Note: Descriptions are shown in the official language in which they were submitted.
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
Apparatus and Methods for Storage and Transfer of Patient Information Using
Biological Sample Cards with Short Range Communications
Background
Newborn infants are commonly screened shortly after birth for a variety of
conditions
via blood testing, particularly using Dried Blood Spot (DBS) cards. Newborn
screening
programs are often implemented on a national scale, and they target treatable
conditions with
the goal of detecting diseases before irreversible damage is done. Typically,
newborn
screening requires blood samples to be taken from a newborn and sent to one of
four hundred
screening laboratories worldwide for processing. Newborn screening programs
collect blood
samples using a specialized filter paper on a Dried Blood Spot (DBS) card.
Blood samples
are typically collected by pricking a newborn baby's heel and placing the
blood samples on
the filter paper portion of the DBS card.
Blood samples may be collected at a variety of times and under a variety of
conditions. For example, in the United States, the blood samples are usually
collected from
the newborn at a medical facility, such as a hospital or doctor's office,
twenty-four to forty-
eight hours after birth. In other countries, such as the United Kingdom, blood
samples are
collected five to eight days after birth. The later samples, may be collected
by midwives or
other medical staff at a location other than a medical facility. The medical
staff collecting the
sample, in these circumstances, may differ from the medical staff attending to
the birth and/or
follow up care of the infant. Thus, the medical facility associated with care
of the infant may
fail to be alerted regarding collection of the sample.
The filter paper used for collection of the sample is often attached to a form
on which
patient information has been entered (e.g., typed, applied by a label, or most
often
handwritten). The filter paper portion and the form portion make up the "blood
card". The
types of blood cards used may vary between hospitals, medical facilities,
and/or regions. For
1
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
example, the arrangement of the information, the particular fields presented
on the blood
card, and/or the labeling of the fields (e.g., names provided for
identification of particular
information, font, font size, etc.) vary based upon the regional rules and
regulations.
The information entered on the blood card is later entered into the computer
system of
a testing laboratory and, often, a hospital, through a manual or semi-
automatic (e.g., optical
character recognition (OCR) followed by staff review) process. Due to
variations in blood
cards, the transfer of information between the blood card and the computer
system can be
prone to difficulties and/or errors. For example, in the event that
handwritten information is
unclear or otherwise difficult to read, errors can result when transferring
the information to
the computer system. If entered separately into both a hospital system and a
laboratory
system, errors can be replicated or a mismatch in patient data can occur.
Oftentimes, a number of samples are collected during a particular period of
time (e.g.,
one day, one shift, etc.), and the batch of samples are then sent to a testing
laboratory for
processing. Upon receipt of the blood card, the testing laboratory may review
the portions
containing patient data (e.g., manually, semi-automatically, OCR, etc.) to
compare to an
inventory list provided by the medical facility. The portions containing
patient data are
provided for entry into the computer system, along with the unique identifier.
At the point of testing, the unique identifier on the filter paper portion may
be scanned
or otherwise entered into the computer system to be associated with the
entered patient data.
Based upon the associated patient data, the computer system can determine one
or more
required tests. Oftentimes, numerous factors derived from the patient
information are
considered in determining which tests to conduct. These factors may include
demographic
information as well as the present state of the infant (e.g., whether the baby
is sick,
premature, using steroids, or using antibiotics).
2
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
The tests conducted may further be based on whether this is the first
screening of the
infant or a repeat screening. In entering patient data into the computer
system, for example,
the testing laboratory may identify the blood sample as being linked to
previous testing (e.g.,
abnormal test results or an unusable sample). Linking of a current sample to a
previously
received sample may be hindered, in some cases, due to discrepancies in
information. For
example, a misspelling may have occurred previously when entering the name of
the infant,
or the infant's name may have changed between the date of birth (e.g. "Baby
Jones") and the
date of collection of the follow up sample (e.g., "Candace Jones"). In this
circumstance,
there is the possibility that proper tests will not be performed due to lack
of recognition of the
patient.
There are a number of inefficiencies associated with current methods and
apparatus
for obtaining and testing biological samples, particularly those involving
blood cards. There
is a need for improved efficiency in obtaining biological samples and
conducting tests on
those samples.
Summary
Described herein are methods and apparatus for storing and transferring
patient
information related to biological sample testing using biological sample cards
and short range
communications. In certain embodiments, the disclosed technology provides for
the
localized data transfer with biological sample cards equipped with a
transmission and storage
device that is capable of storing data such as patient information so that
errors associated with
manually entering handwritten patient information are reduced. The
transmission and storage
device may include a radio frequency identification (RFID) tag/chip such as a
near field
communication (NFC) tag/chip. In some examples, the biological samples include
blood
samples for newborn infant screening, cellular samples for genetics screening,
blood/urine
3
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
samples for drug use screening, or tissue samples for disease screening.
Patient information
is collected and associated with the biological sample. In certain
embodiments, the disclosed
technology reduces labor and improves efficiency and accuracy related to data
entry.
Data may be transferred, in certain embodiments, to or from these biological
sample
cards when the biological sample cards are brought within a specified range of
a computing
device with short range communication capability. In other embodiments, data
may be
transferred to or from these biological sample cards when the biological
sample cards are
tapped against the computing device with short range communication capability.
Data
transfer, in some examples, involves the use of a software application
executing upon a
handheld computing device. For example, a software application for downloading
patient
data to the transmission and storage device of a biological sample card may
include a user
interface for inputting patient data and a routine for interfacing with a
short range
communications feature of a computing device, such as an RFID reader/writer, a
Bluetooth
communicator, or a WiFiTM communicator.
The disclosed technology, in certain embodiments, provides a network
environment
for sharing information between a medical facility and a testing laboratory.
Information,
including patient and sample collection information, may be associated with a
unique
identifier and stored on the network. In some implementations, when a party
provides the
unique identifier to a data center on the network environment, the party is
provided with
information associated with the unique identifier they provided to the data
center. For
example, the testing laboratory may obtain the unique identifier from a
biological sample
card and send the unique identifier to the data center. In response, the data
center may
provide information associated with that unique identifier to the testing
laboratory. The
testing laboratory may use the information to determine what tests to run on a
biological
sample associated with the unique identifier. In certain embodiments, the
testing laboratory
4
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
may also provide status alerts and updates to the data center using the unique
identifier and
the data center can relay these alerts to the medical facility or appropriate
contact.
The biological sample cards, in certain embodiments, include a machine-
readable
indicia. In some implementations, the machine-readable indicia is presented on
a biological
sample card for stably storing the biological sample collected from a patient.
The machine-
readable indicia may include data related to a medical facility, a type of
biological sample,
the unique identifier, and/or sample collection data. Medical facilities and
screening
laboratories may obtain the unique identifier for a given biological sample
card by scanning
the machine-readable indicia.
One feature of the disclosed technology, in certain embodiments, is the
storage of
encrypted or secure patient information on the transmission and storage
device. Moreover,
securing or encrypting the data stored on the biological sample cards reduces
legal and
privacy concerns related to patient information.
The disclosed technology provides a user-friendly method and apparatus that
can
reduce errors and shorten intake time when retrieving patient data associated
with a
biological sample, thus increasing the efficiency of the sample testing
process. Once the
biological sample card is loaded with data such as patient information and
biological samples
have been collected and stably contained therein, the biological sample card
is sent to a
testing laboratory. In certain embodiments, when the testing laboratory
receives the
biological sample card, laboratory staff members may electronically transfer
the data from
the biological sample card to the laboratory computer system without having to
manually
enter the data or read a handwritten biological sample card. As such, this
reduces errors
associated with reading and transcribing handwritten information.
Additionally, in some
implementations, the medical facility collecting the biological sample may
upload the patient
data to a computer system associated with the medical facility and/or download
patient data
5
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
from a computer system associated with the medical facility to the
transmission and storage
device of the biological sample card. Thus, both the laboratory computer
system and the
medical facility computer system may maintain identical patient identification
data.
The disclosed technology, in some implementations, improves communication
between hospitals and screening laboratories. For example, the hospital
computer system
may use the information shared between the hospital and testing laboratory to
track the status
of a biological sample card and any tests associated with the biological
sample card. Status
updates may include whether the sample was received, whether the sample has
been taken,
whether the sample is abnormal, what tests are being performed, the tests
results, and follow-
up activities related to the patient's diagnosis.
In certain embodiments, the disclosed technology also provides medical
facilities
information regarding collection of biological samples such as newborn infant
screening
samples. When the newborn blood sample is collected outside of the hospital or
other
medical facility (e.g., home birth, midwife visit, etc.), for example, the
medical facility may
more accurately monitor when the sample has been collected and the
identification of the
staff member who collected the sample.
Furthermore, the disclosed technology can aid in reducing retest delay that
can occur
between the point at which a testing laboratory determines that a new sample
needs to be
obtained prior to further testing and the collection of the follow-up samples.
For example, a
medical facility may be quickly alerted when an unusable sample has been
provided or when
abnormal results require follow-on testing through automated status updates.
Upon receipt of
a follow-on biological sample, in some implementations, the patient data
electronically
obtained from the transmission and storage device of the follow-on biological
sample card
can be automatically compared to existing patient data in the laboratory
computer system to
link re-testing samples with previous test information. In another example,
retest information
6
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
can be provided within the patient data stored within the transmission and
storage device of
the biological sample card.
In certain embodiments, the testing laboratory may use a biological sample
punching
device to extract blood samples from the filter paper of a biological sample
card. Reading
apparatus for obtaining information from the transmission and storage device
of a biological
sample card, for example, may be located adjacent to or built into the
biological sample
punching device, biological sample testing instrument, or testing laboratory
computing
device. Blood cards may be punched differently depending on the patient
information. A
processor can obtain patient data from the transmission and storage device of
the blood card
and provide punching instructions to either the operator of the biological
sample punching
device or the setup control system of the biological sample punching device.
The punching
instructions, for example, may be based on one or more factors derived from
the patient
information.
In one aspect, the invention is directed to a biological sample card
including: one or
more collection regions, each configured to stably contain a biological
sample; and a
transmission and storage device including: an antenna configured for short
range
communication, and a computer-readable medium, wherein the transmission and
storage
device is configured to: receive patient data transmitted to the antenna,
store the patient data
in the computer-readable medium, and transmit the patient data via the antenna
responsive to
a query signal.
In certain embodiments, the biological sample card further includes a barcode,
wherein the transmission and storage device is programmed with a barcode
identifier
corresponding to the barcode. In certain embodiments, the transmission and
storage device is
further configured to encrypt the patient data. In certain embodiments, the
one or more
collection regions includes filter paper configured to absorb the biological
sample, wherein
7
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
the biological sample includes a blood sample. In certain embodiments, the
patient data is
stored in XML format. In certain embodiments, transmitting the patient data
includes
encrypting the patient data.
In certain embodiments, the biological sample card includes a perforation
configured
to allow separation of a first portion of the biological sample card from a
second portion of
the biological sample card. In certain embodiments, the biological sample card
includes a
first barcode presented upon the first portion and a second barcode presented
upon the second
portion, wherein the first barcode is identical to the second barcode; and the
transmission and
storage device is programmed with a barcode identifier corresponding to the
first barcode. In
certain embodiments, the first portion includes the one or more collection
regions and the
transmission and storage device. In certain embodiments, the second portion
includes one or
more entry fields, wherein each of the entry fields is labeled to accept text
information,
wherein the text information includes a non-electronic rendering of at least a
portion of the
patient data.
In certain embodiments, the transmission and storage device includes an RFID
tag. In
certain embodiments, the transmission and storage device includes an NFC tag.
In another aspect, the invention is directed to a method including: obtaining
patient
data; initiating, by a processor of a computing device, communication with a
transmission
and storage device contained within or upon a biological sample card, wherein
the
transmission and storage device is configured for short range communication;
transmitting,
via an antenna of the computing device, the patient data from the computing
device to the
transmission and storage device, wherein the antenna is configured for short
range
communication; and verifying, by the processor of the computing device,
success of
transmission of the patient data.
8
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In certain embodiments, the computing device is a handheld computing device.
In
certain embodiments, obtaining patient data includes receiving input manually
entered on the
computing device. In certain embodiments, obtaining patient data includes
remotely
receiving at least a portion of the patient data from a patient database
system. In certain
embodiments, obtaining patient data includes scanning a computer-readable
indicia with a
scanner of the computing device. In certain embodiments, initiating
communication with the
transmission and storage device includes tapping the biological sample card to
the computing
device. In certain embodiments, the transmission and storage device includes
an RFID tag.
In certain embodiments, the transmission and storage device includes an NFC
tag.
In certain embodiments, the method includes, prior to transmitting the patient
data,
encoding the patient data in XML format. In certain embodiments, the method
includes,
prior to transmitting the patient data, applying a security algorithm to the
patient data. In
certain embodiments, verifying success of transmission includes presenting, on
a display
region of the computing device, at least a portion of the patient data for
review and
verification. In certain embodiments, verifying success of transmission
includes at least one
of: delivering an audible signal via the computing device; delivering a visual
signal on a
display of the computing device; and delivering a tactile or haptic signal via
the computing
device.
In certain embodiments, the method further includes providing at least a
portion of the
patient data for storage in a patient database system, wherein a remote
computing device
includes the patient database system. In certain embodiments, the method
further includes
providing the at least the portion of the patient data for storage in the
patient database system,
determining, by the processor, whether export criteria is met, wherein the
export criteria
includes at least one of a threshold number of patients and a time period.
9
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In certain embodiments, the biological sample card includes a blood card for
newborn
screening; and the patient data includes patient birth date, patient birth
time, patient birth
weight, patient gender, patient ethnic code, and sample collection date. In
certain
embodiments, obtaining the patient data includes: presenting one or more data
entry fields
related to at least one of national and regional newborn screening information
requirements;
and receiving user input related to each data entry field of the one or more
data entry fields.
In certain embodiments, obtaining the patient data includes associating a new
blood sample
with stored patient data. In certain embodiments, the patient data further
includes retest
information.
In another aspect, the invention is directed to a system including: a
processor; an
antenna, wherein the antenna is configured for short range communication; and
a non-
transitory computer-readable medium storing instructions thereon wherein the
instructions,
when executed, cause the processor to: initiate communication with a
transmission and
storage device, wherein the transmission and storage device is configured for
short range
communication, and a biological sample card includes the transmission and
storage device,
obtain, via the antenna, patient data from the transmission and storage
device, and evaluate
the patient data to identify at least one laboratory test to perform on a
biological sample,
wherein the biological sample card includes the biological sample.
In certain embodiments, evaluating the patient data includes identifying at
least one of
an ethnicity, an age, a sex, an indication of premature birth, an indication
of feeding type, a
medication indication, and a steroid indication. In certain embodiments, the
instructions,
when executed, cause the processor to: identify, from the patient data, a
patient identifier; and
reference a patient database to identify stored patient data associated with
the patient
identifier. In certain embodiments, evaluating the patient data includes
identifying, from the
stored patient data, prior test results or prior attempted testing. In certain
embodiments, the
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
instructions, when executed, cause the processor to identify one or more
discrepancies
between the patient data and the stored patient data. In certain embodiments,
the instructions,
when executed, cause the processor to determine a punching pattern for
punching the
biological sample card, wherein the punching pattern is based in part upon one
or more of the
at least one laboratory test. In certain embodiments, the system further
includes a punching
apparatus, wherein the instructions, when executed, cause the processor to
cause the
punching apparatus to punch the biological sample card in the punching
pattern.
In certain embodiments, the instructions, when executed, cause the processor
to store,
to a patient database, the patient information. In certain embodiments, the
instructions, when
executed, cause the processor to store, to the patient database, a status
indicator, wherein the
status indicator includes an indication regarding at least one of sample
received, sample
unusable, sample abnormal, sample testing ongoing, sample testing completed,
and additional
sample required. In certain embodiments, the instructions, when executed,
cause the
processor to store, to the patient database, one or more test results, wherein
the one or more
test results are associated, within the patient database, with the patient
data. In certain
embodiments, a separate computing device includes the patient database.
In another aspect, the invention is directed to a non-transitory computer-
readable
medium, wherein the computer-readable medium stores instructions that, when
executed by a
processor, cause the processor to: cause the presentation of a user interface
for authentication
of a user; receive a user identifier corresponding to the user; authenticate
the user identifier;
associate user biographic information with the user identifier; obtain patient
information
regarding a patient, wherein a biological sample is being collected from the
patient onto a
biological sample card including a transmission and storage device configured
for short range
communication; determine sample collection information, wherein the sample
collection
information includes at least a portion of the user biographic information and
one or more of
11
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
a date, timestamp, and location; receive a write request; initiate
communication with a
transmission and storage device; write the patient information and the sample
information to
the transmission and storage device; and verify successful transfer of the
patient information
and the biographic information to the transmission and storage device.
In certain embodiments, the user identifier includes biometric data. In
certain
embodiments, receiving the user identifier includes collecting fingerprint
data corresponding
to a fingerprint of the user, wherein the biometric data includes the
fingerprint data. In
certain embodiments, the instructions, when executed, further cause the
processor to: collect,
from a geolocation feature of a computing device, a current location, wherein
the location is
based on the current location. In certain embodiments, the instructions, when
executed,
further cause the processor to determine an address based upon the current
location, wherein
the location includes the address. In certain embodiments, the instructions,
when executed,
further cause the processor to collect fingerprint data corresponding to a
fingerprint of the
patient, wherein the patient information includes the fingerprint data. In
certain
embodiments, the instructions, when executed, further cause the processor to
collect image
data corresponding to a photograph of the patient, wherein the patient
information includes
the photograph. In certain embodiments, the instructions, when executed,
further cause the
processor to determine a current time, wherein the timestamp includes the
current time. In
certain embodiments, the user biographic information includes at least one of
a name and a
unique employee identifier. In certain embodiments, the instructions, when
executed, further
cause the processor to: obtain healthcare facility information, wherein the
healthcare facility
information includes two or more of a name of a health care professional, a
telephone
number, an address, a hospital name, a screening laboratory name, and a
screening laboratory
telephone number; and write the healthcare facility information to the
transmission and
storage device. In certain embodiments, the instructions, when executed,
further cause the
12
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
processor to provide inventory information regarding the biological sample to
a computer
system of a screening laboratory.
In another aspect, the invention is directed to a method including: scanning,
by a
scanner feature of a computing device, machine-readable indicia, wherein the
machine-
readable indicia is presented upon a biological sample card for stably storing
a biological
sample collected from a patient, and the machine-readable indicia includes a
unique
identifier; obtaining patient information regarding the patient; determining,
by a processor of
the computing device, sample collection data, wherein the sample collection
data includes
one or more of a date, timestamp, and location; providing, to a second
computing device via a
network, the patient data, the sample collection data, and the unique
identifier, wherein the
second computing device is configured to: store, associated with the unique
identifier, the
patient data and the sample collection data, and provide, to a third computing
device,
responsive to the third computing device providing the unique identifier, at
least a portion of
the patient data and the sample collection data.
In certain embodiments, the machine-readable indicia includes at least one of
a
barcode, two-dimensional barcode, three-dimensional barcode, QR code, and
matrix barcode.
In certain embodiments, the machine-readable indicia, when translated,
includes card data
related to at least one of a medical facility and a type of biological sample.
In certain
embodiments, the sample collection data includes the card data. In certain
embodiments, a
screening laboratory includes the second computing device.
In certain embodiments, the method further includes: obtaining healthcare
facility
information, wherein the healthcare facility information includes two or more
of a name of a
health care professional, a telephone number, an address, a hospital name, a
screening
laboratory name, and a screening laboratory telephone number, and the sample
collection
data includes the healthcare facility information. In certain embodiments, the
computing
13
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
device is a mobile computing device. In certain embodiments, the biological
sample card
includes a first portion and a second portion, wherein a perforation is
positioned between the
first portion and the second portion, the first portion includes the machine-
readable indicia,
and the second portion includes a copy of the machine-readable indicia.
In another aspect, the invention is directed to a method including: receiving,
via a
network, patient data, sample collection data, and a unique identifier,
wherein the patient data
includes demographic information regarding a patient, wherein a biological
sample collected
from a patient is stably stored on a biological sample card marked with the
unique identifier,
and the sample collection data includes one or more of a date, a timestamp,
and location;
storing, by a processor of a computing device, associated with the unique
identifier, the
patient data and sample collection data; receiving, via the network, a request
including the
unique identifier; and responsive to the request, providing, to a second
computing device
associated with the request, at least a portion of the patient data and the
sample collection
data.
In certain embodiments, the method further includes identifying, based upon a
portion
of the patient data, second sample collection data; and associating the second
sample
collection data with the sample collection data. In certain embodiments,
providing the
portion of the patient data and the sample collection data includes providing
at least a portion
of the second sample collection data. In certain embodiments, the method
further includes
providing, to a third computing device of a screening laboratory, via the
network, inventory
information regarding the biological sample. In certain embodiments, the
sample collection
data includes information regarding the screening laboratory.
In certain embodiments, the method further includes: receiving, from a third
computing device of a screening laboratory, via the network, an alert
regarding the biological
sample; and responsive to receiving the alert, providing a message to one or
more contacts
14
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
regarding the alert, wherein the sample collection data includes the contacts.
In certain
embodiments, the alert includes at least one of an unusable sample, an
availability of
screening results, and an indication of abnormal results. In certain
embodiments, providing
the message includes one or more of an email, a text message, and a pre-
recorded voice mail
message. In certain embodiments, the contacts include one or more of a primary
care
physician, a hospital, and a midwife. In certain embodiments, the method
further includes:
receiving, from the third computing device, via the network, a set of
screening results; and
associating, by the processor, with the unique identifier, the screening
results.
Brief Description of the Fi2ures
The foregoing and other objects, aspects, features, and advantages of the
present
disclosure will become more apparent and better understood by referring to the
following
description taken in conjunction with the accompanying drawings, in which:
FIG. 1 illustrates an example of a biological sample card equipped with a
biological
sample card transmission and storage device for storing patient information;
FIG. 2 illustrates an example system for transferring data between a computing
device
and a biological sample card equipped with a biological sample card
transmission and storage
device;
FIG. 3 is a flow chart describing an example method for localized transfer of
patient
information to a biological sample card equipped with a biological sample card
transmission
and storage device;
FIG. 4 is a flow chart describing an example method for receipt of patient
information
and localized transfer of the patient information to hospital software;
FIG. 5 is a flow chart describing an example method for localized transfer of
patient
information to or from a biological sample card;
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
FIG. 6 is a flow chart describing an example method for exporting patient
information
to or from a remote application;
FIG. 7 is a flow chart describing an example method for transferring patient
information from a biological sample card to a testing laboratory computing
device;
FIG. 8 illustrates an example of a biological sample card that has received
both valid
and invalid biological samples;
FIGS. 9A through 9D illustrate a series of example graphical user interfaces
for
configuring an application for communication with a biological sample card
containing a
biological sample card transmission and storage device;
FIG. 10 illustrates an example of a cloud computing environment for sharing
information related to biological sample testing;
FIG. 11 illustrates a flow chart describing an example method for collecting
and
transmitting information related to a biological sample;
FIG. 12 illustrates a flow chart describing an example method for receiving
and
transmitting information related to a biological sample;
FIG. 13 illustrates a flow chart describing an example method for receiving
and
transmitting information related to a biological sample;
FIG. 14 illustrates a chart describing an example method for communicating
data
regarding a biological sample to and from a data center;
FIG. 15 is a block diagram of an example network environment for communicating
items with a biological sample card transmission and storage device; and
FIG. 16 is a block diagram of a computing device and a mobile computing
device.
The features and advantages of the present disclosure will become more
apparent
from the detailed description set forth below when taken in conjunction with
the drawings, in
16
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
which like reference characters identify corresponding elements throughout. In
the drawings,
like reference numbers generally indicate identical, functionally similar,
and/or structurally
similar elements.
Detailed Description
In some implementations, a device including a short range communications
device
and support, such as radio-frequency identification (RFID) or near field
communication
(NFC) protocols, may be used to initiate communication with one or more
biological sample
cards.
The device, in some implementations, includes a portable computing device
equipped
with the short range communication device, such as, in some examples, a smart
phone,
handheld multimedia entertainment device, personal computer, personal digital
assistant,
tablet computer, notebook computer, or laptop computer. In some
implementations, the short
range communications device is an RFID reader and/or writer, a NFC reader
and/or writer, or
an RF, Bluetooth or WiFiTM transmitter and/or receiver.
The biological sample cards, in some implementations, include a machine-
readable
indicia. The machine-readable indicia may include a barcode, two-dimensional
barcode,
three-dimensional barcode, QR code, and/or matrix barcode. In some
implementations, the
machine-readable indicia is presented on a biological sample card for stably
storing the
biological sample collected from a patient. In some implementations the
machine-readable
indicia includes a unique identifier, data related to a medical facility, a
type of biological
sample, and/or sample collection data.
The biological sample cards, in some implementations, include a biological
sample
card transmission and storage device. Biological sample card transmission and
storage
devices may be passive mode communication devices (e.g., lacking a local power
source)
such as a passive radio transponder or a passive RFID or NFC tag. In some
implementations,
17
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
the biological sample card transmission and storage devices include semi-
passive or active
mode communication devices, such as a battery powered or battery backed up
RFID tag, or a
WiFiTM or Bluetooth transponder. In some examples, active mode communication
devices
include twisted antenna coils, laser embedded technology, or other micro
active tag
technology.
The biological sample card transmission and storage device, in some
implementations, is built into the biological sample card. In some
implementations,
biological sample card transmission and storage devices are applied onto the
packaging of the
biological sample cards using adhesive material.
The biological sample card transmission and storage devices, in some
implementations, include an RFID tag. An RFID tag, in some implementations,
includes an
integrated circuit for storing and processing information, modulating and
demodulating a RF
signal, and other specialized functions. In some implementations, the RFID tag
is an NFC
tag.
An RFID tag, in some implementations, includes an antenna for receiving and
transmitting an RF signal. In some examples, an RFID tag may include an RF
transmitter
including a built in power supply (e.g., battery, etc.) to provide operating
power. In other
examples, an RFID tag may be "field powered", obtaining operating power by
rectifying an
RF signal. In some implementations, the biological sample card transmission
and storage
devices include either passive RFID tags or semi-passive RFID tags which are
provided with
an alternating current (AC) signal.
In some implementations, the biological sample card transmission and storage
devices
include one or more features designed to enhance the security of transmission
between the
biological sample card transmission and storage device and the computing
device. In some
implementations, the biological sample card transmission and storage device
may be
18
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
embedded with one or more encryption features, e.g., an encryption mechanism,
such that the
computing device and the biological sample card transmission and storage
device
communicate through an encrypted transmission. In some implementations, the
biological
sample card transmission and storage device may be embedded with a unique
identifier that
may be included within transmission (e.g., in a packet header). A software
application
executing on the computing device, further to this example, may be designed to
recognize a
particular series or type of identification string. In some implementations,
patient
information may be encrypted by applying a security algorithm to the patient
information,
also known as patient data. In some implementations, the information stored on
the
biological sample card transmission and storage device is compressed.
Throughout the description, where apparatus and systems are described as
having,
including, or comprising specific components, or where processes and methods
are described
as having, including, or comprising specific steps, it is contemplated that,
additionally, there
are apparatus, and systems of the present invention that consist essentially
of, or consist of,
the recited components, and that there are processes and methods according to
the present
invention that consist essentially of, or consist of, the recited processing
steps.
It should be understood that the order of steps or order for performing
certain action is
immaterial so long as the invention remains operable. Moreover, two or more
steps or
actions may be conducted simultaneously.
FIG. 1 illustrates an example of a biological sample card 100 used for newborn
testing. Biological sample cards may be blood sample cards, blood spot cards,
as well as, in
some examples, biological sample cards for collecting cellular samples for
genetics
screening, blood/urine samples for drug use screening, or tissue samples for
disease
screening. In some implementations, biological sample cards are paper cards,
plastic cards,
cardboard cards, glass fiber cards, or they may be made of any other workable
material. In
19
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
certain alternative embodiments, rather than cards, biological samples are
collected and
contained in storage vials, serum sample containers, and/or other storage
media, which have
an attached or embedded RFID/NFC tag, for example.
In some implementations, the biological sample card 100 is used for newborn
screening. Newborns are screened shortly after birth for a variety of
conditions using blood
testing. Typically, newborn screening requires blood samples to be taken from
a newborn
and sent to one of four hundred screening laboratories worldwide for
processing. Blood
samples may be collected by pricking a newborn baby's heel and placing the
blood samples
on a filter paper. The biological sample card 100 may include a portion 112
that is made of
filter paper specifically designed to absorb the blood. Portion 112 may
include collection
regions 106 on the filter paper where the blood samples may be applied.
In some implementations, portion 114 of the biological sample card 100 is
filled out
with patient information, sample collection information, and/or a unique
identifier using entry
fields. The entry fields may be configured to receive text information. In
some
implementations, the text information is a non-electric rendering of at least
a portion of the
patient data. Patient information may include: patient identification number,
patient first
name, patient last name, patient address, patient birth date, patient birth
time, patient birth
weight, patient gender, patient ethnic code, repeat sample, the patient's
parental information,
or whether the patient has had a blood transfusion, received steroids,
received antibiotics,
whether the patient was born prematurely, whether the baby is sick, how the
baby is feeding,
whether the parents provided consent to testing, and/or whether the parents
consent to using
the specimen for research.
In some implementations, the patient information includes biometric data such
as
fingerprint data or data such as photos of the patient, pictures of the blood
spots, or pictures
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
of the biological sample card. Biometric data may include haptic data, image
data, and/or
tactile data.
In some implementations, the portion 114 of the biological sample card 100 is
filled
out with sample collection information. The sample collection information may
include a
date stamp, time stamp, information regarding the device used to collect
information,
information about the submitter of the information such as a name and/or
unique employee
identifier, and/or location information relating to when and where the
biological sample was
collected. In some implementations, the sample collection information includes
information
regarding the testing laboratory such as email information, phone number
information, fax
information, other contact information, and/or a user ID for logging into the
data center, a
web portal, or a software application to access relevant data for testing. In
some
implementations, the sample collection information includes contact
information for the
entity who submitted the biological sample for testing, such as a hospital or
other medical
facility. The contact information may include one or more phone numbers, email
addresses,
and/or fax numbers for contacting the relevant party or parties at the entity
who submitted the
biological sample for testing. The contact information may be for a primary
care physician,
hospital, and/or midwife.
In some implementations, the sample collection information includes healthcare
facility information such as a name of a healthcare professional, a telephone
number, an
address, a hospital name, a testing laboratory name, and/or a testing
laboratory telephone
number.
In some implementations, the patient information, sample collection
information,
and/or unique identifier are loaded into a software application using a
scanner or optical
character recognition tool. The patient information, sample collection
information, and/or
unique identifier may be loaded into a software application manually by a user
who reads the
21
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
information on the biological sample card and enters the information into the
software
program. In some implementations, patient information, sample collection
information,
and/or unique identifier are entered directly into a software application
using a graphical user
interface operating on a laptop, personal computer, smart phone, handheld
multimedia
entertainment device, personal digital assistant, or tablet computer as
explained in reference
to FIG. 9D. In such implementations, portion 114 of the biological sample card
100 may be
omitted from the biological sample card 100 altogether. In some
implementations, the
sample collection data is generated by a computing device such as computing
device 202 as
shown in FIG. 2. In some implementations, at least a portion of the patient
information,
sample collection information, and/or unique identifier is received from a
patient database
system through a medical facility network. In some implementations, at least
the patient
information, sample collection information, and/or unique identifier is
received by scanning a
machine-readable indicia with a scanner of a computing device (e.g. wristband
worn by the
patient or a barcode located near the patient, on a medical chart, or hospital
bed). The data
captured from the machine-readable indicia may include the unique identifier.
The unique
identifier may be used to locate patient information associated with the
unique identifier that
is stored on the medical facility network. The software application may be
connected to the
medical facility network and may receive the patient information after
providing the unique
identifier to the medical facility network.
After the patient information, sample collection information, and/or unique
identifier
are loaded into the software program, the information may be transferred to
the biological
sample card 100 via a transmission and storage device. In some
implementations, the
transmission and storage device is a biological sample card transmission and
storage device
108. In some implementations, the biological sample card transmission and
storage device
includes an antenna configured for short range communication and a computer-
readable
22
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
medium. The biological sample card transmission and storage device may be
configured to
receive patient information, sample collection information, and/or the unique
identifier
transmitted to the antenna, store the information in a computer-readable
medium, and
transmit the information via the antenna in response to a query signal. In
some
implementations, to transfer data to the biological sample card 100 via the
biological sample
card transmission and storage device 108, the biological sample card 100 is
brought within
close proximity of a short range communication device, or vice versa. In some
implementations, the biological sample card 100 may be tapped against the
short range
communication device, or vice versa.
Biological sample card transmission and storage devices may be passive mode
communication devices (e.g., lacking a local power source) such as a passive
radio
transponder or a passive RFID or NFC tag. In some implementations, the
biological sample
card transmission and storage devices include semi-passive or active mode
communication
devices, such as a battery powered or battery backed up RFID tag, or a WiFiTM
or
Bluetooth transponder. In some examples, active mode communication devices
include
twisted antenna coils, laser embedded technology, or other micro active tag
technology.
In some implementations, the biological sample card transmission and storage
device
contains unique barcode information or a barcode identifier. The barcode
information may
be the barcode information associated with the machine-readable indicia 104.
In some
implementations, the biological sample card 100 does not contain a machine-
readable indicia,
however, the biological sample card transmission and storage device 108 may
contain the
unique identifier.
The biological sample card transmission and storage device 108 may be attached
to
the surface of the biological sample card 100 or built into the structure of
the packaging of
the biological sample card 100. In some implementations, the biological sample
card
23
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
transmission and storage device 108 is printed or applied to the biological
sample card 100
during manufacturing. In some other implementations, the biological sample
card
transmission and storage device 108 is printed or applied to the biological
sample card by the
hospital. In some implementations, application of the biological sample card
transmission
and storage device 108 involves printing or applying a label using an
adhesive. In a
particular example, an adhesive biological sample card transmission and
storage device 108
(e.g., a "sticker") may be purchased by a user and applied to a biological
sample card 100 for
use in transferring data from the biological sample card 100 via the software
application 108.
In some implementations, the biological sample card transmission and storage
device
108 is located on portion 112 of the biological sample card 100. In some
implementations,
the biological sample card transmission and storage device 108 is built into
the machine-
readable indicia 104 (e.g., a barcode label, matrix barcode label, Quick
Response (QR) code
label, etc.) of biological sample card 100. The machine-readable indicia 104,
in some
implementations, is optional and may be provided as a back-up for
identification of the
biological sample card 100. In some implementations, machine-readable indicia
104 is
embedded in the biological sample card transmission and storage device 108.
In some implementations, the biological sample card 100 is constructed using a
three
layer structure. The filter paper may be located between two layers, such as
two slotted
cardboard layers. In some implementations, the biological sample card
transmission and
storage device 108 is situated in the middle layer between the two layers. For
example, the
filter paper and the biological sample card transmission and storage device
108 may be
situated between two pieces of slotted cardboard. This configuration may
protect the
biological sample card transmission and storage device 108 against mechanical
damage and
loosening. Additionally, the look, feel, and printings of the biological
sample card 100 could
be exactly the same as it has traditionally been.
24
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
Additionally, biological sample card 100 may include a perforated edge 110. In
such
cases, the biological sample card may be separated along the perforated edge
110 and only
portion 112 of the biological sample card 100 is sent to the testing
laboratory. The hospital
may keep portion 114 of the biological sample card 100 or properly dispose of
it. The
hospital may be required to separate portions 112 and 114 of the biological
sample card 100
before sending the information to the testing laboratory for privacy reasons.
In some
implementations, the entire biological sample card 100 is sent to the testing
laboratory. In
some implementations, as discussed above, the biological sample card 100
consists of portion
112 and does not include portion 114 and/or the perforation 110.
In some implementations, both portions 112 and 114 of the biological sample
card
include the machine-readable indicia 104. The perforation 110 may split the
machine-
readable indicia 104 in half or the machine-readable indicia 104 may be
provided on each
portion 112 and 114 of the biological sample card 100 such that when the
biological sample
card 100 is split along the perforation 110, each portion 112 and 114 contains
a copy of the
machine-readable indicia 104.
When the testing laboratory receives the biological sample card 100, the
testing
laboratory enters the patient information, sample collection information,
and/or unique
identifier into their computer system. In some implementations, the testing
laboratory
manually enters the patient information, sample collection information, and/or
unique
identifier into their computer based on the portion 114 of the biological
sample card 100. In
some implementations, the testing laboratory receives portions 112 and 114 of
a biological
sample card 100 separately. In such cases, the testing laboratory may manually
enter the
information on portion 114 along with the unique identifier of the machine-
readable indicia
104 into their computer system. Before testing, the section of the machine-
readable indicia
104 remaining on portion 112 may be scanned. The testing laboratory computer
system can
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
then instruct the user what tests need to be done based on the patient
information and/or
sample collection information associated with that unique identifier
In some implementations, the biological sample card 100 is tapped against a
short
range communication device to transfer the patient information, sample
collection
information, and/or unique identifier stored in biological sample card
transmission and
storage device 108 to the testing laboratory. In some implementations, the
biological sample
card 100 is brought within a specified distance of the short range
communication device to
transfer the information from the biological sample card 100 to the testing
laboratory
computer system. In these implementations, patient information, sample
collection
information, and/or unique identifier are inputted to the testing laboratory
computer system
without hand entering information that is read from a handwritten form.
In some implementations, multiple biological sample cards may be read at once.
The
testing laboratory may read all biological sample card samples in a given
area. For example,
the testing laboratory may bring all biological sample cards delivered on a
day into a certain
area and all of these biological sample cards may be read at the same time.
After reading the
biological sample cards, the testing laboratory may compare the biological
sample cards
received to an inventory list. In some implementations, the inventory list may
be an
electronic file or list that the testing laboratory receives from one or more
hospitals or other
entities submitting the biological sample cards. The electronic file or list
may be shipped
with the biological sample cards or sent via a network or accessed from a
server. In some
implementations, the testing laboratory manually enters a list into their
computer system
before the comparison can occur.
In some implementations, the testing laboratory may be equipped with
intelligent
shelving to locate individual specimens in a screening lab. Intelligent
shelving may also be
used for determining which samples have been received and comparing which
samples were
26
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
received with the inventory list. In some implementations, intelligent
shelving is used to
automatically provide status updates to the medical facility. For example,
when a biological
sample card is initially received and placed within a specified range of the
intelligent shelf,
the testing laboratory computer system may update the status of the biological
sample card
100 to a "received" status. Similar methods may be used to identify when a
sample is being
tested, when the tests are complete, or to identify other steps completed by
the testing
laboratory during newborn screening.
After receiving the patient information, sample collection information, and/or
unique
identifier into their computer system, the testing laboratory determines
whether certain tests
are to be conducted based on the information on the biological sample card
100. These
factors include, in some examples, demographic information, where the baby is
born, whether
the baby is sick, premature, using steroids, using antibiotics, and/or whether
this is a repeat
test. The testing laboratory also determines whether the blood sample is
linked to previous
test results (e.g. previous unusable or abnormal test results). In some
implementations, the
computer system of the testing laboratory will identify a patient from the
patient information,
sample collection information, and/or unique identifier and reference a
patient database to
identify stored information associated with the patient identified. Stored
information includes
prior test results or prior attempted testing data. In some implementations,
the computer
system of the testing laboratory identifies one or more discrepancies between
the patient
information and the stored patient information.
Once the testing laboratory determines which tests to conduct, a biological
sample
punching device may be used to extract the collection regions 106 from the
biological sample
card 100 and process the samples for testing. Biological sample punching
devices enable
screening laboratories to processor multiple biological sample cards
simultaneously.
Additionally, biological sample punching devices are capable of punching
samples
27
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
differently depending on the quality of the sample, the tests being performed,
and the patient
information and/or sample collection information of a specific biological
sample. The
computer system can read the biological sample card transmission and storage
device 108, in
some implementations, and instruct the biological sample punching device
directly how to
punch the biological sample card 100 based on patient information and/or
sample collection
information stored in the biological sample card transmission and storage
device 108. The
biological sample punching device may determine a punching pattern based in
part on, in
some implementations, the quality of the sample, the tests being performed,
and the patient
information of the specific blood sample. In some implementations, the blood
samples are
extracted from portion 112 manually and the computer system may provide
instructions to
the lab technician regarding how to punch the biological sample card 100.
In implementations where a biological sample punching device or a biological
testing
instrument is used, a short range communication device may be located next to
the biological
sample punching device or the biological testing instrument. In some
implementations, a
short range communication device is integrated, either internally or
externally, with the
biological sample punching device and/or biological testing instrument.
Locating a short
range communication device near the biological sample punching device and/or
biological
testing instrument may reduce any error that occurs between reading the
biological sample
card transmission and storage device 108 and punching the biological sample
card 100. In
some implementations, there may be multiple short range communication devices
in the
testing laboratory.
After the filter paper is punched by the biological sample punching device or
manually separated by a lab technician, the testing laboratory may begin
conducting tests on
the blood sample. As described in reference to FIG. 2, the testing laboratory
may input the
test status and/or results into a software application such as Specimen Gate
Screening
28
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
CenterTM by PerkinElmer of Waltham, Massachusetts. A hospital may be provided
access to
this information so that hospital staff can monitor the status of tests and/or
receive test results
quickly and efficiently.
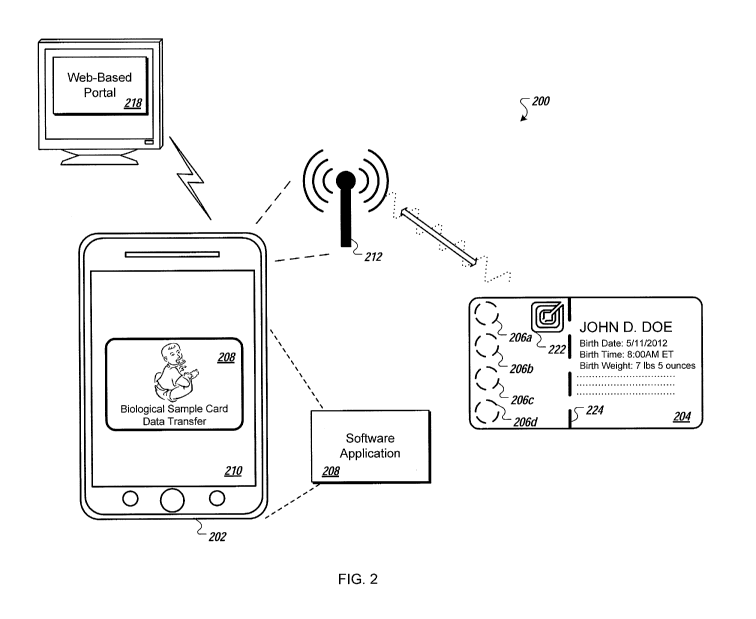
FIG. 2 illustrates an example system 200 for localized data transfer with
biological
sample cards. In some implementations, the system 200 for transferring data to
and from a
biological sample card 204 includes a computing device 202 and the biological
sample card
204 with a biological sample card transmission and storage device 222. The
computing
device 202, as illustrated, may be an electronic device equipped with a short
range
communication device such as, in some examples, a laptop, personal computer,
smart phone,
handheld multimedia entertainment device, personal digital assistant, or
tablet computer. In
some implementations, the short range communications device is an RFID reader
and/or
writer, a NFC reader and/or writer, RF, Bluetooth or WiFiTM transmitter
and/or receiver.
In some implementations, device 202 is a biological sample punching device or
in
communication with the biological sample punching device.
The computing device 202, in some implementations, executes a software
application
208 (e.g., "Biological sample card Data Transfer", as illustrated in a display
210 of the
computing device 202). In some implementations, the software may cause the
presentation
of a user interface for authentication of a user. The user may input a user
identifier
corresponding to the user that can be authenticated by the software to confirm
the identity of
the user. After authentication, the software may associate user biographical
information with
the user identifier. In some implementations, the user identifier is a
username and password,
voiceprint, fingerprint, and/or scan of an employee badge. In some
implementations,
biographical information is a name, contact information such as email, phone
number,
address, employee number, supervisor, and/or medical facility.
29
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
As discussed in reference to FIGS. 9A through 9D below, the software
application
208 may receive patient information, sample collection information, and/or
unique identifier.
The patient information may be stored locally on computing device 202 or on a
network.
Patient information may include: patient identification number, patient first
name, patient last
name, patient address, patient birth date, patient birth time, patient birth
weight, patient
gender, patient ethnic code, sample collection date, sample collection time,
repeat sample, the
patient's parental information, or whether the patient has had a blood
transfusion, received
steroids, received antibiotics, whether the patient was born prematurely,
whether the baby is
sick, how the baby is feeding, the requestor code/name, requestor phone, the
sample collector
name, whether the parents provided consent to testing, and/or whether the
parents consent to
using the specimen for research.
Sample collection information includes at least a portion of the user
biographical
information, a date stamp, time stamp, type of sample, whether the sample is
for an original
test or a retest, the purpose of the sample, information regarding the device
used to collect
information, information about the submitter of patient information such as a
name and/or
unique employee identifier, and/or location information relating to when and
where the
biological sample was collected. In some implementations, the sample
collection information
includes information regarding the testing laboratory such as email
information, phone
number information, fax information, and/or other contact information. In some
implementations, the sample collection information includes contact
information for the
entity who submitted the biological sample for testing, such as a hospital or
other medical
facility. The contact information may include one or more phone numbers, email
addresses,
and/or fax numbers for contacting the relevant party or parties at the entity
who submitted the
biological sample for testing. The contact information may be for a primary
care physician,
hospital, and/or midwife.
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In some implementations, the sample collection information includes healthcare
facility information such as a name of a healthcare professional, a telephone
number, an
address, a hospital name, a testing laboratory name, and/or a testing
laboratory telephone
number.
After receiving patient information, sample collection information, and/or
unique
identifier, the software may transfer the data to the biological sample card
204 via antenna
212 of a short range communication device. The software application 208, in
some
implementations, controls an antenna 212 (e.g., an internal or external
antenna for operation
in a short range communication frequency band) for communication with the
biological
sample card transmission and storage device 222, preferably within a near
field range. In
some implementations, the antenna 212 of the short range communication device
is placed
near the biological sample punching device of the testing laboratory as
discussed above in
reference to FIG. 1.
In some implementations, to transfer data to the biological sample card 204
via the
biological sample card transmission and storage device 222, the computing
device 202 first
establishes a connection with the biological sample card transmission and
storage device 222.
To establish the connection and transfer data with the biological sample card
transmission
and storage device 222, in some implementations, the computing device 202 is
brought
within close proximity of the biological sample card transmission and storage
device 222, or
vice versa. For example, in the circumstance of a NFC tag, the computing
device 202 or
biological sample card 204 may be brought within a range of about ten
centimeters or fewer
for connection purposes. In some implementations, the software application 208
and/or the
computing device 202 provides an indication regarding successful data transfer
(e.g., audible
tone, graphic message, visual cue, blinking lights, tactile feedback, or
haptic feedback, etc.).
31
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In some implementations, after successful data transfer, the software
application
clears the patient information, sample collection information, and/or unique
identifier and
displays the data entry screen and waits for further user action.
In some implementations, the biological sample card 204 is preloaded with the
unique
identifier. In some implementations, the biological sample card 204 may be
loaded with the
unique identifier when the patient information is transferred onto the
biological sample card
204.
In some implementations, the software application 208 includes an export
feature for
transferring patient information, sample collection information, and/or the
unique identifier
stored on the biological sample card 204 to another software application
located locally or on
a network. Examples of such a software program include Specimen Gate ,
eReportsTM, or
LabworksTM Laboratory Information Management System, all by PerkinElmer of
Waltham,
Massachusetts. For example, patient information sent to another software
application, in
some implementations, can then be accessed from a number of locations.
Additionally, the
status of the biological sample card 204 may be monitored so that the medical
facility or user
of the other software application can receive status updates from a testing
laboratory that
receives the biological sample card and associated biological samples. Status
updates may
include whether the sample was received, whether the sample is usable, whether
the testing is
ongoing, whether the testing is complete, whether additional samples are
required, whether
the sample is abnormal, what tests are being performed, the test results, and
follow-up
activities related to the patient's diagnosis. The status information may be
stored in a patient
database that is located locally, on a network, server, or remote storage
device. An example
procedure for transferring patient information, sample collection information,
and/or the
unique identifier to another software application is described in relation to
FIG. 6. In some
implementations, the information is not transferred to another software
application. A
32
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
transfer to the medical facility computer system may involve a secure web
connection or a
web service. Alternatively or additionally, it could involve a hard wire data
transfer.
In some implementations, the software application 208 facilitates a connection
to a
web-based portal 218 for accessing, exporting, or importing information
regarding biological
sample cards. For example, the web-based portal 218 may allow a user to
configure or
manage the information associated with one or more biological sample cards. In
some
implementations, the web-based portal 218 includes patient and status
information regarding
the biological sample card 204.
In some implementations, the biological sample card 204 includes regions 206
on
filter paper where the biological samples may be applied.
In some implementations, the biological sample card 204 may include a
perforated
edge 224. The perforated edge 224 may provide one or more utilities such as
those described
in relation to perforation 110 of FIG. 1 above.
FIG. 3 illustrates a flow chart describing an example method 300 for localized
transfer
of data to a biological sample card. The method 300, for example, may be
performed by the
processor of a computing device such as computing device 202 described in
relation to FIG.
2. For example, one or more steps of method 300 may be performed by software
application
208.
The method 300 begins, in some implementations, when a sample is going to be
collected from a patient (302). When a biological sample is going to be
collected,
information is entered into a computing device (304). The computing device may
be an
electronic device equipped with an short range communication device such as,
in some
examples, a personal computer, laptop, smart phone, handheld multimedia
entertainment
device, personal digital assistant, or tablet computer.
33
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In some implementations, a portion of the data may be captured by scanning an
item
associated with the patient, such as a barcode or RFID tag incorporated in a
wristband worn
by the patient or a tag located near the patient (e.g. a tag on a medical
chart or hospital bed).
The data may be captured by a computing device using a barcode scanner or
short range
communication device. In some implementations, information is derived via a
query of a
medical facility record system to obtain a portion of the patient record.
For example, if blood is being collected by a midwife, the midwife may use a
portable
electronic device like a tablet computer or smart phone running an appropriate
software
application. The midwife would begin by entering the patient information,
sample collection
information, and/or unique identifier into the software application. It should
be appreciated
that the midwife could be performing the sample collection (302) in a hospital
or medical
care facility, in a patient's home, or in any other location. Additionally,
sample collection
(302) could be performed by any doctor, nurse, or other medical personnel.
In some implementations, once data is entered onto a computing device (304),
the
data may be transferred onto a biological sample card (306). In some
implementations, data
is transferred when a user brings the biological sample card within a
threshold distance of a
short range communication device coupled to the computing device. In some
implementations, the data is transferred when the biological sample card is
tapped against the
computing device equipped with the short range communication device. The data
includes at
least a portion of the data entered onto the computing device during step
(304). In some
implementations, the biological sample card is brought within a specified
range of a short
range communication device.
Transferring data onto the biological sample card may begin, in some
implementations, with receiving a request to communicate with a biological
sample card
transmission and storage device located on or in the biological sample card.
In some
34
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
implementations, a user submits a request to the computing device to open a
communication
channel with the biological sample card transmission and storage device. The
user may
request opening the communication channel with the biological sample card
transmission and
storage device using a graphical user interface of a software application
executing upon the
computing device.
In some implementations, the data is stored on the biological sample card in
extensible markup language (XML), comma separated variable format, proprietary
format,
data format, or text format.
In some implementations, after transferring the data to the biological sample
card, a
validation procedure is performed to verify the transfer occurred properly
(308). The
validation procedure verifies that the information was copied properly. For
example, if the
operation is a write operation, the processor can read at least a portion of
the information
from the biological sample card and compare this information with the
information it wrote to
the biological sample card. In some implementations, the validation procedure
ensures that
the data transferred to the biological sample card complies with the business
rules specified
by a testing laboratory or other party.
In some implementations, after transferring the data and/or validating the
data, a
biological sample is obtained from the patient (310). Biological samples taken
from the
patient are applied to the biological sample card storing the patient's
information. After this
process is complete, the biological sample card and associated biological
samples may be
sent to the lab for testing (312).
FIG. 4 illustrates a flow chart describing an example method 400 for localized
transfer
of data to a biological sample card. The method 400, for example, may be
performed by the
processor of a computing device such as computing device 202 described in
relation to FIG.
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
2. For example, one or more steps of method 400 may be performed by software
application
208.
In some implementations, a user logs into a software application (402) to
begin
method 400. The software application may include security measure options such
as
requiring the user to enter a user name and password or enter a secure
identification code.
After a user logs into the software, in some implementations, a data entry
screen is displayed
(404). The data entry screen may be customer specific. Different hospitals
and/or countries
utilize biological sample cards with different information. Therefore, the
data entry screen
may be customized to display the appropriate information input regions. Using
the data entry
screen, patient information, sample collection information, and/or unique
identifier may be
entered into the computing device running the software application (406). In
some
implementations, the processor receives data entered during the check-in
procedure at a
hospital. For example, based on partial data entry (e.g. date of birth and
last name), one or
more fields auto-populate. This eliminates the need to reenter the data.
In some implementations, at least a portion of the data may be captured by
scanning
an item associated with the patient, such as a barcode or an RFID tag that is
incorporated in a
wristband worn by the patient or a tag located near the patient (e.g. a tag on
a medical chart
or hospital bed). The data may be captured by a computing device using a bar
scanner or
short range communication device.
After the processor receives patient information, sample collection
information,
and/or unique identifier, the processor determines whether the data is valid
(408). The
processor verifies that the data entered conforms with established business
rules. For
example, the patient information may be validated with regard to hospital
records such as
validating the patient's birthdate or other patient information based on the
hospital records. If
the validation process fails, the system may prompt the user to correct the
patient
36
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
information. This process could involve determining whether required patient
information
was inputted into the computing device during step (406). If the data is not
valid, the
processor returns to step (406). If the data is valid, the processor may store
the data locally
(410).
In some implementations, the patient information, sample collection
information,
and/or unique identifier are stored in a local memory location accessible to
the computing
device (410). The memory location, in some implementations, includes a network-
accessible
storage area. In this manner, for example, the computing device may be
damaged, replaced,
and/or power cycled without losing the stored information. In some
implementations, a
portion of the information is shared with a separate device. For example,
through a web
portal or through a network-accessible storage location, a separate device
such as a user
personal computer may access the stored information. In some implementations,
the data is
not stored locally. The data may be stored temporarily and transferred on a
batch or periodic
schedule. In some implementations, if the network is not available, a queue
will be created
and the data will be stored in the queue until the network is available. When
the network
returns, the data in the queue can be uploaded one by one based on the order
in the queue or
in a batch.
In some implementations, the processor sends the patient information, sample
collection information, and/or unique identifier to another software
application located
locally or on a network. Examples of such a software program include Specimen
Gate ,
eReports, or LabworksTM Laboratory Information Management System, all by
PerkinElmer
of Waltham, Massachusetts. Patient information, sample collection information,
and/or
unique identifier sent to the other software application can then be accessed
from a number of
locations. Additionally, the status of the biological sample card may be
monitored so that the
hospital or user of the other software application can receive status updates
from a testing
37
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
laboratory that receives the biological sample card and associated biological
samples. Status
updates can include whether the sample was received, whether the sample is
abnormal, what
tests are being performed, and the tests results. An example procedure for
transferring patient
information, sample collection information, and/or unique identifier to
another software
application is described in relation to FIG. 7. In some implementations, the
information is
not transferred to another software application.
In some implementations, if the patient information, sample collection
information,
and/or unique identifier are not stored locally and/or exported to another
software application,
the processor may instruct the user to transfer the patient information,
sample collection
information, and/or unique identifier to the biological sample card (412). The
process of
transferring patient information to the biological sample card is described
above in relation to
FIG. 3. In some implementations, the processor may instruct the user to
transfer the
information to the biological sample card after the information is stored
locally and/or
exported to another software application. In some implementations, the patient
information,
sample collection information, and/or unique identifier may be transferred to
the biological
sample card before or after the information is stored locally or transferred
to another software
application.
In some implementations, if the computing device enters a sleep mode or the
application isn't used for a configurable period of time, the user may be
required to log in
again. If the configurable period of time has not elapsed, the processor may
display the data
entry screen and allow the user to enter another specimen after the data from
the earlier
specimen is validated.
FIG. 5 illustrates a flow chart describing an example method 500 for localized
transfer
of data to/from a biological sample card. The method 500, for example, may be
performed
by the processor of a computing device such as computing device 202 described
in relation to
38
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
FIG. 2. For example, one or more steps of method 500 may be performed by
software
application 208.
In some implementations, once data is received, the data is transferred onto
the
biological sample card. In some implementations, the data is transferred when
the biological
sample card is tapped against the computing device equipped with a short range
communication device. The data may include the data received by the computing
device
about a patient. In some implementations, the computing device receives
patient information,
sample collection information, and/or unique identifier by scanning an item
associated with
the patient, such as a barcode or RFID tag incorporated in a wristband worn by
the patient or
a tag located near the patient (e.g. a tag on a medical chart or hospital
bed).
The method of transferring data onto the biological sample card may begin, in
some
implementations, when the processor detects the availability of a transmission
and storage
device for communication (502). In some implementations, the user brings the
biological
sample card within a threshold distance of a computing device. In some
implementations, the
user requests pairing of the biological sample card with the computing device
through a
graphical user interface of a software application executing upon the
computing device. The
computing device may use any type of short range communication technique for
communicating with the biological sample card transmission and storage device
such as, in
some examples, RFID or other NFC protocols.
In some implementations, once the biological sample card is detected, the
processor
prompts a user via a graphical user interface whether they want to read or
write to the
biological sample card (504). If the user selects a read operation, the
processor will read the
biological sample card and optionally display patient information, sample
collection
information, and/or unique identifier to the user via a display screen (506).
In some
implementations, the display screen is the data entry screen and/or a
confirmation screen. In
39
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
some implementations, the processor secures or blocks sensitive patient
information such as
the mother's social security number and displays only enough patient
information to verify
that the patient information is for the correct patient. If the user selects
the write option, the
processor moves to step (508).
In some implementations, the processor may automatically determine whether the
user wants to read or write based on a number of factors including the
location of the data
transfer, such as whether it is at a hospital or a lab, and whether the
biological sample card
has data already loaded. If the biological sample card has no data loaded, the
processor could
automatically determine that this is a write operation. If the biological
sample card is already
loaded with data, the processor could automatically determine that this is a
read operation.
The software may be configured to only read or only write depending on the
use, such as
whether the software is being used by a hospital or testing laboratory.
If the operation is a write operation, the processor transfers the information
from a
computing device to the biological sample card (508). After writing the
information, the
processor displays the home screen (510).
In some implementations, after writing the information, the processor performs
a
validation procedure. The validation procedure verifies that the information
was written
properly. For example, if the operation is a write operation, the processor
can read at least a
portion of the information from the card and compare this information with the
information it
wrote to the card.
FIG. 6 illustrates a flow chart describing an example method 600 for export of
data to
and from a remote application. The method 600, for example, may be performed
by the
processor of a computing device such as computing device 202 described in
relation to FIG.
2. For example, one or more steps of method 600 may be performed by software
application
208.
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In some implementations, the processor sends the patient information, sample
collection information, and/or unique identifier to a remote software
application located
locally or on a network. Examples of such a software program include Specimen
Gate ,
eReports, or LabworksTM Laboratory Information Management System, all by
PerkinElmer
of Waltham, Massachusetts. In some implementations, patient information,
sample collection
information, and/or unique identifier are accessible by another software
application from a
number of locations. Additionally, the status of the biological sample card
may be monitored
so that the medical facility or user of another software application can
receive status updates
from a testing laboratory that receives the biological sample card and
associated biological
samples. Status updates can include whether the sample was received, whether
the sample is
abnormal, what tests are being performed, and the tests results. In some
implementations, a
hospital monitors when a sample is collected.
In some implementations, the process of exporting data to the remote
application
requires a request by a user. In some implementations, this process operates
autonomously
and no user input is required to start this process.
In some implementations, the processor determines whether a set of export
criteria are
met (602). The criteria may be configurable and can include, but is not
limited to, an
expected period of time or a certain amount of data or patients. For example,
the processor
may export data to the remote application every 24 hours, at the end of the
day, or after
receiving data for a certain number of patients, such as ten patients.
In some implementations, once the triggering event has been satisfied, the
processor
verifies that there is patient data to be exported (604). For example, if the
criteria is set to
export data every 24 hours, the processor verifies that there is data to
export (604). If there is
no data, no export will occur. If data exists, the processor exports data from
the local
computing device to the remote application (606). The export operations may be
specific to
41
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
the applications involved. For instance, a transfer to eReportsTM, by
PerkinElmer of
Waltham, Massachusetts, may involve a secure web connection and a web service.
Other
possible scenarios would involve transfers to LabworksTM Laboratory
Information
Management System, by PerkinElmer of Waltham, Massachusetts. Using the remote
application, the status of the biological sample card may be monitored so that
the hospital or
user of the other software application can receive status updates from a
testing laboratory that
receives the biological sample card and associated biological samples. Status
updates can
include whether the sample was received, whether the sample is abnormal, what
tests are
being performed, and the tests results. In some implementations, the transfer
or export of
data involves a hard wire data transfer/export or a wireless transfer/export.
FIG. 7 illustrates a flow chart describing an example method 700 for
transferring of
data to a testing laboratory. The method 700, for example, may be performed by
the
processor of a computing device such as computing device 202 described in
relation to FIG.
2. For example, one or more steps of method 700 may be performed by software
application
208.
In some implementations, after data, such as patient information, is loaded
onto the
biological sample card and the biological sample is taken, the sample and
biological sample
card are sent to a testing laboratory. The testing laboratory could be a
biological sample
testing lab. After the biological sample card and biological sample arrive at
the testing
laboratory (702), the lab transfers the data on the biological sample card to
a computing
device. In some implementations, before transferring the data on the card to
the computing
device, the envelopes are opened and sorted according to various criteria such
as the type
(initial or repeat) and the quality of the sample.
In some implementations, to transfer data from the biological sample card to
the
computing device, the biological sample card is brought within communication
distance of
42
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
the short range communication device (704). For example, bringing a biological
sample card
within communication distance of the short range communication device may
involve a tap,
bringing the biological sample card near the short range communication device,
or setting the
biological sample card on a platform from which the biological sample card can
be read. The
short range communication device may be a standalone reader or may be built
into the
computing device or biological sample punching device. The short range
communication
device may be located at the computing device or at the biological sample
punching device,
or at both locations. The computing device could be a smart phone, personal
computer,
personal digital assistant, tablet computer, notebook computer, or laptop
computer.
After the biological sample card is tapped against or placed within close
proximity of
the short range communication device, the data containing the patient
information, sample
collection information, and/or unique identifier is imported into the testing
laboratory
computer system (706). At this point, in some implementations, the data may be
imported
into a testing laboratory software program such as Specimen Gate software, by
PerkinElmer
of Waltham, Massachusetts. The import may occur automatically or after
receiving
instruction from a user to begin the import. The import may be a local import,
an import
from a server, or form a local area network.
In some implementations, the patient data is evaluated (708). The evaluation
may
include a comparison of the patient information received from the biological
sample card and
all other previously tested samples to see if the incoming specimen is from an
existing patient
and, if so, whether or not the previous specimen was abnormal or unusable. If
the specimen
is from an existing patient, the processor may obtain data from a server or
other storage
device to make this determination. In some implementations, the name of the
patient may
have changed. In such instances, patient information besides the last name,
such as parental
43
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
information, birth date, etc., may be compared to patient information from the
server or other
storage device.
In some implementations, the type of tests to run against the biological
sample are
determined automatically based on the data received from the biological sample
card. The
processor can make this determination based on demographic information such as
state,
ethnicity, and whether it is a first test or follow-up test.
An application providing instructions to the short range communication device,
for
example, can review data obtained from the biological sample card and provide
instruction on
how to punch the biological sample card based on patient information and/or
sample
collection information stored in the biological sample card transmission and
storage device
on the biological sample card. In some implementations, the application
instructs a user via a
display or submits instructions to the biological sample punching device.
Biological sample
cards may be punched differently depending on the patient information and/or
sample
collection information. Locating the short range communication device near the
biological
sample punching device may reduce any error that occurs between reading the
biological
sample card transmission and storage device in the biological sample card and
punching the
card. When the biological sample is loaded at the biological sample punching
device, the
processor already knows how to punch the biological sample based on the
determination the
processor made regarding the types of tests that need to be run.
After the information is transferred, the testing laboratory can update a
status of the
sample testing to a status identifying that the sample has been received. The
status may also
include whether the sample is abnormal, where the sample is unusable, what
tests are being
performed, whether the test was satisfactory, and the tests results. In some
implementations,
the status information may be loaded into the computing device and associated
with the
relevant patient information and/or sample collection information that was
transferred from
44
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
the biological sample card. Moreover, in some implementations, the sender of
the biological
sample card and biological sample can receive or view the status information
remotely. In
some implementations, if the sample was unsatisfactory, the sender may be
informed via the
status information. The sample may be unsatisfactory, in some examples, if the
volume of
the biological sample is inadequate or the biological sample comes in contact
with certain
substances, such as a lotion or powder.
FIG. 8 illustrates a portion 814 of a biological sample card that includes
both valid
and invalid blood samples. In some implementations, the portion 814 includes a
biological
sample card transmission and storage device 812.
Sometimes during blood collection, blood volumes vary between blood samples.
This
problem occurs, for example, when the filter paper of portion 814 is removed
too quickly or
if the filter paper comes in contact, either before or after collection, with
ungloved hands or
substances such as a lotion or powder. As shown in FIG. 8, blood spot samples
802, 804, and
806 are large and therefore considered valid. However, blood spot samples 808
and 810 are
small and unusable. In the case of unusual blood spot samples, additional
blood spot samples
may need to be taken using a new biological sample card. In some
implementations, the
medical facility may monitor and receive status updates from the testing
laboratory.
Additionally, errors related to manually entering patient information from a
handwritten card
may be eliminated and thus reduce delays associated with manual labor. In this
specific
example, in some implementations, the testing laboratory may notify the
hospital via their
software system. Moreover, the testing laboratory is able to process
biological sample cards
faster and reduce the inefficiencies associated with current technology.
FIGS. 9A through 9D illustrate various implementations of user interfaces for
configuring and using a software application for localized data transfer. As
shown in FIG.
9A, in some implementations, a first screen shot 900 of a software application
executing on a
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
computing device 902 includes an application logo 904 and a series of main
menu controls
906 within a display region 908. A user installs the software application, in
some
implementations, to transfer data to and/or from a biological sample card
within a specified
range of the computing device 902. In some implementations, the software
application
correlates patient information and/or sample collection information with a
unique identifier of
a machine-readable indicia scanned by computing device 902 from the biological
sample
card. In some implementations, a unique identifier may be assigned and
associated with
patient information and/or sample collection information by the user of the
software, by the
software program automatically, or by a data center after the patient
information and/or
sample collection information is uploaded to the data center. As described
below, the
controls provided to a user may vary based on whether the software is being
used by hospital
staff, doctor's office staff, midwives, or testing laboratory.
A first control 906a labeled "Transfer Data To Biological Sample Card" and a
second
control 906b labeled "Transfer Data From Biological Sample Card", in some
implementations, provide a user, upon selection, with the ability to transfer
data between the
biological sample card equipped with a biological sample card transmission and
storage
device and the computing device 902 equipped with a short range communication
device. In
some implementations, to transfer data between a biological sample card and
the computing
device 902, the biological sample card may be brought in close proximity with
the computing
device 902. For example, device 902 may be a smartphone or a tablet computer
that a health
professional uses to, among other things, transfer data to a biological sample
card. In such
implementations, the software may include controls 906a, 906c, and 906d. After
inputting
information from the patient (see discussion regarding FIG. 9D), the health
professional can
select control 906a to transfer the data to the patient's biological sample
card. Accordingly,
the patient information, sample collection information, and/or unique
identifier are stored
46
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
electronically on the biological sample card and the disclosed technology
thereby reduces
errors associated with handwritten patient information on biological sample
cards.
In some implementations, upon transmission of the patient information, sample
collection information, and/or unique identifier on the biological sample card
to the
computing device 902, the computing device 902 provides the user with an
indication of
success. Such alert an mechanism may include, in some examples, audio,
graphic, and/or
tactile feedback.
In some implementations, such as at a screening lab, the software does not
include the
control 906a for transferring data to the biological sample card. Screening
laboratories may
only need to read information from biological sample cards. When the
biological sample
card is received by the screening lab, the testing laboratory may use the
software to transfer
data from the biological sample card, thus eliminating errors associated with
handwritten
patient information. Furthermore, the use of biological sample card
transmission and storage
devices can reduce manual labor at screening laboratories and thereby may
reduce the
amount of time it takes for biological test results to be sent to the
hospital.
In some implementations, a third menu control 906c labeled "Patient
Information
Menu", as shown in FIG. 9A, provides the user, upon selection, with a menu
within the
display region 908 where the user may input or review information associated
with a patient.
Information may be entered using the patient information menu and the
information may be
transferred to a biological sample card via the "Transfer Data To Biological
Sample Card"
control 906a. If data is transferred to the device 902 from a biological
sample card using the
"Transfer Data From Biological Sample Card" control 906b, the data may be
viewed by
accessing the "Patient Information Menu" 906c. Additionally, the "Patient
Information
Menu" 906c may be used to edit this information if there are mistakes or to
input the status of
47
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
any tests being performed on the biological samples associated with the
relevant biological
sample card.
Selection of the "Log Out" control 906d, in some implementations, results in
exiting
the software application.
Turning to FIG. 9B, a screen shot 920 of a software application executing on
the
computing device 902, in some implementations, is presented to a user upon
selection of the
"Transfer Data To Biological Sample Card" menu control 906a or "Transfer Data
From
Biological Sample Card" menu control 906b (as shown in FIG. 9A). The screen
shot 920, in
some implementations, includes a message 922 directing the user to "Press
Button Below to
Transfer Data with this Device." In some implementations, if data is being
transferred to a
biological sample card from the device 902, the data may be displayed on the
display region
908 prior to transfer. Beneath the message 922, in some implementations, a
button-style
touch screen control 924 is presented. In some implementations, upon selection
of the touch
screen control 924, the computing device 902 provides an indication to the
user that transfer
is underway. In some examples, the indication may include audio (e.g., "white
noise",
"elevator music", an audible hum, an egg timer tick, or other consistent
audible feedback
indicating a process is taking place, etc.), graphic (e.g., brightness
variation, change of
screen appearance, change of control appearance, textual message indicating a
process is
taking place, etc.), or tactile (e.g., pulsed or constant vibration, etc.)
feedback to the user from
the computing device 902.
If data transfer between the computing device 902 and the biological sample
card is
successful, the computing device 902, in some implementations, provides an
audio (e.g.,
verbal message, fanfare, one or more chirps, beeps, or other tones, etc.),
graphic (e.g.,
brightness variation, change of screen appearance, change of control
appearance, textual
48
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
message indicating success, image, visual cue, etc.), and/or tactile (e.g.,
pulsed or constant
vibration, etc.) indication of success.
Turning to FIG. 9C, as illustrated in a screen shot 940, a patient information
menu
942 is displayed when the user selects the patient information menu 906c (FIG.
9A). In some
implementations, the patient information menu 942 includes a "View Patient
Information"
control 944, an "Input Patient Information" control 946, an "Input Test Status
and/or Results"
control 948, and a "Log Out" control 950. Selection of the "View Patient
Information"
control 944, in some implementations, provides the user with an interface for
entering or
viewing descriptive information about the patient associated with the relevant
biological
sample card.
The "Input Test Status and/or Results" control 948, upon selection, may
provide the
user with an interface for entering or viewing descriptive information about
the status of a
newborn screening test. The status may include whether the sample was
received, whether
the sample is abnormal, whether the sample is usable, what tests are being
performed, and the
tests results. The hospital may monitor the status of a biological sample card
and related
testing using software application 208 described above in relation to FIG. 2.
The "Log Out" control 950 within the settings menu 942, in some
implementations,
provides the user with a mechanism, upon selection, to log out of the
application.
Turning to FIG. 9D, a screen shot 960 of a software application executing on
the
computing device 902, in some implementations, is presented to a user upon
selection of the
"Input Patient Information" menu control 946 (as described in relation to FIG.
9C). Selection
of the "Input Patient Information" control 946, in some implementations,
provides the user
with a graphical user interface 962 along with a number of data entry regions
for entering or
viewing the patient information described above. The data entry regions may
include one or
more of any number of the following: a window (e.g., collapsible panel,
accordion, modal
49
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
window, dialog box, palette window, inspector window, utility window, or
frame), a text box,
a button, a drop-down list, a list box, a combo box, a check box, a radio
button, a cycle
button, a spinner, a menu (e.g., context menu, pie menu, ribbon), a menu bar,
a tab, a toggle
switch, and/or a scroll bar.
Hospitals, midwives, or other medical personnel may input patient information
into
this interface directly without having to handwrite the information on the
biological sample
card or read handwritten information from the biological sample card. Thus,
errors caused by
misreading handwritten patient information are reduced.
As shown in FIG. 10, in some implementations, a cloud computing environment
1000
is provided for sharing information related to biological sample testing.
In some implementations, a computing device 1002 is used to collect
information
relating to a biological sample. In some implementations, the computing device
1002 is a
smart phone, handheld multimedia entertainment device, personal computer,
personal digital
assistant, tablet computer, notebook computer, or laptop computer.
The information may include patient data 1006a, a unique identifier 1010a,
and/or
sample collection data 1010a. The information may be obtained from scanning a
machine-
readable indicia, manually entering information into the computing device, or
scanning a
biological sample card 1014 equipped with a biological sample card
transmission and storage
device as described in reference to FIG. 1. The various methods of inputting
information into
computing device 102 are described in relation to FIG. 1 above. As described
in relation to
FIG. 1 above, after loading data, such as patient data 1006a, into computing
device 1002, the
computing device may transfer the data to the biological sample card 1014. The
data
transferred to the biological sample card 1014 may include the patient data
1006a, sample
collection data 1010a, and/or the unique identifier 1012a.
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In some implementations, the unique identifier 1012a is marked on the
biological
sample card 1014 in a machine-readable indicia 1016. In some implementations,
the unique
identifier 1012a is stored on a biological sample card transmission and
storage device as
described in relation to FIG. 1. After collecting the data (e.g. 1006a, 1010a)
related to the
biological sample card 1014, the biological sample card 1014 can be sent from
a sample
collection location to a testing laboratory. The biological sample card 1014
may contain a
perforation as described in relation to FIG. 1 (110). The biological sample
card 1014 may be
split along the perforation into two portions. In some implementations, both
portions of the
biological sample card 1014 includes the machine-readable indicia 1016. The
perforation
may split the machine-readable indicia 1016 in half or the machine-readable
indicia 1016
may be provided on each portion of the biological sample card 1014 such that
when the
biological sample card 1014 is split along the perforation, each portion
contains a copy of the
machine-readable indicia 1016 [e.g. 1016a, 1016b1.
In some implementations, the computing device 1002 transfers the patient data
1006a,
sample collection data 1010a, and/or unique identifier to a data center 1004
via a network
1008. The network may be compressed, encrypted, and/or utilize secure
connections. The
data center 1004 may provide a security system for requesting, sending and/or
accessing data
from the data center 1004 such as requiring a user to log into the data center
or be
authenticated.
In some implementations, the computing device 1002 transfers the data to a
computing device located at a hospital or other medical facility via the
network 1008 and
then the computing device located at the hospital or other medical facility
can maintain the
data and/or transfer the data to the data center 1004. The computing device
1002 may
transfer the data to the data center 1104 directly via the network 1008.
51
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
The data center 1004 may be located at the hospital requesting the biological
sample
be tested or at a third party location. The data center 1004 receives, via
network 1008, and
stores patient data 1006 results data 1008, sample collection data 1010,
and/or status
information. The various data including patient data 1006, sample collection
data 1010,
and/or the status information, may be associated using a set of unique
identifiers. (e.g. per
patient, per sample, a barcode). In some implementations, the unique
identifier is a machine-
readable indicia such as a barcode identification. Each type of data may be
stored in a central
database or in its own database. In a particular example, the unique
identifier may be
assigned to a blood sample collected from a newborn named John Doe. The data
center 1004
receives the data and stores the patient data 1006a and sample collection data
1010a with the
unique identifier 1012a. If the data center 1004 receives a request for
information related to
the unique identifier 1012a, the data center 1004 can retrieve the patient
data 1006a and
sample collection data 1010a associated with that unique identifier 1012 as
described below.
The patient information records 1006 relate to information about patient from
whom
biological samples have been collected, such as demographic information, as
described in
relation to FIG. 1. Patient information may include: patient identification
number, patient
first name, patient last name, patient address, patient birth date, patient
birth time, patient
birth weight, patient gender, patient ethnic code, repeat sample, the
patient's parental
information, or whether the patient has had a blood transfusion, received
steroids, received
antibiotics, whether the patient was born prematurely, whether the baby is
sick, how the baby
is feeding, whether the parents provided consent to testing, and/or whether
the parents
consent to using the specimen for research.
The results information records 1008 include the results from testing the
biological
samples. The unique identifiers 1012 are identifiers used to link patient data
records 1006,
results records 1008, and sample data records 1010 [e.g. linking information
provided to the
52
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
data center 1004 by computing device 1002 with results records 1008 provided
to the data
center 1004 by the screening lab] as described below.
The sample collection data 1010a may include a date stamp, time stamp,
information
regarding the device used to collect information, information about the
submitter of patient
information such as a name and/or unique employee identifier, and/or location
information
relating to when and where the biological sample was collected. In some
implementations,
the sample collection information 1010a includes information regarding the
testing laboratory
such as email information, phone number information, fax information, other
contact
information, and/or a user ID for logging into the data center 1004, a web
portal, or a
software application to access relevant data for testing. In some
implementations, the sample
collection information 1010a includes contact information for the entity who
submitted the
biological sample for testing, such as a hospital or other medical facility.
The contact
information may include one or more phone numbers, email addresses, and/or fax
numbers
for contacting the relevant party or parties at the entity who submitted the
biological sample
for testing. The contact information may be for a primary care physician,
hospital, and/or
midwife.
In some implementations, the sample collection information 1010a includes
healthcare facility information such as a name of a healthcare professional, a
telephone
number, an address, a hospital name, a testing laboratory name, and/or a
testing laboratory
telephone number.
The status information may include information provided by a testing
laboratory to
the data center 1004 related to the status of tests for a given biological
sample. Status
information may include whether the sample was received, whether the sample is
usable,
whether the testing is ongoing, whether the testing is complete, whether
additional samples
are required, whether the sample is abnormal, an indication of prior abnormal
results, the
53
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
availability of screening results, what tests are being performed, the tests
results, the location
of the sample, and follow-up activities related to the patient's diagnosis.
In some implementations, the data center 1004 identifies sample data 1010b
related to
previous tests 1008a for a given patient based on a portion of the patient
data 1006a. The
data center 1004 may associate the previous test information 1008a with the
sample
collection data 1010a. In some implementations, the data center 1004 may
associate the
previous test information 1008a with the unique identifier 1012a for the given
patients
current biological sample.
In some implementations, the data center 1004 receives, via the network 1008,
a
request for data. The request may comprise the unique identifier 1012a. In
response to the
request, the data center 1004 may provide at least a portion of the patient
data 1006a and the
sample collection data 1010a associated with the unique identifier 1012a
provided in the
request. In some implementations, the request is sent by a testing laboratory
computing
device 1018. The data center 1004 sends the testing laboratory computing
device 1018 at
least a portion of the patient data 1006a and the sample collection data 1010a
associated with
the unique identifier 1012a provided by the testing laboratory computing
device 1018. In
some implementations, the data center 1004 includes the previous test
information 1008a for
the patient in the data 1006a it sends to the testing laboratory computing
device 1018 in
response to the request. The previous test information 1008a may be used by
the testing
laboratory to identify previous abnormal test results for the patient and/or
determine what
tests need to be run on the current biological sample.
The data center 1004 may include settings that can be set by the data center
1004,
screening lab, hospital, or party requesting the sample be tested. The
settings may include
what type of information is shared with screening laboratories. The
information shared may
54
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
be limited to information necessary to conduct the tests and sensitive
information may be
withheld from the testing laboratory for privacy reasons.
In some implementations, the testing laboratory receives inventory information
from
the hospital or data center 1004 via the network 1008. The inventory
information may be
provided to the testing laboratory before the testing laboratory receives the
biological samples
listed in the inventory information. The inventory information may be used by
the testing
laboratory to verify that the correct samples have been received by the
testing laboratory. In
some implementations, intelligent shelving may be used as described in
relation to FIG. 1.
The inventory information may be sent to the testing laboratory via an email,
a messaging
center, a text message, a letter, or a web portal.
In some implementations, the data center 1004 receives an alert from the
testing
laboratory. The alert may relate to status information the testing laboratory
provides the data
center 1004. Upon receipt of the alert, the data center 1004 may provide a
message to the
contact information associated with the relevant unique identifier (e.g.
derived from patient
information 1006a or sample data 1010a). In some implementations, the message
is a text
message, email, a prerecorded voicemail message, a phone call, a fax, or other
means of
communicating the alert to the contacts. For example, a hospital may receive
an alert from
the data center 1004 after the data center 1004 receives status information
from the testing
laboratory. The status information may be the time when the testing laboratory
received the
sample. The hospital may use this information to see if there are
opportunities to improve the
amount of time it takes for a sample to reach the testing laboratory.
In some implementations, the data center 1004 receives screening results 1008a
from
the testing laboratory. The data center 1004 may associate the screening
results 1008a with
the relevant unique identifier 1012a. The unique identifier 1012a
corresponding to the
screening results 1008a may be sent with the screening results 1008a by the
testing
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
laboratory. The data center 1004 can store the screening results 1008a with
the other data
associated with the unique identifier 1012a. In some implementations, the data
center 1004
alerts the hospital or party that submitted the biological sample for testing.
The alert may
notify the submitting party that the test results have been submitted to the
data center 1004 or
may send the test results to the contact information of the submitting party.
FIG. 11 illustrates a flow chart describing an example method 1100 for
collecting and
transmitting information related to a biological sample to a data center.
As described above, a hospital or other medical facility may need to submit a
biological sample to a testing laboratory for testing. Prior to submission of
the sample to the
screening lab, in some implementations, the submitting party may scan, using a
scanner of a
computing device, a machine-readable indicia (1102). The machine-readable
indicia may
include a barcode, two-dimensional barcode, three-dimensional barcode, QR
code, and/or
matrix barcode. In some implementations, the machine-readable indicia is
presented on a
biological sample card for stably storing the biological sample collected from
a patient. In
some implementations the machine-readable indicia includes a unique
identifier, data related
to a medical facility (e.g. facility name, treating physician, location,
contact information), a
type of biological sample (e.g. newborn blood card, biological tissue card,
biological genetics
card, or a blood/urine sample), and/or sample collection data.
In some implementations, patient data is obtained (1104). The patient
information
may be associated with the unique identifier read from the machine-readable
indicia. Patient
information may include: patient identification number, patient first name,
patient last name,
patient address, patient birth date, patient birth time, patient birth weight,
patient gender,
patient ethnic code, repeat sample, the patient's parental information, or
whether the patient
has had a blood transfusion, received steroids, received antibiotics, whether
the patient was
born prematurely, whether the baby is sick, how the baby is feeding, whether
the parents
56
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
provided consent to testing, and/or whether the parents consent to using the
specimen for
research.
In some implementations, the computing device determines sample collection
data
(1106). The sample collection data may include a date stamp, time stamp,
information
regarding the device used to collect information, information about the
submitter of patient
information such as a name and/or unique employee identifier, and/or location
information
relating to when and where the biological sample was collected. In some
implementations,
the sample collection information includes information regarding the testing
laboratory such
as email information, phone number information, fax information, other contact
information,
and/or a user ID for logging into the data center, a web portal, or a software
application to
access relevant data for testing. In some implementations, the sample
collection information
includes contact information for the entity who submitted the biological
sample for testing,
such as a hospital or other medical facility. The contact information may
include one or more
phone numbers, email addresses, and/or fax numbers for contacting the relevant
party or
parties at the entity who submitted the biological sample for testing. The
contact information
may be for a primary care physician, hospital, and/or midwife.
In some implementations, the sample collection information includes healthcare
facility information such as a name of a healthcare professional, a telephone
number, an
address, a hospital name, a testing laboratory name, and/or a testing
laboratory telephone
number.
In some implementations, the computing device provides the patient data, the
sample
collection data, and the unique identifier to a data center (1108). The data
center may be a
server, server farm, database system, cloud based storage system, or remote
computer. As
described above in reference to FIG. 10, the unique identifier can be
associated with the other
data collected and submitted to the data center so that any party with the
unique identifier
57
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
and, in some implementations, permission, can obtain a portion of the
information associated
with the unique identifier.
FIG. 12 illustrates a flow chart describing an example method 1200 for
receiving and
transmitting information related to a biological sample.
In some implementations, patient data, sample collection data, and a unique
identifier
are received from a computing device (1202). A data center, for example, can
receive and
store the unique identifier and associate the unique identifier with the other
data received by
the data center so that any party with the unique identifier and, in some
implementations,
permission, can obtain a portion of the information associated with the unique
identifier. The
computing device may be a computing device located at a hospital or other
medical facility
that is submitting the biological sample for testing. The computing device
located at the
hospital or other medical facility may be a smart phone, handheld multimedia
entertainment
device, personal computer, personal digital assistant, tablet computer,
notebook computer, or
laptop computer.
After receiving the data, in some implementations, a request for the data is
received
(1204). The request may be from a computing device of a testing laboratory.
The computing
device of the testing laboratory may be a smart phone, handheld multimedia
entertainment
device, personal computer, personal digital assistant, tablet computer,
notebook computer, or
laptop computer. The request may include the unique identifier for the data
the testing
laboratory wants to receive. In some implementations, the data center
validates the
requesting party is authorized to request data associated with the provided
unique identifier
prior to providing the data to the requesting party.
After receiving a request, in some implementations, the data associated with
the
provided unique identifier is provided to the requesting party such as a
testing laboratory
58
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
(1206). The testing laboratory may use this data to determine what tests need
to be done as
described in relation to FIG. 11.
FIG. 13 illustrates a flow chart describing an example method 1300 for
receiving and
transmitting information related to a biological sample.
In some implementations, patient data, sample collection data, and a unique
identifier
are received from a computing device (1302). After receiving the patient data,
sample
collection data, and/or unique identifier, in some implementations, the data
is stored (1304).
The unique identifier can be associated with the other data received by the
data center so that
any party with the unique identifier and, in some implementations, permission,
can obtain a
portion of the information associated with the unique identifier. The
computing device may
be a computing device located at a hospital or other medical facility that is
submitting the
biological sample for testing. The computing device located at the hospital or
other medical
facility may be a smart phone, handheld multimedia entertainment device,
personal computer,
personal digital assistant, tablet computer, notebook computer, or laptop
computer.
In some implementations, previous test information for a patient is identified
(1306).
The data center may identify previous test information based on part of the
patient data
and/or sample collection data received with a unique identifier. The previous
test information
may be used by a testing laboratory to identify previous abnormal test results
for the patient
and/or determine what tests need to be run on the current biological sample.
In some implementations, the previous test information is associated with the
unique
identifier provided with the patient date and sample collection data (1308)
received in step
1302. Accordingly, when a testing laboratory requests information related to a
unique
identifier, the past test information may be sent to the testing laboratory.
In some implementations, a request for the data is recorded (1310). The
request may
be from a computing device of a testing laboratory. The computing device of
the testing
59
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
laboratory may be a smart phone, handheld multimedia entertainment device,
personal
computer, personal digital assistant, tablet computer, notebook computer, or
laptop computer.
The request may include the unique identifier for the data the testing
laboratory wants to
receive. In some implementations, the data center validates the requesting
party is authorized
to request data associated with the provided unique identifier prior to
providing the data to
the requesting party.
After receiving a request, in some implementations, the data associated with
the
provided unique identifier is provided to the requesting party such as a
testing laboratory
(1312). The testing laboratory may use this data to determine what tests need
to be
conducted.
FIG. 14 illustrates a chart describing a system and method 1400 for
communicating
data regarding a biological sample to and from a data center.
In some implementations, a collection device 1402 receives patient data,
sample
collection data, and/or a unique identifier as explained in reference to FIG.
1. In some
implementations, the collection device 1402 generates the sample collection
data itself or
using a software application. The collection device 1402 may be a personal
computer,
laptop, smart phone, handheld multimedia entertainment device, personal
digital assistant, or
tablet computer. The collection device 1402 may transmit a copy of the data
(1412) to a
medical facility 1410 that is requesting a biological sample be tested. The
medical facility
1410 may use the data in a software program such as Specimen Gate ,
eReportsTM, or
LabworksTM Laboratory Information Management System, all by PerkinElmer of
Waltham,
Massachusetts. The software program may be accessed from one or more locations
and may
communicate directly or indirectly with a software program of the testing
laboratory.
In some implementations, the collection device 1402 transmits, via a network,
the
patient data, sample collection data, and/or unique identifier (UID) (1414) to
a data center
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
1404. The data center 1004 receives, via a network, and stores the patient
data, sample
collection data, results data, and/or status information (1416) in a records
database 1406. The
data may be associated using the unique identifier (e.g. a unique identifier
per patient, per
sample, a barcode). The association may be done by the collection device 1402
or the data
center 1404. In some implementations, the unique identifier is a machine-
readable indicia
such as a barcode identification. If the data center 1404 receives a request
for information
related to a specific unique identifier, the data center 1404 can retrieve the
patient data,
sample collection data, and any other data associated with that unique
identifier.
In some implementations, after receiving and storing the data, the data center
1404
may send a status update or alert (1418) to the medical facility 1410 to
notify the medical
facility 1410 of the status of the biological sample testing. The alert or
status update may
notify the medical facility 1410 at the data center has received the patient
date, sample
collection data, and/or unique identifier. The alert or status update may
notify the medical
facility 1410 that a testing laboratory 1408 has received the biological
sample. The data
center 1404 may be notified by the testing laboratory 1408 that the testing
laboratory 1408
has received the biological sample before the data center 1404 sends the alert
to the medical
facility 1410. Alerts and status updates may include a message that is
provided to contact
information associated with the relevant unique identifier (1404). In some
implementations,
the message is a text message, email, a prerecorded voicemail message, a phone
call, a fax, or
other means of communicating the alert to the contacts. The message may be
used to notify a
representative of the party who submitted the biological sample for testing of
the alert.
In some implementations, the testing laboratory 1408 sends a unique identifier
(1420)
to the data center 1404. The unique identifier may be from a biological sample
card the
testing laboratory 1408 received from a medical facility 1410. The unique
identifier may be
stored in a biological sample card transmission and storage device, a machine-
readable
61
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
indicia, or printed on the biological sample card. In response to receipt of
the unique
identifier, the data center 1404 may retrieve records (1422) associated with
that unique
identifier from the records database 1406. After retrieving the records, the
data center 1404
may transmit patient data and/or sample collection data (1424) to the testing
laboratory 1408.
The testing laboratory 1408 may use the data to determine the type of tests to
run against the
biological sample received from the biological sample card. The testing
laboratory 1408 can
make this determination based on demographic information such as state,
ethnicity, and
whether it is a first test or follow-up test.
In some implementations, the data center 1404 updates the status information
(1426)
so that the medical facility 1410 is aware of the status of the biological
sample testing. The
data center 1404 may provide an alert to the medical facility 1410 every time
the status
information is updated. The data center 1404 may set the status (1426) when
the testing
laboratory 1408 receives the patient data and/or sample collection data.
In some implementations, the data center 1404 receives a status alert (1428)
from the
screening center 1408. The status alert may include the status information of
a biological
sample test and the unique identifier of the biological sample for which the
status is being
updated. The status information may include whether the sample was received,
whether the
sample is usable, whether the testing is ongoing, whether the testing is
complete, whether
additional samples are required, whether the sample is abnormal, an indication
of prior
abnormal results, the availability of screening results, what tests are being
performed, the
tests results, the location of the sample, and follow-up activities related to
the patient's
diagnosis.
In some implementations, after receiving a status alert (1428), the data
center 1404
may send a status update or alert (1430) to the medical facility 1410 to
notify the medical
facility 1410 of the status alert (1428).
62
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
In some implementations, the data center 1404 receives test results (1432)
from the
screening center 1408. After receiving the test results, the data center 1404
may update the
records database 1406 to include the test results data and update the status
of the test. The
data center 1404 may send an alert (1434) to the medical facility 1410 to
notify them the test
results have been received by the data center 1404. The data center 1404 may
provide the
medical facility 1410 with the test results automatically or upon request from
the medical
facility 1410. In some implementations, the test results may be accessed by a
member of the
medical facility through a web-based portal.
In some implementations, the collection device 1402 transmits patient data,
second
sample collection data, and a second unique identifier (1436) to the data
center 1404 if a first
test result was unsatisfactory and a new biological sample has been collected
from the same
patient for testing. Test results may be unsatisfactory, in some examples, if
the volume of
the biological sample is inadequate or the biological sample comes in contact
with certain
substances, such as a lotion or powder.
The data center 1004 receives, via a network, and stores the patient data,
sample
collection data, results data, and/or status information (1438) in a records
database 1406. The
data may be associated with the second unique identifier. The association may
be done by
the collection device 1402 or the data center 1404. In some implementations,
the records
database 1406 associates the original unique identifier with the second unique
identifier
(1440). In some implementations, this association is done by the data center
1404. The
original and second unique identifiers are associated so that a party
requesting data associated
with either unique identifier receives the data associated with both unique
identifiers.
In some implementations, a testing laboratory 1408 may send the second unique
identifier (1442) to the data center 1404. The unique identifier may be from a
second
biological sample card for a given patient the testing laboratory 1408
received from a medical
63
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
facility 1410. In response to receipt of the unique identifier, the data
center 1404 may
retrieve records (1444) associated with the second unique identifier and the
first unique
identifier from the records database 1406. After retrieving the records, the
data center 1404
may transmit patient data and/or sample collection data (1446) to the testing
laboratory 1408.
The testing laboratory 1408 may use the data to determine the type of tests to
run against the
biological sample received from the second biological sample card.
Specifically, the testing
laboratory 1408 may use the data pertaining to the first unique identifier,
including whether
and why the first test results were abnormal, to determine the types of tests
to run.
In some implementations, the data center 1404 receives test results (1448)
from the
screening center 1408. After receiving the test results, the data center 1404
may update the
records database 1406 to include the test results data and update the status
of the test. The
data center 1404 may send an alert to the medical facility 1410 to notify them
the test results
have been received by the data center 1404. The data center 1404 may provide
the medical
facility 1410 with the test results automatically or upon request from the
medical facility
1410. In some implementations, the test results may be accessed by a member of
the
medical facility through a web-based portal.
Throughout the method described with reference to FIG. 14, alerts and status
updates
may be provided to and from the collection device 1402 and testing laboratory
1408 at
various steps besides those described above. Alerts and status updates may be
a message that
is provided to contact information associated with the relevant unique
identifier. In some
implementations, the message is a text message, email, a prerecorded voicemail
message, a
phone call, a fax, or other means of communicating the alert to the contacts.
The message
may be used to notify a representative of the party who submitted the
biological sample for
testing of the alert. Status information may include whether the sample was
received,
whether the sample is usable, whether the testing is ongoing, whether the
testing is complete,
64
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
whether additional samples are required, whether the sample is abnormal, an
indication of
prior abnormal results, the availability of screening results, what tests are
being performed,
the tests results, the location of the sample, and follow-up activities
related to the patient's
diagnosis.
As shown in FIG. 15, an implementation of an exemplary cloud computing
environment 1500 for localized transferring data to/from electronically
labeled items is
shown and described. In brief overview, the cloud computing environment 1500
may include
one or more resource providers 1502a, 1502b, 1502c (collectively, 1502). Each
resource
provider 1502 may include computing resources. In some implementations,
computing
resources includes any hardware and/or software used to process data. For
example,
computing resources may include hardware and/or software capable of executing
algorithms,
computer programs, and/or computer applications. In some implementations,
exemplary
computing resources may include application servers and/or databases with
storage and
retrieval capabilities. Each resource provider 1502 may be connected to any
other resource
provider 1502 in the cloud computing environment 1500. In some
implementations, the
resource providers 1502 may be connected over a computer network 1508. Each
resource
provider 1502 may be connected to one or more computing device 1504a, 1504b,
1504c
(collectively, 1504), over the computer network 1508.
The cloud computing environment 1500 may include a resource manager 1506. The
resource manager 1506 may be connected to the resource providers 1502 and the
computing
devices 1504 over the computer network 1508. In some implementations, the
resource
manager 1506 may facilitate the provision of computing resources by one or
more resource
providers 1502 to one or more computing devices 1504. The resource manager
1506 may
receive a request for a computing resource from a particular computing device
1504. The
resource manager 1506 may identify one or more resource providers 1502 capable
of
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
providing the computing resource requested by the computing device 1504. The
resource
manager 1506 may select a resource provider 1502 to provide the computing
resource. The
resource manager 1506 may facilitate a connection between the resource
provider 1502 and a
particular computing device 1504. In some implementations, the resource
manager 1506 may
establish a connection between a particular resource provider 1502 and a
particular
computing device 1504. In some implementations, the resource manager 1506 may
redirect a
particular computing device 1504 to a particular resource provider 1502 with
the requested
computing resource.
FIG. 16 shows an example of a computing device 1600 and a mobile computing
device 1650 that can be used to implement the techniques described in this
disclosure. The
computing device 1600 is intended to represent various forms of digital
computers, such as
laptops, desktops, workstations, personal digital assistants, servers, blade
servers,
mainframes, and other appropriate computers. The mobile computing device 1650
is
intended to represent various forms of mobile devices, such as personal
digital assistants,
cellular telephones, smart-phones, and other similar computing devices. The
components
shown here, their connections and relationships, and their functions, are
meant to be
examples only, and are not meant to be limiting.
The computing device 1600 includes a processor 1602, a memory 1604, a storage
device 1606, a high-speed interface 1608 connecting to the memory 1604 and
multiple high-
speed expansion ports 1610, and a low-speed interface 1612 connecting to a low-
speed
expansion port 1614 and the storage device 1606. Each of the processor 1602,
the memory
1604, the storage device 1606, the high-speed interface 1608, the high-speed
expansion ports
1610, and the low-speed interface 1612, are interconnected using various
busses, and may be
mounted on a common motherboard or in other manners as appropriate. The
processor 1602
can process instructions for execution within the computing device 1600,
including
66
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
instructions stored in the memory 1604 or on the storage device 1606 to
display graphical
information for a GUI on an external input/output device, such as a display
1616 coupled to
the high-speed interface 1608. In some implementations, multiple processors
and/or multiple
buses may be used, as appropriate, along with multiple memories and types of
memory.
Also, multiple computing devices may be connected, with each device providing
portions of
the necessary operations (e.g., as a server bank, a group of blade servers, or
a multi-processor
system).
The memory 1604 stores information within the computing device 1600. In some
implementations, the memory 1604 is a volatile memory unit or units. In some
implementations, the memory 1604 is a non-volatile memory unit or units. The
memory
1604 may also be another form of computer-readable medium, such as a magnetic
or optical
disk.
The storage device 1606 is capable of providing mass storage for the computing
device 1600. In some implementations, the storage device 1606 may be or
contain a
computer-readable medium, such as a floppy disk device, a hard disk device, an
optical disk
device, or a tape device, a flash memory or other similar solid state memory
device, or an
array of devices, including devices in a storage area network or other
configurations.
Instructions can be stored in an information carrier. The instructions, when
executed by one
or more processing devices (for example, processor 1602), perform one or more
methods,
such as those described above. The instructions can also be stored by one or
more storage
devices such as computer- or machine-readable mediums (for example, the memory
1604, the
storage device 1606, or memory on the processor 1602).
The high-speed interface 1608 manages bandwidth-intensive operations for the
computing device 1600, while the low-speed interface 1612 manages lower
bandwidth-
intensive operations. Such allocation of functions is an example only. In some
67
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
implementations, the high-speed interface 1608 is coupled to the memory 1604,
the display
1616 (e.g., through a graphics processor or accelerator), and to the high-
speed expansion
ports 1610, which may accept various expansion cards (not shown). In the
implementation,
the low-speed interface 1612 is coupled to the storage device 1606 and the low-
speed
expansion port 1614. The low-speed expansion port 1614, which may include
various
communication ports (e.g., USB, Bluetooth, Ethernet, wireless Ethernet) may be
coupled to
one or more input/output devices, such as a keyboard, a pointing device, a
scanner, or a
networking device such as a switch or router, e.g., through a network adapter.
The computing device 1600 may be implemented in a number of different forms.
For
example, it may be implemented as a standard server 1620, or multiple times in
a group of
such servers. In addition, it may be implemented in a personal computer such
as a laptop
computer 1622. It may also be implemented as part of a rack server system
1624.
Alternatively, components from the computing device 1600 may be combined with
other
components in a mobile device (not shown), such as a mobile computing device
1650. Each
of such devices may contain one or more of the computing device 1600 and the
mobile
computing device 1650, and an entire system may be made up of multiple
computing devices
communicating with each other.
The mobile computing device 1650 includes a processor 1652, a memory 1664, an
input/output device such as a display 1654, a communication interface 1666,
and a
transceiver 1668, among other components. The mobile computing device 1650 may
also be
provided with a storage device, such as a micro-drive or other device, to
provide additional
storage. Each of the processor 1652, the memory 1664, the display 1654, the
communication
interface 1666, and the transceiver 1668, are interconnected using various
buses, and several
of the components may be mounted on a common motherboard or in other manners
as
appropriate.
68
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
The processor 1652 can execute instructions within the mobile computing device
1650, including instructions stored in the memory 1664. The processor 1652 may
be
implemented as a chipset of chips that include separate and multiple analog
and digital
processors. The processor 1652 may provide, for example, for coordination of
the other
components of the mobile computing device 1650, such as control of user
interfaces,
applications run by the mobile computing device 1650, and wireless
communication by the
mobile computing device 1650.
The processor 1652 may communicate with a user through a control interface
1658
and a display interface 1656 coupled to the display 1654. The display 1654 may
be, for
example, a TFT (Thin-Film-Transistor Liquid Crystal Display) display or an
OLED (Organic
Light Emitting Diode) display, or other appropriate display technology. The
display interface
1656 may include appropriate circuitry for driving the display 1654 to present
graphical and
other information to a user. The control interface 1658 may receive commands
from a user
and convert them for submission to the processor 1652. In addition, an
external interface
1662 may provide communication with the processor 1652, so as to enable near
area
communication of the mobile computing device 1650 with other devices. The
external
interface 1662 may provide, for example, for wired communication in some
implementations,
or for wireless communication in some implementations, and multiple interfaces
may also be
used.
The memory 1664 stores information within the mobile computing device 1650.
The
memory 1664 can be implemented as one or more of a computer-readable medium or
media,
a volatile memory unit or units, or a non-volatile memory unit or units. An
expansion
memory 1674 may also be provided and connected to the mobile computing device
1650
through an expansion interface 1672, which may include, for example, a SIMM
(Single In
Line Memory Module) card interface. The expansion memory 1674 may provide
extra
69
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
storage space for the mobile computing device 1650, or may also store
applications or other
information for the mobile computing device 1650. Specifically, the expansion
memory
1674 may include instructions to carry out or supplement the processes
described above, and
may include secure information also. Thus, for example, the expansion memory
1674 may
be provide as a security module for the mobile computing device 1650, and may
be
programmed with instructions that permit secure use of the mobile computing
device 1650.
In addition, secure applications may be provided via the SIMM cards, along
with additional
information, such as placing identifying information on the SIMM card in a non-
hackable
manner.
The memory may include, for example, flash memory and/or NVRAM memory (non-
volatile random access memory), as discussed below. In some implementations,
instructions
are stored in an information carrier. that the instructions, when executed by
one or more
processing devices (for example, processor 1652), perform one or more methods,
such as
those described above. The instructions can also be stored by one or more
storage devices,
such as one or more computer- or machine-readable mediums (for example, the
memory
1664, the expansion memory 1674, or memory on the processor 1652). In some
implementations, the instructions can be received in a propagated signal, for
example, over
the transceiver 1668 or the external interface 1662.
The mobile computing device 1650 may communicate wirelessly through the
communication interface 1666, which may include digital signal processing
circuitry where
necessary. The communication interface 1666 may provide for communications
under
various modes or protocols, such as GSM voice calls (Global System for Mobile
communications), SMS (Short Message Service), EMS (Enhanced Messaging
Service), or
MMS messaging (Multimedia Messaging Service), CDMA (code division multiple
access),
TDMA (time division multiple access), PDC (Personal Digital Cellular), WCDMA
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
(Wideband Code Division Multiple Access), CDMA2000, or GPRS (General Packet
Radio
Service), among others. Such communication may occur, for example, through the
transceiver 1668 using a radio-frequency. In addition, short-range
communication may
occur, such as using a Bluetooth, WiFi, or other such transceiver (not shown).
In addition, a
GPS (Global Positioning System) receiver module 1670 may provide additional
navigation-
and location-related wireless data to the mobile computing device 1650, which
may be used
as appropriate by applications running on the mobile computing device 1650.
The mobile computing device 1650 may also communicate audibly using an audio
codec 1660, which may receive spoken information from a user and convert it to
usable
digital information. The audio codec 1660 may likewise generate audible sound
for a user,
such as through a speaker, e.g., in a handset of the mobile computing device
1650. Such
sound may include sound from voice telephone calls, may include recorded sound
(e.g., voice
messages, music files, etc.) and may also include sound generated by
applications operating
on the mobile computing device 1650.
The mobile computing device 1650 may be implemented in a number of different
forms. For example, it may be implemented as a cellular telephone 1680. It may
also be
implemented as part of a smart-phone 1682, personal digital assistant, or
other similar mobile
device.
Various implementations of the systems and techniques described here can be
realized
in digital electronic circuitry, integrated circuitry, specially designed
ASICs (application
specific integrated circuits), computer hardware, firmware, software, and/or
combinations
thereof. These various implementations can include implementation in one or
more computer
programs that are executable and/or interpretable on a programmable system
including at
least one programmable processor, which may be special or general purpose,
coupled to
71
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
receive data and instructions from, and to transmit data and instructions to,
a storage system,
at least one input device, and at least one output device.
These computer programs (also known as programs, software, software
applications
or code) include machine instructions for a programmable processor, and can be
implemented
in a high-level procedural and/or object-oriented programming language, and/or
in
assembly/machine language. As used herein, the terms machine-readable medium
and
computer-readable medium refer to any computer program product, apparatus
and/or device
(e.g., magnetic discs, optical disks, memory, Programmable Logic Devices
(PLDs)) used to
provide machine instructions and/or data to a programmable processor,
including a machine-
readable medium that receives machine instructions as a machine-readable
signal. The term
machine-readable signal refers to any signal used to provide machine
instructions and/or data
to a programmable processor.
To provide for interaction with a user, the systems and techniques described
here can
be implemented on a computer having a display device (e.g., a CRT (cathode ray
tube) or
LCD (liquid crystal display) monitor) for displaying information to the user
and a keyboard
and a pointing device (e.g., a mouse or a trackball) by which the user can
provide input to the
computer. Other kinds of devices can be used to provide for interaction with a
user as well;
for example, feedback provided to the user can be any form of sensory feedback
(e.g., visual
feedback, auditory feedback, or tactile feedback); and input from the user can
be received in
any form, including acoustic, speech, or tactile input.
The systems and techniques described here can be implemented in a computing
system that includes a back end component (e.g., as a data server), or that
includes a
middleware component (e.g., an application server), or that includes a front
end component
(e.g., a client computer having a graphical user interface or a Web browser
through which a
user can interact with an implementation of the systems and techniques
described here), or
72
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
any combination of such back end, middleware, or front end components. The
components
of the system can be interconnected by any form or medium of digital data
communication
(e.g., a communication network). Examples of communication networks include a
local area
network (LAN), a wide area network (WAN), and the Internet.
The computing system can include clients and servers. A client and server are
generally remote from each other and typically interact through a
communication network.
The relationship of client and server arises by virtue of computer programs
running on the
respective computers and having a client-server relationship to each other.
A biological sample punching device can be used to automatically or semi-
automatically punch one or more spot samples into microtitration plates. Some
biological
sample punching devices include a variety of punch head sizes so that
microtitration plates
requiring different sample spot sizes can be punched simultaneously. The
punching pattern
used by a biological sample punching device, for example, may be programmed
into the
setup routine of the biological sample punching device.
Short range communications may be used to recognize items or retrieve
information
from items including electronic labels or electronic tags. In some examples, a
radio
frequency (RF) signal, Bluetooth , Near Field Communication (NFC), or WiFiTM
based
wireless connection may be established between an electronic tag and a
separate computing
device. Short range communications standards such as, in some examples,
ISO/IEC 14442,
ISO/IEC 18092, or FeliCa may be used in some circumstances to communicate
information
from an electronic tag to the separate computing device.
Machine readable indicia may be used to label items. The machine-readable
indicia
may provide an item with a unique identification number that may be used to
track items and
provide access to information about an item. In some examples, the machine-
readable indicia
73
CA 02882249 2015-02-17
WO 2014/046646
PCT/US2012/055950
may be a barcode, two-dimensional barcode, three-dimensional barcode, QR code,
or matrix
barcode.
In view of the structure, functions and apparatus of the systems and methods
described here, in some implementations, a system for localized communication
with a
biological sample card transmission and storage device is provided. Having
described certain
implementations of methods and systems for pairing a biological sample card
transmission
and storage device with a software application and communicating with an item
having a
biological sample card transmission and storage device, it will now become
apparent to one
of skill in the art that other implementations incorporating the concepts of
the disclosure may
be used. Therefore, the disclosure should not be limited to certain
implementations, but
rather should be limited only by the spirit and scope of the following claims.
74