Note: Descriptions are shown in the official language in which they were submitted.
CA 02881274 2015-02-05
MIXING DEVICE AND OUTPUT FLUIDS OF SAME
SEQUENCE LISTING
This description contains a sequence listing in electronic form in ASCII text
format. A copy of the sequence listing in electronic form is available from
the Canadian
Intellectual Property Office.
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention is directed generally to mixing devices and more
particularly
to mixing devices that mix two or more materials between surfaces, including
such as
between a rotating rotor and a stationary stator.
Description of the Related Art
Figure 1 provides a partial block diagram, partial cross-sectional view of a
prior art
device 10 for diffusing or emulsifying one or two gaseous or liquid materials
("infusion
materials") into another gaseous or liquid material ("host material")
reproduced from U.S.
Patent No. 6,386,751, incorporated herein by reference in its entirety. The
device 10
includes a housing configured to house a stator 30 and a rotor 12. The stator
30
encompasses the rotor 12. A tubular channel 32 is defined between the rotor 12
and the
stator 30. The generally cylindrically shaped rotor 12 has a diameter of about
7.500
inches and a length of about 6.000 inches providing a length to diameter ratio
of about 0.8.
The rotor 12 includes a hollow cylinder, generally closed at both ends. A gap
exists
between each of the first and second ends of the rotor 12 and a portion of the
housing 34.
A rotating shaft 14 driven by a motor 18 is coupled to the second end of the
rotor 12. The
first end of the rotor 12 is coupled to an inlet 16. A first infusion material
passes through
the inlet 16 and into the interior of the rotor 12. The first infusion
material passes from the
interior of the rotor 12 and into the channel 32 through a plurality of
openings 22 formed in
the rotor 12.
The stator 30 also has openings 22 formed about its circumference. An inlet 36
passes a second infusion material to an area 35 between the stator 30 and
1
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
the housing 34. The second infusion material passes out of the area 35 and
into the
channel 32 through openings 22.
An external pump (not shown) is used to pump the host material into a single
inlet port 37. The host material passes through a single inlet port 37 and
into the
channel 32 where it encounters the first and second infusion materials, which
enter
the channel 32 through openings 22. The infusion materials may be pressurized
at
their source to prevent the host material from passing through openings 22.
The inlet port 37, is configured and positioned such that it is located along
only a relatively small portion (< about 5%) of the annular inlet channel 32,
and is
substantially parallel to the axis of rotation of the rotor 12 to impart an
axial flow
toward a portion of the channel 32 into the host material.
Unfortunately, before entering the tubular channel 32, the host material must
travel in tortuous directions other than that of the axial flow (e.g.,
including in
directions substantially orthogonal thereto) and down into and between the gap
formed between the first end of the rotor 12 and the housing 34 (i.e., down a
portion
of the first end of the rotor adjacent to the inlet 16 between the end of the
rotor 12
and the housing 34). The non-axial and orthogonal flow, and the presence of
the
host material in the gap between the first end of the rotor 12 and the housing
34
causes undesirable and unnecessary friction. Further, it is possible for a
portion of
the host material to become trapped in eddy currents swirling between the
first end
of the rotor and the housing. Additionally, in the device 10, the host
material must
negotiate at least two right angles to enter any aspect of the annual of the
annular
inlet of the tubular channel 32.
A single outlet port 40 is formed in the housing 34. The combined host
material and infusion material(s) exit the channel 32 via the outlet 40. The
outlet
port 40, which is also located along only a limited portion (< about 5%) of
the annular
outlet of tubular channel 32, is substantially parallel to the axis of
rotation of the
rotor 12 to impart or allow for an axial flow of the combined materials away
from the
limited portion of the annular outlet of tubular channel 32 into the outlet
port 40. An
external pump 42 is used to pump the exiting fluid through the outlet port 40.
Unfortunately, before exiting the channel 32, a substantial portion of the
exiting material must travel in a tortuous direction other than that of the
axial flow
(e.g., including in directions substantially orthogonal thereto) and down into
and
2
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
between the gap formed between the second end of the rotor 12 and the housing
34 (i.e.,
down a portion of the second end of the rotor adjacent to the shaft 14 between
the end of
the rotor 12 and the housing 34). As mentioned above, the non-axial and
orthogonal flow,
and the presence of the host material in the other gap between the end (in
this case, the
second end) of the rotor 12 and the housing 34 causes additional undesirable
and
unnecessary friction. Further, it is possible for a portion of the host
material to become
trapped in eddy currents swirling between the second end of the rotor and the
housing.
Additionally, in the device 10, a substantial portion of the exiting combined
material must
negotiate at least two right angles as it exits form the annular exit of the
tubular channel 32
into the outlet port 40.
As is apparent to those of ordinary skill in the art, the inlet port 37
imparts only an
axial flow to the host material. Only the rotor 21 imparts a circumferential
flow into the host
material. Further, the outlet port 40 imparts or provides for only an axial
flow into the exiting
material. Additionally, the circumferential flow velocity vector is imparted
to the material
only after it enters the annular inlet 37 of the tubular channel 32, and
subsequently the
circumferential flow vector must be degraded or eliminated as the material
enters the exit
port 40. There is, therefore, a need for a progressive circumferential
acceleration of the
material as it passes in the axial direction through the channel 32, and a
circumferential
deceleration upon exit of the material from the channel 32. These aspects, in
combination
with the tortuous path that the material takes from the inlet port 37 to the
outlet port 40,
create a substantial friction and flow resistance over the path that is
accompanied by a
substantial pressure differential (26 psi, at 60 gallons/min flow rate)
between the inlet 37
and outlet 40 ports, and these factors, inter alia, combine to reduce the
overall efficiency of
the system.
SUMMARY
Disclosed herein is a mixing device for creating an output mixture by mixing a
first
material and a second material, the device comprising: a first chamber
configured to receive
the first material from a source of the first material; a stator; a rotor
having an axis of
rotation, the rotor being disposed inside the stator and configured to rotate
about the axis of
rotation therein, at least one of the rotor and stator having a plurality of
through-holes; a
3
CA 02881274 2015-02-05
mixing chamber defined between the rotor and the stator, the mixing chamber
being in fluid
communication with the first chamber and configured to receive the first
material therefrom,
and the second material being provided to the mixing chamber via the plurality
of through-
holes formed in the at least one of the rotor and stator; and a first internal
pump housed
inside the first chamber, the first internal pump being configured to pump the
first material
from the first chamber into the mixing chamber.
Also disclosed herein is a mixing device for creating an output mixture by
mixing a
first material and a second material, the device comprising: a stator; a rotor
having an axis
of rotation, the rotor being disposed inside the stator and configured to
rotate about the axis
of rotation therein; a mixing chamber defined between the rotor and the
stator, the mixing
chamber having an open first end through which the first material enters the
mixing
chamber and an open second end through which the output material exits the
mixing
chamber, the second material entering the mixing chamber through at least one
of the rotor
and the stator; a first chamber in communication with at least a majority
portion of the open
first end of the mixing chamber; and a second chamber in communication with
the open
second end of the mixing chamber.
Also disclosed herein is a method of mixing a first material and a second
material in
an arcuate mixing chamber formed between two contoured surfaces to create an
output
mixture, the arcuate mixing chamber having a first end portion opposite a
second end
portion, the method comprising: introducing the first material into the first
end portion of the
arcuate mixing chamber in a flow direction having a first component that is
substantially
tangent to the arcuate mixing chamber and a second component that is directed
toward the
second end portion; and introducing the second material into the arcuate
mixing chamber
though at least one of the two contoured surfaces between the first end
portion of the
arcuate mixing chamber and the second end portion of the arcuate mixing
chamber.
Also disclosed herein is a method of producing an electrokinetically altered
oxygenated aqueous fluid or solution, comprising: providing a flow of a fluid
material
between two spaced surfaces in relative motion and defining a mixing volume
therebetween, wherein the dwell time of a single pass of the flowing fluid
material within and
through the mixing volume is greater than 0.06 seconds or greater than 0.1
seconds; and
introducing oxygen (02) into the flowing fluid material within the mixing
volume under
conditions suitable to dissolve at least 20 ppm.
3a
CA 02881274 2015-02-05
Also disclosed herein is a method of producing an electrokinetically altered
oxygenated aqueous fluid or solution, comprising: providing a flow of a fluid
material
between two spaced surfaces defining a mixing volume therebetween; and
introducing
oxygen into the flowing material within the mixing volume under conditions
suitable to infuse
at least 20 ppm oxygen into the material in less than 400 milliseconds.
Also disclosed herein is a method of producing an electrokinetically altered
oxygenated aqueous fluid or solution, comprising use of a mixing device for
creating an
output mixture by mixing a first material and a second material, the device
comprising: a
first chamber configured to receive the first material from a source of the
first material; a
stator; a rotor having an axis of rotation, the rotor being disposed inside
the stator and
configured to rotate about the axis of rotation therein, at least one of the
rotor and stator
having a plurality of through-holes; a mixing chamber defined between the
rotor and the
stator, the mixing chamber being in fluid communication with the first chamber
and
configured to receive the first material therefrom, and the second material
being provided to
the mixing chamber via the plurality of through-holes formed in the one of the
rotor and
stator; a second chamber in fluid communication with the mixing chamber and
configured to
receive the output material therefrom; and a first internal pump housed inside
the first
chamber, the first internal pump being configured to pump the first material
from the first
chamber into the mixing chamber.
Also disclosed herein is a method of producing an electrokinetically altered
oxygenated aqueous fluid or solution, comprising use of a mixing device for
creating an
output mixture by mixing a first material and a second material, the device
comprising: a
stator; a rotor having an axis of rotation, the rotor being disposed inside
the stator and
configured to rotate about the axis of rotation therein; a mixing chamber
defined between
the rotor and the stator, the mixing chamber having an open first end through
which the first
material enters the mixing chamber and an open second end through which the
output
material exits the mixing chamber, the second material entering the mixing
chamber
through at least one of the rotor and the stator; a first chamber in
communication with at
least a majority portion of the open first end of the mixing chamber; and a
second chamber
in communication with the open second end of the mixing chamber.
Also disclosed herein is a bioreactor system comprising a bioreactor in
combination
with a mixing device as defined above.
3b
CA 02881274 2016-01-26
CA 2881274
Also disclosed herein is an output mixture produced by a mixing device as
defined
above including a gas-enriched fluid. In some embodiments, the output mixture
is an
electrokinetically altered oxygenated aqueous fluid or solution.
Also disclosed herein are electrokinetically altered oxygenated aqueous fluid
or
solutions including ones made by a method as defined above.
Also disclosed herein is a composition, comprising an electrokinetically
altered
oxygenated aqueous fluid or solution, wherein the oxygen in the fluid or
solution is present in
an amount of at least 25 ppm, at least 30, at least 40, at least 50, or at
least 60 ppm oxygen.
Also disclosed herein is a composition, comprising an electrokinetically
altered
oxygenated aqueous fluid or solution, wherein the fluid or solution comprises
at least 25 ppm,
at least 30, at least 40, at least 50, or at least 60 ppm oxygen, wherein the
fluid or solution
facilitates oxidation of pyrogallol to purpurogallin in the presence of
horseradish peroxidase
enzyme (HRP) in an amount above that afforded by a control pressure pot
generated or fine-
bubble generated aqueous fluid or solution having an equivalent dissolved
oxygen level, and
wherein there is no hydrogen peroxide, or less than 0.1 ppm of hydrogen
peroxide present in
the electrokinetic oxygen-enriched aqueous fluid or solution.
The claimed invention relates to an oxygenated ionic aqueous fluid or solution
comprising charge-stabilized oxygen-containing nanobubbles predominantly
having an
average diameter of less than 100 nanometers that persist in the solution for
at least days,
wherein the oxygen in the fluid or solution is present in an amount of at
least 15 ppm. Also
claimed is a method for producing such a fluid or solution comprising
providing a flow of a fluid
material between two spaced surfaces in relative motion and defining a mixing
volume
therebetween; and introducing oxygen (02) into the flowing fluid material
within the mixing
volume under conditions suitable to dissolve at least 15 ppm into the
material.
Also claimed is a method of producing an oxygenated ionic aqueous fluid or
solution
comprising charge-stabilized oxygen-containing nanobubbles predominantly
having an
average diameter of less than 100 nanometers that persist in the solution for
at least days, the
method comprising: providing a flow of a fluid material between two spaced
surfaces in relative
motion and defining a mixing volume therebetween, wherein a single pass of the
flowing fluid
material within and through the mixing volume has a dwell time greater than
0.06 seconds;
and introducing oxygen (02) into the flowing fluid material within the mixing
volume under
conditions suitable to dissolve at least 15 ppm oxygen into the material, and
electrokinetically
alter the material.
3c
CA 02881274 2015-08-20
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWING(S)
Figure 1 is a partial cross-section, partial block diagram of a prior art
mixing device.
Figure 2 is block diagram of an exemplary embodiment of a mixing device.
Figure 3 is an illustration of an exemplary system for delivering a first
material to the
mixing device of Figure 2.
Figure 4 is a fragmentary partial cross-sectional view of a top portion of the
mixing
device of Figure 2.
3d
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
Figure 5 is a fragmentary cross-sectional view of a first side portion of the
mixing device of Figure 2.
Figure 6 is a fragmentary cross-sectional view of a second side portion of the
mixing device of Figure 2.
Figure 7 is a fragmentary cross-sectional view of a side portion of the mixing
device of Figure 2 located between the first side portion of Figure 5 and the
second
side portion of Figure 6.
Figure 8 is a perspective view of a rotor and a stator of the mixing device of
Figure 2.
Figure 9 is a perspective view of an inside of a first chamber of the mixing
device of Figure 2.
Figure 10 is a fragmentary cross-sectional view of the inside of a first
chamber
of the mixing device of Figure 2 including an alternate embodiment of the pump
410.
Figure 11 is a perspective view of an inside of a second chamber of the
mixing device of Figure 2.
Figure 12 is a fragmentary cross-sectional view of a side portion of an
alternate embodiment of the mixing device.
Figure 13 is a perspective view of an alternate embodiment of a central
section of the housing for use with an alternate embodiment of the mixing
device.
Figure 14 is a fragmentary cross-sectional view of an alternate embodiment of
a bearing housing for use with an alternate embodiment of the mixing device.
Figure 15 is a cross-sectional view of the mixing chamber of the mixing device
of Figure 2 taken through a plane orthogonal to the axis of rotation depicting
a rotary
flow pattern caused by cavitation bubbles when a through-hole of the rotor
approaches (but is not aligned with) an aperture of the stator.
Figure 16 is a cross-sectional view of the mixing chamber of the mixing device
of Figure 2 taken through a plane orthogonal to the axis of rotation depicting
a rotary
flow pattern caused by cavitation bubbles when the through-hole of the rotor
is
aligned with the aperture of the stator.
Figure 17 is a cross-sectional view of the mixing chamber of the mixing device
of Figure 2 taken through a plane orthogonal to the axis of rotation depicting
a rotary
flow pattern caused by cavitation bubbles when a through-hole of the rotor
that was
previously aligned with the aperture of the stator is no longer aligned
therewith.
4
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
Figure 18 is a side view of an alternate embodiment of a rotor.
Figure 19 is an enlarged fragmentary cross-sectional view taken through a
plane orthogonal to an axis of rotation of the rotor depicting an alternate
configuration of through-holes formed in the rotor and through-holes formed in
the
stator.
Figure 20 is an enlarged fragmentary cross-sectional view taken through a
plane passing through and extending along the axis of rotation of the rotor
depicting
a configuration of through-holes formed in the rotor and through-holes formed
in the
stator.
Figure 21 is an enlarged fragmentary cross-sectional view taken through a
plane passing through and extending along the axis of rotation of the rotor
depicting
an alternate offset configuration of through-holes formed in the rotor and
through-
holes formed in the stator.
Figure 22 is an illustration of a shape that may be used to construct the
through-holes of the rotor and/or the apertures of the stator.
Figure 23 is an illustration of a shape that may be used to construct the
through-holes of the rotor and/or the apertures of the stator.
Figure 24 is an illustration of a shape that may be used to construct the
through-holes of the rotor and/or the apertures of the stator.
Figure 25 is an illustration of a shape that may be used to construct the
through-holes of the rotor and/or the apertures of the stator.
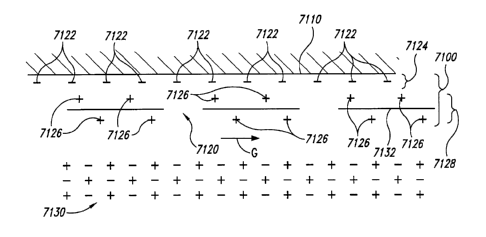
Figure 26 is an illustration of an electrical double layer ("EDL") formed near
a
surface.
Figure 27 is a perspective view of a model of the inside of the mixing
chamber.
Figure 28 is a cross-sectional view of the model of Figure 27.
Figure 29 is an illustration of an experimental setup.
Figure 30 illustrates dissolved oxygen levels in water processed with oxygen
in the mixing device of Figure 2 and stored a 500 ml thin walled plastic
bottle and a
1,000 ml glass bottle each capped at 65 Fahrenheit
Figure 31 illustrates dissolved oxygen levels in water processed with oxygen
in the mixing device of Figure 2 and stored in a 500 ml plastic thin walled
bottle and
a 1,000 ml glass bottle both refrigerated at 39 Fahrenheit
5
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
Figure 32 illustrates the dissolved oxygen levels in GATORADE processed with
oxygen in the mixing device of Figure 2 and stored in 32 oz. GATORADE bottles
having
an average temperature of 55 Fahrenheit.
Figure 33 illustrates the dissolved oxygen retention of a 500 ml braun
balanced salt
solution processed with oxygen in the mixing device of Figure 2.
Figure 34 illustrates a further experiment wherein the mixing device of Figure
2 is
used to sparge oxygen from water by processing the water with nitrogen in the
mixing
device of Figure 2.
Figure 35 illustrates the sparging of oxygen from water by the mixing device
of
Figure 2 at standard temperature and pressure.
Figure 36 is an illustration of a nanocage.
Figure 37 illustrates the Rayleigh scattering effects produced by a sample of
the
water processed with oxygen by the mixing device of Figure 2.
Figures 38-41 illustrate the inventive oxygen-enriched fluid tested positive
for
reactivity with horseradish peroxidase by pyrogallol, while the pressure pot
and fine bubbled
water samples had far less reactivity.
Figure 42 illustrates pyrogallol/HRP assays as described herein, showing that
oxygen is required for the reaction with pyrogallol in the presence of
horseradish
peroxidase, as inventive fluid enriched with other gases (argon and nitrogen )
did not react
in the same manner.
Figure 43 illustrates the hydrogen peroxide positive control showed a strong
reactivity, while none of the other fluids tested reacted with the
glutathione.
Figure 44 illustrates T7 DNA shows a confirmational change at about 50 degrees
Celsius in the control (deionized water), whereas the DNA in the oxygen-
enriched inventive
fluid remains intact until about 60 degrees Celsius.
Figures 45A and 45B illustrate a graphical representation of a exemplary
embodiments of a bioreactor system 3300a.
Figure 46 shows detailed portions of exemplary embodiments of the bioreactor
system 3300a of Figures 45A and 45B.
DETAILED DESCRIPTION
6
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
A mixing device for mixing a first and second material together to create an
output mixture. The device includes a first chamber containing the first
material
coupled to a mixing chamber defined between a rotor and a stator. The rotor is
disposed inside the stator and rotates therein about an axis of rotation. The
first
chamber houses an internal pump configured to pump the first material from the
first
chamber into the mixing chamber. The pump may be configured to impart a
circumferential velocity into the first material before it enters the mixing
chamber. At
least one of the rotor and stator have a plurality of through-holes through
which the
second material is provided to the mixing chamber. Optionally, a second
chamber is
coupled to the mixing chamber. The second chamber may house an internal pump
configured to pump the output material from the mixing chamber into the second
chamber.
Particular aspects provide a composition, comprising an electrokinetically
altered oxygenated aqueous fluid or solution, wherein the oxygen in the fluid
or
solution is present in an amount of at least 25 ppm, at least 30, at least 40,
at least
50, or at least 60 ppm oxygen. In particular embodiments, the
electrokinetically
altered oxygenated aqueous fluid or solution comprises electrokinetically
modified or
charged oxygen species. In certain aspects, the electrokinetically modified or
charged oxygen species are present in an amount of at least 0.5 ppm, at least
1
ppm, at least 3 ppm, at least 5 ppm, at least 7 ppm, at least 10 ppm, at least
15 ppm,
or at least 20 ppm. In particular embodiments, the electrokinetically
altered
oxygenated aqueous fluid or solution comprises solvated electrons stabilized
by
molecular oxygen. In certain aspects, the solvated electrons are present in an
amount of at least 0.01 ppm, at least 0.1 ppm, at least 0.5 ppm, at least 1
ppm, at
least 3 ppm, at least 5 ppm, at least 7 ppm, at least 10 ppm, at least 15 ppm,
or at
least 20 ppm. In particular embodiments, the fluid or solution facilitates
oxidation of
pyrogallol to purpurogallin in the presence of horseradish peroxidase enzyme
(HRP)
in an amount above that afforded by a control pressure pot generated or fine-
bubble
generated aqueous fluid or solution having an equivalent dissolved oxygen
level, and
wherein there is no hydrogen peroxide, or less than 0.1 ppm of hydrogen
peroxide
present in the electrokinetic oxygen-enriched aqueous fluid or solution. In
certain
aspects, the facilitation of oxidation of pyrogallol to purpurogallin persists
for at least
7
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
three hours in an open container, or for at least two months in a closed gas-
tight
container.
Additional aspects provide a composition, comprising an electrokinetically
altered oxygenated aqueous fluid or solution, wherein the fluid or solution
comprises
at least 25 ppm, at least 30, at least 40, at least 50, or at least 60 ppm
oxygen,
wherein the fluid or solution facilitates oxidation of pyrogallol to
purpurogallin in the
presence of horseradish peroxidase enzyme (HRP) in an amount above that
afforded by a control pressure pot generated or fine-bubble generated aqueous
fluid
or solution having an equivalent dissolved oxygen level, and wherein there is
no
hydrogen peroxide, or less than 0.1 ppm of hydrogen peroxide present in the
electrokenetic oxygen-enriched aqueous fluid or solution. In particular
embodiments,
the facilitation of oxidation of pyrogallol to purpurogallin persists for at
least three
hours in an open container, or for at least two months in a closed gas-tight
container.
In certain aspects, the oxygenated aqueous fluid or solution comprises
solvated
electrons stabilized by molecular oxygen. In particular embodiments, the
solvated
electrons are present in an amount of at least 0.01 ppm, at least 0.1 ppm, at
least
0.5 ppm, at least 1 ppm, at least 3 ppm, at least 5 ppm, at least 7 ppm, at
least 10
ppm, at least 15 ppm, or at least 20 ppm.
Further aspects provide a method of producing an electrokinetically altered
oxygenated aqueous fluid or solution, comprising: providing a flow of a fluid
material
between two spaced surfaces in relative motion and defining a mixing volume
therebetween, wherein the dwell time of a single pass of the flowing fluid
material
within and through the mixing volume is greater than 0.06 seconds or greater
than
0.1 seconds; and introducing oxygen (02) into the flowing fluid material
within the
mixing volume under conditions suitable to dissolve at least 20 ppm, at least
25 ppm,
at least 30, at least 40, at least 50, or at least 60 ppm oxygen into the
material, and
electrokinetically alter the fluid or solution. In certain aspects, the oxygen
is infused
into the material in less than 100 milliseconds, less than 200 milliseconds,
less than
300 milliseconds, or less than 400 milliseconds. In particular embodiments,
the ratio
of surface area to the volume is at least 12, at least 20, at least 30, at
least 40, or at
least 50.
Yet further aspects, provide a method of producing an electrokinetically
altered oxygenated aqueous fluid or solution, comprising: providing a flow of
a fluid
8
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
=
WO 2008/052143 PCT/US2007/082578
material between two spaced surfaces defining a mixing volume therebetween;
and
introducing oxygen into the flowing material within the mixing volume under
conditions suitable to infuse at least 20 ppm, at least 25 ppm, at least 30,
at least 40,
at least 50, or at least 60 ppm oxygen into the material in less than 100
milliseconds,
less than 200 milliseconds, less than 300 milliseconds, or less than 400
milliseconds.
In certain aspects, the dwell time of the flowing material within the mixing
volume is
greater than 0.06 seconds or greater than 0.1 seconds. In particular
embodiments,
the ratio of surface area to the volume is at least 12, at least 20, at least
30, at least
40, or at least 50.
Additional embodiments provide a method of producing an electrokinetically
altered oxygenated aqueous fluid or solution, comprising use of a mixing
device for
creating an output mixture by mixing a first material and a second material,
the
device comprising: a first chamber configured to receive the first material
from a
source of the first material; a stator; a rotor having an axis of rotation,
the rotor being
disposed inside the stator and configured to rotate about the axis of rotation
therein,
at least one of the rotor and stator having a plurality of through-holes; a
mixing
chamber defined between the rotor and the stator, the mixing chamber being in
fluid
communication with the first chamber and configured to receive the first
material
therefrom, and the second material being provided to the mixing chamber via
the
plurality of through-holes formed in the one of the rotor and stator; a second
chamber in fluid communication with the mixing chamber and configured to
receive
the output material therefrom; and a first internal pump housed inside the
first
chamber, the first internal pump being configured to pump the first material
from the
first chamber into the mixing chamber. In certain aspects, the first internal
pump is
configured to impart a circumferential velocity into the first material before
it enters
the mixing chamber.
Further embodiments provide a method of producing an electrokinetically
altered oxygenated aqueous fluid or solution, comprising use of a mixing
device for
creating an output mixture by mixing a first material and a second material,
the
device comprising: a stator; a rotor having an axis of rotation, the rotor
being
disposed inside the stator and configured to rotate about the axis of rotation
therein;
a mixing chamber defined between the rotor and the stator, the mixing chamber
having an open first end through which the first material enters the mixing
chamber
9
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
and an open second end through which the output material exits the mixing
chamber, the second material entering the mixing chamber through at least one
of
the rotor and the stator; a first chamber in communication with at least a
majority
portion of the open first end of the mixing chamber; and a second chamber in
communication with the open second end of the mixing chamber.
Additional aspects provide an electrokinetically altered oxygenated aqueous
fluid or solution made according to any of the above methods.
DETAILED DESCRIPTION OF THE INVENTION
OVERVIEW
Figure 2 provides a block diagram illustrating some of the components of a
mixing device 100 and the flow of material into, within, and out of the
device. The
mixing device 100 combines two or more input materials to form an output
material 102, which may be received therefrom into a storage vessel 104. The
mixing device 100 agitates the two or more input materials in a novel manner
to
produce an output material 102 having novel characteristics. The
output
material 102 may include not only a suspension of at least one of the input
materials
in at least one of the other input materials (e.g., emulsions) but also a
novel
combination (e.g., electrostatic combinations) of the input materials, a
chemical
compound resulting from chemical reactions between the input materials,
combinations having novel electrostatic characteristics, and combinations
thereof.
The input materials may include a first material 110 provided by a source 112
of the first material, a second material 120 provided by a source 122 of the
second
material, and optionally a third material 130 provided by a source 132 of the
third
material. The first material 110 may include a liquid, such as water, saline
solution,
chemical suspensions, polar liquids, non-polar liquids, colloidal suspensions,
cell
growing media, and the like. In some embodiments, the first material 110 may
include the output material 102 cycled back into the mixing device 100. The
second
material 120 may consist of or include a gas, such as oxygen, nitrogen, carbon
dioxide, carbon monoxide, ozone, sulfur gas, nitrous oxide, nitric oxide,
argon,
helium, bromine, and combinations thereof, and the like. In preferred
embodiments,
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
,
WO 2008/052143
PCT/US2007/082578
the gas is or comprises oxygen. The optional third material 130 may include
either a
liquid or a gas. In some embodiments, the third material 130 may be or include
the
output material 102 cycled back into the mixing device 100 (e.g., to one or
more of
the pumps 210, 220 or 230, and/or into the chamber 310, and/or 330).
Optionally, the first material 110, the second material 120, and the optional
third material 130 may be pumped into the mixing device 100 by an external
pump 210, an external pump 220, and an external pump 230, respectively.
Alternatively, one or more of the first material 110, the second material 120,
and the
optional third material 130 may be stored under pressure in the source 112,
the
source 122, and the source 132, respectively, and may be forced into the
mixing
device 100 by the pressure. The invention is not limited by the method used to
transfer the first material 110, the second material 120, and optionally, the
third
material 130 into the mixing device 100 from the source 112, the source 122,
and the
source 132, respectively.
The mixing device 100 includes a first chamber 310 and a second
chamber 320 flanking a mixing chamber 330. The three chambers 310, 320,
and 330 are interconnected and form a continuous volume.
The first material 110 is transferred into the first chamber 310 and flows
therefrom into the mixing chamber 330.
The first material 110 in the first
chamber 310 may be pumped into the first chamber 310 by an internal pump 410.
The second material 120 is transferred into the mixing chamber 330.
Optionally, the
third material 130 may be transferred into the mixing chamber 330. The
materials in
the mixing chamber 330 are mixed therein to form the output material 102.
Then,
the output material 102 flows into the second chamber 320 from which the
output
material 102 exits the mixing device 100. The output material 102 in the
mixing
chamber 330 may be pumped into the second chamber 320 by an internal
pump 420. Optionally, the output material 102 in the second chamber 320 may be
pumped therefrom into the storage vessel 104 by an external pump 430 (e.g.,
alone
or in combination with the internal pump 410 and/or 420).
In particular aspects, a common drive shaft 500 powers both the internal
pump 410 and the internal pump 420. The drive shaft 500 passes through the
mixing chamber 330 and provides rotational force therein that is used to mix
the first
11
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
=
,
WO 2008/052143
PCT/US2007/082578
material 110, the second material 120, and optionally, the third material 130
together. The drive shaft 500 is powered by a motor 510 coupled thereto.
Figure 3 provides a system 512 for supplying the first material 110 to the
mixing device 100 and removing the output material 102 from the mixing device
100.
In the system 512, the storage vessel 104 of the output material 102 and the
source 112 of the first material 110 are combined. The external pump 210 is
coupled to the combined storage vessel 104 and source 112 by a fluid conduit
514
such as hose, pipe, and the like. The external pump 210 pumps the combined
first
material 110 and output material 102 from the combined storage vessel 104 and
source 112 through the fluid conduit 514 and into a fluid conduit 516
connecting the
external pump 210 to the mixing device 100. The output material 102 exits the
mixing device 100 through a fluid conduit 518. The fluid conduit 518 is
coupled to
the combined storage vessel 104 and source 112 and transports the output
material 102 exiting the mixing device 100 to the combined storage vessel 104
and
source 112. The fluid conduit 518 includes a valve 519 that establishes an
operating
pressure or back pressure within the mixing device 100.
Referring to Figures 2, 4-10, and 11, a more detailed description of various
components of an embodiment of the mixing device 100 will be provided. The
mixing device 100 is scalable. Therefore, dimensions provided with respect to
various components may be used to construct an embodiment of the device or may
be scaled to construct a mixing device of a selected size.
Turning to Figure 4, the mixing device 100 includes a housing 520 that
houses each of the first chamber 310, the mixing chamber 330, and the second
chamber 320. As mentioned above, the mixing device 100 includes the drive
shaft 500, which rotates during operation of the device. Therefore, the mixing
device 100 may vibrate or otherwise move. Optionally, the mixing device 100
may
be coupled to a base 106, which may be affixed to a surface such as the floor
to
maintain the mixing device 100 in a substantially stationary position.
The housing 520 may be assembled from two or more housing sections. By
way of example, the housing 520 may include a central section 522 flanked by a
first
mechanical seal housing 524 and a second mechanical seal housing 526. A
bearing
housing 530 may be coupled to the first mechanical seal housing 524 opposite
the
central section 522. A bearing housing 532 may be coupled to the second
12
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/1JS2007/082578
mechanical seal housing 526 opposite the central section 522. Optionally, a
housing
section 550 may be coupled to the bearing housings 530.
Each of the bearing housings 530 and 532 may house a bearing
assembly 540 (see Figures 5 and 6). The bearing assembly 540 may include any
suitable bearing assembly known in the art including a model number "202SZZST'
manufactured by SKF USA Inc, of Kulpsville, Pennsylvania, operating a website
at
www.skf.com.
Seals may be provided between adjacent housing sections. For example, o-
ring 560 (see Figure 5) may be disposed between the housing section 550 and
the
bearing housing 530, o-ring 562 (see Figure 5) may be disposed between the
first
mechanical seal housing 524 and the central section 522, and o-ring 564 (see
Figure 6) may be disposed between the second mechanical seal housing 526 and
the central section 522.
MIXING CHAMBER 330
Turning now to Figure 7, the mixing chamber 330 is disposed inside the
central section 522 of the housing 520 between the first mechanical seal
housing 524 and the second mechanical seal housing 526. The mixing chamber 330
is formed between two components of the mixing device 100, a rotor 600 and a
stator 700. The rotor 600 may have a sidewall 604 with an inside surface 605
defining a generally hollow inside portion 610 and an outside surface 606. The
sidewall 604 may be about 0.20 inches to about 0.75 inches thick. In some
embodiments, the sidewall 604 is about 0.25 inches thick. However, because the
mixing device 100 may be scaled to suit a particular application, embodiments
of the
device having a sidewall 604 that is thicker or thinner than the values
provided are
within the scope of the present teachings. The sidewall 604 includes a first
end
portion 612 and a second end portion 614 and a plurality of through-holes 608
formed between the first end portion 612 and the second end portion 614.
Optionally, the outside surface 606 of the sidewall 604 may include other
features
such as apertures, projections, textures, and the like. The first end portion
612 has a
relieved portion 616 configured to receive a collar 618 and the second end
portion 614 has a relieved portion 620 configured to receive a collar 622.
13
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
The rotor 600 is disposed inside the stator 700. The stator 700 has a
sidewall 704 with an inside surface 705 defining a generally hollow inside
portion 710
into which the rotor 600 is disposed. The sidewall 704 may be about 0.1 inches
to
about 0.3 inches thick. In some embodiments, the sidewall 604 is about 1.5
inches
thick. The stator 700 may be non-rotatably coupled to the housing 520 in a
substantially stationary position. Alternatively, the stator 700 may
integrally formed
with the housing 520. The sidewall 704 has a first end portion 712 and a
second end
portion 714. Optionally, a plurality of apertures 708 are formed in the
sidewall 704 of
the stator 700 between the first end portion 712 and the second end portion
714.
Optionally, the inside surface 705 of the sidewall 704 may include other
features
such as through-holes, projections, textures, and the like.
The rotor 600 rotates with respect to the stationary stator 700 about an axis
of
rotation "a" in a direction indicated by arrow "C3" in Figure 9. Each of the
rotor 600
and the stator 700 may be generally cylindrical in shape and have a
longitudinal axis.
The rotor 600 has an outer diameter "D1" and the stator 700 may have an inner
diameter "D2." The diameter "Dl" may range, for example, from about 0.5 inches
to
about 24 inches. In some embodiments, the diameter "Dl" is about 3.04 inches.
In
some embodiments, the diameter "D1" is about 1.7 inches. The diameter "D2,"
which is larger than the diameter "Dl," may range from about 0.56 inches to
about
24.25 inches. In some embodiments, the diameter "D2" is about 4 inches.
Therefore, the mixing chamber 330 may have a ring-shaped cross-sectional shape
that is about 0.02 inches to about 0.125 inches thick (i.e., the difference
between the
diameter "D2" and the diameter "D1"). In
particular embodiments, the mixing
chamber 330 is about 0.025 inches thick. The channel 32 between the rotor 12
and
the stator 34 of prior art device 10 (see Figure 1) has a ring-shaped cross-
sectional
shape that is about 0.09 inches thick. Therefore, in particular embodiments,
the
thickness of the mixing chamber 330 is less than about one third of the
channel 32 of
the prior art device 10.
The longitudinal axis of the rotor 600 may be aligned with its axis of
rotation "a." The longitudinal axis of the rotor 600 may be aligned with the
longitudinal axis of the stator 700. The rotor 600 may have a length of about
3
inches to about 6 inches along the axis of rotation "a." In some embodiments,
the
rotor 600 may have a length of about 5 inches along the axis of rotation "a."
The
14
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
stator 700 may have a length of about 3 inches to about 6 inches along the
axis of
rotation "a." In some embodiments, the stator 700 may have a length of about 5
inches along the axis of rotation "a."
While the rotor 600 and the stator 700 have been depicted as having a
generally cylindrical shape, those of ordinary skill in the art appreciate
that alternate
shapes may be used. For example, the rotor 600 and the stator 700 may be
conically, spherically, arbitrarily shaped, and the like. Further, the rotor
600 and the
stator 700 need not be identically shaped. For example, the rotor 600 may be
cylindrically shaped and the stator 700 rectangular shaped or vise versa.
The apertures 708 of the stator 700 and the through-holes 608 depicted in
Figures 4-7 are generally cylindrically shaped. The diameter of the through-
holes 608 may range from about 0.1 inches to about 0.625 inches. The diameter
of
the apertures 708 may range from about 0.1 inches to about 0.625 inches. One
or
more of apertures 708 of the stator 700 may have a diameter that differs from
the
diameters of the other apertures 708. For example, the apertures 708 may
increase
in diameter from the first end portion 712 of the stator 700 to the second end
portion 714 of the stator 700, the apertures 708 may decrease in diameter from
the
first end portion 712 of the stator 700 to the second end portion 714 of the
stator 700, or the diameters of the apertures 708 may vary in another manner
along
the stator 700. One or more of through-holes 608 of the rotor 600 may have a
diameter that differs from the diameters of the other through-holes 608. For
example, the through-holes 608 may increase in diameter from the first end
portion 612 of the rotor 600 to the second end portion 614 of the rotor 600,
the
through-holes 608 may decrease in diameter from the first end portion 612 of
the
rotor 600 to the second end portion 614 of the rotor 600, or the diameters of
the
through-holes 608 may vary in another manner along the rotor 600.
As described below with reference to alternate embodiments, the
apertures 708 and the through-holes 608 may have shapes other than generally
cylindrical and such embodiments are within the scope of the present
invention. For
example, the through-holes 608 may include a narrower portion, an arcuate
portion,
a tapered portion, and the like. Referring to Figures 7, each of the through-
holes 608
includes an outer portion 608A, a narrow portion 608B, and a tapered portion
608C
providing a transition between the outer portion 608A and the narrow portion
608B.
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
Similarly, the apertures 708 may include a narrower portion, an arcuate
portion, a
tapered portion, and the like.
Figure 8 provides a non-limiting example of a suitable arrangement of the
apertures 708 of the stator 700 and the through-holes 608 of the rotor 600.
The
apertures 708 of the stator 700 may be arranged in substantially parallel
lateral
rows "SLAT-1" through "SLAT-6" substantially orthogonal to the axis of
rotation "a."
The apertures 708 of the stator 700 may also be arranged in substantially
parallel
longitudinal rows "SLONG-1" through "SLONG-7" substantially parallel with the
axis
of rotation "a." In other words, the apertures 708 of the stator 700 may be
arranged
in a grid-like pattern of orthogonal rows (i.e., the lateral rows are
orthogonal to the
longitudinal rows) having the longitudinal rows "SLONG-1" through "SLONG-7"
substantially parallel with the axis of rotation "a."
Like the apertures 708 of the stator 700, the through-holes 608 of the
rotor 600 may be arranged in substantially parallel lateral rows "RLAT-1"
through
"RLAT-6" substantially orthogonal to the axis of rotation "a." However,
instead of
being arranged in a grid-like pattern of orthogonal rows, the through-holes
608 of the
rotor 600 may also be arranged in substantially parallel rows "RLONG-1"
through
"RLONG-7" that extend longitudinally along a helically path. Alternatively,
the
through-holes 608 of the rotor 600 may also be arranged in substantially
parallel
rows "RLONG-1" through "RLONG-7" that extend longitudinally at an angle other
than parallel with the axis of rotation "a."
The apertures 708 of the stator 700 and the through-holes 608 of the
rotor 600 may be configured so that when the rotor 600 is disposed inside the
stator
700 the lateral rows "SLAT-1" to "SLAT-6" at least partially align with the
lateral
rows "RLAT-1" to "RLAT-6," respectively. In this manner, as the rotor 600
rotates
inside the stator 700, the through-holes 608 pass by the apertures 708.
The through-holes 608 in each of the lateral rows "RLAT-1" to "RLAT-6" may
be spaced apart laterally such that all of the through-holes 608 in the
lateral row
align, at least partially, with the apertures 708 in a corresponding one of
the lateral
rows "SLAT-1" to "SLAT-6" of the stator 700 at the same time. The
longitudinally
extending rows "RLONG-1" through "RLONG-6" may be configured such that the
through-holes 608 in the first lateral row "RLAT-1" in each of the
longitudinally
extending rows passes completely by the apertures 708 of the corresponding
lateral
16
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
row "SLAT-1" before the through-holes 608 in the last lateral row "RLAT-6"
begin to
partially align with the apertures 708 of the corresponding last lateral row
"SLAT-6" of
the stator 700.
While, in Figure 8, six lateral rows and six longitudinally extending rows
have
been illustrated with respect to the rotor 600 and six lateral rows and seven
longitudinally extending rows have been illustrated with respect stator 700,
it is
apparent to those of ordinary skill in the art that alternate numbers of
lateral rows
and/or longitudinal rows may be used with respect to the rotor 600 and/or
stator 700
without departing from the present teachings.
To ensure that only one pair of openings between corresponding lateral rows
will be coincident at any one time, the number of apertures 708 in each of the
lateral
rows "SLAT-1" to "SLAT-6" on the stator 700 may differ by a predetermined
number
(e.g., one, two, and the like) the number of through-holes 608 in each of the
corresponding lateral rows "RLAT-1" to "RLAT-6" on the rotor 600. Thus, for
example, if lateral row "RLAT-1" has twenty through-holes 608 evenly spaced
around
the circumference of rotor 600, the lateral row "SLAT-1" may have twenty
apertures 708 evenly spaced around the circumference of stator 700.
Returning to Figure 7, the mixing chamber 330 has an open first end
portion 332 and an open second end portion 334. The through-holes 608 formed
in
the sidewall 604 of the rotor 600 connect the inside portion 610 of the rotor
600 with
the mixing chamber 330.
The rotor 600 is rotated inside the stator 700 by the drive shaft 500 aligned
with the axis of rotation "a" of the rotor 600. The drive shaft 500 may be
coupled to
the first end portion 612 and the second end portion 614 of the rotor 600 and
extend
through its hollow inside portion 610. In other words, a portion 720 of the
drive
shaft 500 is disposed in the hollow inside portion 610 of the rotor 600.
The collar 618 is configured to receive a portion 721 of the drive shaft 500
disposed in the hollow inside portion 610 and the collar 622 is configured to
receive
a portion 722 of the drive shaft 500 disposed in the hollow inside portion
610.
The portion 721 has an outer diameter "D3" that may range from about 0.5
inches to about 2.5 inches. In some embodiments, the diameter "D3" is about
0.625
inches. The portion 722 has an outer diameter "D4" that may be substantially
similar
17
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
to the diameter "03," although, this is not required. The diameter "D4" may
range
from about 0.375 inches to about 2.5 inches.
The rotor 600 may be non-rotationally affixed to the portion 721 and the
portion 722 of the drive shaft 500 by the collar 618 and the collar 622,
respectively.
By way of example, each of the collars 618 and 622 may be installed inside
relieved
portions 616 and 620, respectively. Then, the combined rotor 600 and collars
618
and 622 may be heated to expand them. Next, the drive shaft 500 is inserted
through the collars 618 and 622 and the assembly is allowed to cool. As the
collars 618 and 622 shrink during cooling, they tighten around the portions
722A
and 722B of the drive shaft 500, respectively, gripping it sufficiently
tightly to prevent
the drive shaft 500 from rotating relative to the rotor 600. The collar 618,
which does
not rotate with respect to either the portion 721 or the relieved portion 616,
translates
the rotation of the drive shaft 500 to the first end portion 612 the rotor
600. The
collar 622, which does not rotate with respect to either the portion 722 or
the relieved
portion 620, translates the rotation of the drive shaft 500 to the second end
portion 614 of the rotor 600. The drive shaft 500 and the rotor 600 rotate
together as
a single unit.
The drive shaft 500 may have a first end portion 724 (see Figure 5) and a
second end portion 726 (see Figure 6). The first end portion 724 may have a
diameter "D5" of about 0.5 inches to about 1.75 inches. In particular
embodiments,
the diameter "D5" may be about 1.25 inches. The second end portion 726 may
have
a diameter "06" that may be substantially similar to diameter "05."
The second material 120 may be transported into the mixing chamber 330
through one of the first end portion 724 and the second end portion 726 of the
rotating drive shaft 500. The other of the first end portion 724 and the
second end
portion 726 of the drive shaft 500 may be coupled to the motor 510. In the
embodiment depicted in Figures 5 and 6, the second material 120 is transported
into
the mixing chamber 330 through the first end portion 724 and the second end
portion 726 of the drive shaft 500 is coupled to the motor 510.
Turning to Figure 5, the drive shaft 500 may have a channel 728 formed
therein that extends from first end portion 724 into the portion 720 disposed
in the
inside portion 610 of the rotor 600. The channel 728 has an opening 730 formed
in
18
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
the first end portion 724. When the mixing device 100 is operating, the second
material 120 is introduced into the channel 728 through the opening 730.
A valve 732 may be disposed inside a portion of the channel 728 located in
the first end portion 724 of the drive shaft 500. The valve 732 may restrict
or
otherwise control the backward flow of the second material 120 from inside the
hollow inside portion 610 through the channel 728 and/or the forward flow of
the
second material 120 into the channel 728. The valve 732 may include any valve
known in the art including a check valve. A suitable check valve includes a
part
number "CKFA1876205A," free flow forward check valve, manufactured by The Lee
Company USA having an office in Bothell, WA and operating a website at
www.theleeco.com.
The drive shaft 500 may include an aperture 740 located in the inside
portion 610 of the rotor 600 that connects the channel 728 with the inside
portion 610
of the rotor 600. While only a single aperture 740 is illustrated in Figure 5,
it is
apparent to those of ordinary skill in the art that multiple apertures may be
used to
connect the channel 728 with the inside portion 610 of the rotor 600.
Referring to Figure 2, optionally, the external pump 220 may pump the second
material 120 into the mixing device 100. The pump 220 may include any suitable
pump known in the art. By way of non-limiting example, the pump 220 may
include
any suitable pump known in the art including a diaphragm pump, a chemical
pump, a
peristaltic pump, a gravity fed pump, a piston pump, a gear pump, a
combination of
any of the aforementioned pumps, and the like. If the second material 120 is a
gas,
the gas may be pressurized and forced into the opening 730 formed in the first
end
portion 724 of the drive shaft 500 by releasing the gas from the source 122.
The pump 220 or the source 122 is coupled to the channel 728 by the
valve 732. The second material 120 transported inside the channel 728 exits
the
channel 728 into the inside portion 610 of the rotor 600 through the aperture
740.
The second material 120 subsequently exits the inside portion 610 of the rotor
600
through the through-holes 608 formed in the sidewall 608 of the rotor 600.
Referring to Figure 5, the mixing device 100 may include a seal assembly 750
coupled to the first end portion 724 of the drive shaft 500. The seal assembly
750 is
maintained within a chamber 752 defined in the housing 520. The chamber 752
has
a first end portion 754 spaced across the chamber from a second end portion
756.
19
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
The chamber 752 also includes an input port 758 and an output port 759 that
provide
access into the chamber 752. The chamber 752 may be defined by housing
section 550 and the bearing housing 530. The first end portion 754 may be
formed
in the housing section 550 and the second end portion 756 may be adjacent to
the
bearing housing 530. The input port 758 may be formed in the bearing housing
530
and the output port 759 may be formed in the housing section 550.
The seal assembly 750 includes a first stationary seal 760 installed in the
first
end portion 754 of the chamber 752 in the housing section 550 and the bearing
housing 530. The first stationary seal 760 extends around a portion 762 of the
first
end portion 724 of the drive shaft 500. The seal assembly 750 also includes a
second stationary seal 766 installed in the second end portion 756 of the
chamber 752 in the bearing housing 530. The second stationary seal 766 extends
around a portion 768 of the first end portion 724 of the drive shaft 500.
The seal assembly 750 includes a rotating assembly 770 that is non-rotatably
coupled to the first end portion 724 of the drive shaft 500 between the
portion 762
and the portion 768. The rotating assembly 770 rotates therewith as a unit.
The
rotating assembly 770 includes a first seal 772 opposite a second seal 774. A
biasing member 776 (e.g., a spring) is located between the first seal 772 and
the
second seal 774. The biasing member 776 biases the first seal 772 against the
first
stationary seal 760 and biases the second seal 774 against the second
stationary
seal 766.
A cooling lubricant is supplied to the chamber 752 and around rotating
assembly 770. The lubricant enters the chamber 752 through the input port 758
and
exits the chamber 752 through output port 759. The lubricant may lubricate the
bearing assembly 540 housed by the bearing housing 530. A chamber 570 may be
disposed between the bearing housing 530 and the mechanical seal housing 524.
The bearing housing 530 may also include a second input port 759 connected to
the
chamber 570 into which lubricant may be pumped. Lubricant pumped into the
chamber 570 may lubricate the bearing assembly 540. The seal assembly 750 may
significantly, if not greatly, reduce frictional forces within this portion of
the device
caused by the rotation of the rotor 600 and may increase the active life of
the seals
770. The seals may include surfaces constructed using silicon carbide.
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
Referring to Figure 9, as the rotor 600 rotates about the axis of rotation "a"
in
the direction indicated by arrow "Cl," the rotor expels the second material
120 into
the mixing chamber 330. The expelled bubbles, droplets, particles, and the
like of
the second material 120 exit the rotor 600 and are imparted with a
circumferential
velocity (in a direction indicated by arrow "C3") by the rotor 600. The second
material 120 may forced from the mixing chamber 330 by the pump 220 (see
Figure 2), the centrifugal force of the rotating rotor 600, buoyancy of the
second
material 120 relative to the first material 110, and a combination thereof.
21
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143
PCT/US2007/082578
MOTOR 510
Returning to Figure 6, the second end portion 726 of the drive shaft 500 may
be coupled to a rotating spindle 780 of a motor 510 by a coupler 900. The
spindle 780 may have a generally circular cross-sectional shape with a
diameter "D7" of about 0.25 inches to about 2.5 inches. In particular
embodiments,
the diameter "D7" may be about 0.25 inches to about 1.5 inches. While in the
embodiment depicted in Figure 6, the diameter "D5" of the first end portion
724 of the
drive shaft 500 is substantially equal to the diameter D7" and the spindle
780,
embodiments in which one of the diameter "D5" and the diameter "D7" is larger
than
the other are within the scope of the present invention.
Referring also to Figure 4, it may be desirable to cover or shield the
coupler 900. In the embodiment illustrated in Figures 4 and 6, a drive guard
910
covers the coupler 900. The drive guard 910 may be generally U-shaped having a
curved portion 914 flanked by a pair of substantially linear portions 915 and
916.
The distal end of each of the substantially linear portions 915 and 916 of the
drive
guard 910 may have a flange 918 and 919, respectively. The drive guard 910 may
be fastened by each of its flanges 918 and 919 to the base 106.
The motor 510 may be supported on the base 106 by a support member 920.
The support member 920 may be coupled to the motor 510 near the spindle 780.
In
the embodiment depicted, the support member 920 includes a through-hole
through
which the spindle 780 passes. The support member 920 may be coupled to the
motor 510 using any method known in the art, including bolting the support
member 920 to the motor 510 with one or more bolts 940.
The coupler 900 may include any coupler suitable for transmitting a sufficient
amount of torque from the spindle 780 to the drive shaft 500 to rotate the
rotor 600
inside to the stator 700. In the embodiment illustrated in Figures 4 and 6,
the
coupler 900 is a bellows coupler. A bellows coupler may be beneficial if the
spindle 780 and the drive shaft 500 are misaligned. Further, the bellows
coupler
may help absorb axial forces exerted on the drive shaft 500 that would
otherwise be
translated to the spindle 780. A suitable bellows coupler includes a model
"BC32-8-
8-A," manufactured by Ruland Manufacturing Company, Inc. of Marlborough, MA,
which operates a website at www.ruland.com.
22
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/1JS2007/082578
The motor 510 may rotate the rotor 600 at about 0.1 revolutions per minute
("rpm") to about 7200 rpm. The motor 510 may include any motor suitable for
rotating the rotor 600 inside to the stator 700 in accordance with the present
teachings. By way of non-limiting example, a suitable motor may include a one-
half
horsepower electric motor, operating at 230/460 volts and 3450 per minute
("rpm").
A suitable motor includes a model "C4T34NC4C" manufactured by LEESON Electric
Corporation of Grafton, WI, which operates a website at www.leeson.com.
FIRST CHAMBER 310
Turning to Figures 4 and 7, the first chamber 320 is disposed inside the
central section 522 of the housing 520 between the first mechanical seal
housing 524 and the first end portions 612 and 712 of the rotor 600 and the
stator 700, respectively. The first chamber 310 may be annular and have a
substantially circular cross-sectional shape. The first chamber 310 and the
mixing
chamber 330 form a continuous volume. A portion 1020 of the drive shaft 500
extends through the first chamber 310.
As may best be viewed in Figure 4, the first chamber 310 has an input
port 1010 through which the first material 110 enters the mixing device 100.
The first
material 110 may be pumped inside the first chamber 310 by the external pump
210
(see Figure 2). The external pump 210 may include any pump known in the art
for
pumping the first material 110 at a sufficient rate to supply the first
chamber 310.
The input port 1010 is oriented substantially orthogonally to the axis of
rotation "a." Therefore, the first material 110 enters the first chamber 310
with a
velocity tangential to the portion 1020 of the drive shaft 500 extending
through the
first chamber 310. The tangential direction of the flow of the first material
110
entering the first chamber 310 is identified by arrow "T1." In the embodiment
depicted in Figures 4 and 7, the input port 1010 may be offset from the axis
of
rotation "a." As is apparent to those of ordinary skill in the art, the
direction of the
rotation of the drive shaft 500 (identified by arrow "Cl" in Figure 9), has a
tangential
component. The input port 1010 is positioned so that the first material 110
enters
the first chamber 310 traveling in substantially the same direction as the
tangential
component of the direction of rotation of the drive shaft 500.
23
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
The first material 110 enters the first chamber 310 and is deflected by the
inside of the first chamber 310 about the portion 1020 of the drive shaft 500.
In
embodiments wherein the first chamber 310 has a substantially circular cross-
sectional shape, the inside of the first chamber 310 may deflect the first
material 110
in a substantially circular path (identified by arrow "C2" in Figure 9) about
the
portion 1020 of the drive shaft 500. In such an embodiment, the tangential
velocity
of the first material 110 may cause it to travel about the axis of rotation
"a" at a
circumferential velocity, determined at least in part by the tangential
velocity.
Once inside the first chamber 310, the first material 110 may be pumped from
the first chamber 310 into the mixing chamber 330 by the pump 410 residing
inside
the first chamber 310. In embodiments that include the external pump 210 (see
Figure 2), the external pump 210 may be configured to pump the first material
110
into the first chamber 310 at a rate at least as high as a rate at which the
pump 410
pumps the first material 110 from the first chamber 310.
The first chamber 310 is in communication with the open first end portion 332
of the mixing chamber 330 and the first material 110 inside the first chamber
310
may flow freely into the open first end portion 332 of the mixing chamber 330.
In this
manner, the first material 110 does not negotiate any corners or bends between
the
mixing chamber 330 and the first chamber 310. In the embodiment depicted, the
first chamber 310 is in communication with the entire open first end portion
332 of
the mixing chamber 330. The first chamber 310 may be filled completely with
the
first material 110.
The pump 410 is powered by the portion 1020 of the drive shaft 500
extending through the first chamber 310. The pump 410 may include any pump
known in the art having a rotating pump member 2022 housed inside a chamber
(i.e., the first chamber 310) defined by a stationary housing (i.e., the
housing 520).
Non-limiting examples of suitable pumps include rotary positive displacement
pumps
such as progressive cavity pumps, single screw pumps (e.g., Archimedes screw
pump), and the like.
The pump 410 depicted in Figures 7 and 9, is generally referred to as a single
screw pump. In this embodiment, the pump member 2022 includes a collar
portion 2030 disposed around the portion 1020 of the drive shaft 500. The
collar
portion 2030 rotates with the portion 1020 of the drive shaft 500 as a unit.
The collar
24
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
portion 2030 includes one or more fluid displacement members 2040. In the
embodiment depicted in Figures 7 and 9, the collar portion 2030 includes a
single
fluid displacement member 2040 having a helical shape that circumscribes the
collar
portion 2030 along a helical path.
Referring to Figure 9, the inside of the first chamber 310 is illustrated. The
pump 410 imparts an axial flow (identified by arrow "Al" and arrow "A2") in
the first
material 110 inside the first chamber 310 toward the open first end portion
332 of the
mixing chamber 330. The axial flow of the first material 110 imparted by the
pump 410 has a pressure that may exceed the pressure obtainable by the
external
pump of the prior art device 10 (see Figure 1).
The pump 410 may also be configured to impart a circumferential flow
(identified by arrow "C2") in the first material 110 as it travels toward the
open first
end portion 332 of the mixing chamber 330. The circumferential flow imparted
in the
first material 110 before it enters the mixing chamber 330 causes the first
material 110 to enter the mixing chamber 330 already traveling in the desired
direction at an initial circumferential velocity. In the prior art device 10
depicted in
Figure 1, the first material 110 entered the channel 32 of the prior art
device 10
without a circumferential velocity. Therefore, the rotor 12 of the prior art
device 10
alone had to impart a circumferential flow into the first material 110.
Because the
first material 110 is moving axially, in the prior art device 10, the first
material 110
traversed at least a portion of the channel 32 formed between the rotor 12 and
the
stator 30 at a slower circumferential velocity than the first material 110
traverses the
mixing chamber 330 of the mixing device 100. In other words, if the axial
velocity of
the first material 110 is the same in both the prior art device 10 and the
mixing
device 100, the first material 110 may complete more revolutions around the
rotational axis "a" before traversing the axial length of the mixing chamber
330, than
it would complete before traversing the axial length of the channel 32. The
additional
revolutions expose the first material 110 (and combined first material 110 and
second material 120) to a substantially larger portion of the effective inside
surface 706 (see Figure 7) of the stator 700.
In embodiments including the external pump 210 (see Figure 2), the
circumferential velocity imparted by the external pump 210 combined with the
input
port 1010 being oriented according to the present teachings, may alone
sufficiently
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
increase the revolutions of the first material 110 (and combined first
material 110 and
second material 120) about the rotational axis "a." Further, in some
embodiments,
the circumferential velocity imparted by the pump 210 and the circumferential
velocity imparted by the pump 410 combine to achieve a sufficient number of
revolutions of the first material 110 (and combined first material 110 and
second
material 120) about the rotational axis "a." As is appreciated by those of
ordinary
skill in the art, other structural elements such as the cross-sectional shape
of the first
chamber 310 may contribute to the circumferential velocity imparted by the
pump 210, the pump 410, and a combination thereof.
In an alternate embodiment depicted in Figure 10, the pump 410 may include
one or more vanes 2042 configured to impart a circumferential flow in the
first
material 110 as it travels toward the open first end portion 332 of the mixing
chamber 330.
SECOND CHAMBER 320
Turning now to Figures 4 and 7, the second chamber 320 is disposed inside
the central section 522 of the housing 520 between the second mechanical seal
housing 526 and the second end portions 614 and 714 of the rotor 600 and the
stator 700, respectively. The second chamber 320 may be substantially similar
to
the first chamber 310. However, instead of the input port 1010, the second
chamber 320 may include an output port 3010. A portion 3020 of the drive shaft
500
extends through the second chamber 320.
The second chamber 320 and the mixing chamber 330 form a continuous
volume. Further, the first chamber 310, the mixing chamber 330, and the second
chamber 320 form a continuous volume. The first material 110 flows through the
mixing device 100 from the first chamber 310 to the mixing chamber 330 and
finally
to the second chamber 320. While in the mixing chamber 330, the first material
110
is mixed with the second material 120 to form the output material 102. The
output
material 102 exits the mixing device 100 through the output port 3010.
Optionally,
the output material 102 may be returned to the input port 1010 and mixed with
an
additional quantity of the second material 120, the third material 130, or a
combination thereof.
26
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
The output port 3010 is oriented substantially orthogonally to the axis of
rotation "a" and may be located opposite the input port 1010 formed in the
first
chamber 310. The output material 102 enters the second chamber 320 from the
mixing chamber 330 having a circumferential velocity (in the direction
indicated by
arrow "C3" in Figure 9) imparted thereto by the rotor 600. The circumferential
velocity is tangential to the portion 3020 of the drive shaft 500 extending
through the
second chamber 320. In the embodiment depicted in Figures 4, 6, and 7, the
output
port 3010 may be offset from the axis of rotation "a." The output port 3010 is
positioned so that the output material 102, which enters the second chamber
320
traveling in substantially the same direction in which the drive shaft 500 is
rotating
(identified in Figure 9 by arrow "Cl"), is traveling toward the output port
3010.
The output material 102 enters the second chamber 320 and is deflected by
the inside of the second chamber 320 about the portion 3020 of the drive shaft
500.
In embodiments wherein the second chamber 320 has a substantially circular
cross-
sectional shape, the inside of the second chamber 320 may deflect the output
material 102 in a substantially circular path about the portion 3020 of the
drive
shaft 500.
Referring to Figure 2, optionally, the output material 102 may be pumped from
inside the second chamber 320 by the external pump 430. The external pump 430
may include any pump known in the art for pumping the output material 102 at a
sufficient rate to avoid limiting throughput of the mixing device 100. In such
an
embodiment, the external pump 430 may introduce a tangential velocity (in a
direction indicated by arrow "T2" in Figures 4 and 11) to at least a portion
of the
output material 102 as the external pump 430 pumps the output material 102
from
the second chamber 320. The tangential velocity of the portion of the output
material 102 may cause it to travel about the axis of rotation "a" at a
circumferential
velocity, determined in part by the tangential velocity.
PUMP 420
Turning to Figures 6 and 7, the pump 420 residing inside the second
chamber 320 may pump the output material 102 from the second chamber 320 into
the output port 3010 and/or from the mixing chamber 330 into the second
chamber 320. In embodiments that include the external pump 430, the external
27
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
pump 430 may be configured to pump the output material 102 from the second
chamber 320 at a rate at least as high as a rate at which the pump 420 pumps
the
output material 102 into the output port 3010.
The second chamber 320 is in communication with the open second end
portion 334 of the mixing chamber 330 and the output material 102 inside the
mixing
chamber 330 may flow freely from the open second end portion 334 into the
second
chamber 320. In this manner, the output material 102 does not negotiate any
corners or bends between the mixing chamber 330 and the second chamber 320. In
the embodiment depicted, the second chamber 320 is in communication with the
entire open second end portion 334 of the mixing chamber 330. The second
chamber 320 may be filled completely with the output material 102.
The pump 420 is powered by the portion 3020 of the drive shaft 500
extending through the second chamber 320. The pump 420 may be substantially
identical to the pump 410. Any pump described above as suitable for use as the
pump 410 may be used for the pump 420. While the pump 410 pumps the first
material 110 into the mixing chamber 330, the pump 420 pumps the output
material 102 from the mixing chamber 330. Therefore, both the pump 410 and the
pump 420 may be oriented to pump in the same direction.
As is appreciated by those of ordinary skill in the art, the first material
110
may differ from the output material 102. For example, one of the first
material 110
and the output material 102 may be more viscous than the other. Therefore, the
pump 410 may differ from the pump 420. The pump 410 may be configured to
accommodate the properties of the first material 110 and the pump 420 may be
configured to accommodate the properties of the output material 102.
The pump 420 depicted in Figures 6 and 7, is generally referred to as a single
screw pump. In this embodiment, the pump member 4022 includes a collar
portion 4030 disposed around the portion 3020 of the drive shaft 500. The
collar
portion 4030 rotates with the portion 3020 of the drive shaft 500 as a unit.
The collar
portion 4030 includes one or more fluid displacement members 4040. The collar
portion 4030 includes a single fluid displacement member 4040 having a helical
shape that circumscribes the collar portion 4030 along a helical path.
Referring to Figure 11, the inside of the second chamber 320 is illustrated.
The pump 420 imparts an axial flow (identified by arrow "A3" and arrow "A4")
in the
28
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
output material 102 inside the second chamber 320 away from the open second
end
portion 334 of the mixing chamber 330.
The pump 420 may be configured to impart a circumferential flow (identified
by arrow "C4") in the output material 102 as it travels away from the open
second
end portion 334 of the mixing chamber 330. The circumferential flow imparted
in the
output material 102 may help reduce an amount of work required by the rotor
600.
The circumferential flow also directs the output material 102 toward the
output
port 3010.
In an alternate embodiment, the pump 420 may have substantially the same
configuration of the pump 410 depicted in Figure 10. In such an embodiment,
the
one or more vanes 2042 are configured to impart a circumferential flow in the
output
material 102 as it travels away from the open second end portion 334 of the
mixing
chamber 330.
As is apparent to those of ordinary skill, various parameters of the mixing
device 100 may be modified to obtain different mixing characteristics.
Exemplary
parameters that may be modified include the size of the through-holes 608, the
shape of the through-holes 608, the arrangement of the through-holes 608, the
number of through-holes 608, the size of the apertures 708, the shape of the
apertures 708, the arrangement of the apertures 708, the number of apertures
708,
the shape of the rotor 600, the shape of the stator 700, the width of the
mixing
chamber 330, the length of the mixing chamber 330, rotational speed of the
drive
shaft 500, the axial velocity imparted by the internal pump 410, the
circumferential
velocity imparted by the internal pump 410, the axial velocity imparted by the
internal
pump 420, the circumferential velocity imparted by the internal pump 420, the
configuration of disturbances (e.g., texture, projections, recesses,
apertures, and the
like) formed on the outside surface 606 of the rotor 600, the configuration of
disturbances (e.g., texture, projections, recesses, apertures, and the like)
formed on
the inside surface 706 of the stator 700, and the like.
ALTERNATE EMBODIMENT
Referring to Figure 12, a mixing device 5000 is depicted. The mixing
device 5000 is an alternate embodiment of the mixing device 100.
Identical
reference numerals have been used herein to identify components of the mixing
29
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
device 5000 that are substantially similar corresponding components of the
mixing
device 100. Only components of the mixing device 5000 that differ from the
components of the mixing device 100 will be described.
The mixing device 5000 includes a housing 5500 for housing the rotor 600
and the stator 5700. The stator 5700 may be non-rotatably couple by its first
end
portion 5712 and its second end portion 5714 to the housing 5500. A chamber
5800
is defined between the housing 5500 and a portion 5820 of the stator 5700
flanked
by the first end portion 5712 and the second end portion 5714. The housing
5500
includes an input port 5830 which provides access into the chamber 5800. The
input
port 5830 may be oriented substantially orthogonally to the axis of rotation
ua."
however, this is not a requirement.
The stator 5700 includes a plurality of through-holes 5708 that connect the
chamber 5800 and the mixing chamber 330 (defined between the rotor 600 and the
stator 5700). An external pump 230 may be used to pump the third material 130
(which may be identical to the second material 120) into the chamber 5800 via
the
input port 5830. The third material 130 pumped into the chamber 5800 may enter
the mixing chamber 330 via the through-holes 5708 formed in the stator 5700.
The
third material 130 may forced from the channel 5800 by the pump 230, buoyancy
of
the third material 130 relative to the first material 110, and a combination
thereof. As
the rotor 600 rotates, it may also draw the third material 130 from the
channel 5800
into the mixing chamber 330. The third material 130 may enter the mixing
chamber 330 as bubbles, droplets, particles, and the like, which are imparted
with a
circumferential velocity by the rotor 600.
ALTERNATE EMBODIMENT
An alternate embodiment of the mixing device 100 may be constructed using
a central section 5900 depicted in Figure 13 and a bearing housing 5920
depicted in
Figure 14. Figure 13 depicts the central section 5900 having in its interior
the
stator 700 (see Figure 7). Identical reference numerals have been used herein
to
identify components associated with the central section 5900 that are
substantially
similar corresponding components of the mixing device 100. Only components of
the central section 5900 that differ from the components of the central
section 522
will be described. The central section 5900 and the stator 700 are both
constructed
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143 PCT/US2007/082578
from a conductive material such as a metal (e.g., stainless steel). The input
port 1010 and the output port 3010 are both constructed from a nonconductive
material such as plastic (e.g., PET, Teflon, nylon, PVC, polycarbonate, ABS,
Delrin,
polysulfone, etc.).
An electrical contact 5910 is coupled to the central section 5900 and
configured to deliver a charge thereto. The central section 5900 conducts an
electrical charge applied to the electrical contact 5910 to the stator 700. In
further
embodiments, the central section 5900 may be constructed from a nonconductive
material. In such embodiments, the electrical contact 5910 may pass through
the
central section 5900 and coupled to the stator 700. The electric charge
applied by
the electrical contact 5910 to the stator 700 may help facilitate redox or
other
chemical reactions inside the mixing chamber 330.
Optionally, insulation (not shown) may be disposed around the central
section 5900 to electrically isolate it from the environment. Further,
insulation may
be used between the central section 5900 and the first and second mechanical
seals 524 and 526 that flank it to isolate it electrically from the other
components of
the mixing device.
Turning now to Figure 14, the bearing housing 5920 will be described. The
bearing housing 5920 is disposed circumferentially around the portion 726 of
the
drive shaft 500. An electrical contact 5922 is coupled to the bearing housing
5920.
A rotating brush contact 5924 provides an electrical connection between the
drive
shaft 500 and the electrical contact 5922.
In this embodiment, the drive shaft 500 and the rotor 600 are both constructed
from a conductive material such as a metal (e.g., stainless steel). The
bearing
housing 5920 may be constructed from either a conductive or a nonconductive
material. An electrical charge is applied to the drive shaft 500 by the
electrical
contact 5922 and the rotating brush contact 5924. The
electrical charge is
conducted by the drive shaft 500 to the rotor 600.
The alternate embodiment of the mixing device 100 constructed using the
central section 5900 depicted in Figure 13 and the bearing housing 5920
depicted in
Figure 14 may be operated in at least two ways. First, the electrical contacts
5910
and 5922 may be configured not to provide an electrical charge to the stator
700 and
31
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
the rotor 600, respectively. In other words, neither of the electrical
contacts 5910
and 5922 are connected to a current source, a voltage source, and the like.
Alternatively, the electrical contacts 5910 and 5922 may be configured to
provide an electrical charge to the stator 700 and the rotor 600,
respectively. For
example, the electrical contacts 5910 and 5922 may be coupled to a DC voltage
source (not shown) supplying a steady or constant voltage across the
electrical
contacts 5910 and 5922. The negative terminal of the DC voltage source may be
coupled to either of the electrical contacts 5910 and 5922 and the positive
terminal of
the DC voltage source may be coupled to the other of the electrical contacts
5910
and 5922. The voltage supplied across the electrical contacts 5910 and 5922
may
range from about 0.0001 volts to about 1000 volts. In particular embodiments,
the
voltage may range from about 1.8 volts to about 2.7 volts. By way of another
example, a pulsed DC voltage having a duty cycle of between about 1% to about
99% may be used.
While the above examples of methods of operating the mixing device apply a
DC voltage across the electrical contacts 5910 and 5922, as is apparent to
those of
ordinary skill in the art, a symmetrical AC voltage or non symmetrical AC
voltage
having various shapes and magnitudes may be applied across the electrical
contacts 5910 and 5922 and such embodiments are within the scope of the
present
invention.
MIXING INSIDE THE MIXING CHAMBER 330
As mentioned above, in the prior art device 10 (shown in Figure 1), the first
material 110 entered the channel 32 between the rotor 12 and the stator 30 via
a
single limited input port 37 located along only a portion of the open second
end of
the channel 32. Likewise, the output material 102 exited the channel 32 via a
single
limited output port 40 located along only a portion of the open first end of
the
channel 32. This arrangement caused undesirable and unnecessary friction. By
replacing the single limited inlet port 37 and the single limited outlet port
40 with the
chambers 310 and 320, respectively, friction has been reduced. Moreover, the
first
material 110 does not negotiate a corner before entering the mixing chamber
330
and the output material 102 does not negotiate a corner before exiting the
mixing
32
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143
PCT/US2007/082578
chamber 330. Further, the chambers 310 and 320 provide for circumferential
velocity of the material prior to entering, and after exiting the channel 32.
Accordingly, pressure drop across the mixing device 100 has been
substantially reduced. In the embodiments depicted in Figures 2, 4-9, and 11,
the
pressure drop between the input port 1010 and the output port 3010 is only
approximately 12 psi when the mixing device 100 is configured to produce about
60 gallons of the output material 102 per minute. This is an improvement over
the
prior art device 10 depicted in Figure 1, which when producing about 60
gallons of
output material per minute was at least 26 psi. In other words, the pressure
drop
across the mixing device 100 is less than half that experienced by the prior
art
device 10.
According to additional aspects, the inclusion of pumps 410 and 420, which
are powered by the drive shaft 500, provides a configuration that is
substantially
more efficient in mixing materials and that requires less energy than the
external
pumps used in the prior art.
MICRO-CAVITATION
During operation of the mixing device 100, the input materials may include the
first material 110 (e.g., a fluid) and the second material 120 (e.g., a gas).
The first
material 110 and the second material 120 are mixed inside the mixing chamber
330
formed between the rotor 600 and the stator 700. Rotation of the rotor 600
inside
the stator 700 agitates the first material 110 and the second material 120
inside the
mixing chamber 330. The through-holes 608 formed in the rotor 600 and/or the
apertures 708 formed in the stator 700 impart turbulence in the flow of the
first
material 110 and the second material 120 inside the mixing chamber 330.
Without being limited by theory, the efficiency and persistence of the
diffusion
of the second material 120 into the first material 110 is believed to be
caused in part
by micro-cavitation, which is described in connection with Figures 15-17.
Whenever
a material flows over a smooth surface, a rather laminar flow is established
with a
thin boundary layer that is stationary or moving very slowly because of the
surface
tension between the moving fluid and the stationary surface. The through-holes
608
and optionally, the apertures 708, disrupt the laminar flow and can cause
localized
compression and decompression of the first material 110. If the pressure
during the
33
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143
PCT/US2007/082578
decompression cycle is low enough, voids (cavitation bubbles) will form in the
material. The cavitation bubbles generate a rotary flow pattern 5990, like a
tornado,
because the localized area of low pressure draws the host material and the
infusion
material, as shown in Figure 15. When the cavitation bubbles implode,
extremely
high pressures result. As two aligned openings (e.g., one of the apertures 708
and
one of the through-holes 608) pass one another, a succussion (shock wave)
occurs,
generating significant energy. The energy associated with cavitation and
succussion
mixes the first material 110 and the second material 120 together to an
extremely
high degree, perhaps at the molecular level.
The tangential velocity of the rotor 600 and the number of openings that pass
each other per rotation may dictate the frequency at which the mixing device
100. It
has been determined that operating the mixing device 100 within in the
ultrasonic
frequency range can be beneficial in many applications. It is believed that
operating
the mixing device 100 in the ultrasonic region of frequencies provides the
maximum
succession shock energy to shift the bonding angle of the fluid molecule,
which
enables it to transport an additional quantity of the second material 120
which it
would not normally be able to retain. When the mixing device 100 is used as a
diffuser, the frequency at which the mixing device 100 operates appears to
affect the
degree of diffusion, leading to much longer persistence of the second material
120
(infusion material) in the first material 110 (host material).
Referring now to Figure 18, an alternate embodiment of the rotor 600,
rotor 6000 is provided. The cavitations created within the first material 110
in the
mixing chamber 330 may be configured to occur at different frequencies along
the
length of the mixing chamber 330. The frequencies of the cavitations may be
altered
by altering the number and/or the placement of the through-holes 6608 along
the
length of the rotor 600. Each of the through-holes 6608 may be substantially
similar
to the through-holes 608 (discussed above).
By way of non-limiting example, the rotor 6000 may be subdivided into three
separate exemplary sections 6100, 6200, and 6300. The through-holes 6608
increase in density from the section 6100 to the section 6200, the number of
holes in
the section 6100 being greater than the number of holes in the section 6200.
The
through-holes 6608 also increase in density from the section 6200 to the
section 6300, the number of holes in the section 6200 being greater than the
number
34
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
of holes in the section 6300. Each of the sections 6100, 6200, and 6300 create
succussions within their particular area at a different frequency due to the
differing
numbers of through-holes 6608 formed therein.
By manufacturing the rotor 6000 with a desired number of through-holes 6608
appropriately arranged in a particular area, the desired frequency of the
succussions
within the mixing chamber 330 may be determined. Similarly, the desired
frequency
of the cavitations may be determined by a desired number of apertures 708
appropriately arranged in a particular area upon the stator 700 within which
the
rotor 600 rotates. Further, the desired frequency (or frequencies) of the
succussions
within the mixing chamber 330 may be achieved by selecting both a particular
number and arrangement of the apertures 708 formed in the stator 700 and a
particular number and arrangement of the through-holes 608 formed in the rotor
600.
Figures 19-21, depict various alternative arrangements of the apertures 708
formed in the stator 700 and the through-holes 608 formed in the rotor 600
configured to achieve different results with respect to the cavitations
created.
Figure 19 illustrates a configuration in which the apertures 708 and the
through-
holes 608 are aligned along an axis 7000 that is not parallel with any line
(e.g.,
line 7010) drawn through the axis of rotation "a" of the rotor 600. In other
words, if
the rotor 600 has a cylindrical shape, the axis 7000 does not pass through the
center
of the rotor 600. Thus, the first material 110 within the mixing chamber 330
will not
be oriented perpendicularly to the compressions and decompressions created by
the
apertures 708 and the through-holes 608. The compressions and decompressions
will instead have a force vector that has at least a component parallel to the
circumferential flow (in the direction of arrow "C3" of Figure 9) of first
material 110
within the mixing chamber 330.
Relative alignment of the apertures 708 and the through-holes 608 may also
affect the creation of cavitations in the mixing chamber 330. Figure 20
illustrates an
embodiment in which the apertures 708 are in registration across the mixing
chamber 330 with the through-holes 608. In this embodiment, rotation of the
rotor 600 brings the through-holes 608 of the rotor into direct alignment with
the
apertures 708 of the stator 700. When in direct alignment with each other, the
compressive and decompressive forces created by the apertures 708 and the
through-holes 608 are directly aligned with one another.
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
In the embodiment depicted in Figure 21, the apertures 708 and the through-
holes 608 are offset by an offset amount "X" along the axis of rotation "a.".
By way of
non-limiting example, the offset amount "X" may be determined as a function of
the
size of the apertures 708. For example, the offset amount "X" may be
approximately
equal to one half of the diameter of the apertures 708. Alternatively, the
offset
amount "X" may be determined as a function of the size of the through-holes
608.
For example, the offset amount 'X" may be approximately equal to one half of
the
diameter of the through-holes 608. If features (e.g., recesses, projections,
etc. )
other than or in addition to the through-holes 608 and the apertures 708 are
included
in either the rotor 600 or the stator 700, the offset amount "X" may be
determined as
a function of the size of such features. In this manner, the compressive and
decompressive forces caused by the apertures 708 of the stator 700 and the
through-holes 608 of the rotor 600 collide at a slight offset causing
additional
rotational and torsional forces within the mixing chamber 330. These
additional
forces increase the mixing (e.g., diffusive action) of the second material 120
into the
first material 110 within the mixing chamber 330.
Referring now to Figures 22-25, non-limiting examples of suitable cross-
sectional shapes for the apertures 708 and the through-holes 608 are provided.
The
cross-sectional shape of the apertures 708 and/or the through-holes 608 may be
square as illustrated in Figure 22, circular as illustrated in Figure 23, and
the like.
Various cross-sectional shapes of apertures 708 and/or the through-holes 608
may be used to alter flow of the first material 110 as the rotor 600 rotates
within the
stator 700. For example, Figure 24 depicts a teardrop cross-sectional shape
having
a narrow portion 7020 opposite a wide portion 7022. If the through-holes 608
have
this teardrop shape, when the rotor 600 is rotated (in the direction generally
indicated
by the arrow "F"), the forces exerted on the first material 110, the second
material 120, and optionally the third material 130 within the mixing chamber
330
increase as the materials pass from the wide portion 7022 of the teardrop to
the
narrow portion 7020.
Additional rotational forces can be introduced into the mixing chamber 330 by
forming the apertures 708 and/or the through-holes 608 with a spiral
configuration as
illustrated in Figure 25. Material that flows into and out of the apertures
708 and/or
the through-holes 608 having the spiral configuration experience a rotational
force
36
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
induced by the spiral configuration. The examples illustrated in Figures 22-25
are
provided as non-limiting illustrations of alternate embodiments that may be
employed
within the mixing device 100. By application of ordinary skill in the art,
the
apertures 708 and/or the through-holes 608 may be configured in numerous ways
to
achieve various succussive and agitative forces appropriate for mixing
materials
within the mixing chamber 330.
DOUBLE LAYER EFFECT
The mixing device 100 may be configured to create the output material 102 by
complex and non-linear fluid dynamic interaction of the first material 110 and
the
second material 120 with complex, dynamic turbulence providing complex mixing
that further favors electrokinetic effects (described below). The result of
these
electrokinetic effects may be observed within the output material 102 as
charge
redistributions and redox reactions, including in the form of solvated
electrons that
are stabilized within the output material.
Ionization or dissociation of surface groups and/or adsorption of ions from a
liquid cause most solid surfaces in contact with the liquid to become charged.
Referring to Figure 26, an electrical double layer ("EDL") 7100 forms around
exemplary surface 7110 in contact with a liquid 7120. In the EDL 7100, ions
7122 of
one charge (in this case, negatively charged ions) adsorb to the surface 7120
and
form a surface layer 7124 typically referred to as a Stern layer. The surface
layer 7124 attracts counterions 7126 (in this case, positively charged ions)
of the
opposite charge and equal magnitude, which form a counterion layer 7128 below
the
surface layer 7124 typically referred to as a diffuse layer. The counterion
layer 7128
is more diffusely distributed than the surface layer 7124 and sits upon a
uniform and
equal distribution of both ions in the bulk material 7130 below. For OH- and
H+ ions
in neutral water, the Gouy-Chapman model would suggest that the diffuse
counterion
layer extends about one micron into the water.
According to particular aspects, the electrokinetic effects mentioned above
are caused by the movement of the liquid 7120 next to the charged surface
7110.
Within the liquid 7120 (e.g., water, saline solution, and the like), the
adsorbed
ions 7122 forming the surface layer 7124 are fixed to the surface 7120 even
when
the liquid 7120 is in motion (for example, flowing in the direction indicated
by arrow
37
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
"G"); however, a shearing plane 7132 exists within the diffuse counterion
layer 7128
spaced from the surface 7120. Thus, as the liquid 7120 moves, some of the
diffuse
counterions 7126 are transported away from the surface 7120, while the
absorbed
ions 7122 remain at the surface 7120. This produces a so-called 'streaming
current.'
Within the mixing chamber 330, the first material 110, the second
material 120, and optionally, the third material 130 are subject to an
electromagnetic
field created by the inside surface 705 of the stator 700 and/or the outside
surface 606 of the rotor 600, a voltage between the inside surface 705 and the
outside surface 606, and/or an electrokinetic effect (e.g., streaming current)
caused
by at least one EDL formed in the first material 110. The at least one EDL may
be
introduced into the first material 110 by at least one of the inside surface
705 of the
stator 700 and the outside surface 606 of the rotor 600.
Movement of the first material 110 through the mixing chamber 330 relative to
surface disturbances (e.g., the through-holes 608 and apertures 708) creates
cavitations in the first material 110 within the mixing chamber 330, which may
diffuse
the second material 120 into the first material 110. These cavitations may
enhance
contact between of the first material 110 and/or the second material 120 with
the
electric double layer formed on the inside surface 705 of the stator 700
and/or the
electric double layer formed on the outside surface 606 of the rotor 600.
Larger
surface to volume ratios of the mixing chamber, an increased dwell time of the
combined materials within the mixing chamber, and further in combination with
a
smaller average bubble size (and hence substantially greater bubble surface
area)
provide for effectively imparting EDL-mediated effects to the inventive output
materials.
In embodiments in which the inside surface 705 and the outside surface 606
are constructed from a metallic material, such as stainless steel, the motion
of the
liquid 7120 and/or the streaming current(s) facilitate redox reactions
involving H20,
OH-, H+, and 02 at the inside surface 705 and the outside surface 606.
Referring to Figure 27, without being limited by theory, it is believed a
section 7140 of the mixing chamber 330 between the inside surface 705 and the
outside surface 606 the may be modeled as a pair of parallel plates 7142 and
7144.
If the first material 110 is a liquid, the first material 110 enters the
section 7140
38
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
through an inlet "IN" and exits the section 7140 through an outlet "OUT." The
inlet "IN" and the outlet "OUT" restrict the flow into and out of the section
7140.
Referring to Figure 28, the area between the parallel plates 7142 and 7144
has a high surface area to volume ratio. Hence, a substantial portion of the
counterion layer 7128 (and counterions 7126) may be in motion as the first
material 110 moves between the plates 7142 and 7144. The
number of
counterions 7126 in motion may exceed the number allowed to enter the
section 7140 by the inlet "IN" and the number allowed to exit the section 7140
by the
outlet "OUT." The inlet "IN" and the outlet "OUT" feeding and removing the
first
material 110 from the section 7140, respectively, have far less surface area
(and a
lower surface area to volume ratio) than the parallel plates 7142 and 7144 and
thereby reduce the portion of the counterions 7126 in motion in the first
material 110
entering and leaving the section 7140.
Therefore, entry and exit from the
section 7140 increases the streaming current locally. While a background
streaming
current (identified by arrow "BSC") caused by the flowing first material 110
over any
surface is always present inside the mixing device 100, the plates 7142 and
7144
introduce an increased "excess" streaming current (identified by arrow "ESC")
within
the section 7140.
Without a conductive return current (identified by arrow "RC") in the
plates 7142 and 7144 in the opposite direction of the flow of the first
material 110, an
excess charge 7146 having the same sign as the adsorbing ions 7122 would
accumulate near the inlet "IN," and an excess charge 7148 having the same sign
as
the counterion 7126 would accumulate near the at outlet "OUT." Because such
accumulated charges 7146 and 7148, being opposite and therefore attracted to
one
another, cannot build up indefinitely the accumulated charges seek to join
together
by conductive means. If the
plates 7142 and 7144 are perfectly electrically
insulating, the accumulated charges 7146 and 7148 can relocate only through
the
first material 110 itself. When
the conductive return current (identified by
arrow "RC") is substantially equivalent to the excess streaming current
(identified by
arrow "ESC") in the section 7140, a steady-state is achieved having zero net
excess
streaming current, and an electrostatic potential difference between the
excess
charge 7146 near the inlet "IN," and the excess charge 7148 near the outlet
"OUT"
creating a steady-state charge separation therebetween.
39
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
The amount of charge separation, and hence the electrostatic potential
difference between the excess charge 7146 near the inlet "IN," and the excess
charge 7148 near the outlet "OUT," depends on additional energy per unit
charge
supplied by a pump (e.g., the rotor 600, the internal pump 410, and/or the
external
pump 210) to "push" charge against the opposing electric field (created by the
charge separation) to produce the a liquid flow rate approximating a flow rate
obtainable by a liquid without ions (i.e., ions 7122 and 7126). If the plates
7142
and 7144 are insulators, the electrostatic potential difference is a direct
measure of
the EMF the pump (e.g., the rotor 600, the internal pump 410 and/or the
external
pump 210) can generate. In this case, one could measure the electrostatic
potential
difference using a voltmeter having a pair of leads by placing one of the
leads in the
first material 110 near the inlet "IN," and the other lead in the first
material 110 near
the outlet "OUT."
With insulating plates 7142 and 7144, any return current is purely an ion
current (or flow of ions), in that the return current involves only the
conduction of ions
through the first material 110. If other conductive mechanisms through more
conductive pathways are present between the excess charge 7146 near the
inlet "IN," and the excess charge 7148 near the outlet "OUT," the return
current may
use those more conductive pathways. For example, conducting metal plates 7142
and 7144 may provide more conductive pathways; however, these more conductive
pathways transmit only an electron current and not the ion current.
As is appreciated by those of ordinary skill, to transfer the charge carried
by
an ion to one or more electrons in the metal, and vise versa, one or more
oxidation-
reduction reactions must occur at the surface of the metal, producing reaction
products. Assuming the first material 110 is water (H20) and the second
material 120 is oxygen (02), a non-limiting example of a redox reaction, which
would
inject negative charge into the conducting plates 7142 and 7144 includes the
following known half-cell reaction:
02 + H20 03+2H+ +2e-,
Again, assuming the first material 110 is water (H20) and the second material
120 is
oxygen (02), a non-limiting example of a redox reaction includes the following
known
half-cell reaction, which would remove negative charge from the conducting
plates 7142 and 7144 includes the following known half-cell reaction:
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/1.JS2007/082578
2H+ +e- ¨> H2,
With conducting metal plates 7142 and 7144, most of the return current is
believed to be an electron current, because the conducting plates 7142 and
7144 are
more conductive than the first material 110 (provided the redox reactions are
fast
enough not to be a limiting factor). For the conducting metal plates 7142 and
7144,
a smaller charge separation accumulates between the inlet "IN" and the
outlet "OUT," and a much smaller electrostatic potential exists therebetween.
However, this does not mean that the EMF is smaller.
As described above, the EMF is related to the energy per unit charge the
pump provides to facilitate the flow of the first material 110 against the
opposing
electric field created by the charge separation. Because the electrostatic
potential is
smaller, the pump may supply less energy per unit charge to cause the first
material 110 to flow.
However, the above example redox reactions do not
necessarily occur spontaneously, and thus may require a work input, which may
be
provided by the pump. Therefore, a portion of the EMF (that is not reflected
in the
smaller electrostatic potential difference) may be used to provide the energy
necessary to drive the redox reactions.
In other words, the same pressure differentials provided by the pump to push
against the opposing electric field created by the charge separation for the
insulating
plates 7142 and 7144, may be used both to "push" the charge through the
conducting plates 7142 and 7144 and drive the redox reactions.
Referring to Figure 29, an experimental setup for an experiment conducted by
the inventors is provided. The experiment included a pair of substantially
identical
spaced apart 500 ml standard Erlenmeyer flasks 7150 and 7152, each containing
a
volume of deionized water 7153. A rubber stopper 7154 was inserted in the open
end of each of the flasks 7150 and 7152. The stopper 7154 included three
pathways, one each for a hollow tube 7156, a positive electrode 7158, and a
negative electrode 7160. With respect to each of the flasks 7150 and 7152,
each of
the hollow tube 7156, the positive electrode 7158, and the negative electrode
7160
all extended from outside the flask, through the stopper 7154, and into the
deionized
water 7153 inside the flask. The
positive electrode 7158 and the negative
electrode 7160 were constructed from stainless steel. The hollow tubes 7156 in
both
of the flasks 7150 and 7152 had an open end portion 7162 coupled to a common
41
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
oxygen supply 7164. The positive electrode 7158 and the negative electrode
7160
inserted into the flask 7152 where coupled to a positive terminal and a
negative
terminal, respectively, of a DC power supply 7168. Exactly the same sparger
was
used in each flask.
Oxygen flowed through the hollow tubes 7156 into both of the flasks 7150
and 7152 at a flow rate (Feed) of about 1 SCFH to about 1.3 SCFH (combined
flow
rate). The voltage applied across the positive electrode 7158 and the negative
electrode 7160 inserted into the flask 7152 was about 2.55 volts. This value
was
chosen because it is believed to be an electrochemical voltage value
sufficient to
affect all oxygen species. This voltage was applied continuously over three to
four
hours during which oxygen from the supply 7164 was bubbled into the deionized
water 7153 in each of the flasks 7150 and 7152.
Testing of the deionized water 7153 in the flask 7150 with HRP and pyrogallol
gave an HRP-mediated pyrogallol reaction activity, consistent with the
properties of
fluids produced with the alternate rotor/stator embodiments described herein.
The
HRP optical density was about 20% higher relative to pressure-pot or fine-
bubbled
solutions of equivalent oxygen content. The results of this experiment
indicate that
mixing inside the mixing chamber 330 involves a redox reaction. According to
particular aspects, the inventive mixing chambers provide for output materials
comprising added electrons that are stabilized by either oxygen-rich water
structure
within the inventive output solutions, or by some form of oxygen species
present due
to the electrical effects within the process.
Additionally, the deionized water 7153 in both of the flasks 7150 and 7152
was tested for both ozone and hydrogen peroxide employing industry standard
colorimetric test ampoules with a sensitivity of 0.1 ppm for hydrogen peroxide
and
0.6 ppm for ozone. There was no positive indication of either species up to
the
detection limits of those ampoules.
DWELL TIME
Dwell time is an amount of time the first material 110, the second
material 120, and optionally the third material 130 spend in the mixing
chamber 330.
The ratio of the length of the mixing chamber 330 to the diameter of the
mixing
chamber 330 may significantly affect dwell time. The greater the ratio, the
longer the
42
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
dwell time. As mentioned in the Background Section, the rotor 12 of the prior
art
device 10 (see Figure 1) had a diameter of about 7.500 inches and a length of
about
6.000 inches providing a length to diameter ratio of about 0.8. In contrast,
in
particular embodiments, the length of the mixing chamber 330 of the mixing
device 100 is about 5 inches and the diameter "Dl" of the rotor 600 is about
1.69 inches yielding a length to diameter ratio of about 2.95.
Dwell time represents the amount of time that the first material 110, the
second material 120, and optionally the third material 130 are able to
interact with
the electrokinetic phenomena described herein. The prior art device 10 is
configured
to produce about 60 gallons of the output material 102 per minute and the
mixing
device 100 is configured to produce about 0.5 gallons of the output material
102 per
minute, the prior art device 10 (see Figure 1) had a fluid dwell time of about
0.05
seconds, whereas embodiments of the mixing device 100 have a substantially
greater (about 7-times greater) dwell time of about 0.35 seconds. This longer
dwell
time allows the first material 110, the second material 120, and optionally
the third
material 130 to interact with each other and the surfaces 606 and 705 (see
Figure 7)
inside the mixing chamber 330 for about 7 times longer than was possible in
the prior
art device 10.
With reference to Table I below, the above dwell times were calculated by
first
determining the flow rate for each device in gallons per second. In the case
of the
prior art device 10 was configured to operate at about 60 gallons of output
material
per minute, while the mixing device 100 is configured to operate over a
broader
range of flow rate, including at an optimal range of about 0.5 gallons of
output
material per minute. The flow rate was then converted to cubic inches per
second by
multiplying the flow rate in gallons per second by the number of cubic inches
in a
gallon (i.e., 231 cubic inches). Then, the volume (12.876 cubic inches) of the
channel 32 of the prior art device 10 was divided by the flow rate of the
device (231
cubic inches/second) to obtain the dwell time (in seconds) and the volume
(0.673
cubic inches) of the mixing chamber 330 of the mixing device 100 was divided
by the
flow rate (1.925 cubic inches/second) of the device (in cubic inches per
second) to
obtain the dwell time (in seconds).
43
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
Table 1. Inventive device can accommodate a range of dwell times, including
a substantially increased (e.g., 7-times) dwell time relative to prior art
devices.
Volume
Flow Rate Flow Rate Flow Rate Mixing Dwell
Device Gallons/ Gallons/ Inches/ Cubic Chamber Time
Minute Second
Second (Cubic Inches) (Seconds)
Prior art
60 1.000 231.000 12.876 0.056
device 10
Mixing
device 100 2 0.033 7.700 0.673 0.087
Mixing
device 100 0.5 0.008 1.925 0.673 0.350
Table 1
RATE OF INFUSION
Particular aspects of the mixing device 100 provide an improved oxygen
infusion rate over the prior art, including over prior art device 10 (see
Figure 1).
When the first material 110 is water and the second material 120 is oxygen,
both of
which are processed by the mixing device 100 in a single pass (i.e., the
return block
of Figure 2 is set to "NO") at or near 20 Celsius, the output material 102
has a
dissolved oxygen level of about 43.8 parts per million. In certain aspects, an
output
material having about 43.8 ppm dissolved oxygen is created in about 350
milliseconds via the inventive flow through the inventive non pressurized (non-
pressure pot) methods. In contrast, when the first material 110 (water) and
the
second material 120 (oxygen) are both processed in a single pass at or near 20
Celsius by the prior art device 10, the output material had dissolved oxygen
level of
only 35 parts per million in a single pass of 56 milliseconds.
OUTPUT MATERIAL 102
When the first material 110 is a liquid (e.g., freshwater, saline, GATORADE ,
and the like) and the second material 120 is a gas (e.g., oxygen, nitrogen,
and the
like), the mixing device 100 may diffuse the second material 120 into the
first
material 110. The following discusses results of analyses performed on the
output
44
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
material 102 to characterize one or more properties of the output material 102
derived from having been processed by the mixing device 100.
When the first material 110 is saline solution and the second material 120 is
oxygen gas, experiments have indicated that a vast majority of oxygen bubbles
produced within the saline solution are no greater than 0.1 micron in size.
DECAY OF DISSOLVED OXYGEN LEVELS
Referring now to Figure 30, there is illustrated the DO levels in water
enriched
with oxygen in the mixing device 100 and stored in a 500 ml thin-walled
plastic bottle
and a 1000 ml glass bottle out to at least 365 days. Each of the bottles was
capped
and stored at 65 degrees Fahrenheit. As can be seen in the Figure, the DO
levels of
the oxygen-enriched fluid remained fairly constant out to at least 365 days.
Referring to Figure 31, there is illustrated the DO levels in water enriched
with
oxygen in the mixing device 100 and stored in a 500 ml plastic thin-walled
bottle and
a 1000 ml glass bottle. Both bottles were refrigerated at 39 degrees
Fahrenheit.
Again, DO levels of the oxygen-enriched fluid remained steady and decreased
only
slightly out to at least 365 days.
Referring now to Figure 32, there is illustrated the dissolved oxygen levels
in
GATORADE enriched with oxygen in the mixing device 100 and stored in 32 oz.
GATORADE bottles having an average temperature of 55 degrees Fahrenheit at
capping. The GATORADE bottles were subsequently refrigerated at 38 degrees
Fahrenheit between capping and opening. During the experiment, a different
bottle
was opened at 20, 60, and 90 days, respectively, to measure the DO levels of
the
GATORADE stored therein.
The GATORADE within a first group of GATORADE bottles was
processed with oxygen in the mixing device 100 at approximately 56 degrees
Fahrenheit. The DO levels of the GATORADE at bottling were approximately 50
ppm as indicated by point 8104. A first bottle was opened at approximately 20
days,
and the DO level of the GATORADE was determined to be approximately 47 ppm
as indicated by point 8106. A second bottle was then opened at 60 days, and
the
DO level of the GATORADE was measured to be approximately 44 ppm as
indicated by point 8108. Finally, a third bottle was opened at 90 days, and
the DO
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
level of the GATORADE was determined to be slightly below 40 ppm as indicated
by point 8110.
The GATORADE within a second group of GATORADE bottles was
processed with oxygen in the mixing device 100 at approximately 52 degrees
Fahrenheit. The initial DO level for GATORADE stored in this group of bottles
was
45 ppm as illustrated by point 8112. The GATORADE in the bottle opened at 20
days had a DO level of only slightly lower than 45 ppm as indicated by point
8114.
The second bottle of GATORADE was opened at 60 days and the GATORADE
therein had a DO level of slightly more than 41 ppm. Finally, a third bottle
of
GATORADE was opened at 90 days and the GATORADE therein had a DO level
of approximately 39 ppm as shown by point 8116. As before, with respect to the
water test in the plastic and glass bottles (see Figure 31), it can be seen
that the DO
levels remain at relatively high levels over the 90 day period and
substantially higher
than those levels present in normal (unprocessed) GATORADE stored in 32 oz.
GATORADE bottles. Point 8010 is the level corresponding to inventive output
fluid
in a covered PET bottle.
Figure 33 illustrates the DO retention of 500 ml of braun balanced salt
solution
processed with oxygen in the mixing device 100 and kept at standard
temperature
and pressure in an amber glass bottle. The DO level of the solution before
processing is 5 ppm. After processing in the mixing device 100, the DO level
was
increased to approximately 41 ppm (illustrated as point 8202). An hour after
processing, the DO level dropped to approximately 40 ppm as indicated by point
8204. Two hours after processing, the DO level dropped to approximately 36 ppm
as indicated by point 8206. The DO level dropped to approximately 34 ppm three
hours after processing as indicated by point 8208. At approximately four and a
half
hours after processing, the DO level within the salt solution dropped to
slightly more
than 30 ppm. The final measurement was taken shortly before six hours after
processing whereat the DO level had dropped to approximately 28 ppm. Thus,
each
of the experiments illustrated in Figures 30-33 illustrate that that the DO
levels
remain at relatively high levels over extended periods.
Because the output material 102 may be consumed by human beings, the
materials used to construct the mixing device 100 should be suitable for food
and/or
pharmaceutical manufacture. By way of non-limiting example, the housing 520,
the
46
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
housing 5520, the rotor 600, the stator 700, and the stator 5700 may all be
constructed from stainless steel.
BUBBLE SIZE MEASUREMENTS
Experimentation was performed to determine a size of the bubbles of gas
diffused within the fluid by the mixing device 100. While experiments were not
performed to measure directly the size of the bubbles, experiments were
performed
that established that the bubble size of the majority of the gas bubbles
within the fluid
was smaller than 0.1 microns. In other words, the experiments determined a
size
threshold value below which the sizes of the majority of bubbles fall.
This size threshold value or size limit was established by passing the output
material 102 formed by processing a fluid and a gas in the mixing device 100
through a 0.22 filter and a 0.1 micron filter. In performing these tests, a
volume of
the first material 110, in this case, a fluid, and a volume of the second
material 120,
in this case, a gas, were passed through the mixing device 100 to generate a
volume
of the output material 102 (i.e., a fluid having a gas diffused therein).
Sixty milliliters
of the output material 102 was drained into a 60 ml syringe. The DO level of
the fluid
was measured via the Winkler Titration. The fluid within the syringe was
injected
through a 0.22 micron filter into a 50 ml beaker. The filter comprised the
Milipor
Millex GP50 filter. The DO level of the material in the 50 ml beaker was then
measured. The experiment was performed three times to achieve the results
illustrated in Table II below.
¨ --
DO AFTER 0.22 MICRON
DO IN SYRINGE FILTER
42.1 ppm 39.7 ppm
43.4 ppm 42.0 ppm
43.5 ppm 39.5 ppm
_
Table II
As can be seen, the DO levels measured within the syringe and the DO levels
measured within the 50 ml beaker were not changed drastically by passing the
47
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
output material 102 through the 0.22 micron filter. The implication of this
experiment
is that the bubbles of dissolved gas within the output material 102 are not
larger than
0.22 microns otherwise there would be a significantly greater reduction in the
DO
levels in the output material 102 passed through the 0.22 micron filter.
A second test was performed in which the 0.1 micron filter was substituted for
the 0.22 micron filter. In this experiment, saline solution was processed with
oxygen
in the mixing device 100 and a sample of the output material 102 was collected
in an
unfiltered state. The DO level of the unfiltered sample was 44.7 ppm. The
output
material 102 was filtered using the 0.1 micron filter and two additional
samples were
collected. The DO level of the first sample was 43.4 ppm. The DO level of the
second sample was 41.4 ppm. Then, the filter was removed and a final sample
was
taken from the unfiltered output material 102. The final sample had a DO level
of
45.4 ppm. These results were consistent with those seen using the Millipore
0.22
micron filter. These results lead to the conclusion that there is a trivial
reduction in
the DO levels of the output material 102 passed through the 0.1 micron filter
providing an indication that the majority of the bubbles in the processed
saline
solution are no greater than 0.1 micron in size.
As appreciated in the art, the double-layer (interfacial) (DL) appears on the
surface of an obiect when it is placed into a liquid. This object, for
example, might be
that of a solid surface (e.g., rotor and stator surfaces), solid particles, g.
bubbles,
liquid droplets, or porous body. In the mixing device 100, bubble surfaces
represent
a significant portion of the total surface area present within the mixing
chamber that
may be available for electrokinetic double-layer effects. Therefore, in
addition to the
surface area and retention time aspects discussed elsewhere herein, the
relatively
small bubble sizes generated within the mixer 100 compared to prior art
devices 10,
may also contribute, at least to some extent, to the overall electrokinetic
effects and
output fluid properties disclosed herein. Specifically, in preferred
embodiments, as
illustrated by the mixer 100, all of the gas is being introduced via apertures
on the
rotor (no gas is being introduced through stator apertures. Because the rotor
is
rotating at a high rate (e.g., 3,400 rpm) generating substantial shear forces
at and
near the rotor surface, the bubble size of bubbles introduced via, and
adjacent to the
spinning rotor surface apertures would be expected to be substantially (e.g.,
2 to 3-
times smaller) smaller than those introduced via and near the stationary
stator. The
48
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143 PCT/US2007/082578
average bubble size of the prior art device 10 may, therefore, be
substantially larger
because at least half of the gas is introduced into the mixing chamber from
the
stationary stator apertures. Because the surface area of a sphere surface
varies
with r2, any such bubble component of the electrokinetic surface area of the
mixing
device 100 may be substantially greater than that of the prior art diffusion
device 10.
Therefore, without being bound by theory, not only does the mixing chamber
of the mixing device 100 have (i) a substantially higher surface to volume
ratio than
that of the prior art device 10 (the prior art device 10 has a ratio of
surface to volume
of 10.9, whereas the present mixer 100 has a surface to volume ratio of 39.4),
along
with (ii) a 7-fold greater dwell-time, but (iii) the unique properties of the
current
output solutions may additionally reflect a contribution from the
substantially larger
bubble surface area in the mixing device 100. These distinguishing aspects
reflect
distinguishing features of the present mixing device 100, and likely each
contribute to
the unique electrokinetic properties of the inventive output materials/fluids.
SPARGING EFFECTS
Figures 34-35 illustrate the sparging affects of the mixing device 100 on a
fluid
(e.g., the first material 110) passing therethrough. Sparging refers to
"bubbling" an
inert gas through a solution to remove a different dissolved gas(es) from the
solution.
In each of the examples illustrated in Figures 34 and 35, the second material
120 is
nitrogen. The levels of dissolved oxygen in the output material 102 are
measured at
various points in time. As can be seen in the figures, the nitrogen gas
sparges the
oxygen from the fluid passing through the mixing device 100 causing the DO
levels
in the fluid to decay over a period of time.
The results of another experiment are illustrated in Figure 34 wherein water
is
sparged with nitrogen using the mixing device 100. Two sets of experiments
were
conducted, the first having a gas flow rate of SCFH (Standard Cubic Feet per
Hour)
of 1 and the second having a gas flow rate of SCFH of 0.6 The fluid flow rate
was
about 0.5 gal/min. As can be seen, when the process is begun, the DO levels in
each of the experiments was approximately 9 ppm. After only one minute, the DO
levels had dropped to slightly above 5 ppm. At two minutes the DO levels had
dropped to approximately 2.5 ppm. The DO level appears to level out at a
minimum
49
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
level at approximately 6 minutes wherein the DO level is slightly above zero
(0).
Thus, the nitrogen sparges the oxygen from the water relatively quickly.
Figure 35 illustrates the sparging of oxygenated water in an 8 gallon tank at
standard temperature and pressure. The decay rate of the DO in the water is
illustrated by line 8602. As can be seen, initially the oxygenated water had a
DO
level of approximately 42 ppm. After 2 minutes of processing by the mixing
device 100, the nitrogen sparged the oxygenated water such that the DO level
dropped to slightly more than 20 ppm. At 6 minutes, the DO level dropped from
greater than 40 ppm to only 6 ppm. The DO level of the oxygenated water
reached
a minimum value slightly greater than zero (0) at approximately 14 minutes
after the
beginning of the process. Thus, the above described sparging experiments
illustrate
that the mixing device 100 is capable of quickly sparging oxygen from water
and
replacing the oxygen with another gas such as nitrogen by processing
oxygenated
water with mixing device 100 for a rather short period of time. In other
words,
because total partial gas pressure in the fluid remained at approximately the
same
level despite the decrease in DO, the nitrogen gas replaced the oxygen in the
fluid.
These figures illustrate the manner in which nitrogen may be diffused into
water to sparge the oxygen from the water. However, any gas could be used to
sparge a selected gas from any selected fluid and diffuse into the selected
fluid the
gas used to sparge the selected gas from the selected fluid. For example, the
principals illustrated may also be applicable to sparging nitrogen from water
or
another fluid using oxygen. Further, any gas dissolved within a solution may
be
sparged therefrom using a different gas to take the place of the gas sparged
from the
solution. In other words, by processing a sparging gas and a solution
containing a
dissolved gas through the mixing device 100 for a relatively short period of
time, the
dissolved gas could be quickly and efficiently removed from the solution.
MOLECULAR INTERACTIONS
A number of physicists have begun to describe the quantum properties of
water. Conventionally, quantum properties are thought to belong to elementary
particles of less than 101 meters, while the macroscopic world of our
everyday life is
referred to as classical, in that it behaves according to Newton's laws of
motion.
Between the macroscopic classical world and the microscopic quantum world is
the
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
mesoscopic domain, where the distinction between macroscopic and microscopic
is
becoming increasingly blurred. Indeed, physicists are discovering quantum
properties in large collections of atoms and molecules in the nanometer to
micrometer range, particularly when the molecules are packed closely together
in a
liquid phase.
Recently, chemists have made a surprising discovery that molecules form
clusters that increase in size with dilution. These clusters measure several
micrometers in diameter. The increase in size occurs non-linearly with
dilution and
depends on history, flying in the face of classical chemistry. Indeed, there
is yet no
explanation for this phenomena. It may well be yet another reflection of the
strangeness of water that depends on its quantum properties.
In the mid 1990's, quantum physicist del Giudice and Preparata and other
colleagues at the University of Milan, in Italy, argued that quantum coherent
domains
measuring 100 nanometers in diameter could arise in pure water. They show how
the collective vibrations of water molecules in the coherent domain eventually
become phase locked to the fluctuations of the global electromagnetic field.
In this
way, long lasting, stable oscillations could be maintained in water.
One way in which memory might be stored in water is through the excitation
of long lasting coherent oscillations specific to one or more substances (such
as a
therapeutic agent) dissolved in the water. Interactions between the water
molecules
and the molecules of the substances dissolved in the water change the
collective
structure of the water, which would in turn determine the specific coherent
oscillations that develop. If these oscillations become stabilized and
maintained by
phase coupling between the global field and the excited molecules, then, even
when
the dissolved substances are diluted away, the water may still carry the
coherent
oscillations that can seed other volumes of water on dilution.
The discovery that dissolved substances form increasingly large clusters is
compatible with the existence of a coherent field in water that can transmit
attractive
resonance between molecules when the oscillations are in phase leading to
clumping in dilute solutions. As a cluster of molecules increases in size, its
electromagnetic signature is correspondingly amplified, reinforcing the
coherent
oscillations carried by the water.
51
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
One should expect changes in some physical properties in water that could be
detectible. Unfortunately, all attempts to detect such coherent oscillations
by usual
spectroscopic and nuclear magnetic resonance methods have yielded ambiguous
results. This is not surprising in view of the finding that cluster size of
the dissolved
molecules depends on the precise history of dilution rather than concentration
of the
molecules.
It is possible that despite variations in the cluster size of the dissolved
molecules and detailed microscopic structure of the water, a specificity of
coherent
oscillations may nonetheless exist. Usual detection methods fail because they
depend upon using the microscopic particles of individual molecules, or of
small
aggregates. Instead, what is needed is a method of detecting collective global
properties over many, many molecules. Some obvious possibilities that suggest
themselves are the measurements of freezing points and boiling points,
viscosity,
density, diffusivity, and magnet properties. One possibility for detecting
changes in
collective global properties of water is by means of crystallization. Crystals
are
formed from macroscopic collections of molecules. Like other measurements that
depend on global properties, crystals simplify the subtle changes in the
individual
molecules that would have been undetectable otherwise.
With reference to Figure 36, a simplified protonated water cluster forming a
nanoscale cage 8700 is shown. A protonated water cluster typically takes the
form
of W(H20)n. Some protonated water clusters occur naturally, such as in the
ionosphere. Without being bound by any particular theory, and according to
particular aspects, other types of water clusters or structures (clusters,
nanocages,
etc) are possible, including structures comprising oxygen and stabilized
electrons
imparted to the inventive output materials. Oxygen atoms 8704 may be caught in
the resulting structures 8700. The chemistry of the semi-bound nanocage allows
the
oxygen 8704 and/or stabilized electrons to remain dissolved for extended
periods of
time. .Other atoms or molecules, such as medicinal compounds, can be caged for
sustained delivery purposes. The specific chemistry of the solution material
and
dissolved compounds depend on the interactions of those materials.
Fluids processed by the mixing device 100 have been shown via experiments
to exhibit different structural characteristics that are consistent with an
analysis of the
fluid in the context of a cluster structure.
52
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
RAYLEIGH EFFECTS
If a strong beam of light is passed through a transparent gaseous or liquid
medium containing solid or liquid particles, or even molecules of extremely
high
molecular weight, the light is scattered away from the direction of its
incident path.
The scattering is due to the interference effects that arise from the density
fluctuations in the scattering medium (i.e. the presence of particles or very
high
molecular weight molecules.) There are two types of light scattering. The
first
involves the wavelength of the scattered light differing from that of the
incident light
and is called Raman scattering. The other type scattering involves when the
scattered light has the same wavelength of the incident light and is called
Rayleigh
scattering. In Rayleigh scattering, the intensity of the scattered light is
proportional
to the product of the intensity of the incident light and the attenuation
constant, a
function of the refractive index and the Rayleigh constant. The Rayleigh
constant is
a somewhat involved function of the molecular weight of the scattering
substance
and thus a measurement of the intensity of the scattered light can give a
value for
the molecular weight. This scattering phenomenon is used in a number of liquid
chromatography detectors.
Water processed through the mixing device 100 has been demonstrated to
have detectible structural differences when compared with normal unprocessed
water. For example, processed water has been shown to have more Rayleigh
scattering than is observed in unprocessed water. In the experiments that were
conducted, samples of processed and unprocessed water were prepared (by
sealing
each in a separate bottle), coded (for later identification of the processed
sample and
unprocessed sample), and sent to an independent testing laboratory for
analysis.
Only after the tests were completed were the codes interpreted to reveal which
sample had been processed by the mixing device 100.
At the laboratory, the two samples were placed in a laser beam having a
wavelength of 633 nanometers. The fluid had been sealed in glass bottles for
approximately one week before testing. With respect to the processed sample,
Sample B scattered light regardless of its position relative to the laser
source.
However, "Sample A" did not. After two to three hours following the opening of
the
bottle, the scattering effect of Sample B disappeared. These results imply the
water
53
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/1JS2007/082578
exhibited a memory causing the water to retain its properties and dissipate
over time.
These results also imply the structure of the processed water is optically
different
from the structure of the unprocessed fluid. Finally, these results imply the
optical
effect is not directly related to DO levels because the DO level at the start
was 45
ppm and at the end of the experiment was estimated to be approximately 32 ppm.
GENERATION OF SOLVATED ELECTRONS
Additional evidence indicates that the mixing occurring inside the mixing
device 100 generates solvated electrons within the output material 102. This
conclusion results from conditions observed with respect to the dissolved
oxygen
probe effects used in measuring the DO levels within various processed
solutions.
Due to the experiences viewed with respect to the polarographic dissolved
oxygen
probes, it is a belief that the processed fluid exhibits an electron capture
effect and
thus the fluid includes solvated electrons.
There are two fundamental techniques for measuring dissolved oxygen ("DO")
levels electrically: galvanic measuring techniques and polarographic
measurements.
In both techniques, the DO level sensor includes two electrodes, an anode and
a
cathode, which are both immersed in electrolyte within the sensor body. An
oxygen
permeable membrane separates the anode and cathode from the solution being
tested. The cathode is a hydrogen electrode and carries negative potential
with
respect to the anode. The electrolyte solution surrounds the electrode pair
and is
contained by the membrane. With no oxygen, the cathode becomes polarized with
hydrogen and resists the flow of current. When oxygen passes through the
membrane, the cathode is depolarized and electrons are consumed. In other
words,
oxygen diffuses across the membrane and interacts with the internal components
of
the probe to produce an electrical current. The cathode electrochemically
reduces
the oxygen to hydroxyl ions according to the following equation:
02 + 2H20 + 4E- = 40H-
When attempting to measure DO levels in a solution processed by the mixing
device 100, an overflow condition has been repeatedly experienced wherein the
dissolved oxygen meter actually displays a reading that is higher than the
meter is
capable of reading. Independent means, a Winkler Titration, reveals a much
lower
54
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
DO level for the solution than indicated by the probe. Typically, in a device
such as
the Orion 862, having a maximum reading of 60 ppm, the meter will overflow and
have the high oxygen level indication if left in bulk processed water for
several
minutes.
Because the overload is not caused by dissolved oxygen in the fluid, it is
believed solvated electrons must be causing the overload. In other words,
solvated
electrons are accompanying the processed water across the membrane. These
electrons are attracted to the anode and cause the current observed. It is a
further
belief that these electrons are captured in a cage or cluster mechanism within
the
solution.
Compositions comprising hydrated (solvated) electrons imparted to the
inventive compositions by the inventive processes
In certain embodiments as described herein (see under "Double-layer"), the
gas-enriched fluid is generated by the disclosed electromechanical processes
in
which molecular oxygen is diffused into the fluid and may operate to stabilize
charges (e.g., hydrated (solvated) electrons) imparted to the fluid. Without
being
bound by theory or mechanism, certain embodiments of the present invention
relate
to a oxygen-enriched fluid (output material) comprising charges (e.g.,
hydrated
(solvated) electrons) that are added to the materials as the first material is
mixed
with oxygen in the inventive mixer device to provide the combined output
material.
According to particular aspects, these hydrated (solvated) electrons
(alternately
referred to herein as 'solvated electrons') are stabilized in the inventive
solutions as
evidenced by the persistence of assayable effects mediated by these hydrated
(solvated) electrons. Certain embodiments may relate to hydrated (solvated)
electrons and/or water-electron structures, clusters, etc., (See, for example,
Lee and
Lee, Bull. Kor. Chem. Soc. 2003, v. 24, 6; 802-804; 2003).
Novel HRP based assay. Horseradish peroxidase (HRP) is isolated from
horseradish roots (Amoracia rusticana) and belongs to the ferroprotoporphyrin
group
(Heme group) of peroxidases. HRP readily combines with hydrogen peroxide or
other hydrogen donors to oxidize the pyrogallol substrate. Additionally, as
recognized in the art, HRP facilitates autoxidative degradation of indole-3-
acietic acid
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
in the absence of hydrogen peroxide (see, e.g., Heme Peroxidases, H. Brian
Dunford, Wiley-VCH, 1999, Chapter 6, pages 112-123, describing that
autoxidation
involves a highly efficient branched-chain mechanism; incorporated herein by
reference in its entirety). The HRP reaction can be measured in enzymatic
activity
units, in which Specific activity is expressed in terms of pyrogallol units.
One
pyrogallol unit will form 1.0 mg purpurogallin from pyrogallol in 20 sec at pH
6.0 at
20 C. This purpurogallin (20 sec) unit is equivalent to approx. 18 lifV1 units
per min at
25 C.
OH OH 0
OH
A 044
I at*
HO'
Peroxidw.
PsifogMoi Putpurogallin
According to particular aspects of the present invention, the oxygen-enriched
inventive fluids (output materials) have been described and disclosed herein
to react
with pyrogallol in the presence of horseradish peroxidase. The reaction is
most likely
based on an auto-oxidation of the pyrogallol, since no hydrogen peroxide,
superoxide, or other reactive oxygen species has been detected in oxygen-
enriched
inventive fluid. The extent of this reaction is greater than that of
pressurized oxygen
solutions (pressure-pot oxygen solutions) and less than that of hydrogen
peroxide.
Specifically, the present applicants have determined that while there is no
hydrogen peroxide (none detected at a sensitivity of 0.1 ppm), the inventive
gas-
enriched fluid may be consistently characterized by its facilitation of the
apparent
autoxidation of pyrogallol to purpurogallin in the presence of horseradish
peroxidase
enzyme (HRP). That is, like the case of HRP facilitation of the autoxidative
degradation of indole-3-acietic acid in the absence of hydrogen peroxide,
applicants
have discovered HRP facilitation of the autoxidative degradation of pyrogallol
in the
absence of hydrogen peroxide. According to particular aspects, the presence
and
level of this activity are distinguishing features of the inventive
compositions in view
of the prior art.
In certain embodiments, the inventive gas-enriched fluid facilitates, in the
presence of HRP and absence of hydrogen peroxide, a pyrogallol autoxidation
rate
56
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
(under standard conditions as defined herein under "Definitions") equivalent
to
approximately 0.5 ppm of hydrogen peroxide, approximately 0.8 ppm of hydrogen
peroxide, approximately 1 ppm of hydrogen peroxide, approximately 2 ppm of
hydrogen peroxide, approximately 3 ppm of hydrogen peroxide, approximately 4
ppm of hydrogen peroxide, approximately 5 ppm of hydrogen peroxide,
approximately 6 ppm of hydrogen peroxide, approximately 7 ppm of hydrogen
peroxide, approximately 8 ppm of hydrogen peroxide, approximately 9 ppm of
hydrogen peroxide, approximately 10 ppm of hydrogen peroxide, approximately 11
ppm of hydrogen peroxide, approximately 12 ppm of hydrogen peroxide,
approximately 20 ppm of hydrogen peroxide, approximately 40 ppm of hydrogen
peroxide, approximately 50 ppm of hydrogen peroxide or any value therebetween
or
greater.
It is known that Horseradish peroxidase enzyme catalyzes the auto-oxidation
of pyrogallol by way of facilitating reaction with the molecular oxygen in a
fluid.
(Khajehpour et al., PROTEINS: Struct, Funct, Genet. 53: 656-666 (2003)). It is
also
known that oxygen binds the heme pocket of horseradish peroxidase enzyme
through a hydrophobic pore region of the enzyme (between Phe68 and Phe142),
whose conformation likely determines the accessibility of oxygen to the
interior.
Without being bound by mechanism, because surface charges on proteins are
known in the protein art to influence protein structure, it is possible that
the solvated
electrons present in the inventive gas-enriched fluid act to alter the
conformation of
the horseradish peroxidase such that greater oxygen accessibility results. The
greater accessibility of oxygen to the prosthetic heme pocket of the
horseradish
peroxidase enzyme in turn would allow for increased reactivity with
pyrogallol, when
compared with prior art oxygenated fluids (pressure-pot, fine-bubbled).
Alternatively,
the added or solvated electrons of the present output compositions may be
acting in
other ways to enable facilitation of the apparent autoxidation of pyrogallol
to
purpurogallin in the presence of horseradish peroxidase enzyme (HRP).
In any event, according to particular aspects, production of output material
using the inventive methods and devices comprises a process involving: an
interfacial double layer that provides a charge gradient; movement of the
materials
relative to surfaces pulling charge (e.g., electrons) away from the surface by
virtue of
a triboelectric effect, wherein the flow of material produces a flow of
solvated
57
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143
PCT/US2007/082578
electrons. Moreover, according to additional aspects, and without being bound
by
mechanism, the orbital structure of diatomic oxygen creates charge imbalances
(e.g., the two unpaired electrons affecting the hydrogen bonding of the water)
in the
hydrogen bonding arrangement within the fluid material (water), wherein
electrons
are solvated and stabilized within the imbalances.
The inventive combination of oxygen-enrichment and solvated electrons
imparted by the double-layer effects and configuration of the presently
claimed
devices facilitates the auto-oxidation of pyrogallol in the presence of HRP,
which is a
distinguishing feature of the present inventive output material compositions
that can
be readily monitored and quantified by way of optical density. Typically, the
inventive oxygen-enriched compositions are characterized in that they provide
for
about a 20% higher optical density read-out in the standard assay compared to
either pressurized (pressure pot) or fine-bubbled control fluid have
equivalent
dissolved oxygen concentrations. The HRP is likely providing added oxidative
ability
to the autoxidation.
Pyrogallol Reactivity Test
An aliquot of the inventive oxygen-enriched output material was tested for
peroxidase activity by using a commercially available horseradish peroxidase
and a
pyrogallol assay (Sigma). Briefly, pyrogallol stock solution was prepared with
deionized water. Pyrogallol measures peroxidase activity of the
horseradish
peroxidase enzyme on the fluid as it reacts with a substrate (such as hydrogen
peroxide), to yield purpurogallin and water. Test fluid with horseradish
peroxidase,
pyrogallol and the appropriate potassium phosphate buffer were compared with
other fluids. Hydrogen peroxide served as the positive control. The other
fluids
tested were water that was oxygenated and pressurized in a pressure pot, up to
100
psi to reach the desired dissolved oxygen level (Pressure Pot), while the
other fluid
was oxygenated with an air stone in an open beaker (Fine Bubble). All fluids
tested
were maintained at room temperature, and measured approximately 55 ppm
dissolved oxygen level (by FOXY probe). Water samples were tested by adding
the
enzymatic reagents. Continuous spectrophotometric rate determination was made
at A420 nm, and room temperature (25 degrees Celsius).
As indicated in Figures 38-41, the inventive oxygen-enriched fluid tested
58
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143
PCT/US2007/082578
positive for reactivity with horseradish peroxidase by pyrogallol, while the
pressure
pot and fine bubbled water samples had far less reactivity. As indicated in
Figure 42, oxygen is required for the reaction with pyrogallol in the presence
of
horseradish peroxidase, as inventive fluid enriched with other gases did not
react in
the same manner.
Several chemical tests of the inventive oxygen-enriched fluid for the presence
of hydrogen peroxide were conducted, as described herein, and none of these
tests
were positive (sensitivity of 0.1 ppm hydrogen peroxide). Thus, the inventive
oxygen-enriched fluid of the instant application provides for peroxidase
facilitated
auto-oxidation activity in the absence of hydrogen peroxide.
In particular embodiments, Applicants have determined that the horseradish
peroxidase effect remains at least up to seven hours after opening of the
bottle in
which it is stored. In other embodiments, Applicants have determined that the
horseradish peroxidase effect remains after opening of closed container after
105
days of storage in the closed container. By contrast, in other embodiments,
Applicants have determined that when testing equivalent dissolved oxygen
levels
made with just pressurizing fluid (pressure pot fluids), the decline of a
background
HRP effect takes place rapidly, declining precipitously in under 4 hours.
Glutathione Peroxidase Study
The inventive oxygen-enriched output fluid material was tested for the
presence of hydrogen peroxide by testing the reactivity with glutathione
peroxidase
using a standard assay (Sigma). Briefly, glutathione peroxidase enzyme
cocktail
was constituted in deionized water and the appropriate buffers. Water samples
were tested by adding the enzymatic reagents. Continuous spectrophotometric
rate
determination was made at A340 nm, and room temperature (25 degrees Celsius).
Samples tested were: 1. deionized water (negative control), 2. inventive
oxygen-
enriched fluid at low concentration, 3. inventive oxygen-enriched fluid at
high
concentration, 4. hydrogen peroxide (positive control). As illustrated in
Figure 43,
the hydrogen peroxide positive control showed a strong reactivity, while none
of the
other fluids tested reacted with the glutathione peroxidase.
DIFFERENTIAL NUCLEIC ACID STABILITY
59
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
Particular embodiments of the present invention provide another
distinguishing feature of the present inventive compositions. Specifically,
applicants
have discovered that there is a differential thermostability of nucleic acids
associated
with the inventive output fluids compared to control fluids. For example, the
17
promoter primer 5'-d(TAATACGACTCACTATAGGG)-3' (SEQ ID NO:1) when
measured in the inventive oxygen-enriched output materials relative to non-
enriched
deionized water. As the temperature of the water increases, the DNA oligomeric
structure performs a conformational change. As illustrated in Figure 44,
consent with
the art recognized melting temperature for this oligo of about 48 C, the T7
DNA
begins to denature at about 50 degrees Celsius in the control (deionized
water),
whereas the DNA in the oxygen-enriched inventive fluid remains intact until
about 60
degrees Celsius. Thus, the inventive oxygen-enriched fluid comprising solvated
electrons imparts a higher thermostability for DNA when compared to control
fluid,
and provides a further distinguishing feature of the present inventive output
material
compositions that can be readily monitored and quantified by way of optical
density
measurements.
BIOREACTOR SYSTEMS COMPRISING THE INVENTIVE MIXING DEVICES
Producing significant quantities of target products, such as proteins,
polypeptides, nucleic acids, therapeutic agents, and other products in host
cell
systems are possible due to advances in molecular biology. For
example,
recombinant proteins are produced in a host cell systems by transfecting the
host
cell with nucleic acids (e.g. DNA) encoding a protein of interest. Next, the
host cell is
grown under conditions which allow for expression of the recombinant protein.
Certain host cell systems can be used to produce large quantities of
recombinant
proteins which would be too impractical to produce by other means.
In addition, enzymatic and/or reaction fermentations, with or without host
cells, are utilized for example in producing foodstuff and beverages, in
treating
wastewater, or in environmental cleanup.
Cell culturing processes, or cellular fermentation, typically use prokaryotic
or
eukaryotic host cells to produce recombinant proteins. The fermentation is
typically
conducted in physical containers (e.g. stirred tanks) called fermentors or
tank
bioreactors. The fermentation process itself may comprise (1) discontinuous
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
operation (batch process), (2) continuous operation, or (3) semi-continuous
operations (such as the fed-batch process), or any combination of these.
Since the aim of large scale production of pharmaceutical drugs (e.g.
biologicals) or other target products is to provide improved manufacturing
processes
and reduced costs, there is a need for improved bioreactor equipment, methods,
and
media for preparation of these target products.
The present disclosure sets forth novel gas-enriched fluids, including, but
not
limited to gas-enriched water, saline solutions (e.g., standard aqueous saline
solutions), cell culture media, as well as novel methods and biological and
chemical
reactor systems for use in these application processes, and others.
Certain embodiments disclosed herein relate to systems, media, and methods
for producing a target product, such as a protein.
In certain embodiments, a target product may refer to a protein, peptide,
polypeptide, nucleic acid, carbohydrate, polymer, micelle, and any mixture
thereof.
The target product is typically produced by a vehicle, such as a host cell,
which is associated with the gas-enriched fluid in a chemical or biological
reactor.
Reactors may include standard reactors, such as continuous feed, discontinuous
feed, and/or semi-continuous feed. Reactors may also include a cell culture
vessel
(such as a plate, flask, or tank), a plant, an animal, a fungus, an algae, or
other
organism. For example, a plant that is associated with the gas-enriched fluid
of the
present invention may comprise plant cells acting as vehicles that aid in the
production of the target product (for example, naturally occurring plant
matter or
genetically altered plant matter).
In certain embodiments, the vehicles utilized with the gas-enriched fluids or
solutions (including media) may include prokaryotic cells or eukaryotic cells.
More
specifically, the living cells may include bacterial (e.g. E. coli,
Salmonella,
Streptococcus, Staphylococcus, Neisseria, Nocardia, Mycoplasma, etc.), fungal
(e.g.
yeasts, molds, mushrooms, etc.), plant (tobacco, maize, soybean, fruit or
vegetable,
etc.), animal (mammalian, insect, etc.) archebacterial (blue green algae),
protist,
human embryonic kidney (HEK) cells, HeLa cells, hybridoma cells, Madin-Darby
Canine Kidney (MDCK) cells, stem cells, cell lines (including SP2/0 and NSO),
African Green Monkey Kidney (Vero) cells, Spodoptera trugiperda (army worm),
Trichoplusia ni (cabbage looper), and other cells. In addition, viruses (such
as
61
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
bacteriophage, baculovirus, vaccinia, and other viruses) may be employed in
the
bioreactors of the present invention.
The bioreactor may comprise an airlift reactor, a packed bed reactor, a
fibrous
bed reactor, a membrane reactor, a two-chamber reactor, a stirred-tank
reactor, a
hollow-fiber reactor, or other reactor designed to support suspended or
immobilized
cell growth.
In cases of recombinant or target protein production, a balanced batch and/or
feed medium must encourage optimal cell growth and expression of the
recombinant
protein. The medium, or media, is termed "minimal" if it only contains the
nutrients
essential for growth. For prokaryotic host cells, the minimal media typically
includes
a source of carbon, nitrogen, phosphorus, magnesium, and trace amounts of iron
and calcium. (Gunsalus and Stanter, The Bacteria, V. 1, Ch. 1 Acad. Press
Inc.,
N.Y. (1960)). Most minimal media use glucose as a carbon source, ammonia as a
nitrogen source, and orthophosphate (e.g. PO4) as the phosphorus source. The
media components can be varied or supplemented according to the specific
prokaryotic organism(s) grown, in order to encourage optimal growth without
inhibiting target protein production. (Thompson et al., Biotech. and Bioeng.
27: 818-
824 (1985)). This allows for higher levels of production with lower cost.
In addition to the chemical composition of the media, other factors may affect
cell growth and/or target protein production. These factors include, but are
not
limited to pH, time, cultivation temperature, amount of dissolved oxygen or
other
gas(es), and partial pressure of those dissolved gasses. During the
fermentation
process, the pH of the media is typically altered due to the consumption of
ammonia,
or microorganism synthesis of certain metabolic products, e.g., acetic acid
and lactic
acid. Since altered pH may be unfavorable for optimal cell growth, it may be
necessary or desirable to maintain the medium at a certain pH (i.e. by
addition of
acids or bases). The pH and other process parameters can be monitored manually
or by automatic devices.
Inventive Gas-Enriched Fluids
Enriching a fluid with another fluid may result in a solution or suspension of
the two fluids, depending on the physical and chemical properties of the two
fluids.
In particular, enriching a liquid with a gas (e.g. oxygen) may be beneficial
for certain
62
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
applications, including therapeutic treatments. As
utilized herein, "fluid," may
generally refer to a liquid, a gas, a vapor, a mixture of liquids and/or
gases, a liquid
and/or gas solution, or any combination thereof, for any particular disclosed
embodiment. Furthermore, in certain embodiments a "liquid" may generally refer
to
a pure liquid or may refer to a gel, sol, emulsion, fluid, colloid,
dispersion,
suspension, or mixture, as well as any combination thereof; any of which may
vary in
viscosity.
In particular embodiments, the dissolved gas comprises oxygen. In other
particular embodiments, the dissolved gas comprises nitrogen, carbon dioxide,
carbon monoxide, ozone, sulfur gas, nitrous oxide, nitric oxide, argon,
liquefied
petroleum gas, helium, natural gas, or others.
One particular advantage of embodiments of the present invention relates to
the gas-enriched fluids' long-term diffused gas (particularly oxygen) levels,
which
allows for long-term bio-availability of the gas to cellular or chemical
reactors. The
long-term bio-availability of gasses in the gas-enriched fluids of the present
invention
allow for increased target product production and/or improved enzymatic or
other
chemical reactions that benefit from the gas-enriched fluids (including
oxygenated
media) of the present invention.
In some instances, living cells may be grown in a bioreactor or fermentation
chamber in order to promote cell growth and/or production of the intended
target
product. While some living cells require a mixture of gasses in order to
sustain or
promote their survival or propagation, cell growth may be hindered or ceased
if a
particular gas, such as oxygen, is present at too high of a concentration.
For example, mammalian cells, such as Chinese Hamster Ovary (CHO) cells,
require oxygen in order to proliferate. However, the existing techniques in
the art for
diffusing gasses, such as oxygen, into the bioreactor fluids have a
detrimental effect
on mammalian cell cultures. For example, the cells may be destroyed or
rendered
non-viable in instances where the diffused gas bubbles rupture or coalesce
within
the culture media, which is particularly common at a gas-to-liquid interface.
Accordingly, the present invention represents an advance that would not have
occurred in the ordinary course since the existing knowledge in the art
teaches that
the levels of dissolved gas, particularly the levels of dissolved oxygen, in
the gas-
enriched media disclosed herein is predicted to be harmful or detrimental.
However,
63
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/1JS2007/082578
the gas-enriched fluid media as described herein result in imparting at least
one
beneficial advantage to cell cultures selected from the group consisting of:
enhanced cell growth (e.g. rate and/or number) increased target product yield
(e.g.
amount), increased rate of target product production, improved vehicle cell
viability,
increased efficiency of target product production, increased ease in target
product
purification, and the like. In certain embodiments, one or more of these
beneficial
advantages are conveyed to cell cultures without proving injurious to the
cells
themselves.
In other embodiments, an acellular reaction may utilize the gas-enriched
fluids
and methods of the present invention, including general chemical and/or
enzymatic
reactions. Examples of such reactions include, but are not limited to,
wastewater
treatment, purification of water (such as treating municipal water, home
drinking
purifiers, cleaning swimming pools or aquariums, etc.), homogenization of
milk,
hydrogenation of oils, gas-enriching fuels, and others.
In further embodiments, the gas-enriched fluid maintains a dissolved gas
enrichment level of at least 10 ppm, at least 15 ppm, at least 20 ppm, at
least 25
ppm, at least 30 ppm, at least 35 ppm, at least 40 ppm, at least 45 ppm, at
least 50
ppm, at least 55 ppm, at least 60 ppm, at least 65 ppm, at least 70 ppm, at
least 75
ppm, at least 80 ppm, at least 85 ppm, at least 90 ppm, at least 100 ppm, or
any
value greater or therebetween, at atmospheric pressure. In certain instances,
the
gas-enriched fluid maintains its dissolved gas enrichment level (i.e. the
level of the
gas enriched in the fluid) for a period of at least 10 days, at least 20 days,
at least 30
days, at least 40 days, at least 50 days, at least 60 days, at least 70 days,
at least 80
days, at least 90 days, at least 100 days, at least 110 days, at least 120
days, at
least 130 days, or greater or any value therebetween, within a sealed
container at
atmospheric pressure.
In one particular embodiment, the host material comprises water or water
vapor. In another particular embodiment, the host material comprises other
fluids
(i.e. gasses or liquids) such as wastewater, toxic materials, potable water,
milk, juice,
yogurt, soft drinks (particularly carbonated beverages), ethanol, methanol,
polymers
(such as plastic or rubber compounds), oil (edible or non-edible), emulsions,
suspensions, aqueous carriers, non-aqueous carriers, and the like.
64
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
In certain embodiments, multiple gasses may be used to enrich or infuse a
host fluid. In certain embodiments, ozone and/or oxygen may be used to break
down complex structures into smaller substructures, particularly if used with
sonochemistry techniques, as described herein inter alia.
In certain embodiments, the gas-enriched fluid or other host material of the
present invention has characteristics that may be more similar to the gas that
has
enriched the fluid or other host material, or it may have characteristics that
are more
similar to the fluid (e.g. gas or liquid) or other host material itself.
In certain embodiments, a gas-enriched fluid or solution comprises gas-
enriched culture media. In
particular embodiments, the gas-enriched media
comprises oxygenated or oxygen-enriched media. In certain embodiments, the gas-
enriched fluid or gas-enriched host material may include further processing,
such as
by filtering, separating, modifying or altering various constituents of the
fluid or host
material.
Packaged Gas-Enriched Fluids
Certain embodiments disclosed herein relate to gas-enriched fluids that have
high levels of dissolved or diffused gases (particularly oxygen) that may be
produced
by various methods, including those described herein. In certain embodiments,
the
gas-enriched fluid may be produced in a biomass production facility and
applied
directly to a bioreactor system. Alternatively, the gas-enriched fluid may be
packaged and distributed for use at other locations. In the event that the gas-
enriched fluid is packaged, such packaging may include a sterile package such
as a
glass or plastic container, flexible foil or plastic pouches, sealed boxes
(particularly
waxed boxes), and the like. In the case of sealed packages, the gas-enriched
fluid
may maintain a high level of dissolved or diffused gas for several days,
several
weeks, or several months. In certain embodiments, the sealed container (i.e.
enclosed with a cap, cover or other enclosure that is at least semi-
impermeable to
gas exchange) maintains the diffused nature of the fluid at least 2 weeks, at
least 4
weeks, at least 2 months, at least 4 months, at least 6 months, at least 8
months, at
least 10 months, at least 12 months, or any value greater or therebetween.
Gas-Enriched Fluids in Biological or Chemical Reactors
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
As illustrated in Figures 45A and 45B, a biological or chemical reactor system
3300a may be used for conventional large-scale cell-culturing or chemical
processing to achieve the production of the target product 3318. The target
product
3318 may include, but not be limited to, proteins, antibodies, synthetic
peptides,
active pharmaceutical agents, foodstuff or beverage products (such as wine;
beer;
soft drinks; fruit or vegetable juices); plant products (flowers, cotton,
tobacco, wood,
paper, wood or paper pulp, etc.); ethanol, methanol, paints, fruit or
vegetables or fruit
or vegetable products such as jellies, jams, sauces, pastes, and the like;
cheese or
cheese products; nuts or nut products (such as peanut butter, almond paste,
etc.);
meat or meat products, grain flours or products including bread, cereal,
pasta, and
the like; slurries or mixtures of any of these, processed polymers (including
plastics,
and other polymers), petroleum products, and others.
In certain embodiments, in the case of using a vessel reactor, such as a tank
reactor, the target product resides within inclusion bodies, particularly when
E.coli
cells are utilized. The target product may be obtained by processing the
inclusion
bodies, for example by using high-pressure homogenizers or other techniques.
In particular embodiments in which the reactor is a biological reactor system,
the system 3306 includes a source 3308 of culture cells 3310 to be cultured, a
source 3302 of culture media 3304, a biological reactor 3306, and a harvesting
and
purification system 3316, for producing the target product 3318. The culture
cells
3310 are genetically predetermined to produce proteins or the like that
constitute the
target product 3318, and the culture medium 3304 may comprise a sterile medium
of
a type that provides nourishment for the proliferation of culture cells 3310.
In this
particular exemplary embodiment, the sterile medium 3304 is introduced into
the
internal chamber (which may be referred to as the "fermentation chamber") of
the
reactor 3306 from the source 3302. From the source 3308, the culture cells
3310
are provided such that the cells 3310 and medium 3304 are combined into a
broth
3312 in the fermentation chamber of the bioreactor 3306.
The appropriate base medium 3304 to be utilized in the reactor system 3300a
may be formulated to provide optimal nourishment and growth to the cell
culture
3310. Medium 3304 is preferably a fluid (e.g. liquid or gas) medium, more
preferably
a liquid medium or a solid-liquid medium that is selected based on the certain
variables, such as the characteristics and objectives of the overall
bioreactor system
66
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
3300a, the cost, the type of cells being cultured from the cell culture 3310,
the
desired production parameters, the type of culturing and media management
process used in the reactor3306, the type of downstream harvest and
purification
processes 3316, and the target active pharmaceutical ingredient 3318. Various
cell
culture media presently used may be adapted for use or gas-enrichment by the
present invention.
In certain embodiments, a suitable base medium 3304 may include but not be
limited to a serum-supplemented medium, a hydrolysate medium, chemically-
synthesized medium, chemically-defined medium, a serum-free medium, any
combination of these or other media.
In certain embodiments, the gas-enriched media may be supplemented with
transferrins, albumins, fetuins, protein hydrolysates, or other additives,
preservatives,
nutrients, fillers, shear protectants (such as Pluronic F68), or active or
inactive
agents.
In addition, the medium may be formulated for cells that are attached to some
type of support within the bioreactor 3306, rather than suspended in the broth
3312.
In all embodiments that utilize a medium 3304, the medium 3304 is formulated
to
meet the nutritional requirements of the individual cell type in the cell
culture 3310,
and typically comprise minerals, salts, and sugars.
In certain embodiments, medium 3304 and/or broth 3312 are gas-enriched
using the presently disclosed mixing devices 100, in order to dissolve or
diffuse
gases (such as oxygen) into, for example, the media, both or components
thereof, in
concentrations of at least about 8 ppm, at least about 10 ppm, at least about
20 ppm,
at least about 25 ppm, at least about 30 ppm, at least about 35 ppm, at least
about
40 ppm, at least about 50 ppm, at least about 60 ppm, at least about 70 ppm,
at
least about 80 ppm, at least about 90 ppm, at least about 100 ppm, or any
value
greater or therebetween. In certain embodiments, the gas-enriched medium
and/or
broth contains less than about 160 ppm.
In certain embodiments, the typical biological or chemical reactor is loaded
with sterilized raw materials (nutrients, reactants, etc.) along with air or
specific gas,
as well as cells for a biological reactor. Other agents may be added to the
mixture,
including anti-foaming chemicals or agents, pH controlling agents, and/or
other
agents. The target product is typically recovered by separating the cells,
and/or
67
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
disrupting the cells in order to extract the product, concentrating the
product, and
purifying, drying, or further processing the product.
Many different types of bioreactor systems are in use today, any of which can
be used with the gas-enriched media of the present invention. For example, air-
lift
bioreactors are commonly used with bacteria, yeast and other fungi; fluidized-
bed
bioreactors are commonly used with immobilized bacteria, yeast and other
fungi, as
well as activated sludge; microcarrier bioreactors are commonly used with
mammalian cells immobilized on solid particles; surface tissue propagators are
commonly used with mammalian cells, tissue grown on solid surfaces, and tissue
engineering; membrane bioreactors, hollow fibers and roto-fermentors are
typically
used with bacteria, yeast, mammalian cells, and plant cells; modified stir-
tank
bioreactors are commonly used with immobilized bacteria, yeast, and plant
cells;
modified packed-bed bioreactors are commonly used with immobilized bacteria,
yeast, and other fungi; tower and loop bioreactors are commonly used with
bacteria
and yeast; vacuum and cyclone bioreactors are commonly used with bacteria,
yeast,
and fungi; and photochemical bioreactors are commonly used with photosynthetic
bacteria, algae, cyanobacteria, plant cell culture, and/or DNA plant cells.
Since living cells, including bacteria, yeast, plant cells, mammalian cells,
and
fungal cells require molecular oxygen as an electron acceptor in the
bioxidation of
substrates (such as sugars, fats, and proteins), cell culture media that is
highly
oxygenated is beneficial to the living cells. In a
standard oxidation-reduction
reaction, glucose is oxidized to make carbon dioxide, while oxygen is reduced
to
make water. Molecular oxygen accepts all of the electrons released from the
substrates during aerobic metabolism. Thus, in order to provide the maximum
amount of bio-available oxygen to the growing cells, it is necessary to ensure
that
the oxygen transfer from the air bubbles (gas phase) to the liquid phase
occurs
quickly. When no oxygen accumulates in the liquid phase, the rate of the
oxygen
transfer is the same as the rate of the oxygen uptake by the growing cells.
The oxygen requirements of microorganisms is defined as a standard formula,
that is in units of 002. Where 002 is the mass of oxygen consumed divided by
the
unit weight of dry biomass cells in the bioreactor multiplied by time.
Conversely, the
rate of accumulation of oxygen is equal to the net rate of oxygen supply from
air
bubbles minus the rate of oxygen consumption by cells.
68
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143 PCT/US2007/082578
In addition to a multitude of bioreactor types, each bioreactor may utilize a
particular impeller type or types, such as marine-type propellers, flat-blade
turbines,
disk flat-blade turbines, curved-blade turbines, pitched-blade turbines,
paddles, and
shrouded turbines. The impeller or turbine may create a vortex pattern of
mixing in
the bioreactor, or a uniform mixing.
In certain embodiments, the gas-enriched fluid of the present invention
relates
to a sustained bio-availability of the gas such that a gradual release of the
gas
occurs over time. This gradual or "time" release is beneficial to the
vehicles, such as
cultured cells, particularly when the gas released from the gas-enriched fluid
comprises oxygen. Thus, fermentation, or the biochemical synthesis of organic
compounds by cells 3310, typically involve a relatively fast growth phase,
facilitated
by the concentrations of diffused or dissolved gas in the broth 3312, as well
as by
temperature control and by mixing the medium 3304 and the cell culture 3310 in
the
fermentation chamber of the bioreactor 3306. Particular exemplary embodiments
are depicted in the figures, but may include additional components or tanks.
Mixing
may be enhanced by rotating vanes or the like within bioreactor 3306, and by
reintroduction of fresh and/or freshly re-diffused supplies of medium 3304
from any
of the lines 3332, 3338 or 3334, as described herein inter elle.
In one particular exemplary embodiment depicted in Figure 45A, the
enrichment processing of the medium and/or broth to introduce the gas (e.g.
oxygen)
in a cell culture medium may occur at various points in the system. For
example, the
medium may be gas-enriched prior to introducing the medium 3304 into the
system
3300a at source 3302, or after such introduction at one or more locations "A,"
"B,"
"C," "D," "E" or combinations thereof. Gas-enriched fluid that may be
introduced at
the source 3302, whether enriched at the site of the bioreactor or at a
separate
location. If the gas-enriched fluid is enriched at a separate location, it may
be
transported to the source 3302 in appropriate containers or plumbing.
In certain embodiments, each of the locations "A," "B," and "C," of Figure 45A
represent alternative locations for introduction of a gas-enrichment diffuser
device 1-
within the bioreactor system 3300A. In the event that the introduction occurs
at point
"A," the flow of medium from tank 3304 through the upper section of 3332A of
3332,
the medium may be directed through the gas-enrichment mixer/diffuser device
100
located at position "A," and medium 3304 with dissolved gases therein proceeds
69
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
from the mixer/diffuser device 100 through 3332B and into the fermentation
chamber
of the bioreactor 3306.
With reference to Figure 45B, the medium 3304 from tank 3302 may be
directed through line 3332A into a pump 3410 and, subsequently into the host
material input of the gas-enrichment diffuser device 100. The pump 3410, is
preferably a variable speed pump, which may be controlled by a controller
3390,
based, in part, on pressure readings detected by pressure sensor 3415. While
certain embodiments will utilize manual gauges as pressure detectors, from
which an
operator may manually adjust the speed of the pump 3410, and other components
of
the system 3300a or 3300b, controller 3390 preferably receives an electrical
signal
from sensor 3415 such that controller 3390 will automatically adjust the speed
of
pump 3410. As will be evident from further descriptions herein, the speed of
pump
3410 may also be based on algorithms within the controller 3390 which depend,
in
part, on the state of other components of the system 3400 (such as valves
3420,
3421 and sensors 3425).
Alternatively, the gas-enrichment mixer/diffuser device 100 may be positioned
at location "B" such that the medium 3304 is processed together with medium
3310.
In this particular case, cells 3310 and medium 3304 are mixed in flow using a
conventional mixing nozzle and subsequently introduced into the mixer/diffuser
device 100, where beneficial gases are infused into the mixed liquid of medium
3304
and cells 3310. The resulting gas-enriched medium is then directed into the
fermentation tank of the bioreactor 3306.
As shown in Figure 45A, cells 3310 may be combined with medium 3304, and
following fermentation and/or development of the target product, the contents
of the
bioreactor 3306 may then be directed through line 3336 to a harvesting and
purification stage. Once purified, the target product is directed through line
3339 to a
target production tank 3318.
With reference to Figure 45B, in certain embodiments, the gas-enrichment
mixer/diffuser device 100 combines the flow of a medium 3304 with a flow of a
gas
from line 3426. Preferably, the gas to be combined with medium 3304 flows from
an
oxygen tank 3450 and is metered by a valve 3420, which is controlled by
controller
3390.
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
In certain embodiments, the gas-enrichment mixer/diffuser device 100 is
directed through line 3332b directly into the fermentation tank by a reactor
3306.
Alternatively, the gas-enriched fluid may be directed through line 3332b to
another
blending.
With reference to Figure 45A, a bioreactor system 3300a, may include an
additional system (such as a perfusion system) 3314 that begins processing the
broth from the bioreactor 3306. During the perfusion process 3314, the medium
3304 is continuously added to the broth 3312 to nourish the cell culture 3310,
which
is then mixed throughout the broth 3312. Simultaneously, cell or other waste
may be
continuously removed from the broth 3312, typically at the same rate as new
medium 3304 is added. As indicated herein above, gas-enrichment may also occur
at positions "D" or "E," or at both positions "D" and "E."
The perfusion system can allow for removal of cellular waste and debris, as
well as the target product, while retaining the cells in the bioreactor 3306.
The
perfusion system thus reduces waste accumulation and nutrient fluctuations,
thereby
allowing for higher cell density and productivity. Retention of the cells in
the
bioreactor may be achieved through various methods, including centrifugation,
internal or external spin filters, hollow fiber modules, cross-flow
filtration, depth
filtration, any combination of these or other means. In other embodiments, the
accumulation of waste products may be regulated by use of a glutamine
synthetase
expression system.
With reference to Figure 46, particular exemplary embodiments utilize multiple
gas sources 3502 and 3504 as shown, such that the nature of the gas being
diffused
into the broth 3312 can be changed depending on the stage of fermentation
within
the bioreactor 3306. Hence, in a preferred embodiment, the cell culture medium
is
enriched with oxygen during the proliferative phase of fermentation.
Subsequently,
carbon dioxide, nitrous oxide, or another gas may be substituted to facilitate
other
stages of the fermentation process, particularly with processes that vary from
aerobic
to anaerobic.
The bioreactor may comprise an airlift reactor, a packed bed reactor, a
fibrous
bed reactor, a membrane reactor, a two-chamber reactor, a stirred-tank
reactor, a
hollow-fiber reactor, or other reactor designed to support suspended or
immobilized
cell growth.
71
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
In one particular embodiment, the bioreactor 3306 is a continuous stirred-tank
reactor, comprising heat exchange and refrigeration capabilities, sensors,
controllers, and/or a control system to monitor and control the environmental
conditions within the fermentation chamber. Monitored and controlled
conditions
may include gas (e.g. air, oxygen, nitrogen, carbon dioxide, nitrous oxide,
nitric
oxide, sulfur gas, carbon monoxide, hydrogen, argon, helium, flow rates,
temperature, pH, dissolved oxygen levels, agitation speed, circulation rate,
and
others. Additionally, the bioreactor 3306 may further comprise Cleaning-in-
Place
(CIP) or Sterilization-in-Place (SIP) systems, which may be cleaned and/or
sterilized
without assembly or disassembly of the units.
In one particular embodiment, the bioreactor 3306 performs a continuous
fermentation cycle, continuously adding medium 3304 to the fermentation system
with a balancing withdrawal, or harvest, of the broth 3312 for target product
extraction.
In alternate embodiments, the bioreactor 3306 may perform batch
fermentation cycles, fed-batch fermentation cycles, or fed-batch fermentation
cycles
with the gas-enriched fluids. Typically, batch fermentation cycles¨in which
all of the
reactants are loaded simultaneously-- are used for small scale operations or
for the
manufacture of expensive products or for processes that may be difficult to
convert
into continuous operations. In a typical process, the broth is fermented for a
defined
period to completion, without further additions of the medium. The
concentration
varies with time, but is typically uniform at any one particular time point.
Agitation
serves to mix separate feed lines as well as enhance heat transfer.
For batch fermentation, typically the total mass of each batch is fixed, each
batch is a closed system, and the reaction or residence time for all reactants
of the
medium is the same. After discharging the batch, the fermentation chamber is
cleaned and re-started with the medium 3304 for another batch cycle.
Separation or
purification of the desired product from the other constituents in the harvest
broth
3312, may include further processing, including refolding, altering affinity,
ion
exchange purification, alteration of hydrophobic interactions, gel filtration
chromatography, ultra filtration and/or diafiltration, depending on the target
product.
For fed-batch fermentation, typically an initial, partial charge or aliquot of
medium 3304 is added to the fermentation chamber, and subsequently inoculated
72
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
with cell culture 3304. The medium 3304 may be added at measured rates during
the remainder of the fermentation cycle. The cell mass and the broth 3312 are
typically harvested only at the end of the cycle.
Following harvest and purification of the target product (step 3316),
(typically
once the cell culture 3310 has attained a peak cell growth density within the
bioreactor 3306), the purified product 3318 (in some cases, a pharmaceutical
drug or
Active Pharmaceutical Ingredient, or API) is attained. The purified product
may then
be processed as desired and optionally packaged in appropriate containers
during a
sterile packaging process 3322 for transfer to a pharmaceutical manufacturing
plant,
or other facility. The purified product may then be used for any desired
purpose,
including for prevention, treatment, and/or diagnosis of disease.
Plants and Animals as Reactors
In addition, a reactor may include a plant or animal, which is used to
generate
a plant or animal product, or recombinant product. In certain embodiments, the
plant
or animal target product may be a naturally occurring product (e.g., food
bearing
crops or meat, or textile-related products such as cotton fibers, etc.), or
the target
product may be a genetically altered product (for example, therapeutic agents,
such
human growth hormone or insulin or other biologically active proteins and
polypeptides). A genetically altered or recombinant product may be produced by
a
transgenic or genetically altered plant, animal, or combination thereof.
Fish Culture
Fish (e.g., Tilapia fish) may be grown in aquaculture for food, or as a
transgenic vehicle for production of a target product. The preferred
temperature
range for optimum tilapia growth is 82 -86 F. Growth diminishes significantly
at
temperatures below 68 F and death will typically occur below 50 F. Also, at
temperatures below about 54 F, the immune resistance of tilapia declines and
the
animals are easily subjected to infection by bacteria, fungi, and parasites.
Twenty years ago, aquaculture researchers in Nigeria attempted to correlate
dissolved oxygen concentrations in pond waiter with Tilapia growth rates. UN
FAO
reports: The study was conducted by examining growth rates of young Tialapia
at
high dissolved oxygen levels (approximately 7.0 ppm); at mid-level DO
73
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
(approximately 3.5 ppm); and at low DO levels (less than 2 ppm). The growth
rates
were determined by measuring the weight of the fish. The final increase in
weight at
the end of the research was 19 grams for the high DO level fish; 5 grams for
the mid-
level DO fish; and 1.5 g for the low DO level fish. This represents to a 74%
and 92%
reduction in growth rates correlating to the DO levels. Thus, as the DO levels
decrease, the feeding and waste output also decrease. It was observed that the
Tilapia in the low DO level water break the surface of the water in order to
access
ambient oxygen required for survival.
The gas-enriched fluids of the present invention further include oxygenated
freshwater supplies in which the high dissolved oxygen levels in the water are
maintained for extended periods of time. According to particular aspects,
using the
diffuser device of the present invention in an aquaculture setting, dissolved
oxygen
levels of over 35 ppm can be recorded in 103 F water without significantly
stressing
the aquatic life.
Plant Growth
In addition to animal growth, the gas-enriched fluids of the present invention
may be utilized for plant growth and development. Gases (such as oxygen) are
required for plant root respiration, which allows for the release of energy
for growth,
as well as water and mineral uptake. Plant
growth has been widely and
unequivocally proven to be boosted by maintaining high gas (e.g. oxygen and/or
nitrogen) levels within the root zone. In this regard, increasing gas delivery
to plant
root systems represents a potential for crop improvement through boosting root
activity. Likewise, in embodiments in which transgenic plants are grown,
increasing
gas delivery to the plants may provide for increased production of the target
product
(such as a therapeutic or biopharmaceutical product).
Hydroponic crops represent one exemplary system for production which may
greatly benefit from the gas-enrichment diffuser devices of the present
invention
through direct gas-enrichment (e.g. oxygenation) of the nutrient solution
bathing the
root zone. Hydroponic crops are typically produced in a limited volume of
growing
media or root area and as such need constant replacement of gases (e.g.
oxygen)
within the root zone. Hydroponic crops such as lettuce, spinach, tomatoes, and
cucumbers have already demonstrated a direct and significant response to the
gas-
74
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
enriched nutrient solution. Some of these responses include increases in plant
growth, increases in root volume, increases in plant yield, and higher quality
produce. Thus, hydroponic systems may benefit from the gas-enriched fluids of
the
present invention.
Other hydroponic crops have had similar responses to gas-enrichment in the
root zone. However, at warm temperatures, crop production declines due to the
increased requirement for gases (such as oxygen) in the root zone. Thus,
enrichment is effective for preventing gas-starvation of root cells, as well
as boosting
growth under less than favorable growing conditions.
Typically tropical crops that are able to be grown at high densities due to
high
light levels and rapid rates of development (and high root zone temperatures)
have a
gas requirement that is many times greater than those grown in more temperate
climates. Thus, gas-enrichment will become necessary in many systems of
horticulture production. Highly populated countries, which rely heavily on
producing
intensive horticultural crops for income and sustenance from very limited
areas of
land, will benefit greatly from this technology.
Soil-based cropping systems can also benefit from the gas-enriched solutions
of the present invention. Many crops are fed via drip, trickle, or furrow
irrigation and
could potentially benefit greatly from the use of gas-enriched irrigation
water or
fertigation solutions. Such
crops include, but are not limited to: vegetables
(tomatoes, salad crops such as lettuce, herbs, cucurbits), cut flowers,
ornamental
flowers, turf, vineyards, orchards, and long-term plantings. Gases, such as
oxygen,
can directly impact the health and growth of the plant but can also act
indirectly by
increasing the bio-availability of gases (e.g. oxygen) at the root zone, and
can also
improve the health of the plant by promoting microbial life in the soil.
With regard to the microbial life in the soil, the microbial populations are
essential for mineral conversion in the soil and organic systems and overall
plant
health through suppression of plant diseases. While these microbes are
beneficial
and often essential for crop production, the populations also require gases
(e.g.
oxygen), which can compete with the gases for plant root cells. Thus,
supplying
gases (e.g. oxygen) to the plant roots in order to enable microbial life to
flourish is
vital to both organically grown crops, as well as standard growing conditions.
High
rates of gases supplied to the growing media/soil in organic systems would
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
potentially speed up the rate of organic fertilizer conversion and
mineralization of
plant usable nutrients, thus increasing the health and productivity of highly
profitable
organic crops.
In addition, the available land for growing crops represents a challenge in
many countries with limited resources or unsuitable soils.
In addition to hydroponic crops, the technology disclosed herein may apply to
seed germination, seed raising, cell transplant production, propagation from
cuttings,
sprout production, animal fodder production, soil based cropping, turf
industries,
ornamental plants, and medicinal plants.
Systems for Making Gas-Enriched Fluids
As shown here, exemplary oxygenation systems comprises a supply or
reservoir of fluid which is drawn up and circulated through tubing or other
conduits by
a pump which subsequently delivers the fluid to the mixer/diffuser. The
mixer/diffuser
may be of any number of various embodiments including those set forth and
described herein above. These diffusers significantly increase the amount of
dissolved gas (e.g.oxygen) present in a fluid by introducing, for example,
gaseous
oxygen to the fluid using a diffuser having coaxial cylindrical or frusto
conical stator
and rotor components rotating discs or plates within a housing, Mazzie
diffusers and
impellers to create the desired cavitation and succussion desired for mixing
of the
fluid and the gas. It should be noted that many of the fluids will be aqueous
or water-
based, but that the present invention is not limited to these.
The diffuser is supplied with fluid by the pump and combines this with, for
example, gaseous oxygen from supply and returns the oxygenated (or otherwise
gas-enriched) fluid to the reservoir. The diffuser may employ any number of
possible embodiments for achieving diffusion including, but not limited to,
micro-
membrane, Mazzie injector, fine bubble, vortexing, electromolecular
activation, or
other methods. The oxygen supply may be either a cylinder of compressed oxygen
gas or a system for generating oxygen gas from the air or other chemical
components. The oxygenated fluid produced by the diffuser is returned to the
reservoir and may be recirculated through the pump and/or the diffuser again
to
further increase the dissolved oxygen content. Alternatively, the fluid may be
drawn
off using the oxygenated fluid outlet. Oxygenated fluids which are drawn off
through
76
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
,
WO 2008/052143
PCT/US2007/082578
the outlet may be immediately put to use in various applications or may be
packaged for later use.
The packaging step may enclose gas-enriched (e.g. oxygenated) fluids in a
variety of bottles, bags or other containers formed of plastic, metal, glass,
or other
suitable materials. Although the gas-enriched or oxygenated fluids produced in
accordance with the present invention have a relatively long shelf life under
atmospheric conditions, the shelf life may be further extended by using
packaging
which hermetically seals the gas-enriched fluid. In this manner, dissolved
oxygen
which works its way out of the fluid during storage will form a pressure head
above
the gas-enriched fluid and help to prevent the migration of dissolved oxygen,
or other
gas, out of the fluid and back into the atmosphere. In one preferred
embodiment of
the present invention the gas-enriched fluid is packaged in an air tight
container and
the void space is filled with the gas used for enrichment at a pressure of
greater than
one atmosphere prior to sealing the container. The packaging step may be used
to
produce bottles, bags, pouches, or other suitable containers for holding
oxygenated
solutions.
The presently disclosed systems and/or methods allow oxygen, or other
gases, to be dissolved stably at a high concentration with minimal passive
loss.
These systems and/or methods can be effectively used to dissolve a wide
variety of
gases at heightened percentages into a wide variety of fluids. By way of
example
only, a deionized water at room temperature that typically has levels of about
7-9
ppm (parts per million) of dissolved oxygen can achieve levels of dissolved
oxygen
ranging from about 8-70 ppm using the disclosed systems and/or methods. In
accordance with a particular exemplary embodiment, an oxygenated water or
saline
solution may be generated with levels of about 30-60 ppm of dissolved oxygen.
Culturing Chinese Hamster Ovary Cells
Chinese Hamster Ovary (CHO) cells are mammalian cells that are frequently
utilized in expression and production of recombinant proteins, particularly
for those
that require post-translational modification to express full biological
function.
According to particular aspects, various characteristics of CHO cells can be
improved by integrating either a gas-enriching diffuser device 100 or gas-
enriched
media produced by the device 100 and integrated into a CHO bioreactor.
77
DWT 2157375v1 0083535-000053
CA 02881274 2015-08-20
WO 2008/052143 PCIIUS2007/082578
According to particular aspects, in the cultivation of CHO cells, it is
possible to
utilize the gas-enriched fluids or media of the present invention including
with a cell-
line specific, serum-free medium (for example from SAFC Biosciences, Inc.) for
long-
term growth of transformed CHO cells. According to additional aspects, CHO
cells
are not harmed by passing through the gas-enrichment diffuser device in the
process
of gas-enriching fluids (including media).
A test was conducted that measured the survival of CHO cells in an inline
bioreactor. Briefly, the inline bioreactor was used with 2 L of CHO media, and
CHO
cells at a density of 106 or higher. The bioreactor was run for approximately
10
minutes (including the gas-enriching diffuser), and a 25 mL sample was
removed.
Cells were stained with 0.4% Trypan Blue, and cell viability was assessed with
a
hemacytometer. According to this measure, CHO cells were not significantly
harmed
by passing through the gas-enrichment diffuser device in the process of gas-
enriching fluids (including media).
The foregoing described embodiments depict different components contained
within, or connected with, different other components. It is to be understood
that
such depicted architectures are merely exemplary, and that in fact many other
architectures can be implemented which achieve the same functionality. In a
conceptual sense, any arrangement of components to achieve the same
functionality
is effectively "associated" such that the desired functionality is achieved.
Hence, any
two components herein combined to achieve a particular functionality can be
seen
as "associated with" each other such that the desired functionality is
achieved,
irrespective of architectures or intermedial components.
Likewise, any two
components so associated can also be viewed as being "operably connected," or
"operably coupled," to each other to achieve the desired functionality.
While particular embodiments of the present invention have been shown and
described, it will be obvious to those skilled in the art that, based upon the
teachings
herein, changes and modifications may be made without departing from this
invention and its broader aspects and, therefore, the appended claims are to
encompass within their scope all such changes and modifications as are within
the
scope of this invention. Furthermore, it is to be understood that the
invention is solely defined by the appended claims. It will be understood by
those
within the art that, in general, terms used herein, and especially in the
appended
78
DWT 2157375v1 0083535-000053
CA 02881274 2015-02-05
WO 2008/052143 PCT/US2007/082578
claims (e.g., bodies of the appended claims) are generally intended as "open"
terms
(e.g., the term "including" should be interpreted as "including but not
limited to," the
term "having" should be interpreted as "having at least," the term "includes"
should
be interpreted as "includes but is not limited to," etc.). It will be further
understood
by those within the art that if a specific number of an introduced claim
recitation is
intended, such an intent will be explicitly recited in the claim, and in the
absence of
such recitation no such intent is present. For example, as an aid to
understanding,
the following appended claims may contain usage of the introductory phrases
"at
least one" and "one or more" to introduce claim recitations. However, the use
of
such phrases should not be construed to imply that the introduction of a claim
recitation by the indefinite articles "a" or "an" limits any particular claim
containing
such introduced claim recitation to inventions containing only one such
recitation,
even when the same claim includes the introductory phrases "one or more" or
"at
least one" and indefinite articles such as "a" or "an" (e.g., "a" and/or "an"
should
typically be interpreted to mean "at least one" or "one or more"). the same
holds true
for the use of definite articles used to introduce claim recitations. In
addition, even if
a specific number of an introduced claim recitation is explicitly recited,
those skilled
in the art will recognize that such recitation should typically be interpreted
to mean at
least the recited number (e.g., the bare recitation of "two recitations,"
without other
modifiers, typically means at least two recitations, or two or more
recitations).
Accordingly, the invention is not limited except as by the appended claims.
79
DWT 2157375v1 0083535-000053