Note: Descriptions are shown in the official language in which they were submitted.
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
COMPOSITIONS AND TREATMENTS BASED ON CADHERIN MODULATION
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to US Application Serial No.
13/844,553, filed
on March 15, 2013, which is a non-provisional of and claims the benefit of
U.S. Provisional
Application Serial No. 61/616,393, filed on March 27, 2012, the entire
contents of each of
which are hereby incorporated by reference.
TECHNICAL FIELD
[0002] The inventions relate compositions and methods that involve modulation
of
cadherin proteins. These inventions are useful in various contexts, including
to promote
wound healing and to treat wounds, in particular acute wounds and to wounds
that do not
heal at expected rates, such as delayed-healing wounds, incompletely healing
wounds,
chronic wounds, and dehiscent wounds.
BACKGROUND OF THE INVENTION
[0003] The following includes information that may be useful in understanding
the
present inventions. It is not an admission that any of the information
provided herein is prior
art, or relevant, to the presently described or claimed inventions, or that
any publication or
document that is specifically or implicitly referenced is prior art.
[0004] In humans and other mammals wound injury triggers an organized complex
cascade of cellular and biochemical events that will in most cases result in a
healed wound.
An ideally healed wound is one that restores normal anatomical structure,
function, and
appearance on cellular, tissue, organ, and organism levels. Wound healing,
whether initiated
by trauma, microbes or foreign materials, proceeds via a complex process
encompassing a
number of overlapping phases, including inflammation, epithelialization,
angiogenesis and
matrix deposition. Normally, these processes lead to a mature wound and a
certain degree of
scar formation. Although inflammation and repair mostly occur along a
prescribed course,
the sensitivity of the process is dependent on the balance of a variety of
wound healing
molecules, including for example, a network of regulatory cytokines and growth
factors.
[0005] Cell adhesion and intercellular communication also have a role in wound
healing. Cell adhesion involves a cell binding to surface, extracellular
matrix, and/or one or
more adjacent cells through a variety of cell adhesion molecules such as
cadherins, integrins,
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
and selectins. Intercellular communication includes connexin proteins, which
form gap
junctions. Appropriate cell adhesion is essential not only to maintaining
multicellular
structure and restoring such structure after injury, but also facilitating
intercellular
communication via messenger and signal transduction.
[0006] Cadherins are a class of type-1 transmembrane proteins involved in
binding
cells together to form, maintain, and restore tissues. As a family, cadherins
are adhesion
molecules that mediate Ca2+-dependent cell-cell adhesion in all solid tissues
of multicellular
animals. These proteins modulate a wide variety of processes, including cell
polarization and
migration. Cadherin-mediated cell-cell junctions form as a result of
interaction between
extracellular domains of identical cadherins, which protrude from the
membranes of
adjoining cells. The stability of these adhesive junctions is ensured by
binding of the
intracellular cadherin domain with the actin cytoskeleton.
[0007] The cadherin superfamily includes cadherins, protocadherins,
desmogleins, and
desmocollins, among others. Structurally, each superfamily member has cadherin
repeats,
which are extracellular, calcium ion-binding domains. A number of different
cadherin
isoforms or subtypes exist, and are distributed in a tissue-specific manner in
a wide variety of
organisms. The different cadherin isoforms or subtypes are designated with a
prefix denoting
the tissue with which it is normally associated. For example, N-cadherin
denotes neural
origin, E-cadherin, epithelium, and P-cadherin, placenta. It has been observed
that cells
expressing a specific cadherin subtype tend to cluster together to the
exclusion of other types,
both in cell culture and during development, meaning, for example, that cells
that express N-
cadherin on their surfaces tend to cluster with other N-cadherin-expressing
cells. It has also
been found that cadherin expression causes morphological changes involving the
positional
segregation of cells into layers, indicating that cadherins are important in
the sorting of
different cell types during morphogenesis, histogenesis, and regeneration.
They may also be
involved in the regulation of tight and gap junctions, and in the control of
intercellular
spacing.
[0008] Structurally, members of the cadherin superfamily comprise a number of
domains: a signal sequence; a propeptide of around 130 residues; a single
transmembrane
domain; at least one extracellular domain containing a cadherin repeat motif;
and an N-
terminal cytoplasmic domain. Classical cadherin proteins have an extracellular
domain that
includes five tandemly repeated extracellular cadherin domains, four of which
are cadherin
2
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
repeats, and the fifth of which contains four conserved cysteines. A "cadherin
repeat" is an
independently folding peptide of approximately 110 amino acid residues that
contains motifs
with the conserved sequences DRE, DXNDNAPXF (SEQ ID NO:1), and DXD. Studies of
crystalized extracellular cadherin domains reveal that multiple cadherin
domains form Ca2+-
dependent rod-like structures with a conserved calcium-binding pocket at the
domain-domain
interface. Cadherins depend on calcium for function, as calcium ions bind to
specific
residues in each cadherin repeat to ensure proper folding, to confer rigidity
upon the
extracellular domain, and to prevent protease digestion.
[0009] Cadherin proteins are the primary extracellular components of "adherens
junctions" (also termed "intermediate junctions" or "belt desmosomes") between
cells in
vivo. Adherens junctions are protein complexes that occur at cell¨cell
junctions in epithelial
tissues, and are defined as cell junctions that have a cytoplasmic face linked
to an actin
cytoskeleton. Adherens junctions are usually more basal than "tight
junctions". They can
appear as bands encircling a cell ("zonula adherens") or as spots of
attachment to the
extracellular matrix ("adhesion plaques"). At adherens junctions, the
intrcellular portion of a
cadherin protein interacts with a catenin or vinculin subunit, through which
an actin filament
projects. The extracellular domain of a cadherin protein forms a homodimer
with the
extracellular domain of a cadherin protein of an adjacent cell in a calcium
dependent manner.
[0010] Like adherens junctions, gap junctions are cell membrane structures;
however,
gap junctions facilitate direct cell-cell communication. A gap junction
channel is formed of
two connexons (hemichannels), each composed of six connexin subunits, which
allows direct
connection between the cytoplasm of adjoining cells when the hemichannels are
in an
"open", as opposed to a "closed" configuration. When the connexons forming a
gap junction
channel are "open", molecules (e.g., ions, signaling molecules, etc.) can move
from one cell
to another. Each hexameric connexon docks with a connexon in the opposing
membrane to
form a single gap junction. Gap junction channels are reportedly found
throughout the body.
Tissue such as the corneal epithelium, for example, has six to eight cell
layers, yet expresses
different gap junction channels in different layers with connexin 43 in the
basal layer and
connexin 26 from the basal to middle wing cell layers. In general, connexins
are a family of
proteins, commonly named according to their molecular weight or classified on
a
phylogenetic basis into alpha, beta, and gamma subclasses. Over 20 human
isoforms have
been identified. Different tissues and cell types are reported to have
characteristic patterns of
3
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
connexin protein expression and tissues have been shown to alter connexin
protein expression
pattern following injury or transplantation (Qui, C. et al., (2003) Current
Biology, 13:1967-
1703.
[0011] Polynucleotide-based therapeutic approaches, including antisense
technology
and RNA interference (RNAi), have been proposed for modulating expression of
genes
implicated in viral, fungal, and metabolic diseases. See, e.g., U.S. pat. no.
5,166,195
(oligonucleotide inhibitors of HIV) and U.S. pat. no. 5,004,810 (oligomers for
hybridizing to
herpes simplex virus Vmw65 mRNA and inhibiting replication). See also U.S.
pat. nos.
7,098,190, 7,879,811, 7,902,164, and 7,919,474 (each entitled, "Formulations
comprising
antisense nucleotides to connexins"). Peptide inhibitors of gap junctions and
hemichannels
have also been reported. See, e.g., Berthoud, et al., Am J. Physiol. Lung Cell
Mol. Physiol.
279:L619-L622 (2000); Evans and Boitano, Biochem. Soc. Trans. 29:606-612, and
De
Vriese, et al., Kidney Int. 61:177-185 (2001). See also PCT/U506/04131 ("Anti-
connexin
compounds and uses thereof'), US PG Pub. Nos. 20110217313 ("Treatment of
Orthopedic
Conditions"), 20110144182 ("Treatment of Surgical Adhesions"), 20110136890
("Treatment
of Fibrotic Conditions"), 20110130710 ("Treatment of Abnormal or Excessive
Scars"),
20110065770 ("Formulations comprising antisense nucleotides to connexins"),
20090220450
("Methods and compositions for wound healing"), and 20080249041, 20080221051,
20070078103, 20070072820, 20070072819, 20070066555, 20070060538, and
20070037765
(each entitled, "Formulations comprising antisense nucleotides to connexins").
[0012] Despite advances in the understanding of the principles underlying the
wound
healing process, there remains a significant unmet need in suitable
therapeutic options for
wound care, including wounds that do not heal at expected rates, such as
delayed-healing
wounds, incompletely healing wounds, and chronic wounds. Such therapeutics
compositions
and treatments are described and claimed herein.
BRIEF SUMMARY OF THE INVENTION
[0013] The inventions described and claimed herein have many attributes and
embodiments including, but not limited to, those set forth or described or
referenced in this
Brief Summary. It is not intended to be all-inclusive and the inventions
described and
claimed herein are not limited to or by the features or embodiments identified
in this Brief
Summary, which is included for purposes of illustration only and not
restriction.
4
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[0014] The invention generally relates to the use of an anti-cadherin agent,
preferably
an anti-cadherin polynucleotide species, alone or in combination with one or
more other
agents useful in the treatment of acute, delayed healing and chronic wounds.
[0015] Examples of such other agents include anti-connexin agents, for example
anti-
connexin polynucleotides (for example, connexin inhibitors such as alpha-1
connexin
oligodeoxynucleotides), anti-connexin peptides (for example, antibodies and
antibody
binding fragments) and peptidomimetics (for example, alpha-1 anti-connexin
peptides or
peptidomimetics), gap junction closing or blocking compounds, hemichannel
closing or
blocking compounds, and connexin carboxy-terminal polypeptides, e.g.,
polypeptides that
bind to ZO-1 or a ZO-1 binding site, anti-ZO-1 polynucleotides, as well as
anti-ostepontin
agents, particularly anti-osteopontin polynucleotides. Preferred combinations
include an anti-
N-cadherin polynucleotide species and an anti-connexin polynucleotide species
(particularly
an anti-connexin 43 polynucleotide species) and/or a polynucleotide species
that targets ZO-1
expression.
[0016] Compositions and methods of the invention that employ one or more anti-
cadherin agent species for the treatment of, for example, acute, delayed
healing, and chronic
wounds are described and claimed. Preferred compositions include
therapeutically useful
compositions, particularly pharmaceutical or veterinary compositions that
comprise a
therapeutically acceptable amount of one or more anti-cadherin agent species
in amounts
effective to promote healing or tissue repair in a subject. In preferred
embodiments, such
compositions comprise a therapeutically acceptable amount of one or more anti-
cadherin
agent species in amounts effective to downregulate or otherwise lessen the
expression or
presence of one or more cadherin species; for example, one or more cadherin
species at
and/or around an injury or wound site. As a result, healing of the injury or
wound can be
initiated and/or enhanced, and inflammation and/or scarring reduced.
[0017] Preferred anti-cadherin agents are anti-cadherin polynucleotides. In
one
embodiment the anti-cadherin polynucleotides are anti-N-cadherin
oligodeoxynucleotides
(ODN). Preferred peptides or peptidomimetics, are anti-cadherin peptides or
peptidomimetics, e.g., cadherin complex blocking peptides (for example, anti-
cadherin
antibodies and antibody binding fragments) or peptidomimetics (for example,
peptidometics
directed against one or more regions of cadherin,. Preferred cadherin complex
blocking
compounds are cadherin extracellular polypeptides. Peptidomimetics may be
administered
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
per se, or complexed to one or more other agents, for example, antennapedia in
order to
facilitate membrane transport.
[0018] The compositions of the invention include, for example, topical and
inhaled
delivery forms and formulations. Such delivery forms and formulations include
those for the
treatment of a subject, as described herein.
[0019] Pharmaceutical compositions are also provided in the form of a combined
preparation, for example, as an admixture of one or more distinct anti-
cadherin agent species,
alone in conjunction with and one or more therapeutic agent species that are
not anti-cadherin
agents, for example, one or more anti-connexin or anti-osteopontin agents,
including anti-
connexin and anti-osteopontin polynucleotides, peptide, and or peptidomimetic
species.
[0020] The term "a combined preparation" includes a "kit of parts" in the
sense that
the combination partners as defined herein can be dosed independently or by
use of different
fixed combinations with distinguished amounts of the two or more agent
species, i.e.
simultaneously, separately or sequentially. The parts of the kit can then, for
example, be
administered simultaneously or chronologically staggered, that is, at
different time points,
with equal or different time intervals, and/or in the same or different
numbers of dosings for
any part of the kit of parts.
[0021] In some embodiments, a combined preparation is administered, wherein
two or
more separate compositions are administered to a subject, wherein the first
composition
comprises a therapeutically effective amount of an anti-cadherin agent and the
second
composition comprises a therapeutically effective amount of an anti-connexin
polynucleotide,
peptide, or peptidomimetic. In other embodiments, a third composition is
administered
comprising one or more anti-osteopontin polynucleotides, peptides, or
peptidomimetics.
[0022] Pharmaceutical compositions are provided for combined, simultaneous,
separate sequential, or sustained administration. In some embodiments, a
composition
comprising one or more anti-cadherin agents is administered at or about the
same time as one
or more anti-connexin agents and/or anti-osteopontin agents. In one
embodiment, a
composition comprising one or more anti-cadherin agents is administered within
at least
about thirty, sixty, ninety, or one hundred twenty minutes, or about 3, 4, 5,
6, 8, 12, 24, 48, or
168 hours of one or more anti-connexin agents and/or anti-osteopontin agents.
6
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[0023] In one aspect, the invention includes pharmaceutical compositions,
including
topical, systemic, and inhaled delivery forms and formulations, comprising a
pharmaceutically acceptable carrier and therapeutically effective amounts of
an anti-cadherin
agent species, alone or in combination with a different anti-cadherin agent
species and/or one
or more other therapeutic agent species, e.g., a first anti-connexin agent
species, a second
anti-connexin agent species, a first anti-osteopontin agent species, and/or
and a second anti-
osteopontin agent species. Such compositions are useful, for example, for
wound healing.
[0024] Examples of anti-cadherin agents are anti-cadherin polynucleotides,
including
the anti-cadherin antisense oligodeoxynucleotides ("ODN") described below.
Examples of
anti-cadherin polynucleotides include anti-cadherin oligodeoxynucleotides,
including
antisense (including modified and unmodified backbone antisense), RNAi, and
miRNA and
siRNA. Suitable anti-cadherin peptides include peptides that bind cadherin
extracellular
domains, for example, or cadherin intracellular domains. Suitable anti-
cadherin agents
include, for example, antisense ODNs, peptides, and peptidomimetics against N-
cadherin, E-
cadherin, P-cadherin, cadherin 11, cadherin 12, a protocadherin protein, a
desmoglein
protein, and a desmocollin protein. Included peptides or peptidomimetics are
anti-cadherin
peptides or peptidomimetics, e.g., cadherin complex blocking peptides (for
example, anti-
cadherin antibodies and antibody binding fragments) or peptidomimetics (for
example,
peptidometics directed against one or more extracellular or intracellular
regions of cadherin).
Peptidomimetics may be complexed to one or more other agents, for example,
antennapedia
in order to facilitate membrane transport for binding to intracellular
cadherin regions and
domains.
[0025] The present invention provides for an increase in the rate, extent,
and/or quality
of wound healing through the use of at least one anti-cadherin agent species,
alone or in
combination with one or more therapeutic agent species administered
simultaneously,
separate, or sequentially.
[0026] The present invention provides for a decrease in inflammation through
the use
of at least one anti-cadherin agent species, alone or in combination with one
or more
therapeutic agent species administered simultaneously, separate, or
sequentially.
[0027] The present invention provides for a decrease in scarring and/or an
increased
quality of scar through the use of at least one anti-cadherin agent species,
alone or in
7
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
combination with one or more therapeutic agent species administered
simultaneously,
separate, or sequentially.
[0028] In certain embodiments, the combined use of an anti-cadherin agent in
combination with one or more other therapeutic agents, for example, one or
more anti-
connexin polynucleotides, peptides, or peptidomimetics and/or one or more anti-
osteopontin
polynucleotides, peptides, or peptidomimetics has an additive, synergistic, or
super-additive
effect in the promotion of the desired therapeutic outcome, for example, wound
healing and
for reduced inflammation and scarring. In some of these preferred embodiments,
the
administration of a combined preparation will have fewer administration time
points and/or
increased time intervals between administrations as a result of such combined
use. In other
such preferred embodiments, the combined use allows a reduced frequency of
administration.
In other preferred embodiments, combined use allows the use of reduced doses
of such agents
compared to the dose or doses that may be effective when the agent is
administered alone.
[0029] In another aspect, the invention includes methods for administering a
therapeutically effective amount of an anti-cadherin agent, alone or in
combination with one
or more therapeutic anti-connexin agents. In some embodiments, the
compositions are
formulated, for example, in a delayed release preparation, a slow release
preparation, an
extended release preparation, a controlled release preparation, and/or in a
repeat action
preparation suitable for administration to a subject having a wound, including
chronic
wounds and wounds characterized in whole or in part by slow, delayed, or
incomplete wound
healing. Chronic wounds include diabetic ulcers (e.g., diabetic foot ulcers),
venous ulcers,
venous stasis ulcers, pressure ulcers, decubitus ulcers, vasculitic ulcers,
arterial ulcers,
infectious ulcers, burn ulcers, trauma-induced ulcers, inflammatory ulcers,
and ulcerations
associated with pyoderma gangrenosum. Chronic wounds also include ocular
ulcers,
including persistent epithelial defects. In some embodiments, the subject is
diabetic; in
others, the subject has a cardiovascular disease or condition, for example,
venous
hypertension, venous insufficiency and/or arterial insufficiency.
[0030] In certain other aspects, the invention relates to methods of using the
compounds and compositions of the invention to treat subjects suffering from
or at risk for
various diseases, disorders, and conditions associated with a wound, including
acute wounds
and wounds that do not heal at expected rates, including delayed healing and
chronic wounds.
Treatment of a subject, e.g., for a wound, with one or more pharmaceutical
compositions of
8
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
the invention, e.g. one or more anti-cadherin agents, may comprise their
simultaneous,
separate, sequential or sustained administration.
[0031] In yet another aspect, the invention includes methods for treating a
subject
having or suspected of having or predisposed to, or at risk for, any diseases,
disorders and/or
conditions characterized in whole or in part by a wound or a tissue in need of
repair. Such
compositions include, for example, topical and inhaled delivery forms and
formulations.
[0032] In another aspect, the invention provides methods of treatment
comprising
administering to a subject a pharmaceutical composition of the invention for
use in the
treatment of a wound, including for example, acute, as well as wounds that do
not heal at
expected rates, including delayed healing and chronic wounds.
[0033] In another aspect, the invention provides methods of treatment
comprising
administering to a subject in need thereof a composition comprising
therapeutically effective
amounts of an anti-cadherin agent, alone or in combination with one or more
anti-connexin
and/or anti-osteopontin agents. Also within the scope of the invention is
pretreatment prior to
surgery. This will reduce local damage at points of incision, excision or
revision, for
example, and prime cells for healing.
[0034] In yet another aspect, the invention provides methods of treatment
comprising
administering to a subject in need thereof a first composition and at least
one other
therapeutic composition (e.g., a second composition, second and third
compositions, etc.). In
embodiments of this aspect, the "first" composition comprises a
therapeutically effective
amount of an anti-cadherin agent, although this is not meant to imply that
such composition is
administered before, more frequently, or via a different route than the other
therapeutic
composition(s). In other words, in some of these embodiments, the first
composition is
administered first, while in others, the second composition is administered
first. In
embodiments involving the administration of three different therapeutic
compositions, such
methods, for example, can comprise simultaneous administration of each of the
compositions
according to the same or different dosing or administration regimen.
[0035] In a further aspect, the invention provides methods for improving or
reducing
scar formation in a subject in need thereof, for improving or reducing
fibrosis in a subject,
and for improving or reducing adhesion formation in a subject, comprising
administering to
9
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
said subject a therapeutically effective amount of a pharmaceutical
composition comprising
an anti-cadherin agent, alone or in combination with one or more other
therapeutic agents.
[0036] Preferred methods of combination therapy include the sequential or
simultaneous administration one or more anti-cadherin agents alone or in
combination with
one or more other therapeutic agent species, either, some, or all of which are
provided in
amounts or doses that are less that those used when the agent or agents is/are
administered
alone, i.e., when they are not administered in combination, either physically
or in the course
of treatment of a wound or other condition to be improved. Such lesser amounts
of agents
administered are typically from about one-half, one-third, one-fourth, one-
fifth, one-sixth,
one-eighth, one-tenth, or about one-twentieth the amount when administered
alone.
[0037] In a further aspect, the invention includes transdermal patches,
dressings, pads,
wraps, matrices, and bandages capable of being adhered or otherwise associated
with the skin
of a subject, said articles being capable of delivering a therapeutically
effective amount of an
anti-cadherin agent, alone or in combination with one or more therapeutic
agents, to a
subject.
[0038] In another aspect, the invention includes an article of manufacture
comprising a
vessel containing a therapeutically effective amount of one or more anti-
cadherin agents,
alone or in combination with one or more other therapeutic agents, and
instructions for use,
including use for the treatment of a subject.
[0039] The invention includes an article of manufacture comprising packaging
material containing one or more dosage forms containing one or more anti-
cadherin agents,
alone or together with dosage forms containing one or more other therapeutic
agents, wherein
the packaging material has a label that indicates that the dosage form can be
used for a
subject having or suspected of having or predisposed to any of the diseases,
disorders and/or
conditions described or referenced herein, including diseases, disorders
and/or conditions
characterized in whole or in part by acute, impaired, delayed or chronic wound
healing, by
scarring, by fibrosis, or by adhesions. Such dosage forms include, for
example, topical
delivery forms and formulations, powdered delivery forms and formulations,
delivery forms
and formulations suitable for injection or infusion (including dry or powdered
compositions
that must be reconstituted with a suitable diluent prior to administration),
and delivery forms
and formulations suitable for instillation. Suitable formulations deliver an
amount of a
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
therapeutic agent suitable to achieve a desired therapeutic effect. Preferred
topical
formulations include foams, sprays, and gels. Preferred gels are
polyoxyethylene-
polyoxypropylene copolymer-based gels and carboxymethylcellulose-based and
related
cellulose gels, with pluronic gels being particularly preferred.
[0040] The invention also includes methods for the use of therapeutically
effective
amounts of compositions of the invention in the manufacture of medicaments,
including, for
example, topical delivery forms and formulations. Such medicaments include
those for the
treatment of a subject as described herein.
[0041] In another aspect, the invention provides for the use of one or more
anti-
cadherin agents in the manufacture of pharmaceutical products for the
promotion of wound
healing, improved and/or reduced scarring, improved and/or reduced
inflammation, reduced
fibrosis, or reduced adhesion formation in a patient in need thereof In some
of these
embodiments, the product includes a wound dressing or wound healing promoting
matrix.
Preferably, the wound dressing or matrix is provided in the form of a solid
substrate with a
composition comprising an anti-cadherin agent dispersed on or in the solid
substrate.
[0042] In yet another embodiment, the invention provides for the use of
compounds
and compositions of the invention in conjunction with connective tissue growth
factor
(CTGF) inhibitors, e.g., CTGF antisense compounds. In another embodiment, the
invention
provides for the use of compounds and compositions of the invention in
conjunction with
PDGF receptor inhibitors to, for example, treat wounds and/or to reduce
adhesions and scar
formation. PDGF receptor inhibitors include, for example, receptor blockers,
receptor
antagonists. CTGG and PDGF receptor inhibitors also include monoclonal
antibodies,
polyclonal antibodies, antibody fragments (including, for example, Fab,
F(ab')2 and Fy
fragments; single chain antibodies; single chain Fvs; and single chain binding
molecules such
as those comprising, for example, a binding domain, hinge, CH2 and CH3
domains,
recombinant antibodies and antibody fragments which are capable of binding an
antigenic
determinant (e.g., an epitope) that makes contact with a particular antibody
or other binding
molecule, including antibodies and antibody binding fragments directed against
CTGF or
PDGF receptors.
[0043] In yet another embodiment, the invention provides for the use of
compounds
and compositions of the invention in conjunction with the application of
artificial skin
11
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
products, including, for example Dermagraft (a single-layered cryopreserved
dermal
substitute composed of human fibroblasts, extracellular surrounding substance
and a
bioabsorbable framework), Ap1igraf (living, bilayered skin construct with an
epidermal
layer formed by human keratinocytes and a dermal layer composed of human
fibroblasts in a
bovine Type 1 collagen web), Integra (two-layer membrane system for skin
replacement
comprising a dermal replacement layer made of a porous template of fibers of
bovine tendon
collagen and glycosaminoglycan (chondroitin-6-sulfate) and an epidermal
substitute layer
made of thin silicone to control moisture loss), AlloDerm (acellular dermal
matrix),
CyzactTM (human dermal fibroblasts delivered via a fibrin), ICX-SKN (a
combination of
fibroblasts and fibrin matrix that are remodeled to produce a collagen
matrix), KeragraftTM (a
human stem cell-derived product being developed for wound care as an
autologous epidermal
equivalent), OASIS Wound Matrix (biologically derived extracellular matrix-
based wound
product created from porcine-derived acellular small intestine submucosal),
OrCe1TM (two-
layer cellular template in which human epidermal keratinocytes and dermal
fibroblasts are
cultured in two separate layers onto a bovine collagen sponge), TransCyte
(human
fibroblast-derived temporary skin substitute consisting of a polymer membrane
and neonatal
human fibroblast cells), and so on. The compounds and compositions of the
invention are
also useful in conjunction with the application of other dressings to promote
wound healing,
including, for example, BioBrane. The compounds and compositions of the
invention may
also be used in conjunction with the application of other types of scaffolds
or dressings to
promote wound healing, including, for example, spray on cells being developed
by
HealthPoint (a cell therapy spray suspension known as HP802-247, which
consists of two
components that are sprayed sequentially on the wound bed at the time of
treatment: a
fibrinogen solution and a cell preparation containing a mixture of growth
arrested, living,
allogeneic epidermal keratinocytes and dermal fibroblasts) and cultured
allogenic
keratinocytes.
[0044] The inventions also relate to the use of an anti-ZO-1 agent, including
peptides
and peptido preferably an anti-ZO-1 polynucleotide species, alone or in
combination with one
or more other agents useful in the treatment of acute, delayed healing and
chronic wounds.
[0045] In another aspect, the inventions relate to the use of (a) anti-
connexin agents,
preferably anti-connexin43 agents, and/or (b) anti-cadherin agents, preferably
anti-N-
cadherin agents, most preferably anti-connexin43 and/or anti-N-cadherin
polynucleotides
12
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
(including, for example, antisense polynucleotides) to increase Racl and RhoA
GTPase
activity in vivo and in vitro.
[0046] In another aspect, the inventions relate to the use of (a) anti-
connexin agents,
preferably anti-connexin43 agents, and/or (b) anti-cadherin agents, preferably
anti-N-
cadherin agents, most preferably anti-connexin43 and/or anti-N-cadherin
polynucleotides
(including, for example, antisense polynucleotides) to induce cytoskeletal
changes and
increase lamellipodial protrusions in cells in vitro and in vivo.
[0047] In another aspect, the inventions relate to the use of (a) anti-
connexin agents,
preferably anti-connexin43 agents, and/or (b) anti-cadherin agents, preferably
anti-N-
cadherin agents, most preferably anti-connexin43 and/or anti-N-cadherin
polynucleotides
(including, for example, antisense polynucleotides) to reduce cellular
adhesion.
[0048] In another aspect, the inventions relate to the use of an agent to
reduce calcium,
for example, agents to reduce extracellular calcium for uses in reduction of
cadherin activity,
for example, N-cadherin activity, alone or in conjunction with other anti-
cadherin agents.
[0049] These and other aspects of the present inventions, which are not
limited to or
by the information in this Brief Summary, are provided below.
BRIEF DESCRIPTION OF THE FIGURES
[0050] The patent or application file contains at least one drawing executed
in color.
Copies of this patent or patent application publication with color drawings
will be provided
by the Office upon request and payment of the necessary fee.
[0051] This application contains at least one figure executed in color. Copies
of this
application with color figures will be provided upon request and payment of
the necessary
fee. A brief summary of each of the figures is provided below.
[0052] Figure 1. Dermal Cx43 is greatly upregulated in human chronic VLU. (A)
Cx43 expression levels are reduced at the dermal wound margins 4 hours after
leg punch
biopsy of the skin of healthy volunteers. Scale bar = 25 lam. Blue signal is
Hoechst staining
of nuclei and collagen bundle autofluorescence. The dotted white line shows
the border
between the epidermis and the dermis. (B) Cx43 levels increased with
progressive distance
from the injury site (C). Values represent mean SD (n=3; **p<0.01). (D) A
representative
13
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
picture of a chronic VLU in the lower leg of a patient, from which a wound
edge punch
biopsy has been taken ¨ white dotted circle. (E) Dermal Cx43 expression levels
were
significantly upregulated in chronic VLU in comparison to matched non-wounded
samples
(n=6; *** p<0.005). (F) Graph depicting Cx43 levels in VLU vs. non-wounded
skin. Values
were expressed as mean SEM. Scale bar = 25 lam.
[0053] Figure 2. Increased expression of N-cadherin and ZO-1 in the dermis of
human chronic VLU (A) ZO-1 expression levels are elevated in the dermis of
chronic VLU
compared to matched non-wounded controls (n=6). Scale bar = 25 lam. Higher
magnifications of VLU and intact skin (boxed regions 1 and 2) stained for ZO-1
(green) and
Hoechst (blue) are shown. Scale bar = 10 lam. (B) N-cadherin is significantly
upregulated in
chronic VLU compared to matched non-wounded samples (n=6). Scale bar = 25 lam.
The
boxed regions 1 and 2 show high magnifications of VLU and non-wounded skin
samples
stained for N-cadherin (green) and Hoechst (blue). Scale bar = 10 lam. Values
represent mean
SD. (C and D) Graphs show ZO-1 and N-cadherin expression levels in VLU vs. non-
wounded skin. Values for ZO-1 and N-cadherin were expressed as mean SD;
**p<0.01 and
p<0.005, respectively.
[0054] Figure 3. Targeting Cx43 reduces N-cadherin and ZO-1 expression in
fibroblasts. (A) ZO-1 or (B) N-cadherin expression levels were examined in
mouse skin
wounds treated in vivo with Cx43sODN or Cx43asODN (n=6). Downregulation of ZO-
1 and
N-cadherin was found in the dermis of mice after Cx43 knockdown. Graphs
represent mean
SD; *p<0.05 and **p<0.01. (C-D) ZO-1 and Cx43 levels were analyzed by Western
blot in
Cx43shRNA and p.Sup-transduced fibroblasts. Tubulin was included as loading
control.
Quantification shows that knockdown of Cx43 reduced the expression of ZO-1
(***p<0.005;
n=3). (E) ZO-1 (red) and Cx43 (green) expression and distribution in leading
edge (LE) and
internal areas (IA) were analyzed by confocal microscopy in wounded Cx43shRNA
or p.Sup-
transduced cells (n=4). Cells were also counterstained with Hoechst (blue).
Scale bar = 20
(F) N-cadherin expression levels were significantly reduced after targeting
Cx43
(**p<0.01; n=3). (G) N-cadherin (green) redistribution from membrane
(arrowheads) to
cytosolic compartments (arrows) was analyzed along with F-Actin (red) in p.Sup
and
Cx43shRNA fibroblasts 3 h after scratch wounding of confluent 3T3 monolayers
(n=3). Scale
bar = 20 lam.
14
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[0055] Figure 4. Cx43 and N-cadherin knockdown accelerates the rate of
fibroblast
migration. (A and B) N-cadherin and ZO-1 expression was evaluated by Western
blot in
Mock or N-cadshRNA-transduced cells, and in ZO- 1 asODN or sense-treated (Z0-
1sODN)
cells, respectively. Values represent mean SD (n=3, ***p<0.005; n=3,
*p<0.05). (C)
Cx43shRNA and p.Sup cells, Mock and N-cadshRNA cells, or fibroblasts treated
with ZO-
lsODN and ZO- 1 asODN were allowed to migrate into a wound for 3 h. Pictures
of cells at
the beginning of the migration recording (0 h) and at the end (3 h) are shown.
(D) The graph
shows the velocity of migration for all the aforementioned conditions. Values
represent mean
SEM (***p<0.005).
[0056] Figure 5. Cx43 and N-cadherin contribute to cell polarization,
adhesion, and
proliferation in fibroblasts. (A-B) Cell¨cell adhesion upon suspension in
hanging drops was
assessed for Cx43shRNA and p.Sup, and for Mock and N-cadshRNA cells. The areas
of cell
clusters in six random fields taken from six different hanging drops were
determined for each
condition. Graphs represent the average number of clusters falling into each
of three size
ranges for each condition. Scale bar = 300 p.m. (C-D) Cx43shRNA and p.Sup, and
Mock and
N-cadshRNA cells were wounded, allowed to migrate for 3 h, and then fixed and
immunostained with anti-GM130 (red) and Hoechst (blue). Scale bar = 25 pm. The
percentage of cells with Golgi located in the 120 arc facing the wound were
scored as
positive. Between 66-106 cells were evaluated in n=3 independent experiments.
Data
represent the mean SEM (*P < 0.05). Growth curves showing cell proliferation
of
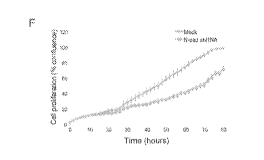
fibroblasts transduced with (E) p.Sup and Cx43shRNA, and (F) Mock and N-
cadshRNA
constructs are shown. Data represent mean SD of three independent
experiments,
performed in duplicate.
[0057] Figure 6. Targeting Cx43 induces cytoskeletal changes in leading-edge
fibroblasts. (A) Representative images showing the distribution of F-actin
(red), and
tyrosinated (green) and acetylated (blue) tubulin (TyrTub and AcetTub,
respectively) in
leading edge Cx43sODN and C43asODN fibroblasts, 3 h after wounding. Cells were
also
counterstained with the nuclear marker Hoechst. Scale bar = 20 pm. (B) The
graph shows
the length of the protrusions of wound edge cells. Data represent the distance
(mean SEM)
from the nucleus to the leading edge in n=3 experiments (***P < 0.005).
[0058] Figure 7. Modulation of Cx43 levels influence wound-edge cytoskeletal
architecture in fibroblasts. Representative images of leading-edge cells
transfected
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
(arrowhead) with (A) a control pGFP construct (B) Cx43-DN, or (C) Cx43-WT,
stained for
TyrTub, F-actin and Hoechst. Scale bar = 20 lam. (D) Graph depicting the
length of
lamelipodial protrusions of fibroblasts transfected with the different
constructs is shown
("p<0.01; ***P < 0.005; n=3).
[0059] Figure 8. Cx43 and N-cadherin knockdown increases Racl and RhoA GTPase
activity in fibroblasts. (A) Rho GTPase (GTP) activities in wounded
fibroblasts were
measured by pull-down assays using the PBD domain of PAK (Racl and Cdc42), or
the RBD
domain of Rhotekin (RhoA), followed by immunoblotting with the respective
antibodies.
Additionally, Racl, Cdc42 and RhoA from total lysates were used as loading
controls. (B)
Graphs show active Racl and RhoA GTPase activity (GTP levels/total levels;
mean SEM,
*P < 0.05); n=3 independent experiments. Representative images of leading edge
Cx43shRNA and p.Sup transduced cells showing (C) Racl, and (D) RhoA GTPase
activities,
3 h after wounding of confluent monolayers. Scale bar = 10 lam. Arrows
indicate the
direction of migration. (E) FRET efficiency analysis show a two-fold increase
in Racl and
RhoA activities in Cx43shRNA vs. p.Sup-transduced cells, while no differences
were
observed for Cdc42. Data is representative of n=3 experiments per condition;
*p < 0.05.
[0060] Figure 9. Cx43 is downregulated in fibroblasts after wounding. (A) The
expression and distribution of Cx43 in mouse's skin dermis was examined by
immunohistochemistry 2 d after excisional wounding. Wound-edge Cx43 was
reduced in
dermal fibroblasts after such wounds. Arrowheads show how Cx43 becomes more
prevalent
with increasing distance from the wound edge. Scale bar = 25 tm. Cx43 levels
were
quantified along the wound site and were significantly lower at the wound
edge; p<0.005.
Values are expressed as mean SD. (B) ZO-1 (green) and Cx43 (red) were
examined in
mouse skin wounds treated in vivo with Cx43sODN or Cx43asODN (n=6). Cells were
also
counterstained with Hoechst (blue). A clear downregulation of ZO-1 was found
in the dermis
of mice treated with the Cx43asODN. Scale bar = 100 lam. (C) Excisional wounds
treated
with Cx43asODN or control Cx43sODN were used to evaluate the distribution of N-
cadherin
(green) by immunohistochemistry. Scale bar = 100 lam.
[0061] Figure 10. Knockdown of Cx43 induces cytoskeletal changes. (A) Cx43
expression levels and (B) cell-cell communication were evaluated in Cx43asODN
or LiC1-
treated 3T3 fibroblasts, and in untreated or sense-treated (Cx43-sODN)
controls. Values are
16
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
expressed as mean SD (*p<0.05; **p<0.01 and ***p<0.005; n=4). (C) Confluent
monolayers treated with Cx43sODN, Cx43asODN or LiC1 were wounded and allowed
to
migrate for 3 hours. Pictures of cells at the beginning of the migration
recording (0 hours)
and at the end (3 hours) are shown. Scale bar = 25 p.m. (D) The graph shows
the velocity of
migration, which was inversely correlated with both dye coupling and Cx43
expression.
Values represent mean SEM (*p<0.01; ***p<0.05; n=6). (E) Cells transfected
with Cx43-
DN, Cx43-WT or pGFP constructs (n=3), as well as Cx43shRNA or p.Sup-infected
cells
(n=6), were allowed to migrate into a wound for 3 hours. Images were taken at
the beginning
of the migration recording (0 hours; arrows pointing to Cx43-DN, Cx43-WT, or
pGFP-
transfected cells) and 3 hours later (arrowheads pointing to Cx43-DN, Cx43-WT
or pGFP).
Cx43shRNA and Cx43-DN cells accelerated migration, while Cx43-WT slowed it.
Scale bar
= 251.im. (F) The graph shows the velocity of migration for each treatment and
confirms that
either silencing of Cx43 or transfection with Cx43-DN induce a significant
increase in the
rate of migration relative to the other conditions analyzed. Data are
expressed as mean SD
(*p<0.05; **p<0.01; n=4). (G) Cx43 levels and dye coupling after LY
microinjection were
assessed in Cx43shRNA or p.Sup-infected 3T3 fibroblasts. Cx43shRNA was
effective in
downregulating Cx43 and eliminating communication with neighbouring cells.
Values
represent mean SEM for Cx43 levels, and mean SD for cell coupling
(***p<0.005; n=4).
(J) Cx43shRNA and p.Sup cells were grown in the presence (FBS) or absence (SS)
of serum
and allowed to migrate into a wound for 4 h. The graph shows the velocity of
migration;
values represent mean SEM of n=3 independent experiments (***p<0.005).
[0062] Figure 11. Analysis of a- and 13-catenin expression and distribution
after
targeting Cx43. (A and C) Protein distribution of a- and 13-catenin was
analyzed in p.Sup and
Cx43shRNA-infected fibroblasts 3 h after wound scratch of confluent 3T3
monolayers.
Arrows in (A) indicate cytoplasmic relocation of 13-catenin from the plasma
membrane to the
cytosol in Cx43shRNA-infected cells. The single arrowhead indicates a site of
putative
nuclear localization of13-catenin in Cx43shRNA-infected fibroblasts. Scale bar
= 25 p.m. (B
and D). The expression levels of these proteins were also evaluated by Western
blot and
values were normalized with respect to actin or tubulin (Tub).
[0063] Figure 12. Knockdown of Cx43 induces cytoskeletal changes. The
distribution of TyrTub, AcetTub, and F-actin was studied 3 h after wounding
confluent
monolayers of Cx43shRNA and p.Sup transduced fibroblasts. Scale bar = 25 p.m.
The graph
17
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
shows the length of the protrusions of wound edge cells and data represent the
distance from
the nucleus to the leading edge (mean SEM; n=3 experiments; ***P < 0.005).
[0064] Figure 13. Knockdown of N-cadherin increases lamellipodial protrusions.
(A)
The distribution of F-actin was studied 3 h after wounding confluent
monolayers of
Cx43shRNA and p. Sup transduced fibroblasts. Targeting N-cadherin induces
cytoskeletal
changes in wound edge cells. Scale bar = 25 um. (B) The graph shows the length
of the
protrusions of control (Mock) and NcadshRNA wound edge cells. Data represent
the
distance from the nucleus to the leading edge (mean SEM; n=3 experiments;
***P < 0.005).
DETAILED DESCRIPTION OF THE INVENTION
Definitions
[0065] As used herein, a "disorder" is any disorder, disease, or condition
that would
benefit from an agent that initiates, accelerates, promotes or enhances wound
healing
(including acute wounds, dehiscent wounds, and slow-healing delayed-healing
and chronic
wounds), reduces inflammation, reduces or lessens scarring, improves scar
quality), reduces
fibrosis, and/or reduces adhesions. For example, diseases, disorders, and
conditions include
acute wounds. Diseases, disorders, and conditions also include dehiscent
wounds, and slow-
healing delayed-healing and chronic wounds. Also included are diseases,
disorders, and
conditions characterized by excess production of fibrous material, including
excess
production of fibrous material within the extracellular matrix. Also included
are diseases,
disorders and conditions characterized by replacement of normal tissue
elements by
abnormal, non-functional, and/or excessive accumulation of matrix-associated
components.
Also included are diseases, disorders and conditions characterized by adhesion
formation.
Also included is any disorder, disease, or condition that would benefit from
an agent that
promotes wound healing and/or reduces swelling, inflammation, and/or scar
formation
(including abnormal and excessive scarring, including keloid scars,
hypertrophic scars,
widespread (stretched) scars, and atrophic (depressed) scars). For example,
included are
wounds resulting from surgery or trauma, wounds that do not heal at expected
rates (such as
delayed-healing wounds, incompletely healing wounds, chronic wounds, and
dehiscent
wounds), and wound associated abnormalities in connection with neuropathic,
ischemic,
microvascular pathology, pressure over bony area (tailbone (sacral), hip
(trochanteric),
buttocks (ischial), or heel of the foot), reperfusion injury, and valve reflux
etiology and
18
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
conditions. Also included are diseases, disorders and conditions characterized
by unwanted
ZO-1 protein or ZO-1 protein activity or that would benefit from reduced ZO-1
protein or
ZO-1 protein activity. Also included are diseases, disorders and conditions
characterized by
unwanted reduced Racl or Racl activity or that would benefit from increased
Racl or Racl
activity. Also included are diseases, disorders and conditions characterized
by unwanted
reduced RhoA GTPase or RhoA GTPase activity or that would benefit from
increased RhoA
GTPase or RhoA GTPase activity. Also included are diseases, disorders and
conditions
characterized that would benefit from enhanced cellular migration, lessened
cellular
adhesion, cellular cytoskeletal changes as described herein, and/or increased
cellular
lamellipodial protrusions as described herein.
[0066] As used herein, "subject" refers to any mammal, including humans,
domestic
and farm animals, and zoo, sports, and pet animals, such as dogs, horses,
cats, sheep, pigs,
cows, etc. The preferred mammal herein is a human, including adults, children,
and the
elderly. A subject may also be a bird, including zoo, sports, and pet birds.
Preferred sports
animals are horses and dogs. Preferred pet animals are dogs and cats.
[0067] As used herein, "preventing" means preventing in whole or in part, or
ameliorating or controlling, or reducing or halting the production or
occurrence of the thing
or event to be prevented.
[0068] As used herein, a "therapeutically effective amount" or "effective
amount" in
reference to the compounds or compositions of the instant invention refers to
the amount
sufficient to induce a desired biological, pharmaceutical, or therapeutic
result. That result can
be alleviation of the signs, symptoms, or causes of a disease or disorder or
condition, or any
other desired alteration of a biological system. In the present invention, the
result will
involve preventing fibrosis. In another aspect of the present invention, the
result will involve
the prevention and/or reduction of adhesions. In another aspect of the present
invention, the
result will involve the prevention and/or reduction of scarring and abnormal
scarring, as well
as prevention and/or reduction of excessive scar formation and other types of
abnormal
proliferation of tissue, including keloid scars, hypertrophic scars,
widespread scars, and
atrophic scars.
[0069] According to a further aspect, the result will involve the promotion
and/or
improvement of wound healing and closure of wounds, in whole or in part,
including
19
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
improvements in rates of healing. Other benefits include reductions in
swelling,
inflammation and/or scar formation, in whole or in part. Still other benefits
reduced ZO-1
protein or ZO-1 protein activity, increased Racl or Racl activity, increased
RhoA GTPase or
RhoA GTPase activity or that would benefit from increased RhoA GTPase or RhoA
GTPase
activity. Also included are diseases, disorders and conditions characterized
by unwanted
reduced RhoA GTPase or RhoA GTPase activity. Still other benefits are cellular
cytoskeletal
changes as described herein, and increased cellular lamellipodial protrusions
as described
herein.
[0070] As used herein, the terms "treating" and "treatment" refer to both
therapeutic
treatment and prophylactic or preventative measures. Those in need of
treatment include
those already with the disorder as well as those prone to having the disorder
or diagnosed
with the disorder or those in which the disorder is to be prevented. Thus, by
way of example,
the promotion of wound healing, the reduction of inflammation, the promotion
of cell
migration, the reduction of cellular adhesion, anti-fibrotic applications of
compounds and
compositions and formulations of the invention administered prior to the
formation of fibrosis
or fibrotic tissue are within the invention, as are anti-adhesion applications
of compounds and
compositions and formulations of the invention administered prior to the
formation of an
adhesion, and anti-scarring applications of compounds and compositions and
formulations of
the invention administered prior to scar formation including, for example, in
a scar reduction
surgery or procedure.
[0071] As used herein, "anti-cadherin agents" are compounds that affect or
modulate
the activity, expression, or formation of a cadherin protein. Anti-cadherin
agents include,
without limitation, anti-cadherin polynucleotides, which include antisense
compounds (e.g.,
antisense polynucleotides), RNAi, miRNA and siRNA compounds; antibodies and
antigen-
binding fragments thereof; and peptides and polypeptides, which include
"peptidomimetics"
and peptide analogs. In addition to anti-cadherin polynucleotides and anti-
cadherin peptides,
peptidomimetics, or adherens junction modifying agents, other anti-cadherin
agents include
adherens junction disrupting compounds (e.g., calcium-ion binding compounds),
and
cadherin carboxy-terminal polypeptides (which can, e.g., block or disrupt
cadherin-cadherin
protein interactions between adjoining cells, thereby disrupting adherens
junction formation
and/or maintenance). Preferred anti-cadherin agents are anti-N-cadherin
agents. Exemplary
anti-cadherin agents are discussed in further detail herein.
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[0072] The terms "peptidomimetic" and "mimetic" include naturally occurring
and
synthetic chemical compounds that may have substantially the same structural
and functional
characteristics of protein regions that they mimic. In the case of cadherin
proteins, these may
mimic, for example, the extracellular loops of cadherin-repeating domains in
the extracellular
region of cadherin proteins involved in cadherin repeat association, adherens
junction
formation and maintenance, and cell-cell adhesion.
[0073] "Peptide analogs" refer to the compounds with properties analogous to
those of
the template peptide and may be non-peptide drugs. "Peptidomimetics" (also
known as
"mimetic peptides"), which include peptide-based compounds, also include such
non-peptide
based compounds such as peptide analogs. Peptidomimetics that are structurally
similar to
therapeutically useful peptides may be used to produce an equivalent or
enhanced therapeutic
or prophylactic effect. Generally, peptidomimetics are structurally identical
or similar to a
paradigm polypeptide (i. e. , a polypeptide that has a biological or
pharmacological function or
activity), but can also have one or more peptide linkages optionally replaced
by a linkage
selected from the group consisting of, for example, -CH2NH-, -CH2S-, -CH2-CH2-
, -
CH=CH- (cis and trans), -COCH2-, -CH(OH)CH2-, and -CH2S0-. The mimetic can be
either
entirely composed of natural amino acids, or non-natural analogues of amino
acids, or, is a
chimeric molecule of partly natural peptide amino acids and partly non-natural
analogs of
amino acids. The mimetic can also comprise any amount of natural amino acid
conservative
substitutions as long as such substitutions also do not substantially alter
mimetic activity. For
example, a mimetic composition may be useful as an anti-cadherin agent if it
is capable of
down-regulating biological actions or activities of cadherin proteins,
cadherin complexes, or
adherens junctions, such as, for example, preventing the head-to-head
association of cadherin
repeats of opposing cadherin extracellular domains on adjoining cells,
association of cadherin
proteins in the same cell, formation of cadherin complexes in cells,
association of cadherin
complexes with the actin cytoskeleton, and/or adherens junction formation..
Peptidomimetics, mimetic peptides, and cadherin-modulating peptides, as well
as
compounds, including cadherin extracellular domains that comprise one or more
cadherin
repeats, encompass those described or referenced herein, as well as those as
may be known in
the art, whether now known or later developed.
[0074] The terms "modulator" and "modulation" of cadherin activity, as used
herein in
its various forms, refers to inhibition in whole or in part of the expression
or action or activity
21
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
of a cadherin protein, cadherin complex, or adherens junction, and may
function as anti-
cadherin agents.
[0075] In general, the term "protein" refers to any polymer of two or more
individual
amino acids (whether or not naturally occurring) linked via peptide bonds, as
occur when the
carboxyl carbon atom of the carboxylic acid group bonded to the alpha-carbon
of one amino
acid (or amino acid residue) becomes covalently bound to the amino nitrogen
atom of the
amino group bonded to the alpha-carbon of an adjacent amino acid. These
peptide bond
linkages, and the atoms comprising them (i. e. , alpha-carbon atoms, carboxyl
carbon atoms
(and their substituent oxygen atoms), and amino nitrogen atoms (and their
substituent
hydrogen atoms)) form the "polypeptide backbone" of the protein. In addition,
as used
herein, the term "protein" is understood to include the terms "polypeptide"
and "peptide"
(which, at times, may be used interchangeably herein). Similarly, protein
fragments, analogs,
derivatives, and variants are may be referred to herein as "proteins," and
shall be deemed to
be a "protein" unless otherwise indicated. The term "fragment" of a protein
refers to a
polypeptide comprising fewer than all of the amino acid residues of the
protein. A "domain"
of a protein is also a fragment, and comprises the amino acid residues of the
protein often
required to confer activity or function.
[0076] As used herein, "simultaneously" is used to mean that the one or more
agents
of the invention are administered concurrently, whereas the term "in
combination" is used to
mean they are administered, if not simultaneously or in physical combination,
then
"sequentially" within a timeframe that they both are available to act
therapeutically. Thus,
administration "sequentially" may permit one agent to be administered within
minutes (for
example, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30) minutes or a matter of hours,
days, weeks, or
months after the other, provided that both are present in effective amounts.
The time delay
between administration or administrations of the components will vary
depending on the
exact nature of the components, the interaction between them, and their
respective half-lives.
[0077] The term "dressing" refers to a dressing for topical application to a
wound and
excludes compositions suitable for systemic administration. For example, the
one or more
anti-cadherin agents may be dispersed in or on a solid sheet of wound
contacting material
such as a woven or nonwoven textile material, or may be dispersed in a layer
of foam such as
polyurethane foam, or in a hydrogel such as a polyurethane hydrogel, a
polyacrylate
hydrogel, gelatin, carboxymethyl cellulose, pectin, alginate, and/or
hyaluronic acid hydrogel,
22
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
for example in a gel or ointment. In certain embodiments the one or more anti-
cadherin
polynucleotides are dispersed in or on a biodegradable sheet material that
provides sustained
release of the active ingredients into the wound, for example a sheet of
freeze-dried collagen,
freeze-dried collagen/alginate mixtures (available under the Registered Trade
Mark
FIBRACOL from Johnson & Johnson Medical Limited) or freeze-dried
collagen/oxidized
regenerated cellulose (available under the Registered Trade Mark PROMOGRAN
from
Johnson & Johnson Medical Limited).
[0078] As used herein, "matrix" includes for example, matrices such as
collagen,
acellular matrices, crosslinked biological scaffold molecules, tissue-based
matrices (including
pig-based wound healing matrices), cultured epidermal autografts, cultured
epidermal
allografts, tissue-engineered skin, collagen and glycosaminoglycan dermal
matrices
inoculated with autologous fibroblasts and keratinocytes, AlloDerm (a
nonliving allogeneic
acellular dermal matrix with intact basement membrane complex), living skin
equivalents
(e.g., Dermagraft (living allogeneic dermal fibroblasts grown on degradable
scaffold),
Transcyte (an extracellular matrix generated by allogeneic human dermal
fibroblasts),
Ap1igraf (a living allogeneic bilayered construct containing keratinocytes,
fibroblasts and
bovine type I collagen), Integra (two-layer membrane system for skin
replacement
comprising a dermal replacement layer made of a porous template of fibers of
bovine tendon
collagen and glycosaminoglycan (chondroitin-6-sulfate) and an epidermal
substitute layer
made of thin silicone to control moisture loss), CyzactTM (human dermal
fibroblasts delivered
via a fibrin), ICX-SKN (a combination of fibroblasts and fibrin matrix that
are remodeled to
produce a collagen matrix), KeragraftTM (a human stem cell-derived product
being developed
for wound care as an autologous epidermal equivalent), OASIS Wound Matrix
(biologically derived extracellular matrix-based wound product created from
porcine-derived
acellular small intestine submucosal), and OrCe1TM (allogeneic fibroblasts and
keratinocytes
seeded in opposite sides of bilayered matrix of bovine collagen), BioBrane,
cultured allogenic
keratinocytes, animal derived dressings (e.g., Oasis's porcine small
intestinal submucosa
acellular collagen matrix; and E-Z Derm's acellular xenogeneic collagen
matrix), tissue-
based bioengineered structural frameworks, scaffolds, biomanufactured
bioprostheses, and
other implanted or applied structures such as for example, vascular grafts
suitable for cell
infiltration and proliferation useful in the promotion of wound healing. A
matrix is also
provided by a cell therapy spray suspension known as HP802-247, being
developed by
HealthPoint, which consists of two components that are sprayed sequentially on
the wound
23
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
bed at the time of treatment: a fibrinogen solution and a cell preparation
containing a mixture
of growth arrested, living, allogeneic epidermal keratinocytes and dermal
fibroblasts.
[0079] Additional suitable biomatrix material may include chemically modified
collagenous tissue to reduce antigenicity and immunogenicity. Other suitable
examples
include collagen sheets for wound dressings, antigen-free or antigen reduced
acellular matrix
(Wilson, et al., Trans Am Soc Artif Intern 1990; 36:340-343), or other
biomatrices that have
been engineered to reduce the antigenic response to the xenograft material.
Other matrices
useful in promotion of wound healing may include for example, processed bovine
pericardium proteins comprising insoluble collagen and elastin (Courtman, et
al., J Biomed
Mater Res 1994; 28:655-666) and other acellular tissue which may be useful for
providing a
natural microenvironment for host cell migration to accelerate tissue
regeneration (Malone, et
al., J Vasc Surg 1984; 1:181-91). In certain embodiments, the matrix material
may be
supplemented with one or more anti-cadherin agents, anti-ZO-1 agents, anti-
connexin43
agents, and/or the one or more therapeutic agents for site-specific release of
such agents.
Wounds and Wound Classification
[0080] Chronic wounds, slow healing wounds, and incomplete healing wounds
often
result in infection and can lead to amputation or death. It has been
discovered that use of
certain compounds, including those described or referenced herein, may block,
inhibit, or
alter cell communications, which may promote closure and healing in chronic,
slow healing,
and incomplete healing wounds.
[0081] By "wound" is meant an injury to any tissue, including, for example,
acute,
delayed, slow, or difficult to heal wounds, and chronic wounds. Examples of
wounds may
include both open and closed wounds. Wounds include, for example, burns,
incisions,
excisions, lacerations, abrasions, puncture or penetrating wounds, surgical
wounds,
contusions, hematomas, crushing injuries, and ulcers. Also included are wounds
that do not
heal at expected rates.
[0082] By a "wound that does not heal at the/an expected rate" is meant an
injury to
any tissue that does not heal in an expected or typical time frame, including
delayed, slow, or
difficult to heal wounds (including delayed or incompletely healing wounds),
and chronic
wounds. Examples of wounds that do not heal at the expected rate include
diabetic ulcers,
diabetic foot ulcers, vasculitic ulcers, arterial ulcers, venous ulcers,
venous stasis ulcers,
24
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
pressure ulcers, decubitus ulcers, infectious ulcers, trauma-induced ulcers,
burn ulcers,
ulcerations associated with pyoderma gangrenosum, and mixed ulcers.
[0083] As described herein, a delayed or difficult to heal wound may include,
for
example, a wound that is characterized at least in part by one or more of 1) a
prolonged
inflammatory phase, 2) a slow forming extracellular matrix, and 3) a stalled
or decreased rate
of epithelialization.
[0084] In the art, the term "chronic wound" refers generally to a wound that
has not
healed within about three months, but can be wounds that have not healed
within about one
or two months. Chronic skin wounds include, for example, pressure ulcers,
diabetic ulcers,
venous ulcers, arterial ulcers, inflammatory ulcers, and mixed ulcers. The
chronic wound
may be an arterial ulcer that can include ulcerations resulting from complete
or partial arterial
blockage. The chronic wound may be a venous stasis ulcer, which can include
ulcerations
resulting from a malfunction of the venous valve and the associated vascular
disease. The
chronic wound may be a trauma-induced ulcer.
[0085] As used herein, chronic wound can also include, for example, a wound
that is
characterized at least in part by 1) a chronic self-perpetuating state of
wound inflammation,
2) a deficient and defective wound extracellular matrix (ECM), 3) poorly
responding
(senescent) wound cells (e.g. fibroblasts), limited ECM production, and 4)
failure of re-
epithelialization due in part to lack of the necessary ECM orchestration and
lack of scaffold
for migration.
[0086] Chronic wounds can also be characterized, for example, by 1) prolonged
inflammation and proteolytic activity, leading to ulcerative lesions,
including, for example,
diabetic, pressure (decubitus), venous, and arterial ulcers, 2) prolonged
fibrosis in the wound
leading to scarring, 3) progressive deposition of matrix in the affected area,
4) longer repair
times, 5) less wound contraction, 6) slower re-epithelialization, and 7)
increased thickness of
granulation tissue.
[0087] Exemplary chronic wounds also include "pressure ulcers." Exemplary
pressure
ulcers may include all four stages of wound classifications based on AHCPR
(Agency for
Health Care Policy and Research, U.S. Department of Health and Human Services)
guidelines, including for example, Stage 1. A stage I pressure ulcer is an
observable pressure
related alteration of intact skin whose indicators as compared to the adjacent
or opposite area
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
on the body may include changes in one or more of the following: skin
temperature (warmth
or coolness), tissue consistency (firm or boggy feel), and/or sensation (pain,
itching). The
ulcer appears as a defined area of persistent redness in lightly pigmented
skin, whereas in
darker skin tones, the ulcer may appear with persistent red, blue, or purple
hues. Stage 1
ulceration may include nonblanchable erythema of intact skin and the heralding
lesion of skin
ulceration. In individuals with darker skin, discoloration of the skin,
warmth, edema,
induration, or hardness may also be indicators of stage 1 ulceration. Stage 2:
stage 2
ulceration may be characterized by partial thickness skin loss involving
epidermis, dermis, or
both. The ulcer is superficial and presents clinically as an abrasion,
blister, or shallow crater.
Stage 3: stage 3 ulceration may be characterized by full thickness skin loss
involving damage
to or necrosis of subcutaneous tissue that may extend down to, but not
through, underlying
fascia. The ulcer presents clinically as a deep crater with or without
undermining of adjacent
tissue. Stage 4: stage 4 ulceration may be characterized by full thickness
skin loss with
extensive destruction, tissue necrosis, or damage to muscle, bone, or
supporting structures
(e.g., tendon, joint capsule, etc.).
[0088] Exemplary chronic wounds also include "decubitus ulcers." Exemplary
decubitus ulcer may arise as a result of prolonged and unrelieved pressure
over a bony
prominence that leads to ischemia. The wound tends to occur in patients who
are unable to
reposition themselves to off-load weight, such as paralyzed, unconscious, or
severely
debilitated persons. As defined by the U.S. Department of Health and Human
Services, the
major preventive measures include identification of high-risk patients;
frequent assessment;
and prophylactic measures such as scheduled repositioning, appropriate
pressure-relief
bedding, moisture barriers, and adequate nutritional status. Treatment options
may include,
for example, pressure relief, surgical and enzymatic debridement, moist wound
care, and
bacterial load control. Certain embodiments of the invention involve treating
a chronic
wound characterized by a decubitus ulcer or ulceration that results from
prolonged,
unrelieved pressure over a bony prominence that leads to ischemia.
[0089] Exemplary chronic wounds also include "arterial ulcers." Arterial
ulcers
include those characterized by complete or partial arterial blockage, which
may lead to tissue
necrosis and/or ulceration. Signs of arterial ulcer can include, for example,
pulselessness of
the extremity; painful ulceration; small, punctate ulcers that are usually
well circumscribed;
cool or cold skin; delayed capillary return time (briefly push on the end of
the toe and release,
26
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
normal color should return to the toe in about 3 seconds or less); atrophic-
appearing skin (for
example, shiny, thin, dry); and loss of digital and pedal hair.
[0090] Exemplary chronic wounds also include "venous ulcers." Exemplary venous
ulcers include the most common type of ulcer affecting the lower extremities
and may be
characterized by malfunction of the venous valve. The normal vein has valves
that prevent
the backflow of blood. When these valves become incompetent, the backflow of
venous
blood causes venous congestion. Hemoglobin from the red blood cells escapes
and leaks into
the extravascular space, causing the brownish discoloration commonly noted. It
has been
shown that the transcutaneous oxygen pressure of the skin surrounding a venous
ulcer is
decreased, indicating that there are forces obstructing the normal vascularity
of the area.
Lymphatic drainage and flow also plays a role in these ulcers. A venous ulcer
can appear
near the medial malleolus and usually occurs in combination with an edematous
and
indurated lower extremity; it may be shallow, not too painful, and may present
with a
weeping discharge from the affected site.
[0091] Exemplary chronic wounds also include "venous stasis ulcers." Exemplary
venous stasis ulcer are characterized by chronic passive venous congestion of
the lower
extremities that results in local hypoxia. One possible mechanism of
pathogenesis of these
wounds includes the impediment of oxygen diffusion into the tissue across
thick perivascular
fibrin cuffs. Another mechanism is that macromolecules leaking into the
perivascular tissue
trap growth factors needed for the maintenance of skin integrity.
Additionally, the flow of
large white blood cells slows due to venous congestion, occluding capillaries,
becoming
activated, and damaging the vascular endothelium to predispose to ulcer
formation.
[0092] Exemplary chronic wounds further include "diabetic foot ulcers."
Diabetic
patients with exemplary diabetic foot ulcer are prone to foot ulcerations due
to both
neurologic and vascular complications. Peripheral neuropathy can cause altered
or complete
loss of sensation in the foot and /or leg. Diabetic patients with advanced
neuropathy lose all
ability for sharp-dull discrimination. Any cuts or trauma to the foot may go
completely
unnoticed for days or weeks in a patient with neuropathy. A patient with
advanced
neuropathy can lose the ability to sense a sustained pressure insult and, as a
result, tissue
ischemia and necrosis may occur leading to, for example, plantar ulcerations.
Additionally,
microfractures in the bones of the foot, if unnoticed and untreated, may
result in
27
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
disfigurement, chronic swelling, and additional bony prominences.
Microvascular disease is
one of the significant complications for diabetics that may also lead to
ulcerations.
[0093] Exemplary chronic wounds can include "traumatic ulcers." Formation of
exemplary traumatic ulcers may occur as a result of traumatic injuries to the
body. These
injuries include, for example, compromises to the arterial, venous, or
lymphatic systems;
changes to the bony architecture of the skeleton; loss of tissue layers -
epidermis, dermis,
subcutaneous soft tissue, muscle or bone; damage to body parts or organs and
loss of body
parts or organs.
[0094] Exemplary chronic wounds can include "burn ulcers" including, for
example,
ulceration that occur as a result of a burn injury, including a first degree
burn (i.e., superficial,
reddened area of skin); a second degree burn (a blistered injury site which
may heal
spontaneously after the blister fluid has been removed); a third degree burn
(burn through the
entire skin and usually require surgical intervention for wound healing);
scalding (may occur
from scalding hot water, grease or radiator fluid); a thermal burn (may occur
from flames,
usually deep burns); a chemical burn (may come from acid and alkali, usually
deep burns); an
electrical burn (either low voltage around a house or high voltage at work);
an explosion flash
(usually superficial injuries); and contact burns (usually deep and may occur
from muffler tail
pipes, hot irons, and stoves).
[0095] As used herein, a delayed or difficult to heal wound may include, for
example,
a wound that is characterized at least in part by 1) a prolonged inflammatory
phase, 2) a slow
forming extracellular matrix (ECM), and 3) a decreased rate of
epithelialization.
[0096] As used herein, "fibrotic" diseases, disorders, or conditions include
those
mentioned herein, and further include acute and chronic, clinical or sub-
clinical presentation,
in which fibrogenic associated biology or pathology is evident. Fibrotic
diseases, disorders,
or conditions include diseases, disorders or conditions characterized, in
whole or in part, by
the excess production of fibrous material, including excess production of
fibrotic material
within the extracellular matrix, or the replacement of normal tissue elements
by abnormal,
non-functional, and/or excessive accumulation of matrix-associated components.
Fibrotic
diseases, disorders, or conditions include, for example, fibrogenic-related
biology or
pathology characterized by fibrosis.
28
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[0097] Exemplary fibrotic diseases, disorders, and conditions include, for
example,
scleroderma (including moThea, generalized morphea, or linear scleroderma),
kidney
fibrosis (including glomerular sclerosis, renal tubulointerstitial fibrosis,
progressive renal
disease or diabetic nephropathy), cardiac fibrosis (e.g., myocardial
fibrosis), pulmonary
fibrosis (e.g., glomerulosclerosis pulmonary fibrosis, idiopathic pulmonary
fibrosis, silicosis,
asbestosis, interstitial lung disease, interstitial fibrotic lung disease, and
chemotherapy/radiation induced pulmonary fibrosis), oral fibrosis,
endomyocardial fibrosis,
deltoid fibrosis, pancreatitis, inflammatory bowel disease, Crohn's disease,
nodular fascilitis,
eosinophilic fasciitis, general fibrosis syndrome characterized by replacement
of normal
muscle tissue by fibrous tissue in varying degrees, retroperitoneal fibrosis,
liver fibrosis, liver
cirrhosis, chronic renal failure; myelofibrosis (bone marrow fibrosis), drug
induced ergotism,
glioblastoma in Li-Fraumeni syndrome, sporadic glioblastoma, myleoid leukemia,
acute
myelogenous leukemia, myelodysplastic syndrome, myeloproferative syndrome,
gynecological cancer, Kaposi's sarcoma, Hansen's disease, collagenous colitis,
and acute
fibrosis.
[0098] Fibrotic diseases, disorders, and conditions can also include
contractures.
Contractures, including post-operative contractures, refer to a permanent or
long term
reduction of range of motion due to tonic spasm or fibrosis, or to loss of
normal tissue
compliance, motion, or equilibrium (e.g., muscle, tendon, ligament, fascia,
synovium, joint
capsule, other connective tissue, or fat). In general, the condition of
contracture may involve
a fibrotic response with inflammatory components, both acute and chronic. Some
of which
may be associated with surgery, including a release procedure. Hereditary
contractures such
as Dupytren's contracture, Peyronie's disease, and Ledderhose's disease are
also included.
[0099] Fibrosis can be either chronic or acute. Fibrotic conditions include
excessive
amounts of fibrous tissue, including excessive amounts of extracellular matrix
accumulation
within a tissue, forming tissue that causes dysfunction and, potentially,
organ failure.
Chronic fibrosis includes fibrosis of the major organs, most commonly lung,
liver, kidney,
and/or heart. Acute fibrosis (usually with a sudden and severe onset and of
short duration)
occurs typically as a common response to various forms of trauma including
injuries,
ischemic illness (e.g. cardiac scarring following heart attack), environmental
pollutants,
alcohol and other types of toxins, acute respiratory distress syndrome,
radiation, and
29
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
chemotherapy treatments. All tissues damaged by trauma can become fibrotic,
particularly if
the damage is repeated.
[00100] Response to injury involves coordinated and temporally regulated
patterns of
mediators and sequence of cellular events in tissues subsequent to injury. The
initial injury
triggers a coagulation cascade and an acute local inflammatory response
followed by
mesenchymal cell recruitment, proliferation, and matrix synthesis.
Uncontrolled matrix
accumulation, often involving aberrant cytokine pathways, can lead to fibrotic
conditions or
disorders. Progressive fibrosis in vital organs such as the lung, kidney,
liver, heart, brain, and
bone marrow, is both a major cause of illness and death.
Adhesions
[00101] Within other aspects of the invention, methods are provided for
treating,
reducing the incidence or severity of, and/or preventing or retarding
adhesions, surgical
adhesions, and/or secondary surgical adhesions by administering to a patient
an anti-connexin
polynucleotide.
[00102] Adhesion formation is a complex process in which bodily tissues that
are
normally separate grow together. For example, post-operative adhesions have
been reported
to occur in about 60% to 90% of patients undergoing major gynecological
surgery. Surgical
trauma as a result of tissue (e.g., epithelial, connective, muscle, and nerve
tissue) drying,
ischemia, thermal injury, infection, or the presence of a foreign body, has
long been
recognized as a stimulus for tissue adhesion formation. These adhesions are a
major cause of
failed surgical therapy and are the leading cause of bowel obstruction and
infertility. Other
adhesion-treated complications include chronic pelvic pain, urethral
obstruction, and voiding
dysfunction.
[00103] Generally, adhesion formation is an inflammatory reaction in which
factors are
released, increasing vascular permeability and resulting in fibrinogen influx
and fibrin
deposition. This deposition forms a matrix that bridges the abutting tissues.
Fibroblasts
accumulate, attach to the matrix, deposit collagen, and induce angiogenesis.
If this cascade
of events can be prevented within 4 to 5 days following surgery, adhesion
formation can be
inhibited.
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00104] Secondary surgical adhesions may also form as a result of a corrective
surgical
procedure designed to correct and existing adhesion. The procedure may be a
release or
separation procedure.
[00105] A wide variety of animal models can be used to assess a particular
therapeutic
composition or treatment regimen for its therapeutic potential. Briefly,
peritoneal adhesions
have been observed to occur in animals as a result of inflicted severe damage
that usually
involves two adjacent surfaces. Injuries may be mechanical, due to ischemia,
or as a result of
the introduction of foreign material. Mechanical injuries include crushing of
the bowel and
stripping or scrubbing away the outer layers of bowel wall. Dividing major
vessels to loops
of the intestine induces ischemia. Foreign material that may be introduced
into the area
includes talcum, gauze sponges, toxic chemicals, bacteria, and feces.
[00106] Presently, typical animal models to evaluate prevention of formation
of
adhesions include the rabbit uterine horn model which involves the abrasion of
the rabbit
uterus, the rabbit uterine horn devascularization modification model which
involves abrasion,
devascularization of the uterus, and the rabbit cecal sidewall model which
involves the
excision of a patch of parietal peritoneum plus the abrasion of the cecum.
Those and other
reported evaluation models are described herein.
Anti-Cadherin A2ents
[00107] Anti-cadherin agents of the invention described herein are capable of
modulating (e.g., blocking or inhibiting or downregulating) or affecting
cadherin activity and
function, cadherin complex formation and maintenance, adherens junction
formation and
maintenance, and cell-cell adhesion. Thus, certain anti-cadherin agents
described herein
modulate cellular adhesion (i.e., cell-to-cell adhesion). Certain anti-
cadherin agents are
adherens junction modulation agents. Such anti-connexin agents are generally
targeted to
messenger RNA (mRNA) molecules (or the genes encoding them) that, when
translated,
result in cadherin protein synthesis and localization to the cell membrane,
where they are
available for adherens junction formation. Other anti-cadherin agents
interfere with cadherin
complex and/or adherens junction formation. Thus, an anti-cadherin agents
provided herein
may directly or indirectly reduce coupling and communication between cells or
reduce or
block communication (or the transmission of molecules) between adjoining
cells. Preferably,
the cadherin is N-cadherin.
31
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00108] Any anti-cadherin agent that is capable of eliciting a desired
modulation of
cadherin activity, cadherin complex formation, and/or adherens junction
formation may be
used in practicing the invention. Such compounds include, for example,
proteins and
polypeptides, polynucleotides, and other organic compounds, and they may, for
example,
block the function or expression of adherens junctions in whole or in part, or
downregulate
the production of one or more cadherin proteins, cadherin complexes, and/or
adherens
junctions in whole or in part.
[00109] Certain anti-cadherin agents provide downregulation of cadherin
expression
(for example, by downregulation of mRNA transcription or translation) or
otherwise decrease
or inhibit the activity of a cadherin protein, a cadherin complex, or adherens
junctions. In the
case of downregulation, this will have the effect of reducing direct cell-cell
adhesion
mediated by adherens junctions.
[00110] Examples of anti-cadherin agents include agents that decrease or
inhibit
expression or function of cadherin mRNA and/or protein or that decrease
activity, expression,
or formation of a cadherin protein species, cadherin complexes, or adherens
junctions. Anti-
cadherin agents include anti-cadherin polynucleotides, such as antisense
polynucleotides and
other polynucleotides (such as miRNAs and polynucleotides having siRNA or
ribozyme
functionalities), as well as antibodies and antigen-binding fragments thereof,
and peptides
and polypeptides, including peptidomimetics and peptide analogs that modulate
cadherin or
adherens junction activity or function, and deoxyribozymes. Anti-cadherin
agents are
preferred, particularly anti-N-cadherin agents.
Anti-Cadherin Polynueleotides
[00111] Anti-cadherin polynucleotides include connexin antisense
polynucleotides as
well as polynucleotides which have functionalities which enable them to
downregulate
cadherin expression. Other suitable anti-cadherin polynucleotides include
miRNAs, RNAi
polynucleotides and siRNA polynucleotides. Anti-N-cadherin polynucleotides are
preferred.
[00112] Synthesis of antisense polynucleotides and other polynucleotides that
can serve
as anti-cadherin polynucleotides, such as miRNA, RNAi, siRNA, and ribozyme
polynucleotides as well as polynucleotides having modified and mixed
backbones, is known
to those of skill in the art. See e.g. Stein C.A. and Krieg A.M. (eds),
Applied Antisense
Oligonucleotide Technology, 1998 (Wiley-Liss). Methods of synthesizing desired
antibodies
32
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
and antigen-binding fragments, as well as desired peptides and polypeptides,
including
peptidomimetics and peptide analogs, are known to those of skill in the art.
See e.g. Lihu
Yang et al., Proc. Natl. Acad. Sci. U.S.A., 1; 95(18): 10836-10841 (Sept 1
1998); Harlow and
Lane (1988) "Antibodies: A Laboratory Manuel" Cold Spring Harbor Publications,
New
York; Harlow and Lane (1999) "Using Antibodies" A Laboratory Manuel, Cold
Spring
Harbor Publications, New York.
[00113] According to one aspect, the downregulation of cadherin expression is
based
generally upon the antisense approach using antisense polynucleotides (such as
DNA or RNA
polynucleotides), and more particularly upon the use of antisense
oligodeoxynucleotides
(ODN). These polynucleotides (e.g., ODN) target mRNA molecules coding for the
cadherin
protein (s) to be downregulated. Typically the polynucleotides are single-
stranded, but may
be double-stranded.
[00114] The antisense polynucleotide may inhibit transcription and/or
translation of a
target cadherin protein species. Preferably, the polynucleotide is a specific
inhibitor of
transcription and/or translation from the cadherin gene or mRNA, and does not
inhibit
transcription and/or translation from other genes or mRNAs. The product binds
to the
cadherin gene or mRNA (i) 5' to the coding sequence, and/or (ii) to the coding
sequence,
and/or (iii) 3' to the coding sequence.
[00115] The antisense polynucleotide is generally antisense to a cadherin
mRNA,
preferably N-cadherin mRNA. Such a polynucleotide may be capable of
hybridizing to the
cadherin mRNA and can thus inhibit the expression of cadherin by interfering
with one or
more aspects of cadherin mRNA metabolism including transcription, mRNA
processing,
mRNA transport from the nucleus, translation, or mRNA degradation. While not
wishing to
be bound to a particular theory, the antisense polynucleotide typically
hybridizes to the
cadherin mRNA to form a duplex which can cause direct inhibition of
translation and/or
destabilization of the mRNA. Such a duplex may be susceptible to degradation
by nucleases.
[00116] The antisense polynucleotide may hybridize to all or part of a
cadherin mRNA.
Typically, the antisense polynucleotide hybridizes to the ribosome binding
region and/or the
coding region of the cadherin mRNA. The polynucleotide may be complementary to
all of or
a region of the target cadherin mRNA. For example, the polynucleotide may be
the exact
complement of all or a part of a cadherin mRNA. However, absolute
complementarity is not
33
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
required and polynucleotides that have sufficient complementarity to form a
duplex having a
melting temperature of greater than about 5 C, 10 C, 20 C, 30 C, or 40 C more
than
physiological temperature are particularly suitable for use in the present
invention.
[00117] Thus the polynucleotide is typically a homologue of a sequence
complementary
to the target cadherin mRNA. The polynucleotide may be a polynucleotide which
hybridizes
to the cadherin mRNA under conditions of medium to high stringency, such as
0.03M sodium
chloride and 0.03M sodium citrate at from about 50 C to about 60 C.
[00118] For certain aspects, suitable polynucleotides are typically from about
6 to 40
nucleotides in length, for example. Preferably a polynucleotide may be from
about 5 and
about 100 nucleotides in length, preferably about 6 to about 40 nucleotides in
length,
preferably about 12 to about 35 nucleotides in length, or alternatively from
about 12 to about
20 nucleotides in length or more preferably from about 18 to about 32
nucleotides in length.
According to alternative embodiments, the polynucleotide is at least about 40,
for example at
least about 60 or at least about 80, nucleotides in length and up to about
100, about 200,
about 300, about 400, about 500, about 1000, about 2000, or about 3000 or more
nucleotides
in length.
[00119] The connexin protein or proteins targeted by an anti-cadherin
polynucleotide
will be dependent upon the site at which downregulation is to be effected.
This reflects the
non-uniform make-up of adherens junction(s) at different sites throughout the
body in terms
of cadherin complex composition. The cadherin is one that naturally occurs in
a human or
animal in one aspect or naturally occurs in the tissue in which cadherin
expression or activity
is to be modulated, preferably decreased. The cadherin gene (including coding
sequence)
generally has homology with the coding sequence of one or more of the specific
cadherins
mentioned herein, such as homology with the cadherin coding sequence shown in
Example 1,
below. The cadherin is typically N-cadherin, E-cadherin, P-cadherin, cadherin
11, cadherin
12, a protocadherin protein, a desmoglein protein, or a desmocollin protein.
Preferably, the
cadherin N-cadherin and is expressed in the tissue to be treated.
[00120] Anti-cadherin polynucleotides include cadherin antisense
polynucleotides as
well as polynucleotides that have functionalities enabling them to
downregulate cadherin
expression. Other suitable anti-cadherin polynucleotides include RNAi
polynucleotides and
siRNA polynucleotides.
34
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00121] In many preferred embodiments, the antisense polynucleotides are
targeted to
the mRNA of one cadherin protein species only. Most preferably, this cadherin
protein is N-
cadherin. It is also contemplated that polynucleotides targeted to separate
cadherin protein
species be used in combination (for example 1, 2, 3, 4, or more different
cadherin
superfamily members can be targeted). For example, polynucleotides targeted to
N-cadherin,
and one or more other members of the cadherin superfamily (e.g., E-cadherin, P-
cadherin,
cadherin 11, cadherin 12, a protocadherin protein, a desmoglein protein, or a
desmocollin
protein) can be used in combination. Alternatively, the antisense
polynucleotides of the
invention may be part of compositions that may comprise polynucleotides
targeted to more
than one cadherin protein. Preferably, one of the cadherin proteins to which
such
polynucleotides are directed is N-cadherin. Thus, individual antisense
polynucleotides may
be specific to mRNA for a particular cadherin protein species, or may target
mRNAs for two
or more different cadherin protein species. Specific polynucleotides will
generally target
sequences in a cadherin gene or mRNA that are not conserved between cadherins,
whereas
non-specific anti-cadherin polynucleotides will target conserved sequences for
various
cadherins.
[00122] The polynucleotides and other agents, including deoxyribozymes, for
use in the
invention can be unmodified phosphodiester oligomers. Such
oligodeoxynucleotides may
vary in length. A 15-18 mer, 20-mer, and 30-mer polynucleotide has been found
to be
suitable. Many aspects of the invention are described with reference to
oligodeoxynucleotides; however, it is understood that other suitable
polynucleotides (such as
RNA polynucleotides) can be used in these embodiments, as well.
[00123] The antisense polynucleotides and other agents, including
deoxyribozymes,
may be chemically modified. This may enhance their resistance to nucleases and
may
enhance their ability to enter cells. Such modifications are known in the art.
For example,
phosphorothioate oligonucleotides can be used. Other deoxynucleotide analogs
include
methylphosphonates, phosphoramidates, phosphorodithioates, N3'P5'-
phosphoramidates, and
oligoribonucleotide phosphorothioates and their 2'-0-alkyl analogs and 2'-0-
methylribonucleotide methylphosphonates. Alternatively, mixed backbone
oligonucleotides
("MBOs") may be used. MBOs contain segments of phosphothioate
oligodeoxynucleotides
and appropriately placed segments of modified oligodeoxy-or
oligoribonucleotides. MBOs
have segments of phosphorothioate linkages and other segments of other
modified
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
oligonucleotides, such as methylphosphonate, which is non-ionic, and very
resistant to
nucleases or 2'-0-alkyloligoribonucleotides. Methods of preparing modified
backbone and
mixed backbone oligonucleotides are known in the art.
[00124] The precise sequence of the antisense polynucleotide(s) used in the
invention
will depend upon the target cadherin protein. In some embodiments, suitable
cadherin
antisense polynucleotides can include polynucleotides such as
oligodeoxynucleotides.
Suitable polynucleotides for the preparation of the combined polynucleotide
compositions
described herein include for example, polynucleotides to N-cadherin and
polynucleotides for
E-cadherin, P-cadherin, cadherin 11, cadherin 12, a protocadherin protein, a
desmoglein
protein, or a desmocollin protein.
[00125] Polynucleotides, including ODN's, directed to cadherin proteins can be
selected in terms of their nucleotide sequence by any convenient, and
conventional, approach.
For example, the computer programs MacVector and OligoTech (from Oligos etc.
Eugene,
Oregon, USA) can be used. Once selected, the ODN's can be synthesized using an
automated
DNA synthesizer.
Polynueleotide Homologues
[00126] Homology and homologues are discussed herein (for example, the
polynucleotide may be a homologue of a complement to a sequence in cadherin
mRNA).
Such a polynucleotide typically has at least about 70% homology, preferably at
least about
80%, at least about 90%, at least about 95%, at least about 97% or at least
about 99%
homology with the relevant sequence, for example, over a region of at least
about 15, at least
about 20, at least about 25, at least about 30, at least about 40, at least
about 50, or at least
about 100 more contiguous nucleotides (of the homologous sequence).
[00127] Homology or sequence identity may be calculated based on any method in
the
art. For example, the UWGCG Package provides the BESTFIT program that can be
used to
calculate homology (Devereux, et al. (1984) Nucleic Acids Research 12, p387-
395). The
PILEUP and BLAST algorithms can also be used to calculate sequence identity or
align
sequences, for example, as described in Altschul, S. F. (1993), J Mol Evol 36:
290-300;
Altschul, et al (1990), J Mol Biol 215: 403-10. Software for performing BLAST
analyses is
publicly available through the National Center for Biotechnology Information
(http://www.ncbi.nlm.nih.gov/). The BLAST algorithm performs a statistical
analysis of the
36
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
similarity between two sequences; see e.g., Karlin and Altschul (1993) Proc.
Natl. Acad. Sci.
USA 90: 5873-5787. The homologous sequence typically differs from the relevant
sequence
by at least about (or by no more than about) 2, 5, 10, 15, 20, or more
nucleotide differences
(which may be substitutions, deletions, or insertions). These differences can
be measured
across any of the regions mentioned above in relation to calculating sequence
identity or
homology.
[00128] The homologous sequence typically hybridizes selectively to the
original
sequence at a level significantly above background. Selective hybridization is
typically
achieved using conditions of medium to high stringency (for example 0.03M
sodium chloride
and 0.03M sodium citrate at from about 50 C to about 60 C). However, such
hybridization
may be carried out under any suitable conditions known in the art (see
Sambrook, et al.
(1989), Molecular Cloning: A Laboratory Manual). For example, if high
stringency is
required, suitable conditions include 0.2 x SSC at 60 C. If lower stringency
is required,
suitable conditions include 2 x SSC at 60 C.
Peptide and Polypeptide Anti-Cadherin Agents
[00129] Cadherin binding proteins, including peptides, peptidomimetics,
antibodies,
antigen-binding antibody fragments, and the like, are also suitable modulators
of adherens
junctions.
[00130] Binding proteins include, for example, monoclonal antibodies,
polyclonal
antibodies, antibody fragments (including, for example, Fab, F(ab')2 and Fy
fragments; single
chain antibodies; single chain Fvs; and single chain binding molecules such as
those
comprising, for example, a binding domain, hinge, CH2 and CH3 domains,
recombinant
antibodies, and antibody fragments which are capable of binding an antigenic
determinant
(i.e., that portion of a molecule, generally referred to as an epitope) that
makes contact with a
particular antibody or other binding molecule. These binding proteins,
including antibodies,
anti-binding antibody fragments, and so on, may be chimeric or humanized or
otherwise
made to be less immunogenic in the subject to whom they are to be
administered, and may be
synthesized, produced recombinantly, or produced in expression libraries. Any
binding
molecule known in the art or later discovered is envisioned, such as those
referenced herein
and/or described in greater detail in the art. For example, binding proteins
include not only
antibodies, and the like, but also ligands, receptors, peptidomimetics, or
other binding
fragments or molecules (for example, produced by phage display) that bind to a
target (e.g., a
37
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
cadherin protein, another protein in a cadherin complex or adherens junction,
or associated
molecules). Fab fragments to N-cadherin extracellular domains have been
reported to inhibit
adherens junction formation. Meyer, et al. (1992) J Cell Biol. 1992 October 1;
119(1): 179-
189.
[00131] Binding molecules will generally have a desired specificity, including
but not
limited to binding specificity, and desired affinity. Affinity, for example,
may be a Ka of
greater than or equal to about 104 M-1, greater than or equal to about 106 M-
1, greater than or
equal to about 107 M-1, greater than or equal to about 108 M-1. Affinities of
even greater than
about 108 M-1 are suitable, such as affinities equal to or greater than about
109 M-1, about 1010
M-1, about 1011 M-1, and about 1012 M-1. Affinities of binding proteins
according to the
present invention can be readily determined using conventional techniques, for
example those
described by Scatchard, et al., 1949 Ann. N.Y. Acad. Sci. 51: 660.
[00132] The extracellular domains of cadherin proteins contributed by two
adjacent
cells "dock" with each other to form complete gap junction channels. Reagents
that interfere
with the interactions of these extracellular domains can impair adherens
junction formation
and/or stability.
[00133] Anti-cadherin agents include peptides comprising an amino acid
sequence
corresponding to a cadherin domain motif from a cadherin protein (e.g., E-
cadherin, N-
cadherin, etc.). Other embodiments are directed to an anti-connexin agent that
is a peptide
having an amino acid sequence that comprises at least about 5, at least about
6, at least about
7, at least about 8, at least about 9, at least about 10, at least about 11,
at least about 12, at
least about 13, at least about 14, at least about 15, at least about 20, at
least about 25, or at
least about 30 contiguous amino acids encoded by a cadherin gene, for example,
an N-
cadherin gene as set forth in Example 1, below. In certain anti-connexin
agents provided
herein, the extracellular domains of N-cadherin may be used to develop the
particular peptide
sequences. The peptides need not have an amino acid sequence identical to
those portions of
naturally occurring N-cadherin, and conservative amino acid changes may be
made such that
the peptides retain binding activity or functional activity. Alternatively,
peptides may target
other regions of the extracellular domain.
[00134] Anti-cadherin peptides may comprise sequences corresponding to a
portion of a
cadherin extracellular domain with conservative amino acid substitutions such
that peptides
38
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
are functionally active anti- cadherin agents. Exemplary conservative amino
acid
substitutions include for example the substitution of a nonpolar amino acid
with another
nonpolar amino acid, the substitution of an aromatic amino acid with another
aromatic amino
acid, the substitution of an aliphatic amino acid with another aliphatic amino
acid, the
substitution of a polar amino acid with another polar amino acid, the
substitution of an acidic
amino acid with another acidic amino acid, the substitution of a basic amino
acid with
another basic amino acid, and the substitution of an ionizable amino acid with
another
ionizable amino acid.
Adherens Junction Modulation Aments
[00135] Certain anti-cadherin agents described herein are capable of
modulation or
affecting (e.g. blocking or inhibiting) adhesion between cells. Thus, certain
adherens
junction modulation agents described herein modulate cellular adhesion. As
used herein,
"adherens junction modulation agent" broadly includes any agent or compound
that prevents,
decreases, or modulate, in whole or in part, the activity, function,
formation, or stability of an
adherens junction. In certain embodiments, an adherens junction modulation
agent prevents
or decreases, in whole or in part, the function of an adherens junction.
Exemplary adherens
junction modulation agents may include, without limitation, polynucleotides,
polypeptides
(e.g. peptiditomimetics, antibodies, binding fragments thereof, and synthetic
constructs), and
other adherens junction modulating agents.
Dosne Forms and Formulations and Administration
[00136] A therapeutically effective amount of each of the agents of the
invention may
be administered simultaneously, separately, or sequentially and in any order.
The agents may
be administered separately or as a fixed combination. When not administered as
a fixed
combination, preferred methods include the sequential administration of one or
more anti-
cadherin agents, alone or in combination with one or more other therapeutic
agents, including
other anti-cadherin agents, anti-connexin agents, anti-ZO-1 agents, and/or
anti-osteopontin
agents.
[00137] Where an anti-cadherin agent and other therapeutic agent are
administered in
combination, either or both are provided in amounts or doses that are less
that those used
when the agent or agents are administered alone, i.e., when they are not
administered in
combination, either physically or in the course of treatment of a wound. Such
lesser amounts
39
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
of agents administered are typically from about one-twentieth to about one-
tenth the amount
or amounts of the agent when administered alone, and may be about one-eighth
the amount,
about one-sixth the amount, about one-fifth the amount, about one-fourth the
amount, about
one-third the amount, and about one-half the amount when administered alone.
Preferably,
the agents are administered sequentially within at least about one-half hour
of each other.
The agents may also be administered with about one hour of each other, with
about one day
to about one week of each other, or as otherwise deemed appropriate.
[00138] The agents of the invention of the may be administered to a subject in
need of
treatment, such as a subject with any of the diseases or conditions mentioned
herein. The
condition of the subject can thus be improved. An anti-cadherin agent may thus
be used in
the treatment of the subject's body by therapy. They may be used in the
manufacture of a
medicament to treat any of the conditions mentioned herein.
[00139] The anti-cadherin agents of the invention are preferably used in the
various
compositions and methods of the invention in a substantially isolated form. It
will be
understood that the product may be mixed with carriers or diluents that will
not interfere with
the intended purpose of the product and still be regarded as substantially
isolated. A product
of the invention may also be in a substantially purified form, in which case
it will generally
comprise about 80%, 85%, or 90%, e.g. at least about 95%, at least about 98%
or at least
about 99% of the polynucleotide (or other anti-cadherin agent) or dry mass of
the preparation.
[00140] Depending on the intended route of administration, the pharmaceutical
products, pharmaceutical compositions, combined preparations and medicaments
of the
invention may, for example, take the form of solutions, suspensions,
instillations, salves,
creams, gels, foams, ointments, emulsions, lotions, paints, sustained release
formulations, or
powders, and typically contain about 0.1 %-95% of active ingredient(s),
preferably about
0.2%-70%. Other suitable formulations include pluronic gel-based formulations,
carboxymethylcellulose(CMC)-based formulations, and
hyroxypropylmethylcellulose(HPMC)-based formulations. Suitable formulations
including
pluronic gel, have for example about 10 to about 15 percent, about 15-20
percent, about 20-
25 percent, and about 25-30 percent, suitably about 22 percent, pluronic gel.
Other useful
formulations include slow or delayed release preparations and instillations.
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00141] Gels or jellies may be produced using a suitable gelling agent
including, but not
limited to, gelatin, tragacanth, alginate, or a cellulose derivative and may
include glycerol as
a humectant, emollient, and preservative. Ointments are semi-solid
preparations that consist
of the active ingredient incorporated into a fatty, waxy, or synthetic base.
Examples of
suitable creams include, but are not limited to, water-in-oil and oil-in-water
emulsions.
Water-in-oil creams may be formulated by using a suitable emulsifying agent
with properties
similar, but not limited, to those of the fatty alcohols such as cetyl alcohol
or cetostearyl
alcohol and to emulsifying wax. Oil-in-water creams may be formulated using an
emulsifying agent such as cetomacrogol emulsifying wax. Suitable properties
include the
ability to modify the viscosity of the emulsion and both physical and chemical
stability over a
wide range of pH. The water soluble or miscible cream base may contain a
preservative
system and may also be buffered to maintain an acceptable physiological pH.
[00142] Foam preparations may be formulated to be delivered from a pressurized
aerosol canister, via a suitable applicator, using inert propellants. Suitable
excipients for the
formulation of the foam base include, but are not limited to, propylene
glycol, emulsifying
wax, cetyl alcohol, and glyceryl stearate. Potential preservatives include
methylparaben and
propylparaben.
[00143] Preferably the agents of the invention are combined with a
pharmaceutically
acceptable carrier or diluent to produce a pharmaceutical composition.
Suitable carriers and
diluents include isotonic saline solutions, for example phosphate-buffered
saline. Suitable
diluents and excipients also include, for example, water, saline, dextrose,
glycerol, or the like,
and combinations thereof In addition, if desired substances such as wetting or
emulsifying
agents, stabilizing, or ph buffering agents may also be present.
[00144] The term "pharmaceutically acceptable carrier" refers to any
pharmaceutical
carrier that does not itself induce the production of antibodies harmful to
the individual
receiving the composition, and which can be administered without undue
toxicity. Suitable
carriers can be large, slowly metabolized macromolecules such as proteins,
polysaccharides,
polylactic acids, polyglycolic acids, polymeric amino acids, and amino acid
copolymers.
[00145] Pharmaceutically acceptable salts can also be present, e.g., mineral
acid salts
such as hydrochlorides, hydrobromides, phosphates, sulfates, and the like; and
the salts of
organic acids such as acetates, propionates, malonates, benzoates, and the
like.
41
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00146] Suitable carrier materials include any carrier or vehicle commonly
used as a
base for creams, lotions, gels, emulsions, lotions, or paints for topical
administration.
Examples include emulsifying agents, inert carriers including hydrocarbon
bases, emulsifying
bases, non-toxic solvents, or water-soluble bases. Particularly suitable
examples include
pluronics, HPMC, CMC and other cellulose-based ingredients, lanolin, hard
paraffin, liquid
paraffin, soft yellow paraffin or soft white paraffin, white beeswax, yellow
beeswax,
cetostearyl alcohol, cetyl alcohol, dimethicones, emulsifying waxes, isopropyl
myristate,
microcrystalline wax, oleyl alcohol, honey (including manuka honey),and
stearyl alcohol.
[00147] Preferably, the pharmaceutically acceptable carrier or vehicle is a
gel, suitably
a nonionic polyoxyethylene-polyoxypropylene copolymer gel, for example, a
Pluronic gel,
preferably Pluronic F-127 (BASF Corp.). This gel is particularly preferred as
it is a liquid at
low temperatures but rapidly sets at physiological temperatures, which
confines the release of
the agent to the site of application or immediately adjacent that site.
Pharmaceutical carriers
also include liposomes, nanosomes, and the like.
[00148] An auxiliary agent such as casein, gelatin, albumin, glue, sodium
alginate,
carboxymethylcellulose, methylcellulose, hydroxyethylcellulose, or polyvinyl
alcohol may
also be included in the formulation of the invention.
[00149] Other suitable formulations include pluronic gel-based formulations,
carboxymethylcellulose(CMC)-based formulations, and
hyroxypropylmethylcellulose(HPMC)-based formulations. The composition may be
formulated for any desired form of delivery, including topical, instillation,
parenteral,
intramuscular, subcutaneous, or transdermal administration. Other useful
formulations
include slow or delayed release preparations.
[00150] Transdermal delivery can be carried out by methods known in the art or
later
discovered, including, for example, methods directed to 1) the use of chemical
penetration
enhancers or skin enhancers; 2) liposome-mediated delivery; 3) iontophoresis;
4)
electroporation; 5) sonophoresis; 6) mechanical (e.g., microporation) devices.
Exemplary
methods suitable for transdermal delivery of the agents disclosed herein can
include, for
example, methods directed to enhancing the transport of material across the
skin pores by
increasing the rate of transport across existing pores or by amplifying the
number of available
skin pores through the creation of artificial pores.
42
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00151] Transdermal delivery can be carried out by the use of chemical or
penetration
enhancers, including for example, an pharmaceutically acceptable oil of
vegetable, nut,
synthetic or animal origin including emu oil, ethoxylated oil, PEG, linoleic
acid, ethanol, 1-
methanol, and/or agents which delipidize the stratum comeum. Suitable oils
include
meadowfoam oil, castor oil, jojoba oil, corn oil, sunflower oil, sesame oil,
and emu oil, all of
which may be optionally ethoxylated. Exemplars include those as described in
US patent
nos. 7291591, 7201919, 7052715, 7033998, 6946144; 6951658, 6759056, 6720001,
6224853, 5695779, and 6750291. In addition, transdermal patches can also be
adapted for
delivery of dry powder or lyophilized drugs, and exemplars include those
described in U.S.
nat. no. 5,983,135.
[00152] Transdermal delivery can be carried out by liposome mediated delivery
methods (e.g., delivery facilitated by application of lipophilic membrane
active agents).
Suitable exemplars may include those described in US patent nos. 5910306,
5718914, and
5064655.
[00153] Transdermal delivery systems can also be employed in conjunction with
a wide
variety of iontophoresis or electrotransport systems. Illustrative
electrotransport drug delivery
systems are disclosed in U.S. US patent nos. 5,147,296, 5,080,646, 5,169,382,
and 5,169383.
[00154] The term "electrotransport" refers, in general, to the passage of a
beneficial
agent, e.g., a drug or drug precursor, through a body surface such as skin,
mucous
membranes, nails, and the like. The transport of the agent is induced or
enhanced by the
application of an electrical potential, which results in the application of
electric current,
which delivers or enhances delivery of the agent, or, for "reverse"
electrotransport, samples
or enhances sampling of the agent. The electrotransport of the agents into or
out of the human
body may be achieved in various manners.
[00155] Transdermal delivery can be carried out by iontophoretic methods
(e.g.,
delivery facilitated by application of low level electrical field to the skin
over time). Suitable
exemplars may include those described in US patent nos. 6731987, 6391015,
6553255,
4940456, 5681580, and 6248349.
[00156] Also, transdermal delivery can be carried out by electroporation
methods (e.g.,
delivery facilitated by brief application of high voltage pulse to create
transient pores in the
43
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
skin). Suitable exemplars may include US patent nos. 7008637, 6706032,
6692456, 6587705,
6512950, 6041253, 5968006, and 5749847.
[00157] Transdermal delivery can be carried out by sonophoresis methods (e.g.,
delivery facilitated by application of pulses of low frequency ultrasound to
increase skin
permeability). Suitable exemplars may include US patent nos. 7232431, 7004933,
6842641,
6868286, 6712805, 6575956, 6491657, 6487447, 623499, and 6190315.
[00158] Transdermal delivery can be carried out by methods comprising the use
of
mechanical devices and/or creation of artificial micropores or microchannels
(e.g.,
microprojections) by inducing mechanical alterations or disruptions in the
structural
elements, thermal stability properties, membrane fluidity and integrity of the
dermal
architecture and substructures. Suitable exemplars may include MicroPor (Altea
Therapeutics), MacroFlux (Alza Corporation), as well as those as described in
US patent nos.
6893655, 6730318, 5484604, 5362308, 5320850, and 5279544, and US re-
examination
certificate RE35474.
[00159] Other suitable formulations are are formulations that may be inhaled.
[00160] Where the anti-cadherin agent (and/or other therapeutic agent(s), if
any) is a
nucleic acid, such as a polynucleotide, uptake of nucleic acids by mammalian
cells is
enhanced by several known transfection techniques for example those including
the use of
transfection agents. Such techniques may be used with certain anti-connexin
agents,
including polynucleotides. The formulation that is administered may contain
such
transfection agents. Examples of these agents include cationic agents (for
example calcium
phosphate and DEAE-dextran) and lipofectants (for example lipofectamTM and
transfectamTm), and surfactants.
[00161] Where the anti-cadherin agent (and/or other therapeutic agent(s), if
any)
comprises a polynucleotide, conveniently, the formulation further includes a
surfactant to
assist with polynucleotide cell penetration or the formulation may contain any
suitable
loading agent. Any suitable non-toxic surfactant may be included, such as
DMSO.
Alternatively a transdermal penetration agent such as urea may be included.
44
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00162] The effective dose for a given subject or condition preferably lies
within the
dose that is therapeutically effective for at least 50% of the population, and
that exhibits little
or no toxicity at this level.
[00163] The effective dosage of each of the anti-cadherin agent (and/or other
therapeutic agent(s), if any) employed in the methods and compositions of the
invention may
vary depending on a number of factors including the particular anti-connexin
agent or agents
employed, the combinational partner (if any), the mode of administration, the
frequency of
administration, the condition being treated, the severity of the condition
being treated, the
route of administration, the needs of a patient sub-population to be treated
or the needs of the
individual patient which different needs can be due to age, sex, body weight,
relevant medical
condition specific to the patient.
[00164] The dose at which an anti-cadherin agent (and/or other therapeutic
agent(s), if
any) is administered to a patient will depend upon a variety of factors such
as the age, weight
and general condition of the patient, the condition that is being treated, and
the particular
anti-connexin agent that is being administered.
[00165] A suitable therapeutically effective dose of an anti-cadherin agent
(and/or other
therapeutic agent(s), if any) may be from about 0.001 to about 1 mg/kg body
weight such as
about 0.01 to about 0.4 mg/kg body weight. A suitable dose may however be from
about
0.001 to about 0.1 mg/kg body weight such as about 0.01 to about 0.050 mg/kg
body weight.
[00166] Therapeutically effective doses of anti-cadherin agent(s) (and/or
other
therapeutic agent(s), if any) from about 1 to 100, 100-200, 100- or 200-300,
100- or 200- or
300-400, and 100- or 200- or 300- or 400-500 micrograms are appropriate. Doses
from about
1-1000 micrograms are also appropriate. Doses up to 2 milligrams may also be
used. Doses
are adjusted appropriately when the anti-cadherin agent(s) (and/or other
therapeutic agent(s),
if any) are provided in the form of a dressing, typically upward to maintain
the desired total
dose administration.
[00167] Alternatively, in the case of anti-cadherin oligonucleotides or anti-
cadherin
proteins or peptides (and/or other therapeutic agent(s), if any), the dosage
of each of the
agents in the compositions may be determined by reference to the composition's
concentration relative to the size, length, depth, area, or volume of the area
to which it will be
applied. For example, in certain topical applications, dosing of the
pharmaceutical
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
compositions may be calculated based on mass (e.g. grams) of or the
concentration in a
pharmaceutical composition (e.g. ug/u1) per length, depth, area, or volume of
the area of
application. Useful doses range from about 1 to about 10 micrograms per square
centimeter
of wound size. Certain doses will be about 1-2, about 1-5, about 2-4, about 5-
7, and about 8-
micrograms per square centimeter of wound size. Other useful doses are greater
than
about 10 micrograms per square centimeter of wound size, including at least
about 15
micrograms per square centimeter of wound size, at least about 20 micrograms
per square
centimeter of wound size, at least about 25 micrograms per square centimeter
of wound size,
about 30 micrograms per square centimeter of wound size, at least about 35
micrograms per
square centimeter of wound size, at least about 40 micrograms per square
centimeter of
wound size, at least about 50 micrograms per square centimeter of wound size,
and at least
about 100 to at least about 150 micrograms per square centimeter of wound
size. Other doses
include about 150-200 micrograms per square centimeter, about 200-250
micrograms per
square centimeter, about 250-300 micrograms per square centimeter, about 300-
350
micrograms per square centimeter, about 350-400 micrograms per square
centimeter, and
about 400-500 micrograms per square centimeter, and 500-1000 micrograms per
square
centimeter, and at least about 600-1000 micrograms per square centimeter.
[00168] In certain embodiments, the anti-cadherin agent composition (and/or
other
therapeutic agent composition(s), if any) may be applied at about 0.01
micromolar ( M) or
0.05 uM to about 200 uM, or up to 300 uM or up to 400, 500, 600, 700, 800, 900
uM or up
to 1000 uM or up to 2000 uM or up to 3200 uM or more final concentration at
the treatment
site and/or adjacent to the treatment site, and any doses and dose ranges
within these dose
numbers. Preferably, antisense polynucleotide compositions are applied at
about 0.05 uM to
about 100 uM or more final concentration, more preferably, the anti-cadherin
polynucleotide
composition is applied at about 1.0 uM to about 50 uM final concentration, and
more
preferably, at about 5-10 uM to about 30-50 uM final concentration.
Additionally, the
combined anti-cadherin agent composition is applied at about 8 uM to about 20
uM final
concentration, and alternatively the anti-cadherin agent composition is
applied at about 10
uM to about 20 uM final concentration, or at about 10 to about 15 uM final
concentration. In
certain other embodiments, the anti-cadherin agent composition is applied at
about 10 uM
final concentration. In yet another embodiment, the anti-cadherin agent
composition is
applied at about 1-15 uM final concentration. In other embodiements, the anti-
cadherin agent
is applied at about a 20 uM, 30 uM, 40 uM, 50 uM, 60 uM, 70 uM, 80 uM, 90 uM,
100
46
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
p.M., 10-200 p.M, 200-300 p.M, 300-400 p.M, 400-500 !LIM, 500-600 !LIM, 600-
700 !LIM, 700-
800 !LIM, 800-900 !LIM, 900-1000 or 1000-1500 [tM , or 1500 litM ¨ 2000 litM
or 2000 litM -
3000 litM or greater.
[00169] Anti-cadherin dose amounts include, for example, about 0.1-1, 1-2, 2-
3, 3-4, or
4-5 micrograms (pg), from about 5 to about 10 pg, from about 10 to about 15
pg, from about
15 to about 20 lag, from about 20 to about 30 pg, from about 30 to about 40
pg, from about
40 to about 50 lag, from about 50 to about 75 pg, from about 75 to about 100
pg, from about
100 pg to about 250 pg, and from 250 lag to about 500 pg. Dose amounts from
0.5 to about
1.0 milligrams or more or also provided, as noted above. Dose volumes will
depend on the
size of the site to be treated, and may range, for example, from about 25-100
pt to about
100-200 pt, from about 200-500 pt to about 500-1000 pt. Milliliter doses are
also
appropriate for larger treatment sites.
[00170] Still other dosage levels between about 1 nanogram (ng)/kg and about 1
mg/kg
body weight per day of each of the agents described herein. In certain
embodiments, the
dosage of each of the subject compounds will generally be in the range of
about 1 ng to about
1 microgram per kg body weight, about 1 ng to about 0.1 microgram per kg body
weight,
about 1 ng to about 10 ng per kg body weight, about 10 ng to about 0.1
microgram per kg
body weight, about 0.1 microgram to about 1 microgram per kg body weight,
about 20 ng to
about 100 ng per kg body weight, about 0.001 mg to about 0.01 mg per kg body
weight,
about 0.01 mg to about 0.1 mg per kg body weight, or about 0.1 mg to about 1
mg per kg
body weight. In certain embodiments, the dosage of each of the subject
compounds will
generally be in the range of about 0.001 mg to about 0.01 mg per kg body
weight, about 0.01
mg to about 0.1 mg per kg body weight, about 0.1 mg to about 1 mg per kg body
weight. If
more than one anti-cadherin agent is used, the dosage of each anti-cadherin
agent need not be
in the same range as the other. For example, the dosage of one anti-cadherin
agent may be
between about 0.01 mg to about 10 mg per kg body weight, and the dosage of
another anti-
cadherin (or other therapeutic agent) may be between about 0.1 mg to about 1
mg per kg
body weight.
[00171] All doses and dose ranges referenced herein are applicable, for
example, to
polynucleotide therapeutic, including anti-cadherin agents that comprise
oligonucleotides.
These dose ranges are also applicable, for example, to therapeutic agents,
including anti-
47
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
cadherin agents, that comprise proteins and peptides, as well as mimetic
peptides and
peptidomimetics.
[00172] Conveniently, an anti-cadherin agent is administered in a sufficient
amount to
downregulate expression of a cadherin protein, or to modulate adherens
junction formation or
stability for at least about 0.5 to 1 hour, at least about 1-2 hours, at least
about 2-4 hours, at
least about 4-6 hours, at least about 6-8 hours, at least about 8-10 hours, at
least about 12
hours, or at least about 24 hours post-administration.
[00173] The dosage of each of the anti-cadherin agent(s) in the compositions
and
methods of the subject invention may also be determined by reference to the
concentration of
the composition relative to the size, length, depth, area or volume of the
area to which it will
be applied. For example, in certain topical and other applications, e.g.,
instillation, dosing of
the pharmaceutical compositions may be calculated based on mass (e.g.
micrograms) of or
the concentration in a pharmaceutical composition (e.g. WA per length, depth,
area, or
volume of the area of application.
[00174] The same dose amounts and frequencies and administrations, described
above
and below herein, are useful for anti-ZO-1 agents.
[00175] As noted herein, the doses of an anti-cadherin polynucleotide,
peptide, or
peptidomimetic administered in combination, or other anti-cadherin agents
(and/or other
therapeutic agents) administered in combination with either or both, can be
adjusted down
from the doses administered when given alone. The combined use of several
agents may
reduce the required dosage for any individual agent because the onset and
duration of effect
of the different agents may be complementary. In a preferred embodiment, the
combined use
of two or more anti-cadherin agents has an additive, synergistic, or super-
additive effect. In
some cases, the combination of one or more anti-cadherin agents and/or one or
more other
therapeutic agents in combination with either or both, have an additive
effect. In other cases,
the combination can have greater-than-additive effect. Such an effect is
referred to herein as
a "supra-additive" effect, and may be due to synergistic or potentiated
interaction.
[00176] The term "supra-additive promotion of wound healing" refers to a mean
wound
healing produced by administration of a combination of one or more anti-
cadherin agents
with one or more therapeutic agents administered in combination with either or
both, is
statistically significantly higher than the sum of the decrease in adhesion
formation by the
48
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
individual administration of either of the agents alone. Whether the result is
"statistically
significantly higher" than the expected additive value of the individual
compounds may be
determined by a variety of statistical methods as described herein and/or
known by one of
ordinary skill in the art. The term "synergistic" refers to a type of supra-
additive inhibition in
which, for example, both an anti-cadherin polynucleotide and anti-cadherin
peptide or
peptidomimetic, or other anti-cadherin agents administered in combination with
either or
both, individually have the ability to prevent or decrease adhesion formation,
for example.
The term "potentiated" refers to type of supra-additive effect in which one of
the anti-
cadherin polynucleotide(s), peptide(s), or peptidomimetic(s), or other
therapeutic agent(s)
administered in combination individually has the increased ability to prevent
or decrease
adhesion formation, for example.
[00177] In general, potentiation may be assessed by determining whether the
combination treatment produces a mean decrease, by way of example, in adhesion
formation
in a treatment group that is statistically significantly supra-additive when
compared to the
sum of the mean decrease in adhesion formation produced by the individual
treatments in
their treatment groups respectively. The mean decrease in adhesion formation,
for example,
may be calculated as the difference between control group and treatment group
mean
decrease in adhesion formation. The fractional decrease in adhesion formation,
for example,
"fraction affected" (Fa), may be calculated by dividing the treatment group
mean decrease in
adhesion formation by control group mean decrease in adhesion formation.
Testing for
statistically significant potentiation requires the calculation of Fa for each
treatment group.
The expected additive Fa for a combination treatment may be taken to be the
sum of mean
Fas from groups receiving either element of the combination. The Two-Tailed
One-Sample
T-Test, for example, may be used to evaluate how likely it is that the result
obtained by the
experiment is due to chance alone, as measured by the p-value. A value of less
than .05 is
considered statistically significant, that is, not likely to be due to chance
alone. Thus, Fa for
the combination treatment group must be statistically significantly higher
than the expected
additive Fa for the single element treatment groups to deem the combination as
resulting in a
potentiated supra-additive effect.
[00178] Whether a synergistic effect results from a combination treatment may
be
evaluated by the median-effect/combination-index isobologram method (Chou, T.,
and
Talalay, P. (1984) Ad. Enzyme Reg. 22:27-55). In this method, combination
index (CI)
49
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
values are calculated for different dose-effect levels based on parameters
derived from
median-effect plots of an anti-osteopontin or anti-connexin agent alone, for
example, the one
or more agents alone, and the combination of the two at fixed molar ratios. CI
values of & lt;
1 indicate synergy, CI-1 indicates an additive effect, and CP1 indicates an
antagonistic effect.
This analysis may be performed using computer software tools, such as
CalcuSyn, Windows
Software for Dose Effect Analysis (Biosoft(D, Cambridge UK).
[00179] Any method known or later developed in the art for analyzing whether a
supra-
additive effect exists for a combination therapy is contemplated for use in
screening for
suitable anti-cadherin agents for use in combination with other anti-cadherin
or other
therapeutic agents.
[00180] In another preferred embodiment, the combined use of one or more anti-
cadherin agents, particularly anti-cadherin polynucleotides, and one or more
anti-cadherin
peptides or peptidomimetics reduces the effective dose of any such agent
compared to the
effective dose when said agent administered alone. In certain embodiments, the
effective
dose of the agent when used in combination is about 1/15 to about 1/2, about
1/10 to about
1/3, about 1/8 to about 1/6, about 1/5, about 1/4, about 1/3 or about 1/2 the
dose of the agent
when used alone.
[00181] In another preferred embodiment, the combined use of one or more anti-
cadherin and one or more anti-cadherin peptides or peptidomimetics, or other
therapeutic
agents in combination with either or both, reduces the frequency in which said
agent is
administered compared to the frequency when said agent is administered alone.
Thus, these
combinations allow the use of lower and/or fewer doses of each agent than
previously
required to achieve desired therapeutic goals.
[00182] The doses may be administered in single or divided applications. The
doses
may be administered once, or application may be repeated. Typically,
application will be
repeated weekly until, for example, wound healing is promoted, or a repeat
application may
be made in the event that, for example, wound healing slows or is stalled.
Doses may be
applied 3-7 days apart, or more. In the case of a chronic wound, for example,
repeat
applications may be made, for example, weekly, or bi-weekly, or monthly or in
other
frequency for example if and when, for example, wound healing slows or is
stalled. For
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
some indications, such as certain ocular uses, more frequent dosing, up to
hourly may
employed.
[00183] In combination therapies, the anti-cadherin agent(s), alone or in
conjunction
with one or more therapeutic agents, can be administered by the same or
different routes.
The various agents can be administered separately at different times during
the course of
therapy, or concurrently in divided or single combination forms.
[00184] In some combination therapy embodiments, the anti-cadherin agent or
anti_ZO-
1 agent is administered in one composition and the second therapeutic agent,
be it a second
anti-cadherin agent, an anti-connexin agent, and/or an anti-osteopontin agent,
is administered
in a second composition. In some of these embodiments, the first composition
is
administered before the second composition. In other embodiments, the first
composition is
administered after the second composition. In still other embodiments, the
first composition
is administered before and after the second composition. In yet other
embodiments, the
second composition is administered before and after the first composition. In
further such
embodiments, the first composition is administered about the same time as the
second
composition.
[00185] Preferably one or more anti-cadherin agents and/or anti_ZO-1 agents is
delivered by topical administration (peripherally or directly to a site),
including but not
limited to topical administration using solid supports (such as dressings and
other matrices)
and medicinal formulations (such as gels, mixtures, suspensions and
ointments). In one
embodiment, the solid support comprises a biocompatible membrane or insertion
into a
treatment site. In another embodiment, the solid support comprises a dressing
or matrix. In
one embodiment of the invention, the solid support composition may be a slow
release solid
support composition in which the anti-cadherin agent, alone or in admixture or
combination
with one or more additional therapeutic agents, is dispersed in a slow release
solid matrix
such as a matrix of alginate, collagen, or a synthetic bioabsorbable polymer.
Preferably, the
solid support composition is sterile or low bio-burden. In one embodiment, a
wash solution
comprising two or more anti-connexin agents can be used.
[00186] The delivery of a formulation of the invention comprising one or more
active
ingredients, over a period of time, in some instances for about 1-2 hours,
about 2-4 hours,
51
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
about 4-6 hours, about 6-8, or about 24 hours or longer, may be a particular
advantage in
more severe injuries or conditions.
[00187] While the delivery period will be dependent upon both the site at
which the
cadherin or cadherin complex modulation is to be induced and the therapeutic
effect which is
desired, continuous or slow-release delivery for about 0.5-1 hour, about 1-2
hours, about 2-4
hours, about 4-6 hours, about 6-8, or about 24 hours or longer is provided. In
accordance
with the present invention, this is achieved by inclusion of one or more anti-
cadherin agent
species, alone or in combination, in a formulation together with a
pharmaceutically
acceptable carrier or vehicle, particularly in the form of a formulation for
continuous or slow-
release administration.
[00188] As noted, the one or more agents of the invention may be administered
before,
during, or immediately following wounding, for example, or following a
procedure likely or
suspected to result in a scar, an adhesion, or fibrosis, for example, or
within about 180, about
120, about 90, about 60, or about 30 days, but preferably within about 10,
about 9, about 8,
about 7, about 6, about 5, about 4, about 3, or about 2 days or less, and most
preferably
within about 24, about 12, about 10, about 9, about 8, about 7, about 6, about
5, about 4,
about 3, about 2 hours or within about 60, about 45, about 30, about 15, about
10, about 5,
about 4, about 3, about 2, about 1 minute following wounding or following a
procedure likely
or suspected to result in an adhesion, for example. The one or more agents of
the invention
may also be administered before and/or during a procedure likely or suspected
to result in an
adhesion, for example.
[00189] The agents and agent combinations of the invention can be administered
in any
manner that achieves a desired result. Preferred methods include peritubular
administration
(either direct application at the time of surgery or with endoscopic,
ultrasound, CT, MRI, or
fluoroscopic guidance); "coating" the surgical implant; and placement of a
drug-eluting
polymeric implant at the surgical site. In a preferred embodiment, 0.5% to 20%
anti-cadherin
agent(s) by weight is loaded into a polymeric carrier (as described in the
following examples)
and applied to the peritubular (mesenteric) surface as a "paste", "film", or
"wrap" which
releases the drug over a period of time such that the incidence of surgical
adhesions is
reduced. During endoscopic procedures, the polymer preparation may be applied
as a
"spray", via delivery ports in the endoscope, to the mesentery of the
abdominal and pelvic
organs manipulated during the operation. In a particularly preferred
embodiment, the
52
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
peritubular composition is about 0.1% to about 5% active ingredient by weight.
In another
preferred embodiment, a polymeric coating containing about 0.1% to about 20%
or more of
active agent(s) is applied to the surface of the surgical implant (e.g.,
breast implant, artificial
joint, vascular graft, etc.) to prevent encapsulation/inappropriate scarring,
for example, in the
vicinity of the implant. In yet another preferred embodiment, a polymeric
implant containing
about 0.01% to about 20% or more of active agent or agents by weight is
applied directly to
the surgical site (e.g., directly into the sinus cavity, chest cavity,
abdominal cavity, or at the
operative site during neurosurgery) such that adhesion formation, for example,
is prevented
or reduced. In one embodiment, one or more active agents can be administered
via
fluoroscopically guided intra-articular injection.
[00190] In another embodiment, lavage fluid containing about 1 to about 100
pg/cm2
(preferably about 10 to about 50 iLig /cm2) of an anti-cadherin agent(s),
would be used at the
time of or immediately following surgery and administered during surgery or
intraperitoneally, by a physician. In all of the embodiments, other anti-
cadherin agents and/or
anti_ZO-1 agents, alone or in combination with other therapeutic agents, would
be
administered at equivalent doses adjusted for potency and tolerability of the
agent.
[00191] The routes of administration and dosages described herein are intended
only as
a guide since a skilled physician will determine the optimum route of
administration and
dosage for any particular patient and condition.
[00192] Any of the agents and methods of treating a subject having a disease,
disorder
or condition referenced or described herein and treating subjects before or
following a
surgical procedure may utilize the administration of any of the doses, dosage
forms,
formulations, and/or compositions herein described.
Dressings and Matrices
[00193] In one aspect, one or more active agents are provided in the form of a
dressing
or matrix. In certain embodiments, the one or more agents of the invention are
provided in
the form of a liquid, semi solid or solid composition for application
directly, or the
composition is applied to the surface of, or incorporated into, a solid
contacting layer such as
a dressing gauze or matrix. The dressing composition may be provided for
example, in the
form of a fluid or a gel. One or more active agents may be provided in
combination with
conventional pharmaceutical excipients for topical application. Suitable
carriers include:
53
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Pluronic gels, Poloxamer gels, Hydrogels containing cellulose derivatives,
including
hydroxyethyl cellulose, hydroxymethyl cellulose, carboxymethyl cellulose,
hydroxypropylmethyl cellulose and mixtures thereof; and hydrogels containing
polyacrylic
acid (Carbopols). Suitable carriers also include creams/ointments used for
topical
pharmaceutical preparations, e.g., creams based on cetomacrogol emulsifying
ointment. The
above carriers may include alginate (as a thickener or stimulant),
preservatives such as benzyl
alcohol, buffers to control pH such as disodium hydrogen phosphate/sodium
dihydrogen
phosphate, agents to adjust osmolarity such as sodium chloride, and
stabilizers such as
EDTA.
[00194] In addition to the biological matrices previously mentioned, suitable
dressings
or matrices may include, for example, the following with one or more anti-
cadherin agents
(or other active agents to be administered alone or in combination therewith):
[00195] 1) Absoiptives: suitable absomtives may include, for example,
absorptive
dressings, which can provide, for example, a semi-adherent quality or a non-
adherent layer,
combined with highly absorptive layers of fibers, such as for example,
cellulose, cotton or
rayon. Alternatively, absorptives may be used as a primary or secondary
dressing.
[00196] 2) Alginates: suitable alginates include, for example, dressings that
are non-
woven, non-adhesive pads and ribbons composed of natural polysaccharide fibers
or xerogel
derived from seaweed. Suitable alginates dressings may, for example, form a
moist gel
through a process of ion exchange upon contact with exudate. In certain
embodiments,
alginate dressings are designed to be soft and conformable, easy to pack, tuck
or apply over
irregular-shaped areas. In certain embodiments, alginate dressings may be used
with a
second dressing.
[00197] 3) Antimicrobial Dressings: suitable antimicrobial dressings may
include, for
example, dressings that can facilitate delivery of bioactive agents, such as,
for example, silver
and polyhexamethylene biguanide (PHMB), to maintain efficacy against
infection, where this
is needed or desirable. In certain embodiments, suitable antimicrobial
dressings may be
available as for example, as sponges, impregnated woven gauzes, film
dressings, absorptive
products, island dressings, nylon fabric, non-adherent barriers, or a
combination of materials.
[00198] 4) Biological & Biosynthetics: suitable biological dressings or
biosynthetic
dressings may include, for example, gels, solutions or semi-permeable sheets
derived from a
54
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
natural source, e.g., pigs or cows. In certain embodiments, a gel or solution
is applied to the
treatment site and covered with a dressing for barrier protection. In another
embodiment, a
biological-based (e.g., pig intestinal mucosa or bladder tissue) or
biosynthetic-based sheet is
placed in situ which may act as membrane, remaining in place after a single
application, or
the may be biological dressings or biosynthetic dressings may be prepared in
advance to
include one or more, preferably two, anti-cadherin agents.
[00199] 5) Collagens: suitable collagen dressings may include, for example,
gels, pads,
particles, pastes, powders, sheets or solutions derived from for example,
bovine, porcine or
avian sources or other natural sources or donors. In certain embodiments, the
collagen
dressing may interact with treatment site exudate to form a gel. In certain
embodiments,
collagen dressing may be used in combination with a secondary dressing.
[00200] 6) Composites: suitable composite dressings may include, for example,
dressings that combine physically distinct components into a single product to
provide
multiple functions, such as, for example, a bacterial barrier, absorption, and
adhesion. In
certain embodiment, the composite dressings are comprised of, for example,
multiple layers
and incorporate a semi-or non-adherent pad. In certain embodiment, the
composite may also
include for example, an adhesive border of non-woven fabric tape or
transparent film. In
certain other embodiment, the composite dressing may function as for example,
either a
primary or a secondary dressing and in yet another embodiment, the dressing
may be used in
combination with topical pharmaceutical composition.
[00201] 7) Contact Layers: suitable contact layer dressings may include, for
example,
thin, non-adherent sheets placed on an area to protect tissue from for
example, direct contact
with other agents or dressings applied to the treatment site. In certain
embodiments, contact
layers may be deployed to conform to the shape of the area of the treatment
site and are
porous to allow exudate to pass through for absorption by an overlying,
secondary dressing.
In yet another embodiment, the contact layer dressing may be used in
combination with
topical pharmaceutical composition.
[00202] 8) Elastic Bandages: suitable elastic bandages may include, for
example,
dressings that stretch and conform to the body contours. In certain
embodiment, the fabric
composition may include for example, cotton, polyester, rayon, or nylon. In
certain other
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
embodiments, the elastic bandage may for example, provide absorption as a
second layer or
dressing, to hold a cover in place, to apply pressure or to cushion a
treatment site.
[00203] 9) Foams: suitable foam dressings may include, for example, sheets and
other
shapes of foamed polymer solutions (including polyurethane) with small, open
cells capable
of holding fluids. Exemplary foams may be for example, impregnated or layered
in
combination with other materials. In certain embodiment, the absorption
capability may be
adjusted based on the thickness and composition of the foam. In certain other
embodiments,
the area in contact with the treatment site may be non-adhesive for easy
removal. In yet
another embodiment, the foam may be used in combination with an adhesive
border and/or a
transparent film coating that can serve as an anti-infective barrier.
[00204] 10) Gauzes & Non-Woven dressings: suitable gauze dressings and woven
dressings may include, for example, dry woven or non-woven sponges and wraps
with
varying degrees of absorbency. Exemplary fabric composition may include, for
example,
cotton, polyester, or rayon. In certain embodiment, gauzes and non-woven
dressing may be
available sterile or non-sterile in bulk and with or without an adhesive
border. Exemplary
gauze dressings and woven dressings may be used for cleansing, packing and
covering a
variety of treatment sites.
[00205] 11) Hydrocolloids: suitable hydrocolloid dressings may include, for
example,
wafers, powders or pastes composed of gelatin, pectin, or
carboxymethylcellulose. In certain
embodiment, wafers are self-adhering and available with or without an adhesive
border and
in a wide variety of shapes and sizes. Exemplary hydrocolloids are useful on
areas that
require contouring. In certain embodiments, powders and pastes hydrocolloids
may use used
in combination with a secondary dressing.
[00206] 12) Hydrogels (Amorphous): suitable amorphous hydrogel dressings may
include, for example, formulations of water, polymers and other ingredients
with no shape,
designed to donate moisture and to maintain a moist healing environments and
or to rehydrate
the treatment site. In certain embodiment, hydrogels may be used in
combination with a
secondary dressing cover.
[00207] 13) Hydrogels: Impregnated Dressings: suitable impregnated hydrogel
dressings may include, for example, gauzes and non-woven sponges, ropes and
strips
saturated with an amorphous hydrogel. Amorphous hydrogels may include for
example,
56
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
formulations of water, polymers and other ingredients with no shape, designed
to donate
moisture to a dry treatment site and to maintain a moist healing environment.
[00208] 14) Hydrogel Sheets: suitable hydrogel sheets may include for example,
three-
dimensional networks of cross-linked hydrophilic polymers that are insoluble
in water and
interact with aqueous solutions by swelling. Exemplary hydrogels are highly
conformable
and permeable and can absorb varying amounts of drainage, depending on their
composition.
In certain embodiment, the hydrogel is non-adhesive against the treatment site
or treated for
easy removal.
[00209] 15) Impregnated Dressings: suitable impregnated dressings may include,
for
example, gauzes and non-woven sponges, ropes and strips saturated with a
solution, an
emulsion, oil, gel or some other pharmaceutically active compound or carrier
agent, including
for example, saline, oil, zinc salts, petrolatum, xeroform, and scarlet red as
well as the
compounds described herein.
[00210] 16) Silicone Gel Sheets: suitable silicone gel sheet dressings may
include, for
example, soft covers composed of cross-linked polymers reinforced with or
bonded to mesh
or fabric.
[00211] 17) Solutions: suitable liquid dressings may include, for example,
mixtures of
multiprotein material and other elements found in the extracellular matrix. In
certain
embodiment, exemplary solutions may be applied to the treatment site after
debridement and
cleansing and then covered with an absorbent dressing or a nonadherent pad.
[00212] 18) Transparent Films: suitable transparent film dressings may include
polymer membranes of varying thickness coated on one side with an adhesive. In
certain
embodiments, transparent films are impermeable to liquid, water and bacteria
but permeable
to moisture vapor and atmospheric gases. In certain embodiments, the
transparency allows
visualization of the treatment site.
[00213] 19) Fillers: suitable filler dressings may include, for example,
beads, creams,
foams, gels, ointments, pads, pastes, pillows, powders, strands, or other
formulations. In
certain embodiment, fillers are non-adherent and may include a time-released
antimicrobial.
Exemplary fillers may be useful to maintain a moist environment, manage
exudate, and for
57
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
treatment of for example, partial- and full- thickness wounds, infected
wounds, draining
wounds, and deep wounds that require packing.
Wound Treatment
General Aspects
[00214] The present invention is directed to pharmaceutical compositions and
their
methods of use wherein the composition comprises therapeutically effective
amounts of one
or more anti-cadherin agents alone or in combination with one or more
therapeutic agents.
The compositions are useful, for example, in enhancing or promoting healing of
wounds, for
example, including acute wounds and wounds that do not heal at expected rates,
such as
chronic wounds and other wounds that may be slow to heal or refractory to
conventional
wound treatment or wound healing promoting therapies, and other diseases,
disorders and
conditions described herein, including diseases, disorders and conditions
characterized by
inflammation or unwanted inflammation. Chronic wounds are often characterized
by
unwanted inflammation.
[00215] Equally, in instances of other tissue damage (particularly wounds) the
methods
and compositions of the invention are effective in promoting the wound healing
process,
reducing swelling and inflammation, and in minimizing scar formation. These
formations are
useful in treating fibrotic diseases, disorders and conditions and in
treating, reducing the
incidence or severity of or preventing or retarding adhesions, surgical
adhesions and/or
secondary surgical adhesions. The formulations have clear benefit in the
treatment of
wounds, whether the result of external trauma (including burns), internal
trauma, or surgical
intervention, as well as chronic wounds.
Compositions
[00216] Accordingly, in one aspect, the invention provides compositions for
use in
therapeutic wound treatment, which comprises an anti-cadherin agent and/or an
anti_ZO-1
agent. In another aspect, the invention provides compositions for use in
therapeutic wound
treatment, which comprises at least one species of anti-cadherin agent and at
least one other
therapeutic agent, for example, an anti-connexin agent and/or an anti_ZO-1
agent. In a
preferred embodiment, the compositions further comprise a pharmaceutically
acceptable
carrier or vehicle.
58
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00217] In one embodiment, the anti-cadherin agent is selected from a group
consisting
of: an anti-cadherin polynucleotide, an anti-cadherin peptide or
peptidomimetic, an adherens
junction modulator, and a cadherin complex modulator for wound treatment. In
another
embodiment, the anti-ZO-1 agent is selected from a group consisting of: an
anti-ZO-1
polynucleotide, an anti-ZO-1 peptide or peptidomimetic, an adherens junction
modulator, and
a ZO-1 complex modulator for wound treatment.
[00218] In preferred embodiments, the anti-cadherin or anti-ZO-1
polynucleotide is an
antisense polynucleotide. In one preferred form, the composition contains one
or more
antisense polynucleotides to the mRNA of one cadherin protein or one ZO-1
protein only.
Most preferably, this cadherin protein is N-cadherin. In another preferred
form, the
composition comprises an anti-cadherin peptide or peptidomimetic and an
antisense
polynucleotide to the mRNA of a cadherin or ZO-1 protein. Most preferably,
this cadherin is
N-cadherin.
[00219] The compositions may comprise polynucleotides or anti-cadherin
peptides or
peptidomimetics, or other anti-cadherin agents with either or both, that are
directed to more
than one cadherin protein. The compositions may comprise polynucleotides or ZO-
1
peptides or peptidomimetics, or other anti-ZO-1 agents with either or both,
that are directed
to more than one ZO-1 protein. One of the cadherin proteins to which
polynucleotides or
anti-cadherin peptides or other anti-cadherin agents are directed is N-
cadherin. Other
cadherins to which the polynucleotides or anti-cadherin peptides or other anti-
cadherin agents
are directed may include, for example, E-cadherin, P-cadherin, cadherin 11,
cadherin 12, a
protocadherin protein, a desmoglein protein, and a desmocollin protein.
Suitable exemplary
polynucleotides (and ODNs) directed to various connexins are set forth in
Table herein.
Suitable anti-cadherin peptides are also provided herein. Suitable adherens
junction or
cadherin complex modulating agents are also described.
[00220] Accordingly, in one aspect, the invention provides compositions for
use in
treating wounds, including chronic and slow or delayed healing wounds. In
another aspect,
the invention provides compositions for use in treating fibrosis or fibrotic
diseases, disorders,
or conditions. In an alternate aspect, the invention provides compositions for
use in
preventing and/or treating abnormal or excessive scarring and/or excessive
tissue
proliferation and related disorders and conditions. In a further aspect, the
invention provides
compositions and methods for their use in preventing and/or decreasing
adhesions, including
59
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
surgical adhesions. In a further aspect, the invention provides compositions
and methods for
their use in preventing and/or decreasing inflammation.
Kits, Medicaments and Articles of Manufacture
[00221] Optionally, one or more anti-cadherin agents and/or anti-ZO-1 agents,
either
alone or in combination with one or more other therapeutic agents, may also be
used in the
manufacture of the medicament.
[00222] In one aspect, the invention provides a kit comprising one or more
compositions or formulations described. For example, the kit may include a
composition
comprising an effective amount of one or more anti-cadherin agents and/or anti-
ZO-1 agents,
either alone or in combination with one or more other anti-cadherin agent
species and/or anti-
ZO-1 agents and/or other therapeutic agents.
[00223] Articles of manufacture are also provided, comprising a vessel
containing a
composition or formulation of the invention as described herein and
instructions for use for
the treatment of a subject. For example, in another aspect, the invention
includes an article of
manufacture comprising a vessel containing a therapeutically effective amount
of one or
more anti-cadherin agents, either alone or in combination with one or more
other therapeutic
agents, and instructions for use, including use for the treatment of a
subject.
[00224] In one aspect, the invention provides for a kit for treating wounds,
including
chronic and slow or delayed healing wounds. In another aspect, the invention
provides a kit
for treating fibrosis or fibrotic diseases, disorders, or conditions.
According to an alternate
aspect, the invention provides a kit for preventing and/or treating abnormal
or excessive
scarring and/or excessive tissue proliferation and conditions comprising one
or more of the
formulations described. In another aspect, the invention provides a kit for
preventing and/or
decreasing adhesions comprising one or more compositions or formulations
described. In
another aspect, the invention provides a kit for preventing and/or decreasing
inflammation
comprising one or more compositions or formulations described.
[00225] Articles of manufacture are provided for preventing and/or treating
wounds,
including chronic and slow or delayed healing wounds. In another aspect,
articles of
manufacture are provided for preventing and/or treating fibrosis or fibrotic
diseases,
disorders, or conditions. Articles of manufacture are also provided for
preventing and/or
treating abnormal or excessive scarring and/or excessive tissue proliferation
and related
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
disorders and conditions. Additional articles of manufacture are provided for
preventing
and/or decreasing adhesions as described herein. Additional articles of
manufacture are
provided for preventing and/or decreasing inflammation as described herein.
Treatment
[00226] The compositions and formulations of the invention may be used in
conjunction or combination with a composition for promoting the healing of
wounds, for
example, and can also be used to reduce swelling, inflammation, and/or
scarring. The
compositions and formulations of the invention may also be used in conjunction
or
combination with a composition for promoting and/or improving the healing of
acute or
chronic wounds, including slow-healing and delayed healing wounds. In one
aspect, the
wound will be the result of surgery or trauma or underlying medical condition,
e.g., diabetes,
peripheral edema, vasculitis, or cardiovascular disease.
[00227] In one aspect the invention is directed to a method of promoting or
improving
wound healing in a subject, comprising administration a therapeutically
effective amount of
one or more anti-cadherin agents, either alone or in combination with one or
more other
therapeutic agents. In certain embodiments, such administration is effective
to reduce
inflammation, promote cell migration to accelerate wound closure and healing,
and/or to
facilitate epithelial growth and surface recovery. In certain embodiments, the
administration
of one or more compositions of the invention is effective to reduce or prevent
scar formation,
including abnormal scar formation.
[00228] In one aspect the invention is directed to a method of promoting or
improving
wound healing in a subject, comprising administration of one or more anti-
cadherin agents
and/or anti-ZO-1 agents in combination with one or more other therapeutic
agents in an
amount effective to regulate adherens junction and/or cadherin complex
formation and/or
stability at wound or other sites of tissue injury or insult.
[00229] In yet a further aspect, the invention provides a method of decreasing
scar
formation and/or improving scar appearance in a patient who has suffered a
wound, e.g., a
surgical wound (such as in, for example, cosmetic, scar revision, and other
surgeries), which
comprises the step of administering one or more anti-cadherin agents and/or
anti-ZO-1
agents, either alone or in combination with one or more other therapeutic
agents, to said
wound to downregulate expression of one or more cadherin protein(s) and/or ZO-
1 protein(s)
61
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
at and immediately adjacent the site of said wound. Again, the wound may be
the result of
trauma or surgery, for example, with the formulation being applied to the
wound immediately
prior to surgical repair and/or closure thereof As noted herein, in methods to
reduce or
improve scar formation or appearance, or prevent or reduce inflammation, the
anti-cadherin
agent and/or anti-ZO-1 agent is preferably administered in combination with,
or after or prior
to, administration of a suitable amount of another wound healing agent, for
example, an anti-
connexin agent or anti-osteopontin agent.
[00230] In one aspect the invention is directed to a method of reducing,
preventing, or
ameliorating tissue damage (including inflammation damage) in a subject,
comprising
administration of one or more anti-cadherin agents and/or anti-ZO-1 agents,
either alone or in
combination with one or more other therapeutic agents.
[00231] In a further aspect, the invention is directed to a method of reducing
swelling
and/or inflammation, for example as part of treating an acute or chronic wound
and/or tissue
(including tissue subjected to physical trauma) which comprises the step of
administering one
or more anti-cadherin agents and/or anti-ZO-1 agents, either alone or in
combination with one
or more other therapeutic agents, to or proximate to said wound or tissue. In
one embodiment
the wound is the result of physical trauma to tissue, including dermal tissue
(leading, for
example, to a pressure ulcer) and neuronal tissue such as the brain, spinal
cord, or optic
nerve, or skin or eye.
[00232] In one aspect the invention is directed to sustained administration of
one or
more anti-cadherin agents and/or anti-ZO-1 agents, either alone or in
combination with one or
more other therapeutic agents. In one embodiment, the agent or agents are
administered for
at least at least about 0.5 hours, about 1- 24 hours, at least about 2, hours,
at least about 3
hours, at least about 4 hours, at least about 5 hours, at least about 6 hours,
at least about 7
hours, at least about 8 hours, at least about 9 hours, at least about 10
hours, at least about 11
hours, at least about 12 hours or at least about 24 hours. In one embodiment,
cadherin
expression is downregulated over a sustained period of time. In another
embodiment,
cadherin complex and/or adherens junction formation and/or stability is
modulated,
preferably inhibited, partially or completely, over a preferred period of
time. Preferably, such
period of time is at least about 1, 2, 4, 6, 8, 10, 12, or 24 hours. According
to one
embodiment, the wound is a chronic wound. Suitable subjects include a diabetic
subject.
Other subjects include, for example, those with peripheral edema, vasculitis,
or
62
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
cardiovascular disease. Other subjects include, for example, those with venous
disease,
including venous insufficiency, or arterial disease, including arterial
insufficiency.
[00233] In one aspect, the present invention provides a method of treating a
subject
having a wound that comprises sustained administration of an effective amount
of one or
more anti-cadherin agents and/or anti-ZO-1 agents, either alone or in
combination with one or
more other therapeutic agents, to the wound.
[00234] In another aspect, methods for treating a subject having a chronic
wound are
provided. Such methods include administering to the subject one or more anti-
cadherin
agents and/or anti-ZO-1 agents, either alone or in combination with one or
more other
therapeutic agents.
[00235] In one aspect the invention is directed to a method for treatment or
prophylaxis
of a chronic wound comprising administering to a subject in need thereof an
effective amount
of an anti-cadherin agent and/or an anti-ZO-1 agent, either alone or in
combination with one
or more other therapeutic agents. In one embodiment, the chronic wound is a
chronic skin
wound and a composition of the present invention is administered to the skin
or a tissue
associated with the skin of said subject for an effective period of time. A
chronic skin wound
suitable for treatment may, for example, be selected from the group consisting
of pressure
ulcers, diabetic ulcers, venous ulcers, arterial ulcers, vasculitic ulcers,
and mixed ulcers, and
other noted herein. The chronic wound may be an arterial ulcer, which
comprises
ulcerations resulting from complete or partial arterial blockage. The chronic
wound may be a
venous stasis ulcer, which comprises ulcerations resulting from a malfunction
of the venous
valve and the associated vascular disease. The chronic wound may be a trauma-
induced
ulcer. The chronic, slow- or delayed-healing wound may be, for example, dermal
or ocular,
associated with another organ tissue (e.g., kidney, bowel, liver, lung), or in
the CNS.
[00236] When not administered as a fixed combination, preferred combination
therapy
methods include the sequential administration of one or more anti-cadherin
agents and/or
anti-ZO-1 agents and one or more other therapeutic agents. Preferably, the
agents are
administered sequentially within at least about one-half hour of each other.
The agents may
also be administered with about one hour of each other, with about one day to
about one
week of each other, or as otherwise deemed appropriate.
63
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00237] In one embodiment the method for treatment or prophylaxis of a chronic
wound
comprises sustained administration of one or more anti-cadherin agents and/or
anti-ZO-1
agents, either alone or in combination with one or more other therapeutic
agents. In one
embodiment, the composition or compositions are administered in a sustained
release
formulation. In another embodiment, the composition or compositions are
administered for a
sustained period of time. Conveniently, the composition is effective to
decrease cadherin
and/or ZO-1 protein levels, or block or reduce cadherin or ZO-1 complexes and
or adherens
junction formation and/or stability, for at least about 1-2 hours, about 2-4
hours, about 4-6
hours, about 4-8 hours, about 12 hours, about 18 hours, or about 24 hours.
Subjects that may
be treated include diabetic subjects, and patients with other ulcers,
including venous ulcers
and others described herein and known in the art.
[00238] In one aspect the invention is directed to a method of preventing
and/or treating
fibrosis or fibrotic diseases, disorders or conditions in a subject,
comprising administration a
therapeutically effective amount of a composition according to the invention.
In certain
embodiments, the administration is effective to reduce fibrosis. In certain
embodiments, the
administration is effective to prevent or reduce contracture.
[00239] In one aspect the invention is directed to a method of preventing
and/or treating
fibrosis or fibrotic diseases, disorders, or conditions in a subject,
comprising administration
of a therapeutically effective amount of one or more anti-cadherin agents
and/or anti-ZO-1
agents effective to reduce fibrosis. In one embodiment, administration of the
anti-cadherin
agent and/or anti-ZO-1 agent and, optionally, one or more anti-connexin
agents, is effective
to prevent or reduce contracture.
[00240] According to one embodiment of the method, the subject has a disorder
selected from the group consisting of scleroderma, kidney fibrosis (including
diabetic
nephropathy), cardiac fibrosis (e.g. myocardial fibrosis), pulmonary fibrosis
(e.g.,
glomerulosclerosis pulmonary fibrosis, idiopathic pulmonary fibrosis,
silicosis, asbestosis,
interstitial lung disease and fibrotic lung disease, and
chemotherapy/radiation induced
pulmonary fibrosis), oral fibrosis, endomyocardial fibrosis, deltoid fibrosis,
pancreatitis,
inflammatory bowel disease, Crohn's disease, nodular fascilitis, eosinophilic
fasciitis, general
fibrosis syndrome characterized by replacement of normal muscle tissue by
fibrous tissue in
varying degrees, retroperitoneal fibrosis, liver fibrosis, liver cirrhosis,
chronic renal failure;
myelofibrosis (bone marrow fibrosis), drug induced ergotism, glioblastoma in
Li-Fraumeni
64
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
syndrome, sporadic glioblastoma, myleoid leukemia, acute myelogenous leukemia,
myelodysplastic syndrome, myeloproferative syndrome, gynecological cancer,
Kaposi's
sarcoma, Hansen's disease, collagenous colitis and acute fibrosis. According
to this
embodiment, the scleroderma may be morphea, generalized morphea, or linear
scleroderma.
Also according to this embodiment, the kidney fibrosis may be glomerular
sclerosis, renal
tubulointerstitial fibrosis or progressive renal disease. Further to this
embodiment, the
pulmonary fibrosis may be diffuse interstitial pulmonary fibrosis.
[00241] According to another embodiment of the method, the fibrosis is acute
fibrosis.
The acute fibrosis may be in response to various forms of trauma including
accidental
injuries, infections, radiation or chemotherapy treatments.
[00242] According to another embodiment of the method, the fibrosis is chronic
fibrosis. The invention also includes methods for treating and/or preventing,
in whole or in
part, various diseases, disorders and conditions, including, for example,
capsular contracture,
Dupytren's contracture, Volkmann's contracture, Ledderhose's contracture,
Peyronie's
contracture or recurrence thereof, comprising administering a effective amount
of a
composition comprising an anti-cadherin agent, preferably an anti-cadherin
polynucleotide.
In some preferred embodiments, the composition is administered to the site of
the injury
before, at the time of and/or after a release procedure (e.g., forced
manipulation, open release,
arthroscopic release, or debulking of scar) to prevent the recurrence of
scarred and abnormal
tissue and/or further contracture.
[00243] In one aspect the invention is directed to a method of for preventing
and/or
treating abnormal or excessive scarring and/or excessive tissue proliferation
and related
disorders and conditions in a subject, comprising administration a
therapeutically effective
amount of one or more one or more anti-cadherin agents and/or anti-ZO-1
agents, either
alone or in combination with one or more other therapeutic agents. In certain
embodiments,
the administration is effective to reduce abnormal or excessive scarring
and/or excessive
tissue proliferation and related disorders and conditions.
[00244] In one aspect the invention is directed to a method of for preventing
and/or
treating abnormal or excessive scarring and/or excessive tissue proliferation
and related
disorders and conditions in a subject, comprising administration a
therapeutically effective
amount of an anti-cadherin and/or anti-ZO-1 agent. In one embodiment, the anti-
-cadherin
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
and/or anti-ZO-1 agent is effective to reduce abnormal or excessive scarring
and/or excessive
tissue proliferation and related disorders and conditions.
[00245] In one aspect the invention is directed to sustained administration of
an anti-
cadherin and/or anti-ZO-1 agent alone or in combination with one or more other
therapeutic
agents.
[00246] According to one embodiment, the subject has an abnormal scar selected
from
the group consisting of keloid scars, hypertrophic scars, widespread scars,
and atrophic scars.
[00247] According to another embodiment, the subjects to be treated include
those
having experienced trauma, surgical intervention, burns, and other types of
injuries that lead,
or can lead, to abnormal or excessive scarring, as well as excessive scar
formation and other
types of abnormal proliferation of tissue, including keloid scars,
hypertrophic scars,
widespread scars, and atrophic scars.
[00248] In certain embodiments, the anti-cadherin and/or anti-ZO-1 agent(s),
alone or in
combination with one or more other therapeutic agents is administered to
epithelial,
connective, muscle, and nerve tissue or other tissue exposed or wounded during
surgery or as
a result of trauma. In some embodiments, the anti-cadherin agent is
administered topically.
In other embodiments, the anti- cadherin and/or anti-ZO-1 agent is implanted
or instilled or
injected.
[00249] The following examples which will be understood to be provided by way
of
illustration only and not to constitute a limitation on the scope of the
invention.
EXAMPLES
EXAMPLE 1
Oligonucleotides Targeting N-cadherin
[00250] This example describes several candidates for N-cadherin AS ODNs
(antisense
oligodeoxtribonucleotides) and shRNAs (small hairpin RNA molecules).
[00251] The nucleotide sequence of human N-cadherin is known. The nucleotide
sequence of full-length N-cadherin cDNA (Genbank accession number EL733845;
SEQ ID
NO:2) comprises 2,721 nucleotide bases and encodes 906 amino acids, and is as
follows:
66
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
atgtgccgga tagcgggagc gctgcggacc ctgctgccgc tgctggcggc cctgcttcag gcgtctgtag
70
aggcttctgg tgaaatcgca ttatgcaaga ctggatttcc tgaagatgtt tacagtgcag tcttatcgaa
140
ggatgtgcat gaaggacagc ctcttctcaa tgtgaagttt agcaactgca atggaaaaag aaaagtacaa
210
tatgagagca gtgagcctgc agattttaag gtggatgaag atggcatggt gtatgccgtg agaagctttc
280
cactctcttc tgaacatgcc aagttcctga tatatgccca agacaaagag acccaggaaa agtggcaagt
350
ggcagtaaaa ttgagcctga agccaacctt aactgaggag tcagtgaagg agtcagcaga agttgaagaa
420
atagtgttcc caagacaatt cagtaagcac agtggccacc tacaaaggca gaagagagac tgggtcatct
490
ctccaatcaa cttgccagaa aactccaggg gaccttttcc tcaagagctt gtcaggatca ggtctgatag
560
agataaaaac ctttcactgc ggtacagtgt aactgggcca ggagctgacc agcctccaac tggtatcttc
630
attatcaacc ccatctcggg tcagctgtcg gtgacaaagc ccctggatcg cgagcagata gcccggtttc
700
atttgagggc acatgcagta gatattaatg gaaatcaagt ggagaacccc attgacattg tcatcaatgt
770
tattgacatg aatgacaaca gacctgagtt cttacaccag gtttggaatg ggacagttcc tgagggatca
840
aagcctggaa catatgtgat gaccgtaaca gcaattgatg ctgacgatcc caatgccctc aatgggatgt
910
tgaggtacag aatcgtgtct caggctccaa gcaccccttc acccaacatg tttacaatca acaatgagac
980
tggtgacatc atcacagtgg cagctggact tgatcgagaa aaagtgcaac agtatacgtt aataattcaa
1050
gctacagaca tggaaggcaa tcccacatat ggcctttcaa acacagccac ggccgtcatc acagtgacag
1120
atgtcaatga caatcctcca gagtttactg ccatgacgtt ttatggtgaa gttcctgaga acagggtaga
1190
catcatagta gctaatctaa ctgtgaccga taaggatcaa ccccatacac cagcctggaa cgcagtgtac
1260
agaatcagtg gcggagatcc tactggacgg ttcgccatcc agaccgacca aaacagcaac gacgggttag
1330
tcaccgtggt caaaccaatc gactttgaag caaataggat gtttgtcctt actgttgctg cagaaaatca
1400
agtgccatta gccaagggaa ttcagcaccc gcctcagtca actgcaacca tgtctgttac agttattgac
1470
67
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
gtaaatgaaa acccttattt tgcccccaat cctaagatca ttcgccaaga agaagggctt catgccggta
1540
ccatgttgac aacattcact gctcaggacc cagatcgata tatgcagcaa aatattagat acactaaatt
1610
atctgatcct gccaattggc taaaaataga tcctgtgaat ggacaaataa ctacaattgc tgttttggac
1680
cgagaatcac caaatgtgaa aaacaatata tataatgcta ctttccttgc ttctgacaat ggaattcctc
1750
ctatgagtgg aacaggaacg ctgcagatct atttacttga tattaatgac aatgcccctc aagtgttacc
1820
tcaagaggca gagacttgcg aaactccaga ccccaattca attaatatta cagcacttga ttatggcatt
1890
gatccaaatg ctggaccatt tgcttttgat cttcctttat ctccagtgac tattaagaga aattggacca
1960
tcactcggct taatggtgat tttgctcagc ttaatttaaa gataaaattt cttgaagctg gtatctatga
2030
agttcccatc ataatcacag attcgggtaa tcctcccaaa tcaaatattt ccatactgcg cgtgaaggtt
2100
tgccagtgtg actccaacgg ggactgcaca gatgtggaca ggattgtggg tgcggggctt ggcaccggtg
2170
ccatcattgc catcctgctc tgcatcatca tcctgcttat ccttgtgctg atgtttgtgg tatggatgaa
2240
acgccgggat aaagaacgcc aggccaaaca acttttaatt gatccagaag atgatgtaag agataatatt
2310
ttaaaatatg atgaagaagg tggaggagaa gaagaccagg actatgactt gagccagctg cagcagcctg
23 80
acactgtgga gcctgatgcc atcaagcctg tgggaatccg acgaatggat gaaagaccca tccacgctga
2450
gccccagtat ccggtccgat ctgcagcccc acaccctgga gacattgggg acttcattaa tgagggcctt
2520
aaagcggctg acaatgaccc cacagacca ccatatgact ccctgttagt gtttgactat gaaggcagtg
2590
gctccactgc tgggtccttg agctccctta attcctcaag tagtggtggt gagcaggact atgattacct
2660
gaacgactgg gggccacggt tcaagaaactt gctgacatg tatggtggag gtgatgactg a 2721
[00252] Any antisense molecule or shRNA that targets a region suitable to
disrupt N-
cadherin expression will be useful in the practicing the invention. One
suitable approach in
this regard concerns RNA interference (RNAi). As is the case with the
expression of many
other genes, N-cadherin expression can be knocked down in vivo or vitro
through the use
68
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
RNAi. A representative class of molecules that can be used for RNAi are short
hairpin RNAs
(shRNAs). One such anti-N-cadherin shRNA molecule can be constructed as
follows using
the vector termed pSuper-Ncad. This vector is assembled using the following
sense and
antisense oligonucleotides (ONs):
Sense ODN: 5'-
GATCCCCGATGTTTACAGCGCAGTCTTTCAAGAGAAGACTGCGCTGTAAACAT
CTTTTTGGAAA-3' (SEQ ID NO:3)
Antisense ON: 5'-
AGCTTTTCCAAAAAGATGTTTACAGCGCAGTCTCTTGAAAGACTGCGCTGTAA
ACATCGGG-3' (SEQ ID NO:4)
The sense and antisense oligos can be annealed and ligated into a linealized
pSuper vector
(OligoEngine: catalog VEC-PBS-0011). This target sequence corresponds to that
of RNAi
against rat Ncad reported by Fairless, et al. ((2005), Mol. Cell. Neurosci.,
vol. 28:253-263).
As a negative control, an shRNA vector consisting of scrambled target sequence
of Ncad can
be designed to construct a negative control. As an example, the following
sense and
antisense ONs can be used to construct a vector termed pSuper-Ncad-scrambled.
The sense
and antisense sequnces are:
Sense ON: 5'-
GATCCCCGCTATCGCTACGTGTAAGTTTCAAGAGAACTTACACGTAGCGATAG
CTTTTTGGAAA-3' (SEQ ID NO:5)
Antisense ON: 5'-
AGCTTTTCCAAAAAGCTATCGCTACGTGTAAGTTCTCTTGAAACTTACACGTA
GCGATAGCGGG-3' (SEQ ID NO:6)
[00253] Antisense and short hairpin RNAs targeted against human N-cadherin
also
include those that target specific sequences. One such representative target
nucleotide
69
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
sequence in the N-cadherin gene is: 5'-GACTGGATTTCCTGAAGAT-3' (SEQ ID NO:7);
nucleotides 215-233 of human N-cadherin GenBank accession number BC036470,
which are
also bases 99-117 of GenBank accession number EL733845; SEQ ID NO:8, above).
EXAMPLE 2
Targeting Cx43 and N-cadherin, Which are Abnormally Up-regulated in Venous Leg
Ulcers, Influences Migration, Adhesion, and Activation of Rho GTPases
Overview
[00254] Venous leg ulcers can be very hard to heal and represent a significant
medical
need with no effective therapeutic treatment currently available. In the
experiments
described in this Example, in wound edge biopsies from human venous leg ulcers
it was
found a striking up-regulation of dermal N-cadherin (N-cad), Zonular Occludens-
1 (Z0-1),
and the gap junction protein Connexin43 (Cx43) compared to these proteins
levels observed
in intact skin, and in stark contrast to the down-regulation of Cx43
expression seen in acute,
healing wounds. The expression of these proteins was targeted in 3T3
fibroblasts to evaluate
their roles in venous leg ulcers healing. Knockdown of Cx43 and N-cad
accelerated cell
migration in a scratch wound-healing assay. Reducing Cx43 increased Golgi
reorientation,
while decreasing cell adhesion and proliferation. Furthermore, Connexin43 and
N-cad
knockdown led to profound effects on fibroblast cytoskeletal dynamics after
scratch-
wounding. The cells exhibited longer lamelipodial protrusions lacking the F-
actin belt seen
at the leading edge in wounded control cells. This phenotype was accompanied
by
augmented activation of Rac-1 and RhoA GTPases, as revealed by Forster
Resonance Energy
Transfer and pull down experiments. Amongst other things, these results show
that Cx43 and
N-cad will be therapeutic targets in promoting healing of venous leg ulcers,
by acting at least
in part through distinct contributions of cell adhesion, migration,
proliferation, and
cytoskeletal dynamics.
Introduction
[00255] Chronic wounds, such as diabetic foot ulcers, pressure ulcers, and
venous leg
ulcers (VLU), are an increasing worldwide problem, with estimates that 1-2% of
the
population in Western countries will develop a chronic wound over the course
of their
lifetimes. Chronic wounds represent a major economic burden on healthcare
services, with
an estimated annual USA expenditure of $25 billion. With growing numbers of
elderly and
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
diabetics in the population, this expenditure figure is expected to rise in
coming years.
Unfortunately, there are few effective therapeutic options for these
debilitating wounds, and
there remains a significant need for effective new treatments.
[00256] Cx43 is the most ubiquitous connexin protein in the skin, expressed in
keratinocytes, fibroblasts, endothelial cells, and dermal appendages. It is
known that the
topical application of a Cx43-specific antisense-containing gel to acute
wounds in rodent
models significantly accelerates the healing process while reducing
inflammation and scar
size.
[00257] In the normal healing process Cx43 protein becomes down-regulated in
keratinocytes in the first 24-48 hours as the cells become migratory and crawl
forward to
close the wound. Following experiments in Cx43 conditional knockout mice it
was later
reported that down-regulation of Cx43 appears to be a prerequisite for the
coordinated
proliferation and mobilization of keratinocytes during wound healing. In
contrast, it was
shown that in STZ diabetic rats, a model for chronic wounds, Cx43 is up-
regulated in wound
edge keratinocytes instead of being down-regulated, and that migration is
delayed until
down-regulation occurs. Application of a Cx43 antisense to STZ diabetic rat
wounds
prevented the abnormal up-regulation of Cx43 and restored wound closure to
normal rates or
better. Over-expression of Cx43 was also shown to inhibit corneal endothelial
wound healing
in an in vivo rat corneal scrape injury model, while knockdown with Cx43
antisense sped it
up. Cx43 was also detected in the cells at the wound margins of the majority
of biopsies
taken from nine mixed and two diabetic leg ulcers.
[00258] One of the key impediments to the healing of chronic wounds is the
failure of
fibroblasts to migrate, proliferate, and generate granulation tissue. Most
previous reports
have concentrated on epidermal Cx43 in wound healing, and little attention has
been paid to
Cx43 in dermal fibroblasts. In the work described in this Example, a
combination of in vivo
and in vitro models were used to analyze the implications of elevated Cx43
expression, which
was discovered to be detrimentally up-regulated in the dermis of human chronic
VLU, and to
correlate with reduced rates of migration of scratch-wounded fibroblasts over-
expressing
Cx43. In addition to Cx43, it was also discovered that ZO-1 and N-cad, which
interact with
Cx43 and each other, are abnormally over-expressed in the dermis of human
chronic VLU.
Targeting Cx43 reduced the expression levels of ZO-1 and N-cad both in vitro
and in vivo.
Knock down of Cx43 and N-cad, but not ZO-1 alone, accelerated cell migration
in a scratch
71
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
wound-healing assay. Reduction of Cx43 or N-cad also increased Golgi
polarization while
reducing proliferation and cell adhesion in fibroblasts. Targeting of Cx43 or
N-cad,
furthermore, was accompanied by cytoskeletal changes, increased lamellipodia
protrusion,
and activation of Rho GTPases. These results support therapeutically targeting
Cx43 and N-
cad to improve wound repair through a mechanism involving remodeling of cell
contacts and
adhesion-dependent cytoskeletal modifications in fibroblasts.
Materials and Methods
Human chronic VLU and matched intact skin samples
[00259] Collection of wound edge punch biopsies from chronic venous ulcer leg
wounds and normal arm biopsies was performed after obtaining written informed
consent and
in accordance with applicable guidelines. Briefly, for the VLUs a single 4 mm
punch biopsy
was taken from the wound edge under local anesthesia in patients with
clinically confirmed
venous ulceration. A second 4 mm punch biopsy was taken from normal skin on
the forearm
in the same subjects. In all, biopsies from 6 subjects with clinically
confirmed VLU (three
male, three female; range 38 ¨ 79 years) were taken. The median ulcer duration
was 6
months (range 1.5 ¨ 36 months), and the median size was 10 cm2 (range 2 ¨ 113
cm2). The
VLU biopsies were initially fixed overnight with 4% paraformaldehyde (PFA),
then
transferred to 25% sucrose, and stored at 4 C until processing. For
immunohistochemistry,
tissue biopsies were embedded in OCT (BDH, UK). Samples were cryo-sectioned
(10 p.m)
for immunohistochemistry analysis. For the normal (acute) wound biopsies, an
initial 6 mm
punch biopsy was performed under local anesthetic on the anterolateral thigh
of three healthy
male volunteers (range 20-36 years) and then 4 hours later the punched wound
site was
excised with a larger 10 mm punch, and the resulting wound sutured closed. The
2mm wide
donut shaped biopsy was immediately imbedded in OCT, snap frozen in liquid
nitrogen, and
then stored at -80 C until being cryo-sectioned before analysis.
Mouse and rat cutaneous wound-healing model and ODNs application
[00260] Male, 8-week-old ICR mice or Sprague-Dawley rats from UCL's Biological
Services Unit were maintained according to UK Home Office animal regulations.
Excisional
lesions were performed as previously described (Mori, et al. (2006), J Cell
Sci, vol. 119:
5193-5203). Single topical applications of 10 p.M unmodified Cx43asODN and
control
Cx43sODN (Sigma-Genosys) were delivered to each of two independent wounds in
30%
72
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Pluronic F-127 gel (Sigma). Two days after wounding, animals were humanely
sacrificed
and the wound tissue was harvested.
Cell cultures and ODNs treatment
[00261] 3T3 fibroblasts were grown in DMEM-G1utaMAXTm-1 (Gibco, Invitrogen)
supplemented with 10% FBS (Gibco, Invitrogen) in 5% CO2 at 37 C. For ODN
treatments
fibroblasts were washed with PBS to eliminate any traces of serum, and were
incubated in
serum free media for 2 hours with 20 litM Cx43asODN or Cx43sODN (Qiu, et al.,
(2003),
Curr Biol, vol. 13: 1697-1703) (Sigma-Genosys) or with 10 litM ZO-lasODN or ZO-
lsODNs
(Underwood, et al. (1999), Am J Physiol, vol. 277: C330-342) (Sigma-Genosys).
Following
this incubation, the medium with the ODNs was removed and replaced by 10% FBS
in
DMEM.
Transfection; retroviral and lentiviral constructs and transduction
[00262] A retroviral pSuper vector containing the Cx43-specific shRNA target
sequence 5'-GGTGTGGCTGTCAGTGCTC-3' (SEQ ID NO:9; van Zeijl, et al. (2007), J
Cell Biol, vol. 177: 881-891), here, designated Cx43shRNA, was used to
establish a stable
knockdown of Cx43 in 3T3 fibroblasts. A pSuppressor (p.Sup) retroviral vector
(Imgenex
Co) was used as a control. The packaging cell line GP2-293 (Clontech) was
transfected with
the p.Sup and Cx43shRNA constructs as previously described (Carr and Whitmore
(2005),
Nat Cell Biol, vol. 7: 319-321). Mock and N-cad shRNA constructs (Hosokawa, et
al.
(2010), Blood, vol. 116: 554-563), were transfected into HEK 293T cells as
described
(Demaison, et al. (2002), Hum Gene Ther, vol. 13: 803-813). 3T3 fibroblasts
were
transduced with retrovirus or lentivirus for 2 days, and Cx43shRNA and N-
cadshRNA and
Mock-transduced cells were selected on the basis of resistance to 2 ng/ml
puromycin or 500
ng/ml of geneticin (for p.Sup).
[00263] Fugene HD (Roche) was used to transfect 70% confluent fibroblasts with
lmg/m1pGFP, Cx43-DN, or Cx43-WT constructs as described (Becker, et al.
(2001), Cell
Commun Adhes, vol. 8: 355-359). Media was changed the following morning and 24
h later
cells were used in different sets of experiments.
73
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Immunohistochemistry of murine and human skin samples
[00264] Immunostaining was carried out on 6-nm cryostat sections of wounded
rat and
mouse skin, or 10-nm human chronic VLU or matched non-wounded skin, fixed in
acetone at
4 C for 5 minutes. Primary antibodies (Abs) for Cx43 (diluted 1:2000; Sigma,
6219), N-
cadherin (diluted 1:100; Abcam, ab18203), and ZO-1 (diluted 1:100; Zymed
Laboratories,
61-7300) were incubated for 1 hour at room temperature. Sections were washed
with PBS
and then incubated with the respective secondary antibodies, swine anti-rabbit
FITC-
conjugated (DAKO) and goat anti-mouse Cy3-conjugated (Pierce). Secondary
antibody
incubation in the absence of primary antibodies was used as a negative
control. Sections
were then counterstained for 10 minutes with 5 ng/m1 of the nuclear dye
Hoechst 33342
(Sigma) and single optical sections were acquired on a Leica 5P2 confocal
microscope (Leica
Microsystems, UK). All parameters during image acquisition were kept constant
throughout
each experiment to allow direct comparison of all of the 8 bit digital images.
Immunostaining
levels were quantified per unit area using a well-established pixel-counting
method (Wang, et
al. (2007), Diabetes, vol. 56: 2809-2817) using ImageJ software (NIH). Images
were
converted to binary images using an identical threshold. Objects greater than
two pixels were
counted in order to generate a readout of the number of positive pixels per
unit area for
comparison between conditions.
Immunostaining
[00265] Confluent fibroblast monolayers were wounded and fixed 3 h later with
4%
PFA for 10 minutes and permeabilized with 0.1% Triton X-100. Primary
antibodies for Cx43
(diluted 1:2000; Sigma), ZO-1 (diluted 1:100; Zymed Laboratories), N-cadherin
(diluted
1:100; Abcam), 13-catenin (diluted 1:200; ab6302 Abcam), a-catenin (diluted
1:200; C2081
Sigma), tyrosinated tubulin (diluted 1:400; YL1/2; Ab6160 Abcam), and
acetylated tubulin
(diluted 1:200; T7451 Sigma) were used according to the manufacturer's
recommendations.
Incubation with the appropriate secondary antibodies (Alexa 488 or 633;
Molecular Probes)
was followed by 10 minutes application of 5 ng/m1 of the nuclear dye Hoechst
33342 (Sigma).
Secondary antibody incubation in the absence of primary antibody was used as
negative
control. TRITC-phalloidin (Sigma) was included in some experiments for
visualization of F-
actin. Cells were imaged using a 63x, 1.25 NA objective on a Leica 5P2
confocal microscope
(Leica Microsystems, UK). All acquisition parameters were kept constant
throughout each
experiment and staining was quantified based on pixel counts (Wang, et al.
(2007), above).
74
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Golgi reorientation measurements
[00266] Measurement of Golgi reorientation was performed as described
previously
(Magdalena, et al. (2003), J Cell Sci, vol. 116: 743-756). Confluent
fibroblasts were
scratched and incubated for 3 h. After this time, cells were fixed with 4% PFA
and stained
with anti-GM130 (BD Biosciences) and Hoechst 33342 nuclear stain (Sigma). One
hundred
cells in ten randomly selected fields were evaluated for Golgi orientation.
Cell proliferation
[00267] 3T3 fibroblasts (1x104 cells) transduced with pSup, Cx43shRNA, Mock,
and
N-cadshRNA constructs were plated into 96-well plates and cell growth was
monitored in
real-time using the IncuCyteTM live-cell imaging system (Essen Instruments,
Ann Arbor, MI).
Experiments were performed at least three times in duplicate and data was
expressed as
percentage of confluence.
Measurement of protrusion length
[00268] Confluent fibroblasts transduced with Cx43shRNA, p.Sup, N-cadshRNA,
and
Mock constructs, or transfected with pGFP, Cx43-DN, or Cx43-WT were wounded
and fixed
with 4% PFA 3 h later. The cells were then stained with Hoechst 33342 nuclear
stain
(Sigma). To quantify protrusion length, the distance from the nuclei to the
leading edge of
wound edge cells was measured. In each of three independent experiments, 3-5
transfected
cells, or 30 transduced cells in randomly selected fields, were analyzed to
calculate the
average protrusion length.
Western blots
[00269] Cell pellets from harvested fibroblasts (Cx43shRNA- or p.Sup-
transduced)
were suspended at 4 C in ice-cold RIPA buffer plus protease inhibitors. Equal
amounts of
protein were resuspended in Laemmli 4x sample buffer, separated by 10% SDS-
PAGE and
visualized with primary Abs to Cx43 (diluted 1:2000; Sigma), ZO-1 (diluted
1:500; Zymed
Laboratories), a-catenin (diluted 1:200; Sigma), 13-catenin 13-catenin
(diluted 1:1000;
Abcam), or N-cadherin (diluted 1:500; Abcam). Antibodies against a-tubulin or
actin (both
from Sigma) were used as loading controls. Secondary antibodies were HRP-
conjugated, and
protein levels were visualized using an enhanced chemiluminescence (ECL)
system
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
(Amersham). The ratio protein/tubulin or /actin was determined by scanning and
quantifying
the bands, using ImageJ software (NIH).
Dye-transfer assays
[00270] Dye-injection was carried out according to the method described
previously
(Becker, et al. (1995), J Cell Sci, vol. 108 ( Pt 4): 1455-1467). Cells were
impaled under
visual control and filled with dye by iontophoresis. Communication was
assessed 1 minute
after cessation of iontophoresis. At least 10 injections were performed for
each set of
experimental conditions. The coupling was tested in cells: a) treated with
Cx43sODN,
Cx43asODN, or control untreated cells; b) transiently transfected with Cx43-
DN, Cx43-WT,
or pGFP; and c) Cx43shRNA or p.Sup-transduced cells. Images of microinjected
cells were
acquired with a 40x 0.8 NA objective on a Leica DMLFS microscope (Leica
Microsystems,
UK) using Volocity acquisition software (Improvision/Perkin Elmer).
Cell migration assays
[00271] Confluent cultures of Cx43sODN, Cx43asODN, ZO-lasODN, and ZO-
lsODN-treated fibroblasts; or Cx43shRNA, p.Sup, N-cadshRNA, and Mock-
transduced
cells, as well as Cx43-WT, Cx43-DN, and pGFP-transfected fibroblasts were
subjected to a
mechanical scratch-wound. In some experiments, Cx43shRNA and p. Sup
fibroblasts were
serum starved (SS) or incubated in the presence of serum (FBS) for 48 has
described
(Francis, et al. (2011), PloS one, vol. 6: e26379). After this period of time,
cells were
wounded and incubated in DMEM supplemented with serum. Time-lapse images were
taken
soon after wounding at intervals of 5 minutes for 3-4 hours, and were acquired
on an inverted
Zeiss LSM AxioPlan 400 fluorescence microscope (Zeiss, UK), equipped with a
Orca CCD
camera (Hamamatsu, UK), using a 40x 1.2NA objective with an incubation chamber
at 37
and 5% CO2. Time-lapse images were captured using OpenLab image acquisition
software
(Improvision/Perkin Elmer, UK). The migration velocities of individual
fibroblasts were
quantified using Volocity4 analysis software (Improvision/Perkin Elmer) by
measuring the
distance between the initial and final positions of leading edge cells 3 hours
after wounding.
For each experimental condition, velocities were presented as mean SD
(lam/sec).
76
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Rho GTPase activation assays
[00272] Fresh lysates were used for Racl/Cdc42 and RhoA-pulldown assays, using
the
Rac/Cdc42-binding domain (p21-binding domain, PBD) of PAK, as previously
described
(Mendoza-Naranjo, et al. (2007), J Cell Sci, vol. 120: 279-288.), or GST-
Rhotekin to detect
relative amounts of RhoA-GTP. Bound protein (GTP-bound Racl, Cdc42, or RhoA)
levels
were detected by western blot. Total Racl, Cdc42, and RhoA from total cell
lysates were
also analyzed as loading controls.
Forster resonance energy transfer (FRET) assays
[00273] Plasmids encoding FRET probes Raichu-Racl, Raichu-Cdc42 (Itoh, et al.
(2002), Mol Cell Biol, vol. 22: 6582-6591), and RhoA biosensor (Pertz, et al.
(2006), Nature,
vol. 440: 1069-1072) were transfected into Cx43shRNA or p.Sup-transduced
fibroblasts
using Fugene HD (Roche). The FRET efficiency of the cells expressing the EYFP-
ECFP
fusion construct was measured using confocal fluorescence microscopy and
acceptor
photobleaching. Leading-edge transfected cells were imaged using an Olympus
FluoView
1000 laser scanning confocal microscope and a 60x 1.4NA oil objective (Olympus
Microscopes, UK). The CFP and YFP channels were excited using the 440 nm and
515 nm
lasers, respectively. The two emission channels were 460-510 nm for CFP and
520-620 nm
for YFP. The gain for each channel was set to approximately 75% of dynamic
range (12-bit,
4096 grey levels) and offsets set such that backgrounds were zero. Pre- and
post-bleach CFP
and YFP images were acquired and the FRET mode was used to collect images for
each
channel after acceptor bleaching with 10-12 scans of the 515 nm argon laser
line at maximum
power (to bleach YFP). Olympus FluoView 1000 software was used to analyze
donor and
acceptor intensity values before (pre) and after (post) bleaching and values
were then
extracted from pixels falling inside the cell of interest, as well as an equal-
sized bleached
region outside the cell, and the mean ratio was determined for each region.
The FRET
efficiency ratio over the whole cell was calculated using the following
formula: FRET
efficiency = [ID (post) - ID (pre)]/ID (post), where Ippre and IDpost refer to
the intensity of the
donor (CFP) before (pre) and after (post) photobleaching of the acceptor
(YFP).
Hanging drop assays
[00274] Approximately 20,000 single cells were suspended in 351.11 drops of
10% FBS
in DMEM from the lids of 24-well dishes for 4 hours. Water was placed at the
bottom of
77
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
each well to maintain humidity. The drops were pipetted five times up and down
with 200 1.11
yellow tips, fixed with 4% paraformaldehyde, and aliquots were spread on
coverslips.
Images of six random fields from six individual samples for each condition
studied (p.Sup
and Cx43shRNA-transduced cells) were taken with a 4x 0.1 NA objective on an
inverted
Axiovert Zeiss LSM microscope. An area of approximately 2.5 x102 ¨ 7.5 x102
m2
corresponds to a cluster of 1-3 cells and 2.5x103 1..t.m2 corresponds to about
10 cells. The area
of each cell cluster was determined using a custom-written plug-in for ImageJ
software
(NIH). The area occupied by single cells was measured in parallel to estimate
the amount of
cells per unit area.
Statistical analysis
[00275] Statistical differences were determined using Wilcoxon Matched-Pairs
Signed-
Ranks Test for paired data, or one-way analysis of variance (ANOVA) for data
sets of multiple
comparisons. A two-tailed Chi-squared test was used for the analysis of cell
cluster sizes in
the hanging drop assay of adhesion. All data are presented as the mean SD
except where
stated. Criterion levels for the individual tests are given in the Results,
below.
Results
Cx43, N-cadherin and ZO-1 are dramatically up-regulated in the dermis of human
VLU
[00276] Skin punch biopsies, 2mm, from healthy human volunteers and chronic
wound
edge biopsy samples, 4mm, from 6 human VLU, together with corresponding intact
skin
samples from the same patients, were used to analyze Cx43 protein levels in
the dermis of
chronic versus acute wounds. As shown in Figure 1, dermal Cx43 proteins levels
are greatly
up-regulated in human chronic VLU. Figure 1, D, is a representative picture of
a chronic
VLU in the lower leg of a patient, from which a wound edge punch biopsy has
been taken
(white dotted circle in photo, Fig. 1, D).
[00277] In acute wounds, down-regulation of Cx43 was seen at dermal wound
margins
4 hours after excisional wounding (Figure 1A; scale bar = 25 p.m). In the
photo shown in
Figure 1A, blue signal is Hoechst staining of nuclei and collagen bundle
autofluorescence,
and the dotted white line shows the border between the epidermis and the
dermis. These
results were similar to those observed in the wound-edge dermis in a murine
model after
excisional wounding (Figure 9, A; scale bar = 25 p.m), where the expression
and distribution
78
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
of Cx43 in mouse skin dermis was examined by immunohistochemistry two days
after
excisional wounding. In these mouse experiments, wound-edge Cx43 was reduced
in dermal
fibroblasts after such wounds. In Figure 9, A, arrowheads show how Cx43
becomes more
prevalent with increasing distance from the wound edge. Cx43 levels were
quantified along
the wound site and were significantly lower at the wound edge (p<0.005).
Values in the
graph shown in Figure 9, A, are expressed as mean SD. On the other hand,
Cx43 has been
reported to persist at the wound edge epidermis of a majority of mixed
diabetic leg ulcers
(Brandner, et al. (2004), J Invest Dermatol, vol. 122: 1310-1320).
[00278] Human and murine skin punch biopsies analysis showed that Cx43 protein
levels were significantly reduced at the wound edge, with levels increasing
towards normal
with progressive distance from the injury site (Figure 1, A, B, and C; p <
0.01; and Figure 9,
A, arrowheads; p < 0.005). In sharp contrast, biopsies from the wound edge of
VLU had not
only failed to down-regulate Cx43, but also showed significantly elevated
expression of Cx43
protein throughout the dermis of the whole 4mm biopsy taken from chronic VLU
in
comparison to matched non-wounded samples (Figure 1, E). The graph shown in
Figure 1, F,
depicts the Cx43 protein levels in VLU versus non-wounded skin (p < 0.005).
[00279] Human and murine skin punch biopsies analysis showed that Cx43 protein
levels were significantly reduced at the wound edge, with levels increasing
towards normal
with progressive distance from the injury site (Figure 1, A, B, and C; p <
0.01; and Figure 9,
A, arrowheads; p < 0.005). In sharp contrast, biopsies from the wound edge of
VLU had not
only failed to down-regulate Cx43, but also showed significantly elevated
expression of Cx43
protein throughout the dermis of the whole 4mm biopsy taken from chronic VLU
in
comparison to matched non-wounded samples (Figure 1, E). The graph shown in
Figure 1, F,
depicts the Cx43 protein levels in VLU versus non-wounded skin (p < 0.005).
[00280] Cx43 can interact with adherens junction proteins such as N-cadherin
(Wei, et
al. (2005), J Biol Chem, vol. 280: 19925-19936; Shaw, et al. (2007), Cell, ol.
128: 547-560),
and with the tight junction-associated protein ZO-1 (Giepmans and Moolenaar
(1998), Curr
Biol, vol. 8: 931-934), which also interact with each other. The expression
and distribution
of these proteins were investigated in the dermis of chronic VLU biopsies and
matched non-
wounded skin. ZO-1 expression levels were elevated in the dermis of chronic
VLU
compared to matched, non-wounded controls (Figure 2, A; n=6; scale bar = 25
lam).
79
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Photographs of higher magnifications of VLU and intact skin (boxed regions 1
and 2) stained
for ZO-1 (green) and Hoechst (blue) are also shown (Figure 2, A; scale bar =
10 lam.
[00281] N-cadherin protein levels were also observed to be significantly up-
regulated in
chronic VLU as compared to matched, non-wounded samples (Figure 2, B; n=6;
scale bar =
25 lam). The boxed regions, numbered 1 and 2, in the far-left photo of Figure
2, B (scale bar
= 10 lam), show higher magnifications of VLU and non-wounded skin samples
stained with
antibodies for N-cadherin (green) and with Hoechst (blue). Scale bar = 10 lam.
[00282] The graphs in Figure 2, C and D, show that ZO-1 and N-cadherin protein
levels
were significantly higher in the dermis of human chronic VLU samples than in
matched, non-
wounded control dermis. In these graphs, values for ZO-1 and N-cadherin were
expressed as
mean SD (p < 0.01 and p < 0.005, respectively). Taken together, these data
indicate that in
human chronic VLUs, Cx43 protein levels are not down-regulated in the dermal
wound
margins, but also that N-cadherin and ZO-1 are abnormally up-regulated
compared to their
levels in intact skin.
Cx43 expression is tightly linked to N-cadherin and ZO-1 expression in
fibroblasts
[00283] It has been previously shown that Cx43 down-regulation significantly
accelerates the skin healing process (Qiu, et al. (2003), Curr Biol, vol. 13:
1697-1703; Mori,
et al. (2006), J Cell Sci, vol. 119: 5193-5203). In the experiments described
in this example,
the effects of Cx43 knockdown were investigated on ZO-1 and N-cadherin protein
expression
in vivo. To this end, an in vivo mouse model of wound healing was used (n =
6), where full
thickness skin excision wounds were treated with Cx43 antisense or sense
control
oligodeoxyribonucleotides (Cx43asODN and Cx43sODN, respectively).
[00284] Immunohistochemical analysis revealed positive ZO-1 staining in dermal
sense-treated wounds, where ZO-1 (green) was frequently co-localized with Cx43
(red)
(Figure 9, B; n = 6; scale bar = 100 lam). Treatments with Cx43asODN, on the
other hand,
induced a significant decline not only of Cx43 protein, but also of ZO-1
protein levels in the
dermal wound margins (Figure 9, B; Figure 3, A, graph represents mean SD, *p
< 0.05). In
the results shown in Figure 9, B, the cells also counterstained with Hoechst
(blue). These
experiments showed a clear down-regulation of ZO-1 protein levels in the
dermis of mice
treated with the Cx43asODN.
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00285] Immunohistochemical analysis of N-cadherin protein expression was also
performed, and revealed a preferential distribution of the protein in the
dermis in sense-
treated wounds and a significant N-cad protein (green) down-regulation after
treatment with
Cx43asODN as compared to treatment with a control oligonucleotides, Cx43sODN
(Figure 9,
C (scale bar = 100 1.im); Figure 3, B, graph represents mean SD, **p <
0.01).
[00286] Together, these data demonstrate that Cx43 expression is tightly
linked to ZO-1
and N-cadherin protein levels in the dermis, and that targeting Cx43 can
reduce ZO-1 and N-
cadherin expression in vivo.
[00287] In order to better understand the effects of Cx43, N-cadherin, and ZO-
1 protein
levels on fibroblast migration in response to a wounding stimulus, the 3T3
fibroblast cell line
was used. This cell line can be easily transfected or transduced with shRNA
constructs, and
it avoids the variability seen when using primary human fibroblasts derived
from different
patients. ZO-1 and N-cadherin protein expression was evaluated in fibroblasts
transduced
with a Cx43 short-hairpin RNA (Cx435hRNA) that suppresses Cx43 expression (van
Zeijl, et
al. (2007), J Cell Biol, vol. 177: 881-891) or with a p.Sup control plasmid.
Cx43 protein
expression and gap junction-mediated intercellular communication (GJIC) were
effectively
prevented in the Cx43shRNA-producing cells, in contrast to p.Sup control
cells, as shown in
the Western blot and accompanying bar graph in Figure 3C and in Figure 10, G,
H, and I,
which show Cx43 protein levels and dye coupling after LY (Lucifer Yellow)
microinjection.
[00288] With regard to ZO-1 expression, ZO-1 protein levels were down-
regulated in
Cx43shRNA-transduced cells (Figure 3D). ZO-1 distribution was also analyzed in
fibroblast
scratch wound assays generated with Cx43shRNA and control cells. The Cx43shRNA-
transduced cells showed a reduction of ZO-1 located at cell contacts at both
the leading edge
(LE) and in regions more internal to the wound (IA), accompanied by a loss of
ZO-1 from the
leading edge lamellipodia (Figure 3, E, arrowheads). In p.Sup-transduced
fibroblasts,
however, ZO-1 was predominantly restricted to the contact margins between
cells, often co-
localizing with Cx43 (Figure 3, E). With regard to N-cadherin, protein levels
were
significantly reduced in Cx43shRNA-transduced fibroblasts (Figure 3, F; p <
0.01) and N-
cadherin was found located predominantly in the cytosol (Figure 3, G, arrows)
rather than the
preferential plasma membrane localization found in p. Sup-transduced control
fibroblasts
(Figure 3, G, arrowheads).
81
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00289] The expression and distribution of the proteins a- and 13-catenin were
also
examined in p.Sup and Cx43shRNA-transduced cells. Whereas total 13-catenin
protein levels
did not change significantly after Cx43 knockdown (Figure 11, B), its cellular
distribution
was altered. In p.Sup-transduced cells, 13-catenin was mainly localized to the
plasma
membrane at sites of cell-cell contact (Figure 11, A), but relocation to the
cytosol was
additionally found in Cx43shRNA-transduced fibroblasts (Figure 11, A, arrows),
with
evidence for some nuclear localization (Figure 11, A, arrowhead). Alpha-
catenin was seen
localized in both the plasma membrane and the cytosol in p.Sup and Cx43shRNA-
transduced
cells (Figure 11, C), and expression levels and location did not change with
Cx43 knockdown
(Figure 11, D).
Cx43 and N-cadherin, but not ZO-1, knockdown accelerate the rate of fibroblast
migration
[00290] Targeting Cx43 for knockdown accelerates the rate of wound closure in
vivo
(Qiu, et al. (2003), above). Here, several experiments investigated whether
the increase in
cell migration following a reduction in Cx43 expression was associated with
reduction of
ZO-1 protein levels or decreased cell adhesion (Z0-1 or N-cadherin down-
regulation,
respectively).
[00291] Initially, the migration rate of fibroblasts transduced with Cx43shRNA
or p.Sup
vectors, or cells treated with Cx43asODN or control Cx43sODN, was evaluated.
Similar to
Cx43shRNA cells, fibroblasts treated with Cx43asODN showed greatly reduced
Cx43
protein levels and GJIC compared to Cx43sODN, or untreated cells (Figure 10, A
and B,
respectively; p < 0.05 and p < 0.01). Fibroblasts were also transiently
transfected with a
bicistronic pIRES-GFP empty vector (pGFP) or with a matching vector encoding
GFP and
either wild-type Cx43 (Cx43-WT), or a dominant negative Cx43 construct (Cx43-
DN) that
interfered with the traffic of endogenous Cx43 protein to the plasma membrane,
thereby
blocking GJIC (Becker, et al. (2001), Cell Commun Adhes, vol. 8: 355-359).
[00292] The velocity of migration of Cx43shRNA and Cx43asODN cells was
significantly faster than p.Sup or Cx43sODN control cells, respectively
(Figure 4, C and D,
p<0.005; Figure 10, C and D). Cx43 knockdown cells also displayed much more
extensive
lamellipodia than control cells (Figure 4C). Similarly, Cx43-DN-transfected
fibroblasts
migrated faster than their untransfected neighbors or pGFP control cells, and
also extended
larger than usual lamellipodia at the leading edge (Figure 10, E and F).
Conversely,
82
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
fibroblasts over-expressing Cx43 (Cx43-WT) migrated significantly slower than
either pGFP
or Cx43-DN constructs (Figure 10, E and F; p < 0.05 and p < 0.01
respectively). These data
provide further evidence for an inverse correlation between Cx43 protein
levels and the rate
of fibroblast migration after scratch wounding. However, there have also been
reports that
siRNA reduction of Cx43 can slow cell migration in 3T3 cell scratch-wound
assays that have
been serum starved for 2 days (Francis, et al. (2011), PloS one, vol. 6:
e26379).
[00293] These experiments were repeated in serum-starved conditions and in the
presence of normal amounts of cell culture serum. It was found that in serum-
starved
conditions, reducing Cx43 protein levels slowed migration, while in more
normal media
conditions with serum, reducing Cx43 significantly sped migration (Figure 10,
J).
[00294] The velocity of migration of fibroblasts transduced with N-cadherin or
Mock
shRNA constructs (N-cadshRNA and Mock, respectively) was then analyzed. The N-
cadshRNA construct effectively suppressed N-cadherin expression in NIH3T3
fibroblasts
(Figure 4, A), which accelerated cell migration in scratch-wound assays almost
to the same
extent as in Cx43shRNA-transduced cells (Figure 4, C and D). To reduce ZO-1
expression in
fibroblasts, cells were treated with ZO-1 sense and antisense
oligodeoxyribonucleotides (ZO-
lsODN and ZO- 1 asODN), previously described as able to effectively reduce ZO-
1
expression (Underwood, et al. (1999), Am J Physiol, vol. 277: C330-342).
Contrary to Cx43
and N-cadherin, ZO-1 down-regulation (Figure 4, B) did not have any effect on
the rate of
fibroblast migration or wound closure (Figure 4, C and D). These results
indicate that
targeting Cx43 may additionally contribute to cell migration by reducing N-
cadherin, and
perhaps ZO-1, protein levels in fibroblasts.
Cx43 and N-cadherin regulate cell adhesion, polarization and proliferation in
fibroblasts
[00295] Down-regulation of the machinery of cell-cell adhesion is one of the
ways in
which different cell types, including skin cells, reactivate themselves to
acquire a migratory
phenotype. Hanging drop assays have previously been used to characterize the
strength of
intercellular adhesion (Elbert, et al. (2006), Mol Biol Cell, vol. 17: 3345-
3355; Redfield, et
al. (1997), J Cell Biol, vol. 138: 1323-1331). This approach was thus used to
compare cell-
cell adhesion properties by measuring the size of cell clusters that formed in
suspended drops,
and which resisted trituration with a micropipette tip. Clusters were
evaluated by
morphometric image analysis and classified into three size groups according to
their area.
83
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Cx43shRNA- and N-cadshRNA-transduced cells formed fewer trituration-resistant
clusters
(area > 2.5x103 m2) and smaller clusters (area < 7.5 x 1021.im2)
characteristic of reduced
cell-cell adhesion (Figure 5, A and B; p < 0.005).
[00296] The redistribution of the Golgi apparatus is an important event in the
polarization and migration of many types of cells, including fibroblasts
(Magdalena, et al.
(2003), Mol Biol Cell, vol. 14: 670-684). To examine the role of Cx43 and N-
cadherin in
cell polarity during cell migration, the localization of the Golgi protein
GM130 was analyzed
in fibroblasts transduced with Cx43shRNA, N-cadshRNA, or their respective
controls.
Increased GM130 polarization towards the wound was observed in Cx43shRNA and N-
cadshRNA cells compared to p.Sup and Mock controls, 3 h after wounding (Figure
5, C and
D; p < 0.05).
[00297] Cell proliferation was also investigated using an IncuCyteTM live-cell
imaging
system. A clear reduction of cell growth was observed in Cx43shRNA and N-
cadshRNA
transduced cells, compared to p.Sup and Mock control cells (Figure 5, E and
F), indicating a
direct correlation between Cx43 and N-cadherin expression, and cell
proliferation in
fibroblasts.
[00298] Altogether these data confirm the important contribution of Cx43 and N-
cadherin to the regulation of cell polarization, adhesion, and proliferation
processes in
fibroblasts during wound repair.
Targeting Cx43 induces cytoskeletal changes in leading-edge fibroblasts
[00299] Using scratch-wound assays it was observed that polarized Cx43
knockdown
cells in the wound edge extended larger than usual lamellipodial protrusions.
Directional cell
locomotion requires complex interactions between actin filaments (F-actin) and
microtubules,
so the distribution of F-actin, and tyrosinated and acetylated tubulin, was
analyzed in
wounded monolayers after targeting Cx43 expression in fibroblasts. Front-row
control
Cx43sODN, pGFP-transfected, and p.Sup fibroblasts displayed the F-actin belt
typical of
polarized migratory cells (Figures 6, A, and 7, A arrowhead; Figure 12, A),
which is
regulated by adherens junctions (Yonemura, et al. (1995), J Cell Sci, vol. 108
( Pt 1): 127-
142). In contrast, Cx43asODN, Cx43-DN, and Cx43shRNA wound-edge cells
developed
rich lamelipodial protrusions oriented in the direction of movement, and
lacked the F-actin
belt found in control cells (Figures 6, A, and 7, B arrowhead; Figure 12, A).
Under control
84
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
conditions, tyrosinated and acetylated microtubules (TyrTub and AcetTub,
respectively) were
arranged very much as previously described in wounded fibroblast monolayer
experiments
(Gundersen and Bulinski (1988), Proc Natl Acad Sci USA, vol. 85: 5946-5950),
fanning out
from the perinuclear region towards the wound margin. In Cx43asODN, Cx43shRNA,
and
Cx43-DN cells, which tended to be more extended, tyrosinated microtubules were
predominantly oriented perpendicularly to the wound edge (Figures 6, A, and 7,
B
arrowhead; Figure 12, A). Cells over-expressing Cx43 (Cx43-WT) showed
microtubules and
lamellipodia that were less extensive than pGFP-transfected control cells
(Figure 7, C).
[00300] Lamellipodial dynamics were analyzed in more detail by transfecting
fibroblasts with a red fluorescent protein (RFP)-actin construct. Sixteen
hours after RFP-
actin transfection a confluent monolayer was scratch-wounded and imaged for
1.5 hours by
confocal microscopy. The p.Sup cells showed active, actin-rich lamellipodia
and filopodia
(Movie 4) and actin remodeling, characterized by dynamic formation and
collapse of
filopodia and actin ruffles in front-row cells. Cx43shRNA-transduced
fibroblasts displayed
considerably more extensive actin-rich membrane protrusions at the front of
the leading-edge
cells. The extent of cell protrusion was quantified, and it was determined
that Cx43asODN,
Cx43-DN, and Cx43shRNA cells displayed almost twice the average protrusion
length
exhibited by Cx43sODN, pGFP, and p.Sup control cells (Figures 6, B, and 7, D;
Figure 12,
B).
[00301] Following demonstration that Cx43 knockdown reduced N-cadherin
expression, which also contributes to accelerate cell migration in fibroblasts
(Figure 4,D), the
distribution of polymerized actin was analyzed in N-cadshRNA- and Mock-
transduced cells 3
h after wound-scratch experiments. Similar to Cx43 knockdown fibroblasts,
wound edge N-
cadherin-targeted cells extended rich lamellipodial protrusions oriented
towards the direction
of cell migration (Figure 13, A), and quantification showed more extensive
lamellipodia
protrusions compared to Mock control cells (Figure 13, B).
Targeting Cx43 and N-cadherin increases Racl and Rho-A GTPase activities in
fibroblasts
[00302] Rho GTPases are key regulators of actin and microtubule dynamics and
play an
essential role in controlling different actin-based structures critical for
cell motility and
chemotactic responses. The effect of Cx43 and N-cadherin knockdown on Rho
family
GTPase activation in fibroblasts was examined using pull-down assays with
specific GST
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
fusion protein-binding domains for activated Racl, Cdc42, and RhoA (GTP-bound
forms).
Quantitative analysis showed enhanced Racl and RhoA activity in Cx43shRNA and
N-
cadshRNA cells compared to p.Sup and Mock controls, respectively (Figure 8, A
and B). In
contrast, no differences in Cdc42 GTPase activity were detected.
[00303] To examine the activation of Rho GTPases in more detail, biosensors
for Racl,
RhoA, and Cdc42 (Itoh, et al. (2002), Mol Cell Biol, vol. 22: 6582-6591;
Pertz, et al. (2006),
Nature, vol. 440: 1069-1072) were transfected into either p.Sup or Cx43shRNA-
transduced
cells and FRET was used to examine the activity of these GTPases in leading
edge fibroblasts
3 hours after scratch-induced migration. Targeting Cx43 with Cx43shRNA induced
a two-
fold increase in Racl and RhoA activity over the p.Sup control (Figure 8, C
and E; p < 0.05),
but had no effect on the activity of Cdc42 (Figure 8E). These findings
indicate a direct role
for Cx43 in the regulation of cytoskeletal dynamics in fibroblasts during cell
migration.
Discussion
[00304] Chronic wounds are a significant and growing global problem and place
a great
burden on both patients and healthcare system resources. While chronic wounds
can occur
anywhere on the body, most fall within the categories of VLUs, diabetic foot
ulcers and
pressure ulcers, which fail to progress through an organized, orderly and
timely sequence of
wound repair. Acceleration or even stimulation of wound closure is important
for these
chronic wounds, which are often infected and inflamed, making them painful and
debilitating
for the growing numbers of elderly and diabetic patients, and putting them at
risk of lower
limb amputation. Here, the experiments show that Cx43 is markedly up-regulated
in the
dermis of human chronic VLU, similar to what was found in the wound edge
epidermis of
STZ diabetic rats, a feature that underlies impaired migration and healing
(Wang, et al.
(2007), Diabetes, vol. 56: 2809-2817).
[00305] Because it would be unethical to biopsy and treat human VLU with Cx43
antisense and then resample these hard to heal wounds with additional
biopsies, a fibroblast
cell line was utilized to reliably manipulate Cx43 protein expression and
explore the effects
on the dynamics of cell migration in a scratch wound healing assay. Such
experiments are
useful for exploring the effect of Cx43 modulation on fibroblast behavior. In
these cell-
culture experiments, fibroblast migration was impaired when Cx43 protein
levels and GJIC
were elevated after transfection with a Cx43-WT construct. These findings lead
to the
86
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
conclusion that the impaired healing seen in human chronic VLU wounds results
from the
reduced fibroblast migration rate caused by elevated Cx43 protein levels.
[00306] However, elevated levels of Cx43 may not be the only factor that
compromises
healing in VLU. Cx43 reportedly forms a multiprotein complex or 'nexus' with
ZO-1, a-
and fl-catenin, and N-cadherin, proteins that likely influence both cell
adhesion and migration
in wound healing. In fact, when compared to intact skin, it was found that
both ZO-1 and N-
cadherin protein levels were significantly up-regulated in the dermis of human
chronic VLU,
along with Cx43 up-regulation. Clinically relevant is the observation that
silencing Cx43
accelerated the velocity of fibroblast migration, which indicates that this
process can be
therapeutically controlled. Using in vitro models it was recently reported
that connexin
mimetic peptides also improve the migration rates of dermal fibroblasts
(Wright, et al.
(2009), Wound Repair Regen, vol. 17: 240-249), as well as keratinocyte and
fibroblast
migration in organotypic models and 2D cultures (Pollok, et al. (2010), J Cell
Mol Med, 2011
Apr;15(4):861-73), which further reinforce these observations. Those studies
reported that
levels of Cx43 protein were not changed by the peptide but phosphorylation of
Cx43 was
increased and cell adhesion decreased. These experiments here, however,
revealed that
directly targeting Cx43 protein production additionally reduced N-cadherin and
ZO-1 protein
levels in vivo, with redistribution of both proteins from the plasma membrane
to the cytosol.
In the case of ZO-1, the protein was noticeably lost from the leading edge
lamellipodia,
which is normally a feature of fibroblast migration in scratch assay wound
healing, which
could bring about changes in the distribution of the cytoskeletal components
to which it
binds.
[00307] The cytoplasmic tail of Cx43 is able to interact with the PDZ2 domain
on ZO-
1, but this interaction can be competed out by the addition of a mimetic
peptide (ACT-1) to
the last 9 amino acids of the Cx43 tail (Hunter, et al. (2005), Mol Biol Cell,
vol. 16: 5686-
5698). This peptide has been reported to stabilize Cx43 in the cell membrane
forming larger
gap junction plaques (Hunter, et al. (2005), Mol Biol Cell, vol. 16: 5686-
5698). Applied to
acute skin lesions this peptide is reported to accelerate wound healing whilst
reducing scar
formation, actions that are similar to those of the Cx43 antisense oligo
(Gourdie, et al.
(2006), Annals of the New York Academy of Sciences, vol 1080: 49-62;
Ghatnekar, et al.
(2009), Regenerative Medicine, vol 4: 205-223; Rhett, et al. (2008), Trends in
Biotechnology, vol. 26: 173-180).
87
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00308] The literature regarding gap junction (GJ) communication and cell
migration
contains conflicting reports. Elevated connexin expression has been associated
with reduced
migration (McDonough et al. (1999), Int J Dev Neurosci, vol. 17: 601-611;
Batten and Haar
(1979), Anat Rec, vol. 194: 125-141), while others have reported the opposite
effect (Xu, et
al. (2006), Development, vol. 133: 3629-3639; Huang, et al. (1998), J Cell
Biol, vol. 143:
1725-1734). Using a different approach, in vitro studies performed on NIH 3T3
cells showed
that the dynamic spreading movement, over an hour, of individual isolated and
non-wounded
cells appeared to be reduced when Cx43 was down-regulated with siRNAs (Wei, et
al.
(2005), J Biol Chem, vol. 280: 19925-19936). This discrepancy may reflect the
different cell
states (wounded versus non-wounded) and different models used (cell migration
into a
scratch-wound as compared to the spreading of non-wounded cells in sparse
cultures). More
recently, the same group reported that Cx43 KO mouse embryonic fibroblasts
migrated more
slowly in scratch wound assays than those with Cx43 (Francis, et al. (2011),
PloS one, vol. 6:
e26379).
[00309] The reasons for these divergent observations are not clear, but may be
related to
serum starving of the cells for 48 hours prior to performing the scratch-wound
assay. In the
experiments described above, the migration experiments were repeated with
control 3T3 cells
and 3T3 cells transduced with Cx43shRNA to reduce Cx43 expression. One batch
was
serum starved for 2 days, while the other was not. Cultures were then scratch-
wounded and
imaged for 4 hours. It was found that serum starved cells with reduced Cx43
migrated more
slowly, whereas reducing Cx43 protein levels in the absence of serum
starvation speeded
migration. As serum starvation would not be expected to match the conditions
found in an
acute wound in vivo, where results have shown that down-regulating Cx43 speeds
migration
of fibroblasts and keratinocytes, it is reasonable to expect that that it is
the abnormal
conditions of serum starvation that triggered the anomalous response.
[00310] Cadherin-mediated cell¨cell adhesion is reported to coordinate
junction
development with cell movement and cell polarization, and to maintain junction
integrity by
forming links with actin filaments (Pokutta and Weis (2002), Curr Opin Struct
Biol, vol. 12:
255-262). Disruption of N-cadherin expression has been reported to increase
the rate of
Schwann cell migration on astrocytes by enhancing both the number of migrating
cells, and
the maximum migration distance (Wilby, et al. (1999), Mol Cell Neurosci, vol.
14: 66-84).
Here, it was demonstrated that targeting Cx43, which consistently reduced N-
cadherin
88
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
expression, significantly diminished cell-cell adhesion, while increasing cell
polarization in
fibroblasts. This was further demonstrated by targeting N-cadherin directly,
which provided
similar results. Direct loss of Cx43¨Cx43 connexon docking in Cx43-targeted
fibroblasts
may also be contribute to decreased cell-cell adhesion, as reported for
cadherin null human
squamous carcinoma cells (Chakraborty, et al. (2010), J Biol Chem, vol. 285:
10761-10776),
and also in other models in which expression of Cx43 increased cell
adhesitivity (Lin, et al.
(2002), J Neurosci, vol. 22: 4302-4311; Cotrina, et al. (2008), Glia, vol. 56:
1791-1798). In
the same way, interfering with Cx43 in individual cells of the 8-16 cell mouse
embryo has
previously been shown to reduce adhesion and produce decompaction of the
targeted cell
(Becker and Davies (1995), Microsc Res Tech, vol. 31: 364-374), whereas ZO-1
has recently
been reported to be essential for the compaction step (Wang, et al. (2008),
Dev Biol, vol.
318: 112-125). Based on the findings described in this Example, it appears
that Cx43 down-
regulation prevents maturation of stable cell¨cell adhesions at least in part
by reducing N-
cadherin expression and/or docking of connexons themselves.
[00311] Here, it has been found that sustained reduction of the expression of
either
Cx43 or N-cadherin in 3T3 cells, by transduction with shRNA, resulted in
reduced cell
proliferation. While this observation does not seem to fit with previous
reports relating to
Cx43 and cell proliferation, it has been found that applying Cx43 antisense to
an excisional
wound on a mouse results in increased proliferation of fibroblasts and nascent
keratinocytes
but this was 1, 2, or 7 days after the treatment by which time the antisense
was no longer
preventing Cx43 protein production (Mori, et al. (2006), J Cell Sci, vol. 119:
5193-5203).
Pollock, et al. (2011, above) reported that the mimetic peptide GAP27 could,
in some cases,
enhance cell proliferation at the leading edge of a scratch-wound assay of
keratinocytes or
fibroblasts. This effect was brought about without apparently reducing Cx43
protein levels,
and so the differences observed when Cx43 protein is significantly reduced
likely reflect the
direct effect of the presence of the Cx43 protein on cell proliferation rather
than the effect of
Cx43-based gap junctional communication.
[00312] Trafficking of Cx43 hemichannels to cell¨cell junctions takes place
through a
pathway that is dependent on microtubules, and it is known that the
cytoplasmic tail of Cx43
is able to interact with various components of the cytoskeleton, including
microtubules and
actin. Here, the experiments described above show that front-row migrating
fibroblasts
lacking N-cadherin or Cx43 protein adopt an elongated phenotype with
lamellipodia
89
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
extended into the wound bed in the direction of migration. In contrast, actin
organization in
control-wounded fibroblasts was characterized by the F-actin belt typical of
polarized
migratory cells, known to be regulated by adherens junctions. Enhanced Racl
and RhoA
activities were also identified in Cx43 and N-cadherin-targeted fibroblasts.
These findings
correlate with a reported role for RhoA in regulating fibroblast protrusion as
an initiator of
actin polymerization at the onset of the protrusion-retraction cycle
(Machacek, et al. (2009)
Nature, vol. 461: 99-103). Racl, on the other hand, can influence the
reinforcement and
stabilization of newly expanded protrusions, which may explain the faster
migration and
more extensive lamellipodia observed in Cx43-targeted fibroblasts.
[00313] The organization of the microtubule cytoskeleton was also investigated
and
found to be altered after reducing Cx43 protein levels, indicating that Cx43
not only interacts
directly with microtubules, but may also affect microtubule dynamics during
fibroblast
migration. See Francis, et al. (2011), PloS one, vol. 6: e26379, reporting
altered microtubule
dynamics in Cx43 KO mouse embryonic fibroblasts).
[00314] Overall, the experiments described in this Example provide insight
into why
chronic VLUs that overexpress Cx43 and cadherin in the dermis are slow to
heal, and the
cellular mechanisms as to how reducing Cx43 and cadherin protein expression
accelerates
fibroblast migration, pointing to a therapeutic solution to this debilitating
problem. These
findings support the role of Cx43 in not simply forming GJ channels, but also
to stabilize a
nexus or multiprotein complex comprising N-cadherin, amongst others, that is
required for
cell-cell adhesion and adhesion-dependent actin dynamics, which must be broken
down to
facilitate efficient fibroblast migration.
EXAMPLE 3
Cx43, N-Cad, and ZO-1 Overexpression In Diabetic Foot Ulcers Retards
Fibroblast
Miuration
Introduction
[00315] Poor healing of diabetic foot ulcers is a major clinical problem that
can be
extremely debilitating and lead to lower limb amputation. In normal acute
wounds, the
Connexin 43 (Cx43) gap junction protein is down-regulated at the wound edge as
a precursor
to cell migration and healing. In this example, experiments that use
fibroblasts from the
human chronic diabetic foot ulcer wound edge show a striking and significant
10-fold
elevation of Connexin 43 protein, as well as a 6-fold increase in N-cadherin
(N-Cad) and a 2-
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
fold increase in Zonular Occludin-1 (ZO-1, a zona occludens protein), as
compared to
unwounded skin. In streptozotocin diabetic rats, Cx43 was found to be
upregulated in intact
dermal fibroblasts in direct proportion to blood glucose levels and increased
2-fold further in
response to wounding. To mimic diabetes, 3T3 fibroblasts were cultured under
different
concentrations of glucose or mannitol and Cx43 protein intercellular
communication and
migration rates were determined. Cultures of fibroblasts in very high (40mM)
glucose
conditions showed significantly elevated Cx43 protein levels, as shown by
immunostaining
and Western Blot, and significantly increasing gap junctional communication,
as shown by
dye transfer. In scratch wound healing assays, increased levels of Cx43 from
high glucose
resulted in repressed filopodial extensions and significantly slower migration
rates than in
either standard conditions (5.5mM glucose) or the osmotic control of mannitol.
Conversely,
when glucose-induced Connexin 43 upregulation was prevented with Cx43shRNA
transduction, the fibroblasts extended long filopodia and migrated
significantly faster.
Connexin 43 protein was upregulated in fibroblasts in diabetic foot ulcers as
well as after
high glucose exposure in culture which correlated with inhibition of
fibroblast migration and
is likely to contribute to impaired wound healing.
Background
[00316] People with diabetes can have wounds that heal poorly and suffer a
high
incidence of non-healing skin ulcers, most frequently found on the feet. Such
ulcers can be
extremely debilitating, and a significant number eventually lead to lower limb
amputations.
[00317] The gap junction protein Connexin 43 (Cx43) plays a central role in
the wound
healing response. In wound edge keratinocytes, Cx43 is normally down-regulated
in the first
24-48 hours after injury, as keratinocytes become migratory and crawl forward
to heal the
wound. An accelerated transition to the migratory state in rodents has been
shown when
Cx43 is more rapidly down-regulated by application of a Cx43 specific
antisense gel to the
wound. In contrast, the Cx43 protein in Streptozotocin (STZ) diabetic rats is
abnormally
over-produced in wound edge keratinocytes after wounding and migration fails
to occur until
Cx43 protein levels are reduced. Abnormal Cx43 protein expression is, at least
in part,
believed to underpin the poor healing observed in diabetic skin ulcers, and
recovery of
normal migratory rates of keratinocytes in STZ diabetic rats can be achieved
by application
of Cx43 antisense to wounds, a treatment that prevents the abnormal elevation
of Cx43.
91
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00318] In the experiments described below, the dermal Cx43 protein levels
have been
quantified in intact human skin biopsies taken from normoglycemic donors, and
from
forearm and ulcer wound edge biopsies in patients with confirmed diabetes.
Complementary
in vivo and in vitro studies are described in which the effects of elevated
glucose on Cx43
protein levels, gap junctional communication, and migration in a scratch wound-
healing
assay, as well as the contribution of Cx43 protein levels to the rate of
migration, were
examined.
Materials and Methods
2.1 Human skin biopsies
[00319] The collection of 4mm punch biopsies from the edges of chronic
diabetic foot
ulcers was approved by the Western Institutional Review Board, Olympia,
Washington State,
USA. This sample was taken from the ulcer wound edge as well as a matched
forearm biopsy
of unwounded skin. A separate non-diabetic cohort had a biopsy of forearm
skin. All biopsies
were taken after written informed consent was obtained.
[00320] The diabetic cohort comprised 10 Caucasian subjects (6 males and 4
females)
with median age 59.5 yrs (range:48-82yrs). Nine subjects were on insulin and
one on oral
medication, with the cohort median HBAlc 6.9 (range 5.3-9.9; n=6 available).
All had
diabetic neuropathy of the extremities and 6 had clinical indications of
peripheral vascular
disease. Each subject had a clinically confirmed diabetic foot ulcer. Median
ulcer size 9.4cm2
(1-81 cm2.) with a duration median of 3.5mths (range1.5-26mths). The control
cohort of non-
diabetic intact skin arm biopsies was taken from Caucasian individuals,
comprising 6 subjects
of median age 48.5 years (2 males and 4 females; range 36-79 years.
Diabetic Rat
[00321] Diabetes was induced in male Sprague-Dawley rats (350-400 g) by
intraperitoneal injection of STZ (65 mg/Kg) and diabetes confirmed using
glucose urinary
strips (Clinistix, Bayer, UK). Wound healing studies were undertaken 2 weeks
after induction
of diabetes and were performed as previously described (Wang, Lincoln (2007),
Diabetes,
vol. 56:2809-17). A single topical application of 50 [1.1 of 10 [tM unmodified
Cx43asODNs
(5'-GTA ATT GCG GCA GGA GGA ATT GTT TCT GTC-3'; SEQ ID NO:10) or control
Cx43sODNs (5'-GAC AGA AAC AAT TCC TCC TGC CGC AAT TAC; SEQ ID NO:11;
Sigma) was delivered to wounds and tissue harvested at 24 hours after
wounding. Blood
92
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
glucose readings were taken and all rats were confirmed to be severely
hyperglycaemic
(blood glucose 27.07 +1.09 mmol/L).
Cell culture
[00322] Confluent monolayers of NIH 3T3 fibroblasts were grown in 5.5% glucose
DMEM (D5796: Invitrogen, UK) supplemented 10% fetal bovine serum and
penicillin/streptomycin. In some experiments the level of glucose was elevated
to 25 or 40
mM or an osmotic control of 19.5 or 34.5 mM mannitol. Cells were cultured in
these
conditions for 2 weeks prior to the experiments being performed. Dye-injection
was carried
out according to the method described by Becker, et al. (Journal of Cell
Science (1995), vol.
108, part 4:1455-67). The scratch wound assay was performed on confluent
monolayers of
fibroblasts as described by Mori, et al. (Journal of Cell Science (2006(, vol.
119:5193-203),
and migration was monitored by time lapse imaging over a 4 hour period on an
Olympus
IX81 microscope.
Immunohistochemistry and imaging
[00323] Immunostaining was carried out on cultured cells or cryostat sections
of
wounded and intact skin as described by Wang, et al. (Diabetes (2007), vol.
56:2809-17).
Primary antibodies for Cx43, diluted 1:2000 (Sigma, Poole, UK), N-cadherin,
diluted 1:100
(Abcam, ab18203), and ZO-1, diluted 1:100 (Zymed Laboratories, 61-7300), were
incubated
for 1 hour at room temperature. Tissues were washed with PBS and then
incubated with the
secondary antibody swine anti-rabbit FITC-conjugated (DAKO). Secondary
antibody
incubation in the absence of primary antibody was used as negative control.
Tissues were
then counterstained for 10 minutes with 51.ig/m1 of the nuclear dye Hoechst
33342 (Sigma,
Poole, UK) and images were acquired on a Leica 5P2 confocal microscope (Leica
Microsystems, UK). All parameters during image acquisition were kept constant
throughout
each experiment to allow direct comparison of all of the 8 bit digital images.
When imaging,
major dermal appendages were avoided in the field of view as they express
considerably
more Cx43 and would have distorted the results. Immunostaining levels were
quantified per
unit area using a well-established pixel-counting method using ImageJ software
(NIH).
Images were converted to binary images using an identical threshold. Objects
greater than 2
pixels were counted in order to generate a readout of the number of positive
pixels per unit
area for comparison between conditions.
93
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00324] Retroviral Constructs: A retroviral pSuper vector contained the
Cx43shRNA
sequence 5'-GGTGTGGCTGTCAGTGCT-3' (SEQ ID NO:12; van Zeijl, et al. (2007), J.
Cell Biol., vol. 177:881-91). A pSuppressor (p.Sup) retroviral vector acted as
control, and
transfection was performed as described by Carr and Whitmore (Nature Cell
biology (2005),
vol. 7:319-21).
Statistical analysis
[00325] Statistical differences were determined using ANOVA followed by
Tukey's
analysis with P<0.05 taken as significant.
Results
Cx43, N-Cadherin and ZO-1 protein levels in human biopsies
[00326] The effect of the underlying diabetic state on the human fibroblast
Cx43 protein
levels from intact non-wounded dermis was negligible in terms of Cx43 protein
levels in
contrast to the effects of the diabetic foot ulcer (DFU). Fibroblasts from
within DFU showed
a striking upregulation of Cx43 protein, which was about 10-fold higher than
the comparable
intact diabetic or non-diabetic skin (P < 0.05). Cx43 protein is often closely
associated with
the tight junction protein ZO-1 and the adhesion protein N-Cadherin, and both
of these
proteins were also found to be elevated in the DFU samples. N-Cadherin protein
levels
increased 6-fold in DFU compared to intact skin (P < 0.05), and mean ZO-1
protein levels
were found to be elevated 2-fold. It was also observed that in the intact
human skin,
autofluorescent bundles of elastin could be seen (but are not seen in rat
skin), and these were
absent from the wound edge of the DFU where they have been degraded by
proteases.
Cx43 protein levels in wound edge fibroblasts of STZ rats
[00327] Cx43 immunostaining of dermal fibroblasts in the wound-edge region of
STZ
diabetic rats was more than double that in control rats 24 hours after
wounding. This
abnormal increase of Cx43 protein in diabetic wound-edge fibroblasts was
efficiently
prevented (P < 0.001) by a single topical application of a Cx43-specific
antisense gel
(Cx43asODNs; SEQ ID NO:13) immediately after wounding. In the intact diabetic
rat skin,
there was found to be a significant increase in Cx43 protein levels in direct
relation to blood
glucose levels r=0.625.
Fibroblast Cx43 expression and communication
94
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00328] 3T3 fibroblasts transduced with p.Sup or Cx43shRNA constructs were
cultured
under increasing glucose (5.5, 25 and 40 mM) conditions for 2 weeks in order
to mimic the
diabetic state in these cells. To control for effects of increased glucose
osmolarity, fibroblasts
were also incubated in 5.5 mM glucose DMEM supplemented with 19.5 or 34.5 mM
mannitol, respectively. The 40 mM levels of glucose resulted in significantly
elevated levels
of Cx43 protein, as shown by Western blotting and immunostaining (P < 0.01).
This
elevation in Cx43 protein levels was largely prevented by transduction with
Cx43shRNA (P
< 0.001). The extent of cell communication was analyzed in fibroblasts
cultured in different
concentrations of glucose and a significant increase in the incidence of dye
coupling was seen
at the highest glucose concentration (40 mM) as compared with lower glucose or
control high
mannitol conditions (P < 0.01).
Fibroblast migration
[00329] Cells cultured under increasing glucose conditions for two weeks
migrated
significantly slower in response to a scratch wound when imaged over a 4 hour
period (P <
0.05 and P < 0.001 for 25 and 40 mM glucose, respectively) than 5.5mM glucose.
There
were no significant changes in the rate of migration for fibroblasts incubated
with 19.5 mM
and 34.5 mM mannitol, ruling out osmotic effects. When fibroblasts stably
transduced with
Cx43shRNA or p.Sup were incubated under increasing glucose and mannitol
conditions, the
Cx43shRNA largely prevented the Cx43 upregulation seen in the 40 mM glucose
conditions
and significantly enhanced the migration rates for all conditions (P < 0.05; P
< 0.001).
Although the velocity of migration for the Cx43shRNA 40 mM high-glucose dose
increased
2-fold over p.Sup 40mM glucose, it did not reach the velocity seen in all of
the other
Cx43shRNA treatment conditions. However, it did match or exceed the rates
obtained in all
p.Sup-transfected fibroblasts culture. In addition, Cx43shRNA also enhanced
the rate of
production and the size of lamellipodial extensions at the leading edge of the
migrating cells
consistent with a more rapid onset of migration and greater motility.
Discussion
[00330] In this study, for the first time a striking 10-fold increase has been
observed in
the expression level of the gap junction protein Cx43 quantified in dermal
fibroblasts from
biopsies of human DFU wound edges. It is believed that this abnormal
expression of Cx43, a
protein that normally must be transiently down-regulated in acute wound
healing, inhibits the
ability of the fibroblasts to migrate and heal such chronic wounds.
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00331] These results show that elevation of Cx43 occurs in wound edge
fibroblasts in
STZ rats, and that this can be prevented by a Cx43asODN. The increase in Cx43
in the intact
STZ rat dermis was found to be in direct proportion to the level of blood
glucose, so glucose
itself may at least in part be a driver of Cx43 expression changes in
fibroblasts. Similarly,
these results also show that raising the level of glucose to 40 mM in the
media of 3T3 cell
cultures elevated Cx43 protein levels and GJIC. This effect was brought about
by glucose,
not increased osmolarity, as similar levels of mannitol did not significantly
increase Cx43
protein or GJIC. The elevated levels of Cx43 had a negative effect on the
migration rate of
fibroblasts, which is consistent with the perturbed wound healing of STZ
diabetic rats that
show abnormally elevated Cx43 in wound edges. Strong supportive evidence that
elevated
levels of Cx43 retard fibroblast migration was additionally provided by
demonstrating that
cells migrated at control rates or faster after Cx43 knockdown. Even at very
high glucose
levels (40mM) the Cx43shRNA migration was 2-fold faster than p.Sup (40mM) and
in excess
of the level seen at a p.Sup control (5.5mM) glucose concentration. While
Cx43shRNA
could easily prevent expression of normal levels of Cx43 protein, it did not
entirely prevent
the elevated levels induced by 40mM glucose. Interestingly, when Cx43shRNA
(40mM
glucose) Cx43 levels are similar to those of p.Sup (5.5mM glucose), the
migration rates are
also similar.
[00332] The importance of down-regulation of Cx43 protein during the wound
healing
process is highlighted by the fact that fibroblast migration is impaired if
Cx43 protein levels
are elevated. It would appear that the more Cx43 protein that is present, the
slower
fibroblasts migrate. The 10-fold elevation of Cx43 protein in fibroblasts of
DFU may explain
why these cells fail to migrate. Precisely how Cx43 inhibits fibroblast
migration is not yet
clear; however, the cytoplasmic tail of Cx43 can interact with a number of
cytoskeletal and
membrane proteins such as a- and 13-catenin, N-cadherin, and ZO-1, and can
form a multi-
protein junctional complex sometimes referred to as a "Proteome" or "nexus",
which may
affect migration. In addition, the Cx43 gene may be a master gene that
controls the
expression of other genes. The discovery here that both ZO-1 and N-cadherin
are
upregulated in the dermis of human diabetic skin, and even more in DFU, also
supports a
regulatory role for Cx43. The increase in adhesion generated by elevated N-
cadherin protein
levels may contribute to the retarded fibroblast migration. Additionally, cell-
cell adhesion
will also increase by virtue of the elevated Cx43-Cx43 hemichannel docking
between DFU
fibroblasts.
96
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
Summary and Conclusions
[00333] In this study 10-fold elevations of Cx43 protein levels were found in
dermal
fibroblasts of human biopsies from diabetic foot ulcers. Additionally, the
levels of N-
cadherin and ZO-1 were discovered to be elevated 6- and 2-fold, respectively,
in DFU. It
was also shown that Cx43 was elevated in the STZ diabetic rat dermis in
proportion to the
level of blood glucose, and that the observed 3-fold elevation of Cx43 in
wound edge dermis
of STZ diabetic rats could be corrected by application of Cx43-specific
antisense to the
wound. It was also shown that in cultured fibroblasts high levels of glucose
can induce
elevated Cx43 protein levels, and that such glucose levels retard fibroblast
migration in vitro.
These results confirm that increased Cx43 expression is a fundamental cause of
poor
fibroblast migration and reduced healing rates in diabetic ulcers.
EXAMPLE 4
Oligonucleotides Targeting ZO-1
[00334] This Example describes several candidates for ZO-1 AS ODNs (antisense
oligodeoxtribonucleotides), shRNAs (small hairpin RNA molecules), and siRNAs
(small
interfering RNA molecules).
[00335] ZO- I was originally been identified at tight junctions, which form a
network
inside cells. This structure is only present at the intersection between two
cells at the cell-cell
contact zone. ZO-1 is a 220-kDa membrane protein, which co-localizes with the
transmembrane proteins claudins and occludin, Later, ZO-1 was demonstrated and
identified
at adherens junctions that zip cells together and thereby maintain cell and
tissue polarity.
These junctions also anchor the cytoskeleton, allowing the formation of large
complexes at
the plasma membrane.
[00336] One sequence selected for synthesis of antisense polynucleotides that
target
ZO-1, 5'-CTGCTTTCTGTTGAGAGGCT-3' (SEQ ID NO:14), corresponds to the segment
from base pair 3154-3169 in MUSZO1 accession number D143401. The complementary
sense polynucleotide, 5'-AGCCTCTCAACAGAAAGCAG-3' (SEQ ID NO: 25), and a
random-order nonsense polynucleotide, 5'-TATGGTACGTGTCGTCCTTG-3' (SEQ ID NO:
26), can be used as controls.
97
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
[00337] Small interfering RNAs (whether now known or later developed) can also
be
used to reduce or eliminate ZO-1 expression. Several such molecules include:
siRNA scrambled 5 -GGGAAGACAGAACITUGUACUCAAAA-3 (SEQ ID NO:15)
3 '-CCCIJUCUGIJCIII_IGAACAUGAGULJUIJ-5' (SEQ D NO:16)
siRNA p53 5'-AAAACUCAUGUIJCAAGACAGAAGGGU-3' (SEQ ID NO:17)
3 '-UITUI_IGAGI_TACAAGULICUGUCULICCCA-5' (SEQ ID NO:18)
siRNA ZO-1 1681 5'-CCAUCUGAUGGUGUCCUACCUAAUU-3' (SEQ ID NO:19)
3'-GGUAGACUACCACAGGAUGGAIJUAA-5' (SEQ ID NO2)
siRNA ZO-1. 2137 5'-GGGCUCUUGGCUUGCUAUUCGAAULF-3' (SEQ ID NO:21)
3 '-CCCGAGAACCGAACGAUAAGCUIJAA-5' (SEQ I NO:22)
siRNA ZO-1 5518 5 '-CCUUCCACCUUUAGAUAAAGAGAAA-3' (SEQ I NO:23)
3 -GGAAGGUGGAAAUCUAUUUCUCUUU-5' (SEQ ID NO:24)
* * *
[00338] All patents, publications, scientific articles, web sites, and other
documents and
materials referenced or mentioned herein are indicative of the levels of skill
of those skilled
in the art to which the invention pertains, and each such referenced document
and material is
hereby incorporated by reference to the same extent as if it had been
incorporated by
reference in its entirety individually or set forth herein in its entirety.
Applicants reserve the
right to physically incorporate into this specification any and all materials
and information
from any such patents, publications, scientific articles, web sites,
electronically available
information, and other referenced materials or documents.
[00339] The specific methods and compositions described herein are
representative of
preferred embodiments and are exemplary and not intended as limitations on the
scope of the
invention. Other objects, aspects, and embodiments will occur to those skilled
in the art upon
consideration of this specification, and are encompassed within the spirit of
the invention as
defined by the scope of the claims. It will be readily apparent to one skilled
in the art that
varying substitutions and modifications may be made to the invention disclosed
herein
without departing from the scope and spirit of the invention. The invention
illustratively
described herein suitably may be practiced in the absence of any element or
elements, or
limitation or limitations, which is not specifically disclosed herein as
essential. Thus, for
example, in each instance herein, in embodiments or examples of the present
invention, any
98
CA 02868534 2014-09-25
WO 2013/148736
PCT/US2013/033948
of the terms "comprising", "consisting essentially of", and "consisting of"
may be replaced
with either of the other two terms in the specification. Also, the terms
"comprising",
"including", containing", etc. are to be read expansively and without
limitation. The methods
and processes illustratively described herein suitably may be practiced in
differing orders of
steps, and that they are not necessarily restricted to the orders of steps
indicated herein or in
the claims. It is also that as used herein and in the appended claims, the
singular forms "a,"
"an," and "the" include plural reference unless the context clearly dictates
otherwise. Under
no circumstances may the patent be interpreted to be limited to the specific
examples or
embodiments or methods specifically disclosed herein. Under no circumstances
may the
patent be interpreted to be limited by any statement made by any examiner or
other official or
employee of government patent office unless such statement is specifically and
without
qualification or reservation expressly adopted in a responsive writing by or
on behalf of the
inventor(s).
[00340] The terms and expressions that have been employed are used as terms of
description and not of limitation, and there is no intent in the use of such
terms and
expressions to exclude any equivalent of the features shown and described or
portions
thereof, but it is recognized that various modifications are possible within
the scope of the
invention as claimed. Thus, it will be understood that although the present
invention has
been specifically disclosed by preferred embodiments and optional features,
modification and
variation of the concepts herein disclosed may be resorted to by those skilled
in the art, and
that such modifications and variations are considered to be within the scope
of this invention
as defined by the appended claims.
[00341] The invention has been described broadly and generically herein. Each
of the
narrower species and subgeneric groupings falling within the generic
disclosure also form
part of the invention. This includes the generic description of the invention
with a proviso or
negative limitation removing any subject matter from the genus, regardless of
whether or not
the excised material is specifically recited herein.
[00342] Other embodiments are within the following claims. In addition, where
features or aspects of the invention are described in terms of Markush groups,
those skilled in
the art will recognize that the invention is also thereby described in terms
of any individual
member or subgroup of members of the Markush group.
99