of the
latter is the
removal of water from the vapor permeable polymer composition.
4
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[0020] Especially when
dealing with applying the vapor permeable polymer
composition and the adhesive composition, it is to be understood that such
applying can
be carried in multiple coatings. Examples are the application of a primer
before applying
the vapor permeable composition, and the application of multiple coats of an
adhesive
composition, such as when a so-called "bottom coat" or "pre-coat" is applied,
or when a
"topcoat" of adhesive is applied over the bottom coat or pre-coat. Sometimes,
the topcoat
and pre-coat are made of the identical composition, so that the multiple
coating results in
a thicker deposition of material than would occur in a single pass. Multiple
coatings are
used to build up a layer or to provide a robust surface for the second coat.
Depending on
the application, a layer can be dried or cured between coats, or the coats can
be applied
and dried or cured together.
[0021] The present invention
relates to a fabric construct including a porous
fabric layer, and a vapor permeable polymer composition applied to the porous
fabric
layer, with at least one of a flocked fiber layer and a non-woven fiber
attached by means
of an adhesive composition to the side of the porous fabric layer opposite the
vapor
permeable polymer. Put another way (and equivalently in the structure that it
describes),
the construct includes 1) a porous fabric layer; 2) an adhesive layer applied
to the fabric
layer; 3) Flock fibers applied on the adhesive and cured by heat; and 4) a
water proof,
moisture vapor permeable polymeric composition applied to the porous fabric on
the side
opposite the fiber layer and cured by heat. The fiber layer is made of one of
a non-
woven fabric and a layer of flocked fibers, as explained in more detail below.
In one
aspect, a flocked fiber layer (also referred to as a layer of flocked fibers)
and a vapor
permeable polymer composition are employed on opposite sides of the porous
fabric
layer. In another aspect, a non-woven fabric and a vapor permeable polymer
composition
are employed on opposite sides of the porous fabric.
[0022] In various
embodiments, features of the individual layers are mixed
and matched to provide a wide range of constructs, all of which share the
feature of
protecting an object from the environment while advantageously removing water
vapor.
Options for selecting suitable materials as the porous fabric, the vapor
permeable
polymer composition, the adhesive composition, the primers, and the flocked
fibers are
provided in the description.
5
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[0023] As noted, in one
embodiment, a fabric construct according to the
invention is made of a porous fabric layer, an adhesive layer applied to the
fabric layer,
and a layer of flocked fibers applied to the adhesive layer. Further, there is
a
substantially waterproof, vapor permeable polymer composition applied to the
porous
fabric on the side opposite the flocked fibers. In various embodiments, the
porous fabric
is a non-woven, a woven, or a combination of a woven and non-woven fabric. In
various
embodiments, the adhesive layer is selected from acrylic latexes, urethanes,
and epoxies,
or is selected from thermoplastic hot melt rubbers, especially non-pressure
sensitive
compositions that lack tackifiers. In preferred embodiments, the adhesive is
hydrophilic
and can contain hydrophilic making additives selected from titanates and
zirconates,
especially hydrophilic titanates and zirconates. The adhesive layer can
further contain,
as needed, one or more additives selected from vapor corrosion inhibitors,
anti-static
agents, antimicrobials, biostatics, and fire resistant additives. The adhesive
preferably
contains a dynamic or a static antimicrobial composition. In preferred
embodiments, the
adhesive layer is foamed.
[0024] In another
embodiment, a non-woven fabric is applied opposite the
vapor permeable polymer composition, using the adhesive compositions described
herein.
[0025] The flocked fibers
(i.e., the fibers that are adhered to the porous fabric
to make up the flocked fiber layer of the construct) are selected from natural
and
synthetic fibers. In various embodiments, the flocked fibers are characterized
by an
average denier of between 0.5 and 90.0 and/or an average length between 10
mils (0.254
mm) and 380 mils (9.65 mm). The flocked fibers are capable of wicking moisture
in the
direction of the porous fabric layer to which they are attached by means of
the adhesive
composition.
[0026] In various
embodiments, the substantially waterproof, moisture vapor
permeable coating is made of a polyamide/polyether block co-polymer, or is
made of a
material that comprises a fatty acid modified ionomer at least partially
neutralized with
potassium or sodium, or is made of an aliphatic polyurethane coating. In a
preferred
embodiment, the vapor permeable polymeric composition comprises a potassium
salt of
a fatty acid and an ionomer comprising a plurality of carboxylate groups, at
least some of
which are modified by a potassium ion.
6
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[0027] In another preferred
embodiment, an aliphatic urethane coating is used
as the vapor permeable polymer composition. Suitable urethane coatings adhere
without
co-extrusion, and can be conveniently applied using extrusion coating
techniques. While
some coatings tend to weaken physical properties such as tear strength of the
underlying
fabric, the urethane coatings have been surprisingly found to actually
increase the tear
strength of the coated fabric and the fabric constructs containing them.
Advantageously,
the urethane coatings have good toughness reflected in good Taber abrasion
numbers. In
a preferred embodiment, the urethane coatings contain an anti-tack additive
that lowers
the abrasion property even further. This leads to a favorable anti-snagging
property of
the fabric construct as well as the other benefits.
[0028] A polyurethane
coating is advantageously applied to a primer layer
after a first layer containing the primer material is applied to the porous
fabric. To build
the fabric construct, this primer is applied to one side of what will later
become the
porous fabric layer of the construct either before or after the flocked fibers
or, as the case
may be, the non-woven fabric is applied to the other side. In one embodiment,
the primer
for the polyurethane coating is a water based acrylic latex primer.
[0029] The vapor permeable
polymeric composition preferably forms a top
layer (or topcoat) of the construct and has an average thickness of 0.5 mils
(0.0127 mm)
to about 10 mils (0.254 mm), in a preferred embodiment. The vapor permeable
polymeric composition forms a "top" layer having a high water vapor
transmission rate,
but it is generally impervious to transmission of liquid water (i.e., it is
said to be
"waterproof"). In a preferred embodiment, the water vapor transmission rate of
the
vapor permeable polymeric composition is at least 100 grams/square meter/day
at 37.8
degrees Celsius and 100% relative humidity. (The coating containing the
permeable
polymeric composition is normally rate limiting as to vapor permeability of
the construct
of which it is a part, such that the same levels of water vapor transmission
obtain for the
construct as a whole.)
[0030] The porous fabric
layer is made of a woven or non-woven material. In
some embodiments of the construct, the porous fabric layer is made of a two-
stage non-
woven fabric having a first side or stage characterized by fibers of a first
hydrophilicity
and a second side or stage characterized by fibers of a second hydrophilicity
different
7
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
from that of the fibers of the first side. The two-stage fabric is optionally
stitch
reinforced.
[0031] In another
embodiment, a method of manufacturing a cover material
that is substantially waterproof and permeable to moisture vapor involves the
steps of
providing a porous fabric layer and applying an adhesive to the fabric layer.
Thereafter,
flocked fibers or a non-woven fabric are applied to the adhesive layer, and
then a
substantially waterproof, moisture vapor permeable polymeric top layer is
applied to the
porous fabric on the side opposite the adhesive and the flocked fibers or non-
woven
fabric. The order of the steps can be changed as discussed further herein.
[0032] The adhesive is
applied to the porous fabric by spray coating, knife
coating, curtain coating, reverse roll coating, gravure coating, rotary screen
coating, and
the like. The adhesive in various embodiments includes vapor corrosion
inhibitors
(VCI). anti-static agents, antibacterial and biostatic additives,
antimicrobial
compositions, and/or fire resistant additives, depending on the conditions of
use.
[0033] Flocked fibers are
applied electrostatically and mechanically to the
adhesive. The flocked fibers are chosen from materials that are capable of
surviving the
conditions of any further manufacturing steps taken after they are flocked. In
non-
limiting examples, the flocked fibers are selected from rayon, acetate, nylon,
polyolefin,
acrylic, polyester, carbon fiber, cotton, hemp, and wool fibers.
[0034] In another
embodiment, a fabric construct is provided that comprises a
stitch reinforced two-stage non-woven entangled fabric having a water vapor
permeable
polymeric composition applied on a major surface of the non-woven fabric. The
two-
stage non-woven entangled fabric has a first major surface characterized by
having fibers
of a first hydrophilicity and a second major surface characterized by having
fibers
characterized by a second hydrophilicity. The hydrophilicity of the fibers on
the second
major surface is greater than the hydrophilicity of fibers on the first major
surface.
Finally, the vapor permeable polymeric composition is in contact with the
second major
surface of the fabric, i.e., the surface characterized by fibers of greater
hydrophilicity.
[0035] In various
embodiments, the non-woven fabric used in the fiber
construct is prepared by carding a layer of fibers having a first
hydrophilicity, placing a
stitch knit fabric on the carded layer, and applying onto the stitch knit
fabric a second
card of fibers having a second hydrophilicity. The hydrophilicities of the
first and
8
CA 2867131 2017-05-30
second card are different. After applying the two cards with the interposed
stitch knit fabric,
the fibers of the non-woven fabric are entangled, such as by hydroentangling
or needle
punching.
19036] In this
embodiment, the non-woven fabric is covered on one side by a
water vapor permeable polymeric composition as described herein. In preferred
embodiments, this permeable polymeric composition includes a fatty acid
modified ionomer
composition partially neutralized by sodium or potassium. Alternatively, the
vapor
permeable polymeric composition includes a polyamide/polyether block copolymer
or other
polymeric composition providing suitable vapor permeability. In another
embodiment, the
vapor permeable polymer composition includes an aliphatic polyurethane
coating.
[0037] In
another embodiment, covers are formed from the vapor permeable,
coated, and optionally flocked constructs. Depending on the nature of the
object to be
protected by the cover, the construct and the cover can be provided with
different vapor
permeabilities at different locations. In this embodiment, where the vapor
permeability in a
particular area of the cover is less than other areas or is essentially zero,
water vapor will
tend to migrate to areas of the cover with greater permeability where it can
escape.
[0037a] In
another embodiment, a fabric construct is provided that comprises a
porous fabric layer; an adhesive layer applied to said fabric layer; a fiber
layer applied to the
adhesive layer; and a waterproof, moisture vapor permeable polymeric
composition applied
to the porous fabric on a side opposite the fiber layer, wherein the fiber
layer comprises
flocked fibers or a non-woven fabric, and wherein the adhesive layer comprises
a static
antimicrobial composition and/or a dynamic antimicrobial composition, and the
static and/or
dynamic antimicrobial composition is chemically bonded to a vapor corrosion
inhibitor.
Structure of the constructs
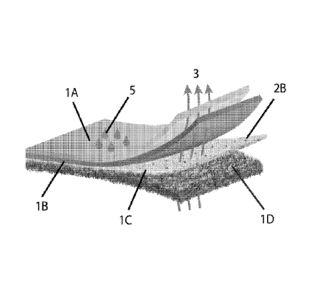
[0038] Referring to FIG.
1, the top layer of a fabric construct is a water proof
highly breathable (i.e. water vapor permeable or moisture vapor permeable)
polymer
composition (1A). Under 1A, there is a porous fabric with high tear and
physical properties,
yet low weight (1B). There is an optional primer layer (not shown) between lA
and 1B. 1B
can be a woven, a nonwoven or a combination thereof. Porous fabric (1B) is
coated by, or in
contact with, an adhesive layer (1C), where the adhesive may contain titanates
and vapor
corrosion inhibitors (2B) and other additives to enhance the properties of the
fabric
construct. Adhesive (1C) also locks the flock fibers (ID) in place,
perpendicular to the
fabric surface (1B). The fabric construct (1) is water proof keeping liquid
water (5) outside,
while allowing water vapor (3) to be pushed out in order to provide a modified
environment
to prevent corrosion.
9
CA 2867131 2017-05-30
[0039] While not shown in Figure 1, the porous fabric can be coated with
another
layer of adhesive on the opposite side of the flocked fiber and then laminated
to a highly
permeable polymer film made from a vapor permeable polymer composition such as
the
kind used for co-extrusion or extrusion coating. Further details of laminating
the highly
permeable polymer film are found in international publication WO 2010/022066.
[0040] In a more
specific and preferred embodiment, the fabric construct of the
present invention is manufactured by first providing a porous fabric and
coating one side of
the porous fabric with a pre-coat adhesive. Then a second coat of adhesive is
applied on top
of the pre-coat adhesive and flock-grade fibers are applied in a flocking
operation. The
fibers are applied by electrostatic and mechanical forces where they are
exposed to certain
amount of charge that keeps the fibers substantially perpendicular to the
adhesive coated
surface. Following the addition of fibers to adhesive layer, the adhesive is
cured in order to
lock the standing fibers in place. Thereafter, the flocked porous fabric
preform is extrusion
coated on the side opposite the flocked fibers with a breathable polymer
composition that is
designed to service outdoor environments.
[0041] Figure 2a
shows an expanded cross-section of a fabric construct of the
invention showing the individual layers. Thus, Figure 2a illustrates the vapor
permeable
polymeric composition 1A, the porous fabric 1B, the adhesive layer 1C, and a
flocked fiber
layer 1D. In an alternative embodiment, layer 1D is a non-woven fabric as
further described.
Although the invention is not to be thereby limited, Figure 2a also shows, in
illustrative
fashion, one example of the relative thicknesses of each of the layers.
[0042] Figure 2b
shows a cross-section of a construct of the invention wherein
the layers 1A, 1B, 1C, and 1D are combined into the construct. Thus, the vapor
permeable
polymeric composition 1A, the porous fabric 1B, the adhesive 1C, and the
flocked fibers (or
non-woven fabric) 1D are shown in cross-section in Figure 2b. Figure 2b also
illustrates that
the thickness of the construct is less than the additive thicknesses of the
individual layers.
This is seen in Figure 2b, for example, in the illustrated "overlap" of the
polymeric
composition 1A and the porous fabric 1B, shown as element 10. Further, the
adhesive 1C
and a flocked fiber layer 1D are shown to "overlap" at region 20 of Figure 2b.
Finally, the
adhesive and the porous fabric are shown to "overlap" at region 30 of Figure
2b. The
structure of the construct shown in Figure 2b is the result of the
manufacturing steps that are
carried out as described herein. In a preferred embodiment, a porous fabric 1B
is coated
with an adhesive 1C and flocked
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
fibers ID are added. The flocked porous fiber then undergoes a coating step,
such as by
extrusion coating to apply the vapor permeable polymeric composition IA.
Methods of making the constructs
[0043] The constructs are
put together in a series of adhesive coating,
extrusion coating, flocking, and other operations using conventional equipment
well
known in the industry. Suitable weights for the fabrics and suitable loadings
for the
applied coatings are chosen depending on the application and the result
required.
Depending on the final structure of the construct, more than one method may be
available to make it. Non-limiting examples of methods A through E are here
briefly
described. Further details are given in the rest of the description herein and
in the
Examples.
Method A
[0044] This method is used
to make a fabric construct with a polyurethane
vapor permeable coating and flocked fibers opposite the vapor permeable
coating. It
involves
applying a coating of aliphatic polyurethane on one side of a porous
fabric;
applying a foamed adhesive composition to the side of the porous fabric
opposite the polyurethane coating; and
applying flocked fibers to the foamed adhesive composition.
[0045] In some embodiments,
applying the foamed adhesive composition
involves applying a pre-coat and a topcoat, where the pre-coat and the topcoat
contain
vapor phase corrosion inhibitors (VCI) and a coupling agent selected from
titanates and
zirconates.
Method B
[0046] This method is used
to make a fabric construct where a vapor
permeable coating containing block copolymer or ionomer is applied opposite a
flocked
fiber layer. the method involves:
applying a foamed adhesive composition onto one side of a porous fabric;
applying flocked fibers to the adhesive composition; and
11
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
extrusion coating the vapor permeable polymer composition onto the
porous fabric on the side opposite the flocked fibers.
[0047] The vapor permeable polymer composition comprises a
polyamide/polyether block copolymer, a fatty acid modified ionomer at least
partially
neutralized with potassium ions and/or sodium ions, or a potassium salt of a
fatty acid
and an ionomer comprising a plurality of carboxylate groups.
[0048] In some embodiments, applying the foamed adhesive composition
involves applying a pre-coat and a topcoat, where the pre-coat and the topcoat
comprise
vapor phase corrosion inhibitors (VCI) and a coupling agent selected from
titanates and
zirconates.
Method C
[0049] This method involves:
applying a hot melt adhesive composition to one side of a porous fabric,
wherein the hot melt adhesive composition comprises a non-pressure sensitive
thermoplastic hot melt rubber and vapor phase corrosion inhibitor (VCI);
applying a foamed adhesive composition onto the adhesive composition
comprising the thermoplastic hot melt rubber;
applying flocked fibers onto the foamed adhesive composition; and
applying the vapor permeable polymer composition onto the porous fabric
on the side opposite the adhesive and the flocked fibers,
and wherein the hot melt adhesive composition is a hot melt block rubber.
[0050] In an embodiment, the foamed adhesive composition is selected from
acrylic latexes, urethanes, and epoxies.
Method D
[0051] This method, like Method A. is used for making a construct with
flocked fibers and a polyurethane permeable coating. It involves
applying a vapor permeable polyurethane composition onto one side of a
porous fabric;
applying a non-pressure sensitive hot melt block rubber composition
comprising VCI onto the side of the porous fabric opposite the polyurethane
coating;
12
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
applying a foamed adhesive composition onto the adhesive composition
comprising the thermoplastic hot melt rubber; and
applying flocked fibers onto the foamed adhesive composition.
Method E
[0052] This method can be
used to make a construct having a non-woven
fabric as the protective soft fiber layer. It involves:
applying a coating of aliphatic polyurethane on one side of the porous
fabric;
applying a hot melt adhesive composition to the side of the porous fabric
opposite the polyurethane, wherein the hot melt adhesive composition comprises
a
pressure sensitive thermoplastic hot melt rubber and vapor phase corrosion
inhibitor
(VCI);
adhering the non-woven fabric to the hot melt adhesive composition.
Method F
[0053] Another method of
making a construct having a non-woven fabric
involves a lamination process, namely:
applying a coating of aliphatic polyurethane on one side of a porous fabric
to make a first lamination part;
applying a layer of adhesive to one side of a non-woven fabric to make a
second lamination part; and
forming the construct by laminating the porous fabric side of the first to
the adhesive side of the second part.
Operation of the constructs
[0054] In one aspect, the
fabric construct of the present invention is useful for
advanced packaging operations. A packaging defines a volume for containing a
product.
The invention provides a method of packaging an object where the flocked side
of the
construct is facing the object to be protected, allowing the flocked fibers to
create a soft
surface where they will not damage the surface of the object being protected.
Yet, this
soft surface is resistant to abrasion, so that it can be placed over objects
that have very
rough surfaces, such as armored vehicles.
13
CA 2867131 2017-05-30
[0055] As will
be described in greater detail below, the material of the non-
woven fabric ID and/or size of the flocked-fibers 1D pull moisture, such as
condensation,
away from the surface of the object covered by the fabric construct or
packaging formed
therefrom. As the water is pulled by capillary action away from the surface
into the interior
of the construct, the cured adhesive composition will provide sufficient
polarity and/or
hydrophilicity by use of hydrophilic titanates or zirconates, by way of non-
limiting example,
to attract water molecules. As the water molecules are attracted towards the
hydrophilic
adhesive layer, a micro environment is created within the layers of the
construct where
water molecules are concentrated within the foamed adhesive where the relative
humidity is
at high levels. As the water moves further away from the surface of the
construct facing the
protected object and through the porous fabric in the middle of the construct,
the vapor
permeable polymer layer on the opposing side will allow the water vapor to
pass though. In
this way, the water inside the packaging will be attracted towards the outside
which has a
lower percent relative humidity than the interior micro environment of the
packaging.
Furthermore the porous fabric, either woven or nonwoven or a combination
thereof, will
allow the breathable (i.e. vapor penneable) polymer coating to penetrate well
into the fabric
during the calendaring process which results in an application sufficient to
pull water away
from the object being protected. As shall be understood by those skilled in
the art,
permeation rates are affected by temperature, humidity and pressure. According
to a
common rule of thumb, permeability increases by 30% to 50% for every 5 degrees
Celsius
rise in temperature (see Permeability Properties of Plastics and Elastanners,
2'd Edition,
L.K. Massey, 2003, ISBN:1-884207-97-9).
[0056] The
permeability or transmission rate of gasses and vapors through the
polymeric material is dependent upon two factors; the solubility of a gas or
vapor and the
rate of diffusion though the polymer matrix. The solubility function is
dependent upon the
chemical relationship between the peimeant molecule and the polymer; and the
rate of
diffusion is dependent up on the size of the permeant molecule and the
amorphous
configuration of the barrier polymer.
[0057] In
another aspect, the adhesive used in the fabric construct carries vapor
corrosion inhibitors, allowing the fabric composition to be used as a
corrosion preventative
cover. Use of vapor corrosion inhibitors is generally discussed in patents
U.S. 5,736,231
(Todt G. L., 1998); U.S. 5,705,566 (Todt G. L., Adhesive With Additive
Delivery System,
1998); WO 2010/022066 A2 (Todt & Ozol, Water Vapor Permeable Shrinkable
Fabric,
2010); PCT/US09/044686 (Todt & Ozol, Adhesive Composition and Method, 2010).
14
CA 2867131 2017-05-30
Suitable vapor corrosion inhibitors are disclosed in the referenced patents
and further below.
100581 Referring to FIG. 3, a general discussion as to the fabric construct of
FIG. 1 in a packaging form, e.g., cover, is also provided. Water vapor (3) is
removed from
the inside of a cover with the assistance of vapor corrosion inhibitors
(2A,2B,2C,2D). As
will now be described, the vapor corrosion inhibitors work essentially in
phases throughout
the moisture removal process. (2A) represents the corrosion inhibitor in its
initial foimat.
The corrosion inhibitors can be encapsulated (4) before application in order
to prevent loss
of VCI vapor (2C). (2B) represents the corrosion inhibitors inside an adhesive
layer. (2C)
represents the volatile corrosion inhibitor after it is volatilized from the
adhesive into the
package / cover; (2D) represents the corrosion inhibitor as it is condensed
onto a metal
surface to prevent corrosion on the object contained therein.
[0059] In a
preferred embodiment, the fabric constructs of the present invention
show a pattern, such as military digital camouflage. One way of doing this is
to mark a
pattern on the porous fabric before the flocking operation is carried out on
the side of the
fabric opposite the marking. One method of marking is to carry out a fabric
printing step.
Alternatively, a patterned fabric can be provided with conventional weaving
techniques.
Either way, the patterned fabric is subjected to a flocking operation to place
flocked fibers
on a side of the fabric opposite the desired print pattern. Then an extrusion
coating or other
process can be used to apply a breathable film coating on the print side
opposite the
flocking. In an embodiment, the breathable film coating is a clear coating
that allows the
marking, pattern, or print to show on the fabric construct opposite of the
flocked side.
Another way of providing constructs showing a pattern is to coat the fabric
with a
breathable polymer before any marking, printing, or flocking step. Then, the
fabric side
containing the permeable polymer composition is treated and printed, or the
polymer
coating can have color added in the form of a master batch.
[0060] With the
above teachings in mind, additional information regarding the
various materials and compositions employed in forming the fabric constructs
of the present
invention, and ultimately packaging formed therefrom will now be described.
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
Vapor permeable Polymeric Coatina
[0061] The fabric constructs
of FIGs 1-3 utilize a polymeric coating on one
side of a porous fabric. The polymeric coating provides good adhesion to the
porous
fabric and is breathable. By "breathable", it is meant that the polymeric
coating is
impermeable to liquids (and especially in many applications to liquid water)
but is
permeable to water vapor. If the breathable coating is impermeable to liquid
water, it is
called a "waterproof' coating. The layer of the construct formed from the
vapor
permeable polymer composition thus acts as a water barrier but allows vapor,
such as
water vapor, to pass through. Under preferred embodiments, the fabric
constructs have a
water vapor transmission rate (WVTR) of at least 100 grams / sq meter / day at
37.8 C
(100.04 F) or at least 120 grams/ sq meter / day at 37.8 C.
[0062] For the constructs
having flocked fibers, the porous fabric can be
coated with the polymeric coating before or after the flocking and printing
operations.
However the most desired application is to coat the porous fabric that has
flocked fibers
on the opposing side and that is printed on the same side as the coating. It
has been
surprisingly found that extrusion coating can be used to coat a vapor
permeable
polymeric composition onto a flocked fabric.
[0063] Suitable polymers for
the vapor permeable film are commercially
available. Polymers that allow high water vapor transmission rates as listed
on
Permeability Properties of Plastics and Elastomers by L.K. Massey include
polyether
amide and polyether ester with 300 to 1,000 and 200 to 900 grams of water per
millimeter / square meter per day are provided as examples.
[0064] Commercial polyether
amide resins are available through Arkema
under the trade name PEBAXO. PEBAXO is a polyether block amide, also called a
polyamide/polyether block copolymer with high physical properties, allowing
the
coating to be waterproof, yet provide high water vapor permeability. Examples
of
suitable resins from Arkema include MX 1205 SA 01; MV 1041 SA 01; MV 3000 SA
01; and MV 1074. These resins can be combined with UV stabilizers to provide a
coated breathable surface for the fabric composite.
[0065] The urethane coating
compositions normally contain other additives in
addition to the water dispersible polyurethanes. Examples are given in the
Table.
16
CA 2867131 2017-05-30
[0066] Another class
of suitable polymers for forming the vapor permeable
film is the fatty acid modified ionomers (FAM1) of Dupont"'. These are
polymers
containing a plurality of carboxyl groups in their backbone, formulated with
fatty acid
salts. In various embodiments, the carboxyl groups of the polymer and fatty
acid are
partially neutralized with sodium (following DuPont jargon, these are known
infon-nally
as "FAMI-Na" in the industry) or with potassium (likewise known as -FAMI-K").
[0067] The fatty
acid modified ionomers contain one or more E/X/Y
copolymers and one or more organic acids or salts thereof with a fraction of
carboxylate
groups being modified or neutralized with an alkali metal. The ionomers are
typically
combined with other polymers to provide suitable vapor permeable polymeric
compositions. A description of suitable ionomers and of vapor permeable
polymeric
compositions containing the ionomers can be found in U.S. Publications
2007/0287019
Al and 2007/0283652 Al.
[0068] Suitable
resins for use in the polymer composition of the present
invention include DuPont'r'E Entira" Breathe. These resins can provide water
vapor
transmission rates at one mil of up to 12,000 grams per square meter.
[0069] As noted in
the foregoing application(s) the disclosed breathable
polymers were intended for lamination to various fabrics. However, it has now
been
determined that these compositions are suitable for extrusion coating Onto
flocked fabrics
in processes of the present invention. Unlike a lamination process; an
extrusion coating
process allows the coating to be embedded into the fabric, allowing the
thickness of the
fabric constructs to be reduced. Blends of water vapor permeable resins which
are
suitable for extrusion coating are also described in US Patent Applications
Serial No.
12/762,818 (Chen, 2010), now U.S. Publication 2010/0272914, published October
28,
2010 and Serial No. 12/762,919 (Chen, Method For Preparing A Selectively
Permeable
Protective Structure, 2010) now U.S. Publication 2010/0272898, published
October 28,
2010. The extrusion coated layer can either be a monolayer application or a co-
extruded
application with two or more layers.
[0070] A non-limiting example
of a polymer blend that can be used as a
coating is a blend of DuPonfrm Elvaloy@AC 1224 - 50% by weight of polymers;
DuPontImEntiralm Breathe - 40% by weight of polymers; DuPontTN1FusabondD FB556
-
17
CA 2867131 2017-05-30
10% by weight of polymers primary. The composition blend will support the
inclusion a
weathering package, such as one containing one or more additives selected from
primary
and secondary antioxidants, UV stabilizers and hindered amine light
stabilizers by way
of non-limiting example. Suitable additives for the weathering package include
Tinuvinfm
TM TM TM
328, Tinuvin 770, Chimassorb 944 and Irganox 100 from BASF (Formerly Ciba
Specialty Chemicals). Preferred loading of the weathering package is preferred
to be 17c
or less of the total polymer by weight. Possible loading combinations can be
seen in the
table. These packages will protect the polymer structure in processing and
outdoor
weathering during its use.
Table 1. Exemplary stabilizer recipes for the polymeric coating
Preferred Range
Package #1 Property % in Film ppm F iii Film ppm
=Ilinavin 770 11ALS - Low Molecular Weight 0.20%
2000 - 0.30% 3000
(11iniassorb 944 lIALS - high _Molecular Weight 0.10% 1000 0.20%
2000
Tinuvin 328 High Performance UVA 0.20% 2000 0.40% 4000
Irganox 1010 Thermal Stability 0. I OF 1000 0.10% 1000
TOTAL 0.60% 6000 1.00% 10000
Preferred Range
Package 2 Property % in Film ppm % in Film ppm
Tilluvin 770 fIAT .5 - I bw Molecular Weight 0.00% 0 0.00% 0
Chimassorb 944 HALS - High Molecultu- Weight 0.25% 2500 0.40%
4000
Tintivin 328 high Performance UVA 0.25ch 2500 0.400'r 4000
Irganox 1010 Thermal Stability 0.10% 1000 0.20% 2000
0.60% 6000 1.00% 10000
[0071] If desired, the vapor permeable polymeric layer of the fabric
composition can be further enhanced by use of other additives including
antimicrobials,
colorants, coupling agents, flame retardants, mold release agents and
antistats, among
others.
[0072] Because the unique properties of the above described resin blends,
additive packages can be limited to no more than 1%. In order to improve the
anti-static
properties of the fabric constructs described herein, the coating blends can
be further
modified as described by European Patent EPI 569 794 B1 (Chen, 2005).
Rendering the
fabric constructs anti-static in nature will allow for its use in certain
highly regulated
18
CA 2867131 2017-05-30
industries such as the aviation industry, where static electricity regulations
are heavily
applied in regards to airplane fueling / de-fueling. En aviation industry
plastic containers
are not to be used for into-aircraft refueling or defueling as the static
electricity charge
potential is sufficient to cause a spark with potential explosive results.
100731 Urethane
("polyurethane" is used interchangeably) coatings can also
be used. Urethane coatings are preferably selected from aliphatic polyether
urethanes
that are water soluble or water dispersible. The aliphatic urethanes are based
on
polymers of saturated isocyanate monomers such as hexamethylene diisocyanate
(HDI)
and isophorone diisocyanate (IPDI) with polyethers such as polyethylene oxide.
In
addition to the hydrophilic polyethers, water dispersible groups such as
carboxvlate and
sulfonate can be incorporated into the urethane polymer system to render them
water
dispersible, using known methods. The coatings preferably exhibit a high
hydrostatic
head measured according to standard industry tests such as ISO 8.11, Suitable
urethane
dispersions are commercially available, for example from Bayer under the
Imprani10.
Baybond , and Dispercoll trade names, and also from Michelman (HydrosizeM,
Lubizol (Sancure8), and Clierntura (Witcobond0). The urethane coatings are
waterproof and highly breathable, providing the vapor permeable polymer
composition
for the -outside" surface of the fabric constructs. Additionally, suitable
polyurethane
vapor permeable coatings are described in US 2010/0266774 Al published October
21,
2010.
[0074] The urethane
coating composition applied to the porous fabric or to a
primer layer on the porous fabric generally contains a urethane dispersion, a
crosslinking
resin, and a rheology modifier. Optional further ingredients include anti-tack
agents,
plasticizers, crosslinking catalysts, defoamer, pH adjusters, mineral
extenders (e.g.
calcium carbonates) as flattening agents, tack modulators, or cost reducers,
dispersants,
antimicrobial agents, pigments, pacifiers, dyes. colorants, antioxidants,
anti-aging.
agents, UV resistance agents, anti-weathering agents, and the like. Further
illustrative
examples arc provided in Table 2 giving a typical formulation.
19
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
Table 2. formulations for the polyurethane vapor permeable polymer composition
Low High Exemplary Component, function, and/or supplier and trade
names
25 75 35 Water
3 Polyurethane dispersions/emulsion, preferably
aliphatic, selected for
1 50 weatherability and to modulate MVTR (Bayer: Impranil, Bay-
bond,
Dispercoll, Michelman: Hydrosize, Lubrizol: Permax, Sancure,
Chemtura: Witcobond)
Non-polyurethane dispersion/emulsion, preferably acrylic,0 selected
0 50 for weatherability and cold flexibility (Lubrizol: Acrylic,
Omnova:
Omnapel, Acrygen, BASF: Acronal, Dow: Rhoplex, UCAR)
5 Paraffin/polyethylene wax emulsion selected for water
repellancy and
0 20 surface tack reduction (Lubrizol: Aquaslip. Michelman: Michem
lube,
BykChemie: Aquacer, Aquamat)
0 20 Plasticizer selected for weatherability and cold flexibility
(phthalates,
benzoate esters, citrates, phosphate esters, adipates)
1 Crosslinkable resin and modified melamines (Cytec:
Cymel, Aerotex,
0.1 10
Ineos: Resimene, Lubrizol: Carbocures)
Crosslinking catalysts selected to depress activation temperature of
0 5
crosslinking resin (pTSA, ammonium chloride, etc)
0.2 Defoamer additive selected to control air entrainment
during
0 1
manufacture (BykChemie: Byk, Emerald: Foam Blast)
0.1 10 1 Rheology modifier (cellulosiic, associative, polyacrylates,
xanthum
gums, bentonites)
0 5 0.8 pII Adjustment (ammonia, caustic)
10 Mineral extender selected as flattening agent, tack
modulator and cost
0 50 reduction (Minex: Nepheline Syenite, Specialty Minerals:
Marblewhite
calcium carbonates)
1 Dispersant/surfactant selected to deagglomerate
slurries, stabilize
0 5 suspensions and viscosity (RT Vanderbilt: Dana, Byk Chemie:
AntiTerra, Byk, BASF: Dispex, Dow: Triton, Rhodia: Igepal)
2 Antimicrobial agent selected for dry state microbial
and fungus
0 5 resistance (Troy: Polyphase, Arch: Proxel, Densil, Omadine,
Dow:
Skane)
0 10 3 Pigmentation, Colorant, pacifier, Dye (BASF: Aurasperse, Sun
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
Chemical: Sun, Ferro: Plasticolors: Colormatch, Keystone: Keysperse)
0
1
Antioxidant optional selected for aging, weathering, and UV resistance
(BASF: Trganox, Tinuvi n)
[0075] The urethane coatings
are applied by direct coating or by transfer
coating, using conventional coating equipment. A water based acrylic latex
basecoat or
primer is preferably used to enhance coverage and adhesion of the urethane
permeable
coating. In a typical embodiment, the urethane is applied at about 0.1 to 10
ounces dry
5 weight per
square yard, for example about 4 ounces per square yard. Wet thickness of
the application is about 3 to about 60 mils or about 8 -20 mils. In particular
embodiments, the thickness is 16-18 mils or about 17 mils.
Extrusion coating
[0076] While aqueous
polyurethane dispersions are applied by direct or
transfer coatings or with suitable methods such as the knife over roll coating
method,
vapor permeable coatings with solid components can be applied by extrusion
coating the
permeable polymer compositions onto a porous fabric that contains flocked
fibers or, in
another embodiment, that contains a non-woven fabric on the side of the porous
fabric
opposite the vapor permeable coating. Advantageously, the constructs are
characterized
by an adhesion peel strength in the preferable range of 500 Win (197 g/cm) and
higher. In
various embodiments, the peel strength is 600 Win (236 g/cm) or higher, 750
Win (295
g/cm) or higher, 800 On (315 g/cm) or higher, 900 On (354 g/cm) or higher, or
1000
On (394 g/cm) or higher. Suitably high peel strengths are required of the
construct when
used in challenging conditions characterized by high and low temperatures,
high and low
humidity, high UV, and rough handling typical of contemplated military uses.
[0077] For conventional
polyolefins, it is known that the modulus changes
greatly with temperatures, it being typical to see differences of up to 600%
from low
temperature extremes to high temperatures. Under these conditions, coatings
with low
bond strength tend to delaminate, especially when subjected to temperature
extremes of -
30 C to 60 C and/or subject to rough and frequent handling. Not only must the
bond
strength be sufficient to survive the six fold variance in modulus during use,
it also must
resist delamination caused by water vapor penetrating between the fabric and
coating.
For these reasons, high bond strengths are required.
21
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[0078] To the extent that
the fabric constructs of the present invention are
extrusion coated, it is contemplated that coatings in the range of 0.5 to 10
mils can be
employed. A range of 3 mils to 7 mils is preferred in some embodiments to
balance the
water vapor transmission rate and the stiffness of the whole structure. As the
polymer
coating thickness gets higher the "hand" or the softness of the fabric
increases and the
water vapor transmission rates go down. The extrusion process can be set up in
various
methods. The polymer blend can be pre-compounded with all resins and additives
or
they can be blended at the time of extrusion coating as should be understood
in the art of
extrusion coatings.
[0079] A preferred method
for applying a breathable polyurethane top coat is
different than extrusion coating. Since the water based polyurethane is liquid
at room
temperature, the top coat is pumped on top of the fabric and the thickness is
adjusted by
using the -knife over roll" method. The most preferred method for applying a
breathable
polyurethane is to apply a pre-coat to improve adhesion to the fabric. In an
example, the
pre-coat is a water based acrylic latex applied in two passes by the knife
over roll
method. This pre-coat is preferred to foam at a 5:1 ratio. Each pass of pre-
coat is at 0.5
oz/sqy, by way of non-limiting example. Following the pre-coat the water based
polyurethane coating is applied, for example, at about 3.2 oz/sqy and the
whole
application is cured by passing through an oven heated up to 340 F.
Porous Fabric:
[0080] One objective of the
porous fabric is to provide a strong core for the
fabric constructs. A high strength to weight ratio is desirable because as the
objects
being covered get larger the covers designed to go over these objects get
heavier and
they require more man power and time for placement and removal. Therefore a
light
weight fabric is desirable. Also, covers made from the fabric constructs of
the present
invention need to have enough physical strength to provide sufficient
protection from the
environmental hazards. Military vehicles, navy equipment on board ships and
some
industrial equipment are often used, transported or stored under undesirable
weather
conditions such as hail storms, high wind storms or hurricanes. Due to these
conditions
the fabric composite must be able to withstand tearing or ripping. If any tear
or ripping
takes place, preferably the fabric construct will be designed so as to limit
their spread.
22
CA 2867131 2017-05-30
[0081] The fabric
constructs of the present invention utilize a woven, a
nonwoven or a combination of woven and non-woven fabric as the porous fabric
layer to
give rise to a so-called core layer. As a non-limiting example, materials
suitable for the
core layer may be made of organic fibers such as cotton or hemp, or made of
synthetic
fibers such as Nylon 6, Polyester, or glass fibers. Polyester fabrics have
been found to be
suitable. The core fabric layer may also be a blend of an organic and a
synthetic fiber as
well. A non-limiting example of a woven fabric that can be used as the porous
fabric
TM
layer is a textured polyester fabric named 380 Greige Fabric available through
Milliken
Company. Depending on the application and the properties of the components of
fabric
construct other than the porous woven fabric, various weaving patterns of the
fabric can
be selected for best performance. For example, in some embodiments it has been
observed that a twill pattern in the porous fabric increases the rate at which
liquid water
is wicked through the fabric construct to be released as vapor on the
permeable coating
side. Other weave types such as plain or satin may be used. = In one
embodiment, a
suitable fabric has a basis weight of 4.5 ounces/square yard and has a tensile
strength of
261 x 205 lbs (Warp x Filling). A preferred fabric would be an 8.5 oz/sqy
fabric with a
twill pattern. In order to improve physical properties the woven fabric may
incorporate
"rip stop"; which is a special reinforcing technique that makes a fabric
resistant against
tearing and ripping. This technique involves reinforcement threads that are
interwoven
typically 0.2 to 0.3 inches apart and creates a better weight to strength
ratio where small
tears and rips are less likely to spread. Threads employed for the rip-stop
can either by a
Nylon 6,6 or a PET fiber, by way of non-limiting example.
[0082] A non-
limiting example of a commercially available non-woven
material suitable for use as the porous fabric core is the Komandaaproduct by
Norafin.
Komanda0 products can have various basis weights, for example from 185 gsm ¨
210
gsm and may have varying ratios of cotton-to-polyester with an integrated
polyzi.unide or
PET scrim in the center of it.
[0083] Regardless of
the material(s) employed for the porous fabric layer,
otherwise referred to herein as the core layer, it is preferable that the
materials be
drapeable, flexible, and be able to hold print. Furthermore the porous fabric
needs to
provide physical properties such as Tensile Strength and Tear Strength in
order to create
a strong fabric construct.
23
CA 2867131 2017-05-30
Adhesives:
[0084] In various
embodiments, the invention provides a fabric construct as
variously described herein wherein the construct contains a special adhesive
component.
An adhesive composition is used to apply a soft fiber based material to the
porous fabric
of the construct. The soft fiber based material in turn is the structure that
faces or is in
contact with an object to be protected in use of the fabric constructs. The
adhesive
provides many benefits to the construct, including vapor corrosion inhibition,
tear
strength, and water/vapor wicking through the construct, to mention some non-
limiting
examples. In prefeiTed embodiments, the adhesive composition is hydrophilic,
it is
based on contact adhesives, and is used in a foamed state.
[0085] In some
embodiments, a single adhesive layer is used. The single
layer is preferably a pressure sensitive layer containing VCI and other
additives. This is
an adhesive suitable for adhering a non-woven fabric fiber layer to the porous
fabric
layer of the construct. The VCI is
optionally encapsulated, for example in a
polypropylene shell. In another embodiment, a two part or two layer adhesive
is used to
adhere the soft fiber based material to the porous fabric. When two layers are
used, the
first is a non-pressure sensitive layer preferably containing VCI chemistry.
In various
embodiments, the non-pressure sensitive layer contains a hot melt
thermoplastic rubber,
also called a hot melt block rubber. It is applied where needed using a
conventional hot
melt application system. If a pressure sensitive adhesive is required, the hot
melt
adhesive can contain suitable amounts of tackifiers. Details of the pressure
sensitive and
non-pressure sensitive adhesive compositions, and of methods of making the
compositions, useful for making the constructs of the invention are given in
international
publication W02009/143251.
[0086] In one embodiment, a
first layer comprising the hot melt is applied to
the porous fabric layer. A top layer of adhesive is then added that is
preferably foamed,
and is an acrylic latex adhesive, in a non-limiting embodiment. The flocked
fibers or the
non-woven fabric of the soft fiber based material are then applied to the
adhesive
topcoat.
[0087] When desirable to
increase the recyclability of the fabric construct, the
material of the adhesive can be selected to match the main polymers present in
the other
layers of the construct, For example, the adhesive material can be based on a
polyolefin
24
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
or polyethylene, especially when the soft fiber layer or the vapor permeable
coating
composition is of the same material.
[0088] Use of an adhesive
and especially use of a foamed adhesive has been
found surprisingly to contribute to a higher tear strength of the porous
fabric on which it
is applied and of the construct of which the porous fabric with the applied
adhesive is a
part. In preferred embodiments, the adhesive composition is rendered
hydrophilic by
incorporation of titanate or zirconate additives, as described further herein.
[0089] The adhesive applied
as a foamed adhesive can be an all solids,
solvent based or water based adhesive. Non-limiting examples for suitable
adhesives are
acrylic latex, urethanes and epoxies. The adhesive can be used either straight
or foamed.
A suitable adhesive is water based acrylic latex adhesive that can be foamed.
A closed
cell foamed adhesive with air pockets is preferred in some embodiments, as it
is believed
to improve the overall breathability of the fabric construct. A non-limiting
example for a
suitable adhesive is 3822 from Key Polymer in Lawrence, MA.
[0090] It is also preferred
that the adhesive be further modified to incorporate
a titanate or a zirconate coupling agent in order to render the adhesive more
hydrophilic,
where the water molecules would be attracted towards the adhesive as they are
being
pulled by the capillary action of the flocked fibers or non-woven fibers of
the soft fiber
based material. Preferred types of titanates and zirconates are available from
Kenrich
Petrochemicals as LICA 38J and KR 44. A suitable loading calculation for the
titanates
is at about 1% of the polymer content plus 0.8% of the solid content in the
adhesive. The
adhesive is preferred to be further modified to carry a vapor corrosion
inhibitor (VCI)
package that is designed to be used in a water based system. The corrosion
inhibitors are
preferably designed in a fashion that they have "multi-metal" corrosion
preventative
chemistries. In a preferred embodiment, the VCI package is a multicomponent,
multimetal package that contains no nitrites or nitrates. An example of a
multi-metal
corrosion inhibitor package is VCI Powder TS 1335 from Desi Kimya in Istanbul,
Turkey. Since the adhesive is preferably in foamed format, the air pockets in
the
adhesive allow the VCI to volatilize. Furthermore the flock fibers or non-
woven fibers
of the soft fiber based material create more air between the metal surface and
the VCI
molecules allowing the VCI to condensate over the metal. The condensation of
VCI' s
allows a better, more uniform coverage of the metal surface, creating
effective corrosion
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
prevention. In a preferred embodiment the adhesive will have between 1% and
10%
"active" VCI chemistry. The VCI chemistry is more active with the high
moisture
content. Since the adhesive is preferably a hydrophilic rendered latex
adhesive, the
moisture captured in the air pockets of the foamed adhesive will improve the
effect of
VCI chemistry on the covered object. The flocking operation, as will be
described in
greater detail below, requires heat setting and drying at high temperatures.
Some vapor
corrosion inhibitors can be heat sensitive by nature. In order to prevent loss
of VCI or
degradation of these chemistries the vapor corrosion inhibitors can be
encapsulated by
protective wax polymers such as polypropylene or polyethylene as is known in
the art.
[0091] It is important that
the adhesive remain flexible in cold weather
environments. A nonflexible adhesive application, one that has a high glass
transition
temperature, would turn rigid, thus covers made from the fabric constructs
would lose
drape-ability.
[0092] The overall average
thickness of the adhesive layer, including the pre-
coat or the top coat combined, ranges from about 0.02 mils to about 4 mils
depending on
the intended application. Flocked fibers anchor into the adhesive from 10% of
its length
to 50% of its length. When flocked fibers are used, the adhesive is preferably
foamed.
[0093] Preferably, the
adhesive contains an antimicrobial composition that
contains a compounds or mixture of compounds having antibacterial activity. In
one
embodiment, the antimicrobial composition is dynamic, meaning that it can
volatilize by
having its own vapor pressure, or it is attached or covalently bonded to the
vapor
corrosion inhibitor (VCI). In embodiments, the volatile VCI will carry the
antimicrobial
and make it dynamic. In embodiments, the dynamic antibacterial is carried to
the metal
surface of the item being protected, where it protects the system from mildew
and mold
formation. One example of a dynamic antimicrobial composition is one that
contains
hexamethylenetetramine (HexamineO) as an antimicrobial compound. In
embodiments,
it is provided in combination with VCI' s selected from primary amines,
secondary
amines, and triazoles. A static antimicrobial composition is one where the
antimicrobial
(e.g. mildicide, fungicide) compound(s) is not volatile and it stays within
the fabric
composition eliminating any microbial ¨ e.g. mold and mildew ¨ growth on the
fabric.
[0094] Additional examples
of the adhesive compositions, and of methods of
making the compositions, useful for making the constructs of the invention are
given in
26
CA 2867131 2017-05-30
international publication W02009/143251.
VCI Particles
[0095] The adhesive
compositions also contain vapor = phase corrosion
inhibitors (VCI). These are provided in powder format, and are dispersed in
the adhesive
polymer along with the coupling agents. In use, a VCI reaches the surfaces
that it must
protect from corrosion through the vapor phase. This transport mechanism
requires that
protective molecules be characterized by having a suitable vapor pressure. In
an
alternative, a VCI compound reacts with moisture or other system components to
generate a volatile species with vapor phase corrosion inhibition 'properties.
In one
aspect, vapor phase corrosion inhibitors are volatile chemistries that an be
adsorbed on
the metal surface. The rate of adsorption of the volatile component and the
temperature
dependent vapor pressure affects the rate and level of inhibition.
[0096] Selection of
suitable VCI's is guided by their final application
environment and the metals that need to be protected. For examples, mixtures
of several
different inhibitors are usually called for, since most articles to be
protected are made up
of different metals and metal alloys. Information on different VCI chemistries
for multi
metal applications can be found on Reviews on Corrosion Inhibitor Science and
Technology, papers given at the CORROSION/89 symposium (NACE Press, 1989), and
in particular the paper of G.E. Fodor entitled "The Inhibition of Vapor-Phase
Corrosion:
a Review" that begins on page 11-17-1 the disclosure of which is useful for
background
information.
[0097] Non limiting
examples of vapor corrosion inhibitors include: primary,
secondary and tertiary aliphatic amines; aliphatic diamines; cycloaliphatic
and aromatic
amines; polymethylimines; long chain ethanolamines; imidazolines; amine salts,
for
example those of carbonic, carbamic, acetic, benzoic, oleic, nitrous and
chromic acids;
acetylenic alcohols; lauric alcohol; alkyl chromates; organic esters= of
nitrous acid;
organic esters of phthalic acid; organic esters of carbonic acid;
nitronaphthalene;
nitrobenzene; amides; mixtures of nitrites with urea, urotropine, or
ethanolamines;
naphthols; thiourea derivatives; heterocyclic compounds such as benzotriazole,
27
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
tolyltriazole, and mercaptobenzothiazole and their respective salts; nitrated
or sulfonated
petroleum derivatives; and organic acid derivatives.
[0098] In
various embodiments, it is desirable to incorporate VCI that have
antimicrobial and especially antifungal activity. For example, it has been
shown that
meta-dinitrobenzene inhibits fungal growth, sporulation and pigmentation of
fungi
including: aspergillus japonicus, curvularia lunata, penicillium pinophilum,
trichoderma
sp., and cladosporum sp.
[0099] In
various embodiments, the VCI in powder format contains particles
that are 100% active corrosion inhibitor with no inert carrier. In this
situation. the VCI
comprises particles that consist of an active corrosion inhibitor or mixture
of corrosion
inhibitors.
[00100] Before incorporation into adhesives in various embodiments, powders
of VCI can be mixed with other powdered materials, including powdered
materials that
do not themselves have the property of being a vapor phase corrosion
inhibitor. As non-
limiting examples they can be mixed with silica powder to improve handling; or
can be
mixed with antioxidants and/or UV stabilizers that are used in adhesive
manufacturing.
In this situation the VCI mixture with the other powder contains particles
consisting of
the VCI and particles consisting of the other material such as silica,
antioxidant,
stabilizer, and the like. Vapor corrosion inhibitors suitable for use in the
present
teachings include those that are available in a powder format at room
temperature and at
temperatures to which the inhibitors are exposed during manufacture of the
adhesive
compositions. In a preferred embodiment, the inhibitors are solids at room
temperature
and remain solids at a temperature up to at least 100 C. The inhibitor
components
themselves have suitable vapor pressure for releasing from the adhesive in
use, or else
they are capable of reacting with moisture and/or other components to generate
a volatile
chemical compound or compounds that can provide the desired corrosion
inhibition.
[00101] To
illustrate sodium nitrite is a suitable VCI, it is a solid up to a
temperature above 100 C. Although the invention is not limited by theory, it
is believed
that NaNO2 provides vapor phase corrosion protection in part by participating
in
reactions in the presence of moisture and other inhibitors to provide volatile
inhibitors
such as formaldehyde, ammonia compounds, amides, and the like.
28
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00102] One class of vapor phase corrosion inhibitors is an organic nitrogen
base salt of nitrous acid, also referred to as nitrite salts. The organic
bases that form
nitrite salts are generally selected from amines, guanidines, alkylated
imidazolines,
nitrosamines, and the like. Examples of nitrites include those of primary
amines,
secondary amines, tertiary amines, cyclic secondary amines (e.g. piperidines,
oxazines,
morpholine, thiazolines, thiaoxazines, diazoles, basic diazole derivatives,
imidazolines,
diazines, basic diazine derivatives, pyrrolidone, basic pyrrolidone
derivatives, ureas,
thioureas, hydrazines, hydroxylamines, amidines, guanamines, guanidine. In any
of the
above nuclei, alkyl, cycloalkyl, terpinyl, bornyl, aralkyl, benzyl, phenyl,
aryl, and
various substituent groups or radicals may be present sol long as the total
basicity of the
organic nitrogenous compound is sufficient that it can form a nitrite salt by
reacting with
nitrous acid.
[00103] Examples of nitrite salts include those of organic nitrogen bases such
as:
) Primary amines such
as: methylamine, isopropyl amine, 2-amino-
butane, tertiary butyl amine, 2-amino-4-methyl-pentane, various amyl, hexyl,
heptyl,
octyl and higher homologous primary amines where the amine group is attached
to a
secondary or tertiary atom; cyclopentyl amine, alkylated cyclopentyl amines,
cyclohexylamine, mono-methyl cyclohexylamines, dimethyl cyclohexylamines,
trimethyl cyclohexylamines, other alkylated cyclohexylamines, bornyl amine,
fenchyl
amine, cycloterpenyl amines, pinyl amine, benzylamine, betaphenylethylamine,
alkylated benzylamines, tetrahydro betanaphthylamine, allyl amine, beta-methyl
allylamine, beta-chloro allylamine, and their homologs and analogs;
2) Secondary amines such
as: di-methyl-, di-ethyl-, di-n-, propyl-, di-
isopropyl-, di-butyl-amines; various secondary amines derived from amyl,
hexyl, heptyl,
oxtyl, and higher homologous alkyl groups; methyl isobutyl amine, N-methyl N-
tertiary-
butyl amine, N-alkyl N-cyclohexyl amine, N-alkyl N-bornyl amine, di-bornyl
amine, N-
methyl N-cycloterpenyl amine, N-isopropyl N-(1)-methyl amine. N-alkyl N-benzyl
amines and their homologs and analogs; dicyclopentyl amine, di-cyclohexyl
amine,
alkylated dicyclohexyl amines; dibenzylamine, di-(beta phenyl ethyl) amine;
piperidine,
piperazine, alkylated piperidines or piperazines: alkylated and unalkylated
oxazines such
29
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
as morpholine and 2,4,4.6-tetramethyl tetra-hydro-1,3-oxazine; alkylated-1,3-
thiazolines
such as 2,4.4,6-tetramethyl tetrahydro-3-thiazoline;
3) Secondary amine type derivatives of alkylene diamines, such as: R1¨
NH¨R2¨NH¨R3 wherein R1 and R2 may be like or different aliphatic, alicyclic,
aralkyl, alkarylalkyl, heterocyclic, terpenic, radicals, and wherein R2 is an
alkylene or
cycloalkylene radical. These R1 and R3 radicals for instance, may be
isopropyl, butyl,
cyclohexyl, benzyl, and/or bornyl radicals. The R2 radical is preferably an
ethylene,
propylene or tetramethylene radical;
4) Tertiary amines such as: trimethyl amine, triethylamine, tri-n-propyl-
amine, tri-isopropylamine, tributylamine, higher homologous and isomeric
trialkylamines, variously N-substituted tertiary amines having different
organic radicals
on the amino nitrogen atom, e.g., alkyl, alicyclic, bornyl, fenchyl, aralkyl,
and like
homologs and analogs; and tertiary amine type derivatives of alkylene
diamines;
5) Quaternary ammonium bases such as, tetramethyl and higher
tetraalkyl ammonium bases; trimethyl benzyl-, trimethyl cyclohexyl-, tributyl
decyl
ammonium bases; various quaternary N-substituted ammonium bases having various
organic radicals (of the type described herein) on the quaternary nitrogen
atom;
pyridinium and alkylated pyridinium or quinolinium quaternary ammonium bases
having
na alkyl cycloalkyl, or aralkyl group on the quaternary nitrogen atom,
including methyl,
butyl, cyclohexyl, benzyl groups, and the like homologs and analogs; and
6) Various organic nitrogenous bases, particularly guanidine, alkylated
guanidines, alkylated thioureas, and also diazoles, imidazolines, diazines,
pyrimidines,
and the basic derivatives of these and other organic nitrogenous-base nuclei.
[00104] Suitable nitrite salts include without limitation betaphenylethylamine
nitrite, piperidine nitrite, 3,3,5-trimethylcyclohexylamine nitrite,
trimethylbenzyl-
ammonium nitrite, di-isopropylamine nitrite, 2,4,4,6-tetramethyl-tetrahydro-3-
oxazine
nitrite. cyclohexylamine nitrite, 2-amino-butane nitrite, di-cyclohexylamine
nitrite,
morpholine nitrite, and dibenzylamine nitrite. Mixtures of nitrite salts can
also be used.
[00105] As noted, it is usually desirable to provide a mixture of different
vapor
phase corrosion inhibitors to provide suitable protection for all of the metal
or alloys
found in the article to be protected. Suitable ferrous inhibitors include for
example
naphthalene and naphthalene derivatives, alkyl amines, alkyl amine salts,
cycloaliphatic
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
amines, dicycloaliphatic amines, dicycloaliphatic amine salts, aromatic
amines,
nitroaromatic acids, aminol salts, fatty acid quaternary ammonium, urea,
thiazoles,
benzimidazoles, benzotriazoles combined with tertiary amines, benzotriazoles
combined
with polyamine, and benzotriazole combined with di(cyclooctyl) amine nitrite.
[00106] Similarly, suitable copper metal vapor phase corrosion inhibitors can
be selected from, without limitation, dicycloaliphatic amine salts, acetylenic
alcohols,
phenol carboxylic acids and esters, fatty acid quaternary ammonium slats,
thiourea,
thiazoles, benzimidazoles, benzotriazoles, benzotriazoles combined with
tertiary amines,
benzotriazoles combined with polyamines, and benzotriazole combined with
di(cyclooctyl) amine nitrite.
[00107] In addition, certain corrosion inhibitors have been found suitable for
protecting aluminum. These
include alkylamines, dicycloaliphatic amines,
dicycloaliphatic amine salts, aminol salts, thiazoles, benzimidazoles, as well
as
combinations of benzotriazoles with tertiary amines, polyamines, or
di(cyclooctyl) amine
nitrite. Suitable copper corrosion inhibitors include tolyltriazole,
benzotriazole, and
mercaptobenzothiazole, as well as their salts.
[00108] Another class of vapor phase corrosion inhibitors includes the salts
of
carboxylic acids such as benzoic acids or aliphatic carboxylic acids of about
3 ¨ 20
carbon atoms. Suitable salts include ammonium, alkyl ammonium, sodium, and the
like.
[00109] A vapor phase corrosion inhibitor suitable for use in the compositions
generally shows a vapor pressure of at least 10-6 ton, at least 2 x 10-5 ton,
or at least 10-4
tom Inhibitors with too high a volatility and vapor pressure are avoided if
the inhibitors
themselves are solids at room temperature or at temperatures up to 100 C or
higher. In
this way, the vapor phase corrosion inhibitors are provided as solids or
powders that can
be formulated into the adhesive with the use of the titanate, zirconate, or
silane coupling
agents as further described herein.
[00110] Vapor phase corrosion inhibitors are incorporated into adhesive
compositions at levels sufficient to supply the adhesive composition with
vapor phase
corrosion inhibitor properties during use in wrapping the protected articles.
In various
embodiments, suitable vapor phase corrosion inhibition by the adhesive
compositions is
measured by a "pass" rating for respective metal (iron, copper, aluminum,
zinc, etc.) in
an industry standard vapor phase corrosion test. Thus, suitable vapor phase
corrosion
31
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
inhibitors include those chemical compounds that, when formulated into an
adhesive
composition as described herein or when formulated for other anti-corrosion
usesõ result
in a "pass" rating in standards such as the German standard TL-8135-002; as
well as
MIL-PRF-22019E.
[00111] In various embodiments, the corrosion inhibitors can be provided as a
part of a masterbatch, where the masterbatch is made of the VCI material and a
carrier or
carriers. Such a masterbatch as part of a proprietary composition sold by
suppliers of
VCIs and can be produced by spray drying, by way of non-limiting example.
Designation of the particles as a "VCI powder" reflects the physical nature of
the
resulting VCI composition. In various embodiments, the carrier polymers are
made of
thermoplastic elastomers or other block copolymers, as long as they are
compatible with
the matrix. For recyclability, the polymeric carriers can be based on a
polymer that is
largely ethylene based.
[00112] VCI in particle or powder form is formulated into adhesive
formulation at a level sufficient to provide suitable corrosion protection in
use. In
general, levels of 0.1 ¨ 20% by weight of the particles are suitable in most
applications.
In some embodiments, the VCI particles are incorporated at a level of about 5%
to about
15% by weight, based on the total weight of the adhesive composition. The VCI
additive
can be a blend of multiple corrosion inhibitors such as a combination of
dicyclohexylamine nitrite, ammonium benzoate, morpholine, sodium benzoate and
benzotriazole. Other examples include mixtures of benzotriazole with benzoates
of
ammonia, guanidine, and hexamethylene-diamine; and a mixture of benzotriazole
with
guanidine benzoate and ammonium benzoate
[00113] In addition to vapor corrosion inhibitors, other materials which may
be added to the adhesive layer and which form a protective or treating vapor
in the cavity
enclosed by the wrapping material include anti-stats (static electricity
removers and
dissipaters), antioxidants, antimicrobials (to protect the product from
bacteria and other
biological contaminants), acid neutralizers, acid or bases (to effect pH
changes),
fragrances, additives that, when exposed to air, change color, thus indicating
that the
product has been tampered with, and others.
[00114] The VCI powder and the adhesive can be mixed together in a batch
process. During the batch process, adhesive is placed in a mixer and the VCI
powder ¨
32
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
either separately or in a form pre-combined with a titanate and/or zirconate
coupling
agent as described herein - is placed slowly in the mixer in pre-set
percentages. While a
batch system produces acceptable results, it has been discovered that
improvements in
uniformity and reproducibility are achieved through the use of a continuous
process,
conveniently carried out in an extrusion apparatus such as a twin screw
extruder.
[00115] The continuous process also produces adhesives of more uniform
viscosity, which tends to minimize the need to run the downstream lamination
process at
variable temperatures to adjust for the higher or lower viscosity of test
samples. Vapor
corrosion inhibitors are heat sensitive by their nature. When the temperature
is elevated
there will be increase in the VCI release to the environment. So when higher
temperatures are used during the lamination process, there could be a higher
rate of VCI
loss to the environment. It is generally preferred to run the lamination
process at a
consistent temperature and preferably as low a temperature as possible.
Coupling Agents
[00116] As noted, another step to improve product uniformity and to gain
some of the lost viscosity characteristics of the hot-melt adhesive is to use
various
titanium and/or zirconium (Ti/Zr) coupling agents. Although the invention is
not to be
limited by theory, it is believed that esters of titanium or zirconium couple
or chemically
bridge two dissimilar species such as inorganic filler/organic
particulate/fiber and an
organic polymer through proton coordination. Proton coordination may be
interpreted as
a form of plasticizing, since the filler is being modified to act more like
the matrix resin.
Under melt compounding shear conditions, the titanate and/or zirconate assists
in the
removal of air voids and moisture from the particle surface, resulting in
complete
dispersion and formation of a true continuous phase, thus optimizing filler
performance.
Titanates and Zirconates
[00117] In various embodiments, the coupling agent includes at least one
compound selected from the group of compounds consisting of a titanate
containing
compound, a zirconate containing compound, and mixtures thereof. Examples
include
ethylenically unsaturated titanate containing compound and neoalkoxy titanate
containing compounds. Non-limiting examples and their commercial designations
from
33
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
Kenrich Petrochemcials, Inc. include tetra (2.2
diallyloxymethyl)butyl-di(ditridecyl)phosphito titanate (KR 55),
neopentyl(diallyl)oxy-trineodecanoyl titanate (LICA 01),
neopentyl(diallyl)oxy-tri(dodec yl)benzene- sulfonyl titanate (LICA
09),
neopentyl(diallyl)oxy-tri(dioctyl)phosphato titanate (LICA
12),
neopentyl(diallyl)oxy-tri(dioctyl)pyro-phosphato titanate
(LICA38),
neopentyl(diallyl)oxy-tri(N-ethylenediamino)ethyl titanate (LICA
44),
neopentyl(diallyl)oxy-tri(m-amino)phenyl titanate (LICA 97),
neopentyl(diallyl)oxy-trihydroxy caproyl titanate (LICA 99), and mixtures
thereof.
[00118] Further examples of coupling agents include ethylenically
unsaturated zirconates and neoalkoxy zirconate containing compounds. Non-
limiting
examples from Kenrich include (2,2 diallyloxymethyl)butyl-
di(ditridecyl)phosphito
zirconate (KZ 55), neopentyl(diallyl)oxy-trineodecanoyl zirconate (NZ 01),
neopentyl(diallyl)oxy- tri(dodecyl)benzene- sulfonyl
zirconate (NZ 09),
neopentyl (di all yl )ox y- tri (di octyl )p hosphato
zirconate (NZ 12),
neopentyl(diallyl)oxy-tri(dioctyl)pyro-phosphato zirconate (NZ
38),
neopentyl(diallyl)oxy-tri(N-ethylenediamino)ethyl zirconate (NZ
44),
neopentyl(diallyl)oxy-tri(m-amino)phenyl zirconate (NZ 97),
neopentyl(diallyl)oxy-trimethacryl zirconate (NZ 33), neopentyl(diallyl)oxy-
triacryl
zirconate (NZ 39), dineopentyl(diallyl)oxy-di-p-aminobenzoyl zirconate (NZ
37),
dineopentyl(diallyl)oxy-di(3-mercapto) propionic zirconate (NZ 66A), and
mixtures
thereof.
[00119] Exemplary titanates include LICA 38J and LICA 09 from Kenrich
Petrochemicals Inc. It has been discovered that use of the Ti/Zr coupling
agents
improves the compatibility of UV stabilized adhesive such as SEBS with the VCI
powder and achieves the required overall flow characteristics for the final
adhesive.
[00120] The Ti/Zr coupling agents are added to the adhesive at levels
sufficient to obtain the noted advantages. In various embodiments, at least
0.1%, at least
0.2%, at least 0.4%, or at least 0.5% by weight is added, relative to the
weight of the
VCI. In various embodiments, up to 5% or up to 6% are used, where all
percentages are
by weight based on the weight of the VCI. Thus in various embodiments, the
adhesive
34
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
contains 0.1-6%, 0.1-5%, 0.5-6%. or 0.5-5% of the Ti/Zr coupling agent, where
the
percentages are based on weight of the VCI particles.
[00121] In compounding, the Ti/Zr coupling agents can be added to the
adhesive separately from the VCI particles, but at the noted weight ratios
relative to the
VCI. Alternatively or in addition, the VCI powder can be first combined with
the
coupling agent, and the resulting so-called "titanated" or "zirconated" VCI
added as a
single component to the adhesive. Thus, in one embodiment, VCI powder is
sprayed
with the Ti/Zr chemistry between 0.5% and 6.0% by weight to provide better
compatibility between the VCI powder and the adhesive. Titanated VCI powder is
termed VCI(T) in the Examples below.
Silanes
[00122] In various embodiments, the coupling agents are selected from those
classified as silanes.
[00123] Examples of amino functional silane coupling agents include
aminopropyltriethoxysilane;
aminopropyltrimethoxysilane;
aminopropylmethyldimethoxysilane;
aminoethylaminopropyltrimethoxysilane;
aminoethylaminopropyltriethoxysilane;
aminoethylaminopropylmethyldimethoxysilane;
diethylenetriaminopropyltrimethoxysilane;
diethylenetriaminopropyltriethoxysilane;
diethylenetriaminopropylmethyldimethoxysilane;
diethylenetriaminopropylmethyldiethoxysilane;
cyclohexylaminopropyltrimethoxysilane;
hexanediaminomethyldiethoxysilane;
anilinomethyltrimethoxysilane;
anilinomethyltriethoxysilane;
diethylaminomethyltriethoxysilane;
(diethylaminoethyl)methyldiethoxysilane; and
methylaminopropyltrimethoxysilane.
[00124] Examples of sulfur functional silane coupling agents include
bis(triethoxysilylpropyl)tetrasulfide; bis(triethoxysilylpropyl)disulfide; bis
(3-
ethoxydimethylsilylpropyl) olig o sulfur ;
mercaptopropyltrimethoxysilane;
mercaptopropyltriethoxysilane; mercaptopropylmethyldimethoxysilane; and 3-
thiocyanatopropyltriethoxysilane.
[00125] Examples of epoxy silane coupling agents include:
glycidoxypropyltrimethox ysi 1 ane; gl ycidox
ypropyltriethoxysil ane;
glycidoxypropylmethyldiethoxysilane; and glycidoxypropylmethyldimethoxysilane.
CA 2867131 2017-05-30
[00126] Examples of (meth)acryl silane coupling agents include:
methacryloxypropyltrimethoxysilane;
methacryloxypropyltriethoxysilane; and
methacryl oxypropylmethyl di meth ox ysilane.
[00127] Examples of chloro silane coupling agents include:
chloropropyltrimethoxysilane; chloropropyltriethoxysilane;
chloromethyltriethoxysilanc;
chloromethyltrimethoxysilane; and dichloromethyltriethoxysilane.
[00128] Examples of vinyl silane coupling agents include:
vinyltrimethoxysilane; vinyltriethoxysilane; and vinyltris(2-methoxyethoxy)si
lane.
Tackifiers
[00129] While non-pressure sensitive adhesives find use in various
embodiments, in other embodiments the adhesive compositions contain tackifiers
(also
called "tackifying agents"). Due to addition of fillers in a hot melt
adhesive, the base
adhesive tends to lose some physical properties, like any other polymer that
contains
fillers. For example, when the VCI powder is added to the hot melt adhesive,
the
adhesive tends to lose -tack." Tack is an important property for laminations,
both for
"initial tack", which bonds the two surfaces together, and for the life of the
laminate so
the laminate does not delaminate over time during its life cycle. In order to
make up for
the loss of tack, one solution is to increase the amount of adhesive put into
a laminate
used to match a "similar" bond strength, that would be achieved with an
unfilled
TM
adhesive. A suitable tackifier is Regalrez 1018 supplied by Eastman Chemical.
[00130] Also adding
tackifying agents can act as a encapsulating agent. In a
mixture of components having different viscosities, a component of lower
viscosity has a
marked tendency to encapsulate the second component in binary blends. Thus in
various
embodiments, tackifiers of low viscosity are added to encapsulate the adhesive
containing dispersed corrosion inhibitors. A suitable tackifying agent has a
viscosity of
10 poise or less at 60 C. Other suitable tackifying agents are characterized
by a
viscosity of 100 poise or less at 40 C and/or by a viscosity of 1000 poise or
less at 30
C. Regalrez 1018
is an example of a tackifying agent having viscosity within these
parameters.
[00131] ln various embodiments,
resins useful as tackifying agents are low
molecular weight amorphous polymers and they are widely used to make adhesives
to
36
CA 2867131 2017-05-30
generate tack and specific adhesion. The resins are of three main groups in
the industry:
Rosin Resins, Terpene Resins and Hydrocarbon Resins. Examples of hydrocarbon
resins
include C5 aliphatic resins, C9 aromatic resins, and cycloaliphatic resins (
such as
dicyclopentadiene or DCPD resin). Hydro genated resins of C9, C5 and/or DCPD
resins are
also suitable. The hydrogenated resins increase the outdoor usage of the
finished adhesive.
Compatibility of tackifying resins with the matrix polymers in the adhesive is
important to
achieve a good product. Color, softening point, molecular weight, glass
transition
temperature, melt viscosity, thermal stability and polarity of the resins are
other criteria to
consider for adhesive applications. Tackifying agents are known in the art.
Regalrcz 1018
has proved to be a good candidate as far as compatibility since it is rated to
be compatible
with various chemistries such as polyethylene, polypropylene, ethylene-
propylene
copolymers, natural rubber, EPDM, butyl rubber, SIS and SEBS blocks.
1001321 When the
VCI adhesive is manufactured by a twin screw method, it is
possible and preferable to add tackifying resins in screw zones downstream
from addition of
the VCI particles and coupling agents. In various embodiments, the tackifying
resins are
saturated hydrocarbon resins, hydrogenated synthetic polyterpenes, natural
hydrogenated
terpenes, and the like. Suitable tackifying resins are described for example
in U.S. Patent
5,204,390. Further
suitable examples include hydrogenated aliphatic petroleum
hydrocarbon resins, aromatic hydrocarbon resins, and hydrogenated derivatives
thereof If
desired, mixtures of two or more tackifying resins can be added. Other
suitable tackifying
resins include hydrocarbon, (e.g. C5 to C9) resins, polyterpenes, and rosin
esters of
pentacrythritol and glycerol. In various embodiments the tackifiers can be
added to reduce
viscosity and/or improve wetting.
[00133] In various
embodiments, the adhesive compositions contain from about
1 A to about 15% tackifying resin, from about 5 to about 10%, or from about 5
to about 7%
tackifying resin, based on the total weight of the adhesive composition. In
certain
embodiments, the base adhesive used to formulate the compositions already
contains a
certain percentage of tackifying resin as part of the commercial product being
37
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
used. In such embodiments, the downstream blending of additional tackifying
resins is
reduced by a corresponding amount.
[00134] The tackifying agent is preferably added downstream of the VCI
particle, and is added at a relatively low shear for a relatively short time
to avoid too high
a degree of mixing or miscibility into the adhesive. It is believed that the
relatively low
degree of mixing of the tackifier leads to encapsulation of the particles in
the
composition, making the tackifier act as a shell. This tends to increase the
pressure
sensitivity of the adhesive (desirable for downstream use in lamination
processes) while
at the same time decreasing the viscosity or at least avoiding an unacceptable
increase in
viscosity, which is also desirable for downstream processing. In one aspect,
the
invention is characterized by an adhesive containing added tackifying agent
(tackifier)
that has a melt index or viscosity no higher than the adhesive before addition
of the
tackifying agent.
Foaming Agent
[00135] A foaming agent can be added to the adhesive composition. In a
continuous process for formulating the adhesive, the foaming agent is
preferably added
downstream of addition of the VCI particle and coupling agent. Alternatively,
foaming
agents can be added to the adhesive compositions in a continuous process
during
lamination or adhesive coating.
[00136] Foaming agents contain an active ingredient that produces a gaseous
decomposition product when subjected to an activating temperature, which is a
characteristic of the agent. In various embodiments, it is preferred to use a
foaming
agent that will not be activated during compounding in any of the stages
described
herein, but that will decompose to provide volatile blowing agent at a later
temperature
of lamination during which the adhesive composition is applied to a substrate.
In a
non-limiting embodiment, a foaming agent is selected that has a decomposition
temperature of 140 C or higher, for example from 140-150 C. A suitable foaming
agent
is Celogen0 780, an activated azodicarbonamide sold by Crompton and having a
decomposition temperature of 140-150 C. It can be formulated in the continuous
process described herein at temperatures below 140 C in all the stages, and
then
38
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
subjected to temperatures of 140-150 C in a subsequent lamination process to
foam the
adhesive.
Flocking
[00137] In general, flocking involves a substrate, an adhesive and flock grade
¨short cut- fibers. U.S. Patents 2,675,330 (Schwartz, 1946) and 4,459,332
(Giglia &
Rye, 1984) provide some initial background on flock processing and application
information.
[00138] According to the teachings of the present invention the porous fabric
serves as an appropriate substrate.
[00139] Flock fibers can be randomly cut or precision cut and can range in
length from 0.25 mm to 25 mm, from 0.25 to 23 mm, from 0.25 mm (about 9-10
mils)
to about 20 mm (about 760 mils), from 0.25 mm to 10 mm, or from 0.25 mm to 5
mm.
They can be in any shape, such as round, trilobal, and dogbone, for example.
Flock
fibers can be synthetic or organic; non-limiting examples of common flock
fibers are
nylon, polyester, rayon, acrylic, cotton, and the like. The fibers can be from
0.5 to 90
denier. The fibers anchor into the adhesive from 10% of its length to 50% of
its length;
10% is more preferred for this application. The flock fibers create a soft-to-
touch
surface, yet are able to withstand degradation caused by wear against surface
contact
over time. By design the flock fibers will not damage painted surfaces.
[00140] Preferably, the flock fibers employed will help wick water or other
solvents away from the surface being protected, by use of capillary action.
The water
gets pulled away from the surface of the article over which the fiber
construct is
employed by the flock fibers. Water or other solvents pass through the flock
fibers and
they are pulled towards the hydrophilic adhesive coating, acting as a bladder.
From there
the porous fabric or nonwoven helps to push water from the foam adhesive to a
lower
moisture content polymeric coating. Working in synergy all the parts act as a
gradient
force to keep water away from the surface of the object to allow further
corrosion
prevention.
[00141] Flocking the porous fabric layer involves several steps. The substrate
gets coated with an adhesive at desired coating levels; then flock fibers are
embedded in
the adhesive using electrostatic and mechanical forces. The electrostatic
force field
39
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
aligns the flock fibers substantially perpendicular to the surface of the
fabric layer. The
adhesive is then cured with heat or by other means ¨ UV, electro-beam etc.
[00142] The adhesive coating methods on the substrate can utilize a variety of
methods including knife coating, curtain coating, reverse roll coating,
gravure coating
and rotary screen coating, by way of non-limiting example.
[00143] Having a flock on at least one side of the porous fabric allows the
finished cover to drag across rough surfaces without snagging the fabric or
sacrificing
any fibers. In some applications, the use of loose fibers such as nonwoven
fibers instead
of flock fibers, could lead to snagging of the fibers on the rough surface of
the object
being protected such as the tank shown in FIG.3. The snagging fibers would
tend to
sacrifice themselves as the fabric construct is being pulled across that
surface. The flock
fibers allow the fabric construct to "glide" over rough surfaces with limited
sacrifice of
the fibers and/or tearing or ripping of the fabric construct as its being
pulled over a rough
surface. The fibers dissipate the energy from the movement when the cover
moves on
the surface.
[00144] The flock fibers are preferably heat set, where the flock fiber is
heated
to its crystalline state and formed into a certain shape. This gives the fiber
a memory and
the return to the ideal orientation. This helps prevent the fiber from being
crushed during
the extrusion process when the breathable polymer is coated on the fabric. It
also allows
faster recovery of the orientation after the cover is folded up.
[00145] The orientation of the fibers, electro-statically and/or
mechanically, in
a substantially perpendicular manner to the substrate also helps with moisture
removal
process from the surface. The fibers help to wick water away from the surface
being
protected, as they act as straws to pull water towards the cover. As the water
moves
towards the adhesive, it is pulled further out; working in conjunction with
the breathable
coating.
Non-woven fabric for the soft fiber layer
[00146] In an alternative embodiment, the soft fiber layer facing the object
to
be protected in use is a non-woven fabric with suitable properties of
softness,
hydrophilicity, water/vapor wicking ability, material compatible during
construction or
recycling, etc. Fibers are made from a suitably soft material, such as a
polyester or
CA 2867131 2017-05-30
polyolefin fiber. In a preferred embodiment, the non-woven fabric contains
polyethylene
fibers. Further detail on the nature of preferred fibers, and the construction
of the soft
fiber layer follows.
[00147] Further
details of a non-woven fabric for use as the soft fiber material
layer of the constructs of the invention are given in international
publication WO
2010/022066.
[00148] In an embodiment, polyethylene fibers for the non-woven fabric are
provided that combine a small denier size (e.g., 1-3 denier or 0.5 to 2
denier) with a heat
distortion or heat deflection temperature higher than 70 C, such that the
fibers are
suitable for the spinning, carding, and other procedures needed in order to
make the
nonwoven fabrics described herein. In various embodiments, polyethylene fibers
with 1-
3 denier have a heat distortion temperature greater than 70 as measured by
ASTM D
648 at a load of 455 kPa. In one embodiment, the fibers are made of LLDPE. The
fibers
in various embodiments are further characterized by one or more of the
following;
- the fibers are of 1 to 1.3 denier;
- the fibers are of 1.3 to 1.7 denier;
- the fibers are in the form of a crimped staple fiber;
- the fibers contain 0.5-6% by weight nanoclay; optionally the nanoclay is
coated with titanate, zireonate, or silane coupling agents;
- a nonwoven fabric is made from the fibers;
- the nonwoven fabric comprises two or more layers wherein the fibers in
the first layer are different denier than fibers of the second layer, when the
first and second layers are adjacent;
- a fabric comprises two or more layers and the fibers of a first layer
have a
different hydrophilicity than fibers of a second layer, when the first and
second layers are adjacent;
- a fabric is a multilayer fabric wherein fibers of a first layer differ
from
fibers of a second layer in both denier and hydrophilicity;
- a layer of the fabric characterized by fibers of a higher denier is also
characterized by fibers of higher hydrophilicity;
- a first layer of a nonwoven fabric comprises fibers of 1 to 1.3 denier
and a
second layer of the fabric comprises fibers of 1.3 to 1.7 denier;
41
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
- the fibers of the nonwoven fabric comprise a hydrophilic titanate or
zirconate coupling agent material;
- the fibers in one or more layers of the nonwoven fabric contain nanoclay
particles to improve HDT:
- fibers of higher denier in a multilayer nonwoven fabric comprise the
hydrophilic titanate material;
- a first layer of a multilayer nonwoven fabric comprises fibers of 1 to
1.3
denier and a second comprises fibers of 1.3 to 1.7 denier; and at least the
fibers of the second layer further comprise a titanate or zirconate coupling
agent;
, 5
- the fabric is made by a spun-bond method;
- the fabric is made by a melt blown method;
- the fabric is made by a spun-laced method
- the nonwoven fabric has an areal weight of 10 to 200 grams per square
meter;
- a laminate is provided comprising any of the nonwoven fiber aspects
shown above;
Low Denier Polyethylene Fibers
[00149] In various aspects, polyethylene fibers are to be used in the nono-
woven fabric of the soft fiber layer because they are soft compared to other
polymers.
This gives the construct the advantage of not scratching the object to be
protected.
However, conventional polyethylene fibers tend to have insufficient heat
deflection
temperature and other physical properties, and in general lack the temperature
stability
for manufacture and use in the contemplated applications. In particular,
conventional
polyethylene fibers generally do not tolerate the heat generated during
spinning. As
staple fibers, they cannot be crimped, and/or tend not to survive the
temperatures of the
carding procedures. Further, because of their low heat distortion temperature,
they tend
not to tolerate conventional needlepunching or hydroentangling processes for
nonwoven
web formation. As such, they must be formed into webs by melt bonding or other
processes that introduce points of bond. These points can scratch a sensitive
and defeat
the purpose of using soft polyethylene fibers.
42
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00150] The drawbacks are overcome by adding components that increase the
heat deflection temperature. The fibers can then be used as stable or filament
to prepare
non-woven fabrics. The components include oxidation and UV stabilizers, as
well as
fillers such as nanoclay; and nucleating agents known for use in polymers
generally but
until now not taught for use in polyethylene fibers. In particular, low denier
polyethylene fibers ( for example, fibers of 1-3 denier) are produced by
spinning
(extruding through spinnerets) a melt that contains
1) a polyethylene polymer or copolymer;
2) a stabilizing package that can contain
a) a primary antioxidant;
b) a secondary antioxidant; and/or
c) a UV stabilizing package such as a HALS (hindered amine light
stabilizer); and
3) a nucleating agent; and
4) a clay or nanoclay
The low denier polyethylene fibers are characterized by heat deflection
temperature
(HDT) greater than 70 C, when measured by ASTM D648 at a load of 455 kPa.
[00151] The primary and secondary antioxidants and the UV stabilizing
package (items 2a-2c) provide protection against oxidation and damage caused
by
ultraviolet radiation. The nucleating agent helps to control the crystallinity
and, it has
been found, the heat distortion temperature of the fiber. In various
embodiments, the
nucleating agent is selected from those of a type conventionally used to
control
crystallinity and nucleation in casting of polyethylene films. An example is
Hyperform
HPN-20E, sold by Milliken. Chemically, the HPN-20E nucleating agent is said to
be a
carboxylic acid salt. The clay is a layered material such as an
aluminosilicate that can be
dispersed or exfoliated into the polyethylene. Because the flakes when
exfoliated have
dimensions on the order of a few tens of Angstroms, the clays can be referred
to as a
nanoclay. That is, nanoclay refers to the dimension of the exfoliated
particles.
[00152] Primary antioxidants (also called free radical scavenging
antioxidants)
inhibit oxidation using chain-terminating reactions. In various embodiments,
they have
reactive OH or NH groups. Non-limiting examples include hindered phenol
antioxidants
43
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
and secondary aromatic amine antioxidants. Inhibition of oxidation occurs via
a transfer
of a proton from the antioxidant to a free radical species formed in a polymer
chain. The
radical resulting from the proton transfer is stable and does not extract a
proton from the
polymer chain.
[00153] Secondary oxidants (also referred to as hydroperoxide decomposers)
decompose hydroperoxides into non-radical, non-reactive and thermally stable
products.
Secondary antioxidants are often used in combination with primary antioxidants
to yield
synergistic stabilization effects. In action, secondary antioxidants prevent
the split of
hydroperoxides into reactive alkoxy and hydroxy radicals. Commonly used
secondary
antioxidants include organophosphorous compounds and thiosynergists.
Thiosynergists
are sulfur-based hydroperoxide decomposers. Non-limiting examples include
esters of
3,3-thiodipropionic acid. The thiosynergists react with a hydroperoxide to
generate
sulfoxides and sulfones. Sulfur-based hydroperoxide decomposers can be used in
combination with hindered phenol antioxidants. The most common commercially
available thiosynergists are based on either lauric or stearic acid.
[00154] Nucleating agents are compounds or compositions that function by
increasing the temperature at which crystallization from the melt begins. In
determining
or assessing the effect of the nucleating agent, the onset of crystallization
can be
determined by differential scanning calorimetry (DSC). The amount of
nucleating agent
to be added to the polyethylene fibers is an amount suitable to raise the
crystallization
temperature of the melt by at least 1 C compared to that without any
nucleating agent.
That is, a measurable rise in the crystallization temperature from using the
nucleating
agent tends to correlate to, or be a proxy for an increase in heat deflection
temperature of
the fiber. The minimum, maximum, or optimum amount of nucleating agent can be
determined in individual cases from correlations of the levels added to the
desired
outcome (i.e., raising the crystallization temperature of the polymer and/or
the deflection
temperature of the fiber). In this aspect, the formulation is not dependent on
an
individual chemistry, but on the power of that chemistry to provide the needed
increase
in the crystallization temperature.
[00155] Incorporation of the clay or nanoclay into the polyethylene resin
results in an exfoliated composition, wherein layers of clay are dispersed
homogenously
throughout the fiber matrix. Exfoliation of clay into the polyethylene results
in a so-
44
= CA 2867131 2017-05-30
called nano-composite. To achieve complete exfoliation into the polymer resin,
the clays
are pre-treated with various coupling chemistries, a compatibilizing resin can
be used
along with the polyethylene, and/or the clays are dispersed into the nano-
composite by
agitation, ultrasound, grinding, and the like.
[00156] Suitable clays include aluminosilicate, which have a sheet-like
(layered) structure, and contain silica SiO4 tetrahedra bonded to alumina A106
octahedra
in a variety of ways. Suitable clays include the smectite clays, which have a
2 to 1 ratio
of tetrahedra to the octahedra. A non-
limiting example of a smectite clay is
rnontimorillonite. In such clays, the thickness of the layers (platelets) is
of the order of
one nanometer. When dispersed or exfoliated, the aspect ratios of the
platelets are high,
typically 100-1500. The exfoliated clays have very high surface areas up to
hundreds of
square meters per gram. Normally, it is necessary to modify the clay to make
it
chemically compatible with the polymeric matrix. A variety of processes is
known to
make the clay "organophilic." Ion exchange with the clay, as well as the use
of
TM
dispersing polymers are two such processes. Two examples of nanoclay are
Closite Na+
TM
and Closite 15A by Southern Clay Products,
[00157] In various
embodiments, the clays are dispersed or exfoliated into the
polymer resin after pre-treatment with coupling agents such as the titanates
and
zirconates described further herein.
100158] In various embodiments, the nanoclay incorporated into the
polyethylene matrix is provided at a treat amount of 0.25 ¨ 15% by weight, 0.5
¨ 10% by
weight, 0.5 - 9.0 percent by weight, 0.5 to 6.0 % by weight, or 2 ¨ 8 % by
weight.
Process conditions are selected in order to form an exfoliated structure,
wherein the
layers of the clay have been completely separated and individual layers are
distributed
throughout the organic matrix,
[00159] In a non-limiting example an LLDPE fiber grade resin, such as
ASPUNTN' 6835A or ASPUN 6850A (two fiber grade polyethylenes produced by Dow
Chemical and differing in melt flow index) is used. The fiber resin is
modified before
being converted into fibers, to improve the heat distortion temperature by
adding anti-
oxidants (e.g. IRGANOX B215), and UV stabilizers (e.g. TINUVIN 111). The heat
and
UV stabilizing package generally make up no more than 1.0% by weight of the
resin to
be made into fibers for the nonwoven. Furthermore the resin can be nucleated
by adding
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
a nucleating agent such as Miliken's Hyper-Form HPN-20E, conveniently by way
of a
masterbatch. This step raises the crystallization temperature and has been
observed to
increase the heat distortion temperature (HDT) of the resulting fibers.
Finally, a
nanoclay such as montmorillonite is exfoliated at a level of about 1 to 15% by
weight
into the polymer matrix. Addition of these packages raises HDT of the fibers
and makes
them suitable both for production and post-production of the laminate. That
is, through
the use of appropriate additives at appropriate levels the heat distortion
temperature can
be raised by several degrees Celsius and preferably to a final value of 70 C
or higher
(e.g., 70-80 C, 70-90 C, or 70-100 C).
[00160] Optionally the fiber resin is modified with a titanate or a silane
chemistry to enhance the physical characteristics of the fiber during
converting and post-
lamination.
Crimped fibers of polyethylene
[00161] Crimped staple fibers made of polyethylene are prepared by
- extruding a molten blend of the ingredients above through spinnerets;
- dressing the extruded fiber , for example with a silane,
- cutting and crimping the fiber to make a coiled fiber, and
- thermally setting the crimped fiber.
Before thermal setting, a low level of surface crosslinking can be applied to
the fiber. It has
been found that fibers made as discussed herein have sufficient heat
distortion properties to
withstand the temperature of crimping and thermal setting to produce a crimped
fiber suitable
for formation of a nonwoven web by needlepunching or hydroentangline.
Non-woven fabrics used opposite the vapor permeable polymer composition in
particular embodiments
[00162] Nonwoven fabrics for use as the layer directly facing the object to be
protected in the constructs described herein are made of suitable fibers that
provide for
transport of water vapor toward the interface and thence through the porous
fabric and
the vapor permeable layer into the outside environment. Non-limiting examples
include
polyester and acrylic fibers. In a particular embodiment, the nonwoven fabric
is
fabricated from polyethylene fibers. For use in the constructs, the
polyethylene fibers are
46
CA 2867131 2017-05-30
preferably treated to increase their heat deflection temperature, as discussed
in an earlier
section of this disclosure.
[00163] The nonwovens
of the present invention can use fiber sizes in the
microfiber range of 3.0 denier or less, more preferably from 1.0 denier to 2.5
denier or a
combination of these sizes. Optionally the fibers used can be hollow to help
with the
flow of vapors and/or gases as stated in U.S. Patent No, 4,838,904.
[00164] The fibers
for the present non-wovens are provided as filaments or as
staple fibers. Fibers in the form of filaments can be spun-bond, melt blown,
or air laid to
provide non-woven webs. In making a multilayer non-woven fabric according to
the
invention, the individual webs can be co-extruded to provide fabrics having
the desired
vapor permeability and wicking capabilities.
[00165] Staple fibers
are normally crimped before further processing to make
the non-woven fabrics.
[00166] In various embodiments,
crimped staple fibers are carded into
multiple layers, wherein the individual layers have the hydrophilicity and
vapor wicking,
liquid wicking, hydrophilicity, and vapor permeability properties further
described
herein.
[00167] In various
embodiments, the successive cards are laid parallel (i.e. at
the same angle, usually the machine direction) or at different angles (e.g.
perpendicular
to one another). After all of the cards are laid down, a non-woven fabric can
be made by
hydroentangling, needle punching, and the like. Alternatively, staple fibers
can be spun
flashed. If cards are laid down in different directions and preferably in a
perpendicular
fashion - for example a first card laid down in the machine direction (MD) and
a second
card at 90 in the transverse direction (TD) - a web is produced having higher
strength in
the machine direction, which is preferred for stretch applications.
[00168] The fibers,
modified as described above to provide suitably high
HDT, are made into a web by suitable processes such as spun laid and melt
blown. An
example is a spun bond/ melt bond/ spun bond web (SMS). In one embodiment the
fibers are cut and crimped into a staple fiber and then formed into a web by
carding.
Any combination of these methods can be used depending on the manufacturing
capabilities to create a multilayer nonwoven structure. After the web
formation, the
47
CA 2867131 2017-05-30
nonwoven can be bonded together by one or a combination of many methods known
in the
art, including but not limited to: chemical bonding (wetlaid), needlefelt,
needlepunching,
ultrasonic pattern bonding, and hydroentangling.
[00169] In various
embodiments, nonwoven fabrics are produced from low
denier crimped polyethylene fibers by setting down at least two layers of
fibers, followed by
needle punching or hydroentangling the fibers to make a fabric. By making the
fabric with
needle punching or hydroentangling, any step of melting the fibers is avoided.
For some
applications, this is advantageous because melting the fibers would provide a
"point of
bond" where the fibers melt and coalesce, and this point of bond would tend to
scratch the
surface of a sensitive object that is being protected by the laminate.
However, the needle
punching or hydroentangling steps subject the fibers to challenging conditions
that require
high heat distortion temperature and other physical properties provided by the
fiber
compositions. Also, for best entangling by needle punching or water jets, the
fibers should
be crimpled, which subjects them to further high temperature and challenging
manufacturing steps that conventional polyethylene fibers have until now been
unsuited for.
[00170] In various
embodiments, the nonwoven fabric has a multilayer structure
such as a three layer structure or a two layer structure. Depending upon the
application, the
multilayer nonwoven can be modified by a scrim material as stated in U.S.
Patent No.
6,696,120 Bl. In various embodiments, a multilayer fabric has different size
fibers in its
individual layers to take advantage of dissimilar wicking characteristics of
these fibers. In
particular, lower denier ("smaller") fibers are used in the "bottom" layer of
the multilayer
nonwoven fabric that is in contact with the object or volume to be protected
when the fabric
construct is in use as a protective wrap. Smaller size fibers tend to wick
water at a faster rate
than larger fibers. The smaller fibers wick at a faster rate at the surface
and turn the
hydrostatic pressure into a hydrokinetic pressure towards the upper layer of
the nonwoven.
The upper layer is normally made of larger fibers.
[00171] In a
preferred embodiment, the fibers are laid sequentially in at least two
layers to make the fabric. The two or more layers contain fibers of different
denier. The low
denier fibers have higher capillary action than the higher denier fibers. As
such,
48
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
the high capillary fiber layer is preferably disposed in use toward the side
subject to a
high relative humidity that needs to be controlled or the water removed.
[00172] When the fiber is in the form of a filament, the layers are separately
formed (by spun bond, melt blown, or air laid processes for example) and
coextruded to
form a multilayer nonwoven fabric. With staple fibers, the multilayer non-
woven fabric
is normally formed by laying individual fibers in separate cards, followed by
needle
punching or hydroentangling to form the non-woven fabric. Various embodiments
involving multilayer non-wovens will be described herein referring to layers
of fibers. It
is to be understood that, where appropriate, the teachings about the layers
refers to cards
formed from crimping staple fibers or to layers formed from filament fibers.
[00173] In an embodiment, the first layer is made of 1.1 - 1.3 denier, and the
second layer is 1.3 - 1.7 denier. Depending on the application a wide variety
of areal
weights of the fibers can be provided in each layer. In various embodiments,
10-70 g/m2
are provided in each layer. In a preferred embodiment, the nonwoven fiber has
a total
areal weight of about 50 g/m2.
[00174] As noted, one function of the low denier fiber is to wick water away
from the surface or the volume being protected. Once the water is wicked away
from the
surface by the low denier fibers of the first layer, the water enters the
higher denier layer.
In order to continue the water in the path away from the protected surface,
the second
layer of fibers is treated so as to be more hydrophilic than the low denier
high capillary
fibers. In this way, the water is led irreversibly in a direction away from
the surface (or
volume) being protected.
[00175] In preferred embodiments, the higher denier fibers of the second layer
are formulated with components or other treatments to make them permanently
hydrophilic. For example, the fibers of the second layer are formulated with
specifically
hydrophilic titanate coupling agents. This renders the fibers permanently
hydrophilic.
Two examples of hydrophilic titanates available through Kenrich Petrochemicals
Inc. are
LICA38J and NZ38J. LICA38J is characterized as soluble in water; and NZ38J is
soluble in water at concentrations equal to, or less than 1%. Suitable
coupling agents
include those described below in the section on adhesives.
49
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00176] In preferred embodiments, the fibers of one or both of the layers
contain fillers such as talc or clay, which acts to make the fibers cheaper
and which acts
as a heat sink, increasing the temperature stability of the fibers.
[00177] In an illustrative embodiment, a multilayer nonwoven fabric contains
a first layer of smaller denier fibers and a second layer of larger denier
fibers, with the
fibers of both layers preferably being in the range of 1-2 denier for
softness. Preferably,
the second layer fibers are further treated to be permanently more hydrophilic
than the
first. The low denier fibers of the first layer have a higher capillary action
than the fibers
of the second layer. The nonwoven fabric optionally has 3rd, 4th, and other
layers, as
long as inclusion of other layers does not adversely affect the direction of
flow of water
vapor through the nonwoven fabric. In various embodiments, each layer is at
least as
hydrophilic as the one before it, measured in the direction from the surface
being
protected to the outside environment. In this way, the multilayer nonwoven
fabric
provides a one way path for moisture. The multilayer non-woven fabric can also
contain
so-called neutral layers for strength. A neutral layer is one that is not
necessarily more
hydrophilic than its neighbor, but it is one with hydrophilic or wicking
properties such
that its presence in the multilayer fabric does not deleteriously affect water
or vapor
flow.
[00178] In preferred embodiments, the fibers can be made with masterbatch
methods. In the first layer a polyethylene masterbatch contains the nucleating
agent, the
primary and secondary antioxidants, and the HALS. In second and subsequent
layers,
the masterbatch can further contain various agents that increase the
hydrophilicity of the
fibers, such as the noted hydrophilic titanates.
[00179] In subsequent fabric layers, if used, the masterbatch contains
increasing amounts of the chemistry that provides the hydrophilic character.
If the
hydrophilic chemistry of subsequent layers is different from that of the
second layer,
then enough of the chemistry is added to the master batch to render each layer
as
hydrophilic, or preferably more hydrophilic, than the previous layer.
[00180] As noted, processes such as melt bonding create a "point of bond" that
can more readily scratch a sensitive surface to be protected. Accordingly, it
is preferred
in some embodiments to form the nonwoven fabric by non-bonding processes such
as
needle punching and hydroentangling.
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00181] Hydrophilicity of a fiber or of a fabric or a single layer of a
multilayer
fabric made from the fiber is determined according to established methods. For
example,
hydrophilicity can be tested by applying drops of water to a fabric surface
from a fixed
height. The time required for the surface to be wetted by the droplet then
provides
information on the hydrophilicity of that fabric/fiber ¨ the lower the wetting
time, the
more hydrophilic the fiber/fabric. As described for example in U.S. Patent
4.073,993
one of the test methods is American Association of Textile Chemists and
Colorists
(AATCC) Standard Test Method 39-1971, Evaluation of Wettability. In the test,
water
droplets (15-25 drops per mL) are dropped every 5 seconds from a height of 3/8
in (1
cm) above the fabric. A stop watch is started from the time a drop falls. The
wetting
time is recorded as the time the water on the fabric loses its specular
reflective power.
An average of 10 droplets can be calculated. A faster wetting time equals to a
higher
level of hydrophilicity. Conveniently, the wetting test can be carried out on
both sides of
a multilayer fabric. Differing hydrophilicity is then indicated when the
fabric has a faster
wetting time on one side than on the other side.
2-stage nonwoven construct with a stitch knit non-woven porous fabric layer
and an
applied vapor permeable composition.
[00182] An alternative embodiment of a fabric construct according to the
teachings of the present invention is shown in FIG.4. This fabric construct is
made up of
a vapor permeable waterproof polymeric coating 1A like that of FIG.1. This
polymer is
coated on to a "stitch-knit" nonwoven structure 40 that is optionally already
printed.
Preferably the "stitch-knit" nonwoven 40 is coated with a VCI composition 50
prior to
coating as shown in FIG.4. The stitch knit nonwoven 40 is made of a layer 44
of first
fibers having a smaller denier and lower hydrophilicity than the fibers in a
second layer
42. A stitch knit fabric or scrim 46 is provided in the nonwoven 40 between
the first
layer 44 and second layer 42. Detailed explanation of the fabric construct
will now be
provided.
[00183] A special non-woven fabric is provided that is coated (by co-extrusion
or by lamination, by way of non-limiting example) with a vapor permeable
breathable
film. In one version of the fabric construct, there is no adhesive and no
flocking. Strength
is provided by the special structure of the non-woven.
51
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00184] In one embodiment, the special non-woven is a strong entangled non-
woven containing a stitch knit fabric as a reinforcement. The non-woven is
further
characterized by at least two layers of fibers having different hydrophilic
and
hydrophobic characteristics. The two layers of fibers are constructed around a
stitch knit
fiber with subsequent treatment such as by hydroentanglement or needle punch
methods
by non-limiting example.
[00185] To illustrate, the non-woven can be made by first carding a fiber of
relatively low denier. After the first layer is carded, a stitch knit fabric
is placed on top of
the card. For purposes of the current description, a stitch knit fabric is
characterized by a
woven structure that is open or is characterized as a loose" stitch, such that
the distance
between the threads of the weave is great enough to give the appearance of a
netting
rather than that of a closed knit fabric. For example, the distance between
threads in the
stitch knit fabric is about 0.5 to about 20 mm or about 1 to 10 mm. A spacing
of about
one eighth of an inch (about 3-4 mm) has been found to be particularly
suitable.
[00186] After providing
the stitch knit fabric in this way, a second card of
fibers is then laid down on top of the stitch knit fabric followed by an
entanglement
process such as hydroentangling or needle punching. The second card consists
of higher
denier fibers than the first card. The smaller fibers of the first card tend
to wick water
and vapor quickly and are suitable for use on the side of the construct which
in
packaging use will face the surface of the object to be protected. Its wicking
properties
will act to quickly absorb vapor from the packaging volume. The second card in
turn is
disposed in use farther from the packaging volume but is physically connected
to the first
card of lower denier fibers. The larger fibers of the second card are more
hydrophilic
than the smaller fibers of the first card, and so are capable of taking the
water vapor
wicked by the first card and delivering it to a vapor permeable film applied
to the second
card side of the non-woven to form the construct of this embodiment.
[00187] Although making the special non-woven has been illustrated by laying
down a first card of smaller denier fibers, it is to be understood it can just
as well be
made by carding the higher denier fibers first, followed by interposing a
reinforcing knit
fabric and subsequent carding of the smaller denier fibers followed by
entangling.
Whichever way the non-woven fabric is made, in use the lower denier fibers
face the
volume or part to be protected, as described further herein.
52
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00188] The non-woven is thus an entangled fabric made of layers of two
different hydrophobicities (or equivalently of two different
hydrophilicities), the whole
thing being reinforced, for example by a stitch knit fabric, to provide
strength. The
material of the fibers and the stitch knit fabric is chosen so that the non-
woven will have
suitable strength and softness and so that the fibers can withstand the
temperature of
subsequent operations such as lamination or co-extrusion of the breathable
polymer
composition onto the non-woven.
[00189] Suitable fibers for the first and second card include polyester and
nylon. Blends of fibers can be used, as well as combinations of natural and
man-made
fibers, as long as they maintain suitable properties at all times under the
conditions of
manufacture and use. The first card side of the non-woven, which in use faces
the object
to be protected, is advantageously soft because of the small denier fibers.
Example
polyester staple fibers that can be used in this application include Dacron
Plus,
HydroPur Fiber, Delcron Hydrotec Fiber and SteriPur AM fibers from DAK
Americas company. In order to provide a soft-to-touch surface it is preferred
that the
fibers used in this application are less than 10 deniers, and more preferably
less than 2
deniers.
[00190] Likewise, the stitch-knit fabric contains threads and fibers made out
of
materials that can withstand all of the process steps. Suitable fibers include
those of the
first and second cards.
[00191] As noted, the fibers of the second card are more hydrophilic than
those of the first card by virtue of their larger size. Advantageously, this
enables the non-
woven to transmit the vapor wicked by the first card through the second card
and deliver
it to the breathable film that is applied to the non-woven on the side of the
second card. If
desired, a difference in hydrophobicity/hydrophilicity can be achieved by
providing a
second card having fibers with additives that increase the hydrophilicity. An
example of
a suitable additive is the hydrophilic titanates discussed elsewhere for use
in the
adhesives of other embodiments of the construct.
[00192] So, the special non-woven is characterized by a first layer of fibers
having a first hydrophilicity in contact with a second layer of fibers having
a second
hydrophilicity. In one embodiment, the structure is further characterized by a
stitch knit
fabric disposed between the first and second card fibers, providing
reinforcement. The
53
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
first and second fiber layers are in contact with one another by virtue of the
fact that the
fabric precursor made up of the card of the first fibers, the stitch knit
fabric, and the card
of the second fibers is subjected to an entanglement process such as
hydroentangling. In
this way, the fibers of the first card and of the second card are entangled
around each
other and around the stitch knit fabric. The entangled non-woven is then
coated, on the
side containing predominantly the second card fibers (i.e. the side with
fibers of greater
hydrophilicity), with a breathable, vapor permeable polymer composition. In
use, the
construct just described is applied with the non-woven facing the object to be
protected,
and more specifically the first card side with the smaller fibers facing the
object to be
protected.
[00193] In another embodiment, a two-stage non-woven is provided as above
by carding a first layer of fibers, followed by carding a second layer, where
the fibers of
the two cards differ in hydrophobic of hydrophilic character. Instead of
having a stitch
knit fiber interposed between the cards as above, in an alternative embodiment
the two
cards can be lightly stitched, either before or after entangling. The two-
stage non-woven
is then provided with a vapor permeable polymeric film composition, as
described
above.
[00194] In another embodiment, the two-stage non-woven with reinforcing
stitching made as described above is used as the porous fabric in the flocked
constructs
described further herein. In this embodiment the two-stage non-woven can be
optionally
printed. The fabric can be flocked before or after printing, and the flocked
fabric can be
provided with a vapor permeable polymer coating such as by laminating or co-
extrusion.
Fire resistant fabric constructs
[00195] The fabric constructs can be made fire resistant by adding known fire
resistant additives to any of the constituent parts to impart a desired
measure of
protection against or resistance to fire. For example, additives can be added
to the porous
fabric, to the fibers making up the porous fabric, to the flocked fibers, to
the flocking
adhesive composition, and to the breathable (water vapor permeable) polymer
coating. In
this way, a fabric construct can keep a fire from spreading from its protected
object
and/or can protect the protected contents from a fire on the outside.
54
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00196] Fire retarding (FR) fabrics include those consisting of glass fibers
(fiberglass) or other inorganic fibers (silica fibers, asbestos, and the
like). Constructs
containing organic and other flammable fabric can be rendered fire resistant
or retardant
by the use of intumescent adhesives such as acrylic, epoxy, melamine, or
urethane
intumescent coatings either in addition to, or as replacements for other
adhesives such as
those used to laminate a vapor permeable polymeric coating or to incorporate
flocked
fibers into the construct.
[00197] If
desired, fire resistant flocking fibers such as fiberglass can be used
in the fabric constructs described herein.
[00198] In a fire, the side of the fabric with the intumescent coating expands
to
create a char barrier. The char helps to protect against the conductive
penetration of hot
gasses and flames. It can also absorb smoke into its matrix, thus lowering by-
products
from the fire. It reduces the flame spread and helps to slow the fire's
progress.
[00199] In an embodiment of a construct that keeps a fire from spreading, the
fabric can be made of fiberglass and have an acrylic intumescent coating on
one side of
the fabric. The flocking adhesive can be applied over the intumescent adhesive
and then
flock can be applied to the flocking adhesive as described herein. The fabric
is then
coated with the highly breathable polymer on the opposite side from the
flocking with a
polymer make-up as described in FIG.1. If fire were to occur inside the
material, the
flock fibers ¨ facing the surface ¨ and the flock adhesive would burn away and
the
intumescent coating would expand and form a char barrier, preventing the fire
from
escaping. The fiberglass fabric would help prevent the fire from breaking
through and
would not readily burn. It would also provide a refractory surface that helps
prevent the
heat from getting through.
[00200] In an embodiment of a construct that keeps a fire on the outside from
damaging the protected contents, the porous fabric can be a fiberglass fabric
and have an
acrylic intumescent coating on one side of the fabric. Here, the flocking
adhesive would
on the fabric side opposite the intumescent and the flock would be applied
into the
flocking adhesive. The vapor permeable polymeric coating would be applied on
the
fabric side opposite the flocking, as usual. In use a cover made from the
fabric construct
would have the flocked fiber on the inside. In case of a fire on the outside,
the
intumescent coating would expand and fonn a char barrier preventing the fire
from
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
entering the cover. The fiberglass fabric would help prevent the fire from
breaking
through and would not readily burn. It would also provide a refractory surface
that helps
prevent the heat from getting through.
Example 1 ¨ flockin2 a porous fabric
[00201] In a non-limiting example, a fabric construct is prepared as outlined
here. The flocking fibers are 1.8 denier round semi-dull nylon 6,6 flock fiber
that is 1
mm (39.37 mils) in length. The fiber density upon flocking in an illustrative
embodiment is about 61 gsm (1.8 oz / sq yard or 0.1125 lbs / sq yard ). The
flocking
operation as it takes place at Spectro Coating Corporation in Leominster, MA
starts with
a fabric ¨ woven or nonwoven ¨ that is preferably printed on one side. An
adhesive pre-
coat is then applied to the fabric on the side opposite the print. The
adhesive pre-coat
most preferably has a blow ratio of 5:1. The pre-coat, FF-3849 by Key Polymer,
add on
rate most preferably is about 0.5 oz / sq yard (0.031 lb/ sq yard or 16.95
gsm).
Following the application the pre-coat gets dried at 280 F. Upon drying the
pre-coating
seals the fabric on the applied side and acts as a primer between the fabric
and the top
coat, FF-3850 by Key Polymer, adhesive. The topcoat is applied on the same
side as the
pre-coat. The adhesive is foamed at a ratio of 1.60 : 1.00 and it is
approximately 4 mils
thick: with an add on rate of 3.20 oz / sq yard (0.2 lbs / sq yard or 108.50
gsm). The pre-
coat and the topcoat optionally contain a static or dynamic antimicrobial
composition.
[00202] After the top
coat adhesive is applied, the fabric goes into the "flock
chamber". Here the fabric is mechanically moved in an "up & down" format as it
moves
through the flock chamber. At the same time pre-cut flocking fibers are
dropped into the
"flock chamber" from hoppers. There are electrically charged metal bars placed
across
the width of the web. These charged metal bars constantly alternate the charge
to align
the fibers perpendicular to the moving fabric. The current flow on the metal
bars is
regulated by providing a voltage between 10,000 volts and 120,000 volts AC or
DC.
[00203] As the fabric is mechanically moved up & down the fibers are pushed
into the adhesive and they are stuck perpendicularly to the adhesive.
Following the
flocking process the fabric enters a drying oven where the adhesive is cured
to set in
place. At this stage the topcoat is dried at 250 F and then cured and cross-
linked at 320
F. The flock fibers are anchored into the adhesive to at least 10% of their
overall length
56
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
or in this case at about 0.10 mm (3.94 mils) deep. The flocking fibers are
heat set at
405 F. During such application the adhesive pre-coat, adhesive top coat or
both layers,
may have Vapor corrosion inhibitors. The preferred VCI add on rate in the top
coat
adhesive is about 2% by weight. In this example there would be about 2.17
grams of
active VCI chemistry and about 1% by weight titanate or zirconate coupling
agents that
renders the adhesive more hydrophilic.
[00204] The thickness of the adhesive is ideal for the addition of VCI and
titanate to work in synergy with the whole system to remove moisture and
provide
corrosion inhibitors into the macro environment of the packaging.
Example 2
[00205] The flocked fabric of Example 1 is extrusion coated by a 3 mil thick
mono-layer polymer blend that is made up of, by non limiting example, 50%
ElvaloyTM
AC 1224, 40% EntiraTM Breathe and 10% FusabondTM FB556 by polymer weight, plus
a
weathering package as within the ranges given in Table 1 above.
[00206] The extrusion process settings were; melt temperature at 473 F, Chill
Roll at 75 F, nip roll at 100 psi, corona treatment at 5kW and line speed of
75 feet per
minute.
Example 3
[00207] Application of polyurethane vapor permeable polymer composition.
Another example of a fabric construct has a polyurethane vapor permeable
coating on
two layers of primer adhesive bonded to a polyester porous fabric, where the
fabric is
further coated with another two layers of adhesive on the opposite side and
flocked. The
urethane coating formulation is provided in Table 2 above.
[00208] First a Polyester fabric with a twill pattern similar to the one in
Example 1 is coated at room temperature by two passes of acrylic primer, FF-
3841
supplied by Key Polymer, at a 5:1 foam ratio. About 0.5 oz/sq yard is applied
on each
pass.
[00209] The primed fabric is then coated with polyurethane, FL-1910 supplied
by Key Polymer, at 3.25 oz /sq yard. It is then treated at a temperature of
340 F. The
temperature increase from ambient is gradual. If a clear top coat is desired
FL-1916
grade could be used.
57
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
[00210] If the
polyurethane coating is not fully cured at this point, it is ok,
because the construct will be run it through the machine again when flocking
at the same
temperatures. It should cure fully during the second pass. All the coatings,
primer,
adhesive and polyurethane are applied with knife over roll method. All the
products are
water based and pumped from drums at room temperature.
[00211] Now the porous fabric with the highly breathable polyurethane
coating is ready for the flocking operation. In this working example a
suitable type of
flock fiber is a 1.5 denier PET that is 30 mils in length, produced by
Palmetto Synthetics
LLC, from South Carolina.
[00212] First a water based acrylic latex pre-coat and topcoat adhesive layer
similar to the one in Example 1 with encapsulated VCI powder is applied. Then
the
fabric goes through the flocking chamber where electrically charged fibers are
applied as
in Example 1. Following the flocking process the fabric enters a drying oven
where the
adhesive is cured to set in place. At this stage the topcoat is dried at 250
F and then
cured and cross-linked at 320 F. The flock fibers are anchored into the
adhesive to at
least 10% of their overall length or in this case at about 0.10 mm (3.94 mils)
deep. The
flocking fibers are heat set at 405 F. Since the breathable coating was
already applied,
the fabric is ready for use.
Example 4 ¨
[00213] The fabric construct in this example is made by having a highly
breathable polyurethane coating on one side of a porous woven fabric, a non-
pressure
sensitive rubber hot melt containing vapor corrosion inhibitors on the
opposite side of the
polyurethane coating, two layers of foamed acrylic latex adhesive applied on
top of the
hot melt adhesive, and flocked fibers applied on top of the acrylic latex
adhesive and
cured.
[00214] Onto a polyester fabric with a twill pattern as in Examples 1 and 3,
there is applied an acrylic primer followed by a polyurethane with the
formulation as in
Table 2. Non-pressure sensitive hot melt adhesive compounded with vapor
conosion
inhibitors is applied to the opposite side of the polyurethane coating. The
hot melt fit for
such VCI compounding and fabric application is a product available by Adherent
Laboratories Inc in Saint Paul, MN. The product is called AffixTM AL-20071.
The hot
58
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
melt adhesive is applied by melting the adhesive at 325 degrees F and pumping
it
through a manifold that feeds the spray nozzles. The spray nozzles with use of
hot air
turn the applied adhesive into a continuous fiber. This method is also called
melt-blown.
The air temperature that helps to create a random adhesive pattern is at 400
degrees F.
The application takes place at 300 feet per minute. The hot melt application
equipment
that can be used in this example can be found at Tufco Technologies Inc. in
Green Bay,
WI. The application can be between 3 gsm and 25 gsm, but 13 gsm is preferred
for this
application. The coated fabric is then laminated to a release liner to protect
the adhesive.
A suitable release liner is on a 60 pound poly-coated paper style 60#C1S BPE
manufactured by Enterprise Coated Products in IL. Before the flocking
operation, the
release liner is peeled off and water based acrylic latex adhesive, for
example FF-3841
supplied by Key Polymer, pre-coat and topcoat ¨both foamed- is applied as
mentioned in
Examples 1 and 2. Since the vapor corrosion inhibitors are in the hot-melt
adhesive
neither the pre-coat nor the topcoat adhesive contains vapor corrosion
inhibitors in this
application. Following the top coat the polyester flock fibers are applied
and the
product is cured by means of heat application as mentioned in Examples 1 and
3.
Example 5 ¨ tear strength measurements
[00215] To illustrate that the vapor permeable coating compositions lead to
improved (increased) tear strength, four fabric or construct samples were
subject to
Elmendorf tear strength measurements in the filling (CD) and warp (MD)
directions.:
5a is a 3x1 twill PET plain fabric with no flocking and no coating;
5b is the fabric of 5a with flocked nylon fibers, but before any vapor
permeable coating is applied;
5c is the flocked fabric of 5b with an ionomer coating as the vapor
permeable coating composition; and
5d is the flocked fabric of 5b with an applied polyurethane coating as the
vapor permeable polymer composition.
Samples 5c and 5d represent fabric constructs of the current invention. Tear
strength test
results are given in Table 3. Each value is the average of 5 sample
measurements.
59
CA 02867131 2019-09-11
WO 2013/137881 PCT/US2012/029165
Table 3. Tear strengths of fabrics and fabric constructs.
Example Tear strength, Tear strength,
Comments
lbs. (grams), lbs.(grams),
filling warp direction
direction
5a 26.1 (11,849) 27.9(12,667) plain 3x1 PET
twill fabric
5b 9.32 (4,231) 17.5 (7945) 5a with nylon
flocked fibers
5c 30.67 (13,910) 21.5 (9,754) 5b with ionomer
coating
5d 28.01 (12,718) 23.55 (10,690) 5b with
polyurethane coating
[00216] The data in Table 3 demonstrate that flocking a porous fabric lowers
the tear strength, and that adding a vapor permeable compositions increases
the tear
strength of the flocked constructs of the invention to a level comparable to
that of the
unflocked and uncoated fabric.