Note: Descriptions are shown in the official language in which they were submitted.
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
1
DEVICE AND PROCESS FOR THE RECOVERY OF INCREASED
VOLUMES OF PURE TERPENES AND TERPENOIDS
FROM SCRAP POLYMERS AND ELASTOMERS
Technical Field.
This inventions relates to the recovery of terpenes and terpenoids from the
pyrolysis of polymers and elastomers.
Background Art.
The continuing accumulation of used tires is one of the worst solid waste
problems
facing industrialized countries. It is estimated that North America discards
approximately
one used tire per person per year. The incineration of tires is both costly
and complex,
while stockpiling used tires is the subject of growing concern. Moreover, the
possibility of
tire fires on these sites poses an ever-increasing threat to the environment.
On the other
hand, tires represent a source of energy and chemicals. By thermal
decomposition, it is
possible to recover useful products in an environmental friendly way.
The presence of terpenes in "Py-oil" or rubber pyrolysis oil has been known
for
decades. However, the concentration is low. Attempts to separate the terpenes
via
distillation have been counterproductive because the heat required for
distillation results in
decomposition of the most valuable terpenes, such as limonene. Simply reducing
the
pressure of the distillation results in very high costs from large columns and
long
processing residence times. Also, there is great difficulty in separating
odiferous
mercaptans, and complications from sulfur content, water content and solids.
Summary of the Invention.
This section provides a general summary of the disclosure, and is not a
comprehensive disclosure of its full scope or all of its features.
In accordance with the various embodiments of the present invention, this
invention
relates to a component mounting system for vacuum pyrolysis of scrap tires to
produce
pyrolytic oil containing such compounds as dl-limonene and pulogene which has
a high
price on the market.
The technology described here provides a combination of a concept totally new
to
the concept of extraction of value from oils derived from pyrolysis of scrap
while
preserving the valuable carbon black solids. This combination yields high
concentrations
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
2
of highly valued fragrance and essential oils that are known for their unique
solvent
properties, their usefulness as precursors for pharmaceuticals, odor masking
capabilities
and "green" character in ultimate disposal. The most obvious example of
application of
these steps is recovery of terpenes and terpenoids from pyrolysis of scrap
tires. It
achieves maximum value recovery from the oils, the solids (carbon black and
metal) and
the gas (light hydrocarbons).
The technology described here provides a combination of a concept totally new
to
the concept of extraction of value from oils derived from pyrolysis of scrap
while
preserving the valuable carbon black solids. This combination yields high
concentrations
of highly valued fragrance and essential oils that are known for their unique
solvent
properties, their usefulness as precursors for pharmaceuticals, odor masking
capabilities
and "green" character in ultimate disposal. The most obvious example of
application of
these steps is recovery of terpenes and terpenoids from pyrolysis of scrap
tires. It
achieves maximum value recovery from the oils, the solids (carbon black and
metal) and
the gas (light hydrocarbons).
Scrap rubber or similar materials are heated under vacuum and in the presence
of
a compound which, upon heating, decomposes into an active species which
accelerates
the de-vulcanization and decomposition of the polymers and elastomers in the
scrap. As
the temperature of the raw material - catalyst mixture rises further, valuable
compounds
are vaporized. The reactor is designed such that there are exhausts in the
immediate
region to carry away vapors, but by the point in the reactions, the catalyst
has
decomposed and the catalytic species is directly in contact with the melting
materials.
Therefore, the catalyst precursor cannot be carried away by escaping vapors.
Brief Description of the Drawings
In the accompanying drawings which form part of the specification:
Figure 1 shows a diagram of one embodiment of the present invention related to
a
continuous reactor process;
Figure 2 shows a system diagram of one embodiment of the present invention
related to a batch reactor process;
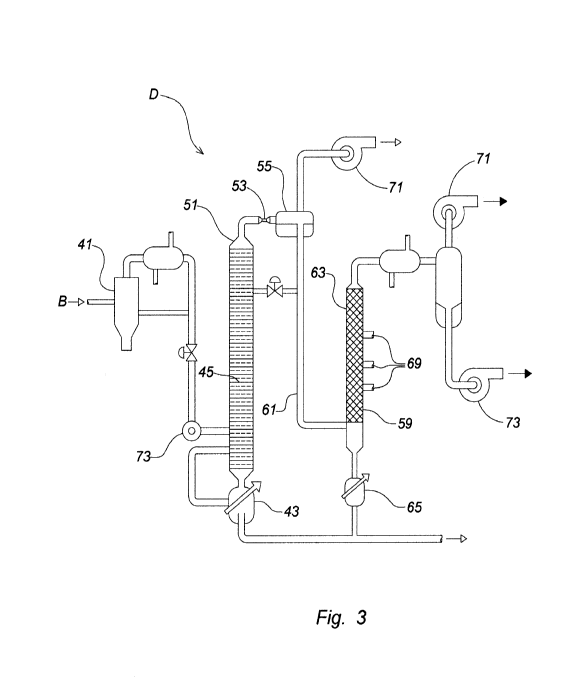
Figure 3 shows a system diagram of one embodiment of the present invention
related to a contactor/separator system; and
Figure 4 shows a system diagram for one embodiment of the present invention
related to a process that uses ultraviolet light.
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
3
Corresponding reference letters and numerals indicate corresponding steps or
parts throughout the several figures of the drawings.
While one embodiment of the present invention is illustrated in the above
referenced drawings and in the following description, it is understood that
the embodiment
shown is merely one example of a single preferred embodiment offered for the
purpose of
illustration only and that various changes in construction may be resorted to
in the course
of manufacture in order that the present invention may be utilized to the best
advantage
according to circumstances which may arise, without in any way departing from
the spirit
and intention of the present invention, which is to be limited only in
accordance with the
claims contained herein.
Best Modes for Carrying Out the Invention
In the following description, numerous specific details are set forth such as
examples of some preferred embodiments, specific components, devices, methods,
in
order to provide a thorough understanding of embodiments of the present
disclosure. It
will be apparent to a person of ordinary skill in the art that these specific
details need not
be employed, and should not be construed to limit the scope of the disclosure.
In the
development of any actual implementation, numerous implementation-specific
decisions
must be made to achieve the developer's specific goals, such as compliance
with system-
related and business-related constraints. Such a development effort might be
complex
and time consuming, but is nevertheless a routine undertaking of design,
fabrication, and
manufacture for those of ordinary skill.
At least one preferred embodiment of the present invention is illustrated in
the
drawings and figures contained within this specification. More specifically,
certain
preferred embodiments of the present invention are generally disclosed and
described in
Figures 1, 2, and 3 for the pyrolysis of tires to obtain various pyrolytic
oils from which
valuable compounds may be extracted.
In that embodiment, two distillation columns are utilized; one at atmospheric
pressure or slightly higher and the second at hard vacuum. Further, this
process
demonstrates the addition of a small volume of an oxidant that contains no new
elements
and adding it only to the final purification step. Mercaptans of the carbon-
chain length
found in the organic liquids downstream of rubber pyrolysis can be removed by
addition of
an oxidant, but the amount of oxidant required is quite high and the
particular oxidants
necessary are expensive. Also, one cannot introduce significant amounts of new
CA 02870183 2014-10-09
WO 2013/155238
PCT/US2013/036052
4
materials without incurring other negative results, such as disposal costs or
reduced value
of remaining materials. That problem is easily handled here by adding small
quantities of
a specific oxidant to the small volume stream containing the terpenes and
terpenoids.
GENERAL PROCESS THEORY.
A. CATALYST/ADDITIVE:
The addition of catalysts to rubber pyrolysis is optional. Some researchers
claim no
catalyst is necessary or even desirable for rubber pyrolysis; other
researchers claim that
whole groups of materials can give a catalytic effect. However, it is often
the case that
observed catalytic effects in laboratory bench-scale batch tests disappear
when the
technology is scaled-up to commercial size and operations are switched from
"batch" to
"continuous." The approach here results from the desire to produce the
catalytic species
in situ, i.e., to convert relatively inert materials to active species just as
the temperature
and reactant state are optimal to utilize the catalyst. The best catalyst to
achieve this is a
Group 1 element, such as Sodium or Potassium. The compounds most able to carry
the
Group 1 element into the high temperature zone are carbonates or bicarbonates.
So, for
scrap rubber containing raw materials, Potassium Carbonate is the best
material to yield
K+ ion directly in the mixture and in intimate contact with the polymeric and
elastomeric
materials in rubber.
B. REACTOR DESIGN.
Full-sized commercial processing units can be "batch" or "continuous." In
either
case, the reactor must be designed so that the additive which is the catalyst
precursor
must be present as the temperature reaches the point where the rubber melts,
the additive
decomposes to release the catalytic species and the organic vapors do not
carry the
precursor additive or valuable carbon black away. This is achieved differently
in
continuous and batch systems.
In a continuous system, the feed materials, e.g., scrap rubber and
catalyst/additive
are blended together and fed via a screw system. The temperature rises as the
mass
passes through the heated reactor. The exhausts for the vapors are located
just beyond
the point where the rubber melts and the additive decomposes to yield the
catalyst.
In a batch system, the catalyst/additive is placed in a container above the
rubber
placed inside the reactor. As the temperature rises, the container is dumped
and its
contents dispersed over the rubber as it reaches the melting temperature.
In either batch or continuous systems, the additive is in contact with the
melting
rubber just as the additive decomposes and releases the catalytic Group 1 ion.
But, this
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
additive decomposition intimate contact occurs before escaping organic vapors
can carry
the additive material away.
C. RECOVERY/PURIFICATION.
Compounds which distill at very similar temperatures can also be separated by
5 operating at different pressures. In this operation, two columns are used
at two pressures,
e.g., atmospheric pressure and so-called "hard" vacuum. The pressure in a
column varies
from high to low, proceeding up the column, the pressure variation being
achieved by the
additive pressure drops of the distillation trays and/or packing. A
significant simplification
and great improvement is achieved by utilizing the concept of a Joule-Thomson
expansion
between two columns. This provides a sharp change in pressure and the
immediate
condensation of a subset of the compounds present because of the temperature
drop.
Thus, a subset can be easily separated in a second, smaller operating at a
greatly
reduced pressure. Mercaptans of the carbon-chain length found in the organic
liquids
downstream of rubber pyrolysis can be removed by addition of an oxidant, but
the amount
of oxidant required is quite high and the particular oxidants necessary are
expensive. One
cannot introduce significant amounts of new materials without incurring other
negative
results, such as disposal costs or reduced value of remaining materials. That
problem is
easily handled here by adding small quantities of a specific oxidant, a Sodium
or
Potassium Percarbonate, to the small volume stream containing the terpenes and
terpenoids. Sodium Percarbonate is the principal ingredient in an existing
consumer
product sold in large volume.
CERTAIN PREFERRED EMBODIMENTS OF THE PRESENT INVENTION.
Referring again to Figure 1, one embodiment of the present invention is
disclosed
that provides a continuous reactor process B.
Shredded scrap material 1, such as automobile tires after being washed and
dried,
is fed into a nitrogen-blanketed bin 3. From nitrogen-blanketed bin 3 the
shredded scrap
material 1 flows through vacuum-lock valves 5 (also called "Double Dump"
valves) and the
additive/catalyst precursor is added at fill point 23. The shredded scrap
material 1 and the
additive/catalyst enters a tubular reactor 7 which has a helical screw 9 which
slowly turns
at between about 0.2 to about 2.0 rpm. so that the mixture of the shredded
material and
the additive/catalyst precursor is conveyed through the tubular reactor which
is heated
electrically heating bands 11.
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
6
At a point approximately 15% of the length of the tubular reactor 7, the
shredded
material 1 reaches its melting point. Organic vapors 21 evolving from the
melting
shredded material 1 are drawn from the first exit port 25 on the tubular
reactor 7. The
temperature continues to increase until the temperature of the shell near the
exit is
approximately 450 degrees Centigrade. The shredded material 1 continues to
decompose/de-vulcanize, and organic materials and residual moisture are drawn
from the
solids of the shredded material 1 as that shredded material proceeds toward
the exit end
13 of the tubular reactor 7. Here, the remainder of the organic materials that
have been
drawn from the shredded material 1 are drawn away through vapor port 15, while
the
solids 19 are removed at solids exit port 17. The solids 19, which are
generally about
80+% carbon black by weight, proceed to other locations for further finishing
and
processing for sale. The organic vapors 21 enter a Contactor/Separator D where
initial
condensation occurs of the organic vapors, utilizing in certain embodiments,
an oil spray
of previous cooled liquid material. From this point in the process, the
recovery process is
the same for either a continuous system or a batch recovery system.
Referring to Figure 2, an alternative embodiment of the present invention is
disclosed that provides a batch reactor process C.
Whole tires, especially whole large, off-the-road and heavy equipment tires 27
are
loaded into a large vacuum-sealed furnace 29. The vacuum sealed furnace 29 is
evacuated using a vacuum pump 31. The heating of the sealed furnace 29 is
initiated by
operating a heating device 33. It is understood that the heating device 33 may
be either
an electric heater or a suitable gas burner. Once a suitable low pressure of
about 0.1
atmosphere is achieved, a pressure valve 35 that is operatively connected to
vacuum
pump 31 is closed and a pressure gauge 37 is monitored to maintain the
suitable low
pressure needed for the process.
When the furnace temperature reaches 200 degrees Centigrade, an
additive/catalyst located in a bin 39 is released and distributed over the
whole tires 27 in
the furnace 29. As the pressure reaches that of the downstream system,
especially at a
spray-tower 41 (Figure 1), the release valve 41 (Figure 2) is opened slowly.
This allows
the organic vapors 21 to flow to the Contactor/Separator D. The operation
continues until
no further organic vapors 21 flow toward the Contactor/Separator D, as would
indicated by
a decline shown on pressure gauge 37. At the completion of the batch
operation, the
heating device 33 is turned off, the valves 35 and 41 are closed and the
furnace 29 is
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
7
allowed to cool. Once cool, the remaining carbon black, ash, and metal wire
are collected
and removed from the furnace 29.
RECOVERY AND PURIFICATION.
The general recovery of the valuable terpene material is accomplished by
inserting
the liquid oil that results form the above described batch or continuous
operation
processes. In the present embodiment, a Contactor/Separator System D (Figure
3) is
used for recovery and purification of the terpene materials. In that recovery
and
purification process, the liquid oil enters a first distillation column 43
where the liquid oil
proceeds downward by gravity. The preferred internal components for the first
distillation
column 43 are filter trays 45. In smaller systems having a internal diameter
of less than
about 20 centimeters to about 30 centimeters, the filter trays 45 would be of
Snyder-type,
floating ball design. In larger columns having an internal diameter of about 2
centimeters
to about 3 centimeters, the filter trays 45 would be bubble cap or "top hat"
design in
preference over sieve trays, however, sieve trays may still be used in the
upper portion of
the larger column. Whichever trays are employed, it is understood that the
vapors in the
first distillation column 43 rise and liquids fall by the operation of
gravity. Heat is supplied
to the first distillation column 43 by means of a re-boiler 47 that will
normally be steam-
heated in larger systems and heated by electricity in smaller systems. In an
integrated
plant, waste heat from unrelated sources may also be used to provide the
necessary heat.
Regardless of the heat source, however, the bottom 49 of first distillation
tube column 43
will be approximately 200 degrees Centigrade.
The liquid material from the bottom 49 of the re-boiler 47 approximates the
properties of crude oil which is taken for sale and constitutes roughly 55% by
volume of
the liquid oil fed to the first distillation column 43. The residual vapors
rising within the first
distillation column 43 are the lighter materials (shorter molecular chains or
smaller
molecular formula weight). The temperature near the top of the first
distillation column 43
is approximately 185 degrees Centigrade at 760 mm Hg or 760 torr. Those
residual
vapors exit the top of the first distillation column 43 where the vapor
pressure is relieved
into a separator 57 through a small orifice 53 and the gases expand rapidly,
whereby they
cool due to the Joule-Thomson effect, i.e., the change of a gas or liquid when
it is forced
through a hole or multiporous plug while kept insulated (so that no heat is
exchanged with
the surroundings). Thus, the name "throttling process" or Joule¨Thomson
process. It is
understood by those of skill in the art that the cooling of a condensable
substance is rapid
enough, the cooling causes some of the organic materials of the residual
vapors 51 to
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
8
become a liquid oil 55. A chiller assembly for reducing the temperature of the
vapor is
provided for each distillation column 43 and 59. That liquid is enriched in
terpenes relative
to the vapors. The vapor temperature should fall almost instantaneously, or
within about
one second.
The light liquid oil 55 from the separator 57 enters the second distillation
column 59
where the light liquid oil 55 partially flashes and the remaining liquid oil
55 proceeds
downward through a set of connective piping 61 by gravity. Preferred internal
components 63 for the second distillation column 59 include sieve trays or
structured
packing. In smaller systems of between about 1 centimeter and about 2
centimeters, the
internal components 63 can be trays and may be of the Snyder-type, floating
ball design.
Heat can be supplied to the column by means of a second re-boiler 65. In an
integrated
plant, waste heat may be used to provide the heat to the second distillation
column 59 as
long as the bottom 67 of the second distillation column will be approximately
100 degrees
Centigrade to ensure minimal degradation of terpenes or terpenoids. The liquid
material
from the bottom of the second distillation column 59 approximates the
properties of light
crude oil. That light crude oil is then combined with the bottom 49 of first
distillation
column 45 and taken away for sale. Auxiliary vacuum pumps 71 and auxiliary
liquid pump
73 are used to generally operate or evacuate the system.
It will be appreciated that several streams or taps 69 are taken from the
second
distillation column 59. Because the vapors rising within the second
distillation column 59
are the lighter materials (shorter molecular chains or smaller molecular
formula weight) the
low boiling terpenes or terpenoids predominate in the upper internal
components 63 of the
second distillation column. It is generally noted that the top portion the
second distillation
column 59 is approximately 85 degrees Centigrade at 60 mm Hg or 60 torr.
The product mixture from the distillation process (Figure 4) 73 can be
irradiated by
a broad spectrum ultraviolet lamp 75 which is triggered by pulses of voltage
increase.
After exposures of two hours, four hours and seven hours to the uv light,
significant
changes in the relative concentrations of terpenes and other valuable
materials takes
place and the appearance of the product material 80 darkens noticeably.
Substantial
cooling is required to remove excess heat from the irradiation chamber and an
additional
pump 79 may be required. Coolant 76 from a refrigeration system removes such
excess
heat which is carried away as a heating fluid 77 to be used elsewhere in the
process.
Analysis by gas chromatography indicates that the concentration of some
terpenes such
as isopulegol has more than doubled while other materials such as toluene may
decrease
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
9
by 25% or more. This entire portion of the process may be located within the
distillation
process.
In the preceding description, numerous specific details are set forth such as
examples of specific components, devices, methods, in order to provide a
thorough
understanding of embodiments of the present disclosure. It will be apparent to
a person of
ordinary skill in the art that these specific details need not be employed,
and should not be
construed to limit the scope of the disclosure. In the development of any
actual
implementation, numerous implementation-specific decisions must be made to
achieve
the developer's specific goals, such as compliance with system-related and
business-
related constraints. Such a development effort might be complex and time
consuming, but
is nevertheless a routine undertaking of design, fabrication and manufacture
for those of
ordinary skill. The scope of the invention should be determined by any
appended claims
and their legal equivalents, rather than by the examples given.
Additionally, it will be seen in the above disclosure that several of the
intended
purposes of the invention are achieved, and other advantageous and useful
results are
attained. As various changes could be made in the above constructions without
departing
from the scope of the invention, it is intended that all matter contained in
the above
descriptions or shown in the accompanying drawings shall be interpreted as
illustrative
and not in a limiting sense.
Terms such as "proximate," "distal," "upper," "lower," "inner," "outer,"
"inwardly,"
"outwardly," "exterior," "interior," and the like when used herein refer to
positions of the
respective elements as they are shown in the accompanying drawings, and the
disclosure
is not necessarily limited to such positions. Terms such as "first," "second,"
and other
numerical terms when used herein do not imply a sequence or order unless
clearly
indicated by the context.
When introducing elements or features and the exemplary embodiments, the
articles "a," "an," "the" and "said" are intended to mean that there are one
or more of such
elements or features. The terms "comprising," "including," and "having" are
intended to be
inclusive and mean that there may be additional elements or features other
than those
specifically noted. The method steps, processes, and operations described
herein are not
to be construed as necessarily requiring their performance in the particular
order
discussed or illustrated, unless specifically identified as an order of
performance. It is also
to be understood that additional or alternative steps may be employed.
CA 02870183 2014-10-09
WO 2013/155238 PCT/US2013/036052
It will also be understood that when an element is referred to as being
"operatively
connected," "connected," "coupled," "engaged," or "engageable" to and/or with
another
element, it can be directly connected, coupled, engaged, engageable to and/or
with the
other element or intervening elements may be present. In contrast, when an
element is
5 referred to as being "directly connected," "directly coupled," "directly
engaged," or "directly
engageable" to another element, there are no intervening elements present.
Other words
used to describe the relationship between elements should be interpreted in a
like fashion
(e.g., "between" versus "directly between," "adjacent" versus "directly
adjacent," etc.).