Note: Descriptions are shown in the official language in which they were submitted.
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
PURIFIED AIR AND METHODS OF MAKING AND USING THE SAME
SPECIFICATION
BACKGROUND OF THE INVENTION
This PCT application claims the benefit under 35 U.S.C. 120 of United States
Application Serial No. 13/554,366, filed on July 20, 2012, which is a
continuation-in-part of
U.S. Patent Application No. 13/244,973, filed on September 26,2011, which is a
continuation
of U.S. Patent Application No. 12/732,246, filed on March 26,2010.
International Application
No. PCT/U S2011/029567, filed on March 23, 2011, claims priority to U.S.
Patent Application
No. 12/732,246.
1. FIELD OF INVENTION
This invention relates to devices and methods for the filtration and
purification of air.
More particularly, this invention relates to air purifiers capable of
providing a level of air quality
suitable for environments that are highly sensitive to airborne contaminants,
e.g., in vitro
fertilization laboratories or other medical environments. Further, the
invention may be adapted
for use in any substantially enclosed environment, including, but not limited
to, homes,
residential buildings, commercial buildings, hotels, cars, buses, trains,
airplanes, cruise ships,
educational facilities, offices, and government buildings. The invention may
also have
applications in, e.g., national security, defense, or airline industries.
2. DESCRIPTION OF RELATED ART
In vitro fertilization ("IVF") is a procedure whereby egg cells are fertilized
by sperm in a
laboratory environment, instead of in the womb. If an egg cell is successfully
fertilized, it may
be transferred into the uterus of a patient wishing to become pregnant.
IVF may be an effective option for patients suffering from infertility,
especially where
other methods of assisted reproduction have failed. However, IVF is very
expensive and is not
typically covered by medical insurance. In 2009, the cost of a single cycle of
IVF was
approximately $10,000 to $15,000 in the United States. It is fmancially
prohibitive for most
people to undergo multiple rounds of IVF. It is therefore imperative that
conditions for
successful pre-implantation embryogenesis are optimized, in order to maximize
the likelihood of
success.
One extremely important factor contributing to the likelihood of successful
pre-
implantation embryogenesis is the air quality of the IVF laboratory. Gametes
and embryos
grown in vitro are highly sensitive to environmental influences. Human embryos
have no means
of protection or filtration against environmental toxins and pathogens. They
are completely at
1
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
the mercy of their environment. The incubators which house the human embryos
often consist
of a significant percentage of room air. Although airborne contaminants can
adversely affect
embryogenesis, surprisingly little emphasis has been placed on optimizing
laboratory air quality
during the last three decades in which IVF has been available as a treatment
for infertility.
Existing filtration devices have been found insufficient to optimize air
quality to truly
acceptable levels for IVF. For example, it has been found that laboratory air
that had been
filtered with only high efficiency particulate air ("HEPA") filters was
actually of lesser quality
than outside air. Additionally, some filters produce by-products or other
contaminants that
actually detract from the quality of the air in an IVF laboratory. For
example, carbon filters can
create carbon dusting that is harmful to the IVF process. This is not to say,
however, that carbon
filters or HEPA filters should not be used to treat air supplied to an IVF
laboratory. On the
contrary, it is preferred that carbon filters, HEPA filters, or their
respective equivalents, are
included among filtration media used to treat air supplied to an IVF
laboratory. Attaining
optimal air quality in an IVF laboratory or other substantially enclosed space
requires proper
selection, combination and sequencing of various filtration media.
BRIEF SUMMARY OF THE INVENTION
Accordingly, air characterized by very high purity and methods of making and
using
such air, are provided.
In one aspect of the present invention, air is provided, characterized by a
TVOC content
of from less than 5 ppb to about 500 ppb, a Biologicals content of from less
than 1 CFU/M3 to
150 CFU/M3 and a Particulate content of from about 1,000 0.3 tim particles per
ft3 to about
50,000 0.3 pm particles per ft3, or from about 600 0.5 1-1,M particles per ft3
to about 500,000 0.5
p.m particles per ft3.
Another aspect of the present invention is a method of achieving an IVF
clinical
pregnancy rate of at least 50%. The method includes performing multiple IVF
cycles in an IVF
laboratory having air characterized by a TVOC content of from less than 5 ppb
to about 500 ppb,
a Biologicals content of from less than 1 CFU/M3 to 150 CFU/M3 and a
Particulate content of
from about 1,000 0.3 p.m particles per ft3 to about 50,000 0.3 pm particles
per ft3, or from about
600 0.5 pun particles per ft3 to about 500,000 0.5 pm particles per ft3.
Another aspect of the present invention is a method of purifying air,
including providing
an air flow path through a housing for the flow of air in a downstream
direction, filtering the air
through oxidizing and adsorbing VOC pre-filtration within the housing,
filtering the air through
UV filtration within the housing, downstream from the oxidizing and adsorbing
VOC pre-
2
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
filtration and filtering the air through final particulate filtration within
the housing, downstream
from the UV filtration.
BRIEF DESCRIPTION OF SEVERAL VIEWS OF THE DRAWINGS
The invention will be described in conjunction with the following drawings in
which like
reference numerals designate like elements and wherein:
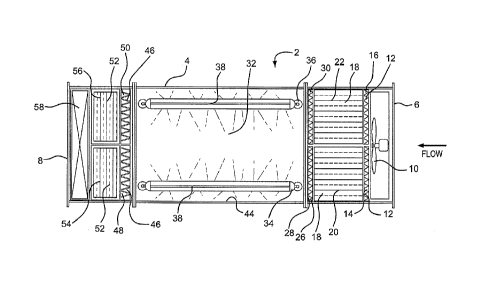
Fig. 1 is a top view of an air purifier according to the present invention.
Fig. 2 is a side view of an air purifier according to the present invention.
Fig. 3 is an internal view of the air purifier along the plane defmed by
section line A - -
A of Fig. 1.
Fig. 4 is an internal view of the air purifier along the plane defined by
section line B - - B
of Fig. 2.
DETAILED DESCRIPTION OF THE INVENTION
Referring now in detail to the various figures of the drawings wherein like
reference
numerals refer to like parts, there are shown in Figs. 1 and 2 top and side
views, respectively, of
an air purifier 2 according to the present invention. As illustrated, the air
purifier 2 includes a
substantially rectangular cuboid housing 4 having an inlet 6 for receiving air
and an outlet 8 for
exhausting air. The term "air" as used herein broadly refers to a gas or
gaseous mixture that may
be safely breathed by mammals and/or that can serve as a source gas or gaseous
mixture towards
an IVF laboratory. The housing 4 provides an air flow path for the flow of air
in a downstream
direction, i.e., from the inlet 6 towards the outlet 8. The term "housing" as
used herein refers to
any conduit, chamber and/or enclosure, or a plurality of conduits, chambers
and/or enclosures
coupled to one another, providing an air flow path within. Thus, the "housing"
could include,
e.g., ductwork of an existing heating, ventilating and air conditioning
("HVAC") system or air
handling unit ("AHU").
Although the housing 4 is preferably substantially rectangular cuboid, as
shown in Figs.
1 and 2, it need not be limited to any particular shape. Moreover, it may
include inner curves,
bends and/or other contours, whereby the air flow path would follow such
curves, bends and/or
other contours. Preferably, however, the air flow path is substantially
straight, as it is in the
embodiment of the housing 4 shown in Figs. 1 and 2.
The air purifier 2 is preferably adapted to be installed into an existing HVAC
system or
AHU. In an alternative embodiment, an air purifier according to the present
invention may
function as a stand-alone unit, i.e., one that is not part of an HVAC system
or AHU. An
exemplary housing 4 may be a substantially rectangular cuboid having
dimensions of
3
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
approximately 11 ft. long by 4 ft. wide by 2 ft. high. Such dimensions would
diffuse or spread
out the air through the air purifier 2 so as to provide sufficient resonance
time for the air through
each of the filtration media discussed infra. A skilled artisan understands,
however, that the
foregoing exemplary shape and size parameters are merely illustrative, and may
be changed,
even substantially, depending on the circumstances or application. For
example, in some
applications, the air purifier 2 may be about 6 ft. long.
Referring now to Fig. 3, there is shown an internal view of the air purifier 2
along the
plane defined by section line A - - A of Fig. 1. In Fig. 4, there is shown an
internal view of the
air purifier 2 along the plane defined by section line B - - B of Fig. 2.
To obtain optimal air quality, e.g., suitable for an IVF laboratory, the air
that is treated by
the air purifier 2 should be pre-conditioned and stable, i.e., moderate both
in terms of
temperature and humidity. Ideally, the air that is treated by the air purifier
2 should have a
temperature of between about 68 F and 75 F, and a humidity of between about
45% and 55%.
Additionally, the air flow rate through the air purifier 2 should preferably
be about 250 ft./min.
and below 2000 CFM. This preferred flow rate is intended to provide sufficient
resonance time
for the air through each of the filtration media discussed infra. The term
"filtration" as used
herein, broadly covers one or more devices that treat air, such as by
trapping, removing,
deactivating and/or destroying contaminants therefrom.
In order to provide an adequate air flow rate through the air purifier 2, it
may be helpful
(although not always necessary) to include a booster fan 10 downstream from
the inlet 6. The
booster fan 10 may be coupled to a control system (not shown) that measures
the air flow rate
and triggers the booster fan 10 as needed, to maintain the desired air flow
rate. In an alternative
embodiment (not shown), a booster fan may not be included, and adequate air
flow rate may be
provided and maintained by other means, e.g., a blower in an HVAC system or
ABU into which
the air purifier 2 is installed.
Downstream from the inlet 6 is particulate pre-filtration 12 for the trapping
of airborne
particulate. The particulate pre-filtration 12 is preferably about 2 inches
thick in one
embodiment, and includes left and right pleated particulate pre-filters 14,16.
The particulate
pre-filters 14,16 trap gross particulate (e.g., dust and bugs) from the
outside air before that air
reaches the other filtration media in the air purifier 2 discussed infra.
Suitable filters for the
particulate pre-filtration 12 are those having a Minimum Efficiency Reporting
Value ("MERV")
of 5 to 13 with an Average ASHRAE Dust Spot Efficiency (Standard 52.1) of 20%
to 80%.
Particularly preferred filters for the particulate pre-filtration 12 are
pleated filters having a
4
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
MERV of 7 to 8, with an Average ASHRAE Dust Spot Efficiency (Standard 52.1) of
30% to
45%.
Proper particulate pre-filter selection should be guided by the need to trap
gross-
particulate without unduly affecting the air flow rate through the air
purifier 2. The particular
type of particulate pre-filter(s) selected for particulate pre-filtration
depends on various factors,
including outside air quality. It is preferred that the particulate pre-
filtration 12 is located
immediately upstream from the additional filtration media discussed infra, as
shown in Figs. 3
and 4. Alternatively (or in addition), however, particulate pre-filtration may
be located further
upstream, e.g., in upstream ductwork of an HVAC system or AHU into which the
air purifier 2
is installed.
Downstream from the particulate pre-filtration 12 is volatile organic compound
("VOC")
pre-filtration 18. Once air passes through the particulate pre-filtration 12,
the air is effectively
free of gross particulate that would otherwise diminish the efficacy and
useful life of the VOC
pre-filtration 18. VOC pre-filtration ideally includes adsorption media, such
as carbon, as well
as oxidation media, such as potassium permanganate ("K1V1n04") or a
photocatalytic oxidizer.
A particularly preferred type of carbon is virgin coconut shell. In a
preferred embodiment, the
VOC pre-filtration 18 is a carbon and KMn04 blend, e.g., in a 50/50
proportion. In some
embodiments, the blend may include additional elements, such as natural
zeolite. The
proportion of the blend may vary depending on the types and levels of VOCs
present in the
source air. Ideally, the source air would be tested for VOCs, and, based on
test results, a custom
blend would be prepared to maximize VOC removal in a given environment. In an
alternative
embodiment of the VOC pre-filtration (not shown), separate (i.e., non-blended)
carbon and
K.Mn04 filters are used.
The embodiment of the VOC pre-filtration 18 shown in Figs. 3 and 4 includes a
total of
twenty stacked filter trays 20,22, whereby ten such trays 20 are on the left
side of the housing 4
and ten such trays 22 are directly adjacent, to the right. The length of the
trays, i.e., the
longitudinal distance over which the air flows, is preferably about 17 inches
in one embodiment,
though it may be shorter or longer. Each tray 20,22 includes two blended
carbon and KMn04
filters 24, arranged in a V-bank along a vertical plane (e.g., the plane of
Fig. 3). The V-bank
arrangement increases the surface area of the filters 24 over which air must
travel, thereby
enhancing the effectiveness of the VOC pre-filtration 18. Once air passes
through the VOC pre-
filtration 18, the VOC load of the air is effectively reduced.
5
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
Downstream from the VOC pre-filtration 18 is particulate post-filtration 26
for the
trapping of airborne particulate, e.g., particulate generated by the VOC pre-
filtration 18 (such as
carbon dusting). The particulate post-filtration 26 includes left and right
pleated particulate
post-filters 28,30. The filters used in the particulate post-filtration 26 may
be identical or similar
to those used in the particulate pre-filtration 12, discussed supra. While
particulate post
filtration 26 downstream from the VOC pre-filtration 18 is preferred, it may
not be necessary in
all applications. For example, if the VOC pre-filtration is of a type that
does not generate air-
borne particulate, such as bonded carbon, particulate post-filtration may be
optional.
Downstream from the particulate post-filtration 26 is ultraviolet ("UV")
filtration 32
which destroys airborne biological contaminants and, in some embodiments,
degrades chemical
contaminants. Whether or not particulate post-filtration 26 is used, the air
reaching the UV
filtration 32 should be effectively free of gross particulate and contain
dramatically reduced
levels of VOCs so as not to diminish the efficacy of the UV filtration 32.
The UV filtration may include one or more UV sources, although a plurality of
UV
sources is preferred. It is further preferred that these UV sources are UVC
sources, capable of
generating UV radiation at a wavelength varying from 220 nm to 288 nm. Most
preferably, the
UVC sources are capable of generating 'UV radiation at a wavelength of 260 nm,
however
commercially available UVC sources capable of generating UV radiation at a
wavelength of 254
nm are adequate. In an alternative embodiment described in U.S. Pat. No.
5,833,740 (Brais),
which is incorporated herein by reference in its entirety, the UV filtration
includes at least one
vacuum UV source, capable of generating UV radiation at a wavelength varying
from 170 nm to
220 nm (preferably 185 mu) and at least one UVC source, capable of generating
UV radiation at
a wavelength varying from 220 nm to 288 nm (preferably 260 nm). In that
embodiment, the
UVC source is preferably downstream from the vacuum UV source. When operating,
the
vacuum UV source breaks oxygen molecules into mono-atomic oxygen which then
reacts with
chemical contaminants present in the air and then degrades them by successive
oxidation to
odorless and inoffensive byproducts. The UVC source kills biological
contaminants present in
the air by irradiation and degrades residual ozone produced by the vacuum UV
source into
molecular oxygen.
Particularly preferred UV filtration 32 shown in Figs. 3 and 4 is the "UV Bio-
wall" made
by Sanuvox. Alternatively, the "Bio 30GX," which is also made by Sanuvox, is a
preferred type
of UV filtration. The UV filtration 32 includes a pair of fixtures 34, 36 each
of which has five
UV lamps 38 (not all five of which are visible in the Figures). The UV lamps
38 are preferably
6
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
about 60 inches long and extend longitudinally through the housing 4 so as to
maximize
exposure time of the air to UV radiation. In one embodiment, the UV lamps are
UVC sources,
providing UV radiation within the UVC wavelength parameters discussed supra.
In an
alternative embodiment, described in U.S. Pat. No. 5,833,740 (Brais), each
lamp 38 is dual-
zoned, having an upstream vacuum UV source and a downstream UVC source. In
that
alternative embodiment, the upstream vacuum UV source may, e.g., be a high
intensity mercury
vapor lamp capable of generating UV radiation having a wavelength in a range
of about 170 nm
to about 220 nm, and the downstream UVC source may, e.g., be a low intensity
mercury vapor
lamp capable of generating radiation having a wavelength in a range of about
220 nm to about
288 nm. The interior 44 of the housing 4 encasing the UV filtration 32 is
highly reflective, with
a preferable coefficient of reflection of at least 60%, so as to enhance the
effectiveness of the
lamps 38.
The kill rate of biological contaminants is a function of the intensity of UVC
radiation
produced by the UV filtration 32 and reflected by the interior 44 of the
housing 4, as well as the
exposure time of such contaminants to the UVC radiation. Thus, the higher the
intensity of the
UVC radiation and the longer the exposure time of such contaminants to the UVC
radiation, the
greater is the level of sterilization achieved. Depending on factors such as
the desired level of
sterilization, the amount of space available to house UV filtration, and costs
of operating and
maintaining UV filtration, the desired total UVC output of the UV filtration
32 may vary. In one
actual embodiment, it was found that a total UVC output ranging from about
33,464 ttJ/cm2 to
about 90,165 1.tJ/cm2, with an average total UVC output of about 43,771
pI/cm2, provided a
desired level of sterilization, given practical constraints of cost and space.
Such total UVC
output killed 100% of numerous biological contaminants including, but not
limited to smallpox,
flu, tuberculosis, anthrax and H1N1 virus.
The UV filtration 32 contained within the housing 4 is likely not visible to a
user of the
air purifier 2 when in use, because direct UV exposure is harmful to humans.
Thus, a user
cannot ascertain visually (i.e., by simply looking at the air purifier 2
itself) whether the lamps 38
are operating at a given time. It cannot be assumed that the air purifier 2 is
effectively
destroying air-borne biological and chemical contaminants, without knowing for
sure that the
UV filtration is operating properly. Accordingly, it is preferred that the
present invention
include sensors and a monitor (not shown) to detect and indicate,
respectively, how much time
each UV lamp 38 has been in use and whether each lamp 38 is operating at a
given time. The
monitor may include, e.g., a scrolling digital clock, which indicates the
length of time each lamp
7
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
38 has been operating. These sensors and monitor would indicate to a user when
it is time to
replace any of the lamps 38.
As a general matter, moisture within the housing 4 can foster the growth of
biological
contaminants. Accordingly, it is preferable to include a UVC source in the
vicinity of areas in
which moisture is generated or gathers. For example, upstream from the
particulate pre-
filtration 12 may be one or more cooling coils (not shown) that help to ensure
that the air which
is treated by the air purifier 2 is moderate in terms of temperature. Such
cooling coils tend to
generate moisture. It is therefore preferable to include a UVC source adjacent
to such cooling
coils. Similarly, it may be appropriate to include a UVC source immediately
upstream from a
filter/diffuser (not shown) from which the air enters into a substantially
enclosed space, e.g., an
IVF laboratory or other room, after leaving the air purifier 2.
Downstream from the UV filtration 32 is VOC post-filtration 46, which capture,
e.g.,
VOC by-products of the irradiation from the UV filtration 32. Possible
embodiments of the
VOC post-filtration 46 include any of those discussed supra regarding the VOC
pre-filtration
18. The VOC post-filtration 46 shown in Figs. 3 and 4 includes left and right
VOC post-filters
48,50 that are arranged in a V-bank along a horizontal plane (e.g., the plane
of Fig. 4). The
VOC post-filters 48,50, like their upstream counterparts, are preferably
blended carbon and
1(Mn04. Although VOC post-filtration 46 is preferred, in some applications, it
may not be
required and may thus be omitted.
Gametes and the human embryo are highly sensitive to VOCs, even in amounts
considered negligible in other applications. It is therefore essential that
the VOC filtration (both
pre-filtration 18 and post-filtration 46) operates effectively to remove VOCs
from air that is fed
into an environment in which IVF is being conducted. Accordingly, one or more
sensors for
detecting VOC levels (not shown), preferably in real time, may be placed in an
IVF laboratory
and coupled to a monitor (not shown) to indicate the VOC levels in the
laboratory at a given
time. With such in-room VOC detection, a user of the air purifier 2 would know
when it is time
to replace the VOC pre-filtration 18 and post filtration 46, and/or whether an
alternative type or
blend of VOC filters would be more suitable. While in-room VOC detection is
particularly
useful in an IVF laboratory, it may be helpful in any environment requiring
low VOC levels.
Downstream from the VOC post-filtration 46 is final particulate filtration 52,
which
traps substantially all remaining particulate in the air before the air exits
the outlet 8. Final
particulate filtration 52 preferably includes one or more filters capable of
trapping fine airborne
particulate, e.g., filters having a MERV of 13 or greater with an average
ASHRAE Dust Spot
8
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
Efficiency (Std. 52.1) of 80% or greater. More preferably, such filters have a
MERV of 16 or
greater with an average ASHRAE Dust Spot Efficiency (Std. 52.1) of 95% or
greater. Most
preferably, such filters have a MERV of 17 or greater with an average ASHRAE
Dust Spot
Efficiency (Std. 52.1) of 99.97%, as do high efficiency particulate air
("HEPA") filters.
Alternatively, ultra low particulate air ("ULPA") filters may be suitable. The
choice of filter(s)
for final particulate filtration should be guided by the potentially competing
needs of
maintaining an optimal air flow rate and effectively removing particulate from
the air.
The final particulate filtration 52 of Figs. 3 and 4 includes left and right
12-inch thick
HEPA filters 54,56. Preferably, magnehelic gauges (not shown) are placed both
upstream and
downstream from the HEPA filters 54, 56 to measure the pressure drop across
those filters. The
degree of pressure drop will assist in the identification of the proper time
in which to change the
HEPA filters 54,56, or other filters used for final particulate filtration.
Downstream from the final particulate filtration 52, is an atomizing
humidifier 58. The
humidifier 58 may or may not be necessary, depending on the needs of the
facility in which the
air purifier 2 is being used. However, if a humidifier 52 is needed, it should
be placed
downstream from the final particulate filtration 52 so that the moisture does
not adversely affect
the performance of the VOC post-filters 48,50, the HEPA filters 54,56, or
other filters used for
final particulate filtration. Humidified air can contain and support the
growth of biological
contaminants. Accordingly, if a humidifier 58 is used, an additional UVC
source (not shown) to
destroy such contaminants should also be included. This additional UVC source
should be
downstream from the humidifier 58, preferably at the last point in ductwork
before entry into a
room served by the purified air.
An air purifier according to the present invention, such as that described in
detail, supra,
will produce air characterized by very high purity, suitable for airborne
contaminant-sensitive
environments such as IVF laboratories or other medical environments, for
example. That said,
an air purifier according to the present invention is not limited to IVF or
other medical
applications. It may be adapted for use in any substantially enclosed
environment, including, but
not limited to, homes, residential buildings, commercial buildings, hotels,
cars, buses, trains,
airplanes, cruise ships, educational facilities, offices, and government
buildings. The invention
may also have applications in, e.g., national security, defense, or airline
industries. The desired
purity of the air may vary depending on application and environment. An air
purifier according
to the present invention, such as that described in detail, supra, may be
adapted accordingly to
achieve a desired level of purity. The sequence and type of air filtration
media in an air purifier
9
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
according to the present invention provides air characterized by a purity that
was unattainable
with prior devices.
Accordingly, another aspect of the present invention includes purified air,
such as that
attainable using an air purifier as described herein. Ideally, such purified
air would be
characterized by a high level of purity as measured by three parameters: (a)
"TVOC," i.e., total
volatile organic compounds, measured in "ppb," or parts per billion; (b)
"Biologicals," i.e.,
biological contaminants, including spores, measured in "CFU/M3," or colony
forming units per
=cubic meter; and (c) "Particulate," i.e., the number of particles per cubic
foot having, e.g.,
nominal sizes of 0.3 pm or 0.5 pm.
TVOC measurements may be made, e.g., using GRAY WOLF SENSING SOLUTIONS,
Model No. TG-502 Toxic Gas Probe with Photo Ionization Detector ("PID")
sensors utilizing a
10.6 eV lamp calibrated to Isobutylene. The lowest detectable limit of TVOCs
using the TG-
502 Toxic Gas Probe is 5 ppb.
To ensure accuracy, measurements of Biologicals are preferably assessed using
two
complementary methods. According to a first method of measuring Biologicals,
ambient air
(i.e., the air being tested) is drawn over ALLERGENCO D spore traps using a
high volume
vacuum pump calibrated to draw 15 liters of air per minute. This is done for
10 minutes, so that
a total of 150 liters of air is drawn through the spore trap cassette. The
traps are then examined
by direct light microscopic observation to determine the identification of
some select types of
biological contaminants present in terms of CFU/M3. According to a second
method of
measuring Biologicals, an ANDERSON N6 sampler is utilized to obtain culturable
air samples
(from the ambient air being tested) on three types of media: malt extract
agar, cellulose agar and
DG-18. The sampler is calibrated pre- and post-collection to draw a rate of
28.3 liters per
minute for a sample time of 5 minutes. Using this second method of measuring
Biologicals
enables determination of the unique identification of any biological
contaminant present in terms
of CFU/M3 due to the three different types of growth media.
The particulate measurements may be made, e.g., using a TSI AEROTRAK 9306
Handheld Particle Counter. The particle counter is preferably calibrated with
NIST traceable
PSL spheres using TSI's Classifier and Condensation Particle Counters, the
recognized standard
for particle measurements. The particle concentrations in the air are measured
at nominal
particle sizes of 0.3 pm, 0.5 pm, 1.0 trn, 3.0 pin, 5.0 pm, and 10.0 pm, per
cubic foot (ft3).
In a preferred embodiment, it is contemplated that purified air attainable
using an air
purifier as described herein, is characterized by: (a) a TVOC content of less
than 5 ppb (or
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
below detectable limits using the GRAY WOLF SENSING SOLUTIONS, Model No. TG-
502
Toxic Gas Probe with PID sensors described supra, or another instrument with
similar
measurement capabilities and tolerances); (b) a Biologicals content of less
than 1 CFU/M3 (or
below detectable limits using the methods of measuring Biologicals described
supra, or other
methods with similar measurement capabilities and tolerances); and (c) a
particulate content of
from about 1,000 0.3 gm particles per ft3 of air to about 10,500 0.3 gm
particles per ft3 of air, or
from about 600 0.5 gm particles per ft3 of air to about 1,000 0.5 gm particles
per ft3of air.
Depending on the application or environment, acceptable levels of TVOCs,
Biologicals
and particulates may vary. For example, in one embodiment, the purified air
may be
characterized by: (a) a TVOC content of from less than 5 ppb to about 500 ppb;
(b) a Biologicals
content of from less than 1 CFU/M3 to 150 CFU/M3; and (c) a particulate
content of from about
1,000 0.3 gm particles per ft3 of air to about 50,000 0.3 gm particles per ft3
of air, or from about
600 0.5 gm particles per ft3 of air to about 500,000 0.5 gm particles per ft3
of air. More
preferable particulate content is from about 1,000 0.3 gm particles per ft3 of
air to about 30,000
0.3 gm particles per ft3 of air, or from about 600 0.5 gm particles per ft3 of
air to about 10,000
0.5 gm particles per ft3 of air. Particularly preferred particulate content is
from about 1,000 0.3
gm particles per ft3 of air to about 10,500 0.3 gm particles per ft3 of air,
or from about 600 0.5
gm particles per ft3 of air to about 1,000 0.5 gm particles per ft3 of air.
Another aspect of the invention includes providing purified air to an IVF
laboratory to
improve IVF clinical pregnancy rates and/or implantation rates. The clinical
pregnancy rate
refers to the presence of a fetal heart beat within an intrauterine sac. The
implantation rate refers
to the ability of a single embryo to implant within the uterus and develop a
fetal heartbeat. A
method of the present invention may comprise providing purified air, such as
air as
characterized supra, to an B/F laboratory, performing multiple cycles of IVF
in the laboratory,
and achieving a clinical pregnancy rate equal to or greater than 50% and/or an
implantation rate
of equal to or greater than 35% based on a minimum patient population of 20
patients. In one
embodiment, it is contemplated that achievable clinical pregnancy rates would
be from 50% to
70% and more preferably from 60% to 70%. In another embodiment, it is
contemplated that
achievable implantation rates would be from 35% to 40%.
Various aspects of the invention will be illustrated in more detail with
reference to the
following Examples, but it should be understood that the present invention is
not deemed to be
limited thereto.
11
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
EXAMPLES
Prior to the air purifier described herein, the national average for clinical
pregnancy rates
was approximately 38%. Couples often had to complete multiple cycles of IVF to
conceive
because the overall success rates were relatively low. As discussed supra, the
cost of a single
IVF cycle is high and multiple cycles are cost prohibitive to many.
Accordingly, there has been
a strong and long-felt need ¨ essentially since the advent of IVF
approximately 30 years ago - to
significantly improve IVF clinical pregnancy rates in order to make IVF a more
viable option for
infertility patients.
Prior to invention of the air purifier described herein, the inventor found
that IVF
laboratory air quality was not conducive to the successful growth of an
embryo, even if extant
filtration systems were utilized. Extant air filtration systems did not
deliver the air quality
necessary to support the human embryo and thus did not noticeably improve IVF
clinical
outcomes. In addition, extant air filtration systems did not protect the IVF
laboratory against
varying concentrations of airborne contaminants from the outside or source
air. For example, if
a nearby road or roof was being tarred, the toxic chemicals released would
potentially enter the
source air and the IVF laboratory and thus, impact the developing embryos.
Below are examples of how the air purifier described herein provides
significant
improvements in the art, representing surprising and unexpected results and
satisfaction of a
long-felt and unmet need. The examples compare the air purifier described
herein with extant
air filtration systems, including the Coda System and Zandair System. The
Coda System and
Zandair System have been the primary air filtration systems used in IVF
laboratories for at least
the last ten years.
EXAMPLE 1
An embodiment of the air purifier described herein was installed in an IVF
laboratory
beta site. Prior to that installation, the laboratory used two Coda Systems.
Each Coda
System included, from an upstream towards a downstream direction: (1)
particulate filtration;
(2) carbon and K1V1n04 filtration; and (3) HEPA filtration. Prior to
installation of the
aforementioned embodiment of the air purifier, clinical pregnancy rates at the
laboratory were
36.4%, which is near the national average of about 38%. The embodiment of the
air purifier
described herein that was installed in the laboratory included, from an
upstream towards a
downstream direction: (1) particulate filtration (located upstream in the air
handler unit); (2)
carbon and KMn04 filtration; (3) UV filtration; (4) carbon and KMn04
filtration; and (5) HEPA
filtration. After installation of the aforementioned embodiment of the air
purifier, clinical
12
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
pregnancy rates at the laboratory jumped to 67.4% based on a patient
population of 191 patients
- representing significant and surprising results in clinical outcomes and
patient care.
"Before" and "after" rvF implantation rates at the laboratory were also
measured. Prior
to installation of the aforementioned embodiment of the air purifier, the
implantation rate at the
laboratory was 21% and the national average was 26.1%. After installation of
the
aforementioned embodiment of the air purifier, the implantation rate at the
IVF laboratory beta
site increased to 39% based on a patient population of 191 patients -
representing significant and
surprising results in clinical outcomes and patient care. The significant and
surprising increase
in implantation rates has allowed the program at the laboratory to return
fewer embryos per
patient thus reducing the chance of multiple pregnancies (e.g., twins,
triplets, etc.) and
improving the overall obstetrical outcome.
In sum, these significant improvements in both clinical pregnancy rates and
implantation
rates demonstrate that the aforementioned embodiment of the air purifier
described herein
achieved unexpected results relative to the closest prior art and satisfied a
long-felt and unmet
need.
EXAMPLE 2
The following three charts provide data from independent third party testing
of air
quality in an IVF laboratory. Common to all three charts is the following
terminology: (1)
"Source Air" - the air going into an IVF laboratory prior to entering a
respective filtration
system; (2) "IVF Laboratory" - the ambient air within the IVF laboratory; (3)
"TVOC" - total
volatile organic compounds, measured in "ppb," or parts per billion; (4)
"Biologicals" ¨
biological contaminants, including spores, measured in "CFU/M3," or colony
forming units per
cubic meter; and (5) "Particulate" - the number of particles per cubic foot
having nominal sizes
of 0.3 p.m and 0.5 rm. These measurements were made using measuring devices
and techniques
described supra.
13
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
Chart No. 1
IVF Laboratory
Using Two (2) CODA Air Filtration Systems
Source Air IVF Laboratory
TVOC 1324 ppb 1372 ppb
Biologicals 469 CFU/M3 1778 CFU/M3
Particulate 2,318,663 11,642
0.3 im 0.3 p.m
particles per ft3 particles per
ft3
1,874,789 9,421
0.5 pm 0.5 1.tm
particles per ft3 particles per
ft3
Chart No. 1 compares the source air quality versus the IVF laboratory air
quality where
the IVF laboratory air had been subjected to two Coda Systems, as they are
described in
Example 1, supra. As Chart No. 1 shows, the air in the IVF laboratory actually
had higher
levels of TVOC and biological contaminants (including spores) than did the
source air. Only the
levels of particulates dropped between the source air and the IVF laboratory
air.
Chart No. 2
IVF Laboratory
Using Three (3) Zandair Filtration Systems
Source Air IVF Laboratory
TVOC 594 ppb 1030 ppb
Biologicals 28 CFU/M3 113 CFU/M3
Particulate 380,098 5,722
0.3 pm 0.3 pm
particles per ft3 particles per
ft3
1,695,377 41,472
0.5 1.tm 0.5 p.m
particles per ft3 particles per
ft3
Chart No. 2 compares the source air quality versus the IVF laboratory air
quality where
the IVF laboratory air had been subjected to three Zandair Systems. Each
Zandair System
included, from an upstream towards a downstream direction: (1) carbon
filtration; (2) HEPA
filtration; and (3) photo-catalytic oxidation along with UV filtration. As
Chart No. 2 shows, the
air in the IVF laboratory actually had higher levels of TVOC and biological
contaminants
(including spores) than did the source air. Only the levels of particulates
dropped between the
14
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
source air and the IVF laboratory air.
Chart No. 3
IVF Laboratory Using a Single (1)
Embodiment of Applicant's Air Purifier Described Herein
Source Air IVF Laboratory
TVOC 1400 ppb Less than 5 ppb
Biologicals 15,240 CFU/M3 Less thanl
CFU/M3
Particulate 1,063,435 5,410
0.3 pm 0.3 jtm
particles per ft3 particles per ft3
98,763 625
0.5 jam 0.5 jtm
particles per ft3 particles per ft3
Chart No. 3 compares the source air quality versus the IVF laboratory air
quality where
the IVF laboratory air had been subjected to only a single embodiment of the
air purifier
described herein. The aforementioned embodiment of the air purifier included,
from an
upstream towards a downstream direction: (1) particulate filtration (located
upstream in the air
handler unit); (2) carbon/KMn04 filtration; (3) UV filtration; (4)
carbon/KMnai filtration; and
(5) HEPA filtration. As shown in Chart No. 3, unlike the air quality results
for the two Coda
Systems and the three Zandair Systems provided in Chart Nos. 1 and 2
respectively, the single
aforementioned embodiment of the air purifier significantly improved air
quality with respect to
all three measured endpoints, i.e., (1) TVOC; (2) Biologicals; and (3)
Particulate.
The Coda System and Zandair System have been the primary air filtration
systems used
in IVF laboratories for at least the last ten years. The independent third
party testing results
provided in Chart Nos. 1, 2 and 3 demonstrate that the air purifier described
herein provided
markedly superior air purity compared to the primary air filtration systems
used in IVF
laboratories for at least the last ten years. The superior air purity
generated by the air purifier
described herein is surprising. Also surprising are the significantly improved
clinical pregnancy
rates and implantation rates described in Example 1, supra, resulting from the
superior air purity
generated by the air purifier described herein.
Taken together, Examples 1 and 2 demonstrate that performing IVF in ambient
air that
has been purified to levels disclosed herein for three parameters - TVOCs,
Biologicals and
Particulate ¨ unexpectedly and significantly improve IVF clinical pregnancy
rates and
implantation rates. In addition, given that IVF has existed for approximately
30 years and that
CA 02879498 2015-01-16
WO 2014/015126
PCT/US2013/051057
the Coda System and Zandair System have been the primary air filtration
systems used in IVF
laboratories for at least the last ten years, there has been a long-felt and
unmet need for an
improved air purifier for, among other things, IVF applications. The air
purifier described
herein has satisfied that need.
EXAMPLE 3
An embodiment of the air purifier described herein was installed in an IVF
laboratory
beta site. This embodiment included, from an upstream towards a downstream
direction: (1)
particulate filtration (located upstream in the air handler unit); (2) carbon
and KMn04 filtration;
(3) UV filtration; (4) carbon and 1(Mn04 filtration; and (5) HEPA filtration.
A catastrophic load of VOCs was accidentally introduced into the building that
housed
the IVF laboratory. In particular, a contractor had poured floor sealant on a
large floor surface
area in a room just adjacent to the IVF laboratory. The floor sealant
comprised 10% xylene and
40% acetone. Both xylene and acetone are highly embryotoxic. While staff
outside of the IVF
laboratory developed nausea and intense headaches from the fumes, the
aforementioned
embodiment of the air purifier protected the embryos and staff within the IVF
laboratory.
TVOC testing before and during the accident demonstrated that despite over
6000 ppb TVOCs
immediately outside of the laboratory ¨ an extremely high level - the TVOC
levels did not
change within the laboratory.
In sum, the significant and surprising results of Applicant's air purifier, as
demonstrated
in Examples 1, 2 and 3, were surprising and unexpected to the inventor and
would be surprising
and unexpected to persons of ordinary skill in the art. Those examples also
help demonstrate
how Applicant's air purifier has satisfied a long-felt and unmet need for an
improved air purifier
which allows for significantly improved clinical pregnancy rates and
implantation rates.
While the invention has been described in detail and with reference to
specific examples
thereof, it will be apparent to one skilled in the art that various changes
and modifications can be
made therein without departing from the spirit and scope thereof.
16