Note: Descriptions are shown in the official language in which they were submitted.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
1
IMPLANT DEVICE AND SYSTEM FOR ABLATION OF A RENAL ARTERIAL
WALL FROM THE INSIDE
TECHNICAL FIELD
The invention pertains to the technical field of the treatment of bodily
vessels by
means of ablation, more specifically to the treatment of conditions such as
arterial
hypertension. In particular, the present invention relates to systems, devices
and
methods for the ablation of a vessel's wall from the inside, more specifically
to
implant devices and to the ablation of the wall of one or more renal arteries
from
the inside, preferably transnnural ablation.
BACKGROUND
The present invention concerns a system of one or more implant devices and
excitation device, an implant device and a method using the system and one or
more devices for the treatment of arterial and venous structures.
The present invention also concerns implant devices, a system of implant
devices
and external excitation means, and a method for positioning one or more
implant
devices in a vessel, and subsequently heating these implant devices,
preferably
simultaneously, thereby transferring heat from implant devices to the vessel's
inner wall.
The general concept of the present invention is to implant one or more implant
devices into vessels, said implant devices making contact with the vessels'
inner
walls at the positions where ablation is deemed necessary. In contrast with
prior
art, ablation is not performed immediately, but the one or more implant
devices
can be heated up to a specified temperature by external energy-providing
means,
which are spatially separated from, i.e. not touching, the implant device and
able
to provide energy remotely to the implant device for increasing the
temperature of
the ablation region of the implant device up to an ablation temperature. In a
preferred embodiment, an implant device comprises an area which is made from a
material which may show magnetic hysteresis and the external energy-providing
means are able to create a time-varying magnetic field at the position of the
implanted device, hereby heating the implant through the phenomenon of
magnetic hysteresis. The maximum temperature the implant device can reach, is
limited by the Curie or Neel temperature of the magnetic material used, above
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
2
which temperature the magnetic hysteresis effect disappears. This Curie or
Neel
temperature can be engineered precisely to the necessary ablation temperature
e.g. by changing the composition of the magnetic alloy that is used. In
another
embodiment, non-magnetic material may be used, and insulation material may
then be used to provide sufficient temperature-controlling means. In an
embodiment, the heating of the implant device is done by Joule heating or
direct
heating, or any other heating system.
The implant device according to the present invention can thus be used in
arterial
and venous structures.
Indeed, the system, device and method according to the present invention can
also be used to perform ablation throughout the renal arteries, to perform
ablation
of the nervous system surrounding the renal arteries, to perform ablation of
the
renal sympathetic nerves, more specific to perform ablation of the renal
sympathetic nerves in the adventitia surrounding the renal arteries, to
perform
ablation of renal afferent and/or efferent nerves.
The device can be used in the renal arteries in which case the device, system
and
method can be used to treat arterial hypertension, norepinephrine spillover,
heart
failure, hypertension related target organ damage, etc.
The device can be used in the renal arteries in which case the device, system
and
method can be used to treat arterial hypertension, more specific, but not
limited
to, mild, moderate and/or severe hypertension, masked hypertension, white-coat
hypertension, reverse dipping hypertension, non-dipping hypertension,
dipping hypertension end neuroadrenergic hypertension.
The devices, systems and methods as described in this document may also be
used in human or animal corpses or in models of human or animal bodies, e.g.
for
practicing or educational purposes, whereby the heating of the implant devices
leaves ablation marks on the vessel's inner wall which can be used to check if
the
implant devices were positioned correctly and sufficient ablation occurred.
The present document focuses its description on the application of the device
inside the renal arteries.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
3
A person skilled in the art will be able to interpret the device, the system
and the
method and to provide them of specific features, components or steps if to be
used in other areas.
Human wellbeing is menaced by numerous disorders which change with time. The
art of medicine continuously needs to innovate and to adapt to these changes.
US patent application 2005/0027306 discloses a catheterization device for
delivering a self-expanding stent. The device has an inner shaft and an outer
shaft
moveable with respect to the inner shaft. The self-expanding stent is received
on
the inner shaft adjacent its distal end. A tapered tip is located on the inner
shaft
distal end and it forms a smooth transition from the delivery device to a
guidewire
extending therethrough. A handle allows a practitioner to deploy the stent
single
handedly. The stent may have its segments in a first radial configuration for
delivery of the stent or the stent may have a plurality of segments in a first
radial
configuration and a plurality of second segments in a second radial position.
US patent application 2005/0101946 discloses a method and system for treating
AF by ablation of a pulmonary vein, using a stent which has a resonant
circuit. The
stent can be implanted at the site of ablation and subsequently activated by
external energy-providing means, in particular by an electromagnetic field
with the
resonating frequency of the resonant circuit of the stent. The application
does not
disclose the possibility of using materials which show magnetic hysteresis for
at
least part of the stent, and to use the hysteresis effect for activating the
stent.
Thereby, it is also in this way not easy to control the ablation temperature
of the
stent. The energy delivered to the stent should be monitored very closely as
it
depends on a multitude of factors and the temperature of the stent is not
under
control, such as the electrical resistance of the stent and the resonant
circuit of
the stent, the magnitude of the RF field at the site of the stent, the quality
of the
thermal contact between stent and vessel wall.
European patent application EP 1 036 574 discloses a device and method for
heating an implanted stent up to a certain temperature, using external energy-
providing means. The stent can be heated up through the effect of magnetic
hysteresis. However, in this patent application, the temperature is controlled
by an
external controlling system which measures the temperature of the stent via
e.g.
an infrared camera, and alters the energy provided with the external energy-
providing means accordingly. Hereby, it is not explicitly disclosed that the
system
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
4
is used for ablation. Furthermore, the temperature is controlled by an
external
feedback system, and not e.g. by the material properties of the stent.
Moreover,
European patent application EP 1 036 574 does not disclose that the stent or
implant may subtend at least a substantially complete circumferential band of
the
vessel's inner wall.
US patent 7,235,096 discloses an implantable stent for treating a damaged body
lumen, which comprises tubular stent body having several interconnected
nnicroholes distributed throughout the body unifornnally along the entire
length of
the body. The tubular stent body has several interconnected nnicroholes
distributed throughout the stent body substantially unifornnally along the
entire
length of the stent body; the several nnicroholes are small so as to promote
an
organized growth pattern of infiltrating cells throughout the stent body, and
the
stent body is otherwise substantially free of holes larger than the
nnicroholes; the
stent body is formed from a fibrous three dimensional non-woven matrix. The
patent also discloses a stent system comprising the stent in spaced
juxtaposition
to an energy source for transcutaneously applying energy to the stent, thereby
causing the temperature of the stent to increase to a temperature above body
temperature (preferably 38 - 49 C). It further discloses an active stent
comprising the stent and further comprising live cells growing in the
interconnected nnicroholes. A method for measuring flow of a fluid through a
body
lumen is disclosed, involving: implanting the stent into a body lumen having a
flow
of fluid through it; energizing the implanted stent transcutaneously to raise
its
temperature above body temperature; monitoring transcutaneously the output
from at least one of the temperature sensors upon cessation of the energizing
to
determine the cooling rate at each of the at least one sensor: and obtaining
the
flow rate of the fluid through the stent from the cooling rate at the at least
one
sensor. Also disclosed is a method for treating a tubular body organ in a
subject
involving: promoting the ingrowth of living cells in the stent; and implanting
the
stent into the tubular organ of the subject prior to or following promoting
the
ingrowth of the living cells so as to treat the tubular organ, whereby the
stent
body is formed from a fibrous three dimensional non-woven matrix.
In US patent 7,235,096, the temperature of the stent can be controlled by an
at
least partially external control system. In this case, the temperature sensor
or
sensors transmit the measured temperature to said external control system,
which
then controls the external energy source. Further, in this patent, the
temperature
of the stent can be controlled by the use of material with a Curie temperature
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
whereby the heating of the stent occurs via hysteresis heating. Hereby the
temperature of the stent is limited to the Curie temperature, since the
mechanism
of hysteresis heating only works below the Curie temperature. Both temperature
control mechanisms, i.e. the external control system and the use of magnetic
5 materials, have their shortcomings.
The mechanism comprising the external control system leads to the necessity of
a
dedicated external energy source, specifically adapted for receiving the
temperature from the temperature sensor. Furthermore, in such a system the
energy source, which in most cases will be a radiofrequent field, will need to
be
controlled in intensity and possibly also in frequency in order for the
implant to be
kept at a desired temperature.
The mechanism of hysteresis heating has a number of difficulties, especially
in
finding the correct alloy with an optimal Curie temperature. As this optimal
temperature may be different case-by-case, a different alloy may need to be
found
for different temperatures.
There remains a need in the art for improved devices, systems and methods for
the ablation of a substantially complete circumferential band or a spiraling
band
around a vessel's wall from the inside. The present invention aims to resolve
at
least some of the problems mentioned above, e.g. to make sure that the
ablation
is performed for a substantially complete circumferential band or a spiraling
band
around a vessel's wall from the inside, that the ablation itself can be
triggered with
external means and this multiple times if necessary, that the ablation
temperature
is well under control and does not depend on less-controlled elements in the
treatment or on an intricate monitoring system, etc.
The present invention tries to overcome the problems by providing an implant
with
a built-in temperature control and/or measuring means, whereby said control
means are capable of keeping the temperature of at least part of the implant
to or
below a desired temperature. The present invention also provides a system and
method for heating an implant to or up to a desired maximal temperature.
SUMMARY OF THE INVENTION
The present invention provides a system of one or more implant devices and
excitation device, an implant device and a method using the system and one or
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
6
more devices for the treatment of arterial and venous structures. The present
invention also concerns implant devices, a system of implant devices and
external
excitation means, and a method for positioning one or more implant devices in
a
vessel, and subsequently heating these implant devices, preferably
simultaneously, thereby transferring heat from implant devices to the vessel's
inner wall.
In a first aspect, the present invention provides a system for ablation of at
least a
part of a vessel's wall from the inside, comprised of
- a self-expanding implant device, adapted to be implanted and deployed
within said vessel; whereby said implant comprises an ablation region
along at least a portion of its length, said ablation region being adapted
for surface contact with said vessel and said ablation region subtending
at least a substantially complete circumferential band or a spiraling
band and being effective to ablate a signal-blocking path within said
vessel upon application of energy to the implant;
- external energy-providing means, which are spatially separated from
the implant device and able to provide energy to the implant device for
increasing the temperature of the ablation region of the implant device
up to an ablation temperature.
In a preferred embodiment, the system comprises more than one implant device,
each of which adapted to be implanted and deployed within one or more vessels.
These implant devices can each be adapted to be implanted and deployed within
one or more renal arteries.
In a particular preferred embodiment, one or more implant devices of the
system
comprise a proximal portion having a first diameter and a distal portion
having a
second diameter that is less than the first diameter and that is sufficient to
enable
said implants to seat within one or more vessels.
In a preferred embodiment, at least part of the one or more implant devices of
the
system is made from at least one material which shows magnetic hysteresis,
such
as a ferromagnetic, ferrinnagnetic or anti-ferromagnetic material.
Furthermore, the
external energy-providing means may create a time-varying magnetic field at
the
position of the one or more implant devices. In a more preferred embodiment,
this
time-varying magnetic field is created by an electric coil through which a
time-
varying electrical current is sent.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
7
In another embodiment, the system also comprises
- a sheath suitable for transporting and delivering the one or more
implant devices to or near the desired position in the one or more
vessels;
- a guidewire suitable for sequentially guiding the sheath with the one or
more implants to the desired position in the one or more vessels.
In a second aspect, the present invention provides a self-expanding implant
device
adapted to be implanted and deployed within a vessel; said implant comprising
an
ablation region along at least a portion of its length, the ablation region
being
adapted for surface contact with the vessel and the ablation region subtending
at
least a substantially complete circumferential band or a spiraling band and
being
effective to ablate a signal-blocking path within the vessel upon application
of
energy to the implant; whereby said ablation region comprises at least one
material which shows magnetic hysteresis, such as a ferromagnetic,
ferrinnagnetic
or anti-ferromagnetic material.
In a similar aspect, the present invention provides a, preferably self-
expanding,
implant device adapted to be implanted and deployed within a vessel, said
implant
comprising an ablation region along at least a portion of its length, the
ablation
region being adapted for surface contact with the vessel and for subtending at
least a substantially complete circumferential band or a spiraling band and
said
ablation region effective to ablate a signal-blocking path within the vessel
upon
application of energy to the implant device, whereby preferably said ablation
region comprises at least one material which shows magnetic hysteresis, such
as a
ferromagnetic, ferrinnagnetic or anti-ferromagnetic material.
In another similar aspect, the present invention provides an implant
comprising an
electrical circuit comprising a pick-up coil, a heater coil and a temperature-
controlled switch which comprises a closed position and an interrupted
position.
Said switch preferably comprises a bi-metallic component and/or a thernnistor,
such as a PTC thernnistor and/or said switch preferably comprises a
temperature
sensor and a, preferably digital, thermostat connected to said sensor and to
said
switch for interrupting said switch and thus said electrical circuit when said
sensor
measures a pre-determined temperature.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
8
In a preferred embodiment, the implant device comprises a shape which is
adapted for a renal artery. In a more preferred embodiment, said ablation
region
of said implant device is adapted for surface contact with said renal arteries
and
for subtending at least a substantially spiraling band, e.g. for treating
arterial
hypertension by ablation and consequential blocking renal sympathetic nerve
signals.
In a particular preferred embodiment, parts of the implant device are made
from
more than one material showing magnetic hysteresis and which have different
Curie or Neel temperatures.
In a more preferred embodiment the implant device is suitable for long-term
implantation. In another preferred embodiment, the implant device is a bio-
resorbable implant device or an implant that disappears, e.g. by evaporation,
after
one or more ablations. Furthermore, the implant device may comprise a proximal
portion having a first diameter and a distal portion having a second diameter
that
is less than the first diameter and that is sufficient to enable said implant
device to
seat within a vessel. The implant device may further comprise anchoring means
at
or near the proximal or distal portion of said implant device, said anchoring
means
being suitable for keeping the device at or near the same position compared to
the
vessel's inner wall.
In a preferred embodiment, part of said implant device which can come into
contact with the patient's blood when said implant device is implanted, is
thermally isolated from the rest of the implant device such that the blood is
not
heated or overheated during the excitation of the implant device. Such part
can
comprise an adlunninal coating or a layer with high isolation characteristics.
In a preferred embodiment, said implant comprises a thernnoactive coating
comprising an activation temperature between 35 C and 37 C so that the body
temperature would trigger activation. In an alternatively preferred
embodiment,
said implant comprises a thernnoactive coating comprising an activation
temperature above 45 C so that activation is triggered only when said ablation
region is heated by said external energy-providing means.
In a preferred embodiment, the implant device comprises a core region of
material
with a certain Curie temperature, surrounded by other material with thermal
and/or elastic properties suitable for the implant device's purpose.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
9
In an embodiment, said implant comprises substances capable of producing a
lesion of limited necrosis and/or neurotoxicity.
In a preferred embodiment, the implant device comprises cavities which are
filled
with one or more substances and which open when the implant is heated. In a
more preferred embodiment, these substances are mixed before being released
into the patient's body or vessel wall, e.g. to deliver a two-component
neurotixine.
In another preferred embodiment, these substances are a selection or a
composition of one or more of the following substances:
- ethanol;
- tetrodotoxin and batrachotoxin;
- nnaurotoxin, agitoxin, charybdotoxin, nnargatoxin, slotoxin, scyllatoxin
or
hefutoxin;
- calciseptine, taicatoxin, calcicludine, or PhTx3;
- botulinunn toxide;
- cytochalasin D, rapannycin, sirolinnus, zotarolinnus, everolinnus,
paclitaxel;
- glutamate;
- isoquinoline;
- N-methyl-(R)-salsolinol;
- Beta-carboline derivates.
In a further aspect, the present invention provides a system comprising one,
two,
three, four or more implant devices, such as 5, 6, 7, 8, 9 or 10 or more
implant
devices according to the present invention. Preferably this system comprises
external energy-providing means, which are spatially separated from said
implant
devices and able to provide energy to said implant devices for increasing the
temperature of the ablation regions of the implant devices up to an ablation
temperature, and/or a sheath suitable for transporting and delivering the one
or
more implant devices to or near the desired position in the one or more
vessels,
and/or a guidewire suitable for sequentially guiding the sheath with the one
or
more implants to the desired position in the one or more vessels. In a
preferred
embodiment, the system comprises one or two implant devices according to the
present invention, each of which adapted for a corresponding renal artery.
In yet a further aspect, the present invention provides a method for the
treatment
of a patient with arterial hypertension by blocking renal sympathetic nerve
signals
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
via ablation of a substantially complete circumferential band or a spiraling
band on
one or more renal arteries' walls from the inside, comprising the steps of
- implanting one or more implant devices in one or more renal arteries by
means
of a sheath and a guidewire, said implant devices each comprising an ablation
5 region along at least a portion of their length, said ablation regions
being adapted
for surface contact with said renal arteries and said ablation regions
subtending at
least a substantially complete circumferential band or a spiraling band and
being
effective to ablate a signal-blocking path within said renal arteries upon
application
of energy to said implant devices;
10 - retracting the sheath and guidewire;
- subsequently heating the ablation region of the one or more implant
devices by
external energy-providing means, which are spatially separated from the
implant
device.
In a similar aspect, the present invention provides a method for heating one,
two
or more implant devices, which are suitable to be implanted in one, two or
more
vessels, comprising the steps of:
- subsequently positioning said implant devices in said vessels by means of
a
sheath and a guidewire, said implant devices each comprising an ablation
region
along at least a portion of their length, said ablation region subtending at
least a
substantially complete circumferential band or a substantially spiraling band,
said
implant devices effective for ablating a signal-blocking path within said
vessels
upon application of energy to said implant devices;
- retracting the sheath and guidewire;
- heating the ablation region of said implant devices by external energy-
providing
means which are spatially separated from said implant devices
characterized in that said heating occurs after said sheath and guidewire are
retracted and said heating of said implant devices occurs simultaneously.
In a preferred embodiment of the method, a recovery period is observed prior
to
heating the ablation region of the one or more implant devices by external
energy-
providing means. Furthermore, this recovery period may be long enough to allow
the one or more implant devices to be integrated into the vessel wall or
endothelialized.
In a particular preferred embodiment of the method, the step of heating the
ablation region of the one or more implant devices by external energy-
providing
means, which are spatially separated from the implant device, is performed
more
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
11
than once, e.g. at well-spaced time-intervals, whenever it is deemed
necessary,
etc.
In a more preferred embodiment of the method, one or more implant devices as
described in this document are being used.
In a still more preferred embodiment of the method, use is made of a system as
described in this document.
DESCRIPTION OF FIGURES
Figures 1 to 5, and 9 to 11 schematically represent different embodiments of
an
implant for the treatment of arterial and venous structures according to the
invention.
Figures 6 to 8 schematically represent a detail of a portion of an implant
according to the invention.
Figure 12 schematically represents an embodiment of a sheath with guidewire
and implant device.
Figure 13 schematically shows the way the catheterization can be done in order
to deliver one or more implants to a vessel.
Figure 14 schematically represents an embodiment of the external energy-
providing means as it can be used for treating a patient.
Figure 15 schematically represents an embodiment of an implant in place in the
vessel.
Figure 16 shows a magnetic hysteresis loop for a ferronnagnet: H is the
intensity
of the magnetic field, M is the magnetic moment of the sample, I-Ic is the
coercive
field, Mr is the residual magnetic moment, and Ms is the saturation magnetic
moment. The nonlinniting hysteresis loop is shown by the dotted line. The
domain
structure of the sample for certain points on the loop is shown schematically.
Figure 17 shows the typical temperature dependence of the hysteresis loop of a
magnetic material with Curie or Neel temperature of 140 C.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
12
Figure 18 shows an embodiment of an implant which has an hour-glass shape,
whereby near the middle region, where the diameter becomes smaller, a set of
heating rings is attached around the hour-glass shaped part of the implant.
Figure 19 shows an embodiment of an implant comprising a fuse, so that at
certain temperatures, the circuit that may be generated gets interrupted.
Figure
19a shows a detailed view of the fuse.
In a different configuration, as shown in figure 20, the metal implant can be
build
up of memory shape alloys. Details of the on and off position of the switch or
fuse
are shown in figures 20a and 20b respectively.
In a still different configuration as shown in fig. 21 and a detail in fig.
21a, the
implant consists of two different materials.
An embodiment of the implant with an extensive coating formed around the
implant, but almost exclusively on the ADLUMINAL side is illustrated in fig.
22.
Figure 23 illustrates the concept of the present invention whereby an implant
device is provided with a built-in thermal switch.
Figure 24 illustrates the dimensions of an implant in an expanded position in
a
vessel.
Figures 25a-g illustrate different embodiments of the present invention,
whereby
the shape and absolute and relevant sizes of the coils may differ between
different
embodiments.
Figure 26 illustrates that the heat is deposited mainly near the winding, but
that
it is possible that also the outer side of the vessel can be heated to an
increased
temperature.
Further embodiments comprising e.g. a PTC or thernnistor switch, are
illustrated in
figs. 27a-b for essentially cylindrical implants.
An AC-DC converter may be part of a larger electronic circuit which can be
attached to a pcb and coupled to the coils as illustrated in fig. 28.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
13
Figures 29a-d illustrate electronic circuits which can be used in embodiments
of
the implant of the present invention.
Figures 30a-34 illustrate embodiments of external energy providing means which
can be used in a system or method of the present invention for providing
energy
to the implant by providing a time-varying magnetic field at the position of
the
implant.
DETAILED DESCRIPTION OF THE INVENTION
The present invention concerns a system of one or more implant devices and
excitation device, an implant device and a method using the system and one or
more devices for the treatment of arterial and venous structures. The present
invention also concerns implant devices, a system of implant devices and
external
excitation means, and a method for positioning one or more implant devices in
a
vessel, and subsequently heating these implant devices, preferably
simultaneously, thereby transferring heat from implant devices to the vessel's
inner wall.
Unless otherwise defined, all terms used in disclosing the invention,
including
technical and scientific terms, have the meaning as commonly understood by one
of ordinary skill in the art to which this invention belongs. By means of
further
guidance, term definitions are included to better appreciate the teaching of
the
present invention.
As used herein, the following terms have the following meanings:
"A", "an", and "the" as used herein refers to both singular and plural
referents
unless the context clearly dictates otherwise. By way of example, "a
compartment" refers to one or more than one compartment.
"About" as used herein referring to a measurable value such as a parameter, an
amount, a temporal duration, and the like, is meant to encompass variations of
+/-20% or less, preferably +/-10% or less, more preferably +/-5% or less, even
more preferably +/-1% or less, and still more preferably +/-0.1 /0 or less of
and
from the specified value, in so far such variations are appropriate to perform
in the
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
14
disclosed invention. However, it is to be understood that the value to which
the
modifier "about" refers is itself also specifically disclosed.
"Comprise," "comprising," and "comprises" and "comprised of" as used herein
are
synonymous with "include", "including", "includes" or "contain", "containing",
"contains" and are inclusive or open-ended terms that specifies the presence
of
what follows e.g. component and do not exclude or preclude the presence of
additional, non-recited components, features, element, members, steps, known
in
the art or disclosed therein.
The recitation of numerical ranges by endpoints includes all numbers and
fractions
subsumed within that range, as well as the recited endpoints.
The expression "% by weight" (weight percent), here and throughout the
description unless otherwise defined, refers to the relative weight of the
respective
component based on the overall weight of the formulation.
The expressions "implant" and "implant device" are used interchangeably in
this
application. An implant device as used in the present context, refers to an
artificial
tube or tube-like device, i.e. a device which has a circumferential wall and
which is
at least partly open at the top and at the bottom, whereby said
circumferential
wall may or may not have openings or holes, said tube or tube-like device
intended to be placed inside a vessel of the body of a patient, e.g. a vein,
or inside
a vessel of a human or animal corpse or model of a human or animal body. In
the
present context, the terms "implant" and "implant device" do not necessarily
mean
that the device is placed inside a vessel to keep this vessel open for fluids,
although this can be one of the effects of the device. The implant device is,
however, meant to be seated in a fixed position compared to the vessel and not
to
move due to fluid flow through the vessel. When using the term "implanted"
device, it is meant that the implant or implant device has been implanted. In
an
embodiment, the implant device is a stent device, meaning that the device has
the
intended effect of keeping the vessel open for fluids when implanted.
The terms "catheter" and "sheath" are used interchangeably in this
application.
The term "guidewire" is used in this application for a device which can be
controllably guided when inserted into a body. In a preferred embodiment, it
is a
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
catheter, i.e. a guiding catheter. In another embodiment, it is solid and does
not
have a lumen.
The terms "Curie temperature" and "Neel temperature" refer to the temperature
5 above which ferromagnetic, anti-ferromagnetic and ferrinnagnetic materials
become para- or diamagnetic, and are used interchangeably in the following.
"Resistive heating" and "Joule heating" here and throughout this text are used
as
synonyms and refer to the process by which the passage of an electric current
10 through a conductor releases heat.
"Thermal switch" and "temperature-controlled switch" here and throughout this
text are used as synonyms and refer to a switch capable of closing or opening
one
or more electrical circuits, depending on the value of a temperature. This
15 temperature may be the temperature at the position of the switch, or
may be the
temperature as obtained on a different position. Specific embodiments of
thermal
switches are presented further in this text.
In a first aspect, the present invention provides a system for ablation of at
least a
part of a vessel's wall from the inside, comprised of
- a self-expanding implant device, adapted to be implanted and deployed
within said vessel; whereby said implant comprises an ablation region
along at least a portion of its length, said ablation region being adapted
for surface contact with said vessel and said ablation region subtending
at least a substantially complete circumferential band or a spiraling
band and being effective to ablate a signal-blocking path within said
vessel upon application of energy to the implant;
- external energy-providing means, which are spatially separated from
the implant device and able to provide energy to the implant device for
increasing the temperature of the ablation region of the implant device
up to an ablation temperature.
The implant device is self-expanding, for example, being formed of a shape
memory alloy, and is configured to lodge against the interior wall of e.g. a
renal
artery. The implant may be formed as a loop, helix, progressively wound helix
or
other suitable shape. It may have anchoring means such as hooks or barbs near
the ends. The implant device may comprise an ablation region which is in
contact
with the ablation region of the vessel's wall. Preferably, the ablation region
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
16
comprises a substantially complete circumferential band or a spiraling band
around
the vessel's wall, or a dotted, dashed or interrupted circumferential or
spiraling
band around the vessel's wall. The ablation region may comprise a complete
circumferential band or a spiraling band around the vessel's inner wall, or
the
ablation region may comprise a complete circumferential band or a spiraling
band
around the vessel's wall and this for the complete thickness of the wall. With
'substantially' is meant that the ablation region is such that all electric
signals
arising at one side of the ablation region do not reach the other side, i.e. a
signal-
blocking path is ablated. Energy can be provided to the implant device by
external
means through electromagnetic radiation, through hysteresis heating via a time-
varying magnetic field, by direct and indirect induction and by Joule heating,
by
acoustic, mechanical-vibrational and chemical energy means, by a
thermal/chemical or mechanical/chemical release system.
One of the advantages of the present invention over prior art techniques, is
that
the one or more implant devices can be heated up simultaneously, i.e. the
delivery
of energy to the implants happens at the same time and does not need to be
done
sequentially. This saves time and increases the patient's comfort. Through
built-in
control of the temperature, e.g. by using magnetic material with a specified
Curie
temperature or by using the proper insulation material in the implant,
additional
energy delivery will not further increase the temperature built-up in the
implant.
By 'external' energy-providing means is meant that these means are spatially
separated from the implant device, i.e. there is no physical connection
between
the energy-providing means and the implant device, or, more specifically, the
energy-providing means are completely outside the patient's body and the
patient's skin can remain intact while the energy is provided.
The temperature of ablation region is specified according to the needs of the
treatment. Depending on the ablation temperature needed, the implant device
can
be engineered to be warmer at certain regions than in other regions by using
the
magnetic and thermal properties of the materials of which the implant device
is
composed. In an embodiment, parts of the implant device may be thermally
isolated from other parts of the implant device or from parts of the body or
bodily
fluids.
In a preferred embodiment, the system comprises more than one implant device,
each of which adapted to be implanted and deployed within one or more vessels.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
17
These implant devices can each be adapted to be implanted and deployed within
one or more renal arteries.
In a particular preferred embodiment, one or more implant devices of the
system
comprise a proximal portion having a first diameter and a distal portion
having a
second diameter that is less than the first diameter and that is sufficient to
enable
said implants to seat within one or more vessels.
In a particular preferred embodiment, one or more implant devices of the
system
comprise a proximal portion having a first diameter and a distal portion
having a
second diameter that is greater than or equal to the first diameter and that
is
sufficient to enable said implants to seat within one or more vessels.
It should be clear that the shape of the implant device can be adapted so as
to fit
into the specific vessel.
In order to make the shape and dimensions of the implants, it is possible by a
scanning technique such as CT-scan or MRI, to collect data on the varying
diameter of the vessel. From these data, one can derive the necessary shape
and
dimensions of the implants e.g. for all two renal arteries of a patient. Again
this
measuring can be done without a surgical procedure, thereby increasing the
patient's comfort and wellbeing and reducing medical risks. After this
measuring,
the implants can be custom-made to fit the patient's vessel or vessels.
In a preferred embodiment, at least part of the one or more implant devices of
the
system is made from at least one material which shows magnetic hysteresis,
such
as a ferromagnetic, ferrinnagnetic or anti-ferromagnetic material.
Furthermore, the
external energy-providing means may create a time-varying magnetic field at
the
position of the one or more implant devices. In a more preferred embodiment,
this
time-varying magnetic field is created by an electric coil through which a
time-
varying electrical current is sent.
Magnetic hysteresis arises in a plethora of materials. Most known and most
used
are the ferromagnetic, anti-ferromagnetic and ferrinnagnetic materials. These
have
highly non-linear magnetic properties, i.e. the magnetic induction field is
not
directly proportional to the applied magnetic field inside the material.
However, all
these material lose their specific magnetic properties above a certain
temperature,
called the Curie or Neel temperature. This temperature is material-specific.
Above
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
18
this temperature, the ferromagnetic, anti-ferromagnetic and ferrinnagnetic
materials become para- or diamagnetic and thereby lose their non-linear
magnetic
properties. The non-linear magnetic properties of ferromagnetic, anti-
ferromagnetic and ferrinnagnetic materials can be deduced from the hysteresis
that is observed when applying a time-varying magnetic field.
Magnetic hysteresis is observed in magnetic materials, such as ferronnagnets.
The
main feature of ferronnagnets is the presence of spontaneous magnetization. A
ferronnagnet usually is not uniformly magnetized but is divided into domains-
regions of uniform spontaneous magnetization whose degree of magnetization
(the magnetic moment per unit volume) is identical, although the directions
are
different. Under the effect of an external magnetic field the number and size
of the
domains magnetized along the field increase at the expense of other domains.
Moreover, the magnetic moments of certain domains may rotate in the direction
of
the field. As a result the magnetic moment of the sample increases.
The dependence of the magnetic moment M of a ferromagnetic sample on the
intensity H of the external magnetic field (the magnetization curve) is shown
in
Fig. 16. In a sufficiently strong magnetic field the sample is magnetized to
saturation (as the field increases further, the value of M remains virtually
unchanged¨point A). Here the sample consists of one domain with a magnetic
moment of saturation Ms oriented along the field. As the intensity H of the
external
magnetic field is reduced, the magnetic moment M of the sample will decline
along
curve I primarily because of the appearance and growth of domains whose
magnetic moment is oriented against the field. The growth of the domains is
due
to the movement of the domain walls. This movement is hindered by the presence
in the sample of various defects (such as impurities or inhonnogeneities) that
strengthen the domain walls at some points; very strong magnetic fields are
required to displace them. Therefore as the field H drops to zero, the so-
called
residual magnetic moment Mr (point 8) is retained. A sample can be completely
demagnetized only in a sufficiently strong field of opposite direction, which
is
called a coercive field (coercive force) Hc (point C). As the magnetic field
of
reverse orientation is further increased, the sample is once again magnetized
along the field to saturation (point D). Magnetic reversal (from point D to
point A)
takes place along curve II. Thus, as the field undergoes a cyclical change,
the
curve characterizing the change in the magnetic moment of the sample forms a
magnetic hysteresis loop. If the field H changes cyclically with such limits
that
magnetization does not reach saturation, a nonlinniting magnetic hysteresis
loop is
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
19
produced (curve III). By reducing the extent of the change in field H to zero,
the
sample can be completely demagnetized (point 0 can be reached). The
magnetization of the sample from point 0 proceeds along curve IV.
In magnetic hysteresis different values of the magnetic moment M correspond to
the same value of the external magnetic field intensity H. This nonuniqueness
is
due to the influence of the states of the sample that precede the given state
(that
is, to the magnetic prehistory of the sample).
The shape and size of magnetic hysteresis loops and the quantity 1-Ic may
range
within wide limits in various ferronnagnets. For example, in pure iron, I-1c=
1
oersted, and in a nnagnico alloy I-1c= 580 oersteds. A magnetic hysteresis
loop is
strongly affected by processing of the material, during which the number of
defects is changed. The area of a magnetic hysteresis loop is equal to the
energy
lost in the sample in one cycle of field change. This energy is also
proportional to
the total volume of ferromagnetic material in the sample. This energy
ultimately is
used to heat the sample. Such energy losses are called hysteresis losses. In
cases
when losses to hysteresis are undesirable (for example, in transformer cores
and
in the stators and rotors of electrical machinery), magnetically soft
materials with
a low 1-Ic and a small hysteresis loop area are used. On the other hand,
magnetically hard materials with a high 1-Ic are required to manufacture
permanent
magnets.
As the frequency of the alternating magnetic field (the number of magnetic
reversal cycles per unit time) increases, other losses caused by eddy currents
and
magnetic viscosity are added to hysteresis losses. At high frequencies the
area of
the hysteresis loop increases correspondingly. Such a loop is sometimes called
a
dynamic loop, in contrast to the static loop described above.
Many other properties of a ferronnagnet, such as electrical resistance and
mechanical deformation, depend on the magnetic moment. A change in magnetic
moment also brings about a change in these properties¨for example,
galvanonnagnetic and nnagnetostrictive hysteresis, respectively, are observed.
The hysteresis loop depends on the temperature. Figure 17 shows the typical
temperature dependence of the hysteresis loop of a magnetic material with
Curie
or Neel temperature of 140 C. Note that only the temperature dependence of
the
shape is characteristic and no units are given on the axes, the figure is
meant for
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
illustration purposes. It is observed that the hysteresis loop changes with
temperature, becoming sharper and thinner, and eventually disappearing at the
Curie or Neel temperature. From this temperature onwards, the material becomes
para- or diamagnetic and no heating losses due to hysteresis are observed.
This
5 means that the material does not heat up anymore, at least not due to
hysteresis
effects, and remains at the Curie or Neel temperature. (In the following, the
two
terms 'Curie' and 'Neel' temperature can be used interchangeably.) It should
be
remarked that heating due to other effects, such as direct or indirect
induction
may still be possible, but these effects are negligible in the present case,
10 especially when compared to the gigantic heating capabilities by
hysteresis effects.
It should be now clear to the skilled person that when the ablation region of
an
implant device comprises material with Curie temperature of e.g. 40 C, the
implant will be heated up to this temperature and not more when being
subjected
15 to a time-varying magnetic field, e.g. by the external energy-providing
means of
the system of the present invention. If the ablation temperature needs to be
42 C
or 45 C, the magnetic material used in the implant may be altered to have this
temperature as Curie temperature. This can be done by e.g. changing the
composition of an alloy of magnetic material. The Curie temperature of a
magnetic
20 material can be very precisely engineered.
In a preferred embodiment, the magnetic materials used in the implant device
are
a combination or alloy of the following materials: MnAs, Gd, Gd with a thin Fe
overlayer, Ni-Fe alloy with around 29.5 at.% Ni which is cooled slowly from
1000 C, Ni-Fe with 30 at.% Ni, Cr, CoO, ZnFe204, are any magnetic material
with
Curie or Neel temperature above 10, 20, 25, 30, 35, 40 C and/or below 75, 70,
65, 60, 55, 50, 45, 40 C.
The Curie or Neel temperatures of alloys or composite materials can depend
highly
on the procedure for making these materials. Especially annealing procedures
may
be important. Also other ways of altering the Curie temperatures such as ion
radiation can be used to provide the desired material. One can use any
magnetic
material, alloy, binary alloy, ternary alloy or quaternary alloy with the
desired
Curie or Neel temperature as specified in standard reference works such as the
LandoIt-Bornstein database.
In another embodiment, the system also comprises
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
21
- a sheath suitable for transporting and delivering the one or more
implant devices to or near the desired position in the one or more
vessels;
- a guidewire suitable for sequentially guiding the sheath with the one or
more implants to the desired position in the one or more vessels.
The sheath in this embodiment includes an implant delivery system capable of
delivering the one or more implant devices as described in this text. An
embodiment of such sheath with delivery device and guidewire is shown in
figure
12.
In a further aspect, the present invention provides a self-expanding implant
device
adapted to be implanted and deployed within a vessel; said implant comprising
an
ablation region along at least a portion of its length, the ablation region
being
adapted for surface contact with the vessel and the ablation region subtending
at
least a substantially complete circumferential band or a spiraling band and
being
effective to ablate a signal-blocking path within the vessel upon application
of
energy to the implant; whereby said ablation region comprises at least one
material which shows magnetic hysteresis, such as a ferromagnetic,
ferrinnagnetic
or anti-ferromagnetic material.
Preferably said material comprises a ferrous fluid, i.e. ferromagnetic,
ferrinnagnetic
and/or anti-ferromagnetic particles suspended in a heat conducting fluidunn,
whereby said material is preferably contained within said implant device. In a
more preferred embodiment, said implant device comprises one or more fluid-
tight
cavities comprising said ferromagnetic, ferrinnagnetic and/or anti-
ferromagnetic
particles in said heat conducting fluidunn. In an even more preferred
embodiment,
said particles comprise any or any combination of the following materials:
SrFe12019, Mea-2W, Mea-2Y, and Mea-2Z, wherein 2W is BaO : 2 Mea0 : 8 Fe203,
2Y is 2 (BaO : Mea0 : 3 Fe203), and 2Z is 3 BaO : 2 Mea0 : 12 Fe203, and
wherein
Mea is a divalent cation, whereby the divalent cation is preferably selected
from
Mg, Co, Mn and Zn, and/or 1 Meb0 : 1 Fe203, where Meb0 is a transition metal
oxide selected from Ni, Co, Mn, and Zn, and/or metal alloys such as
La0.8Sr0.2Mn03, Y3Fe5_xMx012 where M is Al, or Gd and 0<x<2, and/or metal
alloys
of any combination of Pd, Co, Ni, Fe, Cu, Al, and Si and/or metal alloys of
any
combination of Gd, Th, Dy, Ho, Er, and Tnn with any combination of Ni, Co, and
Fe
and/or metal alloys RMn2X where R is a rare earth, such as La, Ce, Pr, or Nb
and X
is either Ge or Si. Particularly preferred is any or any combination of the
following
alloys: NiCu with 28% or 29.6% Ni, NiPd, PdCo with 6.15% Pd, NiSi with 4% Ni,
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
22
(Ni,zno)Fe203, Lao.8Sro.2MnOx, Y3Fe5_xAlx012 with 1.0 x
1.7. The particles can
be of any size, preferably longer than 10 nanonneters, more preferably longer
than
20 nanonneters in the longest dimension, and smaller than 500 micrometers,
preferably smaller than 100 micrometers in the longest dimension. In certain
embodiments, said particles are smaller than 1 micrometer, preferably smaller
than 200 nanonneters in the longest dimension. In other embodiments, said
particles are longer than 1 micrometer, preferably longer than 20 micrometer
in
the longest dimension. Preferably said fluidunn in which said particles are
suspended comprises optimal heat conduction properties. In a preferred
embodiment, said fluidunn comprises a large heat capacity. In another
preferred
embodiment, said fluidunn comprises a low heat capacity. The exact nature,
amount and combination of which magnetic materials to use for the particles
and
which fluidunn to use, depends on the desired temperature and heat for e.g.
inducing complete spiral ablation of the inner wall of a renal artery. In a
preferred
embodiment, said magnetic materials comprise a Curie or Neel temperature of
37,
38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 C or
any
value in between or any combination thereof, preferably said Curie or Neel
temperature is smaller than 75 C, more preferably smaller than 70 C, even more
preferably smaller than 65 C, yet more preferably smaller than 62 C, still
more
preferably smaller than 59 C, yet even more preferably smaller than 57 C,
still
even more preferably smaller than 55 C.
In a preferred embodiment, the implant device is adapted to be implanted and
deployed within a renal artery.
In a particular preferred embodiment, parts of the implant device are made
from
more than one material showing magnetic hysteresis and which have different
Curie or Neel temperatures.
Different temperatures cause different lesions on different places depending
on
which ablation regions of the implant device comprises which material. By
thermally isolating parts of the implant device which consist of material with
different Curie temperatures, a gradation in ablation temperature along the
implant device is possible. Also, parts comprising material with higher heat
capacities will heat up more slowly, but will remain hot longer afterwards,
etc.
Engineering of ablation characteristics can be done by engineering the implant
making use of the magnetic and thermal properties of the materials used in the
implant.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
23
In a more preferred embodiment the implant device is suitable for long-term
implantation. In another embodiment, the implant device is a bio-resorbable
implant device or an implant that disappears, e.g. by evaporation, after one
or
more ablation procedures. In a preferred embodiment, the implant device may
comprise a proximal portion having a first diameter and a distal portion
having a
second diameter that is less than the first diameter and that is sufficient to
enable
said implant device to seat within a vessel. The implant device may further
comprise anchoring means at or near the proximal or distal portion of said
implant
device, said anchoring means being suitable for keeping the device at or near
the
same position compared to the vessel's inner wall. The anchoring means may
comprise hooks or barbs or anything else known in the art for keeping an
implant
device at the desired position.
In a preferred embodiment, the implant device comprises an elastically
compressible body comprising externally triggerable portions and provided of
anchoring means. The body may be mainly made of wires and may, in expanded
or released position, be provided of a narrowing tubular shape and/or a
somewhat
flattened narrowing tubular shape, i.e. that the cross sections at most
positions
along the longitudinal axis are oval or round-shaped or the like, suitable to
be
placed inside a renal artery. The body may also comprise between two and five
circular wires, a first bigger circular or oval wire, and further circular or
oval wires
with decreasing diameter, positioned and maintained at a distance from each
other, at least when the body is in a released or not compressed position. The
body may be built up out of braided metal wires (that have multiple
interconnections, crossings and/or layers which allows for numerous
connections
with the vascular or arterial wall of the vessel). The body may be conceived
as a
spirally shaped wire of which the diameter gradually goes down along its
longitudinal axis. The windings of the implant device may be mutually
connected
with bridging upstanding wire portions providing closed loops to ensure full
and
circular coverage of for example the renal arteries once the device is
released. The
body may show longitudinal metal beads that are outward bending, and that
still
show several interconnections between them, to ultimately form a metal cage.
The
implant device may be characterized in that the greatest distance between two
points that can be measured on the circular or oval wires of the body will
range
from 3 to 30 mm, more specifically from 5 mm to 20 mm, even more specifically
from 9 mm to 13 mm, if to be implanted in the renal artery. The implant device
may be characterized in that the greatest distance between two points that can
be
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
24
measured on the circular or oval wires of the body of the implant device will
range
from 5 to 50 mm, more specifically from 8 mm to 40 mm, even more specifically
from 10 mm to 30 mm, if to be implanted in the vessel. The body of the implant
device may be mainly made of one or more metal alloys. The body of the implant
device may comprise portions which are externally triggerable by means of an
energy field or a combination of energy fields chosen from electromagnetic
radiation, direct or indirect induction, acoustic energy, mechanical
vibration,
heating and/or changing other characteristics of the implant or portions
thereof.
Some of the material used in the implant device may be of the type that
reacts,
for example heats, in response to a remote applied alternating magnetic field.
Energy can be provided to the implant device by external means through
electromagnetic radiation, through hysteresis heating via a time-varying
magnetic
field, by direct and indirect induction and by Joule heating, by acoustic,
mechanical-vibrational and chemical energy means, by a thermal/chemical or
mechanical/chemical release system. The body may comprise portions made of
different metal alloys with optionally different ferromagnetic properties
and/or
absorption coefficients, with specific response to alternating magnetic
fields.
Portions of the body of the implant device may be provided of one or more
coatings with varying properties. The wire or wires or other portions of the
body of
the implant device is/are composed of different layers made of different
alloys
and/or of other materials. The implant device may be further characterized in
that
different coatings or layers represent different responses to externally
applied
energy fields, for example to externally applied alternating magnetic fields.
An
adlunninal coating or layer with high isolation characteristics may be
provided to
the implant device. The body of the implant device may have self-expanding
properties thanks to the elastic characteristics of the material used, and
thanks to
the geometry of the body, and further expansion is stopped when it encounters
a
counter pressure of about 10 to 40, preferably 20 to 30, more preferably 22 to
28,
even more preferably around 25 mm Hg, equal to the distension pressure needed
to alter the vessel's anatomy. The implant device may be characterized in that
it is
provided of toxic substances that are only released upon introduction into the
renal artery, for example after applying an external energy field, which toxic
substances then produce a lesion of limited necrosis/neurotoxicity. These
toxins
may include, but are not limited to ethanol, tetrodotoxin and batrachotoxin,
nnaurotoxin, agitoxin, charybdotoxin, nnargatoxin, slotoxin, scyllatoxin and
hefutoxin, calciseptine, taicatoxin, calcicludine, and PhTx3, botulinunn
toxine,
cytochalasin D, rapannycin, sirolinnus, zotarolinnus, everolinnus, paclitaxel,
glutamate, isoquinoline, N-methyl-(R)-salsolinol, Beta-carboline derivates.
The
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
implant device may be provided of micro pores wherein substances are provided,
which can be released by a triggering energy field. The implant device may be
provided of a thernnoactive coating which is only activated upon temperatures
above 35 C so that the body temperature would trigger activation. The implant
5 device may be provided of a thernnoactive coating which is only activated
upon
temperatures above 45 C so that an external application of an energy field
would
trigger activation. The implant device may be provided of anchoring means
which
mainly consist of the elongated shape and/or the expanding forces optionally
in
combination with the wired structure allowing partial insertion or impression
in the
10 wall of the vessel, e.g. of the renal arteries. These anchoring means
may also
comprise hooks or barbs or the like, optionally provided on the outwardly
directed
portions of the implant.
In a preferred embodiment, said implant device comprises cavities which are
filled
15 with one or more substances and which open when the implant is heated
and/or
which open when the implant acquires body temperature, and/or whereby said
implant comprises a coating comprising one or more substances.
In a preferred embodiment, an implant device according to the present
invention
20 comprises a maximal circumference and a minimal circumference and a
ratio
between maximal and minimal circumference, whereby said ratio is smaller than
10, preferably smaller than 9, more preferably smaller than 8, even more
preferably smaller than 7, yet more preferably smaller than 6 and larger than
1.1,
preferably larger than 1.5, more preferably larger than 2, even more
preferably
25 larger than 2.5, yet more preferably larger than 3. In a preferred
embodiment, the
implant device comprises a variable circumference along a longitudinal
direction of
the implant, said circumference varying between at least 20 mm, preferably at
least 25 mm, more preferably at least 30nnnn, even more preferably at least 36
mm, yet more preferably at least 42 mm, still more preferably at least 48 mm
and
at most 375 mm, more preferably at most 350 mm, even more preferably at most
325 mm, yet more preferably at most 300 mm, still even more preferably at most
275 mm, yet even more preferably at most 250 mm. Such a ratio or dimension
may be necessary to ensure that an essentially circumferential or spiraling
band of
the vessel's inner wall would be subtended, in particular if the vessel is a
renal
artery.
In a particularly preferred embodiment, said circumference may be at most
200%,
preferably at most 190%, more preferably at most 180%, even more preferably at
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
26
most 170%, yet more preferably at most 160%, still more preferably at most
150% of the original diameter of the vessel for which the implant device is
adapted, e.g. of the renal artery, etc. when the self-expanded implant device
is in
an expanded state.
In a preferred embodiment, the implant device comprises an outer surface
comprising zig-zag or woven or braided material, drawn tubes, eccentrically
drawn
tubes, hollow struts, hollow struts filled with fluid, or any combination
thereof.
In a preferred embodiment, an implant device according to the present
invention
comprises an essentially cylindrical shape preferably comprising a diameter
which
is at least 2 mm, preferably at least 3 mm, more preferably at least 4 mm,
even
more preferably at least 5 mm, yet more preferably at least 6 mm and at most
20
mm, preferably at most 16 mm, more preferably at most 13 mm, even more
preferably at most 10 mm, yet more preferably at most 9 mm. Such a shape or
dimension may be necessary to ensure that a circumferential or spiraling band
of
the vessel's inner wall would be subtended, in particular in an essentially
cylindrical portion of said vessel, in particular if the vessel is a renal
artery.
In a preferred embodiment, an implant device according to the present
invention
comprises a distal portion and a proximal portion, whereby said ablation
region is
located within 50%, preferably within 40%, more preferably within 30% of the
implant's total length from the proximal portion. In a preferred embodiment,
an
implant device according to the present invention comprises a distal portion
and a
proximal portion, whereby said ablation region is located within 25 mm,
preferably
within 20 mm, more preferably within 15 mm from the proximal portion.
In a preferred embodiment, the implant device comprises a distal portion and a
proximal portion, and comprising an anchoring device connected to the ablation
region of said implant via a thermally insulating connection for preventing
overheating of said anchoring device, preferably whereby said anchoring device
is
connected to the distal portion. The anchoring device may comprise different
material than the rest of the implant device. In particular, the anchoring
device
may have different thermal characteristics due to its dimensions, shape or
material. The anchoring device may be connected to the distal portion of the
implant device in order to have optimal anchoring in e.g. the lumen of a renal
artery. The thermally insulating connection may comprise thermally insulating
material, or its shape and dimensions may increase thermal insulation, e.g. a
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
27
number of thin straps or wires attaching the anchoring device to the ablation
region.
In a preferred embodiment, the system for the treatment of arterial and venous
structures, comprises an implant device according to any of the above
embodiments and an excitation or energy-providing device preferably conceived
to
be used from the exterior of the patient, after being provided of an implant
device,
whereby the excitation serves to change the characteristics of the implant
device
in order to treat the arterial or venous structure where the implant device is
located. The excitation device for the treatment of arterial and venous
structures,
may be conceived to be used in cooperation with an implant device according to
any embodiment described in this text.
In a preferred embodiment the part of the implant device which can come into
contact with the patient's blood when said implant device is implanted, is
thermally isolated from the rest of the implant device such that the blood is
not
heated or overheated during the excitation of the implant device. Such part
may
comprise an adlunninal coating or a layer with high isolation characteristics.
It is
clear that heating of the blood should be avoided as much as possible for the
benefit and comfort of a patient.
In another embodiment, the implant device comprises a core region of material
with a certain Curie temperature, surrounded by other material with thermal
and/or elastic properties suitable for the implant device's purpose. As such,
one
can engineer the temperature profile through the implant device. It should be
clear that the parts of the implant which are meant to contact the vessel wall
and
the form lesions by ablation, should be heated mostly while other parts of the
implant, which are in contact with the vessel or the blood and are not meant
to
form lesions, should receive as little heat as possible for the wellbeing of a
patient.
In still another embodiment, the implant device comprises cavities which are
filled
with one or more substances and which open when the implant is heated. In a
preferred embodiment, these substances are mixed before being released into
the
patient's body or vessel wall, e.g. to deliver a two-component neurotixine. In
a
more preferred embodiment, these substances are a selection or a composition
of
one or more of the following substances:
- ethanol;
- tetrodotoxin and batrachotoxin;
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
28
- nnaurotoxin, agitoxin, charybdotoxin, nnargatoxin, slotoxin, scyllatoxin
or
hefutoxin;
- calciseptine, taicatoxin, calcicludine, or PhTx3;
- botulinunn toxide;
- cytochalasin D, rapannycin, sirolinnus, zotarolinnus, everolinnus,
paclitaxel;
- glutamate;
- isoquinoline;
- N-methyl-(R)-salsolinol;
- Beta-carboline derivates.
With such implants, it becomes possible to release the desired substances into
the
vessel wall or the blood stream at the desired moment, by heating the implant
with e.g. external energy providing means. Furthermore, the implant and
cavities
in the implant may be designed such that the two components of e.g. a two-
component neurotixine, are mixed before being released into the body.
In a preferred embodiment, an implant device according to the present
invention
comprises one or more deposits of toxin, preferably on an outer surface of
said
device, said deposits covered by a metal layer capable of being resolved by
heating, preferably hysteresis heating.
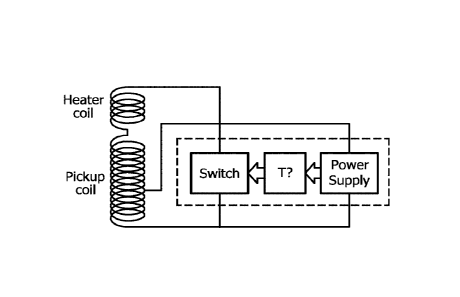
In a similar aspect, the present invention provides an implant comprising an
electrical circuit comprising a pickup coil, a heater coil and a temperature-
controlled switch which comprises a closed position and an interrupted
position.
Said switch preferably comprises a bi-metallic component and/or a thernnistor,
such as a PTC thernnistor and/or said switch preferably comprises a
temperature
sensor and a, preferably digital, thermostat connected to said sensor and to
said
switch for interrupting said switch and thus said electrical circuit when said
sensor
measures a pre-determined temperature. Preferably said switch is arranged to
change from said closed to said open position when a temperature at or near
said
implant is higher than a pre-defined ablation temperature. In a preferred
embodiment, said switch is arranged to change from said open to said closed
position when a temperature at or near said implant is lower than a pre-
defined
switching temperature. In one embodiment, said ablation temperature is equal
to
said switching temperature. In another embodiment, said switching temperature
is
different from said ablation temperature, preferably said switching
temperature is
lower than said ablation temperature, e.g. at least 0.01 C, 0.1 C, 0.5 C, 1 C,
2 C, 3 C, 4 C, 5 C, 6 C, 7 C, 8 C, 9 C, 10 C, 11 C, 12 C, 13 C, 14 C, 15 C,
16 C, 17 C, 18 C, 19 C, 20 C lower than said ablation temperature. In a
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
29
preferred embodiment, said circuit comprises more than one switch, e.g. 2, 3,
4 or
more switches, preferably connected in series, for redundancy, i.e. for
ensuring at
least one switch functions as desired.
In yet another similar aspect, the present invention provides an implant for
treating arterial hypertension by blocking renal sympathetic nerve signals via
ablation by heating, comprising an electrical circuit comprising a pickup
coil, a
heater coil and a temperature-dependent LC-circuit, whereby said LC circuit
comprises a resonant frequency which is temperature-dependent.
The pickup coil of the above implants is arranged for inducing an electrical
current
through an electrical circuit to which it is connected, under the influence of
a time-
varying magnetic flux through said pick-up coil. Thereto, said pickup coil
preferably comprises a low resistance and a high inductance.
Preferably, said pickup coil comprises a resistance which is larger than 0.02
Ohm,
preferably larger than 0.05 Ohm, more preferably larger than 0.1 Ohm, even
more
preferably larger than 0.15 Ohm, yet more preferably larger than 0.2 Ohm,
still
more preferably larger than 0.3 Ohm, still even more preferably larger than
0.5
Ohm, and/or smaller than 30 Ohm, preferably smaller than 30 Ohm, more
preferably smaller than 25 Ohm, even more preferably smaller than 20 Ohm, yet
more preferably smaller than 15 Ohm, still more preferably smaller than 10
Ohm,
yet even more preferably smaller than 5 Ohm, most preferably about 1 Ohm.
Preferably, said pickup coil comprises an inductance which is larger than 0.02
pH,
preferably larger than 0.05 pH, more preferably larger than 0.1 pH, even more
preferably larger than 0.15 pH, yet more preferably larger than 0.2 pH, still
more
preferably larger than 0.3 pH, still even more preferably larger than 0.5 pH,
and/or smaller than 30 pH, preferably smaller than 30 pH, more preferably
smaller
than 25 pH, even more preferably smaller than 20 pH, yet more preferably
smaller
than 15 pH, still more preferably smaller than 10 pH, yet even more preferably
smaller than 7 pH, most preferably about 4 pH, e.g. 1 pH, 2 pH, 3 pH, 4 pH, 5
pH,
6 pH, 7 pH, or any value therebetween.
In a preferred embodiment, said heater coil is arranged for subtending a
substantially complete circumferential or a spiraling ablation region in a
vessel,
preferably in a renal artery, for obtaining a substantially complete
circumferential
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
or spiralling signal-blocking lesion on the inner wall of said vessel.
Preferably said
heater coil comprises a high resistance and a low inductance.
Preferably, said heater coil comprises a resistance which is larger than 0.4
Ohm,
5 preferably larger than 1 Ohm, more preferably larger than 2 Ohm, even
more
preferably larger than 3 Ohm, yet more preferably larger than 4 Ohm, still
more
preferably larger than 6 Ohm, still even more preferably larger than 10 Ohm,
and/or smaller than 150 Ohm, preferably smaller than 100 Ohm, more preferably
smaller than 80 Ohm, even more preferably smaller than 60 Ohm, yet more
10 preferably smaller than 50 Ohm, still more preferably smaller than 40
Ohm, yet
even more preferably smaller than 30 Ohm, most preferably about 25 Ohm.
Preferably, said heater coil comprises an inductance which is larger than 0.02
pH,
preferably larger than 0.05 pH, more preferably larger than 0.1 pH, even more
15 preferably larger than 0.15 pH, yet more preferably larger than 0.2 pH,
still more
preferably larger than 0.3 pH, still even more preferably larger than 0.5 pH,
and/or smaller than 30 pH, preferably smaller than 30 pH, more preferably
smaller
than 25 pH, even more preferably smaller than 20 pH, yet more preferably
smaller
than 15 pH, still more preferably smaller than 10 pH, yet even more preferably
20 smaller than 7 pH, most preferably about 4 pH, e.g. 1 pH, 2 pH, 3 pH, 4
pH, 5 pH,
6 pH, 7 pH, or any value there between.
In a particular preferred embodiment, the resistance of the heater coil is
larger
than the resistance of the pickup coil and/or the inductance of the pickup
coil is
25 larger than the inductance of the heater coil.
In a preferred embodiment, the current flowing through the heater coil, when
the
implant is activated e.g. by external energy-providing means such as by an
imposed time-varying magnetic field via inductance, is larger than 0.1A,
preferably
30 larger than 0.2A, more preferably larger than 0.3A, even more preferably
larger
than 0.4A, yet more preferably larger than 0.5A, still more preferably larger
than
0.6A, yet even more preferably larger than 0.7A, still even more preferably
larger
than 0.8A,and smaller than 10A, more preferably smaller than 8A, even more
preferably smaller than 6A, yet more preferably smaller than 4A, still more
preferably smaller than 2A, yet even more preferably smaller than 1.5A, still
even
more preferably smaller than 1A, most preferably about than 0.9A.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
31
The pre-determined temperature in the present invention is preferably an
ablation
temperature for the inner wall of a vessel into which the implant is to be
placed.
Preferably said ablation temperature is 37, 38, 39, 40, 41, 42, 43, 44, 45,
46, 47,
48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66,
67, 68,
69, 70, 71, 72, 73, 74, 75 C or any value in between.
In a preferred embodiment, said pick-up coil, said heater coil and said
temperature-controlled switch comprising said bi-metallic component are
connected in series, whereby said bi-metallic component is in an open position
when heated above a pre-defined temperature, thereby interrupting said circuit
and stopping the heater coil from heating up, and whereby said bi-metallic
component is in a closed position when its temperature is below said
temperature,
thereby closing said circuit such that a current, e.g. an induced current, can
flow
through the heater coil.
Preferably said implant comprises a circuit supply coil capable of picking up
an AC
current via induction from an externally applied time-varying magnetic field,
said
supply coil coupled to an AC-DC converter for providing a DC current or
voltage,
preferably to said switch or to other electronic components of said implant.
In a preferred embodiment, the implant comprises a source electrical circuit
and a
heating electrical circuit, which can be separately closed and/or open,
whereby
said source electrical circuit is arranged for providing a DC voltage and/or
current
output from an induced AC current in said pick-up loop. Preferably said DC
output
is connected to said switch for providing said switch with energy. Preferably
said
heating electrical circuit is arranged for heating said heater coil via
resistive
heating when said switch is closed, thereby allowing a heating current to flow
through said heater coil.
In a preferred embodiment, said pickup coil and said heater coil are connected
in
series when said switch is closed, thereby allowing an electrical current
picked up
by induction by said pickup coil to flow through said heater coil, thereby
heating
said heat coil through resistive heating.
Preferably said heating current comprising an AC current, which may be induced
in
said pick-up coil and transferred to said heater coil if said switch is
closed, and/or
said AC current may be induced in said heater coil by external energy
providing
means such as an external generator. Said heating current may comprise a DC
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
32
current, e.g. a DC current as provided for by a supply coil coupled to an AC-
DC
convertor.
In a preferred embodiment, said implant is at least partly self-expanding. In
a
preferred embodiment, said implant comprises a cylindrical shape for
implantation
into a renal artery.
Preferably, said pickup coil comprises a length which is larger than 10nnnn,
more
preferably larger than 12nnnn, even more preferably larger than 14nnnn, yet
more
preferably larger than 15nnnn, still more preferably larger than 16nnnn, yet
still
more preferably larger than 17nnnn, yet even more preferably larger than
18nnnn,
still even more preferably larger than 19nnnn, most preferably larger than
20nnnn,
and smaller than 95nnnn, more preferably smaller than 90nnnn, more preferably
smaller than 85nnnn, even more preferably smaller than 80nnnn, yet more
preferably smaller than 75nnnn, still more preferably smaller than 70nnnn, yet
still
more preferably smaller than 65nnnn, yet even more preferably smaller than
60nnnn, still even more preferably smaller than 55nnnn, most preferably
smaller
than 50nnnn.
Preferably, said pickup coil comprises a maximal diameter which is larger than
10nnnn, more preferably larger than 12nnnn, even more preferably larger than
15nnnn, yet more preferably larger than 18nnnn, still more preferably larger
than
20nnnn, yet still more preferably larger than 22nnnn, yet even more preferably
larger than 24nnnn, still even more preferably larger than 26nnnn, most
preferably
larger than 28nnnn, and smaller than 70nnnn, more preferably smaller than
65nnnn,
even more preferably smaller than 60nnnn, yet more preferably smaller than
50nnnn, still more preferably smaller than 40nnnn, yet still more preferably
smaller
than 35nnnn, yet even more preferably smaller than 30nnnn, still even more
preferably smaller than 25nnnn, most preferably smaller than 20nnnn when said
implant is in an expanded position.
Preferably, said heater coil comprises a length which is larger than 1nnnn,
more
preferably larger than 2nnnn, even more preferably larger than 3nnnn, yet more
preferably larger than 4nnnn, still more preferably larger than 5nnnn, most
preferably larger than 6nnnn, and smaller than 30nnnn, more preferably smaller
than 27nnnn, still more preferably smaller than 25nnnn, yet even more
preferably
smaller than 24nnnn, still even more preferably smaller than 22nnnn, most
preferably smaller than 20nnnn.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
33
Preferably, said heater coil comprises a maximal diameter which is larger than
2nnnn, more preferably larger than 4nnnn, even more preferably larger than
6nnnn,
yet more preferably larger than 8nnnn, still more preferably larger than
10nnnn, yet
still more preferably larger than 12nnnn, yet even more preferably larger than
13nnnn, still even more preferably larger than 14nnnn, most preferably larger
than
15nnnn, and smaller than 90nnnn, more preferably smaller than 80nnnn, even
more
preferably smaller than 70nnnn, yet more preferably smaller than 60nnnn, most
preferably smaller than 50nnnn when said implant is in an expanded position.
Preferably, said implant comprises a distance between said pickup coil and
said
heater coil, said distance being larger than 1nnnn more preferably larger than
3nnnn, even more preferably larger than 5nnnn, yet more preferably larger than
6nnnn, still more preferably larger than 7nnnn, yet still more preferably
larger than
8nnnn, yet even more preferably larger than 9nnnn, still even more preferably
larger
than 10nnnn, most preferably larger than 12nnnn, and smaller than 80nnnn, more
preferably smaller than 70nnnn, even more preferably smaller than 60nnnn, yet
more preferably smaller than 50nnnn, most preferably smaller than 40nnnn.
The present invention further provides a system for treating arterial
hypertension
by blocking renal sympathetic nerve signals via ablation by heating,
comprising an
implant comprising an electrical circuit comprising a pickup coil, a heater
coil and a
temperature-controlled switch as described in this text, and a magnetic field
generator for generating a time-varying magnetic field at the position of the
implant device, whereby preferably said magnetic field generator comprises
orientation means for changing the orientation of the magnetic field generated
by
said generator. "Changing the orientation of the magnetic field" hereby refers
to a
change in the polarization of the time-varying magnetic field and/or direction
of
propagation of accompanying electromagnetic waves. By using the orientation
means, the generator can be arranged to provide a magnetic field which varies
in
time maximally along a longitudinal axis of the pickup coil, thereby
efficiently
inducing a current in said pickup coil. The orientation means may comprise a
movable and/or rotatable arm or antenna-like structure, such as a U-shaped
electromagnet. Preferably, said system comprises two implants such as
disclosed
here above.
In a similar aspect, the present invention provides a system for treating
arterial
hypertension by blocking renal sympathetic nerve signals via ablation by
heating,
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
34
comprising an implant as disclosed in this document, comprising a temperature-
dependent LC-circuit, whereby said LC circuit comprises a resonant frequency
which is temperature-dependent; a magnetic field generator for generating a
time-varying magnetic field at the position of the implant device; a
temperature
measurement apparatus arranged for measuring said resonant frequency of said
LC circuit and arranged for relating a measured resonant frequency to an
implant
temperature; temperature controlling means arranged for:
- receiving said implant temperature from said temperature
measurement apparatus;
- comparing said implant temperature to a pre-determined ablation
temperature;
- controlling the time-varying magnetic field generated by said magnetic
field generator on the basis of said comparison.
Preferably, said system comprises two implants such as disclosed here above.
In a further aspect, the present invention provides a method for the treatment
of
a patient with arterial hypertension by blocking renal sympathetic nerve
signals
via ablation of a substantially complete circumferential band or a spiraling
band on
one or more renal arteries' inner walls, comprising the steps of
- implanting one or more implant devices in one or more renal arteries by
means
of a sheath and a guidewire, said implant devices each comprising an ablation
region along at least a portion of their length, said ablation regions being
adapted
for surface contact with said renal arteries and said ablation regions
subtending at
least a substantially complete circumferential band or a spiraling band and
being
effective to ablate a signal-blocking path within said renal arteries upon
application
of energy to said implant devices;
- retracting the sheath and guidewire;
- subsequently heating the ablation region of the one or more implant
devices by
external energy-providing means, which are spatially separated from the
implant
device.
In a related aspect, the present invention provides a method for treating
arterial
hypertension by blocking renal sympathetic nerve signals via ablation by
heating,
comprising the steps of:
- implanting one or more implants as disclosed in the present document,
preferably comprising a pickup coil, a heater coil and a temperature-
controlled
switch or preferably comprising a temperature-dependent LC-circuit, whereby
said
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
LC circuit comprises a resonant frequency which is temperature-dependent, in
one
or more renal arteries;
- applying a time-varying magnetic field at the position of said implants,
thereby
heating up said one or more implants to a pre-determined ablation temperature.
5
It should be stressed that in the above method, the heating of the implant
device
occurs after the surgical procedure. This improves the ease of the heating
procedure and the comfort of the patient.
10 In a similar aspect, the present invention provides a method for heating
one, two
or more implant devices, which are suitable to be implanted in one, two or
more
vessels, comprising the steps of:
- subsequently positioning said implant devices in said vessels by means of
a
sheath and a guidewire, said implant devices each comprising an ablation
region
15 along at least a portion of their length, said ablation region
subtending at least a
substantially complete circumferential band or a substantially spiraling band,
said
implant devices effective for ablating a signal-blocking path within said
vessels
upon application of energy to said implant devices;
- retracting the sheath and guidewire;
20 - heating the ablation region of said implant devices by external energy-
providing
means which are spatially separated from said implant devices
characterized in that said heating occurs after said sheath and guidewire are
retracted and said heating of said implant devices occurs simultaneously.
25 In a preferred embodiment of the method, a recovery period is observed
prior to
heating the ablation region of the one or more implant devices by external
energy-
providing means. Furthermore, this recovery period is long enough to allow the
one or more implant devices to be overgrown by bodily tissue. This recovery
period may also be long enough to test if the implant devices are well
positioned
30 and do not move substantially within the vessel.
The advantages of observing a waiting period are multiple: the patient has
time to
recover from the surgical procedure, extra tests can be performed during the
waiting period to check whether the implant device was well implanted, bodily
35 tissue can overgrow the implant device, thereby improving the contact of
implant
device with the vessel's inner wall and thus improving the efficiency of the
ablation
procedure, etc.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
36
In a particular preferred embodiment of the method, the step of heating the
ablation region of the one or more implant devices by external energy-
providing
means, which are spatially separated from the implant device, is performed
repeatedly at well-spaced time-intervals.
The presented method has the main advantage that in case multiple ablation
procedures are necessary, no second surgical procedure is needed, i.e. the
implanted implant device or devices can be reused for a second, third, ...
ablation
procedure.
In a more preferred embodiment of the method, one or more implant devices as
described in this document are being used. Thereby the implant devices can be
engineered in order to produce the required effects by engineering their
shape,
size, material composition, magnetic and thermal properties, etc.
In a still more preferred embodiment of the method, use is made of a system as
described in this document. In this case, the implant device can be heated by
the
external energy-providing means and a highly-controlled temperature of the
ablation region of implant can be reached.
In a preferred embodiment, at least one implant device comprises a shape which
is adapted for a renal artery.
In a preferred embodiment, said vessels comprise one or more renal arteries
and
said ablation regions of said implant devices being adapted for surface
contact
with said renal arteries and subtending at least a substantially spiraling
band for
ablating a signal-blocking path within said renal arteries upon application of
energy to said implant devices.
In an embodiment of the method, the patient's vessels into which an implant is
to
be implanted are scanned using a 3D scanning technique such as CT or MRI to
collect data on the varying diameter of the vessel e.g. when going from a
distal to
a proximal end of the vessel. From these data, one can derive the necessary
shape and dimensions of the implants e.g. for all two renal arteries of a
patient.
This measuring can be done without a surgical procedure, thereby increasing
the
patient's comfort and wellbeing and reducing medical risks. After this
measuring,
the implants can be custom-made to fit the patient's vessel or vessels.
Obviously,
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
37
custom-made implants, in contrast with standard-sized implants, increase the
success rate of any medical procedure.
The invention is further described by the following non-limiting examples
which
further illustrate the invention, and are not intended to, nor should they be
interpreted to, limit the scope of the invention.
EXAMPLES
Figure 1 represents a preferred embodiment of an implant 1 according to the
present invention with circular cross-section (figure 1B) and elliptic cross-
section
(figure 1C). It should be clear that in the other figures showing embodiments
of
implants, the cross-sections can also be circular or elliptical, or basically
any other
shape which fits the vessel into which it is to be implanted best.
The represented implant 1 comprises a body 2, in this case shaped as narrowing
tubular cage and made of metal wires 3 or the like, suitable to be placed
inside a
renal artery.
More in particular, the body 2 is provided of in this case three circular
wires, a first
bigger circular wire 4, an intermediate mid-sized second circular wire 5, and
a
third smaller circular wire 6.
The outlook of the body 2 may though be provided of more or less than three
rings, for example two to five, more specifically three to four, or even more
than
five rings.
The first bigger circular wire 4 is connected with the intermediate mid-sized
second circular wire 5 by means of in this case three straight inclined but
upstanding wire portions 7.
In a similar manner, the intermediate mid-sized second circular wire 5 is
connected with the third smaller circular wire 6 by means of in this case also
three
straight inclined but upstanding wire portions 8.
The wire portions 8 are here located at intermediate positions with respect to
the
wire portions 7.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
38
The result is a narrowing tubular cage, which could also be described as a
mainly
conical or funnel shaped body 2, provided of three circular wires positioned
at a
distance from each other, at least when the body 2 is in a released or not
compressed position.
In general terms, the self-expanding body 2 is preferably provided of a shape
that
fits the anatomy of the vein, for example a renal artery.
The circular wires may, according to a preferential embodiment, be provided of
a
mainly oval shape such that the body 2 is built up of different oval rings of
converging diameter, preferably adapted to the anatomy of the renal arteries.
These rings of the body 2 will typically range in diameter from 3 to 30 mm,
more
specifically between 5 mm and 20 mm, even more specifically between 9 mm and
13 mm.
These rings of the body 2 will typically range from 5 mm to 50 mm, more
specifically from 8 mm to 40 mm, even more specifically between 10 mm and 30
mm.
The body 2 has self-expanding properties thanks to the elastic characteristics
of
the material used, and thanks to the geometry of the body 2.
If a metal is used, it may be nitinol-based known for its superior self-
expansion
properties.
The self-expanding body 2 is conceived to stop expanding when it encounters a
pressure of about 1 to 150 mm Hg, more specifically 3 to 80 mm Hg, more
specifically 5 to 60 mm Hg, more specifically 10 to 40 mm Hg, equal to the
distension pressure needed to alter the renal artery's anatomy.
Alternatively, a self-expanding cage may be conceived existing of different
circular
or oval shape rings that are interconnected. The rings may be formed so that a
spiral form is created. The different rings of the spiral will also be
interconnected
so that upon heating or upon release of substances from the cage, no openings
for
recurrence of electrical signals are left open.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
39
In this case, the material used is of the type that reacts, for example heats,
in
response to a remote applied alternating magnetic field.
The principle of hysteresis causes the metal of the cage to heat up, depending
on
the absorptive properties of the metal alloy that builds up the body 2.
Alternative is an electromagnetic field generator that changes polarity and
therefore induces hysteresis heating in materials put inside the field. The
system
may use the Curie temperatures (the temperature to which a certain material
can
be heated, upon which further energy delivery does no longer change the
temperature) that certain materials possess, as to the target temperature that
should and can be reached. For example, ZnFe204 is a material that has a Curie
temperature between 30 and 45 degrees Celsius.
A metal alloy cage that comprises ZnFe204 in its structure may therefore be
heated to exactly 45 degrees, close to the target temperature desirable for
appropriate ablation purposes.
Another alternative to deliver energy to the cage could be direct induction,
using a
magnetic core, again making use of hysteresis heating but in a more directive
way.
Another alternative to deliver energy to the cage could be to use
electromagnetic
radiation through a thernnical chemical-release system with external trigger,
where
the chemical is only released on demand and at the appropriate sites.
It is clear that still alternative energy field can be applied, such as
electromagnetic
radiation, hysteresis heating, reaching Curie temperature, direct induction,
thermal/chemical release system, mechanical/chemical release system, indirect
induction, Joule heating, acoustic energy, mechanical vibration, chemical
release
system.
Alternatively, the body 2 can be provided of toxic substances that are only
released upon introduction into the renal artery, for example after applying
an
external energy field, which toxic substances then produce a lesion of limited
necrosis/neurotoxicity.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
In figure 2, an alternative embodiment of the implant 1 according to the
invention
is represented.
The body is built up out of braided metal wires 9 that have multiple
5 interconnections, crossings and layers. This iteration allows for numerous
connections with the renal vascular or other wall.
In figure 3, the implant 1 is conceived as a spirally shaped wire 10 of which
the
diameter gradually goes down along its longitudinal axis.
The windings 10A-10D, in this case four, are mutually connected with bridging
upstanding wire portions 8.
This embodiment thus differs from the embodiment as represented in figure 1 by
the single or continuous spirally shaped wire instead of the different
circular wires
4-6.
The bridging upstanding wire portions 8, apart from giving structure and
strength
to the implant 1, also provide closed loops.
Indeed, the different windings 10A-10D are still interconnected to ensure,
once
the device is released, full and circular lesions in the renal artery.
In figure 4 the implant shows longitudinal metal beads 11 that are outward
bending, and that still show several interconnections between them, to
ultimately
form a metal cage.
An implant 1 according to the present invention may comprise portions made of
different metal alloys with optionally different ferromagnetic properties
and/or
absorption coefficients, with specific response to alternating magnetic
fields.
Alternatively, the basic structure of the implant 1 may be made of one and the
same material, which may be provided of coating portions with varying
properties.
The embodiment illustrated in figure 5 shows an implant conceived as a
spirally
shaped wire 10 of which the diameter gradually goes down along its
longitudinal
axis, but where, as opposed to the embodiment represented in figure 3, the
windings 10A-10E, in this case five, are mutually connected by means of
bridging
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
41
upstanding wires 12 which reach from the biggest winding 10A up till the
smallest
winding 10E.
The biggest winding 10A of the implant 1 is built up of a metallic alloy with
self-
expanding properties, and covered with a layer of a metal that has minimal
ferromagnetic properties 100.
The next winding 10B is built up of the same self-expanding alloy, covered
with a
layer of material that has a higher rate of absorption of energy during the
hysteresis phenomenon, and thus with altering magnetic fields will reveal
different
thermal heating properties 200.
The windings 10D and 10E most distal from the biggest winding 10A, to be
located
in a portion of the renal artery, is provided of a layer of material that has
still a
higher rate of absorption of energy during the hysteresis phenomenon 400.
According to another embodiment of the implant 1, a portion of which is
schematically represented in figure 6, the wire building up the body 2 of the
implant 1 is composed of different layers, in this case three layers made of
different alloys 15, 16 and 17.
These different alloys are in contact with each other, and depending on
different
magnetic fields to be applied, they will exhibit different properties.
It is clear that alternatively or in combination with the above or other
features,
one or more layers can have high thermal isolation characteristics, in order
to
direct heat where needed, and to isolate portions to prevent undesired heating
of
blood or tissue.
According to another embodiment of the implant 1, a portion of which is
schematically represented in figure 7, the body 2 is provided of nnicropores
18 at
the ablunninal side 13 (in contrast with the adlunninal side 14) wherein
substances
are provided.
Such substances may for example be a selection or a composition of one or more
of the following substances:
- ethanol;
- tetrodotoxin and batrachotoxin;
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
42
- nnaurotoxin, agitoxin, charybdotoxin, nnargatoxin, slotoxin, scyllatoxin
or
hefutoxin;
- calciseptine, taicatoxin, calcicludine, or PhTx3;
- botulinunn toxide;
- cytochalasin D, rapannycin, sirolinnus, zotarolinnus, everolinnus,
paclitaxel;
- glutamate;
- isoquinoline;
- N-methyl-(R)-salsolinol;
- Beta-carboline derivates.
The nnicropores 18 are closed when the body is coiled together, for example
prior
to the provision in a guiding catheter system, and upon release into its site
of
destination, upon expansion, these nnicropores 18 open so that the substances
inside the metal arms of the cage can be released.
According to another embodiment of the implant 1, a portion of which is
schematically represented in figure 8, the body 2 is covered by a
thernnoactive
coating 19 which is only activated upon temperatures above 35 C so that the
body
temperature would trigger activation.
Alternatively, a thernnoactive coating 19 can be provided which is only
activated
upon temperatures above 45 C so that an external application of an energy
field
would trigger activation.
Alternatively, an insulating material 44 can be provided at the parts of the
implant
which comes into contact with parts of the body which are preferably not
heated
such as some parts of the vessel wall or the blood. Hereby the parts of the
implant
which are heated are thermally insulated from e.g. the blood.
The energy field could for example be a remote applied alternating magnetic
field,
heating the body 2 of the implant 1 thanks to a hysteresis effect.
At the said activation temperature, the coating 19 gets absorbed, and the
active
component that is residing below the coating 20 is released into the vascular
or
arterial wall.
Note that the elongated shape and/or the expanding forces provide can be
considered as anchoring means of the implant 1.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
43
Alternatively, hooks or barbs or the like 29 can be provided as in figure 11
on the
outwardly directed portions of the implant 1, providing guaranteed anchoring
of
the implant 1 once it put in place.
According to still another embodiment, the outer rings or other cage
structures
that are fitted into the vessel, or the whole cage may be equipped with
structures
to increase the solidity of cage immobility, to ascertain the fixed position
of the
implant, to reduce the possibility of movement of the implant after the
implantation.
The method of placing the implant is easy and can be performed as hereafter
described.
According to the known practices, a catheter 30 with guidewire 31 is
introduced up
till the place where the implant 1 is to be left. This is shown schematically
in
figures 12 and 13.
Pull-back of the catheter while leaving the implant 1 in position causes the
implant
1 to expand.
As the shape and/or elastic characteristics of the implant 1 is/are suitably
adapted
to fit and optionally press against the arterial or venous structure, for
example in
the renal artery, the implant 1 is left in a safe and self-anchoring manner.
After full pullback of the catheter, the implant 1 is fully released.
Alternatively, the well-known balloon expansion can be applied.
An appropriate period can be awaited prior to applying an external energy
field in
order to trigger the triggerable portions of the implant 1.
Various consecutive treatments by simply applying an appropriate energy field
can
be considered, without the need to perform renewed invasive surgery, which is
the
main advantage of the implant and the system according to the present
invention.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
44
Furthermore, in case where the implant 1 is provided of varying substructures,
each with their own response to an externally applied energy field, varying
treatments can be considered, for example with increasing intensity.
Each portion can for example be triggered with a remote applied alternating
magnetic field characterized with a specific frequency.
It is clear that when reference is made to lesions, these may concern
transnnural
lesions extending up till the exterior wall, and that lesions may be
continuous, as
opposed to discrete or composed partial lesions.
The present invention is by no means limited to the embodiments described by
way of example and represented in the accompanying drawings; on the contrary,
such an implant and system of an implant and excitation device for the
treatment
of arterial and venous structures according to the invention can be made in
all
sorts of shapes and dimensions while still remaining within the scope of the
invention.
Figure 9 shows a circular braided implant 21 in which one circumferential
region
22 comprises an alloy with a specific Curie temperature and in which a second
circumferential region 23 comprises an alloy with another specific Curie
temperature.
Figure 10 shows a funnel-shaped braided implant 24 in which one
circumferential
region 25 comprises an alloy with a specific Curie temperature, in which a
second
circumferential region 26 comprises an alloy with another specific Curie
temperature, and in which a third circumferential region 27 comprises an alloy
with still another specific Curie temperature.
Figure 11 shows a funnel-shaped braided implant 28 with anchoring means 29 in
the form of small barbs.
Figure 13 shows how a catheter tip can be guided, e.g. through an insertion
vein
or artery, to e.g. a renal artery 38.
Figure 14 represents an embodiment of the external energy-providing means 42
as it can be used for treating a patient during the ablation procedure 43.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
Figure 15 shows an implant in place 40 and the ablation region in cross
section 41
in a conical section of a vessel.
Figure 18 shows another embodiment of an implant which has an hour-glass
5 shape 45, whereby near the middle region, where the diameter becomes
smaller,
a set of heating rings 46 is attached around the hour-glass shaped part of the
implant. The heating rings are attached to the hour-glass shaped part in a
thermally insulating manner such that little heat is transferred to the blood
stream
when the heating rings are heated. Furthermore the heating rings are meant to
be
10 completely separated from this blood stream, since the hour-glass shaped
part
may be covered by a blood-tight tissue and may be clamped into the vessel at
or
near the implant ends 47 and 48.
Figure 19 shows an embodiment of an implant comprising a fuse, so that at
15 certain temperatures, more specific the temperature that is reached to
achieve an
optimal ablation, between 40 and 80 degrees Celsius, more specifically between
45 and 60 degrees Celsius, the circuit that may be generated gets interrupted.
This comes from the phenomenon that when an implant, more specifically a
metallic implant, more specifically a nitinol implant is brought into the
alternating
20 magnetic field, an electrical current is generated through the metal
implant itself,
thus generating accelerated heating by itself (induction and Joule heating).
This
phenomenon results in extremely rapid heating of the implant, that can be
stopped by interrupting the electrical current that may run through the
implant.
This stopping of the current may be caused by a fuse that is mounted within
the
25 metallic implant, and that for instance may exist of a resistance, that
breaks when
it is heated above a certain temperature. In this case, the fuse would stop
heating
up further above a temperature of 45-60 degrees, more specifically 50-55
degrees. Figure 19a shows a detailed view of the fuse.
30 In a different configuration, as shown in figure 20, the metal implant
can be build
up of memory shape alloys, so that upon heating of the device, the different
metallic parts take up another configuration, thereby interrupting the
electrical
current that can run through the implant. Details of the on and off position
of the
switch or fuse are shown in figures 20a and 20b respectively. This different,
i.e.
35 open, configuration consists potentially of the original form of the
metal, so that it
goes back to its "memory shape". This is called a "shape memory metal".
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
46
In a still different configuration as shown in fig. 21 and a detail in fig.
21a, the
implant consists of two different materials, where upon heating the bondage
between the two different metals gets interrupted, so to stop the electrical
current
from running through the implant.
Another addition of this application is that the heating needs to be
unidirectional.
The blood needs to be isolated from the heating because of two reasons: first,
blood should not be heated because proteins in the blood can denature and form
clots, and second, because blood is a huge heat dissipator that may substract
too
much heat away from the implant, it would need too much energy to get the
ablation region of the implant to the desired temperature. Therefore, an
extensive
coating is formed around the implant, but almost exclusively on the ADLUMINAL
side as illustrated in fig. 22, so that when the implant is heated, no heat is
dissipated towards the blood stream.
Figure 23 illustrates the concept of the present invention whereby an implant
device (55) is provided with a built-in thermal switch (54). Hereby, said
implant
may be activated by applying a time-varying magnetic field cp, e.g. a
radiofrequent
field as can be produced by an electromagnet or electromagnetic coil or
antenna
(51). Said time-varying magnetic field cp may induce a current in said
electrical
circuit, through said pick-up coil (53) and said heating coil (52), if said
switch (54)
is closed. Whether the switch is closed or open, depends on the temperature at
the position of the switch or at a position of a temperature sensor attached
to said
switch, preferably via a thermostat.
Figure 24 illustrates the dimensions of an implant in an expanded position in
a
vessel. Hereby, the vessel (65) is typically between 5nnnn and 50nnnn wide,
e.g.
20nnnn in diameter. The heater coil (62) can be about 20nnnn long, while the
pickup
coil (63) can be, and preferably is, longer than 20nnnn. The thermal switch
(64) in
fig. 24 is positioned near the heater coil (62) and is open or closed
depending on
the temperature at or near said heater coil. The heater coil subtends a
circumferential ablation region of the vessel over the heater coil length.
Figures 25a-g illustrate different embodiments of the present invention,
whereby
the shape and absolute and relevant sizes of the coils may differ between
different
embodiments. A heater coil (72) and a pickup coil (73) can be clearly
identified,
the pickup coils (73) as presented comprising a large amount of windings to
increase their inductances. A thermal switch (74), in figs. 25a-e attached to
a
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
47
printed circuit board and coated, is coupled to heater coil (72) and pickup
coil
(73). In figs. 25d-g, a pcb (75) comprising one or more electronic circuitry,
possibly including a thermal switch and/or a supply circuit coil is coupled to
the
pickup coil (fig. 25f-g) or switch (fig. 25d-e). The shape of the coils can be
arranged to fit into a specific vessel, e.g. a cylindrical vein or artery
(fig. 25a) or a
cone-shaped vein or artery (fig. 25b-g). In particular for renal arteries, a
cylindrical-shaped heater coil for implantation in the lumen of the artery is
preferred.
A winding of the heater coil (76) induces a temperature profile (77) in the
wall
(78) of the vessel upon activation of the implant. This is illustrated in fig.
26,
where it is illustrated that the heat is deposited mainly near the winding,
but that
it is possible that also the outer side of the vessel (79) can be heated to an
increased temperature. Appropriate modelling of the vessel and testing of the
setup allows to set the optimal temperature for the implant to ablate a signal-
blocking path on the inner wall of the vessel, without unnecessary damaging
tissue which should remain intact.
Further embodiments comprising e.g. a PTC (80) or thernnistor switch, are
illustrated in figs. 27a-b for essentially cylindrical implants.
In some embodiments, it is necessary or advisable to use electrical components
which need DC current or voltage to operate. In such embodiments, it is
necessary
that the implant comprises an AC-DC converter in order to convert AC current
flowing via induction in at least part of the circuitry of the implant, to DC
current.
This converter may obtain an AC input current from the pickup coil or from a
supply circuit coil. Such a converter may be part of a larger electronic
circuit which
can be attached to a pcb (81) and coupled to the coils as illustrated in fig.
28.
Figures 29a-d illustrate electronic circuits which can be used in embodiments
of
the implant of the present invention. If a large current is sent through a
heater
wire or heater coil, the heater coil generates heat and the temperature around
the
heater coil rises. If there is no current through the heater coil, the
temperature
drops because of cooling inside a bloodstream. For ablation, a target
temperature
around 55 C may need to be reached and held for an amount of time. A digital
thermostat PCB (IC1) measures the temperature by means of a temperature
sensor and switches the large current through the heater coil on or off by
means
of a switch (IC3), hereby forcing the temperature around the heater coil to
rise or
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
48
drop. The heater coil is powered by means of the energy induced in a large
pickup
coil. The control circuit is powered by a separate coil.
The temperature sensor measures the internal temperature and compares it to
55 C with a hysteresis loop of 2 C. This chip provides a high output voltage
level
(5 V) if the measured temperature is higher than 55 C, and a low output
voltage
level (0 V) if the temperature is lower than the target temperature of 55 C.
This
thermostat chip (IC1) is used to control a switch (IC3), which in this case is
a
solid-state relay (SSR) with integrated optocoupler, as this SSR is able to
switch
the alternating current induced by the pickup coil. As this switch (IC3)
requires a
larger current than the temperature sensor can provide (the thermostat chip
may
have a very low output current driving capability), a buffer needs to be used.
As a
high switch input voltage results in a closed state and a low voltage results
in an
open state, this buffer is realised by means of an inverter (IC2) with large
output
current. A resistor (R1) of e.g. 330 Ohm between switch (IC3) and inverter
(IC2)
limits the switch drive current, hereby protecting the switch input. This
chain (fig.
29b) provides the temperature control. As this chain consists of active
devices,
this can only work if adequately supplied. The chain in fig. 29a provides a
stable 5
V supply voltage out of an input AC-voltage delivered by a separate circuit
supply
coil. This coil provides a lower AC-voltage than required for the power chain.
The
input AC-voltage is transformed into a DC-voltage by a half-wave rectifier
(D1)
higher than the target 5V. Then a 5V linear regulator (IC4) is used to
transform
this voltage into a stable 5 V power supply. The capacitors, e.g. of 100nF,
visible
throughout the design are placed for decoupling (local stabilization of the
supply
voltage).
The symbols GND, VAC and 5V each represent a net, connecting the symbols with
the same name. These have no physical meaning other than just another
connection. GND is the universal sign for a voltage reference (as all voltages
need
to be referred to some point inside a circuit). This is not to be mistaken for
an
earth connection. GND is often chosen to be a connection with very low
impedance, and therefore often realized as reference plane.
The connector CON1 is the interface for the heater coil (pin 1), the circuit
supply
coil (pin 2) and the voltage reference GND (pin 3).
Another embodiment of the electrical circuitry is illustrated in figs. 29c-d.
This
board is a smaller version as the one presented in figs. 29a-b. The
functionality
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
49
remains identical, but it uses smaller components. The regulator (IC4) is
different
and the switch and resistor are combined into one component (IC3). Also the
large
connector is changed into three smaller connectors CON1 - CON3. The extra
connectors CONVCC, CONGND and CONTout can be used to split the board into a
small board with a temperature sensor and another board with the remainder of
the chips. This way, the small temperature sensor can be brought much closer
to
the heater coil.
In figures 29a-d, embodiments of an implant according to the present invention
are illustrated, whereby the implants comprise a separate supply coil with a
dedicated supply circuit for providing the other components such as the
thermostat, inverter and switch with a constant DC voltage of e.g. 5V, as
illustrated in fig. 29e. In other embodiments of an implant according to the
present invention, a center tap may be used at the pickup coil side to obtain
the
circuit power instead of a separate circuit power supply coil (fig. 29f). This
seems
easier to integrate in an implant instead of using a third coil. This latter
embodiment may pose the following extra problem: The center tap may be
situated inside the switching chain. If a large current is flowing through the
coil
combination, this current generates a magnetic field counteracting the
external
magnetic field that actually caused this current to flow. This results in a
voltage
drop across the coil terminals. This means that in case of a closed switch,
the
voltage across the coil terminals can be much lower than in case of an open
switch. This voltage difference could cause regulators to fail due to high
power
dissipation. To solve this problem, extra components need to be added to the
circuit to prevent this. Such components may be added in e.g. a miniaturised
chip
design.
Figures 30a-34 illustrate embodiments of external energy providing means which
can be used in a system or method of the present invention for providing
energy
to the implant by providing a time-varying magnetic field at the position of
the
implant.
Figures 30a-b and also figures 33-34 illustrate an embodiment whereby a
patient
with an implant may sit down during the heating procedure, as a time-varying
magnetic field is produced preferentially within the arc-shaped arms (90) of
the
generator (91). The arms are capable of being rotated, preferably around a
horizontal axis (92) and the patient chair (93) may also be rotated,
preferably
around a vertical axis, and may be moved up and down, such that an optimal
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
induction coupling between magnetic field of the generator and induced field
in the
implant is reached. The optimal position of the generator may depend on the
patient, and on the orientation of the treated vessels in the patient.
Therefore a
generator as illustrated in these figures, whereby both the orientation and
5
magnitude of the magnetic field may be varied in time, is particularly
preferred in
the systems and methods presented in this document.
Figure 31 illustrates the possibility of using a large magnetic field
generator (91)
around a table (94) onto which a patient can lie down for treatment. The table
can
10 move horizontally through the generator.
Figures 32a-b illustrate a magnetic field generator (91) which is capable of
generating fields in different orientations, whereby the orientation which is
optimal
for inducing a current in the implant can be adapted in a patient-dependent
15 manner.
It is supposed that the present invention is not restricted to any form of
realization
described previously and that some modifications can be added to the presented
example of fabrication without reappraisal of the appended claims. For
example,
20 the
present invention has been described referring to renal arteries, but it is
clear
that the invention can be applied to other vessels for instance.
The present invention concerns, but is not limited to:
25 1.
Method for heating one, two or more implant devices which are suitable to
be implanted in one, two or more vessels, comprising the steps of:
- subsequently positioning said implant devices in said vessels by
means
of a sheath and a guidewire, said implant devices each comprising an
ablation region along at least a portion of their length, said ablation
30 region
subtending at least a substantially complete circumferential band
or a substantially spiraling band, said implant devices effective for
ablating a signal-blocking path within said vessels upon application of
energy to said implant devices;
- retracting the sheath and guidewire;
35 -
heating the ablation region of said implant devices by external energy-
providing means which are spatially separated from said implant
devices
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
51
characterized in that said heating occurs after said sheath and guidewire
are retracted and said heating of said implant devices occurs
simultaneously.
2. Method according to point 1, whereby at least part of each of said implant
devices is made from at least one material which shows magnetic
hysteresis, such as a ferromagnetic, ferrinnagnetic or anti-ferromagnetic
material.
3. Method according to point 2, whereby said implant devices comprise a
ferrous fluid.
4. Method according to any of the points 1 to 3, whereby heating occurs by
external energy-providing means which create a time-varying magnetic
field at the position of said implant devices.
5. Method according to any of the points 1 to 4, whereby at least one of said
implant devices comprises a thernnoactive coating comprising an activation
temperature between 35 C and 37 C so that the body temperature would
trigger activation.
6. Method according to any of the points 1 to 4, whereby at least one of said
implant devices comprises a thernnoactive coating comprising an activation
temperature above 45 C so that activation is triggered only when said
ablation region is heated by said external energy-providing means.
7. Method according to any of the points 1 to 6, whereby said implant devices
comprise substances capable of producing a lesion of limited necrosis
and/or neurotoxicity.
8. Method according to point 6, whereby at least one of said implant devices
comprises cavities which are filled with said substances and which open
when the implant is heated.
9. Method according to any of the points 6 to 8, whereby at least two
substances are mixed before being released, e.g. to deliver a two-
component neurotoxine.
10. Method according to any of the points 6 to 9, whereby said substances are
a selection or a composition of one or more of the following substances:
- ethanol;
- tetrodotoxin and batrachotoxin;
- nnaurotoxin, agitoxin, charybdotoxin, nnargatoxin, slotoxin, scyllatoxin
or hefutoxin;
- calciseptine, taicatoxin, calcicludine, or PhTx3;
- botulinunn toxide;
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
52
- cytochalasin D, rapannycin, sirolinnus, zotarolinnus, everolinnus,
paclitaxel;
- glutamate;
- isoquinoline;
- N-methyl-(R)-salsolinol;
- Beta-carboline derivates.
11. Method according to any of the points 1 to 10, whereby at least one
implant device comprises a shape which is adapted for a renal artery.
12. Method according to any of the points 1 to 11, whereby said vessels
comprise one or more renal arteries and said ablation regions of said
implant devices being adapted for surface contact with said renal arteries
and subtending at least a substantially spiraling band for ablating a signal-
blocking path within said renal arteries upon application of energy to said
implant devices.
13. Method according to any of the points 1 to 12, whereby a recovery period
is observed prior to heating the ablation region of the one or more implant
devices by external energy-providing means, whereby said recovery period
is long enough to allow the implant devices to be incorporated into the
vessel's wall.
14. Method according to any of the points 1 to 13, whereby the step of heating
the ablation region of the implant devices by external energy-providing
means, which are spatially separated from the implant device, is performed
repeatedly at well-spaced time-intervals.
15.A self-expanding implant device adapted to be implanted and deployed
within a vessel, said implant comprising an ablation region along at least a
portion of its length, the ablation region being adapted for surface contact
with the vessel and for subtending at least a substantially complete
circumferential band or a spiraling band and said ablation region effective
to ablate a signal-blocking path within the vessel upon application of
energy to the implant device.
16. An implant according to point 15, whereby said ablation region comprises
at least one material which shows magnetic hysteresis, such as a
ferromagnetic, ferrinnagnetic or anti-ferromagnetic material.
17. An implant according to point 16, whereby said implant devices comprise a
ferrous fluid.
18.An implant according to any of the points 15 to 17, comprising a
thernnoactive coating comprising an activation temperature between 35 C
and 37 C so that the body temperature would trigger activation.
CA 02869334 2014-10-02
WO 2013/149684 PCT/EP2012/069382
53
19.An implant according to any of the points 15 to 17, comprising a
thernnoactive coating comprising an activation temperature above 45 C so
that activation is triggered only when said ablation region is heated by said
external energy-providing means.
20. An implant according to any of the points 15 to 19, comprising substances
capable of producing a lesion of limited necrosis and/or neurotoxicity.
21. An implant according to point 20, comprising cavities which are filled
with
said substances and which open when the implant is heated.
22.An implant according to any of the points 20 or 21, whereby said
substances are mixed before being released, e.g. to deliver a two-
component neurotoxine.
23.An implant according to any of the points 20 to 22, whereby said
substances are a selection or a composition of one or more of the following
substances:
- ethanol;
- tetrodotoxin and batrachotoxin;
- nnaurotoxin, agitoxin, charybdotoxin, nnargatoxin, slotoxin, scyllatoxin
or hefutoxin;
- calciseptine, taicatoxin, calcicludine, or PhTx3;
- botulinunn toxide;
- cytochalasin D, rapannycin, sirolinnus, zotarolinnus, everolinnus,
paclitaxel;
- glutamate;
- isoquinoline;
- N-methyl-(R)-salsolinol;
- Beta-carboline derivates.
24.An implant according to any of the points 15 to 23, comprising a shape
which is adapted for a renal artery.
25.An implant according to point 24, whereby said ablation region of said
implant device is adapted for surface contact with said renal arteries and
for subtending at least a substantially spiraling band.
26. An implant according to any of the points 15 to 25, comprising a maximal
circumference and a minimal circumference and a ratio between maximal
and minimal circumference, whereby said ratio is smaller than 7 and larger
than 3.
27. An implant according to any of the points 15 to 26, comprising a variable
circumference along a longitudinal direction of the implant, said
circumference varying between at least 36 mm and at most 250 mm.
CA 02869334 2014-10-02
WO 2013/149684
PCT/EP2012/069382
54
28.An implant according to any of the points 15 to 25, comprising an
essentially cylindrical shape comprising a diameter which is at least 5 mm
and at most 10 mm.
29.An implant according to any of the points 15 to 28, comprising a distal
portion and a proximal portion, whereby said ablation region is located
within 50% of the implant's total length from the proximal portion.
30.An implant according to any of the points 15 to 29, comprising a distal
portion and a proximal portion, whereby said ablation region is located
within 15 mm from the proximal portion.
31.An implant according to any of the points 15 to 30, comprising a distal
portion and a proximal portion, and comprising an anchoring device
connected to the ablation region of said implant via a thermally insulating
connection for preventing overheating of said anchoring device, whereby
said anchoring device is connected to the distal portion.
32.A system comprising one, two, three, four or more implant devices
according to any of the points 15 to 31.
33.A system according to point 32, comprising external energy-providing
means, which are spatially separated from said implant devices and able to
provide energy to said implant devices for increasing the temperature of
the ablation regions of the implant devices up to an ablation temperature.
34.A system according to any of the points 32 or 33, comprising
- a sheath suitable for transporting and delivering the one or more
implant devices to or near the desired position in the one or more
vessels; and
- a guidewire suitable for sequentially guiding the sheath with the one or
more implants to the desired position in the one or more vessels.
35.A system according to any of the points 32 to 34, comprising one or two
implant devices according to any of the points 15 to 34, each of which
adapted for a corresponding renal artery.