Note: Descriptions are shown in the official language in which they were submitted.
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
IN-SITU METHOD AND SYSTEM FOR REMOVING HEAVY METALS FROM
PRODUCED FLUIDS
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims benefit under 35 USC 119 of US Patent
Application
Serial Nos. 61/647,983 and 61/647,999, both with a filing date of May 16,
2012. This
application claims priority to and benefits from the foregoing, the
disclosures of which are
incorporated herein by reference.
TECHNICAL FIELD
[002] The invention relates generally to a process, method, and system for
removing
heavy metals including mercury from hydrocarbon fluids such as crude oil and
gases.
BACKGROUND
[003] Heavy metals can be present in trace amounts in all types of produced
fluids
such as hydrocarbon gases and crude oils. The amount can range from below the
analytical
detection limit to several thousand ppbw (parts per billion by weight)
depending on the
source.
[004] Methods have been disclosed for in-situ treatment of fluid for heavy
metal
removal. US Patent Publication No. 2011/0253375 discloses an apparatus and
related
methods for removing mercury from reservoir effluent by placing materials
designed to
adsorb mercury into the vicinity of a formation at a downhole location, and
letting the
reservoir effluent flow through the volume of the adsorbing material. US
Patent Publication
No. 2012/0073811 discloses a method for mercury removal by injecting a solid
sorbent into a
wellbore intersecting a subterranean reservoir containing hydrocarbon
products.
[005] Production of oil and gas is usually accompanied by the production of
water.
The produced water may consist of formation water (water present naturally in
the reservoir),
or water previously injected into the formation. As exploited reservoirs
mature, the quantity
of water produced increases. Produced water is the largest single fluid stream
in exploration
and production operations. Every day, U.S. oil and gas producers bring to the
surface 60
million barrels of produced water.
[006] There is still a need for improved methods for the removal of heavy
metals
from produced streams right at the production source, particularly for the
removal of
mercury.
1
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
SUMMARY OF THE INVENTION
[007] In one aspect, the invention relates to a method for recovering
hydrocarbons
from a subterranean hydrocarbon-bearing formation while simultaneously
removing heavy
metals from the hydrocarbons. The method comprises: exposing the heavy metals
in the
hydrocarbons to a fixing agent in a dilution fluid for the fixing agent to
react with the heavy
metals forming heavy metal complexes in the dilution fluid; and recovering the
hydrocarbons
and the dilution fluid containing the heavy metal complexes from the formation
via a
production well as a mixture.
io [008] In another aspect, the invention relates to a method for
recovering
hydrocarbons from a subterranean hydrocarbon-bearing formation while
simultaneously
removing heavy metals from the hydrocarbons. The method comprises: exposing
the heavy
metals in the hydrocarbons to a fixing agent in a dilution fluid for the
fixing agent to react
with the heavy metals forming insoluble heavy metal complexes that precipitate
and remain
in the reservoir; and recovering the hydrocarbons and the dilution fluid
containing the heavy
metal complexes from the formation via a production well as a mixture.
[009] In another aspect, the invention relates to another method for
recovering
hydrocarbons from a subterranean hydrocarbon-bearing formation while
simultaneously
removing heavy metals from the hydrocarbons. The method comprises: fracturing
the
formation to generate fractures; providing a dilution fluid containing a
fixing agent for the
fixing agent to react with the heavy metals in the formation, forming heavy
metal complexes
in the dilution fluid; recovering the dilution fluid containing the heavy
metal complexes; and
recovering hydrocarbons having a reduced concentration of heavy metals from
the formation
via a production well.
[010] In another aspect, the invention relates to an in-situ method for
removing
heavy metals from the hydrocarbons while recovering the hydrocarbons from a
subterranean
hydrocarbon-bearing formation. The method comprises: fracturing the formation
to generate
fractures; providing a dilution fluid containing a fixing agent for at least a
portion of the
fixing agent to be adsorbed into fractures and rocks in the formation;
reducing the pressure
for the dilution fluid to flow back through a well bore; allowing the
hydrocarbons to pass
through the fractures and rocks having the fixing agent adsorbed thereon,
wherein heavy
metals in the hydrocarbons react with the fixing agent forming heavy metal
complexes; and
recovering the hydrocarbons from the formation via a well bore.
2
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
[011] In another aspect, the invention relates to a method for recovering
hydrocarbons from a subterranean hydrocarbon-bearing formation while
simultaneously
removing heavy metals from the hydrocarbons. The method comprises: fracturing
the
formation to generate fractures; providing a dilution fluid containing a
fixing agent for the
fixing agent to diffuse into fractures and rocks in the formation to react
with the heavy metals
in the hydrocarbons; recovering the dilution fluid containing the heavy metal
complexes; and
recovering hydrocarbons having a reduced concentration of heavy metals from
the formation
via a production well.
[012] In yet another aspect, the invention relates to a system for the in-situ
removal
of heavy metals hydrocarbons in recovering the hydrocarbons from a
subterranean
hydrocarbon-bearing formation. The system comprises: a well drilled into an
underground
formation comprising hydrocarbons and a topside production facility. The
topside production
facility is for the storage and treatment of produced water recovered from a
subterranean
formation, and the injection of the treated produced water containing the
fixing agent into the
well.
BRIEF DESCRIPTION OF THE FIGURE
[013] FIG. 1 is a diagram of an embodiment of an in-situ system for the
removal of
heavy metals from a produced fluid.
[014] FIG. 2 is a diagram of a second embodiment of an in-situ system for the
simultaneous recovery of oil and removal of heavy metals from the recovered
oil.
DETAILED DESCRIPTION
[015] The following terms will be used throughout the specification and will
have
the following meanings unless otherwise indicated.
[016] "Hydrocarbons" refers to hydrocarbon streams such as crude oils and / or
natural gases.
[017] "Produced fluids" refers hydrocarbon gases and / or crude oil. Produced
fluids may be used interchangeably with hydrocarbons.
[018] "Crude oil" refers to a hydrocarbon material, including to both crude
oil and
condensate, which is typically in liquid form. Under some formation conditions
of
temperature and/or pressure, the crude may be in a solid phase. Under some
conditions, the
oil may be in a very viscous liquid phase that flows slowly, if at all.
3
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
[019] "Production well" is a well through which produced fluids are carried
from an
oil-bearing geological formation to the earth's surface, whether the surface
is water or land.
Surface facilities are provided for handling and processing the crude from the
formation as it
arrives on the surface.
[020] "Topside production facility" refers to the surface hardware on an
offshore oil
platform or connected group of platforms, such as the oil production plant and
the drilling rig.
[021] "Injection well" is a well through which at least a treatment agent is
passed
from the surface facilities into the geological formation. In one embodiment,
a well is
alternatively employed in a producing and an injection mode. The well is
alternatively
employed for injecting a material into the formation for some period of time.
The process
conditions within the well are then adjusted to permit crude to flow into the
well, from where
it is withdrawn to surface facilities.
[022] "Trace amount" refers to the amount of heavy metals in a produced fluid.
The
amount varies depending on the source of the fluid and the type of heavy
metal, for example,
ranging from a few ppb to up to 30,000 ppb for mercury and arsenic.
[023] "Heavy metals" refers to gold, silver, mercury, osmium, ruthenium,
uranium,
cadmium, tin, lead, selenium, and arsenic. While the description described
herein refers to
mercury removal, in one embodiment, the treatment removes one or more of the
heavy
metals.
[024] "Flow-back water" refers to water that flows back to the surface after
being
placed into a subterranean formation as part of an enhanced oil recovery
operation, e.g., a
hydraulic fracturing operation.
[025] "Produced water" refers to the water generated in the production of oil
and
gas, including formation water (water present naturally in a reservoir), as
well as water
previously injected into a formation either by matrix or fracture injection,
which can be any
of connate water, aquifer water, seawater, desalinated water, flow-back water,
industrial by-
product water, and combinations thereof
[026] "Mercury sulfide" may be used interchangeably with HgS, referring to
mercurous sulfide, mercuric sulfide, or mixtures thereof, which can be in any
common phases
of cinnabar, meta-cinnabar, hyper-cinnabar and combinations thereof Mercury
sulfide is
typically present as mercuric sulfide with a stoichiometric equivalent of one
mole of sulfide
ion per mole of mercury ion.
[027] The invention relates to a method for the in-situ removal of heavy
metals such
as mercury, arsenic, etc., from produced fluids such as gases and crudes from
a subterranean
4
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
hydrocarbon bearing formation. In the course of extracting the produced fluids
from the
formation, a fixing agent is injected in the formation, which reacts with the
heavy metals and
forms precipitates and / or soluble heavy metal compounds. The amount of
precipitates or
soluble heavy metal compounds formed depends on the type of mercury present in
the
formation, as well as well as the amount and type of fixing agent(s) employed.
[028] Produced Fluids Containing Heavy Metals: Heavy metals such as lead,
zinc,
mercury, silver, selenium, arsenic and the like can be present in trace
amounts in all types of
hydrocarbon streams such as crude oils and natural gases. Producers may desire
to remove
heavy metals such as mercury and lead from crude oil. The amount of mercury
and / or
arsenic can range from below the analytical detection limit to several
thousand ppb
depending on the feed source.
[029] Arsenic species can be present in produced fluids in various forms
including
but not limited to triphenylarsine (Ph3As), triphenylarsine oxide (Ph3As0),
arsenic sulfide
minerals (e.g., As4S4 or AsS or As2S3), metal arsenic sulfide minerals (e.g.,
FeAsS; (Co, Ni,
Fe)AsS; (Fe, Co)AsS), arsenic selenide (e.g., As25e5, As25e3), arsenic-
reactive sulfur
species, organo-arsenic species, and inorganic arsenic held in small water
droplets.
[030] Mercury can be present in produced fluids as elemental mercury Hg ,
ionic
mercury, inorganic mercury compounds, and / or organic mercury compounds.
Examples
include but are not limited to: mercuric halides, mercurous halides, mercuric
oxides,
mercuric sulfide, mercuric sulfate, mercurous sulfate, mercury selenide,
mercury hydroxides,
organo-mercury compounds and mixtures of thereof. Mercury can be present as
particulate
mercury, which can be removed from hydrocarbons by filtration or
centrifugation. The
particulate mercury in one embodiment is predominantly non-volatile.
[031] In one embodiment, the produced fluid is a crude oil containing at least
50
ppbw mercury. In another embodiment, the mercury level is at least 100 ppbw.
In one
embodiment of a mercury-containing crude, less than 50% of the mercury can be
removed by
stripping (or more than 50% of the mercury is non-volatile). In another
embodiment, at least
65% of the mercury in the crude is non-volatile. In a third embodiment, at
least 75% of the
mercury is of the particulate or non-volatile type.
[032] In-situ Removal of Heavy Metals: In one embodiment, the removal of heavy
metals such as mercury and arsenic is simultaneous with the recovery of a
produced fluid in
a subterranean reservoir with the injection of a dilution fluid. In this
method, a sufficient
amount of fixing agent is added to the formation for the removal of heavy
metals as oil and /
or gas is being produced in the well.
5
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
[033] The in-situ removal occurs simultaneously with a water flooding in one
embodiment, and with a fracturing process in another embodiment. Fracturing is
a method
for increasing the production of crude oil and gas from a fractured reservoir.
Fractures can
be generated in formations by means known in the art, e.g., pulsed power
energy, gas
fracturing, explosion, plasma stimulation, hydraulic fracturing, etc. Water
injection or
waterflooding is a widely applied method of improved oil recovery, wherein
water is used as
the dilution fluid for injecting into the rock formation through a system of
injection boreholes
to facilitate recovery of hydrocarbons from subsurface formations. In one
embodiment, a
fracturing fluid is injected into the well at a rate and pressure sufficient
to propagate a
fracture adjacent to or in the well. The fracturing fluid is allowed to soak
into the formation
rock for a period of time, ranging from hours to days. The fracturing fluid is
a dilution fluid
which contains propping agents to maintain the fracture in a propped condition
when the
applied pressure is relieved, as well as a sufficient amount of a fixing agent
for the removal of
heavy metals. The fracturing fluid can also be an acid, e.g., HC1, to etch the
fracture faces in
the formation to form conductive channels facilitating the oil recovery.
[034] In one embodiment, at least a portion of the fixing agent diffuses into
the
formation fractures and reacts with the heavy metals embedded in the
formation, forming
heavy metal complexes in the fracturing (dilution) fluid. In one embodiment,
after the
pressure is reduced and the direction of the fluid flow is reversed, the fluid
containing
extracted heavy metals flows back to the surface for recovery and subsequent
treatment to
remove extracted heavy metals and other contaminants. In another embodiment,
at least a
portion of the fixing agent adsorbs onto the reservoir rock in the soaking
process, for "treated
rock" with embedded fixing agent.
[035] When the flow is reversed and the hydrocarbons pass over the treated
rock, the
heavy metal reacts with the embedded fixing agent forming heavy metal
complexes. In some
embodiment, the heavy metal complexes are embedded and stay in the formation
fractures for
a produced fluid when recovered from the production well to effectively have a
lower heavy
metal concentration than a produced fluid from a well without the fixing agent
in the fracture
fluid.
[036] At least 25% of the heavy metal complexes stay in the formation
fractures in
one embodiment, at least 50% of the heavy metal complexes remain in the
formation
fractures in a second embodiment; and at least 75% in a third embodiment. When
the
fixing reagent is exhausted from the formation, increasing amounts of heavy
metals will be
detected in the recovered produced fluids so that a new supply of fixing agent
can be injected
6
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
into the formation. In one embodiment, the amount of heavy metals such as
mercury
remaining in the formation can be determined by measuring concentration of in-
situ
formation material before and after drilling and coring. The amount can be
determined by
analyses of adsorption on samples from the formation, e.g., core samples,
cutting waste,
produced water from the formation, etc.
[037] In one embodiment, the fixing agent is added to a dilution fluid such as
water
for injection into the well, during any stage of the recovery, and on a
continuous or
intermittent basis. It can be added to the dilution fluid along with other
additives, e.g.,
proppants, surfactants, electrolytes, etc. The fixing agent can also be added
to the production
well as a separate feed from the dilution fluid. It can be injected into the
production well
within less than thirty days of the injection of the dilution fluid or
periodically over a period
of a few months to allow for the soaking of the reservoir. The fixing agent
can be provided
in a dispenser with perforations positioned in the production tubing for
continuous slow
dissolution into the injected dilution fluid, as disclosed in US Patent
Publication No.
2011/0162841, the relevant disclosure is included herein by reference.
[038] In one embodiment after the injection of the fixing agent into the
reservoir, the
well can be shut-in for some period of time to allow the fixing agent and
optionally, other
additives such as surfactants, etc., to imbibe into the matrix rock and
thereby react with the
heavy metals present in the oil, as the dilution fluid displaces the oil into
the fracture system.
The shut-in time can range from 2 hours to hundreds of days in one embodiment,
and 2-10
days in another embodiment, and less than 30 days in a third embodiment.
[039] In another embodiment of another in-situ removal process, the fixing
agent as
dissolved in the injected dilution fluid flows through the subsurface or
formation
passageways reacts with the heavy metals forming metal complexes, where the
heavy metal
complexes are extracted from the produced fluid into the dilution fluid for
subsequent
recovery. The injected dilution fluid such as water contains a sufficient
amount of fixing
agent, so as the water flows through subsurface or formation passageways may
include pores
in the formation matrix, fractures, voids, cavities, perforations and fluid
passages through the
wells, including cased and uncased wells, tubings and other fluid paths in the
wells, causing
the hydrocarbons trapped in the formation to move toward the production well.
In the
process, the fixing agent in the injected water reacts and extracts the heavy
metals from the
produced fluids into the injected water. The injected water travels through
the rock
formation at a speed of 0.1 to 20 m/day in one embodiment. In another
embodiment, the
7
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
water is heated while within the formation which facilitates the in-situ
removal of heavy
metals.
[040] After the in-situ reaction and recovery of the produced fluid and
injected water
from the reservoir, the wastewater containing the heavy metal complexes is
separated from
the crude in a phase separation device known in the art, resulting in a crude
oil with a
significantly reduced level of heavy metals and a wastewater stream. In one
embodiment
after the recovery of a mixture of produced fluid such as crude oil and
dilution fluid
containing heavy metal complexes from the formation, additional chemical
reagents such as
complexing agents can be added to the mixture to facilitate the oil / water
separation.
io
[041] For an onshore or in sensitive near-shore environments, the water phase
after
separation is diverted to treatment systems before re-injection back into the
same reservoir or
a different reservoir (after depletion), re-used for drilling or stimulation,
or discharged where
applicable or feasible. The water treatment is carried out to control any of
excessive solids,
dissolved oil, corrosion, chemical reactions, or growth of microbes. For an
offshore
application, the wastewater can be treated to remove oil and followed by
discharge to the sea
in compliance with relevant regulations.
[042] Recovery of the treated crude oil with reduced levels of heavy metals,
and
treatment of the recovered water phase can be carried out using processes and
equipment
known in the art, including separators, hydroclone, mesh coalescer, filter,
membrane,
centrifuge and the like for the oil / water separation; ion exchange,
electrodialysis,
electrodialysis reversal, electrochemical, deionization, evaporation, electro-
deionization,
reverse osmosis, membrane separation, oxidation reactor, filtration, and
combinations thereof
can be used for the treatment of recovered water.
[043] Diluent Fluid for the In-situ Reaction: The diluent fluid to be used for
the in-
situ reaction depends on the production fluids to be recovered, the state of
the production, the
location of the production well, amongst other factors.
[044] In one embodiment for the in-situ removal of heavy metals in a produced
fluid
from wells in low permeability formations, the dilution fluid is a lighter
hydrocarbon, e.g.,
pentane, diesel oil, gas oil, kerosene, gasoline, benzene, toluene, heptane,
and the like. In
one embodiment, the dilution fluid is non-potable water, e.g., connate water,
aquifer water,
seawater, desalinated water, oil field produced water, industrial by-product
water, or
combinations thereof. , e.g., connate water, aquifer water, seawater,
desalinated water, oil
fields produced water, industrial by-product water, or combinations thereof In
one
embodiment, the dilution fluid may be a mixture comprising a mixture of an oil
phase in
8
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
water. Besides the fixing agent, the dilution fluid may be augmented with
other additives
such as scale inhibitors, surfactants, proppants, etc. In one embodiment, the
dilution fluid is
from a water storage / treatment facility connected to a topside production
facility, wherein
produced water, seawater, etc., is recovered and prepared with the addition of
additives, e.g.,
fixing agents needed for the removal of the heavy metals. The dilution fluid
may be injected
into the production well at cold, heated, or ambient temperature.
[045] In one embodiment, the produced fluid such as crude oil is recovered in
the
same injection well for the dilution fluid and / or the fixing agent. In
another embodiment,
the recovery is through a second well located some distance from the injection
well referred
113 to above. In another embodiment, at least a portion of fixing agent may
adsorb to the rock
downhole or packing materials around the well. When hydrocarbons pass over the
treated
rock or the packing material, the fixing agent reacts with and extracts the
heavy metals into
the passing the dilution fluid for subsequent removal from the same production
well, or a
second well located some distance from the injection well. Dilution fluids are
driven to the
production well by formation re-compaction, fluid expansion and gravity.
[046] The well-servicing amount of injected dilution fluid depends on a number
of
factors including but not limited to the composition and salinity of the
dilution fluid
employed, the properties of the produced fluid to be recovered, the amount of
produced fluids
to be recovered, the characteristics of the formation rock, and the maturity
of the field. The
well-servicing amount as the volume ratio of dilution fluid to produced fluid
ranges from 1:3
to 60:1 in on embodiment, from 2:1 to 40:1 in a second embodiment, and from
10:1 to 30:1 in
a third embodiment.
[047] Fixing Agent: In one embodiment for the removal of arsenic and / or
mercury, the fixing agent is a sulfur-based compound for forming sulfur
complexes with the
heavy metals. Examples include organic and inorganic sulfide materials
(including
polysulfides), which in some embodiments, convert the heavy metal complexes
into a form
which is more soluble in an aqueous dilution fluid than in a produced fluid
such as shale oil.
In one embodiment, the fixing agent is a water-soluble monatomic sulfur
species, e.g.,
sodium sulfides and alkaline sulfides such as ammonium sulfides and
hydrosulfides, for the
extract of mercury into an aqueous dilution fluid as soluble mercury sulfur
complexes, such
as HgS22-. In another embodiment, the sulfur-based compound is any of hydrogen
sulfide,
bisulfide salt, or a polysulfide, for the formation of precipitates which
require separated from
the treated produced fluid by filtration, centrifugation, and the like. In yet
another
embodiment, the fixing agent is an organic polysulfide such as di-tertiary-
nonyl-polysulfide.
9
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
In another embodiment, the sulfur-based compound is an organic compound
containing at
least a sulfur atom that is reactive with mercury as disclosed in US Patent
No. 6,685,824, the
relevant disclosure is included herein by reference. Examples include but are
not limited to
dithiocarbamates, sulfurized olefins, mercaptans, thiophenes, thiophenols,
mono and dithio
organic acids, and mono and dithiesters.
[048] In another embodiment, the fixing agent is an oxidizing agent which
converts
the heavy metal to an oxidation state that is soluble in water. Examplary
fixing agents
include elemental halogens or halogen containing compounds, e.g., chlorine,
iodine, fluorine
or bromine, alkali metal salts of halogens, e.g., halides, chlorine dioxide,
etc; iodide of a
heavy metal cation; ammonium iodide; iodine-potassium iodide; an alkaline
metal iodide;
etheylenediamine dihydroiodide; hypochlorite ions (Oa such as Na0C1, Na0C12,
Na0C13,
Na0C14, Ca(0C1)2, NaC103,NaC102, etc.); vanadium oxytrichloride; Fenton's
reagent;
hypobromite ions; chlorine dioxine; iodate 103 (such as potassium iodate KI03
and sodium
iodate NaI03); monopersulfate; alkali salts of peroxide like calcium
hydroxide; peroxidases
that are capable of oxidizing iodide; oxides, peroxides and mixed oxides,
including
oxyhalites, their acids and salts thereof In one embodiment, the fixing agent
is selected
from KMn04, K2S208, K2Cr07, and C12. In another embodiment, the fixing agent
is selected
from the group of persulfates. In yet another embodiment, the fixing agent is
selected from
the group of sodium perborate, potassium perborate, sodium carbonate
perhydrate, potassium
peroxymonosulfate, sodium peroxocarbonate, sodium peroxodicarbonate, and
mixtures
thereof
[049] In one embodiment in addition to at least a fixing agent, a complexing
agent is
also added to the fixing agent to form strong complexes with the heavy metal
cations in the
produced fluids, e.g., Hg2', extracting heavy metal complexes from the oil
phase and / or the
interface phase of the oil-water emulsion into the dilution fluid by forming
soluble
complexes. Examples of complexing agents to be added to an oxidizing fixing
agent include
hydrazines, sodium metabisulfite (Na2S205), sodium thiosulfate (Na2S203),
thiourea,
thiosulfates (such as Na2S203), ethylenediaminetetraacetic acid, and
combinations thereof
In one embodiment with the addition of a complexing agent to a fixing agent,
the fixing agent
is added to the dilution fluid for injection into formation first to oxidize
the heavy metal, then
the complexing agent is subsequently added to form a complex that is soluble
in water. The
complexing agent can be injected at intervals into the formation, or it can be
subsequently
added after the introduction of the fixing agent to the formation for the in-
situ reaction.
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
[050] In one embodiment, the fixing agent reacts with heavy metals such
mercury,
forming insoluble heavy metal complexes, e.g., mercury sulfide, which
precipitate out of the
hydrocarbons and dilution fluid and at least a portion remains in the
reservoir. Examples of
fixing agents of this type may include sodium polysulfide, or polymeric
compounds
containing sulfide functional groups.
[051] The fixing agent can be added as in a solid form, or slurried /
dissolved in a
diluent, e.g., water, alcohol (such as methanol, ethanol, propanol), a light
hydrocarbon
diluent, or combinations thereof, in an amount sufficient for a molar ratio of
fixing agent to
heavy metals ranging from 1:1 to 20,000:1 in one embodiment; from 50:1 to
10,000:1 in a
second embodiment; from 100:1 to 5,000:1 in a third embodiment; and from 150:1
to 500:1
in a fourth embodiment. If a complexing agent is to be added to the in-situ
reaction to
effectively stabilize (forming complexes with) soluble heavy metals, e.g.,
mercury, in the oil-
water mixture, the amount as molar ratio of complexing agent to soluble
mercury ranges from
2:1 to about 3,000:1 from one embodiment; from 5:1 to about 1,000:1 in a
second
embodiment; and from 20:1 to 500:1 in a third embodiment.
[052] Figures Illustrating Embodiments: Reference will be made to the figures
to
further illustrate embodiments of the invention.
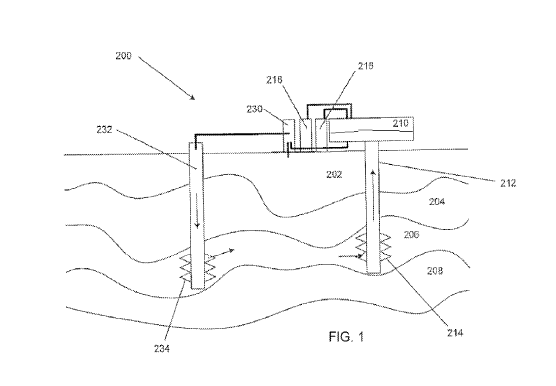
[053] Referring now to FIG. 1 for an embodiment of an in-situ mercury removal
system 200. In-situ system 200 includes body of water 202, formation 204,
formation 206,
and formation 208. Production facility including processing equipment for the
separation of
water containing mercury complexes from the treated crude may be provided at
the surface of
body of water 202. Dilution fluid such as water containing a fixing agent is
pumped down
well 232, to fractured portions 234 of formation 206. Water containing a
fixing agent
traverses formation 206 to aid the in-situ removal of mercury and the
production of oil and
gas going to well 212 and subsequently to production facility 210.
[054] Well 212 traverses body of water 202 and formation 204, and has openings
at
formation 206. Portions of formation may be fractured and/or perforated as
shown at 214.
Water containing fixing agent(s) may be injected under pressure into injection
zones 234
formed in the subsurface formation 206 to stimulate hydrocarbon production
through the
production wells in a field, and facilitate the mixing of the produced fluids
with the fixing
agent for the in-situ removal of mercury. Instead of or in addition to water
storage facility
230, sea water (for offshore wells) and brine produced from the same or nearby
formations
(for onshore wells) may be used as the water source to pump down well 232.
Produced
fluids from the earth's subsurface formation 206 can be recovered through
production
11
CA 02872808 2014-11-05
WO 2013/173634
PCT/US2013/041433
wellbore 212 with perforations 206 that penetrate hydrocarbon-bearing
formations or
reservoirs, facilitating the flow of the "treated" produced fluids as well
from the hydrocarbon-
bearing formations to the production wellbores.
[055] As oil and gas is produced from formation 206 it enters portions 214,
with
mercury being extracted from the oil and gas into the water 202 in the
process, and travels up
well 212 to separation facility 210. Gas and liquid may be separated, with gas
being sent to
gas storage 216, and treated crude to liquid storage 218, and water to water
storage 230.
[056] In one embodiment, water production facility includes equipment to
process
water, for example from body of produced water 202 and/or waste water
containing extracted
lo mercury from well 212. The recycled water may be processed and stored in
water storage
230 for recycle, for example by re-injection into well 232.
[057] FIG. 2 illustrates a second embodiment of a system 100 for the in-situ
removal
of heavy metals from a produced fluid. A vertical wellbore 101 comprising an
outer sleeve
102 and an inner bore 103 driven into reservoir 105 is connected to a bottom
wellbore portion
106. The bottom wellbore portion 106 comprises a perforated liner section 107
and an inner
bore 108.
[058] In operation, dilution fluid, e.g., produced water from water source 109
and
the fixing agent is pumped down outer sleeve 102 to perforated liner section
107, where the
injected water percolates into reservoir 105 and penetrates reservoir
materials to yield a
reservoir penetration zone. Crude oil in the formation flows down and collects
at or around
the toe 111 and may be pumped by a surface pump through inner bores 108 and
103 through
a motor at the wellhead 114 to a production tank 115 where oil and the water
mixture
containing extracted heavy metal complexes are separated. The wastewater may
be treated
and recycled back into the reservoir as shown.
[059] EXAMPLES: The following examples are given to illustrate the present
invention. However, that the invention is not limited to the specific
conditions or details
described in these examples.
[060] Example 1: 100 gram sample of formation material obtained from a
drilling
operation is crushed to 8-16 mesh and soaked in a solution of 1 wt% sodium
sulfide
(equivalent to 0.4 wt% sulfur) for at least 48 hours. The sample is placed
into a glass tube,
and a crude oil containing 444 ppb of mercury is pumped through the tube at
room
temperature at an equivalent rate of 0.1 m/day. Samples of the treated crude
are collected
and analyzed for mercury. It is anticipated that the mercury content in the
crude to be
reduced to at least 75%.
12