Note: Descriptions are shown in the official language in which they were submitted.
CA 02877979 2014-12-24
1
Reactor for carrying out an exothermic reaction in the gas phase
Description
The invention proceeds from a reactor for carrying out an exothermic reaction
in the
gas phase, which comprises a vessel having an outer wall composed of a
metallic
material.
Reactors of this type are used, for example, in the case of reactions which
are carried
out at elevated temperatures. Here, the material of which the reactor is made
has to be
selected so that it is stable at the temperatures which prevail in the
interior of the
reactor. Particularly in the case of corrosive media, there is the additional
problem that
-- the material is attacked by the media used and additional weakening occurs
as a result
of the high temperatures at which the reaction is carried out. In particular,
it has to be
taken into account that the outer wall of the reactor is generally a load-
bearing part on
which the mass of the reactor additionally rests.
-- A reaction which is carried out at elevated temperature using corrosive
media is, for
example, the oxidation of sulfur dioxide to sulfur trioxide.
The reactors used at present for this oxidation are usually made of stainless
steel.
However, it has been found that the material is damaged due to the
temperatures
-- occurring during the oxidation, which leads to a reduction in the creep
strength and
thus also to a reduced life of the reactor. Thus, for example, the stainless
steels having
the material numbers 1.4878 or 1.4541 which are generally used are subject to
creep
damage at temperatures above 560 C. The damage results from a change in the
mechanical materials properties which, depending on the progress of the
damage, can
-- lead to failure.
It is therefore an object of the present invention to provide a reactor which,
compared
to the reactors known from the prior art, has an increased operating life when
carrying
out an exothermic reaction in the gas phase or permits higher gas-phase
temperatures.
This object is achieved by a reactor for carrying out an exothermic reaction
in the gas
phase, which comprises a vessel having an outer wall composed of a metallic
material,
wherein an inner shell is accommodated in the interior of the reactor and the
inner shell
has a spacing of at least 50 mm to the inside of the outer wall.
CA 02877979 2014-12-24
2
The use of the inner shell results in an additional gas layer being formed
between the
inner shell and the outer wall of the reactor. The gas layer has an insulating
effect, so
that the temperatures which act on the outer wall are lower than the
temperatures in
the interior of the reactor. This prevents the outer wall from being subjected
to
temperatures which have an adverse effect on the stability of the outer wall,
so that the
operating life of the reactor is increased. In particular, the use of the
inner shell
prevents the material of the outer wall becoming brittle because of the
temperatures
within the reactor and the stability and strength of the outer wall decreasing
as a result.
Embrittlement of the inner shell has a far less dramatic effect since the
inner shell does
not have a load-bearing function. Unlike embrittlement of the outer wall,
embrittlement
of the inner shell does not lead to possible failure of the reactor.
The reactor of the invention is particularly suitable for carrying out
exothermic reactions
in the gas phase which are carried out at elevated temperatures, for example
at
temperatures above 300 C, preferably at temperatures above 500 C. In
particular, the
reactor is suitable for carrying out reactions which contain media which are
aggressive
toward the material of the outer wall, for example for the reaction of sulfur
dioxide with
oxygen to form S03. The SO3 produced in this way is used, for example, in the
preparation of sulfuric acid.
In one embodiment, the inner shell is made of the same material as the outer
wall. A
suitable material for manufacturing outer wall and inner shell is, for
example, stainless
steel. Here, the stainless steel is selected so that it is stable toward the
media
comprised in the reactor. When the reactor is used for preparing sulfur
trioxide by
oxidation of sulfur dioxide, stainless steels having the material numbers
1.4878 or
1.4541, for example are suitable. These are stable toward the sulfur dioxide
and sulfur
trioxide comprised in the reactor and also have a sufficient long-term
stability when the
temperature to which the stainless steel is exposed can be kept to below 560
C. Since
the inner shell does not have a load-bearing function, unlike the outer wall,
embrittlement and an associated decrease in the strength of the material does
not lead
to failure of the reactor, and under customary operating conditions also not
to damage
to the inner shell.
A further advantage of the inner shell is that in the case of damage,
replacement of the
inner shell can be carried out without the entire reactor having to be
replaced.
As an alternative to producing outer wall and inner shell from the same
material, it is
also possible to use different materials for producing outer wall and inner
shell. For
example, it is possible to use different steels. It is also possible to make
the outer wall
of a steel and the inner shell of a heat-resistive material which is inert
toward the
materials comprised in the reactor. As material for the inner shell, it is
also possible to
CA 02877979 2014-12-24
3
use, for example, nonmetals, for example a ceramic or glass. It is also
possible to coat
the inner shell in order to reduce heat radiation. A suitable coating material
for the inner
shell is, for example, high-temperature-resistant mineral wools.
It is also possible to make the outer wall of a material other than stainless
steel. Here
too, it is necessary to use a material which is stable toward the materials
comprised in
the reactor. Owing to the inner shell and the gas phase between the inner
shell and
outer wall, the temperature to which the outer wall is exposed is less than
the
temperature in the interior of the reactor. It is therefore also possible to
use a material
which is less thermally stable than stainless steel for the outer wall.
However, the use
of stainless steel as material for the outer wall is preferred. Furthermore,
particular
preference is given to making the outer wall and inner shell of the same
material.
In a further preferred embodiment, there is a gap between the inner shell and
the
bottom and/or lid of the reactor. Gas can flow from the reactor through the
gap between
inner shell and outer wall. This ensures, in particular, that the same
pressure prevails in
the gap between inner shell and outer wall as in the reactor. As a result, the
inner shell
is not pressurized on one side, namely the inside, but instead the pressure
acts
uniformly on the inner shell from all sides.
The gap between the inner shell and bottom and/or lid of the reactor is kept
so small
that although pressure equalization occurs, only a small gas flow through the
gap
between the inner shell and outer wall is generated. The less the gas moves in
the gap
between the inner shell and outer wall, the better the insulating effect of
the gas. In the
case of uniform gas flow, on the other hand, hot gas is normally conveyed into
the gap
so that the intended insulating effect by the gap does not occur. In the case
of cold,
introduced gas, it is possible to cool the inner shell and the outer wall by
means of the
gas flow.
In one embodiment, the reactor comprises internals. For the purposes of the
present
invention, the internals are, for example, trays, structured or unstructured
packets or
beds of packing elements. Suitable trays which can be accommodated in the
reactor
are, for example sieve trays, bubble cup trays or any other desired trays
known to
those skilled in the art. Particular preference is given to at least one tray
being
accommodated as internal in the reactor.
In a further embodiment, at least one catalyst bed is comprised in the
reactor. The
catalyst bed can be configured, for example, as fixed bed or as fluidized bed.
When the
catalyst bed is a fluidized bed, at least one tray which serves as gas
distributor in the
fluidized bed is preferably accommodated in the reactor. Between the granular
material
for the fluidized bed and the tray above it, which acts as lid, a sufficient
spacing is left
CA 02877979 2014-12-24
4
to allow the fluidized bed material to be sufficiently fluidized by a gas
flowing through it.
The catalyst bed is preferably a fixed bed. For this purpose, the catalyst
forming the
fixed bed can, for example, rest on a tray. Unlike the case of a fluidized
bed, a fixed
bed is independent of the flow direction. Thus, flow through this can also be,
for
example, from the top downward. When a catalyst bed is comprised in the
reactor, the
tray on which the catalyst rests is, for example, a grating or a metal support
sheet for
the catalyst.
In the preparation of SO3 by oxidation of sulfur dioxide, preference is given
to a catalyst
bed in the form of a fixed bed.
In a particularly preferred embodiment, the reactor is divided into a
plurality of
segments, with each segment having at least one inlet and at least one outlet
and each
segment comprising a catalyst bed and a gas space above the catalyst bed. The
division of the reactor into a plurality of segments is preferably achieved by
means of
intermediate trays. In the case of a catalyst bed configured as a fixed bed,
the inlet is
located, for example, above the catalyst bed in the gas space so that the gas
flowing
through the catalyst bed can be introduced via the inlet. In a gas space below
the
catalyst bed, the gas flowing through the catalyst bed is collected and can
then be
taken off from the gas space below the catalyst best via an outlet.
When a catalyst bed is used, the chemical reaction usually takes place in the
catalyst
bed. In a particularly preferred embodiment, the reactor of the invention is
used for the
oxidation of sulfur dioxide to sulfur trioxide. For this purpose, gaseous
sulfur dioxide
and an oxygen-comprising gas are fed in and the sulfur dioxide reacts with the
oxygen
to form sulfur trioxide. As oxygen-comprising gas, it is possible to use, for
example,
oxygen or air. When oxygen is used, an inert gas can be additionally
comprised. As an
alternative, it is also possible for the air to be additionally enriched in
oxygen. However,
particular preference is given to the use of air.
In the oxidation of sulfur dioxide to sulfur trioxide, the gases are
introduced at a
temperature in the range from 400 to 460 C. The reaction occurs in the
presence of a
catalyst at a gauge pressure of 0.4 bar. Owing to the exothermic nature of the
reaction,
the gas comprising sulfur trioxide, sulfur dioxide and, when air is used,
oxygen and
nitrogen which leaves the reactor has a temperature of from 550 to 650 C.
Accordingly,
temperatures in this range are also generated in the reactor. Above a
temperature of
560 C, the use of steels 1.4878 or 1.4541 results in a reduced life due to
alteration of
the mechanical materials properties with a decrease in the strength. To avoid
failure of
the reactor, the inner shell is therefore accommodated according to the
invention in the
reactor. The inner shell results in formation of an insulating layer between
inner shell
and outer wall of the reactor, so that the temperature acting on the outer
wall of the
CA 02877979 2014-12-24
reactor is reduced. Thus, for example, it is possible to bring the temperature
of the
outer wall to a temperature in the range from 400 to 560 C by means of the
inner shell.
In this way, the creep strength is not reduced and the life of the reactor is
increased.
Since, unlike the outer wall of the reactor, the inner shell has no load-
bearing function,
5 embrittlement of the inner shell does not lead to damage to the reactor
so as to
adversely affect operation.
When the reaction, for example the oxidation of sulfur dioxide to sulfur
trioxide, is
carried out in the presence of a catalyst and the reactor additionally
comprises a
fluidized bed, particular preference is given to the material of the fluidized
bed being
catalytically active. For this purpose, the entire granular material of the
fluidized bed
can be catalytically active or, as an alternative, the fluidized bed can
comprise a
heterogeneous catalyst in addition to an inert granular material. The catalyst
can, for
example, likewise be mixed in granular form into the inert granular material
of the
fluidized bed. However, particular preference is given to the entire granular
material of
the fluidized bed being catalytically active.
In the case of a fixed bed, it is possible, for example, to use catalytically
active packing
or catalytically active packing elements. Particular preference is given to
making the
packing or packing elements of a support material onto which catalytically
active
material is applied.
The catalyst suitable for the reaction to be carried out in the reactor is in
each case
used as catalyst.
When a reaction other than the oxidation of sulfur dioxide to sulfur trioxide
is carried out
in the reactor, it is also possible for the reactor to be made of a material
other than
stainless steel. The material from which the outer wall of the reactor is
produced is
dependent on the reaction. It is usual to employ a material which is inert
toward the
materials to be reacted in the reactor. Regardless of the material of the
outer wall,
preference is also given to making the inner shell of the same material as the
outer
wall. Such an inner shell is preferably used when the temperature which acts
on the
material of the outer wall is so high that damage to the outer wall can occur.
As a result
of the inner shell, an insulating layer is formed between inner shell and
outer wall, so
that the temperature which acts on the outer wall of the reactor can be
reduced.
Examples of the invention are depicted in the figures and are explained in
more detail
in the following description.
The figures show:
CA 02877979 2014-12-24
6
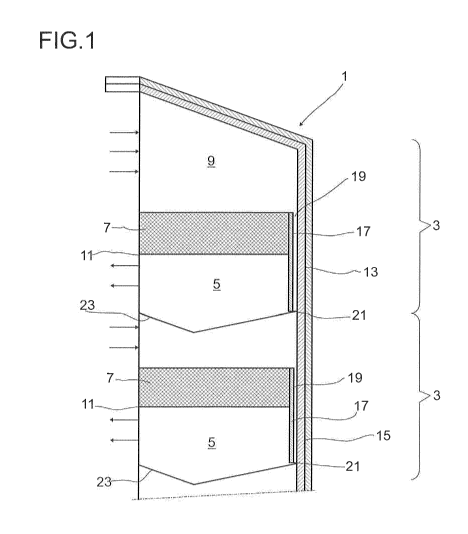
Figure 1 a section of a reactor constructed according to the invention,
Figure 2 a temperature distribution without inner shell,
Figure 3 a temperature distribution with inner shell.
Figure 1 depicts a section of a reactor.
The section shows a right-hand half of a reactor 1 which is divided into two
segments
3. In addition to the two segments 3 shown here, further segments 3 can also
be
comprised. These are then arranged appropriately above and/or below.
When the reactor is used for the oxidation of sulfur dioxide to sulfur
trioxide, each
segment 3 usually has a lower gas space 5, a catalyst bed 7 and an upper gas
space
9. The catalyst bed 7 is usually configured in the form of a fixed bed and
rests on a tray
11. In this case, the tray 11 is, for example, a grating or a metal support
sheet for the
catalyst.
In operation, a gas stream comprising the starting materials necessary for the
reaction
is fed into the upper gas space 9. From the upper gas space 9, the gas stream
is
introduced into the catalyst bed 7. In the catalyst bed 7, the starting
materials of the
gas stream are converted into the product. The product-comprising gas collects
in the
lower gas space 5 and can be taken off from the latter. When the reaction of
the gas is
incomplete, the gas comprising the product in the lower gas space 5 also
comprises
starting materials.
In the case of an exothermic reaction, heat is liberated during the reaction
and, in
particular, the catalyst bed 7 is heated up as a result. Since the hot gas
exits from the
catalyst bed 7, at least the outer wall below the catalyst bed is also heated
up.
A reactor usually has a metallic outer wall 13. Owing to the high temperature
in the
interior of the reactor, the outer wall 13 is provided on its outside with
insulation 15.
Owing to the high temperatures in the interior of the reactor which occur due
to the
exothermic reactions, the outer wall 13 is subjected to a correspondingly high
temperature. This can in the case of some materials lead to thermal damage to
the
material of the outer wall 13. Thus, for example, the steels 1.4878 or 1.4541
usually
used for the outer wall 13 in the oxidation of sulfur dioxide to sulfur
trioxide are
subjected to creep damage at the temperatures occurring in the reaction and
this leads
to a reduced life of the overall reactor 1. The reduced life results from
embrittlement
with a decrease in the strength of the outer wall 13.
CA 02877979 2014-12-24
7
According to the invention, an inner shell 17 is therefore accommodated in the
interior
of the reactor 1 and is positioned with a defined spacing to the outer wall
13. Thus, the
inner shell 17 forms a gap between inner shell 17 and outer wall 13. The inner
shell 17
is, in particular, arranged in the positions at which temperatures above the
temperature
which could lead to creep damage to the material of the outer wall 13 occur in
the
reactor.
The gap 19 between outer wall 13 and inner shell 17 is filled with a gas. The
gas has
an insulating effect and the temperature acting on the outer wall 13 is
therefore lower
than without use of the inner shell 17. In this way, the temperature which
acts on the
outer wall 13 can be kept below the critical temperature which leads to creep
damage.
The inner shell 17 is in this case preferably made of the same material as the
outer wall
13.
The gas comprised in the gap 19 between outer wall 13 and inner shell 17 is
preferably
the gas fed into the reactor. For this purpose, it is possible, for example,
to provide a
gap 21 between an intermediate tray 23 by means of which the segments 3 are
divided
and the inner shell 17. Gas can then exit through the gap 21 from the gap 19
between
inner shell 17 and outer wall 13. On the opposite side, the gap 19 is, for
example, open
to the upper gas space 9. When an additional tray is provided between the
upper gas
space 9 and the catalyst bed 7, preference is given to the inner shell 17
being provided
in the region of the catalyst bed 7 and the lower gas space 5 and an inlet gap
being
provided between the catalyst bed 7 to the tray separating off the upper gas
space 9
through which the gas can enter the gap 19 between inner shell 17 and outer
wall 13.
However, it is usual not to provide a tray above the catalyst bed 7 so that
the gas can
flow directly from the upper gas space 9 into the gap 19 between inner shell
17 and
outer wall 13.
When cold gas enters the gap, it is not necessary to keep the gas flow very
low since
the gas itself likewise has a cooling action.
A temperature distribution without inner shell is shown by way of example in
figure 2.
Here, the temperature of the gas in the interior of the reactor is shown by
one line and
the temperature at the outer wall is shown by the other. The temperature
profile in the
interior is denoted by the reference numeral 25 and the temperature profile at
the outer
wall is denoted by the reference numeral 27.
The position in segment 3 from entry of the gas to exit of the gas is shown in
the x axis
and the temperature is shown on the y axis.
=
CA 02877979 2014-12-24
8
The gas is fed in with a temperature of 450 C, and flows through the upper gas
space
9 until it reaches the catalyst bed 7. In the catalyst bed 7, the chemical
reaction
commences and, owing to its exothermic nature, leads to a temperature
increase. The
temperature rises to 630 C. The gas is taken off with a corresponding
temperature
from the lower gas space 5. The gas is once again introduced into the upper
gas space
9 of the second segment at a temperature of 450 C and the temperature once
again
increases in the catalyst bed. Owing to the sulfur dioxide which has been
reacted in the
first segment, the maximum temperature in the second segment is lower than
that in
the first segment and the temperature rises only to 560 C.
Owing to the high temperature of the gas stream, the metal of the outer wall
also heats
up. However, as a result of convective heat transfer and heat conduction, the
maximum
temperature at the outer wall is lower than the temperature in the interior of
the gas
stream. In addition, the temperature decreases again in the region of the
lower gas
space 5 until it reaches the upper gas space 9 of a segment located
underneath, since
the outer wall is cooled in the region of the upper gas space 9 of the
subsequent
segment. This leads, owing to heat conduction, to a temperature decrease in
the lower
gas space 5 of the segment 3 further up.
However, the maximum temperature at the outer wall which occurs as a result of
the
temperature of the gas stream is in the case of a steel outer wall when the
reactor is
used for the oxidation of sulfur dioxide to sulfur trioxide above the critical
temperature
above which creep damage to the steel occurs.
Figure 3 shows, by way of example, the temperature profile in the gas stream
and at
the outer wall when an inner shell is used.
The temperature profile in the gas stream corresponds to that which also
occurs
without use of the inner shell. However, the temperature acting on the outer
wall 13 is
significantly lower as a result of the use of the inner shell 17. Thus, in
this example, the
temperature maxima in each case in the upper segment are about 525 C and in
the
lower segment about 500 C. Thus, the temperatures remain below the critical
temperature at which the creep strength of the steel of which the outer wall
13 is made
is reduced.
= CA 02877979 2014-12-24
9
List of reference numerals
1 Reactor
3 Segment
5 Lower gas space
7 Catalyst bed
9 Upper gas space
11 Tray
13 Outer wall
Insulation
17 Inner shell
19 Gap
21 Gap
15 23 Intermediate tray
Temperature profile in the interior
27 Temperature profile at the outer wall