Note: Descriptions are shown in the official language in which they were submitted.
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
DEPOSITION OF ULTRA-THIN INORGANIC OXIDE COATINGS ON PACKAGING
BACKGROUND OF THE INVENTION
Technical Field
[0001] The present invention relates to an elemental layer on a packaging
substrate
and the method and apparatus for applying the elemental layer. More
specifically, the invention
disclosed herein pertains to an ultra-thin inorganic metal oxide layer that
serves as an oxygen and
water vapor barrier layer and/or to serve as an interface for future
functionalization when applied
to a packaging substrate. This layer can be formed during the manufacture of
the packaging
substrate or in later processing stages by use of known chemical vapor
deposition apparatus and
methods in a commercial packaging substrate manufacturing context.
Description of Related Art
[0002] Multi-layered packaging substrates made from petroleum-based products,
polymers, copolymers, bio-polymers and/or paper structures are often used
where there is a need
for advantageous barrier, sealant, and graphics-capability properties. Barrier
properties in one or
more layers comprising the packaging substrate are important in order to
protect the product
inside the package from light, oxygen and/or moisture. Such a need exists, for
example, for the
protection of foodstuffs that may run the risk of flavor loss, staling, or
spoilage if sufficient
barrier properties are not present to prevent transmission of light, oxygen,
or moisture into or out
of the package. A graphics capability may also be required so as to enable a
consumer to quickly
identify the product that he or she is seeking to purchase, which also allows
food product
manufacturers a way to label information such as the nutritional content of
the packaged food,
and present pricing information, such as bar codes, to be placed on the
product.
1
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0003] In the packaged food industry, protecting food from the effects of
moisture and
oxygen is important for many reasons, including health, safety, and consumer
acceptability (i.e.,
preserving product freshness and taste). Conventional methods to protect food
contents
incorporate specialized coatings or layers within or on a surface of the
packaging substrate,
which function as an impervious barrier to prevent the migration of light,
water, water vapor,
fluids and foreign matter. These coatings may consist of coextruded polymers
(e.g., ethyl vinyl
alcohol, polyvinyl alcohol, polyimides, polyamides (i.e. nylons and polyvinyl
acetate) and/or a
thin layer of metal or metal oxide, depending on the level of barrier
performance required to
preserve the quality of the product stored within the package volume.
[0004] Coatings produced by chemical vapor deposition are known to provide
certain
barrier characteristics to the coated substrate. For example, an organic
coating such as
amorphous carbon can inhibit the transmission of elements including water,
oxygen and carbon
dioxide. Accordingly, carbon coatings have been applied to substrates, for
example, polymeric
films, to improve the barrier characteristics exhibited by the substrate.
Another example of
coatings applied to substrates to improve barrier adhesion performance
includes coatings
comprised of inorganic materials such as inorganic metal oxides. Ethyl vinyl
alcohol and other
polymer skin layers are widely used to prime or improve the wettability of
film substrates for the
application of a barrier layer also referred to herein as "metallization
primer". Aluminum metal,
aluminum oxide, and silicon oxide are widely used for the application of
barrier layer(s) directly
to the substrates also referred to herein as "metallization".
[0005] The inorganic coatings described above may be deposited onto substrates
through various techniques as known in the art. Such techniques include
physical vapor
deposition (PVD) or chemical vapor deposition (CVD) processes. Examples of PVD
include ion
2
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
beam sputtering and thermal evaporation. Examples of CVD include glow
discharge, combustion
chemical vapor deposition (CCVD) and plasma enhanced chemical vapor deposition
(PECVD)
by generation of flame plasma or in strong electric fields.
[0006] The most commonly known and utilized method for depositing barrier
layers
on packaging substrates for metallization requires the use of a vacuum chamber
to provide the
vacuum environment for the deposition of inorganic atoms/ions on to the film
substrate surface.
This known technique, as used in the food packaging industry, consists of
processing packaging
substrate rolls that are from less than one to three meters wide and 500 to
150,000 meters in
length running at industry speeds of 60-600 meters/min and higher in a vacuum
metallization
chamber. This equipment is highly specialized, requires a great deal of
electrical power and
requires large capital expense. Current vacuum chamber processes for
metalizing films is
inefficient in many respects due to the high operational costs and limited
production capacity
associated with the use of such equipment. Moreover, higher quality film
substrates, requiring
additional capital expenditure, must typically be used to achieve the desired
barrier properties.
[0007] Combustion chemical vapor deposition (CCVD) and plasma enhanced
chemical vapor deposition (PECVD) apparatus and methods are known in the art,
as disclosed in
U.S. Patent Nos. 5,997,996 and 7,351,449, the disclosures of which are hereby
incorporated by
reference. Typically, a combustion flame or plasma field provides the
environment required for
the deposition of the desired coating (via the vapors and gases generated by
the combustion or
plasma) onto the substrate. The elemental precursors (e.g. organometallics)
may be vaporous or
dissolved in a solvent that may also act as a combustible fuel. The deposition
of organic and
inorganic oxides may then be carried out under standard and/or open
atmospheric pressures and
temperatures without the need of a vacuum chamber, furnace and/or pressure
chamber.
3
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0008] As described above, the application of barrier to food packaging is
required to
protect food and food products from the effects of moisture and oxygen. It is
well known in the
art that metalizing a petroleum-based polyolefin such as OPP or PET reduces
the moisture vapor
and oxygen transmission through specialty film by approximately three orders
of magnitude.
Conventional technology employs an inorganic layer of metal or ceramic on a
specialized
polymer film. The inorganic layer may be aluminum, silicon, zinc, or other
desired element in a
metal or oxide form. However, the surface of the substrate onto which the
barrier layer will be
applied is typically primed to increase its surface energy so as to be
receptive to the metal barrier
to be deposited thereon and/or to "smooth" the surface to be metalized so as
to reduce the surface
gauge variation or surface roughness of the film to be metalized. The term
"wettability" is
defined herein to include surface energy, metal adhesion bond strength, and
any other associated
characteristic that would increase the receptiveness of the film layer surface
for deposition of an
inorganic ultra-thin as disclosed herein.
[0009] For example, the utilization of aluminum metal as a barrier layer on
low energy
plastics, such as biaxially oriented polypropylene (BOPP) film, requires a
metallization primer to
reduce the gauge variation of the film substrate surface and/or to improve the
adhesion or bond
between the metal and film substrate. Various chemical methods are employed to
prime the
substrate surface layer for improving the substrate surface and/or bonding of
the metal barrier
layer to the film substrate. With polymer film substrates, one method to prime
the substrate for
metallization is to co-extrude a specialized polymer as a skin layer on the
substrate film. These
skin layers may comprise ethyl vinyl alcohol (EVOH), polyvinyl alcohol (PVOH),
and polyvinyl
acetate (PVA), ethyl vinyl acetate (EVA), polyethylene terephthalate glycol
(PETG), amorphous
polyethylene terephthalate (aPET), among other polymers used in the industry.
Unfortunately,
4
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
these materials are quite expensive and add additional cost to the manufacture
of metallization
ready films. Plastic film cores, such as oriented polypropylene (OPP),
polystyrene (PS), and
polyethylene terephthalate (PET) are typically treated with corona discharge
or flame treatment.
However, these treatments tend to create undesired, adverse impacts on film
substrate
characteristics such as the formation of pin holes, chemical degradation of
the surface through
cross linking or intra-molecular chain scission that can adversely affect
downstream
metallization and heat sealing processes.
[0010] As such, there exists a need for an improved apparatus and method for
depositing an ultra-thin inorganic oxide layer onto a packaging substrate to
prime a substrate for
metallization. Likewise, a need exists in the art for an improved apparatus
and method for
depositing multiple ultra-thin layers of an inorganic oxide layer on to a
packaging substrate to
enhance the barrier properties of a packaging substrate, which is less
expensive and more energy
efficient than tradition metallization while achieving and maintaining high
quality barrier
characteristics.
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
SUMMARY OF THE INVENTION
[0011] The inventive embodiments disclosed herein include a packaging
substrate with
an ultra-thin barrier layer and an apparatus and method for applying an ultra-
thin inorganic metal
oxide barrier layer to a film substrate. In one embodiment, the apparatus and
method disclosed
herein use the direct combustion of liquids, gases and/or vapors that contain
the chemical
precursors or reagents capable of producing inorganic oxides which are
deposited on to the
surface of a film substrate at open atmosphere. Chemical precursors, for
example tetraethyl
orthosilicate, tetramethyl disiloxane, silicon tetrachloride, silane,
trimethylaluminium,
triethylaluminium, methylaluminiumdichlorid-diethyletherate,
trimethylaluminium-
diethyletherate, ethylaluminiumdichlorid-diethyletherate, diethylaluminium-
dimethylamide,
aluminum trichloride, and other aluminum halides may be sprayed or atomized in
an oxidant and
combusted resulting in a vapor and/or gas that is directed on to the surface
of the substrate via
one or more flame heads for forming the desired coating or multiple coatings
thereon. Multiple
coating layers may be deposited onto the substrate by passing the substrate
through the system in
either a stand-alone or in-line manufacturing environment, or by passing the
substrate over
various one or more flame head configurations in either a stand-alone or in-
line manufacturing
environment as disclosed herein.
[0012] One embodiment of the present invention comprises a packaging substrate
surface with an inorganic metal oxide layer of less than 50 nm thickness that
is constructed by
depositing multiple ultra-thin layers of inorganic metal oxide on to a surface
of the packaging
substrate. In various embodiments, a preferred process that can accomplish
deposition of an
inorganic oxide layer onto the packaging substrate surface is CCVD or PECVD in
an open
atmosphere utilizing novel flame head assembly designs and orientations to
provide and adjust as
6
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
for various precursor concentrations and coating thicknesses that are
deposited on to the film
substrate.
[0013] In one embodiment of the invention, a method of coating a film
substrate with
at least one inorganic oxide layers comprises pretreating said substrate by
passing said substrate
through at least one flame treatment flame head assembly supplied with no
inorganic oxide
precursor, and after said pretreating step, depositing one or more inorganic
oxide layers on said
substrate by passing said substrate through one or more deposition flame heads
on at least one
deposition flame head assembly supplied with at least one inorganic oxide
precursor, wherein
said pretreating and depositing steps occur at open atmosphere.
[0014] In another embodiment, the at least one inorganic oxide precursor
comprises at
least one of tetraethyl orthosilicate, tetramethyl disiloxane, silicon
tetrachloride, silane,
trimethylaluminium, triethylaluminium, methylaluminiumdichlorid-
diethyletherate,
trimethylaluminium-diethyletherate, ethylaluminiumdichlorid-diethyletherate,
diethylaluminium-
dimethylamide, aluminum trichloride, and aluminum halides.
[0015] In one embodiment, the pretreating step comprising passing said
substrate over
a portion of at least one chill roll. In another embodiment, the pretreating
step comprises passing
said substrate over a portion of multiple chill rolls. The chill roll can
comprise a temperature of
40 C to 80 C.
[0016] In one embodiment, the depositing step comprises depositing multiple
inorganic oxide layers on said substrate by passing said substrate through two
or more deposition
flame heads in series. In another embodiment, the pretreating and depositing
steps occur as said
film substrate is unwound from one roll and wound onto a second roll. The
pretreating and
depositing steps may occur in-line during manufacturing of said film
substrate.
7
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0017] In one embodiment, the film substrate is cooled during said pretreating
step by
spraying cooling fluid on said film substrate.
[0018] In one embodiment of the invention, a system for coating a packaging
film
substrate with an inorganic oxide layer comprises at least one flame treatment
flame head
assembly supplied with no inorganic oxide precursor, one or more deposition
flame heads
supplied with at least one inorganic oxide precursor placed in series on at
least one deposition
flame head assembly, wherein said substrate passes through said flame
treatment flame head
assembly before said substrate passes through said deposition flame head
assembly, and wherein
said at least one flame treatment flame head assembly and said one or more
deposition flame
heads are at open atmosphere.
[0019] In another embodiment, the at least one flame treatment flame head
assembly
or said at least one deposition flame head assembly comprises multiple flame
head assemblies
oriented in parallel rows perpendicular to a substrate movement direction.
[0020] In one embodiment, the at least one flame treatment flame head assembly
or
said at least one deposition flame head assembly comprises a square or
rectangular shaped flame
head assembly. In another embodiment, the at least one flame treatment flame
head assembly or
said at least one deposition flame head assembly comprises multiple flame
heads assemblies
oriented in rows parallel to a substrate movement direction. In still another
embodiment, the at
least one flame treatment flame head assembly or said at least one deposition
flame head
assembly comprises a curved flame head assembly.
[0021] In one embodiment, the at least one flame treatment flame head assembly
or
said at least one deposition flame head assembly is oriented at an angle
relative to a surface of
8
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
said substrate. In another embodiment, the substrate passes through said flame
head assemblies
as it passes over a portion of said at least one chill roll.
[0022] In one embodiment, the inorganic precursors are fed into a flame fuel
line of
said deposition flame heads prior to being mixed with air from an air line and
combusted at said
flame heads, into an air line of said deposition flame heads prior to being
mixed with fuel from a
fuel line and combusted at said flame heads, into an air line and a fuel line
of said deposition
flame heads prior to being mixed and combusted at said flame heads, or mixed
with an air/fuel
mixture prior to being fed to said deposition flame heads. In another
embodiment, the inorganic
precursors is injected into a flame produced by said deposition flame heads.
[0023] The inventive embodiments described herein may be implemented in stand-
alone configurations, retrofitted to existing film production lines, or
installed into an in-line film
substrate manufacturing and/or processing system. The substrate material to be
coated does not
need to be heated or treated in a furnace or reaction chamber, or placed under
vacuum or non-
standard atmospheric conditions to effect coating deposition. The heat of
combustion provides
the needed conditions for the reaction of the chemical precursors. The
substrate material being
coated is likewise heated by the combustion flame, which creates and/or
enhances the kinetic
environment for surface reactions, wettability, diffusion, film (coating)
nucleation and film
(coating) growth. The chemical precursors utilized need to be properly
reactive to form the
desired coating. While inorganic metal oxides are the preferred material for
the coating applied
to the packaging substrate, other elemental coatings and compounds, for
example metals,
nitrides, carbides, and carbonates may also be used as desired.
[0024] Other aspects, embodiments and features of the invention will become
apparent
from the following detailed description of the invention when considered in
conjunction with the
9
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
accompanying figures. The accompanying figures are schematic and are not
intended to be
drawn to scale. For purposes of clarity, not every component is labeled in
every figure, nor is
every component of each embodiment of the invention shown where illustration
is not necessary
to allow those of ordinary skill in the art to understand the invention. All
patent applications and
patents incorporated herein by reference are incorporated by reference in
their entirety. In case
of conflict, the present specification, including definitions, will control.
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
BRIEF DESCRIPTION OF THE FIGURES
[0025] The novel features believed characteristic of the invention are set
forth in the
appended claims. The invention itself, however, as well as a preferred mode of
use, further
objectives and advantages thereof, will be best understood by reference to the
following detailed
description of illustrative embodiments when read in conjunction with the
accompanying figures,
wherein:
[0026] Figure 1 depicts a cross-section view of a typical prior art food
packaging film
substrate;
[0027] Figures 2A-21 depict various embodiments of the apparatus and method
employed in the present invention disclosed herein;
[0028] Figures 3A-3E are depictions of the apparatus and method as integrated
into
in-line packaging substrate production and manufacturing equipment according
to one
embodiment of the invention disclosed herein;
[0029] Figure 4 is a cross-sectional depiction of a film substrate with
multiple coating
nanolayers according to one embodiment of the invention disclosed herein; and,
[0030] Figures 5A-51 are depictions of various apparatus embodiments which may
be
employed in the present invention disclosed herein.
[0031] Figure 6 is a graph showing, for a single deposition pass of silica,
the amount
of silica deposited as determined by signal strength via information collected
by XPS.
[0032] Figure 7 is a graph showing a signal strength (CPS) vs. binding energy
(eV)
from XPS for multiple passes; and
[0033] Figure 8 is a graph showing the atomic percentage of silicon atoms on
the film
surface, WVTR, and OTR values plotted versus the number of silica deposition
passes.
11
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
DETAILED DESCRIPTION
[0034] Figure 1 depicts a schematic cross-section of a typical, currently used
food
packaging multi-layer or composite film substrate 10. Film 10 is constructed
of various
intermediate layers that act in concert to provide the film 10 with the
required performance
characteristics. For example, a graphics layer 14 allows a graphic to be
printed or otherwise
disposed thereon and is protected by transparent exterior base layer 12 which
may consist of
oriented polypropylene (OPP) or polyethylene terephthalate (PET). A glue or
laminate layer 16,
which is typically a polyethylene extrusion, acts to bind the exterior base
layer 12 with the inner,
product-side base layer 18. A metal layer may be disposed upon inner base
layer 18 by means of
metallization known in the art. Sealant layer 20 is disposed upon the OPP or
PET interior base
layer 18 to enable a hermetic seal to be formed at a temperature lower than
the melt temperature
of the interior base layer 18. Each layer described is formed as a roll of
film that is then unwound
and laminated together to form the composite film. Each film being laminated
together forms
the composite films, which are film structures composed of multiple layers
when originally
extruded or fabricated.
[0035] Alternative materials used in the construction of packaging film
substrates may
include polyesters, polyolefin extrusions, cellulosic polymers, acetate
polymers, adhesive
laminates, bio-films such as polylactic acid (PLA) films and polyhydroxy-
alkanoate (PHA)
films, produced in various combinations resulting in composite, multi-layered
film structures.
The film substrate may be formed by typical coextrusion, lamination, or
extrusion coating
techniques as known in the art. The film substrate can also be composed of
polyimide, liquid
crystal, polyethylene, or other materials normally used in electronic, optic
or specialty packaging
or multilayer applications.
12
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0036] In both PECVD and CCVD processes described herein, the localized
environment required for coating deposition to occur is provided by the flame,
plasma or other
energy means. With CCVD and PECVD no furnace, auxiliary heating, or reaction
chamber is
necessary for the reaction to occur. Further, both CCVD and PECVD can be
carried out in open-
atmosphere conditions. The plasma or flame supplies the energy needed for
coating deposition
in the forms of the kinetic energy of the species present and radiation. This
energy creates the
appropriate thermal environment to form reactive species and coincidentally
heats the substrate,
thus providing the kinetic conditions for surface reactions, diffusion,
nucleation, and growth to
occur. When using combustible solutions, the solvent plays two primary roles.
First, the solvent
conveys the coating reagents into the vicinity of the substrate where coating
deposition occurs,
thereby allowing the use of low cost soluble precursors. Uniform feed rates of
any reagent
stoichiometry can be produced easily by simply varying the reagents'
concentrations in solution
and the solution flow rate. Second, the combustion of the solvent produces the
flame required
for CCVD and PECVD.
[0037] In general, the deposition processes described herein are performed
under
ambient conditions in the open atmosphere to produce an inorganic film on a
substrate. The film
preferably is amorphous, but may be crystalline, depending on the reagent and
deposition
conditions. The reagent, or chemically reactive compound, is dissolved or
carried in a solvent,
typically a liquid organic solvent, such as an alkene, alkide or alcohol. The
resulting solution is
sprayed from a nozzle using oxygen-enriched air as the propellant gas and
ignited. A substrate is
positioned at or near the flame's end. Flame blow-off may be prevented by use
of a hot element
such as a small pilot light. The reactants are combusted in the flame and the
ions or radicals
generated from the combustion are deposited on the substrate as a coating. For
the present
13
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
invention, the formation and rate of deposition of the inorganic oxide
layer(s) are important to
the quality of the coating produced and the invention disclosed herein
describes in various
embodiments and examples of the equipment and processes for producing such
quality coatings.
[0038] The methods and apparatus utilized to perform the inventive methods
disclosed
herein provide a less-energy intensive and more efficient method for the
surface treatment of
film substrates for a variety of applications. For example, priming a
substrate for metallization is
usually required to enhance the wettability of the substrate surface for the
reception of a
metalized layer. As previously discussed, prior art methods of priming a
substrate for
metallization typically require the addition of a skin layer via coextrusion
or solution coating of
chemical additives such as EVOH and/or treatment by flame or Corona discharge
prior to
metallization. The apparatus and methods herein provide a novel method by
which the surface
energy of the film substrate is raised typically between 1 and 10 dynes by the
addition of the
inorganic primer nanolayer, thereby enhancing the wettability of the substrate
surface and thus
improving the adhesion between the deposited metal barrier coating and the
substrate.
[0039] It is also important for the inorganic oxide layer(s) to enable future
vapor
deposition of barrier, printing or adhesive layers applied to the film
substrate to adhere well and
for hot seal processes to still function as desired. An integral aspect of the
invention includes
application of the inorganic oxide layer to the film substrate so as to
improve the surface
wettability of the substrate surface for future applications.
[0040] By using different inorganic materials, additional properties can be
created to
enhance the use of the film for various applications. For example, use of
silver can provide
antimicrobial/disinfection properties. In other embodiments, ultraviolet
radiation blocking
inorganics, including zinc oxides and tin oxides may be utilized to form a
clear ultra-violet light
14
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
and gas barrier layer. Other transparent materials, for example silica oxide,
may be used to form
and/or act as ultra-thin barrier layer(s).
[0041] A key economic feature in using polymer-based products is maintaining
low
cost. As a result, the inorganic materials used as nanolayer coatings are
typically selected from
low cost inorganic elements. Also, the health aspect of the materials used in
the formation of
films for packaging is very important since the polymer films are used most
often in consumer
products including food and medical packaging. Thus, health safe materials,
for example silica-
based inorganics, may be utilized in various embodiments. Silica is the most
common oxide of
the earth's crust and soil and long-term storage in glass containers has
extensive proven history
as a safe and effective storage medium with regard to human health
requirements.
[0042] The use of current surface modifying materials in film production
represents a
significant volume and weight fraction of the end product thus reducing its
recyclability. The
present invention greatly reduces the material required to form the desired
barrier thickness,
resulting in a more recyclable and/or compostable product. In one an
embodiment, the inorganic
oxide layer is generally less than lOnm thick and more preferably less than
5nm average
thickness. Due to the small thickness of such a layer, the inorganic oxide
layer more readily
breaks into smaller pieces resulting in a higher grade of recyclable material.
In fact, silica is
often used as an enhancement additive to polymers to improve strength and
durability. One
embodiment of the invention includes an inorganic oxide layer that alters the
bulk physical
properties of film base polymer, as compared to reprocessing of neat polymer,
by less than 1%.
[0043] For biodegradable polymers, such as PLA and PHA, a barrier layer
applied to a
film substrate incorporating PLA and/or PHA or other bio-polymer may in fact
detract from the
desired degradability of the packaging material resulting therefrom. Such a
barrier layer reduces
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
the transmission of moisture or oxygen that can affect the degradation process
of the film
package. Multiple layers of barrier can result in a package that does not
degrade due to the core
film substrate material (barrier on both sides) never being exposed to the
proper environment for
decomposition. An embodiment of the present invention includes forming an
inorganic oxide
coating that alone does not provide an impervious barrier, but enables
subsequent printing,
adhesion layers, or quality barrier layer(s) to be deposited upon the
inorganic oxide coating in an
online manufacturing context or a secondary downstream facility. In one
embodiment, the
inorganic oxide layer can be deposited on both sides of the packaging
substrate for a variety of
contemplated end uses.
[0044] One of the key uses of the smooth inorganic ultra-thin layer is
subsequent
barrier layer formation thereon. Thin film metallization or oxide barrier
layers adhere to and
perform better on smooth surfaces with low defects. Polymer films readily form
such surfaces
during manufacturing, but the addition of anti-block agents as currently used
in the industry
cause an increase in the film's surface roughness and defects, with RMS
generally greater than
100nm. A key aspect of the present invention results in packaging substrates
with surface
roughness than 30nm RMS, and more preferably less than lOnm RMS, and in some
cases less
than 5nm RMS.
[0045] In another embodiment, the invention disclosed herein produces the
ability to
maintain low surface RMS values while controlling the surface wetting
properties. The surface
tension can be controlled by a combination of the inorganic ultra-thin layer's
surface roughness
and also the termination material on the surface. To improve the adhesion of
inorganic barrier
layer materials to the substrate, it is desired that a surface of the
substrate be receptive to metal or
inorganic oxide ionic or covalent bonding. Inorganic oxide surfaces provide
excellent bonding
16
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
sites for both metal and oxide layers, along with a smooth surface coating. It
has been
discovered that surface smoothness enhances the formation of barrier layer(s)
on the substrate.
For barrier deposition applications, it is preferred that the substrate
surface to be coated has a
smooth, low texture surface on both the nanometer and micrometer scale.
[0046] One key to successful application of such interface layers is to form
and apply
the primer and barrier layers to the substrate prior to winding or rolling of
the film. Films are
made by a number of processes including cast and blown films. These processes
are typically
performed at ambient atmosphere and pressure on large production lines. The
introduction of
prior art vacuum deposition equipment into such a line makes such processes
economically
impractical. Thus, a method for forming films online with an inorganic ultra-
thin layer at
ambient pressure on low temperature polymers is a better pathway to accomplish
such an
inventive ultra-thin layer. Aspects of how to do this with a process such as
CCVD are disclosed
in U.S. Patent No.5,652,021 (Hunt et al.) and U.S. Patent No. 5,863,604 (Hunt
et al.), the
disclosures of which are incorporated herein by reference.
[0047] In order to form an effective barrier layer in subsequent processing
operations,
it is important for the film substrate surface to be smooth. Thin film barrier
requires a smooth
substrate surface without features that can shadow or inhibit the thin film
material from being
deposited onto the vast majority of the entire surface. It is preferred that
at least 90% of the
substrate surface be coated and even more preferred that over 99% be
accessible to vapor
deposition material without surface roughness that can cause shadowing or thin
film defects. It
is also important that the inorganic primer layer is very smooth so that it
will not impact the
dense uniform continuous growth of additional inorganic oxide layer(s)
deposited thereon to
build an effective thin film barrier layer. Columnar growth on the inorganic
primer layer will
17
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
have a negative impact on the subsequent growth of a vacuum deposited or other
thin film barrier
layer applied thereto. The end effect is that a subsequent barrier layer can
be grown to yield a
Oxygen Transmission Rate (OTR) of less than 10 cc/m2/day @ 23 C and 0% RH and
a Water
Vapor Transmission Rate (WVTR) of less than 2 g/m2/day @ 38 C and 90% RH ,
more
preferably OTR <2 cc/m2/day @ 23 C and 0% RH and WVTR <1 g/m2/day @ 38 C and
90%
RH, and even more preferably OTR <1 cc/m2/day @ 23 C and 0% RH and WVTR <0.2
g/m2/day @ 38 C and 90% RH on substrates where an inorganic primer layer is
deposited prior
to the barrier layer. In one embodiment, the primer and/or barrier layer is
transparent to light in
the visible spectrum. In alternative embodiments, the subsequent primer and/or
barrier layers
may be translucent or opaque as appropriate for effective utilization of the
coated substrate for
flexible packaging or other contemplated end uses.
[0048] The current invention also has minimal environmental impact and yields
a safer
packaging material as a result of the reduction in the number of organic
chemicals blended into
the polymer film substrate. Such additives can cause health concerns or can
reduce the quality of
recyclable material. Silica, alumina, and the other elements of the present
invention are common
in the earth's crust, are often used as food additives, and have been used
safely in glass
containers for many years. As a result, the invention disclosed herein
utilizes plentiful and non-
toxic, safe inorganic materials with essentially no detrimental environmental
impact.
[0049] Multilayer packaging substrates may be produced with excellent bonding
characteristics provided by application of one or more ultra-thin inorganic
oxide layers as
described herein. In various embodiments, moisture, oxygen and light can pass
through the
inorganic oxide layer(s) so that compostable polymer film structures can still
be decomposed
under typical environmental conditions. The inorganic oxide coating with
proper selection of
18
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
metalloid or metal element, such as silicon or aluminum, creates a thin
coating that will not
inhibit composting of the film substrate and has absolute minimal impact on
the environment.
[0050] In one embodiment disclosed herein, a PECVD or CCVD apparatus is used
to
deposit one or more ultra-thin layers of silica oxide (Si0x) and/or other
inorganic oxides on the
surface of the substrate in an open atmosphere environment thereby increasing
the substrate
surface energy and improving the adhesion of the metal barrier layer with the
substrate,
effectively "priming" the substrate for metallization. In one embodiment
disclosed herein, a
PECVD or CCVD apparatus is integrated "in-line" with a packaging substrate
manufacturing
line there for priming the substrate for metallization before being wound into
a roll.
[0051] Various embodiments of the present invention disclosed herein also
comprise
apparatus and methods for applying a barrier layer to the surface of a
packaging substrate at open
atmosphere. The apparatus and method disclosed herein provide for the direct
combustion of
liquids and/or vapors that contain the chemical precursors or reagents to be
deposited on to the
surface of a substrate material at open atmosphere. Metal oxides, for example,
aluminum oxides,
are formed from the combustion of materials, such as organo-aluminum compounds
with an
oxidant, and combusted resulting in a vapor and/or gas at open atmosphere that
is directed on to
the surface of the substrate and resulting in the deposition of the desired
coating thereon.
[0052] In accordance with an embodiment of the invention disclosed herein,
Figure
2A depicts a flame CCVD apparatus that is supplied with combustible chemical
precursors for
the deposition of an inorganic oxide coating on to a substrate. The system
operates to break the
chemical precursors into micron and sub-micron sized droplets in the
combustion zone for the
application of the ultra-thin coating process disclosed herein.
19
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0053] Turning to Figure 2A, a general schematic of the apparatus 40 that is
utilized
to carry out the coating deposition process is shown. Chemical precursors 42
may comprise a
solvent-reagent solution of flammable or non-flammable solvents mixed with
liquid, vaporous,
or gaseous reagents supplied to flame head assembly 44 or other flame-
producing device. The
term "flame head assembly" is used to refer generally to describe any
apparatus that is capable of
producing a flame from a fuel feed, including flame treaters, flame burners
and flame head
devices as described herein and which are commercially available from various
manufacturers.
Chemical precursors 42 are ignited in the presence of an oxidant 46 resulting
in a flame 48. As
the chemical precursors 42 solution or mixture burn, the reagent reacts to
form an inorganic
vapor and leaves the flame 48 along with other hot gases 50 and combustion
products. The
substrate 52 to be coated is located proximal to flame 48 within the region of
gases 50. In one
embodiment, substrate 52 is oriented tangentially to the flame 48, or as shown
in Figure 2B
substrate 52 is oriented obliquely to the flame 48, or at any angle facing the
flame end 54 of
flame 48 such that the hot gases 50 containing the reagent vapor will contact
the substrate
surface 56 to be coated. In various embodiments, substrate 52 may consist of a
film or composite
film comprising oriented polypropylene (OPP), polyethylene (PE), polylactic
acid (PLA),
polyhydroxy-alkanoate (PHA), polyethylene terephthalate (PETP), other
polyesters, or other
known polymer, biopolymer, paper or other cellulosic substrates, alone or in
combination, as
known in the art.
[0054] Figure 2B is similar to the apparatus 40 shown in Figure 2A, but is
configured
for a non-turbulent flame methodology, suitable for chemical precursors
comprising gaseous
precursors 42 and non-flammable carrier solutions 46. Flame 48 produced by the
flame head
assembly 44a typically has the flame characteristics of an inner flame 48a
defining the reducing
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
region where the majority of oxidizing gas supplied with the reagent burns and
an outer flame
48b defining the oxidizing region where the excess fuel oxidizes with any
oxidizing gas in the
atmosphere. In this example embodiment, the substrate is positioned at an
oblique angle
proximate to the flame end 54 of the flame 48 such that the hot gases and/or
vapors 50
containing the reagent vapor will contact the substrate surface 56 of
substrate 52.
[0055] Referring back to Figure 2A, the precursor mixture 46 is supplied to
the flame
head assembly 44. Oxidant 46 is also supplied to the flame head assembly 44 in
some fashion,
via a separate feed, or is present in the process atmosphere, or the oxidant
may be supplied by a
separate feed to the process atmosphere or flame ignition point, or the
oxidant may be present in
the reagent mixture. In the depicted embodiment, the chemical precursor
solution 42 is ignited in
the presence of oxidant 46 and combust in flame 48 resulting in the generation
of heat, gases
and/or vapors 50. The generation of heat causes any liquid reagent solutions
present to vaporize
and increase the temperature of the substrate 52 so as to result in improved
surface diffusion of
the coating resulting in a more uniform coating deposited onto the substrate
surface 56.
[0056] In performing CCVD or PECVD coating deposition on film substrates,
certain
deposition conditions are preferred. First, the substrate needs to be located
in a zone such that it
is heated by the flame's radiant energy and the hot gases produced by the
flame sufficiently to
allow surface diffusion. This temperature zone is present from about the
middle of the flame to
some distance beyond the flame's end. The temperature of the flame can be
controlled to some
extent by varying the oxidant-to-fuel ratio as well as by adding non-reactive
gases to the feed gas
or non-combustible miscible liquids to the feed solution. Secondly, the metal-
based precursors
need to be vaporized and chemically changed into the desired state. For
oxides, this will occur in
the flame if sufficient oxygen is present. The high temperatures, radiant
energy (infrared,
21
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
ultraviolet and other radiant energy), and plasma of the flame also aid in the
reactivity of
precursors. Finally, for single crystal films, the material being deposited
should be in the vapor
phase, with little or no stable particle deposition. Particle formation can be
suppressed by
maintaining a low concentration of solutes, and by minimizing the distance,
and therefore time,
between locations where the reagents react and where the substrate is
positioned. Combining
these different factors predicts the best deposition zone to be located in
proximity of the flame's
tip. If a solution is sprayed, droplets can strike a substrate located too far
into the flame
proximity, possibly resulting in some spray pyrolysis characteristics in the
resulting film. In fact,
in some configurations, with large droplets or with some reactants, it may be
impossible to not
have some spray pyrolysis occur.
[0057] In various embodiments of the invention disclosed herein, a plasma
torch may
also be used in a manner similar to a flame apparatus to achieve similar
results. Chemical
precursors are sprayed through a plasma torch and deposited on to the
substrate. The reagents
and other matter fed through the plasma torch are heated and, in turn, heat
the substrate surface,
much in the same manner by the flame embodiment described herein. In plasma
enhanced
chemical vapor deposition, lower plasma temperatures may be used as compared
to conventional
plasma spraying, as lower heat is required to cause the chemical precursors to
react. As a result,
the chemical precursor reactions occur at lower temperatures thereby allowing
substrates with
low melt points to take advantage of PECVD. The deposition of the coating on
to the substrate
results from directing of the plasma gas vapor containing the charged ions in
the direction of the
substrate. For example, a chemical precursor gas mixture or solution is fed
into a plasma flame
resulting in the formation of a chemical vapor. The chemical precursor
solution may comprise
inorganic metal oxides such as aluminum oxide or silicon oxide. Once oxidized,
the resulting
22
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
ions in substantially vapor form are directed onto the surface of the
substrate resulting in the
formation of a solid coating formed on the surface of the substrate and which
are typically
formed with thicknesses in the 1 to 50 nanometer range.
[0058] In general, as long as a flame is produced, CCVD or PECVD can occur
independently of the flame temperature or substrate surface temperature. The
flame temperature
is dependent on the type and quantity of reagent, solvent, fuel and oxidant
used, and the substrate
shape and material, and can be determined by one skilled in the art when
presented with the
particular reagent, solvent, fuel, oxidant and other components and conditions
for deposition.
The preferred flame temperature near the deposition surface on a moving web
line is between
about 800 C and 1300 C. As flames may exist over a wide pressure range, CCVD
can be
accomplished at a pressure from about 10 torr to about thousands of ton, but
it is preferred to be
at ambient pressure to ease its use on the polymer film processing line.
Likewise, if plasma is
formed for depositing the coating, the temperature of the plasma can range
from about 400 C to
about 1200 C. The temperature of the substrate during the CCVD process also
can vary
depending on the type of coating desired, the substrate material, and the
flame characteristics.
Generally, a substrate surface temperature of between about 40 C and 80 C is
preferred for
temperature sensitive polymer films.
[0059] The deposition rate of the coating onto the substrate can vary widely
depending
on, among other factors, the coating quality, the coating thickness, the
reagent, the substrate
material and the flame characteristics. For example, increasing the exposure
period of the film
substrate to the vapor stream emanating from a flame head can result in
thicker coatings
deposited on the film substrate, assuming a relatively constant precursor feed
flow rate to the
flame generated at the flame nozzle. Less porous coatings are possible
assuming a relatively
23
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
lower feed flow rate to the flame generated at the flame nozzle or more porous
coatings
assuming a relatively greater feed flow rate to the flame generated at the
flame nozzle. Likewise,
if a higher quality coating is desired, a longer exposure time at a lower
precursor feed flow rate
may be necessary, while a gross or textured coating can be produced relatively
quickly using a
greater precursor feed flow rate. One skilled in the art can determine the
precursor feed flow
rates and exposure periods necessary to produce a desired coating on the film
substrate. Typical
deposition rates on product made using the apparatus and methods disclosed
herein range from
about 10 nm/min to about 1000 nm/min with the film surface being normally
exposed to the
flame for 0.1 to 10 seconds. As discussed above, the chemical precursor
solution in one
embodiment is a liquid reagent dissolved in a liquid solvent. However, solid,
liquid, vaporous
and gaseous reagents can be used, with a liquid or gaseous solvent, as long as
the chemical
precursor feed to the flame is typically liquid or gaseous in nature.
[0060] Referring to Figure 2C, one embodiment of the invention disclosed
herein is
shown wherein a flame redirect source is shown. The flame redirect technique
employs an air
knife 49 situated at an angle to the flame 48 to redirect the gases and/or
vapors 50 from the
process. The air knife 49 directs an air stream into the vapor stream 50
coming from the flame
48. This effectively redirects the vapor stream 50 in the desired direction of
the substrate surface
56 while at the same time deflecting the heat stream associated with flame 48
from overheating
or melting the substrate 52 being coated with the vapor 50. This method
results in the dissipation
of heat directed on to the substrate 52 from the flame 48 heat stream thereby
resulting in the
deposition of desired coating on to the substrate surface 56 at lower
temperatures.
[0061] The redirect flame embodiment also acts to disperse the gas and/or
vapor
stream 50 emanating from the flame 48 resulting in a wider deposition stream
50 being directed
24
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
on to the substrate surface 56 and enlarging the coating area of same. In an
alternative
embodiment, an electromagnetic or "electro-redirect" method may be employed to
redirect the
deposition of ions and/or particles emanating from a flame and/or plasma
source on to the
substrate surface. In this embodiment, the flame and/or plasma source
initially directs the ion
and/or particle stream and any associated heat in a substantially parallel
direction to the film
substrate to be coated. A field with an electrical potential is generated by
means as is known in
the art that passes through a portion of the film substrate resulting in the
redirection and/or
acceleration of the ion and/or particle stream emanating from the flame or
plasma source on to
the film surface. The chemical bonds within the polymer molecules are more
readily broken
which results in the rapid formation of free radicals. This results in the
deposition of the desired
ultra-thin coating on to the film surface without the associated heat being
transferred to the film
surface thereby preventing potential melting of the film substrate during the
deposition process.
[0062] With reference to Figure 2D, one embodiment of the invention disclosed
herein is shown with multi-flame head system 60. In this embodiment, system 60
includes a
flame head assembly 62 comprising a pipe with spaced holes or nozzles for
emitting flames and
referred to as flame heads 64 integrated therewith. In various embodiments,
such flame head
assembly 62 may comprise commercially available flame burner heads
manufactured by Flynn
Burner Corporation of New Rochelle, New York. Chemical precursors 61, which
may also
include an oxidant, are fed into flame head assembly 62 and when ignited
result in lit flames
emanating from flame heads 64 resulting in the generation of hot gases and/or
vapors 66. The
substrate 52 to be coated is located proximal to flame heads 64 within the
region of hot gases
and/or vapors 66, such that hot gases and/or vapors 66 containing the reagent
vapor will contact
the substrate surface 56 resulting in a coating deposited thereon. The multi-
head flame head
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
deposition system 60 improves the continuity and thickness of coating
deposition across the
substrate surface 56 as the hot gas and/or vapor region 66 is expanded by the
use of multiple
flame sources. System 60 depicted in Figure 2D is shown with flame head
assembly 62 aligned
with multiple flame heads positioned in a planar and/or linear orientation.
However, other
embodiments are contemplated wherein one or more flame head assemblies may be
designed in
various two-dimensional and three-dimensional geometries such as square,
rhomboid, cylindrical
shapes which may be fashioned and positioned relative to the film being
processed according to
the necessity of the user as depicted in Figures 2E, 2F, 2G, 2H and 21. In
these alternative
contemplated embodiments, one or more precursor(s) may be fed to select flame
heads in the
individual flame head assembly providing the user with the ability to vary the
type,
characteristics and thickness of the coating deposited on to a substrate. As
can be readily seen in
these figures, the shape of the individual flame heads and flame head
assemblies and their
orientation relative to the substrate may be configured to achieve
differential types,
concentrations and/or thicknesses of ultra-thin coating deposition on to the
substrate by the
apparatus and methods described herein.
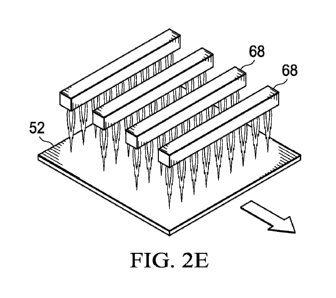
[0063] For example, Figure 2E discloses multiple flame head assemblies 68
oriented
in parallel rows perpendicular to the direction of the packaging substrate 52
movement. By
orienting the flame head assemblies 68 in this fashion, multiple coatings may
be deposited on the
substrate 52 in one pass along the indicated direction of substrate 52 travel.
In one embodiment,
various concentrations, gradients of precursor concentrations or different
precursors may be fed
to each individual flame head assembly 68, or to each individual flame head
integrated into each
flame head assembly 68 to vary the type of coating layers and/or concentration
of coating layers
and/or thickness of coating layers deposited on to substrate 52. In one
embodiment, one or more
26
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
of the flame head assemblies 68 emit a flame for purposes of priming the film
substrate 52 via
flame treatment. After passing through the flame treatment flame head
assemblies, the substrate
encounters one or more of the latter positioned flame head assemblies 68 which
may be supplied
with a precursor or various precursors for application of an ultra-thin
coating on the flame-
treated substrate 52 as desired by the user.
[0064] Figure 2F discloses a curved flame head assembly 70 that provides for
deposition of an ultra-thin inorganic oxide layer on to a substrate 52 as it
passes over a portion of
chill roll 72 and is held in relative contact with chill roll 72 via placement
of nip rollers 74. In
one embodiment, various concentrations, gradients of precursor concentrations
or different
precursors may be fed to the curved flame head assembly 70, or to each
individual flame head
integrated into the curved flame head assembly 70, to vary the type of coating
layers and/or
concentration of coating layers and/or thickness of coating layers deposited
on to substrate 52.
[0065] Figure 2G depicts a square or rectangular shaped flame head assembly 76
that
provides for deposition of an ultra-thin inorganic oxide layer on to substrate
52. In one
embodiment, various concentrations, gradients of precursor concentrations or
different
precursors may be fed to the flame head assembly 76, or to each individual
flame head integrated
into the flame head assembly 76, to vary the type of coating layers and/or
concentration of
coating layers and/or thickness of coating layers deposited on to substrate
52.
[0066] Figure 2H discloses multiple flame heads integrated into flame head
assemblies 68 oriented in parallel rows parallel to the direction of the
packaging substrate 52
travel. In one embodiment, various concentrations, gradients of precursor
concentrations or
different precursors may be fed to each individual flame head assembly 68, or
to each individual
27
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
flame head integrated into each flame head assembly 68 to vary the type of
coating layers and/or
concentration of coating layers and/or thickness of coating layers deposited
on to substrate 52.
[0067] Turning to Figure 21, one embodiment of the invention disclosed herein
depicts a flame head assembly 78 oriented at an angle relative to the
substrate 52 surface. In this
configuration, one end of the flame head assembly 78 is closer to the
substrate surface as the
substrate 52 moves in the longitudinal direction parallel to the flame head
assembly 78. In one
embodiment, the "lower" end of the flame head assembly 78 is positioned at
substantially 20 mm
above the surface of substrate 52 and serves to precondition the substrate 52
as it provides a
more intensive heat treatment upon introduction of the substrate 52 to the
proximity of flame
head assembly 78 and would serve to clean off dirt, dust and other
contaminants that may be on
the substrate surface. As the substrate 52 moves along, the "upper" end of the
flame head
assembly 78 is positioned substantially 40 mm above the surface of substrate
52 and resulting in
lower intensity heat treatment applied to the substrate 52 due to the
increasing distance between
the surface of substrate 52 and the flame head assembly 78. Therefore, various
concentrations of
precursor could be fed to select or all of the remaining flame heads in the
flame head assembly
78 resulting in the differential application of inorganic oxide layers to the
surface of substrate 52
as it moves along the length of the flame head assembly 78. In one embodiment,
the flame head
assembly 78 is oriented at a 2 mm distance from the substrate 52 surface at
the initial encounter
between the flame/plasma with the substrate 52 surface and oriented at an
inclined angle to
produce a 4 mm distance between the substrate 52 and the last flame head in
the flame head
assembly 78 as shown. In alternative embodiments, the flame head assembly 78
may be oriented
at inclined angles perpendicular or along a radial arc relative to the
direction of the substrate 52
28
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
to achieve flame pretreatment or variegated organic oxide layer deposition on
the substrate 52 as
desired.
[0068] Such configurations and shapes would increase the film surface area
exposed to
the flame in a single pass of the film substrate past the burner. In turn,
these geometric
configurations increase the dwell time the flame or plasma has in contact with
the film substrate
surface thereby altering the coating properties imparted to the film
substrate. Therefore, the
embodiments depicted herein are not to be construed as limiting to the
disclosure herein.
[0069] Turning to Figure 3A, one embodiment of a CCVD and/or PECVD coating
assembly as described herein is shown "in-line" with a roll-to-roll
winding/coating assembly 80
in a typical manufacturing context. In the depicted embodiment, an unwinding
unit 86 unwinds
film 88 from roller 96 as winding unit 84 winds film 88 on to winding core 94.
A flame chamber
82 housing a CCVD and/or PECVD coating assembly 92 as described herein is
integrated in-line
with the unwinding/winding units 86 and 84. The flame chamber 82 constitutes
an unpressurized
enclosure in which at least one CCVD and/or PECVD flame head assembly 92 is
housed for the
safety of the user and surrounding equipment and materials. During the
unwinding/winding
process, a film substrate 88 is drawn from unwinding unit 86 through various
rollers and on to
drum 90. After receiving a coating and exiting the flame chamber 82, film
substrate 88 is wound
around winding core 94. Drum roller 90 rotates and winds and/or draws
substrate 88 in proximity
to the hot gases and/or vapors generated by the flame head assembly 92. In the
depicted
embodiment, drum roller 90 is positioned above flame head assembly 92 so as to
maximize the
surface area contact between the rising hot gases and/or vapors generated by
flame head
assembly 92 thereby resulting in efficient deposition of the coating material
carried by the hot
gases and/or vapors on to substrate 88. In various contemplated embodiments,
drum roller 90
29
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
may comprise a temperature control roll or "chill roll" so as to impart a
thermal temperature to
the substrate and a differential between the substrate 88 to be coated and the
heat generated by
the flame head assembly 92 which would facilitate coating substrates with low
melt points
without heat damage to the substrate according the inventive method and
apparatus disclosed
herein. In the embodiment depicted in Figure 3B, multiple flame assemblies 82
are integrated
in-line to provide multiple coating layers to the substrate 88. In this
configuration, multiple
layers of ultra-thin inorganic coatings of variable type, concentration and/or
thickness may be
applied to the substrate at each flame head assembly 82 station as desired and
configured by the
user.
[0070] With reference to Figures 3A and 3B and without being bound by theory,
it has
been discovered that better quality deposition coatings (i.e. improved coating
layer coverage
uniformity over the substrate surface and enhanced RMS smoothness of the
deposited coating
layer) may be achieved by passing the substrate film multiple times through
the flame treatment
system or past multiple flame heads and/or flame head assemblies, with low
concentrations of
precursor, as opposed to a single pass of the substrate through a flame
treatment system using a
high concentration of precursor resulting in one thick deposition layer. In
one example
embodiment, a stand alone roll-to-roll coater was equipped with a single
burner plasma flame
treatment system. A combustible inorganic precursor, tetraethyl orthosilicate
(TEOS), was
metered into the fuel stream at a controlled rate. As the film was unwound and
passed over the
plasma flame, low concentration levels of silica were deposited onto the
surface of the film
substrate. Collected data revealed that the Si02 deposition quality was poor
where the TEOS
concentration was greater than 22 mg/min, 5i02 deposition quality was rated as
good where the
TEOS concentration was less than 11 mg/min, and 5i02 deposition quality was
rated as excellent
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
where the TEOS concentration level was less than 2 mg/min. As the film was
passed multiple
times (between two to five passes) over a plasma flame fed with low
concentration TEOS,
multiple layers of Si02 were deposited on the film substrate which resulted in
the development
of a barrier layer with a thickness of 50 nm and exhibiting an OTR< 10
cc/m2/day and a WVTR
<0.5 g/m2/day.
[0071] The metallization primer process described herein may be conducted
either
during ("in-line") or after film manufacturing. The film surface manufacture
in-line is
commonly pristine and free of contaminants thereby making it ideal for surface
priming due to
the challenges of keeping the film surface clean after the manufacturing
process is complete. For
example, dust, anti-block particles, or additives in the polymer film may
"bloom" to the surface
of the film substrate in a post-manufacturing environment. These conditions
can make it
difficult to achieve a uniform primer coating during the priming process
conducted after the film
has been manufactured and stored for a period of time. Blooming additives can
also migrate
over the inorganic nanolayer, as it is not a barrier layer in itself, thus it
is desired not to have
these additives in the film.
[0072] Turning to Figure 3C, one embodiment of the invention disclosed herein
is
shown wherein a flame CCVD or PECVD unit is installed in-line with a biaxial
film substrate
production line 100. In the depicted embodiment, a biaxial film substrate 102
is formed by an
extrusion unit 104. The film substrate 102 is then passed through a cooling
unit 106 and is
stretched in the machine (longitudinal) direction in machine stretching unit
108 and in the
transverse direction in transverse stretching unit 110. The film substrate is
then passed through
the flame head assembly 112 wherein it is coated with the desired inorganic
primer and/or barrier
31
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
coating according to the apparatus and processes described herein. The coated
film substrate is
then wound into a transportable roll in winding unit 114 for further
processing or distribution.
[0073] With reference to Figure 3D, an embodiment of the invention disclosed
herein
is depicted wherein a flame CCVD or PECVD coating tower unit 118 is installed
in-line with a
biaxial film substrate production line 100 as similar to the production line
depicted in Figure 3C.
In this embodiment, multiple flame head assemblies 120 are placed in series
with each delivering
a low concentration of inorganic precursor as the substrate 102 passes through
the line over
various chill rolls and nip rolls in a single pass through the system. The
flame head assembly
geometry, substrate line speed, chill roll temperature and precursor types and
concentration could
be configured in various contexts to produce the desired type, concentration
and/or thickness(es)
of ultra-thin inorganic coating(s) to be deposited on the particular packaging
substrate. Typical
processing conditions are as follows: line speeds from 200 to 1500 ft/min (60
m/min to 450
m/min); chill roll temperatures of 40 to 80 C; the flame pretreatment with
burner to film distance
of 5 mm for flame pretreatment, a fuel to air ratio of 0.90 to 0.95 for the
flame treatment step, a
natural gas flow rate of 100 liters/min for a 1 meter wide line; the
deposition step with burner to
film distances of 5 to 45 mm, a fuel to air ratio 1.0, gas flow rates of 70 to
105 liters/min for a 1
meter wide line, a precursor concentration of 0.0001 mole% to 0.01 mole % of
the gas stream.
The plasma temperatures have exhibited good results at 1200 C with a range
covering 650 C to
1450 C. The above conditions will produce a coating with a WVTR of < 0.2
g/m2/day and an
OTR < 20 cc/m2/day.
[0074] Managing heat buildup in the substrate from exposure to PECVD or CCVD
flame head is of great concern as such heat buildup will distort or melt the
substrate being
coated. As described in various embodiments shown and disclosed herein, chill
roll technology
32
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
is used to dissipate heat buildup in the substrate. However, the diameter of
the chill roll or
number of multiple chill rolls required to accomplish certain coatings may be
cost or space
prohibitive due to size/space limitation in the manufacturing environment.
Alternatively, spray
coolants may be utilized to dissipate heat buildup in the substrate as it is
treated according to the
apparatus and methods herein that are practiced in limited space environments.
As depicted in
Figure 3E, one embodiment of the invention disclosed herein depicts an "off
line" inorganic
coating deposition apparatus that could be used to coat a substrate produced
at a different
facility. For example, in one embodiment the equipment design shown in Figure
3E may be
incorporated into a stand-alone process step at a converter. In this
embodiment, a packaging
substrate 102 is unwound from unwind roll 96, passed over a series of flame
head assemblies 82
which may flame treat and/or deposit an ultra-thin coating(s) on to the
exposed surface of the
substrate 102, while concurrently the opposite exposed surface of the
substrate 102 is being
cooled with spray coolant from coolant nozzles 130 to dissipate heat and
control or prevent
degradation or melting of the substrate 102. In this embodiment, chill rolls
or other thermal
applicators are not required to keep the substrate 102 from degrading or
overheating due to the
thermal inputs from exposure to the burners 82. The flame head assembly
geometry, substrate
line speed, coolant spray temperature and precursor concentration could be
configured in various
contexts to produce the desired thickness(es) of ultra-thin coating(s) to be
deposited on the
particular packaging substrate. Industrial spray coolants that may be utilized
in this embodiment
may include aromatics, silicate-ester (COOLANOL 25R), Aliphatic (PAO),
silicone
(SYLTHERM XLT) or others as known in the art. Typical processing conditions
are as follows:
line speeds from 200 to 1500 ft/min (60 m/min to 450 m/min); chill roll
temperatures of 40 to
80 C; the flame pretreatment with burner to film distance of 5 mm for flame
pretreatment, a fuel
33
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
to air ratio of 0.90 to 0.95 for the flame treatment step, a natural gas flow
rate of 100 liters/min
for a 1 meter wide line; the deposition step with burner to film distances of
5 to 45 mm, a fuel to
air ratio 1.0, gas flow rates of 70 to 105 liters/min for a 1 meter wide line,
a precursor
concentration of 0.0001 mole% to 0.01 mole % of the gas stream. The plasma
temperatures have
exhibited good results at 1200 C with a range covering 650 C to 1450 C. The
above conditions
will produce a coating with a WVTR of < 0.2 g/m2/day and an OTR < 20
cc/m2/day.
[0075] It should be noted that the embodiments shown in Figures 2A - 3E and
Figures 5A-51 may utilize plasma-enhanced chemical vapor deposition (PECVD)
apparatus and
methods to accomplish the coating process as described herein. As such, the
depicted
embodiments are not be construed as being limited to flame CCVD methods.
Whenever the term
"flame" or its analogues such as "flame head" or "flame head assembly" are
used herein, it is
interpreted as including "plasma" and its analogues, and equivalent laser
ablation equipment.
The plasma may be manipulated by an electromagnetic field in proximity to the
plasma source so
as to direct the ions generated from the plasma reaction on to the substrate
surface to be coated.
Thus CCVD is not limiting to the product made, but is just one enabling method
used to
accomplish making of the described product on the film fabrication line. As
previously
described herein, alternative embodiments of the apparatus and systems
disclosed in Figures 2A-
3E may be independently be configured to provide flame treatment of the
substrate, to apply a
primer coating and/or to apply barrier coatings at open atmosphere to the
substrate as it moves
along the manufacturing line.
[0076] Figure 4 is a structural diagram depicting an embodiment of a coated
substrate
120. In the depicted embodiment, a film or paper substrate 122 is primed with
a pure or
substantially pure silica layer 124. The substrate 122 with silica layer 124
is then coated with
34
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
additional oxide layer 126 and a subsequent metal or oxide layer 128. Oxide
layers 126, 128 may
be comprised of silica mixed with an additional chemical additive or "dopant"
for purposes of
enhancing the reactivity of the primed surface 124 with additional desired
coatings. In one
embodiment, the metal barrier layer deposited by the apparatus and method
described herein has
a thickness between 5 and 50 nm, with an optical density of over 30%. The
metal barrier layer
may comprise aluminum, copper, iron, manganese, zinc and/or other metals as
dictated by the
needs of the user. In other embodiments, layer 128 is an oxide layer deposited
via CCVD or
layer 128 is a metal layer deposited by conventional vacuum metallization
technology.
[0077] Figures 5A-51 depict various apparatus in which various embodiments of
the
invention disclosed herein may be configured as desired by the user. Figure 5A
discloses a
configuration wherein the chemical precursors 504 are fed into the flame fuel
line 502 prior to
being mixed with air from the air line 506 and combusted at the flame head 508
as shown.
Figure 5B depicts a configuration wherein the chemical precursors 504 are fed
into the air line
506 prior to being mixed with fuel from the fuel line 502, which in this
embodiment is natural
gas, and combusted at the flame head 508 as shown. Figure 5C discloses a
configuration were
chemical precursors 504 are fed into an air line 506 and a fuel line 502 prior
to being mixed at a
fuel/air mixer 510 and combusted at the flame head 508 as shown. In this
embodiment, different
chemical precursors may be utilized and fed into the air line and fuel line
prior to mixing at the
fuel/air mixer. Figure 5D discloses wherein a chemical precursor is introduced
after the fuel and
air constituents have mixed at the fuel/air mixer as shown. The resulting
mixture is then
combusted at the flame head as described herein. Figure 5E discloses a
configuration wherein
one or more chemical precursors may be mixed at the fuel/air mixer prior to
the introduction of
an additional chemical precursor downstream and which is thereafter combusted
at the flame
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
head as shown. Figure 5F discloses a configuration where the chemical
precursor is introduced
at the point where the fuel and air are mixed. The resulting mixture is then
combusted at the
flame head as described herein. Figure 5G discloses a configuration wherein
the chemical
precursor is sprayed or otherwise injected into the existing flame produced by
the flame head as
shown. Figure 5H discloses a configuration where in the chemical precursor is
combusted into
the flame head burner as shown. Figure 51 discloses a configuration wherein a
laser ablation
apparatus 512 is used to generate the vapors and/or ion stream 514 which is
directed to a
substrate for coating thereon. In the embodiments disclosed in Figures 5A-5I,
it will be evident
to one of ordinary skill in the art that various fuel, air and chemical
precursor species may be
utilized to generate the desired coatings upon the film substrate passing in
the desired proximity
of the flame head as described herein. The various embodiments shown in
Figures 5A-51 may be
integrated into the various in-line and standalone film substrate
manufacturing and processing
environments as disclosed herein.
[0078] To describe certain embodiments of the inventive apparatus and methods
disclosed herein, the following examples are provided. Once having understood
the examples set
forth herein, one of ordinary skill in the art should be able to apply the
apparatus and methods
disclosed herein to other chemical deposition methods, and such applications
are deemed to fall
within the scope of the invention disclosed herein. The following examples are
for illustrative
purposes and are not to be construed as limiting the scope of the invention.
In the examples, the
primer coating deposition was performed using CCVD in an open atmosphere
environment. The
chemical precursors consisted of TEOS in a methane air feed through a film
flame treater with a
flame temps of 800 C to 1200 C unless otherwise indicated.
[0079] Example 1 Si02 Deposition on OPP Polymer by Roll Coater
36
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0080] As an example and for comparative purposes, a biaxially oriented OPP
polymer film substrate was flame treated first on the inside surface of the
roll. Conditions for the
flame treatment of film include: line speed of about 184 feet/min, a burner to
film distance of
about 5 mm, and a fuel to air ratio of about 1Ø Following flame treatment
step, the film was run
through the roll coater a second time to deposit a silica layer. Conditions
for the silica layer
deposition include: line speed of about 184 feet/min, a burner to film
distance of about 5 mm,
and a fuel to air ratio of about 1.0, TEOS concentration of about 0.00379 mole
percentage for
both of the flame treatment and silica deposition runs.
[0081] The deposition of silica is greatly enhanced by the flame treatment
step prior to
the treatment with silica. This is demonstrated in Figure 6 for a single
deposition pass of silica
via information collected by XPS. The amount of silica, as determined by
signal strength, has a
70% increase in silica deposited. Signal without flame pretreatment is 290
counts per second
(CPS) at a peak maximum, while the max signal is 500 counts per second for a
single pass of
silica after flame treatment. In other words, the silica content increased
from 0.18 atomic %
silicon without flame pretreatment to 0.23 atomic % silicon.
[0082] The pretreatment was so successful that multiple laps of silica were
deposited
after a flame pretreatment was utilized. This is shown in Figure 7 for signal
strength (CPS) vs.
binding energy (eV) from XPS. The amount of silica increase during each pass,
as can be clearly
seen. In terms of atomic % of silicon present, 1, 2, and 3 deposition passes
of silica result in
0.23%, 0.26%, and 0.44%, respectively.
[0083] The ultimate arbiter of effectiveness is barrier of the deposited
silica layer. All
of the samples from above in this example were metallized under standard
vacuum metallization
conditions to an optical density of 2.3. The atomic percentage of silicon
atoms on the film
37
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
surface, WVTR, and OTR values are shown in Figure 8 and plotted versus the
number of silica
deposition passes. All samples were flame treated before the silica deposition
passes except for
the first sample labeled with a black oval, which had no flame pretreatment
before a single silica
deposition. Flame treatment and increasing number of silica passes result in
lower WVTR and
OTR, or increasing barrier. This increasing barrier results from a higher
quality or more
effective layer of aluminum metal deposited on the silica primed film.
[0084] Example 2 High Speed Deposition on OPP Film
[0085] The current example is a biaxially oriented polypropylene (BOPP) placed
on a
roll to roll coater as disclosed herein for a single pass flame treatment and
single layer silica
coating deposited in one pass. Typical processing conditions are as follows:
line speed at about
900 ft/min (275m/min); chill roll temperatures at about 54 degrees Celsius;
the flame
pretreatment with burner to film substrate distance of about 5 mm for flame
pretreatment, a fuel
to air ratio of about 0.95 for the flame pretreatment step, a natural gas flow
rate of about 100
liters/min for a 1 meter wide line; the silica coating deposition step with
burner to film distances
of about 5 to 10 mm, a fuel air ratio of about1.0, gas flow rates of about 75
to 100 liters/min for a
1 meter wide line, a precursor concentration of in the range of about 0.0001
mole% to 0.01
mole% of the gas stream and plasma temperatures at 1200 degrees Celsius. The
film samples
were then metallized under standard conditions to an optical density of 2.5.
The above described
operating conditions produced a film substrate with a WVTR of < 0.2 g/m2/day
and an OTR of <
20 cc/m2/day. WVTR and OTR data for a variety of working distances (flame
burner to film
substrate distance), gas flow rate, and precursor concentration (TEOS) are
given in Table 1.
Table 1
38
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
\===
sktskt scs
5.00 .... 0.0038 ... 75.00 ...... 0.05 10.9
2 5.00 0.0038 100.00 0.12 12.8
10.00 . 0.0038 75.00 0.08 10.5
4 10.00 0.0038 100.00 0.08 6.86
. 5.00 0.0049 75.00 Av... 0.09 9.09
6 5.00 0.0049 100.00 0.12 17.7
10.00 .. 0.0049 75.00 .... 0.15 0.5
8 10.00 0.0049 100.00 0.09 9.75
. 5.00 .. 0.0095 Hi 75.00 .... L 0.07
5.00 0.0095 100.00 0.13 10.1
10.00 :. 0.0095 Hi 75.00 L .. 0.09
12 10.00 0.0095 100.00 0.16 25.2
[0086] The data in Table 1 demonstrates the robustness of the silica
deposition and
primer process. The speeds employed in this example are similar, if not
identical, to the line
speeds in the typical film substrate production process.
[0087] Example 3 Multiple Layer Silica Deposition on OPP Film
[0088] Experiments were conducted with multiple laps over a film to produce
pure
silica coating between 10 to 50 nm. The coatings were produced under the
following conditions:
line speeds of about 600 to 900 FPM (180m/min to 275 m/min), the flame
treatment with flame
burner to film substrate distance of about 5mm for the flame treatment step, a
fuel to air ratio of
about 0.95, and a natural gas flow rate of about 100 liters/min for a 0.3
meter wide line. For the
coating deposition step, a flame burner to film substrate distance of about 5
to 15 mm, a fuel to
air ratio of about 1.0, gas flow rates of about 75 to 100 liters/min for a 0.3
meter wide line, and a
precursor concentration in the range of 0.0001 mole% to 0.01 mole% of the gas
stream. The
plasma temperatures were about 1250 degrees Celsius. A number of silica laps
were made
between about 36 and 72.
39
CA 02872318 2014-10-30
WO 2013/192560 PCT/US2013/047128
[0089] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
properties such as molecular weight, reaction conditions, and so forth used in
the specification
and claims are to be understood as being modified in all instances by the term
"about."
Accordingly, unless indicated to the contrary, the numerical parameters set
forth in the following
specification and attached claims are approximations that may vary depending
upon the desired
properties sought to be obtained by the present invention. At the very least,
and not as an attempt
to limit the application of the doctrine of equivalents to the scope of the
claims, each numerical
parameter should at least be construed in light of the number of reported
significant digits and by
applying ordinary rounding techniques. While the invention has been
particularly shown and
described with reference to a preferred embodiment, it will be understood by
those skilled in the
art that various changes in form and detail may be made therein without
departing from the spirit
and scope of the invention.