Note: Descriptions are shown in the official language in which they were submitted.
METHODS FOR SEPARATING AND DEWATERING FINE PARTICLES
FIELD OF THE INVENTION
[002] The instant invention pertains to methods of cleaning fine particles,
particularly
hydrophobic particles such as coal, of its impurities in aqueous media and
removing process
water from products to the levels that can usually be achieved by thermal
drying.
BACKGROUND OF THE INVENTION
[003] Coal is an organic material that is burned to produce heat for power
generation
and for industrial and domestic applications. It has inclusions of mineral
matter and may contain
undesirable elements such as sulfur and mercury. Coal combustion produces
large amounts of
ash and fugitive dusts that need to be handled properly. Therefore, run-of-the
mine coal is
cleaned of the mineral matter before utilization, which also helps increase
combustion
efficiencies and thereby reduces CO2 emissions. In general, coarse coal (50 x
0.15 mm) can be
cleaned efficiently by exploiting the specific gravity differences between the
coal and mineral
matter, while fine coal (approximately 0.15 mm and smaller) is cleaned by
froth flotation.
1
CA 2875024 2019-07-24
CA 02875024 2014-11-27
WO 2013/188419
PCT/US2013/045199
[004] In flotation, air bubbles are dispersed in water in which fine coal
and mineral
matter are suspended. Hydrophobic coal particles are selectively collected by
a rising stream of
air bubbles and form a froth phase on the surface of the aqueous phase,
leaving the hydrophilic
mineral matter behind. Higher-rank coal particles are usually hydrophobic and,
therefore, can be
attracted to air bubbles that are also hydrophobic via a mechanism known as
hydrophobic
interaction. The hydrophobic coal particles reporting to the froth phase and
subsequently to final
product stream are substantially free of mineral matter but contain a large
amount of process
water. Wet coal is difficult to handle and incurs high shipping costs and
lower combustion
efficiencies. Therefore, the clean coal product is dewatered using various
devices such as
cyclones, thickeners, filters, centrifuges, and/or thermal dryers.
[005] Flotation becomes inefficient with finer particles. On the other
hand, low-grade
ores often require fine grinding for sufficient liberation. In mineral
flotation, its efficacy
deteriorates rapidly below approximately 10 to 151.1m, while coal flotation
becomes difficult
below approximately 44 pm. Furthermore, it is difficult to dewater flotation
products due to the
large surface area and the high-capillary pressure of the water trapped in
between fine particles.
Flotation also becomes inefficient when particle size is larger than
approximately 150 lam for
minerals and 500 lam for coal.
[0061 Many
investigators explored alternative methods of separating mineral matter
from fine coal, of which selective agglomeration received much attention. In
this process, which
is also referred to as oil agglomeration or spherical agglomeration, oil is
added to an aqueous
suspension while being agitated. Under conditions of high-shear agitation, the
oil breaks up into
small droplets, collide with particles, adsorb selectively on coal by
hydrophobic interaction, form
pendular bridges with neighboring coal particles, and form agglomerates. The
high-shear
2
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
agitation is essential for the formation of agglomerates, which is also known
as phase inversion.
Nicol et al. (U.S. Patent No. 4,209,301) disclose that adding oil in the form
of unstable oil-in-
water emulsions can produce agglomerates without intense agitation. The
agglomerates formed
by these processes are usually large enough to be separated from the mineral
matter dispersed in
water by simple screening. One can increase the agglomerate size by subjecting
the slurry to a
low-shear agitation after a high-shear agitation.
[007] In general, selective agglomeration gives lower-moisture products and
higher coal
recoveries than froth flotation. On the other hand, it suffers from high
dosages of oil.
[008] The amounts of oil used in the selective agglomeration process are
typically in the
range of 5 to 30% by weight of feed coal (S,C. Tsai, in Fundamentals of Coal
Beneficiation and
Utilization, Elsevier, 2982, p. 335). At low dosages, agglomerates have void
spaces in between
the particles constituting agglomerates that are filled-up with water, in
which fine mineral matter,
e.g., clay, is dispersed, which in turn makes it difficult to obtain low
moisture- and low-ash
products. Attempts were made to overcome this problem by using sufficiently
large amounts of
oil so that the void spaces are filled-up with oil and thereby minimize the
entrapment of fine
mineral matter. Capes et al. (Powder Technology, vol. 40. 1 84, pp. 43-52)
disclose that the
moisture contents are in excess of 50% by weight when the amount of oil used
is less than 5%.
By increasing the oil dosage to 35%, the moisture contents are substantially
reduced to the range
of 17-18%.
[009] Keller et al. (Colloids and Surfaces, vol. 22, 1987, pp. 37-50)
increase the dosages
of oil to 55-56% by volume to fill up the void spaces more completely, which
practically
eliminated the entrapment problem and produced super-clean coal containing
less than 1-2% ash.
However, the moisture contents remained high. Keller (Canadian Patent No.
1,198,704) obtains
3
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
40% moisture products using fluorinated hydrocarbons as agglomerants.
Depending on the types
of coal tested, approximately 7-30% of the moisture was due to the water
adhering onto the
surface of coal, while the rest was due to the massive water globules trapped
in the agglomerates
(Keller et al., Coal Preparation, vol. 8, 1990, pp.1-17).
[0010] Smith et al (U.S. Patent No. 4,244,699) and Keller (U.S. Patent No.
4,248,698;
Canadian Patent No. 1,198,704) use fluorinated hydrocarbon oils with low
boiling points (40-
159 F) so that the spent agglomerants can be readily recovered and be
recycled, These reagents
are known to have undesirable effect on the atmospheric ozone layer.
Therefore, Keller (U.S.
Patent No. 4,484,928) and Keller et al. (U.S. Patent No, 4,770,766) disclose
methods of using
short chain hydrocarbons, e,g., 2-methyl butane, pentane, and heptane as
agglomerants. Like the
fluorinated hydrocarbons, these reagents have relatively low boiling points,
which allowed them
to be recovered and recycled.
[0011] Being able to recycle an agglomerant would be a significant step
toward
eliminating the barrier to commercialization of the selective agglomeration
process. Another way
to achieve this goal would be to substantially reduce the amount of the oils
used. Capes (in
Challenges in Mineral Processing, ed. by K.V.S. Sastry and M.C. Fuerstenau,
Society of Mining
Engineers, Inc., 1989, pp. 237-251) developed the low-oil agglomeration
process, in which the
smaller agglomerates (<1 mm) formed at low dosages of oil (0.5-5%) are
separated from mineral
matter by flotation rather than by screening. Similarly, Wheelock et al.,
(U.S. Patent No.
6,632,258) developed a method of selectively agglomerating fine coal using
microscopic gas
bubbles to limit the oil consumption to 0.3-3% by weight of coal.
[0012] Chang et al. (U.S. Patent No. 4,613,429) disclose a method of
cleaning fine coal
of mineral matter by selective transport of particles across the water/liquid
carbon dioxide
4
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
interface. The liquid CO2 can be recovered and recycled. A report shows that
the clean coal
products obtained using this liquid carbon dioxide (LICADO) process contained
5-15% moisture
after filtration (Cooper et al., Proceedings of the 25th Intersociety Energy
Conversion
Engineering Conference, 1990, August 12-17, 1990, pp. 137-142).
[0013] Yoon et al. (U.S. Patent No. 5,459,786) disclose a method of
dewatering fine coal
using recyclable non-polar liquids. The dewatering is achieved by allowing the
liquids to
displace surface moisture. Yoon et al. report that the process of dewatering
by displacement
(DBD) is capable of achieving the same or better level of moisture reduction
than thermal drying
at substantially lower energy costs, but do not show the removal of mineral
matter from coal.
[0014] As noted above, Keller (Canadian Patent No. 1,198,704) attributed
the high
moisture contents of the clean coal products obtained from his selective
agglomeration process to
the presence of massive water globules. Therefore, there remains a need for a
process that can
be used to clean hydrophobic particles, especially coal, of hydrophilic
impurities with low water
content.
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
SUMMARY OF THE INVENTION
[0015] It is an object of the instant invention to provide methods for
cleaning
hydrophobic particulate materials of hydrophilic contaminants. It is also an
object to provide a
clean hydrophobic fine particulate material that contains moisture levels that
is substantially
lower than can be achieved by conventional devvatering methods. In this
invention, the
particulate materials include, but are not limited to, minerals and coal
particles smaller than
about 1 mm in diameter, preferably smaller than about 0.5 mm, more preferably
smaller than
about 0.15 mm. Significant benefits of the present invention can be best
realized with the
ultrafine particles that are difficult to be separated by flotation.
[0016] In the instant invention, a hydrophobic liquid is added to an
aqueous medium, in
which a mixture (or slurry) of hydrophobic and hydrophilic particles are
suspended. The
hydrophobic liquid is added under conditions of high-shear agitation to
produce small droplets.
As used herein, "high shear", or the like, means a shear rate that is
sufficient to form large and
visible agglomerates, which is referred to phase inversion. As noted above,
under conditions of
high-shear agitation, oil breaks up into small droplets, which collide with
the fine particles, and
selectively form pendular bridges with neighboring hydrophobic particles, and
thereby produce
agglomerates of hydrophobic particles. The intensity of agitation required to
form the
agglomerates should vary depending on particle size, particle hydrophobicity,
particle shape,
particle specific gravity (S.G.), the type and amounts of hydrophobic liquid
used, etc. Ordinarily,
agglomerate formation typically occurs at impeller tip speeds above about 35
ft/s, preferably
above about 45 ft/s, more preferably above about 60 ft/s. In certain
embodiments, the aqueous
slurry is subjected to a low-shear agitation after the high-shear agitation to
allow for the
6
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
agglomerates to grow in size, which will help separate the agglomerates from
the hydrophilic
particles dispersed in the aqueous phase.
[0017] The agglomerated hydrophobic particles are separated from the
dispersed
hydrophilic particles using a simple size-size separation method such as
screening. At this stage,
the agglomerates are substantially free of the hydrophilic particles, but
still contain considerable
amount of the process water entrapped in the interstitial spaces created
between the hydrophobic
particles constituting the agglomerates. The entrapped water also contains
dispersed hydrophilic
particles dispersed in it.
[0018] To remove the entrained water, a second hydrophobic liquid is added
to the
agglomerates to disperse the hydrophobic particles in the liquid. The
dispersion liberates the
entrapped process water and the hydrophilic particles dispersed in it from the
agglomerates. The
hydrophobic particles dispersed in the second hydrophobic liquid are then
separated from the
hydrophobic liquid. The hydrophobic particles obtained from this final step
are practically free
of surface water and entrained hydrophilic particles. Typically, the amount of
hydrophilic
particles associated with the clean hydrophobic particles are less than 10 %
by weight, preferably
less than about 7 %, more preferably less than about 3 %; and less than about
10 % water,
preferably less than about 7 % water, more preferably less than about 5 %
water. Importantly, the
present invention is able to remove over 90 % of hydrophilic particles from
the hydrophobic
particles, preferably 95 %, more preferably 98%; and 95 % of water from the
hydrophobic
particles, preferably 95 %, more preferably 99 %.
[0019] It is, therefore, an object of the invention to separate hydrophobic
particles from
hydrophilic particles and simultaneously remove the water from the product
using a hydrophobic
liquid. The hydrophobic-hydrophilic separation (HHS) process described above
can also be used
7
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
to separate of one type of hydrophilic particles from another by
hydrophobizing a selected
component using an appropriate method. The invention, for example, may be
practiced with
different types of coal including without limitation bituminous coal,
anthracite, and
subbituminous coal.
[0020] It is another object of this invention to further reduce the
moisture of clean coal
product to the extent that they can be dried without using excessive heat, and
thus energy.
[0021] It is still another object to recover the spent hydrophobic liquid
for recycling
purposes.
8
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
BRIEF DESCRIPTION OF THE DRAWINGS
[0022] Figure 1 is a graph showing the contact angles of n-alkanes on a
hydrophobic coal
immersed in water (Yoon et al., PCT Application No. 61/300,270, 2011) that are
substantially
larger than those (-65 ) of water droplets on most hydrophobic coal (Gutierrez-
Rodriguez, et al.,
Colloids and Surfaces, 12, p.1, 1984).
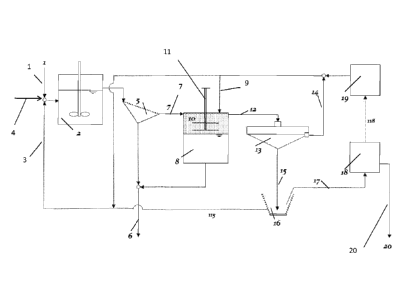
[0023] Figure 2 is a schematic of one embodiment of the process as
disclosed in the
present invention.
9
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0024] The present invention provides methods of separating a mixture of
hydrophobic
fine particulate materials suspended in water. It is also an object to dewater
at least one of the
products to a level that is substantially lower than can be achieved by
conventional dewatering
methods. In this invention, the fine particulate materials include but not
limited to minerals and
coal particles, smaller than about 1 mm in diameter, preferably smaller than
about mm, more
preferably smaller than about 0.5 mm more preferably less than about 0.15 mm.
The
hydrophobic particulate materials amenable to the present invention include,
but are not limited
to, coal, base-metal sulfides, precious metallic minerals, platinum group
metals, rare earth
minerals, non-metallic minerals, phosphate minerals, and clays.
[0025] The present invention provides a method of separating hydrophobic
and
hydrophilic particles from each other in two steps: 1) agglomeration of the
hydrophobic particles
in a first hydrophobic liquid/aqueous mixture; followed by 2) dispersion of
the agglomerates in a
second hydrophobic liquid to release the water trapped within the agglomerates
along with the
entrained hydrophilic particles. The second hydrophobic liquid can be the same
as the first
hydrophobic liquid in many cases. Essentially, the agglomeration step removes
the bulk of
hydrophilic particles and the water from the fine hydrophobic particles by
selectively
agglomerating the latter; and the dispersion step removes the residual process
water entrapped
within the structure of the agglomerates.
[0026] In the agglomeration step, a hydrophobic liquid is added to an
aqueous medium,
in which a mixture (or slurry) of fine hydrophobic (usually the product of
interest) and
hydrophilic (the contaminants) particles are suspended. The hydrophobic liquid
is added under
conditions of high-shear agitation to produce small droplets. The agitation
must be sufficient to
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
induce agglomeration of the hydrophobic particles. In general, the probability
of collision
between oil droplets and fine particles increases with decreasing droplet
size. Further, the high-
shear agitation helps prevent and/or minimize the formation of oil-in-water
emulsions stabilized
by hydrophobic particles. The hydrophobic liquid is chosen such that its
contact angle (0) on the
surface, as measured through aqueous phase, is larger than 90 . Use of such a
liquid allows it to
spontaneously displace the moisture from the surface. High shear agitation
produces small oil
droplets that are more efficient than larger droplets for collecting the
hydrophobic fine particles
and forming agglomerations of those particles. The hydrophilic particles
(usually undesired
material) remain in the aqueous phase.
[0027] When oil and water are mixed in the presence of spherical particles,
water-in-oil
emulsions are formed when B > 90 , and oil-in-water emulsions are formed when
0 < 90 (Binks,
B.P., Current Opinion in Colloid and Interface Science, 7, p.21, 2002). The
former is likely the
case when using the hydrophobic liquids that give contact angles greater than
90 . In the instant
invention, this problem is eliminated and/or minimized by adding a hydrophobic
liquid to
aqueous slurry under conditions of high-shear agitation.
[0028] While high-shear agitation can minimize the formation of water-in-
oil emulsions,
it may not prevent the residual process water from being entrapped in the
interstitial spaces
created in between the particles constituting agglomerates. In the dispersion
step, the entrapped
water can be removed by breaking the agglomerates and dispersing the
hydrophobic particles in
a hydrophobic liquid. The hydrophobic particles readily disperse in a
hydrophobic liquid due to
the strong attraction between hydrophobic particles and hydrophobic liquid. On
the other hand,
water has no affinities toward either the hydrophobic particles or the
hydrophobic liquid;
therefore, it is released (or liberated) from the agglomerates and are
separated from the
11
CA 02875024 2014-11-27
WO 2013/188419
PCT/US2013/045199
hydrophobic particles. During the dispersion step, the hydrophilic particles
in the entrained water
are also removed, providing an additional mechanism of separating hydrophobic
and hydrophilic
particles from each other.
[0029] The bulk of the hydrophobic liquid used in the instant invention is
recovered for
recycle purpose without involving phase changes by using appropriate solid-
liquid separation
means such as settling, filtration, and centrifugation. Only the small amount
of the residual
hydrophobic liquid adhering onto the surface of hydrophobic particles can be
recovered by
vaporization and condensation. Thermodynamically, the energy required to
vaporize and
condense the recyclable hydrophobic liquids disclosed in the instant invention
is only a fraction
of what is required to vaporize water from the surface of hydrophobic
particulate materials.
[0030] In floatation, for a bubble to collect a hydrophobic particle on
its surface, the thin
liquid film (TLF) of water (or wetting film) formed in between must thins and
ruptures rapidly
during the short time frame when the bubble and particle are in contact with
each other. In a
dynamic flotation cell, the contact times are very short typically in the
range of tens of
milliseconds or less. If the film thinning kinetics is slow, the bubble and
particle will be
separated from each other before the film ruptures. It has been shown that the
kinetics of film
thinning increases with increasing particle hydrophobicity (Pan et al.,
Faraday Discussion, 146,
p.325, 2010). Therefore, various hydrophobizing agents, called collectors, are
used to increase
the particle hydrophobicity and facilitate the film thinning process.
[0031] At the end of a film thinning process, the film must rupture to
form a three-phase.
A wetting film can rupture when the following thermodynamic condition is met,
rYsw < rYW [11
12
CA 02875024 2014-11-27
WO 2013/188419
PCT/US2013/045199
where 7s is the surface free energy of a solid (or particle) in contact with
air, while 7sw and 7w
are the same at the solid/water and water/air interfaces, respectively. The
term on the left, i.e.,
ys--ys.v, is referred to as wetting tension. Eq. [1] suggests that a particle
can penetrate the TLF
and from a three-phase contact if the film tension is less than the surface
tension of water. The
free energy gained during the film rupture process (26) is given by ys ¨
ysr,v¨ therefore, the
lower the wetting tension, the easier it is to break the film.
[0032] It
follows also that for a wetting tension to be small, it is necessary that Ysw
be
large. According to the acid-base interaction theory (van Oss, C.J.,
Interfacial Forces in Aqueous
Media, CRC Taylor and Francis, 2nd Ed., p.160), the solid/water interfacial
tension can be
calculated by the following relation,
isw = + y. ¨ 21 y ¨ 2,1r;y ¨ 2nK [2]
where 7sT'w is the Lifshitz-van der Waals component of y5 and 7WE'w is the
same of yw; 4and ys-
are the acidic and basic components of ys,, respectively; and and
yi-õ, are the same for water.
Essentially, the acidic and basic components represent the propensity for
hydrogen bonding.
According to Eq. [2], it is necessary to keep 4-and 1757 small to increase
ysw, which can be
accomplished by rendering the surface more hydrophobic. When a surface becomes
more
hydrophobic, ys decreases also, which helps decrease the wetting tension and
hence improve
flotation.
[0033] In
the present invention, a hydrophobic liquid (oil), rather than air, is used to
collect hydrophobic particles. In this case, oil-particle attachment can occur
under the following
condition.
[3]
13
CA 02875024 2014-11-27
WO 2013/188419
PCT/US2013/045199
where no represents the interfacial tension between solid and oil. According
to the acid-base
theory,
)(so= is + ro ro" ¨2117,+ro- ¨2117,-ro+ [4]
where the subscript 0 represents hydrophobic liquid phase. The hydrophobic
liquids that can be
used in the instant invention include, but are not limited to, n-alkanes (such
as petane, hexane,
and heptanes), n-alkenes, unbranched and branched cycloalkanes and
cycloalkenes with carbon
numbers of less than eight, ligroin, naphtha, petroleum naptha, petroleum
ether, liquid carbon
dioxide, and mixtures thereof. The acidic and basic components of these
hydrophobic liquids,
i.e., yo- and y6+, are zero as they cannot form hydrogen bonds with water,
which makes the last
two terms of Eq. [4] to drop out. Since yois nonzero, one may be concerned
that yso > is.
However, the value of the third term of Eq. [4], i.e., is is
substantial. For n-pentane
interacting with Teflon, for example, yo = 16.05 mJ/m2 and ys = 17.9 mJ/m2.
Since both of these
substances are completely non-polar, y = rgiv. and ys = yr, From those values,
one obtains the
fourth term to be -33.9 mJ/m2, the magnitude of which is larger than that of
yo. Therefore, in
reality yso < is and hence,
2'so Ysw < 75vv [5]
which suggests that the wetting film formed between n-pentane and a
hydrophobic surface can
more readily rupture than the same formed between air bubble and a hydrophobic
surface.
[0034]
According to the inequality of Eq. [5], an oil droplet placed on a hydrophobic
surface immersed in water should give a higher contact angle than an air
bubble can. Figure 1
shows the contact angles of various n-alkane hydrocarbons placed on a
hydrophobic coal. As
14
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
shown, all of the contact angles are larger than 900 and increase with
decreasing hydrocarbon
chain length. In comparison, the maximum contact angles of the air bubbles
adhering on the
surface of the most hydrophobic bituminous coal placed in water is
approximately 65
(Gutierrez-Rodriguez, et al., Colloids and Surfaces, 12, p.1, 1984). The large
differences
between the oil and air contact angles supports the thermodynamic analysis
presented above and
clearly demonstrates that oil is better than air bubble for collecting
hydrophobic particles from an
aqueous medium.
[0035] When an air bubble encounters a particle during flotation, it
deforms and causes a
change in curvature, which in turn creates an excess pressure (p) in the
wetting film. The excess
pressure created by the curvature change (pew) can be predicted using the
Laplace equation;
therefore, it is referred to as Laplace pressure or capillary pressure. The
excess pressure causes a
wetting film to drain. When its film thickness (h) reaches ¨200 nm, the
surface forces (e.g.,
electrical double-layer and van der Waals forces) present at the air/water and
bitumen/water
interfaces interact with each other and give rise to a disjoining pressure
(II). A pressure balance
along the direction normal to a film shows that the excess pressure becomes
equal to the Laplace
pressure minus disjoining pressure, i.e., p
= Pcur - n. Under most flotation conditions, both the
double-layer and van der Waals forces are repulsive (or positive) in wetting
films, causing the
excess pressure to decrease and hence the film thinning process be retarded.
[0036] The disjoining pressure can become negative when the particle
becomes
sufficiently hydrophobic by appropriate chemical treatment. In this case, the
excess pressure (p)
in the film will increase and hence accelerate the film thinning process. It
has been shown that
the negative disjoining pressures (II < 0) are created by the hydrophobic
forces present in
wetting films. In general, hydrophobic forces and hence the negative
disjoining pressures
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
increase with increasing particle hydrophobicity or contact angle (Pan et al.,
Faraday Discussion,
vol. 146, 325-340, 2010).
[0037] Thus, it is essential to render a particle sufficiently hydrophobic
for successful
flotation. An increase in particle hydrophobicity should cause the wetting
film to thin faster,
while at the same time cause the wetting tension to decrease. If the wetting
tension becomes less
than the surface tension of water, then the wetting film ruptures, which is
the thermodynamic
criterion for bubble-particle attachment.
[0038] A fundamental problem associated with the forced air flotation
process as
disclosed by Sulman et al. (U.S. Patent No. 793,808) is that the van der Waals
force in wetting
films are always repulsive, contributing to positive disjoining pressures
which is not conducive
to film thinning. When using oil to collect hydrophobic particles, on the
other hand, the van der
Waals forces in wetting films are always attractive, causing the disjoining
pressures to become
negative. As discussed above, a negative disjoining pressure causes an
increase in excess
pressure in the film and hence facilitates film thinning. For the reasons
discussed above, oil
agglomeration should have faster kinetics and be thermodynamically more
favorable than air
bubble flotation. An implication of the latter is that oil agglomeration can
recover less
hydrophobic particles, has higher kinetics, and gives higher throughput.
[0039] In the instant invention, the hydrophobic liquid is dispersed in
aqueous slurry. In
general, the smaller the air bubbles or oil droplets, the higher the
probability of collision, which
is a prerequisite for bubble-particle or oil-particle attachment. At a given
energy input, it would
be easier to disperse oil in water than to disperse air in water. The reason
is simply that the
interfacial tensions at the oil-water interfaces are in the range of 50 mJ/m2,
while the same at the
air/water interface is 72.6 mJ/m2.
16
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
[0040] In the instant invention, hydrophobic liquid, rather than air, is
used to collect
hydrophobic particles to take advantage of the thermodynamic and kinetic
advantages discussed
above. On the other hand, hydrophobic liquid is generally more expensive than
air to use.
Further, oil flotation products have high moistures. In the instant invention,
the first problem is
overcome by using hydrophobic oils that can be readily recovered and recycled
after use, while
the second problem is addressed as discussed below.
[0041] There are three basic causes for the high moisture content in oil
agglomeration
products (the agglomerated fine particles recovered by hydrophobic/hydrophilic
separation).
They include i) the film of water adhering on the surface of the hydrophobic
particles recovered
by oil flotation; ii) the water-in-oil emulsions (or Pickering emulsions)
stabilized by the
hydrophobic particles; and iii) the water entrapped in the interstitial void
spaces created by the
hydrophobic particles constituting agglomerates. In the instant invention, the
water from i and ii
are removed in the agglomeration stage by selecting a hydrophobic liquid with
contact angle
greater than 90 . The surface moisture (mentioned in i) is removed by using a
hydrophobic liquid
that can displace the water from the surface. Thermodynamically, the surface
moisture can be
spontaneously displaced by using a hydrophobic liquid whose contact angles are
greater than
90 .
[0042] The water entrainment in the form of water-in-oil emulsions
(mentioned in ii) is
eliminated by not allowing large globules of water to be stabilized by
hydrophobic particles. This
is accomplished by subjecting aqueous slurries to high-shear agitation.
Preferably, the high shear
agitation produces hydrophobic liquid droplet sizes to be smaller than the air
bubbles used in
flotation, which allows the process of the instant invention to be more
efficient than flotation.
Typically, the droplet sizes are in the range of 0.1 to 400 lam, preferably 10
to 300 11M, more
17
CA 02875024 2014-11-27
WO 2013/188419
PCT/US2013/045199
preferably 100 to 200 ium. The agitation can be accomplished by using a
dynamic mixer or an in-
line mixer known in the art. In-line mixers are designed to provide a
turbulent mixing while
slurries are in transit.
[0043] Under conditions of high-shear agitation, hydrophobic particles can
be detached
from oil-water interface and, thereby, destabilize water-in-oil emulsions or
prevent them from
forming. The amount of energy (E) required to detach hydrophobic particles
from the interface
can be calculated by the following relation (Binks, B.P., Current Opinion in
Colloid and
Interface Science, 7. 2002, p.21),
E = 2y,,, (1 cos 0) [6]
where yom is the interfacial tension, r is particle radius, and El is the
contact angle. The sign
inside the bracket is positive for removal into hydrophobic phase and is
negative for removal into
water phase. Therefore, the higher the contact angle, the easier it is to
remove particles to the
hydrophobic phase. Conversely, the lower the contact angle, the easier it is
to remove particles to
water phase. Thus, the high-shear agitation employed in the instant invention
offers a mechanism
by which less hydrophobic particles are dispersed in water phase, while more
hydrophobic
particles are dispersed in oil phase. Eq. [6] suggests also that the smaller
the particles, the easier
it is to detach particles from the oil-water interface and achieve more
complete dispersion.
[0044] The interstitial water trapped in between hydrophobic particles
(mentioned in iii)
is removed by dispersing the agglomerates in a second hydrophobic liquid. Upon
dispersion, the
trapped interstitial water is liberated from the agglomerates and are
separated from the
hydrophobic particles and subsequently from the hydrophobic liquid. As has
already been noted
in conjunction with Eq. [6], the smaller the particles and the higher the
contact angles, the easier
it is to disperse agglomerates into the hydrophobic liquid in which the
hydrophobic particles are
18
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
dispersed. The second hydrophobic liquid (used for dispersion) can be the same
of different
from the hydrophobic liquid used in the agglomeration step. The second
hydrophobic liquid can
be, but is not limited to, n-alkanes (such as petane, hexane, and heptanes), n-
alkenes, unbranched
and branched cycloalkanes and cycloalkenes with carbon numbers of less than
eight, ligroin,
naphtha, petroleum naptha, petroleum ether, liquid carbon dioxide, and
mixtures thereof.
[0045] The hydrophobic liquid recovered from the process is preferably
recycled. The
hydrophobic particles obtained from the solid/liquid separation step are
substantially free of
surface moisture. However, a small amount of the hydrophobic liquid may be
present on the coal
surface, in which case the hydrophobic particles may be subjected to a
negative pressure or
gentle heating to recover the residual hydrophobic liquid as vapor, which is
subsequently
condensed back to a liquid phase and recycled.
[0046] Figure 2 shows an embodiment of the instant invention. A mixture of
hydrophobic and hydrophilic particulate materials dispersed in water (stream
1) is fed to a
mixing tank 2, along with the hydrophobic liquid recovered downstream (stream
3) and a small
amount of make-up hydrophobic liquid (stream 4). The aqueous slurry and
hydrophobic liquid in
the mixing tank 2 is subjected to a high-shear agitation, e.g. by means of a
dynamic mixer as
shown to break the hydrophobic liquid into small droplets and thereby increase
the efficiency of
collision between particles and hydrophobic liquid droplets. As noted above,
collision efficiency
with fine particles should increase with decreasing droplet size. Further,
high-shear agitation is
beneficial for preventing entrainment of water into the hydrophobic liquid
phase in the form of
water-in-oil emulsions. Upon collision, the wetting films between oil droplets
and hydrophobic
particles thin and rupture quickly due to the low wetting tensions and form
agglomerates of the
hydrophobic particulate material, while hydrophilic particles remain dispersed
in water. The
19
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
agitated slurry flows onto a screen 5 (or a size separation device) by which
hydrophilic particles
(stream 6) and agglomerated hydrophobic particles (stream 7) are separated.
The latter is
transferred to a tank 8, to which additional (or a second) hydrophobic liquid
9 is introduced to
provide a sufficient volume of the liquid in which hydrophobic particles can
be dispersed. A set
of vibrating meshes 10 installed in the hydrophobic liquid phase provides a
sufficient energy
required to break the agglomerates and disperse the hydrophobic particles in
the hydrophobic
liquid phase. Vibrational frequencies and amplitudes of the screens are
adjusted by controlling
the vertical movement of the shaft 11 holding the screens. Other mechanical
means may be used
to facilitate the breakage of agglomerates. The hydrophobic particles
dispersed in hydrophobic
liquid (stream 12) flows to a thickener 13, in which hydrophobic particles
settle to the bottom
while clarified hydrophobic liquid (stream 14) is returned to the mixer 2 (in
this case, the
hydrophobic liquids in the agglomeration and dispersion steps are the same).
The thickened oily
slurry of hydrophobic particles 15 at the bottom of the thickener 13 is sent
(stream 15) to a solid-
liquid separator 16, such as centrifuge or a filter. The hydrophobic particles
(stream 17) exiting
the solid-liquid separator 16 are fed to a hydrophobic liquid recovery system
consisting of an
evaporator 18 and/or a condenser 19. The condensate is recycled back to the
mixer 2. The
hydrophobic particles (stream 20) exiting the evaporator 18 are substantially
free of both
moisture and of hydrophilic impurities. The hydrophilic particles recovered
from the screen 5
and the disperser 8 may be rejected or recovered separately.
[0047] The hydrophobic liquids that can be used in the process described
above include
shorter-chain n-alkanes and alkenes, both unbranched and branched, and
cycloalkanes and
cycloalkenes, with carbon numbers less than eight. These and other hydrophobic
liquids such as
ligroin (light naphtha), naphtha and petroleum naphtha, and mixtures thereof
have low boiling
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
points, so that they can be readily recovered and recycled by vaporization and
condensation.
Liquid carbon dioxide (CO2) is another that can be used as a hydrophobic
liquid in the instant
invention. When using low-boiling hydrophobic liquids, it may be necessary to
carry out the
process described in Figure 2 in appropriately sealed reactors to minimize the
loss of the
hydrophobic liquids by vaporization.
[0048] When processing high-value fine particulate materials, such as
precious metals,
platinum group metals (PGM), and rare earth minerals, it may not be necessary
to recycle the
spent hydrophobic liquids. In this case, hydrocarbons of higher carbon
numbers, such as
kerosene, diesel, and fuel oils may be used without provisions for recycling.
When using those
hydrophobic liquids, the instant invention can be similar to the conventional
oil agglomeration
process, except that agglomeration products are dispersed in a suitable
hydrophobic liquid to
obtain lower-moisture and lower-impurity products.
[0049] In the process diagram presented in Figure 2, a hydrophobic
particulate material
(e.g., high-rank coals) is separated from hydrophilic materials (e.g., silica
and clay), with the
resulting hydrophobic materials having very low surface moistures.
[0050] The processes as described in the instant invention can also be used
for separating
one-type of hydrophilic materials from another by selectively hydrophobizing
one but not the
other(s). For example, the processes can be used to separate copper sulfide
minerals from
siliceous gangue minerals by using an alkyl xanthate or a thionocarbamate as
hydrophobizing
agents for the sulfide minerals. The hydrophobized sulfide minerals are then
separated from the
other hydrophilic minerals using the process of the present invention.
[0051] Further, the process disclosed in the instant invention can be used
for further
reducing the moisture of the hydrophobic particulate materials dewatered by
mechanical
21
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
dewatering methods. For example, a filter cake consisting of hydrophobic
particles can be
dispersed in a hydrophobic liquid to remove the water entrapped in between the
void spaces of
the particles constituting the filter cake, and the hydrophobic liquid is
subsequently separated
from the dispersed hydrophobic particles and recycled to obtain low-moisture
products.
[0052] In addition, the process disclosed in the instant invention can be
used for
dewatering low-rank coals. This can be accomplished by heating a coal in a
hydrothermal reactor
in the presence of CO2. The water derived from the low-rank coal is displaced
by liquid CO) in
accordance to the DBD and the HHS mechanisms disclosed above. The product coal
obtained
from this novel process will be substantially free of water and can be
transported under CO2
atmosphere to minimize the possibility of spontaneous combustion.
[0053] Further, low-rank coals can be dewatered and upgraded by the present
invention
by derivatizing the low-rank coal to make it hydrophobic. It is well known
that low-rank coals
are not as hydrophobic as high-rank coals, such as bituminous coal and
anthracite. Some are so
hydrophilic that flotation using conventional coal flotation reagents, such as
kerosene and diesel
oils do not work. Part of the reasons is that various oxygen containing groups
such as carboxylic
acids are exposed on the surface. When a low-rank coal is upgraded in
accordance to the present
invention, it is preferably derivatized to render the surface hydrophilic
surface hydrophobic. In
one embodiment, the low-rank coal is first esterified with an alcohol, e.g.
methanol, ethanol, and
the like, using methods known in the art. The esterification renders the low-
rank coal more
hydrophobic (than before esterification). The reaction between the carboxyl
groups (R-COOH)
of the low-rank coal and alcohol (R-OH) is indicated as follows:
o H+ \ 0
R H 0 -R R + H20
/ OH / 0 - R
22
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
The reaction produces esters (R-COOR) on the surface of the low-rank coal and
water.
Preferably, the reaction takes place at about 25-75 C, more preferably about
45-55 C, and most
preferably at about 50 C. A catalyst, such as H+ ions may also be used for the
esterification. The
production of water by the condensation reaction represents a mechanism by
which "chemically-
bound" water is removed, while the substitution of the hydrophilic carboxyl
groups with short
hydrocarbon chains (R) renders the low-rank coal hydrophobic. Once esterified,
the low-rank
coal can be subjected to the HHS process disclosed in the instant invention to
remove the
residual process water and the entrained hydrophilic mineral using the
agglomeration/dispersion
steps as disclosed in the present invention.
[0054]
Without further description, it is believed that one of ordinary skill in the
art can,
using the preceding description and the following illustrative examples, make
and utilize the
present invention. The following examples are given to illustrate the present
invention. It
should be understood that the invention is not to be limited to the specific
conditions or details
described in the examples.
Example 1
[0055] A
sample of rougher concentrate was received from a chalcopyrite flotation plant
operating in the U.S. The sample assaying 15.9 %Cu was wet ground in a
laboratory ball mill for
12.5 hours to reduce the particle size to 80% finer than 20 The
mill product was subjected to
a standard flotation test, and the results were compared with those obtained
from an oil
agglomeration test. In each test, a 100 g mill product was treated with 4
lb/ton of potassium amyl
xanthate (KAX) to selectively hydrophobize chalcopyrite.
23
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
[0056] The flotation test was conducted using a Denver laboratory flotation
cell. The oil
agglomeration test was conducted using a kitchen blender with 100 g mill
product, 80 nil n-
pentane, and 400 ml tap water. The mixture was subjected initially to a high-
shear agitation for
40 s and subsequently to a low-shear agitation for another 40 s. Here, the
dividing line between
the high- and low-shear agitations is the impeller speed that can create
agglomerates of
hydrophobic (and/or hydrophobized) particles, which is referred to as phase
inversion. For the
case of bituminous coal, the phase inversion occurs at the rotational speeds
above approximately
8,000 r.p.m. The slurry in the blender was then poured over a screen to
separate the
agglomerated hydrophobized chalcopyrite particles from the dispersed
hydrophilic siliceous
gangue. The agglomerates recovered as screen overflow were then dispersed in n-
pentane, while
being agitated by means of an ultrasonic vibrator to assist dispersion. The
hydrophobized
chalcopyrite particles dispersed in pentane were then separated from pentane
and analyzed for
copper and moisture.
[0057] As shown in Table 1, oil agglomeration gave 92.3% copper recovery,
as
compared to 55.4% recovery obtained by flotation. The large improvement can be
attributed to
the differences in wetting tensions and the nature of the van der Waals forces
present in the
respective wetting films. On the other hand, the oil agglomeration test gave a
little lower copper
grade than the flotation test.
24
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
[0058] A problem associated with the oil agglomeration process was that the
moisture content of
the agglomerates was high (48.6%) due to the presence of the water trapped
within the
agglomerate structure. It was possible, however, to overcome this problem by
dispersing the
agglomerates in a hydrophobic liquid (n-pentane) and thereby liberating the
residual process
water entrapped within the agglomerate structure. The moisture content of the
chalcopyrite
concentrate obtained in this mariner was only 0.6 %, as shown in Tale 1.
Table 1
Copper Moisture (%wt)
Recovery Grade
Agglomerates Dispersed
(%) (%Cu)
Flotation 55.4 28.0
Agglomeration 92.3 23.1 48.6 0.6
[0059] This example shows that oil droplets are more efficient than air
bubbles for the recovery
of ultrafine hydrophobic particles from aqueous media, and that that the HHS
process can be
used to overcome the high moisture problem associated with the oil
agglomeration process.
Example 2
[0060] In this example, the process of the present invention was compared
with flotation.
The copper rougher concentrate assaying 15.9 %Cu was wet ground in a ball mill
using tap
water. The grinding times were varied to obtain mill products of different
particle sizes, and the
products were subjected to both flotation and HHS tests.
[0061] Table 2 compares the flotation and HHS test results obtained on a
mill product
with a particle size distribution of 80% finer than 22 rim. Each test was
conducted using ¨250 g
samples with 17.6 lb/ton potassium amyl xanthate (KAX) as a selective
hydrophobizing agent
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
(collector) for the copper mineral (chalcopyrite). As shown, flotation gave a
concentrate assaying
28.0 %Cu with a 67.4 % copper recovery, while the HHS process gave a
concentrate assaying
23.1 %Cu with a 91.9 % recovery. In the latter, the mill product was first
agglomerated with
pentane in a kitchen blender, which provided a high-shear agitation, and the
agglomerates were
subsequently separated from dispersed materials by means of a screen. The
agglomerates were
then dispersed in pentane so that the residual process water entrapped within
the agglomerate
structure is liberated from the agglomerates. A gentle mechanical agitation
facilitated the
dispersion by breaking the agglomerates.
Table 2
Weight Assays (%wt) Copper
Products
grams % wt Cu Moistur Recovery
Concentrate 151.1 68.6 28.0 67.4
Flotation
Tailing 69.2 31.4 8.4
Feed 220.3 100.0 15.9
Hydrophobic- Concentrate 238.2 98.3 23.1 0.14 91.9
Hydrophilic
Tailing 4.0 1.7 3.5
Separation (HHS)
Feed 242.2 100.0 15.9
[0062] The results presented in the table demonstrated that the present
invention is more
efficient in recovering fine particles. That the present process gave a
slightly lower copper grade
than the flotation process can be attributed to high recovery. Since the
droplets of hydrophobic
liquid (pentane) are more efficient than air bubbles in collecting hydrophobic
particles, the
former can recover composite particles that are less hydrophobic than fully
liberated chalcopyrite
particles, resulting in a lower-grade product. When the present process (HHS)
was conducted at
lower dosages of xanthate, the concentrate grade was improved.
26
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
Example 3
[0063] Monosized silica spheres of 11 im in diameter were hydrophobized and
subjected
to oil agglomeration, followed by a dispersion step as described in the
foregoing examples. The
silica particles were hydrophobized by immersing them in a 0.002 moles/liter
octadecyltrichlorosilane (OTS) solution. After a 10 minute immersion time, the
particles were
washed with toluene and subsequently with ethanol to remove the residual OTS
molecules
adhering on the surface.
[0064] An aqueous suspension containing 50 g of the hydrophobized silica at
10% solids
was placed in a kitchen blender and subjected to a high-shear agitation for 40
s in the presence of
20 ml of n-pentane, followed by 40 s of low-shear agitation. The agglomerates
showed 19.5%
moisture by weight.
[0065] The agglomerates were then dispersed in n-pentane while being
agitated
mechanically to facilitate the breakage of the agglomerates and thereby
release the water trapped
in between hydrophobic particles. The mechanical device that was used to help
break the
agglomerates was a set of vibrating meshes located in the pentane phase. The
tiny water droplets
liberated from the agglomerates fall to the bottom, while the hydrophobic
particles remain
dispersed in the organic phase. The hydrophobic particles separated from the
organic phase were
practically dry containing only 0.7% by weight of moisture. This example
clearly demonstrates
that the process of the present invention is efficient for recovering and
dewatering ultrafine
particles.
27
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
Example 4
[0066] Fundamentally, dewatering is a process in which solid/water
interface is replaced
by solid/air interface. For hydrophobic solids, the interfacial free energies
at the solid/oil
interface (y) is lower than the same at the solid/air interface (n) as
discussed in view of Eqs.
[4] and [5]. It should, therefore, be easier to displace the solid/water
interface with solid/oil
interface than with solid/air interface.
[0067] In this example, 200 ml of tap water and 50 g of monosized silica
particles of 71
[tm were agitated in a kitchen blender for a short period of time to
homogenize the mixture. A
known volume of a cationic surfactant solution, i.e., 4x10-6 M dodecylaminium
hydrochloride
(DAH), was then added to the mixture. The slurry was agitated for 3 minutes at
a low speed to
allow for the surfactant molecules to adsorb on the surface and render the
silica surface
hydrophobic. A volume of n-pentane (25 ml) was then added before agitating the
slurry at a high
speed for 40 s, followed by another 40 s of agitation at a low speed. The
agitated slurry was
poured over a screen to separate the agglomerates, formed in the presence of
the hydrocarbon oil,
from the water. The agglomerates were analyzed for surface moisture after
evaporating the
residual n-pentane adhering on the silica surface. The tests were conducted at
different DAH
dosages, with the results being presented in Table 3. As shown, the moisture
of the agglomerates
decreased with increasing DAH dosages. Nevertheless, the moistures remained
high due to the
presence of the water trapped in between the particles constituting the
agglomerates.
28
CA 02875024 2014-11-27
WO 2013/188419 PCT/1JS2013/045199
Table 3
DAH Dosage Moisture (%wt)
(lb/ton) Agglomerate Dispersed
2.2 24.20 7.8
4.4 23.67 0.9
15 22.5 0.06
[0068] Another set of agglomeration tests were conducted under identical
conditions. In
this set of experiments, the agglomeration step was followed by another step,
in which the
agglomerates were added to a beaker containing 100 ml of n-pentane. After a
gentle agitation by
hand, the hydrophobic silica particles dispersed in pentane was transferred to
a Buchner filter for
solid-liquid separation. Additional pentane was added to ensure that most of
the entrapped water
was displaced by the hydrophobic liquid. The filter cake was analyzed for
moisture after
evaporating the residual n-pentane from the surface. As shown in Table 3, the
moisture contents
of the filtered silica were substantially lower than those of the
agglomerates.
Example 5
[0069] Screen-bowl centrifuges are widely used to dewater clean coal
products from
flotation. However, the process loses ultrafine particles smaller than 44 tm
as effluents. In this
example, a screen-bowl effluent received from an operating bituminous coal
cleaning plant was
first subjected to two stages of flotation to remove hydrophilic clay, and the
froth product was
dewatered by vacuum filtration. The cake moisture obtained using
sorbitanmonooleate as a
dewatering aid was 20.2% by weight. The filter cake was then dispersed in a
hydrophobic liquid
29
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
(n-pentane) while the slurry was being agitated by sonication to facilitate
the breakage of the
agglomerate. Since the bituminous coal particles are hydrophobic, they can
readily be dispersed
in the hydrophobic liquid, while the water droplets trapped in between the
particles were released
and fall to the bottom. The ultrafine coal particles dispersed in the
hydrophobic liquid phase
contained only 2.3% moisture, as analyzed after appropriately separating the n-
pentane from the
coal. The results obtained in this example showed that most of the moisture
left in the filter cake
was due to the water trapped in the void spaces in between the particles
constituting the cake,
and that it can be substantially removed by the method disclosed in the
instant invention.
Example 6
[0070] Recognizing the difficulty in cleaning and dewatering ultrafine
coal, many
companies in the U.S. remove ultrafine coal by cyclone prior to flotation and
subsequently
dewater the froth product using screen-bowl centrifuges. A sample of cyclone
overflow
containing particles finer than 400 mesh (37 um) and 53.6% ash was subjected
to a series of
selective agglomeration tests using n-pentane as agglomerant. The tests were
conducted by
varying oil dosages, agitation speed, and agitation time. As shown in Table 4,
low-shear agitation
resulted in high-ash and high-moisture products. Combination of high- and low-
shear agitation
gave better results. In general, selective oil agglomeration did an excellent
job in ash rejection.
However, product moistures were high due to the entrapment of water within the
structure of the
agglomerates as has already been discussed.
CA 02875024 2014-11-27
WO 2013/188419 PCT/1JS2013/045199
Table 4
Product (%wt) Combustible Oil Dosage Agitation Speed &
Moisture Ash Recovery (% wt) (%wt) Time (min)
61.2 19.1 74.1 25 low shear (2)
24.8 11.1 67.0 50 high shear (0.5) & low shear (2)
43.1 10.4 66.1 30 high shear (0.5) & low shear (2)
50.9 11.0 72.2 20 high shear (0.5) & low shear (2)
45.8 13.2 75.8 10 high shear (0.5) & low shear (2)
[0071] The same coal sample was subjected to a series of oil agglomeration
tests as
described above. The amount of n-pentane used in each test was 20% by weight
of feed, and the
mixture was agitated for 30 s at a high speed and then for 2 min at a low
speed. The results
presented in Table 5 show that the moistures of the clean coal products were
substantially
reduced further from those obtained in the agglomeration tests (Table 4). The
improvements can
be attributed to the liberation of the interstitial water by dispersing the
agglomerates in a
hydrophobic liquid. Note also that by releasing the interstitial water, the
mineral matter dispersed
in it was also removed, resulting in a further reduction in ash content beyond
what was
obtainable using the selective agglomeration process alone. Thus, the process
of the instant
invention can improve both moisture and ash rejections.
31
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
Table 5
Product (% wt) Combustible
Moisture Ash Recovery (% wt)
3.1 2.8 78.8
3.5 3.9 84.7
3.8 2.9 83.4
10.6 3.0 78.8
10.0 2.5 78.7
4.4 3.0 80.1
9.1 3.7 86.7
Example 7
[0072] A sample of screen bowl effluent was received from a metallurgical
coal
processing plant and used for the process of the present invention. The
effluent, containing 11%
ash, was processed at 5% solids as received without thickening. The procedure
was the same as
described in the preceding examples. The amount of n-pentane used was 20% by
weight of feed,
and the slurry was agitated for 20 s in a kitchen blender at a high agitation
speed. The results
presented in Table 6 show that low-moisture and low-ash products were obtained
from the screen
bowl effluent. Since the coal was very hydrophobic, it was not necessary to
have a low-shear
agitation after the high-shear agitation.
[0073] The fourth column of Table 6 gives the %solids of the coal dispersed
in n-
pentane. The data presented in the table show that product moistures become
higher at higher
%solids. However, other operating conditions such as the amount of mechanical
energy used to
break agglomerates and facilitate dispersion also affected the moisture. In
this example, the
mechanical energy was provided by a set of two vibrating meshes moving up and
down in the
32
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
pentane phase. The solid content in dispersed phase is important in continuous
operation, as it
affects throughput and product moisture.
Table 6
Product (/0 wt) Reject Ash %Solid Combustible
Moisture Ash (% wt) Pentane Recovery %
8.1 2.3 84.0 7.1 98.0
6.1 2.0 84.3 6.3 98.0
6.8 2.7 83.8 7.3 98.1
2.8 2.2 83.0 1.7 97.8
Example 8
[0074] A bituminous coal processing plant is cleaning a 100 mesh x 0 coal
assaying
approximately 50% ash by flotation. Typically, clean coal products assay 9 to
11% ash. A coal
sample was taken from the plant feed stream and subjected to the method of the
present
invention. As shown in Table 7, the process produced low-ash (3.2 to 4.2%) and
low-moisture
(-1%) products with approximately 90% combustible recoveries. Without the
additional
dispersion step, the agglomerates assayed 37.2 to 45.1% moistures.
33
CA 02875024 2014-11-27
WO 2013/188419 PCT/1JS2013/045199
Table 7
Feed Product Moisture (% wt) Ash (%wt)
Combustible
Ash (%wt) Agglomerate Dispersed Clean Coal Reject
Recovery (%)
51.0 45.1 1.1 4.2 90.0 88.9
52.6 45.2 0.7 3.5 91.4 89.9
52.6 37.2 1.0 3.6 91.7 90.3
Example 9
[0075] Two different bituminous coal samples were subjected to continuous
process of
the present invention. n-pentane was used as a hydrophobic liquid. The process
was substantially
the same as described in Figure 2, except that an ultrasonic vibrator rather
than a set of vibrating
mesh was used to break the agglomerates and facilitate dispersion in n-
pentane. As shown in
Table 8, the oil agglomeration followed by a dispersion step reduced the ash
content of a
metallurgical coal from 51 to 3.6% ash with a 92% combustible recovery. With
another coal
sample assaying 40.4% ash, the ash contents were reduced to 3.3 to 5.0% with
combustible
recoveries in the neighborhood of 80%.
34
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
[0076] The bulk of the spent pentane was recycled without phase changes.
However, a
small amount of the hydrophobic liquid adhering onto coal surfaces was
recycled by evaporation
and condensation. The amount of n-pentane that was lost due to adsorption or
incomplete
removal from coal was in the range of 1.5 to 4 lb/ton of clean coal. The
energy cost for
evaporating n-pentane is substantially less than that for water in view of the
large differences in
boiling points (36.1 C vs. 100 C) and heats of vaporization (358 kJ/kg vs.
2,257 kJ/kg) for
pentane and water.
Table 8
Feed Product (%wt) Reject Combustible
Ash (%wt) Moisture Ash Ash (%wt) Recovery (%wt)
51.0 2.9 3.6 92.6 92.0
40.4 1.0 5.0 80.6 84.8
40.4 3.8 3.3 80.1 83.9
Example 10
[0077] In this example, a subbituminous coal (-1.18 + 0.6 mm) from Wyoming
was dry
pulverized and hydrophobized in water using sorbitanmonooleate (Reagent U) in
the presence of
water. The coal sample assayed 28% moisture by weight of as-received moisture,
8.5% ash, and
8,398 Btu/lb. As shown in Table 9, the process of the present invention
substantially reduced the
moisture and hence increased the heating values. In general, the moisture
reductions were higher
at higher reagent dosages and longer agitation times. As has been the cases
with bituminous
coals, the hydrophobized subbituminous coal also formed agglomerates in the
presence of a
hydrophobic liquid (n-pentane) but the agglomerate moistures were high due to
the entrapment
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
mechanism discussed in the foregoing examples. When the agglomerates were
dispersed in n-
pentane, however, the moisture contents were substantially reduced and the
heading values
increased accordingly.
Table 9
Reagent U Agglomerate Product
Dosage Agtn. Time Moisture Moisture Ash Heating Value
(lb/ton) (min) (%wt) (%wt) (%wt) (Btu/lb)
33.3 15 44.6 38.2 6.2 7,562
33.3 30 27.1 20.8 5.8 9,814
50 5 46.2 6.0 5.8 11,560
50 30 28.1 4.1 6.0 11,759
Example 11
[0078] In this example, a Wyoming coal sample was hydrophobized by
esterification
with ethanol and then subjected to the process of the present invention. The
reaction took place
at 50 C in the presence of a small amount of Fr ions as a catalyst. As has
already been
discussed, the esterification reaction removes the chemically bound water by
condensation and
renders the coal hydrophobic. The hydrophobized coal sample was then subjected
to the process
of the present invention (HHS) as discussed above to remove the water
physically entrapped
within the agglomerate structure and the capillaries of low-rank coals. As is
well known, much
of the 'inherent moistures' in low-rank coals is due to the water trapped in
macropres
(Katalambula and Gupta, Energy and Fuels, vol. 23, p. 3392, 2009).
[0079] The ethanol molecules may be small enough to penetrate the pore
structures and
remove the water by condensation and the displacement mechanisms involved in
the HHS
process. A strong evidence for this possibility may be that even the coarse
particles were readily
dewatered as shown in Table 10. Also shown in that table is that the
hydrophobized low-rank
36
CA 02875024 2014-11-27
WO 2013/188419
PCT/US2013/045199
coals form agglomerates, which trap large amount of moistures. When they were
dispersed in n-
pentane, however, the moisture was substantially reduced.
Table 10
Top Size of Agglomerate HHS Product
Coal Samples Moisture Moisture Ash Heating Value
(mm) (%wt) (%wt) (%wt) (Btu/lb)
0.350 40.3 3.20 9.92 10,827
0.600 25.62 3.20 9.82 11,019
1.180 28.34 2.87 8.4 11,216
6.300 37.63 2.30 6.27 11,529
[0080] Table 11 shows the results obtained with different alcohols for
esterification. As
shown, the shorter the hydrocarbon chains of the alcohols, the lower the
moistures of the
Wyoming coal samples treated by the HHS process. This finding suggests that
smaller molecules
can more readily enter the pores and remove the chemically-bound water by the
mechanisms
discussed above.
Table 11
Agglomerate HHS Product .
Alcohol Moisture Moisture Ash Heating Value
(%wt) (%wt) (%wt) (Btu/lb)
Methanol 25.39 8.32 2.35 11,625
Ethanol 30.92 9.14 3.20 11,125
2-Propanol 29.82 10.12 0.93 10,693
1-Pentanol 31.05 15.12 3.8 10,092
[0081] Although
certain presently preferred embodiments of the invention have been
specifically described herein, it will be apparent to those skilled in the art
to which the invention
37
CA 02875024 2014-11-27
WO 2013/188419 PCT/US2013/045199
pertains that variations and modifications of the various embodiments shown
and described
herein may be made without departing from the spirit and scope of the
invention. Accordingly, it
is intended that the invention be limited only to the extent required by the
appended claims and
the applicable rules of law.
38