Note: Descriptions are shown in the official language in which they were submitted.
CA 02880062 2015-01-28
ACID GAS ABSORBENT, ACID GAS REMOVAL METHOD, AND
ACID GAS REMOVAL DEVICE
This is a divisional application of Canadian Patent
Application Serial No. 2,762,180 filed on December 14, 2011.
FIELD
[0002]
Embodiments described herein relate generally to an
acid gas absorbent, an acid gas removal device, and an acid
gas removal method using the acid gas absorbent.
It should be understood that the expression "the
invention" and the like used herein may refer to subject matter
claimed in either the parent or the divisional applications.
BACKGROUND
[0003]
In recent years, a greenhouse effect resulting from an
increase of a carbon dioxide (CO2) concentration is pointed out
as a cause of global warming phenomena, and there is an urgent
need to device an international countermeasure to protect
environment in a global scale. Industrial activities have a large
responsibility as a generation source of CO2, and there is a trend
to suppress discharge of CO2.
[0004]
As technologies to suppress the increase of an acid gas
concentration starting with CO2, there are a development of energy
savingproducts , a separation and recovery technology of discharged
1
CA 02880062 2015-01-28
acid gas, technologies to use the acid gas as a resource and to
isolate and store the acid gas, a switching to alternate energies
such as natural energy, atomic energy, and so on which do not
discharge the acid gas, and so on.
[0005] As a
separation technology of the acid gas studied up
to now, there are an absorption process, a suction process, a
membrane separationprocess , a cryogenic process, and so on . Among
them, the absorption process is suitable for processing a large
amount of gas, and an application for a factory, a power station
is considered.
[0006]
Accordingly, a method in which exhaust gas generated
when fossil fuel (coal, coal oil, natural gas, and so on) is burned
is brought into contact with a chemical absorbent to remove and
recover CO2 in exhaust combustion gas, and further a method storing
the recovered CO2 are performed throughout the world in a facility
such as a thermal power station using the fossil fuel. Besides,
acid gas such as hydrogen sulfide (H2S) in addition to CO2 are
removed by using the chemical absorbent is proposed.
[0007] In general, alkanolamines represented by
monoethanolamine (MEA) have been developed from 1930 years as the
chemical absorbent used in the absorption process, and it is sLill
used at present. This method is economical and it is easy to increase
the removal device in size.
[0008] As existing and widely used alkanolamines, there are
monoethanolamine, 2-amino-2-methylpropanolamine,
methylaminoethanol, ethylaminoethanol, propylaminoethanol,
diethanolamine, methyldiethanolamine, dimethylethanolamine,
diethylethanolamine, triethanolamine,
2
CA 02880062 2015-01-28
dimethylamino-1 -methylethanol, and so on.
[0009] In part i
cular , primary monoethanolamine and so on are widely
used because their reaction rates are fast. However, there are
problems in which this compound has corrosiveness, is easy to be
deteriorated, and requires high energy for regeneration. On the
other hand, tertiary methyldiethanolamine has low corrosiveness,
and requires low energy for regeneration, but has a defect that an
absorption speed is low. Accordingly, a development of a new
absorbent improving these points is required.
[0010] In recent years,
a study for alkanolamine particularly
having structural steric hindrance is vigorously tried as the
absorbent of acid gas among amino based compounds. The alkanolamine
having the steric hindrance has merits in which selectivity of acid
gas is very high, and the energy required for regeneration is small.
[0011] The reaction
speed of the amine based compound having the
steric hindrance depends on a degree of reaction hindrance determined
by a steric structure thereof. The reaction speed of the amine based
compound having the steric hindrance is lower than the secondary
amine, for example, such as methylethanolamine, diethanolamine, but
higher than the tertiary amine . Besides, 2 -amino-2 -methylpropanol ,
2-piperidineethanol, and so on are known as the alkanolamine to be
compounded in the absorbent.
[0012] On the other
hand, a method using a cyclic amine as the
absorbent as the amine based compound having a structure different
from the alkanolamines is also known.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] FIG. 1 is a
schematic diagram of an acid gas removal
3
CA 02880062 2015-01-28
device according to an embodiment.
DETAILED DESCRIPTION
[0014] However, these technologies are still insufficient
relating to absorption capacities of acid gas such as an absorption
amount of acid gas, an absorption speed of acid gas, and further
improvement of gas absorption capacities is required. Besides,
the one of which absorption amount of acid gas is higher is required
to further enhance recovery efficiency of acid gas.
[0015] A
problem to be solved by the present embodiments is
to provide an acid gas absorbent of which absorption amount of
acid gas such as carbon dioxide is large and a recovery amount
of acid gas is high, an acid gas removal device and an acid gas
removal method using the acid gas absorbent.
[0016] An
acid gas absorbent according to a first embodiment
comprising at least one type of tertiary amine compound represented
by the following general formula (1) .
R1
\
-----i
R2 -N ,--
N
1
R3 . . . (1)
(In the above-stated formula (1) , either one of the R1, R2 represents
a substituted or non-substituted alkyl group of which carbon number
is 2 to 5, and the other one represents a substituted or
non-substituted alkyl group of which carbon number is 1 to 5. The
R3 represents a methyl group or an ethyl group, and the R4 represents
a hydroxyalkyl group. The RI-, R2may either be the same or di f ferent,
4
CA 02880062 2015-01-28
and they may be coupled to form a cyclic structure.)
[0017]
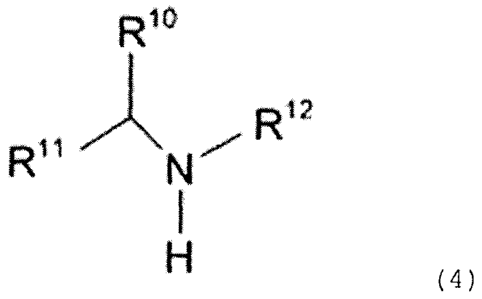
Besides, the acid gas absorbent according to a second
embodiment comprising at least one type of secondary amine compound
represented by the following general formula (4) .
[0018]
R" \N"-
... (4)
(In the above-stated formula (4) , either one of the R1 , Ril
represents a substituted or non-substituted alkyl group of which
carbon number is 2 to 5, and the other one represents a substituted
or non-substituted alkyl group of which carbon number is 1 to 5.
The R12 represents the hydroxyalkyl group. The R1 , R11 may either
be the same or different, and they may be coupled to form the cyclic
structure. When the R10, R11 form the cyclic structure, the R1 ,
R11 each represent the substituted or non-substituted alkyl group
of which carbon number is 1 to 5.)
[0019] An
acid gas removal method according to the embodiment
is to remove the acid gas from gas containing the acid gas by bringing
the gas containing the acid gas into contact with the acid gas
absorbent according to the embodiment.
[0020] An acid
gas removal device according to the embodiment
comprising:an absorption tower bringing the gas containing the
acid gas into contact with the acid gas absorbent according to
the embodiment to remove the acid gas from the gas; and a regeneration
tower removing the acid gas from the acid gas absorbent absorbing
5
CA 02880062 2015-01-28
the acid gas and regenerating the acid gas absorbent to be reused
at the absorption tower.
[0021]
Hereinafter, embodiments are described in detail. An
acid gas absorbent according to the first embodiment is
characterized in that it comprises at least one type of tertiary
amine compound represented by the following general formula (1) .
[0022]
RI
R2
W
... (1)
(In the above- stated formula (1) , either one of the R1, R2 represents
a substituted or non- substituted alkyl group of which carbon number
is 2 to 5, and the other one represents a substituted or
non-substituted alkyl group of which carbon number is 1 to 5. The
R3 represents a methyl group or an ethyl group, and the R4 represents
a hydroxyalkyl group . The R1, R2may either be the same or different,
and they may be coupled to form a cyclic structure.)
[0023]
Conventionally, it is known that a steric hindrance held
by the amine compound has a large influence on a product at a carbon
dioxide absorption time, and plays an advantageous role on a
generation of bicarbonate ion showing low heat of reaction. For
example, it is reported that N- (isopropyl) -N-methylaminoethanol
having a branch structure shows low heat of reaction for an
absorption reaction of carbon dioxide. The present inventors
examined based on the above-stated information to obtain a larger
6
CA 02880062 2015-01-28
effect of the steric hindrance, and as a result, they found that
it is possible to obtain further lower heat of reaction by using
the compound represented in the above-stated general formula (1)
(for example, N- (sec-butyl) -N-methylaminoethanol) than the
conventional amino compound having the branch structure.
[0024] Namely, in
the tertiary amine compound of the general
formula (1) , the methyl group or the ethyl group (R3) and the
hydroxyalkyl group (R4) are each coupled to a nitrogen atom. The
tertiary amine compound of the general formula (1) further has
the branch structure in which two alkyl groups (121, R2) are coupled
to one carbon atom which is coupled to the nitrogen atom.
[0025] As stated above, the tertiary amine compound of the
general formula (1) in which the branched alkyl groups are directly
coupled to the nitrogen atom has a structure of which steric
hindrance is large. Accordingly, it is conceivable that the
bicarbonate ion is generated and the heat of reaction is reduced
in a reaction between the tertiary amine compound of the general
formula (1) and carbon dioxide (CO2) .
[0026] The tertiary
amine compound represented by the general
formula (1) (hereinafter, it is referred to as the tertiary amine
compound (1) ) is dissolved in a solvent, for example, such as water,
and thereby, an acid gas absorbent of which absorption capacity
for the acid gas is high can be obtained. In the following
embodiment, a case when the acid gas is carbon oxide is described
as an example , but the acid gas absorbent according to the embodiment
is able to obtain similar effect as for the other acid gas such
as hydrogen sulfide.
[0027]
The Rl, R2 are groups coupling to the carbon atom which
7
CA 02880062 2015-01-28
is coupled to the nitrogen atom. Either one of the R1-, R2 is the
substituted or non-substituted alkyl group of which carbon number
is 2 to 5, and the other one is the substituted or non-substituted
alkyl group of which carbon number is 1 to 5. The 121, R2 may either
be the same or different. For example, branched or linear
hydrocarbon groups such as the methyl group, the ethyl group, a
propyl group, an isopropyl group, a butyl group, an s-butyl group
can be used as the substituted or non-substituted alkyl group of
which carbon number is 1 to 5, and these hydrocarbon groups may
contain a hetero atom such as Si, 0, N, S. It is more preferable
to use the methyl group or the ethyl group as the substituted or
non-substituted alkyl group of which carbon number is 1 to 5.
[0028]
For example, branched or linear hydrocarbon groups such
as the ethyl group, the propyl group, the isopropyl group, the
butyl group, the s-butyl group can be used as the substituted or
non-substituted alkyl group of which carbon number is 2 to 5, and
these hydrocarbon groups may contain the hetero atom such as Si,
0, N, S. It is more preferable to use the ethyl group as the
substituted or non-substituted alkyl group of which carbon number
is 2 to 5.
[0029]
The tertiary amine compound (1) in which at least either
one of the
R2 is the alkyl group of which carbon number is
2 or more has a small heat of reaction in a reaction with the acid
gas, and has an excellent reactivity for the acid gas. Besides,
the tertiary amine compound (1) in which at least either one of
the RI-, R2 is the alkyl group of which carbon number is 2 or more
has a higher boiling point and volatile from absorbing liquid is
difficult to occur compared to the tertiary amine compound in which
8
CA 02880062 2015-01-28
both of the R3-, R2 are the methyl groups.
[0030] The
R2 may form the cyclic structure in which the
substituted alkyl group or the non-substituted alkyl group of which
carbon number is 2 to 5 and the substituted alkyl group or the
non-substituted alkyl group of which carbon number is 1 to 5 are
coupled. A cyclopropyl group, a cyclobutyl group, a cyclopentyl
group, a cyclohexyl group, a cycloheptyl group, a cyclooctyl group,
a cyclononyl group can be cited as the cyclic structure.
[0031]
The volatile of the tertiary amine compound of the formula
(1) is suppressed by the cyclic structure formed by the R1, R2.
Accordingly, it is possible to make an acid gas absorbent in which
an amount of the amine component discharged into the atmosphere
is reduced during the exhaust gas is processed. Besides, the heat
of reaction of the tertiary amine compound of the formula (1) at
the reaction time with the acid gas is reduced by the cyclic structure
formed the Rl, R2. The cyclopentyl group and the cyclohexyl group
are more preferable among the above-stated cyclic structures from
a point of view of solubility.
[0032]
The R3 is the methyl group or the ethyl group. The R3
coupled to the nitrogen atom is set to be the methyl group or the
ethyl group, and thereby, it is possible to reduce the heat of
reaction of the tertiary amine compound (1) with the acid gas,
and to improve the reactivity of the acid gas absorbent with carbon
dioxide. The R3 is more preferable to be the methyl group.
[0033] The R4
is the hydroxyalkyl group. It is preferable to
be the hydroxyalkyl group of which carbon number is 2 to 4 from
a point of view of improving the reactivity with carbon dioxide.
The hydroxyalkyl group of the R4 is more preferable to be a
9
CA 02880062 2015-01-28
2-hydroxyethyl group.
[0034] For
example, N-(2-buty1)-N-methylaminoethanol,
N-(2-penty1)-N-methylaminoethanol,
N-(2-hexyl)-N-methylaminoethanol,
N-(3-penty1)-N-methylaminoethanol,
N-(3-hexyl)-N-methylaminoethanol,
N-(3-hepty1)-N-methylaminoethanol,
N-(4-hepty1)-N-methylaminoethanol,
N-(4-octy1)-N-methylaminoethanol,
N-(5-nony1)-N-methylaminoethanol,
N-(2-buty1)-N-ethylaminoethanol,
N-(2-penty1)-N-ethylaminoethanol,
N-(2-hexyl)-N-ethylaminoethanol,
N-(3-penty1)-N-ethylaminoethanol,
N-(3-hexyl)-N-ethylaminoethanol,
N-(3-hepty1)-N-ethylaminoethanol,
N-(4-hepty1)-N-ethylaminoethanol,
N-(4-octy1)-N-ethylaminoethanol,
N-(5-nony1)-N-ethylaminoethanol,
N-(2-buty1)-N-methylaminopropanol,
N-(2-penty1)-N-methylaminopropanol,
N-(2-hexyl)-N-methylaminopropanol,
N-(3-penty1)-N-methylaminopropanol,
N-(3-hexyl)-N-methylaminopropanol,
N-(3-hepty1)-N-methylaminopropanol,
N-(4-hepty1)-N-methylaminopropanol,
N-(4-octy1)-N-methylaminopropanol,
N-(5-nony1)-N-methylaminopropanol,
CA 02880062 2015-01-28
N-(2-buty1)-N-ethylaminopropanol,
N-(2-penty1)-N-ethylaminopropanol,
N-(2-hexyl)-N-ethylaminopropanol,
N-(3-penty1)-N-ethylaminopropanol,
N-(3-hexyl)-N-ethylaminopropanol,
N-(3-hepty1)-N-ethylaminopropanol,
N-(4-hepty1)-N-ethylaminopropanol,
N-(4-octy1)-N-ethylaminopropanol,
N-(5-nony1)-N-ethylaminopropanol,
N-(2-buty1)-N-methylaminobutanol,
N-(2-penty1)-N-methylaminobutanol,
N-(2-hexyl)-N-methylaminobutanol,
N-(3-penty1)-N-methylaminobutanol,
N-(3-hexyl)-N-methylaminobutanol,
N-(3-hepty1)-N-methylaminobutanol,
N-(4-hepty1)-N-methylaminobutanol,
N-(4-octy1)-N-methylaminobutanol,
N-(5-nony1)-N-methylaminobutanol,
N-(2-buty1)-N-ethylaminobutanol,
N-(2-penty1)-N-ethylaminobutanol,
N-(2-hexyl)-N-ethylaminobutanol,
N-(3-penty1)-N-ethylaminobutanol,
N-(3-hexyl)-N-ethylaminobutanol,
N-(3-hepty1)-N-ethylaminobutanol,
N-(4-hepty1)-N-ethylaminobutanol,
N-(4-octy1)-N-ethylaminobutanol,
N- (5-nonyl) -N-ethylaminobutanol canbe cited as the tertiaryamine
compound (1) in which the branched alkyl group is coupled to the
11
CA 02880062 2015-01-28
nitrogen atom.
[0035] As the
tertiary amine compound (1) in which the R1, R2
form the cyclic structure, N-cyclopropyl-N-methylaminoethanol,
N-cyclobutyl-N-methylaminoethanol,
N-cyclopentyl -N-methylaminoethanol ,
N-cyclohexyl-N-methylaminoethanol,
N-cycloheptyl-N-methylaminoethanol,
N-cyclooctyl-N-methylaminoethanol, and so on can be cited.
[0036] Note that one type of compound selected from the
above-stated group can be used as the tertiary amine compound (1) ,
and the one in which two or more type of compounds selected from
the above-stated group are mixed can be used as the tertiary amine
compound (1) .
[0037] It is
preferable that a content of the tertiary amine
compound (1) contained in the acid gas absorbent is 10 mass% to
55 mass. In general, the absorption amount, a desorption amount
of carbon dioxide per a unit capacity are larger and an absorption
speed, a desorption speed of carbon dioxide are faster as a
concentration of the amine component is higher, and therefore,
it is preferable in an energy consumption side, a size of a plant
facility, and a process efficiency side. However, it becomes
impossible for the water contained in the absorbing liquid to fully
exhibit a function as an activator relative to the absorption of
carbon dioxide when the concentration of the amine component in
the absorbing liquid is too high. Besides, defects such as an
increase of viscosity of the absorbing liquid become unable to
disregard when the concentration of the amine component in the
absorbing liquid is too high. When the content of the tertiary
12
CA 02880062 2015-01-28
amine compound (1) is 55 mass% or less, phenomena such as the increase
of the viscosity of the absorbing liquid, the deterioration of
the function of water as the activator are not recognized. Besides,
the content of the tertiary amine compound (1) is set to be 10
mass % or more, and thereby, it is possible to obtain the enough
absorption amount, absorption speed of carbon dioxide, and to
obtain excellent process efficiency.
[0038] Not only the absorption amount of carbon dioxide and
the absorption speed of carbon dioxide are high but also the
desorption amount of carbon dioxide and the desorption speed of
carbon dioxide are high when the acid gas absorbent of which content
of the tertiary amine compound (1) is within a range of 10 mass%
to 55 mass% is used for recovery of carbon dioxide. Accordingly,
it is advantageous in a point that the recovery of carbon dioxide
can be performed effectively. The content of the tertiary amine
compound (1) is more preferable to be 20 mass% to 50 mass%.
[0039] It is preferable that the tertiary amine compound (1)
is used while being mixed with a reaction accelerator composed
of alkanolamines and/or a hetero cyclic amine compound represented
by the following general formula (2) (hereinafter referred to as
the hetero cyclic amine compound (2) ) .
[0040]
¨ ¨13
R6
H2C I NR5
\ /
/ . . . (2)
13
CA 02880062 2015-01-28
[0041]
In the formula (2), the R5 represents a hydrogen atom
or a substituted or non-substituted alkyl group of which carbon
number is 1 to 4. The R6 represents the substituted or
non-substituted alkyl group of which carbon number is 1 to 4 coupled
to the carbon atom. The "n" represents an integer number of 1
to 3, the "m" represents an integer number of 1 to 4, and the "p"
represents an integer number of "0" (zero) to 12. When the "n"
is 2 to 3, the nitrogen atoms are not directly coupled with each
other.
[0042]
In the present embodiment, it is possible to mix, for
example, the tertiary amine compound (1) and the reaction
accelerator composed of the alkanolamines and/or the hetero cyclic
amine compound (2) . In addition, it is possible to use the one
in which the mixture of the tertiary amine compound (1) and the
alkanolamines and/or the hetero cyclic amine compound (2) is made
into, for example, a water solution as the acid gas absorbent.
The tertiary amine compound (1) is used while being mixed with
the alkanolamines and/or the hetero cyclic amine compound (2) ,
and thereby, it is possible to further improve the absorption amount
of carbon dioxide per unit mol of the tertiary amine compound (1) ,
the absorption amount of carbon dioxide per unit volume of the
acid gas absorbent and the absorption speed of carbon dioxide.
Besides, the tertiary amine compound (1) is used while being mixed
with the alkanolamines and/or the hetero cyclic amine compound
(2) , and thereby, an energy separating the acid gas after the
absorption of carbon dioxide (acid gas desorption energy) is
lowered, and it becomes possible to reduce the energy when the
14
CA 02880062 2015-01-28
acid gas absorbent is regenerated.
[0043] For example, monoethanolamine,
2-amino-2-methylpropanolamine, 2-amino-2-methyl-1,
3-dipropanolamine, methylaminoethanol, ethylaminoethanol,
propylaminoethanol, diethanolamine,
bis(2-hydroxy-l-methylethyl)amine, methyldiethanolamine,
dimethylethanolamine, diethylethanolamine, triethanolamine,
dimethylamino-l-methylethanol, 2-methylaminoethanol,
2-ethylaminoethanol, 2-propylaminoethanol,n-butylaminoethanol,
2-(isopropylamino)ethanol, 3-ethylaminopropanol,
triethanolamine, diethanolamine, and so on can be cited as
alkanolamine.
[0044] Among them, it is preferable to be at least one type
selected from a group consisting of 2-(isopropylamino)ethanol,
2-(ethylamino)ethanol, and 2-amino-2-methyl-l-propanol as the
alkanolamines from a point of view of improving the reactivity
between the tertiary amine and the acid gas.
[0045] As the hetero cyclic amine compound (2), azetidine,
1-methylazetidine, 1-ethylazetidine, 2-methylazetidine,
2-azetidinemethanol, 2-(2-aminoethyl)azetidine, pyrrolidine,
1-methylpyrrolidine, 2-methylpyrrolidine, 2-butylpyrrolidine,
2-pyrrolidinemethanol, 2- (2-aminoethyl)pyrrolidine, piperidine,
1-methylpiperidine, 2-ethylpiperidine, 3-propylpiperidine,
4-ethylpiperidine, 2-piperidinemethanol, 3-piperidineethanol,
2-(2-aminoethyl)pyrrolidine, hexahydro-1H-azepine,
hexamethylenetetramine, piperazine, piperazine derivatives, and
so on can be cited.
[0046] Among them, the piperazine derivative is particularly
CA 02880062 2015-01-28
desirable from points of view of improvements of the absorption
amount and the absorption speed of carbon dioxide of the acid gas
absorbent. The piperazine derivative is the secondary amine
compound, and in general, the nitrogen atom of the secondary amino
group is coupled to carbon dioxide to form carbamate ion, and thereby,
it contributes to the improvement of the absorption speed at an
initial stage of the reaction. Further, the nitrogen atom of the
secondary amino group has a role of converting carbon dioxide
coupled thereto into bicarbonate (HCO3-), and contributes to the
improvement of speed at a half stage after the reaction.
[0047]
It is more preferable that at least one type from among
2-methylpiperazine, 2,5-dimethylpiperazine,
2,6-dimethylpiperazine is the piperazine derivative.
[0048] It is preferable that the content of the reaction
accelerator (the alkanolamines and/or the hetero cyclic amine
compound (2) ) contained in the acid gas absorbent is 1 mass % to
mass% . There is a possibility that the effect improving the
absorption speed of carbon dioxide cannot be fully obtained when
the content of the reaction accelerator contained in the acid gas
20 absorbent is less than 1 mass% . There is a possibility that the
reactivity conversely deteriorates because the viscosity of the
absorbent becomes excessively high when the content of the reaction
accelerator contained in the acid gas absorbent exceeds 20 mass.
The content of the reaction accelerator (the alkanolamines and/or
the hetero cyclic amine compound (2) ) is more preferable to be
5 mass % to 15 mass% .
[0049]
Besides, an acid gas absorbent according to a second
embodiment is characterized in that it comprises at least one kind
16
CA 02880062 2015-01-28
of secondary amine compound represented by the following general
formula (4) .
[0050]
R12
Ri---- \
. . . (4)
(In the above-stated formula (4) , either one of the R1 , R11
represents a substituted or non-substituted alkyl group of which
carbon number is 2 to 5, and the other one represents a substituted
or non-substituted alkyl group of which carbon number is 1 to 5.
The R12 represents a hydroxyalkyl group. The R10, R11 may either
be the same or different, and they may be coupled to form the cyclic
structure. When the R1 , R11 form the cyclic structure, the R1 ,
R11 each represent the substituted or non-substituted alkyl group
of which carbon number is 1 to 5.)
[0051] The secondary amine compound of the general formula (4)
has a branch structure in which two alkyl groups (R10, R11) are
coupled to one carbon atom which is coupled to a nitrogen atom.
[0052] As stated above, the secondary amine compound of the
general formula (4) in which the branched alkyl group is directly
coupled to the nitrogen atom has a structure of which steric
hindrance is large. Accordingly, it has high reactivity for the
acid gas such as carbon dioxide (CO2) and the high acid gas absorption
amounL can be obtained.
[0053] The secondary amine compounds represented by the general
17
CA 02880062 2015-01-28
formula (4) (hereinafter, referred to as the secondary amine
compound (4)) is dissolved into a solvent, for example, such as
water, and thereby, the acid gas absorbent of which absorption
capacity of the acid gas is high can be obtained.
[0054]
The 121 , x are groups coupled to the carbon atom which
is coupled to the nitrogen atom in the formula (4) . Either one
of the R.1 , R11 represents the substituted or non-substituted alkyl
group of which carbon number is 2 to 5, and the other one represents
the substituted or non-substituted alkyl group of which carbon
number is 1 to 5. The R10, R11 may either be the same or different.
For example, branched or linear hydrocarbon groups such as the
methyl group, the ethyl group, the propyl group, the isopropyl
group, the butyl group, the s-butyl group can be used as the
substituted or non-substituted alkyl group of which carbon number
is 1 to 5, and these hydrocarbon groups may contain the hetero
atom such as Si, 0, N, S. It is more preferable to use the methyl
group or the ethyl group as the substituted or non-substituted
alkyl group of which carbon number is 1 to 5.
[0055] It
is possible to use the branched or linear hydrocarbon
group such as, for example, the ethyl group, the propyl group,
the isopropyl group, the butyl group, the s-butyl group as the
substituted or non-substituted alkyl group of which carbon number
is 2 to 5, and these hydrocarbon groups may contain the hetero
atom such as Si, 0, N, S. It is more preferable to use the ethyl
group as the substituted or non-substituted alkyl group of which
carbon number is 2 to 5.
[0056]
The secondary amine compound (4) in which at least uiLhe.t.
one of the Rn, is
the alkyl group of which carbon number is
18
CA 02880062 2015-01-28
2 or more has small heat of reaction in the reaction with the acid
gas and has excellent reactivity for the acid gas. Besides, the
secondary amine compound (4) in which either one of the R10,
is the alkyl group of which carbon number is 2 or more has the
higher boiling point and the volatile from the absorbing liquid
is difficult to occur compared to the secondary amine compound
in which both of the Rl , R11 are the methyl groups.
[0057] The R3- , Ril may be coupled to form the cyclic structure.
When the Rl , Rll form the cyclic structure, the R1 , Ril each represent
the substituted or non-substituted alkyl group of which carbon
number is 1 to 5. The cyclopropyl group, the cyclobutyl group,
the cyclopentyl group, the cyclohexyl group, the cycloheptyl group,
the cyclooctyl group, the cyclononyl group can be cited as the
cyclic structure.
[0058] The volatile of the secondary amine compound (4) is
suppressed by the cyclic structure formed by the Rl ,
Accordingly, it is possible to make the acid gas absorbent in which
an amount of the amine component discharged in the atmosphere is
reduced during the exhaust gas is processed. Besides, the heat
of reaction of the secondary amine compound of the formula (4)
at the reaction time with the acid gas is reduced by the cyclic
structure formed by the R1 , R11. The cyclopentyl group and the
cyclohexyl group are more preferable among the above-stated cyclic
structures from a point of view of solubility.
[0059] The R12 is the hydroxyalkyl group. It is preferable to
be a hydroxyalkyl group of which carbon number is 2 to 4 from a
point of view of improving the reactivity with carbon dioxide.
The hydroxyalkyl group of the R12 is more preferable to be the
19
CA 02880062 2015-01-28
2-hydroxyethyl group.
[0060]
The following compounds can be cited as the secondary
amine compound (4) in which the branched alkyl group is coupled
to the nitrogen atom. Namely, 2-(2-butylamino)ethanol,
2-(2-pentylamino)ethanol, 2-(2-hexylamino)ethanol,
2-(3-pentylamino)ethanol, 2-(3-hexylamino)ethanol,
2-(3-heptylamino)ethanol, 2-(4-heptylamino)ethanol,
2-(4-octylamino)ethanol, 2-(5-nonylamino)ethanol,
3-(2-butylamino)propanol, 3-(2-pentylamino)propanol,
3-(2-hexylamino)propanol, 3-(3-pentylamino)propanol,
3-(3-hexylamino)propanol, 3-(3-heptylamino)propanol,
3-(4-heptylamino)propanol, 3-(4-octylamino)propanol,
3-(5-nonylamino)propanol, 4-(2-butylamino)butanol,
4-(2-pentylamino)butanol, 4-(2-hexylamino)butanol,
4-(3-pentylamino)butanol, 4-(3-hexylamino)butanol,
4-(3-heptylamino)butanol, 4-(4-heptylamino)butanol,
4-(4-octylamino)butanol, 4-(5-nonylamino)butanol,
2-(cyclopropylamino)ethanol, 2-(cyclobutylamino)ethanol,
2-(cyclopentylamino)ethanol, 2-(cyclohexylamino)ethanol,
2-(cycloheptylamino)ethanol, 2-(cyclooctylamino)ethanol,
3-(cyclopropylamino)propanol, 3-(cyclobutylamino)propanol,
3-(cyclopentylamino)propanol, 3-(cyclohexylamino)propanol,
3-(cycloheptylamino)propanol, 3-(cyclooctylamino)propanol,
4-(cyclopropylamino)propanol, 4-(cyclobutylamino)butanol,
4-(chiclopentylamino)butanol, 4-(cyclohexylamino)butanol,
4- (cycloheptylamino)butanol, 4- (cyclooctylamino)butanol, and so
on can be cited as the secondary amine compound (4). Note LhdL
the one in which one type of compound or two types or more compounds
CA 02880062 2015-01-28
selected from the above-stated group are mixed can be used as the
secondary amine compound (4) .
[0061]
The secondary amine compound (secondary aminoalcohols)
having the high boiling point is preferable as the secondary amine
compound (4) . The acid gas absorbent absorbing CO2 is heated at
a high-temperature range of approximately 120 C to be regenerated.
Accordingly, it is preferable to use the high-boiling point
secondary amine compound as the secondary amine compound (4) which
is difficult to be discharged from the regeneration tower when
it is heated. It is therefore preferable to use the alkyl group
as the secondary amine compound (4) having many carbon atoms. In
particular, the secondary amine compound having the cyclic
structure is preferable.
[0062]
Note that it is possible to use one type of compound
selected from the above-stated group as the secondary amine
compound (4) , or to use the one in which two or more types of compounds
selected from the above-stated group are mixed.
[0063] It
is preferable that a content of the secondary amine
compound (4) contained in the acid gas absorbent is preferable
to be 10 mass% to 55 mass. In general, the absorption amount,
the desorption amount of carbon dioxide per the unit capacity are
larger and the absorption speed, the desorption speed of carbon
dioxide are faster as the concentration of the amine component
is higher, and therefore, the high concentration is preferable
in an energy consumption side, a size of a plant equipment, and
a process efficiency side. However, it becomes impossible for
the water contained in the absorbing liquid Lu fully exhibiL a
function as an activator relative to the absorption of carbon
21
CA 02880062 2015-01-28
dioxide when the concentration of the amine component in the
absorbing liquid is too high. Besides, defects such as an increase
of viscosity of the absorbing liquid become unable to disregard
when the concentration of the amine component in the absorbing
liquid is too high.
[0064]
When the content of the secondary amine compound (4)
is 55 mass% or less, phenomena such as the increase of the viscosity
of the absorbing liquid, the deterioration of the function of water
as the activator are not recognized. Besides, the content of the
secondary amine compound (4) is set to be 10 mass% or more, and
thereby, it is possible to obtain the enough absorption amount,
absorption speed of carbon dioxide, and to obtain excellent process
efficiency.
[0065]
Not only the absorption amount of carbon dioxide and
the absorption speed of carbon dioxide are high but also the
desorption amount of carbon dioxide and the desorption speed of
carbon dioxide are high when the acid gas absorbent of which content
of the secondary amine compound (4) is within the range of 10 mass%
to 55 mass% is used for carbon dioxide recovery. Accordingly,
it is advantageous in a point that the recovery of carbon dioxide
can be performed effectively. The content of the secondary amine
compound (4) is more preferable to be 20 mass% to 50 mass%.
[0066] It
is preferable that the secondary amine compound (4)
is used by mixing with the reaction accelerator composed of the
alkanolamines and/or a hetero cyclic amine compound represented
by the following general formula (2) (hereinafter referred to as
the hetero cyclic amine compound (2) ) .
[0067]
22
CA 02880062 2015-01-28
R5
H2C NR5
- \\/./- n
. . . (2)
[0068]
In the formula (2) , the R5 represents a hydrogen atom
or a substituted or non-substituted alkyl group of which carbon
number is 1 to 4. The R6 represents the substituted or
non-substituted alkyl group of which carbon number is 1 to 4 coupled
to the carbon atom. The "n" represents an integer number of 1
to 3, the "m" represents an integer number of 1 to 4, and the "p"
represents an integer number of "0" (zero) to 12. When the "n"
is 2 to 3, the nitrogen atoms are not directly coupled with each
other.
[0069]
In the present embodiment, it is possible to mix, for
example, the secondary amine compound (4) and the reaction
accelerator composed of the alkanolamines and/or the hetero cyclic
amine compound (2) . In addition, it is possible to use the one
in which the mixture of the secondary amine compound (4) and the
alkanolamines and/or the hetero cyclic amine compound (2) is made
to be, for example, a water solution as the acid gas absorbent.
[0070] The secondary amine compound (4) is mixed with the
alkanolamines and/or the hetero cyclic amine compound (2) to be
used, and thereby, it is possible to further improve the absorption
amount of carbon dioxide per unit mol of the secondary amine compound
(4) , the absorption amount of carbon dioxide per unit volume of
the acid gas absorbent and the absorption speed of carbon dioxide.
23
CA 02880062 2015-01-28
Besides, the secondary amine compound (4) is mixed with the
alkanolamines and/or the hetero cyclic amine compound (2) to be
used, and thereby, an energy separating the acid gas after the
absorption of carbon dioxide (acid gas desorption energy) is
lowered, and it becomes possible to reduce the energy when the
acid gas absorbent is regenerated.
[0071] As concrete examples and preferable examples of
alkanolamine used as the reaction accelerator in the second
embodiment, the similar ones as the concrete examples and the
preferable examples cited in the first embodiment can be cited.
[0072] As
concrete examples and preferable examples of the
hetero cyclic amine compound (2) used as the reaction accelerator
in the second embodiment, the similar ones as the concrete examples
and the preferable examples cited in the first embodiment can be
cited.
[0073] It is preferable that a content of the reaction
accelerator (the alkanolamines and/or the hetero cyclic amine
compound (2)) contained in the acid gas absorbent according to
the second embodiment is 1 mass-% to 20 mass% . There is apossibility
that the effect improving the absorption speed of carbon dioxide
cannot be fully obtained when the content of the reaction
accelerator contained in the acid gas absorbent is less than 1
mass%. There is a possibility that the reactivity conversely
deteriorates because the viscosity of the absorbent becomes
excessively high when the content of the reaction accelerator
contained in the acid gas absorbent exceeds 20 mass% . The content
of the reaction accelerator (the alkanolamines and/or the hetero
cyclic amine compound (2)) contained in the acid gas absorbent
24
CA 02880062 2015-01-28
according to the second embodiment is more preferable to be 5 mass%
to 15 mass%.
[0074] The acid gas absorbent may contain an anticorrosive of
phosphoric acid based and so on to prevent a corrosion of the plant
equipment, a defoamer of silicon based and so on to prevent
effervescence, an antioxidant to prevent deterioration of the acid
gas absorbent, and so on, in addition to the amine compound and
the reaction accelerator as stated above.
[0075] An acid gas removal method according to the present
embodiment is the one in which exhaust gas containing acid gas
is brought into contact with an acid gas absorbent made up by
dissolving the amine compound described in the above-stated
embodiment in a solvent, and the acid gas is absorbed and separated
to be removed from the exhaust gas containing the acid gas.
[0076] A basic constitution of an absorbing and separating
process of carbon dioxide comprises: a process bringing exhaust
gas containing carbon dioxide into contact with an acid gas
absorbent to make the acid gas absorbent absorb carbon dioxide
(carbon dioxide absorbing process) ; and a process heating the acid
gas absorbent to which carbon dioxide is absorbed obtained at the
carbon dioxide absorbing process to desorb and recover carbon
dioxide (carbon dioxide separating process) .
[0077] A method to bring the gas containing carbon dioxide into
contact with a water solution containing the acid gas absorbent
is not particularly limited, but for example, it is performed by
a method in which the gas containing carbon dioxide is bubbled
in the acid gas absorbent to absorb carbon dioxide, a method in
which the acid gas absorbent is atomized and sprayed in a gas flow
CA 02880062 2015-01-28
containing carbon dioxide (atomizing and spraying method) , a method
in which the gas containing carbon dioxide is brought into
countercurrent contact with the acid gas absorbent in an absorption
tower containing filler made of a porcelain or a metal net, or
the like.
[0078] A
temperature of the acid gas absorbent when the gas
containing carbon dioxide is absorbed in the water solution is
generally set within a range from a room temperature to 60 C or
less. It is preferable to be set at 50 C or less, andmore preferable
to be set at approximately 20 C to 45 C. The absorption amount
of the acid gas increases as it is performed at a lower temperature,
but a lower limit value of the process temperature is determined
by a gas temperature and a heat recovery target and so on in the
process. A pressure at the carbon dioxide absorption time is
generally set at approximately the atmospheric pressure. It is
possible to pressurize up to higher pressure to enhance the
absorption performance, but it is preferable to set under the
atmospheric pressure to suppress energy consumption required for
compression.
[0079] In the
carbon dioxide absorption process, the carbon
dioxide absorption amount at the carbon dioxide absorption time
(40 C) of the acid gas absorbent containing the amine compound
according to the above-stated embodiment for 10 mass % to 55 mass%
is approximately 0.26 mol to 0.62 mol per 1 mol of amine contained
in the absorbent. Besides, in the carbon dioxide absorption
process, the carbon dioxide absorption speed of the acid gas
absorbent containing the amine compound according to the embodiment
for 10 mass % to 55 mass% after a few minutes have passed since
26
CA 02880062 2015-01-28
the absorption of carbon dioxide is started is approximately 0.029
mol/L/min to 0.038 mol/L/min.
[0080]
Here, a carbon dioxide saturation absorption amount is
a value in which an inorganic carbon amount in the acid gas absorbent
is measured by an infrared gas concentration measurement device.
Besides, the carbon dioxide absorption speed is a value measured
by using an infrared carbon dioxide sensor at a time when a few
minutes have passed since the absorption of carbon dioxide is
started.
[0081] A method
desorbing carbon dioxide by heating the acid
gas absorbent as same as distillation and beating in an iron pot,
a method heating by extending a liquid interface in a plate tower,
a spray tower, and the regeneration tower containing filler made
of a porcelain or a metal net, or the like, and so on can be cited
as a method separating carbon dioxide from the acid gas absorbent
absorbing carbon dioxide, and recovering pure or
high-concentration carbon dioxide. Carbon dioxide is thereby
released and discharged from anionic carbamate and bicarbonate.
[0082] A
temperature of the acid gas absorbent at the carbon
dioxide separation time is normally set to be 70 C or more, it
is preferable to be 80 C or more, and more preferable to be
approximately 90 C to 120 C. The absorption amount increases as
the temperature is higher, but the energy required for the heating
of the absorbing liquid increases if the temperature is increased.
Accordingly, the temperature of the acid gas absorbent at the carbon
dioxide separation time is determined by the gas temperature, the
heat recovery target and so on in the process. The pressure at
the carbon dioxide desorption time is generally set at
27
CA 02880062 2015-01-28
approximately the atmospheric pressure. It is possible to
decrease the pressure to a lower pressure to enhance the desorption
performance, but it is preferable to be set under the atmospheric
pressure to suppress energy consumption required to decrease the
pressure.
[0083] The carbon dioxide desorption amount at the carbon
dioxide desorption time (80 C) of the water solution containing
the amine compound according to the above-stated embodiment for
mass% to 55 mass % is approximately 0.15 mol to 0.47 mol per
10 1 mol of amine contained in the absorbent.
[0084]
The acid gas absorbent after carbon dioxide is separated
is transferred to the carbon dioxide absorption process again to
be cyclic used (recycled) . Besides, the heat generated at the
carbon dioxide absorption time is generally heat exchanged by a
heat exchanger for preheating the water solution injected into
the regeneration tower during a recycle process of the water
solution and cooled.
[0085]
Purity of carbon dioxide recovered as stated above is
normally extremely high such as approximately 95 vol% to 99 vol%.
This pure carbon dioxide or carbon dioxide in high concentration
are used as chemicals, synthetic raw materials of high polymer,
a coolant for freeze foods, and so on. In addition, it is possible
to isolate and store the recovered carbon dioxide to an underground
or the like by means which is currently technically developed.
[0086] The
process separating carbon dioxide from the acid gas
absorbent and regenerating the acid gas absorbent is a part
consuming the largest amount of energy among the above-stated
processes, and the energy of approximately 50% to 80% within the
28
CA 02880062 2015-01-28
whole process is consumed at the process. Accordingly, it is
possible to reduce a cost of the absorbing and separating process
of carbon dioxide and to perform the removal of the acid gas from
the exhaust gas advantageously from a economy standpoint by
reducing the consumption energy at the regeneration process of
the acid gas absorbent. from a point of view of
[0087] According to
the present embodiment, it is possible to
reduce the energy required for the desorption of carbon dioxide
(regeneration process) by using the acid gas absorbent according
to the above-stated
embodiment. Accordingly, it is possible to
perform the absorbing and separating process of carbon dioxide
under an economically advantageous condition.
[0088] Besides, the
amine compound according to the embodiment
has extremely high corrosion resistance relative to a metal
material such as a carbon steel compared to alkanolamines such
as 2-aminoethanol which is conventionally used as the acid gas
absorbent. Accordingly, it is costly advantageous by using the
acid gas removal method using the acid gas absorbent as stated
above because it is not necessary to use expensive anticorrosion
steel in, for example, a plant construction.
[0089] An acid gas removal device according to the present
embodiment comprises: an absorption tower in which gas containing
acid gas is brought into contact with an acid gas absorbent according
to the embodiment to remove the acid gas from the gas; and a
regeneration tower removing the acid gas from the acid gas absorbent
absorbing the acid gas to regenerate the acid gas absorbent reused
at the absorption tower.
[0090] FIG. 1 is a
schematic diagram of an acid gas removal
29
CA 02880062 2015-01-28
device according to the embodiment. An acid gas removal device
1 includes: an absorption tower 2 in which gas containing acid
gas (hereinafter, referred to as exhaust gas) is brought into
contact with an acid gas absorbent to absorb and remove the acid
gas from the exhaust gas; and a regeneration tower 3 separating
the acid gas from the acid gas absorbent absorbing the acid gas
to regenerate the acid gas absorbent. Hereinafter, a case when
the acid gas is carbon dioxide is described as an example.
[0091] As illustrated in FIG. 1, exhaust gas containing carbon
dioxide such as exhaust combustion gas discharged from a thermal
power station is introduced to a lower part of the absorption tower
2 by passing through a gas supply port 4. This exhaust gas is
shut in the absorption tower 2, and it is brought into contact
with an acid gas absorbent supplied from an acid gas absorbent
supply port 5 at an upper part of the absorption tower 2 The
acid gas absorbent according to the above-stated embodiment is
used as the acid gas absorbent.
[0092] A pH value of the acid gas absorbent is to be adjusted
at least at 9 or more, but an optimal condition may be appropriately
selected depending on a kind or a concentration of harmful gas
contained in the exhaust gas, a flow rate, and so on. Besides,
the other compounds such as nitrogen-containing compound improving
the absorption performance of carbon dioxide, antioxidant, pH
adjusting agent may be contained in the acid gas absorbent with
an arbitrary rate in addition to the above-stated amine based
compound, and the solvent such as water.
[0093] As stated above, the exhaust gas is brought into contact
with the acid gas absorbent, and thereby, carbon dioxide within
CA 02880062 2015-01-28
the exhaust gas is absorbed by the acid gas absorbent and removed.
The exhaust gas after carbon dioxide is removed is discharged toward
outside of the absorption tower 2 from a gas discharge port 6.
[0094] The acid gas absorbent absorbing carbon dioxide is
transferred to a heat exchanger 7, a heater 8 to be heated, and
thereafter, transferred to the regeneration tower 3. The acid
gas absorbent transferred to the regeneration tower 3 is moved
from an upper part to a lower part of the regeneration tower 3.
Carbon dioxide within the acid gas absorbent is desorbed during
the moving, and the acid gas absorbent is regenerated.
[0095]
The acid gas absorbent regenerated in the regeneration
tower 3 is transferred to the heat exchanger 7, an absorbing liquid
cooler 10 by a pump 9, and returned to the absorption tower 2 from
the acid gas absorbent supply port 5.
[0096] On
the other hand, carbon dioxide separated from the
acid gas absorbent is brought into contact with reflux water
supplied from a reflux drum 11 at the upper part of the regeneration
tower 3, and discharged toward outside of the regeneration tower
3.
The reflux water in which carbon dioxide is dissolved is cooled
in a reflux condenser 12, and thereafter, it is separated from
liquid component in which vapor with carbon dioxide is condensed
in the reflux drum 11. This liquid component is introduced to
the carbon dioxide recovery process by a recovery carbon dioxide
line 13. On the other hand, the reflux water from which carbon
dioxide is separated is transferred to the regeneration tower 3
by a reflux water pump 14.
[0097]
According to the acid gas removal device 1 of the present
embodiment, it becomes possible to perform the absorption and the
31
CA 02880062 2015-01-28
removal of carbon dioxide with high efficiency by using the acid
gas absorbent excellent in the absorption feature and desorption
feature of carbon dioxide.
[0098] Hereinabove,
the embodiments of the present invention
are described with reference to concrete examples, but the
above-stated examples are cited just as an example of the present
invention, and not to intend to limit the invention. Besides,
a description relating to portions and so on which are not directly
necessary for the explanation of the present invention is not given
in the description of each embodiment in the acid gas absorbent,
the acid gas removal device, and the acid gas removal method.
However, each element required thereto may be appropriately
selected to be used.
[0100] Hereinafter, the embodiments are described in more
detail with reference to examples, a comparative example, but the
present invention is not limited to these examples.
(Example 1)
[0101] A water
solution of 50 ml is prepared by dissolving 45
mass% of N- (sec-butyl) -N-methylaminoethanol, and 5 mass% of
piperidine inwater (hereinafter, referred to as absorbing liquid) .
This absorbing liquid is filled in a test tube, heated to be 40 C,
32
CA 02880062 2015-01-28
then mixed gas containing carbon dioxide (CO2) for 10 vol%, nitrogen
(N2) gas for 90 vol% is aerated at a flow rate of 500 mL/min . The
absorptionperformance is evaluated bymeasuring the carbon dioxide
(CO2) concentration within the gas at an exit of the test tube
by using an infrared gas concentration measurement device
(manuf actured by Shimadzu Corporation, name of article: "CGT- 700" ) .
A Teflon (registered trademark) tube (inside diameter; 1.59 mm,
outside diameter: 3.17 mm) of 1/8 inches is set at a gas introducing
port to the amine solution in the test tube. Besides, the solution
after the mixed gas is absorbed at 40 C as stated above is heated
to be 80 C, 100% nitrogen (N2) gas is aerated at a flow rate of
500 mL/min, and the CO2 concentration in the absorbing liquid is
measured byusing the infrared gas concentrationmeasurement device
to evaluate a release performance.
[0102] The absorption speed of carbon dioxide of the absorbing
liquid is the speed measured at a time after two minutes have passed
since the absorption of carbon dioxide is started. The heat of
reaction is measured by using a calorimeter "DRC Evolution"
(product name, manufactured by SETRAM company) .
[0103] A diffusion performance of the amine compound is
evaluated as stated below. Namely, the absorbing liquid is put
into a flask with a cooling tube, and thereafter, it is heated
to 120 C together with the flask. A gas component diffused from
the cooling tube is collected, and an amount of the amine compound
contained in the collected gas is measured.
[0104] The absorption amount of carbon dioxide of the absorbing
liquid at 40 C is 0.47 mol per 1 mol of the amine compound in the
absorbing liquid. Besides, the absorption amount of carbon
33
CA 02880062 2015-01-28
dioxide (CO2) of the absorbing liquid at 80 C is 0.20 mol per 1
mol of the amine compound. During a process absorbing carbon
dioxide (CO2) at 40 C and desorbing carbon dioxide (CO2) at 80 C,
CO2 of 0.27 mol per 1 mol of the amine compound is recovered. The
absorption speed of carbon dioxide is 0.034 mol/L/min.
(Example 2)
[0105]
The absorbing liquid is prepared as same as the example
1 except that 2-ethylpiperazine is used instead of piperidine.
The absorption amount of carbon dioxide and the absorption speed
of carbon dioxide are measured under the same conditions by using
the same devices as the example 1. The absorption amount of carbon
dioxide at 40 C is 0.53 mol, and the absorption amount of carbon
dioxide at 80 C is 0.18 mol per 1 mol of the amine compound in
the absorbing liquid. Carbon dioxide of 0.35 mol per 1 mol of
the amine compound in the absorbing liquid is recovered. The
absorption speed of carbon dioxide is 0.035 mol/L/min.
(Example 3)
[0106]
The absorbing liquid is prepared as same as the example
1 except that piperazine is used instead of piperidine. The
absorption amount of carbon dioxide and the absorption speed of
carbon dioxide are measured under the same conditions by using
the same devices as the example 1. The absorption amount of carbon
dioxide at 40 C is 0.58 mol, and the absorption amount of carbon
dioxide at 80 C is 0.11 mol per 1 mol of the amine compound in
the absorbing liquid. Carbon dioxide of 0.47 mol per 1 mol of
the amine compound in the absorbing liquid is recovered. The
absorption speed of carbon dioxide is 0.037 mol/L/min.
(Example 4)
34
CA 02880062 2015-01-28
[0107]
The absorbing liquid is prepared as same as the example
1 except that 2,5 -dimethylpiperazine is used instead of piperidine.
The absorption amount of carbon dioxide and the absorption speed
of carbon dioxide are measured under the same conditions by using
the same devices as the example 1. The absorption amount of carbon
dioxide at 40 C is 0.49 mol, and the absorption amount of carbon
dioxide at 80 C is 0.18 mol per 1 mol of the amine compound in
the absorbing liquid. Carbon dioxide of 0.31 mol per 1 mol of
the amine compound in the absorbing liquid is recovered. The
absorption speed of carbon dioxide is 0.035 mol/L/min.
(Example 5)
[0108]
The absorbing liquid is prepared as same as the example
1 except that N- (3-pentyl) -N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.48 mol, and the
absorption amount of carbon dioxide at 80 C is 0.13 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.45 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 6)
[0109] The
absorbing liquid is prepared as same as the example
1 except that N- (2-hexyl) -N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and
2,5-dimethylpiperazine is used instead of piperidine. The
CA 02880062 2015-01-28
absorption amount of carbon dioxide and the absorption speed of
carbon dioxide are measured under the same conditions by using
the same devices as the example 1. The absorption amount of carbon
dioxide at 40 C is 0.44 mol, and the absorption amount of carbon
dioxide at 80 C is 0.14 mol per 1 mol of the amine compound in
the absorbing liquid. Carbon dioxide of 0.30 mol per 1 mol of
the amine compound in the absorbing liquid is recovered. The
absorption speed of carbon dioxide is 0.034 mol/L/min.
(Example 7)
[0110] The
absorbing liquid is prepared as same as the example
1 except that N- (2-heptyl) -N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and
2,5-dimethylpiperazine is used instead of piperidine. The
absorption amount of carbon dioxide and the absorption speed of
carbon dioxide are measured under the same conditions by using
the same devices as the example 1. The absorption amount of carbon
dioxide at 40 C is 0.41 mol, and the absorption amount of carbon
dioxide at 80 C is 0.14 mol per 1 mol of the amine compound in
the absorbing liquid. Carbon dioxide of 0.27 mol per 1 mol of
the amine compound in the absorbing liquid is recovered. The
absorption speed of carbon dioxide is 0.034 mol/L/min.
(Example 8)
[0111]
The absorbing liquid is prepared as same as the example
1 except that N-cyclopentyl-N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
36
CA 02880062 2015-01-28
absorption amount of carbon dioxide at 40 C is 0.43 mol, and the
absorption amount of carbon dioxide at 80 C is 0.04 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.39 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 9)
[0112]
The absorbing liquid is prepared as same as the example
1 except that N-cyclohexyl-N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.37 mol, and the
absorption amount of carbon dioxide at 80 C is 0.10 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.27 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 10)
[0113]
The absorbing liquid is prepared as same as the example
1 except that N-cyclobutyl-N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.39 mol, and the
absorption amount of carbon dioxide at 80 C is 0.05 mol per 1 mol
37
CA 02880062 2015-01-28
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.34 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 11)
[0114]
The absorbing liquid is prepared as same as the example
1 except that 3- (N-cyclopentyl-N-methylamino) -1-propanol is used
instead of N- (sec-butyl) -N-methylaminoethanol, and piperazine is
used instead of piperidine. The absorption amount of carbon
dioxide and the absorption speed of carbon dioxide are measured
under the same conditions by using the same devices as the example
1. The absorption amount of carbon dioxide at 40 C is 0.38 mol,
and the absorption amount of carbon dioxide at 80 C is 0.04 mol
per 1 mol of the amine compound in the absorbing liquid. Carbon
dioxide of 0.34 mol per 1 mol of the amine compound in the absorbing
liquid is recovered. The absorption speed of carbon dioxide is
0.035 mol/L/min.
(Example 12)
[0115]
The absorbing liquid is prepared as same as the example
1 except that 30 mass% of N-cyclopentyl-N-methylaminoethanol is
used instead of N- (sec-butyl) -N-methylaminoethanol, and
piperazine is used instead of piperidine. The absorption amount
of carbon dioxide and the absorption speed of carbon dioxide are
measured under the same conditions by using the same devices as
the example 1. The absorption amount of carbon dioxide at 40 C
is 0.39 mol, and the absorption amount of carbon dioxide at 80 C
is 0.04 mol per 1 mol of the amine compound in the absorbing liquid.
Carbon dioxide of 0.35 mol per 1 mol of the amine compound in the
38
CA 02880062 2015-01-28
absorbing liquid is recovered. The absorption speed of carbon
dioxide is 0.035 mol/L/min.
(Example 13)
[0116]
The absorbing liquid is prepared as same as the example
1 except that N-cyclopentyl-N-methylaminoethanol is used instead
of N- (sec-butyl) -N-methylaminoethanol, and 2.5 mass% of
piperazine and 2.5 mass% of 2-amino-2-methyl-l-propanol are used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.48 mol, and the
absorption amount of carbon dioxide at 80 C is 0.10 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.38 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 14)
[0117]
The absorbing liquid is prepared as same as the example
1 except that 2- (cyclopentylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.56 mol, and the
absorption amount of carbon dioxide at 80 C is 0.25 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.31 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
39
CA 02880062 2015-01-28
mol/L/min.
(Example 15)
[0118]
The absorbing liquid is prepared as same as the example
1 except that 2- (2-butylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1 The
absorption amount of carbon dioxide at 40 C is 0.57 mol, and the
absorption amount of carbon dioxide at 80 C is 0.24 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.33 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 16)
[0119]
The absorbing liquid is prepared as same as the example
1 except that 2- (2-pentylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.55 mol, and the
absorption amount of carbon dioxide at 80 C is 0.25 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.30 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 17)
CA 02880062 2015-01-28
[0 12 0]
The absorbing liquid is prepared as same as the example
1 except that 2- (3-pentylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.53 mol, and the
absorption amount of carbon dioxide at 80 C is 0.25 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.28 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 18)
[0121]
The absorbing liquid is prepared as same as the example
1 except that 2- (2-hexylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.51 mol, and the
absorption amount of carbon dioxide at 80 C is 0.26 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.25 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.036
mol/L/min.
(Example 19)
[0122]
The absorbing liquid is prepared as same as the example
1 except that 2- (3-hexylamino) ethanol is used instead of
41
CA 02880062 2015-01-28
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.50 mol, and the
absorption amount of carbon dioxide at 80 C is 0.27 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.23 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.034
mol/L/min.
(Example 20)
[0123]
The absorbing liquid is prepared as same as the example
1 except that 30 mass% of 2- (cyclopentylamino) ethanol is used
instead of N- (sec-butyl) -N-methylaminoethanol, and piperazine is
used instead of piperidine. The absorption amount of carbon
dioxide and the absorption speed of carbon dioxide are measured
under the same conditions by using the same devices as the example
1. The absorption amount of carbon dioxide at 40 C is 0.56 mol,
and the absorption amount of carbon dioxide at 80 C is 0.24 mol
per 1 mol of the amine compound in the absorbing liquid. Carbon
dioxide of 0.32 mol per 1 mol of the amine compound in the absorbing
liquid is recovered. The absorption speed of carbon dioxide is
0.037 mol/L/min.
(Example 21)
[0124] The
absorbing liquid is prepared as same as the example
1 except that 2- (cyclobutylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
42
CA 02880062 2015-01-28
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.56 mol, and the
absorption amount of carbon dioxide at 80 C is 0.25 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.31 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.037
mol/L/min.
(Example 22)
[0125] The absorbing
liquid is prepared as same as the example
1 except that 2- (cyclopentylamino) -1-propanol is used instead of
N- (sec-butyl) -N-methylaminoethanol. The absorption amount of
carbon dioxide and the absorption speed of carbon dioxide are
measured under the same conditions by using the same devices as
the example 1. The absorption amount of carbon dioxide at 40 C
is 0.54 mol, and the absorption amount of carbon dioxide at 80 C
is 0.24 mol per 1 mol of the amine compound in the absorbing liquid.
Carbon dioxide of 0.30 mol per 1 mol of the amine compound in the
absorbing liquid is recovered. The absorption speed of carbon
dioxide is 0.034 mol/L/min.
(Example 23)
[0126] The absorbing
liquid is prepared as same as the example
1 except that 3- (cyclohexylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and piperazine is used
instead of piperidine. The absorption amount of carbon dioxide
and the absorption speed of carbon dioxide are measured under the
same conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.50 mol, and the
43
CA 02880062 2015-01-28
absorption amount of carbon dioxide at 80 C is 0.24 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.26 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.034
mol/L/min.
(Example 24)
[0127]
The absorbing liquid is prepared as same as the example
1 except that 2- (cyclopentylamino) ethanol is used instead of
N- (sec-butyl) -N-methylaminoethanol, and 2.5 mass% of piperazine,
2.5 mass% of 2-amino-2-methyl-1-propanol is used instead of
piperidine. The absorption amount of carbon dioxide and the
absorption speed of carbon dioxide are measured under the same
conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.58 mol, and the
absorption amount of carbon dioxide at 80 C is 0.26 mol per 1 mol
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.32 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.037
mol/L/min.
(Example 25)
[0128]
The absorbing liquid is prepared as same as the example
1 except that 30 mass % of 2- (cyclopentylamino) ethanol is used
instead of N- (sec-butyl) -N-methylaminoethanol , and piperidine is
not used. The absorption amount of carbon dioxide and the
absorption speed of carbon dioxide are measured under the same
conditions by using the same devices as the example 1. The
absorption amount of carbon dioxide at 40 C is 0.59 mol, and the
absorption amount of carbon dioxide at 80 C is 0.25 mol per 1 mol
44
CA 02880062 2015-01-28
of the amine compound in the absorbing liquid. Carbon dioxide
of 0.34 mol per 1 mol of the amine compound in the absorbing liquid
is recovered. The absorption speed of carbon dioxide is 0.038
mol/L/min.
(Example 26)
[0129]
The absorbing liquid is prepared as same as the example
1 except that 30 mass% of 2- (cyclopentylamino) ethanol is used
instead of N- (sec-butyl) -N-methylaminoethanol, and 5 mass % of
2-amino-2-methyl-l-propanol is used instead of piperidine . The
absorption amount of carbon dioxide and the absorption speed of
carbon dioxide are measured under the same conditions by using
the same devices as the example 1. The absorption amount of carbon
dioxide at 40 C is 0.61 mol, and the absorption amount of carbon
dioxide at 80 C is 0.28 mol per 1 mol of the amine compound in
the absorbing liquid. Carbon dioxide of 0.33 mol per 1 mol of
the amine compound in the absorbing liquid is recovered. The
absorption speed of carbon dioxide is 0.038 mol/L/min.
(Comparative Example 1)
[0130] A
water solution of 50 ml is prepared by dissolving 60
mass% of n-butyldiethanolamine and 5 mass% of piperazine in water
(hereinafter, referred to as absorbing liquid) . After that, the
absorption amount of carbon dioxide and the absorption speed of
carbon dioxide are measured under the same conditions as the example
1 by using the same devices as the example 1. The absorption amount
of carbon dioxide at 40 C is 0.20 mol, and the absorption amount
of carbon dioxide at 80 C is 0.08 mol per 1 mol of the amine compound
in the absorbing liquid. Carbon dioxide of 0.12 mol per 1 mol
of the amine compound in the absorbing liquid is recovered. The
CA 02880062 2015-01-28
absorption speed of carbon dioxide is 0.023 mol/L/min.
[0131] The measurement results of the absorption amount of
carbon dioxide at 40 C, the absorption amount of carbon dioxide
at 80 C, the recovery amount of carbon dioxide, the absorption
speed of carbon dioxide, and the heat of reaction as for the examples
1 to 26, and the comparative example 1 are represented at Tables
1 to 2 together with the content of the amine compound and the
reaction accelerator in the absorbing liquid. Note that in Tables
1 to 2, the absorption amount of carbon dioxide and the recovery
amount of carbon dioxide are the ones representing the absorption
amount and the recovery amount per 1 mol of the amine compound
contained in the absorbing liquid by the number of moles.
20
46
[0132] [Table 1]
reaction accelerator
CO2 CO2
AMINE CO2 ABSORPTION CO2 ABSORPTION
HEAT OF
AMINE HETERO CYCLIC
RECOVERY ABSORPTION
COMPOUND AMOUNT (40 C) AMOUNT (80
C) REACTION
COMPOUND AMINE COMPOUND
AMOUNT SPEED
[ MAS .9% ] [mol] [mol]
[kJ/mol]
[MASSM [MASSN
[nol] [mol/L/min]
EXAMPLE 1 45 5 0.47 0.20
0.27 0.034 67
EXAMPLE 2 45 5 0.53 0.18
0.35 0.035 67
EXAMPLE 3 45 5 0.58 0.11
0.47 ' 0.037 66 o
EXAMPLE 4 45 5 0.49 0.18
, 0.31 0.035 67 0
.
iv
EXAMPLE 5 45 5 0.48 0.13
, 0.45 0.036 67 m
m
, 0
,
EXAMPLE 6 45 5 - 0.44 0.14
0.30 0.034 67 0
m
EXAMPLE 7 , 45 5 0.41 0.14
0.27 0.034 67 iv
N.,
EXAMPLE 8 , 45 5 0.43 0.04
0.39 0.036 66 0
1-,
01
1
EXAMPLE 9 45 5 0.37 0.10
0.27 0.036 , 66 1 0
1-,
1
EXAMPLE 10 45 5 0.39 0.05
0.34 0.036 66
iv
EXAMPLE 11 45 5 0.38 0.04
0.34 0.035 66 m
EXAMPLE 12 30 5 0.39 0.04
0.35 0.035 66
EXAMPLE 13 45 2.5 2.5 0.48 0.10
0.38 0.036 , 71
47
[0133] [Table 2]
reaction accelerator
CO2 co2
AMINE CO2 ABSORPTION CO2 ABSORPTION
HEAT OF
AMINE HETERO CYCLIC
RECOVERY ABSORPTION
COMPOUND AMOUNT (40 C) AMOUNT (80
C) REACTION
COMPOUND AMINE COMPOUND
AMOUNT SPEED
[MASSN [mo1] [mol]
[kJ/mo1] .
[MAD] [MASON]
[mol] [mol/L/min]
EXAMPLE 14 45 5 0.56 0.25
0.31 0.036 74
EXAMPLE 15 45 5 0.57 0.24
0.33 0.036 75
EXAMPLE 16 45 5 0.55 0.25
0.30 0.036 75 o
EXAMPLE 17 45 5 0.53 0.25
0.28 0.036 75 0
iv
m
EXAMPLE 18 45 5 0.51 0.26
0.25 0.036 75 m
0
EXAMPLE 19 45 5 0.50 0.27
0.23 0.034 75 0
m
iv
EXAMPLE 20 30 5 0.56 0.24
0.32 0.037 74 iv
0
EXAMPLE 21 45 , 5 0.56 0.25
0.31 0.037 75
01
1
EXAMPLE 22 45 5 0.54 0.24
0.30 0.034 75 0
1-,
1
EXAMPLE 23 45 5 0.50 0.24
0.26 0.034 75 N.)
m
EXAMPLE 24 45 2.5 2.5 0.58 0.26
0.32 0.037 74
EXAMPLE 25 30 0.59 0.25
0.34 0.038 76
EXAMPLE 26 30 5 0.61 0.28
0.33 0.038 76
COMPARATIVE
60 5 0.20 0.08
0.12 0.023 66
EXAMPLE 1 -
48
CA 02880062 2015-01-28
[0134] As
it is obvious from Tables 1 to 2, in the absorbing
liquid of the examples 1 to 13 using the tertiary amine compound
having the branched alkyl group or the cyclic alkyl group, the
recovery amount of carbon dioxide is high, the heat of reaction
is suppressed to be low, the absorption speed of carbon dioxide
is high, and the absorption performance of carbon dioxide is
excellent. In particular, in each of the examples 8 to 13 using
the tertiary amine compound having the cyclic alkyl group, the
heat of reaction is generally 66 kJ/mol, and the heat of reaction
is lower compared to the examples 1 to 7 using the tertiary amine
compound having the branched alkyl group. Besides, in the
evaluation test of the diffusion performance, the amine compound
of approximately 1 mass -% is recovered in each of the examples 1
to 7 using the tertiary amine compound having the branched alkyl
group, but the amine compound is seldom recovered in each of the
examples 8 to 13 using the tertiary amine compound having the cyclic
alkyl group. It is recognized from the above that the tertiary
amine compound having the cyclic alkyl group has low diffusion
performance, and the volatile thereof is suppressed. Further,
in each of the examples 8 to 13, it is recognized that the recovery
amount of carbon dioxide and the recovery speed of carbon dioxide
equivalent to the examples 1 to 7 can be obtained.
[0135] On
the other hand, in the comparative example 1 using
the amine compound which does not have the branched alkyl group
or the cyclic alkyl group, it is recognized that the recovery amount
of carbon dioxide is low such as 0.12 mol, and the absorption speed
of carbon dioxide is small.
[0136] Besides, as it is obvious from Tables 1 to 2 in the
49
CA 02880062 2015-01-28
absorbing liquid of each of the examples 14 to 26 using the secondary
amine compound having the branched alkyl group or the cyclic alkyl
group, the absorption amount of carbon dioxide, the recovery amount
of carbon dioxide are high. Besides, in each of the examples 14
to 26, the absorption speed of carbon dioxide is also high, and
the absorption performance of carbon dioxide is excellent. In
particular, in each of the examples 14, 20, 24 using the secondary
amine compound having the cyclic alkyl group, it is recognized
that the heat of reaction is lower compared to each of the examples
15 to 19 using the secondary amine compound having the branched
alkyl group. Besides, in the evaluation test of the diffusion
performance, the amine compound of approximately 1 mass% is
recovered in each of the examples 15 to 19 using the secondary
amine compound having the branched alkyl group, but the amine
compound is seldom recovered in each of the example 14 and examples
to 26 using the secondary amine compound having the cyclic alkyl
group. It is recognized from the above that the secondary amine
compound having the cyclic alkyl group has low diffusion
performance, and the volatile thereof is suppressed. Further,
20 in each of the example 14 and examples 20 to 26, it is recognized
that the recovery amount of carbon dioxide, the recovery speed
of carbon dioxide can be obtained equivalent to the examples 15
to 19.
[0137] On the other hand, in the comparative example 1 using
the amine compound which does not have the branched alkyl group
or the cyclic alkyl group, it is recognized that the recovery amount
of carbon dioxide is low such as 0.12 mol, and the absorption speed
of carbon dioxide is also small.