Note: Descriptions are shown in the official language in which they were submitted.
1
METHODS FOR UPGRADING OF CONTAMINATED HYDROCARBON STREAMS
[0001] Deleted
BACKGROUND
[0002] The present disclosure is directed to systems and methods for upgrading
crude oil,
refinery intermediate streams, and refinery products to substantially decrease
the content of undesired
heteroatom contaminants, including, but not limited to, sulfur, nitrogen,
phosphorus, nickel, vanadium,
iron, with the added benefit of decreasing the total acid number and
increasing the API gravity. A
heteroatom contaminated hydrocarbon feed stream is subjected to heteroatom
oxidizing conditions to
produce an oxidized-heteroatom-containing hydrocarbon intermediate stream and
then contacting said
stream with a selectivity promoter and caustic thereby removing the heteroatom
contaminants from the
hydrocarbon stream and thereby increasing the API gravity and decreasing the
total acid number relative
to the initial contaminated hydrocarbon feed stream.
[0003] As is well known in the industry, crude oil contains heteroatom
contaminants
including, but not limited to, sulfur, nitrogen, phosphorus, nickel, vanadium,
and iron and acidic
oxygenates in quantities that negatively impact the refinery processing of the
crude oil fractions. Light
crude oils or condensates contain heteroatoms in concentrations as low as
0.001 wt %. In contrast, heavy
crude oils contain heteroatoms as high as 5-7 wt %. The heteroatom content of
crude oil increases with
increasing boiling point and the heteroatom content increases with decreasing
API gravity. These
contaminants must be removed during refining operations to meet the
environmental regulations for the
final product specifications (e.g., gasoline, diesel, fuel oil) or to prevent
the contaminants from decreasing
catalyst activity, selectivity, and lifetime in downstream refining
operations. Contaminants such as sulfur,
nitrogen, phosphorus, nickel, vanadium, iron, and total acid number (TAN) in
the crude oil fractions
negatively impact these downstream processes, and others, including
hydrotreating, hydrocracking and
FCC to name just a few. These contaminants are present in the crude oil
fractions in various organic
hydrocarbon molecules and in various concentrations.
CA 2868851 2019-12-19
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
2
[0004] Sulfur is widely recognized as the most egregious heteroatom
contaminant as a result
of the environmental hazard caused by its release into the environment after
combustion. It is believed,
sulfur oxides from combustion (known collectively as SOõ emissions) contribute
to the formation of acid
rain and also to the reduction of the efficiency of catalytic converters in
automobiles. Furthermore, sulfur
compounds are thought to ultimately increase the particulate content of
combustion products. Nitrogen,
phosphorus, and other heteroatom contaminants present similar environmental
risks.
[0005] A variety of methods have been implemented for removing sulfur
compounds either
from fuels before combustion or from emission gases afterward. Most refineries
employ
hydrodesulfurization (HDS) as the predominant process for removing sulfur from
hydrocarbon streams.
HDS remains a cost-effective option for light streams with sulfur levels up to
about 2% (w/w) elemental
sulfur, but the environmental and economic benefits of HDS are offset in very
heavy and sour (>2%
elemental sulfur) streams because the energy input to the reaction, the high
pressures and the amount of
hydrogen necessary to remove the sulfur paradoxically create a substantial CO2
emission problem.
[0006] Because of these issues, reduction of contaminants and, in
particular, of the sulfur
content in hydrocarbon streams has become a major objective of environmental
legislation worldwide.
Sulfur is regulated in the United States for on-road diesel at a maximum
concentration of 15 ppm. By
October 2012, sulfur specifications will be 15 ppm for non-road, locomotive,
and marine diesel fuel. In
the European Union that specification is expected to tighten to 10 ppm in
January 2011 for diesels
intended for inland waterways and for on-road and off-road diesel operated
equipment. In China, the on-
road diesel specification will be 10 ppm by 2012. Currently the tightest
specifications in the world are in
Japan, where the on-road diesel specification is 10 ppm.
[0007] Refiners typically use catalytic hydrodesulfurizing ("HDS", commonly
referred to as
"hydrotreating") methods to lower the sulfur content of hydrocarbon fuels,
decrease the total acid number,
and increase the API gravity. In HDS, a hydrocarbon stream that is derived
from petroleum distillation is
treated in a reactor that operates at temperatures ranging between 575 and 750
F. (about 300 to about
400 C.), a hydrogen pressure that ranges between 430 to 14,500 psi (3000 to
10,000 kPa or 30 to 100
atmospheres) and hourly space velocities ranging between 0.5 and 411'.
Dibenzothiophenes in the feed
react with hydrogen when in contact with a catalyst arranged in a fixed bed
that comprises metal sulfides
from groups VI and VIII (e.g., cobalt and molybdenum sulfides or nickel and
molybdenum sulfides)
supported on alumina. Because of the operating conditions and the use of
hydrogen, these methods can be
costly both in capital investment and operating costs.
[0008] As is currently known, HDS or hydrotreating may provide a treated
product in
compliance with the current strict sulfur level targets. However, due to the
presence of sterically hindered
refractory sulfur compounds such as substituted dibenzothiophenes, the process
is not without issues. For
example, it is particularly difficult to eliminate traces of sulfur using such
catalytic processes when the
sulfur is contained in molecules such as dibenzothiophene with alkyl
substituents in position 4-, or 4- and
6-positions of the parent ring. Attempts to completely convert these species,
which are more prevalent in
heavier stocks such as diesel fuel and fuel oil, have resulted in increased
equipment costs, more frequent
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
3
catalyst replacements, degradation of product quality due to side reactions,
and continued inability to
comply with the strictest sulfur requirements for some feeds.
[0009] This has prompted many to pursue non-hydrogen alternatives to
desulfurization, such
as oxydesulfurization. One attempt at solving the problem discussed above
includes selectively
desulfurizing dibenzothiophenes contained in the hydrocarbon stream by
oxidizing the dibenzothiophenes
into a sulfone in the presence of an oxidizing agent, followed by optionally
separating the sulfone
compounds from the rest of the hydrocarbon stream and further reacting the
sulfones with a caustic to
remove the sulfur moiety from the hydrocarbon fragment.
[0010] Oxidation has been found to be beneficial because oxidized sulfur
compounds can be
removed using a variety of separation processes that rely on the altered
chemical properties such as the
solubility, volatility, and reactivity of the sulfone compounds. An important
consideration in employing
oxidation is chemical selectivity. Selective oxidation of sulfur heteroatom
moieties without oxidizing the
plethora of olefins and benzylic hydrocarbons found in crude oils, refinery
intermediates, and refinery
products remains a significant challenge. One selective sulfoxidation method
and system is disclosed in
International Publication Number WO 2009/120238 Al, to Litz et al. The
inventors of the present
disclosure have further discovered that the catalyst of the above-mentioned
international publication
number is further capable of oxidizing additional heteroatoms, including, but
not limited to nitrogen and
phosphorus found as naturally abundant contaminants in crude oils, refinery
intermediates, and refinery
products as organic heteroatom-containing compounds. Figure 1 describes a
table of available oxidation
states for organic heteroatom compounds.
[0011] Another concern with heteroatom oxidation lies in the fate of the
oxidized organic
heteroatom compounds produced. If the oxidized organic heteroatom compounds
are hydrotreated, they
may be converted back to the original heteroatom compounds thereby
regenerating the original problem.
The feed heteroatom content may be likely to be in the range of 0% to 10% by
weight heteroatom.
Heteroatoms, on average, comprise about 15 wt % of substituted and
unsubstituted organic heteroatom
molecules. Therefore, up to 67 wt % of the oil may be removed as oxidized
organic heteroatom extract if
not removed from the organic molecules. For a typical refinery processing
40,000 barrels per day of crude
oil, up to 27,000 barrels per day of oxidized organic heteroatom oil will be
generated, which is believed to
be too much to dispose of conventionally as a waste product. Further, the
disposal of oxidized organic
heteroatom oil wastes valuable hydrocarbons, which could theoretically be
recycled if an efficient process
were available.
[0012] A considerable challenge presented to heteroatoin removal remains
the removal of the
oxidized heteroatom fragment from the oxidized organic heteroatom compounds
created by oxidation of
the initial organic heteroatom species. Therefore, a need exists for methods
and systems for upgrading
heteroatom-contaminated hydrocarbon feed streams by removing heteroatom
contaminants from
hydrocarbon streams with the added benefit of decreasing the total acid number
and increasing the API
gravity of the resulting product relative to the contaminated hydrocarbon feed
stream.
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
4
SUMMARY OF THE DISCLOSURE
[0013] The present invention relates to a method of upgrading a heteroatom-
containing
hydrocarbon feed by removing heteroatom contaminants, the method comprising:
contacting the
heteroatom-containing hydrocarbon feed with at least one oxidant and at least
one immiscible acid;
contacting the oxidized heteroatom-containing hydrocarbon feed with at least
one caustic and at least one
selectivity promoter; and removing the heteroatom contaminants from the
heteroatom-containing
hydrocarbon feed. The oxidant may be used in the presence of a catalyst.
[0014] The invention further provides a method of upgrading a heteroatom-
containing
hydrocarbon feed by removing heteroatom contaminants, the method comprising:
contacting the
heteroatom-containing hydrocarbon feed with an oxidant to oxidize at least a
portion of the heteroatom
contaminants to form a first intermediate stream; contacting the first
intermediate stream with at least one
oxidant and at least one immiscible acid to oxidize at least a portion of any
remaining heteroatom
contaminants to form a second intermediate stream, contacting the second
intermediate stream with at
least one caustic and at least one selectivity promoter, said at least one
selectivity promoter comprising an
organic compound having at least one acidic proton, to form a third
intermediate stream; separating a
substantially heteroatom- free hydrocarbon product from the third intermediate
stream; recovering the at
least one caustic and at least one selectivity promoter from the second
intermediate stream; and recycling
the recovered at least one caustic and at least one selectivity promoter.
[0015] The invention still further provides a method of upgrading a
heteroatom-containing
hydrocarbon feed by removing heteroatom contaminants, the method comprising
oxidizing
dibenzothiophenes in the heteroatom-containing feed to sulfones, contacting
the sulfones under oxidizing
biphasic conditions with an immiscible acid and an oxidant to remove at least
a portion of the heteroatom
contaminants, then reacting the sulfones with caustic and a selectivity
promoter, and separating a
substantially heteroatom-free hydrocarbon product for fuel.
[0016] Other features, aspects, and advantages of the present invention
will become better
understood with reference to the following description.
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] The features of the disclosure are set forth in the appended
claims. The disclosure
itself, however, will be best understood by reference to the following
detailed description of illustrative
embodiments when read in conjunction with the accompanying drawings, wherein:
[0018] Figure 1 is a graphic representation of the various oxidation
states of certain
heteroatoms, in accordance with embodiments of the present disclosure.
[0019] Figure 2 is a generic process flow diagram of an embodiment of a
combination
heteroatom oxidation process followed by heteroatom cleavage, in accordance
with embodiments of the
present disclosure.
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
[0020] Figure 3A is a more detailed process flow diagram of an embodiment of a
combination
heteroatom oxidation process followed by heteroatom cleavage, in accordance
with embodiments of the
present disclosure.
[0021] Figure 3B is an alternative more detailed process flow diagram of an
embodiment of a
combination heteroatom oxidation process followed by heteroatom cleavage, in
accordance with
embodiments of the present disclosure.
[0022] Figure 4 is an even more detailed process flow diagram of an embodiment
of a
combination heteroatom oxidation process followed by heteroatom cleavage, in
accordance with
embodiments of the present disclosure.
[0023] Figure 5 is an alternative even more detailed process flow diagram of
an embodiment
of a combination heteroatom oxidation process followed by heteroatom cleavage,
in accordance with
embodiments of the present disclosure.
DETAILED DESCRIPTION OF THE PRESENTLY PREFERRED EMBODIMENTS
[0024] While this disclosure contains many specific details, it should be
understood that
various changes and modifications may be made without departing from the scope
of the technology
herein described. The scope of the technology shall in no way be construed as
being limited to the
number of constituting components, the concentration of constituting
components, the materials thereof,
the shapes thereof, the relative arrangement thereof, the temperature
employed, the order of combination
of constituents thereof, etc., and are disclosed simply as examples. The
depictions and schemes shown
herein are intended for illustrative purposes and shall in no way be construed
as being limiting in the
number of constituting components, connectivity, reaction steps, the materials
thereof, the shapes thereof,
the relative arrangement thereof, the order of reaction steps thereof, etc.,
and are disclosed simply as an
aid for understanding. The examples described herein relate to the oxidation
of heteroatom contaminates
in hydrocarbon streams including crude oil, refinery intermediate streams, and
refinery products, and they
relate to systems and methods for the removal of said oxidized heteroatoms
from said hydrocarbon
streams.
[0025] Unless otherwise indicated, all numbers expressing quantities of
ingredients, properties
such as molecular weight, reaction conditions, and so forth used in this
specification and claims are to be
understood as being modified in all instances by the term "about."
Accordingly, unless indicated to the
contrary, the numerical parameters set forth in the following specification
and attached claims are
approximations that may vary depending upon the desired properties sought to
be obtained by the present
disclosure. At the very least, and not as an attempt to limit the application
of the doctrine of equivalents
to the scope of the claims, each numerical parameter should at least be
construed in light of the number of
reported significant digits and by applying ordinary rounding techniques.
[0026] Notwithstanding that the numerical ranges and parameters setting forth
the broad scope
of the disclosure are approximations, the numerical values set forth in the
specific examples are reported
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
6
as precisely as possible. Any numerical value, however, inherently contain
certain errors necessarily
resulting from the standard deviation found in their respective testing
measurements.
[0027] As used in this application, the term "biphasic" means a chemical
system that contains
two separate and distinct immiscible chemical phases.
[0028] As used in this application, the term "promoted-caustic visbreaker"
means a heated
reactor that contains a caustic and a selectivity promoter that react with
oxidized heteroatoms to remove
sulfur, nickel, vanadium, iron and other heteroatoms, increase API gravity and
decrease total acid
number.
[0029] As used in this application, the term "contaminated hydrocarbon stream"
is a mixture of
hydrocarbons containing heteroatom constituents. "Heteroatoms" is intended to
include all elements
other than carbon and hydrogen.
[0030] Oxidation may be carried out in a single step using at least one
oxidant, optionally in
the presence of a catalyst, and at least one immiscible acid. The reaction
mixture will be biphasic,
comprising a hydrocarbon oil phase, and an acid phase. The purpose of the
immiscible acid and oxidant
treatment is to remove a portion of the heteroatom contaminants from the feed.
Upon being oxidized by
the immiscible acid and oxidant, these heteroatoms will become soluble in the
acid phase, and be
subsequently removed.
[0031] In another embodiment, oxidation may also be carried out in two
steps; an initial
oxidation using at least one oxidant, optionally in the presence of a
catalyst, followed by a secondary
oxidation using at least one oxidant, optionally in the presence of a
catalyst, and at least one immiscible
acid. The oxidant and the optional catalyst in each step may be the same or
different.
[0032] The initial oxidation step is more selective towards sulfur and/or
nitrogen-containing
heteroatom contaminants, although other heteroatom contaminants may be
oxidized. The secondary
oxidation step is more selective towards oxidizing other heteroatom
contaminants, such as metal-
containing heteroatom containing contaminants. By targeting specific
heteroatoms in the first oxidation,
alternative oxidation reactions can be utilized to oxidize more heteroatom
contaminants in the second
improving chemical process efficiency.
[0033] The oxidation reaction(s) may be carried out at a temperature of about
20 C to about
120 C, at a pressure of about 0.5 atmospheres to about 10 atmospheres, with a
contact time of about 2
minutes to about 180 minutes. The oxidant employed may be any oxidant which,
optionally in the
presence of a catalyst, oxidizes heteroatoms in the heteroatom-containing
hydrocarbon feed, for example,
but not limited to, hydrogen peroxide, peracetic acid, benzyl hydroperoxide,
ethylbenzene hydroperoxide,
cumyl hydroperoxide, sodium hypochlorite, oxygen, air, etc, and more presently
preferably an oxidant
which does not oxidize the heteroatom-free hydrocarbons in the contaminated
hydrocarbon feed. Even
more preferably, the catalyst employed therein may be any catalyst capable of
utilizing an oxidant to
oxidize heteroatoms in the heteroatom-containing hydrocarbon feed
[0034] Suitable catalysts include, but are not limited to, catalyst
compositions represented by
the formula Min0,,(0R)n, where M is a metal complex, such as, for example,
titanium or any metal,
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
7
including, but not limited to, rhenium, tungsten or other transition metals
alone or in combination that
causes the chemical conversion of the sulfur species, as described herein. R
is carbon group having at
least 3 carbon atoms, where at each occurrence R may individually be a
substituted alkyl group
containing at least one OH group, a substituted cycloalkyl group containing at
least one OH group, a
substituted cycloalkylalkyl group containing at least one OH group, a
substituted heterocyclyl group
containing at least one OH group, or a heterocyclylalkyl containing at least
one OH group. The subscripts
m and n may each independently be integers between about 1 and about 8. R may
be substituted with
halogens such as F, Cl, Br, and I. In some embodiments, the metal alkoxide
comprises
bis(glycerol)oxotitanium(IV)), where M is Ti, m is 1, n is 2, and R is a
glycerol group. Other examples of
metal alkoxides include bis(ethyleneglycol)oxotitanium (IV),
bis(erythritol)oxotitanium (IV), and
bis(sorbitoBoxotitanium (IV), as disclosed in International Publication Number
WO 20091120238 Al , to
Litz et al.
[0035] Other suitable catalysts include, but are not limited to, catalyst
compositions prepared
by the reaction of Q-R-Q' with a bis(polyol)oxotitanium(IV) catalyst, wherein
Q and Q' each
independently comprise an isocyanate, anhydride, sulfonyl halide, benzyl
halide, carboxylic acid halide,
phosphoryl acid halide, silyl chloride, or any chemical functionality capable
of reacting with the -OH
pendant group of the catalyst, and wherein R comprises a linking group. The R
linking group is selected
from the group consisting of alkyl groups (including linear, branched,
saturated, unsaturated, cyclic, and
substituted alkyl groups, and wherein hetero atoms, such as oxygen, nitrogen,
sulfur, silicon, phosphorus,
and the like can be present in the alkyl group), typically with from 1 to
about 22 carbon atoms, preferably
with from 1 to about 12 carbon atoms, and more preferably with from 1 to about
7 carbon atoms, although
the number of carbon atoms can be outside of these ranges, aryl groups
(including substituted aryl
groups), typically with from about 6 to about 30 carbon atoms, preferably with
from about 6 to about 15
carbon atoms, and more preferably with from about 6 to about 12 carbon atoms,
although the number of
carbon atoms can be outside of these ranges, arylalkyl groups (including
substituted arylalkyl groups),
typically with from about 7 to about 30 carbon atoms, preferably with from
about 7 to about 15 carbon
atoms, and more preferably with from about 7 to about 12 carbon atoms,
although the number of carbon
atoms can be outside of these ranges, such as benzyl or the like, alkylaryl
groups (including substituted
alkylaryl groups), typically with from about 7 to about 30 carbon atoms,
preferably with from about 7 to
about 15 carbon atoms, and more preferably with from about 7 to about 12
carbon atoms, although the
number of carbon atoms can be outside of these ranges, silicon or phosphorus,
typically with from 1 to
about 22 carbon atoms, preferably with from 1 to about 12 carbon atoms, and
more preferably with from
1 to about 7 carbon atoms, although the number of carbon atoms can be outside
of these ranges,
polyalkyleneoxy groups (including substituted polyalkyleneoxy groups), such as
polyethyleneoxy groups,
polypropyleneoxy groups, polybutyleneoxy groups, and the like, typically with
from about 3 to about 60
repeat alkyleneoxy units, preferably with from about 3 to about 30 repeat
alkyleneoxy units, and more
preferably with from about 3 to about 20 repeat alkyleneoxy units, although
the number of repeat
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
8
alkyleneoxy units can be outside of these ranges, as disclosed in
International Publication Number WO
2009/120238 Al, to Litz et al.
[0036] The immiscible acid used may be any acid which is insoluble in the
hydrocarbon oil
phase. Suitable immiscible acids may include, but are not limited to,
carboxylic acids, sulfuric acid,
hydrochloric acid, and mixtures thereof, with or without varying amounts of
water as a diluent. Suitable
carboxylic acids may include, but are not limited to, formic acid, acetic
acid, propionic acid, butyric acid,
lactic acid, benzoic acid, and the like, and mixtures thereof, with or without
varying amounts of water as a
diluent.
[0037] The solvent used in extracting the heteroatom-containing hydrocarbon
stream after the
oxidation reaction (e.g. in a liquid-liquid extractor) may be any solvent with
relatively low solubility in
oil but relatively high solubility of oxidized heteroatom-containing
hydrocarbons, including, but not
limited to, acetone, methanol, ethanol, ethyl lactate, N-methylpyrollidone,
dimethylacetamide,
dimethylformamide, gamma-butyrolactone, dimethyl sulfoxide, propylene
carbonate, acetonitrile, acetic
acid, sulfuric acid, and liquid sulfur dioxide, which is capable of extracting
the heteroatoms from the
heteroatom containing hydrocarbon stream and producing a substantially
heteroatom-free hydrocarbon
product.
[0038] The caustic of the present invention may be any compound which exhibits
basic
properties including, but not limited to, metal hydroxides and sulfides, such
as alkali metal hydroxides
and sulfides, including, but not limited to, Li0H, NaOH, KOH and Na2S; alkali
earth metal hydroxides,
such as Ca(OH)2, Mg(OH)7 and Ba(OH); carbonate salts, such as alkali metal
carbonates, including, but
not limited to, Na,CO3 and K2CO3; alkali earth metal carbonates, such as
CaCO3, MgCO3 and BaCO3;
phosphate salts, including, but not limited to, alkali metal phosphates, such
as sodium pyrophosphate,
potassium pyrophosphate, sodium tripolyphosphate and potassium
tripolyphosphate; and alkali earth
metal phosphates, such as calcium pyrophosphate, magnesium pyrophosphate,
barium pyrophosphate,
calcium tripolyphosphate, magnesium tripolyphosphate and barium
tripolyphosphate; silicate salts, such
as, alkali metal silicates, such as sodium silicate and potassium silicate,
and alkali earth metal silicates,
such as calcium silicate, magnesium silicate and barium silicate, organic
alkali compounds expressed by
the general formula: R-En MniCri, where R is hydrogen or an organic compound
(which may be further
substituted) including, but not limited to, straight, branched and cyclic
alkyl groups; straight, branched
and cyclic alkenyl groups; and aromatic or polycyclic aromatic groups. Further
substituents where R is
an organic may include hydroxide groups, carbonyl groups, aldehyde groups,
ether groups, carboxylic
acid and carboxylate groups, phenol or phenolate groups, alkoxide groups,
amine groups, imine groups,
cyano groups, thiol or thiolate groups, thioether groups, disulfide groups,
sulfate groups, and phosphate
groups. En- represents an atom with a negative charge (where n = -1, -2, -3, -
4 etc.) such as oxygen,
sulfur, selenium, tellurium, nitrogen, phosphorus, and carbon; and Min is any
cation (m = +1, +2, +3, +4
etc.), such as a metal ion, including, but not limited to, alkali metals, such
as Li, Na, and K, alkali earth
metals, such as Mg and Ca, and transition metals, such as Zn, and Cu. When in
> +1, Q may be the same
CA 02868851 2014-09-26
WO 2013/188144
PCT/US2013/043843
9
as Ell-R or an atom with a negative charge such as Br-, Cl-, I, or an anionic
group that supports the charge
balance of the cation Mm' including but not limited to, hydroxide, cyanide,
cyanate, and carboxylates.
[0039] Examples of the straight or branched alkyl groups may include methyl,
ethyl, n-,
sec- and t-butyl, octyl, 2-ethylhexyl and octadecyl. Examples of the straight
or branched alkenyl groups
may include vinyl, propenyl, allyl and butenyl. Examples of the cyclic alkyl
and cyclic alkenyl groups
may include cyclohexyl, cyclopentyl, and cyclohexene. Examples of the aromatic
or polycyclic aromatic
groups may include aryl groups, such as phenyl, naphthyl, andanthracenyl;
aralkyl groups, such as benzyl
and phenethyl; alkylaryl groups, such as methylphenyl, ethylphenyl,
nonylphenyl, methylnaphthyl and
ethylnaphthyl.
[0040] Preferred
caustic compounds, based on reaction conversion and selectivity, are alkali
metal hydroxides and sulfides, such as NaOH, KOH, Na2S, and/or mixtures
thereof.
[0041] In one embodiment of the present invention, the caustic may be in the
molten phase.
Presently preferred molten phase caustics include, but are not limited to,
eutectic mixtures of the
inorganic hydroxides with melting points less than 350 C, such as, for
example, a 51 mole % NaOH / 49
mole % KOH eutectic mixture which melts at about 170 C.
[0042] In another embodiment of the present invention, the caustic may be
supported on an
inorganic support, including, but not limited to, oxides, inert or active,
such as, for example, a porous
support, such as talc or inorganic oxides.
[0043] Suitable
inorganic oxides include, but are not limited to, oxides of elements of groups
TB, II-A and II-B, III-A and II-B, IV-A and IV-B, V-A and V-B, VI-B, of the
Periodic Table of the
Elements. Examples of oxides preferred as supports include copper oxides,
silicon dioxide, aluminum
oxide, and/or mixed oxides of copper, silicon and aluminum. Other suitable
inorganic oxides which may
be used alone or in combination with the abovementioned preferred oxide
supports may be, for example,
MgO, ZrO2, TiO2 CaO and/or mixtures thereof.
[0044] The support materials used may have a specific surface area in the
range from 10 to
1000 m 2/g, a pore volume in the range from 0.1 to 5 ml/g and a mean particle
size of from 0.1 to 10 cm.
Preference may be given to supports having a specific surface area in the
range from 0.5 to 500 m 2 a
pore volume in the range from 0.5 to 3.5 ml/g and a mean particle size in the
range from 0.5 to 3 cm.
Particular preference may be given to supports having a specific surface area
in the range from 200 to 400
m 2/g, and a pore volume in the range from 0.8 to 3.0 ml/g.
[0045] The selectivity promoter of the present invention may be any organic
compound having
at least one acidic proton. Generally, the selectivity promoter has a pKa (log
of the acid dissociation
constant) value, as measured in DMSO (dimethylsulfoxide), in the range of from
about 9 to about 32,
preferably in the range of from about 18 to about 32. Examples of the
selectivity promoter include, but
are not limited to, hydroxyl-functional organic compounds; straight, branched,
or cyclic amines having at
least one H substituent; and/or mixtures thereof The selectivity promoter may
further include crown
ethers.
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
[0046] Suitable hydroxyl-functional organic compounds include, but are
not limited to: (i)
straight-, branched-, or cyclic-alkyl alcohols (which may be further
substituted) such as methanol,
ethanol, isopropanol, ethylhexanol, cyclohexanol, ethanolamine, di-, and tri-
ethanolamine, mono- and di-
methylaminoethanol; including -diols such as ethylene glycol, propylene
glycol, 1,3-propanediol, and 1,2-
cyclohexanediol; and ¨polyols, such as glycerol, erythritol, xylitol,
sorbitol, etc; -monosaccharides, such
as glucose, fructose, galactose, etc; -disaccharides, such as sucrose,
lactose, and maltose; -
polysaccharides, such as starch, cellulose, glycogen, chitan, wood chips and
shavings; (ii) straight-,
branched-, or cyclic-alkenyl alcohols (which may be further substituted), such
as vinyl alcohol, and allyl
alcohol; (iii)aryl- and aralkyl- alcohols (which may be further substituted),
such as phenol, and benzyl
alcohol; (iv) polycyclic aryl- and aralkyl- alcohols (which may be further
substituted), such as naphthol,
and -tetralol; and (v) ammonium salts, such as choline hydroxide, and
benzyltrimethylammonium
hydroxide.
[0047] Examples of straight or branched alkyls may include: methyl,
ethyl, n-, sec- and t-
butyl, octyl, 2-ethylhexyl and octadecyl. Examples of the straight or branched
alkenyls may include:
vinyl, propenyl, allyl and butenyl. Examples of the cyclic-alkyls may include:
cyclohexyl, and
cyclopentyl. Examples of aryls, aralkyls and polycyclics include: aryls, such
as phenyl, naphthyl,
anthracenyl; aralkyls, such as benzyl and phenethyl; alkylaryl, such as
methylphenyl, ethylphenyl,
nonylphenyl, methylnaphthyl and ethylnaphthyl.
[0048] Suitable amines, include, but are not limited to, straight-,
branched-, and cyclic-amines
having at least one H substituent, which may be further substituted,
including, but not limited to, mono-,
or di-substituted amines, such as methylamine, ethylamine, 2-ethylhexylamine,
piperazine, 1,2-
diaminoethane and/or mixtures thereof.
[0049] Suitable crown ethers, which may be further substituted, include,
but are not limited to,
18-crown-6, 15-crown-5, etc; and/or mixtures thereof.
[0050] Preferred selectivity promoters, based on reaction conversion and
selectivity, are
ethylene glycol, propylene glycol, triethanolamine, and/or mixtures thereof.
[0051] In one embodiment of the present invention the at least one
caustic and the at least one
selectivity promoter may be different components. In another embodiment of the
present invention the at
least one caustic and the at least one selectivity promoter may be the same
component When the at least
one caustic and the at least one selectivity promoter are the same component
they may be referred to as a
caustic selectivity promoter. Moreover, a suitable caustic selectivity
promoter may possess the properties
of both the at least one caustic and the at least one selectivity promoter.
That is, combinations of caustics
with selectivity promoters may react (in situ or a priori) to form a caustic
selectivity promoter which has
the properties of both a caustic and a selectivity promoter.
[0052] The caustic selectivity promoter may react with the oxidized heteroatom-
containing
compounds, such as dibenzothiophene, sulfoxides, dibenzothiophene sulfones,
and/or mixtures thereof, to
produce substantially non-oxygenated hydrocarbon products, such as biphenyls.
Non-limiting examples
of caustic selectivity promoters include, but are not limited to, sodium
ascorbate, sodium erythorbate,
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
11
sodium gluconate, 4-hydroxyphenyl glycol, sodium salts of starch or cellulose,
potassium salts of starch
or cellulose, sodium salts of chitan or chitosan, potassium salts of chitan or
chitosan, sodium glycolate,
glyceraldehyde sodium salt, 1-thio-beta-D-glucose sodium salt, and/or mixtures
thereof.
[0053] For example, the caustic, such as sodium hydroxide and/or potassium
hydroxide and
the selectivity promoter, such as ethylene glycol, may react in situ or prior
to contacting with the oxidized
heteroatoin-containing hydrocarbon feed, to fonn water and a caustic
selectivity promoter, such as the
sodium or potassium salt of ethylene glycol. Generally, an excess molar ratio
of selectivity promoter
hydroxyl groups to caustic cations is preferred for conversion and
selectivity.
[0054] The promoted-caustic visbreaker reaction may take place at a
temperature in the range
of from about 150 C to about 350 C, at a pressure in the range of from about 0
psig to about 2000 psig,
with a contact time in the range of from about 2 minutes to about 180 minutes.
Without being limited to
any particular theory, the reaction mechanism is believed to include a
solvolysis reaction; particularly
alcoholysis when the selectivity promoter is an alcohol, and aminolysis when
the selectivity promoter is
an amine; without the selectivity promoter of the present invention, the
reaction mechanism may involve
hydrolysis which leads to the undesirable formation of substantially
oxygenated product.
[0055] Generally, the mole ratio of caustic to selectivity promoter is in
the range of from about
10:1 to about 1:10, preferably the mole ratio of caustic to selectivity
promoter is in the range of from
about 3:1 to about 1:3, and more preferably the mole ratio of caustic to
selectivity promoter is in the range
of from about 2:1 to about 1:2.
[0056] Generally, the mole ratio of caustic and selectivity promoter to
heteroatom in the
heteroatom-containing hydrocarbon feed oil is in the range of from about 100:1
to about 1:1, preferably
the mole ratio of caustic and selectivity promoter to heteroatom in the
heteroatom-containing hydrocarbon
feed oil is in the range of from about 10:1 to about 1:1, and more preferably
the mole ratio of caustic and
selectivity promoter to heteroatom in the heteroatom-containing hydrocarbon
feed oil is in the range of
from about 3:1 to about 1:1.
[0057] Separation of the heavy caustic phase from the light oil phase may be
by gravity. Other
suitable methods include, but are not limited to, solvent extraction of the
caustic or oil phases, such as by
washing with water, centrifugation, distillation, vortex separation, and
membrane separation and
combinations thereof. Trace quantities of caustic and selectivity promoter may
be removed according to
known methods by those skilled in the art.
[0058] As a result of removing the heteroatom contaminants from the heteroatom-
containing
hydrocarbon feed, the light oil phase product has a lower density and
viscosity than the untreated,
contaminated feed. The heavy caustic phase density is generally in the range
of from about 1.0 to about
3.0 g/mL and the light product oil phase density is generally in the range of
from about 0.7 to about 1.1
g/mL.
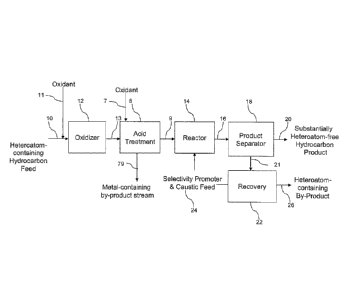
[0059] As illustrated in Figure 2, a heteroatom-containing hydrocarbon feed 10
may be
combined with an oxidant 11 and subjected to an oxidizing process in an
oxidizer vessel 12 in order to
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
12
meet current and future environmental standards. The oxidizer vessel 12 may
optionally contain a
catalyst or oxidation promoter (not shown).
[0060] After subjecting a hydrocarbon stream to oxidation conditions in
oxidizer vessel 12,
thereby oxidizing at least a portion of the heteroatom compounds (e.g.,
oxidizing dibenzothiophenes to
sulfones), intermediate stream 13 may be generated. The intermediate stream 13
may be combined with
an oxidant 7 and an immiscible acid and subjected to an oxidizing process in
acid treatment reactor 8,
thereby oxidizing a further portion of the heteroatom compounds (e.g.,
oxidizing metalloporphyrins to
generate porphyrins and metal salts), generating intermediate stream 9 and
metal-containing acidic by-
product stream 79. The intermediate stream 9 may be reacted with caustic
(e.g., sodium hydroxide,
potassium hydroxide, eutectic mixtures thereof etc.) and a selectivity
promoter 24 in reactor 14 to produce
a biphasic intermediate stream 16.
[0061] Intermediate stream 16 may be transferred to a product separator 18
from which a
substantially heteroatom-free hydrocarbon product 20 may be recovered from the
light phase. The denser
phase 21 containing the selectivity promoter and caustic and heteroatom by-
products may be transferred
to a recovery vessel 22 in which the selectivity promoter and caustic 24 may
be recovered and recycled to
reactor 14 and the heteroatom-containing byproduct 26 may be sent to a
recovery area for further
processing, as would be understood by those skilled in the art.
[0062] In a more specific embodiment, as illustrated in Figure 3A, a
heteroatom-containing
hydrocarbon feed 30 may be combined with a hydroperoxide 32 in a catalytic
oxidizer 34 thereby
oxidizing the heteroatoms yielding intermediate stream 36. Intermediate stream
36 may be fed to a by-
product separator 38 from which the hydroperoxide by-product may be recovered
and recycled for reuse
in catalytic oxidizer 34 (as would be understood by those skilled in the art)
yielding intermediate stream
39. The intermediate stream 39 may be reacted with an oxidant 7 and an
immiscible acid feed 77 in acid
treatment column 71 producing intermediate stream 73 from the hydrocarbon
phase and intermediate
stream 75 from the acid phase. Intermediate stream 75 may be fed to a solvent
recovery unit 81 from
which the acid 77 may be recovered and recycled for reuse in acid treatment
column 71 producing a
metal-containing by-product stream 79.
[0063] The intermediate stream 73 may be reacted with a selectivity promoter
and caustic feed
42 in promoted-caustic visbreaker 40 producing intermediate biphasic stream 44
that may be separated in
product separator 46 to produce a substantially heteroatom-free hydrocarbon
product 48 from the light
phase. The dense phase 49 from product separator 46 may be transferred to
heteroatom by-product
separator 50 from which a heteroatom-containing byproduct stream 52 and
selectivity promoter and
caustic feed 42 may be independently recovered, as would be known by those
skilled in the art.
[0064] In still another embodiment, as illustrated in Figure 3B, the
heteroatom-containing
hydrocarbon feed 30 may be combined with hydroperoxide 32 and contacted with a
catalyst in catalytic
oxidizer 34 yielding intermediate stream 36 which may be reacted with an
oxidant 7 and an immiscible
acid feed 77 in acid treatment column 71 producing intermediate stream 73 from
the hydrocarbon phase
and intermediate stream 75 from the acid phase. Intermediate stream 75 may be
fed to a solvent recovery
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
13
unit 81 from which the acid 77 may be recovered and recycled for reuse in acid
treatment column 71
producing a metal-containing by-product stream 79.
[0065] Intermediate stream 73 may be transferred to a promoted-caustic
visbreaker 40 where it
reacts with selectivity promoter and caustic feed 42 producing a biphasic
intermediate stream 62.
Intermediate stream 62 may be transferred to a product separator 38 from which
a substantially
heteroatom-free hydrocarbon product stream 48 may be removed as the light
phase and transported to
storage or commercial use. The byproduct separator 54 may separate the dense
phase 64 into two
streams: a heteroatom-containing by-product stream 52 (which may be
transported to storage or
commercial use) and a by-product mixture stream 66 containing the selectivity
promoter, caustic, and
hydroperoxide by-products for recovery and recycle, as would be known by those
skilled in the art.
[0066] In yet another embodiment, as illustrated in Figure 4, the
heteroatom-containing
hydrocarbon feed 30 may be mixed with a hydroperoxide feed 32 and may be
reacted with a catalyst or
promoter (not shown) in the catalytic oxidizer 34 producing intermediate
stream 36. Stream 36 may be
transferred to a by-product separator 38 from which the hydroperoxide by-
product 37 may be separated
producing intermediate stream 70. Stream 70 may be extracted by solvent 78 in
product separator 46
(e.g. a liquid-liquid extraction column) from which a substantially heteroatom-
free hydrocarbon product
72 may be withdrawn resulting in intermediate stream 74. Stream 74 may be fed
to solvent recovery 76
from which solvent 78 may be recovered and recycled to product separator 46,
producing intermediate
stream 80. Intermediate stream 80 may be reacted with an oxidant 7 and an
immiscible acid feed 77 in
acid treatment column 71 producing intermediate stream 73 from the hydrocarbon
phase and intermediate
stream 75 from the acid phase. Intermediate stream 75 may be fed to a solvent
recovery unit 81 from
which the acid 77 may be recovered and recycled for reuse in acid treatment
column 71 producing a
metal-containing by-product stream 79.
[0067] Intermediate stream 73 may be treated in the promoted-caustic
visbreaker 40
containing selectivity promoter and caustic feed 42 producing a biphasic
intermediate stream 82. The two
phases of stream 82 may be separated in product separator 84 as a light phase
48 and a dense phase 86.
The light phase 48 may comprise a substantially heteroatom-free hydrocarbon
product that may be
shipped to storage or commercial use. The dense phase 86 may be transferred to
a heteroatom by-product
separator 88 from which a beteroatom-containing byproduct stream 52 may be
separated from resulting in
a stream 42 containing a selectivity promoter and caustic that may be
recovered and recycled for reuse in
the promoted-caustic visbreaker 40, as would be understood by those skilled in
the art.
[0068] In still another embodiment, as illustrated in Figure 5, the
heteroatom-containing
hydrocarbon feed 30 may be fed to a catalytic oxidizer 34 where it may be
reacted with catalyst stream 90
in the catalytic oxidizer 34 producing intermediate stream 92. Stream 92 may
be transferred to catalyst
separator 94 from which intermediate stream 70 and a depleted catalyst stream
96 may be separated.
Stream 96 may be fed to catalyst regenerator 98 for regeneration by oxidant
feed 100 producing catalyst
stream 90 and an oxidant by-product stream 102. Oxidant by-product stream 102
may be optionally
recovered, recycled, and reused as would be understood by those skilled in the
art. Stream 70 may be
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
14
extracted by solvent 78 in product separator 46 (e.g. a liquid-liquid
extraction column) from which a
substantially heteroatom-free hydrocarbon product 72 may be withdrawn
resulting in intermediate stream
74. Stream 74 may be fed to solvent recovery 76 from which solvent 78 may be
recovered and recycled
to product separator 46, producing intermediate stream 80. Intermediate stream
80 may be reacted with
an oxidant 7 and an immiscible acid feed 77 in acid treatment column 71
producing intermediate stream
73 from the hydrocarbon phase and intermediate stream 75 from the acid phase.
Intermediate stream 75
may be fed to a solvent recovery unit 81 from which the acid 77 may be
recovered and recycled for reuse
in acid treatment column 71 producing a metal-containing by-product stream 79.
[0069] Stream 73 may be treated in the promoted-caustic visbreaker 40
containing selectivity
promoter and caustic feed 42 producing biphasic intermediate stream 82. The
two phases of stream 82
may be separated in product separator 84 as a light phase 48 and a dense phase
86. The light phase 48
may comprise a substantially heteroatom-free hydrocarbon product that may be
shipped to storage or
commercial use. The dense phase 86 may be transferred to a heteroatom by-
product separator 88 from
which a heteroatom-containing byproduct stream 52 may be separated from
resulting in a stream 42
containing a selectivity promoter and caustic that may be recovered and
recycled for reuse in the
promoted-caustic visbreaker 40, as would be understood by those skilled in the
art.
[0070] The following non-limiting examples illustrate certain aspects of
the present invention.
[0071] Examples
[0072] Example 1
[0073] Preparation of Pelletized Polymeric Titanyl Catalyst
[0074] A dimethyl sulfoxide (DMSO) solution of co-monomer (e.g. 4,4'-bisphenol
A
dianhydride (BPADA)) is prepared and is combined with a DMSO solution of the
titanyl (e.g.
bis(glycerol)oxotitanium(IV)) with stirring at 70 C for about 4 hrs to produce
a copolymer solution.
Then, the solution is cooled to room temperature, and the polymer product is
precipitated with excess
acetone. The polymeric precipitate is collected by vacuum filtration and is
dried. The yield of
precipitated polymeric titanyl catalyst is greater than 90%.
[0075] A blend of bonding agent (Kynart), optional inert filler (silica or
alumina), and the
polymeric titanyl catalyst is prepared in a solid mixer or blender. The
blended mixture is then extruded or
pelletized by compression producing uniform catalyst pellets with hardness
test strength preferably
greater than 2 kp.
[0076] Example 2
[0077] Continuous Catalytic Removal of Heteroatoms from a Heteroatom-
contaminatecl Light
Atmospheric Gas Oil
[0078] Straight- run light atmospheric gas oil (LAGO) (3.45 % sulfur) and
cumene
hydroperoxide (30% in cumene, fed at a rate of 2.1 mole equivalents to sulfur
in LAGO feed) are fed to a
fixed bed reactor containing pelletized titanyl polymeric catalyst, prepared
in accordance with Example 1,
at about 85 C with a combined LHSV of about 1.0 hr-1 producing a first
intermediate stream. The first
intermediate stream is then fed into a heated reactor at 50 C wherein it
combines with a feed stream
CA 02868851 2014-09-26
WO 2013/188144 PCT/US2013/043843
containing acetic acid, hydrogen peroxide, and residual cumene hydroperoxide
to produce a biphasic
mixture that exits the reactor. The biphasic mixture is then separated by
gravity to produce a second
intermediate stream of a light phase comprising substantially heteroatom-
decreased light atmospheric gas
oil, and a heavy phase by-product stream comprising essentially acetic acid,
oxidant, and heteroatom-
containing salts. The second intermediate stream is vacuum distilled at -25 in
Hg to remove and recover a
low boiling distillate comprising cumene, cumyl alcohol, alpha-methylstyrene,
acetophenone, and
residual acetic acid from a heavy second intermediate stream. The heavy second
intermediate stream
essentially comprises light atmospheric gas oil with oxidized heteroatom
compounds. The second
intermediate stream is then fed into a heated reactor wherein it combines with
a feed stream containing
caustic and ethylene glycol (the combined liquid residence time is 1.0 hr-')
to produce a biphasic mixture
that exits the reactor. The biphasic mixture is then separated by gravity to
produce a light phase product
comprising essentially heteroatom-free LAGO and a heavy phase by-product
stream comprising
essentially caustic, ethylene glycol, and heteroatom-containing salts. Sulfur
removal from the light phase
product is greater than 50%, nitrogen removal is greater than 50%, vanadium
removal is greater than
50%, nickel removal is greater than 50%, and iron removal is greater than 50%
when the samples are
measured for elemental composition and compared against the LAGO feed
composition. The heavy
phase by-product is further treated according to known methods to recover and
recycle the caustic and
ethylene glycol from the heteroatom by-products.
[0079] The foregoing description of the embodiments of this invention has
been presented for
purposes of illustration and description. It is not intended to be exhaustive
or to limit the invention to the
precise faun disclosed, and obviously, many modifications and variations are
possible. Such
modifications and variations that may be apparent to a person skilled in the
art are intended to be included
within the scope of the above described invention.