Note: Descriptions are shown in the official language in which they were submitted.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
1
Magnetic separation of particles including one-step-conditioning of a pulp
Description
The present invention relates to a process for separating at least one first
material from
a mixture comprising this at least one first material and at least one second
material,
which comprises the following steps
(A) contacting of the mixture comprising at least one first material and at
least one
second material with at least one magnetic particle, if appropriate in the
presence
of at least one dispersant,
or
contacting of the mixture comprising at least one first material and at least
one
second material with at least one magnetic particle and at least one surface-
modifying substance at the same time, so that the at least one first material,
the
at least one surface-modifying substance and the at least one magnetic
particle
become attached to one another, if appropriate in the presence of at least one
dispersant,
(B) if appropriate, addition of at least one dispersant to the mixture
obtained in step
(A) to give a dispersion having a suitable concentration,
(C) contacting of the mixture from step (A) or (B) with at least one surface-
modifying
substance, if this has not been done in step (A), so that the at least one
first ma-
terial, the at least one surface-modifying substance and the at least one
magnetic
particle become attached to one another,
(D) separation of the addition product from step (A), (B) or (C) from the
mixture by
application of a magnetic field,
(E) if appropriate, cleavage of the addition product which has been separated
off in
step (D) to obtain the at least one first material and the at least one
magnetic par-
tide separately.
In particular, the present invention relates to a process for the enrichment
of ores in the
presence of the gangue.
Processes for separating ores from mixtures comprising these are already known
from
the prior art.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
2
WO 02/0066168 Al relates to a process for separating ores from mixtures
comprising
these, in which suspensions or slurries of these mixtures are treated with
particles
which are magnetic and/or capable of floating in aqueous solutions. After
addition of
the magnetic particles and/or particles capable of floating, a magnetic field
is applied so
that the agglomerates are separated off from the mixture. However, the extent
to which
the magnetic particles are bound to the ore and the strength of the bond is
not suffi-
cient for the process to be carried out with a satisfactorily high yield and
effectiveness.
US 4,657,666 discloses a process for the enrichment of ores, in which the ore
present
in the gangue is treated with magnetic particles, as a result of which
agglomerates are
formed due to the hydrophobic interactions. The magnetic particles are
hydrophobized
on the surface by treatment with hydrophobic compounds, so that attachment to
the
ore occurs. The agglomerates are then separated off from the mixture by means
of a
magnetic field. The cited document also discloses that the ores are treated
with a sur-
face-activating solution of 1% sodium ethylxanthogenate before the magnetic
particle is
added. In this process, separation of ore and magnetic particle is effected by
the de-
struction of the surface-activating substance which has been applied in the
form of the
surface-activating solution to the ore. Furthermore, in this process only 04-
hydrophobising agents are used for the ore.
US 4,834,898 discloses a process for separating off nonmagnetic materials by
bringing
them into contact with magnetic reagents which are enveloped by two layers of
sur-
face-modifying substances. US 4,834,898 also discloses that the surface charge
of the
nonmagnetic particles which are to be separated off can be influenced by
various types
and concentrations of electrolytes reagents. For example, the surface charge
is altered
by addition of multivalent anions, for example tripolyphosphate ions.
S. R. Gray, D. Landberg, N. B. Gray, Extractive Metallurgy Conference, Perth,
2 - 4
October 1991, pages 223 - 226, disclose a process for recovering small gold
particles
by bringing the particles into contact with magnetite. Before contacting, the
gold parti-
cles are treated with potassium amylxanthogenate. A process for separating the
gold
particles from at least one hydrophilic material is not disclosed in this
document.
WO 2007/008322 Al discloses a magnetic particle which is hydrophobized on the
sur-
face for separating impurities from mineral substances by magnetic separation
pro-
cesses. According to WO 2007/008322 Al, a dispersant selected from among
sodium
silicate, sodium polyacrylate and sodium hexametaphosphate can be added to the
so-
lution or dispersion.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
3
W02009/030669 Al discloses a process for separating at least one first
material from
a mixture comprising this at least one first material and at least one second
material, in
which the first material is firstly brought into contact with a surface-
modifying substance
to hydrophobize it, this mixture is then brought into contact with at least
one magnetic
particle so that the magnetic particle and the hydrophobized first material
become at-
tached to one another and this agglomerate is separated from the at least one
second
material by application of a magnetic field. The at least one first material
is subsequent-
ly separated, preferably quantitatively, from the magnetic particle, with the
magnetic
particle preferably being able to be recirculated to the process. This
document does not
disclose that the addition of magnetic particles prior to a surface-modifying
substance
or the addition of magnetic particles and a surface-modifying substance at the
same
time gives rise to certain advantages like accelerated agglomeration.
It is an object of the present invention to provide a process by means of
which at least
one first material can be efficiently separated from mixtures comprising at
least one first
material and at least one second material. A further object of the present
invention is to
treat the first particles to be separated off in such a way that the addition
product of
magnetic particle and first material is sufficiently stable to ensure a high
yield of the first
material in the separation.
A further object is to provide a process for separating particles from a
mixture compris-
ing them and other particles, using magnetic particles to obtain magnetic
agglomerates
of particles to be separated and magnetic particles, wherein the agglomeration
occurs
faster and more evenly than in processes of the prior art.
These objects are achieved by a process for separating at least one first
material from
a mixture comprising this at least one first material and at least one second
material,
which comprises the following steps:
(A) contacting of the mixture comprising at least one first material and at
least one
second material with at least one magnetic particle, if appropriate in the
presence
of at least one dispersant,
or
contacting of the mixture comprising at least one first material and at least
one
second material with at least one magnetic particle and at least one surface-
modifying substance at the same time, so that the at least one first material,
the
at least one surface-modifying substance and the at least one magnetic
particle
become attached to one another, if appropriate in the presence of at least one
dispersant,
1
, ,
4
(B) if appropriate, addition of at least one dispersant to the mixture
obtained in step
(A) to give a dispersion having a suitable concentration,
(C) contacting of the mixture from step (A) or (B) with at least one surface-
modifying
substance, if this has not been done in step (A), so that the at least one
first
material, the at least one surface-modifying substance and the at least one
magnetic particle become attached to one another,
(D) separation of the addition product from step (A), (B) or (C) from the
mixture by
application of a magnetic field,
(E) if appropriate, cleavage of the addition product which has been separated
off in
step (D) to obtain the at least one first material and the at least one
magnetic
particle separately.
Another embodiment of the invention relates to a process for separating at
least one
first material from a mixture comprising this at least one first material and
at least one
second material, which comprises the following steps:
(A) contacting of the mixture comprising the at least one first
material and the at
least one second material with at least one magnetic particle and at least one
surface-modifying substance at the same time, so that the at least one first
material, the at least one surface-modifying substance and the at least one
magnetic particle become attached to one another, or
(A) contacting of the mixture comprising the at least one first material
and the at
least one second material with at least one magnetic particle and at least one
surface-modifying substance at the same time, so that the at least one first
material, the at least one surface-modifying substance and the at least one
magnetic particle become attached to one another, and
(B) optionally, adding at least one dispersion medium to the mixture
obtained in step
(A) to give a dispersion having a suitable concentration; and then
I CA 2869226 2019-05-22
1
4a
(D)
separating the addition product from step (A) or (B) from the mixture by
application of a magnetic field.
Another embodiment of the invention relates to the process defined
hereinabove,
wherein step (A) is further carried out in the presence of at least one
dispersant.
Another embodiment of the invention relates to the process defined
hereinabove,
wherein step (D) is further followed by a step (E) cleaving the addition
product which
has been separated off in step (D) to obtain the at least one first material
and the at
least one magnetic particle separately.
The process of the invention is preferably employed for separating at least
one first,
hydrophobic material from a mixture comprising this at least one first,
hydrophobic
material and at least one second, hydrophilic material.
For the purposes of the present invention, "hydrophobic" means that the
corresponding
particle can subsequently be hydrophobized by treatment with the at least one
surface-
modifying substance.
It is also possible for a particle which is hydrophobic per se to be
additionally
hydrophobized by treatment with the at least one surface-modifying substance.
Within the scope of the present invention, "hydrophobic" means that the
surface of
corresponding "hydrophobic substances", and, respectively, of a "hydrophobized
substance" has a contact angle with water against air of > 900. In the scope
of the
present invention, "hydrophilic" means that the surface of corresponding
"hydrophilic
substance" has a contact angle with water against air of < 900
.
In a preferred embodiment of the process of the invention, the at least one
first material
is at least one hydrophobic metal compound or coal and the at least one second
material is preferably at least one hydrophilic metal compound.
In a further preferred embodiment of the process according to the present
invention, the
at least one hydrophobic metal compound is selected from the group consisting
of
sulfidic ores, oxidic ores, carbonate-comprising ores, noble metals in
elemental form,
compounds comprising noble metals and mixtures thereof.
CA 2869226 2019-05-22
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
The present invention therefore preferably relates to the process according to
the pre-
sent invention, wherein the at least one hydrophobic metal compound is
selected from
the group consisting of sulfidic ores, oxidic ores, carbonate-comprising ores,
noble
5 metals in elemental form, compounds comprising noble metals and mixtures
thereof.
In a further preferred embodiment of the process according to the present
invention,
the at least one hydrophilic metal compound is selected from the group
consisting of
oxidic metal compounds, hydroxidic metal compounds and mixtures thereof.
The present invention therefore preferably relates to the process according to
the pre-
sent invention, wherein the at least one hydrophilic metal compound is
selected from
the group consisting of oxidic metal compounds, hydroxidic metal compounds and
mix-
tures thereof.
Examples of the at least one first material to be separated off are preferably
metal
compounds selected from the group consisting of sufidic ores, oxidic and/or
carbonate-
comprising ores, for example azurite [Cu3(CO3)2(OH)2] or malachite
[Cu2[(OH)21CO3]],
rare earth metals comprising ores like bastnaesite (Y, Ce, La)CO3F, monazite
(RE)PO4
(RE = rare earth metal) or chrysocolla (Cu,A1)2H2Si205(OH)4 = n H20, noble
metals in
elemental form and their compounds to which a surface-modifying compound can
be-
come selectively attached to produce hydrophobic surface properties. Examples
of
noble metals that may be present as at least first material are Au, Pt, Pd,
Rh, etc.,
preferably in the native state or as sulphides, phosphides, selenides,
tellurides or as
alloys with bismuth, antimony and/or other metals.
Examples of sulfidic ores which can be separated according to the invention
are, for
example, selected from the group of copper ores consisting of covellite CuS,
molyb-
denum(IV) sulfide, chalcopyrite (cupriferous pyrite) CuFeS2, bornite Cu5FeS4,
chalco-
cite (copper glass) Cu2S, pendlandite (Fe,Ni)9S8, and mixtures thereof.
Suitable oxidic metal compounds which may be present as at least one second
materi-
al according to the invention are preferably selected from the group
consisting of silicon
dioxide S102, silicates, aluminosilicates, for example feldspars, for example
albite
Na(S13AI)08, mica, for example muscovite KAI2[(OH,F)2AISi3010], garnets (Mg,
Ca,
Fe11)3(Al, Fe111)2(SiO4)3 and further related minerals and mixtures thereof.
Accordingly, untreated ore mixtures obtained from mines are preferably used as
mix-
ture comprising at least one first material and at least one second material
in the pro-
cess of the invention.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
6
In a preferred embodiment of the process of the invention, the mixture
comprising at
least one first material and at least one second material in step (A) is in
the form of
particles having a size of from 100 nm to 100 pm, see for example US
5,051,199. In a
preferred embodiment, this particle size is obtained by milling. Suitable
processes and
apparatuses are known to those skilled in the art, for example wet milling in
a ball mill.
The mixture comprising at least one first material and at least one second
material is
therefore milled to particles having a size of from 100 nm to 100 pm before or
during
step (A) in a preferred embodiment of the process of the invention. Preferred
ore mix-
tures have a content of sulfidic minerals of at least 0.4% by weight,
particularly prefera-
bly at least 10% by weight.
Examples of sulfidic minerals which are present in the mixtures which can be
used ac-
cording to the invention are those mentioned above. In addition, sulfide of
metals other
than copper, for example, sulfides of iron, lead, zinc or molybdenum, i.e.
FeS, FeS2,
PbS, ZnS or MoS2, can also be present in the mixtures. Furthermore, oxidic com-
pounds of metals and semimetals, for example silicates or borates or other
salts of
metals and semimetals, for example phosphates, sulfates or ox-
ides/hydroxides/carbonates, and further salts, for example azurite
[Cu3(CO3)2(0 El )2] ,
malachite [Cu2[(OH)2(CO3)]], barite (BaSO4), monazite ((La-Lu)PO4), spinels
(Mg, Ca,
Fe(II))(Fe(III), Al, Cr)204, can be present in the ore mixtures to be treated
according to
the invention.
A typical ore mixture which can be separated by means of the process of the
invention
has the following composition: about 30% by weight of SiO2 as an example of a
pre-
ferred at least one second material, about 30% by weight of feldspar (e.g.
Na(Si3A1)08),
about 3% by weight of CuFeS2 as an example of a preferred at least one first
material,
about 0,05% by weight of MoS2, balance chromium, iron, titanium and magnesium
ox-
ides.
The individual steps of the process of the invention are described in detail
below:
Step (A):
Step (A) of the process according to the present invention can be conducted
according
to two alternative embodiments.
According to the first embodiment of the process according to the present
invention,
step (A) comprises contacting of the mixture comprising at least one first
material and
at least one second material with at least one magnetic particle, if
appropriate in the
presence of at least one dispersant.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
7
According to this first embodiment only at least one magnetic particle is
added in step
(A), and at least one surface-modifying substance is added in step (C) of the
process
according to the present invention.
According to the second embodiment of the process according to the present
invention,
step (A) comprises contacting of the mixture comprising at least one first
material and
at least one second material with at least one magnetic particle and at least
one sur-
face-modifying substance at the same time, so that the at least one first
material, the at
least one surface-modifying substance and the at least one magnetic particle
become
attached to one another, if appropriate in the presence of at least one
dispersant.
According to this second embodiment, both, at least one magnetic particle and
at least
one surface active substance are added in step (A). According to this
embodiment,
step (C) of the process according to the present invention is not necessary
and is pref-
erably not conducted.
Suitable preferred first and second materials have been mentioned above.
According to the present invention at least one magnetic particle is added in
step (A).
In a preferred embodiment of the process according to the present invention,
at least
one hydrophobic magnetic particle is added in step (A). In a preferred
embodiment of
the present invention, the at least one magnetic particle is hydrophobized at
the sur-
face.
In step (A) of the process of the invention, it is in general possible to use
all magnetic
substances and materials known to those skilled in the art. In a preferred
embodiment,
the at least one magnetic particle is selected from the group consisting of
magnetic
metals, for example iron, cobalt, nickel and mixtures thereof, ferromagnetic
alloys of
magnetic metals, for example NdFeB, SmCo and mixtures thereof, magnetic iron
ox-
ides, for example magnetite, maghemite, cubic ferrites of the general formula
(II)
M2+,<Fe2+1_1Fe3+204 (II)
where
M is selected from among Co, Ni, Mn, Zn and mixtures thereof and
x is 5 1,
hexagonal ferrites, for example barium or strontium ferrite MFe6019 where M =
Ca, Sr,
Ba, or a mixture thereof. The magnetic particles can additionally have an
outer layer,
for example of 5i02.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
8
In a particularly preferred embodiment of the present invention, the at least
one mag-
netic particle is magnetite or cobalt ferrite Co2',<Fe2'1,Fe3+204 where x 1.
Very prefer-
ably magnetite is used as at least one magnetic particle.
In a further preferred embodiment, in step (A) of the process according to the
present
invention, magnetic particles are present in the size of 100 nm to 100 pm,
particularly
preferred 1 to 50 pm. The magnetic particles may be brought into the adequate
size by
processes known to the skilled artisan, for example by milling. Furthermore,
the parti-
cles, obtained from precipitation reaction, can be brought to the adequate
particle size
by setting up the reaction parameters (for example pH, reaction time,
temperature).
In a further preferred embodiment, the at least one magnetic particle is
hydrophobized
at the surface by at least one hydrophobic compound. The hydrophobic compound
is
preferably selected from among compounds of the general formula (III)
B-Y (III),
where
B is selected from among linear or branched 03-C30-alkyl, C3-030-
heteroalkyl, op-
tionally substituted 06-C30-aryl, optionally substituted C6-C30-heteroaryl, 06-
030-
aralkyl, and
Y is a group by means of which the compound of the general formula (III)
binds to
the at least one magnetic particle.
In a particularly preferred embodiment, B is a linear or branched 06-C18-
alkyl, prefera-
bly linear 08-C12-alkyl, very particularly preferably a linear 012-alkyl.
Heteroatoms which
may be present according to the invention are selected from among N, 0, P, S
and
halogens such as F, Cl, Br and I.
In a further particularly preferred embodiment, Y is selected from the group
consisting
of -(X)n-SiHal3, -(X)n-SiHHal2, -(X)n-SiH2Hal where Hal is F, CI, Br, I, -(X)n-
Si(OR1)3-n
wherein n is 1, 2 or 3 and R1 is 01-C6-alkyl, and anionic groups such as -(X)n-
Si033-, -
(X)9-002-, -(X)n-P032-, -(X)n-P02S2-, -(X)n-P0S22-, -(X)n-PS32-, -(X)n-PS2-, -
(X)n-POS-, -
(X)9-P02-, -(X)n-0O2-, -(X)n-CS2-, -(X)-COS, -(X)n-C(S)NHOH, -(X)n-S- where X
= 0, S,
NH, CH2 and n = 0, 1 or 2, and, if appropriate, cations selected from the
group consist-
ing of hydrogen, NR4+ where the radicals R are each, independently of one
another,
hydrogen or 01-08-alkyl, an alkali metal, an alkaline earth metal or zinc,
also -(X)n-
Si(OZ)3 where n = 0, 1 or 2 and Z = charge, hydrogen or short-chain alkyl
radical.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
9
If, in the mentioned formulas n = 2, two equal or different, preferably equal,
groups B
are attached to one group Y.
Very preferred hydrophobizing substances of general formula (III) are alkyltri-
chlorosilane (alkyl group having 1 to 12 carbon atoms), alkyldialkoxysilane
(alkyl group
having 1 to 12 carbon atoms and alkoxy group having 1 to 6 carbon atoms), for
exam-
ple alkyldimethoxysilane (alkyl group having 1 to 12 carbon atoms),
alkyltrialkoxysilane
(alkyl group having 1 to 12 carbon atoms and alkoxy group having 1 to 6 carbon
at-
oms), for example alkyltrimethoxysilane (alkyl group having 6 to 12 carbon
atoms),
octylphosphonic acid, lauric acid, oleic acid, stearic acid or mixtures
thereof. Using al-
kyltrialkoxysilanes as hydrophobizing substances of general formula (III),
preferably a
polymeric hydrophobic layer is obtained at the surface of the magnetic
particle.
The at least one magnetic particle is in general added in an amount that is
high enough
to have the complete amount of at least one first material embedded in
agglomerates,
preferably the at least one magnetic particle is added in excess. For example,
the at
least one magnetic particle is added in an amount of 0.1 to 20% by weight,
preferably
0.5 to 5% by weight, in each case based on the amount of the complete mixture
to be
treated with the process according to the present invention.
According to the second embodiment of step (A) of the process according to the
pre-
sent invention, at least one surface-modifying substance is added. In general,
all sur-
face-modifying substances can be used according to the present invention that
are
able to modify the surface of the at least one first material in a way that
agglomeration
with at least one magnetic particle is possible.
For the purposes of the present invention, a "surface-modifying substance" is
a sub-
stance which is able to modify the surface of the particle to be separated off
in the
presence of the other particles which are not to be separated off in such a
way that
attachment of a hydrophobic particle by means of hydrophobic interactions
occurs.
Surface-modifying substances which can be used according to the invention
become
attached to the at least one first material and thereby produce a suitable
hydrophobicity
of the first material.
In the process of the invention, preference is given to using a surface-
modifying sub-
stance of the general formula (I)
A-Z (I)
which becomes attached to the at least one first material, where
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
A is
selected from among linear or branched 02-030-alkyl, 02-030-heteroalkyl, op-
tionally substituted 06-C30-aryl, optionally substituted 06-030-heteroaryl, 06-
030-
aralkyl, and
5
Z is a group by means of which the compound of the general formula (I)
binds to
the at least one hydrophobic material.
In a particularly preferred embodiment, A is a linear or branched 02-C14-
alkyl, very par-
10 ticularly preferably a linear C4- or C8-alkyl. Heteroatoms which may be
present accord-
ing to the invention are selected from among N, 0, P, S and halogens such as
F, Cl, Br
and I.
In a further preferred embodiment, A is preferably a branched, 02-C20-alkyl,
particularly
preferably a branched 08-C14-alkyl, wherein preferably the at least one
substituent,
preferably having 1 to 6 carbon atoms, is attached in 2-position, for example
2-
ethylhexyl and/or 2-propylheptyl. Corresponding compounds being substituted in
2-
position are, for example, obtained using the Guerbet reaction that is known
to the
skilled artisan as one reaction step.
In a further particularly preferred embodiment, X is selected from the group
consisting
of anionic groups -(X)n-P032-, -(X)n-POS22-, -(X)n-PS32-,
-(X)-PO, -(X)n-P032- -(X)-CO2, -(X)n-CS2-, -(X)9-
C(S)NHOH, -(X)0-
S- where X is selected from the group consisting of 0, S, NH, CH2 and n = 0, 1
or 2,
with, if appropriate, cations selected from the group consisting of hydrogen,
NR4+ with
R being independently of one another hydrogen and/or C1-C8-alkyl, hydroxy-
substituted
C1-C8-alkyl or -heteroalkyl, like 2-hydroxy-ethyl HO-CH2CH2- or 2-hydroxy-
ethoxy-ethyl
HO-CH2CH2-0-CH2CH2-, alkali- or earth alkali metals, preferably sodium or
potassium,
are present. The anions mentioned and the corresponding cations form,
according to
the invention, uncharged compounds of the general formula (I).
If, in the mentioned formulas n = 2, two equal or different, preferably equal,
groups A
are attached to one group Z.
In a further preferred embodiment, compounds are applied, selected from the
group
consisting of xanthates
dialkyldithiophosphates (A-0)2-PS2-, dialkyldithio-
phosphinates (A)2-PS2-, dialkyldithiocarbamates, alkyltrithiocarbamates,
dithiophos-
phates and mixtures thereof, wherein A independently of one another is a
linear or
branched, preferably linear, 06-020-alkyl, for example n-octyl, or a branched
06-C14-
alkyl, wherein the branch is preferably located in 2-position, for example 2-
ethylhexyl
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
11
and/or 2-propylheptyl. As counterions, in these compounds preferably cations
selected
from the group consisting of hydrogen, NR4+ with R being independently of one
another
hydrogen and/or C1-C8-alkyl, hydroxy-substituted C1-C8-alkyl or -heteroalkyl,
like 2-
hydroxy-ethyl HO-CH2CH2- or 2-hydroxy-ethoxy-ethyl HO-CH2CH2-0-CH2CH2-, alkali-
or earth alkali metals, preferably sodium or potassium, are present.
Exceptionally preferred compounds of general formula (I) are selected from the
group
consisting of sodium- or potassium-n-octylxanthate, sodium- or potassium-
butylxanthate, sodium- or potassium-di-n-octyldithiophosphinate, sodium- or
potassi-
um-di-n-octyldithiophosphate, sodium- or potassium-di-n-octyldithiocarbamates
and
mixtures of these compounds.
In the case of noble metals, for example Au, Pd, Rh, etc., particularly
preferred surface-
modifying substances are monothiols, dithiols and trithiols, or 8-
hydroxyquinolines, for
example as described in EP 1200408 B1.
In the case of metal oxides, for example Fe0(OH), Fe304, ZnO, etc.,
carbonates, for
example azurite [Cu(CO3)2(OH)2], malachite [Cu2ROH)2C031 particularly
preferred
surface-modifying substances are octylphosphonic acid (0 PS), (Et0)3Si-A,
(Me0)35i-
A, with the abovementioned meanings of A.
In the case of metal sulfides, for example Cu2S, MoS2, FeS2 etc., particularly
preferred
surface-modifying substances are monothiols, dithiols and trithiols,
xanthogenates or
dithiophosphates.
Therefore, according to the above mentioned, in a further preferred embodiment
of the
process of the invention, Z is -(X)9-CS2-, -(X)9-PS2- or -(X)n-S- where X is 0
and n is 0 or
1, and a cation is selected from among hydrogen, sodium, potassium and
NHx(CH2CH20H)4,, wherein x is 0, 1, 2, 3, or 4.
The at least one surface-modifying substance is generally used in an amount
which is
sufficient to achieve the desired effect. In a preferred embodiment, the at
least one
surface-modifying substance is added in an amount of from 0.001 to 1% by
weight,
preferably 0.001 to 0.1% by weight in each case based on the total mixture to
be treat-
ed.
According to the first embodiment of step (A) of the process according to the
present
invention, the contacting of the mixture with at least one magnetic particle
in step (A) of
the process of the invention can be carried out by all methods known to those
skilled in
the art, for example in bulk or in dispersion, preferably in suspension. In a
preferred
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
12
first embodiment, a dispersion of the at least one magnetic particle is added
to the mix-
ture.
In a preferred embodiment, the at least one magnetic particle is dispersed in
a suitable
dispersion medium. Suitable dispersion media are all dispersion media in which
the at
least one magnetic particle is not completely soluble. Suitable dispersion
media are for
example selected from the group consisting of water, water-soluble organic com-
pounds, for example alcohols having from 1 to 4 carbon atoms, particularly
preferably
water.
According to the invention, the amount of dispersion medium for predispersing
the
magnetic particles can generally be selected so that a slurry or dispersion
which is
readily stirrable and/or conveyable is obtained. In a preferred embodiment,
the amount
of mixture to be treated based on the total slurry or dispersion is up to 100%
by weight,
.. preferably up to 60% by weight, for example 0.5 to 60% by weight,
preferably 0.5 to
20% by weight, particularly preferably 0.5 to 10% by weight.
In one embodiment of the present invention, a high amount of mixture to be
treated in
the slurry or dispersion is preferred. Therefore, the present invention
preferably relates
to the process according to the present invention, wherein the amount of
mixture to be
treated based on the total slurry or dispersion is up to 60% by weight, for
example 20 to
60% by weight.
According to the invention, the dispersion of the magnetic particles can be
produced by
all methods known to those skilled in the art. In a preferred embodiment, the
magnetic
particles to be dispersed and the appropriate amount of dispersion medium or
mixture
of dispersion media are combined in a suitable reactor, for example a stirring
tank, and
stirred by means of devices known to those skilled in the art, for example in
a stirring
tank by means of a magnetically operated propeller stirrer, for example at a
tempera-
ture of from 1 to 80 C, preferably at room temperature.
According to the second embodiment of step (A) of the process according to the
pre-
sent invention, the contacting of the mixture with at least one magnetic
particle and at
least one surface-modifying substance at the same time is generally carried
out by
combining the components by methods known to those skilled in the art. For
example,
this second embodiment of step (A) can be carried out in bulk or in
dispersion, prefera-
bly in suspension. Suitable dispersion media are for example selected from the
group
consisting of water, water-soluble organic compounds, for example alcohols
having
from 1 to 4 carbon atoms, and mixtures thereof. In a particularly preferred
embodiment,
the dispersion medium is water. In a preferred second embodiment, a dispersion
of the
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
13
at least one magnetic particle and at least one surface-modifying substance is
added to
the mixture.
The present invention therefore preferably relates to the process according to
the pre-
.. sent invention, wherein contacting of the mixture comprising at least one
first material
and at least one second material with at least one magnetic particle or with
at least one
magnetic particle and at least one surface-modifying substance at the same
time in
step (A) is achieved by addition of a mixture, preferably a dispersion, of
least one mag-
netic particle and at least one surface-modifying substance.
In a further embodiment of the process of the invention, both embodiments of
step (A)
can be carried out in bulk, i.e. in the absence of a dispersion medium.
For example, the mixture to be treated and the at least one magnetic particle
or at least
one magnetic particle and the at least one surface-modifying substance are
combined
and mixed in the appropriate amounts without a further dispersion medium.
Suitable
mixing apparatuses are known to those skilled in the art, for example mills
such as ball
mills.
Step (A) is generally carried out at a temperature of from 1 to 80 C,
preferably from 10
to 30 C.
According to the second embodiment of step (A), wherein at least one magnetic
parti-
cle and at least one surface-modifying substance are added at the same time,
the at
least one magnetic particle becomes attached to the at least one first
material that is
more hydrophobic than the at least one second material by nature and that is
further
hydrophobized at its surface by the at least one surface-modifying substance.
The
bond between the two components is based on hydrophobic interactions. There is
generally no bonding interaction between the at least one magnetic particle
and the
.. hydrophilic component of the mixture, so that these components do not
become at-
tached to one another. Thus, addition products of the at least one first,
hydrophobic
material and the at least one magnetic particle are present alongside the at
least one
second, hydrophilic material in the mixture after the second embodiment of
step (A) of
the process according to the present invention.
40
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
14
Step (B):
The optional step (B) of the process of the invention comprises addition of at
least one
dispersion medium to the mixture obtained in step (A) to give a dispersion
having a
suitable concentration.
In one embodiment, if step (A) is carried out in bulk, the mixture obtained in
step (A)
comprises at least one first material and at least second material, at least
one magnetic
particle and optionally at least one surface-modifying substance. If step (A)
is carried
out in bulk, step (B) of the process of the invention is preferably carried
out, i.e. at least
one suitable dispersion medium is added to the mixture obtained in step (A) in
order to
obtain a dispersion.
In the embodiment in which step (A) of the process of the invention is carried
out in
dispersion, step (B) is preferably not carried out. However, in this
embodiment, too, it is
possible to carry out step (B), i.e. to add further dispersion medium in order
to obtain a
dispersion having a lower concentration.
Suitable dispersion media are all dispersion media which have been mentioned
above
in respect of step (A). In a particularly preferred embodiment, the dispersion
medium in
step (B) is water.
Thus, step (B) comprises either converting the mixture present in bulk from
step (A)
into a dispersion or converting the mixture which is already in dispersion
from step (A)
into a dispersion of lower concentration by addition of dispersion media.
According to the invention, the amount of dispersion medium added in step (A)
and/or
step (B) can generally be selected so that a dispersion is obtained which is
readily stir-
rable and/or conveyable. In a preferred embodiment, the amount of mixture to
be treat-
ed based on the total slurry or dispersion is up to 100% by weight, preferably
up to
60% by weight, for example 0.5 to 60% by weight, preferably 0.5 to 20% by
weight,
particularly preferably 0.5 to 10% by weight.
In one embodiment of the present invention, a high amount of mixture to be
treated in
the slurry or dispersion is preferred. Therefore, the present invention
preferably relates
to the process according to the present invention, wherein the amount of
mixture to be
treated based on the total slurry or dispersion is up to 60% by weight, for
example 20 to
60% by weight.
In a preferred embodiment of the process of the invention, step (B) is not
carried out
but instead step (A) is carried out in aqueous dispersion so that a mixture in
aqueous
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
dispersion having the correct concentration for use in step (C) of the process
of the
invention is obtained directly in step (A).
The addition of dispersion medium in step (B) of the process of the invention
can, ac-
5 cording to the invention, be carried out by all methods known to those
skilled in the art.
Step (C):
Step (C) of the process of the invention comprises contacting of the mixture
from step
10 (A) or (B) with at least one surface active substance, if this has not
been done in step
(A), so that the at least one first material, the at least one surface-
modifying substance
and the at least one magnetic particle become attached to one another.
Step (C) of the process according to the present invention is conducted, if at
least one
15 surface-modifying active substance is not added in step (A) of the
process according to
the present invention. According to this first embodiment of the process
according to
the present invention, a mixture comprising the at least one first material,
the at least
one second material, the at least one magnetic particle and optionally at
least one dis-
persion medium, that is obtained in step (A) or (B), is introduced in step
(C).
The at least one surface-modifying substance that is added in step (C) of the
process
according to the present invention and preferred embodiments thereof can be
selected
from the group of compounds of general formula (I) as mentioned in respect of
step (A)
of the process according to the present invention.
According to step (C) of the process according to the present invention, the
at least one
surface-modifying substance is generally used in an amount which is sufficient
to
achieve the desired effect. In a preferred embodiment, the at least one
surface-
modifying substance is added in step (C) of the process according to the
present inven-
tion in an amount of from 0.001 to 1% by weight, preferably 0.001 to 0.1% by
weight in
each case based on the total mixture to be treated.
Step (C) of the process according to the present invention can in general be
conducted
according to all methods that are known to the skilled artisan. In particular,
the addition
according to step (C) of the process according to the present invention can be
con-
ducted as mentioned in respect of step (A) of this process.
Step (D):
Step (D) of the process of the invention comprises separation of the addition
product
from step (A), (B) or (C) from the mixture by application of a magnetic field.
According
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
16
to the present invention, the "addition product" in the sense of step (D) is
the agglom-
erate that is obtained in step (A) or (C) containing at least one first
material, at least
one surface active substance and at least one magnetic particle.
In general, step (D) can be carried out with any magnetic equipment that is
suitable to
separate magnetic particles from dispersion, e. g. drum separators, high or
low intensi-
ty magnetic separators, continuous belt type separators or others.
Step (D) can be carried out by introducing a permanent magnet into the reactor
in
which the mixture from step (A), (B) or (C) is present. In a preferred
embodiment, a
dividing wall composed of nonmagnetic material, for example the wall of the
reactor, is
present between permanent magnet and mixture to be treated. In a further
preferred
embodiment of the process of the invention, an electromagnet which is only
magnetic
when an electric current flows is used in step (D). Suitable apparatuses are
known to
those skilled in the art.
In a preferred embodiment, the magnetic separation equipment allows to wash
the
magnetic concentrate while the separation with a dispersant, preferably water.
This
washing preferably allows removing inert material from the magnetic
concentrate lead-
ing to higher grades of the valuables.
In a preferred embodiment, step (D) is conducted continuously or semi-
continuously,
wherein preferably the mixture to be treated flows through a separator,
preferably in
dispersion. Flow velocities of the dispersion to be treated are in general
adjusted to
obtain an advantageous yield of magnetic agglomerates separated. In a
preferred em-
bodiment, flow velocities of the dispersion to be treated are 10 mm/sec. to
1000
mm/sec.
The pH-value of the dispersion which is treated according to step (D) is in
general neu-
tral or weakly basic, being a pH-value of 6 to 13, preferably 8 to 12. In a
preferred em-
bodiment, no adjustment of pH-value of the dispersion obtained in step (A) or
(B) is
necessary.
Step (D) of the process of the invention can be carried out at any suitable
temperature,
for example from 10 to 60 C, preferably at ambient temperature.
In a continuous or semi-continuous process the mixture is preferably mixed by
turbu-
lent flow, and is preferably not additionally stirred.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
17
The magnetic agglomerates can be separated from the magnetic surface and/or
the
unit wherein magnetic separation is conducted according to the present
invention by all
methods known to those skilled in the art.
In a preferred embodiment the magnetic agglomerates are removed by flushing
with a
suitable dispersion medium. Suitable dispersion media have been mentioned
above. In
a preferred embodiment, water is used to flush the separated magnetic
agglomerates.
After step (D) of the process according to the present invention, the
agglomerate of at
least one first material that is to be separated according to the present
invention, at
least one surface-modifying substance and at least one magnetic particle is
separated
from the at least one second material. Preferably both fractions that are
obtained are
present as dispersions in at least one dispersion medium, preferably in water.
Step (E):
Optional step (E) of the process of the invention comprises cleavage of the
addition
product which has been separated off in step (D) to obtain the at least one
first material
and the at least one magnetic particle separately.
In a preferred embodiment of the process of the invention, the cleavage in
step (E) is
carried out in a nondestructive manner, i.e. the individual components present
in the
dispersion are not changed chemically. For example, the cleavage according to
the
invention is preferably not affected by oxidation of the hydrophobizing agent,
for exam-
ple to give the oxidation products or degradation products of the
hydrophobizing agent.
Cleavage can be carried out by all methods known to those skilled in the art
which are
suitable for cleaving the addition product in such a way that the at least one
magnetic
particle can be recovered in reusable form. In a preferred embodiment, the
magnetic
particle which has been cleaved off is reused in step (A) of the process
according to
the present invention.
In a preferred embodiment, the cleavage in step (E) of the process of the
invention is
affected by treatment of the addition product with a substance selected from
the group
consisting of organic solvents, basic compounds, acidic compounds, oxidants,
reducing
agents, surface-active compounds and mixtures thereof.
Examples of basic compounds which can be used according to the invention are
ague-
ous solutions of basic compounds, for example aqueous solutions of alkali
metal and/or
alkaline earth metal hydroxides, for example KOH, NaOH, lime water, aqueous
ammo-
nia solutions, aqueous solutions of organic amines of the general formula
R23N, where
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
18
the radicals R2 are selected independently from the group consisting of
CrCralkyl
which may optionally be substituted by further functional groups.
Examples of surface-active compounds which can be used according to the
invention
are nonionic, anionic, cationic and/or zwitterionic surfactants. In a
preferred embodi-
ment, the cleavage is made by the use of biodegradable, preferably nonionic,
surfac-
tants with concentrations in the range of the critical micelle concentrations.
In a pre-
ferred embodiment, the addition product of hydrophobic material and magnetic
particle
is cleaved by means of biodegradable nonionic surfactants, further preferably
added in
an amount slightly, for example up o 5%, more preferably up to 3%, above the
critical
micelle concentration of the surfactant.
After optional cleavage according to step (E), the at least one first material
and the at
least one magnetic particle are, according to the invention, present as
dispersion in the
abovementioned cleavage reagent, preferably in a mixture of water and
surfactant.
For example, the at least one magnetic particle is separated from the
dispersion com-
prising this at least one magnetic particle and the at least one first
material by means of
a permanent magnet or electromagnet. Details of the separation are analogous
to step
(D) of the process of the invention.
The first material to be separated off, preferably the metal compound to be
separated
off, is preferably separated from the dispersion medium by drying.
The process according to the present invention comprises steps (A) to (D),
wherein
particles or agglomerates are obtained comprising at least one magnetic
particle and at
least one metal. In a particularly preferred embodiment these particles or
agglomerates
are suitable for direct work-up without optional step (E) according to the
present inven-
tion to obtain the at least one metal in pure form.
The present invention further relates to the process according to the present
invention,
wherein after step (D) or step (E) the following step (F) is conducted:
(F) further processing of the particles or of the agglomerate from step
(D) or (E) via
smelting, extracting and/or wet chemical refining.
The magnetic particles or agglomerates obtained in step (D) preferably
comprise iron
comprising magnetic substances or magnetic particles in addition to at least
one first
material, being for example at least one precious metal. Because iron is
essentially
necessary for melting and/or smelting processes to obtain the at least one
first material
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
19
in pure or enriched form, the particles or agglomerates that are obtained in
step (D) of
the process according to the present invention can directly be treated in a
smelting
and/or melting process.
In the case that noble metals are used as first material in combination with
iron com-
prising magnetic particles, no need for further addition of other iron
containing com-
pounds may exist. Instead, the magnetic iron oxide particles loaded with
precious met-
als are added to the furnace feed in place of iron oxide otherwise added to
the process.
In a further embodiment of the process according to the present invention,
step (F) is
conducted according to the present invention after step (E).
Smelting, extracting and/or wet chemical refining are conducted according to
methods
that are known to the skilled artisan.
Figures:
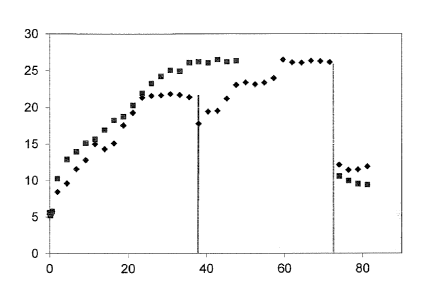
Figure 1 shows a diagram, wherein a process for separating valuables from the
gangue
of an ore according to the prior art, wherein surface-modifying substance and
magnet-
ite are added in two steps (diamonds) is compared to the process according to
the pre-
sent invention, wherein surface-modifying substance and magnetite are added in
one
step (square). The x-axis shows time in minutes, the y-axis shows a value that
is corre-
sponding to the particle size in pm.
In the case of the process according to the present invention, both, surface-
modifying
substance and magnetite are added at t = 0 min. In the case of the process
according
to the prior art, surface-modifying substance is added at t = 0 min, and
magnetite is
added at t = about 37 min. (left vertical line). At about 72 min (right
vertical line), a sur-
factant is added to separate the agglomerates, in both cases.
Examples
A roughly premilled porhyric copper ore from south America (0.66 wt% Cu, 0.029
wt%
Mo) is milled to d80 = about 40 pm without the addition of any additive. After
milling,
the pulp having a solid content of 60 wt% is treated with octyl xanthate (400
g/t) and
hydrophobic magnetite (3 wt%) in varying orders. Treatment is conducted in a
beaker
under stirring using an inertly coated paddle mixer. Subsequently, the pulp is
diluted to
a solid content of 15 wt% und is separated magnetically. Results in respect of
recovery
and grade of copper and molybdenum are shown in table 1.
CA 02869226 2014-10-01
WO 2013/160219 PCT/EP2013/058245
Table 1
Examples No. 1 2 3 4 5 6
Time of addition
15 15*
of xanthate [min]
15 10 5
Time of addition
15 15*
of magnetite [min]
Cu Recovery
93 92 89 95 95 95
[wt%]
Mo Recovery [wt%] 94 90 88 94 94 91
Cu Grade [%] 18.2 17.8 17.9 17.9 16.9 17.1
Mo Grade [%] 0.62 0.56 0.61 0.61 0.55 0.53
*reversed order
5
In examples 1 and 2 at t = 0 min, the first substance (xanthate or magnetite)
is added,
after 15 min the other substance (xanthate or magnetite) is added, then it is
stirred for
15 min, before agglomerates are treated as mentioned above.
10 In examples 3 to 6 both substances (xanthate and magnetite) are added at
t = 0 min,
after stirring for the time as mentioned in table 1, the agglomerates are
treated as men-
tioned above.