Note: Descriptions are shown in the official language in which they were submitted.
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
NOVEL COMPOSITION FOR EXTRACORPOREAL REDUCTION OF
BETA-AMYLOIDS AND PROCESS FOR PRODUCING THEREOF
FIELD OF THE INVENTION
The present invention generally relates to a composition intended
for reducing Beta-Amyloid levels in a subject. More particularly, the
present invention relates to use of a composition for the preparation of
a dialysis fluid formulation, intended for the method and system of
extracorporeal treatment, through a blood filtration process, of a Beta-
Amyloid associated pathological condition in the subject.
io BACKGROUND OF THE INVENTION
The hallmark of Alzheimer's disease (AD) is the presence in the
brain of senile plaques, which are primarily composed of a central
deposition of Beta-Amyloid peptides. Genetic, neuropathological and
biochemical evidences have shown that these deposits of Beta-Amyloid
is peptide play an important role in the pathogenesis of AD. The amyloid
cascade hypothesis for AD, as presented by Karran et al, postulates
that the deposition of Beta-Amyloid peptide in the brain is the central
event in the pathology of AD. This hypothesis has been very influential
in research works in the field of both academe and pharmacy, as the
20 same synthesizes histopathological and genetic information. It also
provides evidence that the deposition of Beta-Amyloid peptide in the
brain parenchyma initiates a sequence of events that ultimately lead to
AD dementia. Beta-Amyloid peptide refers to a 39-43 amino acid
peptide derived from the amyloid precursor protein (APP) by
25 proteolytic processing as specifically disclosed in U.S. Patent
Publication Nos. 2007/0092508 and 2006/0069010, the contents of
which are herein incorporated in their entirety. Both Beta-Amyloid 1-40
1
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
and Beta-Amyloid 1-42 SEQ ID NOS: 1 and 2, respectively, are
components of the deposits of amyloid fibrils found in the brain tissue
of AD patients. Beta-Amyloid 1-40 and Beta-Amyloid 1-42 aggregate
into insoluble beta-sheet structures which are considered neurotoxic
(such as, for example, ADDLs (Amyloid Beta-Derived Diffusible
Ligand)). Essentially, ADDLs are soluble oligomers of Beta-Annyloid
that accumulate and cause functional deficits prior to overt neuronal
cell death or plaque deposition. The process of beta-sheet formation is
initiated at Beta-Amyloid residues 16-20 (Beta-Amyloid 16-20 of
KLVFF, SEQ ID NOS: 3 and 4) which then nucleates conversion of the
entire Beta-Amyloid 1-40 and aggregation of these monomeric Beta-
Amyloid peptides into toxic fibrils and plagues has a rate-limiting
nucleation phase followed by rapid extension. Indeed, Beta-Amyloid 1-
42 is believed to play a more important role in the early nucleation
stage.
A Beta-Amyloid peptide sequence comprising residues KLVFF
(SEQ ID NO: 4, as mentioned above) is able to bind to the homologous
sequence in Beta-Amyloid 1-40 and Beta-Amyloid 1-42 peptides and
interferes with fibril formation in vitro and in vivo. KLVFF is found to be
the ideal binding site for Beta-Amyloids such that, potentially, there's
no organic/natural antibody that could be more efficient than a
mechanism that provides targeted capturing and binding action. More
specifically, association of the two homologous sequences leads to the
formation of an atypical anti-parallel beta-sheet structure stabilize
primarily by interaction between Lys, Leu, and Phe residues. This self-
recognition property of the KLVFF peptide and the retro-inverso version
thereof, FFVLK (SEQ ID NO: 5 of the previously cited U.S. Patent
Publications) has been employed in the design of subcutaneous
hydrogel "detoxification depots" or "sinks" for the capture of circulating
Beta-Amyloid peptides in vivo. See, e.g., Zhang et al., Bioconjugate
2
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
Chem., 2003, 14,86-92; Sundaram et at., c Alzheimer Res., 2008 Feb;
5(1) 26-32; US 2006/0069010; US 2007/0092508 the entire contents
of each of which are incorporated by reference herein as if fully set
forth herein.
Although the patterns of symptoms vary among subjects with
induced level of neurotoxic Beta-Amyloid peptides, the potential threat
of the accumulation of these neurotic peptides which in turn may result
in the development of several pathological diseases such as, for
example, Alzheimer's Disease (AD) justifies a need for suitable therapy
io
utilizing a composition, as described in the previously cited U.S. Patent
Publications, in the great majority of cases. Traditional
biopharmaceutical therapy, such as in the form of antibodies
administered in a systemic method, although effective during the early
phase of treatment, is known to become progressively less effective
and efficient if its administration is not consistent throughout a series
of treatment schedules. Generally, such anti-bodies come in large
molecular sizes. Unlike substances with low molecular weight which
can be administered in many different ways depending upon the
condition of patient and method of treatment required, the large-sized
molecules of most anti-bodies have a limited route for administration.
Although anti-bodies are specific to substrates, they are not
necessarily specific to targeted site because of the aforementioned
limited route for administration. In one instance, it has been known in
the art that engineered anti-bodies are too large to cross the blood
brain barrier which separates the circulating blood from the brain
extracellular fluid (BECF) in the central nervous system, serving as
physiological defense mechanism. Among other notable disadvantages
of using anti-bodies are their tendencies to provide undesired biological
response, cross-reactions with unrelated antigens, and economic
burden of patients who have to make commitments with expensive
3
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
therapeutic procedures over a long period. In view of these
disadvantages that are apparent in the art for utilizing anti-bodies, a
need for extracorporeal treatment for reducing Beta-Amyloid levels is
strongly desired.
Nobuya and Kazunori (2012), through their U.S. Patent
Publication No. 2012/0031840 which was filed on 08 July 2012,
discloses a method for reducing a Beta-Amyloid concentration in blood,
comprising the steps of; removing blood out of a body, passing the
blood that is removed through a hollow fiber membrane, and returning
the blood that is passed through into the body, wherein the blood
containing a 13-amyloid-albumin complex is passed through the hollow
fiber membrane to allow beta-amyloid to adsorb to the hollow fiber
membrane so that the beta-amyloid concentration in blood is reduced.
Nobuya and Kazunori do not disclose a composition having a capturing
and binding agent which mainly consists of KLVFF peptide sequence or
any variant thereof. In their disclosure, it is apparent that the
capturing agent used is a polymer selected from the group not
consisting of KLVFF or any variant thereof. It is worth noting that the
absorbent (itself serving as a Beta-Annyloid binding agent), as
disclosed by Nobuya and Kazunori, is introduced in the hollow fiber
membrane. After which, the blood is passed through the hollow fiber
membrane to remove Beta-Amyloids, and the blood is ultimately
returned back to the body. Generally, Nobuya and Kazunori disclose a
process that is laborious and hard to maintain. As one having ordinary
skill in the art would know, the degree to which a particular binding
action occurs in the membrane depends on a large extent and
significant considerations. As a result, a substantial amount of effort
has to be exerted to study and focus on the characteristics of the
membrane. The characteristics of the dialysis membrane, whether it is
porous or non-porous, hydrophobic or hydrophilic, polar or non-polar,
4
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
have to be studied carefully in order to achieve an optimum filtering
capacity.
Moreover, Bias et al (2007), through their U.S. Patent Publication
No. 20070010435 which was filed on 18 December 2003, teach a
method of treating an amyloid disease in a patient in need of such
treatment comprising filtering the blood of the patient through a filter,
membrane or column, thereby removing circulating beta-amyloid from
the patient. Bias et al teach a method that behaves substantially
similar to the process described by Nobuya and Kazunori. The
io similarities lie on the fact that both of the two prior art documents
utilize respective Beta-Amyloid binding agents that are introduced into
the membrane portion of a dialysis machine. Bias et al only claims a
compound for the binding agent that is selected from apolipoprotein E,
apolipoprotein 3, serum amyloid P component, a RNA aptamer directed
against beta-amyloid, al-antichymotrypsin, a proteoglycan, a
ganglioside, vimentin, vitronectin, albumin, transthyretin, amyloid-
beta-binding fragments thereof, and combinations thereof. Apparently,
the use of KLVFF as capturing and binding agent is not disclosed by
Bias et al. In view of the disclosure from Nobuya and Kazunori and
from Bias et al, there is still a need for a process that is configured to
remove Beta-Amyloids without having to rely mainly on the
performance of the semi-permeable membrane or, more specifically,
columns. As a matter of fact, Santoro and Guadagni (2009), in their
publication entitled, "Dialysis Membrane: from Convection to
Adsorption," specify that membrane performance is difficult to
evaluate, and different membranes can only be compared by
establishing adequate points of comparison. They further elaborate on
the count that the points of comparison themselves may change
depending on the type of co-morbidities of the specific subject or
patient who is considered for membrane selection.
5
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
Finally, Kitaguchi et at (2011), in their journal which was
published on 24 February 2011 and entitled "Reduction of Alzheimer's
Disease Amyloid-P in Plasma by Hemodialysis and Its Relation to
Cognitive Functions," reveal that dialyzers, being a primary component
of a hemodialysis process, effectively reduce Beta-Amyloids in whole
body circulation, and that repeated rapid decrease of plasma Beta-
Amyloids may maintain cognitive state. With more particularity,
Kitaguchi et al, in the discussion portion of their published journal,
identify at least three (3) possible mechanisms to achieve fairly high
removal efficacies of dialyzers, namely, filtration, adsorption, or
filtration and adsorption (a combination). The authors also emphasize
that their preliminary in vitro experiments indicate that the adsorption
may have a major contribution in Beta-Amyloid removal activity of the
dialyzer. Plasma filtration requires dialysate formulated at certain
conductivity value based on input variables such as plasma ultrafiltrate
conductivity for feeding on a computer program that is developed to
generate conductivity kinetic model. It bears stressing that Kitaguchi
et al do not provide description for the process associated with the
aforementioned filtration and adsorption. Nor do they disclose an
amyloid capturing and binding process associated with any amyloid
binding agent, if there is indeed any. Thus, a related disclosure by
Nalesso and Ronco (2006) is herein incorporated and quoted. Nalesso
and Ronco, in the book edited by Jean-Louis Vincent and entitled
"Intensive Care Medicine: Annual Update 2006," describe that plasma
filtration and adsorption are two processes which may be coupled
together to improve the effectiveness of extracorporeal purification.
They further describe that such plasma filtration and adsorption is
directed to the utilization of vehicle of pathological molecules. As such,
molecules in the whole blood circulation are transported by plasma
water if they are soluble or by carriers in the plasma (e.g., albumin or
other specific carriers) if they are insoluble. Therefore, the foregoing
6
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
observation indicates that the real vehicle of toxic molecules is none
other than plasma, and the best extracorporeal purification technique
could act directly on this plasma. By identifying the carriers and solute
composition of the plasma, the plasma itself can become a medium of
blood purification. This concept matured into the development of the
plasma filtration adsorption dialysis (PFAD). It is believed that
Kitaguchi et al have adapted the concept that the plasma is the only
carrier of all molecules, including those that are considered toxic. As
disclosed by Nalesso and Ronco, all molecules are transported either in
the plasma water or bound to albumin (or other protein carrier for that
matter). Albumin and other carriers are often present in the solution in
plasma water. Thus, transport of molecules depends on their
hydrophobic characteristics and their molecular weights. Molecules
with elevated solubility are transported in solution found in plasma
water; whereas, hydrophobic molecules are transported bound to
specific or non-specific carriers in plasma water. Hence, physio-
chemical equilibrium can be achieved among tissues and plasma
through the capillary endothelium. This results in the establishment of
the dynamic equilibrium in the plasma. Consequently, an
extracorporeal treatment acting directly on plasma is able to remove
toxic molecules from tissues operating on this dynamic equilibrium. As
a summary, the process described by Kitaguchi et al involves removal
of Beta-Amyloid through the use of ultra-filtrated water to instigate
capturing of Beta-Amyloid having low molecular weight. Hence, the
described process does not, in any way, involve targeting of specific
Beta-Amyloids.
By further analyzing the foregoing concept as explained in full
detail by Nalesso and Ronco, a person having ordinary skill in the art
can understand the potential of a subject's plasma to be used as
dialysate after purification into its component such as electrolyte
7
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
composition, add-base status, water, and protein carrier. According to
the authors, plasma does not contain cells so they can be used in
extracorporeal purification systems using non-biocompatible materials
such as some sorbents for specific molecules. To summarize, the
patient's own plasma plays a very important function in purifying blood
and, consequently, in removing all molecules with high molecular
weight or those with hydrophobic characteristics. Kitaguchi et al
specify that hydrophobicity may be a key factor of their disclosed
dialysis process. Indeed, the plasma is the medium through which all
molecules can be transported to the site of purification. Due to the
inherent capacity of plasma to bind and transport toxins, the
regenerated plasma from the patient can be used. Through the use of
regenerated, filtered plasma in a suitable compartment of a dialysis
machine, the dialysis procedure based on the processes of diffusion
and binding to remove certain toxins may be performed. However,
regeneration of plasma may take a longer while. Most notably, one of
the major disadvantages that can be observed in plasma regeneration
is the potential loss of vital physiological substances from the plasma,
and, in most cases, these substances have to be infused back into the
patient. This infusion without a doubt provides a more tedious,
complicated and expensive process, not to mention that the health risk
of the patient is also at stake. Through this process, there is also no
guarantee that the escape of trapped Beta-Amyloids back into the
blood plasma can be prevented.
In view of all the foregoing limitations of the prior art, it is clear
that there is still a need for a compact, inexpensive, and simple
standard dialysis machine-assisted process that extracorporeally
removes Beta-Amyloids without allowing escape of the Beta-Amyloids
back into a subject's body, without having to intricately evaluate the
8
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
performance and characteristics of a dialysis membrane, and without
putting the health condition of the subjects at risk.
SUMMARY OF THE INVENTION
In view of the foregoing limitations of the prior art, the present
invention primarily provides use of a composition that can be utilized
for treating a subject with pathological condition related to induced
levels of Beta-Amyloids in which this composition is used as part of a
dialysis process, characterized in that the composition specifically
targets and binds to Beta-Amyloids, that the binding reaction to Beta-
Amyloids of the composition occurs extracorporeally, that the
composition does not allow escape of captured Beta-Amyloids, and that
the composition is mixed with a dialysate for use in dialysis process
and, by means of which, the dialysis process results in highly reduced
Beta-Amyloid levels in the subject.
It is therefore the primary object of the present invention to
provide a composition which can be used to prepare a dialysis fluid or
dialysate formulation intended for extracorporeally treating, assisted
by a blood filtration process, a pathological condition associated with
induced level of Beta-Amyloids. In accordance with the present
invention, the composition mainly consists of a binding agent which is
designed and prepared to capture and bind target neurotoxic Beta-
Amyloids. The capturing agent, also serving as binding agent, is
directly introduced into the dialysis fluid or dialysate together with
other standard components of an ordinary dialysate required to
achieve' the equilibrium necessary for the dialysis procedure to take
place.
9
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
It is another object of the present invention to treat a subject
having a pathological condition related to induced level of Beta-
Amyloids and one or more of the symptoms of such pathological
condition as mentioned above by providing a composition comprising
the KLVFF peptide, or any variant thereof such as its reverse analog
FFVLK peptide, in an amount sufficient to capture the majority of
prevalent neurotoxic Beta-Amyloids.
It is yet another object of the present invention to provide a
capturing and binding agent that includes peptides consisting of an
amino acid sequence selected from the group consisting of: Lys-Leu-
Val-Phe-Phe-Cys; phe-phe-val-leu-lys-cys; [phe-
ph0-val-leu-lys-
13Ala]2-lys-cys; [phe-phe-val-leu-lys-PEG-lys-]3-cys; and [phe-phe-val-
leu-lys-PEG-lys-]3-cys. An amino acid sequence may include D-isomers
which may be prepared as a linear, branched, or cross-linked
polypeptide. KLVFF-related peptides could be monomers, dimers,
trimers or higher oligomers linked to one another in a linear or
branched form, such as, but not limited to the following table:
Structure of conjugate Copies of peptide
Lys-Leu-Val-Phe-Phe-Cys 1 (native)
phe-phe-val-leu-lys-cys 1 (retro-inverso)
[Phe-phe-val-leu-lys-f3Ala]2-lys-cys (branched) 2 (retro-inverso)
[Phe-Phe-val-leu-lys-13Ala]4-lys2-lys-cys
4 (retro-inverso)
(branched)
[phe-phe-val-leu-lys-PEG-lys-]3-cys (linear) 3 (retro-inverso)
Lower case is for D-amino acids. 13Ala is beta-alanine, C-terminus is
amidated, uncharged form, N-terminus is free, positive charged form,
PEG can be terminated by an amino group at one end and a
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
carboxylate group at the other end. In a preferred embodiment, the
cysteine residue is linked via its side chain thiol to the gel matrix.
It is yet another object of the present invention to use the
above-mentioned composition in preparing a dialysis fluid or dialysate
intended for further use in a standard or typical dialysis device. This
blood filtration device is configured to separate plasma constituents
from other cellular components of the blood. The blood filtration device
(or a dialyzer) includes three (3) primary components, namely, a blood
circuit side adapted to receive extracted blood from a subject, a
dialysate side adapted to receive the dialysis fluid formulation, and a
permeable membrane that separates the blood circuit side and the
dialysate side. In a straightforward sense, the composition has to be
introduced in the dialysate contained in the dialysate side of the blood
filtration device so the entire object of the invention can be achieved.
It is a further object of the present invention to provide a
process of producing a composition, wherein at least one capturing and
binding agent, as described above, or one of its pharmaceutically
acceptable derivative or analogue, is brought into dosage form
together with at least one solid, liquid, or semi-liquid carrier and/or
auxiliary substance. With regard to this specific aspect of the present
invention, the carrier includes polyethylene glycol polymer chains that
can be cross-linked to increase the total molecular weight of the
composition. The carrier provides a framework by which the avidity of
binding can be increased by linking together multiple copies of the
binding element.
It is a further object of the present invention to provide a system
for an extracorporeal system for reducing the level of targeted Beta-
Amyloid peptides in blood. The system primarily comprises a blood
11
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
filtration device and a composition which consists mainly of a capturing
and binding agent for capturing and binding targeted Beta-Amyloid
peptide. In a preferred embodiment of the present invention, the blood
filtration device is composed of a blood circuit side, a dialysate side,
and a semi-permeable membrane configured to separate plasma
constituents from other cellular components of the blood. In another
preferred embodiment, the composition is introduced directly into the
dialysate contained in the dialysate side.
It is a further object of the present invention to provide a
method for reducing Beta-Amyloid levels in the blood of a subject
requiring such treatment by extracorporeal circulation. The method
primarily comprises of at least four (4) steps. The second step is
extracting blood from the subject at a pre-determined flow rate
through a blood filtration device having mainly a dialysate side and a
blood circuit side wherein the blood is routed to the blood circuit side
only. The first step is introducing an effective amount of a composition
consisting mainly of a capturing and binding agent for Beta-Amyloid
peptides directly into the dialysis fluid contained in the dialysate side.
The third step is circulating the blood through the blood circuit side
'that is located along the periphery of the dialysate side, wherein the
circulation is facilitated by any standard dialysis process or, more
specifically, hemodialysis process. Finally, the fourth step is returning
the other cellular components and treated plasma constituents of the
blood back to the subject.
It is a further object of the present invention to provide a
package for treatment of patients suffering pathological conditions
associated with induced level of Beta-Arnyloids. The kit may comprise
a quantity of a carrier carrying the Beta-Amyloid capturing and binding
agent, in combination with a dialysate material for use in a dialysis
12
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
procedure. In one embodiment, the composition is combined with
sodium bicarbonate in the form of either powder or liquid. In another
embodiment, the composition is combined with dialysate acid mixture.
In another embodiment, the composition is introduced directly to the
dialysate.
Yet another object of the present invention is treating a subject
suffering from a pathological condition selected from a group
consisting of: Alzheimer's disease (AD), diabetes, Parkinson's Disease,
Huntington's Disease, cataracts, muscular dystrophy and Down's
Syndrome.
Further objects and accompanying advantages afforded by the
present invention will become apparent from the detailed descriptions
of the embodiments, as set forth hereunder; to wit:
BRIEF DESCRIPTION OF THE DRAWINGS
The appended drawings are presented to further describe the
present invention and to provide a clear understanding of various
embodiments of the present invention through illustrative examples.
These examples, being said as illustrative, should not be regarded as
limiting with respect to the scope of the present invention.
FIG. 1 shows an illustration of a partial sequence of APP770
wherein KLVFF, primarily serving as capturing and binding agent for
mixing with a dialysate solution, is underlined;
FIG. 1A shows a tetramer peptide of the capturing and binding
agent as described in FIG. 1;
13
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
FIG. 1B shows an illustration of the tetramer peptide of the
capturing and binding agent linked to an 8-arm polyethylene glycol
maleimide;
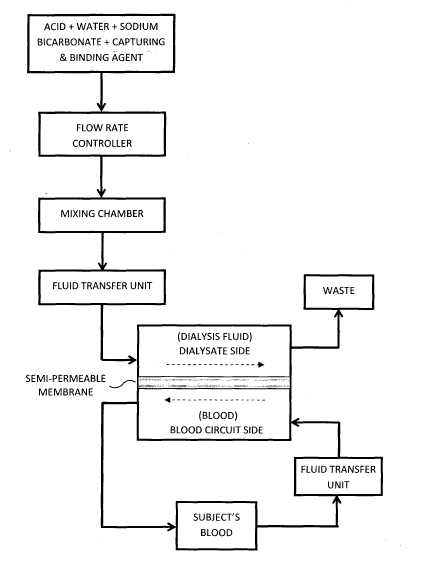
FIG. 2 shows a block diagram illustrating the use of a capturing
and binding agent, such as that illustrated in FIGS. 1A and 1B, in the
preparation of a dialysis fluid formulation for use in a dialysis process;
FIG. 3 shows a block diagram illustrating an extracorporeal
system for reducing the level of targeted Beta-Amyloid peptides in
blood; and
FIG. 4 shows a flowchart illustrating method of reducing Beta-
Amyloid level in the blood of a subject requiring such treatment by
extracorporeal blood circulation.
DETAILED DESCRIPTION OF THE INVENTION
The present invention primarily provides a use of a composition
for the preparation of a dialysis fluid formulation intended for the
extracorporeal treatment, through a blood filtration process, of a
pathological condition associated with the induction of Beta-Amyloid
level in a subject. The composition advantageously consists of a
capturing and binding agent for capturing and binding targeted Beta-
Amyloids, the use of which has been described in the cited prior art
documents to be clinically safe and effective in reducing Beta-Amyloid
levels in blood of subjects that are suffering from pathological
conditions related to induced level of Beta-Amyloids and one or more
of the clusters of symptoms that are characteristically exhibiting high
level of beta amyloids.
14
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
Referring to FIG. 1, there is shown an illustration of partial
sequence of APP770. The P-amyloid peptide, A131-42, (SEQ ID NO: 1)
is shown in bold italics. On the other hand, A131-40 (SEQ ID NO: 2)
would have IAT truncated from the C-terminus. Lastly, KLVFF (SEQ ID
NO. 4) is underlined. The KLVFF peptide, or any of its variants, is the
primary component of the claimed composition of the present
invention. This composition would be superior to attract, capture, and
bind neurotoxic Beta-Amyloid peptides that are often present in blood
or, more specifically, in the plasma component of the blood. The
characteristics of KLVFF peptide and its reverse analog form, FFVLK
peptides, have been described by Zhang et al in their prior
publications.
The KLVFF-related peptides could be monomers, dimers, trimers
or higher oligomers linked to one another in a linear or branched form,
such as, but not limited to the following Table:
Table 1.
Structure of conjugate Copies of peptide
Lys-Leu-Val-Phe-Phe-Cys 1 (native)
phe-phe-val-leu-lys-cys 1 (retro-inverso)
[phe-phe-val-leu-lys-f3Ala]2-lys- 2 (retro-inverso) 4 (retro-
cys (branched) inverso)
[phe-phe-val-leu-lys-PEG-lys-]3- 3 (retro-inverso)
cys (linear)
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
Lower case is for D-amino acids. 13Ala is beta-alanine, C-terminus
is amidated, uncharged form, N-terminus is free, positive charged
form, PEG can be terminated by an amino group at one end and a
carboxylate group at the other end. In a preferred embodiment, the
cysteine residue is linked via its side chain thiol to the carrier.
Referring to FIG. 1A, there is shown the composition of the
capturing and binding agent having a tetramer peptide containing four
copies of the monomer peptide in reverse sequence (retro-inverso),
ffv1k, as shown below:
y s -cys -amid e
NHAys--phe-phe-val-leu-lys-Pala-lys/
Nh12-lys--phe-phe-val-leu-lys-Palai
The above structure is preferably provided in a bicarbonate
powder which may be mixed with a standard dialysis fluid. A standard
dialysis fluid is further preferred to take the form of ultrapure
dialysate. An ultrapure type of dialysate is a combination of water and
other chemicals that wash waste out of the blood. The primary
advantage of using an ultrapure dialysis fluid is that there would be
less risk of blood pressure drops during the process of treatment. This
ensures that the circulation process is taking place consistently, and
there is a stable environment for the capturing and binding agent,
'KLVFF or FFVLK peptides, to capture and bind the Beta-Amyloids which
may be potentially present in the plasma component of the blood.
16
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
Referring to FIG. 1B, depicted is a the tetramer capturing and
binding agent that is linked, through its cys side chain, to an 8-arm
polyethylene glycol maleimide to form a molecule of the capturing and
binding agent with 32 Beta-Arnyloid capturing arms, as shown below:
8ARM-PEG-OH: 8-arm Polyethylene Glycol (trioentaerythritol core) MW: 40,000 Da
OH
PEG
0
02
HO ¨PE G¨ 0 -(CH2¨ C ¨CH2-0)¨PEG -OH
3
CH,
0
PEG
OH
Referring now to FIG. 2, there is shown a block diagram
illustrating the use of a binding agent, such as that illustrated in FIG.
1A and 1B, in the preparation of a dialysis fluid formulation. The
composition, which mainly consists of the binding agent as described in
FIG. 1, may further consist of an effective amount of each of the
following components: acid, water, and sodium bicarbonate. The
resulting composition may then be suitably mixed with a standard
dialysate solution in a mixing chamber. In order to ensure that a
suitable amount of each of the resulting composition and the dialysate
concentrate solution, individual flow rate controllers may then be used.
The resulting mixture now forms a dialysis fluid which may be
transferred from the mixing chamber to the dialysate side through a
fluid transfer unit with sufficient power to pump an amount of dialysis
fluid required in a preferred mode of operation of the present
invention. Adjacent the dialysate side is the blood circuit side. The
17
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
blood circuit side is configured to receive blood from another fluid
transfer unit which is designed to pump the blood from a subject at a
pre-determined flow rate. In between the dialysate side and the blood
circuit side is the permeable membrane. This membrane is
characterized by a porous material having pores of sufficient size to
permit neurotoxic Beta-Amyloid peptides to pass through it but of
certain size so as not to allow the passage of other cellular components
of the blood, most of which are generally important as the same is
required in maintaining the physiological state thereof. It is the
attracting and binding capacity of the capturing and binding agent that
plays an important role in the process of substantially eliminating any
amount of neurotoxic Beta-Amyloid peptides that may be present in
the blood freshly extracted from a patient. While the blood containing
neurotoxic Beta-Amyloids is passed through the blood circuit side in a
first uniform direction, the capturing and binding agent, being used in
the preparation of the dialysis fluid flowing inside the dialysate side in
a uniform direction opposite to the first direction, is capturing
substantially most of the Beta-Amyloids therein. The capturing and
binding agent attracts the Beta-Amyloids which pass through the
permeable membrane. The capturing and binding agent itself may be
formulated to become large enough so it may not pass through the
porous material of the permeable membrane. In this regard, only the
Beta-Amyloids having sizes (or molecular weights) that are smaller
than the porous material of the permeable membrane can pass
through it.
Referring now to FIG. 3, there is shown a block diagram
illustrating an extracorporeal system for reducing the level of targeted
Beta-Amyloid peptides in blood. The system primarily comprises of two
components, namely, a blood filtration device and the composition
mainly consisting of a capturing and binding agent, as described in
18
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
FIG. 1A & 1B. The blood filtration device is configured to separate
plasma constituents from other cellular components of the blood.
Furthermore, the blood filtration device includes a blood circuit side
adapted to receive the extracted blood, a dialysate side adapted to
receive a dialysis fluid, and a permeable membrane that separates the
blood circuit side and the dialysate side. On the other hand, the second
component, being the composition as previously mentioned and
described, consists mainly of a binding agent for targeted Beta-
Amyloid peptides. The composition is introduced directly into the
dialysis fluid contained in the dialysate side. The binding agent has a
binding capacity sufficient to capture and bind the neurotoxic Beta-
Amyloid peptides from the plasma 'constituent transfer from the blood
circuit side to the dialysate side. In a preferred embodiment, the
permeable membrane has pore sizes that are substantially larger than
the sizes of the Beta-Amyloid peptides (in oligomer form) thereby
enabling the Beta-Amyloid peptides to pass through the permeable
membrane while the binding agent attracts them for disposal. In the
entire blood filtration process, auxiliary components may be added in
the system to ensure reliability in operation. Some of the known
auxiliary components which are known in the art are pressure monitor
equipment and fluid transfer units which are capable or pumping
blood. In another preferred embodiment, air detector and air trap,
including clamps, may also be employed in the system so that,
substantially, no amount of air can get through the blood. As one
having ordinary skill in the art, even a very small amount of air that
penetrates into a blood circulation may cause air embolism or gas
embolism. This is a pathological condition that is primarily caused by
the presence of gas bubbles in a vascular system. Through the use of
air trap, the vascular system can still function desirably. Furthermore,
a fluid volume controller (not shown) may also be employed in the
system. This type of controller is commonly used to achieve fluid
19
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
balance which, in turn, affects the efficiency of the entire system of
providing the dialysis process, as preferably described above.
Generally speaking, dialysis machines are used in the treatment
of various diseases, such as kidney related diseases. When the kidneys
are functioning normally, they participate in the removal of toxic
substances from the body. However, when the kidneys are not
functioning properly because of diseases, it is often necessary to
remove toxins from the body by a technique known as dialysis. The
patient is connected to a dialysis machine so that either blood or
peritoneal fluid can flow from the patent, into the machine, and then
be returned back to the patient. During this standard process of
dialysis, the fluid comes in contact with a dialysis membrane. The
dialysis membrane is porous and allows low molecular weight
substances, including toxins, to pass through. Such machines, and the
use of such machines, are familiar to one having ordinary skill in the
art.
In one embodiment of the present invention, a dialysis machine
similar to that used in the removal of toxic substances from a patient
with kidney disease is used. Using a typical dialysis machine, the
inventive technique comprises the incorporation of a "capturing and
binding agent" into the dialysis buffer solution which is then used in
conjunction with a dialysis machine for treatment of Alzheimer's
Disease (AD) by Beta-Amyloid extraction therapy as contemplated
herein and, for example, according to the basic scientific principles
described in the cited prior art documents. In another preferred
embodiment, this capturing and binding agent is the KLVFF peptide.
As familiar to one of ordinary skill in the art, there are two flow
paths in a typical dialysis machine. One path provides a circuitous flow
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
of blood or peritoneal fluid along one face of the standard permeable
dialysis membrane. The circuitous path takes the blood or fluid from
the patient, through the machine and along the face of the membrane,
and finally back to the subject. The other path provides for contact
along the other face of the dialysis membrane by the dialysate or
buffer solution (along with, typically, an appropriate amount of water)
that will receive the toxins. Normally, the two paths run "counter-
current" to one another, such that the blood or fluid flows along the
membrane in a first direction and the dialysate or buffer solution flows
io along the other face of the membrane in a direction generally opposite
to the first direction. As contemplated herein, the dialysate or buffer
solution may comprise a capturing and binding agent. Although the
two solutions will be separated at all times by the dialysis membrane,
all substances below the cut-off molecular weight of the dialysis
membrane (e.g., the pore size of the permeable membrane) will be
able to transfer back and forth. In a standard dialysis machine, the
buffer solution may be continually replaced to increase the rate of
extraction of toxins. If a capturing and binding agent having a
particularly powerful binding strength is used however, it may not be
necessary to replace the buffer solution. As previously described, the
capturing and binding agent may contain polypeptide which may be
prepared to consist three or more amino acid sequences which are
linked together through a linker with one or more capturing arms.
Increasing the number of capturing arms of the linker increases the
total length thereof. The total length of the capturing arms is directly
proportional to the binding capacity of the capturing and binding agent
such that a longer chain of capturing arms yields a greater capacity of
the binding agent to capture and bind targeted Beta-Amyloids. In an
embodiment above, the present invention advantageously provides an
8-arm assembly to achieve optimum binding capacity.
21
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
The standard dialysis membrane may be one of a generally
synthetic based membrane or a traditional cellulose based dialysate
membrane. Moreover, high flux hemodialysis membranes have new
technologies that allow passage of larger size molecules through the
membrane through larger pores supported by diffusion and
convention. Alternatively, more recent nanotechnology versions are
becoming available. Nanotechnology is being used in some of the most
recent high-flux membranes to create a uniform pore size. The goal of
high-flux membranes is to pass relatively large molecules such as
beta-2 microglobulin (having an approximate molecular weight of
11,600 Daltons), but not to pass albumin (having an approximate
molecular weight of 66,400 Daltons).
A single Beta-Amyloid strand is has an approximate molecular
weight of 4,200 Daltons. However, we have identified that ADDLS,
which is neurotoxic form Beta-Amyloids most prevalent in the body
when there are plagues, are an aggregate of mostly 8 Beta-Amyloid
peptides combined to have a resulting molecular weight of 33,600
Daltons. Thus, it is preferable to use a membrane which can allow
passage to these larger molecules, such as membranes which may
allow molecules with molecular weight as high as 45,000 Daltons- to
50,000 Daltons to pass through them.
In one embodiment, the capturing and binding agent comprises
the peptide sequence of KLVFF or a variant thereof, e.g., a retro or a
reverse analog (see, e.g., Table 1 in Applicant's attached related
application). This peptide may be linked to a poly(ethylene glycol)
cross-linker/carrier gel to increase the total molecular weight of the
linked carrier gel and said peptide sequence, thereby preventing its
transport across the dialysis membrane. Such a capturing and binding
agent and carrier can successfully bind to Beta-Arnyloids, which may
22
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
thus remove Beta-Arnyloids from the blood or fluid passing through the
dialysis machine and along the membrane. About 10mg to about
100mg of the carrier gel should be used per treatment and would
suffice, though these quantities may vary, e.g., depending on various
parameters of each particular patient. The capturing and binding
agent, gel, and water combination may further comprise a standard
dialysate since a high-flux hemodialysis treatment requires electrolytes
and other elements to be put into the blood (the electrolytes and other
elements may pass through the membrane and into the blood or fluid,
as is commonly done in a typical dialysis treatment for pH balance and
other considerations and as understood by one of ordinary skill in the
art).
In a further embodiment, the capturing and binding agent may
be configured such that the capturing and binding agent itself cannot
pass through the pores of the selected membrane. However, a
molecular size of approximately 33,600 Da!tons is not necessarily
required to achieve this since the synthetic gel does not fold like the
Beta-Amyloid peptides (or ADDLS) found in the body. Thus, the
capturing and binding agent may only need to have a molecular weight
of approximately 15,000 Daltons to 20,000 Da!tons to prevent passage
through the pores of the membrane, since its hydrodynamic volume
makes its apparent molecular weight large.
Such an enhanced capturing and binding agent may added to
water or other conventional dialysis solution or buffer solution for use
in the dialysis machine for dialysis treatment of a subject or patient
suffering from AD or other pathological condition associated with
abnormal in vivo levels of beta amyloid peptides. Alternatively, the
carrier gel may still be used such that the enhanced capturing and
binding agent is linked to the carrier gel. The combination of the
23
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
capturing and binding agent and water (and optionally carrier gel) may
further comprise a standard dialysate since a high-flux hemodialysis
treatment requires electrolytes and other elements to be out into the
blood (the electrolytes and other elements may pass through the
membrane and into the blood or fluid, as is commonly done in a typical
dialysis treatment for pH balance and other useful and related
considerations).
In another embedment, the present invention may include a
modified version of a typical dialysis machine for the treatment of AD,
wherein a buffer solution is used with a dialysis machine, the buffer
solution comprising a capturing and binding agent as described herein.
The capturing and binding agent may be, for example, the KLVFF
peptide sequence. Such a capturing and binding agent may increase
the effectiveness of such a dialysis treatment to extract toxins found in
patients with Alzheimer's Disease (AD) having Beta-Amyloid peptides.
Such as system may be referred to as, for example, a Beta-Amyloid
detoxification flush-style therapy.
In yet another embodiment, the present invention may include a
system for treating a patient in need thereof, comprising a dialysis
machine which comprises a highly permeable membrane; and a
dialysate comprising at least a carrier gel and a capturing and binding
agent linked to the carrier gel. The dialysis machine may be any
hemodialysis machine as that known in the art. The permeable
membrane may be a synthetic membrane, including but not limited to
membranes suitable for high flux dialysis. The carrier gel may be a
poly(ethylene glycol) cross-linker/carrier gel, or a carrier protein as it
is known in the art, or any other carrier molecule or device designed to
retrieve the hydrodynamic volume or molecular weight of the trapping
reagent. The capturing and binding agent may be a KLVFF peptide or
24
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
a retro or a reverse analog or other variant thereof. Alternatively, the
capturing and binding agent may have a larger molecular size than the
KLVFF peptide. Further, such a capturing and binding agent may have
a molecular size equivalent to a molecular weight of approximately
15,000 Daltons to 20,000 Daltons. The dialysate may further comprise
water or other suitable buffer for dialysis. Such as a system may be
used to treat a subject in need thereof, e.g., a patient with Alzheimer's
Disease (AD) or any other Beta-Amyloid-related pathology wherein
extraction of Beta-Amyloid peptides from a subject is desired.
As contemplated herein, a subject in need thereof includes a
human suffering from a pathological condition associated with
abnormal in vivo levels of Beta-Amyloid peptides (beta amyloids). In a
particular embodiment, such pathological conditions are selected from
the group consisting of Alzheimer's Disease, diabetes, Parkinson's
Disease, Huntington's Disease, cataracts, muscular dystrophy, and
Down's Syndrome.
Referring now to FIG. 4, where is shown a flowchart illustrating a
method of reducing Beta-Amyloid levels in the blood of a subject
requiring such treatment by extracorporeal circulation. The method of
reducing Beta-Amyloid levels in the blood of a subject requiring such
treatment by extracorporeal circulation mainly comprises of four (4)
primary steps. The first step is extracting blood from the subject at a
pre-determined flowrate using a blood filtration device configured to
separate plasma constituents from other cellular components of the
blood. The blood filtration device includes a blood circuit side adapted
to receive the extracted blood, a dialysate side adapted to receive a
dialysis fluid, and a permeable membrane that separates the blood
circuit side and the dialysate side. The second step, moving forward, is
directed to the process of introducing an effective amount of a
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
composition consisting mainly of a capturing and binding agent for
Beta-Amyloid peptides directly into the dialysis fluid contained in the
dialysate side. The capturing and binding agent has a binding capacity
sufficient to attract and capture the neurotoxic Beta-Amyloid peptides
from the plasma constituents circulating in the blood circuit side. In
one preferred embodiment of the present invention, the permeable
membrane is a semi-permeable type that has pore sizes that are
substantially larger than the sizes of the Beta-Amyloid peptides, (i.e.
50,000 Daltons) thereby enabling the Beta-Amyloid peptides to pass
through the semi-permeable membrane. Following this step is the third
step, wherein the blood contained in the blood circuit side is being
circulated therein. The circulation takes a substantially uniform
direction or flow path. The blood circuit side, as it is known in art, may
be situated in along the periphery of the side for the dialysis fluid or
dialysate. This dialysate side is where the composition consisting of the
capturing and binding agent is being introduced. The capturing and
binding agent may then capture and bind the Beta-Amyloids, and as
well as dimers and oligomers thereof, which are often present in the
plasma composition of the blood. The circulation of the blood, through
a regular hemodialysis process, provides the separation of the plasma.
This enables the capturing and binding agent to capture and bind the
Beta-Amyloids which pass through the semi-permeable membrane.
Finally, the fourth step is returning the other cellular components and,
subsequently, treated plasma constituents of the blood without Beta-
Amyloids back to the subject. This step involves a path to the same
membrane. The blood goes back into the body of the subject is now
substantially free from Beta-Amyloids. The therapeutic effect of this
method would then to prevent any pathological condition which is
closely associated with abnormal level of Beta-Amyloids in vivo.
26
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387 PCT/PH2013/000005
In one preferred embodiment, the present invention relates to a
method of treating a subject suffering from a pathological condition
associated with abnormal in vivo levels of Beta-Amyloid peptide
comprising administering to said subject the dialysis detoxification
procedure of the present invention to extract Beta-Amyloid peptides
from the subject, and said extraction reduces levels of Beta-Amyloid
peptides in the subject. In a particular embodiment, the pathological
condition is selected from the group consisting of Alzheimer's Disease,
Down's Syndrome, diabetes, Parkinson's Disease, Huntington's
io Disease, cataracts, and muscular dystrophy.
In another aspect, the invention relates to a method of reducing
the likelihood of a subject developing a pathological condition
associated with abnormal levels of Beta-Amyloid peptides comprising
administering to said subject the dialysis detoxification procedure of
is the present invention for a time and in an amount sufficient to extract
Beta-Amyloid peptides from the subject and said procedure results in
reduced in vivo levels of Beta-Amyloid peptides in the subject.
The present invention also includes additional methods, aspects
and embodiments., for the treatment, diagnosis or monitoring of a
20 subject in need thereof which comprise lowering or monitoring in vivo
levels of Beta-Amyloid peptides such as provided in detail in
Applicant's attached related applications which are attached hereto and
incorporated by reference in its entirety herein.
Such a system and method, as both described above, may be
25 used on the blood of a patient in need thereof, and further may be
used as often as required, and may even be used repeatedly or
continuously to remove such toxins from the blood. Moreover, the high
27
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
efficiency of detoxification by the capturing and binding agent may also
result in fewer frequency of treatments required.
In a further embodiment, the present invention may include a
package for treatment of patients in need thereof, and the package
may comprise a quantity of a carrier gel comprising a capturing and
binding agent. The package may also comprise a dialysate solution or
a dialysis fluid.
Under treatment procedures as described above, the Beta-
Amyloids in the blood, along with its oligomer form (especially ADDLS),
are bound to capturing and binding agent-gel enhanced dialysate as
the Beta-Amyloids and ADDLS pass through the highly permeable
membrane, thereby cleansing the blood of these toxins as the blood
passes through the dialysis membrane.
Throughout this discussion, the term "dialysate" has been used
is synonymously with the terms "buffer solutions" and "dialysis fluid" to
define the fluid which attracts and captures toxins from the blood or
fluid as the blood or fluid pass through the dialysis machine.
28
SUBSTITUTE SHEET (RULE 26)
CA 02871585 2014-10-24
WO 2013/162387
PCT/PH2013/000005
ADVANTAGES
The present invention provides the following advantages:
(1) It provides a binding action that is specific to Beta-
Amyloids;
(2) It does not rely on the mechanical nature (e.g., filtration
using a membrane) of Beta-Amyloid removal; instead, it simply utilizes
a binding agent to capture Beta-amyloids from blood components;
(3) It
provides a process by which highest binding potential is
systematically created; and
(4) It provides a process which does not involve introducing of
foreign substances in the body thereby eliminating potential immune
system response which, in turn, may be translated into adverse risk
events.
20
29
SUBSTITUTE SHEET (RULE 26)