Note: Descriptions are shown in the official language in which they were submitted.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
1
APPLICATOR ASSEMBLY FOR APPLYING A COMPOSITION
FIELD OF THE INVENTION
The present invention relates to an applicator assembly and a method for
applying a
composition. More particularly, the invention relates to an applicator
assembly having a
container, an extended tip actuator with a hollow tine, and an engine for
applying a composition,
and methods thereof.
BACKGROUND OF THE INVENTION
Various devices have been made for applying compositions to the scalp. Such
devices
have been used to apply compositions for the purposes of conditioning,
cleansing, dying, and/or
applying one or more benefit agents. However, the designs of currently
marketed devices can
lead to consumer confusion as to how to use the device, resulting in decreased
efficacy and
inefficient delivery of the composition.
Based on the foregoing, there is a need for a unique delivery system which
effectively
communicates to the consumer how to use the product, improves the efficacy of
the composition
applied, and delivers optimal dosage of the composition to the scalp.
SUMMARY OF THE INVENTION
According to an embodiment of the invention, there is provided a method for
delivering a
composition to the scalp comprising: (a) providing a composition in an
applicator assembly, the
applicator assembly comprising: (i) a container for holding the composition;
(ii) an extended tip
actuator in fluid communication with the container, the extended tip actuator
comprising: (1) a
base portion configured to fluidly connect the extended tip actuator to the
container; and (2) a
body portion configured to fluidly connect the base portion to a hollow tine,
wherein the tine
comprises a face located distally from the body portion, wherein the tine
comprises an aperture
in fluid communication with the container, and wherein the tine has a
protrusion length of from
about 0.5 mm to about 100 mm; and (iii) an engine for delivering the
composition from the
container through the extended tip actuator; and (b) dispensing the
composition from the
applicator assembly directly onto the scalp.
According to yet another embodiment of the invention, there is provided an
applicator
assembly for delivering a composition to the scalp comprising: (a) a container
for holding the
composition; (b) an extended tip actuator in fluid communication with the
container, the
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
2
extended tip actuator comprising: (i) a base portion configured to fluidly
connect the extended tip
actuator to the container; and (ii) a body portion configured to fluidly
connect the base portion to
a hollow tine, wherein the tine comprises a face located distally from the
body portion, wherein
the tine comprises an aperture in fluid communication with the container, and
wherein the tine
has a protrusion length of from about 0.5 mm to about 100 mm; and (c) an
engine for delivering
the composition from the container through the extended tip actuator to the
scalp.
These and other features, aspects, and advantages of the present invention
will become
evident to those skilled in the art from a reading of the present disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
While the specification concludes with the claims particularly pointing out
and distinctly
claiming the invention, it is believed that the present invention will be
better understood from the
following description taken in conjunction with the accompanying drawings in
which:
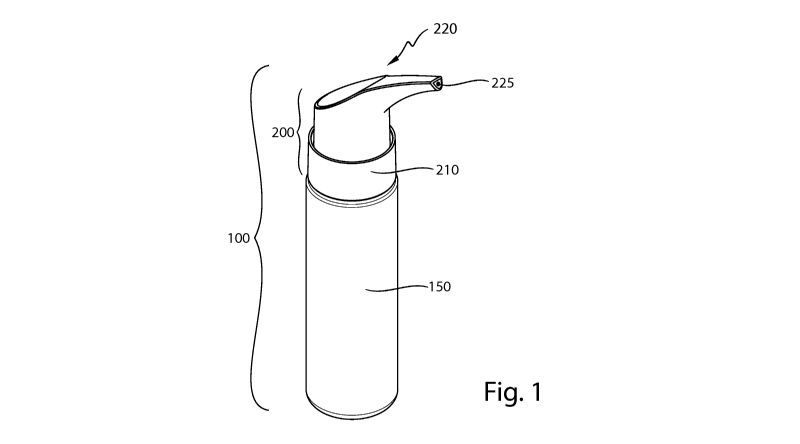
Fig. 1 is a perspective front view of one embodiment of the applicator
assembly;
Fig. 2 is a front view of the applicator assembly of Fig. 1;
Fig. 2A is an enlarged front view of the extended tip actuator, taken of the
area included
in circle 2A in Fig. 2;
Fig. 3 is a top view of the applicator assembly of Fig. 1;
Fig. 4 is a right side view of the applicator assembly of Fig. 1.
DETAILED DESCRIPTION OF THE INVENTION
In all embodiments of the present invention, all percentages are by weight of
the total
composition, unless specifically stated otherwise. All ratios are weight
ratios, unless specifically
stated otherwise. All ranges are inclusive and combinable. The number of
significant digits
conveys neither a limitation on the indicated amounts nor on the accuracy of
the measurements.
All numerical amounts are understood to be modified by the word "about" unless
otherwise
specifically indicated. Unless otherwise indicated, all measurements are
understood to be made
at 25 C and at ambient conditions, where "ambient conditions" means
conditions under about
one atmosphere of pressure and at about 50 % relative humidity. All such
weights as they
pertain to listed ingredients are based on the active level and do not include
carriers or by-
products that may be included in commercially available materials, unless
otherwise specified.
The term "comprising," as used herein, means that other steps and other
ingredients
which do not affect the end result can be added. This term encompasses the
terms "consisting
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
3
of' and "consisting essentially of." The compositions and methods/processes of
the present
invention can comprise, consist of, and consist essentially of the elements
and limitations of the
invention described herein, as well as any of the additional or optional
ingredients, components,
steps, or limitations described herein.
The terms "include," "includes," and "including," as used herein, are meant to
be non-
limiting and are understood to mean "comprise," "comprises," and "comprising,"
respectively.
The term "scalp," as used herein, includes the roots of the hair.
The test methods disclosed in the Test Methods Section of the present
application should
be used to determine the respective values of the parameters of Applicants'
inventions.
Unless otherwise noted, all component or composition levels are in reference
to the active
portion of that component or composition, and are exclusive of impurities, for
example, residual
solvents or by-products, which may be present in commercially available
sources of such
components or compositions.
All percentages and ratios are calculated by weight unless otherwise
indicated. All
percentages and ratios are calculated based on the total composition unless
otherwise indicated.
The term "weight percent" may be denoted as "wt.%" herein.
It should be understood that every maximum numerical limitation given
throughout this
specification includes every lower numerical limitation, as if such lower
numerical limitations
were expressly written herein. Every minimum numerical limitation given
throughout this
specification will include every higher numerical limitation, as if such
higher numerical
limitations were expressly written herein. Every numerical range given
throughout this
specification will include every narrower numerical range that falls within
such broader
numerical range, as if such narrower numerical ranges were all expressly
written herein.
A. Container
Referring to Fig. 1, the applicator assembly 100 may comprise a container 150.
The
container 150 may be of any type that is suitable for holding a composition.
In one embodiment,
the container 150 may be substantially rigid. The container 150 is
substantially rigid if it does
not collapse under external atmospheric pressure when it is subject to an
interior partial vacuum.
In an embodiment, the container 150 may comprise a non-rigid material. The non-
rigid material
may be designed for being squeezed.
The container 150 may be made out of any suitable material selected from the
group
consisting of plastic, metal, alloy, laminate, and combinations thereof. The
container 150 may
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
4
be any shape that fits the holding structure and may comprise at least one
interior compartment
for containing at least one fluid. The container 150 may be a refillable
container such as a pour-
in or screw-on container, or the container 150 may be for one-time use. The
container 150 may
also be removable from the applicator assembly 100. Alternatively, the
container 150 may be
integrated with applicator assembly 100.
B. Extended Actuator
Still referring to Fig. 1, the applicator assembly 100 may comprise an
extended tip
actuator 200. The extended tip actuator 200 may be in fluid communication with
the container
150. The extended tip actuator 200 may comprise a base portion 210, a body
portion 220, and a
hollow tine 225. The extended tip actuator 200 may be made out of any suitable
material
selected from the group consisting of plastic, metal, alloy, and combinations
thereof. The
extended tip actuator 200 may be removable from the applicator assembly 100.
Alternatively,
the extended tip actuator 200 may be integrated with the applicator assembly
100.
1. Base Portion
Referring to Fig. 1, the extended tip actuator 200 may comprise a base portion
210. The
base portion 210 may be configured to fluidly connect the extended tip
actuator 200 to the
container 150. The base portion 210 may be removable from the extended tip
actuator 200.
Alternatively, the base portion 210 may be integrated with the extended tip
actuator 200.
2. Body Portion
Still referring to Fig. 1, the extended tip actuator 200 may comprise a body
portion 220.
The body portion 220 may be configured to fluidly connect the base portion 210
to a hollow tine
225. The body portion 220 may be removable from the extended tip actuator 200.
Alternatively,
the body portion 210 may be integrated with the extended tip actuator 200.
3. Hollow Tine
Still referring to Fig. 1, the extended tip actuator 200 may comprise a hollow
tine 225.
The hollow tine 225 may comprise a face located distally from the body portion
220. The tine
may also comprise an aperture in fluid communication with the container 150.
The tine may be
removable from the extended tip actuator 200. Alternatively, the tine may be
integrated with the
extended tip actuator 200. In an embodiment, the extended tip actuator 200 may
only have one
hollow tine 225.
Now referring to Fig. 3, the hollow tine 225 may have a protrusion length 420.
The
protrusion length 420 is the distance that hollow tine 225 protrudes from the
outer contours of
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
the container 150. In one embodiment, the hollow tine 225 may have a
protrusion length 420 of
from about 0.5 mm to about 100 mm, alternatively from about 5 mm to about 80
mm,
alternatively from about 10 mm to about 60 mm, alternatively from about 15 mm
to about 55
mm, alternatively from about 20 mm to about 50 mm, and alternatively from
about 25 mm to
5 about 45 mm. In another embodiment, the hollow tine 225 may have a
protrusion length 420 of
from about 0.5 mm to about 30 mm, and alternatively from about 10 mm to about
25 mm.
Referring to Fig. 4, the applicator assembly 100 may further comprise a
longitudinal axis
300 therethrough. In one embodiment, the hollow tine 225 is angled from about
20 to about
100 from the longitudinal axis 300, alternatively from about 25 to about
70 from the
longitudinal axis 300, alternatively from about 30 to about 60 from the
longitudinal axis 300,
alternatively from about 35 to about 55 from the longitudinal axis 300,
and alternatively at
about 45 from the longitudinal axis 300. In another embodiment, the hollow
tine 225 is angled
from about 70 to about 110 from the longitudinal axis 300, alternatively
from about 80 to
about 100 from the longitudinal axis 300, alternatively from about 85 to
about 95 from the
longitudinal axis 300, and alternatively at about 90 from the longitudinal
axis 300. The angle
measurement may be calculated counterclockwise from the upper portion of the
longitudinal axis
300 shown in Fig. 4.
a. Face
Now referring to Figs. 2 and 2A, the hollow tine 225 may comprise a face 226
located
distally from the body portion 220 of the extended tip actuator 200. As
described above, the
applicator assembly 100 may further comprise a longitudinal axis 300
therethrough. In one
embodiment, the face 226 is angled from about 10 to about 30 from the
longitudinal axis 300,
alternatively from about 15 to about 25 from the longitudinal axis 300,
alternatively from
about 20 to about 25 from the longitudinal axis 300, and alternatively at
about 23 from the
longitudinal axis 300. In another embodiment, the face 226 is angled from
about 35 to about
55 from the longitudinal axis 300, alternatively from about 40 to about 50
from the
longitudinal axis 300, alternatively from about 43 to about 48 from the
longitudinal axis 300,
and alternatively at about 45 from the longitudinal axis 300. The face 226
angle measurement
may be calculated counterclockwise from the upper portion of the longitudinal
axis 300 shown in
Fig. 4.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
6
b. Aperture
Still referring to Figs. 2 and 2A, the hollow tine 225 may comprise an
aperture 227 in
fluid communication with the container 150. The aperture 227 may be of any
shape, including
but not limited to, circular or square.
In one embodiment, each aperture 227 may have a diameter of from about 0.1 mm
to
about 5 mm. In another embodiment, each aperture 227 may have a diameter of
from about 0.2
mm to about 2 mm. In yet another embodiment, each aperture 227 may have a
diameter of from
about 0.5 mm to about 1.5 mm.
C. Engine
The applicator assembly 100 may also comprise an engine for delivering a
composition
from the container 150 through the extended tip actuator 200. The engine may
be of any type
suitable for dispensing a composition from the container 150, including but
not limited to a
mechanical pump, aerosol, or squeezing. In one embodiment, the engine may be
powered by
any means capable of delivering electricity.
In one embodiment, the engine may dispense from about 0.05 mL to about 4 mL of
a
composition per complete stroke. In another embodiment, the engine may
dispense from about
0.1 mL to about 1 mL of the composition per complete stroke. In yet another
embodiment, the
engine may dispense from about 0.3 mL to about 0.5 mL per complete stroke.
In another embodiment, the engine may deliver an unmetered dose. In this
embodiment,
the consumer may control the dosage delivered by the applicator assembly 100
by deciding, for
example, how long to hold down a button.
D. Optional Composition
The applicator assembly 100 may further comprise a composition. The
composition may
be a rinse-off product or a leave-on product, and can be formulated in a wide
variety of product
forms, including but not limited to liquids, foams, creams, gels, emulsions,
powders, and
mousses.
In one embodiment, the composition may have a neat viscosity of from about
2,000 cps
to about 45,000 cps, alternatively from about 9,000 cps to about 25,000 cps,
alternatively from
about 9,000 cps to about 12,000 cps, alternatively from about 20,000 cps to
about 25,000 cps,
alternatively from about 3,000 to about 10,000 cps, and alternatively from
about 5,000 to about
8,000 cps.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
7
The neat viscosity of the composition is determined by measuring the viscosity
of the
composition at a shear rate of 2 1/sec. Scientifically, neat viscosity is the
ratio of shear stress to
shear rate. Neat viscosity of the composition can be measured with a
rheometer. A TA
Instrument AR2000 may be used to measure the shear stress curve of the
composition.
In one embodiment, the composition may be of any type suitable for application
to
human skin, including the scalp. In another embodiment, the composition may be
of any type
suitable for application to pet skin, including the roots of pet hair. In yet
another embodiment,
the composition may be of any type suitable for application to fabric and/or
carpet.
In one embodiment, the composition may comprise one or more components known
for
use in hair care or personal care products, provided that the additional
components do not
otherwise unduly impair product stability, aesthetics, or performance. Such
optional ingredients
are most typically those described in reference books such as the CTFA
Cosmetic Ingredient
Handbook, Second Edition, The Cosmetic, Toiletries, and Fragrance Association,
Inc. 1988,
1992.
Non-limiting examples of components for use in the composition include
conditioning
agents (e.g., silicones, hydrocarbon oils, fatty esters), natural cationic
deposition polymers,
synthetic cationic deposition polymers, anti-dandruff agents, particles,
particulate tapioca starch,
suspending agents, paraffinic hydrocarbons, propellants, viscosity modifiers,
dyes, non-volatile
solvents or diluents (water-soluble and water-insoluble), pearlescent aids,
foam boosters,
surfactants or nonionic cosurfactants, pediculocides, pH adjusting agents,
perfumes,
preservatives, proteins, skin active agents, sunscreens, UV absorbers, and
vitamins.
1. Conditioning Agent
In one embodiment, the composition may comprise one or more conditioning
agents.
Conditioning agents include materials that are used to give a particular
conditioning benefit to
hair and/or scalp. The conditioning agents that may be useful in the
composition typically
comprise a water-insoluble, water-dispersible, non-volatile, liquid that forms
emulsified, liquid
particles. Suitable conditioning agents for use in the composition are those
conditioning agents
characterized generally as silicones (e.g., silicone oils, cationic silicones,
silicone gums, high
refractive silicones, and silicone resins), organic conditioning oils (e.g.,
hydrocarbon oils,
polyolefins, and fatty esters) or combinations thereof, or those conditioning
agents which
otherwise form liquid, dispersed particles in the aqueous surfactant matrix.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
8
One or more conditioning agents may be present from about 0.01 wt% to about 10
wt%,
alternatively from about 0.1 wt% to about 8 wt%, and alternatively from about
0.2 wt% to about
4 wt%, by weight of the composition.
a. Silicones
The conditioning agent of the composition may be an insoluble silicone
conditioning
agent. The silicone conditioning agent particles may comprise volatile
silicone, non-volatile
silicone, or combinations thereof. If volatile silicones are present, it will
typically be incidental
to their use as a solvent or carrier for commercially available forms of non-
volatile silicone
materials ingredients, such as silicone gums and resins. The silicone
conditioning agent particles
may comprise a silicone fluid conditioning agent and may also comprise other
ingredients, such
as a silicone resin to improve silicone fluid deposition efficiency or enhance
glossiness of the
hair.
The concentration of the silicone conditioning agent may range from about
0.01% to about
10%, by weight of the composition, alternatively from about 0.1% to about 8%,
alternatively
from about 0.1% to about 5%, and alternatively from about 0.2% to about 3%.
Non-limiting
examples of suitable silicone conditioning agents, and optional suspending
agents for the
silicone, are described in U.S. Reissue Pat. No. 34,584, U.S. Pat. No.
5,104,646, and U.S. Pat.
No. 5,106,609, which are incorporated herein by reference. The silicone
conditioning agents for
use in the composition may have a viscosity, as measured at 25A C., from
about 20 to about
2,000,000 centistokes ("csk"), alternatively from about 1,000 to about
1,800,000 csk,
alternatively from about 50,000 to about 1,500,000 csk, and alternatively from
about 100,000 to
about 1,500,000 csk.
The dispersed silicone conditioning agent particles typically have a volume
average
particle diameter ranging from about 0.01 micrometer to about 50 micrometer.
For small particle
application to hair, the volume average particle diameters typically range
from about 0.01
micrometer to about 4 micrometer, alternatively from about 0.01 micrometer to
about 2
micrometer, and alternatively from about 0.01 micrometer to about 0.5
micrometer. For larger
particle application to hair, the volume average particle diameters typically
range from about 5
micrometer to about 125 micrometer, alternatively from about 10 micrometer to
about 90
micrometer, alternatively from about 15 micrometer to about 70 micrometer, and
alternatively
from about 20 micrometer to about 50 micrometer.
Background material on silicones including sections discussing silicone
fluids, gums, and
resins, as well as manufacture of silicones, are found in Encyclopedia of
Polymer Science and
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
9
Engineering, vol. 15, 2d ed., pp 204-308, John Wiley & Sons, Inc. (1989),
incorporated herein
by reference.
i. Silicone Oils
Silicone fluids include silicone oils, which are flowable silicone materials
having a
viscosity, as measured at 25 C, less than 1,000,000 csk, alternatively from
about 5 csk to about
1,000,000 csk, and alternatively from about 100 csk to about 600,000 csk.
Suitable silicone oils
for use in the hair care composition include polyalkyl siloxanes, polyaryl
siloxanes, polyalkylaryl
siloxanes, polyether siloxane copolymers, and mixtures thereof. Other
insoluble, non-volatile
silicone fluids having hair conditioning properties may also be used.
Silicone oils include polyalkyl or polyaryl siloxanes which conform to the
following
Formula (I):
R ........ 0
_it
wherein R is aliphatic, in some embodiments alkyl, alkenyl, or aryl, R can be
substituted or
unsubstituted, and x is an integer from 1 to about 8,000. Suitable R groups
for use in the
compositions include, but are not limited to: alkoxy, aryloxy, alkaryl,
arylalkyl, arylalkenyl,
alkamino, and ether-substituted, hydroxyl-substituted, and halogen-substituted
aliphatic and aryl
groups. Suitable R groups also include cationic amines and quaternary ammonium
groups.
Possible alkyl and alkenyl substituents include C1 to C5 alkyls and alkenyls,
alternativelyfrom C1 to C4, and alternatively from C1 to C2. The aliphatic
portions of other alkyl-,
alkenyl-, or alkynyl-containing groups (such as alkoxy, alkaryl, and alkamino)
can be straight or
branched chains, and may be from C1 to C5, alternatively from C1 to C4,
alternatively from C1 to
C3, and alternatively from C1 to C2. As discussed above, the R substituents
can also contain
amino functionalities (e.g. alkamino groups), which can be primary, secondary
or tertiary amines
or quaternary ammonium. These include mono-, di-and tri-alkylamino and
alkoxyamino groups,
wherein the aliphatic portion chain protrusion length may be as described
herein.
ii. Amino and Cationic Silicones
Cationic silicone fluids suitable for use in the composition include, but are
not limited to,
those which conform to the general formula (II):
(R1)aG3_a-Si--(--0SiG2) .-(--0SiGb(R1)2_b)m--0--SiG3_a (Z1)a
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
wherein G is hydrogen, phenyl, hydroxy, or Ci-C8 alkyl, in some embodiments,
methyl; a is 0 or
an integer having a value from 1 to 3; b is 0 or 1; n is a number from 0 to
1,999, alternatively
from 49 to 499; m is an integer from 1 to 2,000, alternatively from 1 to 10;
the sum of n and m is
a number from 1 to 2,000, alternatively from 50 to 500; R1 is a monovalent
radical conforming to
5 the
general formula CqH2qL, wherein q is an integer having a value from 2 to 8 and
L is selected
from the following groups:
--N(R2)CH2--CH2--N(R2)2
--N(R2)2
10 --N(R2)3 A-
--N(R2)CH2--CH2--NR2H2 A-
wherein R2 is hydrogen, phenyl, benzyl, or a saturated hydrocarbon radical, in
some
embodiments an alkyl radical from about C1 to about C20, and A- is a halide
ion.
In one embodiment, the cationic silicone corresponding to formula (II) is the
polymer
known as "trimethylsilylamodimethicone", which is shown below in formula
(III):
' m'' ¨ . :::.u., ¨
i . .
KR:wes---o.-µsss'S 1:$ -swlii- WM:A:
I :t
,
*.=
Mh.
I
NA:
¨
,..
Other silicone cationic polymers which may be used in the composition are
represented
by the general formula (IV):
riZtVess,CW$iNsssCR;.:-,w;VM%<):'
0 ¨
I
iX).3;i.i.'"'-a' :ii'x's0. :=.:c"'N'O''''"::::'""O'x'xx:S:....e.
1
i
It.3 = L. i
..,
,
- ,
wherein R3 is a monovalent hydrocarbon radical from C1 to C18, in some
embodiments an alkyl
or alkenyl radical, such as methyl; R4 is a hydrocarbon radical, in some
embodiments a C1 to C18
alkylene radical or a C10 to C18 alkyleneoxy radical, alternatively a C1 to C8
alkyleneoxy radical;
Q is a halide ion, in some embodiments chloride; r is an average statistical
value from 2 to 20, in
some embodiments from 2 to 8; s is an average statistical value from 20 to
200, in some
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
11
embodiments from 20 to 50. One polymer of this class is known as UCARE
SILICONE ALE
56 , available from Union Carbide.
iii. Silicone Gums
Other silicone fluids suitable for use in the composition may be insoluble
silicone gums.
These gums are polyorganosiloxane materials having a viscosity, as measured at
25 C, of
greater than or equal to 1,000,000 csk. Silicone gums are described in U.S.
Pat. No. 4,152,416;
Noll and Walter, Chemistry and Technology of Silicones, New York: Academic
Press (1968);
and in General Electric Silicone Rubber Product Data Sheets SE 30, SE 33, SE
54 and SE 76, all
of which are incorporated herein by reference. Specific non-limiting examples
of silicone gums
for use in the hair care include polydimethylsiloxane,
(polydimethylsiloxane)(methylvinylsiloxane)copolymer,
poly(dimethylsiloxane)(diphenyl
siloxane)(methylvinylsiloxane)copolymer and mixtures thereof.
iv. High Refractive Index Silicones
Other non-volatile, insoluble silicone fluid conditioning agents that are
suitable for use in
the hair care composition are those known as "high refractive index
silicones," having a
refractive index of at least about 1.46, altemativelyy at least about 1.48,
alternatively at least
about 1.52, and alternatively at least about 1.55. The refractive index of the
polysiloxane fluid
will generally be less than about 1.70, typically less than about 1.60. In
this context,
polysiloxane "fluid" includes oils as well as gums. The high refractive index
polysiloxane fluid
includes those represented by general Formula (I) above, as well as cyclic
polysiloxanes such as
those represented by Formula (V) below:
wherein R is as defined above, and n is a number from about 3 to about 7,
alternatively from
about 3 to about 5.
The high refractive index polysiloxane fluids contain an amount of aryl-
containing R
substituents sufficient to increase the refractive index to the desired level,
which is described
herein. Additionally, R and n may be selected so that the material is non-
volatile.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
12
Aryl-containing substituents include those which contain alicyclic and
heterocyclic five
and six member aryl rings and those which contain fused five or six member
rings. The aryl
rings themselves can be substituted or unsubstituted.
Generally, the high refractive index polysiloxane fluids will have a degree of
aryl-
containing substituents of at least about 15%, alternatively at least about
20%, alternatively at
least about 25%, alternatively at least about 35%, and alternatively at least
about 50%.
Typically, the degree of aryl substitution will be less than about 90%, more
generally less than
about 85%, alternativelyfrom about 55% to about 80%. In some embodiments, the
high
refractive index polysiloxane fluids have a combination of phenyl or phenyl
derivative
substituents, with alkyl substituents, in some embodiments C1-C4 alkyl,
hydroxy, or Ci-C4
alkylamino (especially¨R4NHR5NH2 wherein each R4 and R5 independently is a C1-
C3 alkyl,
alkenyl, and/or alkoxy).
When high refractive index silicones are used in the composition, they may be
used in
composition with a spreading agent, such as a silicone resin or a surfactant,
to reduce the surface
tension by a sufficient amount to enhance spreading and thereby enhance the
glossiness
(subsequent to drying) of hair treated with the compositions.
Silicone fluids suitable for use in the composition are disclosed in U.S. Pat.
No.
2,826,551, U.S. Pat. No. 3,964,500, U.S. Pat. No. 4,364,837, British Pat. No.
849,433, and
Silicon Compounds, Petrarch Systems, Inc. (1984), all of which are
incorporated herein by
reference.
v. Silicone Resins
Silicone resins may be included in composition. These resins are highly cross-
linked
polymeric siloxane systems. The cross-linking is introduced through the
incorporation of
trifunctional and tetrafunctional silanes with monofunctional or difunctional,
or both, silanes
during manufacture of the silicone resin.
Silicone materials and silicone resins in particular, can conveniently be
identified
according to a shorthand nomenclature system known to those of ordinary skill
in the art as
"MDTQ" nomenclature. Under this system, the silicone is described according to
presence of
various siloxane monomer units which make up the silicone. Briefly, the symbol
M denotes the
monofunctional unit (CH3)35i005; D denotes the difunctional unit (CH3)25i0; T
denotes the
trifunctional unit (CH3)5i01 5; and Q denotes the quadra-or tetra-functional
unit 5i02. Primes of
the unit symbols (e.g. M', D', T', and Q') denote substituents other than
methyl, and must be
specifically defined for each occurrence.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
13
Silicone resins for use in the composition may include, but are not limited to
MQ, MT,
MTQ, MDT and MDTQ resins. Methyl is a possible silicone substituent. In some
embodiments,
silicone resins are MQ resins, wherein the M:Q ratio is from about 0.5:1.0 to
about 1.5:1.0 and
the average molecular weight of the silicone resin is from about 1000 to about
10,000.
The weight ratio of the non-volatile silicone fluid, having refractive index
below 1.46, to
the silicone resin component, when used, may be from about 4:1 to about 400:1,
alternatively
from about 9:1 to about 200:1, and alternatively from about 19:1 to about
100:1, particularly
when the silicone fluid component is a polydimethylsiloxane fluid or a mixture
of
polydimethylsiloxane fluid and polydimethylsiloxane gum as described herein.
Insofar as the
silicone resin forms a part of the same phase in the compositions hereof as
the silicone fluid, i.e.
the conditioning active, the sum of the fluid and resin should be included in
determining the level
of silicone conditioning agent in the composition.
b. Organic Conditioning Oils
The conditioning agent of the composition may also comprise at least one
organic
conditioning oil, either alone or in combination with other conditioning
agents, such as the
silicones described above.
i. Hydrocarbon Oils
Suitable organic conditioning oils for use as conditioning agents in the
composition may
include, but are not limited to, hydrocarbon oils having at least about 10
carbon atoms, such as
cyclic hydrocarbons, straight chain aliphatic hydrocarbons (saturated or
unsaturated), and
branched chain aliphatic hydrocarbons (saturated or unsaturated), including
polymers and
mixtures thereof. Straight chain hydrocarbon oils may be from about C12 to
about C19. Branched
chain hydrocarbon oils, including hydrocarbon polymers, typically will contain
more than 19
carbon atoms.
ii. Polyolefins
Organic conditioning oils for use in the composition may also include liquid
polyolefins,
alternatively liquid poly-a-olefins, alternatively hydrogenated liquid poly-a-
olefins. Polyolefins
for use herein are prepared by polymerization of C4 to about C14 olefenic
monomers, in some
embodiments from about C6 to about C12.
iii. Fatty Esters
Other suitable organic conditioning oils for use as the conditioning agent in
the
composition may include fatty esters having at least 10 carbon atoms. These
fatty esters include
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
14
esters with hydrocarbyl chains derived from fatty acids or alcohols. The
hydrocarbyl radicals of
the fatty esters hereof may include or have covalently bonded thereto other
compatible
functionalities, such as amides and alkoxy moieties (e.g., ethoxy or ether
linkages, etc.).
iv. Fluorinated Conditioning Compounds
Fluorinated compounds suitable for delivering conditioning to hair or skin as
organic
conditioning oils include perfluoropolyethers, perfluorinated olefins,
fluorine based specialty
polymers that may be in a fluid or elastomer form similar to the silicone
fluids previously
described, and perfluorinated dimethicones.
v. Fatty Alcohols
Other suitable organic conditioning oils for use in the composition may
include, but are
not limited to, fatty alcohols having at least about 10 carbon atoms,
altemativelyfrom about 10 to
about 22 carbon atoms, and alternatively from about 12 to about 16 carbon
atoms.
vi. Alkyl Glucosides and Alkyl Glucoside Derivatives
Suitable organic conditioning oils for use in the composition may include, but
are not
limited to, alkyl glucosides and alkyl glucoside derivatives. Specific non-
limiting examples of
suitable alkyl glucosides and alkyl glucoside derivatives include Glucam E-10,
Glucam E-20,
Glucam P-10, and Glucquat 125 commercially available from Amerchol.
c. Other Conditioning Agents
i. Quaternary Ammonium Compounds
Suitable quaternary ammonium compounds for use as conditioning agents in the
composition may include, but are not limited to, hydrophilic quaternary
ammonium compounds
with a long chain substituent having a carbonyl moiety, like an amide moiety,
or a phosphate
ester moiety or a similar hydrophilic moiety.
Examples of useful hydrophilic quaternary ammonium compounds include, but are
not
limited to, compounds designated in the CTFA Cosmetic Dictionary as
ricinoleamidopropyl
trimonium chloride, ricinoleamido trimonium ethylsulfate, hydroxy
stearamidopropyl
trimoniummethylsulfate and hydroxy stearamidopropyl trimonium chloride, or
combinations
thereof.
ii. Polyethylene Glycols
Additional compounds useful herein as conditioning agents include polyethylene
glycols
and polypropylene glycols having a molecular weight of up to about 2,000,000
such as those
with CTFA names PEG-200, PEG-400, PEG-600, PEG-1000, PEG-2M, PEG-7M, PEG-14M,
PEG-45M and mixtures thereof.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
iii. Cationic Deposition Polymers
The composition may further comprise a cationic deposition polymer. Any known
natural or synthetic cationic deposition polymer can be used herein. Examples
include those
polymers disclosed in U.S. Patent No. 6,649,155; U.S. Patent Application
Publication Nos.
5 2008/0317698; 2008/0206355; and 2006/0099167, which are incorporated
herein by reference in
their entirety.
The cationic deposition polymer may be included in the composition at a level
from
about 0.01 wt% to about 2 wt%, in one embodiment from about 1.5 wt% to about
1.9 wt%, in
another embodiment from about 1.8 wt% to about 2.0 wt%, in view of providing
the benefits of
10 the composition.
The cationic deposition polymer may be a water soluble polymer with a charge
density
from about 0.5 milliequivalents per gram to about 12 milliequivalents per
gram. The cationic
deposition polymer used in the composition may have a molecular weight of
about 100,000
Daltons to about 5,000,000 Daltons. The cationic deposition polymer may be a
low charge
15 density cationic polymer.
In one embodiment, the cationic deposition polymer is a synthetic cationic
deposition
polymer. A variety of synthetic cationic deposition polymers can be used
including mono- and
di-alkyl chain cationic surfactants. In one embodiment, mono-alkyl chain
cationic surfactants
are chosen including, for example, mono-alkyl quaternary ammonium salts and
mono-alkyl
amines. In another embodiment, di-alkyl chain cationic surfactants are used
and include, for
example, dialkyl (14-18) dimethyl ammonium chloride, ditallow alkyl dimethyl
ammonium
chloride, dihydrogenated tallow alkyl dimethyl ammonium chloride, distearyl
dimethyl
ammonium chloride, dicetyl dimethyl ammonium chloride, and mixtures thereof.
In another embodiment, the cationic deposition polymer is a naturally derived
cationic
polymer. The term, "naturally derived cationic polymer" as used herein, refers
to cationic
deposition polymers which are obtained from natural sources. The natural
sources may be
polysaccharide polymers. Therefore, the naturally derived cationic polymer may
be selected
from the group comprising starches, guar, cellulose, Cassia, locust bean,
Konjac, Tara,
galactomannan, tapioca, and synthetic polymers. In a further embodiment,
cationic deposition
polymers are selected from Mirapol 100S (Rhodia), Jaguar C17, polyDADMAC,
Tapioca
starch (Akzo), TriquatTm, and mixtures thereof.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
16
d. Anionic Emulsifiers
A variety of anionic emulsifiers can be used in the composition as described
below. The
anionic emulsifiers include, by way of illustrating and not limitation, water-
soluble salts of alkyl
sulfates, alkyl ether sulfates, alkyl isothionates, alkyl carboxylates, alkyl
sulfosuccinates, alkyl
succinamates, alkyl sulfate salts such as sodium dodecyl sulfate, alkyl
sarcosinates, alkyl
derivatives of protein hydrolyzates, acyl aspartates, alkyl or alkyl ether or
alkylaryl ether
phosphate esters, sodium dodecyl sulphate, phospholipids or lecithin, or
soaps, sodium,
potassium or ammonium stearate, oleate or palmitate, alkylarylsulfonic acid
salts such as sodium
dodecylbenzenesulfonate, sodium dialkylsulfosuccinates, dioctyl
sulfosuccinate, sodium
dilaurylsulfosuccinate, poly(styrene sulfonate) sodium salt, isobutylene-
maleic anhydride
copolymer, gum arabic, sodium alginate, carboxymethylcellulose, cellulose
sulfate and pectin,
poly(styrene sulfonate), isobutylene-maleic anhydride copolymer, gum arabic,
carrageenan,
sodium alginate, pectic acid, tragacanth gum, almond gum and agar; semi-
synthetic polymers
such as carboxymethyl cellulose, sulfated cellulose, sulfated methylcellulose,
carboxymethyl
starch, phosphated starch, lignin sulfonic acid; and synthetic polymers such
as maleic anhydride
copolymers (including hydrolyzates thereof), polyacrylic acid, polymethacrylic
acid, acrylic acid
butyl acrylate copolymer or crotonic acid homopolymers and copolymers,
vinylbenzenesulfonic
acid or 2-acrylamido-2-methylpropanesulfonic acid homopolymers and copolymers,
and partial
amide or partial ester of such polymers and copolymers, carboxymodified
polyvinyl alcohol,
sulfonic acid-modified polyvinyl alcohol and phosphoric acid-modified
polyvinyl alcohol,
phosphated or sulfated tristyrylphenol ethoxylates.
In addition, anionic emulsifiers that have acrylate functionality may also be
used in the
composition. Anionic emulsifiers useful herein include, but aren't limited to:
poly(meth)acrylic
acid; copolymers of (meth)acrylic acids and its (meth)acrylates with C1-22
alkyl, C1-C8 alkyl,
butyl; copolymers of (meth)acrylic acids and (meth)acrylamide;
Carboxyvinylpolymer; acrylate
copolymers such as Acrylate/C10-30 alkyl acrylate crosspolymer, Acrylic
acid/vinyl ester
copolymer/Acrylates/Vinyl Isodec ano ate crosspolymer, Acrylates/Palmeth-25
Acrylate
copolymer, Acrylate/Steareth-20 Itaconate copolymer, and Acrylate/Celeth-20
Itaconate
copolymer; Polystyrene sulphonate, copolymers of methacrylic acid and
acrylamidomethylpropane sulfonic acid, and copolymers of acrylic acid and
acrylamidomethylpropane sulfonic acid; carboxymethycellulose; carboxy guar;
copolymers of
ethylene and maleic acid; and acrylate silicone polymer. Neutralizing agents
may be included to
neutralize the anionic emulsifiers herein. Non-limiting examples of such
neutralizing agents
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
17
include sodium hydroxide, potassium hydroxide, ammonium hydroxide,
monoethanolamine,
diethanolamine, triethanolamine, diisopropanolamine, aminomethylpropanol,
tromethamine,
tetrahydroxypropyl ethylenediamine, and mixtures thereof. Commercially
available anionic
emulsifiers include, for example, Carbomer supplied from Noveon under the
tradename
Carbopol 981 and Carbopol 980; Acrylates/C10-30 Alkyl Acrylate Crosspolymer
having
tradenames Pemulen TR-1, Pemulen TR-2, Carbopol 1342, Carbopol 1382, and
Carbopol ETD
2020, all available from Noveon; sodium carboxymethylcellulose supplied from
Hercules as
CMC series; and Acrylate copolymer having a tradename Capigel supplied from
Seppic. In
another embodiment, anionic emulsifiers are carboxymethylcelluloses.
2. Benefit Agents
In an embodiment, the composition further comprises one or more additional
benefit
agents. The benefit agents comprise a material selected from the group
consisting of anti-
dandruff agents, vitamins, lipid soluble vitamins, chelants, perfumes,
brighteners, enzymes,
sensates, attractants, anti-bacterial agents, dyes, pigments, bleaches, hops,
caffeine, resorcinol,
cleaning agents, and mixtures thereof.
a. Vitamin B3 Compounds
The composition may include a vitamin B3 compound. In one embodiment, the
vitamin
B3 compound is niacinamide. Vitamin B3 compounds may be useful for regulating
skin
conditions, as described in U.S. Patent No. 5,939,082. In some embodiments,
the composition
may comprise from about 0.1% to about 25% of a vitamin B3 compound, in another
embodiment
from about 0.5% to about 15% of a vitamin B3 compound, and in yet another
embodiment from
about 3.5% to about 7.5% of a vitamin B3 compound.
As used herein, "vitamin B3
compound" means a one or more compounds having the formula:
N
wherein R is - CONH2 (i.e., niacinamide), - COOH (i.e., nicotinic acid) or -
CH2OH (i.e.,
nicotinyl alcohol); derivatives thereof; mixtures thereof; and salts of any of
the foregoing.
Exemplary derivatives of the foregoing vitamin B3 compounds include nicotinic
acid
esters, including non-vasodilating esters of nicotinic acid (e.g, tocopherol
nicotinate, myristyl
nicotinate), nicotinyl amino acids, nicotinyl alcohol esters of carboxylic
acids, nicotinic acid N-
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
18
oxide and niacinamide N-oxide. Additional exemplary derivatives of vitamin B3
compounds are
set forth in U.S. Patent App. No. 11/897084, which is incorporated herein by
reference.
b. Alcohol
In one embodiment, the composition may comprise an alcohol. Alcohol may be
used for
faster drying and skin penetration of the composition. In a particular
embodiment, the
composition comprises from about 10 % to about 90 % alcohol, alternatively
from about 15 % to
about 75 % alcohol, or alternatively from about 25 % to about 50 % alcohol.
Any suitable
alcohol, such as ethanol, can be used.
c. Anti-Dandruff Agent
In one embodiment, the composition may comprise an anti-dandruff agent, which
may be
an anti-dandruff active particulate. Such anti-dandruff particulate should be
physically and
chemically compatible with the components of the composition, and should not
otherwise unduly
impair product stability, aesthetics or performance.
In an embodiment, the anti-dandruff agent may be selected from the group
consisting of:
pyridinethione salts; azoles, such as ketoconazole, econazole, and elubiol;
selenium sulphide;
particulate sulfur; keratolytic agents such as salicylic acid; and mixtures
thereof.
Pyridinethione salts may be suitable anti-dandruff active particulates. In an
embodiment,
the anti-dandruff active may be a 1-hydroxy-2-pyridinethione salt and is in
particulate form. In
an embodiment, the concentration of pyridinethione anti-dandruff particulate
ranges from about
0.01 wt% to about 5 wt%, or from about 0.1 wt% to about 3 wt%, or from about
0.1 wt% to
about 2 wt%. In an embodiment, the pyridinethione salts are those formed from
heavy metals
such as zinc, tin, cadmium, magnesium, aluminium and zirconium, generally
zinc, typically the
zinc salt of 1-hydroxy-2-pyridinethione (known as "zinc pyridinethione" or
"ZPT"), commonly
1-hydroxy-2-pyridinethione salts in platelet particle form. In an embodiment,
the 1-hydroxy-2-
pyridinethione salts in platelet particle form have an average particle size
of up to about 20
microns, or up to about 5 microns, or up to about 2.5 microns. Salts formed
from other cations,
such as sodium, may also be suitable. Pyridinethione anti-dandruff actives are
described, for
example, in U.S. Pat. No. 2,809,971; U.S. Pat. No. 3,236,733; U.S. Pat. No.
3,753,196; U.S. Pat.
No. 3,761,418; U.S. Pat. No. 4,345,080; U.S. Pat. No. 4,323,683; U.S. Pat. No.
4,379,753; and
U.S. Pat. No. 4,470,982.
In an embodiment, in addition to the anti-dandruff active selected from
polyvalent metal
salts of pyrithione, the composition may further comprise one or more anti-
fungal and/or anti-
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
19
microbial actives. In an embodiment, the anti-microbial active is selected
from the group
consisting of: coal tar, sulfur, charcoal, whitfield's ointment, castellani's
paint, aluminum
chloride, gentian violet, octopirox (piroctone olamine), ciclopirox olamine,
undecylenic acid and
its metal salts, potassium permanganate, selenium sulphide, sodium
thiosulfate, propylene
glycol, oil of bitter orange, urea preparations, griseofulvin, 8-
hydroxyquinoline ciloquinol,
thiobendazole, thiocarbamates, haloprogin, polyenes, hydroxypyridone,
morpholine,
benzylamine, allylamines (such as terbinafine), tea tree oil, clove leaf oil,
coriander, palmarosa,
berberine, thyme red, cinnamon oil, cinnamic aldehyde, citronellic acid,
hinokitol, ichthyol pale,
Sensiva SC-50, Elestab HP-100, azelaic acid, lyticase, iodopropynyl
butylcarbamate (IPBC),
isothiazalinones such as octyl isothiazalinone, and azoles, and mixtures
thereof. In an
embodiment, the anti-microbial is selected from the group consisting of
itraconazole,
ketoconazole, selenium sulphide, coal tar, and mixtures thereof.
In an embodiment, the azole anti-microbials is an imidazole selected from the
group
consisting of: benzimidazole, benzothiazole, bifonazole, butaconazole nitrate,
climbazole,
clotrimazole, croconazole, eberconazole, econazole, elubiol, fenticonazole,
fluconazole,
flutimazole, isoconazole, ketoconazole, lanoconazole, metronidazole,
miconazole, neticonazole,
omoconazole, oxiconazole nitrate, sertaconazole, sulconazole nitrate,
tioconazole, thiazole, and
mixtures thereof, or the azole anti-microbials is a triazole selected from the
group consisting of:
terconazole, itraconazole, and mixtures thereof. When present in the
composition, the azole anti-
microbial active may be included in an amount of from about 0.01 wt% to about
5 wt%, or from
about 0.1 wt% to about 3 wt%, or from about 0.3 wt% to about 2 wt%. In an
embodiment, the
azole anti-microbial active is ketoconazole. In an embodiment, the sole anti-
microbial active is
ketoconazole.
Embodiments of the composition may also comprise a combination of anti-
microbial
actives. In an embodiment, the combination of anti-microbial actives is
selected from the group
of combinations consisting of: octopirox and zinc pyrithione, pine tar and
sulfur, salicylic acid
and zinc pyrithione, salicylic acid and elubiol, zinc pyrithione and elubiol,
zinc pyrithione and
climbasole, octopirox and climbasole, salicylic acid and octopirox, and
mixtures thereof.
In an embodiment, the composition comprises an effective amount of a zinc-
containing
layered material. In an embodiment, the composition comprises from about 0.001
wt% to about
10 wt%, or from about 0.01 wt% to about 7 wt%, or from about 0.1 wt% to about
5 wt% of a
zinc-containing layered material, by total weight of the composition.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
Zinc-containing layered materials may be those with crystal growth primarily
occurring
in two dimensions. It is conventional to describe layered structures as not
only those in which all
the atoms are incorporated in well-defined layers, but also those in which
there are ions or
molecules between the layers, called gallery ions (A.F. Wells "Structural
Inorganic Chemistry"
5 Clarendon Press, 1975). Zinc-containing layered materials (ZLMs) may have
zinc incorporated
in the layers and/or be components of the gallery ions. The following classes
of ZLMs represent
relatively common examples of the general category and are not intended to be
limiting as to the
broader scope of materials which fit this definition.
Many ZLMs occur naturally as minerals. In an embodiment, the ZLM is selected
from
10 the group consisting of: hydrozincite (zinc carbonate hydroxide),
aurichalcite (zinc copper
carbonate hydroxide), rosasite (copper zinc carbonate hydroxide), and mixtures
thereof. Related
minerals that are zinc-containing may also be included in the composition.
Natural ZLMs can
also occur wherein anionic layer species such as clay-type minerals (e.g.,
phyllosilicates) contain
ion-exchanged zinc gallery ions. All of these natural materials can also be
obtained synthetically
15 or formed in situ in a composition or during a production process.
Another common class of ZLMs, which are often, but not always, synthetic, is
layered
double hydroxides. In an embodiment, the ZLM is a layered double hydroxide
conforming to
the formula [1\42+1_xM3 x(OH)21x Am-xim=nH20 wherein some or all of the
divalent ions (M2 ) are
zinc ions (Crepaldi, EL, Pava, PC, Tronto, J, Valim, JB J. Colloid Interfac.
Sci. 2002, 248, 429-
20 42).
Yet another class of ZLMs can be prepared called hydroxy double salts
(Morioka, H.,
Tagaya, H., Karasu, M, Kadokawa, J, Chiba, K Inorg. Chem. 1999, 38, 4211-6).
In an
embodiment, the ZLM is a hydroxy double salt conforming to the formula
)01+ An-(1,3y)/n' nH20 where the two metal ions (M2 ) may be the same or
different. If they are the
same and represented by zinc, the formula simplifies to Vni x(OH)212x 2x K.
nH20. This latter
formula represents (where x=0.4) materials such as zinc hydroxychloride and
zinc
hydroxynitrate. In an embodiment, the ZLM is zinc hydroxychloride and/or zinc
hydroxynitrate.
These are related to hydrozincite as well wherein a divalent anion replace the
monovalent anion.
These materials can also be formed in situ in a composition or in or during a
production process.
In embodiments having a zinc-containing layered material and a pyrithione or
polyvalent
metal salt of pyrithione, the ratio of zinc-containing layered material to
pyrithione or a
polyvalent metal salt of pyrithione is from about 5:100 to about 10:1, or from
about 2:10 to about
5:1, or from about 1:2 to about 3:1.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
21
The on-scalp deposition of the anti-dandruff active is at least about 1
microgram/cm2.
The on-scalp deposition of the anti-dandruff active is important in view of
ensuring that the anti-
dandruff active reaches the scalp where it is able to perform its function. In
an embodiment, the
deposition of the anti-dandruff active on the scalp is at least about 1.5
microgram/cm2, or at least
about 2.5 microgram/cm2, or at least about 3 microgram/cm2, or at least about
4 microgram/cm2,
or at least about 6 microgram/cm2, or at least about 7 microgram/cm2, or at
least about 8
microgram/cm2, or at least about 8 microgram/cm2, or at least about 10
microgram/cm2. The on-
scalp deposition of the anti-dandruff active is measured by having the hair of
individuals washed
with a composition comprising an anti-dandruff active, for example a
composition pursuant to
the present invention, by trained a cosmetician according to a conventional
washing protocol.
The hair is then parted on an area of the scalp to allow an open-ended glass
cylinder to be held
on the surface while an aliquot of an extraction composition is added and
agitated prior to
recovery and analytical determination of anti-dandruff active content by
conventional
methodology, such as HPLC.
d. Fatty Alcohol Gel Network
Embodiments of the composition may also comprise fatty alcohol gel networks,
which
have been used for years in cosmetic creams and hair conditioners. These gel
networks are
formed by combining fatty alcohols and surfactants in the ratio of about 1:1
to about 40:1
(alternatively from about 2:1 to about 20:1, and alternatively from about 3:1
to about 10:1). The
formation of a gel network involves heating a dispersion of the fatty alcohol
in water with the
surfactant to a temperature above the melting point of the fatty alcohol.
During the mixing
process, the fatty alcohol melts, allowing the surfactant to partition into
the fatty alcohol
droplets. The surfactant brings water along with it into the fatty alcohol.
This changes the
isotropic fatty alcohol drops into liquid crystalline phase drops. When the
mixture is cooled
below the chain melt temperature, the liquid crystal phase is converted into a
solid crystalline gel
network. The gel network contributes a stabilizing benefit to cosmetic creams
and hair
conditioners. In addition, they deliver conditioned feel benefits for hair
conditioners.
Thus according to an embodiment, the fatty alcohol is included in the fatty
alcohol gel
network at a level by weight of from about 0.05 wt% to about 14 wt%. For
example, the fatty
alcohol may be present in an amount ranging from about 1 wt% to about 10 wt%,
and
alternatively from about 6 wt% to about 8 wt%.
The fatty alcohols useful herein are those having from about 10 to about 40
carbon atoms,
from about 12 to about 22 carbon atoms, from about 16 to about 22 carbon
atoms, or about 16 to
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
22
about 18 carbon atoms. These fatty alcohols can be straight or branched chain
alcohols and can
be saturated or unsaturated. Nonlimiting examples of fatty alcohols include,
cetyl alcohol,
stearyl alcohol, behenyl alcohol, and mixtures thereof. Mixtures of cetyl and
stearyl alcohol in a
ratio of from about 20:80 to about 80:20, are suitable.
The fatty alcohols useful herein are those having from about 10 to about 40
carbon atoms,
from about 12 to about 22 carbon atoms, from about 16 to about 22 carbon
atoms, or about 16 to
about 18 carbon atoms. These fatty alcohols can be straight or branched chain
alcohols and can
be saturated or unsaturated. Nonlimiting examples of fatty alcohols include,
cetyl alcohol,
stearyl alcohol, behenyl alcohol, and mixtures thereof. Mixtures of cetyl and
stearyl alcohol in a
ratio of from about 20:80 to about 80:20, are suitable.
E. Method for Dispensing
The applicator assembly 100 described above may also be used in a method for
dispensing a composition. The method for dispensing a composition may comprise
providing
the applicator assembly 100 described above, disposing a composition in the
applicator assembly
100, and activating the applicator assembly 100. In one embodiment, the
activating of the
applicator assembly 100 may include actuating a mechanical pump, an aerosol
container, or a
squeeze container.
In one embodiment, the method may be used for dispensing the composition from
the
applicator assembly directly onto the scalp.
In another embodiment, the method may also be used for improving the efficacy
of a
composition after being delivered to the scalp.
F. Data
Referring to Table 1, 30 consumers ages 22 to 55 were asked to rate the
following scalp
applicators. The spray applicator is a common in-store applicator used for
applying
compositions to the scalp. The single tine applicator is an embodiment of the
present invention
used for applying compositions to the scalp. The consumers were asked to rate
the scalp
applicators on a scale of 1 to 10, 10 being most useful for scalp application,
and 1 being most
useful for hair application. The mean values were then calculated and listed
in Table 1. Using
the Student T method at a 95% confidence level, there was a statistically
significant difference in
the consumers' preference for the single tine applicator for being most useful
for scalp
application.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
23
Table 1: Usefulness for Scalp Application
Applicator Mean
Single Tine 5.90
Spray 2.83
Referring to Table 2, 30 consumers ages 22 to 55 were asked to rate the
following scalp
applicators. The spray applicator is a common in-store applicator used for
applying
compositions to the scalp. The single tine applicator is an embodiment of the
present invention
used for applying compositions to the scalp. The consumers were asked to rate
the scalp
applicators on a scale of 1 to 10, 10 being easiest to apply to the
composition to the scalp, and 1
being hardest to apply the composition to the scalp. The mean values were then
calculated and
listed in Table 2. Using the Student T method at a 95% confidence level, there
was a statistically
significant difference in the consumers' preference for the single tine
applicator for being easiest
to apply a composition to the scalp.
Table 2: Easy to Apply
Applicator Mean
Single Tine 4.10
Spray 2.67
Referring to Table 3, 236 consumers were asked to use and rate the spray
applicator on
ability to deliver product benefits. The spray applicator is a common in-store
applicator used for
applying compositions to the scalp. 100 consumers were asked to use and rate
the single tine
applicator on ability to deliver product benefits. The single tine applicator
is an embodiment of
the present invention used for applying compositions to the scalp. The
consumers were asked to
rate the scalp applicators on a scale of 1 to 100, 100 being the most able to
deliver product
benefits, and 1 being least able to deliver product benefits. The mean values
were then
calculated and listed in Table 3. Using an 85% confidence level, there was a
statistically
significant difference in the consumers' ratings for the single tine
applicator for being most able
to deliver product benefits.
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
24
Table 3: Delivers Product Benefits
Applicator Mean
Single Tine 52
Spray 43
Referring to Table 4, 10 consumers were asked to use either the spray
applicator or the
single tine applicator. The spray applicator is a common in-store applicator
used for applying
compositions to the scalp. The single tine applicator is an embodiment of the
present invention
used for applying compositions to the scalp. Product spread on each consumer's
scalp was then
quantified by using florescent formula and image analysis. The mean values are
listed in Table
4.
The results show that the single tine applicator delivered substantially more
product to the
scalp than the spray applicator ¨ an increase in coverage of 62.9 % versus the
spray applicator.
Table 4: Area Intensity Coverage
Applicator Mean
Single Tine 6,830,561.4
Spray 4,192,614.3
G. Area Intensity Coverage Test Method
1) Panelists use a delivery system on their own scalp with wet hair to apply a
product
with a fluorescent agent (curcumin).
2) Panelists rub the product into their scalp as would be instructed during
normal use.
3) Panelists have their hair sectioned by a stylist ¨ sectioning must be
consistent over all
panelists.
4) Pictures are taken utilizing a regular camera with a black light to pick up
on the
fluorescent agent (curcumin) at four different angles to determine the scalp
coverage
from the delivery system.
5) The computer program Image J is used to perform analysis on all of the
images,
measuring the area (reported in pixels) and intensity of the fluorescent agent
in that
area. The value for area intensity coverage is calculated by area*intensity.
The dimensions and values disclosed herein are not to be understood as being
strictly
limited to the exact numerical values recited. Instead, unless otherwise
specified, each such
dimension is intended to mean both the recited value and a functionally
equivalent range
CA 02870208 2014-10-09
WO 2013/163491
PCT/US2013/038311
surrounding that value. For example, a dimension disclosed as "40 mm" is
intended to mean
"about 40 mm."
Every document cited herein, including any cross referenced or related patent
or
application, is hereby incorporated herein by reference in its entirety unless
expressly excluded
5 or otherwise limited. The citation of any document is not an admission
that it is prior art with
respect to any invention disclosed or claimed herein or that it alone, or in
any combination with
any other reference or references, teaches, suggests, or discloses any such
invention. Further, to
the extent that any meaning or definition of a term in this document conflicts
with any meaning
or definition of the same term in a document incorporated by reference, the
meaning or definition
10 assigned to that term in this document shall govern.
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
modifications can be made without departing from the spirit and scope of the
invention. It is
therefore intended to cover in the appended claims all such changes and
modifications that are
15 within the scope of this invention.