Note: Descriptions are shown in the official language in which they were submitted.
CA 02875640 2014-12-03
BASF Coatings GmbH 27 Aug. 2013
PF0074123/S012952
Polymer in multicoat color and/or effect paint systems
The invention relates to an innovative polymer. It
further relates to a pigmented aqueous basecoat
material which comprises this polymer, and to the use
of said polymer in pigmented aqueous basecoat
materials. It further relates to a method for producing
multicoat paint systems, and also to the multicoat
paint systems producible by means of said method.
Moreover, the present invention relates to the refinish
of defect areas on multicoat paint systems.
The prior art has disclosed a host of methods for
producing multicoat color and/or effect paint systems.
Known from the prior art (cf., e.g., German patent
application DE 199 48 004 Al, page 17, line 37, to page
19, line 22, or German patent DE 100 43 405 Cl, column
3, paragraph [0018], and column 8, paragraph [0052], to
column 9, paragraph [0057], in conjunction with column
6, paragraph [0039], to column 8, paragraph [0050]) is
the following method, which involves
(1) applying a pigmented aqueous basecoat
material to a substrate,
(2) forming a polymer film from the coating
CA 02875640 2014-12-03
BASF Coatings GmbH 2 27 Aug. 2013
PF0074123/S012952
material applied in stage (1),
(3) applying a clearcoat material to the
resulting basecoat film, and subsequently
(4) curing the basecoat film together with the
clearcoat film.
This method is widely practiced, for example, both for
the original (OEM) finishing of motor vehicles, and for
the painting of metal and plastic parts for
installation in or on vehicles. In the course of these
operations, under certain conditions, adhesion problems
occur, particularly between basecoat and clearcoat.
Furthermore, the method is also used for the
refinishing of motor vehicle bodies. This is a
reference not only to OEM motor vehicle refinishing,
but also to the motor vehicle refinishing which takes
place, for example, in a workshop. A particular problem
here is the adhesion between the original finish and
the basecoat that is used for refinishing.
It was an object of the present invention, therefore,
to provide a polymer which can be used to produce
coatings which do not have the above-identified
disadvantages of the prior art. This relates to an
improvement in adhesion both in the painting of
CA 02875640 2014-12-03
BASF Coatings GmbH 3 27 Aug. 2013
PF0074123/S012952
metallic and plastics substrates and in automotive
refinish. An important factor in the painting of
metallic and plastics substrates, as well as the
adhesion of the basecoat to the substrate, is the
adhesion between basecoat and clearcoat. In the case of
automotive refinish, an important factor, alongside the
adhesion between basecoat and clearcoat, is the
adhesion between basecoat and original finish. This is
to be improved especially for use in OEM automotive
refinish.
The problems with adhesion are especially marked when
the coated substrates are exposed to weathering. The
object of the present invention was therefore also to
provide coatings which still possess outstanding
adhesion properties even after having been exposed to
weathering.
In the case of exposure by weathering, poor adhesion is
also manifested in particular in an incidence of
blisters and swelling. A further object of the present
invention, furthermore, was to prevent or reduce the
incidence of blisters and swelling.
This object has surprisingly been achieved by means of
4
a polymer which is prepared by reacting
(a) dimer fatty acids with
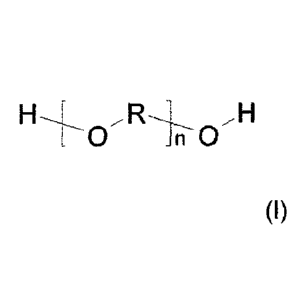
(b) at least one polyether of general structural
=
formula (I)
,H
L 0 Jn 0
(I)
where R is a C4 alkylene radical and n is selected
accordingly such that said polyether has a number-
average molecular weight of 450 to 2200 g/mol,
components (a) and (b) are used in a molar ratio of
0.7/2.3 to 1.3/1.7, and the resulting polymer has a
number-average molecular weight of 1500 to 5000 g/mol
and an acid number < 10 mg KOH/g.
The condition whereby n is selected such that said
polyether has a number-average molecular weight of 450
to 2200 g/mol may be illustrated as follows: Where, for
example, R is a tetramethylene radical and the number-
average molecular weight is to be 1000 g/mol, n is on
average between 13 and 14.
Component (a) :
Dimer fatty acids are mixtures prepared by
CA 2875640 2020-02-05
. ,
CA 02875640 2014-12-03
.=
BASF Coatings GmbH S
27 Aug. 2013
PF0074123/S012952
oligomerization of unsaturated fatty acids. Starting
materials used may be unsaturated C12 to C22 fatty
acids.
In the context of the present invention it is preferred
to use C18 fatty acids. Particular preference is given
to using C18 fatty acids, and very particular preference
to using linolenic, linoleic and/or oleic acid.
Depending on the reaction regime, the oligomerization
referred to above produces a mixture comprising chiefly
dimeric, but also trimeric molecules, and also
monomeric molecules and other by-products. Purification
is normally by distillation. Commercial dimer fatty
acids generally contain at least 80% by weight of
dimeric molecules, up to 19% by weight of trimeric
molecules, and not more than 1% by weight of monomeric
molecules and other by-products.
In the context of the present invention it is preferred
to use dimer fatty acids which consist to an extent of
at least 90% by weight of dimeric molecules, less than
5% by weight of trimeric molecules, and less than 5% by
weight of monomeric molecules and other by-products. It
is particularly preferred to use dimer fatty acids
1
. .
CA 02875640 2014-12-03
.,
BASF Coatings GmbH 6
27 Aug. 2013
PF0074123/S012952
which consist to an extent of 95% to 98% by weight of
dimeric molecules, less than 5% by weight of trimeric
molecules, and less than 1% by weight of monomeric
molecules and other by-products. Likewise particularly
preferred is the use of dimer fatty acids which consist
to an extent of at least 98% by weight of dimeric
molecules, less than 1.5% by weight of trimeric
molecules, and less than 0.5% of monomeric molecules
and other by-products.
Depending on reaction regime, dimer fatty acids include
both aliphatic and aromatic molecular fragments. The
aliphatic molecular fragments can be divided further
into linear and cyclic fragments, which in turn may be
saturated or unsaturated. By hydrogenation it is
possible to convert the aromatic and the unsaturated
aliphatic molecular fragments into corresponding
saturated aliphatic molecular fragments.
In the context of the present invention it is preferred
to use those dimer fatty acids which almost exclusively
comprise saturated aliphatic molecular fragments and
thus preferably have an iodine number 10 g/100 g.
Particularly preferred dimer fatty acids are those
7
which consist to an extent of at least 98% by weight of
dimeric molecules, less than 1.5% by weight of trimeric
molecules, and less than 0.5% by weight of monomeric
molecules and other by-products, and, furthermore, have
an iodine number 10 g/100 g.
Dimer fatty acids whose use is especially preferred
include Radiacid 0970, Radiacid 0971, Radiacid 0972,
Radiacid 0975, Radiacid 0976, and Radiacid 0977 from
Oleon, Pripol 1006, Pripol 1009, Pripol 1012, and
Pripol 1013 from Unichema, Empol 1009, Empol 1061, and
Empol 1062 from Cognis, and Unidyme 10 and Unidyme TI
from Arizona Chemical.
Component (b):
As component (b) at least one polyether is used of
general structural formula (I)
_ n-0
(I)
where R is a C3 to C6 alkylene radical. The index n
should be selected in each case such that said
polyether has a number-average molecular weight of 450
to 2200 g/mol. More preferably it has a number-average
molecular weight of 700 to 1400 g/mol, and very
preferably of 800
CA 2875640 2020-02-05
CA 02875640 2014-12-03
BASF Coatings GmbH 8 27 Aug. 2013
PF0074123/S012952
to 1200 g/mol. The number-average molecular weight is
measured by means of gel permeation chromatography
against a polymethyl methacrylate standard; eluent:
tetrahydrofuran.
In the polyether for use in accordance with the
invention, all n radicals R may be the same. Similarly,
however, it is also possible for different kinds of
radicals R to be present. Preferably all of the
radicals R are the same.
R is preferably a C3 or C4 alkylene radical. More
preferably it is an isopropylene radical or a
tetramethylene radical.
With very particular preference the polyether for use
in accordance with the invention is polypropylene
glycol or polytetrahydrofuran.
The preparation of the polymer of the invention has no
peculiarities. The esterification is accomplished
typically by means of a water separator. In that case
components (a) and (b) are used in a molar ratio of
0.7/2.3 to 1.3/1.7, preferably of 0.8/2.2 to 1.2/1.8,
and very preferably 0.9/2.1 to 1.1/1.9. The reaction is
I
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 9
27 Aug. 2013
PF0074123/S012952
discontinued when the polymer of the invention has an
acid number of 10 mg KOH/g. Preferably it has an acid
number of < 7.5 mg KOH/g, and very preferably of < 5 mg
KOH/g. The acid number here is determined by means of
DIN 53402.
The resulting polymer has a number-average molecular
weight of 1500 to 5000 g/mol, preferably 2000 to
4500 g/mol, and very preferably 3000 to 4000 g/mol. The
number-average molecular weight is measured by gel
permeation chromatography against a polymethyl
methacrylate standard; eluent: tetrahydrofuran.
The water solubility of the polymers of the invention
is low. If they are used in aqueous systems, they
accumulate at the interfaces, owing to their
incompatibility, and are therefore capable of
contributing to an improvement in the adhesion to
adjacent layers.
If a number-average molecular weight of 5000 g/mol is
exceeded, then the solubility of the polymers of the
invention in aqueous systems becomes so low that they
tend towards crystallization and may undergo
precipitation. If the number-average molecular weight
CA 02875640 2014-12-03
BASF Coatings GmbH 10 27 Aug. 2013
PF0074123/S012952
is below 1500 g/mol, then the water solubility of said
polymer is increased to such an extent that it is no
longer able to accumulate in sufficient concentration
at the interfaces. In that case an improvement in
adhesion is no longer achievable.
Particularly preferred embodiments are specified below:
a) In one particularly preferred embodiment of the
polymer of the invention, the dimer fatty
acid is prepared from linolenic, linoleic
and/or oleic acid, consists to an extent of
at least 98% by weight of dimeric molecules,
less than 1.5% by weight of trimeric
molecules, and less than 0.5% by weight of
monomeric molecules and other by-products,
and has an iodine number of 10 g/100 g.
b) In another particularly preferred embodiment of
the polymer of the invention, the polyether
for use in accordance with the invention is
polypropylene glycol or polytetrahydrofuran
and furthermore, has a number-average
molecular weight of 800 to 1200 g/mol.
CA 02875640 2014-12-03
BASF Coatings GmbH 11 27 Aug. 2013
PF0074123/S012952
C) In another particularly preferred embodiment of
the polymer of the invention, components (a)
and (b) are used in a molar ratio of 0.9/2.1
to 1.1/1.9.
d) In another particularly preferred embodiment of
the polymer of the invention, it has an acid
number of < 5 mg KOH/g.
e) In another particularly preferred embodiment of
the polymer of the invention, it has a
number-average molecular weight of 3000 to
4000 g/mol.
In one especially preferred embodiment of the polymer
of the invention, all of the features indicated under
a) to e) are realized in combination.
The present invention further relates to a pigmented
aqueous basecoat material which comprises at least one
polymer of the invention.
A basecoat material is a color-imparting intermediate
coating material which is used in motor vehicle
finishing and general industrial coating. It is applied
CA 02875640 2014-12-03
BASF Coatings GmbH 12 27 Aug. 2013
PF0074123/S012952
generally to a metallic or plastics substrate which has
been pretreated with a primer or primer-surfacer, and
occasionally is even applied directly to the plastics
substrate. Substrates used may also include existing
finishes, which may also need to be pretreated (by
sanding, for example). It is now entirely customary for
more than one basecoat film to be applied. In such a
case, accordingly, a first basecoat film constitutes
the substrate for a second. In order to protect a
basecoat film against environmental effects, in
particular, at least one additional clearcoat film is
applied over it.
The sum total of the weight-percentage fractions, based
on the total weight of the pigmented aqueous basecoat
material, of all of the polymers according to the
invention is preferably 0.1% to 30% by weight, more
preferably 1% to 20% by weight, and very preferably
1.596 to 15% by weight or even 2% to 12% by weight.
Where the amount of the polymer of the invention is
below 0.1% by weight, it may be that no improvement in
adhesion is achieved any longer. Where the amount is
more than 30% by weight, there may under certain
circumstances be disadvantages, such as an
CA 02875640 2014-12-03
BASF Coatings GmbH 13 27 Aug. 2013
PF0074123/S012952
incompatibility of said polymer in the basecoat
material, for example. Such incompatibility may be
manifested, for example, in uneven flow and also in
floating or sedimentation.
As already described above, the polymer of the
invention is of low solubility in aqueous systems. It
is therefore preferably used directly in the
preparation of the pigmented aqueous basecoat material,
and not only added, following preparation, to the other
wise completed basecoat material.
In one preferred embodiment, the sum total of the
weight-percentage fractions of all of the polymers
according to the invention is 0.1% to 30% by weight,
based on the total weight of the pigmented aqueous
basecoat material. Where preferred embodiments of the
polymers of the invention are used, the sum total of
the weight-percentage fractions of all preferred
embodiments of the polymers of the invention is
preferably likewise 0.1% to 30% by weight, based on the
total weight of the pigmented aqueous basecoat
material. With particular preference, the pigmented
aqueous basecoat material comprises exclusively, as
polymers of the invention, preferred embodiments of the
. .
CA 02875640 2014-12-03
,
DASF Coatings GmbH 14
27 Aug. 2013
PF0074123/S012952
polymers of the invention.
In one preferred embodiment, the sum total of the
weight-percentage fractions of all of the polymers
according to the invention is 1% to 20% by weight,
based on the total weight of the pigmented aqueous
basecoat material. Where preferred embodiments of the
polymers of the invention are used, the sum total of
the weight-percentage fractions of all preferred
embodiments of the polymers of the invention is
preferably likewise 1% to 20% by weight, based on the
total weight of the pigmented aqueous basecoat
material. With particular preference, the pigmented
aqueous basecoat material comprises exclusively, as
polymers of the invention, preferred embodiments of the
polymers of the invention.
In one especially preferred embodiment, the sum total
of the weight-percentage fractions of all of the
polymers according to the invention is 1.5% to 15% by
weight, based on the total weight of the pigmented
aqueous basecoat material. Where preferred embodiments
of the polymers of the invention are used, the sum
total of the weight-percentage fractions of all
preferred embodiments of the polymers of the invention
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 15 27
Aug. 2013
PF0074123/S012952
is preferably likewise 1.5% to 15% by weight, based on
the total weight of the pigmented aqueous basecoat
material. With particular preference, the pigmented
aqueous basecoat material comprises exclusively, as
polymers of the invention, preferred embodiments of the
polymers of the invention.
In one likewise especially preferred embodiment, the
sum total of the weight-percentage fractions of all of
the polymers according to the invention is 2% to 12% by
weight, based on the total weight of the pigmented
aqueous basecoat material. Where preferred embodiments
of the polymers of the invention are used, the sum
total of the weight-percentage fractions of all
preferred embodiments of the polymers of the invention
is preferably likewise 2% to 12% by weight, based on
the total weight of the pigmented aqueous basecoat
material. With particular preference, the pigmented
aqueous basecoat material comprises exclusively, as
polymers of the invention, preferred embodiments of the
polymers of the invention.
Examples of embodiments of the polymers of the
invention that are preferred in this context include
the following particularly preferred embodiments:
CA 02875640 2014-12-03
BASF Coatings GmbH 16 27 Aug. 2013
PF0074123/S012952
a) In one particularly preferred embodiment of the
polymer of the invention, the dimer fatty
acid is prepared from linolenic, linoleic
and/or oleic acid, consists to an extent of
at least 98% by weight of dimeric molecules,
less than 1.5% by weight of trimeric
molecules, and less than 0.5% by weight of
monomeric molecules and other by-products,
and has an iodine number of 10 g/100 g.
b) In another particularly preferred embodiment of
the polymer of the invention, the polyether
for use in accordance with the invention is
polypropylene glycol or polytetrahydrofuran
and, furthermore, has a number-average
molecular weight of 800 to 1200 g/mol.
c) In another particularly preferred embodiment of
the polymer of the invention, components (a)
and (b) are used in a molar ratio of 0.9/2.1
to 1.1/1.9.
d) In another particularly preferred embodiment of
the polymer of the invention, it has an acid
CA 02875640 2014-12-03
BASF Coatings GmbH 17 27 Aug. 2013
PF0074123/S012952
number of < 5 mg KOH/g.
e) In another particularly preferred embodiment of
the polymer of the invention, it has a
number-average molecular weight of 3000 to
4000 g/mol.
As a further example of embodiments of the polymers of
the invention that are preferred in this context,
mention may be made of that embodiment in which all of
the features indicated under a) to e) are realized in
combination.
The basecoat materials used in accordance with the
invention comprise color and/or effect pigments. Such
color pigments and effect pigments are known to the
skilled person and are described for example in Rompp-
Lexikon Lacke und Druckfarben, Georg Thieme Verlag,
Stuttgart, New York, 1998, pages 176 and 451. The
fraction of the pigments may be situated for example in
the range from 1% to 40% by weight, preferably 2% to
30% by weight, more preferably 3% to 25% by weight,
based on the total weight of the pigmented aqueous
basecoat material.
CA 02875640 2014-12-03
BASF Coatings GmbH 18 27 Aug. 2013
PF0074123/S012952
For the purposes of the present invention it is
preferred to use basecoat materials which as binders
comprise binders curable physically, thermally or both
thermally and with actinic radiation.
Besides the polymer of the invention, the pigmented
aqueous basecoat materials of the invention comprise
with particular preference at least one polyurethane
resin. Coating materials of this kind comprising
polyurethane resin may likewise be typically cured
physically, thermally, or both thermally and with
actinic radiation.
In the context of the present invention, the term
"physical curing" denotes the formation of a film by
release of solvent from polymer solutions or polymer
dispersions. Typically, no crosslinking agents are
necessary for this purpose.
In the context of the present invention, the term
"thermal curing" denotes the heat-
initiated
crosslinking of a coating film where in the parent
coating material either a separate crosslinking agent
or else self-crosslinking binders are employed. The
crosslinking agent comprises reactive functional groups
1
. .
CA 02875640 2014-12-03
,
BASF Coatings GmbH 19
27 Aug. 2013
PF0074123/S012952
which are complementary to the reactive functional
groups present in the binders. This is typically
referred to by those in the art as external
crosslinking. Where the complementary reactive
functional groups or autoreactive functional groups,
i.e., groups which react with groups of the same kind,
are already present in the binder molecules, the
binders are self-crosslinking. Examples of suitable
complementary reactive functional groups and
autoreactive functional groups are known from German
patent application DE 199 30 665 Al, page 7, line 28 to
page 9, lines 24.
In the context of the present invention, actinic
radiation means electromagnetic radiation such as near
infrared (NIR), UV radiation, more particularly UV
radiation, and particulate radiation such as electron
radiation. Curing by UV radiation is typically
initiated by radical or cationic photoinitiators.
Where thermal curing and curing with actinic light are
employed jointly, the term "dual cure" is also used.
In the present invention, preferred basecoat materials
are those which are curable physically and those which
CA 02875640 2014-12-03
BASF Coatings GmbH 20 27 Aug. 2013
PF0074123/S012952
are curable thermally or both thermally and with
actinic radiation, in other words by "dual cure".
Preferred thermally curing basecoat materials are those
which comprise a polyurethane resin binder and as
crosslinking agent an amino resin or a blocked or
nonblocked polyisocyanate, preferably an amino resin.
Among the amino resins, melamine resins are preferred.
The polyurethane resin preferably present may be
ionically and/or nonionically hydrophilically
stabilized. In preferred embodiments of the present
invention, the polyurethane resin is ionically
hydrophilically stabilized. The preferred polyurethane
resins are linear or contain branches. Particular
preference is given to a polyurethane resin in whose
presence olefinically unsaturated monomers have been
polymerized. The polyurethane resin (A) here may be
present in addition to the polymer originating from the
polymerization of the olefinically unsaturated
monomers, without these monomers being bonded
covalently to one another. Also possible, however, is
for the polyurethane resin (A) to be bonded covalently
to the polymer originating from the polymerization of
the olefinically unsaturated monomers. The olefinically
CA 02875640 2014-12-03
BASF Coatings GmbH 21 27 Aug. 2013
PF0074123/S012952
unsaturated monomers are preferably monomers containing
acrylate and/or methacrylate groups. It is likewise
preferred for the monomers containing acrylate and/or
methacrylate groups to be used in combination with
further olefinically unsaturated compounds that do not
contain any acrylate or methacrylate groups.
Olefinically unsaturated monomers attached to the
polyurethane resin (A) are more preferably monomers
containing acrylate groups or methacrylate groups,
producing polyurethane (meth)acrylates. With very
particular preference the polyurethane resin is a
polyurethane (meth)acrylate. The polyurethane resin
preferably present is curable physically, thermally, or
thermally and with actinic radiation. More particularly
it is curable thermally or both thermally and with
actinic radiation. With particular preference the
polyurethane resin comprises reactive functional groups
through which external crosslinking is possible.
Suitable saturated or unsaturated polyurethane resins
are described for example in
German patent application DE 199 14 896 Al, column
1, lines 29 to 49 and column 4, line 23 to column
µ
CA 02875640 2014-12-03
BASF Coatings GmbH 22 27
Aug. 2013
PF0074123/S012952
11, line 5;
- German patent application DE 199 48 004 Al, page
4, line 19 to page 13, line 48;
- European patent application EP 0 228 003 Al, page
3, line 24 to page 5, line 40;
- European patent application EP 0 634 431 Al, page
3, line 38 to page 8, line 9; or
- international patent application WO 92/15405, page
2, line 35 to page 10, line 32.
For the preparation of the polyurethane resin it is
preferred to make use of the aliphatic, cycloaliphatic,
aliphatic-cycloaliphatic, aromatic, aliphatic-aromatic
and/or cycloaliphatic-aromatic polyisocyanates known to
the skilled person.
As an alcohol component for preparing the polyurethane
resins it is preferred to use the saturated and
unsaturated, relatively high molecular mass and low
f
CA 02875640 2014-12-03
BASF Coatings GmbH 23
27 Aug. 2013
PF0074123/S012952
molecular mass, polyols and also, optionally,
monoalcohols in minor amounts, that are known to the
skilled person. Low molecular mass polyols used are
more particularly diols and, in minor amounts, triols
for the purpose of introducing branches. Examples of
suitable polyols of relatively high molecular mass are
saturated or olefinically unsaturated polyester polyols
and/or polyether polyols. Relatively high molecular
mass polyols used more particularly are polyester
polyols, especially those having a number-average
molecular weight of 400 to 5000 g/mol (measured by gel
permeation chromatography against a polymethyl
methacrylate standard, with tetrahydrofuran as eluent).
For the hydrophilic stabilization and/or for increasing
the dispersibility in aqueous medium, the polyurethane
resin preferably present may comprise particular ionic
groups and/or groups which can be converted into ionic
groups (potentially ionic groups). Such polyurethane
resins are referred to in the context of the present
invention as ionically hydrophilically stabilized
polyurethane resins. Likewise present may be nonionic
hydrophilically modifying groups. Preference is given,
however, to the ionically hydrophilically stabilized
polyurethanes. More specifically the modifying groups
I
. ,
CA 02875640 2014-12-03
BASF Coatings GmbH 24
27 Aug. 2013
PF0074123/S012952
are
- functional groups which can be converted into
cations by neutralizing agents and/or quaternizing
agents and/or cationic groups
(cationic
modification)
or
- functional groups which can be converted into
anions by neutralizing agents, and/or anionic
groups (anionic modification)
and/or
- nonionic hydrophilic groups
(nonionic
modification).
As the skilled person is aware, the functional groups
for cationic modification are, for example, primary,
secondary and/or tertiary amino groups, secondary
sulfide groups and/or tertiary phosphine groups, more
particularly tertiary amino groups and secondary
sulfide groups (functional groups which can be
converted into cationic groups by neutralizing agents
and/or quaternizing agents). Also noteworthy are the
cationic groups prepared from the aforementioned
functional groups using neutralizing agents and/or
quaternizing agents known to the skilled person, such
as primary, secondary, tertiary and/or quaternary
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 25
27 Aug. 2013
PF0074123/S012952
ammonium groups, tertiary sulfonium groups and/or
quaternary phosphonium groups, more particularly
quaternary ammonium groups and tertiary sulfonium
groups.
The functional groups for anionic modification are, as
is known, for example, carboxylic, sulfonic and/or
phosphonic acid groups, more particularly carboxylic
acid groups (functional groups which can be converted
into anionic groups by neutralizing agents), and also
anionic groups prepared from the aforementioned
functional groups using neutralizing agent known to the
skilled person, such as carboxylate, sulfonate and/or
phosphonate groups.
The functional groups for nonionic hydrophilic
modification are preferably poly(oxyalkylene) groups,
more particularly poly(oxyethylene) groups.
The ionically hydrophilic modifications may be
introduced into the polyurethane resin by monomers
which comprise the (potentially) ionic groups. The
nonionic modifications are introduced, for example,
through the incorporation of poly(ethylene) oxide
polymers as side groups or terminal groups of the
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 26
27 Aug. 2013
PF0074123/S012952
polyurethane molecules. The hydrophilic modifications
are introduced, for example, via compounds which
comprise at least one group that is reactive toward
isocyanate groups, preferably at least one hydroxyl
group. For introducing the ionic modification it is
possible to use monomers which as well as the modifying
groups comprise at least one hydroxyl group. For
introducing the nonionic modifications it is preferred
to use the alkoxypoly(oxyalkylene) alcohols and/or
polyetherdiols that are known to the skilled person.
The polyurethane resin may preferably be a graft
polymer. More particularly it is a polyurethane resin
grafted using olefinically unsaturated compounds,
preferably olefinically unsaturated monomers. In this
case, therefore, the polyurethane is grafted, for
example, with side groups and/or side chains that are
based on olefinically unsaturated monomers. The groups
or chains in question are more particularly side chains
based on poly(meth)acrylates. Poly(meth)acrylates in
the context of the present invention are polymers or
polymeric radicals which comprise monomers containing
acrylate and/or methacrylate groups, and preferably
consist of monomers containing acrylate and/or
methacrylate groups. Side chains based on
CA 02875640 2014-12-03
BASF Coatings GmbH 27 27 Aug. 2013
PF0074123/S012952
poly(meth)acrylates are side chains which are
constructed during graft polymerization using monomers
containing (meth)acrylate groups. In this case, during
the graft polymerization, preferably more than 50 mol%,
more particularly more than 75 mol%, more particularly
100 mol% of monomers containing (meth)acrylate groups
are used, based on the total amount of the monomers
used in the graft polymerization.
The side chains described are introduced into the
polymer preferably after the preparation of a primary
polyurethane resin dispersion. In this case the
polyurethane resin present in the primary dispersion
may comprise pendant and/or terminal olefinically
unsaturated groups, via which the graft polymerization
with the olefinically unsaturated compounds then
proceeds. The polyurethane resin for grafting may
therefore be an unsaturated polyurethane resin (A). The
graft polymerization is in that case a radical
polymerization of olefinically unsaturated reactants.
Also possible, for example, is for the olefinically
unsaturated compounds used for the graft polymerization
to comprise at least one hydroxyl group. In that case
it is also possible initially for there to be
attachment of the olefinically unsaturated compounds
CA 02875640 2014-12-03
BASF Coatings GmbH 28 27 Aug. 2013
PF0074123/S012952
via these hydroxyl groups, by reaction with free
isocyanate groups of the polyurethane resin. This
attachment occurs instead of or in addition to the
radical reaction of the olefinically unsaturated
compounds with any pendant and/or terminal olefinically
unsaturated groups that may be present in the
polyurethane resin. This is then followed, again, by
the graft polymerization via radical polymerization as
described earlier on above. In each case, polyurethane
resins are obtained that are grafted with olefinically
unsaturated compounds, preferably olefinically
unsaturated monomers.
As olefinically unsaturated compounds with which the
polyurethane resin (A) is preferably grafted it is
possible to use virtually all radically polymerizable,
olefinically unsaturated and organic monomers that are
available to the skilled person for these purposes. A
number of preferred monomer classes may be cited as
examples:
- hydroxyalkyl esters of (meth)acrylic acid or of
other alpha,beta-ethylenically unsaturated
carboxylic acids,
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 29
27 Aug. 2013
PF0074123/S012952
- (meth)acrylic acid alkyl esters and/or cycloalkyl
esters having up to 20 carbon atoms in the alkyl
radical,
- ethylenically unsaturated monomers, comprising at
5 least one acid
group, more particularly precisely
one carboxyl group, such as (meth)acrylic acid,
- vinyl esters of monocarboxylic acids branched in
alpha position and having 5 to 18 carbon atoms,
- reaction products of (meth)acrylic acid with the
10 glycidyl ester of
a monocarboxylic acid branched
in alpha position and having 5 to 18 carbon atoms,
- other ethylenically unsaturated monomers such as
olefins (for example ethylene), (meth)acrylamides,
vinylaromatic hydrocarbons (styrene for example),
15 and vinyl
compounds such as vinyl chloride and/or
vinyl ethers such as ethyl vinyl ethers.
Preference is given to using monomers containing
(meth)acrylate groups, and so the grafted-on side
20 chains are poly(meth)acrylate-based side chains.
The pendant and/or terminal olefinically unsaturated
groups in the polyurethane resin, via which graft
polymerization with the olefinically unsaturated
25 compounds is able to proceed, are introduced into the
CA 02875640 2014-12-03
BASF Coatings GmbH 30 27 Aug. 2013
PF0074123/5012952
polyurethane resin preferably by way of certain
monomers which as well as an olefinically unsaturated
group also comprise, for example, at least one group
reactive toward isocyanate groups. Hydroxyl groups and
also primary and secondary amino groups are preferred.
Hydroxyl groups are especially preferred.
Of course, the monomers described by which the pendant
and/or terminal olefinically unsaturated groups may be
introduced into the polyurethane resin may also be
employed without the polyurethane resin being
additionally grafted thereafter with olefinically
unsaturated compounds. It is preferred, however, for
the polyurethane resin to be grafted with olefinically
unsaturated compounds.
The polyurethane resin preferably present may be a
self-crosslinking and/or externally crosslinking
binder. The polyurethane resin preferably comprises
reactive functional groups through which external
crosslinking is possible. In this case, the pigmented
aqueous basecoat material preferably comprises at least
one crosslinking agent. More particularly, the reactive
functional groups through which external crosslinking
is possible are hydroxyl groups. For the purposes of
CA 02875640 2014-12-03
BASF Coatings GmbH 31 27 Aug. 2013
PF0074123/S012952
the method of the invention it is possible with
particular advantage to use polyhydroxy-functional
polyurethane resins. This means that the polyurethane
resin contains on average more than one hydroxyl group
per molecule.
The polyurethane resin is prepared by the typical
methods of polymer chemistry. This means, for example,
the polymerization of polyisocyanates and polyols to
polyurethanes, and the graft polymerization that
preferably then follows with olefinically unsaturated
compounds. These techniques are known to the skilled
person and may be adapted individually. Exemplary
preparation processes and reaction conditions are found
in European patent EP 0 521 928 Bl, page 2, line 57 to
page 8, line 16.
By film-forming solids is meant the nonvolatile weight
fraction of the basecoat material, excluding pigments
and any fillers. The film-forming solids can be
determined as follows: A sample of the pigmented
aqueous basecoat material (approximately 1 g) is
admixed with 50 to 100 times the amount of
tetrahydrofuran and then stirred for about 10 minutes.
The insoluble pigments and any fillers are then removed
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 32 27
Aug. 2013
PF0074123/S012952
by filtration, and the residue is rinsed with a little
THF, after which the THF is removed from the resultant
filtrate on a rotary evaporator. The filtrate residue
is dried at 120 C for two hours and the film-forming
solids that results in this drying operation is
weighed.
The polyurethane resin content is preferably between 5%
and 80%, more preferably between 8% and 70%, and very
preferably between 10% and 60%, by weight, based in
each case on the film-forming solids of the basecoat
material.
The polyurethane resin preferably present possesses
preferably a number-average molecular weight of 200 to
30 000 g/mol, more preferably of 2000 to 20 000 g/mol
(measured by means of gel permeation chromatography
against a polymethyl methacrylate
standard;
tetrahydrofuran is employed as eluent). It additionally
possesses, for example, a hydroxyl number of 0 to 250
mg KOH/g, but more particularly of 20 to 150 mg KOH/g.
The acid number of the polyurethane resin is preferably
5 to 200 mg KOH/g, more particularly 10 to 40 mg KOH/g.
The hydroxyl number is determined in accordance with
26 DIN/ISO 4629, the acid number in accordance with DIN
CA 02875640 2014-12-03
BASF Coatings GmbH 33 27 Aug. 2013
PF0074123/5012952
53402.
The pigmented aqueous basecoat material to be used may
further comprise at least one polyester, more
particularly a polyester having a number-average
molecular weight of 400 to 5000 g/mol (measured by
means of gel permeation chromatography against a
polymethyl methacrylate standard; tetrahydrofuran is
used as eluate). Corresponding polyesters are described
in DE 4009858 in column 6, line 53 to column 7, line 61
and column 10, line 24 to column 13, line 3.
Preferably, moreover, a thickener is present. Suitable
thickeners are inorganic thickeners from the group of
the phyllosilicates. Besides the inorganic thickeners,
however, it is also possible to use one or more organic
thickeners. These organic thickeners are preferably
selected from the group consisting of (meth)acrylic
acid-(meth)acrylate copolymer thickeners, such as, for
example, the commercial product Viscalex HV30 (Ciba,
BASF), and polyurethane thickeners, such as, for
example, the commercial product DSX 1550 from Cognis.
The thickeners used are different from the binders
used.
CA 02875640 2014-12-03
BASF Coatings GmbH 34 27 Aug. 2013
PF0074123/S012952
The pigmented aqueous basecoat material may also,
furthermore, comprise at least one additive. Examples
of such additives are salts which can be decomposed
thermally without residue or substantially without
residue, resins as binders, which are curable
physically, thermally and/or with actinic radiation and
are different from polyurethane resins, further
crosslinking agents, organic solvents, reactive
diluents, transparent pigments, fillers, molecularly
dispersely soluble dyes, nanoparticles, light
stabilizers, antioxidants, deaerating agents,
emulsifiers, slip additives, polymerization inhibitors,
radical-polymerization initiators, adhesion promoters,
flow control agents, film-forming assistants, sag
control agents (SCAs), flame retardants, corrosion
inhibitors, waxes, siccatives, biocides, and matting
agents.
Suitable additives of the aforementioned kind are known
for example from
German patent application DE 199 48 004 Al, page
14, line 4, to page 17, line 5,
- German patent DE 100 43 405 Cl, column 5,
CA 02875640 2014-12-03
BASF Coatings GmbH 35 27 Aug. 2013
PF0074123/S012952
paragraphs [0031] to [0033].
They are used in the customary and known amounts.
The solids content of the basecoat materials used in
accordance with the invention may vary according to the
requirements of the individual case. First and foremost
the solids content is guided by the viscosity that is
required for application, more particularly spray
application, and so it may be set by the skilled person
on the basis of his or her general art knowledge, with
the assistance where appropriate of a few rangefinding
tests.
The solids content of the basecoat materials is
preferably 5% to 70%, more preferably 8% to 60%, and
with particular preference 12% to 55%, by weight.
By solids content is meant that weight fraction which
remains as a residue on evaporation under defined
conditions. In the present specification, the solids
has been determined in accordance with DIN EN ISO 3251.
For this the coating material is evaporated at 130 C
for 60 minutes.
CA 02875640 2014-12-03
BASF Coatings GmbH 36 27 Aug. 2013
PF0074123/S012952
The basecoat materials used in accordance with the
invention can be prepared using the mixing methods and
mixing assemblies that are customary and known for
producing basecoat materials.
A further aspect of the present invention is a method
for producing a multicoat paint system by
(1) applying a pigmented aqueous basecoat material
to a substrate,
(2) forming a polymer film from the coating
material applied in stage (1),
(3) applying a clearcoat material to the resulting
basecoat film, and subsequently
(4) curing the basecoat film together with the
clearcoat film,
which is characterized in that in stage (1) a pigmented
aqueous basecoat material is used that comprises the
polymer of the invention. All details given above for
the polymer of the invention and for the pigmented
aqueous basecoat material are also applicable to the
CA 02875640 2014-12-03
BASF Coatings GmbH 37 27 Aug. 2013
PF0074123/S012952
inventive use. This applies additionally, not least, to
all preferred, more preferred, and very preferred
features.
The said method is used preferably for producing
multicoat color paint systems, effect paint systems,
and color and effect paint systems.
The pigmented aqueous basecoat material used in
accordance with the invention is applied typically to
metallic or plastics substrates which have been
pretreated with a primer or primer-surfacer. The said
basecoat material may optionally also be applied
directly to the plastics substrate.
If a metallic substrate is to be coated, it is
preferably also coated with an electrodeposition coat
before the primer or primer-surfacer is applied.
If a plastics substrate is being coated, it is
preferably also pretreated before the primer or primer-
surfacer is applied. The techniques most frequently
employed for such pretreatment are flaming, plasma
treatment, and corona discharge. It is preferred to use
flaming.
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 38
27 Aug. 2013
PF0074123/S012952
The application of the pigmented aqueous basecoat
material used in accordance with the invention to a
metallic substrate may take place in the film
thicknesses that are customary in the context of the
motor vehicle industry, in the range from, for example,
5 to 100 micrometers, preferably 5 to 60 micrometers.
This is done by employing spray application methods,
such as, for example, compressed air spraying, airless
spraying, high speed rotation, or electrostatic spray
application (ESTA), alone or in conjunction with hot
spray application such as hot air spraying, for
example.
After the pigmented aqueous basecoat material has been
applied, it may be dried by known techniques. For
example, 1-component basecoat materials may be flashed
at room temperature for 1 to 60 minutes and
subsequently dried preferably at optionally slightly
elevated temperatures of 30 to 80 C. Flashing and
drying for the purposes of the present invention mean
the evaporation of organic solvents and/or water,
whereby the coating material becomes drier, but is not
yet cured, or there is as yet no formation of a fully
crosslinked coating film.
I
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 39
27 Aug. 2013
PF0074123/S012952
A commercially customary clearcoat material is then
applied likewise by common techniques, the film
thicknesses again being situated within the customary
ranges, such as 5 to 100 micrometers, for example.
Following the application of the clearcoat material, it
may be flashed at room temperature for 1 to 60 minutes,
for example, and optionally dried. The clearcoat
material is then cured together with the pigmented
basecoat material applied. Here, for example,
crosslinking reactions take place, to produce a
multicoat color and/or effect paint system of the
invention on a substrate. Curing takes place preferably
thermally at temperatures from 60 to 200 C. Thermally
curing basecoat materials are preferably those which
comprise additionally a polyurethane resin binder and
as crosslinking agent an amino resin or a blocked or
nonblocked polyisocyanate, preferably an amino resin.
Among the amino resins, melamine resins are preferred.
Plastics substrates are coated basically in the same
way as for metallic substrates. Here, however, curing
takes place generally at much lower temperatures, of 30
to 90 C. It is therefore preferred to use two-component
I
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 40
27 Aug. 2013
PF0074123/3012952
clearcoat materials. In addition, it is preferred to
use basecoat materials which comprise a polyurethane
resin binder but no crosslinker.
With the aid of the method of the invention it is
possible to coat metallic and nonmetallic substrates,
especially plastics substrates, preferably motor
vehicle bodies or parts thereof.
The method of the invention can be used, in addition,
for the double coating in OEM finishing. This means
that a substrate which has been coated using the method
of the invention is painted a second time, again using
the method of the invention.
The invention further relates to multicoat paint
systems which are producible by the method described
above. These multicoat paint systems will be referred
to below as multicoat paint systems of the invention.
All of the abovementioned details relating to the
polymer of the invention, to the pigmented aqueous
basecoat material, and to the method of the invention
also apply correspondingly in respect of the said
multicoat paint system. This also applies not least in
CA 02875640 2014-12-03
BASF Coatings GmbH 41 27 Aug. 2013
PF0074123/S012952
respect of all preferred, more preferred, and very
preferred features.
The multicoat paint systems of the invention are
preferably multicoat color paint systems, effect paint
systems, and color and effect paint systems.
A further aspect of the invention relates to the method
of the invention where said substrate from stage (1) is
a multicoat paint system that has defect areas.
The method of the invention is suitable, accordingly,
for remedying defect areas on multicoat paint systems.
Defect areas or film defects are, generally,
disruptions on and in the coating, usually named
according to their shape or their appearance. The
skilled person knows a multiplicity of possible kinds
of such film defects. They are described for example in
Rompp-Lexikon Lacke und Druckfarben, Georg Thieme
Verlag, Stuttgart, New York, 1998, page 235,
"Filmfehler" ["Film defects"].
The multicoat paint systems produced using the method
of the invention may likewise exhibit such defect
areas. In one preferred embodiment of the method of the
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 42
27 Aug. 2013
PF0074123/S012952
invention, therefore, the substrate from stage (1) is a
multicoat paint system of the invention that exhibits
defect areas.
These multicoat paint systems are produced preferably
on motor vehicle bodies or parts thereof, by means of
the above-designated method of the invention, as part
of automotive OEM finishing. Where such defect areas
appear directly after OEM finishing, they are remedied
directly. Hence the term OEM automotive refinish is
also used. Where only small defect areas require
remedy, a repair is carried out not to the whole body
(double coating) but only to the "spot", as it is
called. This operation is called "spot repair".
Particularly preferable, therefore, is the use of the
method of the invention for remedying defect areas on
multicoat paint systems of the invention in OEM
automotive refinish.
So that the area remedied does not differ, in terms of
color, from the rest of the original finish, it is
preferred for the aqueous basecoat material used in
stage (1) of the method of the invention to remedy
defect areas to be the same as that used in the method
of the invention for producing the multicoat paint
CA 02875640 2014-12-03
BASF Coatings GmbH 43 27 Aug. 2013
PF0074123/S012952
system of the invention.
The details given above concerning the polymer of the
invention and concerning the aqueous pigmented basecoat
material therefore also apply in respect of the as-
discussed use of the method of the invention for
remedying defect areas on a multicoat paint system.
This applies in particular also for all preferred, more
preferred, and very preferred features specified. It is
preferred, moreover, for the multicoat paint systems of
the invention that are to be remedied to be multicoat
color paint systems, effect paint systems, and color
and effect paint systems.
The above-described defect areas on the multicoat paint
system of the invention can be remedied using the
method of the invention described above. For this
purpose, the surface of the multicoat paint system that
is to be remedied may initially be sanded. This is
followed by application of the pigmented aqueous
basecoat material to the defect area in the original
finish, by pneumatic spraying. Following the
application of the pigmented aqueous basecoat material,
it can be dried by known techniques. For example, the
basecoat material can be dried at room temperature for
CA 02875640 2014-12-03
BASF Coatings GmbH 44 27 Aug. 2013
PF0074123/S012952
1 to GO minutes, and can subsequently be dried at
slightly elevated temperatures, if desired, of 30 to
80 C. Flashing and drying in the context of the present
invention mean the evaporation of organic solvents
and/or water, but not so as to fully cure the coating
material. In the context of the present invention it is
preferred for the basecoat material to comprise a
polyurethane resin binder and as crosslinking agent an
amino resin, preferably a melamine resin.
Subsequently a commercial clearcoat material is
applied, by techniques that are likewise commonplace.
Following the application of the clearcoat material, it
can be flashed at room temperature for 1 to 60 minutes,
for example, and optionally dried. The clearcoat
material is then cured together with the applied
pigmented basecoat material.
In the case of what is known as low-temperature baking,
curing takes place preferably at temperatures from 20
to 90 C. Here it is preferred to use two-component
clearcoat materials. If, as described above, a
polyurethane resin is used as further binder and an
amino resin is used as crosslinking agent, then at
these temperatures there is only a low level of
I
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 45
27 Aug. 2013
PF0074123/S012952
crosslinking in the basecoat film, as a result of the
amino resin. Besides its function as a curing agent,
the amino resin in this case also serves for
plasticizing and is able to assist in pigment wetting.
As well as the amino resins, nonblocked isocyanates can
also be used. Depending on the nature of the isocyanate
used, they crosslink from temperatures as low as 20 C.
In the case of what is called high-temperature baking,
curing is accomplished preferably at temperatures from
130 to 150 C. Here, both one-component and two-
component clearcoat materials are used. If, as
described above, a polyurethane resin is used as
further binder and an amino resin is used as
crosslinking agent, then at these temperatures there is
crosslinking in the basecoat film, as a result of the
amino resin.
A further aspect of the present invention is the use of
the polymer of the invention in pigmented aqueous
basecoat materials for the purpose of promoting
adhesion. By this is meant the promotion of adhesion
with respect to those pigmented aqueous basecoat
materials which do not contain any polymer of the
invention.
CA 02875640 2014-12-03
BASF Coatings GmbH 46 27 Aug. 2013
PF0074123/S012952
The polymer of the invention can be used for promoting
adhesion in the finishing of metallic and plastics
substrates. It can also be used in automotive refinish.
By automotive refinish is meant both the OEM automotive
refinish and the automotive refinish of the kind which
takes place in a workshop, for example.
Where said pigmented aqueous basecoat materials are
used in the finishing of metallic and plastics
substrates, the use of the polymer of the invention
results more particularly in an improvement of the
adhesion between the basecoat film and the clearcoat
film immediately adjacent to it. The polymer of the
invention is therefore used preferably for promoting
adhesion between basecoat film and clearcoat film in
the coating of metallic and plastics substrates.
Where the said pigmented aqueous basecoat materials are
used in automotive refinish, the use of the polymer of
the invention results more particularly in an
improvement in adhesion between basecoat material and
original finish. The polymer of the invention is
therefore also used with preference for promoting the
adhesion between basecoat and original finish in
CA 02875640 2014-12-03
BASF Coatings GmbH 47 27 Aug. 2013
PF0074123/S012952
automotive refinishing, more preferably in OEM
automotive refinishing.
The adhesion problem is especially striking when the
coated substrates are exposed to weathering. Such
weathering conditions can be simulated by heat-
and-humidity storage conditions. This refers to the
storage of coated substrates in a climatic chamber in
accordance with test conditions CH of DIN EN ISO 6270-
2:2005-09.
Consequently the polymers of the invention are also
used more particularly for improving adhesion after
heat-and-humidity storage. The adhesion is investigated
preferably by means of a steam jet test in accordance
with test method A of DIN 55662:2009-12.
If coated substrates are exposed to weathering, any
poor adhesion is manifested not least in the occurrence
of blisters and swelling. The polymers of the invention
are therefore also used more particularly in order to
reduce or prevent the occurrence of blisters and
swelling. The presence of such blisters and swelling
may be appraised visually.
, .
CA 02875640 2014-12-03
BASF Coatings GmbH 48 27
Aug. 2013
PF0074123/S012952
The invention is elucidated below, using examples.
CA 02875640 2014-12-03
BASF Coatings GmbH 49 27 Aug. 2013
PF0074123/S012952
Examples
The dimer fatty acid used contains less than 1.5% by
weight of trimeric molecules, 98% by weight of dimeric
molecules, and an iodine number of < 10 g/100 g. It is
prepared on the basis of linolenic, linoleic, and oleic
acids.
Polyester 1 (P1):
Prepared as per Example D, column 16, lines 37 to 59 of
DE 4009858 A.
Polyester 2 (P2):
Prepared as per Example 5, page 18, paragraph 150,
DE 102009018249 Al.
Inventive binder 1 (BI1):
In a 4 1 stainless steel reactor, equipped with anchor
stirrer, thermometer, condenser, thermometer for
overhead temperature measurement, and water separator,
2 mol of po1yTHF1000, 579.3 g of dimer fatty acid
(1 mol) and 51 g of cyclohexane were heated to 100 C in
the presence of 2.1 g of di-n-butyltin oxide (Axion CS
2455, from Chemtura). Heating was continued slowly
until the onset of condensation. At a maximum overhead
CA 02875640 2014-12-03
BASF Coatings GmbH 50 27 Aug. 2013
PF0074123/5012952
temperature of 85 C, heating was then continued
gradually up to 220 C. The progress of the reaction was
monitored via determination of the acid number. When an
acid number of 3 mg KOH/g was
reached, remaining
cyclohexane was removed by distillation under vacuum.
This gave a viscous resin.
Condensate quantity (water): 34.9 g
Acid number: 2.7 mg KOH/g
Solids content (60 min at 1300C): 100.0%
Molecular weight (calibration: PMMA standards):
Mn: 3900 g/mol
Mw: 7200 g/mol
Viscosity: 5549 mPas
(measured at 23 C with a Brookfield CAP 2000+ rotary
viscometer, spindle 3, shear rate: 1333 s-1)
Inventive binder 2 (BI2):
In the same was as for the synthesis of binder BIl, 2
mol of polypropylene glycol having an average molecular
weight of 900 g/mol (Pluriol P900, BASF SE) and 1 mol
of dimer fatty acid were esterified in the presence of
1.9 g of di-n-butyltin oxide (Axione CS 2455, from
Chemtura). This gave a viscous resin.
I
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 51
27 Aug. 2013
PF0074123/5012952
Condensate quantity (water): 35.2 g
Acid number: 0.3 mg KOH/g
Solids content (60 min at 130 C): 100.0%
Molecular weight (calibration: PMMA standards):
Mn: 3400 g/mol
Mw: 7000 g/mol
Viscosity: 2003 mPas
(measured at 23 C with a Brookfield CAP 2000+ rotary
viscometer, spindle 3, shear rate: 1333 s-1)
Noninventive binder 1 (BC1):
In analogy to the above experiment, 2 mol of dimer
fatty acid and 1 mol of po1yTHF1000 were esterified in
the presence of 1.7 g of di-n-butyltin oxide (Axion CS
2455, from Chemtura). This gave a viscous resin.
Condensate quantity (water): 34.7 g
Acid number: 54.6 mg KOH/g
Solids content (60 min at 130 C): 100.0%
Molecular weight (calibration: PMMA standards):
Mn: 2800 g/mol
Mw: 8100 g/mol
Viscosity: 19 793 mPas
(measured at 23 C with a Brookfield CAP 2000+ rotary
viscometer, spindle 3, shear rate: 307 s-1)
1
CA 02875640 2014-12-03
BASF Coatings GmbH 52
27 Aug. 2013
PF0074123/5012952
Noninventive binder 2 (BC2):
In analogy to the above experiment, 1 mol of
polypropylene glycol having an average molecular weight
of 900 g/mol (Pluriol P900, BASF SE) and 2 mol of dimer
fatty acid were esterified in the presence of 1.6 g of
di-n-butyltin oxide (Axions CS 2455, from Chemtura).
This gave a viscous resin.
Condensate quantity (water): 34.6 g
Acid number: 57.2 mg KOH/g
Solids content (60 min at 130 C): 100.0%
Molecular weight (calibration: PMMA standards):
Mn: 2400 g/mol
Mw: 5800 g/mol
Viscosity: 7790 mPas
(measured at 23 C with a Brookfield CAP 2000+ rotary
viscometer, spindle 3, shear rate: 507 s1)
Examples of paint formulations
1. Preparation of a silver waterborne basecoat
material 1
The components listed in Table A under "aqueous phase"
were stirred together in the order stated to form an
CA 02875640 2014-12-03
BASF Coatings GmbH 53 27 Aug. 2013
PF0074123/S012952
aqueous mixture. In the next step, an organic mixture
was prepared from the components listed under "Organic
phase". The organic mixture was added to the aqueous
mixture. The resulting mixture was then stirred for
10 minutes and adjusted using deionized water and
dimethylethanolamine to a pH of 8 and a spray viscosity
of 58 mPas under a shearing load of 1000 s-1, measured
using a rotary viscometer (Rheomat RM 180 instrument
from Mettler-Toledo) at 23 C.
Table A:
Component Parts by
Aqueous phase weight
3% strength Na Mg phyllosilicate solution 26
Deionized water 13.6
Butyl glycol 2.8
Polyurethane-modified polyacrylate; prepared 4.5
as per page 7, line 55 to page 8, line 23 of
DE 4437535 A
50% strength by weight solution of DSX 1550 0.6
(BASF), rheological agent
P1 3.2
Tensid S (BASF), surfactant 0.3
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 54 27 Aug.
2013
PF0074123/S012952
Melamine-formaldehyde resin (Cymel 203 from 4.1
Cytec
10% strength dimethylethanolamine in water 0.3
Graft copolymer based on polyurethane; 20.4
prepared as per page 19, line 44 to page 20,
line 21 of DE 19948004 A
Tensid S (BASF), surfactant 1.6
3% strength by weight aqueous solution of 3.9
Viscalex HV 30; rheological agent, available
from BASF
Organic phase
Mixture of two commercial aluminum pigments, 6.2
available from Altana-Eckart
Butyl glycol 7.5
P1 5
Waterborne basecoat material El:
To prepare the inventive waterborne basecoat material
El, a coating material was prepared in analogy to the
preparation of waterborne basecoat material 1, using
BIl rather than the polyester P1 in both the aqueous
phase and the organic phase.
1
. ,
CA 02875640 2014-12-03
BASF Coatings GmbH 55
27 Aug. 2013
PF0074123/S012952
Waterborne basecoat material E2:
To prepare the noninventive waterborne basecoat
material E2, a coating material was prepared in analogy
to the preparation of waterborne basecoat material 1,
using BC1 rather than the polyester P1 in both the
aqueous phase and the organic phase.
Waterborne basecoat material E3:
To prepare the inventive waterborne basecoat material
E3, a coating material was prepared in analogy to the
preparation of waterborne basecoat material 1, using
BI2 rather than the polyester P1 in both the aqueous
phase and the organic phase.
Waterborne basecoat material E4:
To prepare the noninventive waterborne basecoat
material E4, a coating material was prepared in analogy
to the preparation of waterborne basecoat material 1,
using BC2 rather than the polyester P1 in both the
aqueous phase and the organic phase.
Table 1: Compositions of waterborne basecoat materials
1 and El to E4
1
. .
CA 02875640 2014-12-03
,
BASF Coatings GmbH 56 27 Aug. 2013
PF0074123/S012952
WBM [96 by weight] Polymer
1 4.92 P1
El 4.92 BIl
E2 4.92 BC1
E3 4.92 3I2
E4 4.92 BC2
The weight percentage figures in Table 1 are based on
the total weight of the waterborne basecoat material.
Comparison between waterborne basecoat material 1 and
waterborne basecoat materials El to E4
In order to determine the stability toward occurrence
of blisters and swelling after heat-and-humidity
storage, the multicoat paint systems were produced in
accordance with the following general procedure:
A steel panel with dimensions of 10 x 20 cm, coated
with a standard cathodic electrocoat (Cathoguarde 800
from BASF Coatings GmbH), was coated with a standard
medium-gray primer-surfacer (ALG 670173
from
Hemmelrath). After preliminary drying of the aqueous
primer-surfacer over a period of 10 minutes at 80 C, it
1
CA 02875640 2014-12-03
BASF Coatings GmbH 57 27
Aug. 2013
PF0074123/S012952
was baked at a temperature of 190 C over a period of
30 minutes.
Each waterborne basecoat material of Table 1 was then
applied pneumatically. The resulting waterborne
basecoat film was flashed at room temperature for
2 minutes and then dried for 10 minutes in a forced-air
oven at 70 C. Over the dried waterborne basecoat film,
a customary two-component clearcoat material was
applied (Proglosse 345 from BASF Coatings GmbH). The
resulting clearcoat film was flashed for 20 minutes at
room temperature. Subsequently, the waterborne basecoat
film and the clearcoat film were cured in a forced-air
oven at 160 C for 30 minutes. The present system
represents an overbaked original system and is referred
to below as original finish.
This original finish is sanded with an abrasive paper,
after which each of the waterborne basecoat materials
from Table 1 is pneumatically applied to this sanded
original finish. The resulting waterborne basecoat film
was flashed at room temperature for 2 minutes and then
dried for 10 minutes in a forced-air oven at 70 C. Over
the dried waterborne basecoat film, a so-called 80 C
two-component clearcoat material (FF230500 2K refinish
1
. .
CA 02875640 2014-12-03
,
BASF Coatings GmbH 58 27
Aug. 2013
PF0074123/S012952
clearcoat, scratch-resistant from BASF Coatings GmbH)
was applied. The resulting clearcoat film was flashed
at room temperature for 20 minutes. Subsequently, the
waterborne basecoat film and the clearcoat film were
cured in a forced-air oven at 80 C for 30 minutes.
The steel panels thus treated were then stored over a
period of 10 days in a climatic chamber in accordance
with test conditions CH of DIN EN ISO 6270-2:2005-09.
Subsequently, 24 hours following their removal from the
climatic chamber, the panels were inspected for
blistering and swelling.
The occurrence of blisters was assessed as follows
through a combination of 2 values:
- The number of blisters was evaluated by a quantity
rating of 1 to 5, with ml denoting very few
blisters and m5 very many blisters.
- The size of the blisters was evaluated by a size
rating, again from 1 to 5, with gl denoting very
small blisters and g5 denoting very large
blisters.
- The designation mOgO, accordingly, denotes a
blister-free finish after heat-and-humidity
storage, and represents a satisfactory result in
1
. .
CA 02875640 2014-12-03
BASF Coatings GmbH 59 27 Aug. 2013
PF0074123/5012952
terms of blistering.
Table 2: Blistering and swelling of waterborne basecoat
material 1 and waterborne basecoat materials El to E4
WBM Blistering Swelling Assessment
1 m5g1 none unsat.
El m0g0 none sat.
E2 m2g1 none unsat.
E3 m0g0 none sat.
E4 m3g1 none unsat.
Key:
m . number of blisters
g = size of blisters
sat. = satisfactory result
unsat. = unsatisfactory result
The results confirm that when using the polyesters of
the invention there are no longer any blisters after
heat-and-humidity exposure and there is no longer any
visible swelling.
2. Preparation of a red metallic waterborne basecoat
material 2
CA 02875640 2014-12-03
BASF Coatings GmbH 60 27 Aug. 2013
PF0074123/S012952
The components listed in Table B under "aqueous phase"
were stirred together in the order stated to form an
aqueous mixture. In the next step, an organic mixture
was prepared from the components listed under "Organic
phase". The organic mixture was added to the aqueous
mixture. The resulting mixture was then stirred for
minutes and adjusted using deionized water and
dimethylethanolamine to a pH of 8 and a spray viscosity
of 85 mPas under a shearing load of 1000 s-1, measured
10 using a rotary viscometer (Rheomat RN 180 instrument
from Mettler-Toledo) at 23 C.
Table B:
Component Parts by
Aqueous phase weight
3% strength Na Mg phyllosilicate solution 19.0
Polyurethane dispersion; prepared as per page 15.0
14, lines 38 to 53 of EP1358278 B1
Butyl glycol 2.0
Polyester P1 3.6
10% strength dimethylethanolamine in water 1.0
Resimene HM-2608; melamine-formaldehyde 3.0
resin, available from Ineos
Polyurethane-modified polyacrylate; prepared 3.0
1
CA 02875640 2014-12-03
BASF Coatings GmbH 61 27 Aug.
2013
PF0074123/S012952
as per page 7, line 55 to page 8, line 23 of
DE-A-4437535
Deionized water 19.7
50% strength by weight solution of DSX 1550 0.5
(BASF), rheological agent
2-Ethylhexanol 1.0
Plurio10 P900; polypropylene glycol, 0.8
available from BASF
3% strength by weight aqueous solution of 4.0
Viscalex HV 30; rheological agent, available
from BASF, in water
Red paste 14.0
Carbon black paste 0.1
Effect substance suspensions
PALIOCROMO ORANGE L2804; coated aluminum 3.0
pigment, available from BASF
Mixture of two commercial aluminum pigments, 0.4
available from Altana-Eckart
Butyl glycol 3.5
Mixing varnish; prepared as per page 14, 6.4
lines 15-27 of EP1799783 Al
Preparation of the red paste:
The red paste was prepared from 45.0 parts by weight of
I
. .
CA 02875640 2014-12-03
,
BASF Coatings GmbH 62 27
Aug. 2013
PF0074123/S012952
an acrylated polyurethane dispersion, prepared in
accordance with international patent application
WO 91/15528, binder dispersion A, and from 21.0 parts
by weight of Paliogeng red L 3885, 0.7 part by weight
of dimethylethanolamine, 2.5 parts by weight of 1,2-
propylene glycol and 30.8 parts by weight of deionized
water.
Preparation of the carbon black paste:
The carbon black paste was prepared from 25 parts by
weight of an acrylated polyurethane dispersion,
prepared in accordance with international patent
application WO 91/15528, binder dispersion A, and from
10 parts by weight of carbon black, 0.1 part by weight
of methyl isobutyl ketone, 1.36 parts by weight of
dimethylethanolamine, 2 parts by weight of a commercial
polyether (Pluriolg P900 from BASF Aktiengesellschaft)
and 61.45 parts by weight of deionized water.
Waterborne basecoat material E5:
To prepare the noninventive waterborne basecoat
material E5, a coating material was prepared in analogy
to the preparation of waterborne basecoat material 2,
using BIl rather than the Resimene HM-2608 melamine-
formaldehyde resin, available from Ineos in the aqueous
CA 02875640 2014-12-03
BASF Coatings GmbH 63 27 Aug. 2013
PF0074123/5012952
phase.
Waterborne basecoat material E6:
To prepare the noninventive waterborne basecoat
material E6, a coating material was prepared in analogy
to the preparation of waterborne basecoat material 2,
using polyester P1 rather than the Resimene HM-2608
melamine-formaldehyde resin, available from Ineos in
the aqueous phase.
Waterborne basecoat material E7:
To prepare the noninventive waterborne basecoat
material E7, a coating material was prepared in analogy
to the preparation of waterborne basecoat material 2,
using polyester P2 rather than the Resimene HM-2608
melamine-formaldehyde resin, available from Ineos in
the aqueous phase.
Table 3: Compositions of waterborne basecoat materials
2 and E5 to E7
WBM 196 by weight] MFR or MFR substitute
2 2.7 Resime HM-2608
2.16 P1
E5 2.7 BIl
CA 02875640 2014-12-03
BASF Coatings GmbH 64 27 Aug. 2013
PF0074123/S012952
2.16 P1
E6 2.7 P1
2.16 P1
E7 2.7 P2
2.16 P1
The weight percentage figures in Table 3 are based on
the total weight of the waterborne basecoat material.
MFR = melamine-formaldehyde resin
Comparison between waterborne basecoat material 2 and
waterborne basecoat materials E5-E7
In order to determine the stability toward occurrence
of blisters and swelling after heat-and-humidity
storage, the finishes on plastic were produced in
accordance with the following general procedure:
A plastics substrate made of PP-EPDM (Hifax EKC 112X
from Lyondell-Basell) was cleaned with a degreaser and
then briefly flamed using a laboratory flaming device
with a blue oxidizing flame.
Flaming parameter settings:
CA 02875640 2014-12-03
BASF Coatings GmbH 65 27 Aug. 2013
PF0074123/S012952
Speed 0.13 m/s
Flame-to-substrate distance 5 cm
Gas (propane) 258 L/h
Air 6600 L/h
Gas/air mix 1:26
Then a 2-component (2K) primer-surfacer (slate-gray,
R1471, with curing agent WW60738 100:10, from Worwag)
was applied to the panels, which were flashed at room
temperature for 10 minutes and then dried for
30 minutes in a forced-air oven at 80 C. The waterborne
basecoat material was applied pneumatically. The
resulting waterborne basecoat film was flashed at room
temperature for 2 minutes and then dried for 10 minutes
in a forced-air oven at 70 C. Over the dried waterborne
basecoat film, a customary low-bake 80 C two-component
clearcoat material (EverGloss 905 from BASF Coatings
GmbH) was applied. The resulting clearcoat film was
flashed at room temperature for 10 minutes. Then the
waterborne basecoat film and the clearcoat film were
cured in a forced-air oven at 80 C for 40 minutes. The
present system represents an original system for
plastic parts for installation in or on vehicles.
The panels were stored at room temperature for seven
CA 02875640 2014-12-03
BASF Coatings GmbH 66 27 Aug. 2013
PF0074123/S012952
days. They were then stored under heat-and-humidity
conditions, test conditions CH according to DIN EN ISO
6270-2:2005-09 (test duration 10 days).
30 minutes and 24 hours after removal from the climatic
chamber, the panels were inspected for blistering and
swelling.
Additionally, after 24 hours, a steam jet test was
carried out in accordance with test method A of DIN
55662:2009-12. Following steam jet exposure, the test
specimen is inspected for detachment of the paint
surface and detachment at the diagonal cross. An
evaluation is made of the degree of damage, in
accordance with the diagrams in Figure 4, section 9.2
of DIN 55662:2009-12. For the purposes of the
evaluation, minor delaminations of up to 1 mm,
resulting from adverse cutting at the cross-cut, are
disregarded. After the steam jet test there must be no
delamination of the coating films.
Table 4: Blisters/swelling/steam jet test of waterborne
basecoat material 2 and waterborne basecoat materials
E5 to E7
I
* CA 02875640 2014-12-03
BASF Coatings GmbH 67 27
Aug. 2013
PF0074123/S012952
WBM Blistering/ Blistering/ Steam jet
swelling 0.5 h swelling 24 H
2 m2g1/severe mlgl/moderate unsat. 4a BC/CC
E5 mOgO/slight mOgO/none sat.
E6 m351/severe m2g1/moderate unsat. 3a BC/CC
E7 m2g1/severe mlgl/moderate unsat. 2a BC/CC
Key:
m . number of blisters
g . size of blisters
sat. = satisfactory result
unsat. = unsatisfactory result
BC/CC . basecoat-clearcoat separation plane
4a, 3a and 2a refer to the characteristic values in
DIN 55662.
The results show clearly that when using the polyesters
of the invention there are no longer any blisters after
heat-and-humidity exposure and there is no longer any
visible swelling. Furthermore, the basecoat-clearcoat
adhesion has significantly improved.
3. Preparation of a silver waterborne basecoat
material 3
The components listed in Table C under "aqueous phase"
were stirred together in the order stated to form an
CA 02875640 2014-12-03
BASF Coatings GmbH 68 27 Aug. 2013
PF0074123/S012952
aqueous mixture. In the next step, an organic mixture
was prepared from the components listed under "Organic
phase". The organic mixture was added to the aqueous
mixture. The resulting mixture was then stirred for
10 minutes and adjusted using deionized water and
dimethylethanolamine to a pH of 8 and a spray viscosity
of 58 mPas under a shearing load of 1000 s-1, measured
using a rotary viscometer (Rheomat RN 180 instrument
from Mettler-Toledo) at 23 C.
Table C:
Component Parts by
Aqueous phase weight
3% strength Na Mg phyllosilicate solution 26
Deionized water 21.7
Butyl glycol 2.8
Polyurethane-modified polyacrylate; prepared 4.5
as per page 7, line 55 to page 8, line 23 of
DE 4437535 A
50% strength by weight solution of DSX 1550 0.6
(BASF), rheological agent
P1 13.3
Tensid S (BASF), surfactant 0.3
CA 02875640 2014-12-03
BASF Coatings GmbH 69 27 Aug. 2013
PF0074123/S012952
Melamine-formaldehyde resin (Cymel 203 from 4.1
Cytec
10% strength dimethylethanolamine in water 0.3
Graft copolymer based on polyurethane; 1.8
prepared as per page 19, line 44 to page 20,
line 21 of DE 19948004 A
Tensid S (BASF), surfactant 1.6
3% strength by weight aqueous solution of 3.9
Viscalex HV 30; rheological agent, available
from BASF
Organic phase
Mixture of two commercial aluminum pigments, 6.2
available from Altana-Eckart
Butyl glycol 7.5
P1 5
Waterborne basecoat material E8:
To prepare the inventive waterborne basecoat material
E8, a coating material was prepared in analogy to the
preparation of waterborne basecoat material 3, using
BIl rather than the polyester P1 in both the aqueous
phase and the organic phase.
Waterborne basecoat material E9:
To prepare the noninventive waterborne basecoat
CA 02875640 2014-12-03
BASF Coatings GmbH 70 27 Aug. 2013
PF0074123/5012952
material E9, a coating material was prepared in analogy
to the preparation of waterborne basecoat material 3,
using BC1 rather than the polyester P1 in both the
aqueous phase and the organic phase.
Waterborne basecoat material E10:
To prepare the inventive waterborne basecoat material
E10, a coating material was prepared in analogy to the
preparation of waterborne basecoat material 3, using
BI2 rather than the polyester P1 in both the aqueous
phase and the organic phase.
Waterborne basecoat material Ell:
To prepare the noninventive waterborne basecoat
material Ell, a coating material was prepared in
analogy to the preparation of waterborne basecoat
material 3, using BC2 rather than the polyester P1 in
both the aqueous phase and the organic phase.
Table 5: Compositions of waterborne basecoat materials
3 and E8 to Ell
WBM (% by weight] Polymer
3 10.98 P1
. .
CA 02875640 2014-12-03
,
BASF Coatings GmbH 71 27 Aug. 2013
PF0074123/S012952
E8 10.98 BIl
E9 10.98 BC1
El0 10.98 BI2
Ell 10.98 BC2
The weight percentage figures in Table 1 are based on
the total weight of the waterborne basecoat material.
Comparison between waterborne basecoat material 3 and
waterborne basecoat materials E8 to Ell
In order to determine the stability toward occurrence
of blisters and swelling after heat-and-humidity
storage, the multicoat paint systems were produced in
accordance with the following general procedure:
A steel panel with dimensions of 10 x 20 cm, coated
with a standard cathodic electrocoat (Cathoguare 800
from BASF Coatings GmbH), was coated with a standard
medium-gray primer-surfacer (ALG 670173 from
Hemmelrath). After preliminary drying of the aqueous
primer-surfacer over a period of 10 minutes at 80 C, it
was baked at a temperature of 190 C over a period of
30 minutes.
r
' CA 02875640 2014-12-03
,
BASF Coatings GmbH 72 27
Aug. 2013
PF0074123/S012952
Each waterborne basecoat material of Table 5 was then
applied pneumatically. The resulting waterborne
basecoat film was flashed at room temperature for
2 minutes and then dried for 10 minutes in a forced-air
oven at 70 C. Over the dried waterborne basecoat film,
a customary two-component clearcoat material was
applied (Proglossa 345 from BASF Coatings GmbH). The
resulting clearcoat film was flashed for 20 minutes at
room temperature. Subsequently, the waterborne basecoat
film and the clearcoat film were cured in a forced-air
oven at 160 C for 30 minutes. The present system
represents an overbaked original system and is referred
to below as original finish.
This original finish is sanded with an abrasive paper,
after which each of the waterborne basecoat materials
from Table 5 is applied pneumatically to this sanded
original finish. The resulting waterborne basecoat film
was flashed at room temperature for 2 minutes and then
dried for 10 minutes in a forced-air oven at 70 C. Over
the dried waterborne basecoat film, a so-called 80 C
two-component clearcoat material (FF230500 2K refinish
clearcoat, scratch-resistant from BASF Coatings GmbH)
was applied. The resulting clearcoat film was flashed
CA 02875640 2014-12-03
BASF Coatings GmbH 73 27 Aug. 2013
PF0074123/5012952
at room temperature for 20 minutes. Subsequently, the
waterborne basecoat film and the clearcoat film were
cured in a forced-air oven at 80 C for 30 minutes.
The steel panels thus treated were then stored over a
period of 10 days in a climatic chamber in accordance
with test conditions CH of DIN EN ISO 6270-2:2005-09.
Subsequently, 24 hours following their removal from the
climatic chamber, the panels were inspected for
blistering and swelling.
The occurrence of blisters was assessed as follows
through a combination of 2 values:
- The number of blisters was evaluated by a quantity
rating of 1 to 5, with ml denoting very few
blisters and m5 very many blisters.
- The size of the blisters was evaluated by a size
rating, again from 1 to 5, with gl denoting very
small blisters and g5 denoting very large
blisters.
- The designation mOgO, accordingly, denotes a
blister-free finish after heat-and-humidity
storage, and represents a satisfactory result in
terms of blistering.
I
. ,
CA 02875640 2014-12-03
BASF Coatings GmbH 74 27 Aug. 2013
PF0074123/S012952
Table 6: Blistering and swelling of waterborne basecoat
material 3 and waterborne basecoat materials E8 to Ell
WBM Blistering Swelling Assessment
3 m5g4 none unsat.
E8 m0g0 none sat.
E9 m3g2 none unsat.
E10 m0g0 none sat.
Ell m2g2 none unsat.
Key:
m . number of blisters
g = size of blisters
sat. = satisfactory result
unsat. . unsatisfactory result
The results confirm that when using the polyesters of
the invention there are no longer any blisters after
heat-and-humidity exposure and there is no longer any
visible swelling.