Note: Descriptions are shown in the official language in which they were submitted.
CA 02867480 2015-09-15
METHOD AND DEVICE FOR PREVENTING
CORROSION IN HOT WATER SYSTEMS
[001] (This paragraph intentionally left blank).
TECHNICAL FIELD
[002] This invention relates generally to methods of reducing or inhibiting
corrosion in hot water systems, More specifically, the invention relates to
measuring real-
time oxidation-reduction potential at operating temperature and pressure in
one or more
operational protective zones and using those measurements to control the
adjustment of
parameters that affect the oxidation-reduction potential. The invention has
particular
relevance to locally and/or globally reducing or inhibiting corrosion in
simple or complex
hot water systems.
BACKGROUND
[003] Hot water systems are generally composed of all-ferrous metallurgy or
mixed
metallurgy, such as copper or copper alloy systems, nickel and nickel-based
alloys, and stainless
steel and may also be mixed with mild steel components. Many general
classes/components of
hot water systems exist, such as boilers, hot water heaters, heat exchangers,
steam generators,
nuclear power electric systems combustion engine and diesel coolant systems,
evaporator
systems, thermal desalination systems, papermaking operations, fermentation
processes, the like,
and attached ancillary devices. They are dynamic operating systems that
undergo a myriad of
REDOX Stress events (i.e., any electrochemical event in the hot water system
related to changes
in oxidative or reductive potential). Such events generally
1
CA 02867480 2014-09-15
WO 2013/155036 PCT/US2013/035713
include any process that implicates the oxidation-reduction potential ("ORP")
space
or regime in the system.
[0041 These events result from a multitude of factors including leaks from
various components, contamination from air in-leakage, malfunctioning pumps,
seals,
vacuum lines, and gauges. Further, increased use of oxygen-enriched water,
such as
boiler make-up water, returned steam condensate, and/or raw surface or
subsurface
water, dcaerator malfunctions, steam and turbine load swings, and problems
with
chemical feed pumps cause unplanned reduction or increase in chemical
treatment
feed rates. Uncontrolled REDOX Stress events can cause serious corrosion
problems,
such as localized corrosion, stress corrosion, corrosion fatigue, and/or flow
accelerated corrosion problems in hot water systems, By their nature, these
problems
tend to be electrochemical and thus tied-in to the oxidative-reductive
properties of the
environment and structural material interaction.
[005] Although some conventional methods are practiced today to identify
REDOX Stress events in hot water systems, because of hot water system dynamics
most REDOX Stress events are unpredictable. These methods are not widely
practiced because they have inherent drawbacks (see below). As a consequence,
the
majority of REDOX Stress events go undetected and thus uncorrected,
Uncontrolled
REDOX Stress events can lead to serious corrosion problems in these systems,
which
negatively impact plant equipment life expectancy, reliability, production
capability,
safety, environmental regulations, capital outlay, and total plant operation
costs,
[006] Identifying REDOX Stress events currently includes both online
instruments and grab sample wet chemical analysis test methods. In both
approaches,
the sample has to first undergo sample conditioning, such as cooling, prior to
measurement. Examples of online instruments include dissolved oxygen meters,
cation conductivity instruments, room temperature ORP instruments, pH
instruments,
sodium analyzers, hardness analyzers, specific conductivity meters, silica
analyzers,
particle and turbidity meters, reductant analyzers, and the like. General
corrosion
monitoring, such as coupon and electrochemical analysis, is typically
performed after
2
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
cooling a sample or at elevated temperatures. Grab sample test methods include
analyzing for dissolved oxygen, pH, hardness, silica conductivity, total and
soluble
iron, copper, and silica, reductant excess, and the like.
[007] Some drawbacks of these methods include the following. Grab
sample analysis gives a single point in time measurement and consequently is
not a
viable continuous monitoring method for REDOX Stress events. It also often has
inadequately low-level detection limits. Online monitors do not provide a
direct
measurement of REDOX Stress and thus cannot indicate whether or not a REDOX
Stress event is occurring at any particular time. Corrosion monitors detect
general
corrosion, but are not capable of measuring changes in local corrosion rates
caused by
REDOX Stress events. Online reductant analyzers measure the amount of
reductant,
but not the net REDOX Stress a system is undergoing at system temperature and
pressure. That REDOX Stress can occur in. the apparent presence of a reductant
is
thus another drawback of this technique.
[008] Dissolved oxygen ("DO") meters have similar drawbacks.
Measuring the amount of DO (an oxidant) but not necessarily the net REDOX
Stress a
system is undergoing is not an accurate indicator of corrosion stress. The
sample also
must be cooled prior to DO measurement thus increasing the lag time in
detecting
when the REDOX Stress event started. Further, the potential for oxygen
consumption
in the sample line could cause inaccurate readings. REDOX Stress can also
occur in
the apparent absence of DO and little or no DO in the sample could potentially
be a
false negative. in addition, all of the instruments described above are
relatively costly
to purchase, and require frequent calibration and maintenance.
[009] Corrosion coupons give a time-averaged result of general system
corrosion. Again, this technique does not offer a real-time indication or
control of
REDOX Stress events. Online electrochemical corrosion tools are inadequate for
localized corrosion determinations and cannot be used in low conductivity
environments.
3
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
[00101 Room temperature ORP is a direct measurement of the net ORP of a
sample taken from the system. A drawback of this technique is that it fails to
indicate
what is happening at system temperature and pressure, REDOX Stress events that
occur at operating temperature and pressure often cannot be observed at room
temperature, as process kinetics and thermodynamics vary with temperature. In
addition, room temperature ORP measuring devices are more sluggish and more
likely to become polarized. Reliability of such devices is poor and they need
frequent
calibration and maintenance.
[0011] There thus exists an ongoing need to develop methods of accurately
monitoring and controlling real-time ORP in hot water systems at operating
temperature and pressure.
SUMMARY
[00121 This disclosure accordingly provides a method of monitoring and
controlling ORP in a hot water system in real-time at operating temperature
and
pressure. A myriad of processes occurring in a hot water system contribute to
the
ORP, which in tarn acts as a REDOX Stress indicator for the hot water system,
In
contrast to conventional room temperature measurements, ORP measurements taken
in real-time at system operating temperature and pressure are capable of
indicating
primary and secondary REDOX Stress events occurring in the system in real-
time,
Such real-time ORP monitoring may be used to measure, identify, and assess
REDOX
Stress demands in the system and can act as a direct or indirect corrosion
process
indicator.
[0013] In an aspect, the invention provides a method of controlling a real-
time ORP in a hot water system to reduce or inhibit corrosion in the hot water
system.
The method includes defining one or more operational protective zones ("zone"
or
"zones") in the hot water system, At least one (eg., one, two, or more) of the
defined.
zones is selected and one or more (e.g., one, two, or more) of the selected
zones
includes at least one ORP probe operable to measure the real-time ORP and
4
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
communicate with a controller. The real-time ORP is measured either
continuously
or intermittently at one or more (e.g., one, two, or more) of the selected
zones while
the hot water system is at operating temperature and pressure. The method
further
includes transmitting the measured real-time ORP to the controller and
assessing
whether the measured real-time ORP or a calculated ORP based upon the measured
real-time Ore conforms to an ORP setting. The ORP setting may either be a same
ORP setting for each of the selected zones or a different ORP setting for at
least two
of the selected zones. If the measured real-time ORP or the calculated ORP
does not
conform to the ORP setting, the method includes changing a parameter in the
hot
water system In an embodiment, the method includes performing at least one of
the
following actions if the measured real-time ORP or the calculated ORP does not
conform to the ORP setting: (i) feeding an effective amount of one or more
active
chemical species into the hot water system, (ii) removing an effective amount
of one
or more active chemical species from the hot water system, and (iii) changing
a
system parameter.
[00141 In another aspect, the invention provides a corrosion control device
for a hot water system. The hot water system has one or more (e.g., one, two,
or
more) operational protective zones, where a subset of the zones (preferably
two or
more zones) is selected, In an embodiment, the device includes a receiver that
is in
communication with one or more ORP probes. A subset of the ORP probes is
activated and each activated ORP probe is operable to measure a real-time ORP
at
operating temperature and pressure. At least one ORP probe is installed at one
or
more of the selected zones. In an embodiment, the device also includes a
processor
operable to interpret the measured real-time ORP communicated to the receiver
from
each activated ORP probe. The processor interprets either the measured real-
time
01U3 directly or a calculated ORP based upon the measured real-time ORP, In an
embodiment, the corrosion control device is operable to change or adjust a
parameter
based upon one or more iterations of the measured and interpreted ORP.
[00I5] According to at least one embodiment, in communication with a
transmitter is a feeding device that is operable to manage introduction of one
or more
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
active chemical species into the hot water system to affect changes in the
real-time
ORP. In at least one embodiment, a chemical removal device operable to remove
one, two, or more chemical species from the hot water system is in
communication
with the corrosion control device. The processor is operable to send an output
signal
through the transmitter to the feeding device or the chemical removal device,
if the
interpreted real-time ORP does not conform to an ORP setting,
[0016] It is an advantage of the invention to provide a method of inhibiting
corrosion in a hot water system based upon measuring a real-time ORP at
operating
temperature and pressure in the hot water system and reacting to the measured
ORP
by feeding one or more active chemical species into the hot water system to
maintain
an ORP setting.
[0017] Another advantage of the invention is to provide a hot water system
corrosion control device including a receiver, a processor, a transmitter, and
a feeding
device, which work in unison to control a real-time ORP in one or more
operational
protective zones in the hot water system.
[0018] A further advantage of the invention is to increase hot water system
efficiency by enabling improved maintenance and control of system parameters.
[0019] Yet another advantage of the invention is to decrease operating costs
for a variety of hot water systems and components by accurately preventing
corrosion.
[0020] The foregoing has outlined rather broadly the features and technical
advantages of the present invention in order that the detailed description of
the
invention that follows may be better understood. Additional features and
advantages
of the invention will be described hereinafter that form the subject of the
claims of the
invention. It should be appreciated by those skilled in the art that the
conception and
the specific embodiments disclosed may be readily utilized as a basis for
modifying or
designing other embodiments for carrying out the same purposes of the present
invention. It should also be realized by those skilled in the art that such
equivalent
6
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
embodiments do not depart from the spirit and scope of the invention as set
forth in
the appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
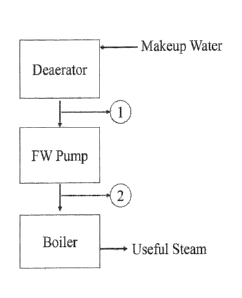
[0021] Figure 1 depicts a simplified 3-component hot water system, where
make-up water flows through a "Decorator," a "FW Pump," and into a "Boiler"
and
the boiler in turn generates "Useful Steam" for subsequent use in various
processes.
[0022] Figure 2. illustrates a more complex boiler configuration, including a
plurality of feed water pumps, a plurality of heat exchangers, and a steam
producer
[0023] Figure 3 depicts various "ORP Control Zones," where the ORP
setting may be different for systems at various temperatures.
[0024] Figure 4 illustrates feeding multiple REDOX active species at
various locations to control the @T ORP (trademark of Nalco Company) at a
single
location.
DETAILED DESCRIPTION
[0025] As used herein, "hot water system," "system," and like terms refer to
any system where hot water is in contact with metallic surfaces. "Hot water"
means
water having a temperature from about 37C up to about 370 C. The system may
operate at or below atmospheric pressure or a pressure up to about 4,000 psi,
[0026] "ORP," ORP," "at-T ORP," and "real-time ORP" refer to
oxidation-reduction potential for an industrial water system at operating
temperature
and pressure, in certain instances herein, ORP is indicated as room
temperature ORP.
[0027] "ORP probe" refers to any device capable of measuring and
transmitting a real-time ORP signal. Though any suitable device may be used, a
preferred device is disclosed in U.S. Pat. App. Ser. No, 11/668,048, filed
"High
7
CA 02867480 2015-09-15
Temperature and Pressure Oxidation-Reduction Potential Measuring and
Monitoring Device for
Hot Water Systems," now pending. Typically, the ORP probe includes a
temperature detector, a
noble metal electrode, and a reference electrode.
[0028] "Active chemical species" refers to oxidants, reductants, corrosion-
inhibitors,
corrodants, and other species that have an affect on or influence the ORP in a
hot water system.
Such species are described in more detail below.
[0029] "REDOX Stress" refers to any electrochemical event in a hot water
system related
to changes in oxidative or reductive potential, either directly or indirectly.
[0030] "Controller system," "controller," and similar terms refer to a manual
operator or
an electronic device having components such as a processor, memory device,
digital storage
medium, cathode ray tube, liquid crystal display, plasma display, touch
screen, or other monitor,
and/or other components. In certain instances, the controller may be operable
for integration with
one or more application-specific integrated circuits, programs, computer-
executable instructions,
or algorithms, one or more hard-wired devices, wireless devices, and/or one or
more mechanical
devices. Some or all of the controller system functions may be at a central
location, such as a
network server, for communication over a local area network, wide area
network, wireless
network, internet connection, microwave link, infrared link, and the like. In
addition, other
components such as a signal conditioner or system monitor may be included to
facilitate signal-
processing algorithms.
[0031] In one embodiment, the method includes an automated controller. In
another
embodiment, the controller is manual or semi-manual, where an operator-
interprets the signals
and determines feed water ("INV") chemistry, such as oxygen or other oxidant,
oxygen
scavenger or other reductant, corrosion-inhibitor, and/or corrodant dosage. In
an embodiment,
the measured ORP signal is interpreted by a controller system that controls FW
chemistry
according to the described method. In
8
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
an embodiment, the controller system also interprets measured temperature to
determine the amount of active chemical to add or remove, if any. The
controller
system is also operable to determine if changing or adjusting a system
parameter is
needed in addition to or instead of adding or removing one or more chemical
species
from the hot water system. The temperature detector might also be used for
information purposes, such as in alarm schemes and/or control schemes, It
should be
appreciated that the control scheme may incorporate pump limiters, alarming,
intelligent control, and/or the like, based off further inputs, such as pH, DO
levels,
and other water constituents/properties.
[0032] It is contemplated that the disclosed method is applicable in a variety
of hot water systems, including both direct and satellite active chemical
feeding
designs, "Direct" feeding typically refers to measuring real-time ORP at a
zone and
feeding active chemical to the same zone. "Satellite" feeding usually refers
to
measuring real-time ORP at a zone and feeding active chemical to a different
zone.
Representative systems and system components include condensers, both tube and
shell side; heat exchangers; pumps; seals; mild steel or copper-based FW
heaters;
copper-based alloy surface condensers; dearators; water tube and fire tube
boilers;
paper machines; condensate receivers; steam condensate transfer lines with or
without
steam traps; process liquid heat exchangers; evaporators; desalination
systems; sweet-
water condensers; attemperated water sources; flow-accelerated corrosion
protection;
air heaters; engine coolant systems for diesel and gasoline; and the like.
[0033] Other exemplary processes include papermaking process, such as
Kraft pulping and bleaching processes; wafer polishing and planarization
processes
(e.g., silicon wafer polishing); combustion gas emission (e.g., 502, NOx,
mercury);
fermentation processes; geothermal processes; and aqueous organic redox
synthesis
(i.e., polymerization processes that require redox initiators).
[0034] Conventional corrosion control regimes use one point feed. The
disclosed invention uses targeted feed by precisely determining the needed
active
chemicals and the proper amount/dosage of those chemicals. For example,
relatively
9
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
oxidizing zones, such as low-pressure FW heaters (copper-based metallurgy),
and
more reducing zones, with high-pressure FW heaters (non copper-based
metallurgy),
may be differentiated to alleviate flow-accelerated corrosion-related issues.
Relatively oxidizing conditions within all ferrous FW heaters at sections of
pressurized water reactors versus relatively reducing final FW heater regimes
for
stress corrosion cracking mitigation in steam generators.
[0035] The invention is capable of detecting and reacting to both primary
and secondary REDOX Stress events. Typically, the implementer knows the system
corrosion control implications and possible REDOX stressors and is able to
accordingly select one or more of the defined operational protective zones to
appropriately monitor a given system's gT ORP space. In this way, it is
possible to
control corrosion by feeding or removing REDOX active species based off local
and/or remote @,.T ORP readings as a primary REDOX Stress indicator, The fg.T
ORP space is monitored and measured to assess and identify system demands,
which
are then compared to known/formulated metrics to react, solve, and control
REDOX
Stress events. As an indicator of secondary REDOX Stress, the invention can
detect
corrosion processes resulting from prior, primary REDOX Stress, where the
primary
REDOX stressor is no longer evident.
[0036] The ORP probe may detect several different factors that contribute to
REDOX Stress events in the hot water system. For example, an ORP probe in a
selected zone can act as a direct indicator of corrosion in that zone or in
another zone.
In an embodiment, the real-time ORP is measured in a first selected zone and
one or
more active chemical species are fed to the first selected zone, if the
measured real-
time ORP at the first selected zone or the calculated ORP does not conform to
the
ORP setting for the first selected zone, in another embodiment, the real-time
ORP is
measured at a first selected zone and one or more active chemical species are
fed at
one or more other selected zones, if the measured real-time ORP or the
calculated
ORP does not conform to the ORP setting for the first selected zone. In a
further
embodiment, one or more real-time ORPs are measured at one or more of the
selected
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
zones and one or more other real-time ORPs are calculated for one or more
other
selected zones, based upon one or more of the measured real-time ORP(s),
10037] As described above, in some cases, the measured ORP in a first zone
is used to calculate an ORP for another zone. Such calculations may be done by
making various assumptions regarding system dynamics or by measuring
temperature/water chemistry differences between zones, Using mixed potential
theory and thermodynamic principles known to those skilled in the art also
allows for
approximating conditions in other zones. However, such calculations are
typically
subject to inherent inaccuracies; thus, the preferred method is to measure the
real-time
ORP in situ in selected zones.
[003S] Several important factors exist for determining or defining specific
operational protective/control zones for a system. The goal for any particular
system
is to achieve @.õ-T ORP "Plant Specific Boiler Best Practices" for that
system. For
instance, certain plants are limited to certain chemistries due to control
philosophy,
environmental constraints, economics, industry standards, etc. System
temperatures
also may dramatically vary from one plant to another, which requires adjusting
the
specific control philosophy employed, explained in more detail in the below
Examples. Different plants may also have a unique REDOX Stress baseline and
insipient changes to the baseline may need to be determined.
[0039] Other factors include, specific ORP altering species purposefully
added or inherently present; engineering alloys of construction for various
portions/entities in the system; desired general and local corrosion
mitigation; dosing
limitations; other system design specifics; special considerations, such as
flow-
accelerated corrosion, stress, and corrosion cracking; system variability.
Those
skilled in the art would understand how to assess these and other system
variables/specifies to implement the invention for a specific plant or system.
[0040] Ideally, any portion of a plant can have its P,T ORP REDOX Stress
measured and controlled using (ifilr ORP, That is, the .REDOX active species
is fed
I
CA 02867480 2015-09-15
directly to a specific piece of equipment (or groups of equipment) and the @T
ORP of the water
in that piece of equipment is measured in situ and controlled for corrosion
mitigation, This
invention more specifically addresses corrosion local to the part(s) being
protected and transport
of corrosion products with concomitant deleterious effects of that corrosion
transport elsewhere
in the system, including fouling, heat transfer surface coating, turbine
deposition, etc. This type
of full equipment monitoring and control approach is often not possible due to
system limitations
and economics. As such, parts of systems typically need to be handled as whole
entities. In some
cases, the entire feed water train of a boiler system might he the entity.
Alternatively, only small
portions of the system or groups of portions of the system are the entity. it
is contemplated that
any portion, component, or entity (including the entire system viewed as one
entity) may be
selected and monitored/controlled.
[0041] In an aspect, the ORP setting for one selected zone may overlap with
another
defined or selected zone. In another aspect, the ORP setting for one selected
zone is completely
independent of each and every other defined or selected zone, In a further
aspect, the ORP
setting for one selected zone is partially dependent upon factors in one or
more other defined or
selected zones.
[0042] In an embodiment, the ORP setting is determined for a first selected
zone and
additional ORP settings are optionally determined for additional selected
zones, if any. In one
embodiment, each additional ORP setting is independently determined
Alternatively, one or
more of the ORP settings may be dependent upon one or more other ORP settings,
ORP settings
are generally dependent and based upon operational limitations of the hot
water system.
[0043] Determining the ORP setting for any particular system may he
accomplished by
any suitable method. A preferred method is described in U.S, Patent No.
7,666,312, "Method of
Inhibiting Corrosion in Industrial Hot Water Systems by Monitoring and
Controlling
Oxidant/Reductant Feed through a Nonlinear Control Algorithm". It is
contemplated, however,
that any method known to those skilled in the art may be
12
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
employed to ascertain the ORP setting. In an embodiment, the ORP setting is an
ORP
set point that is chosen from one or more single values. In another
embodiment, the
ORP setting is an ORP set range chosen from one or more ranges of values. Over
time, the ORP setting for any selected zone may be adjusted or changed. For
example, a given plant may have a timetable outlining ORP settings for
different
parts/components of the system at different times. This timetable would
typically be
based upon operational factors in the system that may change as demands on the
system change.
[00441 Some zones might be kept relatively reducing and other zones might
be relatively more oxidizing. For example, referring to FIG 2, Heat Exchangers
I and
2 might be manufactured from an alloy that exhibits low corrosion rates under
more
reducing conditions, Whereas, Heat Exchanger 3 might be manufactured from a
different metallurgy that exhibits lower corrosion rates under more oxidizing
conditions, The "Steam Producer" might then again need to be kept under more
reducing conditions. The AT ORP control zones would he accordingly adjusted
and
monitored to compensate for these differences,
[00451 In one embodiment, one or more of the selected zones may be in a
monitoring and/or alarm mode, while one or more other selected zones is in a
control
mode. A selected zone in a monitoring and/or alarm mode is capable, in an
embodiment, of switching between these modes. Such switching may either he
manually controlled or automatic. Several examples are presented below of how
AT
ORPTM system design can be used for REDOX stress control.
[0046] In another embodiment, the AT ORP is measured across any pump
to detect pump or seal corrosion or failure. In another embodiment, the method
may
be used to detect heat exchanger tube leaks as one active chemical species
might
transfer through the leak in the heat exchanger to the other side (e.g., shell
side to tube
side or visa versa). Another example would be a surface condenser cooling
water
leak into a FW condensate hot well. In a further embodiment, the method may be
used to detect any unwanted intrusion of external active chemical species
system
13
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
contaminants), In an alternative embodiment, @?,T ORP can be used to form a
"fingerprint" of specific REDOX stressors in a system. In this way, it can be
used as
an early warning system for boiler tube rupture as more boiler makeup water is
added
to the system from time to time with a concomitant increase in the REDOX
stress.
[00471 Measured or calculated ORP values may indicate amounts of
electrochemically active species in one or more of the selected zones. Such an
indication may either be directly seen in the zone where the ORP was measured
or
inferred in another zone where the ORP was not directly measured. In certain
cases,
the measured or calculated ORP indicates an amount of chemical that indirectly
affects an amount of electrochemically active species in one or more selected
zones,
in a more typical case, the electrochemically active species directly
influences the
measured or calculated ORP.
[0048] in one embodiment, the method includes ramping from one of the
selected zones to another one of the selected zones after a triggering event.
Any event
that causes a shift or change in the real-time ORP in one or more control
zones may
be a triggering event. A person having ordinary skill in the art would be able
to
analyze such options and choose one or more triggering events for a system.
For
example, bringing pumps or other parts of the system online (or taking
offline) may
be a triggering event. Steam pressure changes due to downstream use changes,
such
as between turbine driving and other lower pressure uses, may also be chosen
as a
triggering event. Triggering may also be based on activating various
condensate
streams, which could introduce specific REDOX stressors in the system. Such
triggering events could be detected by probes, relays, monitors, etc., while
remaining
detectable by changes in the real-time ORP in one or more control zones.
Moreover,
the rate of change of these and other events may dictate the ramping rate from
one
control zone to another control zone, including instantaneous, timed, step-
wise, or
other suitable ramping modes.
[0049] Representative triggering events may also include numerous timed
operations or timetables or other plant dynamics. A timetable could be a
.fixed startup
14
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
time followed by ramp up in some system operations over time. For example, 30
minutes after initiating VW flow, the real-time ORP should be within 100 mV of
the
desired ORP setting. After 20 minutes of full load firing of the boiler, the
real-time
ORP should be ramped up to the ORP setting, The ramping may also be triggered
when an ORP setting has been achieved elsewhere in the system, such as
upstream
components. For example, once an upstream control zone has achieved its ORP
setting (or is within, for instance, 50 mV), a downstream control zone is
activated or
brought into a control mode, Such sequencing of real-time ORP control is one
preferred method of triggering,
[0050] Changing plant dynamics may also initiate triggering and/or
ramping. in an embodiment, the triggering event can include plant power output
changes. For example, a 5% power output decrease may be the triggering event
that
initiates real-time ORP changes in one or more control zones in the system.
The
procedure used to initiate the real-time ORP changes might be, for example, an
immediate signal to change the ORP setting for one or more control zones or a
gradual ramp to a new ORP setting. This procedure may be based upon the rate
or
magnitude of power decline. Furthermore, the triggering and/or ramping
mechanisms
might be complex interconnections of multiple signals and timing.
[0051] /n a preferred embodiment, changes and adjustments to }NV
chemistry includes adding or removing (when possible) oxygen or other oxidant,
oxygen scavenger or other reductant, corrosion-inhibitor, corrodant, and/or
other
active chemicals to the FIN. By definition, oxygen scavengers are reducing
agents,
although not all reducing agents are necessarily oxygen scavengers. Reducing
agents,
suitable as oxygen scavengers, satisfy the thermodynamic requirements that an
exothermic heat of reaction exists with oxygen. For practical applications,
reasonable
reactivity is typically required at low temperatures. That is, there should be
some
favorable kinetics of reaction. Furthermore, other changes and adjustments to
FW
chemistry, such as for system control and corrosion control may include
adding/removing other oxidizing agents (oxidants), other reducing agents
(reductants), and/or other active or inert chemicals,
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
[0052] It is also highly desirable that the reducing agent and its oxidation
products are not corrosive and do not form products that are corrosive when
they form
in steam generating equipment. Typically, certain oxygen scavengers function
optimally in certain pH ranges, temperatures, and pressures and are also
affected by
catalysis in one way or another. The selection of the proper oxygen scavengers
for a
= given system can be readily determined based on the criteria discussed
herein and.
knowledge of those skilled in the art.
[0053] Preferred reductants (i.e., oxygen scavengers) include hydrazine,
sulfite, bisulfite, carbohyrazide, N,N-diethylhydroxylamine, hydroquinone,
erythorbate or erythorbic acid, methyl ethyl ketoxime, hydroxylamine,
tartronic acid,
ethoxyCitkill, methyltetrazone, tetramethylphenylenediamine, semi-=carbazides,
diethylaminoethanol, monoethanolamine, 2-ketogluconate, ascorbic acid;
borohydrides, N-isopmpylhydroxylamine, gallic acid, dihydroxyaeetone, tannic
acid
and its derivatives, food-grade antioxidants, the like, and any combinations.
It should
be appreciated that any active chemical species may be used in the method of
the
invention.
[0054] Representative oxidants include oxygen, hydrogen peroxide, organic
(alkyl and aryl) peroxides and peraeids, ozone, quinone, acid and base forms
of
nitrates and nitrites, the like, and combinations.
[00551 Representative cormdants include mineral acids (e.g., HC1, 1-12SO4,
HNO3, H3PO4) and their salts/derivatives, caustics (e,g, Na, K, Li,
hydroxides);
ammonium hydroxide; chelants, such as EDTA, NIA, HEDP; phosphonic acid and
polyphosphonie acids; phosphonates; water soluble and/or dispersable organic
polymeric complexing agents, such as acrylic acid homopolymers, copolymers,
and
terpolymers; acrylamide; acrylonitrile, methacrylic acid; styrene sulfonic
acids; the
like; and combinations.
16
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
[0056] Representative corrosion inhibitors include alkali and amine salts of
phosphate and polyphosphates; neutralizing amines; molybdates; tungstates;
borates;
ben.zoates; filming inhibitors, such as alkyl, alkenyl, and aryl polyamines
and their
derivatives; surfactant compositions, such as that disclosed in U.S. Pat. No.
5,849,220; oligomeric phosphinosuccinic acid chemistries; such as that
disclosed in
U.S. Pat. No. 5,023,000; the like; and combinations.
[00571 In another embodiment of the invention, one or more chemical
species are removed from the hot water system. For example, oxygen may be
removed from a. main process water sidestream via a membrane process. Any
suitable
membrane may be used for .such removal and one skilled in the art would select
a
suitable membrane and sidestream procedure. Nitrogen or a lower oxygen
concentration carrier gas (or main process water sidestream) may be present on
one
side of a gas permeable membrane and the process water is on the other side of
the
membrane. The oxygen present in the main process water sidestream would
diffuse
out of the main process water sidestream to equilibrate its partial pressure
across the
membrane which would then lower the oxygen content in the main process water
and
lower the ORP. .n an embodiment, a dearator (see e.g,, the configuration of
FIG 1) or
similar deaeration process may be incorporated to mechanically remove or strip
non-
condensable gases (e.g., oxygen) out of the main system with counter flowing
steam
(having a lower dissolved oxygen value). The main system flow thus has its ORP
lowered by the lowering of its inherent dissolved oxygen value. Such removal
of
chemical species may occur without or in conjunction with the addition of
other
chemical species into the hot water system.
[0058] In another embodiment of the invention, a non-chemical technique to
change at least one system parameter may be -used either alone or in
conjunction with
chemical addition/removal to adjust or conform the measured ORP. The ORP in
any
one actual zone (or linked zone) might be affected by non-chemical-addition
techniques, upstream of the ORP control zone. Representative non-chemical
techniques and system parameters include, for example, choosing a particular
type of
feed pump or condensate pump; partitioning flow of the system process stream;
17
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
blending or combining streams; selecting materials of construction of various
parts of
the hot water system to control the rate of oxidation; cathodic protection;
electromagnetic wave production; physical property changes; the like; and
combinations thereof.
[00591 The foregoing may be better understood by reference to the
following examples, which are intended for illustrative purposes and are not
intended
to limit the scope of the invention or its application in any way,
Example 1
WWI FIG 1 depicts a simplified 3-component hot water system. Make-
up
water flows through a "Deaerator," a "FW Pump," and into a "Boiler." The
boiler in
turn generates "Useful Steam" that is used for various downstream processes.
in this
Example, ORP may be monitored/controlled at the Deaerator exit, labeled as "1"
in
FIG 1, or at the FW Pump exit, labeled as "2" in FIG 1, REDOX Stress may be
reacted to in real-time as it occurs in the Deaerator and/or FW Pump
independently,
Active chemical species may also be fed into the Deaerator, after the
Deaerator,
and/or after the FW Pump for more specific corrosion control.
Example 2
[0061] FIG 2 illustrates a more complex boiler configuration, including a
plurality of feed water pumps, a plurality of heat exchangers, and a steam
producer
(i.e., boiler), In such a configuration, any number (i.e., one, two, or more)
of
condensers, heat exchangers, pumps, boilers, process steam applications, etc.
could be
used. In FIG 2, flowing feed water is shown as solid arrowed lines as it moves
toward
the "Use of Process Steam" areas 1 and 2. Condensed steam is shown as dotted
arrowed lines as it is fed to various plant locations, which could include the
shell side
of heat exchangers or directly back to the condensate areas. If desired,
condensate
that does not meet plant water specifications for boiler feed water could be
drained
out of the system as blow down,
18
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
[0062] Examples of positions where ORP could be monitored/controlled
and/or feed locations for active chemical species are labeled as "22" in FIG
2. Such
user-controlled positioning allows local corrosion protection capabilities for
a specific
units and/or groups of units as well as global corrosion protection.
Example 3
[0063] FIG 3 depicts how the ORP setting may be different for systems at
different temperatures. The temperatures shown in FIG 3 may represent, tbr
example,
different plants or different operational protective/control zones in the same
plant. In
this Example, the ORP setting is an ORP set range selected from a series of
ranges,
shown as vertical lines labeled "Preferred," "Broader," and "Broadest."
Depending
upon the sophistication of equipment in the plant (i.e., operational
limitations), the
useable ORP set range or point may vary. That is, some plants are able to
handle a
narrow, or preferred, ORP set range, whereas other plants are able to handle
only a
broader ORP set range.
[0064] The (EDT ORP numbers would typically be recorded against an
external pressure balanced reference electrode (designated as "EPBRE" in FIG
3)
having 0.1 normal potassium chloride filling solution, The first 180 F control
zone
might be measured and controlled by an wr ORP probe positioned after "Heat
Exchanger 2" (FIG 2) in the feed water, and the active chemical species might
be fed
into the feed water just after the "Condenser" (FIG 2) in the feed water.
[0065.] The second 350 F control zone might be measured and controlled by
an @T ORP probe positioned after "Heat Exchanger 3" (FIG 2) in the feed water,
and
the active chemical species might be fed into the feed water just prior to
"Heat
Exchanger 3" (FIG 2) in the feed water.
[0066] The third 500 F control zone might be measured and controlled by
an gr ORP probe positioned after "Heat Exchanger 4" (FIG 2) in the feed water,
and
the active chemical species might be fed into the feed water just prior to
"Heat
Exchanger 4" (FIG 2) in the feed water.
19
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
Example 4
[0067] This Example illustrates feeding multiple REDOX active species at
various locations to control the @)T ORP at a single location, as shown in FIG
4, The
controlling (ip;IC ORP probe was placed directly upstream of the feed location
for
REDOX active species #2. The @T ORP probe was used to measure the @T ORP
prior to the feed of REDOX active species #2, The ,(6-0- ORP probe was then
switched
to control the feed of another REDOX active species (#1), being fed upstream
of the
single @T ORP probe. It should be noted that when REDOX active species #2
(that
was being manually controlled) was turned off, the effect of that loss quickly
permeated the plant water chemistry and was sensed by the (ci3T ORP probe. The
controller (in this Example, the controller was automated for REDOX active
species
41) immediately initiated additional feed of REDOX active species #1 to make-
up for
the shortfall in REDOX active species 42.
[0068] The controlled feed of REDOX active species 41 was able to achieve
and maintain the @,`F ORP setting thus minimizing corrosion in the heat
exchangers
during this event. Note that as soon as the REDOX active species #2 was
manually
turned back on, the corrosion control device (i.e., the äT ORP probe system)
immediately compensated by cutting feed of REDOX active species #1 to maintain
the desired ren ORP setting for corrosion control.
Example 5
[0069] This Example illustrates an unpredicted response of the @T ORPTM
probe to measure corrosion events directly and how real-time ORP measurements
act
as a direct indicator of corrosion in hot water systems from REDOX Stress
events,
[0070] The @,,T ORP probe reacts to the formation of corrosion products in
the FW. The REDOX stresses in the FAY include the complex conjugate ionic
corrosion pairs like Fe2+/Fe3+ or Ctr-F/Cu2+, for example. In an all iron-
based ENV
heater, water of high DO (i.e., greater than 500 ppb) starts to enter the FW
heater,
The room temperature ORP and real-time ORP at the heater inlet were initially
¨125
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
trill and ---280 triV, respectively. On experiencing the added REDOX stress
event, the
room temperature ORP and real-time ORP at the heater inlet rose to --70 mV and
--30
mV, respectively. The sensitivity of the @,-)T ORP probe (real-time ORP
increases 250
mV) is clearly seen as compared to the room temperature ORP probe (increased
only
55 mV). The real-time and room temperature ORP probes at the FW heater exit
were
initially ¨540 mV and ¨280 mV, respectively. After the high REDOX stress event
the
real-time and room temperature ORP probes at the FW heater exit became -140
and ¨
280 mV, respectively. It is important to note that the real-time ORP rose by
400 inV,
whereas the room temperature ORP showed no change.
[0071] It is not intended to be bound to any particularly theory; however,
one theory that the room temperature ORP measurements at the exit of the FW
heater
showed no change was that the DO exiting the FW heater remained unchanged
throughout the DO ingress event at the inlet of the ENV heater, The reason the
real--
time ORP numbers rose so dramatically at the PNV heater exit was most likely
because
of the corrosion that had occurred in the FW heater itself. This event
generated a
plentiful supply of ionic oxidized iron species, which the (AT ORP probe
detected; but
the room temperature ORP probe did not,
[0072] The same effect was seen across copper based FAN heaters where the
dissolved oxygen was consumed within the FIN heaters. Once again, room
temperature ORP measurements showed no change at the exit of the FW heaters,
hut
OR.P probe responses showed elevated numbers as oxidized copper ionic species
(conjugate pairs) were released into the FW and exited the FW heater, only to
be
sensed by the (AT ORP probes and not the room temperature ORP instruments.
Example 6 --- Non-Chemical Techniques
[00731 The paragraphs below provide several examples of non-chemical
techniques to change a system parameter that could be used to control measured
ORP
in a hot water system. One of skill in the art would be able to utilize these
techniques
without undue experimentation,
21
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
[0074] Pump Choice: Pumps can be notoriously had actors for air ingress
(often an undesirable affect) and can add to redox stress in systems.
Depending on
whether such ingress was desired or conversely its exclusion was desired the
choice
of feed pump or condensate pump type could affect the ORP measurements quite
drastically. For example, pump design parameters, such as piston packing,
check
valves, diaphragms, seals, glands, impellers, etc. are all zones of possible
failure and
air ingress. Air ingress typically occurs on the lower pressure side of a pump
during
the suction phase of pumping.
[0075] Partitioning: Stream flow and quantity can just be partitioned so that
some or part of the system flow is diverted via sidestream to pieces of
apparatus that
can affect the inherent ORP and return it to the main system stream. For
example,
electrochemical ionization processes could be used to affect its chemical
properties
and thus ORP properties in the sidestream,
[0076] Blending: System or process streams with different ORP properties
could be blended together in known/controlled/calculated ratios to affect the
ORP and
thus corrosion of the downstream system,
[0077] Materials: Separate sections of system made from different materials
that could affect the ORP and corrosion properties could he incorporated and
used in a
specific combination to achieve the desired ORP for the hot water system. For
example, a material that would have a great affinity for dissolved oxygen
(e.g., any
material that can actively oxidize, such as aluminum, chrome, the like, and
combinations thereof, and even, more reactive would he lithium, sodium,
magnesium,
zinc, the like, and combinations thereof) to reduce locally the dissolved
oxygen values
in the process water by oxidizing at a controlled rate. Later in the process
the water
would then have lower ORP values and lowered propensity to corrode other
materials
which would then be better protected. This is somewhat similar to anodic
protection
except that in this case an entire zone, or piece of equipment, might be the
anodic
zone to protect a later zone from corrosive forces. One or more ORP-affecting
species (e.g., a piece of hardware or system component) are added upstream
from a
22
CA 02867480 2014-09-15
WO 2013/155036
PCT/US2013/035713
later zone that requires specific ORP numbers for corrosion protection. While
the
pieces of hardware are generally thought to be metallic they need not be. For
example
activated carbon might prove to be an effective chemistry altering species,
and thus
ORP altering species as well.
[0078] Cathodic Protection: Impressed current similar to cathodic protection
may be used to alter the ORP space whereby sections of equipment or zones
contacting process water can be cathodically protected. In one extreme case
the
cathodic protection could be run at high enough impressed electrochemical
voltages
to introduce chemical altering species, like hydrogen. Hydrogen would then in
and of
itself lower ORP values and could combine with oxygen locally (or downstream)
to
lower the measured ORP values,
[0079] Electromagnetic Waves: Pieces of apparatus could be in a zone of
electromagnetic wave production, such as light sources, ultra-violet
additions,
microwave-inducing waves, the like, and combinations thereof. The
electromagnetic
wave sources could be on continuously or intermittently such as in a
controlled
fashion, pulsed, etc. The wave sources, via their specific action could be
used to
affect the ORP species either directly or indirectly in any zone. For example,
UV
light may activate a cobalt-catalyzed reaction between oxygen and sulfite in
the water.
[0080] Physical Properties: Purposeful and localized changes in physical
properties, for example temperature, pressure, flow, turbulence, and the like
might be
designed to locally affect the system ORP and thus the resultant corrosivity,
[0081] All of the compositions and methods disclosed and claimed herein
can be made and executed without undue experimentation in light of the present
disclosure. While this invention may be embodied in many different forms,
there are
described in detail herein specific preferred embodiments of the invention.
The
present disclosure is an exemplification of the principles of the invention
and is not
intended to limit the invention to the particular embodiments illustrated. In
addition,
unless expressly stated to the contrary, use of the term "a" is intended to
include "at
CA 02867480 2015-09-15
least one" or "one or more." For example, "a device" is intended to include
"at least one device"
or "one or more devices."
[0082] Any ranges given either in absolute terms or in approximate terms are
intended to
encompass both, and any definitions used herein are intended to be clarifying
and not limiting.
Notwithstanding that the numerical ranges and parameters setting forth the
broad scope of the
invention are approximations, the numerical values set forth in the specific
examples are reported
as precisely as possible. Any numerical value, however, inherently contains
certain errors
necessarily resulting from the standard deviation found in their respective
testing measurements.
Moreover, all ranges disclosed herein are to be understood to encompass any
and all subranges
(including all fractional and whole values) subsumed therein.
[0083] Furthermore, the invention encompasses any and all possible
combinations of
some or all of the various embodiments described herein. It should also be
understood that
various changes and modifications to the presently preferred embodiments
described herein will
be apparent to those skilled in the art. Such changes and modifications can he
made without
departing from the scope of the invention and without diminishing its intended
advantages. It is
therefore intended that such changes and modifications be covered by the
appended claims.
24