Note: Descriptions are shown in the official language in which they were submitted.
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
METHOD OF MAKING A MANGANESE CONTAINING SUPPORTED
SILVER CATALYST INTERMEDIATE
FIELD OF INVENTION
[0001] The invention relates to methods of making a manganese-containing
supported
silver catalyst.
BACKGROUND
[0002] Ethylene oxide can be commercially produced by the direct
epoxidation of
ethylene over a supported silver-containing catalyst at elevated temperature.
As the catalyst
is an important element in the direct oxidation of ethylene to ethylene oxide,
much effort
has been expended to improve catalyst stability, efficiency, selectivity,
and/or other aspects
of the performance of the catalyst in producing ethylene oxide.
[0003] Using suitable promoters is an effective and proven way to enhance
the
performance of the catalyst in the production of ethylene oxide, and is well
known to those
skilled in the art. There are at least two types of promoters--solid promoters
and gaseous
promoters. A solid promoter can be incorporated into the catalyst prior to its
use, either as a
part of the carrier (i.e., support) or as a part of the silver component
applied thereto.
Typically, the silver-containing supported catalyst is prepared by
impregnating the support
in an impregnation solution containing silver and optionally one or more
promoters.
[0004] U.S. Pat. No. 5,504,053 describes a silver-containing, supported
catalyst
containing a stability, efficiency and/or activity enhancing amount of a
manganese-
containing component. The manganese is present in the silver-containing
supported catalyst
in an amount of at least 20 parts per million weight (ppmw), or at least 60
ppmw, preferably
70 to 1000 ppmw, more preferably 80 to 500 ppmw, ppmw calculated as the weight
of
manganese based on the total weight of the catalyst.
[0005] W02005/023417A1, W02008/054564A1 and US2007/0111886 describe adding
diammonium ethylenediaminetetraacetic acid with the manganese-containing
component in
order to stabilize the manganese-containing component in an impregnation
solution.
1
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0006] In US2007/0032670, promoters and solubilizers are added to an
impregnating
solution which includes neat potassium nitrate, manganese EDTA (K2MnEDTA)
solution
and diammonium EDTA solution. One equivalent of diammonium EDTA is added with
the
manganese promoter in order to increase the stability of the manganese-
containing ion in
the impregnation solution.
[0007] EP 480,537A1 discloses preparing a solid manganese complex of
tetrahydrate of
ethylene diamine tetraacetatomanganic II-acid (H2MnEDTA), which can be then
introduced
into the impregnation solution. EP 480,537A1 discloses that the metal-
containing
promoter(s), including manganese may be present as complexes in the
impregnating
solution containing silver, prior to being associated with the carrier. Such
complexes may
conveniently be derived by including one or more complexing agents effective
to form a
complex with at least one metal species (a) in the silver-containing
impregnating solution or
(b) in a solution containing a metal-containing promoter precursor in an
amount effective to
enhance the solubility and/or solubility stability of the metal-containing
promoter in the
impregnating solution or solution precursor. The term "solubility stability"
is defined as the
measure of the ability of a metal-containing promoter to remain in solution
over time: the
longer the time in solution, the more solubility stable the metal-containing
promoter is. The
enhancement in solubility and/or solubility stability of the metal-containing
promoter
solutions, as described in EP 480,537A1, refers to solutions not containing
metal-containing
promoters in the complexed form.
[0008] Typically a stoichiometric amount of manganese-containing component
corresponding to the desired target level is provided in an impregnation
solution for
impregnating a support. However, many times the impregnated support or the
catalyst may
not have the desired target level of manganese or they exhibit variability in
the amount of
manganese. If the resultant catalyst exhibits variability of the order of 10%
or more from
the desired target level, the performance of the catalyst is adversely
affected. Therefore a
much simplified, commercially viable, and yet reliable way of providing
manganese
component in supported silver catalyst is desirable.
BRIEF DESCRIPTION
2
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0009] According to embodiments of the present invention, variability in
the amount of
manganese on the manganese-containing supported silver catalyst can be lowered
by
following the inventive method of making a manganese-containing supported
silver catalyst
intermediate. The method includes the step (i) of preparing a first solution
comprising a
manganese component and a complexing agent. The pH of the first solution at
any time
during or after step (i) is less than or equal to 7. At step (ii), the first
solution is combined
with a second solution comprising silver to form an impregnation solution. At
step (iii), a
support is subsequently impregnated with at least a portion of the
impregnation solution to
form the catalyst intermediate. The impregnation solution has a pH of greater
than 7. By
decreasing the variability in the amount of manganese on the catalyst, the
catalyst
performance such as efficiency, activity, aging and/or other aspects of
catalyst performance
is improved.
DRAWINGS
[0010] FIG. 1 is a plot of variability in the amount of manganese in an
impregnation
solution against impregnation solution batches prepared using a prior art
method and is
expressed as the percentage variation in manganese content from the desired
target levels;
[0011] FIG. 2 is a plot of variability in the amount of manganese in an
impregnation
solution against impregnation solution batches prepared using embodiments of
the present
invention and is expressed as the percentage variation in manganese content
from the
desired target levels;
[0012] FIG. 3 is a comparison of performance of catalyst batches prepared
using a prior
art method example 4* and an inventive method example 3; and
[0013] FIG. 4 is a comparison of variation in the amount of manganese in
manganese-
containing silver-amine-oxalate solution batches prepared using a prior art
method example
5* and an inventive method example 4.
DETAILED DESCRIPTION
[0014] Supported silver catalysts containing manganese promoters show
enhanced
stability, activity and/or selectivity upon ethylene epoxidation to produce
ethylene oxide,
3
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
when compared to silver catalysts not having manganese promoters in them. We
have
surprisingly found that when a first solution comprising a manganese component
and a
complexing agent is combined with a second solution comprising silver to form
an
impregnation solution, a catalyst obtained by impregnation of this
impregnation solution
shows better performance characteristics than compared to a catalyst obtained
using an
impregnation solution prepared using a method which does not include preparing
the first
solution comprising the manganese component and the complexing agent. In one
embodiment, the inventive method provides lower variability in the amount of
manganese
on the manganese-containing supported silver catalyst compared to a prior art
method
which does not include preparing the first solution.
[0015] In a typical epoxidation reaction, an alkylene, such as ethylene,
reacts with
oxygen or an oxygen-containing gas in presence of a supported silver catalyst
in a reactor to
form an alkylene oxide such as ethylene oxide. The epoxidation reaction can be
characterized in terms of "activity", "productivity" and/or "selectivity" of
the epoxidation
reaction.
[0016] The activity of the epoxidation reaction can be quantified in a
number of ways,
one being the mole percent of alkylene oxide contained in an outlet stream of
the reactor
relative to that in the inlet stream (the mole percent of alkylene oxide in
the inlet stream
typically, but not necessarily, approaches zero percent) while the reactor
temperature is
maintained substantially constant; and another being the temperature required
to maintain a
given rate of alkylene oxide production. In many instances, activity is
measured over a
period of time in terms of the mole percent of alkylene oxide produced at a
specified
constant temperature. The activity can be defined as the reaction rate towards
the alkylene
oxide formation per unit of catalyst volume in the reactor. Alternatively,
activity can be
measured as a function of the temperature required to sustain production of a
specified
constant mole percent of alkylene oxide, such as ethylene oxide, given other
conditions such
as pressure and total moles in the feed.
[0017] The productivity of the reaction is a measure of the reaction rate
normalized by
the amount of catalyst. In many instances, productivity can be expressed as
moles or
kilograms of alkylene oxide produced per hour per volume of the catalyst
measured as the
4
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
packed volume of the reactor. In certain instances, the productivity can be
expressed as
mole percent of alkylene oxide in the outlet stream of the reactor at
specified process
conditions such as a space velocity.
[0018] The "selectivity" of the epoxidation reaction, which is synonymous
with
"efficiency," refers to the relative amount (as a fraction or in percent) of
converted or
reacted olefin that forms a particular product. For example, the "efficiency
to alkylene
oxide" refers to the percentage on a molar basis of converted or reacted
alkylene that forms
alkylene oxide.
[0019] "Deactivation" or "aging", as used herein, refers to a permanent
loss of activity
and/or efficiency that is a decrease in activity and/or efficiency that cannot
be recovered.
Lower rates of deactivation are generally desirable.
[0020] As used herein, the term "compound" refers to the combination of a
particular
element with one or more different elements by surface and/or chemical
bonding, such as
ionic and/or covalent and/or coordinate bonding. The term "ionic" or "ion"
refers to an
electrically charged chemical moiety; "cationic" or "cation" being positive
and "anionic" or
"anion" being negative. The term "oxyanionic" or "oxyanion" refers to a
negatively charged
moiety containing at least one oxygen atom in combination with another
element. An
oxyanion is thus an oxygen-containing anion. It is understood that ions do not
exist in
vacuo, but are found in combination with charge-balancing counter ions when
added as a
compound to the catalyst.
[0021] As used herein, the term "solution" refers to clear solutions and
also includes
suspensions and colloidal solutions.
[0022] The term "support", as used herein, refers to a support or a carrier
that is
commonly used for preparing epoxidation catalysts. An "impregnated support"
refers to a
support that has been impregnated with silver or upon which silver has been
deposited. The
term "catalyst intermediate", as used herein, refers to a support which has
been impregnated
or deposited with at least manganese and silver, according to embodiments of
the present
invention by following steps (i) to (iii) of the method of making a manganese-
containing
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
supported silver catalyst intermediate. The "catalyst intermediate" is
otherwise also
referred to as "manganese-containing supported silver catalyst intermediate".
[0023] As used herein, the term "catalyst" refers to the finished catalyst
obtained after
further processing of the "catalyst intermediate". The "catalyst" is also
otherwise termed as
the "manganese-containing supported silver catalyst" prepared according to
embodiments of
the present invention and that which can be directly charged in the reactor
for use in
commercial ethylene oxide production.
[0024] As used herein, the term "variability" is defined as the variation
or the deviation
in the amount of manganese deposited or present on a catalyst intermediate, or
a finished
catalyst or in an impregnation solution from the desired target level. In one
embodiment,
variability is expressed as the percentage variation in the amount of
manganese from the
desired target level.
[0025] As used herein, the term the "pH of the first solution at any time
during or after
step (i)" means the pH of the first solution at least one point in time after
the combination of
the manganese component and the complexing agent. It does not mean "at any and
all
times" subsequent to the combination of the manganese component and the
complexing
agent.
[0026] The manganese component (manganese promoter) can be provided in various
forms, e.g., as a covalent compound such as manganese dioxide, as a cation or
as an anion
such as a manganate anion. Manganese components present in the first solution,
can
include, but are not limited to, manganese acetate, manganese ammonium
sulfate,
manganese citrate, manganese dithionate, manganese oxalate, manganous nitrate,
manganous sulfate, and manganate anion, e.g., permanganate anion, manganate
anion, and
the like. Mixtures of manganese components may also be used. The manganese
species
that provides enhanced activity and/or stability is not certain and may be the
component
added or that generated either during catalyst preparation or during use as a
catalyst.
Although the manganese species that provide the beneficial properties to the
catalysts are
not known with specificity, generally acceptable results are obtained when the
manganese
6
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
component is added to the first solution in the form of permanganate ion
(Mn04)1- and/or as
manganous cation, e.g., as in Mn(NO3)2. Moreover, different added manganese
components
may also have different optimum concentrations to achieve the results. Often,
the
manganese in the manganese component has an oxidation state of +2, +3, +4
and/or +7,
preferably +2, +3, and/or +7.
[0027] The desired amount of the manganese promoter on the catalyst
intermediate or
the catalyst may be decided based upon the silver content of the catalyst
intermediate or the
catalyst, the amounts and types of other promoters present and the chemical
and physical
properties of the support. In one embodiment, the manganese is present on the
catalyst
intermediate or the catalyst in an amount of at least 20 ppmw, more preferably
at least 60
ppmw calculated as the weight of manganese. In some embodiments, the amount of
manganese on the catalyst intermediate or the catalyst falls within the range
of 70 ppmw to
1000 ppmw, preferably 80 ppmw to 500 ppmw calculated as the weight of
manganese. If
too much manganese is present, the catalyst performance, e.g., stability,
efficiency and/or
activity, may suffer. If too little manganese is present, it is also possible
that the
performance of the catalyst will suffer. In determining desired amounts of
manganese, a
traverse of manganese concentrations in the catalyst composition can be
effected with the
catalysts being evaluated for performance. In some instances, it may be
desirable to vary
the amounts of other components, e. g., silver and other promoters, to achieve
beneficial
combinations of effects and optimal catalyst performance.
[0028] Examples of complexing agents in the first solution include
ethylenediaminetetraacetic acid (EDTA); N, N'-ethylenediaminediacetic acid; N-
hydroxyethylethylenediaminetriacetic acid; diethylenetriaminepentaacetic acid
(DTPA);
nitrilotriacetic acid; 1,2-cyclohexylenedinitrilotetraacetic acid (CDTA); N-
hydroxyethyliminodiacetic acid; N-dihydroxyethylglycine and any derivatives
thereof. In
one embodiment, the complexing agent is EDTA.
[0029] The amount of complexing agent employed varies widely, for example,
depending on the specific complexing agent and specific manganese component to
be
complexed, as well as on the amount of manganese component to be complexed.
Preferably, the amount of complexing agent is at least 50%, more preferably at
least 100%,
7
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
of that needed to form complexes with the manganese component in the first
solution.
Excesses of complexing agent over that needed to form the desired complexes
may be
employed, for example, so that the complexes can be maintained over a
relatively long
period of time. For example, the complexing agent may be included in an amount
of at least
150% or at least 200% or at least 400% or more of that needed to form the
desired
complexes.
[0030] At step (i), the manganese component solution and the complexing
agent solution
can be combined simultaneously, or sequentially, to form the first solution.
In one
embodiment, a complexing agent solution is combined with an aqueous solution
containing
manganese component. In another embodiment, the manganese component is in
solid form
and can be added to the complexing agent solution. Additionally, heating may
be required
for dissolving the complexing agent, the manganese component or both. The pH
of the first
solution at any time during or after the preparation of the first solution is
less than or equal
to 7. The pH of the first solution can be measured using conventional pH
meters or using
pH papers. In one embodiment, the pH of the first solution is less than or
equal to 7 after
combining the manganese component and the complexing agent or during the
preparation of
the first solution. In another embodiment, following the preparation of the
first solution it is
stored at a pH of less than or equal to 7. It is believed, without being bound
to any theory,
that the manganese component may exhibit enhanced solubility in the first
solution
comprising the complexing agent at pH of less than or equal to 7. As is known
to those of
skill in the art, the pH of the first solution can be adjusted if necessary to
a pH of less than
or equal to 7 through the use of an acid. Examples of suitable acids include
acetic acid and
formic acid and other acids that do not leave a residue upon subsequent
roasting of the
impregnated support. In yet other embodiments, the pH of the first solution
prepared
according to the invention may subsequently be increased above 7 prior to step
(ii), through
addition of a basic compound, such as an amine, for example, monoethanolamine.
[0031] The first solution may additionally include one or more other
promoters other
than manganese. In one embodiment, the one or more other promoters does not
comprise
potassium.
8
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0032] At
step (ii), a second solution comprising silver is combined with the first
solution to form an impregnation solution. In one embodiment, the pH of the
first solution
at the time of combining with the second solution is less than or equal to 7.
In another
embodiment, the pH of the first solution at the time of combining with the
second solution
is greater than 7. The second solution comprising silver includes a silver
compound in a
solvent or a solubilizing agent such as the silver solutions disclosed in the
art. The
particular silver compound employed may be chosen, for example, from among
silver
complexes, silver nitrate, silver oxide or silver carboxylates, such as silver
acetate, oxalate,
citrate, phthalate, lactate, propionate, butyrate and higher fatty acid salts.
In one
embodiment, the silver oxide compound complexed with amines is the preferred
form of
silver in the second solution.
[0033] A wide
variety of solvents or solubilizing agents may be employed to solubilize
silver compound to the desired concentration in the second solution. Among
those disclosed
as being suitable for this purpose are lactic acid (U.S. Pat. Nos. 2,477,436
and 3,501,417);
ammonia (U.S. Pat. No. 2,463,228); alcohols, such as ethylene glycol (U.S.
Pat. Nos.
2,825,701 and 3,563,914); and amines and aqueous mixtures of amines (U.S. Pat.
Nos.
2,459,896; 3,563,914; 3,215,750; 3,702,259; 4,097,414; 4,374,260 and
4,321,206). In a
preferred embodiment of the invention, the solubilizing agent is an
amine/oxalate
combination or aqueous mixtures of amines and oxalate and the resulting
impregnation
solution has a pH that is greater than 7.
[0034] Silver
oxide (Ag20) can be dissolved in a solution of oxalic acid and
ethylenediamine to an extent of approximately 30% by weight of silver. Vacuum
impregnation of such a solution onto an alpha alumina support of approximately
0.7 cc/g
porosity results in a catalyst containing approximately 25% by weight of
silver based on the
entire weight of the catalyst.
[0035] In
some embodiments, the catalyst intermediate or catalyst contain a high
concentration of silver, generally at least 25 or 30 percent by weight, based
on the total
weight of the catalyst, more generally in the range of from 25 or 30 percent
to 60 percent by
weight. Accordingly, in order to obtain catalysts having a silver loading of
greater than 25
or 30 weight %, and more, it may be necessary to subject the support or the
catalyst
9
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
intermediate to at least one or more sequential impregnations of silver, with
or without
promoters, until the desired amount of silver is deposited on the support, as
will be
described in detail.
[0036] In one embodiment, the silver particle size on manganese-containing
silver
catalyst is in the range of 10 angstroms to 10,000 angstroms in diameter. A
preferred silver
particle size ranges from greater than 100 angstroms to less than 5,000
angstroms in
diameter. It is desirable that the silver be relatively uniformly dispersed
within, throughout,
and/or on the manganese-containing silver catalyst.
[0037] The second solution may additionally include one or more promoters
other than
manganese, and these may also be added subsequent to the combining of the
first and
second solutions, before impregnation of the support. These promoters are
provided in a
promoting amount. As used herein, the term "promoting amount" refers to an
amount of a
component of the catalysts that works effectively to provide an improvement in
one or more
of the catalytic properties of that catalyst when compared to a catalyst not
containing said
component. Examples of catalytic properties include, inter alia, operability
(resistance to
run-away), efficiency, activity, conversion, stability and yield. It is
understood by one
skilled in the art that one or more of the individual catalytic properties may
be enhanced by
the "promoting amount" while other catalytic properties may or may not be
enhanced or
may even be diminished. It is further understood that different catalytic
properties may be
enhanced at different operating conditions. For example, a catalyst having
enhanced
efficiency at one set of operating conditions may be operated at a different
set of conditions
wherein the improvement shows up in the activity rather than the efficiency.
[0038] The promoting effect provided by the promoters can be affected by a
number of
variables such as for example, operating conditions, catalyst preparative
techniques, surface
area and pore structure and surface chemical properties of the support, the
silver and other
promoter content of the catalyst, the presence of other cations and anions
present on the
catalyst. The presence of other activators, stabilizers, promoters, enhancers
or other catalyst
improvers can also affect the promoting effects. During the reaction to make
ethylene
oxide, the specific form of the promoter on the catalyst may be unknown, and
the promoter
may be present without the counterion added during the preparation of the
catalyst. For
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
example, a catalyst made with cesium hydroxide may be analyzed to contain
cesium but not
hydroxide in the finished catalyst. Likewise, compounds such as alkali metal
oxide, for
example cesium oxide, or transition metal oxides, for example Mo03, while not
being ionic,
may convert to ionic compounds during catalyst preparation or in use. For the
sake of ease
of understanding, the promoters will be referred to in terms of cations and
anions regardless
of their form in the catalyst under operating conditions.
[0039] Examples of solid promoter compositions and their characteristics as
well as
methods for incorporating the promoters as part of the catalyst are described
in U.S. Patent
No. 5,187,140, particularly at columns 11 through 15; U.S. Patent Nos.
6,511,938,
5,102,848, 4,916,243, 4,908,343, 5,059,481 4,761,394, 4,766,105, 4,808,738,
4,820,675 and
4,833,261.
[0040] Examples of well-known promoters other than manganese for catalysts
used to
produce ethylene oxide include halides and/or oxyanions of elements other than
oxygen
having an atomic number of 5 to 83 and being from the groups 3b to 7b, and 3a
to 7a, of the
Periodic Table. One or more of the oxyanions of nitrogen, sulfur, tantalum,
molybdenum,
tungsten and rhenium may be preferred for some applications. In some
embodiments, the
promoters include compounds of rhenium, rubidium, cesium, sulfur, molybdenum,
and
tungsten. In one embodiment, the one or more promoters is selected from a
group
consisting of Group IA metals, Group HA metals, phosphorus, boron, sulfur,
molybdenum,
tungsten, chromium, titanium, hafnium, zirconium, vanadium, thallium, thorium,
tantalum,
niobium, gallium, germanium and any mixtures thereof, and in another
embodiment,
excluding potassium. In yet another embodiment, the one or more promoters
comprises
Group IA metals selected from cesium, lithium, sodium and any mixtures
thereof.
[0041] The types of anion promoters other than manganates suitable for use
in the
catalysts of this invention comprise, by way of example only, oxyanions such
as sulfate,
504-2, phosphates, for example, PO4-3, titanates, e g., TiO3-2, tantalates,
for example, Ta206-
2, molybdates, for example, Mo04-2, vanadates, for example, V204-2, chromates,
for
example, Cr04-2, zirconates, for example, Zr03-2, polyphosphates, nitrates,
chlorates,
bromates, borates, silicates, carbonates, tungstates, thiosulfates, cerates
and the like. The
halides may also be present, including fluoride, chloride, bromide and iodide.
11
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0042] It is
well recognized that many anions have complex chemistries and may exist in
one or more forms, for example, orthovanadate and metavanadate; and the
various
molybdate oxyanions such as Moat- and 1µ40 o
2, _24 6
and Mo207 2. The oxyanions may also
include mixed metal-containing oxyanions including polyoxyanion structures.
For instance,
manganese and molybdenum can form a mixed metal oxyanion. Similarly, other
metals,
whether provided in anionic, cationic, elemental or covalent form may enter
into anionic
structures.
[0043] While an oxyanion, or a precursor to an oxyanion, may be used in
solutions for
impregnating the support, it is possible that during the conditions of
preparation of the
catalyst and/or during use, the particular oxyanion or precursor initially
present may be
converted to another form. Indeed, the element may be converted to a cationic
or covalent
form. In many instances, analytical techniques may not be sufficient to
precisely identify
the species present. The invention is not intended to be limited by the exact
species that
may ultimately exist on the catalyst during use.
[0044] The amount of anion promoter other than manganates may vary widely, for
example, from 0.0005 weight percent to 2 weight percent, preferably from 0.001
weight
percent to 0.5 weight percent based on the total weight of the catalyst.
[0045] The
catalyst prepared using embodiments of the present invention may comprise
a rhenium promoter. The rhenium promoter can be provided in various forms, for
example,
as the metal, as a covalent compound, as a cation or as an anion. Rhenium
promoted
supported silver containing catalysts are known from U.S. Pat. No. 4,761,394
and U.S. Pat.
No. 4,766,105. The catalysts comprise silver, rhenium or compound thereof, and
in some
embodiments, a second promoter such as a further metal or compound thereof and
optionally a third promoter such as one or more of sulfur, phosphorus, boron,
and
compounds thereof, on the support material.
[0046] The
rhenium species that provides the enhanced efficiency and/or activity is not
certain and may be the component added or that generated either during
preparation of the
catalyst or during use as a catalyst. Examples of rhenium compounds include
the rhenium
salts such as rhenium halides, the rhenium oxyhalides, the rhenates, the
perrhenates, the
oxides and the acids of rhenium. However, the alkali metal perrhenates,
ammonium
12
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
perrhenate, alkaline earth metal perrhenates, silver perrhenates, other
perrhenates and
rhenium heptoxide can also be suitably utilized. Rhenium heptoxide, Re207,
when
dissolved in water, hydrolyzes to perrhenic acid, HRe04, or hydrogen
perrhenate. Thus, for
purposes of this specification, rhenium heptoxide can be considered to be a
perrhenate, that
is, Re04. Similar chemistries can be exhibited by other metals such as
molybdenum and
tungsten.
[0047] When used, the rhenium component is often provided in an amount of at
least 1
ppmw, say, at least 5 ppmw, for example, 10 ppmw to 2000 ppmw, often between
20 ppmw
and 1000 ppmw, calculated as the weight of rhenium based on the total weight
of the
catalyst.
[0048] In some instances, the one or more promoters other than manganese
comprise a
mixture of cations, for example cesium and at least one other alkali metal to
obtain a
synergistic efficiency enhancement, as disclosed in U.S. No. 4,916,243. In
some
embodiments of the present invention, potassium is not one of these alkali
metal promoters.
[0049] The concentration of the alkali metal promoters in the finished
catalyst is not
narrow and may vary over a wide range. The optimum alkali metal promoter
concentration
for a particular catalyst will be dependent upon performance characteristics,
such as catalyst
efficiency, rate of catalyst aging and reaction temperature.
[0050] The concentration of alkali metal (based on the weight of cation,
for example
cesium) in the finished catalyst may vary from 0.0005 to 1.0 wt. %, preferably
from 0.005
to 0.5 wt. %. The preferred amount of cation promoter deposited on or present
on the
surface of the support or catalyst generally lies between 10 ppm and 4000 ppm,
preferably
15 ppm and 3000 ppm, and more preferably between 20 ppm and 2500 ppm by weight
of
cation calculated on the total support material. Amounts between 50 ppm and
2000 ppm are
frequently most preferable. When the alkali metal cesium is used in mixture
with other
cations, the ratio of cesium to any other alkali metal and alkaline earth
metal salt(s), if used,
to achieve desired performance is not narrow and may vary over a wide range.
The ratio of
cesium to the other cation promoters may vary from 0.0001:1 to 10,000:1,
preferably from
0.001:1 to 1,000:1.
13
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0051] At step (iii), a support or impregnated support is impregnated with
at least a
portion of the impregnation solution to form the catalyst intermediate.
According to
preferred embodiments of the present invention, the impregnation solution used
for
impregnating the support has a pH of greater than 7. Suitable support
materials of the
catalyst intermediate can include porous refractory carrier or materials that
are relatively
inert in the presence of the reaction mixture introduced for epoxidation and
the product
epoxide, and are able to withstand preparation conditions when converted into
catalyst. For
example, the support can comprise alpha-alumina, silicon carbide, silicon
dioxide, zirconia,
magnesia, pumice, zeolites, charcoal, various clays, alkaline earth metal
carbonates, such as
calcium carbonate and mixtures thereof. In one embodiment, the support
comprises alpha-
alumina.
[0052] There are many well-known methods of preparing supports suitable for
use in
alkylene oxide catalysts. Some of such methods are described in, for example,
U.S. Patent
Nos. 4,379,134, 4,806,518, 5,063,195, 5,384,302, 6,831,037 and the like. For
example, an
alpha-alumina support of at least 95% purity can be prepared by compounding
(mixing) the
raw materials, extrusion, drying and a high temperature calcination. In this
case, the
starting raw materials usually include one or more alpha-alumina powder(s)
with different
properties, a clay-type material which may be added as binder to provide
physical strength,
and a burnout material (usually an organic compound) used in the mix to
provide desired
porosity and/or pore size distribution after its removal during the
calcination step. The
levels of impurities in the finished support are determined by the purity of
the raw materials
used, and their degree of volatilization during the calcination step. Common
impurities may
include silica, alkali and alkaline earth metal oxides and trace amounts of
metal and/or non-
metal-containing additives. Another method for preparing a support having
particularly
suitable properties for alkylene oxide catalyst usage comprises optionally
mixing zirconium
silicate with boehmite alumina (A100H) and/or gamma-alumina, peptizing the
aluminas
with a mixture containing an acidic component and halide anions (preferably
fluoride
anions) to provide peptized halogenated alumina, forming (for example, by
extruding or
pressing) the peptized halogenated alumina to provide formed peptized
halogenated
alumina, drying the formed peptized halogenated alumina to provide dried
formed alumina,
and calcining the dried formed alumina to provide pills of modified alpha-
alumina support.
14
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0053] There have been employed alumina which has a very high purity, that
is, at least
98 wt. % alpha-alumina, any remaining components being silica, alkali metal
oxides (for
example, sodium oxide) and trace amounts of other metal-containing and/or non-
metal-
containing additives or impurities. Likewise, there have been employed alumina
of lower
purity, that is, 80 wt. % alpha-alumina, the balance being one or more of
amorphous and/or
crystalline alumina and other alumina oxides, silica, silica alumina, mullite,
various alkali
metal oxides (for example, potassium oxide and cesium oxide), alkaline earth
metal oxides,
transition metal oxides (for example, iron oxide and titanium oxide), and
other metal and
non-metal oxides. In addition, the material used to make the support may
comprise
compounds which have been known for improving catalyst performance, for
example,
rhenium, (such as rhenates) and molybdenum.
[0054] In one embodiment, the support material comprises at least 80 weight
percent
alpha-alumina and comprises less than 30 parts per million acid-leachable
alkali metals by
weight, the weight percent of the alpha-alumina and the concentration of the
acid-leachable
alkali metals being calculated on the weight of the support, where the acid-
leachable alkali
metals are selected from lithium, sodium, potassium, and mixtures thereof.
[0055] The alpha-alumina support preferably has a pore volume of at least
0.3 cubic
centimeters per gram (cm3/g), and more preferably, from 0.4 cm3/g to 2.0
cm3/g; and a
median pore diameter from 1 to 50 microns.
[0056] The alpha-alumina support preferably has a specific surface area of
at least 0.5
square meters per gram (m2/g), and more preferably, at least 0.7 m2/g. The
surface area is
typically less than 10 m2/g, and preferably, less than 5 m2/g.
[0057] In one embodiment, the alpha-alumina support includes particles each
of which
has at least one substantially flat major surface having a lamellate or
platelet morphology
which approximates the shape of a hexagonal plate (some particles having two
or more flat
surfaces), at least 50 percent of which (by number) have a major dimension of
less than 50
microns.
[0058] The alpha-alumina support can be of any suitable shape. Exemplary
shapes of
the support includes pills, chunks, tablets, pieces, pellets, rings, spheres,
wagon wheels,
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
toroids having star shaped inner and/or outer surfaces, and the like. The
support can be of
any size suitable for employment in reactors. For example, in a fixed bed
ethylene oxide
reactor having a plurality of parallel elongated tubes (in a suitable shell) 1
to 3 inches (2.5 to
7.5 cm) outer diameter and 15 to 45 feet (4.5 to 13.5 m) long filled with
catalyst, it is
desirable to employ alpha alumina support having a rounded shape, such as, for
example,
spheres, pellets, rings, cross-partitioned rings, penta-rings, tablets, and
the like, having
diameters from 0.1 inch (0.25 cm) to 0.8 inch (2 cm).
[0059] The support or impregnated support is impregnated with at least a
portion of the
impregnation solution to form the catalyst intermediate. Impregnation of
similar supports is
given in U.S. Patent Nos. 6,511,938 and 5,187,140. Following impregnation, the
catalyst
intermediate is separated from any remaining non-absorbed impregnation
solution. This is
conveniently accomplished by draining the excess impregnation solution or,
alternatively,
by using another separation technique, such as filtration or centrifugation.
[0060] The impregnation step (iii) may be followed by roasting or other
procedures to
render the silver insoluble, after separating the non-absorbed impregnation
solution.
Generally in roasting process, the catalyst intermediate is heat treated to
effect
decomposition and reduction of the catalytic material, for example, silver
metal compound
(complexes in most cases), to metallic form and the deposition of manganese
and any other
promoters. Such roasting may be carried out at a temperature of from 100 C to
900 C,
preferably from 200 C to 700 C, for a period of time sufficient to, for
example, convert
substantially all of any salt, for example, silver salt, to metal, for
example, silver metal.
[0061] Although a wide range of heating periods have been suggested in the
art to
thermally treat impregnated support (for example, U.S. Pat. No. 3,563, 914
suggests heating
for less than 300 seconds to dry, but not roast to reduce, the catalytic
material; U.S. Pat. No.
3,702,259 discloses heating from 2 to 8 hours at a temperature of from 100 C
to 375 C to
reduce silver salt in the catalyst), it is only important that the reduction
time be correlated
with temperature such that substantially complete reduction of, for example,
the silver salt
to metal is accomplished. A continuous or step-wise heating program is
desirably used for
this purpose. Continuous roasting of the catalyst intermediate for a short
period of time,
such as for not longer than 1 hour is preferred and can be effectively done in
making the
16
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
catalysts of this invention. When more than one roasting is carried out, it is
not necessary
that the roasting conditions be the same in each roasting.
[0062] Heat treatment is preferably carried out in air, but nitrogen,
hydrogen, carbon
dioxide or other atmospheres may also be employed. The equipment used for such
heat
treatment may use a static or flowing atmosphere of such gases to effect
reduction, but a
flowing atmosphere is much preferred. In some embodiments, the catalyst
intermediate can
be chemically treated to reduce any silver compounds to metallic silver.
[0063] In one embodiment, the method of making the manganese-containing
supported
silver catalyst includes following steps (i) to (iii) to form the catalyst
intermediate which is
then roasted or chemically treated to form the manganese-containing silver
catalyst. In
some embodiments, two or more impregnation steps are used to make the
manganese-
containing supported silver catalysts. For example in a sequential
impregnation, a support
is impregnated with at least a portion of a first impregnation solution
containing silver,
manganese and optionally one or more promoters other than manganese to form a
first
catalyst intermediate, by following steps (i) to (iii). The first catalyst
intermediate is then
roasted or chemically treated to form a second catalyst intermediate. For
subsequent
impregnation, the second catalyst intermediate is impregnated with at least a
portion of a
second impregnation solution by following steps (i) to (iii), or by any known
impregnation
process. In another embodiment, a support is impregnated with at least a
portion of a first
impregnation solution containing silver to form a first impregnated support.
The first
impregnated support is roasted to form a silver-impregnated support. The
silver-
impregnated support is subjected to a second impregnation step following steps
(i) to (iii) to
form a first catalyst intermediate.
[0064] In embodiments where sequential impregnation is followed, a
concentration of
the silver may be higher in the second impregnation solution than in the first
impregnation
solution. For example, if a total silver concentration of 30% were desired in
the catalyst, a
low amount of silver of 10% by weight would be deposited on the support as a
result of the
first impregnation followed by a second silver impregnation on the support
depositing the
remaining 20% by weight, all percentages being calculated on the basis of the
finished
catalyst. In other embodiments, approximately equal amounts of silver are
deposited during
17
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
each impregnation step. Often, to effect the equal deposition in each
impregnation step, the
silver concentration in the subsequent impregnation solutions may need to be
greater than
that in the initial impregnation solutions. In further embodiments, a greater
amount of silver
is deposited on the support in the initial impregnation than that deposited in
subsequent
impregnations. Impregnation of the catalyst support may be effected using one
or more
solutions containing silver and promoters in accordance with well-known
procedures for
coincidental or sequential depositions. For coincidental deposition, following
impregnation
the impregnated support is heat or chemically treated to reduce the silver
compound to
silver metal and deposit the salts onto the catalyst surfaces.
[0065] The manganese-containing supported silver catalysts of the invention
are
particularly suitable in the vapor phase process for the continuous production
of ethylene
oxide by contacting in vapor phase ethylene with oxygen or an oxygen
containing gas. The
epoxidation reaction can be air-based or oxygen-based, see Kirk-Othmer's
Encyclopedia of
Chemical Technology, 3rd ed., Vol. 9, 1980, p. 445-447. The commercially-
practiced
processes are carried out by continuously introducing a feed stream containing
ethylene and
oxygen to a manganese-containing supported silver catalyst containing reactor
at a
temperature of from 200 C to 300 C, and a pressure which may vary from five
atmospheres to 30 atmospheres depending upon the mass velocity and
productivity desired.
Residence times in large-scale reactors are generally on the order of 0.1 to 5
seconds. The
feed stream may also include gas-phase modifiers such as organic chlorides;
ethane; carbon
dioxide; and water.
[0066] The oxygen can be provided to the process as pure molecular oxygen,
or
alternatively, as an oxygen-containing gas, wherein the gas may further
contain one or more
gaseous components, for example, gaseous diluents, such as nitrogen, helium,
methane, and
argon, which are essentially inert with respect to the oxidation process. The
raw ethylene
feed stream may also contain other hydrocarbons, such as ethane, present as an
impurity.
Ethane can also be added to a commercial reactor to provide better control of
the organic
chloride's inhibitor action. The relative volumetric ratio of ethylene to
oxygen in the
reaction mixture can range in accordance with any of such known conventional
values.
18
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0067] The gas-phase modifiers are also otherwise termed as inhibitors
and/or enhancers.
Suitable gas-phase modifiers can be selected from a group containing C1-C8
chlorohydrocarbons. It is believed that the ability of the gas-phase modifiers
to enhance the
efficiency and/or activity of the epoxidation process depends on the extent to
which the gas-
phase modifiers chlorinates the surface of the catalyst, for example, by
depositing particular
chlorine species such as atomic chlorine or chloride ions on the catalyst.
However,
hydrocarbons lacking chlorine atoms are believed to strip chlorides from the
catalyst, and
therefore, detract from the overall efficiency enhancement provided by the
gaseous
chlorine-containing promoter species. Discussions of this phenomenon may be
found in
Berty, "Inhibitor Action of Chlorinated Hydrocarbons in the Oxidation of
Ethylene to
Ethylene Oxide," Chemical Engineering Communications, Vol. 82 (1989) at 229-
232 and
Berty, "Ethylene Oxide Synthesis," Applied Industrial Catalysis, Vol. I (1983)
at 207-238.
Paraffinic compounds, such as ethane or propane, are believed to be especially
effective at
stripping chlorides from the catalyst. However, olefins such as ethylene and
propylene, are
also believed to act to strip chlorides from the catalyst. Some of these
hydrocarbons may
also be introduced as impurities in the ethylene feed or may be present for
other reasons
(such as the use of recycle stream) in the feed stream.
[0068] Carbon dioxide is generally considered an inhibitor, and the
inhibitor effect of
carbon dioxide on process efficiency may be variable with its concentration.
With different
types of promoters used in preparation of the catalysts of this invention,
different
concentrations of carbon dioxide may be more desirable in certain commercial
processes.
Typically, the amount of carbon dioxide used in commercial processes can vary
from less
than 2 to 15 mole percent for achieving optimization under both air process
conditions and
oxygen process conditions. The amount of carbon dioxide may also be dependent
on the
size and type of carbon dioxide scrubbing system employed.
[0069] Typically, the volumetric ratio of alkylene to oxygen in the
reaction mixture can
vary from 1/1 to 10/1. Likewise, the quantity of inert gases, diluents, or
other gaseous
components, such as water, carbon dioxide, gas-phase modifiers and gaseous by-
product
inhibitors, can vary in accordance with known conventional ranges as found in
the art.
19
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
[0070] Suitable reactors for the epoxidation reaction include fixed bed
reactors, fixed
bed tubular reactors, continuously stirred tank reactors (CSTR), fluid bed
reactors and a
wide variety of reactors that are well known to those skilled in the art. The
reaction
conditions for carrying out the epoxidation reaction are well-known and
extensively
described in the prior art. This applies to reaction conditions, such as
temperature, pressure,
residence time, concentration of reactants, gas phase diluents (e.g.,
nitrogen, methane and
carbon dioxide), gas phase inhibitors (e.g., organic chlorides), and the like.
The desirability
of recycling unreacted feed, or employing a single-pass system, or using
successive
reactions to increase ethylene conversion by employing reactors in series
arrangement can
be readily determined by those skilled in the art. The particular mode of
operation selected
will usually be dictated by process economics. The ethylene oxide produced
according to
embodiments of the present invention is separated and recovered from the
reaction products
using conventional methods.
[0071] The ethylene oxide produced by the present epoxidation process may
typically be
processed to provide further downstream products, such as, for example,
ethylene glycol,
ethylene glycol ether, ethylene carbonate, and ethanol amine. The conversion
of ethylene
oxide into ethylene glycol or ethylene glycol ether may comprise, for example,
reacting the
desired ethylene oxide with water, suitably in the presence of an acidic or
basic catalyst.
For example, for preferential production of the ethylene glycol over the
ethylene glycol
ether, the ethylene oxide may be reacted with a tenfold molar excess of water,
in a liquid
phase reaction in the presence of an acid catalyst, e.g., 0.5-1.0 wt% sulfuric
acid, based on
the total reaction mixture, at 50-70 C at 1 bar absolute, or in a gas phase
reaction, at 130-
240 C and 20-40 bar absolute, preferably in the absence of a catalyst. If the
proportion of
water is lowered, the proportion of the ethylene glycol ether in the reaction
mixture will be
increased. Alternatively ethylene glycol ether may be prepared by converting
the ethylene
oxide with an alcohol, such as methanol or ethanol, or by replacing at least a
portion of the
water with the alcohol. The resulting ethylene glycol and ethylene glycol
ether may be
utilized in a wide variety of end-use applications in the food, beverage,
tobacco, cosmetic,
thermoplastic polymer, curable resin system, detergent, heat transfer system,
etc., industries.
[0072] The conversion of ethylene oxide produced via the method of the
present
invention into ethanolamine may comprise, for example, reacting the ethylene
oxide with
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
ammonia. Anhydrous or aqueous ammonia may be used. The resulting ethanolamine
may
be used, for example, in the treatment of natural gas. The ethylene oxide may
be converted
into the corresponding ethylene carbonate by reacting ethylene oxide with
carbon dioxide.
If desired, ethylene glycol may be prepared by subsequently reacting ethylene
carbonate
with water or an alcohol to form the ethylene glycol. For applicable methods,
reference is
made to U. S. Patent No. 6,080,897.
[0073] Ethylene glycol is used in two significant applications: as a raw
material for
poly(ethylene terephthalate) for use in polyester fiber, film, and containers,
and as an
automotive antifreeze. Di-, tri-, and tetraethylene glycols are coproducts of
ethylene glycol.
EXAMPLES
[0074] Preparation of silver-amine-oxalate solution: An amine solution is
prepared by
mixing 11.47 weight parts of ethylenediamine (high purity grade) with 20.00
weight parts of
distilled water. Then 11.60 weight parts of oxalic acid dihydrate (reagent
grade) is slowly
added to the amine solution at ambient conditions. The addition of the oxalic
acid dihydrate
is at a rate that the exotherm does not cause the temperature of the amine-
oxalate solution to
rise above 40 C. Then 19.82 weight parts of silver oxide are added followed by
4.01
weight parts of monoethanolamine (Fe and Cl free). Distilled water is then
added to adjust
the solution weight to 70.00 weight parts to form the silver-amine-oxalate
solution. The
silver-amine-oxalate solution has a pH in the range between 11 and 12.
Stability studies of manganese in solutions
COMPARATIVE EXAMPLE 1
[0075] Preparation of Solution A for stability study of manganese in
solution: About
0.293 grams of aqueous manganese(II) nitrate solution (0.157 grams Mn/gram
solution) are
added to 588.3 grams of the above silver-amine-oxalate solution. Other (non-Mn-
containing) promoter-containing compounds are then added as aqueous solutions
(about
16.14 grams in total), followed by stirring for 30 minutes at 20 C to obtain
solution A. A
sample of Solution A is withdrawn and filtered through a 0.111 filter paper
and the
concentration of manganese in the filtrate is analyzed by X-Ray Fluorescence
(XRF). The
21
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
XRF analysis error is typically about 3 ppm and the result is given in Table
1. In the
Table, the calculated Mn (manganese) refers to the stoichiometric amount of
manganese in
the solution. XRF Mn is the amount of manganese remaining in the solution
after filtration
measured by XRF. XRF Mn value is about 60.9 3 ppm indicating precipitation
of
manganese from the solution A.
Solution Calculated Mn XRF Mn
(ppm) (ppm)
A 76 60.9 3
B 76 54.6 3
C 76 80 3
Table 1: XRF analyses of Solution A, Solution B and Solution C
COMPARATIVE EXAMPLE 2
[0076] Preparation of Solution B for stability study of manganese in
solution: About
1.0724 grams of aqueous diammonium EDTA solution (46 wt% EDTA) is added to
solution
A and stirred for 30 minutes at 20 C to obtain Solution B. A sample of
Solution B is
withdrawn for XRF analysis and is analyzed according to the method described
previously.
Solution B has an XRF value of about 54.6 3 ppm, as shown in Table 1,
indicating higher
precipitation of manganese compared to Solution A.
EXAMPLE 1
[0077] Preparation of Solution C for stability study of manganese in
solution: The
experiment of Comparative Example 2 is repeated except that prior to addition
to the silver-
amine-oxalate solution, about 0.293 grams of aqueous manganese (II) nitrate
solution
(0.157 grams Mn/gram solution) is combined with about 1.072 grams of aqueous
diammonium EDTA solution (46 wt% EDTA) and mixed thoroughly to form a first
solution. The first solution thusly prepared has a pH of less than or equal to
7. The first
solution is then added to the silver-amine-oxalate solution, followed by the
addition of the
22
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
other (non-Mn-containing) promoter solutions and stiffing for 30 minutes at 20
C to obtain
Solution C. Solution C has a pH which is greater than 7. Solution C shows a
XRF Mn
value of about 80.0 3 ppm which matches the calculated Mn value indicating
minimal
precipitation of manganese from the solution.
Variability studies on the amount of manganese in impregnation solution
batches
COMPARATIVE EXAMPLE 3
Preparation of catalyst batches according to prior art method
[0078] Preparation of first impregnated support: An alpha-alumina support
is vacuum
impregnated with a first silver impregnation solution typically containing 31
weight percent
silver oxide, 18 weight percent oxalic acid, 18 weight percent
ethylenediamine, 6 weight
percent monoethanolamine and 27 weight percent distilled water. The first
silver
impregnation solution is prepared by mixing 1.14 parts of ethylenediamine
(high purity
grade) with 1.75 parts of distilled water to form aqueous ethylenediamine
solution. This is
followed by slow addition of 1.16 parts of oxalic acid dihydrate (reagent
grade) to the
ethylenediamine solution such that the temperature of the solution does not
exceed 40 C,
followed by addition of 1.98 parts of silver oxide and 0.40 parts of
monoethanolamine (Fe
and Cl free) to form the first silver impregnation solution. The first silver
impregnation
solution has a pH which is in the range between 11 and 12.
[0079] The alpha-alumina support is impregnated with the first silver
impregnation
solution. The support remains immersed in the first silver impregnation
solution at ambient
conditions for 5 to 30 minutes. The impregnated support is then taken out and
thereafter
drained of excess solution for 10 to 30 minutes.
[0080] The impregnated support is then roasted to effect reduction of
silver on the
support surface to form a first impregnated support. For roasting, the
impregnated support
is spread out in a single layer on stainless steel wire mesh trays which is
placed on a belt
and transported to a heating zone for 2.5 minutes. The heating zone is
maintained at 500 C
by passing hot air upward through the belt and the impregnated support. After
roasting in
the heating zone, the first impregnated support is kept in the open and
brought to room
23
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
temperature and weighed. Preparation of first catalyst intermediate: The first
impregnated
support is vacuum impregnated with a second silver impregnation solution to
form a first
catalyst intermediate. The second silver impregnation solution comprises
drained solutions
from previous silver impregnation solution(s) and fresh aliquots of each of
the manganese
nitrate and diammonium EDTA in separate additions directly into the silver-
amine-oxalate
solution. The second impregnation solution includes one or more promoters
selected from
cesium, lithium, sodium and any mixtures thereof. Following impregnation, the
first
catalyst intermediate is drained of excess solution and roasted as described
previously with
reference to the first impregnated support to form a first catalyst. The
weight percent of
silver is calculated based on the weight of first catalyst and the support.
The concentration
of the promoters is calculated, assuming a similar rate of deposition for the
promoters as is
for the silver. The manganese content of the first catalyst can be correlated
to the
manganese content of the impregnation solution and is determined using XRF, as
described
in previous examples on stability studies of manganese in solutions. Various
first catalyst
batches are prepared following this method having a wide range of desired
target levels and
also at various scale-up levels. The wide range of desired target levels can
be achieved by
varying the stoichiometry of the impregnating solutions. FIG. 1 is a plot of
variability in the
amount of manganese as determined by XRF analyses in the silver impregnation
solution
batches prepared for use in preparing first catalyst batches and is expressed
as the
percentage variation in manganese content from the desired target levels. This
Example
shows large variability in the amount of manganese in the impregnation
solution ranging
from about +20% to about -90% relative to the target values. The catalysts
prepared using
such impregnation solutions are expected to reflect this variability in
manganese as well.
EXAMPLE 2
Preparation of catalyst batches according to inventive method
[0081] Preparation of second catalyst intermediate according to the
inventive method:
The first impregnated support is prepared according to the method of
Comparative Example
3 and then is vacuum impregnated with a third silver impregnation solution to
form a
second catalyst intermediate. The third silver impregnation solution comprises
drained
solution from previous silver impregnation solution(s) and fresh aliquots of
each of the first
24
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
solution and the second solution. The first solution comprises manganese(II)
nitrate and
diammonium EDTA and at any time, i.e. at least one point in time during or
after the
preparation of the first solution it has a pH which is less than or equal to
7. The second
solution comprises silver-amine-oxalate solution. The third silver
impregnation solution
includes one or more promoters selected from cesium, lithium, sodium and any
mixtures
thereof. The third silver impregnation solution has a pH of greater than 7.
Following
impregnation, the second catalyst intermediate is drained of excess solution
and roasted as
described previously with reference to the first impregnated support to form a
second
catalyst. The weight percent of silver and the concentrations of the promoters
are
calculated. The manganese content in the second catalyst can be correlated to
the
manganese content of third silver impregnation solution and is measured using
XRF.
Various second catalyst batches are prepared having a wide range of desired
target levels
and also at various scale-up levels. FIG. 2 is a plot of variability in the
amount of
manganese as determined by XRF in the impregnation solution batches prepared
according
to the invention for use in preparing second catalyst batches and is expressed
as the
percentage variation in manganese content from the desired target levels. The
variability in
the manganese concentration is within 10% relative to the targets. This
Example indicates
that the inventive method of forming a first solution by combining manganese
nitrate and
diammonium EDTA, prior to addition to second solution comprising silver,
lowers the
variability in the amount of manganese in the resulting impregnation solution.
The catalysts
prepared using such impregnation solutions are expected to reflect this lower
variability as
well.
COMPARATIVE EXAMPLE 4
[0082] Manganese-containing first catalyst batches 4-1 to 4-5 are prepared
in large-scale
generally according to the procedure as described in Comparative Example 3.
The
concentrations of manganese on the first catalyst batches 4-1 to 4-5 are
determined using
XRF and are provided in Table 2.
[0083] Catalyst performance studies: Multiple 80-cc samples are withdrawn
from each
of the first catalyst batches and evaluated in backmixed (CSTR) Berty-type
autoclave
reactors under the following conditions: inlet concentrations of 8.0 vol%
oxygen, 6.5 vol%
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
carbon dioxide, 30.0 vol% ethylene, 0.50 vol% ethane, 3.5 ppmv ethyl chloride,
balance
nitrogen; with a pressure of 1900 kPa gauge; a total flow of 640 standard
liters per hour
(measured as nitrogen); and a startup temperature of 230 C. Following startup,
the
temperature is adjusted to produce an outlet concentration of 2.0 vol%
ethylene oxide. For
each batch, the average autoclave temperature required to attain an outlet
concentration of
2.0 vol% ethylene oxide after seven days of testing is shown in Table 2.
Catalyst batch Manganese in ppm T in C
4-1 91 240.2
4-2 82 238.8
4-3 84 238.3
4-4 92 236.7
4-5 87 238.7
Table 2 Manganese content and average activity of the first catalyst batches 4-
1 to 4-5
EXAMPLE 3
[0084] This Example illustrates the performance improvement in a manganese-
containing silver catalyst prepared using the inventive method.
[0085] Manganese-containing second catalyst batches 3-1 to 3-6 are prepared
in large-
scale generally according to the procedure outlined in Example 2. The
concentrations of
manganese on the second catalyst batches 3-1 to 3-6 are determined using XRF
and are
provided in Table 3. The second catalyst batches 3-1 to 3-6 are evaluated
following the
procedure for catalyst performance studies provided in Comparative Example 4.
The
manganese concentrations of the second catalyst batches and the temperature,
which is the
average autoclave temperature required to attain an outlet concentration of
2.0 vol%
ethylene oxide after seven days of testing, are shown in Table 3.
26
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
Catalyst batch Manganese in ppm T in C
3-1 91 234.9
3-2 87 235.4
3-3 83 236.7
3-4 88 236.5
3-5 95 235.6
3-6 91 233.2
Table 3 Manganese content and average activity of the second catalyst batches
3-1 to 3-6
[0086] Figure 3 is a comparison of the activity of second catalyst batches
of Example 3
and first catalyst batches of Comparative Example 4. The significantly lower
temperatures
required to produce 2.0 vol% ethylene oxide for the second catalyst batches of
Example 3
indicates an improvement in catalyst activity over the first catalyst batches
of Comparative
Example 4. Advantageously, the improvement in catalyst activity may also
benefit a
lifetime of the second catalyst batches of Example 3.
COMPARATIVE EXAMPLE 5
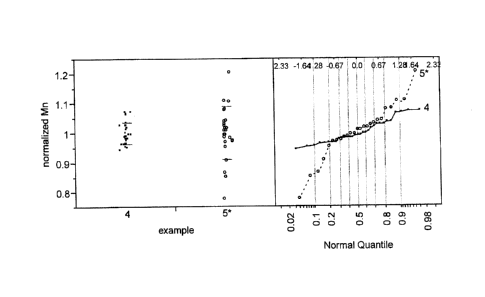
[0087] This example highlights the variability in the manganese content
during the
course of preparing the large scale first catalyst batches (4-1 to 4-5), as
described in
Comparative Example 4. Figure 4 is a plot of normalized manganese content of
manganese-containing silver-amine-oxalate solution batches prepared for use in
the
preparation of first catalyst batches 4-1 to 4-5 (marked as 5*), where
normalized manganese
content is the ratio of the measured manganese content in the solution batches
(as analyzed
by XRF) to the target manganese content, divided by the average of the ratio
across all the
solution batches. Multiple points on the plot of Figure 4 thus correspond to
each of the
27
CA 02867506 2014-09-15
WO 2013/148417
PCT/US2013/033032
large-scale first catalyst batches of Comparative Example 4 and is indicative
of the variation
in manganese content from solution-batch to solution-batch even within each
first catalyst
batch when preparing manganese-containing silver catalysts according to the
prior art
method.
EXAMPLE 4
[0088] This example illustrates the benefit of the inventive method
observed during
preparation of the large scale second catalyst batches (3-1 to 3-6) as
described in Example
3. Figure 4 is a plot of normalized manganese content of Mn-containing silver-
amine-
oxalate solution batches prepared in accordance with inventive method for use
during
preparation of second catalyst batches 3-1 to 3-6 (marked as 4), where
normalized
manganese content is the ratio of the measured manganese content in the
solution batches
(as analyzed by XRF) to the target manganese content, divided by the average
of the ratio
across all the solution batches. Multiple points on the plot of Figure 4
therefore correspond
to each of the large-scale second catalyst batches of Example 3. When compared
to Mn-
containing silver-amine-oxalate solution batches prepared using a prior art
method (marked
as 5*), the solution batches prepared using inventive method show lower
variability in the
amount of manganese from batch to batch.
28